-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMaternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
The basic head-to-tail polarity of an animal is established very early in development. In dipteran insects (flies, midges, and mosquitoes), polarity is established with the help of so-called morphogen gradients. Morphogens are regulatory proteins that are distributed as a concentration gradient, often involving diffusion from a localised source. This graded distribution then leads to the concentration-dependent activation of different target genes along the embryo’s axis. We examine this process, which differs to a surprising extent between dipteran species, in the scuttle fly Megaselia abdita, and compare our results to the model organism Drosophila melanogaster. In this way, we not only gain insights into how the mechanisms that establish polarity function differently in different species, but also how the system has evolved since these two flies shared a common ancestor. Specifically, we pin down the main difference between Drosophila and Megaselia in the altered function of the maternal Hunchback morphogen gradient, which activates target genes in the former, but not the latter species, where it has been completely replaced by the Bicoid morphogen during evolution.
Published in the journal: . PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005042
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005042Summary
The basic head-to-tail polarity of an animal is established very early in development. In dipteran insects (flies, midges, and mosquitoes), polarity is established with the help of so-called morphogen gradients. Morphogens are regulatory proteins that are distributed as a concentration gradient, often involving diffusion from a localised source. This graded distribution then leads to the concentration-dependent activation of different target genes along the embryo’s axis. We examine this process, which differs to a surprising extent between dipteran species, in the scuttle fly Megaselia abdita, and compare our results to the model organism Drosophila melanogaster. In this way, we not only gain insights into how the mechanisms that establish polarity function differently in different species, but also how the system has evolved since these two flies shared a common ancestor. Specifically, we pin down the main difference between Drosophila and Megaselia in the altered function of the maternal Hunchback morphogen gradient, which activates target genes in the former, but not the latter species, where it has been completely replaced by the Bicoid morphogen during evolution.
Introduction
Axis formation and segment determination in the vinegar fly Drosophila melanogaster are among the most thoroughly studied developmental processes today [1–5]. They offer an ideal starting point for the comparative study of development and the evolution of pattern-forming gene regulatory networks. Axis formation in flies is based on the graded distribution of morphogens established through a number of different maternal regulatory systems. In this study, we will be focusing on two of those in particular: the anterior and posterior systems [4].
In D. melanogaster, maternal protein gradients are either formed by localisation of mRNA at the anterior or posterior pole of the embryo, or by regionally specific translational repression of ubiquitous maternal transcripts [4,5]. The anterior system centres around the anterior determinant Bicoid (Bcd). bcd mRNA is localised to the anterior pole of the embryo and an antero-posterior (A–P) protein gradient forms through diffusion from that source [6–8]. Bcd regulates the translation of uniformly distributed maternal mRNA of caudal (cad) [9,10], which leads to a graded distribution of Cad protein with high concentration levels in the posterior [6,9,11–14]. In addition, Bcd acts as a concentration-dependent transcriptional regulator of zygotically expressed segmentation genes—such as gap or pair-rule genes [6,15–20]. In the case of the posterior maternal system, nanos (nos) mRNA is localised in the posterior pole region forming the source of the Nos protein gradient [21–23]. Unlike Bcd, Nos is not a transcriptional regulator: its only role is to translationally regulate ubiquitous maternal hunchback (hb) mRNA, leading to an anterior gradient of maternal Hb protein [24–26].
Evidence for the presence of localised determinants in dipterans goes back to early studies that utilised UV irradiation or RNAse treatment on embryos of chironomid midges (Fig. 1, Nematocera: Culicomorpha). These experiments produced mirror-duplicated abdomens, so-called bicaudal phenotypes, in which anterior structures are missing and replaced by duplicated organs usually found in the posterior [27–29]. The observed effects were attributed to the destruction of an anteriorly localised mRNA. However, the identity of the anterior determinant is still unknown in the majority of dipteran infraorders. The bcd gene arose through a duplication of the hox3 factor zerknüllt (zen) at the base of the cyclorrhapha (Fig. 1) [30–33]. While its spatial distribution and role as transcriptional regulator are highly conserved among cyclorrhaphans [30–32,34–39], it is not present in other flies.
Fig. 1. Phylogenetic position of M. abdita. 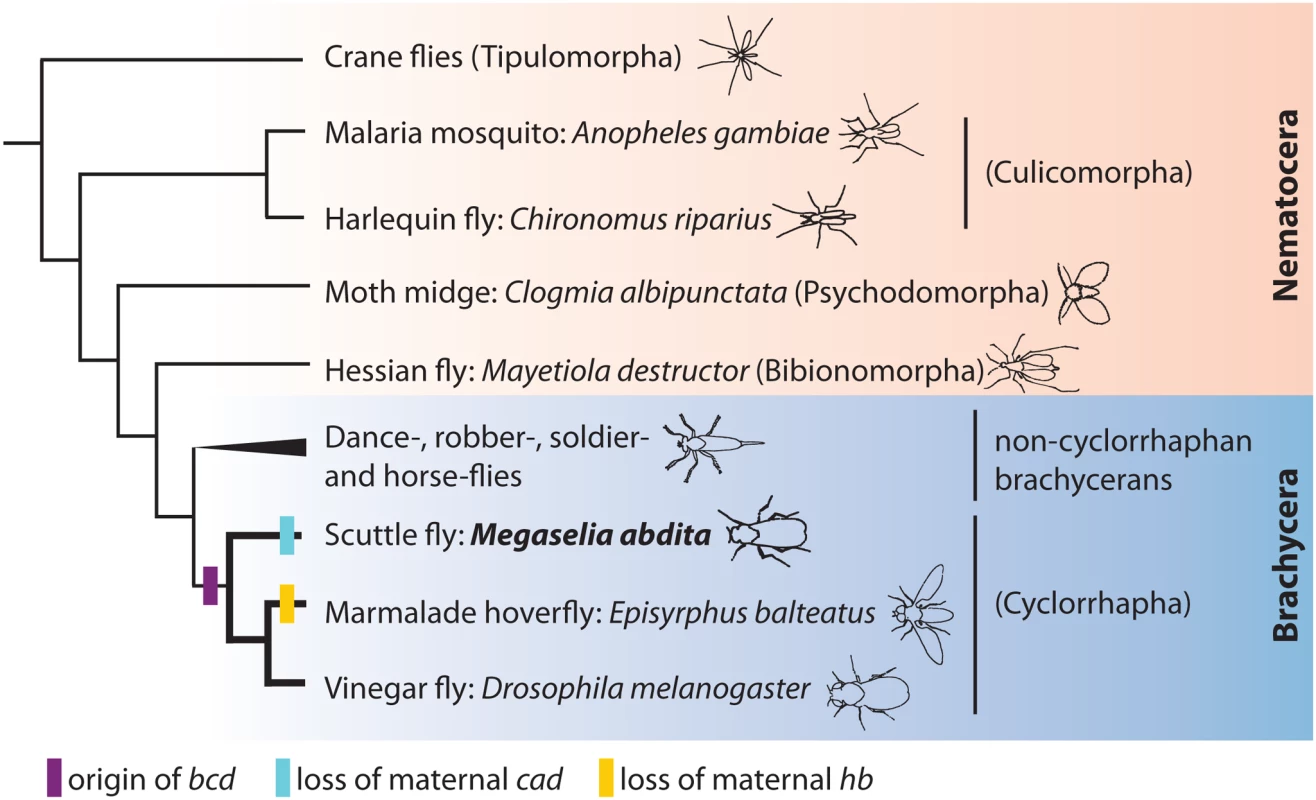
This figure shows a simplified phylogenetic tree of the order Diptera (based on [45,46]), subdivided into the monophyletic suborder Brachycera (blue background) and a paraphyletic assemblage of basally branching lineages, the Nematocera (pink background). Infraorder names are shown in parentheses. Non-cyclorraphan brachycerans include the infraorders Asiloidea, Stratiomyomorpha, Tabanomorpha (robber, soldier, and horse flies), and the likely sister group of the Cyclorrhapha, the Empidoidea (dance flies). Gain and loss of maternal factors within the Cyclorrhapha are indicated by coloured bars. Interestingly, anterior UV irradiation of D. melanogaster embryos—or mutations to the bcd gene—never produce bicaudal phenotypes [40,41]. This hints at the presence of an additional non-localised factor. This factor is hb, which contributes to axis specification and A–P polarity in D. melanogaster. The ubiquitous distribution of its maternal mRNA may explain why it is resistant to localised UV irradiation. This interpretation is consistent with the fact that only embryos lacking both bcd and hb show bicaudal phenotypes in this species [24,42,43].
While the roles of bcd and hb in axis specification appear to be somewhat redundant in D. melanogaster, the situation is different in other cyclorrhaphan flies. The hoverfly Episyrphus balteatus, for example, has secondarily lost maternal hb expression (Fig. 1) [38,44]. Consequently, knock-down of bcd by RNA interference (RNAi) leads to bicaudal phenotypes in this species [38].
In this paper, we study axis specification and maternal regulation of segmentation genes in another non-drosophilid cyclorrhaphan species, the scuttle fly Megaselia abdita (Fig. 1). M. abdita belongs to the most basally branching cyclorrhaphan lineage, the Phoridae [45,46]. While maternal cad expression has been lost in this species (Fig. 1) [47], hb retains its maternal contribution [31]. In light of this, it is surprising that knock-down of bcd does lead to bicaudal phenotypes. We investigate the regulatory causes of this phenomenon through a detailed study of the establishment and regulatory role of maternal gradients in M. abdtia. Our results reveal that the anterior and posterior systems are much less redundant compared to D. melanogaster. In particular, the difference between the two species can be explained by the loss of gap gene activation through maternal hb in M. abdita. Our results indicate that the role of the posterior system in axis specification has been lost in E. balteatus and M. abdita, while it still retains some of its ancestral functionality in D. melanogaster. In this general scenario, the anterior system is gradually replacing the posterior one during the evolution of the cyclorrhaphan flies.
Results and Discussion
The posterior system establishes a maternal Hb gradient in M. abdita
The posterior maternal system is based on maternal gradients of Nos and Hb protein. In M. abdita, nos mRNA is localised posteriorly during early cleavage stages (Fig. 2A) becoming restricted to the pole cells by C10 (Fig. 2B) as in D. melanogaster. Previous reports have documented ubiquitous maternal hb mRNA [31] as well as conserved zygotic hb expression in an anterior and a posterior domain [48]. Antibody stainings reveal a distribution of Hb protein very similar to the zygotic mRNA pattern during the late blastoderm (Fig. 2C,D). Furthermore, an anterior Hb protein gradient is present at cleavage and early blastoderm stages (Fig. 2E). In order to investigate the role of the posterior system in the formation of this gradient, we treated M. abdita embryos with nos RNAi. These embryos show no effect on hb mRNA, while ectopic Hb protein is present in the posterior of the embryo (effect detectable in 15 out of 16 RNAi-treated embryos; Fig. 2F). We conclude that the maternal Hb gradient is set up through translational repression by Nos in M. abdita as in D. melanogaster.
Fig. 2. The posterior maternal system and formation of the maternal Hb gradient in M. abdita. 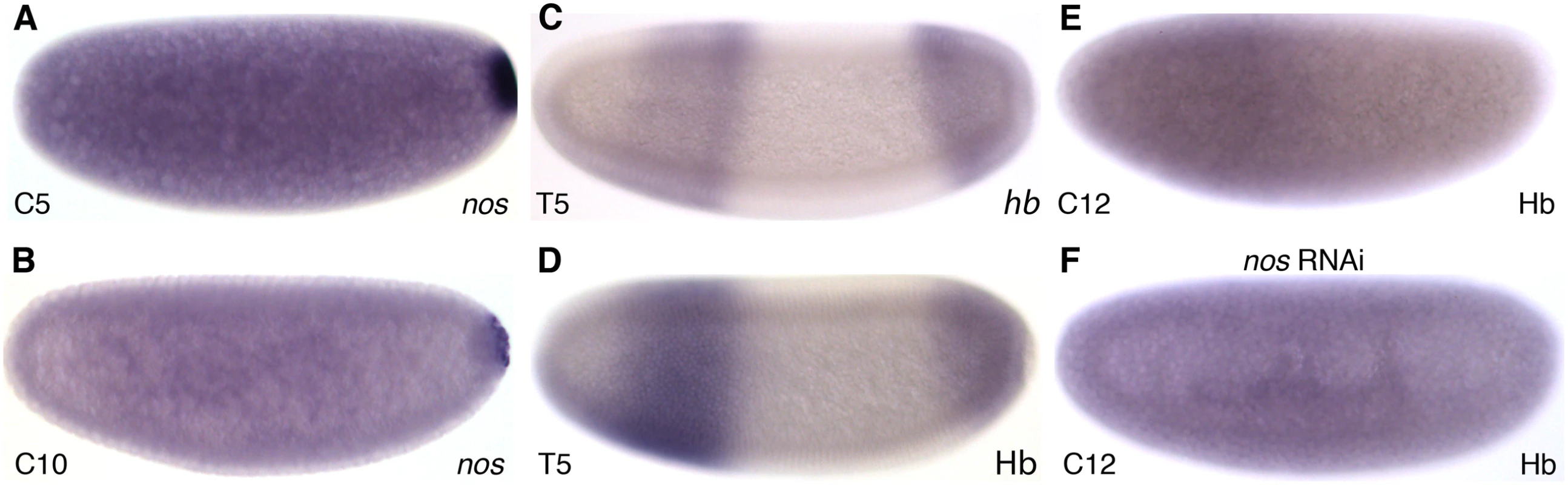
(A,B) Expression of nos mRNA in wild-type embryos at C5 (A) and C10 (B). (C,D) Hb protein expression (D) resembles the mRNA pattern (C) at late blastoderm stage (C14A-T5). (E) An anterior gradient of Hb protein can be observed during cleavage and early blastoderm stages (shown for C12). (F) This gradient is abolished in embryos treated with nos RNAi. Images show colorimetric in situ hybridisation (A–C) or antibody staining (D–F). Embryo images show lateral views: anterior is to the left, dorsal is up. Time classes according to [56] are indicated at bottom left in each panel. Regulation of cad in M. abdita
The anterior maternal system of M. abdita is less conserved than the posterior one. Unlike D. melanogaster [9,10] and E. balteatus [44], M. abdita lacks maternal cad transcripts [47] and consequently maternal Cad protein. Zygotic expression of cad, on the other hand, is qualitatively similar in D. melanogaster, E. balteatus, and M. abdita [9–12,14,37,42,47–49]. The only notable difference is that abdominal cad expression reaches further anterior in the latter two species compared to Drosophila [44,48].
In order to test how zygotic cad expression is regulated in M. abdita, we knocked down bcd, hb, and the head gap gene orthodenticle (otd). In bcd RNAi-treated embryos, we observe a derepression of cad transcripts in the anterior (38/48; Fig. 3A–F). At cleavage cycle 13 (C13), cad expression appears uniform throughout the embryo (Fig. 1D). During early C14A (time class 2, T2), cad becomes expressed at higher levels in the anterior than in the posterior (Fig. 3E). This effect is specifically confined to the region that is free of cad expression in wild-type embryos (compare to Fig. 3B). At later stages, an ectopic domain resembling the posterior cad stripe forms in the anterior (Fig. 3F). Similar ectopic cad stripes have been observed in the anterior of D. melanogaster bcd mutants [14], cad reporter assays in D. melanogaster [47], and E. balteatus embryos treated with bcd RNAi [38].
Fig. 3. Zygotic cad mRNA expression in wild-type and RNAi-treated embryos. 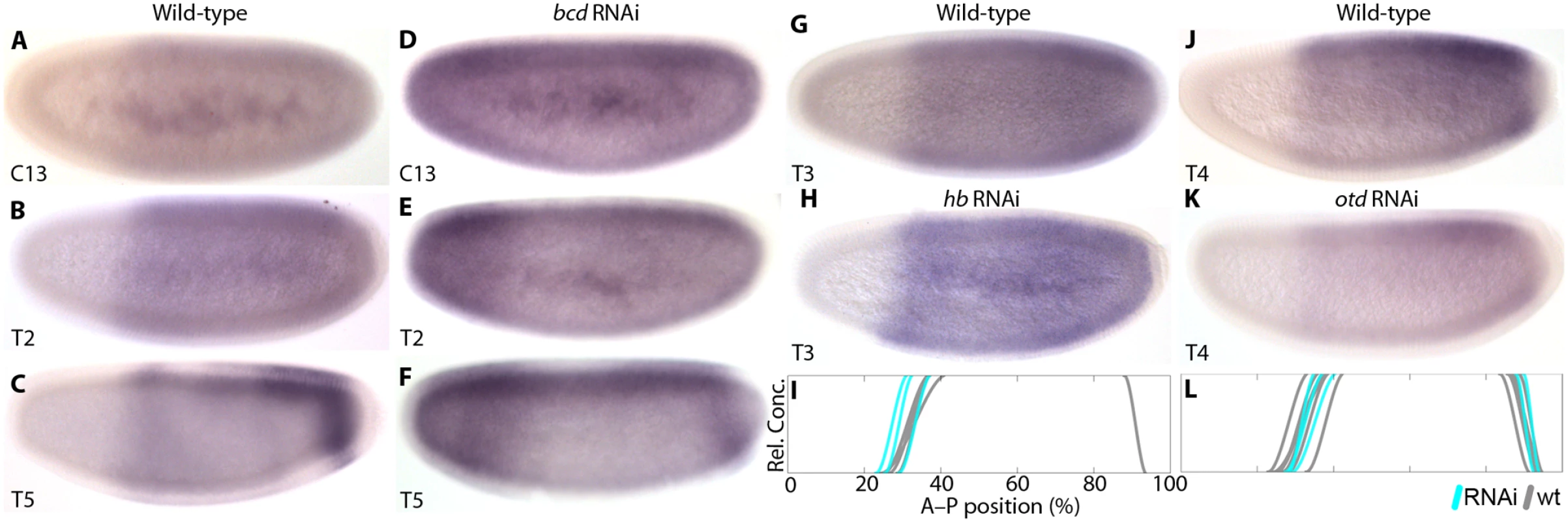
(A–F) The effect of bcd knock-down on cad expression. (A–C) show wild-type and (D–F) bcd RNAi-treated embryos. (G–I) hb knock-down. (G) shows wild-type, (H) hb RNAi-treated embryos. (I) compares the position of anterior cad expression boundaries between wild-type (wt; grey) and hb knock-down embryos (cyan). (J–L) otd knock-down. (J) shows wild-type, (K) otd RNAi-treated embryos. (L) compares the position of anterior cad expression boundaries between wild-type (wt; grey) and otd knock-down embryos (cyan). Embryo images show lateral views: anterior is to the left, dorsal is up. Time classes according to [56] are indicated at bottom left in each panel. Summary graphs in (I) and (L): horizontal axes represent % A–P position (with 0% at the anterior pole); vertical axes indicate relative mRNA concentration in arbitrary units (au). In hb knock-down embryos, we observe a small anterior expansion of cad expression in a minority of specimens (4/13; Fig. 3G–I; S1 File). Anterior derepression is much more subtle in this case than in bcd knock-downs (Fig. 3D–F). This effect is similar to hb mutants of D. melanogaster [42].
Given the difference between bcd and hb knock-downs, we investigated potential additional contributions by otd, a factor known to act as a transcriptional repressor of cad in the jewel wasp Nasonia vitripennis [50]. otd expression is lost in bcd RNAi-treated embryos (12/16; S1 Fig). However, expression of cad appears normal in embryos treated with otd RNAi (25/25; Fig. 3J–L; S1 File). This indicates that otd is not involved in cad regulation, consistent with results from D. melanogaster [50] and E. balteatus [44].
In summary, anterior repression of cad in D. melanogaster is due mainly to a combination of translational repression by Bcd—acting on ubiquitous maternal cad mRNA—and transcriptional repression by hb—acting on the zygotic abdominal cad domain [14,42]. Transcriptional regulation of cad by Bcd plays a minor role, if any [47]. In contrast, repression of cad by Bcd occurs predominantly at the transcriptional rather than the translational level in M. abdita, similar to E. balteatus [38]. Our evidence does not conclusively establish whether this interaction is direct. However, we have shown that potential intermediate factors such as Otd and Hb are not involved in cad regulation, or show regulatory effects that are far too subtle to account for anterior repression in M. abdita.
The role of bcd in M. abdita
Previous work has shown that bcd mRNA is localised anteriorly in M. abdita [30–32,48], and that it regulates hb transcription through the P2 promoter [31,37]. To assess the effect of bcd on gap gene regulation and embryo polarity in general, we characterised expression patterns of the trunk gap genes hb, giant (gt), knirps (kni), Krüppel (Kr), and the pair-rule gene even-skipped (eve) in M. abdita embryos treated with bcd RNAi. We used single - and double-stained embryos to assess severity of the knock-down and spatial registration of expression patterns—between gap domains (Fig. 4) as well as between Kr and the pair-rule gene eve (Fig. 5). We take advantage of the variable knock-down efficiency in RNAi experiments, which acts similar to an allelic series in classical genetics, to measure the sensitivity of specific gap domain boundaries towards decreasing levels of Bcd. In general, we find that all of these boundaries are highly sensitive to changes in Bcd concentration (Figs. 4 and 5; see also S1 File).
Fig. 4. Gap gene expression in response to bcd RNAi knock-down. 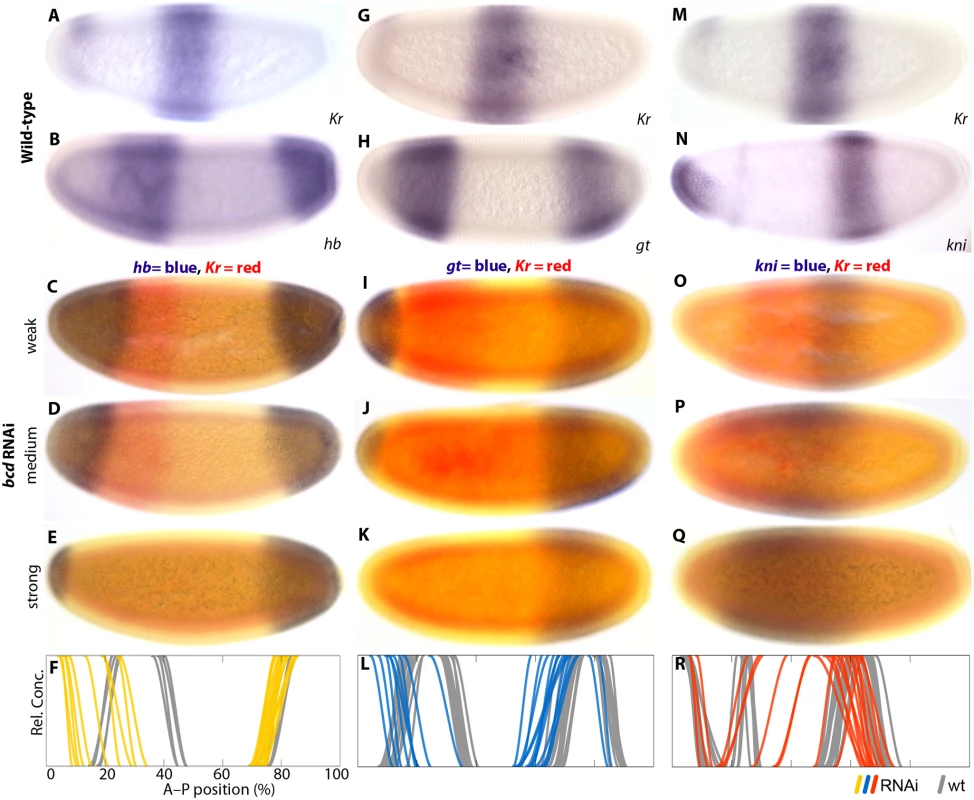
Columns show wild-type (A,B,G,H,M,N) or bcd RNAi embryos (C–E,I–K,O–Q) single- or double-stained for Kr (A,C–E,G,I–K,M,O–Q) along with hb (B–F), gt (H–L), and kni (N–R) as indicated. (F,L,R) Summary graphs comparing wild-type boundary positions (wt; grey lines) to boundary positions affected by RNAi (coloured lines). Kr is stained in red (C–E, I–K, O–Q) or blue (A,G,M). Other stains as indicated. All embryos are at time class T4 (hb and kni columns) or T3 (gt column). Embryo images show lateral views: anterior is to the left, dorsal is up. Fig. 5. eve and Kr expression in response to bcd knock-down. 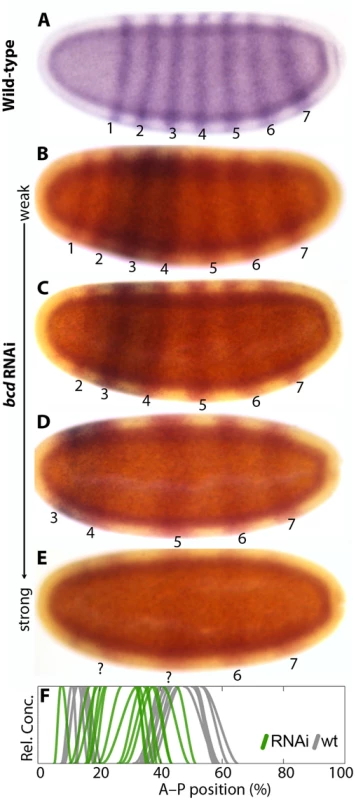
(A) Wild-type eve expression. (B–E) Weak to strong phenotypes in embryos treated with bcd RNAi: embryos were double-stained for eve (red) and Kr (blue). Numbers indicate eve stripes 1–7. (F) Summary graph comparing wild-type Kr boundary positions (wt; grey lines) to boundary positions affected by RNAi (green lines). All embryos are at time class T5. Embryo images show lateral views: anterior is to the left, dorsal is up. Wild-type embryos of M. abdita show a broad, bcd-dependent, anterior domain of zygotic hb expression, which gradually retracts from the pole (Fig. 4B) [31,37]. The posterior boundary of this domain shifts in anterior direction over time [48], unlike its equivalent in D. melanogaster. In embryos treated with bcd RNAi, we observe an anterior cap of hb expression which never retracts from the pole (35/42; Fig. 4C–F; S1 File). It reduces in size with the severity of the bcd knock-down (Fig. 4C–E) indicating dependence on Bcd concentration. Similar anterior domains have been observed in embryos derived from bcd mutant mothers in D. melanogaster [51] and in bcd RNAi-treated embryos of E. balteatus [38]. In both of these cases, the anterior cap of hb expression has been interpreted as an anterior mirror duplication of the posterior hb domain [38,51]. The posterior hb domain is also conserved in M. abdita (Fig. 4B) [48]. It exhibits a slight anterior expansion in some embryos treated with bcd RNAi (Fig. 4C–F; S1 File). In contrast, the posterior hb domain remains unaffected in D. melanogaster embryos lacking bcd [51].
Wild-type embryos of M. abdita have a broad anterior domain of gt, with a stationary posterior boundary, plus a posterior domain that shifts anteriorly over time (Fig. 4H) [48]. In embryos treated with bcd RNAi, we observe either loss (10/18) or strong reduction (8/18) of the anterior gt domain at early stages (before T3), while most embryos exhibit expression in a small anterior cap at later time points (14/15; Fig. 4I–L; S1 File). This anterior cap retracts from the pole around T8 (1/1). As for hb, the extent of anterior gt expression decreases with increasing strength of the knock-down effect (Fig. 4I–K). We interpret these observations as follows: delay and reduction of anterior gt expression are due to a lack of activation by Bcd, while the late anterior cap domain may be induced by ectopically expressed Cad (see Fig. 3E, F). The effect of bcd knock-down on the posterior gt domain is more modest. This domain is always present in bcd RNAi embryos but exhibits some anterior displacement of both its boundaries (Fig. 4I–L; S1 File). D. melanogaster embryos from bcd mutant mothers show a similar anterior displacement of the posterior gt domain, but no expression of gt in the anterior [52,53]. In contrast, E. balteatus embryos treated with bcd RNAi show broad derepression of gt, whose expression is only excluded from the anterior and posterior tip of the embryo [38].
In wild-type embryos of M. abdita, kni is expressed in an L-shaped anterior head domain, plus an abdominal domain that shifts to the anterior over time (Fig. 4N) [48]. In embryos treated with bcd RNAi, the head domain disappears, while the abdominal domain of kni expands and becomes displaced towards the anterior (38/38; Fig. 4O–R; S1 File). As in the case of hb and gt, the amount of expansion depends on the severity of the knock-down. This is qualitatively similar to embryos derived from bcd mutant mothers in D. melanogaster, but the effect is more severe in M. abdita and resembles kni expression in bcd mutants which are also heterozygous for maternal hb [24]. The effect of Bcd on kni is even more pronounced in E. balteatus where kni becomes drastically derepressed—showing ubiquitous expression in extreme cases—in embryos treated with bcd RNAi [38].
Wild-type M. abdita embryos have a central Kr domain, which is wider than its equivalent in D. melanogaster (Fig. 4A, G, M) [48]. As is the case for other gap domains, it shifts anteriorly and contracts over time. In embryos treated with bcd RNAi, the central domain of Kr expands towards the anterior (94/116; Fig. 4C–E, I–K, O–Q; Fig. 5B–F; S1 File). Yet again, the extent of the expansion is correlated with the strength of the knock-down. In the strongest cases, Kr expression is entirely missing (22/116; Fig. 4E, K, Q). A similar expansion of the central Kr domain has been observed in embryos from bcd mutant mothers in D. melanogaster [24]. However, these embryos never show a complete lack of Kr expression; it is only abolished by the additional removal of maternal hb [24,54]. Knock-down of bcd in E. balteatus, which lacks maternal hb expression altogether, leads to a complete absence of Kr expression in all RNAi-treated embryos [38].
In summary, our results suggest that Bcd is a concentration-dependent transcriptional regulator of gap genes in M. abdita. The observed effects of Bcd on gap gene expression are more severe than in D. melanogaster (resembling gap gene patterns in mutants affecting both bcd and hb), but milder than in E. balteatus.
Kr expression and polarity reversal in M. abdita
M. abdita embryos treated with bcd RNAi can exhibit a bicaudal phenotype with complete axis polarity reversal and mirror-duplicated posterior structures in the anterior [31]. These severe knock-down phenotypes have their plane of symmetry at abdominal segment 5 (A5), and express four eve stripes—the two anterior ones probably being mirror-duplicated stripes 6 and 7 [31]. Such polarity reversal is never observed in embryos derived from bcd mutant mothers in D. melanogaster [41], only in embryos that lack both bcd and maternal hb [24,43,54]. While the former still have a residual Kr domain, the latter lack Kr expression completely. Polarity reversal is also observed in E. balteatus embryos treated with bcd RNAi, which show no Kr expression at all [38].
We tested the relationship between the bicaudal phenotype and the presence or absence of Kr by co-staining bcd knock-down embryos for both eve and Kr (Fig. 5). The pair-rule gene eve is expressed in seven stripes in wild-type M. abdita embryos (Fig. 5A) [44,55,56]. Weak bcd knock-down phenotypes show a full complement of seven eve stripes that are displaced towards the anterior, with a correspondingly mild anterior displacement of Kr (Fig. 5B; compare to Fig. 4C, I, O). Increasing severity of the knock-down results in the progressive loss of anterior eve stripes and more pronounced anterior displacement of the central Kr domain (Fig. 5C–E; compare to Fig. 4D, J, P). In the strongest cases, we detect four eve stripes only (as in [31]), and no or very little Kr expression (Fig. 5F; compare to Fig. 4E, K, Q). This suggests that the absence of Kr expression is correlated with polarity reversal in bcd knock-down embryos.
Differing roles of maternal hb in M. abdita and D. melanogaster
Why does lack of Bcd induce a bicaudal phenotype in M. abdita if it has a maternal Hb gradient very similar to D. melanogaster? To answer this question, we compared the role of maternal Hb in gap gene regulation in both species.
We have previously characterised the effect of Hb on Kr, kni, and gt in M. abdita [48]. Expression of kni and gt in embryos treated with hb RNAi is very similar to the corresponding patterns in hb mutants of D. melanogaster. In contrast, the effect of Hb on Kr differs between the two species: both show an anterior expansion of the central Kr domain (24 out of 53 RNAi-treated embryos in M. abdita), but only D. melanogaster embryos lacking maternal Hb exhibit a decrease in Kr expression levels [24,54]. We never observe such down-regulation in M. abdita embryos treated with hb RNAi (S2 Fig) [48]. Together with the absence of Kr expression in strong bcd knock-down phenotypes (Fig. 4E, K, Q, Fig. 5E), this indicates that Hb is unable to activate Kr in M. abdita.
In contrast, several authors have interpreted the reduced levels of Kr expression in hb mutants as evidence for activation of Kr by Hb in D. melanogaster [24,42]. However, it has never been shown whether this activating effect is direct or indirect—via repression of the repressor Kni by Hb (see [5], for a detailed discussion). To distinguish between these two possibilities, it is necessary to suppress kni in a background lacking maternal and zygotic hb. Direct activation is supported if levels of Kr expression remain low in embryos lacking both hb and kni, while an indirect effect via kni is supported if Kr levels are restored in these embryos compared to hb mutants alone. Unfortunately, it is not straightforward to create such double mutants, since both hb and kni are located on the same chromosome in the D. melanogaster genome, and germ line clones must be induced to eliminate both maternal and zygotic activities of hb. This may be the reason why this experiment has never been carried out. To overcome this challenge, we used RNAi-mediated double knock-down of hb and kni, and knock-down of hb in a kni mutant background.
In D. melanogaster hb knock-down embryos, we observe anterior expansion and strong down-regulation of Kr (5/9; Fig. 6A, B; S2 File), as well as considerable anterior displacement of kni (3/5; S3 Fig; S2 File). These patterns correspond precisely to Kr and kni expression in embryos mutant for both maternal and zygotic hb [24]. Similarly, kni knock-down embryos show a Kr pattern which is identical to that observed in kni null mutants: we observe no posterior expansion of Kr (Fig. 6E; S2 File), in accordance with a recent quantitative study [57], but in disagreement with earlier qualitative reports [58–60]. These results indicate that our early embryonic RNAi knock-downs mimic strong null mutant phenotypes.
Fig. 6. Kr is activated by maternal Hb in D. melanogaster. 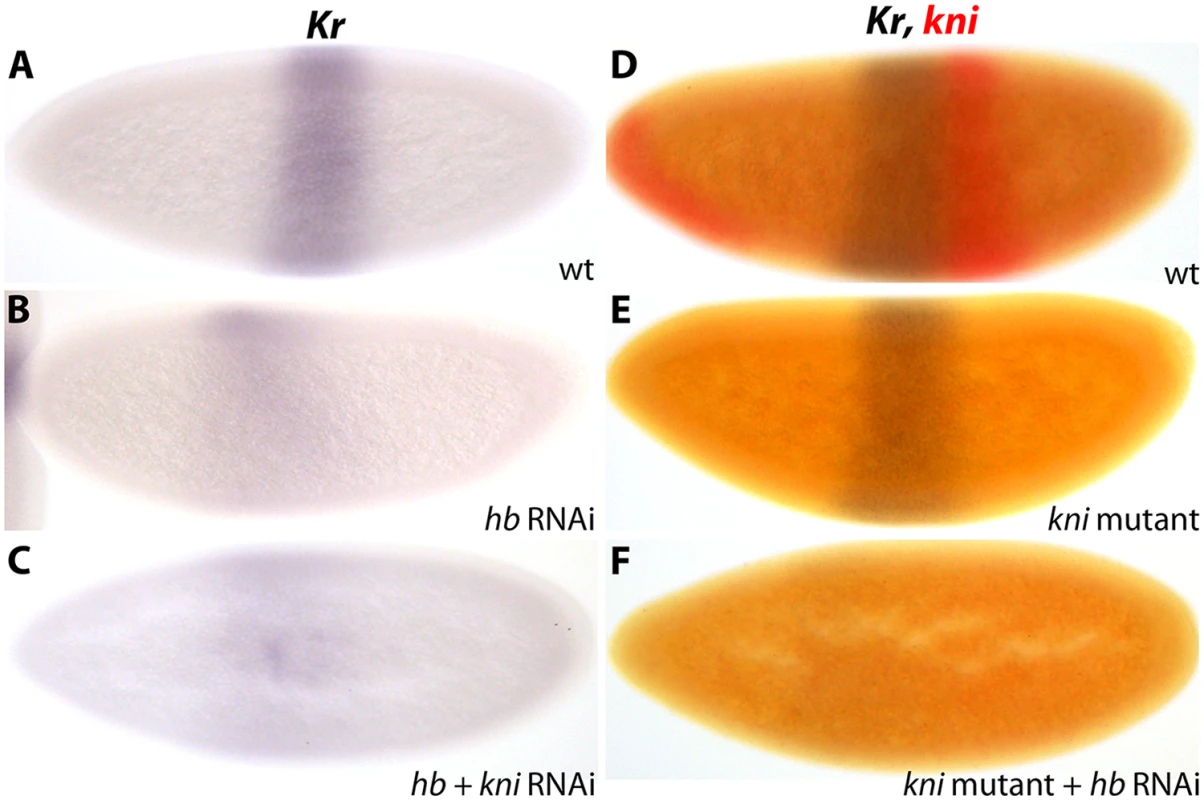
(A–C) Kr expression in wild-type embryos (wt; A) versus embryos treated with hb RNAi (B) and hb/kni double RNAi (C). (D–F) Kr (blue) and kni (red) expression in wild-type (wt; D) versus kni mutants (E) and kni mutants treated with hb RNAi (F). See text for details. All embryos are at time class T4. Embryo images show lateral views: anterior is to the left, dorsal is up. In D. melanogaster hb/kni double knock-down embryos, we observe an anterior expansion of Kr, but no restoration of expression levels (12/18; Fig. 6C; S2 File). We confirm this result in kni mutant embryos treated with hb RNAi, which exhibit an identical anterior expansion of Kr and no restoration of expression levels (12/14; Fig. 6F; S2 File). Taken together, these results demonstrate that kni is not responsible for Kr down-regulation in D. melanogaster embryos lacking maternal and zygotic Hb. Therefore, activation of Kr by Hb is direct in this species. In contrast, this activatory role is absent in M. abdita where Hb acts as a repressor only, which leads to a lack of Kr expression and mirror symmetrical expression of the remaining gap genes in bcd knock-down embryos (see also Conclusions).
The role of zygotic cad in M. abdita
In D. melanogaster, maternal and zygotic Cad contribute to the activation of posterior gap domains [13,42] and—at least partially independently of gap gene regulation—activate posterior stripes of pair rule gene expression [11,50,61–64]. To investigate the exclusively zygotic contribution of Cad to gap and pair-rule gene expression in M. abdita, we characterised the expression patterns of hb, gt, Kr, kni, and eve (Fig. 7A–L; S1 File) as well as the cuticle phenotype (Fig. 7O) of embryos treated with cad RNAi. The cad knock-down phenotype of M. abita exhibits deletions of all segments posterior of T3, and T3 itself is also disrupted in some embryos (Fig. 7O). This phenotype is more similar to D. melanogaster than to E. balteatus. Embryos of the latter treated with cad RNAi exhibit a strongly reduced cephalopharyngeal skeleton, in addition to an almost complete loss of abdomen and thorax [44]. In contrast, D. melanogaster embryos mutant for both maternal and zygotic cad have an intact head and thorax and, although there is extensive loss of abdominal segments, often even retain some abdominal structures [11]. The fact that the M. abdita phenotype is stronger than that of D. melanogaster suggests that cad still plays an essential role in posterior segmentation in this species despite the loss of its maternal contribution.
Fig. 7. RNAi knock-down of M. abdita cad. 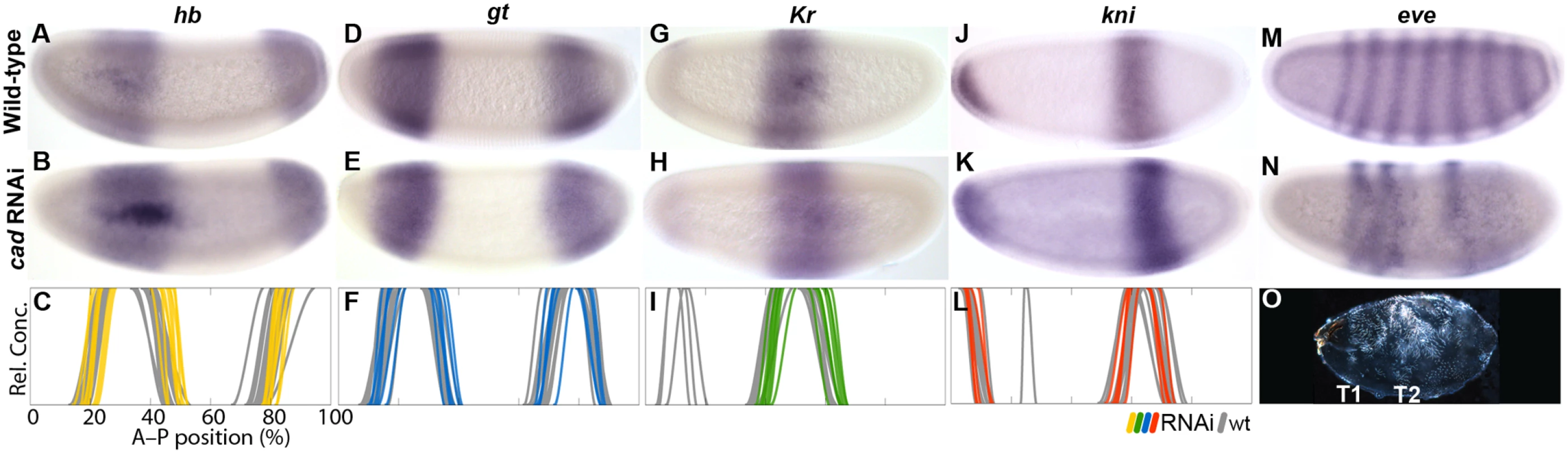
Columns show the expression of hb (A–C; yellow), gt (D–F; blue), Kr (G–I; green), kni (J–L; red), and eve (M,N) in wild-type embryos (top row: A,D,G,J,M), in embryos treated with cad RNAi (middle row: B,E,H,K,N), and as summary graphs comparing wild-type (wt) boundary positions (grey) to those affected by RNAi (coloured lines) (bottom row: C,F,I,L). (O) Cuticle phenotype of an embryo treated with cad RNAi. All embryos are at time class T4. Embryo images show lateral views: anterior is to the left, dorsal is up. Graphs: horizontal axes indicate % A–P position (where 0% is the anterior pole); vertical axes represent relative mRNA concentration in arbitrary units. In light of this, it is surprising that knock-down of cad in M. abdita does not have a strong effect on gap gene expression. The only clearly detectable defect is a slightly reduced posterior hb domain (7/16; Fig. 7A–C). All other domains of hb, gt, Kr, and kni seem unaffected (Fig. 7A–L; see also S1 File). Expression levels of Kr, kni, and gt appear similar to wild-type, although we cannot completely rule out a marginal decrease due to lack of sensitivity of our enzymatic detection method. This stands in contrast to D. melanogaster, where expression levels in the abdominal domain of kni and the posterior domain of gt are reduced in mutants lacking both zygotic and maternal cad (while hb and Kr are expressed as in wild-type) [13,42,50]. In E. balteatus cad knock-down embryos, anterior hb and Kr are normal, while the posterior kni, gt, and hb domains are absent or severely reduced [38,44].
To test if activation of gap genes by Cad is present but redundant with the complementary contribution by Bcd, we characterised the expression of kni and gt in embryos treated with RNAi against both bcd and cad (Fig. 8; see also S1 File). We observe a large anterior displacement in the position of the abdominal kni domain (36/36), as is seen in bcd RNAi-treated embryos. This was associated with a strong reduction in expression levels, particularly before T3 (Fig. 8B; 14/15), though after this stage levels of expression begin to resemble those in the wild-type. Embryos treated with bcd or cad RNAi alone, never show such reduction (Fig. 4N–Q, Fig. 7J, K). Expression of gt is absent before T2 (6/10), and only becomes detectable as a weak posterior domain at later stages (Fig. 8D, F). In contrast to bcd RNAi-treated embryos (Fig. 4H–L), we do not observe any anterior displacement of this domain (see S1 File). In the anterior, we observe a cap of gt expression at the late blastoderm stage (Fig. 8H), which closely resembles the anterior cap in embryos treated with RNAi against bcd alone (Fig. 4I,J; S1 File). Our observations stand in contrast to those from D. melanogaster mutants lacking both maternal and zygotic cad and bcd. Such mutants show complete absence of both kni and gt expression [13].
Fig. 8. RNAi double knock-down of M. abdita bcd and cad. 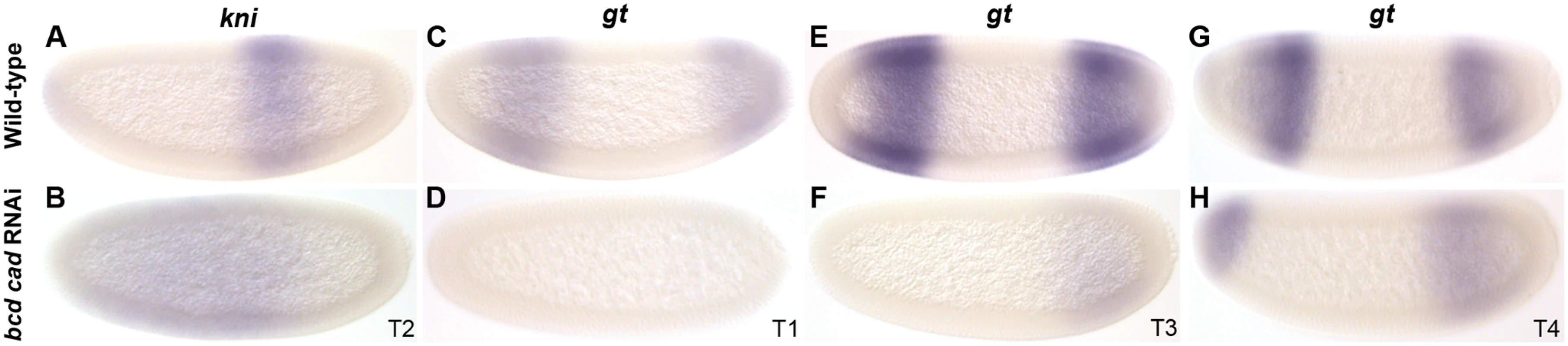
Columns show the expression of kni (A, B), and gt (C–H) in wild-type embryos (top row: A, C, E, G), and in embryos treated simultaneously with bcd and cad RNAi (bottom row: B, D, F, H). Embryo time classes as indicated at bottom right of each column. Embryo images show lateral views: anterior is to the left, dorsal is up. Taken together, our results suggest that Cad contributes to early activation of both abdominal kni and posterior gt in M. abdita, in a way which is largely redundant with activation by Bcd. Surprisingly, late expression of both kni and gt in the posterior of the embryo seems to be at least partially independent of both Bcd and Cad activation. This suggests that a third, yet unknown, factor must contribute to gap gene activation in this species.
Finally, we investigated the contribution of M. abdita cad to pair-rule expression. In embryos treated with cad RNAi, we observe a reduction in the number of eve stripes: 2 out of 12 embryos showed three, 5/12 four, and 5/12 five eve stripes (Fig. 7M,N). Similarly, D. melanogaster embryos mutant for both maternal and zygotic cad have four eve stripes [50]. The most drastic effect of cad on pair-rule gene expression is observed in E. balteatus, where embryos treated with cad RNAi exhibit the loss of all but the first stripe of eve [44].
Taken together, our evidence demonstrates that zygotic cad still plays an important role in the determination of posterior segments of M. abdita. In contrast to D. melanogaster and E. balteatus, where eliminating cad has a clearly detectable effect on gap gene expression [13,42,44], it is largely redundant for gap gene activation in M. abdita. This implies that cad performs its pattern-forming role mainly at the level of the pair-rule genes in this species.
Conclusions
In this study, we have investigated the establishment of maternal gradients and their role in gap gene regulation in the scuttle fly M. abdita. We compare our results with the evidence from the vinegar fly D. melanogaster as well as the marmalade hoverfly E. balteatus (Fig. 9). On the one hand, we find that important aspects of maternal regulation are highly conserved among cyclorrhaphan flies. Bcd acts as a concentration-dependent transcriptional regulator, and Cad is involved in posterior patterning in all three species. On the other hand, we find a number of interesting differences between M. abdita, E. balteatus, and D. melanogaster.
Fig. 9. Summary of maternal gene regulatory networks and gap gene expression in embryos with bicaudal phenotypes. 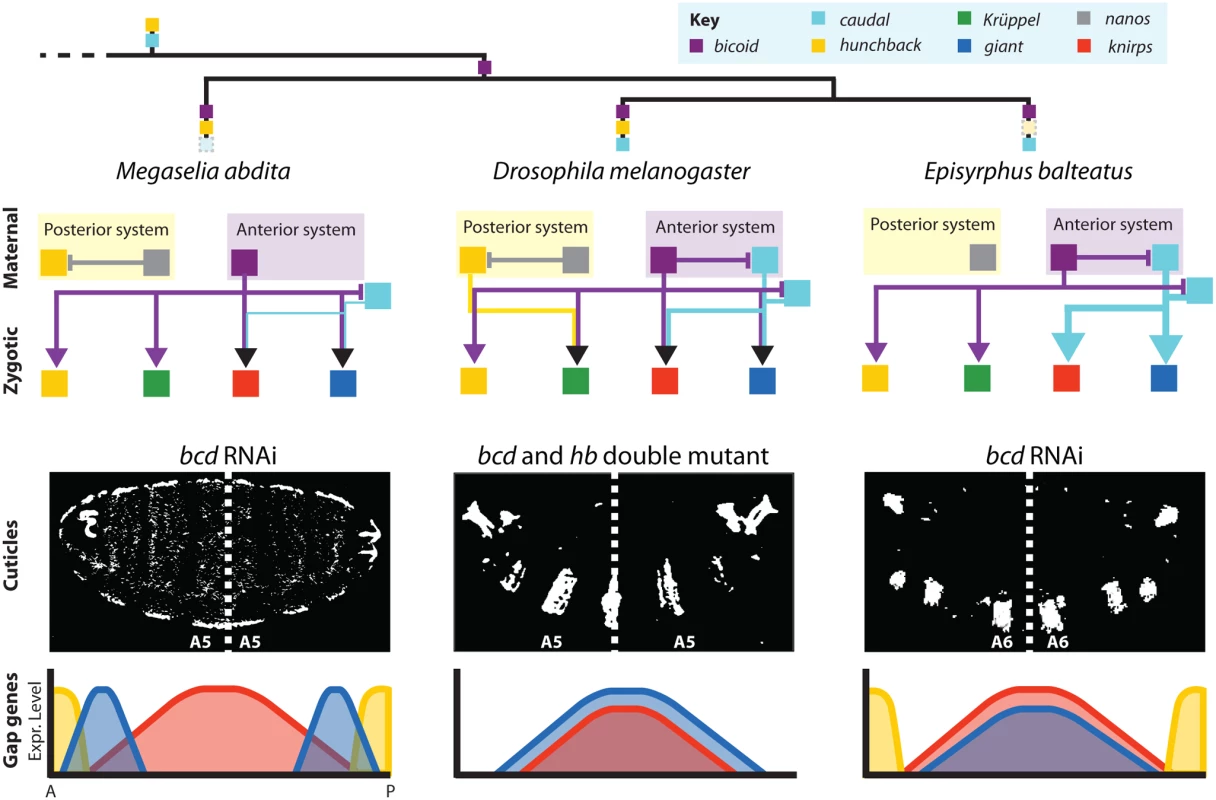
Top: phylogeny of species discussed in this paper indicating ancestral maternal factors (cad and hb), the origin of bcd, and the loss of maternal factors in different lineages (faded dashed boxes). Middle: schematic graphs showing gene regulatory networks implementing maternal contributions to gap gene activation. Translational and/or transcriptional cross-repression among maternal genes represented by T-bars, activating inputs to gap genes shown by arrows. Cross-regulation between gap genes has been analysed previously [48] and is omitted for clarity. Bottom: double-abdomen cuticles (upper row) and corresponding gap gene expression patterns (bottom row) are shown for M. abdita and E. balteatus embryos treated with bcd RNAi, as well as bcd/hb double mutants in D. melanogaster. Dashed lines indicate the plane of symmetry. D. melanogaster and E. balteatus cuticle images adapted from [24,38]. The first difference concerns the regulation of cad. Even though maternal cad expression can be detected in nematocerans, and basally branching non-cyclorrhaphan brachycerans (Fig. 1), maternal expression of cad has been lost in M. abdita [47]. Zygotic expression of cad is qualitatively similar between species, but reaches further anterior in M. abdita and E. balteatus than in D. melanogaster, creating a large overlap of cad and hb in these flies. Consistent with the absence of strong repression between these two genes, hb only weakly affects cad expression in M. abdita. In contrast, cad is completely de-repressed anteriorly in bcd knock-down embryos (see Fig. 3). There is some evidence from reporter assays that Bcd may regulate cad transcriptionally in D. melanogaster as well [47]. The situation is much less ambiguous in the case of E. balteatus, where cad is strongly up-regulated in the anterior upon bcd RNAi knock-down [38]. This similarity between M. abdita and E. balteatus suggests that transcriptional repression of cad by Bcd is much more prominent in these flies compared to D. melanogaster. Whether this interaction is direct in any of the three species remains to be shown.
The second difference concerns the roles of bcd and hb in axis specification and gap gene patterning. Knock-down of bcd in M. abdita and E. balteatus leads to bicaudal phenotypes, as observed in bcd/hb double mutants but not in bcd mutants in D. melanogaster [24,41–43]. It is important to note that the situation in M. abdita is distinct from both D. melanogaster and E. balteatus (Fig. 9). More positional information is retained in bicaudal embryos, resulting in a more anterior (A5) plane of symmetry, compared to A6 in the latter two species [24,31,38]. This difference is also reflected at the level of gap gene expression. Severe M. abdita knock-down phenotypes for bcd, which lack Kr expression, show a sequence of hb-gt-kni-gt-hb domains along the antero-posterior axis (Fig. 9) (this paper and [31]). D. melanogaster hb/bcd double mutants only have overlapping central gt and kni domains (Fig. 9) [24,51,52,65]. E. balteatus knock-down embryos show an almost complete de-repression of gt and kni throughout the embryo (Fig. 9) [38].
The anterior gradient of Bcd is an evolutionary innovation of the cyclorrhaphan lineage (Fig. 1) [30–33]. The evidence suggests that it is completely sufficient for axis specification and embryo polarity in M. abdita and E. balteatus. In contrast, both maternal Bcd and Hb contribute synergistically to axis specification and gap gene patterning in D. melanogaster. While differences in the effect of Bcd between D. melanogaster and E. balteatus are easily explained by the absence of maternal hb in the latter [38], it is less straightforward to pinpoint the cause for polarity reversal in bcd knock-down embryos of M. abdita. Our evidence suggests that this difference lies in the ability of maternal Hb to activate Kr in D. melanogaster, but not M. abdita (see Fig. 4, and [48]). Kr expression in the anterior of the embryo is correlated with the maintenance of polarity in D. melanogaster bcd mutants, and weak bcd knock-down phenotypes in M. abdita (Figs. 4 and 5). In D. melanogaster, maternal Hb is required for Kr expression in the absence of Bcd [24,42], and we have shown here that this activating interaction is indeed direct and not caused by the indirect repression of the Kni repressor (Fig. 6).
It remains unclear whether activation of Kr by maternal Hb has been gained in D. melanogaster or lost in M. abdita. However, there is some evidence that favours the latter scenario. Maternal hb expression is strongly conserved across arthropods far beyond the cyclorrhaphan lineage [66–74], and hb is involved in axis patterning in many of the species where it has been studied [67,68,70–72,75,76]. Most interestingly in our context, Hb activates Kr in the flour beetle Tribolium castaneum [75], the honeybee Apis mellifera [72], the hemipteran milkweed bug Oncopeltus fasciatus [76], and the cricket Gryllus bimaculatus [69]. The fact that this activating role of hb is conserved, and is only present in the one cyclorrhaphan species that retains some activity of maternal Hb in axis formation, seems to suggest that it may represent the ancestral state, and that activation of Kr by Hb has been lost in M. abdita and E. balteatus.
We have previously demonstrated that the gap gene system of M. abdita compensates for the significant differences in the distribution of maternal factors compared to D. melanogaster, such that gap gene expression converges to equivalent patterns in both species by the onset of gastrulation [48]. Such compensatory evolution is called developmental system drift or phenogenetic drift [77–81]. At the level of the gap genes, this is achieved through quantitative changes in the strength of otherwise wholly conserved gap-gap interactions [48]. In contrast, our study shows that system drift at the level of maternal-to-gap interactions is mediated by both quantitative and qualitative differences in gene regulation. While inter-species differences in the effect of Bcd and Cad mainly consist in changes in activation strength, the activating role of Hb on Kr has changed in a qualitative way: while Hb activates Kr in D. melanogaster, this activating role is absent in both M. abdita and E. balteatus (Fig. 9).
In summary, we observe a trend towards replacing the role of maternal Hb with activity of the anterior maternal system—Bcd and Cad—in non-drosophilid cyclorrhaphan lineages through the process of developmental system drift. This is reflected by the stronger phenotypes of bcd and cad knock-downs in both E. balteatus and M. abdita compared to D. melanogaster. In this view, axis formation and gap gene patterning in D. melanogaster retains more ancestral characteristics than these early-branching non-drosophilid cyclorrhaphans. Further corroboration of these insights will have to come from functional studies of axis specification and gap gene patterning in an appropriate outgroup (Fig. 1): non-cyclorrhaphan brachycerans or emerging nematoceran model systems such as the chironomid midge Chironomus riparius or the moth midge Clogmia albipunctata.
Methods
M. abdita fly culture, embryo collection and fixation were carried out as described in [82,83]. Enzymatic mRNA in situ hybridisation, image acquisition, and data processing were carried out as described in [84,85]. We use an embryonic staging scheme—homologous to the one already established for D. melanogaster [86]—which is described in detail in [56]. Embryo morphology and developmental timing are remarkably similar in both species. Embryos are classified into cleavages cycles C1–C14A according to nuclei number; C14A is further subdivided into eight time classes T1–8 based on nuclear and membrane morphology.
Polyclonal antiserum was raised against M. abdita Hb protein expressed by means of a pET-DEST42 vector (Invitrogen) containing a full length cDNA insert. Purified Hb protein dissolved in 6M urea was used to raise rat antibodies by Primm Biotech (primmbiotech.com) using standard protocols. For antibody stains, wild-type blastoderm-stage embryos were collected after 4 hrs of egg laying and stained with a colorimetric protocol adapted from the in situ protocol published in [85]. In brief, fixed and dehydrated embryos were re-hydrated by washing 1x5min in PBT/methanol (embryos were allowed to sink before the solution was removed), 2x in PBT, and 1x20 min in PBT. Embryos were washed 1x, then blocked with 2x30 min in western blocking reagent (Roche) in PBT followed by incubation with primary antibodies in blocking solution overnight. Unbound antibody was removed washing 3x in PBT followed by 4x15 min washes in PBT. Embryos were then re-blocked and incubated with secondary antibodies conjugated with alkaline phosphatase (Roche) at 1 : 3000 in blocking solution for 1 hr. Unbound antibody was removed as before. To prepare for staining, embryos were washed 2x5 min in AP buffer (100 mM NaCl, 50 mM MgCl, 100 mM Tris pH 9.5, 0.1% tween-20). Staining was carried out in the dark by the addition of AP buffer containing 0.1 mg/ml NBT and 0.05 mg/ml BCIP. Staining was stopped with 3x1 min followed by 3x10 min washes in PBT. Nuclei were counter-stained by a 10-min incubation in PBT containing 0.3 μM DAPI, followed by 3x washes and 3x10 min washes in PBT. Embryos were cleared through a series into 70% glycerol:PBS, of which 30 μl were mounted per slide. All washes were done on a nutator.
We used RNAi knock-down protocols adapted from [31,37,87]. See [48] for further details.
All expression boundaries plotted as graphs were extracted from NBT/BCIP stained embryos, except for Kr expression in M. abdita bcd RNAi-treated embryos, where boundaries were extracted from FastRed stains. Differences in expression levels in Fig. 6 and S2 Fig were assessed through simultaneous staining of wild-type and RNAi-treated embryos using NBT/BCIP to ensure a robust signal.
Quantified expression data for M. abdita wild-type and RNAi knock-down embryos are available online through figshare (http://dx.doi.org/10.6084/m9.figshare. 1252195; [88], and the SuperFly database (http://superfly.crg.eu; [89]). Plots of gene expression boundaries from RNAi-treated or mutant embryos can be found in S1 File (M. abdita) and S2 File (D. melanogaster).
nos (KP232978) was cloned from cDNA using data from our published early embryonic transcriptome (http://diptex.crg.es; MAB_comp4961) [46]. All other genes were cloned as described in [48].
Embryo collection, fixation, RNAi treatment, and in situ hybridisation in D. melanogaster was carried out as for M. abdita [85,87]. D. melanogaster kni mutants correspond to deletion strain 3127 (Bloomington Drosophila Stock Center) with genotype Df(3L)ri-79c/TM3, Sb1. Homozygous mutants were detected by an absence of FastRed kni staining during in situ hybridisation.
Supporting Information
Zdroje
1. Nüsslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287 : 795–801. 6776413
2. Akam M (1987) The molecular basis for metameric pattern in the Drosophila embryo. Development 101 : 1–22. 2896587
3. Ingham PW (1988) The molecular genetics of embryonic pattern formation in Drosophila. Nature 335 : 25–34. 2901040
4. St Johnston D, Nüsslein-Volhard C (1992) The origin of pattern and polarity in the Drosophila embryo. Cell 68 : 201–219. 1733499
5. Jaeger J (2011) The gap gene network. Cell Mol Life Sci 68 : 243–274. doi: 10.1007/s00018-010-0536-y 20927566
6. Driever W, Nüsslein-Volhard C (1988) The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell 54 : 95–104. 3383245
7. Little SC, Tkačik G, Kneeland TB, Wieschaus EF, Gregor T (2011) The formation of the Bicoid morphogen gradient requires protein movement from anteriorly localized mRNA. PLoS Biol 9: e1000596. doi: 10.1371/journal.pbio.1000596 21390295
8. Gregor T, Wieschaus EF, McGregor AP, Bialek W, Tank DW (2007) Stability and Nuclear Dynamics of the Bicoid Morphogen Gradient. Cell 130 : 141–152. 17632061
9. Mlodzik M, Gehring WJ (1987) Expression of the caudal gene in the germ line of Drosophila: formation of an RNA and protein gradient during early embryogenesis. Cell 48 : 465–478. 2433048
10. Mlodzik M, Fjose A, Gehring WJ (1985) Isolation of caudal, a Drosophila homeo box-containing gene with maternal expression, whose transcripts form a concentration gradient at the pre-blastoderm stage. EMBO J 4 : 2961–2969. 16453641
11. Macdonald PM, Struhl G (1986) A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature 324 : 537–545. 2878369
12. Surkova S, Kosman D, Kozlov K, Manu, Myasnikova E, et al. (2008) Characterization of the Drosophila segment determination morphome. Dev Biol 313 : 844–862. 18067886
13. Rivera-Pomar R, Lu X, Perrimon N, Taubert H, Jäckle H (1995) Activation of posterior gap gene expression in the Drosophila blastoderm. Nature 376 : 253–256. 7617036
14. Mlodzik M, Gehring W (1987) Hierachy of the genetic interactions that specify the anterior-posterior segmentation pattern of the Drosophila embryo monitored by caudal protein expression. Development 101 : 421–435.
15. Driever W, Thoma G, Nüsslein-Volhard C (1989) Determination of spatial domains of zygotic gene expression in the Drosophila embryo by the affinity of binding sites for the bicoid morphogen. Nature 340 : 363–367. 2502714
16. Driever W, Nüsslein-Volhard C (1989) The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature 337 : 138–143. 2911348
17. Struhl G, Struhl K, Macdonald PM (1989) The gradient morphogen bicoid is a concentration-dependent transcriptional activator. Cell 57 : 1259–1273. 2567637
18. Gregor T, Tank DW, Wieschaus EF, Bialek W (2007) Probing the Limits to Positional Information. Cell 130 : 153–164. 17632062
19. Ochoa-Espinosa A, Yucel G, Kaplan L, Pare A, Pura N, et al. (2005) The role of binding site cluster strength in Bicoid-dependent patterning in Drosophila. Proc Natl Acad Sci U S A 102 : 4960–4965. 15793007
20. Gao Q, Wang Y, Finkelstein R (1996) Orthodenticle regulation during embryonic head development in drosophila. Mech Dev 56 : 3–15. 8798143
21. Wang C, Lehmann R (1991) Nanos is the localized posterior determinant in Drosophila. Cell 66 : 637–647. 1908748
22. Forrest KM, Gavis ER (2003) Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr Biol 13 : 1159–1168. 12867026
23. Lehmann R, Nüsslein-Volhard C (1991) The maternal gene nanos has a central role in posterior pattern formation of the Drosophila embryo. Development 112 : 679–691. 1935684
24. Hülskamp M, Pfeifle C, Tautz D (1990) A morphogenetic gradient of hunchback protein organizes the expression of the gap genes Krüppel and knirps in the early Drosophila embryo. Nature 346 : 577–580. 2377231
25. Struhl G (1989) Differing strategies for organizing anterior and posterior body pattern in Drosophila embryos. Nature 338 : 741–744. 2716822
26. Irish V, Lehmann R, Akam M (1989) The Drosophila posterior-group gene nanos functions by repressing hunchback activity. Nature 338 : 646–648. 2704419
27. Yajima H (1964) Studies on embryonic determination of the harlequin-fly, Chironomus dorsalis. II. Effects of partial irradiation of the egg by UV light. J Embryol exp Morph 12 : 89–100. 14155410
28. Kalthoff K, Sander K (1968) Der Entwicklungsgang der Missbildung Doppelabdomen im partiell UV-bestrahlten Ei von Smittia parthenogenetica (Dipt., Chironomidae). Wilhelm Roux Arch EntwMech Org 161 : 129/278.
29. Kalthoff K (1978) Pattern formation in early insect embryogenesis—data calling for modification of a recent model. J Cell Sci 29 : 1–15. 627599
30. Stauber M, Jäckle H, Schmidt-Ott U (1999) The anterior determinant bicoid of Drosophila is a derived Hox class 3 gene. Proc Natl Acad Sci U S A 96 : 3786–3789. 10097115
31. Stauber M, Taubert H, Schmidt-Ott U (2000) Function of bicoid and hunchback homologs in the basal cyclorrhaphan fly Megaselia (Phoridae). Proc Natl Acad Sci U S A 97 : 10844–10849. 10995461
32. Stauber M, Prell A, Schmidt-Ott U (2002) A single Hox3 gene with composite bicoid and zerknüllt expression characteristics in non-Cyclorrhaphan flies. Proc Natl Acad Sci U S A 99 : 274–279. 11773616
33. Schmidt-Ott U, Rafiqi AM, Lemke S (2010) Hox Genes. Deutsch JS, editor New York, NY: Springer New York. doi: 10.1007/978-1-4419-6673-5
34. Bonneton F, Shaw PJ, Fazakerley C, Shi M, Dover GA (1997) Comparison of bicoid-dependent regulation of hunchback between Musca domestica and Drosophila melanogaster. Mech Dev 66 : 143–156. 9376318
35. McGregor AP, Shaw PJ, Hancock JM, Bopp D, Hediger M, et al. (2001) Rapid restructuring of bicoid-dependent hunchback promoters within and between Dipteran species: implications for molecular coevolution. Evol Dev 3 : 397–407. 11806635
36. Shaw PJ, Wratten NS, McGregor AP, Dover GA (2002) Coevolution in bicoid-dependent promoters and the inception of regulatory incompatibilities among species of higher Diptera. Evol Dev 4 : 265–277. 12168619
37. Lemke S, Stauber M, Shaw PJ, Rafiqi AM, Prell A, et al. (2008) Bicoid occurrence and Bicoid-dependent hunchback regulation in lower cyclorrhaphan flies. Evol Dev 10 : 413–420. doi: 10.1111/j.1525-142X.2008.00252.x 18638318
38. Lemke S, Busch SE, Antonopoulos D a., Meyer F, Domanus MH, et al. (2010) Maternal activation of gap genes in the hover fly Episyrphus. Development 137 : 1709–1719. doi: 10.1242/dev.046649 20430746
39. Shaw PJ, Salameh A, McGregor AP, Bala S, Dover GA (2001) Divergent structure and function of the bicoid gene in Muscoidea fly species. Evol Dev 3 : 251–262. 11478522
40. Bownes M, Kalthoff K (1974) Embryonic defects in Drosophila eggs after partial u.v. irradiation at different wavelengths. J Embryol Exp Morphol 31 : 329–345. 4211978
41. Frohnhöfer HG, Lehmann R, Nüsslein-Volhard C (1986) Manipulating the anteroposterior pattern of the Drosophila embryo. J Embryol Exp Morphol 97 Suppl: 169–179. 3625112
42. Schulz C, Tautz D (1995) Zygotic caudal regulation by hunchback and its role in abdominal segment formation of the Drosophila embryo. Development 121 : 1023–1028. 7743918
43. Simpson-Brose M, Treisman J, Desplan C (1994) Synergy between the hunchback and bicoid morphogens is required for anterior patterning in Drosophila. Cell 78 : 855–865. 8087852
44. Lemke S, Schmidt-Ott U (2009) Evidence for a composite anterior determinant in the hover fly Episyrphus balteatus (Syrphidae), a cyclorrhaphan fly with an anterodorsal serosa anlage. Development 136 : 117–127. doi: 10.1242/dev.030270 19060334
45. Wiegmann BM, Trautwein MD, Winkler IS, Barr NB, Kim J-W, et al. (2011) Episodic radiations in the fly tree of life. Proc Natl Acad Sci U S A 108 : 5690–5695. doi: 10.1073/pnas.1012675108 21402926
46. Jiménez-Guri E, Huerta-Cepas J, Cozzuto L, Wotton KR, Kang H, et al. (2013) Comparative transcriptomics of early dipteran development. BMC Genomics 14 : 123. doi: 10.1186/1471-2164-14-123 23432914
47. Stauber M, Lemke S, Schmidt-Ott U (2008) Expression and regulation of caudal in the lower cyclorrhaphan fly Megaselia. Dev Genes Evol 218 : 81–87. doi: 10.1007/s00427-008-0204-5 18214532
48. Wotton KR, Jimenez-Guri E, Crombach A, Janssens H, Alcaine Colet A, et al. (2014) Quantitative System Drift Compensates for Altered Maternal Inputs to the Gap Gene Network of the Scuttle Fly Megaselia abdita. eLIFE 4: e04785.
49. Moreno E, Morata G (1999) Caudal is the Hox gene that specifies the most posterior Drosophila segment. Nature 400 : 873–877. 10476966
50. Olesnicky EC, Brent AE, Tonnes L, Walker M, Pultz MA, et al. (2006) A caudal mRNA gradient controls posterior development in the wasp Nasonia. Development 133 : 3973–3982. 16971471
51. Tautz D (1988) Regulation of the Drosophila segmentation gene hunchback by two maternal morphogenetic centres. Nature 332 : 281–284. 2450283
52. Eldon ED, Pirrotta V (1991) Interactions of the Drosophila gap gene giant with maternal and zygotic pattern-forming genes. Development 111 : 367–378. 1716553
53. Kraut R, Levine M (1991) Spatial regulation of the gap gene giant during Drosophila development. Development 111 : 601–609. 1893877
54. Schulz C, Tautz D (1994) Autonomous concentration-dependent activation and repression of Krüppel by hunchback in the Drosophila embryo. Development 120 : 3043–3049. 7607091
55. Bullock SL, Stauber M, Prell A, Hughes JR, Ish-Horowicz D, et al. (2004) Differential cytoplasmic mRNA localisation adjusts pair-rule transcription factor activity to cytoarchitecture in dipteran evolution. Development 131 : 4251–4261. 15280214
56. Wotton KR, Jiménez-Guri E, García Matheu B, Jaeger J (2014) A staging scheme for the development of the scuttle fly Megaselia abdita. PLoS ONE 9: e84421. doi: 10.1371/journal.pone.0084421 24409295
57. Surkova S, Golubkova E, Manu, Panok L, Mamon L, et al. (2013) Quantitative dynamics and increased variability of segmentation gene expression in the Drosophila Krüppel and knirps mutants. Dev Biol 376 : 99–112. doi: 10.1016/j.ydbio.2013.01.008 23333947
58. Jäckle H, Tautz D, Schuh R, Seifert E, Lehmann R (1986) Cross-regulatory interactions among the gap genes of Drosophila. Nature 324 : 668–670.
59. Harding K, Levine M (1988) Gap genes define the limits of antennapedia and bithorax gene expression during early development in Drosophila. EMBO J 7 : 205–214. 2896123
60. Gaul U, Jäckle H (1987) Pole region-dependent repression of the Drosophila gap gene Krüppel by maternal gene products. Cell 51 : 549–555. 3119224
61. Dearolf CR, Topol J, Parker CS (1989) The caudal gene product is a direct activator of fushi tarazu transcription during Drosophila embryogenesis. Nature 341 : 340–343. 2571934
62. Mlodzik M, Gibson G, Gehring WJ (1990) Effects of ectopic expression of caudal during Drosophila development. Development 109 : 271–277. 1976085
63. La Rosée A, Häder T, Taubert H, Rivera-Pomar R, Jäckle H (1997) Mechanism and Bicoid-dependent control of hairy stripe 7 expression in the posterior region of the Drosophila embryo. EMBO J 16 : 4403–4411. 9250684
64. Häder T, La Rosée A, Ziebold U, Busch M, Taubert H, et al. (1998) Activation of posterior pair-rule stripe expression in response to maternal caudal and zygotic knirps activities. Mech Dev 71 : 177–186. 9507113
65. Kraut R, Levine M (1991) Mutually repressive interactions between the gap genes giant and Krüppel define middle body regions of the Drosophila embryo. Development 111 : 611–621. 1893878
66. Rohr KB, Tautz D, Sander K (1999) Segmentation gene expression in the mothmidge Clogmia albipunctata (Diptera, psychodidae) and other primitive dipterans. Dev Genes Evol 209 : 145–154. 10079357
67. Liu PZ, Kaufman TC (2004) hunchback is required for suppression of abdominal identity, and for proper germband growth and segmentation in the intermediate germband insect Oncopeltus fasciatus. Development 131 : 1515–1527. 14998925
68. Pultz MA, Westendorf L, Gale SD, Hawkins K, Lynch J, et al. (2005) A major role for zygotic hunchback in patterning the Nasonia embryo. Development 132 : 3705–3715. 16077090
69. Mito T, Sarashina I, Zhang H, Iwahashi A, Okamoto H, et al. (2005) Non-canonical functions of hunchback in segment patterning of the intermediate germ cricket Gryllus bimaculatus. Development 132 : 2069–2079. 15788457
70. He Z, Cao Y, Yin Y, Wang Z, Chen B, et al. (2006) Role of hunchback in segment patterning of Locusta migratoria manilensis revealed by parental RNAi: 439–445.
71. Schwager EE, Pechmann M, Feitosa NM, McGregor AP, Damen WGM (2009) hunchback functions as a segmentation gene in the spider Achaearanea tepidariorum. Curr Biol 19 : 1333–1340. doi: 10.1016/j.cub.2009.06.061 19631543
72. Wilson MJ, Dearden PK (2011) Diversity in insect axis formation: two orthodenticle genes and hunchback act in anterior patterning and influence dorsoventral organization in the honeybee (Apis mellifera). Development 138 : 3497–3507. doi: 10.1242/dev.067926 21771808
73. Wolff C, Sommer R, Schröder R, Glaser G, Tautz D (1995) Conserved and divergent expression aspects of the Drosophila segmentation gene hunchback in the short germ band embryo of the flour beetle Tribolium. Development 121 : 4227–4236. 8575322
74. Patel NH, Hayward DC, Lall S, Pirkl NR, DiPietro D, et al. (2001) Grasshopper hunchback expression reveals conserved and novel aspects of axis formation and segmentation. Development 128 : 3459–3472. 11566852
75. Marques-Souza H, Aranda M, Tautz D (2008) Delimiting the conserved features of hunchback function for the trunk organization of insects. Development 135 : 881–888. doi: 10.1242/dev.018317 18216167
76. Ben-David J, Chipman AD (2010) Mutual regulatory interactions of the trunk gap genes during blastoderm patterning in the hemipteran Oncopeltus fasciatus. Dev Biol 346 : 140–149. doi: 10.1016/j.ydbio.2010.07.010 20643118
77. Weiss K, Fullerton S (2000) Phenogenetic Drift and the Evolution of Genotype Phenotype Relationships. Theor Popul Biol 195 : 187–95.
78. True JR, Haag ES (2001) Developmental system drift and flexibility in evolutionary trajectories. Evol Dev 3 : 109–19. 11341673
79. Weiss KM (2005) The phenogenetic logic of life. Nat Rev Genet 6 : 36–45. 15630420
80. Haag ES (2007) Compensatory vs. pseudocompensatory evolution in molecular and developmental interactions. Genetica 129 : 45–55. 17109184
81. Pavlicev M, Wagner GP (2012) A model of developmental evolution: selection, pleiotropy and compensation. Trends Ecol Evol 27 : 316–22. doi: 10.1016/j.tree.2012.01.016 22385978
82. Rafiqi AM, Lemke S, Schmidt-Ott U (2011) Megaselia abdita: fixing and devitellinizing embryos. Cold Spring Harb Protoc 2011: pdb.prot5602. doi: 10.1101/pdb.prot5602 21460051
83. Rafiqi AM, Lemke S, Schmidt-Ott U (2011) Megaselia abdita: culturing and egg collection. Cold Spring Harb Protoc 2011: pdb.prot5600. doi: 10.1101/pdb.prot5600 21460049
84. Crombach A, Wotton KR, Cicin-Sain D, Jaeger J (2012) Medium-throughput processing of whole mount in situ hybridisation experiments into gene expression domains. PLoS ONE 7: e46658. doi: 10.1371/journal.pone.0046658 23029561
85. Crombach A, Wotton KR, Cicin-Sain D, Ashyraliyev M, Jaeger J (2012) Efficient reverse-engineering of a developmental gene regulatory network. PLoS Comput Biol 8: e1002589. doi: 10.1371/journal.pcbi.1002589 22807664
86. Surkova S, Myasnikova E, Janssens H, Kozlov KN, Samsonova AA, et al. (2008) Pipeline for acquisition of quantitative data on segmentation gene expression from confocal images. Fly 2 : 1–9. 18849648
87. Rafiqi A, Lemke S, Schmidt-Ott U (2011) The scuttle fly Megaselia abdita (Phoridae): a link between Drosophila and mosquito development. Cold Spring Harb Protoc 4 : 349–353.
88. Wotton KR, Jimenez-Guri E, Crombach A, Cicin-Sain D, Jaeger J (2014) High-resolution gene expression data from blastoderm embryos of the scuttle fly Megaselia abdita. Sci Data doi: 10.1038/sdata.2015.5.
89. Cicin-Sain D, Hermoso Pulido A, Crombach A, Wotton KR, Jiménez-Guri E, et al. (2015) SuperFly: a comparative database for quantified spatio-temporal gene expression patterns in early dipteran embryos. Nucleic Acids Res 43 (database issue): D752–756.
Štítky
Genetika Reprodukční medicína
Článek NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY ModuleČlánek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in NematodesČlánek The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse SpermatocytesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 3
-
Všechny články tohoto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



