-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
Eukaryotic genomes from yeast to man express numerous non-coding RNAs (ncRNAs) that regulate the expression of messenger RNAs (mRNAs) encoding the proteins vital for cell and body function. As faulty ncRNAs impair mRNA expression and contribute to cancers and neurodegenerative disease, it is imperative to understand how ncRNAs are processed and/or degraded. In budding yeast, a conserved RNA shredding machine known as the exosome nibbles at or destroys ncRNAs. The exosome is assisted by a conserved TRAMP exosome cofactor that recruits the exosome to ncRNAs for processing/ degradation. To better understand TRAMP function, we performed a genetic screen to identify genes that improve the growth of TRAMP mutant yeast cells that grow poorly at high temperature. We find that overexpression of the Nab3 RNA binding protein, which belongs to another exosome cofactor, the Nrd1-Nab3-Sen1 (NNS) complex, improves the growth of TRAMP mutant cells. Importantly, Nab3 requires the exosome to improve the growth and ncRNA processing of TRAMP mutant cells. We therefore suggest that Nab3 facilitates TRAMP function by recruiting the exosome to ncRNAs for processing/degradation. We also show that the human RNA binding protein, RALY, like Nab3, can improve the growth of TRAMP mutant cells.
Published in the journal: . PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005044
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005044Summary
Eukaryotic genomes from yeast to man express numerous non-coding RNAs (ncRNAs) that regulate the expression of messenger RNAs (mRNAs) encoding the proteins vital for cell and body function. As faulty ncRNAs impair mRNA expression and contribute to cancers and neurodegenerative disease, it is imperative to understand how ncRNAs are processed and/or degraded. In budding yeast, a conserved RNA shredding machine known as the exosome nibbles at or destroys ncRNAs. The exosome is assisted by a conserved TRAMP exosome cofactor that recruits the exosome to ncRNAs for processing/ degradation. To better understand TRAMP function, we performed a genetic screen to identify genes that improve the growth of TRAMP mutant yeast cells that grow poorly at high temperature. We find that overexpression of the Nab3 RNA binding protein, which belongs to another exosome cofactor, the Nrd1-Nab3-Sen1 (NNS) complex, improves the growth of TRAMP mutant cells. Importantly, Nab3 requires the exosome to improve the growth and ncRNA processing of TRAMP mutant cells. We therefore suggest that Nab3 facilitates TRAMP function by recruiting the exosome to ncRNAs for processing/degradation. We also show that the human RNA binding protein, RALY, like Nab3, can improve the growth of TRAMP mutant cells.
Introduction
Non-coding RNAs (ncRNAs) are fast emerging as key regulators of gene expression in eukaryotic cells [1,2]. Dysregulation of ncRNAs is linked to cancer and neurodegeneration [3,4]. Understanding how ncRNAs are produced, processed, and destroyed is therefore critical to elucidating gene regulation and has become an area of major research focus in recent years. Transcriptional termination and 3’-end processing of ncRNAs are functionally linked but how they are coupled is poorly characterized [5]. In Saccharomyces cerevisiae, unlike most mRNAs, ncRNAs are not terminated and 3’-end processed by the conventional cleavage and polyadenylation machinery, but by a trio of evolutionarily conserved complexes: the RNA exosome, the TRAMP complex, and the NNS complex [5]. Critically, the interactions and mechanisms employed by these complexes to terminate and process/degrade ncRNAs are not well understood.
In budding yeast, ncRNAs are processed and/or degraded by the evolutionarily conserved ribonuclease complex, the RNA exosome [6,7]. The nuclear and cytoplasmic core exosome, which is composed of ten essential subunits, including the key catalytic 3’-5’-riboexonuclease Dis3/Rrp44, forms a ring-shaped structure through which RNA is threaded for processing or degradation [6,7]. The nuclear exosome contains an additional key nuclear catalytic subunit, the 3’-5’-riboexonuclease, Rrp6 [6,7]. The nuclear exosome processes and/or degrades several classes of ncRNAs, including rRNAs, snRNAs, snoRNAs, and tRNAs, as well as some mRNAs [6,7]. In addition, the nuclear exosome rapidly degrades a novel class of ncRNAs, termed cryptic unstable transcripts (CUTs) [8].
The exosome is recruited to RNAs by exosome cofactors that recognize the RNA and regulate exosome function [9,10]. Certain exosome cofactors also facilitate transcriptional termination of ncRNAs [5,10]. Exosome cofactors are usually single RNA binding proteins or protein complexes containing RNA binding proteins that recognize RNAs in a sequence or structure-specific manner and interact with core exosome and/or Rrp6 [5,10]. Three examples of exosome cofactors in Saccharomyces cerevisiae are the Rrp47 protein, the TRAMP complex, and the Nrd1-Nab3-Sen1 (NNS) complex [9,10].
The TRAMP (Trf4/5-Air1/2-Mtr4 Polyadenylation) complex is a key nuclear exosome cofactor that oligoadenylates the 3’-ends of ncRNAs and recruits the exosome to process/degrade these RNAs [11–13]. TRAMP is composed of a non-canonical poly(A) polymerase, Trf4 or Trf5, a zinc-knuckle RNA-binding protein, Air1 or Air2, and an essential RNA helicase, Mtr4 [11–13]. TRAMP targets include ncRNAs, such as snoRNAs and CUTs, and some mRNAs [11–13]. Air1 and Air2, which are critical for TRAMP assembly and function, contain five CCHC zinc knuckles that bind to RNA and Trf4 [11–16]. While tΔrག, trf5Δ, air1Δ, and air2Δ single mutants are viable, the trf4ΔtrΔdouble mutant is inviable and the trf4Δsingle mutant and air1Δair2Δ double mutant are growth impaired, indicating that Trf and Air activity is critical for cell function [13,16,17]. Importantly, human orthologues of Trf4/5, PAPD5/hTRF4–2, Air1/2, ZCCHC7, and Mtr4, hMTR4, have been identified, suggesting a TRAMP-like complex also functions in human cells [16,18].
The NNS (Nrd1-Nab3-Sen1) complex is a second nuclear exosome cofactor, composed of two sequence-specific RNA binding proteins, Nrd1 and Nab3, and an RNA/DNA ATP-dependent helicase, Sen1, that interacts with RNA polymerase II (RNA Pol II) and the exosome to stimulate termination and processing/degradation of ncRNAs [19–22]. The NNS subunits bind to different classes of ncRNAs, including snoRNAs, CUTs, tRNAs, and mRNAs, via NNS-dependent terminators [23,24]. All NNS components are essential, but nrd1, nab3, and sen1 mutants show transcriptional readthrough of NNS terminators in ncRNAs and elevated levels of CUTs [20,21,25–28]. Nrd1 and Nab3 are single RNA recognition motif (RRM)-containing proteins that recognize the RNA sequences GUAG/A and UCUU, respectively, in the NNS terminators [21]. Nrd1 and Nab3 also directly interact with one another via Nab3/Nrd1-binding domains [29,30]. In addition, Nrd1 coprecipitates with the exosome and TRAMP and Nab3 interacts with Sen1 [19,31]. Specifically, Nrd1 directly interacts Rrp6 and Trf4 [32].
In current models for NNS function, Nrd1 binds to the carboxy-terminal domain of RNA Pol II during early transcription of a ncRNA and the Nrd1-Nab3 heterodimer recognizes the ncRNA terminator, recruits the Sen1 helicase to dissociate the elongation complex and terminate transcription in a mechanism involving Sen1 RNA binding and ATP hydrolysis, and recruits the exosome to process/degrade the ncRNA [5,19,29,30,33,34]. Notably, the human Sen1 orthologue, Senataxin, associated with neurodegenerative disorders, promotes termination in human cells, suggesting human counterparts of Nrd1 and Nab3 could exist [35–37].
To better understand the termination/processing of ncRNAs by exosome cofactors and the exosome, cryptic unstable transcripts (CUTs) have become important model ncRNA substrates. CUTs were first identified in budding yeast because they show elevated levels upon deletion of the gene encoding the nuclear exosome subunit, Rrp6 [13]. CUTs are short RNA Pol II-derived transcripts that can regulate gene expression by transcriptional interference or chromatin modification [8]. Two of the best-characterized CUTs are the NEL025c CUT and the IMD2 CUT which contain Nrd1/Nab3 binding sites in their NNS terminators and are terminated/degraded by the NNS complex, TRAMP and the exosome [8,25–28,38]. Importantly, NEL025c and IMD2 CUT levels and terminator readthrough are increased in nrd1, nab3, trf4Δ, and rrp6Δmutants [8,25–28,38]. In mammalian cells, novel ncRNAs termed promoter upstream transcripts (PROMPTs) that share similarity with CUTs are also degraded by the exosome [39].
The interactions between exosome cofactors and the exosome that are required to orchestrate proper termination/processing of ncRNAs are not well characterized. Determining the specific and shared functions of exosome cofactors and whether components of exosome cofactor complexes function only within the complex or can function separately are key areas of current investigation. To provide insight into TRAMP/exosome function, we performed a high copy suppressor screen with a thermosensitive air1/2 TRAMP mutant [16]. Here, we report that Nab3 of the NNS complex is a potent suppressor of air1/2 and trf4ΔTRAMP mutants. Our results suggest that Nab3 facilitates TRAMP function by recruiting Rrp6 to ncRNAs for processing/degradation independent of Nrd1. We also find that the human RNA binding protein, RALY, which shares identity with Nab3, can suppress TRAMP mutants. Combined, these data raise the intriguing possibility that Nab3 and Nrd1 RNA binding proteins may function independently to recruit the exosome to specific ncRNA targets, providing combinatorial flexibility in RNA processing dependent on the number of Nab3/Nrd1 binding sites.
Results
NAB3 suppresses the thermosensitive growth of air1/2 cells
To study the function of TRAMP, we exploited thermosensitive air1-C178R air2ΔTRAMP mutant cells, hereafter referred to as air1/2 cells, containing an integrated C178R substitution in AIR1 zinc knuckle 5 combined with deletion of AIR2 [16]. The air1/2 cells are thermosensitive, show impaired growth at 30°C, and can be suppressed by TRAMP components [16] (Fig. 1A).
Fig. 1. NAB3 suppresses, but NRD1 and SEN1 do not suppress, air1/2 thermosensitive growth. 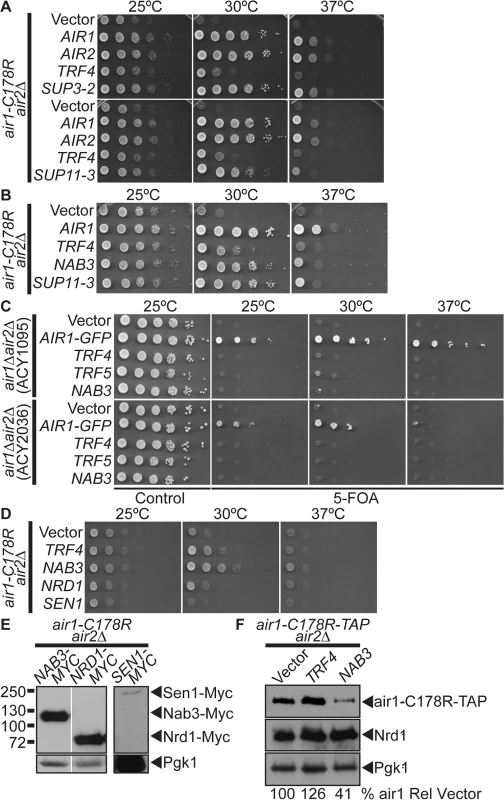
(A) A high copy suppressor screen identifies suppressor plasmid, SUP11–3, containing NAB3, as suppressor of air1-C178R air2Δthermosensitive growth at 30°C. The screen identified forty-four AIR1/2 suppressor plasmids, including SUP3–2 (AIR1). NAB3 suppressor plasmid, SUP11–3 (NAB3), suppresses air1-C178R air2Δ growth at 30°C. The air1-C178R air2Δ cells containing vector, TRAMP component or suppressor 2μ URA3 plasmid were spotted and grown at indicated temperatures. (B) NAB3 suppresses the air1-C178R air2Δ thermosensitive growth at 30°C to the same degree as NAB3 suppressor plasmid, SUP11–3. The air1-C178R air2Δ cells containing vector, AIR1, TRF4, SUP11–3 or NAB3 μ URA3 plasmids were spotted and grown at indicated temperatures. See also S1 Fig. (C) NAB3 is not a bypass suppressor of AIR1/2. NAB3 cannot improve air1Δ air2Δ growth. Two air1Δ air2Δ strains (ACY1095, ACY2036) containing AIR2 URA3 maintenance plasmid and vector, AIR1-GFP, TRF4, TRF5 or NAB3 2μ HIS3 plasmid were spotted on control/5-FOA and grown at indicated temperatures. (D) NRD1 and SEN1 do not suppress the air1-C178R air2Δ thermosensitive growth at 30°C. The air1-C178R air2Δ cells containing vector, TRF4, NAB3, NRD1 or SEN1 2μ URA3 plasmid were spotted and grown at indicated temperatures. See also S1 Fig. (E) Exogenous Nrd1 and Sen1 proteins are expressed in air1-C178R air2Δ cells with Nrd1 expressed to a similar level as Nab3. Lysates of air1-C178R air2Δ cells expressing Myc-tagged Nab3, Nrd1 or Sen1 at 30°C were analyzed by immunoblotting to detect Myc-tagged proteins and 3-phosphoglycerate kinase (Pgk1) as a loading control. Nonadjacent lanes in the same immunoblot are separated by white space. Different immunoblots are outlined by black boxes. See also S2 Fig. (F) Nab3 expression does not increase the level of air1-C178R mutant protein. Lysates of air1-C178R-TAP air2Δ cells containing vector or expressing Trf4 or Nab3 protein grown at 25°C were analyzed by immunoblotting to detect air1-C178R-TAP protein and 3-phosphoglycerate kinase (Pgk1) as a loading control. Percentage of air1-C178R-TAP relative to Pgk1 loading control and cells containing vector alone (% air1-C178R Rel Vector) is shown below lanes and was calculated as described in Materials and Methods. Quantitation refers to specific experiment shown but is representative of multiple experiments. To identify additional factors that facilitate TRAMP function, we performed a high-copy suppressor screen with air1/2 cells. From 60 suppressor (SUP) plasmids that improved air1/2 growth at 30°C, 44 plasmids contained either AIR1 (e.g. SUP3–2) or AIR2 (Fig. 1A). Four other plasmids, including SUP11–3, all contained a large insert including the NAB3 gene and potently suppress air1/2 growth at 30°C (Fig. 1A). A plasmid clone of NAB3 suppresses air1/2 growth at 30°C to a similar degree as suppressor SUP11–3 (Fig. 1B), confirming that suppression is conferred by NAB3. NAB3 suppression of air1/2 growth could mean that Nab3 specifically facilitates Air1 function or more generally facilitates TRAMP function. To this point, NAB3 can suppress the slow growth of another TRAMP mutant, trf4Δ, at 25°C (S1A Fig), suggesting that Nab3 facilitates TRAMP function.
As TRAMP is an exosome cofactor and Air1/2 facilitate RNA recognition, the Nab3 RNA binding protein could simply replace RNA binding function of the Air proteins in air1/2 cells and target TRAMP/exosome to RNA substrates. This suppression mechanism predicts that NAB3 should bypass the requirement for AIR1/2. However, NAB3 cannot improve air1Δair2Δgrowth (Fig. 1C) and thus is not a bypass suppressor of AIR1/2 function.
To assess the specificity of NAB3 suppression, we examined whether other RNA-binding protein genes can suppress air1/2 growth. NAB3 was identified in a screen for nuclear polyadenylated RNA-binding (Nab) proteins [40]. NAB/hnRNP genes, NPL3/NAB1, HRP1/NAB4, PUB1 or NAB2 do not suppress air1/2 growth (S1B Fig), indicating that air1/2 suppression is specific to NAB3 and that Nab3 possesses a unique function, as compared to other RNA binding proteins.
NRD1 and SEN1 do not suppress air1/2 thermosensitive cell growth
Nab3 is part of the Nrd1-Nab3-Sen1 (NNS) complex that functions in the termination and 3’-end processing/degradation of ncRNAs [19,20]. Nab3 directly binds to Nrd1 [29,30], and together, Nab3 and Nrd1 recognize terminators in ncRNAs [29]. Nab3 also binds to the RNA helicase, Sen1 [31], which is thought to unwind the RNA-DNA hybrid to terminate transcription [5]. We tested whether NRD1 and SEN1 could suppress air1/2 growth. Unlike NAB3, NRD1 and SEN1 do not suppress air1/2 growth at 30°C (Fig. 1D), even though both exogenous Nrd1 and Sen1 are expressed in air1/2 cells (Fig. 1E). NRD1 and SEN1 also do not suppress trf4Δ slow growth (S1A Fig). Importantly, both exogenous Nrd1 and Nab3 are overexpressed by at least 8-fold relative to endogenous Nrd1 and Nab3 in air1/2 cells (S2 Fig). As overexpression of exogenous Sen1 could not be verified at this time, it remains possible that SEN1 does not suppress air1/2 cells because it is not significantly overexpressed. These results indicate that air1/2 suppression is specific to NAB3 and highlight a functional difference between Nab3 and Nrd1.
NAB3 does not increase air1 mutant or Nrd1 protein levels in air1/2 cells
Previous studies revealed that the air1-C178R mutant protein is unstable and impacts the integrity of TRAMP in air1/2 cells [16]. Trf4 increases the air1-C178R level and suppresses air1/2 growth, suggesting that Trf4-mediated suppression involves air1-C178R stabilization [16]. To determine whether Nab3 suppression also involves air1-C178R stabilization, we examined the air1-C178R level in air1/2 cells that overexpress Nab3. Unlike Trf4, Nab3 expression does not increase the air1-C178R level in air1/2 cells (Fig. 1F). Nab3 also does not appreciably alter the level of endogenous Nrd1 in air1/2 cells (Fig. 1F).
The Nab3 Nrd1-binding domain is not essential for air1/2 suppression
Nab3 and Nrd1 directly interact and are thought to work together to recognize RNAs for termination/processing [5,10]. However, only NAB3 suppresses air1/2 cells, even though Nab3 and Nrd1 are expressed at similar levels (see Fig. 1E). This result suggests that Nab3 might not require interaction with Nrd1 to suppress air1/2 cells and could function independent of Nrd1. To address this point, we tested whether a nab3 mutant that lacks the Nrd1-binding domain can still suppress air1/2 cells. Previously, the Nrd1-binding domain (NBD) of Nab3 was mapped to residues 204–248 [30] (Fig. 2A). We thus generated a Nab3 deletion mutant that lacks the Nrd1-binding residues 204–248, nab3-Δ ΔNBD, to test for air1/2 suppression. To assess whether Nab3 RNA binding is important for air1/2 suppression, we also tested a Nab3 RNA recognition motif (RRM) mutant, nab3–11 [22] (Fig. 2A). To confirm that nab3-ΔNBD binding to Nrd1 is impaired, we precipitated TAP-tagged Nrd1 from lysates of NRD1-TAP cells expressing Myc-tagged Nab3, nab3–11, and nab3-ΔNBD and analyzed the bound fractions by immunoblotting. The nab3-ΔNBD mutant protein shows greatly reduced binding to Nrd1 (8% bound) compared to wild-type Nab3 (2B and S3A Figs). In contrast, the nab3–11 RRM mutant protein shows no decrease in binding to Nrd1 (Fig. 2B).
Fig. 2. The Nab3 Nrd1-binding domain is not essential for suppression of air1/2 thermosensitive growth. 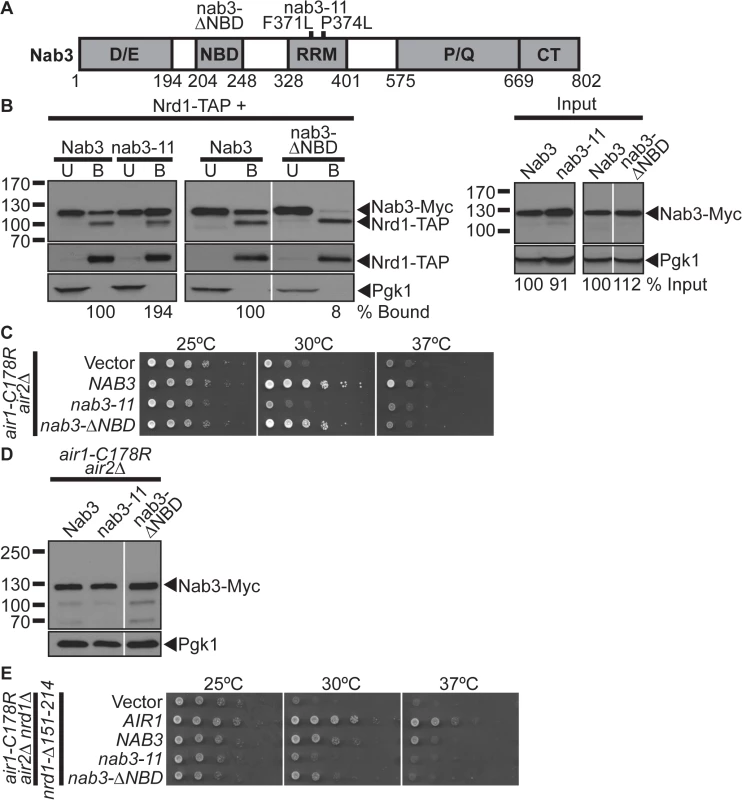
(A) Schematic of Nab3 depicting domain structure. Nab3 contains five domains: an Asp/Glu-rich domain (D/E; residues 1–194), a Nrd1-binding domain (NBD; 204–248), an RNA recognition motif (RRM; 328–401), a Pro/Gln-rich domain (P/Q; 575–668), and a carboxy-terminal self-association domain (CT; 669–802). Residue deletion in nab3-ΔNBD mutant and residue substitutions, F371L and P374L, in the nab3 RRM mutant, nab3–11, are depicted above. Residue positions are depicted below. See also S6 Fig for sequence. (B) The nab3-ΔNBD mutant protein shows greatly reduced binding to Nrd1. TAP-tagged Nrd1 was precipitated from lysates of NRD1-TAP cells expressing Myc-tagged Nab3, nab3–11 or nab3-ΔNBD and bound (B), unbound (U), and input fractions were analyzed by immunoblotting to detect Nab3-Myc proteins and 3-phosphoglycerate kinase (Pgk1) as a loading control. Samples were also probed with anti-Nrd1 antibody to detect Nrd1-TAP proteins. The percentage of bound Nab3 protein relative to input protein and bound wild-type Nab3 (% Bound) is shown below the bound lanes. The percentage of input Nab3 protein relative to input wild-type Nab3 protein (% Input) is shown below the input lanes. The percentages of protein were calculated as described in Materials and Methods. Quantitation refers to specific experiment shown but is representative of multiple experiments. Nonadjacent lanes in the same immunoblot are separated by white space. Different immunoblots are outlined by black boxes. The original immunoblot for the spliced immunoblot on the right is shown in S3A Fig. (C) The nab3-ΔNBD mutant suppresses, but the nab3–11 RRM mutant does not suppress, air1-C178R air2Δ thermosensitive growth at 30°C. The air1-C178R air2Δ cells containing vector, NAB3, nab3–11 or nab3-ΔNBD 2μ URA3 plasmid were spotted and grown at indicated temperatures. See also S1 and S3B Figs. (D) nab3-ΔNBD mutant protein is expressed in air1-C178R air2Δ cells to a similar level as Nab3. Lysates of air1-C178R air2Δ cells expressing Myc-tagged Nab3, nab3–11 or nab3-ΔNBD at 30°C were analyzed by immunoblotting detect Myc-tagged proteins and 3-phosphoglycerate kinase (Pgk1) as a loading control. Nonadjacent lanes in the same immunoblot are separated by white space. (E) NAB3 and nab3-ΔNBD suppress air1-C178R air2Δ nrd1-Δ151–214 thermosensitive growth at 30°C. The air1-C178R air2Δ nrd1Δ cells containing nrd1-Δ151–214 CEN HIS3 plasmid and vector, AIR1, NAB3, nab3–11, or nab3-ΔNBD 2μ URA3 plasmid were spotted and grown at indicated temperatures. We then tested whether the nab3-ΔNBD and nab3–11 RRM mutants can suppress air1/2 growth. The nab3-ΔNBD mutant suppresses air1/2 growth similarly but not identically to NAB3 at 30°C (Fig. 2C). The nab3-ΔNBD mutant protein is expressed at a similar level to wild-type Nab3 protein (Fig. 2D). This result suggests that the Nab3 Nrd1-binding domain is not essential for air1/2 suppression. In contrast, the nab3–11 mutant cannot suppress air1/2 growth at 30°C (Fig. 2C). Notably, nab3-ΔNBD, but not nab3–11, also suppresses trf4Δ growth (S1A Fig).
To further examine whether Nab3 requires Nrd1 interaction to suppress air1/2 cells, we also tested whether NAB3 and nab3-ΔNBD can suppress air1/2 nrd1Δ cells that only express a nrd1-Δ151–214 variant that lacks the Nab3-binding domain. NAB3 and nab3-ΔNBD suppress air1/2 nrd1-Δ151–214 growth at 30°C (Fig. 2E). Combined, these data suggest that Nab3 does not require interaction with Nrd1, but must interact with RNA, to suppress air1/2 growth.
As the Nab3 Nrd1-binding domain is not essential for air1/2 suppression, we assessed whether this domain is essential for Nab3 function. We tested whether the nab3-ΔNBD mutant can function as the sole copy of the essential NAB3 gene. The nab3-ΔNBD mutant cells are viable, but show a moderate growth defect, while nab3–11 mutant cells exhibit a severe growth defect (Fig. 3E), indicating that the Nab3 Nrd1-binding domain and thus Nab3 interaction with Nrd1 is not essential for the cellular function of Nab3.
Fig. 3. Nab3 RNA recognition motif is essential for suppression of air1/2 thermosensitive growth. 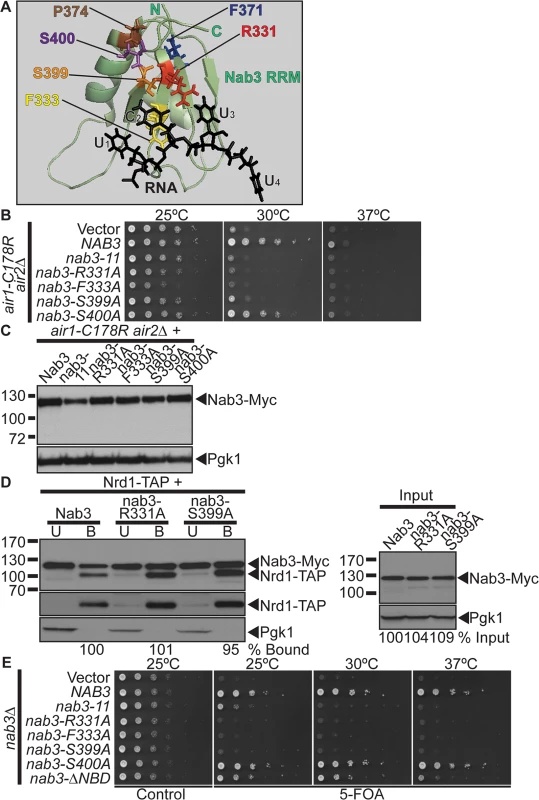
(A) NMR structure of Nab3 RRM in complex with UCUU RNA, which highlights key Nab3 RRM residues that contact the RNA [41]. The Nab3 RRM-RNA NMR structure (PDB ID: 2L41) shows that the Nab3 RRM forms a four-stranded β-sheet packed against two α–helices [41]. The Nab3 RRM (green; Nab3 residues 321–415) interacts with the U1C2U3U4 RNA oligonucleotide (black). Nab3 RRM β-strand residues, Arg331 (red), Phe333 (yellow), and Ser399 (orange), make specific contacts with the C2 nucleotide. Ser399 (orange) also contacts the U3 nucleotide. The Nab3 RRM residues, Phe371 (blue) and Pro374 (brown), mutated in the nab3–11 (nab3-F371L-P374L) RRM mutant, are also highlighted. The Nab3 RRM-RNA NMR structure was reproduced from the PDB file 2L41 [41] using MacPyMOL software [62] and altered and annotated using Adobe Photoshop and Illustrator CS4 (Adobe). (B) nab3 RRM mutants, nab3-R331A, nab3-F333A, nab3-S399A do not suppress the air1-C178R air2Δ thermosensitive growth at 30°C. nab3 RRM mutant, nab3-S400A, suppresses air1-C178R air2Δ thermosensitive growth at 30°C. The air1-C178R air2Δ cells containing vector, NAB3 or nab3 RRM mutant 2μURA3 plasmid were spotted and grown at indicated temperatures. See also S1 and S2 Figs. (C) All nab3 RRM mutant proteins, except nab3–11, are expressed in air1-C178R air2Δ cells to similar levels as Nab3. Lysates of air1-C178R air2Δ cells expressing Myc-tagged Nab3 or nab3 RRM mutants at 30°C were analyzed by immunoblotting to detect Myc-tagged proteins and 3-phosphoglycerate kinase (Pgk1) as a loading control. (D) The nab3-R331A and nab3-S399A RRM mutant proteins show binding to Nrd1 similar to wild-type Nab3. TAP-tagged Nrd1 was precipitated from lysates of NRD1-TAP cells expressing Myc-tagged Nab3, nab3-R331A or nab3-S399A and bound (B), unbound (U), and input fractions were analyzed by immunoblotting to detect Nab3-Myc proteins, Nrd1-TAP proteins and 3-phosphoglycerate kinase (Pgk1) as a loading control. The percentage of bound Nab3 relative to input protein and bound wild-type Nab3 (% Bound) is shown below the bound lanes. The percentage of input Nab3 protein relative to input wild-type Nab3 protein (% Input) is shown below the input lanes. The percentages of protein were calculated as described in Materials and Methods. Quantitation refers to specific experiment shown but is representative of multiple experiments. The original immunoblot is shown in S3A Fig. (E) nab3Δcells expressing nab3 RRM mutants, nab3-R331A, nab3-F333A or nab3-S399A, are not viable, but nab3Δ cells expressing nab3-S400A RRM mutant or nab3-Δ NBD mutant are viable. nab3Δ cells expressing nab3–11 RRM mutant show a severe growth defect. nab3Δ cells containing NAB3 URA3 maintenance plasmid and vector, NAB3, nab3 RRM mutant or nab3-Δ NBD mutant 2μ HIS3 plasmid were spotted on control/5-FOA and grown at indicated temperatures. The Nab3 RNA recognition motif is essential for air1/2 suppression
The inability of the nab3–11 RRM mutant to suppress air1/2 growth suggests that the Nab3 RRM and RNA interaction is critical for suppression. To further analyze the importance of the Nab3 RRM in air1/2 suppression, we took advantage of a recent NMR structure of the Nab3 RRM in complex with UCUU RNA, the Nab3 RNA binding site [41]. The Nab3 RRM bound to RNA forms a four-stranded β-sheet packed against two α-helices similar to canonical RRMs [41]. Key residues in the β-strands interact specifically with the U1C2U3U4 RNA sequence [41] (Fig. 3A). In particular, Nab3 residues Arg331, Phe333, and Ser399 make contacts with the C2 nucleotide and Ser399 also contacts the U3 nucleotide [41] (Fig. 3A). Importantly, Nab3 RRM mutants R331A and S399A show a 3–4-fold decrease in binding to RNA in vitro [41]. We thus generated nab3 RRM mutants, nab3-R331A, nab3-F333A, and nab3-S399A and tested if these nab3 RRM mutants can suppress air1/2 growth. We also generated and tested the nab3 RRM mutant, nab3-S400A, as the Ser400 residue neighbors the key Ser399 nucleotide-binding residue in the Nab3 RRM. The nab3-R331A, nab3-F333A, and nab3-S399A RRM mutants, like nab3–11, do not suppress air1/2 growth at 30°C (Fig. 3B). These nab3 RRM mutants also do not suppress trf4 Δ growth (S1A Fig). The nab3-S400A RRM mutant, like NAB3, however, suppresses air1/2 growth at 30°C (Fig. 3B). Thus, Nab3 RRM residues, Arg331, Phe333, and Ser399, are critical for air1/2 suppression, supporting the notion that the Nab3 RNA interaction is essential for suppression.
Critically, all the nab3 RRM mutants, except nab3–11, are expressed at similar levels to Nab3 (Fig. 3C). Expression of nab3 RRM mutants in wild-type cells does not impair growth, showing that the inability of most nab3 RRM mutants to suppress air1/2 growth is not due to toxicity (S3B Fig).
The nab3 RRM mutants could fail to suppress air1/2 growth because mutations in the Nab3 RRM disrupt the overall folding of Nab3, rather than the Nab3 RRM alone. To test whether nab3 RRM mutants are folded correctly, we examined the interaction between nab3 RRM mutants, nab3-R331A and nab3-S399A, and Nrd1. We precipitated TAP-tagged Nrd1 from lysates of NRD1-TAP yeast cells expressing Myc-tagged nab3-R331, nab3-S399A or Nab3, and analyzed the bound fractions by immunoblotting. The nab3-R331A and nab3-S399A RRM mutants show binding to Nrd1 similar to wild-type Nab3 (95–101% bound; Fig. 3D). These results show that Nab3 RRM mutations do not impair interaction of Nab3 with Nrd1, indicating that overall Nab3 folding is not disrupted in these mutants.
To determine if the nab3 RRM mutants are functional in vivo, we tested whether the nab3 RRM mutants can function as the sole copy of the essential NAB3 gene. The nab3-R331A, nab3-F333A, and nab3-S399A mutants are not viable, whereas nab3-S400A mutant has growth comparable to NAB3 (Fig. 3E). Thus, Nab3 RNA binding is essential for Nab3 function in vivo.
NAB3 reduces IMD2 CUT terminator readthrough product from a reporter in air1/2 cells
Nab3 and Nrd1 recognize elements in the terminators of ncRNAs/CUTs and mRNAs to facilitate termination and processing/degradation [5,20,21]. Notably, nrd1/nab3 mutants exhibit readthrough of NNS terminators in ncRNAs and Nrd1/Nab3 binding site mutations within these NNS terminators also cause readthrough [5,8,20,21]. Moreover, trf4 Δ cells show readthrough of RNA terminators [42,43]. To begin to assess termination of CUTs in air1/2 cells and test the impact of NAB3 on CUT termination, we employed a pREF-GFP reporter for CUT terminator readthrough [38]. The pREF-GFP reporter contains a galactose-inducible promoter and the IMD2 CUT intergenic terminator (IT) upstream of GFP [38] (Fig. 4A). In wild-type cells, upon galactose induction, little GFP is observed as transcription is efficiently terminated at the terminator before GFP. In termination defective cells, GFP is produced as transcription is not terminated at the terminator and readthrough to GFP occurs. We galactose-induced the pREF-GFP reporter in wild-type, air1/2, and other TRAMP mutants and examined the GFP level in the cells by immunoblotting. GFP expression is increased in air1/2 and TRAMP mutants containing pREF-GFP (Fig. 4B).
Fig. 4. NAB3 reduces the level of IMD2 CUT terminator readthrough product from a reporter and native IMD2 CUT and readthrough RNA, but NAB3 does not significantly affect the termination of native IMD2 CUT in air1/2 cells. 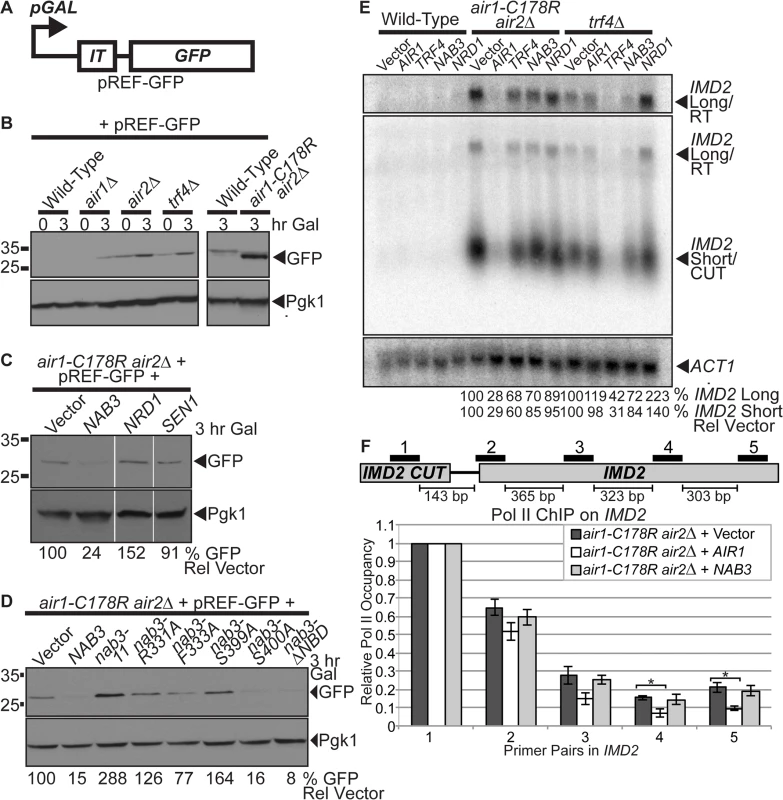
(A) Schematic of pREF-GFP IMD2 CUT terminator reporter plasmid [38]. The pREF-GFP reporter contains the IMD2 CUT intergenic terminator (IT) upstream of GFP open reading frame (GFP) under the control of a galactose-inducible promoter (pGAL). (B) Level of GFP readthrough product from pREF-GFP reporter is increased in air1-C178R air2Δ cells and other TRAMP mutants. Wild-type, air1Δ, air2 Δ, trf4Δ, and air1-C178R air2Δ cells containing pREF-GFP reporter were grown in the presence of galactose (Gal) to induce to GFP expression for 3hr at 30°C and lysates were analyzed by immunoblotting to detect GFP and 3-phosphoglycerate kinase (Pgk1) as a loading control. Samples of cells just after addition of galactose (0 hour time point) were also analyzed. Different immunoblots are outlined by black boxes. (C) NAB3, but not NRD1 or SEN1, decreases level of GFP readthrough product from pREF-GFP reporter in air1-C178R air2 Δ cells. air1-C178R air2 Δ cells containing pREF-GFP reporter plasmid and vector, NAB3, NRD1 or SEN1 were grown in the presence of galactose (Gal) to induce to GFP expression for 3hr at 30°C and lysates of cells were analyzed by immunoblotting as described previously. Nonadjacent lanes in the same immunoblot are separated by white space. (D) nab3-Δ NBD mutant, like NAB3, decreases level of GFP readthrough product from pREF-GFP reporter in air1-C178R air2Δ cells. nab3-R331A, nab3-F333A, and nab3-S399A RRM mutants do not decrease level of GFP from pREF-GFP reporter in air1/2 cells. air1-C178R air2Δ cells containing pREF-GFP reporter plasmid and vector, NAB3, nab3-ΔNBD mutant or nab3 RRM mutants were grown in the presence of galactose (Gal) to induce to GFP expression for 3hr at 30°C and lysates were analyzed by immunoblotting as described previously. The percentage of GFP relative to Pgk1 loading control and GFP in cells containing vector alone (% GFP Rel Vector) is shown below the lanes and was calculated as described in Materials and Methods. Quantitation refers to specific experiment shown but is representative of multiple experiments. (E) NAB3 reduces the levels of native IMD2 CUT and readthrough RNA in air1-C178R air2 Δ and trf4Δ mutant cells. Northern blot of total RNA from wild-type, air1-C178R air2Δ, and trf4Δ cells containing vector, AIR1, TRF4, NAB3 or NRD1 grown at 30°C were probed with an IMD2 CUT-specific probe. Northern blot was probed with ACT1 probe as a loading control. The IMD2 CUT (IMD2 Short/CUT) and IMD2 CUT readthrough RNA (IMD2 Long/RT) are labeled. A longer exposure of IMD2 CUT readthrough RNA is shown above. The percentage of IMD2 CUT and readthrough RNA relative to ACT1 loading control and cells containing vector alone (% IMD2 Short Rel Vector; % IMD2 Long Rel Vector) is shown below lanes and was calculated as described in Materials and Methods. Quantitation refers to specific experiment shown but is representative of multiple experiments. See also S4A Fig. (F) NAB3 does not significantly affect Pol II occupancy downstream of the IMD2 CUT at Primer Pair 2–5 positions in air1-C178R air2Δ cells relative to air1/2 cells containing vector alone (p-value ≥ 0.4), suggesting that Nab3 overexpression does not significantly affect IMD2 CUT termination in air1/2 cells. As a control, AIR1 significantly decreases Pol II occupancy downstream of the IMD2 CUT in air1-C178R air2Δ cells at Primer Pair 4 and 5 positions compared to air1/2 cells containing vector alone (p-value ≤ 0.05), indicating that Air1 significantly affects termination and suggesting that air1/2 cells have a termination defect. Anti-Pol II ChIP was performed on air1-C178R air2Δ cells containing vector, AIR1 or NAB3 and relative Pol II occupancy was measured across IMD2 gene by qPCR with IMD2 Primer Pair 1–5 as described in Material and Methods. Mean RNA Pol II occupancy values from three independent experiments normalized to Primer Pair 1 within IMD2 CUT are shown with error bars that represent standard error of the mean. Statistical significance of differences in mean Pol II occupancy values was determined using unpaired t test and significant differences in mean values (p-value ≤ 0.05) are denoted with asterisks. Schematic of IMD2 CUT and downstream IMD2 gene is shown with positions of IMD2 qPCR Primer Pairs 1–5 above and base pair distances between primer pairs below. See also S4B Fig. Having established that air1/2 cells express GFP from pREF-GFP, suggesting readthrough of the IMD2 CUT terminator and/or impaired degradation of the readthrough RNA, we wished to determine if NAB3 impacts the GFP level in air1/2 cells containing pREF-GFP. NAB3 greatly reduces the GFP level (24% GFP), NRD1 increases the GFP level (152% GFP), and SEN1 does not affect the GFP level (91% GFP) in air1/2 cells (Fig. 4C). These data suggest that NAB3 reduces the readthrough of the IMD2 CUT terminator and/or increases the degradation of the IMD2 readthrough RNA in air1/2 cells, suggesting that NAB3 can improve the termination/degradation of CUTs in air1/2 cells.
To determine whether the NAB3 effect on IMD2 terminator readthrough and/or IMD2 readthrough RNA degradation in air1/2 cells requires Nab3 interaction with RNA or Nrd1, we tested if the nab3 RRM mutants and nab3–204–248 NBD mutant affect the GFP level. Most nab3 RRM mutants do not reduce the GFP level (126–288% GFP), but the nab3-ΔNBD mutant greatly decreases the GFP level (8% GFP) in air1/2 cells (Fig. 4D). These data suggest that the NAB3-mediated decrease in IMD2 CUT terminator readthrough and/or increase in IMD2 readthrough RNA degradation in air1/2 cells requires the Nab3 RRM, but does not require Nrd1 interaction.
NAB3 decreases the levels of native IMD2 CUT and readthrough RNA in air1/2 and trf4Δ cells
NAB3 reduction of the GFP level from the pREF-GFP reporter in air1/2 cells suggests that Nab3 decreases IMD2 CUT readthrough, but the decrease in GFP level could also result from downstream effects of Nab3 on RNA processing/degradation or translation. To test if NAB3 affects the native IMD2 CUT in air1/2 or trf4Δ cells, we probed a Northern blot of total RNA from air1/2, trf4Δ or wild-type cells containing vector, AIR1, TRF4, NAB3 or NRD1, with an IMD2 CUT-specific probe. The level of the short IMD2 CUT is greatly increased in air1/2 and trf4Δ cells with vector alone, compared to wild-type cells with vector alone, as expected for TRAMP mutants that impair exosome degradation of CUTs (Fig. 4E). Importantly, the level of a long IMD2 CUT readthrough RNA is also increased in air1/2 and trf4Δ cells with vector alone, relative to wild-type cells, consistent with the increased GFP from pREF-GFP in these cells and supporting the notion that TRAMP mutants also have termination defects (Fig. 4E). AIR1 greatly reduces to 28–29% and TRF4 partially reduces to 60–68% the levels of the IMD2 CUT and readthrough RNA in air1/2 cells, relative to cells containing vector alone, consistent with AIR1 and TRF4 reactivating TRAMP degradation/termination (Fig. 4E). NAB3 reduces to 85% the level of the IMD2 CUT, whereas NRD1 reduces to 95% the level of the IMD2 CUT, in air1/2 cells (Fig. 4E). Moreover, NAB3 decreases to 70% the IMD2 CUT readthrough RNA, whereas NRD1 decreases to 89% the readthrough RNA, in air1/2 cells (Fig. 4E). In addition, NAB3 reduces to 72–84% the level of IMD2 CUT and readthrough RNA, but NRD1 does not decrease the level of the IMD2 CUT and readthrough RNA in trf4Δ cells (Fig. 4E). These data indicate that NAB3 can reduce both the IMD2 CUT and readthrough RNA in air1/2 cells and does so to a greater extent than NRD1, suggesting that Nab3 affects the degradation/termination of the IMD2 CUT and readthrough RNA to a larger degree than Nrd1. NAB3 suppression of TRAMP mutant growth therefore correlates with NAB3 reduction of IMD2 CUT and readthrough RNA in TRAMP mutant cells.
To determine if the NAB3 decrease of the IMD2 CUT and readthrough RNA in air1/2 cells is dependent on Nab3 interaction with RNA or Nrd1, we probed a Northern blot of total RNA from air1/2 cells containing vector, NAB3, nab3 RRM mutants or nab3-Δ NBD mutant with an IMD2 CUT-specific probe. nab3-Δ NBD decreases the level of IMD2 CUT and readthrough RNA similar to NAB3 in air1/2 cells (S4A Fig). In contrast, the nab3 RRM mutants, nab3–11, nab3-R331A, and nab3-S399A, decrease the level of the IMD2 CUT and readthrough RNA to a lesser degree than NAB3 (S4A Fig). These data support the idea that Nab3 requires RNA interaction, but does not require Nrd1 binding, to terminate/degrade and decrease the IMD2 CUT readthrough RNA.
NAB3 does not significantly affect termination of native IMD2 CUT in air1/2 cells
The Nab3-mediated decrease in the level of the IMD2 CUT readthrough RNA detected in air1/2 cells could be due to increased termination and/or increased degradation. To assess whether Nab3 affects the termination of the native IMD2 CUT in air1/2 cells, we examined RNA Pol II occupancy on the IMD2 gene in air1/2 cells containing vector, AIR1 or NAB3 by Pol II ChIP. We employed five primer pairs to examine Pol II occupancy and normalized all data back to Primer Pair 1 located within the IMD2 CUT (Fig. 4F). Given that the chromatin was sheared to a size ranging from 300–500 base pairs and the distances between the primer pairs (Fig. 4F), we expected to detect differences in Pol II occupancy most readily with Primer Pair 4 and 5. The air1/2 cells expressing AIR1 show a statistically significant decrease in Pol II occupancy downstream of the IMD2 CUT at Primer Pair 4 and 5 positions relative to the air1/2 cells containing vector alone (p-value ≤ 0.05; Fig. 4F). In contrast, the air1/2 cells expressing NAB3 show no statistically significant change in Pol II occupancy downstream of the IMD2 CUT at Primer Pair 2–5 positions relative to air1/2 cells containing vector alone (p-value ≥ 0.4; Fig. 4F). These data suggest that overexpression of Nab3 does not significantly affect termination of the IMD2 CUT in air1/2 cells. To extend this analysis, we also performed Pol II ChIP on the native snR13 snoRNA gene, which contains a well-characterized NNS-dependent terminator [20,21,44], in air1/2 cells containing vector or NAB3. As described for the IMD2 CUT, air1/2 cells expressing NAB3 do not exhibit a statistically significant change in Pol II occupancy downstream of snR13 compared to air1/2 cells containing vector alone (p-value ≥ 0.3; S4B Fig), suggesting overexpression of Nab3 does not significantly affect termination of the snR13 gene in air1/2 cells. These results indicate that Nab3 overexpression does not have a significant effect on the termination of the IMD2 CUT, suggesting that Nab3 suppression of air1/2 cells and reduction of IMD2 CUT readthrough RNA predominantly involves Nab3 rescue of degradation.
NAB3 suppression of air1/2 and reduction of IMD2 CUT and readthrough RNA is dependent on RRP6
The TRAMP and NNS complexes are intimately linked to the catalytic exosome subunit, Rrp6, which is critical for processing/degrading ncRNAs/CUTs [10]. In particular, TRAMP stimulates Rrp6-mediated degradation of RNA in vitro [45], Nrd1 co-precipitates with Rrp6 [19], and the nab3–11 RRM mutant exhibits a negative genetic interaction with RRP6 [46]. We therefore examined whether NAB3 suppression of air1/2 cells depends on RRP6. We deleted RRP6 from the air1/2 cells and tested if NAB3 could suppress the growth of air1/2 rrp6Δ cells at 30°C. We also tested if AIR1, AIR2, TRF4, nab3–11 RRM mutant or nab3-Δ NBD mutant could suppress the air1/2 rrp6Δ mutant. AIR1, AIR2, and TRF4 suppress air1/2 rrp6Δ growth at 30°C (Fig. 5A). However, strikingly, both NAB3 and nab3-Δ NBD do not suppress air1/2 rrp6Δ growth at 30°C (Fig. 5A). nab3–11 also does not suppress the air1/2 rrp6Δ cells (Fig. 5A). These results indicate that NAB3 suppression of air1/2 growth requires RRP6.
Fig. 5. NAB3 suppression of air1/2 thermosensitive growth and reduction of IMD2 CUT and readthrough RNA in air1/2 cells is dependent on RRP6 and Nab3 interacts with Rrp6 independent of Nrd1. 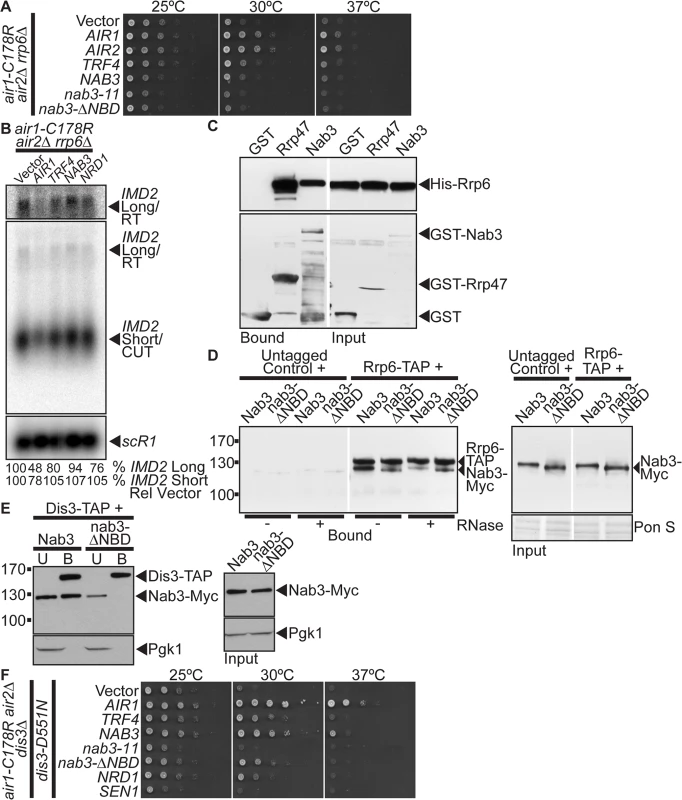
(A) NAB3 and nab3-Δ NBD mutant do not suppress the thermosensitive growth of air1-C178R air2Δ rrp6Δ cells that contain a deletion of RRP6 at 30°C. air1-C178R air2Δ rrp6Δ cells containing vector, AIR1, AIR2, TRF4, NAB3, nab3–11, nab3-Δ NBD or NRD1 2 μ URA3 plasmid were spotted and grown at indicated temperatures. See also S5A Fig. (B) NAB3 does not decrease the levels of native IMD2 CUT and only weakly decreases the IMD2 CUT readthrough RNA in air1-C178R air2Δ rrp6Δ cells. Northern blot of total RNA from air1-C178R air2Δ rrp6Δ cells expressing vector, AIR1, TRF4, NAB3 or NRD1 grown at 30°C were probed with an IMD2 CUT-specific probe. Northern blot was probed with scR1 probe as a loading control. The IMD2 CUT (IMD2 Short/CUT) and IMD2 CUT readthrough RNA (IMD2 Long/RT) are labeled. A longer exposure of IMD2 CUT readthrough RNA is shown above. The percentage of IMD2 CUT and readthrough RNA relative to sCR1 loading control and cells containing vector alone (% IMD2 Short Rel Vector; % IMD2 Long Rel Vector) is shown below lanes and was calculated as described in Materials and Methods. Quantitation refers to specific experiment shown but is representative of multiple experiments. (C) Nab3 directly interacts with Rrp6 in vitro. Recombinant GST-Nab3, GST-Rrp47 or GST was incubated with recombinant His-tagged Rrp6 and glutathione Sepharose beads and bound and input fractions were analyzed by SDS-PAGE and immunoblotting to detect His-Rrp6 and GST fusion proteins. Nonadjacent lanes in the same immunoblot are separated by white space. (D) nab3-ΔNBD mutant protein binds to Rrp6 in an RNA-independent manner. TAP-tagged Rrp6 was precipitated from lysates of RRP6-TAP or untagged control cells expressing Myc-tagged Nab3 or nab3-ΔNBD in the absence or presence of RNase A. Bound and input fractions were analyzed by immunoblotting to detect Nab3-Myc proteins and membrane was stained with Ponceau S (Pon S) stain to detect total protein as a loading control. Nonadjacent lanes in the same immunoblot are separated by white space. Different immunoblots are outlined by black boxes. See also S5B Fig. (E) nab3-ΔNBD mutant protein, unlike wild-type Nab3, does not bind to Dis3. TAP-tagged Dis3 was precipitated from lysates of DIS3-TAP cells expressing Myc-tagged Nab3 or nab3-ΔNBD and bound (B), unbound (U), and input fractions were analyzed by immunoblotting to detect Nab3-Myc proteins and 3-phosphoglycerate kinase (Pgk1) as a loading control. Different immunoblots are outlined by black boxes. (F) NAB3 and nab3-ΔNBD mutant suppress the thermosensitive growth of air1-C178R air2Δdis3Δcells expressing a catalytically inactive dis3 mutant, dis3-D551N. air1-C178R air2Δdis3Δcells containing dis3-D551N and vector, AIR1, TRF4, NAB3, nab3–11, nab3-ΔNBD, NRD1 or SEN1 2 μ URA3 plasmid were spotted and grown at indicated temperatures. To ascertain if the catalytic activity of Rrp6 is required for NAB3 suppression of the air1/2 growth, we tested whether NAB3 can suppress air1/2 rrp6Δcells expressing the catalytically inactive mutant of Rrp6, rrp6-D238A [47]. NAB3 does not suppress air1/2 rrp6Δ cells containing rrp6-D238A indicating that NAB3 suppression is dependent on the catalytic activity of Rrp6 (S5A Fig).
As NAB3 reduces the levels of IMD2 CUT and CUT readthrough RNA in air1/2 cells, we determined if NAB3 reduction of these RNA levels is dependent on RRP6. We probed a Northern blot of total RNA from air1/2 rrp6Δ cells containing vector, NAB3, AIR1, TRF4 or NRD1 with an IMD2 CUT-specific probe. AIR1 decreases to 48–78% the levels of the IMD2 CUT and readthrough RNA in air1/2 rrp6Δ cells (Fig. 5B). In contrast, NAB3 does not decrease (107%) the level of the IMD2 CUT and only weakly decreases to 94% the IMD2 readthrough RNA in air1/2 rrp6Δ cells (Fig. 5B). These data indicate that NAB3 reduction of the IMD2 CUT and readthrough RNA depends on RRP6, suggesting Nab3 enhanced degradation of the IMD2 CUT requires Rrp6.
Nab3 interacts with Rrp6 independent of Nrd1
The requirement of RRP6 for NAB3 suppression of air1/2 growth and reduction of the IMD2 CUT and readthrough RNA suggests that Nab3 may physically interact with Rrp6. Nrd1 coprecipitates with Rrp6 [19], raising the possibility, given the Nrd1-Nab3 interaction, that Nab3 could interact with Rrp6. To test if Nab3 directly interacts with Rrp6, we examined the binding of recombinant GST-tagged full-length Nab3 to His-tagged full-length Rrp6 in an in vitro protein binding assay. As controls, we also tested the binding of GST and GST-tagged Rrp47, an exosome cofactor known to directly interact with Rrp6 [48], to His-Rrp6. GST-Nab3 binds to His-Rrp6 but not as strongly as GST-Rrp47 binds to His-Rrp6 (Fig. 5C). This result shows that Nab3 directly interacts with Rrp6 and indicates that Nab3 can bind Rrp6 independent of Nrd1.
To determine if Nab3 can bind to Rrp6 independent of interactions with Nrd1 or RNA in yeast, we precipitated TAP-tagged Rrp6 from lysates of RRP6-TAP yeast cells expressing Myc-tagged Nab3 or nab3-ΔNBD mutant in the absence or presence of RNase A, and analyzed the bound fractions by immunoblotting. In the absence of RNase, binding of nab3-Δ NBD to Rrp6 is reduced but not abolished compared to Nab3 (Fig. 5D). In the presence of RNase, binding of nab3-Δ NBD to Rrp6 is similar to Nab3 (Fig. 5D). Importantly, the Nab3-Rrp6 interaction is reduced but not abolished by RNase-treatment and the nab3-NBD-Rrp6 interaction is not decreased by RNase-treatment (Fig. 5D). These results indicate that a proportion of Nab3 can interact with Rrp6 in the absence of the Nrd1-binding domain and RNA, suggesting that Nab3 can bind to Rrp6 independent of Nrd1. In further support, we performed a reverse Nab3-Rrp6 coprecipitation and found that Rrp6 binds to both Nab3 and a nab3-Δ1–248 NBD mutant (S5B Fig).
As Nab3 interacts with the catalytic exosome subunit, Rrp6, we determined if Nab3 can also interact with the other main catalytic subunit of the core exosome, Dis3/Rrp44. Notably, Nrd1 coprecipitates with Dis3 [19]. To assess interaction between Nab3 and Dis3, we precipitated TAP-tagged Dis3 from cells expressing Myc-tagged Nab3 and examined the bound fraction by immunoblotting. Nab3 co-purifies with Dis3 (Fig. 5E). To assess whether Nab3 can interact with Dis3 independent of Nrd1, we precipitated TAP-tagged Dis3 from cells expressing the nab3-ΔNBD mutant and assayed for co-purification of Nab3. Strikingly, unlike Nab3, the nab3-ΔNBD mutant does not bind to Dis3 (Fig. 5E). These results indicate that the Nab3 interaction with Dis3 is dependent upon the Nab3 Nrd1-binding domain and therefore likely interaction with Nrd1.
Given that NAB3 suppression of air1/2 cells is dependent on Rrp6 catalytic activity, we tested whether NAB3 suppression of air1/2 cells also requires Dis3 catalytic activity. NAB3 and the nab3-ΔNBD mutant suppress the thermosensitive growth of air1/2 dis3Δcells that express an exonucleolytically inactive mutant of Dis3, dis3-D551N [49] (Fig. 5F). This result indicates that NAB3 suppression of air1/2 cells is not dependent on Dis3 exonucleolytic activity.
Human RALY protein suppresses the thermosensitive growth of air1/2 cells
To address the question of functional conservation of Nab3, we performed a BLAST search with the Nab3 RRM and identified the human RALY (hRALY) protein that contains an RRM and C-terminal domain with homology to Nab3 (Figs. 6A and S6). The hRALY RRM shares 31% identity (23/74 residues) and a similar predicted β1α1β2β3α2β4 secondary structure with the Nab3 RRM (Fig. 6B). Moreover, the Nab3 RRM RNA-interacting residues Arg331 and Phe333 are conserved in the hRALY RRM as Arg22 and Phe24 (Fig. 6B). Importantly, hRALY can suppress the growth defect of air1/2 TRAMP mutant cells (Fig. 6C). In contrast, hRALY RRM mutants, hRALY-R22A and hRALY F24A, cannot suppress the growth of air1/2 cells (Fig. 6D). However, the hRALY RRM mutants are expressed to lower levels than the wild-type hRALY protein (Fig. 6E), leaving open the caveat that the inability of hRALY RRM mutants to suppress air1/2 cells could be due to low expression levels and not to impairment of the RRM/RNA binding function of hRALY. At this time, it is therefore not possible to definitively conclude whether RNA binding by hRALY is required for suppression. Although Nab3 and hRALY share similar RRMs, the Nab3 RRM alone is not sufficient to suppress air1/2 cells. A Nab3 truncation mutant nab3–1–448, which lacks the C-terminal 354 amino acids, but retains the intact RRM, also does not suppress air1/2 cells (S7A Fig), even though the truncated protein is expressed (S7B Fig) and properly localized to the nucleus (S7C Fig). The C-terminal region of hRALY with homology to the C-terminal domain of Nab3 could therefore contribute to hRALY suppression of air1/2 cells.
Fig. 6. Human RALY RNA binding protein suppresses air1/2 thermosensitive growth. 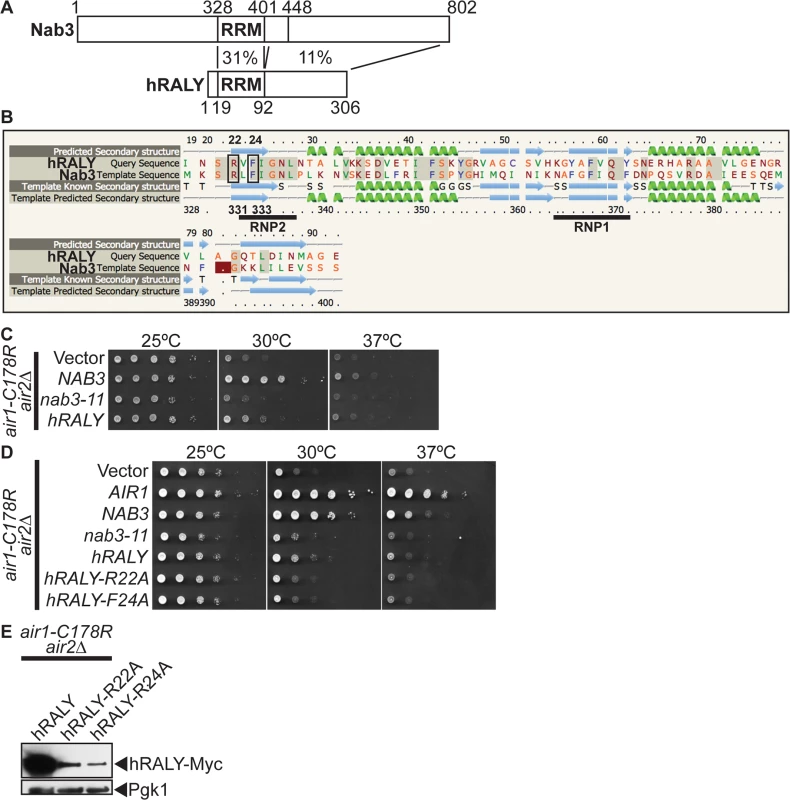
(A) Schematic comparison of Nab3 and human RALY (hRALY) Isoform 1 (GenBank accession number Q9UKM9) RNA binding protein showing percentage sequence identity between RNA recognition domain (RRM) and C-terminal domains. Residue positions are depicted above and below proteins. See also S6 Fig. (B) Alignment of hRALY and Nab3 RRMs showing that the hRALY RRM has 31% identity (identical residues shaded in gray) and a similar predicted β1α1β2β3 α2β4 secondary structure (99.8% confidence; α-helices in green; β-sheets in blue) to the Nab3 RRM. Nab3 RRM RNA-binding residues R331 and F333 that are conserved in the hRALY RRM as R22 and F24 are boxed. RRM consensus motifs RNP1 and RNP2 are underlined. Alignment and secondary structure prediction of hRALY RRM based on Nab3 RRM crystal structure (PDB ID: 2XNQ [63]) was generated by Phyre2 server [64] (C) hRALY suppresses the air1-C178R air2Δ thermosensitive growth at 30°C. The air1-C178R air2Δ cells containing vector, NAB3, nab3–11 or hRALY 2μ URA3 plasmid were spotted and grown at indicated temperatures. (D) hRALY RRM mutants hRALY-R22A and hRALY-F24A do not suppress the air1-C178R air2Δ thermosensitive growth at 30°C. The air1-C178R air2Δ cells containing vector, AIR1, NAB3, nab3–11, hRALY, hRALY-R22A or hRALY-F24A 2μ URA3 plasmid were spotted and grown at indicated temperatures. (E) hRALY-R22A and hRALY-F24A RRM mutant proteins are expressed in air1-C178R air2 Δ cells but not to the same level as hRALY. Lysates of air1-C178R air2∆ cells expressing Myc-tagged hRALY, hRALY-R22A or hRALY-F24A at 30°C were analyzed by immunoblotting to detect Myc-tagged proteins and 3-phosphoglycerate kinase (Pgk1) as a loading control. See also S7A–S7C Fig. Discussion
Non-coding RNAs (ncRNAs) play key roles in gene regulation and disease [1–4]. Understanding how the TRAMP and NNS complex exosome cofactors and the exosome coordinate the processing of ncRNAs is therefore critically important. Here, we find that the Nab3 RNA-binding protein but not Nrd1 of the NNS complex facilitates the function of TRAMP in ncRNA processing/degradation, suggesting a key functional difference between Nab3 and Nrd1. Nab3 suppresses the thermosensitive growth and reduces ncRNA levels of TRAMP mutants independent of Nrd1 interaction. Moreover, Nab3 improvement of TRAMP mutant growth is dependent on the catalytic activity of the nuclear exosome subunit Rrp6 but not the core exosome subunit Dis3. In addition, Nab3 directly binds to Rrp6 and Nab3 coprecipitates with Rrp6 independent of Nrd1 interaction. In the established Nrd1-dependent model for Nab3 function, Nab3 works together with Nrd1 in the NNS complex to recognize ncRNA terminators, interact with TRAMP, and recruit Rrp6/core exosome to terminate and process/degrade ncRNAs (Fig. 7A) [5,19,29,30]. The data presented here suggest a Nrd1-independent model for Nab3 function in which Nab3 facilitates TRAMP by recruitment of Rrp6 to ncRNAs for processing/degradation independent of Nrd1 (Fig. 7B). This model raises the possibility that Nrd1 and Nab3 can function independently to recruit the exosome to ncRNA targets, allowing combinatorial flexibility in processing of RNAs. We also find that the human RALY protein that shares homology with Nab3 improves the function of TRAMP mutants.
Fig. 7. Model for Nrd1-dependent and Nrd1-independent roles of Nab3 in facilitating TRAMP function in the recruitment of the exosome for RNA processing/degradation. 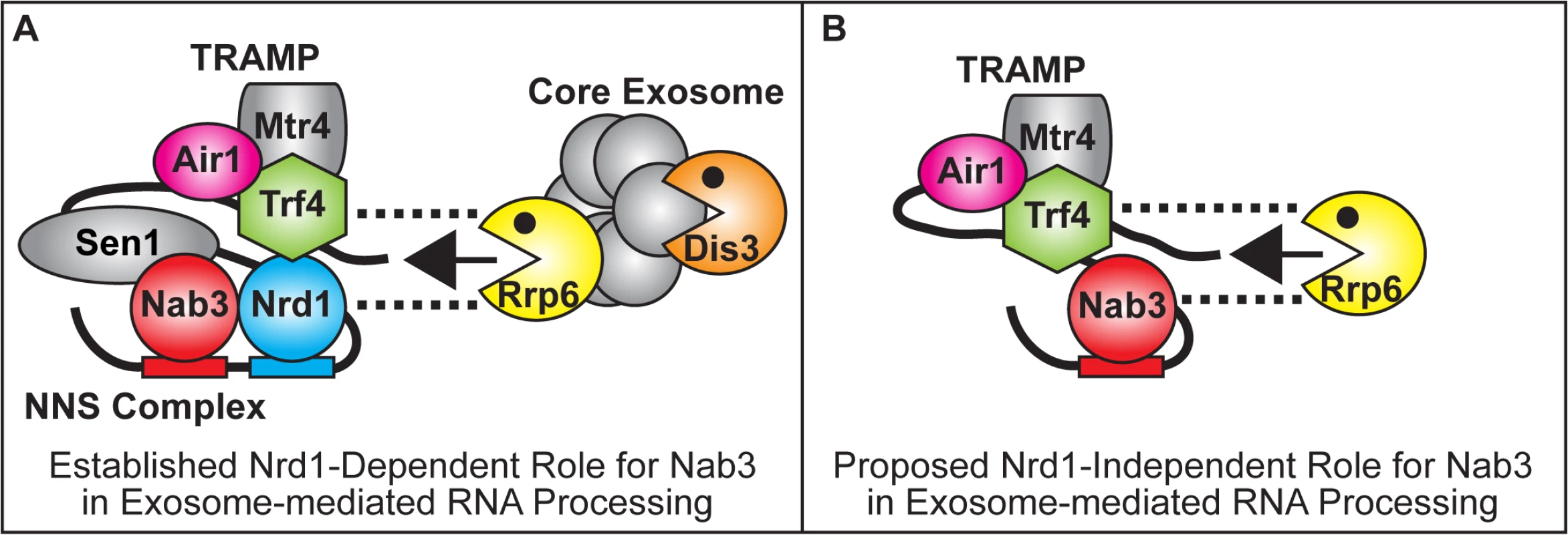
(A) In the established Nrd1-dependent model, Nab3 works in partnership with Nrd1 in the NNS complex to bind to ncRNA terminators, interact with TRAMP and recruit Rrp6 and the core exosome to terminate and process/degrade ncRNAs [5,19,29,30] (B) In the proposed Nrd1-independent model, Nab3 facilitates TRAMP by recruitment of Rrp6 to ncRNAs for processing/degradation independent of Nrd1. Dashed lines reflect direct physical interactions between Nrd1, Trf4 or Nab3 and Rrp6 [32; This Study]. The NNS complex and TRAMP exosome cofactors facilitate termination of ncRNAs and recruit/stimulate the exosome to process/degrade these ncRNAs (Fig. 7A). However, the mechanisms and interactions employed by the NNS and TRAMP complexes to recruit Rrp6 and the core exosome are not fully characterized. Nrd1 coprecipitates with Rrp6, the core exosome, and TRAMP and stimulates the activity of purified exosome in vitro [19]. Moreover, Nrd1 directly interacts weakly with Rrp6 and strongly with Trf4 [32]. Putative human TRAMP components (hTRF4–2, ZCCHC7, hMTR4) also coprecipitate strongly with hRRP6 [18]. In addition, Trf4 also directly interacts strongly with Rrp6 and TRAMP stimulates the activity of Rrp6 in vitro [32,45]. Both Nrd1 and TRAMP therefore interact with Rrp6. We now find that Nab3 directly interacts with Rrp6 and a nab3 mutant that lacks the Nrd1-binding domain coprecipitates with Rrp6 but not with Dis3/core exosome. Nab3 and Nrd1 of the NNS complex and TRAMP may thus independently recruit Rrp6 to ncRNA targets, providing flexibility in processing/degradation. The NNS complex and TRAMP may also interact together via Nrd1-Trf4 interaction to enhance recruitment of Rrp6 and the core exosome to ncRNA targets for rapid processing/degradation.
To better understand TRAMP function, we utilized air1/2 TRAMP mutant cells [16]. In air1/2 cells, the air1-ZnK5 protein shows reduced stability and decreased binding to Trf4 that leads to reduced integrity of TRAMP [16]. The primary defect in air1/2 cells, as in other TRAMP mutants, is reduced TRAMP integrity/function that leads to decreased recruitment/stimulation of Rrp6/core exosome and thus defective processing/degradation of ncRNAs and some mRNAs [12,13,16]. Impaired TRAMP function in trf4Δ mutant cells also causes terminator readthrough of snoRNA, CUT, and some mRNA genes [13,42,43], suggesting TRAMP plays a role in transcription termination. In support, we find that Air1 significantly reduces RNA Pol II occupancy downstream of the IMD2 CUT in air1/2 cells, indicating that Air1 and TRAMP affect termination and suggesting that air1/2 cells have a termination defect. Exactly how TRAMP affects transcription termination is an important question for future study. Impaired growth of TRAMP mutant cells is thus correlated with undegraded and/or non-terminated RNAs. Notably, polyadenylation defective TRAMP mutant cells are viable [13], indicating that polyadenylation is not the essential function of TRAMP and suggesting that exosome recruitment and other activities, such as termination, are the more vital functions of TRAMP.
In this study, we identified NAB3 as a potent suppressor of the impaired growth of air1/2 and trf4Δ TRAMP mutant cells, linking Nab3 of the NNS complex to TRAMP function. Surprisingly, NRD1 does not suppress air1/2 cells and a nab3 mutant that lacks the Nrd1-binding domain still suppresses air1/2 cells, suggesting Nab3 harbors a Nrd1-independent function that it shares with TRAMP. As NAB3 suppression and decrease of IMD2 CUT RNA in air1/2 cells is dependent on Rrp6, and Nab3 directly interacts with Rrp6, we suggest that the Nab3 mechanism of suppression of air1/2 cells involves Nab3 recruitment of Rrp6 to target ncRNAs for processing/degradation (Fig. 7B). Given that TRAMP recruits/stimulates Rrp6, this Nab3 suppression mechanism would seem logical. In support of a Nab3 role in RNA degradation, mutation of a Nab3 binding site in the IMD2 CUT terminator leads to an increase in IMD2 CUT RNA [28]. Importantly, NAB3 does not significantly affect RNA Pol II occupancy downstream of the IMD2 CUT or the snR13 gene in air1/2 cells, suggesting that the Nab3 suppression mechanism does not involve Nab3 improvement of termination. Conceivably, the Nab3 suppression mechanism could also involve an as yet uncharacterized function of Nab3 in TRAMP activity or the ability of Nab3 to reduce the cellular requirement for TRAMP.
The finding that NAB3, but not NRD1, suppresses the growth of TRAMP mutants is surprising, given that Nrd1 directly interacts with Nab3, Trf4, and Rrp6 [29,32]. As the Nab3 RRM is essential for suppression of TRAMP mutants and Nab3 and Nrd1 RRMs recognize different RNA sequences, one intriguing explanation is that Nab3 recognizes important ncRNAs with NNS terminators composed predominantly or exclusively of Nab3 binding sites that Nrd1 cannot recognize in TRAMP mutant cells. On this note, AU-rich sequences (e.g. UAAA; AAAU) and extended Nrd1/Nab3 binding sites have recently been identified in artificial NNS-dependent terminators that are critical for Nrd1-Nab3 interaction, present in native ncRNAs, and could serve as supermotifs for NNS recognition [50]. As Air2 binds to adenosine RNA [15], TRAMP could help Nrd1-Nab3 to cooperatively recognize these AU-rich sequences. If critical ncRNAs contained terminators with AU-rich-Nab3 site supermotifs, this could explain why Nab3, but not Nrd1, specifically suppresses TRAMP mutants. This possibility implies that Nab3 and TRAMP process/degrade a common set of ncRNAs that are critical for cell growth, but are not regulated by Nrd1.
In support of the idea of Nab3 - and Nrd1-specific RNA targets, two RNA cross-linking and RNA-Seq studies that mapped Nab3 and Nrd1 sites transcriptome-wide found that a greater percentage of reads for Nab3 RNAs map to RNA Pol II transcripts than that for Nrd1 RNAs (Nab3–73% Pol II vs Nrd1–59% Pol II [23]; Nab3–42% Pol II vs Nrd1–36% Pol II [24]). As yeast cells express more Nrd1 (∼20,000 mols/cell) than Nab3 (∼6,000 mols/cell) [51], this result suggests that Nab3 may bind to more RNA Pol II transcripts than Nrd1. Notably, comparison of the top 100 Nab3 and Nrd1 cross-linked sites from one study reveals that Nab3 cross-links more efficiently to snoRNAs than Nrd1 (Nab3–59/100 sites are snoRNAs vs Nrd1–33/100 sites are snoRNAs [52]). Studies have also reported that the processing/degradation of certain ncRNAs is more prominently altered in nab3 mutants compared to nrd1 mutants. In particular, the level of an FLC1-FMP40 intergenic CUT with three Nab3 binding sites is elevated in nab3–11 RRM mutant cells, but unchanged in nrd1–102 RRM mutant cells [25]. The processing of 23S/20S pre-rRNA is also impacted in nab3–11 cells, but unaltered in nrd1–102 cells [46]. Finally, the level of 5’-extended pre-tRNAArg(UCU) is greater in Nab3-depleted cells than it is in Nrd1-depleted cells [23].
The importance of the NNS complex in regulating the exosome, TRAMP, and RNA processing in yeast and conservation of the exosome and TRAMP components in humans raises the question of whether the NNS exosome cofactor is also conserved in humans. In strong support, the human Sen1 helicase, Senataxin, catalyzes termination in human cells [35]. The human SCAF8 RRM protein has also been proposed to be a Nrd1 orthologue based on sequence identity [53]. However, to date, no human Nrd1 or Nab3 orthologue has been functionally characterized. Here, we find that expression of the human RALY (hRALY) RRM-containing protein, which shares homology with Nab3 and contains an RRM with 31% identity to the Nab3 RRM, like Nab3, can improve the growth of air1/2 TRAMP mutant cells, suggesting that hRALY can modulate a function performed by TRAMP in yeast cells. Consistent with a potential role for hRALY in processing of ncRNAs, nuclear hRALY interacts with numerous RNA binding proteins involved in ncRNA processing/stability in human cells [54].
Combined, the data presented here indicate that Nab3 and Nrd1 can work independently to recruit the exosome to ncRNA targets, providing combinatorial flexibility in RNA processing.
Materials and Methods
Chemicals and media
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO), United States Biological (Swampscott, MA), or Fisher Scientific (Pittsburgh, PA) unless otherwise noted. All media were prepared by standard procedures [55].
Plasmids
All plasmids used in this study are listed in S1 Table. All DNA manipulations were performed according to standard methods [56]. The URA3 2μ NAB3 (pAC2880), NRD1 (pAC2869), SEN1 (pAC3235), NPL3 (pAC1726), NAB2 (pAC1813), HRP1 (pAC1745), and PUB1 (pAC1759) plasmid were constructed by amplification of NAB3, NRD1, SEN1, NPL3, NAB2, HRP1, and PUB1 genes by polymerase chain reaction (PCR) from S. cerevisiae genomic DNA with oligonucleotides (Integrated DNA Technologies) and cloning into pRS426 plasmid [57]. The URA3 2 μ nab3 RRM mutant nab3–11 (F371L-P374L; pAC2915), nab3-R331A (pAC3231), nab3-F333A (pAC3232), nab3-S399A (pAC3233), and nab3-S400A (pAC3234) plasmid, nab3–1–448 plasmid (pAC3280), and nab3 Nrd1-binding domain (NBD) mutant nab3-ΔNBD (Δ204–248; pAC3236) plasmid were generated by site-directed mutagenesis with nab3 oligonucleotides encoding F371L-P374L, R331A, F333A, S399A, S400A, R449X residue substitutions or 204–248 (NBD) residue deletion, NAB3 (pAC2880) plasmid template, and QuikChange Site-Directed Mutagenesis Kit (Stratagene). The HIS3 2 μ NAB3 (pAC3246), nab3 RRM mutants, nab3–11 (F371L-P374L; pAC3247), nab3-R331A (pAC3248), nab3-F333A (pAC3249), nab3-S399A (pAC3250), nab3-S400A (pAC3251), nab3 NBD mutant nab3-ΔNBD (Δ204–248; pAC3252), NRD1 (pAC3255), SEN1 (pAC3256), TRF4 (pAC2940), and TRF5 (pAC2930) plasmid were constructed by subcloning NAB3, nab3 mutant, NRD1, SEN1, TRF4, TRF5 genes from pRS426 plasmids into pRS423 [57]. The URA3 2 μ C-terminally Myc-tagged NAB3 (pAC3237), nab3 RRM mutant nab3–11 (P371L-F374L; pAC3240), nab3-R331A (pAC3241), nab3-F333A (pAC3242), nab3-S399A (pAC3243), and nab3-S400A (pAC3244), and nab3-ΔNBD (Δ204–248; pAC3245) plasmid were constructed by PCR amplification of NAB3 using oligonucleotides containing 2xMyc tag and NAB3 (pAC2880), nab3 RRM mutant (pAC2915, pAC3231–3234) or nab3-ΔNBD (pAC3236) template and cloning into pRS426. The URA3 2 μ C-terminally Myc-tagged NRD1 (pAC3238), SEN1 (pAC3239), and RRP6 (pAC3034) plasmids were constructed by PCR amplification of NRD1, SEN1, and RRP6 using oligonucleotides containing 2xMyc tag and NRD1 (pAC2869), SEN1 (pAC3235) or W303 gDNA templates and cloning into pRS426. The LEU2 2 μ C-terminally TAP-tagged NAB3 (pAC3253) and nab3-Δ1–248 (pAC3254) plasmids were constructed by PCR amplification of NAB3 5’-UTR, full-length NAB3, and nab3 249–802 using oligonucleotides and NAB3 (pAC2880) plasmid template and cloning into YEp351 plasmid containing C-terminal TAP tag. The LEU2 2 μ GFP-tagged NAB3 (pAC3281) and nab3–1–448 (pAC3282) plasmids were constructed by PCR amplification of NAB3 5’-UTR, full-length NAB3, and nab3–1–448 using oligonucleotides and NAB3 (pAC2880) template and cloning into YEp351 plasmid containing C-terminal GFP tag. The URA3 2 μ NAB3–5’-UTR-hRALY Isoform 1 (pAC3279) plasmid was constructed by PCR amplification of NAB3 5’-UTR using oligonucleotides and NAB3 (pAC2880) template, PCR amplification of hRALY Isoform 1 ORF (GenBank accession number Q9UKM9) using oligonucleotides and human cDNA from HeLa cells, and cloning into pRS426 plasmid. The URA3 2 μ NAB3–5’-UTR-hRALY-R22A (pAC3306) and NAB3–5’-UTR-hRALY-F24A (pAC3307) mutant plasmids were generated by site-directed mutagenesis with hRALY oligonucleotides encoding R22A and F24A residue substitutions and hRALY (pAC3279) plasmid template. The URA3 2 μ C-terminally Myc-tagged hRALY (pAC3308), hRALY-R22A (pAC3309), and hRALY-F24A (pAC3310) plasmids were constructed by PCR amplification of NAB3–5’-UTR-hRALY using oligonucleotides containing 2xMyc tag and hRALY (pAC3279), hRALY-R22A (pAC3306) or hRALY-F24A (pAC3307) template and cloning into pRS426. The URA3 CEN6 NAB3 (pAC3285), NRD1 (pAC3314) and DIS3 (pAC2861) plasmids were constructed by PCR amplification of NAB3, NRD1, and DIS3 genes by PCR from S. cerevisiae genomic DNA with oligonucleotides and cloning into pRS316 plasmid [58]. The HIS3 CEN6 AIR1-GFP (pAC2224) plasmid was constructed by PCR amplification of AIR1 gene from AIR1 (pAC1613) plasmid template with oligonucleotides and cloning into pRS313 containing C-terminal GFP tag. The TRP1 CEN6 RRP6 (pAC2301) plasmid was constructed by PCR amplification of RRP6 gene from S. cerevisiae genomic DNA with oligonucleotides and cloning into pRS314 plasmid [58]. The TRP1 CEN6 rrp6-D238A (pAC2302) plasmid was generated by site-directed mutagenesis with rrp6 oligonucleotides encoding D238A residue substitution and pRS314-RRP6 (pAC2301) plasmid template. The TRP1 CEN6 dis3-D551N (pAC2675) plasmid was generated by site-directed mutagenesis with dis3 oligonucleotides encoding D551N residue substitution and pRS314-DIS3 plasmid template. The HIS3 CEN6 nrd1-Δ151–214 (pAC3223) plasmid was generated by site-directed mutagenesis with nrd1 oligonucleotides encoding 151–214 residue deletion and pRS313-NRD1 plasmid template. The GST-RRP47 (pAC3311) and GST-NAB3 (pAC3312) bacterial expression plasmids were constructed by PCR amplification of NAB3 and RRP47 genes using oligonucleotides and NAB3 (pAC2880) or W303 gDNA template and cloning into pGEX-TEV. The His6-RRP6 (pAC3313) bacterial expression plasmid was constructed by PCR amplification of RRP6 gene using oligonucleotides and RRP6 (pAC2301) template and cloning into pET30a-TEV. All constructs were sequenced to ensure the absence of any undesired mutations and the presence of each desired mutation.
Strains
All strains used in this study are listed in S1 Table. The air1 (ACY1090), air2Δ (ACY1091), and trf4Δ (ACY2149) strains were obtained from Research Genetics. The NRD1-TAP (ACY2293), RRP6-TAP (ACY1063), and DIS3-TAP (ACY1926) strains were obtained from Thermo Scientific (Open Biosystems). The air1-C178R air2Δ (ACY2020) strain was constructed by insertion of the C178R mutation into the AIR1 ORF in the W303 strain by the ‘delitto perfetto’ method [59] and deletion of the AIR2 ORF by homologous recombination with AIR2-NATMX PCR product. The trf4Δ (ACY2154) strain was constructed by deletion of the TRF4 ORF in the W303 strain by homologous recombination with TRF4-NATMX PCR product. The nab3Δ (ACY2181) strain was constructed by transformation of a URA3 NAB3 (pAC3285) plasmid into the W303 strain and deletion of the NAB3 ORF by homologous recombination with NAB3-NATMX PCR product. The air1-C178R air2Δ rrp6Δ strain (ACY2294) was constructed by deletion of the RRP6 ORF in the ACY2020 strain by homologous recombination with RRP6-UTR KANMX PCR product. The air1-C178R air2Δ nrd1Δ strain (ACY2320) was constructed by transformation of a URA3 NRD1 (pAC3285) plasmid into the ACY2020 strain and deletion of the NRD1 ORF by homologous recombination with NRD1-UTR KANMX PCR product. The air1-C178R air2Δ dis3Δ strain (ACY2119) was constructed by transformation of a URA3 DIS3 (pAC2681) plasmid into the ACY2020 strain and deletion of the DIS3 ORF by homologous recombination with DIS3-UTR KANMX PCR product. The air1Δ air2Δ (ACY2036) strain was constructed by transformation of a URA3 AIR2 (pAC1614) plasmid into the W303 strain and consecutive deletion of AIR1 and AIR2 ORFs by homologous recombination with AIR1-NATMX and AIR2-HPHMX PCR products. The air1-C178R-TAP air2Δ strain (ACY2051) was constructed by insertion of C-terminal TAP-Sphis5+ PCR product into the air1-C178R ORF in the air1-C178R air2Δ (ACY2020) strain by homologous recombination.
High copy suppressor screen
To identify high copy suppressors of the temperature sensitive growth of air1-C178R air2Δ cells at 30°C, air1-C178R air2Δ cells (ACY2020) were transformed with a 2 μ URA3 yeast genomic DNA plasmid library and plated on Ura− minimal media plates. As controls, the cells were also transformed with 2 μ URA3 vector (pRS426) or AIR1 (pAC1613). The cells were grown at 25°C for one day and then shifted 30°C for 2–4 days select for suppressors. Approximately 18,000 transformants containing library plasmids were screened at 30°C. Library plasmids from transformants that showed improved growth at 30°C relative to vector alone were isolated and retransformed into air1-C178R air2Δ cells to confirm that these plasmids cause suppression of the thermosensitive growth of the cells at 30°C. The confirmed suppressor plasmids were sequenced to identify the genomic DNA inserts.
High copy suppression growth assay
To assess suppression of the temperature sensitive growth of air1-C178R air2Δ cells, air1-C178R air2Δ nrd1Δ cells containing nrd1-Δ151–214, air1-C178R air2Δ rrp6Δ cells, air1-C178R air2Δ rrp6Δ cells containing RRP6 or rrp6-D238A, air1-C178R air2Δ dis3Δ cells containing dis3-D551N or the slow growth of Δtrf4 (ACY2154) cells by TRAMP components, gDNA suppressors, NAB3, nab3 mutants, NRD1, SEN1, and NAB/hnRNP, hRALY, hRALY mutants, air1-C178R air2Δ cells (ACY2020), air1-C178R air2Δ nrd1Δ (ACY2320) transformed with nrd1-Δ151–214 (pAC3223) CEN HIS3 plasmid, air1-C178R air2Δ rrp6Δ (ACY2294), air1-C178R air2Δ rrp6Δ cells (ACY2294) transformed with RRP6 (pAC2301) or rrp6-D238A (pAC2302) CEN TRP1 plasmid, air1-C178R air2Δ dis3Δ cells transformed with dis3-D551N (pAC2675) or trf4Δ cells (ACY2154) were transformed with vector (pRS426), AIR1 (pAC1613), AIR2 (pAC1614), TRF4 (pAC2147), (SUP3–2 (pAC3227), SUP11–3 (pAC3229), NAB3 (pAC2880), nab3–11 (pAC2915), nab3-R331A (pAC3231), nab3-F333A (pAC3232), nab3-S399A (pAC3233), nab3-S400A (pAC3234), nab3-ΔNBD (pAC3236), nab3–1–448 (pAC3280), NRD1 (pAC2869), SEN1 (pAC3235), NPL3 (pAC1726), NAB2 (pAC1813), HRP1 (pAC1745), PUB1 (pAC1759), NAB3 5’UTR-hRALY Isoform 1 (pAC3279), NAB3 5’UTR-hRALY-R22A (pAC3306) or NAB3 5’UTR-hRALY-F24A (pAC3307) 2μ URA3 plasmid and selected on Ura−, Ura−Trp− or Ura−His− minimal media. Cells were grown overnight at 25°C to saturation in Ura−, Ura−Trp− or Ura−His− minimal media, cell concentrations were normalized by OD600, and cells were serially diluted and spotted onto Ura−, Ura−Trp− or Ura−His− minimal media plates and grown at 25, 30 and 37°C. To assess bypass suppression of the slow growth of air1Δ air2Δ cells by NAB3, two strains of air1Δ air2Δ cells (ACY1095, ACY2036), containing AIR2 URA3 maintenance plasmid (pAC1614), were transformed with vector (pRS423), AIR1-GFP (pAC2224), TRF4 (pAC2940), TRF5 (pAC2930) or NAB3 (pAC3246) 2 μ HIS3 plasmids and selected on Ura−His− minimal media. Cells were grown overnight at 25°C to saturation in Ura−His− minimal media, cell concentrations were normalized by OD600, and cells were serially diluted and spotted onto control Ura−His− minimal media or His− minimal media containing 5-FOA. Growth of air1Δ air2Δ cells harboring only AIR1-GFP, TRF4, TRF5 or NAB3 was examined at 25, 30 and 37°C.
Functional growth assay
To test the in vivo function of nab3 mutants, a standard plasmid shuffle assay combined with serial dilution and spotting was employed. nab3Δ cells (ACY2181), containing NAB3 URA3 maintenance plasmid (pAC3285) were transformed with vector (pRS423), NAB3 (pAC3246), nab3–11 (pAC3247), nab3-R331A (pA3248), nab3-F333A (pAC3249), nab3-S399A (pAC3250), nab3-S400A (pAC3251), or nab3-Δ NBD (pAC3252) 2 μ HIS3 plasmids and selected on Ura−His− minimal media. Cells were grown overnight at 25°C to saturation in Ura−His− minimal media, cell concentrations were normalized by OD600, and cells were serially diluted and spotted onto control Ura−His− minimal media, where the NAB3 URA3 maintenance plasmid is maintained, or His-minimal media containing 5-FOA, which selects for cells that have lost the NAB3 URA3 maintenance plasmid. Growth of nab3Δ cells, harboring NAB3 or nab3 mutants as the sole copy of NAB3, was examined at 25, 30 and 37°C.
Analysis of protein expression levels
For analysis of Myc-tagged Nab3, nab3 mutant, Nrd1, and Sen1, hRALY, hRALY mutant protein levels, air1-C178R air2Δ cells (ACY2020) expressing Myc-tagged Nab3 (pAC3237), nab3–11 (pAC3240), nab3-R331A (pAC3241), nab3-F333A (pAC3242), nab3-S399A (pAC3243), nab3-S400A (pAC3244), nab3-ΔNBD (pAC3245), Nrd1 (pAC3238) Sen1 (pAC3239), hRALY (pAC3308), hRALY-R22A (pAC3309) or hRALY-F24A (pAC3310) protein were grown in minimal media overnight at 25°C, 10 ml cultures with an OD600 = 0.4 were prepared and grown for 2 hr at 25°C, and then shifted to 30°C for 4 hr. For analysis of nab3–1–448 mutant protein level, air1-C178R air2Δ cells (ACY2020) containing vector or expressing nab3–1–448 (pAC3280) mutant protein were grown in minimal media overnight at 25°C, 10 ml cultures with an OD600 = 0.4 were prepared and grown for 2 hr at 25°C, and then shifted to 30°C for 4 hr. For analysis of TAP-tagged air1-C178R protein levels upon expression of Trf4 or Nab3 protein, air1-C178R-TAP air2Δ (ACY2051) cells containing vector (pAC426), TRF4 (pAC2147) or NAB3 (pAC2880) were grown in minimal media overnight at 25°C, 10 ml cultures with an OD600 = 0.4 were prepared and grown at 25°C for 6 hr. Whole cell lysates of cells were then prepared and 30–50 μ g of protein was analyzed by immunoblotting with an anti-Myc monoclonal antibody to detect Myc-tagged proteins, anti-Nab3 monoclonal antibody to detect nab3–1–448, and peroxidase anti-peroxidase antibody to detect TAP-tagged air1-C178R.
Immunoblotting
Protein samples (30–50 μ g lysate; total bound) were resolved on Criterion 4–20% gradient gels (Bio-Rad), transferred to nitrocellulose membranes (Bio-Rad) and Myc-tagged proteins were detected with anti-Myc monoclonal antibody 9B11 (1 : 2000; Cell Signaling), TAP-tagged proteins were detected with peroxidase anti-peroxidase antibody (1 : 5000; Sigma), Nrd1 was detected with anti-Nrd1 rabbit polyclonal antibody (1 : 3,000; a gift from David Brow), Nab3 was detected with anti-Nab3 monoclonal antibody (1 : 2000; a gift from Maurice Swanson), GFP was detected with anti-GFP rabbit polyclonal antibody (1 : 3000; Sigma), His-tagged Rrp6 was detected with anti-His monoclonal antibody coupled to horseradish peroxidase (1 : 2000; Invitrogen), and GST fusion proteins were detected with anti-GST monoclonal antibody (1 : 2000; Santa Cruz Biotechnology).
Quantitation of immunoblots and Northern blots
The band intensities/areas from all immunoblots and Northern blots were quantitated using ImageJ v1.4 software (National Institute of Health, MD; http://rsb.info.nih.gov/ij/) and relevant percentages of protein or RNA were calculated in Microsoft Excel for Mac 2011 (Microsoft Corporation). To quantitate the fold overexpression of Nab3 and Nrd1 protein in air1-C178R air2Δ cells containing NAB3 or NRD1 relative to cells containing vector alone, the Nab3/Nrd1 intensity in cells containing NAB3 or NRD1 was normalized to Pgk1 intensity and Nab3/Nrd1 intensity in cells containing vector alone. To quantitate the percentage of air1-C178R-TAP protein in air1-C178R-TAP air2Δ cells containing TRF4 or NAB3 relative to cells containing vector alone, the air1-C178R-TAP intensity in cells containing TRF4 or NAB3 was normalized to Pgk1 intensity and air1-C178R-TAP intensity in cells containing vector alone. To quantitate the percentage of bound nab3-Myc mutant protein relative to bound Nab3-Myc wild-type protein in Nrd1-TAP binding assays, the bound Nab3/nab3-Myc intensity was normalized to bound Nrd1-TAP intensity, input Nab3/nab3-Myc intensity (normalized to Pgk1 intensity), and bound Nab3-Myc wild-type protein intensity. To quantitate the percentage of input nab3-Myc mutant protein relative to input Nab3-Myc wild-type protein, input nab3-Myc intensity were normalized to Pgk1 intensity and Nab3-Myc intensity. To quantitate the percentage of GFP protein in air1-C178R air2Δ cells containing pREF-GFP reporter and NAB3, nab3 mutants, NRD1 or SEN1, relative to cells containing pREF-GFP reporter and vector alone in terminator readthrough reporter assays, the GFP intensity in cells containing NAB3, nab3 mutants, NRD1 or SEN1 was normalized to Pgk1 intensity and GFP intensity in cells containing vector alone. To quantitate the percentage of IMD2 CUT and readthrough RNA in W303, air1-C178R air2Δ, trf4Δ, and air1-C178R air2Δ rrp6Δ containing vector, AIR1, TRF4, NAB3 or NRD1 in Northern blots, IMD2 CUT and readthrough RNA intensity was normalized to ACT1 or scR1 RNA intensity and IMD2 CUT/readthrough intensity in cells containing vector alone. Quantitation refers to specific experiments shown in Figures but is representative of multiple experiments.
Analysis of RNA expression levels
For analysis of native IMD2 CUT and readthrough RNA levels, wild-type (W303; ACY233), air1-C178R air2Δ (ACY2020), air1-C178R air2Δ rrp6Δ (ACY2294), and trf4Δ (ACY2154) cells containing vector (pRS426), AIR1 (pAC1613), TRF4 (pAC2147), NAB3 (pAC2880), nab3 mutants (pAC2915, pAC3231–3233, pAC3236) or NRD1 (pAC2869) were grown in minimal media overnight at 25°C, 10 ml cultures with an OD600 = 0.4 were prepared and grown for 2 hr at 25°C, and then shifted to 30°C for 4 hr. Total RNA was isolated from cells and analyzed by Northern blotting with an IMD2 CUT and ACT1 or scR1 loading control oligonucleotide probe (S2 Table).
Total RNA isolation
To prepare S. cerevisiae total RNA from cell pellets of 10 ml cultures grown to OD600 = 0.5–0.7, glass beads (2–3 x 100 μ l) were added to each cell pellet in 2 ml screw-cap tube, 1 ml TRIzol (Invitrogen) was added and cell sample was vigorously disrupted in Mini Bead Beater 16 Cell Disrupter (Biospec) for 2 min at 25°C. For each sample, 100 μ l of 1-bromo-3-chloropropane (BCP) was added, the sample was vortexed for 15 sec, and incubated at 25°C for 2 min. Each sample was then centrifuged at 16,300 x g for 8 min at 4°C and the upper layer was transferred to a fresh microfuge tube. RNA was precipitated with 500 μ l isopropanol and the sample was vortexed for 10 sec to mix. Total RNA was pelleted by centrifugation at 16,300 x g for 8 min at 4°C. Supernatant was decanted and 1 ml of 75% ethanol was added to wash RNA pellet. Sample was centrifuged at 16,300 x g for 5 min at 4°C. Supernatant was decanted, remaining ethanol was removed, and the RNA pellet was air dried for 15 min. Total RNA was resuspended in 50 μ l diethylpyrocarbonate (DEPC (Sigma))-treated water and stored at-80°C.
Northern blotting
To detect native IMD2 CUT and readthrough RNA in wild-type, air1-C178R air2Δ, air1-C178R air2Δ rrp6Δ, and trf4Δ cells containing vector, AIR1, TRF4, NAB3, nab3 mutants or NRD1, total RNA from cells was resolved on an agarose gel, blotted to a nylon membrane and membrane was probed with radiolabeled IMD2 CUT and ACT1 or scR1 loading control oligonucleotides (S2 Table). Total RNA (10 μ g) was mixed with equal volume of RNA loading dye (70% formamide; 2X MOPS; 10% glycerol; 0.1 μ g/μ l ethidium bromide; 0.03% bromophenol blue) and resolved on 1.2% agarose/1.8% formaldehyde/1X MOPS gel at 70V for 2–3 hr. RNA was blotted to Hybond-N+ nylon membrane (Amersham, GE Healthcare) overnight by standard procedure and cross-linked to membrane with UV light (120,000 μ Joules) using UV Stratalinker 2400 (Stratagene). Ethidium bromide-stained rRNA on membrane was imaged. Membrane was incubated in Rapid-hyb hybridization buffer (Amersham, GE healthcare) at 42°C for 1 hr. DNA oligonucleotide (100 ng) was 5’-end labeled with [γ-P32]-ATP (PerkinElmer) using polynucleotide kinase (New England Biolabs) at 37°C for 30 min. [P32]-Labeled oligonucleotide probe was purified through Centrispin-20 spin column (Princeton Separations), heated at 100°C for 5 min, and added to hybridization buffer. Oligonucleotide probe was hybridized to membrane in hybridization buffer at 42°C overnight. Following removal of hybridization buffer, membrane was washed once in 5 x SSC; 0.1% SDS at 25°C for 20 min and washed twice in 1 x SSC; 0.1% SDS at 42°C for 15 min each. Membrane was exposed to phosphoscreen overnight and imaged using Typhoon FLA 7000 phosphoimager (GE Healthcare).
TAP-tagged protein binding assay
To assess binding between TAP-tagged Nrd1, Rrp6 or Dis3 protein and Myc-tagged Nab3 or nab3 mutant protein or TAP-tagged Nab3 or nab3-Δ1–248 protein and Myc-tagged Rrp6 protein, NRD1-TAP (ACY2293), RRP6-TAP (ACY1063), DIS3-TAP (ACY1926) or untagged BY4741 (ACY1105) cells expressing Myc-tagged Nab3 (pAC3237), nab3 RRM mutants (pAC3240–44), nab3 NBD mutant (pAC3245) or Nrd1 (pAC3238), and W303 cells containing NAB3-TAP (pAC3253) or nab3- Δ1–248-TAP (pAC3254) and expressing Myc-tagged Rrp6 (pAC3034) were inoculated into 50 ml Ura− or Ura−Leu− minimal media and grown overnight at 25°C to an OD600 = 2. For NRD1-TAP, DIS3-TAP, and W303 cells, each cell pellet was resuspended in 1 ml IPP150 buffer (10 mM Tris-HCl, pH 8; 150 mM NaCl; 0.1% NP-40) supplemented with protease inhibitors (1 mM phenylmethanesulfonylfluoride (PMSF); 3 ng/ml pepstatin A, leupeptin, aprotinin, and chymostatin). For RRP6-TAP and untagged BY4741 cells, each cell pellet was resuspended in 1ml IPP100 buffer (10 mM Tris-HCl, pH 8; 100 mM NaCl; 0.1% NP-40) supplemented with protease inhibitors. To each cell resuspension, glass beads (8 x 100 μ l scoops) were added and sample was vigorously disrupted in a Mini Bead Beater 16 Cell Disrupter (Biospec) for 4 x 1 min at 25°C. Protein lysate was cleared by centrifugation at 16,000 x g for 20 min at 4°C. Protein lysate supernatant was transferred to a new microfuge tube, centrifuged at 16,000 x g for an additional 10 min at 4°C, and transferred to a new microfuge tube using a 1 ml syringe. Protein lysate concentration was determined by Bradford assay using Bio-Rad Protein Assay. For Nrd1-TAP, Dis3-TAP, and W303 containing Nab3-TAP samples, protein lysate (2–5 mg) in 1 ml IPP150 buffer was incubated with 25 μ l IgG Sepharose beads (GE Healthcare) overnight at 4°C with mixing and beads were washed three times with 1 ml IPP150 buffer for 5 min each. For Rrp6-TAP and untagged BY4741 samples, protein lysate (2–5 mg) in 1 ml IPP100 buffer was incubated with 25 μ l IgG Sepharose beads (GE Healthcare) in the presence or absence of 5 μ l Purelink RNase A (20 mg/ml; Invitrogen) overnight at 4°C with mixing and beads were washed three times with 1 ml IPP100 buffer for 1 min each. Input lysate (30–50 μ g), unbound supernatant (50 μ g) and total bound Nrd1-TAP-, Dis3-TAP-, Nab3-TAP-, Rrp6-TAP-, and untagged BY4741-bead sample were analyzed by SDS-PAGE and immunoblotting with anti-Myc monoclonal antibody to detect Nab3-Myc proteins and loading control anti-Pgk1 monoclonal antibody to detect 3-phosphoglycerate kinase.
Recombinant protein expression and purification
Recombinant GST fusion and His-tagged proteins were expressed in bacteria and purified. GST (pGEX-TEV), GST-Rrp47 (pAC3311), GST-Nab3 (pAC3312), and His-Rrp6 (pAC3313) were expressed in E. coli DE3 cells and purified by batch purification. Overnight cultures were used to inoculate 100 ml LB media supplemented with 100 μ g/ml ampicillin or 50 μ g/ml kanamycin. Cultures were grown at 37°C to an OD600 of 0.6–0.8, induced with 200 μ M IPTG and grown at 25°C overnight. For batch purification of GST fusion proteins, cells were collected and lysed in 10 ml phosphate buffered saline (PBS) supplemented with protease inhibitor mixture (1 mM phenylmethylsulfonyl fluoride, 3 ng/ml pepstatin A, leupeptin, aprotinin, and chymostatin) and 2 mM dithiothreitol (DTT) by incubation with 10 mg lysozyme for 30 min on ice and sonication. Lysates were cleared by centrifugation at 12,000 x g for 10 min and incubated with glutathione Sepharose 4B (GE Healthcare) for 2 hr at 4°C with mixing. The beads were then washed once with 10 ml PBS supplemented with 0.5% Triton-X-100 and 2 mM DTT and twice with 10 ml PBS supplemented with 2 mM DTT. GST proteins were eluted from beads with 1 ml 50 mM Tris-HCl, pH 8 containing 10 mM reduced glutathione and dialyzed into PBS supplemented with 2 mM DTT. For batch purification of His-tagged Rrp6 protein, cells were collected and lysed in 10 ml lysis buffer (50 mM NaH2PO4, pH 7.4, 300 mM NaCl, 10 mM imidazole) supplemented with protease inhibitor mixture and 2 mM DTT by incubation with 10 mg lysozyme and sonication. Lysates were cleared by centrifugation at 12,000 x g for 10 min and incubated with nickel-NTA agarose (Qiagen) in lysis buffer for 2 hr at 4°C with mixing. The beads were then washed twice with 10 ml wash buffer (50 mM NaH2PO4, pH 7.4, 300 mM NaCl, 20 mM imidazole). His-tagged Rrp6 protein was eluted from agarose with 1 ml elution buffer (50 mM NaH2PO4, pH 7.4, 300 mM NaCl, 250 mM imidazole) and dialyzed into PBS supplemented with 2 mM DTT.
Recombinant protein binding assay
For GST-Nab3 binding to His-Rrp6, purified soluble GST, GST-Rrp47 or GST-Nab3 (8 μ g) was incubated with 14 μ g of purified soluble His-Rrp6 and 10 μ l glutathione Sepharose 4B (GE Healthcare) in 1 ml IPP100 buffer (10 mM Tris-HCl, pH 8, 100 mM NaCl, 0.1% NP-40) supplemented with 10 mg/ml BSA, 2 mM DTT, and protease inhibitor mixture (0.2 mM phenylmethylsulfonyl fluoride, 3 ng/ml pepstatin A, leupeptin, aprotinin, and chymostatin) for 2 hr at 4C with mixing. Beads were washed three times with 1 ml IPP100 buffer supplemented with 2 mM DTT for 10 sec each. Total bound and 25 μ l (2.5%) input samples were analyzed by SDS-PAGE followed by immunoblotting with an anti-His antibody coupled to horseradish peroxidase (Invitrogen) to detect His-Rrp6 and anti-GST antibody (Santa Cruz Biotechnology) to detect GST, GST-Rrp47, and GST-Nab3.
Terminator readthrough reporter assay
To examine GFP protein expression from the pREF-GFP IMD2 terminator reporter plasmid in TRAMP mutant cells alone or air1-C178R air2Δ cells expressing NAB3, nrd1 mutants, NRD1, or SEN1, cells containing the pREF-GFP plasmid were grown in the presence of galactose to induce GFP expression, and the level of GFP protein in lysates was detected by immunoblotting. BY4741 Wild-type (ACY402), air1Δ (ACY1090), air2Δ (ACY1091), trf4Δ (ACY2149), W303 wild-type (ACY233), air1-C178R air2Δ (ACY2020) cells alone or air1-C178R air2Δ (ACY2020) cells containing vector (pAC423), NAB3 (pAC3246), nab3–11 (pAC3247), nab3-R331A (pAC3248), nab3-F333A (pAC3249), nab3-S399A (pAC3250), nab3-S400A (pAC3251), or nab3-ΔNBD (pAC3252), NRD1 (pAC3255) or SEN1 (pAC3256) 2 μ HIS3 plasmid, were transformed with pREF-GFP (pAC3225; [38]) and selected on Ura− or Ura−His− minimal media plates. Cells were grown overnight at 25°C to saturation in Ura− or Ura−His− minimal media containing 2% glucose. Cells were then diluted to OD600 = 0.4 in 10 ml Ura− or Ura−His− minimal media containing 2% raffinose and grown at 30°C for 2 hr. To induce GFP expression from pREF-GFP, 2% galactose was added to cells, 5 ml samples were removed for 0 hr time point, and remaining 5 ml samples were grown at 30°C for 3 hr. Protein lysates from cells at 0 hr and 3 hr time points were analyzed by immunoblotting with an anti-GFP antibody.
Chromatin immunoprecipitation
To analyze RNA Pol II association with IMD2 or snR13 gene in air1/2 cells, chromatin was prepared from yeast cells and immunoprecipitated with an RNA Pol II antibody according to the protocols of Keogh and Buratowski [60] and Chen et al. [61]. The relative Pol II occupancy across IMD2 or snR13-TRS31 locus was determined by quantitative PCR on chromatin immunoprecipitation and input samples using five IMD2 or snR13 primer pairs (S2 Table) and calculation of percentage input. Triplicate 100 ml cultures of air1-C178R air2Δ (ACY2020) cells containing vector (pRS426), AIR1 (pAC1613) or NAB3 (pAC2880) in Ura− media were grown to OD600 = 0.5 at 25°C. air1-C178R air2Δ cultures were shifted to 30°C for 1 hr 30 min and grown to OD600 = 0.8. Cells were cross-linked with 2.7 ml 37% formaldehyde (1% final) at 25°C for 20 min, incubated with 10 ml glycine stop solution (3 M glycine, 20 mM Tris base) for 5 min at 25°C, collected by centrifugation at 1500 x g for 4 min, washed twice with 50 ml TBS (20 mM Tris-HCl, pH 7.5, 150 mM NaCl), washed once with 10 ml FA lysis buffer (50 mM HEPES: KOH, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate) supplemented with 0.1% SDS, and collected by centrifugation. Cross-linked cell pellets were lysed in 300 μ l 300 mM FA Lysis buffer (FA buffer containing 300 mM NaCl) by bead beating. Chromatin extract was sonicated using a Bioruptor sonicator (set to high) for 7 min in 30 sec pulses. Sonicated chromatin extract was centrifuged at 4,000 x g for 15 min at 4°C and 500 μg chromatin extract was utilized per immunoprecipitation with an anti-Pol II monoclonal antibody (Clone 4H8; Active Motif). Immune complexes were captured using Protein A-conjugated agarose beads (Santa Cruz Biotechnology) and washed for 5 min each in 300 mM FA lysis buffer, 500 mM FA buffer (FA buffer containing 500 mM NaCl), LiCl buffer (10 mM Tris-HCl, pH 8.0, 250 mM LiCl, 0.5% NP-40, 0.5% sodium deoxycholate, 1 mM EDTA), and TE. Samples were digested with RNase A and eluted from the Protein A-agarose beads in elution buffer (0.1 M sodium bicarbonate, 1% SDS) by two 200 μ l room temperature incubations with rotation. IP samples and input samples (10% total chromatin extract) were incubated at 65°C for at least 4 hr to reverse cross-links. DNA was purified using miniprep columns (Qiagen), IP DNA was eluted in 50 μ l distilled water, and input DNA in 100 μ l distilled water. Quantitative PCR (qPCR) on technical duplicates of IP or input DNA with IMD2 or snR13 primers (S2 Table) was performed in 20 μ l reactions containing QuantiTect SYBR Green PCR master mix (Qiagen), 0.5 mM primers and 2 μ l of IP or input DNA. Each qPCR experiment was performed on a StepOnePlus Real-Time PCR machine (Applied Biosystems) using Tanneal = 55°C and 44 cycles. Relative Pol II occupancy was calculated as a percentage of input using the equation: ΔCt = 2∧-(IPCt—InputCt). The mean relative occupancy values from three independent experiments were calculated and the mean occupancy values at Primer Pair 1–5 locations were normalized to the value obtained with Primer Pair 1 at the IMD2 CUT or snR13 gene in each strain. The normalized mean Pol II occupancy (relative Pol II occupancy) is displayed with error bars that represent the standard error of mean.
Localization of GFP-tagged proteins
To localize Nab3-GFP and nab3–1–448 proteins, air1-C178R air2Δ cells (ACY2020) expressing GFP-tagged Nab3 protein (pAC3281) or nab3–1–448 (pAC3282) were grown in Leu− media with 2% glucose overnight at 25°C, transferred to Ura−Leu− media with 2% glucose and grown to log phase at 25°C. GFP fusion proteins were visualized by direct fluorescence microscopy using an Olympus BX60 direct fluorescence microscope equipped with a photometric Quantix digital camera from Roper Scientific (Tucson, AZ) and filters from Chroma Technology (Brattleboro, VT). All images were captured using IP Lab Spectrum software.
Supporting Information
Zdroje
1. Houseley J (2012) Form and function of eukaryotic unstable non-coding RNAs. Biochem Soc Trans 40 : 836–841. doi: 10.1042/BST20120040 22817744
2. Knowling S, Morris KV (2011) Non-coding RNA and antisense RNA. Nature's trash or treasure? Biochimie 93 : 1922–1927. doi: 10.1016/j.biochi.2011.07.031 21843589
3. Shi X, Sun M, Liu H, Yao Y, Song Y (2013) Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett 339 : 159–166. doi: 10.1016/j.canlet.2013.06.013 23791884
4. van D II, Gordebeke PM, Khoshab N, Tiesinga PH, Buitelaar JK, et al. (2013) Long non-coding RNAs in neurodevelopmental disorders. Front Mol Neurosci 6 : 53. doi: 10.3389/fnmol.2013.00053 24415997
5. Kuehner JN, Pearson EL, Moore C (2011) Unravelling the means to an end: RNA polymerase II transcription termination. Nat Rev Mol Cell Biol 12 : 283–294. doi: 10.1038/nrm3098 21487437
6. Sloan KE, Schneider C, Watkins NJ (2012) Comparison of the yeast and human nuclear exosome complexes. Biochem Soc Trans 40 : 850–855. doi: 10.1042/BST20120061 22817747
7. Chlebowski A, Lubas M, Jensen TH, Dziembowski A (2013) RNA decay machines: the exosome. Biochim Biophys Acta 1829 : 552–560. doi: 10.1016/j.bbagrm.2013.01.006 23352926
8. Rougemaille M, Libri D (2011) Control of cryptic transcription in eukaryotes. Adv Exp Med Biol 702 : 122–131. doi: 10.1007/978-1-4419-7841-7_10 21713682
9. Schmidt K, Butler JS (2013) Nuclear RNA surveillance: role of TRAMP in controlling exosome specificity. Wiley Interdiscip Rev RNA 4 : 217–231. doi: 10.1002/wrna.1155 23417976
10. Butler JS, Mitchell P (2011) Rrp6, rrp47 and cofactors of the nuclear exosome. Adv Exp Med Biol 702 : 91–104. doi: 10.1007/978-1-4419-7841-7_8 21713680
11. Vanacova S, Wolf J, Martin G, Blank D, Dettwiler S, et al. (2005) A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol 3: e189. 15828860
12. LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, et al. (2005) RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 121 : 713–724. 15935758
13. Wyers F, Rougemaille M, Badis G, Rousselle JC, Dufour ME, et al. (2005) Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121 : 725–737. 15935759
14. Hamill S, Wolin SL, Reinisch KM (2010) Structure and function of the polymerase core of TRAMP, a RNA surveillance complex. Proc Natl Acad Sci U S A 107 : 15045–15050. doi: 10.1073/pnas.1003505107 20696927
15. Holub P, Lalakova J, Cerna H, Pasulka J, Sarazova M, et al. (2012) Air2p is critical for the assembly and RNA-binding of the TRAMP complex and the KOW domain of Mtr4p is crucial for exosome activation. Nucleic Acids Res 40 : 5679–5693. doi: 10.1093/nar/gks223 22402490
16. Fasken MB, Leung SW, Banerjee A, Kodani MO, Chavez R, et al. (2011) Air1 Zinc Knuckles 4 and 5 and a Conserved IWRXY Motif Are Critical for the Function and Integrity of the Trf4/5-Air1/2-Mtr4 Polyadenylation (TRAMP) RNA Quality Control Complex. J Biol Chem 286 : 37429–37445. doi: 10.1074/jbc.M111.271494 21878619
17. Inoue K, Mizuno T, Wada K, Hagiwara M (2000) Novel RING finger proteins, Air1p and Air2p, interact with Hmt1p and inhibit the arginine methylation of Npl3p. J Biol Chem 275 : 32793–32799. 10896665
18. Lubas M, Christensen MS, Kristiansen MS, Domanski M, Falkenby LG, et al. (2011) Interaction profiling identifies the human nuclear exosome targeting complex. Mol Cell 43 : 624–637. doi: 10.1016/j.molcel.2011.06.028 21855801
19. Vasiljeva L, Buratowski S (2006) Nrd1 interacts with the nuclear exosome for 3' processing of RNA polymerase II transcripts. Mol Cell 21 : 239–248. 16427013
20. Steinmetz EJ, Conrad NK, Brow DA, Corden JL (2001) RNA-binding protein Nrd1 directs poly(A)-independent 3'-end formation of RNA polymerase II transcripts. Nature 413 : 327–331. 11565036
21. Carroll KL, Pradhan DA, Granek JA, Clarke ND, Corden JL (2004) Identification of cis elements directing termination of yeast nonpolyadenylated snoRNA transcripts. Mol Cell Biol 24 : 6241–6252. 15226427
22. Conrad NK, Wilson SM, Steinmetz EJ, Patturajan M, Brow DA, et al. (2000) A yeast heterogeneous nuclear ribonucleoprotein complex associated with RNA polymerase II. Genetics 154 : 557–571. 10655211
23. Wlotzka W, Kudla G, Granneman S, Tollervey D (2011) The nuclear RNA polymerase II surveillance system targets polymerase III transcripts. EMBO J 30 : 1790–1803. doi: 10.1038/emboj.2011.97 21460797
24. Jamonnak N, Creamer TJ, Darby MM, Schaughency P, Wheelan SJ, et al. (2011) Yeast Nrd1, Nab3, and Sen1 transcriptome-wide binding maps suggest multiple roles in post-transcriptional RNA processing. RNA 17 : 2011–2025. doi: 10.1261/rna.2840711 21954178
25. Arigo JT, Eyler DE, Carroll KL, Corden JL (2006) Termination of cryptic unstable transcripts is directed by yeast RNA-binding proteins Nrd1 and Nab3. Mol Cell 23 : 841–851. 16973436
26. Thiebaut M, Kisseleva-Romanova E, Rougemaille M, Boulay J, Libri D (2006) Transcription termination and nuclear degradation of cryptic unstable transcripts: a role for the nrd1-nab3 pathway in genome surveillance. Mol Cell 23 : 853–864. 16973437
27. Kuehner JN, Brow DA (2008) Regulation of a eukaryotic gene by GTP-dependent start site selection and transcription attenuation. Mol Cell 31 : 201–211. doi: 10.1016/j.molcel.2008.05.018 18657503
28. Jenks MH, O'Rourke TW, Reines D (2008) Properties of an intergenic terminator and start site switch that regulate IMD2 transcription in yeast. Mol Cell Biol 28 : 3883–3893. doi: 10.1128/MCB.00380-08 18426909
29. Carroll KL, Ghirlando R, Ames JM, Corden JL (2007) Interaction of yeast RNA-binding proteins Nrd1 and Nab3 with RNA polymerase II terminator elements. RNA 13 : 361–373. 17237360
30. Vasiljeva L, Kim M, Mutschler H, Buratowski S, Meinhart A (2008) The Nrd1-Nab3-Sen1 termination complex interacts with the Ser5-phosphorylated RNA polymerase II C-terminal domain. Nat Struct Mol Biol 15 : 795–804. doi: 10.1038/nsmb.1468 18660819
31. Nedea E, Nalbant D, Xia D, Theoharis NT, Suter B, et al. (2008) The Glc7 phosphatase subunit of the cleavage and polyadenylation factor is essential for transcription termination on snoRNA genes. Mol Cell 29 : 577–587. doi: 10.1016/j.molcel.2007.12.031 18342605
32. Tudek A, Porrua O, Kabzinski T, Lidschreiber M, Kubicek K, et al. (2014) Molecular basis for coordinating transcription termination with noncoding RNA degradation. Mol Cell 55 : 467–481. doi: 10.1016/j.molcel.2014.05.031 25066235
33. Porrua O, Libri D (2013) A bacterial-like mechanism for transcription termination by the Sen1p helicase in budding yeast. Nat Struct Mol Biol 20 : 884–891. doi: 10.1038/nsmb.2592 23748379
34. Kim HD, Choe J, Seo YS (1999) The sen1(+) gene of Schizosaccharomyces pombe, a homologue of budding yeast SEN1, encodes an RNA and DNA helicase. Biochemistry 38 : 14697–14710. 10545196
35. Skourti-Stathaki K, Proudfoot NJ, Gromak N (2011) Human senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent termination. Mol Cell 42 : 794–805. doi: 10.1016/j.molcel.2011.04.026 21700224
36. Moreira MC, Klur S, Watanabe M, Nemeth AH, Le Ber I, et al. (2004) Senataxin, the ortholog of a yeast RNA helicase, is mutant in ataxia-ocular apraxia 2. Nat Genet 36 : 225–227. 14770181
37. Chen YZ, Bennett CL, Huynh HM, Blair IP, Puls I, et al. (2004) DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am J Hum Genet 74 : 1128–1135. 15106121
38. Loya TJ, O'Rourke TW, Reines D (2012) A genetic screen for terminator function in yeast identifies a role for a new functional domain in termination factor Nab3. Nucleic Acids Res 40 : 7476–7491. doi: 10.1093/nar/gks377 22564898
39. Preker P, Nielsen J, Kammler S, Lykke-Andersen S, Christensen MS, et al. (2008) RNA exosome depletion reveals transcription upstream of active human promoters. Science 322 : 1851–1854. doi: 10.1126/science.1164096 19056938
40. Wilson SM, Datar KV, Paddy MR, Swedlow JR, Swanson M (1994) Characterization of nuclear polyadenylated RNA-binding proteins in Saccharomyces cerevisiae. J Cell Biol 127 : 1173–1184. 7962083
41. Hobor F, Pergoli R, Kubicek K, Hrossova D, Bacikova V, et al. (2011) Recognition of transcription termination signal by the nuclear polyadenylated RNA-binding (NAB) 3 protein. J Biol Chem 286 : 3645–3657. doi: 10.1074/jbc.M110.158774 21084293
42. Arigo JT, Carroll KL, Ames JM, Corden JL (2006) Regulation of yeast NRD1 expression by premature transcription termination. Mol Cell 21 : 641–651. 16507362
43. Grzechnik P, Kufel J (2008) Polyadenylation linked to transcription termination directs the processing of snoRNA precursors in yeast. Mol Cell 32 : 247–258. doi: 10.1016/j.molcel.2008.10.003 18951092
44. Steinmetz EJ, Brow DA (2003) Ssu72 protein mediates both poly(A)-coupled and poly(A)-independent termination of RNA polymerase II transcription. Mol Cell Biol 23 : 6339–6349. 12944462
45. Callahan KP, Butler JS (2010) TRAMP complex enhances RNA degradation by the nuclear exosome component Rrp6. J Biol Chem 285 : 3540–3547. doi: 10.1074/jbc.M109.058396 19955569
46. Lepore N, Lafontaine DL (2011) A functional interface at the rDNA connects rRNA synthesis, pre-rRNA processing and nucleolar surveillance in budding yeast. PLoS One 6: e24962. doi: 10.1371/journal.pone.0024962 21949810
47. Phillips S, Butler JS (2003) Contribution of domain structure to the RNA 3' end processing and degradation functions of the nuclear exosome subunit Rrp6p. RNA 9 : 1098–1107. 12923258
48. Stead JA, Costello JL, Livingstone MJ, Mitchell P (2007) The PMC2NT domain of the catalytic exosome subunit Rrp6p provides the interface for binding with its cofactor Rrp47p, a nucleic acid-binding protein. Nucleic Acids Res 35 : 5556–5567. 17704127
49. Dziembowski A, Lorentzen E, Conti E, Seraphin B (2007) A single subunit, Dis3, is essentially responsible for yeast exosome core activity. Nat Struct Mol Biol 14 : 15–22. 17173052
50. Porrua O, Hobor F, Boulay J, Kubicek K, D'Aubenton-Carafa Y, et al. (2012) In vivo SELEX reveals novel sequence and structural determinants of Nrd1-Nab3-Sen1-dependent transcription termination. EMBO J 31 : 3935–3948. doi: 10.1038/emboj.2012.237 23032188
51. Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, et al. (2003) Global analysis of protein expression in yeast. Nature 425 : 737–741. 14562106
52. Creamer TJ, Darby MM, Jamonnak N, Schaughency P, Hao H, et al. (2011) Transcriptome-wide binding sites for components of the Saccharomyces cerevisiae non-poly(A) termination pathway: Nrd1, Nab3, and Sen1. PLoS Genet 7: e1002329. doi: 10.1371/journal.pgen.1002329 22028667
53. Yuryev A, Patturajan M, Litingtung Y, Joshi RV, Gentile C, et al. (1996) The C-terminal domain of the largest subunit of RNA polymerase II interacts with a novel set of serine/arginine-rich proteins. Proc Natl Acad Sci U S A 93 : 6975–6980. 8692929
54. Tenzer S, Moro A, Kuharev J, Francis AC, Vidalino L, et al. (2013) Proteome-wide characterization of the RNA-binding protein RALY-interactome using the in vivo-biotinylation-pulldown-quant (iBioPQ) approach. J Proteome Res 12 : 2869–2884. doi: 10.1021/pr400193j 23614458
55. Adams A, Gottschling DE, Kaiser CA, Stearns T (1997) Methods in Yeast Genetics. Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
56. Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press.
57. Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P (1992) Multifunctional yeast high-copy-number shuttle vectors. Gene 110 : 119–122. 1544568
58. Sikorski RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122 : 19–27. 2659436
59. Storici F, Lewis LK, Resnick MA (2001) In vivo site-directed mutagenesis using oligonucleotides. Nat Biotechnol 19 : 773–776. 11479573
60. Keogh MC, Buratowski S (2004) Using chromatin immunoprecipitation to map cotranscriptional mRNA processing in Saccharomyces cerevisiae. Methods Mol Biol 257 : 1–16. 14769992
61. Chen H, Fan M, Pfeffer LM, Laribee RN (2012) The histone H3 lysine 56 acetylation pathway is regulated by target of rapamycin (TOR) signaling and functions directly in ribosomal RNA biogenesis. Nucleic Acids Res 40 : 6534–6546. doi: 10.1093/nar/gks345 22553361
62. Schrodinger, LLC (2010) The PyMOL Molecular Graphics System, Version 1.3r1.
63. Lunde BM, Horner M, Meinhart A (2011) Structural insights into cis element recognition of non-polyadenylated RNAs by the Nab3-RRM. Nucleic Acids Res 39 : 337–346. doi: 10.1093/nar/gkq751 20805243
64. Kelley LA, Sternberg MJ (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4 : 363–371. doi: 10.1038/nprot.2009.2 19247286
Štítky
Genetika Reprodukční medicína
Článek NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY ModuleČlánek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in NematodesČlánek The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse SpermatocytesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 3
-
Všechny články tohoto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



