-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaResistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
Gray leaf spot (GLS), a necrotrophic, foliar fungal disease of maize, contributes to maize yield losses worldwide. We identified and characterized regions of the maize genome that confer resistance to GLS and gained insight into the mechanisms associated with these quantitative trait loci (QTL). We provide evidence for structural and detoxification-related mechanisms underlying quantitative resistance. The distance between major veins of the maize leaf was positively correlated with the quantity of fungal conidiophores (reproductive structures) produced. Four of the GLS QTL were associated with inter-vein distance, and co-localization was confirmed for one of these QTL in near-isogenic lines. In addition, up-regulation of a putative detoxification-related flavin-monooxygenase gene was correlated with a fine-mapped QTL. Plant breeding decisions regarding development and deployment of disease resistance traits can be improved with better understanding of the mechanisms underlying quantitative disease resistance.
Published in the journal: . PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005045
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005045Summary
Gray leaf spot (GLS), a necrotrophic, foliar fungal disease of maize, contributes to maize yield losses worldwide. We identified and characterized regions of the maize genome that confer resistance to GLS and gained insight into the mechanisms associated with these quantitative trait loci (QTL). We provide evidence for structural and detoxification-related mechanisms underlying quantitative resistance. The distance between major veins of the maize leaf was positively correlated with the quantity of fungal conidiophores (reproductive structures) produced. Four of the GLS QTL were associated with inter-vein distance, and co-localization was confirmed for one of these QTL in near-isogenic lines. In addition, up-regulation of a putative detoxification-related flavin-monooxygenase gene was correlated with a fine-mapped QTL. Plant breeding decisions regarding development and deployment of disease resistance traits can be improved with better understanding of the mechanisms underlying quantitative disease resistance.
Introduction
Plants resist attack by many plant pathogens by inducing localized cell death. This strategy can provide effective defense against pathogens that require living host tissue (biotrophs or hemibiotrophs) and is the basis for most monogenic resistance. However, it is not effective for pathogens that feed on dead tissue (necrotrophs) [1]. For this reason, complete, single-gene resistance is typically unavailable for diseases caused by necrotrophic pathogens [2,3]. Gray leaf spot (GLS), a foliar disease of maize caused by the polycyclic pathogens Cercospora zeae-maydis and Cercospora zeina [4,5], is a necrotrophic pathogen that is mostly controlled by quantitative forms of host plant resistance. Understanding the mechanisms underlying resistance to GLS may further elucidate the biology of host-pathogen interactions since there are contrasting mechanisms of defense against necrotrophic and biotrophic pathogens [6]. GLS is one of the most important maize diseases in the United States and in maize-growing regions worldwide [7]. It can cause yield losses over 70% due to associated severe blighting, stalk deterioration and lodging [8,9].
Although it is critical for the management of most plant diseases (not only those caused by necrotrophic pathogens), the genetic basis of quantitative disease resistance is not well understood. A better understanding of the genetic architecture and the underlying genes and mechanisms could contribute to crop improvement and disease management. Previous QTL studies have improved understanding of the genetic architecture by identifying regions of the genome that confer resistance to GLS [4,10–22]. The aim of this study was to improve understanding of the locations, sizes and interactions among loci controlling GLS resistance using nested association mapping (NAM) [23] and to develop hypotheses concerning the underlying mechanisms of resistance.
Quantitative resistance can be divided into a range of sub-phenotypes at the macroscopic and microscopic levels. These phenotypes include reduction in total number of infections, reduction in lesion expansion, reduction of sporulation, lengthening of the latent period, and increasing the number of propagules necessary to establish infection [24]. Individual QTL may differentially affect specific sub-phenotypes [25]. Poland et al. [26] provided six hypotheses regarding mechanisms that underlie QDR loci: (1) genes that underlie plant development and architecture, (2) genes with mutations or allelic changes in genes involved in basal defense, (3) genes involved in secondary metabolite production known to fend off pathogen attacks, (4) genes involved in signal transduction, (5) weak forms of R-genes, and (6) genes previously unassociated with pathogen defense. Cloning of several disease QTL has provided hints about the underlying molecular mechanisms involved in resistance [27–32].
Poland’s third hypothesis above indicates that such mechanisms might include those involved in chemical warfare between plant pathogens and their hosts. For example, genes for resistance might mitigate the damaging effects of microbial compounds on host tissues. Plant pathogens produce an array of toxic molecules and enzymes that aid in host infection [33]. These products come in diverse chemical forms and are either host non-specific or specific. Cercospora zeae-maydis, the predominant causal agent of GLS in the United States, produces the non-host-selective toxin cercosporin. Cercosporin is a photo-activated perylenequinone that converts molecular oxygen to active oxygen species, including singlet oxygen [34]. Plant carotenoids play a key role in quenching singlet oxygen before it damages the chloroplast or other machinery within the plant cell [35] and are hypothesized to play a role in defense against toxins that produce reactive oxygen species [36]. Additional enzymes and metabolites, such as oxidoreductases and secondary metabolites with antioxidant properties, may be involved in cercosporin detoxification or in reducing damage caused by cercosporin [37–39]. We explored the hypothesis that detoxification could play a role in GLS resistance by analyzing the annotation and expression of genes underlying one of the detected GLS loci.
It has also been proposed that some genes conferring quantitative disease resistance are related to morphological and developmental processes such as leaf structure and flowering time [40,41]. GLS symptoms include tan-colored, rectangular-shaped lesions that elongate parallel to the leaf venation. Lesions appear to be bounded by major veins [42]. We hypothesized that inter-vein distance (IVD) could act as a resistance-related parameter for GLS, because the width of GLS lesions is defined by IVD. Although there is evidence that lesion expansion can contribute significantly to disease epidemics caused by Cercospora spp., to our knowledge, no studies have examined the relationship between leaf venation structure and lesion development.
Results
Heritability of resistance
The NAM population was screened for GLS in Blacksburg, VA, and exhibited a wide range of disease levels among parental lines within and among populations (Table 1). Parental line AUDPC varied from 35.2 to 51.5 at the point of maximum variance identified among BLUPs. Mean NAM population AUDPC was 42.6 (σ2 = 13.2). Broad sense heritability on an individual plot basis was 0.72, while on a line mean basis, correcting for the unbalanced design of the experiment, it was 0.83 [43]. Additive genetic variance was responsible for 52% of the phenotypic variance as determined by calculating the correlation between the sum of all parental effects and the parental phenotypic BLUPs.
Tab. 1. AUDPC summary statistics for the nested association mapping population. 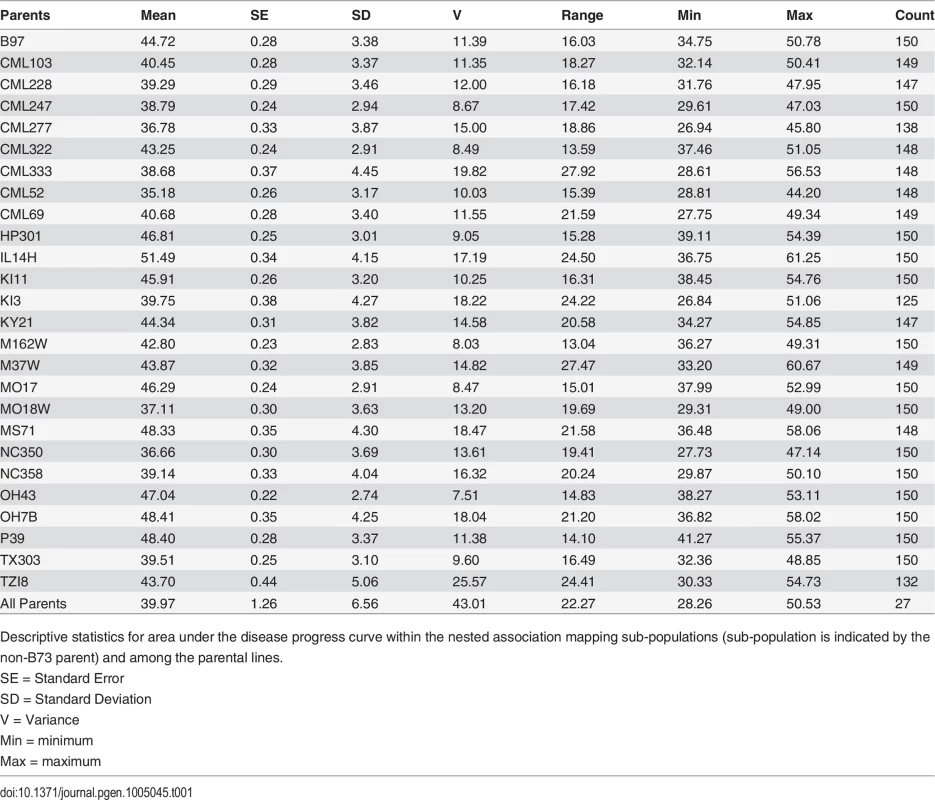
Descriptive statistics for area under the disease progress curve within the nested association mapping sub-populations (sub-population is indicated by the non-B73 parent) and among the parental lines. Disease resistance loci and interactions
A model selection approach to QTL mapping was used to identify loci that significantly described GLS disease progress. GLS QTL were designated as qGLSbin#x, where “q” indicates a QTL, bin# is replaced with the genomic bin location within which the selected markers were located, and “x” denotes the genotype source of the specified allele. Sixteen markers, located on nine of the 10 chromosomes, were selected by the model at p<4.3x10–4 (Table 2). The selection threshold was based on the q-value calculation for false discovery rate (FDR). Effect sizes across parental lines varied at each locus. The estimated effects on disease levels of each parental allele at each QTL relative to the B73 allele are listed in Fig. 1. Across the set of QTL, each parental line had susceptible, resistant and neutral allelic effects relative to B73. Significant model-selected QTL explained 78.5% of the phenotypic variation within the population, while inclusion of all QTL (p<0.05) explained 82.2% of the variation. The parental LSM of AUDPC for each locus was used to estimate the effect of each allele on disease levels. Alleles at three loci, qGLS1.04, qGLS2.09 and qGLS4.05, were predicted to confer disease reduction of greater than 10% (Fig. 2). The greatest change in disease reduction was predicted to be 11.7% for the TZI8 allele at qGLS2.09.
Fig. 1. Parental allelic effects at quantitative trait loci. 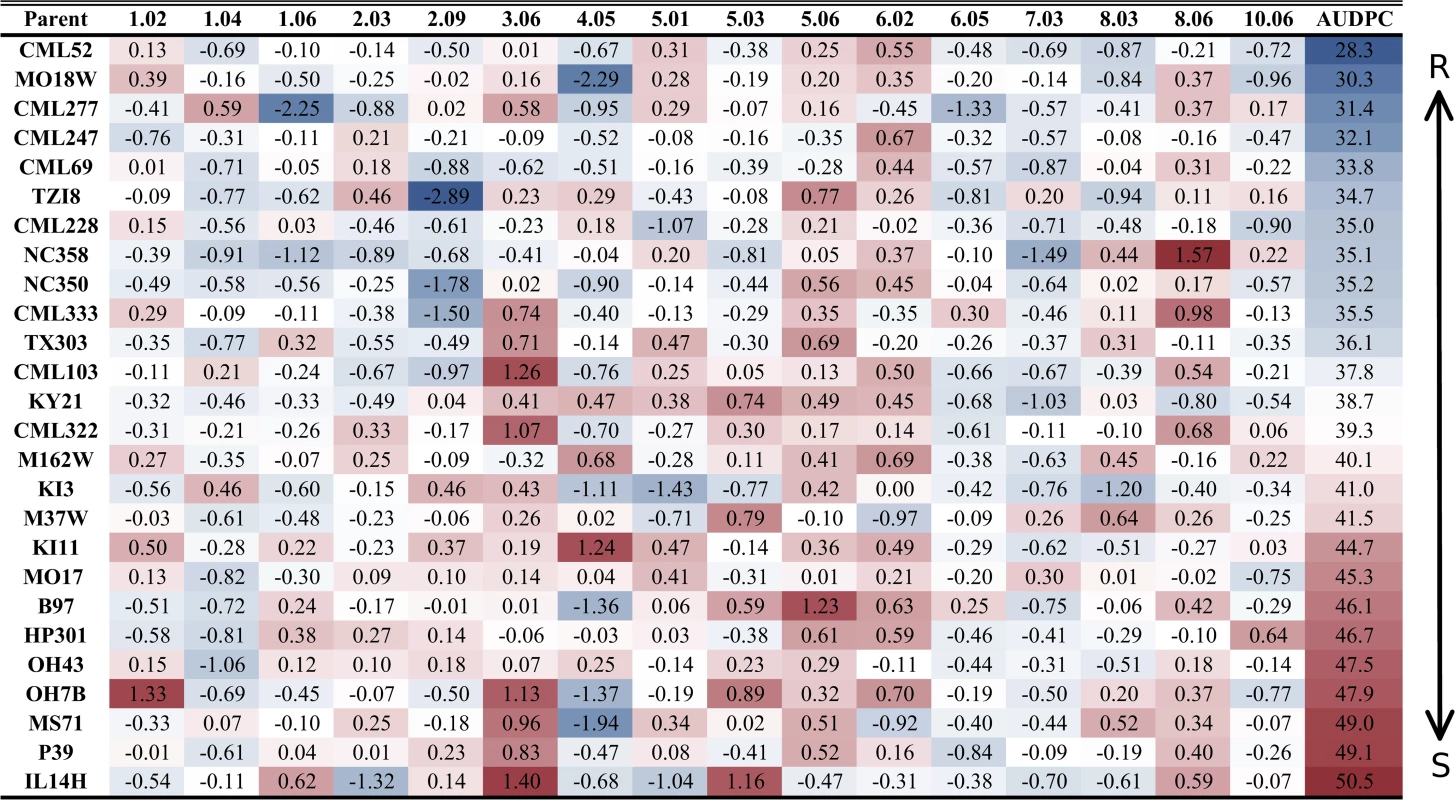
The bin number is listed in the respective column header between the first and last columns. Two sets of data are presented in this figure. Estimated allelic effects relative to the B73 allele of parental lines at qGLSbin for relative change in disease are listed. The values in the table are the coefficients of the general linear model parameter while the color coding is indicative of area under the disease progress curve. R = resistant; S = susceptible. Fig. 2. General linear model predicted percent change in disease across significant disease quantitative trait loci (QTL). 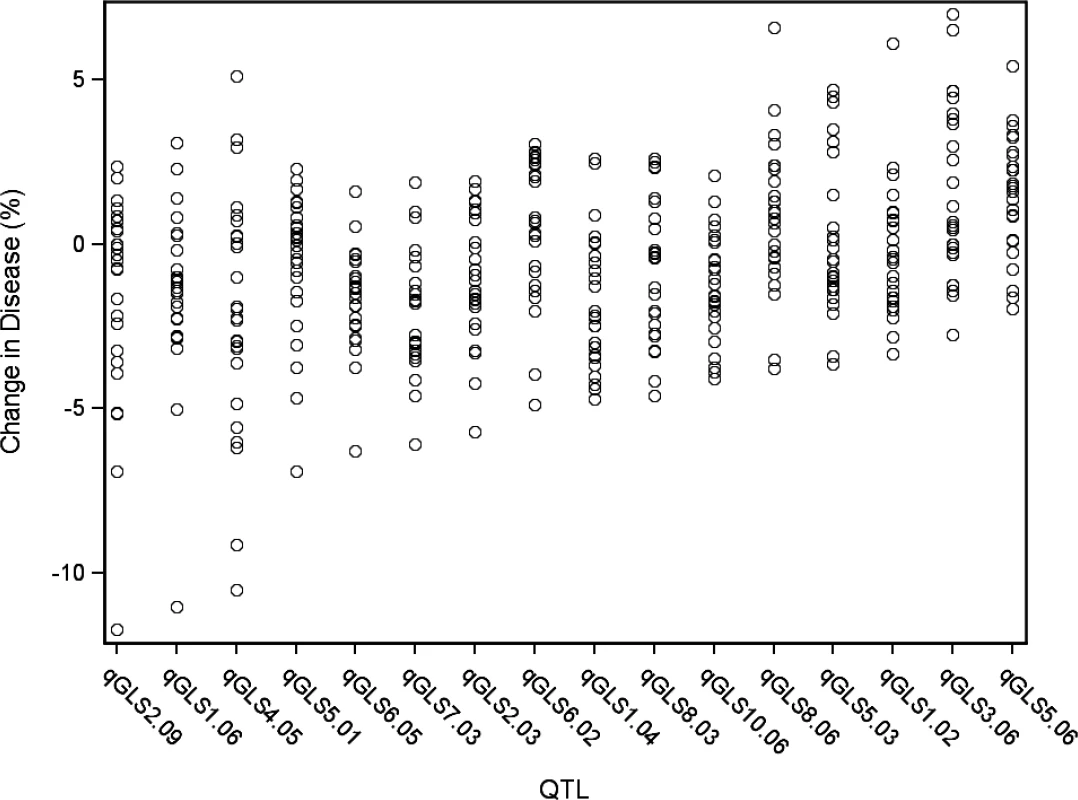
Each circle indicates the predicted change in disease of a single allele. There are 26 circles for each QTL, each representating the allele from one of the nested association mapping parental sources. Tab. 2. Summary of resistance to gray leaf spot (GLS) in the nested association mapping population. 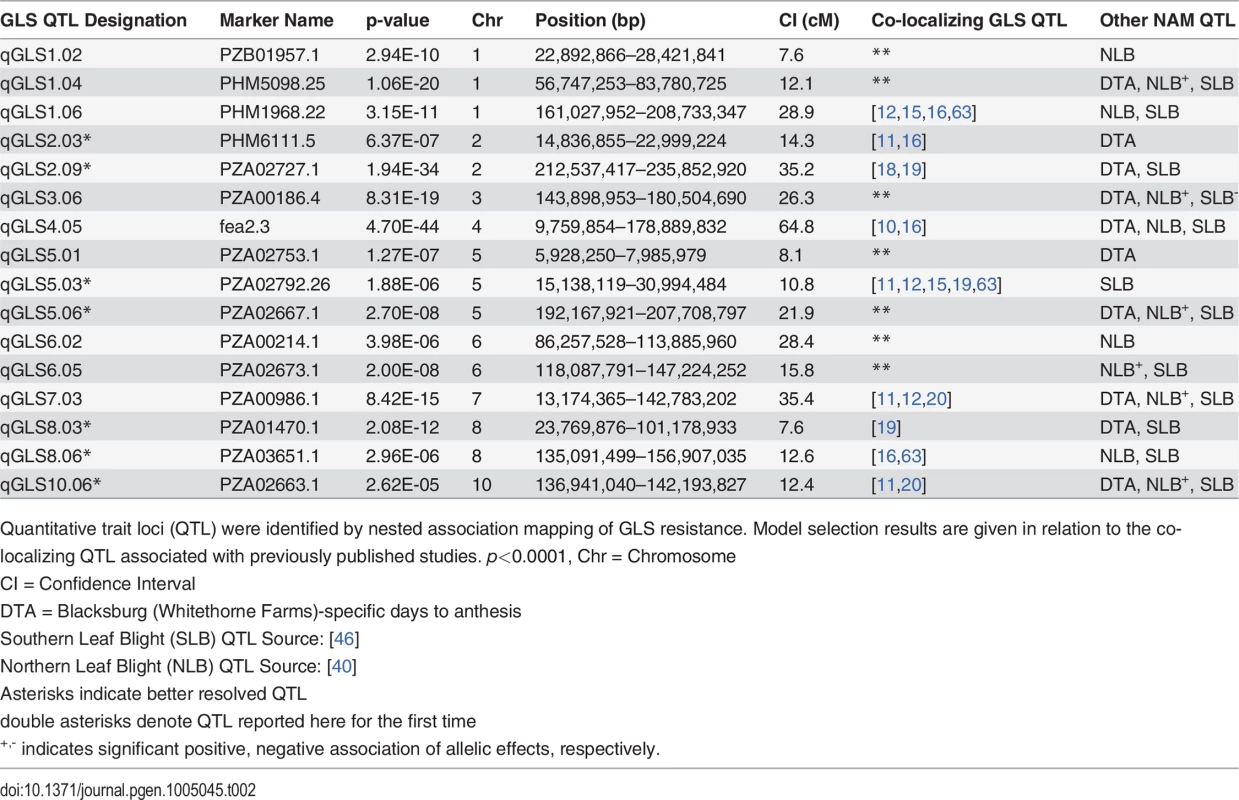
Quantitative trait loci (QTL) were identified by nested association mapping of GLS resistance. Model selection results are given in relation to the co-localizing QTL associated with previously published studies. p<0.0001, Chr = Chromosome All possible two-way interactions were entered one-by-one as predictors of AUDPC in the GLM model containing main-effect QTL and the parameters DTA and population. Three allelic interactions were detected. One pair of interacting loci involved two markers within significant disease QTL confidence intervals. There was a significant interaction between the two QTL significant for disease at qGLS4.05 and qGLS7.03. The other two pairs involved interactions between significant and non-significant markers at p<1.0x10–5. One of these interactions was between qGLS2.03 and maize bin 7.06 (175.8 Mb) while the other was between qGLS6.02 and 8.02 (12.3 Mb).
In addition to joint linkage, The NAM population was used to identify loci associated with resistance to GLS based on a genome-wide association study (GWAS). GWAS revealed a total of 145 single-nucleotide polymorphisms (SNPs) that were significantly associated with the GLS phenotype (S1 Table). One or more genes were identified within a 20 kb window of the significant association (hit) for 63 of the 145 GWAS hits (S1 Table). Characterized functional annotations based on the gene sequence provided in the maize B73 reference genome (V2) were detected for 41 of the 63 genes located within 20 kb of a GWAS hit. A proportions test was conducted for the gene ontology (GO) terms in each of these 41 genes to determine whether the observed frequency of the evaluated gene type associated with GLS QTL exceeded the frequency that would be expected at random, based on the frequency of the evaluated gene type in the maize genome (Table 3). Genes related to cellular energy, development, and secondary metabolites were statistically overrepresented categories with p-values of 2.22 x 10–10, 2.24 x 10–15, and 1.23x10–3, respectively.
Tab. 3. Summary statistics of functionally annotated and categorized genome wide association hits. 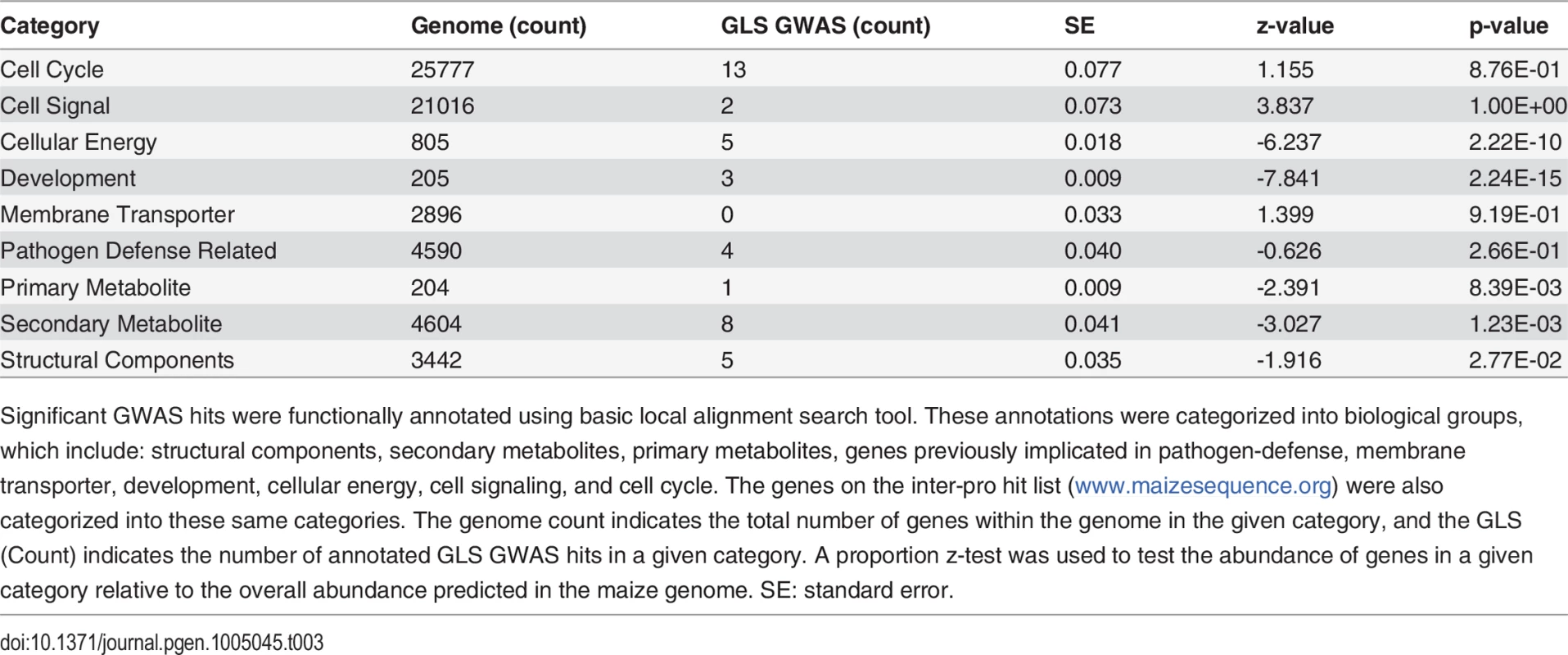
Significant GWAS hits were functionally annotated using basic local alignment search tool. These annotations were categorized into biological groups, which include: structural components, secondary metabolites, primary metabolites, genes previously implicated in pathogen-defense, membrane transporter, development, cellular energy, cell signaling, and cell cycle. The genes on the inter-pro hit list (www.maizesequence.org) were also categorized into these same categories. The genome count indicates the total number of genes within the genome in the given category, and the GLS (Count) indicates the number of annotated GLS GWAS hits in a given category. A proportion z-test was used to test the abundance of genes in a given category relative to the overall abundance predicted in the maize genome. SE: standard error. Disease QTL confirmation and pleiotropic loci
The three most significant QTL based on the predictive model, qGLS1.04, qGLS2.09, qGLS4.05, were confirmed using near-isogenic line pairs (NILs) extracted from heterogeneous inbred families (HIFs; [44]) (Table 2). Two of these QTL for GLS were previously reported, while qGLS1.04 is reported here for the first time. The families were composed of RIL that had been selfed for seven generations. Lines of the original S5 generation that were heterozygous at one of the three loci of interest were identified. Corresponding S7 lines were genotyped. For each HIF, at least six lines resulting from independent recombination events (three with the B73 allele and three with the alternate allele) were identified for further analysis of the three loci of interest.
The observed levels of disease were significantly different among the NILs for the B73 and other parent alleles (Fig. 3; p<0.05). The observed allelic effects were higher than those expected based on model predictions. NILs with the CML228 allele at qGLS1.04 exhibited an average of 12% less disease compared to NILs carrying the B73 allele at the same locus, while the model predicted a 2.5% disease reduction. Lines with the CML333 allele at qGLS2.09 exhibited an average of 22% less disease while the model predicted a 5.2% reduction. Finally, lines with the KI11 allele at qGLS4.05 exhibited an average disease increase of 8.4% relative to lines with the B73 allele at the same locus, while the model predicted a 5.1% increase in disease.
Fig. 3. Confirmation of three disease quantitative trait loci using near isogenic lines. 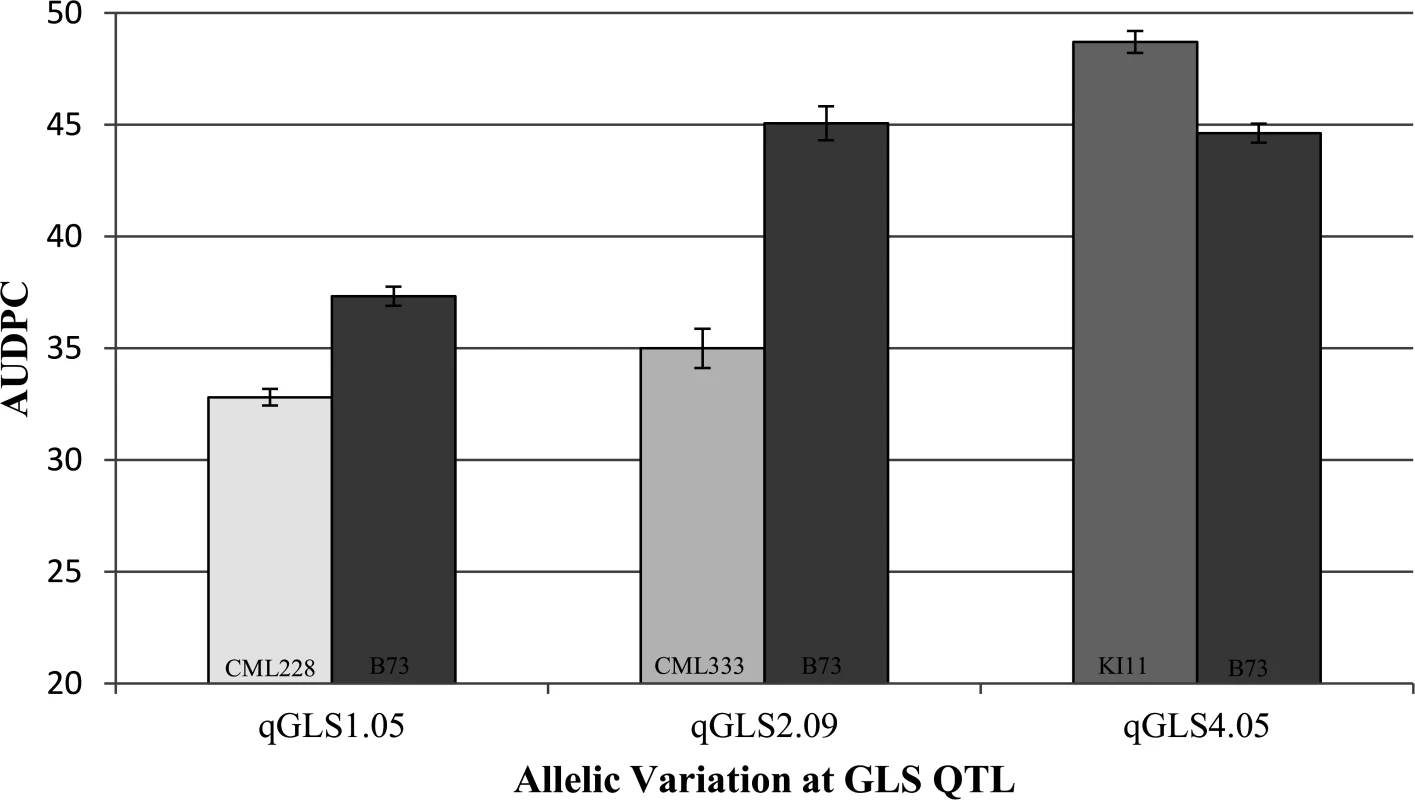
Disease development [area under the disease progress curve (AUDPC)]is indicated among heterogeneous inbred family lines across three gray leaf spot quantitative trait loci. Buckler et al. [45] previously identified flowering time QTL using the NAM population. Days to anthesis (DTA) data from that study (obtained from Aurora, NY) as well as from the present study were analyzed in relation to GLS disease progress data. A quadratic relationship was identified when the DTA data from Buckler et al. [45] were associated with disease progress collected in Blacksburg, while the relationship between DTA and disease progress in Blacksburg was linear. A linear relationship is preferred for general linear modeling, so the only DTA dataset used in this study was collected from the Blacksburg location. Ten of the GLS QTL had confidence intervals that overlapped with those of DTA QTL (Table 2). The DTA allelic effects were predicted to be significant at qGLS1.04CML228 (p = 0.0402) and qGLS4.05KI11 (p = 0.0244). Although DTA differences were significant among the NILs (p = 0.0263), the LSM differences in disease between the two alleles at qGLS4.05KI11 did not change when DTA was removed from the model.
In addition to DTA, QTL for northern leaf blight (NLB) and southern leaf blight (SLB) were previously identified using the NAM population [40,46]. The QTL count and absolute value of the QTL effect size were analyzed across GLS, NLB, SLB and DTA. There were significant differences in QTL effect size across all four traits (Fig. 4; p = 6.33x10–30). Fewer, significantly larger effect QTL were identified for GLS than for NLB and SLB. NLB and SLB QTL counts were similar, but effect sizes for SLB were significantly smaller. There was evidence of pleiotropy at several loci, based on correlations among allele effects for the different diseases. Allelic effects of GLS QTL were positively correlated with those of NLB QTL at six loci (Table 2; p<0.05). GLS QTL were negatively associated with allelic effects of SLB QTL at one locus (Table 2; p<0.05).
Fig. 4. Absolute value of quantitative trait loci (QTL) effect size across three diseases and flowering time. 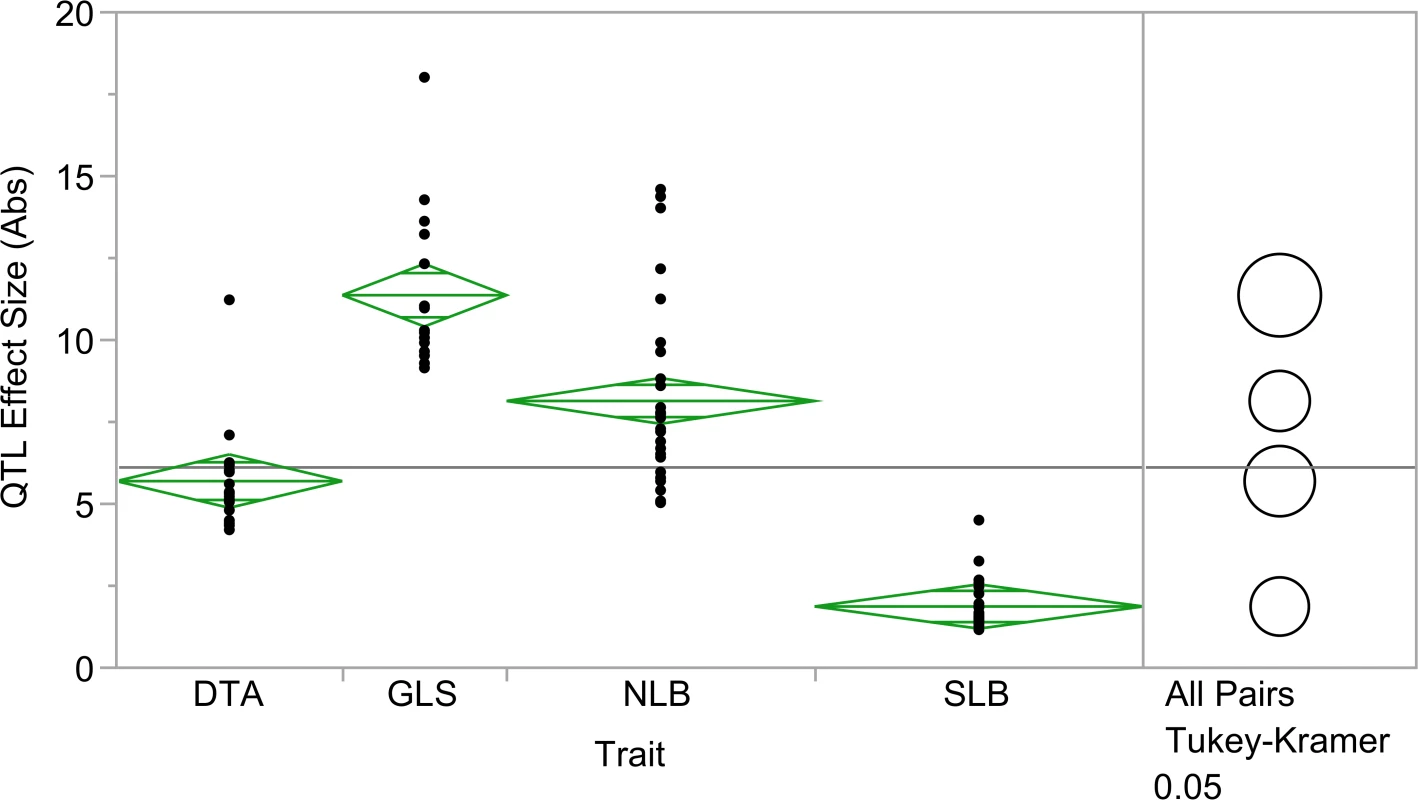
Effect sizes of QTL for gray leaf spot, northern leaf blight, southern leaf blight and Blacksburg specific days to anthesis were significantly different (p = 6.33x10–30). qGLS1.04CML228: fine-mapping and flavin-monooxygenase expression
NIL pairs were developed for the qGLS1.04CML228 locus. The allelic effect of the locus on GLS disease progress was determined, as noted above, and the locus was subsequently fine-mapped using derivatives of the same HIF population. The QTL interval spanned from 56,747,253 Mb to 83,780,725 Mb. The estimated recombination rate at this locus was 0.236 cM/Mb. The QTL was fine-mapped to two intervals of 77,242,690 to 83,780,725 Mb and 88,849,284 to 94,085,195 Mb, referred to as qGLS1.04_1 and qGLS1.04_2 (Table 4). The qGLS1.04_1 interval contained 99 genes based on version 2 of the maize genome (www.maizesequence.org), while the qGLS1.04_2 interval contained 51 genes. The genes within these regions were functionally annotated using BLAST. Both intervals in the 1.04 region were observed to have a high density of defense response (DR) genes. Genes implicated in detoxification constituted 13% of genes at the qGLS1.04_1 locus and 9.8% at the qGLS1.04_2 locus. Proportion tests revealed that putative glutathione-S-transferase genes were significantly more numerous than expected (Table 5).
Tab. 4. Markers and associated p-values from the qGLS1.04 fine-mapping analysis. 
Tab. 5. Summary statistics for detoxification-related genes underlying qGLS1.04. 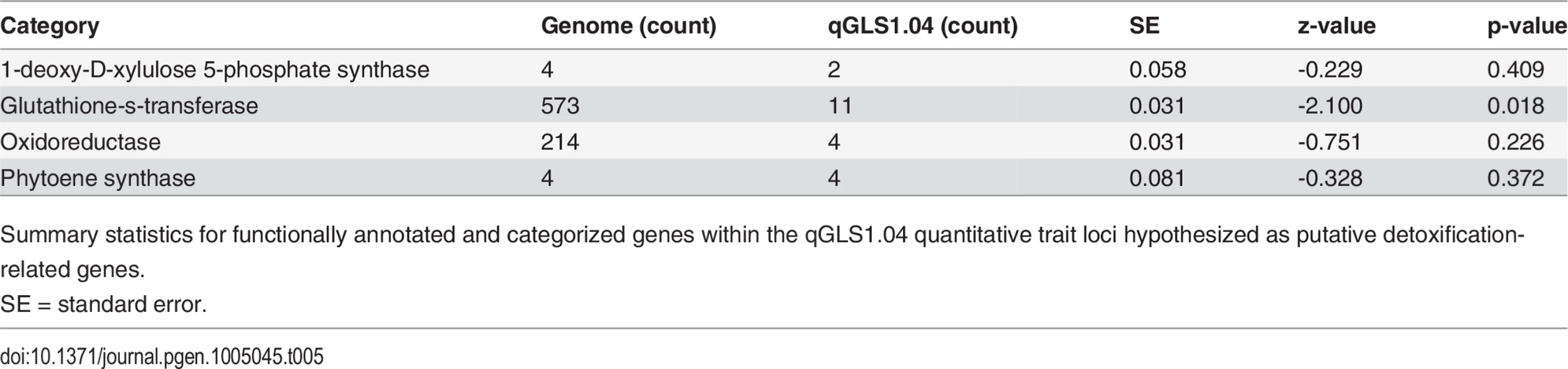
Summary statistics for functionally annotated and categorized genes within the qGLS1.04 quantitative trait loci hypothesized as putative detoxification-related genes. NILs were treated with cercosporin to test for DR genes playing a role in disease resistance. Expression was up-regulated 3.4-fold for GRMZM2G425719, a putative flavin-monooxygenase (FMO; Fig. 5). Up-regulation of other DR genes in the regions was not detected. Forty-four polymorphisms within the FMO were identified using HMPv2. Twenty of these polymorphisms were identified in the promoter region of the gene. G to A substitution was identified using a plant promoter algorithm to detect functional changes between the B73 and CML228 promoter regions. This change led to the detection of a putative functional TATA box within the CML228 allele. No significant difference was detected in carotenoid levels among the treated NILs.
Fig. 5. Analysis of flavin-monooxygenase expression at qGLS1.04. 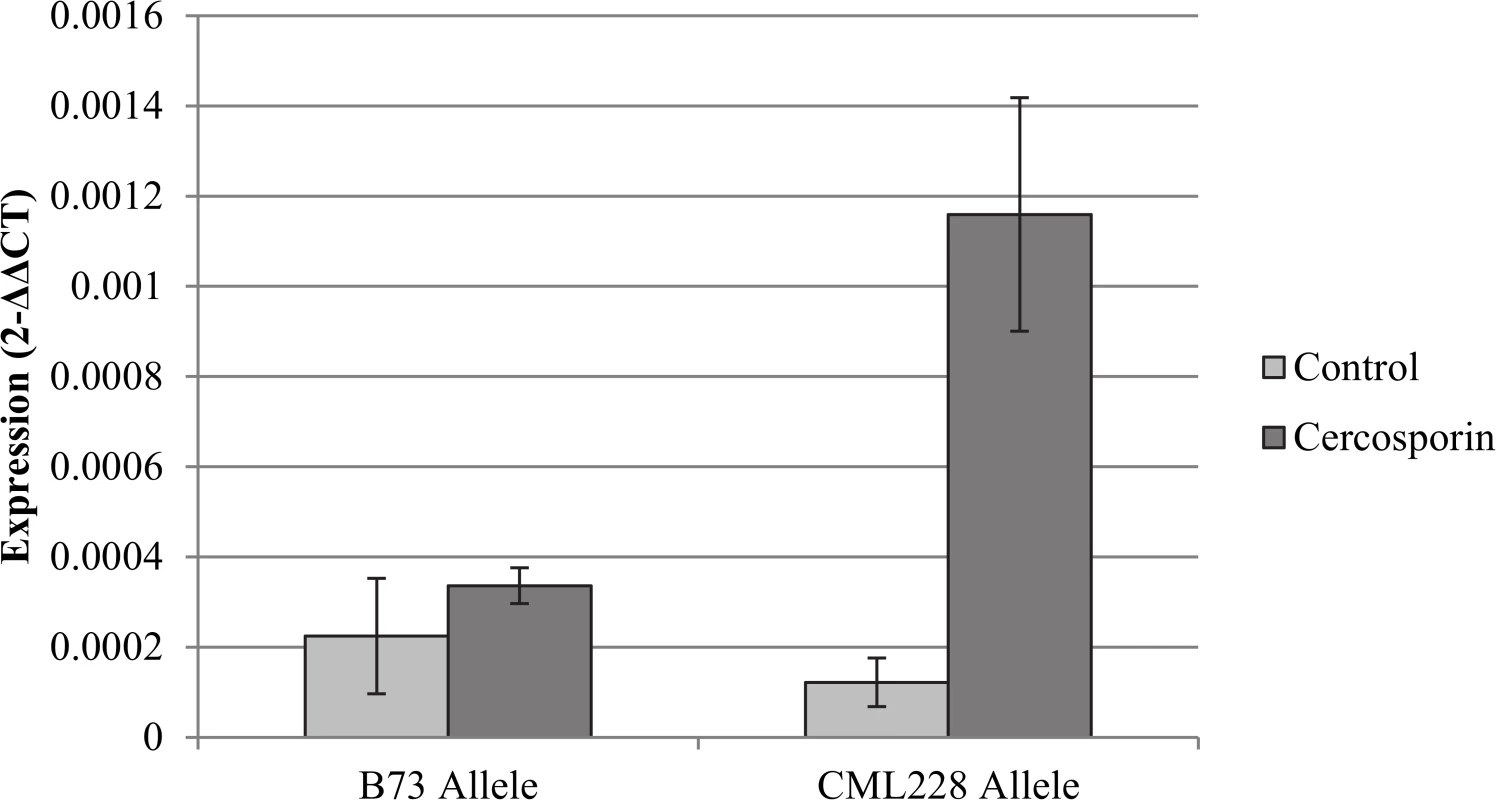
Expression differences were detected for the putative flavin-monooxygenase among heterogeneous inbred family lines segregating at qGLS1.04 for the B73 or CML228 maize alleles. The heterogenous inbred family lines were treated with cercosporin or the acetone control on either side of the maize leaf midrib. There was a significant difference between the CML228 samples treated with cercosporin and the other samples in the experiment (p = 0.0012). qGLS2.09CML333 and qGLS4.05KI11: fine-mapping
NIL pairs developed to be 99% isogenic were used to confirm and further fine-map qGLS2.09CML333 and qGLS4.05KI11. The fine-mapping population for qGLS2.09CML333 had poor seed set and was susceptible to drought stress. Only 375 plants survived from the 2450 kernels planted for fine-mapping during the 2011 field season. Irrigation needs were more closely monitored in 2012 to ensure higher survival rates (> 95%). The GLS QTL interval was reduced from 13 Mb to 4 Mb. This region had a recombination frequency of 0.908 cM/Mb and was predicted to contain 290 protein-coding genes.
qGLS4.05KI11 was initially considered an attractive target for fine-mapping because of its maximum LOD score of 44. The breakpoint density of this centromeric QTL could not be increased, however, due to the low recombination frequency of 0.05cM/Mb. This region had an estimated size of 140 Mb and contained as many as 7,500 protein-coding genes.
Loci affecting inter-vein distance and conidiophore development
Scanned images of GLS infection on maize leaves were analyzed using image analysis software. The distance between three major and three minor veins was measured for each leaf sample at the widest point of the leaf blade. A significant positive relationship between disease development (AUDPC) and the distance between major veins (IVD) was detected in the NAM population (p<0.0001). A significant relationship was not observed between minor vein distance and disease development. The observation that narrow IVD was associated with lower disease levels supported the hypothesis that this morphological trait could influence disease progression [26], and further suggested that QTL for disease and IVD might co-localize. To test this hypothesis, loci affecting IVD were analyzed using a model selection approach. Nine markers, located on six of the 10 chromosomes, were found to be associated with IVD. Similar to the disease QTL, effect sizes for IVD QTL across parental lines varied at each locus, and there were allelic effects significantly above and below the effect of the B73 allele. QTL for IVD and disease development co-localized at four intervals (Fig. 6). One of these QTL co-localized with qGLS4.05KI11. NILs for this locus were planted in the greenhouse and the average distance between veins was measured. A significant difference was detected between NILs containing the KI11 allele and those containing the B73 allele at the 4.05 locus (p<0.05).
Fig. 6. Gray leaf spot (GLS) and venation quantitative trait loci. 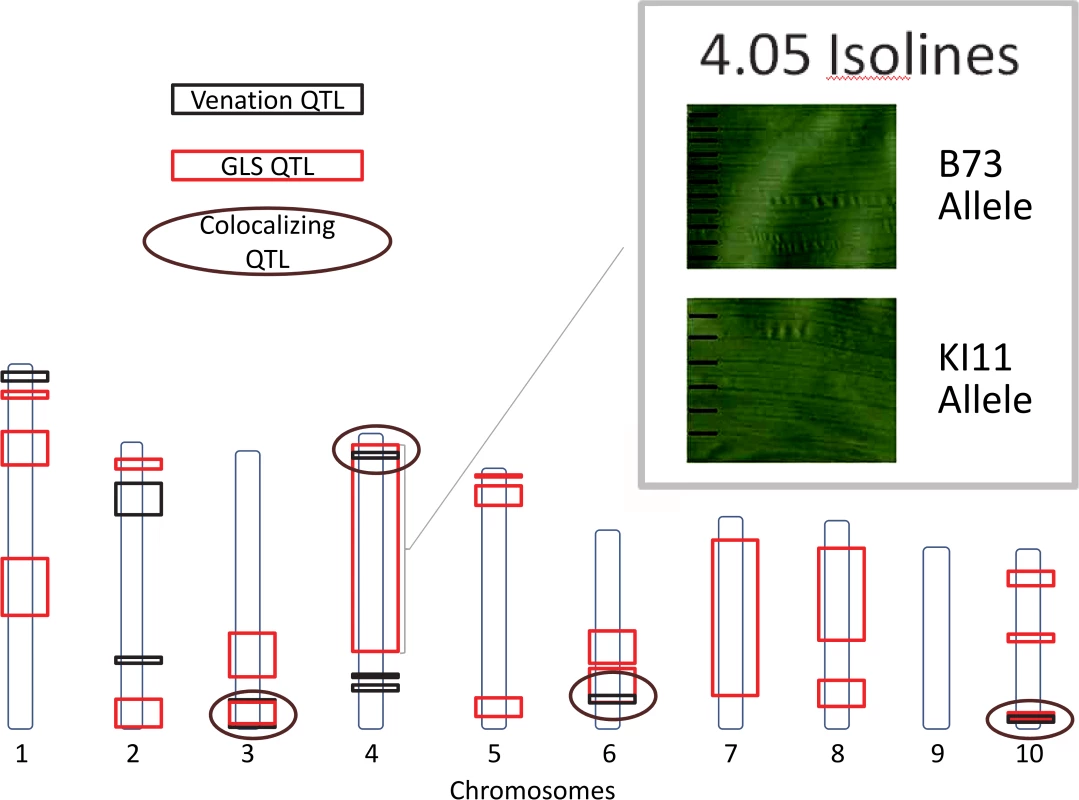
Quantitative trait loci for GLS and inter-vein distance (IVD) are indicated. Circles designate co-localizing areas. Scanned leaf images (upper-right corner) demonstrate the difference between IVD from maize leaves with B73 and KI11 alleles at qGLS4.05. To assess the epidemiological relevance of narrow IVD, lesion parameters and conidiophore counts were collected from 2011 lesion samples. The conidiophore counts and IVDs for NAM parental lines were compared (Fig. 7). IVD accounted for 46% of variation in conidiophore count. A significant positive correlation was detected between IVD and the number of conidiophores per lesion (p = 2.34x10–14). This correlation suggests that the smaller the distance between the major veins, the lower the conidiophore count. IVD remained significant in a model predicting conidiophore count, with lesion length, length*IVD and pedigree as additional fixed effects (Table 6). There was a strong relationship between pedigree and IVD, such that IVD became less significant in a model with pedigree (p<0.0001). Lesion length and IVD were entered into the model to determine the effect of IVD when conidiophore variation attributed to length was taken into account. In this case, IVD accounted for 41% of the variation for conidiophore count.
Fig. 7. Relationship between conidiophore count and intervein distance. 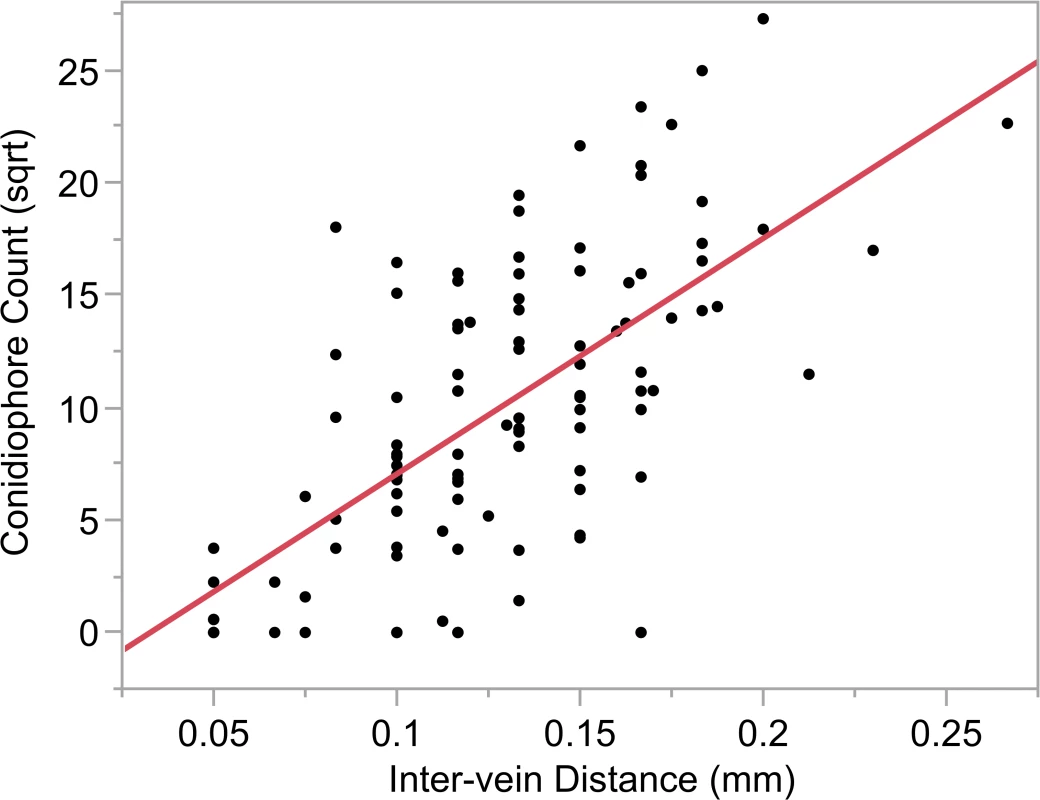
Significant relationship was detected between inter-vein distance and square root (sqrt) of the conidiophore counts (r2 = 0.43; p<0.0001). Tab. 6. Model results with conidiophore count (square root) as the response variable and lesion parameter predictors. 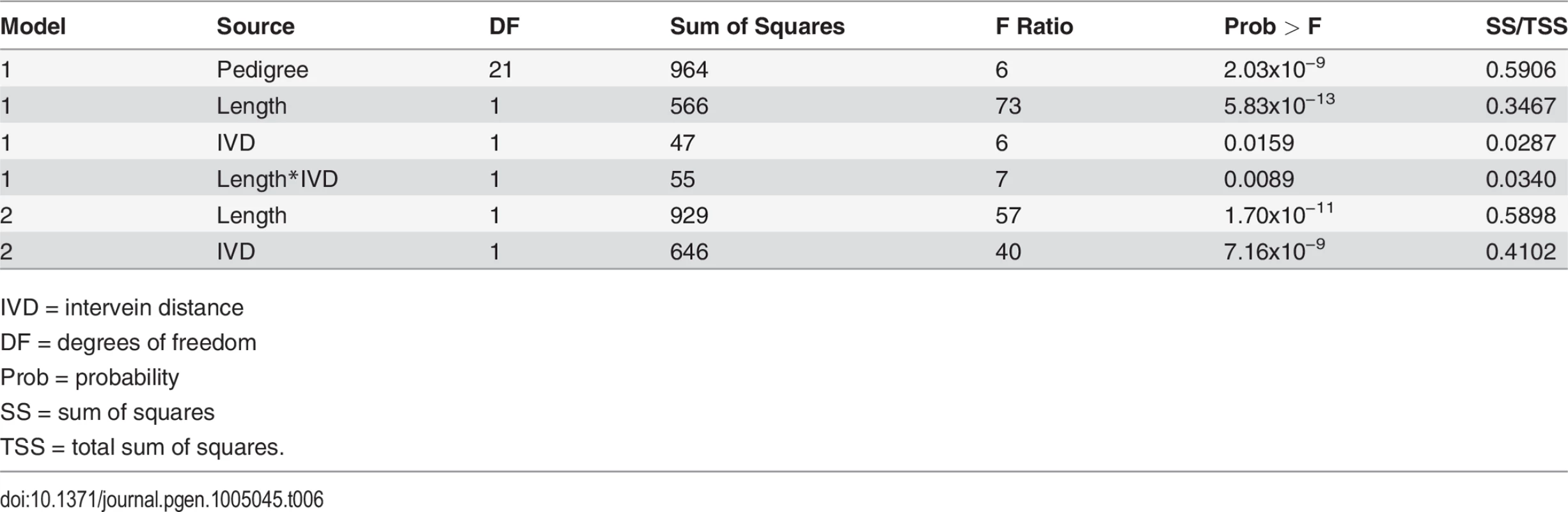
IVD = intervein distance Discussion
In this study, seven novel GLS disease resistance loci were identified by nested association mapping in a diverse sample of maize germplasm. Another nine loci were identified that co-localized with GLS QTL previously identified from other studies (Table 2); seven of these were more precisely localized in this study than in previous analyses. Other QTL (e.g., qGLS4.05KI11) were not finely resolved due to the low recombination rates in the intervals. McMullen et al. [23] identified heavy segregation distortion on chromosome 4 within the B73 x KI11 family, and found that this region had a significantly greater proportion of B73 alleles than KI11 alleles. All QTL mapped by Maroof et al. [16] were identified using the NAM strategy. Our study was performed on the same field site over a decade later and, although it appears that the pathogen population had changed from one with both Cercospora zeae-maydis and C. zeina to one dominated by C. zeae-maydis, similar QTL and presumably similar plant resistance mechanisms appear to function.
Evidence was obtained for pleiotropic responses across diseases. Several loci appeared to condition resistance to both GLS and NLB; six of the 16 QTL showing positively correlated allelic effects. A locus may condition resistance to one or more diseases while acting as a susceptibility factor for another disease. For example, GLS parental allelic effects at qGLS3.06 were negatively associated with SLB effects while positively associated with NLB.
Heritability of GLS resistance was high across the NAM population, and the QTL effects were generally large. Similar heritability estimates have been reported for other foliar diseases scored across the NAM population [40,46], despite differences in disease and rating methodology. For the three loci tested both in the NAM and in NILs, the estimated allelic effects were much larger based on NILs. The BLUPs of disease index ratings reduced the estimated variance, and allelic effect estimates were further deflated when maturity was taken into account. There were fewer QTL with larger effect estimates for GLS than for NLB and SLB, although each of these diseases was scored in the one location across three sequential field seasons. This may reflect the more recent emergence of GLS; it is possible that the pathogen population has had less time to co-evolve to partially overcome the resistance QTL compared to NLB and SLB. Similarly, the shorter time since emergence of the disease may mean that breeders have had less opportunity to accumulate minor resistance factors in maize germplasm.
In the NAM analyses of SLB and NLB, no interactions among disease QTL were detected [40,46]. Here we report interactions involving loci not directly contributing to resistance. In other systems, interactions have been reported to exist among functionally similar genes, often those that act in the same pathway [47] and can be elucidated using RNA interference methodologies [48], among others. Without further fine mapping, there were too many genes underlying the interacting loci to allow us to speculate on the pathways involved.
This study revealed a relationship between leaf structure (the distance between major veins) and GLS disease development. The 4.05 locus had pleiotropic effects on both disease and IVD. There was a positive correlation between IVD and disease development, suggesting that IVD-based lesion restriction may serve as a host resistance mechanism. Tropical germplasm tended to have greater IVD than temperate germplasm among the inbreds observed. These findings suggest that a lesion on an inbred with narrow veins produces fewer conidiophores than a lesion on an inbred with wide veins, resulting in reduced production of inoculum. The effect of reduced conidiophore production would be compounded across multiple reproductive cycles within one season, because Cercospora zeae-maydis is a polycyclic pathogen. Shorter IVD may decrease fungal reproduction via reduced lesion size (lower area available for fungal nourishment) and may also have indirect effects on fungal development via other morphological traits such as stomatal density (this could influence conidial production). A leaf width QTL identified in bin 4.05 [49] co-localized with GLS and venation QTL found in this study. Correlated effects of IVD and leaf width might influence yield through effects on photosynthetic capacity and/or harvest index. The pleiotropic effects of morphological phenotypes influencing disease and other traits merit greater investigation.
qGLS1.04, which was identified for the first time in this study, was validated and fine-mapped. Fine-mapping this locus provides breeders with markers that are closely linked to gene(s) conditioning the disease resistance phenotype. The novel QTL interval was fine-mapped from an initial interval of 27.0 Mb to two intervals of 6.5 Mb and 5.2 Mb, suggesting that multiple genes may underlie original QTL identified by NAM. Similar QTL fractionation has been identified in other quantitative disease resistance studies [50–52]. Increased marker density may help resolve one QTL into two (or more) if there is sufficient recombination in the region among the NAM founders.
Treatment of the qGLS1.04 NILs with cercosporin increased expression of a putative flavin-monooxygenase (FMO) gene. FMOs are a family of oxidoreductases that have been previously implicated in disease resistance. For example, FMOs play a role in glucosinolate production [53,54]. Glucosinolates are a class of secondary metabolites noted for their role in fungal disease resistance among the brassicaceae [55]. Mishina and Zeier [56] determined that FMOs play a role in biologically-induced systemic acquired resistance. Oxidoreductases have also been implicated in the degradation of cercosporin into non-toxic xanosporic acid. Taylor et al. [57] identified mutant strains of Xanthomonas campestris that could no longer degrade cercosporin, while its wild-type progenitor had this ability. All mutants in the Taylor et al. [57] study could be complemented with a genomic clone with homologous sequence to a transcriptional regulator and an oxidoreductase. The mutants had point mutations in the oxidoreductase and not in the regulator, but expression of both was necessary for complementation [57]. The putative FMO may play a role in cercosporin degradation, because it was upregulated by cercosporin application. In future studies, this might be tested by transforming X. campestris wild-type strains with sequences coding for the putatative FMO to verify the gene’s involvement in cercosporin degradation.
Our findings lend preliminary support to the hypothesis that genes underlying quantitative disease resistance are involved in mitigating the effects of microbial compounds deployed during the pathogenesis process [26]. Other studies have implicated genes related to detoxification of microbial compounds in resistance to GLS. In a multivariate analysis of resistance to GLS, NLB and SLB, Wisser et al. [58] identified a significant association across all three diseases with a glutathione-S-transferase. This gene lies within the confidence interval of qGLS7.03 and is considered responsible for reducing oxidative stress and detoxification of microbial compounds. The development of a qGLS1.04 FMO mutant for disease resistance studies would provide more evidence needed to validate the findings. Multiple genes are likely to be involved in conferring resistance at qGLS1.04, because there are multiple fine-mapping intervals underlying resistance at this locus.
The results of this study provide a more thorough and higher-resolution understanding of the genetic architecture of GLS resistance and provide initial support for the hypothesis that structural and detoxification mechanisms underlie quantitative resistance to GLS of maize. Plant breeding decisions regarding development and deployment of resistance will be improved with better understanding of the mechanisms underlying quantitative disease resistance, especially as the mechanisms relate to important agronomic traits such as leaf anatomy and days to maturity.
Materials and Methods
Plant materials and field site
The NAM population was developed by the Maize Diversity Project as a public resource (www.panzea.org) [23]. The NAM population consists of 25 recombinant inbred line (RIL) families derived from crossing each of 25 diverse maize lines with a single reference parent (B73) [23]. The majority of the NAM population (3,678 lines) was planted at Virginia Polytechnic Institute’s Whitethorne Research Farm located in Blacksburg, VA. In addition, 150 lines of the Intermated B73 x Mo17 population (IBM) were included. Line selection was based on seed availability and predicted experimental power [59]. Three replications of the populations were planted in 2008, 2009 and 2010 (one replication/year). Sixteen kernels were planted in 2.4-m long rows with 0.3-m row spacing for each line. The population was arranged in an incomplete block design, augmented by blocks that contained two parental checks. The blocks were arranged by families within the NAM.
The Whitethorne Research Farm was chosen for high and consistent disease pressure that is routinely observed from natural inoculum. Maize had been continuously planted in the field under no-till conditions since 1985. The field had been manually inoculated for three seasons (1985, 1986, and 1987) prior to dependence on natural inoculum. The isolates originally used to inoculate the field (VA-1, VA-2 and VA-3) were collected from maize fields located in Montgomery County and Wythe County, Virginia, in 1985. These isolates were initially identified as only Cercospora zeae-maydis, but were later found to be a mixture of C. zeae-maydis I and C. zeae-maydis II. C. zeae-maydis II has subsequently been reclassified as C. zeina [4]. Sporulation of C. zeae-maydis and C. zeina on the residues from the previous seasons likely provided primary inoculum for disease development on the maize plants. Diseased leaf samples were collected at random from a subset of the parental lines at the conclusion of the 2009 and 2010 seasons. For each year, 25–50 isolations were made. Isolates were identified at the genus level based on conidial morphology and identified at the species level using colony traits when grown on potato dextrose agar. Isolates producing the characteristic purple halo (cercosporin) and exhibiting faster colony growth were inferred to be C. zeae-maydis [60]. These tests have been extensively compared with molecular typing in our laboratory and found to be reliable for distinguishing the two species [60]. The majority of samples (90–95%) were identified as C. zeae-maydis.
Phenotypic assessment
A disease rating methodology was modified to include increments of 0.25 on a 0–5 scale [16]. Using this 21-point scale, each line was scored three times at seven-day intervals. Ratings were made after flowering time (dehiscence) for most lines (S2 Table, S1 Dataset). Area under the disease progress curve (AUDPC) was calculated from the resulting disease scores for each line. Days to anthesis (DTA) data, defined as days from planting to anthesis of 50% of row plants, were also collected (S2 Dataset).
Scanned images of GLS infection on maize leaves were collected in 2009 and 2010. Ear leaves were sampled from each RIL three times at 10-d intervals. These leaves were collected after flowering time, when the GLS symptoms began to develop. Leaves were transferred to the lab on the day of sampling, mounted on a white sheet of paper, and scanned with the corresponding identification. We developed digital image analysis software to analyze scanned leaf images. The software measured the distance between three major and three minor veins on each leaf sample, and the values were averaged for each NAM line. Venation structure was measured at the widest part of the leaf right above the midrib, because leaves within the population varied in size and shape (S3 Table, S3 Dataset). The software also measured the dimensions of each lesion and the number of lesions within a defined area.
Lesion samples were collected in 2011 from the parents of the NAM population to assess sporulation in relation to lesion size. Samples were boiled in 1 M KOH and then rinsed with fresh, sterile, autoclaved water. The rinse was repeated several times over a two week period, resulting in cleared leaf samples that were devoid of chlorophyll and other pigments. Each sample was then mounted on a slide and examined under a light microscope. Conidiophores within the lesions were counted using a manual counter and the computer-projected image (S4 Table). Distance between the major veins was measured using a standard metric ruler.
Analysis
Best linear unbiased predictions (BLUPs) of the GLS disease scores were acquired using ASReml3 statistical software as described by Poland et al. [40]. BLUPs extracted from this model were used to calculate AUDPC, which was used as the response variable in the PROC GLMSELECT stepwise selection procedure in SAS 9.3 (SAS Institute Inc., Cary, NC). Covariates included family and DTA. Common-parent-specific markers (n = 1,106) were also used as predictor variables with a selection threshold (p = 1 x 10–4). QTL mapping was undertaken using a general linear modeling approach with PROC GLM in SAS 9.3 (SAS Institute Inc., Cary, NC).
Markers selected as significant contributors in the stepwise selection model were identified as QTL, while the associated model estimates were considered allelic effects (S5 Table). The sum of allelic effects in the parental lines was compared to the parental phenotypic BLUP in order to determine the percent phenotypic variation explained by the QTL [40]. A general linear model (GLM) was constructed using the selected markers associated with the QTL as predictors, as well as family and flowering time as covariates. Variations of this model were used to construct confidence intervals, identify interactions, and determine least squared means (LSM) of AUDPC for a given allelic effect of a given QTL (S6 Table). The variance components of family and RIL were used to describe genetic variance for the NAM population. Heritabilities on an individual plot basis and on a line mean basis were estimated for the entire NAM population as described in Hung et al. [43]. A mid-parent offspring regression was used to predict narrow sense heritability. Confidence intervals were identified by removing one marker at a time from the full linear model and inserting the associated flanking markers individually until the flanking markers failed to significantly describe the response variable at p<0.0001 [40]. QTL-QTL interactions and interactions between QTL-associated markers and non-significant markers were included in the GLM to identify significant interactions (p<0.00001). Pleiotropic loci and alleles affecting both flowering time and disease development were identified by substituting DTA for AUDPC as the response variable in the GLM described above.
Residuals for each chromosome were extracted from the GLM by removing physically linked markers, one linkage group at a time, from the model (S4 Dataset). These residuals were submitted to NAM-Genome Wide Association Study (NAM-GWAS) at BioHPC for genome-wide single nucleotide polymorphisms (SNP) association using the bootstrap regression analysis option [49]. Significant GWAS hits were functionally annotated using basic local alignment search tool (BLAST). The sequence of each gene associated with a significant GWAS hit was pulled from the maize B73 reference genome (V2). The gene sequences were then populated in the gene ontology BLAST search and run using the default settings of 0.1 Expect threshold, 50 Max # alignments and “all” selected for gene product types, data sources, species, ontology and evidence code (AmiGO 1.8; http://amigo1.geneontology.org/cgi-bin/amigo/blast.cgi?). These annotations were categorized into biological groups, which included: structural components, secondary metabolites, primary metabolites, genes previously implicated in pathogen defense, membrane transporter, development, cellular energy, cell signaling, and cell cycle. The genes on the inter-pro hit list (www.maizesequence.org) were also categorized into the same groups. A proportion z-test was used to test the abundance of genes in a given category relative to the overall abundance predicted in the maize genome.
Heterogenous inbred family development, QTL confirmation & fine-mapping
Specific RILs composing the NAM were selected for heterogenous inbred family (HIF) development [44]. Forty-three lines met the criteria of segregating at one of three QTL and being fixed at all other significant QTL. The three QTL of interest were those that corresponded to the three markers that most significantly described GLS AUDPC. These lines were selected from six subpopulations of the NAM based on the predicted effect of the allele at the specific locus on disease development. The derived lines contrasting for each QTL are hereafter referred to as near isogenic lines (NILs).
The selected lines were selfed and then genotyped in 2009 at Cornell University’s Musgrave Research Farm in Aurora, NY. Heterozygous plants at the loci of interest were selfed in 2010 winter nursery. Fixed lines were selected for random placement in one of six pedigree-based Latin square designs on Whitethorne Farm in summer 2010. These lines were genotyped and scored using the disease rating methodology described above. Lines within the same HIF were analyzed for significant phenotype and genotype association at loci of interest. Heterozygous lines in the region implicated in disease resistance were again advanced in a winter field season, genotyped and planted in an incomplete block design that included both heterozygous and fixed lines in the 2011 and 2012 field seasons. A total of 1,750 and 6,175 plants were screened and genotyped in the 2011 and 2012 field seasons, respectively, in order to increase statistical power and to increase the likelihood of identifying an advantageous recombination breakpoint.
Experimental units (rows in 2010 and individual plants in 2011 and 2012) were scored three times for disease at seven day intervals, which were used to calculate AUDPC. Flowering time data were also collected for each experimental unit in 2010 and 2011. Experimental units with like haplotypes were analyzed together. Like haplotypes were identified as having the same genotypes flanking the segregating regions. The experimental units were analyzed using PROC GLM in SAS 9.3 (SAS Institute Inc., Cary, NC). Disease development (AUDPC) was the response variable and predictors were the genotypes within the QTL confidence or fine-mapping interval. False discovery rate (FDR) was calculated independently for each year based on the number of markers analyzed and used as a threshold for significant associations between the disease phenotypes and marker genotypes.
Venation confirmation at the 4.05 locus
One of the venation QTL co-localized with qGLS4.05KI11 and with a fine-mapping population already developed for this locus, the NILs were planted in the greenhouse to measure average distance between veins. The experiment was set up in a complete randomized block with 10 replications of 7 HIF lines. The plants were grown to just beyond the R1stage when all lines had reached the point of anthesis. As with the scanning protocol described above, leaves were transferred to the lab on the day of sampling, mounted on a white sheet of paper, and scanned with the corresponding identification. The software measured the distance between three major and three minor veins and the values were averaged for each NAM line. Venation structure was measured at the widest part of the leaf right above the midrib, because leaves within the population varied in size and shape. A t-test was used to detect a significant difference between the isolines.
Functional annotation of NAM-GWAS and genes within the fine-mapping interval
A list of genes within the qGLS1.04 fine-mapping intervals (1 : 77,242,690 to 1 : 83,780,725 Mb and 1 : 88,849,284 to 1 : 94,085,195 Mb) was exported from the maize genome browser (www.maizesequence.org). These genes were functionally annotated using BLAST and compared to the maize top 500 inter-pro hit list. An initial inspection indicated that the region was rich in detoxification-related genes. The z-test is used to determine if the hypothesized population proportion is significantly different from the sampled proportions and as such, it was used to evaluate the genome abundance of detoxification-related (DR) genes compared to the observed sample differences. Specific gene families identified by sequence inspection were tested for enrichment using the z-test.
Cercosporin treatment of 1.04 isolines
Twenty-four near isogenic lines (F6 : 8NILs) developed using the HIF strategy were grown in the greenhouse under standard maize growing conditions. Lines were organized in a randomized complete block design (4 blocks). Three of the six plants in each block contained the susceptible B73 allele, and the other three contained the resistant CML228 allele. Different lines in the same HIF family were used to account for residual background effect resulting from regions that may still have been segregating (estimated at less than 0.5%).
At flowering time, two ear leaves on each plant were treated with 0.1 ml of 100 μM cercosporin in acetone and an acetone control using a procedure modified from Batchvarova et al. [61]. Treatment and control were infiltrated using a needleless syringe. The control and cercosporin treatments were applied to the same leaf on either side of the midrib. Plants were placed in constant light for 24 h in order to activate the cercosporin. After this time period, 10 leaf punches of 6-mm diameter were collected directly around each treated site using a paper punch (2 controls and 2 treatments per plant). Samples collected from the lower ear leaf were used for carotenoid detection and samples from the upper ear leaf were used for expression analysis.
Carotenoids were extracted and measured from maize leaf tissue using modified procedures based on those of Alba et al. (2005) and Bushway (1986). Under low light conditions, 100 mg of pre-weighed tissue was homogenized with 50 μl of a 0.3% MgCO3 solution (w:v) and 300 μl of tetrahydrofuran (THF). Homogenization was repeated after addition of 300 μl of 0.5% butylated hydroxyl-toluene/methanol (w:v). An additional 600 μl of THF was added to the extract, which was then filtered. To the filtered extract, 50 μl of 25% NaCl and 600 μl of petroleum ether were added and the sample was vortexed well. The upper phase was dried down and 500 μL HPLC grade ethyl acetate was added in preparation for the column; this was mixed well and filtered. A sample of the extract was added to a YMC C30 column for reverse-phase high performance liquid chromatography (RP-HPLC).
Real-time quantitative PCR (qRT-PCR) was performed on cercosporin - and control-treated HIFs to test for expression differences across genes hypothesized to play a role in cercosporin detoxification. Total RNA was purified from maize leaf tissue using the RNEasy Mini Kit (Qiagen). The same kit was used for DNase I digestion. The cDNA was prepared using the SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen). Primer pairs were designed for candidate detoxification-related genes (Table 7) and used for RT-PCR with Power SYBR Green PCR Master Mix (Invitrogen). Data were analyzed using the Comparative CT method [62] (S7 Table).
Tab. 7. Primer sequences used for qGLS1.04CML228 expression tests. 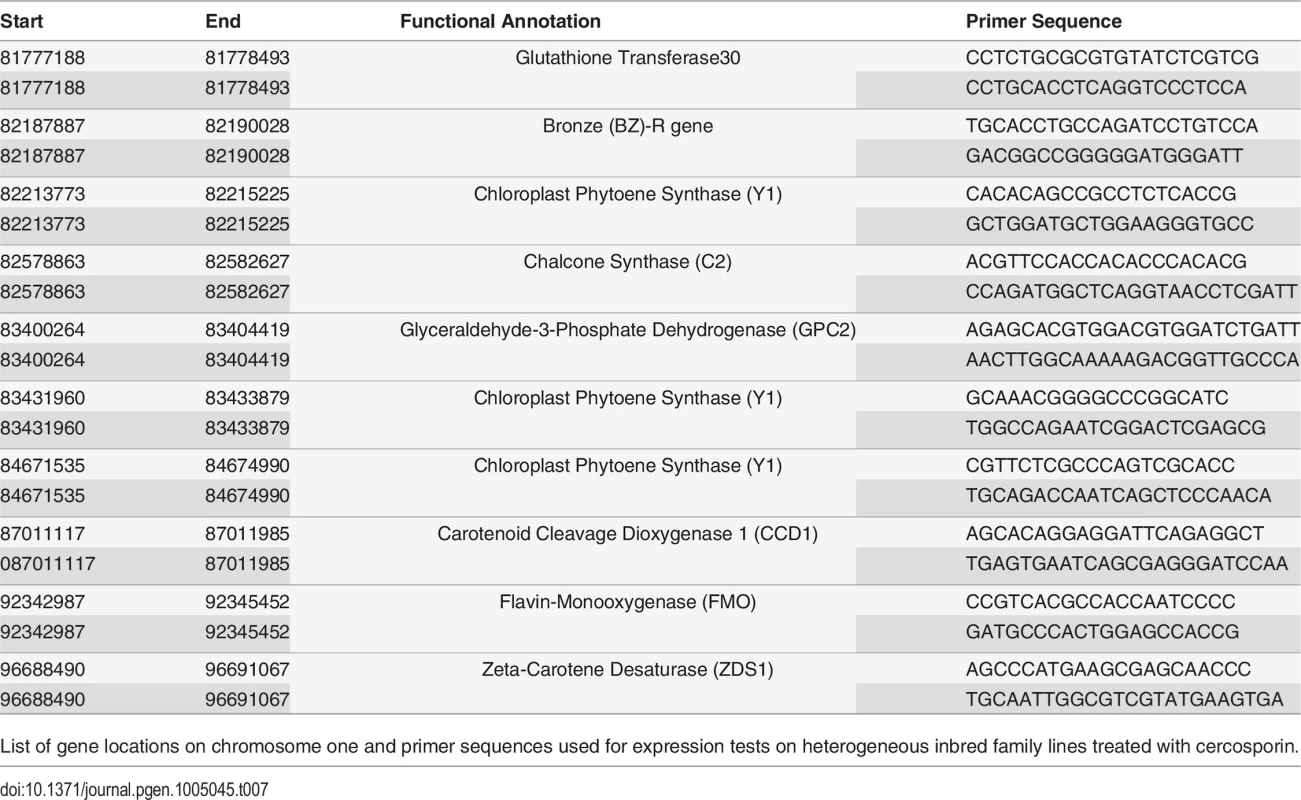
List of gene locations on chromosome one and primer sequences used for expression tests on heterogeneous inbred family lines treated with cercosporin. Flavin-monooxygenase single nucleotide polymorphisms
SNPs between B73 and CML228 within the putative flavin-monooxygenase gene were identified using maize Haplotype Map Version 2 (HMPv2; www.panzea.org). The B73 and CML228 gene sequences were developed by aligning SNP calls with the sequence provided by the Maize Genome Sequence Consortium. The B73 SNP calls from HMPv2 matched the B73 reference genome. The promoter region sequence for the B73 and CML228 alleles was analyzed using plant promoter prediction software (Prediction of PLANT Promoters using RegSite Plant DB, Softberry Inc.) to detect putative functional differences in the promoter region between the parental lines.
Supporting Information
Zdroje
1. Wen L (2013) Cell death in plant immune response to necrotrophs. J Plant Biochem Physiol 1 : 1–3.
2. Lai Z, Wang F, Zheng Z, Fan B, Chen Z (2011) A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J 66 : 953–968. doi: 10.1111/j.1365-313X.2011.04553.x 21395886
3. Sivasithamparam K, Barbetti MJ, Li H (2005) Recurring challenges from a necrotrophic fungal plant pathogen: a case study with Leptosphaeria maculans (causal agent of blackleg disease in Brassicas) in Western Australia. Ann Bot 96 : 363–377. 15994842
4. Crous PW, Groenewald JZ, Groenewald M, Caldwell P, Braun U, et al. (2006) Species of Cercospora associated with grey leaf spot of maize. Stud Mycol: 189–197.
5. Tehon L, Daniels E (1925) Notes on parasitic fungi of Illinois. Mycologia 71 : 240–249.
6. Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43 : 205–227. 16078883
7. Ward JMJ, Stromberg EL, Nowell DC, Nutter FW (1999) Gray leaf spot—A disease of global importance in maize production. Plant Dis 83 : 884–895.
8. Latterell FM, Rossi AE (1983) Gray leaf spot of corn: A disease on the move. Plant Dis 67.
9. Ngoko Z, Cardwell KF, Marasas WFO, Wingfield MJ, Ndemah R, et al. (2002) Biological and physical constraints on maize production in the Humid Forest and Western Highlands of Cameroon. Eur J Plant Pathol 108 : 893–902.
10. Balint-Kurti PJ, Wisser R, Zwonitzer JC (2008) Use of an advanced intercross line population for precise mapping of quantitative trait loci for gray leaf spot resistance in maize. Crop Sci 48 : 1696–1704.
11. Bubeck DM, Goodman MM, Beavis WD, Grant D (1993) Quantitative trait loci controlling resistance to gray leaf spot in maize. Crop Sci 33 : 838–847.
12. Clements MJ, Dudley JW, White DG (2000) Quantitative trait loci associated with resistance to gray leaf spot of corn. Phytopathology 90 : 1018–1025. doi: 10.1094/PHYTO.2000.90.9.1018 18944528
13. Danson J, Lagat M, Kimani M, Kuria A (2008) Quantitative trait loci (QTLs) for resistance to gray leaf spot and common rust diseases of maize. Afr J Biotechnol 7 : 3247–3254.
14. Juliatti FC, Pedrosa MG, Silva HD, da Silva JVC (2009) Genetic mapping for resistance to gray leaf spot in maize. Euphytica 169 : 227–238.
15. Lehmensiek A, Esterhuizen AM, van Staden D, Nelson SW, Retief AE (2001) Genetic mapping of gray leaf spot (GLS) resistance genes in maize. Theor Appl Genet 103 : 797–803.
16. Maroof MAS, Yue YG, Xiang ZX, Stromberg EL, Rufener GK (1996) Identification of quantitative trait loci controlling resistance to gray leaf spot disease in maize. Theor Appl Genet 93 : 539–546. doi: 10.1007/BF00417945 24162345
17. Pozar G, Butruille D, Silva HD, McCuddin ZP, Penna JCV (2009) Mapping and validation of quantitative trait loci for resistance to Cercospora zeae-maydis infection in tropical maize (Zea mays L.). Theor Appl Genet 118 : 553–564. doi: 10.1007/s00122-008-0920-2 18989654
18. Gordon SG, Bartsch M, Matthies I, Gevers HO, Lipps PE, et al. (2004) Linkage of molecular markers to Cercospora zeae-maydis resistance in maize. Crop Sci 44 : 628–636.
19. Zhang Y, Xu L, Fan X, Tan J, Chen W, et al. (2012) QTL mapping of resistance to gray leaf spot in maize. Theor Appl Genet: 1–12.
20. Berger DK, Carstens M, Korsman JN, Middleton F, Kloppers FJ, et al. (2014) Mapping QTL conferring resistance in maize to gray leaf spot disease caused by Cercospora zeina. BMC genetics 15 : 60. doi: 10.1186/1471-2156-15-60 24885661
21. Chung CL, Poland J, Kump K, Benson J, Longfellow J, et al. (2011) Targeted discovery of quantitative trait loci for resistance to northern leaf blight and other diseases of maize. Theor Appl Genet 123 : 307–326. doi: 10.1007/s00122-011-1585-9 21526397
22. Zwonitzer JC, Coles ND, Krakowsky MD, Arellano C, Holland JB, et al. (2010) Mapping resistance quantitative trait Loci for three foliar diseases in a maize recombinant inbred line population-evidence for multiple disease resistance? Phytopathology 100 : 72–79. doi: 10.1094/PHYTO-100-1-0072 19968551
23. McMullen MD, Kresovich S, Villeda HS, Bradbury P, Li H, et al. (2009) Genetic properties of the maize nested association mapping population. Science 325 : 737–740. doi: 10.1126/science.1174320 19661427
24. Berger R (1977) Application of epidemiological principles to achieve plant disease control. Annu Rev Phytopathol 15 : 165–181.
25. Chung CL, Longfellow JM, Walsh EK, Kerdieh Z, Van Esbroeck G, et al. (2010) Resistance loci affecting distinct stages of fungal pathogenesis: use of introgression lines for QTL mapping and characterization in the maize-Setosphaeria turcica pathosystem. BMC plant biology 10 : 103. doi: 10.1186/1471-2229-10-103 20529319
26. Poland JA, Balint-Kurti PJ, Wisser RJ, Pratt RC, Nelson RJ (2009) Shades of gray: the world of quantitative disease resistance. Trends Plant Sci 14 : 21–29. doi: 10.1016/j.tplants.2008.10.006 19062327
27. Fu D, Uauy C, Distelfeld A, Blechl A, Epstein L, et al. (2009) A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 323 : 1357–1360. doi: 10.1126/science.1166289 19228999
28. Fukuoka S, Saka N, Koga H, Ono K, Shimizu T, et al. (2009) Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 325 : 998–1001. doi: 10.1126/science.1175550 19696351
29. Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, et al. (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323 : 1360–1363. doi: 10.1126/science.1166453 19229000
30. Manosalva PM, Davidson RM, Liu B, Zhu X, Hulbert SH, et al. (2009) A germin-like protein gene family functions as a complex quantitative trait locus conferring broad-spectrum disease resistance in rice. Plant Physiol 149 : 286–296. doi: 10.1104/pp.108.128348 19011003
31. Cook DE, Lee TG, Guo X, Melito S, Wang K, et al. (2012) Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science 338 : 1206–1209. doi: 10.1126/science.1228746 23065905
32. St. Clair DA(2010) Quantitative disease resistance and quantitative resistance loci in breeding. Annu Rev Phytopathol 48 : 247–268. doi: 10.1146/annurev-phyto-080508-081904 19400646
33. Kimura M, Anzai H, Yamaguchi I (2001) Microbial toxins in plant-pathogen interactions: Biosynthesis, resistance mechanisms, and significance. J Gen Appl Microbiol 47 : 149–160. 12483615
34. Daub M (1982) Cercosporin, a photosensitizing toxin from Cercospora species. Phytopathology 72 : 370–374.
35. Ramel F, Birtic S, Cuiné S, Triantaphylidès C, Ravanat JL, et al. (2012) Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol 158 : 1267–1278. doi: 10.1104/pp.111.182394 22234998
36. Daub ME, Payne GA (1989) The role of carotenoids in resistance of fungi to cercosporin. Phytopathology 79 : 180–185.
37. Daub ME, Leisman GB, Clark RA, Bowden EF (1992) Reductive detoxification as a mechanism of fungal resistance to singlet oxygen-generating photosensitizers. Proc Natl Acad Sci 89 : 9588–9592. 1409670
38. Daub ME (1987) The fungal photosensitizer cercosporin and its role in plant disease. In: Heitz JR, Downum KR, editors. Light-activated pesticides. Washington, DC: ACS Symposium Series, American Chemical Society. pp. 271–280.
39. Ververidis P, Davrazou F, Diallinas G, Georgakopoulos D, Kanellis A, et al. (2001) A novel putative reductase (Cpd1p) and the multidrug exporter Snq2p are involved in resistance to cercosporin and other singlet oxygen-generating photosensitizers in Saccharomyces cerevisiae. Curr Genet 39 : 127–136. 11409174
40. Poland JA, Bradbury PJ, Buckler ES, Nelson RJ (2011) Genome-wide nested association mapping of quantitative resistance to northern leaf blight in maize. Proc Natl Acad Sci 108 : 6893. doi: 10.1073/pnas.1010894108 21482771
41. Wisser RJ, Balint-Kurti PJ, Nelson RJ (2006) The genetic architecture of disease resistance in maize: a synthesis of published studies. Phytopathology 96 : 120–129. doi: 10.1094/PHYTO-96-0120 18943914
42. Berger R, Filho AB, Amorim L (1997) Lesion expansion as an epidemic component. Phytopathology 87 : 1005–1013. doi: 10.1094/PHYTO.1997.87.10.1005 18945033
43. Hung H, Browne C, Guill K, Coles N, Eller M, et al. (2012) The relationship between parental genetic or phenotypic divergence and progeny variation in the maize nested association mapping population. Heredity 108 : 490–499. doi: 10.1038/hdy.2011.103 22027895
44. Tuinstra M, Ejeta G, Goldsbrough P (1997) Heterogeneous inbred family (HIF) analysis: a method for developing near-isogenic lines that differ at quantitative trait loci. Theor Appl Genet 95 : 1005–1011.
45. Buckler ES, Holland JB, Bradbury PJ, Acharya CB, Brown PJ, et al. (2009) The genetic architecture of maize flowering time. Science 325 : 714–718. doi: 10.1126/science.1174276 19661422
46. Kump KL, Bradbury PJ, Wisser RJ, Buckler ES, Belcher AR, et al. (2011) Genome-wide association study of quantitative resistance to southern leaf blight in the maize nested association mapping population. Nature Genet 43 : 163–168. doi: 10.1038/ng.747 21217757
47. Tong AHY, Lesage G, Bader GD, Ding H, Xu H, et al. (2004) Global mapping of the yeast genetic interaction network. Sci STKE 303 : 808. 14764870
48. Byrne AB, Weirauch MT, Wong V, Koeva M, Dixon SJ, et al. (2007) A global analysis of genetic interactions in Caenorhabditis elegans. J Biol 6. doi: 10.1186/jbiol60 18177504
49. Tian F, Bradbury PJ, Brown PJ, Hung H, Sun Q, et al. (2011) Genome-wide association study of leaf architecture in the maize nested association mapping population. Nature Genet 43 : 159–162. doi: 10.1038/ng.746 21217756
50. Studer AJ, Doebley JF (2011) Do large effect QTL fractionate? A case study at the maize domestication QTL teosinte branched1. Genetics 188 : 673–681. doi: 10.1534/genetics.111.126508 21515578
51. Johnson EB, Haggard JE, Clair DAS (2012) Fractionation, stability, and isolate-specificity of QTL for resistance to Phytophthora infestans in cultivated tomato (Solanum lycopersicum). G3 (Bethesda) 2 : 1145–1159. doi: 10.1534/g3.112.003459 23050225
52. Jamann TM, Poland JA, Kolkman JM, Smith LG, Nelson RJ (2014) Unraveling genomic complexity at a quantitative disease resistance locus in maize. Genetics: In press.
53. Hansen BG, Kliebenstein DJ, Halkier BA (2007) Identification of a Flavin-monooxygenase as the S-oxygenating enzyme in aliphatic glucosinolate biosynthesis in Arabidopsis. Plant J 50 : 902–910. 17461789
54. Li J, Hansen BG, Ober JA, Kliebenstein DJ, Halkier BA (2008) Subclade of flavin-monooxygenases involved in aliphatic glucosinolate biosynthesis. Plant Physiol 148 : 1721–1733. doi: 10.1104/pp.108.125757 18799661
55. Mithen R (1992) Leaf glucosinolate profiles and their relationship to pest and disease resistance in oilseed rape. Euphytica 63 : 71–83.
56. Mishina TE, Zeier J (2006) The Arabidopsis flavin-dependent monooxygenase FMO1 is an essential component of biologically induced systemic acquired resistance. Plant Physiol 141 : 1666–1675. 16778014
57. Taylor TV, Mitchell TK, Daub ME (2006) An oxidoreductase is involved in cercosporin degradation by the bacterium Xanthomonas campestris pv. zinniae. Appl Environ Microbiol 72 : 6070–6078. 16957231
58. Wisser RJ, Kolkman JM, Patzoldt ME, Holland JB, Yu J, et al. (2011) Multivariate analysis of maize disease resistances suggests a pleiotropic genetic basis and implicates a GST gene. Proc Natl Acad Sci 108 : 7339–7344. doi: 10.1073/pnas.1011739108 21490302
59. Yu J, Holland JB, McMullen MD, Buckler ES (2008) Genetic design and statistical power of nested association mapping in maize. Genetics 178 : 539–551. doi: 10.1534/genetics.107.074245 18202393
60. Hsieh L-S (2011) Coexistence of sibling species of Cercospora causing gray leaf spot on maize in southern New York State [Master's]. Ithaca, NY: Cornell University. 90 p.
61. Batchvarova R, Reddy V, Bennett J (1992) Cellular resistance in rice to cercosporin, a toxin of Cercospora. Phytopathology 82 : 642–646.
62. Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3 : 1101–1108. 18546601
63. Shi L-Y, Li X-H, Hao Z-F, Xie C-X, Ji H-L, et al. (2007) Comparative QTL mapping of resistance to gray leaf spot in maize based on bioinformatics. Agric Sci China 6 : 1411–1419.
Štítky
Genetika Reprodukční medicína
Článek NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY ModuleČlánek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in NematodesČlánek The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse SpermatocytesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 3
-
Všechny články tohoto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



