-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
BP is a class-I KNOX transcription factor that controls normal inflorescence architecture development by repressing the expression of two KNOX genes, KNAT2 and KNAT6. In this study, we showed that Arabidopsis BP directly interacts with the SWI2/SNF2 chromatin remodeling ATPase BRM. brm and bp mutants displayed similar inflorescence architecture defects, with clustered inflorescences, horizontally orientated pedicels, and short pedicels and internodes. Furthermore, BP and BRM co-target to KNAT2 and KNAT6 genes and repress their expression. This work reveals a new regulatory mechanism that BP associates with BRM in control of inflorescence architecture development.
Published in the journal: . PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005125
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005125Summary
BP is a class-I KNOX transcription factor that controls normal inflorescence architecture development by repressing the expression of two KNOX genes, KNAT2 and KNAT6. In this study, we showed that Arabidopsis BP directly interacts with the SWI2/SNF2 chromatin remodeling ATPase BRM. brm and bp mutants displayed similar inflorescence architecture defects, with clustered inflorescences, horizontally orientated pedicels, and short pedicels and internodes. Furthermore, BP and BRM co-target to KNAT2 and KNAT6 genes and repress their expression. This work reveals a new regulatory mechanism that BP associates with BRM in control of inflorescence architecture development.
Introduction
In flowering plants, internode patterning and pedicel characteristics are two important determinants of inflorescence architecture, which is highly diversified among flowering plant species [1,2]. Inflorescence architecture results from the activity of the shoot apical meristem (SAM), a cluster of pleuripotent stem cells located at the apex of the primary shoot. In Arabidopsis, determining the SAM function is mainly controlled by overlapping activities of two protein family members, the class-I KNOTTED1-like homeobox (KNOX) transcription factor subfamily and the BELL1-like (BELL) transcription factor subfamily. Both KNOX and BELL proteins belong to the three-amino-acid loop extension (TALE) homeodomain superfamily and are able to form heterodimers in determining meristem maintenance [1,2,3,4,5,6,7].
The class-I KNOX family contains four members, SHOOT MERISTEMLESS (STM), BREVIPEDICELLUS (BP, also called KNAT1), KNAT2, and KNAT6 [8]. STM is required for the initiation of SAM during embryogenesis and maintenance of proliferation of the cells in SAM [2,9]. BP, together with STM, contributes to SAM maintenance as loss of function of BP reduces the residual meristematic activity of the weak allele stm-2 [10]. Furthermore, mutations of BP in Arabidopsis cause severe inflorescence architecture defects, with downward-pointing pedicels, short and abnormal internodes with pronounced node bending [1,2], suggesting that BP may play crucial roles in inflorescence architecture development. Further studies showed that PENNYWISE (PNY), a member of the BELL subfamily, could physically interact with BP [4,6,11]. bp pny double mutants showed a synergistic phenotype of extremely short internodes interspersed with long internodes and increased branching, suggesting that BP-PNY complex is essential for proper inflorescence architecture development. Moreover, a genetic study showed that inactivation of both KNAT2 and KNAT6 could rescue inflorescence architecture defects caused by the bp or pny single mutation [12]. Increased expression of KNAT2 and KNAT6 was detected in bp and pny mutants, indicating that BP and PNY may restrict KNAT2 and KNAT6 expression to promote correct inflorescence architecture development. Taken together, these studies revealed that the BP-PNY complex regulates inflorescence architecture development mainly by repressing the expression of KNAT2 and KNAT6. However, the molecular mechanism of BP-mediated transcription regulation remains largely unknown.
In eukaryotic cells, gene activity is controlled not only by DNA but also by epigenetic marks. Epigenetic changes involve the modification of DNA activity by methylation, histone modification, and chromatin remodeling [13,14,15,16]. ATP-dependent chromatin remodeling factors use the energy derived from ATP hydrolysis to change the interaction between histone octamer and DNA, and alter the accessibility of genomic regions to transcription factors or the general transcriptional machinery in the context of chromatin [17,18]. BRAHMA (BRM), a member of SWI/SNF ATPases, plays an essential role in reprogramming of transcription in vegetative, embryonic and reproductive plant development in Arabidopsis [19,20,21,22]. Mutation 27of BRM in Arabidopsis causes many morphological defects, such as reduced plant sizes with short roots and small leaves, floral homeotic defects, and earlier flowering [23,24,25]. More recently, BRM was shown to interact with LEAFY and SEPALLATA3, two key transcription factors involved in controlling floral organ identity by regulating APETALA3 (AP3) and AGAMOUS (AG) expression [21]. Furthermore, BRM associates with the transcription factor TCP4 in regulation of leaf maturation by modulating the cytokine responsive gene expression [26]. In addition, an interactome screen revealed that BRM interacts with a larger subset of transcription factors, including MYB, bHLH and zinc finger proteins [26]. Collectively, these findings suggest that the SWI/SNF ATPase BRM may act together with different transcription factors in modulating gene expression in plant development processes.
In present work, we demonstrated a direct protein-protein interaction between BRM and BP both in vitro and in vivo. Furthermore, BRM and BP co-repressed KNAT2 and KNAT6 expression in control of inflorescence architecture development.
Results
BRM Interacts with BP In Vitro and In Vivo
To identify the interaction proteins of BRM, we performed a yeast two-hybrid library screening. BP was identified as a candidate BRM-interacting partner. Yeast cells co-transformed with AD-BRM (full-length of BRM fused to pGAKT7) and BD-BP (full-length of BP fused to pGBKT7) could grow on selective medium QDO (synthetic medium lacking tryptophan, leucine, histidine and adenine) (Fig. 1A-C), indicating that BRM could directly interact with BP in yeast. Further deletion analysis showed that the DII domain of BRM (amino acids 689–952) and the MEINOX domain (amino acids 130–240) of BP (Fig. 1A-C) were responsible for their interaction. We further detected the interaction between BRM and BP by pull-down assays. Purified BRM (amino acids 689–952)-His was pulled down by GST-BP proteins (Fig. 1D), confirming that BRM physically interacts with BP in vitro.
Fig. 1. BP interacts with BRM in yeast two-hybrid and in vitro pull-down assays. 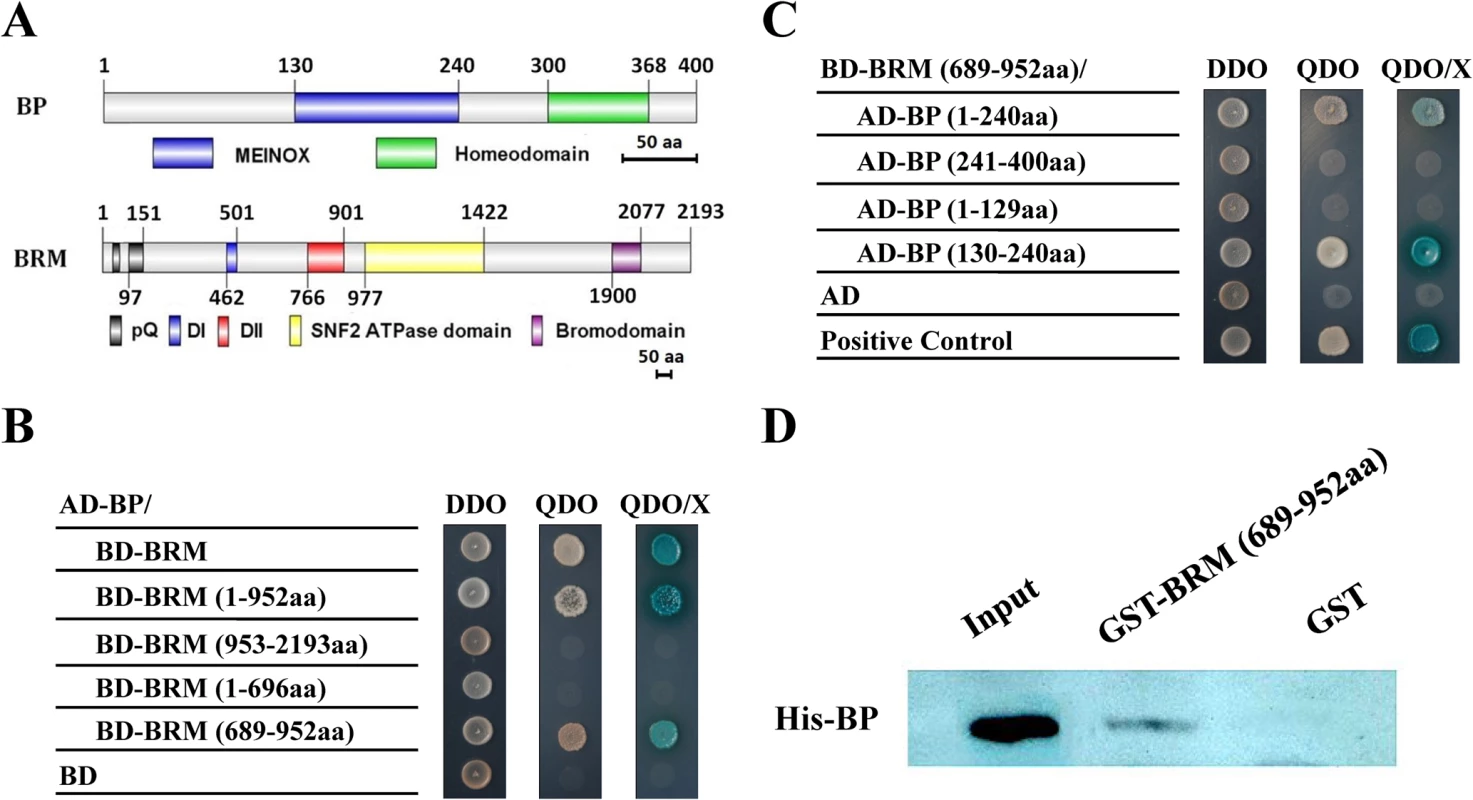
(A) Schematic structures of BP and BRM protein domains. (B,C) Different BRM and BP deletion constructs were cotransformed into the yeast cells GOLD Y2H and plated in DDO. The transformants were also plated on QDO to test for possible interaction. DDO, SD/–Leu/–Trp. QDO, SD/-Leu/-Trp/-His/-Ade. X, x-a-gal. (D) GST-BRM (689-952aa) or GST was incubated with His-BP and His resin, and the bounded proteins were then detected by western blotting using an anti-His antibody. Equal amounts of input His-BP protein were used for pull-down assays. The interaction of BRM and BP was further examined in vivo by bimolecular fluorescence complementation (BiFC) and co-immunoprecipitation (Co-IP) assays. For the BiFC assay, BRM and BP were fused to the YN vector pUC-pSPYNE or the YC vector pUC-pSPYCE [27]. The constructs were co-delivered into tobacco Bright Yellow 2 (BY-2) suspension cells by polyethylene glycol (PEG) mediated transformation. As shown in Fig. 2A, BRM interacted with BP in BiFC assays. Among the cells observed, about 10% cells showed positive signals and similar results were obtained in four different experiments. For the Co-IP assay, we transiently expressed BRM and BP proteins in tobacco (Nicotiana benthamiana) [14]. As the full length BRM protein could not be well expressed in tobacco cells, we made a construct with the DII domain (amino acids 689–952) of BRM fused with three FLAG tags (BRM-Δ-FLAG). The full length of BP was fused with a GFP tag (BP-GFP). These constructs were co-transformed into tobacco epidermal cells by Agrobacterium-mediated infiltration assays. We showed that BRM-Δ-FLAG protein was co-immunoprecipitated by BP-GFP (Fig. 2B). Taken together, these data indicate that BRM interacts with BP both in vitro and in vivo.
Fig. 2. BRM interacts with BP in vivo detected by BiFC and Co-IP assays. 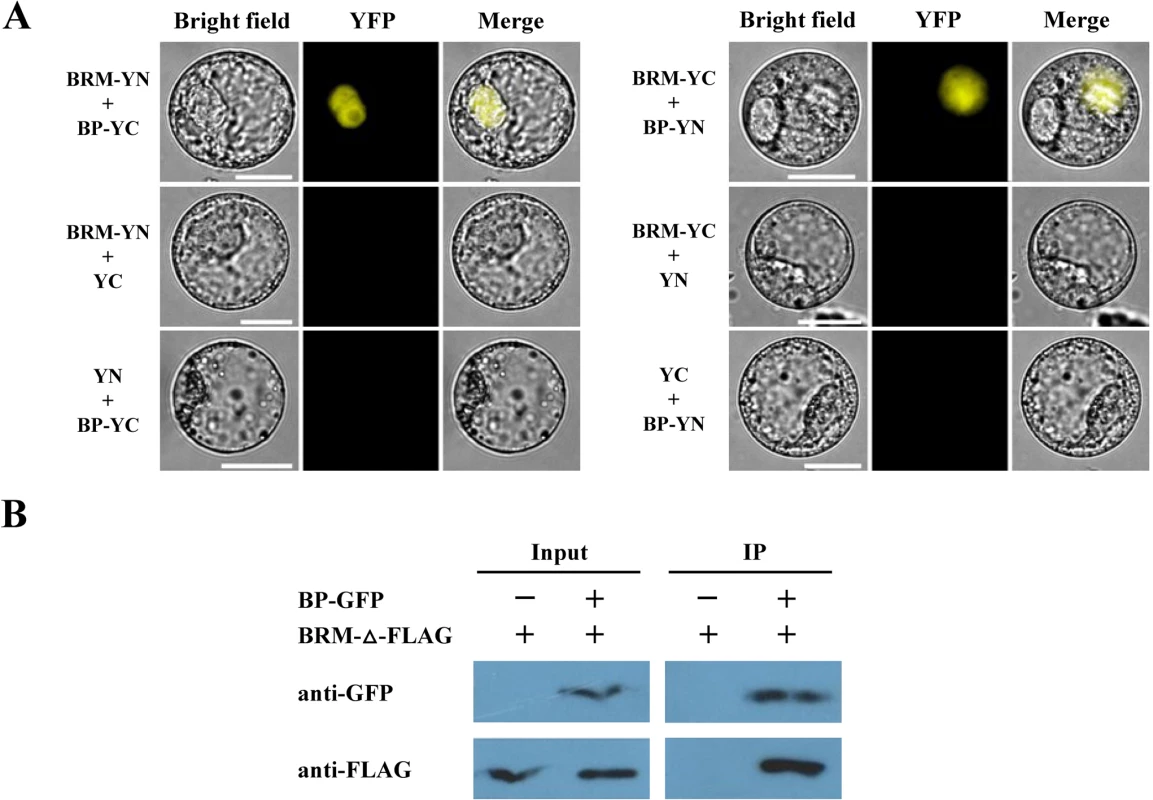
(A) Full length of BRM and BP fused with the C terminus (YC) or the N terminus (YN) of YFP were co-transformed into tobacco cells. As a negative control, BRM and BP fused with YC or YN and empty vectors were also cotransformed into tobacco cells. (B) The amino acids 689–952 of BRM fused with three FLAG tags (BRM-Δ-FLAG), and the full length of BP was fused with a GFP tag. These constructs were co-transformed into tobacco cells by Agrobacterium mediated infiltration assays. Transiently expressed BP-GFP and BRM-Δ-FLAG was immunoprecipitated with an anti-GFP antibody, and then detected by western-blotting assay with an anti-Flag antibody. BRM Is Required for the Inflorescence Development
Previous studies indicated that BP is strongly expressed in inflorescences including pedicels and internodes [1]. GUS-staining analyses with pBRM:GUS plants showed that BRM is also expressed in the florescence in Arabidopsis (S1 Fig.). Furthermore, expression patterns from the public Arabidopsis microarray databases (http://www.bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi) revealed that both BRM and BP are expressed in shoot apex, stems and internodes in Arabidopsis (S2 Fig.). These findings suggested an overlapping expression pattern of BP and BRM in the inflorescences.
To study the genetic interaction of BRM and BP, several brm alleles, brm-1 [19], brm-3 [28], brm-4 and brm-5 [29], and the null bp allele, bp-9 [1,2], were analyzed. bp-9 contains a dSpm transposon insertion in the 1st intron of BP [6]. The transcript of BP was not detected in the bp-9 mutant (S3 Fig.), confirming that bp-9 is a null allele. Furthermore, the expression level of BP was not significantly altered in brm-3 compared with wild-type (S3 Fig.), suggesting that BRM may not affect BP expression in inflorescence. We observed that brm-3 and bp-9 plants displayed similar inflorescence architecture defects, with horizontally orientated pedicels (Fig. 3A and 3B), clustered inflorescences (Fig. 3C), shorter internodes and pedicels (Fig. 3D-F) compared to wild-type plants. Similar inflorescence architecture defects were also observed in brm-1, brm-4 and brm1-5 mutant alleles (S4A–S4D Fig.). Interestingly, loss-of-function mutants of SWITCH/SUCROSE NONFERMENTING 3C (SWI3C) encoding an interaction partner of BRM [25] also showed inflorescence architecture defects as bp-9 (Fig. 3A).
Fig. 3. Inflorescence patterns of brm-3, bp-9, and brm-3 bp-9 double mutants. 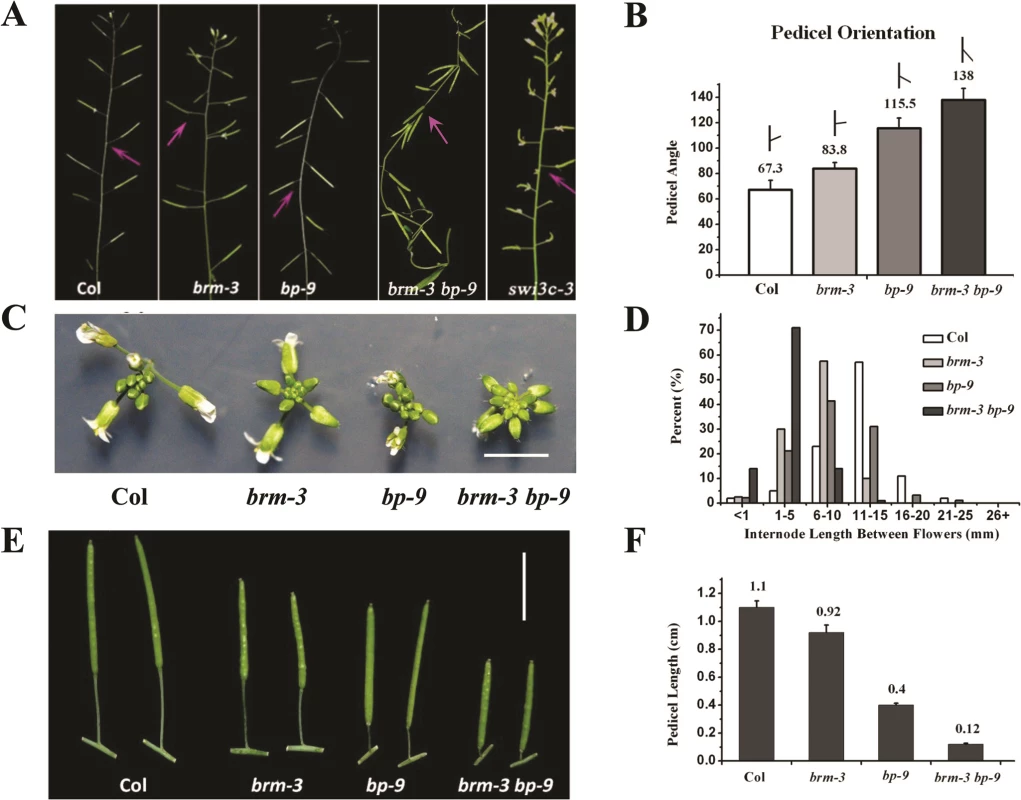
(A) Phenotypes of brm-3, bp-9 and brm-3 bp-9 double mutants in pedicel orientation. The arrows indicate the typical pedicel orientation of the mutants. 35-day-old plants were used for phenotype observation. (B) Quantitative analysis of the pedicel orientation in Col, brm-3, bp-9, and brm-3 bp-9 plants (N≥100). (C) Top view of inflorescence in Col, brm-3, bp-9, and brm-3 bp-9 plants (bar = 0.5 cm). (D) Distribution of the internode length between two successive siliques. Ten internodes between the 1st and 11th siliques were analyzed. (E) Phenotype of pedicle elongation of the mature siliques in Col, brm-3, bp-9, and brm-3 bp-9 plants (bar = 1 cm). 35-day-old plants were used for phenotype observation. (F) Quantitative analysis of the pedicle length of mature siliques in 35-day-old Col, brm-3, bp-9, and brm-3 bp-9 plants. The null allele brm-1 was completely sterile [19]. Therefore, we generated the double mutant by crossing the weak allele brm-3 with bp-9. The brm-3 bp-9 double mutants displayed more severe inflorescence architecture defects compared with brm-3 and bp-9 single mutants, with more compacted inflorescences, shorter internodes and pedicels, downward-oriented siliques (Figs. 3A-F and S5). The brm-3 bp-9 double mutant showed synergistic interaction in inflorescence architecture development, suggesting that additional factors other than BP likely interact with BRM to regulate the same processes. Previous studies indicated that the BELL subfamily transcription factor PNY interacts with BP and is involved in repression of KNAT2 and KNAT6 [12]. It is possible that PNY may also interact with BRM in regulating inflorescence architecture development.
In addition, we also showed that the internodes of brm-3 bp-9 plants were severely bent (Fig. 4A and 4B). Chlorenchyma are the specialized parenchyma cells, which contain chloroplasts and are distributed in the outer cortex of stems. Bends in stems correlate with a loss of chlorenchyma tissue at the node adjacent to lateral organs [1]. The chlorenchyma density was dramatically reduced in the internodes of brm-3 bp-9 plants compared with the bp-9 single mutant (Fig. 4C and 4D), suggesting an involvement of BRM in control of internode patterns. Taken together, our findings indicate that BRM is required for the inflorescence architecture development in Arabidopsis.
Fig. 4. Phenotype of the node in brm-3, bp-9, and brm-3 bp-9 mutants. 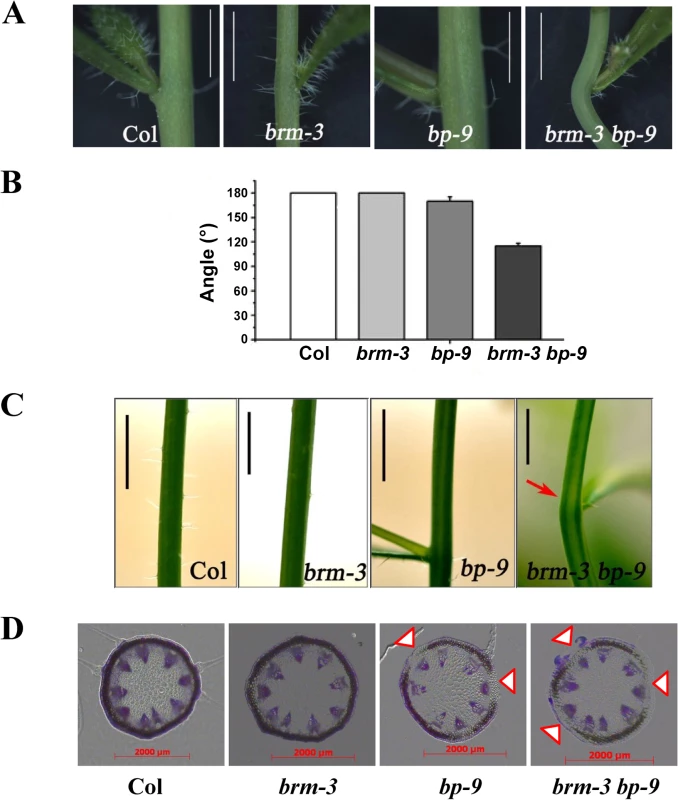
(A) brm-3 bp-9 showed obvious bend at node (bar = 0.5cm). (B) Quantitative analysis of the angles at the node in Col, brm-3, bp-9, and brm-3 bp-9 plants. 50 plants were analyzed. (C) brm-3 bp-9 displayed chlorenchyma-deficient (as indicated with red arrow) in the stem (bar = 0.5cm). (D) Transverse section of the stem at the nodes of the wild type and mutants. Arrows indicate the regions in which chlorenchyma development is repressed (bar = 2000 μm). BRM and BP Repress the Transcription of KNAT2 and KNAT6
Previous studies indicated that inflorescence architecture defects of bp mutants are caused by increased expression of two class-I KNOX genes, KNAT2 and KNAT6 [12]. We further examined the expression levels of KNAT2 and KNAT6 in brm-3, bp-9 and brm-3 bp-9 plants. The expression levels of KNAT2 and KNAT6 in inflorescences of Col, brm-3, bp-9, brm-3 bp-9 were analyzed. Compared with wild-type, the expression of KNAT2 and KNAT6 was increased in brm-3, bp-9 and brm-3 bp-9 mutants (Fig. 5). Furthermore, the transcription of KNAT2 and KNAT6 was up-regulated in brm-1 and brm-4 mutants compared to wild-type plants (S6 Fig.). Much higher expression levels of KNAT2 and KNAT6 were detected in the brm-3 bp-9 double mutant compared to brm-3 and bp-9 single mutants (Fig. 5), indicating that BRM may function synergistically with BP in repression of KNAT2 and KNAT6 expression.
Fig. 5. BRM and BP repress KNAT2 and KNAT6 expression in inflorescences. 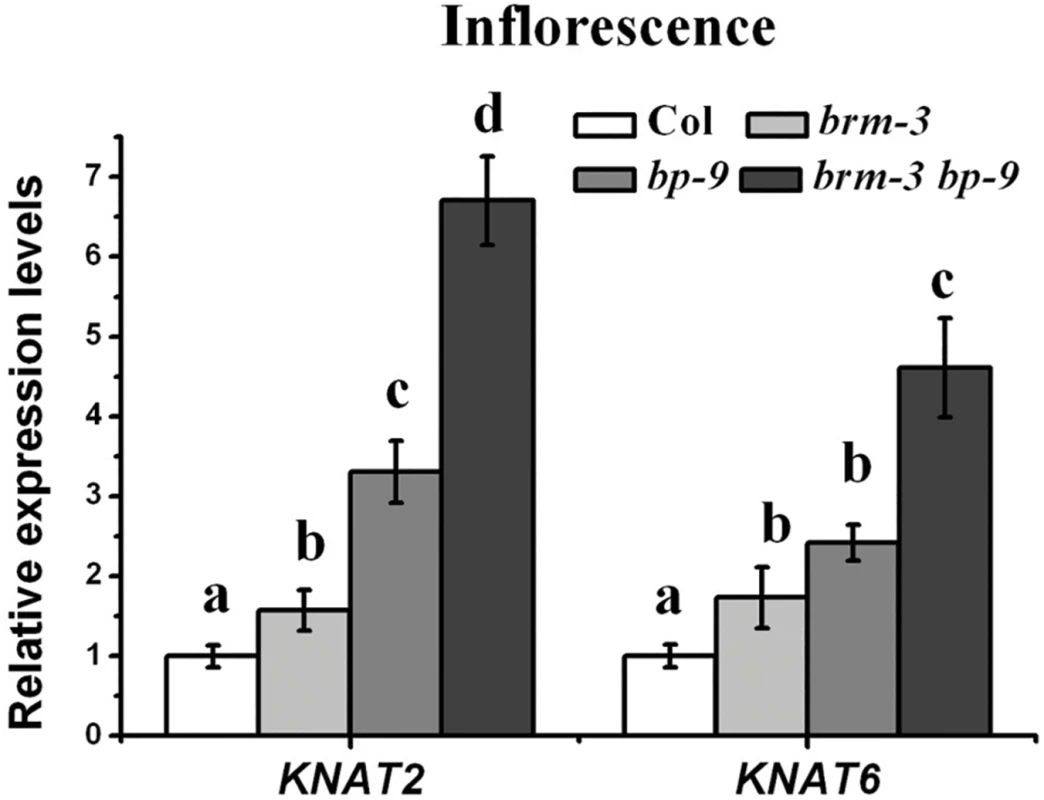
qRT-PCR analysis of KNAT2 and KNAT6 expression in inflorescences of Col, brm-3, bp-9, and brm-3 bp-9 mutants. Data shown are means±SD. UBQ was used as an internal control. 35-day-old plants were used for analysis. One-way ANOVA (Tukey-Kramer test) analysis was performed, and statistically significant differences (P < 0.01) were indicated by different lowercase letters (a, b, c, d). Equivalent means have the same letter; different letters indicate statistically significant differences. The H3K4me3 Levels of KNAT2 and KNAT6 Were Increased in brm-3, bp-9 and brm-3 bp-9 Plants
We further determined the levels of the activation marker H3K4me3 and the repression marker H3K27me3 of KNAT2 and KNAT6 in brm-3, bp-9 and brm-3 bp-9 mutants by chromatin immunoprecipitation (ChIP) assays. The relative enrichment of H3K4me3 and H3K27me3 levels was determined by real-time PCR using gene specific primers (Fig. 6A). Increased H3K4me3 levels were detected in both proximal promoter regions (region P of KNAT2 and region Y of KNAT6) and transcription starting sites (region S of KNAT2 and region Z of KNAT6) of KNAT2 and KNAT6 in brm-3, bp-9 and brm-3 bp-9 plants. Elevated H3K4me3 levels were also detected in the intron of KNAT6 (region E) in brm-3 and brm-3 bp-9 mutants compared with wild-type (Fig. 6B and 6C). Increased H3K4me3 levels of KNAT2 and KNAT6 observed in brm-3, bp-9 and brm-3 bp-9 plants are consistent with the up-regulation of these genes in these mutants. Increased expression and H3K4me3 levels of KNAT2 and KNAT6 in brm-3 bp-9 plants were observed compared to bp-9 plants. The enhanced brm-3 bp-9 phenotype relative to bp-9 suggests that additional factors other than BP likely interact with BRM to regulate KNAT2 and KNAT6. By contrast, the H3K27me3 levels of KNAT2 and KNAT6 were not significantly altered in brm-3, bp-9 and brm-3 bp-9 mutants (S7 Fig.).
Fig. 6. BRM and BP decrease H3K4Me3 levels of KNAT2 and KNAT6 in inflorescences. 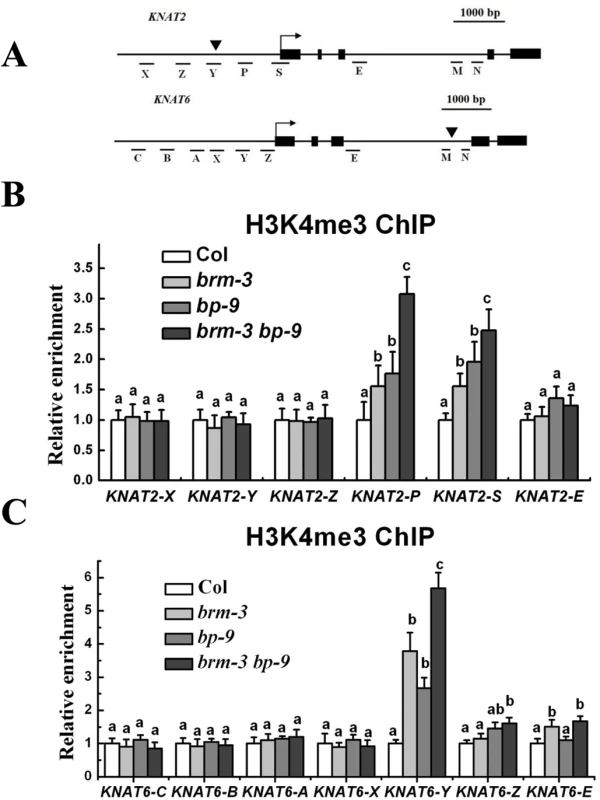
(A) Schematic diagram of KNAT2 and KNAT6 for ChIP-qPCR analysis. Black boxes indicate the exons. Arrows indicate transcriptional starting sites, whereas black triangles indicate the positions of the TAGC motifs. (B) ChIP-qPCR analysis of relative H3K4me3 levels of KNAT2 chromatin in Col, brm-3, bp-9, and brm-3 bp-9 mutants. (C) ChIP-qPCR analysis of relative H3K4me3 levels of KNAT6 chromatin in Col, brm-3, bp-9, and brm-3 bp-9 mutants. The amounts of DNA after ChIP were quantified and normalized to TUB2, the relative enrichment refers to the H3K4me3 enrichment versus the histone H3 occupancy. The values are shown as means±SD. 35-day-old plants were used for analysis. One-way ANOVA (Tukey-Kramer test) analysis was performed, and statistically significant differences (P < 0.01) were indicated by different lowercase letters (a, b, c). BP Binds to KNAT2 and KNAT6 In Vitro
To examine whether BP protein could directly bind to KNAT2 and KNAT6 in vitro, we performed electrophoretic mobility shift assays (EMSA). The target sequences of KNOX proteins have been identified previously with a core motif of TGAC [30,31]. In maize, the KNOX protein KN1 binds to an intron of GA2ox1 through a cis-regulatory element containing two adjacent TGAC motifs [32]. We identified two TAGC motifs in the promoter of KNAT2 (-1039 to -991 bp, the Y region as indicated in Fig. 6A) and two TAGC motifs in the third intron of KNAT6 (4269 to 4319 bp between M and N regions as indicated in Fig. 6A) (Fig. 7A). EMSA assays showed that BP bound strongly to the TAGC motifs of KNAT2 and KNAT6 (Fig. 7B). We further showed that the mutated competitor probes could not affect the binding of BP to the TAGC motifs of KNAT2 and KNAT6 (S8 Fig.), indicating that BP specifically binds to the TAGC motifs of KNAT2 and KNAT6 in vitro (S8 Fig.).
Fig. 7. BP binds to KNAT2 and KNAT6 in vitro. 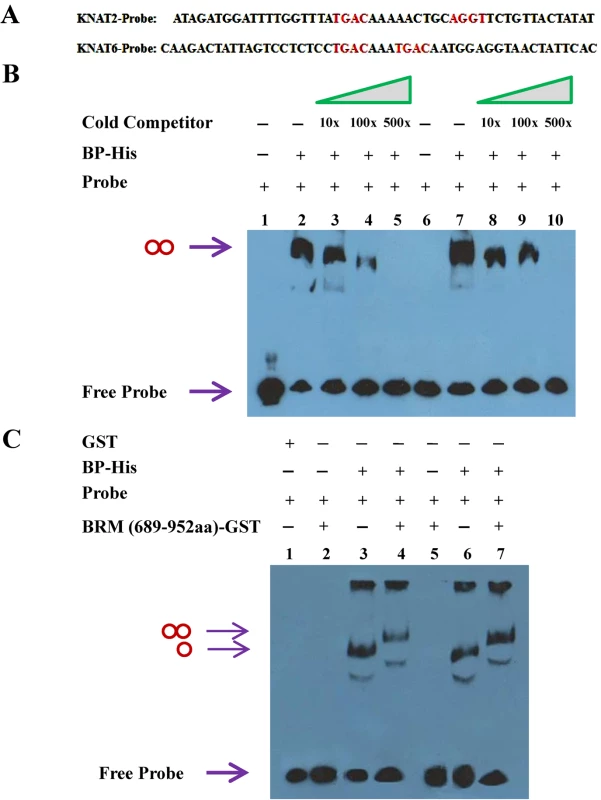
(A) Biotin–labeled probe sequence of KNAT2 (-1039 to -991 bp) and KNAT6 (4269 to 4319 bp). The core TGAC/AGGT motifs are indicated in red. (B) EMSA assay using purified BP-His fusion protein. Lane 1, 2, 3, 4 and 5 were added with KNAT2 probe (20 fmol), whereas lane 6, 7, 8, 9 and 10 were added with KNAT6 probe (20 fmol). 500 ng protein was added in lane 2, 3, 4, 5, 7, 8, 9 and 10, and no protein was added in lane 1 and 6 as negative controls. Non-biotin-labeled probe was added as cold competitor. The double circle indicates binding of the probe id by BP-His. (C) EMSA assay showing that BRM (689-952aa)-GST binds to KNAT2 and KNAT6 probes requiring BP. Lane 1 was added with mixed KNAT2 and KNAT6 probes, the lane 2, 3 and 4 were added with KNAT2 probe, and lane 5, 6 and 7 were added with KNAT6 probe. Lane 1 with GST protein (~500 ng) only was served as a negative control. The single circle indicates the probe is bound by BP-His, and the double circle indicates the probe is bound by BP-BRM (689-952aa) complex. To determine whether BRM proteins can also directly bind to KNAT2 and KNAT6, purified BRM (689-952aa)-GST protein was incubated with the KNAT2 and KNAT6 probes. BRM (689-952aa)-GST alone could not directly bind to KNAT2 and KNAT6 (Fig. 7C). When BRM (689-952aa)-GST, BP-His proteins and the KNAT2 and KNAT6 probes were incubated together in EMSA assays, two slower shifted bands were detected (Fig. 7C), indicating that BRM may form a complex with BP thus bind to KNAT2 and KNAT6 in vitro.
BP and BRM Co-Target to KNAT2 and KNAT6 In Vivo
To study whether KNAT2 and KNAT6 are direct targets of BP in vivo, ChIP assays were performed using transgenic plants expressing green fluorescent protein (GFP)-Tagged BP driven by the native BP promoter (ProBP:BP-GFP). Expression of ProBP:BP-GFP in bp-9 background fully rescued the inflorescence architecture defects of bp-9 (Fig. 8A-C), suggesting that BP-GFP is functional in vivo. BP strongly bound to the proximal promoter region (Y) of KNAT2 and the third intron (M and N) of KNAT6 (Fig. 8D and 8E), indicating that KNAT2 and KNAT6 are direct target genes of BP.
Fig. 8. BRM and BP co-target to KNAT2 and KNAT6 in inflorescences. 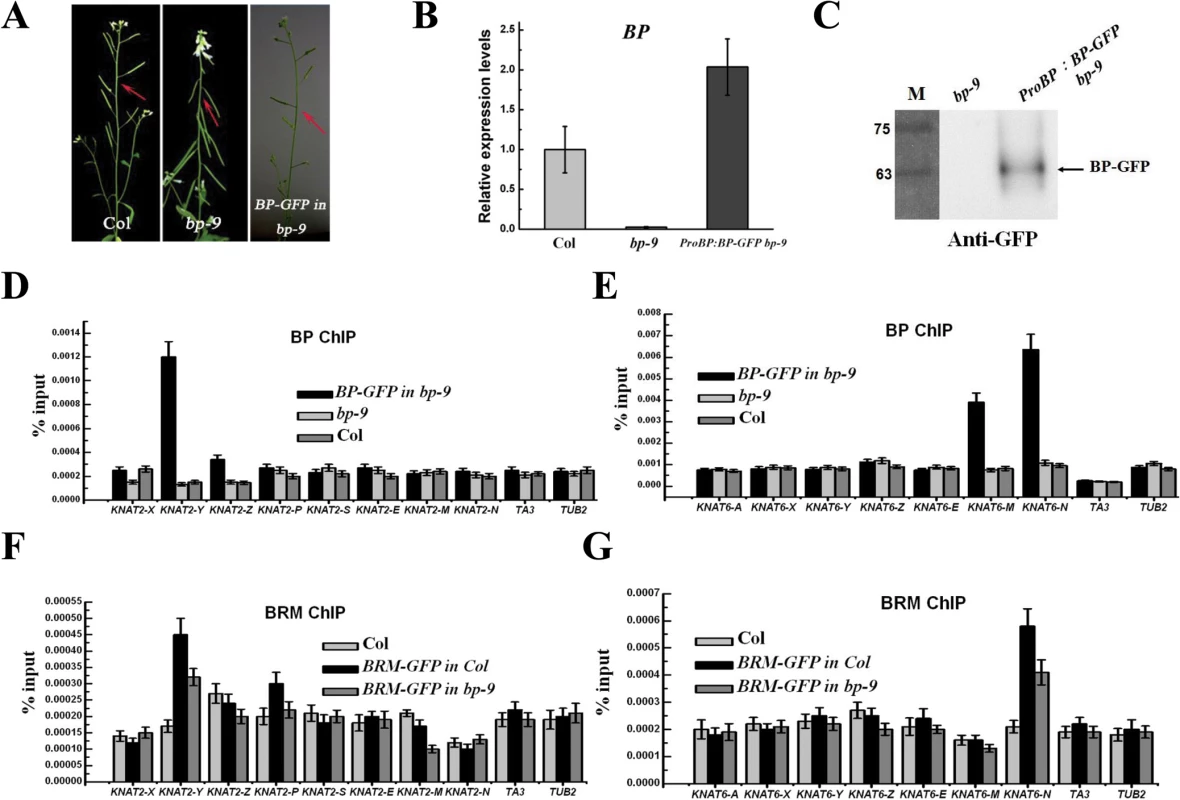
(A) Phenotypes of the ProBP:BP-GFP bp-9 (BP-GFP in bp-9) transgenic plants. Arrows indicate the typical pedicel orientation of the plants. (B) Detection of the expression level of BP in ProBP:BP-GFP bp-9 transgenic plants. (C) Western blotting analysis of BP protein in ProBP:BP-GFP bp-9 transgenic plants. The arrow indicates BP-GFP protein; the numbers indicate the molecular mass in kilodaltons. (D, E) ChIP-qPCR analysis of BP-GFP DNA fragments co-immunoprecipitated with the anti-GFP antibody in KNAT2 and KNAT6 chromatin. Relative enrichment was calculated based on IP/input for each sample. TA3 and TUB2 were used as negative control. The values are shown as means±SD. (F, G) ChIP-qPCR analysis of BRM-GFP DNA fragments co-immunoprecipitated with the anti-GFP antibody in KNAT2 and KNAT6 chromatin. BRM-GFP in Col is a transgenic line expressing GFP-tagged BRM in Col background, while BRM-GFP in bp-9 is a transgenic line expressing GFP-tagged BRM in bp-9 mutants. Relative enrichment was calculated based on IP/input for each sample. TA3 and TUB2 were used as negative control. The values are shown as means±SD. We further analyzed whether KNAT2 and KNAT6 are also direct targets of BRM in vivo. The transgenic plants expressing GFP-tagged BRM driven by the BRM native promoter (ProBRM:BRM-GFP) [33] was used to perform the ChIP assay. ProBRM:BRM-GFP brm-1 and ProBRM:BRM-GFP brm-3 plants were generated by crossing ProBRM:BRM-GFP plants with brm-1 and brm-3 plants, respectively. The growth defects of brm-1 and brm-3 were rescued by ProBRM:BRM-GFP, indicating that BRM-GFP is functional in vivo (S9 Fig.). In addition, ProBRM:BRM-GFP bp-9 plants were also generated by crossing ProBRM:BRM-GFP plants with bp-9. Similar to the previous studies [20], we showed that BRM bound to the promoter region of ABI5, but not to the control genes, TA3 and TUB2 (S10 Fig.).
Similar to BP, BRM also bound to the proximal promoter region (Y) of KNAT2 and the third intron (N) of KNAT6 (Fig. 8F and 8G), suggesting that BRM and BP co-target to KNAT2 and KNAT6 in vivo. Compared to ProBRM:BRM-GFP plants, a decrease of binding of BRM to KNAT2 and KNAT6 was observed in ProBRM:BRM-GFP bp-9 plants (Fig. 8F and 8G). Taken together, these analyses suggest that BP is required for the binding of BRM to KNAT2 and KNAT6.
Removal of KNAT2 and KNAT6 Activity Partially Rescues the brm-3 Phenotype
We further analyzed the genetic interaction of BRM with KNAT2 and KNAT6 in inflorescence architecture development. We generated brm-3 knat2-5 and brm-3 knat6-1 double mutants as well as brm-3 knat2-5 knat6-1 triple mutants by genetic crossing brm-3 with knat2-5 and knant6-1 alleles [34]. The pedicel angel, internode and pedicel length were determined in brm-3, brm-3 knat2-5, brm-3 knat6-1 and brm-3 knat2-5 knat6-1 plants. Similar to a previous report [12], no difference was found in the pedicel and internode length of the knat2-5, knat6-1 and knat2 knat6 mutants compared to wild-type. Compared to brm-3 plants, a significant decrease of average pedicel angel was found in brm-3 knat2-5 knat6-1 but not in brm-3 knat2-5 and brm-3 knat6-1 plants (Fig. 9A and 9B). Quantitative phenotype analysis showed that knat2 and knat6 mutations could fully rescue the pedicel orientation defect of brm-3, indicating a requirement of both KNAT2 and KNAT6 in control of pedicel orientation. The distribution of internodes along the main inflorescence was also determined. The brm-3 knat2-5, brm-3 knat6-1 and brm-3 knat2-5 knat6-1 mutants showed longer internodes compared to brm-3 plants (Fig. 9C). However, removal of both KNAT2 and KNAT6 activity could not rescue the pedicel length of brm-3, since brm-3, brm-3 knat2-5, brm-3 knat6-1 and brm-3 knat2-5 knat6-1mutants displayed a similar pedicel length (Fig. 9D). Taken together, our findings suggest that inactivation of KNAT2 and KNAT6 partially rescues the brm-3 phenotype.
Fig. 9. Removal of KNAT2 and KNAT6 rescues the brm-3 phenotype in pedicel orientation and internode length. 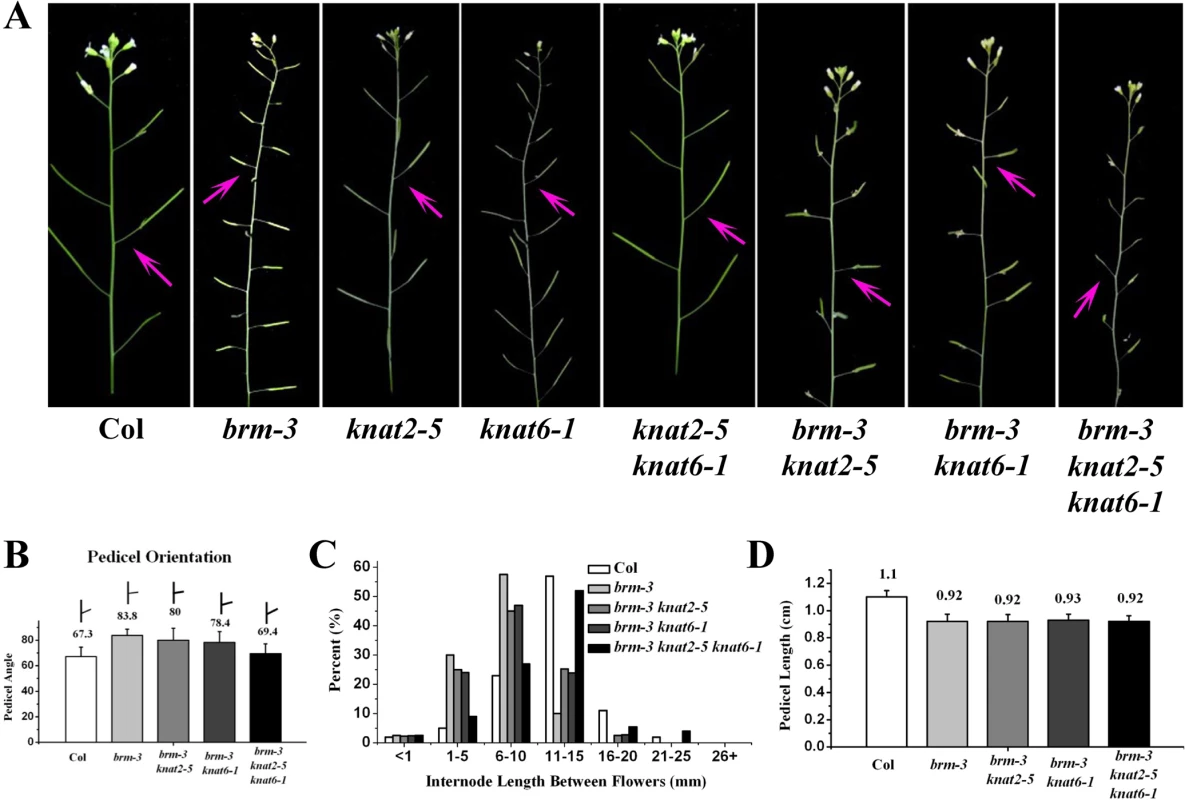
(A) Phenotypes of brm-3, brm-3 knat2-5, brm-3 knat6-1, and brm-3 knat2-5 knat6-1 plants in pedicel orientation. The arrows indicate the typical pedicel orientation of the mutants. 35-day-old plants were used for phenotype observation. (B) Quantitative analysis of the pedicel orientation in Col, brm-3, brm-3 knat2-5, brm-3 knat6-1, and brm-3 knat2-5 knat6-1 plants. (C) Distribution of the internode length between two successive siliques. Ten internodes between the 1st and 11th siliques were analyzed. (D) Quantitative analysis of the pedicle length of mature siliques in 35-day-old Col, brm-3, brm-3 knat2-5, brm-3 knat6-1, and brm-3 knat2-5 knat6-1 plants. Discussion
BRM Is Required for Inflorescence Architecture Development
In eukaryotes, the ATP dependent SWI/SNF chromatin remodeling complexes use energy from ATP hydrolysis to alter the interaction between histones and DNA and control accessibility of cis-regulatory DNA regions to transcription machinery [35]. BRM, a member of SWI/SNF ATPases, plays an essential role in reprogramming of transcription in vegetative, embryonic and reproductive development in Arabidopsis [19,20,21,22,29]. In present work, we showed that BRM is required for inflorescence architecture development. Loss of function BRM mutants display inflorescence architecture defects, with clustered inflorescences and horizontally orientated pedicels. Mutations of SWI3C, another SWI2/SNF2 chromatin remodeling ATPase gene in Arabidopsis, also cause a horizontally-pointing pedicel phenotype. BRM was shown to interact with SWI3C and they function in the same protein complex [25]. The similar pedicel orientation defect of brm and swi3c mutants supports an involvement of the SWI/SNF ATPases chromatin remodeling complex in inflorescence architecture development.
BRM Represses KNAT2 and KNAT6 Expression
The SWI/SNF complex has a co-activator function, catalyzing chromatin remodeling and recruiting activator determinants to gene sequences [36]. Furthermore, SWI/SNF can remodel chromatin resulting in either activation or repression of gene expression [37]. In present work, increased expression of two KNOX genes, KNAT2 and KNAT6, was detected in brm-3, brm-1 and brm-4 plants. EMSA and ChIP experiments showed that KNAT2 and KNAT6 are the direct target genes of BRM both in vitro and in vivo. These findings suggest that BRM may act as a repressor in regulation of KNAT2 and KNAT6 expression in Arabidopsis. The human BRM was shown to associate with Methyl CpG Binding Protein 2 (MeCP2) in vivo and is functionally linked with gene repression [38]. Moreover, a direct association of BRM with the histone demethylase UTX was also reported in Drosophila melanogaster [39]. Increasing levels of H3K4me3 in KNAT2 and KNAT6 in brm-3 indicate that BRM may associate with a histone H3K4 demethylase in repression of gene expression.
Previous studies showed that SWI/SNF ATPases act antagonistically with Polycomb-group (PcG) proteins in gene expression in mammalian [40]. PcG proteins are subunits of two multi-protein complexes, Polycomb Repressive Complex 1 (PRC1) and PRC2 [41,42]. PRC2 catalyses the trimethylation of lysine 27 of histone H3 (H3K27me3) [43,44]. More recently, it was reported that KNAT2 is repressed by ASYMMETRIC LEAVES 1 (AS1) and AS2 via recruitment of PRC2 [45]. However, the H3K27me3 levels of KNAT2 and KNAT6 were not changed in brm mutants. Further research is required to investigate the interaction between BRM and PcG proteins in repression of KNAT2 and KNAT6.
BP Associates with BRM in Regulation of Inflorescence Architecture
A previous study showed that knat2 knat6 bp mutants rescue the pedicel orientation and internode length defects of the bp mutant [12]. Similarly, we found that introduction of knat2-5 and knat6-1 into brm-3 can also rescue the pedicel orientation and internode length phenotypes of brm-3. Increased expression of KNAT2 and KNAT6 was found in both brm and bp mutants. These findings indicate that BRM and BP act upstream of KNAT2 and KNAT6 in regulation of inflorescence architecture. ChIP analysis indicated that BRM and BP co-target to KNAT2 and KNAT6 genes, suggesting that BRM and BP directly regulate KNAT2 and KNAT6 expression in the inflorescences. Furthermore, brm-3 bp-9 double mutants displayed more severe inflorescence architecture defects compared with brm-3 and bp-9 single mutants, supporting that BRM acts synergistically with BP in regulation of inflorescence development. knat2 and knat6 mutations did not rescue the shorter pedicel phenotype of brm-3 and bp mutants [12], suggesting that other genes are also involved in the control of pedicel growth. KNOX proteins promote shoot apical meristem activity by coordinately regulating cytokinin (CK) and gibberellin (GA) biosynthesis genes [46]. Furthermore, BRM could directly regulate GA and CK responsive genes to promote leaf growth and shoot apical meristem activity [26,47]. Further research is required to identify additional target genes regulated by BRM and BP in promotion of cell proliferation and elongation in Arabidopsis.
Accurate initiation of gene transcription requires multiple factors, including transcription cofactors (coactivator or corepressors) and chromatin remodeling factors [13]. In vitro studies have shown that transcription factors recruit chromatin remodeling factors and histone modification factors to affect the chromatin status of specific loci [48]. For example, two Jumonji N/C (JmjN/C) domain-containing proteins, ELF6 and REF6, are recruited by their interacted transcription factor BES1 to regulate their co-target genes and coordinate BR responses [49]. Histone deacetylase HDA15 is recruited by its interacted partner PIF3 to repress chlorophyll biosynthetic and photosynthetic genes in etiolated seedlings [50]. In addition, HDA6 and HDA19 are recruited by AS1 and HSL2, respectively, to regulate gene expression involved in leaf and seed development [51,52,53]. More recently, the chromatin remolding factor BBM was shown to interact with transcription factors in yeast two-hybrid assays [26], indicating that BRM may be associated with different transcription factors involved in regulation of gene expression. In present work, we showed that BRM physically interacted with BP both in vitro and in vivo, suggesting that BP may associate with BRM to regulate gene expression. Furthermore, the binding of BRM to the target genes depended on the presence of BP, indicating that BRM may be recruited by BP through the protein-protein interaction. PNY, a member of the BELL subfamily protein, has been shown to interact with BP physically [6]. In addition, PNY was also shown to play a role in repressing of KNAT2 and KNAT6 expression. It remains to be determined whether PNY is also associated with BRM in regulating inflorescence patterning by epigenetic regulation of KNAT2 and KNAT6 expression.
Materials and Methods
Plant Materials
brm-1, brm-3 (SALK_088462), brm-4 (WiscDsLox436E9), brm-5 and swi3c-3 (SAIL_224_B10) were obtained from the Arabidopsis Biological Resource Center (http://www.arabidopsis.org/). knat6-1 (SALK_047931) and knat2-5 (SALK_099837) were obtained from Nottingham Arabidopsis Stock Centre (NASC). bp-9 was kindly provided by Prof. Lin Xu (Shanghai Institute of Plant Physiology and Ecology, Chinese Academy of Sciences).
ProBP:BP-GFP bp-9 transgenic plants were generated by transforming the ProBP:BP-GFP construct into bp-9 plants using the floral dip method [54]. The ProBRM:BRM-GFP bp-9 plants were generated by crossing ProBRM:BRM-GFP plants [33] with bp-9 plants. All Arabidopsis plants were grown in 22°C under long-day (16 h light/8 h dark) conditions.
Phenotypic Analysis
The pedicel orientation, pedicel length and internode (the stem between two nodes) length between siliques were measured in 35-day-old plants. 10 individual plants were used for quantitative analysis, and 8–10 pedicels were measured for each plant. The minimum age of pedicel selected for analysis is 15 days after flowering. A protractor was used to determine the angle of pedicels. Bend at node was imaged using a stereoscope (ZEISS, SV11). The thin sections of chlorenchyma tissue were prepared with a razor blade and observed under a microscope.
Quantitative RT-PCR Analysis
Total RNA was isolated from inflorescences (0.15 g) of 35-day-old plants using 1 mL Trizol reagent (Invitrogen). The first strand cDNA synthesis was generated using 2 μg total RNA according to the manufacturer’s instructions of TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (TransGen, Beijing). 100 ng synthesized cDNA was used as a template to perform quantitative RT-PCR analysis. PCR reactions were performed in the total volume of 20 μL, with 0.5 μL for each primer (10 mm, final concentration 100 nm) and 10 μL for SYBR Green PCR Supermix (Bio-Rad Laboratories) on a ABI7500 Real-Time PCR System (Applied Biosystems). The PCR program included an initial denaturation step at 94°C for 3 min, followed by 40 cycles of 5 s at 94°C and 1 min at 60°C. Each sample was quantified at least triplicate and normalized using Ubiquitin 10 (UBQ) as an internal control. The gene-specific primer pairs for quantitative Real-Time PCR are listed in S1 Table. All PCR reactions were normalized using Ct value corresponding to the reference gene UBQ. The relative expression levels of target gene were calculated with formula 2-ddCt [55]. Values represented the average of three biological replicates.
Yeast Two-Hybrid Assays
Yeast two-hybrid assays were performed as described in the manual of Matchmaker Gold Yeast Two-Hybrid Systems (Clontech). Full length and different deletion coding regions of BRM and BP were subcloned into pGBKT7 and pGADT7 vectors to construct different bait and prey constructs (primers are listed in S1 Table). Then, different pairs of bait and prey constructs were co-transformed into yeast strain Gold Y2H by PEG, and yeast cells were grown on DDO medium (minimal media double dropouts, SD medium with-Leu/-Trp) for 3 days. Transformed colonies were dropped onto QDO medium (minimal media quadruple dropouts, SD medium with-Leu/-Trp/-Ade/-His) containing 4 mg mL-1 X-a-Gal (QDO/ X) to test for possible interactions between BRM and BP according to their growth status.
In Vitro Pull-Down Assays
In vitro pull-down assays were performed as described [50]. His-BP recombinant protein was incubated with 30 mL His resin (QIAGEN) in a phosphate buffer (10 mM Na2HPO4, 10 mM NaH2PO4, 500 mM NaCl, and 10 mM imidazole) for 2 h at 4°C, the binding reaction was washed three times with the phosphate buffer, and then BRM (689-952aa)-GST or GST was added and incubated for an additional 2 h at 4°C. After washing three times with the phosphate buffer, the pulled-down proteins were eluted by boiling, separated by 10% SDS-PAGE, and detected by western blotting using an anti-His antibody.
BiFC Assays
For BiFC assays, full length coding regions of BRM and BP were subcloned into YN vector pUC-pSPYNE and the YC vector pUC-pSPYCE, respectively [27]. Then fused YN and YC constructs were transformed into tobacco cells by polyethylene glycol for transient expression [56]. Transfected protoplast cells were imaged using a TCS SP5 confocal spectral microscope imaging system (Leica).
Co-IP Assays
Co-IP assays were performed as described previously [14]. Two days after infiltration, tobacco (Nicotiana benthamiana) leaves were harvested and ground in liquid nitrogen. Proteins were extracted in an extraction buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 20% glycerol, and 1% NP-40) containing protease inhibitor cocktail (Roche). Cell debris was pelleted by centrifugation at 14,000g for 20 min. The supernatant was incubated with 30 μL of GFP-Trap A beads (Chromo Tek) at 4°C for 4 h, then the beads were centrifuged and washed six times with a washing buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM MgCl2, 1mM DTT, 10% glycerol, and 1% NP-40). Proteins were eluted with 40 μL of 2×loading buffer and analyzed by western blotting using anti-GFP (Roche) and anti-Flag antibodies (Life Tein).
ChIP Assays
ChIP assays were performed as previously described [57]. Chromatin was extracted from the inflorescence tissues (0.3 g) bearing the first 10 siliques of 35-d-old flowering plants, after fixation with formaldehyde, the chromatin was extracted and then sheared to an average length of 500 bp by sonication. The chromatin was immunoprecipitated with specific antibodies including anti-H3K27me3 (Millipore, 07–449), anti-H3K4me3 (Millipore, 07–473), and anti-GFP (Abcam, ab290). The histone H3 occupancy at specific gene loci was analyzed by using an anti-H3 antibody (Millipore 06–775). Equal amount of the sonicated chromatin solution was set aside as the input sample. After cross-linking reversed, the amount of precipitated DNA fragments and input DNA was detected by quantitative Real-Time PCR using specific primers listed in S1 Table. The relative enrichments of various regions of KNAT2 and KNAT6 in brm-3, bp-9 and brm-3 bp-9 over Col were calculated after normalization to TUB2. The percentage of input was calculated by determining 2-ΔCt (= 2-[Ct(ChIP)-Ct(Input)]). The exon region of retrotransposon TA3 [58] was used as negative control.
EMSAs
In EMSAs, purified recombinant BP-His and BRM (689-952aa)-GST proteins are used. Oligonucleotide probes of KNAT2 (-1039 to -991 bp) and KNAT6 (4269 to 4319 bp) sequences were commercially synthesized with 5'-end biotin-labeled as single-stranded DNA (Invitrogen). To generate double-stranded oligonucleotides, equal amounts of complementary single-stranded oligonucleotides were mixed, heated to 95°C for 5 min, and slowly cooled down to 25°C. For a binding reaction, the Light Shift Chemiluminescent EMSA kit (Pierce) was used. For BP-His or BRM (689-952aa)-GST binding, the purified protein is incubated with binding buffer (2.5% glycerol, 5 mM MgCl2, 50 ng/μL poly [dI.dC], 0.05% Nonidet P-40) mixed with the labeled probe for 1 h at 4°C in 20 μL reaction volume. For cold competition, the non-labeled probe is added first for 1 h at 4°C followed by the labeled probe added. For BP-His and BRM (689-952aa)-GST interaction complex binding, first purified BP-His and BRM (689-952aa)-GST proteins were incubated together as the GST-pull down assay, then the mixed proteins were used for the EMSA assay. After the binding incubation, the reaction mixture is loaded on a 5% polyacrylamide gel (acrylamide:bisacrylamide, 29 : 1; Bio-Rad) and run in 0.5×Tris-borate-EDTA buffer at 4°C. The DNA-protein complex was transferred to a Hybond-N+ membrane, and the membrane was cross-linked. Detection was performed according to the manufacturer’s instructions (Pierce).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome initiative or GenBank/EMBL databases under the following accession numbers: BRM (AT2G46020), BP (AT4G08150), KNAT2 (AT1G70510), KNAT6 (AT1G23380), SWI3C (AT1G21700), TUB2 (AT5G62690), TA3 (AT1G37110) and PNY (AT5G02030).
Supporting Information
Zdroje
1. Douglas SJ, Chuck G, Dengler RE, Pelecanda L, Riggs CD (2002) KNAT1 and ERECTA regulate inflorescence architecture in Arabidopsis. Plant Cell 14 : 547–558. 11910003
2. Venglat SP, Dumonceaux T, Rozwadowski K, Parnell L, Babic V, et al. (2002) The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc Natl Acad Sci U S A 99 : 4730–4735. 11917137
3. Bhatt AM, Etchells JP, Canales C, Lagodienko A, Dickinson H (2004) VAAMANA—a BEL1-like homeodomain protein, interacts with KNOX proteins BP and STM and regulates inflorescence stem growth in Arabidopsis. Gene 328 : 103–111. 15019989
4. Hamant O, Pautot V (2010) Plant development: a TALE story. C R Biol 333 : 371–381. doi: 10.1016/j.crvi.2010.01.015 20371112
5. Rutjens B, Bao D, van Eck-Stouten E, Brand M, Smeekens S, et al. (2009) Shoot apical meristem function in Arabidopsis requires the combined activities of three BEL1-like homeodomain proteins. Plant J 58 : 641–654. doi: 10.1111/j.1365-313X.2009.03809.x 19175771
6. Smith HM, Hake S (2003) The interaction of two homeobox genes, BREVIPEDICELLUS and PENNYWISE, regulates internode patterning in the Arabidopsis inflorescence. Plant Cell 15 : 1717–1727. 12897247
7. Byrne ME, Groover AT, Fontana JR, Martienssen RA (2003) Phyllotactic pattern and stem cell fate are determined by the Arabidopsis homeobox gene BELLRINGER. Development 130 : 3941–3950. 12874117
8. Hake S, Smith HM, Holtan H, Magnani E, Mele G, et al. (2004) The role of knox genes in plant development. Annu Rev Cell Dev Biol 20 : 125–151. 15473837
9. Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379 : 66–69. 8538741
10. Byrne ME, Simorowski J, Martienssen RA (2002) ASYMMETRIC LEAVES1 reveals knox gene redundancy in Arabidopsis. Development 129 : 1957–1965. 11934861
11. Kanrar S, Onguka O, Smith HM (2006) Arabidopsis inflorescence architecture requires the activities of KNOX-BELL homeodomain heterodimers. Planta 224 : 1163–1173. 16741748
12. Ragni L, Belles-Boix E, Gunl M, Pautot V (2008) Interaction of KNAT6 and KNAT2 with BREVIPEDICELLUS and PENNYWISE in Arabidopsis inflorescences. Plant Cell 20 : 888–900. doi: 10.1105/tpc.108.058230 18390591
13. Berger SL (2007) The complex language of chromatin regulation during transcription. Nature 447 : 407–412. 17522673
14. Yu CW, Liu X, Luo M, Chen C, Lin X, et al. (2011) HISTONE DEACETYLASE6 interacts with FLOWERING LOCUS D and regulates flowering in Arabidopsis. Plant Physiol 156 : 173–184. doi: 10.1104/pp.111.174417 21398257
15. Liu X, Yu CW, Duan J, Luo M, Wang K, et al. (2012) HDA6 directly interacts with DNA methyltransferase MET1 and maintains transposable element silencing in Arabidopsis. Plant Physiol 158 : 119–129. doi: 10.1104/pp.111.184275 21994348
16. Yuan L, Liu X, Luo M, Yang S, Wu K (2013) Involvement of histone modifications in plant abiotic stress responses. J Integr Plant Biol 55 : 892–901. doi: 10.1111/jipb.12060 24034164
17. Cairns BR (2005) Chromatin remodeling complexes: strength in diversity, precision through specialization. Current opinion in genetics & development 15 : 185–190.
18. Ho L, Crabtree GR (2010) Chromatin remodelling during development. Nature 463 : 474–484. doi: 10.1038/nature08911 20110991
19. Kwon CS, Hibara K, Pfluger J, Bezhani S, Metha H, et al. (2006) A role for chromatin remodeling in regulation of CUC gene expression in the Arabidopsis cotyledon boundary. Development 133 : 3223–3230. 16854978
20. Han SK, Sang Y, Rodrigues A, Wu MF, Rodriguez PL, et al. (2012) The SWI2/SNF2 chromatin remodeling ATPase BRAHMA represses abscisic acid responses in the absence of the stress stimulus in Arabidopsis. Plant Cell 24 : 4892–4906. doi: 10.1105/tpc.112.105114 23209114
21. Wu MF, Sang Y, Bezhani S, Yamaguchi N, Han SK, et al. (2012) SWI2/SNF2 chromatin remodeling ATPases overcome polycomb repression and control floral organ identity with the LEAFY and SEPALLATA3 transcription factors. Proc Natl Acad Sci U S A 109 : 3576–3581. doi: 10.1073/pnas.1113409109 22323601
22. Zhu Y, Rowley MJ, Bohmdorfer G, Wierzbicki AT (2013) A SWI/SNF chromatin-remodeling complex acts in noncoding RNA-mediated transcriptional silencing. Molecular cell 49 : 298–309. doi: 10.1016/j.molcel.2012.11.011 23246435
23. Farrona S, Hurtado L, Bowman JL, Reyes JC (2004) The Arabidopsis thaliana SNF2 homolog AtBRM controls shoot development and flowering. Development 131 : 4965–4975. 15371304
24. Farrona S, Hurtado L, March-Diaz R, Schmitz RJ, Florencio FJ, et al. (2011) Brahma is required for proper expression of the floral repressor FLC in Arabidopsis. PLoS One 6: e17997. doi: 10.1371/journal.pone.0017997 21445315
25. Hurtado L, Farrona S, Reyes JC (2006) The putative SWI/SNF complex subunit BRAHMA activates flower homeotic genes in Arabidopsis thaliana. Plant Mol Biol 62 : 291–304. 16845477
26. Efroni I, Han SK, Kim HJ, Wu MF, Steiner E, et al. (2013) Regulation of leaf maturation by chromatin-mediated modulation of cytokinin responses. Dev Cell 24 : 438–445. doi: 10.1016/j.devcel.2013.01.019 23449474
27. Walter M, Chaban C, Schutze K, Batistic O, Weckermann K, et al. (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40 : 428–438. 15469500
28. Farrona S, Hurtado L, Reyes JC (2007) A nucleosome interaction module is required for normal function of Arabidopsis thaliana BRAHMA. J Mol Biol 373 : 240–250. 17825834
29. Tang X, Hou A, Babu M, Nguyen V, Hurtado L, et al. (2008) The Arabidopsis BRAHMA chromatin-remodeling ATPase is involved in repression of seed maturation genes in leaves. Plant Physiol 147 : 1143–1157. doi: 10.1104/pp.108.121996 18508955
30. Smith HM, Boschke I, Hake S (2002) Selective interaction of plant homeodomain proteins mediates high DNA-binding affinity. Proc Natl Acad Sci U S A 99 : 9579–9584. 12093897
31. Viola IL, Gonzalez DH (2006) Interaction of the BELL-like protein ATH1 with DNA: role of homeodomain residue 54 in specifying the different binding properties of BELL and KNOX proteins. Biol Chem 387 : 31–40. 16497162
32. Bolduc N, Hake S (2009) The maize transcription factor KNOTTED1 directly regulates the gibberellin catabolism gene ga2ox1. Plant Cell 21 : 1647–1658. doi: 10.1105/tpc.109.068221 19567707
33. Smaczniak C, Immink RG, Muino JM, Blanvillain R, Busscher M, et al. (2012) Characterization of MADS-domain transcription factor complexes in Arabidopsis flower development. Proc Natl Acad Sci U S A 109 : 1560–1565. doi: 10.1073/pnas.1112871109 22238427
34. Belles-Boix E, Hamant O, Witiak SM, Morin H, Traas J, et al. (2006) KNAT6: an Arabidopsis homeobox gene involved in meristem activity and organ separation. Plant Cell 18 : 1900–1907. 16798887
35. Kwon CS, Chen C, Wagner D (2005) WUSCHEL is a primary target for transcriptional regulation by SPLAYED in dynamic control of stem cell fate in Arabidopsis. Genes Dev 19 : 992–1003. 15833920
36. Wang W, Xue Y, Zhou S, Kuo A, Cairns BR, et al. (1996) Diversity and specialization of mammalian SWI/SNF complexes. Genes Dev 10 : 2117–2130. 8804307
37. Battaglioli E, Andres ME, Rose DW, Chenoweth JG, Rosenfeld MG, et al. (2002) REST repression of neuronal genes requires components of the hSWI.SNF complex. J Biol Chem 277 : 41038–41045. 12192000
38. Harikrishnan KN, Chow MZ, Baker EK, Pal S, Bassal S, et al. (2005) Brahma links the SWI/SNF chromatin-remodeling complex with MeCP2-dependent transcriptional silencing. Nature genetics 37 : 254–264. 15696166
39. Tie F, Banerjee R, Conrad PA, Scacheri PC, Harte PJ (2012) Histone demethylase UTX and chromatin remodeler BRM bind directly to CBP and modulate acetylation of histone H3 lysine 27. Molecular and cellular biology 32 : 2323–2334. doi: 10.1128/MCB.06392-11 22493065
40. Wilson BG, Wang X, Shen X, McKenna ES, Lemieux ME, et al. (2010) Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer cell 18 : 316–328. doi: 10.1016/j.ccr.2010.09.006 20951942
41. Schwartz YB, Pirrotta V (2007) Polycomb silencing mechanisms and the management of genomic programmes. Nature reviews Genetics 8 : 9–22. 17173055
42. Simon JA, Kingston RE (2009) Mechanisms of polycomb gene silencing: knowns and unknowns. Nature reviews Molecular cell biology 10 : 697–708. doi: 10.1038/nrm2763 19738629
43. Margueron R, Reinberg D (2011) The Polycomb complex PRC2 and its mark in life. Nature 469 : 343–349. doi: 10.1038/nature09784 21248841
44. Gutierrez L, Oktaba K, Scheuermann JC, Gambetta MC, Ly-Hartig N, et al. (2012) The role of the histone H2A ubiquitinase Sce in Polycomb repression. Development 139 : 117–127. doi: 10.1242/dev.074450 22096074
45. Lodha M, Marco CF, Timmermans MC (2013) The ASYMMETRIC LEAVES complex maintains repression of KNOX homeobox genes via direct recruitment of Polycomb-repressive complex2. Genes Dev 27 : 596–601. doi: 10.1101/gad.211425.112 23468429
46. Jasinski S, Piazza P, Craft J, Hay A, Woolley L, et al. (2005) KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr Biol 15 : 1560–1565. 16139211
47. Archacki R, Buszewicz D, Sarnowski TJ, Sarnowska E, Rolicka AT, et al. (2013) BRAHMA ATPase of the SWI/SNF chromatin remodeling complex acts as a positive regulator of gibberellin-mediated responses in arabidopsis. PLoS One 8: e58588. doi: 10.1371/journal.pone.0058588 23536800
48. Eberharter A, Becker PB (2002) Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO reports 3 : 224–229. 11882541
49. Yu X, Li L, Guo M, Chory J, Yin Y (2008) Modulation of brassinosteroid-regulated gene expression by Jumonji domain-containing proteins ELF6 and REF6 in Arabidopsis. Proc Natl Acad Sci U S A 105 : 7618–7623. doi: 10.1073/pnas.0802254105 18467490
50. Liu X, Chen CY, Wang KC, Luo M, Tai R, et al. (2013) PHYTOCHROME INTERACTING FACTOR3 associates with the histone deacetylase HDA15 in repression of chlorophyll biosynthesis and photosynthesis in etiolated Arabidopsis seedlings. Plant Cell 25 : 1258–1273. doi: 10.1105/tpc.113.109710 23548744
51. Luo M, Yu CW, Chen FF, Zhao L, Tian G, et al. (2012) Histone deacetylase HDA6 is functionally associated with AS1 in repression of KNOX genes in arabidopsis. PLoS genetics 8: e1003114. doi: 10.1371/journal.pgen.1003114 23271976
52. Zhou Y, Tan B, Luo M, Li Y, Liu C, et al. (2013) HISTONE DEACETYLASE19 interacts with HSL1 and participates in the repression of seed maturation genes in Arabidopsis seedlings. Plant Cell 25 : 134–148. doi: 10.1105/tpc.112.096313 23362207
53. Liu X, Yang S, Zhao M, Luo M, Yu CW, et al. (2014) Transcriptional repression by histone deacetylases in plants. Mol Plant 7 : 764–772. doi: 10.1093/mp/ssu033 24658416
54. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 : 735–743. 10069079
55. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 : 402–408. 11846609
56. Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nature protocols 2 : 1565–1572. 17585298
57. Gendrel AV, Lippman Z, Martienssen R, Colot V (2005) Profiling histone modification patterns in plants using genomic tiling microarrays. Nature methods 2 : 213–218. 16163802
58. Johnson L, Cao X, Jacobsen S (2002) Interplay between two epigenetic marks. DNA methylation and histone H3 lysine 9 methylation. Curr Biol 12 : 1360–1367. 12194816
Štítky
Genetika Reprodukční medicína
Článek NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY ModuleČlánek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in NematodesČlánek The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse SpermatocytesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 3
-
Všechny články tohoto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



