-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPersistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
The evolution of resistance is a universal challenge in antimicrobial chemotherapy. A key driver of resistance is that drug resistance mutations often persist even in the absence of drugs and despite the fact that resistance mutations are often associated with reduced pathogen replication (“fitness costs”). Such persistence may occur because fitness costs are low, especially if they are compensated by additional mutations in their “genetic background”. Here we assessed the role of fitness-cost and the genetic background for resistance in a real-world epidemiological setting by studying the persistence behavior of transmitted antiretroviral resistance mutations of HIV. This persistence behavior was associated with the predicted fitness cost of a given resistance mutation in the particular genetic background in which it occurred. We found that persistence behavior varied strongly across both mutation types and genetic backgrounds and that persistence was significantly associated with predicted fitness costs. In particular we found that even mutations of the same type tended to persist longer if they occurred in a genetic background where they caused weak fitness costs. Overall our results underline the variability of persistence behavior as well as the important role of fitness costs and the genetic background in the evolution of antimicrobial resistance.
Published in the journal: . PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004722
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004722Summary
The evolution of resistance is a universal challenge in antimicrobial chemotherapy. A key driver of resistance is that drug resistance mutations often persist even in the absence of drugs and despite the fact that resistance mutations are often associated with reduced pathogen replication (“fitness costs”). Such persistence may occur because fitness costs are low, especially if they are compensated by additional mutations in their “genetic background”. Here we assessed the role of fitness-cost and the genetic background for resistance in a real-world epidemiological setting by studying the persistence behavior of transmitted antiretroviral resistance mutations of HIV. This persistence behavior was associated with the predicted fitness cost of a given resistance mutation in the particular genetic background in which it occurred. We found that persistence behavior varied strongly across both mutation types and genetic backgrounds and that persistence was significantly associated with predicted fitness costs. In particular we found that even mutations of the same type tended to persist longer if they occurred in a genetic background where they caused weak fitness costs. Overall our results underline the variability of persistence behavior as well as the important role of fitness costs and the genetic background in the evolution of antimicrobial resistance.
Introduction
Drug-resistant pathogens represent one of the major public health and clinical challenges in infectious diseases (http://www.who.int/drugresistance/en/). It is an almost universal observation that as soon as a chemotherapeutic agent against a given pathogen is introduced, resistant pathogen strains emerge, which reduce the clinical benefits conferred by that agent. One crucial obstacle in curbing drug resistance is that once it has emerged it often persists even in the absence of drug pressure. The central concept here is pathogen fitness: whereas the resistant pathogen has a very strong advantage over the sensitive one in the presence of drug pressure, its disadvantages in the absence of treatment are typically weaker and can be compensated by other mechanisms such as compensatory mutations or selection at linked loci. Despite this key role of pathogen fitness for a conceptual understanding of the spread and persistence of drug resistance, real-world epidemiological examples documenting its role are rare. An ideal opportunity to assess this role of fitness is provided by the dynamics of antiretroviral resistance in HIV-1.
In the case of HIV, combinations of modern anti-retroviral treatment (ART) have successfully reduced the morbidity and mortality of HIV-1 infected individuals [1]. Though drug resistance prevalence has been shown to decrease or to stabilize in various industrialized countries due to successful ART, it still remains a major concern jeopardizing treatment success [2,3].
Transmission of a drug-resistant virus has been observed in most countries where ART is available [4–10]. After transmission, viruses with transmitted drug resistance mutations (TDRM) persist either as the dominant species or as minority variants, which are difficult to detect by population sequencing techniques [11–17]. Consequently, patients harboring TDRM have a higher chance to fail their first-line therapy [12,18–20].
Several studies have illustrated that the persistence time of individual TDRM in the absence of drug pressure exhibits substantial variance [11,13,15,17,21,22]. Persistence times have been suggested to be associated with fitness costs [18], which are typically measured as the reduction of replicative capacity of the virus caused by a given mutation [21]. It is generally assumed that transmitted drug-resistant viruses revert more rapidly to wild-type viruses if the fitness is reduced to a larger extent by the TDRM (high fitness cost) because then reversion of TDRM confers correspondingly high fitness gains [23]. Several studies have measured the fitness of some specific TDRM using phenotypic replicative capacity assays [6,17,21]. However, evidence for the impact of such fitness costs on the dynamics of TDRM at an in vivo and epidemiological level is largely lacking. Here, we aimed to determine the persistence times of TDRM in an epidemiological approach in vivo and to determine whether these persistence times depend on the fitness costs of TDRM.
Methods
Study population
The SHCS is a prospective, nationwide, clinic-based study including a biobank. The SHCS is very representative of the HIV epidemiology in Switzerland; it includes at least 53% of all HIV cases ever diagnosed in Switzerland, 72% of all patients receiving ART, and 69% of the nationwide registered AIDS cases [24,25]. Since 1996, the SHCS includes approximately 85% of the newly diagnosed HIV infected individuals in Switzerland. This number was obtained when we compared the estimated numbers of newly diagnosed HIV cases published by the Swiss Federal Office of Public Health to the numbers of patients enrolled in the SHCS annually since 1996. Genotypic resistance data stem from routine clinical testing and from systematic retrospective sequencing before routine genotyping was introduced (over 11000 sequences were retrospectively generated). Genotyping is performed by four laboratories in Switzerland authorized by the Federal Office of Public Health. All laboratories perform population-based sequencing of the full protease gene and at least codons 28–225 of the reverse transcriptase gene using commercial assays such as Viroseq Vs.1 PE Biosystems; Virsoseq Vs. 2, Abbott AG; VircoTYPE HIV-1 Assay, Virco Lab or in-house methods [4] and has participated in the yearly quality control evaluation by the Agence Nationale de la Recherche du SIDA(ANRS) since 2002. All sequences are stored the SHCS drug-resistance database using SmartGene’s Integrated Dababase Network System (SmartGene, Zug, Switzerland, IDNS version 3.6.3) [12]. For details on the sequencing procedure, see [12]. To increase coverage, we have systematically selected all treatment-naïve individuals carrying TDRM and retrieved their sequential plasma samples before therapy from the SHCS biobank.
For this study we considered genotypic resistance test (GRT) performed for a patient when being treatment-naïve. All sequential GRTs were included for individuals having ≥ 2 GRTs and harboring TDRM at baseline before ever starting any antiretroviral therapy.
TDRM was defined according to the WHO surveillance list of transmitted HIV drug resistance [11]. We studied mutations to the major three drug classes: nucleoside and nucleotide analogue reverse transcriptase inhibitors (NRTIs), protease inhibitors (PIs), and nonnucleoside reverse transcriptase inhibitors (NNRTIs). Additionally, we excluded 17 potential super-infections based on phylogenetic distance and the lack of phylogenetic clustering. Finally, since TDRM in HIV-1 CTL epitopes can disrupt binding to the HLA allele and such CTL-escape may essentially influence the reversion dynamics, we screened the list of optimal HIV-1 CTL epitopes (according to the Los Alamos HIV database, http://www.hiv.lanl.gov/content/immunology/pdf/2013/optimal_ctl_article.pdf) for epitopes containing TDRM and excluded from our analysis those mutations that disrupted binding to the epitope according to NetMHCcons (http://www.cbs.dtu.dk/services/NetMHCcons/).
Ethics statement
The SHCS, enrolling HIV-infected adults aged ≥ 16 years old, has been approved by ethics committees of all participating institutions. The data collection was anonymous and written informed consent was obtained from all participants [24].
Survival analysis
Our goal was to assess systematically the persistence of TDRM in the absence of drug pressure. In particular we considered the persistence across different mutations and viral genetic backgrounds (for a given mutation occurring in a given virus, the viral genetic background is given by the entire amino acid sequence in which this mutation is observed). To allow inter-patient comparisons we included TDRM that were present in at least five individuals at baseline.
We quantified the persistence via calculating reversion rates of individual TDRMs. Reversion of a TDRM was defined as an event at which a TDRM becomes undetectable by population sequencing assays. In other words, a TDRM has reversed when the HIV variant carrying that TDRM has decreased to the level below the detection limit of population sequencing assays (∼20–30% [26]). Therefore, reversion is not necessarily always to wild type. We fitted our data with an interval-censored survival model using exponential waiting times. We chose an interval-censored model because the data did not allow to determine the exact time point of reversion; instead a GRT not detecting a given resistance mutation preceded by a GRT with that mutation informs that the reversion event must have occurred in the time interval between those two tests.
Our results were expressed with 95% CI and two-sided p-values with p<0·05 being statistically significant. We analyzed our data with Stata 13.1 SE (StataCorp, Texas, USA).
Estimation of fitness costs of TDRM
We estimated fitness costs based on a previously published approach to predict HIV replicative fitness from amino acid sequences [27]. This approach uses a machine-learning algorithm (ridge regression) trained on >70000 data points, each consisting of a pol-amino-acid sequence and an in vitro replicative capacity. Specifically, the algorithm predicts replicative capacity (pRC) from an amino acid sequence by a quadratic fitness model of the form
where xi denotes the presence (1) or absence (0) of a given mutation i and Mij the epistatic effects (i<j) and the main effects (i = j) characterizing the fitness landscape. These coefficients were derived in [27] by fitting the model to the >70000 data points. Since the number of parameters of the above model exceeds the number of data points, this model was fitted using an approach based on ridge regression. In essence, in this approach the data set was split into a “training”, “training-test”, and “true-test” data set. Then assuming a given penalty weight for model parameters, the model parameters are determined such that for the “training” data set, the sum of squared residuals plus the sum of squares of parameters times the penalty weight are minimized. In this specific case the approach was modified to a generalized linear ridge regression to take the non-normal error structure into account. The model was evaluated on the “test-training” data set, and the penalty weight was determined such that the predictive power on the “test-training” test was optimized. This final model was then evaluated on the “true-test” data set (which was used neither in deriving the model parameters nor in determining the penalty weight). Details on the method and validation on in vitro and clinical data can be found in [27] and [28]. Using this model, we estimated the fitness cost of a mutation in a given genetic background as follows. If A denotes the partial pol-amino-acid sequence (first 404 amino acid used in the reference [27]) with a given resistance mutation m and A’ the same amino acid sequence but with the mutation reverted to its wild-type allele, then the fitness cost of the mutation m in the background A can be estimated asA negative fitness cost was set to zero.
The impact of this fitness cost was assessed in univariable and multivariable versions of the interval-censored model. The multivariable models were adjusted for whether a given TDRM was present as a mixture with another amino acid at this position. Specifically, this was considered to be the case if the nucleotide sequence coding for this mutation contained at least one ambiguous nucleotide that affects the amino acid encoded.
Results
Study population
From 7920 treatment-naïve patients enrolled in the SHCS from May 1995 to February 2013, we could identify 987 sequential GRTs from 197 patients, who had ≥ 2 GRT while being treatment-naïve and presented with ≥ 1 TDRM at baseline. See S1 Table for all types and numbers of mutations and reversions observed from these 197 patients. The criterion that a given mutation must have been present in at least 5 individuals at baseline reduced the number of sequential GRTs and patients to 857 and 168, respectively.
From our studied population most individuals were male (80%), white (87.5%), and infected with subtype-B viruses (81.5%; Table 1). The median (IQR) number of GRT performed per person was 7 (4, 11) and the median (IQR) of test interval was 193 (170, 243) days. Baseline CD4 count was relatively high (494 [347, 656]), suggesting that patients were tested relatively early on after infection. 60.1% of patients had a single mutation detected at their first GRT. Detailed patient characteristics were shown in Table 1.
Tab. 1. Basic characteristics of study population. 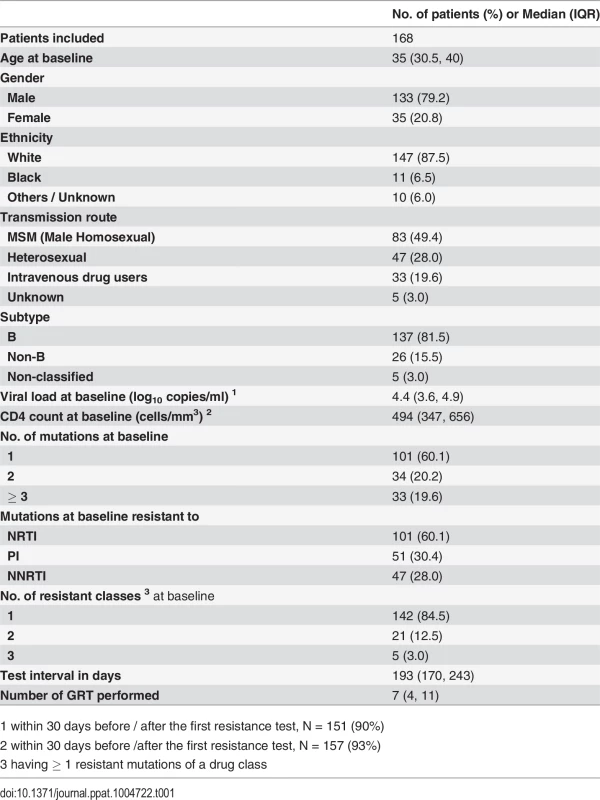
1 within 30 days before / after the first resistance test, N = 151 (90%) Reversion rate of individual TDRM varies
In total, 21 TDRM were analyzed. One mutation (190A of NNRTI) was excluded because we observed no reversion at all from the studied patients and three mutations (101E, 181C, 210W) were further excluded because they were located in the HLA epitopes (see Methods). Thus we could obtain reversion rates for 17 TDRM (Fig. 1). Among them, 10 were mutations associated with resistance to NRTI, 6 to PI, and 1 to NNRTI. The quantified linear reversion rate showed that persistence time varied strongly among mutations. Among three drug classes, NRTI mutations showed the largest variability. Both the fastest and the slowest reversion rates, 174.0/100-person-years [confidence interval = 51.4, 588.8] from 184V and 2.7/100-person-years [0.7, 10.9] from 215D, respectively, belonged to this drug class.
Fig. 1. Reversion rate of individual TDRM. 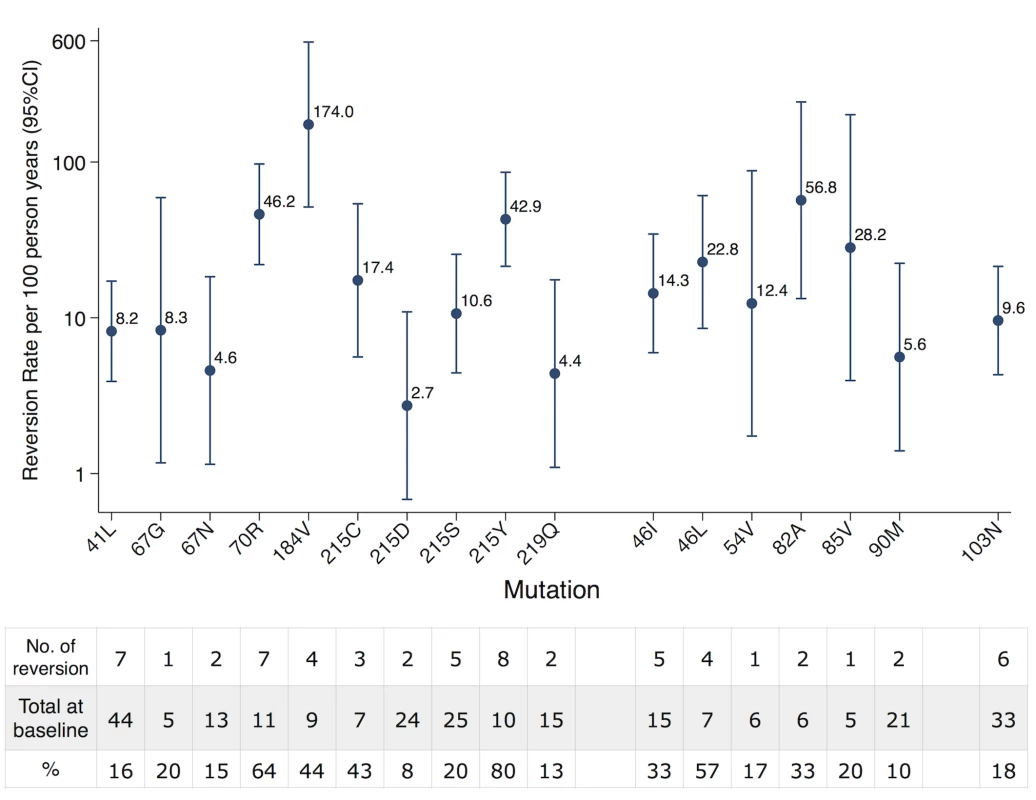
Reversion rate was quantified via an interval-censored survival model using an exponential distribution. The table below showed the number of reversion and total number observed at baseline for each TDRM. NRTI resistance mutations showed the largest variability that included both the fastest (184V) and the slowest (215D) reverting TDRM. Predicted fitness cost is associated with TDRM persistence
We found that reversion rates were associated significantly with the predicted fitness costs of resistance mutations (Fig. 2). Specifically, the survival analysis with predicted fitness cost as an explanatory variable yielded that reversion rates increased by a factor 1.6[1.3,2.0] (p<0.001) if fitness is increased by one standard deviation. Thus predicted fitness has a considerable and highly significant impact on reversion rates. Since this analysis included different fitness costs of mutations, each in at least five patients, the observed effect of fitness can be caused by two mechanisms: On the one hand, by overall differences in costs among mutations (“main effects”) and, on the other hand, by different costs of the same mutation in different backgrounds (“epistatic effects”). In order to distinguish between these two effects, we further analyzed the data with two alternative approaches:
Fig. 2. Impact of fitness cost on reversion rates. 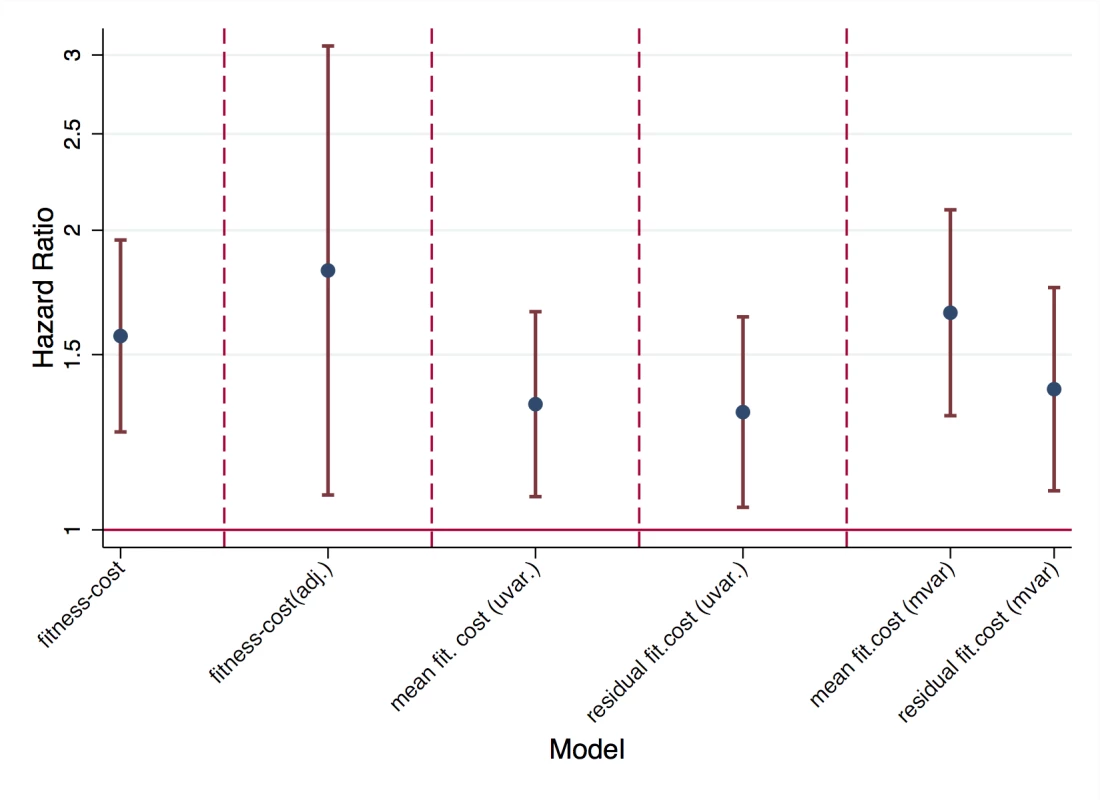
In unadjusted survival analysis (“fitness cost”), in survival analysis adjusted for type of mutation (“fitness cost adj.”). Impact of mean fitness cost and residual fitness cost in univariable analysis (“uvar.”) and in multivariable analysis including both mean and residual fitness cost (“mvar.”). In the first approach, we still used predicted fitness cost as the explanatory variable but adjusted for the identity of the resistance mutation (i.e. the type of resistance mutation was included as a categorical variable). In this approach, the estimated effect of fitness corresponded to the impact of fitness within a given type of mutation. Since this approach introduced 17 variables for 264 data points and 62 events (and hence carries the risk of over-parameterization), we considered an alternative second approach, which only included two parameters. Specifically, we divided fitness cost into two components: the mean fitness cost of a mutation (across backgrounds) and the residual fitness cost, which is given as the difference between the predicted fitness cost in a given background and the mean fitness cost. In the first approach, reversion rate was increased by a factor 1.8[1.1,3.1] (p<0.001) if fitness cost was increased by one standard deviation (after adjusting for type of mutation). In the second approach, both mean fitness cost and residual fitness cost increased the reversion rate significantly by a factor 1.7[1.3, 2.1] (p<0.001) and 1.4[1.1,1.8] (p = 0.007) per standard deviation, respectively. Thus our models predict that a typical difference in fitness cost among resistance mutations (i.e. one standard deviation of the fitness costs observed in our data set), causes a 40%-80% increase in the rate with which resistance mutations revert. Moreover, both approaches showed that both types of fitness cost (different overall costs of drug resistance mutations, and different costs in different backgrounds) are associated with higher reversion rates.
These multivariable models also showed that, as can be expected, reversion occurs much faster if a given TDRM is present as a mixture (see Methods, Table 2).
Tab. 2. Hazard ratios (HR) reported in univariable and multivariable models. 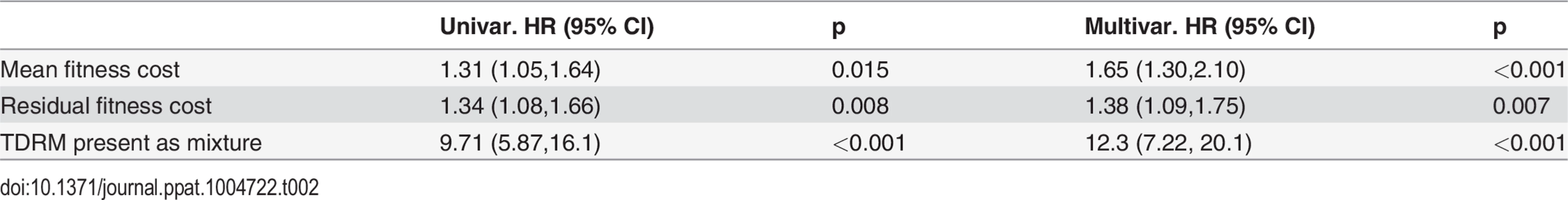
Discussion
In this study we investigated the differential persistence behaviors of TDRM in the absence of drug pressure and analyzed the association of the reversion rate with the predicted fitness cost of a given mutation. We used an interval-censored survival model to quantify the reversion rate of each mutation that was at least harbored by five individuals at baseline. We observed that the reversion rate of individual mutations varied substantially. Moreover, the reversion rates were significantly associated with the differential fitness costs of the TDRM: We showed that both the fitness-cost differences among mutations and among viral genetic backgrounds for the same mutation contributed to the variation in reversion rates. Thus, the novelty of this study is that we compared in total 17 TDRM from patients in a single cohort and could associate the persistence times with fitness costs of mutations predicted by a machine-learning model. An additional strength of this study is the high frequency and the number of resistance tests performed per patient.
Our results were consistent with most studies showing that M184V disappeared rapidly [15,21,29] whereas most thymidine analogue associated mutations (TAMs: 41L, 67N, 70R, 215Y, 219Q) disappeared at a slower rate [21,29,30] with the exception of 70R and 215Y. It is known however that 215Y has a high impact on fitness [21] and is rapidly replaced by intermediate 215S or atypical variants 215C/D [31]. Additionally, the fitness cost of 70R was shown to be higher when combined with other mutations in vitro [21,24]. This could explain the observed high reversion rate of 70R regardless of its low fitness cost because in our data set 7 from 11 patients harboring 70R had at least one other mutation. Our data showed that most TDRM to PI reverted more rapidly, compared to NRTI mutations.
From a more general perspective our findings have important implications for understanding the epidemic spread of drug-resistant pathogens. One of the general problems with drug resistance is that it can be quickly selected by drug pressure, but upon transmission it reverts only slowly if at all in the absence of drug pressure [32]. The intuition behind this is that drugs cause an enormous reduction in the replicative capacity of wild-type virus and hence lead to a strong relative fitness benefit for resistant mutants. By contrast, the fitness cost in the absence of drugs is typically weak. Our results highlight the large variability in reversion rates and the central role of fitness cost in governing the speed of reversion in the in vivo setting within the SHCS. In particular, they show that the genetic background of a resistance mutation substantially modulates the fitness cost and thereby the reversion rate of the mutation. This implies heritable variation in the fitness cost of resistance and thereby the danger that such fitness costs are reduced by evolutionary selection, i.e. mutations in genetic backgrounds causing lower fitness cost will have larger chances to spread to other patients and hence may dominate the population in the long run. Assessing the impact of the genetic background on reversion rates is central for understanding the spread of antimicrobial resistance in general. For example, theoretical models and in vitro evidence suggest a crucial role of compensatory mutations in boosting antibiotic resistance for a broad range of bacterial pathogens [33]. However, real-world epidemiological evidence for an impact of the genetic backgrounds found in natural pathogen populations on reversion of resistance in patients is largely lacking. In this context our approach offers a proof of principle for using machine learning approaches to bridge the gap between epidemiological data on resistance reversion and in vitro fitness measurements and thereby to address this crucial issue.
In the context of HIV epidemiology in Switzerland, such a scenario of mutation evolution can be probably prevented by the good surveillance and the early treatment of HIV-infected individuals, implying that resistant strains have only limited opportunity to cause new infections and hence to select backgrounds with lower fitness cost. By contrast, this scenario is a very real danger in settings with poorer surveillance and hence ampler opportunities for resistant viruses to spread. In those settings evolution might indeed successfully act on the variation of fitness costs and lead in the long term to resistant viruses with a low fitness cost.
Previous work [18] has assessed fitness costs of some antiretroviral resistance mutations in vitro by site directed mutations (SDM). Since these studies did not consider the impact of different genetic backgrounds, we can only compare the average fitness cost of a mutation determined by our method with the fitness costs determined by SDM. This comparison reveals a good qualitative but not perfect agreement to our estimates with SDM data (as summarized in [18]). Estimates were available in both data sets for the RT mutations 184V, 70R, 41L, 103N, and 215Y; in agreement with [18] we found a high fitness cost for 184V (1.8 standard deviations above mean fitness cost = +1.8s.d) and a moderate fitness cost for 70R, 41L, and 103N (+0.58 s.d., −0.16 s.d., and +0.48 s.d., respectively). In agreement with [18] we also found moderate fitness costs for 210W and 181C (−0.85 s.d. and −0.69 s.d. respectively), which were excluded from our analysis because they lie in HLA epitopes and disrupt binding. The main discrepancy was found for 215Y, where our methods predicted low fitness costs (−0.86 s.d.) in contrast to the SDM data [18]. The fact that reversion rates are high for this mutation indicates that our estimator has underestimated the real fitness cost of this mutation. This failure may be also related to the complexity of the mutational pathways at this position, which may have been oversimplified by our approach (in which we do not distinguish which amino acid a TDRM reverts to). This deviation is also not surprising since the computational predictor underlying our approach is not perfect (42% of deviance in in vitro fitness were explained in [27]). Overall this comparison thus validates our method but also reveals that there is potential for improvement and hence our approach should be best viewed as a proof of principle of using machine-learning approaches in conjunction with in vitro fitness measurements to assess reversion of TDRM in vivo.
This assessment of the fitness predictor is confirmed by considering the quality of fit of the different models summarized in Fig. 2: Starting from an interval-censored survival model without explanatory variables, adding the information of whether a given TDRM is present as a mixture reduces the model deviance by 22%. Adding TDRM-fitness as an explanatory variable reduces the model’s deviance by a further 9%. If we separate fitness cost into the mean fitness cost of a given mutation type and the corresponding residual fitness cost (as in Fig. 2), this 9% results from a 6% of deviance-reduction explained by the mean fitness cost and 3% by the residual fitness cost. This indicates an important role of fitness for TDRM reversion; especially given that, firstly, the fitness predictor used here is not perfect (it explains 42% of deviance of in vitro replicative capacity [27]) and that, secondly, being a mixture implies that a nucleotide has already started to revert and hence the corresponding variable represents a very strong determinant of reversion. Finally, these numbers suggest that the differential fitness-costs of the same mutation in different genetic backgrounds contribute half as much to the population-level variability in reversion than different fitness-costs of different mutations. Given the well-described and strong differences in reversion rates across mutation types this therefore implies an important role of the genetic background. However, these fractions of deviance explained by our predicted fitness costs imply that reversion rates also depend on other factors not captured by in vitro replicative capacity. This includes interactions between host-viral factors such as HLA escape. Even though we excluded TDRMs known to mediate CTL escape (see Methods), it is likely that this does not encompass all such escape mutations or more generally all mutations that affect the interaction of a virus with a given patient’s immune system.
Our study had several limitations. One of the limitations of this study was the lack of information before the first GRT was performed. More specifically, we could not determine how long a TDRM had already persisted before the first GRT. We studied the reversion of TDRM from the baseline GRT instead of the infection date of a patient because an exact infection date was not known for most of the patients and because GRTs at infection time are typically not available. This approach increased the sample size considerably in exchange for missing some TDRM that had reverted before the first GRT was performed. This could explain why K65R or T215F, which are known to revert rapidly, were not identified in our study. The fast reverting TDRM such as M184V were either missed or detected right after the infection by GRT, thus the estimated reversion rates were not altered to a large extent and only the sample size may be lower. Another limitation was that around 40% (67 / 168) of patients carried > 1 TDRM at baseline. Although combinations of mutations could modulate the fitness costs substantially [21], causing that a given mutation has varying fitness costs when having different genetic backgrounds, the number of mutations detected at the first GRT was not found to be associated with the reversion of TDRM [29]. Additionally we adjusted for different genetic backgrounds including the residual fitness costs in our model and still found positive associations of reversion rates with average fitness costs.
In conclusion, our study demonstrated that TDRM showed substantial variation in reversion rates, which were positively associated with the fitness costs these mutations had in their genetic background.
Supporting Information
Zdroje
1. Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. The new England journal of medicine 1998; 338 : 853–860. 9516219
2. von Wyl V, Yerly S, Bürgisser P, Klimkait T, Battegay M, Bernasconi E, et al. Long‐Term Trends of HIV Type 1 Drug Resistance Prevalence among Antiretroviral Treatment–Experienced Patients in Switzerland. Clin Infect Dis 2009; 48 : 979–987. doi: 10.1086/597352 19228107
3. Vercauteren J, Wensing AM, van de Vijver DA, Albert J, Balotta C, Hamouda O, et al. Transmission of Drug‐Resistant HIV‐1 Is Stabilizing in Europe. J Infect Dis 2009; 200 : 1503–1508. doi: 10.1086/644505 19835478
4. Yerly S, Vora S, Rizzardi P, Chave JP, Vernazza PL, Flepp M, et al. Acute HIV infection: impact on the spread of HIV and transmission of drug resistance. AIDS 2001; 15 : 2287–2292. 11698702
5. Yerly S, von Wyl V, Ledergerber B, Böni J, Schüpbach J, Bürgisser P, et al. Transmission of HIV-1 drug resistance in Switzerland: a 10-year molecular epidemiology survey. AIDS 2007; 21 : 2223–2229. 18090050
6. Little SJ. Transmission and prevalence of HIV resistance among treatment-naive subjects. Antiviral Therapy 2000;
7. Jakobsen MR, Tolstrup M, Søgaard OS, Jørgensen LB, Gorry PR, Laursen A, et al. Transmission of HIV-1 drug-resistant variants: prevalence and effect on treatment outcome. Clin Infect Dis 2010; 50 : 566–573. doi: 10.1086/650001 20085464
8. Wensing AMJ, van de Vijver DA, Angarano G, Asjö B, Balotta C, Boeri E, et al. Prevalence of drug-resistant HIV-1 variants in untreated individuals in Europe: implications for clinical management. J Infect Dis 2005; 192 : 958–966. 16107947
9. Manasa J, Katzenstein D, Cassol S, Newell M-L, de Oliveira T. Southern Africa Treatment And Resistance Network (SATuRN). Primary drug resistance in South Africa: data from 10 years of surveys. AIDS Res Hum Retroviruses 2012; 28 : 558–565. doi: 10.1089/AID.2011.0284 22251009
10. Aghokeng AF, Kouanfack C, Laurent C, Ebong E, Atem-Tambe A, Butel C, et al. Scale-up of antiretroviral treatment in sub-Saharan Africa is accompanied by increasing HIV-1 drug resistance mutations in drug-naive patients. AIDS 2011; 25 : 2183–2188. doi: 10.1097/QAD.0b013e32834bbbe9 21860346
11. Bennett DE, Camacho RJ, Otelea D, Kuritzkes DR, Fleury H, Kiuchi M, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS ONE. 2009; 4:e4724. doi: 10.1371/journal.pone.0004724 19266092
12. von Wyl V, Yerly S, Böni J, Bürgisser P, Klimkait T, Battegay M, et al. Emergence of HIV-1 drug resistance in previously untreated patients initiating combination antiretroviral treatment: a comparison of different regimen types. Arch. Intern. Med. 2007; 167 : 1782–1790. 17846398
13. Little SJ, Frost SD, Wong JK, Smith DM, Pond SL, Ignacio CC, et al. Persistence of Transmitted Drug Resistance among Subjects with Primary Human Immunodeficiency Virus Infection. Journal of Virology 2008; 82 : 5510–5518. doi: 10.1128/JVI.02579-07 18353964
14. Metzner KJ, Scherrer AU, Preiswerk B, Joos B, von Wyl V, Leemann C, et al. Origin of Minority Drug-Resistant HIV-1 Variants in Primary HIV-1 Infection. J Infect Dis 2013; 208 : 1102–1112. doi: 10.1093/infdis/jit310 23847055
15. Jain V, Sucupira MC, Bacchetti P, Hartogensis W, Diaz RS, Kallas EG, et al. Differential Persistence of Transmitted HIV-1 Drug Resistance Mutation Classes. J Infect Dis 2011; 203 : 1174–1181. doi: 10.1093/infdis/jiq167 21451005
16. Metzner KJ, Leemann C, Di Giallonardo F, Grube C, Scherrer AU, Braun D, et al. Reappearance of Minority K103N HIV-1 Variants after Interruption of ART Initiated during Primary HIV-1 Infection. PLoS ONE 2011;
17. Barbour JD, Hecht FM, Wrin T, Liegler TJ, Ramstead CA, Busch MP, et al. Persistence of primary drug resistance among recently HIV-1 infected adults. AIDS 2004; 18 : 1683–1689. 15280779
18. Martinez-Picado J, Martínez MA. HIV-1 reverse transcriptase inhibitor resistance mutations and fitness: A view from the clinic and ex vivo. 2008; 134 : 104–123. doi: 10.1016/j.virusres.2007.12.021 18289713
19. Wittkop L, Günthard HF, de Wolf F, Dunn D, Cozzi-Lepri A, de Luca A, et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. The Lancet Infectious Diseases 2011; 11 : 363–371. doi: 10.1016/S1473-3099(11)70032-9 21354861
20. Grant RM, Hecht FM, Warmerdam M, Liu L, Liegler T, Petropoulos CJ, et al. Time Trends in Primary HIV-1 Drug Resistance Among Recently Infected Persons. JAMA 2002; 288 : 181–188. 12095382
21. Cong M-E, Heneine W, García-Lerma JG. The fitness cost of mutations associated with human immunodeficiency virus type 1 drug resistance is modulated by mutational interactions. Journal of Virology 2007; 81 : 3037–3041. 17192300
22. Little SJ, Holte S, Routy J-P, Daar E, Markowitz M, Collier A, et al. Antiretroviral-drug resistance among patients recently infected with HIV. The New England Journal of mMedicine 2002; 347 : 385–394. 12167680
23. Hirsch MS, Günthard HF, Schapiro JM, Vezinet F-B, Clotet B, Hammer S, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2008; 47 : 266–285. doi: 10.1086/589297 18549313
24. Swiss HIV Cohort Study. Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol 2010; 39 : 1179–1189. doi: 10.1093/ije/dyp321 19948780
25. Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, Battegay M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet 1999; 353 : 863–868. 10093977
26. Gunthard HF, Wong JK, Ignacio CC, Havlir DV, Richman DD. Comparative performance of high-density oligonucleotide sequencing and dideoxynucleotide sequencing of HIV type 1 pol from clinical samples. AIDS Res Hum Retroviruses 1998; 14 : 869–876. 9671215
27. Hinkley T, Martins J, Chappey C, Haddad M, Stawiski E, Whitcomb JM, et al. A systems analysis of mutational effects in HIV-1 protease and reverse transcriptase. Nature Genetics 2011; 43 : 487–489. doi: 10.1038/ng.795 21441930
28. Kouyos RD, von Wyl V, Hinkley T, Petropoulos CJ, Haddad M, Whitcomb JM, et al. Assessing predicted HIV-1 replicative capacity in a clinical setting. PLoS Pathog. 2011; 7:e1002321. doi: 10.1371/journal.ppat.1002321 22072960
29. Castro H, Pillay D, Cane P, Asboe D, Cambiano V, Phillips A, et al. Persistence of HIV-1 transmitted drug resistance mutations. J Infect Dis 2013; 208 : 1459–1463. doi: 10.1093/infdis/jit345 23904291
30. Yerly S, Rakik A, De Loes SK, Hirschel B, Descamps D, Brun-Vézinet F, et al. Switch to unusual amino acids at codon 215 of the human immunodeficiency virus type 1 reverse transcriptase gene in seroconvertors infected with zidovudine-resistant variants. Journal of Virology 1998; 72 : 3520–3523. 9557630
31. Garcia-Lerma JG, Nidtha S, Blumoff K, Weinstock H, Heneine W. Increased ability for selection of zidovudine resistance in a distinct class of wild-type HIV-1 from drug-naive persons. Proc. Natl. Acad. Sci. U.S.A. 2001; 98 : 13907–13912. 11698656
32. Wiesch zur PA, Kouyos RD, Engelstädter J, Regoes RR, Bonhoeffer S. Population biological principles of drug-resistance evolution in infectious diseases. The Lancet Infectious Diseases 2011; 11 : 236–247. doi: 10.1016/S1473-3099(10)70264-4 21371657
33. Andersson DI, Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat. Rev. Microbiol. 2010; 8 : 260–271. doi: 10.1038/nrmicro2319 20208551
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





