-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIdentification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
Experimental evidence has established that bacteria do not always exist as isolated single celled organisms, but instead communicate with each other through the secretion of small molecules that enable individual cells to coordinate complex traits and behaviors. Gram-positive bacteria rely on the secretion of small peptide pheromones to coordinate activities that include biofilm formation, exogenous DNA uptake via competence mechanisms, conjugal transfer of plasmid DNA, and expression of gene products that promote bacterial virulence. Here we provide evidence of a novel use of bacterial peptide pheromone signaling, that being the use of the pPplA peptide by L. monocytogenes to detect the confines of host cell vacuoles. Secretion and import of pPplA is required for efficient escape of L. monocytogenes from its initial membrane-bound host cell compartment, and bacteria lacking the peptide pheromone are severely attenuated for virulence in mice. The pPplA peptide pheromone is thus used by individual bacteria within a confined membrane bound space to coordinate the expression of gene products required by L. monocytogenes for intracellular growth and survival.
Published in the journal: . PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004707
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004707Summary
Experimental evidence has established that bacteria do not always exist as isolated single celled organisms, but instead communicate with each other through the secretion of small molecules that enable individual cells to coordinate complex traits and behaviors. Gram-positive bacteria rely on the secretion of small peptide pheromones to coordinate activities that include biofilm formation, exogenous DNA uptake via competence mechanisms, conjugal transfer of plasmid DNA, and expression of gene products that promote bacterial virulence. Here we provide evidence of a novel use of bacterial peptide pheromone signaling, that being the use of the pPplA peptide by L. monocytogenes to detect the confines of host cell vacuoles. Secretion and import of pPplA is required for efficient escape of L. monocytogenes from its initial membrane-bound host cell compartment, and bacteria lacking the peptide pheromone are severely attenuated for virulence in mice. The pPplA peptide pheromone is thus used by individual bacteria within a confined membrane bound space to coordinate the expression of gene products required by L. monocytogenes for intracellular growth and survival.
Introduction
It has become increasingly apparent that bacteria do not simply exist as isolated, single celled organisms but instead have evolved a variety of communication systems that enable them to interact with other bacterial cells within a population. Bacterial cell-to-cell communication occurs through the secretion and sensing of small signal molecules that coordinate complex behaviors such as light emission, biofilm formation, DNA uptake and conjugation, bacteriocin synthesis, and virulence factor secretion [1–4]. For Gram positive bacteria, one important method of communication involves the synthesis of small peptide pheromones that are secreted by bacterial cells and trigger changes within the same cell or in receiver cells either via peptide import into the receiver or by stimulating membrane receptors that initiate signaling cascades within the receiver cell [1,3]. Four related but distinct peptide pheromone signaling systems have been described, and these include cyclical peptides exemplified by th e Agr signaling system, sensory systems of the RNPP family, signaling via peptides that contain Gly-Gly processing motifs, and peptides associated with the Rgg-like family of peptide-binding proteins that regulate gene expression [1].
Limited information has thus far been available regarding the use of peptide pheromone sensing to coordinate cellular functions of the Gram-positive environmental pathogen Listeria monocytogenes [5–9]. This bacterium survives as a saprophyte in soil but is capable of transitioning into life as an intracellular pathogen following ingestion by susceptible mammalian hosts [10–15]. Consumption of L. monocytogenes contaminated food products is the primary route of exposure in humans [16–20]; this normally leads to mild gastroenteritis in healthy individuals but can manifest into more serious invasive disease and even death in those who are immunocompromised, such as the elderly, individuals with HIV, chemotherapy and transplant patients, and in pregnant women, where infections can lead to stillbirths [12,15,21]. The ability for L. monocytogenes to cause disease in a mammalian host depends upon the expression of a number of gene products that enable L. monocytogenes to gain entry into host cells, escape from host cell vacuoles, replicate within the cytosol, and spread to infect adjacent cells [22–24]. Gene products contributing to host infection are up-regulated following bacterial entry into the host however the signal(s) that governs the induction of gene products associated with bacterial virulence remains unclear. It has been postulated that L. monocytogenes senses environmental cues that inform the bacterium as to its intracellular location [13].
One key factor that coordinates the transition of L. monocytogenes from a soil dweller into an intracellular pathogen is the central virulence regulatory protein, PrfA [10,13,25]. PrfA is a 27 kD transcriptional activator that regulates the expression of the majority of L. monocytogenes gene products associated with bacterial virulence [25–27]. It is a member of the cAMP receptor protein (Crp)-Fnr family of transcriptional regulators, many of which require the binding of a small-molecule cofactor for full activity [28–30]. PrfA regulates the expression of gene products that include those contributing to bacterial entry into non-professional phagocytic cells (InlA and InlB); those that mediate the perforation and disruption of host cell vacuoles [listeriolysin O (LLO) and the phospholipases PlcA and PlcB]; a protease that processes proPlcB to its active form (Mpl); a bacterial surface protein that directs host cell actin polymerization for bacterial movement (ActA); a hexose phosphate transporter that contributes to cytosolic growth (Hpt); and PrsA2, a post-translocation secretion chaperone that contributes to the folding and activity of L. monocytogenes secreted virulence factors [26,27]. PrfA activation and the induction of virulence gene expression occurs following bacterial entry into host cells, but the identity of the small molecule cofactor that is thought to trigger PrfA activation has not yet been determined. However, constitutively activated forms of the protein, known as PrfA* mutants, have been isolated and have been shown to have activity comparable to the fully activated wild type form that occurs within infected host cells [31–37].
We have examined changes in the profiles of secreted L. monocytogenes proteins following PrfA activation via prfA* mutations [38], and have recently identified a lipoprotein encoded by lmo2637 whose secretion is increased in prfA* strains and that shares homology with a peptide pheromone-encoding lipoprotein whose associated pheromone regulates conjugal plasmid transfer in Enterococcus faecalis [39]. The Lmo2637 lipoprotein, or PplA (peptide pheromone-encoding lipoprotein A) is most homologous to the E. faecalis Cad lipoprotein; proteolytic processing of the Cad N-terminal secretion signal sequence gives rise to the cAD1 peptide that is imported into receiver cells and stimulates the expression of a number of gene products that result in bacterial aggregation and plasmid conjugal transfer [39–43]. Examination of the L. monocytogenes genome reveals a number of potential gene products that share homology with E. faecalis proteins that are required for peptide pheromone processing, import, and transcriptional regulation. Here we describe the functional analysis of the pPplA peptide pheromone in L. monocytogenes and its contributions to intracellular growth and bacterial virulence.
Results
Investigation of PplA as a peptide pheromone-encoding lipoprotein whose secretion is increased following PrfA activation
Mutational activation of the virulence regulatory protein PrfA (PrfA*) results in an increase in the abundance of a number of L. monocytogenes secreted proteins during growth in broth culture, including a gene product encoded by lmo2637 [38], also known as lmrg_02182 in strain 10403S. Lmo2637 is a putative peptide pheromone-encoding lipoprotein that shares significant homology over its entire sequence (50% identity, 66% similarity) with the E. faecalis Cad lipoprotein and its associated peptide pheromone cAD1 [39,43] (Fig. 1A). The functional role of the Cad lipoprotein is not known, however signal peptidase II-directed cleavage of the Cad N-terminal secretion signal sequence releases a 22 amino acid polypeptide that is further processed down to the eight amino acid peptide pheromone cAD1 [43]. cAD1 is imported via a peptide transport system into an E. faecalis receiver cell, where it binds to the TraA transcriptional repressor to relieve repression and stimulate expression of TraE1 [20,39,44]. TraE1 induces the expression of gene products that contribute to bacterial aggregation and conjugal transfer of plasmids into cAD1-peptide producer cells. In addition to Lmo2637, predicted gene products with homology to other E. faecalis components of the cAD1 pathway are also present, including one homologue of the Eep protease involved in the processing of cAD1 (Lmo1318, 50% identity and 66% similarity), two peptide transport systems (OppA and CtaP), and Lmo0833, a protein of which the first 60 amino acids are 34% identical and 49% similar to the N terminal DNA binding domain of the TraA repressor, while the C terminal region shares homology with Rgg peptide binding regulatory proteins (20% identity, 44% similarity to Streptococcus pyogenes Rggs). The Listeria genome encodes four additional Rgg-like proteins that all appear to have an N terminal DNA binding domain, however TraA shares the most homology with Lmo0833. In addition, the Listeria genome encodes a gene product that shares homology with the TraE1 activator, Lmo0618. Lmo0618 encodes a predicted protein of 380 amino acids, of which the first 115 are 24% identical and 45% similar to the entire 118 amino acid TraE1 protein, while the remaining C terminal portion of Lmo0618 contains a DUF872 domain, a conserved eukaryotic domain of unknown function.
Fig. 1. L. monocytogenes secretes a PrfA-induced lipoprotein that shares homology with Enterococcus faecalis Cad and its N-terminal encoded peptide-pheromone. 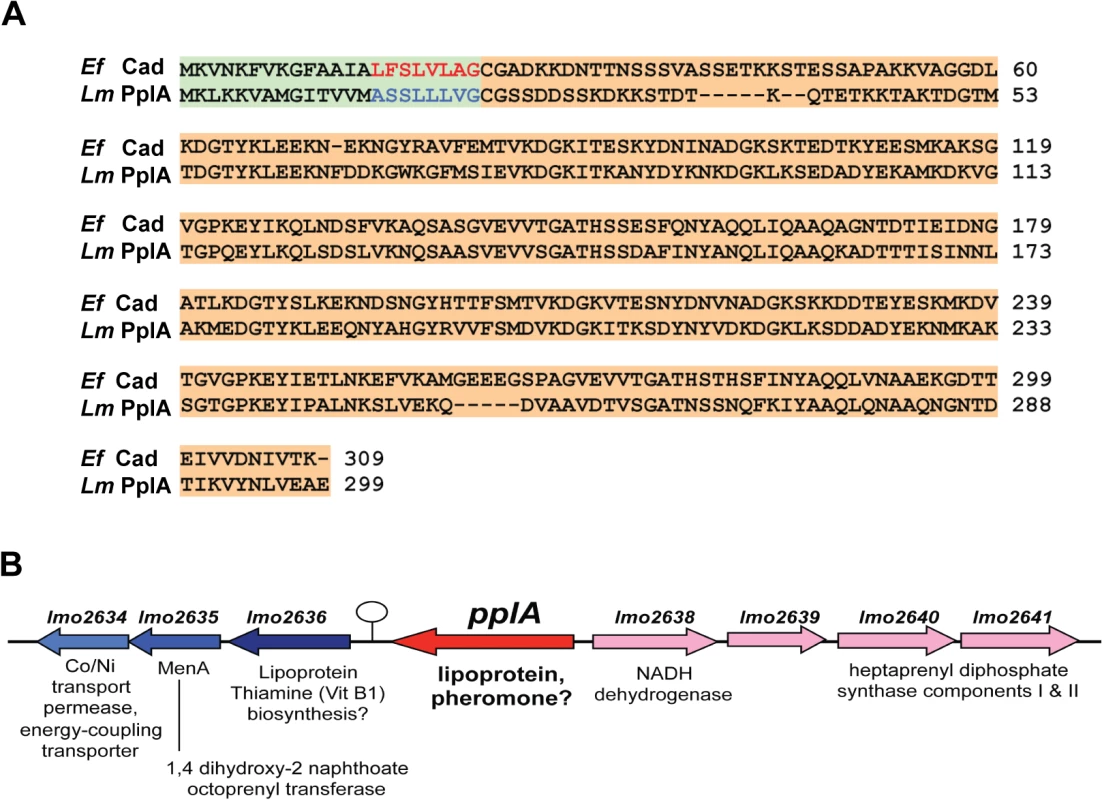
(A) Protein alignment of E. faecalis Cad to L. monocytogenes Lmo2637 (PplA) using ClustalW2 software program (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The signal sequence region encoding the cAD1 peptide-pheromone and the predicted pPlpA peptide are respectively highlighted in green and red for Ef (E. faecalis) or blue for Lm (L. monocytogenes), and the lipoprotein portion of the proteins is highlighted in tan. (B) Gene organization of the pplA coding region. The presence of a putative transcriptional terminator downstream of pplA is indicated by the circle with stem. Based on its shared homology with the Cad lipoprotein and cAD1 peptide pheromone, we have designated lmo2637 as pplA for peptide pheromone-encoding lipoprotein A, encoding the mature lipoprotein PplA and the putative peptide pheromone pPplA. pplA is divergently transcribed relative to its neighbor lmo2638, which encodes a predicted NADH dehydrogenase (Fig. 1B). Within this region are genes that are predicted to encode subunits involved in the biosynthesis of menaquinone-7, a Vitamin K2 derivative and a thiamine (Vitamin B1) biosynthesis lipoprotein, a gene product similar to the E. coli MenA protein which is also involved in Vitamin K synthesis, a predicted membrane Co/Ni transport permease gene product similar to B. subtilis YbaF, and a large number of genes encoding both small and large ribosomal subunit proteins.
Peptide-pheromones secreted by E. faecalis coordinate conjugal plasmid transfer and contribute to bacterial virulence [1,39,45]. Given that the secretion of the PplA lipoprotein was increased following PrfA activation and that the majority of gene products associated with PrfA regulation contribute to bacterial virulence, we sought to investigate the function of the PplA lipoprotein and/or the pPplA peptide in L. monocytogenes physiology and mammalian infection. To differentiate between peptide pheromone secretion and production of the lipoprotein, two mutant constructs were generated: an in-frame deletion of the entire pplA coding sequence as well as a pplA mutant containing a stop codon introduced 50 amino acids downstream of the signal sequence cleavage site (G72STOP, Fig. 2A). To facilitate the transfer of the pplA deletion into different genetic backgrounds, the ermB gene encoding resistance to erythromycin was introduced in place of pplA coding sequences to enable the generation of pplA deletion mutants via phage transduction. The pplA mutant containing the engineered stop codon 50 amino acids downstream of the signal sequence cleavage site (named pplA-G72STOP) was designed so as to express only the peptide-pheromone and not the lipoprotein portion of PplA. A related mutation within E. faecalis Cad has been shown to truncate the lipoprotein while still enabling cAD1 peptide secretion [43]. In addition, an in-frame deletion of eep (lmo1318, encoding a predicted membrane-bound protease) was constructed to determine if Eep had any functional role associated with the proteolytic processing of the PplA N terminal signal sequence into mature pPplA peptide.
Fig. 2. Construction of L. monocytogenes mutants that lack the PplA lipoprotein but retain peptide pheromone secretion. 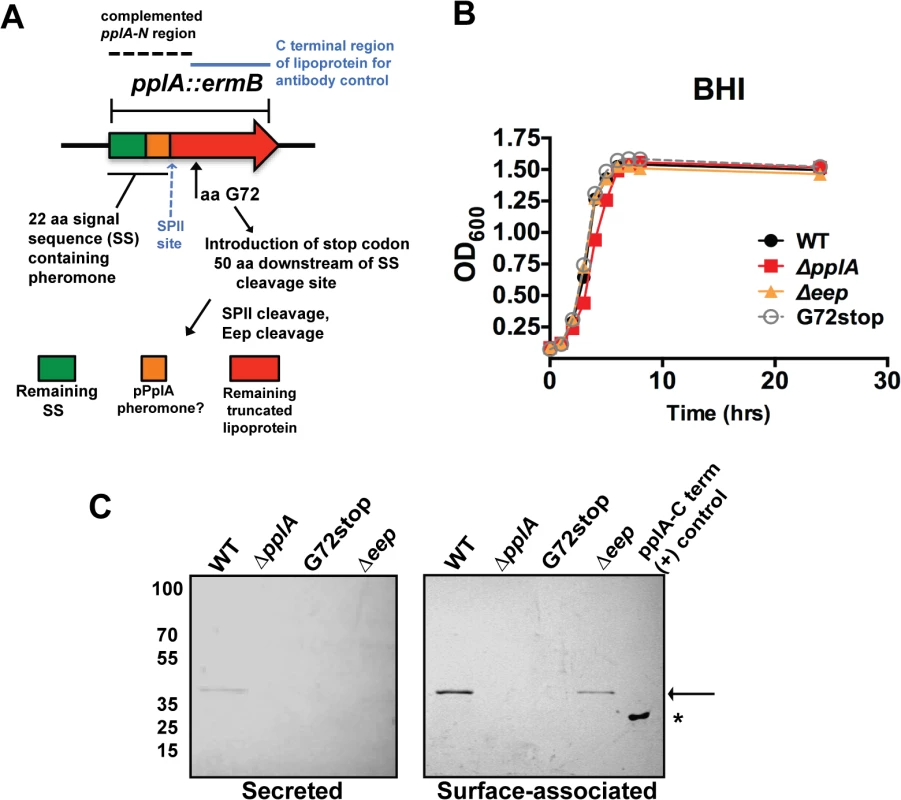
(A) Strategy for the construction of the in-frame pplA deletion and pplA-G72stop mutants that retain peptide pheromone secretion. The region used for complementation of the pplA deletion is indicated by the dashed lines. The C terminal lipoprotein region of PplA expressed and purified from E.coli that was used for affinity purification of the PplA antibody and also as the (+) control in western blots is indicated by the blue solid line. (B) Assessment of growth of the lipoprotein and pheromone mutants in BHI broth culture media. Overnight cultures of each strain grown shaking in BHI at 37°C were diluted 1:20 in fresh BHI media and the OD600 was measured at the indicated time points. (C) Western blot analysis of both surface-associated and secreted PlpA lipoprotein isolated from stationary phase cultures grown overnight shaking at 37°C. Samples were normalized to OD600. Secreted PplA was TCA extracted from the culture supernatant and surface-associated PplA was isolated by boiling in SDS-boiling buffer. Arrow indicates the position of full length PplA. ‘*’ indicates truncated and purified PplA lipoprotein used as positive control for antibody recognition. PplA lipoprotein is primarily detected in the surface-associated preparations versus the supernatant of wild-type L. monocytogenes. For panels (B) and (C), data is representative of at least three-independent experiments. All mutants exhibited normal patterns of growth in BHI media (Fig. 2B) indicating that neither the lipoprotein, the lipoprotein and associated pheromone, or the Eep protease were required for growth in broth culture. Using an antibody directed against the C-terminal region of the PplA lipoprotein, western blot analysis confirmed the secretion of the lipoprotein in both surface-associated and secreted protein preparations derived from the wild-type strain 10403S (Fig. 2C). The PplA lipoprotein was not detected in protein preparations isolated from the ΔpplA mutant or the pplA-G72STOP mutant (Fig. 2C). The PplA lipoprotein appeared more abundant in cell associated fractions versus bacterial supernatants (Fig. 2C, S1A Fig.). Both cell associated and secreted activities of Enterococcal lipoprotein-derived peptide-pheromones have been reported [46]. The presence of the PplA lipoprotein appeared modestly reduced in the Δeep mutant, suggesting that Eep protease may directly or indirectly contribute to the stability of the mature PplA lipoprotein but is not absolutely required for PplA secretion.
The E. faecalis cAD1 peptide pheromone induces a mating response in plasmid-containing donor cells to stimulate conjugal transfer of plasmids to the recipient cells [40,47]. Production of a pheromone-induced aggregation substance (Asa1) on the cell surface of plasmid-containing cells enhances bacterial aggregation and conjugal transfer [48,49]. E. faecalis aggregation has been used as the basis for assays designed to detect the relative abundance of peptide-pheromone in culture supernatants from recipient cells or to test the activity of exogenously added synthetic peptides [40,47]. We and others have previously noted that prfA* cultures exhibit bacterial settling and/or aggregation when bacterial cultures are allowed to sit without shaking [37,50]. The physiological significance of this phenotype is not clear, although it has been recently reported that bacterial aggregation of prfA* strains is mediated by the surface protein ActA and contributes to persistence of intestinal colonization [50].
To determine whether the pPplA pheromone contributed to bacterial aggregation of prfA* strains in broth culture, the ΔpplA::erm mutation was introduced into the prfA* L140F genetic background via phage transduction, and bacterial aggregation was determined by measuring aggregation-associated decreases in the optical density (Fig. 3B). Bacterial cultures were grown overnight at 37°C in BHI with shaking followed by static incubation at room temperature. prfA*L140F strains formed bacterial aggregates at the bottom of culture tubes after 24 hours of static incubation at room temperature, whereas the wild-type 10403S cultures remained in suspension (Fig. 3A). Interestingly, the prfA* ΔpplA::erm mutant exhibited reduced aggregation when compared to the parental prfA* strain (Fig. 3B). Although the ActA protein has been reported to contribute to the aggregation of prfA* cultures, the levels of ActA protein associated with the prfA* ΔpplA::erm mutant compared to the parental prfA*L140F strain were similar (S2A Fig.), suggesting that the presence of ActA was not sufficient for mediating aggregation of the prfA* ΔpplA::erm mutant. Introduction of the prfA* allele into strains containing the pplA-G72STOP mutation resulted in aggregation patterns that were similar to the prfA* L140F strain (Fig. 3B). Spent culture media derived from a pheromone-producing strain (pplA-G72STOP), but not from ΔpplA::erm, could restore bacterial settling of the prfA* ΔpplA::erm mutant, consistent with the presence of a secreted substance derived from the N-terminal region of PplA enhancing bacterial aggregation in broth culture (Fig. 3C). Spent media treated with protease K did not restore bacterial aggregation to prfA* ΔpplA::erm cultures (S2B Fig.).
Fig. 3. pPplA enhances bacterial aggregation in broth culture. 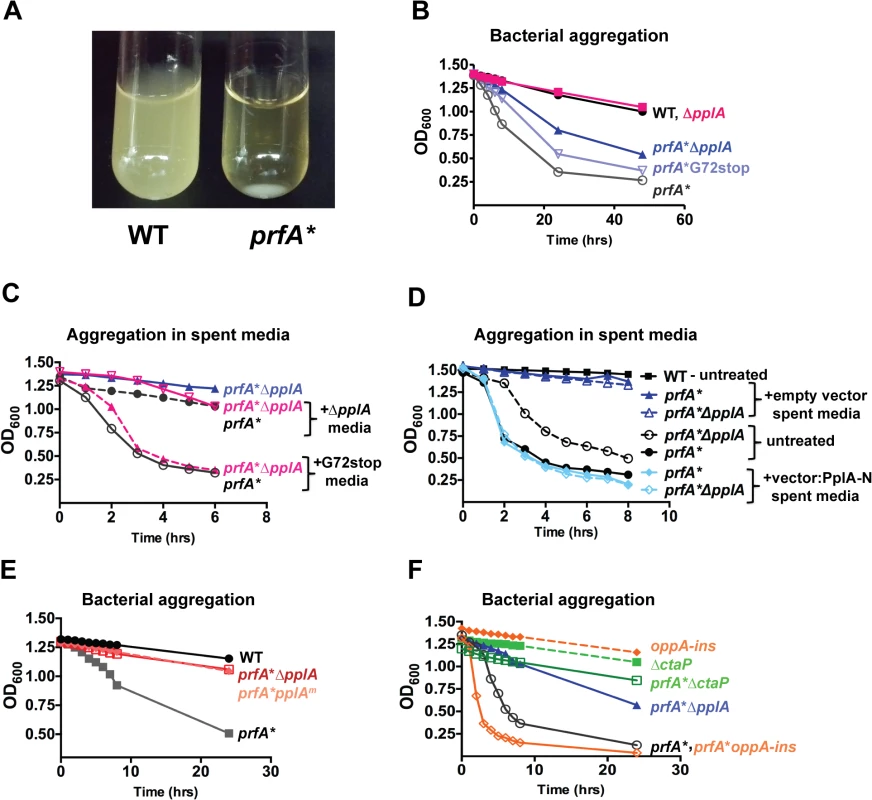
(A) Image of bacterial aggregation observed between the wild-type L. monocytogenes 10403S strain versus a prfA* mutant when bacterial cultures grown in BHI are left statically overnight at room-temperature (RT). (B) Measurement of the rate of bacterial aggregation in BHI. The optical-density at 600nm was monitored at the indicated time points for 1 mL of an overnight culture initially grown in BHI with shaking at 37°C then left statically at RT, where bacterial aggregation is measured as the decrease in the optical-density of the culture supernatant as the bacterial aggregate out of solution. (C) Measurement of bacterial aggregation of the indicated mutant strains resuspended in 1 mL of spent media derived from overnight stationary phase cultures either containing the pPplA peptide (G72stop) or lacking it (ΔpplA). The ability of the pPplA containing media (G72stop) to restore bacterial aggregation indicates the presence of a secreted substance (potentially pPplA) that enhances bacterial aggregation in broth culture. (D) Measurement of bacterial aggregation as done in panel C, except strains were resuspended in 1 mL of BHI spent media derived from E. coli containing the complementation vector construct expressing the N-terminal 72 amino acids of PplA as described in Fig. 2A or the empty vector. The ability of spent media derived from an E.coli strain containing the first 72 amino acids of pPplA supports the secretion of a PplA N-terminus derived peptide that enhances bacterial aggregation. (E) Assessment of bacterial aggregation in a strain containing three amino acid substitutions within the predicted peptide sequence (referred to as prfA*pplAm). Reduced aggregation of prfA*pplAm indicates the importance of these amino acids within the pPplA pheromone. (F) Bacterial aggregation of two oligopeptide transport mutants, a prfA* ΔctaP compared to a prfA*-oppA insertion mutant. A prfA* ΔctaP is impaired for bacterial aggregation, indicating a possible link between the pPplA peptide and import of the peptide through the CtaP transport system. For panels (B), (C) and (D), data is representative of at least three independent experiments. It has been previously shown that the expression of an E. faecalis N-terminal peptide pheromone coding region in Escherichia coli resulted in active pheromone secreted into the E. coli supernatant [51,52]. We therefore examined whether spent media derived from an E.coli strain containing a plasmid vector encoding the first 72 amino acids of the N-terminal region of PplA could restore aggregation to L. monocytogenes prfA* ΔpplA strains. Supernatants derived from E. coli containing the plasmid encoding pplA-G72STOP restored aggregation to L. monocytogenes prfA* ΔpplA strains whereas supernatants derived from E. coli containing the plasmid vector alone did not (Fig. 3D). In addition, L. monocytogenes chromosomal mutants encoding the substitution of three residues within the predicted active peptide region of pPplA (ASSLLLVG to ASATLAVG, pplAm mutants) were observed to have reduced bacterial aggregation (Fig. 3E) while still maintaining secretion of the PplA lipoprotein (S1B Fig.). Confirmation of PplA secretion was important in this experiment given that the L20A substitution occurred within the conserved lipobox motif recognized by SPII [53] however sufficient conservation was retained to allow SPII cleavage and PplA secretion. Taken together, these results are consistent with the existence of a secreted pPplA peptide pheromone derived from the N-terminal secretion sequence of the PplA lipoprotein.
The cAD1 signaling peptide of E. faecalis is actively transported into recipient cells via the TraC oligopeptide transport system to stimulate the induction of gene products required for plasmid conjugation [39,54]. L. monocytogenes is predicted to encode five oligopeptide transport systems, two of which encode ABC transporter systems that are involved in the uptake of oligopeptides and three of which import di - and tri-peptides [55–58]. The oligopeptide OppA transport system (lmo2196) transports peptides that are 4–8 amino acids in length and is required for bacterial growth at low temperatures and for intracellular survival at early stages of infection in cultured macrophages and in mice [55]. The CtaP-associated system is an oligopeptide ABC transport system that also functions for high-affinity cysteine transport [58]. Oligopeptide-binding proteins associated with ABC transport systems have been reported to have either broad-specificity for peptide substrates or high sequence specificity [45,55,56,59]. We therefore examined whether the OppA or CtaP oligopeptide transport systems contributed to the transport of the pPplA pheromone-peptide by generating loss of function mutations within oppA and ctaP and examining patterns of bacterial aggregation in broth culture. prfA* oppA mutants exhibited patterns of bacterial aggregation that were similar to those of prfA*L140F strains, whereas the prfA* ΔctaP mutant exhibited no bacterial aggregation (Fig. 3F). Production and secretion of the PplA lipoprotein was not impaired in the ΔctaP mutant, although more lipoprotein was detected in the culture supernatant in comparison to cell associated proteins; this may be a reflection of changes surface hydrophobicity noted previously for this mutant (S1B Fig.) [58]. These results suggest that the CtaP ABC transporter system may be involved in the import of the pPplA pheromone into the bacterial cell to stimulate bacterial aggregation following PrfA activation.
pPplA, but not the PplA lipoprotein, is required for bacterial virulence within mice
The secretion of the PplA lipoprotein is increased following PrfA activation [38]. Given that a number of gene products directly or indirectly regulated by PrfA are known to contribute to bacterial virulence, we examined the phenotypes of mutant strains lacking the PplA lipoprotein, the pheromone and lipoprotein, and the Eep protease in a mouse infection model. Six to 8-week old female Swiss Webster mice were intravenously infected with 2 x104 colony forming units of either wild-type 10403S, ΔpplA::erm, pplA-G72STOP, Δeep, or the ΔpplA::erm mutant strain complemented with either the entire pplA open-reading frame or the N-terminal 72 amino acids encoded by pplA using the pIMK2 complementation vector, which integrates in single copy at a neutral tRNAArg site within the L. monocytogenes chromosome [35]. At three days post-infection the livers and spleens of infected mice were harvested to determine bacterial burdens. The ΔpplA::erm mutant was severely attenuated for virulence as mice infected with the mutant exhibited bacterial burdens that were two to three logs lower in comparison to mice infected with the wild-type strain (Fig. 4A). The Δeep mutant was also significantly reduced for virulence in mice with bacterial burdens that were approximately one log lower in comparison to animals infected with wild-type L. monocytogenes (Fig. 4A). As Δeep strains are less attenuated than ΔpplA::erm strains, this suggests that Eep may contribute to pPplA processing but is not absolutely required. In contrast, the pplA-G72STOP mutant was fully virulent and exhibited no defects in colonization or replication within the liver or the spleen. Consistent with this result, the complemented ΔpplA::erm mutant containing either the entire pplA ORF or, most importantly, just the coding region for the N-terminal 72 amino acids fully complemented the virulence defect associated with the loss of pplA (Fig. 4B). These results strongly support a role for the N-terminal peptide pheromone of pplA (and not the PplA lipoprotein) in L. monocytogenes pathogenesis within the host.
Fig. 4. pPplA significantly contributes to bacterial virulence in vivo. 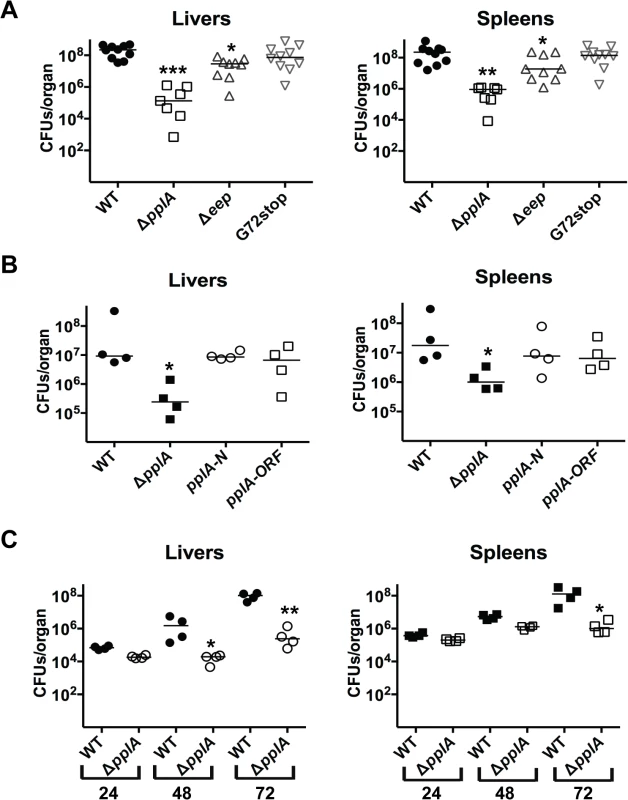
(A) Swiss Webster female mice were intravenously inoculated with 2 x 104 CFUs through the tail vein, and the livers and spleens were harvested 72 hours post-infection (p.i.) and homogenized to determine bacterial burdens. (B) Same as panel (A) except mice were also infected with the ΔpplA mutant complemented with either N-terminal of pplA (pplA-N) or the entire ORF (pplA-ORF). (C) Same as in panel (A), except the livers and spleens were harvested at 24, 48 and 72 hours p.i. to enable a comparison of bacterial burdens over time. Each datum point represents one mouse, and the solid horizontal line denotes the median for each data group. Data was obtained from two independent experiments. Asterisks indicated statistical significance of mutant strains in comparison to wild type L. monocytogenes using an unpaired two-tail student t-test (GraphPad Prism V.5.0A). *p≤0.05, **p≤0.005, and ***p≤0.0005. Given that a virulence defect was detected for the pplA deletion mutant at three days post-infection, we sought to determine whether the mutant was defective for initial organ colonization, or whether the mutant was capable of colonizing host tissues but defective for replication. Female Swiss Webster mice were infected intravenously with wild-type L. monocytogenes or the ΔpplA mutant, and livers and spleens were harvested at 24, 48 and 72 hours post-infection. At 24 hours post-infection, the bacterial burdens detected within the livers and spleens were similar for mice infected with either wild-type 10403S or the ΔpplA mutant, indicating that the ΔpplA mutant demonstrated no significant defect in its ability to establish infections in target organs (Fig. 4C). However, differences in bacterial burdens became evident at 48 hours and increased by 72 hours post-infection, suggesting that the ΔpplA mutant was either defective for replication within tissues and/or cleared more rapidly by the host immune system.
The pPplA pheromone enhances cytosolic bacteria replication within mammalian host cells
The significant virulence attenuation observed for ΔpplA but not pplA-G72STOP mutant strains was consistent with a role for pPplA in L. monocytogenes pathogenesis. We next examined the ability of mutants lacking pPplA to invade, replicate, and spread within host cell monolayers. No discernable defects were detected for mutant strains with respect to the invasion of PtK2 epithelial cells (Fig. 5A). However, the ΔpplA mutant exhibited a significant delay in intracellular replication following cell entry but by 24 hours post-infection reached intracellular numbers that were similar to those observed for wild type bacteria (Fig. 5A). In contrast to ΔpplA, the pplA-G72STOP mutant and the ΔpplA strains complemented with either the full length pplA gene or just the N-terminal first 72 amino acids exhibited patterns of intracellular growth that were indistinguishable from cells infected with wild-type L. monocytogenes (Fig. 5AB). Similarly, the ΔpplA mutant exhibited a delay in cell-to-cell spread based on the formation of small zones of clearing or plaques in L2 fibroblast monolayers, whereas the pplA-G72STOP mutant exhibited normal patterns of plaque formation (S3A and S3B Fig.). Interestingly, the Δeep mutant displayed no significant defects for intracellular growth despite being attenuated for virulence (Fig. 5A). These data suggest that while the pPplA peptide pheromone is required for optimal bacterial replication within infected host cells, limited processing of the PplA N-terminal signal sequence-derived peptide may occur through the action of other proteases during intracellular infection.
Fig. 5. pPplA enhances L. monocytogenes vacuolar escape in host cells. 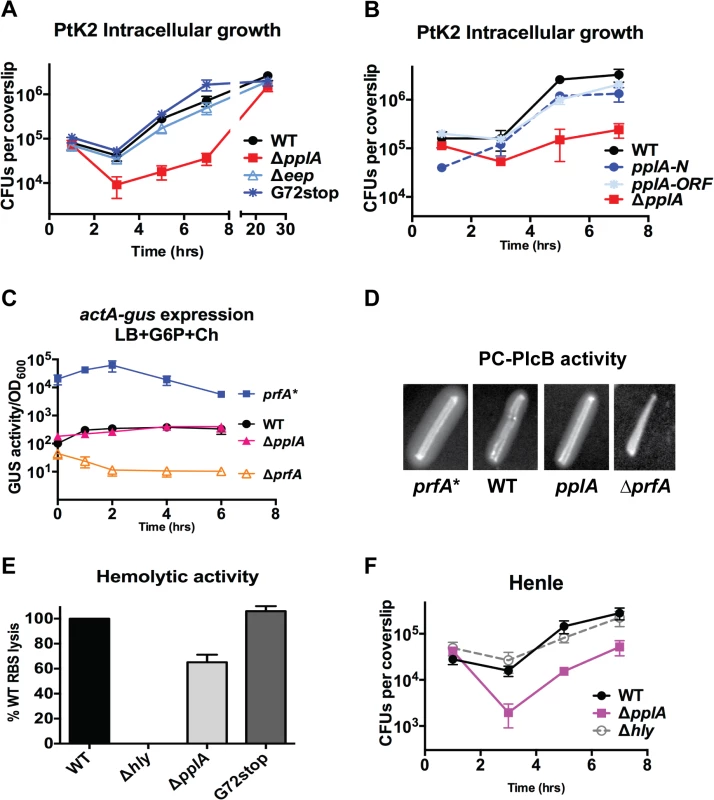
(A) Intracellular growth of the indicated L. monocytogenes strains in PtK2 epithelial cells. PtK2 monolayers grown on glass coverslips (cs) were infected with bacteria at an MOI of 100:1. Gentamicin was added one hour p.i. to kill extracellular bacteria, cs were removed at indicated time points, host cells were lysed and the amounts of intracellular bacteria were enumerated. Loss of pplA delayed the initial stages of intracellular growth, suggestive of a vacuolar escape defect. (B) Intracellular growth in PtK2 cells (as described for panel A) of the ΔpplA mutant complemented with either the entire pplA open reading frame (pplA-ORF) or just the first 72 amino acids of the N terminal region (pplA-N). (C) Measurement of the level of actA and plcB expression in broth culture as assessed by monitoring strains containing actA-gus-plcB transcriptional reporter fusions. Bacterial strains were grown under in vitro inducing conditions for PrfA activity at 37°C with shaking, and GUS activity was measured from normalized samples collected at the indicated time points. Each data point represents the mean±SEM of GUS activity measured in duplicate from at least two independent experiments. (D) The production of PlcB-dependent phospholipase was assessed on egg yolk agar plates. Bacteria were streaked onto egg yolk plates and incubated overnight at 37°C. Zone of opacity surrounding bacterial streaks is indicative of PlcB activity. Loss of pplA did not impair PlcB-dependent phospholipase activity. Data shown is representative of three independent experiments. (E) Measurement of LLO-associated hemolytic activity as assessed by lysis of sheep red blood cells from serial dilutions of culture supernatants of bacterial strains grown shaking in LB for 5 hours at 37°C. Hemolytic activity was determined as the reciprocal of supernatant dilution at which 50% lysis was observed, the data is reported as the percentage of WT, with WT values set to 100%. (F) Intracellular growth assay in human Henle epithelial cell line, where vacuole escape is independent of LLO activity, done as described in panel (B) for PtK2 cells. For panels (A), (C) and (D), data shown represents the mean±SEM of three independent experiments done in triplicate. Loss of pPplA inhibits L. monocytogenes escape from host cell vacuoles in non-professional phagocytic cells
The delay observed in ΔpplA intracellular replication (versus pplA-G72STOP) suggested the possibility that strains lacking the pPplA peptide were defective in mediating escape from host cell vacuoles. Vacuole escape requires the secretion of the cholesterol-dependent pore-forming cytolysin listeriolysin O (LLO) and is assisted by two phospholipases, PlcA and PlcB [60–63]. Loss of pplA did not affect plcB expression based on an actA-gus-plcB transcriptional reporter gene fusion for which β-glucuronidase (GUS) activity serves as a read-out for actA and plcB expression (Fig. 5C), or secretion of PlcB as determined by examination of lecithinase activity on egg yolk agar plates (Fig. 5D). Modest reductions in secreted LLO activity were observed for the ΔpplA mutant as indicated by the reduced lysis of sheep red blood cells (about 70% of wild-type levels) (Fig. 5E). Modest reductions in secreted LLO activity have generally not been associated with significant vacuole escape defects as bacterial strains that exhibit approximately 10% LLO activity in comparison to wild type strains still exhibit normal patterns of vacuole escape and intracellular growth [64]. In addition, a delayed pattern of intracellular growth was also observed for the ΔpplA mutant in human Henle kidney epithelial cells for which vacuole lysis is not dependent on the activity of LLO but can be mediated by the activities of PlcB and PlcA (Fig. 5F) [65–68]. These results are consistent with a L. monocytogenes vacuole escape defect that is not restricted to variations in secreted LLO activity.
L. monocytogenes mutant strains that exhibit vacuole escape defects are delayed in gaining access to the host cytosol, delayed in initiating bacterial cytosolic replication, and delayed in the acquisition and polymerization of host cell actin, a process that is dependent on the expression of the bacterial surface protein ActA [31,69–71]. Defects in vacuole escape can be monitored based on the timing of bacterial accumulation of host cell actin filaments within infected cells using fluorescent indicators for F-actin. Following the infection of PtK2 epithelial cells, ΔpplA exhibited a significant delay in vacuolar escape based both on its reduced rate of host actin accumulation as well as its failure to initiate bacterial replication (replication does not occur within host vacuoles but is restricted to the cytosol [72,73]) (Fig. 6A). In contrast, at 2 hours post-infection cells infected with the wild-type strain co-localized with host cell actin as indicated by the formation of actin clouds surrounding individual bacteria. A small percentage of wild-type bacteria formed short actin tails indicative of subsequent steps of polar ActA localization and bacterial movement. In contrast, the ΔpplA mutant either failed to co-localize with host cell actin or exhibited reduced numbers of bacteria associated with actin clouds and minimal actin tail formation. Quantification of the total numbers of actin clouds formed versus tails over time conclusively demonstrated the delay of the ΔpplA mutant in accumulating host cell actin and initiating movement (Fig. 6B).
Fig. 6. Loss of the pPplA pheromone delays escape from host cell vacuoles but does not impair vacuole perforation. 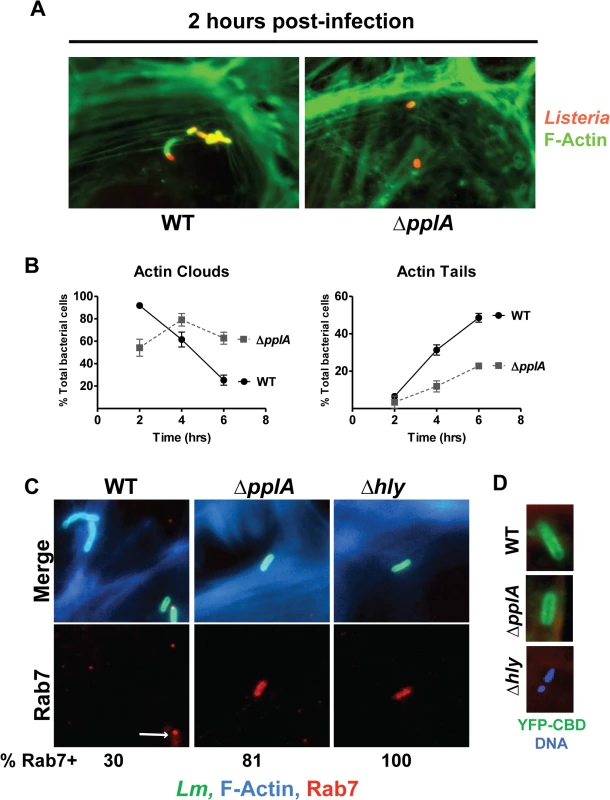
(A) Host cell actin localization as a measure of cytosolic L. monocytogenes during infection of PtK2 epithelial cells. Monolayers of PtK2s were infected as described for Fig. 5, except that an MOI of 20:1 was used. At 2, 4, and 6 hours p.i., Listeria infected host cells were fixed and bacteria were stained using a Listeria specific polyclonal antibody, followed by a secondary goat anti-rabbit antibody conjugated to rhodamine (red stain). Host cell actin was stained using Alexa Fluor 488 phalloidin (green stain), which is a toxin that binds actin. Immunofluorescently labeled coverslips were then visualized on a Zeiss Axio Imager A2 microscope. Data shown is for 2 hours p.i. and is representative of 10 different fields from two independent experiments. (B) Quantification of actin clouds and actin tails formed by wild-type bacteria versus the ΔpplA mutant during intracellular growth in PtK2 cells. A total of 10 different fields, containing a total of 100 bacteria were assessed for clouds and tails. A ΔpplA mutant was delayed in recruitment of host-cell actin, consistent with a vacuole escape defect. (C) Co-localization studies of bacterial cells with host cell Rab7, a small GTPase associated with the late endosome. Ptk2 cells were infected and processed for microscopy as described above except that coverslips were removed at 1.5 hours post-infection and host cell F-actin was stained with phalloidin conjugated to Alexa-350 (blue), the secondary antibody used to stain Listeria cells was conjugated to Alexa-488 (green), and host cell Rab7 was stained with goat anti-Rab7 followed by a secondary donkey anti-goat antibody conjugated to Texas Red (red). Both the Δhly and ΔpplA mutants stained robustly with Rab7 and the majority of ΔpplA bacteria counted co-localized with Rab7, whereas only a small number of wild-type Listeria were positive for Rab7. A population of wild type bacteria also formed actin clouds at this early time. These results suggest the loss of the pPplA peptide results in bacterial mutants that are retained within vacuoles and which are delayed for entry into the cytosol. A minimum of 10 different fields containing a total of at least 100 bacteria in two independent experiments were counted for each strain. (D) The contribution of the pPplA peptide to vacuole membrane perforation was assessed during intracellular growth in PtK2 cells. PtK2 cells were transfected with a mammalian expression vector containing a yellow fluorescent protein (YFP) fused to the cell wall binding domain of a phage endolysin Ply118 (CBD) that binds with high affinity to the Listeria cell surface. YFP-CBD is stably expressed in the host cytosol and nucleus, and once intracellular L. monocytogenes perforates the vacuole membrane, YFP-CBD enters the vacuole and binds bacteria prior to escape into the cytosol. Transfected PtK2 cells were infected with bacteria as described for panel A, except coverslips were removed at 15 minutes post-infection and host cell-F-actin was stained with phalloidin conjugated to Texas Red (red), DNA with DAPI (blue), and bacteria were green if bound with YFP-CBD. Data shown is representative of three independent experiments. YFP-CBD binding of wild-type and the ΔpplA mutant but not Δhly bacteria indicates that vacuole perforation was not impaired by the loss of the pPplA peptide. A defect in vacuolar escape for mutants lacking pheromone was further verified by observing that the ΔpplA mutants co-localized with Rab7 (a small GTPase associated with late endosomal vacuoles) at an early time point post-infection in infected PtK2 cells. Examination of infected cells at 1.5 hours post-infection revealed co-localization of Rab7 with the ΔpplA mutant as well as with a L. monocytogenes Δhly mutant that lacks LLO and remains trapped within host vacuoles (Fig. 6C). 100% of the Δhly mutants co-localized with Rab7, whereas approximately 81% of ΔpplA mutants were positive for Rab7 and only 30% of wild type bacteria co-localized with Rab7 at 1.5 hours post-infection. Furthermore, wild type bacteria that did stain positive for Rab7 exhibited reduced or punctate staining patterns, suggesting that the bacteria were losing the endosomal marker (Fig. 6C). These data support a role for the pPplA peptide in mediating efficient vacuole escape.
Loss of the pPplA peptide does not impair vacuole membrane perforation
While the loss of the pPplA peptide delayed host cell vacuole escape, it appeared that significant levels of proteins associated with vacuole lysis, LLO and phospholipase, were secreted in broth culture (Fig. 5C-F). LLO in particular is associated with rapid perforation of host cell vacuoles [74], thus we sought to determine whether membrane perforation itself was impaired by loss of pPplA, or alternatively if membrane perforation occurred but bacterial escape was inhibited. Based an approach developed by Henry et al [74], PtK2 cells were transfected with a mammalian expression vector encoding a fusion protein of yellow fluorescent protein (YFP) fused the cell wall binding domain (CBD) of the phage endolysin Ply118 which binds to the L. monocytogenes cell wall with high affinity [74]. YFP-CBD protein is stably expressed in the host cell cytosol and nucleus in transfected cells; when vacuole perforation occurs as a result of L. monocytogenes host cell infection, YFP-CBD from the cytosol enters the vacuole and binds to the bacterium. Binding of YFP-CBD to intracellular L. monocytogenes is thus an early readout of vacuolar membrane perforation and can be detected prior to bacterial entry into the cytosol [74].
Transfected PtK2 cells expressing YFP-CBD were grown on glass coverslips and infected with either wild type L. monocytogenes, the ΔpplA or the Δhly mutant. Cells were examined using fluorescence-based microscopy at 15 and 30 minutes post-infection. In macrophages, LLO-dependent vacuole membrane perforation can be detected within 5 minutes following bacterial internalization, whereas complete vacuole escape generally occurs within about 30–45 minutes [74,75]. Both wild type L. monocytogenes and the ΔpplA mutant were observed to bind YFP-CBD by 15 minutes post-infection, indicating that ΔpplA mutant was capable of perforating the vacuole membrane (Fig. 6D). Mutants trapped within host vacuoles due to lack of LLO secretion (Δhly) did not stain with YFP-CBD (Fig. 6D). These results indicate that the pPplA peptide contributes to L. monocytogenes escape from host cell vacuoles at a step that is subsequent to initial vacuole membrane perforation.
pPplA is dispensable for intracellular growth inside professional phagocytic cells
Resident macrophage populations are often the first cells to encounter Listeria circulating in the blood [76]. Surprisingly, when bacterial growth was examined following the infection of J774 murine macrophage-like cells, the growth pattern of the ΔpplA mutant resembled that of wild type bacteria, with no discernable defect in phagosomal escape (Fig. 7A). Similarly, the infection of primary bone-marrow derived macrophages revealed no phenotypic difference between mutant and wild type bacterial strains, even following macrophage activation with IFNγ(Fig. 7B and S4 Fig.). As mentioned above, resident macrophages are usually the cells associated with the initial contact of L. monocytogenes circulating in the bloodstream. Therefore, these results support the in vivo time course data that indicated that the ΔpplA mutant was not impaired in its ability to initially colonize target organs. The pPplA peptide thus enhances bacterial escape from the vacuoles of non-professional phagocytic cells but appears dispensable for growth within professional phagocytic cells.
Fig. 7. Loss of pplA pheromone does not delay intracellular growth or vacuolar escape in professional phagocytic cells. 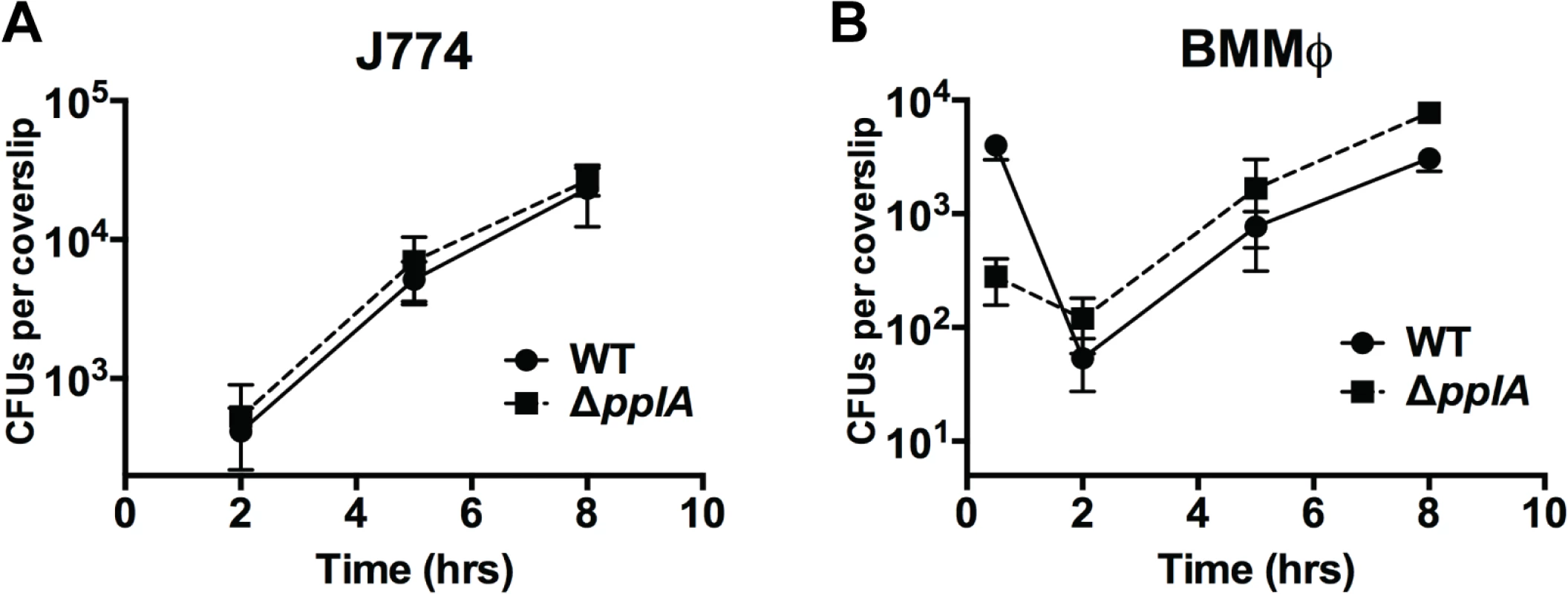
Intracellular growth of the wild-type strain compared to the ΔpplA mutant in (A) J774 macrophage-like cells and (B) murine bone-marrow derived macrophages (BMMØ) using an MOI of 01:1. Loss of pplA did not impair intracellular growth inside professional phagocytic cells. Data shown is representative of three independent experiments. Constitutive activation of the PrfA regulator restores virulence to strains lacking the pPplA peptide pheromone
The transcriptional activator PrfA regulates the expression of a number of gene products required for L. monocytogenes pathogenesis, including the process of vacuole escape [25–27]. PrfA is a member of the cAMP receptor protein (Crp)-Fnr family of transcriptional regulators that require the binding of small molecule cofactors to become fully activated for transcription [28,30,32]. The putative PrfA cofactor is unknown, but it has been determined that PrfA activation occurs following the entry of L. monocytogenes into host cells. Given the apparent vacuole escape defect of strains lacking the pPplA secreted peptide, we investigated whether mutational activation of PrfA could compensate for the intracellular growth defect resulting from the loss of pplA and/or the virulence defect observed in mice. The introduction of the mutationally activated prfA*L140F allele completely rescued the growth defect observed for the pplA deletion mutation within infected host cells and also fully restored virulence in mice to the levels of prfA* strains, which are hypervirulent in comparison to wild type L. monocytogenes (Fig. 8AB). Mutational activation of prfA therefore fully compensated for the loss of the pPplA secreted peptide, suggesting a possible linkage between pPplA function and PrfA activation.
Fig. 8. Constitutive activation of prfA* rescues virulence defects associated with loss of pPplA pheromone. 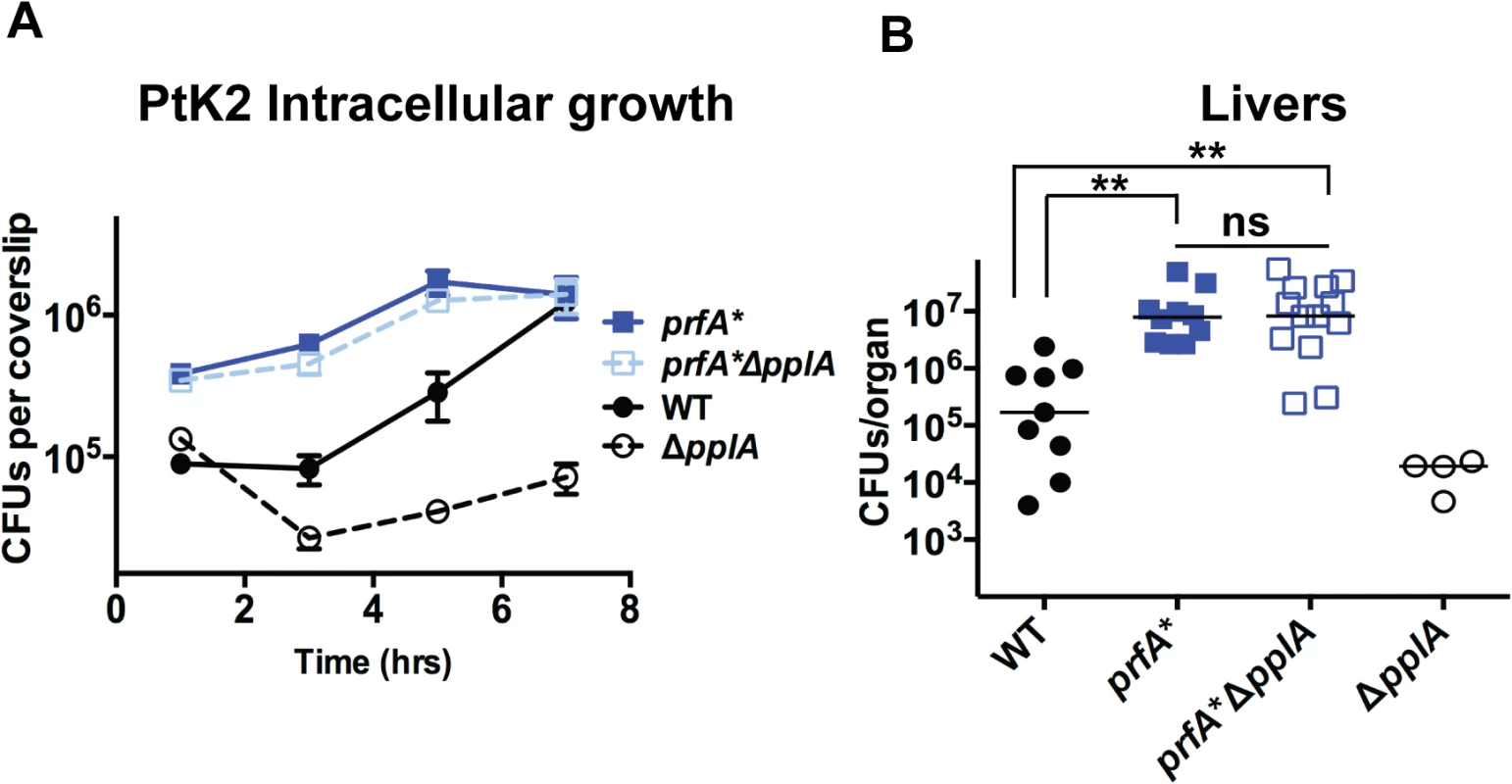
(A) Intracellular growth of the prfA* mutant, the wild-type strain and a prfA*ΔpplA mutant in PtK2 epithelial cells. Gentamicin was added one hour p.i. to kill extracellular bacteria, coverslips were removed at the indicated time points, host cells were lysed and intracellular bacteria were enumerated. (B) Swiss Webster female mice were intravenously inoculated with 2 x 104 CFUs through the tail vein, and the livers and spleens were harvested 48 hours post-infection (p.i.) and homogenized to determine bacterial burdens. The ΔpplA single mutant data is from data presented in Figs. 4 and 5 and is meant to represent a point of reference. The addition of a prfA* mutation to the ΔpplA mutant is able to completely restore any virulence defects associated with loss of pplA, suggesting a link between the pPplA pheromone function and PrfA activation. L. monocytogenes mutants that lack pPplA exhibit altered profiles of surface and secreted proteins
Peptide pheromone secretion and signaling pathways have been associated with the induction of selected gene products in a variety of Gram positive bacteria. For example, peptide pheromone signaling has been shown to regulate competence and sporulation in Bacillus subtilis [77,78], conjugation [39,40,45,47,79] and virulence factors/biofilm formation [80–82] in Enterococcus, competence and fratricide in Streptococcus pneumoniae [83,84], and virulence factor secretion in Staphylococcus aureus [4,85,86]. We therefore investigated whether the pPplA peptide pheromone influenced patterns of L. monocytogenes secreted proteins by examining polypeptide profiles following two-dimensional gel electrophoresis with subsequent peptide identification using mass spectroscopy. Proteins were isolated from strains grown in BHI to stationary phase as stationary phase-derived supernatants were observed to contain more peptide activity to promote bacterial aggregation, and also based on the abundance of the PplA lipoprotein as monitored by Western blot analyses using antibody directed against PplA. The comparison of secreted protein profiles for wild type, the pplA-G72STOP mutant, and ΔpplA facilitated the identification of changes in protein profiles specific to the loss of the peptide pheromone versus the PplA lipoprotein.
Dramatic differences in the profiles of secreted proteins were observed for the pplA in-frame deletion mutant in comparison to wild-type 10403S and pplA-G72STOP strains (Fig. 9). A number of proteins secreted by 10403S and pplA-G72STOP were absent in the supernatants derived from the ΔpplA mutant, while the secreted protein profiles of wild-type 10403S and pplA-G72STOP were similar. Complementation of the ΔpplA mutant with the first 72 amino acids of PplA restored secreted protein profiles to those resembling wild type (S1A Fig.). In contrast, the protein profiles of supernatants derived from prfA* L140F cultures appeared similar to those of prfA*L140F ΔpplA and prfA*L140FxpplA-G72STOP (S5A Fig.), a finding consistent with the ability of the constitutively activated prfA* allele to compensate for loss of the secreted pPplA peptide within cells and in vivo.
Fig. 9. Loss of pPplA affects bacterial protein secretion. 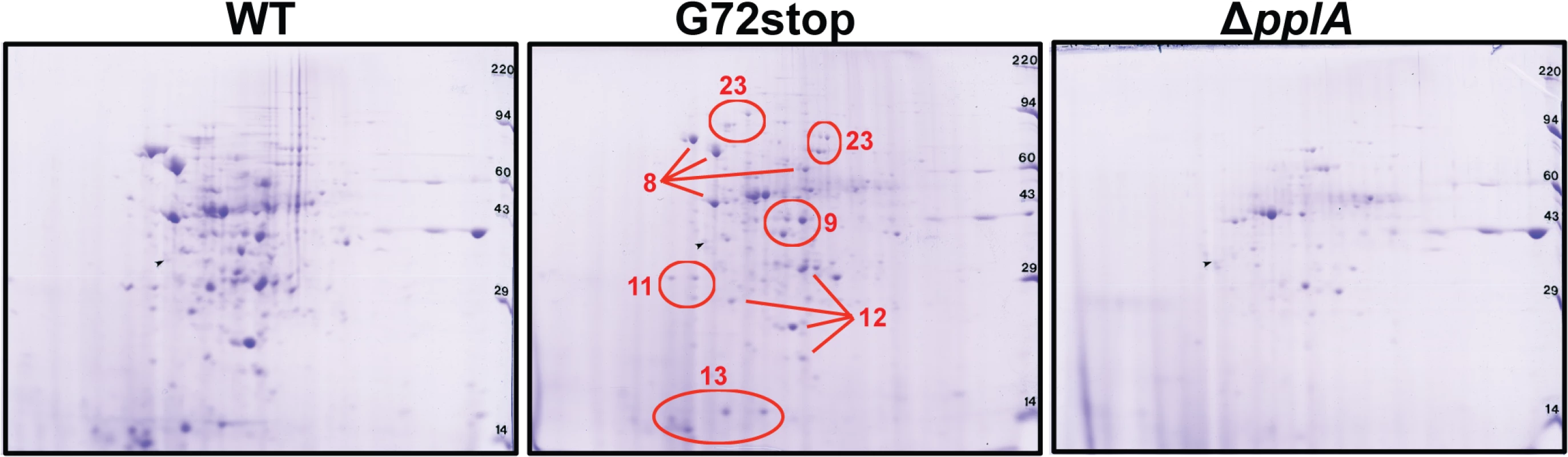
Two dimensional gel electrophoresis of secreted protein preparations isolated from overnight stationary phase cultures grown in BHI. Secreted proteins were TCA precipitated from bacterial culture supernatants and processed for 2-D gel analysis by Kendrick Labs (Madison, WI). The wild-type and the pplA-G72stop mutant protein profiles displayed similar patterns, whereas loss of pplA resulted in the loss of a number of secreted proteins. As loss of the pplA-encoded pheromone and not the encoded lipoprotein significantly diminished L. monocytogenes pathogenesis, we chose to focus on most significant differences in secreted protein profiles between the pplA-G72STOP mutant versus the pplA in-frame deletion mutant (summarized in Table 1). Secreted proteins absent from the culture supernatants of strains lacking pPplA were associated with a range of functional groups that included substrate-binding lipoproteins involved in metal and oligopeptide ABC transport (OppA), protein folding, adaptation to atypical conditions, detoxification, membrane bioenergetics, and also a number of metabolic and amino acid biosynthetic proteins. The majority of these proteins do not appear to be associated with SecA1-dependent protein secretion as they lack the presence of a classical secretion signal peptide, however a significant number have been associated with SecA2-dependent secretion [87–89]. We confirmed that the presence of proteins with cytosolic function was not the result of increased bacterial cell lysis as cell viability based on bacterial CFU was identical between cultures, and there was no indication of compromised membrane integrity as visualized by cell impermeant dyes (S5B Fig.). While it is not yet clear as to how the pPplA-dependent secretion of these proteins enhances L. monocytogenes vacuolar escape, it is evident that pPplA-dependent signaling leads to significant changes in the repertoire of L. monocytogenes secreted and surface-associated proteins.
Tab. 1. Secreted protein spots present in both wild-type 10403S and the pplA-G72STOP mutant but absent in the in-frame pplA deletion mutant. 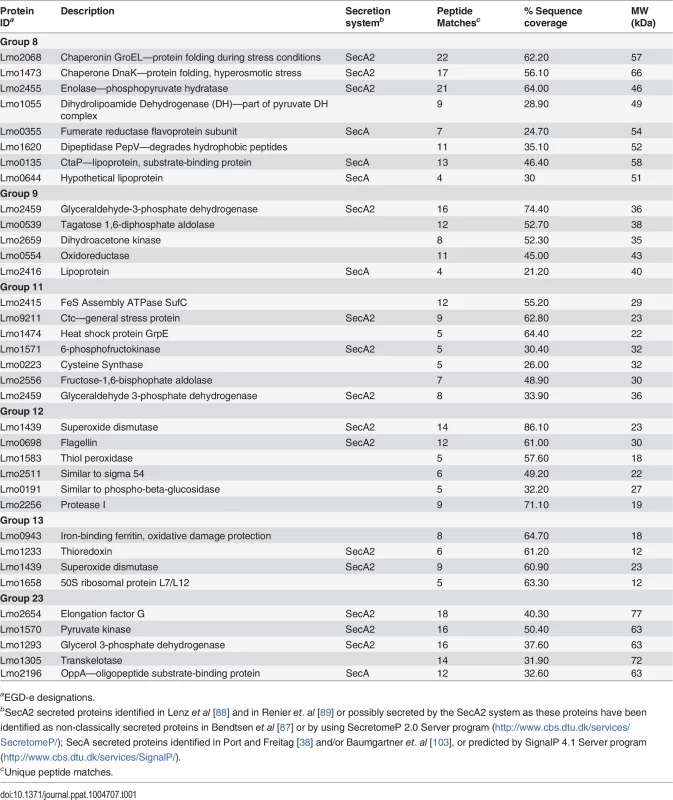
aEGD-e designations. Discussion
Bacterial peptide pheromones have been described as signaling molecules that coordinate bacterial communication and complex multicellular processes that include biofilm formation, DNA transfer via conjugation, as well as virulence factor secretion [1,2,4]. Here we present evidence for a novel signaling role for a bacterial peptide pheromone, namely facilitating the escape of an individual bacterial cell from the confines of a host cell vacuole. Experimental evidence suggests that the pPplA peptide pheromone is released by L. monocytogenes following its cleavage and processing from the secretion signal sequence of the PplA lipoprotein; production of the pPplA peptide enhances L. monocytogenes escape from host cell vacuoles and is required for full bacterial virulence. Our data suggests that pPplA may serve to signal to L. monocytogenes that the bacterium is within the confines of a membrane-bound vacuole, such that the secretion of pPplA leads to its rapid uptake to initiate a signaling cascade that ultimately enhances vacuolar membrane dissolution and bacterial escape into the cytosol. Mutational activation of PrfA, the transcriptional activator that induces the expression of LLO and the phospholipases that mediate vacuole membrane disruption, eliminates the need for pPplA, suggesting that pheromone signaling may be functionally linked to PrfA activation. To our knowledge, this is the first indication of a bacterial peptide pheromone being adapted to coordinate gene expression for an individual bacterium.
Interestingly, the escape defect associated with the loss of the pPplA pheromone appears thus far to be limited to non-professional phagocytic cells, as normal patterns of escape were observed in both primary macrophages as well as macrophage cell lines. Cell line-dependent differences in vacuole escape have been previously noted for L. monocytogenes. For example, bacterial escape from human epithelial cell lines does not require the activity of LLO, whereas escape is absolutely dependent upon this pore forming toxin in other cell types [65,66,68]. Rabinovich et al recently reported that gene products sharing homology with competence proteins involved in formation of the competence apparatus contribute to the escape of L. monocytogenes from professional phagocytes but not from non-professional phagocytic cells [90]. These observations indicate that the composition and/or properties of vacuolar membranes differ between different cell types, with the difference between professional phagocytes and non-professional phagocytic cells perhaps being most prominent. Based on their findings, Rabinovich et. al speculated that L. monocytogenes com genes may contribute to the formation of a pseudopilus structure that enhances phagosome lysis [90]. Bacterial competence and the formation of a Com-dependent psuedopilus are regulated by peptide pheromone-dependent signaling in other bacteria [91], however no peptide has thus far been associated with L. monocytogenes com gene expression. Our findings that the pPplA pheromone appears to enhance escape in non-professional phagocytic cells and not within professional phagocytes (where com genes are required) suggests that either the gene products that contribute to bacterial escape are differentially regulated by either pPplA or Com-associated regulation in specific cell types, or each system regulates a different set of gene products that contribute to escape within specific cell types. L. monocytogenes encodes gene products that share homology with those involved in Type IV secretion and the assembly of a conjugal DNA transfer system [92]; given the potential similarity of pPplA signaling pathways with those of cAD1 and Type IV secretion in E. faecalis, it is tempting to speculate that pPplA regulates the expression of a Type IV effector molecule and/or secretion apparatus that aides in vacuole escape.
An alternative hypothesis for the dependence of L. monocytogenes on pPplA expression is the possibility that the peptide itself enhances membrane lysis. Some bacterial cationic peptides have the ability to insert within lipid bilayers and disrupt membrane integrity [93]. While we have not yet been able to identify the precise composition of the pPplA peptide, it is likely to be hydrophobic in nature given the composition of the PplA signal sequence. We do not favor direct disruption of vacuole membranes by pPplA as we have not observed that the addition of pPplA-containing culture supernatants enhances LLO-dependent lysis of red blood cells for strains lacking pplA, or any indication that pPplA can disrupt membranes in the absence of LLO (S6 Fig.). Our data suggests that pPplA enhances vacuole escape through a mechanism that is not restricted to LLO activity as escape defects were still evident in Henle human epithelial cells, for which LLO is dispensable. Based on (1) the homology pPplA with cAD1 and its associated signaling components, and (2) the observation that pPplA induces the expression of a number of secreted gene products, we favor a model in which import of the pPplA pheromone stimulates the expression of L. monocytogenes gene products that enhance vacuole escape, possibly through the induction of a factor or factors that stabilize membrane perforations or pores.
Given that mutational activation of PrfA eliminates the requirement for pPplA-dependent signaling (Fig. 8), it is possible that pPplA contributes in some way to the process of PrfA activation. PrfA is postulated to require the binding of a small molecule cofactor for full activity, and pPplA could potentially be involved in: (1) the induction of a small molecule cofactor; (2) the induction of a transport system for import of the cofactor; or alternatively, (3) the induction of bacterial factors that stabilize or extend vacuole membrane perforations generated by LLO, allowing influx into the vacuole of a host-derived environmental signal. A role for pPplA in vacuole dissolution would be consistent with the fact that the pheromone is not required in strains containing constitutively activated PrfA*, as these strains secrete high levels of LLO and phospholipase which may be capable of mediating vacuole membrane disruption in the absence of any additional factors. While membrane perforation has been shown to occur rapidly within infected cells [60,74], the subsequent steps leading to complete membrane disruption and L monocytogenes cytosolic entry are less clearly defined. Finally, it should be noted that the PrfA cofactor binding pocket appears to have a high positive charge [94], thus we feel it is unlikely that the pPplA peptide itself serves as cofactor, although this remains a possibility.
Thus far, the principal in vitro phenotype we have identified in association with the secretion of the pPplA pheromone outside of cellular infection is the tendency for mutationally activated prfA* strains to aggregate in stationary phase static cultures (Fig. 3). While the relevance of this phenotype to L. monocytogenes physiology or virulence is not yet clear, bacterial aggregation has been associated with biofilm formation and virulence for a number of bacteria. In the case of E. faecalis, bacterial aggregation contributes to adhesion to mammalian host cells and the formation of large Enterococcal vegetative aggregates, consisting of a mix of bacteria, fibrin, platelets, and host cells in target organs such as the heart, where the bacterium causes infective endocarditis [95–99]. E. faecalis aggregation in culture has been used as a read out for the assessment of the synthesis and levels of the peptide-pheromones present in culture supernatants [47]. E. faecalis aggregation depends on the production of a surface protein known as the Aggregation substance (AS), and is required for the facilitation of conjugal DNA transfer of plasmids by bringing plasmid donor and recipient cells into close proximity for the formation of the conjugal bridge. L. monocytogenes is not known to contain a surface protein analogous to AS, however a recent study has reported that ActA, the surface protein required for host actin polymerization, promotes bacterial aggregation in broth culture in prfA* strains [50]. ActA-dependent bacterial aggregation was also associated with long term colonization of the intestines of mice. Interestingly, we have found that the aggregation of prfA* strains requires pPplA secretion in addition to surface expression of the ActA protein, as ΔpplA prfA* strains exhibited similar levels of ActA protein on their surface in comparison to prfA* strains (S2A Fig.), yet the ΔpplA prfA* strains were reduced for aggregation (Fig. 3B). Inhibition of protein translation through the addition of chloramphenicol to spent media containing pPplA resulted in reduced aggregation of ΔpplA prfA* strains (S7 Fig.), suggesting that translation is required in response to pheromone for efficient aggregation to occur. Finally, the reduced bacterial aggregation observed for prfA*ΔpplA strains is not due to any reduction in flagellar based swimming motility as this mutant exhibited the same swimming motility pattern on soft agar plates as the parental prfA* strain (S8 Fig.).
Given the important contributions of pPplA to L. monocytogenes pathogenesis, it would be useful to identify the amino acid composition of the pPplA peptide pheromone. To date, identification of the definitive amino acid sequence of pPplA has been extremely challenging. As described above, the only in vitro phenotype identified thus far in association with the pPplA pheromone is aggregation of prfA* strains in high density cultures grown in rich media. We have tried adding synthetic peptides based on the cAD1 sequence homology (ASSLLLVG) as well as the full length PplA signal sequence peptide to prfA* ΔpplA cultures to see if aggregation can be stimulated, however this approach has not been successful. It is possible that the L. monocytogenes pPplA peptide differs in length and/or composition in comparison to cAD1, and/or that the pPplA peptide is post-translationally modified. The precise amino acid composition and length of the active peptide of cAD1 is important for activity as the introduction of synthetic peptides containing additional N-terminal amino acids did not induce aggregation activity in E. faecalis [43]. Similarly, in Streptococcus pyogenes it has been recently demonstrated that single amino acid differences in peptide length or composition dramatically affect the activation of gene expression by Rgg proteins [100]. Peptide pheromones may be present and functional at very low concentrations (1 to 5 molecules of peptide are sufficient to stimulate the E. faecalis mating response [45,101]) and we are working to optimize a more sensitive assay that may enable us to purify pPplA from culture supernatants. In addition, there have been challenges associated with the cloning of pplA in E. coli plasmid vectors, suggesting that this gene may be somewhat toxic for E. coli host strains. Similar difficulties in cloning and overexpression of some of the Enterococcal peptide-pheromones have been reported [43,51] [102]. Purification of pPplA is likely to require the development of sensitive in vitro assays for the detection of peptide along with the optimization of the conditions for peptide expression.
In summary, we have described a novel peptide-based signaling system in L. monocytogenes that is required for bacterial virulence. The pPplA peptide pheromone, processed from the N-terminal secretion signal sequence of pplA, has functionally evolved to enhance vacuolar escape in non-professional phagocytic cells. Based on data presented here, we propose the following as a working model for the role of pPplA in L. monocytogenes pathogenesis (Fig. 10): L. monocytogenes produces a basal level of PplA that is secreted through bacterial cellular membrane by the general secretory pathway. Following secretion, the N-terminal secretion signal peptide of PplA gets cleaved by Signal Peptidase II releasing the lipoprotein of PplA, which is modified with diacyl glycerol and anchored to the cellular membrane by Lgt [103]. The membrane embedded signal peptide is further processed by Eep and possibly other proteases to release the mature pPplA peptide into the extracellular space. Once L. monocytogenes enters a host cell and is contained within a vacuole, the pPplA peptide accumulates in the confines of the vacuolar space and is transported back into the bacterial cell through the CtaP transport system, thus initiating a signaling cascade to induce the expression of gene products that contribute to vacuolar escape. These gene products may serve to stabilize membrane pores induced by LLO and phospholipases, facilitating the influx into the vacuole of a host-derived environmental signal that stimulates PrfA activation. Fully activated PrfA induces the expression of additional LLO and phospholipase as well as gene products required for bacterial replication within the host cytosol and for spread to adjacent host cells. While much remains to be elucidated about this intriguing pheromone signaling pathway, it serves to illustrate the diversity of cellular processes regulated by small peptides in Gram positive bacteria.
Fig. 10. Model of L. monocytogenes pPplA signaling within the host vacuole. 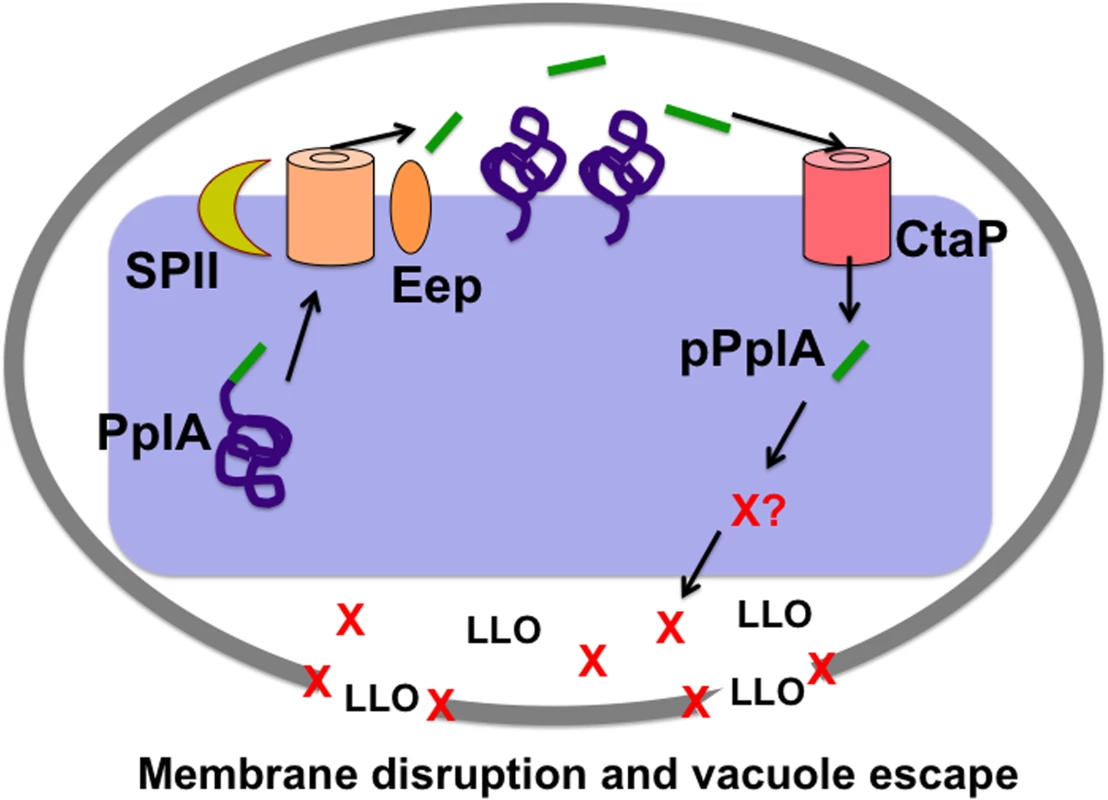
Modeling of the predicted L. monocytogenes pPlpA signaling pathway involved in enhancing vacuole escape from the host cell vacuoles in non-professional phagocytic cells. In wild-type L. monocytogenes, pplA encodes a lipoprotein (PplA) with a peptide pheromone (pPplA) located within the N terminal secretion signal peptide (shown in green). The signal sequence of prePplA is processed by signal peptidase II (SPII) and the released signal peptide is further cleaved by the protease Eep releasing the pPplA pheromone, while the PplA protein becomes lipid modified and associated with the membrane or secreted. Upon entry of wild-type L. monocytogenes into non-professional phagocytic host cell, the confined space of the vacuole leads to import of the secreted pPplA pheromone through the CtaP peptide transporter. pPplA accumulation in the bacterial cytoplasm stimulates a signaling cascade that results in the production of an unknown factor (X) that contributes to vacuole lysis. Factor X may function by helping to stabilize the LLO generated membrane pore, facilitating eventual vacuole membrane dissolution as well as the influx of mammalian cytosol components that may promote PrfA activation and the expression of gene products required for intracellular growth and cell-to-cell spread. In the absence of pPplA (ΔpplA strains), bacterial escape is delayed until sufficient LLO and phospholipase accumulate to disrupt the vacuole membrane in the absence of factor X function. For strains containing constitutively activated PrfA*, the substantially increased secretion of LLO and the phospholipases is sufficient to disrupt the vacuole membrane in the absence of factor X. Materials and Methods
Bacterial strains, plasmids and growth conditions
L. monocytogenes and E. coli strains used in this study are listed in Table 2. E. coli XL1-Blue (Agilent Technologies, Santa Clara, CA), One Shot TOP10 (Invitrogen Corp., Carlsbad, CA), NEB 5αF’Iq (New England Biolabs, Ipswich, MA), SM10s, S17-1 and BH10s (both kind gifts of Nicholas Cianciotto, Northwestern University) were used as host strains for maintenance and propagation of recombinant plasmids. L. monocytogenes and E. coli strains were grown at 37°C in brain heart infusion (BHI) media (Difco Laboratories, Detroit, MI) and Luria broth (LB) (Invitrogen Corp., Carlsbad, CA). Maintenance of the integration plasmid pPL2 was selected for using 25 μg/ml of chloramphenicol in E. coli and 5 μg/ml in L. monocytogenes. Maintenance of the integration plasmid pIMK2 (a kind gift of Dr. Colin Hill, University College Cork) was selected for using 50 μg/ml of kanamycin in E. coli and in L. monocytogenes. Bacteria containing the 6X Histidine tagged expression vector pQE30 (Qiagen Inc., Valencia, CA) were maintained in E. coli with 100 μg/ml ampicillin. Streptomycin 200 μg/ml was used in selection of L. monocytogenes following bacterial conjugation and isolation from organs of infected mice.
Tab. 2. Bacterial strains and plasmids used in this study. 
Plasmid and bacterial mutant construction
Primer pairs used for construction of in-frame deletions of lmo2637 (pplA) and lmo1318 (eep), introduction of a premature stop codon at amino acid G72 of pplA, and complementation of the pplA deletion mutant are listed in Table 3. Mutants were generated by cloning 600 base pairs of the immediate upstream and downstream regions of their respective coding regions into the temperature-sensitive shuttle plasmid pKSV7 [104], leaving only the translational start and stop of the open reading frames. Due to apparent difficulties in the cloning of DNA sequences toxic to E. coli, only 250 base pairs of the flanking regions of pplA were amplified for construction of the pplA deletion mutant vector construct. The flanking regions were joined using SOEing PCR, and the SOEing PCR products were digested with the appropriate restriction enzymes and ligated into pKSV7 to generate the mutant constructs. The ermB gene encoding erythromycin (Em) resistance and containing its native promoter was PCR amplified from pHY-304 (a kind gift from Dr. Craig Rubens and Dr. Amanda Jones, Seattle Children’s Hospital Research Foundation) and inserted in between the internal KpnI sites of the flanking regions in the pKSV7-based pplA deletion construct. All pKSV7-pplA containing plasmids had to be maintained in the E. coli host restrictive strain BH10s to maintain stable plasmid constructs. Gene-disruption mutants were constructed by amplification of a 500 base pair internal fragment of the appropriate coding region that was then ligated into pKSV7. The resulting plasmid constructs were transformed into the L. monocytogenes wild-type strain 10403S. pplA deletion mutants were selected based on EmR and CmS, and confirmed by PCR amplification of products from L. monocytogenes chromosomal DNA. eep and pplA-G72stop mutants were screened for CmS and confirmed by PCR for the eep deletion mutant, and sequencing for the pplA-G72stop codon mutant. oppA gene disruption mutants were maintained by selecting for CmR.
Tab. 3. Oligonucleotides used in this study. 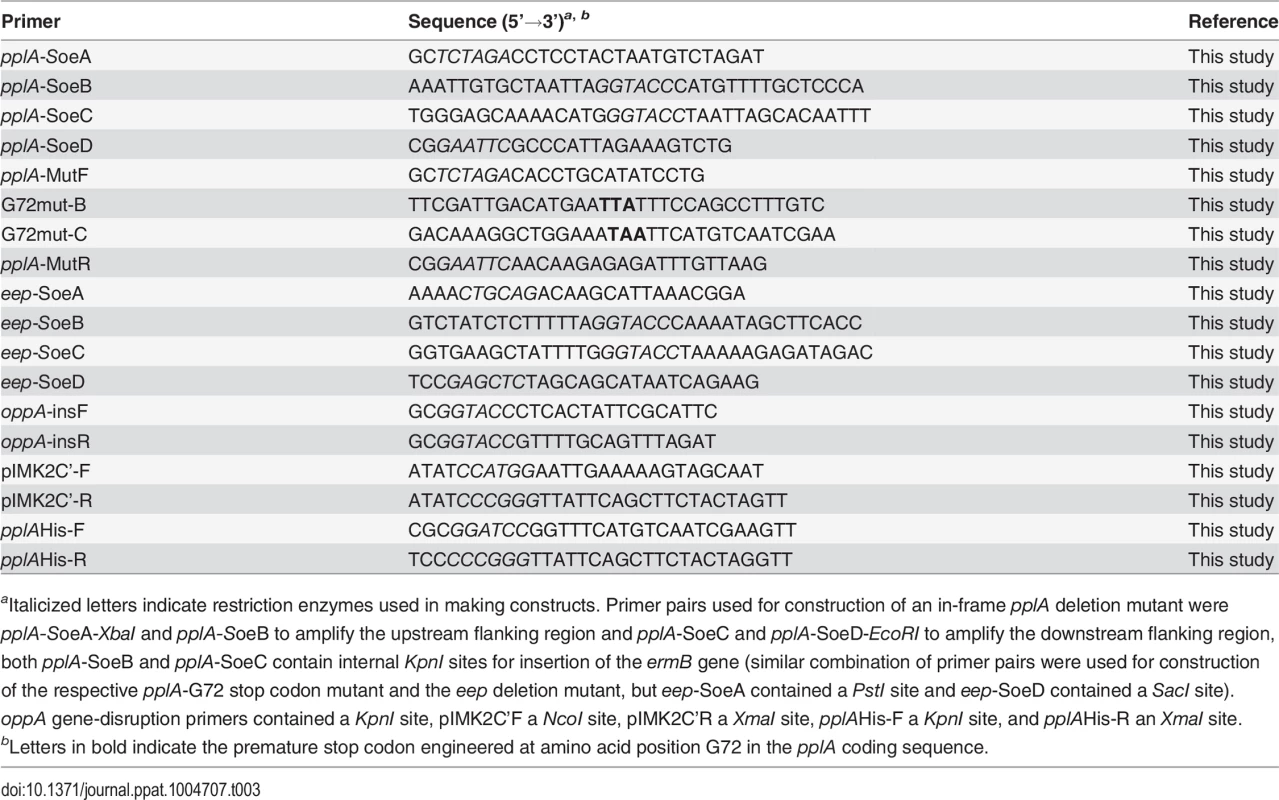
aItalicized letters indicate restriction enzymes used in making constructs. Primer pairs used for construction of an in-frame pplA deletion mutant were pplA-SoeA-XbaI and pplA-SoeB to amplify the upstream flanking region and pplA-SoeC and pplA-SoeD-EcoRI to amplify the downstream flanking region, both pplA-SoeB and pplA-SoeC contain internal KpnI sites for insertion of the ermB gene (similar combination of primer pairs were used for construction of the respective pplA-G72 stop codon mutant and the eep deletion mutant, but eep-SoeA contained a PstI site and eep-SoeD contained a SacI site). oppA gene-disruption primers contained a KpnI site, pIMK2C’F a NcoI site, pIMK2C’R a XmaI site, pplAHis-F a KpnI site, and pplAHis-R an XmaI site. The integration plasmid, pIMK2, was used for complementation of the pplA deletion mutant. This plasmid is a derivative of the conjugative plasmid, pPL2, and integrates in single copy into the L. monocytogenes 10403S chromosome at a phage attachment site within the tRNAArg gene following conjugation [35]. For construction of the complementation vectors, the entire open reading of pplA beginning with the second codon and ending with the stop codon or just up to the first 72 amino acids were PCR amplified and digested with NcoI and XmaI and subsequently ligated together with pIMK2 vector and transformed into E. coli XL1-Blues. The resulting construct was initially electroporated into the wild-type background strain using the method as described by Monk et al., resulting in a strain containing two copies of pplA. Tranduction of the ΔpplA::erm mutation into this pIMK2-pplA integrated strain or different background strains was performed as previously described [33,105]. pPL2-derived plasmids were conjugated into the various L. monocytogenes strains.
For generation of purified truncated PplA protein used for antibody affinity purification and as a control standard for Western blot analyses, the lipoprotein region of pplA from the G72 amino acid position to the stop codon was PCR amplified from L. monocytogenes strain 10403S (wild-type) using the primer pairs listed in Table 3 and subsequently cloned into a pQE30 Expression vector (Qiagen Inc., Valencia, CA), which contains an N-terminal 6x-Histidine tag and an isoproyl-β-D-thiogalactopyranoside (IPTG) inducible promoter. The resulting construct was initially propagated in E. coli TOP10 cells, isolated and then transformed into NEB 5αF’Iq (New England Biolabs, Ipswich, MA). An overnight culture containing the expression construct was diluted 1 : 50 in fresh LB broth and the culture was incubated at 37°C (with shaking) until an optimal density of 0.5 was reached. To induce expression of the PplA protein, 1 mM IPTG (Inalco, Paris, France) was added to the culture and induction was allowed to proceed for 3 to 4 hours. The bacterial cells were recovered by centrifugation, resuspended in 1 ml of PBS and 20 mg of lyzozyme (Sigma-Aldritch, St. Louis, MO), and incubated at 37°C for 30 minutes. Prior to sonication with 5 repeated 10 second bursts and 1 minute cooling on ice, 10 ul of 100X protease inhibitor cocktail was added. The soluble fraction containing the N-His-PplA protein was collected and purified using the His-Pur Purification Kit (Thermo Scientific, Rockford, IL). Protein concentration was determined using a BCA Protein Assay Kit (Thermo Scientific; Rockford, IL).
Western blot analysis of PplA lipoprotein
PplA lipoprotein was detected from both secreted protein preparations isolated from culture supernatants and surface-associated fractions isolated from bacterial whole cells as previously described with slight modifications [38,106,107]. In brief, 100 ml culture of each L. monocytogenes strain was grown to mid-log and stationary phase in BHI at 37°C with shaking. Samples were all normalized to equivalent cell densities (OD600 nm). Proteins present in the culture supernatants were precipitated with Trichloroacetic acid (TCA, Fisher Scientific, Hanover Park, IL), and pellets were resuspended in 100μl of 2X SDS-boiling buffer (Bio-Rad, Hercules, CA). Surface-associated (SA) proteins were extracted by boiling of the bacterial pellet in 200 μl of 2X SDS-boiling buffer (Bio-Rad, Hercules, CA). For detection of PplA lipprotein, 10 μl of the isolated secreted proteins sample and 20 μl of SA proteins were separated using SDS-polyacrylamide gel electrophoresis. Protein samples were transferred onto PVDF membranes. PplA lipoprotein was detected using a 1 : 200 dilution of a affinity-purified polyclonal antibody directed against the C-terminal of the PplA lipoprotein (FKIYAAQLQN), in 1X PBST (Phosphate buffered saline solution plus 0.05% Tween-20) followed by incubation with a 1 : 2500 dilution of a polyclonal Goat-anti rabbit secondary antibody conjugated to alkaline-phosphatase (SouthernBiotech, Birmingham, AL). Bands were visualized colormetrically with the addition of 10ml of a BCIP/NBT Plus solution (SouthernBiotech, Birmingham, AL). Densitometry was determined using ImageJ software (http://rsbweb.nih.gov/ij/download.html).
Bacterial aggregation assay
Three mls of various L. monocytogenes strains were grown in BHI at 37°C with shaking overnight. One ml of the overnight stationary phase culture was removed and left static at room-temperature and the optical-density at 600nm was measured at the indicated time points. Bacterial aggregation was indicated via the evident decrease of the optical-density of the culture supernatant as the bacteria aggregated out of solution.
Mouse infections
All animal procedures were IACUC approved and performed in the Biological Resources Laboratory at the University of Illinois at Chicago. Overnight bacterial overnight cultures were diluted 1 : 20 into fresh media and grown to an OD600 ∼0.6. 1 ml of culture (corresponding to 6x108 CFU/ml) was washed, diluted and resuspended in PBS to a final concentration of 1x105 CFU/ml. 8–10 week old female Swiss Webster mice (Charles River Laboratories, Chicago, IL) were injected with 200 ul PBS containing 2x104 CFU L. monocytogenes via the tail vein. At 24, 48 and 72 hrs post-infection, mice were sacrificed and livers and spleens were harvested. Organs were homogenized with a Tissue Master 125 homogenizer (Omni International, Kennesaw, GA) and dilutions were plated onto BHI streptomycin (200 ug/ml) plates. Non-paired student t-test was used for statistical analysis.
Bacterial intracellular growth assays
Bacterial intracellular growth assays in Potoroo tridactylis kidney epithelial cells (PtK2) were performed as previously described [37,68,108]. In brief, monolayers of cells were grown on glass coverslips to confluency and infected with bacterial strains with an MOI of 100 : 1. One hour post-infection, monolayers were washed 3X in PBS and 5 μg/ml of gentamicin was added to kill extracellular bacteria. At indicated time points, coverslips were removed and lysed in 5 mls of sterile H2O to release intracellular bacteria for enumeration of intracellular growth or were processed for microscopy. Actin localization studies were done as previously described [31] except that host cell actin was stained with Alexa Fluor 488 phalloidin (Invitrogen Corp.) at 2, 4, and 6 hours p.i.
For staining of host cell Rab7, PtK2 cells grown on glass coverslips were infected with bacteria as described above. Coverslips were removed at 1.5 hours post-infection, and cells were fixed and permeabilized as previously described for host F-Actin staining but with phalloidin conjugated to Alexa-350. Coverslips were then blocked for 30 minutes in PBS with 10% FBS, washed 5 times in PBS, stained with goat anti-Rab7 (Santa Cruz Biotechnology) for 30 minutes at RT, washed 5 times in PBS, followed by staining with donkey anti-goat conjugated to Texas Red (Santa Cruz Biotechnology) for 30 minutes at RT. Listeria in the infected cells was stained with a rabbit anti-Listeria (BD Biosciences) for 30 minutes at RT, washed in PBS, followed by incubation with a goat anti-rabbit secondary antibody conjugated to Alexa-488 (Invitrogen Corp.) for 30 minutes at RT.
For detection of bacteria contained in vacuoles by binding with YFP-CBD (yellow fluorescent protein fused to the cell wall binding domain of the phage endolysin Ply118, which binds to the Listeria cell surface) [74], semi-confluent monolayers of PtK2 cells grown in 24 well plates were transfected with the YFP-CBD plasmid (a kind gift from Joel A. Swanson, University of Michigan, Ann Arbor, MI) using Fugene 6 (Promega) as per manufactures protocol. Transfected cells were then infected with L. monocytogenes and at 15 and 30 minutes post-infection, coverslips were removed and processed for microscopy. Host cell F-actin was stained using phalloidin conjugated to Texas Red and DNA was stained with DAPI. Bacteria bound with YFP-CBD appeared green.
For infection of murine bone-marrow derived macrophages (BMMØ), macrophages were isolated from the femurs of mice and maintained as described [109] and intracellular growth was performed with an MOI of 0.1 : 1 as described above for J774 cells. Where indicated, 1 ηg/ml of recombinant mouse IFN-γ(Invitrogen Corp., Carlsbad, CA) was added to monolayers of BMMØ 24 hours prior to infection to activate the macrophages.
Measurement of bacterial cell-to-cell spread
Plaque assays were conducted as previously described [110]. Briefly, murine L2 fibroblasts were grown to confluency in 6-well microtiter plates and infected with 20 μl of a normalized 1 : 20 dilution of overnight culture grown at 37°C in BHI with shaking (MOI 10 : 1). One hour post-infection, L2 infected monolayers were washed and 10 μg/ml of gentamicin was added to kill extracellular bacteria. Three days post-infection, Neutral Red (Sigma-Aldrich, St. Louis, MO) was added and plaques were visualized and measured using a micrometer (Finescale, Orange County, CA).
Measurement of β-glucuronidase activity
Overnight cultures of L. monocytogenes were diluted 1 : 20 in fresh BHI or LB containing 25 mM glucose-6-phosphate (Sigma-Aldrich, St. Louis, MO) and 0.2% activated charcoal and grown with shaking at 37°C. At various time points, the OD600 was determined for each culture and 1 ml of each sample was centrifuged. Bacterial pellets were resuspended in 1 ml of ABT buffer [1M potassium phosphate (pH 7.0), 0.1 M NaCl, 1% Triton] and ß-Glucuronidase (GUS) activity was measured as described by Youngman [111] with the substitution of 4-methylumbelliferyl-ß-D-glucuronided (Sigma-Aldrich, St. Louis, MO) in place of 4-methylumbelliferyl-ß-D-galactoside.
Assessment of PlcB-associated phospholipase activity
plcB-dependent phospholipase production was assayed on egg yolk agar plates [66,112]. Antibiotic-free chicken egg yolk was added in a 1 : 1 (vol/vol) ratio to PBS and vortexed to form a suspension. 5 ml of egg yolk suspension was added to 100 ml of molten LB medium plus 0.2% activated charcoal (Sigma-Aldrich, St. Louis, MO) and 25mM glucose-6-phosphate (Sigma-Aldrich, St. Louis, MO), [68,113] and 10 ml of this mixture was poured into Petri dishes. Bacterial strains were gently streaked onto the surface of the plate and incubated at 37°C for 24h. Phospholipase activity was visualized as a zone of opacity surrounding bacterial streaks.
Measurement of LLO-associated hemolytic activity
Stationary-phase bacterial cultures were diluted 1 : 10 into LB medium and grown at 37°C for 5h with shaking. Optical density OD600 was determined, and 1 ml of each culture was normalized and centrifuged at 13,000 x g for 5 min. The supernatant was collected and was assayed for LLO-associated hemolytic activity using phosphate-buffered saline (PBS)-washed sheep erythrocytes (Cocalico Biologicals Inc., Reamstown, PA) as previously described [114]. Hemolytic activity was determined as the reciprocal of the supernatant dilution at which 50% lysis of erythrocytes was observed.
The amount of secreted LLO protein present in the culture supernatants was detected as previously described [68].
2-Dimensional gel electrophoresis and mass spectrometry
Isolation of L. monocytogenes secreted proteins, 2-dimensional gel electrophoresis (Kendrick Labs, Madison, WI), and identification of protein spot differences (Proteomics Core Facility, Research Resources Center at the University of Illinois at Chicago) were all processed as previously described with one minor modification [68,107]. Proteins isolated for this study were obtained from stationary phase cultures grown overnight versus exponential phase cultures that were previously assessed.
Membrane integrity assessments
Membrane permeability differences between the varying strains were assessed using the LIVE/DEAD BacLight Bacterial Viability Kit (Invitrogen Corp., Carlsbad, CA) as previously described [58].
Supporting Information
Zdroje
1. Cook LC, Federle MJ (2013) Peptide pheromone signaling in Streptococcus and Enterococcus. FEMS Microbiol Rev. 38 : 473–492. doi: 10.1111/1574-6976.12046 24118108
2. Parker CT, Sperandio V (2009) Cell-to-cell signalling during pathogenesis. Cell Microbiol 11 : 363–369. doi: 10.1111/j.1462-5822.2008.01272.x 19068097
3. Sturme MH, Kleerebezem M, Nakayama J, Akkermans AD, Vaugha EE, et al. (2002) Cell to cell communication by autoinducing peptides in gram-positive bacteria. Antonie Van Leeuwenhoek 81 : 233–243. 12448722
4. Rutherford ST, Bassler BL (2012) Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2.
5. Garmyn D, Gal L, Lemaitre JP, Hartmann A, Piveteau P (2009) Communication and autoinduction in the species Listeria monocytogenes: A central role for the agr system. Commun Integr Biol 2 : 371–374. 19721895
6. Riedel CU, Monk IR, Casey PG, Waidmann MS, Gahan CG, et al. (2009) AgrD-dependent quorum sensing affects biofilm formation, invasion, virulence and global gene expression profiles in Listeria monocytogenes. Mol Microbiol 71 : 1177–1189. doi: 10.1111/j.1365-2958.2008.06589.x 19154329
7. Autret N, Raynaud C, Dubail I, Berche P, Charbit A (2003) Identification of the agr locus of Listeria monocytogenes: role in bacterial virulence. Infect Immun 71 : 4463–4471. 12874326
8. Rieu A, Weidmann S, Garmyn D, Piveteau P, Guzzo J (2007) agr system of Listeria monocytogenes EGD-e: role in adherence and differential expression pattern. Appl Environ Microbiol 73 : 6125–6133. 17675424
9. Rieu A, Briandet R, Habimana O, Garmyn D, Guzzo J, et al. (2008) Listeria monocytogenes EGD-e biofilms: no mushrooms but a network of knitted chains. Appl Environ Microbiol 74 : 4491–4497. doi: 10.1128/AEM.00255-08 18502930
10. Xayarath B, Freitag NE (2012) Optimizing the balance between host and environmental survival skills: lessons learned from Listeria monocytogenes. Future Microbiol 7 : 839–852. doi: 10.2217/fmb.12.57 22827306
11. Czuprynski CJ (2005) Listeria monocytogenes: silage, sandwiches and science. Anim Health Res Rev 6 : 211–217. 16583783
12. Drevets DA, Bronze MS (2008) Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol Med Microbiol 53 : 151–165. doi: 10.1111/j.1574-695X.2008.00404.x 18462388
13. Freitag NE, Port GC, Miner MD (2009) Listeria monocytogenes—from saprophyte to intracellular pathogen. Nat Rev Microbiol 7 : 623–628. doi: 10.1038/nrmicro2171 19648949
14. Lecuit M (2007) Human listeriosis and animal models. Microbes Infect 9 : 1216–1225. 17720601
15. Swaminathan B, Gerner-Smidt P (2007) The epidemiology of human listeriosis. Microbes Infect 9 : 1236–1243. 17720602
16. Gottlieb SL, Newbern EC, Griffin PM, Graves LM, Hoekstra RM, et al. (2006) Multistate outbreak of Listeriosis linked to turkey deli meat and subsequent changes in US regulatory policy. Clin Infect Dis 42 : 29–36. 16323088
17. Mead PS, Dunne EF, Graves L, Wiedmann M, Patrick M, et al. (2006) Nationwide outbreak of listeriosis due to contaminated meat. Epidemiol Infect 134 : 744–751. 16318652
18. Swaminathan B, Gerner-Smidt P, Whichard JM (2006) Foodborne disease trends and reports. Foodborne Pathog Dis 3 : 316–318. 17199513
19. McCollum JT, Cronquist AB, Silk BJ, Jackson KA, O'Connor KA, et al. (2013) Multistate outbreak of listeriosis associated with cantaloupe. N Engl J Med 369 : 944–953. doi: 10.1056/NEJMoa1215837 24004121
20. (2013) Vital signs: Listeria illnesses, deaths, and outbreaks—United States, 2009–2011. MMWR Morb Mortal Wkly Rep 62 : 448–452. 23739339
21. Bortolussi R (2008) Listeriosis: a primer. Cmaj 179 : 795–797. doi: 10.1503/cmaj.081377 18787096
22. Camejo A, Carvalho F, Reis O, Leitao E, Sousa S, et al. (2011) The arsenal of virulence factors deployed by Listeria monocytogenes to promote its cell infection cycle. Virulence 2 : 379–394. doi: 10.4161/viru.2.5.17703 21921683
23. Dussurget O, Pizarro-Cerda J, Cossart P (2004) Molecular determinants of Listeria monocytogenes virulence. Annu Rev Microbiol 58 : 587–610. 15487949
24. Mostowy S, Cossart P (2012) Virulence factors that modulate the cell biology of Listeria infection and the host response. Adv Immunol 113 : 19–32. doi: 10.1016/B978-0-12-394590-7.00007-5 22244577
25. Freitag NE (2006) From hot dogs to host cells: how the bacterial pathogen Listeria monocytogenes regulates virulence gene expression. Future Microbiol 1 : 89–101. 17661688
26. de las Heras A, Cain RJ, Bielecka MK, Vazquez-Boland JA (2011) Regulation of Listeria virulence: PrfA master and commander. Curr Opin Microbiol 14 : 118–127. doi: 10.1016/j.mib.2011.01.005 21388862
27. Scortti M, Monzo HJ, Lacharme-Lora L, Lewis DA, Vazquez-Boland JA (2007) The PrfA virulence regulon. Microbes Infect 9 : 1196–1207. 17764998
28. Korner H, Sofia HJ, Zumft WG (2003) Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol Rev 27 : 559–592. 14638413
29. Lampidis R, Gross R, Sokolovic Z, Goebel W, Kreft J (1994) The virulence regulator protein of Listeria ivanovii is highly homologous to PrfA from Listeria monocytogenes and both belong to the Crp-Fnr family of transcription regulators. Mol Microbiol 13 : 141–151. 7984088
30. Vega Y, Dickneite C, Ripio MT, Bockmann R, Gonzalez-Zorn B, et al. (1998) Functional similarities between the Listeria monocytogenes virulence regulator PrfA and cyclic AMP receptor protein: the PrfA* (Gly145Ser) mutation increases binding affinity for target DNA. J Bacteriol 180 : 6655–6660. 9852011
31. Miner MD, Port GC, Bouwer HG, Chang JC, Freitag NE (2008) A novel prfA mutation that promotes Listeria monocytogenes cytosol entry but reduces bacterial spread and cytotoxicity. Microb Pathog 45 : 273–281. doi: 10.1016/j.micpath.2008.06.006 18675335
32. Ripio MT, Dominguez-Bernal G, Lara M, Suarez M, Vazquez-Boland JA (1997) A Gly145Ser substitution in the transcriptional activator PrfA causes constitutive overexpression of virulence factors in Listeria monocytogenes. J Bacteriol 179 : 1533–1540. 9045810
33. Shetron-Rama LM, Mueller K, Bravo JM, Bouwer HG, Way SS, et al. (2003) Isolation of Listeria monocytogenes mutants with high-level in vitro expression of host cytosol-induced gene products. Mol Microbiol 48 : 1537–1551. 12791137
34. Xayarath B, Smart JI, Mueller KJ, Freitag NE (2011) A novel C-terminal mutation resulting in constitutive activation of the Listeria monocytogenes central virulence regulatory factor PrfA. Microbiology. 157 : 3138–3149. doi: 10.1099/mic.0.049957-0 21835879
35. Monk IR, Gahan CG, Hill C (2008) Tools for functional postgenomic analysis of Listeria monocytogenes. Appl Environ Microbiol 74 : 3921–3934. doi: 10.1128/AEM.00314-08 18441118
36. Vega Y, Rauch M, Banfield MJ, Ermolaeva S, Scortti M, et al. (2004) New Listeria monocytogenes prfA* mutants, transcriptional properties of PrfA* proteins and structure-function of the virulence regulator PrfA. Mol Microbiol 52 : 1553–1565. 15186408
37. Wong KK, Freitag NE (2004) A novel mutation within the central Listeria monocytogenes regulator PrfA that results in constitutive expression of virulence gene products. J Bacteriol 186 : 6265–6276. 15342597
38. Port GC, Freitag NE (2007) Identification of novel Listeria monocytogenes secreted virulence factors following mutational activation of the central virulence regulator, PrfA. Infect Immun 75 : 5886–5897. 17938228
39. Clewell DB (2007) Properties of Enterococcus faecalis plasmid pAD1, a member of a widely disseminated family of pheromone-responding, conjugative, virulence elements encoding cytolysin. Plasmid 58 : 205–227. 17590438
40. Dunny GM, Craig RA, Carron RL, Clewell DB (1979) Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid 2 : 454–465. 113798
41. Mori M, Sakagami Y, Narita M, Isogai A, Fujino M, et al. (1984) Isolation and structure of the bacterial sex pheromone, cAD1, that induces plasmid transfer in Streptococcus faecalis. FEBS Lett 178 : 97–100. 6437872
42. An FY, Sulavik MC, Clewell DB (1999) Identification and characterization of a determinant (eep) on the Enterococcus faecalis chromosome that is involved in production of the peptide sex pheromone cAD1. J Bacteriol 181 : 5915–5921. 10498702
43. An FY, Clewell DB (2002) Identification of the cAD1 sex pheromone precursor in Enterococcus faecalis. J Bacteriol 184 : 1880–1887. 11889094
44. Tanimoto K, Clewell DB (1993) Regulation of the pAD1-encoded sex pheromone response in Enterococcus faecalis: expression of the positive regulator TraE1. J Bacteriol 175 : 1008–1018. 8432694
45. Chandler JR, Dunny GM (2004) Enterococcal peptide sex pheromones: synthesis and control of biological activity. Peptides 25 : 1377–1388. 15374642
46. Buttaro BA, Antiporta MH, Dunny GM (2000) Cell-associated pheromone peptide (cCF10) production and pheromone inhibition in Enterococcus faecalis. J Bacteriol 182 : 4926–4933. 10940037
47. Dunny GM, Brown BL, Clewell DB (1978) Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A 75 : 3479–3483. 98769
48. Galli D, Lottspeich F, Wirth R (1990) Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol Microbiol 4 : 895–904. 2120541
49. Galli D, Wirth R, Wanner G (1989) Identification of aggregation substances of Enterococcus faecalis cells after induction by sex pheromones. An immunological and ultrastructural investigation. Arch Microbiol 151 : 486–490. 2505719
50. Travier L, Guadagnini S, Gouin E, Dufour A, Chenal-Francisque V, et al. (2013) ActA promotes Listeria monocytogenes aggregation, intestinal colonization and carriage. PLoS Pathog 9: e1003131. doi: 10.1371/journal.ppat.1003131 23382675
51. Chandler JR, Dunny GM (2008) Characterization of the sequence specificity determinants required for processing and control of sex pheromone by the intramembrane protease Eep and the plasmid-encoded protein PrgY. J Bacteriol 190 : 1172–1183. 18083822
52. Fixen KR, Chandler JR, Le T, Kozlowicz BK, Manias DA, et al. (2007) Analysis of the amino acid sequence specificity determinants of the enterococcal cCF10 sex pheromone in interactions with the pheromone-sensing machinery. J Bacteriol 189 : 1399–1406. 17098891
53. Kovacs-Simon A, Titball RW, Michell SL (2011) Lipoproteins of bacterial pathogens. Infect Immun 79 : 548–561. doi: 10.1128/IAI.00682-10 20974828
54. Tanimoto K, An FY, Clewell DB (1993) Characterization of the traC determinant of the Enterococcus faecalis hemolysin-bacteriocin plasmid pAD1: binding of sex pheromone. J Bacteriol 175 : 5260–5264. 8349566
55. Borezee E, Pellegrini E, Berche P (2000) OppA of Listeria monocytogenes, an oligopeptide-binding protein required for bacterial growth at low temperature and involved in intracellular survival. Infect Immun 68 : 7069–7077. 11083832
56. Verheul A, Hagting A, Amezaga MR, Booth IR, Rombouts FM, et al. (1995) A di - and tripeptide transport system can supply Listeria monocytogenes Scott A with amino acids essential for growth. Appl Environ Microbiol 61 : 226–233. 7887604
57. Wouters JA, Hain T, Darji A, Hufner E, Wemekamp-Kamphuis H, et al. (2005) Identification and characterization of Di - and tripeptide transporter DtpT of Listeria monocytogenes EGD-e. Appl Environ Microbiol 71 : 5771–5778. 16204487
58. Xayarath B, Marquis H, Port GC, Freitag NE (2009) Listeria monocytogenes CtaP is a multifunctional cysteine transport-associated protein required for bacterial pathogenesis. Mol Microbiol 74 : 956–973. doi: 10.1111/j.1365-2958.2009.06910.x 19818015
59. Verheul A, Rombouts FM, Abee T (1998) Utilization of oligopeptides by Listeria monocytogenes Scott A. Appl Environ Microbiol 64 : 1059–1065. 9501445
60. Schnupf P, Portnoy DA (2007) Listeriolysin O: a phagosome-specific lysin. Microbes Infect 9 : 1176–1187. 17720603
61. Mengaud J, Braun-Breton C, Cossart P (1991) Identification of phosphatidylinositol-specific phospholipase C activity in Listeria monocytogenes: a novel type of virulence factor? Mol Microbiol 5 : 367–372. 1645839
62. Camilli A, Goldfine H, Portnoy DA (1991) Listeria monocytogenes mutants lacking phosphatidylinositol-specific phospholipase C are avirulent. J Exp Med 173 : 751–754. 1847723
63. Kathariou S, Metz P, Hof H, Goebel W (1987) Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol 169 : 1291–1297. 3029033
64. Freitag NE, Youngman P, Portnoy DA (1992) Transcriptional activation of the Listeria monocytogenes hemolysin gene in Bacillus subtilis. J Bacteriol 174 : 1293–1298. 1735720
65. Marquis H, Doshi V, Portnoy DA (1995) The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect Immun 63 : 4531–4534. 7591098
66. Mueller KJ, Freitag NE (2005) Pleiotropic enhancement of bacterial pathogenesis resulting from the constitutive activation of the Listeria monocytogenes regulatory factor PrfA. Infect Immun 73 : 1917–1926. 15784531
67. Portnoy DA, Jacks PS, Hinrichs DJ (1988) Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med 167 : 1459–1471. 2833557
68. Alonzo F 3rd, Port GC, Cao M, Freitag NE (2009) The posttranslocation chaperone PrsA2 contributes to multiple facets of Listeria monocytogenes pathogenesis. Infect Immun 77 : 2612–2623. doi: 10.1128/IAI.00280-09 19451247
69. Smith GA, Portnoy DA (1997) How the Listeria monocytogenes ActA protein converts actin polymerization into a motile force. Trends Microbiol 5 : 272–276. 9234509
70. Portnoy DA, Auerbuch V, Glomski IJ (2002) The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J Cell Biol 158 : 409–414. 12163465
71. Lambrechts A, Gevaert K, Cossart P, Vandekerckhove J, Van Troys M (2008) Listeria comet tails: the actin-based motility machinery at work. Trends Cell Biol 18 : 220–227. doi: 10.1016/j.tcb.2008.03.001 18396046
72. O'Riordan M, Portnoy DA (2002) The host cytosol: front-line or home front? Trends Microbiol 10 : 361–364. 12160633
73. Joseph B, Goebel W (2007) Life of Listeria monocytogenes in the host cells' cytosol. Microbes Infect 9 : 1188–1195. 17719818
74. Henry R, Shaughnessy L, Loessner MJ, Alberti-Segui C, Higgins DE, et al. (2006) Cytolysin-dependent delay of vacuole maturation in macrophages infected with Listeria monocytogenes. Cell Microbiol 8 : 107–119. 16367870
75. Shaughnessy LM, Hoppe AD, Christensen KA, Swanson JA (2006) Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell Microbiol 8 : 781–792. 16611227
76. Drevets DA (1999) Dissemination of Listeria monocytogenes by infected phagocytes. Infect Immun 67 : 3512–3517. 10377133
77. Grossman AD (1995) Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu Rev Genet 29 : 477–508. 8825484
78. Pottathil M, Lazazzera BA (2003) The extracellular Phr peptide-Rap phosphatase signaling circuit of Bacillus subtilis. Front Biosci 8: d32–45. 12456319
79. Clewell DB, Francia MV, Flannagan SE, An FY (2002) Enterococcal plasmid transfer: sex pheromones, transfer origins, relaxases, and the Staphylococcus aureus issue. Plasmid 48 : 193–201. 12460535
80. Qin X, Singh KV, Weinstock GM, Murray BE (2000) Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect Immun 68 : 2579–2586. 10768947
81. Hancock LE, Perego M (2004) The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J Bacteriol 186 : 5629–5639. 15317767
82. Bourgogne A, Hilsenbeck SG, Dunny GM, Murray BE (2006) Comparison of OG1RF and an isogenic fsrB deletion mutant by transcriptional analysis: the Fsr system of Enterococcus faecalis is more than the activator of gelatinase and serine protease. J Bacteriol 188 : 2875–2884. 16585749
83. Havarstein LS, Coomaraswamy G, Morrison DA (1995) An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci U S A 92 : 11140–11144. 7479953
84. Perry JA, Jones MB, Peterson SN, Cvitkovitch DG, Levesque CM (2009) Peptide alarmone signalling triggers an auto-active bacteriocin necessary for genetic competence. Mol Microbiol 72 : 905–917. doi: 10.1111/j.1365-2958.2009.06693.x 19400789
85. Novick RP, Geisinger E (2008) Quorum sensing in staphylococci. Annu Rev Genet 42 : 541–564. doi: 10.1146/annurev.genet.42.110807.091640 18713030
86. Thoendel M, Kavanaugh JS, Flack CE, Horswill AR (2011) Peptide signaling in the staphylococci. Chem Rev 111 : 117–151. doi: 10.1021/cr100370n 21174435
87. Bendtsen JD, Kiemer L, Fausboll A, Brunak S (2005) Non-classical protein secretion in bacteria. BMC Microbiol 5 : 58. 16212653
88. Lenz LL, Mohammadi S, Geissler A, Portnoy DA (2003) SecA2-dependent secretion of autolytic enzymes promotes Listeria monocytogenes pathogenesis. Proc Natl Acad Sci U S A 100 : 12432–12437. 14527997
89. Renier S, Chambon C, Viala D, Chagnot C, Hebraud M, et al. (2013) Exoproteomic analysis of the SecA2-dependent secretion in Listeria monocytogenes EGD-e. J Proteomics 80C: 183–195.
90. Rabinovich L, Sigal N, Borovok I, Nir-Paz R, Herskovits AA (2012) Prophage excision activates Listeria competence genes that promote phagosomal escape and virulence. Cell 150 : 792–802. doi: 10.1016/j.cell.2012.06.036 22901809
91. Claverys JP, Prudhomme M, Martin B (2006) Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu Rev Microbiol 60 : 451–475. 16771651
92. Bhatty M, Laverde Gomez JA, Christie PJ (2013) The expanding bacterial type IV secretion lexicon. Res Microbiol 164 : 620–639. doi: 10.1016/j.resmic.2013.03.012 23542405
93. Last NB, Schlamadinger DE, Miranker AD (2013) A common landscape for membrane-active peptides. Protein Sci 22 : 870–882. doi: 10.1002/pro.2274 23649542
94. Xayarath B, Volz KW, Smart JI, Freitag NE (2011) Probing the Role of Protein Surface Charge in the Activation of PrfA, the Central Regulator of Listeria monocytogenes Pathogenesis. PLoS One 6: e23502. doi: 10.1371/journal.pone.0023502 21858145
95. Kozlowicz BK, Dworkin M, Dunny GM (2006) Pheromone-inducible conjugation in Enterococcus faecalis: a model for the evolution of biological complexity? Int J Med Microbiol 296 : 141–147. 16503196
96. Hirt H, Schlievert PM, Dunny GM (2002) In vivo induction of virulence and antibiotic resistance transfer in Enterococcus faecalis mediated by the sex pheromone-sensing system of pCF10. Infect Immun 70 : 716–723. 11796604
97. Chow JW, Thal LA, Perri MB, Vazquez JA, Donabedian SM, et al. (1993) Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob Agents Chemother 37 : 2474–2477. 8285637
98. Kreft B, Marre R, Schramm U, Wirth R (1992) Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun 60 : 25–30. 1729187
99. Sussmuth SD, Muscholl-Silberhorn A, Wirth R, Susa M, Marre R, et al. (2000) Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect Immun 68 : 4900–4906. 10948103
100. Aggarwal C, Jimenez JC, Nanavati D, Federle MJ (2014) Multiple length peptide-pheromone variants produced by Streptococcus pyogenes directly bind Rgg proteins to confer transcriptional regulation. J Biol Chem 289 : 22427–22436. doi: 10.1074/jbc.M114.583989 24958729
101. Mori M, Sakagami Y, Ishii Y, Isogai A, Kitada C, et al. (1988) Structure of cCF10, a peptide sex pheromone which induces conjugative transfer of the Streptococcus faecalis tetracycline resistance plasmid, pCF10. J Biol Chem 263 : 14574–14578. 3139658
102. Flannagan SE, Clewell DB (2002) Identification and characterization of genes encoding sex pheromone cAM373 activity in Enterococcus faecalis and Staphylococcus aureus. Mol Microbiol 44 : 803–817. 11994160
103. Baumgartner M, Karst U, Gerstel B, Loessner M, Wehland J, et al. (2007) Inactivation of Lgt allows systematic characterization of lipoproteins from Listeria monocytogenes. J Bacteriol 189 : 313–324. 17041050
104. Smith K, Youngman P (1992) Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie 74 : 705–711. 1391050
105. Hodgson DA (2000) Generalized transduction of serotype 1/2 and serotype 4b strains of Listeria monocytogenes. Mol Microbiol 35 : 312–323. 10652092
106. Alonzo F 3rd, Freitag NE (2010) Listeria monocytogenes PrsA2 is required for virulence factor secretion and bacterial viability within the host cell cytosol. Infect Immun 78 : 4944–4957. doi: 10.1128/IAI.00532-10 20823208
107. Alonzo F 3rd, Xayarath B, Whisstock JC, Freitag NE (2011) Functional analysis of the Listeria monocytogenes secretion chaperone PrsA2 and its multiple contributions to bacterial virulence. Mol Microbiol 80 : 1530–1548. doi: 10.1111/j.1365-2958.2011.07665.x 21545417
108. Moors MA, Levitt B, Youngman P, Portnoy DA (1999) Expression of listeriolysin O and ActA by intracellular and extracellular Listeria monocytogenes. Infect Immun 67 : 131–139. 9864207
109. Portnoy DA, Jacks PS, Hinrichs DJ (1988) Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med 167 : 1459–1471. 2833557
110. Sun AN, Camilli A, Portnoy DA (1990) Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun 58 : 3770–3778. 2172168
111. Youngman P (1987) Plasmid vectors recovering and exploiting Tn917 transposons in Bacillus and other Gram-positive bacteria. Plasmids: A Practical Approach: 79–103.
112. Alonzo F, 3rd, Port GC, Cao M, Freitag NE (2009) The post-translocation chaperone PrsA2 contributes to multiple facets of Listeria monocytogenes pathogenesis. Infect Immun.
113. Bitar AP, Cao M, Marquis H (2008) The metalloprotease of Listeria monocytogenes is activated by intramolecular autocatalysis. J Bacteriol 190 : 107–111. 17965168
114. Camilli A, Paynton CR, Portnoy DA (1989) Intracellular methicillin selection of Listeria monocytogenes mutants unable to replicate in a macrophage cell line. Proc Natl Acad Sci U S A 86 : 5522–5526. 2501788
115. Bishop DK, Hinrichs DJ (1987) Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol 139 : 2005–2009. 3114382
116. Jones S, Portnoy DA (1994) Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect Immun 62 : 5608–5613. 7960143
117. Miner MD, Port GC, Freitag NE (2008) Functional impact of mutational activation on the Listeria monocytogenes central virulence regulator PrfA. Microbiology 154 : 3579–3589. doi: 10.1099/mic.0.2008/021063-0 18957610
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



