-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInduction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
Interferon Regulatory Factor 3 (IRF3) is an essential transcription factor for the expression of antiviral genes, including type I IFNs and ISGs. The coordinated action of the ISGs leads to the inhibition of one or multiple steps of viral life cycle. In contrast to the well-known antiviral function of IRF3, we report here an unexpected pro-parasitic role of IRF3 in supporting the replication of the protozoan parasite, Toxoplasma gondii, in both cells and mice. The IRF3-deficient mice did not support T. gondii replication and, therefore, were protected from T. gondii-induced pathogenesis. The novel pro-Toxoplasma role of IRF3 was type I IFN-independent, but required its transcriptional function that induced the effector ISGs. Using cells deficient in known components of the IRF3 activation pathways, we have delineated the nature of the pro-parasitic signaling pathway, which we named ‘PISA’. Our detailed genetic and biochemical analyses revealed that PISA is activated by a T. gondii-triggered cytoplasmic cGAS/STING/TBK1-dependent pathway that activates IRF3 for the induction of the pro-parasitic ISGs.
Published in the journal: . PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004779
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004779Summary
Interferon Regulatory Factor 3 (IRF3) is an essential transcription factor for the expression of antiviral genes, including type I IFNs and ISGs. The coordinated action of the ISGs leads to the inhibition of one or multiple steps of viral life cycle. In contrast to the well-known antiviral function of IRF3, we report here an unexpected pro-parasitic role of IRF3 in supporting the replication of the protozoan parasite, Toxoplasma gondii, in both cells and mice. The IRF3-deficient mice did not support T. gondii replication and, therefore, were protected from T. gondii-induced pathogenesis. The novel pro-Toxoplasma role of IRF3 was type I IFN-independent, but required its transcriptional function that induced the effector ISGs. Using cells deficient in known components of the IRF3 activation pathways, we have delineated the nature of the pro-parasitic signaling pathway, which we named ‘PISA’. Our detailed genetic and biochemical analyses revealed that PISA is activated by a T. gondii-triggered cytoplasmic cGAS/STING/TBK1-dependent pathway that activates IRF3 for the induction of the pro-parasitic ISGs.
Introduction
Toxoplasma gondii, an obligate intracellular protozoan, is responsible for severe Toxoplasmosis [1]. Roughly one-third of the world's population is infected with T. gondii, which may lead to a mononucleosis-like syndrome with fever, lymph node enlargement, asthenia and headache. T. gondii is a major cause of blindness [1], and infection in pregnant women, transmitted transplacentally, can cause congenital fetal toxoplasmosis, leading to miscarriage, microcephaly, hydrocephalus, and seizures. To date, there is no vaccine against T. gondii for human use and no permanent cure for chronic toxoplasmosis; moreover, therapies such as pyrimethamine and clindamycin have significant side effects, including bone marrow suppression, rashes, and male infertility [2,3]. T. gondii evades adaptive immunity by transforming into dormant cysts that cause an asymptomatic chronic infection [4]. For this reason, the innate immune response of the host against T. gondii has received considerable attention, focused almost exclusively on cells of the immune system, such as macrophages and dendritic cells (DCs), and several key cytokines produced by these cells in response to T. gondii infection [5–10]. In contrast, little is known about the innate immune response that T. gondii elicits in non-immune cells, such as the epithelia, fibroblasts, the central nervous system (CNS) and ocular cells, which together represent important host organs for the parasite. In the current study, we investigated the role of the type I interferon (IFN) system, the most prominent antiviral innate immune response, in T. gondii infection of cells of immune and non-immune origins.
Microbial infection of mammalian hosts elicits a variety of immune responses that are temporally regulated. An early response is the activation of the innate immune signaling pathways that lead to the transcriptional induction of many cellular genes, including those encoding cytokines; the cytokines are then secreted and act upon as yet uninfected cells to forearm them against oncoming microbial infection. The IFN system is a good example of such a circuitry [11], whereby virus infection induces the synthesis of type I IFN that is secreted and activates immune cells to eliminate the infected cells. In addition, IFN can directly induce an antiviral state in a cell by inducing hundreds of genes, called IFN-stimulated genes (ISG), which encode intracellular proteins, some with the ability to interfere with different stages of virus replication. Surprisingly, ISGs can also be induced by many other signaling pathways activated by microbial infection, without any involvement of IFN, indicating a much broader physiological role of these genes [12].
Much is known about how ISGs are induced by microbes. Microbial pathogen-associated molecular patterns (PAMP) are recognized by cellular pattern recognition receptors (PRR), such as membrane-bound Toll-like receptors (TLR), cytoplasmic RIG-I-like receptors (RLRs), and various cytoplasmic DNA receptors [13,14]. One such receptor, STING, can be activated either by direct DNA-binding or by cyclic dinucleotides produced by the cyclic GMP-AMP synthase (cGAS), which is also activated by cytoplasmic DNA [15–19]. The PRRs use adaptor proteins, such as MyD88, TRIF or MAVS, to assemble different multi-protein signaling complexes, including specific protein kinases. One such protein kinase is TBK1, used by TLR3, TLR4, RLRs and STING to directly phosphorylate the latent transcription factor, IRF3, and activate it [20]. Activation causes nuclear translocation of IRF3, where it induces transcription of ISGs by binding to a specific promoter sequence, called ISRE. Thus, any signaling pathway that can activate TBK1 and IRF3 has the ability to induce ISGs. Other genes, such as that of IFN-β itself, need in addition to IRF3, other transcription factors for induction. Because the ISRE is recognized by all nine members of the IRF family, some signaling pathways use other IRFs to induce ISGs. For example, type I IFN-signaling uses a transcription complex containing IRF9 for this purpose. Thus, in an infected cell, ISGs are directly induced by IRF3, but concomitant synthesis of type I IFN can reinforce the ISG induction.
Here, we report that ISG induction by IRF3 is not only not detrimental to the parasite but it actually promotes efficient T. gondii replication, in cell cultures or in mice, although type I IFN itself has no role in it. We present evidence that this novel interaction between the parasite and IRF3, which we have termed "parasite-IRF3 signaling activation" (PISA), is achieved by parasite-mediated activation of TBK1 through the cGAS/STING pathway.
Results
Efficient replication of T. gondii requires host IRF3
To investigate whether the type I IFN system regulates the replication of unicellular parasites, we used the virulent T. gondii RH strain as a model, and measured parasite replication by immunoblot of parasitic SAG1 protein as well as quantitative PCR (qPCR) of the parasitic genomic DNA. Two types of mouse cells, mouse embryonic fibroblasts (MEFs) and bone marrow-derived DCs (BMDCs), and two human cell lines, HT1080 (fibrosarcoma) and M17 (neuroblastoma), were used, and in all cell types TgSAG1 expression increased over time after parasite infection. Using this assay, we observed that T. gondii RH replicated poorly in cells deficient in IRF3 (Fig. 1A, 1B, 1C; S1 Fig.). Specifically, in MEFs (Fig. 1A) and BMDCs (Fig. 1B) from IRF3 -/ - mice, or in human cells in which IRF3 was knocked down by shRNA (KD) (Fig. 1C), T. gondii replicated poorly. Expression of recombinant IRF3 in the HT1080 KD cells improved their ability to support parasite replication (Fig. 1C).
Fig. 1. T. gondii replication is stimulated by IRF3. 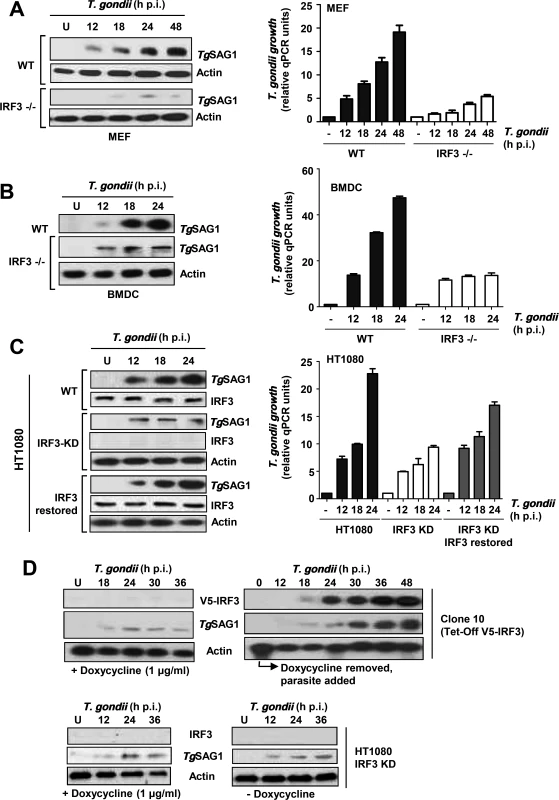
A/B/C, Three types of cells (MEF: mouse embryonic fibroblasts, DC: mouse dendritic cells, HT1080: human fibroblasts) were infected with T. gondii RH (a virulent type I strain). Total infected cell proteins at the indicated times post-infection were analyzed by immunoblotting to detect T. gondii SAG1 protein. U = uninfected cell control. The IRF3-deficient (KO = knockout; KD = knockdown with stably expressed shRNA) cells were compared with the corresponding wild type (WT), or with IRF3- restored, cells as noted. Parasite growth was also quantified by qPCR of genomic DNA using the invariant ITS-1 primers and normalized against GAPDH. D, Use of Tet (Dox)-off IRF3 expression system (Clone 10 cells) shows parasitic growth kinetics in response to IRF3. Immunoblot of the V5-tagged Tet-regulated recombinant IRF3 (top panels), T. gondii SAG1 (middle panels), and control actin (bottom panels) are shown. Left: Poor parasite growth in IRF3-repressed cells. Right: Growth kinetics of the parasite in the Clone 10 cells, in which IRF3 was induced by the withdrawal of Dox. In all panels, actin serves as the loading control. V5-IRF3 was detected by anti-V5 antibody. To gain an understanding of the kinetics of IRF3 action, we used an engineered cell line, Clone 10, expressing recombinant V5-tagged IRF3 under Doxycycline (Dox) control [21]. To perform this experiment, we first determined the inhibitory concentration of Dox against T. gondii. Both the Western blot and the quantification of parasitic DNA showed an IC50 of 10–12 μg/ml in HT1080 host cells under our growth conditions (S2 Fig.), and essentially no inhibition at 1 μg/ml, the concentration of Dox we used to shut-down IRF3 expression in Clone 10 cells. When grown in the presence of Dox (1 μg/ml), these cells did not express IRF3 and did not support efficient T. gondii replication (Fig. 1D, left). Removal of Dox resulted in the appearance of IRF3 protein around 18 h and promoted robust T. gondii growth that closely followed on the heels of IRF3 appearance (Fig. 1D, right), consistent with parasitic growth stimulation by IRF3.
We further confirmed the stimulatory role IRF3 on T. gondii by two independent techniques: microscopy and flow cytometry, both of which clearly revealed stunted parasite replication in the IRF3-/ - cells. In confocal microscopy, more parasites are seen the PVs in the wild type MEFs than in the IRF3-/ - MEF at any time point of infection (Fig. 2A). For flow cytometry (Fig. 2B), we set the baseline to detect cells containing two or more parasites. With this gating, nearly all initially infected WT BMDCs scored as positive (30.91% of total, matching the m.o.i. of 0.3), whereas the number was significantly lower (6.28%) in the IRF3-/ - BMDCs (Fig. 2B). When gated for 1 parasite per cell, KO and WT cells showed the same percentage of parasite-positive cells (30–33% of the total population), which was equal to the m.o.i (as shown for WT) (Fig. 2B). These results also indicated that IRF3 deficiency affected the intracellular replication of the parasite, but not the entry or the formation of PV.
Fig. 2. IRF3 facilitates intracellular T. gondii replication regardless of parasite virulence type. 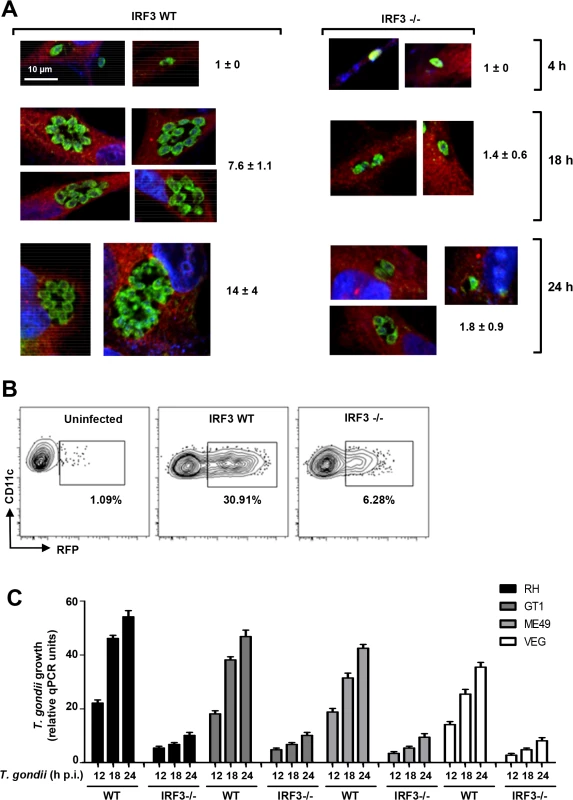
A, Stunted parasite replication in IRF3 -/- cells. Isogenic wild type and IRF3 -/- MEF cells were infected by T. gondii RH at an m.o.i. of 0.2, and at the indicated times (4, 18, 24 h) stained for parasites (green), cytoplasmic actin (red) and nucleus (DAPI, blue) and visualized by confocal microscopy. For each sample, several individual PVs are shown; number of parasites in 12–14 PVs was counted and the mean ± SD values are shown. B, Flow cytometry measurement of parasite growth. DCs isolated from wild type and IRF3 -/- mice were infected with T. gondii RH at 0.3 m.o.i., and cells were permeabilized and dual-stained for CD11c and parasitic SAG1 at 18 h post-infection, with uninfected wild type cells as control. Gating was set to count infected cells with 2 or more parasites per PV, as marked by contour plot (left), and the percentage of such cells in the total population is shown. C, IRF3 is important for optimal growth of diverse T. gondii strains. The indicated T. gondii strains, represented the three major types: GT1 (type I), ME49 (type II), and VEG (type III). Infection was performed as in Fig. 1A, and total DNA was isolated at the indicated times. The amount of T. gondii DNA was quantified by qPCR of the genomic DNA as in Fig. 1. The experiments described thus far used T. gondii RH, a highly laboratory-passaged non-cyst-forming strain. To inquire if the need for IRF3 extends to other types of T. gondii, we tested the growth of three more strains by qRT-PCR, which showed that IRF3 is needed not only for RH, but also for another type I strain (GT1) as well as type II (ME49) and type III (VEG) strains, although the extent of IRF3-dependence varied (Fig. 2C).
Together, using four independent parasite growth analyses, e.g. immunoblot, qRT-PCR, flow cytometry and confocal microscopy, we demonstrated a specific role of IRF3 in augmenting T. gondii replication that is independent of host cell and parasite types.
Cellular gene induction by IRF3 is essential for parasite growth
Although IRF3 is well known as a transcription factor, we have shown that it can trigger apoptosis by a transcription-independent mechanism [22]. To inquire which function of IRF3 is critical for supporting parasite growth, we capitalized on the fact that the transcriptional function but not the pro-apoptotic function of IRF3 absolutely requires the presence of HDAC6, which deacetylates β-catenin, an obligatory co-activator of IRF3-driven transcription [23]. T. gondii in fact replicated very poorly in HDAC6-/ - MEF cells (Fig. 3A), reinforcing our conclusion that in order to support parasite replication IRF3 was acting as a transcription factor of its target genes. Indeed, in infected cells, there was strong induction of ISGs, as manifested by the presence of the ISG56 protein (Fig. 3B, upper half). ISG56 induction did not require induced IFN as an intermediate because in IFNAR-/ - cells, which cannot respond to type I IFN, infection caused similar induction of ISG56; moreover, T. gondii replicated well in these cells (Fig. 3B, lower half). As stated above, IFN can also induce ISGs, but not its own gene. Hence, we treated IRF3 KO MEF cells, which supported T. gondii growth poorly, with exogenous IFN-β to induce the ISGs and then challenged them with the parasite. As shown, T. gondii could replicate efficiently in IFN-β-treated cells, even when the cells did not express any IRF3 (Fig. 3C). These results strongly suggest that one or more ISG-encoded proteins, induced by activated IRF3, facilitate parasite replication; however, type I IFN is not required for this action of IRF3, although it can substitute for IRF3 by virtue of the fact that IFN and IRF3 can induce the expression of an overlapping set of genes, the ISGs.
Fig. 3. Requirement of IRF3-induced host genes for T. gondii replication. 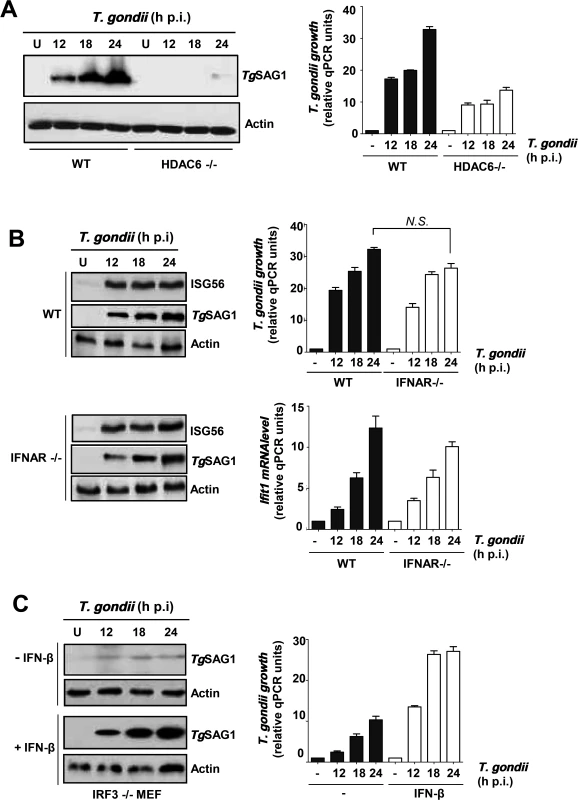
A, Infection and immunoblotting was performed with HDAC6 KO and wild type MEF cells. B, MEF cells (wild type or IFNAR KO) were infected with T. gondii and the indicated proteins were measured by immunoblotting. C, T. gondii growth measured in IRF3 KO MEF cells; where indicated, recombinant murine IFN-β (2,000 U/ml, R&D Systems) was added, and parasite was added 6 h later. Immunoblotting was performed as above. Where shown, parasite growth was also quantified by qPCR of genomic DNA, and ISG56 (Ifit1) mRNA was quantified by qRT-PCR as described before [66]. PISA activates IRF3 by its TBK1-mediated phosphorylation
Activation of IRF3, as a transcription factor, requires phosphorylation of at least two of its serine residues (Ser396, Ser398 in human IRF3 and Ser388, Ser390 in mouse IRF3) [24]. The known signaling pathways activate IRF3 through its phosphorylation by the protein kinase, TBK1, which in turn is activated by signal-dependent auto-phosphorylation [25]. The same scenario held true for IRF3 activation in T. gondii-infected cells. IRF3 was phosphorylated upon infection in all cell lines tested and the phosphorylation was sustained, indicating the continuous presence of active IRF3 in infected cells (Fig. 4A, left). In all cases, endogenous TBK1 was also phosphorylated with similar kinetics (Fig. 4A, right). The need for the two target Ser residues, 396 and 398, was confirmed by using an IRF3 mutant in which they were mutated; in IRF3 KD cells, expression of wild type IRF3 promoted efficient replication of T. gondii but the mutant IRF3 was ineffective (Fig. 4B). Finally, the essential role of TBK1 was confirmed by using TBK1 KO cells, in which IRF3 was not phosphorylated and the parasite replicated poorly; however, expression of recombinant TBK1 in these cells caused IRF3 phosphorylation as well as efficient parasite replication (Fig. 4C).
Fig. 4. IRF3 phosphorylation is essential for PISA and T. gondii replication. 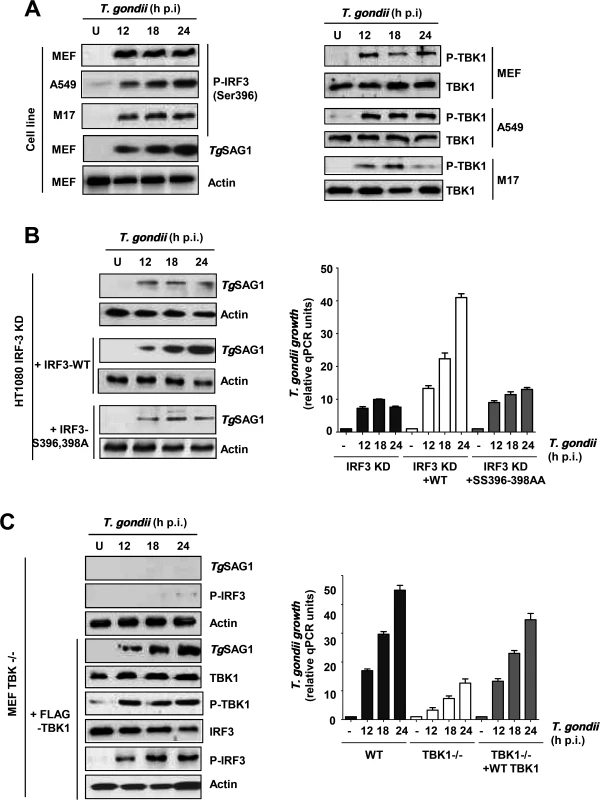
Parasite infection and growth analyses were performed as described in Fig. 1A. Immunoblotting detected the indicated proteins, including phospho-IRF3 (P-IRF3), using a phospho-Ser396-specific antibody. A, The cell lines used are indicated. B, IRF3 KD (knocked-down) HT1080 cell line or these cells, reconstituted with WT or 396/98AA mutant of IRF3, were used for T. gondii infection. C, The indicated TBK1 KO and FLAG-TBK1-restored MEFs were used for T. gondii infection. Where shown, parasite growth was quantified by qPCR of genomic DNA. PISA is TLR-independent
To identify the specific signaling pathway used by PISA, we used various knockout cell lines devoid of strategic signaling molecules (Fig. 5). Without the knowledge of the nature of the PAMP used by T. gondii to activate PISA, we tested the requirements of the major PRRs and their adaptor proteins (Fig. 5A). RLRs were ruled out, because PISA was activated in RIG-I KO cells, as manifested by TgSAG1 synthesis and IRF3 phosphorylation (Fig. 5A, panel 2); the same was true for all TLRs that use MyD88 as the obligatory adaptor protein, since loss of MyD88 also had no effect on PISA (Fig. 5A, panel 3). TLR3 and TLR4 can signal via TRIF instead of MyD88; however, neither of these TLRs were required for PISA (Fig. 5A, panels 4, 5), thus indicating a potentially novel signaling branch in PISA-related cellular signaling.
Fig. 5. Essential role of a TLR3/4-independent cGAS-STING signaling axis in PISA and optimal T. gondii replication. 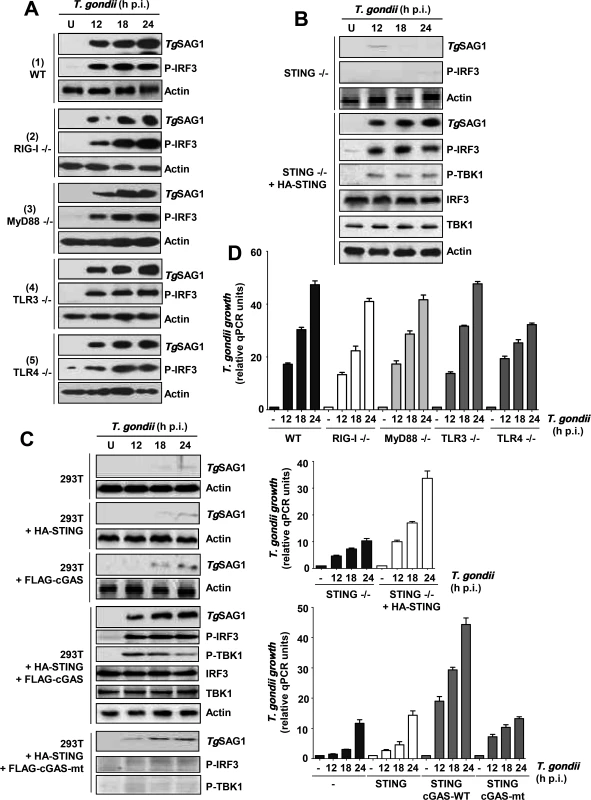
Parasite infection and growth analyses were performed as in Fig. 1A. A, MEFs (WT, RIG-I-/-, TLR3-/-) and primary macrophages (MyD88-/-, TLR4-/-) were used for comparing PISA (TgSAG1 expression or IRF3 phosphorylation) upon T. gondii infection. B/C, STING-/- MEF cells (B) or human 293T cells (C) were transiently transfected with wild type or mutant (mt) FLAG-cGAS and/or HA-STING plasmids, and infected as shown. D, Parasite growth in various conditions used in the other panels, quantified by qPCR; error bars are from three data sets. STING / cGAS signaling pathway is essential for PISA
Since neither RLRs nor TLRs were needed for PISA, we tested the DNA-sensing STING pathway, and found that PISA is indeed defective in STING KO MEF cells and that parasite growth as well as phosphorylation of TBK1 and IRF3 in these cells could be enhanced by recombinant expression of STING (Fig. 5B). STING can directly respond to cytoplasmic DNA; alternatively, the enzyme cGAS is activated by this PAMP and produces cyclic dinucleotides (CDNs), which in turn activate STING. To distinguish between these possibilities, we resorted to 293T cells, which are known to express very little cGAS and STING [18]. As expected, these cells did not support T. gondii replication (Fig. 5C). Ectopic expression of either cGAS or STING alone could not promote parasite growth; however, co-expression of both proteins fully triggered PISA, supporting efficient T. gondii replication as well as TBK1 and IRF3 phosphorylation. In contrast to wild type cGAS, an enzymatically inactive mutant of cGAS failed to trigger PISA. These results suggest that cGAS recognizes a PAMP, possibly parasitic DNA, and produces a CDN that activates STING and consequently TBK1 and IRF3.
Since the naturally low level of STING in 293T cells appeared to correlate with poor T. gondii growth, we inquired whether this may be true for some other commonly used cell lines. We first quantified the STING mRNA levels in the following cell lines, which were either immortalized or cancerous: A549, H196, H1048 (all lung carcinoma cells), HeLa (cervical cancer), and three kinds of 293 cells (also known as HEK293, human embryonic kidney cells), viz. 293T (expressing the T-antigen) and two 293 cell lines of unknown origin, obtained from different laboratories (ours and Dr. George Stark's). Quantitative RT-PCR results (Fig. 6A) revealed that the STING levels in these cells varied widely; A549 and H196 contained the highest amount, the two 293 cell lines contained slightly lower levels, and H1048, HeLa, HME, and 293T contained very small amounts. While the mechanism of the natural variation in STING expression is unknown, these results reveal the diversity that may exist within established cell lines, even those bearing the same name. When tested for PISA in terms of T. gondii growth (Fig. 6B, 6C), there was a general correlation with STING expression. For example, A549 and H196 cells supported robust parasite growth, the two 293 cells supported moderate growth, and H1048, HME and 293T supported poor parasite growth. As seen by others [26–28], our HeLa cell line supported decent parasite growth (Fig. 6B), even though it had a low level of STING, lending further support to the variability of cell lines and the contribution of multiple host determinants besides STING in parasite replication.
Fig. 6. STING levels and T. gondii replication in selected human cells. 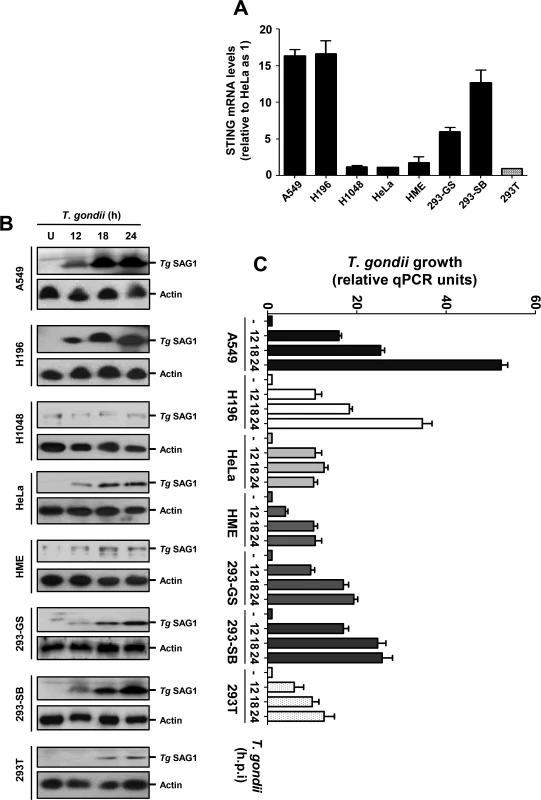
A, STING mRNA levels of the indicated cell lines, determined by qRT-PCR, were plotted. B/C, T. gondii RH growth in cell lines of diverse STING levels, measured respectively by Tg SAG1 immunoblot (panel B, with actin as loading control) and by qPCR of parasite DNA (panel C). IRF3 promotes parasite infection and resultant pathogenesis in vivo
Upon establishing the need of IRF3 for promoting parasite growth in cell culture, we wanted to determine whether the same is true in vivo as well. For this purpose, mice of different genetic backgrounds were infected with T. gondii by intraperitoneal injections, and their rates of survival were monitored. Infection by the parasite killed WT mice in a dose-dependent fashion; in contrast, IRF3-/ - mice were quite resistant to death at every dose of infection tested (Fig. 7A). For example, at the lowest dose of 10 parasites per mouse, all WT mice either died or suffered severe weight loss by 15 days post infection, whereas none of the IRF3-/ - mice died even after 25 days post infection. The pathogenicity in the WT mice correlated with weight loss (Fig. 7B), high parasite loads in the key organs tested, including spleen, liver and brain, whereas parasites were nearly undetectable in the same organs of IRF3-/ - mice (Fig. 7C). Since IL-12 has been shown to be a protective cytokine in the mouse model of Toxoplasma infection, we tested its level in IRF3-/ - and WT dendritic cells (DCs), following parasite infection in culture, as well as in the serum of the mice upon infection. Whereas WT DCs and wild type mice produced IL-12 in response to T. gondii infection, the levels were much higher when IRF3 was absent (Fig. 7D). When we tested IL-12 induction in nonimmune cell types, no post-infection induction of IL-12 could be detected in the non-immune cells, such as A549, MEF and M17 cells (S3 Fig.). Thus, IRF3 is needed for parasitic replication in a cell-intrinsic manner in many cell-types, although it is possible that the higher systemic IL-12 levels in the IRF3 KO animal, likely produced by infected immune cells, plays an important or even dominant role in the lower parasitic lethality. The higher level of IL-12 could be due to the non-transcriptional IL-12-suppressive role of IRF3, as shown in elegant recent studies [29, 30]. Recent studies have also revealed that three members of the IFN-γ-inducible p47 GTPase family are induced upon T. gondii infection in mouse; they are IGTP, IRG-47 and LRG-47 [29]. Of these, IGTP and LRG-47 play a role in resistance to acute T. gondii infection [29,30]. However, all three were expressed in similar amounts in T. gondii-infected WT and IRF3 -/ - mice, as measured by qRT-PCR (S4 Fig.), suggesting that the parasite resistance of IRF3 -/ - mice is unlikely due to overexpression of these IFN-γ-inducible GTPases.
Fig. 7. T. gondii growth and virulence in mice is promoted by IRF3. 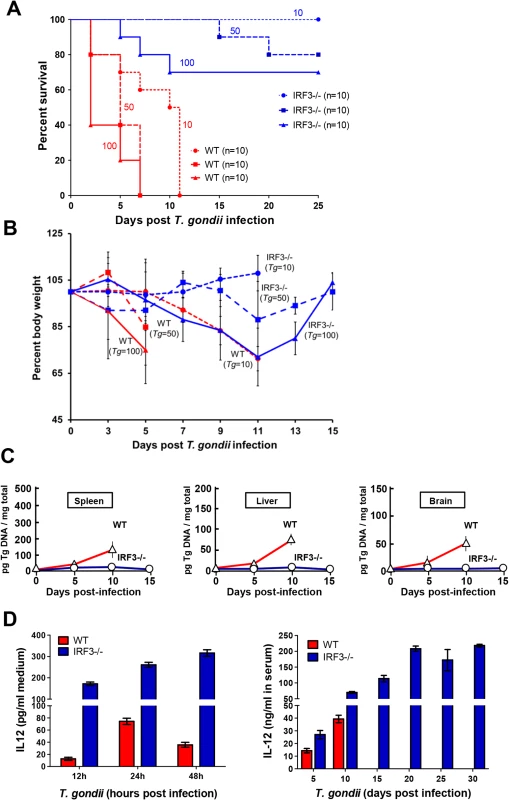
A, C57BL/6 wild type (WT, red lines) and IRF3−/− (blue lines) mice (of the indicated number, n) were infected with 10 (O), 50 (□), or 100 tachyzoites (Δ) (the number of parasites are shown as well), and survival was monitored. B, Body weight profile of WT and IRF3-/- mice upon T. gondii infection. C57BL/6 wild type (WT, red lines) and IRF3 KO (blue lines) mice (10 mice in each group) were infected with T. gondii (number of parasites shown) and monitored for their body weight for the indicated time. Due to severe weight loss (>25% of the original), WT mice were withdrawn at various days of infection and were euthanized. C, Mice were infected with 10 parasites as in panel A, and the indicated organs from live animals were harvested on the days indicated, followed by DNA isolation and real-time qPCR. Relative quantification was performed using standard curve analysis of purified parasite DNA, and results were expressed as pg of parasite DNA per mg of total tissue DNA, as shown. D, Left panel: Bone-marrow derived DC from IRF3 -/- and isogenic WT mice were infected with T. gondii RH at m.o.i. 10 in tissue culture, and IL-12 released in the media were assayed by ELISA using a commercial kit (R&D Systems). Right panel: IRF3-/- and isogenic WT mice were infected 10 parasites as in panel A and IL-12 in the serum of the live animals was assayed by ELISA. Discussion
We have uncovered a new signaling pathway, PISA, which is activated in mammalian cells upon infection with T. gondii and follows the scheme: Parasitic PAMP → cGAS → STING → TBK1 → P-IRF3 → ISG(s) → Parasite replication (Fig. 8). All the cellular proteins of the PISA pathway have been previously identified as signaling components of the host’s innate immune defense responses that protect it from viral or bacterial infection. In contrast, PISA is pro-microbial, not anti-microbial, and hence, should not be viewed as the host’s defense response; rather, it is the first example of a parasite co-opting an innate antiviral pathway for its replication. It remains to be seen whether this new paradigm is true for other intracellular protozoa as well. It is interesting to note that type I IFN was not needed for efficient parasite growth indicating that intracellular proteins, induced by IRF3 in the infected cells, promote this process.
Fig. 8. Our working model for PISA. 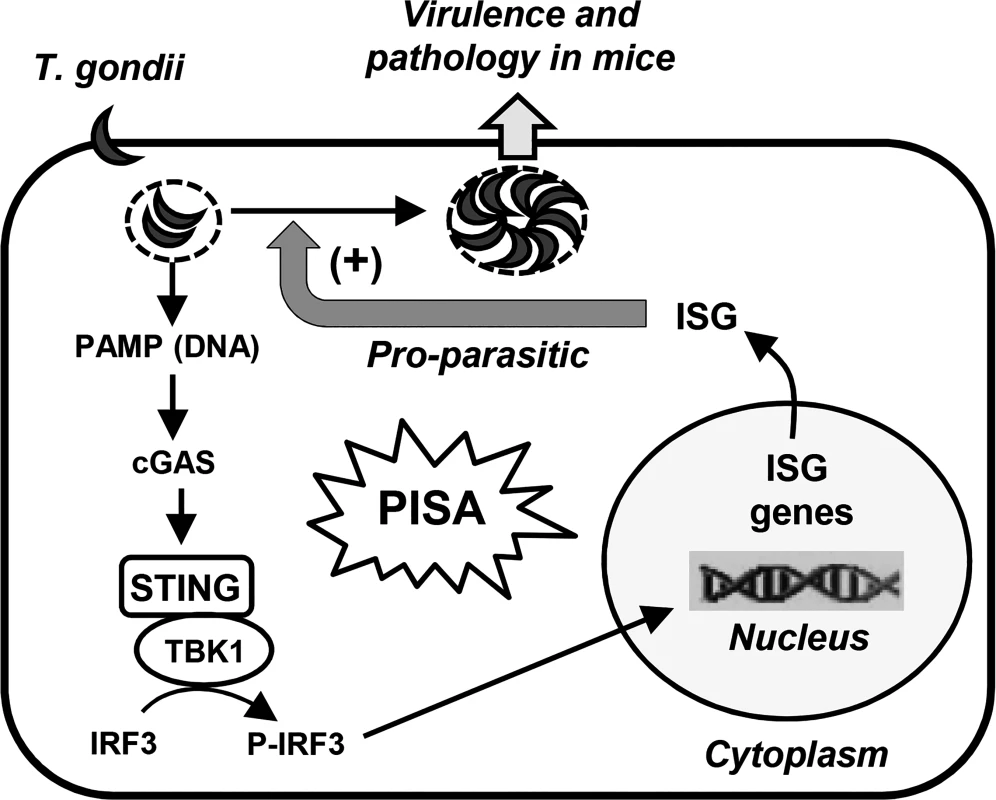
In this model, a parasitic PAMP (possibly DNA) is sensed by the PRR, cGAS; the CDN product of cGAS then activates the STING-TBK1 kinase complex, which phosphorylates IRF3. The phospho-IRF3 translocates into the nucleus and leads to the induction of specific, parasite-friendly host gene(s), which facilitate T. gondii replication, thereby causing pathology and death. A recent study [31] concluded that intracellular death of T. gondii results in the release of its nucleic acids, leading to the activation of RNA or DNA receptors, thereby activating IRF3 and inducing IFN-β. Pioneering studies by Beiting et al [32] recently showed that in macrophages, heat-killed T. gondii induce ISGs more efficiently than live parasites. The loss of this induction in TLR3 -/ - macrophages suggested that a T. gondii RNA, perhaps released by phagocytosis of the dead parasites, is the PAMP. We do not know if a fraction of the parasites in our preparation was dead, but it appears that UV-killed and heat-killed parasites interact differently with the host. We cannot rule out a contribution of RNA in PISA, but the lack a role of TLR3 and the need for cGAS suggest that DNA is likely the major PAMP.
Although the exact structural features of the DNA recognized by the cytoplasmic DNA sensors remain to be defined, unmethylated DNA of prokaryotic pathogens is a major PAMP. Interestingly, genomes of three Apicomplexa parasites, namely Plasmodium falciparum, T. gondii and Cryptosporidium parvum, contain very low or little methylation [33,34]. Purified genomic DNA of P. falciparum and P. berghei in fact activates the STING pathway [35] and type I IFN in an IRF3-dependent manner [36]. A new concept of PAMP, named 'viability-associated PAMP' or vita-PAMP, has been defined as an entity generated by viable pathogens and not by dead ones. Although the vita-PAMP of cytoplasmic bacteria was identified as mRNA generated from bacterial transcription [37], any de novo synthesized PAMP could potentially be a vita-PAMP. This concept may also hold true for T. gondii since we have observed that UV-inactivated, non-replicative parasite is unable to activate PISA. Unfortunately, our knowledge of the molecular exchange between the parasite and its host is highly limited. Nonetheless, an increasing number of T. gondii macromolecules are found to access the host cytoplasm, such as kinases and pseudokinases, injected into the host cytoplasm promptly after parasitic invasion, whereby they regulate specific host signaling pathways [38–49]. The parasitic rhoptry kinase (ROP16) and the rhoptry protein phosphatase 2C (PP2C) actually travel all the way to the host nucleus and likely regulate host gene expression [40,45]. As mentioned, it will be important to know if infecting parasites actually release DNA or RNA at any stage of growth and whether they reach the host cytoplasm to activate the cGAS-STING pathway.
We provide evidence that PISA promotes parasite replication but do not know whether additional pathways are also stimulatory. It remains possible that genes induced by other transcription factors, such as NF-κB and AP-1, that are known to be activated by STING signaling [50], co-operate with IRF3-induced genes in promoting parasite replication. A distinct feature of PISA is that it requires the adaptor protein, STING, and it appears that STING and TBK1 form a signal-dependent complex that activates IRF3; indeed, STING has been reported to bind and activate TBK1 in vitro [51].
Recent studies have shown that under certain conditions T. gondii is highly vulnerable to autophagy [52] and, in the absence of a documented apoptotic cascade in T. gondii, autophagy has been suggested to be the primary mechanism of programmed cell death in T. gondii and potentially other related parasites. It is tempting to speculate that parasite-induced ISGs suppress an autophagic response that might otherwise be triggered by a host defense mechanism.
PISA appears to be stimulatory to T. gondii replication in all human and mouse cell lines and primary cells that we tested. More importantly, without IRF3 and PISA, the parasite replicated poorly and was poorly pathogenic in mice. Pathogenesis in T. gondii-infected mice has been shown to be indirectly inhibited by IRF3 through its action on specific cytokine synthesis. Thus, in this context, IRF3 inhibits pathogenesis by T. gondii, in contrast to the cell-intrinsic pro-parasitic effect of IRF3 reported here. Not surprisingly, in IRF3-/ - mice, the latter effect was overriding and little pathogenesis was observed because the parasite could not replicate in infected cells of these mice. We established the pivotal role of IRF3 and PISA in inducing ISGs to promote parasite replication but they can also be induced by many other pathways, activated by other stimulants that do not use IRF3. For example, the IFN-activated Jak-STAT pathway uses IRF9 whereas TLR7 and TLR9 use IRF7 to induce the same genes. Thus, even in the absence of IRF3, if IFN synthesis is induced by any viral or bacterial infection, circulating IFN will induce ISGs systemically and facilitate efficient T. gondii replication. Even for WT mouse, IFN produced by prior viral infection will make it a better host for subsequent T. gondii infection because the as-yet uninfected cells will already be loaded with ISG products. Therefore, in the natural context, viral or bacterial infection of an organism may set it up to be a better host for T. gondii.
The ISGs have so far been studied in the context of their specific antiviral activities; this report reveals an unexpected new facet of their physiological significance in promoting microbial growth. The new knowledge can, in principle, be exploited to inhibit toxoplasmosis; inhibitors of any components of PISA, including IRF3 and ISGs, will be attractive candidates for this purpose. For good reasons, much of the related research so far has focused on formulating potent agonists of TLR and RLR signaling to boost the innate immune response and host defense [53]. However, inhibitors for these pathways are also known; for example, we discovered several inhibitors of ISG induction by TLR3 through the screening of a chemical library [54]. A similar approach can be taken to identify small molecule inhibitors of PISA, which can target multiple ISGs that may be involved in promoting various steps of parasitic cell division [55].
Materials and Methods
Cells and reagents
H196, H1048, HME, and HEK293 cell lines were kindly provided by Dr. George Stark, Lerner Research Institute (LRI). All cells, with the exception of the primary macrophages and DCs, were grown in Dulbecco's minimum essential media (DMEM) supplemented with 10% FBS (fetal bovine serum), 20 mM L-glutamine, 100 U/mL of penicillin and 100 μg/mL of streptomycin (Life Technologies). Primary bone-marrow-derived DCs were obtained as follows. Bone marrow from the femurs and hind legs of mice of the appropriate genotype was collected in 1 ml of RPMI1640 medium (without serum) and single cell suspensions were made using 26G needle and syringe. Cells were washed twice with RPMI by centrifugation at 5,000 x g for 10 min in at 4°C, resuspended in RPMI containing 10% FBS, recombinant GM-CSF (PeproTech; Cat# 315–03) (10 ng/ml) and recombinant IL-4 (eBioscience; Cat# 14-8041-62) (10 ng/ml), and then grown for 8 days. The mature DCs were resuspended in RPMI plus 10% FBS and transferred to appropriate multi-well plates for infection with T. gondii. The details of the Clone 10 cells were described before [21]. Briefly, these cells were generated from the parental HT1080/shIRF3 cells by expressing N-terminally V5-tagged human IRF3, subcloned into pTRE2hyg vector (Clontech) and co-transfected with pTet-Off (Clontech). The resultant cells were selected against G418 (400 μg/ml), puromycin (1 μg/ml), and hygromycin (100 μg/ml). Where mentioned, MEF cells were treated with murine IFN-β (R&D Systems). Primary antibodies were obtained against the following: FLAG, HA and V5 epitope tags (Millipore), SAG1 and actin (Santa Cruz Biotechnology sc-52255, sc-8432, respectively), P-IRF3 (Ser 396), IRF3, P-TBK1 (Ser 172), TBK1 (Cell Signaling mAb #4947, mAb #4302, mAb #5483, #3013, respectively), and murine ISG56 (LRI Hybridoma Core). cGAMP (3'3' cyclic GAMP) was from Invivogen. Lipofectamine 2000 and Lipofectamine LTX with Plus reagent were from Invitrogen/Life Technologies.
Plasmids and transfection
N-terminally V5-tagged human IRF3 and the deletion mutants were described before [22]. N-terminally HA-tagged STING and N-terminally Flag-tagged TBK1 were used for transfection experiments. Flag-tagged wild type (WT) cGAS and its enzymatically defective mutant were kindly provided by Robert Silverman (LRI). Human cell lines were transfected using Lipofectamine 2000, and mouse cells (MEFs), with Lipofectamine LTX with Plus reagent. Unless otherwise stated, 0.8 μg plasmid was used for transfection per 5 x 105 cells (each well of a 12-well plate) using the manufacturers' instructions. After 8 h post transfection, cells were infected with T. gondii at an MOI of 2.
Gene knockdown experiments
IRF3 knockdown HT1080 cells were described previously [22,56]. Briefly, these cells were generated by lentivirally expressing the shRNAs against the 5’ and 3’ UTRs of human IRF3 and selecting the resultant transduced cells under G418. These cells were used to express IRF3 by transfecting a cDNA of human IRF3 without the UTR. Clone 10 cells were described before and were derived from these parental cells.
Growth of T. gondii stock and infection of cells
All T. gondii strains were grown in hTERT-immortalized human foreskin cells as described before [57,58] and purified by differential centrifugation (3,000 x g, 10 min), followed by filtration through 3 μm Whatman filter. Parasites were resuspended in phosphate-buffered saline, counted in a hemocytometer under microscope, and used for infection of cells at an MOI of 2, as described in Fig. legends. The infected cells were processed for immunoblot as described later. All experiments in this paper, except those indicated in Fig. 2C, used the T. gondii RH strain, which is a highly virulent, type I strain.
Quantitative RT-PCR analyses
RT-PCR primers for cGAS and STING (Fig. 6A) were kind gifts of Dr. George Stark. Real-time qRT-PCR was performed as described with the following primer pair, against the ITS-1 region conserved in all T. gondii strains [59]: AATATTGGAAGCCAGTGCAGG (forward), CAATCTTTCACTCTCTCTCAA (reverse). Results were normalized against GAPDH gene, amplified with the following primers: CTGGAAAACCCTGCCAAATA (forward), TGCTCAGTTTAGCCCAGGAT (reverse). The primers for IGTP and LRG-47 have been described [60,61]; those for IRG-47 (GenBank M63630.1), designed by us, were: GCCAAACCCATAGCTTTCAAG (forward) and GAAATCAAACGCACCCAGATC (reverse). PCRs were setup in a final volume of 20 μl using 5 ng of template DNA, 200 ng of each primer and 1x of the iQ SYBR Green Supermix (BioRad). Quantitative RT-PCR analysis was performed on a DNA Engine Opticon 2 Real-Time Cycler (MJ Research). Primers directed against the T. gondii ITS-1 gene did not generate a product when the template genomic DNA was derived from organs of uninfected mice.
Cell lysis and immunoblotting (IB)
Immunoblotting procedures were performed as described preciously [62,63]. Briefly, at the indicated times post infection (p.i.), cells were washed twice with PBS. For IB, cells were lysed by the addition of 1.5 x Laemmli sample buffer containing protease inhibitor (Roche, Product # 04693116001) and phosphatase inhibitor cocktails (Cell Signaling Technology, Cat# 5870). Cells were fully lysed by pipetting followed by sonication. Samples were heated at 95°C and equal amounts of proteins were analyzed on denaturing SDS-polyacrylamide gels. The proteins were transferred to PVDF Immobilon-P membrane (Millipore, Cat# IPVH00010) and probed with specific primary antibody followed by secondary antibody conjugated to horseradish peroxidase. Bands were visualized by chemiluminescence-based detection system (LI-COR Biosciences).
Confocal microscopy
Cells (2.0 × 105 /well) were plated onto cover glasses in 6-well plates, grown overnight (to ∼5 x 105 cells), then infected with T. gondii at an m.o.i. of 0.2. At indicated times p.i., cells were fixed in ice-cold methanol for 5 min and permeabilized with PBS containing 0.1% Triton X-100. Fixed cells were blocked in PBS containing 1% BSA for 1 hr and labeled with anti-SAG1 as primary antibody (1 : 50) for 3 h, and Alexa Fluor 488-conjugated secondary antibody (1 : 200). Cytosol was stained with anti-actin antibody and Alexa Fluor 647-conjugated secondary. Nuclei were stained with DAPI. Cells were visualized at a 60× magnification in a Nikon A1RSI confocal microscope.
Flow cytometry
DCs were prepared from wild type and IRF3-/ - mice as described earlier and infected with freshly egressed RFP-expressing T. gondii RH strain at an m.o.i. of 0.3. At 18 h post-infection, the DCs were extensively washed to remove any free parasites, and 5 × 105 cells were collected in 300 μl cold PBS and stained with anti-CD11c-FITC antibody (BD Pharmingen) for 1 hr at 4°. DCs were then washed with PBS, pelleted at 300 x g for 6 min at 4° C, fixed with 1% formalin, and analyzed for RFP-positive cells by flow cytometry in a FacsCanto II cell analyzer (BD Biosciences) using FlowJo software (Ashland, OR).
Infection of mice with T. gondii
For in vivo infection, we used the IRF3-/ - mice, as described before [23]. The stated number of RH parasites (e.g., 10, 50, or 100 as indicated in Fig. 7) in phosphate-buffered saline was intraperitoneally injected in mice [64,65]. For survival analyses, mice were monitored for the indicated time post-infection. For various tissue analyses, animals were sacrificed on the indicated days; organs were homogenized and total DNA isolated by the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA). All animal procedures were approved by the Institutional Animal Care and Use Committee.
Statistical analysis
Changes between treatment groups were analyzed by one-way ANOVA and by Student's t-test with Bonferroni correction. Numerical data were derived from three experiments, and results expressed as mean ± SEM or SD as stated (presented as error bars in graphs). P < 0.05 was considered significant.
Supporting Information
Zdroje
1. Torgerson PR, Mastroiacovo P (2013) The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ 91 : 501–508. doi: 10.2471/BLT.12.111732 23825877
2. Cosentino MJ, Pakyz RE, Fried J (1990) Pyrimethamine: an approach to the development of a male contraceptive. Proc Natl Acad Sci U S A 87 : 1431–1435. 2304908
3. Kongsaengdao S, Samintarapanya K, Oranratnachai K, Prapakarn W, Apichartpiyakul C (2008) Randomized controlled trial of pyrimethamine plus sulfadiazine versus trimethoprim plus sulfamethoxazole for treatment of toxoplasmic encephalitis in AIDS patients. J Int Assoc Physicians AIDS Care (Chic) 7 : 11–16. 17517949
4. Frenkel JK (1988) Pathophysiology of toxoplasmosis. Parasitol Today 4 : 273–278. 15463000
5. Sher A, Collazzo C, Scanga C, Jankovic D, Yap G, et al. (2003) Induction and regulation of IL-12-dependent host resistance to Toxoplasma gondii. Immunol Res 27 : 521–528. 12857995
6. Yarovinsky F (2014) Innate immunity to Toxoplasma gondii infection. Nat Rev Immunol 14 : 109–121. doi: 10.1038/nri3598 24457485
7. Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, et al. (2002) Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol 168 : 5997–6001. 12055206
8. Denkers EY, Gazzinelli RT, Martin D, Sher A (1993) Emergence of NK1.1+ cells as effectors of IFN-gamma dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J Exp Med 178 : 1465–1472. 8228800
9. Khan IA, Matsuura T, Kasper LH (1994) Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect Immun 62 : 1639–1642. 7909536
10. Pifer R, Yarovinsky F (2011) Innate responses to Toxoplasma gondii in mice and humans. Trends Parasitol 27 : 388–393. doi: 10.1016/j.pt.2011.03.009 21550851
11. Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, et al. (2007) Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov 6 : 975–990. 18049472
12. Noyce RS, Taylor K, Ciechonska M, Collins SE, Duncan R, et al. (2011) Membrane perturbation elicits an IRF3-dependent, interferon-independent antiviral response. J Virol 85 : 10926–10931. doi: 10.1128/JVI.00862-11 21813605
13. Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34 : 637–650. doi: 10.1016/j.immuni.2011.05.006 21616434
14. Medzhitov R (2007) Recognition of microorganisms and activation of the immune response. Nature 449 : 819–826. 17943118
15. Xiao TS, Fitzgerald KA (2013) The cGAS-STING pathway for DNA sensing. Mol Cell 51 : 135–139. doi: 10.1016/j.molcel.2013.07.004 23870141
16. Barber GN (2014) STING-dependent cytosolic DNA sensing pathways. Trends Immunol 35 : 88–93. doi: 10.1016/j.it.2013.10.010 24309426
17. Gao D, Wu J, Wu YT, Du F, Aroh C, et al. (2013) Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341 : 903–906. doi: 10.1126/science.1240933 23929945
18. Sun L, Wu J, Du F, Chen X, Chen ZJ (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339 : 786–791. doi: 10.1126/science.1232458 23258413
19. Liang Q, Seo GJ, Choi YJ, Kwak MJ, Ge J, et al. (2014) Crosstalk between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host Microbe 15 : 228–238. doi: 10.1016/j.chom.2014.01.009 24528868
20. Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, et al. (2003) IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 4 : 491–496. 12692549
21. Chattopadhyay S, Fensterl V, Zhang Y, Veleeparambil M, Yamashita M, et al. (2013) Role of interferon regulatory factor 3-mediated apoptosis in the establishment and maintenance of persistent infection by Sendai virus. J Virol 87 : 16–24. doi: 10.1128/JVI.01853-12 23077293
22. Chattopadhyay S, Marques JT, Yamashita M, Peters KL, Smith K, et al. (2010) Viral apoptosis is induced by IRF-3-mediated activation of Bax. EMBO J 29 : 1762–1773. doi: 10.1038/emboj.2010.50 20360684
23. Chattopadhyay S, Fensterl V, Zhang Y, Veleeparambil M, Wetzel JL, et al. (2013) Inhibition of viral pathogenesis and promotion of the septic shock response to bacterial infection by IRF-3 are regulated by the acetylation and phosphorylation of its coactivators. MBio 4: e00636–12. doi: 10.1128/mBio.00636-12 23532979
24. Servant MJ, Grandvaux N, tenOever BR, Duguay D, Lin R, et al. (2003) Identification of the minimal phosphoacceptor site required for in vivo activation of interferon regulatory factor 3 in response to virus and double-stranded RNA. J Biol Chem 278 : 9441–9447. 12524442
25. Ma X, Helgason E, Phung QT, Quan CL, Iyer RS, et al. (2012) Molecular basis of Tank-binding kinase 1 activation by transautophosphorylation. Proc Natl Acad Sci U S A 109 : 9378–9383. doi: 10.1073/pnas.1121552109 22619329
26. Evans R, Chatterton JM, Ashburn D, Joss AW, Ho-Yen DO (1999) Cell-culture system for continuous production of Toxoplasma gondii tachyzoites. Eur J Clin Microbiol Infect Dis 18 : 879–884. 10691199
27. Gaji RY, Huynh MH, Carruthers VB (2013) A novel high throughput invasion screen identifies host actin regulators required for efficient cell entry by Toxoplasma gondii. PLoS One 8: e64693. doi: 10.1371/journal.pone.0064693 23741372
28. Moser LA, Pollard AM, Knoll LJ (2013) A genome-wide siRNA screen to identify host factors necessary for growth of the parasite Toxoplasma gondii. PLoS One 8: e68129. doi: 10.1371/journal.pone.0068129 23840822
29. Collazo CM, Yap GS, Sempowski GD, Lusby KC, Tessarollo L, et al. (2001) Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection. J Exp Med 194 : 181–188. 11457893
30. Taylor GA, Collazo CM, Yap GS, Nguyen K, Gregorio TA, et al. (2000) Pathogen-specific loss of host resistance in mice lacking the IFN-gamma-inducible gene IGTP. Proc Natl Acad Sci U S A 97 : 751–755. 10639151
31. Melo MB, Nguyen QP, Cordeiro C, Hassan MA, Yang N, et al. (2013) Transcriptional analysis of murine macrophages infected with different Toxoplasma strains identifies novel regulation of host signaling pathways. PLoS Pathog 9: e1003779. doi: 10.1371/journal.ppat.1003779 24367253
32. Beiting DP, Peixoto L, Akopyants NS, Beverley SM, Wherry EJ, et al. (2014) Differential induction of TLR3-dependent innate immune signaling by closely related parasite species. PLoS One 9: e88398. doi: 10.1371/journal.pone.0088398 24505488
33. Gissot M, Choi SW, Thompson RF, Greally JM, Kim K (2008) Toxoplasma gondii and Cryptosporidium parvum lack detectable DNA cytosine methylation. Eukaryot Cell 7 : 537–540. doi: 10.1128/EC.00448-07 18178772
34. Ponts N, Fu L, Harris EY, Zhang J, Chung DW, et al. (2013) Genome-wide mapping of DNA methylation in the human malaria parasite Plasmodium falciparum. Cell Host Microbe 14 : 696–706. doi: 10.1016/j.chom.2013.11.007 24331467
35. Sharma S, DeOliveira RB, Kalantari P, Parroche P, Goutagny N, et al. (2011) Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity 35 : 194–207. doi: 10.1016/j.immuni.2011.05.016 21820332
36. Liehl P, Zuzarte-Luis V, Chan J, Zillinger T, Baptista F, et al. (2014) Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat Med 20 : 47–53. doi: 10.1038/nm.3424 24362933
37. Sander LE, Davis MJ, Boekschoten MV, Amsen D, Dascher CC, et al. (2011) Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 474 : 385–389. doi: 10.1038/nature10072 21602824
38. Bradley PJ, Sibley LD (2007) Rhoptries: an arsenal of secreted virulence factors. Curr Opin Microbiol 10 : 582–587. 17997128
39. Fentress SJ, Behnke MS, Dunay IR, Mashayekhi M, Rommereim LM, et al. (2010) Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe 8 : 484–495. doi: 10.1016/j.chom.2010.11.005 21147463
40. Gilbert LA, Ravindran S, Turetzky JM, Boothroyd JC, Bradley PJ (2007) Toxoplasma gondii targets a protein phosphatase 2C to the nuclei of infected host cells. Eukaryot Cell 6 : 73–83. 17085638
41. Hakansson S, Charron AJ, Sibley LD (2001) Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J 20 : 3132–3144. 11406590
42. Ngo HM, Yang M, Joiner KA (2004) Are rhoptries in Apicomplexan parasites secretory granules or secretory lysosomal granules? Mol Microbiol 52 : 1531–1541. 15186406
43. Ong YC, Reese ML, Boothroyd JC (2010) Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. J Biol Chem 285 : 28731–28740. doi: 10.1074/jbc.M110.112359 20624917
44. Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP (2011) Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci U S A 108 : 9625–9630. doi: 10.1073/pnas.1015980108 21436047
45. Saeij JP, Boyle JP, Coller S, Taylor S, Sibley LD, et al. (2006) Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314 : 1780–1783. 17170306
46. Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, et al. (2007) Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445 : 324–327. 17183270
47. Steinfeldt T, Konen-Waisman S, Tong L, Pawlowski N, Lamkemeyer T, et al. (2010) Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol 8: e1000576. doi: 10.1371/journal.pbio.1000576 21203588
48. Taylor S, Barragan A, Su C, Fux B, Fentress SJ, et al. (2006) A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 314 : 1776–1780. 17170305
49. Yamamoto M, Standley DM, Takashima S, Saiga H, Okuyama M, et al. (2009) A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J Exp Med 206 : 2747–2760. doi: 10.1084/jem.20091703 19901082
50. Abe T, Barber GN (2014) Cytosolic DNA-Mediated, STING-Dependent Pro-Inflammatory Gene Induction Necessitates canonical NF-kappaB activation Through TBK1. J Virol.
51. Tanaka Y, Chen ZJ (2012) STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal 5: ra20. doi: 10.1126/scisignal.2002521 22394562
52. Ghosh D, Walton JL, Roepe PD, Sinai AP (2012) Autophagy is a cell death mechanism in Toxoplasma gondii. Cell Microbiol 14 : 589–607. doi: 10.1111/j.1462-5822.2011.01745.x 22212386
53. Hennessy EJ, Parker AE, O'Neill LA (2010) Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov 9 : 293–307. doi: 10.1038/nrd3203 20380038
54. Zhu J, Smith K, Hsieh PN, Mburu YK, Chattopadhyay S, et al. (2010) High-throughput screening for TLR3-IFN regulatory factor 3 signaling pathway modulators identifies several antipsychotic drugs as TLR inhibitors. J Immunol 184 : 5768–5776. doi: 10.4049/jimmunol.0903559 20382888
55. Francia ME, Striepen B (2014) Cell division in apicomplexan parasites. Nat Rev Microbiol 12 : 125–136. doi: 10.1038/nrmicro3184 24384598
56. Peters K, Chattopadhyay S, Sen GC (2008) IRF-3 activation by Sendai virus infection is required for cellular apoptosis and avoidance of persistence. J Virol 82 : 3500–3508. doi: 10.1128/JVI.02536-07 18216110
57. Roos DS (1996) Molecular genetic tools for the identification and analysis of drug targets in Toxoplasma gondii. Curr Top Microbiol Immunol 219 : 247–259. 8791705
58. Musiyenko A, Majumdar T, Andrews J, Adams B, Barik S (2012) PRMT1 methylates the single Argonaute of Toxoplasma gondii and is important for the recruitment of Tudor nuclease for target RNA cleavage by antisense guide RNA. Cell Microbiol 14 : 882–901. doi: 10.1111/j.1462-5822.2012.01763.x 22309152
59. Rahumatullah A, Khoo BY, Noordin R (2012) Triplex PCR using new primers for the detection of Toxoplasma gondii. Exp Parasitol 131 : 231–238. doi: 10.1016/j.exppara.2012.04.009 22561042
60. Bafica A, Feng CG, Santiago HC, Aliberti J, Cheever A, et al. (2007) The IFN-inducible GTPase LRG47 (Irgm1) negatively regulates TLR4-triggered proinflammatory cytokine production and prevents endotoxemia. J Immunol 179 : 5514–5522. 17911638
61. Bernstein-Hanley I, Coers J, Balsara ZR, Taylor GA, Starnbach MN, et al. (2006) The p47 GTPases Igtp and Irgb10 map to the Chlamydia trachomatis susceptibility locus Ctrq-3 and mediate cellular resistance in mice. Proc Natl Acad Sci U S A 103 : 14092–14097. 16959883
62. Swedan S, Andrews J, Majumdar T, Musiyenko A, Barik S (2011) Multiple functional domains and complexes of the two nonstructural proteins of human respiratory syncytial virus contribute to interferon suppression and cellular location. J Virol 85 : 10090–10100. doi: 10.1128/JVI.00413-11 21795342
63. Swedan S, Musiyenko A, Barik S (2009) Respiratory syncytial virus nonstructural proteins decrease levels of multiple members of the cellular interferon pathways. J Virol 83 : 9682–9693. doi: 10.1128/JVI.00715-09 19625398
64. Howe DK, Sibley LD (1995) Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 172 : 1561–1566. 7594717
65. Sibley LD, Mordue DG, Su C, Robben PM, Howe DK (2002) Genetic approaches to studying virulence and pathogenesis in Toxoplasma gondii. Philos Trans R Soc Lond B Biol Sci 357 : 81–88. 11839185
66. Fensterl V, Wetzel JL, Ramachandran S, Ogino T, Stohlman SA, et al. (2012) Interferon-Induced Ifit2/ISG54 Protects Mice from Lethal VSV Neuropathogenesis. PLoS Pathog 8: e1002712. doi: 10.1371/journal.ppat.1002712 22615570
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



