-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaUbiquitin-Mediated Response to Microsporidia and Virus Infection in
Microbial pathogens have two distinct lifestyles:
some pathogens live outside of host cells, and others live inside of host cells and are called intracellular pathogens. Microsporidia are fungal-related intracellular pathogens that can infect all animals, but are poorly understood. We used the roundworm C. elegans as a host to show that ubiquitin pathways provide defense against both a natural microsporidian infection of C. elegans, as well as a natural viral infection. Our study shows that ubiquitin, the proteasome and autophagy components are all important to control intracellular infection in C. elegans, although microsporidia seem to partially evade this defense. We also show that SCF ubiquitin ligases help control both microsporidia and virus infection. Furthermore, we find that C. elegans upregulates expression of SCF ligases when ubiquitin-related degradation machinery is inhibited, indicating that C. elegans monitors the functioning of this core cellular process and upregulates ligase expression when it is perturbed. Altogether, our findings describe ubiquitin-mediated pathways that are involved in host response and defense against intracellular pathogens, and how this machinery is regulated by infection to increase defense against intracellular pathogens such as microsporidia and viruses.
Published in the journal: . PLoS Pathog 10(6): e32767. doi:10.1371/journal.ppat.1004200
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004200Summary
Microbial pathogens have two distinct lifestyles:
some pathogens live outside of host cells, and others live inside of host cells and are called intracellular pathogens. Microsporidia are fungal-related intracellular pathogens that can infect all animals, but are poorly understood. We used the roundworm C. elegans as a host to show that ubiquitin pathways provide defense against both a natural microsporidian infection of C. elegans, as well as a natural viral infection. Our study shows that ubiquitin, the proteasome and autophagy components are all important to control intracellular infection in C. elegans, although microsporidia seem to partially evade this defense. We also show that SCF ubiquitin ligases help control both microsporidia and virus infection. Furthermore, we find that C. elegans upregulates expression of SCF ligases when ubiquitin-related degradation machinery is inhibited, indicating that C. elegans monitors the functioning of this core cellular process and upregulates ligase expression when it is perturbed. Altogether, our findings describe ubiquitin-mediated pathways that are involved in host response and defense against intracellular pathogens, and how this machinery is regulated by infection to increase defense against intracellular pathogens such as microsporidia and viruses.Introduction
The Microsporidia phylum contains over 1400 species of obligate intracellular pathogens most closely related to fungi [1]. These pathogens can infect a wide variety of animal hosts including humans, where they can cause significant disease. Infections in humans can cause lethal diarrhea in immunocompromised people such as AIDS patients, and microsporidia are considered priority pathogens at the National Institutes of Health [2], [3]. Microsporidia can also plague agriculturally significant animals such as fish and honeybees [4], [5], [6]. Treatment options for microsporidia infections are limited and often ineffective [7], [8]. In mammals, studies have shown that T cells and dendritic cells provide protection against infection, but little is known about the innate and/or intracellular responses to these pathogens [9], [10], [11].
Previously, we described Nematocida parisii, a microsporidian species isolated from a wild-caught C. elegans near Paris, which causes a lethal intestinal infection in its host [12], [13]. N. parisii infection of the simple nematode C. elegans provides a convenient system in which to investigate host responses and defense against microsporidia infection. Interestingly, canonical C. elegans defense pathways, such as the conserved PMK-1 p38 MAPK pathway that provides defense against bacterial and fungal infections, are not important for defense against N. parisii [12], [14]. Thus, distinct immunity mechanisms may be involved in the C. elegans response to microsporidia. In addition to microsporidia, another natural intracellular infection has recently been described in C. elegans: wild-caught animals from Orsay, France, were shown to harbor a viral infection [15]. The Orsay virus is a positive strand RNA virus of the family Nodaviridae, and like N. parisii it appears to undergo its entire replicative cycle inside C. elegans intestinal cells. The RNAi pathway has been shown to provide defense against viral infections in C. elegans [15], [16], [17], [18], [19], but little else is known about host defense against this natural intracellular pathogen of C. elegans.
Defense against intracellular pathogens in diverse animal hosts is increasingly appreciated to involve ubiquitin-mediated degradation pathways [20], [21], [22], [23]. Ubiquitylation is the process by which an E3 ubiquitin ligase catalyzes the conjugation of a ubiquitin tag onto substrates, which can be further ubiquitylated to generate poly-ubiquitin chains [24]. Ubiquitylated substrates have a number of different fates, two of which involve degradation. The most well characterized fate is degradation by the proteasome, but larger substrates can be targeted for degradation by the process of autophagy, which is termed 'xenophagy' when it involves degradation of intracellular microbes [25], [26]. Recently, ubiquitin ligases that mediate ubiquitin targeting to human bacterial pathogens Salmonella enterica [21] and Mycobacterium tuberculosis [22] have been identified, and they, together with the autophagy pathway, are important for controlling levels of these intracellular pathogens [23], [27], [28], [29]. However, while several ubiquitin-mediated defense components and mechanisms have been defined, there are many unanswered questions about which host ubiquitin ligases are involved in targeting ubiquitin to different pathogens, how these systems are regulated, and their overall importance for defense in vivo.
One major class of E3 ubiquitin ligases includes the Skp1−Cul1−F-box protein (SCF) multi-subunit RING-finger type, which is a modular complex found throughout eukaryotes [30]. SCF ligases are usually composed of three core components (a cullin protein, Skp1, and a RING-containing subunit) and a variable F-box protein component, which enables recognition of different substrates depending on which F-box protein is associated with the complex [31]. Interestingly, the C. elegans genome has a greatly expanded and diversified family of F-box proteins (∼520 genes compared to 69 genes in humans), as well as other SCF components (21 Skp1-related genes compared to 1 in humans), suggesting they use SCF ligases to recognize an extremely diverse array of substrates [32], [33]. In particular, it has been proposed that C. elegans uses these SCF ligases to target toxins and intracellular pathogen proteins for degradation, and that the expanded C. elegans SCF ligase repertoire is the manifestation of a host/pathogen arms race between nematodes and their natural intracellular pathogens [32]. At the time this intriguing idea was proposed however, there were no known intracellular pathogens of C. elegans to test the role of ubiquitin-mediated responses in defense.
Here we describe the C. elegans host response to the natural intracellular pathogens N. parisii and the Orsay virus, and find a role for ubiquitin-mediated defense against both infections. We perform gene expression analyses of the transcriptional response to microsporidia infection and find that the response is strikingly similar to the response to viral infection, but not to extracellular pathogens. We see upregulation of SCF ligase components, which help to restrict microsporidia growth, and find that defense against microsporidia appears to rely on the proteasome, as well as the autophagy pathway. We find a subset of parasite cells targeted by host-derived ubiquitin, which relies partly on the SCF cullin component CUL-6. Notably, this ubiquitin targeting, as well as the role for ubiquitin-mediated defense, increases upon inhibition of microsporidia growth by anti-microsporidia drugs. These results suggest that N. parisii may suppress or evade ubiquitin-mediated host defenses. Interestingly, expression of specific infection-upregulated SCF ligase components is also upregulated by genetic or pharmacological inhibition of UPS function, suggesting that stress placed upon the UPS may be a hallmark of intracellular infection, and that hosts monitor UPS function to upregulate appropriate defenses during intracellular infection. Finally, we show that SCF ligase components, in particular CUL-6, promote defense against viral infection in C. elegans. Altogether, these studies show the involvement of ubiquitin-mediated defense and xenophagy against natural intracellular pathogens in a whole animal host, and provide insight into their regulation in response to infection in vivo.
Results
C. elegans transcriptional response to N. parisii infection is distinct from response to extracellular infection, but similar to response to viral infection
We examined the C. elegans transcriptional response over the course of an infection with N. parisii using strand-specific deep sequencing of RNA (RNA-seq). Like other microsporidia, the life cycle of N. parisii is complex and its growth and replication takes place entirely inside the host cell (Figure 1A). Microsporidian spores initiate an intracellular infection by firing an infection apparatus called a polar tube, which pierces the host cell membrane and then injects into the host cell a nucleus and sporoplasm, which replicates as a stage called a meront. In the case of N. parisii, meronts become very large, multi-nucleate cells that replicate in direct contact with the cytoplasm. Meronts will eventually differentiate into spores and these spores then exit from infected cells to infect new hosts. We collected and sequenced cDNA from age-matched uninfected controls and infected animals at 8, 16, 30, 40 and 64 hours post inoculation (hpi) (Figure 1A, B), which are timepoints that correspond to specific stages of N. parisii infection as described in our previous study [34] (Table S1). A large number of C. elegans genes had significantly altered expression during N. parisii infection (edgeR, FDR<0.05, Table S2). The overall number of upregulated genes was relatively stable throughout infection, while the number of downregulated genes increased markedly with time (Figure 1C). To validate our RNA-seq studies, we also performed Affymetrix microarrays, which had substantial agreement in the genes found to be regulated by N. parisii infection (see Supplemental Text S1, Table S3). Notably, we found that a significant number of genes upregulated by infection were associated with the intestine, which is the site of N. parisii infection (Figure 1D).
Fig. 1. C. elegans gene expression during infection with N. parisii. 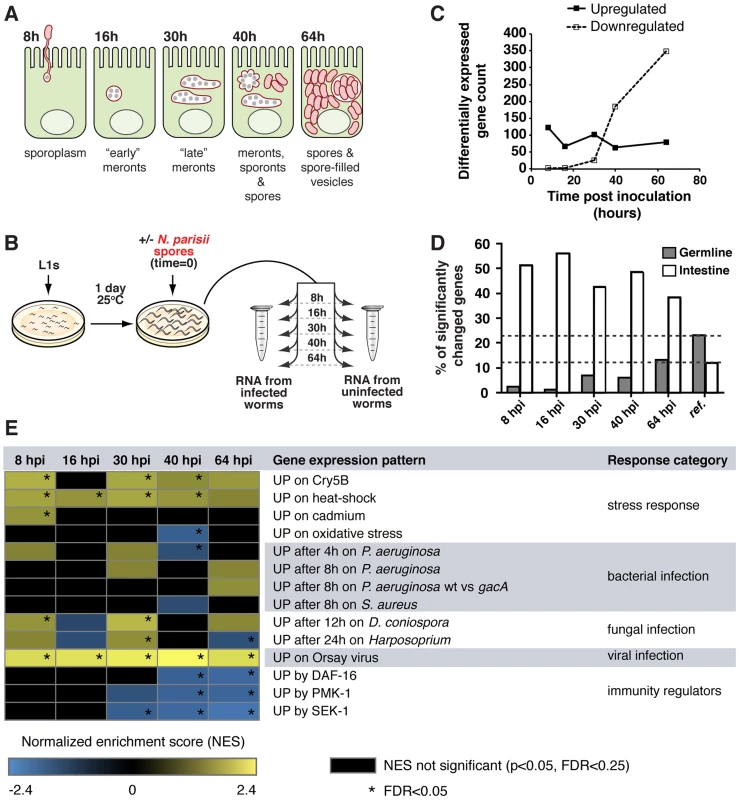
A) Diagram of N. parisii infection stages in C. elegans intestinal cells. B) Synchronized populations of fer-15;fem-1 sterile animals were inoculated with N. parisii spores and collected for RNA extraction at timepoints corresponding to specific stages of infection. Uninfected controls were included for each timepoint. C) Number of significantly (FDR<0.05) up- or downregulated C. elegans genes during infection with N. parisii. D) Proportion of intestine- and germline-associated C. elegans genes with significantly altered expression at each timepoint. The “reference” bars indicate intestine or germline associated genes as a percentage of the C. elegans genome (20,404 genes). At each timepoint, 39% to 56% of all highly regulated genes were associated with the intestine, which represents a significant enrichment (chi-squared test, p<1.03E-26, all comparisons), while germline genes were significantly underrepresented (chi-squared test, p<1.51E-05, all comparisons) (Figure 1D, Table S2). E) Correlations between genes regulated by N. parisii infection and genes upregulated by other pathogens, stressors and immunity pathways. Gene sets were compared using the GSEA software (see Table S5 for detailed summary of results) and normalized enrichment scores (NESs) with a relaxed significance threshold (FDR<0.25, p<0.05) are reported in the figure. A positive NES (yellow) indicates a correlation with genes upregulated in response to N. parisii infection, while a negative NES (blue) indicates a correlation with genes downregulated in response to N. parisii infection (see Materials and Methods for analysis details). Black indicates no significant (FDR<0.25, p<0.05) correlation, and an NES with FDR<0.05 is indicated with an asterisk. Next, we compared genes regulated by N. parisii (Table S4) to gene sets regulated by infection with other pathogenic microbes, by treatment with non-biotic stressors, and by known immunity and stress-response pathways in C. elegans [17], [35], [36], [37], [38], [39], [40], [41] (Figure 1E, Table S5, Table S6). Here, we used a well-established analytical method called Gene Set Enrichment Analysis (GSEA), which analyzes gene expression data at the level of gene sets instead of individual genes (see Materials and Methods) [42]. We found limited but significant correlations with gene sets upregulated by heat shock treatments, the pore-forming toxin Crystal protein-5B (Cry5B), and Drechmeria coniospora fungal infection, predominantly at the 30 hpi timepoint (Figure 1E). The heat shock pathway has been shown to play a role in resistance to bacterial pathogens as well as other stresses [43], [44]. However, despite the overlap between genes induced by heat shock and microsporidia, we found that N. parisii infection upregulated only two canonical heat shock protein-encoding genes, hsp-17 at 30 hpi and hsp-16.1/hsp-16.11 (which have identical sequence and are indistinguishable in RNA-seq data) at 64 hpi (Table S7, Figure S1A). Notably, there was almost no correlation between C. elegans genes upregulated in response to N. parisii infection compared to infections with the extracellular bacterial pathogens Pseudomonas aeruginosa and Staphylococcus aureus, the fungal pathogen Harposporium, or to genes affected by known C. elegans immunity regulators (Figure 1E). However, there was extensive correlation between genes downregulated by N. parisii and genes downregulated by other pathogens - for further discussion of this correlation, and other comparisons to previously published gene expression analyses see Supplemental Text S1 and Figure S2. Strikingly, we found a very strong correlation between genes most strongly upregulated by N. parisii, (e.g. genes of unknown function C17H1.6 and F26F2.1) and genes upregulated by viral infection (Figure 1E, Figure S1B). Thus, N. parisii induces robust gene expression changes that are largely distinct from changes induced by extracellular pathogens, but share similarity to changes induced by the Orsay virus, which is another natural intracellular pathogen of C. elegans.
N. parisii and viral infection upregulates expression of genes involved in ubiquitylation
To understand the nature of the C. elegans response to microsporidia infection, we analyzed the enrichment of gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) terms for the significantly induced and repressed genes [45], [46] (Table 1 and S8). Early during infection upregulated genes were enriched for GO terms associated with regulation of growth, while at later timepoints they were enriched for GO terms associated with the nucleosome, defense response, and structural components. At 30 hpi, upregulated genes were enriched for association with the ubiquitin-mediated proteolysis KEGG pathway (Table 1). To extend our analysis, we also identified specific enrichment of Pfam protein domains among N. parisii regulated genes (Table 1 and S8). At early times following infection these included two Caenorhabditis domains of unknown function, DUF713 and DUF684. Notably, genes upregulated at 8, 16 and 30 hpi were also enriched for the F-box, FTH (fog-2-homology), and MATH (meprin and Traf homology) protein-protein interaction domains, which are domains associated with ubiquitin-mediated proteolysis. For more details on regulated proteins containing these domains, analysis of gene enrichment at later time points, and analysis of downregulated genes, see Supplemental Text S1.
Tab. 1. Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Pfam domain (PF) enrichment analysis of C. elegans genes upreguated by N. parisii infection. 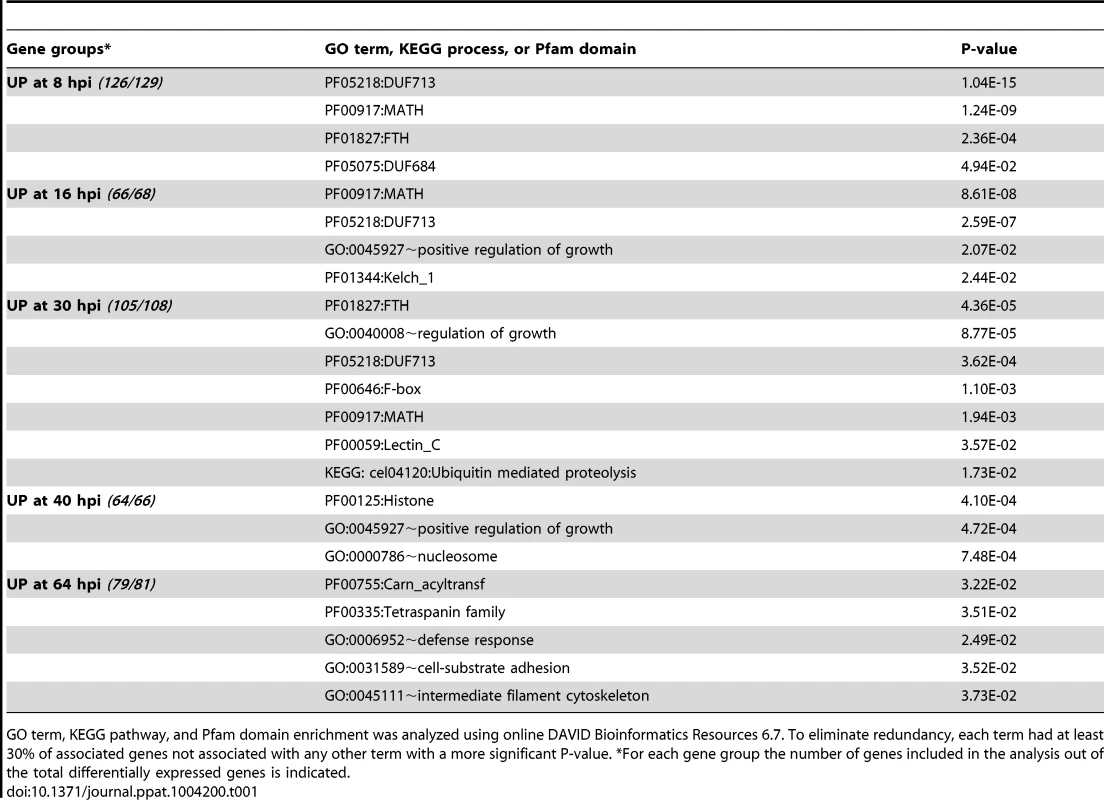
GO term, KEGG pathway, and Pfam domain enrichment was analyzed using online DAVID Bioinformatics Resources 6.7. To eliminate redundancy, each term had at least 30% of associated genes not associated with any other term with a more significant P-value. *For each gene group the number of genes included in the analysis out of the total differentially expressed genes is indicated. Previously it had been hypothesized that F-box and MATH domain-containing proteins could function in C. elegans to target foreign pathogen proteins for proteasomal degradation, as part of SCF multi-subunit E3 ubiquitin ligases [32]. Indeed, we found that C. elegans SCF ligase components, Skp1-related (skr) genes skr-4 and skr-5, were significantly upregulated at 30 hpi with N. parisii (Table S2), while skr-3 and the cullin gene cul-6 were also upregulated at 30 hpi over 6.5 - and 5.5-fold respectively, although the difference was not significant (Table S4). While these SCF ligase components were not reported to be significantly upregulated in a published dataset of the wild-type C. elegans response to viral infection [17], we found that in the virus-susceptible rde-1 strain of C. elegans, the SCF ligase components cul-6, skr-3, skr-4, and skr-5 were upregulated in response to viral infection (data not shown). Overall, this increased expression of genes encoding SCF ligase components (see Table S9 for list of significantly upregulated ubiquitylation-associated genes) is consistent with ubiquitylation being upregulated in virus and microsporidia-infected animals.
N. parisii growth is limited by SCF ligase components, the proteasome and autophagy
To examine a functional role for genes induced by N. parisii infection we used RNAi to knock-down expression of specific genes, then infected these animals with N. parisii and measured pathogen load at 24 hpi by quantifying N. parisii rRNA FISH signal (Figure 2A, S3). We tested several genes highly induced by infection, as well as genes that belong to gene classes identified through our GO term and Pfam domain analysis. Knock-down of most genes showed little to no effect on pathogen load (see Supplemental Text S1 and Figure S4). When we examined whether the upregulated SCF ligase components have a functional role in defense against N. parisii infection we found a more substantial role. Because there are a large number of F-box proteins in the C. elegans genome (∼520 proteins), we focused on the core SCF ligase components that belong to smaller families, namely the Skp1-related skr family (21 proteins) and the cullin family (6 proteins), which likely have less functional redundancy than F-box proteins. In particular, we knocked down expression of cul-6, skr-3, skr-4 and skr-5 because these were upregulated upon N. parisii infection. Here, we found a modest but significant increase in pathogen load in cul-6, skr-3 and skr-5 RNAi-treated animals (Figure 2B), suggesting that these SCF ligase components limit the growth of N. parisii during infection.
Fig. 2. The SCF ligases, UPS and autophagy limit the growth of N. parisii in the C. elegans intestine. 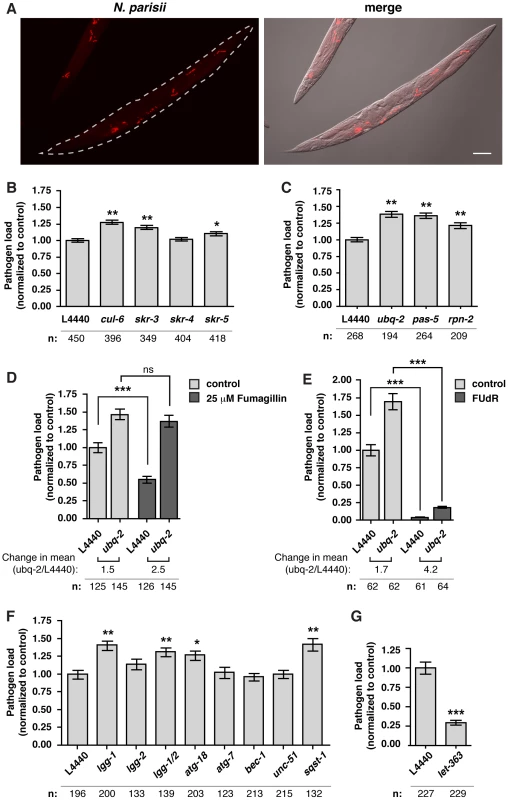
A) Fluorescence and bright field images demonstrating FISH staining with a probe against N. parisii rRNA used to quantify pathogen load in the C. elegans intestine following 24 hours of infection with N. parisii. Scale bar = 100 µm. B–F) Quantification of pathogen load (see Materials and Methods) in nematodes treated with RNAi against SCF ligase components (B), against ubiquitin (ubq-2) and two components of the proteasome (pas-5 and rpn-2) (C), against ubq-2, +/− fumagillin (D), against ubq-2, +/− FUdR (E), against autophagy components (F), and the C. elegans TOR ortholog let-363 (G). Pathogen area occupying each RNAi-treated animal was normalized to mean L4440 control values. The number of animals analyzed for each condition (n) is indicated. Mean +/− SEM is shown for all analyzed animals (data for B, C, F are from three independent experiments, data for D, G are from two independent experiments and data for E are from one experiment). Each independent experiment was comprised of two separate populations of animals. Statistical significance was assessed using a one-way ANOVA with Dunnett's Multiple Comparisons Test for B, C, and F, with Bonferroni Multiple Comparison Test for D and E, and with student's t-test for G (***p<0.001, **p<0.01, *p<0.05). After substrates have been ubiquitylated by ubiquitin ligases, they can either be degraded by the proteasome or by the autophagy pathway. First, we examined whether components of the proteasome may be acting downstream of SCF ligase components in limiting growth of N. parisii. We reduced expression of ubiquitin itself with RNAi against ubq-2, as well as two components of the proteasome: pas-5 and rpn-2. In order for animals to develop properly, we introduced the RNAi in a diluted form at a late larval stage, infected animals and measured pathogen load. We found that reducing expression of any of these three genes led to an increase in pathogen load, suggesting that the UPS is important for defense against N. parisii (Figure 2C).
Because the effect of ubiquitin knock-down on pathogen load was modest, we hypothesized that, like other intracellular pathogens, N. parisii may suppress this defense system or subvert some aspects of the UPS to promote its replication. To test this hypothesis, we treated animals with drugs that block N. parisii growth but have minimal effects on adult C. elegans (see Supplementary Text S1) [47], [48]. First, we treated animals with a low dose of the anti-microsporidia drug fumagillin [49], [50], [51], [52], which limits N. parisii growth (Figure 2D and data not shown). After fumagillin treatment we found that ubq-2 RNAi had a more robust effect on pathogen load (150% increase) than in the absence of this drug (50% increase) (Figure 2D). Similarly, ubq-2 RNAi had a stronger effect on pathogen load when N. parisii growth was repressed with a DNA synthesis inhibitor, FUdR, (320% increase) than in the absence of this drug (70% increase) (Figure 2E). Taken together, these results suggest that the host UPS plays a greater role in controlling infection when pathogen growth is inhibited.
We next investigated a role for the autophagy pathway in response to N. parisii infection. We used RNAi to knock-down expression of different autophagy components, infected these animals with N. parisii, and quantified pathogen load. Similar to the effects of knocking down components of the UPS, we found a modest but significant increase in pathogen load when expression of several key autophagy components was reduced (Figure 2F). Furthermore, RNAi of the C. elegans nutrient sensor TOR (Target Of Rapamycin) ortholog let-363, which activates autophagy in C. elegans [53], caused a dramatic 70% decrease in pathogen load (Figure 2G). To determine whether autophagy machinery was directed toward N. parisii cells, we examined localization of GFP-tagged LGG-1 (homolog of Atg8/LC3 in yeast/mammals) [54], a protein whose distribution is often used to assess autophagy [55]. We found that early during infection only 7% of parasite cells (25/360 parasite cells, n = 6 animals) were targeted by GFP::LGG-1 (Figure 3A–C). When animals were treated with let-363 RNAi, we found that there was a greater than 2-fold increase in parasite cells targeted by GFP::LGG-1 (Figure 3D), consistent with this treatment causing an upregulation of the autophagy machinery directed toward N. parisii cells. Thus, the autophagy machinery appears to be targeted to N. parisii cells, and promotes resistance against infection.
Fig. 3. Targeting of N. parisii cells by the autophagy marker GFP::LGG-1 increases upon let-363/TOR RNAi. 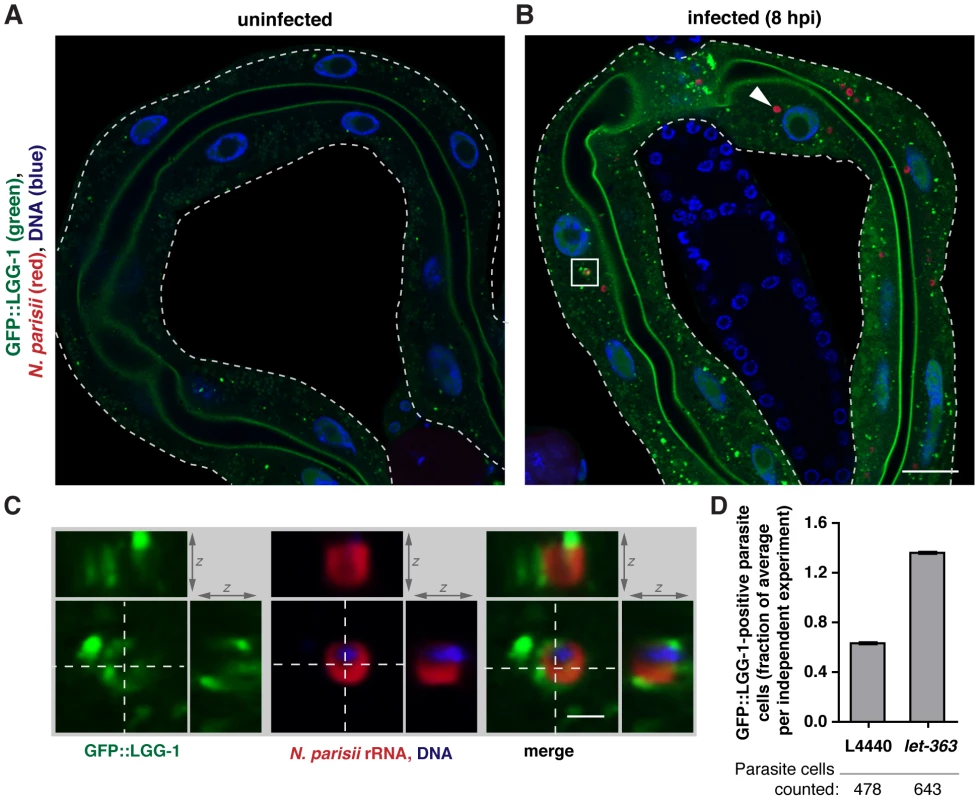
GFP::LGG-1-expressing transgenic animals were fixed and stained with a FISH probe against N. parisii rRNA (red) and DAPI for DNA (blue). A) Intestine of an uninfected nematode, and B) an N. parisii-infected nematode, 8 hpi, are shown. N. parisii parasite cells not colocalizing with GFP::LGG-1 (arrowhead) and surrounded by GFP::LGG-1 (boxed in area) are indicated. Scale bars = 20 µm. C) Enlarged view of boxed in area from panel B, showing cross-section from two other dimensions. Scale bar = 2 µm. D) Quantification of parasite cell colocalization at 8 hpi with wild-type GFP::LGG-1 following knockdown of let-363 compared to L4440 vector control. Data are normalized for the average level of targeting in each independent experiment. Mean +/− SEM of two independent experiments is shown. Number of individual parasite cells assessed for colocalization is indicated. One potential caveat to the results described above is that specific RNAi treatments might affect the feeding rates of nematodes, which could then result in changes in pathogen load simply due to differences in the initial dose of N. parisii spores ingested by these animals. To address this concern, we measured the accumulation of fluorescent beads in the intestinal lumen of animals fed dsRNA against the genes described above (Figure S5A–C). Importantly, RNAi against let-363/TOR, which causes decreased pathogen load, did not cause a decrease in the accumulation of fluorescent beads. In addition, RNAi against most autophagy genes that caused increased pathogen load did not cause an increase in accumulation of fluorescent beads. Furthermore, UPS RNAi, which increases pathogen load, did not increase fluorescent bead accumulation, and to the contrary, knock-down of ubq-2 or pas-5 marginally inhibited accumulation. Finally, feeding rates as measured by pharyngeal pumping were not affected by RNAi treatments, with the exception of ubq-2 RNAi, which caused a decrease in feeding (Figure S5D–F). For further details on these controls, see Supplemental Text S1. Altogether, our data support the model that defense against N. parisii infection involves ubiquitylation components, the proteasome, and the autophagy pathway, although microsporidia appears to partially evade or suppress this ubiquitin-mediated response.
Host ubiquitin targets some N. parisii cells, but most parasite cells escape this targeting
To examine whether N. parisii itself is targeted by ubiquitin, we stained infected animals with the FK2 antibody, which recognizes ubiquitin that is conjugated to a substrate, and with a FISH probe against N. parisii rRNA to label the pathogen. Because N. parisii is a eukaryote, it contains its own ubiquitin, which is recognized by the FK2 antibody. However, distinct from this staining, we observed very strong accumulation of conjugated ubiquitin surrounding a subset of N. parisii meronts, with signal far above background of the microsporidia-derived ubiquitin (Figure 4A). To confirm that this ubiquitin was host-derived, we created a transgenic C. elegans strain that expresses a GFP::ubiquitin fusion protein under the control of an intestinal-specific promoter. Using these transgenic animals, we observed targeting of GFP::ubiquitin to parasite cells (Figure 4B). In contrast, we did not observe significant targeting to parasite cells by a conjugation defective GFP::ubiquitinΔGG fusion protein (Figure 4C, Figure S6). Altogether these experiments demonstrate that host ubiquitin is specifically targeted to N. parisii cells, where it is conjugated to a substrate.
Fig. 4. N. parisii cells are targeted by host ubiquitin early during infection. 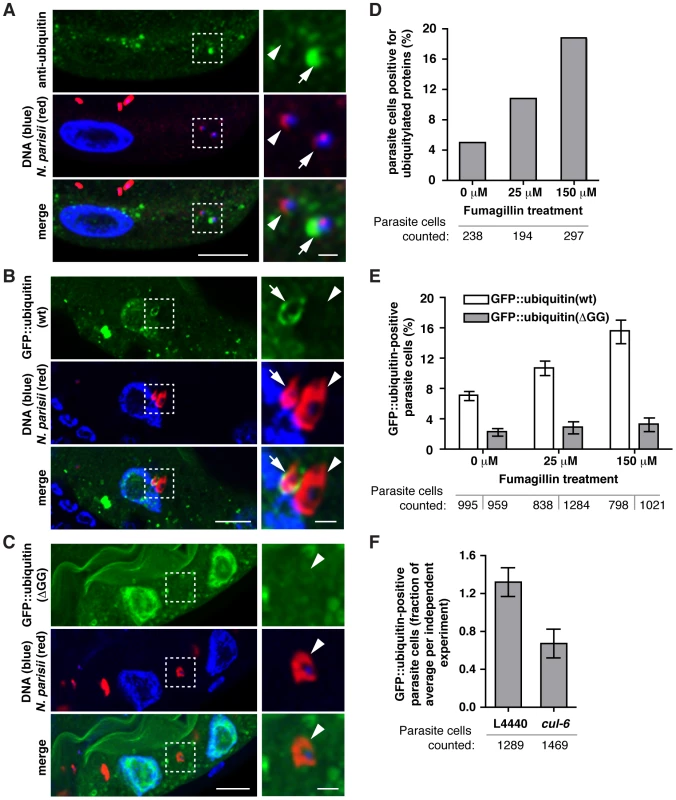
A–C) C. elegans intestines stained with a FISH probe against N. parisii rRNA (red), and DAPI for DNA (blue). A section of the image outlined by a dotted square is enlarged on the right and shows both an N. parisii parasite cell that colocalizes with ubiquitin (arrow), and one that does not (arrowhead). A) Animals were stained with an anti-conjugated-ubiquitin antibody, FK2 (green), and B, C) Transgenic C. elegans intestines expressing wild-type (B) or conjugation-defective mutant (C) GFP::ubiquitin (green). For A–C, scale bar = 10 µm in main images and 2 µm in enlarged sections. D) Quantification of parasite cell colocalization at 12 hpi (see Materials and Methods for timepoint information) with FK2 antibody in the presence of increasing doses of fumagillin. Difference is significant: chi-squared test, p<6.3E-28, all comparisons. E) Quantification of parasite cell colocalization at 15 hpi with wild-type or mutant GFP::ubiquitin in the presence of increasing doses of fumagillin. Mean +/− SEM of two independent experiments is shown. F) Quantification of parasite cell colocalization at 15 hpi with wild-type GFP::ubiquitin following knockdown of cul-6 RNAi compared to L4440 vector control. Targeting of ubiquitin to parasite cells was less robust and more variable in animals feeding on HT115 RNAi bacteria compared to OP50-1 E. coli, ranging from 10.6% to 2.2% in control animals, and 2.1% to 1.8% in cul-6 RNAi treated animals and the data presented are normalized for the average level of targeting in each independent experiment. Mean +/− SEM of three independent experiments is shown. Number of individual parasite cells assessed for colocalization with ubiquitin is indicated. The percentage of N. parisii cells specifically targeted by ubiquitin was relatively low: using the FK2 antibody we found only about 5% of pathogen cells were targeted by ubiquitin at 12 hpi (Figure 4D). Similarly, we found only about 7% of pathogen cells were targeted by GFP::ubiquitin (Figure 4E). Therefore, we examined whether N. parisii is suppressing or evading ubiquitin targeting by the host. If so, inhibiting the growth/vigor of the pathogen should cause an increased level of ubiquitin targeting. Indeed, we found increased targeting of ubiquitin to parasite cells after fumagillin treatment, with 16–18% of cells targeted (Figure 4D, E). This effect was dose-dependent, and was apparent both with the FK2 antibody, as well as the GFP::ubiquitin fusion protein. These results support the hypothesis that N. parisii is actively suppressing or evading ubiquitin targeting by C. elegans, and that after inhibition of N. parisii growth with an anti-microsporidia drug, the host is better able to target pathogen cells with ubiquitin.
Because the SCF ubiquitin ligase components cul-6, skr-3 and skr-5 serve to limit N. parisii growth (Figure 2B) we hypothesized that they could be responsible for ubiquitin targeting of parasite cells. Thus, we examined ubiquitin targeting to N. parisii cells in animals that had been treated with cul-6 RNAi compared to the RNAi control (Figure 4F). Indeed, we found that cul-6 RNAi had significantly reduced targeting of ubiquitin to N. parisii cells (two-tailed unpaired t-test, p<0.05). Thus, cul-6 is important for efficient ubiquitylation of parasite-associated proteins, suggesting that cul-6-containing SCF ligases may mediate recognition of N. parisii infection by the host.
The ubiquitin targeting of parasite cells described above was only observed at early timepoints of infection, when pathogen cells were small and mono-nucleate. When the pathogen cells grew bigger and became multi-nucleate meronts, we observed virtually no parasite cells targeted by ubiquitin or by autophagy (data not shown). Similarly, once meronts have differentiated into spores at later stages of infection, we found exceedingly few spores targeted by ubiquitin (Figure 5A). Although there was virtually no specific ubiquitin targeting to the parasite at these later stages of infection, we did observe an increased number of clusters of ubiquitylated proteins (Figure 5B–F). These clusters were dispersed throughout the infected intestinal cells, but in some cases were closely associated with N. parisii, although not encircling the parasite cells (Figure 5D). In addition, we found that infection caused increased clustering of the autophagy marker GFP::LGG-1 in regions distinct from the pathogen cells (Figure S7A–C) and found that GFP::LGG-1 partially colocalized with ubiquitylated protein clusters (Figure S7E–F). In order to determine whether this is a specific response, we examined GFP::LGG-1 upon infection with the extracellular bacterial pathogen P. aeruginosa, and did not find a significant increase in clustering (Figure S7D). Thus, as infection proceeds, an increased amount of conjugated ubiquitin and GFP::LGG-1 clusters accumulate in the host cytosol, and these markers are almost never seen specifically surrounding the pathogen cells.
Fig. 5. N. parisii cells are almost never targeted by ubiquitin later during infection, but ubiquitin forms clusters that accumulate in the C. elegans intestine. 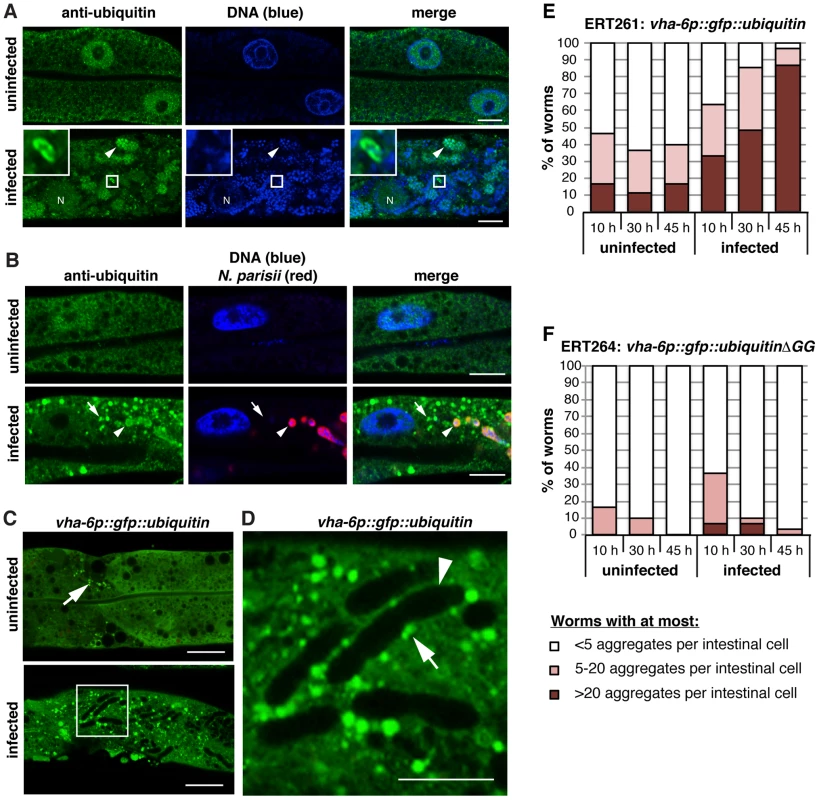
A,B) C. elegans intestines stained an anti-conjugated-ubiquitin antibody FK2 (green), and DAPI for DNA (blue): Panel B also includes a FISH probe against N. parisii rRNA (red). A) Sections of uninfected and N. parisii-infected C. elegans intestines. In the infected intestine an N. parisii spore labeled with the anti-conjugated-ubiquitin antibody is enlarged in box inset in upper left, and meront (arrowhead), and host nucleus (N) are indicated. Scale bar = 10 µm. B) Sections of uninfected and N. parisii-infected C. elegans intestines (30 hpi) shown with ubiquitin cluster (arrow) and ubiquitin staining within an N. parisii meront (arrowhead) indicated. Scale bar = 10 µm. C) Intestines of uninfected and infected animals expressing an intestinal GFP::ubiquitin transgene (48 hpi, grown at 20°C to prevent construct aggregation) are shown. Small GFP::ubiquitin aggregates are sometimes observed in uninfected animals (arrow). Scale bar = 20 µm. D) Enlarged portion of box in panel C. Oblong N. parisii meronts are visible through the absence of green (arrowhead) and ubiquitin clusters associating with the meronts (arrow) are indicated. Scale bar = 10 µm. E) Animals expressing the intestinal GFP::ubiquitin construct were infected with N. parisii and, together with control uninfected animals, fixed at the indicated times. Fixed animals were stained with a FISH probe against N. parisii rRNA to mark the infection and their intestinal cells were inspected for visible GFP::ubiquitin aggregates (30 transgenic animals were inspected per timepoint and condition). F) Animals expressing the intestinal control GFP::ubiquitinΔGG construct were treated and analyzed as in E. Perturbation of the UPS induces expression of SCF ligase components and other infection response genes in the intestine
Recent studies have indicated that host cells monitor the functioning of core processes that are commonly perturbed by pathogen infection and that disruption of these processes can trigger defense-related gene expression by the host [56], [57], [58], [59], [60]. Because intracellular infection by N. parisii leads to an increase in ubiquitylated protein clusters, which may reflect an increase in demand on the UPS, we investigated whether perturbation of the UPS might be responsible for inducing gene expression changes upon N. parisii infection. To conveniently monitor gene expression in vivo and to examine where genes are induced upon N. parisii infection, we made promoter-GFP fusions for C17H1.6 and F26F2.1, two genes of unknown function that are among the most highly upregulated genes at all infection timepoints (eg. at 8 hpi, C17H1.6 and F26F2.1 are upregulated 1.2×1011 - and 1441-fold, respectively) (Table S2, S3). Expression of GFP driven by promoters of these genes was strongly induced in intestinal cells of infected animals by 8 hpi and even more robustly by 24 hpi (Figure 6A). These GFP reporters indicated that N. parisii infection drives expression of genes in intestinal cells of infected animals and provided convenient tools for monitoring expression of infection response genes.
Fig. 6. UPS perturbation induces similar gene expression responses to N. parisii infection. 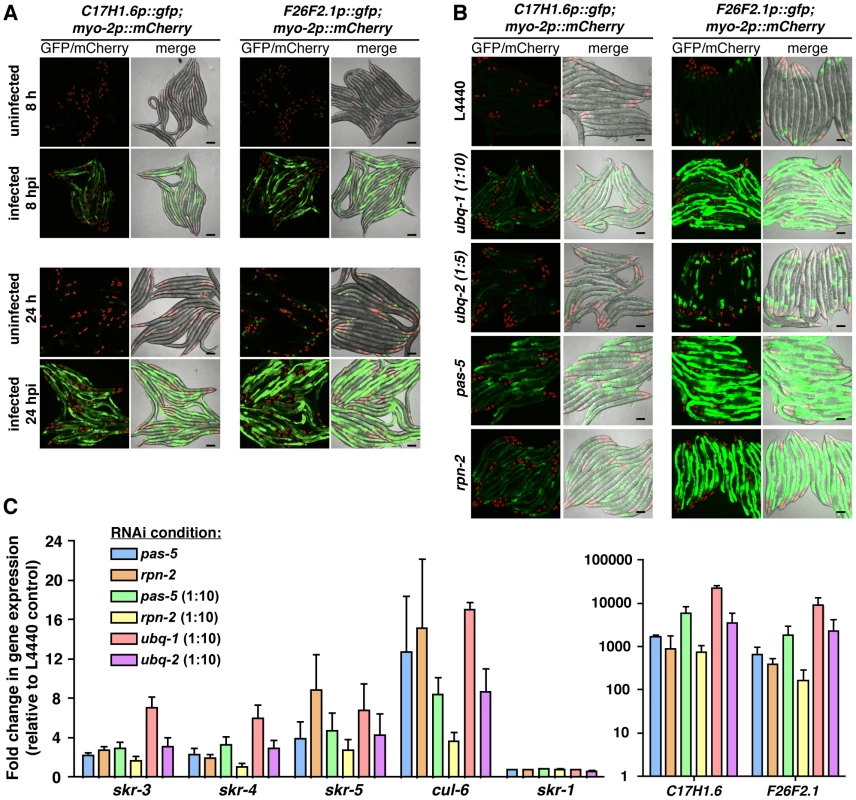
A) Expression of C17H1.6p::gfp and F26F2.1p::gfp in the intestine (pharyngeal myo-2p::mCherry expression is a marker for the presence of the transgene) following infection with N. parisii (8 and 24 hpi). Scale bars = 100 µm. B) Expression of C17H1.6p::gfp and F26F2.1p::gfp following RNAi against ubq-1, ubq-2, pas-5 and rpn-2 in the absence of infection. C) Expression of endogenous mRNA of C17H1.6 and F26F2.1, as well as the SCF ligase components skr-1, skr-3, skr-4, skr-5 and cul-6 following RNAi against ubq-1, ubq-2, pas-5 and rpn-2 in the absence of infection, as assessed by qRT-PCR. Due to the very large changes in expression of C17H1.6 and F26F2.1 genes, these are presented on a separate graph to allow for expansion of the y-axis and easier observation of expression changes in SCF ligase components. Mean +/- SEM of two to three independent experiments. To disrupt UPS function, we first performed RNAi knock-down of ubiquitin, pas-5 and rpn-2 in C17H1.6p::gfp and F26F2.1p::gfp transgenic animals. Strikingly, RNAi against the UPS components dramatically induced GFP expression in the intestine in both of these strains (Figure 6B). To confirm these results we performed qRT-PCR and saw levels of endogenous C17H1.6 and F26F2.1 mRNA transcripts also increased by UPS RNAi (Figure 6C). To perturb UPS function pharmacologically, we used the proteasome inhibitor MG-132 and similarly saw that this led to dramatic increase in C17H1.6 and F26F2.1 expression (Figure S8A–C). Because C17H1.6 and F26F2.1 are genes of unknown function, we extended these analyses to genes upregulated by intracellular infection that have predicted function, namely the genes that encode the SCF ubiquitin ligase components skr-3, skr-4, skr-5 and cul-6 (Figure 6C, Figure S8C). Similar to other infection response genes, we found that these genes were also induced by RNAi against the UPS, while another SCF component, skr-1, whose expression was not altered during microsporidia infection, was not affected (Figure 6C, Figure S8C). Thus, C. elegans appears to monitor efficacy of the UPS, and when this core process is disrupted it can trigger expression of a number of specific genes, including SCF components such as cul-6 that are used by C. elegans to limit intracellular infection.
SCF ligases promote anti-viral defense, although host UPS is required for viral replication
The C. elegans gene expression response to N. parisii was most similar to its response to viral infection, including the upregulation of SCF ligase components (Figure 1E, Table S5, Table S6). Because of this similarity, we investigated whether the SCF ligases implicated in response to N. parisii also played a role in response to viral infection. Indeed, we found that cul-6 RNAi caused a 13-fold increase in viral load, and skr-3 and skr-4 RNAi caused 5 - and 4-fold increases in viral load respectively (Figure 7A), indicating that these SCF ligase components promote anti-viral defense. However, contrary to N. parisii infection, global inhibition of the UPS by RNAi-mediated knockdown of UPS components drastically reduced viral replication (Figure 7B). Many viruses exploit host UPS in order to replicate, for example to degrade host RNAi and immune signaling machinery, or to control function and stability of viral proteins [61], [62], [63], [64], [65], and thus the Orsay virus may likewise be hijacking this host pathway. Importantly, this result also suggests that the increased susceptibility to N. parisii infection of UPS-compromised nematodes (Figure 2C) is not likely just a result of general 'sickness' in these animals. Thus, the UPS appears to play two different roles in response to the Orsay virus, involving an unknown ligase(s) that promotes susceptibility to viral infection, and the cul-6, skr-3 and skr-4 SCF ubiquitin ligases promoting anti-viral defense.
Fig. 7. Ubiquitin-mediated host response and defense against Orsay viral infection in C. elegans. 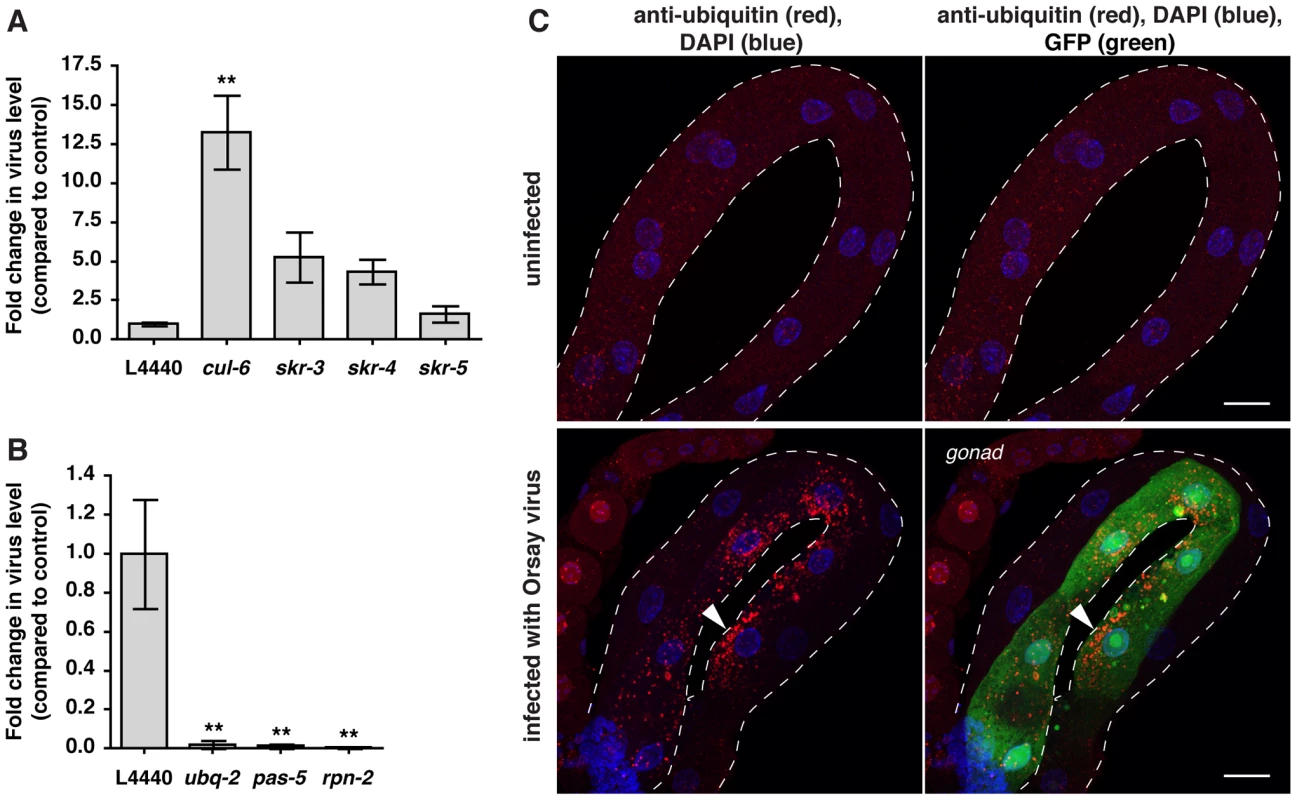
A) Viral pathogen load in nematodes treated with RNAi against the SCF ligase components skr-3, skr-4, skr-5, and cul-6 compared to vector control RNAi (L4440), as assessed by qRT-PCR for viral transcript. Mean +/− SEM of three independent experiments shown. B) Viral pathogen load in nematodes treated with RNAi against ubq-2, pas-5 and rpn-2 analyzed as above. Mean +/− SEM of three independent experiments shown. C) Intestines of F26F2.1p::gfp transgenic animals were stained with the FK2 antibody against conjugated ubiquitin (red), and DAPI for DNA (blue). Intestines outlined with white dotted line. Animals infected with virus have increased ubiquitin clustering in some intestinal cells compared to uninfected animals (arrowhead), and also express the GFP reporter. Scale bar = 20 µm. ** p<0.01. Because N. parisii infection caused increased clustering of ubiquitylated proteins in C. elegans intestine, and robust gene expression changes in response to infection appeared to be a reflection of increased demand on the UPS, we investigated whether similar host responses occurred upon viral infection. Indeed, we found that infection with Orsay virus caused clustering of ubiquitylated proteins (Figure 7C), and clustering of GFP::LGG-1 (Figure S7A–D). Thus, infection with the Orsay virus induces similar cell biological changes as N. parisii infection. Furthermore, we found that viral infection induced the GFP reporter F26F2.1p::gfp (Figure 7C), which is also induced when the UPS is perturbed. Thus, it appears that the C. elegans transcriptional response to viral infection, like the response to N. parisii infection, involves surveillance pathways that detect perturbation of the UPS caused by infection, to upregulate defense gene expression.
Discussion
Ubiquitin-mediated defense against intracellular infection by N. parisii and the Orsay virus
Based on our results we propose a model for the C. elegans intestinal response to intracellular infection (Figure 8), which highlights an important role for ubiquitin-mediated defense. In response to N. parisii infection, C. elegans upregulates expression of SCF ligase components, which restrict growth of the microsporidian pathogen N. parisii, as well as the Orsay virus. Restriction of N. parisii growth appears to also depend on the proteasome, as well as the autophagy pathway. While SCF ligase components such as CUL-6 have a substantial role in restricting growth of the virus, their more modest role in defense against N. parisii may be due to functional redundancy and/or the relatively inefficient targeting of ubiquitin to this pathogen. Inefficient targeting may be a result of suppression or evasion of host defenses by the parasite, as we find increased ubiquitin targeting of pathogen cells and a greater role for ubiquitin-mediated defense after treatment with drugs that inhibit N. parisii growth. Furthermore, we observe an increase in autophagy machinery targeting to N. parisii cells after activation of autophagy by inhibition of the TOR pathway. Interestingly, the increased demand on the UPS caused by intracellular pathogens like N. parisii and the Orsay virus may induce gene expression in response to infection, because genetic or pharmacological perturbation of the UPS upregulates expression of SCF ligase components and other genes that are induced by these intracellular infections.
Fig. 8. Model for SCF E3 ligases and ubiquitin-mediated responses to intracellular infection in C. elegans. 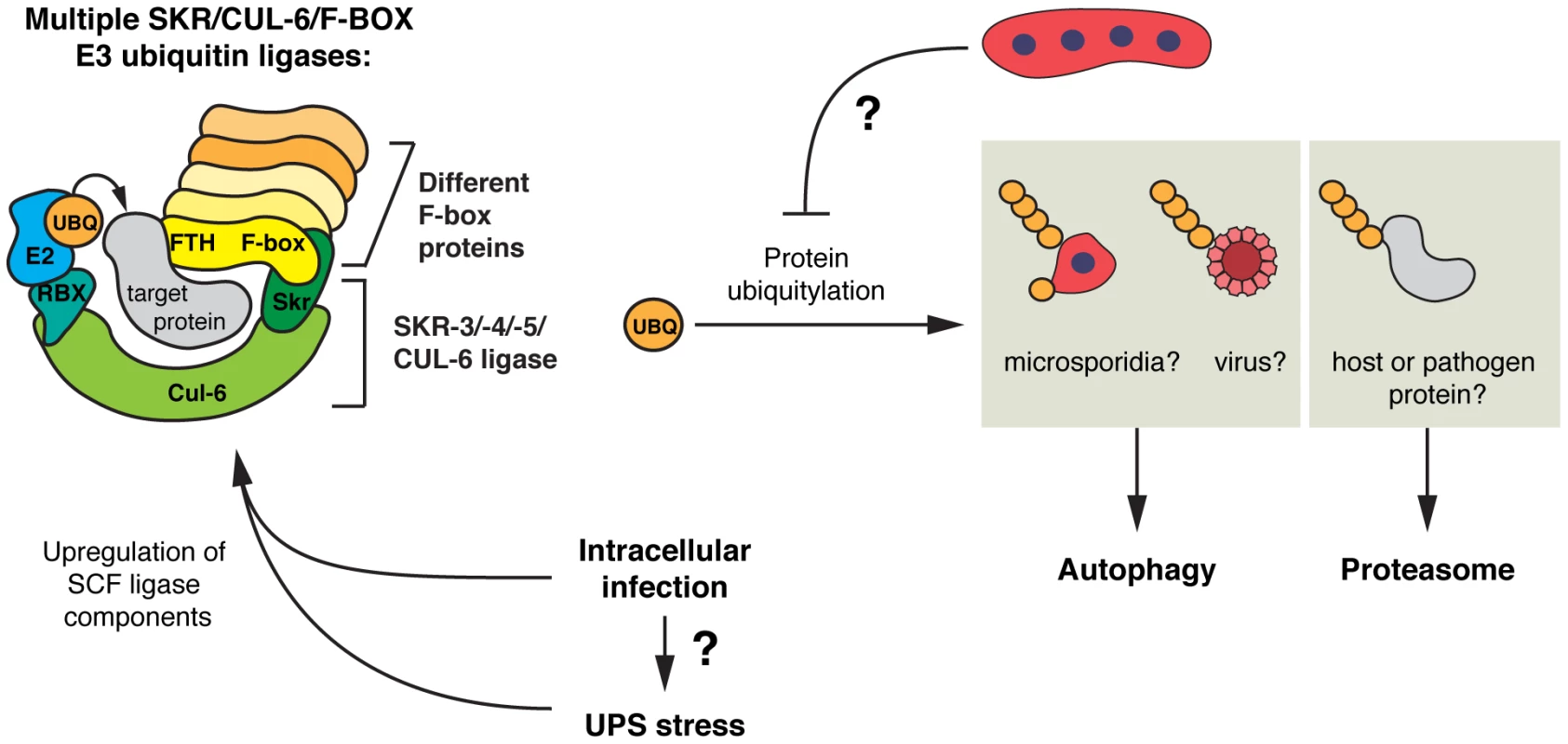
Intracellular microsporidia or viral infection triggers the expression of SCF ligase components in C. elegans, including a large number of F-box genes, the cullin cul-6, and Skp1-related genes, skr-3, -4, and -5. Due to the modularity of the SCF ligase complex, many SCF ligases with vast substrate recognition potential may be formed, which could recognize pathogen-derived proteins or host proteins. Ubiquitylation of substrates leads to their degradation by the proteasome or by autophagy, with large substrates such as microsporidia cells, potentially viral particles, and protein aggregates (not shown), targeted by autophagy, and individual pathogen or host proteins by the proteasome. N. parisii parasite cells may be able to suppress or evade ubiquitylation. Both intracellular infection and UPS stress can induce SCF ligase components, and greater demand on the UPS during intracellular infection may contribute to upregulation of SCF ligase components. See Discussion for more details. SCF ligases comprise one of the major classes of E3 ubiquitin ligases that catalyze transfer of ubiquitin onto substrates. These ligases have very well characterized roles in controlling levels of endogenous proteins that regulate the cell cycle and development. Intriguingly, the expanded and diversified repertoire in C. elegans and plants of ubiquitin ligase adaptors such as F-box and BTB-MATH domain proteins, as well as other SCF components, has led to the hypothesis that these ligases may also be involved in recognition of foreign substrates. Our study with microsporidia and virus infection provides the first experimental support for this hypothesis. In particular, we see that the C. elegans SCF ligase components cul-6 and skr-3, skr-4, and skr-5, mediate a defense response of C. elegans to N. parisii and virus infection. Previous reports indicated that CUL-6 and SKR-3 interact physically in a yeast two-hybrid assay, indicating these components could assemble in vivo to produce a functioning SCF ligase [33]. Moreover, we see targeting of ubiquitin to N. parisii cells that depends on the cul-6 cullin component of the SCF ligase, which could conjugate host ubiquitin onto pathogen proteins or to host proteins that are associated with the pathogen cell. SCF ligases may also be involved in processing of proteins distinct from the pathogen cells, such as virulence factors that are secreted out of the pathogen cell into the cytosol. Another intriguing possibility is that SCF ligases are important for degrading inhibitory host proteins to trigger host innate immunity, analogous to ubiquitin-mediated degradation of IκB in NFκB signaling in mammals. However, the actual signaling proteins in C. elegans would be different because the NFκB transcription factor has been lost in this lineage [66]. Processing of host signaling proteins could occur in the clusters of conjugated ubiquitin we see later during infection, which are not associated with pathogen cells. Indeed, all of these possibilities are not mutually exclusive, and there are likely many roles for the SCF ligases and ubiquitin-mediated responses to intracellular infection in C. elegans.
Perturbation of UPS function triggers upregulation of SCF ligases and other intracellular infection response genes
Our analysis of the gene expression response to N. parisii infection indicated that C. elegans has a very distinct response to this pathogen compared to previously described extracellular pathogens. Responses to extracellular pathogens like S. aureus and P. aeruginosa are marked by upregulation of secreted anti-microbials and detoxifying enzymes [37], [41], [67], [68], [69], which did not comprise a substantial part of the gene sets upregulated by N. parisii. Instead we found enrichment for genes associated with ubiquitylation (Table 1, S9), and that the response to N. parisii shared greatest similarities with the response to Orsay virus infection. The commonality of transcriptional response to these two very distinct pathogens (N. parisii is a eukaryotic organism with 2661 genes and the Orsay virus has only 3 genes) is quite striking, and our data indicate that some genes induced by infection such as SCF ligases can also be induced by perturbation of UPS function. Indeed, inhibition of the proteasome has been shown to induce stress response genes in other C. elegans studies as well [58], [59]. These results fit with the growing theme that C. elegans epithelial defense relies on monitoring of core host processes as an important cue to indicate the presence of pathogen attack [56], [57], [70], [71]. Such surveillance pathways are increasingly appreciated in mammalian defense as well, and may constitute a major mode by which hosts discriminate pathogens from other microbes [72], [73]. It is possible that surveillance of UPS function is responsible for controlling the transcriptional response to intracellular infection, although it is possible that UPS perturbation and infection are distinct triggers that converge to upregulate the same response genes.
Intracellular infection as well as perturbation of the UPS would be expected to cause substantial stress on the protein homeostasis (proteostasis) network of intestinal cells [74], [75], [76]. Intracellular infection by both N. parisii and virus should introduce a suite of foreign proteins into the host cell, may also cause damage to host proteins, and lead to activation of inducible immune responses. Any and all of these physiological changes may cause stress on the protein degradation and/or chaperone/folding systems of the host. This stress could explain the partial overlap we saw between the transcriptional response to intracellular infection and prolonged heat shock, a condition known to disrupt cellular proteostasis, although we saw an upregulation of only two hsp chaperones in response to infection (Table S7). In particular, hsp-16.1, which was significantly upregulated at 64 hpi when animal intestines are filled with parasite spores and large vacuoles (Figure 1A), has been shown to act in the Golgi where it helps to maintain cellular Ca2+ balance and protects cells against necrotic cell death triggered by heat as well as insults unrelated to thermal stress [43]. Further comparison between the responses to UPS stress and intracellular infection will likely shed light on mechanisms of cytosolic quality control and how they regulate defense against intracellular infection.
N. parisii may suppress and/or evade recognition by the host ubiquitylation machinery
While ubiquitin-mediated defense does play a role in limiting N. parisii growth, it appears to be only a minor one. There are several reasons that could account for this small effect. First, because UPS components are essential for animal development and overall health we relied on partial knockdown of UPS components to compromise UPS function. Second, in analyses of genes that are not essential, such as SCF ligase components, there may be redundancy in the proteins involved in defense. Third, we anticipate that like other intracellular pathogens [77], [78] (for example the Orsay virus in this study), N. parisii may subvert host ubiquitylation machinery to promote its own growth. In this case, compromised host UPS would negatively impact both the replication of N. parisii as well as the ability of C. elegans to clear infection, yielding a small net change in pathogen load. Lastly, it is possible that N. parisii suppresses or evades the host ubiquitin-mediated defense. Consistent with this idea, C. elegans is better able to target ubiquitin to pathogens and induce their degradation when N. parisii is treated with drugs that slow its growth. Additionally, if N. parisii were suppressing C. elegans ubiquitin-mediated defenses, then genetic inhibition of these processes in the context of infection would only have a minor effect on pathogen resistance, while genetic activation could have a greater effect. Indeed, we found that activating autophagy through RNAi against let-363/TOR led to improved targeting and clearance of N. parisii cells, with a greater effect on resistance than autophagy inhibition. However, it is important to note that let-363/TOR is upstream of several other processes, including protein synthesis [79], which may also account for the increased resistance of this strain.
Other pathogens have been shown to actively suppress ubiquitin-mediated defenses of other eukaryotic hosts [21], [78], [80], [81], [82]. For example, in human cells, the bacterial pathogen Salmonella enterica suppresses ubiquitin-mediated host defenses with the GogB effector, which inhibits a human SCF ligase by interacting with Skp1 and the human F-box only 22 (FBXO22) protein, an interaction that impedes NFκB signaling and limits inflammation in infected cells [83]. Similarly, N. parisii might deploy effectors that block ubiquitylation of meronts, which are in direct contact with the cell cytosol of C. elegans intestinal cells and should be accessible to host ubiquitylation machinery. N. parisii might also evade ubiquitylation by the host by masking or simply lacking host-recognizable cues present during other intracellular pathogen infections. In particular, because N. parisii is itself a eukaryote, it may possess fewer pathogen-associated molecular patterns (e.g. bacterial peptidoglycan or lipopolysaccharide), which can be used by eukaryotes to recognize pathogens.
Microsporidia are increasingly recognized as natural pathogens of nematodes [84], [85], and Nematocida strains in particular have been isolated from multiple wild-caught Caenorhabditis nematodes [12]. It will be interesting to examine the interaction between other Nematocida pathogens and Caenorhabditis hosts to determine whether ubiquitin-mediated defenses have a greater or lesser role in those encounters, as part of the ever-shifting landscape of the host/pathogen arms race. Because microsporidia are obligate intracellular pathogens (which by definition cannot grow outside of host cells), it is imperative that they evade or suppress host defense pathways such as ubiquitylation to propagate the species. Thus suppression or evasion of host defense, together with extremely rapid intracellular replication [34], may be at the heart of why the Microsporidia have grown to be such a large and successful phylum able to infect virtually all animal hosts.
Materials and Methods
C. elegans and N. parisii culture conditions
All C. elegans strains were maintained on nematode growth media (NGM) and fed with E. coli strain OP50-1, as described [86]. N. parisii spores were prepared as previously described [87]. Briefly, N. parisii was cultured by infecting large-scale cultures of C. elegans, followed by mechanical disruption of worms and then filtering to isolate spores away from worm debris. The temperature-sensitive sterile strain CF512 fer-15(b26);fem-1(hc17) was used for RNA-seq and other experiments to prevent internal hatching of progeny at later infection time points. This strain was maintained using standard laboratory techniques at the permissive temperature of 15°C and shifted to 25°C for pathogen infection experiments [39]. The DA2123 adIs2122[lgg-1p::gfp::lgg-1] strain was a kind gift from Dr. Malene Hansen [88], [89].
Generation of transgenic C. elegans strains
Promoter-GFP fusions for the N. parisii induced genes C17H1.6 and F26F2.1 were made using overlap PCR. Briefly, genomic DNA upstream of the predicted start for these genes was amplified (1273 bp for C17H1.6 and 796 bp for F26F2.1) with PCR and then fused in frame to GFP amplified from pPD95.75. These promoter-GFP fusions were co-injected with the myo-2p::mCherry marker that labels pharyngeal muscle. Several independent transgenic lines carrying extrachromosomal arrays for these fusions were isolated and these lines induced GFP upon infection with N. parisii. One line for each fusion was integrated using psoralen/UV-irradiation to generate the integrated transgenic strains ERT54 jyIs8[C17H1.6p::gfp; myo-2p::mCherry] × and ERT72 jyIs15[F26F2.1p::gfp; myo-2::mCherry].
A GFP-tagged ubiquitin construct pET341 was generated using three-part Gateway recombination by fusing the intestinal-specific vha-6 promoter to GFP at the N-terminus of ubiquitin (amplified from the C. elegans ubq-1 gene), with a unc-54 3'UTR, introduced into destination vector pCFJ150 that encodes for a wild-type copy of C. briggsae unc-119 gene under the control of the unc-119 promoter. This construct was injected into EG6699 ttTi5605 II; unc-119(ed9) III mutant animals and transgenic progeny were recovered, to generate a multi-copy array strain ERT261 jyEx128[vha-6p::gfp::ubiquitin cb-unc-119(+)];ttTi5605 II; unc-119(ed9). Likewise, construct pET346 was generated, which contains a mutant version of ubiquitin without its last two C-terminal glycines. This construct was injected into EG6699 to generate multi-copy array strain ERT264 jyEx131[vha-6p::gfp::ubiquitinΔGG cb-unc-119(+)]ttTi5605 II; unc-119(ed9).
C. elegans infections, RNA isolation and RNA-seq library construction
C. elegans infections, RNA isolation, and library construction are previously described [34]. Briefly, synchronized fer-15(b26);fem-1(hc17) L1s were grown for 24 hours at 25°C on 10-cm NGM plates seeded with OP50-1 E. coli and then infected with N. parisii ERTm1 spores. Infected and control C. elegans were harvested at appropriate times and total RNA was extracted using TriReagent (Molecular Research Center, Inc.). RT-qPCR and the Bioanalyzer assessed quality of RNA samples. Strand-specific libraries were constructed using the dUTP second strand marking method [90], [91].
RNA-seq analysis
Reads were aligned using Bowtie[92] and transcript abundance estimated using RSEM [93]. Differentially expressed transcripts were identified using the edgeR Bioconductor package (Empirical analysis of digital gene expression data in R, v 3.0.8) [94]. FDR [95] cutoff was set to <0.05, which yielded lists of genes with >4-fold difference in expression. C. elegans reads comprised the majority of the infected sample reads, ranging from over 99% early during infection (8 and 16 hpi) to 71.6% at 40 hpi (Table S1). The progressive reduction in the fraction of C. elegans reads corresponded to replication of microsporidia in the C. elegans intestine resulting in increased contribution of parasite RNA to total RNA of each infected sample [34]. The number of expressed C. elegans genes in all samples ranged from 55.4% (64 hpi) to 62.1% (16 hpi) of the total genome (Table S1). Despite the growing input of parasite RNA, global C. elegans gene expression remained comparable between infected samples and uninfected controls, with the greatest absolute difference (3.61%) in total number of expressed genes, which occurred at 64 hpi (infected vs uninfected control).
Tissue enrichment analysis
Based on previous studies, genes were classified as either intestinal-associated (as determined by fluorescence-activated nuclei sorting) [96], germline-associated (as determined by SAGE) [97], or neither. Very few germline specific/enriched genes were among the differentially expressed genes (Table S2) and therefore we used all genes expressed in germ lines detected by SAGE as the germline-associated class. We then compared the number of differentially expressed genes from each category to the number expected from the classification using the chi-squared test.
GSEA analysis
Gene Set Enrichment Analysis (GSEA) v2.0 [42] was used to compare gene sets from relevant C. elegans expression studies to our RNA-seq data. The RNA-seq expression dataset file used to generate ranked gene lists (from most upregulated to most downregulated) based on changes in expression between infected and uninfected conditions is summarized in Table S4 while the compiled gene sets used for analysis are described in detail in Table S5. Genes from other studies were converted where necessary to WBGeneIDs according to Wormbase version WS235. Five independent analyses were performed, one for each infection timepoint, with 1000 permutations for each analysis. Results for gene sets with FDR<0.25 and nominal p-value<0.05 were compiled into a graphical representation based on their NES-values, and for gene sets where the NES was not considered significant a value of zero was assigned (Table S6).
N. parisii pathogen load measured by FISH
Experiments were performed at 25°C and for each condition two biological replicates were included. About 200 synchronized fer-15(b26);fem-1(hc17) L1s were grown on 6-cm plates for two days, feeding on a lawn of E. coli RNAi clones from the Ahringer library or the skr-4 RNAi clone generated through amplification of C. elegans skr-4 genomic sequence (using primers 5′ CCGAATTCGTCTCACGAAAAGTGATC - and 5′ - CCGAATTCGGCGTTATACATTTATTCAA) and cloned into the L4440 RNAi vector using EcoRI restriction sites. Animals were then infected with 2 million spores, fixed in 4% paraformaldehyde (PFA) 24 hpi, and stained with MicroB FISH probe against N. parisii rRNA as previously described [12], [34]. Stained animals were mounted on glass slides in Vectashield with DAPI (Vector Laboratories) and imaged using a Zeiss AxioImager microscope with a 10× objective. Exposure times were kept the same for all samples within a single experiment. For all experiments except for ones in Figures 2B, 2G and S4, where a custom fully automatic method for estimating pathogen load written in Matlab was used (see Figure S2 and Supplemental Methods in Text S1), images were analyzed semi-manually using ImageJ software, where the nematode body area, and the area of pathogen contained within were determined using two different thresholds of the MicroB FISH signal (a relaxed threshold to recognize the background staining of the animal body, and a stringent threshold to specifically recognize the pathogen). Due to developmental defects caused by knockdown of UPS components, for experiments targeting the UPS, animals were first grown for one day on E. coli strain OP50-1, and then transferred to plates seeded with UPS RNAi clones diluted with the L4440 RNAi vector control (1∶10 for ubq-2, 1∶5 for pas-5, and 1∶20 for rpn-2). C. elegans has two genes encoding for ubiquitin, ubq-1 and ubq-2. The ubq-2 RNAi clone was chosen for majority of experiments because it had less pronounced developmental defects then animals fed with RNAi against ubq-1 (data not shown). After one day on RNAi, animals were infected and processed as described above. For fumagillin and FUdR experiments, animals were grown, infected, and processed as described above, except at 8 hpi, 0 or 25 µM of fumagillin (Medivet Pharmaceuticals Ltd.) or 0 or 2.6 µg/µL of FUdR (Acros Organics) in 250 µL of M9 with 0.1% Triton-X was spread onto plates containing the animals for a final concentration of 0 to 0.26 µg/mL (fumagillin) and 0 to 59 µg/mL (FUdR) present for the remainder of the experiment (an additional 16 hours).
Conjugated-ubiquitin immunofluorescence and quantification of colocalization with N. parisii
To quantify ubiquitin colocalization with microsporidia, about 200 synchronized fer-15(b26);fem-1(hc17) L1s were grown on 6-cm plates for 2 days at 25°C, and then were infected with 5 million N. parisii spores. At 8 hpi, the infected animals were treated with 250 µL of 0 µM, 25 µM, or 150 µM of fumagillin in M9 with 0.1% Triton-X (fumagillin final plate concentrations of 0 µg/mL, 0.26 µg/mL, or 1.56 µg/mL). At 12 hpi, animals were anesthetized with 10 mM levamisole, their intestines dissected out, and fixed for 15–30 min in 4% PFA. The intestines were stained with MicroB FISH probe against N. parisii rRNA, followed by staining with FK2 antibody (Millipore), and secondary antibody staining with FITC goat anti-mouse IgG (Jackson ImmunoResearch). Stained intestines were mounted in Vectashield with DAPI (Vector Laboratories) and imaged. For each condition, z-stacks spanning the width of twelve intestines were taken, and colocalization between each imaged parasite cell and the FK2 antibody was determined. All images, unless specified otherwise, were captured using a laser scanning confocal microscope with a 40× oil immersion objective (Zeiss LSM 700, equipped with an AxioCam digital camera and Zen 2010 acquisition software). Images were imported into Adobe Photoshop and assembled using Adobe Illustrator.
For ubiquitin immunofluorescence at different stages of infection, animals were infected with N. parisii as described for RNA-seq. After 30 or 40 hpi, animals were anesthetized with 10 mM levamisole, their intestines dissected out, and fixed for 30 min in 4% PFA. The intestines from the 30 hpi infected and uninfected control samples were stained as described above. Intestines from the 40 hpi infected and control samples were stained directly with antibodies without FISH staining. Stained intestines were mounted in Vectashield with DAPI (Vector Laboratories) and imaged.
GFP::ubiquitin imaging and quantification of colocalization with N. parisii
To quantify GFP::ubiquitin colocalization with microsporidia, about 200 synchronized ERT261 or ERT264 L1s were grown on 6-cm plates, seeded either with OP50-1 E. coli or control L4440 and cul-6 RNAi clone, for 36 hours at 20°C and then infected with 5 million N. parisii spores. At 10 hpi, the infected animals were treated with 250 µL of 0 µM, 25 µM, or 150 µM of fumagillin in M9 with 0.1% Triton-X, and at 15 hpi animals were fixed in 4% PFA, stained with MicroB FISH probe against N. parisii rRNA, mounted in Vectashield with DAPI, and imaged as described above. For each condition and experiment, z-stacks spanning the width of twenty to eleven ERT261 and seven to ten ERT264 intestines were taken, and colocalization between each imaged parasite cell and GFP was determined. For RNAi experiments, eight to ten ERT261 animals were imaged for each condition and experiment.
For imaging of GFP::ubiquitin in live animals, synchronized ERT261 animals expressing the intestinal GFP::ubiquitin construct were grown and infected at 20°C to minimize ubiquitin aggregate formation in uninfected controls. Synchronized animals were grown for 24 hours on 6-cm plates prior to inoculation with 2 million N. parisii spores and 48 hpi were mounted on agarose pads, anesthetized with 1 mM levamisole and imaged. For quantification of GFP::ubiquitin aggregates, synchronized ERT261 or ERT264 animals were grown at 20°C for 31 hours on 6-cm plates prior to inoculation with 1 million spores. At 10, 30 and 45 hpi, animals were fixed with PFA and stained with MicroB FISH probe as described above. Stained animals were mounted in Vectashield with DAPI and viewed directly with a laser scanning confocal microscope with a 40× oil immersion objective (Zeiss LSM 700).
Imaging of promoter-GFP reporter strains
To image promoter-GFP reporter strains, synchronized ERT54 and ERT72 L1s were grown for 24 hours at 25°C and infected with 10 million N. parisii spores on 6-cm plates. Infected and control worms were anesthetized with 1 mM levamisole, mounted on agar pads, and imaged at 8 and 24 hpi using a Zeiss AxioImager microscope. For RNAi experiments, synchronized ERT54 and ERT72 L1s were grown for 48 hours at 20°C on plates seeded with RNAi clones and imaged as described above. For MG-132 experiments, synchronized ERT54 and ERT72 L1s were grown for 24 hours at 20°C, incubated on a nutator at room temperature for six hours in M9 with 0.1% Triton-X and 0 µM, 500 µM, or 1mM MG-132, and then imaged as described above.
qRT-PCR
To measure endogenous mRNA expression changes due to UPS component knockdown, synchronized fer-15(b26);fem-1(hc17) L1s were grown at 20°C for 48 hours on RNAi bacteria, and then collected in TriReagent (Molecular Research Center, Inc.) for RNA extraction. To measure endogenous mRNA expression changes due to pharmacological proteasome inhibition, synchronized fer-15(b26);fem-1(hc17) L1s were grown 24 h at 20°C, incubated on a nutator at room temperature for six hours in M9 with 0.1% Triton-X and 0 µM or 500 µM MG-132, and then collected in TriReagent for RNA extraction. RNA extraction, reverse transcription, and qRT-PCR were performed as previously described [41]. qRT-PCR primer sequences are available upon request. Each biological replicate was measured in duplicate and normalized to the snb-1 control gene, which did not change upon conditions tested. The Pffafl method was used for quantifying data [98].
Orsay virus preparation, infections, measurements of viral load, and immunofluorescence
Virus stock for infections was prepared as described previously [5], with minor modifications. Briefly, the virus-susceptible rde-1(ne219) nematodes were grown in large-scale cultures until just starved, mechanically disrupted, and filtered through a 0.2 µm filter to separate the virus away from nematode debris. When spread on a 6-cm plate in a 250 µL volume, the 1∶50 dilution of this filtrate was the maximum dilution tested that turned on the F26F2.1p::gfp reporter in all animals 24 hpi at 25°C (data not shown). These conditions were used for all viral infections. To measure changes in viral load upon RNAi-mediated knockdown of C. elegans genes of interest, the viral RNA1 levels were measured using primers GW195 and GW194 [5] and compared to those found in L4440 controls. For these experiments, fer-15(b26);fem-1(hc17) animals were grown and treated with RNAi the same as for the N. parisii pathogen load experiments, except about 300 synchronized L1 animals were used per 6-cm plate and following 24 hours of infection with the virus, animals were collected for RNA extraction and qRT-PCR. Intestine dissections from ERT72 animals and immunofluorescence with FK2 anti-conjugated-ubiquitin antibody were performed as described above, except the secondary antibody used was the Cy3 goat anti-mouse IgG (Jackson ImmunoResearch).
Accession numbers/gene names
C. elegans genes analyzed:
cul-6, skr-3, skr-4, skr-5, ubq-1, ubq-2, pas-5, rpn-2, lgg-1, lgg-2, atg-18, sqst-1, let-363, C17H1.6, F26F2.1, skr-1, C17H1.14, F26F2.4, Y39G8B.5, sdz-6, T08E11.1, W08A12.4, ZC196.3, Y94H6A.2, his-10, his-16
RNA-seq data are part of NCBI BioProject #PRJNA163569.
Supporting Information
Zdroje
1. WilliamsBA (2009) Unique physiology of host-parasite interactions in microsporidia infections. Cellular microbiology 11 : 1551–1560.
2. DidierES (2005) Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta tropica 94 : 61–76.
3. DidierES, WeissLM (2011) Microsporidiosis: not just in AIDS patients. Current opinion in infectious diseases 24 : 490–495.
4. HigesM, Martin-HernandezR, BotiasC, BailonEG, Gonzalez-PortoAV, et al. (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environmental microbiology 10 : 2659–2669.
5. KentML, SpeareDJ (2005) Review of the sequential development of Loma salmonae (Microsporidia) based on experimental infections of rainbow trout (Oncorhynchus mykiss) and Chinook salmon (O. tshawytscha). Folia parasitologica 52 : 63–68.
6. TroemelER (2011) New models of microsporidiosis: infections in Zebrafish, C. elegans, and honey bee. PLoS pathogens 7: e1001243.
7. AnaneS, AttouchiH (2010) Microsporidiosis: epidemiology, clinical data and therapy. Gastroenterologie clinique et biologique 34 : 450–464.
8. DidierES, MaddryJA, BrindleyPJ, StovallME, DidierPJ (2005) Therapeutic strategies for human microsporidia infections. Expert review of anti-infective therapy 3 : 419–434.
9. ValencakovaA, HalanovaM (2012) Immune response to Encephalitozoon infection review. Comparative immunology, microbiology and infectious diseases 35 : 1–7.
10. MorettoMM, KhanIA, WeissLM (2012) Gastrointestinal cell mediated immunity and the microsporidia. PLoS pathogens 8: e1002775.
11. Roxstrom-LindquistK, TereniusO, FayeI (2004) Parasite-specific immune response in adult Drosophila melanogaster: a genomic study. EMBO reports 5 : 207–212.
12. TroemelER, FelixMA, WhitemanNK, BarriereA, AusubelFM (2008) Microsporidia are natural intracellular parasites of the nematode Caenorhabditis elegans. PLoS Biol 6 : 2736–2752.
13. HodgkinJ, PartridgeFA (2008) Caenorhabditis elegans meets microsporidia: the nematode killers from Paris. PLoS biology 6 : 2634–2637.
14. KimDH, FeinbaumR, AlloingG, EmersonFE, GarsinDA, et al. (2002) A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297 : 623–626.
15. FelixMA, AsheA, PiffarettiJ, WuG, NuezI, et al. (2011) Natural and experimental infection of Caenorhabditis nematodes by novel viruses related to nodaviruses. PLoS biology 9: e1000586.
16. LuR, MaduroM, LiF, LiHW, Broitman-MaduroG, et al. (2005) Animal virus replication and RNAi-mediated antiviral silencing in Caenorhabditis elegans. Nature 436 : 1040–1043.
17. SarkiesP, AsheA, Le PenJ, McKieMA, MiskaEA (2013) Competition between virus-derived and endogenous small RNAs regulates gene expression in Caenorhabditis elegans. Genome research 23 : 1258–1270.
18. WilkinsC, DishonghR, MooreSC, WhittMA, ChowM, et al. (2005) RNA interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature 436 : 1044–1047.
19. AsheA, BelicardT, Le PenJ, SarkiesP, FrezalL, et al. (2013) A deletion polymorphism in the Caenorhabditis elegans RIG-I homolog disables viral RNA dicing and antiviral immunity. eLife 2: e00994.
20. CollinsCA, BrownEJ (2010) Cytosol as battleground: ubiquitin as a weapon for both host and pathogen. Trends in cell biology 20 : 205–213.
21. HuettA, HeathRJ, BegunJ, SassiSO, BaxtLA, et al. (2012) The LRR and RING domain protein LRSAM1 is an E3 ligase crucial for ubiquitin-dependent autophagy of intracellular Salmonella Typhimurium. Cell host & microbe 12 : 778–790.
22. ManzanilloPS, AyresJS, WatsonRO, CollinsAC, SouzaG, et al. (2013) The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature 501 : 512–516.
23. PerrinAJ, JiangX, BirminghamCL, SoNS, BrumellJH (2004) Recognition of bacteria in the cytosol of Mammalian cells by the ubiquitin system. Curr Biol 14 : 806–811.
24. FangS, WeissmanAM (2004) A field guide to ubiquitylation. Cellular and molecular life sciences: CMLS 61 : 1546–1561.
25. KnodlerLA, CelliJ (2011) Eating the strangers within: host control of intracellular bacteria via xenophagy. Cellular microbiology 13 : 1319–1327.
26. Mansilla ParejaME, ColomboMI (2013) Autophagic clearance of bacterial pathogens: molecular recognition of intracellular microorganisms. Frontiers in cellular and infection microbiology 3 : 54.
27. BirminghamCL, BrumellJH (2006) Autophagy recognizes intracellular Salmonella enterica serovar Typhimurium in damaged vacuoles. Autophagy 2 : 156–158.
28. BirminghamCL, SmithAC, BakowskiMA, YoshimoriT, BrumellJH (2006) Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. The Journal of biological chemistry 281 : 11374–11383.
29. ThurstonTL, RyzhakovG, BloorS, von MuhlinenN, RandowF (2009) The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nature immunology 10 : 1215–1221.
30. HuaZ, VierstraRD (2011) The cullin-RING ubiquitin-protein ligases. Annual review of plant biology 62 : 299–334.
31. SkaarJR, PaganJK, PaganoM (2013) Mechanisms and function of substrate recruitment by F-box proteins. Nature reviews Molecular cell biology 14 : 369–381.
32. ThomasJH (2006) Adaptive evolution in two large families of ubiquitin-ligase adapters in nematodes and plants. Genome Res 16 : 1017–1030.
33. NayakS, SantiagoFE, JinH, LinD, SchedlT, et al. (2002) The Caenorhabditis elegans Skp1-related gene family: diverse functions in cell proliferation, morphogenesis, and meiosis. Current biology: CB 12 : 277–287.
34. CuomoCA, DesjardinsCA, BakowskiMA, GoldbergJ, MaAT, et al. (2012) Microsporidian genome analysis reveals evolutionary strategies for obligate intracellular growth. Genome research 22 : 2478–2488.
35. EngelmannI, GriffonA, TichitL, Montanana-SanchisF, WangG, et al. (2011) A comprehensive analysis of gene expression changes provoked by bacterial and fungal infection in C. elegans. PLoS One 6: e19055.
36. HuffmanDL, AbramiL, SasikR, CorbeilJ, van der GootFG, et al. (2004) Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proceedings of the National Academy of Sciences of the United States of America 101 : 10995–11000.
37. IrazoquiJE, TroemelER, FeinbaumRL, LuhachackLG, CezairliyanBO, et al. (2010) Distinct pathogenesis and host responses during infection of C. elegans by P. aeruginosa and S. aureus. PLoS pathogens 6: e1000982.
38. MongkoldhumrongkulN, SwainSC, JayasingheSN, SturzenbaumS (2010) Bio-electrospraying the nematode Caenorhabditis elegans: studying whole-genome transcriptional responses and key life cycle parameters. Journal of the Royal Society, Interface/the Royal Society 7 : 595–601.
39. MurphyCT, McCarrollSA, BargmannCI, FraserA, KamathRS, et al. (2003) Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424 : 277–283.
40. ShinH, LeeH, FejesAP, BaillieDL, KooHS, et al. (2011) Gene expression profiling of oxidative stress response of C. elegans aging defective AMPK mutants using massively parallel transcriptome sequencing. BMC research notes 4 : 34.
41. TroemelER, ChuSW, ReinkeV, LeeSS, AusubelFM, et al. (2006) p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS genetics 2: e183.
42. SubramanianA, TamayoP, MoothaVK, MukherjeeS, EbertBL, et al. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America 102 : 15545–15550.
43. KourtisN, NikoletopoulouV, TavernarakisN (2012) Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature 490 : 213–218.
44. SinghV, AballayA (2006) Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proceedings of the National Academy of Sciences of the United States of America 103 : 13092–13097.
45. Huang daW, ShermanBT, LempickiRA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols 4 : 44–57.
46. Huang daW, ShermanBT, LempickiRA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic acids research 37 : 1–13.
47. BoxemM, TsaiCW, ZhangY, SaitoRM, LiuJO (2004) The C. elegans methionine aminopeptidase 2 analog map-2 is required for germ cell proliferation. FEBS letters 576 : 245–250.
48. MitchellDH, StilesJW, SantelliJ, SanadiDR (1979) Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. Journal of gerontology 34 : 28–36.
49. BaileyL (1953) Effect of fumagillin upon Nosema apis (Zander). Nature 171 : 212–213.
50. KatinkaMD, DupratS, CornillotE, MetenierG, ThomaratF, et al. (2001) Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414 : 450–453.
51. SinN, MengL, WangMQ, WenJJ, BornmannWG, et al. (1997) The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proceedings of the National Academy of Sciences of the United States of America 94 : 6099–6103.
52. WilliamsGR, SampsonMA, ShutlerD, RogersRE (2008) Does fumagillin control the recently detected invasive parasite Nosema ceranae in western honey bees (Apis mellifera)? Journal of invertebrate pathology 99 : 342–344.
53. HansenM, ChandraA, MiticLL, OnkenB, DriscollM, et al. (2008) A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS genetics 4: e24.
54. Manil-SegalenM, LefebvreC, JenzerC, TrichetM, BoulogneC, et al. (2014) The C. elegans LC3 acts downstream of GABARAP to degrade autophagosomes by interacting with the HOPS subunit VPS39. Developmental cell 28 : 43–55.
55. KlionskyDJ, AbeliovichH, AgostinisP, AgrawalDK, AlievG, et al. (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4 : 151–175.
56. DunbarTL, YanZ, BallaKM, SmelkinsonMG, TroemelER (2012) C. elegans detects pathogen-induced translational inhibition to activate immune signaling. Cell host & microbe 11 : 375–386.
57. McEwanDL, KirienkoNV, AusubelFM (2012) Host translational inhibition by Pseudomonas aeruginosa Exotoxin A Triggers an immune response in Caenorhabditis elegans. Cell host & microbe 11 : 364–374.
58. MeloJA, RuvkunG (2012) Inactivation of conserved C. elegans genes engages pathogen - and xenobiotic-associated defenses. Cell 149 : 452–466.
59. GuisbertE, CzyzDM, RichterK, McMullenPD, MorimotoRI (2013) Identification of a tissue-selective heat shock response regulatory network. PLoS genetics 9: e1003466.
60. LiX, MatilainenO, JinC, Glover-CutterKM, HolmbergCI, et al. (2011) Specific SKN-1/Nrf stress responses to perturbations in translation elongation and proteasome activity. PLoS genetics 7: e1002119.
61. HorvathCM (2004) Weapons of STAT destruction. Interferon evasion by paramyxovirus V protein. European journal of biochemistry/FEBS 271 : 4621–4628.
62. KomuroA, BammingD, HorvathCM (2008) Negative regulation of cytoplasmic RNA-mediated antiviral signaling. Cytokine 43 : 350–358.
63. RandowF, LehnerPJ (2009) Viral avoidance and exploitation of the ubiquitin system. Nature cell biology 11 : 527–534.
64. RajsbaumR, Garcia-SastreA (2013) Viral evasion mechanisms of early antiviral responses involving regulation of ubiquitin pathways. Trends in microbiology 21 : 421–429.
65. ChoiAG, WongJ, MarchantD, LuoH (2013) The ubiquitin-proteasome system in positive-strand RNA virus infection. Reviews in medical virology 23 : 85–96.
66. IrazoquiJE, UrbachJM, AusubelFM (2010) Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nature reviews Immunology 10 : 47–58.
67. CouillaultC, PujolN, ReboulJ, SabatierL, GuichouJF, et al. (2004) TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nature immunology 5 : 488–494.
68. O'RourkeD, BabanD, DemidovaM, MottR, HodgkinJ (2006) Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome research 16 : 1005–1016.
69. ShapiraM, HamlinBJ, RongJ, ChenK, RonenM, et al. (2006) A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proceedings of the National Academy of Sciences of the United States of America 103 : 14086–14091.
70. KleinoA, SilvermanN (2012) UnZIPping mechanisms of effector-triggered immunity in animals. Cell host & microbe 11 : 320–322.
71. LiuY, SamuelBS, BreenPC, RuvkunG (2014) Caenorhabditis elegans pathways that surveil and defend mitochondria. Nature 508 : 406–410.
72. StuartLM, PaquetteN, BoyerL (2013) Effector-triggered versus pattern-triggered immunity: how animals sense pathogens. Nature reviews Immunology 13 : 199–206.
73. LemaitreB, GirardinSE (2013) Translation inhibition and metabolic stress pathways in the host response to bacterial pathogens. Nature reviews Microbiology 11 : 365–369.
74. PowersET, BalchWE (2013) Diversity in the origins of proteostasis networks–a driver for protein function in evolution. Nature reviews Molecular cell biology 14 : 237–248.
75. AballayA (2013) Role of the nervous system in the control of proteostasis during innate immune activation: insights from C. elegans. PLoS pathogens 9: e1003433.
76. TaylorRC, BerendzenKM, DillinA (2014) Systemic stress signalling: understanding the cell non-autonomous control of proteostasis. Nature reviews Molecular cell biology 15 : 211–217.
77. AltoNM, OrthK (2012) Subversion of cell signaling by pathogens. Cold Spring Harbor perspectives in biology 4: a006114.
78. Steele-MortimerO (2011) Exploitation of the ubiquitin system by invading bacteria. Traffic 12 : 162–169.
79. WullschlegerS, LoewithR, HallMN (2006) TOR signaling in growth and metabolism. Cell 124 : 471–484.
80. ZhengYT, ShahnazariS, BrechA, LamarkT, JohansenT, et al. (2009) The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. Journal of immunology 183 : 5909–5916.
81. AndersonDM, FrankDW (2012) Five mechanisms of manipulation by bacterial effectors: a ubiquitous theme. PLoS pathogens 8: e1002823.
82. VeigaE, CossartP (2005) Ubiquitination of intracellular bacteria: a new bacteria-sensing system? Trends in cell biology 15 : 2–5.
83. PilarAV, Reid-YuSA, CooperCA, MulderDT, CoombesBK (2012) GogB is an anti-inflammatory effector that limits tissue damage during Salmonella infection through interaction with human FBXO22 and Skp1. PLoS pathogens 8: e1002773.
84. Ardila-GarciaAM, FastNM (2012) Microsporidian infection in a free-living marine nematode. Eukaryotic cell 11 : 1544–1551.
85. FelixMA, DuveauF (2012) Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae. BMC biology 10 : 59.
86. BrennerS (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94.
87. EstesKA, SzumowskiSC, TroemelER (2011) Non-lytic, actin-based exit of intracellular parasites from C. elegans intestinal cells. PLoS pathogens 7: e1002227.
88. MelendezA, TalloczyZ, SeamanM, EskelinenEL, HallDH, et al. (2003) Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 301 : 1387–1391.
89. KangC, YouYJ, AveryL (2007) Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes & development 21 : 2161–2171.
90. ParkhomchukD, BorodinaT, AmstislavskiyV, BanaruM, HallenL, et al. (2009) Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic acids research 37: e123.
91. LevinJZ, YassourM, AdiconisX, NusbaumC, ThompsonDA, et al. (2010) Comprehensive comparative analysis of strand-specific RNA sequencing methods. Nature methods 7 : 709–715.
92. LangmeadB, TrapnellC, PopM, SalzbergSL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology 10: R25.
93. LiB, DeweyCN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC bioinformatics 12 : 323.
94. RobinsonMD, McCarthyDJ, SmythGK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26 : 139–140.
95. StoreyJD, TibshiraniR (2003) Statistical significance for genomewide studies. Proceedings of the National Academy of Sciences of the United States of America 100 : 9440–9445.
96. HaenniS, JiZ, HoqueM, RustN, SharpeH, et al. (2012) Analysis of C. elegans intestinal gene expression and polyadenylation by fluorescence-activated nuclei sorting and 3'-end-seq. Nucleic acids research 40 : 6304–6318.
97. WangX, ZhaoY, WongK, EhlersP, KoharaY, et al. (2009) Identification of genes expressed in the hermaphrodite germ line of C. elegans using SAGE. BMC genomics 10 : 213.
98. PfafflMW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research 29: e45.
99. GuhaThakurtaD, PalomarL, StormoGD, TedescoP, JohnsonTE, et al. (2002) Identification of a novel cis-regulatory element involved in the heat shock response in Caenorhabditis elegans using microarray gene expression and computational methods. Genome research 12 : 701–712.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA SplicingČlánek Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance GenesČlánek The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- BdlA, DipA and Induced Dispersion Contribute to Acute Virulence and Chronic Persistence of
- Morphotype Transition and Sexual Reproduction Are Genetically Associated in a Ubiquitous Environmental Pathogen
- A Nucleic-Acid Hydrolyzing Single Chain Antibody Confers Resistance to DNA Virus Infection in HeLa Cells and C57BL/6 Mice
- HopW1 from Disrupts the Actin Cytoskeleton to Promote Virulence in Arabidopsis
- Ly6C Monocytes Become Alternatively Activated Macrophages in Schistosome Granulomas with Help from CD4+ Cells
- Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA Splicing
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- Antibody Responses to : Role in Pathogenesis and Diagnosis of Encephalitis?
- Discovery of a Novel Compound with Anti-Venezuelan Equine Encephalitis Virus Activity That Targets the Nonstructural Protein 2
- Activation of Focal Adhesion Kinase by Suppresses Autophagy via an Akt/mTOR Signaling Pathway and Promotes Bacterial Survival in Macrophages
- Crossing the Interspecies Barrier: Opening the Door to Zoonotic Pathogens
- Catching Fire: , Macrophages, and Pyroptosis
- IscR Is Essential for Type III Secretion and Virulence
- Selective Chemical Inhibition of Quorum Sensing in Promotes Host Defense with Minimal Impact on Resistance
- The Glycosylated Rv1860 Protein of Inhibits Dendritic Cell Mediated TH1 and TH17 Polarization of T Cells and Abrogates Protective Immunity Conferred by BCG
- A Genome-Wide Tethering Screen Reveals Novel Potential Post-Transcriptional Regulators in
- Structural Insights into SraP-Mediated Adhesion to Host Cells
- Human IGF1 Regulates Midgut Oxidative Stress and Epithelial Homeostasis to Balance Lifespan and resistance in
- Cycling Empirical Antibiotic Therapy in Hospitals: Meta-Analysis and Models
- Rab11 Regulates Trafficking of -sialidase to the Plasma Membrane through the Contractile Vacuole Complex of
- Mitogen and Stress Activated Kinases Act Co-operatively with CREB during the Induction of Human Cytomegalovirus Immediate-Early Gene Expression from Latency
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- A Central Role for Carbon-Overflow Pathways in the Modulation of Bacterial Cell Death
- An Invertebrate Warburg Effect: A Shrimp Virus Achieves Successful Replication by Altering the Host Metabolome via the PI3K-Akt-mTOR Pathway
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
- A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees () Prevails after -Mediated, or , Transmission
- Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance Genes
- Ubiquitin-Mediated Response to Microsporidia and Virus Infection in
- Preclinical Detection of Variant CJD and BSE Prions in Blood
- Toll-Like Receptor 8 Agonist and Bacteria Trigger Potent Activation of Innate Immune Cells in Human Liver
- Progressive Proximal-to-Distal Reduction in Expression of the Tight Junction Complex in Colonic Epithelium of Virally-Suppressed HIV+ Individuals
- The Triggering Receptor Expressed on Myeloid Cells 2 Inhibits Complement Component 1q Effector Mechanisms and Exerts Detrimental Effects during Pneumococcal Pneumonia
- Differential Activation of Acid Sphingomyelinase and Ceramide Release Determines Invasiveness of into Brain Endothelial Cells
- Forward Genetic Screening Identifies a Small Molecule That Blocks Growth by Inhibiting Both Host- and Parasite-Encoded Kinases
- Defining Immune Engagement Thresholds for Control of Virus-Driven Lymphoproliferation
- Growth Factor and Th2 Cytokine Signaling Pathways Converge at STAT6 to Promote Arginase Expression in Progressive Experimental Visceral Leishmaniasis
- Multimeric Assembly of Host-Pathogen Adhesion Complexes Involved in Apicomplexan Invasion
- Biogenesis of Influenza A Virus Hemagglutinin Cross-Protective Stem Epitopes
- Adequate Th2-Type Response Associates with Restricted Bacterial Growth in Latent Mycobacterial Infection of Zebrafish
- Protective Efficacy of Passive Immunization with Monoclonal Antibodies in Animal Models of H5N1 Highly Pathogenic Avian Influenza Virus Infection
- Fructose-Asparagine Is a Primary Nutrient during Growth of in the Inflamed Intestine
- The Calcium-Dependent Protein Kinase 3 of Influences Basal Calcium Levels and Functions beyond Egress as Revealed by Quantitative Phosphoproteome Analysis
- A Translocated Effector Required for Dissemination from Derma to Blood Safeguards Migratory Host Cells from Damage by Co-translocated Effectors
- Functional Characterization of a Novel Family of Acetylcholine-Gated Chloride Channels in
- Both α2,3- and α2,6-Linked Sialic Acids on O-Linked Glycoproteins Act as Functional Receptors for Porcine Sapovirus
- The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
- MicroRNA-146a Provides Feedback Regulation of Lyme Arthritis but Not Carditis during Infection with
- Recombination in Enteroviruses Is a Biphasic Replicative Process Involving the Generation of Greater-than Genome Length ‘Imprecise’ Intermediates
- Cytoplasmic Viral RNA-Dependent RNA Polymerase Disrupts the Intracellular Splicing Machinery by Entering the Nucleus and Interfering with Prp8
- and Are Associated with Murine Susceptibility to Infection and Human Sepsis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



