-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMorphotype Transition and Sexual Reproduction Are Genetically Associated in a Ubiquitous Environmental Pathogen
Sex, despite its cost, is an important means to maximize species fitness in coping with unpredictable environmental challenges. In the human fungal pathogen Cryptococcus neoformans, sexual reproduction has yielded hyper virulent and drug resistant variants, and produces airborne infectious spores. Developmentally, sexual spores are generated from fruiting bodies that are differentiated from aerial hyphae. Cryptococcus cells typically grow as yeast cells with a subpopulation that respond to mating stimulation and switch to hyphal growth after mating. However, mechanisms that connect sexual reproduction and multiple differentiation events to ensure the developmental continuality are unknown. Here we revealed a network of yeast-to-hypha transition in Cryptococcus. From this network we identified a Pumilio-family RNA binding protein Pum1 that acts in concert with the matricellular signal Cfl1 in regulating the yeast-to-hyphal transition following mating. Interestingly, Pum1 is also important in sustaining hyphal growth and in directing the progression from aerial hyphal morphogenesis to the formation of fruiting bodies. Intriguingly, mutations of Pum1 affect the spatiotemporal expression pattern of the filament - and meiosis-specific proteins Fas1 and Dmc1. Our study opens a new avenue to investigate how a microbe controls development continuity while maintaining population heterogeneity.
Published in the journal: . PLoS Pathog 10(6): e32767. doi:10.1371/journal.ppat.1004185
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004185Summary
Sex, despite its cost, is an important means to maximize species fitness in coping with unpredictable environmental challenges. In the human fungal pathogen Cryptococcus neoformans, sexual reproduction has yielded hyper virulent and drug resistant variants, and produces airborne infectious spores. Developmentally, sexual spores are generated from fruiting bodies that are differentiated from aerial hyphae. Cryptococcus cells typically grow as yeast cells with a subpopulation that respond to mating stimulation and switch to hyphal growth after mating. However, mechanisms that connect sexual reproduction and multiple differentiation events to ensure the developmental continuality are unknown. Here we revealed a network of yeast-to-hypha transition in Cryptococcus. From this network we identified a Pumilio-family RNA binding protein Pum1 that acts in concert with the matricellular signal Cfl1 in regulating the yeast-to-hyphal transition following mating. Interestingly, Pum1 is also important in sustaining hyphal growth and in directing the progression from aerial hyphal morphogenesis to the formation of fruiting bodies. Intriguingly, mutations of Pum1 affect the spatiotemporal expression pattern of the filament - and meiosis-specific proteins Fas1 and Dmc1. Our study opens a new avenue to investigate how a microbe controls development continuity while maintaining population heterogeneity.
Introduction
Selective pressures from the environment shape microbial evolution. To cope with the challenges presented by both predictable and erratic environmental fluctuations, microbes employ various adaptation and bet-hedging strategies, like coordinated community behaviors and morphotype transition [1], [2], [3], [4], [5], [6], [7]. The transition between different morphotypes confers genetically identical cells the distinct ability in responding to different environmental stimuli. This maximizes the community fitness and enhances species survival under disparate conditions [3], [4], [6], [8], [9]. Similarly, morphotype transition is widely adopted by evolutionally divergent pathogens to assist their survival both inside and outside of the host [3], [4], [6], [7], [9]. The causal agent of the most common fungal disease of the central nervous system, Cryptococcus neoformans, can undergo the transition between yeast and hypha states [6], [10]. This morphotype transition is linked with its virulence potential [7].
Hyphal growth (filamentation) in this ubiquitous pathogen generally occurs as a cellular response to environmental stimuli that induce sexual reproduction [11], [12], [13]. Sexual reproduction in Cryptococcus has been known for decades to take place between cells of opposite mating types, a and α (bisexual mating). Such bisexual mating generates an equal number of a and α meiotic progeny [14], [15]. However, the Cryptococcus population worldwide is sharply skewed towards the α mating type (>99%) and the chance of locating a compatible mating partner nearby is slim. Yet, many natural and clinical isolates maintain the ability to mate [14]. The discovery of the unisexual life cycle in C. neoformans that involves cells of only a single mating type, most often the α mating type [16], [17], [18], offers a plausible explanation for the observed dominance by α isolates [12], [16], [17], [19]. Besides the fact that spores produced by sexual reproduction are infectious propagules [20], [21], unisexual mating may have played a variety of roles in cryptococcal infections [22], [23], [24]. For instance, α unisexual mating is proposed to have yielded hyper-virulent Cryptococcus isolates [22], and may have assisted this pathogen in adapting to host environment [25]. Unisex in C. neoformans can also lead to aneuploidy [24], which could offer fitness benefit under certain stress conditions [26].
Sexual reproduction, including both unisexual and bisexual reproduction, occurs only in a subpopulation of a Cryptococcus mating community. It involves sequential morphological differentiation events in a stochastic manner [23]. The life cycle of this pathogen commences with early mating events controlled by the pheromone signaling pathway and is followed by the transition from the yeast to the hyphal form. Hyphae generated from this morphological transition are mostly concentrated at the periphery of the mating colony and they can invade solid substrates, extend on the surface, or expand into air. Some of the aerial hyphae further develop into fruiting bodies on their apexes, which form cup-shaped basidia that eventually give rise to four chains of spores (Figure 1A). The spores disperse and germinate into yeasts and a new life cycle begins.
Fig. 1. Identification of the genetic network in hyphal and yeast populations. 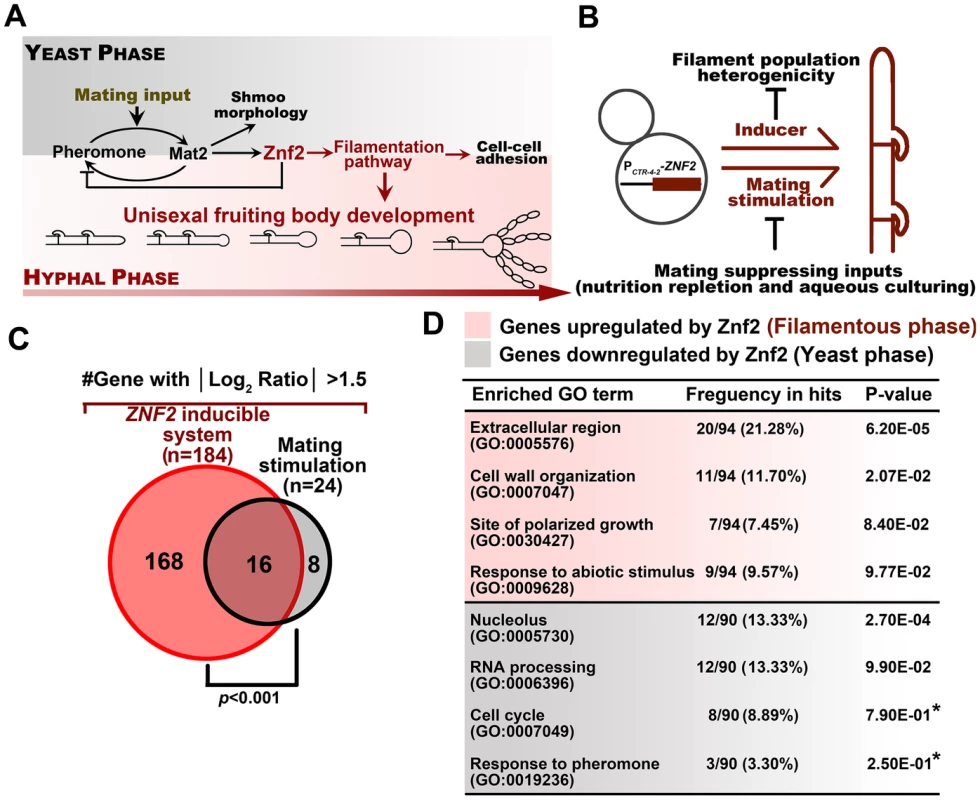
(A) A diagram depicting the pheromone and filamentation signaling pathways and their downstream cellular processes in Cryptococcus. (B) The PCTR4-2-ZNF2 strain and the wildtype strain H99 were grown under the condition that inhibits mating activation in order to perform comparative transcriptome analyses of homogeneous yeast and hyphal populations without the complication pheromone stimulation. (C) Histogram and the Venn diagram depicting the difference in two transcriptome analyses. The current one (red) used the homogeneous morphotypes and yielded a higher number of genes with significantly differential expression between the yeast and filamentous growth. The former one (black) used wildtype population in response to mating stimulation and the population showed pronounced heterogeneity in cell morphotype, which likely obstructed the previous effort in identifying many of the potential Znf2 downstream targets. The significant overlap of the lists from this study and the previous one verified our current approach and also indicated some factors that are likely to be specific to the growth conditions (hypergeometric p-value of significant overlap <0.001). (D) The list of enriched GO terms with differentially expressed genes in different morphotypes. The DAVID gene ontology program was used for the analysis and gene-enrichment in the annotated terms was evaluated based on the EASE Score. The EASE Score is a modified Fisher Exact P-Value and the default threshold is 0.1 [29]. Asterisks indicate GO terms with values above the default threshold. These GO terms are included because of their potential biological significance based on previous studies in C. neoformans or other fungi [11], [12], [30], [47]. The extremely mating-suppressive conditions used in this study may result in the under-representation of these classes and their lower statistical significance. The architecture of the upstream signaling pathways engaged in unisexual reproduction resembles that of bisexual reproduction [11], [16]. In this regard, the pheromone signaling (MAPK pathway) plays a prominent role in integrating external inputs into the initiation of the mating development [11], [23], [27] (Figure 1A). The ultimate decision maker for the morphological transition from yeasts to hyphae is the C2H2 zinc finger regulator Znf2. Upregulation of Znf2 initiates the formation of dikaryotic (bisexual) or monokaryotic (unisexual) hyphae and Znf2 is also required to sustain hyphal growth [18]. Overexpression of Znf2 can drive the yeast-to-hypha transition independent of environment cues, which generates a homogenous hyphal population [7]. Thus, Znf2, as a master regulator of filamentation, links the upstream signaling activation, including the ones from the pheromone pathway, to the yeast-hypha cellular response.
Like many other human fungal pathogens, the yeast-to-hypha transition is linked with virulence potential in Cryptococcus, and Znf2 plays a crucial role in this connection [7], [28]. Znf2 orchestrates these two behaviors (morphogenesis and virulence) partially through its downstream target Cfl1 [7]. Cfl1 is a cell-wall associated adhesion protein and it also functions as a signaling molecule upon its release into the extracellular matrix [2]. This matrix protein plays a similar but less prominent role than Znf2 in regulating filamentation in C. neoformans [7]. These observations indicate the existence of additional players in coordinating hyphal development.
In this study, we elucidated a genetic network controlling morphotype transition in C. neoformans. We discovered that Pum1, a RNA-binding protein, acts in concert with Cfl1 to direct Znf2-dependent filamentation. Pum1 plays a pleiotropic role in cryptococcal development: it regulates the initiation and the extension of hyphal growth; it directs the progression from aerial hyphal morphogenesis to the formation of fruiting body; and intriguingly, it also controls meiosis and sporulation during bisexual and unisexual mating. Pum1 regulates filamentation and meiosis partly through its control of the spatiotemporal expression pattern of filament-specific and meiosis-specific proteins Fas1 and Dmc1. Not surprisingly, Pum1 is critical for filamentation and sporulation in both laboratory and clinical isolates. Hence, this investigation offers a new prospective in our understanding of forces that shape cell fate and sexual reproduction in environmental pathogens.
Results
Identification of potential components in the filamentation pathway using an engineered strain that produces a homogeneous hyphal population
We previously demonstrated that Znf2 is the decision maker of filamentation in Cryptococcus and it is sufficient and necessary to direct hyphal formation [7], [11], [28]. Firstly, Znf2 is required for initiating filamentation and the deletion of ZNF2 locks cells in the yeast form [11]. Secondly, the expression level of ZNF2 is positively correlated with the amplitude of filamentation [7], [28]. Thirdly, overexpression of ZNF2 stimulates robust filamentation irrespective of environmental stimulation or pheromone signaling [7]. Lastly, attempts of UV mutagenesis in the znf2Δ mutant background failed to recover any suppressor mutations that could restore hyphal growth (data not shown). By comparison, from the 60,000 mutants that we screened (19 Mb genome, ∼7,000 genes), mutants that could form other cell types (e.g. pseudohypha or shmoo) were isolated. Given the central role of Znf2 in filamentation, we hypothesize that Znf2 coordinates the expression of multiple factors that are critical for hyphal development. Thus, characterization of Znf2 regulon is expected to provide a global insight into the molecular bases for hyphal development.
Filamentation in C. neoformans usually occurs in response to mating cues (Figure 1B) and the morphological state in a mating community is highly heterogeneous (Figure S1). Even in a mature mating colony, the yeast sub-population dominates over the hyphal sub-population (Figure S1). Such heterogeneity in morphotype presents a challenge to identify filamentation-specific molecules. To circumvent this complication, we took advantage of a strain with the ZNF2 gene under the control of the promoter of the copper transporter gene CTR4 (PCTR4-2-ZNF2) [7]. The proportion of filaments generated by this PCTR4-2-ZNF2 strain is positively correlated to the degree of copper-limitation, which induces its expression [7]. To eliminate potential noise caused by other cell types (e.g. shmoo cells) generated in response to mating-inducing cues, we cultured this engineered strain under conditions that are known to be extremely mating-suppressive [7]. Under such conditions, the PCTR4-2-ZNF2 strain generated a nearly homogenous filamentous population (Figures 1B and S1).
We then compared the transcription profiles of the two populations with homogeneous morphotypes cultured under the above mentioned conditions: the wild type (yeasts) and the PCTR4-2-ZNF2 strain (hyphae) (Figure S1). The genes that were differentially expressed between the two morphotypes (populations) were selected. A much larger number of differentially expressed genes were identified with a wider range of expression amplitudes by this current approach compared to the previous data generated from a comparison between the wildtype and the znf2Δ mutant in response to mating stimulation (Figure S1) [11]. This current approach of using the two morphologically homogenous populations allowed us to identify 184 genes that exhibited more than 3 fold differences in the expression level (Figure 1C and Table S1). In comparison, only 24 genes above that threshold were identified by our previous approach [11]. Although different culture conditions were used, we believe that the lower sensitivity of the previous approach was largely attributable to the morphological heterogeneity of the mating-colony (Figure S1). Thus, this current approach provides us with a more comprehensive regulon of Znf2.
Functional prediction of this expanded regulon indicates that genes related to secretion and cell wall organization are the two functional groups overrepresented in the filamentous state (Figure 1D). As expected, some genes that are highly induced by Znf2 were also identified and confirmed in our previous study [7]. A gene ontology analysis of the new data by the DAVID program [29] revealed additional biological processes associated with Znf2-stimulated filamentous growth, including polarized growth and stress responses (Figure 1D). Both are also implicated in filamentous growth in other dimorphic fungi [30], [31], [32]. “Response to pheromone” was also identified as a distinctive class, although this class was not found to be statistically significant (Figure 1D). However, given that the condition used for the transcriptome experiment was extremely suppressive to the pheromone sensing pathway or mating [7], we speculate that this identification is biologically significant even though the P-value for this category might be underwhelming. This finding likely reflects the unique feature of Cryptococcus among human fungal pathogens in that filamentation in this organism is typically a cellular response to mating signals. The indication that Znf2, a filamentation master regulator, may actually repress pheromone signaling genes is seemingly contradictory to the above notion. However, it is consistent with an inhibitory effect of Znf2 on the expression of early mating genes and early mating events noted in two earlier studies [11], [12]. Collectively, these observations suggest negative feedback from hyphal growth on the mating stimulation that initiates the hyphal formation. Such prohibition of cell fusion and pheromone-response during hyphal differentiation could be important to confine the cell lineage for the proper development progression.
Pum1 is an important regulator in the filamentation pathway
To assess the biological roles of Znf2 targets in filamentation, we chose to overexpress part of the Znf2 regulon (29 genes) that encode potential regulators or extracellular products (Figure 2A). To identify genes that are specifically involved in filamentation but not important in activating pheromone signaling, we screened this overexpression library for filamentation on YPD medium, a culture condition that suppresses mating behaviors [7]. We found that overexpression of CNB04870 (serotype D JEC21/XL280 background) resulted in a robust filamentation phenotype under the mating-suppressing condition (Figure 2B). Conversely, the deletion of this gene dramatically reduced filamentation in the hyper-filamentous XL280 background under filamentation-inducing condition (Figure 2B). Thus both the gain of function (overexpression) and the loss of function (gene knockout) demonstrated the significance of this gene in initiating filamentation. Motif searches using the Motif Scan and the InterProScan programs revealed a CLN-rich region at its N-terminus and a Pumilio-family RNA binding domain at its C-terminus (Figure S2A). The Pumilio domain is conserved throughout the eukaryotic domain and its family members are known for their regulatory roles in a wide range of processes, including mitochondrial biogenesis, cell division, development, and differentiation [33], [34]. We therefore named this gene PUM1 (Pumilio protein 1) (Figure S2A). Consistent with the idea that ZNF2 regulates PUM1, the expression of PUM1 was considerably increased by the overexpression of ZNF2 (Figure S2B). To examine the cell type that expresses Pum1 during the development of a mating community, we fused the fluorescence protein mCherry with Pum1 that is driven by the PUM1's native promoter. In agreement with the notion that Pum1 belongs to the Znf2-controled filamentation pathway, Pum1 is expressed in the hyphal population at a significantly higher level than that in the yeast population (Figure 2C).
Fig. 2. The Znf2 downstream target Pum1 directs filamentation. 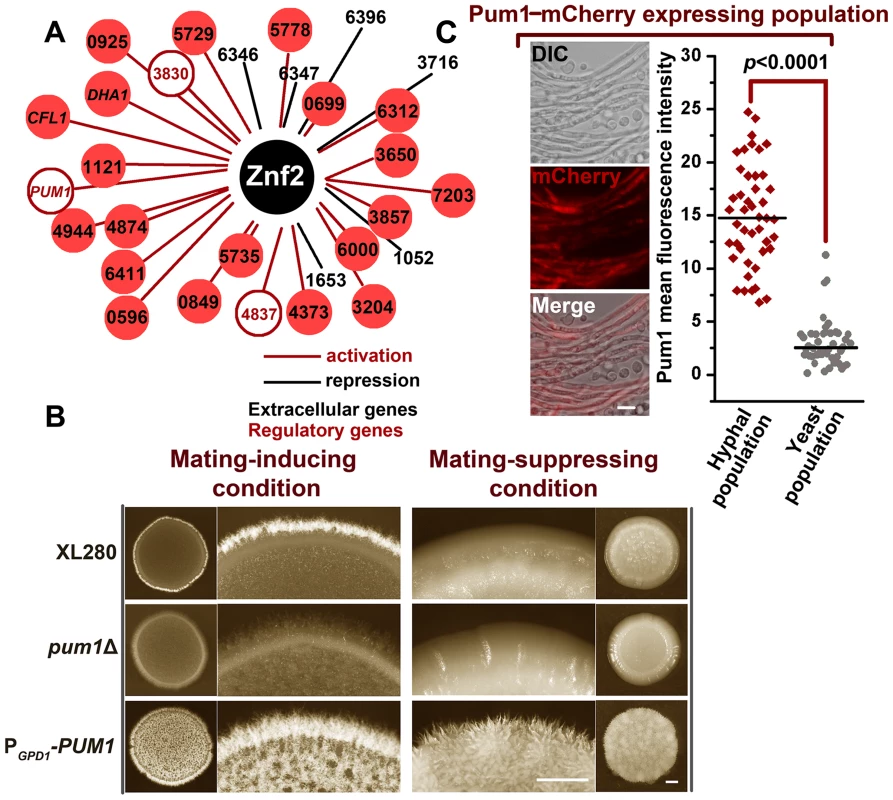
(A) The 29 Znf2 downstream targets were placed under the control of the constitutive PGDP1 promoter to construct a Cryptococcus overexpression library. The screen for their effects on filamentation was conducted under filamentation-suppressing or filamentation-inducing conditions with a 5-day incubation period. CNAG_0‘number’ indicates the gene locus name used in Broad Institute C. neoformans H99 sequence annotation, and these locus names were used because they are previously uncharacterized genes. The gene locus number of PUM1 is CNAG_04003. (B) Pum1 positively controls filamentation. The overnight cultures of the wildtype strain XL280, the pum1Δ mutant, and the PUM1 overexpression strain of the same cell density were dropped onto the V8 agar (mating-inducing) or the YPD agar (mating-suppressing) and photographed after 5 days of growth. Scale bar: 1 mm. (C) The Pum1-mCherry shows a biased expression in the hyphal subpopulation. The images of the fluorescence labeled wildtype strain were taken at 72 hrs post mating stimulation. The localization patterns of Pum1 in the figure are representative for each given cell types (at least 40 cells for each cell type were examined). See Materials and Methods for the detailed description of the experimental condition for the sub-cellular localization of Pum1-mCherry. Scale bar: 10 µm. It is known that Znf2 and its previously characterized downstream factor Cfl1 do not control filamentation through feedback activation of the upstream pheromone signaling circuit or early mating events [2], [11]. Rather, Znf2 and Cfl1 are dedicated morphogenesis/filamentation regulators [2], [11]. Pum1 is also a filamentation regulator, as overexpression of PUM1 initiated hyphal formation even under the condition that was extremely inhibitory to mating activation (Figure 2B and Figure 3D). The observation that the transcriptional induction of PUM1 was delayed compared to early mating genes (e.g. the pheromone gene MF1α) during mating development (Figure 3A), as we previously observed for ZNF2 and CFL1 [2], [7], led us to speculate that Pum1 is also a filamentation specific regulator and it is not critical for early mating events. Indeed, the overexpression of PUM1 did not increase the expression of MAT2, a key transcription factor that controls pheromone sensing and response [11](Figure 3B). On the contrary, we observed a reduction in expression of the early mating genes that are involved in the synthesis (MF1α), secretion (STE6), and response (STE3) of the pheromone signal in the PUM1 overexpression strain (Figure 3B). This finding echoes the inhibitory effect of Znf2 and Cfl1 on the early mating genes [2], [11] and suggests that Pum1 is likely involved in suppressing the pheromone circuit in an inhibitory feedback manner. Consistently, the deletion of PUM1 enhanced cell fusion efficiency, an early cellular response to mating induction (Figure 3C).
Fig. 3. Pum1 controls filamentation but not early mating processes. 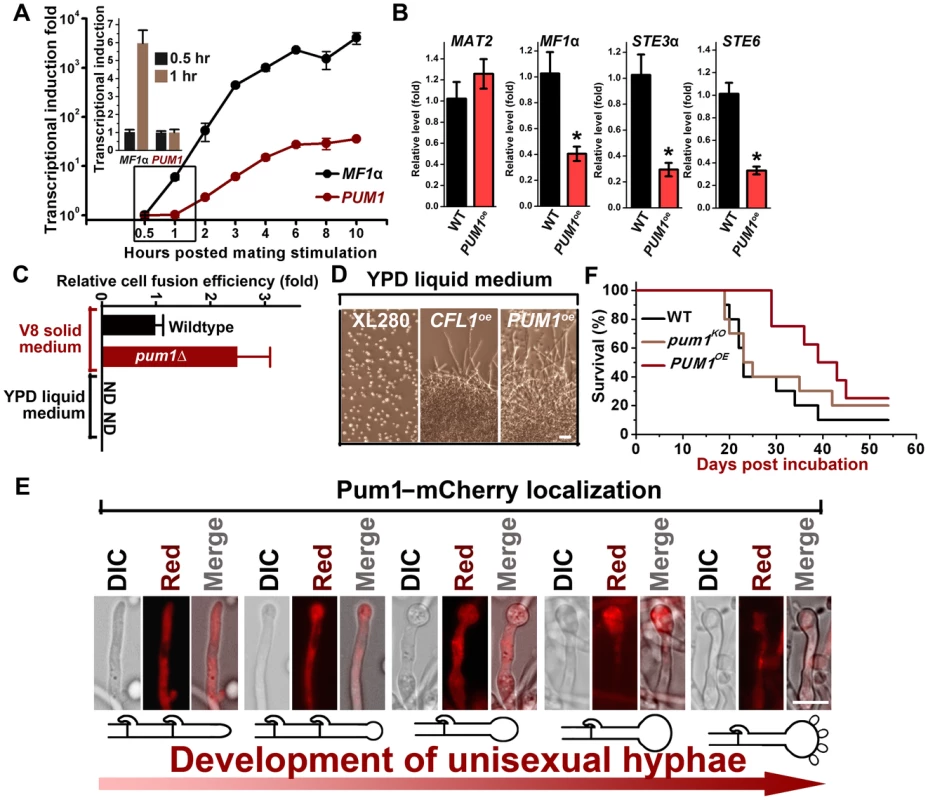
(A) Transcriptional dynamics of MF1α and PUM1 during mating. The RNA from the a+α mixture co-cultured on V8 agar at 22°C in the dark at indicated time points was subjected to the transcriptional analysis by real time PCR. There was a delay in the transcriptional induction of PUM1 compared to that for the pheromone gene MF1α. (B) Overexpression of PUM1did not increase the transcript level of the genes in the pheromone pathway. The expression level of the corresponding genes in the wildtype strain XL280 grown under the same condition was arbitrarily set as 1 for comparison. (C) Pum1 is modestly inhibitory for cell fusion. The α and a partners from wildtype strains or the pum1Δ mutant marked with different auxotrophic markers were co-cultured on V8 agar or YPD liquid medium for 22 hours at 22°C in the dark. The cocultures were then collected, washed, and plated onto YNB minimal medium to select for fusion products. The cell fusion efficiency of the wildtype coculture was arbitrarily set as 1 for comparison. (D) Overexpression of PUM1 and CFL1 are sufficient to trigger filamentation even under mating-suppressive conditions. The wildtype strain, the PCTR4-2-PUM1 strain, and the PCTR4-2-CFL1 strain were grown in the YPD liquid medium. Filamentation and cell aggregation (cell-cell adhesion) were observed in the CFL1oe and PUM1oe strains but not in the wildtype strain. Scale bar: 20 µm. (E) The PGPD1-PUM1 strain was significantly less virulent in a murine intranasal inhalation infection model compared with the wildtype strain XL280 and the pum1Δ mutant (P<0.01). (F) Pum1-mCherry is expressed at a relatively constant level throughout hyphal growth and basidial development (>40 hyphae for each stage were examined). Scale bar: 10 µm. To study Pum1's spatiotemporal regulation of filamentation, we examined the localization of Pum1-mCherry driven by its native promoter throughout hyphal development (Figure 3E). Pum1 exhibited a relatively constant expression level during the progression of hyphal development, and the majority of Pum1 proteins were localized in the cytoplasm. Such subcellular localization of Pum1 is consistent with its predicted function as a mRNA-binding protein [33]. Intriguingly, Pum1 accumulated at the aerial hyphal apexes where basidia form upon the initiation of fruiting body development (Figure 3E). An intense patch of Pum1-mCherry was observed in the basidial heads and the signal gradually became dimmer after spore formation. The spatiotemporal expression pattern of Pum1 implicates its involvement in the regulation of fruiting body formation in addition to its role in filamentation.
Morphotype has long been associated with virulence in Cryptococcus, with the yeast form being pathogenic and the filamentous form attenuated in virulence [11], [28]. Our previous study demonstrated that Znf2 links cryptococcal morphotype and virulence potential. The deletion of ZNF2 locks cells in the yeast phase and enhances virulence while the overexpression of ZNF2 leads to filamentation in vivo and abolishes cryptococcal virulence in the clinical isolate H99 background and significantly attenuates the virulence in the XL280 background in a mouse model of cryptococcosis [7], [28]. Given that Pum1 regulates filamentation, we decided to test the impact of mutations of Pum1 on cryptococcal virulence. Here we examined the wildtype XL280, the pum1Δ mutant, and the PGPD1-PUM1 strain in the inhalation infection model of murine cryptococcosis. The PGPD1-PUM1 strain harbors the PUM1 gene driven by the promoter of the house keeping gene GPD1. This strain was considerably attenuated in pathogenicity (Figure 3F). The comparable level of virulence between the wildtype and the pum1Δ mutant was likely due to a low level of expression of Pum1 in the wildtype during infection and it is known that mammalian physiological conditions are extremely inhibitory to the filamentation program [7]. Consistently, the Pum1-mCherry was below detectable level under the conditions that mimic host environments.
Pum1 and Cfl1 represent two branches downstream of Znf2 in controlling aerial structure development
During the development of a mating colony, a portion of cryptococcal cells switch from the yeast to the hyphal form and subsequently generate two types of hyphae: invasive hyphae and aerial hyphae [7]. Invasive hyphae penetrate and grow beneath the solid substrate in search of nutrient while aerial hyphae expand into the air for reproduction and spore dispersal. Znf2 is required for the formation of both types of hyphae [7]. Surprisingly, the deletion of PUM1 considerably reduced the formation of aerial hyphae but exerted a much more modest effect on invasive filamentation under different conditions (Figures 4A, S3A and S3B). Interestingly, the previously characterized extracellular matrix signal protein Cfl1 [2] is also more critical for aerial hyphal development than for invasive hyphal growth (Figure S3A). The similar effect of Pum1 and Cfl1 on the three-dimensional architecture of a mating colony prompted us to further investigate their genetic interactions.
Fig. 4. Cfl1 and Pum1 represent two major circuits downstream of Znf2 in directing aerial hyphae formation. 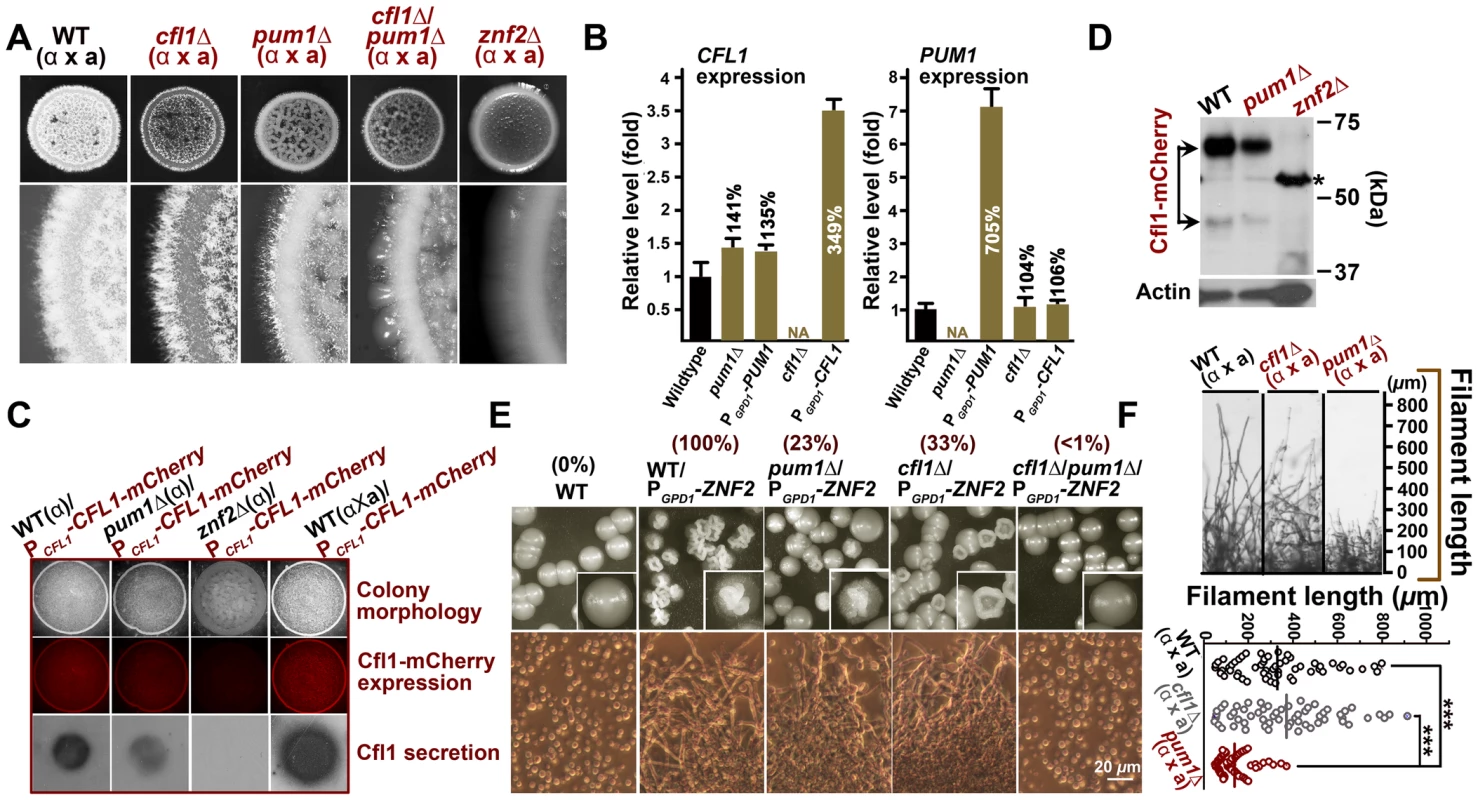
(A) Transcriptional analysis by qPCR indicates that alterations of Cfl1 or Pum1 do not affect the transcript level of each other. The expression level of genes in the wildtype strain XL280 was arbitrarily set as 1 for comparison. (B) The production or the release of Cfl1 proteins was not drastically affected in the pum1Δ mutant. The expression of Cfl1 proteins was abolished in the znf2Δ mutant and enhanced when the compatible mating partner was present. The mCherry-labeled Cfl1 was used to measure the Cfl1 protein level by fluorescence microscopy and released Cfl1 proteins was detected by colony immunoblot. (C) Pum1 is dispensable for the expression or the processing of Cfl1. By comparison, the Cfl1 protein expression was below the detectable level in the znf2Δ mutant. This served as a negative control. The asterisk indicates the non-specific band, which is also shown in control strain XL280 without mCherry. (D) Double deletion of CFL1 and PUM1 resulted in a much more severe defect in aerial filamentation during bisexual mating compared to either of the single deletion. (E) Pum1 and Cfl1 represent two major downstream regulatory branches of Znf2 in initiating filamentation. (F) Pum1 is also involved in hyphal extension. The deletion of PUM1 resulted in shorter filaments during bisexual mating. Such a phenotype was not observed in the cfl1Δ mutant. Scale bar: 20 µm. To interrogate the relationship between Pum1 and Cfl1, we first examined that the reciprocal effect of the disruption or the overexpression of one gene on the transcript level of the other. We did not observe any significant changes in expression of one gene when the other gene was altered (deletion or overexpression) (Figure 4B). Thus it appears that Cfl1 and Pum1 do not modulate each other at the transcript level in a major way. We next examined the effect of Pum1 on the abundance of Cfl1 protein within a community. We introduced Cfl1-mCherry under the control of CFL1's native promoter into the wildtype strain, the pum1Δ mutant, and the znf2Δ mutant. As expected, the Cfl1 protein expression was almost abolished in the znf2Δ mutant (Figure 4C). By comparison, the Cfl1 protein expression level in the pum1Δ mutant was only slightly affected, suggesting that Pum1 has no major impact on the protein level of Cfl1. Western blot analyses further supported this conclusion (Figure 4D). Thus, unlike Znf2, Pum1 is not required for the expression of the Cfl1 protein.
We previously established that Cfl1 is secreted extracellularly and that some of the Cfl1 proteins are cleaved and released into the surrounding milieu [2]. The released Cfl1 proteins can induce the expression of endogenous Cfl1 in nearby cells and stimulate their filamentation in a paracrine manner [2]. Thus an impairment of Cfl1's secretion or its release could lead to deficient filamentation, a phenotype observed in the pum1Δ mutant. To assess the impact of the deletion of PUM1 on the secretion and releasing of Cfl1 from the cell wall, we employed the colony immunoblot assay, which detects released proteins from an intact colony [2]. As shown in Figure 4C (bottom panel), released Cfl1 was not detected in the znf2Δ mutant while the deletion of PUM1 only modestly reduced the level of released Cfl1 proteins. Furthermore, some of the Cfl1 proteins were correctly cleaved into a smaller product in the pum1Δ mutant as in the wildtype strain (Figure 4D). Taken together, these observations suggest that Pum1 is not critical for the expression, secretion, or the processing of Cfl1. Because the pum1Δ cfl1Δ double mutant displayed a much more severe defect in aerial hypha development than either of the single mutant alone (Figure 4A), Pum1 and Cfl1 represent two parallel branches modulating Znf2-mediated filamentation program.
To examine if Pum1 and Cfl1 represent the major targets of Znf2 in eliciting filamentation, we overexpressed ZNF2 in the cfl1Δ, the pum1Δ, and the pum1Δ cfl1Δ mutants. The resulting overexpression strains were plated for single colonies under a mating-suppressive condition and were evaluated for the incidence of forming wrinkled colonies. The formation of wrinkled colonies reflects strong cell-cell adhesion and filamentation induced by the Znf2 controlled regulon [7]. The existence of a threshold level of Znf2 appears to control the colony morphological heterogeneity, with the whole population filamentous at a high expression level [7]. A population with a modest level of Znf2 displays a heterogeneous phenotype, likely reflecting the inherent fluctuation in the expression of Znf2's downstream genes in the population [7]. Similar phenomena are observed in another fungal pathogen Candida albicans in terms of the white-opaque switch controlled by the regulator Wor1 [35], [36], and in the bacterium Bacillus subtilis in terms of the motile-chained state switch controlled by the regulator SlrR [37]. In Cryptococcus, the overexpression of ZNF2 in the wildtype strain evoked homogeneous wrinkled colony morphology in the population (Figure 4E), consistent with our previous study [7]. However, when ZNF2 was overexpressed in the pum1Δ mutant or the cfl1Δ mutant, less than a third of the population showed wrinkled colony morphology. Double deletion of PUM1 and CFL1 almost abolished the ability of the population to form wrinkled colony morphology (<1%) in response to the ZNF2 overexpression (Figure 4E). Thus, the fluctuation of the expression level of Pum1 and Clf1 likely contributes to the phenotypic heterogeneity in the population. Taken together, Pum1 and Cfl1 represent the major targets of Znf2 in directing filamentation and cell-cell adhesion in C. neoformans.
Pum1 directs the initiation and the extension of hyphae partially through a secretory protein Fas1
The results presented earlier highlighted the major roles of Pum1 and Cfl1 in directing hyphal morphogenesis. Next we wanted to examine if Pum1 and Cfl1 are functionally redundant or they have overlapping but distinct roles. A closer examination of the hyphal morphology in the absence of CFL1 or PUM1 suggests that Pum1 and Cfl1 have overlapping but distinct roles in hyphal growth. Although both mutants showed a reduction in the abundance of aerial hyphal production, hyphae produced in the pum1Δ mutant were notably shorter than that in the wildtype strain or the cfl1Δ mutant (Figures 4F and S4) during both bisexual and unisexual reproductions. Conversely, overexpression of Pum1 elicited a phenotype of longer hyphae compared with the wildtype (Figure S4). Thus Pum1 is not only important for hyphal initiation, but also for hyphal extension. Interestingly, overexpression of ZNF2 could not bypass the defect of hyphal extension caused by the disruption of Pum1 (Figure S5), indicating that other Znf2 targets could not replace the role of Pum1 in controlling hyphal extension. To discover Pum1-mediated biological processes during hyphal development, we performed the whole-transcriptome microarray analysis of the hyper-filamentous PUM1OE strain. There were 85 genes exhibiting more than a 3 fold difference in response to PUM1 overexpression (Table S2). In agreement with the RT-PCR result (Figure 4B), CFL1 expression is not affected by the overexpression of PUM1. Gene ontology analyses indicated enrichment of two functional groups among the Pum1 regulon: one involved in meiosis and the other in extracellular proteins. The latter functional group (GO: 0005576) was also identified in the Znf2 regulon (Figure 1D). A comparison between the regulons of these two regulators revealed 3 genes encoding extracellular products [CNAG_00925 (named as FAD1), CNAG_05729 (named as FAS1) and Dha1] that were upregulated by overexpression of ZNF2 and PUM1 (Figure 5A). To evaluate their biological role in filamentation, we deleted these three genes. Only the deletion of CNAG_05729/FAS1 impaired filamentation and recapitulated the phenotypes produced by the disruption of PUM1 (Figure 5B). Conversely, overexpression of CNAG_05729/FAS1 stimulated robust aerial morphogenesis and longer hyphal growth (Figure 5C), confirming its position as the major Pum1 target in quantitatively controlling various aspects of filamentation.
Fig. 5. Pum1 directs hyphal initiation and extension partially through a novel hypha-specific extracellular protein Fas1. 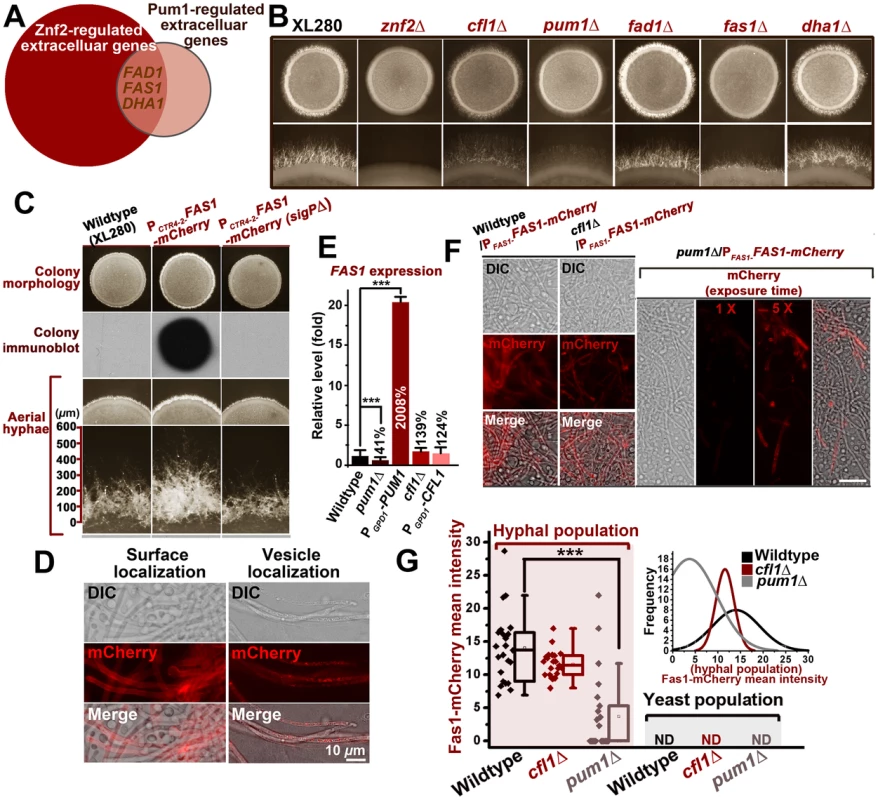
(A) FAS1, FAD1, and DHA1 are the genes encoding extracellular proteins overlapped in the regulons of Znf2 and Pum1. (B) Fas1 recapitulates the role of Pum1 in unisexual hyphal production. The fas1Δ mutant, like the pum1Δ mutant, showed reduced abundance of aerial hyphae. (C) The indicated strains were grown on V8 agar plate containing 200 µM BCS in the dark at 22°C. Images of the colonies were photographed after 5 days of incubation. Released Fas1 proteins were detected by colony immune-blot. Only overexpression of Fas1 with intact signal peptide led to a more robust filamentation and longer hyphae. Thus, the secretion of Fas1 is crucial for its biological function. (D) Fas1-mCherry is observed on hyphal surface and in vesicles at 72 hrs post unisexual mating stimulation. (E) The FAS1 expression level was positively regulated by Pum1 based on the transcriptional analysis of its transcript level in the pum1Δ mutant and the PGPD1-PUM1 strain by qPCR. The deletion or the overexpression of CFL1 did not significantly affect the FAS1 expression level. (F and G) Pum1 increased the intensity of Fas1 expression and reduces its stochasticity. The average intensity of Fas1-mCherry fluorescence was dramatically reduced in the absence of Pum1, but not Cfl1. The deletion of PUM1 led to a much higher variability in the Fas1-mCherry level among hyphal cells during unisexual filamentation (Inset of Figure? 5G). The frequencies of Fas1-mCherry's mean intensity in the hyphal population were plotted for the wildtype strain, the cfl1Δ mutant, and the pum1Δ mutant (software: ZEN 2011). CNAG_05729 is predicted to encode a small secreted protein (154 aa) with a signal peptide for secretion at its N-terminus. The mCherry-fused protein was almost exclusively expressed in the filamentous subpopulation during mating colony differentiation (Figure 5D and F), which corroborates its involvement in the filamentation pathway. Thus, we named CNAG_05729 FAS1 (Filament-Associated Secretory Protein 1). To investigate whether Fas1 is specially controlled by Pum1 or/and Cfl1, we assessed the impact on the expression of FAS1 when PUM1 or CFL1 was either absent or overexpressed. Neither the absence nor the overexpression of CFL1 altered the expression level of FAS1. By comparison, the transcript level of FAS1 was positively correlated with that of PUM1 (Figure 5E). At the protein level measured based on the fluorescence intensity of Fas1-mCherry, the deletion of PUM1 led to a marked reduction in Fas1 expression in the hyphal subpopulation (Figure 5F). However, the level of Fas1-mCherry in hyphae was not significantly different in the presence or absence of CFL1, suggesting that Fas1 belongs to the Pum1 pathway but not the Cfl1 pathway. Interestingly, the variation in Fas1 expression among individual hyphae in the absence of Pum1 was noticeably higher, and some hyphal cells in the pum1Δ mutant did not produce any measurable Fas1 proteins (Figure 5F and G). This suggests that Pum1 may mediate a stochastic buffering-like regulatory mechanism [38].
To gain the clue for the molecular functions of Fas1, we employed a variety of bioinformatic tools to identify potential functional domains or motifs harbored in this small protein. However, no annotated domain was identified in Fas1. A PSI-BLAST analysis with regions of low complexity masked suggested that there are Fas1 homologues present in other fungal species, nearly all of them belong to the family of tremellaceae from the phylum of Basidiomycota. This suggests that Fas1 proteins may have arisen relatively recently. Most of the Fas1 homologues contain a signal peptide for secretion and are predicted to be secreted proteins.
We previously showed that the Basidiomycota-specific adhesion protein Cfl1 displays two patterns of subcellular localization: cell surface and intracellular vesicles that are morphologically similar in appearance to known fungal secretory vesicles [39], [40]. Similar localization patterns were also observed for Fas1-mCherry during hyphal development (Figure 5D). Like Cfl1, Fas1 can be also released into extracellular matrix, as released Fas1 from the community was easily detected by the colony immunoblot assay (Figure 5C). The deletion of the N-terminal signal peptide predicted for secretion abolished its secretion (Figure 5C). As expected, the ability of Fas1 to be secreted is critical for its function in enhancing filamentation and extending filamentous growth (Figure 5C).
Pum1 bridges filamentation and meiosis during unisexual and bisexual mating
The aforementioned results demonstrate the major roles of Pum1 and Cfl1 in directing aerial hyphal morphogenesis, a process that could lead to the formation of fruiting bodies (sexual sporulation) critical for species survival and expansion into new niches. The enrichment of meiosis and sporulation genes in the Pum1 regulon in addition to the filamentation genes (e.g. FAS1) raises the possibility that Pum1 may bridge the progression from filamentation to meiosis and to post-meiotic sporulation during sexual reproduction. One meiosis-specific gene that was significantly upregulated in the PUM1OE strain is DMC1. Dmc1 is the recombinase specifically involved in homologous recombination at an early stage of meiosis. Earlier investigations have demonstrated that DMC1 is important for the formation of tetrad spore chains in Cryptococcus [12], [16]. Overexpression of PUM1 resulted in a greater than 4 fold increase in the DMC1 transcript level whereas the deletion of PUM1 led to a two-fold reduction in its expression (Figure 6A). By contrast, we did not observe any significant impact on DMC1 expression by altering the expression level of CFL1 (Figure 6A). The results reinforce the notion that Pum1 and Cfl1 are functionally different and support our hypothesis that Pum1 may control subsequent development following filamentation through its regulation of factors such as DMC1.
Fig. 6. Unisexual meiotic progression is only observed in the Pum1-controlled aerial hyphal population. 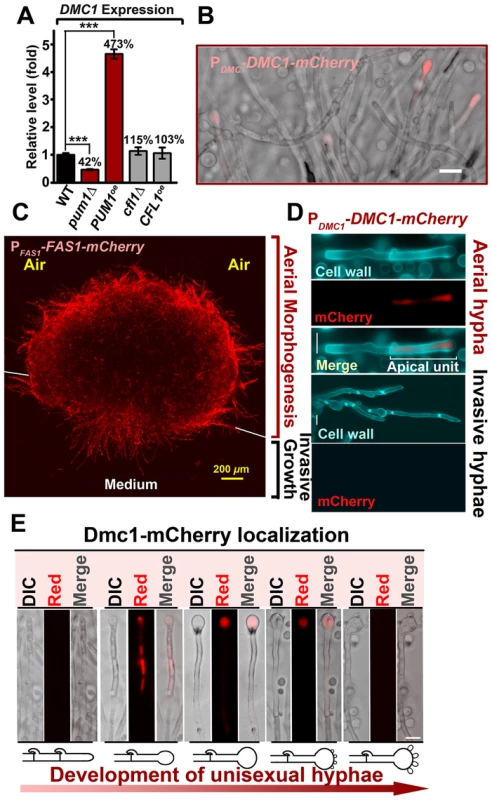
(A) Pum1, but not Cfl1, affects the expression of DMC1 during unisexual reproduction. (B) Dmc1 expression is associated with a portion of hyphal cells but not yeast cells. Scale bar: 20 µm. (C) Fas1 is exclusively expressed in the hyphal population and it is used as a molecular indicator to visualize the hyphal population in a mating colony. A side-view of a single-cell derived colony (3 days old) shows the topology of Fas1-expressing hyphal population in the community. (D) Details of the morphology and Dmc1 expression for aerial hyphae and root hyphae (invasive hyphae) from the unisexual mating community are shown. Fungal cell wall was stained blue with calcofluor white. Dmc1 expression was exclusively observed in aerial hyphae but not in invasive hyphae (more than 200 invasive hyphae were examined). Scale bar: 10 µm. (E) The dynamics of Dmc1-mCherry monitored during unisexual development. Scale bar: 10 µm. The localization patterns of Dmc1 in the figure are representative for each given stage of development (>40 hyphae for each stage were examined). The function of Dmc1 in the progression of meiosis is extremely conserved among evolutionally divergent eukaryotes [41] and it serves as a molecular marker for the meiotic process [42]. Therefore, we decided to use Dmc1-mCherry controlled by the DMC1's native promoter to identify the cell type associated with Dmc1 and monitor meiotic progression during the development of a unisexual mating community. Dmc1-mCherry was only detected in apical cells in a portion of hyphae and undetectable in yeast cells (Figure 6B and D). To further specify the subpopulation of hyphae associated with Dmc1 expression, we probed the topological pattern of the hypha-specific protein Fas1 in a unisexual community derived from a single cell using confocal microscopy (Figure 6C). The vertical transverse cross section of the colony indicated that the aerial hyphal population was enriched in the peripheral region of the upper colony while invasive filaments radially rooted the colony into the agar (Figure 6C). Interestingly, Dmc1-mCherry was detected only in some of the aerial hyphae, and not in any of the invasive hyphae (Figure 6B and D). A closer examination of aerial hyphae showed that Dmc1-mCherry was not detected in actively growing hyphae with no sign of differentiation into basidia. Rather, an intense signal was detected in the apical compartment of aerial hyphae when their apexes began to differentiate into basidia (Figure 6E). The signal gradually became focused in the basidial head during basidial maturation until four pre-spores started to emerge. The signal disappeared from the basidia when spores matured (Figure 6E). The dynamic expression of Dmc1 mirrors the progression from vegetative aerial hyphal growth to unisexual meiosis and sporulation. Given that mutations of PUM1 affect the transcript level of DMC1 (Figure 6A), we decided to examine the impact of the deletion of PUM1on Dmc1 protein expression. Remarkably, many aerial hyphal apexes differentiating into basidia lost the signal of Dmc1-mCherry in the pum1Δ mutant during unisexual colony development (Figure 7A). For those that had the Dmc1-mCherry signal, the fluorescent intensity was significantly lower (Figure 7B). Again, the loss of Pum1 increased the stochasticity of Dmc1 dynamics in basidia (Figure 7B) as observed for Fas1 in filaments (Figure 5G).
Fig. 7. Pum1 coordinates filamentation and sexual reproduction. 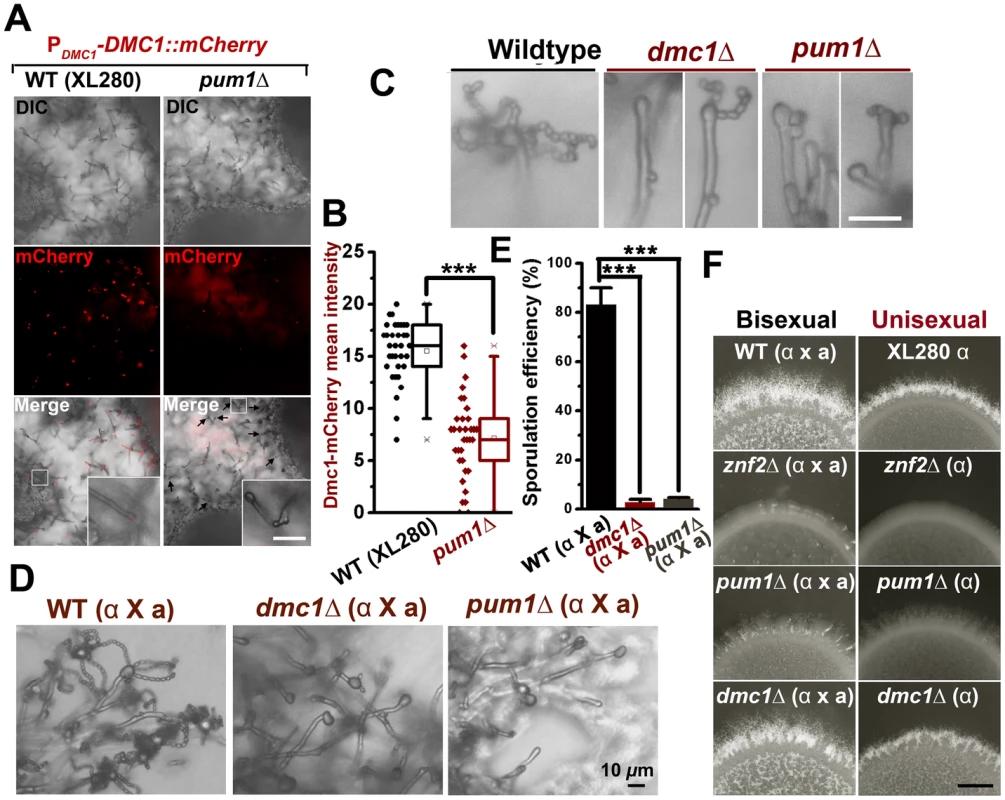
(A and B) The deletion of PUM1 impairs the expression of Dmc1 in basidia in a stochastic manner. Some but not all differentiating basidial heads lost the expression of Dmc1-mCherry without Pum1. Those that did expressed Dmc1at lower levels. Scale bars: 20 µm. (C) The unisexual sporulation was perturbed in the absence of the meiosis-specific protein Dmc1 and the global regulator Pum1. The wildtype strain XL280, the dmc1Δ mutant, and the pum1Δ mutant were diluted on V8 agar to form single colonies. Typical mature basidia observed from these strains were shown. Scale bar: 10 µm. (D and E) The deletion of PUM1 and DMC1 also dramatically reduced the sporulation efficiency during bisexual mating. (F) The effect of the deletion of ZNF2, PUM1, or DMC1 on aerial hyphal morphogenesis at the colony level during both bisexual and unisexual development. Scale bar: 1 mm. Previous studies indicated that mutations of meiosis-specific genes perturb sporulation [12], [16]. The effect of such mutations are highly specific, as the deletion of DMC1 or SPO11(an endonuclease creating double-stranded breaks to initiate meiosis) did not affect hyphal initiation, hyphal extension, or the terminal differentiation of some aerial hyphae into basidia (Figure 7F)[12]. The deletion of PUM1 likewise resulted in a severe defect in the formation of basidia and appropriate tetrads of spore chains during either unisexual or bisexual reproduction (Figure 7C, D, E and F). However, disruption of Pum1 also drastically reduced the ability of this fungus to initiate and to maintain hyphal growth (Figures 2B and 4F). These results suggest that Pum1 links sexual reproduction with filamentation, and meiosis per se does not influence earlier morphogenesis events. Although most of the studies were performed in a laboratory strain XL280 background, we confirmed that Pum1 plays a similar role in bridging sex and filamentation in a clinical isolate H99 (Figure S6). Our data presented here indicate that Pum1 plays a conserved role in genetically orchestrating the transition from yeasts to hyphae and the subsequent transition from aerial hyphae to sexual reproduction in this eukaryotic pathogen.
Discussion
Facing unpredictable changes in the environment, terrestrial microbes have evolved sophisticated adaptation strategies. One well-known example is morphotype transition [3], [6], [10], which essentially achieves a functional transition by changing cell shape or size [3]. This strategy is commonly adopted by environmental pathogens to survive in the environment and inside a host, conditions that could differ drastically from each other [3], [6]. As the transition between yeasts and hyphae is linked to pathogenesis in many environmental fungal pathogens [3], [6], [43], [44], [45], and that morphogenesis is an integral part of fungal development, understanding the molecular mechanisms controlling fungal morphogenesis is crucial in our understanding of fungal pathogenic strategies and fungal biology. Because morphotype transition in Cryptococcus is a stochastic process, identification of phase-specific genes and their regulation systems in this organism has been challenging.
In this study, we took advantage of an engineered cryptococcal strain to generate homogeneous yeast and hyphal populations. This approach enabled a more sensitive comparison between the two cryptococcal morphotypes and allowed us to uncover a more reliable Znf2 regulon. Functional classification of this regulon revealed similar genetic programs related to filamentous growth controlled by Znf2 in Cryptococcus as the ones regulated by Rbf1 in the basidiomycete plant pathogen Ustilago maydis [46], [47]. Although Rbf1 is much smaller than Znf2, both regulators contain the similar C2-H2 Zinc-finger DNA binding domain [47] (data not shown). A considerable proportion of Rbf1's regulon also encode secretory proteins and factors involved in cell cycle progression [47], [48]. Such a striking parallelism in Cryptococcus and Ustilago suggests that Znf2 and Rbf1 might have evolved from a common regulator, which controlled the morphological transition in an ancient Basidiomycota species.
The inherent properties of yeasts or hyphae might dictate the necessary genetic programs common in diverse fungi regardless of the upstream species-specific regulatory systems. For instance, extracellular proteins and cell wall-modifying proteins are required to reconstruct different cell shapes [30], [32]. Cell cycle progression is overrepresented in a yeast population compared to a filamentous population [30] likely because all but the apical compartments are quiescent in the hyphal population. Given that the yeast-to-hypha transition is observed in major fungal phyla [3], [5], [30], [32], [49], the ability to undergo morphological switches might have existed in original fungi prior to the demarcation of these phyla. Species-specific features could be wired later into the regulation of morphological transition to optimize the adaptation of each fungal species to their unique natural niches, as in the case of mating-initiated yeast-hyphal transition in C. neoformans and in U. maydis. In both organisms, filamentation is developmentally associated with the progression of sex, which helps ensure species' long term success through the creation of genetic variants and infectious spores [22], [23], [24], [50], [51].
In the development of a cryptococcal mating community, the yeast-to-hypha transition is followed by sustained hyphal growth and subsequent formation of fruiting bodies from aerial hypha apexes. Although it is clear that Znf2 controls filamentation in Cryptococcus and a few proteins like Dmc1 and Spo11 are involved in meiosis, the factor that connects filamentation with sexual reproduction remains elusive (Figure 8). From the Znf2 regulon, we found two major regulatory branches downstream of Znf2 controlling the yeast-to-hypha transition: Pum1 and the matricellular signal Cfl1. Cfl1 does not play a major role in promoting sustained hyphal growth or the fruiting body formation, suggesting that this signal probably exerts a dedicated control at the stage of hyphal initiation [2] (Figure 8). By contrast, Pum1 is important for multiple developmental stages. Pum1 promotes yeast-to-hypha transition in a mating colony through its regulation of hypha-specific proteins (e.g. Fas1) (Figures 5F and 8); Pum1 bridges the progression from aerial hyphal growth to meiosis through its additional regulation on multiple meiosis-related genes (Figure 8). The representation of genes involved in diverse functions in the Pum1 regulon (Table S2) is also consistent with its pleiotropic role. One of the interesting finding of this study is the dynamic expression pattern of the meiosis-specific recombinase Dmc1. Dmc1 is undetectable in undifferentiating growing hyphae (none in invasive hyphae), and is only expressed in aerial hypha apexes that are differentiating into fruiting bodies (Figures 6B and 7A). The concurrence of the onset of meiosis and the terminal differentiation of hyphal growth represents a culminating feature for the committed sexual reproduction. The dynamics of the expression of the highly conserved meiosis-specific protein during the development of basidia (Figure 6E) in a unisex colony provides yet another strong piece of evidence for the meiotic nature of this novel life cycle [16].
Fig. 8. Filamentation pathway coordinates sexual reproduction and morphogenesis in Cryptococcus neoformans. 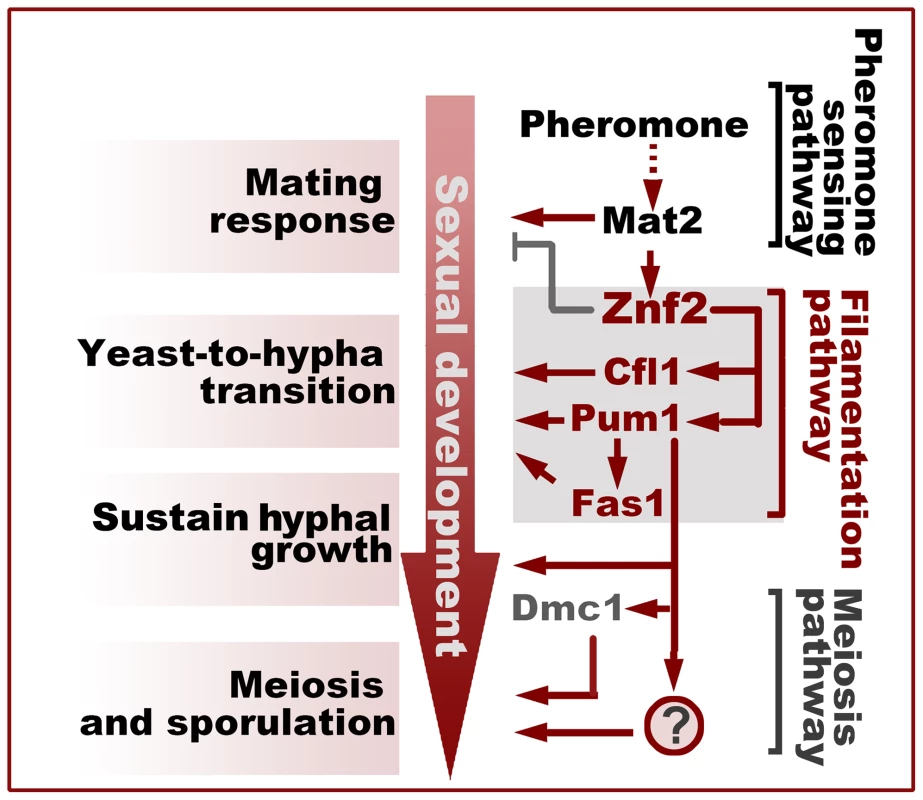
Activation of Znf2 promotes the filamentous growth and modestly inhibits the early mating behaviors that initiate the transition from the yeast form to the hypha form [7], [12]. Cfl1 and Pum1 represent two parallel major branches downstream of Znf2 in the orchestration of this morphological switch. Pum1 plays additional important roles in sustaining hyphal growth and in bridging hyphal development and sexual reproduction partially through its control over the expression of DMC1 and FAS1. The question mark indicates unidentified factors controlled by Pum1 that regulate meiosis and sporulation. What remains to be established is the mode of action of Pum1. Pum1 contains an mRNA-binding Pumilio domain. The members of this family control a variety of biological processes through modulating the stability and the translation of their target transcripts [33]. Pumilio proteins are generally considered repressors and they recruit the deadenylase to decay their target mRNAs [33]. However, accumulating evidence indicates that regulators of this family can also function as activators [33]. One such example is Puf9 from the parasite Trypanosoma brucei [52]. Puf9 in T. brucei stabilize its mRNA targets, likely through a competition with a repressor for the mRNA binding [52]. Here, we show that Pum1 also plays a positive role in the control of the gene expression (e.g. FAS1 and DMC1). Loss of Pum1 decreases the overall expression levels of FAS1 and DMC1 but increases the variations of these protein expression levels among cells in the same population. This suggests that Pum1 may mediate a stochastic buffering-like regulatory mechanism [38]. It remains to be determined if Pum1 positively controls the expression level of its targets through promoting their transcription, stabilizing their mRNAs, or facilitating their translation. Further investigations into Pum1-interating factors and the dynamics of target mRNAs at different developmental stages would help obtain a mechanistic understanding of how Pum1 coordinates hyphal and sexual development.
Materials and Methods
Ethics statement
All the animal experiments were performed according to the guidelines of NIH and Texas A&M University Institutional Animal Care and Use Committee (IACUC permit # 2011–22).
Statistics
The programs Originpro and GraphPad Prism were used for statistical analyses in this study. A two-tailed unpaired Student t test was performed to compare the mean florescence intensity or transcript levels from two groups. P values less than 0.05 were considered statistically significant.
Strains and growth conditions
Strains used in this study are listed in Table S3. For both unisexual and bisexual mating assays, strains were maintained on V8 agar unless indicated otherwise. The optical density of cell suspensions measured at 600 nm was used to represent cell density. When the inducible promoter of the copper transporter gene CTR4 (e.g. PCTR4-2-FAS1) was used to drive the genes, the gene expression level was manipulated by the addition of 25 µM CuSO4 (inhibitor) or 200 µM the copper chelator Bathocuproine disulphonate (BCS; inducer) to the media as described previously [7], [53], [54].
UV mutagenesis and suppressor screen for filamentation mutant
Overnight culture of the znf2Δ mutant in YPD liquid medium was plated on YPD agar. The plates were dried and then explored to UV light (300 J/m2) for 3 seconds by using the UV cross linker. The cells were then collected, washed by PBS and plated on V8 agar medium for 7 days in the dark at 22°C to form visible colonies. The colonies were screen under a stereoscope for the formation of hyphae. The mutants with hypha-like morphology were furthered examined with a compound microscope. No true hyphae were observed in any of the mutants screened but mutants with pseudohyphae and/or shmoo cells were observed.
Mating and self-filamentation assays
For bisexual mating assays, a and α cells of equal number (original optical density of 1.5 at OD600) were cocultured on V8 juice agar medium (pH = 7.0 for serotype D strains, pH = 5.0 for serotype A strains) in the dark at 22°C [55]. Random basidiospores were isolated using a micromanipulator. For unisexual mating assay (serotype D XL280 strain background), cells were spotted on V8 medium alone. Both bisexual and unisexual matings were examined microscopically for formation of mating hyphae and chains of basidiospores.
Gene knockout and overexpression
Primers used in this study are listed in Table S4. To disrupt the PUM1, FAS1, FAD1, DHA1 or the ZNF2 genes, an overlap PCR products were generated with the NAT or the NEO dominant drug marker amplified from plasmid pAI1 or pJAF1 [11] and 5′ and 3′ flanking sequences (1∼1.5 kb) of the coding genes from strain JEC21α or H99α as we described previously [11]. The PCR product was directly introduced into strains JEC21α, XL280α, JEC20a, or H99α by biolistic or electronic transformation. The resulting deletion mutants generated via homologous recombination were confirmed by PCR and genetic crosses. For overexpression, an overlap PCR product with the NEO or NAT marker and the wild-type genes containing their native promoter (∼1.2 kb) and GPD1 terminator (∼0.5 kb) from strain JEC21 or H99 was generated. The PCR products were directly introduced into appropriate backgrounds as indicated in the text. Mutant strains in the MATa background were obtained by crossing the corresponding α mutants with the widltype congenic a strains. For overexpression, genes (ORF) were amplified by PCR and the amplified fragments were digested and inserted into pXL1 or pXC after the GPD1 or the CTR4-2 promoters respectively as we previously described [7]. Overexpression plasmids were linearized via restriction enzyme digestion before being introduced into the relevant Cryptococcus strains as we previously described [7].
Murine models of cryptococcosis
Animals were intranasally infected as previously described [7], [56], [57]. Nine A/J mice (6 - to 8-week-old female; Jackson Labs) per group were used for survival studies. Statistical significance (P≤0.05) of the survival data between different groups was assessed by the Mantel-Cox log-rank test [58].
Cell fusion assay
The coculture of α and a cells with different auxotrophic markers (lys for α; ade2 for a) were inoculated on V8 agar or in YPD liquid medium. After 22 hrs of incubation, the coculture was collected, washed by cold water twice, and plated on minimum YNB agar to select fusion products at 37°C as described previously [11].
Construction of fluorescent proteins and microscopic examination
The mCherry was fused to the C-terminus of the interested protein used in this study. Overlap PCR was used to piece together the fragments including the coding region of the gene with 1∼1.2 kb upstream sequences and the mCherry. The resulting products were introduced into plasmid pXL1 as described previously [7]. The FAS1-mCherry without the promoter was amplified and introduced to pXC to generate the plasmid PCTR4-2-FAS1-mCherry. To overexpress Fas1-mCherry that lacks the N-terminal signal peptide [Fas1 (sigPΔ)-mCherry], primers Linlab 1581 and Linlab864 were used to generate FAS1(sigPΔ)-mCherry allele. The PCTR4-2-FAS1-mCherry construct was used as the template. The resulting PCR product was cloned into pXC to produce PCTR4-2-FAS1(sigPΔ)-mCherry. To examine the sub-cellular localization of morphogenesis or meiosis-related proteins during filamentation, the fluorescence strains were grown on V8 agar medium at 22°C for 72 hrs before being examined microscopically. Images were acquired and processed with a Zeiss Axioplan 2 imaging system with the AxioCam MRm camera software Zen 11 (Carl Zeiss Microscopy). To assess the expression level of PCTR4-2 -FAS1-mCherry and PCTR4-2-FAS1(sigPΔ)-mCherry in a community, the overexpression strains were grown on V8 agar medium containing the copper chelator BCS before examined with the fluorescence stereoscope.
RNA purification, qPCR analyses, and microarray analyses
The purelink RNA kit (Invitrogen) and the Superscript III cDNA synthesis kit (Invitrogen) were used for total RNA purification and the first strand cDNA synthesis respectively according to the manufacture instruction. The house-keeping gene TEF1 was used as the endogenous control for normalizing gene expression levels. The relative transcript levels were determined using the comparative CT method as described previously [7].
For transcriptional profiling of different morphotypes, the yeast H99 wildtype strain and the filamentous PCTR4-2-ZNF2 strain were grown in YPD liquid medium containing BCS at 22°C for 48 hrs. The cell morphology and the proportion of hyphal and yeast populations were measured before the total RNAs were extracted. RNAs were processed for microarray analyses as described previously [7]. For the transcript analysis to identify the Pum1 regulon using microarray, XL280 and the PGPD1-PUM1 strain were grown on V8 agar medium at 22°C for 72 hrs. Unisexual filamentation and sporulation were confirmed microscopically before extraction of total RNAs. The arrays were analyzed similarly as described previously [11].
Protein extraction and western-blot analyses
For Western Blot analysis of the Cfl1 expression, overnight cultures of indicated strains in YPD liquid medium were spotted onto V8 agar for 3 days at 22°C. The cells were removed and suspended in cold PBS for protein extraction. Protein extraction and western analysis were performed as described previously [2]. Briefly, the cell suspension was subjected to centrifuging at 13,000xg for 10 min at 4°C. The pellet was freeze dried and disrupted. The disrupted cells were subsequently suspended for western immunoblotting analysis of whole-cell proteins. The house keeping protein β-actin was used as the endogenous control.
Colony immunoblot for the detection of secreted Fas1 and Cfl1 proteins
For colony immunoblot assays, 3 microliters of cell suspension with the density of 1.5×107 cells/mL were spotted onto the same plate for the assay as described previously [2]. The strains were grown on V8 agar medium containing BCS at 22°C for 24 hrs to form visible colonies and the plate with the colonies was laid over by a sterile nitrocellulose membrane (Millipore, Billerica, MA). The membrane was removed from the plate after incubation for additional 3 days. The membrane was subsequently washed with 1× Tris-buffered saline (TBS) to remove attached cells. The blots were incubated with the anti-mCherry primary antibody (1/1000 dilution) and then a rabbit anti-mouse secondary antibody (1/10,000 dilution). Detection was performed using the ECL system according to the manufacture's instruction.
Confocal microscope analyses
PDMC1-DMC1-mCherry or PFAS1-FAS1-mCherry were grown on V8 (pH7.0) plates for 5 days at room temperature in dark to form visible colonies. For probing DMC1 expression in different hyphal subpopulations, 50 µg/ml calcofluor white (stain cell wall) was added into the medium to enable the visualization of all fungal cell types. For sample preparation, colonies were embedded in low-gelling agarose (sigma-Aldrich) on the plates [59]. Immediately after gelling, agarose-embedded colonies were cut vertically in the middle and transferred to the cover glass with the longitudinal section facing the cover glass for the side view of the colonies. Cover the sample with 1∶9 PBS-Glycerol. An Olympus Fv1000 confocal laser scanning microscope and an Olympus IX81 spinning disk confocal microscope were used for the image acquisition. For Olympus Fv1000 confocal laser scanning microscope, calcoflour white and mCherry were excited with 488 nm argon and 543 nm He-Ne laser lines respectively using a dry 40× objective. For Olympus IX81 spinning disk confocal microscope, calcoflour white and mCherry were excited with 405 nm argon and 561 nm laser lines respectively with dry 40× objective. The data analyses were carried out with MetaMorph Microscopy Automation & Image Analysis Software (Molecular Devices, PA).
Supporting Information
Zdroje
1. DietrichLE, TealTK, Price-WhelanA, NewmanDK (2008) Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science 321 : 1203–1206.
2. WangL, TianX, GyawaliR, LinX (2013) Fungal adhesion protein guides community behaviors and autoinduction in a paracrine manner. Proc Natl Acad Sci U S A 110 : 11571–11576.
3. KleinBS, TebbetsB (2007) Dimorphism and virulence in fungi. Curr Opin Microbiol 10 : 314–319.
4. MitchellAP (1998) Dimorphism and virulence in Candida albicans. Curr Opin Microbiol 1 : 687–692.
5. LiCH, CervantesM, SpringerDJ, BoekhoutT, Ruiz-VazquezRM, et al. (2011) Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathog 7: e1002086.
6. WangL, LinX (2012) Morphogenesis in fungal pathogenicity: shape, size, and surface. PLoS Pathog 8: e1003027.
7. WangL, ZhaiB, LinX (2012) The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS Pathog 8: e1002765.
8. NemecekJC, WuthrichM, KleinBS (2006) Global control of dimorphism and virulence in fungi. Science 312 : 583–588.
9. LopezCE (2006) [Dimorphism and pathogenesis of Histoplasma capsulatum]. Rev Argent Microbiol 38 : 235–242.
10. LinX (2009) Cryptococcus neoformans: morphogenesis, infection, and evolution. Infect Genet Evol 9 : 401–416.
11. LinX, JacksonJC, FeretzakiM, XueC, HeitmanJ (2010) Transcription factors Mat2 and Znf2 operate cellular circuits orchestrating opposite - and same-sex mating in Cryptococcus neoformans. PLoS Genet 6: e1000953.
12. FeretzakiM, HeitmanJ (2013) Genetic Circuits that Govern Bisexual and Unisexual Reproduction in Cryptococcus neoformans. PLoS Genet 9: e1003688.
13. HullCM, CoxGM, HeitmanJ (2004) The alpha-specific cell identity factor Sxi1alpha is not required for virulence of Cryptococcus neoformans. Infect Immun 72 : 3643–3645.
14. Kwon-ChungKJ (1976) Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68 : 821–833.
15. Kwon-ChungKJ (1976) A new species of Filobasidiella, the sexual state of Cryptococcus neoformans B and C serotypes. Mycologia 68 : 943–946.
16. LinX, HullCM, HeitmanJ (2005) Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434 : 1017–1021.
17. LinX, HeitmanJ (2006) The biology of the Cryptococcus neoformans species complex. Annu Rev Microbiol 60 : 69–105.
18. LinX, HuangJC, MitchellTG, HeitmanJ (2006) Virulence attributes and hyphal growth of C. neoformans are quantitative traits and the MATalpha allele enhances filamentation. PLoS Genet 2: e187.
19. BuiT, LinX, MalikR, HeitmanJ, CarterD (2008) Isolates of Cryptococcus neoformans from infected animals reveal genetic exchange in unisexual, alpha mating type populations. Eukaryot Cell 7 : 1771–1780.
20. VelagapudiR, HsuehYP, Geunes-BoyerS, WrightJR, HeitmanJ (2009) Spores as infectious propagules of Cryptococcus neoformans. Infect Immun 77 : 4345–4355.
21. BottsMR, HullCM (2010) Dueling in the lung: how Cryptococcus spores race the host for survival. Curr Opin Microbiol 13 : 437–442.
22. FraserJA, GilesSS, WeninkEC, Geunes-BoyerSG, WrightJR, et al. (2005) Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437 : 1360–1364.
23. WangL, LinX (2011) Mechanisms of unisexual mating in Cryptococcus neoformans. Fungal Genet Biol 48 : 651–660.
24. NiM, FeretzakiM, LiW, Floyd-AveretteA, MieczkowskiP, et al. (2013) Unisexual and heterosexual meiotic reproduction generate aneuploidy and phenotypic diversity de novo in the yeast Cryptococcus neoformans. PLoS Biol 11: e1001653.
25. HeitmanJ (2006) Sexual reproduction and the evolution of microbial pathogens. Curr Biol 16: R711–725.
26. MorrowCA, FraserJA (2013) Ploidy variation as an adaptive mechanism in human pathogenic fungi. Semin Cell Dev Biol 24 : 339–346.
27. HsuehYP, XueC, HeitmanJ (2009) A constitutively active GPCR governs morphogenic transitions in Cryptococcus neoformans. EMBO J 28 : 1220–1233.
28. ZhaiB, ZhuP, FoyleD, UpadhyayS, IdnurmA, et al. (2013) Congenic strains of the filamentous form of Cryptococcus neoformans for studies of fungal morphogenesis and virulence. Infect Immun 81 : 2626–2637.
29. Huang daW, ShermanBT, LempickiRA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4 : 44–57.
30. BeyhanS, GutierrezM, VoorhiesM, SilA (2013) A temperature-responsive network links cell shape and virulence traits in a primary fungal pathogen. PLoS Biol 11: e1001614.
31. LorenzMC, CutlerNS, HeitmanJ (2000) Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol Biol Cell 11 : 183–199.
32. AriyachetC, SolisNV, LiuY, PrasadaraoNV, FillerSG, et al. (2013) SR-like RNA-binding protein Slr1 affects Candida albicans filamentation and virulence. Infect Immun 81 : 1267–1276.
33. QuenaultT, LithgowT, TravenA (2011) PUF proteins: repression, activation and mRNA localization. Trends Cell Biol 21 : 104–112.
34. JiangH, GuanW, GuZ (2010) Tinkering evolution of post-transcriptional RNA regulons: puf3p in fungi as an example. PLoS Genet 6: e1001030.
35. HuangG, WangH, ChouS, NieX, ChenJ, et al. (2006) Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc Natl Acad Sci U S A 103 : 12813–12818.
36. ZordanRE, GalgoczyDJ, JohnsonAD (2006) Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc Natl Acad Sci U S A 103 : 12807–12812.
37. NormanTM, LordND, PaulssonJ, LosickR (2013) Memory and modularity in cell-fate decision making. Nature 503 : 481–486.
38. JoshiA, BeckY, MichoelT (2012) Post-transcriptional regulatory networks play a key role in noise reduction that is conserved from micro-organisms to mammals. FEBS J 279 : 3501–3512.
39. SchusterM, TreitschkeS, KilaruS, MolloyJ, HarmerNJ, et al. (2012) Myosin-5, kinesin-1 and myosin-17 cooperate in secretion of fungal chitin synthase. EMBO J 31 : 214–227.
40. Santiago-TiradoFH, Legesse-MillerA, SchottD, BretscherA (2011) PI4P and Rab inputs collaborate in myosin-V-dependent transport of secretory compartments in yeast. Dev Cell 20 : 47–59.
41. KagawaW, KurumizakaH (2010) From meiosis to postmeiotic events: uncovering the molecular roles of the meiosis-specific recombinase Dmc1. FEBS J 277 : 590–598.
42. DevisettyUK, MayesK, MayesS (2010) The RAD51 and DMC1 homoeologous genes of bread wheat: cloning, molecular characterization and expression analysis. BMC Res Notes 3 : 245.
43. NemecekJC, WuthrichM, KleinBS (2007) Detection and measurement of two-component systems that control dimorphism and virulence in fungi. Methods Enzymol 422 : 465–487.
44. NguyenVQ, SilA (2008) Temperature-induced switch to the pathogenic yeast form of Histoplasma capsulatum requires Ryp1, a conserved transcriptional regulator. Proc Natl Acad Sci U S A 105 : 4880–4885.
45. PasrichaS, PayneM, CanovasD, PaseL, NgaosuwankulN, et al. (2013) Cell-Type-Specific Transcriptional Profiles of the Dimorphic Pathogen Penicillium marneffei Reflect Distinct Reproductive, Morphological, and Environmental Demands. G3 (Bethesda) 3 : 1997–2014.
46. HeimelK, SchererM, SchulerD, KamperJ (2010) The Ustilago maydis Clp1 protein orchestrates pheromone and b-dependent signaling pathways to coordinate the cell cycle and pathogenic development. Plant Cell 22 : 2908–2922.
47. HeimelK, SchererM, VranesM, WahlR, PothiratanaC, et al. (2010) The transcription factor Rbf1 is the master regulator for b-mating type controlled pathogenic development in Ustilago maydis. PLoS Pathog 6: e1001035.
48. HeimelK, FreitagJ, HampelM, AstJ, BolkerM, et al. (2013) Crosstalk between the unfolded protein response and pathways that regulate pathogenic development in Ustilago maydis. Plant Cell 25 : 4262–4277.
49. NadalM, Garcia-PedrajasMD, GoldSE (2008) Dimorphism in fungal plant pathogens. FEMS Microbiol Lett 284 : 127–134.
50. NielsenK, HeitmanJ (2007) Sex and virulence of human pathogenic fungi. Adv Genet 57 : 143–173.
51. HeitmanJ (2010) Evolution of eukaryotic microbial pathogens via covert sexual reproduction. Cell Host Microbe 8 : 86–99.
52. ArcherSK, LuuVD, de QueirozRA, BremsS, ClaytonC (2009) Trypanosoma brucei PUF9 regulates mRNAs for proteins involved in replicative processes over the cell cycle. PLoS Pathog 5: e1000565.
53. ChayakulkeereeM, RudeTH, ToffalettiDL, PerfectJR (2007) Fatty acid synthesis is essential for survival of Cryptococcus neoformans and a potential fungicidal target. Antimicrob Agents Chemother 51 : 3537–3545.
54. OryJJ, GriffithCL, DoeringTL (2004) An efficiently regulated promoter system for Cryptococcus neoformans utilizing the CTR4 promoter. Yeast 21 : 919–926.
55. Kwon-ChungKJ, BennettJE, RhodesJC (1982) Taxonomic studies on Filobasidiella species and their anamorphs. Antoine Van Leeuwenhoek 48 : 25–38.
56. CoxGM, MukherjeeJ, ColeGT, CasadevallA, PerfectJR (2000) Urease as a virulence factor in experimental cryptococcosis. Infect Immun 68 : 443–448.
57. LinX, NielsenK, PatelS, HeitmanJ (2008) Impact of mating type, serotype, and ploidy on the virulence of Cryptococcus neoformans. Infect Immun 76 : 2923–2938.
58. LitvintsevaAP, MitchellTG (2009) Most environmental isolates of Cryptococcus neoformans var. grubii (Serotype A) are not lethal for mice. Infect Immun 77 : 3188–3195.
59. VachovaL, ChernyavskiyO, StrachotovaD, BianchiniP, BurdikovaZ, et al. (2009) Architecture of developing multicellular yeast colony: spatio-temporal expression of Ato1p ammonium exporter. Environ Microbiol 11 : 1866–1877.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA SplicingČlánek Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance GenesČlánek The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- BdlA, DipA and Induced Dispersion Contribute to Acute Virulence and Chronic Persistence of
- Morphotype Transition and Sexual Reproduction Are Genetically Associated in a Ubiquitous Environmental Pathogen
- A Nucleic-Acid Hydrolyzing Single Chain Antibody Confers Resistance to DNA Virus Infection in HeLa Cells and C57BL/6 Mice
- HopW1 from Disrupts the Actin Cytoskeleton to Promote Virulence in Arabidopsis
- Ly6C Monocytes Become Alternatively Activated Macrophages in Schistosome Granulomas with Help from CD4+ Cells
- Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA Splicing
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- Antibody Responses to : Role in Pathogenesis and Diagnosis of Encephalitis?
- Discovery of a Novel Compound with Anti-Venezuelan Equine Encephalitis Virus Activity That Targets the Nonstructural Protein 2
- Activation of Focal Adhesion Kinase by Suppresses Autophagy via an Akt/mTOR Signaling Pathway and Promotes Bacterial Survival in Macrophages
- Crossing the Interspecies Barrier: Opening the Door to Zoonotic Pathogens
- Catching Fire: , Macrophages, and Pyroptosis
- IscR Is Essential for Type III Secretion and Virulence
- Selective Chemical Inhibition of Quorum Sensing in Promotes Host Defense with Minimal Impact on Resistance
- The Glycosylated Rv1860 Protein of Inhibits Dendritic Cell Mediated TH1 and TH17 Polarization of T Cells and Abrogates Protective Immunity Conferred by BCG
- A Genome-Wide Tethering Screen Reveals Novel Potential Post-Transcriptional Regulators in
- Structural Insights into SraP-Mediated Adhesion to Host Cells
- Human IGF1 Regulates Midgut Oxidative Stress and Epithelial Homeostasis to Balance Lifespan and resistance in
- Cycling Empirical Antibiotic Therapy in Hospitals: Meta-Analysis and Models
- Rab11 Regulates Trafficking of -sialidase to the Plasma Membrane through the Contractile Vacuole Complex of
- Mitogen and Stress Activated Kinases Act Co-operatively with CREB during the Induction of Human Cytomegalovirus Immediate-Early Gene Expression from Latency
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- A Central Role for Carbon-Overflow Pathways in the Modulation of Bacterial Cell Death
- An Invertebrate Warburg Effect: A Shrimp Virus Achieves Successful Replication by Altering the Host Metabolome via the PI3K-Akt-mTOR Pathway
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
- A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees () Prevails after -Mediated, or , Transmission
- Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance Genes
- Ubiquitin-Mediated Response to Microsporidia and Virus Infection in
- Preclinical Detection of Variant CJD and BSE Prions in Blood
- Toll-Like Receptor 8 Agonist and Bacteria Trigger Potent Activation of Innate Immune Cells in Human Liver
- Progressive Proximal-to-Distal Reduction in Expression of the Tight Junction Complex in Colonic Epithelium of Virally-Suppressed HIV+ Individuals
- The Triggering Receptor Expressed on Myeloid Cells 2 Inhibits Complement Component 1q Effector Mechanisms and Exerts Detrimental Effects during Pneumococcal Pneumonia
- Differential Activation of Acid Sphingomyelinase and Ceramide Release Determines Invasiveness of into Brain Endothelial Cells
- Forward Genetic Screening Identifies a Small Molecule That Blocks Growth by Inhibiting Both Host- and Parasite-Encoded Kinases
- Defining Immune Engagement Thresholds for Control of Virus-Driven Lymphoproliferation
- Growth Factor and Th2 Cytokine Signaling Pathways Converge at STAT6 to Promote Arginase Expression in Progressive Experimental Visceral Leishmaniasis
- Multimeric Assembly of Host-Pathogen Adhesion Complexes Involved in Apicomplexan Invasion
- Biogenesis of Influenza A Virus Hemagglutinin Cross-Protective Stem Epitopes
- Adequate Th2-Type Response Associates with Restricted Bacterial Growth in Latent Mycobacterial Infection of Zebrafish
- Protective Efficacy of Passive Immunization with Monoclonal Antibodies in Animal Models of H5N1 Highly Pathogenic Avian Influenza Virus Infection
- Fructose-Asparagine Is a Primary Nutrient during Growth of in the Inflamed Intestine
- The Calcium-Dependent Protein Kinase 3 of Influences Basal Calcium Levels and Functions beyond Egress as Revealed by Quantitative Phosphoproteome Analysis
- A Translocated Effector Required for Dissemination from Derma to Blood Safeguards Migratory Host Cells from Damage by Co-translocated Effectors
- Functional Characterization of a Novel Family of Acetylcholine-Gated Chloride Channels in
- Both α2,3- and α2,6-Linked Sialic Acids on O-Linked Glycoproteins Act as Functional Receptors for Porcine Sapovirus
- The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
- MicroRNA-146a Provides Feedback Regulation of Lyme Arthritis but Not Carditis during Infection with
- Recombination in Enteroviruses Is a Biphasic Replicative Process Involving the Generation of Greater-than Genome Length ‘Imprecise’ Intermediates
- Cytoplasmic Viral RNA-Dependent RNA Polymerase Disrupts the Intracellular Splicing Machinery by Entering the Nucleus and Interfering with Prp8
- and Are Associated with Murine Susceptibility to Infection and Human Sepsis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



