-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAntibody Responses to : Role in Pathogenesis and Diagnosis of Encephalitis?
article has not abstract
Published in the journal: . PLoS Pathog 10(6): e32767. doi:10.1371/journal.ppat.1003983
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1003983Summary
article has not abstract
The pathogenesis of encephalitis associated with the respiratory pathogen Mycoplasma pneumoniae is not well understood. A direct infection of the central nervous system (CNS) and an immune-mediated process have been discussed [1]. Recent observations suggest that intrathecally detectable antibodies against the bacterium, which can serve to establish the etiology of encephalitis, may indeed mediate the disease.
Mycoplasma pneumoniae is a major cause of upper and lower respiratory tract infections in humans worldwide, particularly in children [2], [3]. Up to 40% of community-acquired pneumonia in children admitted to the hospital are attributed to M. pneumoniae infection [4]–[7]. Although the infection is rarely fatal, patients of every age can develop severe and fulminant disease. Apart from the respiratory tract infection, M. pneumoniae can cause extrapulmonary manifestations. They occur in up to 25% of manifest M. pneumoniae infections and may affect almost every organ, including the skin as well as the hematologic, cardiovascular, musculoskeletal, and nervous system [8]. Encephalitis is one of the most common and severe complications [1]. M. pneumoniae infection is established in 5%–10% of pediatric encephalitis patients [9], [10], and up to 60% of them show neurologic sequelae [10], [11].
It is important to establish the cause of encephalitis at an early stage in order to specifically treat what can be treated and to avoid unnecessary treatment. The diagnosis of M. pneumoniae encephalitis is challenging. The current diagnostic algorithm of the “Consensus Statement of the International Encephalitis Consortium” [12] recommends for the diagnosis of M. pneumoniae infection in children with encephalitis (1) serology and polymerase chain reaction (PCR) from throat samples (routine studies), and if positive test results and/or respiratory symptoms are present, then (2) additionally PCR in cerebrospinal fluid (CSF) (conditional studies).
However, M. pneumoniae serology and PCR in the respiratory tract cannot discern between colonization and infection in a clinically relevant time frame [13]. The main reason for this is the relatively high prevalence of M. pneumoniae in the upper respiratory tract of healthy children (up to 56%) [13], [14]. The demonstrated positive serological results in such asymptomatic PCR-positive children (positive immunoglobulin (Ig) M in 17%, IgG in 24%, and IgA in 6% of 66 cases) [13] may simply reflect one or more previous encounters with M. pneumoniae and are not necessarily related to the presence of M. pneumoniae in the respiratory tract. It is clear that this may give rise to an overestimation of the M. pneumoniae-related disease burden. A more reliable diagnosis of M. pneumoniae infection may be achieved by using paired patient sera in order to detect seroconversion and/or a 4-fold increase in antibody titers in addition to PCR (Table 1; table references: [13], [15]–[24]). However, such procedures are time-consuming and are therefore neither practicable nor useful in an acutely ill patient.
Tab. 1. Overview of diagnostic tests for M. pneumoniae. 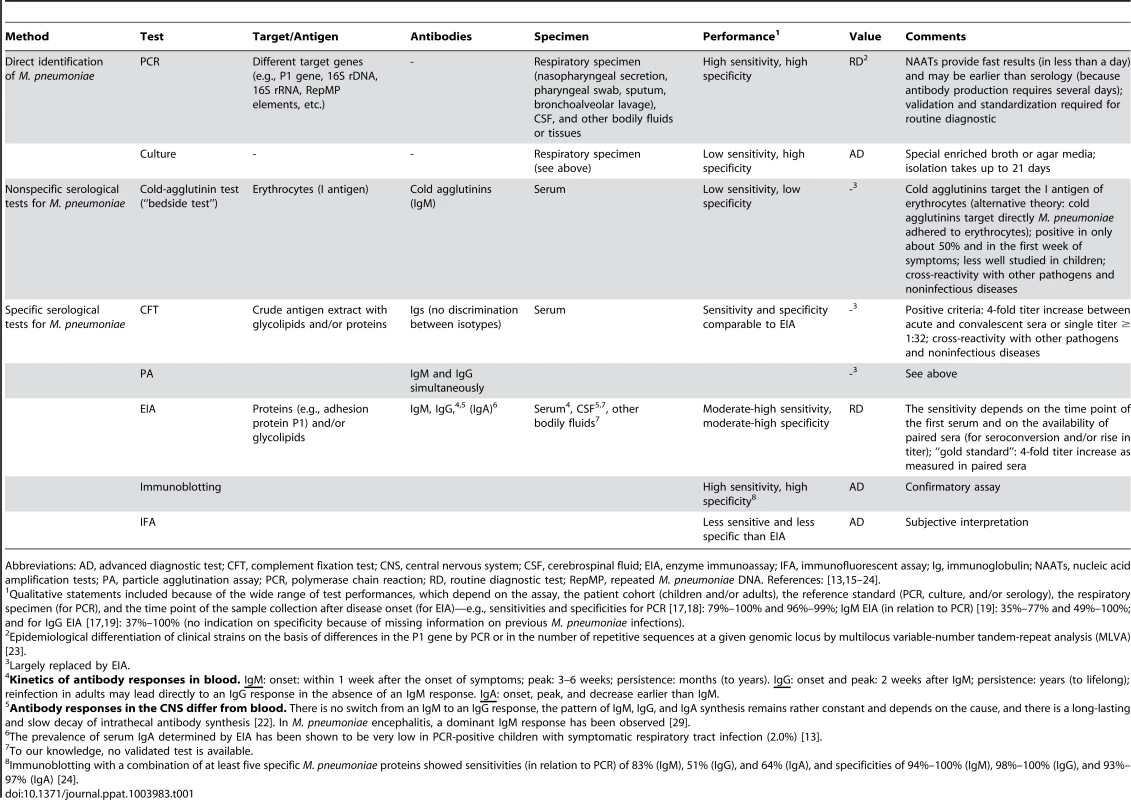
Abbreviations: AD, advanced diagnostic test; CFT, complement fixation test; CNS, central nervous system; CSF, cerebrospinal fluid; EIA, enzyme immunoassay; IFA, immunofluorescent assay; Ig, immunoglobulin; NAATs, nucleic acid amplification tests; PA, particle agglutination assay; PCR, polymerase chain reaction; RD, routine diagnostic test; RepMP, repeated M. pneumoniae DNA. References: [13], [15]–[24]. The detection rate of M. pneumoniae by PCR in the CSF of M. pneumoniae encephalitis patients is relatively low (0%–14%) [9], [10], [25], [26]. Moreover, various cases with M. pneumoniae encephalitis in which bacterial DNA could not be detected in the CSF had a more prolonged duration of respiratory symptoms before the onset of encephalitis (>5–7 days) [10], [25], [27]. These cases indicate that M. pneumoniae encephalitis may exemplify a postinfectious phenomenon that manifests after clearance of the bacteria from the CNS or respiratory tract by the immune system. The immune response to M. pneumoniae in the CNS or other sites may also contribute to the encephalitis (Figure 1; figure references: [1]).
Fig. 1. Proposed schematic pathomechanisms in M. pneumoniae encephalitis. 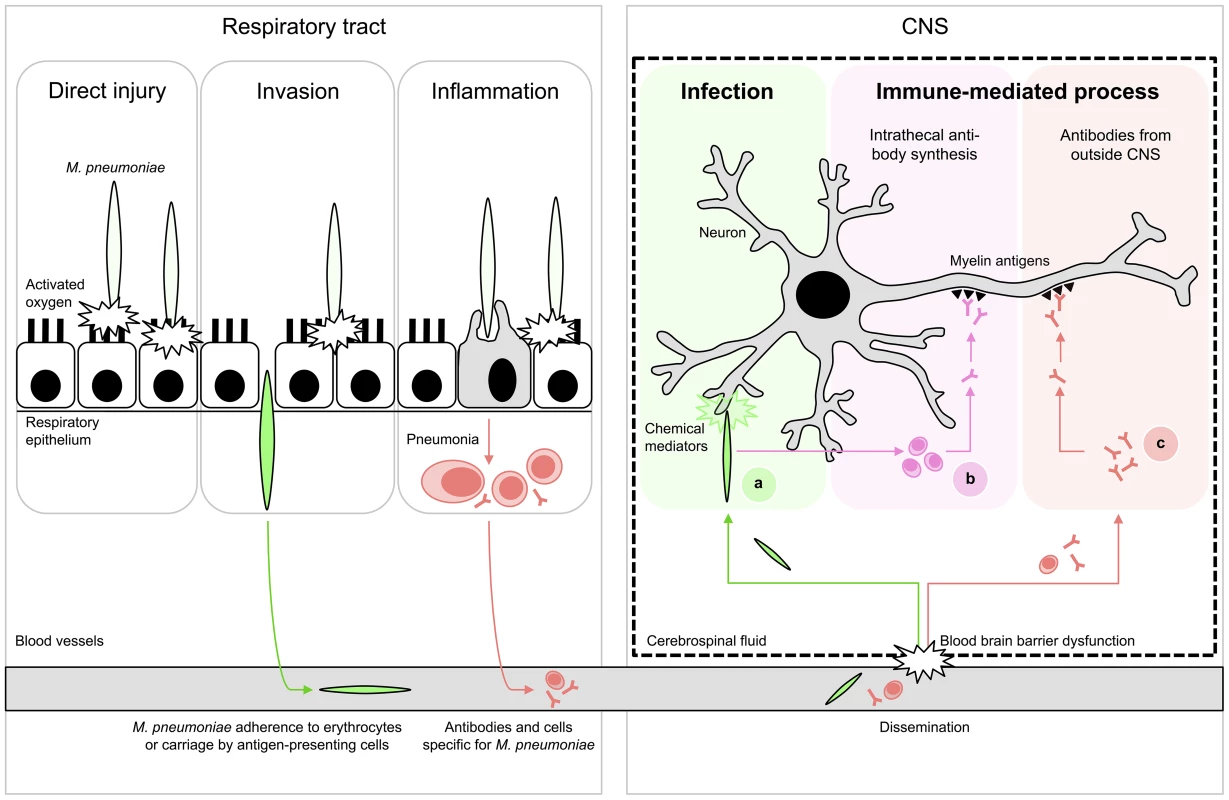
(Left) Respiratory tract infection. M. pneumoniae resides mostly extracellularly on epithelial surfaces. Its close association allows the production of direct injury by a variety of local cytotoxic effects. Furthermore, it can induce inflammatory responses, elicited by both adhesion proteins and glycolipid epitopes that result in pneumonia. (Right) Encephalitis. Extrapulmonary disease of the CNS is characterized by systemic dissemination with resultant direct infection and local tissue injury (A) or immune-mediated injury (B,C). The latter may occur as a result of cross-reactive antibodies against myelin components, e.g., gangliosides and galactocerebroside C. These antibodies could theoretically have originated from intrathecal synthesis (B) or from outside the CNS (C). Figure adapted from [1]; see references in the text. Interestingly, a promising diagnostic marker for M. pneumoniae encephalitis has recently emerged from a few case studies. In one study, intrathecal synthesis of antibodies to M. pneumoniae was reported in 14 cases of M. pneumoniae encephalitis (74%) [28]. The intrathecal production of antibodies is generally considered a highly specific marker for infection of the CNS [22]. All cases that underwent PCR testing (93%) indeed had a positive PCR targeting M. pneumoniae in the CSF [28] even though it has been recently demonstrated that intrathecal antibody responses to M. pneumoniae but not bacterial DNA can be present at the onset of clinical encephalitis [29]. In another study, it was reported that intrathecal antibodies to M. pneumoniae were found to cross-react with galactocerebroside C (GalC) in eight out of 21 (38%) of M. pneumoniae encephalitis cases [30]. All eight cases showed a negative PCR targeting M. pneumoniae in CSF. The cross-reactivity in these cases is likely induced by molecular mimicry between bacterial glycolipids and host myelin glycolipids, including GalC and gangliosides (Figure 2; figure references: [31]–[34]). Cross-reactive, anti-GalC antibodies have previously been detected in patients with Guillain-Barré syndrome (GBS) who suffered from a preceding M. pneumoniae infection [32], [35]–[38]. GBS is a typical postinfectious immune-mediated peripheral neuropathy [39]. In GBS, cross-reactive antibodies cause complement activation and formation of a membrane attack complex at the peripheral nerves, resulting in neuromuscular paralysis. Anti-GalC antibodies have been associated with demyelination in patients with GBS [35], [38]. Moreover, these anti-GalC antibodies cause neuropathy in rabbits that are immunized with GalC [40]. Such antibodies may also be involved in demyelination of central nerve cells in M. pneumoniae encephalitis, as a significant correlation was found between the presence of anti-GalC antibodies in the CSF and demyelination (p = 0.026) [30].
Fig. 2. Schematic structures responsible for molecular mimicry between M. pneumoniae and neuronal cells. 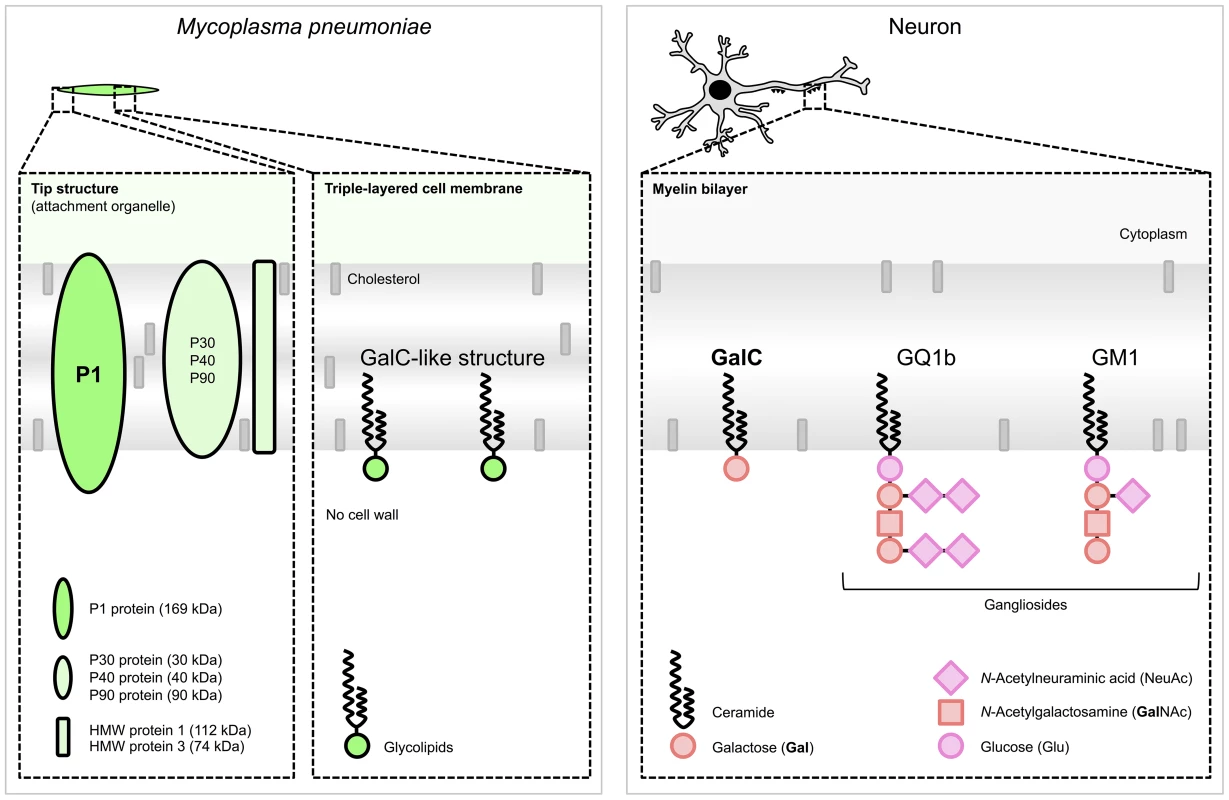
(Left) M. pneumoniae adhesion proteins and glycolipids. The immunogenic and major cytadherence proteins P1 and P30 are densely clustered at the tip structure. The P1 protein [31] and glycolipids, e.g., those forming a GalC-like structure [32], elicit cross-reactive antibodies induced by molecular mimicry. (Right) Host myelin glycolipids, to which antibodies were found in patients with M. pneumoniae encephalitis. Glycolipids are organized in specialized functional microdomains called “lipid rafts” and play a part in the maintenance of the cell membrane structure. Abbreviations: GalC, galactocerebroside C; GQ1b, ganglioside quadrosialo 1b; GM1, ganglioside monosialo 1 (the numbers stand for the order of migration on thin-layer chromatography, and the lower-case letters stand for variations within basic structures); HMW, high-molecular-weight. Structures of M. pneumoniae adhesion proteins and host glycolipids are adapted from [33] and [34], respectively. Anti-GalC antibodies have not only been detected in CSF but also in the serum of M. pneumoniae encephalitis patients [30], [36], [41]–[43], including rates from 13% (2/15) [30] to 100% (3/3) [41], respectively. It is possible that during inflammation the blood-brain barrier (BBB) can become permeable, which would thereby enable antibodies to cross the BBB and cause disease. As a consequence, the cross-reactive antibodies in the CSF of M. pneumoniae encephalitis patients do not necessarily have to be produced intrathecally (Figure 1).
M. pneumoniae infections may also be followed by the production of antibodies to gangliosides, both in patients with GBS and in those with encephalitis. In M. pneumoniae encephalitis, such antibodies were directed against GQ1b [44], [45] or GM1 [46] (Figure 2). Interestingly, anti-GQ1b antibodies are associated with a distinct and severe encephalitis variant, referred to as Bickerstaff brain stem encephalitis [47].
In conclusion, while PCR and serology may be of limited value in the diagnosis of M. pneumoniae encephalitis, the detection of intrathecal antibodies to M. pneumoniae, including cross-reactive antibodies against GalC and gangliosides, may be regarded as a promising new diagnostic tool.
The routine diagnostic workup of M. pneumoniae encephalitis should therefore aim for the detection of M. pneumoniae antibodies in both CSF and serum, in addition to M. pneumoniae PCR in CSF. Intrathecal antibodies can be detected by widely accessible enzyme immunoassays (EIAs) or immunoblotting (Table 1), while intrathecal antibody synthesis can be established either by calculation of an antibody index [22] or through parallel immunoblotting of simultaneously collected CSF and serum samples [48], [49]. Antiganglioside antibodies can be detected routinely by some specialized laboratories, but their detection together with cross-reactive antibodies against GalC primarily serve scientific purposes and may help to clarify M. pneumoniae antibodies' immune target(s). Furthermore, their hypothesized role in the pathogenesis might provide a basis for immunomodulatory treatment in M. pneumoniae encephalitis.
Zdroje
1. NaritaM (2009) Pathogenesis of neurologic manifestations of Mycoplasma pneumoniae infection. Pediatr Neurol 41 : 159–166.
2. FoyHM (1993) Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients. Clin Infect Dis 17 Suppl 1S37–S46.
3. LindK, BenzonMW, JensenJS, ClydeWAJr (1997) A seroepidemiological study of Mycoplasma pneumoniae infections in Denmark over the 50-year period 1946–1995. Eur J Epidemiol 13 : 581–586.
4. MichelowIC, OlsenK, LozanoJ, RollinsNK, DuffyLB, et al. (2004) Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics 113 : 701–707.
5. BaerG, EngelckeG, Abele-HornM, SchaadUB, HeiningerU (2003) Role of Chlamydia pneumoniae and Mycoplasma pneumoniae as causative agents of community-acquired pneumonia in hospitalised children and adolescents. Eur J Clin Microbiol Infect Dis 22 : 742–745.
6. PrincipiN, EspositoS, BlasiF, AllegraL (2001) Mowgli study group (2001) Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin Infect Dis 32 : 1281–1289.
7. JuvenT, MertsolaJ, WarisM, LeinonenM, MeurmanO, et al. (2000) Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J 19 : 293–298.
8. NaritaM (2010) Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother 16 : 162–169.
9. ChristieLJ, HonarmandS, TalkingtonDF, GavaliSS, PreasC, et al. (2007) Pediatric encephalitis: what is the role of Mycoplasma pneumoniae? Pediatrics 120 : 305–313.
10. BitnunA, Ford-JonesEL, PetricM, MacGregorD, HeurterH, et al. (2001) Acute childhood encephalitis and Mycoplasma pneumoniae. Clin Infect Dis 32 : 1674–1684.
11. BitnunA, Ford-JonesE, BlaserS, RichardsonS (2003) Mycoplasma pneumoniae encephalitis. Semin Pediatr Infect Dis 14 : 96–107.
12. VenkatesanA, TunkelAR, BlochKC, LauringAS, SejvarJ, et al. (2013) Case Definitions, Diagnostic Algorithms, and Priorities in Encephalitis: Consensus Statement of the International Encephalitis Consortium. Clin Infect Dis 57 : 1114–1128.
13. SpuesensEB, FraaijPL, VisserEG, HoogenboezemT, HopWC, et al. (2013) Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med 10: e1001444.
14. WoodPR, HillVL, BurksML, PetersJI, SinghH, et al. (2013) Mycoplasma pneumoniae in children with acute and refractory asthma. Ann Allergy Asthma Immunol 110 : 328–334.e1.
15. JacobsE (1993) Serological diagnosis of Mycoplasma pneumoniae infections: a critical review of current procedures. Clin Infect Dis 17 Suppl 1S79–S82.
16. Waites KB, Talkington DF (2004) Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 17: : 697–728, table of contents.
17. LoensK, GoossensH, IevenM (2010) Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods. Eur J Clin Microbiol Infect Dis 29 : 1055–1069.
18. NadalD, BossartW, ZucolF, SteinerF, BergerC, et al. (2001) Community-acquired pneumonia in children due to Mycoplasma pneumoniae: diagnostic performance of a seminested 16S rDNA-PCR. Diagn Microbiol Infect Dis 39 : 15–19.
19. BeersmaMF, DirvenK, van DamAP, TempletonKE, ClaasEC, et al. (2005) Evaluation of 12 commercial tests and the complement fixation test for Mycoplasma pneumoniae-specific immunoglobulin G (IgG) and IgM antibodies, with PCR used as the "gold standard". J Clin Microbiol 43 : 2277–2285.
20. OzakiT, NishimuraN, AhnJ, WatanabeN, MutoT, et al. (2007) Utility of a rapid diagnosis kit for Mycoplasma pneumoniae pneumonia in children, and the antimicrobial susceptibility of the isolates. J Infect Chemother 13 : 204–207.
21. GavranichJB, ChangAB (2005) Antibiotics for community acquired lower respiratory tract infections (LRTI) secondary to Mycoplasma pneumoniae in children. Cochrane Database Syst Rev 2005: CD004875.
22. ReiberH (1994) Flow rate of cerebrospinal fluid (CSF) — a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci 122 : 189–203.
23. JacobsE (2012) Mycoplasma pneumoniae: now in the focus of clinicians and epidemiologists. Euro Surveill 17 : 1–3.
24. DumkeR, StrubelA, CyncynatusC, NuyttensH, HerrmannR, et al. (2012) Optimized serodiagnosis of Mycoplasma pneumoniae infections. Diagn Microbiol Infect Dis 73 : 200–203.
25. DaxboeckF, BlackyA, SeidlR, KrauseR, AssadianO (2004) Diagnosis, treatment, and prognosis of Mycoplasma pneumoniae childhood encephalitis: systematic review of 58 cases. J Child Neurol 19 : 865–871.
26. DomenechC, LevequeN, LinaB, NajioullahF, FloretD (2009) Role of Mycoplasma pneumoniae in pediatric encephalitis. Eur J Clin Microbiol Infect Dis 28 : 91–94.
27. NaritaM, YamadaS (2001) Two distinct patterns of central nervous system complications due to Mycoplasma pneumoniae infection. Clin Infect Dis 33 : 916–917.
28. BencinaD, DovcP, Mueller-PremruM, Avsic-ZupancT, SocanM, et al. (2000) Intrathecal synthesis of specific antibodies in patients with invasion of the central nervous system by Mycoplasma pneumoniae. Eur J Clin Microbiol Infect Dis 19 : 521–530.
29. Meyer SauteurPM, RellyC, HackenbergA, StahrN, BergerC, et al. (2014) Mycoplasma pneumoniae Intrathecal Antibody Responses in Bickerstaff Brain Stem Encephalitis. Neuropediatrics 45 : 61–63.
30. ChristieLJ, HonarmandS, YagiS, RuizS, GlaserCA (2007) Anti-galactocerebroside testing in Mycoplasma pneumoniae-associated encephalitis. J Neuroimmunol 189 : 129–131.
31. JacobsE, BartlA, OberleK, SchiltzE (1995) Molecular mimicry by Mycoplasma pneumoniae to evade the induction of adherence inhibiting antibodies. J Med Microbiol 43 : 422–429.
32. KusunokiS, ShiinaM, KanazawaI (2001) Anti-Gal-C antibodies in GBS subsequent to mycoplasma infection: evidence of molecular mimicry. Neurology 57 : 736–738.
33. RottemS (2003) Interaction of mycoplasmas with host cells. Physiol Rev 83 : 417–432.
34. WillisonHJ, YukiN (2002) Peripheral neuropathies and anti-glycolipid antibodies. Brain 125 : 2591–2625.
35. AngCW, Tio-GillenAP, GroenJ, HerbrinkP, JacobsBC, et al. (2002) Cross-reactive anti-galactocerebroside antibodies and Mycoplasma pneumoniae infections in Guillain-Barré syndrome. J Neuroimmunol 130 : 179–183.
36. SusukiK, OdakaM, MoriM, HirataK, YukiN (2004) Acute motor axonal neuropathy after Mycoplasma infection: Evidence of molecular mimicry. Neurology 62 : 949–956.
37. ArakawaH, YuharaY, TodokoroM, KatoM, MochizukiH, et al. (2005) Immunoadsorption therapy for a child with Guillain-Barré syndrome subsequent to Mycoplasma infection: a case study. Brain Dev 27 : 431–433.
38. SamukawaM, HamadaY, KuwaharaM, TakadaK, HiranoM, et al. (2013) Clinical features in Guillain-Barré syndrome with anti-Gal-C antibody. J Neurol Sci 337 : 55–60.
39. van DoornPA, RutsL, JacobsBC (2008) Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol 7 : 939–950.
40. SaidaT, SaidaK, DorfmanSH, SilberbergDH, SumnerAJ, et al. (1979) Experimental allergic neuritis induced by sensitization with galactocerebroside. Science 204 : 1103–1106.
41. NishimuraM, SaidaT, KurokiS, KawabataT, ObayashiH, et al. (1996) Post-infectious encephalitis with anti-galactocerebroside antibody subsequent to Mycoplasma pneumoniae infection. J Neurol Sci 140 : 91–95.
42. KumadaS, KusakaH, OkaniwaM, KobayashiO, KusunokiS (1997) Encephalomyelitis subsequent to mycoplasma infection with elevated serum anti-Gal C antibody. Pediatr Neurol 16 : 241–244.
43. SugenoN, KawaguchiN, HasegawaT, KurodaT, NakashimaI, et al. (2012) A case with anti-galactocerebroside antibody-positive Mycoplasma pneumoniae meningoencephalitis presenting secondary hypersomnia. Neurol Sci 33 : 1473–1476.
44. KikuchiM, TagawaY, IwamotoH, HoshinoH, YukiN (1997) Bickerstaff's brainstem encephalitis associated with IgG anti-GQ1b antibody subsequent to Mycoplasma pneumoniae infection: favorable response to immunoadsorption therapy. J Child Neurol 12 : 403–405.
45. SteerAC, StarrM, KornbergAJ (2006) Bickerstaff brainstem encephalitis associated with Mycoplasma pneumoniae infection. J Child Neurol 21 : 533–534.
46. FuscoC, BoniniE, SonciniG, FrattiniD, GiovanniniS, et al. (2010) Transient basal ganglia and thalamic involvement following Mycoplasma pneumoniae infection associated with antiganglioside antibodies. J Child Neurol 25 : 1029–1033.
47. OdakaM, YukiN, YamadaM, KogaM, TakemiT, et al. (2003) Bickerstaff's brainstem encephalitis: clinical features of 62 cases and a subgroup associated with Guillain-Barré syndrome. Brain 126 : 2279–2290.
48. MonteyneP, AlbertF, WeissbrichB, ZardiniE, CiardiM, et al. (1997) The detection of intrathecal synthesis of anti-herpes simplex IgG antibodies: comparison between an antigen-mediated immunoblotting technique and antibody index calculations. European Union Concerted Action on Virus Meningitis and Encephalitis. J Med Virol 53 : 324–331.
49. GranerodJ, CunninghamR, ZuckermanM, MuttonK, DaviesNW, et al. (2010) Causality in acute encephalitis: defining aetiologies. Epidemiol Infect 138 : 783–800.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA SplicingČlánek Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance GenesČlánek The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- BdlA, DipA and Induced Dispersion Contribute to Acute Virulence and Chronic Persistence of
- Morphotype Transition and Sexual Reproduction Are Genetically Associated in a Ubiquitous Environmental Pathogen
- A Nucleic-Acid Hydrolyzing Single Chain Antibody Confers Resistance to DNA Virus Infection in HeLa Cells and C57BL/6 Mice
- HopW1 from Disrupts the Actin Cytoskeleton to Promote Virulence in Arabidopsis
- Ly6C Monocytes Become Alternatively Activated Macrophages in Schistosome Granulomas with Help from CD4+ Cells
- Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA Splicing
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- Antibody Responses to : Role in Pathogenesis and Diagnosis of Encephalitis?
- Discovery of a Novel Compound with Anti-Venezuelan Equine Encephalitis Virus Activity That Targets the Nonstructural Protein 2
- Activation of Focal Adhesion Kinase by Suppresses Autophagy via an Akt/mTOR Signaling Pathway and Promotes Bacterial Survival in Macrophages
- Crossing the Interspecies Barrier: Opening the Door to Zoonotic Pathogens
- Catching Fire: , Macrophages, and Pyroptosis
- IscR Is Essential for Type III Secretion and Virulence
- Selective Chemical Inhibition of Quorum Sensing in Promotes Host Defense with Minimal Impact on Resistance
- The Glycosylated Rv1860 Protein of Inhibits Dendritic Cell Mediated TH1 and TH17 Polarization of T Cells and Abrogates Protective Immunity Conferred by BCG
- A Genome-Wide Tethering Screen Reveals Novel Potential Post-Transcriptional Regulators in
- Structural Insights into SraP-Mediated Adhesion to Host Cells
- Human IGF1 Regulates Midgut Oxidative Stress and Epithelial Homeostasis to Balance Lifespan and resistance in
- Cycling Empirical Antibiotic Therapy in Hospitals: Meta-Analysis and Models
- Rab11 Regulates Trafficking of -sialidase to the Plasma Membrane through the Contractile Vacuole Complex of
- Mitogen and Stress Activated Kinases Act Co-operatively with CREB during the Induction of Human Cytomegalovirus Immediate-Early Gene Expression from Latency
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- A Central Role for Carbon-Overflow Pathways in the Modulation of Bacterial Cell Death
- An Invertebrate Warburg Effect: A Shrimp Virus Achieves Successful Replication by Altering the Host Metabolome via the PI3K-Akt-mTOR Pathway
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
- A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees () Prevails after -Mediated, or , Transmission
- Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance Genes
- Ubiquitin-Mediated Response to Microsporidia and Virus Infection in
- Preclinical Detection of Variant CJD and BSE Prions in Blood
- Toll-Like Receptor 8 Agonist and Bacteria Trigger Potent Activation of Innate Immune Cells in Human Liver
- Progressive Proximal-to-Distal Reduction in Expression of the Tight Junction Complex in Colonic Epithelium of Virally-Suppressed HIV+ Individuals
- The Triggering Receptor Expressed on Myeloid Cells 2 Inhibits Complement Component 1q Effector Mechanisms and Exerts Detrimental Effects during Pneumococcal Pneumonia
- Differential Activation of Acid Sphingomyelinase and Ceramide Release Determines Invasiveness of into Brain Endothelial Cells
- Forward Genetic Screening Identifies a Small Molecule That Blocks Growth by Inhibiting Both Host- and Parasite-Encoded Kinases
- Defining Immune Engagement Thresholds for Control of Virus-Driven Lymphoproliferation
- Growth Factor and Th2 Cytokine Signaling Pathways Converge at STAT6 to Promote Arginase Expression in Progressive Experimental Visceral Leishmaniasis
- Multimeric Assembly of Host-Pathogen Adhesion Complexes Involved in Apicomplexan Invasion
- Biogenesis of Influenza A Virus Hemagglutinin Cross-Protective Stem Epitopes
- Adequate Th2-Type Response Associates with Restricted Bacterial Growth in Latent Mycobacterial Infection of Zebrafish
- Protective Efficacy of Passive Immunization with Monoclonal Antibodies in Animal Models of H5N1 Highly Pathogenic Avian Influenza Virus Infection
- Fructose-Asparagine Is a Primary Nutrient during Growth of in the Inflamed Intestine
- The Calcium-Dependent Protein Kinase 3 of Influences Basal Calcium Levels and Functions beyond Egress as Revealed by Quantitative Phosphoproteome Analysis
- A Translocated Effector Required for Dissemination from Derma to Blood Safeguards Migratory Host Cells from Damage by Co-translocated Effectors
- Functional Characterization of a Novel Family of Acetylcholine-Gated Chloride Channels in
- Both α2,3- and α2,6-Linked Sialic Acids on O-Linked Glycoproteins Act as Functional Receptors for Porcine Sapovirus
- The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
- MicroRNA-146a Provides Feedback Regulation of Lyme Arthritis but Not Carditis during Infection with
- Recombination in Enteroviruses Is a Biphasic Replicative Process Involving the Generation of Greater-than Genome Length ‘Imprecise’ Intermediates
- Cytoplasmic Viral RNA-Dependent RNA Polymerase Disrupts the Intracellular Splicing Machinery by Entering the Nucleus and Interfering with Prp8
- and Are Associated with Murine Susceptibility to Infection and Human Sepsis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



