-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
Reklamaand Are Associated with Murine Susceptibility to Infection and Human Sepsis
Staphylococcus aureus causes life-threatening infections in humans. Host genetic determinants influence the outcome of S. aureus infection, yet are poorly understood. Susceptible A/J and resistant C57BL/6J mice provide a unique platform to study the genetic difference responsible for variable host response to S. aureus infection. We showed that chromosome 11 in A/J was responsible for susceptibility to S. aureus. We further identified a QTL locus on Chromosome 11 significantly associated with S. aureus susceptibility. Five genes in the QTL (Dcaf7, Dusp3, Fam134c, Psme3, and Slc4a1) were significantly differently expressed in a) susceptible vs. resistant mice, and b) humans with S. aureus blood stream infection vs. healthy human subjects. Three genes (Dusp3, Psme3, and Dcaf7) were down-regulated in susceptible A/J mice. siRNA-mediated knockdown of Dusp3 and Psme3 in bone marrow derived macrophage (BMDMs) significantly enhanced cytokine responses through NF-κB activity upon S. aureus challenge in a pattern that was also present in S. aureus-challenged BMDMs from susceptible CSS11 (chr. 11 from A/J but otherwise C57BL/6J) mice, but not resistant C57BL/6J mice. These findings suggest that Dusp3 and Psme3 contribute to S. aureus infection susceptibility in A/J mice and play a role in human S. aureus infection.
Published in the journal: . PLoS Pathog 10(6): e32767. doi:10.1371/journal.ppat.1004149
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004149Summary
Staphylococcus aureus causes life-threatening infections in humans. Host genetic determinants influence the outcome of S. aureus infection, yet are poorly understood. Susceptible A/J and resistant C57BL/6J mice provide a unique platform to study the genetic difference responsible for variable host response to S. aureus infection. We showed that chromosome 11 in A/J was responsible for susceptibility to S. aureus. We further identified a QTL locus on Chromosome 11 significantly associated with S. aureus susceptibility. Five genes in the QTL (Dcaf7, Dusp3, Fam134c, Psme3, and Slc4a1) were significantly differently expressed in a) susceptible vs. resistant mice, and b) humans with S. aureus blood stream infection vs. healthy human subjects. Three genes (Dusp3, Psme3, and Dcaf7) were down-regulated in susceptible A/J mice. siRNA-mediated knockdown of Dusp3 and Psme3 in bone marrow derived macrophage (BMDMs) significantly enhanced cytokine responses through NF-κB activity upon S. aureus challenge in a pattern that was also present in S. aureus-challenged BMDMs from susceptible CSS11 (chr. 11 from A/J but otherwise C57BL/6J) mice, but not resistant C57BL/6J mice. These findings suggest that Dusp3 and Psme3 contribute to S. aureus infection susceptibility in A/J mice and play a role in human S. aureus infection.
Introduction
Staphylococcus aureus is an important cause of potentially lethal human infections [1]–[3]. It is generally accepted that host genetic variation influences susceptibility to S. aureus colonization and infection [4], [5].
A significant body of evidence supports the importance of human genetic variation on host susceptibility to a variety of infectious diseases. For example, TNF gene SNP rs1800629 is strongly associated with susceptibility to severe sepsis in the Chinese Han population [6], while genetic variants in TRAF6 are significantly associated with susceptibility to sepsis-induced acute lung injury [7]. In addition, a genetic variant of β2-adrenocepter gene increases susceptibility to bacterial meningitis [8], while genetic variations in Toll-like receptors have been linked with both infectious and autoimmune diseases [9]. More interestingly, genetic variation of IL17A gene is associated with altered susceptibility to Gram-positive infection and mortality of severe sepsis [10].
Far less is known about the specific genes associated with host susceptibility to S. aureus. Our group [11], [12] and others [5], [13] have shown that different inbred mouse strains exhibit variable susceptibility to S. aureus infection. For example, A/J is highly susceptible to S. aureus infection, whereas C57BL/6J is resistant [5]. These susceptible and resistant strains provide an attractive approach to investigate the host genetic determinants of susceptibility to S. aureus infection.
Using A/J donor to C57BL/6J host chromosomal substitution strains (CSS) we recently discovered that chromosomes 8, 11, and 18 from A/J account for its high susceptibility to S. aureus infection [11]. However, the genes on chromosome 11 that influence susceptibility to S. aureus remain unknown.
In the present investigation, we used a multi-step selection process to identify genes on A/J chromosome 11 contributing to susceptibility to S. aureus infection. Because human and murine response to sepsis can differ significantly [14], we used whole blood gene expression data from a cohort of patients with S. aureus blood stream infection (BSI) to verify the potential biological relevance of all candidate genes identified in mice. Genes shown to be involved in host response to S. aureus in both mice and humans were evaluated for biological function. Using this cross-species validation approach, we identified Dusp3 and Psme3 as relevant in both human and murine inflammatory response to S. aureus infection, and demonstrated these genes were involved in NF-κB signaling.
Results
Susceptibility to S. aureus localizes to A/J Chromosome 11
In the peritoneal S. aureus sepsis experiment, C57BL/6J, A/J and Chromosomal Substitution Strain 11 (CSS11) (A/J chromosome 11 in C57BL6/J background) mice were infected with S. aureus by an intraperitoneal (IP) route. Survival was observed for five days. C57BL/6J mice were resistant to S. aureus sepsis (median survival >5 days), while CSS11 mice demonstrated a susceptible phenotype (median survival <2 days) (Figure 1A) (p<0.05). In the intravenous S. aureus sepsis experiment, C57BL/6J, A/J and CSS11 mice were infected by direct inoculation of S. aureus by tail-vein route. Survival was observed every 6 hours, both A/J and CSS11 were susceptible to S. aureus infection (median survival <24 hours, p<0.05) as compared with C57BL/6J (Figure 1B).
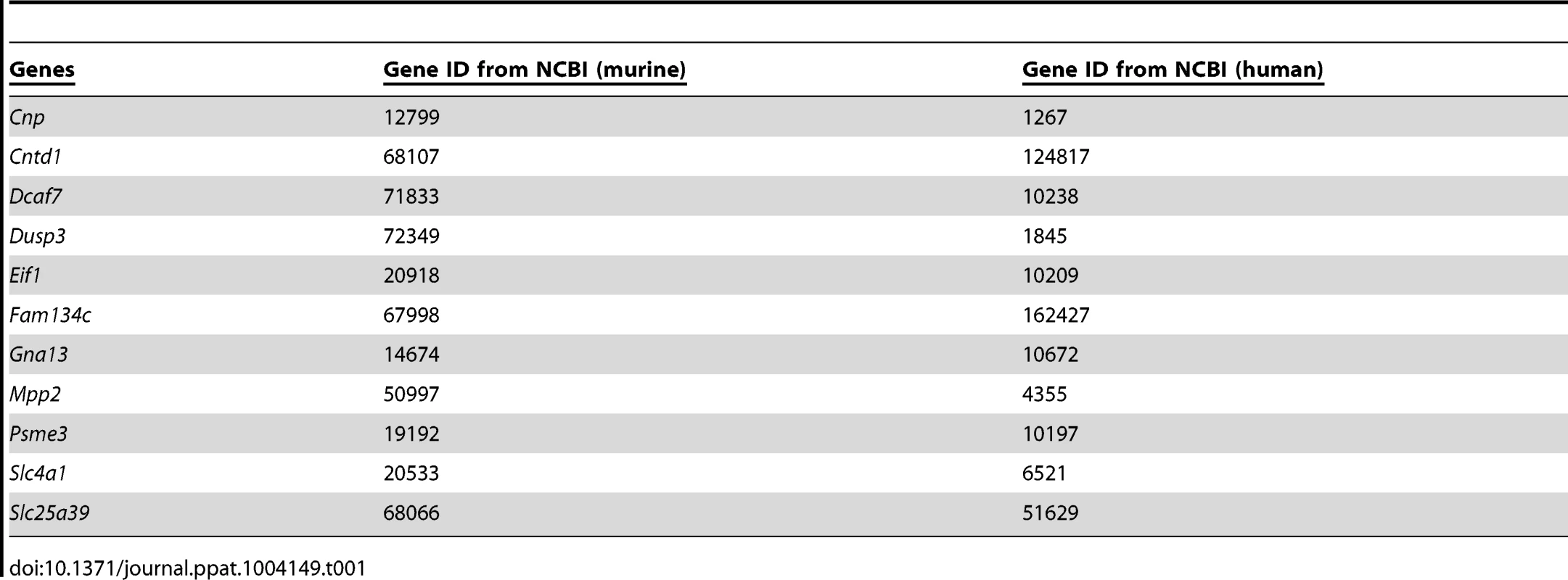
Fig. 1. Chromosome substitution strain 11 was susceptible to S. aureus infection, and QTL mapping found eleven putative candidate genes on Chr11. 
(A) CSS11 was susceptible to S. aureus peritoneal sepsis. C57BL/6J, A/J, or CSS11 mice were injected (i.p.) with S. aureus Sanger 476 at 107 CFU/g (n = 10 for each strain). Comparison of survival curves was performed by Mann-Whitney u test. The difference between C57BL/6J and CSS11 mice was significant (p<0.05). (B) CSS11 was susceptible to S. aureus intravenous sepsis. C57BL/6J, A/J, or CSS11 were intravenous injected with S. aureus Sanger 476 at 2×106 CFU/g (n = 10 for each strain). Comparison of survival curves was performed by Mann-Whitney u test. The difference between C57BL/6J and CSS11 mice was significant (p<0.05). (C) Bacterial load in kidneys were significantly higher in CSS11 mice after S. aureus injection. C57BL/6J, A/J and CSS11 were injected (i.p.) with S. aureus Sanger 476 at 107 CFU/g and euthanized 24 hours post infection (n = 10 for each group). The bacterial load in CSS11 kidneys were significantly higher than C57BL/6J (2.0±1.32×106 CFU/ml vs 200±158 CFU/ml, p<0.0001). (D) Chromosome 11 LOD score plot for susceptibility to S. aureus in F2 intercross mice (F1 [C11A]×F1 [C11A]). Six to eight-week-old intercross mice were injected i.p. with 107 CFU/g S. aureus Sanger 476 and observed every 8 hours continuously for 5 days. Thresholds for significant (p = 0.05) and suggestive (p = 0.63) linkage are indicated by the horizontal dashed lines. LOD score was determined by the J/qtl permutation test using 1,000 permuted data sets. The microsatellite markers for determining genotypes of F2 intercross mice are marked along the X-axis. The differentially expressed genes are indicated. Genes identified within significant or suggestive QTL were indicated with *** or *, respectively. Footnote: Figure 1A and 1C have been presented in Ahn S, et al., 2010 PLoS Pathogens, e1001088. The kidney bacterial load in CSS11 was also significantly higher after S. aureus challenge as compared with C57BL/6J (2.0±1.32×106 CFU/ml vs 200±158 CFU/ml, p<0.0001) (Figure 1C), indicating the higher mortality of CSS11 strain was associated with higher S. aureus burden in tissue.
C57BL/6J, A/J, and CSS11 mice were also infected with Escherichia coli by IP injection. Survival was observed every 6 hours. Both A/J and CSS11 mice were susceptible to E. coli sepsis as compared with C57BL/6J (median survival <2 days; p<0.05) (Figure S1).
Quantitative Trait Loci (QTL) mapping identifies locus on chromosome 11 linked to susceptibility to S. aureus
To localize the determinants on A/J chromosome 11 that are responsible for susceptibility to S. aureus infection, QTL analysis was performed. Previously, we established that the traits conferring susceptibility to S. aureus on A/J chromosome 11 were transmitted in an autosomal recessive fashion [11]. Thus, a total of 208 F2 intercross mice were generated by mating F1 (C11A) to F1 (C11A) and infected with methicillin susceptible S. aureus strain Sanger 476. Survival times were analyzed by J/qtl software (Jackson Labs). One significant QTL region containing 422 genes and located between 97006867 and 110713165 was significantly linked to susceptibility to S. aureus infection (Figure 1D).
Microarray gene expression data identifies genes within the QTL region that are associated with susceptibility to S. aureus infection
Next, we employed the previous murine microarray expression data to further identify candidate genes located within the identified QTL region [11] (Figure 2). Genes within the identified QTL region that were differentially expressed between susceptible A/J and resistant C57BL/6J at all pre-infection and post-infection time points were considered to be putative candidate genes. A total of 11 genes met these criteria: Eif1, Cnp, Fam134c, Cntd1, Psme3, Dusp3, Mpp2, Slc4a1, Slc25a39, Dcaf7, and Gna13 (Figure 1D). The accession numbers for each gene was listed (Table 1).
Fig. 2. Overall strategy for identifying genes associated with S. aureus susceptibility on chromosome 11 of A/J mice. 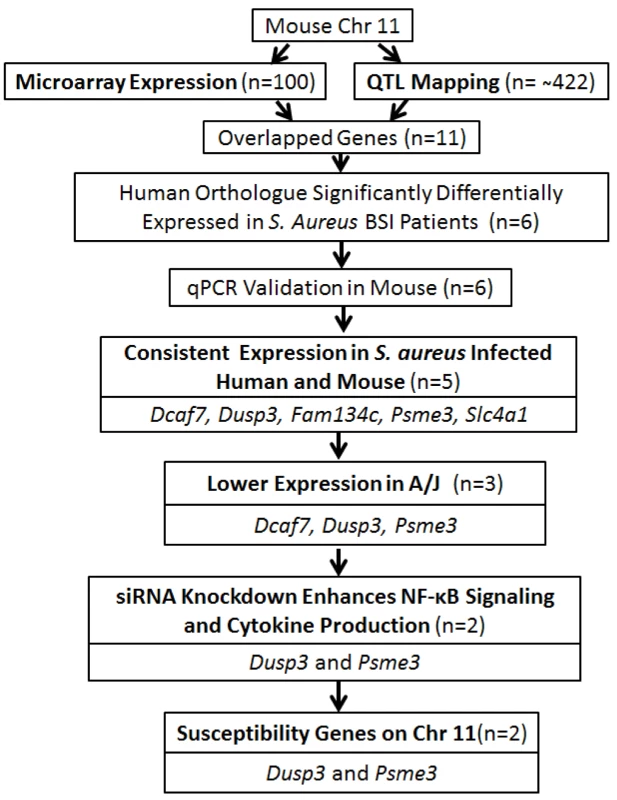
Flow chart of the strategy for identifying S. aureus susceptible genes on chromosome 11 of A/J mice. Consistent expression pattern between mouse candidate genes and human orthologues
To further evaluate the relevance of our candidate genes in human staphylococcal disease, we next used expression data from patients with S. aureus BSI (n = 32) or Escherichia coli BSI (n = 19) to evaluate the potential clinical relevance of our identified candidate genes. Among the 11 putative genes identified in the murine model, six were found to have human orthologues that exhibited significantly different levels of expression in patients with S. aureus BSI as compared to healthy subjects with no infection (n = 43): Dcaf7 (0.85fold; p = 0.003), Dusp3 (1.73 fold; p<0.0001), Fam134c (0.75fold; p<0.0001), Psme3 (0.78fold; p<0.0001), Mpp2 (1.21fold; p = 0.004), and Slc4a1 (0.81fold; p = 0.012) (Figure 3A). The gene expression patterns for the six genes were also significantly different among the patients with E. coli BSI vs. healthy subjects (Figure 3A).
Fig. 3. Human orthologues of six candidate genes were significantly differentially expressed between patients with S. aureus blood stream infection (BSI) and healthy subjects, and five of these genes (Dcaf7, Dusp3, Fam134c, Psme3, and Slc4a1) demonstrated consistent uninfected vs infected expression patterns between mouse and human. 
(A) Human orthologues of six candidate genes (Dcaf7, Dusp3, Fam134c, Psme3, Slc4a1, and Mpp2) were significantly differentially expressed between patients with S. aureus BSI and healthy subjects by microarray. Human blood RNA from patients with S. aureus BSI (n = 32) and healthy subjects with no infection (n = 43) were extracted and analyzed and applied to microarray. The expression of Dusp3(1.73 fold; p<0.0001) and Mpp2 (1.21fold; p = 0.004) were significantly higher in S. aureus BSI patients as compared with healthy controls, and the expression of Dcaf7(0.85fold; p = 0.003), Fam134c(0.75fold; p<0.0001), Psme3(0.78fold; p<0.0001), and Slc4a1(0.81fold; p = 0.012) were significantly lower in S. aureus BSI patients. Six genes showed similar significant expression changes in E. coli BSI (n = 19) patients as compared with healthy subjects (n = 43) (B) Quantitative-PCR validation of the six genes identified five candidate genes (Dcaf7, Dusp3, Fam134c, Psme3 and Slc4a) with consistent uninfected vs infected expression patterns between mouse and human. Dcaf7 (0.83fold), Dusp3(1.31fold), Fam134c (0.65fold), Psme3 (0.86fold), and Slc4a1(0.85fold). Both eight-week-old male A/J and C57BL/6J mice were i.p. injected with S. aureus Sanger 476 at 107 CFU/g (n = 6 for each strain), at two hours post infection all mice were sacrificed by CO2 inhalation and whole blood were obtained through cardiac puncture. Blood RNA were extracted by QIAGEN RNeasy Protect Animal Blood Kit, and then subjected to reverse-transcription PCR and SYBR-green quantitative-PCR. The expression of all target genes were normalized to 18s rRNA. (C) Scatter plot of fold changes of the five candidate genes showed a consistent pattern between mouse and human. For human data analysis, the expression level of the non-infection healthy controls was set to 1, and the expression level of S. aureus BSI patients was normalized to the non-infection level to get the fold change. For mouse data, the fold changes were average between A/J and C57BL/6J mice. Using qPCR on the murine samples, five of the 6 genes identified by both human and murine gene expression also exhibited consistent expression changes under infectious vs. non-infectious conditions : Dcaf7 (0.83fold), Dusp3 (1.31fold), Fam134c (0.65fold), Psme3 (0.86fold), and Slc4a1(0.85fold) (Figure 3B). The expression patterns for these five genes were highly consistent between S. aureus-infected mice and S. aureus-infected humans (Figure 3C). The expression patterns of these five genes also remained consistent when mice were infected with E. coli instead of S. aureus (Figure S2). qPCR primers for candidate genes were listed (Table S1).
qPCR of candidate genes
Using qPCR in susceptible (A/J) vs resistant (C57BL/6J) mice at baseline (0 hr), three of the five genes demonstrated significantly lower expression: Dcaf7 (0.81 fold; p<0.05), Dusp3 (0.27 fold; p<0.01), and Psme3 (0.83 fold; p<0.05), while two genes exhibited significantly higher expression: Fam134c (1.82 fold; p<0.01), Slc4a1 (1.31fold; p<0.05) (Figure 4A). The baseline difference in expression between susceptible (A/J) and resistant (C57BL/6J) mice for all the five genes remained unchanged at 2 hr post-infection of S. aureus (Figure 4A).
Fig. 4. Down-regulation of Dusp3 and Psme3 in A/J are responsible for increased NF-κB signaling activity. 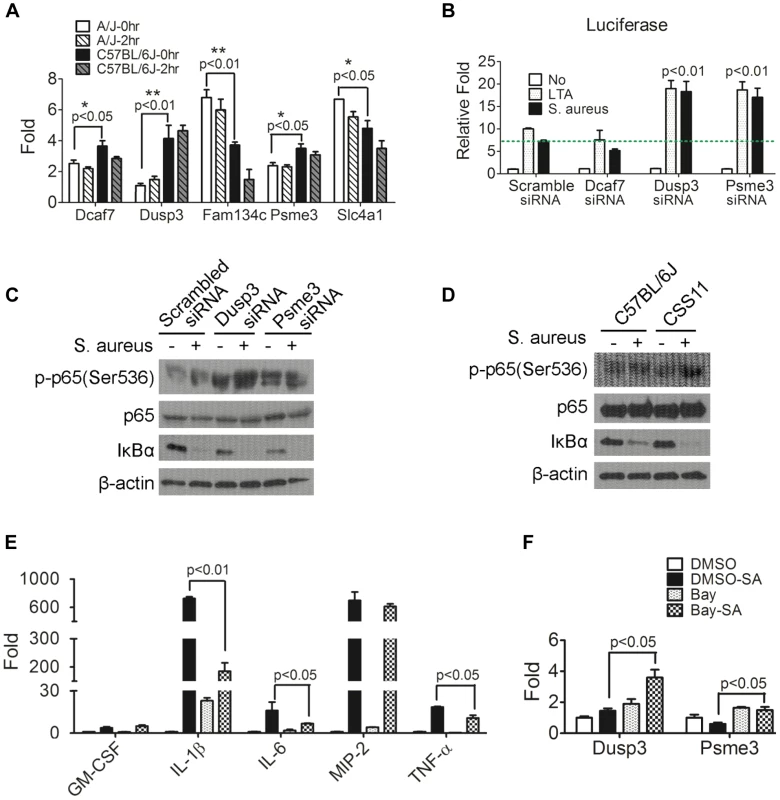
(A) The expression of Dcaf7, Dusp3, and Psme3 in A/J was significantly lower than C57BL/6J under both non-infected and S. aureus infected conditions. Eight-week-old male A/J and C57BL/6J mice (n = 6) were challenged (i.p.) by S. aureus Sanger 476 at 107 CFU/g or DPBS. At two hours post-infection whole blood RNAs was extracted by RNeasy followed by RT-PCR and qPCR. Dcaf7 (0.81 fold; p<0.05), Dusp3 (0.27 fold; p<0.01), and Psme3 (0.83 fold; p<0.05) were down-regulated in A/J mice at baseline (0 hr) as compared with resistant C57BL/6J. Two genes exhibited elevated expression in susceptible A/J mice (baseline): Fam134c (1.82 fold; p<0.01) and Slc4a1 (1.31fold; p<0.05). The baseline difference in expression between susceptible (A/J) and resistant (C57BL/6J) mice of all the five genes remained unchanged at 2 hr post S. aureus-infection. (B) Dusp3 and Psme3 inhibit NF-κB signaling activity in RAW264.7 macrophages. RAW264.7 cells co-transfected with NF-κB-luciferase and pRL-TK plasmids were then transfected with siRNA of each individual candidate genes (Dcaf7, Dusp3, Psme3) or scrambled siRNA. Then transfected RAW cells were stimulated by either medium alone, medium containing LTA (10 µg/ml) or medium containing S. aureus particles (10 µg/ml) for 7 hours. Cells were directly lysed by 1× passive lysis buffer and luciferase activity was assayed and normalized to renilla activity as previously described [15]. As shown, knockdown of both Dusp3 and Psme3 significantly up-regulates NF-κB luciferase activity (p<0.01). (C) Knockdown of Dusp3 or Psme3 enhanced the activation of NF-κB signaling upon S. aureus stimulation. BMDMs from C57BL/6J were transfected with either scrambled, Dusp3 or Psme3 siRNA, then stimulated with S. aureus for 15 minutes. Whole cell lysate was loaded for western-blot. Knockdown of Dusp3 or Psme3 dramatically increased degradation of IκBα and phosphorylation of p65 (Ser536) as compared with scrambled siRNA control. (D) Enhanced NF-κB signaling upon S. aureus stimulation in CSS11 BMDMs. BMDMs from either C57BL/6J or CSS11 were stimulated with S. aureus for 15 minutes. Whole cell lysate was loaded for Western blot. BMDMs from CSS11 exhibited increased degradation of IκBα and phosphorylation of p65 (Ser536) as compared with BMDMs from C57BL/6J. (E) Bay inhibition of NF-κB dramatically suppressed cytokine production. RAW264.7 macrophages were pre-treated with 4 µM Bay 11-7085 for one hour, then stimulated with 10 µg/ml S. aureus particles in 2 µM Bay for 3 hours. RNA was extracted and subjected to reverse-transcription PCR and qPCR. Inhibition of NF-κB by Bay inhibitor dramatically suppressed cytokine production upon S. aureus stimulation, including IL-1β (p<0.01), IL-6 (p<0.05) and TNF-α (p<0.05). (F) Inhibition of NF-κB enhanced Dusp3 and Psme3 expression. The inhibition of NF-κB activity by Bay inhibitor significantly enhanced the expression of both Dusp3 (p<0.05) and Psme3 (p<0.05), which indicated a reciprocal relationship between NF-κB signaling activity and Dusp3 or Psme3 expression. Candidate genes influence NF-κB signaling pathway
Since NF-κB signaling plays a crucial rule in host defense to various pathogens [15]–[17] including S. aureus [18], [19], the impact of the five putative genes on NF-κB signaling was analyzed by co-transfection of NF-κB-luciferase reporter plasmid and siRNA of each gene into RAW264.7 murine macrophage cell line. The siRNAs used in this study were listed (Table S2). The siRNA of each candidate gene efficiently reduced gene expression in RAW 264.7 cells (Figure S3). Knockdown of two genes, Dusp3 (p<0.01) and Psme3 (p<0.01), significantly enhanced NF-κB signaling upon stimulation with either lipoteichoic acid (LTA) or S. aureus particles (Figure 4B). Because both of these genes (Dusp3 and Psme3) were down-regulated in A/J at baseline and S. aureus infection (Figure 4A), siRNA-mediated knockdown mimicked the status of susceptible A/J in RAW264.7 murine macrophages. These results suggest that lower expression of Dusp3 and Psme3 in A/J mice could explain the observed susceptibility to S. aureus.
To better understand how Dusp3 and Psme3 affect NF-κB signaling activity, phosphorylation of p65 at Ser536 and degradation of IκBα were analyzed by western blot. BMDMs from C57BL/6J were transfected with either scrambled, Dusp3, or Psme3 siRNA one day before and then subjected to S. aureus challenge for 15 minutes. The knock-down efficiency was tested in a parallel experiment (Figure S4). Western blot results showed that knockdown of Dusp3 or Psme3 dramatically increased phosphorylation of p65 at Ser536 as compared with scrambled siRNA (Figure 4C, top panel). The degradation of IκBα was also increased in either Dusp3 or Psme3 knockdown cells (Figure 4C, second bottom panel). The antibodies used in this study were listed (Table S3).
Similarly, BMDMs from CSS11 exhibited increased phosphorylation of p65 (Ser536) and degradation of IκBα after S. aureus stimulation as compared with BMDMs from C57BL/6J (Figure 4D). These data indicate that the suppressive function of Dusp3 and Psme3 on NF-κB signaling happens prior to the inhibitory cytosolic complex of IκBα-p65-p50. The signaling event should happen within the cytosol or at the cell membrane rather than in the nucleus, and probably in the proximal signaling stage before IκBα degradation.
NF-κB signaling is responsible for inflammatory cytokine production
Next, we analyzed cytokine and chemokine production after inhibiting NF-κB signaling. Either Bay 11-7085 or DMSO was applied to RAW264.7 cells before S. aureus challenge. qPCR illustrated that Bay inhibition of NF-κB signaling significantly suppressed inflammatory cytokine and chemokine production upon S. aureus stimulation in RAW264.7 macrophages, including IL-1β (p<0.01), IL-6 (p<0.05), and TNF-α (p<0.05) (Figure 4E). Inhibition of NF-κB signaling also enhanced the expression of Dusp3 (p<0.05) and Psme3 (p<0.05), suggesting a negative feedback regulatory loop between NF-κB and both Dusp3 and Psme3 (Figure 4F). qPCR primers for cytokines and chemokines applied in this study were listed (Table S4).
Suppressive regulation of cytokine production by Dusp3 and Psme3
Neutrophils and macrophages from A/J and C57BL/6J exhibit similar bacterial killing capacity [11], suggesting that other host characteristics account for differences in the S. aureus susceptibility phenotype. To evaluate these factors, we used a well-established macrophage differentiation system [15] to differentiate the bone-marrow progenitor cells from A/J and C57BL/6J into macrophages and analyzed macrophage markers by flow cytometry. No significant differences in either CD11b or F4/80 expression were observed (Figure S5). Using flow cytometry, bone marrow derived macrophages (BMDMs) from A/J and C57BL/6J exhibited a similar phagocytosis capacity for S. aureus (Figure 5A).
Fig. 5. siRNA knockdown of Dusp3 and Psme3 result in significant elevation of cytokine production, consistent with the pattern of bone marrow derived macrophages from CSS11 as compared with C57BL/6J (GM-CSF, IL-1β, IL-6 and TNF-α). 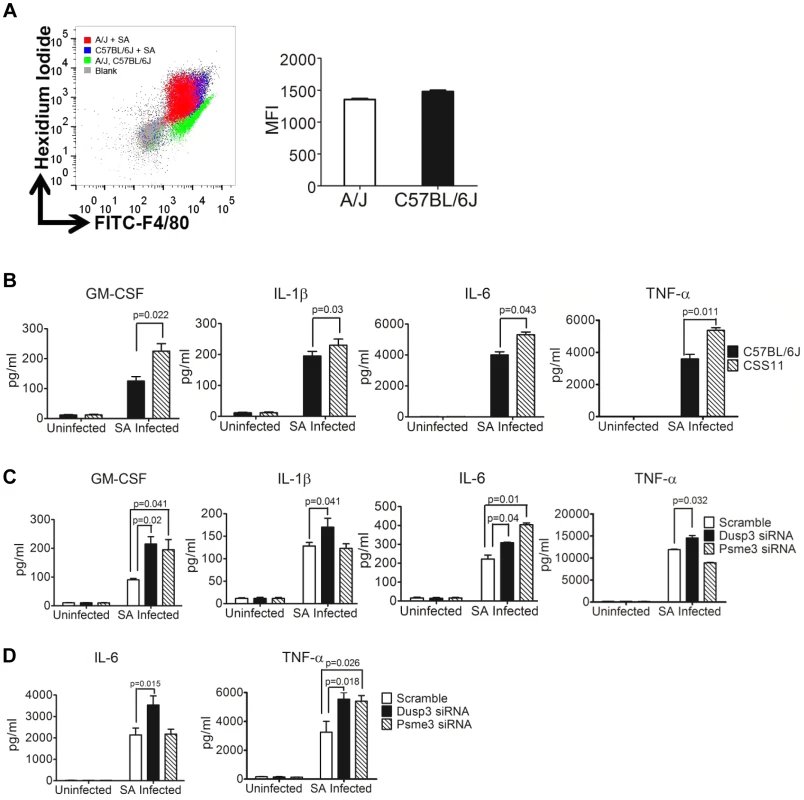
(A) Bone-marrow derived macrophages (BMDMs) from A/J and C57BL/6J have similar S. aureus phagocytosis ability. 2×106 BMDMs were seeded to single wells in a 6-well plate the day before phagocytosis and incubated with hexidium iodide stained S. aureus. Phagocytic efficiency as determined by the mean fluorescence intensity (MFI) is not significantly different in BMDMs from C57BL/6J and CSS11 mice. Representative histogram of 3 separate experiments. (B) BMDMs from CSS11 mice produced significantly higher cytokine levels as compared to C57BL/6J. 4×105 BMDMs from both C57BL/6J and CSS11 mice were seeded to single-wells of a 24-well plate the day before infection. Infection was simulated by adding S. aureus particles at 10 µg/ml. At 24 hours post-infection the supernatants were harvested and subjected to Luminex cytokine assaying. BMDMs from CSS11 mice significantly enhanced cytokine production, including GM-CSF, IL-1β, IL-6, and TNF-α. (C) Down-regulation of Dusp3 and Psme3 by siRNA led to up-regulation of cytokine production upon S. aureus challenge in RAW264.7 macrophages. RAW264.7 cells were transfected by either scramble or Dusp3 or Psme3 siRNA, and then infected with S. aureus particles at 10 µg/ml as before [11]. At 24 hours post-infection, the supernatants were harvested and subjected to Luminex-multiplex cytokine assaying. The down-regulation of Dusp3 significantly enhanced cytokine production, including GM-CSF, IL-1β, IL-6, and TNF-α, as compared to scramble siRNA control. The down-regulation of Psme3 also significantly elevated GM-CSF and IL-6 production. (D) Down-regulation of Dusp3 or Psme3 by siRNA led to up-regulation of cytokine production upon S. aureus challenge in BMDMs. BMDMs from C57BL/6J were transfected by either scrambled, Dusp3 or Psme3 siRNA, and then infected with S. aureus particles at 10 µg/ml. At 24 hours post-infection, the supernatants were harvested and subjected to cytokine analysis. The down-regulation of Dusp3 significantly enhanced cytokine production, including IL-6 and TNF-α, as compared to scrambled siRNA control. The down-regulation of Psme3 also significantly elevated TNF-α production. Luminex cytokine profiling showed that BMDMs from CSS11 produced more inflammatory cytokines as compared with C57BL/6J in response to stimulation with S. aureus, including GM-CSF (p = 0.022), IL-1β (p = 0.03), IL-6 (p = 0.043), and TNF-α (p = 0.011) (Figure 5B, and S6). This elevation in cytokine production is likely to be due to the down-regulation of Dusp3 and Psme3 in CSS11. siRNA knockdown of these two genes in S. aureus-challenged RAW264.7 macrophages and BMDMs greatly enhanced NF-κB signaling (Figure 4B and 4C), which in turn directly activated cytokine and chemokine production (Figure 4E). Indeed, in Dusp3 siRNA transfected S. aureus-challenged RAW264.7 macrophages, Luminex-multiplex profiling detected significantly enhanced cytokine production as compared with scrambled siRNA, including GM-CSF (p = 0.02), IL-1β (p = 0.041), IL-6 (p = 0.04), and TNF-α (p = 0.032) (Figure 5C and S7). Similarly, Psme3 siRNA transfected, S. aureus-challenged RAW264.7 cells also produced significantly more GM-CSF (p = 0.041) and IL-6 (p = 0.01) than control (Figure 5C and S7). Similarly, in S. aureus challenged BMDMs of C57BL/6J, knockdown of Dusp3 dramatically enhanced the production of IL-6 (p = 0.015) and TNF-α (p = 0.018) (Figure 5D and S8). Likewise, knockdown of Psme3 enhanced the production of TNF-α (p = 0.026) as compared with scrambled siRNA (Figure 5D and S8).
Patterns of increased cytokine production were also consistent between mRNA production from Dusp3 or Psme3 siRNA transfected RAW 264.7 macrophages or BMDMs and primary CSS11 BMDMs. Knockdown of Dusp3 in RAW264.7 macrophages significantly increased cytokine mRNA production upon S. aureus challenge as compared with scrambled control, including GM-CSF (p = 0.013), IL-1β (p = 0.048), and TNF-α (p = 0.031) (Figure 6A and S9). Knockdown of Psme3 in RAW264.7 macrophages also significantly increased IL-6 (p = 0.032) mRNA production (Figure 6A and S9). Primary BMDMs from CSS11, which contains chr. 11 from A/J in an otherwise C57BL/6J background, expressed more cytokine mRNA upon S. aureus challenge as compared with C57BL/6J, including GM-CSF (p = 0.019), IL-1β (p = 0.039), IL-6 (p = 0.027), and TNF-α (p = 0.043). This observation is likely to reflect the down-regulation of both Dusp3 and Psme3 in the A/J chromosome 11 contained by CSS11 mouse lineage (Figure 6B and 4A). Similarly, in S. aureus challenged BMDMs of C57BL/6J, knockdown of Dusp3 dramatically increased IL-6 RNA (p = 0.0078) and TNF-α RNA (p = 0.048) (Figure 6C). Knockdown of Psme3 significantly increased TNF-α RNA (p = 0.046) as compared with scrambled siRNA (Figure 6C).
Fig. 6. Quantitative-PCR confirmed elevation of cytokine production in macrophages transfected by Dusp3 and Psme3 siRNA or BMDMs from CSS11(GM-CSF, IL-1β, IL-6, and TNF-α). 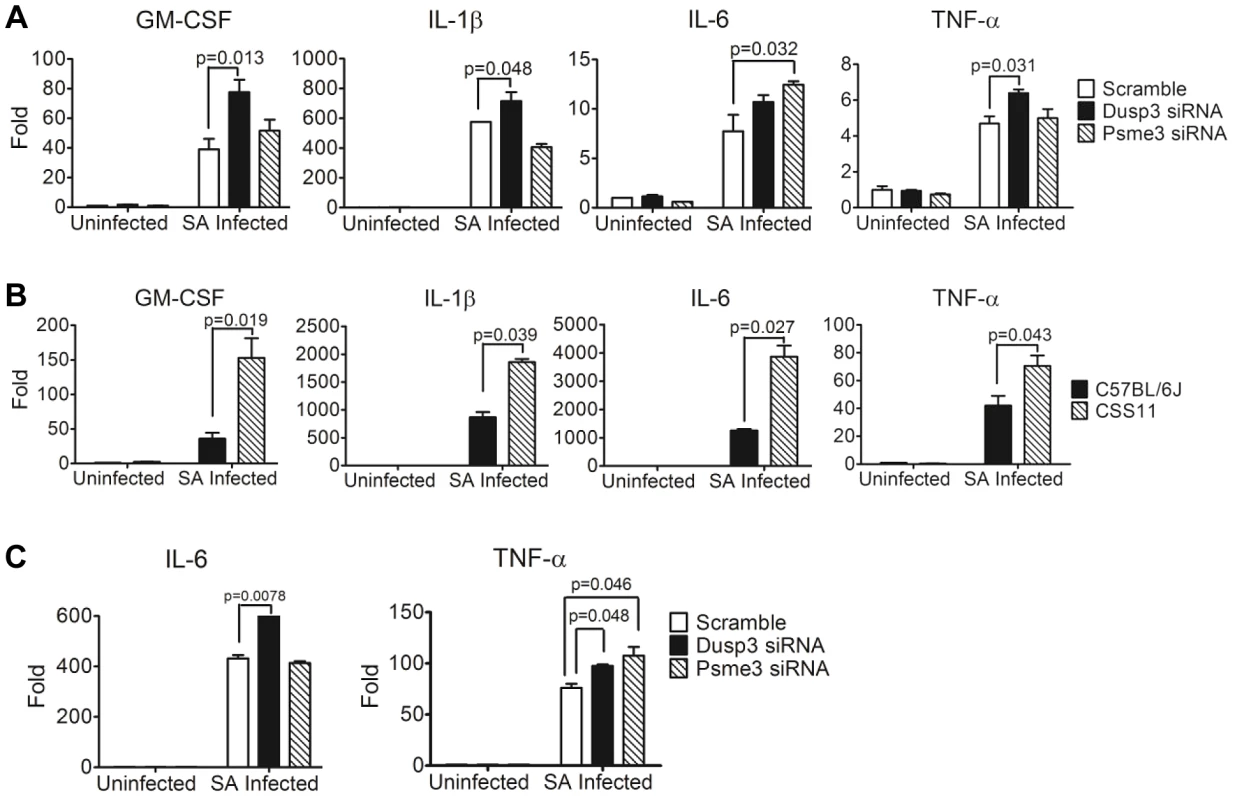
(A) Down-regulation of Dusp3 and Psme3 by siRNA led to increased cytokine RNA expression upon S. aureus challenge in RAW264.7 macrophages. At three hours post-infection total RNA was extracted followed by reverse-transcription PCR and SYBR-Green qPCR. The expression of all genes were normalized to 18s rRNA. The expression level of GM-CSF, IL-1β, IL-6, and TNF-α was higher in Dusp3 knockdown RAW cells, and the level of GM-CSF and IL-6 was higher in Psme3 knockdown RAW cells. p-value smaller than 0.05 was considered significant. (B) BMDMs cytokine RNA production in CSS11 mice was significantly higher than in C57BL/6J upon S. aureus infection. 2×106 BMDMs were seeded to single wells in a 6-well plate the day before infection. At three hours post-infection, RNA was extracted using RNeasy followed by RT-PCR and qPCR. The expression levels of GM-CSF, IL-1β, IL-6, and TNF-α were significantly higher in BMDMs from CSS11 mice. The expression of all genes were normalized to 18s rRNA. p-value smaller than 0.05 was considered significant. (C) Down-regulation of Dusp3 and Psme3 by siRNA led to increased cytokine RNA expression upon S. aureus challenge in BMDMs of C57BL/6J. The expression level of IL-6 was higher in Dusp3 siRNA transfected BMDMs, and the expression of TNF-α was higher in both Dusp3 and Psme3 siRNA transfected BMDMs. Pre-exposure to S. aureus impairs phagocytosis ability
Since NF-κB signaling mediates host defenses generally in a positive way, we hypothesized that persistent, unbated stimulation of production of antimicrobial effectors from immune cells, such as antimicrobial peptides, would eventually lead to “immune paralysis” or “immune exhaustion” that impeded further defense against S. aureus challenge. To test this hypothesis, BMDMs from both C57BL/6J and A/J were pre-exposed to either TNF-α (100 ng/ml) or S. aureus particles (10 µg/ml), then subjected to phagocytosis analysis. Pre-exposure to TNF-α enhanced the phagocytosis capacity of BMDMs from both strains (Figure S10). However, pre-exposure to S. aureus particles reduced phagocytosis ability of BMDMs from both strains, and the reduction was more extensively in BMDMs from A/J (Figure S10). These results suggest that prolonged simultaneous activation of all pathways in the host by S. aureus, and not isolated stimulation of the TNFα-TNF receptor pathway alone, impaired the immune system's ability to further respond to S. aureus infection.
Expression of Dusp3 and Psme3 in human neutrophils and macrophages with S. aureus infection
Next, we analyzed the role of Dusp3 and Psme3 upon S. aureus challenge in the immortalized human monocyte cell line U-937. Despite efficient knock-down of Dusp3 and Psme3, S. aureus stimulation of U-937 led to cell death rather than activation of NF-κB signaling and cytokine production (data not shown). We therefore concluded that U-937 was not suitable for our current analysis, and instead evaluated function of these two genes in human samples. To do this, we used publically available datasets of various human immune cells challenged by S. aureus, including human neutrophils (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE16837) and human macrophages (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE13670). In the human neutrophil dataset (GEO:GSE16837), Dusp3 increased to 9.88 fold at 3 hr (p<0.001) and 7.95 fold at 6 hr (p<0.05) as compared with 0 hr after S. aureus stimulation; and Psme3 decreased to 0.68 fold at 3 hr (p<0.0001) and 0.45 fold at 6 hr (p<0.0001) as compared with 0 hr (Figure S11). In the human macrophage dataset (GEO:GSE13670), Dusp3 increased to 1.62 fold at 8 hr following S. aureus challenge compared with controls (p<0.005) and Psme3 decreased to 0.73 fold at 8 hr following S. aureus challenge as compared with each control (p<0.001) (Figure S11). Collectively, these data and our human and murine data together strongly support that Dusp3 and Psme3 are candidate genes highly associated with S. aureus susceptibility.
Discussion
The genetic basis for host susceptibility to S. aureus is largely unknown. In the current report, we have identified two genes, Dusp3 and Psme3, which are strongly associated with S. aureus sepsis in mice and humans, and have proposed a potential biological mechanism as negative feedback components in NF-κB-mediated signaling (Figure 7). These factors are likely to contribute to host susceptibility to S. aureus sepsis in mouse, and potentially in humans.
Fig. 7. Down-regulation of Dusp3 and Psme3 in A/J is associated with over-production of pro-inflammatory cytokines. 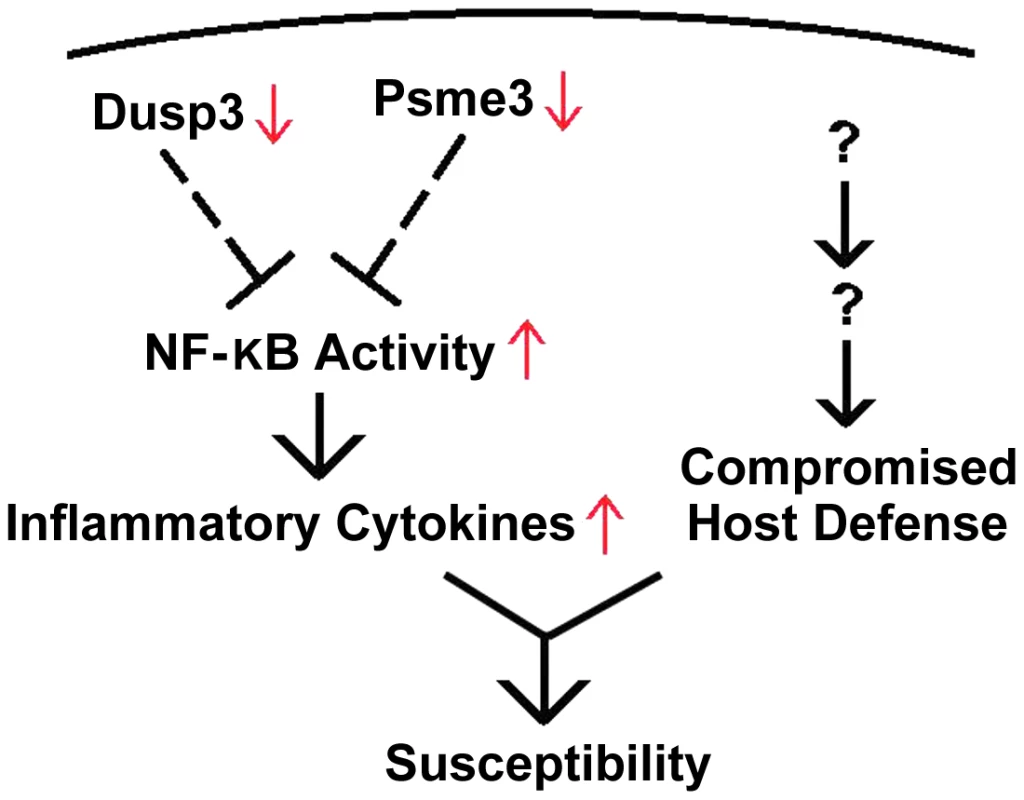
Both Dusp3 and Psme3 inhibit NF-κB activity, which is responsible for the production of inflammatory cytokines. The reduced expression of Dusp3 and Psme3 in A/J mice is associated with elevated NF-κB activity, which leads to increased inflammatory cytokines. Dusp3 and Psme3, together with other factors from murine chromosome 8 and 18, contribute to S. aureus susceptibility of A/J strain. We identified a QTL locus on chromosome 11 that was significantly linked to S. aureus susceptibility in A/J mice. We found five candidate genes (Dcaf7, Dusp3, Fam134c, Psme3, and Slc4a1) within the QTL locus that were differentially expressed between susceptible and resistant mouse strains and had human orthologues with the same significant expression patterns in patients with both S. aureus BSI and E. coli BSI. The results suggest that one or more of these five genes are involved in a common host response to S. aureus infection in both humans and mice. qPCR demonstrated that two of these five genes, Dusp3 and Psme3, were down-regulated in susceptible vs. resistant mice, and exhibited a significantly enhanced inflammatory cytokine response when knocked down with siRNA in both RAW264.7 macrophages and BMDMs. The over-production of inflammatory cytokines encountered with siRNA-mediated knockdown of these two genes was consistent with that encountered in S. aureus-challenged bone marrow derived macrophages from CSS 11 (C57BL/6J background with A/J chromosome 11) mice. Taken together, these data provide strong evidence that Dusp3 and Psme3 may be key genes involved in the host susceptibility to S. aureus.
Previous investigations have shown inconsistencies between murine and human response to sepsis [20]–[23]. For example, a recent study by Seok and colleagues demonstrated significant differences between murine and human genomic responses to several acute inflammatory diseases, including burns, trauma, and endotoxemia [14]. Our candidate gene selection approach ensured that all murine candidate genes were also relevant in humans with S. aureus BSI. In this way, almost two-thirds of the more than 1000 genes on A/J chromosome 11 were excluded from contributing to susceptibility to S. aureus. Using this trans-species comparative genomic approach, only Dusp3 and Psme3 were identified as putative contributors to S. aureus susceptibility and both exhibited a significant biological effect on NF-κB signaling. Because NF-κB is centrally involved in the inflammatory response in both mouse and human [24], [25], these two genes are likely to be involved in the host inflammatory response to S. aureus in both humans and mice.
The diverse function of Dusp3 requires further detailed investigation on its biological relevance to S. aureus susceptibility. First, it is greatly involved in signaling transduction pathways regulating protein de-phosphorylation. For example, Dusp3 has been reported to be the main protein tyrosine phosphatase in macrophage mediating cellular processes, including immune response [26]. It is a redox-sensitive and ERK-specific phosphatase. Bacterial colonization results in oxidative inactivation of Dusp3 and consequently stimulation of ERK [27]. Dusp3 also regulates cell death and cell proliferation, exhibiting anti-apoptotic ability in prostate cancer cells and promoting cell cycle progression in carcinoma of the cervix [28], [29]. Perhaps most interestingly, Dusp3 has been shown to affect the expression of vascular endothelial growth factor (VEGF) [30]–[32]. VEGF has been reported to increase cardiovascular collapse and vascular permeability during S. aureus sepsis pathogenesis and has been proposed as a major determinant of vascular hyperpermeability in MRSA sepsis [30], [31]. In our experiment, VEGF was also significantly elevated in Dusp3 siRNA transfected RAW264.7 macrophages in both non-infection and S. aureus infection condition. Finally, Dusp3 homozygous mutant mice exhibited susceptibility to Gram negative bacterial infection in screening results from the Knockout Mouse Consortium program [33], [34]. Collectively, this evidence suggests the Dusp3 pathway may be an important candidate pathway in resolving S. aureus related pathogenesis.
Psme3 is a subunit of a proteasome responsible for the generation of peptides loaded onto MHC class I molecules [35], [36]. MHC class I molecules are crucial components of host response to infection, contributing to host recognition of viral and bacterial infected cells by host cytotoxic CD8-T cells for the final killing and degradation of pathogens [37], [38]. The down-regulation of Psme3 in A/J but not C57BL/6J may result in less degradation of phagocytosed S. aureus, less S. aureus antigen presented on MHC class I molecules, and ultimately less degradation of infected host cells by cytotoxic CD8-T cells. Interestingly, siRNA-mediated down-regulation of Psme3 also dramatically increased VEGF production by RAW264.7, further suggesting that VEGF enrichment affected by down-regulation of Dusp3 and Psme3 in murine chromosome 11 contributes to the S. aureus susceptible phenotype of A/J.
In the process of sepsis, harmful molecular mechanisms contribute to the high mortality seen in severely ill patients [39], [40]. In these cases, a pathogenic role of excessive immunity, also known as “cytokine storm” and reduced immunity through immune paralysis are highly associated with death induced by acute bacterial infection. Referred to as “compensatory anti-inflammatory response syndrome” (CARS), this “immune-exhaustion” phenomenon is the consequence of counter-regulatory mechanisms initiated to limit the over-activated inflammatory response in sepsis patients [41], [42]. In patients with CARS, over-activation of inflammatory response ultimately leads to changes in expression of genes associated with phagocytosis, antigen presentation, cell migration and apoptosis [43]. In the current investigation, downregulation of Dusp3 and Psme3 in A/J increased the production of pro-inflammatory cytokines, which may partially account for the cytokine storm observed in that mouse lineage when infected with S. aureus. These two genes, combined with other factors from chromosome 8 and 18, would together contribute to susceptibility to S. aureus in A/J mice, and potentially in humans.
Further, our studies showed that pre-exposure of macrophages to S. aureus particles compromised their ability to further take up the bacteria. This finding suggests that persistent, unabated stimulation of the immune system by S. aureus infection can eventually lead to immune paralysis or exhaustion of antimicrobial peptides. Since the overproduction of cytokines is the major phenotype of CSS11, we hypothesize that cytokine storm could account for the increased susceptibility of A/J to dying of S. aureus sepsis. Given these findings, we hypothesize that down-regulation of Dusp3 and Psme3 in A/J result in hyper-responsiveness of host immune system, which in turn leads to “immune paralysis” of the host to further defense against prolonged S. aureus challenge. Collectively, these finding also indicate that immediate bacterial clearance is important for host defense against S. aureus.
Several of the other genes identified in our experiments were also promising candidates. Dcaf7 is a scaffold protein for activating MEKK1 kinase [44] and is involved in the human TNFα/NF-κB signal transduction pathway [45]. A dominant negative mutant of MEKK1 was reported to abolish T-cell receptor activation by super-antigen staphylococcus enterotoxin E [46] while NF-κB mediated innate immune defense against S. aureus through TLR2 and NOD2 [16], [47], [48]. Fam134c is a family with sequence similarity to 134, member C. Little is known of the putative function of Fam134c or related family members [49]. However, mice with Fam134c homozygous mutations have demonstrated bacterial susceptible phenotype based on the screening results from the Knockout Mouse Consortium program [33], [34]. Slc4a1 is a membrane protein or protein of membrane related organelles mediating small molecule transporting and intracellular metabolism [50]–[52].
The current study has limitations. First, our cohort of patients did not include subjects who were colonized, but not infected, with S. aureus. Second, the two model systems used in this manuscript (intraperitoneal sepsis, tail vein sepsis) fail to fully represent the diversity of human infections caused by S. aureus (e.g., endocarditis, osteomyelitis, visceral abscesses, pneumonia, soft tissue infection). Our approach does not consider the impact of post-translational modification [53], [54] and single nucleotide polymorphisms on genes and their products. Thus, there may be additional candidate genes on chromosome 11 beyond Dusp3 and Psme3. Moreover, our approach may have missed genes that contribute to the susceptible phenotype by way of a joint or additive effect. In support of this possibility is the fact that our original discovery found that three chromosomes, 8, 11, and 18, were each independently associated with susceptibility to S. aureus [11]. Thus, additional experiments are underway in our lab, including QTL mapping analysis for A/J chromosome 8, defining the pathogenesis of Dusp3 and Psme3 using knockout mice, and evaluating the impact of Dusp3 and Psme3 on host susceptibility to different pathogens.
Despite these limitations, this study makes several key observations. First, we have identified one QTL on chromosome 11 that is significantly linked to survival time after infection with S. aureus. Eleven differentially expressed genes mapped to the significant - or suggestive - threshold of this QTL. Five of these 11 genes exhibited significant evidence of involvement in patients with S. aureus BSI that was consistent with the pattern encountered in the murine model. Two of these five genes, Dusp3 and Psme3, responded to S. aureus challenge by negatively regulating NF-κB signaling, leading to enhanced cytokine response (GM-CSF, IL-1β, IL-6, and TNFα). Consistent with the hypothesis of enhanced A/J susceptibility caused by unchecked inflammatory response, Dusp3 and Psme3 were less expressed in susceptible A/J as compared with resistant C57BL/6J. All of our results support a potential role of these two genes in host response to S. aureus. Dusp3 and Psme3 represent promising candidates for the genetic basis of host susceptibility to S. aureus.
Materials and Methods
Ethics statement
All animal experiments were carried out in strict accordance with the recommendations of NIH guidelines, the Animal Welfare Act, and US federal law. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC Protocol A191-12-07) of Duke University which has been accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. All animals were housed in a centralized and AAALAC accredited research animal facility that is fully staffed with trained husbandry, technical, and veterinary personnel. The Institutional Review Boards from all involved hospitals approved the human studies referenced in this work. Written informed consent was obtained for all subjects after the nature and possible consequences of the studies were explained.
Human subjects
Subjects were enrolled at Duke University Medical Center (DUMC; Durham, NC), Durham VAMC (Durham, NC), and Henry Ford Hospital (Detroit, Michigan) as part of a prospective, NIH-sponsored study to develop novel diagnostic tests for severe sepsis and community-acquired pneumonia as mentioned before [55]–[57]. All participants were adults. RNA was obtained from blood drawn at the time patients initially presented to the Emergency Department with sepsis. RNA expression data from patients who were ultimately found to have BSI with either S. aureus (n = 32) or E. coli (n = 19) were used in this study. Healthy controls were defined as uninfected human (n = 43), enrolled as part of a study on the effect of aspirin on platelet function among healthy volunteers. Subjects were recruited through advertisements posted on the Duke campus. Blood used to derive gene expression data in these healthy controls was drawn prior to aspirin challenge. Human orthologs of murine genes were identified by Chip comparer (http://chipcomparer.genome.duke.edu/) as reported before [58]. When there were multiple orthologs, we preferentially used the anti-sense target probes that shared the fewest probes with other genes as identified by the probe label.
Mouse strains
C57BL/6J, A/J, and CSS11 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All the mice were allowed to acclimate for more than 7 days before experiments. For generation of F1 progeny, CSS11 mice were mated with C57BL/6J in reciprocal crosses [C57BL/6J male×CSS11 female and C57BL/6J female×CSS11 male] to generate an F1 population with heterozygous chromosome 11 due to homologous recombination. To generate F2 intercross mice for QTL linkage analysis, F1 (C11A) mice were intercrossed with F1 (C11A) to produce more than 200 progeny.
Preparation of bacteria
S. aureus clinical strain, Sanger 476 was used in the mortality and infection studies. For preparation of S. aureus for injection, overnight culture of S. aureus was diluted 100 folds with fresh tryptic soy broth (TSB) and shake at 37°C with aeration to log-phase (OD600≈0.8). S. aureus was harvested by centrifugation at 3000 rpm for 10 minutes at 4°C, washed once in DPBS and re-suspended in DPBS.
Murine sepsis experiment and bacterial load quantification
For murine peritonitis-sepsis experiments, 8-week-old male mice (n = 10) in each strain of C57BL/6J, A/J, and CSS11 were i.p. injected with 107 CFU/g S. aureus (Sanger 476) or 2×105 CFU/g E. coli (K1H7) and observed every 6 hours for morbidity continuously for 5 days. For murine intravenous sepsis, 8-week-old male mice (n = 10) in each strain of C57BL/6J, A/J, and CSS11 were i.v. injected with 2×106 CFU/g S. aureus (Sanger 476) and observed every 6 hours for morbidity continuously for 3 days. For bacterial load quantification, kidneys were collected from euthanized mice at 24 hours post-infection, then homogenized in DPBS and serially diluted (10 fold). The dilutions were plated in Tryptic Soy Agar (TSA) plates and incubated at 37°C overnight for counting colony forming units (CFU).
QTL linkage analysis
Polymorphic microsatellite markers on chromosome 11 between C57BL/6J and A/J were chosen from a database maintained by Mouse Genomic Informatics (http://www.informatics.jax.org/). Twelve microsatellite markers were selected with an average inter-marker distance of 3.1 cM covering chromosome 11. A total of 208 F2 intercross were generated, all of which were genotyped for each microsatellite marker by PCR amplification and gel electrophoresis. J/qtl software was used to analyze phenotype and genotype data for linkage of survival time after infection with S. aureus Sanger 476 and marker location. Phenotypes were defined as either sensitive or resistant based on the dichotomization of survival data (survival of less than 2 day is “0” and survival of longer than 2 days is “1”, respectively). All linkage analysis results were expressed as LOD scores. LOD score was considered “suggestive” if > = 1.6 (p = 0.63) and “significant” if > = 3.55 (p = 0.05). Threshold values for linkage were determined by a 1,000 permutation test by using J/qtl.
Culture of bone-marrow derived macrophage and RAW264.7 macrophage cell line
To generate bone marrow-derived macrophages (BMDMs), bone marrow progenitor cells were harvested from mice and cultured for 7 days in 70% (vol/vol) D10 (DMEM containing 10% (vol/vol) FBS, 2 mM glutamine, 100 µg/ml streptomycin, and 100 units/ml penicillin) and 30% (vol/vol) L-929 cell culture supernatant. Mature BMDMs were washed twice with cold DPBS, collected with 5 mM EDTA in DPBS, and re-plated on tissue-culture plates as reported before [15]. The murine RAW 264.7 macrophage cells (ATCC) were cultured in D10 in tissue culture plates before downstream experiments.
Macrophage phagocytosis assay
The day before treatment, 2×106 BMDMs from either C57BL/6J or A/J were seeded to 6-well plate for analysis of S. aureus phagocytosis ability. Next day BMDMs were either treated with fresh medium, TNF-α at 100 ng/ml, or S. aureus particles at 10 µg/ml for 24 hours. On the day of the phagocytosis experiment, S. aureus Sanger 476 were grown to exponential period (OD600≈0.8) and harvested by centrifugation. After washing in DPBS, S. aureus were stained by Hexidium Iodide (100 µg/ml) for 15 minutes at room temperature followed by washing once in DPBS and re-suspended in DPBS on ice [59]. Then multiplicity of infection (MOI) 10 was applied for S. aureus infection. Briefly, old medium were removed and replaced with fresh medium containing 2×107 S. aureus to each well of 6-well plate with BMDMs, followed by quick spin at 500 rpm for 5 minutes at room temperature. Cells were then incubated at 37°C for 30 min in CO2 incubator to allow bacterial uptake by macrophages. After introduction of S. aureus, trypsin was added at a final concentration of 0.25% for 10 minutes at room temperature to remove any residual bacteria at the macrophage surface. Macrophages were next washed three times with DPBS to remove remaining bacteria and floated by 5 mM EDTA in DPBS. Macrophages were then stained by FITC-F4/80 and analyzed by Flow cytometer [60]. The fluorescence produced from hexidium iodide staining falls into FL2 channel (Excitation 488/Emmision 575) in FACSCanto [59]. Experiments were repeated at least three times.
Small interfering RNA (siRNA) experiments
To test the role of each candidate gene on cytokine production by host defense cells, we transfected siRNAs into the mouse macrophages. All siRNAs were purchased from Invitrogen. RAW264.7 cells or bone-marrow derived macrophages from C57BL/6J were transfected with 50 nM siRNA by Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. Twenty-four hours post-transfection, cells were treated with S. aureus Bioparticles (Invitrogen) to a final concentration of 10 µg/ml. At 24 hours post-infection, supernatants were collected and stored at −80°C for Luminex-multiplex cytokine assay. In parallel experiments, cells at 3 hours post-infection were harvested for RNA extraction by RNeasy (QIAGEN), RT-PCR by SuperScript II (Invitrogen) and SYBR Green qPCR analysis (ABI) respectively. Experiments were repeated at least three times. A full list of gene names and siRNA ID numbers were in Table S1.
Luciferase assay
The day before transfection, 5×106 RAW264.7 macrophages were seeded into 10 cm dishes. On the second day, 10 µg NF-κB-Luc (Clontech) and 0.5 µg pRL-TK (Promega) plasmids were co-transfected by Lipofectamine LTX (Invitrogen) according to manufacturer's instruction. At 6 hours post-transfection, RAW264.7 were split into 6-well plate (1×106/well) and cultured overnight in CO2 incubator. On the third day, scrambled siRNA or siRNA for each candidate gene was transfected into 6-well plate by Lipofectamine RNAiMAX according to manufacturer's instruction. At 6 hours post-transfection, cells were split into 24-well plate (4×105/well). On the fourth day, cells were stimulated by either D10 or D10 containing S. aureus lipoteichoic acid (LTA) (10 µg/ml) or S. aureus particles (10 µg/ml) for seven hours. Then cells were lysed by 1×Passive Lysis Buffer (Promega) and luciferase activities were analyzed by dual-luciferase reporter assay system (Promega). All of Firefly luciferase activity was normalized by the Renilla luciferase activity and relative fold changes were compared. Experiments were repeated at least three times.
Inhibition of NF-κB signaling
4×105 RAW264.7 macrophages were seeded into each well in 24-well plate and cultured overnight in CO2 incubator. Then cells were treated by D10 with DMSO or 4 µM Bay 11-7085 (EMD Millipore) for one hour. Afterwards, the cells were stimulated by four different ways including D10+DMSO, D10+DMSO+S. aureus particles (10 µg/ml), D10+2 µM Bay and D10+2 µM Bay+S. aureus particles (10 µg/ml) for 3 hours. Then RNA was extracted, and reverse-transcription PCR and qPCR were applied. Experiments were repeated at least three times.
Measurement of cytokine/chemokine production
Cytokine production was assayed from the collected supernatant of both S. aureus-challenged siRNA transfected RAW cells and the S. aureus-challenged BMDM from C57BL/6J and CSS11 using multiplex cytokine assay kit (Invitrogen) and Luminex technology available at Duke Human Vaccine Institute.
Quantitative PCR
Total RNA was isolated using RNeasy kits (Qiagen) primed with random hexamer oligonucleotides and reversely transcribed using Invitrogen SuperScript II. Real-time quantitative PCR was performed using SYBR Green Mastermix (ABI). All data were normalized to 18s rRNA.
Western blot
Cells were lysed in RIPA buffer with cocktail of proteinase and protein phosphatase inhibitors. Then 20 µg whole cell lysate was loaded to SDS-PAGE and transferred to PVDF membrane. Blotting was followed according to manufacturer's instruction.
Statistical analyses
The differences in candidate gene expression, mRNA and protein of cytokines and chemokines and luciferase activities were analyzed by two-tailed Student's t test. The difference in mice survival rate was analyzed by Mann-Whitney u test. P-values smaller than 0.05 were considered to be statistically significant.
Supporting Information
Zdroje
1. BayerAS (1982) Staphylococcal bacteremia and endocarditis: state of the art. Arch Intern Med 142 : 1169–1177.
2. EadyEA, CoveJH (2003) Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus–an emerging problem for the management of skin and soft tissue infections. Curr Opin Infect Dis 16 : 103–124.
3. MarchantB, BrownJ (1987) Toxic shock syndrome and staphylococcal pneumonia. Lancet 2 : 578.
4. VerhoefJ, VerbrughHA (1981) Host determinants in staphylococcal disease. Annu Rev Med 32 : 107–122.
5. von Kockritz-BlickwedeM, RohdeM, OehmckeS, MillerLS, CheungAL, et al. (2008) Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model. Am J Pathol 173 : 1657–1668.
6. SongZ, SongY, YinJ, ShenY, YaoC, et al. (2012) Genetic variation in the TNF gene is associated with susceptibility to severe sepsis, but not with mortality. PLoS One 7: e46113.
7. SongZ, YaoC, YinJ, TongC, ZhuD, et al. (2012) Genetic variation in the TNF receptor-associated factor 6 gene is associated with susceptibility to sepsis-induced acute lung injury. J Transl Med 10 : 166.
8. AdrianiKS, BrouwerMC, BaasF, ZwindermanAH, van der EndeA, et al. (2012) Genetic variation in the beta2-adrenocepter gene is associated with susceptibility to bacterial meningitis in adults. PLoS One 7: e37618.
9. NeteaMG, WijmengaC, O'NeillLA (2012) Genetic variation in Toll-like receptors and disease susceptibility. Nat Immunol 13 : 535–542.
10. NakadaTA, RussellJA, BoydJH, WalleyKR (2011) IL17A genetic variation is associated with altered susceptibility to Gram-positive infection and mortality of severe sepsis. Crit Care 15: R254.
11. AhnSH, DeshmukhH, JohnsonN, CowellLG, RudeTH, et al. (2010) Two genes on A/J chromosome 18 are associated with susceptibility to Staphylococcus aureus infection by combined microarray and QTL analyses. PLoS Pathog 6: e1001088.
12. JohnsonNV, AhnSH, DeshmukhH, LevinMK, NelsonCL, et al. (2012) Haplotype Association Mapping Identifies a Candidate Gene Region in Mice Infected With Staphylococcus aureus. G3 (Bethesda) 2 : 693–700.
13. SugiN, WhistonEA, KsanderBR, GregoryMS (2013) Increased resistance to Staphylococcus aureus endophthalmitis in BALB/c mice: Fas ligand is required for resolution of inflammation but not for bacterial clearance. Infect Immun 81 : 2217–2225.
14. SeokJ, WarrenHS, CuencaAG, MindrinosMN, BakerHV, et al. (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 110 : 3507–3512.
15. YanQ, CarmodyRJ, QuZ, RuanQ, JagerJ, et al. (2012) Nuclear factor-kappaB binding motifs specify Toll-like receptor-induced gene repression through an inducible repressosome. Proc Natl Acad Sci U S A 109 : 14140–14145.
16. KarraschT, KimJS, MuhlbauerM, MagnessST, JobinC (2007) Gnotobiotic IL-10−/−;NF-kappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J Immunol 178 : 6522–6532.
17. Hedengren-OlcottM, OlcottMC, MooneyDT, EkengrenS, GellerBL, et al. (2004) Differential activation of the NF-kappaB-like factors Relish and Dif in Drosophila melanogaster by fungi and Gram-positive bacteria. J Biol Chem 279 : 21121–21127.
18. MitchellG, GrondinG, BilodeauG, CantinAM, MalouinF (2011) Infection of polarized airway epithelial cells by normal and small-colony variant strains of Staphylococcus aureus is increased in cells with abnormal cystic fibrosis transmembrane conductance regulator function and is influenced by NF-kappaB. Infect Immun 79 : 3541–3551.
19. GjertssonI, HultgrenOH, CollinsLV, PetterssonS, TarkowskiA (2001) Impact of transcription factors AP-1 and NF-kappaB on the outcome of experimental Staphylococcus aureus arthritis and sepsis. Microbes Infect 3 : 527–534.
20. PoundP, EbrahimS, SandercockP, BrackenMB, RobertsI, et al. (2004) Where is the evidence that animal research benefits humans? BMJ 328 : 514–517.
21. HackamDG, RedelmeierDA (2006) Translation of research evidence from animals to humans. JAMA 296 : 1731–1732.
22. van der WorpHB, HowellsDW, SenaES, PorrittMJ, RewellS, et al. (2010) Can animal models of disease reliably inform human studies? PLoS Med 7: e1000245.
23. RiceJ (2012) Animal models: Not close enough. Nature 484: S9.
24. BaltimoreD (2011) NF-kappaB is 25. Nat Immunol 12 : 683–685.
25. KarinM (2009) NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol 1: a000141.
26. ArimuraY, YagiJ (2010) Comprehensive expression profiles of genes for protein tyrosine phosphatases in immune cells. Sci Signal 3: rs1.
27. WentworthCC, AlamA, JonesRM, NusratA, NeishAS (2011) Enteric commensal bacteria induce extracellular signal-regulated kinase pathway signaling via formyl peptide receptor-dependent redox modulation of dual specific phosphatase 3. J Biol Chem 286 : 38448–38455.
28. ArnoldussenYJ, LorenzoPI, PretoriusME, WaehreH, RisbergB, et al. (2008) The mitogen-activated protein kinase phosphatase vaccinia H1-related protein inhibits apoptosis in prostate cancer cells and is overexpressed in prostate cancer. Cancer Res 68 : 9255–9264.
29. HenkensR, DelvenneP, ArafaM, MoutschenM, ZeddouM, et al. (2008) Cervix carcinoma is associated with an up-regulation and nuclear localization of the dual-specificity protein phosphatase VHR. BMC Cancer 8 : 147.
30. JonkamCC, BansalK, TraberDL, HamahataA, MaybauerMO, et al. (2009) Pulmonary vascular permeability changes in an ovine model of methicillin-resistant Staphylococcus aureus sepsis. Crit Care 13: R19.
31. JonkamCC, LangeM, TraberDL, MaybauerDM, MaybauerMO, et al. (2009) Cardiovascular collapse and vascular permeability changes in an ovine model of methicillin-resistant Staphylococcus aureus sepsis. Shock 32 : 621–625.
32. EcsediS, TothL, BalazsM (2012) Array CGH analysis of the rare laryngeal basaloid squamous cell carcinoma: a case report. Int J Clin Exp Pathol 5 : 834–839.
33. van der WeydenL, WhiteJK, AdamsDJ, LoganDW (2011) The mouse genetics toolkit: revealing function and mechanism. Genome Biol 12 : 224.
34. The Wellcome Trust Sanger Institute Mouse Portal [http://www.sanger.ac.uk/mouseprotal/]
35. KohdaK, IshibashiT, ShimbaraN, TanakaK, MatsudaY, et al. (1998) Characterization of the mouse PA28 activator complex gene family: complete organizations of the three member genes and a physical map of the approximately 150-kb region containing the alpha - and beta-subunit genes. J Immunol 160 : 4923–4935.
36. KanaiK, AramataS, KatakamiS, YasudaK, KataokaK (2011) Proteasome activator PA28{gamma} stimulates degradation of GSK3-phosphorylated insulin transcription activator MAFA. J Mol Endocrinol 47 : 119–127.
37. ChenZW, CraiuA, ShenL, KurodaMJ, IrokuUC, et al. (2000) Simian immunodeficiency virus evades a dominant epitope-specific cytotoxic T lymphocyte response through a mutation resulting in the accelerated dissociation of viral peptide and MHC class I. J Immunol 164 : 6474–6479.
38. Schutze-RedelmeierMP, GournierH, Garcia-PonsF, MoussaM, JoliotAH, et al. (1996) Introduction of exogenous antigens into the MHC class I processing and presentation pathway by Drosophila antennapedia homeodomain primes cytotoxic T cells in vivo. J Immunol 157 : 650–655.
39. RittirschD, FlierlMA, WardPA (2008) Harmful molecular mechanisms in sepsis. Nat Rev Immunol 8 : 776–787.
40. SriskandanS, AltmannDM (2008) The immunology of sepsis. J Pathol 214 : 211–223.
41. Adib-ConquyM, CavaillonJM (2009) Compensatory anti-inflammatory response syndrome. Thromb Haemost 101 : 36–47.
42. van der PollT, MeijersJC (2010) Systemic inflammatory response syndrome and compensatory anti-inflammatory response syndrome in sepsis. J Innate Immun 2 : 379–380.
43. XuPB, LouJS, RenY, MiaoCH, DengXM (2012) Gene expression profiling reveals the defining features of monocytes from septic patients with compensatory anti-inflammatory response syndrome. J Infect 65 : 380–391.
44. RitterhoffS, FarahCM, GrabitzkiJ, LochnitG, SkuratAV, et al. (2010) The WD40-repeat protein Han11 functions as a scaffold protein to control HIPK2 and MEKK1 kinase functions. EMBO J 29 : 3750–3761.
45. BouwmeesterT, BauchA, RuffnerH, AngrandPO, BergaminiG, et al. (2004) A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol 6 : 97–105.
46. TaoL, WadsworthS, MercerJ, MuellerC, LynnK, et al. (2002) Opposing roles of serine/threonine kinases MEKK1 and LOK in regulating the CD28 responsive element in T-cells. Biochem J 363 : 175–182.
47. Oviedo-BoysoJ, Barriga-RiveraJG, Valdez-AlarconJJ, Bravo-PatinoA, Carabez-TrejoA, et al. (2008) Internalization of Staphylococcus aureus by bovine endothelial cells is associated with the activity state of NF-kappaB and modulated by the pro-inflammatory cytokines TNF-alpha and IL-1beta. Scand J Immunol 67 : 169–176.
48. LiB, LiJ, PanX, DingG, CaoH, et al. (2010) Artesunate protects sepsis model mice challenged with Staphylococcus aureus by decreasing TNF-alpha release via inhibition TLR2 and Nod2 mRNA expressions and transcription factor NF-kappaB activation. Int Immunopharmacol 10 : 344–350.
49. KurthI, PammingerT, HenningsJC, SoehendraD, HuebnerAK, et al. (2009) Mutations in FAM134B, encoding a newly identified Golgi protein, cause severe sensory and autonomic neuropathy. Nat Genet 41 : 1179–1181.
50. Al-SamirS, PapadopoulosS, ScheibeRJ, MeissnerJD, CartronJP, et al. (2013) Activity and distribution of intracellular carbonic anhydrase II and their effects on the transport activity of anion exchanger AE1/SLC4A1. J Physiol 591(Pt 20): 4963–82.
51. Barneaud-RoccaD, EtchebestC, GuizouarnH (2013) Structural Model of the Anion Exchanger 1 (SLC4A1) and Identification of Transmembrane Segments Forming the Transport Site. J Biol Chem 288 : 26372–26384.
52. HaitinaT, LindblomJ, RenstromT, FredrikssonR (2006) Fourteen novel human members of mitochondrial solute carrier family 25 (SLC25) widely expressed in the central nervous system. Genomics 88 : 779–790.
53. YanQ, GongL, DengM, ZhangL, SunS, et al. (2010) Sumoylation activates the transcriptional activity of Pax-6, an important transcription factor for eye and brain development. Proc Natl Acad Sci U S A 107 : 21034–21039.
54. YanQ, LiuWB, QinJ, LiuJ, ChenHG, et al. (2007) Protein phosphatase-1 modulates the function of Pax-6, a transcription factor controlling brain and eye development. J Biol Chem 282 : 13954–13965.
55. ZaasAK, BurkeT, ChenM, McClainM, NicholsonB, et al. (2013) A host-based RT-PCR gene expression signature to identify acute respiratory viral infection. Sci Transl Med 5 : 203ra126.
56. GlickmanSW, CairnsCB, OteroRM, WoodsCW, TsalikEL, et al. (2010) Disease progression in hemodynamically stable patients presenting to the emergency department with sepsis. Acad Emerg Med 17 : 383–390.
57. LangleyRJ, TsalikEL, van VelkinburghJC, GlickmanSW, RiceBJ, et al. (2013) An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med 5 : 195ra195.
58. AhnSH, TsalikEL, CyrDD, ZhangY, van VelkinburghJC, et al. (2013) Gene expression-based classifiers identify Staphylococcus aureus infection in mice and humans. PLoS One 8: e48979.
59. MasonDJ, ShanmuganathanS, MortimerFC, GantVA (1998) A fluorescent Gram stain for flow cytometry and epifluorescence microscopy. Appl Environ Microbiol 64 : 2681–2685.
60. ThurlowLR, HankeML, FritzT, AngleA, AldrichA, et al. (2011) Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 186 : 6585–6596.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA SplicingČlánek Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance GenesČlánek The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- BdlA, DipA and Induced Dispersion Contribute to Acute Virulence and Chronic Persistence of
- Morphotype Transition and Sexual Reproduction Are Genetically Associated in a Ubiquitous Environmental Pathogen
- A Nucleic-Acid Hydrolyzing Single Chain Antibody Confers Resistance to DNA Virus Infection in HeLa Cells and C57BL/6 Mice
- HopW1 from Disrupts the Actin Cytoskeleton to Promote Virulence in Arabidopsis
- Ly6C Monocytes Become Alternatively Activated Macrophages in Schistosome Granulomas with Help from CD4+ Cells
- Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA Splicing
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- Antibody Responses to : Role in Pathogenesis and Diagnosis of Encephalitis?
- Discovery of a Novel Compound with Anti-Venezuelan Equine Encephalitis Virus Activity That Targets the Nonstructural Protein 2
- Activation of Focal Adhesion Kinase by Suppresses Autophagy via an Akt/mTOR Signaling Pathway and Promotes Bacterial Survival in Macrophages
- Crossing the Interspecies Barrier: Opening the Door to Zoonotic Pathogens
- Catching Fire: , Macrophages, and Pyroptosis
- IscR Is Essential for Type III Secretion and Virulence
- Selective Chemical Inhibition of Quorum Sensing in Promotes Host Defense with Minimal Impact on Resistance
- The Glycosylated Rv1860 Protein of Inhibits Dendritic Cell Mediated TH1 and TH17 Polarization of T Cells and Abrogates Protective Immunity Conferred by BCG
- A Genome-Wide Tethering Screen Reveals Novel Potential Post-Transcriptional Regulators in
- Structural Insights into SraP-Mediated Adhesion to Host Cells
- Human IGF1 Regulates Midgut Oxidative Stress and Epithelial Homeostasis to Balance Lifespan and resistance in
- Cycling Empirical Antibiotic Therapy in Hospitals: Meta-Analysis and Models
- Rab11 Regulates Trafficking of -sialidase to the Plasma Membrane through the Contractile Vacuole Complex of
- Mitogen and Stress Activated Kinases Act Co-operatively with CREB during the Induction of Human Cytomegalovirus Immediate-Early Gene Expression from Latency
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- A Central Role for Carbon-Overflow Pathways in the Modulation of Bacterial Cell Death
- An Invertebrate Warburg Effect: A Shrimp Virus Achieves Successful Replication by Altering the Host Metabolome via the PI3K-Akt-mTOR Pathway
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
- A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees () Prevails after -Mediated, or , Transmission
- Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance Genes
- Ubiquitin-Mediated Response to Microsporidia and Virus Infection in
- Preclinical Detection of Variant CJD and BSE Prions in Blood
- Toll-Like Receptor 8 Agonist and Bacteria Trigger Potent Activation of Innate Immune Cells in Human Liver
- Progressive Proximal-to-Distal Reduction in Expression of the Tight Junction Complex in Colonic Epithelium of Virally-Suppressed HIV+ Individuals
- The Triggering Receptor Expressed on Myeloid Cells 2 Inhibits Complement Component 1q Effector Mechanisms and Exerts Detrimental Effects during Pneumococcal Pneumonia
- Differential Activation of Acid Sphingomyelinase and Ceramide Release Determines Invasiveness of into Brain Endothelial Cells
- Forward Genetic Screening Identifies a Small Molecule That Blocks Growth by Inhibiting Both Host- and Parasite-Encoded Kinases
- Defining Immune Engagement Thresholds for Control of Virus-Driven Lymphoproliferation
- Growth Factor and Th2 Cytokine Signaling Pathways Converge at STAT6 to Promote Arginase Expression in Progressive Experimental Visceral Leishmaniasis
- Multimeric Assembly of Host-Pathogen Adhesion Complexes Involved in Apicomplexan Invasion
- Biogenesis of Influenza A Virus Hemagglutinin Cross-Protective Stem Epitopes
- Adequate Th2-Type Response Associates with Restricted Bacterial Growth in Latent Mycobacterial Infection of Zebrafish
- Protective Efficacy of Passive Immunization with Monoclonal Antibodies in Animal Models of H5N1 Highly Pathogenic Avian Influenza Virus Infection
- Fructose-Asparagine Is a Primary Nutrient during Growth of in the Inflamed Intestine
- The Calcium-Dependent Protein Kinase 3 of Influences Basal Calcium Levels and Functions beyond Egress as Revealed by Quantitative Phosphoproteome Analysis
- A Translocated Effector Required for Dissemination from Derma to Blood Safeguards Migratory Host Cells from Damage by Co-translocated Effectors
- Functional Characterization of a Novel Family of Acetylcholine-Gated Chloride Channels in
- Both α2,3- and α2,6-Linked Sialic Acids on O-Linked Glycoproteins Act as Functional Receptors for Porcine Sapovirus
- The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
- MicroRNA-146a Provides Feedback Regulation of Lyme Arthritis but Not Carditis during Infection with
- Recombination in Enteroviruses Is a Biphasic Replicative Process Involving the Generation of Greater-than Genome Length ‘Imprecise’ Intermediates
- Cytoplasmic Viral RNA-Dependent RNA Polymerase Disrupts the Intracellular Splicing Machinery by Entering the Nucleus and Interfering with Prp8
- and Are Associated with Murine Susceptibility to Infection and Human Sepsis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání