-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSystematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance Genes
Clinical infections by the yeast-like pathogen Candida glabrata have been ever-increasing over the past years. Importantly, C. glabrata is one of the most prevalent causes of drug-refractory fungal infections in humans. We have generated a novel large-scale collection encompassing 619 bar-coded C. glabrata mutants, each lacking a single gene. Extensive profiling of phenotypes reveals a number of novel genes implicated in tolerance to antifungal drugs that interfere with proper cell wall function, as well as genes affecting fitness of C. glabrata both during normal growth and under environmental stress. This fungal deletion collection will be a valuable resource for the community to study mechanisms of virulence and antifungal drug tolerance in C. glabrata, which is particularly relevant in view of the increasing prevalence of infections caused by this important human fungal pathogen.
Published in the journal: . PLoS Pathog 10(6): e32767. doi:10.1371/journal.ppat.1004211
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004211Summary
Clinical infections by the yeast-like pathogen Candida glabrata have been ever-increasing over the past years. Importantly, C. glabrata is one of the most prevalent causes of drug-refractory fungal infections in humans. We have generated a novel large-scale collection encompassing 619 bar-coded C. glabrata mutants, each lacking a single gene. Extensive profiling of phenotypes reveals a number of novel genes implicated in tolerance to antifungal drugs that interfere with proper cell wall function, as well as genes affecting fitness of C. glabrata both during normal growth and under environmental stress. This fungal deletion collection will be a valuable resource for the community to study mechanisms of virulence and antifungal drug tolerance in C. glabrata, which is particularly relevant in view of the increasing prevalence of infections caused by this important human fungal pathogen.
Introduction
Candida glabrata, a small, asexual, haploid yeast, is the second most frequent cause of candidiasis after Candida albicans, accounting for approximately 15%–25% of clinical cases [1]–[4]. C. glabrata forms part of the normal microbial flora in humans, but can cause serious infections in immunocompromised and hospitalized patients; antibiotic exposure and presence of central venous catheter devices, being additional important risk factors for disease development [2]. In contrast to the pleomorphic diploid C. albicans [5], C. glabrata is found clinically, exclusively as monomorphic yeast cells. It also lacks several attributes considered key mediators of fungal pathogenicity in other Candida spp, such as secretion of proteases and lipases [6], [7]. Despite the apparent absence of these well-known fungal virulence traits, C. glabrata remains highly pathogenic to humans. Hence, C. glabrata may rely upon distinct strategies and other virulence attributes to initiate infection, as well as to persist in infected patients.
Some traits that have been linked to the clinical importance and virulence of C. glabrata include an inherently elevated tolerance to azole antifungals [8]–[11]; the presence of a large repertoire of telomere-associated adhesins [12]–[18]; melanin-like pigment production [19]; adaptation to the acidic phagosomal environment and intra-phagosomal survival [20]–[22]; and Ace2-dependent components of the cell wall [23]. However, it is clear that the molecular basis of C. glabrata virulence is far from being completely understood.
C. glabrata clinical isolates generally exhibit a high inherent tolerance level to azole drugs [9]. While this trait has been extensively studied, the underlying mechanisms remain incompletely explained. Azole resistance can be acquired through increased expression of genes encoding ABC transporters (Cdr1, Pdh1, Snq2) or changes in their transcriptional regulatory system (Pdr1, Gal11) [24]–[28]. Mitochondrial dysfunction [29] and serum utilization via the putative sterol transporter Aus1 [30], [31] also impact the ability of C. glabrata to tolerate high azole levels. Notably, calcineurin signaling has been implicated in azole tolerance in C. glabrata [32]. It remains unclear if these are the only mechanisms driving azole resistance in C. glabrata. However, the clinical implications of this resistance demand alternative antifungals for effective treatment of C. glabrata infections in patients, especially since C. glabrata infections are globally rising, sometimes accounting for more than 30% of clinical cases [33].
Echinocandins such as caspofungin (CF), anidulafungin and micafungin are a relatively new class of effective yet high-cost antifungal drugs targeting fungal 1,3-β-D-glucan synthases and thereby impairing cell wall integrity [34]. Mutations in subunits of glucan synthases can render fungi resistant to echinocandin [34]–[37]. Surprisingly, even ectopic overexpression of the C. albicans Cdr2 efflux ABC transporter gene in both laboratory strains and clinical isolates markedly increases CF tolerance [38]. Furthermore, the Hsp90 heat shock protein has also been identified as a regulator of echinocandin tolerance acting through calcineurin signaling [36], [39]. However, a better understanding of molecular mechanisms modulating echinocandin susceptibility is necessary, since it may facilitate targeted drug discovery, especially in the case of emerging resistant strains [40]. Importantly, an increase in the number of echinocandin resistant C. glabrata clinical isolates has been reported recently, implying that many genes can contribute to echinocandin tolerance [41].
Reverse genetics coupled with global functional profiling has proven a powerful approach to identify genes required for specific phenotypes. Functional genomics studies in the non-pathogenic yeast S. cerevisiae have provided the starting point to decipher genotype-phenotype relations as they enabled answers about fundamentally important questions concerning complex genetic interactions and the genetic landscape of yeast. These approaches also unraveled stress response mechanisms and provided new insights into drug susceptibility and morphogenesis [42]–[49]. Heroic efforts by a few groups have recently resulted in highly useful genome-scale deletion collections of the major pathogen C. albicans [50]–[52] and Cryptococcus neoformans [53], enabling the identification of novel virulence genes and further demonstrating the power of a functional genomics approach.
Here, we have adapted a semi-automated approach [51] for constructing gene deletions to generate a collection of individually bar-coded strains in the sequenced C. glabrata strain ATCC2001 [54], each lacking one defined open reading frame. We took advantage of this library to undertake the first systematic functional-genomic and phenotypic analysis of C. glabrata, in particular examining the response to traits putatively implicated in virulence and antifungal tolerance of this human pathogen of increasing importance and prevalence. We performed a series of growth assays in distinct media to determine the impact of gene deletion on fitness. We then determined the susceptibility of the collection to major antifungal compounds (including azoles and caspofungin) and various other cell wall-damaging compounds. Finally, we investigated the effect on cellular morphology on fitness in vitro, and the ability to form biofilms. This enabled us to generate the first large-scale chemogenetic and phenotypic profile of C. glabrata. Our analysis revealed numerous novel genes implicated in stress response, cell wall homeostasis, growth morphology and fitness. Most importantly, we discovered numerous novel genes implicated in susceptibility to echinocandins, demonstrating the usefulness of this deletion collection for the functional analysis of virulence-related, as well as clinically relevant traits, including the discovery of novel antifungal target genes.
Results
Gene selection
Using a large-scale phylogenetic approach across many fungal species [55], we identified 1047 putatively non-essential candidate genes in C. glabrata representing functional GO categories such as environmental stress sensing and signaling (MAPK pathways, TOR, RIM, PKA), transcriptional regulation, antifungal drug resistance (PDR network, membrane permeases), cell wall structure and homeostasis (glucan, mannan, chitin synthesis, glycosylation, adhesins, glycosylphosphatidylinositol (GPI)-anchor), chromatin and histone modification, iron metabolism and metal sensitivity, as well as peroxisome biogenesis. We also selected genes lacking obvious orthologues in S. cerevisiae (Table S1).
Parental recipient strains
To enable the rescue of deletion phenotypes, and to facilitate double - or triple mutant construction, we engineered a triple-auxotrophic recipient strain in the sequenced strain C. glabrata ATCC 2001 [54]. We used the dominant recyclable nourseothricin resistance marker SAT1 [56] to replace the coding sequences of HIS3, LEU2 and TRP1 (Figure S1A). The repeated use of this marker cassette resulted in the new C. glabrata background recipient strain for deletions referred to as HTL (his3Δ::FRT leu2Δ::FRT trp1Δ::FRT), as well as all possible isogenic single deletions and all combinations of double deletion strains (Table S2). We also constructed a bar-coded version of the HTL strain, C. glabrata HTL reference, by inserting 20 bp barcodes flanking the trp1 locus (Table S2).The transcription factor (TF) mutants were made in a his3 derivative of ATCC2001, the majority using a codon-optimized version of the NAT1 marker [57]. We avoided the use of the URA3 marker in C. glabrata, since it is known to alter virulence properties of C. albicans [58], [59]. The HTL strain displayed similar growth properties and rates as the parental strain, on both minimal and full media (Figure S1C). Importantly, the auxotrophic markers did not significantly influence growth in vitro, or the survival in immunocompetent mice when compared to the parental wild type strain [60]. While the growth behavior of C. glabrata HTL was largely unchanged, these cells reached a slightly lower maximal cell density when growing in minimal media when compared to wild type cells (Figure S1C).
Gene deletion strategy
Targeted gene disruption with short-homology flanking regions, as was done to construct the S. cerevisiae knock-out library, is inefficient in C. glabrata. Higher targeting efficiency requires the use of ≥500 base pair (bp) flanking regions [61]. Therefore, to maximize efficiency of gene replacement, we adapted and automated the fusion PCR technique [51] to generate gene deletion constructs containing ∼500 bp homologous flanking regions for every gene, fused to the dominant marker NAT1 and flanked by unique barcode identifiers (Figure 1A). We employed a limited set of barcode sequences selected from those successfully used in the S. cerevisiae genome deletion project [43], [49]. These barcodes enable quantification and tracking of single mutants in pool experiments. A complete list of all barcode sequences corresponding to each deleted ORF in the collection is given in Table S3.
Fig. 1. Generation of C. glabrata mutants and systematic phenotypic analysis. 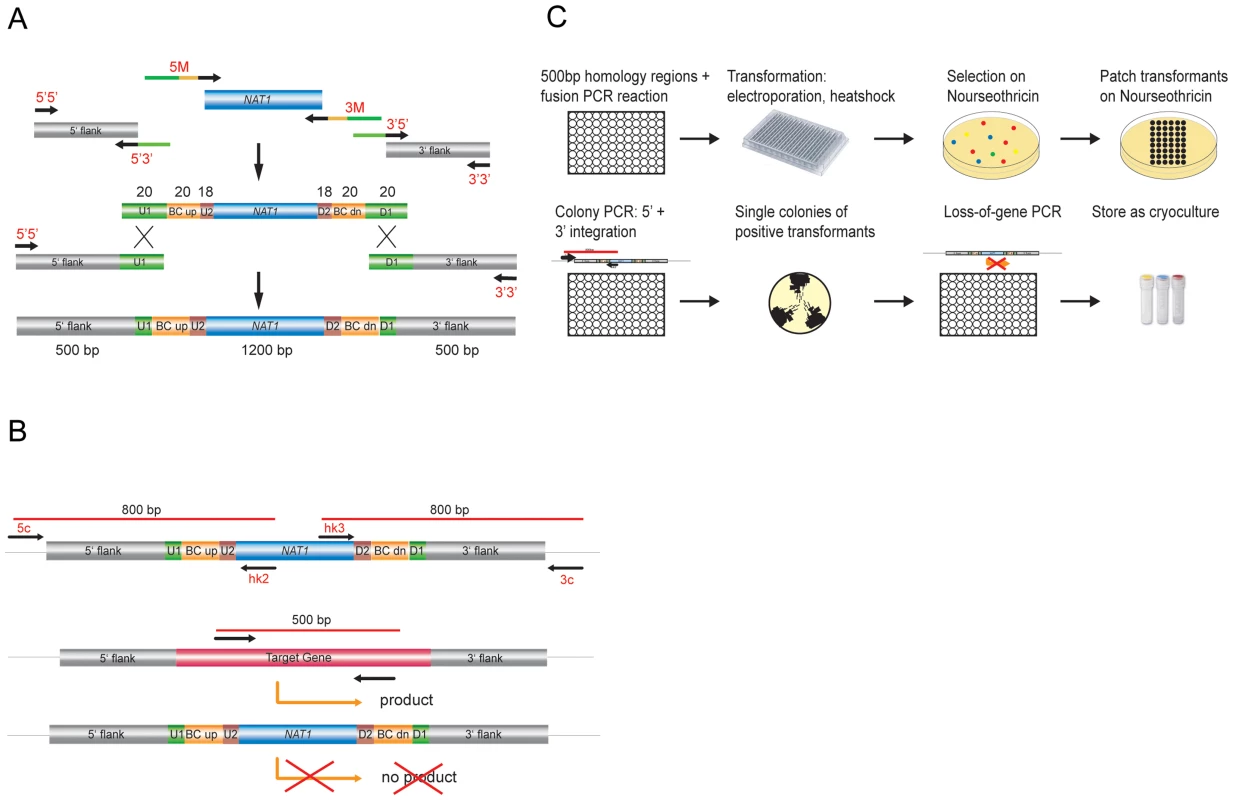
(a) Generation of gene deletion constructs by fusion PCR using the dominant selectable marker NAT1. A set of two times 96 unique barcode sequences was integrated in oligonucleotides to amplify the marker fragment and to add overlap sequences. (b) Transformants were verified by colony PCR for correct integration on the 5′ and 3′ junction and checked for absence of the target ORF. (c) Overview of the construction of the gene deletion strain library. C. glabrata recipient strains were transformed with these deletion cassettes using a modified 96-well format electroporation protocol. All resulting nourseothricin-resistant transformants were tested for correct genomic integration by colony PCR to verify both 5′ and 3′ junctions (Figure 1B). Single colonies of up to six verified transformants for each gene were isolated; the absence of the corresponding gene from the genome was confirmed using another PCR-based step and confirmed gene deletion strains were cryo-preserved (Figure 1B, C). A total of 24 PCR-verified gene deletion strains were randomly selected and subjected to Southern blot analysis to confirm correct genomic replacements (data not shown).
In all, we successfully deleted 619 of the 1047 genes initially selected for inactivation, yielding a total of 1601 independent unique deletion strains (Table S3; http://funpath.cdl.univie.ac.at), representing 59% of the selected genes and about 12% of the entire C. glabrata genome. Notably, for 77 genes only one deletion was obtained, while the majority of deletions result from two (246) or three (224) independent transformation and thus genomic removal events (Table S3). We assume that a large fraction of the genes that we failed to inactivate is a consequence of inefficient homologous targeting, rather than a true representation of the frequency of essential genes.
Validation of screening conditions
Fungal pathogens need to adapt to diverse host immune defense mechanisms and environmental stresses. Relevant stress conditions employed include perturbations of cell wall integrity, osmostress during phagolysosome maturation and growth at elevated temperature as well as often exposure to antifungal drugs. An efficient adaptation to particular stress conditions may also include formation of biofilms. We therefore phenotypically profiled the mutant collection to identify genes implicated in the response to host-mimicking adverse conditions. First, we carried out a preliminary pilot screen using a small set of selected deletion mutants displaying known phenotypes. Mutants lacking genes of the high osmolarity glycerol (HOG) pathway, the cell integrity protein kinase C (PKC) pathway and the pleiotropic drug resistance (PDR) network were tested for growth under conditions known to affect the corresponding mutants in S. cerevisiae. As expected, lack of PBS2, encoding the central MAPK (mitogen-activated protein kinase) of the HOG pathway [62], resulted in severe osmosensitivity (Figure S2A). Likewise, azole hypersensitivity was observed in cdr1 and pdr1 strains (Figure S2B) [24]–[26], [28], [63], [64]. Finally, as previously shown cells lacking the Slt2 kinase of the PKC pathway [65], [66] displayed drastic hypersensitivities to CF (Figure S2C).
Following these initial experiments, which served to establish and validate screening parameters, the collection of 1601 gene deletion mutants was subjected to extensive phenotypic profiling in four independent laboratories using the same conditions. The deletion library was screened for various phenotypes, including growth defects in YPD at 30°C, defects in biofilm formation, sensitivities against antifungal drugs (azoles, amphotericin B (AmB)) in liquid medium, and cell wall-perturbing agents (Congo Red (CR), Calcofluor White (CW) and CF), as well as heat stress (42°C) and osmostress (NaCl) on solid media. Furthermore, colonies were also inspected for obvious morphology alterations. A total number of 196 mutants showed phenotypes different from the wild type control for at least one condition tested. A summary of all of these mutants with their phenotypes is provided in Table S4.
Growth and fitness defects
Changes in the pathogenic potential of a fungus may be associated with a gain or loss of in vitro or in vivo growth, influencing the ability to efficiently replicate in the host or to withstand antifungal treatment [67]. Thus, we measured the growth rates of individual mutants in the deletion collection in YPD at 30°C. For each deletion strain, a relative fitness index [68] was calculated using the doubling times from at least two independent cultures of 1–6 independent mutants, and compared to the doubling time of all other strains. Notably, we identified 57 deletion mutants showing strong variations in relative fitness between independent cultures (Table S5), all of which were omitted from further analysis along with deletions that were present in duplicate. Hence, reproducible fitness data could be obtained for 503 unique mutants (representing 1125 deletions). Out of these gene knock-outs, 402 had a relative fitness index within two standard deviations (SD) of the average relative fitness (Figure 2A, Table S6). These data suggested that the corresponding genes are not necessary for efficient planktonic growth of C. glabrata in YPD at 30°C. However, 70 strains showed a significant fitness defect (doubling time of ≥2 SD below the average fitness). Interestingly, another 34 deletions showed a gain in fitness (doubling time of ≥2 SD above the average fitness) under these growth conditions (Figure 2A, Table S7).
Fig. 2. Relative fitness distribution and morphology of C. glabrata gene deletion strains. 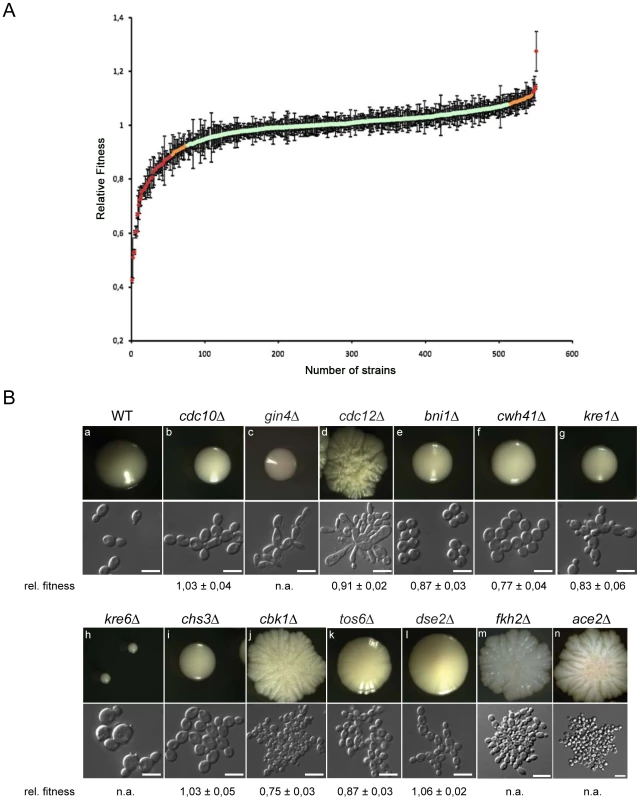
(a) Wild type and mutant strains were grown in rich medium at 30°C and doubling times were recorded. The median doubling time of the wild type C. glabrata ATCC2001 strain under these conditions was 63.9 min while the median for mutant strains was 68.1 min. For each strain, the relative fitness was calculated using doubling times from at least two independent cultures of 1–6 independent mutants. Strains that showed strong variations in relative fitness between independent mutants or independent cultures were omitted from further analysis (Table S5). Data were obtained for 503 knock-out mutants (Table S6). (b) Colony and cell morphologies of C. glabrata deletion strains. Different types of distinct cell and colony morphologies were found. Cell morphology classes: ellipsoid (a), chains (b, f, g, i, k, l), elongated (c, d), large clumps (d, j, m, n), round (e), large and round (h); colony morphologies: smooth (a, b, c, e, f, g, i), small (h), slightly wrinkled (k, l), wrinkled (d, j, m, n). WT = HTL background strain; white bars correspond to 10 µm. A total of 13 deletion strains showed obvious alterations of both colony and cellular morphology (Figure 2B). Five of these mutants were also shown to exhibit fitness defects in vitro (Table S6). Most morphology mutants grew as small or large wrinkled or smooth colonies. Microscopic inspection of deletion mutant phenotypes allowed for further classification into different cell morphology classes, including round cells (dse2, tos6, ace2, chs3, bni4, cwh41), giant cells with obvious structural defects (cdc21, cbk1, kre6), cells with pseudohyphal-like elongated morphologies (gin4), and as pearl-string-like cells connected to each other as for dse2 mutants (Figure 2B). Notably, except for pseudohyphal morphologies [69], [70] or small round cells as for ace2 [23], many of these morphological alterations have not been described in C. glabrata to date.
Biofilm formation
Biofilm formation on indwelling medical devices represents a significant risk for invasive infections by Candida spp, since biofilms display both increased drug tolerance and represent a persistent source of shedded cells that disseminate via the blood stream [71]–[73]. Hence, mutants in the deletion collection were scored for their ability to form biofilms. Strains were induced to form biofilms in 96-well polystyrene plates in minimal medium at 37°C; the biofilm biomass was quantified by determining the metabolic activity using a fluorescein diacetate (FDA) accumulation assay [74], [75]. For each strain, a relative biofilm-forming index was calculated using FDA hydrolysis data from at least two independent cultures of the independent deletion mutants for each gene (see Materials & Methods; Table S8, S9, S10). Independent deletions of the same gene including duplicates showing strong variations in relative biofilm formation between independent assays or independent cultures (Table S8) were omitted from further analysis, leaving 420 deletions for which biofilm production was analyzed in detail (Figure 3; Table S9).
Fig. 3. Relative biofilm fitness distribution. 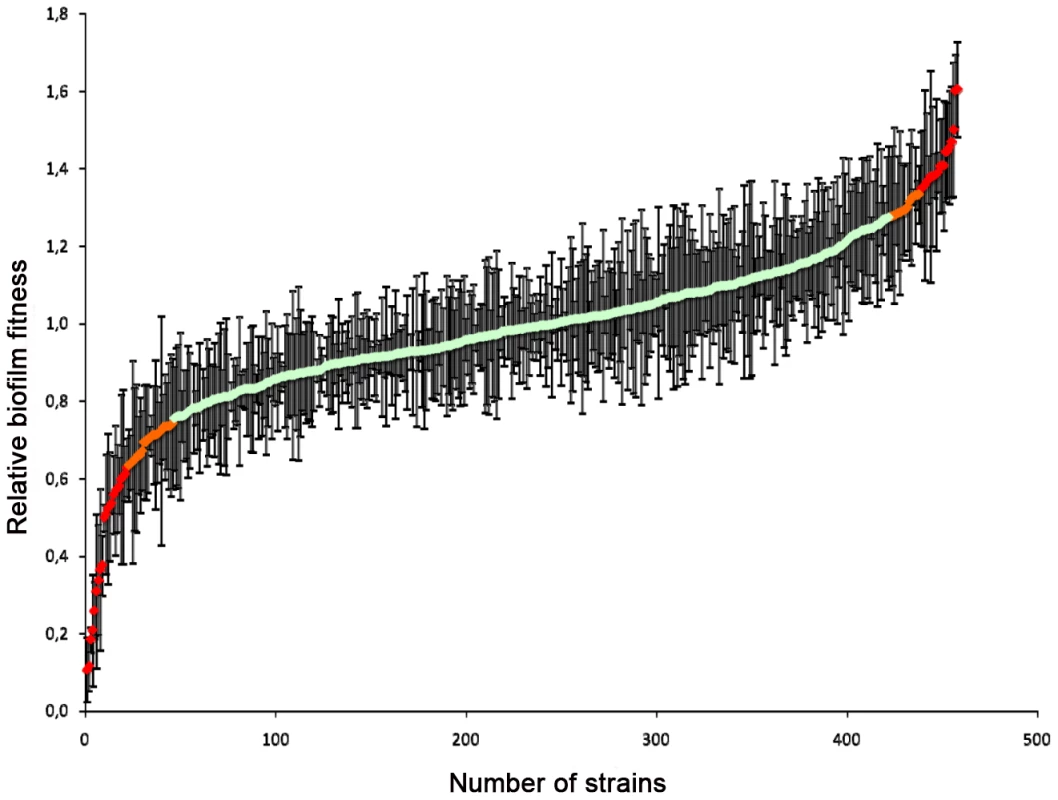
Wild type and mutant strains were induced to form biofilms in 96-well polystyrene plates in minimal medium at 37°C and the biofilm biomass was quantified using fluorescein diacetate (FDA). For each strain, a relative biofilm fitness was calculated based on FDA hydrolysis data from at least two independent cultures. Data were obtained for 420 knock-out mutants (Table S9). Strains that showed strong variations in relative fitness between independent mutants or independent cultures were omitted from further analysis (Table S8). Out of these, 341 gene deletions resulted in a relative biofilm forming index within two standard deviations (SD) of the average relative biofilm-forming capacity (Figure 3; Table S10), suggesting that the corresponding genes do not contribute to biofilm formation in minimal medium at 37°C. Isolates for the 46 gene deletions that resulted in an alteration in biofilm formation (i.e. a biofilm fitness score 2 SD below or above the average relative biofilm-forming index) but not of planktonic growth at 30°C were retested in quadruplicate for their ability to form biofilms. Moreover, the planktonic growth rate of these strains in minimal medium at 37°C was monitored to assess possible effects of the higher incubation temperature on biofilm formation. Notably, four of the corresponding deletion strains, namely those corresponding to FKH2, PKH2, SNF1 and ACE2, also displayed temperature-sensitive phenotypes and may therefore not be solely biofilm-specific. Yet, this analysis identified 14 gene deletions that resulted in significant defects in biofilm formation but no significant defect in planktonic growth, including AVO2, BCY1, CCW12, CCH1, CNB1, DCW1, GAS1, MHP1, PKH2, SLM1, SUB1, UTP14, YNL300W and YOR1 (Table S9). Remarkably, we identified 11 gene knock-outs resulting in a gain in biofilm formation without an increase in planktonic growth, including BPH1, GAL11, GPB2, MIG1, PEX2, SSN2, SSN8, STE20, YAP6, YDR134C and YVC1 (Table S9).
Susceptibility to azole and AmB antifungal drugs
Azoles and AmB remain the most common drugs for treating fungal infections. The inherently reduced azole susceptibilities of most C. glabrata clinical isolates is considered a major contributor to the increasing clinical prevalence of this pathogen [76]. While this is mainly the consequence of transcriptional upregulation of the CDR1 and PDH1 (CDR2) encoding membrane efflux pumps or gain-of-function mutations in the PDR1 regulator [28], additional mechanisms may play a role. The polyene AmB is thought to impair membrane function by binding to ergosterol, resulting in cellular leakage of cytoplasm [77]. While C. glabrata can develop AmB tolerance [78], [79], the underlying molecular mechanisms remain obscure. We have thus used the C. glabrata deletion collection to identify genes modulating azole as well as AmB susceptibility.
A total of 14 deletion strains displayed marked hypersensitivities to azoles such as fluconazole and voriconazole albeit to a different extent (Figure S5A, C; Figure 4B). Moreover, 13 mutants were hypersusceptible to AmB (Figure S6A, Figure 4B). The corresponding mutants were retested using microdilution assays to quantify their IC50 values (Figure S5B, D; Figure S6B). The majority of the 14 azole-sensitive strains were sensitive to both fluconazole and voriconazole (Figure S5A, C), while 6 strains (ktr2, cwh41, ssd1, ktr6, hap1 and slt2) appeared more sensitive to voriconazole. Notably, the strain lacking the KTR2 gene encoding a mannosyltransferase [80] displayed the most significant voriconazole-specific hypersensitivity (Figure S5C, D). As expected, deletion of either the PDR1 transcription factor or its target CDR1 efflux pump resulted in marked azole hypersensitivity (Figure 4B, Figure S2, S8D). Furthermore, calcineurin pathway mutants such as cna1 and cnb1 also displayed pronounced azole hypersensitivities, as also shown by previous reports [32], [81]. Several additional signaling mutants (slt2, wsc1, ypk1, cka2), as well as cell wall mutants (ktr2, cwh41, ssd1, ktr6) displayed slight to intermediate azole hypersensitivities (Figure S5A, B; Figure 4B). Among the 13 AmB-sensitive strains, the five genes displaying the most pronounced susceptibilities play diverse roles in phospho - and sphingolipid signaling, including YPK1, CKA2, DEP1, SNF6 and VPS15.
Fig. 4. Classification of C. glabrata chemogenetic profiles. 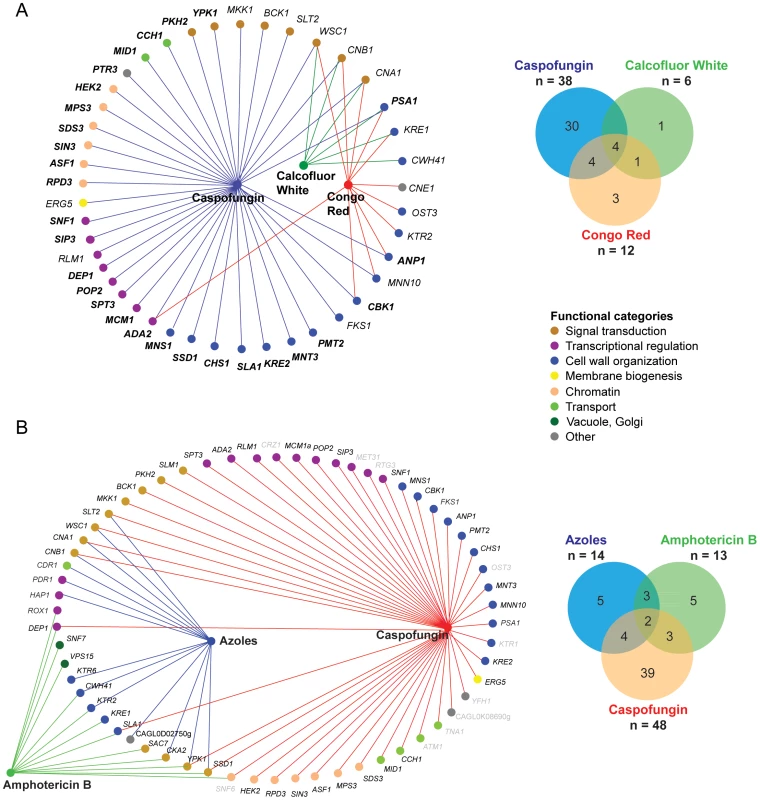
(A) Overlap between the chemical-genetic profile of CF (38 genes), CR (12 genes) and CW (6 genes). 28 genes (labeled in bold letters) were novel CF tolerance genes, since they have not been previously associated with echinocandin hypersensitivity in S. cerevisiae or C. albicans. (B) Overlap between the chemical-genetic profile of CF, azoles and AmB. 61 genes displayed an increased sensitivity to CF (38 genes plus 10 genes with weak sensitivity), azoles (14 genes; fluconazole and voriconazole) and AmB (13 genes). The mutant strain collection was screened in a 96 well microplate format using an endpoint assay and medium was supplemented with 5 µg/ml fluconazole, 100 ng/ml voriconazole and 1,5 µg/ml AmB. The OD600 was determined after 24 and 48 hours of incubation at 30°C. Grey-colored genes only display a weak sensitivity phenotype or were excluded from further analysis due to strong variations in the screening. Nodes represent compounds or genes and edges indicate chemical-genetic interactions. Gene nodes are color-coded according to GO annotation. Venn diagrams summarize distribution of genes affecting resistance to one of the three compounds. Cell wall stress, osmostress and heat sensitivity
The fungicidal echinocandins stand out as the most efficient clinically used drugs that block cell wall glucan biogenesis. Thus, we subjected the deletion collection to profiling for susceptibilities to the caspofungin (CF) echinocandin, as well as other cell wall stressors such as CR and CW (Figure 4, Figure 5, Figure S7). A total of 12 mutants were strongly hypersensitive to CR, and six to CW (Figure 4A; Figure S7C). Unsurprisingly, deletion of genes encoding functions implicated in cell wall integrity or polarity (PSA1, KRE1, CWH41, OST3, KTR2, MNN10, ANP1, CBK1) conferred hypersensitivity to CR and/or CW. Strikingly, we identified 48 mutants with altered CF susceptibilities (Figure 4A, B), 38 of which were strongly hypersensitive, while another 10 were mildly CF-sensitive, including RTG3, YFH1, TNA1, ATM1, SNF6, CRZ1 (Figure 4, Figure S7).
Fig. 5. Clustering of susceptibility data of the C. glabrata gene deletion collection. 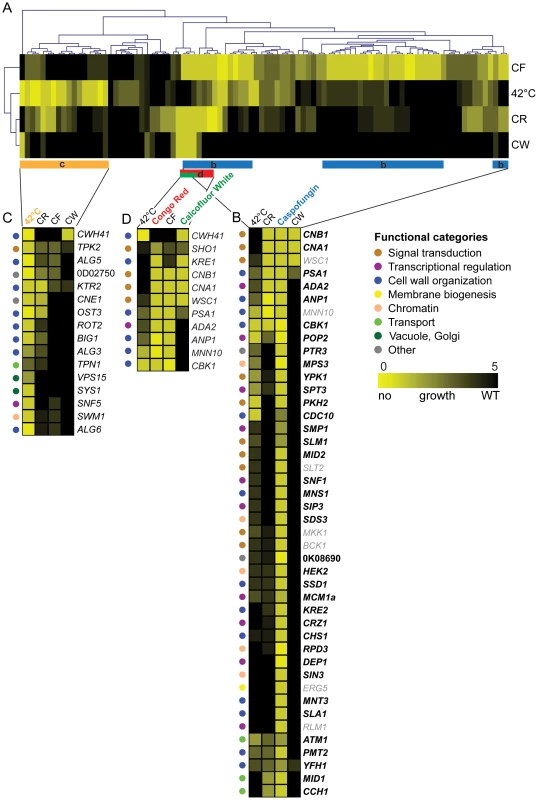
The set of mutants was screened on plates for hypersensitivity to four distinct stress conditions (CF, CR, CW and 42°C heat stress) using serial dilution assays on agar plates. (A) Two-dimensional hierarchical cluster plot of chemical-genetic profiles. On the horizontal axis genes are listed and stress conditions on the vertical axis. Interactions are shown in yellow depending on the degree of growth sensitivity (yellow = no growth, black = WT growth). Stress conditions and genes are clustered by the similarity of their interactions. (B) The clusters of genes are enlarged to highlight the chemical-genetic interaction profile of Caspofungin (blue bar in ‘a’ labeled ‘b’). (C) A section of the cluster (orange bar labeled ‘C’) involved in heat stress (42°C) is enlarged. (D) Genes implicated in sensitivity to the cell wall-perturbing agents CR and CW (red and green bar labeled ‘D’). To identify the functionally overlapping mutants, we used hierarchical clustering of stress-induced phenotypes, including heat, CF, CR and CW, identifying some 106 putative chemical-genetic interactions (Figure 5). The clustering approach identified three subsets of deletion mutants displaying distinct but partially overlapping hypersensitivities to high temperature, CR, CW and CF (Figure 5). Remarkably, the profiling analysis revealed some 28 novel CF tolerance genes, none of which had previously been associated with echinocandin hypersensitivities in S. cerevisiae, C. albicans or in other fungal pathogens (Figure 4, Figure 5). Importantly, CF sensitivity phenotypes of a subset of these mutants were confirmed in different strain backgrounds, including unrelated clinical isolates (Figure 6, Figure S8). Furthermore, restoration of the wild type phenotypes upon reintroduction of the corresponding genes confirmed that the observed caspofungin sensitivity in deletion strains is specifically caused by the lack of the respective gene (Figure S9). Notably, the group of genes affecting CF sensitivity contained several genes operating in the PKC cell integrity signaling pathway (WSC1, SLT2, BCK1, MKK1), in calcium/calcineurin signaling (CNA1, CNB1, MID1, CCH1), general cell wall homeostasis, including mannosylation and glycosylation (MNN10, ANP1, MNS1, MNT3, PMT2, PSA1, KRE2), as well as transcriptional regulators (RLM1, DEP1, POP2, SPT3, MCM1, SIP3, SNF1). Interestingly, deletion of several genes encoding components of the chromatin and histone modification machinery (RPD3, HEK2, MPS3, SDS3, SIN3, ASF1) also modulated CF susceptibility, suggesting an important regulatory role for chromatin in controlling surface homeostasis and CF susceptibility, as recently demonstrated for the C. albicans Hat1 acetyltransferase [82]. The removal of only four genes (WSC1, CNB1, CNA1 and PSA1) resulted in sensitivity to all three cell wall stressors (Figure 4A), confirming the pivotal roles PKC and calcineurin signaling pathways play in sensing and maintaining cell wall homeostasis in fungal pathogens [36], [83], [84].
Fig. 6. Caspofungin sensitivity of C. glabrata clinical isolate deletion strains. 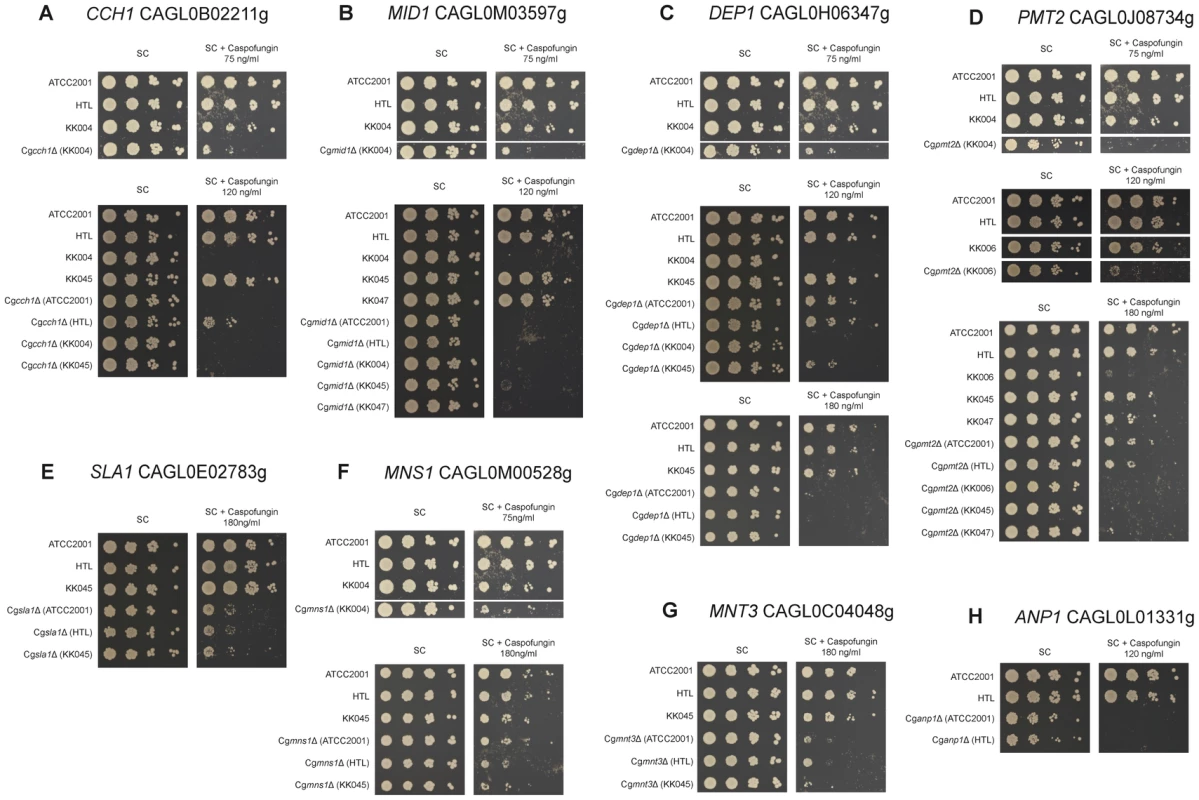
Sensitivities of deletion strains constructed in clinical isolates (KK004, KK006, KK045 and KK047) or in the ATCC2001 strain were tested for caspofungin (CF) susceptibility on plates. Strains were spotted in serial dilutions on synthetic agar medium supplemented with the indicated CF concentrations and growth was monitored for 3 days at 30°C. Screening was performed for (A) CCH1, (B) MID1, (C) DEP1, (D) PMT2, (E) SLA1, (F) MNS1, (G) MNT3 and (H) ANP1. Notably, the heat stress profiling on plates identified several genes implicated in cell wall biogenesis and organization, including CWH41, ALG5, OST3, ALG6, KTR2, BIG1, CBK1 and ALG3. Interestingly enough, this gene set showed some overlap with the CF, CR or CW gene clusters (Figure 5), suggesting that heat stress triggers partially overlapping signaling response pathways, ranging from stress and cell integrity signaling to cell wall homeostasis and membrane lipid perturbation.
Moreover, we also identified remarkably strong osmosensitivity phenotypes caused by the loss of genes that have never been linked to osmostress in other fungi before, including ATM1, ARB1, KRE1, ANP1, MPS3 and PTR3 (Figure S3). These data suggest that even the highly conserved fungal osmosensing pathway has acquired additional and/or novel components in C. glabrata.
Discussion
C. glabrata is an important human fungal pathogen and, after C. albicans, the second-most frequent cause of candidiasis, causing 15–30% of infections in humans [1]–[4]. Although C. glabrata appears similar to S. cerevisiae concerning gene synteny and conservation it is an obligate haploid, and the lack of a sexual cycle and elevated rates of non-homologous recombination have prevented a systematic genetic analysis. Importantly, the decreased sensitivity of C. glabrata clinical isolates to azole antifungals is at least in part responsible for its clinical significance [33], and recent clinical reports suggest that azole antifungal resistance in C. glabrata, including multidrug resistance, is emerging at a rapid pace, thus posing an important challenge to therapeutic management [40], [85].
To aid in addressing these issues, as well as to better understand virulence properties, we have generated a large-scale deletion collection of C. glabrata representing almost 12% of the genome. This deletion mutant collection that comprises 619 unique strains each lacking a single gene has several advantages over known transposon-based mutant collections [86], since each ORF is completely removed. Thus, gene dosage effects or dominant phenotypes from truncated protein variants are excluded. Likewise, aberrantly expressed proteins due to insertion in promoters, which may result in partial loss or gain-of-function for the corresponding gene product, are precluded. The deletion collection was subjected to extensive phenotypic profiling, with a particular emphasis on susceptibility to major antifungal drugs, growth and morphology phenotypes, stress response pathways, cell wall biogenesis as well as biofilm formation, which has been associated with the initiation and development of candidiasis [87]. Importantly, we show here the feasibility to generate a genome-scale bar-coded gene deletion library in C. glabrata, revealing novel genes contributing to fitness, biofilm formation, antifungal drug resistance and several genes implicated in general cell wall homeostasis. We found 196 mutants sensitive to at least one of the tested conditions. In summary, 21% of the tested mutants display altered fitness phenotypes and 19% show alterations in biofilm formation, while only 2% exhibit abnormal cell and colony morphology. Approximately 16% of the mutants show severe stress-related phenotypes, including hypersensitivity to several antifungal drugs, heat and osmostress conditions.
General fitness defects
C. glabrata and S. cerevisiae are phylogenetically closely related [54]. Hence, many orthologous gene functions may have been conserved between the two species. In our study, however, we found several mutants with reduced or increased fitness in rich media corresponding to genes whose inactivation in S. cerevisiae resulted in entirely different or opposing phenotypes. These include homologous genes from the RAS-PKA pathway (RAS2, GPA2 and GPR1) and genes involved in N-glycosylation and outer chain elongation (ALG5, KTR2 and MNN4). Moreover, several genes known to be essential in S. cerevisiae were non-essential in C. glabrata, although their inactivation often confers reduced fitness or morphology defects. These include MEC1, encoding a master DNA damage checkpoint kinase [88], and CBK1, although the latter is only essential in the yeast background S288c [89]. CBK1 encodes a RAM network kinase that is central to the establishment of cell polarity and involved in septum formation and cell separation and morphogenesis [90]–[93]. Notably, inactivation of the orthologous genes in C. albicans is not lethal [93]–[95]. Hence, Mec1 - and Cbk1-dependent regulatory networks may operate differently between S. cerevisiae and C. glabrata and, possibly, other hemiascomycetous yeasts. Many biological processes and regulatory networks in fungi have evolved under distinct evolutionary pressures [96], [97]. Because Candida spp. have evolved as opportunistic pathogens of mammals, including humans, it is fair to propose that adaptation to the host environment and immunity surveillance may have been driving the evolution of functionally rewired genetic regulatory networks [98].
Biofilm genes
Deletion strains displaying altered ability to form biofilms include, as expected, several mutants lacking cell wall-related genes, whose absence disturb normal cell wall homeostasis; not surprisingly, these mutants also strongly altered CF susceptibility. Lack of the putative kinase gene PKH2/CAGL0I07513g, whose orthologue in S. cerevisiae encodes a component of the alternative cell wall integrity pathway affected biofilm formation. In C. glabrata, PKH2 has two additional paralogues, CAGL0G04609g and CAGL0K06479g. While pkh2 mutants are defective in both biofilm formation and show temperature-defective growth on solid media, lack of the other two putative paralogues leaves biofilm formation and temperature sensitivity unaffected (data not shown). These data suggest that apparent PKH kinase homologues in C. glabrata have acquired some specialization concerning their roles in sensing cell wall integrity or regulating cell surface homeostasis.
Antifungal drug sensitivity
We generated a chemical-genetic profile of C. glabrata relevant to the understanding of drug susceptibilities in vitro. In addition to known genes implicated in azole resistance (PDR1, CDR1, PDH1), the profiling for azoles revealed only a small number of novel genes (YPK1, KTR2) mediating azole tolerance, with KTR2 even showing a significant azole-specificity for voriconazole. Our data, as well as data from a wealth of clinical isolates, imply that C. glabrata utilizes a limited set of mechanisms to mount azole resistance, with ABC transporter-mediated efflux by Cdr1 and Pdh1 being the most important one, both in vitro and in vivo [32], [67], [99]–[102]. However, it has been noticed that genomic deletions conferring azole resistance when otherwise mutated or overexpressed do not necessarily change sensitivities in the wild type strain [103], perhaps due to genetic (functional) redundancy or compensatory mechanisms. Thus, we cannot exclude the existence of other mechanisms and genes promoting azole resistance in C. glabrata.
The results from our screen, as anticipated, showed overlap with phenotypic screens in S. cerevisiae [104] and C. albicans [105] regarding sensitivity to CF, which belongs to the family of the fungicidal echinocandin drugs that inhibit the fungal 1,3-β-D-glucan synthase. Out of 38 mutants showing strong alterations in echinocandin sensitivities, 16 correspond to genes whose orthologues in S. cerevisiae and in Candida spp (at least for FKS1) have been linked to CF tolerance [36], [104]–[106]. Hyperresistance to echinocandins can result from mutations in the glucan synthase genes FKS1 or FKS2 [35], as well as through the PKC pathway that mediates CF tolerance in S. cerevisiae [66] and C. albicans [107]. Accordingly, C. glabrata wsc1, slt2, mkk1, bck1, rlm1 and fks1 mutants, all lacking key genes of this central pathway, are also hypersensitive to CF in our assays. This overlap points to commonalities in the response to CF between the three species, and also serves to validate the phenotypic profiling for echinocandin susceptibility. Remarkably, 28 genes whose deletion resulted in marked hypersensitivity to CF in C. glabrata have not previously been associated with CF susceptibility in S. cerevisiae or other fungi including C. albicans [105]. Some of these apparent differences between species likely relates to difficulties in determining CF hypersensitivity phenotypes, given that some strains (cdc12, ace2, fkh2, cbk1) display aberrant cell morphologies or strong growth fitness defects (kre6), all of which interfere with IC-50 quantifications. Regardless, our data clearly identify novel genes implicated in CF tolerance, and imply that these genes may be players in as yet undiscovered CF resistance mechanisms.
Interestingly, cells lacking chitin synthesis genes such as CHS1 were also hypersensitive to the glucan synthase inhibitor, completely consistent with reports from S. cerevisiae [105]. Interestingly, recent data from C. albicans demonstrate a role for chitin in regulating cell wall susceptibility to CF [108], [109]. Likewise, the absence of calcineurin subunit genes CNA1 or CNB1 leads to CF hypersensitivity, indicating that calcineurin signaling is necessary for buffering or compensating cell wall stress, perhaps by affecting FKS1 transcript levels through the transcription factor Crz1 [35]. In the mould Aspergillus fumigatus, a mutation or inhibition of calcineurin enhances the antifungal potency of CF [110]. Thus, calcineurin inhibitors may exert synergistic effects on cell wall mutants in other fungal species. The impact of CF on calcium signaling in C. glabrata is further confirmed by the hypersensitivity of mutants lacking CCH1 and MID1, both of which encode stress-induced calcium channel proteins.
In S. cerevisiae, the Ypk1-mediated signaling pathway, which is activated by lipid-sensing [111], constitutes an alternative cell integrity signaling pathway connected to the classical PKC pathway [112], [113]. Remarkably, C. glabrata cells lacking YPK1 and PKH2 are CF-hypersensitive, suggesting that the Ypk1-mediated signaling plays a major role in regulating CF tolerance or cell wall homeostasis in C. glabrata (Figure 4, Figure S7). Taken together, these results indicate that C. glabrata employs several signaling pathways to respond to CF-induced cell wall damage and the required subsequent cell wall remodeling. It is therefore not unexpected that mutants displaying CF hypersensitivities include genes that affect trafficking of proteins or surface carbohydrate homeostasis, including chitin deposition and biogenesis.
Genes implicated in the susceptibility to both CF and azoles include those encoding the calcineurin subunits Cna1 and Cnb1 and the PKC pathway components Wsc1 and Slt2. These results confirm that both pathways are necessary for a response to both drug classes and are consistent with data in many fungi showing the synergistic action of calcineurin inhibitors and azoles or CF [36], [81], [84], [114], [115]. Comparison of azole-sensitive, AmB-sensitive and CF-sensitive mutants show that only a small number of gene deletion strains are susceptible to all three or to at least two compounds, which is consistent with their distinct mechanism of action. The few deletions conferring sensitivity to all three drugs occur in genes implicated in stress signaling, and include kinases such as Ypk1 and the mRNA-binding protein Ssd1, which is thought to control expression of surface genes in concert with Cbk1 [116]–[120]. Our results are consistent concerning the phenotypes for the corresponding mutants in S. cerevisiae. However, the exact molecular function of Ypk1, a mammalian SGK kinase homologue [121], which regulates sphingolipid biosynthesis and cell integrity pathways in S. cerevisiae, remains unclear in C. glabrata. It is tempting to speculate though that Ypk1 is part of a kinase network implicated in sensing and regulating membrane perturbations, drug sensitivity and lipid-mediated stress signaling [97], [122].
AmB is thought to interfere with normal membrane bilayer function by forming complexes with ergosterol [123]. Indeed, cells deficient in membrane biogenesis or organelle dynamics may show a synthetic fitness loss upon perturbation of the lipid composition, potentially explaining the AmB hypersensitivity of snf7, vps15 and sla1 mutants. A similar reason may explain the sensitivity of cells lacking DEP1, which, in S. cerevisiae, regulates transcription of structural phospholipid biosynthesis genes [124]. Notably, the profiling analysis reveals a strong genetic interaction between AmB action and genes implicated in cell wall function. AmB-sensitive strains include mutants lacking KRE1 and SAC7, which encode proteins implicated in glucan homeostasis, as well as KTR6, KTR2, CWH41, whose products affect surface protein glycosylation. Remarkably, the latter three mutants show pronounced azole hypersensitivity, demonstrating a direct link between membrane lipid perturbation, cell wall function and antifungal sensitivity.
The hierarchical clustering of stress-induced phenotypes caused by heat, CF, CR and CW, identifies some 106 putative chemical-genetic interactions (Figure 5). We expected to discover distinct patterns for each compound, since CR and CW mainly affect cell wall structure and composition, whereas CF targets Fks1. All compounds strongly activate cell integrity signaling, which together drives cell wall remodeling and regulates surface homeostasis. Accordingly, the CF profile was enriched for genes involved in cell wall organization, signaling and transcriptional regulators, reflecting the activity of CF as an inhibitor of fungal cell wall biosynthesis (Figure 4). We were surprised that only a few genes in the CF cluster overlap with genes associated with CR and CW sensitivity (Figure 4A), most of which are involved in stress and cell wall signaling (CNA1, CNB1, WSC1) or cell wall biogenesis (KRE1, PSA1, ANP1, MNN10), indicating that distinct signaling pathways must cooperate to ensure maintenance of a functional cell wall under various adverse conditions.
As expected, our screens for drug susceptibility and cell wall integrity identified C. glabrata orthologues of genes implicated in related processes in other fungi. In addition, we identified novel genes whose functions may be specific to C. glabrata, since none of them has been associated with drug sensitivity or cell wall homeostasis. Because the phenotypes are in general hypersensitivities, these data suggest that at least some genes may represent feasible targets for drug discovery. The discovery of a large number of deletion mutants affecting CF tolerance expands our knowledge about plausible mechanisms regulating CF sensitivity. For example, the fact that several CF-sensitive mutants are implicated in exocytic delivery of cell wall components such as chitin, glucan or mannan, implies a constant cross-talk of distinct signaling pathways to control proper cell wall remodeling upon CF-induced surface damage. As the number of chemical-genetic and genomic data in baker's yeast [45], [46] is steadily increasing, our data add novel information concerning the function of related genes in an important human fungal pathogen. Hence, these data represent an important contribution towards a better understanding of drug resistance mechanisms, as well as species-specific differences.
These large-scale phenotypic profiling data also demonstrate the power of the C. glabrata knock-out collection, which is, in addition to the C. albicans and C. neoformans collections [50], [53], to the best of our knowledge, among the three largest academic deletion collections for a human fungal pathogen. We anticipate that this library will facilitate studies on virulence factors and other aspects of C. glabrata biology. The use of sequence barcodes, which were adopted from the S. cerevisiae gene deletion collection [43], allows for the functional analysis of pools of mutants either in vitro or in vivo [50], [53]. The use of multiple auxotrophic markers, which do not affect in vivo dissemination of strains in standard mouse models of fungal virulence [60] will allow for construction of double or triple mutants, and facilitate genetic interaction studies as well as epistasis analysis of pathway architectures. Notably, screens of the collection in different animal models and the understanding of virulence phenotypes (or lack thereof) can be challenging and laborious requiring a very large number of animals, due to the potential impact of fitness defects on growth in vivo or possible genetic redundancy. We suggest that interpretation of virulence phenotypes will be aided by the in vitro phenotypic analysis presented here, permitting correlation of in vitro phenotypes and in vivo fitness effects for C. glabrata.
Similar to existing C. albicans [50] or C. neoformans [53] collections, the C. glabrata mutant library constitutes a valuable tool for the fungal research community to study the function of virulence and drug resistance genes in C. glabrata. In view of rapid changes in the epidemiology of fungal infections, with C. glabrata infections showing ever-increasing clinical importance reaching up to 30% prevalence in some countries [33], [125], this work is the first large-scale contribution to the systematic analysis of mechanisms implicated in antifungal drug resistance and C. glabrata pathogenicity. Notably, much of the previous work aimed at unraveling the molecular basis of drug resistance mechanisms in C. glabrata have been based on what is known from baker's yeast [104], [126] or other Candida pathogens [105]. Although gene synteny has been largely conserved between pathogenic an non-pathogenic yeasts such as C. glabrata and S. cerevisiae, extensive rewiring of signaling pathways generated distinct and species-specific functions for seemingly orthologous genes. Indeed, our work clearly demonstrates that comparing and predicting drug resistance or virulence phenotypes for C. glabrata based on data from even related yeasts requires extensive experimental verification and the use of loss-of-function approaches. This fungal pathogen deletion collection will pave the way for these future efforts.
Materials and Methods
Ethics statement
The use of clinical C. glabrata isolates was approved through respective ethics committees according to national regulations.
Media and growth conditions
YPD (1% yeast extract, 2% peptone, 2% dextrose) media were prepared as described elsewhere [127]. Synthetic complete (SC) medium contained 0,67% YNB, 2% glucose supplemented with 1×CSM (ForMedium; complete synthetic mixture) containing histidine, tryptophane and leucine, which are required for growth of the deletion strains. Plates contained 2% agar.
Bioinformatic analysis and gene selection
Genes of different functional categories were manually selected based on potential function in virulence and drug resistance. The categories involved genes of signaling pathways, kinases, ABC transporters and permeases, GPI-anchored proteins, cell wall associated genes, genes involved in glycosylation, phospholipid biosynthesis, histone modification, iron metabolism, and several genes with no obvious homologue in S. cerevisiae. The genes were selected by their homology to S. cerevisiae based on these functional categories (SGD annotations; http://www.yeastgenome.org). C. glabrata orthologues of the selected genes were first identified using a BLAST approach. The three best-aligned hits for each gene were saved and the C. glabrata homologue with the highest P-value was arbitrarily defined as the C. glabrata orthologue of a given gene in baker's yeast and named accordingly. In addition, a complete catalogue of orthology and paralogy relationships between C. glabrata genes and their homologues in 16 other fully-sequenced fungi was derived using a phylogenetic approach [128]. For this a complete collection of Maximum Likelihood phylogenetic trees for all C. glabrata genes, the so-called phylome, was generated using the automated pipeline described elsewhere [129]. Gene phylogenies, alignments and orthology and paralogy predictions are publicly available through PhylomeDB (http://www.phylomedb.org).
Primer design and generation
Oligonucleotide sequences for generation of the deletion cassettes and strain verification were automatically designed, using a custom-written Perl script called PrimerList (W. Glaser, unpublished data). PrimerList utilizes Bioperl to read and process nucleotide sequences and uses the EMBOSS [130] programs eprimer 3 and stssearch to find suitable primersets. For PCR-based generation of knock-out constructs, upstream and downstream fragments for genomic recombination were chosen to have a size between 450 and 550 nucleotides. 5′5′ (forward) and 3′3′ (reverse) primers were chosen to have a length of 20 to 30 bp, a GC content between 30 and 60% and a melting temperature of 50°C±4°C with a GC clamp. 5′3′, 3′5′ primers were chosen to have a length of 20 to 30 bp plus the 20 bp constant overlap sequence (Figure 1, Table S3, ‘barcode sequence sheet’), a GC content between 30 and 60% and a melting temperature of 50°C±4°C. 5′3′ (reverse), 3′5′ (forward) primers were chosen to bind exactly adjacent to the coding sequence, including the start codon ATG or the stop codon, respectively. 5c and 3c control primers have a length of 20 to 25 bp, a GC content between 40 and 60% and a melting temperature between 50°C to 60°C. The product size of the control PCR is between 750 and 900 bp. Internal control primers (5i and 3i) were designed to bind inside the coding sequence and to give a product of 400 to 500 bp in size. The primers have a length of 20 to 25 bp, a GC content between 40 and 60% and a melting temperature between 55°C to 60°C. Oligonucleotides were commercially purchased in 96-well plate format (Eurogentec, Belgium). Six plates were needed for each set. Each of the six plates (5′5′, 5′3′, 3′5′, 3′3′, 5c, 3c) contained the primers for a specific gene at the exact same well position.
Generation of gene deletion cassettes by fusion PCR
The dominant marker NAT1 was amplified from plasmid pJK863 [57] using the primers fp_NAT1-U2 and rp_NAT1-D2 to add the constant 20 bp sequences U2 and D2. The PCR product was ligated into a pGEM-T vector (Promega), generating plasmid pTS50. For the fusion PCR step, deletion cassettes were generated using a modified fusion PCR protocol [51]. Briefly, 500 bp long flanking homology regions were amplified from ATCC2001 genomic DNA with primer pairs 5′5′/5′3′ and 3′5′/3′3′ adding the constant overlap sequence (U1/D1) of 20 bp and purified by ethanol precipitation. The conditions for a 50 µl reaction were as follows: 1× Taq buffer (50 mM KCl, 10 mM Tris-HCl (pH 9.0, 25°C), 0.1% TritionX-100, 1.5 mM MgCl2), 0.2 µM dNTPs, 0.5 µM each primer, 1 µl Taq-Polymerase and genomic wild type DNA from strain ATCC2001; 93°C for 5 minutes, 35 cycles 93°C for 30 s, 45°C for 30 s, 72°C for 90 s, finally 10 minutes at 72°C.
The dominant marker NAT1 was amplified from plasmid pTS50 in a separate PCR reaction using primers 5M and 3M, adding unique barcode tags and constant complementary U1 and D1 sequences. Marker fragments were gel-purified in 0.7% agarose gels. The conditions for a 50 µl reaction were as follows: 1× Taq buffer, 0.2 µM dNTPs, 0,5 µM each primer, 1 µl Taq-Polymerase and plasmid TS50; 93°C for 3 minutes, 32 cycles 93°C for 30 s, 49°C for 30 s, 72°C for 2,5 minutes, finally 10 minutes at 72°C. Fusion PCR was carried out in a 50 µl volume with the same conditions as above: 1× ExTaq buffer, 0.2 µM dNTPs, 0.5 µM each primer, 0.5 µl ExTaq-Polymerase (TaKaRa) and 3 µl marker fragment, 1.25 µl each flanking homology fragment; 93°C for 3 minutes, 35 cycles 93°C for 30 s, 45°C for 30 s, 72°C for 3 minutes, finally 10 minutes at 72°C. The final deletion construct was purified by ethanol precipitation.
Complementation of C. glabrata deletion mutants
Cloning of C. glabrata ORFs in the pDONR207 vector was done as described in [131]. Briefly, for each of the selected ORFs, a forward primer including the attB1 site and the first 10 codons of the ORF and a reverse primer including the attB2 site and the last ten codons of the ORF were designed and synthesized at Pasteur-Genopole-Ile-de-France oligonucleotide synthesis platform (Table S13). ORFs were amplified from genomic DNA of C. glabrata strain ATCC2001 [54] using Phusion High-Fidelity DNA Polymerase (New England Biolabs) and 30 cycles of amplification with elongation times varying from 1 to 3 min. according to the ORF size. The resulting PCR products were checked by agarose gel electrophoresis, ethanol precipitated and, following resuspension in Tris-EDTA, mixed with the donor plasmid pDONR207 (Invitrogen), and subjected to a recombination reaction with Invitrogen Gateway BP Clonase. The recombination mixes were transformed into E. coli strain DH5α and one transformant per ORF was used for plasmid preparation. The cloned ORFs were sequenced from the 5′ - and 3′-ends using Sanger sequencing.
To construct a replicative, Gateway-compatible C. glabrata expression vector, the C. glabrata TDH3 promoter amplified from genomic DNA of C. glabrata strain ATCC2001 using oligos CgTDH3p-fwd (5′-GCGCCCGGTACCCAGGTGATCATATCACTCACA-3′) and CgTDH3p-rev (5′-GGGCCGACTAGTGTTATGTTTGTTGTGATTTGTA-3′), and a Gateway cassette amplified from plasmid CIp10-PTET-GTW [132] were cloned into the replicative vector pCgACT-14 [133], yielding the Destination vector pCgACT-PTDH3-GTW. Transfer of C. glabrata ORFs and the GFP ORF from pDONR207 into pCgACT-PTDH3-GTW was as described in [131]. An aliquot of each Entry plasmid was mixed with 50 ng of the Destination plasmid and subjected to a recombination reaction with Invitrogen Gateway LR Clonase. The recombination mixes were transformed into E. coli strain DH5α and one transformant was used for plasmid preparation. EcoRV digestion was used to verify the cloning of the appropriate ORF. A list of the plasmids used in this study is shown in Table S11. The expression plasmids were transformed into the corresponding C. glabrata deletion strain according to [134]. Transformants were selected for prototrophy. The resulting strains are listed in Table S12.
Transformation of C. glabrata by electroporation
For transformation of the background strain HTL, we used a modified electroporation protocol [56]. Aliquots of 50 ml of a C. glabrata culture in YPD at an optical density of 600 nm (OD600) of 1.3 were harvested, washed with H2O, resuspended in 1× TE buffer, 100 mM LiAc and incubated at 30°C for 30 minutes with slow shaking (130×rpm). After addition of 250 µl 1M DTT and further incubation at 30°C for 60 minutes (130×rpm), 40 ml of H2O were added and the cells were harvested at 1000 g for 5 minutes at 4°C. The cells were washed with 25 ml H2O, subsequently with 5 ml 1 M cold sorbitol, finally resuspended in 550 µl 1 M sorbitol and kept on ice until use. Sterile electroporation cuvettes were precooled on ice and loaded with a mix of 40–45 µl electrocompetent cells and 5–10 µl linear DNA deletion construct (app. 2–3 µg DNA). Cells were left on ice for 10 minutes and electroporation was carried out with a BioRad GenePulser (200Ω, 1.5 kV, 25 µF). For recovery, 950 µl YPD was added and cells incubated for 4 h shaking at 30°C, before plating on YPD supplemented with 200 µg/ml Nourseothricin (Werner Bioagents, Jena). The plates were incubated for 48 hours at 30°C. For auxotrophic marker constructs the cells were recovered for 1 h at 30°C before plating on selective SC medium. Transformants were patched on YPD/Nourseothricin plates for colony PCR. For 96-well parallel electroporation, 300 ml of culture were grown to OD600 of 1.3, split into 50 ml aliquots and treated as described above. For electroporation, we used a BTX Harvard Apparatus ECM630 electroporation device with a HT-100 plate handler.
Verification by yeast colony PCR
Strains were verified by colony PCR to confirm correct genomic integration of the deletion cassette, as well as loss of the wild type allele according to the following protocol (Figure 1B). Transformants were patched on selective plates and incubated at 30°C for 24 h. Cells were resuspended in 40 µl PCR mix 1 (0.2 µM dNTPs, 0.5 µM of each gene specific primer 5c/3c up-/downstream of the homology region and marker specific primer 5M/3M) and heated for 10 minutes at 93°C. After cooling on ice, 10 µl polymerase mix (5 µl 10× PCR buffer and 1 µl Taq-Pol.) were added per reaction and a regular PCR was performed (93°C for 5 min, 25 cycles 93°C for 30 s, 45°C for 30 s, 72°C for 90 s, final 10 min 72°C). To verify the loss of the coding sequence (CDS), colony PCR was essentially performed the same way. Oligonucleotides used to screen for CDS loss bind inside the CDS to generate a product of 500 bp. All internal primers were also checked for functionality in a separate PCR reaction, amplifying the fragment from genomic wild type DNA.
Phenotypic profiling
For phenotypic analysis of the deletion collection, mutant cells were re-streaked from frozen stocks and grown for 48 h at 30°C on fresh YPD plates. Each of the three plates containing independent transformants of the same set of genes was arrayed into a 384-spot format serving as source plates. Phenotypic profiling of the deletion collection was performed using a robot on YPD plates (RoToR HDA, Singer Ltd., Roadwater, UK) or by manually spotting (two 1∶10 dilutions from 24 h culture in SC) on SC plates supplemented with the compounds to be tested. Unless otherwise indicated, we added 120 ng/ml CF, 50 µg/ml CW (Sigma-Aldrich) or 250 µg/ml CR (Sigma-Aldrich) as supplements to media from sterile stock solutions after autoclaving. Plates were routinely incubated at 30°C for up to 3 days and scanned photographed with S&P Imaging system (S&P Imaging, Canada), after 24, 48 and 72 h for documentation. Primary hits were manually rescreened for confirmation in 1∶10 serial dilutions. Exponentially growing cells were adjusted to an OD600 of 0.1. Equal volumes of serial dilutions (1∶10, 1∶100 and 1∶1000) were spotted on YPD plates containing drugs and incubated as described above.Hypersensitive mutants identified as primary hits by robotic screening were independently re-screened manually in 96-well format, to verify growth phenotypes on agar plates containing various concentrations of xenobiotics. All manual re-screening assays were carried out independently at least in biological triplicates in four different laboratories, including the confirmation of hits by serial-dilution spot assays on agar plates or as appropriate by microdilution assays in liquid cultures.
Azole and drug susceptibility screenings
Azole susceptibility screenings were carried out by a modified endpoint method [135] in liquid culture in microtiter plates, using the following drug concentrations: 4 µg/ml Fluconazole, 0.1 µg/ml Voriconazole, (all azoles from Discovery Fine Chemicals), 3 µg/ml AmB (Discovery Fine Chemicals). Cells were grown overnight in deep well plates to stationary phase, diluted 100-fold in sterile water and 100 µl suspension mixed with 100 µl of 2× YPD containing a 2× drug concentration (app. 105 cells/well). After incubation at 30°C for 24 h and 48 h, cells were resuspended and the OD600 was measured with a Victor plate reader (Perkin Elmer, USA).
Microbroth dilution assay for IC50 determination
To determine the IC50 of antifungal drugs a modified protocol of the microbroth dilution assay was used [136]. Briefly, an overnight culture was diluted 1∶100 in YPD, regrown to an OD600 of 1 and an inoculum of 2.5×104 cells/ml was prepared. Antifungal stock solutions were prepared in DMSO. Two fold serial dilutions of the drugs were then prepared in water in a deep well plate and stored at −20°C until use. 100 µl of two fold serial drug dilutions were distributed in triplicates into a flat bottom microtiter plate. The last wells free of antifungal drugs served as a growth control. After adding 100 µl of the inoculum (200 µl total volume), plates were incubated at 30°C for 24 h and 48 h in a humid environment to avoid evaporation. OD600 was determined with a plate reader. Endpoint readings were set as the antifungal concentrations, causing at least 90% growth inhibition after 24 h of growth when compared to the control. The IC50 was determined by linear regression using Graph Pad Prism software.
Fitness analysis
Growth curves were performed in 96-well plates in a Tecan Infinite M200 microplate reader or a Tecan Sunrise microplate reader. C. glabrata strains were grown in YPD at 30°C. For each 96-well plate, the doubling times of each of the 96 tested strains were calculated based on the time necessary for a given strain to go from OD 0.15 to OD 0.6. The relative fitness of a strain was then calculated as the ratio of the average doubling time of all strains within the third to eighth deciles to the doubling time of the strain of interest [68]. Means and standard deviations are presented for fitness values determined for one or two independent knock-out mutants in two biological replicates. Strains with standard deviation above 0.1 or an absolute difference between the relative fitness of two independent knock-out mutants above 0.1 were not considered further. In order to identify among the remaining strains those that showed significantly increased or decreased fitness, the average and standard deviation of the fitness for strains within the second to ninth deciles were calculated. Strains were classified based on the number of standard deviation between their fitness and the average fitness. Strains with decreased fitness had a fitness at least two standard deviations below the average fitness. Strains with increased fitness had a fitness at least two standard deviations above the average fitness.
Biofilm formation assays
Biofilms were produced in 96-well plates as previously described [75]. Briefly, saturated cultures in YPD were pin-inoculated diluted in 100 µl SD 0.4% glucose medium in 96-well polystyrene plates and incubated at 37°C for 24 h. The 96-well plates were then washed with PBS using a HydroFlex platform (Tecan) and 100 µl of a 1× FDA solution (50× stock: fluorescein diacetate, 2 g l−1 in acetone; diluted to 1× in PBS) was added per well [74]. Plates were wrapped in aluminium foil and incubated for 1 h at 37°C before measuring fluorescence in a Tecan Infinite M200 microplate reader using an excitation filter of 486±9 nm and an emission filter of 535±20 nm. The relative biofilm fitness of a strain was then calculated as the ratio of the OD535 nm recording for the strain of interest to the average of the OD535 nm recordings obtained for strains within the third to eighth deciles of all OD535 nm recordings obtained within the 96-well plate to which the strain of interest belonged. Means and standard deviations are presented for fitness values determined for one or two independent knock-out mutants in two biological replicates. Strains with standard deviation above 0.3 or an absolute difference between the 2–4 relative biofilm fitness values above 0.5 were not considered further. In order to identify among the remaining strains those that showed significantly increased or decreased ability to form biofilm, the average and standard deviation of the relative biofilm fitness for strains within the second to ninth deciles were calculated. Strains were classified based on the number of standard deviation between their biofilm fitness and the average biofilm fitness. Strains with decreased biofilm fitness had a relative biofilm fitness at least two standard deviations below the average relative biofilm fitness. Strains with increased biofilm fitness had a relative biofilm fitness at least two standard deviations above the average fitness. Strains with decreased or increased biofilm fitness were further evaluated by performing a biofilm assay in quadruplicate for each of two independent isolates. Data obtained for the 58 candidate strains were compared to those obtained for wild type isolates (96 independent values) using the Wilcoxon test. A p value below 0.01 was considered as indicative of a significant difference with the wild type strain. In this assay, a yak1Δ mutant was found significantly impaired in biofilm formation while a sir3Δ mutant showed significantly elevated biofilm production as previously shown [75].
Supporting Information
Zdroje
1. MeanM, MarchettiO, CalandraT (2008) Bench-to-bedside review: Candida infections in the intensive care unit. Crit Care 12 : 204.
2. PerlrothJ, ChoiB, SpellbergB (2007) Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol 45 : 321–346.
3. PfallerMA, DiekemaDJ, GibbsDL, NewellVA, BartonR, et al. (2010) Geographic variation in the frequency of isolation and fluconazole and voriconazole susceptibilities of Candida glabrata: an assessment from the ARTEMIS DISK Global Antifungal Surveillance Program. Diagn Microbiol Infect Dis 67 : 162–171.
4. RichardsonM, Lass-FlorlC (2008) Changing epidemiology of systemic fungal infections. Clin Microbiol Infect 14 Suppl 4 : 5–24.
5. GowNA, van de VeerdonkFL, BrownAJ, NeteaMG (2011) Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol 10 : 112–122.
6. AlbrechtA, FelkA, PichovaI, NaglikJR, SchallerM, et al. (2006) Glycosylphosphatidylinositol-anchored proteases of Candida albicans target proteins necessary for both cellular processes and host-pathogen interactions. J Biol Chem 281 : 688–694.
7. GhannoumMA (2000) Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev 13 : 122–143 table of contents.
8. PanackalAA, GribskovJL, StaabJF, KirbyKA, RinaldiM, et al. (2006) Clinical significance of azole antifungal drug cross-resistance in Candida glabrata. J Clin Microbiol 44 : 1740–1743.
9. PfallerMA, DiekemaDJ, GibbsDL, NewellVA, EllisD, et al. (2010) Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol 48 : 1366–1377.
10. PfallerMA, MesserSA, HollisRJ, BoykenL, TendolkarS, et al. (2009) Variation in susceptibility of bloodstream isolates of Candida glabrata to fluconazole according to patient age and geographic location in the United States in 2001 to 2007. J Clin Microbiol 47 : 3185–3190.
11. RuanSY, ChuCC, HsuehPR (2008) In vitro susceptibilities of invasive isolates of Candida species: rapid increase in rates of fluconazole susceptible-dose dependent Candida glabrata isolates. Antimicrob Agents Chemother 52 : 2919–2922.
12. CastanoI, PanSJ, ZupancicM, HennequinC, DujonB, et al. (2005) Telomere length control and transcriptional regulation of subtelomeric adhesins in Candida glabrata. Mol Microbiol 55 : 1246–1258.
13. CormackBP, GhoriN, FalkowS (1999) An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 285 : 578–582.
14. de GrootPW, KraneveldEA, YinQY, DekkerHL, GrossU, et al. (2008) The cell wall of the human pathogen Candida glabrata: differential incorporation of novel adhesin-like wall proteins. Eukaryot Cell 7 : 1951–1964.
15. De Las PenasA, PanSJ, CastanoI, AlderJ, CreggR, et al. (2003) Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1 - and SIR-dependent transcriptional silencing. Genes Dev 17 : 2245–2258.
16. DomergueR, CastanoI, De Las PenasA, ZupancicM, LockatellV, et al. (2005) Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science 308 : 866–870.
17. KaurR, DomergueR, ZupancicML, CormackBP (2005) A yeast by any other name: Candida glabrata and its interaction with the host. Curr Opin Microbiol 8 : 378–384.
18. KaurR, MaB, CormackBP (2007) A family of glycosylphosphatidylinositol-linked aspartyl proteases is required for virulence of Candida glabrata. Proc Natl Acad Sci U S A 104 : 7628–7633.
19. BrunkeS, SeiderK, AlmeidaRS, HeykenA, FleckCB, et al. (2010) Candida glabrata tryptophan-based pigment production via the Ehrlich pathway. Mol Microbiol 76 : 25–47.
20. RoetzerA, GratzN, KovarikP, SchullerC (2010) Autophagy supports Candida glabrata survival during phagocytosis. Cell Microbiol 12 : 199–216.
21. SeiderK, BrunkeS, SchildL, JablonowskiN, WilsonD, et al. (2011) The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J Immunol 187 : 3072–3086.
22. RaiMN, BalusuS, GorityalaN, DanduL, KaurR (2012) Functional genomic analysis of Candida glabrata-macrophage interaction: role of chromatin remodeling in virulence. PLoS Pathog 8: e1002863.
23. KamranM, CalcagnoAM, FindonH, BignellE, JonesMD, et al. (2004) Inactivation of transcription factor gene ACE2 in the fungal pathogen Candida glabrata results in hypervirulence. Eukaryot Cell 3 : 546–552.
24. IzumikawaK, KakeyaH, TsaiHF, GrimbergB, BennettJE (2003) Function of Candida glabrata ABC transporter gene, PDH1. Yeast 20 : 249–261.
25. MiyazakiH, MiyazakiY, GeberA, ParkinsonT, HitchcockC, et al. (1998) Fluconazole resistance associated with drug efflux and increased transcription of a drug transporter gene, PDH1, in Candida glabrata. Antimicrob Agents Chemother 42 : 1695–1701.
26. SanglardD, IscherF, CalabreseD, MajcherczykPA, BilleJ (1999) The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob Agents Chemother 43 : 2753–2765.
27. ThakurJK, ArthanariH, YangF, PanSJ, FanX, et al. (2008) A nuclear receptor-like pathway regulating multidrug resistance in fungi. Nature 452 : 604–609.
28. VermitskyJP, EarhartKD, SmithWL, HomayouniR, EdlindTD, et al. (2006) Pdr1 regulates multidrug resistance in Candida glabrata: gene disruption and genome-wide expression studies. Mol Microbiol 61 : 704–722.
29. BrunS, BergesT, PoupardP, Vauzelle-MoreauC, RenierG, et al. (2004) Mechanisms of azole resistance in petite mutants of Candida glabrata. Antimicrob Agents Chemother 48 : 1788–1796.
30. NakayamaH, TanabeK, BardM, HodgsonW, WuS, et al. (2007) The Candida glabrata putative sterol transporter gene CgAUS1 protects cells against azoles in the presence of serum. J Antimicrob Chemother 60 : 1264–1272.
31. NagiM, TanabeK, UenoK, NakayamaH, AoyamaT, et al. (2013) The Candida glabrata sterol scavenging mechanism, mediated by the ATP-binding cassette transporter Aus1p, is regulated by iron limitation. Mol Microbiol 88 : 371–381.
32. MiyazakiT, YamauchiS, InamineT, NagayoshiY, SaijoT, et al. (2010) Roles of calcineurin and Crz1 in antifungal susceptibility and virulence of Candida glabrata. Antimicrob Agents Chemother 54 : 1639–1643.
33. DiekemaD, ArbefevilleS, BoykenL, KroegerJ, PfallerM (2012) The changing epidemiology of healthcare-associated candidemia over three decades. Diagn Microbiol Infect Dis 73 : 45–48.
34. PerlinDS (2011) Current perspectives on echinocandin class drugs. Future Microbiol 6 : 441–457.
35. PerlinDS (2007) Resistance to echinocandin-class antifungal drugs. Drug Resist Updat 10 : 121–130.
36. Singh-BabakSD, BabakT, DiezmannS, HillJA, XieJL, et al. (2012) Global analysis of the evolution and mechanism of echinocandin resistance in Candida glabrata. PLoS Pathog 8: e1002718.
37. KatiyarSK, Alastruey-IzquierdoA, HealeyKR, JohnsonME, PerlinDS, et al. (2012) Fks1 and Fks2 are functionally redundant but differentially regulated in Candida glabrata: implications for echinocandin resistance. Antimicrob Agents Chemother 56 : 6304–6309.
38. Schuetzer-MuehlbauerM, WillingerB, KrapfG, EnzingerS, PresterlE, et al. (2003) The Candida albicans Cdr2p ATP-binding cassette (ABC) transporter confers resistance to caspofungin. Mol Microbiol 48 : 225–235.
39. SinghSD, RobbinsN, ZaasAK, SchellWA, PerfectJR, et al. (2009) Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog 5: e1000532.
40. AlexanderBD, JohnsonMD, PfeifferCD, Jimenez-OrtigosaC, CataniaJ, et al. (2013) Increasing Echinocandin Resistance in Candida glabrata: Clinical Failure Correlates With Presence of FKS Mutations and Elevated Minimum Inhibitory Concentrations. Clin Infect Dis 56 : 1724–1732.
41. AlexanderBD, JohnsonMD, PfeifferCD, Jimenez-OrtigosaC, CataniaJ, et al. (2013) Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56 : 1724–1732.
42. CostanzoM, BaryshnikovaA, BellayJ, KimY, SpearED, et al. (2010) The genetic landscape of a cell. Science 327 : 425–431.
43. GiaeverG, ChuAM, NiL, ConnellyC, RilesL, et al. (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418 : 387–391.
44. HillenmeyerME, EricsonE, DavisRW, NislowC, KollerD, et al. (2010) Systematic analysis of genome-wide fitness data in yeast reveals novel gene function and drug action. Genome Biol 11: R30.
45. HillenmeyerME, FungE, WildenhainJ, PierceSE, HoonS, et al. (2008) The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science 320 : 362–365.
46. ParsonsAB, BrostRL, DingH, LiZ, ZhangC, et al. (2004) Integration of chemical-genetic and genetic interaction data links bioactive compounds to cellular target pathways. Nat Biotechnol 22 : 62–69.
47. RyanO, ShapiroRS, KuratCF, MayhewD, BaryshnikovaA, et al. (2012) Global gene deletion analysis exploring yeast filamentous growth. Science 337 : 1353–1356.
48. TongAH, LesageG, BaderGD, DingH, XuH, et al. (2004) Global mapping of the yeast genetic interaction network. Science 303 : 808–813.
49. WinzelerEA, ShoemakerDD, AstromoffA, LiangH, AndersonK, et al. (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285 : 901–906.
50. NobleSM, FrenchS, KohnLA, ChenV, JohnsonAD (2010) Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet 42 : 590–598.
51. NobleSM, JohnsonAD (2005) Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot Cell 4 : 298–309.
52. RoemerT, JiangB, DavisonJ, KetelaT, VeilletteK, et al. (2003) Large-scale essential gene identification in Candida albicans and applications to antifungal drug discovery. Mol Microbiol 50 : 167–181.
53. LiuOW, ChunCD, ChowED, ChenC, MadhaniHD, et al. (2008) Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell 135 : 174–188.
54. DujonB, ShermanD, FischerG, DurrensP, CasaregolaS, et al. (2004) Genome evolution in yeasts. Nature 430 : 35–44.
55. Marcet-HoubenM, GabaldonT (2009) The tree versus the forest: the fungal tree of life and the topological diversity within the yeast phylome. PLoS ONE 4: e4357.
56. ReussO, VikA, KolterR, MorschhäuserJ (2004) The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341 : 119–127.
57. ShenJ, GuoW, KöhlerJR (2005) CaNAT1, a heterologous dominant selectable marker for transformation of Candida albicans and other pathogenic Candida species. Infect Immun 73 : 1239–1242.
58. BrandA, MacCallumDM, BrownAJ, GowNA, OddsFC (2004) Ectopic expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot Cell 3 : 900–909.
59. LayJ, HenryLK, CliffordJ, KoltinY, BulawaCE, et al. (1998) Altered expression of selectable marker URA3 in gene-disrupted Candida albicans strains complicates interpretation of virulence studies. Infect Immun 66 : 5301–5306.
60. JacobsenID, BrunkeS, SeiderK, SchwarzmullerT, FironA, et al. (2010) Candida glabrata persistence in mice does not depend on host immunosuppression and is unaffected by fungal amino acid auxotrophy. Infect Immun 78 : 1066–1077.
61. UenoK, UnoJ, NakayamaH, SasamotoK, MikamiY, et al. (2007) Development of a highly efficient gene targeting system induced by transient repression of YKU80 expression in Candida glabrata. Eukaryot Cell 6 : 1239–1247.
62. BoguslawskiG (1992) PBS2, a yeast gene encoding a putative protein kinase, interacts with the RAS2 pathway and affects osmotic sensitivity of Saccharomyces cerevisiae. J Gen Microbiol 138 : 2425–2432.
63. SanglardD, IscherF, BilleJ (2001) Role of ATP-binding-cassette transporter genes in high-frequency acquisition of resistance to azole antifungals in Candida glabrata. Antimicrob Agents Chemother 45 : 1174–1183.
64. VermitskyJP, EdlindTD (2004) Azole resistance in Candida glabrata: coordinate upregulation of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrob Agents Chemother 48 : 3773–3781.
65. CotaJM, GrabinskiJL, TalbertRL, BurgessDS, RogersPD, et al. (2008) Increases in SLT2 expression and chitin content are associated with incomplete killing of Candida glabrata by caspofungin. Antimicrob Agents Chemother 52 : 1144–1146.
66. Reinoso-MartinC, SchullerC, Schuetzer-MuehlbauerM, KuchlerK (2003) The yeast protein kinase C cell integrity pathway mediates tolerance to the antifungal drug caspofungin through activation of Slt2p mitogen-activated protein kinase signaling. Eukaryot Cell 2 : 1200–1210.
67. FerrariS, IscherF, CalabreseD, PosteraroB, SanguinettiM, et al. (2009) Gain of function mutations in CgPDR1 of Candida glabrata not only mediate antifungal resistance but also enhance virulence. PLoS Pathog 5: e1000268.
68. St OngeRP, ManiR, OhJ, ProctorM, FungE, et al. (2007) Systematic pathway analysis using high-resolution fitness profiling of combinatorial gene deletions. Nat Genet 39 : 199–206.
69. CsankC, HaynesK (2000) Candida glabrata displays pseudohyphal growth. FEMS Microbiol Lett 189 : 115–120.
70. VandeputteP, TronchinG, BergesT, HennequinC, ChabasseD, et al. (2007) Reduced susceptibility to polyenes associated with a missense mutation in the ERG6 gene in a clinical isolate of Candida glabrata with pseudohyphal growth. Antimicrob Agents Chemother 51 : 982–990.
71. FinkelJS, MitchellAP (2011) Genetic control of Candida albicans biofilm development. Nat Rev Microbiol 9 : 109–118.
72. NobileCJ, NettJE, HerndayAD, HomannOR, DeneaultJS, et al. (2009) Biofilm matrix regulation by Candida albicans Zap1. PLoS Biol 7: e1000133.
73. RieraM, MogensenE, d'EnfertC, JanbonG (2012) New regulators of biofilm development in Candida glabrata. Res Microbiol 163 : 297–307.
74. HonraetK, GoetghebeurE, NelisHJ (2005) Comparison of three assays for the quantification of Candida biomass in suspension and CDC reactor grown biofilms. J Microbiol Methods 63 : 287–295.
75. IraquiI, Garcia-SanchezS, AubertS, DromerF, GhigoJM, et al. (2005) The Yak1p kinase controls expression of adhesins and biofilm formation in Candida glabrata in a Sir4p-dependent pathway. Mol Microbiol 55 : 1259–1271.
76. PfallerMA, CastanheiraM, LockhartSR, AhlquistAM, MesserSA, et al. (2012) Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol 50 : 1199–1203.
77. CowenLE (2008) The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat Rev Microbiol 6 : 187–198.
78. KhanZU, AhmadS, Al-ObaidI, Al-SweihNA, JosephL, et al. (2008) Emergence of resistance to amphotericin B and triazoles in Candida glabrata vaginal isolates in a case of recurrent vaginitis. J Chemother 20 : 488–491.
79. Krogh-MadsenM, ArendrupMC, HesletL, KnudsenJD (2006) Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin Infect Dis 42 : 938–944.
80. LussierM, SdicuAM, CamirandA, BusseyH (1996) Functional characterization of the YUR1, KTR1, and KTR2 genes as members of the yeast KRE2/MNT1 mannosyltransferase gene family. J Biol Chem 271 : 11001–11008.
81. OnyewuC, BlankenshipJR, Del PoetaM, HeitmanJ (2003) Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob Agents Chemother 47 : 956–964.
82. TschernerM, StapplerE, HniszD, KuchlerK The histone acetyltransferase Hat1 facilitates DNA damage repair and morphogenesis in Candida albicans. Mol Microbiol
83. MiyazakiT, InamineT, YamauchiS, NagayoshiY, SaijoT, et al. (2010) Role of the Slt2 mitogen-activated protein kinase pathway in cell wall integrity and virulence in Candida glabrata. FEMS Yeast Res 10 : 343–352.
84. ChenYL, KonieczkaJH, SpringerDJ, BowenSE, ZhangJ, et al. (2012) Convergent Evolution of Calcineurin Pathway Roles in Thermotolerance and Virulence in Candida glabrata. G3 (Bethesda) 2 : 675–691.
85. LiuW, TanJ, SunJ, XuZ, LiM, et al. (2014) Invasive candidiasis in intensive care units in China: in vitro antifungal susceptibility in the China-SCAN study. J Antimicrob Chemother 69 : 162–167.
86. CastanoI, KaurR, PanS, CreggR, PenasAdeL, et al. (2003) Tn7-based genome-wide random insertional mutagenesis of Candida glabrata. Genome Res 13 : 905–915.
87. KojicEM, DarouicheRO (2004) Candida infections of medical devices. Clin Microbiol Rev 17 : 255–267.
88. FriedelAM, PikeBL, GasserSM (2009) ATR/Mec1: coordinating fork stability and repair. Curr Opin Cell Biol 21 : 237–244.
89. JorgensenP, NelsonB, RobinsonMD, ChenY, AndrewsB, et al. (2002) High-resolution genetic mapping with ordered arrays of Saccharomyces cerevisiae deletion mutants. Genetics 162 : 1091–1099.
90. BidlingmaierS, WeissEL, SeidelC, DrubinDG, SnyderM (2001) The Cbk1p pathway is important for polarized cell growth and cell separation in Saccharomyces cerevisiae. Mol Cell Biol 21 : 2449–2462.
91. RackiWJ, BecamAM, NasrF, HerbertCJ (2000) Cbk1p, a protein similar to the human myotonic dystrophy kinase, is essential for normal morphogenesis in Saccharomyces cerevisiae. EMBO J 19 : 4524–4532.
92. BharuchaN, Chabrier-RoselloY, XuT, JohnsonC, SobczynskiS, et al. (2011) A large-scale complex haploinsufficiency-based genetic interaction screen in Candida albicans: analysis of the RAM network during morphogenesis. PLoS Genet 7: e1002058.
93. McNemarMD, FonziWA (2002) Conserved serine/threonine kinase encoded by CBK1 regulates expression of several hypha-associated transcripts and genes encoding cell wall proteins in Candida albicans. J Bacteriol 184 : 2058–2061.
94. LegrandM, LephartP, ForcheA, MuellerFM, WalshT, et al. (2004) Homozygosity at the MTL locus in clinical strains of Candida albicans: karyotypic rearrangements and tetraploid formation. Mol Microbiol 52 : 1451–1462.
95. Gutierrez-EscribanoP, ZeidlerU, SuarezMB, Bachellier-BassiS, Clemente-BlancoA, et al. (2012) The NDR/LATS kinase Cbk1 controls the activity of the transcriptional regulator Bcr1 during biofilm formation in Candida albicans. PLoS Pathog 8: e1002683.
96. LavoieH, HoguesH, WhitewayM (2009) Rearrangements of the transcriptional regulatory networks of metabolic pathways in fungi. Curr Opin Microbiol 12 : 655–663.
97. LiH, JohnsonAD (2010) Evolution of transcription networks–lessons from yeasts. Curr Biol 20: R746–753.
98. CasadevallA (2012) Fungi and the rise of mammals. PLoS Pathog 8: e1002808.
99. BennettJE, IzumikawaK, MarrKA (2004) Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob Agents Chemother 48 : 1773–1777.
100. BorstA, RaimerMT, WarnockDW, MorrisonCJ, Arthington-SkaggsBA (2005) Rapid acquisition of stable azole resistance by Candida glabrata isolates obtained before the clinical introduction of fluconazole. Antimicrob Agents Chemother 49 : 783–787.
101. BoucharaJP, ZouhairR, Le BoudouilS, RenierG, FilmonR, et al. (2000) In-vivo selection of an azole-resistant petite mutant of Candida glabrata. J Med Microbiol 49 : 977–984.
102. CostaC, PiresC, CabritoTR, RenaudinA, OhnoM, et al. (2013) Candida glabrata drug:H+ antiporter CgQdr2 (ORF CAGL0G08624g) confers imidazole drug resistance, being activated by the CgPdr1 transcription factor. Antimicrob Agents Chemother 57 : 3159–3167.
103. MorschhäuserJ, BarkerKS, LiuTT, BlaBWJ, HomayouniR, et al. (2007) The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog 3: e164.
104. LesageG, SdicuAM, MenardP, ShapiroJ, HusseinS, et al. (2004) Analysis of beta-1,3-glucan assembly in Saccharomyces cerevisiae using a synthetic interaction network and altered sensitivity to caspofungin. Genetics 167 : 35–49.
105. XuD, JiangB, KetelaT, LemieuxS, VeilletteK, et al. (2007) Genome-wide fitness test and mechanism-of-action studies of inhibitory compounds in Candida albicans. PLoS Pathog 3: e92.
106. Ben-AmiR, KontoyiannisDP (2012) Resistance to echinocandins comes at a cost: the impact of FKS1 hotspot mutations on Candida albicans fitness and virulence. Virulence 3 : 95–97.
107. SussmanA, HussK, ChioLC, HeidlerS, ShawM, et al. (2004) Discovery of cercosporamide, a known antifungal natural product, as a selective Pkc1 kinase inhibitor through high-throughput screening. Eukaryot Cell 3 : 932–943.
108. LeeKK, MaccallumDM, JacobsenMD, WalkerLA, OddsFC, et al. (2012) Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrob Agents Chemother 56 : 208–217.
109. WalkerLA, GowNA, MunroCA (2013) Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrob Agents Chemother 57 : 146–154.
110. SteinbachWJ, CramerRAJr, PerfectBZ, HennC, NielsenK, et al. (2007) Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob Agents Chemother 51 : 2979–2981.
111. RoelantsFM, BreslowDK, MuirA, WeissmanJS, ThornerJ (2011) Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 108 : 19222–19227.
112. InagakiM, SchmelzleT, YamaguchiK, IrieK, HallMN, et al. (1999) PDK1 homologs activate the Pkc1-mitogen-activated protein kinase pathway in yeast. Mol Cell Biol 19 : 8344–8352.
113. RoelantsFM, TorrancePD, BezmanN, ThornerJ (2002) Pkh1 and Pkh2 differentially phosphorylate and activate Ypk1 and Ykr2 and define protein kinase modules required for maintenance of cell wall integrity. Mol Biol Cell 13 : 3005–3028.
114. KaurR, CastanoI, CormackBP (2004) Functional genomic analysis of fluconazole susceptibility in the pathogenic yeast Candida glabrata: roles of calcium signaling and mitochondria. Antimicrob Agents Chemother 48 : 1600–1613.
115. WiederholdNP, KontoyiannisDP, PrinceRA, LewisRE (2005) Attenuation of the activity of caspofungin at high concentrations against Candida albicans: possible role of cell wall integrity and calcineurin pathways. Antimicrob Agents Chemother 49 : 5146–5148.
116. JansenJM, WanlessAG, SeidelCW, WeissEL (2009) Cbk1 regulation of the RNA-binding protein Ssd1 integrates cell fate with translational control. Curr Biol 19 : 2114–2120.
117. GankKD, YeamanMR, KojimaS, YountNY, ParkH, et al. (2008) SSD1 is integral to host defense peptide resistance in Candida albicans. Eukaryot Cell 7 : 1318–1327.
118. Colman-LernerA, ChinTE, BrentR (2001) Yeast Cbk1 and Mob2 activate daughter-specific genetic programs to induce asymmetric cell fates. Cell 107 : 739–750.
119. KaeberleinM, GuarenteL (2002) Saccharomyces cerevisiae MPT5 and SSD1 function in parallel pathways to promote cell wall integrity. Genetics 160 : 83–95.
120. KurischkoC, KuraviVK, HerbertCJ, LucaFC (2011) Nucleocytoplasmic shuttling of Ssd1 defines the destiny of its bound mRNAs. Mol Microbiol 81 : 831–849.
121. SunY, TaniguchiR, TanoueD, YamajiT, TakematsuH, et al. (2000) Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Mol Cell Biol 20 : 4411–4419.
122. RoelantsFM, BaltzAG, TrottAE, FereresS, ThornerJ (2010) A protein kinase network regulates the function of aminophospholipid flippases. Proc Natl Acad Sci U S A 107 : 34–39.
123. ParksLW, SmithSJ, CrowleyJH (1995) Biochemical and physiological effects of sterol alterations in yeast–a review. Lipids 30 : 227–230.
124. LampingE, LucklJ, PaltaufF, HenrySA, KohlweinSD (1994) Isolation and characterization of a mutant of Saccharomyces cerevisiae with pleiotropic deficiencies in transcriptional activation and repression. Genetics 137 : 55–65.
125. PfallerM, NeofytosD, DiekemaD, AzieN, Meier-KriescheHU, et al. (2012) Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance(R)) registry, 2004–2008. Diagn Microbiol Infect Dis 74 : 323–331.
126. MorschhauserJ (2010) Regulation of multidrug resistance in pathogenic fungi. Fungal Genet Biol 47 : 94–106.
127. Kaiser C, Michaelis S, Mitchell AP (1994) Methods in yeast genetics. A laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
128. GabaldonT (2008) Large-scale assignment of orthology: back to phylogenetics? Genome Biol 9 : 235.
129. Huerta-CepasJ, Capella-GutierrezS, PryszczLP, DenisovI, KormesD, et al. (2011) PhylomeDB v3.0: an expanding repository of genome-wide collections of trees, alignments and phylogeny-based orthology and paralogy predictions. Nucleic Acids Res 39: D556–560.
130. RiceP, LongdenI, BleasbyA (2000) EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16 : 276–277.
131. CabralV, ChauvelM, FironA, LegrandM, NesseirA, et al. (2012) Modular gene over-expression strategies for Candida albicans. Methods Mol Biol 845 : 227–244.
132. ChauvelM, NesseirA, CabralV, ZnaidiS, GoyardS, et al. (2012) A versatile overexpression strategy in the pathogenic yeast Candida albicans: identification of regulators of morphogenesis and fitness. PLoS One 7: e45912.
133. KitadaK, YamaguchiE, ArisawaM (1996) Isolation of a Candida glabrata centromere and its use in construction of plasmid vectors. Gene 175 : 105–108.
134. CormackBP, FalkowS (1999) Efficient homologous and illegitimate recombination in the opportunistic yeast pathogen Candida glabrata. Genetics 151 : 979–987.
135. RexJH, PfallerMA, GalgianiJN, BartlettMS, Espinel-IngroffA, et al. (1997) Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin Infect Dis 24 : 235–247.
136. SanglardD, KuchlerK, IscherF, PaganiJL, MonodM, et al. (1995) Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother 39 : 2378–2386.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA SplicingČlánek The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- BdlA, DipA and Induced Dispersion Contribute to Acute Virulence and Chronic Persistence of
- Morphotype Transition and Sexual Reproduction Are Genetically Associated in a Ubiquitous Environmental Pathogen
- A Nucleic-Acid Hydrolyzing Single Chain Antibody Confers Resistance to DNA Virus Infection in HeLa Cells and C57BL/6 Mice
- HopW1 from Disrupts the Actin Cytoskeleton to Promote Virulence in Arabidopsis
- Ly6C Monocytes Become Alternatively Activated Macrophages in Schistosome Granulomas with Help from CD4+ Cells
- Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA Splicing
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- Antibody Responses to : Role in Pathogenesis and Diagnosis of Encephalitis?
- Discovery of a Novel Compound with Anti-Venezuelan Equine Encephalitis Virus Activity That Targets the Nonstructural Protein 2
- Activation of Focal Adhesion Kinase by Suppresses Autophagy via an Akt/mTOR Signaling Pathway and Promotes Bacterial Survival in Macrophages
- Crossing the Interspecies Barrier: Opening the Door to Zoonotic Pathogens
- Catching Fire: , Macrophages, and Pyroptosis
- IscR Is Essential for Type III Secretion and Virulence
- Selective Chemical Inhibition of Quorum Sensing in Promotes Host Defense with Minimal Impact on Resistance
- The Glycosylated Rv1860 Protein of Inhibits Dendritic Cell Mediated TH1 and TH17 Polarization of T Cells and Abrogates Protective Immunity Conferred by BCG
- A Genome-Wide Tethering Screen Reveals Novel Potential Post-Transcriptional Regulators in
- Structural Insights into SraP-Mediated Adhesion to Host Cells
- Human IGF1 Regulates Midgut Oxidative Stress and Epithelial Homeostasis to Balance Lifespan and resistance in
- Cycling Empirical Antibiotic Therapy in Hospitals: Meta-Analysis and Models
- Rab11 Regulates Trafficking of -sialidase to the Plasma Membrane through the Contractile Vacuole Complex of
- Mitogen and Stress Activated Kinases Act Co-operatively with CREB during the Induction of Human Cytomegalovirus Immediate-Early Gene Expression from Latency
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- A Central Role for Carbon-Overflow Pathways in the Modulation of Bacterial Cell Death
- An Invertebrate Warburg Effect: A Shrimp Virus Achieves Successful Replication by Altering the Host Metabolome via the PI3K-Akt-mTOR Pathway
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
- A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees () Prevails after -Mediated, or , Transmission
- Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance Genes
- Ubiquitin-Mediated Response to Microsporidia and Virus Infection in
- Preclinical Detection of Variant CJD and BSE Prions in Blood
- Toll-Like Receptor 8 Agonist and Bacteria Trigger Potent Activation of Innate Immune Cells in Human Liver
- Progressive Proximal-to-Distal Reduction in Expression of the Tight Junction Complex in Colonic Epithelium of Virally-Suppressed HIV+ Individuals
- The Triggering Receptor Expressed on Myeloid Cells 2 Inhibits Complement Component 1q Effector Mechanisms and Exerts Detrimental Effects during Pneumococcal Pneumonia
- Differential Activation of Acid Sphingomyelinase and Ceramide Release Determines Invasiveness of into Brain Endothelial Cells
- Forward Genetic Screening Identifies a Small Molecule That Blocks Growth by Inhibiting Both Host- and Parasite-Encoded Kinases
- Defining Immune Engagement Thresholds for Control of Virus-Driven Lymphoproliferation
- Growth Factor and Th2 Cytokine Signaling Pathways Converge at STAT6 to Promote Arginase Expression in Progressive Experimental Visceral Leishmaniasis
- Multimeric Assembly of Host-Pathogen Adhesion Complexes Involved in Apicomplexan Invasion
- Biogenesis of Influenza A Virus Hemagglutinin Cross-Protective Stem Epitopes
- Adequate Th2-Type Response Associates with Restricted Bacterial Growth in Latent Mycobacterial Infection of Zebrafish
- Protective Efficacy of Passive Immunization with Monoclonal Antibodies in Animal Models of H5N1 Highly Pathogenic Avian Influenza Virus Infection
- Fructose-Asparagine Is a Primary Nutrient during Growth of in the Inflamed Intestine
- The Calcium-Dependent Protein Kinase 3 of Influences Basal Calcium Levels and Functions beyond Egress as Revealed by Quantitative Phosphoproteome Analysis
- A Translocated Effector Required for Dissemination from Derma to Blood Safeguards Migratory Host Cells from Damage by Co-translocated Effectors
- Functional Characterization of a Novel Family of Acetylcholine-Gated Chloride Channels in
- Both α2,3- and α2,6-Linked Sialic Acids on O-Linked Glycoproteins Act as Functional Receptors for Porcine Sapovirus
- The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
- MicroRNA-146a Provides Feedback Regulation of Lyme Arthritis but Not Carditis during Infection with
- Recombination in Enteroviruses Is a Biphasic Replicative Process Involving the Generation of Greater-than Genome Length ‘Imprecise’ Intermediates
- Cytoplasmic Viral RNA-Dependent RNA Polymerase Disrupts the Intracellular Splicing Machinery by Entering the Nucleus and Interfering with Prp8
- and Are Associated with Murine Susceptibility to Infection and Human Sepsis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



