-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCatching Fire: , Macrophages, and Pyroptosis
article has not abstract
Published in the journal: . PLoS Pathog 10(6): e32767. doi:10.1371/journal.ppat.1004139
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004139Summary
article has not abstract
Introduction
Candida albicans is a commensal organism of the human gastrointestinal and genitourinary systems as well as the most common human fungal pathogen [1]. The organism causes mucosal infections such as oropharyngeal or vulvo-vaginal candidiasis, but it can also cause life-threatening invasive disease. Healthy individuals readily maintain the organism in its commensal state but individuals with defects in the anti–C. albicans immune response are at high risk for developing disease. Phagocytes, particularly macrophages and neutrophils, are critical to the host's ability to prevent invasive candidiasis [2].
C. albicans poses a particular challenge to host defenses because it is polymorphic; round yeast forms and filamentous pseudohyphae and hyphae forms are all present during infection [3], [4]. Yeast and filamentous C. albicans have a number of important physiological, structural, and biochemical differences. Accordingly, the immune response to these forms differs substantially [4], [5]. The type of recognition receptors used to detect C. albicans, as well as the phagocyte type, anatomic site of infection, and course of infection, all serve to modulate the host response to the organism [2], [6]. Macrophages are particularly important because they can both limit C. albicans burden early in infection and recruit and activate other immune effector cells [4], [6]. Our understanding of the mechanisms and consequences of the interaction between macrophages and C. albicans is improving, but much remains to be learned.
What Does the Macrophage–C. albicans Interaction “Look” Like?
Much of our understanding of the interaction between C. albicans and macrophages arose from observations using wide-field, confocal, and fluorescence microscopy. Macrophages readily ingest the round yeast form of C. albicans as well as relatively short C. albicans filaments [7]. After ingestion, some C. albicans are killed; however, most survive and form hyphae in response to the phagosome environment (morphogenesis) [8]. Time-lapse microscopy suggests that some macrophages are able to withstand the stress of elongating C. albicans filaments without apparent loss of integrity, whereas other macrophages that have ingested C. albicans undergo lysis [9]. As lysis is temporally linked to filament elongation, the filaments appear to puncture through macrophage membrane [2], [9], [10]. Thus, while macrophages are able to damage or kill C. albicans, the fungus also has a significant cytotoxic effect on macrophages.
In addition to their role in ingestion and possible clearance of C. albicans, macrophages make a critical contribution to the innate and adaptive anti–C. albicans immune response. Macrophages produce a variety of pro - and anti-inflammatory cytokines in response to C. albicans; the type of response is governed both by morphology and other organisms factors as well as by the host pathogen recognition receptors (PRR) that are engaged (see PLOS Pathogens Pearls [11] and [3] or, for a more in-depth review, [4]). In particular, hyphae formation is a strong trigger for production of the pro-inflammatory cytokine interleukin-1β (IL-1β) [5], [12].
How Do C. albicans Filaments Kill Macrophages?
Because of the visual/temporal association of intracellular filament growth with macrophage lysis, a logically appealing hypothesis is that C. albicans filaments simply grow so long that the macrophage membrane is stretched to the point of failure, resulting in lysis [2], [5]. Two additional findings support this hypothesis: First, killed or inactivated C. albicans yeast, which obviously do not form filaments within macrophages, trigger minimal levels of macrophage lysis [13]. Second, C. albicans mutant strains that do not form filaments also do not trigger macrophage lysis [10].
Despite the appealing simplicity of the physical rupture hypothesis, conflicting data has emerged. Several laboratories have identified C. albicans mutant strains that form normal hyphae within macrophages yet induce significantly lower levels of lysis [10], [14], [15]. Furthermore, recent time lapse microscopy experiments have observed “non-lytic expulsion/exocytosis,” in which C. albicans hyphae appear to be expelled from within macrophages without loss of macrophage viability [16]. Thus, hyphal formation alone is not sufficient to trigger macrophage lysis.
Are Macrophage Programmed Cell Death Pathways Activated by C. albicans?
An alternative to the long-held idea that C. albicans physically destroys macrophages is that macrophage lysis in response to C. albicans is actually a macrophage-driven response. In the last decade, there has been an explosion of new data describing programmed cell death pathways in response to infection [17]. The designation “programmed” refers to cell death that is specifically induced by host-cell signaling pathways; thus, programmed cell death is host-driven. The archetypal programmed cell death pathway apoptosis may occur in macrophages responding to Candida [18]; however, apoptosis is non-lytic and cannot account for C. albicans–induced lysis. In contrast, several newly described programmed cell death pathways result in lytic cell death, including: pyroptosis, pyronecrosis, and necroptosis [17]. The most well studied of these is pyroptosis, which results in cell swelling, lysis, and release of inflammatory cytokines (see the PLOS Pathogens Pearl [19], or [20] for more detail). This pathway was originally identified in macrophages infected with intracellular bacteria such as Salmonella, Legionella, and possibly Mycobacteria. By undergoing pyroptosis, infected macrophages deprive intracellular bacteria of their immune-protected niche as well as intracellular nutrients.
A critical hallmark of pyroptosis is its dependence on the cysteine protease caspase-1 [17]. Caspase-1 is activated via formation of the inflammasome, a multiprotein complex that forms in response to a variety of inflammatory signals. Inflammasome formation is initiated through activation of either a nod-like receptor protein (NLRP1, NLRP3, or NLRC4) or the absent in melanoma protein AIM2 [21]. Subsequently, the adaptor molecule ASC (apoptosis-associated speck-like protein containing a CARD) is recruited to the complex; this is followed by binding and activation of caspase-1. In addition to its role in triggering cell lysis, activation of caspase-1 results in cleavage of the pro-forms of the cytokines IL-1β and IL-18 into their mature forms [20]. Thus, pyroptosis is a lytic, inflammatory form of cell death. When considering the role of caspase-1 in pyroptosis, it should be noted that the caspase-1 deficient mice used in the majority of research studies are not only deficient for caspase-1 but also have a dysfunctional caspase-11 [22].
C. albicans triggers the activation of NLRP3, NLRC4, and noncanonical inflammasomes [23]–[27], suggesting that C. albicans might trigger macrophage lysis via pyroptosis. Studies from our laboratory and from Uwamahoro et al., recently demonstrated that the majority of C. albicans–induced macrophage lysis requires caspase-1 [13], [15]. We also found that NLRP3 and ASC, but not NLRC4, were required for this process [13]. Thus, host cell components are required for C. albicans–induced lysis; this would not be expected if lysis were due to physical disruption of the macrophage by C. albicans filaments. Furthermore, C. albicans-induced macrophage lysis can be substantially suppressed by the addition of glycine to the culture medium [13]. Glycine, which has no effect on C. albicans growth or filamentation, suppresses pyroptotic lysis, presumably via blocking membrane pores [28]. Taken together, these data clearly demonstrate that pyroptosis occurs in response to C. albicans via the NLRP3 inflammasome (Figure 1). Thus, most of the “cytotoxicity” seen in macrophages exposed to C. albicans is controlled not by C. albicans but rather by host cell pathways [13]. The role of other lytic programmed cell death pathways, such as pyronecrosis or necroptosis, has not been studied, and it remains possible that these pathways are also triggered by C. albicans. Nevertheless, pyroptosis appears to play a major role in the lytic response of macrophages to ingested C. albicans cells.
Fig. 1. C. albicans–mediated NLRP3 inflammasome activation. 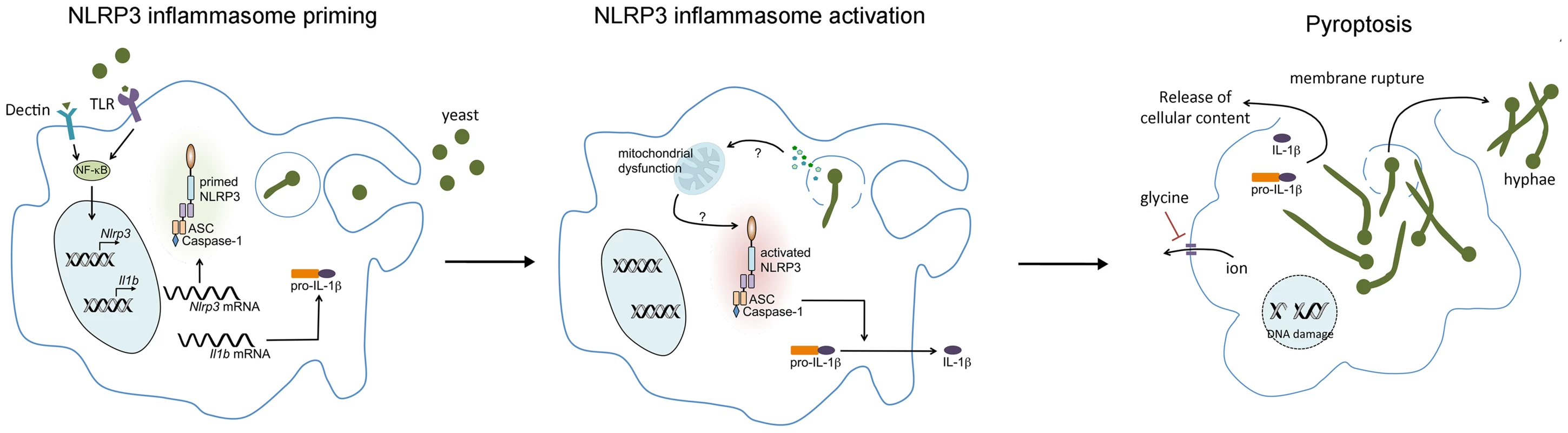
Upon encountering C. albicans, pattern recognition receptors (PRR) on the macrophage, such as TLR2 and Dectin 1 and 2, activate NF-κB, leading to the transcription and translation of NLRP3 and pro-IL-1β. Phagocytosis of C. albicans yeast forms triggers hyphal formation which may result in lysosomal rupture. NLRP3 inflammasome activation is then triggered through an as-yet-undefined mechanism. Although morphogenesis appears to be necessary for inflammasome activation, it is not sufficient. Activation of the NLRP3 inflammasome results in activation of the cysteine protease caspase-1, which mediates the processing and secretion of pro-IL-1β and pro-IL-18. Caspase-1 activation also induces pyroptotic cell death of the macrophage, resulting in cell swelling, DNA fragmentation, and the lytic release of intracellular inflammatory contents. Osmotic lysis of the macrophage during pyroptosis can be inhibited by the addition of extracellular glycine. Our results with mutant C. albicans strains demonstrated that pyroptotic lysis in response to C. albicans does not require hyphal formation [14]. However, we have also observed that the non-albicans Candida species and Saccharomyces cerevisiae strains that are capable of forming filaments are stronger inducers of pyroptosis than those that do not form filaments [13]. Thus, morphogenesis appears to be necessary but not sufficient for triggering pyroptosis. We expect to be able to use this set of Candida and Saccharomyces strains and mutants as a powerful tool for future investigations into the mechanisms through which C. albicans triggers pyroptosis.
How Does Inflammasome Activation Trigger Inflammation in Response to C. albicans?
Another important consequence of pyroptosis is the release of IL-1β/IL-18 [20]. The finding that pyroptosis occurs in response to C. albicans is quite consistent with the ability of C. albicans to trigger IL-1β production in macrophages. Production of mature IL-1β via the NLRP3 inflammasome is tightly regulated in a two-step process: The first, or priming signal, triggers activation of NFκB and transcription of pro-IL-1β [12]. The second signal results in inflammasome assembly, caspase-1 activation, and cleavage of pro-IL-1β into mature IL-1β. As with most biological systems, the two signal “pathways” are not completely separated; priming signals also increase the level of NLRP3 [29].
Macrophages have a variety of PRR that recognize C. albicans and may provide the first or priming signal for inflammasome activation. These include complement receptors; the C-type lectins dectin-1, dectin-2 and mannose receptor; and Toll-like receptors, particularly TLR2 [3], [4], [11]. The mechanism(s) by which C. albicans provides the second signal for inflammasome activation is less clear. The NLRP3 inflammasome is activated in response to a wide range of stimuli including cellular stress, tissue damage, and many types of infection [29]. It appears that NLRP3 responds to these myriad conditions through their convergence on mitochondrial damage; potassium efflux, calcium influx and increased mitochondrial reactive oxygen species production are common triggers of activation. In addition, lysosomal rupture is required for NLRP3 inflammasome activation in response to particulate agonists. Any combination of these signals may occur in C. albicans exposed macrophages; furthermore, the mechanisms of C. albicans mediated NLRP3 activation may vary in different environmental conditions and phagocyte types. As with C. albicans–induced macrophage lysis, activation of NLRP3 was thought to occur in direct response to hyphal formation [5]. However, as with our studies on pyroptosis, our data suggests that, at least for macrophages, morphogenesis is necessary but not sufficient to trigger NLRP3 activation [14].
In addition to the NLRP3 inflammasome, C. albicans activates the NLRC4 inflammasome as well as a noncanonical caspase-8 containing inflammasome [26], [27]. Some of the variation in inflammasome responses to C. albicans may be related to the type of host cell that encounters the organism, the PRR used, and/or the type of infection. This is exemplified by the finding that NLRC4 expressed in mucosal stromal cells is important in defense against oropharyngeal candidiasis [26]. In addition, activation of the 1,3-β-glucan lectin dectin-1 on dendritic cells leads to production of IL-1β via a noncanonical inflammasome that utilizes caspase-8 rather than caspase-1 [27]. The role of caspase-8 in production of IL-1β raises particularly interesting questions about caspase activation and programmed cell death pathways as caspase-8 also has a prominent role in initiating apoptosis. Clearly, there is still much to learn about the role of cell death pathways, inflammasome activation, and cytokine production in C. albicans infections.
IL-1β production in response to C. albicans is important for recruiting additional phagocytes to the site of infection and stimulating protective immune responses via the Th17 and/or Th1 pathway [30]. Less is known about the role of IL-18 in the host response to C. albicans, but it has been associated with recruitment of monocytes to the site of infection, the development of protective Th1 responses, and modest increases in the ability of neutrophils to damage C. albicans pseudohyphae [30], [31].
Pyroptosis also triggers inflammation through IL-1β/IL-18 independent pathways, including production of IL-1α, HMGB1, and eicosanoids [19]; these danger signals may be important in anti–C. albicans defenses. Inflammasome activation in response to C. albicans has been implicated in elaboration of IL-6, CXCL1 (a murine chemokine similar to human IL-8), and antimicrobial peptides [26]. Furthermore, cell lysis results in global release of intracellular molecules such as ATP, DNA, RNA, which are inflammatory when found in the extracellular environment [19]. Very little is known about the role that these molecules and processes play in the inflammatory response to C. albicans, but they may represent additional mechanisms through which pyroptosis contributes to the anti–C. albicans host defense.
Summary: A New Paradigm for Host–C. albicans Interactions
Although C. albicans hyphae formation clearly plays a role in macrophage lysis, the death of macrophages that have ingested C. albicans is not simply the result of the hyphae physically rupturing the macrophage [13]. Rather, the current data supports a new model in which C. albicans–induced macrophage lysis occurs via pyroptosis, a host-cell programmed death pathway. These findings represent the first demonstration that pyroptosis occurs in response to a fungal pathogen. One important question raised by these findings is whether pyroptosis is beneficial to the host, C. albicans, or both. The components of the NLRP3 inflammasome as well as IL-1β, IL-18, and the IL-1α/IL-1β receptor IL-1RI, are important for host survival from systemic candidiasis and prevention of dissemination of oropharyngeal candidiasis [12], [32]. Thus, the NLRP3 inflammasome is clearly important to the host for its role in triggering inflammation; it may also benefit the host by triggering pyroptosis. Alternatively, the host program of pyroptosis could have been “conscripted” during the evolution of C. albicans to provide a mechanism of escape from the macrophage. In that case, triggering pyroptotic macrophage lysis could be a “cost” to the host that is outweighed by the other benefits of inflammasome activation.
Future studies may identify additional mechanisms of host cell death that are triggered in response to C. albicans. Indeed, it seems likely that the cytotoxic effect of C. albicans on phagocytes is a function of multiple mechanisms of cell death with factors such as phagocyte type, local environment, organism burden, and host cell activation influencing which pathway(s) is most strongly activated. Although much remains to be learned about C. albicans–triggered phagocyte lysis, the finding that macrophages are catching fire in response to C. albicans represents a paradigm shift in our understanding of C. albicans–phagocyte interactions. As therapies that modulate specific components of the immune response continue to be developed, a fuller understanding of programmed cell pathways in response to C. albicans may allow us to develop more effective treatment for this life-threatening pathogen.
Zdroje
1. Moran GP, Coleman D, Sullivan D (2012) An introduction to the medically important Candida species. In: Calderone R, Clancy CJ, editors. Candida and Candidiasis. 2nd edition. Washington, D.C.: ASM Press. pp. 11–25.
2. SeiderK, HeykenA, LuttichA, MiramonP, HubeB (2010) Interaction of pathogenic yeasts with phagocytes: survival, persistence and escape. Curr Opin Microbiol 13 : 392–400.
3. LionakisMS, NeteaMG (2013) Candida and host determinants of susceptibility to invasive candidiasis. PLoS Pathog 9: e1003079.
4. FillerSG (2006) Candida-host cell receptor-ligand interactions. Curr Opin Microbiol 9 : 333–339.
5. GowNA, van de VeerdonkFL, BrownAJ, NeteaMG (2012) Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol 10 : 112–122.
6. LionakisMS, LimJK, LeeCC, MurphyPM (2011) Organ-specific innate immune responses in a mouse model of invasive candidiasis. J Innate Immun 3 : 180–199.
7. LewisLE, BainJM, LowesC, GillespieC, RudkinFM, et al. (2012) Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog 8: e1002578.
8. Jimenez-LopezC, ColletteJR, BrothersKM, ShepardsonKM, CramerRA, et al. (2013) Candida albicans induces arginine biosynthetic genes in response to host-derived reactive oxygen species. Eukaryot Cell 12 : 91–100.
9. LewisLE, BainJM, OkaiB, GowNA, ErwigLP (2013) Live-cell video microscopy of fungal pathogen phagocytosis. J Vis Exp doi:10.3791/50196
10. McKenzieCG, KoserU, LewisLE, BainJM, Mora-MontesHM, et al. (2010) Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect Immun 78 : 1650–1658.
11. LevitzSM (2010) Innate recognition of fungal cell walls. PLoS Pathog 6: e1000758.
12. JolyS, SutterwalaFS (2010) Fungal pathogen recognition by the NLRP3 inflammasome. Virulence 1 : 276–280.
13. WellingtonM, KoselnyK, SutterwalaFS, KrysanDJ (2014) Candida albicans Triggers NLRP3-Mediated Pyroptosis in Macrophages. Eukaryot Cell 13 : 329–340.
14. WellingtonM, KoselnyK, KrysanDJ (2012) Candida albicans morphogenesis is not required for macrophage interleukin 1beta production. MBio 4: e00433–00412.
15. UwamahoroN, Verma-GaurJ, ShenHH, QuY, LewisR, et al. (2014) The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. MBio 5: e00003–00014.
16. BainJM, LewisLE, OkaiB, QuinnJ, GowNA, et al. (2012) Non-lytic expulsion/exocytosis of Candida albicans from macrophages. Fungal Genet Biol 49 : 677–678.
17. GalluzziL, VitaleI, AbramsJM, AlnemriES, BaehreckeEH, et al. (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19 : 107–120.
18. Ibata-OmbettaS, IdziorekT, TrinelPA, PoulainD, JouaultT (2003) Candida albicans phospholipomannan promotes survival of phagocytosed yeasts through modulation of bad phosphorylation and macrophage apoptosis. J Biol Chem 278 : 13086–13093.
19. LaRockCN, CooksonBT (2013) Burning down the house: cellular actions during pyroptosis. PLoS Pathog 9: e1003793.
20. MiaoEA, RajanJV, AderemA (2011) Caspase-1-induced pyroptotic cell death. Immunol Rev 243 : 206–214.
21. FranchiL, Munoz-PlanilloR, NunezG (2012) Sensing and reacting to microbes through the inflammasomes. Nat Immunol 13 : 325–332.
22. KayagakiN, WarmingS, LamkanfiM, WalleLV, LouieS, et al. (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479 : 117–121.
23. GrossO, PoeckH, BscheiderM, DostertC, HannesschlagerN, et al. (2009) Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459 : 433–436.
24. HiseAG, TomalkaJ, GanesanS, PatelK, HallBA, et al. (2009) An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5 : 487–497.
25. JolyS, MaN, SadlerJJ, SollDR, CasselSL, et al. (2009) Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol 183 : 3578–3581.
26. TomalkaJ, GanesanS, AzodiE, PatelK, MajmudarP, et al. (2011) A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog 7: e1002379.
27. GringhuisSI, KapteinTM, WeversBA, TheelenB, van der VlistM, et al. (2012) Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol 13 : 246–254.
28. FinkSL, CooksonBT (2006) Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol 8 : 1812–1825.
29. WenH, MiaoEA, TingJP (2013) Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity 39 : 432–441.
30. van de VeerdonkFL, JoostenLA, ShawPJ, SmeekensSP, MalireddiRK, et al. (2011) The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur J Immunol 41 : 2260–2268.
31. NeteaMG, StuytRJ, KimSH, Van der MeerJW, KullbergBJ, et al. (2002) The role of endogenous interleukin (IL)-18, IL-12, IL-1beta, and tumor necrosis factor-alpha in the production of interferon-gamma induced by Candida albicans in human whole-blood cultures. J Infect Dis 185 : 963–970.
32. NeteaMG, VonkAG, van den HovenM, VerschuerenI, JoostenLA, et al. (2003) Differential role of IL-18 and IL-12 in the host defense against disseminated Candida albicans infection. Eur J Immunol 33 : 3409–3417.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA SplicingČlánek Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance GenesČlánek The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- BdlA, DipA and Induced Dispersion Contribute to Acute Virulence and Chronic Persistence of
- Morphotype Transition and Sexual Reproduction Are Genetically Associated in a Ubiquitous Environmental Pathogen
- A Nucleic-Acid Hydrolyzing Single Chain Antibody Confers Resistance to DNA Virus Infection in HeLa Cells and C57BL/6 Mice
- HopW1 from Disrupts the Actin Cytoskeleton to Promote Virulence in Arabidopsis
- Ly6C Monocytes Become Alternatively Activated Macrophages in Schistosome Granulomas with Help from CD4+ Cells
- Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA Splicing
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- Antibody Responses to : Role in Pathogenesis and Diagnosis of Encephalitis?
- Discovery of a Novel Compound with Anti-Venezuelan Equine Encephalitis Virus Activity That Targets the Nonstructural Protein 2
- Activation of Focal Adhesion Kinase by Suppresses Autophagy via an Akt/mTOR Signaling Pathway and Promotes Bacterial Survival in Macrophages
- Crossing the Interspecies Barrier: Opening the Door to Zoonotic Pathogens
- Catching Fire: , Macrophages, and Pyroptosis
- IscR Is Essential for Type III Secretion and Virulence
- Selective Chemical Inhibition of Quorum Sensing in Promotes Host Defense with Minimal Impact on Resistance
- The Glycosylated Rv1860 Protein of Inhibits Dendritic Cell Mediated TH1 and TH17 Polarization of T Cells and Abrogates Protective Immunity Conferred by BCG
- A Genome-Wide Tethering Screen Reveals Novel Potential Post-Transcriptional Regulators in
- Structural Insights into SraP-Mediated Adhesion to Host Cells
- Human IGF1 Regulates Midgut Oxidative Stress and Epithelial Homeostasis to Balance Lifespan and resistance in
- Cycling Empirical Antibiotic Therapy in Hospitals: Meta-Analysis and Models
- Rab11 Regulates Trafficking of -sialidase to the Plasma Membrane through the Contractile Vacuole Complex of
- Mitogen and Stress Activated Kinases Act Co-operatively with CREB during the Induction of Human Cytomegalovirus Immediate-Early Gene Expression from Latency
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- A Central Role for Carbon-Overflow Pathways in the Modulation of Bacterial Cell Death
- An Invertebrate Warburg Effect: A Shrimp Virus Achieves Successful Replication by Altering the Host Metabolome via the PI3K-Akt-mTOR Pathway
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
- A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees () Prevails after -Mediated, or , Transmission
- Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance Genes
- Ubiquitin-Mediated Response to Microsporidia and Virus Infection in
- Preclinical Detection of Variant CJD and BSE Prions in Blood
- Toll-Like Receptor 8 Agonist and Bacteria Trigger Potent Activation of Innate Immune Cells in Human Liver
- Progressive Proximal-to-Distal Reduction in Expression of the Tight Junction Complex in Colonic Epithelium of Virally-Suppressed HIV+ Individuals
- The Triggering Receptor Expressed on Myeloid Cells 2 Inhibits Complement Component 1q Effector Mechanisms and Exerts Detrimental Effects during Pneumococcal Pneumonia
- Differential Activation of Acid Sphingomyelinase and Ceramide Release Determines Invasiveness of into Brain Endothelial Cells
- Forward Genetic Screening Identifies a Small Molecule That Blocks Growth by Inhibiting Both Host- and Parasite-Encoded Kinases
- Defining Immune Engagement Thresholds for Control of Virus-Driven Lymphoproliferation
- Growth Factor and Th2 Cytokine Signaling Pathways Converge at STAT6 to Promote Arginase Expression in Progressive Experimental Visceral Leishmaniasis
- Multimeric Assembly of Host-Pathogen Adhesion Complexes Involved in Apicomplexan Invasion
- Biogenesis of Influenza A Virus Hemagglutinin Cross-Protective Stem Epitopes
- Adequate Th2-Type Response Associates with Restricted Bacterial Growth in Latent Mycobacterial Infection of Zebrafish
- Protective Efficacy of Passive Immunization with Monoclonal Antibodies in Animal Models of H5N1 Highly Pathogenic Avian Influenza Virus Infection
- Fructose-Asparagine Is a Primary Nutrient during Growth of in the Inflamed Intestine
- The Calcium-Dependent Protein Kinase 3 of Influences Basal Calcium Levels and Functions beyond Egress as Revealed by Quantitative Phosphoproteome Analysis
- A Translocated Effector Required for Dissemination from Derma to Blood Safeguards Migratory Host Cells from Damage by Co-translocated Effectors
- Functional Characterization of a Novel Family of Acetylcholine-Gated Chloride Channels in
- Both α2,3- and α2,6-Linked Sialic Acids on O-Linked Glycoproteins Act as Functional Receptors for Porcine Sapovirus
- The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
- MicroRNA-146a Provides Feedback Regulation of Lyme Arthritis but Not Carditis during Infection with
- Recombination in Enteroviruses Is a Biphasic Replicative Process Involving the Generation of Greater-than Genome Length ‘Imprecise’ Intermediates
- Cytoplasmic Viral RNA-Dependent RNA Polymerase Disrupts the Intracellular Splicing Machinery by Entering the Nucleus and Interfering with Prp8
- and Are Associated with Murine Susceptibility to Infection and Human Sepsis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



