-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTransfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
All organisms need to respond quickly to sudden environmental changes. Translational regulation can occur in response to environmental stresses within minutes, which is much faster than transcriptional regulation, and thus normally provides immediate adaptation. Eukaryotic cells can manipulate their tRNA molecules, mainly in a reversible manner, to suppress translation. Here, we showed for the first time that bacteria respond to oxidative stress by adjusting the translational system in a manner that differs from that of eukaryotes. The bacteria nonspecifically, irreversibly, and enzymatically degrade tRNAs to block protein synthesis. Interestingly, we showed that elevated tRNA concentrations lead to opposing effects by causing increased protein aggregation, which impairs fitness under normal conditions but facilitates adaptation under oxidative stress, including that caused by antibiotics. Our results provide a new understanding of the role of global adjustments to the entire translation system during stress adaptation in bacteria. This mechanism may also be involved in the development of antibiotic resistance in bacteria.
Published in the journal: . PLoS Genet 11(6): e32767. doi:10.1371/journal.pgen.1005302
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005302Summary
All organisms need to respond quickly to sudden environmental changes. Translational regulation can occur in response to environmental stresses within minutes, which is much faster than transcriptional regulation, and thus normally provides immediate adaptation. Eukaryotic cells can manipulate their tRNA molecules, mainly in a reversible manner, to suppress translation. Here, we showed for the first time that bacteria respond to oxidative stress by adjusting the translational system in a manner that differs from that of eukaryotes. The bacteria nonspecifically, irreversibly, and enzymatically degrade tRNAs to block protein synthesis. Interestingly, we showed that elevated tRNA concentrations lead to opposing effects by causing increased protein aggregation, which impairs fitness under normal conditions but facilitates adaptation under oxidative stress, including that caused by antibiotics. Our results provide a new understanding of the role of global adjustments to the entire translation system during stress adaptation in bacteria. This mechanism may also be involved in the development of antibiotic resistance in bacteria.
Introduction
Reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), the hydroxyl radical (·OH), and superoxide (O2-), are mainly generated as byproducts of the respiratory chain or introduced on exposure to a hazardous environment [1]. These ROSs can damage proteins and nucleic acids by oxidation, leading to cellular oxidative stress (reviewed in [1–3]). To counteract oxidative stress, bacteria have evolved diverse systems, such as the OxyR system and SoxRS systems, to activate the transcription of a series of enzymes including superoxide dismutases (reviewed in [3]). However, the transcription of the stress-response genes requires 10 min to reach maximal production levels, and protein translation requires additional time for synthesis [4,5], which takes ~20–30 min to take effect in bacteria and 45 min in yeast (reviewed in [6]). Thus, cells need an alternative mechanism(s) to response to environmental stresses within minutes, i.e., at the translation level (reviewed in [7]).
Translational regulation under oxidative stress has been intensively studied in eukaryotic cells. Early studies suggested that translation is globally inhibited in 5 min in Saccharomyces cerevisiae cells [8]. This process is mediated by specific tRNA and rRNA cleavage [9,10]. The same phenomenon has also been observed in mammalian cell lines [11–13]. Cleavage of tRNAs leads to the formation of small RNA fragments that can repress translation initiation and can regulate cellular functions, such as proliferation [14,15]. However, recent studies have provided increasing evidence that a considerable fraction of genes is more actively translated under oxidative stress in eukaryotic cells. In fission yeast, 26 genes were translationally upregulated in 15 min, and 191 genes were translationally upregulated in 60 min [5]. Results from a time-resolved transcriptome and proteome study in S. cerevisiae revealed that >80% of proteins diverged from their mRNA expression profiles, suggesting a widespread translational control mechanism involving both upregulation and downregulation. Approximately 25% of the proteins were upregulated with their mRNA expression levels nearly unchanged[16]. The tRNALeu(CAA) hypermodified at the wobble position increases the translation of TTG codons after H2O2 exposure and thus enhanced the protein expression of TTG-enriched genes [17].
In contrast, little is known about translational regulation in response to oxidative stress in prokaryotes, although the available data have provided clues suggesting that this response differs substantially from that of eukaryotes. For example, specific endoribonucleases such as colicin and PrrC specifically cleave tRNA into 2 fragments at anticodon loop in prokaryotes; however, this phenomenon is not as universal in prokaryotes as it is in eukaryotes, occurring only under specific conditions with a select few tRNAs such as tRNA-Arg(ICG) [18] and tRNA-Lys [19,20].
Recent “omics” reports investigating alterations in bacteria have been focused on steady-state conditions. The translational fidelity is slightly lowered due to increased tRNA mischarging [21]. However, the mild effects of mischarging can be easily overcome by an adequate supply of amino acids [21]. Modified Escherichia coli strains can adapt to their oxidative cytoplasmic environments to yield growth rates similar to those of the wild-type [22]. Such “oxidative” E. coli strains have been used in industrial applications to produce recombinant proteins that contain disulfide bonds, showing its strong fitness (reviewed in [23]). The chaperone system (e.g., DnaK, Tig, and GroEL) remains nearly constant or only marginally decreased under oxidative stress, indicating that oxidative stress does not lead to as much protein unfolding or damage as the heat stress[24], which is in sharp contrast to the effects observed following heat shock [25,26]. Although it has been proposed that polynucleotide phosphorylase may globally degrade oxidized RNAs containing 8-oxo-G to protect E. coli from oxidative damage [27], results from multiple transcriptome and proteome studies have shown that transcriptomes and proteomes are nearly unchanged by such activity. For example, only 6 differentially expressed proteins were found in E. coli when exposed to oxidative stress, only 2 of which are transcriptionally regulated in response to oxidative stress [24]. Similar results were found in Bacillus subtilis [28]. Notably, ~200 differentially expressed mRNAs were detected in B. subtilis under oxidative stress, but only 16 of the corresponding proteins showed differential expression. Discrepancies between the numbers of differentially expressed genes and proteins suggest that a remarkable translational regulation process occurs in response to oxidative stress in prokaryotes. However, a general translational response remains elusive.
The protein synthesis rate is a critical index for representing the translational efficiency, especially for a nearly steady-state proteome, and can be measured using stable isotope labeling by amino acids in cell culture mass spectrometry (SILAC-MS) [29–31]. Briefly, cells are transferred into SILAC media containing amino acids (usually lysine or arginine) labeled with heavy isotopes, and the newly synthesized proteins are labeled with the heavy isotopes. The ratio of heavy to light peptides can be accurately measured by mass spectrometry (reviewed in [32]). Thus, the protein synthesis rate can be deduced by monitoring the production of heavy peptides in a time-course assay. This strategy works well for higher eukaryotic systems, but is not applicable in prokaryotes due to their complex metabolic pathways that convert labeled lysine or arginine residues to other amino acids, which deviates from the principle of metabolic labeling [33]. Metabolic labeling of all nitrogen atoms with 15N can label entire proteins very effectively (>99%) [34], but creates complex m/z shifts due to the large number of nitrogen atoms present in peptides, which severely challenges data interpretation. Therefore, poor identification is expected, and the turnover of only 40 proteins in the green alga Ostreococcus tauri could be analyzed using this method [35]. To bypass this disadvantage, Maier et al. used 30 external isotope-labeled peptides as references and determined half-lives of 231 proteins in Mycoplasma pneumoniae [26], which is a more effective approach than those used in previous studies, but is relatively complicated.
In this study, we improved upon our previously developed ProVerB algorithm [36] to identify and quantify 15N-metabolically labeled proteins in time-course assays using MS. Thus, we could measure protein synthesis in E. coli under normal conditions and oxidative stress at the proteome-wide level. Surprisingly, we found that translation elongation nearly halted, minutes after the oxidant was applied. We detected no specific tRNA and rRNA degradation, but levels of full-length tRNAs were rapidly reduced. Interestingly, eleva-ted tRNA concentrations promoted the fitness of E. coli under oxidative stress, making it more resistant to oxidation-generating antibiotics. These results depict a unique tRNA-centric scheme of global translational responses during oxidative stress in prokaryotes, which is different from the regulatory mechanisms used in eukaryotes.
Results
Optimal condition of oxidative stress
To determine optimal conditions for inducing oxidative stress, we measured the growth of the E. coli BL21 strain in M9 minimal medium with various concentrations of H2O2, ranging from 0.2 mM to 2 mM. Exposing cells to 0.5 mM H2O2 resulted in marked transient growth arrest (10-fold decrease in the growth rate), while higher concentrations (1 mM and 2 mM) led to considerable cell death (Fig 1A). This concentration is comparable to that used in previous studies [37,38]. In parallel, we performed propidium iodide (PI) staining and flow cytometry to examine the degree of cell death caused by oxidative reagents. Treating cells with 0.5 mM H2O2 resulted in a nearly identical profile of low-stained cells (representing live cells) under normal conditions after at least 90 min following incubation with H2O2 (Fig 1B). In contrast, the cells killed at 65°C for 15 min showed a single peak with high PI staining. These results showed that treating cells with 0.5 mM H2O2 does not cause detectable cell death.
Fig. 1. Optimization of the oxidative stress conditions. 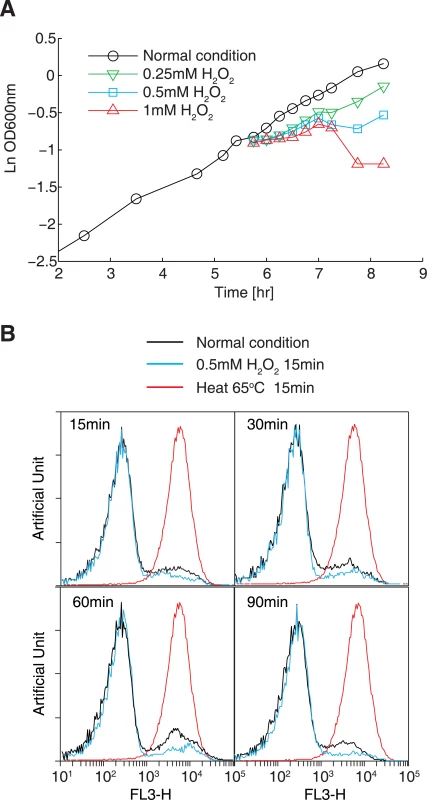
(A) Cell growth in the presence of different concentrations of H2O2. E. coli BL21(DE3) cells were cultivated in M9 minimal medium. Cell growth in the presence of 0.25–1 mM H2O2 was monitored by measuring OD600 values. (B) Cell viability assay using propidium iodide staining and flow cytometry. Histograms showing cells exposed to 0.5 mM H2O2 for 15, 30, 60, or 90 min were obtained (cyan lines). Cells grown under normal condition was used as a positive control (black line), and cells killed by incubation at 65°C for 15 min were used as a negative control (red line). Proteome synthesis measured by 15N metabolic labeling
To study the translational response of E. coli cells under oxidative stress, we investigated protein synthesis (amino acids incorporation) under normal and oxidative stress conditions. To achieve accurate quantification, 15N metabolic labeling was used, as SILAC labeling is not applicable for bacterial studies [33]. However, it has been reported previously that 15N metabolic labeling can cause a significant decrease in growth rates and significant alterations in metabolite production in the E. coli BL21star strain. These findings indicate that the metabolism is remarkably changed just because of the single extra neutron in heavy nitrogen atoms and that this method can thus introduce bias in analysis of protein synthesis [39]. To examine this effect in our system, we measured the growth curve of the E. coli BL21(DE3) strain in M9 minimal medium with 14NH4Cl or 15NH4Cl as the only nitrogen source. Interestingly, we could not detect any significant differences in growth rates during log-phase growth using these two isotopes (P = 0.738, two-tailed Student t-test; Fig 2A). This indicated that our 15N metabolic-labeling protocol did not create any growth defects and that it can be used for analyzing protein synthesis.
Fig. 2. Protein synthesis measurement under normal condition and oxidative stress using 15N metabolic labeling and mass spectrometry. 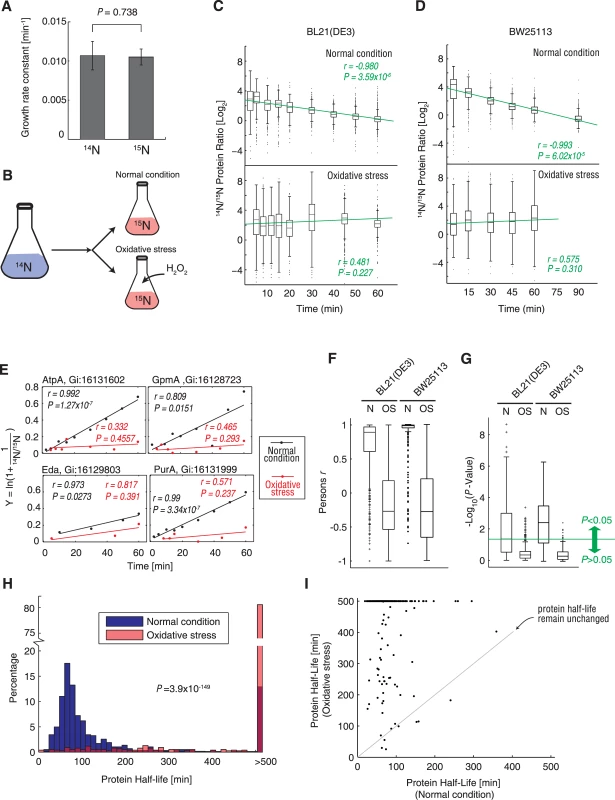
(A) Growth rate constant of E. coli BL21(DE3) cells grown under normal conditions in M9 minimal medium containing 14NH4Cl (14N) or 15NH4Cl (15N). (B) Experimental design for pulse labeling with 15N under normal and oxidative stress conditions. (C) Bacterial cells were transferred to M9 medium containing 15N, after which the 14N/15N ratio of the E. coli BL21(DE3) proteome was determined by mass spectrometry under normal conditions (upper plot) and oxidative stress induced by exposure to 0.5 mM H2O2 (lower plot). The green line indicates the best linear fit of the dataset. The Pearson r correlation coefficient and P-value of the fit are indicated in green text. (D) The same experiment represented in (C) was repeated using the wild-type E. coli BW25113 strain. (E) Examples showing changes in the 14N/15N ratio of 4 randomly selected proteins (AtpA, GpmA, Eda, and PurA) in BL21(DE3) cells over time. The fit was determined according to Eq 6 (Materials and Methods section). The slope of the linear fit is the synthesis rate constant (ksyn). The black dots indicate the actual data points following protein quantification under normal condition, while the red dots indicate the data points measured under oxidative stress. Linear fits were determined for each dataset, and the Pearson correlation coefficients (r) are indicated in the diagram. (F) The Pearson correlation coefficients (r) values of all proteins were fitted using Eq 6, under normal condition and oxidative stress. Data generated using the BL21(DE3) and wild-type BW25113 strains are shown. (G) P values (in -log10 scale) of linear fits of all proteins using Eq 6 under normal and oxidative stress conditions. The green line denotes the significance threshold (P = 0.05). The data generated using the BL21(DE3) and wild-type BW25113 strains are shown. (H) Histograms of protein half-lives under normal (blue) and oxidative stress (red) conditions. (I) Comparison of protein half-lives measured under normal and oxidative stress conditions. The grey line is at a 45° angle, which demarks the positions where the protein half-lives were unchanged. Half-lives longer than 500 min are shown as 500 min. To overcome the drawbacks of traditional MS identification and quantification algorithms in the context of 15N metabolic labeling, we used our ProVerB algorithm [36], which is capable of handling complex m/z shifts caused by 15N metabolic labeling, as described in the Materials and Methods section. Peptides were identified from spectra matches to our tandem MS/MS spectra. Two spectral matches are shown in S1 Fig. These spectra demonstrated the reliable identification of peptides, even when noise spectra peaks were present. The 14N/15N ratios of peptides were determined based on the peak areas of the MS1 scans. Using a stringent criterion of the false discovery rate (FDR) <1% at the protein level, we quantified ~300–1000 proteins from single E. coli soluble protein samples (quantifications shown in S1 Table), which is markedly higher than results obtained using previous algorithms. This allowed us to assess the protein-synthesis rate of E. coli on a proteome-wide scale in high resolution for the first time.
We cultured E. coli BL21(DE3) cells until the optical density at 600 nm (OD600) reached 0.4 in 14N M9 minimal medium and then quickly changed the medium to 15N labeled minimal medium, with or without 0.5 mM H2O2. Next, we collected the cells at various time points and analyzed their proteomes by MS (Fig 2B). We quantified the 14N/15N ratios for each protein at each time point with our new algorithm. Without oxidative stress, 15N-labeled proteins were continuously synthesized and replaced the 14N proteins, resulting in a constant decrease of the 14N/15N ratio over time (Fig 2C, upper plot), indicating that the 15N-labeled proteins gradually substituted for their 14N counterparts. This finding was confirmed by performing linear regression of the data points, which showed a very significant and negative slope (Pearson correlation coefficient r = -0.980, P = 3.59 × 10-6; Fig 2C, upper plot). In sharp contrast, the 14N/15N ratio of the proteome showed negligible differences over time under oxidative stress (insignificant linear fit with P = 0.227; Fig 2C, lower plot). S2 Fig shows the MS1 scans obtained for 2 peptides after 60 min growth under oxidative stress and normal conditions, as examples. Under normal condition, the quantity of the newly synthesized 15N peptides exceeded their 14N counterparts (S2A Fig). In contrast, under oxidative stress, the quantities of newly synthesized 15N peptides were at least one order of magnitude lower than the corresponding 14N peptides (S2B Fig). These results indicate that protein synthesis is almost completely blocked under oxidative stress. We performed the same proteomic measurements in the wild-type E. coli BW25113 strain and reproduced the same trend (Fig 2D).
To validate these findings, we calculated the synthesis rate constant (ksyn) of those proteins that were quantified in at least 3 time points, using Eq 6. The linear fits for 4 randomly selected proteins are demonstrated in Fig 3E, showing that the ksyn (equal to the slope) of most proteins under oxidative stress was much lower than that observed under normal conditions. The 4 fits under normal conditions were all significant (P < 0.05), while none of the fits under oxidative stress were significant (P = ~0.237–0.457), indicating that we could not reliably detect the protein synthesis rate of these proteins, i.e., the protein synthesis was blocked. The linear fits (Eq 6) of most proteins yielded Pearson correlation coefficient (r) >0.6 in BL21(DE3) cells, and approached 1 in the wild-type BW25113 strain, indicating a good linear fit. In sharp contrast, the Pearson r values of most proteins were <0.2 in both strains (Fig 2F). Moreover, more than half of the fits under normal conditions were statistically significant (P < 0.05), while 95% of the fits obtained under oxidative stress were insignificant (P > 0.05), indicating that oxidative stress caused an overall shutdown in proteome synthesis in both strains (Fig 2G).
Fig. 3. Protein biogenesis under oxidative stress. 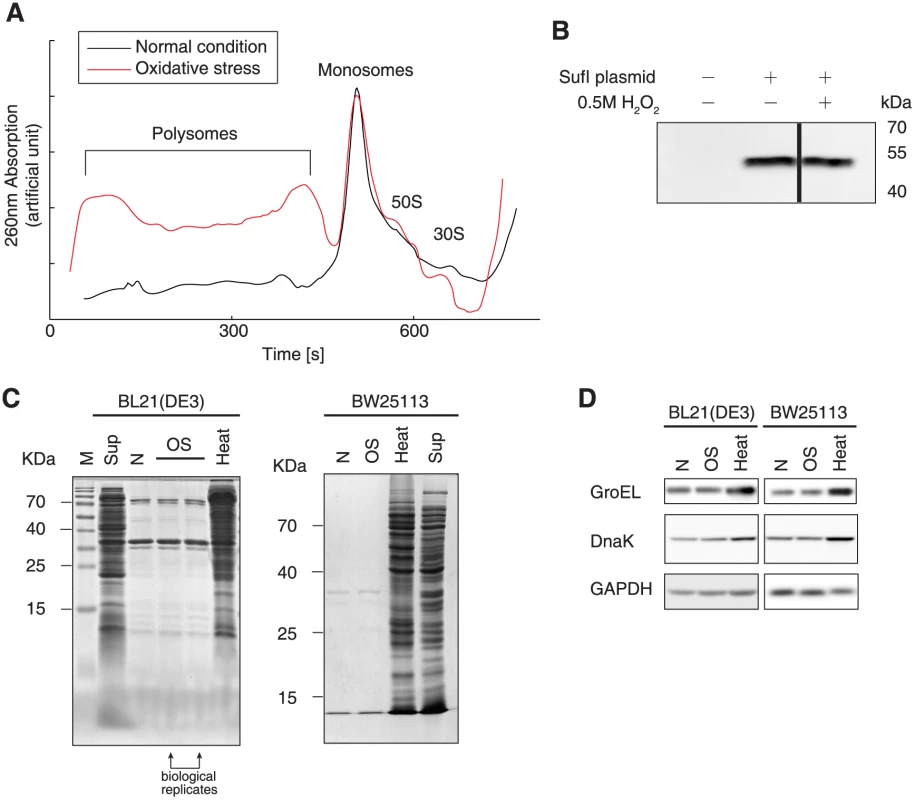
(A) Polysome profiles under normal and oxidative stress conditions. (B) SufI synthesis in an in vitro translation system, with or without oxidative stress. The SufI gene fused with a C-terminal His6-tag sequence constructed in a pET-28b plasmid (Novagen) was used as a template in an in vitro translation reaction. The protein product was detected by western blotting with an anti-His6 antibody. (C) Detergent-insoluble proteins under normal (N) and oxidative stress (OS) conditions. Insoluble proteins extracted from E. coli BL21(DE3) and wild-type BW25113 cells grown at 47°C for 10 min were used as positive controls (Heat). Soluble proteins (Sup) were loaded on the same gel as controls. (D) Western blot analysis of the molecular chaperones GroEL and DnaK in E. coli BL21(DE3) and wild-type BW25113 cells grown under normal (N) conditions, oxidative stress (OS) condition, or heat stress (heat) at 47°C for 30 min. We then calculated the half-lives for each protein using Eq 7. The average half-life of the proteome under normal conditions was 100.38 min, with >69% of the individual proteins having a half-life <100 min (Fig 2H). In sharp contrast, >80% of the proteins showed a half-life exceeding 500 min under oxidative stress, which significantly deviated from the half-life distributions under normal conditions (P = 3.9 × 10-149, Kolmogorov-Smirnov test; Fig 2H). Half-lives of >500 min could not be reliably detected (P > 0.05) using the linear fit of Eq 6. The vast majority of proteins were regenerated much more slowly under oxidative stress (Fig 2I). In sum, these data revealed that protein synthesis was inhibited or nearly abolished under conditions of oxidative stress.
Oxidative stress slows down translation elongation, but does not affect translation initiation
To investigate whether inhibited protein synthesis occurs in the initiation or elongation phase, we measured polysome profiles in E. coli grown with and without oxidative stress. Cells under oxidative stress showed remarkably higher fractions of polysomes than observed under normal conditions, indicating that the translation initiation frequency may be unchanged, but that elongation was considerably decelerated (Fig 3A). This finding agreed with our previous data showing that an unchanged translation initiation and faster elongation speed results in a decrease in the polysome fraction [40].
To further validate this hypothesis, we performed cell-free translation of the E. coli protein SufI expressed with a C-terminal His6-tag, in the presence or absence of 0.5 mM H2O2. The reaction mixtures contained only the target gene and the necessary components required for translation, and no protease or ribonuclease was present. Therefore, the protein production rate is determined solely by translation initiation in this system. The SufI protein synthesized in the cell-free translation system was detected by western blot analysis, which showed that H2O2 incubation did not inhibit in vitro protein production (Fig 3B). This suggested that translation initiation is not hindered by oxidative reagents, i.e., the oxidative reagents used in this study did not cause significant oxidative damage to the proteins.
To test this postulation, we examined protein aggregation, as oxidative damage to proteins results in protein misfolding and aggregation in cells [41]. Under normal conditions, we observed a pattern of protein aggregation (Fig 3C) that was in agreement with results from a previous report [40]. Under the oxidative stress condition used in this study, we did not observe increased protein aggregation in either E. coli strain tested (Fig 3C). As a positive control, we showed that heat stress at 47°C led to severe protein misfolding and aggregation. We also tested the molecular chaperones GroEL and DnaK, which serve as markers for heat shock and protein aggregation responses. The levels of these chaperones remained approximately equal under normal and oxidative stress conditions, but were remarkably elevated at a high temperature (Fig 4D). These results suggested that oxidative stress did not generate massive protein aggregation.
Fig. 4. Decrease of full-length tRNAs under oxidative stress. 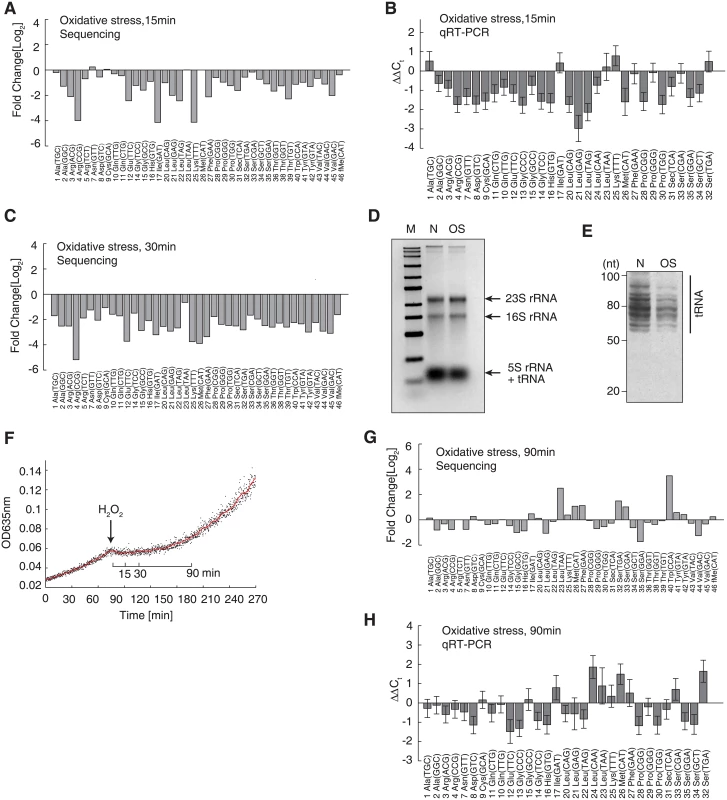
The tRNAs are displayed in alphabetical order and named according to their amino acids and anticodons. (A) Decreased tRNA levels following 15 min of H2O2-induced oxidative stress, relative to tRNA levels observed under normal conditions. The data were generated by next-generation sequencing. (B) qRT-PCR validation of the tRNA decreases induced by 15 min of oxidative stress, using independent biological replicates. The data are shown as the mean ± SD. (C) Decreased tRNA levels following 30 min of OS, measured by next-generation sequencing. (D) Agarose gel electrophoresis of the total RNA extracted from the same number of cells grown under normal conditions (N) or under 15 min of oxidative stress (OS). (E) Polyacrylamide gel electrophoresis of the sample represented in panel (D) to resolve the tRNAs. The size range of tRNAs is indicated. (F) Growth curves before and after the application of oxidative stress. The red line shows the moving average of the OD635 observed over 5 min. (G) The tRNA concentration after 90 min of oxidative stress, after which the bacteria had fully adapted to the stress and growth was resumed. (H) qRT-PCR validation of the results shown in panel (G), using an independent biological replicate. The data are shown as the mean ± SD. Given that the proteome was not damaged, we hypothesized that the inhibition of translation elongation may have been caused by the downregulation of tRNA abundances in vivo.
Full-length tRNA levels are globally downregulated under oxidative stress in vivo
To study in vivo variations in tRNA abundances upon oxidative stress, we quantitatively analyzed the tRNA pool by next-generation sequencing and qRT-PCR. We added an in vitro transcribed single-stranded RNA as a spike-in RNA. This spike-in contained a 3′-CCA tail like tRNA and deviates by least 3 nucleotides from any E. coli tRNA (S3 Fig). We obtained ~8–10 million sequencing reads for cells under normal and oxidative conditions. A total of 1.6~2.0 million reads were aligned to 47 tRNA species using a modified Smith–Waterman algorithm, which considered modified bases according to the Modomics database and allowed for a maximum of 3 errors [42,43]. All 47 tRNA species were detected under both conditions with high reproducibility (R = 0.998, independent biological replicates; S4A Fig), except for 2 methionine tRNAs (S2 Table). The lack of detection of the 2 methionine tRNAs may have been due to the higher number of mismatches occurring in the sequencing reads. After normalization to the spike-in RNA, we detected a global downregulation of full-length tRNA (on average, a 2.47-fold decrease) 15 min after the application of oxidative stress (Fig 4A). This trend was in general validated by qRT-PCR performed using an independent biological replicate, with which 38 tRNA species were successfully and specifically amplified (Fig 4B). Notably, the qRT-PCR results obtained with independent biological replicates showed very high reproducibility (R ≥ 0.99; S4B Fig). These tRNA levels were further decreased after 30 min of oxidative stress (Fig 4C). With ribosomal RNA levels remaining constant under oxidative stress, the decrease in low molecular weight RNAs could be clearly seen (Fig 4D), indicating a decrease in tRNA levels. This global decrease in tRNA was then visually confirmed by polyacrylamide gel electrophoresis, which can resolve tRNAs into multiple bands (Fig 4E). To rule out possible technical artifacts, we collected the cells 90 min after the application of oxidative stress, during which time the cells adapted the new environment and cell growth was resumed (Fig 4F), implying that protein synthesis was restored. Both next-generation sequencing and qRT-PCR showed that tRNA production was restored to normal or even slightly higher levels (Fig 4G and 4H). To rule out a potential bias occurring during quantitative reverse-transcription reactions associated with the CCA-specific reverse-transcription method, we used a traditional method with random hexamers to prime the reverse-transcription reactions, which is not 3’-CCA specific [44,45]. We could reproduce the global and significant downregulation of tRNAs 15 min after oxidative stress, which was reversed after 90 min (S5 Fig). To demonstrate that this trend was not strain-specific, we reproduced the effect of global tRNA downregulation after 15 min of oxidative stress (with reversal after 90 min) in the wild-type E. coli BW25113 strain (S6 Fig). These results showed that the abundances of full-length tRNAs were indeed decreased under oxidative stress, and thus inhibited the translation elongation.
Enzymatic and nonspecific tRNA degradation decreases full-length tRNA concentrations under oxidative stress
To determine how the full-length tRNAs were degraded, we first examined whether tRNAs were specifically cleaved into halves in E. coli, similar to the phenomenon observed in eukaryotes [9]. However, no bands or smears could be detected at ~30–40 nt in a polyacrylamide gel, suggesting that specific tRNA degradation did not occur in E. coli under oxidative stress (Fig 4E). The lack of smaller bands or smear below the tRNA bands also suggested that RNAs in general was not degraded.
We then investigated the non-full-length tRNA reads in the sequencing datasets. Three tRNAs, namely, 1-Ala(TGC), 7-Asn(GTT), and 12-Glu(TTC), possess tandem CCA sequences at their 3′-end (i.e., CCACCA-3′). Therefore, if the 3′-CCA sequences were specifically cleaved, the remaining sequence could be detected by our method. However, in contrast to eukaryotes, in which the 3′-CCA tails can be reversibly cleaved to temporarily block translation [13], no accumulation of 3′-CCA cleavage intermediates was detected in E. coli cells under oxidative stress. Reads that were ~1–10 nucleotides shorter than the full-length sequence were detected, and the versions without 3′-CCA were not the most prevalent species for most tRNAs (Fig 5A). These results indicated that tRNA degradation under oxidative stress is nonspecific and irreversible, which stands in contrast to the situation observed in eukaryotic cells.
Fig. 5. Under oxidative stress, tRNAs are degraded in vivo, but not in the cell-free in vitro translation system. 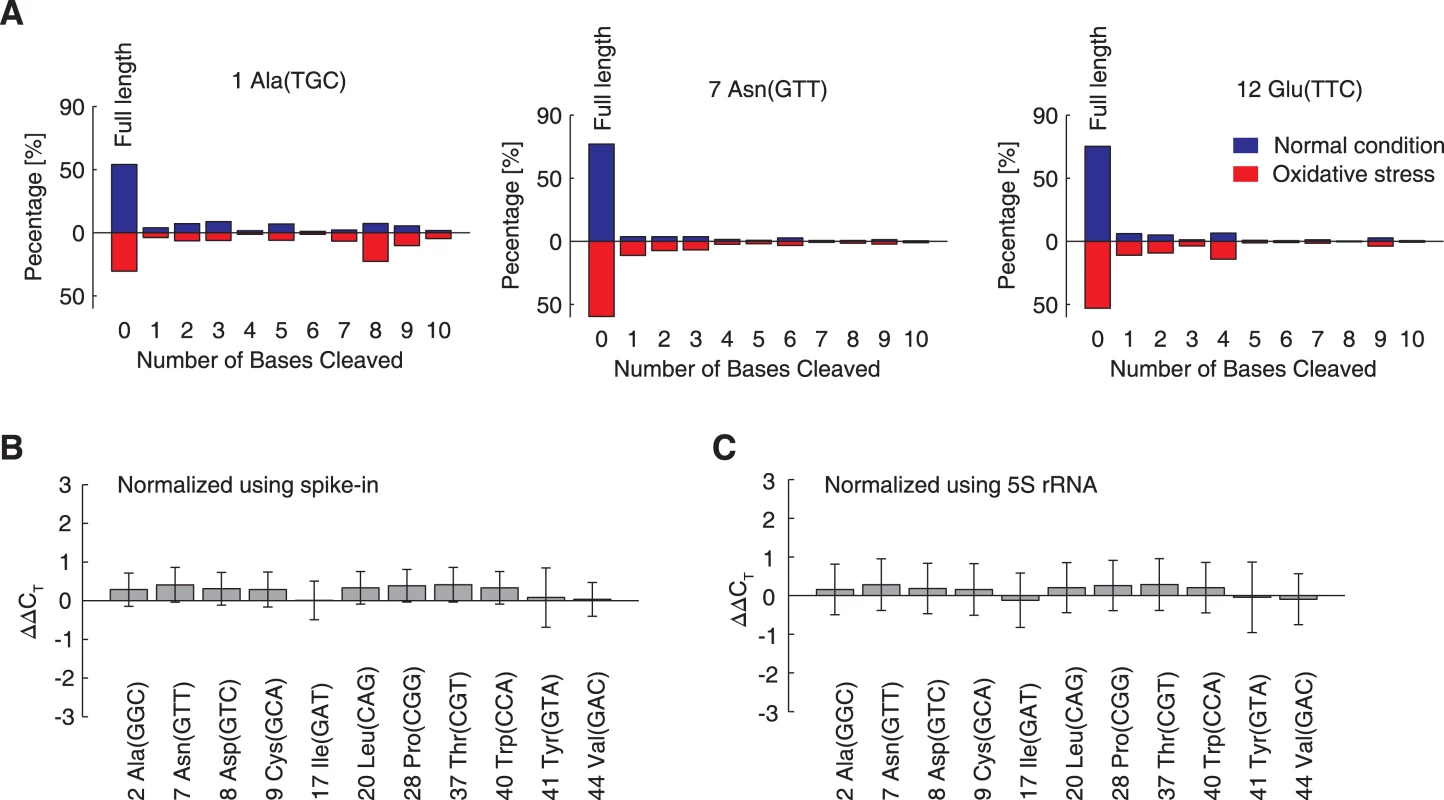
(A) Percentages of cleaved tRNA reads. X-axes indicate the number of bases cleaved from the 3′-termini. The Y-axes denote the fraction of such tRNA reads among all tRNA reads relative to each specific tRNA species. Three tRNAs are shown as examples. The observed distributions of the cleavage lengths were compared between normal (blue bars) and oxidative stress conditions (red bars). (B,C) The degradation of full-length tRNA in in vitro translation system. Eleven tRNAs were randomly selected and quantified by qRT-PCR. The difference observed between the oxidative stress and normal conditions are expressed in terms of ΔΔCT values, normalized using spike-in RNA (B) or 5S rRNA (C). The data are shown as the mean ± SD. The next open question was whether the observed nonspecific tRNA degradation relied upon cellular enzymes like RNases. To address this question, we added 0.5 mM H2O2 in the E. coli cell-free in vitro translation system with an RNase inhibitor and quantified 11 tRNAs by qRT-PCR. Compared to the in vitro translation results found without H2O2, none of the tRNAs in the oxidative environment were degraded; their quantities remained unchanged (Fig 5B and 5C). This conclusion was validated when qRT-PCR results were normalized according to the quantity of spike-in tRNA (without nucleotide modifications, Fig 5B) and 5S rRNA (with nucleotide modifications, Fig 5C), showing that the oxidation of the modified nucleotides did not affect the cDNA-based quantitation methods. Therefore, the nonspecific degradation observed in vivo under oxidative stress is dependent on cellular enzymes.
Elevated tRNA levels protects E. coli against oxidative stress
Translation elongation was remarkably inhibited due to nonspecific tRNA degradation. Therefore, we posited that excess tRNAs may rescue translation elongation under oxidative stress. To test this hypothesis, we elevated the levels of low-abundance tRNAs in the wild-type E. coli BW25113 strain and the BL21(DE3) strain by transforming them with the pRIL plasmid. This plasmid was isolated from the E. coli BL21(DE3)-CodonPlus RIL (BL21-RIL) strain, which carries extra copies of tRNAs that decode AGA, AGG, AUA, and CUA codons and, thus, can significantly accelerate translation elongation [40,45]. Control E. coli cells were transformed with the empty pBAD33 vector, which is chloramphenicol resistant like pRIL, to exclude any potential influence caused by chloramphenicol selection [40]. The oxidative environment did not cause elevated cell death in BL21-RIL cells or control cells without tRNA supplementation (Fig 6A). The pRIL plasmid speed up the translation, resulting in augmented protein misfolding and aggregation [40], thus significantly impairing the fitness and growth rate, compared to control cells (P = 2.39 × 10-4, two-tailed Student t-test; Fig 6B). In the wild-type BW25113 strain, tRNA supplementation also slightly decreased the growth rate, but this difference was not significant (P = 0.201, two-tailed Student t-test; Fig 6B), perhaps because the wild-type E. coli strains expresses the OmpT and Lon proteases and can degrade the aggregated misfolded proteins more efficiently than the BL21 strain. In contrast, when oxidative stress was applied, both the wild-type BW25113 and the BL21 cells with augmented tRNAs grew significantly faster than the control cells (P = 0.0154 and 1.96 × 10-3, respectively, two-tailed Student t-test; Fig 6C), which supported our hypothesis.
Fig. 6. Higher tRNA concentrations improve adaptation under oxidative stress. 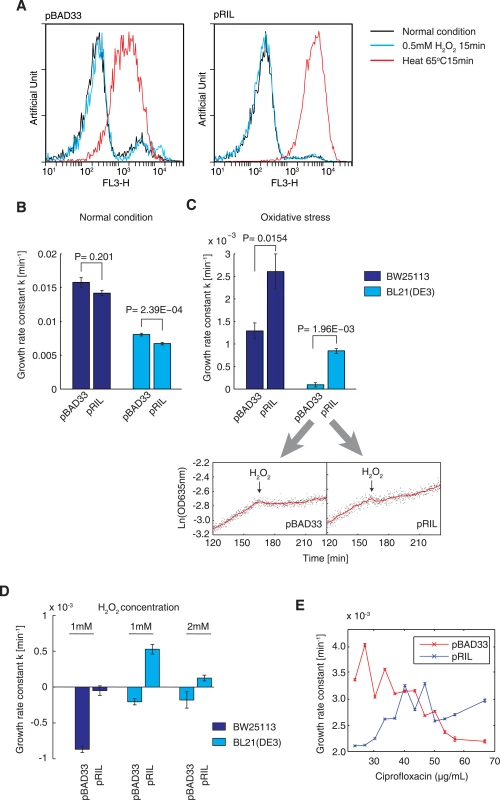
(A) Cell viability testing results obtained using the PI staining method. E. coli BL21(DE3) cells carrying the empty vector pBAD33 and the pRIL plasmid were tested. A histogram showing cells under 0.5 mM H2O2-induced oxidative stress for 15 min was prepared. Cells grown under normal conditions were used as a positive control, and cells killed by incubation at 65°C for 15 min were used as a negative control. (B) Growth rate constants of E. coli BW25113 and BL21(DE3) carrying the pBAD33 and pRIL plasmids, under normal condition. The values shown are the mean ± SD. (C) Growth rate constants of E. coli BW25113 and BL21(DE3) carrying the pBAD33 and pRIL plasmids, under oxidative stress. The actual growth curves of BL21(DE3) carrying these two plasmids are shown respectively. The red line is the moving average observed over 5 min. (D) Cell growth-rate constant under harsh oxidative stress (~1–2 mM H2O2). A negative growth rate constant represents the occurrence of cell death. The data shown are the mean ± SD. (E) The growth-rate constant of E. coli BW25113 cells carrying the pBAD33 and pRIL plasmid, cultured in the presence of different concentrations of ciprofloxacin. We then posited that the protective effect afforded by tRNA upregulation increases the survival of E. coli under harsh oxidative stress. Therefore, we increased the H2O2 concentration to ~1–2 mM. In the presence of ~1–2 mM H2O2, the control BL21 cells died (Fig 6D), in agreement with the results shown in Fig 1A. In contrast, the tRNA-supplemented BL21-RIL cells continued to grow, although at a slower rate (Fig 6D). In the presence of 1 mM H2O2, the BW25113 control cells died rapidly, while the tRNA-supplemented BW25113-RIL cells survived (Fig 6D). These results showed that elevated tRNA concentrations alone could increase the resistance of E. coli cells to harsh oxidative stress.
To validate the phenomenon of tRNA-mediated resistance using another oxidative reagent, we incubated E. coli cells with ciprofloxacin, an antibiotic that induces extensive intracellular oxidative radical formation in prokaryotes [46,47], and examined whether increased tRNA concentrations improves the fitness of E. coli in the presence of ciprofloxacin. We determined the minimal inhibitory concentration (MIC) of ciprofloxacin on control E. coli BW25113 cells (carrying the pBAD33 plasmid) as 70 ng/ml. In the presence of low ciprofloxacin concentrations (<40 ng/ml), BW25113 cells carrying the pRIL plasmid grew slower under oxidative stress than did the control cells (Fig 6E). The growth rate of the control cells decreased with the increasing antibiotic concentrations, while the BW25113-RIL cells grew faster under higher ciprofloxacin concentrations (Fig 6E). These results further validated our hypothesis that enhanced tRNA concentrations can help to maintain the translation elongation speed and thus increase resistance to oxidative stress.
Discussion
All organisms need to adapt environmental oxidative stress. The regulation of translational response is one of the fastest mechanisms whereby cells can guard against sudden environmental stress factors before more specific, persistent mechanisms come into play, such as the OxyR and SoxRS systems. Although some previous studies have shown that the oxidation of certain aminoacyl-tRNA synthetases can affect the fidelity of translation (reviewed in [48]), a general translational response has remained elusive due to the nearly unchanged transcriptomes and translatomes observed under oxidative stress. Indeed, our results showed that no significant inhibition of protein synthesis could be detected in the in vitro translation system in the presence of 0.5 mM H2O2. This finding suggests that the oxidation of translational machinery components, such as initiation/elongation factors, tRNAs, ribosomes, and mRNAs, are not the direct causal factors in the immediate arrest of protein synthesis in vivo during sudden oxidative stress, at least under the stress conditions employed in our study. Of note, this translational arrest was found for nearly all proteins examined, a phenomenon that cannot be caused by the stress translation machinery, which enhances the production of a small group of “leaderless” stress proteins (reviewed in [49]).
The main novelty of this study lays on our discovery of the direct causal factor leading to this rapid and global translation arrest: the enzymatic degradation of tRNA. Our results showed that bacteria respond differently from eukaryotes under oxidative stress in terms of tRNA manipulation. In eukaryotes, tRNAs can be retrotransported into the nucleus and cleaved into halves, or the 3′-CCA tail can be reversibly digested. In contrast, bacteria degrade tRNAs rapidly, without the accumulation of residual tRNA fragments or CCA-lacking tRNAs. These findings indicated that bacteria have a simpler and more direct mechanism of tRNA manipulation than eukaryotes.
Technically, our modified ProVerB MS identification algorithm enabled the efficient identification and quantification of peptides in the full 15N metabolic-labeling experiments. We identified ~300–1000 proteins from single E. coli soluble protein samples, which is at least one order of magnitude higher than previous studies. This improved algorithm provides a general tool for accurate and practical assessments of protein synthesis in prokaryotes and algae, a field, which has lagged far behind eukaryotic studies.
The reduction of tRNA levels under oxidative stress significantly slows down the translation elongation speed. This could be beneficial for prokaryotes because oxidative stress elevates the probability of protein misfolding, and the accumulation of misfolded proteins can be toxic [50,51]. This may be partially compensated by the deceleration of translation elongation, which coordinates protein synthesis and co-translational, domain-wise folding [45]. Further, oxidative stress increases the probability of amino-acid misincorporation (reviewed in [48]), and the slowdown of translation elongation effectively reduces the production rate of mis-synthesized proteins. In sum, tRNA degradation ensures the quality of protein production under oxidative stress, providing a prompt and basal persistence to the bacteria until more specific and effective adaptation mechanism begin to commence.
Interestingly, tRNA supplementation has a dual impact on bacterial fitness. Under normal growth conditions, augmented tRNA levels globally accelerate translation elongation, thereby suppressing translational pausing. This leads to massive protein misfolding and thus impairs fitness [40]. However, under oxidative stress, the tRNA concentration is generally decreased, and the translation elongation is thus largely decelerated. Our results showed that the polysome fraction is remarkably increased under oxidative stress, indicating a much higher ribosome density and thus, an elevated probability of ribosome jamming. Yet, this slow elongation facilitates co-translational folding and effectively rescues the fitness perturbation caused by tRNA augmentation. Higher tRNA concentration also protects cells by decreasing the probability of ribosome jamming that causes the pre-mature dissociation of RNAs from ribosomes [44]. Therefore, the elevated tRNA levels serves to protect against oxidative stress, resulting in enhanced cell growth under mild oxidative stress and survival under harsh oxidative stress.
A natural question is why cells do not increase their tRNA concentrations immediately after oxidative stress. This is unproductive because transcription and complex tRNA processing need a prolonged period of time [52,53]. Results from a previous study showed that the tRNA genes were transcriptionally regulated after 30 min in E. coli MG1655 cells under stimulus, while no tRNA changes could be detected after 10 min [54]. However, specific oxidation-counteracting systems, such as the OxyR and SoxRS systems, can exert an effect ~20–30 min after oxidative stress, making tRNA upregulation shortly after oxidative stress less effective. In addition, synthesizing proteins under oxidative stress may elevate the risk for producing oxidized proteins, thereby wasting valuable energy [1]. After cells adapt to oxidative stress, restoring tRNA levels (and thus protein synthesis) is beneficial for further growth. This is exactly what we observed after 90 min of oxidative stress, with many tRNAs reaching higher concentrations than observed under normal conditions (Fig 4, S5 Fig and S6 Fig). In sum, it is an efficient strategy for bacteria to maintain tRNAs at low levels under normal growth conditions and degrade them shortly after encountering oxidative stress.
In addition, we showed that the protective effect of elevated tRNA concentrations also protects bacteria against oxidative stress induced by the antibiotic ciprofloxacin. At a sub-MIC concentration of ciprofloxacin, tRNA-augmented bacteria grew significantly faster than control cells, thereby providing the bacteria with elevated tRNA concentrations a survival advantage in the presence of an antibiotic. Therefore, the global regulation of tRNAs may serve as a general mechanism of antibiotic resistance.
Another question of interest is why eukaryotes tend to reversibly manipulate tRNAs (including the retrotransport of tRNAs into the nucleus and the rapid and reversible cleavage of 3′-CCA) under oxidative stress, while bacteria tend to simply degrade the tRNA irreversibly. A possible answer to this question may relate to the complexity and cost of tRNA production. First, the prokaryotes usually possess ~40–90 tRNA genes in their genomes, while eukaryotic cells express hundreds or thousands of tRNA genes. For example, 506 tRNA genes have been found in human genome hg19, and 12,794 tRNA genes have been found in zebra fish genome zv9, according to the Genomic tRNA Database [55,56]. Complex regulatory information and machineries are needed to control so many tRNA genes separately and efficiently [57]. Second, eukaryotic cells need many more enzymes to process tRNAs than prokaryotes. In S. cerevisiae, 85 genes are known to process tRNAs to their mature forms and export them to the cytoplasm (reviewed in [58]). In Arabidopsis thaliana, 94 genes have been implicated in tRNA modifications [59]. In contrast, E. coli cells require only 35 enzymes for tRNA modifications [59] and 15 enzymes for tRNA precursor digestion [53]. Third, tRNA processing and transport in eukaryotic cells are spatially localized (reviewed in [58,60]), whereas prokaryotic cells do not have nuclei, and the enzymatic reactions are less spatially organized. Fourth, the transcription speed in prokaryotic cells (~25–65 nt/s in E. coli [61]) is faster than that in eukaryotes (~18–42 nt/s in yeast [62]). Collectively, these data indicate that regulation in eukaryotic cells involves a much higher energy cost and a much more complex system to produce tRNAs ab initio than do prokaryotic cells. Therefore, complex but reversible tRNA manipulation allows eukaryotes to save energy and resume translation elongation after full stress adaptation by avoiding synthesizing and processing tRNAs ab initio. In contrast, prokaryotic cells can quickly produce tRNAs and thus tend to maintain a much simpler and irreversible degradation system.
In sum, we found that the global regulation of tRNA mediates rapid adaptation under oxidative stress. This general mechanism provides new insights into the translational responses of bacteria to stress conditions and may offer new views for understanding antibiotic resistance in bacteria.
Materials and Methods
Bacterial strains and plasmids
E. coli BL21(DE3) and wild-type BW25113 strains were used in this study. To increase the tRNA concentrations, bacteria were transformed with the pRIL plasmid that was isolated from the E. coli BL21(DE3)-CodonPlus-RIL strain (Stratagene). This plasmid confers chloramphenicol resistance and encodes the tRNA genes argU, ileY, and leuW that recognize AGA, AGG, AUA, and CUA codons. In this study, these three tRNAs were designated as 5-Arg(TCT), 17-Ile(GAT), and 22-Leu(TAG), respectively (Fig 4). We used the empty vector pBAD33, which also confers chloramphenicol resistance as a control for the pRIL plasmid.
Growth curve measurement
To accurately measure bacterial growth rates in batches, we assembled in house a fully automatic device to measure ODs. Bacteria were grown in rectangular, sterilized cuvettes, referred to here as “measuring units.” A narrow 635-nm laser beam was used as a light source, and the penetrating light was measured by a digital illuminometer connected to computer (S7A Fig). Optical densities were calculated according to the Beer-Lambert Law. Data were recorded automatically via the computer with a minimal interval of 10 s. During cell growth, measurements were continuously obtained without interfering with the shaker, which prevented temperature fluctuations caused by opening and closing the lid. Six such measuring units were fixed on a 30° slope and installed in an orbital shaker for culturing purposes (S7B Fig). No sedimentation of E. coli cells was noted after 12 h of culturing with shaking at 200 rpm.
We validated the measurements obtained with our in-house constructed device by comparison with the Genesys 10S UV-Vis spectrophotometer (Thermo). Our device yielded comparable results compared to the Genesys 10S UV-Vis spectrophotometer between an OD range of ~0–0.45, with a correlation coefficient approaching 1 (S7C Fig).
For each growth curve measurement, the E. coli cells were pre-cultured in LB medium at 37°C overnight and then collected by centrifugation, resuspended, and added in M9 minimal medium at a 1 : 1000 dilution, yielding an initial OD of <0.005. The measurements were performed within the linear range of our device (OD = 0~0.45).
Cell viability assessments
Cell viabilities were determined via propidium iodide (PI) staining [63,64]. Briefly, E. coli cells were harvested at OD = 0.05 by centrifugation and diluted in phosphate-buffered saline (PBS) to ~106 cells/mL. A 100-μl suspension was supplemented with PI (Sigma) to a final concentration of 10 μg/mL and incubated for 30 min at 4°C. After centrifugation at 12,000 × g for 2 min at 4°C, the cell pellet was resuspended in 1 mL of PBS twice to remove unbound PI. Flow cytometry was performed using a C6 flow cytometer (BD Accuri), using previously described settings [64].
Metabolic labeling with 15N
E. coli BL21(DE3) cells were cultivated in M9 minimal medium with 0.2% glucose at 37°C in an orbital shaker until the OD reached 0.6, and cells were harvested at room temperature by centrifugation at 5000 × g and washed twice with M9 minimal medium to remove any remaining 14NH4Cl. The cells were then resuspended in an equal volume of 15NH4Cl-containing M9 medium. To generate oxidative stress, H2O2 (Sigma) was also added to a final concentration of 0.5 mM. Cells were further cultured, and samples were obtained at the indicated time points.
Mass spectrometry of proteins
Harvested cells were washed with PB) and resuspended in urea lysis buffer (8 M urea, 2 M thiourea, 4% CHAPS, 15 mM DTT, and 1 mM PMSF). Cell lysates were sonicated in an ice bath for 5 min and centrifuged 15,000 × g for 15 min at 4°C to remove cell debris. In-gel digestion was performed, as described previously [65]. Briefly, 40 μg of protein was resolved on a 12% SDS-PAGE gel. The gel bands were cut into 4 slices and in-gel digestion was performed with MS-grade trypsin (Promega). MS was performed on an LTQ-Orbitrap mass spectrometer (Thermo Scientific), equipped with a nanospray source as described previously [66].
Protein identification and quantification
dta-format files were generated using BioWorks software, version 3.3.1 (Thermo-Finnigan, San Jose, CA) and searched against real and decoy E. coli reference protein databases, using a modified version of ProVerB software [36]. We modified the ProVerB program while considering the following terms: 1) the amino acid table contains 40 types of amino acids (20 15N-labeled amino acids and 20 14N (unlabeled) amino acids), 2) the theoretical peptide database contains 15N peptides and 14N peptides, 3) candidate peptides for each spectrum were selected from the theoretical E. coli peptide database according to precursor ion mass, and 4) all candidates were assessed by determining ProVerB scoring functions. The following search criteria were employed: full tryptic specificity was required, 2 missed cleavages were allowed, and carbamidomethylation was set as a fixed modification, whereas oxidation (M) was considered a variable modification. Precursor ion mass tolerances were set at 10 ppm for all MS spectra acquired in an LTQ-Orbitrap mass analyzer, and the fragment ion mass tolerance was 0.5 Da for all MS2 spectra acquired. All top peptides from each spectrum were filtered using a FDR of ≤0.01, and the protein group was built as the set of all filtered peptides.
To quantify the 14N/15N ratio for each protein, all filtered peptides and grouped proteins were converted to the dtaselect (version 1.9)-output format. The raw files from the LTQ-Orbitrap instrument were converted to MS1 format using RawXtract software, version 1.93. Census software (version 1.7) was used to the calculate area ratio, defined as the ratio of the 14N peptide peak area over the 15N peptide peak area in a given chromatogram.
Protein synthesis and half-life calculations
After cells were transferred from 14N medium to 15N medium (time point t0), the 15N-labeled proteins began to be synthesized. The 14N protein decay can be described as shown in Eq 1
where N0 is the amount of protein at t0, N14(t) is the amount of 14N protein at time t, and kdeg is the degradation rate constant. Given that the proteome is generally in steady state during exponential growth under normal and oxidative-stress conditions (see [24,28,67] and data in Fig 2A from this study), we have N0 = N14(t)+N15(t) and kdeg = ksyn, where ksyn is the synthesis rate constant. Therefore, Eq 1 can be reformulated as follows:Thus,
Let R be the ratio between the 14N and 15N proteins: R=N14(t)N15(t), which is the value measured by MS. Eq 2 can be then rewritten as follows:
By letting
and substitute Y into Eq 3, we derive the equation:Therefore,
According to Eq 6, ksyn can be fitted using the dataset of Y and t. Rapid protein synthesis is represented by a high ksyn value, which corresponds to a high slope for dY/dt.
The half-life time of the protein is then calculated as
Cell-free expression
The S30 T7 High-Yield Cell-Free Protein Expression System (Promega) was used for cell-free expression tests under oxidative stress, according to the manufacturer’s recommended protocol. The E. coli SufI gene inserted into pET-28b plasmid with C-terminal His6 tag was expressed according to the manufacturer’s recommended protocol. The reactions were incubated for 20 min, stopped, and then assessed by western blot analysis.
Isolation of detergent-insoluble proteins
E. coli cells were harvested at OD635nm = 0.05. The detergent-insoluble proteins (aggregated proteins) were isolated as described in our previous report [40].
Polysome profiles
Polysome profiles were generated as described previously [44]. Briefly, debris-free cell lysates were layered onto 30 mL 15–40% sucrose gradients and centrifuged at 30,000 rpm for 6 h in an SW32Ti rotor (Beckman). Gradients were slowly pumped out and detected at 254 nm with a UV spectrophotometer.
Spike-in preparation and total RNA extraction
Spike-in RNA (5′ - CGAAAUUAAUACGACUCACUAUAGGGGAAUUGUGAGCGGAUAACUGACUGACUGACUAAAUAAUUUUGUUUAACUUUAAGAAGGAGAUAUACCA-3′) was produced using the RiboMAX Large-Scale RNA Production System-T7 (Promega) using a synthetic DNA template with a T7 promoter. The spike-in was purified using TRIzol (Ambion), according to the manual and quantified using a NanoDrop 2000 Spectrophotometer (Thermo).
Cells were harvested by centrifugation 8000 × g for 15 min at 4°C and resuspended in 1 mL TRIzol (Ambion). Spike-in RNA was added to each sample at 500 ng/(OD•mL). RNA extraction was then performed according to the product manual, with the modification that the RNA was precipitated overnight in isopropanol at -20°C. The remaining DNA was removed by DNaseI treatment (Fermentas), according to the product manual.
qRT-PCR analysis of tRNAs
tRNA deacylation was performed as described previously [68], except that deacylation was performed at a pH of 10. A poly(A) tail was added to the deacylated 3′-CCA terminus using E. coli poly(A) polymerase (New England BioLabs), according to the product manual. Prior to reverse transcription, polyadenylated tRNA were denatured for 5 min with a TG primer (5′-T15GG-3′; 100 μM) and dNTPs (25 mM each) at 80°C and then chilled on ice. Reverse transcription was performed with RevertAid Premium Reverse Transcriptase (Fermentas), according to the product manual.
Specific primers for amplifying 39 E. coli tRNAs were designed using Primer-Premier 5 software and are listed in S3 Table. The specificity of these primers was verified both by in silico analysis (NCBI Primer-Blast) and melting-curve analysis after qPCR amplification. qPCR was then performed with each of the 39 tRNA-specific primer sets and SsoFast Evagreen Supermix (Bio-Rad) on a Bio-Rad MiniOpticon Real-Time PCR system (Bio-Rad), according to the manufacturer’s instructions.
Next-generation sequencing of tRNAs and data processing
After reverse transcribing tRNA to cDNA, second-strand synthesis was performed using RNase H, DNA Polymerase I, and T4 DNA Polymerase (Fermentas) according to the manufacturer’s recommended procedure. The double-stranded cDNA was then purified using a HiPure PCR Kit (Magen). A sequencing library was constructed using the NEBNext Ultra DNA Library Prep Master Mix Set for Illumina (NEB). Next-generation sequencing was performed on an Illumina HiSeq-2000 sequencer for 100 cycles. High-quality reads passing the Illumina filter were kept for subsequent data analysis. The sequencing dataset was deposited in the Gene Expression Omnibus database under Accession Number GSE62995.
Adapter sequences were trimmed from the reads. The reads were aligned to tRNA reference sequences using the modified Smith–Waterman algorithm that considered modified nucleotides matching any nucleotide [43]. A maximum of 3 errors (including mismatches, insertions, and deletions) was allowed in the alignments, and the minimal alignment length was set to 25 bp. The number of aligned tRNA reads were then normalized using the spike-in reads.
Supporting Information
Zdroje
1. Cabiscol E, Tamarit J, Ros J (2000) Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol 3 : 3–8. 10963327
2. Kryston TB, Georgiev AB, Pissis P, Georgakilas AG (2011) Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res 711 : 193–201. doi: 10.1016/j.mrfmmm.2010.12.016 21216256
3. Imlay JA (2013) The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11 : 443–454. doi: 10.1038/nrmicro3032 23712352
4. Jozefczuk S, Klie S, Catchpole G, Szymanski J, Cuadros-Inostroza A, et al. (2010) Metabolomic and transcriptomic stress response of Escherichia coli. Mol Syst Biol 6 : 364. doi: 10.1038/msb.2010.18 20461071
5. Lackner DH, Schmidt MW, Wu S, Wolf DA, Bahler J (2012) Regulation of transcriptome, translation, and proteome in response to environmental stress in fission yeast. Genome Biol 13: R25. doi: 10.1186/gb-2012-13-4-r25 22512868
6. Davies KJ (1999) The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress. IUBMB Life 48 : 41–47. 10791914
7. de Nadal E, Ammerer G, Posas F (2011) Controlling gene expression in response to stress. Nat Rev Genet 12 : 833–845. doi: 10.1038/nrg3055 22048664
8. Shenton D, Smirnova JB, Selley JN, Carroll K, Hubbard SJ, et al. (2006) Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J Biol Chem 281 : 29011–29021. 16849329
9. Thompson DM, Lu C, Green PJ, Parker R (2008) tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA 14 : 2095–2103. doi: 10.1261/rna.1232808 18719243
10. Yamasaki S, Ivanov P, Hu GF, Anderson P (2009) Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol 185 : 35–42. doi: 10.1083/jcb.200811106 19332886
11. Fu H, Feng J, Liu Q, Sun F, Tie Y, et al. (2009) Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett 583 : 437–442. doi: 10.1016/j.febslet.2008.12.043 19114040
12. Saikia M, Krokowski D, Guan BJ, Ivanov P, Parisien M, et al. (2012) Genome-wide identification and quantitative analysis of cleaved tRNA fragments induced by cellular stress. J Biol Chem 287 : 42708–42725. doi: 10.1074/jbc.M112.371799 23086926
13. Czech A, Wende S, Morl M, Pan T, Ignatova Z (2013) Reversible and rapid transfer-RNA deactivation as a mechanism of translational repression in stress. PLoS Genet 9: e1003767. doi: 10.1371/journal.pgen.1003767 24009533
14. Lee YS, Shibata Y, Malhotra A, Dutta A (2009) A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes Dev 23 : 2639–2649. doi: 10.1101/gad.1837609 19933153
15. Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P (2011) Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell 43 : 613–623. doi: 10.1016/j.molcel.2011.06.022 21855800
16. Vogel C, Silva GM, Marcotte EM (2011) Protein expression regulation under oxidative stress. Mol Cell Proteomics 10: M111 009217.
17. Chan CT, Pang YL, Deng W, Babu IR, Dyavaiah M, et al. (2012) Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat Commun 3 : 937. doi: 10.1038/ncomms1938 22760636
18. Tomita K, Ogawa T, Uozumi T, Watanabe K, Masaki H (2000) A cytotoxic ribonuclease which specifically cleaves four isoaccepting arginine tRNAs at their anticodon loops. Proc Natl Acad Sci U S A 97 : 8278–8283. 10880568
19. Jiang Y, Meidler R, Amitsur M, Kaufmann G (2001) Specific interaction between anticodon nuclease and the tRNA(Lys) wobble base. J Mol Biol 305 : 377–388. 11152597
20. Meineke B, Shuman S (2012) Determinants of the cytotoxicity of PrrC anticodon nuclease and its amelioration by tRNA repair. RNA 18 : 145–154. doi: 10.1261/rna.030171.111 22101242
21. Ling J, Soll D (2010) Severe oxidative stress induces protein mistranslation through impairment of an aminoacyl-tRNA synthetase editing site. Proc Natl Acad Sci U S A 107 : 4028–4033. doi: 10.1073/pnas.1000315107 20160114
22. Bessette PH, Aslund F, Beckwith J, Georgiou G (1999) Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc Natl Acad Sci U S A 96 : 13703–13708. 10570136
23. Sorensen HP, Mortensen KK (2005) Advanced genetic strategies for recombinant protein expression in Escherichia coli. J Biotechnol 115 : 113–128. 15607230
24. Tang Y, Quail MA, Artymiuk PJ, Guest JR, Green J (2002) Escherichia coli aconitases and oxidative stress: post-transcriptional regulation of sodA expression. Microbiology 148 : 1027–1037. 11932448
25. Luders S, Fallet C, Franco-Lara E (2009) Proteome analysis of the Escherichia coli heat shock response under steady-state conditions. Proteome Sci 7 : 36. doi: 10.1186/1477-5956-7-36 19772559
26. Maier T, Schmidt A, Guell M, Kuhner S, Gavin AC, et al. (2011) Quantification of mRNA and protein and integration with protein turnover in a bacterium. Mol Syst Biol 7 : 511. doi: 10.1038/msb.2011.38 21772259
27. Wu J, Jiang Z, Liu M, Gong X, Wu S, et al. (2009) Polynucleotide phosphorylase protects Escherichia coli against oxidative stress. Biochemistry 48 : 2012–2020. doi: 10.1021/bi801752p 19219992
28. Mostertz J, Scharf C, Hecker M, Homuth G (2004) Transcriptome and proteome analysis of Bacillus subtilis gene expression in response to superoxide and peroxide stress. Microbiology 150 : 497–512. 14766928
29. Doherty MK, Hammond DE, Clague MJ, Gaskell SJ, Beynon RJ (2009) Turnover of the human proteome: determination of protein intracellular stability by dynamic SILAC. J Proteome Res 8 : 104–112. doi: 10.1021/pr800641v 18954100
30. Westman-Brinkmalm A, Abramsson A, Pannee J, Gang C, Gustavsson MK, et al. (2011) SILAC zebrafish for quantitative analysis of protein turnover and tissue regeneration. J Proteomics 75 : 425–434. doi: 10.1016/j.jprot.2011.08.008 21890006
31. Cambridge SB, Gnad F, Nguyen C, Bermejo JL, Kruger M, et al. (2011) Systems-wide proteomic analysis in mammalian cells reveals conserved, functional protein turnover. J Proteome Res 10 : 5275–5284. doi: 10.1021/pr101183k 22050367
32. Mann M (2006) Functional and quantitative proteomics using SILAC. Nat Rev Mol Cell Biol 7 : 952–958. 17139335
33. Hinkson IV, Elias JE (2011) The dynamic state of protein turnover: It's about time. Trends Cell Biol 21 : 293–303. doi: 10.1016/j.tcb.2011.02.002 21474317
34. Snijders AP, de Koning B, Wright PC (2005) Perturbation and interpretation of nitrogen isotope distribution patterns in proteomics. J Proteome Res 4 : 2185–2191. 16335965
35. Martin SF, Munagapati VS, Salvo-Chirnside E, Kerr LE, Le Bihan T (2012) Proteome turnover in the green alga Ostreococcus tauri by time course 15N metabolic labeling mass spectrometry. J Proteome Res 11 : 476–486. doi: 10.1021/pr2009302 22077659
36. Xiao CL, Chen XZ, Du YL, Sun X, Zhang G, et al. (2013) Binomial probability distribution model-based protein identification algorithm for tandem mass spectrometry utilizing peak intensity information. J Proteome Res 12 : 328–335. doi: 10.1021/pr300781t 23163785
37. Imlay JA, Linn S (1986) Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J Bacteriol 166 : 519–527. 3516975
38. Imlay JA, Linn S (1987) Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Bacteriol 169 : 2967–2976. 3298208
39. Filiou MD, Varadarajulu J, Teplytska L, Reckow S, Maccarrone G, et al. (2012) The 15N isotope effect in Escherichia coli: a neutron can make the difference. Proteomics 12 : 3121–3128. doi: 10.1002/pmic.201200209 22887715
40. Fedyunin I, Lehnhardt L, Bohmer N, Kaufmann P, Zhang G, et al. (2012) tRNA concentration fine tunes protein solubility. FEBS Lett 586 : 3336–3340. doi: 10.1016/j.febslet.2012.07.012 22819830
41. Tamarit J, Cabiscol E, Ros J (1998) Identification of the major oxidatively damaged proteins in Escherichia coli cells exposed to oxidative stress. J Biol Chem 273 : 3027–3032. 9446617
42. Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, et al. (2013) MODOMICS: a database of RNA modification pathways--2013 update. Nucleic Acids Res 41: D262–267. doi: 10.1093/nar/gks1007 23118484
43. Puri P, Wetzel C, Saffert P, Gaston KW, Russell SP, et al. (2014) Systematic identification of tRNAome and its dynamics in Lactococcus lactis. Mol Microbiol.
44. Zhang G, Fedyunin I, Miekley O, Valleriani A, Moura A, et al. (2010) Global and local depletion of ternary complex limits translational elongation. Nucleic Acids Res 38 : 4778–4787. doi: 10.1093/nar/gkq196 20360046
45. Zhang G, Hubalewska M, Ignatova Z (2009) Transient ribosomal attenuation coordinates protein synthesis and co-translational folding. Nat Struct Mol Biol 16 : 274–280. doi: 10.1038/nsmb.1554 19198590
46. Goswami M, Mangoli SH, Jawali N (2006) Involvement of reactive oxygen species in the action of ciprofloxacin against Escherichia coli. Antimicrob Agents Chemother 50 : 949–954. 16495256
47. Dwyer DJ, Kohanski MA, Hayete B, Collins JJ (2007) Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol Syst Biol 3 : 91. 17353933
48. Katz A, Orellana O (2012) Protein Synthesis and the Stress Response. In: Biyani M, editor. Cell-Free Protein Synthesis: InTech. pp. 111–134.
49. Moll I, Engelberg-Kulka H (2012) Selective translation during stress in Escherichia coli. Trends Biochem Sci 37 : 493–498. doi: 10.1016/j.tibs.2012.07.007 22939840
50. Nakamura T, Cho DH, Lipton SA (2012) Redox regulation of protein misfolding, mitochondrial dysfunction, synaptic damage, and cell death in neurodegenerative diseases. Exp Neurol 238 : 12–21. doi: 10.1016/j.expneurol.2012.06.032 22771760
51. Bhattacharya A, Wei R, Hamilton RT, Chaudhuri AR (2014) Neuronal cells but not muscle cells are resistant to oxidative stress mediated protein misfolding and cell death: role of molecular chaperones. Biochem Biophys Res Commun 446 : 1250–1254. doi: 10.1016/j.bbrc.2014.03.097 24685484
52. Collins LJ, Biggs PJ (2011) RNA networks in prokaryotes II: tRNA processing and small RNAs. Adv Exp Med Biol 722 : 221–230. doi: 10.1007/978-1-4614-0332-6_14 21915792
53. Deutscher MP (1984) Processing of tRNA in prokaryotes and eukaryotes. CRC Crit Rev Biochem 17 : 45–71. 6094100
54. Durfee T, Hansen AM, Zhi H, Blattner FR, Jin DJ (2008) Transcription profiling of the stringent response in Escherichia coli. J Bacteriol 190 : 1084–1096. 18039766
55. Lowe TM, Eddy SR (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25 : 955–964. 9023104
56. Chan PP, Lowe TM (2009) GtRNAdb: a database of transfer RNA genes detected in genomic sequence. Nucleic Acids Res 37: D93–97. doi: 10.1093/nar/gkn787 18984615
57. Zhang G, Lukoszek R, Mueller-Roeber B, Ignatova Z (2011) Different sequence signatures in the upstream regions of plant and animal tRNA genes shape distinct modes of regulation. Nucleic Acids Res 39 : 3331–3339. doi: 10.1093/nar/gkq1257 21138970
58. Phizicky EM, Hopper AK (2010) tRNA biology charges to the front. Genes Dev 24 : 1832–1860. doi: 10.1101/gad.1956510 20810645
59. Chen P, Jager G, Zheng B (2010) Transfer RNA modifications and genes for modifying enzymes in Arabidopsis thaliana. BMC Plant Biol 10 : 201. doi: 10.1186/1471-2229-10-201 20836892
60. Hopper AK, Phizicky EM (2003) tRNA transfers to the limelight. Genes Dev 17 : 162–180. 12533506
61. Sneppen K, Dodd IB, Shearwin KE, Palmer AC, Schubert RA, et al. (2005) A mathematical model for transcriptional interference by RNA polymerase traffic in Escherichia coli. J Mol Biol 346 : 399–409. 15670592
62. Perez-Ortin JE, Alepuz PM, Moreno J (2007) Genomics and gene transcription kinetics in yeast. Trends Genet 23 : 250–257. 17379352
63. Jepras RI, Carter J, Pearson SC, Paul FE, Wilkinson MJ (1995) Development of a robust flow cytometric assay for determining numbers of viable bacteria. Appl Environ Microbiol 61 : 2696–2701. 16535078
64. Rault A, Beal C, Ghorbal S, Ogier JC, Bouix M (2007) Multiparametric flow cytometry allows rapid assessment and comparison of lactic acid bacteria viability after freezing and during frozen storage. Cryobiology 55 : 35–43. 17577587
65. Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1 : 2856–2860. 17406544
66. Sun X, Jia HL, Xiao CL, Yin XF, Yang XY, et al. (2011) Bacterial proteome of streptococcus pneumoniae through multidimensional separations coupled with LC-MS/MS. OMICS 15 : 477–482. doi: 10.1089/omi.2010.0113 21699404
67. Bore E, Hebraud M, Chafsey I, Chambon C, Skjaeret C, et al. (2007) Adapted tolerance to benzalkonium chloride in Escherichia coli K-12 studied by transcriptome and proteome analyses. Microbiology 153 : 935–946. 17379704
68. Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, et al. (2006) Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell 21 : 87–96. 16387656
Štítky
Genetika Reprodukční medicína
Článek Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and ThrombocytopeniaČlánek Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of andČlánek Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on PhenotypeČlánek Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human CancersČlánek A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic BacteriaČlánek The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System inČlánek The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological LifespanČlánek The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 6
-
Všechny články tohoto čísla
- Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Orphan Genes Find a Home: Interspecific Competition and Gene Network Evolution
- Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
- Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia
- Leveraging Identity-by-Descent for Accurate Genotype Inference in Family Sequencing Data
- Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
- Epigenetic Aging Signatures Are Coherently Modified in Cancer
- Silencing of DNase Colicin E8 Gene Expression by a Complex Nucleoprotein Assembly Ensures Timely Colicin Induction
- A Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
- The Orphan Gene Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation
- 9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
- The DnaA Protein Is Not the Limiting Factor for Initiation of Replication in
- FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling
- Multiple Changes of Gene Expression and Function Reveal Genomic and Phenotypic Complexity in SLE-like Disease
- Directed Evolution of RecA Variants with Enhanced Capacity for Conjugational Recombination
- The Regulatory T Cell Lineage Factor Foxp3 Regulates Gene Expression through Several Distinct Mechanisms Mostly Independent of Direct DNA Binding
- MreB-Dependent Inhibition of Cell Elongation during the Escape from Competence in
- DNA Damage Regulates Translation through β-TRCP Targeting of CReP
- Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of and
- The Analysis of () Mutants Reveals Differences in the Fusigenic Potential among Telomeres
- The Causative Gene in Chanarian Dorfman Syndrome Regulates Lipid Droplet Homeostasis in .
- Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype
- The . Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units
- AKTIP/Ft1, a New Shelterin-Interacting Factor Required for Telomere Maintenance
- Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
- Transfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
- Connecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
- Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Response to the Formal Letter of Z. Chrzanowska-Lightowlers and R. N. Lightowlers Regarding Our Article “Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers
- Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins
- Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential
- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level
- Genetic Changes to a Transcriptional Silencer Element Confers Phenotypic Diversity within and between Species
- Functional Assessment of Disease-Associated Regulatory Variants Using a Versatile Dual Colour Transgenesis Strategy in Zebrafish
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy
- The Human Blood Metabolome-Transcriptome Interface
- A Common Cancer Risk-Associated Allele in the Locus Encodes a Dominant Negative Inhibitor of Telomerase
- A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
- The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
- The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological Lifespan
- Dissection of a Complex Disease Susceptibility Region Using a Bayesian Stochastic Search Approach to Fine Mapping
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
- Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in
- Abnormal Activation of BMP Signaling Causes Myopathy in Null Mice
- Reproductive Mode and the Evolution of Genome Size and Structure in Nematodes
- Replication and Active Partition of Integrative and Conjugative Elements (ICEs) of the SXT/R391 Family: The Line between ICEs and Conjugative Plasmids Is Getting Thinner
- Motor and Sensory Deficits in the Mice Result from Mutation of the ESCRT Component HGS
- Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
- Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake
- Separable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4
- Multilayered Organization of Jasmonate Signalling in the Regulation of Root Growth
- Necrotic Cells Actively Attract Phagocytes through the Collaborative Action of Two Distinct PS-Exposure Mechanisms
- A Novel Feedback Loop That Controls Bimodal Expression of Genetic Competence
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Human Blood Metabolome-Transcriptome Interface
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání











