-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaConnecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
article has not abstract
Published in the journal: . PLoS Genet 11(6): e32767. doi:10.1371/journal.pgen.1005266
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1005266Summary
article has not abstract
Life expectancy has dramatically increased in recent human history. As a result, neurodegenerative diseases have become a primary health issue. Circadian clocks are found in all mammalian tissues, including neurons and glia, and are, thus, likely to have an impact on the onset and progression of neurodegenerative diseases. However, the link between circadian clocks and neurodegeneration is poorly understood. Means et al. now report an intriguing connection between circadian genes and pathways implicated in neurodegenerative processes [1].
Circadian rhythms are fundamental adaptive mechanisms that enable organisms to optimize most of their bodily functions with the day/night cycle. A critical circadian output in animals is the sleep/wake cycle. Circadian and sleep disruptions are frequently associated with neurodegenerative diseases [2]. Furthermore, sleep abnormalities can precede the onset of other neurological symptoms. For example, patients carrying a pathogenic allele of Ataxin-2 causing Spinocerebellar Ataxia Type 2 (SCA-2) experience sleep disruptions prior to suffering from ataxic symptoms [3,4]. Interestingly, in fruit flies, the ATXN2 homolog dATX2 plays a critical role in the expression of the circadian gene period (per) in circadian pacemaker neurons. Indeed, dATX2 collaborates with the translation factor TWENTYFOUR (TYF) to promote per mRNA translation [5,6]. Thus, the homolog of a gene involved in neurodegeneration contributes to the control of circadian rhythms in fruit flies. But what about the opposite connection: does the clock, or at least some circadian genes, contribute to the onset and progression of neurodegenerative diseases? Means et al. also turned to Drosophila to try to answer this important question [1].
The Price lab has had a long interest in a critical circadian kinase called DOUBLETIME (DBT), which is the fly homolog of Casein Kinase 1 δ/ε. DBT regulates PER phosphorylation, as well as the activity of the circadian transactivator CLOCK (CLK) [7]. DBT is, thus, critical to determining the period of circadian rhythms. Looking for DBT regulators, Means et al. identified SPAGHETTI (SPAG). SPAG downregulation leads to a long period phenotype or to arrhythmic behavior. SPAG has recently been shown to be part of a multimeric co-chaperone that works with HSP70 and HSP90 to regulate the assembly of large protein complexes [8]. HSP proteins play an important role in the progression of neurodegeneration [9]. Moreover, SPAG was found to affect aggregation of Huntingtin (HTT), the protein that causes Huntington disease when its polyQ domain is expanded [10].
Means et al. uncovered a novel pathway connecting SPAG to neurodegenerative mechanisms in flies, which is modulated by circadian genes and light (Fig 1). Their results indicate that SPAG protects DBT from proteasomal degradation at specific time points: during the day about seven hours after the lights are turned on, and seven hours into the night. DBT disappearance during the day is very closely associated with activation of the caspase DRONC [11,12], but not at night. However, light exposure during the night can also cause DRONC activation, demonstrating that both light and loss of DBT are required. DRONC promotes cell death, and Means et al. show that it can also trigger TAU cleavage, which is observed in Alzheimer disease [13]. Loss of DBT also worsened the neurodegenerative phenotype observed when human TAU is overexpressed in the fly eye. Thus, SPAG and DBT protect flies against activation of caspases that can ultimately lead to neuronal degeneration and cell death.
Fig. 1. Connection between circadian proteins (in blue) and proteins causing cell death or neuronal degeneration (in red). 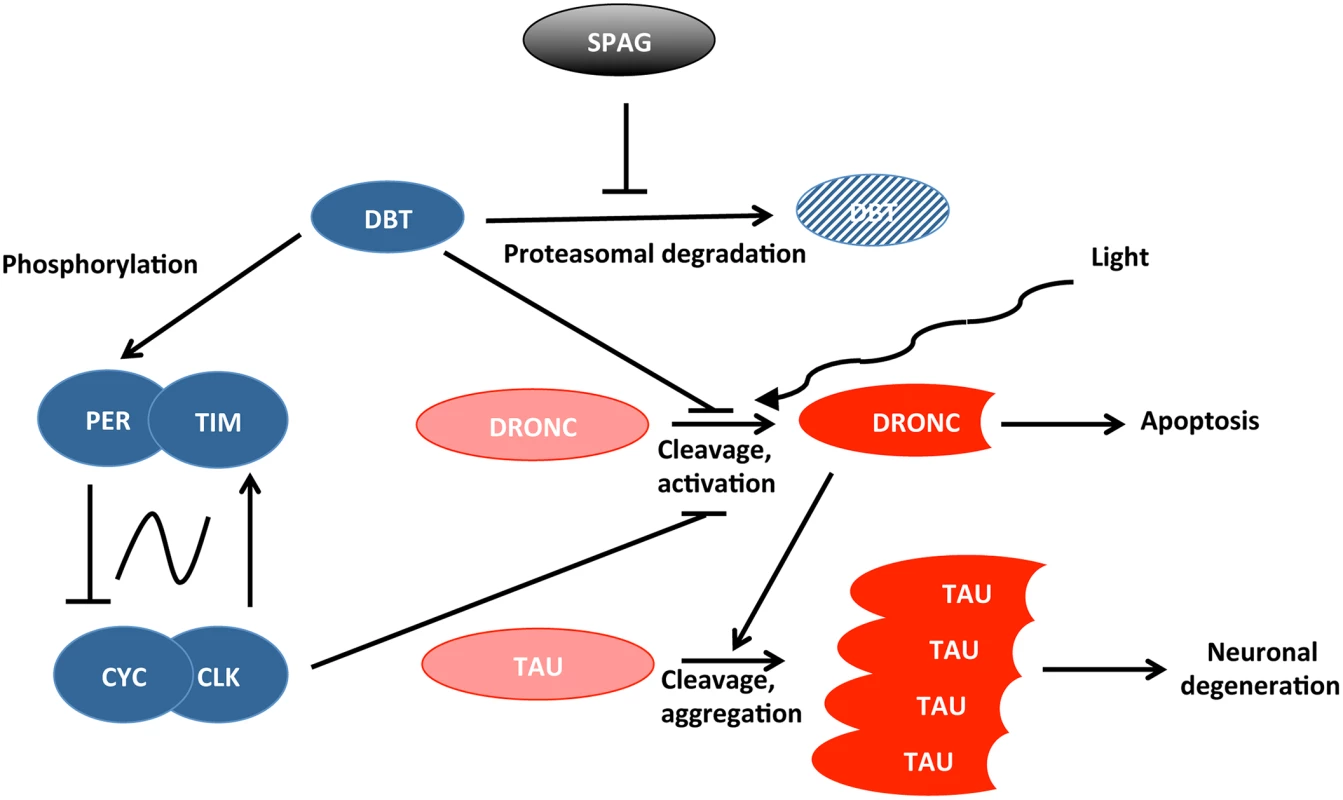
SPAG and light modulate this protein network. Interestingly, the SPAG pathway appears to be entirely cell-autonomous since it can be recapitulated beginning from SPAG inactivation to TAU cleavage in Drosophila S2 cell culture. However, in the brain, a more complicated mechanism is at play. Indeed, DRONC activation is very broad in the brain, even when SPAG/DBT are inactivated in only 16 circadian neurons called ventral lateral neurons (LNvs), which express the neuropeptide Pigment Dispersing Factor (PDF). Moreover, the receptor for PDF (PDFR) is required for broad DRONC activation, which, in the brain at least, is largely non-autonomous. How a similar set of proteins can be involved in what appears to be rather different mechanisms of DRONC activation will need to be determined. Also surprising is the fact that downregulating DRONC only in tissues expressing circadian rhythms in the brain (clock neurons and glia) appears to block DRONC activation in non-circadian neurons. It seems that DRONC expression in circadian neurons is required for the spread of its activation. These are very intriguing observations that certainly warrant further study since this could shed light on how neurodegeneration spreads to large regions of the brain.
To come back to the initial question, what is the actual role of the circadian clock in this process? In vivo, DRONC activation implicates DBT, a key circadian protein, and PDF positive circadian neurons. It would, therefore, seem likely that the circadian molecular pacemaker is implicated. Unexpectedly however, although a strong dominant negative clk mutant caused DRONC to be activated, a null per mutation—which makes flies completely arrhythmic—did not. Moreover, DRONC activation happened in clk mutant flies at the same time as in wild-type flies. Finally, short and long period per mutants had no effect on the phase of DRONC activation. Thus, although at least two circadian proteins (CLK, DBT) control DRONC activation, it does not appear that the circadian molecular clock itself impacts this process. This conclusion could seem particularly unexpected since well-characterized circadian neurons and their neuropeptidic output PDF are critical for DRONC activation in the brain. However, there are two types of PDF positive circadian neurons. The small LNvs are the actual pacemaker for circadian behavior, which means that they determine the pace and phase of circadian locomotor rhythms [14]. Then there are the large LNvs, which have been shown to mediate various behavioral light responses, including acute light-induced arousal [15–17]. These neurons send numerous projections into the optic lobe and, thus, probably receive light input from the eyes. Moreover, they are themselves directly acutely light sensitive through the photoreceptor CRYPTOCHROME [18]. It seems therefore plausible that prolonged light exposure would cause the large LNvs to secrete PDF and, thus, trigger the broad activation of DRONC when the protective SPAG/DBT pathway is disrupted. Importantly, Means et al. show that this protective pathway is defective in aging wild-type flies, with DRONC becoming activated even under dark conditions. Neuronal aging in flies could be linked to SPAG/DBT pathway disruption. Whether similar mechanisms are at play in aging or diseased mammalian neurons now needs to be determined.
Zdroje
1. Means JC, Venkatesan A, Gerdes B, Fan J-Y, Bjes ES, Price JL. (2015) Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy. PLoS Genet 11(5): e1005171. doi: 10.1371/journal.pgen.1005171 25951229
2. Videnovic A, Lazar AS, Barker RA, Overeem S (2014) 'The clocks that time us'—circadian rhythms in neurodegenerative disorders. Nat Rev Neurol 10 : 683–693. doi: 10.1038/nrneurol.2014.206 25385339
3. Velazquez-Perez L, Voss U, Rodriguez-Labrada R, Auburger G, Canales Ochoa N, et al. (2011) Sleep disorders in spinocerebellar ataxia type 2 patients. Neurodegener Dis 8 : 447–454. doi: 10.1159/000324374 21494015
4. Rodriguez-Labrada R, Velazquez-Perez L, Ochoa NC, Polo LG, Valencia RH, Cruz GS, et al. (2011) Subtle rapid eye movement sleep abnormalities in presymptomatic spinocerebellar ataxia type 2 gene carriers. Mov Disord 26 : 347–350. doi: 10.1002/mds.23409 20960485
5. Zhang Y, Ling J, Yuan C, Dubruille R, Emery P (2013) A role for Drosophila ATX2 in activation of PER translation and circadian behavior. Science 340 : 879–882. doi: 10.1126/science.1234746 23687048
6. Lim C, Allada R (2013) ATAXIN-2 activates PERIOD translation to sustain circadian rhythms in Drosophila. Science 340 : 875–879. doi: 10.1126/science.1234785 23687047
7. Zhang Y, Emery P (2012) Molecular and Neural Control of Insects Circadian Rhythms. In: Gilbert LI, editor. Insect Molecular Biology and Biochemistry: Academic Press. pp. 513–551.
8. Benbahouche Nel H, Iliopoulos I, Torok I, Marhold J, Henri J, Kajava AV, et al. (2014) Drosophila Spag is the homolog of RNA polymerase II-associated protein 3 (RPAP3) and recruits the heat shock proteins 70 and 90 (Hsp70 and Hsp90) during the assembly of cellular machineries. J Biol Chem 289 : 6236–6247. doi: 10.1074/jbc.M113.499608 24394412
9. Paul S, Mahanta S (2014) Association of heat-shock proteins in various neurodegenerative disorders: is it a master key to open the therapeutic door? Mol Cell Biochem 386 : 45–61. doi: 10.1007/s11010-013-1844-y 24096700
10. Zhang S, Binari R, Zhou R, Perrimon N (2010) A genomewide RNA interference screen for modifiers of aggregates formation by mutant Huntingtin in Drosophila. Genetics 184 : 1165–1179. doi: 10.1534/genetics.109.112516 20100940
11. Quinn LM, Dorstyn L, Mills K, Colussi PA, Chen P, Coombe M, et al. (2000) An essential role for the caspase dronc in developmentally programmed cell death in Drosophila. J Biol Chem 275 : 40416–40424. 10984473
12. Dorstyn L, Colussi PA, Quinn LM, Richardson H, Kumar S (1999) DRONC, an ecdysone-inducible Drosophila caspase. Proc Natl Acad Sci U S A 96 : 4307–4312. 10200258
13. Rohn TT, Rissman RA, Davis MC, Kim YE, Cotman CW, Head E. (2002) Caspase-9 activation and caspase cleavage of tau in the Alzheimer's disease brain. Neurobiol Dis 11 : 341–354. 12505426
14. Stoleru D, Peng Y, Nawathean P, Rosbash M (2005) A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature 438 : 238–242. 16281038
15. Shang Y, Griffith LC, Rosbash M (2008) Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci U S A 105 : 19587–19594. doi: 10.1073/pnas.0809577105 19060186
16. Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, et al. (2008) Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol 18 : 1537–1545. doi: 10.1016/j.cub.2008.08.033 18771923
17. Cusumano P, Klarsfeld A, Chelot E, Picot M, Richier B, Rouyer F. (2009) PDF-modulated visual inputs and cryptochrome define diurnal behavior in Drosophila. Nat Neurosci 12 : 1431–1437. doi: 10.1038/nn.2429 19820704
18. Fogle KJ, Parson KG, Dahm NA, Holmes TC (2011) CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science 331 : 1409–1413. doi: 10.1126/science.1199702 21385718
Štítky
Genetika Reprodukční medicína
Článek Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and ThrombocytopeniaČlánek Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of andČlánek Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on PhenotypeČlánek Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human CancersČlánek A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic BacteriaČlánek The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System inČlánek The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological LifespanČlánek The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 6
-
Všechny články tohoto čísla
- Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Orphan Genes Find a Home: Interspecific Competition and Gene Network Evolution
- Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
- Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia
- Leveraging Identity-by-Descent for Accurate Genotype Inference in Family Sequencing Data
- Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
- Epigenetic Aging Signatures Are Coherently Modified in Cancer
- Silencing of DNase Colicin E8 Gene Expression by a Complex Nucleoprotein Assembly Ensures Timely Colicin Induction
- A Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
- The Orphan Gene Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation
- 9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
- The DnaA Protein Is Not the Limiting Factor for Initiation of Replication in
- FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling
- Multiple Changes of Gene Expression and Function Reveal Genomic and Phenotypic Complexity in SLE-like Disease
- Directed Evolution of RecA Variants with Enhanced Capacity for Conjugational Recombination
- The Regulatory T Cell Lineage Factor Foxp3 Regulates Gene Expression through Several Distinct Mechanisms Mostly Independent of Direct DNA Binding
- MreB-Dependent Inhibition of Cell Elongation during the Escape from Competence in
- DNA Damage Regulates Translation through β-TRCP Targeting of CReP
- Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of and
- The Analysis of () Mutants Reveals Differences in the Fusigenic Potential among Telomeres
- The Causative Gene in Chanarian Dorfman Syndrome Regulates Lipid Droplet Homeostasis in .
- Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype
- The . Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units
- AKTIP/Ft1, a New Shelterin-Interacting Factor Required for Telomere Maintenance
- Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
- Transfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
- Connecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
- Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Response to the Formal Letter of Z. Chrzanowska-Lightowlers and R. N. Lightowlers Regarding Our Article “Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers
- Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins
- Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential
- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level
- Genetic Changes to a Transcriptional Silencer Element Confers Phenotypic Diversity within and between Species
- Functional Assessment of Disease-Associated Regulatory Variants Using a Versatile Dual Colour Transgenesis Strategy in Zebrafish
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy
- The Human Blood Metabolome-Transcriptome Interface
- A Common Cancer Risk-Associated Allele in the Locus Encodes a Dominant Negative Inhibitor of Telomerase
- A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
- The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
- The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological Lifespan
- Dissection of a Complex Disease Susceptibility Region Using a Bayesian Stochastic Search Approach to Fine Mapping
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
- Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in
- Abnormal Activation of BMP Signaling Causes Myopathy in Null Mice
- Reproductive Mode and the Evolution of Genome Size and Structure in Nematodes
- Replication and Active Partition of Integrative and Conjugative Elements (ICEs) of the SXT/R391 Family: The Line between ICEs and Conjugative Plasmids Is Getting Thinner
- Motor and Sensory Deficits in the Mice Result from Mutation of the ESCRT Component HGS
- Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
- Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake
- Separable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4
- Multilayered Organization of Jasmonate Signalling in the Regulation of Root Growth
- Necrotic Cells Actively Attract Phagocytes through the Collaborative Action of Two Distinct PS-Exposure Mechanisms
- A Novel Feedback Loop That Controls Bimodal Expression of Genetic Competence
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Human Blood Metabolome-Transcriptome Interface
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



