-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNon-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
Changes in mating system are supposed to change levels of sexual conflict, causing reduced conflict in inbreeding compared to outbreeding species. The recently diverged species pair of the inbreeding Capsella rubella and the outbreeding C. grandiflora provides the opportunity to test this hypothesis. While hybridizations of C. rubella maternal plants with C. grandiflora pollen donors gave rise to seeds with phenotypic similarities to paternal excess hybridizations in Arabidopsis thaliana, the reciprocal hybridization had similarities to maternal excess hybridizations. These results lend support to the hypothesis that selfing reduces sexual conflict, causing the outcrossing parent to have an increased effective ploidy compared to the selfing parent, resulting in unbalanced genome ratios after hybridization and seed failure. Seed failure correlates with either precocious or delayed endosperm cellularization, in agreement with the endosperm being the battleground for sexual conflict in flowering plants. C. grandiflora and C. rubella have only recently diverged, suggesting that the genes building the hybridization barrier are rapidly evolving as a consequence of sexual conflict.
Published in the journal: . PLoS Genet 11(6): e32767. doi:10.1371/journal.pgen.1005295
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005295Summary
Changes in mating system are supposed to change levels of sexual conflict, causing reduced conflict in inbreeding compared to outbreeding species. The recently diverged species pair of the inbreeding Capsella rubella and the outbreeding C. grandiflora provides the opportunity to test this hypothesis. While hybridizations of C. rubella maternal plants with C. grandiflora pollen donors gave rise to seeds with phenotypic similarities to paternal excess hybridizations in Arabidopsis thaliana, the reciprocal hybridization had similarities to maternal excess hybridizations. These results lend support to the hypothesis that selfing reduces sexual conflict, causing the outcrossing parent to have an increased effective ploidy compared to the selfing parent, resulting in unbalanced genome ratios after hybridization and seed failure. Seed failure correlates with either precocious or delayed endosperm cellularization, in agreement with the endosperm being the battleground for sexual conflict in flowering plants. C. grandiflora and C. rubella have only recently diverged, suggesting that the genes building the hybridization barrier are rapidly evolving as a consequence of sexual conflict.
Introduction
The highly selfing species Capsella rubella separated about 100,000 years ago from the obligate outcrosser C. grandiflora [1,2]. While C. rubella is found throughout much of southern and western Europe, C. grandiflora is restricted primarily to the northwest of Greece [3,4]. The breakdown of self-incompatibility in Capsella was concurrent with species divergence [1,3,4], which was associated with a rapid loss of diversity in the newly founded C. rubella [5].
Changes in mating system have been proposed to change levels of sexual conflict, with parental conflict being less intense in self-pollinating plants than in outcrossers [6]. The sexual conflict theory implies that maternally and paternally inherited genes are not equal in relation to maternal investment in offspring, while paternally inherited genes promote maternal provisioning of the progeny, maternally inherited genes counteract this activity [7,8]. Based on this theory, if such genes evolved under different levels of selection pressure in two different species, hybridization should lead to unbalanced maternal investment to offspring, leading to the establishment of a postzygotic hybridization barrier.
Nonequivalence of maternal and paternal genomes can be explained by genomic imprinting, an epigenetic phenomenon causing differential expression of genes depending on their parent-of-origin [9]. In flowering plants, genomic imprinting plays a predominant role in the endosperm, a terminal nutritive tissue supporting embryo growth that is consumed by the embryo during seed development or after germination [10]. Most angiosperms follow a nuclear-type of endosperm development, where the endosperm initially develops as a syncytium and cellularization is triggered after a defined number of mitotic cycles [11,12]. Endosperm cellularization is a crucial developmental transition, which in case of failure triggers embryo arrest [13–15]. Studies on different plant species consistently reported interspecies hybrid seed lethality due to endosperm cellularization failure [7,16–21]. Similar endosperm defects have been observed in hybridizations of plants that differ in ploidy, suggesting a common mechanistic basis [13,21,22]. Specifically in Arabidopsis, hybridization between a maternal tetraploid plant and a paternal diploid plant results in precocious endosperm cellularization, while the reciprocal hybridization causes increased endosperm growth and delayed or failure of endosperm cellularization [13]. Interspecies postzygotic hybridization barriers that are established in the endosperm can often be bypassed by changing the ploidy of one parental species [23–25]. This suggests that these barriers have a quantitative basis and that endosperm-based hybridization barriers are a consequence of deregulated imprinted genes [26–28]. These theoretical considerations are supported by the imprinted gene ADMETOS, which is causally responsible for triploid seed abortion [29]. As parental conflict is predictably less intense in self-pollinating plants than in outcrossers [7], in hybridizations of plants with differing mating systems, outcrossing parents are expected to behave like plants of increased ploidy. Therefore, there should be symptoms of maternal excess when the outcrosser is the seed parent and symptoms of paternal excess in the reciprocal cross [6]. While endosperm phenotypes in response to interspecies crosses lent support for this hypothesis [6], molecular evidence has been lacking thus far. To close this knowledge gap, we have investigated the interspecies hybridization barrier between the closely related species pair C. rubella and C. grandiflora on a morphological, genetic, and molecular level. Our results provide strong support for the theory that crosses between plants of different mating systems will be unbalanced, with the outcrosser behaving like a plant of increased ploidy, evoking a response that resembles an interploidy-type seed failure.
Results
The postzygotic barrier between C. rubella and C. grandiflora non-reciprocally affects seed development
The recently diverged C. rubella and C. grandiflora species are reproductively separated by different mating systems [1,3,4]. However, whether there are additional reproductive barriers between both species has not yet been investigated. We therefore performed reciprocal crosses between C. grandiflora and C. rubella and analyzed the resulting seed set. Pollinations in both directions were successful and resulted in similar numbers of seeds per silique between reciprocal crosses and intra-species crosses, revealing that there were no pollen incompatibilities between C. rubella and C. grandiflora (S1 Table). However, hybrid seeds developed abnormally and aborted at different frequencies; while about 40% of seeds after crosses of C. grandiflora × C. rubella (female × male) were abnormal (Fig 1A and 1B), corresponding to a germination rate of about 60% (Fig 1C), in the reciprocal cross all seeds aborted (Fig 1A, 1B and 1C). Hybrid seedlings of the cross C. grandiflora × C. rubella were variable in size and germination time point (Fig 1D) but developed into fertile adult plants. Intra-species crosses resulted in seed abortion rates below 5% and germination rates above 80% (Fig 1A and 1C). The seeds of the parental species were similar in size and weight (Fig 1B, 1E and 1F; P > 0.1, t-test). Seeds of the cross C. rubella × C. grandiflora were significantly larger than C. rubella seeds but lighter compared to both parental seeds (Fig 1E and 1F; P < 0.05, t-test), while seeds of the reciprocal cross were significantly smaller and lighter (Fig 1E and 1F; P < 0.01, t-test).
Fig. 1. Cross direction-dependent incompatibility affects development of Capsella rubella and C. grandiflora hybrid seeds. 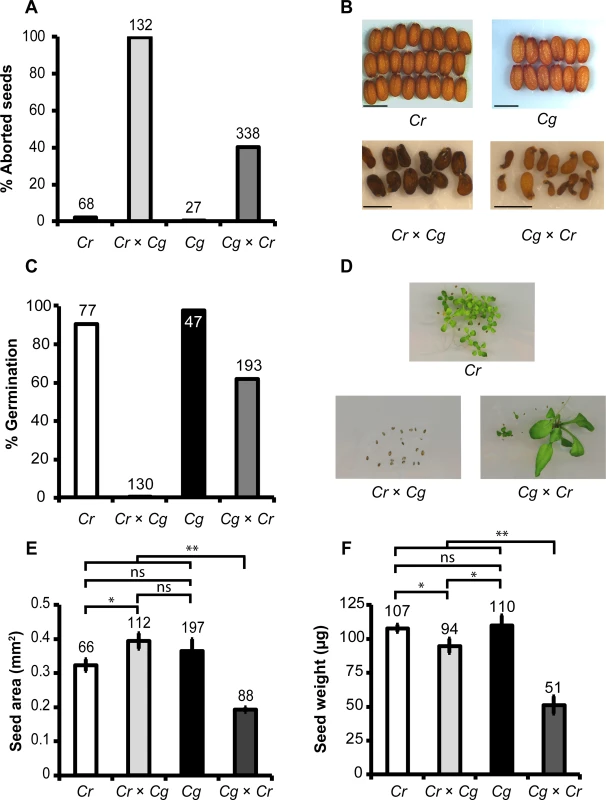
Percentage (A) and phenotypes (B) of aborted and non-aborted seeds of C. rubella, C. grandiflora and reciprocal hybrids of both species. Scale bars reflect 1 mm. (C) Percentage of germinated seeds of indicated crosses. (D) Seedlings of indicated crosses 10 days after germination. Seed area (E) and seed weight (F) of indicated crosses. Error bars show standard deviation. Significance was determined by t test analysis. * P < 0.05, ** P < 0.01 ns, not significant. In all graphs numbers on top of the bars correspond to number of analyzed seeds. Abortion of C. rubella × C. grandiflora hybrid seeds is a consequence of abnormal endosperm development
Detailed analysis of parental and hybrid seeds at defined time points (4–7 days after pollination, DAP) revealed differences in the timing of endosperm cellularization in hybrid seeds compared to parental seeds (Fig 2). In both parental species cellularization was completed at 6 DAP, with the embryo having reached the torpedo stage. In contrast, the endosperm of C. rubella × C. grandiflora hybrid seeds was not cellularized at 7 DAP and embryo development was substantially delayed (Fig 2). Conversely, in the reciprocal cross, cellularization happened precociously at 4 DAP and was completed at 5 DAP. The seed coat did not expand properly, leaving insufficient space for the embryo to bend.
Fig. 2. Hybrid seed incompatibility between C. rubella and C. grandiflora correlates with endosperm cellularization defects. 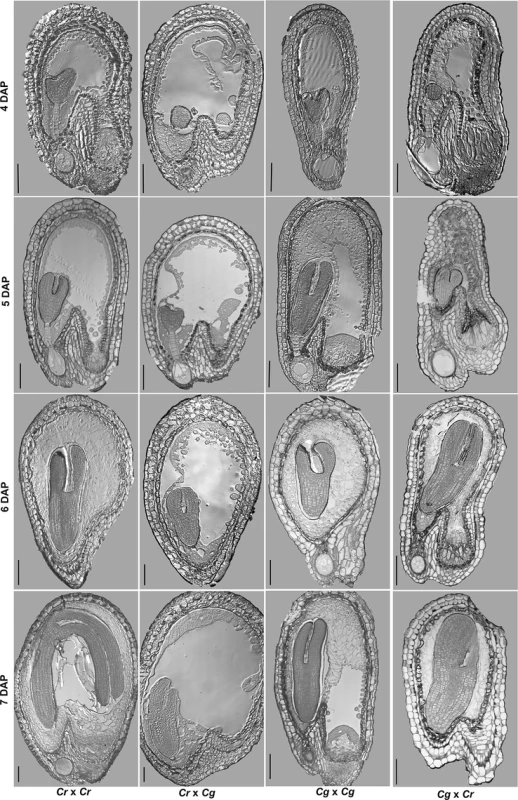
Sections of C. rubella, C. grandiflora and reciprocal hybrid seeds at 4–7 days after pollination (DAP). Scale bar 100μm. Delayed or complete block of endosperm cellularization has been correlated with embryo arrest [15]. We therefore tested whether abortion of hybrid C. rubella × C. grandiflora seeds is a consequence of abnormal endosperm development or rather caused by an embryo defect. To distinguish between both possibilities we isolated hybrid embryos at 13 DAP, when the endosperm was already completely collapsed, but the embryo was still viable (Fig 3A and 3B). Of 14 isolated embryos 9 developed normally and based on flower size the F1 plants were clearly recognized as hybrids (Fig 3C). C. grandiflora × C. rubella hybrids were easily obtained and resembled in flower size the reciprocal hybrid (Fig 3C). Together we conclude that abortion of C. rubella × C. grandiflora hybrid seeds is a consequence of abnormal endosperm development and most likely endosperm cellularization failure, which can be bypassed by embryo rescue.
Fig. 3. C. rubella × C. grandiflora hybrid embryos are viable, revealing a major role of endosperm defects in hybrid seed incompatibility. 
(A) C. rubella × C. grandiflora hybrid seeds at 13 days after pollination (DAP). (B) Section through seeds derived from crosses of C. rubella × C. rubella (left) and C. rubella × C. grandiflora (right) at 13 DAP. The sections reveal that hybrid embryos reach the torpedo stage at which development arrests. (C) Comparison of adult plants of all crosses (C. grandiflora × C. grandiflora (Cg), C. grandiflora × C. rubella (Cg × Cr), C. rubella × C. grandiflora (Cr × Cg), C. rubella × C. rubella (Cr)). Flower phenotypes of the respective genotypes are shown on top. Endosperm cellularization failure is not coupled to proliferation abnormalities
In interploidy crosses of A. thaliana, increased paternal ploidy causes increased endosperm proliferation and cellularization failure [13], mimicking crosses of C. rubella × C. grandiflora. Conversely, in seeds with increased maternal ploidy, endosperm proliferation is decreased and cellularization occurs precociously [13]. We therefore tested whether the endosperm cellularization defects in reciprocal Capsella crosses were reflected by changes in endosperm proliferation. Crosses of C. rubella × C. grandiflora resulted in similar proliferation rates compared to proliferation rates in the maternal C. rubella species, with nuclei numbers doubling between 3–5 DAP (Figs 4 and S1). Nuclei numbers decreased at 5–7 DAP in C. rubella, correlating with progression of embryo development. Failure of endosperm cellularization and arrest of embryo development correlated with unchanged nuclei numbers in C. rubella × C. grandiflora hybrid seeds between 5 and 7 DAP (Fig 4).
Fig. 4. Hybrid seed incompatibility is not a consequence of endosperm proliferation defects. 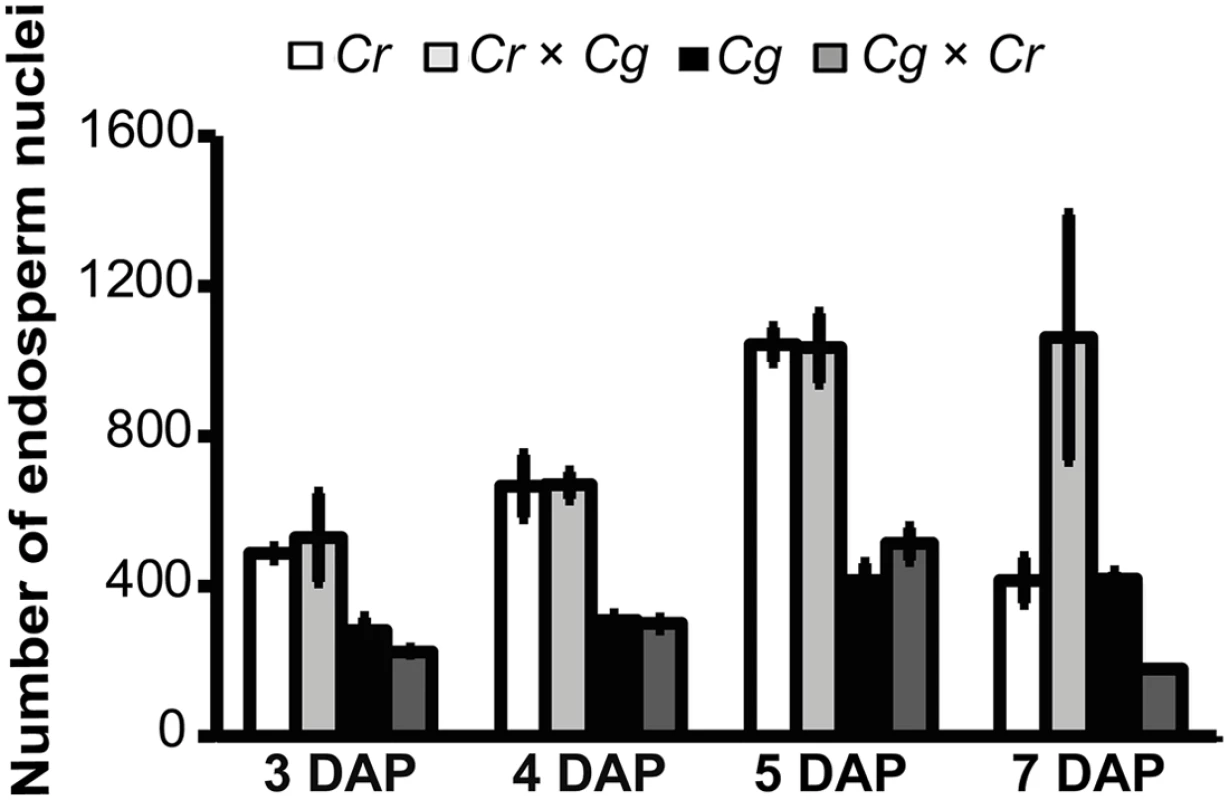
Endosperm nuclei numbers for each cross (C. grandiflora × C. grandiflora (Cg), C. grandiflora × C. rubella (Cg × Cr), C. rubella × C. grandiflora (Cr × Cg), C. rubella × C. rubella (Cr)) at indicated days after pollination (DAP). Three seeds per cross where counted. Error bars show standard error. In the reciprocal cross, C. grandiflora × C. rubella nuclei proliferation followed that of the maternal C. grandiflora parent and nuclei numbers also doubled between 3–5 DAP. However, C. grandiflora as well as C. grandiflora x C. rubella hybrid seeds had only half the nuclei numbers compared to C. rubella and C. rubella x C. grandiflora seeds, revealing different endosperm proliferation rates between both parental species. At 7 DAP nuclei numbers in C. grandiflora x C. rubella seeds were only half that compared to C. grandiflora seeds, which is likely a consequence of increased endosperm consumption by the hybrid embryo. Together, we conclude that endosperm proliferation in the hybrids followed that of their maternal parental species, suggesting that the hybridization block caused by failure in endosperm cellularization is not a consequence of abnormal endosperm proliferation.
Interspecies and interploidy hybridizations cause similar molecular defects
The observed non-reciprocal defects in Capsella hybrid seeds resembled defects in response to interploidy hybridizations in Arabidopsis [13]. To test whether the similarity at the phenotypic level was also reflected at the molecular level, we generated genome-wide expression data of C. rubella and C. grandiflora parental seeds and reciprocal hybrid seeds. We filtered for genes exhibiting transgressive expression towards both parents, thus having either increased or decreased expression levels compared to both parents. For each gene we identified the closest homolog in Arabidopsis and analyzed the expression of those genes in interploidy hybrid seeds generated using the omission of second division1 (osd1) mutant [30,31]. Loss of OSD1 causes the formation of unreduced male and female gametes at high frequency [32], allowing to mimic interploidy hybridizations. A large proportion of genes were similarly deregulated in C. rubella × C. grandiflora hybrid seeds and seeds of paternal excess hybridizations in Arabidopsis (Fig 5A, S2 Table), while substantially fewer genes overlapped with deregulated genes in maternal excess seeds (S2 Fig). Conversely, in the reciprocal cross a substantial number of downregulated genes overlapped with downregulated genes in maternal excess hybridizations in Arabidopsis (Fig 5A, S2 Table), whereas the overlap with deregulated genes in paternal excess seeds was less pronounced (S2 Fig). These data reveal that interspecies and interploidy hybridizations cause a similar molecular response; while C. rubella × C. grandiflora hybrid seeds mimic a paternal excess phenotype, C. grandiflora × C. rubella hybrid seeds mimic a maternal excess phenotype. Notably, genes related to microtubular activity were enriched among downregulated genes in both C. rubella × C. grandiflora and Arabidopsis paternal excess hybrid seeds, while upregulated genes were enriched for genes involved in cell wall modification and specifically in glycosyl hydrolyzing activity (S3 Table). Microtubules are required in the phragmoplast for the transport of vesicles to construct a new cell wall [33], while glycosyl hydrolyzing enzymes can degrade pectin, which is assumed to be a key step in the deconstruction of plant cell walls [34]. Therefore, reduced expression of genes that are potentially required to build the phragmopast together with increased expression of genes that degrade cell walls correlates with the observed cellularization failure in interploidy and interspecies hybrid seeds (S3 Table). Conversely, in the reciprocal cross C. grandiflora × C. rubella, pectinesterase encoding genes were significantly downregulated, suggesting that inhibition of pectin degradation promotes cellularization.
Fig. 5. Molecular response to reciprocal hybridizations of C. rubella × C. grandiflora is similar to interploidy hybridizations in Arabidopsis. 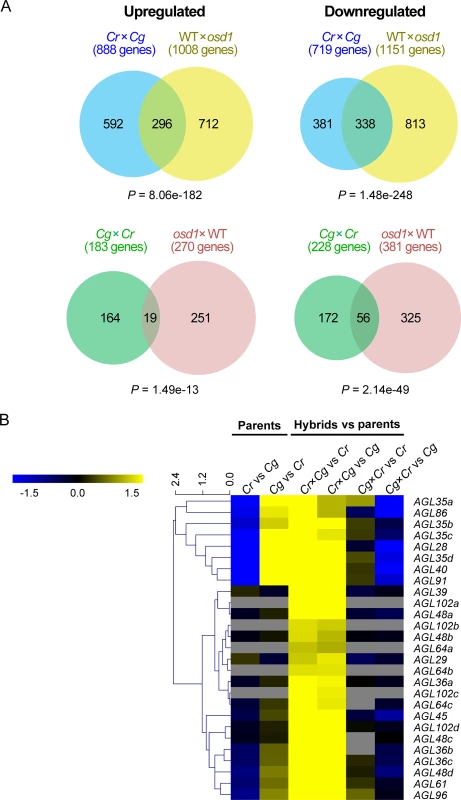
(A) Genes up- and downregulated in C. rubella × C. grandiflora reciprocal hybrid seeds compared to both parents overlap with genes deregulated in Arabidopsis interploidy seeds. The Arabidopsis osd1 mutant produces unreduced gametes, mimicking an interploidy hybridization when crossed with wild-type (WT). P values reflecting significance of overlap were calculated using a hypergeometric test. (B) Heatmap of expression log2 fold changes of selected AGL genes between samples. Capsella AGLs with several homologs in Arabidopsis are marked by small letters. Increased expression of type I MADS-box AGAMOUS-LIKE (AGL) genes has been functionally connected to interploidy and interspecies seed arrest [15,25,35–37]. To test whether the same concept applies to Capsella interspecies hybrids, we isolated type I AGLs in the C. rubella genome according to the Phytozome annotation (S4 Table). Consistently, we identified a large number of AGLs being upregulated in C. rubella × C. grandiflora seeds compared to both parents (Fig 5B, S4 Table) and confirmed these results for selected AGLs (homologs of AGL28, AGL36a, AGL61) in independent experiments (S3 Fig). AGL62 and PHE1 have been functionally connected to interspecies seed abortion [25,35], however, read numbers were too low to detect significant expression changes for either gene. Nevertheless, by qPCR analysis we could detect strongly increased expression levels of both genes in C. rubella × C. grandiflora hybrid seeds (S3 Fig). We also tested expression of ADM, which is causally responsible for triploid seed abortion in Arabidopsis [29]. However, increased ADM expression in C. rubella × C. grandiflora hybrid seeds was only detected quite late during seed development at 7 DAP (S3 Fig), making it unlikely that increased ADM expression is causally responsible for interspecies seed arrest.
Multiple genetic loci control incompatibility of C. rubella × C. grandiflora hybrid seeds
Seeds derived from selfed F1 plants aborted at a frequency of 5.7% (n = 7 plants, total number of seeds = 207), suggesting a multilocus genetic interaction. To estimate the number of loci involved in postzygotic hybrid incompatibility in the cross combination C. rubella × C. grandiflora we generated an F2 population of plants from selfed F1 individuals and backcrossed them as pollen donors to C. rubella plants (C. rubella × F2). We developed a genetic model assuming that one C. rubella maternal locus negatively interacts with two or three C. grandiflora paternally derived loci in a Bateson–Dobzhansky–Muller-type interaction, whereby divergent alleles at two or more loci negatively interact in hybrids to reduce fitness [38,39] (S4 Fig, S5 Table). Thus, based on this model hybrid seed abortion only occurs when two or three unlinked C. grandiflora paternal loci are inherited together. The frequency distribution of F2 plants (n = 250) triggering specific seed abortion rates in the backcrosses to C. rubella (Fig 6A) fitted the prediction of the model with three C. grandiflora paternal loci negatively interacting with one maternal C. rubella locus (Fig 6B), revealing a multilocus negative genetic interaction underlying hybrid incompatibility between C. rubella and C. grandiflora.
Fig. 6. C. rubella × C. grandiflora hybrid seed incompatibility involves multiple genetic loci. 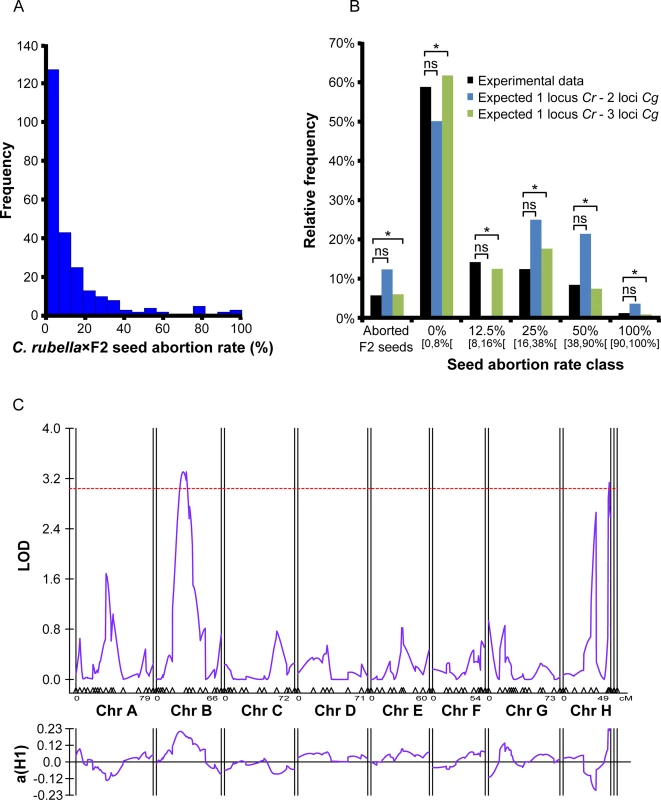
(A) Frequency of F2 plants derived from crosses of C. grandiflora × C. rubella producing defined rates of seed abortion when backcrossed to C. rubella maternal plants. (B) Theoretical distribution of F2 plants producing defined rates of seed abortion when assuming that one maternal locus from C. rubella negatively interacts with two or three paternal loci from C. grandiflora. Experimental data correspond to data shown in (A). Significance of the observed distributions with the predictions of the models has been tested by Chi-square and P>0.05 is marked by an asterisk. Ns, non significant. (C) LOD scores for paternal C. grandiflora QTLs affecting abortion of C. rubella × C. grandiflora hybrid seeds. The purple line (top) represents the LOD score of each marker and the purple line (bottom) represents the effects. The red line represents the significance threshold as estimated by 1,000 permutations. To identify the paternal C. grandiflora genetic loci contributing to postzygotic hybrid incompatibility with a C. rubella seed parent, we determined seed abortion rates after crossing a RIL population of C. grandiflora / C. rubella [40] to C. rubella maternal plants (S5A Fig). The parental accessions of the RIL population gave rise to 100% seed abortion in the C. rubella × C. grandiflora cross and 50% seed abortion in the reciprocal cross (S5B Fig).
The majority of RILs did not trigger seed abortion (S5A Fig), suggesting that hybrid incompatibility genes are purged from this population. Nevertheless, we could identify two significant QTLs located on chromosomes 2 and 8 (Fig 6C) that showed a positive correlation between C. grandiflora alleles and seed abortion. Both QTLs explain about 30% of the phenotypic variance, supporting the idea that there are more than two genes involved in conferring hybrid seed abortion and that incompatibility genes likely have been lost in the RIL population.
Discussion
Recent divergence of C. rubella and C. grandiflora has been associated with the loss of self-incompatibility in C. rubella [1,2]. This drastic change in mating system most probably decreased gene flow between the two species, increasing genetic divergence and allowing additional reproductive barriers to be established. Our data lend support to this hypothesis by revealing a cross direction-dependent, non-reciprocal effect on hybrid seed development. Hybrid seeds resulting from C. rubella × C. grandiflora hybridizations were completely unviable; while the other cross direction resulted in smaller seeds that however were partially viable. Similar examples of unidirectional hybrid incompatibility were previously reported [6,41,42] and have been conceptionalized in the "weak inbreeder/strong outbreeder" (WISO) hypothesis [6]. Based on this hypothesis, in crosses between plants with differing mating systems outcrossing parents are expected to cause a stronger detrimental effect on seed development than selfing parents, as parental conflict is less intense in self-pollinating plants than in outcrossers [6]. Our data provide strong support for this hypothesis, suggesting that genes evolving under parental conflict may build the interspecies hybridization barrier between C. rubella and C. grandiflora. In angiosperms, the endosperm is the battleground for parental conflict [7], consistent with our data revealing that the endosperm is causally responsible for interspecies seed arrest. In C. rubella × C. grandiflora hybrid seeds, endosperm cellularization fails, which likely causes embryo arrest [15]. Supporting this idea, viable hybrids of C. rubella × C. grandiflora could easily be generated using in vitro embryo rescue. Conversely, arrest of C. grandiflora × C. rubella hybrid seeds is likely caused by precocious endosperm cellularization, in agreement with data showing that precocious endosperm cellularization causes decreased seed size and in extreme cases seed arrest [13,43]. Similar to data from rice interspecies hybrids revealing that endosperm cellularization and endosperm proliferation are uncoupled [21], we did not detect obvious endosperm proliferation defects that could explain differences in endosperm cellularization. Therefore, the observed correlation between precocious or delayed endosperm cellularization with respectively decreased or increased endosperm proliferation in response to interploidy hybridizations [13,44] seems unlikely to reflect a general mechanism coupling endosperm proliferation to cellularization. Our transcriptome data revealed that endosperm cellularization failure in response to interspecies and interploidy hybridizations correlated with increased expression of enzymes that hydrolyze glycosidic bonds between carbohydrates and could therefore degrade cell walls [45]. This suggests that the timing of endosperm cellularization is transcriptionally controlled and requires suppression of cell wall degrading enzymes.
Our data suggest that there are three or more C. grandiflora paternal loci that confer seed abortion in combination with a C. rubella seed parent. Similarly, the hybridization barrier between A. thaliana and A. arenosa is controlled by many genes participating in a complex genetic network [46], suggesting that postzygotic hybrid incompatibility that manifests in the endosperm is built by rather complex genetic interactions. Consistent with a scenario that there are many small effect genes contributing to hybrid seed abortion in Capsella, the identified QTLs explained only a low percentage of the phenotypic variation. Nevertheless, it is possible that major QTLs contributing to hybrid seed lethality have been purged from the RIL population and only small effect QTLs are maintained. The Bateson-Dobzhansky-Muller (BDM) model provides a theoretical framework explaining postzygotic reproductive isolation and substantial evidence in support of this model have accumulated over recent years [47–49]. This model stipulates that the interaction of independently evolving loci is innocuous in their native genomic context, however, the interaction between them is deleterious in hybrids [38,39]. Several studies in multiple plant species indicate that the genetic basis of reproductive isolation may involve relatively few loci [49–52]. This is contrasted by other studies supporting a scenario of multiple small incompatibilities accumulating over time [46,53–56]. Our study lends support to the latter, suggesting that multiple small incompatibilities can quickly evolve. A role of imprinted genes in building the interspecies hybridization barrier could provide an explanation for the fast evolution of hybridization barriers.
In conclusion, this study supports the “weak inbreeder/strong outcrosser” hypothesis [6] and suggests that a fast evolving, multi-locus mechanism underlies the post-zygotic barrier affecting C. rubella × C. grandiflora seed survival.
Material and Methods
Plant material and growth conditions
All seeds were surface sterilized using 5% Sodium Hypochlorite solution under the fume hood. After sterilization seeds were plated on MS media containing 1% sucrose. After stratification for two days in the dark at 4°C seedlings were grown in a growth room under long-day photoperiod (16 hrs light and 8 hrs darkness) at 22°C light and 20°C darkness temperature and a light intensity of 110 μE. All seedlings were transferred to pots and plants were grown in a growth chamber at 60% humidity and daily cycles of 16 hrs light at 21°C and 8 hrs darkness at 18°C.
For all crosses, designated female partners were emasculated, and the pistils were hand-pollinated 2 days after emasculation (same procedure for selfing and outcrossing plants).
Capsella accessions Cr48.21, Cg88.14 and Cg4a were used for the phenotypic analysis and Cr48.21 and Cg4a for the gene expression and QTL analyses. All accessions originated from wild collections. An F2 hybrid population of C. grandiflora × C. rubella individuals was generated based on an F1 interspecies cross (C. grandiflora × C. rubella; accessions: Cg4a × Cr48.21). F1 hybrid plants were selfed and the resulting F2 seeds were grown. Capsella QTL lines were described in [40]. Hybrid embryos derived from seeds of crosses of C. rubella (Cr48.21) × C. grandiflora (Cg4a) were isolated at 13 days after pollination (DAP). After a short incubation of siliques in 70% ethanol, the embryo was isolated using fine needles and placed on MS medium containing 2% sucrose. Plates were covered with a tissue and incubated in a growth room under conditions as indicated above. Surviving seedlings were transferred to soil.
Seed size and seed weight analysis
Seeds were arranged on white plastic dishes and pictures were taken using a Leica Z16apoA microscope. Images were converted to black and white using the ‘‘threshold” function in ImageJ (http://rsbweb.nih.gov/ij/) and seed size was measured using the ‘‘Analyze Particles” function. For seed weight, 30–150 seeds per replicate (three replicates per cross) were weighed with an Ohaus GA110 balance and the total weight was divided by the number of seeds.
Microscopy
Clearing analysis was performed as previously described [57].
Endosperm nuclei counts were conducted on cleared seeds at defined time points. After one day incubation in chloral hydrate solution (glycerol/chloral hydrate/water in a ratio of 1 : 8:3) at 4°C pictures in all possible planes of the seed were taken and endosperm nuclei were subsequently counted in all possible seed planes. Endosperm nuclei were counted using Adobe Photoshop CS5. For tissue sections, seeds were fixed and embedded with Technovit 7100 (Heraeus Kulzer, Hanau, Germany) following the procedure described in [15]. Treatment of the embedded seeds was conducted following [30]. Pictures of embedded and cleared samples were taken using a Leica DMI 4000B microscope and Leica DFC360 FX camera. Pictures of mature seeds were taken using a Leica Z16apoA microscope and a Leica DFC425C camera. All pictures were processed using Adobe Photoshop CS5.
RNA sequencing and expression analysis
Two biological replicates with 300–500 seeds per replicate were generated. Seeds were harvested at 6 DAP and stored at -20°C in RNAlater (Sigma-Aldrich, St Louis, USA). RNA extraction was done using RNAqueous Kit with Plant RNA Isolation Aid (Life Technologies, Carlsbad, USA). The RNA-sequencing libraries were prepared using TruSeq RNA Sample Preparation Kit v2 (Illumina, San Diego, USA) according to the manufacturer's instructions.
For quantitative RT-PCR analysis five siliques at each time point were harvested and frozen in liquid nitrogen (accessions: Cr48.21, Cg4a). Glass beads (1.25–1.55 mm) were added, and the samples were ground in a Silamat S5 (IvoclarVivadent, Ellwangen, Germany). RNA was extracted using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Residual DNA was removed using the RNase-free DNase kit (Qiagen) and cDNA was synthesized using the Fermentas First strand cDNA synthesis kit (Fermentas, Burlington, Canada) according to the manufacturer’s instruction.
Quantitative RT-PCR was performed using a Bio-Rad MyiQ single colour Real-Time PCR Detection System (BioRad, Hercules, USA) and Maxima SYBR green qPCR master mix (Fermentas) according to the manufacturer’s instruction. Specificity of the PCR reactions was confirmed by melting curve analysis (55°-95°C). Results were analyzed as described by [58] using PP2A as a reference gene (S6 Table).
High-throughput RNA sequence analysis
Sequencing reads were aligned to the C. rubella genome v1.0 (Phytozome) using Stampy v1.0.23 [59]. Reads mapping to multiple locations were discarded using Samtools [60], by invoking view–q 4 option. The number of reads for each annotated gene were counted using the default option in htseq-count command from the HTSeq Python package [61] (S7 Table). Paired logarithmic fold changes between each replicate were calculated using the R/Bioconductor package DESeq2 [62]. Differentially regulated genes across the two replicates were detected using the rank product method [63] as implemented in the Bioconductor RankProd Package [64]. The test was run with 100 permutations and gene selection was corrected for multiple comparison errors using a pfp (percentage of false prediction) <0.05. Arabidopsis closest homologues of C. rubella genes were determined according to the Phytozome v1.0 annotation of the C. rubella genome. For overlaps between misregulated genes in Arabidopsis and Capsella, only genes having a homologue in the other species were kept. The significance of the overlap between deregulated genes was estimated using a hypergeometric test. Enrichment of GO categories was determined using ATCOECIS [65]. Homologues of A. thaliana type I AGLs were identified in the C. rubella genome according to the Phytozome C. rubella v1.0 genome annotation. A. thaliana type I AGLs were determined as in [66]. Sequencing reads are deposited as fastq files in the Gene Expression Omnibus (GSE67359).
QTL analysis
The QTL analysis was done using Windows QTL Cartographer v2.5 [67]. The crossing type was set to selfing RILs line (Ri1). Genotype data were coded as follows: two (2) for homozygous C. grandiflora alleles, one (1) for heterozygous alleles, and zero (0) for homozygous C. rubella alleles. Marker information and linkage map were obtained from [40]. The number of RILs used in the analysis was 101. Composite Interval Mapping method (CIM) was applied by setting walking speed to 1 cM to recover maximum resolution (the average distance between markers is 6 cM). The background control parameter was set to standard model with backward regression to select any available possible QTLs with standard 5 control markers. Trait values to be tested were entered as binary value (1 for plants with >2% seed abortion and 0 for plants with <2% seed abortion). The 2% threshold was defined based on the log transformed distribution of seed abortion frequencies that showed two distinct distributions bordering at 2%. The LR significant threshold was determined by running 1000 permutation tests with p = 0.05.
Pollen viability assay
Pollen viability among 20 F2 individuals was assessed using Alexander stain [68]. Collected inflorescences were incubated in 10% ethanol. The anthers were dissected on a slide prior to adding a few drops of Alexander stain. The samples were incubated at room temperature for 15 min and then examined under a Zeiss Axioplan microscope. Viable (pink) and aborted (green) pollen grains were counted for each sample (190–320 pollen grains per individual).
Supporting Information
Zdroje
1. Guo Y-L, Bechsgaard JS, Slotte T, Neuffer B, Lascoux M, Weigel D, et al. Recent speciation of Capsella rubella from Capsella grandiflora, associated with loss of self-incompatibility and an extreme bottleneck. PNAS. 2009;106 : 5246–5251. doi: 10.1073/pnas.0808012106 19307580
2. Foxe JP, Slotte T, Stahl EA, Neuffer B, Hurka H, Wright SI. Recent speciation associated with the evolution of selfing in Capsella. PNAS. 2009;106 : 5241–5245. doi: 10.1073/pnas.0807679106 19228944
3. Hurka H, Neuffer B. Evolutionary processes in the genusCapsella (Brassicaceae). Pl Syst Evol. 1997;206 : 295–316. doi: 10.1007/BF00987954
4. Paetsch M, Mayland-Quellhorst S, Neuffer B. Evolution of the self-incompatibility system in the Brassicaceae: identification of S-locus receptor kinase (SRK) in self-incompatible Capsella grandiflora. Heredity. 2006;97 : 283–290. doi: 10.1038/sj.hdy.6800854 16773120
5. Brandvain Y, Slotte T, Hazzouri KM, Wright SI, Coop G. Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species Capsella rubella. PLoS Genet. 2013;9: e1003754. doi: 10.1371/journal.pgen.1003754 24068948
6. Brandvain Y, Haig D. Divergent Mating Systems and Parental Conflict as a Barrier to Hybridization in Flowering Plants. The American Naturalist. 2005;166 : 330–338. doi: 10.1086/an.2005.166.issue-3 16224688
7. Haig D, Westoby M. Genomic Imprinting in Endosperm: Its Effect on Seed Development in Crosses between Species, and between Different Ploidies of the Same Species, and Its Implications for the Evolution of Apomixis. Phil Trans R Soc Lond B. 1991;333 : 1–13. doi: 10.1098/rstb.1991.0057
8. Haig D. GENOMIC IMPRINTING AND KINSHIP: How Good is the Evidence? Annual Review of Genetics. 2004;38 : 553–585. doi: 10.1146/annurev.genet.37.110801.142741 15568986
9. Gehring M. Genomic imprinting: insights from plants. Annu Rev Genet. 2013;47 : 187–208. doi: 10.1146/annurev-genet-110711-155527 24016190
10. Li J, Berger F. Endosperm: food for humankind and fodder for scientific discoveries. New Phytologist. 2012;195 : 290–305. doi: 10.1111/j.1469-8137.2012.04182.x 22642307
11. Boisnard-Lorig C, Colon-Carmona A, Bauch M, Hodge S, Doerner P, Bancharel E, et al. Dynamic Analyses of the Expression of the HISTONE::YFP Fusion Protein in Arabidopsis Show That Syncytial Endosperm Is Divided in Mitotic Domains. Plant Cell. 2001;13 : 495–509. doi: 10.1105/tpc.13.3.495 11251092
12. Berger F. Endosperm: the crossroad of seed development. Curr Opin Plant Biol. 2003;6 : 42–50. doi: 10.1016/S1369526602000043 12495750
13. Scott RJ, Spielman M, Bailey J, Dickinson HG. Parent-of-origin effects on seed development in Arabidopsis thaliana. Development. 1998;125 : 3329–3341. 9693137
14. Pignocchi C, Minns GE, Nesi N, Koumproglou R, Kitsios G, Benning C, et al. ENDOSPERM DEFECTIVE1 Is a Novel Microtubule-Associated Protein Essential for Seed Development in Arabidopsis. Plant Cell. 2009;21 : 90–105. doi: 10.1105/tpc.108.061812 19151224
15. Hehenberger E, Kradolfer D, Köhler C. Endosperm cellularization defines an important developmental transition for embryo. Development. 2012;139 : 2031–2039. doi: 10.1242/dev.077057 22535409
16. Watkins AE. Hybrid sterility and incompatibility. Journ of Genetics. 1932;25 : 125–162. doi: 10.1007/BF02983249
17. Cooper DC, Brink RA. The Endosperm as a Barrier to Interspecific Hybridization in Flowering Plants. Science. 1942;95 : 75–76. doi: 10.1126/science.95.2455.75 17791072
18. Stebbins GL. The Inviability, Weakness, and Sterility of Interspecific Hybrids. In: Demerec M., editor. Advances in Genetics. Academic Press; 1958. pp. 147–215. 13520442
19. Valentine DH, Woodell SRJ. Seed Incompatibility in Primula. Nature. 1960;185 : 778–779. doi: 10.1038/185778b0
20. Bushell C, Spielman M, Scott RJ. The basis of natural and artificial postzygotic hybridization barriers in Arabidopsis species. Plant Cell. 2003;15 : 1430–1442. 12782734
21. Ishikawa R, Ohnishi T, Kinoshita Y, Eiguchi M, Kurata N, Kinoshita T. Rice interspecies hybrids show precocious or delayed developmental transitions in the endosperm without change to the rate of syncytial nuclear division. The Plant Journal. 2011;65 : 798–806. doi: 10.1111/j.1365-313X.2010.04466.x 21251103
22. Schatlowski N, Köhler C. Tearing down barriers: understanding the molecular mechanisms of interploidy hybridizations. J Exp Bot. 2012;63 : 6059–6067. doi: 10.1093/jxb/ers288 23105129
23. Johnston SA, Hanneman RE. Manipulations of Endosperm Balance Number Overcome Crossing Barriers Between Diploid Solanum Species. Science. 1982;217 : 446–448. doi: 10.1126/science.217.4558.446 17782980
24. Carputo D, Frusciante L, Peloquin SJ. The Role of 2n Gametes and Endosperm Balance Number in the Origin and Evolution of Polyploids in the Tuber-Bearing Solanums. Genetics. 2003;163 : 287–294. 12586716
25. Josefsson C, Dilkes B, Comai L. Parent-Dependent Loss of Gene Silencing during Interspecies Hybridization. Curr Biol. 2006;16 : 1322–1328. doi: 10.1016/j.cub.2006.05.045 16824920
26. Gutierrez-Marcos JF, Pennington PD, Costa LM, Dickinson HG. Imprinting in the endosperm: a possible role in preventing wide hybridization. Philos Trans R Soc Lond, B, Biol Sci. 2003;358 : 1105–1111. doi: 10.1098/rstb.2003.1292 12831476
27. Birchler JA, Veitia RA. The Gene Balance Hypothesis: implications for gene regulation, quantitative traits and evolution. New Phytol. 2010;186 : 54–62. doi: 10.1111/j.1469-8137.2009.03087.x 19925558
28. Birchler JA. Interploidy hybridization barrier of endosperm as a dosage interaction. Front Plant Sci. 2014;5 : 281. doi: 10.3389/fpls.2014.00281 25018757
29. Kradolfer D, Wolff P, Jiang H, Siretskiy A, Köhler C. An imprinted gene underlies postzygotic reproductive isolation in Arabidopsis thaliana. Dev Cell. 2013;26 : 525–535. doi: 10.1016/j.devcel.2013.08.006 24012484
30. Kradolfer D, Hennig L, Köhler C. Increased Maternal Genome Dosage Bypasses the Requirement of the FIS Polycomb Repressive Complex 2 in Arabidopsis Seed Development. PLoS Genet. 2013;9: e1003163. doi: 10.1371/journal.pgen.1003163 23326241
31. Schatlowski N, Wolff P, Santos-González J, Schoft V, Siretskiy A, Scott R, et al. Hypomethylated Pollen Bypasses the Interploidy Hybridization Barrier in Arabidopsis. Plant Cell. 2014; tpc.114.130120. doi: 10.1105/tpc.114.130120
32. D’ Erfurth I, Jolivet S, Froger N, Catrice O, Novatchkova M, Mercier R. Turning Meiosis into Mitosis. PLoS Biol. 2009;7. doi: 10.1371/journal.pbio.1000124
33. Murata T, Sano T, Sasabe M, Nonaka S, Higashiyama T, Hasezawa S, et al. Mechanism of microtubule array expansion in the cytokinetic phragmoplast. Nat Commun. 2013;4. doi: 10.1038/ncomms2967
34. Xiao C, Somerville C, Anderson CT. POLYGALACTURONASE INVOLVED IN EXPANSION1 Functions in Cell Elongation and Flower Development in Arabidopsis. Plant Cell. 2014;26 : 1018–1035. doi: 10.1105/tpc.114.123968 24681615
35. Walia H, Josefsson C, Dilkes B, Kirkbride R, Harada J, Comai L. Dosage-dependent deregulation of an AGAMOUS-LIKE gene cluster contributes to interspecific incompatibility. Curr Biol. 2009;19 : 1128–1132. doi: 10.1016/j.cub.2009.05.068 19559614
36. Erilova A, Brownfield L, Exner V, Rosa M, Twell D, Mittelsten Scheid O, et al. Imprinting of the polycomb group gene MEDEA serves as a ploidy sensor in Arabidopsis. PLoS Genet. 2009;5: e1000663. doi: 10.1371/journal.pgen.1000663 19779546
37. Tiwari S, Spielman M, Schulz R, Oakey RJ, Kelsey G, Salazar A, et al. Transcriptional profiles underlying parent-of-origin effects in seeds of Arabidopsis thaliana. BMC Plant Biology. 2010;10 : 72. doi: 10.1186/1471-2229-10-72 20406451
38. Dobzhansky T. Studies on Hybrid Sterility. II. Localization of Sterility Factors in Drosophila Pseudoobscura Hybrids. Genetics. 1936;21 : 113–135. 17246786
39. Muller HJ. Isolating mechanisms, evolution, and temperature. Biol Symp. 1942;6 : 71–125.
40. Sicard A, Stacey N, Hermann K, Dessoly J, Neuffer B, Bäurle I, et al. Genetics, Evolution, and Adaptive Significance of the Selfing Syndrome in the Genus Capsella. Plant Cell. 2011;23 : 3156–3171. doi: 10.1105/tpc.111.088237 21954462
41. Valentine DH, Woodell SRJ. Studies in British Primulas. X. Seed Incompatibility in Intraspecific and Interspecific Crosses at Diploid and Tetraploid Levels. New Phytol. 1963;62 : 125–143.
42. Kato J, Mii M. Production of Interspecific Hybrids in Ornamental Plants. In: Loyola-Vargas VM, Ochoa-Alejo N, editors. Plant Cell Culture Protocols. Humana Press; 2012. pp. 233–245.
43. Garcia D, Fitz Gerald JN, Berger F. Maternal Control of Integument Cell Elongation and Zygotic Control of Endosperm Growth Are Coordinated to Determine Seed Size in Arabidopsis. Plant Cell. 2005;17 : 52–60. doi: 10.1105/tpc.104.027136 15598800
44. Sekine D, Ohnishi T, Furuumi H, Ono A, Yamada T, Kurata N, et al. Dissection of two major components of the post-zygotic hybridization barrier in rice endosperm. Plant J. 2013;76 : 792–799. doi: 10.1111/tpj.12333 24286595
45. Wagner GK, Pesnot T. Glycosyltransferases and their assays. Chembiochem. 2010;11 : 1939–1949. doi: 10.1002/cbic.201000201 20672277
46. Burkart-Waco D, Josefsson C, Dilkes B, Kozloff N, Torjek O, Meyer R, et al. Hybrid incompatibility in Arabidopsis is determined by a multiple-locus genetic network. Plant Physiol. 2012;158 : 801–812. doi: 10.1104/pp.111.188706 22135429
47. Bomblies K, Lempe J, Epple P, Warthmann N, Lanz C, Dangl JL, et al. Autoimmune response as a mechanism for a Dobzhansky-Muller-type incompatibility syndrome in plants. PLoS Biol. 2007;5: e236. doi: 10.1371/journal.pbio.0050236 17803357
48. Moyle LC, Nakazato T. Hybrid Incompatibility “Snowballs” Between Solanum Species. Science. 2010;329 : 1521–1523. doi: 10.1126/science.1193063 20847271
49. Chae E, Bomblies K, Kim S-T, Karelina D, Zaidem M, Ossowski S, et al. Species-wide Genetic Incompatibility Analysis Identifies Immune Genes as Hot Spots of Deleterious Epistasis. Cell. 2014;159 : 1341–1351. doi: 10.1016/j.cell.2014.10.049 25467443
50. Moyle LC, Graham EB. Genetics of hybrid incompatibility between Lycopersicon esculentum and L. hirsutum. Genetics. 2005;169 : 355–373. doi: 10.1534/genetics.104.029546 15466436
51. Fishman L, Willis JH. A cytonuclear incompatibility causes anther sterility in Mimulus hybrids. Evolution. 2006;60 : 1372–1381. 16929654
52. Sweigart AL, Fishman L, Willis JH. A simple genetic incompatibility causes hybrid male sterility in mimulus. Genetics. 2006;172 : 2465–2479. doi: 10.1534/genetics.105.053686 16415357
53. Orr HA, Coyne JA. The genetics of postzygotic isolation in the Drosophila virilis group. Genetics. 1989;121 : 527–537. 2714637
54. Coyne JA, Orr HA. “Patterns of Speciation in Drosophila” Revisited. Evolution. 1997;51 : 295. doi: 10.2307/2410984
55. Sasa MM, Chippindale PT, Johnson NA. Patterns of Postzygotic Isolation in Frogs. Evolution. 1998;52 : 1811. doi: 10.2307/2411351
56. Presgraves DC. Patterns of postzygotic isolation in Lepidoptera. Evolution. 2002;56 : 1168–1183. 12144018
57. Roszak P, Köhler C. Polycomb group proteins are required to couple seed coat initiation to fertilization. PNAS. 2011;108 : 20826–20831. doi: 10.1073/pnas.1117111108 22143805
58. Simon P. Q-Gene: processing quantitative real-time RT–PCR data. Bioinformatics. 2003;19 : 1439–1440. doi: 10.1093/bioinformatics/btg157 12874059
59. Lunter G, Goodson M. Stampy: A statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res. 2010; doi: 10.1101/gr.111120.110
60. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25 : 2078–2079. doi: 10.1093/bioinformatics/btp352 19505943
61. Anders S, Pyl PT, Huber W. HTSeq—A Python framework to work with high-throughput sequencing data. bioRxiv. 2014; 002824. doi: 10.1101/002824
62. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. bioRxiv. 2014; 002832. doi: 10.1101/002832
63. Breitling R, Armengaud P, Amtmann A, Herzyk P. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Letters. 2004;573 : 83–92. doi: 10.1016/j.febslet.2004.07.055 15327980
64. Hong F, Breitling R, McEntee CW, Wittner BS, Nemhauser JL, Chory J. RankProd: a bioconductor package for detecting differentially expressed genes in meta-analysis. Bioinformatics. 2006;22 : 2825–2827. doi: 10.1093/bioinformatics/btl476 16982708
65. Vandepoele K, Quimbaya M, Casneuf T, De Veylder L, Van de Peer Y. Unraveling transcriptional control in Arabidopsis using cis-regulatory elements and coexpression networks. Plant Physiol. 2009;150 : 535–546. doi: 10.1104/pp.109.136028 19357200
66. Bemer M, Heijmans K, Airoldi C, Davies B, Angenent GC. An Atlas of Type I MADS Box Gene Expression during Female Gametophyte and Seed Development in Arabidopsis. Plant Physiol. 2010;154 : 287–300. doi: 10.1104/pp.110.160770 20631316
67. Wang Shengchu, Basten CJ, Zeng Z-B. Windows QTL Cartographer 2.5. In: Department of Statistics, North Carolina State University, Raleigh, NC http://statgen.ncsu.edu/qtlcart/WQTLCart.htm
68. Alexander MP. Differential Staining of Aborted and Nonaborted Pollen. Biotech Histochem. 1969;44 : 117–122. doi: 10.3109/10520296909063335
Štítky
Genetika Reprodukční medicína
Článek Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and ThrombocytopeniaČlánek Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of andČlánek Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on PhenotypeČlánek Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human CancersČlánek A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic BacteriaČlánek The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System inČlánek The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological LifespanČlánek The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 6
-
Všechny články tohoto čísla
- Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Orphan Genes Find a Home: Interspecific Competition and Gene Network Evolution
- Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
- Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia
- Leveraging Identity-by-Descent for Accurate Genotype Inference in Family Sequencing Data
- Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
- Epigenetic Aging Signatures Are Coherently Modified in Cancer
- Silencing of DNase Colicin E8 Gene Expression by a Complex Nucleoprotein Assembly Ensures Timely Colicin Induction
- A Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
- The Orphan Gene Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation
- 9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
- The DnaA Protein Is Not the Limiting Factor for Initiation of Replication in
- FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling
- Multiple Changes of Gene Expression and Function Reveal Genomic and Phenotypic Complexity in SLE-like Disease
- Directed Evolution of RecA Variants with Enhanced Capacity for Conjugational Recombination
- The Regulatory T Cell Lineage Factor Foxp3 Regulates Gene Expression through Several Distinct Mechanisms Mostly Independent of Direct DNA Binding
- MreB-Dependent Inhibition of Cell Elongation during the Escape from Competence in
- DNA Damage Regulates Translation through β-TRCP Targeting of CReP
- Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of and
- The Analysis of () Mutants Reveals Differences in the Fusigenic Potential among Telomeres
- The Causative Gene in Chanarian Dorfman Syndrome Regulates Lipid Droplet Homeostasis in .
- Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype
- The . Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units
- AKTIP/Ft1, a New Shelterin-Interacting Factor Required for Telomere Maintenance
- Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
- Transfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
- Connecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
- Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Response to the Formal Letter of Z. Chrzanowska-Lightowlers and R. N. Lightowlers Regarding Our Article “Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers
- Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins
- Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential
- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level
- Genetic Changes to a Transcriptional Silencer Element Confers Phenotypic Diversity within and between Species
- Functional Assessment of Disease-Associated Regulatory Variants Using a Versatile Dual Colour Transgenesis Strategy in Zebrafish
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy
- The Human Blood Metabolome-Transcriptome Interface
- A Common Cancer Risk-Associated Allele in the Locus Encodes a Dominant Negative Inhibitor of Telomerase
- A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
- The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
- The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological Lifespan
- Dissection of a Complex Disease Susceptibility Region Using a Bayesian Stochastic Search Approach to Fine Mapping
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
- Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in
- Abnormal Activation of BMP Signaling Causes Myopathy in Null Mice
- Reproductive Mode and the Evolution of Genome Size and Structure in Nematodes
- Replication and Active Partition of Integrative and Conjugative Elements (ICEs) of the SXT/R391 Family: The Line between ICEs and Conjugative Plasmids Is Getting Thinner
- Motor and Sensory Deficits in the Mice Result from Mutation of the ESCRT Component HGS
- Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
- Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake
- Separable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4
- Multilayered Organization of Jasmonate Signalling in the Regulation of Root Growth
- Necrotic Cells Actively Attract Phagocytes through the Collaborative Action of Two Distinct PS-Exposure Mechanisms
- A Novel Feedback Loop That Controls Bimodal Expression of Genetic Competence
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Human Blood Metabolome-Transcriptome Interface
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



