-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
Plants have evolved elaborated mechanisms to monitor microbial presence and to control their infection, therefore only particular microbes, so called “endophytes,” are able to colonise the internal tissues with minimal or no host damage. The legume root nodule is a unique environmental niche induced by symbiotic bacteria, but where multiple species, symbiotic and endophytic co-exist. Genetic studies of the binary interaction legume-symbiont led to the discovery of key components evolved in the two partners allowing mutual recognition and nodule infection. In contrast, there is limited knowledge about the endophytic nodule infection, the role of the legume host, or the symbiont in the process of nodule colonisation by endophytes. Here we focus on the early stages of nodule infection in order to identify which molecular signatures and genetic components favour/allow endophyte accommodation, and multiple species co-existence inside nodules. We found that colonisation of Lotus japonicus nodules by endophytic bacteria is a selective process, that endophyte nodule occupancy is host-controlled, and that exopolysaccharides are key bacterial features for chronic infection of nodules. Our strategy based on model legume genetics and co-inoculation can thus be used for identifying mechanisms operating behind host-microbes compatibility in environments where multiple species co-exist.
Published in the journal: . PLoS Genet 11(6): e32767. doi:10.1371/journal.pgen.1005280
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005280Summary
Plants have evolved elaborated mechanisms to monitor microbial presence and to control their infection, therefore only particular microbes, so called “endophytes,” are able to colonise the internal tissues with minimal or no host damage. The legume root nodule is a unique environmental niche induced by symbiotic bacteria, but where multiple species, symbiotic and endophytic co-exist. Genetic studies of the binary interaction legume-symbiont led to the discovery of key components evolved in the two partners allowing mutual recognition and nodule infection. In contrast, there is limited knowledge about the endophytic nodule infection, the role of the legume host, or the symbiont in the process of nodule colonisation by endophytes. Here we focus on the early stages of nodule infection in order to identify which molecular signatures and genetic components favour/allow endophyte accommodation, and multiple species co-existence inside nodules. We found that colonisation of Lotus japonicus nodules by endophytic bacteria is a selective process, that endophyte nodule occupancy is host-controlled, and that exopolysaccharides are key bacterial features for chronic infection of nodules. Our strategy based on model legume genetics and co-inoculation can thus be used for identifying mechanisms operating behind host-microbes compatibility in environments where multiple species co-exist.
Introduction
Plants are the major manufacturers of carbohydrates in ecosystems, and their roots develop in soil environments rich in heterotrophic microorganisms that require carbon for their growth. To adapt to this habitat, plants have evolved sophisticated surveillance systems for monitoring microbial presence, or invasion and corresponding response strategies [1–4]. As a consequence, only a limited range of microbes, endophytes and symbionts have the ability to colonise internal plant tissues with minimal or no host damage [5, 6].
The legume-symbiotic rhizobia interaction is a well-studied example of a very selective and clearly defined host/non-host plant-microbe association. Rhizobial-produced lipochitin oligosaccharide (Nod factors) are recognised by receptors in the host that subsequently trigger cell dedifferentiation, organogenesis and infection of root nodules [7–9]. In most legumes the infection starts at the stage of bacterial entrapment within curled root hairs. This is followed by initiation and elongation of infection threads (ITs), which are plant-derived tubular structures that guide the microbe through the plant’s epidermal and cortical cell layers towards the nodule primordia, in which the bacteria are endocytosed in organelle-like symbiosomes where they develop into bacteroids and fix nitrogen. Characterization of plant mutants impaired at different stages of the symbiotic process has identified genes required to establish and regulate this microbial infection. In Lotus japonicus, SymRK, Nup133, Nup85, Nena, Castor and Pollux act upstream of the nuclear calcium spiking induced by the symbionts and are required for nodule organogenesis [10–14]. A calcium-calmodulin dependent kinase, CCaMK, subsequently interprets these calcium oscillations and interacts with CYCLOPS to coordinate infection with organogenesis [15–17]. Activation of cytokinin signalling via the LHK1 receptor leads to cell division, and downstream transcriptional activators Nsp1, Nsp2 and Nin, control both the infection and the organogenesis [18–21]. In Lotus, which has spherical determinate nodules with a transient meristem, genes involved in actin rearrangement or nucleation (Nap1, Pir1, ArpC1), a putative ubiquitin E3 ligase (Cerberus) and a pectate lyase (Npl1) are required for IT initiation and progression towards primordia, a process which also appears to be controlled by two genes, Alb1 and Crinkle, whose products await identification [22–27]. Later in the developmental process several genes, for example Sst1, encoding a sulphate transporter, are required for bacterial persistence inside the plant cell [28], and Medicago truncatula, which develops indeterminate nodules with a persistent meristem, produces nodule-specific cysteine-rich (NCR) peptides to control the irreversible terminal differentiation of bacteria [29].
From the bacterial side, Nod factors are the main signals recognised by the host, but lipopolysaccharides (LPS), exopolysaccharides (EPS) and cyclic beta-glucans are also critical for infection and bacterial release inside the plant cells [30–32]. In addition, an array of species-specific bacterial effectors orchestrates another level of the specificity identified in the legume-rhizobia symbiosis [33–35].
Given that the final outcome of this highly controlled host-microbe interaction is the bacterial fixation of atmospheric nitrogen in exchange for plant-produced carbohydrates, it is surprising, that, as far as it is currently known, the host selects its symbiont on the basis of bacterial features that are not correlated with their capacity to fix nitrogen [36–39]. In accordance with this notion, inventories of bacterial species retrieved from nodules of legumes growing in a variety of environmental conditions and soils revealed a bacterial community composed of both symbionts and endophytes [40–46]. The presence of poor or even nonsymbionts within nodules of economically important legumes may thus negatively affect the efficiency of their symbiotic nitrogen fixation, and hence plant growth [47, 48]. However, to date there has been no evaluation of the role of endophytic bacteria in pioneer legumes grown in poor soils where fully compatible, highly efficient nitrogen fixing symbionts are either low in titre, or which might need to evolve into more effective symbionts [49]. Interestingly, recent experimental evolution studies have revealed that in the presence of the legume host as a selective environment, a more rapid evolution of symbiont compatibility takes place in a bacterial community [50]. Nevertheless, the co-habitation of diverse bacteria inside nodules raises questions with respect to endophyte recognition by the plant, their infection path(s), and the mechanisms employed by the host-symbiont-endophyte interacting partners leading to access and accommodation of endophytes. Currently there is limited information regarding the entry mode of endophytic bacteria and the role of the legume host, or the proficient symbiont, in the process of nodule colonisation by endophytes.
Here we report that in Lotus the colonisation of nodules by endophytic bacteria follows a selective process with at least three steps, that endophyte nodule occupancy is host-controlled, and that exopolysaccharides represent key bacterial features for chronic infection of nodules.
Results
Lotus japonicus nodules induced by M. loti can accommodate endophytes
In order to test the ability of endophytic bacteria to colonise and multiply inside Lotus nodules we chose to: i) investigate endophytic bacteria that were previously found inside plant roots, as endophytes or presumptive endophytes, and ii) monitor their ability to colonise nodules by visualising their presence inside primordia induced by the M. loti symbiont. In our tests we included Herbaspirillum frisingense GSF30, Herbaspirillum sp. B501 endophytic bacteria from Miscanthus and rice (Oryza sativa), respectively [51, 52], Rhizobium giardinii sp. 129E isolated from Arabidopsis roots [53], and Burkholderia sp. KAW25 (KAW25), R. mesosinicum KAW12 (KAW12) isolated from Lotus roots (see Material and Methods). None of these bacterial strains induced nodule formation when applied individually to Lotus roots. Fluorescently labelled endophytes and M. loti were mixed in a 1 : 1 inoculum, which was applied to Lotus seedlings. After nodule development, whole nodules, or hand sections were inspected microscopically for the presence of the two bacterial strains (Table 1). We found that, with the exception of H. frisingense, the other four strains were present inside the nodules or the cortical ITs induced by M. loti (Figs 1A and S1), but endophyte amplification and effective colonisation of the nodule interior was observed only for Burkholderia KAW25 and Rhizobium KAW12 (Figs 1B and S1C). These results show that in Lotus, the infection threads induced by M. loti can be inhabited by an endophyte (Fig 1A and 1C), and that particular bacteria have the capacity to employ this route for access into the nodules in which they multiply.
Fig. 1. Endophytic colonisation of Lotus japonicus roots and nodules by R. mesosinicum KAW12. 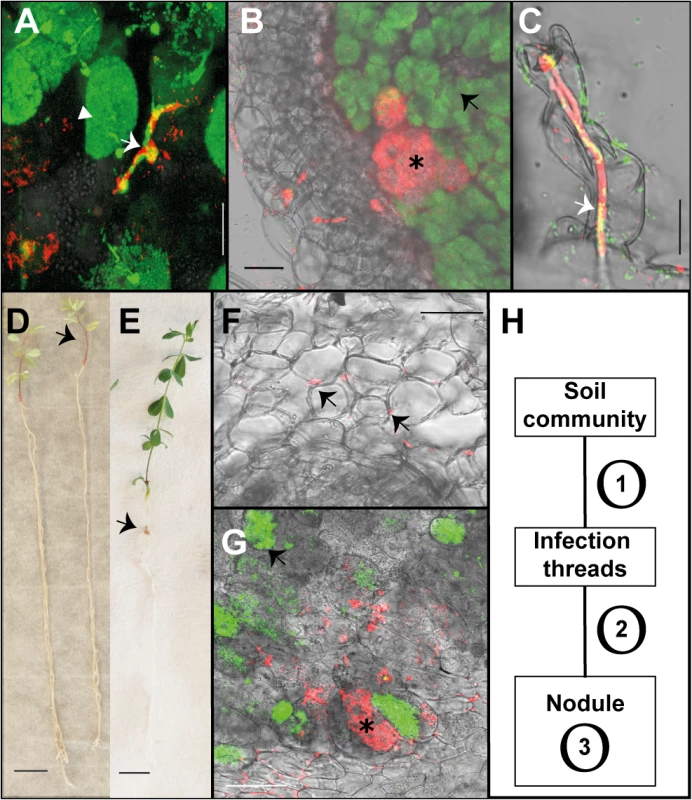
A) Nodule section displaying a cortical infection thread (arrow) that contains both the M. loti wild-type and KAW12 bacteria, among fully infected nodule cells (arrow head) containing the M.loti symbiont. B) Nodule section showing that KAW12 (*) multiplication inside nodules is limited to small sectors compared to M. loti wild-type (arrow), which is the predominant coloniser. C) Root hair infection thread (arrow) containing both the M. loti wild-type symbiont and the KAW12 endophytic bacteria. D) Lotus plants inoculated with KAW12 endophyte display a nod-minus and nitrogen-starved phenotype (arrow) in comparison to E) Lotus plants that form nodules (arrow) and establish a nitrogen-fixing symbiosis after inoculation with M. loti wild-type. F) Root section illustrating the capacity of KAW12 (arrow) to colonise the intercellular space of Lotus roots. G) Section of an M. loti nodZ-induced nodule presenting KAW12 (*) and M. loti nodZ (arrow) infection. H) The infection and accommodation of compatible endophytes within Lotus nodules is regulated in at least three steps. Scale bars: A) and C) 20 μm, B) F) and G) 50 μm, D) and E) 1 cm. Mesorhizobium loti bacteria are visualized in green, and KAW12 in red. Mixed inoculum has been used in A) to C) and G), and single inocula with the aforementioned bacteria have been used for E) to F). Tab. 1. Colonisation of Lotus japonicus nodules by endophytic bacteria. 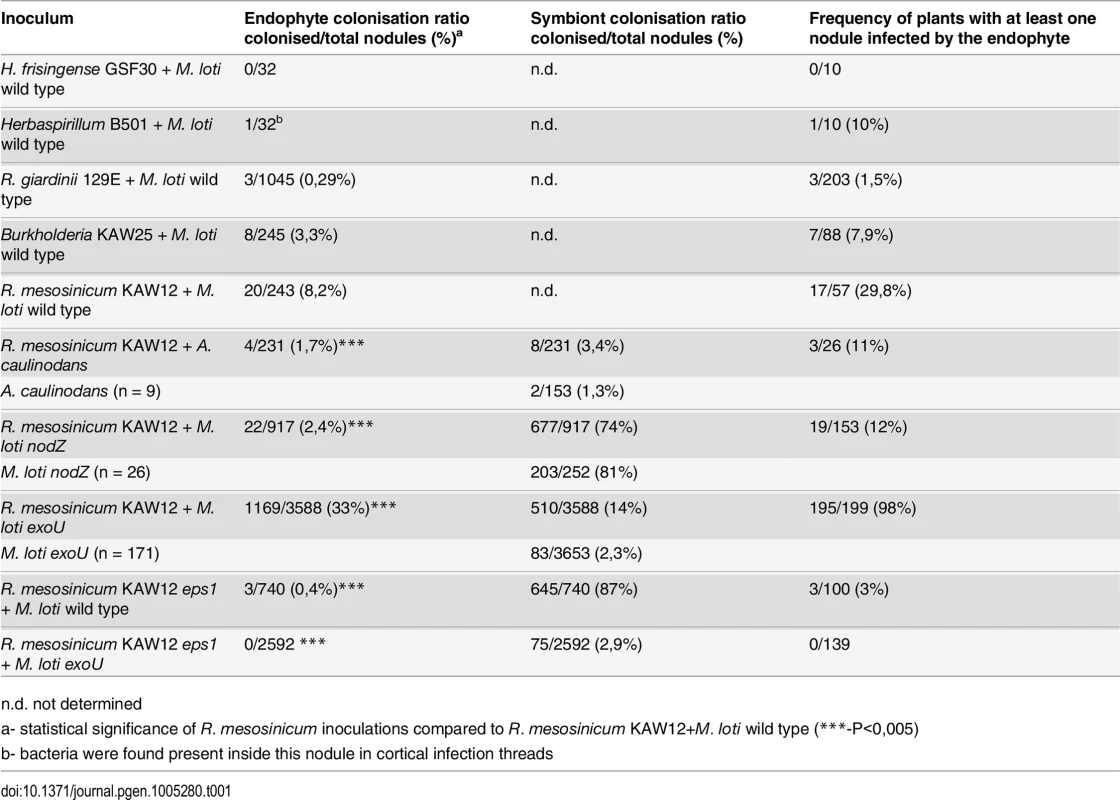
n.d. not determined We observed that even when both symbiotic and endophytic bacteria were able to infect the nodule, the well-adapted symbiont, M. loti, occupied most of the nodule interior, while KAW12 or KAW25 remained within small, distinct sectors (Figs 1B and S1). Interestingly, the host response to the endophytic infection by KAW12 and KAW25 was different. Nodule sectors containing KAW25 bacteria were found to show signs of necrosis (S1C Fig), whilst no similar response was detected in the nodules containing KAW12 (Fig 1B). This indicates that infection and multiplication of endophytic bacteria within Lotus nodules is based on host-microbe compatibility.
Among the five different bacteria included in our study, KAW12 presented the highest level of nodule infection. One third of tested plants (29.8%) contained at least one KAW12-infected nodule (Table 1), and 20 out of the 243 analysed nodules (8.2%) were co-infected by KAW12, demonstrating the ability of this endophyte to colonise Lotus nodules.
These results based on analysis of a limited, but diverse set of endophytes show their differential capacity for nodule infection in the presence of M. loti, and that a sequential selection process shapes the community of bacterial inhabitants inside the nodules, i.e. i) access and/or persistence inside the IT, ii) within the nodule, iii) multiplication within the nodule without causing damage to the host.
KAW12 is a nonsymbiotic Rhizobium with endophytic features
KAW12 was identified as a root-inhabiting bacterium in Lotus plants grown in Japanese forest soil (see Material and Methods), that causes no obvious effect (positive or negative) on its host (S3 Fig). A comparison of its 16S rRNA sequence against known bacteria revealed a close relationship to nodulating Rhizobium species (S2A Fig). In spite of this similarity to symbiotic bacteria, Nod and Nif gene clusters, including key symbiotic genes such as nodC, which is required for Nod-factor synthesis, and nifH, which encodes the Fe subunit of nitrogenase, were not found in the KAW12 genome (S2C Fig). In order to determine the type of infection that this bacterium, which neither produces Nod factors nor fixes atmospheric nitrogen, establishes with Lotus, the KAW12 derivative constitutively expressing DsRED was used for detailed analyses. Confirming the absence of symbiotic genes, KAW12 alone, or co-inoculated with M.loti nodC was unable to induce root hair curling or microcolony formation and was unable to nodulate Lotus (S3 Fig). A nitrogen-starved phenotype was observed when KAW12-inoculated plants were grown under low nitrogen conditions (1 mM KNO3) compared to plants inoculated with the effective symbiont M. loti (Fig 1D and 1E). However, careful inspection of KAW12-inoculated tissue revealed that KAW12 colonised the intercellular spaces of Lotus roots (Figs 1F and S3A). These results illustrate that KAW12 is a nonsymbiotic Rhizobium with endophytic features and a capacity for infecting symbiotic nodules.
Endophytic invasion of nodules by KAW12 depends on a functional Nod factor-induced infection pathway
Research into the binary interaction between legumes and nitrogen fixing rhizobia has revealed that a number of molecular components produced by the bacteria are required and/or contribute to a successful symbiotic association. Nod factor, EPS, LPS, cyclic beta-glucans and Type-III Secretion System (T3SS) effectors have been shown to be major modulators of the host response [31]. The increased capacity of KAW12 to infect Lotus nodules, together with its apparent acceptance by the Lotus host, provided us with a unique opportunity to study the interplay between the legume host and various bacterial partners during mixed infections, and to identify molecular and genetic components contributing to nodule infection by endophytic bacteria.
In order to investigate if KAW12 has the capacity to launch an active infection once the symbiotic Nod factor signalling has been initiated in Lotus roots, we used two different symbiotic bacteria as co-inoculating partners i.e. Azorhizobium caulinodans ORS571, and a M. loti nodZ mutant. Azorhizobium caulinodans ORS571, a symbiont of Sesbania rostrata [54] induced root hair curling, microcolony formation, and a large number of nodule primordia (17 per plant), but approximately 99% of them remained uninfected (Table 1). Furthermore, ITs penetrating the primordia were not observed, indicating that the infection pathway induced by A. caulinodans Nod factors is only partly effective. This restricted symbiotic development was used as a background for assaying the contributions from KAW12 during infection. At 6 weeks after inoculation with A. caulinodans and KAW12 only 3 of 26 plants had nodules colonised by KAW12, and the overall frequency of colonisation was also very limited (4 out of 231 primordia) (Table 1 and S4 Fig). We then tested the ability of KAW12 to colonise the nodules induced by the M. loti nodZ mutant. This mutant strain produces Nod factors lacking the acetylated-fucosyl decoration, and as a consequence the induction of primordia and the infection process are delayed and less effective [55]. Inspection of plants inoculated with the M. loti nodZ and KAW12, showed that only 2.4% of the induced nodules were infected by the endophyte. This is more than 3-fold fewer than in the co-inoculation with the M. loti wild-type (8.2%). The frequency of plants containing at least one KAW12-colonised nodule was also reduced; 12% compared to 29.8% in the wild-type M. loti co-inoculation (Fig 1G and Table 1).
This lower frequency of bacterial infection in the absence of a fully functional Nod factor signalling indicates that signalling components possessed by KAW12 cannot complement nor bypass an ineffective Nod factor-dependent infection pathway.
Exopolysaccharides are critical for symbiotic and endophytic nodule colonisation
In addition to Nod factor-induced signalling, host perception of compatible bacterial polysaccharides, such as EPS, is also important for symbiont recognition and efficient nodule infection [37, 56]. For example, in Lotus, perception of incompatible EPS produced by M. loti R7A exoU mutant severely impairs IT initiation and elongation is reduced, and consequently infected nodules are rare [32] (Table 1 and Fig 2A and 2B and S5A). Considering that Nod factor signalling is functional in the Lotus-exoU interaction [32], we investigated the ability of KAW12 to colonise nodules in the presence of incompatible symbiotic EPS signalling. Analysis of plants co-inoculated with exoU and KAW12 revealed that KAW12 had the ability to colonise the primordia and the ITs initiated by exoU bacteria (Table 1 and Fig 2A and 2C and Fig 2D). Infection threads colonised by KAW12 reached the base of the root hair where they expanded into an infection pocket, and from there, bacterial infection progressed into the underlying nodule primordium (Fig 2E). This indicates that the KAW12 endophyte has the capacity to rescue, and progress the arrested infection process induced by the exoU. Nodules infected by the exoU, KAW12, or by both bacteria, could be observed based on fluorescence marker screening (Fig 2A–2C). Unexpectedly, the majority of plants (98%) had at least one nodule containing KAW12, and overall 33% of primordia (1169 of 3588 nodules) were infected by KAW12, suggesting that molecular features of KAW12 may substitute for the lack of compatible M. loti EPS (Table 1). The increased frequency of KAW12-colonised nodules (33% compared to 8.2% in M. loti wild-type co-inoculation) also indicates that KAW12 infection is competitively restricted by the fully compatible EPS produced by wild-type M. loti.
Fig. 2. R. mesosinicum KAW12 colonisation pattern in Lotus japonicus nodules induced by M. loti exoU. 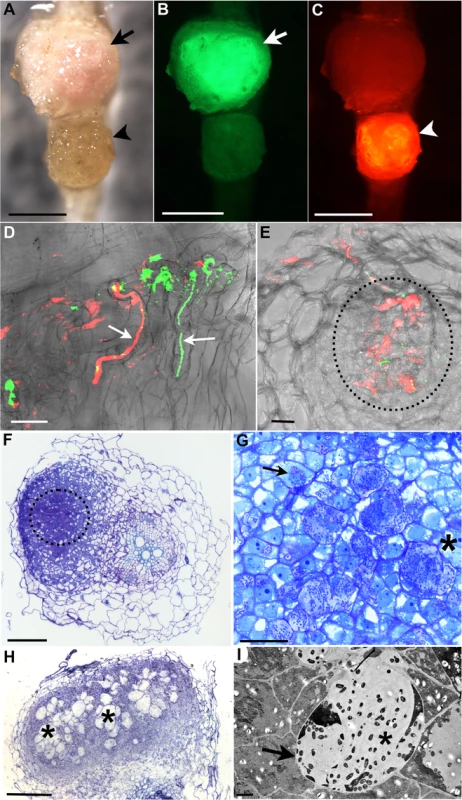
A) to C) Nodules colonised by M. loti exoU-GFP (arrow) or KAW12-DsRed (arrowhead). The two nodules were visualized in bright field (A) with the GFP filter (B) or with the DsRed filter (C). D) Root hair infection threads (arrows) colonised by M. loti exoU (green) and KAW12 (red). E) Confocal laser scanning microscopy (CLSM) image of a nodule section illustrating internal nodule infection (dashed line) by KAW12 (red) and M. loti exoU (green). F) Thin section of a nodule primordium showing KAW12 infection (dashed line) in the inner zone. G) Detailed view of the same nodule as in (F) illustrating the inter- (*) and intra-cellular (arrow) KAW12-containing lagoons. H) Section of a mature nodule presenting multiple and enlarged lagoons colonised by KAW12 (*). (I) Transmission electron micrograph of an intercellular lagoon (arrow) containing bacteria surrounded by a white, undefined matrix (*). Scale bars = 500 μm (A to C), 20 μm (D, G), 50 μm (E), 100 μm (F, H), and 2 μm (I). The M. loti exoU is visualized in green and KAW12 in red (A to E). The ability of KAW12 to overcome the arrested infection of the exoU suggested that KAW12 EPS might act as an important factor for its nodule colonisation ability. We tested this hypothesis by investigating the capacity of EPS-defective KAW12 to colonise the exoU-induced nodules. An EPS mutant of KAW12 was isolated from a random mutagenesis screen utilising the transposon mTn5-GNm [57]. The gene disrupted in this mutant encodes for a protein that shows high similarity (70%) to PssN from R. leguminosarum (S6A Fig), which is involved in polymerisation and export of EPS [58–60]. In contrast to the wild-type KAW12, the eps mutant displayed a non-mucoid colony growth phenotype, a typical characteristic of EPS deficiency (S6B Fig). In planta analyses of the colonisation phenotype revealed that this mutant, despite its presence inside root hair ITs when co-inoculated with the exoU (S6C Fig), was unable to infect and multiply within nodules, while exoU maintained its low infection ability (Table 1). In the reciprocal experiment, we found that co-inoculation of the KAW12 eps mutant with M. loti wild type enabled access of endophytes inside nodules, albeit to a very low frequency compared to EPS proficient KAW12 wild type bacteria (S6D Fig and Table 1). These results show that EPS is an important molecular feature of KAW12 allowing it to colonise the symbiont-induced primordium, and that co-infecting bacteria may complement each other for the lack of compatible EPS.
These co-inoculation studies pinpoint the critical role of EPS during nodule infection by symbiotic and endophytic bacteria, and have revealed that compatible EPS provides the wild-type symbiont with a clear advantage over the endophyte during mixed nodule infection.
KAW12 is capable of intra - and inter-cellular colonisation of nodules
The nodule is a unique root organ where the intracellular accommodation and multiplication of compatible symbionts is permitted. Many of the nodules infected by KAW12 were abundantly colonised by endophytic bacteria in comparison to their sparse infection of the root intercellular spaces (Fig 1B and 1F). In spite of this increased nodule colonisation no signs of hypersensitive reactions or necrosis were observed in KAW12-colonised nodules (Fig 2A). This apparent acceptance of KAW12 endophytic bacteria by the host might be due to their distinct colonisation pattern within nodules that involves inter - and/or intracellular accommodation. To investigate this we studied the infection pattern of KAW12 in more detail using light and transmission electron microscopy (TEM) applied to selected nodules (Material and Methods). We observed that KAW12 multiplied extensively in the central zone of the nodules where bulbous structures accommodating numerous bacteria were observed between and within the plant cells (Figs 2F–2I and S5B). These disorganised structures differed in size and shape from the fully colonised nodule cells containing the exoU symbiont (Fig 3A and 3C) and from the finely defined ITs induced and occupied by symbiotic rhizobia (Figs 1C and 3B and 3D and S5C–S5H). Similar to the ITs induced by symbiotic bacteria, the KAW12-containing structures were encapsulated within cell wall material, as illustrated by the presence of a homogalacturonan epitope which is present in the plant cell wall and which was detected by the monoclonal antibody JIM5 (Figs 3E–3G and S5E and S5F). Glycoproteins, usually present in the IT matrix containing symbiotic bacteria and detected by the MAC236 antibody [61], were rarely observed in the matrix of KAW12-containing lagoons, but instead were found in the surrounding plant cells (Figs 3H and 3I and S5G and S5H). Localised cell wall degradation was observed leading to singular or multiple bacterial entrapments in the plant cell (Figs 3F and S5E–S5H). No membrane-like structure was observed around the internalised KAW12 indicating that symbiosomes were not formed. The infected plant cells contained KAW12 bacteria that were clustered together and surrounded by a white, undefined matrix (S5C–S5H Fig). The infected plant cells appeared to be viable, based on their apparently normal internal structure (S5F Fig), however, collapsed plant cells with massive intracellular infection of un-clustered KAW12 bacteria were also observed.
Fig. 3. Transmission electron micrographs of Lotus japonicus nodules colonised by M. loti exoU or by R. mesosinicum KAW12. 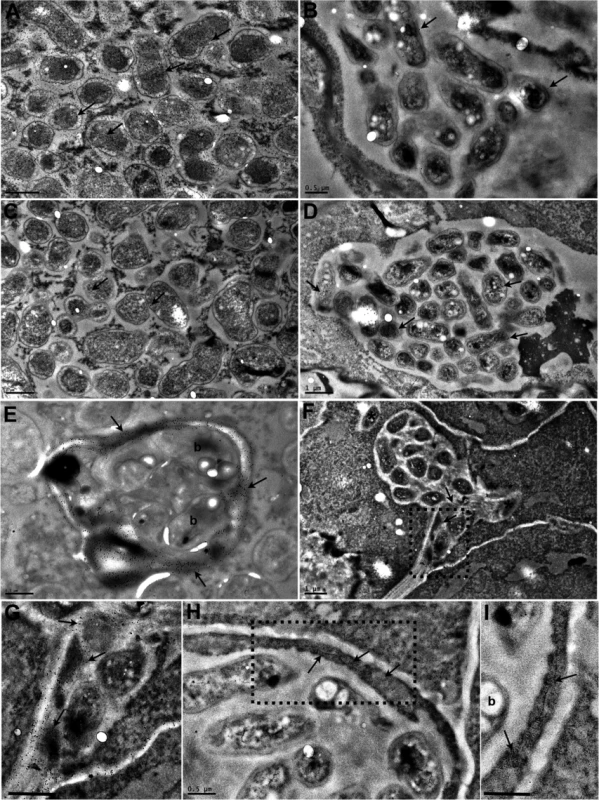
The nodule sections were immunogold labelled (arrows) with an antibody against the GFP protein (A, B), or against the DsRED protein (C, D). GFP was detected (arrows) in the M. loti exoU-selected nodule (A), and DsRED (arrows) in the KAW12-selected nodule (D). There is some minor nonspecific labelling by the GFP antibody (arrows) in the nodule colonised by the DsRed-tagged KAW12 (B) and by the DsRED antibody (arrows) in the nodule colonised by the GFP-tagged M. loti exoU (C). Immunogold labelling of homogalacturonan by the JIM5 monoclonal antibody shows the presence of cell wall material (arrow) in the infection thread that contains M. loti exoU (E), and in the lagoons containing KAW12 (F, G). KAW12 is released inside the plant cell (*) (F). Immunogold labelling of glycoproteins (arrows) by the MAC236 monoclonal antibody reveals their location within the plant cells containing the KAW12-containing lagoons (H, I). Detailed images of the regions marked by rectangles in F) and H) are shown in G) and I), respectively. Scale bars = 1 μm (A to D, and F), 0.5 μm (E, G, I). b = bacteria. These results show that KAW12 is able to multiply within the nodules both intra - and inter-cellularly, and to a higher extent than that observed in the root tissue, indicating that nodules offer a competent biological niche for microbial accommodation.
Plant symbiotic genes control invasion of nodules by endophytic bacteria
The results obtained from co-inoculation of Lotus wild-type plants showed that KAW12 has the ability to colonise ITs and nodules induced by M. loti and to progress the infection initiated by the M. loti exoU toward nodule primordia. In order to determine if plant genes required for infection by symbionts would also be necessary for the progression of the KAW12 infection, a panel of plant mutants impaired at different stages during symbiotic infection were analysed for their ability to sustain KAW12 colonisation. Mutation of a non-essential plant gene was assumed to result in a KAW12 infection frequency of nodule primordia similar to that of wild-type plants (i.e. 33%).
First, we analysed the Cyclops, Cerberus, Nap1 and ArpC1 genes involved in the signalling pathway controlling IT initiation and elongation. After the co-inoculation of mutants impaired in these Lotus genes by exoU and KAW12 only a negligible KAW12 infection of primordia was detected (Table 2), revealing that these plant symbiotic genes are essential for KAW12 infection of symbiotic nodules. These results confirm the dependency of KAW12 infection on the root hair IT initiation that is host-symbiont controlled. We then analysed the involvement of Npl1, Alb1 and Crinkle, controlling symbiotic infection at the stage of IT passage through the epidermal/cortical barrier. After co-inoculation KAW12 was found impaired in infection of nodules induced by exoU on npl1 and alb1 mutants, but not on crinkle mutants where the infection frequency was similar to Lotus Gifu wild-type plants (Table 2). This indicates that the Npl1 and Alb1 genes, together with Cyclops, Cerberus, Nap1 and ArpC1 are required for both M. loti and KAW12 nodule infection via ITs. On the other hand, the mutation present in crinkle, which limits M. loti wild-type infection [23], does not affect KAW12 colonisation. This observation is interesting, since alb1 and crinkle have been reported to have similar mutant phenotypes in the presence of wild-type M. loti, and, therefore, have been suggested to be impaired at corresponding stages of infection [26]. Identification of the Alb1 and Crinkle genes would likely help to explain the observed differences. Finally, we investigated the role of the symbiotic gene Sst1, involved in the later stages of the Lotus-M. loti symbiosis. We observed that the sst1 mutation had a limited effect on KAW12 colonisation, indicating that this gene is not required for KAW12 multiplication inside nodules (Table 2).
Tab. 2. Colonisation of M. loti exoU induced nodules by R. mesosinicum KAW12 on Lotus japonicus wild type and symbiotic mutants. 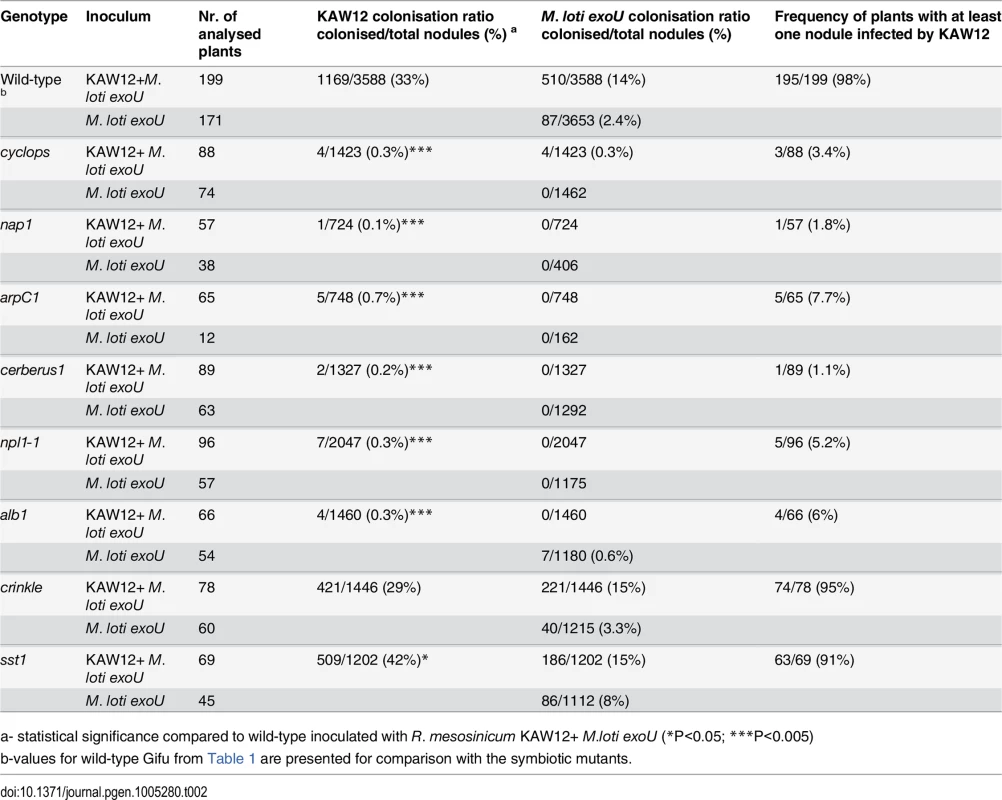
a- statistical significance compared to wild-type inoculated with R. mesosinicum KAW12+ M.loti exoU (*P<0.05; ***P<0.005) Taken together, our analyses revealed that the legume host controls the access into nodules for both symbionts and endophytes when ITs are used as entry route, and that selective mechanisms may exist to control the accommodation of compatible symbionts and/or endophytes.
Discussion
Land plants develop their root systems in a microbe-rich soil environment and have sophisticated mechanisms for microbial surveillance. In addition to the selection pressure imposed from the plant host, differences in the physiology of microbes and their ability to establish various microbe-microbe interactions, contribute to the composition of microbial communities in the soil, rhizosphere and in planta [4, 62–65]. There is a large diversity and wealth of diazotrophs in the soil, but it has become clear in the last decades that legumes select the infecting root nodule symbionts on the basis of molecular signatures, such as Nod factors, EPS, and LPS, that are unrelated to their symbiotic function performed within nodules [31]. As a consequence of this indirect selection mechanism, legumes that grow in natural habitats end up hosting a varied bacterial community inside nodules [45]. Experimental data support this suggestion; efficient nitrogen fixing bacteria, but also poor nitrogen fixers and endophytes have been shown to co-exist as part of the nodule bacterial community [42]. Likewise, laboratory studies using defined mixed symbiotic inocula and field studies monitoring the symbionts within nodules have revealed that mutants or poor nitrogen-fixing symbionts can infect and colonise nodules together with compatible strains [32, 66, 67]. Previous reports presented theoretical models or experimental evidence for the various mechanisms employed by the host to sanction the non/poor symbionts after establishment within nodules [68–70]. Our study focuses on the early stages of nodule infection by the endophytes in order to identify which molecular signatures and genetic components favour/allow an endophytic nodule infection.
Using co-inoculation experiments with a panel of endophytic bacteria together with the efficient symbiont M. loti, we show that complex host-microbe and microbe-microbe interactions can be captured and studied in Lotus plants grown under controlled conditions. Additional information may be gained from similar studies in legumes where rhizobial infection doesn’t follow the well-characterised root hair infection pathway. Using fluorescently labelled bacteria we monitored microbial infection patterns, and found that in the presence of M. loti the infection and accommodation of compatible endophytes within Lotus nodules is regulated in at least three steps (Fig 1H). Four of the tested endophytes were able to colonise cortical ITs induced by M. loti while only two infected and multiplied inside the nodules. Finally, R. mesosinicum KAW12 persisted inside nodules without inducing necrosis. Since KAW12 lacks the crucial genetic basis for establishing a nitrogen-fixing symbiosis a tempting explanation for this competence could reside in the endophytic features that enable KAW12 to colonise the intercellular spaces of Lotus roots in the absence of M. loti. Rhizobial species are frequently found as endophytes in a wide range of plant species [53, 71–75], indicating either an improved fitness compared to other soil bacteria or a better communication with the plant host. Nevertheless, the fact that the two Rhizobium species included in our study differ in their ability to colonise the nodules demonstrates that specific bacterial determinants contribute to their acceptance by the host.
Co-inoculation studies revealed that even if the endophyte had the competence to infect and multiply within nodules it was the M. loti symbiont which occupied most of the nodule interior, demonstrating its adapted ability to compete with other bacteria and to efficiently communicate with the host during infection (Fig 1B). The ability of KAW12 to co-infect the nodules in the presence of M. loti provided the opportunity to study the role/contribution of the symbiont and the host to endophyte infection.
Rhizobium KAW12 utilises M. loti-induced ITs as a route for access into the nodules (Fig 1C) and this infection pattern prompted us to investigate the role of the Nod factor signalling induced by the M. loti symbiont for the endophyte infection. Symbiotic rhizobia produce Nod factors continuously during root and nodule infection, and previous studies have revealed that fully compatible Nod factor signalling is important for the initiation and fast progression of ITs towards nodule primordia to ensure rapid infection and symbiotic development [54, 76, 77]. Our results from the co-inoculation experiments of Lotus with KAW12 and symbionts, such as A. caulinodans ORS571 or the M. loti nodZ mutant that produces less-compatible Nod factors, revealed a lower rate of KAW12 infection when compared to its co-inoculation with wild-type M. loti R7A. Based on these results we conclude that fully compatible Nod factor signalling is important for nodule infection by KAW12, as it allows rapid access of the endophyte into the nodule primordium.
We then investigated the role of bacterial exopolysaccharides, and demonstrated that during nodule infection compatible EPS provides the symbiotic bacteria with an advantage over the co-infecting endophyte. Both the frequency and the nodule volume presenting endophytic infection increased after co-inoculation with KAW12 and the M. loti exoU (Table 1 and Fig 2). Furthermore, KAW12 had the ability to rescue the exoU-containing aborted ITs within the root hairs and thus to progress the infection towards the nodule primordia in a manner similar to a nodA mutant of M. loti defective for Nod factor production [32]. Using a KAW12 eps mutant we show that this ability to bypass the requirement for compatible symbiotic EPS is dependent on KAW12 EPS. This is consistent with results showing EPS to be crucial for legume colonisation by symbiotic nitrogen-fixing bacteria, where both a protective activity against host defence responses and a positive signalling role have been proposed [32, 37, 56]. Interestingly, genes involved in EPS biosynthesis or export were found as targets of selection among several Sinorhizobium medicae and S. meliloti strains that share host plants [78]. Our results demonstrate that EPS represent a key molecular feature during nodule infection by both symbiotic and endophytic bacteria, and opens up the possibility that nodule infection by KAW12 is facilitated by perceptions of endophytic, yet compatible, EPS by the host.
Additional indications for a compatible host-endophyte interaction came from the analyses aimed to determine the role of the legume host for KAW12 infection. We found that the ability of KAW12 to progress the exoU-arrested ITs and to infect nodule primordia is dependent on early symbiotic genes. Lotus mutants impaired in the early events required for IT initiation and elongation (Cyclops, Cerberus, Nap1, ArpC1, Npl1, Alb1) were also defective for the endophyte infection (Table 2). We conclude that these host symbiotic genes gate the access of both symbiotic and endophytic microbes. On the other hand, genes like Crinkle that are required for M. loti infection, and Sst1 which supports the symbiotic function of M. loti within nodules, did not seem to be required for KAW12 colonisation, indicating specific mechanisms operating inside host nodules to control the persistence of both symbiotic and endophytic bacteria.
Maintaining populations of highly competitive symbionts in the soil, and ensuring predominant occupancy by effective nitrogen fixing bacteria represents a major challenge that limits legume cultivation [79–82]. So far, most progress was obtained from the application of selected bioinoculants that are edaphically adapted [82, 83]. However, conventional application of superior nitrogen-fixing rhizobia did not prove to be a consistent solution for this challenge [84–86]. On the other hand, when the legume-breeding programme was performed in the presence of elite-selected rhizobia, and in conditions that favoured biological nitrogen fixation, a significant and consistent increase in plant yields was obtained as a result of selection for improved host-symbiont compatibility [87]. These practical results, together with those provided by the biodiversity studies corroborate with the results we present here and provide an explanation i.e.; the legume nodule is a unique environmental niche with an adapted program for accommodation of host-selected compatible soil microbes, and layers of compatibility determine access and colonisation efficiencies, symbiotic or not.
Our study shows that genetic resources available for the model legumes, in combination with co-inoculation strategies provide a reliable framework for identifying the genetic mechanisms operating behind this compatibility at the plant root interface, thus allowing developments to further address this challenge in a targeted manner.
Material and Methods
A detailed version of material and methods is presented in the S1 Text.
Plant material and bacterial strains
Plant genotypes and bacterial strains used in this work are listed in S1 Table and S2 Table.
Isolation of R. mesosinicum KAW12 and Burkholderia sp. KAW25
Forest soil (0-4cm) was sampled from the Botanical Garden of Tohoku University (12–2 Kawauchi Aoba-ku Sendai Miyagi, Japan) on December 2006. Sterilized seeds of the L. japonicus cCaMK (Ljsym72) mutant were incubated with the soil in Magenta containers for 3 months. Plants were grown in 16h/light and 8h/dark conditions at 25°C. Whole plants were surface sterilized using 0.5% bleach and homogenized with 10 ml sterilized water. 500μl of the homogenated samples were inoculated onto sterilized seeds of the same mutant in Magenta containers with sterilized vermiculite supplemented with B&D medium, and were incubated for two months. Whole plants were sterilized and homogenized. These homogenized samples were plated onto TY medium, and KAW12 together with KAW25 were isolated among the bacteria growing on the plates. The 16S rRNA from KAW12 and KAW25 has been PCR-amplified, sequenced and analysed for similarity to other bacterial sequences present in the NCBI database (S2A and S2B Fig). According to the results of the 16SrRNA-gene sequences, the Burkholderia sp. KAW25 belongs to the plant-associated branch of the genus Burkholderia, [88] while the Rhizobium sp. KAW12 is within the Rhizobium mesosinicum species.
Isolation of R. mesosinicum KAW12 eps1 mutant
The KAW12 eps1 mutant was isolated from a random mutagenesis screen utilising the transposon mTn5-GNm [57]. The transposon insertions site was identified by arbitrary PCR and sequencing. The KAW12 eps1 was found to harbour an insertion in a gene encoding a polysaccharide export protein with 70% amino acid identity to PssN of R. leguminosarum bv. trifolii (S6A Fig). The mutant strain distinguished from wild-type KAW12 by displaying nonmucoid colony growth on YMB and G/RDM media.
Plant assays and analyses
Plants were grown under nitrogen-limited conditions (1mM KNO3) and analysed for infection after 5 to 6 weeks. For the co-inoculation experiments a 1 : 1 mixture of bacteria (OD600 - 0.02) was used. Screening for colonised nodules was performed by whole plant inspection on a Leica M165FC stereomicroscope in bright field and using filters for GFP and DsRED. Selected nodules were fine-sectioned (50 μm) using a Leica VT1000S vibratome, and analysed for internal colonisation with a Zeiss LSM510 Meta microscope. Semithin nodule sections were analysed by light microscopy.
Transmission electron microscopy (TEM)
Ultrathin nodule sections were analysed by transmission electron microscopy (TEM) as previously described (Madsen et al., 2010). Commercially available DsRED and GFP antibodies were used to identify the bacteria on TEM sections (Fig 3A to 3D) via immunogold labelling [89].
Supporting Information
Zdroje
1. Jones JD, Dangl JL. The plant immune system. Nature. 2006 Nov 16;444(7117):323–9. 17108957
2. Kiers ET, Denison RF. Sanctions, Cooperation, and the Stability of Plant-Rhizosphere Mutualisms. Annual Review of Ecology Evolution and Systematics. Palo Alto: Annual Reviews; 2008. p. 215–36.
3. Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol. 2008 Oct;6(10):763–75. doi: 10.1038/nrmicro1987 18794914
4. Turner TR, Ramakrishnan K, Walshaw J, Heavens D, Alston M, Swarbreck D, et al. Comparative metatranscriptomics reveals kingdom level changes in the rhizosphere microbiome of plants. The ISME journal. 2013 Dec;7(12):2248–58. doi: 10.1038/ismej.2013.119 23864127
5. Bulgarelli D, Schlaeppi K, Spaepen S, Ver Loren van Themaat E, Schulze-Lefert P. Structure and functions of the bacterial microbiota of plants. Annual review of plant biology. 2013;64 : 807–38. doi: 10.1146/annurev-arplant-050312-120106 23373698
6. Sessitsch A, Hardoim P, Doring J, Weilharter A, Krause A, Woyke T, et al. Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Molecular plant-microbe interactions: MPMI. 2012 Jan;25(1):28–36. doi: 10.1094/MPMI-08-11-0204 21970692
7. Broghammer A, Krusell L, Blaise M, Sauer J, Sullivan JT, Maolanon N, et al. Legume receptors perceive the rhizobial lipochitin oligosaccharide signal molecules by direct binding. P Natl Acad Sci USA. 2012 Aug 21;109(34):13859–64. doi: 10.1073/pnas.1205171109 22859506
8. Radutoiu S, Madsen LH, Madsen EB, Felle HH, Umehara Y, Gronlund M, et al. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature. 2003 Oct 9;425(6958):585–92. 14534578
9. Radutoiu S, Madsen LH, Madsen EB, Jurkiewicz A, Fukai E, Quistgaard EM, et al. LysM domains mediate lipochitin-oligosaccharide recognition and Nfr genes extend the symbiotic host range. The EMBO journal. 2007 Sep 5;26(17):3923–35. 17690687
10. Charpentier M, Bredemeier R, Wanner G, Takeda N, Schleiff E, Parniske M. Lotus japonicus CASTOR and POLLUX are ion channels essential for perinuclear calcium spiking in legume root endosymbiosis. Plant Cell. 2008 Dec;20(12):3467–79. doi: 10.1105/tpc.108.063255 19106374
11. Groth M, Takeda N, Perry J, Uchida H, Draxl S, Brachmann A, et al. NENA, a Lotus japonicus homolog of Sec13, is required for rhizodermal infection by arbuscular mycorrhiza fungi and rhizobia but dispensable for cortical endosymbiotic development. Plant Cell. 2010 Jul;22(7):2509–26. doi: 10.1105/tpc.109.069807 20675572
12. Kanamori N, Madsen LH, Radutoiu S, Frantescu M, Quistgaard EM, Miwa H, et al. A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. P Natl Acad Sci USA. 2006 Jan 10;103(2):359–64. 16407163
13. Saito K, Yoshikawa M, Yano K, Miwa H, Uchida H, Asamizu E, et al. NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell. 2007 Feb;19(2):610–24. 17307929
14. Stracke S, Kistner C, Yoshida S, Mulder L, Sato S, Kaneko T, et al. A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature. 2002 Jun 27;417(6892):959–62. 12087405
15. Madsen LH, Tirichine L, Jurkiewicz A, Sullivan JT, Heckmann AB, Bek AS, et al. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nature communications. 2010;1 : 10. doi: 10.1038/ncomms1009 20975672
16. Miller JB, Pratap A, Miyahara A, Zhou L, Bornemann S, Morris RJ, et al. Calcium/Calmodulin-dependent protein kinase is negatively and positively regulated by calcium, providing a mechanism for decoding calcium responses during symbiosis signaling. Plant Cell. 2013 Dec;25(12):5053–66. doi: 10.1105/tpc.113.116921 24368786
17. Singh S, Katzer K, Lambert J, Cerri M, Parniske M. CYCLOPS, A DNA-Binding Transcriptional Activator, Orchestrates Symbiotic Root Nodule Development. Cell Host Microbe. 2014 Feb 12;15(2):139–52. doi: 10.1016/j.chom.2014.01.011 24528861
18. Desbrosses GJ, Stougaard J. Root nodulation: a paradigm for how plant-microbe symbiosis influences host developmental pathways. Cell Host Microbe. 2011 Oct 20;10(4):348–58. doi: 10.1016/j.chom.2011.09.005 22018235
19. Heckmann AB, Lombardo F, Miwa H, Perry JA, Bunnewell S, Parniske M, et al. Lotus japonicus nodulation requires two GRAS domain regulators, one of which is functionally conserved in a non-legume. Plant Physiol. 2006 Dec;142(4):1739–50. 17071642
20. Murray JD, Karas BJ, Sato S, Tabata S, Amyot L, Szczyglowski K. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science. 2007 Jan 5;315(5808):101–4. 17110535
21. Tirichine L, Sandal N, Madsen LH, Radutoiu S, Albrektsen AS, Sato S, et al. A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science. 2007 Jan 5;315(5808):104–7. 17110537
22. Hossain MS, Liao J, James EK, Sato S, Tabata S, Jurkiewicz A, et al. Lotus japonicus ARPC1 is required for rhizobial infection. Plant Physiol. 2012 Oct;160(2):917–28. doi: 10.1104/pp.112.202572 22864583
23. Tansengco ML, Hayashi M, Kawaguchi M, Imaizumi-Anraku H, Murooka Y. crinkle, a novel symbiotic mutant that affects the infection thread growth and alters the root hair, trichome, and seed development in Lotus japonicus. Plant Physiol. 2003 Mar;131(3):1054–63. 12644658
24. Xie F, Murray JD, Kim J, Heckmann AB, Edwards A, Oldroyd GE, et al. Legume pectate lyase required for root infection by rhizobia. P Natl Acad Sci USA. 2012 Jan 10;109(2):633–8. doi: 10.1073/pnas.1113992109 22203959
25. Yano K, Shibata S, Chen WL, Sato S, Kaneko T, Jurkiewicz A, et al. CERBERUS, a novel U-box protein containing WD-40 repeats, is required for formation of the infection thread and nodule development in the legume-Rhizobium symbiosis. The Plant journal: for cell and molecular biology. 2009 Oct;60(1):168–80. doi: 10.1111/j.1365-313X.2009.03943.x 19508425
26. Yano K, Tansengco ML, Hio T, Higashi K, Murooka Y, Imaizumi-Anraku H, et al. New nodulation mutants responsible for infection thread development in Lotus japonicus. Molecular plant-microbe interactions: MPMI. 2006 Jul;19(7):801–10. 16838792
27. Yokota K, Fukai E, Madsen LH, Jurkiewicz A, Rueda P, Radutoiu S, et al. Rearrangement of actin cytoskeleton mediates invasion of Lotus japonicus roots by Mesorhizobium loti. Plant Cell. 2009 Jan;21(1):267–84. doi: 10.1105/tpc.108.063693 19136645
28. Krusell L, Krause K, Ott T, Desbrosses G, Kramer U, Sato S, et al. The sulfate transporter SST1 is crucial for symbiotic nitrogen fixation in Lotus japonicus root nodules. Plant Cell. 2005 May;17(5):1625–36. 15805486
29. Van de Velde W, Zehirov G, Szatmari A, Debreczeny M, Ishihara H, Kevei Z, et al. Plant peptides govern terminal differentiation of bacteria in symbiosis. Science. [Research Support, Non-U.S. Gov't]. 2010 Feb 26;327(5969):1122–6. doi: 10.1126/science.1184057 20185722
30. D'antuono AL, Casabuono A, Couto A, Ugalde RA, Lepek VC. Nodule development induced by Mesorhizobium loti mutant strains affected in polysaccharide synthesis. Mol Plant Microbe In. 2005 May;18(5):446–57. 15915643
31. Downie JA. The roles of extracellular proteins, polysaccharides and signals in the interactions of rhizobia with legume roots. FEMS microbiology reviews. 2010 Mar;34(2):150–70. doi: 10.1111/j.1574-6976.2009.00205.x 20070373
32. Kelly SJ, Muszynski A, Kawaharada Y, Hubber AM, Sullivan JT, Sandal N, et al. Conditional requirement for exopolysaccharide in the Mesorhizobium-Lotus symbiosis. Molecular plant-microbe interactions: MPMI. 2013 Mar;26(3):319–29. doi: 10.1094/MPMI-09-12-0227-R 23134480
33. Marchetti M, Capela D, Glew M, Cruveiller S, Chane-Woon-Ming B, Gris C, et al. Experimental evolution of a plant pathogen into a legume symbiont. PLoS Biol. [Research Support, Non-U.S. Gov't]. 2010 Jan;8(1):e1000280. doi: 10.1371/journal.pbio.1000280 20084095
34. Okazaki S, Kaneko T, Sato S, Saeki K. Hijacking of leguminous nodulation signaling by the rhizobial type III secretion system. P Natl Acad Sci USA. 2013 Oct 15;110(42):17131–6. doi: 10.1073/pnas.1302360110 24082124
35. Okazaki S, Okabe S, Higashi M, Shimoda Y, Sato S, Tabata S, et al. Identification and functional analysis of type III effector proteins in Mesorhizobium loti. Molecular plant-microbe interactions: MPMI. 2010 Feb;23(2):223–34. doi: 10.1094/MPMI-23-2-0223 20064065
36. Gibson KE, Kobayashi H, Walker GC. Molecular determinants of a symbiotic chronic infection. Annu Rev Genet. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. 2008;42 : 413–41. doi: 10.1146/annurev.genet.42.110807.091427 18983260
37. Jones KM, Sharopova N, Lohar DP, Zhang JQ, VandenBosch KA, Walker GC. Differential response of the plant Medicago truncatula to its symbiont Sinorhizobium meliloti or an exopolysaccharide-deficient mutant. P Natl Acad Sci USA. 2008 Jan 15;105(2):704–9. doi: 10.1073/pnas.0709338105 18184805
38. Lerouge P, Roche P, Faucher C, Maillet F, Truchet G, Prome JC, et al. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–4. 2330031
39. Spaink HP, Sheeley DM, van Brussel AA, Glushka J, York WS, Tak T, et al. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.]. 1991 Nov 14;354(6349):125–30. 1944592
40. Gyaneshwar P, Hirsch AM, Moulin L, Chen WM, Elliott GN, Bontemps C, et al. Legume-nodulating betaproteobacteria: diversity, host range, and future prospects. Molecular plant-microbe interactions: MPMI. 2011 Nov;24(11):1276–88. doi: 10.1094/MPMI-06-11-0172 21830951
41. Lei X, Wang ET, Chen WF, Sui XH, Chen WX. Diverse bacteria isolated from root nodules of wild Vicia species grown in temperate region of China. Arch Microbiol. 2008 Dec;190(6):657–71. doi: 10.1007/s00203-008-0418-y 18704366
42. Li L, Sinkko H, Montonen L, Wei G, Lindstrom K, Rasanen LA. Biogeography of symbiotic and other endophytic bacteria isolated from medicinal Glycyrrhiza species in China. FEMS Microbiol Ecol. [Research Support, Non-U.S. Gov't]. 2012 Jan;79(1):46–68. doi: 10.1111/j.1574-6941.2011.01198.x 22066910
43. Lorite MJ, Donate-Correa J, del Arco-Aguilar M, Perez Galdona R, Sanjuan J, Leon-Barrios M. Lotus endemic to the Canary Islands are nodulated by diverse and novel rhizobial species and symbiotypes. Systematic and applied microbiology. 2010 Aug;33(5):282–90. doi: 10.1016/j.syapm.2010.03.006 20447791
44. Martinez-Romero E. Diversity of Rhizobium-Phaseolus vulgaris symbiosis: overview and perspectives. Plant Soil. 2003 May;252(1):11–23.
45. Muresu R, Polone E, Sulas L, Baldan B, Tondello A, Delogu G, et al. Coexistence of predominantly nonculturable rhizobia with diverse, endophytic bacterial taxa within nodules of wild legumes. FEMS Microbiol Ecol. 2008 Mar;63(3):383–400. doi: 10.1111/j.1574-6941.2007.00424.x 18194345
46. Saidi S, Mnasri B, Mhamdi R. Diversity of nodule-endophytic agrobacteria-like strains associated with different grain legumes in Tunisia. Systematic and applied microbiology. 2011 Nov;34(7):524–30. doi: 10.1016/j.syapm.2011.01.009 21621936
47. Kiers ET, Hutton MG, Denison RF. Human selection and the relaxation of legume defences against ineffective rhizobia. Proceedings Biological sciences / The Royal Society. 2007 Dec 22;274(1629):3119–26. 17939985
48. Terpolilli JJ, Hood GA, Poole PS. What Determines the Efficiency of N-2-Fixing Rhizobium-Legume Symbioses? Adv Microb Physiol. 2012;60 : 325–89. doi: 10.1016/B978-0-12-398264-3.00005-X 22633062
49. Sullivan JT, Ronson CW. Evolution of rhizobia by acquisition of a 500-kb symbiosis island that integrates into a phe-tRNA gene (vol 95, pg 5145, 1998). P Natl Acad Sci USA. 1998 Jul 21;95(15):9059-.
50. Remigi P, Capela D, Clerissi C, Tasse L, Torchet R, Bouchez O, et al. Transient Hypermutagenesis Accelerates the Evolution of Legume Endosymbionts following Horizontal Gene Transfer. PLoS Biol. 2014 Sep;12(9):e1001942. doi: 10.1371/journal.pbio.1001942 25181317
51. Elbeltagy A, Nishioka K, Sato T, Suzuki H, Ye B, Hamada T, et al. Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp isolated from wild rice species. Appl Environ Microb. 2001 Nov;67(11):5285–93. 11679357
52. Rothballer M, Eckert B, Schmid M, Fekete A, Schloter M, Lehner A, et al. Endophytic root colonization of gramineous plants by Herbaspirillum frisingense. FEMS Microbiol Ecol. 2008 Oct;66(1):85–95. doi: 10.1111/j.1574-6941.2008.00582.x 18761671
53. Bulgarelli D, Rott M, Schlaeppi K, Ver Loren van Themaat E, Ahmadinejad N, Assenza F, et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature. 2012 Aug 2;488(7409):91–5. doi: 10.1038/nature11336 22859207
54. Den Herder J, Vanhee C, De Rycke R, Corich V, Holsters M, Goormachtig S. Nod factor perception during infection thread growth fine-tunes nodulation. Mol Plant Microbe In. 2007 Feb;20(2):129–37. 17313164
55. Rodpothong P, Sullivan JT, Songsrirote K, Sumpton D, Cheung KW, Thomas-Oates J, et al. Nodulation gene mutants of Mesorhizobium loti R7A-nodZ and nolL mutants have host-specific phenotypes on Lotus spp. Molecular plant-microbe interactions: MPMI. 2009 Dec;22(12):1546–54. doi: 10.1094/MPMI-22-12-1546 19888820
56. Lehman AP, Long SR. Exopolysaccharides from Sinorhizobium meliloti Can Protect against H2O2-Dependent Damage. J Bacteriol. 2013 Dec;195(23):5362–9. doi: 10.1128/JB.00681-13 24078609
57. Reeve WG, Tiwari RP, Worsley PS, Dilworth MJ, Glenn AR, Howieson JG. Constructs for insertional mutagenesis, transcriptional signal localization and gene regulation studies in root nodule and other bacteria. Microbiol-Uk. 1999 Jun;145 : 1307–16.
58. Mazur A, Krol JE, Skorupska A. Isolation and sequencing of Rhizobium leguminosarum bv. Trifolii PssN, PssO and PssP genes encoding the proteins involved in polymerization and translocation of exopolysaccharide. DNA Sequence. 2001;12(1):1–12. 11697141
59. Skorupska A, Janczarek M, Marczak M, Mazur A, Krol J. Rhizobial exopolysaccharides: genetic control and symbiotic functions. Microbial cell factories. 2006 Feb 16;5.
60. vanWorkum WAT, Cremers HCJC, Wijfjes AHM, vanderKolk C, Wijffelman CA, Kijne JW. Cloning and characterization of four genes of Rhizobium leguminosarum bv trifolii involved in exopolysaccharide production and nodulation. Mol Plant Microbe In. 1997 Mar;10(2):290–301. 9057334
61. James EK, Sprent JI. Development of N2-fixing nodules on the wetland legume Lotus uliginosus exposed to conditions of flooding. New Phytologist. 1999;142(2):219–31.
62. Geddes BA, Oresnik IJ. Inability To Catabolize Galactose Leads to Increased Ability To Compete for Nodule Occupancy in Sinorhizobium meliloti. J Bacteriol. 2012 Sep;194(18):5044–53. doi: 10.1128/JB.00982-12 22797764
63. Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol. 2010 Jan;8(1):15–25. doi: 10.1038/nrmicro2259 19946288
64. Ma LS, Hachani A, Lin JS, Filloux A, Lai EM. Agrobacterium tumefaciens Deploys a Superfamily of Type VI Secretion DNase Effectors as Weapons for Interbacterial Competition In Planta. Cell Host Microbe. 2014 Jul 9;16(1):94–104. doi: 10.1016/j.chom.2014.06.002 24981331
65. Vorholt JA. Microbial life in the phyllosphere. Nat Rev Microbiol. 2012 Dec;10(12):828–40. doi: 10.1038/nrmicro2910 23154261
66. Kiers ET, Rousseau RA, West SA, Denison RF. Host sanctions and the legume-rhizobium mutualism. Nature. 2003 Sep 4;425(6953):78–81. 12955144
67. Relic B, Perret X, Estradagarcia MT, Kopcinska J, Golinowski W, Krishnan HB, et al. Nod Factors of Rhizobium Are a Key to the Legume Door. Mol Microbiol. 1994 Jul;13(1):171–8. 7984092
68. Friesen ML, Mathias A. Mixed infections may promote diversification of mutualistic symbionts: why are there ineffective rhizobia? J Evolution Biol. 2010 Feb;23(2):323–34. doi: 10.1111/j.1420-9101.2009.01902.x 20002933
69. Fujita H, Aoki S, Kawaguchi M. Evolutionary Dynamics of Nitrogen Fixation in the Legume-Rhizobia Symbiosis. PloS one. 2014 Apr 1;9(4).
70. Weyl EG, Frederickson ME, Yu DW, Pierce NE. Economic contract theory tests models of mutualism. P Natl Acad Sci USA. 2010 Sep 7;107(36):15712–6. doi: 10.1073/pnas.1005294107 20733067
71. Brown SD, Utturkar SM, Klingeman DM, Johnson CM, Martin SL, Land ML, et al. Twenty-one genome sequences from Pseudomonas species and 19 genome sequences from diverse bacteria isolated from the rhizosphere and endosphere of Populus deltoides. J Bacteriol. 2012 Nov;194(21):5991–3. doi: 10.1128/JB.01243-12 23045501
72. Hardoim PR, Andreote FD, Reinhold-Hurek B, Sessitsch A, van Overbeek LS, van Elsas JD. Rice root-associated bacteria: insights into community structures across 10 cultivars. FEMS Microbiol Ecol. 2011 Jul;77(1):154–64. doi: 10.1111/j.1574-6941.2011.01092.x 21426364
73. Ikeda S, Okubo T, Anda M, Nakashita H, Yasuda M, Sato S, et al. Community - and genome-based views of plant-associated bacteria: plant-bacterial interactions in soybean and rice. Plant & cell physiology. 2010 Sep;51(9):1398–410.
74. Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, et al. Defining the core Arabidopsis thaliana root microbiome. Nature. 2012 Aug 2;488(7409):86–90. doi: 10.1038/nature11237 22859206
75. Tan Z, Hurek T, Vinuesa P, Muller P, Ladha JK, Reinhold-Hurek B. Specific detection of Bradyrhizobium and Rhizobium strains colonizing rice (Oryza sativa) roots by 16S-23S ribosomal DNA intergenic spacer-targeted PCR. Applied and environmental microbiology. 2001 Aug;67(8):3655–64. 11472944
76. Schlaman HRM, Horvath B, Vijgenboom E, Okker RJH, Lugtenberg BJJ. Suppression of Nodulation Gene-Expression in Bacteroids of Rhizobium-Leguminosarum Biovar Viciae. J Bacteriol. 1991 Jul;173(14):4277–87. 1712355
77. Timmers ACJ, Auriac MC, de Billy F, Truchet G. Nod factor internalization and microtubular cytoskeleton changes occur concomitantly during nodule differentiation in alfalfa. Development. 1998 Feb;125(3):339–49. 9425130
78. Epstein B, Branca A, Mudge J, Bharti AK, Briskine R, Farmer AD, et al. Population Genomics of the Facultatively Mutualistic Bacteria Sinorhizobium meliloti and S. medicae. PLoS genetics. 2012 Aug;8(8).
79. Den Herder G, Parniske M. The unbearable naivety of legumes in symbiosis. Curr Opin Plant Biol. 2009 Aug;12(4):491–9. doi: 10.1016/j.pbi.2009.05.010 19632141
80. Schumpp O, Deakin WJ. How inefficient rhizobia prolong their existence within nodules. Trends Plant Sci. 2010 Apr;15(4):189–95. doi: 10.1016/j.tplants.2010.01.001 20117958
81. Triplett EW, Sadowsky MJ. Genetics of Competition for Nodulation of Legumes. Annu Rev Microbiol. 1992;46 : 399–428. 1444262
82. Yates RJ, Howieson JG, Reeve WG, O'Hara GW. A re-appraisal of the biology and terminology describing rhizobial strain success in nodule occupancy of legumes in agriculture. Plant Soil. 2011 Nov;348(1–2):255–67.
83. Howieson J, Ballard R. Optimising the legume symbiosis in stressful and competitive environments within southern Australia—some contemporary thoughts. Soil Biol Biochem. 2004 Aug;36(8):1261–73.
84. Botha WJ, Jaftha JB, Bloem JF, Habig JH, Law IJ. Effect of soil bradyrhizobia on the success of soybean inoculant strain CB 1809. Microbiol Res. 2004;159(3):219–31. 15462522
85. Streeter JG. Failure of Inoculant Rhizobia to Overcome the Dominance of Indigenous Strains for Nodule Formation. Can J Microbiol. 1994 Jul;40(7):513–22.
86. Vlassak KM, Vanderleyden J. Factors influencing nodule occupancy by inoculant rhizobia. Crit Rev Plant Sci. 1997;16(2):163–229.
87. Alves BJR, Boddey RM, Urquiaga S. The success of BNF in soybean in Brazil. Plant Soil. 2003 May;252(1):1–9.
88. Angus AA, Agapakis CM, Fong S, Yerrapragada S, Estrada-de los Santos P, Yang P, et al. Plant-Associated Symbiotic Burkholderia Species Lack Hallmark Strategies Required in Mammalian Pathogenesis. PloS one. 2014 Jan 8;9(1).
89. Chen WM, James EK, Prescott AR, Kierans M, Sprent JI. Nodulation of Mimosa spp. by the beta-proteobacterium Ralstonia taiwanensis. Molecular plant-microbe interactions: MPMI. 2003 Dec;16(12):1051–61. 14651338
Štítky
Genetika Reprodukční medicína
Článek Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and ThrombocytopeniaČlánek Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of andČlánek Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on PhenotypeČlánek Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human CancersČlánek The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System inČlánek The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological LifespanČlánek The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 6
-
Všechny články tohoto čísla
- Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Orphan Genes Find a Home: Interspecific Competition and Gene Network Evolution
- Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
- Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia
- Leveraging Identity-by-Descent for Accurate Genotype Inference in Family Sequencing Data
- Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
- Epigenetic Aging Signatures Are Coherently Modified in Cancer
- Silencing of DNase Colicin E8 Gene Expression by a Complex Nucleoprotein Assembly Ensures Timely Colicin Induction
- A Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
- The Orphan Gene Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation
- 9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
- The DnaA Protein Is Not the Limiting Factor for Initiation of Replication in
- FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling
- Multiple Changes of Gene Expression and Function Reveal Genomic and Phenotypic Complexity in SLE-like Disease
- Directed Evolution of RecA Variants with Enhanced Capacity for Conjugational Recombination
- The Regulatory T Cell Lineage Factor Foxp3 Regulates Gene Expression through Several Distinct Mechanisms Mostly Independent of Direct DNA Binding
- MreB-Dependent Inhibition of Cell Elongation during the Escape from Competence in
- DNA Damage Regulates Translation through β-TRCP Targeting of CReP
- Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of and
- The Analysis of () Mutants Reveals Differences in the Fusigenic Potential among Telomeres
- The Causative Gene in Chanarian Dorfman Syndrome Regulates Lipid Droplet Homeostasis in .
- Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype
- The . Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units
- AKTIP/Ft1, a New Shelterin-Interacting Factor Required for Telomere Maintenance
- Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
- Transfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
- Connecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
- Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Response to the Formal Letter of Z. Chrzanowska-Lightowlers and R. N. Lightowlers Regarding Our Article “Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers
- Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins
- Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential
- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level
- Genetic Changes to a Transcriptional Silencer Element Confers Phenotypic Diversity within and between Species
- Functional Assessment of Disease-Associated Regulatory Variants Using a Versatile Dual Colour Transgenesis Strategy in Zebrafish
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy
- The Human Blood Metabolome-Transcriptome Interface
- A Common Cancer Risk-Associated Allele in the Locus Encodes a Dominant Negative Inhibitor of Telomerase
- A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
- The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
- The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological Lifespan
- Dissection of a Complex Disease Susceptibility Region Using a Bayesian Stochastic Search Approach to Fine Mapping
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
- Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in
- Abnormal Activation of BMP Signaling Causes Myopathy in Null Mice
- Reproductive Mode and the Evolution of Genome Size and Structure in Nematodes
- Replication and Active Partition of Integrative and Conjugative Elements (ICEs) of the SXT/R391 Family: The Line between ICEs and Conjugative Plasmids Is Getting Thinner
- Motor and Sensory Deficits in the Mice Result from Mutation of the ESCRT Component HGS
- Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
- Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake
- Separable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4
- Multilayered Organization of Jasmonate Signalling in the Regulation of Root Growth
- Necrotic Cells Actively Attract Phagocytes through the Collaborative Action of Two Distinct PS-Exposure Mechanisms
- A Novel Feedback Loop That Controls Bimodal Expression of Genetic Competence
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Human Blood Metabolome-Transcriptome Interface
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



