-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
Telomerase is a highly regulated enzyme whose activity is essential for long-term cellular proliferation. In the presence of DNA double-strand breaks (DSBs), telomerase activity must be curtailed to promote faithful DNA repair. We previously showed that the flowering plant Arabidopsis thaliana rapidly down-regulates telomerase in response to DSBs, and further that this mode of regulation is dependent on TER2, a non-canonical telomerase RNA subunit. Here we demonstrate that the unique regulatory properties of TER2 are conveyed by a transposable element (TE) embedded in the TER2 gene. A comparison of A. thaliana accessions with and without the TE revealed that the element increases the binding affinity of TER2 for the telomerase catalytic subunit TERT relative to the canonical telomerase RNA subunit. The TE also increases TER2 turnover. In response to DSBs, TER2 is induced and accumulates in TERT containing complexes in vivo. Thus, invasion of a TE endows TER2 with a DNA damage sensor to rapidly and reversibly modulate enzyme activity in response to genotoxic stress. These findings provide an example of how exaptation of a TE altered the function of a long noncoding RNA. In this case, a duplicated gene (TER2) was used as the platform, and the TE as the tool to engineer a novel mode of telomerase regulation.
Published in the journal: . PLoS Genet 11(6): e32767. doi:10.1371/journal.pgen.1005281
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005281Summary
Telomerase is a highly regulated enzyme whose activity is essential for long-term cellular proliferation. In the presence of DNA double-strand breaks (DSBs), telomerase activity must be curtailed to promote faithful DNA repair. We previously showed that the flowering plant Arabidopsis thaliana rapidly down-regulates telomerase in response to DSBs, and further that this mode of regulation is dependent on TER2, a non-canonical telomerase RNA subunit. Here we demonstrate that the unique regulatory properties of TER2 are conveyed by a transposable element (TE) embedded in the TER2 gene. A comparison of A. thaliana accessions with and without the TE revealed that the element increases the binding affinity of TER2 for the telomerase catalytic subunit TERT relative to the canonical telomerase RNA subunit. The TE also increases TER2 turnover. In response to DSBs, TER2 is induced and accumulates in TERT containing complexes in vivo. Thus, invasion of a TE endows TER2 with a DNA damage sensor to rapidly and reversibly modulate enzyme activity in response to genotoxic stress. These findings provide an example of how exaptation of a TE altered the function of a long noncoding RNA. In this case, a duplicated gene (TER2) was used as the platform, and the TE as the tool to engineer a novel mode of telomerase regulation.
Introduction
The discovery of long noncoding RNA (lncRNA) has challenged the prevailing paradigm of protein-mediated regulation of gene expression and cell behavior. lncRNAs play essential roles in epigenetic regulation, stem cell biology and signal transduction and are emerging as key targets in human disease [1–3]. Unlike small regulatory RNAs (e.g. miRNAs, siRNAs), lncRNAs are not subjected to purifying selection, and as a consequence they are very poorly conserved, tending to emerge quickly and evolve swiftly [4]. Although transcriptome analyses have uncovered a vast array of lncRNAs, just a tiny fraction of these have an assigned biological function, and fewer still an ascribed molecular mechanism. Little is known about the evolutionary pathways via which lncRNAs gain new functions.
The telomerase RNA subunit TER is a lncRNA and an integral component of the telomerase enzyme. TER functions as template to direct the synthesis of telomeric DNA by the telomerase reverse transcriptase TERT. Telomerase continually synthesizes telomeric DNA in stem and germline cells to avert cellular senescence. Conversely, in cells with limited proliferation programs telomerase activity is repressed, an outcome in vertebrates that may have evolved to avert tumorigenesis [5,6]. Mechanisms of telomerase regulation are varied and complex, and include modulation of telomerase localization, recruitment to the telomere and enzymology at the chromosome terminus [7]. Within the telomerase ribonucleoprotein itself, the major target of enzyme regulation is TERT. However, TER is also implicated in telomerase control. In addition, different isoforms of core telomerase components influence telomerase behavior [8,9].
In conjunction with modulating telomerase action at natural chromosome ends, the enzyme must also be restrained from acting at sites of DNA double-strand breaks (DSBs). Barbara McClintock coined the term “chromosome healing” to describe the acquisition of telomeres on broken chromosomes in maize [10]. Although de novo telomere formation (DNTF) protects the terminus from subsequent repair activities, it leads to loss of the centromere distal chromosome fragment. Thus, DSBs must be sheltered from telomerase action to prevent gross chromosomal rearrangements and loss of heterozygosity. Multiple pathways evolved to prevent the establishment of telomeres at DSBs in yeast [11]. For example, phosphorylation of the Cdc13 telomere binding protein decreases its affinity for DSBs [12]. In addition, the Pif1 helicase is activated by DSBs, resulting in removal of telomerase from DNA [13]. Less is known about how DNTF is repressed in multicellular eukaryotes. In mammals, DSBs trigger TERT phosphorylation leading to decreased telomerase activity [14]. In addition, ionizing radiation causes transient sequestration of TERT in the nucleolus [15]. In Arabidopsis thaliana, a non-canonical TER represses telomerase activity in response to DSBs [16].
TER ranges in size from 150 nt in Tetrahymena to >2 kb in certain fungi, and while the nucleotide sequence is highly variable across species, core secondary and tertiary structures are conserved and essential for TER interaction with TERT and for telomerase catalysis [17–21]. TER is transcriptionally regulated in mammals [22], but the transcript is highly stable with a half-life of several days [23]. Recent data show that that 3’ terminus of Schizosaccharomyces pombe TER is generated by an additional RNA processing step termed slicing, which involves only the first step in mRNA splicing [24,25]. Conventional introns have not been associated with TER.
Arabidopsis thaliana is unusual in that it harbors two TER genes, TER1 (784 nt) and TER2 (748 nt) [26]. Within TER1 and TER2, there are two regions of high similarity spanning ~219 nt termed conserved region 1 (CR1) and conserved region 2 (CR2). In TER2, CR1 and CR2 are separated by a 529 nt intervening sequence. An additional unique 36 nts lie at the 3’ end of the TER2 CR2 termed 3’R. The intervening sequence and 3’R are removed in vivo to create a truncated isoform called TER2S [16]. Sequences flanking the intervening sequence do not adhere to consensus splice donor and acceptor sites, suggesting that removal of this element may not proceed via conventional mRNA splicing.
Although the function of TER2S is unclear, TER1 and TER2 play opposing roles in the control of telomerase enzyme activity. TER1 serves as the canonical telomere repeat template necessary for telomere length maintenance in vivo [26]. Plants deficient in TER1 exhibit progressive telomere shortening, and mutations in the TER1 template alter the telomere repeat sequence in vivo. In contrast, TER2 does not direct telomere repeat incorporation in vivo. Instead, this RNA negatively regulates TER1-mediated enzyme activity. Telomerase activity is elevated in plants lacking TER2, while in plants over-expressing TER2, telomerase activity is decreased and telomeres shorten [16].
TER2 is regulated by DNA damage. Under standard growth conditions, the steady state levels of TER1 and TER2S are similar, and 10-20-fold higher than TER2 [16]. However, in response to DSBs, TER2 is rapidly induced and becomes the predominant TER isoform. The increase in TER2 is coincident with a reduction in telomerase activity. Indeed telomerase inhibition is dependent on TER2: ter2 mutants do not down-regulate telomerase in response to DNA damage [16]. Telomerase repression is not elicited by replication stress or telomere dysfunction, indicating that TER2-mediated telomerase regulation is specific for DSBs and thus may play a role in repressing DNTF. While the mechanism of TER2-mediated telomerase inhibition is not known, TERT has a higher affinity for TER2 than for TER1 or TER2S, and preferentially assembles into TER2 containing RNP complexes in vivo. Therefore, TER2 may serve as a molecular sponge to sequester TERT in a non-functional RNP in response to DSBs [16].
TER is evolving rapidly in Arabidopsis and its relatives. Analysis of sixteen closely related species within the Brassicaceae lineage revealed that these species contain a single locus that bears similarity to the 3’ end of TER1 and the 5’ end of TER2 from A. thaliana [27]. Remarkably, several of these TER-like loci lack a template domain altogether, indicating that a functional TER must be encoded elsewhere in the genome. The intervening sequence associated with A. thaliana TER2 is missing from the TER-like genes of other Brassicaceae. Thus, the appearance of TER2 and its intervening sequence represent recent events likely generated during a massive genome rearrangement that occurred on the branch leading to A. thaliana [28].
In this study we employ a comparative genomics approach to investigate the regulatory function of TER2. Using data acquired from the 1,001 Arabidopsis genomes project, we show that the intervening sequence in TER2 has the characteristics of a solo long terminal repeat (LTR) from a Copia-like retrotransposon. The element is associated with most, but not all of the TER2 loci. We report that the unique regulatory functions of TER2, including its responsiveness to DSBs, are derived from this transposable element. Consequently, invasion of the TER2 locus by a transposon transformed this lncRNA into a highly sensitive DNA damage sensor that modulates telomerase enzyme activity.
Results
The intervening sequence within TER2 is retained in most but not all A. thaliana accessions
Since a clear TER2 ortholog could not be discerned in other members of the Brassicaceae, we analyzed genomic sequence data for different A. thaliana accessions, natural strains of A. thaliana collected from the wild. A. thaliana diverged from its closest relative 10 million years ago [29]. It is estimated that Col-0 and Ler-0, the two best studied A. thaliana accessions, are approximately 200,000 years divergent from one another [30]. We retrieved TER1 and TER2 loci from 853 accessions compiled by the 1001 Arabidopsis genomes project (http://signal.salk.edu/atg1001) and analyzed them for variation against Col-0, the A. thaliana reference genome where a regulatory function for TER2 was first described [16]. The TER1 locus is highly conserved, including the 5’ and 3’ regions flanking CR1 and CR2 (Fig 1A), which lie upstream of the RAD52 coding region or within a predicted intron [27,31]. TER1 exhibits 92% identity across the sequenced accessions, but a few polymorphisms are scattered across the RNA (Fig 1A and 1B, S1A Fig). The most notable variations lie within the TER1 template domain (S2A Fig). A transition of A to C occurred three times while a T-A transversion appeared in 44/853 accessions. In neither instance are the two variations found within the same TER1 gene. Because the A. thaliana TER template is 11 nt in length and encodes one and a half copies of the telomere repeat, these TER1 RNAs retain the potential to direct synthesis of TTTAGGG repeats. More intriguing is the C to T mutation in the middle of the template in Bela-1 (S2A Fig). Whether this variation reflects a sequencing error or indicates that an alternative TER1 locus is present in this accession is unknown.
Fig. 1. Analysis of TER1 and TER2 loci across A. thaliana accessions. 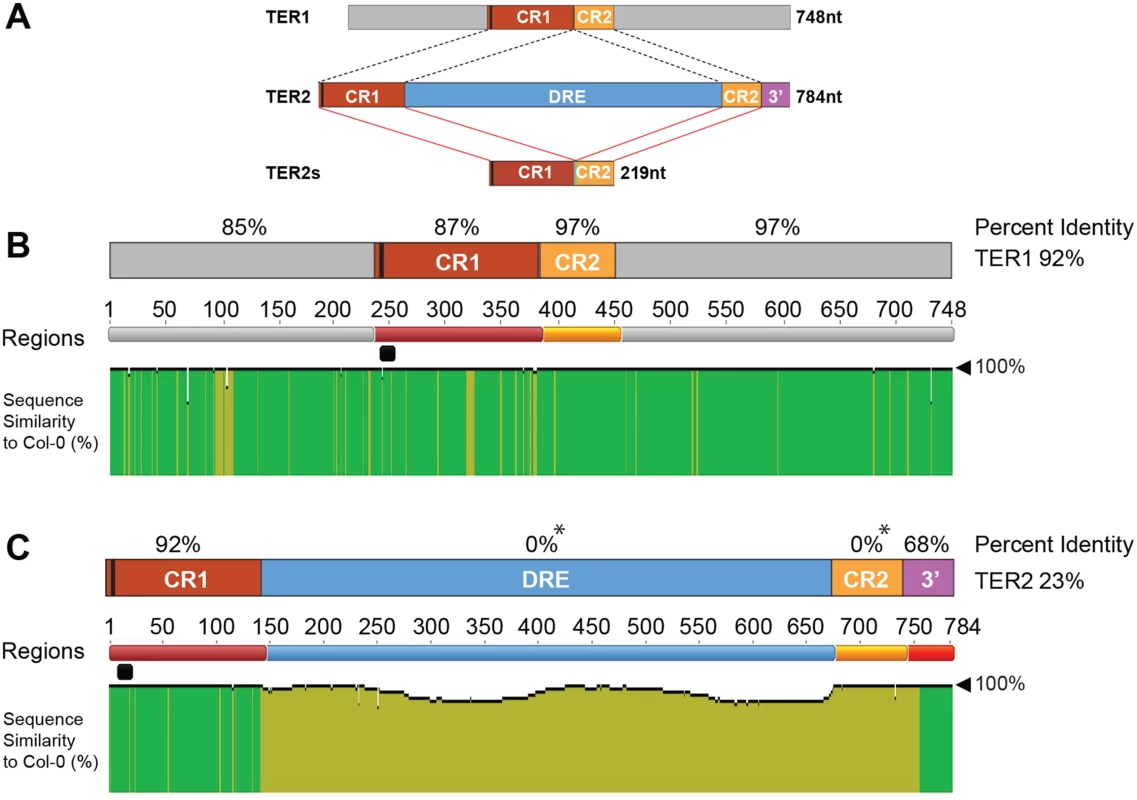
(A) Schematic diagram of TER1, TER2, and TER2S. TER1 and TER2 share a core region of ~219 nt comprised of conserved regions 1 and 2 (CR1 and CR2). The telomere template is denoted by a vertical black bar in CR1. TER2S is formed by splicing to remove the DSB responsive element (DRE) and elimination of the 3’ terminus (3’ R). (B) Analysis of TER1 among 853 A. thaliana accessions. Identity shown in green denotes regions 100% nucleotide similarity whereas mustard yellow indicates variation. There is one colored line for each nucleotide. The height of the bar indicates the degree of variation. Percent identity for each region is denoted in % above each RNA region or for the entire RNA to the right. The telomere template region is indicated by the horizontal black bar. (C) Analysis of TER2 in 853 accessions. Color scheme is the same as in (B). Asterisk indicates that for percent identity to be calculated in a given region, sequence data must be present in all accessions. Sequence was missing for DRE and CR2 for some accessions, and hence these regions are listed as having 0% ID. However, >60% of the accessions were 100% conserved in DRE, and 98% of accessions were 99% conserved in CR2. See S2 Fig for complete alignment of TER2 in 853 accessions. Like TER1 much of TER2 is strongly conserved. CR1 retains high percent identity among the accessions (92%) (Fig 1C). CR2 and the 3’R are also very well conserved with complete conservation in >60% of the accessions analyzed (S3 Fig). Conservation of 3’R was unanticipated since this segment of TER2 is eliminated in the production of TER2S (Fig 1A). Nevertheless, the high degree of conservation in CR1, CR2 and 3’R argues that these regions are important for TER2 function.
Although the intervening sequence within TER2 is completely conserved in more than 60% of the accessions, striking sequence divergence was observed in many of the other accessions. Two islands of conservation with ≥ 50% identity were identified within the intervening sequence, one corresponding to 63 nt and a second of 123 nt (S2B Fig). Hyper-variable sequences flank these regions within the 65 accessions bearing an incomplete intervening sequence. To verify the TER2 sequencing data, we performed PCR genotyping on a sampling of accessions predicted to harbor an intact intervening sequence (Col-0, Ws-2), a partial intervening sequence (Aa-0, Ang-0, Co-1 and Ei-2) or no intervening sequence (Ler-0). PCR primers were positioned within CR1 and 3’R (S4A Fig). A 784 bp PCR product is expected for accessions bearing an intact intervening sequence, a 255 nt product for accessions completely lacking the intervening sequence, and an intermediate size product for accessions with a partial intervening sequence. Products of the expected sizes were obtained for loci predicted to contain an intact or no intervening sequence, but for all TER2 loci predicted to contain a partial intervening sequence, the genotyping results indicated that this element was completely absent (S4B Fig). Genotyping repeated with siblings from accessions predicted to contain a partial intervening sequence gave the same result (S4C Fig). Genotyping was performed on several additional accessions reported to contain a partial intervening sequence (S1 Table). In all cases, the intervening sequence was absent. Finally, PCR products were sequenced from TER1 and TER2 reactions, with TER1 polymorphisms serving as a control to ensure that seed stocks were as expected (S4B and S4D Fig). The sequencing results confirmed the PCR genotyping data. For all partial intervening sequence accessions tested, there was complete loss of this element. The sequencing data also revealed a substantial deletion (~20 bp) within CR2 in two accessions (S4D Fig).
The simplest explanation for these genotyping results is that the TER2 locus was mis-annotated in some of the A. thaliana accessions. However, we cannot exclude the possibility that the intervening sequence within TER2 is extremely labile and between the time the genome sequencing was performed and our acquisition of seeds, partially deleted elements were completely eliminated.
The intervening sequence within TER2 is derived from a Copia-like solo LTR
For reasons discussed below, we named the intervening sequence within TER2 DSB responsive element (DRE). BLAST analyses against the A. thaliana genome using DRE as a query returned two hits, one on the left arm of chromosome 3 (adjacent to At3G30120) bearing 94.6% identity to DRETER2 termed DRE3L, and another on the right arm of chromosome 3 (adjacent to At3G50120) showing 63.4% identity called DRE3R (Fig 2A). Both DRE3L and DRE3R are found within intergenic regions and display a number of single-nucleotide polymorphisms among A. thaliana accessions (S5A Fig).
Fig. 2. The TER2 intervening sequence has the properties of a Copia-like solo LTR. 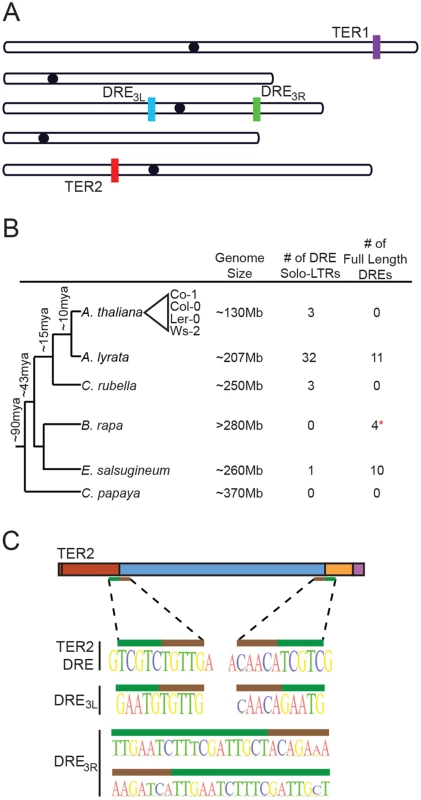
(A) Schematic of the five chromosomes in A. thaliana Col-0 illustrating the locations of TER1, TER2 and DRE on the left arm of chromosome 3 (DRE3L) and the right arm of chromosome 3 (DRE3R) (schematic adapted from TAIR). (B) Phylogenetic tree of select Brassicaceae members (including the Brassicales member Carica papaya). The number of solo and full-length DREs identified by BLAST are shown to the right. Approximate time of divergence was adapted from [29]. Representative A. thaliana accessions re indicated by the triangle. (C) Sequences at the 5’ and 3’ boundary elements of DRE in TER2 (top), DRE3L (middle), and DRE3R (bottom) are shown. Nucleotides within the target site duplication are denoted by the green bar and tandem inverted repeats of DRE are represented by the brown bar. BLAST was performed to determine if the DRE is present in other species within the Brassicaceae family. Arabidopsis lyrata, A. thaliana’s closest relative, contains 32 copies of DRE dispersed throughout the genome (Fig 2B). A significant fraction of these elements exhibit a high degree of similarity within the 5’ 200nt of DRETER2, and are associated with open reading frames encoding typical retrotransposon proteins (S6 Fig). Three DREs were also detected in Capsella rubella, four in Brassica rapa, and ten in Eutrema salsugineum (Fig 2B). The presence of multiple copies of DRE in A. thaliana and its relatives suggests that it is a transposable element (TE). Consistent with this conclusion, sequences at the 5’ and 3’ borders of DRETER2 contain a 5 nt tandem inverted repeat of TGTTG/ACAAC (Fig 2C, brown bar). The tandem inverted repeat at the 5’ and 3’ boundaries of DRETER2 and DRE3L are highly conserved across the A. thaliana accessions and are present at the boundaries of DREs detected in other species (S6 Fig). In addition, a target site duplication of TCGTC is present at the 3’ end of CR1 and the 5’ end of CR2 of TER2 (Fig 2C, green bar). Tandem site duplications flank all three DREs in A. thaliana, ranging in length from 5 nt for DRETER2 and DRE3L to 18nt for DRE3R (Fig 2C, green bar). The tandem site duplication sequence varies, consistent with the hypothesis that these insertions represent unique TE insertion events rather than gene duplications. The small size of DRE and its association with tandem inverted repeats and target site duplications suggest that DRE is derived from a solo LTR of the abundant Copia family. Based on synteny mapping with Arabidopsis lyrata we confirmed that all three Copia-like solo LTRs in A. thaliana (TER2DRE, DRE3L, and DRE3R) are unique insertion events and are of approximately the same age (S7 and S8 Figs). Since the large majority of A. thaliana accessions apparently harbor an intact DRE within the TER2 locus, it is likely that the element was inserted soon after the TER duplication and was subsequently lost in a small subset of accessions.
Differential expression of TER2 and TER2Δ
The presence of two distinct TER2 alleles in A. thaliana provided us with an opportunity to study the functional impact of DRE. We previously showed that two RNA transcripts are derived from the Col-0 TER2 locus: the primary TER2 transcript and a processed isoform, TER2S, in which DRETER2 is removed along with 3’R [16,26]. In the Ler-0 accession, the TER2 locus lacks DRE, and thus the primary transcript is predicted to be TER2Δ. To assay for TER2Δ, RT-PCR was performed on RNA from Ler-0 seedlings using primers directed at CR1 and 3’R, which is unique to TER2 (Fig 3A and 3B). A product of the expected size was generated, indicating that a Ler-0 transcript containing CR1, CR2 and 3’R is present. Sequence analysis confirmed this conclusion. Notably, the CR1/CR2 junction in Ler-0 TER2Δ is distinct from Col-0 TER2S [26] as it contains only a single 5’ TCGTC 3’ motif instead of the two found in Col-0 (Fig 3B bottom, underlined sequence). Although a faint signal for TER2 was observed in Col-0 using our PCR conditions, TER2Δ was not (Fig 3A), suggesting that TER2Δ is either a transient processing intermediate, or is not generated during the conversion of TER2 to TER2S.
Fig. 3. Expression of TER2Δ and association with TERT. 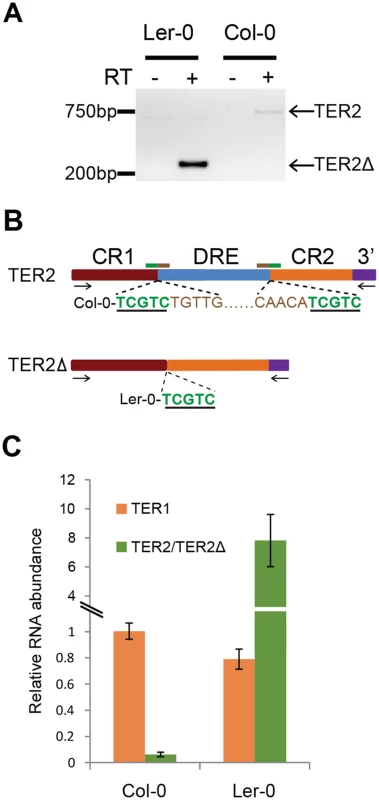
(A) RT-PCR results for TER2Δ in Ler-0 and TER2 in Col-0. Primer positions are indicated by arrows in panel B. (B) Schematic showing sequencing results for TER2 and TER2Δ PCR products from Col-0 and Ler-0 obtained from (A). The target site duplication is indicated by the green underlined nucleotides. Tandem inverted repeats are indicated by brown nucleotides. (C) qPCR results for TER1, TER2 and TER2Δ in Col-0 and Ler-0. For comparison, the Col-0 TER1 level was set to 1. Col-0 TER2 is a poorly expressed transcript (Fig 3A) and is substantially less abundant than TER1 or TER2S [16]. To assess the relative abundance of Ler-0 TER2Δ, we performed qPCR (Fig 3C). The steady state level of TER1 was similar in Ler-0 and Col-0. However, Ler-0 TER2Δ was approximately 6–8 fold more abundant than Ler-0 TER1. By comparison, Col-0 TER2 was 15–20 fold less abundant than Col-0 TER1 (Fig 3C). Thus, Col-0 TER2 and Ler-0 TER2Δ are differentially regulated in vivo.
In Col-0, TER2 but not TER1 or TER2S is rapidly induced by DSBs [16]. Therefore, we asked if regulation is confined to TER2 by examining TER2 and TER2Δ in other A. thaliana accessions (Fig 4A). Seven day-old Ler-0 and Col-0 seedlings were treated with 20μM zeocin and qPCR was performed. In control reactions, BRCA1 mRNA was induced in both accessions after 2 hours and peaked at 4 hours, confirming that a DNA damage response was elicited (Fig 4B). As expected, the level of TER1 was unchanged in Ler-0 and Col-0 following zeocin treatment (S9 Fig). In addition, Col-0 TER2 increased 2.5 fold after 2 hours in zeocin relative to untreated seedlings (Fig 4C). In marked contrast, there was no significant change in TER2Δ over the 4 hour zeocin treatment (Fig 4C).
Fig. 4. DSB-mediated RNA induction and telomerase inhibition are associated with DRE. 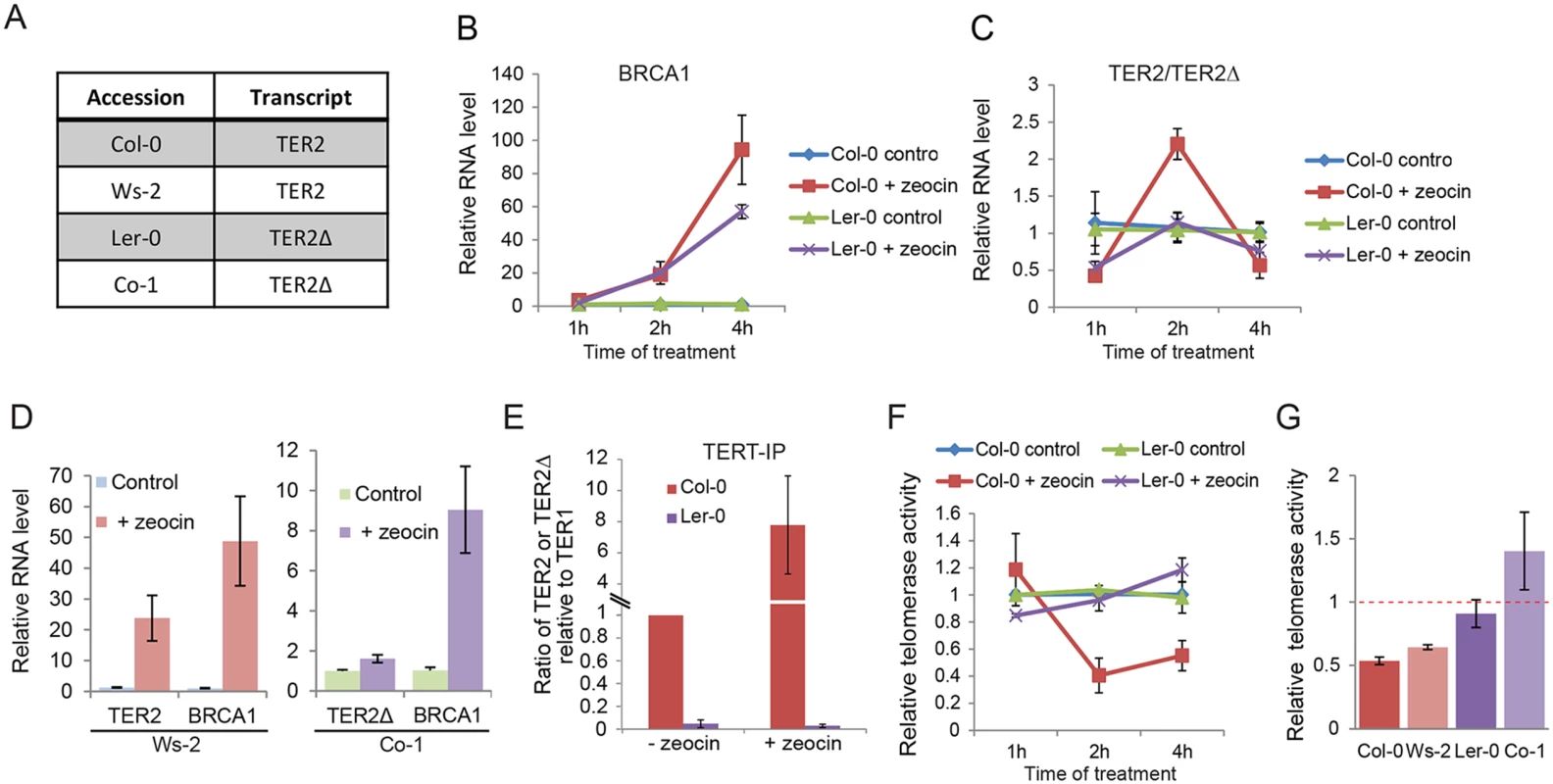
(A) Table indicating the TER2 transcript status for four A. thaliana accessions. (B) and (C) show qPCR results for Col-0 and Ler-0 seedlings treated with zeocin for the time points indicated. Data for the BRCA1 control (B) and TER2 (Col-0) or TER2Δ (Ler-0) transcripts (C) are shown. (D) qPCR results for accessions with TER2 (Ws-2) and TER2Δ (Co-1) submitted to the zeocin regimen for 2 h. (E) qPCR results following TERT immunoprecipitation in Col-0 and Ler-0 seedlings treated with or without zeocin (time point). The TER2:TER1 ratio in Col-0 and the TER2Δ:TER1 ratio in Ler-0 are shown. Values were normalized to Col-0 TER2:TER1 ratio in the absence of zeocin (set to 1). (F) qTRAP results for Col-0 and Ler-0 seedlings with or without zeocin treatment. (G) qTRAP results for the samples in (D) and the 2 h time point from (C). Telomerase activity was normalized to the corresponding untreated controls and set to 1. Red dashed bar indicates no change between treated and untreated samples. The changes in telomerase activity in Col-0 and Ws-2 were statistically significant (p-value< 0.05). Significance was calculated relative to untreated samples using a Student’s t-test. For all experiments, n > 3. To test if DSB-mediated regulation of TER2 is a peculiarity of the Col-0 accession, we examined TER2/TER2Δ transcripts in two additional accessions: Ws-2, which contains DRETER2 and Co-1, which lacks it (Fig 4D). Consistent with the findings in Ler-0 and Col-0, there was no change in Co-1 TER2Δ, while Ws-2 TER2 was induced (Fig 4D). We conclude that the effect of DSBs on TER2 varies across A. thaliana accessions, and correlates with the presence of DRETER2.
We next asked if transcripts were derived from the other two DRE-like sequences in Col-0, and if so whether they responded to DSBs. Semi-quantitative RT-PCR was performed with primers specific for DRE3L and DRE3R on seedlings in the presence or absence of zeocin (S5B Fig). DRE3L transcripts could not be detected under either condition. However, transcripts from DRE3R were observed in the presence of zeocin (S5B Fig), indicating that a DNA damage-sensing element resides within DRETER2 as well as DRE3R
TERT preferentially associates with TER2 over TER2Δ in vivo
We previously showed that Col-0 TERT displays a hierarchy of binding favoring TER2 > TER1 >> TER2S both in vitro and in vivo [16]. The molecular basis for the enhanced affinity of TERT for TER2 is known. Since DRETER2 and the 3’R are unique to TER2, it seems likely that one of these elements influences TERT binding. To investigate this possibility, we examined the relative affinity of TERT for TER2Δ. Col-0 and Ler-0 seedlings were subjected to immunoprecipitation with TERT antibody followed by qPCR (Fig 4E). We set the ratio of TER2 to TER1 in the Col-0 TERT IP to 1, and then assessed the change in TERT-bound TER2 following zeocin treatment. The relative abundance of TER2 containing TERT complexes increased ~ 7-fold in response to DSBs (Fig 4E). Since the input level of TER2 increased by only 2.5-fold under these conditions (Fig 4C), the data raise the interesting possibility that other DNA damage-induced factors promote TER2 assembly with TERT. In marked contrast to TER2, we found that TER2Δ is not a preferred binding partner for TERT in vivo, and further zeocin treatment did not change the relative abundance of TER2Δ containing TERT complexes (Fig 4E). These results argue that the increased affinity of TERT for TER2 in Col-0 reflects the presence of DRETER2 and not 3’R.
Accessions lacking DRETER2 do not exhibit DSB-induced telomerase inhibition
Since Col-0 plants lacking TER2 do not down-regulate telomerase activity in response to DSBs [16], we asked if DSB-induced telomerase regulation is dependent on DRETER2 by comparing the level of telomerase activity in Ler-0 and Col-0 in the presence of zeocin. As expected application of quantitative telomere repeat amplification protocol (qTRAP) to Col-0 seedlings treated with zeocin for 2 or 3 hours showed reduced telomerase activity (70% decrease) compared to untreated seedlings (Fig 4F and S10 Fig). Although there was an alleviation of the inhibitory effect after 3–4 hours of treatment, enzyme activity was maintained at 50% of untreated level. In contrast, under the same treatment regime, telomerase activity was unaltered in Ler-0 (Fig 4F). Similar results were obtained with Ws-2 (plus DRETER2) and Co-1 (minus DRETER2) accessions, respectively (Fig 4G). These findings imply that DRE is necessary for DSB-induced telomerase repression.
To further assess the role of DRE in telomerase regulation, we generated two transgenic A. thaliana lines. First we asked if the presence of TER2 was sufficient to alter the level of telomerase activity in Ler-0 by expressing TER2 from its native promoter in this accession. In one of the transformants, the steady state level of transgenic TER2 was higher (2.5 fold) than the basal level of endogenous TER2 in wild type Col-0 (Fig 5A). qTRAP revealed a small, but statistically significant decrease in telomerase activity in the transformant (Fig 5B), indicating that Ler-0 telomerase can be down-regulated by Col-0 TER2. Next we asked if over-expression of TER2Δ altered telomerase activity in Col-0. TER2Δ expression was driven by the powerful CaMV promoter in wild type Col-0. As expected, there were no change in TER1 or TER2, but the steady state level of transgenic TER2Δ was ~8-fold higher than endogenous TER2Δ in wild type Ler-0. However, qTRAP showed no change in telomerase activity relative to untransformed Col-0 controls (Fig 5A and 5B). We conclude that the regulation of telomerase by TER2 is dependent on DRETER2.
Fig. 5. TER2 not TER2Δ represses telomerase activity. 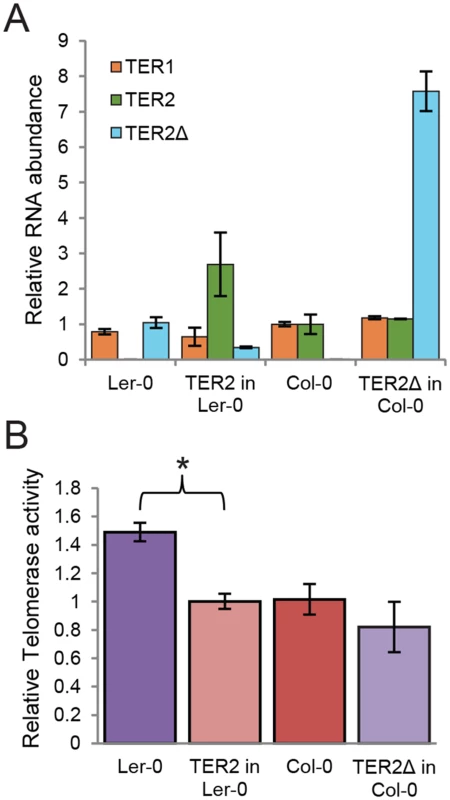
(A) qPCR results are shown for transgenic seedlings expressing TER2 in Ler-0 or TER2Δ in Col-0. TER1 and TER2 levels were normalized to the values in wild type Col-0 (set to 1). TER2Δ was normalized to the value in wild type Ler-0 (set to 1). GAPDH served as a reference gene. (B) qTRAP results are shown for the seedlings analyzed in (A). Relative telomerase activity was normalized to wild type Col-0. The change in telomerase activity in Ler-0 transformants expressing TER2 relative to wild type Ler-0 is statistically significant (p-value<0.005). Significance was calculated relative to untreated samples using a Student’s t-test. For all experiments, n > 3. TER2 is an unstable RNA stabilized in response to DSBs
The rapid induction of Col-0 TER2 in response to DSBs could occur through increased TER2 transcription or increased RNA stability. Because the sequences upstream of all TER2 genes are highly conserved, we considered the former possibility less likely. Indeed, when TER2 transcription was monitored in seedlings expressing a fused GUS reporter to a TER2 or TER2Δ promoter in Col-0 and Ler-0, respectively, approximately the same level of GUS staining was observed in the presence or absence of zeocin (S11 Fig). Hence, TER2 induction in response to DNA damage is not caused by increased transcription.
We assessed TER2 stability using six day-old seedlings treated with the transcription elongation inhibitor cordycepin. TER1 and TER2 RNA levels assessed by qPCR showed that Col-0 and Ler-0 TER1 have similar half-lives, t1/2 = 75 and 84 min, respectively (Fig 6A). The stability of TER2Δ was even greater with t1/2 = 244 min (Fig 6B). TER2, on the other hand, had a much shorter half-life than either TER2Δ or TER1: TER2 t1/2 = 13 min (Fig 6B). Thus, TER2 is an intrinsically unstable transcript.
Fig. 6. TER2 is a labile RNA transcript stabilized by DNA damage. 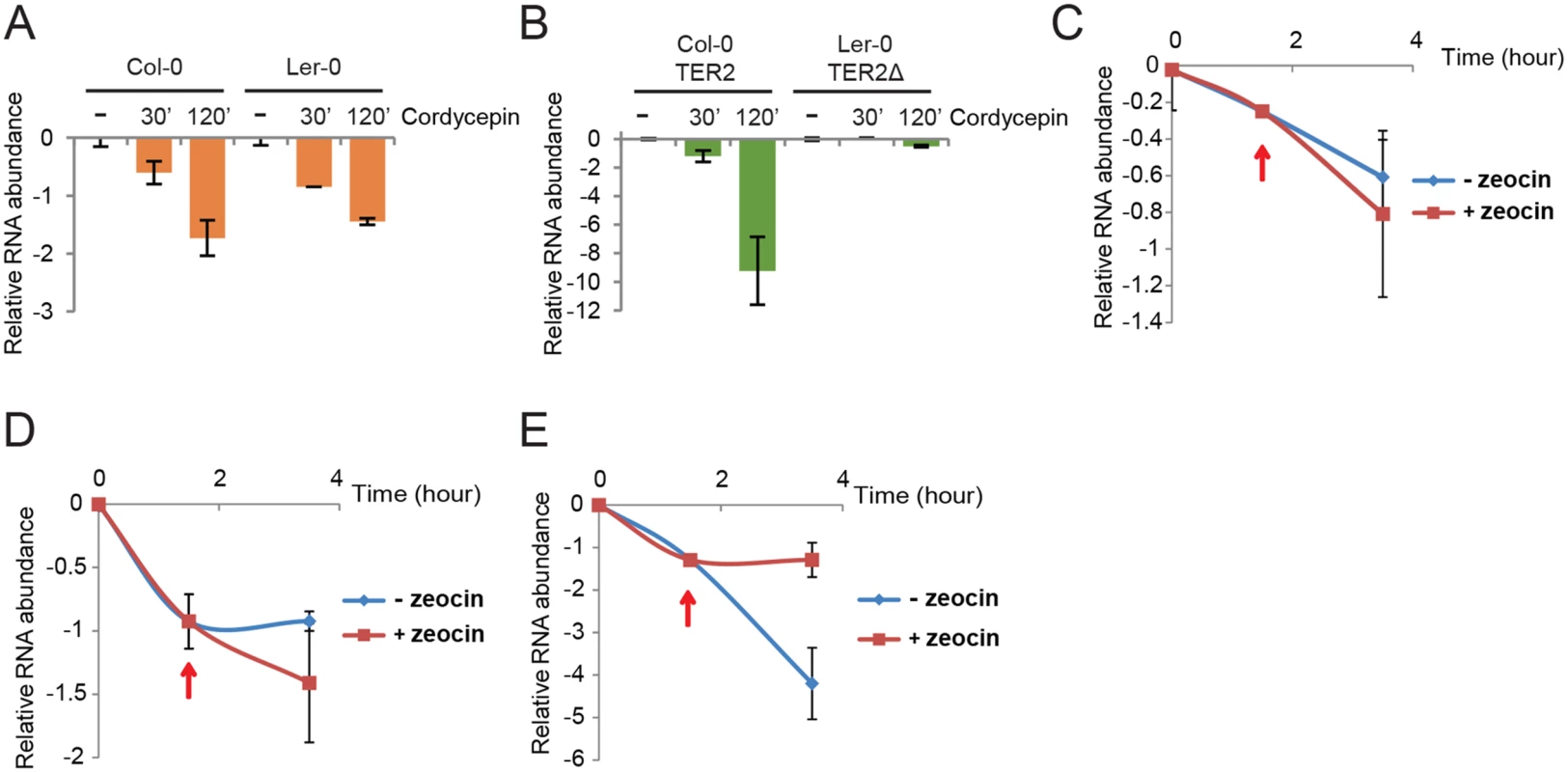
qPCR results are shown for TER1 and TER2/TER2Δ from Col-0 and Ler-0 in the presence of cordycepin. Col-0 and Ler-0 seedlings were treated with cordycepin (100μg/μl) for the times indicated followed by qPCR to monitor TER1 (A) and TER2/ TER2Δ (B). The values obtained for untreated RNA samples were set to 0 and the fold decrease is shown. eIF-4a was used as reference gene for normalization. (C-E) qPCR results from a time course experiment of Col-0 seedlings treated with cordycepin followed by zeocin. Seedlings were incubated with cordycepin for 1.5 h to shut down transcription, and zeocin was added (red arrows). The incubation continued for a total of 3.5 h. Results for BRCA1 (C), TER1 (D), TER2 (E) are shown. To test if DSBs reduce TER2 turnover, Col-0 seedlings were treated with cordycepin to pause transcription and then zeocin was added after 90 min to produce DSBs. Although there was a slight change in the abundance of TER1 and BRCA1 mRNA in the presence of zeocin, this change was not statistically significant (Fig 6C and 6D). In contrast, TER2 abundance declined sharply over the 3.5 hour time course, but immediately after the introduction of zeocin, TER2 was stabilized (Fig 6E). These data implicate DRETER2 as the causal factor in destabilizing TER2 and in turn negatively regulating telomerase activity during bouts of DNA damage.
Discussion
When the insertion of a TE within or adjacent to a gene leads to a change in gene function the process is termed “exaptation” [32]. Exaptation can alter gene regulation through myriad different mechanisms. A prominent example in plants is the insertion of multiple TEs adjacent to teosinte branched1 (tb1), which gave rise to domesticated maize [33]. One of the TEs disrupts a regulatory region of tb1, leading to increased expression and enhanced apical dominance. In vertebrates, exaptation of TEs is more prevalent at lncRNA loci than in protein-coding genes [34]. Approximately 41% of vertebrate lncRNA sequence is derived from TEs [35,36], leading Johnson and Guigo to propose that TEs can behave as pre-formed functional RNA domains, and further that exaptation of TEs is a major driving force in lncRNA evolution [36]. A recent systematic survey in vertebrates catalogued multiple instances of TEs altering lncRNA promoters, splice sites, and polyadenylation sites [37]. LncRNAs can also acquire novel interaction partners as a direct result of TE exaptation [32]. For instance, TEs within XIST facilitate interaction with a host of protein complexes including PRC2 and splicing factor ASF2 [38].
Here we show that invasion of a small TE (DRE) into the A. thaliana TER2 locus profoundly altered the function of this lncRNA (Fig 7). This exaptation event is not fixed, as the TER2 genes in 9% of the 853 accessions examined lack DRE. Insertion and subsequent loss of TEs is not uncommon in Arabidopsis. Some 80% of the annotated TEs in A. thaliana were lost in one or more accessions [39]. In the 200,000 years since Col-0 and Ler-0 diverged, at least 200 TEs have been active, and the unique insertions/deletions between the two accessions have biological implications [30]. One illustrative example of TE exaptation occurred at the Flowering Locus C (FLC) in Ler-0. Insertion of a Mutator-like transposon in this accession decreased FLC transcription, causing early flowering [40]. In this study we exploited the natural genetic heterogeneity within the TER2 locus, and discovered that many of the unique functions ascribed to this lncRNA derive from DRE.
Fig. 7. Model for exaptation of a TE into TER2 and the emergence of a telomerase regulatory lncRNA. 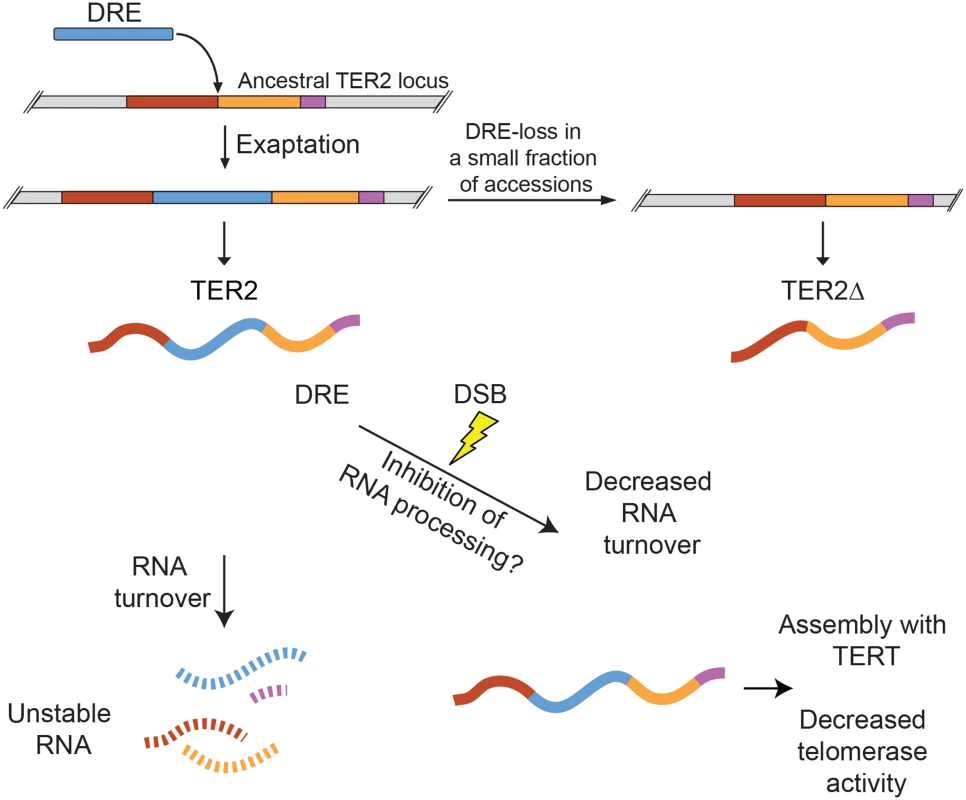
Duplication of the single copy ancestral TER gene was followed soon thereafter by exaptation of DRE into the A. thaliana TER2 locus. The majority of A. thaliana accessions retain DRE (e.g. Col-0), but a small subset lost it (e.g. Ler-0). TER2Δ is produced by accessions lacking DRE. DRE acts as a post-transcriptional sensor that modulates TER2 abundance in response to DNA damage. Under normal physiological conditions, TER2 is an unstable RNA. However, in the presence of DSBs, TER2 is rapidly induced. Whether this is due to direct RNA stabilization or inhibition of TER2 processing (to yield TER2s) is unknown. TER2 has a higher affinity for TERT than TER1 or TER2Δ and following induction by DNA damage accumulates in TERT containing complexes in vivo. TER2-mediated telomerase inhibition may reflect competitive inhibition of TER1 for TERT. The transient decrease in telomerase activity may promote DSB repair rather than de novo telomere formation, thereby stabilizing the genome. First, DRE destabilizes TER2. A survey of ~800 lncRNAs in mouse revealed that only a small fraction are unstable, defined as RNAs with a half-life of less than 60 minutes [41]. By this criterion, TER2 is a highly unstable transcript with a half-life of only 13 minutes (Fig 7). TER1 (t1/2 = 80 min) and TER2Δ (t1/2 = 240 min), on the other hand, are categorized as stable RNAs. Unstable lncRNAs, like their unstable mRNA counterparts, are typically associated with regulatory functions, while stable RNAs are thought to serve housekeeping roles [42]. With Col-0 A. thaliana TER1 and TER2, this paradigm also holds.
A second key observation is that the instability of TER2 arising from DRE is reversed in response to DNA damage (Fig 7). The abundance of TER2, but not TER1 or TER2Δ is elevated in response to DSBs, and this change is largely, if not entirely, dependent on RNA stabilization rather than new transcription. Exaptation of a TE is known to endow host genes with the capacity to respond to environmental cues. For example, a cold-sensitive TE was inserted into the promoter of Ruby, a transcription factor that regulates flesh color in Citrus sinensis (blood orange). Cold activates the transposon, which in turn activates Ruby and downstream anthocyanin production [43]. In the case of TER2, DRE imparts DNA damage sensitivity, which increases TER2 abundance. How TER2 is regulated in response to DSBs is unknown. One possibility is that DRE carries binding sites for one or more interaction partners responsive to DNA damage, which then stabilize TER2. RNA binding proteins can play a significant role in the DNA damage response by regulating specific target genes post-transcriptionally [44]. TER2 turnover might be controlled through the small RNA regulatory pathway. A 24 nt RNA is associated with DRETER2 [45]. This finding is particularly intriguing given the recent discovery that small RNAs modulate the response to DSBs in both vertebrates and Arabidopsis [46]. Finally, it is possible that DNA damage blocks the RNA processing steps (e.g. splicing) that lead to production of TER2S (Fig 7). Splicing machinery has emerged as a target of the DDR [47].
The third key observation from this work is that DRE increases the affinity of TER2 for TERT (Fig 7), and correlates with the down-regulation of telomerase activity. DRE could modify TER2 structure in a manner that enhances its inherit affinity for TERT. Alternatively, DRE may make independent contacts with TERT to increase TER2 affinity. Intriguingly, zeocin treatment causes an even greater enrichment of TER2 containing TERT complexes than expected based on the fold induction of TER2, suggesting that a TER2 associated factor that is also responsive to DNA damage might drive the assembly of TER2-TERT RNPs.
Altogether, our data are consistent with a model in which exaptation of a TE into the A. thaliana TER2 locus gave rise to a new mode of telomerase regulation. Specifically, we propose that the DRE converted TER2 into a DNA damage sensor that controls telomerase enzyme activity through sequestration of TERT. Furthermore, because this regulatory pathway is regulated by changes in RNA stability, it is both rapidly responsive and reversible, allowing the A. thaliana accessions that carry DRE to fine-tune telomerase activity when the plant is under genome assault. These discoveries provide a fresh perspective on the role of TE exaptation in shaping lncRNA function and evolution.
Materials and Methods
Plant material, growth conditions and transformation
For experiments with seedlings, seeds from different accessions (Col-0, Ler-0, Ws-2, etc) were sterilized in 50% bleach with 0.1% Triton X-100 and then stored in 4°C for 2–4 days. Liquid Murashige and Skoog (MS) medium were used for germination and growing [16]. After transferring cold-treated seeds to MS, plants were grown at 22°C under long day light condition for ~7 days. The Col-0 TER2 gene including 3kb upstream sequence and 300bp downstream sequence was cloned in the pMDC99 vector for transformation in the Ler-0 background. Hygromycin MS plates were used for selection. For Col-0 transformation, TER2Δ together with 300 bp downstream flanking region was cloned into the pBA002 vector with 35S promoter. BASTA MS plates were used for the selection.
Sequence acquisition and analysis
Sequences corresponding to TER2 (Genbank accession number: HQ401285.1) were obtained using the genome browser at http://signal.salk.edu/atg1001. The search query AT5G24660 was used to pinpoint the region of interest, and all available tracks (accessions) were selected. Two sequences were removed from our analysis. Hov 3–2 was removed because it was the only accession with two deletions in the 5’ end, corresponding to 20 nt from the 5’ start of TER2, and a 100 nt deletion starting at nucleotide #101. The template region was not disturbed in this accession, possibly indicating a functional TER2 is generated. The Tottarp-2 accession was removed because the sequence corresponding to our search region did not contain sequences corresponding to TER2, most importantly, a template region.
Sequences were trimmed in MEGA5, and then analyzed using Geneious v6.0 (Biomatters). Sequence conservation and alignments were performed using Geneious. DRE-like sequences were obtained by BLAST searches of the A. thaliana (www.arabidopsis.org), A. lyrata, Capsella rubella, Brassica rapa, and Thellungiella halophila genomes accessed via www.phytozome.net v9.1 [48,49].
DNA damage treatment and assays
A. thaliana seedlings (5–7 day old) were transferred to fresh MS liquid medium with 20 μM zeocin (Invitrogen) as described [16]. Seedlings were kept in the dark with gentle agitation for 1, 2 or 4 h. Multiple seedlings were combined and flash frozen in liquid nitrogen for RNA extraction or protein extraction for TRAP. The combined sample was treated as a single biological replicate.
Nucleic acid extraction, genotyping and PCR
DNA samples were prepared from the leaves of different accessions. Both TER1 and TER2 loci were used for genotyping. PCR samples were resolved in 1% agrose and gel purified and sequenced. RNA was extracted from seedlings using the Direct-zol RNA MiniPrep kit (Zymo Research, Epigenetics) according to the manufacturer’s instructions. 1 μg total RNA was used for preparing cDNA. For RT-PCR, cDNA was synthesized by SuperscriptIII Reverse Transcriptase (Invitrogen) using random primers. For qRT-PCR, reverse transcription was performed using the Superscript cDNA master mix (Quanta), according to the manufacturer’s instructions. 1 : 5 diluted cDNA was used for qPCR. qPCR was performed on a Bio-Rad CFX-1000 using the following primers: qTER2Δ F: 5’-AGAACGTTGACGGCTAAAGG-3’; qTER2Δ R: 5’ - TGTGGCATAAGGCAAACTGA-3’; TER2, BRCA1, TER1 and GAPDH primers are used as described before [16]. Data were analyzed using Bio-Rad’s CFX manager software. ΔΔCT values were obtained by comparing against GAPDH levels.
qTRAP and immunoprecipitation (IP) qRT-PCR
qTRAP assays were performed as described [50]. Data were normalized against untreated Col-0. For immunoprecipitation, TERT antibody [50] was conjugated with Dynabeads Protein A (Invitrogen) then incubated with protein extracts in 4°C. RNA was recovered from the IP sample using phenol/chloroform followed by ethanol precipitation [16]. qPCR was performed on TER1 and TER2/TER2Δ. The ΔCT value was used to determine the relative level of TER2 or TER2Δ against TER1.
RNA stability assays
5–7 day old seedlings were treated with cordycepin (100 ng/μl as a working concentration) for 2 h before RNA extraction. RNA was analyzed by qPCR normalized to eIF-4a [51]. RNA abundance was converted to the decreased level relative to untreated. RNA half-life was determined by the absolute value of inverse of the slope of the equation plotted by untreated and treated data. For the combined cordycepin/zeocin experiment, seedlings were pre-incubated with cordycepin for 1.5 h followed by zeocin and the incubation was continued for 2 h. RNA extraction and qPCR were used to determine RNA abundance. RNA half-life was determined by plotting RNA abundance versus time as described in [51].
GUS staining
3 kb of sequence upstream of the TER2 5’ terminus was cloned in a GUS reporter vector pMDC163. The construct was transformed into A. thaliana Col-0 and Ler-0 as described [52]. After selection in hygromycin, transformants seedlings were treated with zeocin for 2 h and then subjected to GUS histochemical staining as described [53].
Supporting Information
Zdroje
1. Lee JT, Strauss WM, Dausman JA, Jaenisch R (1996) A 450 kb transgene displays properties of the mammalian X-inactivation center. Cell 86 : 83–94. 8689690
2. Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, et al. (2011) lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 477 : 295–300. doi: 10.1038/nature10398 21874018
3. Scheuermann JC, Boyer LA (2013) Getting to the heart of the matter: long non-coding RNAs in cardiac development and disease. EMBO J 32 : 1805–1816. doi: 10.1038/emboj.2013.134 23756463
4. Ponting CP, Oliver PL, Reik W (2009) Evolution and functions of long noncoding RNAs. Cell 136 : 629–641. doi: 10.1016/j.cell.2009.02.006 19239885
5. Gunes C, Rudolph KL (2013) The role of telomeres in stem cells and cancer. Cell 152 : 390–393. doi: 10.1016/j.cell.2013.01.010 23374336
6. Bernardes de Jesus B, Blasco MA (2013) Telomerase at the intersection of cancer and aging. Trends Genet 29 : 513–520. doi: 10.1016/j.tig.2013.06.007 23876621
7. Cifuentes-Rojas C, Shippen DE (2012) Telomerase regulation. Mutat Res 730 : 20–27. doi: 10.1016/j.mrfmmm.2011.10.003 22032831
8. Karamysheva Z, Wang L, Shrode T, Bednenko J, Hurley LA, et al. (2003) Developmentally programmed gene elimination in Euplotes crassus facilitates a switch in the telomerase catalytic subunit. Cell 113 : 565–576. 12787498
9. Wong MS, Wright WE, Shay JW (2014) Alternative splicing regulation of telomerase: a new paradigm? Trends Genet 30 : 430–438. doi: 10.1016/j.tig.2014.07.006 25172021
10. McClintock B (1941) The stability of broken ends of chromosomes in Zea mays. Genetics 26 : 234–282. 17247004
11. Ribeyre C, Shore D (2013) Regulation of telomere addition at DNA double-strand breaks. Chromosoma 122 : 159–173. doi: 10.1007/s00412-013-0404-2 23504035
12. Zhang W, Durocher D (2010) De novo telomere formation is suppressed by the Mec1-dependent inhibition of Cdc13 accumulation at DNA breaks. Genes Dev 24 : 502–515. doi: 10.1101/gad.1869110 20194442
13. Makovets S, Blackburn EH (2009) DNA damage signalling prevents deleterious telomere addition at DNA breaks. Nat Cell Biol 11 : 1383–1386. doi: 10.1038/ncb1985 19838171
14. Kharbanda S, Kumar V, Dhar S, Pandey P, Chen C, et al. (2000) Regulation of the hTERT telomerase catalytic subunit by the c-Abl tyrosine kinase. Curr Biol 10 : 568–575. 10837221
15. Wong JM, Kusdra L, Collins K (2002) Subnuclear shuttling of human telomerase induced by transformation and DNA damage. Nat Cell Biol 4 : 731–736. 12198499
16. Cifuentes-Rojas C, Nelson AD, Boltz KA, Kannan K, She X, et al. (2012) An alternative telomerase RNA in Arabidopsis modulates enzyme activity in response to DNA damage. Genes Dev 26 : 2512–2523. doi: 10.1101/gad.202960.112 23109676
17. Romero DP, Blackburn EH (1991) A conserved secondary structure for telomerase RNA. Cell 67 : 343–353. 1840508
18. Chen JL, Blasco MA, Greider CW (2000) Secondary structure of vertebrate telomerase RNA. Cell 100 : 503–514. 10721988
19. Tzfati Y, Knight Z, Roy J, Blackburn EH (2003) A novel pseudoknot element is essential for the action of a yeast telomerase. Genes Dev 17 : 1779–1788. 12832393
20. Chappell AS, Lundblad V (2004) Structural elements required for association of the Saccharomyces cerevisiae telomerase RNA with the Est2 reverse transcriptase. Mol Cell Biol 24 : 7720–7736. 15314178
21. Qi X, Li Y, Honda S, Hoffmann S, Marz M, et al. (2013) The common ancestral core of vertebrate and fungal telomerase RNAs. Nucleic Acids Res 41 : 450–462. doi: 10.1093/nar/gks980 23093598
22. Cairney CJ, Keith WN (2008) Telomerase redefined: integrated regulation of hTR and hTERT for telomere maintenance and telomerase activity. Biochimie 90 : 13–23. 17854971
23. Yi X, Tesmer VM, Savre-Train I, Shay JW, Wright WE (1999) Both transcriptional and posttranscriptional mechanisms regulate human telomerase template RNA levels. Mol Cell Biol 19 : 3989–3997. 10330139
24. Box JA, Bunch JT, Tang W, Baumann P (2008) Spliceosomal cleavage generates the 3' end of telomerase RNA. Nature 456 : 910–914. doi: 10.1038/nature07584 19052544
25. Tang W, Kannan R, Blanchette M, Baumann P (2012) Telomerase RNA biogenesis involves sequential binding by Sm and Lsm complexes. Nature 484 : 260–264. doi: 10.1038/nature10924 22446625
26. Cifuentes-Rojas C, Kannan K, Tseng L, Shippen DE (2011) Two RNA subunits and POT1a are components of Arabidopsis telomerase. Proc Natl Acad Sci U S A 108 : 73–78. doi: 10.1073/pnas.1013021107 21164032
27. Beilstein MA, Brinegar AE, Shippen DE (2012) Evolution of the Arabidopsis telomerase RNA. Front Genet 3 : 188. 23015808
28. Lysak MA, Koch MA, Beaulieu JM, Meister A, Leitch IJ (2009) The dynamic ups and downs of genome size evolution in Brassicaceae. Mol Biol Evol 26 : 85–98. doi: 10.1093/molbev/msn223 18842687
29. Beilstein MA, Nagalingum NS, Clements MD, Manchester SR, Mathews S (2010) Dated molecular phylogenies indicate a Miocene origin for Arabidopsis thaliana. Proc Natl Acad Sci U S A 107 : 18724–18728. doi: 10.1073/pnas.0909766107 20921408
30. Ziolkowski PA, Koczyk G, Galganski L, Sadowski J (2009) Genome sequence comparison of Col and Ler lines reveals the dynamic nature of Arabidopsis chromosomes. Nucleic Acids Res 37 : 3189–3201. doi: 10.1093/nar/gkp183 19305000
31. Samach A, Melamed-Bessudo C, Avivi-Ragolski N, Pietrokovski S, Levy AA (2011) Identification of plant RAD52 homologs and characterization of the Arabidopsis thaliana RAD52-like genes. Plant Cell 23 : 4266–4279. doi: 10.1105/tpc.111.091744 22202891
32. de Souza FS, Franchini LF, Rubinstein M (2013) Exaptation of transposable elements into novel cis-regulatory elements: is the evidence always strong? Mol Biol Evol 30 : 1239–1251. doi: 10.1093/molbev/mst045 23486611
33. Studer A, Zhao Q, Ross-Ibarra J, Doebley J (2011) Identification of a functional transposon insertion in the maize domestication gene tb1. Nat Genet 43 : 1160–1163. doi: 10.1038/ng.942 21946354
34. Sela N, Mersch B, Hotz-Wagenblatt A, Ast G (2010) Characteristics of transposable element exonization within human and mouse. PLoS One 5: e10907. doi: 10.1371/journal.pone.0010907 20532223
35. Kelley D, Rinn J (2012) Transposable elements reveal a stem cell-specific class of long noncoding RNAs. Genome Biol 13: R107. doi: 10.1186/gb-2012-13-11-r107 23181609
36. Johnson R, Guigo R (2014) The RIDL hypothesis: transposable elements as functional domains of long noncoding RNAs. RNA 20 : 959–976. doi: 10.1261/rna.044560.114 24850885
37. Kapusta A, Kronenberg Z, Lynch VJ, Zhuo X, Ramsay L, et al. (2013) Transposable elements are major contributors to the origin, diversification, and regulation of vertebrate long noncoding RNAs. PLoS Genet 9: e1003470. doi: 10.1371/journal.pgen.1003470 23637635
38. Wutz A, Rasmussen TP, Jaenisch R (2002) Chromosomal silencing and localization are mediated by different domains of Xist RNA. Nat Genet 30 : 167–174. 11780141
39. Cao J, Schneeberger K, Ossowski S, Gunther T, Bender S, et al. (2011) Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat Genet 43 : 956–963. doi: 10.1038/ng.911 21874002
40. Gazzani S, Gendall AR, Lister C, Dean C (2003) Analysis of the molecular basis of flowering time variation in Arabidopsis accessions. Plant Physiol 132 : 1107–1114. 12805638
41. Clark MB, Johnston RL, Inostroza-Ponta M, Fox AH, Fortini E, et al. (2012) Genome-wide analysis of long noncoding RNA stability. Genome Res 22 : 885–898. doi: 10.1101/gr.131037.111 22406755
42. Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, et al. (2011) Global quantification of mammalian gene expression control. Nature 473 : 337–342. doi: 10.1038/nature10098 21593866
43. Butelli E, Licciardello C, Zhang Y, Liu J, Mackay S, et al. (2012) Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in blood oranges. Plant Cell 24 : 1242–1255. doi: 10.1105/tpc.111.095232 22427337
44. Dutertre M, Lambert S, Carreira A, Amor-Gueret M, Vagner S (2014) DNA damage: RNA-binding proteins protect from near and far. Trends Biochem Sci 39 : 141–149. doi: 10.1016/j.tibs.2014.01.003 24534650
45. Rajagopalan R, Vaucheret H, Trejo J, Bartel DP (2006) A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev 20 : 3407–3425. 17182867
46. Wei W, Ba Z, Gao M, Wu Y, Ma Y, et al. (2012) A role for small RNAs in DNA double-strand break repair. Cell 149 : 101–112. doi: 10.1016/j.cell.2012.03.002 22445173
47. Lenzken SC, Loffreda A, Barabino SM (2013) RNA splicing: a new player in the DNA damage response. Int J Cell Biol 2013 : 153634. doi: 10.1155/2013/153634 24159334
48. Hu TT, Pattyn P, Bakker EG, Cao J, Cheng JF, et al. (2011) The Arabidopsis lyrata genome sequence and the basis of rapid genome size change. Nat Genet 43 : 476–481. doi: 10.1038/ng.807 21478890
49. Cheng F, Liu S, Wu J, Fang L, Sun S, et al. (2011) BRAD, the genetics and genomics database for Brassica plants. BMC Plant Biol 11 : 136. doi: 10.1186/1471-2229-11-136 21995777
50. Kannan K, Nelson AD, Shippen DE (2008) Dyskerin is a component of the Arabidopsis telomerase RNP required for telomere maintenance. Mol Cell Biol 28 : 2332–2341. doi: 10.1128/MCB.01490-07 18212040
51. Golisz A, Sikorski PJ, Kruszka K, Kufel J (2013) Arabidopsis thaliana LSM proteins function in mRNA splicing and degradation. Nucleic Acids Res 41 : 6232–6249. doi: 10.1093/nar/gkt296 23620288
52. Zhang X, Henriques R, Lin SS, Niu QW, Chua NH (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1 : 641–646. 17406292
53. Pecinka A, Rosa M, Schikora A, Berlinger M, Hirt H, et al. (2009) Transgenerational stress memory is not a general response in Arabidopsis. PLoS One 4: e5202. doi: 10.1371/journal.pone.0005202 19381297
Štítky
Genetika Reprodukční medicína
Článek Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and ThrombocytopeniaČlánek Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of andČlánek Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on PhenotypeČlánek Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human CancersČlánek A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic BacteriaČlánek The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System inČlánek The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological LifespanČlánek The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 6
-
Všechny články tohoto čísla
- Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Orphan Genes Find a Home: Interspecific Competition and Gene Network Evolution
- Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
- Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia
- Leveraging Identity-by-Descent for Accurate Genotype Inference in Family Sequencing Data
- Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
- Epigenetic Aging Signatures Are Coherently Modified in Cancer
- Silencing of DNase Colicin E8 Gene Expression by a Complex Nucleoprotein Assembly Ensures Timely Colicin Induction
- A Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
- The Orphan Gene Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation
- 9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
- The DnaA Protein Is Not the Limiting Factor for Initiation of Replication in
- FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling
- Multiple Changes of Gene Expression and Function Reveal Genomic and Phenotypic Complexity in SLE-like Disease
- Directed Evolution of RecA Variants with Enhanced Capacity for Conjugational Recombination
- The Regulatory T Cell Lineage Factor Foxp3 Regulates Gene Expression through Several Distinct Mechanisms Mostly Independent of Direct DNA Binding
- MreB-Dependent Inhibition of Cell Elongation during the Escape from Competence in
- DNA Damage Regulates Translation through β-TRCP Targeting of CReP
- Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of and
- The Analysis of () Mutants Reveals Differences in the Fusigenic Potential among Telomeres
- The Causative Gene in Chanarian Dorfman Syndrome Regulates Lipid Droplet Homeostasis in .
- Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype
- The . Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units
- AKTIP/Ft1, a New Shelterin-Interacting Factor Required for Telomere Maintenance
- Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
- Transfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
- Connecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
- Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Response to the Formal Letter of Z. Chrzanowska-Lightowlers and R. N. Lightowlers Regarding Our Article “Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers
- Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins
- Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential
- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level
- Genetic Changes to a Transcriptional Silencer Element Confers Phenotypic Diversity within and between Species
- Functional Assessment of Disease-Associated Regulatory Variants Using a Versatile Dual Colour Transgenesis Strategy in Zebrafish
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy
- The Human Blood Metabolome-Transcriptome Interface
- A Common Cancer Risk-Associated Allele in the Locus Encodes a Dominant Negative Inhibitor of Telomerase
- A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
- The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
- The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological Lifespan
- Dissection of a Complex Disease Susceptibility Region Using a Bayesian Stochastic Search Approach to Fine Mapping
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
- Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in
- Abnormal Activation of BMP Signaling Causes Myopathy in Null Mice
- Reproductive Mode and the Evolution of Genome Size and Structure in Nematodes
- Replication and Active Partition of Integrative and Conjugative Elements (ICEs) of the SXT/R391 Family: The Line between ICEs and Conjugative Plasmids Is Getting Thinner
- Motor and Sensory Deficits in the Mice Result from Mutation of the ESCRT Component HGS
- Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
- Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake
- Separable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4
- Multilayered Organization of Jasmonate Signalling in the Regulation of Root Growth
- Necrotic Cells Actively Attract Phagocytes through the Collaborative Action of Two Distinct PS-Exposure Mechanisms
- A Novel Feedback Loop That Controls Bimodal Expression of Genetic Competence
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Human Blood Metabolome-Transcriptome Interface
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



