-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDNA Damage Regulates Translation through β-TRCP Targeting of CReP
Approximately 600 human genes encode enzymes that act as ubiquitin ligases, which facilitate the transfer of the small protein ubiquitin to thousands of substrate proteins; “tagging” with ubiquitin often promotes the degradation of the substrate by the proteasome. In this paper, we adapt a technique called Ligase Trapping for use in mammalian cells. Ligase Trapping is a highly accurate method for determining which substrates are targeted by a ubiquitin ligase. Here we use it to identify new substrates of the human cell cycle regulator βTRCP. Our screen was indeed highly accurate, as we were able to validate 88% of the candidate substrates we identified by mass spectrometry. Some of these new substrates were unstable proteins that were stabilized by inhibition of βTRCP, or of the entire class of ubiquitin ligases of which βTRCP is a part. However, others appear to be stable or redundantly-targeted substrates, which have been more difficult to identify with current techniques. This suggests that Ligase Trapping will be able to reliably identify new substrates of human ubiquitin ligases. Further, one of the new βTRCP substrates, CReP, is specifically depleted upon DNA damage, and depletion of CReP contributes to inactivation of the translational machinery upon DNA damage.
Published in the journal: . PLoS Genet 11(6): e32767. doi:10.1371/journal.pgen.1005292
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005292Summary
Approximately 600 human genes encode enzymes that act as ubiquitin ligases, which facilitate the transfer of the small protein ubiquitin to thousands of substrate proteins; “tagging” with ubiquitin often promotes the degradation of the substrate by the proteasome. In this paper, we adapt a technique called Ligase Trapping for use in mammalian cells. Ligase Trapping is a highly accurate method for determining which substrates are targeted by a ubiquitin ligase. Here we use it to identify new substrates of the human cell cycle regulator βTRCP. Our screen was indeed highly accurate, as we were able to validate 88% of the candidate substrates we identified by mass spectrometry. Some of these new substrates were unstable proteins that were stabilized by inhibition of βTRCP, or of the entire class of ubiquitin ligases of which βTRCP is a part. However, others appear to be stable or redundantly-targeted substrates, which have been more difficult to identify with current techniques. This suggests that Ligase Trapping will be able to reliably identify new substrates of human ubiquitin ligases. Further, one of the new βTRCP substrates, CReP, is specifically depleted upon DNA damage, and depletion of CReP contributes to inactivation of the translational machinery upon DNA damage.
Introduction
E3 ubiquitin ligases, which facilitate the attachment of anywhere from one to a long chain of the small protein ubiquitin to substrate proteins, are important regulators of the cell cycle and the response to stress. The best-studied outcome of ubiquitination is destruction of the substrate by the proteasome. There has been a great deal of interest in the discovery of ubiquitin ligase substrates, with the recent introduction of techniques that either look for proteins whose levels change when a particular ubiquitin ligase is inhibited [1–5], or those that use mass spectrometry to look for proteins that interact physically with the ubiquitin ligase [6–11]. Unfortunately, some ligase-substrate interactions are likely too weak to purify by affinity. Moreover, once a list of associated proteins is identified, it is not always clear which are direct substrates. To address this, most studies have determined whether the half-life of the substrate is significantly altered upon inhibition of the ligase [11]. However, in many instances, only a select fraction of substrate is targeted. In addition, some substrates are targeted redundantly by multiple ligases [12]. These facts often make it impossible to verify candidates merely by examining their half-life. For ubiquitin ligases for which a consensus binding sequence is known, the presence of this sequence has been used frequently to separate true substrates from non-substrate or non-specific interactors. However, this method is not useful to discover substrates of the vast majority of ubiquitin ligases, for which no consensus sequence is known. To eliminate these problems, we developed a technique called Ligase Trapping [13] (Fig 1A), in which an E3 ubiquitin ligase is fused to a ubiquitin-associated (UBA) domain. This mediates an extended interaction between the E3 ligase and its ubiquitinated substrates, allowing their co-immunoprecipitation. To distinguish between substrates and other associated proteins, this immunoprecipitate is subjected to a second purification for 6xHIS-ubiquitin under denaturing conditions. These purifications can be used both for substrate identification and as a diagnostic for candidate confirmation, in cases where the bulk level of a protein is stable.
Fig. 1. Establishing Ligase Trapping in human cells. 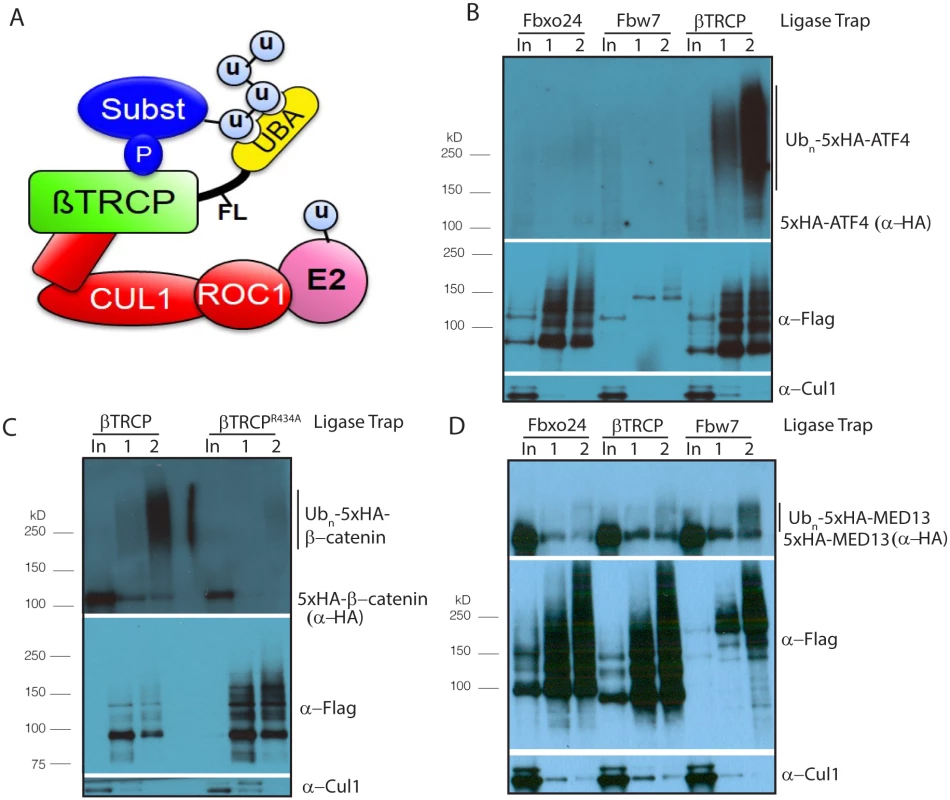
(A) The SCF includes the scaffolds Skp1 (unlabeled, in red) and Cul1, which connect the E2-binding protein Roc1 to an F box protein such as βTRCP, which recruits substrates. Ligase Trapping is a two-step process in which ubiquitinated substrates are first precipitated under native conditions by a ubiquitin ligase fused to a UBA domain and then purified further under denaturing conditions via a 6xHis tag on ubiquitin. (B) βTRCP Ligase Trap purifies ubiquitinated species of the known substrate ATF4. Stable cell lines expressing the βTRCP Ligase Trap or a negative control (FBXO24 or Fbw7) were induced to express 6xHisUb for 3 days, transfected with 5xHA-tagged ATF4 for 24 hours, treated with 5 μM MG132 for 4 hours, lysed and subjected to a two-step precipitation. First, the Ligase Traps were purified under native conditions with anti-Flag antibody and eluted with Flag peptide. Then, the eluate was denatured in 6M urea and ubiquitinated proteins purified with NiNTA beads and eluted with imidazole. Loading was 1X input (In), 250X 1st step (1), and 5,000X 2nd step (2). (C) The interaction between the βTRCP Ligase Trap and the known substrate β-catenin depends on conserved substrate-binding regions in βTRCP. The pulldown in B was repeated, but without MG132 and with the substrate β-catenin as prey and both wt and mutant βTRCP as bait. (D) Fbw7 Ligase Trap specifically purifies ubiquitinated species of the known Fbw7 substrate MED13. Performed as in Fig 1B. The SCF is a cullin-RING ligase (CRL) containing 3 core catalytic subunits: the RING finger protein RBX1, the cullin CUL1 and the adaptor SKP1 [14–17]. This catalytic base associates with a substrate adaptor called an F box protein, of which humans encode at least 69. F box proteins are thought to recognize their substrates only after substrate modification, typically by phosphorylation [14,17]. Several of these F box proteins have been characterized due to their well-established roles as tumor suppressors and oncogenes. βTRCP[18] is an F box protein that turns over substrates to control the G2/M transition (e.g. WEE1 [19]/CDC25 [20,21]), as well as the response to DNA damage (e.g. CDC25 [20,21], claspin [7,22]).
In this paper, we establish ubiquitin ligase trapping in mammalian cells. Of the 28 candidates identified using this technique, 12 were well-established substrates [6,20,21,23–33]. For the 16 remaining candidates, we examined 14 and found that 11 of these confirmed. Thus, 23 of the 26 known/tested candidates, (88%) appear to be substrates, suggesting that Ligase Trapping is a robust discovery technique. Further characterization showed that turnover of one of the βTRCP substrates, CReP, is exacerbated by DNA damage. CReP is a protein phosphatase 1 (PP1) specificity subunit that counteracts the phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α) on serine-51 [34], a stress-induced modification that inhibits translation initiation on most transcripts [35,36]. Inhibiting the turnover of CReP after DNA damage significantly reduced the accumulation of serine-51 phosphorylated eIF2α, and increased translation after DNA damage, suggesting that CReP turnover is an important mechanism by which DNA damage regulates translation.
Results
To establish Ligase Trapping in human cells, we created a stable HEK293 line in which 6xHIS-ubiquitin is expressed upon treatment with doxycycline. In this cell line, tagged ubiquitin accounts for a significant portion of the total ubiquitin pool when cells are treated with doxycycline (S1A Fig). In yeast, fusion of F box proteins, via a 3xFlag linker, to the UBA of Dsk2 or the two tandem UBAs of Rad23, led to enhanced purification of nascent ubiquitinated F box protein substrates [13]. We fused the human F box protein βTRCP to the human homologs of these UBA-containing proteins, and found that the RAD23B fusion increased the poly-ubiquitinated species purified by the βTRCP fusion most strongly (S1B Fig). Accordingly, we made a stable cell line that expressed both doxycycline-inducible 6xHIS-Ub and a Ligase Trap consisting of βTRCP fused on its C-terminus to 3xFlag and the C-terminal UBAs of RAD23B.
To determine whether the βTRCP trap was functional, we expressed an epitope-tagged allele of the βTRCP substrate ATF4 in our stable cell line. We were able to immunoprecipitate poly-ubiquitinated ATF4 with the βTRCP trap, but not with the Ligase Traps of two unrelated F box proteins, FBXO24 and Fbw7 (Fig 1B). We obtained a similar result with β-catenin (S2 Fig). We also purified ubiquitinated forms of the Ligase Traps, which was unsurprising as many ubiquitin ligases are themselves ubiquitinated. We also purified substantial unmodified forms of the Ligase Traps. This is likely a result of the very large amount of IP loaded relative to input (5,000 : 1 for the 2nd step), which is necessary to see the very small percentage of substrate that is poly-ubiquitinated. Even in cases where the unmodified band is equal in intensity in the input and 2nd step IP, this represents only 0.02% IP background. This phenomenon also occurs frequently with unmodified substrates, while the relevant purification of poly-ubiquitinated substrates is highly specific to the relevant Ligase Trap. To examine further whether the purification of β-catenin was specific, we made a stable cell line identical to our βTRCP ligase trap line, but with a mutation in the WD40 domain of βTRCP predicted to prevent binding to β-catenin [37]. As expected, this mutant trap failed to purify polyubiquitinated β-catenin (Fig 1C), showing that β-catenin purification by βTRCP represents a specific interaction. To make certain that the βTRCP Ligase Trap didn’t simply bind all ubiquitinated proteins more efficiently, we made a similar stable cell line expressing Fbw7-3xFlag-RAD23. Poly-ubiquitinated forms of the known Fbw7 substrate MED13 [10] were preferentially precipitated with the Fbw7 Ligase Trap (Fig 1D).
Having established the functionality of the βTRCP ligase trap cell line, we performed a large-scale, two-step purification and identified ubiquitinated co-precipitating proteins by mass spectrometry. Before collection, we treated cells with the proteasome inhibitor MG132 for four hours, as we had shown that this treatment increases the amount of poly-ubiquitinated material purified by the βTRCP ligase trap (S1C Fig). We defined candidate βTRCP substrates as those proteins identified in at least two of three purifications of the βTRCP ligase trap, but not in any of the negative control purifications. Twenty-eight proteins met these criteria (Table 1). Of these, twelve were previously-validated βTRCP substrates, and many others had been shown to interact with βTRCP in previously published large data sets, but had not been individually examined to determine if they were substrates [4,8,11,38–40]. SUN2 was purified in a large-scale screen for βTRCP substrates, and shown to be stabilized by the proteasome inhibitor MG132 [39] while this manuscript was under review. In addition, several other known βTRCP substrates, such as ß-catenin [41–45], were selectively identified in the βTRCP purification, but as some peptides were also identified in control purifications, these did not meet the stringent criteria that we had chosen for this initial analysis (bottom of Table 1). The large fraction of previously-published substrates (43%) that we purified confirms that Ligase Trapping accurately identified true substrates.
Tab. 1. Discovery and validation summary for identified βTRCP substrates. 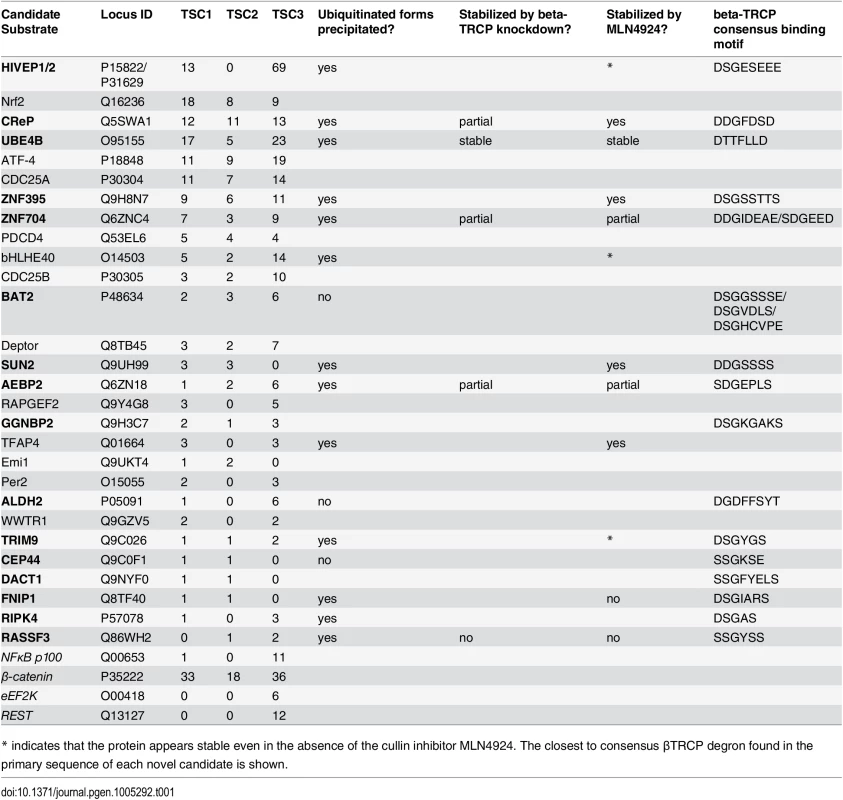
List of all proteins purified uniquely and at least twice by the βTRCP Ligase Trap, with total spectral counts (TSC) for each of three purifications. Two unique HLA alleles were excluded, as other HLA alleles were identified in negative control purifications. Substrates in normal text were previously well-described, and those in bold are novel, although some have previously been isolated in large-scale experiments. The substrates below the black bar are known βTRCP substrates that were isolated, but did not meet our criteria for candidates. For the validation experiments, a blank box means the experiment was not performed. We also purified substrates of Fbw7 using a Ligase Trap. The Fbw7 Ligase Trap was expressed at a low level, suggesting that this trap was less stable. However, the proteins pulled down most abundantly and specifically by the Fbw7 Ligase Trap were MED13 and MED13L, two members of the Mediator complex shown to be Fbw7 substrates in a recent screen[10] in which Fbw7 interactors were precipitated and identified by mass spectrometry. (Our purification of MED13 is shown in Fig 1D) In that screen, the entire 26-member Mediator complex was purified, and MED13 and MED13L had to be identified as the direct Fbw7 substrates by a combination of degron prediction and careful validation; we did not purify any other members of the Mediator complex.
Ligase Trapping also provided a method to validate candidates beyond simply examining substrate turnover. Ligase Trapping is able to show that a ubiquitinated substrate specifically purifies with a particular ligase even if the substrate is redundantly targeted by multiple ligases, or if only a small fraction of the substrate (such as that in a particular complex) is ubiquitinated. To fully assay the accuracy of the Ligase Trap technique, we decided to validate candidate βTRCP substrates. Out of fourteen of the previously unknown/unvalidated candidates that we examined, eleven showed specific purification of polyubiquitinated material by the βTRCP ligase trap (Table 1 and Figs 2, S4 and S5). This strongly suggested that these candidates are true substrates of βTRCP, and that this technique accurately identified substrates with low background and thus will be an efficient way of identifying and validating substrates of other ubiquitin ligases in the future. Two βTRCP candidate substrates were not examined due to technical difficulties.
Fig. 2. Validation of novel βTRCP substrates. 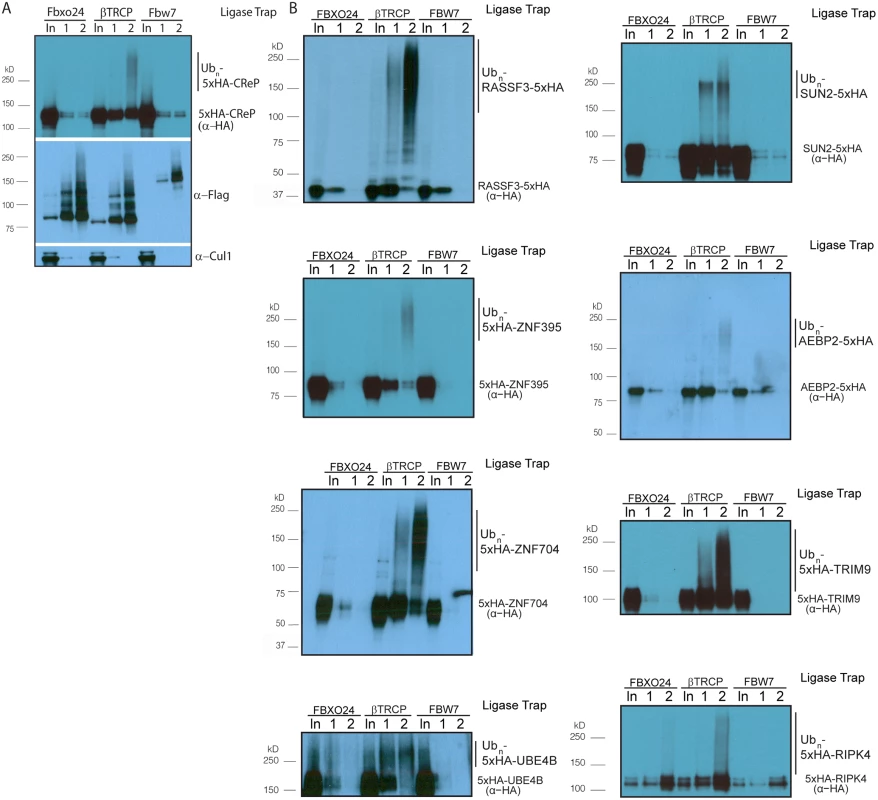
(A) βTRCP Ligase Trap specifically purifies ubiquitinated species of the novel βTRCP substrate CReP. Performed as in Fig 1, without MG132 treatment. Loading was 1X for input, 250X for the 1st step, and 5,000X for the 2nd step. (B) Validation of additional candidate substrates. Loading controls and the rest of the substrates are in S3 and S4 Figs. In order to determine whether βTRCP could bind its candidate substrates in the absence of the UBA domains present in the Ligase Traps, we co-expressed Flag-tagged versions of these F box proteins in HEK293 cells with HA-tagged versions of a subset of their candidate substrates. In all cases, the substrate was purified more efficiently by its cognate ligase than by the negative control ligase (Fig 3A).
Fig. 3. Ubiquitin ligase binding and turnover of a subset of novel βTRCP substrates. 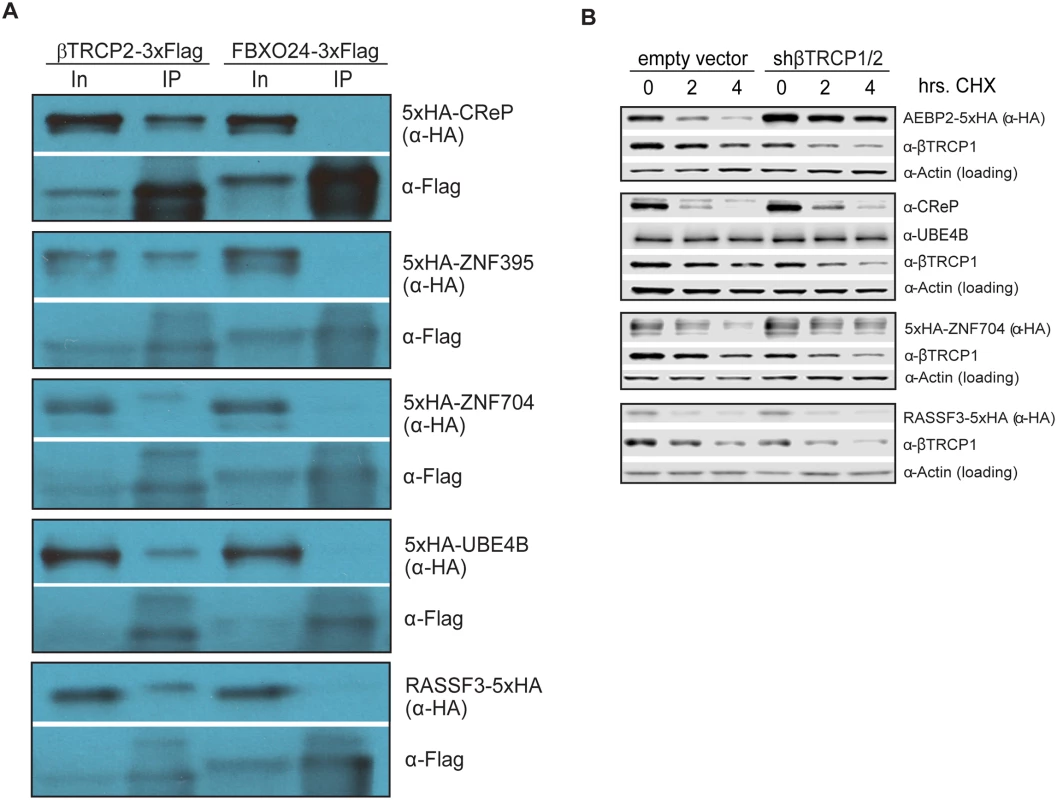
(A) βTRCP binds to its candidate substrates in vivo. HEK293 cells were transfected with 3xFlag-tagged F box proteins and 5xHA-tagged substrates for 1 day, lysed and subjected to a one-step precipitation. The F box proteins were purified under native conditions with anti-Flag antibody and eluted with Flag peptide. Loading was 1X input (In) and 75.3X IP for CReP, and 1X input (In) and 83.7X IP for other substrates. (B) Effect of βTRCP knockdown on candidate substrate half-life. HEK293 cells were co-transfected with a negative control plasmid, or a plasmid encoding an shRNA targeting βTRCP1 and 2, and a plasmid encoding a tagged βTRCP candidate substrate. Cells were treated with 100 μg/mL cycloheximide for the indicated time before collection. Because a common outcome of ubiquitination by the SCF is proteasomal degradation of the ubiquitinated protein, we assayed whether a subset of the candidate substrates were degraded in a way that depended on the cognate ligase. For five of the βTRCP candidate substrates, we co-transfected cells with DNA encoding tagged substrate, as well as a negative control plasmid or a plasmid expressing an shRNA targeting both paralogs of βTRCP, then inhibiting bulk protein translation with cycloheximide and assaying substrate levels. Although the knockdown we achieved was quite modest, three of the five substrates were significantly stabilized (Fig 3B). One, RASSF3, was not stabilized, suggesting either that it is a better βTRCP substrate than the others, or that it is targeted by other ubiquitin ligases. UBE4B is a stable protein. (Note that we detected UBE4B with a specific antibody against this protein, and did not ectopically express it, so its stability is unlikely to be an artifact.) It is possible that either only a small pool of the substrate was targeted, or that the outcome of ubiquitination of UBE4B is not proteasomal degradation.
Several commonly-used approaches identify ubiquitin ligase substrates as those proteins whose abundance is increased by inhibition of the relevant ligase. One key advantage of ligase trapping is that, in contrast to these techniques, it can identify substrates whose bulk turnover is not affected by inhibition of the ligase. To determine more universally which substrates were quantitatively targeted for degradation by βTRCP, we expressed tagged versions of the substrates, inhibited protein synthesis with cycloheximide, and followed the turnover of the substrate in the absence or presence of MLN4924 (Table 1 and S6 Fig). Of the ten substrates examined, three (CReP, ZNF395, and SUN2) were unstable proteins that were stabilized by MLN4924, suggesting that their turnover is mediated by βTRCP alone or in combination with other cullin-RING ligases. (CReP was previously shown to be an unstable protein [34], as was SUN2.) Four (ZNF704, FNIP, RASSF3 and AEBP2) were not or only partially stabilized by MLN4924, suggesting that these might be redundantly targeted by βTRCP and a non-CRL ligase. Three proteins (HIVEP2, UBE4B, and TRIM9) appeared to be constitutively stable, although we cannot rule out that overexpression or epitope tagging of HIVEP2 and TRIM9 led to an artifactual stabilization. βTRCP could be promoting non-degradative ubiquitination of these substrates, or may only ubiquitinate a specific pool.
We were initially concerned that treating cells with MG132 would lead to increased background, or skewing of the results. Therefore, we performed two purifications of the βTRCP ligase trap in the absence of MG132. This purification generated a list with several of the same substrates, but lacking a subset, especially those shown to be unstable in Figs 3B and S6 (S7 Fig). In addition, all of our validations were performed in the absence of MG132 (Figs 2, S4 and S5).
We wished to further explore the biological significance of CReP turnover. First, we verified that the ubiquitinated CReP pulled down by the βTRCP ligase trap required SCF activity. Indeed, pre-treatment of cells with MLN4924 eliminated the ubiquitinated CReP (but not unmodified CReP) pulled down by the βTRCP ligase trap (Fig 4A). Second, we mutated CReP’s single well-conserved βTRCP-binding consensus, as well as the amino acids immediately downstream, which form a second less-well-conserved consensus. The βTRCP consensus is DpSGX(1–4)pS [46], with some substitution of acidic amino acids for phosphorylations tolerated. The sequence we mutated in CReP is DDGFDSDSSLSDSD (marked in S11 Fig). Although this sequence lacks the most-conserved DSG motif, many well-documented βTRCP substrates have variations in this part of the degron [18], and human CDC25A and CDC25B have well-validated degrons that contain DDG, just like CReP [25] (shown in Fig 4B). This mutant, CReP11A, was significantly stabilized relative to wild type CReP (Fig 4C and 4D), strongly suggesting that CReP turnover is dependent on βTRCP. The notable downshift of the mutant is likely due to mutation of several negatively-charged residues. Mutation of a portion of the same region was independently shown to stabilize CReP while our manuscript was in the review process[47].
Fig. 4. CReP ubiquitination is dependent on CRLs and turnover is regulated by a βTRCP consensus degron. 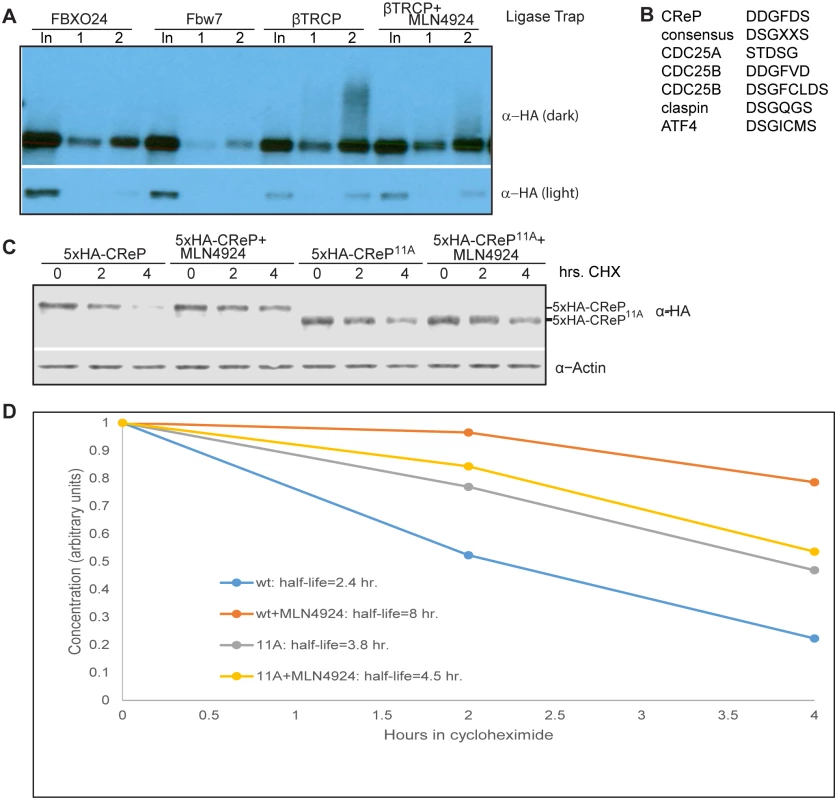
(A) Ubiquitinated CReP precipitated by the βTRCP ligase trap depends on cullin activity. Tagged CReP was transiently expressed in the βTRCP or negative control ligase trap cell lines, as in Fig 2B. Where indicated, 1 μM MLN4924 was added 4 hours before cell collection to inhibit cullin activity. (B) A near-consensus βTRCP degron in CReP, compared to well-validated degrons. (C) CReP turnover depends on βTRCP consensus sites. Two consensus sites in CReP were mutated to generate the 11A mutant. Wildtype or mutant CReP was expressed transiently in 293 cells, which were then treated with 100 μg/mL cycloheximide for the time indicated to monitor degradation in the absence of new protein synthesis. Where indicated, cells were treated with 1 μM MLN4924 coincident with cycloheximide addition. (D) Quantitation of the average of two independent replicates of (B). Because both protein-folding stress and DNA damage have been shown to regulate eIF2α phosphorylation, we tested whether these stresses also regulated CReP levels. The proteostatic stress inducer thapsigargin had a very minor effect on CReP levels, consistent with a previous report showing no effect [34]. However, DNA damage provoked by either ultraviolet light (UV) or the topoisomerase inhibitor camptothecin (CPT) led to complete depletion of CReP (Fig 5A). Suggestively, the disappearance of CReP was coincident with the induction of eIF2α phosphorylation by these stressors. The depletion of CReP was not due merely to inhibition of translation by eIF2α phosphorylation, as DNA damage also decreases the half-life of CReP compared to no treatment or treatment with proteostatic stressors in a cycloheximide chase (S11 Fig), and CReP still disappears upon DNA damage in mouse embryonic fibroblasts in which Ser51 of eIF2α has been mutated to alanine (data not shown). CReP turnover and subsequent eIF2α phosphorylation is at least partially dependent on βTRCP, as transfection with shRNA against both paralogs of this ligase delays DNA damage-dependent induction of both CReP turnover and eIF2α phosphorylation (Fig 5B). CReP depletion is fully dependent on CRL-mediated degradation, because treatment of cells with the CRL inhibitor MLN4924 prevents CReP depletion (Fig 5C). The residual CReP turnover seen even in cells treated with βTRCP shRNA may reflect our inability to achieve sufficient knockdown of βTRCP, or additional turnover mediated by another CRL. Cullin-mediated turnover of CReP in response to DNA damage was not restricted to HEK293 cells, since it occurs in both primary human fibroblasts (Fig 5D) and immortalized mouse embryonic fibroblasts (MEFs) (S12 Fig).
Fig. 5. Regulation of CReP turnover and impact on eIF2α phosphorylation. 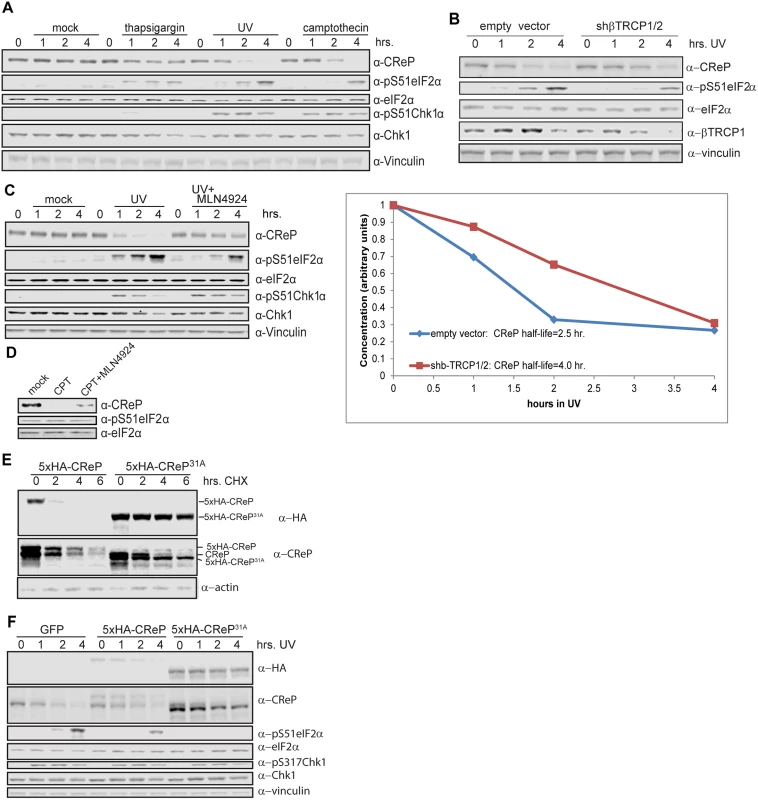
(A) CReP is depleted upon DNA damage but not proteostatic stress. Cells were treated with 1 μM thapsigargin, 3 μg/mL camptothecin, or 300 J/m2 UV for the indicated time; all samples not treated with UV were mock-treated and all samples were given the same total volume of the solvent DMSO. (B) CReP turnover upon DNA damage depends at least in part on βTRCP. Cells were transfected for 48 hours with an empty vector or shRNA targeting βTRCP1 and 2, then irradiated with 300 J/m2 UV-C. CReP levels are quantitated below, and the half-life calculated from the linear (0–2 hr.) part of the timecourse. (C) CReP depletion and full eIF2α phosphorylation in UV depends on CRLs. Cells were treated with UV with or without MLN4924 for the times indicated. (D) CReP depletion in primary human fibroblasts depends on CRLs. Primary human fibroblasts were treated with 1 μg/mL camptothecin for 6 hours, with 1 μM MLN4924 where indicated. (E) The 31A allele of CReP is stable even upon treatment with DNA damage and cycloheximide. Cells were transfected with wildtype or mutant CReP, then pre-treated for 2 hours with 3 μg/mL camptothecin before addition of cycloheximide. (F) Expression of a stable allele of CReP prevents phosphorylation of eIF2α in response to UV treatment. Cells were transfected with tagged wild type or mutant CReP, then treated with UV for the indicated times. The CReP11A mutant was not completely stabilized upon DNA damage (data not shown), possibly because DNA damage promotes βTRCP binding to additional sites on CReP. βTRCP has been shown to interact with non-consensus phosphodegrons in MDM2, suggesting that it may be difficult to identify degrons by sequence alone[48]. Therefore, we mapped phosphorylated residues on CReP to identify any additional degron sequences (S9 Fig). Notably, most phosphosites were observed both with and without CPT. It is possible that the increase in CReP turnover observed upon DNA damage is not due to increased phosphorylation, but to a change in a targeting factor or localization of CReP. However, phosphosites are still likely to be required for turnover. For clustered phosphosites and phosphosites that were near short acidic stretches, we mutated both the phospho-acceptor and all acidic and potential phospho-acceptors in the region. In addition, we mutated one additional weak βTRCP consensus site that was not covered in the phospho-mapping. We then tested the stability of these mutants, in various combinations, in DNA damage (data not shown). CReP31A (S10 Fig) was the least mutated allele that was completely stable upon treatment with DNA damage (Fig 5E and 5F). Importantly, this stabilization was not merely an artifact of high starting levels resulting from prioritized transcription or translation, as CReP31A is stable even upon pre-treatment with camptothecin followed by cycloheximide chase (Fig 5E). Like the 11A mutant, CReP31A migrates much more quickly than the endogenous protein, likely due to mutation of many negatively-charged amino acids.
To examine the physiologic role of the turnover of CReP upon DNA damage, we determined whether CReP stabilization had an effect on eIF2α phosphorylation. When CReP turnover was inhibited by knockdown of βTRCP, treatment with MLN4924, or mutation of CReP, phosphorylation of eIF2α was delayed or inhibited to an equivalent degree (Fig 5B, 5C and 5F). This is not specific to HEK293 cells, as MLN4924 also reduced eIF2α phosphorylation after UV treatment in immortalized mouse embryonic fibroblasts (MEFs) (S12 Fig). However, primary human fibroblasts (Fig 6D) had constitutively high levels of eIF2α phosphorylation, so the effect of CReP turnover was only subtle. This may reflect a greater need for this pathway in fast-growing cells, or the fact that these primary cells were under constant stress.
Fig. 6. Consequences of CReP turnover downstream of eIF2α phosphorylation. 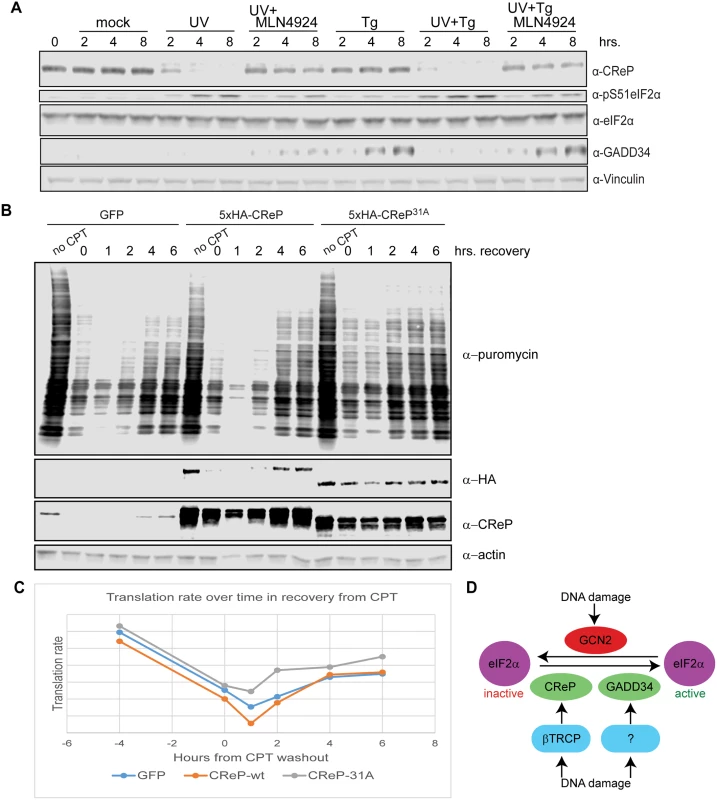
(A) UV dominantly prevents the induction of GADD34. 293 cells were treated with 300 J/m2 UV-C, 1 μM thapsigargin, or a combination of the two, and with 1 μM MLN4924 where indicated. All treatments were added simultaneously. (B) CReP turnover reduces bulk translation after DNA damage. HEK293 cells were transfected with plasmids expressing GFP, wildtype CReP, or stable mutant CReP31A, then, as indicated, were untreated, treated with 1 μg/mL camptothecin (CPT) for 4 hours, or treated with CPT and then washed in medium to initiate DNA damage recovery for the indicated time. 10 minutes before collection, puromycin was added to cells at a final concentration of 10 μg/mL to label nascent polypeptide chains, and cells were collected in cold PBS, on ice, before flash-freezing. (C) Quantitation of (B) by densitometry. (D) A model for the role of CRLs in regulating eIF2α after DNA damage. Upon proteostatic stress, eIF2α phosphorylation promotes the translation of the transcription factor ATF4 [49]. ATF4 activates the expression of the transcription factor CHOP [49], which in turn promotes the transcription of GADD34 [50]. Like CReP, GADD34 is a PP1 targeting subunit that acts on Ser51 of eIF2α [51,52]. These PP1 subunits appear to have a dedicated role in regulating eIF2α, since the lethal phenotype of knockout mice lacking both GADD34 and CReP can be rescued by mutating eIF2α Ser51 [51]. Previous reports suggested that GADD34 is induced at late time points after DNA damage in some cell types [53]. We were especially interested in whether DNA damage promoted the destruction of CReP only to replace it with GADD34. However, we found that UV treatment did not promote GADD34 protein expression, while ER stress induced by thapsigargin did (Fig 6A). This may reflect a cell-type difference between HEK293 cells and cells previously used to show GADD34 induction. Surprisingly, treating cells with UV and thapsigargin simultaneously blocked the thapsigargin-mediated increase in GADD34 protein levels, suggesting that DNA damage somehow dominantly prevents expression of this protein. Inhibition of GADD34 expression by UV treatment could be rescued by simultaneously treating cells with MLN4924, suggesting that a CRL is involved in blocking GADD34 accumulation.
Finally, we examined whether CReP turnover after DNA damage affected rates of translation. After treatment with DNA damage, translation rate was assayed via the SUnSET method [54], by adding puromycin to the cells for 10 minutes, then detecting the degree of puromycin incorporation into newly translating polypeptides via western blotting with an anti-puromycin antibody. We found that expression of CReP31A, which led to high CReP levels even after treatment with camptothecin and initial recovery from this damage, accelerated the recovery of translation after DNA damage, doubling the translation rate at 2 hours after CPT washout (Fig 6B and 6C). Notably, this effect was not seen with the unstable, ectopically expressed wildtype CReP, although it was expressed at the same level as CReP31A. This effect reproduced several times, although the exact timing varies, likely due to subtle variations in CReP expression levels during transfection.
Discussion
We have identified and validated thirteen novel substrates of the well-studied ubiquitin ligase βTRCP via Ubiquitin Ligase Trapping. While we were unable to test two of the twenty-eight candidate substrates identified, 88% of the remaining twenty-six were either known or validated novel substrates. While affinity chromatography is often able to identify ligase substrates, these data suggest that Ligase Trapping provides an unprecedented hit rate, making it an especially efficient way to identify new ubiquitin ligase substrates. Moreover, this technology has allowed us to easily validate substrates even if their bulk stability is not affected by βTRCP ubiquitination.
Our results for FBW7 suggest another way in which Ligase Trapping can complement currently available techniques. In a previous study, the Clurman lab pulled out all 26 members of the Mediator complex with FBW7. They used degron prediction and follow-up experiments to identify MED13 and MED13L as the ubiquitylated Fbw7 substrates and carefully confirmed that they are direct substrates. Our mass spec of the Fbw7 ligase trap immunoprecipitation specifically purified MED13 (and MED13L) uniquely in the Fbw7 Ligase Trap, and not in any of the other purifications. Moreover, we pulled out none of the other 25 subunits. This underscores the usefulness of our technique, especially for the great majority of F box proteins for which no degron consensus is known. Thus, even in cases where Ligase Trapping identifies similar numbers of substrates compared to other techniques, it allows one to quickly identify the directly ubiquitylated substrates.
In addition to the substrate CReP, which we followed up in detail, turnover of several of the other substrates is likely to be regulated in response to cell cycle position or stress. Sun2 is a transmembrane protein that spans the inner nuclear envelope and has been implicated in the maintenance of nuclear structure and the regulation of DNA damage. Its turnover by βTRCP may regulate these processes, and its removal from the membrane after ubiquitination may also be a regulated step. Strikingly, four of the eleven novel substrates we validated, ZNF395, HIVEP1/2, ZNF704, and AEBP2, are transcription factors, as are several known βTRCP substrates, such as Nrf2 and ATF4. We also identified two substrates that are themselves ubiquitin ligases, UBE4B and TRIM9, which opens up the possibility of complex mutual regulation. While UBE4B ubiquitination depends on the SCF (data not shown), it is not highly ubiquitinated (Fig 2), and it appears that the majority of the UBE4B population is stable (Fig 3B). RASSF3 is a candidate tumor suppressor protein that activates p53-dependent apoptosis under appropriate conditions, including DNA damage [55]. Its regulation by βTRCP is consistent with the known role of βTRCP in responding to DNA damage, and may help explain the oncogenic effect of βTRCP overexpression [18] (along with other known tumor suppressor substrates of βTRCP, such as REST[45]). RASSF3 appears to have both stable and unstable pools. This may reflect the relatively small pool of cells undergoing stress at any particular time in an untreated culture. Perturbations such as DNA damage might drive RASSF3 turnover.
Our previous studies in yeast [13] showed that 56% of newly-identified SCF substrates were strongly stabilized when the F box in question was mutated. 25% showed small or moderate stabilization, but were still unstable in the F box gene mutant. Finally, 19% appeared stable even in wildtype. We find here that 45% of confirmed novel substrates were stabilized by treatment with a pan-CRL inhibitor, 18% showed no stabilization, and 27% were stable in wildtype. Thus, in both cases only half or fewer novel substrates were quantitatively turned over by the single ligase, although this is likely an underestimate overall, since previously characterized substrates may be biased for this category. While some of these effects could be due to the population assay employed, as noted above, substrates such as Cln3 and Gal4 in yeast, as well as PIP box-containing substrates in humans, are targeted in a way that is dependent upon the sub-cellular localization/context of the substrate [12,56]. Alternatively, some turnover events occur as part of quality control pathways that only target those proteins that are in some way defective.
We have implicated βTRCP in the regulation of translation initiation after DNA damage through its turnover of CReP, and shown that DNA damage-induced phosphorylation of eIF2α, because it uniquely requires the depletion of CReP, occurs via a different mechanism from the other stresses known to promote eIF2α phosphorylation, which all promote kinase activation. Previous work has shown that the phosphorylation of eIF2α after UV treatment depends on the kinase Gcn2 [57,58]. We propose that this phosphorylation requires both Gcn2 activation and CReP turnover.
Why does phosphorylation of eIF2α require CReP depletion after DNA damage, but not in response to proteostatic stress? One possibility is that eIF2alpha kinases are less active after DNA damage than after proteostatic stress. We observed that, once CReP levels begin to drop, eIF2α phosphorylation is much higher upon our UV treatment than after proteostatic stress (Fig 5A). This likely reflects both continued CReP activity and the induction of GADD34 upon proteostatic stress. We showed in Fig 6B and 6C that CReP turnover has a significant effect on translation rates after DNA damage, but substantial inhibition of translation happens even in the absence of CReP turnover. Translation rates are highly redundantly regulated, both via control of eIF2α phosphorylation and via regulation of eIF4. Our results are consistent with a model in which CReP turnover is important to enforce continued low levels of translation at later timepoints. Moreover, the high levels of eIF2α phosphorylation enabled by CReP turnover in response to DNA damage may allow translational reprograming that leads to induction of DNA damage repair proteins, even as global translation is downregulated. Indeed, translation of several DNA repair proteins has been shown to be resistant to inhibition of CAP-dependent translational inhibition by eIF2α phosphorylation [58].
Finally, how do CRLs prevent the induction of GADD34 after UV treatment? One possibility is that CReP turnover upon DNA damage (which requires CRLs) drives such strong eIF2α phosphorylation that translation of GADD34 or one of its upstream regulators ATF4 or CHOP is inhibited. Another possibility is that a CRL is turning over a specific protein to keep GADD34 levels low. βTRCP is known to target ATF4 [24] and the Cul3-associated ligase SPOP is reported to target CHOP [59]. GADD34 is also a known proteasome target, consistent with its being a substrate of βTRCP or another CRL [60]. Targeting of both CReP and Gadd34 for degradation upon DNA damage underscores the importance of limiting eIF2α phosphatase activity during DNA damage.
Methods
Plasmids and tissue culture
All plasmids were transfected into the 293 FlpIn TRex cell line (Life Technologies, Grand Island, NY, USA), which contains both a site for FRT-mediated recombination (which we did not use in this work) and expresses the tet repressor, which allows doxycycline-inducible expression from promoters that include tet operators. Mouse embryonic fibroblasts (MEFs) were immortalized by transduction with the SV40 large T antigen (kind gift of Morgan Truitt and Davide Ruggero). All cells were grown in DMEM with 10% heat-inactivated fetal bovine serum. For large-scale purifications, medium was supplemented with 500 U/mL penicillin and 500 μg/mL streptomycin.
6xHis-ubiquitin was expressed from pTB30, a modified pcDNA3.1 vector with a pCMV/TetO promoter expressing 6xHis-Uba52-IRES-6xHis-RPS27A. The parent of this construct was the kind gift of Zhijian Chen, UT Southwestern. The construct was linearized with Pvu I and transfected into 293 FlpIn TRex cells. Stable transfectants were selected with G418 and a clone was selected that expressed at a high level only upon treatment with doxycycline.
To make the ligase trap fusion proteins, F box proteins were fused on the C-terminus to 3xFlag followed by the C terminal half of human RAD23B (Accession #BC020973.2, amino acids 185–410), encoding two UBA domains. Ligase traps βTRCP2 (FBXW11; Accession #BC026213.1, pTB53), Fbxo24 (Accession #NM033506.2, pBEN20), and Fbxo6 (Accession #NM018438.5, pBEN5) were expressed as hygromycin resistance-T2A-ligase trap fusions driven by the mouse PGK1 promoter. Each of these constructs also expresses an shRNA against the relevant F box protein (to which the fusion protein is resistant), driven by the mouse U6 promoter. These cassettes were linearized by digestion with Pac I. Fbw7 (Accession# NM_033632.3, pTB59) Ligase Trap was expressed from a pcDNA3.1 vector, under the control of the CMV promoter. The vector was linearized with BglII. All linearized plasmids were transfected into the HisUb cell line and stable transfectants were selected with hygromycin. We selected clonal cell lines that expressed moderate levels of the relevant ligase trap.
All substrate proteins were tagged on the N-terminus with the 5xHA epitope, and expressed from the CMV promoter in pcDNA3.1, except SUN2, AEBP2, ALDH2, and RASSF3, which were tagged on the C-terminus. They were transiently transfected into the relevant cell line using Fugene HD at 3 μL/μg DNA (Promega Corporation, Madison, WI, USA) or polyethyleneimine (at 18 μg/μg DNA) 24–48 hours before the experiment. βTRCP was knocked down with an shRNA targeting both BTRC and FBXW11, expressed from the pSUPER-puro-retro vector (under the H1 promoter)[61].
Drugs
MG132 is used at 5 μM. MLN4924 is used at 1 μM. Camptothecin is used at 3 μg/mL, unless otherwise specified.
UV treatment
Medium was removed from adherent cells and set aside. Cells were covered in 37°C 1X PBS with 0.9 mM CaCl2 and 0.5 mM MgCl2, then exposed to 300 J/m2 UV-C, PBS was aspirated, and medium was replaced.
Antibodies and western blotting
For western blotting, cells were lysed in 1X RIPA buffer with protease and phosphatase inhibitors for 30 minutes on ice, insoluble material was spun out, then protein concentrations were measured with BCA Reagent (Pierce, Thermo Scientific, Rockford, IL, USA) and normalized before addition of SDS sample buffer with DTT. For Figs S7 (except for RASSF3) and 5C, cells were lysed directly in SDS sample buffer with DTT or βMe.
All gels were Criterion Tris-HCl 4–20% gradients (cat. #345–0034, BioRad, Hercules, CA, USA), except for the gel for the α-HA blot in Fig 2C, which was a 7.5% gel (BioRad cat. #345–0007).
Antibodies used were α-HA 16B12 at 1 : 1,000–1 : 2,000 (cat. #MMS-101R, Covance, Emeryville, CA, USA), α-6xHis at 1 : 1,000–1 : 2,000, α-ubiquitin P4D1 at 1 : 100, α-Flag M2 at 1 : 2,000 (cat. #F3165, Sigma, St. Louis, MO, USA), α-Cul1 at 1 : 1,000, α-vinculin at 1 : 1,000–1 : 5,000, α-βactin at 1 : 1,000–1 : 10,000 (Sigma cat. #A5441 for Fig 4A, Abcam, Cambridge, UK, cat.#ab8226 for all others), α-PPP1R15B (CReP) at 1 : 1,000–1 : 5,000 (cat. #14634-1-AP, Proteintech, Chicago, IL, USA), and α-GADD34 (cat. # 10449-1-AP, Proteintech, Chicago, IL, USA). α-phosphoS51-eIF2α (cat. #9721), α-eIF2α (cat. #9722), α-phosphoS317Chk1 (cat. #2344), and α-Chk1 (cat. #2360) antibodies were all from Cell Signaling Technologies, Danvers, MA, USA. The α-puromycin antibody 12D10 was from EMD Millipore (cat. #MABE343).
Western blots in Figs 1, 2A, 2B and 3A were incubated with secondary antibodies fused to horseradish peroxidase and visualized by treatment with Western Lightning ECL (Perkin Elmer, Waltham, MA, USA). Western blots in Figs 2C, 3B and 4 were incubated with fluorescent secondary antibodies and visualized with an Odyssey scanner (Licor, Lincoln, NE, USA).
Immunoprecipitations of Ligase Traps
Unless otherwise noted, stable cell lines expressing Ligase Traps were treated with 5 μM MG132 for 4 hours before collection. We grew 100–200 barely sub-confluent 15 cm dishes for each purification, representing approximately 1–3 x 109 cells. Pellets were lysed in 25 mM Hepes-KOH, pH8, 150 mM K Oac,10 mM MgCl2, 5 mM CaCl2, 20 mM iodoacetamide, 30 μM MG132, protease inhibitors, and phosphatase inhibitors by sonication, then treated with DNase (660 U/mL) at 4°C for 30 minutes before addition of Nonidet P-40 to 0.1%. Samples were spun to remove insoluble material, then incubated with α-Flag M2 magnetic beads (Sigma, St. Louis, MO, USA) at 4°C overnight. Beads were washed 5 times in 1X PBS+0.1% Nonidet P-40, then eluted in this wash buffer+0.5 mg/mL 3xFlag peptide. The eluate was denatured by addition of 2X volume Buffer B (216 mM NaH2PO4, 16 mM Tris, 9.37 M urea, pHed to 8). The sample was then incubated with NiNTA agarose for 3 hours at room temperature. The beads were washed 3X in Buffer B diluted to 8M urea+10 mM imidazole, then 2X in Buffer B diluted to 1 M urea+10mM imidazole. Samples were eluted in 0.5 M urea, 300 mM imidazole, 0.1% rapigest (or Nonidet P-40 if not to be used for mass spectrometry), 108 mM NaH2PO4, 8 mM Tris (pHed to 8 before adding imidazole).
Mass spectrometry analysis
The immunopurified protein complexes were mixed in a ratio of 1 : 1 with digestion buffer (100 mM Tris-HCl, pH 8.5, 8M urea), reduced, alkylated and digested by sequential addition of lys-C and trypsin proteases as previously described[62,63]. For identification of phosphorylation site, proteins were digested directly in the excised gel slice using trypsin[62]. Peptide digests desalted and fractionated online using a 50 μM inner diameter fritted fused silica capillary column with a 5 μM pulled electrospray tip and packed in-house with 15 cm of Luna C18(2) 3 μM reversed phase particles. The gradient was delivered by an easy-nLC 1000 ultra high pressure chromatography system (Thermo Scientific). MS/MS spectra were collected on a Q-Exactive mass spectrometer (Thermo Scientific) [64,65]. Data analysis was performed using the ProLuCID, DTASelect2, and Ascore algorithms as implemented in the Integrated Proteomics Pipeline—IP2 (Integrated Proteomics Applications, Inc., San Diego, CA) [66–69]. Phosphopeptides were identified using a differential modification search that considered a mass shift of +79.9663 on serines, threonines and tyrosines. Protein and peptide identifications were filtered using DTASelect and required at least two unique peptides per protein and a peptide-level false positive rate of less than 5% as estimated by a decoy database strategy[70]. Normalized spectral abundance factor (NSAF) values were calculated as described and multiplied by 105 to improve readability [71].
Puromycin incorporation assay
We followed the SUnSET protocol [54]. Puromycin was added to culture medium at a final concentration of 10 μg/mL, incubated for 10 minutes at 37°C and 8% CO2, then medium was replaced with ice-cold PBS with 5 mM EDTA, and cells were sprayed from the dish on ice, spun down at 4°C and flash-frozen. Samples were normalized by protein concentration, and puromycin incorporation was detected by western blotting with a monoclonal anti-puromycin antibody (12D10) and quantified by densitometry.
Supporting Information
Zdroje
1. Benanti JA, Cheung SK, Brady MC, Toczyski DP (2007) A proteomic screen reveals SCFGrr1 targets that regulate the glycolytic-gluconeogenic switch. Nat Cell Biol 9 : 1184–1191. 17828247
2. Yen HC, Elledge SJ (2008) Identification of SCF ubiquitin ligase substrates by global protein stability profiling. Science 322 : 923–929. doi: 10.1126/science.1160462 18988848
3. Yen HC, Xu Q, Chou DM, Zhao Z, Elledge SJ (2008) Global protein stability profiling in mammalian cells. Science 322 : 918–923. doi: 10.1126/science.1160489 18988847
4. Emanuele MJ, Elia AE, Xu Q, Thoma CR, Izhar L, et al. (2011) Global identification of modular cullin-RING ligase substrates. Cell 147 : 459–474. doi: 10.1016/j.cell.2011.09.019 21963094
5. Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, et al. (2011) Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell 44 : 325–340. doi: 10.1016/j.molcel.2011.08.025 21906983
6. Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, et al. (2006) S6K1 - and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 314 : 467–471. 17053147
7. Peschiaroli A, Dorrello NV, Guardavaccaro D, Venere M, Halazonetis T, et al. (2006) SCFbetaTrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response. Mol Cell 23 : 319–329. 16885022
8. Sowa ME, Bennett EJ, Gygi SP, Harper JW (2009) Defining the human deubiquitinating enzyme interaction landscape. Cell 138 : 389–403. doi: 10.1016/j.cell.2009.04.042 19615732
9. Vashisht AA, Zumbrennen KB, Huang X, Powers DN, Durazo A, et al. (2009) Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science 326 : 718–721. doi: 10.1126/science.1176333 19762596
10. Davis MA, Larimore EA, Fissel BM, Swanger J, Taatjes DJ, et al. (2013) The SCF-Fbw7 ubiquitin ligase degrades MED13 and MED13L and regulates CDK8 module association with Mediator. Genes Dev 27 : 151–156. doi: 10.1101/gad.207720.112 23322298
11. Tan MK, Lim HJ, Bennett EJ, Shi Y, Harper JW (2013) Parallel SCF adaptor capture proteomics reveals a role for SCFFBXL17 in NRF2 activation via BACH1 repressor turnover. Mol Cell 52 : 9–24. doi: 10.1016/j.molcel.2013.08.018 24035498
12. Landry BD, Doyle JP, Toczyski DP, Benanti JA (2012) F-box protein specificity for g1 cyclins is dictated by subcellular localization. PLoS Genet 8: e1002851. doi: 10.1371/journal.pgen.1002851 22844257
13. Mark KG, Simonetta M, Maiolica A, Seller CA, Toczyski DP (2014) Ubiquitin ligase trapping identifies an SCF(Saf1) pathway targeting unprocessed vacuolar/lysosomal proteins. Mol Cell 53 : 148–161. doi: 10.1016/j.molcel.2013.12.003 24389104
14. Skaar JR, Pagan JK, Pagano M (2013) Mechanisms and function of substrate recruitment by F-box proteins. Nat Rev Mol Cell Biol 14 : 369–381. doi: 10.1038/nrm3582 23657496
15. Willems AR, Schwab M, Tyers M (2004) A hitchhiker's guide to the cullin ubiquitin ligases: SCF and its kin. Biochim Biophys Acta 1695 : 133–170. 15571813
16. Deshaies RJ (1999) SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol 15 : 435–467. 10611969
17. Reed SI (2006) The ubiquitin-proteasome pathway in cell cycle control. Results Probl Cell Differ 42 : 147–181. 16903211
18. Frescas D, Pagano M (2008) Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer 8 : 438–449. doi: 10.1038/nrc2396 18500245
19. Watanabe N, Arai H, Nishihara Y, Taniguchi M, Watanabe N, et al. (2004) M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc Natl Acad Sci U S A 101 : 4419–4424. 15070733
20. Busino L, Donzelli M, Chiesa M, Guardavaccaro D, Ganoth D, et al. (2003) Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage. Nature 426 : 87–91. 14603323
21. Jin J, Shirogane T, Xu L, Nalepa G, Qin J, et al. (2003) SCFbeta-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev 17 : 3062–3074. 14681206
22. Mamely I, van Vugt MA, Smits VA, Semple JI, Lemmens B, et al. (2006) Polo-like kinase-1 controls proteasome-dependent degradation of Claspin during checkpoint recovery. Curr Biol 16 : 1950–1955. 16934469
23. Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, et al. (2011) SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol 31 : 1121–1133. doi: 10.1128/MCB.01204-10 21245377
24. Lassot I, Segeral E, Berlioz-Torrent C, Durand H, Groussin L, et al. (2001) ATF4 degradation relies on a phosphorylation-dependent interaction with the SCF(betaTrCP) ubiquitin ligase. Mol Cell Biol 21 : 2192–2202. 11238952
25. Kanemori Y, Uto K, Sagata N (2005) Beta-TrCP recognizes a previously undescribed nonphosphorylated destruction motif in Cdc25A and Cdc25B phosphatases. Proc Natl Acad Sci U S A 102 : 6279–6284. 15845771
26. Uchida S, Watanabe N, Kudo Y, Yoshioka K, Matsunaga T, et al. (2011) SCFbeta(TrCP) mediates stress-activated MAPK-induced Cdc25B degradation. J Cell Sci 124 : 2816–2825. doi: 10.1242/jcs.083931 21807946
27. Wang Z, Zhong J, Gao D, Inuzuka H, Liu P, et al. (2012) DEPTOR ubiquitination and destruction by SCF(beta-TrCP). Am J Physiol Endocrinol Metab 303: E163–169. doi: 10.1152/ajpendo.00105.2012 22454292
28. Magliozzi R, Low TY, Weijts BG, Cheng T, Spanjaard E, et al. (2013) Control of epithelial cell migration and invasion by the IKKbeta - and CK1alpha-mediated degradation of RAPGEF2. Dev Cell 27 : 574–585. doi: 10.1016/j.devcel.2013.10.023 24290981
29. Margottin-Goguet F, Hsu JY, Loktev A, Hsieh HM, Reimann JD, et al. (2003) Prophase destruction of Emi1 by the SCF(betaTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev Cell 4 : 813–826. 12791267
30. Yang X, Wood PA, Ansell CM, Ohmori M, Oh EY, et al. (2009) Beta-catenin induces beta-TrCP-mediated PER2 degradation altering circadian clock gene expression in intestinal mucosa of ApcMin/+ mice. J Biochem 145 : 289–297. doi: 10.1093/jb/mvn167 19106159
31. Huang W, Lv X, Liu C, Zha Z, Zhang H, et al. (2012) The N-terminal phosphodegron targets TAZ/WWTR1 protein for SCFbeta-TrCP-dependent degradation in response to phosphatidylinositol 3-kinase inhibition. J Biol Chem 287 : 26245–26253. doi: 10.1074/jbc.M112.382036 22692215
32. D'Annibale S, Kim J, Magliozzi R, Low TY, Mohammed S, et al. (2014) Proteasome-dependent degradation of transcription factor activating enhancer-binding protein 4 (TFAP4) controls mitotic division. J Biol Chem 289 : 7730–7737. doi: 10.1074/jbc.M114.549535 24500709
33. Kim J, D'Annibale S, Magliozzi R, Low TY, Jansen P, et al. (2014) USP17 - and SCFbetaTrCP--regulated degradation of DEC1 controls the DNA damage response. Mol Cell Biol 34 : 4177–4185. doi: 10.1128/MCB.00530-14 25202122
34. Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, et al. (2003) Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol 163 : 767–775. 14638860
35. Pavitt GD, Ron D (2012) New insights into translational regulation in the endoplasmic reticulum unfolded protein response. Cold Spring Harb Perspect Biol 4.
36. Holcik M, Sonenberg N (2005) Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6 : 318–327. 15803138
37. Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW, et al. (2003) Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Mol Cell 11 : 1445–1456. 12820959
38. Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, et al. (2005) Towards a proteome-scale map of the human protein-protein interaction network. Nature 437 : 1173–1178. 16189514
39. Kim TY, Siesser PF, Rossman KL, Goldfarb D, Mackinnon K, et al. (2015) Substrate Trapping Proteomics Reveals Targets of the betaTrCP2/FBXW11 Ubiquitin Ligase. Mol Cell Biol 35 : 167–181. doi: 10.1128/MCB.00857-14 25332235
40. Low TY, Peng M, Magliozzi R, Mohammed S, Guardavaccaro D, et al. (2014) A systems-wide screen identifies substrates of the SCFbetaTrCP ubiquitin ligase. Sci Signal 7: rs8. doi: 10.1126/scisignal.2005882 25515538
41. Latres E, Chiaur DS, Pagano M (1999) The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene 18 : 849–854. 10023660
42. Qu Z, Qing G, Rabson A, Xiao G (2004) Tax deregulation of NF-kappaB2 p100 processing involves both beta-TrCP-dependent and-independent mechanisms. J Biol Chem 279 : 44563–44572. 15310758
43. Kruiswijk F, Yuniati L, Magliozzi R, Low TY, Lim R, et al. (2012) Coupled activation and degradation of eEF2K regulates protein synthesis in response to genotoxic stress. Sci Signal 5: ra40. doi: 10.1126/scisignal.2002718 22669845
44. Guardavaccaro D, Frescas D, Dorrello NV, Peschiaroli A, Multani AS, et al. (2008) Control of chromosome stability by the beta-TrCP-REST-Mad2 axis. Nature 452 : 365–369. doi: 10.1038/nature06641 18354482
45. Westbrook TF, Hu G, Ang XL, Mulligan P, Pavlova NN, et al. (2008) SCFbeta-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature 452 : 370–374. doi: 10.1038/nature06780 18354483
46. Paul M, Jabbar MA (1997) Phosphorylation of both phosphoacceptor sites in the HIV-1 Vpu cytoplasmic domain is essential for Vpu-mediated ER degradation of CD4. Virology 232 : 207–216. 9185604
47. Coyaud E, Mis M, Laurent EM, Dunham WH, Couzens AL, et al. (2015) BioID-based identification of SCF beta-TrCP1/2 E3 ligase substrates. Mol Cell Proteomics.
48. Inuzuka H, Tseng A, Gao D, Zhai B, Zhang Q, et al. (2010) Phosphorylation by casein kinase I promotes the turnover of the Mdm2 oncoprotein via the SCF(beta-TRCP) ubiquitin ligase. Cancer Cell 18 : 147–159. doi: 10.1016/j.ccr.2010.06.015 20708156
49. Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, et al. (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6 : 1099–1108. 11106749
50. Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, et al. (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18 : 3066–3077. 15601821
51. Harding HP, Zhang Y, Scheuner D, Chen JJ, Kaufman RJ, et al. (2009) Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2alpha) dephosphorylation in mammalian development. Proc Natl Acad Sci U S A 106 : 1832–1837. doi: 10.1073/pnas.0809632106 19181853
52. Novoa I, Zeng H, Harding HP, Ron D (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol 153 : 1011–1022. 11381086
53. Fornace AJ Jr, Jackman J, Hollander MC, Hoffman-Liebermann B, Liebermann DA (1992) Genotoxic-stress-response genes and growth-arrest genes. gadd, MyD, and other genes induced by treatments eliciting growth arrest. Ann N Y Acad Sci 663 : 139–153. 1482047
54. Schmidt EK, Clavarino G, Ceppi M, Pierre P (2009) SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods 6 : 275–277. doi: 10.1038/nmeth.1314 19305406
55. Kudo T, Ikeda M, Nishikawa M, Yang Z, Ohno K, et al. (2012) The RASSF3 candidate tumor suppressor induces apoptosis and G1-S cell-cycle arrest via p53. Cancer Res 72 : 2901–2911. doi: 10.1158/0008-5472.CAN-12-0572 22593196
56. Muratani M, Kung C, Shokat KM, Tansey WP (2005) The F box protein Dsg1/Mdm30 is a transcriptional coactivator that stimulates Gal4 turnover and cotranscriptional mRNA processing. Cell 120 : 887–899. 15797387
57. Deng J, Harding HP, Raught B, Gingras AC, Berlanga JJ, et al. (2002) Activation of GCN2 in UV-irradiated cells inhibits translation. Curr Biol 12 : 1279–1286. 12176355
58. Powley IR, Kondrashov A, Young LA, Dobbyn HC, Hill K, et al. (2009) Translational reprogramming following UVB irradiation is mediated by DNA-PKcs and allows selective recruitment to the polysomes of mRNAs encoding DNA repair enzymes. Genes Dev 23 : 1207–1220. doi: 10.1101/gad.516509 19451221
59. Zhang P, Gao K, Tang Y, Jin X, An J, et al. (2014) Destruction of DDIT3/CHOP Protein by Wild-Type SPOP but Not Prostate Cancer-Associated Mutants. Hum Mutat 35 : 1142–1151. doi: 10.1002/humu.22614 24990631
60. Brush MH, Shenolikar S (2008) Control of cellular GADD34 levels by the 26S proteasome. Mol Cell Biol 28 : 6989–7000. doi: 10.1128/MCB.00724-08 18794359
61. Popov N, Schulein C, Jaenicke LA, Eilers M (2010) Ubiquitylation of the amino terminus of Myc by SCF(beta-TrCP) antagonizes SCF(Fbw7)-mediated turnover. Nat Cell Biol 12 : 973–981. doi: 10.1038/ncb2104 20852628
62. Kaiser P, Wohlschlegel J (2005) Identification of ubiquitination sites and determination of ubiquitin-chain architectures by mass spectrometry. Methods in Enzymology 399 : 266–277. 16338362
63. Wohlschlegel JA (2009) Identification of SUMO-conjugated proteins and their SUMO attachment sites using proteomic mass spectrometry. Methods in molecular biology 497 : 33–49. doi: 10.1007/978-1-59745-566-4_3 19107409
64. Kelstrup CD, Young C, Lavallee R, Nielsen ML, Olsen JV (2012) Optimized fast and sensitive acquisition methods for shotgun proteomics on a quadrupole orbitrap mass spectrometer. Journal of proteome research 11 : 3487–3497. doi: 10.1021/pr3000249 22537090
65. Michalski A, Damoc E, Hauschild JP, Lange O, Wieghaus A, et al. (2011) Mass spectrometry-based proteomics using Q Exactive, a high-performance benchtop quadrupole Orbitrap mass spectrometer. Molecular & cellular proteomics: MCP 10: M111 011015.
66. Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP (2006) A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nature biotechnology 24 : 1285–1292. 16964243
67. Cociorva D, D LT, Yates JR (2007) Validation of tandem mass spectrometry database search results using DTASelect. Curr Protoc Bioinformatics Chapter 13: Unit 13 14.
68. Tabb DL, McDonald WH, Yates JR 3rd (2002) DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res 1 : 21–26. 12643522
69. Xu T, Venable JD, Park SK, Cociorva D, Lu B, et al. (2006) ProLuCID, A Fast and Sensitive Tandem Mass Spectra-based Protein Identification Programs. Molecular & Cellular Proteomics 5: S174.
70. Elias JE, Gygi SP (2007) Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods 4 : 207–214. 17327847
71. Florens L, Carozza MJ, Swanson SK, Fournier M, Coleman MK, et al. (2006) Analyzing chromatin remodeling complexes using shotgun proteomics and normalized spectral abundance factors. Methods 40 : 303–311. 17101441
Štítky
Genetika Reprodukční medicína
Článek Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and ThrombocytopeniaČlánek Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of andČlánek Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on PhenotypeČlánek Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human CancersČlánek A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic BacteriaČlánek The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System inČlánek The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological LifespanČlánek The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 6
-
Všechny články tohoto čísla
- Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Orphan Genes Find a Home: Interspecific Competition and Gene Network Evolution
- Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
- Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia
- Leveraging Identity-by-Descent for Accurate Genotype Inference in Family Sequencing Data
- Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
- Epigenetic Aging Signatures Are Coherently Modified in Cancer
- Silencing of DNase Colicin E8 Gene Expression by a Complex Nucleoprotein Assembly Ensures Timely Colicin Induction
- A Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
- The Orphan Gene Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation
- 9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
- The DnaA Protein Is Not the Limiting Factor for Initiation of Replication in
- FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling
- Multiple Changes of Gene Expression and Function Reveal Genomic and Phenotypic Complexity in SLE-like Disease
- Directed Evolution of RecA Variants with Enhanced Capacity for Conjugational Recombination
- The Regulatory T Cell Lineage Factor Foxp3 Regulates Gene Expression through Several Distinct Mechanisms Mostly Independent of Direct DNA Binding
- MreB-Dependent Inhibition of Cell Elongation during the Escape from Competence in
- DNA Damage Regulates Translation through β-TRCP Targeting of CReP
- Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of and
- The Analysis of () Mutants Reveals Differences in the Fusigenic Potential among Telomeres
- The Causative Gene in Chanarian Dorfman Syndrome Regulates Lipid Droplet Homeostasis in .
- Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype
- The . Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units
- AKTIP/Ft1, a New Shelterin-Interacting Factor Required for Telomere Maintenance
- Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
- Transfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
- Connecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
- Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Response to the Formal Letter of Z. Chrzanowska-Lightowlers and R. N. Lightowlers Regarding Our Article “Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers
- Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins
- Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential
- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level
- Genetic Changes to a Transcriptional Silencer Element Confers Phenotypic Diversity within and between Species
- Functional Assessment of Disease-Associated Regulatory Variants Using a Versatile Dual Colour Transgenesis Strategy in Zebrafish
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy
- The Human Blood Metabolome-Transcriptome Interface
- A Common Cancer Risk-Associated Allele in the Locus Encodes a Dominant Negative Inhibitor of Telomerase
- A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
- The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
- The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological Lifespan
- Dissection of a Complex Disease Susceptibility Region Using a Bayesian Stochastic Search Approach to Fine Mapping
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
- Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in
- Abnormal Activation of BMP Signaling Causes Myopathy in Null Mice
- Reproductive Mode and the Evolution of Genome Size and Structure in Nematodes
- Replication and Active Partition of Integrative and Conjugative Elements (ICEs) of the SXT/R391 Family: The Line between ICEs and Conjugative Plasmids Is Getting Thinner
- Motor and Sensory Deficits in the Mice Result from Mutation of the ESCRT Component HGS
- Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
- Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake
- Separable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4
- Multilayered Organization of Jasmonate Signalling in the Regulation of Root Growth
- Necrotic Cells Actively Attract Phagocytes through the Collaborative Action of Two Distinct PS-Exposure Mechanisms
- A Novel Feedback Loop That Controls Bimodal Expression of Genetic Competence
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Human Blood Metabolome-Transcriptome Interface
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



