-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSeparable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
At the heart of reproductive cell formation is a nuclear division process (meiosis) whereby homologous chromosomes segregate from one another. Meiotic partner chromosomes establish exclusive associations via a patterned distribution of crossover recombination events. During the maturation of recombination intermediates into crossovers, homologous axes are aligned in the context of a striking proteinaceous structure, the synaptonemal complex (SC). While genetic data link the SC with crossovers, it is unclear whether the mature SC structure facilitates crossover formation. Here we describe an interspecies complementation experiment in which we replace the S. cerevisiae version of an SC structural protein with an ancestrally related version from K. lactis. Our experiment reveals that, while SC proteins are required, mature full-length SC is dispensable for the formation of SC-associated crossovers in budding yeast. We furthermore discovered that most, but not all, members of a conserved meiotic crossover pathway are required for the crossovers that form in this interspecies context. Our findings strengthen the notion that a primary function of many SC proteins is to facilitate crossover recombination, independent of a role in building the larger SC structure. Furthermore, these data suggest that during normal meiosis in S. cerevisiae the assembled SC may act to functionally couple key crossover recombination proteins to one another.
Published in the journal: . PLoS Genet 11(6): e32767. doi:10.1371/journal.pgen.1005335
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005335Summary
At the heart of reproductive cell formation is a nuclear division process (meiosis) whereby homologous chromosomes segregate from one another. Meiotic partner chromosomes establish exclusive associations via a patterned distribution of crossover recombination events. During the maturation of recombination intermediates into crossovers, homologous axes are aligned in the context of a striking proteinaceous structure, the synaptonemal complex (SC). While genetic data link the SC with crossovers, it is unclear whether the mature SC structure facilitates crossover formation. Here we describe an interspecies complementation experiment in which we replace the S. cerevisiae version of an SC structural protein with an ancestrally related version from K. lactis. Our experiment reveals that, while SC proteins are required, mature full-length SC is dispensable for the formation of SC-associated crossovers in budding yeast. We furthermore discovered that most, but not all, members of a conserved meiotic crossover pathway are required for the crossovers that form in this interspecies context. Our findings strengthen the notion that a primary function of many SC proteins is to facilitate crossover recombination, independent of a role in building the larger SC structure. Furthermore, these data suggest that during normal meiosis in S. cerevisiae the assembled SC may act to functionally couple key crossover recombination proteins to one another.
Introduction
The segregation of homologous chromosomes at meiosis I is essential for the formation of haploid reproductive cells. Accurate segregation is dependent on the establishment of one or more associations between homologous chromosomes [1,2]. For most organisms, crossover recombination events in conjunction with sister chromatid cohesion provide the temporary associations needed between homologous chromosomes for their proper alignment and segregation on the meiosis I spindle. Interhomolog crossovers arise via the resolution of joint molecule (JM) intermediates, such as double Holliday junctions (dHJs), that form between homologous partner chromosomes during the repair of programmed, double-stranded DNA breaks (DSBs). The formation of interhomolog crossovers during meiosis depends on meiosis-specific proteins and, in a number of organisms, is temporally and functionally linked to a conserved meiotic chromosomal structure called the synaptonemal complex (SC).
Recombination-based associations between homologs can be cytologically detected and are referred to as chiasmata [1,3–5]. During the maturation of recombination intermediates into crossovers, however, such sites are often obscured by the presence of SC, a prominent, proteinaceous structure assembled along the entire lengthwise interface of aligned homologous chromosomes. The SC has a tripartite organization. One component of the larger structure is established via the multimeric assembly of coiled-coil containing proteins that form transverse filaments [6–8]. Transverse filaments are oriented perpendicular to the long axis of an aligned homolog pair and span the width of the SC, bridging the proteinaceous axes of each chromosome. Chromosome axes are referred to as lateral elements within the context of the mature SC. Additional proteins that make up the mature SC’s “central element” substructure assemble at the midline of the SC’s central region, apparently associating with and perhaps organizing transverse filament proteins. Zip1 is a coiled-coil protein component of the transverse filaments of the budding yeast SC [9,10], while Ecm11, SUMO and Gmc2 are proteins that are incorporated into the central element substructure [11–13].
Several additional proteins that are critical for the elaboration of SC along chromosomes in budding yeast do not appear to form structural components of the complex. These so-called “Synapsis Initiation Complex” (SIC) proteins [14], which include Zip2, Zip3, Zip4 and Spo16, localize at SC assembly (synapsis initiation) sites on meiotic chromosomes, many of which are thought to correspond to sites of ongoing recombination, and remain predominantly distributed as foci on full-length SCs after synapsis is complete [11–13,15–18].
The SC structure is established downstream of initial homology recognition and mediates the close apposition of homologous chromosomes (synapsis) during mid-meiotic prophase; the SC thus forms the context in which the majority of meiotic crossovers mature. The characterization of meiotic mutants has revealed a tight correlation between the presence of SC and the establishment of a proper number and distribution of interhomolog crossover recombination events, raising the possibility that SC structure itself plays a functional role in promoting crossover formation [1,7,19,20]. However, the molecular relationship between SC proteins, SC structure and the processing of recombination intermediates remains uncertain. The SC has also been linked to meiotic checkpoint signaling during meiosis, which can delay or arrest meiotic progression [21,22].
In many species, mutants defective in SC assembly (synapsis) exhibit a deficit in a genetically defined subset of crossovers, sometimes referred to as “class I” events [7,23–29]. SC-associated crossovers rely on SC proteins (SIC proteins and SC structural proteins in budding yeast) and often also rely on specific eukaryotic homologs of the bacterial MutS and MutL mismatch repair proteins (the Msh4/Msh5 and Mlh1/Mlh3 heterodimers, which comprise MutSγ and MutLγ, respectively) to promote the formation, maturation and resolution of the majority of dHJ intermediates that arise during meiosis [23,27,30–39]. The Msh4/Msh5 heterodimer (MutSγ) is capable of forming a “clamp” on double-stranded DNA and can recognize HJ structures [36]; these observations in conjunction with other data have led to the idea that Msh4/Msh5 acts to protect a dHJ intermediate from the anti-crossover activity of helicases such as Sgs1 [40,41]. Alternatively, or in addition, Msh4/Msh5 might promote the formation of a JM structure that can be recognized by a MutLγ-associated resolvase complex (in budding yeast this resolvase complex appears to involve MutLγ and Exo1 [23]), or may directly recruit MutLγ complexes to dHJs [32]. Once targeted, the MutLγ-Exo1 complex presumably resolves dHJ intermediates through its endonuclease activity [23,33,38,42]. The MutSγ complex can be detected cytologically at chromosomal sites where SIC proteins (Zip2, Zip3, Zip4) localize, and although MutSγ is dispensable per se for Zip1 elaboration along chromosomes, mutants missing MSH4 have been reported to exhibit delayed SC formation [30], suggesting the possibility of a complex interplay between the SC assembly process and discrete steps in the processing of DNA intermediates at recombination sites. Precisely how MutSγ and MutLγ complexes collaborate with one another and with SC-associated proteins to process recombination intermediates into interhomolog crossover products is not well understood.
On the other hand, MutSγ-MutLγ-independent crossovers can be detected in many organisms, including budding yeast [26]. Such so-called “class II” crossovers, in budding yeast, are genetically unlinked to SC protein activity and resolution of recombination intermediates associated with this class rely on the Mus81-Mms4, Slx1-Slx4, and/or Yen1 structure-selective endonuclease complexes [23,24,26,28,43]. While these observations suggest a conserved and perhaps functional relationship between the SC and MutSγ-MutLγ, it should be noted that SC-associated crossovers, MutSγ and MutLγ might not be strictly linked in all organisms. C. elegans, for example, relies on SC proteins and Msh4/Msh5 (MutSγ) for processing recombination intermediates toward an interhomolog crossover fate [44–47] but apparently employs predominantly MUS-81 and XPF-1 endonuclease complexes (presumably instead of MutLγ) to resolve such intermediates [48–50]. Drosophila also relies on SC proteins for crossover formation [51] but does not appear to have msh4 nor msh5 homologs, and instead uses a meiosis-specific version of mini-chromosome maintenance proteins to perform at least some of roles of MutSγ in processing recombination intermediates [52]. For any of these scenarios, how SC proteins and/or the SC structure might interface with DNA repair enzymes to facilitate a crossover event is poorly understood.
At least in budding yeast, several SC-associated proteins appear to facilitate early steps in MutSγ-MutL-associated crossover formation, prior to the elaboration of full-length SC. Mutant meiotic cells missing either ZIP2 or ZIP3 exhibit the same deficit as msh5 mutants in the accumulation of single end invasion (SEI) and dHJ intermediates, early crossover recombination intermediates that occur largely prior to full-length SC formation [27,35,53]. Although at one recombination hotspot, zip1 mutants showed a distinctly weaker defect in the accumulation of SEI and dHJ intermediates relative to zip2, zip3 and msh5 mutants [27], the altered kinetics of SEI and dHJ formation observed in zip1 mutants has been used to argue that even the SC transverse filament protein Zip1 acts early, prior to its elaboration along chromosomes, to facilitate the formation of qualitatively normal dHJ structures [27,54,55]. Consistent with the idea that SC proteins are involved in recombination independent of their role in elaborating an SC structure along the chromosome, SIC proteins localize to chromosomal sites that are correlated with class I crossover-designated recombination events, even in the absence of full-length SC [14,15,56]. How SC proteins functionally interface with the processes mediated by MutSγ, MutLγ and/or other recombination proteins during crossover formation is unknown: Are SC proteins, particularly SC structural proteins like Zip1, merely forming a scaffold upon which recombination enzymes dock, or do these proteins have a more specialized role in the processing of recombination intermediates?
Furthermore, is there any role for the fully assembled SC structure per se in MutSγ-MutLγ-associated crossover formation? Later steps in the maturation of crossovers occur in the context of full-length SC, and MutLγ-mediated resolution occurs concomitant with SC disassembly in budding yeast; the latter two events are triggered by Cdc5 activity at a mid-late prophase transition marked by elevated Ndt80 activity [3,19]. As the relevant protein targets of Cdc5 with respect to these events remain unknown, it is unclear whether the process of SC disassembly is normally mechanistically linked to the resolution of recombination intermediates into crossovers.
As noted above, mutants lacking ZIP1 appear to have a weaker defect in accumulating JM structures (presumed to be dHJs) relative to mutants missing ZIP2, ZIP3 or MSH5 [27], but zip1 mutants nevertheless lack MutSγ-MutLγ-associated crossovers [23,25,27,30,34]. This observation is consistent with a role for the mature SC in facilitating later steps in the successful maturation of MutSγ-MutLγ interhomolog recombination events. Other studies support the possibility that SC is dispensable for generating meiotic crossovers in budding yeast. These studies describe mutant situations in which SC assembly is disrupted yet crossovers form (i.e. in the absence of normal SUMOylation [11,13] and in the absence of the meiosis-specific chromosomal axis protein Red1 [55]. However, these prior investigations did not explore whether the apparently SC-independent crossovers form through a canonical SIC protein/ MutSγ-MutLγ–dependent mechanism. Thus the question remains: Is the assembled, full-length SC structure required for MutSγ-MutLγ-dependent crossover formation in budding yeast?
Here we describe an interspecies complementation experiment that reveals intriguing features about the relationship between the full-length SC structure, the Zip1 transverse filament protein, and MutLγ-mediated crossover events in S. cerevisiae. We generated S. cerevisiae strains that express Kluyveromyces lactis ZIP1 in place of Saccharomyces cerevisiae ZIP1, and observed that spores from K. l. ZIP1-expressing budding yeast display wild-type crossover levels despite a failure in SC formation. Our in-depth analysis of this separation-of-function version of Zip1 reveals several interesting findings. In particular, our study demonstrates that the full-length SC structure is dispensable for Zip-protein mediated and Mlh3-dependent crossover formation in budding yeast. Our data strongly suggest that crossover recombination activity, independent of SC elaboration, is sufficient to overcome a checkpoint-induced block to meiotic progression. Furthermore, we describe the surprising result that K. lactis Zip1 promotes crossovers in S. cerevisiae cells that are SC protein - and Mlh3-dependent, but largely independent of the MutSγ proteins Msh4 and Msh5. MutLγ activities are thus uncoupled from MutSγ in the context of K. l. ZIP1, suggesting that at least one aspect of S. c. Zip1 normally mediates a constraint that couples MutLγ-dependent resolvase activity to MutSγ-associated crossover intermediates. We discuss the idea that SC assembly itself could be involved in establishing such a constraint.
Results
K. lactis Zip1 rescues meiotic chromosome segregation functions of S. cerevisiae Zip1
Kluyveromyces lactis ZIP1 encodes a protein that shares 25% identity and 16.7% homology with S. cerevisiae’s Zip1, and the two versions of Zip1 are predicted to share overall structural characteristics including an extended central coiled-coil domain flanked by non-coiled coil segments (Fig 1A). To determine whether K.l. Zip1 can rescue the meiotic functions of S. c. Zip1, we created an S. cerevisiae strain (CO9) in which the S. c. ZIP1 ORF is replaced by the K. l. ZIP1 ORF (Fig 1B).
Fig. 1. Alignment of K. lactis and S. cerevisiae Zip1 and experimental design. 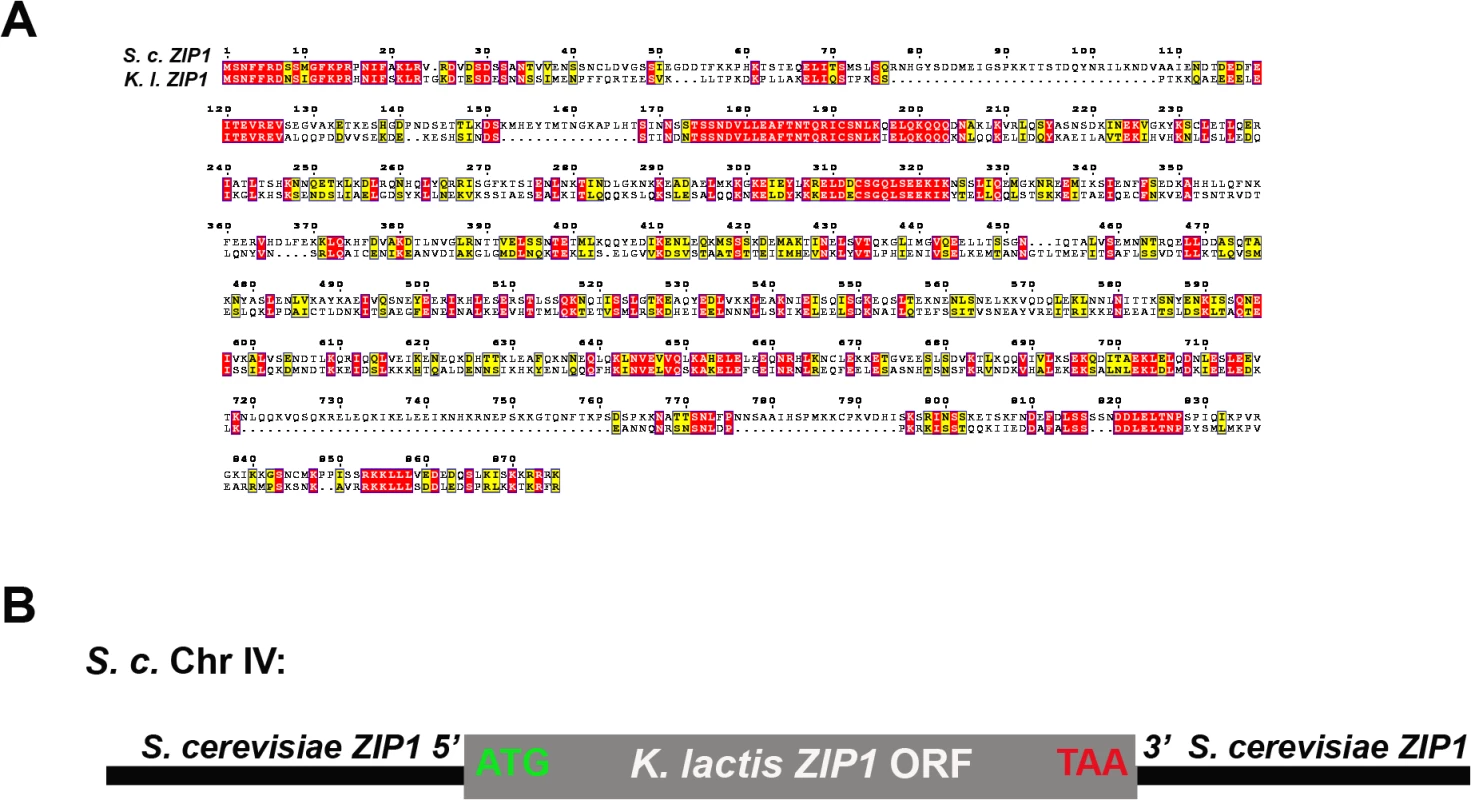
(A) S. cerevisiae and K. lactis Zip1 sequence alignment. ClustalW (http://www.ch.embnet.org) was used to align amino acid sequences for the translated products of ZIP1 from the S. cerevisiae and K. lactis genomes, and the alignment was processed for presentation using MEGA tool [94]. The alignment suggests ~25% identity (red boxes), and ~16.7% homology (yellow boxes) between the two sequences. The approximate boundaries of an extended central region of the two proteins that is predicted to form coiled-coil are marked with blue arrow brackets (COILS program, [95]). (B) Cartoon depicting the chromosome IV genotype of S. cerevisiae strains expressing K. l. ZIP1. The diploid cells used in our study are homozygous for the locus illustrated in the cartoon. The start and stop codons of the K. l. ZIP1 open reading frame are indicated in green and red, respectively. The success of homologous chromosome segregation at meiosis I in budding yeast correlates with the viability of the haploid spore products formed. Accordingly, when we assessed spore viability among S. cerevisiae strains, greater than 90% of spores from meiotic cells carrying S. c. ZIP1 (YAM1252) were viable while only 56% of spores were viable from diploids missing ZIP1 (and therefore missing class I crossovers; Table 1). We found that 77% of spores from diploids expressing K. l. ZIP1 were viable. Thus, K. l. Zip1 is able to promote successful meiotic chromosome segregation to some extent, even in an S. cerevisiae cell context.
Tab. 1. Sporulation efficiency and spore viability in S. cerevisiae cells expressing S. c. or K. l. ZIP1. 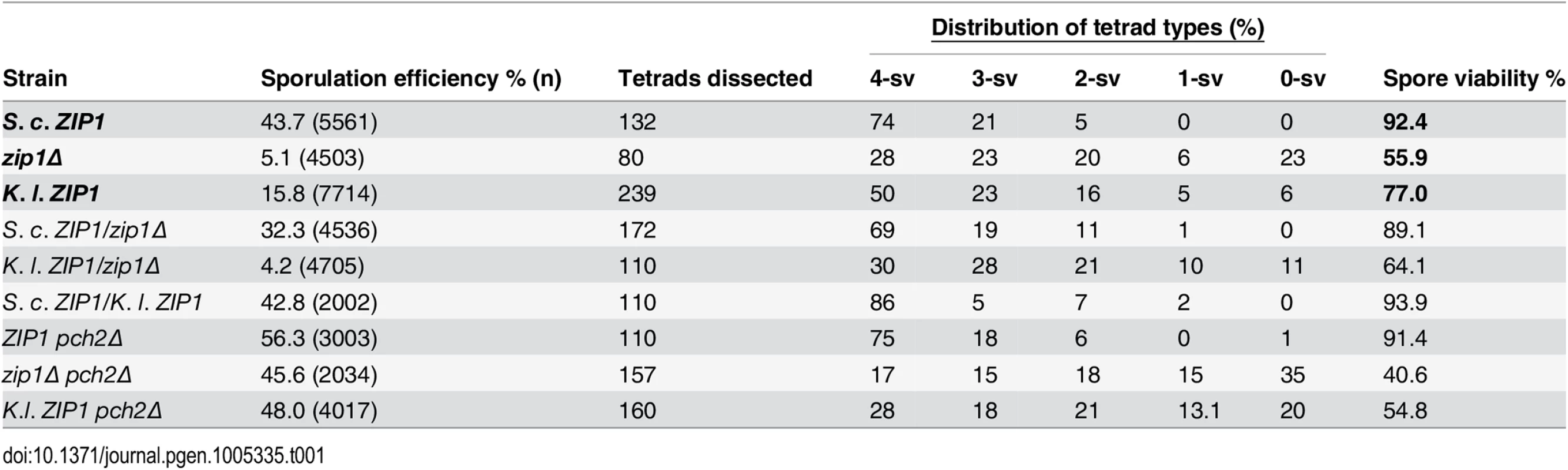
Sporulation efficiency and viability of spores produced by strains expressing either S. c. ZIP1 or K. l. ZIP1. Sporulation efficiency is the fraction of total sporulating cells that are 2, 3 or 4-spore asci. The far right column shows the overall spore viability of each strain. Displayed in each “Distribution of tetrad types” column is the frequency of tetrads containing four viable spores (4-sv), three viable spores (3-sv), two viable spores (2-sv), one viable spore (1-sv) or no viable spores (0-sv). Full strain genotypes are listed in S6 Table. S. cerevisiae cells expressing K. l. ZIP1 as the sole source of Zip1 also display an intermediate sporulation efficiency. About half of sporulating diploid cells from wild-type S. cerevisiae of the BR1919-8B background progress to form spores (44% in the experiment shown in Table 1). Due to a Pch2-mediated checkpoint [57], only ~5% of sporulating diploids from zip1 null S. cerevisiae strains form spores (Table 1). We found that K.l. ZIP1-expressing cells exhibit ~16% sporulation efficiency. PCH2 removal from K. l. ZIP1-expressing S. cerevisiae cells resulted in a nearly wild-type (45%, n = 3002) sporulation efficiency, indicating that the Pch2-mediated prophase checkpoint is responsible for the diminished spore formation by K. l. ZIP1-expressing S. cerevisiae cells.
K. lactis Zip1 localizes to meiotic chromosomes but fails to assemble synaptonemal complex in S. cerevisiae
We investigated the localization of K. l. Zip1 on S. cerevisiae meiotic chromosomes using antisera raised against K. l. Zip1 (kindly provided by Abby Dernburg) as well as antisera raised against S. c. Zip1 [10,58]. Both sets of antisera gave similar results, but because anti—S. c. Zip1 antisera gave a more robust and consistent signal, this latter antibody was used for the analyses presented here.
To assess the distribution of S. c. or K. l. Zip1 on meiotic prophase chromosomes, meiotic nuclei from S. c. cells expressing either S. c. ZIP1 (control) or K. l. ZIP1 were harvested at two-hour time points between 12 and 24 hours after transfer to sporulation medium, and surface-spread on glass slides for examination by immunofluorescence. Each strain expressed a single copy of ECM11-MYC; Ecm11 localizes uniformly along the length of the budding yeast SC central element substructure, and a fraction of Ecm11 protein in the SC is SUMOylated [12,13]. Strains were additionally missing NDT80 activity, which is required for meiotic nuclei to progress beyond the pachytene stage of meiotic prophase [59]. Chromosome spreads from control and experimental nuclei were stained with anti-Zip1, anti-SUMO and anti-MYC antisera in order to assess whether SC structure is properly established.
At the 22 and 24 hour time points, a majority (> 80%) of chromosome spreads from ndt80Δ mutants appeared to be at the pachytene stage where homologous chromosomes are aligned and exhibit nearly full synapsis (Figs 2 and 3A). The DAPI-stained DNA morphology of surface-spread pachytene chromosomes from wild-type S. cerevisiae reveals distinct, individualized chromosome pairs with Zip1, Ecm11-MYC, and SUMO coinciding as linear structures at the interface of each chromosome pair (Figs 2 and 3A, [11–13]). In contrast, while the DAPI-stained DNA morphology of our K.l. ZIP1 meiotic time course nuclei suggested normal progression into the pachytene stage, none of the meiotic chromosome spreads from cells expressing K. l. ZIP1 at any of the seven time points (n = 560) exhibited full-length linear structures of Zip1, SUMO, or Ecm11-MYC. Across time points, the vast majority of nuclei displayed either no detectable K. l. Zip1 or a handful of K. l. Zip1 foci dispersed along meiotic chromosomes (Figs 2 and 3), accompanied by a punctate distribution of SUMO and Ecm11 on chromosomes. The number of K. l. Zip1 chromosome-associated foci exhibited by these nuclei ranged from 1–36, with an average of 11 K. l. Zip1 foci per nucleus. The most prominent K.l. Zip1 structure found associated with S. cerevisiae meiotic nuclei was an aggregate of K. l. Zip1, Ecm11-MYC and SUMO proteins (examples in Figs 2 and 3A and S1). K. l. Zip1 polycomplexes also contained the SIC protein, Zip3 (Fig 4). Such “polycomplex” aggregates of synapsis proteins are a characteristic feature of meiotic nuclei in S. cerevisiae mutants that fail to assemble SC [12,15,18,20]. K. l. Zip1 polycomplexes were exhibited by over half (358/560) of the surface-spread nuclei, and were observed at both early and later meiotic time points, regardless of whether they displayed detectable chromosomal K. l. Zip1 foci.
Fig. 2. K. lactis Zip1 fails to assemble mature SC on S. cerevisiae meiotic chromosomes. 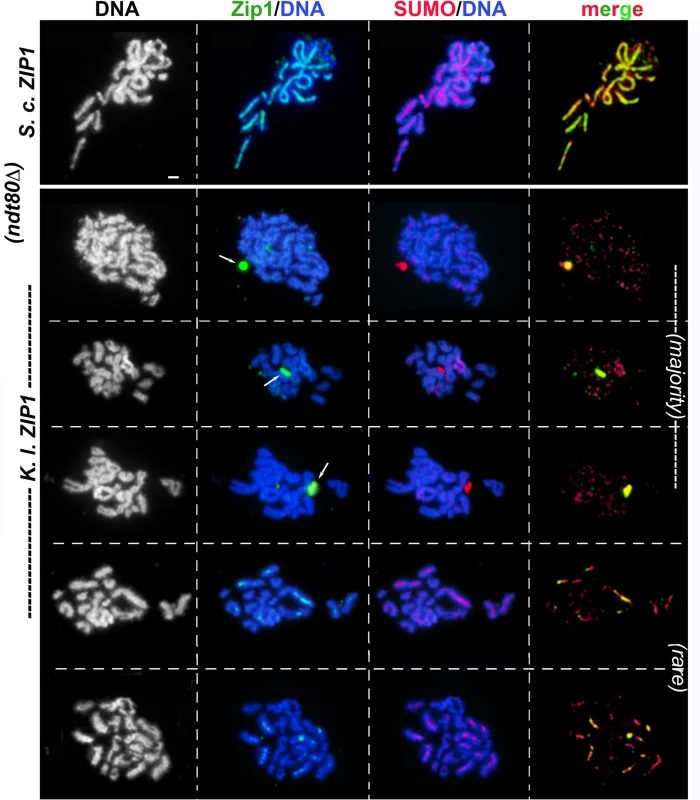
S. cerevisiae meiotic cells expressing S. c. ZIP1 (K375; top row) or K. l. ZIP1 (YT12) were surface-spread at 2 hour intervals during sporulation, beginning at 12 hours after entry into sporulation medium and ending at 24 hours. These strains are homozygous for an ndt80 null allele, and thus will not progress beyond the pachytene stage of meiotic prophase. Immunolocalization was used to label S. c. or K. l. Zip1 (green) and SUMO (red) on meiotic chromosomes (which are labeled with DAPI, white in first column and blue in second and third columns). Arrows point to polycomplex aggregates of Zip1. Sparse K. l. Zip1 foci and dotty SUMO staining was typically observed in S. cerevisiae cells expressing K. l. ZIP1 (rows 2–4). Rarely (less than 6% of the total spreads, n = 560) SUMO assembled short linear structures, often overlapping a short linear stretch or several foci of K. l. Zip1 (rows 5 and 6). Scale, 1 micron. Fig. 3. Ecm11-MYC predominantly assembles as foci on meiotic chromosomes and is partially SUMOylated in S. cerevisiae cells expressing K. lactis ZIP1. 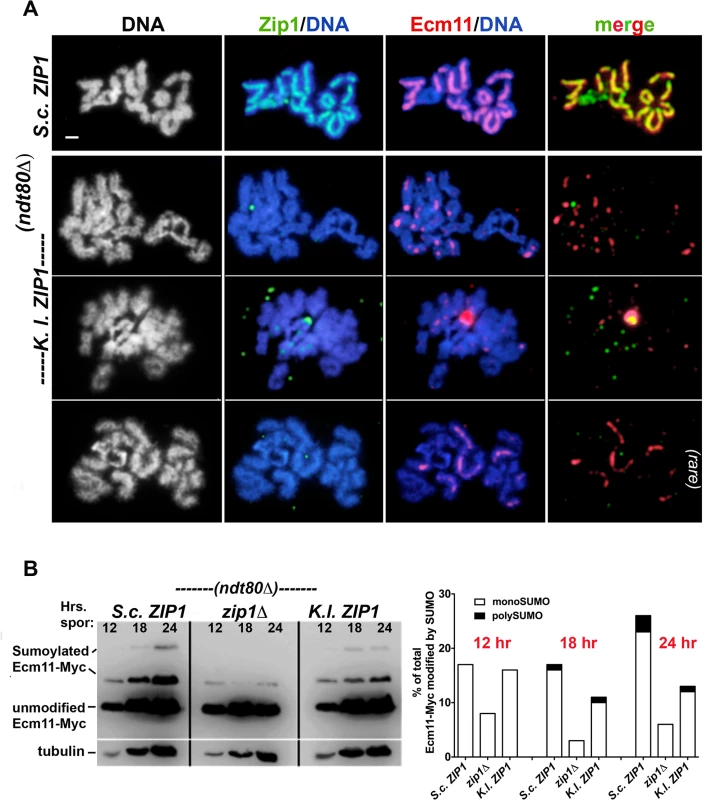
A) S. cerevisiae meiotic cells carrying one copy of ECM11-MYC and expressing S. c. ZIP1 (K292; top row) or K. l. ZIP1 (K268) were surface-spread at 2 hour intervals during sporulation, beginning at 12 hours after entry into sporulation medium and ending at 24 hours. These strains are homozygous for an ndt80 null allele, and thus will not progress beyond the pachytene stage of meiotic prophase. Immunolocalization was used to label S. c. or K. l. Zip1 (green) and Ecm11-MYC (red) on meiotic chromosomes (labeled with DAPI, white in first column and blue in second and third columns). Sparse K. l. Zip1 foci and dotty Ecm11-MYC staining was typically observed in S. cerevisiae cells expressing K. l. ZIP1 (rows 2–3). Similar to our observations of SUMO localization on meiotic chromosomes in S. cerevisiae cells expressing K. l. ZIP1, Ecm11-MYC occasionally (less than 6% of the total spreads) assembled short linear structures. Scale, 1 micron. (B) Trichloroacetic acid (TCA) extracts from sporulating cultures of S. cerevisiae homozygous for ECM11-MYC, and carrying S. c. ZIP1 (AM2712), a zip1 null allele (AM2784), or K. l. ZIP1 (AM2711) were run on a 10% polyacrylamide gel and western blotting was used to detect unSUMOylated, monoSUMOylated and polySUMOylated forms of Ecm11-MYC, as described in [12,13]. These strains are homozygous for an ndt80 null allele, and thus will not progress beyond the pachytene stage of meiotic prophase. For each strain, the fraction of total Ecm11-MYC found in the mono-SUMOylated (open bar) and poly-SUMOylated (shaded bar) forms at three sporulation time points is plotted in the graph below. Fig. 4. Relative distribution of Zip3-MYC and K. l. Zip1 on meiotic chromosomes in K. l. ZIP1-expressing cells. 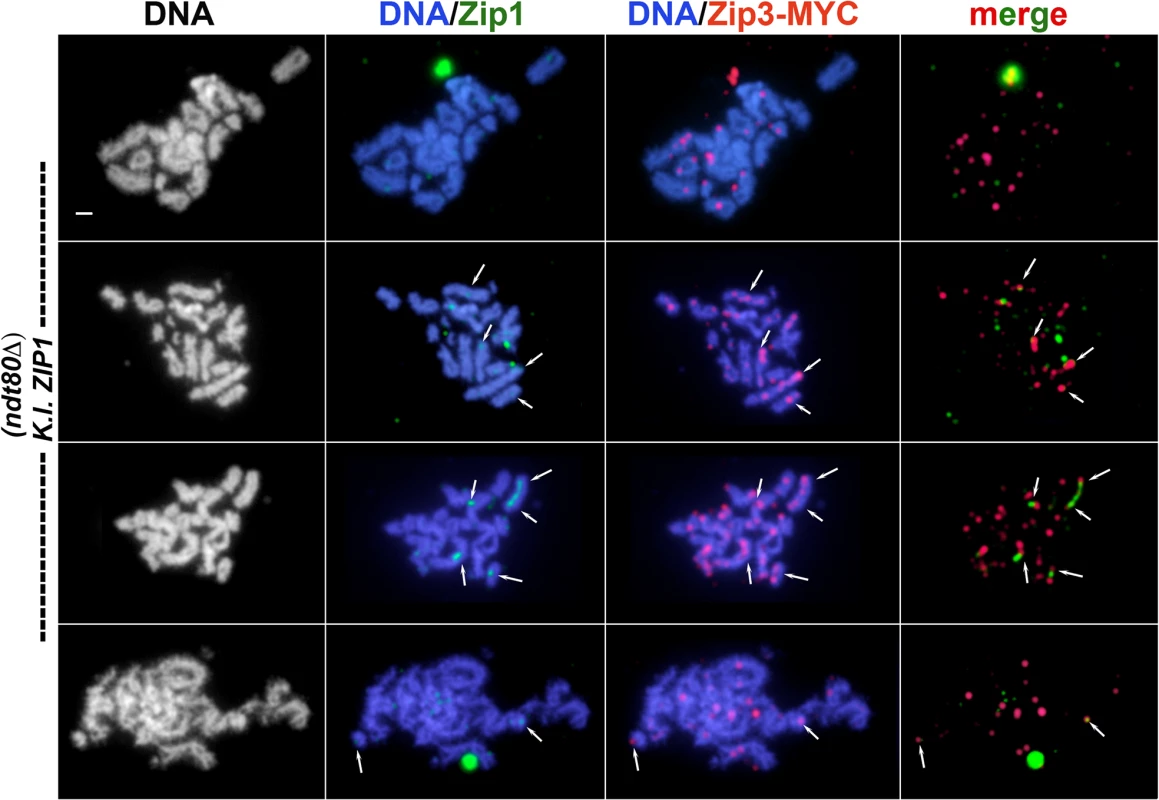
S. cerevisiae meiotic cells carrying one copy of ZIP3-MYC and expressing K. l. ZIP1 (K375) were surface-spread at 2 hour intervals during sporulation, beginning at 12 hours after entry into sporulation medium and ending at 24 hours. These strains are homozygous for an ndt80 null allele, and thus will not progress beyond the pachytene stage of meiotic prophase. Immunofluorescence was used to label K. l. Zip1 (green) and Zip3-MYC (red) on meiotic chromosomes (labeled with DAPI, white in first column and blue in second and third columns). Note in the top row images, the polycomplex aggregate of K. l. Zip1 overlaps an aggregate of Zip3-MYC. Arrows point to a subset of apparent co-localization or adjacency events between K. l. Zip1 foci and Zip3-MYC. Scale, 1 micron. An additional Zip1 staining pattern was rarely observed, in which a single or a small number of short linear Zip1 structures appear on chromosomes (examples in Figs 2 and 3). Such short linear structures may result from bona fide but aborted elaborations of an SC precursor, or could be the result of several K. l. Zip1 foci assembled side-by-side on the chromosome. Interestingly, especially in those nuclei that showed robust Zip1 foci or short linear stretches, Ecm11 and SUMO often appeared as short linear assemblies that encompass but surpass the Zip1 structures in length (Figs 2 and 3). Short linear Zip1, Ecm11 and/or SUMO assemblies were rarely found in any nuclei among all time points examined, indicating that these structures are not stable; we observed an apparently linear Zip1, Ecm11 or SUMO structure in 0/75 nuclei at 12 hours, 3/91 nuclei at 14 hours, 4/92 nuclei at 16 hours, 10/85 nuclei at 18 hours, 6/89 nuclei at 20 hours, 1/84 nuclei at 22 hours and 3/44 nuclei at 24 hours. Taken together, our data for three readouts of SC structure (Zip1, Ecm11, SUMO), across a 12-hour meiotic prophase time course, indicate that K. l. Zip1 fails to assemble mature SC in S. cerevisiae cells.
To guard against the possibility that our antibody recognizes only a subset of potentially detectable K. l. Zip1 protein, we examined the distribution of an epitope-tagged version of K. l. Zip1, which retains function. Insertion of a V5 epitope tag just after asparagine at position 647 in the K. l. Zip1 protein failed to rescue the spore viability defect of zip1 null S. cerevisiae cells, despite the fact that YFP, inserted at the equivalent position (amino acid 700) of S. c. Zip1, creates a functional S. c. Zip1-YFP protein [60]. However, insertion of the V5 epitope tag just after arginine at position 472 generated a K. l. Zip1 protein that rescues the spore viability defect of S. c. zip1 null diploids to the same extent as untagged K. l. Zip1: Diploids carrying the K. l. ZIP1-V5 cassette in place of the S. c. ZIP1 ORF exhibited 20.6% sporulation efficiency and the spore products exhibited 79.9% viability (307 viable out of 384 spores dissected). Immunolocalization of the V5 tag in S. cerevisiae meiotic nuclei expressing K. l. ZIP1-V5 in conjunction with ECM11-MYC revealed a distribution of K. l. Zip1 on S. cerevisiae meiotic chromosomes indistinguishable from that observed using anti-Zip1 antisera (S1 Fig). Importantly, linear V5 structures were never observed among the meiotic pachytene nuclei we screened. Instead, V5 staining most often appeared as a small polycomplex structure, which typically contained Ecm11-MYC (S1 Fig). Occasionally, meiotic chromosomes displayed a limited number of faint K. l. Zip1-V5 foci on chromosomes; these V5 foci often, but not always, co-localized with Ecm11-MYC foci.
Further support for the conclusion that K. l. Zip1 fails to assemble SC in S. cerevisiae came from staining of the axial element protein, Red1, on surface-spread meiotic chromosomes. Red1 labels the axes of meiotic prophase chromosomes [61]; because the SC structure brings homolog axes into intimate alignment along their entire lengths, the closely apposed Red1-labeled axes of partner homologs in wild-type meiotic pachytene bivalents appear as a single linear structure along their full-lengths (Fig 5, top left). In contrast, meiotic pachytene chromosomes from zip1 null cells exhibit loosely-associated chromosome axes labeled by Red1 (Fig 5, bottom left) [10]. The Red1-labeled “loops” apparent in such synapsis-defective mutants correspond to homolog axes joined in intimate alignment only at sporadic positions along the chromosomes (these “axial associations” are presumably where a crossover event has been established) [62]. The Red1-stained chromosome axis patterns exhibited by surface-spread meiotic chromosomes from K. l. ZIP1-expressing S. cerevisiae cells appeared indistinguishable from those seen in zip1 null cells, consistent with an absence of mature SC structure (Fig 5, middle left).
Fig. 5. Zip3-MYC and Zip4-HA levels exhibited by K. l. ZIP1-expressing cells resemble zip1 null levels. 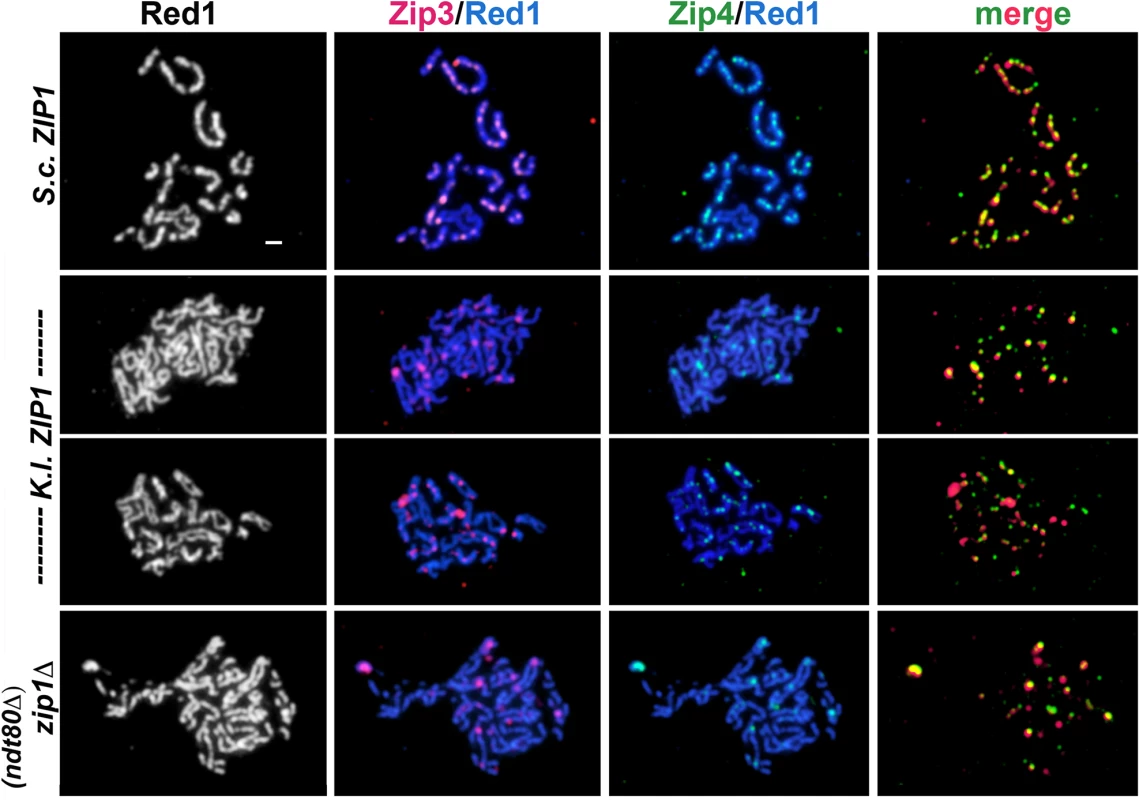
Sporulating cultures of S. cerevisiae meiotic cells carrying one copy of ZIP3-MYC and ZIP4-HA and expressing S. c. ZIP1 (AM3362), a zip1 null allele (AM3363) or K. l. ZIP1 (AM3361) were surface-spread on glass slides at 24 hours. AM3362 and AM3361 strains are homozygous for an ndt80 null allele, and thus will not progress beyond the pachytene stage of meiotic prophase. AM3363 sporulating cultures are enriched for pachytene owing to the fact that zip1null meiotic cells trigger the meiotic prophase checkpoint. Immunolocalization was used to label the chromosomal axis protein Red1 (white, blue), Zip3-MYC (red) and Zip4-HA (green) on surface-spread meiotic chromosomes. See (S2 Fig) for quantification of Zip3-MYC and Zip4-HA foci and frequency of co-localization. Scale, 1 micron. The relationship between K. lactis Zip1 and S. cerevisiae synapsis proteins
As described above, proteins that appear to have a structural role in building SC (such as Ecm11 and SUMO) fail to assemble normal linear structures in S. cerevisiae cells expressing K. l. ZIP1. However, evidence that K. l. Zip1 is able to interface, at least to some extent, with components of the SC in S. cerevisiae cells was revealed by an examination of SUMOylated forms of Ecm11-MYC in wild-type, zip1 null, and K. l. ZIP1-expressing cells. Humphryes et al. reported that the SUMOylation of Ecm11-MYC during meiosis is largely dependent on Zip1 [12]. Consistent with their report, we found that levels of mono - and poly-SUMOylated forms of Ecm11-MYC were severely diminished in meiotic cell extracts from zip1 null cells, relative to wild-type meiotic cell extracts (Fig 3B). In meiotic cell extracts from S. cerevisiae cells expressing K. l. ZIP1, mono - and poly-SUMOylated Ecm11-MYC rose to near wild-type levels between the 0 and 12 hour time points, and appeared intermediate between wild-type and the zip1 null at the 18 and 24 hour time points (Fig 3B). These data demonstrate that K. l. Zip1 can support partial levels of Ecm11-MYC SUMOylation in S. cerevisiae meiotic cells. K. l. Zip1 might promote the SUMOylation of Ecm11 within complexes on chromosomes and/or within the polycomplex structure (where K. l. Zip1, Ecm11-MYC, and SUMO co-localize) [11,12].
We also examined the distribution of SIC proteins on meiotic chromosomes in K.l. ZIP1-expressing cells. SIC proteins, such as Zip2, Zip3 and Zip4, are required for SC assembly, but localize as multiple foci along the length of SCs instead of displaying a linear, Zip1-like distribution ([14–16,18] and Figs 4 and 5). We first examined Zip3-MYC and Zip1 on surface-spread meiotic chromosomes from S. cerevisiae cells expressing K. l. ZIP1 (Fig 4). Nuclei were harvested every two hours from 12 to 24 hours after transfer to sporulation medium. At each time point, the number of Zip3-MYC foci on surface-spread chromosomes from K.l. ZIP1 expressing cells ranged from ~10–40, which is diminished relative to the range of foci (50–70) observed on wild-type pachytene chromosomes (S2 Fig). A fraction of K. l. Zip1 foci in each nucleus (arrows in Fig 4) appeared to overlap or localize adjacent to a Zip3-MYC focus. Taking nuclei from all time points into account, 50% (1059/2108, n = 184 nuclei) of Zip1 foci overlapped or localized adjacent to a Zip3-MYC focus. However, the low number of Zip1 relative to Zip3-MYC foci exhibited by each nucleus prevents a rigorous assessment of whether the apparent adjacency events are significantly different from what one would observe from a random distribution of Zip3-MYC and K. l. Zip1.
Next we analyzed the co-localization of Zip3-MYC and Zip4-HA on pachytene stage meiotic chromosomes from S. cerevisiae cells expressing S. c. ZIP1 or K.l. ZIP1, or in cells missing ZIP1 altogether (Fig 5). As has been previously reported [14–16,18,58], we observed that the number of Zip3 and Zip4 foci on wild-type pachytene chromosomes ranged between 50–70, and well over 90% of Zip3 and Zip4 foci co-localize with one another (Figs 5 and S2). The number of Zip3-MYC and Zip4-HA foci observed on pachytene chromosomes from zip1 null cells was diminished, relative to wild type, ranging from 8–31 with an average of 19 +/ - 0.95 Zip3-MYC and from 4–35 with an average of 16 +/ - 1.00 Zip4-HA foci per nucleus (n = 39 nuclei). This observation is in contrast to a prior report stating that normal numbers of Zip3 foci are observed on meiotic chromosomes in zip1 null cells [15] but is consistent with the lower number of Zip3 foci that were observed on meiotic pachytene chromosomes from zip1 null cells in other studies [63,64]. The diminished number of SIC foci on chromosomes from zip1 null cells indicates a role for the SC or Zip1 in either the formation or persistence of SIC complexes during meiotic prophase.
As previously reported [18], the co-localization between Zip3-MYC and Zip4-HA on meiotic chromosomes from zip1 null strains is high, although in our experiments not as high as that observed on wild-type pachytene chromosomes (S2B Fig). In the 39 zip1 null pachytene chromosome spreads examined, the number of Zip3-Zip4 coincident localization events ranged from 38%-100% with an average of 70 +/ - 3% (S2 Fig).
We found that the number of Zip3-MYC and Zip4-HA foci observed on surface-spread meiotic chromosomes from cells expressing K. l. Zip1 resembled the levels observed on pachytene-stage chromosomes from zip1 null cells. We counted between 5–40, with a mean of 16 +/ - 0.83 Zip3-MYC foci, and between 3–35, with a mean of 15 +/ - 0.90 Zip4-HA foci on meiotic pachytene stage chromosomes from K. l. ZIP1-expressing cells (n = 47). Apparent co-localization events observed between Zip3-MYC and Zip4-HA on meiotic chromosomes from S. cerevisiae expressing K. l. Zip1 ranged from 33%-100% with an average of 63 +/ - 3% (S2B Fig). The percent Zip3-Zip4 co-localization values for zip1 null and for K. l. ZIP1-expressing cells are not significantly different from one another, as evaluated by an unpaired t test using Welch’s correction (two-tailed P = 0.1). Our data indicate that expression of K. l. ZIP1 is not sufficient to restore a wild-type number of cytologically-detectable SIC foci to pachytene chromosomes in S. cerevisiae meiotic cells missing S. c. ZIP1.
K. lactis Zip1 provides partial function at centromeres
Zip1 has been found to associate with the centromere regions of meiotic prophase chromosomes and centromeres mark sites where many of the earliest SC assembly events occur in S. cerevisiae [63,65]. Furthermore, S. c. Zip1 promotes pairwise associations between centromeres outside of the context of the SC, during early and late meiotic prophase [65–67]. To investigate whether K. l. Zip1 plays a role at centromeres in S. cerevisiae meiotic nuclei, we monitored an epitope-tagged version of the kinetochore protein, Ctf19-MYC, which localizes to the centromere regions on meiotic prophase chromosomes [65,68].
In order to assess co-localization between K. l. Zip1 and meiotic centromeres, we harvested sporulating cells at two-hour intervals that spanned 12 to 24 hours following transfer to sporulation medium. Across all time points, surface-spread meiotic chromosomes from cells expressing K. l. ZIP1 and CTF19-MYC exhibited an average of 10 K. l. Zip1 foci and 22 Ctf19-MYC foci (n = 120 nuclei). Despite the fact that centromere foci typically far outnumbered detectable K.l. Zip1 foci, K. l. Zip1 foci appeared co-localized or adjacent to Ctf19-MYC foci only 46% of the time (539/1163 Zip1 foci) (S3 Fig). From an analysis of exclusively pachytene stage nuclei (classified based on DAPI-stained DNA morphology) we measured an average of eight K. l. Zip1 foci and 21 Ctf19-MYC foci (n = 68 nuclei); in this subgroup, K. l. Zip1 foci appeared co-localized or adjacent to Ctf19-MYC foci 59% of the time (325/555 Zip1 foci). Thus, while K. l. Zip1 and centromeres do not exhibit a strong co-localization pattern, these data do not rule out the possibility that K. l. Zip1 may have some preferential affinity for centromere sites on S. cerevisiae meiotic chromosomes.
We additionally explored the relationship between K. l. Zip1 and centromeres in S. cerevisiae meiotic cells through a functional assay. Zip1 facilitates two-by-two associations between meiotic prophase centromeres, independent of SC formation [65–67]. For example, spo11 mutant meiotic cells fail to initiate recombination and also fail to assemble SC, but centromeres nevertheless tend to associate in pairs. Thus, surface-spread meiotic prophase nuclei from spo11 strains exhibit fewer than 32, and often an average of 16, centromere groups. In contrast, surface spread nuclei from spo11 meiotic cells that are also missing ZIP1 exhibit closer to 32 centromere foci, demonstrating that Zip1 is required for the observed Spo11-independent centromere associations. Zip1-dependent centromere associations can also be observed outside of the context of SC, in haploid cells capable of entry into meiosis. In the haploid cell context, Zip1-dependent centromere associations are found in both spo11 null and SPO11 contexts (neither of which supports extensive SC formation); the mechanisms used for Zip1-dependent centromere associations in spo11 null versus SPO11 cells may involve distinct (yet overlapping) mechanisms since only the latter is dependent on the Pph3 phosphatase [65–67].
We assessed the capacity of K. l. Zip1 to facilitate centromere associations in both diploid and haploid spo11 null meiotic cells as well as in SPO11 haploid meiotic cells expressing CTF19-MYC. Haploids capable of progressing through meiotic prophase were created by targeting a MATa locus cassette to an ectopic location in the genome (the THR1 locus) in MATα haploids [69]. We compared the number of Ctf19-MYC foci observed on surface-spread meiotic chromosomes when such strains carried S. c. ZIP1, K. l. ZIP1, or the zip1 null genotype.
Consistent with the observations described in the initial report on “centromere coupling” [65], spo11 diploid meiotic cells expressing S. c. ZIP1 exhibited a variable number of Ctf19-MYC foci per nucleus, ranging from 4–27 with an average of 17 (n = 245 total nuclei over 5 experiments; S4 Fig), while spo11 haploid meiotic cells exhibited from 4–14, with an average of 9 Ctf19-MYC foci per nucleus (n = 143 total nuclei over 3 experiments). In contrast, spo11 diploid cells missing ZIP1 exhibited between 16–35 with an average of 26 Ctf19-MYC foci (n = 258 total nuclei over 5 experiments), and spo11 haploid cells missing ZIP1 exhibited between 9–22 with an average of 15 Ctf19-MYC foci (n = 168 total nuclei over 3 experiments; S4 Fig).
In spo11 diploid meiotic cells expressing K. l. ZIP1, we observed between 5–36 with an average of 20 Ctf19-MYC foci (n = 288 total nuclei over 5 experiments, S4 Fig), suggesting that K. l. Zip1 may weakly restore the centromere association function of S. c. Zip1 in the context of a diploid spo11 cell. In contrast, however, spo11 null haploid meiotic cells expressing K. l. ZIP1 displayed no capacity for centromere association: spo11 null haploid meiotic cells expressing K. l. ZIP1 exhibited an average of 15 Ctf19-MYC foci (n = 152 total nuclei over 3 experiments).
As reported in [66], SPO11 haploid meiotic cells exhibited between 6–12 with an average of 8 Ctf19-MYC foci, while SPO11 zip1 null haploid meiotic cells exhibited between 8–22 with an average of 14 Ctf19-MYC foci. We found that SPO11 K. l. ZIP1-expressing haploid meiotic cells exhibited between 7–22 with an average of 14 Ctf19-MYC foci.
Taken together, our findings suggest that while K. l. Zip1 may maintain a weak capacity to mediate SPO11-independent centromere associations in diploid spo11 meiotic cells, K. l. Zip1 fails to facilitate persistent centromere associations in a haploid meiotic cell context (with or without Spo11 activity). The basis for the difference observed between diploid and haploid cell contexts may reflect a sensitivity (on the part of centromeres) to the dosage of K. l. Zip1.
K. lactis Zip1 promotes a wild-type level of crossing over in a subset of S. cerevisiae meiotic cells
Since crossover recombination events are critical for the formation of the stable connections between homologs that ensure proper chromosome disjunction at meiosis I, it is reasonable to speculate that the basis for the diminished viability of spore products from K. l. Zip1-expressing S. cerevisiae strains lies in a failure of K. l. Zip1 to rescue S. c. Zip1’s crossover function. We therefore assessed crossover formation in four consecutive intervals on chromosome III, one interval on chromosome VIII and one interval on chromosome XI in S. cerevisiae cells expressing K. l. ZIP1 (Fig 6B and Table 2).
Fig. 6. Four-spore viable tetrads from K. l. ZIP1 meioses exhibit wild-type crossover levels, largely independent of Msh4, with diminished interference. 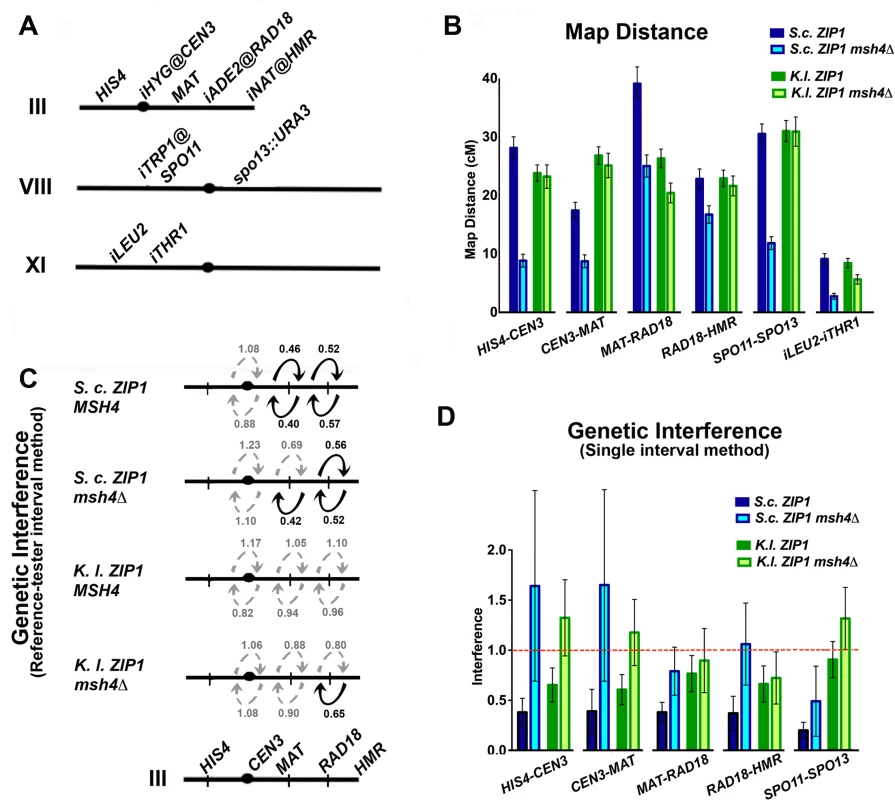
Cartoon in (A) displays the markers used to define six genetic intervals in which crossing over was assessed in spores from S. c. ZIP1 and K. l. ZIP1-expressing S. cerevisiae strains (YT131, YT125, AM3313 and YT152). Graph in (B) plots the map distances (+/- S. E.) for each of the six intervals (labeled on the x axis) that were calculated from linkage analysis in 4-spore viable tetrads from each of the four strains analyzed (S. c. ZIP1 +/- MSH4 are indicated in darker and lighter blue, respectively while K. l. ZIP1 +/- MSH4 are indicated in darker and lighter green.). The specific values for map distance are listed in Table 2. Cartoon in (C) depicts observable (solid dark arrow) or undetectable (gray, dotted arrow) interference acting between adjacent genetic intervals on chromosome III, as measured by the “interference ratio” method (see Text for details) [74,75]. Strain genotypes are indicated at left. The interference ratio gives an estimate of the strength of interference; P values from chi-square analysis of the distribution of tetrad types derived from recombinant versus non-recombinant groups (Instat, Graphpad.com; S5 Table), as well as statistical analysis of the significance of differences between map lengths calculated by tetrad types (Stahl Online Tools; S5 Table) were used to determine whether adjacent intervals exhibited interference. Graph in (D) shows the genetic interference values obtained for each genetic interval (labeled on the x axis) when the number of four-chromatid double crossovers observed (NPDs) are compared to the number expected if there was no interference [76]. The red line marks an interference value of 1, which is equal to an absence of positive interference. The specific values for both map distance and interference measured in this manner are listed in Table 2. Tab. 2. Map distances from 4 spore-viable tetrads carrying S. cerevisiae or K. lactis ZIP1. 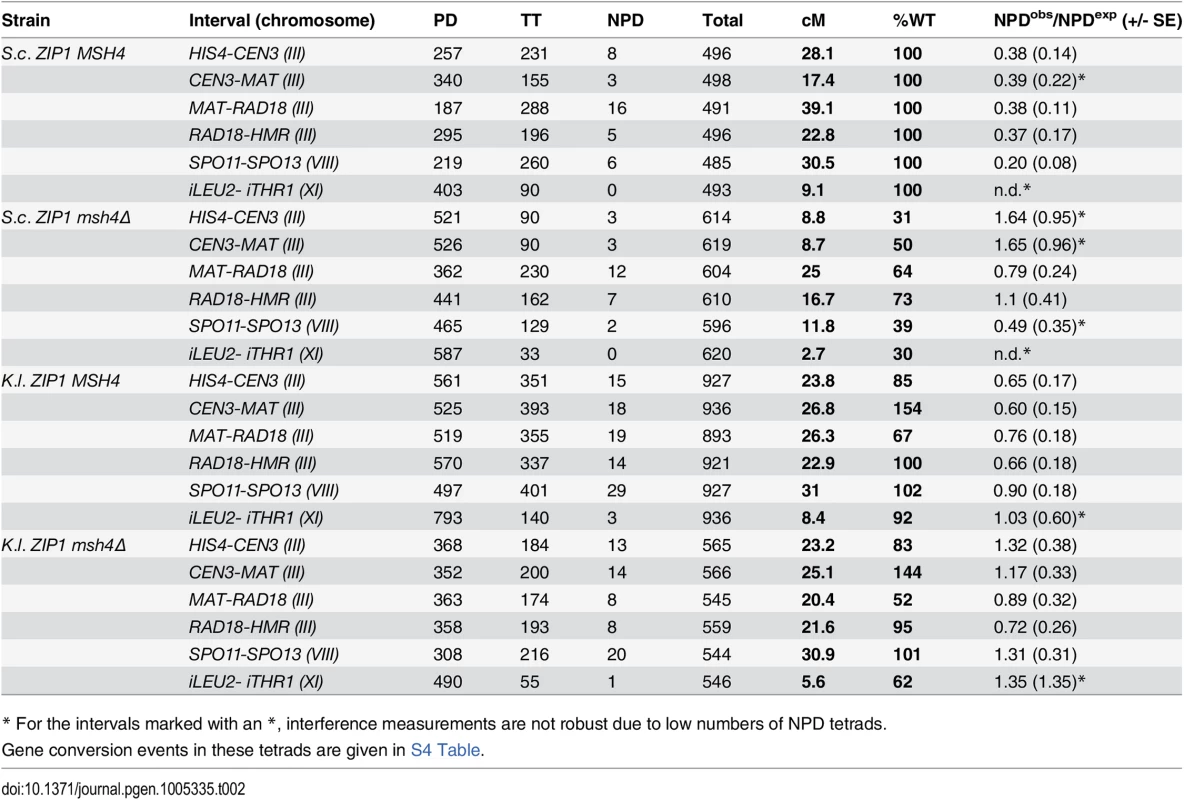
Map distances were calculated using tetrad analysis (as indicated in Methods), in S. c. ZIP1- expressing and K. l. ZIP1-expressing strains (YT131, YT125, AM3313 and YT152). Tetrad analysis to generate genetic distances, interference (and standard error (S.E.) values using tetrad data were calculated using the Stahl lab online tools: http://molbio.uoregon.edu/~fstahl/. Interference was measured by calculating the ratio of Non Parental Ditype tetrads (NPDs) observed over the NPDs expected (values less than one reflect positive interference). NPDs expected for each interval were calculated using the fraction of tetratypes (TT) observed in each dataset according to the formula: NPDexp = 1/2(1-fTT-[1-3fTT/2]2/3 (where fTT = fraction of tetratypes) [76]. To our surprise, crossover recombination levels measured using genetic marker segregation analysis on spores from S. cerevisiae cells expressing K. l. ZIP1 were nearly indistinguishable from wild-type levels (Table 2). Crossovers are typically reduced by 30–60% in mutant budding yeast strains that are missing a “class I” crossover pathway protein [20,53]. Accordingly, in our experiments cells missing the MutS component, Msh4, displayed 30%-73% (depending on the interval) of the wild-type level of crossovers (Fig 6B and Table 2). On the other hand, the map distances derived from four-spore viable tetrads of K.l. ZIP1-expressing strains were found to be within 90–105% of wild-type values. Two exceptions to this general finding existed in a pair of adjacent intervals on chromosome III: one of the two intervals exhibited 147% of the wild type map distance and the adjacent interval showed 69% of the wild-type map distance (Fig 6B and Table 2). The addition of these exceptional intervals thus gives a map distance that is 108% of our control (S.c. ZIP1-expressing) meiotic cells. Overall our data indicate that, for meioses resulting in four-spore viable tetrads (~8%, n = 7714, Table 1) the crossover recombination deficit of a zip1 null [55,70] is completely rescued by expression of K. l. ZIP1.
To ask whether the rescue in crossover formation observed for K.l. ZIP1-expressing cells is specific to four-spore viable tetrads, we used random spore analysis to assess crossing over in the three-spore viable, two-spore viable, and one-spore viable tetrads that arose in the same crossover experiment described above. Like the four-spore viable tetrads, analysis of spores from three-, two - and one-spore viable K. l. ZIP1-expressing cells gave wild-type map distances (S1 Table). Furthermore, the frequency of chromosomes III displaying zero, single, double, triple and quadruple crossovers is similar between meiotic cells expressing S. c. ZIP1 and cells expressing K. l. ZIP1 (S2 Table). Thus, in meioses that are productive for spore formation, regardless of whether four-spore viable tetrads are produced, K. l. Zip1 rescues the crossover function of S. c. Zip1.
A question that our genetic data raises is why meioses in K. l. ZIP1-expressing cells with a wild-type crossover map (at least in the intervals measured) nevertheless result in reduced spore viability (Tables 1 and S3). One explanation for reduced spore viability despite wild-type crossover levels in K. l. ZIP1-expressing S. cerevisiae cells is that the K. l. Zip1 protein fails to provide a function at centromeres that normally supports proper MI segregation; such a function could provide centromere associations between the rare chromosome pairs that fail to sustain a crossover, or alternatively could ensure that crossover events do not occur within centromeric regions [67,71,72]. An additional or alternative possibility involves the distribution of crossovers on meiotic chromosomes, which normally exhibits measureable positive interference. The interfering distribution displayed by meiotic crossovers in wild type means that two crossover events are less likely to occur close to one another than expected from a random distribution of crossover events. In the case of weakened interference, some chromosomes (especially small chromosomes) will more frequently fail to establish stable chiasmata, relative to when strong interference is imposed [73]. Consistent with reduced interference, K. l. ZIP1-expressing strains exhibited a significantly elevated frequency of viable spores carrying a chromosome III with zero interhomolog crossovers among the intervals measured (P = 0.0004) (S2 Table).
We assessed interference among the crossovers detected in S. c. ZIP1 and K. l. ZIP1-expressing strains in two distinct ways. First, we measured an “interference ratio” [74,75] by comparing the map distances of an interval when an adjacent interval had, or had not, experienced crossover recombination. To do this for intervals along chromosome III, we parsed tetrads that showed no evidence of recombination in a “reference” interval (Parental Ditype (PD) tetrads) from those tetrads containing a single or double crossover in that reference interval (Tetratype (TT) and Non-Parental Ditype (NPD) tetrads). Next we compared the distributions of tetrad types and map distances for an adjacent, “test” interval between the parsed groups—those associated with a non-recombinant reference interval versus those associated with a recombinant reference interval. The “interference ratio” is derived from the ratio of two map distances associated with the same test interval: the map distance calculated from tetrads in which the adjacent reference interval is recombinant (contains NPD or TTs) divided by the map distance calculated from tetrads that are PD for the reference interval. Since the two map distance values should approximate 1 in the case that a recombination event in an adjacent reference interval has no interfering effect on the frequency of crossing over in a test interval, the interference ratio gives an estimate of the strength of interference; a ratio of less than one can signify positive interference. The significance of differences between map lengths calculated for an interval in either the case of the recombinant or the non-recombinant reference interval was determined using Stahl Online Tools (http://molbio.uoregon.edu/~fstahl/), and a chi-square test was employed to determine if the distribution of tetrad types is considered significantly different in test intervals associated with the recombinant versus the non-recombinant reference interval (S5 Table). When both 1) the P value associated with comparing the distribution of tetrad types between test intervals and 2) the difference in the calculated map lengths were found to reflect statistical significance, we associated the interference ratio with positive interference (dark arrows in Fig 6C).
By this “interference ratio” method, positive interference was observed between two sets of genetic intervals on the right arm of chromosome III in wild-type strains (Fig 6C, top row, S5 Table). In contrast to previously obtained measurements of interference for msh4Δ mutant strains [25,30], this method did not indicate a strong diminishment in interference over these intervals in msh4Δ mutant strains. However, the method identified a uniform loss in positive interference for the two intervals examined in S. cerevisiae cells expressing K. l. ZIP1 (Fig 6C, third line, S5 Table). The interference ratio values associated with K. l. ZIP1-expressing msh4Δ cells (Fig 6C, fourth line, S5 Table) appeared broadly similar to K. l. ZIP1-expressing, MSH4 cells. Thus, according to this method for estimating the strength of interference, the wild-type levels of Msh4-independent crossovers promoted by K. l. Zip1 exhibit little interference, while (unexpectedly) the Msh4-independent crossovers observed in S. c. ZIP1-expressing meiotic cells exhibit significant levels of positive interference. The reason that interference among crossovers in S. c. ZIP1 msh4Δ strains was detected by the “interference ratio” method is unknown.
Interference can also be detected by a lower-than-expected incidence of NPDs, which normally arise from a double crossover within a single interval. The observed number of NPDs is compared to the number expected in the case of a random distribution of crossovers (i.e. no interference), using the equation of Papazian (1952) [76]. Using this latter method for analyzing interference we found that, compared with MSH4 S. c. ZIP1-expressing strains, crossover interference in msh4Δ mutants is nearly ablated in all intervals assessed, while crossover interference appears reduced (although not ablated) for every interval assessed in S. cerevisiae cells expressing K. l. ZIP1 (Table 2 and Fig 6D).
In summary, both measurements of interference identified a defect in crossover patterning in K. l. ZIP1-expressing cells. The basis for why the interference defect (for both msh4Δ mutant, and K. l. ZIP1-expressing cells) appears stronger in one versus the other measurement remain unclear.
Our genetic analysis of interhomolog recombination in spores from K. l. ZIP1-expressing cells uncovered one additional deviation from wild-type: The frequency of gene conversion events in K. l. ZIP1-expressing S. cerevisiae meiotic cells that were productive in spore formation was elevated at eight out of nine loci (S4 Table). This result, in conjunction with the absence of SCs in K. l. ZIP1-expressing cells, is consistent with the idea that the SC structure prevents additional interhomolog recombination events in budding yeast, perhaps through a mechanism involving a downregulation of DSBs [77]. It is also possible that an altered gene conversion tract length for K. l. Zip1-mediated recombination events contributes to the elevated gene conversion frequency observed. We note that the 2–3 fold elevated gene conversion frequencies in K. l. ZIP1-expressing cells is not accompanied by an increase in the frequency interhomolog crossover events (over wild-type levels).
K. lactis Zip1-promoted crossovers form largely independently of the MutSγ component, Msh4
The MutSγ heterodimer Msh4/Msh5 is required for the class I crossovers mediated by S. c. Zip1 [27,30,34,53,78]. Consistent with prior reports, we observed that the loss of MSH4 in wild-type cells resulted in 30–70% reductions in crossover levels (Table 2). In contrast, our genetic analysis revealed that the bulk of the crossovers mediated by K. l. Zip1 in S. cerevisiae cells occur in a Msh4-independent manner. In K. l. ZIP1 msh4Δ strains, map distances are reduced, relative to K. l. ZIP1 MSH4 strains, by less than seven percent in every interval measured with two exceptions: a 22% reduction in the MAT-RAD18 interval in chromosome III and a 33% reduction in the iLEU2-iTHR1 interval on chromosome XI (Fig 6 and Table 2). Overall, the crossover reductions observed when Msh4 is removed from K. l. ZIP1-expressing strains are dramatically less pronounced than the crossover reductions resulting from the removal of Msh4 in S. c. ZIP1–expressing strains. These data indicate that K. l. Zip1 rescues crossover formation in S. cerevisiae cells through a mechanism that does not rely heavily on the MutSγ component, Msh4.
Perhaps not surprisingly given its dispensability in crossover formation, the abundance of Msh4 on mid-meiotic prophase chromosomes in K. l. ZIP1-expressing cells is severely diminished relative to Msh4’s abundance on meiotic chromosomes in S. c. ZIP1-expressing cells (S5 Fig). Consistent with previous reports, we observed ~40–65 Msh4-HA foci co-localized with Zip3-MYC protein on mid-meiotic prophase chromosomes from S. c. ZIP1-expressing meiotic cells (at a stage when chromosomes normally exhibit full-length SC). In contrast, only 0–20 Msh4-HA foci were observed on similarly staged meiotic chromosomes from K. l. ZIP1-expressing cells; such low levels of Msh4 on meiotic chromosomes resembled the level detected in a zip1 null mutant (S5 Fig).
Msh4-independent K. lactis Zip1-mediated crossovers in S. cerevisiae cells rely on SIC proteins and Mlh3
In order to measure crossover recombination among all meiotic cells regardless of their capacity to successfully form spores, we turned to a physical assay for recombination on chromosome III. In this “circle-linear” assay, meiotic nuclei harboring one linear and one circular chromosome III are subjected to pulsed-field gel electrophoresis followed by a Southern blot to detect the position of chromosome III on the gel [79]. The circular chromosome III fails to enter the gel and thus is not detectable. However, the non-recombinant and recombinant forms of linear chromosome III fall into three size categories that are detectable on these gels: A single crossover between linear and circular chromosomes III runs at twice the molecular weight of the parental linear chromosome III, whereas a double crossover involving three chromatids runs at three times the molecular weight of the parental chromosome III. The proportion of trimer and dimer chromatids relative to the total (detectable) chromatids can be used to generate a relative measure of crossing over on chromosomes III in the population.
In wild-type strains, crossover recombination values estimated using this physical assay for crossovers on chromosome III were at nearly 100% by 40 and 70 hours of sporulation (Fig 7A). In zip1 null mutants, on the other hand, approximately 20% and 30% recombination was measured at 40 and 70 hours after transfer to sporulation medium, respectively. In strains expressing K.l. ZIP1, approximately 50% and 65% crossover recombination was measured at 40 and 70 hours of sporulation, respectively (Fig 7A). Because K. l. ZIP1-expressing meiocytes that go on to form spores display wild-type levels of crossing over on chromosome III, the intermediate level of crossing over measured by this physical assay indicates that K. l. ZIP1-expressing meiocytes that fail to form spores are crossover-deficient.
Fig. 7. Crossovers mediated by K. lactis Zip1 are dependent on Zip3, Zip4, Spo16 and Mlh3 but independent of Msh4, Msh5 and non-MutSγ-MutLγ crossover pathway components. 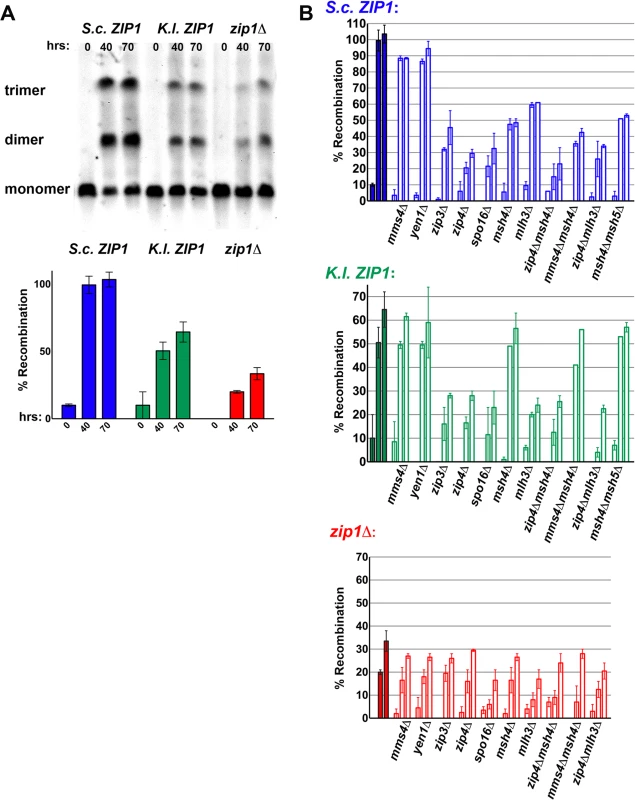
Sporulating cultures of S. cerevisiae strains carrying one linear and one circular chromosome III and carrying either S. c. ZIP1 (K479), K. l. ZIP1 (K457) or a zip1 null (TY521, [18]) allele were embedded in agarose plugs, processed, run on a pulsed-field gel and analyzed by southern blot using a probe to chromosome III sequences (see Methods). Aliquots of sporulating cells were taken at 0, 40, and 70 hours after placement in sporulation medium. (A) shows an example blot that displays bands corresponding to different sized versions of linear chromosome III for the three strains indicated above. Circular chromosomes III present in these strains do not enter the gel. The lowest molecular weight band represents the size of endogenous (linear) III, while the middle and upper bands represent crossover products between linear and circular III; a single crossover event results in a linear chromatid III that runs at the size of the middle band (“dimer”) while a double crossover event involving 3 sister chromatids (of which 2 are circular) produces a “trimer” chromatid III which migrates at the position of the upper band. Plotted on the bar graph below is a value estimating % recombination observed on chromosome III (see Methods) for S. c. ZIP1 (blue), K. l. ZIP1 (green) or zip1 null (red) strains at each meiotic time point (see Methods). Open bars displayed by the three graphs in (B) plot the relative % recombination measured for S. c. ZIP1 (blue, top), K. l. ZIP1 (green, middle) or zip1 null (red, bottom) strains that are additionally missing the function of a class I or class II meiotic crossover pathway gene (listed on x axis). Each set of three adjacent bars represents samples harvested at 0, 40 and 70 hours (left to right) after placement in sporulation medium. Solid bars at far left of graphs in (B) are the % recombination values for S. c. ZIP1, K. l. ZIP1 or zip1 null strains from (A). Graphs plot an average and range for data from at least two independent experiments. We used this assay to explore whether K.l. Zip1-mediated crossovers are dependent on synapsis-associated proteins and MutLγ, or on the so-called “class II” crossover pathway components (Fig 7B). In control strains expressing S.c. ZIP1, removal of MMS4 or YEN1 (which encode proteins that have been genetically linked to the “class II” crossover pathway) resulted in a modest decrease (~10%) in the percentage of recombinant chromosomes III. In contrast, the individual removal of ZIP3, ZIP4, SPO16, MSH4 or MLH3 (each encoding a protein that has been linked to a discrete “class I” pathway for meiotic crossovers) resulted in a larger (50%-70%) reduction in crossover formation on chromosome III. zip1 null strains missing MMS4, YEN1, or any of the “class I” crossover genes tested displayed similarly low levels of crossover recombination on chromosome III.
Analysis of K. l. ZIP1-expressing strains missing these crossover-associated genes revealed strong evidence that K. l. Zip1 functionally interfaces with a canonical Zip1/SC–associated crossover pathway in S. cerevisiae cells. Crossover recombination in K.l. ZIP1-expressing strains is strongly reduced (to nearly zip1 null levels) in the absence of ZIP3, ZIP4, SPO16, or the MutLγ protein-encoding gene, MLH3 (Fig 7B). On the other hand, crossover recombination on chromosome III was reduced only modestly (by ~10%) in K.l. ZIP1-expressing strains missing either MMS4 or YEN1 (Fig 7B), indicating that these DNA repair-associated factors are dispensable for the bulk of meiotic interhomolog crossovers in both wild-type and K. l. ZIP1-expressing strains.
Consistent with our genetic analysis, recombination on chromosome III was reduced only modestly (by ~10%) in K. l. ZIP1-expressing strains missing MSH4, and a similar result was obtained for K. l. ZIP1-expressing strains missing both MSH4 and MSH5 (Fig 7B). We did not find evidence that class II crossover pathway components rescue Msh4 function when it is absent from K. l. ZIP1-expressing cells, as the small reduction in crossovers on chromosome III measured in K.l. ZIP1-expressing strains missing both MSH4 and MMS4 was similar to that observed in either the msh4Δ or mms4Δ single mutant (Fig 7B).
Taken together, our data clearly indicate that K.l. Zip1, like S.c. Zip1, functionally interfaces with other synapsis-associated proteins in order to facilitate the maturation of MutLγ-associated crossovers in budding yeast, but that K. l. Zip1-mediated crossovers can largely bypass a requirement for MutSγ.
S. c. Zip1 and K. l. Zip1 promote Msh4-independent joint molecule formation in S. cerevisiae meiotic cells
We examined the capacity for K. l. Zip1 to facilitate MutSγ-independent recombination in greater detail by asking whether K. l. Zip1 can rescue the JM deficit reported for cells missing MutSγ complex function [27]. We analyzed six strains, each carrying S.c. ZIP1, K.l. ZIP1 or a zip1 null allele, in either a MSH4 or a msh4 null background. As our strains (BR1919-8B-derived [69]) progress through meiosis in an asynchronous manner, we reasoned that we would be more likely to detect JMs if we prevent their resolution. Thus, each of our strains is also missing NDT80 activity, which is normally required to promote the molecular pathways that resolve JMs into crossovers in S. cerevisiae [3,19], and is indeed required for crossover formation in K. l. ZIP1-expressing cells (S6 Fig). Cells were harvested at 0, 24, 32 and 40 hours after being introduced into sporulation medium, then subjected to psoralen crosslinking to preserve JM structures. Crosslinked DNA was extracted, digested with HindIII, and DNA fragments were separated by two-dimensional (2D) electrophoresis. The branched nature of crosslinked JMs causes them to migrate to a position on the 2D gel which is displaced from the arc of the bulk of crosslinked genomic DNA [5,35] (see cartoon in Fig 8). The positions of all DNA fragments that correspond to the ERG1 and YCR047c loci, which are associated with DSB hotspots [35,77,80,81], were analyzed by Southern blot hybridization.
Fig. 8. S. c. Zip1 and K. l. Zip1 promote Msh4-independent JM formation in S. cerevisiae meiotic cells. 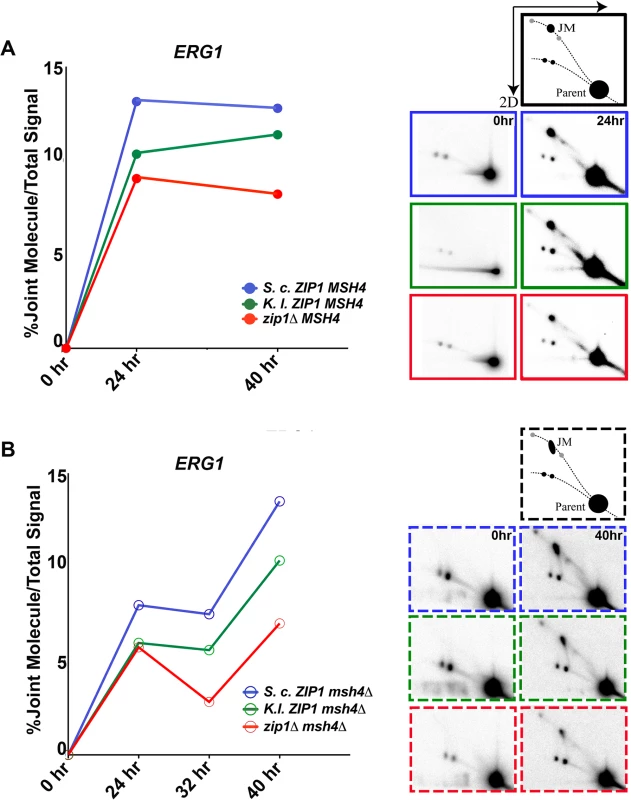
Sporulating cultures of S. cerevisiae strains carrying either S. c. ZIP1 (K663), K. l. ZIP1 (K666) or a zip1 null (K669) allele in the MSH4 background or S. c. ZIP1 (K672), K. l. ZIP1 (K675) or a zip1 null (K678) allele in the msh4 background were subject to psoralen crosslinking to preserve recombination intermediates (JMs; see Methods). Aliquots of sporulating cells were taken at 0, 24, 32, and 40 hours after placement in sporulation medium and crosslinked DNA was separated by 2D gel electrophoresis. In this assay, the linear DNA travels as an arc while branched recombination intermediates (including JMs) are slower migrating and are retarded from the arc of linear fragmented, crosslinked DNA. These molecules can be detected by Southern hybridization as shown in the schematic on the right and images below. The line graphs (A and B) are from a representative time course experiment and plot the percentage of JM/total DNA exhibited by each strain at the ERG1 locus as a function of time. For any given time point, all three strains were analyzed on the same blot; time course experiments were analyzed at least twice with similar trends observed in each experiment. We note that although we do not know the true molecular nature of these JMs, the position of the strong signal–black intermediate labeled “JM” in the schematic—is consistent with that of the dHJ, while the faster migrating signal on the arc of branched molecules (lower grey spot in the schematic) may reflect single-end invasions (SEIs), and the slower migrating signal (higher grey spot in the schematic) could be from multi-chromatid JMs [23,41]. Signals representing JM structures were undetectable at the t = 0 time points in any of our strains. However, JMs were detectable at both ERG1 and YCR047c sites in all strains at 24 hours after introduction into sporulation medium (Figs 8 and S7). Quantification of the percentage of DNA that was present in the JM spot in either MSH4 or msh4 strains is shown for the ERG1 locus in Fig 8. In MSH4 strains, we observed that both K. l. ZIP1-expressing samples and zip1 null samples exhibited a diminished JM signal relative to S. c. ZIP1 samples at the 24 hour time point. However, at the 40 hour time point the JM signal in K. l. ZIP1 samples appeared closer to that of S. c. ZIP1, and elevated above the JM level exhibited by the zip1 null. These data are consistent with the crossover data we obtained with the circle-linear chromosome III assay (Fig 7) and suggest that K. l. Zip1 has an (albeit diminished) capacity to facilitate stable JM formation, and that K. l. Zip1-dependent JMs accumulate over time in an ndt80Δ mutant background.
Support for the idea that MutSγ is critical for the bulk of JM formation in S. cerevisiae meiosis comes from the observation of strongly diminished JMs at the HIS4-LEU2 artificial hotspot in msh5 mutants (using the SK1 strain background) [27]. As Mlh3-dependent crossovers form in K. l. ZIP1-expressing cells despite the absence of Msh4, we wondered whether K. l. Zip1 rescues the deficit in JM formation presumed to occur in the absence of Msh4. We asked this question by analyzing JM formation in S. c. ZIP1, K.l. ZIP1 and zip1 null strains that were also missing MSH4.
In light of the strong reduction in JMs at the HIS4-LEU2 artificial hotspot in msh5 mutants, we were surprised to observe a robust JM signal at both ERG1 and YCR047c in our S. c. ZIP1 msh4Δ ndt80Δ strains (Fig 8B, blue line, and S7 Fig). Furthermore, the JM signal at the ERG1 site appeared to increase between 24 and 40 hours of sporulation in the ndt80-arrested, msh4 mutants. We presume that the extensive period of late prophase arrest performed for our analysis facilitated the slow but steady accumulation of JMs even in the absence of Msh4. Consistent with this possibility, a prior report demonstrated that msh5Δ ndt80Δ mutants of the SK1 background exhibit JM accumulation over time, ultimately achieving ~1/3 of the peak wild-type JM level by a late (8 hour) time point [41].
Our analysis thus reveals the existence of Msh4-independent JMs that accumulate in an ndt80Δ, meiotic prophase-arrested cell population at ERG1 and YCR047c sites in the BR1919 strain. Interestingly, we observed that a substantial fraction of the Msh4-independent JM signal at ERG1 and YCR047c sites in S. cerevisiae is dependent on Zip1. In zip1 msh4 double mutants (Fig 8B, red line), JMs do not accumulate to the same high levels as seen in the ZIP1 msh4 strain. Thus, our data indicate that S. c. Zip1 can promote Msh4-independent JM formation.
Since msh4 mutants are missing the same set of crossovers as zip1 mutants [30], the bulk of the Msh4-independent JMs promoted by S. c. Zip1 (the set of JMs present in S. c. ZIP1-expressing cells but not present in zip1 null cells) are not likely resolved to form interhomolog crossovers. This observation raises the possibility that the crossover defects of S. c. ZIP1 msh4 mutants may not be solely the result of a deficit in JM formation per se, but rather could be in part the result of a function for Msh4 in channeling SIC protein-associated recombination intermediates into an interhomolog JM pathway that is resolved by Mlh1/Mlh3.
Finally, our analysis revealed that, like S. c. Zip1, K. l. Zip1 promotes Msh4-independent JM formation in S. cerevisiae cells, albeit with a reduced capacity relative to S. c. Zip1 (Fig 8B, green line, and S7 Fig). While the Msh4-independent JMs in S. c. ZIP1-expressing cells presumably do not resolve to give interhomolog crossovers, in light of our genetic and physical crossover data (Figs 6 and 7) we propose that a substantial fraction of the Msh4-independent JMs in K. l. ZIP1-expressing cells are successfully resolved into interhomolog crossovers via an Mlh3-dependent mechanism.
Although experiments aimed at understanding the molecular nature of Msh4-independent JMs are outside the scope of the current work and will be the subject of a future study, we note that the shape of the JM signal in msh4 mutants appears elongated relative to that of the JMs observed in MSH4 strains (illustrations in Fig 8A and 8B). The elongated shape of the observed signal for Msh4-independent JMs in our strains suggest the presence of JM species with a similar molecular mass but different branched pattern, possibly the result of an altered dHJ structure or perhaps from junction migration in the msh4 mutants. An alteration in the structure of dHJs in the absence of Msh4 is consistent with the finding that hMSH4-hMSH5 recognizes Holliday Junctions and can potentially form a clamp which “embraces” partner DNA molecules of homologous chromosomes [36,82].
Discussion
A version of Zip1 that promotes Mlh3-dependent crossovers but not SC assembly
Here we report on the capacity of the Kluyveromyces lactis Zip1 protein to carry out S. cerevisiae Zip1 functions in the S. cerevisiae meiotic cell context. Kluyveromyces lactis and S. cerevisiae last shared a common ancestor well over 100 million years ago, prior to the fungal lineage’s whole genome duplication event [83]. The K. lactis genome encodes apparent homologs of most if not all synapsis-related proteins that have been thus far characterized in S. cerevisiae (including SUMO, Hop1, Red1, Ecm11, Gmc2, Zip2, Zip3, Zip4, Spo16, and Pch2), as well as the Msh4, Msh5, Mlh1 and Mlh3 proteins (http://www.genome.jp/kegg-bin/show_organism?org=kla). Whether K. lactis meiotic cells assemble an SC is unknown.
K. l. Zip1 exhibits ~ 40% overall homology with S. c. Zip1 at the primary amino acid level, and K. l. Zip1 and S. c. Zip1 share predicted structural characteristics. In particular, both K. l. Zip1 and S. c. Zip1 have a ~550 residue, centrally located group of amino acids that have a high probability of forming coiled-coil. The N - and C - terminal, non-coiled-coil regions of S. c. Zip1 are ~30–40% larger than the corresponding regions of K. l. Zip1. Several ~5–20 residue blocks of conserved sequence identity exist between the two ancestrally related proteins (Fig 1).
K. l. Zip1 fails to assemble mature SC structures in S. cerevisiae cells, as indicated by the absence of full-length linear Zip1, Ecm11 or SUMO assemblies on meiotic prophase chromosomes at any time point during meiotic prophase, and by the asynapsis phenotype of Red1-labeled chromosome axes (Figs 2, 3 and 5). Apart from a distinct polycomplex aggregate, little K. l. Zip1 was detectable on S. cerevisiae meiotic prophase chromosomes in our experiments, including those that assessed the distribution of an epitope-tagged version of K. l. Zip1 (Figs 2–5 and S1). Moreover, levels of the SC - and/or crossover-associated Zip3, Zip4, and Msh4 proteins on S. cerevisiae prophase chromosomes appeared similar to the levels of these proteins in zip1 null cells (Figs 4 and S2 and S5).
However, evidence that K. l. Zip1 can interface, at least to some extent, with S. cerevisiae SC-associated proteins stems from the observation that K. l. Zip1 polycomplex structures are decorated by S. c. Ecm11, SUMO and Zip3 proteins (Figs 2–5 and S1). Furthermore, K. l. Zip1 promotes SUMOylation of the S. cerevisiae Ecm11 protein (Fig 3), an activity that normally also largely relies on the function of synapsis proteins Zip2 and Zip4 [12]. Finally, the interhomolog crossover events that are promoted by K. l. Zip1 in S. cerevisiae cells are dependent on other so-called SIC proteins, namely Zip3, Zip4 and Spo16 (Fig 7). These observations suggest that at least some molecular features of S. c. Zip1 responsible for interfacing with SC-associated proteins are preserved in the K. l. Zip1 protein. Such molecular features could be represented by the short segments of identical sequence shared by the two Zip1 proteins, and/or may be based in a shared secondary structure.
The sparse distribution of detectable K. l. Zip1 and the reduced number of Zip3 and Zip4 proteins observed on meiotic prophase chromosomes in S. cerevisiae expressing K. l. ZIP1 suggests that SC precursor structures and/or their associations with chromosomes are unstable in this context. Because zip1 loss-of-function mutants and other S. cerevisiae mutant meiotic cells with such a dramatic asynapsis phenotype typically also exhibit a deficit in crossovers [16,18,27,70], we were surprised to measure wild-type levels of crossover recombination in spores derived from K. l. ZIP1-expressing S. cerevisiae meiotic cells (Fig 6). A combination of genetic and physical assays to measure crossing over revealed that K. l. Zip1-mediated crossovers are dependent on the SC-associated proteins Zip3, Zip4 and Spo16, and are dependent on the MutLγ protein Mlh3, but are relatively unaffected by the loss of Mms4 and Yen1, which is as expected for Zip1-mediated (SC-associated) crossover events (Fig 7). Furthermore, the resolution of K. l. Zip1-mediated repair intermediates into crossovers is, like most if not all meiotic crossovers in S. cerevisiae, dependent on the Ndt80 transcription factor (S6 Fig). Our data strengthen the notion that at least one pro-crossover function of Zip1 is separate from its role in assembling SC, a possibility previously raised by an analysis of the red1 mutant in the presence and absence of Zip1 and to a certain extent by analysis of the recombination phenotype of zip1 mutants [27,55]. K. l. Zip1’s behavior in S. cerevisiae cells demonstrates that these independent activities of Zip1 can be uncoupled at the protein level.
If K. l. ZIP1-expressing cells rely on other SC-associated proteins to promote crossing over, why is the level of Zip3 and Zip4 on meiotic chromosomes in K. l. ZIP1-expressing cells at the low level seen in the zip1 null? One possibility is that nascent SC-initiation structures are dynamic in the absence of elaborated SC, and thus only a subset of the so-called SIC complexes are detectable on meiotic chromosomes at a given time in the zip1 null or the K. l. ZIP1 context. The discrepancy between the low observed level of K. l. Zip1 protein on S. cerevisiae meiotic chromosomes and the high level of crossovers observed in at least a subset of S. cerevisiae meiotic cells expressing K. l. ZIP1 raises the important point that the abundance and spatial distribution of a protein that is minimally sufficient to provide crossover function may not necessarily be detectable by immunostaining.
Mlh3-dependent crossover levels, independent of the SC, are tightly correlated with overcoming a checkpoint-imposed block to spore formation
A discrepancy exists between our genetic and physical analyses of crossing over in S. cerevisiae cells expressing K. l. ZIP1. When measured genetically in spores, K.l. ZIP1-expressing strains exhibit wild-type map distances within intervals across chromosome III, and within intervals on two additional chromosomes (VIII and XI; Fig 6 and Table 2). On the other hand, by our physical assay we observed an intermediate crossover level across chromosome III in strains expressing K.l. ZIP1, relative to the levels exhibited by S.c. ZIP1 and zip1 null strains (Fig 7). Similarly, K.l. ZIP1 msh4Δ double mutants exhibit significantly higher crossover levels relative to S.c. ZIP1 msh4Δ strains when measured genetically, but crossover levels across chromosome III are at comparable levels in K.l. ZIP1 msh4Δ and S.c. ZIP1 msh4Δ strains by our physical assessment.
The discrepancy between crossover levels measured genetically versus a physical assay is likely due to a Pch2-mediated, prophase surveillance system that blocks spore formation in the majority of K. l. ZIP1-expressing meiotic cells. The triggers that activate a Pch2-mediated checkpoint have been associated with defects in both synapsis and in DSB repair, and can be modulated by environmental factors in budding yeast [27,57,84–87]. Our data suggest that this meiotic prophase checkpoint activity is more robust in K.l. ZIP1 msh4Δ than in S. c. ZIP1 msh4Δ cells as the sporulation efficiency of K.l. ZIP1 msh4 strains is lower than the sporulation efficiency of S.c. ZIP1 msh4 strains (16.6% for K.l. ZIP1 msh4 versus 30.0% for S.c. ZIP1 msh4; n > 1000).
Overcoming a block to meiotic progression could occur either by removing or by bypassing the insult that triggered the checkpoint. The phenotype observed in K.l. ZIP1-expressing, S. cerevisiae strains, where only those meiocytes with a nearly wild-type interhomolog crossover level progress to form spores, appears to underscore the strong influence that SC protein-associated, MutLγ-mediated recombination can have on overcoming the Pch2-associated checkpoint (regardless of how the checkpoint is triggered in these cells). Our data indicate that a capacity to overcome the prophase checkpoint in K. l. ZIP1-expressing cells tightly correlates with crossover recombination outcomes: Wild-type crossover levels are measured for K. l. ZIP1-expressing meiotic nuclei that succeed in forming spores, but crossover recombination is lower (intermediate between the levels exhibited by zip1 null and wild-type) when examined by a physical assay, an analysis that includes meiotic cells that are blocked from progressing to form spores. Since mature SC is absent in S. cerevisiae cells expressing K. l. ZIP1, these data suggest that crossover levels alone, independent of the SC, may be sufficient to overcome the Pch2-mediated prophase checkpoint block to spore formation.
On the other hand, the S. c. ZIP1 msh4 mutant phenotype is difficult to explain with the simple model that crossover levels overcome the prophase checkpoint to allow spore formation, since msh4 meiotic cells with strongly diminished crossovers can nevertheless successfully form spores. Perhaps an absence of MutLγ-associated recombination intermediates in S. c. ZIP1 msh4 cells results in a less stringent checkpoint activity than that observed in K. l. ZIP1-expressing cells. Alternatively, perhaps the SC structure is capable of modulating the prophase checkpoint [21,88]. Previous reports indicate that SC formation is delayed but ultimately occurs to some extent in msh4 mutants [17,25,30]; thus the increased capacity of cells deficient in MutSγ-associated crossovers to complete spore formation could be a consequence of signals from the SC structure itself that overcome the checkpoint.
The relationship between Zip1, the SC, and MutLγ crossover formation
While prior studies indicated that Zip1 might play a role in recombination separate from its role in SC assembly, the question remained whether the SC structure has a mechanistic role in the formation of a set of crossovers that are normally associated with synapsis. The presence of K. l. Zip1 as the sole source of Zip1 in S. cerevisiae cells fails to support SC assembly but promotes the formation of a set of crossovers that are Mlh3-dependent. Thus, this unique separation-of-function version of Zip1 demonstrates that an elaborated SC structure is not required per se for the formation of Mlh3-dependent crossovers in S. cerevisiae.
By what mechanism is Zip1 (including K. l. Zip1) involved in MutLγ-associated crossover recombination? The notion that Zip1 acts early in the pathway leading to stable crossover recombination intermediates could account for Zip1’s entire role in promoting MutLγ-dependent events, via a function in shaping or stabilizing proper JM structures that are recognizable and/or accessible to MutLγ and its companion resolvase-promoting factors. On the other hand, current data does not rule out the idea that Zip1 protein acts at later stages in the JM maturation process to facilitate the targeting of MutLγ proteins to MutSγ-associated crossover intermediates. Our analysis of JM formation in S. c. ZIP1, K. l. ZIP1 and zip1 null cells (Figs 8 and S7) supports either model: K.l. Zip1 appears to promote some stable JM formation above the level seen in the zip1 null, consistent with the idea that K. l. Zip1 might act early to promote the formation of a stable JM structure. Our observation of S. c. or K. l. Zip1-dependent JMs in msh4 mutants also supports a role for Zip1 in establishing a stable JM structure. On the other hand, the fact that (in the S. c. ZIP1 context) S. c. Zip1-dependent JMs form in the absence of Msh4 but do not resolve properly highlights the possibility that an S. c. Zip1-mediated constraint linking MutLγ resolvase activity to MutSγ-associated recombination intermediates may act downstream of or in parallel to stable JM formation, (see below).
Importantly, while spores from K. l. ZIP1-expressing cells exhibit nearly wild-type crossover levels over multiple genetic intervals, this result is influenced heavily by a stringent meiotic checkpoint; it is certainly not the case that all meiotic cells enjoy wild-type crossover levels in K. l. ZIP1-expressing S. cerevisiae strains (as indicated by our physical assays of crossover recombination on chromosome III). We imagine that the capacity of K. l. Zip1 to promote crossover recombination in S. cerevisiae cells is diminished relative to S. c. Zip1 because of suboptimal protein function and/or diminished protein levels.
Our physical and genetic crossover analyses indicate that a small subset of S. cerevisiae meiotic cells exhibit nearly wild-type levels of crossing over on chromosome III. If K. l. Zip1 only partially rescues S. c. Zip1’s crossover function, why do some cells experience a wild-type level of crossing over in the context of K. l. Zip1? Thacker et al. (2014) reported that zip1 mutants fail to properly down-regulate DSBs at later meiotic prophase stages [77]. With the idea in mind that the presence of SC could participate in down-regulating recombination-based interhomolog interactions, we propose that ongoing DSB-initiated interhomolog interactions allowed because of the absence of SC in K. l. ZIP1-expressing cells may be critical for the gradual establishment of a class of K. l. ZIP1-expressing cells that achieve wild-type interhomolog crossover levels.
K. l. Zip1 bypasses the requirement for Msh4/Msh5 in Mlh3-dependent crossover formation
Data presented in this study indicate that K. l. Zip1 retains a robust pro-crossover activity that functionally interfaces with several canonical Zip1 crossover pathway factors, including the so-called SIC proteins Zip3, Zip4, Spo16, and the MutLγ protein Mlh3 in S. cerevisiae cells. However, K. l. Zip1 crossovers in the context of the S. cerevisiae cell are different from S. c. Zip1-associated crossovers in two ways: First, K. l. Zip1 crossovers are unassociated with SC formation. Second, in the context of K. l. Zip1, MutLγ-mediated crossovers bypass a requirement for the MutSγ complex.
In K. l. ZIP1-expressing cells, Mlh3 promotes the resolution of recombination intermediates into crossovers even in the absence of MutSγ complex proteins Msh4/Msh5. While evidence from both Tetrahymena and C. elegans indicates that eukaryotic versions of bacterial MutS may not always be functionally linked to MutL proteins [48–50], K. l. ZIP1-expressing S. cerevisiae meiotic cells reflect the first example, to the authors’ knowledge, of MutLγ-dependent crossover formation that does not rely on MutSγ. The result indicates that MutLγ is not intrinsically constrained to act on MutSγ-associated DNA structures in S. cerevisiae nuclei, but that a constraint is normally active in the context of S. cerevisiae ZIP1 that normally couples MutLγ to MutSγ-associated recombination intermediates. Our study furthermore demonstrates that K. l. Zip1 can bypass this constraint. Understanding how K. l. Zip1 bypasses the requirement for Msh4/Msh5 in generating MutLγ-associated crossovers will provide a useful framework for understanding the molecular mechanism normally used by budding yeast to couple MutLγ-associated resolvase activity to MutSγ-associated intermediates.
It is noteworthy that Zip1 appears to be central to the mechanism that normally links MutLγ-associated resolvase activity to MutSγ-associated intermediates in budding yeast. As raised in the Introduction, one explanation for the role of SC structural proteins such as Zip1 in meiotic interhomolog recombination is that SC proteins or the SC itself act as a recruitment platform upon which specialized recombination enzymes can dock. While the data presented here do not rule out this model, the fact that an alternate version of Zip1 can bypass the requirement for MutSγ in MutLγ-mediated crossover formation raises the possibility that Zip1 may play a more specialized role in the processing of joint molecule intermediates.
Does K. l. Zip1 promote Msh4-independent crossovers by replacing Msh4/Msh5’s function in JM formation? The Msh4/Msh5 heterodimer is thought to form a sliding clamp on DNA and thus could recognize and stabilize both SEI and dHJ structures [27,36] in order to protect them from disassembly by helicases [40] and/or to facilitate their resolution by MutLγ-Exo1 [23,33,38,40–42]. Interestingly, the pro-crossover function(s) of Msh4/Msh5 in stabilizing meiotic crossover recombination intermediates are replaced by novel minichromosome maintenance protein complex in Drosophila [52]. Furthermore, proteins that promote SC formation have been implicated in antagonizing the anti-crossover activity of the Sgs1 helicase, consistent with an the idea that these proteins may share functionality with Msh4-5 in protecting JM structures from dissolution by helicases [40]. Prior observations of the DNA intermediates that accumulate at the HIS4-LEU2 artificial DSB hotspot in msh5 mutant strains (of the SK1 background) are consistent with the idea that Msh4/Msh5 activity is required for the accumulation of the bulk of stable dHJ recombination intermediates [27,41], although Msh5-independent JMs were found to accumulate over time in an ndt80Δ mutant background [41]. If K. l. Zip1 can bypass the need for Msh4 through rescuing a function of Msh4 in promoting stable JMs, we expected to observe a larger abundance of JMs in K. l. ZIP1 msh4 mutants, relative to S. c. ZIP1 msh4. Surprisingly, our analysis of JM formation at two natural hotspots (ERG1 and YCR047c) in MSH4 and msh4 mutant strains of the BR1919 background indicate that S. c. Zip1 and K. l. Zip1 (to a lesser extent) both promote the formation of a population of Msh4-independent JMs, although the elongated shape of the observed signal suggests the possibility that JMs that form in the absence of Msh4 in our strains have altered structure.
While these data reveal the interesting result that Msh4 and Zip1 may indeed share overlapping roles upstream of JM formation, JM formation activity per se is not likely to be the reason that Msh4 is dispensable for MutLγ-dependent crossovers in K. l. ZIP1-expressing strains, since both S. c. Zip1 and K. l. Zip1 promote Msh4-independent JM formation. We presume that only in the context of K. l. ZIP1 can such Msh4-independent JMs resolve via MutLγ.
Perhaps the critical crossover function of MutSγ in S. cerevisiae, instead of JM formation per se, is in ensuring that MutLγ-associated resolvase activity is successfully targeted to SC-associated JMs. When K. l. Zip1 is present, MutLγ is targeted to SIC protein-dependent crossover intermediates independently of MutSγ. Evidence that mammalian MutSγ and MutLγ components can directly interact [89] raises the possibility that Msh4/Msh5 might directly recruit Mlh1/Mlh3 complexes to JM structures. If the major mechanism for targeting MutLγ to MutSγ-associated JMs involves a direct protein-protein interaction between MutSγ and MutLγ components, one might propose that K. l. Zip1 bypasses the normal requirement for Msh4/Msh5 via a capacity to directly interact with Mlh1 or Mlh3 in S. cerevisiae cells. On the other hand, S. cerevisiae MutLγ complex can recognize and bind preferentially to JM structures in vitro [38]. Thus, perhaps the critical role of Msh4/Msh5 in coupling SC-associated recombination intermediates with MutLγ-associated resolvase activity is not through its potential capacity to interact directly with Mlh1/Mlh3, but through a capacity to promote the formation of a JM structure that is recognizable and/or accessible to the S. cerevisiae MutLγ complex. In this case, a simple explanation for the bypass of MutSγ provided by K. l. Zip1 is that K. l. Zip1 activity is functionally redundant with Msh4/Msh5 in S. cerevisiae meiotic nuclei (this idea is reminiscent of the functional redundancy with MutSγ proposed for the minichromosome maintenance protein complex in Drosophila [52]) and can facilitate the processing of JM intermediates in a manner that allows their resolution by a MutLγ-mediated mechanism.
On the other hand, Fig 9 presents an alternative model to explain both the mechanism that normally constrains MutLγ activity to target MutSγ-associated recombination intermediates in S. cerevisiae and how K. l. Zip1 bypasses this constraint. In our alternative model, we propose that S. c. Zip1 is normally associated with both pro-crossover and anti-crossover activities, and that Msh4/Msh5 counters the anti-crossover activity of Zip1 at JMs. A possible anti-crossover aspect of Zip1 activity could be an action that destabilizes dHJ structures, or one that prevents the accessibility of dHJ structures to MutLγ-associated resolvase activity. The presence of MutSγ at JMs might protect them or directly counter Zip1’s anti-crossover activity. In the context of S. cerevisiae cells expressing K. l. ZIP1, K. l. Zip1 retains S. c. Zip1’s pro-crossover activity but lacks its anti-crossover activity, thus rendering MutSγ dispensable for the MutLγ-mediated resolution of Zip1/SC protein-associated recombination intermediates.
Fig. 9. One model to explain the constrained relationship between MutSγ, SIC/Zip1-associated JMs, and MutLγ proteins and how K. l. Zip1 might bypass the constraint. 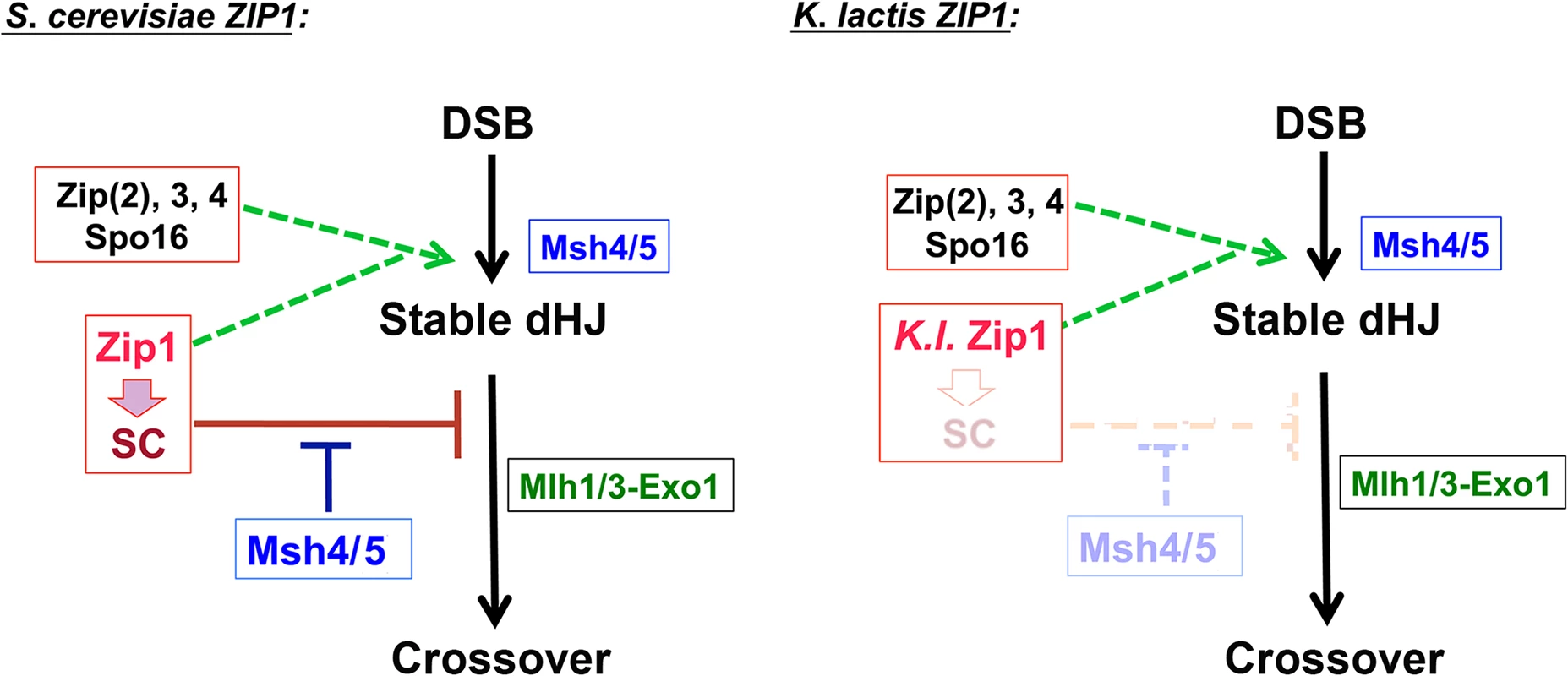
Cartoons depict potential pathways for SC protein-MutSγ-MutLγ associated crossovers in budding yeast. At left is depicted S. cerevisiae expressing S. c. ZIP1, while at right is depicted S. cerevisiae expressing K. l. ZIP1. In these models, Zip1 maintains a pro-crossover function along with other SIC proteins and Msh4/Msh5, upstream of stabilized dHJs. Both S. c. Zip1 and K. l. Zip1 collaborate with other SIC proteins and Msh4/Msh5 to promote the establishment of stable JMs. In this model, S. c. Zip1 also has an anti-crossover activity that Msh4/Msh5 normally counters (left cartoon). This anti-crossover activity might be an intrinsic feature of the Zip1 protein or perhaps is an activity coming from SC formation. An antagonistic relationship between Msh4/Msh5 and S. c. Zip1 constrains Zip1-mediated, MutLγ-associated resolvase activity to act exclusively on MutSγ-associated recombination intermediates. In the context of S. cerevisiae cells expressing K. l. ZIP1 (right cartoon), K. l. Zip1 retains the pro-crossover activity but lacks the anti-crossover activity of S. c. Zip1, rendering MutSγ dispensable for MutLγ-dependent crossover recombination. Since K. l. Zip1 uncouples Mlh3-mediated crossover formation from both SC assembly and from a reliance on Msh4, we are drawn to the possibility that SC assembly normally constrains Zip1 crossovers–ensuring that they occur successfully only on Msh4-associated intermediates. We emphasize that the anti-crossover aspects of Zip1 activity could either be an action that destabilizes JM structures or one that prevents the accessibility of JM structures to MutLγ and other resolvase proteins. The proposed antagonistic relationship between Msh4/Msh5 and S. c. Zip1 that this model proposes would effectively constrain MutLγ-mediated resolvase activity to act exclusively on MutSγ-associated recombination intermediates: Although S. c. Zip1 may be able to promote the formation of JM structures in the absence of MutSγ, only MutSγ-associated crossover intermediates are processed during meiosis in a manner that protects them from S. c. Zip1’s anti-crossover activity and allows their resolution by a MutLγ-mediated mechanism.
It is tantalizing to suggest that the putative “crossover constraining” activity of S. c. Zip1 proposed by this model is the process of SC assembly or the assembled SC itself. Perhaps the Msh4/Msh5 complex is required to protect the integrity of SEI and dHJ recombination intermediates during the process of SC elaboration, or to maintain the accessibility of such intermediates to resolvases in the context of full length SC later on. Under this scenario, the absence of SC in K. l. ZIP1-expressing cells renders MutSγ complexes dispensable for MutLγ-dependent crossover formation.
A role for Zip1 or the SC in crossover patterning?
In budding yeast, Zip1, Msh4/Msh5, and Mlh1/Mlh3 are associated with the successful resolution of crossover recombination intermediates that exhibit interference [25,27,30,34,70,90]. However, SC proteins likely play no role in the initial establishment of an interfering distribution pattern of SC-associated (MutSγ-MutLγ-mediated) crossover recombination events. Such a conclusion is supported by the fact that early crossover-correlated recombination intermediates form at a stage of meiotic prophase that is prior to when full-length SCs are present [27]. Furthermore, Zip2 and Zip3 chromosomal foci, which co-localize at SIC structures and are presumably cytological manifestations of SC-associated crossovers, exhibit an interfering distribution on meiotic pachytene chromosomes even when Zip1 is absent [14,91]. However, while the initial establishment of interfering crossover events may not require SC-associated proteins, the fact that Mlh3-dependent crossovers exhibit diminished interference in K. l. ZIP1-expressing S. cerevisiae cells indicates that SC protein activity may well influence not only the resolution of, but the ultimate pattern of MutLγ-associated crossover events.
Indeed, taken to the extreme, a model postulating that SC proteins and/or SC structures play no role in crossover interference predicts that if MutSγ-MutLγ-dependent crossovers could successfully mature in the absence of SC, these crossovers would exhibit normal interference. Our data, however, show that K. l. Zip1 can promote wild-type levels of Mlh3-dependent crossovers in a subset of S. cerevisiae meiotic cells, but these crossovers exhibit a substantially weakened interference pattern (Table 2 and Fig 6). As the cytological manifestation of MutLγ-associated crossovers (Zip2 or Zip3 foci) exhibit an interfering distribution pattern even in strains missing Zip1 altogether [14,91], we assume that K. l. Zip1-mediated crossovers are designated with proper interference in K.l. ZIP1–expressing S. cerevisiae meiotic cells. If the designation of interfering crossovers is intact in strains expressing K. l. ZIP1, then the preservation of an interfering distribution pattern of crossover-designated recombination events apparently requires a Zip1 activity that is separable from its crossover promoting function per se.
The influence of Zip1 on crossover patterning could be explained if an interfering pattern of designated crossover events is different depending on the stage in meiotic prophase when those events are initiated. Perhaps the presence of S. c. Zip1 (and/or the SC) preserves the integrity of a discrete set of “earliest designated” crossover intermediates, which exhibit a robust interference pattern. When K. l. Zip1 is present, perhaps fewer of these earliest-designated intermediates undergo successful maturation into stable JM intermediates. Since the SC structure may possibly be a barrier to the formation of ongoing interhomolog recombination-based interactions [77], it is reasonable to speculate that SC protein-mediated interhomolog recombination events may initiate in an ongoing manner and occur at later meiotic prophase stages in a K. l. ZIP1 (synapsis-defective) context, relative to normal S. cerevisiae meiosis. Thus, that subset of K. l. ZIP1-expressing meiotic cells carrying a wild-type crossover level may ultimately exhibit a crossover landscape that includes both early - and late-designated crossover intermediates. If the distribution of later prophase-designated recombination intermediates is less subject to interference, the result would be an overall weakening of the interfering distribution pattern of Mlh3-resolved crossovers in K. l. ZIP1-expressing cells.
Methods
Strains
All strains used in this study (S6 Table) are isogenic to BR1919-8B [69]. Strain variants were created by standard genetic crosses and transformation procedures. Every strain in which the K. l. ZIP1 open reading frame replaced the S. c. ZIP1 open reading frame was derived from the same parent, CO1. CO1 was created by first inserting URA3 in place of S. c. ZIP1 open reading frame sequences. Next, a PCR product containing the K. l. ZIP1 open reading frame (amplified off of genomic DNA extracted from K. lactis cells) flanked by ~50 bp of homology to the 5’ and 3’ sequences of the S. c. ZIP1 endogenous locus was transformed into the zip1::URA3 strain, in order to replace the URA3 sequences at the ZIP1 locus with K. l. ZIP1 sequences. Primers used for this step were: 5’TTCTTTGAGATTCGGAAGTAAAATACCCTCGGCGGCTAAATTTTTAGAGAATGTCTAACTTCTTCAGAGACAACTCG 3’ and 5’ACAAAATGAAATGTATTCGCACAAAACGATTTCAAATTTTCCATTATCCTTTATCTGAATCTTTTGGTCTTTTTTAATCGAGG 3’ (underlined regions correspond to K. l. ZIP1 sequences). Counterselection against Ura+ was carried out using 5-FOA medium.
The K.lactis ZIP1-V5 fusion cassette was created by first inserting URA3 between the codons for amino acids 472 and 473 of K. lactis Zip1. Next, a PCR product with flanking homology to K.lactis ZIP1 but carrying an in-frame V5 sequence was used to counterselect against Ura+ cells on 5-FOA medium. DNA sequencing confirmed the position of V5 coding sequences in frame with the codon for amino acid 472 in an otherwise complete K.lactis ZIP1 gene.
To construct a haploid strain capable of sporulation, MATa was integrated at the THR1 locus in a haploid MATα strain, using the B211 plasmid from Beth Rockmill [69].
Strains used for crossover analysis in spores carry a hphMX cassette inserted near the chromosome III centromere, ADE2 inserted upstream of the RAD18 locus, a natMX cassette inserted near the HMR locus, TRP1MX4 was inserted just downstream of the SPO11 locus, and LEU2 and THR1 were inserted on chromosome XI at 152kb, and at 193,424bp, respectively.
Chromosome III circular MATα strains as well as TY521 and TY522 [18] were received from the Roeder lab.
Cytological analysis and imaging
Meiotic nuclei were surface spread on glass slides and imaged as described in [13]. The following antibodies were used: rabbit anti-Zip1 (created as described in [10]), rabbit anti-Red1 [61], guinea pig anti-SUMO [11], chicken anti-HA (Abcam), mouse anti-MYC (clone 9E10, Invitrogen), rabbit anti-V5 (Abcam). Secondary antibodies conjugated with Alexa Fluor dyes were purchased from Life Technologies and used at a 1 : 200 dilution.
Calculations and statistical analysis
Genetic crossover data was compiled and processed using an Excel Linkage Macro program, created by Jonathan Greene (Rhona Borts, pers. comm.) and donated by Eva Hoffmann (University of Sussex, UK). Final crossover and interference values (and their standard errors) were obtained using the Stahl lab online tools (http://molbio.uoregon.edu/~fstahl/), with the method of Perkins [92]. All other statistical analyses were carried out using Graphpad Prism or Graphpad InStat (www.graphpad.com).
Pulsed field gel electrophoresis, Southern blotting, western blot
Agarose plugs were prepared from meiotic cultures at 0, 40 and 70 hours of sporulation and subjected to pulsed-field gel analysis [18,79]. For Southern blotting, a 1 kb probe from the THR4 region of chromosome III was prepared using a DIG High Prime DNA Labeling and Detection Kit (Roche). A Syngene “G:Box” was used to detect chemiluminescence and the Syngene “Gene-Tools” program was used to analyze the data. A value for % recombination (Fig 7) was calculated by summing twice the intensity of the trimer band (a double crossover product) plus the dimer band (product of a single crossover) over the total intensity of the three bands (trimer, dimer and monomer). Note that circular chromosome III chromatids do not enter the gel, and thus are not included in the calculation to estimate recombination. The average of two experiments is presented.
Western blotting was performed as described previously [13].
2D gel electrophoresis and JM analysis
2D gel electrophoresis followed by Southern analysis to assay JMs was performed as previously described [35,66,93] Probes for detection of JMs at the ERG1 locus [77,81] were amplified from yeast genomic DNA with primers - 5’-GGCAGCAACATATCTCAAGGCC-3’ and 5’-TCAATGTAGCCTGAGATTGTGGCG-3’. Probes for detection of JMs at YCR047c [35,80] were amplified from yeast genomic DNA using primers 5’-GGAATTCCGAGAGAATCGACTTGCTAA-3’ and 5’-GGAATTCCAGCCACCAGTGGGCTTTTC-3’. Hybridization signal was detected and quantified using a Typhoon FLA 9000 (GE) and the ImageJ software (http://imagej.nih.gov/ij/).
Supporting Information
Zdroje
1. Zickler D, Kleckner N (1999) Meiotic chromosomes: integrating structure and function. Annu Rev Genet 33 : 603–754. 10690419
2. Bhalla N, Dernburg AF (2008) Prelude to a division. Annu Rev Cell Dev Biol 24 : 397–424. doi: 10.1146/annurev.cellbio.23.090506.123245 18597662
3. Allers T, Lichten M (2001) Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106 : 47–57. 11461701
4. De Muyt A, Jessop L, Kolar E, Sourirajan A, Chen J, et al. (2012) BLM helicase ortholog Sgs1 is a central regulator of meiotic recombination intermediate metabolism. Mol Cell 46 : 43–53. doi: 10.1016/j.molcel.2012.02.020 22500736
5. Schwacha A, Kleckner N (1995) Identification of double Holliday junctions as intermediates in meiotic recombination. Cell 83 : 783–791. 8521495
6. Moses MJ (1968) Synaptonemal complex. Annu Rev Genet 2 : 363–412.
7. Page SL, Hawley RS (2004) The genetics and molecular biology of the synaptonemal complex. Annu Rev Cell Dev Biol 20 : 525–558. 15473851
8. de Boer E, Heyting C (2006) The diverse roles of transverse filaments of synaptonemal complexes in meiosis. Chromosoma 115 : 220–234. 16523321
9. Dong H, Roeder GS (2000) Organization of the yeast Zip1 protein within the central region of the synaptonemal complex. J Cell Biol 148 : 417–426. 10662769
10. Sym M, Engebrecht J, Roeder GS (1993) Zip1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell 72 : 365–378. 7916652
11. Hooker GW, Roeder GS (2006) A role for SUMO in meiotic chromosome synapsis. Curr Biol 16 : 1238–1243. 16782016
12. Humphryes N, Leung WK, Argunhan B, Terentyev Y, Dvorackova M, et al. (2013) The Ecm11-Gmc2 complex promotes synaptonemal complex formation through assembly of transverse filaments in budding yeast. PLoS Genet 9: e1003194. doi: 10.1371/journal.pgen.1003194 23326245
13. Voelkel-Meiman K, Taylor LF, Mukherjee P, Humphryes N, Tsubouchi H, et al. (2013) SUMO localizes to the central element of synaptonemal complex and is required for the full synapsis of meiotic chromosomes in budding yeast. PLoS Genet 9: e1003837. doi: 10.1371/journal.pgen.1003837 24098146
14. Fung JC, Rockmill B, Odell M, Roeder GS (2004) Imposition of crossover interference through the nonrandom distribution of synapsis initiation complexes. Cell 116 : 795–802. 15035982
15. Agarwal S, Roeder GS (2000) Zip3 provides a link between recombination enzymes and synaptonemal complex proteins. Cell 102 : 245–255. 10943844
16. Chua PR, Roeder GS (1998) Zip2, a meiosis-specific protein required for the initiation of chromosome synapsis. Cell 93 : 349–359. 9590170
17. Shinohara M, Oh SD, Hunter N, Shinohara A (2008) Crossover assurance and crossover interference are distinctly regulated by the ZMM proteins during yeast meiosis. Nat Genet 40 : 299–309. doi: 10.1038/ng.83 18297071
18. Tsubouchi T, Zhao H, Roeder GS (2006) The meiosis-specific Zip4 protein regulates crossover distribution by promoting synaptonemal complex formation together with Zip2. Dev Cell 10 : 809–819. 16740482
19. Sourirajan A, Lichten M (2008) Polo-like kinase Cdc5 drives exit from pachytene during budding yeast meiosis. Genes Dev 22 : 2627–2632. doi: 10.1101/gad.1711408 18832066
20. Roeder GS (1997) Meiotic chromosomes: it takes two to tango. Genes Dev 11 : 2600–2621. 9334324
21. MacQueen AJ, Hochwagen A (2011) Checkpoint mechanisms: the puppet masters of meiotic prophase. Trends Cell Biol 21 : 393–400. doi: 10.1016/j.tcb.2011.03.004 21531561
22. Bhalla N, Dernburg AF (2005) A conserved checkpoint monitors meiotic chromosome synapsis in Caenorhabditis elegans. Science 310 : 1683–1686. 16339446
23. Zakharyevich K, Tang S, Ma Y, Hunter N (2012) Delineation of joint molecule resolution pathways in meiosis identifies a crossover-specific resolvase. Cell 149 : 334–347. doi: 10.1016/j.cell.2012.03.023 22500800
24. Kohl KP, Sekelsky J (2013) Meiotic and mitotic recombination in meiosis. Genetics 194 : 327–334. doi: 10.1534/genetics.113.150581 23733849
25. Nishant KT, Chen C, Shinohara M, Shinohara A, Alani E (2010) Genetic analysis of baker's yeast Msh4-Msh5 reveals a threshold crossover level for meiotic viability. PLoS Genet 6. e1001083. doi: 10.1371/journal.pgen.1001083 20865162
26. de los Santos T, Hunter N, Lee C, Larkin B, Loidl J, et al. (2003) The Mus81/Mms4 endonuclease acts independently of double-Holliday junction resolution to promote a distinct subset of crossovers during meiosis in budding yeast. Genetics 164 : 81–94. 12750322
27. Borner GV, Kleckner N, Hunter N (2004) Crossover/noncrossover differentiation, synaptonemal complex formation and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell 1127 : 29–45.
28. Argueso JL, Wanat J, Gemici Z, Alani E (2004) Competing crossover pathways act during meiosis in Saccharomyces cerevisiae. Genetics 168 : 1805–1816. 15611158
29. Mercier R, Jolivet S, Vezon D, Huppe E, Chelysheva L, et al. (2005) Two meiotic crossover classes cohabit in Arabidopsis: one is dependent on MER3, whereas the other one is not. Curr Biol 15 : 692–701. 15854901
30. Novak JE, Ross-Macdonald P, Roeder GS (2001) The budding yeast Msh4 protein functions in chromosome synapsis and the regulation of crossover distribution. Genetics 158 : 1013–1025. 11454751
31. Hunter N, Borts RH (1997) Mlh1 is unique among mismatch repair proteins in its ability to promote crossing-over during meiosis. Genes Dev 11 : 1573–1582. 9203583
32. Santucci-Damanin S, Walpita D, Lespinasse F, Desnuelle C, Ashley T, et al. (2000) MSH4 acts in conjunction with MLH1 during mammalian meiosis. FASEB J 1539–1547.
33. Rogacheva MV, Manhart CM, Chen C, Guarne A, Surtees J, et al. (2014) Mlh1-Mlh3, a meiotic crossover and DNA mismatch repair factor, is a Msh2-Msh3-stimulated endonuclease. J Biol Chem 289 : 5664–5673. doi: 10.1074/jbc.M113.534644 24403070
34. Sonntag Brown M, Lim E, Chen C, Nishant KT, Alani E (2013) Genetic analysis of mlh3 mutations reveals interactions between crossover promoting factors during meiosis in baker's yeast. G3 (Bethesda) 3 : 9–22.
35. Hunter N, Kleckner N (2001) The single-end invasion: an asymmetric intermediate at the double-strand break to double-Holliday junction transition of meiotic recombination. Cell 106 : 59–70. 11461702
36. Snowden T, Acharya S, Butz C, Berardini M, Fishel R (2004) hMSH4-hMSH5 recognizes Holliday Junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol Cell 15 : 437–451. 15304223
37. Wang TF, Kleckner N, Hunter N (1999) Functional specificity of MutL homologs in yeast: evidence for three Mlh1-based heterocomplexes with distinct roles during meiosis in recombination and mismatch correction. Proc Natl Acad Sci U S A 96 : 13914–13919. 10570173
38. Ranjha L, Anand R, Cejka P (2014) The Saccharomyces cerevisiae Mlh1-Mlh3 heterodimer is an endonuclease that preferentially binds to Holliday junctions. J Biol Chem 289 : 5674–5686. doi: 10.1074/jbc.M113.533810 24443562
39. Oke A, Anderson CM, Yam P, Fung JC (2014) Controlling Meiotic Recombinational Repair—Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation. PLoS Genet 10: e1004690. doi: 10.1371/journal.pgen.1004690 25329811
40. Jessop L, Rockmill B, Roeder GS, Lichten M (2006) Meiotic chromosome synapsis-promoting proteins antagonize the anti-crossover activity of Sgs1. PLoS Genet 2: e155. 17002499
41. Oh SD, Lao JP, Hwang PY, Taylor AF, Smith GR, et al. (2007) BLM ortholog, Sgs1, prevents aberrant crossing-over by suppressing formation of multichromatid joint molecules. Cell 130 : 259–272. 17662941
42. Zakharyevich K, Ma Y, Tang S, Hwang PY, Boiteux S, et al. (2010) Temporally and biochemically distinct activities of Exo1 during meiosis: double-strand break resection and resolution of double Holliday junctions. Mol Cell 40 : 1001–1015. doi: 10.1016/j.molcel.2010.11.032 21172664
43. Villeneuve AM, Hillers KJ (2001) Whence meiosis? Cell 106 : 647–650. 11572770
44. Colaiacovo MP, MacQueen AJ, Martinez-Perez E, McDonald K, Adamo A, et al. (2003) Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev Cell 5 : 463–474. 12967565
45. Kelly KO, Dernburg AF, Stanfield GM, Villeneuve AM (2000) Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics 156 : 617–630. 11014811
46. MacQueen AJ, Colaiacovo MP, McDonald K, Villeneuve AM (2002) Synapsis-dependent and-independent mechanisms stabilize homolog pairing during meiotic prophase in C. elegans. Genes Dev 16 : 2428–2442. 12231631
47. Zalevsky J, MacQueen AJ, Duffy JB, Kemphues KJ, Villeneuve AM (1999) Crossing over during Caenorhabditis elegans meiosis requires a conserved MutS-based pathway that is partially dispensable in budding yeast. Genetics 153 : 1271–1283. 10545458
48. Saito TT, Lui DY, Kim HM, Meyer K, Colaiacovo MP (2013) Interplay between structure-specific endonucleases for crossover control during Caenorhabditis elegans meiosis. PLoS Genet 9: e1003586. doi: 10.1371/journal.pgen.1003586 23874210
49. Agostinho A, Meier B, Sonneville R, Jagut M, Woglar A, et al. (2013) Combinatorial regulation of meiotic holliday junction resolution in C. elegans by HIM-6 (BLM) helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 nucleases. PLoS Genet 9: e1003591. doi: 10.1371/journal.pgen.1003591 23901331
50. O'Neil NJ, Martin JS, Youds JL, Ward JD, Petalcorin MI, et al. (2013) Joint molecule resolution requires the redundant activities of MUS-81 and XPF-1 during Caenorhabditis elegans meiosis. PLoS Genet 9: e1003582. doi: 10.1371/journal.pgen.1003582 23874209
51. Page SL, Hawley RS (2001) c(3)G encodes a Drosophila synaptonemal complex protein. Genes Dev 15 : 3130–3143. 11731477
52. Kohl KP, Jones CD, Sekelsky J (2012) Evolution of an MCM complex in flies that promotes meiotic crossovers by blocking BLM helicase. Science 338 : 1363–1365. doi: 10.1126/science.1228190 23224558
53. Lynn A, Soucek R, Borner GV (2007) ZMM proteins during meiosis: crossover artists at work. Chromosome Res 15 : 591–605. 17674148
54. Storlazzi A, Xu L, Cao L, Kleckner N (1995) Crossover and noncrossover recombination during meiosis: Timing and pathway relationships. Proc Natl Acad Sci USA 92 : 8512–8516. 7667321
55. Storlazzi A, Xu L, Schwacha A, Kleckner N (1996) Synaptonemal complex (SC) component Zip1 plays a role in meiotic recombination independent of SC polymerization along the chromosomes. Proc Natl Acad Sci USA 93 : 9043–9048. 8799151
56. Rockmill B, Fung JC, Branda SS, Roeder GS (2003) The Sgs1 helicase regulates chromosome synapsis and meiotic crossing over. Curr Biol 13 : 1954–1962. 14614820
57. San-Segundo P, Roeder GS (1999) Pch2 links chromatin silencing to meiotic checkpoint control. Cell 97 : 313–324. 10319812
58. Voelkel-Meiman K, Moustafa SS, Lefrancois P, Villeneuve AM, MacQueen AJ (2012) Full-length synaptonemal complex grows continuously during meiotic prophase in budding yeast. PLoS Genet 8: e1002993. doi: 10.1371/journal.pgen.1002993 23071451
59. Xu L, Ajimura M, Padmore R, Klein C, Kleckner N (1995) NDT80, a meiosis-specific gene required for exit from pachytene in Saccharomyces cerevisiae. Mol Cell Biol 15 : 6572–6581. 8524222
60. Scherthan H, Wang H, Adelfalk C, White EJ, Cowan C, et al. (2007) Chromosome mobility during meiotic prophase in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 104 : 16934–16939. 17939997
61. Smith AV, Roeder GS (1997) The yeast Red1 protein localizes to the cores of meiotic chromosomes. J Cell Biol 136 : 957–967. 9060462
62. Rockmill B, Sym M, Scherthan H, Roeder GS (1995) Roles for two RecA homologs in promoting meiotic chromosome synapsis. Genes Dev 9 : 2684–2695. 7590245
63. Tsubouchi T, Macqueen AJ, Roeder GS (2008) Initiation of meiotic chromosome synapsis at centromeres in budding yeast. Genes Dev 22 : 3217–3226. doi: 10.1101/gad.1709408 19056898
64. Serrentino ME, Chaplais E, Sommermeyer V, Borde V (2013) Differential association of the conserved SUMO ligase Zip3 with meiotic double-strand break sites reveals regional variations in the outcome of meiotic recombination. PLoS Genet 9: e1003416. doi: 10.1371/journal.pgen.1003416 23593021
65. Tsubouchi T, Roeder GS (2005) A synaptonemal complex protein promotes homology-independent centromere coupling. Science 308 : 870–873. 15879219
66. Falk JE, Chan AC, Hoffmann E, Hochwagen A (2010) A Mec1 - and PP4-dependent checkpoint couples centromere pairing to meiotic recombination. Dev Cell 19 : 599–611. doi: 10.1016/j.devcel.2010.09.006 20951350
67. Newnham L, Jordan P, Rockmill B, Roeder GS, Hoffmann E (2010) The synaptonemal complex protein, Zip1, promotes the segregation of nonexchange chromosomes at meiosis I. Proc Natl Acad Sci U S A 107 : 781–785. doi: 10.1073/pnas.0913435107 20080752
68. Hyland KM, Kingsbury J, Koshland D, Hieter P (1999) Ctf19p: A novel kinetochore protein in Saccharomyces cerevisiae and a potential link between the kinetochore and mitotic spindle. J Cell Biol 145 : 15–28. 10189365
69. Rockmill B, Roeder GS (1998) Telomere-mediated chromosome pairing during meiosis in budding yeast. Genes Dev 12 : 2574–2586. 9716409
70. Sym M, Roeder GS (1994) Crossover interference is abolished in the absence of a synaptonemal complex protein. Cell 79 : 283–292. 7954796
71. Chen SY, Tsubouchi T, Rockmill B, Sandler JS, Richards DR, et al. (2008) Global analysis of the meiotic crossover landscape. Dev Cell 15 : 401–415. doi: 10.1016/j.devcel.2008.07.006 18691940
72. Rockmill B, Voelkel-Meiman K, Roeder GS (2006) Centromere-proximal crossovers are associated with precocious separation of sister chromatids during meiosis in Saccharomyces cerevisiae. Genetics 174 : 1745–1754. 17028345
73. Chua PR, Roeder GS (1997) Tam1, a telomere-associated meiotic protein, functions in chromosome synapsis and crossover interference. Genes Dev 11 : 1786–1800. 9242487
74. Malkova A, Swanson J, German M, McCusker JH, Housworth EA, et al. (2004) Gene conversion and crossing over along the 405-kb left arm of Saccharomyces cerevisiae chromosome VII. Genetics 168 : 49–63. 15454526
75. Martini E, Diaz RL, Hunter N, Keeney S (2006) Crossover homeostasis in yeast meiosis. Cell 126 : 285–295. 16873061
76. Papazian HP (1952) The analysis of tetrad data. Genetics 37 : 175–188. 17247384
77. Thacker D, Mohibullah N, Zhu X, Keeney S (2014) Homologue engagement controls meiotic DNA break number and distribution. Nature 510 : 241–246. doi: 10.1038/nature13120 24717437
78. Pochart P, Woltering D, Hollingsworth N (1997) Conserved properties between functionally distinct MutS homologs in yeast. J Biol Chem 272 : 30345–30349. 9374523
79. Game JC, Sitney KC, Cook VE, Mortimer RK (1989) Use of a ring chromosome and pulsed-field gels to study interhomolog recombination, double-strand DNA breaks and sister-chromatid exchange in yeast. Genetics 123 : 695–713. 2693206
80. Joshi N, Brown MS, Bishop DK, Borner GV (2015) Gradual Implementation of the Meiotic Recombination Program via Checkpoint Pathways Controlled by Global DSB Levels. Mol Cell 57 : 797–811. doi: 10.1016/j.molcel.2014.12.027 25661491
81. Lao JP, Cloud V, Huang CC, Grubb J, Thacker D, et al. (2013) Meiotic crossover control by concerted action of Rad51-Dmc1 in homolog template bias and robust homeostatic regulation. PLoS Genet 9: e1003978. doi: 10.1371/journal.pgen.1003978 24367271
82. Snowden T, Shim KS, Schmutte C, Acharya S, Fishel R (2008) hMSH4-hMSH5 adenosine nucleotide processing and interactions with homologous recombination machinery. J Biol Chem 283 : 145–154. 17977839
83. Kellis M, Birren BW, Lander ES (2004) Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces cerevisiae. Nature 428 : 617–624. 15004568
84. Mitra N, Roeder GS (2007) A novel nonnull ZIP1 allele triggers meiotic arrest with synapsed chromosomes in Saccharomyces cerevisiae. Genetics 176 : 773–787. 17435220
85. Wu H-Y, Burgess SM (2006) Two distinct surveillance mechanisms monitor meiotic chromosome metabolism in budding yeast. Curr Biol 16 : 2473–2479. 17174924
86. Borner GV, Barot A, Kleckner N (2008) Yeast Pch2 promotes domainal axis organization, timely recombination progression, and arrest of defective recombinosomes during meiosis. Proc Natl Acad Sci U S A 105 : 3327–3332. doi: 10.1073/pnas.0711864105 18305165
87. Ho HC, Burgess SM (2011) Pch2 acts through Xrs2 and Tel1/ATM to modulate interhomolog bias and checkpoint function during meiosis. PLoS Genet 7: e1002351. doi: 10.1371/journal.pgen.1002351 22072981
88. Daniel K, Lange J, Hached K, Fu J, Anastassiadis K, et al. (2011) Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1. Nat Cell Biol 13 : 599–610. doi: 10.1038/ncb2213 21478856
89. Santucci-Darmanin S, Neyton S, Lespinasse F, Saunieres A, Gaudray P, et al. (2002) The DNA mismatch-repair MLH3 protein interacts with MSH4 in meiotic cells, supporting a role for this MutL homolog in mammalian meiotic recombination. Hum Mol Genet 11 : 1697–1706. 12095912
90. Chan AC, Borts RH, Hoffmann E (2009) Temperature-dependent modulation of chromosome segregation in msh4 mutants of budding yeast. PLoS One 4: e7284. doi: 10.1371/journal.pone.0007284 19816584
91. Zhang L, Wang S, Yin S, Hong S, Kim KP, et al. (2014) Topoisomerase II mediates meiotic crossover interference. Nature 511 : 551–556. doi: 10.1038/nature13442 25043020
92. Perkins DD (1949) Biochemical Mutants in the Smut Fungus Ustilago Maydis. Genetics 34 : 607–626. 17247336
93. Schwacha A, Kleckner N (1994) Identification of joint molecules that form frequently between homologs but rarely between sister chromatids during yeast meiosis. Cell 76 : 51–63. 8287479
94. Kumar S, Nei M, Dudley J, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9 : 299–306. doi: 10.1093/bib/bbn017 18417537
95. Lupas A, Dyke MV, Stock J (1991) Predicting coiled-coils from protein sequences. Science 252 : 1162–1164. 2031185
Štítky
Genetika Reprodukční medicína
Článek Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and ThrombocytopeniaČlánek Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of andČlánek Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on PhenotypeČlánek Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human CancersČlánek A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic BacteriaČlánek The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System inČlánek The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological LifespanČlánek The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 6
-
Všechny články tohoto čísla
- Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Orphan Genes Find a Home: Interspecific Competition and Gene Network Evolution
- Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
- Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia
- Leveraging Identity-by-Descent for Accurate Genotype Inference in Family Sequencing Data
- Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
- Epigenetic Aging Signatures Are Coherently Modified in Cancer
- Silencing of DNase Colicin E8 Gene Expression by a Complex Nucleoprotein Assembly Ensures Timely Colicin Induction
- A Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
- The Orphan Gene Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation
- 9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
- The DnaA Protein Is Not the Limiting Factor for Initiation of Replication in
- FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling
- Multiple Changes of Gene Expression and Function Reveal Genomic and Phenotypic Complexity in SLE-like Disease
- Directed Evolution of RecA Variants with Enhanced Capacity for Conjugational Recombination
- The Regulatory T Cell Lineage Factor Foxp3 Regulates Gene Expression through Several Distinct Mechanisms Mostly Independent of Direct DNA Binding
- MreB-Dependent Inhibition of Cell Elongation during the Escape from Competence in
- DNA Damage Regulates Translation through β-TRCP Targeting of CReP
- Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of and
- The Analysis of () Mutants Reveals Differences in the Fusigenic Potential among Telomeres
- The Causative Gene in Chanarian Dorfman Syndrome Regulates Lipid Droplet Homeostasis in .
- Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype
- The . Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units
- AKTIP/Ft1, a New Shelterin-Interacting Factor Required for Telomere Maintenance
- Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
- Transfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
- Connecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
- Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Response to the Formal Letter of Z. Chrzanowska-Lightowlers and R. N. Lightowlers Regarding Our Article “Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers
- Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins
- Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential
- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level
- Genetic Changes to a Transcriptional Silencer Element Confers Phenotypic Diversity within and between Species
- Functional Assessment of Disease-Associated Regulatory Variants Using a Versatile Dual Colour Transgenesis Strategy in Zebrafish
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy
- The Human Blood Metabolome-Transcriptome Interface
- A Common Cancer Risk-Associated Allele in the Locus Encodes a Dominant Negative Inhibitor of Telomerase
- A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
- The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
- The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological Lifespan
- Dissection of a Complex Disease Susceptibility Region Using a Bayesian Stochastic Search Approach to Fine Mapping
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
- Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in
- Abnormal Activation of BMP Signaling Causes Myopathy in Null Mice
- Reproductive Mode and the Evolution of Genome Size and Structure in Nematodes
- Replication and Active Partition of Integrative and Conjugative Elements (ICEs) of the SXT/R391 Family: The Line between ICEs and Conjugative Plasmids Is Getting Thinner
- Motor and Sensory Deficits in the Mice Result from Mutation of the ESCRT Component HGS
- Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
- Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake
- Separable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4
- Multilayered Organization of Jasmonate Signalling in the Regulation of Root Growth
- Necrotic Cells Actively Attract Phagocytes through the Collaborative Action of Two Distinct PS-Exposure Mechanisms
- A Novel Feedback Loop That Controls Bimodal Expression of Genetic Competence
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Human Blood Metabolome-Transcriptome Interface
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



