-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMechanisms for the Evolution of a Derived Function in the Ancestral Glucocorticoid Receptor
Understanding the genetic, structural, and biophysical mechanisms that caused protein functions to evolve is a central goal of molecular evolutionary studies. Ancestral sequence reconstruction (ASR) offers an experimental approach to these questions. Here we use ASR to shed light on the earliest functions and evolution of the glucocorticoid receptor (GR), a steroid-activated transcription factor that plays a key role in the regulation of vertebrate physiology. Prior work showed that GR and its paralog, the mineralocorticoid receptor (MR), duplicated from a common ancestor roughly 450 million years ago; the ancestral functions were largely conserved in the MR lineage, but the functions of GRs—reduced sensitivity to all hormones and increased selectivity for glucocorticoids—are derived. Although the mechanisms for the evolution of glucocorticoid specificity have been identified, how reduced sensitivity evolved has not yet been studied. Here we report on the reconstruction of the deepest ancestor in the GR lineage (AncGR1) and demonstrate that GR's reduced sensitivity evolved before the acquisition of restricted hormone specificity, shortly after the GR–MR split. Using site-directed mutagenesis, X-ray crystallography, and computational analyses of protein stability to recapitulate and determine the effects of historical mutations, we show that AncGR1's reduced ligand sensitivity evolved primarily due to three key substitutions. Two large-effect mutations weakened hydrogen bonds and van der Waals interactions within the ancestral protein, reducing its stability. The degenerative effect of these two mutations is extremely strong, but a third permissive substitution, which has no apparent effect on function in the ancestral background and is likely to have occurred first, buffered the effects of the destabilizing mutations. Taken together, our results highlight the potentially creative role of substitutions that partially degrade protein structure and function and reinforce the importance of permissive mutations in protein evolution.
Published in the journal: . PLoS Genet 7(6): e32767. doi:10.1371/journal.pgen.1002117
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002117Summary
Understanding the genetic, structural, and biophysical mechanisms that caused protein functions to evolve is a central goal of molecular evolutionary studies. Ancestral sequence reconstruction (ASR) offers an experimental approach to these questions. Here we use ASR to shed light on the earliest functions and evolution of the glucocorticoid receptor (GR), a steroid-activated transcription factor that plays a key role in the regulation of vertebrate physiology. Prior work showed that GR and its paralog, the mineralocorticoid receptor (MR), duplicated from a common ancestor roughly 450 million years ago; the ancestral functions were largely conserved in the MR lineage, but the functions of GRs—reduced sensitivity to all hormones and increased selectivity for glucocorticoids—are derived. Although the mechanisms for the evolution of glucocorticoid specificity have been identified, how reduced sensitivity evolved has not yet been studied. Here we report on the reconstruction of the deepest ancestor in the GR lineage (AncGR1) and demonstrate that GR's reduced sensitivity evolved before the acquisition of restricted hormone specificity, shortly after the GR–MR split. Using site-directed mutagenesis, X-ray crystallography, and computational analyses of protein stability to recapitulate and determine the effects of historical mutations, we show that AncGR1's reduced ligand sensitivity evolved primarily due to three key substitutions. Two large-effect mutations weakened hydrogen bonds and van der Waals interactions within the ancestral protein, reducing its stability. The degenerative effect of these two mutations is extremely strong, but a third permissive substitution, which has no apparent effect on function in the ancestral background and is likely to have occurred first, buffered the effects of the destabilizing mutations. Taken together, our results highlight the potentially creative role of substitutions that partially degrade protein structure and function and reinforce the importance of permissive mutations in protein evolution.
Introduction
A central goal in studies of molecular evolution is to reveal the genetic, structural, and biophysical mechanisms by which protein functions have evolved [1]–[6]. Ancient proteins and DNA are seldom directly available, but the traces of their evolutionary history are found in their extant descendants [7]. Direct comparisons among present-day proteins can sometime yield insights into the sequence and structural mechanisms that underlie functional differences [8]–[11]. Such “horizontal” comparisons, however, cannot determine which protein features are ancestral and which are derived, so they are not suited to reconstructing the events that produced functional diversity [12]. Further, because the effect of a mutation on protein structure and function often depends on the residues present at other sequence sites [13]–[17], studies of extant proteins may often be unsuited to revealing the effects of mutations in the historical backgrounds in which they occurred [12].
Ancestral sequence reconstruction (ASR) allows the forms and functions of ancient proteins to be studied experimentally. Beginning with an alignment of extant sequences, the maximum likelihood phylogeny and best-fit probabilistic model of evolution are inferred; the most likely ancestral sequence at any node – defined as the sequence with the highest probability of delivering all the observed extant sequences – can then be identified [18]. These ancestral protein sequences can be “resurrected” using gene synthesis and cell culture or in vitro expression systems and then characterized using the same methods typically applied to study extant proteins. This approach allows hypotheses about the ancestral and derived characteristics of proteins to be tested experimentally. It also allows the historical interval during which structure and function changed to be identified and the causal role of specific historical mutations in the ancestral background to be determined.
The glucocorticoid and mineralocorticoid receptors (GR and MR) are paralogous hormone-regulated transcription factors that have served as useful models for studying protein evolution [15], [16], [19]. GR and MR have a modular domain structure that includes a well-conserved DNA-binding domain (DBD) and a moderately conserved ligand-binding domain (LBD) – which binds the hormone, changes conformation, and attracts coactivator proteins that potentiate transcription of nearby target genes; they also contain poorly conserved hinge and N-terminal domains. In most bony vertebrates, the intrinsic functions of the GR and MR LBDs differ in both specificity and sensitivity. GR is more specific, being activated by high doses of the adrenal hormone cortisol to regulate aspects of immunity, glucose metabolism, and the long-term stress response [20], [21]. MR, in contrast, is activated by the adrenal mineralocorticoids aldosterone or deoxycorticosterone, as well as cortisol (albeit with somewhat lower sensitivity), and primarily regulates osmotic homeostasis. GR is also considerably less sensitive than MR, often requiring concentrations several orders of magnitude higher for activation [19], [22], [23].
Some information is available on GR and MR evolution. The two paralogs descend by duplication from a single ancestral corticosteroid receptor (AncCR), which existed in an ancient jawed vertebrate ∼450 million years ago, before the divergence of bony vertebrates from cartilaginous fishes (Figure 1) [19], [24]. Reconstruction and experimental analysis showed that AncCR, like the extant MRs, was extremely sensitive to both mineralocorticoids and glucocorticoids, and its structure was MR-like, as well [19]. Subsequent work revealed that GR's specificity for glucocorticoids evolved later in the lineage leading to bony vertebrates, after the divergence of cartilaginous fishes but before the split of ray-finned fish from the lineage leading to tetrapods and lobe-finned fish, due to a small specific set of historical mutations [15], [16], [19] (Figure 1).
Fig. 1. Simplified phylogeny of corticosteroid receptors. 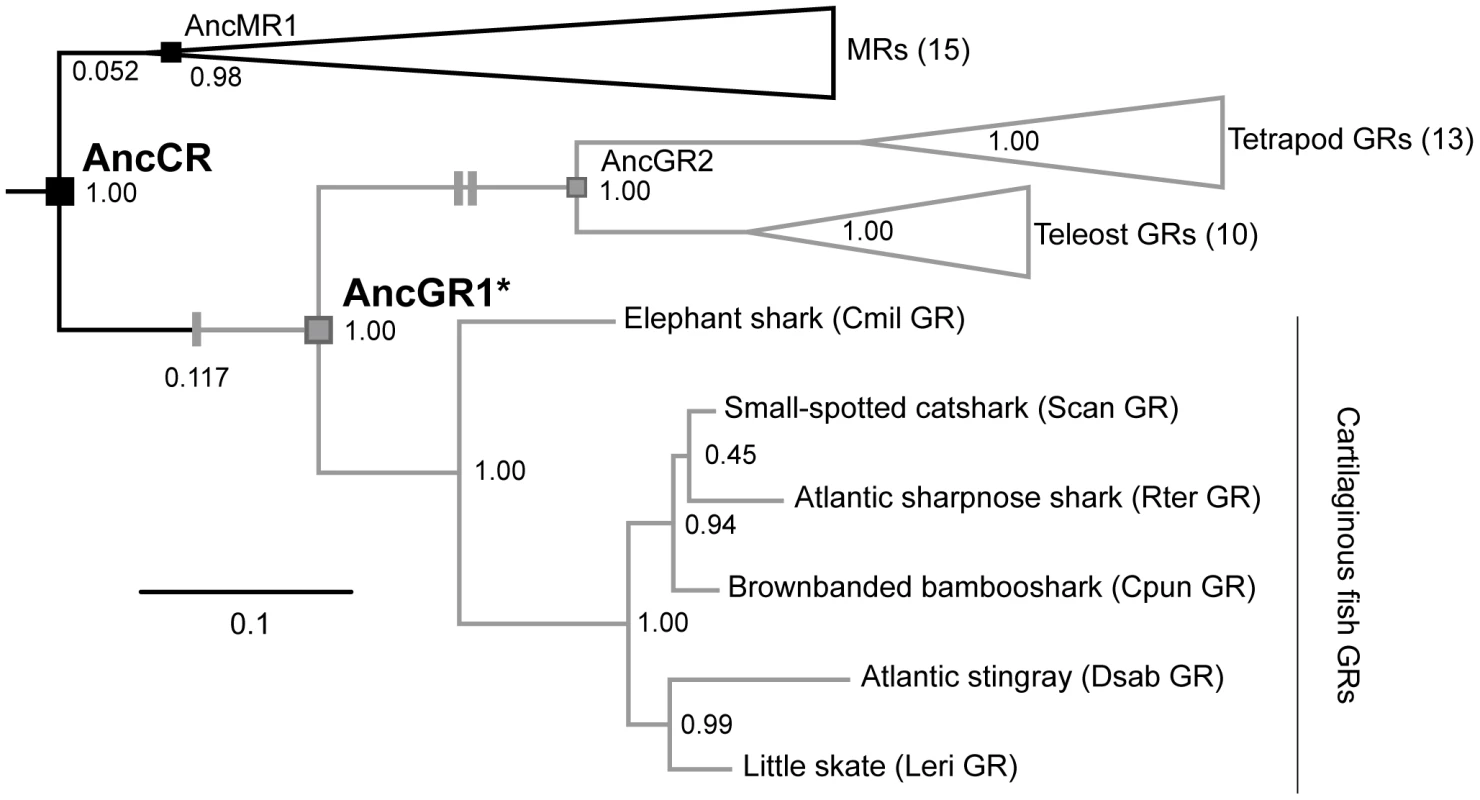
Ancestral sequences are shown at relevant nodes: AncCR, the last common ancestor of all MRs and GRs; AncGR1, the GR ancestor of cartilaginous fishes and bony vertebrates; AncGR2, the GR ancestor of ray- and lobe-finned fishes (including tetrapods); AncMR1, the MR ancestor of cartilaginous fishes and bony vertebrates. (AncGR1.0 and AncGR1.1 are different reconstructions of node AncGR1, inferred from datasets with different taxon sampling.) Black, high sensitivity receptors; gray, low sensitivity receptors. Single and double gray dashes mark functional shifts towards reduced sensitivity and increased specificity, respectively. Support values are the chi-square statistic (1 – p, where p equals the estimated probability that a node could occur by chance alone) calculated from approximate likelihood ratios. The length of branches from AncCR to AncMR1 and to AncGR1, expressed as the mean number of substitutions per site, are indicated in parentheses. The evolutionary causes of GR's reduced hormone sensitivity are not known. In the little skate – the only cartilaginous fish studied to date – GR is a low-sensitivity, broad-spectrum receptor: like MR, it responds to both glucocorticoids and mineralocorticoids, but it is unique in requiring high concentrations of either type of hormone to activate it. The difference in receptor sensitivity between the GR and MR is thought to have physiological consequences: in several elasmobranch species, the same corticosteroids appear to regulate both stress and osmolarity [25]–[28], and the highest titres are associated with stress conditions [29], [30]. These observations suggest that GR regulates stress in response to high doses of hormones, while MR regulates osmolarity in response to much lower doses [23].
Based on these data, we hypothesize that GRs' reduced sensitivity to all hormones was an independent evolutionary event that occurred before cartilaginous fishes split from bony vertebrates, and before glucocorticoid specificity evolved in the GRs of bony vertebrates [15], [19]. Here we report on experiments to test this hypothesis and determine the genetic, structural, and biophysical mechanisms by which GR's reduced hormone sensitivity evolved. We first resurrected the LBD of AncGR1 (Figure 1) – the GR protein present in the common ancestor of bony and cartilaginous vertebrates and the earliest node after the GR-MR split – and then used functional assays, X-ray crystallography, site-directed mutagenesis, and computational predictions of biophysical parameters to dissect the mechanisms by which GR evolved. We show that after its initial birth by gene duplication, a small number of mutations that partially degraded its structure, stability, and function caused GR to become a novel low-sensitivity receptor.
Results
Isolation and Characterization of Cartilaginous Fish GRs
Statistical confidence in ASR depends in part on taxon sampling in groups descending directly from the node of interest [31]–[33]. Although GR sequences are available from many bony vertebrates, only a single GR sequence from cartilaginous fishes has been previously sequenced. We therefore isolated additional GRs sampled from throughout the cartilaginous fishes and characterized the functions of their LBDs. Specifically, we isolated GRs from four elasmobranch species – the Atlantic sharpnose shark (Rhizoprionodon terraenovae), brownbanded bambooshark (Chiloscyllium punctatum), small-spotted catshark (Scyliorhinus canicula), and the Atlantic stingray (Dasyatis sabina) – and one holocephalan, the elephant shark (Callorhincus milii) (Figure 1).
We used a luciferase reporter gene expression assay to characterize the sensitivity of each LBD to four major corticosteroids present in elasmobranchs – 11-deoxycorticosterone (DOC), corticosterone, 1alpha-hydroxycorticosterone, and 11-dehydrocorticosterone [34], [35]. All elasmobranch GRs were low-sensitivity receptors activated by multiple corticosteroids, except for the D. Sabina GR, which did not activate transcription in the presence of any hormone. All hormone-activated cartilaginous fish GRs were most sensitive to DOC and corticosterone. The receptors had EC50 values (the hormone concentration required to elicit half-maximal activation) for these steroids in the 10−8 to 10−6 M range (Figure 2, Table S1), typical of the EC50s of bony fish GRs for glucocorticoids but two to four orders of magnitude greater than AncCR or the MRs of bony vertebrates [19]. These observations are consistent with a model that after duplication of AncCR – which was highly sensitive to a broad array of corticoisteroids – GR evolved reduced sensitivity without a shift in specificity, explaining the observed characteristics of AncGR1 and the GRs of extant elasmobranchs; later – after elasmobranchs diverged from bony vertebrates – the narrower specificity for glucocorticoids that characterizes the GRs of present-day tetrapods and teleosts evolved (Figure 1).
Fig. 2. AncGR1 and its descendents evolved reduced hormone sensitivity. 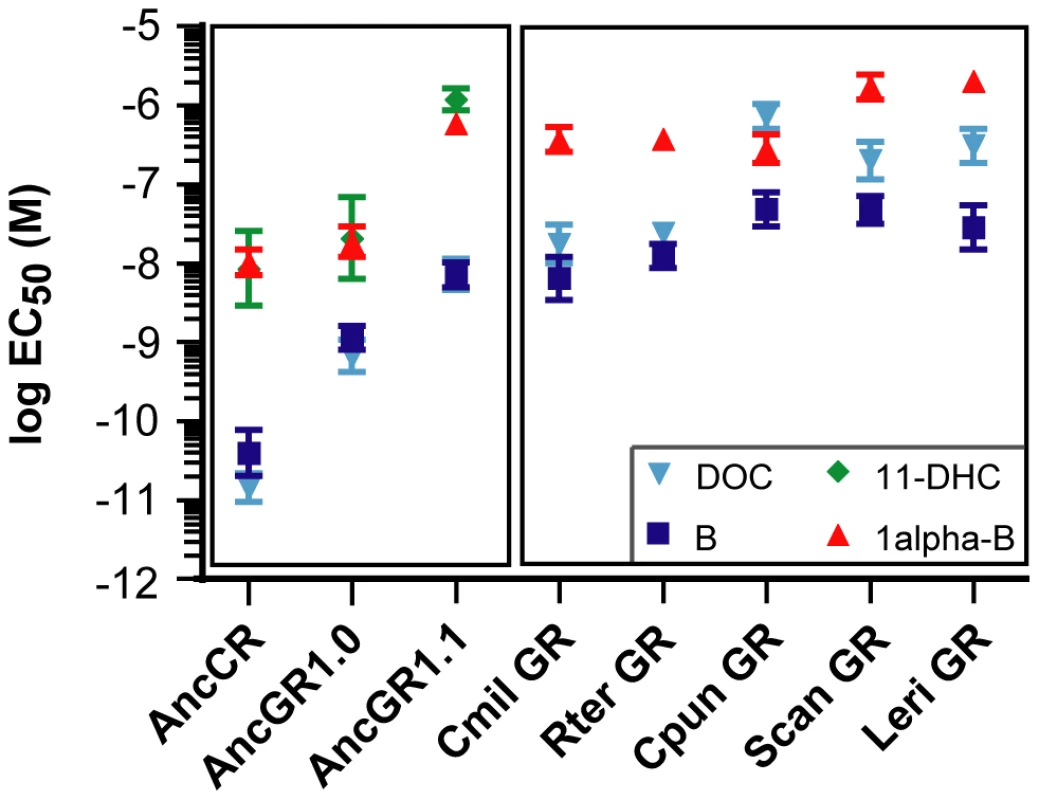
Ligand-dependent transcriptional activation of receptors was measured in the presence of increasing concentrations of hormone using a luciferase reporter gene assay. Dose-response curves were calculated for receptor-hormone pairs and plotted as the log effective concentration of hormone for half maximal activation (log EC50) in molar with standard error. Larger values, lower sensitivity; smaller values, higher sensitivity. Hormones are: 11-deoxycorticosterone (DOC), corticosterone (B), 11-dehydrocorticosterone (11-DHC), and 1α-hydroxycorticosterone (1α-B). Cartilaginous fish species are shown in (Figure 1). 11-DHC did not activate cartilaginous fish GRs in our assay (defined as <2-fold activation for EC50>1 µM of hormone) and is not shown for these receptors. Reconstruction and Functional Analysis of the Ancestral GR
The new cartilaginous fish GR sequences were added to a dataset of 97 other steroid receptor sequences and aligned for phylogenetic analyses and ancestral sequence reconstruction. The maximum likelihood phylogeny was generally well supported and in agreement with previously published trees [19], except for the placement of the agnathan receptors (Figure S1, Table S2).
The hypothesis that GR's functions are derived can be tested experimentally and by sequence analysis of evolutionary rates. This hypothesis predicts that the rate of amino acid evolution after duplication of AncCR should be faster in the lineage leading to the GRs than in that leading to the MRs, which retain the ancestral functions [3]. Branch lengths between two nodes represent the mean probability of substitution per site, which equals the product of evolutionary rate times time. The branch leading from AncCR to AncGR1 and the branch leading from AncCR to AncMR1 (MR in the same ancestral species—the common ancestor of jawed vertebrates) cover exactly the same period of time, so any authentic differences in length must be due to differences in evolutionary rate. As predicted, there are 36 differences between the AncCR and AncGR1.1, compared to 16 between AncCR and AncMR1, and the estimated amino acid replacement rate 2.25 times greater on the GR branch than on the MR branch (Figure 1), but this difference did not reach formal statistical significance (p = 0.09) using a likelihood ratio test.
To more decisively test the hypothesis that GR's functions changed between AncCR and AncGR1, we used ancestral reconstruction. We inferred the sequence of AncGR1 assuming the best-fit model and integrating over plausible phylogenies weighted by their posterior probabilities [36]. The denser taxon sampling of this study was found to improve confidence in the inferred AncGR1 sequence compared to the previously published version, which was inferred from an alignment that included only a single cartilaginous fish [15]. The updated reconstruction, which we named AncGR1.1, differs at 7% of sites from the original reconstruction (AncGR1.0), with a higher mean posterior probability across all sites (0.951 vs. 0.930), a greater number of sites reconstructed with 100% posterior probability, and fewer sites reconstructed with plausible alternate states (Figure S2, Table S3). Other previously reconstructed ancestral steroid receptor sequences, such as AncCR and AncGR2 (the GR gene in the last common ancestor of ray - and lobe-finned fishes, including tetrapods) [19], were affected to a much lesser extent by including additional cartilaginous fish sequences.
We then characterized the functions of the AncGR1.1 LBD by synthesizing a nucleic acid sequence that codes for it, subcloning that sequence into an expression construct, and assaying its sensitivity to the same suite of corticosteroids using the luciferase reporter assay. As predicted, we found that AncGR1.1 is activated by the same broad suite of hormones as AncCR, but markedly higher doses are required. For all ligands tested – including the classic mineralocorticoid DOC – AncGR1.1 was 25 - to 530-fold less responsive to hormone than AncCR (Figure 2, Table S1). These data allow us to trace on the phylogeny two separate shifts in the evolution of GRs from a high-sensitivity, promiscuous corticosteroid receptor: first, the evolution of reduced hormone sensitivity, and later a loss of sensitivity to mineralocorticoids (Figure 1).
To determine whether AncGR1.1′s reduced sensitivity could be an artifact of uncertainty in the ancestral sequence reconstruction, we introduced plausible alternate states into the maximum likelihood ancestral sequence and repeated the experimental characterization. None of these contradicted the finding that AncGR1.1 has markedly reduced hormone sensitivity compared to AncCR (Table S1). Taken together, these observations indicate that reduced sensitivity evolved in the GR lineage after duplication of AncCR but before the split of cartilaginous from bony vertebrates, and this conclusion is robust to uncertainty about the ancestral reconstruction.
Genetic Basis of Reduced AncGR1.1 Sensitivity
We next sought to identify the genetic mechanisms that caused reduced hormone sensitivity to evolve. Because the shift in function occurred on the branch between AncCR and AncGR1.1, the initial set of candidate mutations includes the 36 historical substitutions that occurred on this same branch. At 17 of these sites, the same derived state is present in both AncGR1.1 and AncGR1.0: AncGR1.0 is much more similar in sensitivity to AncCR than AncGR1.1 is (Figure 2), so substitutions at these sites are unlikely to represent the major-effect mutations. Of the 19 substitutions that are unique to AncGR1.1, twelve represent biochemically conservative replacements (e.g., D/E, I/L, K/R, S/T). Only one of the others is in a position predicted to contact ligand based on the crystal structures of other steroid receptors [15], [37]; this substitution (A36G) was previously tested in AncCR and found to have no significant effect on sensitivity to DOC or other corticosteroids [19]. We therefore prioritized the six remaining biochemically radical replacements as the best candidates for having caused the evolution of reduced sensitivity.
We introduced each candidate mutation into the maximum likelihood (ML) AncCR background using site-directed mutagenesis and tested its effect on hormone sensitivity in the luciferase reporter gene assay with increasing concentrations of DOC. Two substitutions – V43A and R116H – markedly reduced AncCR's sensitivity to hormone, increasing the receptor's EC50 of DOC by at least two orders of magnitude to AncGR1.1-like values (Figure 3, Table 1). The others had much weaker effects on sensitivity. The double mutant V43A/R116H was severely compromised, with an EC50 for DOC ∼10,000 times greater than AncCR and more than 50 times greater than even AncGR1.1. These results indicate that V43A and R116H are large-effect historical mutations that are more than sufficient to recapitulate the evolution of the low-sensitivity AncGR1.1. They also indicate that the effects of these two mutations on receptor sensitivity must have been partially buffered by additional substitutions that occurred during the same interval.
Fig. 3. Three historical substitutions in AncCR recapitulate the evolution of reduced sensitivity. 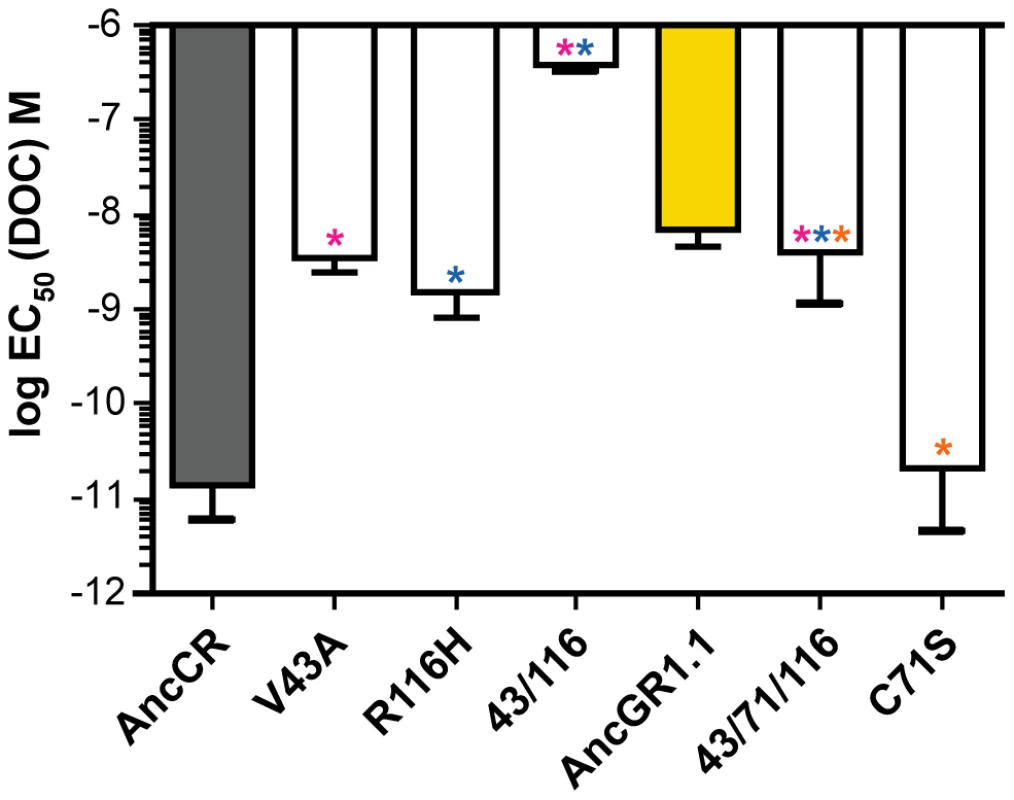
Shown is the log EC50 of molar DOC with the highly sensitive ancestor (AncCR, gray), the low sensitivity descendent (AncGR1.1, yellow), and AncCR mutants. Single and combination mutants are denoted with colored asterisks: V43A, pink; R116H, blue; C71S, orange. Tab. 1. Response of ancestral and mutant receptors to 11-deoxycorticosterone (DOC). 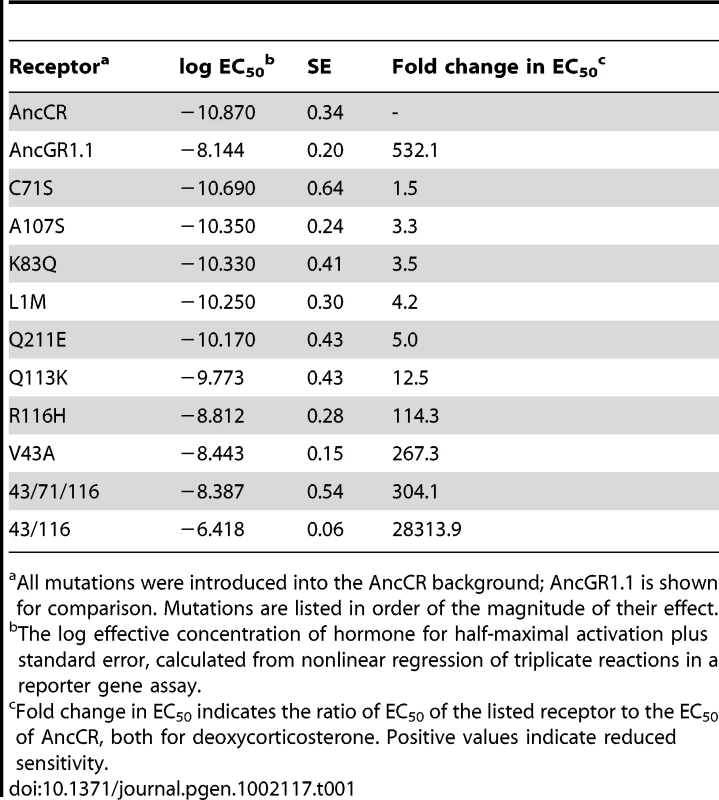
All mutations were introduced into the AncCR background; AncGR1.1 is shown for comparison. Mutations are listed in order of the magnitude of their effect. Crystal Structure of the AncGR1:DOC Complex
To understand the structural basis of reduced GR sensitivity and identify other important substitutions, we purified AncGR1.1 expressed in E. coli and used X-ray crystallography to determine its atomic structure in complex with DOC at 1.95 Å resolution (see Table S4). AncGR1.1 adopts the classic steroid receptor active conformation [38], consisting of three helical layers, an internal ligand cavity bounded by helices 3, 5, 6, 7, and 10, well-defined ligand density within the cavity, and a surface for coactivator binding formed by helices H3, H5, and H12 [39].
The conformation of AncGR1.1 is very similar to the previously determined AncCR crystal structure, with a root mean square deviation (RMSD) in backbone atom position of only 0.66 Å (Figure 4A), and most of the larger deviations are far from the ligand. The side chain identity of all residues within 4 Å of DOC are conserved except for A36G, which alters a ligand-contacting residue but has no discernible effect on hormone sensitivity [19]. These results indicate that AncGR1.1′s reduced sensitivity to hormone must be due to indirect mechanisms not involving contacts with the ligand, such as changes to intraprotein contacts that affect the stability of the protein-hormone complex.
Fig. 4. The crystal structure of AncGR1.1-LBD in complex with DOC (yellow, PDB 3RY9) compared to the previously solved structure of AncCR with DOC (gray, PDB 2Q3Y). 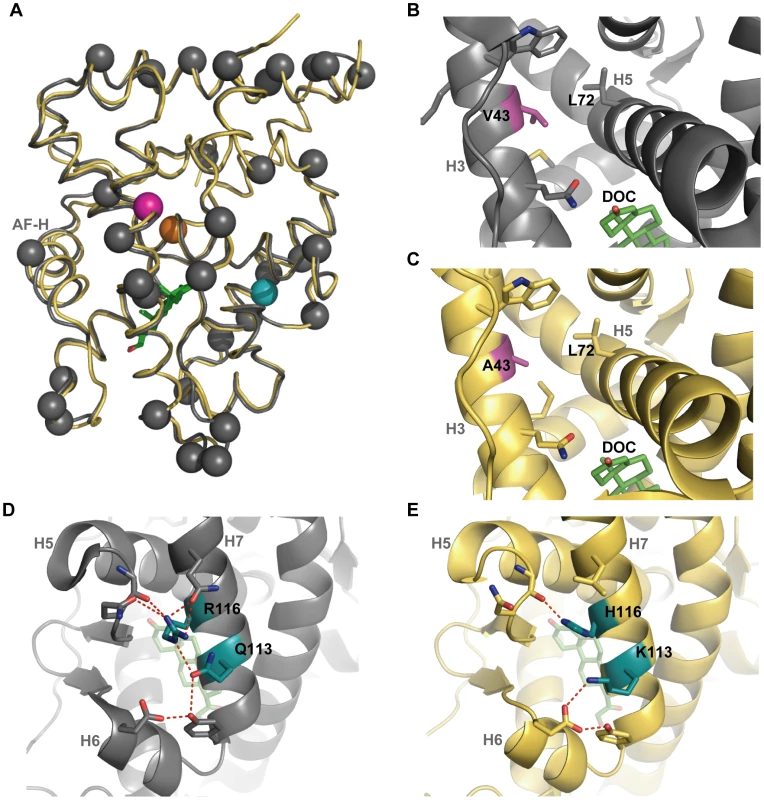
Green sticks indicate DOC; possible hydrogen bonding indicated by red dashes. Helices are indicated by light grey text; AF-H equals H12, the activation function helix. A) AncCR and AncGR1.1 are highly structurally conserved despite 36 substitutions between them. Spheres show sequence differences: pink, position 43; blue, position 116; orange, position 71. B) and C) show the structural environment around position 43. The substitution of V43 in AncCR (B) in place of A43 in AncGR1.1 (C) is thought to weaken the hydrophobic interactions of the surrounding helices. D) and E) show the structural neighborhood of position 116. Replacing R116 (D) with H116 (E), along with a Q113K substitution, significantly reduces and rearranges ancestral hydrogen bonding. Desensitizing Mutations Caused a Loss of Intra-Protein Interactions
To understand the mechanisms by which mutations V43A and R116H reduced hormone sensitivity, we first examined the apparent roles of these residues in the AncCR and AncGR1.1 crystal structures. Position 43 faces inward on the middle of H3, just above the ligand where H3 packs against H5 and forms part of the coactivator-binding cleft. In AncCR, Val43 packs tightly against neighboring hydrophobic residues, making van der Waals contacts with Leu72, presumably stabilizing H3 and H5, which participate in forming both the coactivator interface and the ligand pocket (Figure 4B). In AncGR1.1, the smaller side chain of Ala43 loses its van der Waals contacts to Leu72, opening a small cavity in this region (Figure 4C). The poor packing that results is expected to destabilize the receptor-ligand complex.
Position 116 is situated on H7, the opposite side of the protein from site 43. In AncCR, R116 is a hub in a network of hydrogen bonds between H7 and residues in H5 and H6 (Figure 4D). In AncGR1.1, this hydrogen-bond network is much sparser, largely due to the replacement of Arg116 with His (Figure 4E). The loss of favorable interactions presumably destabilizes these helices, the ligand pocket, and possibly the coactivator interface.
We also noted that a third historical substitution in this region, Q113K, abolishes other hydrogen bonds in the same network as Arg116H. When we introduced Q113K into AncCR, it also reduced sensitivity, though its effect was considerably smaller than those of V43A and R116H (Table 1). The loss of favorable interactions in the atomic structure due to substitutions at sites 43, 116 and 113, together with the experimental finding that these mutations recapitulate the evolutionary decline in AncCR's hormone sensitivity, suggests that AncGR1's novel function – its reduced sensitivity to corticosteroids – evolved because of the partial degeneration of ancestral structures and functions.
C71S Buffers against Desensitizing Mutations
Because introducing mutations V43 and R116H together into AncCR reduces sensitivity to an extent greater than the historical difference between AncCR and AncGR1, other historical substitutions during the same interval must have buffered the impact of these large-effect mutations. Of the remaining candidate mutations, one – C71S – occurred at a site already known to have a strong positive effect on receptor function in extant steroid hormone receptors: introducing serine at the homologous site in mammalian GRs (F602S) dramatically improves bacterial expression, solubility, and crystallization [15], [16], [37], [40]–[2]. To test the hypothesis that the historical acquisition of Ser71 buffered the effect of mutations at sites 43 and 116, we introduced mutation C71S into AncCR-V43A/R116H background. As predicted, this additional change improved sensitivity by ∼90-fold, yielding a receptor with DOC sensitivity similar to that of AncGR1.1. In isolation, however, the C71S substitution has no discernable effect on AncCR sensitivity (Figure 3, Table 1).
The biophysical mechanism for this buffering effect is not clear. All previously crystallized corticosteroid receptors, ancestral and extant, have had Ser71 engineered into them to aid in protein expression and crystallization [15], [16], [37], [40]–[2]; comparison to receptor agonist structures lacking Ser71 is therefore not possible. In both the AncCR and AncGR1.1 structures, this site is located on H 5 in the central core of the protein, just above the ligand-binding pocket, bordering a distinctive kink in H5 (Figure 5). Ser71 is adjacent to a highly solvated channel next to the hydrophobic core of the receptor, and a serine substitution would increase the hydrophilicity of the region compared to the ancestral cysteine. It appears that Ser71 in Chain B of AncGR1.1 might stabilize the receptor through direct and water-mediated hydrogen bonds that a cysteine would not form (Figure 5). This is not a strictly conserved mechanism, however, because in the structures of AncCR-C71S (Figure 5, inset) and Chain A of AncGR1.1, the polar Ser71 side chain occupies an alternate conformation: it interacts with water molecules in the channel, but the bond network varies among the structures. An alternate explanation for the buffering effect of C71S is that it may facilitate proper folding and solubility of the protein, an effect that could have a more beneficial effect on receptors with less stable native conformations, such as those carrying the V43A or R116H substitutions.
Fig. 5. The structural context of site 71. 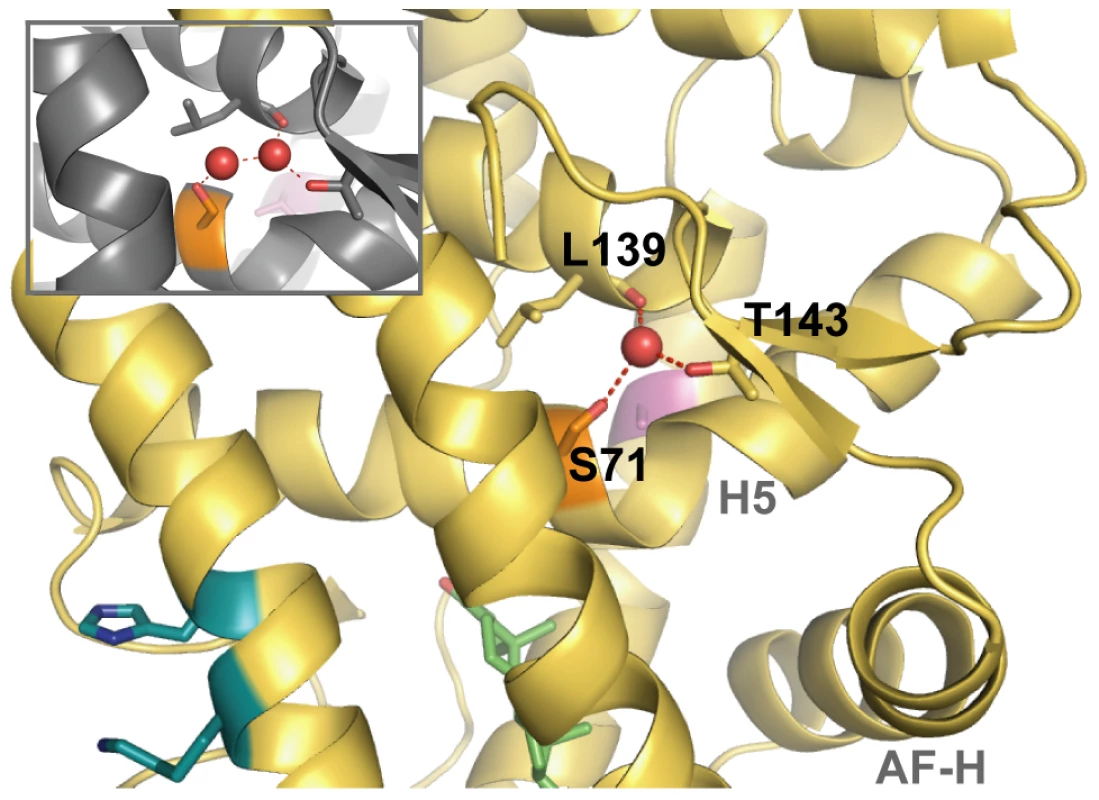
S71 (orange) in AncGR1.1 (Chain B, yellow) forms a water-mediated hydrogen bond with the carbonyl carbon of L139 and the T143 side chain. In AncCR, the side chain of an engineered S71 mutant adopts a slightly different conformation (inset). The C71S substitution increases the hydrophilicity of the region. Also shown are the relative locations of the ligand, DOC (green); V43A (pink); the R116H and Q113K substitutions (blue); possible hydrogen bonding (red dashes); and relevant helices (light grey text). Desensitizing Mutations Are Predicted to Be Thermodynamically Destabilizing
To test the hypothesis that the evolutionary reduction in GR sensitivity was due to mutations that destabilized the receptor-hormone complex, we used a computational approach to predict the effects of historical GR mutations on the stability of AncCR. Using the AncCR:DOC crystal structure as a starting-point, we used FoldX software [43] to generate single and combination AncCR mutants, optimize the predicted structures, and calculate the predicted change in free energy of folding (ΔΔG) for mutant receptors. We calculated the distribution of stability effects for 29 single-substitution mutants and found that V43A and R116H are, as predicted, the most destabilizing substitutions, reducing stability by 1.62 and 2.81 kcal/mol, respectively (Figure 6A, Table S5). The buffering mutation C71S had a very weakly destabilizing effect (0.29 kcal/mol). The combination V43A/R116H is predicted to be extremely destabilizing in AncCR (5.31 kcal/mol); addition of C71S to this background causes a slight additional decrease in stability (5.41 kcal/mol).
Fig. 6. The effect of historical GR substitutions on AncCR protein stability, predicted by FoldX [43]. ![The effect of historical GR substitutions on AncCR protein stability, predicted by FoldX <em class="ref">[<b>43</b>]</em>.](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/2e9aee402738b63fb1215a7e54a34809.png)
A) The distribution of stability effects (ΔΔG) for almost all (n = 29 of 36) single GR substitutions in the AncCR background. Colored asterisks indicate bins with notable mutants: V43A (pink), R116H (blue), and C71S (orange). B) For those mutants in which hormone sensitivity was assessed (n = 10, plus AncCR), the predicted loss of stability correlates well with the observed loss of sensitivity towards DOC. Linear regression with C71S mutants, r2 = 0.78; without, r2 = 0.89. Single and combination mutants are colored as in (A). X values are the average of five FoldX runs with standard deviation; Y values are the mean of triplicate reactions with standard error. The intercept lies at values for AncCR (0, −10.870). We found a strong overall correlation between the predicted effects of mutations on protein stability and the observed reduction in receptor sensitivity to hormone (r2 = 0.78; Figure 6B, Table 1, Table S5). The relationship is even tighter (r2 = 0.89) when C71S-containing mutants are excluded. These results corroborate the hypothesis that V43A, R116H, and Q113K caused the evolution of reduced receptor sensitivity by destabilizing the protein-hormone complex, while C71S partially buffered the effects of these mutations through mechanisms not directly related to protein stability.
Discussion
Our analyses allow a detailed description of the genetic, structural, and biophysical mechanisms by which AncGR1 evolved its derived function – sensitivity to only high concentrations of corticosteroid hormone – after duplication of an ancestral receptor that was sensitive to very low doses of the same hormones. The shift appears to have been driven primarily by two large-effect mutations that caused partial degradation of the receptor's structure and function. The mechanism for the functional change appears to be that these mutations compromised favorable hydrophobic interactions and hydrogen bonds in the ancestral protein, destabilizing the hormone-receptor complex. Although the combined effect of the two large-effect mutations is so great that they nearly abolish hormone sensitivity in the double mutant, a third mutation – which occurred during the same historical interval strongly buffers their effect. This buffering mutation causes no apparent effect on function in isolation; we therefore conjecture that this mutation occurred as a permissive mutation before V43A and R116H were established. Additional substitutions subsequently tuned the sensitivity of AncGR1 and its descendants. Our results do not allow us to determine the roles of selective and neutral processes in the fixation of these mutations, and the physiological significance of the GR's reduced sensitivity in the ancestral organism is unknown; it is possible, however, that the advent of a low-sensitivity GR, along with the high-sensitivity MR, allowed a greater degree of endocrine control by different doses of corticosteroids, as appears to be the case in extant elasmobranchs.
Modulating the stability of a protein:ligand complex represents one way to alter the effective dose of ligand required to produce some specific quantity of active complex. During the evolution of AncGR1, this outcome was achieved through mutations that disrupted favorable contacts among structural elements of the peptide itself, without directly affecting receptor-ligand contacts. Our findings add to a growing literature on the evolution of protein stability. Random mutations are more likely to reduce than increase protein stability [44]–[46]. Periods of mutation accumulation without purifying selection can quickly degrade proteins structures so they fall below the minimum threshold for folding and activity [13], [14], [47], [48]. Although most proteins are marginally stable, a protein's precise distance from this threshold is dynamic, depending on the specific balance of stabilizing and destabilizing mutations that have occurred [13], [49]. A stability threshold can also be relaxed by mechanisms such as the overexpression of chaperone proteins [50] or decreased selection for optimal protein function [14]. “Global suppressor” mutations that increase protein stability allow a greater number of destabilizing mutations to accumulate than would otherwise be allowed [51]–[53], including those that generate novel functions [15], [17], [49], [51], [54]–[56].
Our observations indicate that destabilizing mutations can be buffered not only by permissive mutations that increase stability but also by those that affect protein structure and function via other biophysical mechanisms, such as folding or solubility [57], [58]. The extreme reduction in stability conferred on AncGR1.1 by mutations at sites 43 and 116 are strongly buffered by historical mutation C71S, which does not affect function in isolation and is not predicted to alter protein stability. Previous studies in extant proteins have shown that introduction of a serine at the homologous site in rat and human GRs increases transcriptional activity and ligand affinity [59] and dramatically improves protein solubility and expression in bacterial cells [15], [16], [37], [40]–[42]. It has been proposed that the serine maintains the receptor in an “agonist-like” conformation, preventing the collapse and aggregation of the LBD [60]. Effects on protein folding and aggregation may explain why C71S by itself is neutral in AncCR and yet, in the context of strongly destabilizing mutations, is required to maintain the active conformation and function. An alternative explanation is that C71S may play a local role in maintaining secondary structural elements and the spatial relations between them in the active conformation; the location of C71S on H5, directly opposite where V43A packs against H5, lends credence to this possibility. Additional experiments will be necessary to directly measure the effects of mutations at sites 43, 71, and 116 on the protein's biophysical properties.
Our study highlights a creative role for partial loss-of-function mutations in the evolution of novel genes and gene functions. This aspect of GR evolution is related to the process described by Bridgham et al. [61] during post-duplication evolution of a different steroid receptor: in that case, a loss-of-function mutation abolished the modular LBD's ligand-activated transcriptional function and generated a competitive repressor that retained its ability to compete with its paralog for DNA and dimerization partners. The mechanism we observed during AncGR1 evolution, in contrast, involved a partial loss of activity, leading to densensitization of the receptor and a novel response to existing hormone levels. Steroid signaling relies on very precise molecular cues, and changes in receptor sensitivity can have noticeable effects on biological response [62]. After duplication of AncCR, MRs retained the ancestral receptor's sensitivity, while the evolution of reduced sensitivity in the GR created a distinctly different transcriptional regulator that responded only to high doses of hormone. These observations demonstrate how mutations that abolish or impair native protein functions can drive the evolution of novel functional roles after gene duplication [3].
Methods
Receptor Isolation
The little skate (Leucoraja erinacea) GR ligand-binding domain (LBD) was isolated previously using degenerate PCR and RACE with liver cDNA [19]. The skate GR protein sequence was used in a tblastn search of the elephant shark genome (http://esharkgenome.imcb.a-star.edu.sg/) to identify its GR LBD, and gene-specific primers were designed to amplify the coding sequence from cDNA. All other cartilaginous fish GR LBDs were isolated by hemi-degenerate PCR from cDNA using a degenerate primer in the GR DNA-binding domain (DBD) in combination with a gene-specific primer for a ∼25 bp sequence conserved in the 3′-UTR of the elephant shark and skate (5′-TCATATGCACTACATATGGTTTACAGA-3′). In total, GR LBDs were amplified using high-fidelity PCR from five cartilaginous fish species: elephant shark (Callorhincus milii), Atlantic sharpnose shark (Rhizoprionodon terraenovae), brownbanded bambooshark (Chiloscyllium punctatum), small-spotted catshark (Scyliorhinus canicula), and Atlantic stingray (Dasyatis sabina). Template cDNA for PCR was graciously provided by B. Venkatesh (C. milii and C. punctatum) and B.S. Nunez (R. terraenovae, S. canicula, and D. sabina).
Phylogenetic Analysis
The conserved DNA - and/or ligand-binding domains of 97 steroid receptor protein sequences were aligned using Clustal X [63]. Maximum likelihood phylogenetics was performed using PhyML_aLRT [64] assuming the Jones model of evolution [65] and a four-category discrete gamma distribution of among-site rate variation, with the shape parameter estimated from the data; the JTT model was previously shown to be highly supported, with 100% posterior probability, when this and other models are compared in a Bayesian analysis [19]. Support at nodes was calculated as the chi-square statistic using an approximate likelihood ratio test [64]; the chi-square statistic represents 1-p, where p is the estimated probability that the given node would occur by chance alone.
Ancestral Sequence Reconstruction and Gene Resurrection
The maximum likelihood tree topology differed from previously published SR phylogenies with respect to the placement of jawless fish receptors [19]; to account for this uncertainty, ancestral receptor sequences were reconstructed over both the experimental and published trees weighted by their inferred posterior probability [36]. Ancestral states were inferred using PAML version 3.15 [66] and the ancestral reconstruction tool Lazarus [36], given the sequence alignment, phylogenies, and the JTT model. For any ancestor relevant to our study, no site in the inferred sequence possessed amino acid states that differed between trees. A nucleic acid sequence coding for the LBD of the last common ancestor of all GRs (AncGR1.1) was optimized for expression in mammalian cells, synthesized de novo (Genscript, Piscataway, NJ), and characterized as described below.
Tests of Evolutionary Rates
Rates of protein evolution were analyzed in HyPhy [67]. A likelihood ratio test (LRT) was used to compare the relative branch lengths from the last common GR/MR ancestor (AncCR) to the last common ancestors of all GRs (AncGR1.1) or MRs (AncMR1). Under the null hypothesis of equal rates, all branch lengths were unconstrained and optimized independently; under the alternate hypotheses, the branch leading from AncCR to AncGR1 was constrained to have the same length as that leading from AncCR to MR1 (MR in the ancestor of all jawed vertebrates). The likelihood ratio of alternate and null models was determined and a p-value calculated using a chi-squared distribution with one degree of freedom.
Receptor Characterization
LBDs were cloned as fusion proteins into a pSG5-Gal4DBD expression vector (gift of D. Furlow) and cotransfected using Lipofectamine and Plus Reagents (Invitrogen, Carlsbad, CA) with a UAS-driven luciferase reporter gene (pFRluc) into mammalian cell culture (CHO-K1), and grown in phenol red-free α-MEM plus 10% dextran-charcoal-stripped fetal bovine serum (Hyclone, Logan, UT). Cells were incubated with transfection reagents for four hours, after which they were treated with fresh medium; after recovery, cells were treated in triplicate with hormone or vehicle control, and incubated for one day. Reporter expression was measured using Dual-Glo (Promega, Madison, WI) and dose-response relationships analyzed using Prism4 (GraphPad, La Jolla, CA). Site-directed mutagenesis was carried out using QuickChange II (Stratagene, La Jolla, CA) and clones verified by DNA sequencing. Plausible alternate states were defined as non-maximum likelihood amino acid states with posterior probability >0.20; we reasoned that residues that are present in one or more extant high-sensitivity receptors are the most likely to increase receptor sensitivity, whereas those that are present only in low-sensitivity receptors are unlikely to confer high sensitivity. Each such alternate state was introduced singly into the ML AncGR1.1, and the experimental characterization was repeated.
Protein Growth, Purification, and X-Ray Crystallography
AncGR1.1 was subcloned into the pMCSG7-MBP-His expression vector, transformed into BL21 (DE3) pLysS cells, and grown to an OD600 of 0.8–1.0. Cultures were induced with 0.1 µM IPTG plus 50 µM of the steroid 11-deoxycorticosterone (DOC) and grown overnight at 16°C. Purification of AncGR1.1 was performed using nickel affinity chromatography and a sizing column. Pure AncGR1.1 was concentrated to 3.7 mg/mL and dialyzed into a crystallization buffer consisting of 20 mM Tris, pH 6.5, 150 mM NaCl, 5% glycerol, 50 µM CHAPS, and 50 µM hormone (DOC).
Multiple sparse matrix screens were set with AncGR1.1 protein using a Phoenix crystallization robot (Art Robbins Instruments, Sunnyvale, CA); hits formed at 22°C from the Salt Rx screen (Hampton Research, Aliso Viejo, CA). Crystals were optimized at 22°C in hanging drop diffusion plates with: 2.5–2.8 M sodium acetate trihydrate, pH 7.0, 0.1 M BIS-TRIS propane, pH 7.0, and a small peptide designed from the TIF2 Box3 steroid receptor coactivator protein. Crystals were soaked in a cryoprotectant solution containing 20% glycerol and flash-frozen in liquid nitrogen. Data was collected to 1.95 Å resolution at the South East Regional Collaborative Access Team (SER-CAT) at the Advanced Photon Source (Argonne National Laboratory) and data was processed and scaled with HKL2000 [68]. Initial phasing of the AncGR1.1 plus DOC structure was determined using molecular replacement of the AncCR with DOC (2Q3Y); model building and refinement of the structure was carried out using COOT version 0.5 [69] and REFMAC [70] in the CCP4 suite [71]. The root mean square deviation (RMSD), a measure of the overall similarity between protein backbones, was calculated using CaspR [72]. Interpretation was focused on Chain B, which displayed lower overall b-factors and had fewer crystal-packing contacts. Coordinates have been deposited in PDB with accession 3RY9.
Analyses of Protein Stability
We used FoldX version 3.0 Beta 4 [43] to predict protein stability of AncCR and its mutational variants, using the empirical AncCR structure (PDB 2Q3Y, which contains an engineered C71S mutation to facilitate expression and crystallization) as a template. The receptor was optimized using the ‘Repair PDB’ function with sites Q39 and S76 fixed, as these side chains moved considerably in the absence of ligand (which is not modeled in FoldX). The ancestral state Cys71 was re-introduced into the AncCR sequence, and the structure was energy-minimized. GR substitutions were analyzed singly or in combination using the ‘Build Model’ function, and the change in protein stability estimated as the average difference in the free energies between the maximum likelihood and mutant AncCR structures (ΔΔG) for five runs. Several substitutions were excluded from the dataset because they generated errors (“segmentation fault” at sites Y27R, Q213K, and K246Q), bordered gaps in the electron density of the AncCR structure (A171V, K173R, and N175G), or contacted ligand (A36G).
Supporting Information
Zdroje
1. AnfinsenCB 1959 The molecular basis of evolution. New York Wiley
2. PaulingLZuckerkandlE 1963 Chemical paleogenetics: molecular "restoration studies" of extinct forms of life. Acta Chem Scand 17 S9 S16
3. OhnoS 1970 Evolution by gene duplication. BerlinNew York Springer-Verlag
4. KimuraM 1974 Gene pool of higher organisms as a product of evolution. Cold Spring Harb Symp Quant Biol 38 515 524
5. PerutzMF 1983 Species adaptation in a protein molecule. Mol Biol Evol 1 1 28
6. GoldingGBDeanAM 1998 The structural basis of molecular adaptation. Mol Biol Evol 15 355 369
7. ZuckerkandlEPaulingL 1965 Molecules as documents of evolutionary history. J Theor Biol 8 357 366
8. SerranoLDayAGFershtAR 1993 Step-wise mutation of barnase to binase. A procedure for engineering increased stability of proteins and an experimental analysis of the evolution of protein stability. J Mol Biol 233 305 312
9. CapraJASinghM 2008 Characterization and prediction of residues determining protein functional specificity. Bioinformatics 24 1473 1480
10. DonaldJEShakhnovichEI 2009 SDR: a database of predicted specificity-determining residues in proteins. Nucleic Acids Res 37 D191 4
11. GerltJABabbittPC 2009 Enzyme (re)design: lessons from natural evolution and computation. Curr Opin Chem Biol 13 10 18
12. HarmsMJThorntonJW 2010 Analyzing protein structure and function using ancestral gene reconstruction. Curr Opin Struct Biol 20 360 366
13. BloomJDSilbergJJWilkeCDrummondDAAdamiC 2005 Thermodynamic prediction of protein neutrality. P Natl Acad Sci USA 102 606 611
14. BershteinSSegalMBekermanRTokurikiNTawfikDS 2006 Robustness-epistasis link shapes the fitness landscape of a randomly drifting protein. Nature 444 929 932
15. OrtlundEABridghamJTRedinboMRThorntonJW 2007 Crystal structure of an ancient protein: Evolution by conformational epistasis. Science 317 1544 1548
16. BridghamJTOrtlundEAThorntonJW 2009 An epistatic ratchet constrains the direction of glucocorticoid receptor evolution. Nature 461 515 519
17. TomatisPEFabianeSMSimonaFCarloniPSuttonBJ 2008 Adaptive protein evolution grants organismal fitness by improving catalysis and flexibility. Proc Natl Acad Sci U S A 105 20605 20610
18. ThorntonJW 2004 Resurrecting ancient genes: experimental analysis of extinct molecules. Nat Rev Genet 5 366 375
19. BridghamJTCarrollSMThorntonJW 2006 Evolution of hormone-receptor complexity by molecular exploitation. Science 312 97 101
20. BeatoM 1989 Gene regulation by steroid hormones. Cell 56 335 344
21. BentleyPJ 1998 Comparative vertebrate endocrinology. New York Cambridge University Press
22. ArrizaJLWeinbergerCCerelliGGlaserTMHandelinBL 1987 Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237 268 275
23. CarrollSMBridghamJTThorntonJW 2008 Evolution of hormone signaling in elasmobranchs by exploitation of promiscuous receptors. Mol Biol Evol 25 2643 2652
24. ThorntonJW 2001 Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci U S A 98 5671 5676
25. GelsleichterJMusickJA 1999 Effects of insulin-like growth factor-I, corticosterone, and 3,3′, 5-tri-iodo-L-thyronine on glycosaminoglycan synthesis in vertebral cartilage of the clearnose skate, Raja eglanteria. J Exp Zool 284 549 556
26. NunezBSEvansANSimpsonMAWongWPIpYK 2006 Characterization of cDNAs encoding cholesterol side chain cleavage and 3beta-hydroxysteroid dehydrogenase in the freshwater stingray Potamotrygon motoro. Comp Biochem Physiol B Biochem Mol Biol 145 306 317
27. NunezSTrantJM 1999 Regulation of interrenal gland steroidogenesis in the Atlantic stingray (Dasyatis sabina). J Exp Zool 284 517 525
28. ManireCARasmussenLELMaruskaKPTricasTC 2007 Sex, seasonal, and stress-related variations in elasmobranch corticosterone concentrations. Comp Biochem Phys A 148 926 935
29. HazonNHendersonIW 1984 Secretory dynamics of 1 alpha-hydroxycorticosterone in the elasmobranch fish, Scyliorhinus canicula. J Endocrinol 103 205 211
30. ArmourKJO'TooleLBHazonN 1993 The effect of dietary protein restriction on the secretory dynamics of 1 alpha-hydroxycorticosterone and urea in the dogfish, Scyliorhinus canicula: a possible role for 1 alpha-hydroxycorticosterone in sodium retention. J Endocrinol 138 275 282
31. HillisDM 1998 Taxonomic sampling, phylogenetic accuracy, and investigator bias. Syst Biol 47 3 8
32. HeathTAZwicklDJKimJHillisDM 2008 Taxon sampling affects inferences of macroevolutionary processes from phylogenetic trees. Syst Biol 57 160 166
33. PollockDDZwicklDJMcGuireJAHillisDM 2002 Increased taxon sampling is advantageous for phylogenetic inference. Syst Biol 51 664 671
34. IdlerDRTruscottB 1966 1-alpha-hydroxycorticosterone from cartilaginous fish: a new adrenal steroid in blood. Journal of the Fisheries Research Board of Canada 23 615 619
35. TruscottBIdlerDR 1972 Corticosteroids in plasma of elasmobranchs. Comp Biochem Physiol A 42 41 50
36. Hanson-SmithVKolaczkowskiBThorntonJW 2010 Robustness of ancestral sequence reconstruction to phylogenetic uncertainty. Mol Biol Evol 27 1988 1999
37. BledsoeRKMontanaVGStanleyTBDelvesCJApolitoCJ 2002 Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 110 93 105
38. NagyLSchwabeJW 2004 Mechanism of the nuclear receptor molecular switch. Trends Biochem Sci 29 317 324
39. FengWRibeiroRCWagnerRLNguyenHAprilettiJW 1998 Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science 280 1747 1749
40. KauppiBJakobCFarnegardhMYangJAholaH 2003 The three-dimensional structures of antagonistic and agonistic forms of the glucocorticoid receptor ligand-binding domain: RU-486 induces a transconformation that leads to active antagonism. J Biol Chem 278 22748 22754
41. BledsoeRKMadaussKPHoltJAApolitoCJLambertMH 2005 A ligand-mediated hydrogen bond network required for the activation of the mineralocorticoid receptor. J Biol Chem 280 31283 31293
42. LiYSuinoKDaughertyJXuHE 2005 Structural and biochemical mechanisms for the specificity of hormone binding and coactivator assembly by mineralocorticoid receptor. Mol Cell 19 367 380
43. SchymkowitzJBorgJStricherFNysRRousseauF 2005 The FoldX web server: an online force field. Nucleic Acids Res 33 W382 8
44. MatthewsBW 1987 Genetic and structural analysis of the protein stability problem. Biochemistry 26 6885 6888
45. MatthewsBW 1993 Structural and genetic analysis of protein stability. Annu Rev Biochem 62 139 160
46. TokurikiNStricherFSchymkowitzJSerranoLTawfikDS 2007 The stability effects of protein mutations appear to be universally distributed. Journal of molecular biology 369 1318 1332
47. BloomJDArnoldFHWilkeC 2007 Breaking proteins with mutations: threads and thresholds in evolution. Mol Syst Biol 3 1 2
48. BloomJDArnoldF 2009 In the Light of Evolution III: Two Centuries of Darwin Sackler Colloquium: In the light of directed evolution: Pathways of adaptive protein evolution. Proc Natl Acad Sci U S A 106 9995 10000
49. BloomJDLabthavikulSTOteyCRArnoldFH 2006 Protein stability promotes evolvability. Proc Natl Acad Sci U S A 103 5869 5874
50. TokurikiNTawfikDS 2009 Chaperonin overexpression promotes genetic variation and enzyme evolution. Nature 459 668 673
51. BershteinSTawfikDS 2008 Advances in laboratory evolution of enzymes. Current opinion in chemical biology 12 151 158
52. MitrakiAFaneBHaase-PettingellCSturtevantJKingJ 1991 Global suppression of protein folding defects and inclusion body formation. Science 253 54 58
53. ShortleDLinB 1985 Genetic analysis of staphylococcal nuclease: identification of three intragenic "global" suppressors of nuclease-minus mutations. Genetics 110 539 555
54. BershteinSGoldinKTawfikDS 2008 Intense neutral drifts yield robust and evolvable consensus proteins. Journal of Molecular Biology 379 1029 1044
55. TokurikiNStricherFSerranoLTawfikDS 2008 How protein stability and new functions trade off. PLoS Comput Biol 4 e1000002 doi:10.1371/journal.pcbi.1000002
56. FieldSFMatzMV 2010 Retracing evolution of red fluorescence in GFP-like proteins from Faviina corals. Mol Biol Evol 27 225 233
57. DrummondDABloomJDAdamiCWilkeCOArnoldFH 2005 Why highly expressed proteins evolve slowly. Proc Natl Acad Sci U S A 102 14338 14343
58. BloomJDGongLIBaltimoreD 2010 Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 328 1272 1275
59. GarabedianMJYamamotoKR 1992 Genetic dissection of the signaling domain of a mammalian steroid receptor in yeast. Mol Biol Cell 3 1245 1257
60. RicketsonDHostickUFangLYamamotoKRDarimontBD 2007 A conformational switch in the ligand-binding domain regulates the dependence of the glucocorticoid receptor on Hsp90. J Mol Biol 368 729 741
61. BridghamJTBrownJERodrÌguez-MarÌACatchenJMThorntonJW 2008 Evolution of a new function by degenerative mutation in cephalochordate steroid receptors. PLoS Genet 4 e1000191 doi:10.1371/journal.pgen.1000191
62. SimonsSSJ 2006 How much is enough? Modulation of dose-response curve for steroid receptor-regulated gene expression by changing concentrations of transcription factor. Curr Top Med Chem 6 271 285
63. LarkinMABlackshieldsGBrownNPChennaRMcGettiganPA 2007 Clustal W and Clustal X version 2.0. Bioinformatics 23 2947 2948
64. AnisimovaMGascuelO 2006 Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst Biol 55 539 552
65. JonesDTTaylorWRThorntonJM 1992 The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8 275 282
66. YangZ 1997 PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci 13 555 556
67. PondSLFrostSDMuseSV 2005 HyPhy: hypothesis testing using phylogenies. Bioinformatics 21 676 679
68. OtwinowskiZMinorW 1997 Processing of X-ray diffraction data collected in oscillation mode. Methods in enzymology 276 307 326
69. EmsleyPCowtanK 2004 Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60 2126 2132
70. MurshudovGNVaginAADodsonEJ 1997 Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallographica Section D: Biological Crystallography 53 240 255
71. PottertonEBriggsPTurkenburgMDodsonE 2003 A graphical user interface to the CCP4 program suite. Acta Crystallogr D Biol Crystallogr 59 1131 1137
72. ClaudeJBSuhreKNotredameCClaverieJMAbergelC 2004 CaspR: a web server for automated molecular replacement using homology modelling. Nucleic Acids Res 32 W606 9
Štítky
Genetika Reprodukční medicína
Článek Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding SitesČlánek Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene SilencingČlánek Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse GutČlánek FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field SizeČlánek Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian SpermatogenesisČlánek Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese PopulationČlánek Differential Effects of and Risk Variants on Association with Diabetic ESRD in African AmericansČlánek Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 6
-
Všechny články tohoto čísla
- Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding Sites
- Statistical Inference on the Mechanisms of Genome Evolution
- Revisiting Heterochromatin in Embryonic Stem Cells
- A Two-Stage Meta-Analysis Identifies Several New Loci for Parkinson's Disease
- Identification of a Sudden Cardiac Death Susceptibility Locus at 2q24.2 through Genome-Wide Association in European Ancestry Individuals
- Genomic Prevalence of Heterochromatic H3K9me2 and Transcription Do Not Discriminate Pluripotent from Terminally Differentiated Cells
- Epistasis between Beneficial Mutations and the Phenotype-to-Fitness Map for a ssDNA Virus
- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Telomere DNA Deficiency Is Associated with Development of Human Embryonic Aneuploidy
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Unexpected Role for DNA Polymerase I As a Source of Genetic Variability
- Transportin-SR Is Required for Proper Splicing of Genes and Plant Immunity
- How Chromatin Is Remodelled during DNA Repair of UV-Induced DNA Damage in
- Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene Silencing
- Two Evolutionary Histories in the Genome of Rice: the Roles of Domestication Genes
- Natural Allelic Variation Defines a Role for : Trichome Cell Fate Determination
- Multiple Common Susceptibility Variants near BMP Pathway Loci , , and Explain Part of the Missing Heritability of Colorectal Cancer
- Pathogenic Mechanism of the FIG4 Mutation Responsible for Charcot-Marie-Tooth Disease CMT4J
- A Functional Variant in Promoter Modulates Its Expression and Confers Disease Risk for Systemic Lupus Erythematosus
- Drift and Genome Complexity Revisited
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
- Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse Gut
- Pathways of Distinction Analysis: A New Technique for Multi–SNP Analysis of GWAS Data
- Web-Based Genome-Wide Association Study Identifies Two Novel Loci and a Substantial Genetic Component for Parkinson's Disease
- Chk2 and p53 Are Haploinsufficient with Dependent and Independent Functions to Eliminate Cells after Telomere Loss
- Exome Sequencing Identifies Mutations in High Myopia
- Distinct Functional Constraints Partition Sequence Conservation in a -Regulatory Element
- CorE from Is a Copper-Dependent RNA Polymerase Sigma Factor
- A Single Sex Pheromone Receptor Determines Chemical Response Specificity of Sexual Behavior in the Silkmoth
- FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field Size
- Maps of Open Chromatin Guide the Functional Follow-Up of Genome-Wide Association Signals: Application to Hematological Traits
- Increased Susceptibility to Cortical Spreading Depression in the Mouse Model of Familial Hemiplegic Migraine Type 2
- Differential Gene Expression and Epiregulation of Alpha Zein Gene Copies in Maize Haplotypes
- Parallel Adaptive Divergence among Geographically Diverse Human Populations
- Genetic Analysis of Genome-Scale Recombination Rate Evolution in House Mice
- Mechanisms for the Evolution of a Derived Function in the Ancestral Glucocorticoid Receptor
- Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian Spermatogenesis
- Interferon Regulatory Factor 8 Regulates Pathways for Antigen Presentation in Myeloid Cells and during Tuberculosis
- High-Resolution Analysis of Parent-of-Origin Allelic Expression in the Arabidopsis Endosperm
- Specific SKN-1/Nrf Stress Responses to Perturbations in Translation Elongation and Proteasome Activity
- Graded Nodal/Activin Signaling Titrates Conversion of Quantitative Phospho-Smad2 Levels into Qualitative Embryonic Stem Cell Fate Decisions
- Genome-Wide Analysis Reveals PADI4 Cooperates with Elk-1 to Activate Expression in Breast Cancer Cells
- Trait Variation in Yeast Is Defined by Population History
- Meiosis-Specific Loading of the Centromere-Specific Histone CENH3 in
- A Genome-Wide Survey of Imprinted Genes in Rice Seeds Reveals Imprinting Primarily Occurs in the Endosperm
- Multiple Regulatory Mechanisms to Inhibit Untimely Initiation of DNA Replication Are Important for Stable Genome Maintenance
- SIRT1 Promotes N-Myc Oncogenesis through a Positive Feedback Loop Involving the Effects of MKP3 and ERK on N-Myc Protein Stability
- Bacteriophage Crosstalk: Coordination of Prophage Induction by Trans-Acting Antirepressors
- Role of the Single-Stranded DNA–Binding Protein SsbB in Pneumococcal Transformation: Maintenance of a Reservoir for Genetic Plasticity
- Genomic Convergence among ERRα, PROX1, and BMAL1 in the Control of Metabolic Clock Outputs
- Genome-Wide Association of Bipolar Disorder Suggests an Enrichment of Replicable Associations in Regions near Genes
- Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese Population
- DNA Ligase III Promotes Alternative Nonhomologous End-Joining during Chromosomal Translocation Formation
- Differential Effects of and Risk Variants on Association with Diabetic ESRD in African Americans
- Finished Genome of the Fungal Wheat Pathogen Reveals Dispensome Structure, Chromosome Plasticity, and Stealth Pathogenesis
- Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
- Extracellular Matrix Dynamics in Hepatocarcinogenesis: a Comparative Proteomics Study of Transgenic and Null Mouse Models
- Integrating 5-Hydroxymethylcytosine into the Epigenomic Landscape of Human Embryonic Stem Cells
- Vive La Différence: An Interview with Catherine Dulac
- Multiple Loci Are Associated with White Blood Cell Phenotypes
- Nuclear Accumulation of Stress Response mRNAs Contributes to the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- A New Mutation Affecting FRQ-Less Rhythms in the Circadian System of
- Cryptic Transcription Mediates Repression of Subtelomeric Metal Homeostasis Genes
- A New Isoform of the Histone Demethylase JMJD2A/KDM4A Is Required for Skeletal Muscle Differentiation
- Genetic Determinants of Lipid Traits in Diverse Populations from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- A Genome-Wide RNAi Screen for Factors Involved in Neuronal Specification in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Statistical Inference on the Mechanisms of Genome Evolution
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



