-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Genome-Wide RNAi Screen for Factors Involved in Neuronal Specification in
One of the central goals of developmental neurobiology is to describe and understand the multi-tiered molecular events that control the progression of a fertilized egg to a terminally differentiated neuron. In the nematode Caenorhabditis elegans, the progression from egg to terminally differentiated neuron has been visually traced by lineage analysis. For example, the two gustatory neurons ASEL and ASER, a bilaterally symmetric neuron pair that is functionally lateralized, are generated from a fertilized egg through an invariant sequence of 11 cellular cleavages that occur stereotypically along specific cleavage planes. Molecular events that occur along this developmental pathway are only superficially understood. We take here an unbiased, genome-wide approach to identify genes that may act at any stage to ensure the correct differentiation of ASEL. Screening a genome-wide RNAi library that knocks-down 18,179 genes (94% of the genome), we identified 245 genes that affect the development of the ASEL neuron, such that the neuron is either not generated, its fate is converted to that of another cell, or cells from other lineage branches now adopt ASEL fate. We analyze in detail two factors that we identify from this screen: (1) the proneural gene hlh-14, which we find to be bilaterally expressed in the ASEL/R lineages despite their asymmetric lineage origins and which we find is required to generate neurons from several lineage branches including the ASE neurons, and (2) the COMPASS histone methyltransferase complex, which we find to be a critical embryonic inducer of ASEL/R asymmetry, acting upstream of the previously identified miRNA lsy-6. Our study represents the first comprehensive, genome-wide analysis of a single neuronal cell fate decision. The results of this analysis provide a starting point for future studies that will eventually lead to a more complete understanding of how individual neuronal cell types are generated from a single-cell embryo.
Published in the journal: . PLoS Genet 7(6): e32767. doi:10.1371/journal.pgen.1002109
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002109Summary
One of the central goals of developmental neurobiology is to describe and understand the multi-tiered molecular events that control the progression of a fertilized egg to a terminally differentiated neuron. In the nematode Caenorhabditis elegans, the progression from egg to terminally differentiated neuron has been visually traced by lineage analysis. For example, the two gustatory neurons ASEL and ASER, a bilaterally symmetric neuron pair that is functionally lateralized, are generated from a fertilized egg through an invariant sequence of 11 cellular cleavages that occur stereotypically along specific cleavage planes. Molecular events that occur along this developmental pathway are only superficially understood. We take here an unbiased, genome-wide approach to identify genes that may act at any stage to ensure the correct differentiation of ASEL. Screening a genome-wide RNAi library that knocks-down 18,179 genes (94% of the genome), we identified 245 genes that affect the development of the ASEL neuron, such that the neuron is either not generated, its fate is converted to that of another cell, or cells from other lineage branches now adopt ASEL fate. We analyze in detail two factors that we identify from this screen: (1) the proneural gene hlh-14, which we find to be bilaterally expressed in the ASEL/R lineages despite their asymmetric lineage origins and which we find is required to generate neurons from several lineage branches including the ASE neurons, and (2) the COMPASS histone methyltransferase complex, which we find to be a critical embryonic inducer of ASEL/R asymmetry, acting upstream of the previously identified miRNA lsy-6. Our study represents the first comprehensive, genome-wide analysis of a single neuronal cell fate decision. The results of this analysis provide a starting point for future studies that will eventually lead to a more complete understanding of how individual neuronal cell types are generated from a single-cell embryo.
Introduction
When establishing the nematode C.elegans as a genetic model system, Sydney Brenner's stated goal was the identification of genes required to build a nervous system [1], [2]. Several decades of investigation, mainly using the classic forward screening methods established by Brenner [1], have indeed revealed genes that are required to generate a diversity of individual neuron types [3]. Yet our understanding of nervous system development still remains spotty. The advent of RNAi technology in C.elegans has opened an alternative path to classic forward analysis to study the genetic bases of various processes [4], [5]. However, this approach has not been undertaken in C.elegans to comprehensively study neuronal development and fate determination. In this paper we utilize the RNAi approach to screen, at a genome-wide level, for factors involved in all aspects of neuronal development.
We are using the ASE gustatory neuron class, composed of two bilaterally symmetric neurons, ASEL and ASER, as a paradigm to understand how neuronal fate is specified. Each neuron is produced by two distinct descendants of the embryonic blastomere AB (Figure 1; [6]) called ABa (generates ASEL) and ABp (generates ASER). There is a progressive restriction in the types of cells produced by the AB blastomere. The great-granddaughter of AB, ABalp, still produces cells from two different germ layers (meso - and ectoderm), while one of its daughters, ABalpp only produces neurons and glia. ABalpp's posterior daughter, ABalppp, then becomes committed to only produce neurons, one of them the left ASE neuron. Similar progressive restrictions of fate occur along the ABp lineage that generates the right ASE neuron. Little is known about genes that control these lineage restrictions. The RNAi screen we present here has uncovered a basic helix-loop-helix (bHLH) transcription factor, hlh-14, that we characterize in detail in this study. We find hlh-14 to be crucial for the specification of neurons that derive from several lineage branches, including the ASE neurons.
Fig. 1. The embryonic origins and specification of the ASE neurons. 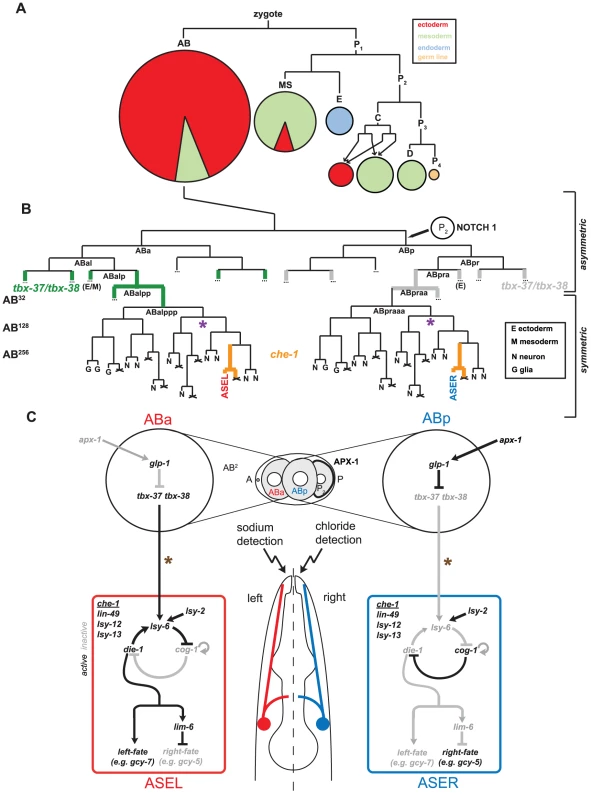
A: The early lineage of the C. elegans embryo leading to the formation of the five founder blastomeres (AB, MS, E, C and P3). The pie-charts indicate the relative number of cells and tissue types that are produced from each founder blastomere. The majority of neurons, including ASE, descend form the AB blastomere. B: Lineage of the ASEL and ASER neurons indicating they descend from asymmetric lineage origins which first diverge at the AB2-cell stage (adapted from [6]). The Notch signal that specifies the difference between ABa and ABp blastomeres is indicated and leads to the down-regulation of tbx-37/tbx-38 (expression indicated in green) in the ABp lineage. This early lineage difference is crucial for the specification of ASE left-right asymmetry [12]. The molecular link between the expression of tbx-37/tbx-38 and the specification of ASE asymmetry is unclear. From the ABalpp/ABpraa stage onwards the lineage produces exclusively ectodermal tissue. From the ABalpppp/ABpraaap stage onwards the lineage produces exclusively neurons, including ASE. Purple asterisks indicate the point at which the proneural gene hlh-14, described in this paper, acts to regulate neuronal differentiation in the ASE lineage. C: Summary of the previously described bi-stable feedback loop that regulates ASE left-right asymmetry. The bi-stable feedback loop consists of both transcription factors and micro-RNAs and controls the expression of downstream terminal differentiation genes. All factors in the loop and the downstream terminal factors are regulated in addition by the ASE terminal selector che-1 (expression indicated in B in orange). The molecular link between the early lineage difference and the bi-stable feedback loop is not known. Brown asterisks indicate that the COMPASS histone-modifying complex, also identified in this paper, acts earlier than che-1 and before the birth of the ASE neurons to regulate lsy-6 expression and control ASE asymmetry. Once a lineage is committed to only produce neurons (ABalpppp for ASEL and ABpraaap for ASER), fates have to be differentially segregated to generate distinct types of neurons. At this stage, as well as at earlier stages, a binary Wnt-signaling system operates to differentially segregate fates via the anterior-posterior differential distribution of the transcriptional regulators pop-1 and sys-1 [7]. These factors presumably interact with stage - and lineage-specific transcription factors to produce distinct cell types [7], [8].
One intriguing aspect of neuronal lineage specification in C. elegans is that even though many neurons in the nervous system come as bilaterally symmetric pairs, the lineage of the individual cells that make up a pair can be remarkably distinct [9]. In other words, cells that have limited lineage relation can produce remarkably similar neurons. The pair of ASE neurons falls into this category. As stated above, the left ASE neuron derives from ABa and the right neuron from ABp, which are asymmetrically localized in the early embryo. However, 4 cell divisions later, at the beginning of gastrulation, the ABa and ABp descendants that generate ASEL and ASER (called ABalppp and ABpraaa), begin to undergo left/right symmetric cleavage patterns to generate ASEL and ASER (Figure 1B). During gastrulation the descendants of these cells migrate into left/right bilaterally symmetric positions [10]. How this bilateralization process is regulated at the levels of cell fate and cell morphogenesis is not known.
The correct specification of the ASE neurons involves not only the imposition of ASE neuron identity that is shared by both the left and the right ASE neuron, but also involves the retention of the distinct lineage history of the two neurons. Even though morphologically largely bilaterally symmetric, both ASE neurons express a distinct set of chemosensory receptors and respond differently to distinct sensory cues [11]. The differential, left/right asymmetric expression of these receptors depends on a Notch-mediated signal, received in the early embryo by the lineage that produces ASEL (Figure 1B–1C; [12]). This Notch-mediated signal results in a differential and transient expression of a pair of paralogous T-box genes, tbx-37/38 [13], and then, several rounds of cell division later, in the expression of the miRNA lsy-6 in the ASEL, but not the ASER neuron [12]. How the information is transmitted from Notch, via tbx-37/38, to lsy-6 restriction is not known.
The lsy-6 miRNA has been uncovered through genetic screens for mutants in which the expression of ASEL - and ASER-specific chemoreceptors is disrupted [14]. These screens have uncovered an important principle of ASEL/R laterality through the identification of so-called Class I “2-ASEL” and Class II “2-ASER” mutants [15]. In Class I mutants, both neurons adopt ASEL fate (as assessed by expression of ASEL-specific gcy chemoreceptors), while losing ASER fate; in Class II mutants the opposite happens in that both neurons adopt ASER fate and lose ASEL fate [16]. The molecular basis for this bistability is a double-negative feedback loop of transcription factors and at least one miRNA, the above-mentioned lsy-6 miRNA (Figure 1C; [16], [17]). Through genetic epistasis analysis, we have found the earliest trigger of asymmetry to be the expression of the lsy-6 miRNA [16]. Yet, it is not known how its expression is restricted to ASEL. Our screen has uncovered a novel regulator of ASE asymmetry, the chromatin methlytransferase complex COMPASS. Through analysis of known and novel mutants in components of this complex we show that the COMPASS complex acts during embryogenesis, before the birth of the ASE neurons, to regulate lsy-6 expression. As such, it provides a potential molecular link between the early Notch-signal, tbx-37/38 expression and ASEL-specific lsy-6 expression in the maturing ASE neurons.
Taken together, studying the development of the ASE neurons provides a window to understanding a host of questions relevant to neuronal differentiation – how are early lineage decisions made, how are symmetry and asymmetry imposed, how are individual neuronal cell fate decisions controlled? Previous genetic mutant screens that used ASE differentiation markers as a read-out have begun to address these questions, but these genetic screens have not yet been saturated [18]. We sought to further expand the list of genes required to build an ASE neuron. To facilitate the recovery of lethal genes and to avoid the problem of mutational hotspots, we have used a genome-wide RNAi screen to reach this goal. To our knowledge, this is the first genome-wide RNAi analysis of a single neuron cell fate decision.
Results
Establishing conditions for a genome-wide neuronal RNAi screen
RNAi is known to work less efficiently in the nervous system than in other tissues [19]. Indeed, we find that dsRNA directed against gfp barely reduces gfp expression levels in transgenic animals that drive a gfp reporter in the ASEL neuron (Figure 2A). Several genetic backgrounds have been described in which animals are more sensitive to RNAi. We tested three of these backgrounds, nre-1 lin-15b [20], eri-1; lin-15b [21] and rrf-3 [22]. We find that in all of them ASE-specific gfp expression is now significantly reduced, with nre-1 lin-15b and eri-1; lin-15b mutants allowing more effective gfp knockdown than rrf-3 (Figure 2A).
Fig. 2. Testing hypersensitive strains and validating the screen with visible phenotypes. 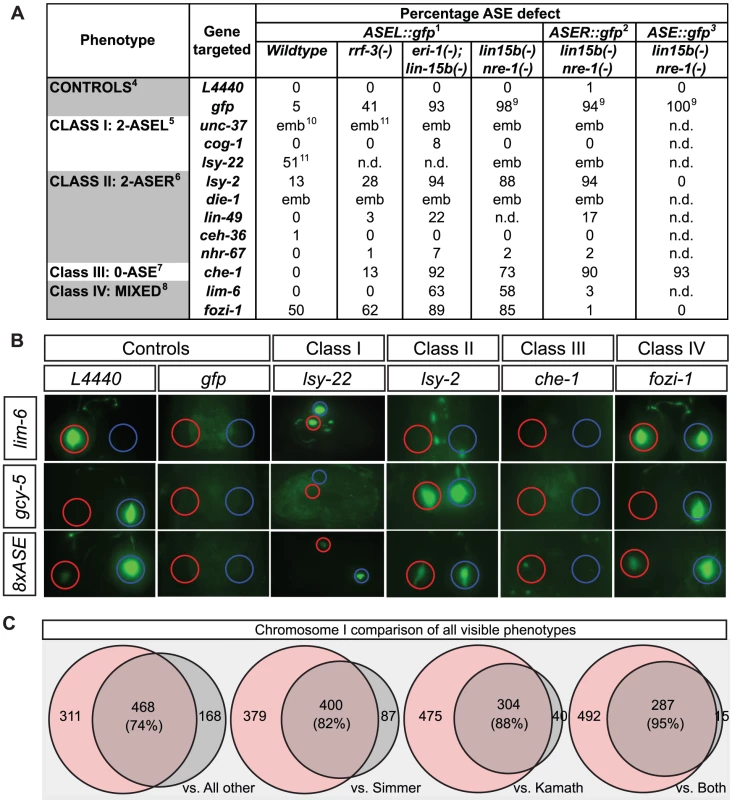
A: Table indicating the quantification of the laterality defects observed following RNAi knock-down of the indicated genes in wild-type (otIs114) and 3 different RNAi sensitized backgrounds. nre-1(-) lin-15b(-) and eri-1(-); lin-15b are the most sensitive strains for ASE. In all cases n>40. 1Scored with otIs114; 2Scored with otIs186; 3Scored with otIs299; 4ASE defect refers to loss or dimming of ASEL::gfp, ASER::gfp or ASE::gfp; 5ASE defect refers to ectopic expression of ASEL::gfp in ASER or loss or dimming of ASER::gfp or ASE::gfp; 6ASE defect refers to loss or dimming of ASEL::gfp or ASE::gfp and ectopic expression of ASER::gfp in ASEL; 7ASE defect refers to loss or strong dimming of ASEL::gfp, ASER::gfp or ASE::gfp; 8For fozi-1 ASE defect refers to ectopic expression of ASEL::gfp in ASER or loss of ASER::gfp or ASE::gfp. For lim-6 it refers to loss of ASEL::gfp expression or ectopic expression of ASER::gfp in ASEL; 9ASEL::gfp when still present appeared much dimmer than in any other strain; 106/6 escapers displayed ectopic expression of ASEL::gfp in ASER; 111/1 escaper displayed ectopic expression of ASEL::gfp in ASER; 12Taken from Flowers and Poole et al. 2010. B: Representative pictures of the ASE laterality that result from RNAi knock-down of the indicated known ASE factors in an nre-1(-) lin-15b(-) background. ASEL is labeled with lim-6prom::gfp and ASER is labeled with gcy-5prom::gfp. L4440 refers to feeding with an empty RNAi vector. Examples of the 4 main classes of ASE phenotype as described in [18]. Class I is 2-ASEL, class II is 2-ASER, class III is 0-ASE (loss of ASE cell fate) and class IV is mixed (in the case of fozi-1 ASEL markers are depressed in ASER but ASER markers are unaffected). C: Comparison of the visible phenotypes observed in our screen with previous screens for all genes on Chr I. Ovals represent the amount of bacterial clones that gave an RNAi phenotype in an experiment with results from our screen shaded in pink and previous published results shaded in grey. Areas that overlap represent clones (in %) for which in both experiments an RNAi phenotype was detected. Differences and overlap between an RNAi experiment done with the lin-15b nre-1 mutant strain and (in the following order): (1) all data previously reported for an RNAi experiment on Chr. I (whether as part of a genome wide screen, subscreen or a single experiment, exported from wormbase.org WS190); (2) the data obtained by screen done with the hypersensitive rrf-3 strain [22]; (3) the data obtained by screen done with the standard laboratory strain Bristol N2 [23]; (4) the data obtained by an overlap of phenotypes reported in both Simmer and Kamath screens. Previous mutant analysis from our lab has identified a number of genes required for the correct terminal differentiation of the ASE neuron (Figure 2A–2B; [18]). We tested whether RNAi recapitulates the mutant phenotype of 11 of these genes. We find that in a non-sensitized background, RNAi of only 3 of the genes partially recapitulates the mutant phenotype. In rrf-3 mutants, the effects of knockdown improved for only some genes, but in both nre-1 lin-15b and eri-1; lin-15b mutants, RNAi phenocopies the effect of the respective mutant in most cases examined (Figure 2A–2B). Since eri-; lin-15b mutants generally seem less healthy than nre-1 lin-15b mutants, we chose the latter strain for our genome-wide screen.
As the nre-1 lin-15b mutant background is relatively untested for its overall sensitivity to RNAi in general, we screened all RNAi clones on chromosome I not just for ASE neuronal phenotypes (as we did for all chromosomes), but also for obviously visible phenotypes, (sterility, embryonic or larval lethality, slow post-embryonic growth, or post-embryonic morphological defects; see Materials and Methods) and compared those to previously reported chromosome I screens of visible phenotypes, conducted either in a wildtype or an rrf-3 mutant background (Figure 2C; [22], [23]). We found a total of 779 clones with at least one visible phenotype detected, with an overlap of 82% and 88% for the rrf-3 and wildtype Bristol N2 screens respectively. When compared to the group of clones for which phenotypes were reported in both rrf-3 and wildtype N2 screens, our screen reveals 95% overlap. When we specifically compared the phenotypic categories reported by others and us, we found a striking trend of genes being shifted into more severe phenotypic categories in the nre-1 lin-15b background. For example, of the 303 clones reported previously to produce a Gro phenotype (slow growth), we found 57% to also be Gro in our screen but an additional 22% were found in more severe non-viable categories (Figure S1).
A genome wide RNAi screen identifies 245 genes that affect expression of an ASEL-specific neuronal fate marker
Using the nre-1 lin-15b sensitized background and the ASEL-marker lim-6::gfp (otIs114 transgene), we then went on to conduct a primary screen of all available clones. We were able to screen 15,395 RNAi clones for ASEL defects, equaling 81% of genes in the genome. In the primary screen, all clones were scored in duplicate, revealing that 748 RNAi clones affect development of the ASEL neuron, as evidenced by loss or ectopic expression of the lim-6::gfp marker. These 748 clones were re-screened 6 additional times and we set the arbitrary threshold of considering any clone that repeated at least 3/8 times as positive. These criteria identified a total of 245 genes that affect the development of the ASEL neuron (Figure 3). Examples of some of the knockdown phenotypes are shown in Figure 4 (showing either the RNAi phenotype or mutant alleles that confirm the RNAi phenotype). The vast majority of these genes (237/245) cause embryonic lethality, in addition to the ASEL phenotype. Of these 245 genes, 21 genes resulted in ectopic expression of the ASEL marker and 210 genes resulted in a loss of ASEL marker expression when knocked-down. 14 genes displayed a mixed phenotype, i.e. individual animals within a population of treated animals show either no phenotype, loss or ectopic expression of the ASEL marker (this category was not further pursued; Table S1).
Fig. 3. Overview of the screening strategy. 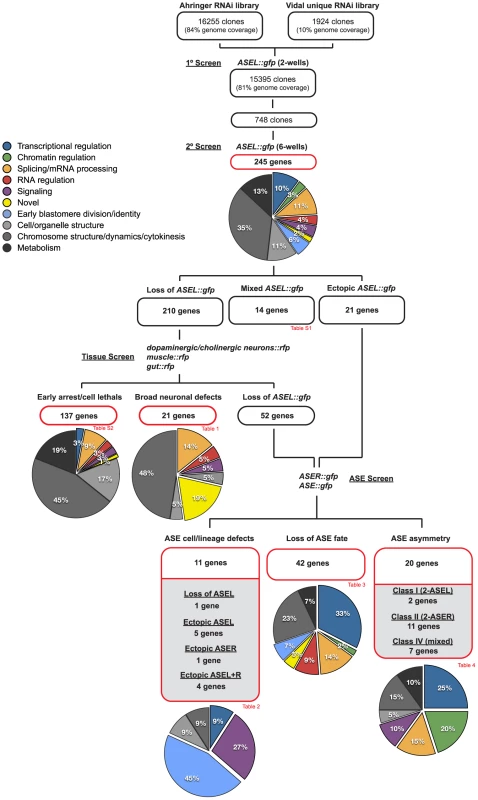
A flow chart illustrating the screening strategy. The number of RNAi clones screened is indicated and at each step the number of genes identified in each phenotypic category is depicted. The pie-charts illustrate the percentage of genes in each of several defined functional categories at each step (see color key). See the text for details on categorization criteria and see the indicated tables (Table 1, Table 2, Table 3, Table 4, and Tables S1 and S2) for the list of genes in each category, a more detailed phenotypic description and their functional categorization. Fig. 4. Examples of phenotypic classes uncovered by the screen. 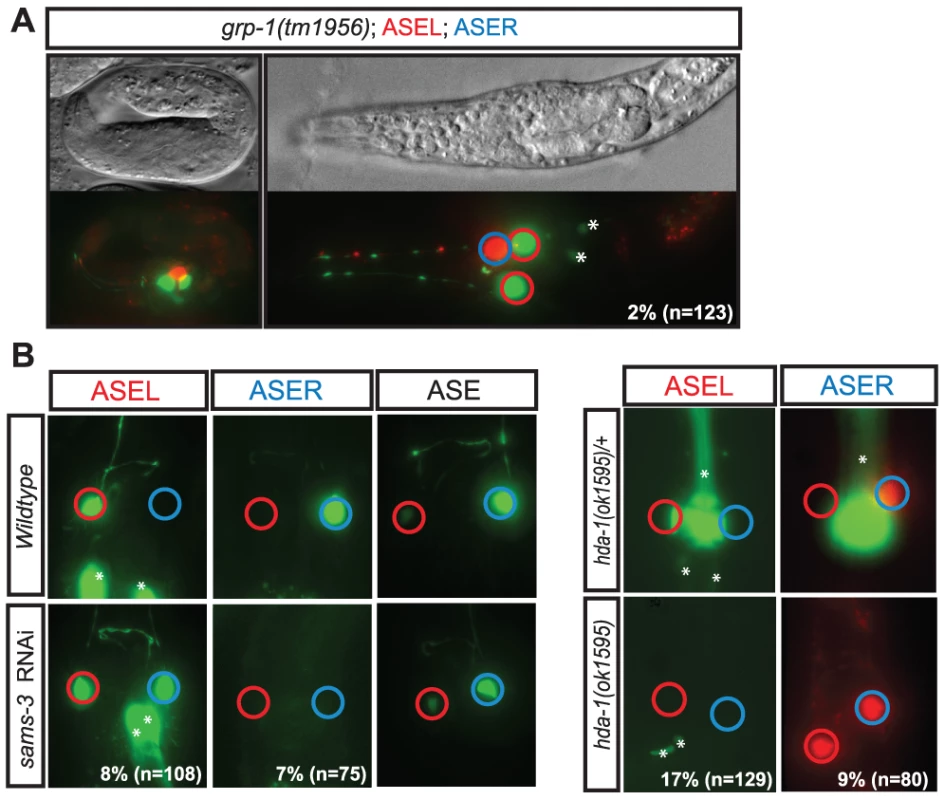
A: ASE cell/lineage defect—grp-1(tm1956) mutants, a GTP exchange factor, display ASE lineage defects in embryonic and larval stages. In the figure shown the animal has a total of 3 ASEs. One that displays ASER fate (blue circle), and instead of one that displays ASEL fate as seen in wild-type animals, grp-1(-) animals display two ASEL fated cells (red circles), suggesting an ectopic ASEL lineage has formed in these mutants. B: ASE asymmetry defects—Panel on the left shows RNAi knockdown of gene S-adenosylmethionine synthetase (sams-3) results in a class I “2-ASEL” phenotype in which ASER (blue circle) looses its sub-type specific fate and transforms to ASEL (red circle). Overall ASE fate is unaffected. We observed a similar phenotype with similar penetrance for sams-4 (data not shown). Panel on the right shows the phenotype of histone deacetylase hda-1(ok1595) mutants which display a class II “2-ASER” defect. ASEL fate is reported by lim-6prom::gfp expression, ASER fate is reported by gcy-5prom::gfp or gcy-5prom::mCherry and ASE fate is reported by 8XASE::gfp. *Indicates expression unrelated to the ASE neurons. Early arrest
To determine whether loss of ASEL marker expression was due to (a) a specific loss of ASEL, (b) a more broad neuronal differentiation defect or (c) general early embryonic arrest, we re-screened the 210 genes that resulted in a loss of ASEL with fluorescently labeled differentiation markers for ectoderm, mesoderm or endoderm. We used two neuronal markers that label 6 different classes (each class composed of a pair of symmetric neurons) of dopaminergic and cholinergic neurons with limited lineage relation (dat-1::rfp; ttx-3::rfp, both contained on one array), a collagen marker for hypodermis (dpy-7::yfp), a myosin muscle marker (myo-3::gfp) and a gut-specific GATA factor marker (elt-2::gfp). We considered all those genes that by RNAi led to a loss of all tissue markers tested as “early arrest” genes (87 genes; Table S2). Additionally, we included in this list those genes that have previously been characterized as arresting before the 200-cell stage (11 genes; [24]) and those genes whose knock-down resulted in a loss of all markers tested except the dopaminergic neuron marker, as we presume this to result from non-reproducibility of RNAi (8 genes; we note that as expected, knock-down of these genes also led to a loss of expression of an ASER marker and a bilateral ASE marker). Finally, we also considered as early arrest those genes whose knock-down led to a loss of neurons and muscle but not gut, since the latter marker is expressed early in development before terminal neuronal differentiation (31 genes). In this manner we classified a total of 137 of those genes that led to a loss of lim-6::gfp expression as “early arrest” genes (Figure 3 and Table S2). More than 80% of genes in this category classify as genes involved in metabolism, cell/organelle structure and basic cellular processes like cell division.
Broad neuronal defects
Interestingly, we were also able to identify from this tissue screen 21 genes that when knocked-down by RNAi led to a broad loss of neurons (Figure 3 and Table 1). In all cases, in addition to a loss of the ASEL marker, the dopaminergic/cholinergic markers were absent, yet either muscle, or muscle and gut, were unaffected. We note that in all cases the expression of the hypodermal marker was absent, which is consistent with a loss of all ectodermal tissue. Genes in this category include two mex genes, known to be involved in determining the identity of the AB blastomere (which produces mainly ectodermal tissue), with their loss leading to excess P-cell descendants such as muscle, being generated ectopically from the AB-lineage (hence muscle excess for mex [25]; Table 1). We suspect that other genes in this category are also involved in establishing early blastomere asymmetry and identity. We examined these and several other genes in this category in more detail by performing RNAi in the background of a pan-neuronally expressed gene (rab-3::yfp). While both mex-3 and mex-5 led to an almost complete loss of neurons, no other gene examined generated such a complete loss of neurons, suggesting they generate broad neuronal but not pan-neuronal differentiation defects (Figure S2).
Tab. 1. Genes that elicit broad neuronal defects when knocked-down by RNAi. 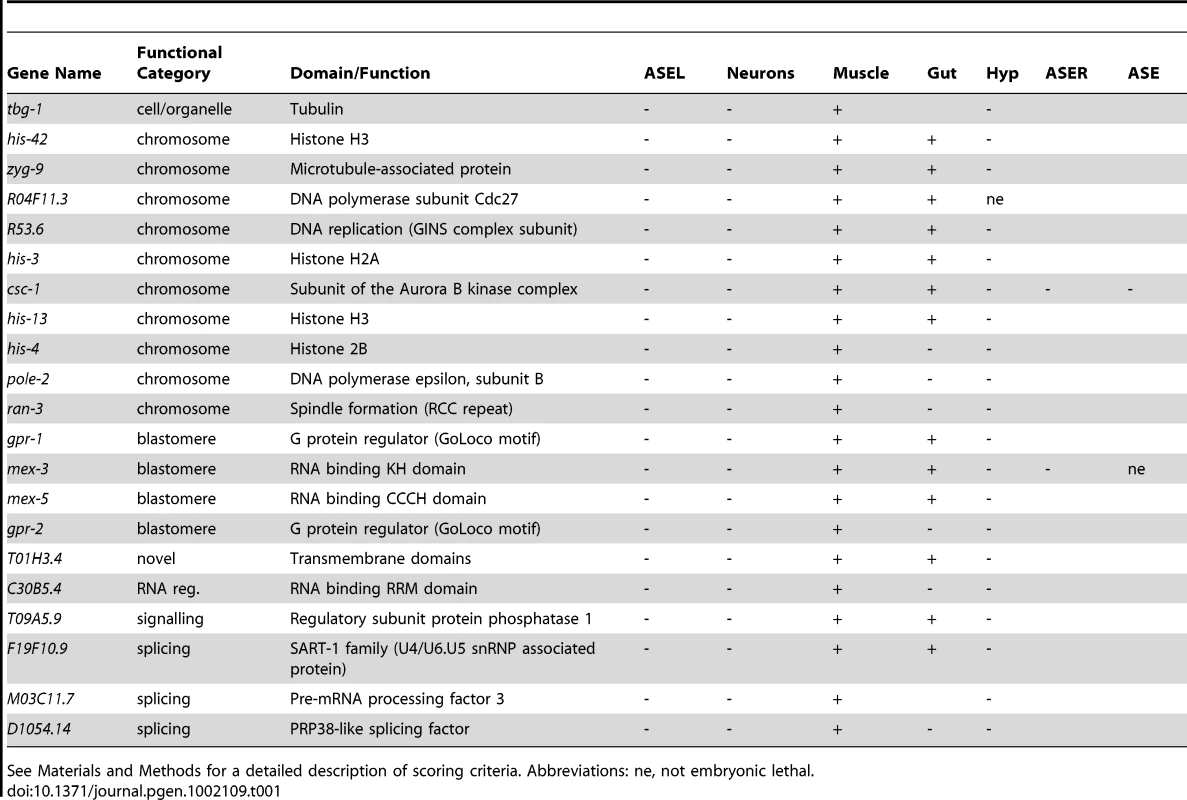
See Materials and Methods for a detailed description of scoring criteria. Abbreviations: ne, not embryonic lethal. Neuron-type specific defects
We then re-screened the remaining genes, that show specific ASEL defects (52 genes with a loss of ASEL marker and 21 genes that showed ectopic ASEL marker expression), with an ASER specific marker and an ASE bilateral marker in order to distinguish between the following three RNAi knock-down phenotypic categories: (1) genes that cause ASE cell/lineage defects (loss or ectopic ASEL and/or ASER cells – but not a complete loss of ASE cell fate); (2) genes that cause bilateral loss of ASE cell fate; (3) genes that cause ASEL versus ASER asymmetry defects. Firstly, we classified those genes that showed ectopic expression of the ASEL marker in additional cells, with the ASER marker being unaffected (or vice versa) as having an ectopic ASEL (or ASER) lineage and we classified those genes that showed a loss of the ASEL marker with the ASER being unaffected (or vice versa) as having lost an ASEL (or ASER) lineage. These genes may alter the fate of entire lineage branches or they may cause individual cells to lose/acquire an ASE fate – importantly ASE fate in general is not lost. Altogether we identified 11 genes with ASE cell/lineage defects (Figure 3 and Table 2). Nearly half of the genes that fall into this class are genes that are previously described as affecting early blastomere identities, including, for example, par genes [26].
Tab. 2. Genes that elicit ASE cell/lineage defects when knocked-down by RNAi. 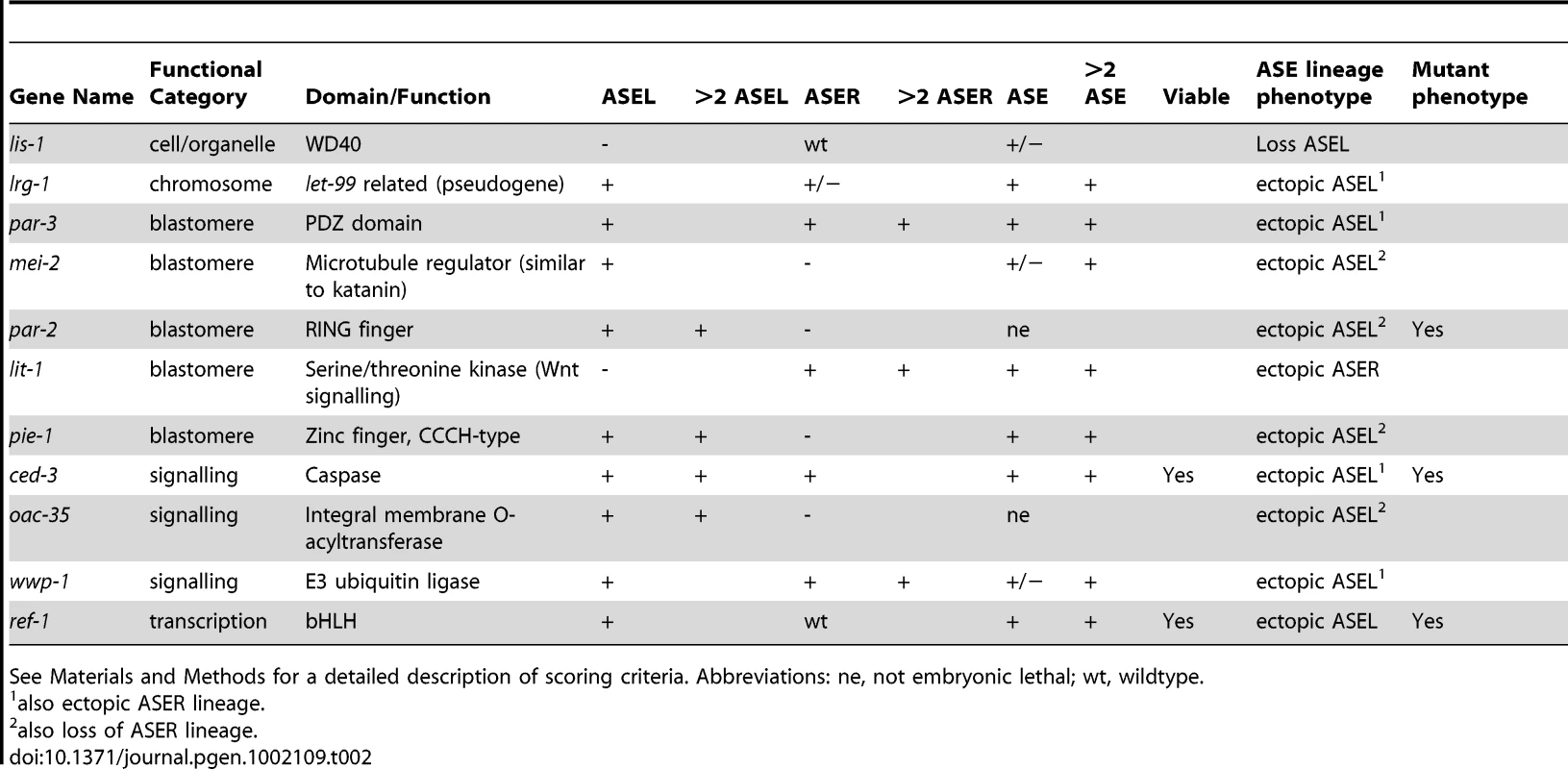
See Materials and Methods for a detailed description of scoring criteria. Abbreviations: ne, not embryonic lethal; wt, wildtype. Secondly, we classified those genes that result in a loss of both the ASEL and the ASER cell fate markers, as loss of ASE fate (as expected, knock-down of the majority of these genes also resulted in no expression of a bilateral ASE marker). In addition, one gene, although seemingly wildtype for the ASER marker did not express a bilateral ASE marker and was added to this group. A total of 42 genes fell into this group (Figure 3 and Table 3). Note that, as stated above, dopaminergic and cholinergic neuron markers are unaffected by these genes. A majority of genes in this list have a role in gene regulation, either on the DNA or RNA level. The list includes a known regulator of ASE specification, the ASE terminal selector che-1 [15], [27], and the bHLH gene hlh-14 that we describe in more detail later in this paper.
Tab. 3. Genes that elicit a loss of ASE fate when knocked-down by RNAi. 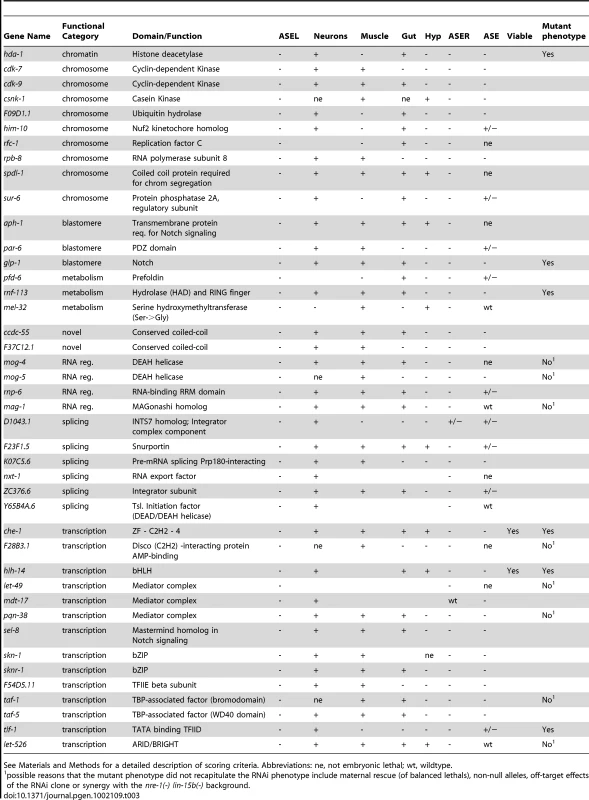
See Materials and Methods for a detailed description of scoring criteria. Abbreviations: ne, not embryonic lethal; wt, wildtype. L/R asymmetry defects
Finally, we identified 20 genes, knock-down of which caused an ASE asymmetry defect (Figure 3 and Table 4). We classified genes as having an asymmetry defect when the bilateral ASE marker is unaffected but ectopic expression of either the ASEL or ASER marker is observed. In addition, 4 genes for which the result from the bilateral marker was ambiguous, but which clearly showed loss of the ASEL marker and expression of the ASER marker in one extra cell or vice versa were added to this list. Ten genes showed ectopic expression of the ASEL marker, in 2/10 the ASER marker was lost, indicating a Class I (2-ASEL) phenotype, while in 7/10 the ASER is unaffected indicating a Class IV (mixed) phenotype (see Figure 2B). 11 genes showed a Class II (2-ASER) phenotype. This list includes a number of previously described regulators of ASE laterality, such as die-1, lsy-22, lsy-2, fozi-1 and lim-6 and, in addition, some novel genes that we describe later on in this paper.
Tab. 4. Genes that elicit ASE asymmetry defects when knocked-down by RNAi. 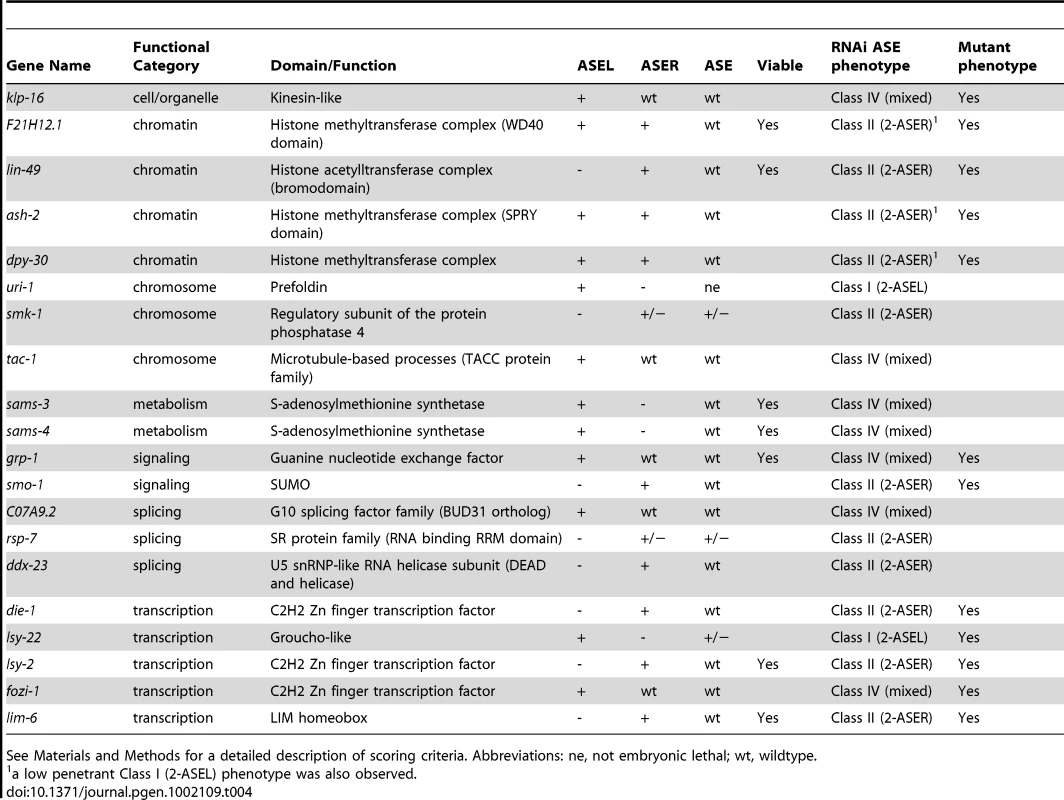
See Materials and Methods for a detailed description of scoring criteria. Abbreviations: ne, not embryonic lethal; wt, wildtype. Taken together, our RNAi screen uncovered a rich set of genes that affect early development, broad neuronal development, lineage specification and terminal ASE differentiation. Functionally, genes fall into a variety of different categories, from signaling to gene regulatory factors. In the second and third parts of this paper we undertake a more in-depth analysis of two sets of genes retrieved from our screen: (1) One specific transcription factor, the bHLH transcription factor hlh-14; (2) several genes that code for members of a specific histone-methylation complex, called COMPASS.
The Achaete-Scute-like bHLH gene hlh-14 controls neuronal fate induction
The RNAi screen identified the hlh-14 gene as affecting overall differentiation of ASEL/R. hlh-14 RNAi animals are viable and display a loss of asymmetrically expressed and bilaterally expressed genes in ASE to varying extents (Table 5). We first set out to corroborate the RNAi phenotype using available mutant alleles of hlh-14. We found that two different alleles of hlh-14 recapitulated the ASE differentiation phenotype observed upon RNAi and were larval lethal as previously reported [28]. Asymmetrically expressed genes are lost in hlh-14 mutants, as is the expression of che-1, a terminal selector of ASE neuron differentiation (Figure 5A and Table 5). The defects are almost completely penetrant in hlh-14(tm295), a deletion allele that removes the entire bHLH domain and is likely a functional null.
Fig. 5. The achaete-scute homolog hlh-14 is required to specify neurons in the ASE lineage. 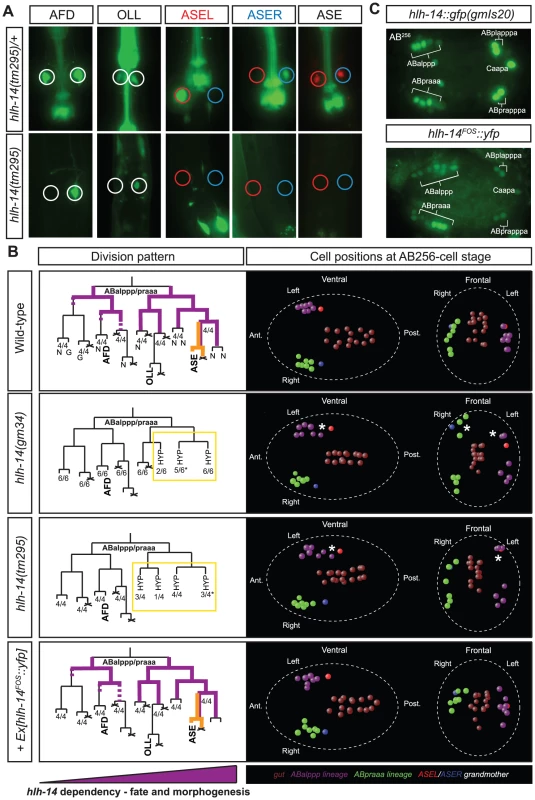
A: hlh-14 is required for the terminal specification of several neuron pairs from the ABalppp/praaa lineage branch. Images of hlh-14(tm295) mutants indicating a loss of ASE (ASEL: lim-6prom::gfp, ASER: gcy-5prom::gfp and ASEL/ASER: che-1prom::rfp) OLL (ser-2prom2:gfp) and AFD (gcy-8::gfp) cell fates. Heterozygous balanced siblings are unaffected (presence of balancer indicated by GFP in the pharynx). B: Lineage, 3D-morphogenesis and hlh-14 expression analysis in the ABalppp/praaa lineage of wild-type (containing the ntIs1 transgene), hlh-14(gm34); otIs114, hlh-14(tm295); ntIs1 and hlh-14(tm295); otEx4507, Ex[hlh-14FOS::yfp]; ntIs1 embryos. The left panel shows the division pattern and expression (purple lines) of hlh-14 in the ABalppp/praaa lineage as determined by 4D-lineage analysis. hlh-14 expression was assessed in both gmIs20 and hlh-14(tm295); Ex[hlh-14FOS::yfp] animals. In wild-type embryos the cells divided as expected. In hlh-14(gm34) mutants the 3 most posterior great-granddaughters of the ABalppp/praaa blastomere fail to divide. In hlh-14(tm295) mutants these defects extend to the 4 most posterior granddaughter. The number of lineages that divided as indicated by the lineage and the total number of individual left or right lineages followed to terminal division (and assessed for 3D cell positions) are shown at the bottom of each lineage diagram. The asterisks indicate that on one occasion the lineages still divided but aberrantly, much later than normal. N indicates neurons and G indicates glia. The right panel shows representative examples of the 3D-positions of cells from the ABalppp/ABpraaa lineage at the AB256-cell stage as assessed by 4D-lineage analysis. Both ventral and frontal views are presented. The posterior descendants of the ABalppp/praaa blastomere are dorsally and posteriorly mispositioned in hlh-14 mutants (asterisks), a position normally occupied by hypodermal cells. The fosmid array rescues the division and morphogenesis defects. The gut lineage is indicated for positional information. Cell coloring, except C-lineage, after [10]. The purple triangle under the panel indicates that hlh-14 expression is lost more rapidly in the anterior of the lineage and this correlates with more penetrant defects in posterior parts of lineage in hlh-14 mutants. C: Images of hlh-14 expression in embryos at the pre-morphogenesis (AB256-cell) stage. Top panel shows the expression of hlh-14 from an integrated translational hlh-14::gfp reporter [28]. Bottom panel shows the expression of hlh-14 from a rescuing YFP-tagged hlh-14 fosmid array. Expression in the ASE, PVQ/HSN/PHB, and DVC/PVR lineages is indicated. The full expression pattern of hlh-14 in the ABalppp/praaa lineage is indicated by purple lines in the lineage diagram in panel C and full expression in the C-lineage is indicated in Figure 6. Tab. 5. hlh-14 is required for the ASE neurons and several other neurons in the same lineage branch. 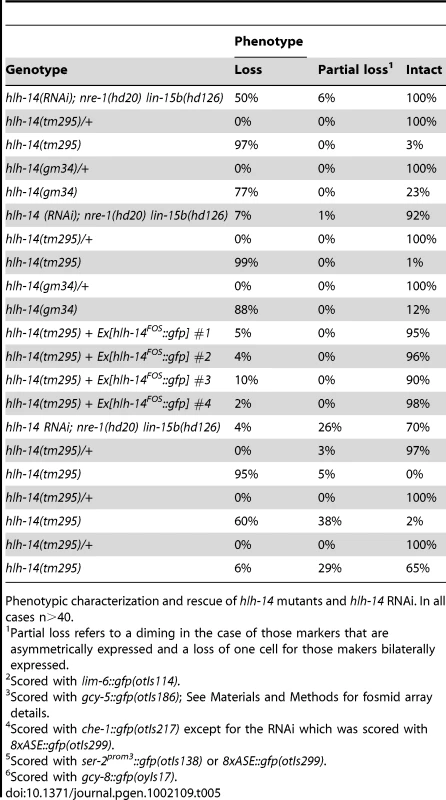
Phenotypic characterization and rescue of hlh-14 mutants and hlh-14 RNAi. In all cases n>40. We find that other neurons that are lineally related to ASE are also affected in hlh-14 mutants, as assessed by loss of terminal fate marker expression of the OLL and AFD neuron pairs. These broader neuronal defects were not apparent during initial RNAi screening since the dopaminergic and cholinergic neuron marker was unaffected and hlh-14(RNAi) animals are viable, unlike hlh-14 mutants which are larval lethal. Intriguingly, the effect on fate appears to be more pronounced the more posterior a neuron is located in the lineage (Figure 5A and Table 5).
We analyzed the hlh-14 mutant phenotype in more detail using a classic lineaging approach, in which we monitored cell position, cleavage and morphology, using 4D microscopy [10]. We find that from the AB64-cell stage onwards, during gastrulation, the ABalpppp/ABpraaap neuroblasts and their descendants migrate dorsally, towards a location usually populated by hypodermal cells [6], rather than remaining more ventrally positioned as they do in wild-type animals. By the AB256-cell stage these descendants are both dorsally and posteriorly mislocalised (Figure 5B). At this stage, the time at which hypodermal cells normally cease division and differentiate, these descendants fail to divide further, unlike wild-type cells, which undergo one or two more rounds of division before differentiating into neurons. Furthermore, in certain cases where we were able to follow the cells for a sufficient period of time, we observed that they acquired a hypodermal-like cell morphology. We observe more frequent hypodermal transformations and mis-positioning defects in more posterior descendants of the ABalpppp/ABpraaap neuroblasts (Figure 5B). Additionally, we examined the hypodermal cell fate marker dpy-7 in hlh-14(tm295) mutants at the comma stage (Figure S3). Although we were able to easily observe additional cells in the posterior of the embryo, consistent with previously reported cell fate transformations [28] and novel tranformations that we describe below, we were unable to unambiguously identify extra hypodermal cells in the anterior of the embryo due to the dim expression of dpy-7 in certain anterior hypodermal cells (we note that several anterior hypodermal cells, in addition to the hypodermal seam cells, do not appear to express dpy-7 at this stage). Nevertheless we were able to observe mispositioned hypodermal cells in the anterior of the hlh-14(tm295) mutants, where the transformed cells of the ABalpppp/ABpraaap reside (Figure S3). The division and dorsal mispositioning defects are more severe in the null allele of hlh-14 (Figure 5B). Together, these data are consistent with a neuron-to-hypodermal transformation of these cells and suggest that, in addition to cell fate, hlh-14 regulates aspects of cell migration.
To examine hlh-14 gene expression, we generated a YFP-tagged hlh-14 transgene through fosmid recombineering (Figure 5C; [29]). This fosmid reporter fully rescues the lethality, division, morphogenesis and ASE cell fate defects of hlh-14 mutants (Figure 5B and Table 5). We find, using 4D-lineage analysis, that this reporter and a previously published hlh-14 translational reporter [28] show similar expression in the ASE lineage. hlh-14 is first expressed at the AB64-cell stage (Figure 5B). This is just shortly after the point at which the ASE-lineages, which descend from distinct asymmetric blastomeres, become symmetric in terms of division pattern and daughter cell fates; the descendants will form exclusively neurons and glia, producing the left-right bilateral homologs of eleven pairs of neurons and two pairs of glia (Figure 1). It is also the time we begin to see morphogenesis defects and together is consistent with a role of hlh-14 being a bilateral binary switch at, or shortly after the ABalpppp/ABpraaap stage, to determine neuronal versus hypodermal fate.
hlh-14 expression appears to persist longer in posterior parts of the ABalppp/praaa lineage than in anterior parts, thereby mirroring the differential phenotypic severity of hlh-14 removal (Figure 5A–5B). In the ASE branch, located at the posterior part of the lineage, hlh-14 expression is maintained throughout the lineage until shortly before the terminal division of the ASE neurons, suggesting not only early neuronal versus hypodermal decision roles for hlh-14 but perhaps also a later role in differentiation. Consistent with such a notion we find that on examining ASEL, partial removal of hlh-14, through RNAi in animals that express two distinct markers for ASEL fate simultaneously, sometimes leads to loss of one but not the other marker (13/28 embryos in which ASEL was still present, as assessed by a lsy-6::yfp reporter, lost ceh-36::mCherry expression) suggesting the ASE cell is still produced but fails to differentiate correctly.
Symmetric and asymmetric expression of hlh-14 in other neuronal lineages
In addition to its expression in the ASE lineage, we observe by 4D-lineage analysis that our hlh-14 fosmid reporter is expressed in several other neuronal lineages. In some instances this expression is bilaterally symmetric, such as in the neuroblasts that give rise to the L/R bilaterally symmetric PLM/ALN neuronal pairs, PVQ/HSN/PHB neuron pairs and the ALM/BDU neuron pairs (Figure S4). Surprisingly, in other instances this expression is L/R asymmetric, such as in neuroblasts of the C lineage (Figure 6). We observe asymmetric expression in two asymmetrically generated neuroblasts, the Caapa neuroblast that gives rise to the DVC neuron and a cell death, and the Caappv cell, which differentiates into the neuron PVR. The bilateral homologs of these hlh-14-expressing neuroblasts are non-neuronal hypodermal cells (Figure 6C). In both hlh-14(gm34) and hlh-14(tm295) mutants we observe that the Caapa neuroblast divides precociously, indicative of a hypodermal transformation. In the position of Caapaa, we observe an ectopic cell expressing the dpy-7 hypodermal cell marker, corroborating that PVR has been transformed into hyp11, the normal fate of Caappv's bilateral homolog (Figure 6C). These division defects are rescued by the hlh-14FOS::yfp transgene (Figure 6C). Several other lineages in which we observe hlh-14 expression also give rise mainly to hypodermal cells, the P-cells in the case of the PLM/ALN and PVQ/HSN/PHB lineages (Figure S4B) and the V-cells in the case of the ALM/BDU lineage (Figure S4C). In the PVQ/HSN/PHB lineage we also observe precocious division of the neuroblast that gives rise to these neurons and ectopic dpy-7 expressing cells in the tail in the absence of hlh-14 (Figure S3; data not shown). We suggest that hlh-14 acts as a neural/hypodermal switch in the neuroblasts of all these lineages.
Fig. 6. Asymmetric expression and function of hlh-14 in the C-lineage. 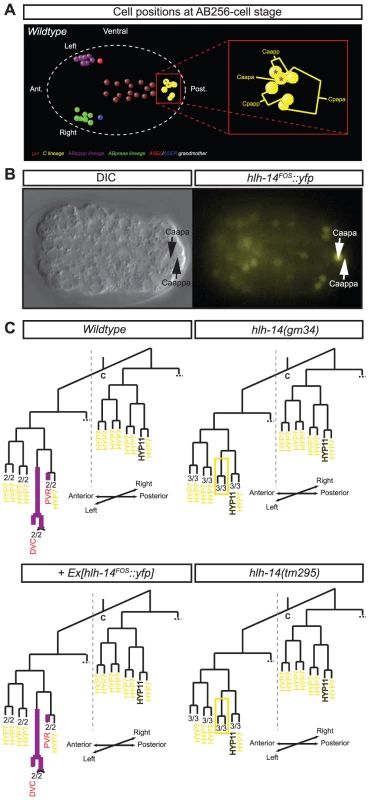
A: Ventral view of the 3D-positions of certain cells of the C-lineage of a representative wild-type embryo at the AB256-cell stage (shortly after the division of the ASE grandmothers), as assessed by 4D-lineage analysis. The left-sided asymmetric neuroblast Caapa and the neuron PVR express hlh-14 (purple asterisks). Cells of the gut and the ASE lineages are shown for positional information. Cell coloring, except C-lineage, after [10]. B: Ventral view in both DIC and fluorescence of a representative lineaged hlh-14(tm295); Ex[hlh-14FOS::yfp]; ntIs1 embryo at the AB256-cell stage. C: Panel shows the expression (purple lines) of hlh-14 and the division pattern of part of the C-lineage as determined by 4D-lineage analysis of wild-type (containing the ntIs1 transgene, hlh-14(gm34); otIs114, hlh-14(tm295); ntIs1 and hlh-14(tm295); otEx4507, Ex[hlh-14FOS::yfp]; ntIs1 embryos. hlh-14 expression was assessed in both gmIs20 and hlh-14(tm295); Ex[hlh-14FOS::yfp] animals. The Caapa blastomere divides early in hlh-14 mutants, at a time when closely related cells produce hypodermal cells. This defect is rescued by the hlh-14FOS::yfp transgene. The extra hyp11 cell that can be observed expressing dpy-7 (Figure S3), is indicated. The number of lineages that divided as indicated and the total number of individual left or right lineages followed to terminal division are shown at the bottom of each lineage diagram. Red cell names indicate neurons/glia and yellow cell names indicate hypodermis. Intriguingly, we also observe expression in the bilaterally symmetric neuroblasts that produce a pair of pharyngeal interneurons known as I1L/I1R (Figure S5). The lineages that generate these neurons are unusual for two reasons: (1) they derive from L/R asymmetric origins and (2) they are multi-potent, giving rise to mesodermal, epithelial and neuronal cells of the pharynx. We find that in hlh-14(tm295) mutants the division of this neuroblast is again premature, more closely matching the division time of its sister, which does not express hlh-14 and normally gives rise to two myoepithelial cells, a muscle and a marginal cell (Figure S5B). While it is currently unclear what fate these cells acquire in the absence of hlh-14, it suggests that in addition to regulating neural/hypodermal fate decisions, hlh-14 regulates more general neural/non-neural cell fate decisions and that “non-clonally” derived neurons use the same basic neural specification mechanisms as clonally derived neurons.
Finally, we observe later expression of hlh-14 in currently unidentified lineages, at a time in which all terminally differentiated cells present at hatching are already formed. This is consistent with hlh-14 acting during terminal cell fate decisions in other lineages. Taken together, hlh-14 acts as a proneural gene to regulate early neuroblast cell fate, morphogenesis and certain aspects of terminal differentiation in multiple L/R symmetric and L/R asymmetric neuronal lineages. In the ASE lineage, its proneural activity is graded in an anterior-to-posterior manner.
The COMPASS histone methyltransferase complex regulates ASE laterality
Our RNAi screen revealed ASE laterality defects upon knockdown of two different components of the histone methyltransferase complex COMPASS [30], ash-2 (a PHD/SPRY domain protein) and dpy-30. A third locus, F21H12.1, also generated ASE laterality defects and we find that this locus corresponds to the worm ortholog of an additional member of the COMPASS complex, the WD40 protein RbBP5, here-on referred to as rbbp-5 (Table 4 and Table S3; Figure 7). We confirmed the ash-2(RNAi) and dpy-30(RNAi) ASE laterality defects observed during our RNAi screen using mutant alleles of the respective genes (Figure 7 and Table S3). In regard to rbbp-5, we noted that it resides in the mapping interval of the previously identified but uncloned lsy-15 gene, an ASE laterality mutant retrieved from a large-scale mutagenesis screen [18]. As previously described, lsy-15(ot86) mutants show a conversion of ASEL to ASER fate [18]; this phenotype, like those of the other COMPASS members, is maternally rescued (see below). Additionally, lsy-15(ot86) mutants, like other COMPASS complex mutants, display a partially penetrant dumpy phenotype, a low brood size and an egg-laying defect (data not shown). The latter is likely the result of a uterine attachment defect (Cog phenotype) that can be observed in lsy-15(ot86) mutants along with ectopic expression of the ceh-2 vulval cell marker (data not shown). Through whole genome sequencing, rescue, and analysis of deletion alleles provided by the C. elegans knockout consortia, we find that lsy-15 indeed corresponds to rbbp-5 (Figure 8, Table S3 and see below).
Fig. 7. The evolutionary conserved methyltransferase COMPASS complex is required for ASE asymmetric cell fate specification. 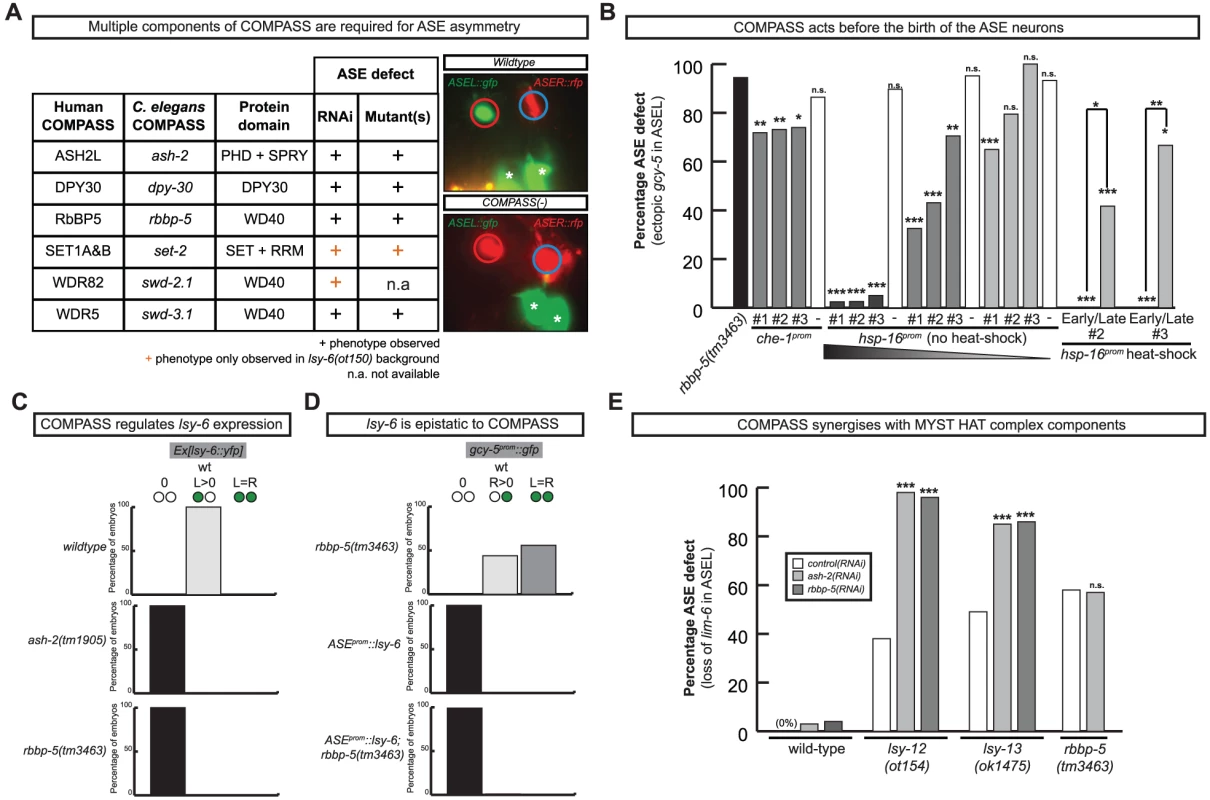
A: Table and representative images of COMPASS complex indicating the ASE phenotype in multiple components of the COMPASS complex (for detailed numbers see Figure S3). The image shown on the right is of the lsy-15(tm3463) mutant; all alleles of several members of the COMPASS complex show an identical phenotype. The strain contains a reporter that marks ASEL (lim-6prom::gfp) and a reporter that marks ASER (gcy-5prom::mcherry). The phenotype displayed is loss of ASEL (red circle) fate and a concomitant gain of ASER (blue circle) fate in ASEL. Asterisks denote expression unrelated to ASE. B: Rescue of the rbbp-5 mutant phenotype with heterologous promoter lines (see Materials and Methods). ‘Early’ heat-shocks were performed on embryos younger than the AB256-cell stage, before the birth of ASE and ‘late’ heat-shocks on embryos older than the AB256-cell stage, after the birth of ASE. The che-1::rbbp-5::gfp construct was injected at 20 ng/ul and the hsp-16::rbbp-5::gfp construct was injected at 50, 20 and 2 ng/ul. Three lines of each were tested. Statistical significance (in comparison to the mutant alone unless otherwise indicated) was assessed using a z-test and a Bonferroni correction was applied for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001). The ASE phenotype was scored with ASER marker gcy-5prom::gfp (otIs220). In all cases n>30 except for the staged heat-shocks where n>8. C: Graphs indicating the left-right ASE expression of lsy-6 in wild-type and COMPASS complex mutants. Ex[lsy-6::yfp] is an extrachromosomal array, otEx4410 Ex[lsy-6::yfp; ttx-3::mcherry]. Only array carrying worms were scored. In all cases n>50, see Table S3 for more detailed numbers. D: Graphs indicating the left-right ASE expression of gcy-5prom::gfp (ntIs1) in lsy-6 overexpressing, rbbp-5(tm3463) and double-mutant animals. Similar results were obtained with rbbp-5(ot86) (Table S3). In all cases n>50. ASE::lsy-6 is ceh-36prom::lsy-6 (otIs204). E: Graph indicating the percentage ASE defect following control (empty RNAi vector), ash-2 and rbbp-5 RNAi in wild-type, lsy-12(ot154), lsy-13(ok1475) and rbbp-5(tm3463) mutant animals. The ASE defect was scored with the ASEL marker lim-6prom::gfp (otIs114). Statistical significance (in comparison to control RNAi in relevant mutant background unless otherwise indicated) was assessed using a z-test (*p<0.05, **p<0.01, ***p<0.001). In all cases n>70. Numbers for control (empty vector) RNAi are taken from Figure 1. For more detailed numbers see Table S3. For the ASEL scoring of ash-2 and rbbp-5 RNAi in a wild-type background, RNAi was performed in both a strain containing either the lim-6::gfp(otIs114) reporter to match the reporter present in lsy-12(ot154) strain and in a strain containing both the lim-6::gfp(otIs114) and gcy-5::rfp(otIs220) reporter to match the lsy-13(ok1417) strain. No difference was observed and the numbers were pooled. Fig. 8. The cloning and expression of lsy-15/rbbp-5(ot86). 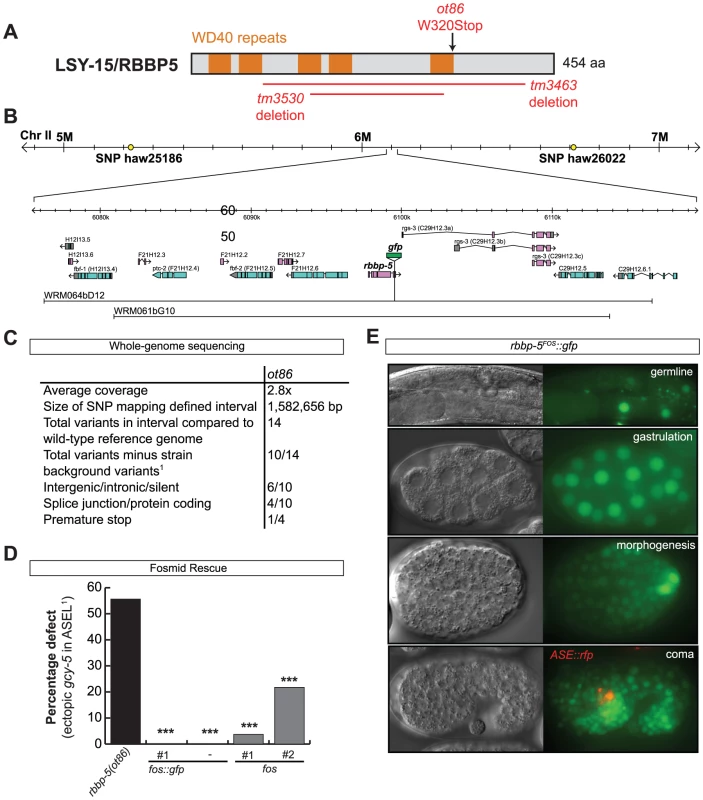
A: Protein schematic of LSY-15/RBBP-5 with the location and nature of mutant alleles identified and/or used in this study. B: Schematic illustrating the SNP-mapping and genomic region of lsy-15/rbbp-5, and the fosmids used to for expression and rescue analysis. lsy-15(ot86) was mapped to a ∼15 MB interval on Chr II between the polymorphisms haw25186 and haw26022. Two different fosmids were used to rescue lsy-15/rbbp-5(ot86) mutants, one of which was recombineered to express a translational lsy-15/rbbp-5::gfp fusion for expression analysis. C: Whole genome sequencing (WGS) data for the lsy-15(ot86) allele. Only 4 protein coding variants resided in the interval to which lsy-15(ot86) had been mapped, one of which was a premature stop in the rbbp-5 locus which was confirmed by Sanger sequencing. 1Variants were considered as background if they were also found in other WGS datasets from the lab (see Material and Methods). D: Rescue of the rbbp-5 mutant phenotype with fosmid lines (see Materials and Methods). Statistical significance (in comparison to the mutant alone) was assessed using a z-test and a Bonferroni correction was applied for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001). E: Representative images of the expression of the rbbp-5FOS::gfp transgene. The top three images are in a lsy-15/rbbp-5(ot86) background and the bottom image is in a che-1prom::mcherry background that is expressed specifically in ASE neurons. Overlapping expression can be observed. We analyzed several other members of the COMPASS complex that were not revealed by our RNAi screen, using RNAi, mutant alleles or both. We identified a SET-type methyltransferase component, set-2 (a SET1A&B ortholog) and another two WD40 proteins, swd-3.1 and swd-2.2 (the WDR5 and WDR82 orthologs) as having ASE laterality defects similar to those observed in rbbp-5, dpy-30 and ash-2 mutants (Figure 7 and Table S3). The ASE laterality defects of set-2 and swd-2 were only revealed in a genetically sensitized lsy-6(ot150) background. Additionally, this background enhanced the penetrance of the RNAi defects observed in several other COMPASS complex components (Table S3). Together we find that all core components of the COMPASS complex regulate ASE laterality.
The COMPASS complex acts during embryogenesis and upstream of the miRNA lsy-6 to regulate ASE laterality
To determine when and where COMPASS acts to regulate ASE asymmetry we focused on rbbp-5 and generated several reporter gene and rescue constructs. A GFP-tagged fosmid array that fully rescues the ASE laterality defects of rbbp-5(ot86) mutants is expressed ubiquitously during early embryogenesis and is predominantly nuclear localized (Figure 8D–8E). We observe similar expression of a translational reporter gene construct containing the intergenic region upstream of rbbp-5 (data not shown). This is consistent with previously reported broad expression of several other components of the COMPASS complex [31], [32]. At later stages, during and after the birth of the ASE neurons, rbbp-5 expression is significantly reduced throughout the embryo. We are able to observe overlapping expression with a reporter gene expressed bilaterally in the ASE neurons, however, we note that expression of the transgenic array in the ASE neurons was relatively infrequent despite the fact that array carrying animals are fully rescued (Figure 8D–8E). Additionally, we also observe complete rescue of the ASE laterality defects in non-array carrying worms derived from array positive mothers suggesting that the fosmid array can maternally rescue rbbp-5(ot86) mutants. Consistent with this we observe maternal germline expression of the rbbp-5 fosmid (Figure 8E) and the mutants themselves are maternally rescued (data not shown). These observations provide a hint that rbbp-5 and the COMPASS complex may act early during embryogenesis to regulate ASE asymmetry. To examine this possibility in more detail, we attempted to rescue the rbbp-5 mutant phenotype by driving an rbbp-5::gfp translational fusion using two heterologous promoters: (1) the che-1 promoter, which is exclusively expressed bilaterally in the mother of the ASE neurons and then in the mature ASE neurons throughout the life of the animal or (2) the ubiquitous hsp-16 heat-shock promoter. We find that ASE-specific expression of rbbp-5 only minimally rescues the rbbp-5(tm3463) mutant phenotype, despite being strongly expressed in the ASE neurons as expected (Figure 7B and Figure S6). In contrast, leaky expression from lines carrying the hsp-16 construct and maintained at room temperature strongly rescues the ASE laterality defect in a dose dependent manner, despite rare expression in the post-mitotic ASE neurons (Figure 7B and Figure S6). We next performed staged heat-shocks using those lines with minimal rescue ability of the rbbp-5 laterality defect at room temperature. We find that when the heat-shock is performed early during embryogenesis, before the birth of the ASE neurons, the ASE laterality defect is fully rescued (Figure 7B). In contrast, if the heat-shock is performed at or after the birth of the ASE neurons the defect is only weakly rescued. We conclude that rbbp-5 acts before the generation of the ASE neurons.
The most upstream component of the previously described network of laterality factors is the ASEL-expressed miRNA lsy-6. We find that lsy-6 expression is lost upon abrogation of various COMPASS components (Figure 7C and Table S3). Moreover, the “2 ASEL” fate inducing activity of lsy-6, expressed from a heterologous promoter, is epistatic to the loss of COMPASS activity (rbbp-5 mutants), corroborating the notion that COMPASS acts upstream of lsy-6 (Figure 7D and Table S3).
Previous genetic screens have revealed that a MYST-type histone acetyltransferase (HAT) complex also shows ASE laterality defects that are indistinguishable from those of the COMPASS histone methyltransferase (HMT) complex, in that the ASEL neurons convert into ASER neurons and that lsy-6 expression is lost in the post-mitotic ASE neurons [33]. One of the components of the MYST HAT complex was also retrieved from our RNAi screen (the PHD/Bromodomain lin-49 locus). Given the phenotypic similarity between the MYST HAT and COMPASS HMT complex, we asked whether they genetically interact. This hypothesis was born out of biochemical studies that suggest that histone methylation is often a prerequisite for ensuing histone acetylation and transcriptional activation [34]. We tested for a genetic interaction by asking for synergies in gene function. We chose conditions in which RNAi against two different HMT components (ash-2 and lsy-15) resulted in essentially no laterality defect (in a non-sensitized, rather than nre-1; lin-15b RNAi hypersensitive background) and tested the effect of RNAi against these genes either in a wildtype background or in a MYST HAT mutant background (the lsy-12 MYST-like HAT gene and the lsy-13 ING-like gene). We observed strikingly synergistic phenotypes in both lsy-12 and lsy-13 mutant backgrounds (Figure 7E). In contrast, ash-2 RNAi was unable to enhance the defects of rbbp-5 mutants (Figure 7E) and only additive or mild synergy at best was observed in ceh-36 mutants (Table S3). All together, this data demonstrates that rbbp-5 and the COMPASS complex acts early during embryogenesis, before the birth of the ASE neurons and in conjunction with the MYST complex to regulate the expression of the miRNA lsy-6 and therefore ASE laterality.
Discussion
We described here the first comprehensive, genome-wide RNAi screen that monitors a single neuron-type cell fate decision. The screen has been remarkably successful in retrieving genes known to be involved in controlling ASE neuron specification. Six genes previously known to be involved in ASE neuron specification have been found in an unbiased manner (che-1, die-1, lsy-2, lin-49, lim-6, fozi-1) and only two have not (cog-1, nhr-67: these genes also display no laterality defects with sequence-verified RNAi clones). The RNAi screen also revealed a number of genes that we would have predicted to result in ASE lineage defects, including genes involved in the segregation of early patterning cues (e.g. par and mex- genes) and involved in a re-iteratively used binary fate decision system (lit-1 gene)[7]. Moreover, the RNAi screen assisted the cloning of two previously unknown lsy mutants, lsy-15/rbbp-5 (this paper) and lsy-22 [35] by identifying genes with laterality defects in the genetic intervals to which lsy-15 and lsy-22 had been mapped, facilitating the identification of the phenotype-inducing mutation within the whole-genome sequencing data.
The screen identified an as yet elusive proneural patterning gene for the ASE lineage, the bHLH gene hlh-14. Previous forward genetic screens for ASE differentiation mutants have failed to retrieve alleles in this gene, possibly because these screens are intrinsically biased against alleles in mutants with lethal phenotypes, illustrating a key conceptual advantage of the RNAi screening approach. Even though previous genetic screens for ASE developmental defects have successfully retrieved hypomorphic alleles of essential genes [18], in the case of hlh-14, alleles that separate essential functions from its proneuronal function in the ASE lineage may not exist. We have demonstrated that hlh-14 is required for the production of neurons from several distinct lineages. Besides lin-32/Atonal [36], hlh-14 is only the second bHLH gene in C. elegans described with such a canonical neural versus hypodermal switch function. It is possible that hlh-14 may also regulate more general neural/non-neural decisions, such as the “non-clonal” production of neurons from the multi-potent pharyngeal lineages. Additionally, we have shown that the loss of hlh-14 leads to neuronal sub-type differentiation defects, suggesting that, in a similar fashion to lin-32 [37], it may act at multiple stages of neuronal differentiation.
How hlh-14 is regulated remains to be discovered. Although early pre-patterning mechanisms that regulate proneural gene expression have been well described in both vertebrates and Drosophila, few direct activators of proneural genes have been identified [38], [39]. In C. elegans, lin-32 is activated by Hox genes and is also repressed by ref-1, a downstream effector of Notch signaling [40], [41]. This suggests parallels to the Notch-mediated regulation of vertebrate proneural genes [39]. Intriguingly, an ectopic ASE lineage is generated from the ABara blastomere in ref-1 mutants [12] and it will be interesting to determine whether this is due to a de-repression of hlh-14.
We observe L/R bilaterally symmetric expression of hlh-14 in neuroblasts of symmetric origin but remarkably we also observe L/R bilaterally symmetric expression of hlh-14 in lineages that derive from asymmetric lineage origins. This expression is initiated at an identical moment in time on both the left and right sides, despite the neuroblasts deriving from L/R asymmetric origins and is the case in both the ASEL/ASER lineages and the I1L/I1R lineages. In both cases, expression coincides with the point at which these lineages become bilaterally symmetric in that they undergo an identical set of divisions to generate the left-right homologs of bilateral pairs of neurons. Future identification of upstream regulators of hlh-14 and other factors that are expressed during this “symmetrization phase” will begin to address how left-right bilateral symmetry is established in the nervous system. Intriguingly, we also observe left/right asymmetric expression of hlh-14, which is required to induce the fate of certain asymmetric neuroblasts/neurons of the C-lineage. It will be interesting to determine how this asymmetric expression is induced, how it is coordinated with bilaterally symmetric expression programs and whether, given the anterior-posterior lineage difference (Figure 6C), asymmetric expression is regulated by the binary Wnt-signaling system [7].
Given that hlh-14, like lin-32, fulfills its proneural function in several very distinct neuronal lineages [28](this paper), it is obvious that additional co-factors must exist to determine specificity of bHLH gene action. With this thought in mind, we were surprised to find essentially no other transcriptional regulator that is involved at the neuronal versus ectodermal decision step - or any later or earlier step in controlling the generation of the ASE lineage branch. This is also surprising from the perspective of the binary, Wnt-mediated a/p decision system that works broadly throughout the developing C. elegans embryo [7], [42], [43]. Through mutant analysis we have previously shown that this system also operates in the ASE lineage [44] and, moreover, we have also identified in this screen the lit-1 gene, an important signal transducer of the binary system [43]. This binary specification system, whose critical output is a differential distribution of a POP-1/LEF transcription factor in dividing cells, is generally thought of as needing to pair with lineage-specific transcriptional regulators to instruct development [8], [44]. In other words, differentially distributed POP-1/LEF-1 is thought to interact with cell-intrinsic transcriptional “codes” (i.e. combinations of transcription factors) to define regulatory states that are characteristic and specific for any cell at any stage of development. Since the pop-1 system presumably cooperates with different transcription factors at each of the a/p divisions that generate ASE, we expected to reveal a wealth of transcriptional regulators. Even if such factors had pleiotropic functions early in the embryo, our screen should have nevertheless revealed them as non-gfp-positive, dead embryos. Yet, with the exception of the known transcription factors (TFs) and hlh-14, we identified no novel TF in the set of 801 TFs screened in our RNAi library that affect ASE specification. Moreover, we noted that only about 12% of TFs in the genome-wide TF clone library resulted in the embryonic arrest phenotype that one would expect from strong lineage patterning defects. In all these arrested embryos, the ASE neurons are generated normally, with the exception of the known embryonic patterning genes pie-1 and skn-1. Finally, 59 TFs generated a P0 sterile phenotype preventing the analysis of F1 phenotypes in the nre-1 lin-15b background. We re-screened these genes in wildtype and rrf-3 or eri-1; lin-15b RNAi hypersensitive backgrounds, but uncovered no additional regulators of ASE fate (data not shown).
There are several possible and non mutually-exclusive explanations for the paucity of TF phenotypes. The simplest is a technical one, RNAi may not sufficiently knock down specific TFs. Supporting this notion, the cog-1 homeobox gene does not display an ASE laterality defect by RNAi and was not retrieved by the screen. The other, more complex and perhaps more interesting one is well exemplified with the T box TFs tbx-37 and tbx-38 [13]. We have previously shown that both are required at an early point in the lineage that generates the ASEL neuron for the correct establishment of ASE asymmetry [12]. Yet both genes act redundantly; removal of either gene alone has no effect [13]. The C. elegans genome contains a good number of closely related, paralogous TF pairs from various families and it is conceivable that particularly during early patterning, TFs may work in redundant pairs. In addition to the tbx-37/38 case, there are several other reported cases for such redundant TF pairs (pes-1/fkh-2 [45], med-1/med-2 [46]; end-1/end-3 [47] tbx-8/tbx-9 [48] and ref-family genes [41]).
The early lineage determination of ASE left-right asymmetry led us to postulate the existence of either an “asymmetry mark” that can be remembered through several rounds of division to regulate expression of the miRNA lsy-6 expression or a cascade of TFs that leads from tbx-37/38 to lsy-6 [12]. Previous work from our lab has identified a MYST-type histone acetylase complex that controls ASEL/R asymmetry through regulation of the lsy-6 miRNA [33] and in this study we have shown that the histone methyltransferase complex COMPASS also regulates ASE laterality through regulation of lsy-6 (Figure 7). We have shown that COMPASS may be directly linked to the MYST HAT complex activity, as supported by phenotypic similarity as well as genetic interaction tests. Mechanistically, histone methylation may be a step directly upstream of and preceding histone acetylation, leading to transcriptional activation in several contexts (reviewed in [49]). In the context of ASE asymmetry, this is supported by two components of the MYST HAT complex containing a PHD domain (the LIN-49 protein and the LSY-13 protein), which is known to interact with methylated histones [34], [50]. Indeed, biochemical studies show that the PHD finger of lsy-13 ortholog ING, recognizes the H3K4 trimethylation mark of COMPASS and serves as a link in site specific recruitment of HATs [51], [52]. Our synergistic data corroborate this idea in an in vivo context and demonstrate that the COMPASS complex acts before the birth of the ASE neurons, providing a potential molecular link between the early Notch signal, the expression of tbx-37/38 and the ASEL specific expression of lsy-6. Together, these histone-modifying complexes may form part of a lineage-specific “chromatin mark” that acts, in cohort with specific TFs to bias lsy-6 expression, such that it is only expressed in ASEL.
What other genes and processes has our screen revealed? Many of the genes revealed in our RNAi screen as being involved in neural development code for proteins with apparently very general cellular functions, such as general splicing factors and core components of RNA polymerases. The remarkably specific effects of some of these factors suggest that some of these proteins may not have as broad roles as previously thought or it may argue that some of the substrates (e.g. those of splicing factors) relevant to specific neuronal lineage decisions are sensitive to gene dosage (and therefore more easy to functionally impair) than other, more generally acting substrates. In addition, early C. elegans embryogenesis is characterized by very specific signaling events between specific individual blastomeres [53]. Several of the genes that we identified as having cell/lineage defects are likely a reflection of disrupting early blastomere divisions and positions resulting in aberrant signaling events and lineage misspecifications. For yet other genes - many of them with intriguing molecular identities - their function in determining ASE remains to be determined and will likely provide novel insights into the mechanisms of neural specification.
Materials and Methods
Strains and transgenes
Strains and transgenes used
N2 Bristol wild type [1] and CB4856 Hawaiian wild type [54]; OH2281, rrf-3(pk1426); otIs114; him-5(e1490); OH7704, eri-1(mg366); lin-15b(n744); otis114; him-5(e1490), and OH7082, nre-1(hd20) lin-15b(hd126); otis114; him-5(e1490) [20]. VC1137, hda-1(ok1595) V/nT1[qIs51] (IV;V); VC954, rnf-113(ok1401) III/hT2[bli-4(e937) let-?(q782) qIs48](I;III); JK907, mog-4(q233)/mnC1 dpy-10(e128) unc-52(e444)II; VC2127, mog-4(ok2708)/mT1 II; +/mT1[dpy-10(e128)] III; JK2321, mog-5(q449) unc-4(e120)/mIn1[dpy-10(e128)] II; VC724, mog-5(ok1101)/mIn1[mIs14 dpy-10(e128)] II; RB1593, klp-15(ok1505) I; RW3199, lev-11(x12) let-49(st44)/lev-11(x12) unc054(e1152) I; VC186 smo-1(ok359) I/szT1 [lon-2(e678) (I;X); NG2295, hlh-14(gm34)/mnC1 dpy-10(e128) unc-52(e444) II [28]; NG4280, hlh-14(tm295)/mIn1 [mIs14 dpy-10(e128)] II [28]; RB979, F28B3.1(ok885) I; SM1017, tlf-1(ok389)/unc-55(e402) dpy-24(s71) I; KR499, let-526(h185) dpy-5(e61) unc-13(e450) I; sDp2(I;f); VC1395, pqn-38(ok1791) III/hT2[bli-4(e937) let-?(q782) qIs48] (I;III); RB1025, set-2(ok952) III; MT14851, set-2(n4589) III; RB1304 swd-3.1(ok1417). dpy-30(y228) and dpy-30(y130) III courtesy of B.J. Meyer. F21H12.1(tm3463) and F21H12.1(tm3530) II, grp-1(tm1956) III, ash-2(tm1905) II, mag-1(tm645) I: all obtained from Mitani Laboratory, NBP, Japan. lsy-15(ot86) was isolated as previously described [18].
Transgenes to label ASEL and ASER fates
otIs3 V, Is[gcy-7prom::gfp; lin-15 (+)]; otIs114 I, Is[lim-6prom::gfp; rol-6(d)]; otIs220 IV, Is[gcy-5prom::mCherry; rol-6(d)]; otIs186 Is[gcy-5prom::gfp]; ntIs1 V, Is[gcy-5prom::gfp; lin-15 (+)]; and the ASEL/ASER markers otIs151 V, Is[ceh-36prom::DsRed2; rol-6(d)] and otIs232 V, Is[che-1prom::mCherry; rol-6(d)], otIs217 Is[che-1prom::HIS-3::mCherry; rol-6(d)] and otIs299 Is[8xASE::gfp; elt-2::dsRed].
Reporters for other cell types
OLL otIs138 Is[ser-2prom3::gfp; lin-15(+)] [55], AFD oyIs17 Is[gcy-8prom::gfp; lin-15(+)] [56]; muscle ccIs4251, Is[myo-3prom::DFP-LacZ(NLS); myo-3prom::mitochondrial GFP; dpy-20(+)] [4]; gut (elt-2::gfp/LacZ) (personal communication A. Grishok); dopaminergic and cholinergic neurons otIs181, Is[dat-1prom::mCherry; ttx-3prom::mCherry] [57]; hypodermis arIs99, Is[dpy-7prom::2Xnls::yfp; ceh-22::gfp] [58]; pan-neuronal otIs287, Is[rab-3prom::nls::yfp]; gmIs20 Is[hlh-14prom::gfp] [28].
Fosmid recombineering and transgenic arrays
Fosmids were tagged as described [29] to generate OH10173-OH10176 otEx4507-otEx45010, Ex[hlh-14FOS(WRM0627dH07)::yfp; rol-6(d)]; hlh-14(tm295); ntIs1 (4 independent lines); OH10171 otEx4506, Ex[rbbp-5FOS(WRM064bD12)::gfp; ttx-3prom::mCherry]; otIs232.
Rescue of lsy-15(ot86): In addition to the tagged fosmid line (above) the following untagged fosmid arrays were generated: OH9335-OH9336, otEx4135-otEx4136 Ex[F21H12.1FOS (WRM061bG10);elt-2::gfp] line 1 (2 independent lines).
Rescue of lsy-15(tm3463): Heterologous promoter constructs were made by PCR fusion ([59]; primers available upon request) to generate OH10177-OH10179 otEx4511-otEx4513, Ex[che-1prom::rbbp-5::gfp; elt-2prom::gfp] (3 independent lines); OH10180-OH10182 otEx4514-otEx4516, Ex[hsp-16prom::rbbp-5::gfp; elt-2prom::gfp] (3 independent lines; 50 ng/ul); OH10183-OH10185 otEx4517-otEx4519, Ex[hsp-16prom::rbbp-5::gfp; elt-2prom::gfp] (3 independent lines; 20 ng/ul); OH10186-OH10188 otEx4520-otEx4522, Ex[hsp-16prom::rbbp-5::gfp; elt-2prom::gfp] (3 independent lines; 2 ng/ul).
Overexpression: OH7805 otIs204 Is[ceh-36prom2::lsy-6, elt-2prom::gfp].
RNA interference
RNAi was performed using a bacterial feeding protocol [23] with the following modifications. NGM agar plates containing 6 mM IPTG and 100 µg/ml ampicillin were seeded with bacteria expressing dsRNA. L4 staged hermaphrodites were placed onto these plates and grown at 22°C. After five days, the F1 progeny of these worms were scored for neuronal and pleiotropic defects. All inserts of clones for the known ASE factors were sequenced to confirm identity of the clone. The visible phenotypes screened for chromosome I: Emb (embryonic lethal), P0 Ste (P0 sterile), Stp (sterile progeny), Brd (low brood size), Gro (slow postembryonic growth), Lva (larval arrest), Let (larval lethal), Adl (adult lethal), Bli (blistering of cuticle), Bmd (body morphological defects), Clr (clear), Dpy (dumpy), Egl (egg - laying defective), Lon (long), Mlt (molt defects), Muv (multivulva), Prz (paralyzed), Pvl (protruding vulva), Rol (roller), Rup (ruptured), Sck (sick) and Unc (uncoordinated). Emb was defined as greater than 30% dead embryos. Each phenotype was required to be present among at least 10% of the analyzed worms for the visible pleiotropies and neuronal defects. Non-viable (nonV) category encompasses P0 Ste, Emb, Stp and Let phenotypes. The JA library of RNAi clones was purchased from Geneservice (http://www.lifesciences.sourcebioscience.com/) and was supplemented with the ORFeome based RNAi clones [60] courtesy of John Kim [61]. Based on sequencing of several randomly picked clones from the library we estimate the faithfulness of the library to be 80–90% (data not shown).
Phenotypic scoring criteria
Primary screen
Primary screen was conducted by screening two wells for the phenotypic categories “loss of ASEL” and “ectopic ASEL”. A positive clone was defined as having a phenotype in at least 3/8 wells scored (or > = 0.4). If clone had a phenotype (at least one well), it was then re-screened an additional 6 times (see below). An ASEL loss score was generated by dividing the number of wells showing “loss of ASEL” over the total number of wells scored. An ASEL ectopic score was generated by dividing the number of wells showing “ectopic ASEL” over total number of wells scored. Thus, in Tables 1, 2, 3, 4 and Tables S1 and S2 (see Results):
ASEL off (-) = ASEL off score >0, ASEL ectopic score 0;
ASEL ectopic (+) = ASEL ectopic score >0, ASEL off score 0;
ASEL mixed (+/−) = score in both ASEL off and ASEL ectopic >0. For ASEL mixed category the following adjustment was made: based on the ratio of (ASEL ectopic/ASEL off score) if score < = 0.25 clones were moved to ASEL off. Based on ratio (ASEL off/ASEL ectopic score) if score < = 0.25 clones were moved to ASEL ectopic.
The ASE screen
The ASE screen was conducted by screening 6 wells with an ASER specific reporter and bilateral ASE reporter. Phenotypic scoring for ASER reporter consisted of “loss of ASER” and “ectopic ASER” while for bilateral ASE reporter was “off”, “1-on”, “2-on”, “ectopic ASEs” and “mixed” where “mixed” category was any combination of the above phenotypes. The ASER phenotypic categorization was generated in the same way as the ASEL above. Thus, in Tables 1, 2, 3, 4 and Tables S1 and S2 (see Results):
ASER off (-) = ASER off score >0, ASER on score 0, > = 1 wells screened;
ASEL ectopic (+) = ASER on score >0, ASER off score 0, > = 1 wells screened;
ASER mixed (+/−) = ASER off score >0, ASER ectopic score >0, > = 1 wells screened;
ASER wt = ASER score = 0, > = 1 wells screened.
Blank means not done.
The ASE bilateral score was generated as follows:
ASE wt = ASE score = 0, > = 1 wells screened;
ASE 2-off (-) = ASE 2-off score >0, all other scores 0, > = 1 wells screened;
ASE 1-off (-) = ASE 1-off score >0, all other scores 0, > = 1 wells screened;
ASE ectopic (+) = ASE ectopic score >0, all other scores 0, > = 1 wells screened;
ASE mixed (+/−) = ASE off scores (either of two) >0, ASE ectopic score >0; or if scored >0 in the ASE “mixed”, > = 1 wells screened.
The tissue marker screen
The tissue marker screen was conducted by screening two wells, for phenotypic categories “tissue OFF” “tissue Mixed” “tissue ON”, which were assigned an arbitrary score of 0, 0.25 and 0.5 respectively. The final tissue score of the clone was calculated by adding the individual scores of the two wells, resulting in a range from minimum 0 (0+0) to maximum 1 (0.5+0.5). If the score >0.5, the clone is “ON” for that tissue; if the score is < = 0.5, the clone is designated “OFF”. In the Tables 1, 2, 3, 4 and Tables S1 and S2 (see Results Section) “ON” denoted “+”, OFF denoted “−”.
“ne” means clone not embryonic lethal. Clones that were “ne” in 3 or more strains (out of 6 strains screened) were taken off the list under the premise of non-repeatable embryonic phenotype. Categorization in groups is described in Results.
Lineage and 3D cell position analysis of hlh-14 expression and hlh-14 mutants
Cells were identified using 4D microscopy and SIMI BioCell software as described in [10]. Briefly, wild-type (containing the ntIs1 transgene), hlh-14:gfp (gmIs20), hlh-14(gm34); otIs114, hlh-14(tm295); ntIs1; otEx4507 Ex[hlh-14FOS::YFP] gravid adults were dissected and single two-cell embryos were mounted and visualized on a Zeiss Axioplan 2 compound microscope. To lineage hlh-14(tm295) mutants, non-array carrying embryos from the hlh-14(tm295); ntIs1; otEx4507 Ex[hlh-14FOS::YFP] strain were examined. Nomarski stacks were taken every 35 seconds and images within a stack at ∼1 µm apart. Fluorescent images were acquired at pre-determined time points during the course of embryonic development. Movies were lineaged using the SIMI BioCell program. The positions of cells at different time-points were monitored using the 3D-feature of SIMI BioCell. All additional DIC and fluorescent images were acquired using the open source Micro-manager software [62].
Heat-shock rbbp-5 rescue experiments
Embryos were dissected from array carrying mothers, staged and then subjected to a 15 minute heat-shock at 37°C after which the embryos were placed at 15°C and allowed to develop to the L1 larval stage before scoring. Embryos were divided into two categories for heat-shocks: ‘early’ = younger than AB256-cell stage; ‘late’ = older than AB256-cell stage.
Mapping and whole-genome sequencing
The lsy-15(ot86) allele was mapped to a region of approximately 15 MB (Figure 6) through classic three-factor and single-nucleotide polymorphism (SNP) mapping. Then, genomic DNA was prepared from a population of lsy-15(ot86); otIs114 animals using the Gentra GenePure DNA extraction kit. This genomic DNA (5 µg) was genome sequenced as previously described [63] using an Illumina Genome Analyzer II according to the manufacturers specification. The resultant sequence data was analyzed with MAQGene [64]. The following stringency criteria were used to filter variants: quality score > = 3, loci multiplicity < = 1, sequencing depth > = 3. All detected sequence variants were analyzed for whether they were present in any one of several additional genomes of different genotypes that we sequenced in the laboratory; if so, they were considered background and eliminated from further analysis.
Supporting Information
Zdroje
1. BrennerS 1974 The genetics of Caenorhabditis elegans. Genetics 77 71 94
2. WoodWB 1988 The nematode Caenorhabditis elegans: Cold Spring Harbor Laboratory Press
3. HobertO 2010 Neurogenesis in the nematode Caenorhabditis elegans. WormBook 1 24
4. FireAXuSMontgomeryMKKostasSADriverSE 1998 Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans [see comments]. Nature 391 806 811
5. KamathRSAhringerJ 2003 Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30 313 321
6. SulstonJESchierenbergEWhiteJGThomsonJN 1983 The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100 64 119
7. BertrandVHobertO 2010 Lineage programming: navigating through transient regulatory states via binary decisions. Curr Opin Genet Dev
8. LinRHillRJPriessJR 1998 POP-1 and anterior-posterior fate decisions in C. elegans embryos. Cell 92 229 239
9. SulstonJE 1983 Neuronal cell lineages in the nematode Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol 48 443 452
10. SchnabelRHutterHMoermanDSchnabelH 1997 Assessing normal embryogenesis in Caenorhabditis elegans using a 4D microscope: variability of development and regional specification. Dev Biol 184 234 265
11. OrtizCOFaumontSTakayamaJAhmedHKGoldsmithAD 2009 Lateralized gustatory behavior of C. elegans is controlled by specific receptor-type guanylyl cyclases. Curr Biol 19 996 1004
12. PooleRJHobertO 2006 Early embryonic programming of neuronal left/right asymmetry in C. elegans. Curr Biol 16 2279 2292
13. GoodKCioskRNanceJNevesAHillRJ 2004 The T-box transcription factors TBX-37 and TBX-38 link GLP-1/Notch signaling to mesoderm induction in C. elegans embryos. Development 131 1967 1978
14. JohnstonRJHobertO 2003 A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature 426 845 849
15. ChangSJohnstonRJJrHobertO 2003 A transcriptional regulatory cascade that controls left/right asymmetry in chemosensory neurons of C. elegans. Genes Dev 17 2123 2137
16. JohnstonRJJrChangSEtchbergerJFOrtizCOHobertO 2005 MicroRNAs acting in a double-negative feedback loop to control a neuronal cell fate decision. Proc Natl Acad Sci U S A 102 12449 12454
17. DidianoDCochellaLTursunBHobertO 2010 Neuron-type specific regulation of a 3′UTR through redundant and combinatorially acting cis-regulatory elements. RNA 16 349 363
18. SarinSO'Meara MMFlowersEBAntonioCPooleRJ 2007 Genetic Screens for Caenorhabditis elegans Mutants Defective in Left/Right Asymmetric Neuronal Fate Specification. Genetics 176 2109 2130
19. FireA 1999 RNA-triggered gene silencing. Trends Genet 15 358 363
20. SchmitzCKingePHutterH 2007 Axon guidance genes identified in a large-scale RNAi screen using the RNAi-hypersensitive Caenorhabditis elegans strain nre-1(hd20) lin-15b(hd126). Proc Natl Acad Sci U S A 104 834 839
21. KennedySWangDRuvkunG 2004 A conserved siRNA-degrading RNase negatively regulates RNA interference in C. elegans. Nature 427 645 649
22. SimmerFMoormanCVan Der LindenAMKuijkEVan Den BerghePV 2003 Genome-Wide RNAi of C. elegans Using the Hypersensitive rrf-3 Strain Reveals Novel Gene Functions. PLoS Biol 1 e12 doi:10.1371/journal.pbio.0000012
23. KamathRSFraserAGDongYPoulinGDurbinR 2003 Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421 231 237
24. ZipperlenPFraserAGKamathRSMartinez-CamposMAhringerJ 2001 Roles for 147 embryonic lethal genes on C.elegans chromosome I identified by RNA interference and video microscopy. Embo J 20 3984 3992
25. CioskRDePalmaMPriessJR 2006 Translational regulators maintain totipotency in the Caenorhabditis elegans germline. Science 311 851 853
26. WattsJLEtemad-MoghadamBGuoSBoydLDraperBW 1996 par-6, a gene involved in the establishment of asymmetry in early C. elegans embryos, mediates the asymmetric localization of PAR-3. Development 122 3133 3140
27. UchidaONakanoHKogaMOhshimaY 2003 The C. elegans che-1 gene encodes a zinc finger transcription factor required for specification of the ASE chemosensory neurons. Development 130 1215 1224
28. FrankCABaumPDGarrigaG 2003 HLH-14 is a C. elegans achaete-scute protein that promotes neurogenesis through asymmetric cell division. Development 130 6507 6518
29. TursunBCochellaLCarreraIHobertO 2009 A toolkit and robust pipeline for the generation of fosmid-based reporter genes in C. elegans. PLoS ONE 4 e4625 doi:10.1371/journal.pone.0004625
30. TenneyKShilatifardA 2005 A COMPASS in the voyage of defining the role of trithorax/MLL-containing complexes: linking leukemogensis to covalent modifications of chromatin. J Cell Biochem 95 429 436
31. SimonetTDulermoRSchottSPalladinoF 2007 Antagonistic functions of SET-2/SET1 and HPL/HP1 proteins in C. elegans development. Dev Biol 312 367 383
32. XuLStromeS 2001 Depletion of a novel SET-domain protein enhances the sterility of mes-3 and mes-4 mutants of Caenorhabditis elegans. Genetics 159 1019 1029
33. O'MearaMMZhangFHobertO 2010 Maintenance of Neuronal Laterality in Caenorhabditis elegans Through MYST Histone Acetyltransferase Complex Components LSY-12, LSY-13 and LIN-49. Genetics
34. TavernaSDIlinSRogersRSTannyJCLavenderH 2006 Yng1 PHD finger binding to H3 trimethylated at K4 promotes NuA3 HAT activity at K14 of H3 and transcription at a subset of targeted ORFs. Mol Cell 24 785 796
35. FlowersEBPooleRJTursunBBashllariEPe'erI 2010 The Groucho ortholog UNC-37interacts with the short Groucho-like protein LSY-22 to control developmental decisions in C. elegans. Development 137 1799 1805
36. ZhaoCEmmonsSW 1995 A transcription factor controlling development of peripheral sense organs in C. elegans. Nature 373 74 78
37. PortmanDSEmmonsSW 2000 The basic helix-loop-helix transcription factors LIN-32 and HLH-2 function together in multiple steps of a C. elegans neuronal sublineage. Development 127 5415 5426
38. SkeathJBCarrollSB 1994 The achaete-scute complex: generation of cellular pattern and fate within the Drosophila nervous system. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology 8 714 721
39. BertrandNCastroDSGuillemotF 2002 Proneural genes and the specification of neural cell types. Nat Rev Neurosci 3 517 530
40. RossJMKalisAKMurphyMWZarkowerD 2005 The DM domain protein MAB-3 promotes sex-specific neurogenesis in C. elegans by regulating bHLH proteins. Developmental cell 8 881 892
41. NevesAPriessJR 2005 The REF-1 family of bHLH transcription factors pattern C. elegans embryos through Notch-dependent and Notch-independent pathways. Dev Cell 8 867 879
42. SawaH 2010 Specification of neurons through asymmetric cell divisions. Curr Opin Neurobiol 20 44 49
43. KalettaTSchnabelHSchnabelR 1997 Binary specification of the embryonic lineage in Caenorhabditis elegans. Nature 390 294 298
44. BertrandVHobertO 2009 Linking asymmetric cell division to the terminal differentiation program of postmitotic neurons in C. elegans. Dev Cell 16 563 575
45. MolinLMounseyAAslamSBauerPYoungJ 2000 Evolutionary conservation of redundancy between a diverged pair of forkhead transcription factor homologues. Development 127 4825 4835
46. MaduroMFMeneghiniMDBowermanBBroitman-MaduroGRothmanJH 2001 Restriction of mesendoderm to a single blastomere by the combined action of SKN-1 and a GSK-3beta homolog is mediated by MED-1 and -2 in C. elegans. Molecular cell 7 475 485
47. MaduroMFRothmanJH 2002 Making worm guts: the gene regulatory network of the Caenorhabditis elegans endoderm. Dev Biol 246 68 85
48. PocockRAhringerJMitschMMaxwellSWoollardA 2004 A regulatory network of T-box genes and the even-skipped homologue vab-7 controls patterning and morphogenesis in C. elegans. Development 131 2373 2385
49. LathamJADentSY 2007 Cross-regulation of histone modifications. Nat Struct Mol Biol 14 1017 1024
50. VezzoliABonadiesNAllenMDFreundSMSantiveriCM 2010 Molecular basis of histone H3K36me3 recognition by the PWWP domain of Brpf1. Nat Struct Mol Biol 17 617 619
51. MartinDGBaetzKShiXWalterKLMacDonaldVE 2006 The Yng1p plant homeodomain finger is a methyl-histone binding module that recognizes lysine 4-methylated histone H3. Mol Cell Biol 26 7871 7879
52. PenaPVDavrazouFShiXWalterKLVerkhushaVV 2006 Molecular mechanism of histone H3K4me3 recognition by plant homeodomain of ING2. Nature 442 100 103
53. SchnabelRPriessJR 1997 Specification of Cell Fates in the Early Embryo. RiddleDLBlumenthalTMeyerBJPriessJR Celegans II Cold Spring Harbor Cold Spring Harbor Laboratory Press 361 382
54. HodgkinJDoniachT 1997 Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics 146 149 164
55. TsalikELNiacarisTWenickASPauKAveryL 2003 LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol 263 81 102
56. LanjuinAVanHovenMKBargmannCIThompsonJKSenguptaP 2003 Otx/otd Homeobox Genes Specify Distinct Sensory Neuron Identities in C. elegans. Dev Cell 5 621 633
57. FlamesNHobertO 2009 Gene regulatory logic of dopamine neuron differentiation. Nature 458 885 889
58. MyersTRGreenwaldI 2005 lin-35 Rb acts in the major hypodermis to oppose ras-mediated vulval induction in C. elegans. Developmental cell 8 117 123
59. HobertO 2002 PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32 728 730
60. RualJFCeronJKorethJHaoTNicotAS 2004 Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res 14 2162 2168
61. KimJKGabelHWKamathRSTewariMPasquinelliA 2005 Functional genomic analysis of RNA interference in C. elegans. Science 308 1164 1167
62. EdelsteinAAmodajNHooverKValeRStuurmanN 2010 Computer Control of Microscopes Using µManager: John Wiley & Sons, Inc
63. SarinSPrabhuSO'MearaMMPe'erIHobertO 2008 Caenorhabditis elegans mutant allele identification by whole-genome sequencing. Nat Methods 5 865 867
64. BigelowHDoitsidouMSarinSHobertO 2009 MAQGene: software to facilitate C. elegans mutant genome sequence analysis. Nat Methods 6 549
Štítky
Genetika Reprodukční medicína
Článek Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding SitesČlánek Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene SilencingČlánek Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse GutČlánek FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field SizeČlánek Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian SpermatogenesisČlánek Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese PopulationČlánek Differential Effects of and Risk Variants on Association with Diabetic ESRD in African AmericansČlánek Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 6
-
Všechny články tohoto čísla
- Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding Sites
- Statistical Inference on the Mechanisms of Genome Evolution
- Revisiting Heterochromatin in Embryonic Stem Cells
- A Two-Stage Meta-Analysis Identifies Several New Loci for Parkinson's Disease
- Identification of a Sudden Cardiac Death Susceptibility Locus at 2q24.2 through Genome-Wide Association in European Ancestry Individuals
- Genomic Prevalence of Heterochromatic H3K9me2 and Transcription Do Not Discriminate Pluripotent from Terminally Differentiated Cells
- Epistasis between Beneficial Mutations and the Phenotype-to-Fitness Map for a ssDNA Virus
- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Telomere DNA Deficiency Is Associated with Development of Human Embryonic Aneuploidy
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Unexpected Role for DNA Polymerase I As a Source of Genetic Variability
- Transportin-SR Is Required for Proper Splicing of Genes and Plant Immunity
- How Chromatin Is Remodelled during DNA Repair of UV-Induced DNA Damage in
- Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene Silencing
- Two Evolutionary Histories in the Genome of Rice: the Roles of Domestication Genes
- Natural Allelic Variation Defines a Role for : Trichome Cell Fate Determination
- Multiple Common Susceptibility Variants near BMP Pathway Loci , , and Explain Part of the Missing Heritability of Colorectal Cancer
- Pathogenic Mechanism of the FIG4 Mutation Responsible for Charcot-Marie-Tooth Disease CMT4J
- A Functional Variant in Promoter Modulates Its Expression and Confers Disease Risk for Systemic Lupus Erythematosus
- Drift and Genome Complexity Revisited
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
- Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse Gut
- Pathways of Distinction Analysis: A New Technique for Multi–SNP Analysis of GWAS Data
- Web-Based Genome-Wide Association Study Identifies Two Novel Loci and a Substantial Genetic Component for Parkinson's Disease
- Chk2 and p53 Are Haploinsufficient with Dependent and Independent Functions to Eliminate Cells after Telomere Loss
- Exome Sequencing Identifies Mutations in High Myopia
- Distinct Functional Constraints Partition Sequence Conservation in a -Regulatory Element
- CorE from Is a Copper-Dependent RNA Polymerase Sigma Factor
- A Single Sex Pheromone Receptor Determines Chemical Response Specificity of Sexual Behavior in the Silkmoth
- FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field Size
- Maps of Open Chromatin Guide the Functional Follow-Up of Genome-Wide Association Signals: Application to Hematological Traits
- Increased Susceptibility to Cortical Spreading Depression in the Mouse Model of Familial Hemiplegic Migraine Type 2
- Differential Gene Expression and Epiregulation of Alpha Zein Gene Copies in Maize Haplotypes
- Parallel Adaptive Divergence among Geographically Diverse Human Populations
- Genetic Analysis of Genome-Scale Recombination Rate Evolution in House Mice
- Mechanisms for the Evolution of a Derived Function in the Ancestral Glucocorticoid Receptor
- Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian Spermatogenesis
- Interferon Regulatory Factor 8 Regulates Pathways for Antigen Presentation in Myeloid Cells and during Tuberculosis
- High-Resolution Analysis of Parent-of-Origin Allelic Expression in the Arabidopsis Endosperm
- Specific SKN-1/Nrf Stress Responses to Perturbations in Translation Elongation and Proteasome Activity
- Graded Nodal/Activin Signaling Titrates Conversion of Quantitative Phospho-Smad2 Levels into Qualitative Embryonic Stem Cell Fate Decisions
- Genome-Wide Analysis Reveals PADI4 Cooperates with Elk-1 to Activate Expression in Breast Cancer Cells
- Trait Variation in Yeast Is Defined by Population History
- Meiosis-Specific Loading of the Centromere-Specific Histone CENH3 in
- A Genome-Wide Survey of Imprinted Genes in Rice Seeds Reveals Imprinting Primarily Occurs in the Endosperm
- Multiple Regulatory Mechanisms to Inhibit Untimely Initiation of DNA Replication Are Important for Stable Genome Maintenance
- SIRT1 Promotes N-Myc Oncogenesis through a Positive Feedback Loop Involving the Effects of MKP3 and ERK on N-Myc Protein Stability
- Bacteriophage Crosstalk: Coordination of Prophage Induction by Trans-Acting Antirepressors
- Role of the Single-Stranded DNA–Binding Protein SsbB in Pneumococcal Transformation: Maintenance of a Reservoir for Genetic Plasticity
- Genomic Convergence among ERRα, PROX1, and BMAL1 in the Control of Metabolic Clock Outputs
- Genome-Wide Association of Bipolar Disorder Suggests an Enrichment of Replicable Associations in Regions near Genes
- Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese Population
- DNA Ligase III Promotes Alternative Nonhomologous End-Joining during Chromosomal Translocation Formation
- Differential Effects of and Risk Variants on Association with Diabetic ESRD in African Americans
- Finished Genome of the Fungal Wheat Pathogen Reveals Dispensome Structure, Chromosome Plasticity, and Stealth Pathogenesis
- Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
- Extracellular Matrix Dynamics in Hepatocarcinogenesis: a Comparative Proteomics Study of Transgenic and Null Mouse Models
- Integrating 5-Hydroxymethylcytosine into the Epigenomic Landscape of Human Embryonic Stem Cells
- Vive La Différence: An Interview with Catherine Dulac
- Multiple Loci Are Associated with White Blood Cell Phenotypes
- Nuclear Accumulation of Stress Response mRNAs Contributes to the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- A New Mutation Affecting FRQ-Less Rhythms in the Circadian System of
- Cryptic Transcription Mediates Repression of Subtelomeric Metal Homeostasis Genes
- A New Isoform of the Histone Demethylase JMJD2A/KDM4A Is Required for Skeletal Muscle Differentiation
- Genetic Determinants of Lipid Traits in Diverse Populations from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- A Genome-Wide RNAi Screen for Factors Involved in Neuronal Specification in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Statistical Inference on the Mechanisms of Genome Evolution
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



