-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIdentification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese Population
White blood cells (WBCs) mediate immune systems and consist of various subtypes with distinct roles. Elucidation of the mechanism that regulates the counts of the WBC subtypes would provide useful insights into both the etiology of the immune system and disease pathogenesis. In this study, we report results of genome-wide association studies (GWAS) and a replication study for the counts of the 5 main WBC subtypes (neutrophils, lymphocytes, monocytes, basophils, and eosinophils) using 14,792 Japanese subjects enrolled in the BioBank Japan Project. We identified 12 significantly associated loci that satisfied the genome-wide significance threshold of P<5.0×10−8, of which 9 loci were novel (the CDK6 locus for the neutrophil count; the ITGA4, MLZE, STXBP6 loci, and the MHC region for the monocyte count; the SLC45A3-NUCKS1, GATA2, NAALAD2, ERG loci for the basophil count). We further evaluated associations in the identified loci using 15,600 subjects from Caucasian populations. These WBC subtype-related loci demonstrated a variety of patterns of pleiotropic associations within the WBC subtypes, or with total WBC count, platelet count, or red blood cell-related traits (n = 30,454), which suggests unique and common functional roles of these loci in the processes of hematopoiesis. This study should contribute to the understanding of the genetic backgrounds of the WBC subtypes and hematological traits.
Published in the journal: . PLoS Genet 7(6): e32767. doi:10.1371/journal.pgen.1002067
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002067Summary
White blood cells (WBCs) mediate immune systems and consist of various subtypes with distinct roles. Elucidation of the mechanism that regulates the counts of the WBC subtypes would provide useful insights into both the etiology of the immune system and disease pathogenesis. In this study, we report results of genome-wide association studies (GWAS) and a replication study for the counts of the 5 main WBC subtypes (neutrophils, lymphocytes, monocytes, basophils, and eosinophils) using 14,792 Japanese subjects enrolled in the BioBank Japan Project. We identified 12 significantly associated loci that satisfied the genome-wide significance threshold of P<5.0×10−8, of which 9 loci were novel (the CDK6 locus for the neutrophil count; the ITGA4, MLZE, STXBP6 loci, and the MHC region for the monocyte count; the SLC45A3-NUCKS1, GATA2, NAALAD2, ERG loci for the basophil count). We further evaluated associations in the identified loci using 15,600 subjects from Caucasian populations. These WBC subtype-related loci demonstrated a variety of patterns of pleiotropic associations within the WBC subtypes, or with total WBC count, platelet count, or red blood cell-related traits (n = 30,454), which suggests unique and common functional roles of these loci in the processes of hematopoiesis. This study should contribute to the understanding of the genetic backgrounds of the WBC subtypes and hematological traits.
Introduction
White blood cells (WBCs) mediate immune systems, and play essential roles in defending the body against invading foreign microorganisms [1]. WBCs consist of a variety of cells that mediate diverse roles, and are morphologically classified into 5 main subtypes: neutrophils, lymphocytes, monocytes, basophils, and eosinophils [1]. A number of previous studies have demonstrated significant contributions of these WBC subtypes to the regulation of innate and adaptive immune systems [2]–[6]. Since the number of WBC subtypes circulating in peripheral blood are tightly regulated, and abnormality in their numbers are closely linked to the presence and prognosis of diseases [2]–[6], the counts of WBC subtypes are widely used as important blood markers in medical treatment. Therefore, elucidation of the mechanism(s) that regulates the counts of WBC subtypes would have substantial clinical impact and would provide new insights into the etiology of the immune system.
WBC subtypes are known to be heritable traits and several epidemiological studies have suggested the existence of genetic factors that explain the variations in the counts of WBC subtypes, as well as a number of common environmental factors such as age, sex, and smoking [7]–[10]. Recently, genome-wide association studies (GWAS) have identified a number of genetic loci that affect hematological traits, but most of these identified loci were determined to be associated with red blood cell (RBC) or platelet (PLT) -related traits or total WBC count [11]–[17]. However, studies investigating WBC subtypes have yet to be further assessed [18]–[21]. Moreover, it is also of interest to evaluate whether ethnic differences underlie the genetic backgrounds that affect hematological traits.
Previous studies for hematological traits have also suggested that several genetic loci have pleiotropic associations with other hematological traits [15]–[18]. Therefore, it is of interest whether the WBC subtype-associated genetic loci have pleiotropic associations with counts of other WBC subtypes, RBCs, and PLTs, when considering the biological roles of the loci in the processes of hematopoiesis.
In this study, we report a large-scale GWAS for the counts of the WBC subtypes in 14,792 Japanese subjects enrolled in the BioBank Japan Project [22]. Subsequently, we performed pleiotropic association analysis of the identified WBC subtype-associated loci. We evaluated the associations of the loci identified in the Japanese population using data obtained by cohorts of Caucasian populations [23], in order to highlight the ethnically common and divergent genetic backgrounds of WBC subtypes.
Results
GWAS
In the GWAS for the WBC subtypes, we enrolled 8,794 Japanese subjects. The counts of the 5 main WBC subtypes (neutrophils, lymphocytes, monocytes, basophils, and eosinophils) of the subjects were collected from medical records and summarized in Table S1. We found moderate degree of correlation (R2>0.1) between the neutrophil and monocyte counts, between the basophil and lymphocyte counts, and between the basophil and eosinophil counts (Table S2). These results were considered to be compatible with previous reports [7]–[9]. To relatively compare the effect sizes on the traits, we carried out normalization of the counts of the respective WBC subtypes. The subjects with normalized values beyond ±4SD were discarded, which accounted for less than 0.5% of the total subjects.
Genotyping was performed with over 590,000 SNP markers using Illumina610-Quad Genotyping BeadChip (Illumina, CA, USA). We applied stringent quality control criteria, including principal component analysis (PCA) [24] to evaluate potential population stratification, and obtained genotype data for 481,110 autosomal SNPs. To extend the genomic coverage, we subsequently performed the whole-genome imputation of the SNPs, using HapMap Phase II genotype data of Japanese (JPT) and Han Chinese (CHB) individuals as references [25]. After the imputation, 2,178,645 autosomal SNPs that satisfied the criteria of a minor allele frequency (MAF) ≥0.01 and an imputation score (Rsq value by MACH software [26]) ≥0.7 were obtained. The associations of these imputed SNPs with the transformed values of the counts of WBC subtypes were evaluated using a linear regression model.
Quantile-Quantile plots of P-values indicated remarkable departures from the null hypothesis in their tails, except for the lymphocyte count (Figure S1). Inflation factors of P-values, λGC [27], were as low as 1.024–1.038, which suggested no substantial population stratification existed in our study population as previously anticipated for the Japanese population [28]. We identified 10 significant associations that satisfied the genome-wide significance threshold of P<5.0×10−8 in the GWAS for the counts of neutrophils, monocytes, basophils and eosinophils (Figure 1 and Table S3). We also evaluated the associations in the previously-reported WBC subtype-associated loci, and observed significant associations in six of these loci (the PSMD3-CSF3 and PLCB4 loci for the neutrophil counts [21], the MHC region for the lymphocyte counts [20], the IL1RL1, IKZF2, HBS1L-MYB loci for the eosinophil counts [18], P<0.005; Table S4).
Fig. 1. Manhattan plots of the GWAS for the WBC subtypes. 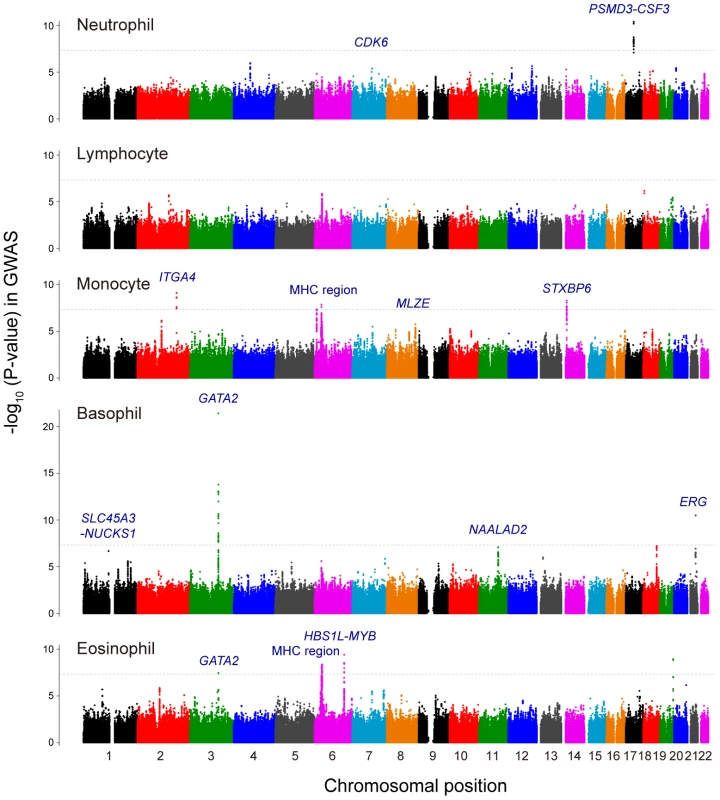
Manhattan plots showing the -log10 (P-values) of the SNPs in the GWAS for neutrophil, lymphocyte, monocyte, basophil, and eosinophil counts. The genetic loci that satisfied the genome-wide significance threshold of P<5.0×10−8 in the combined study of the GWAS and the replication study were labeled in each of the traits. The gray horizontal line represents the threshold of P = 5.0×10−8. Combined analysis of GWAS and replication study
We subsequently performed a replication study using independent 5,998 Japanese subjects, and further evaluated the associations of the loci by combining the results of the GWAS and the replication study. We selected a total of 36 genetic loci that showed P<5.0×10−6 in the GWAS for any of the WBC subtypes as the candidates for inclusion in the replication study. As a result of combined study, we finally identified 12 genetic loci that satisfied genome-wide significance threshold of P<5.0×10−8. Of these, the top-associated SNPs in 2 loci were genotyped and the SNPs in 10 loci were imputed with imputation score of Rsq>0.90. Specifically, we found 2, 4, 4, and 3 loci for neutrophil, monocyte, basophil, and eosinophil counts, respectively (Table 1, Table S3, and Figure 1). One locus was shared between basophil and eosinophil counts. On the other hand, no significant association was detected for the lymphocyte count in the combined study.
Tab. 1. Significantly associated SNPs in the GWAS for WBC subtypes. 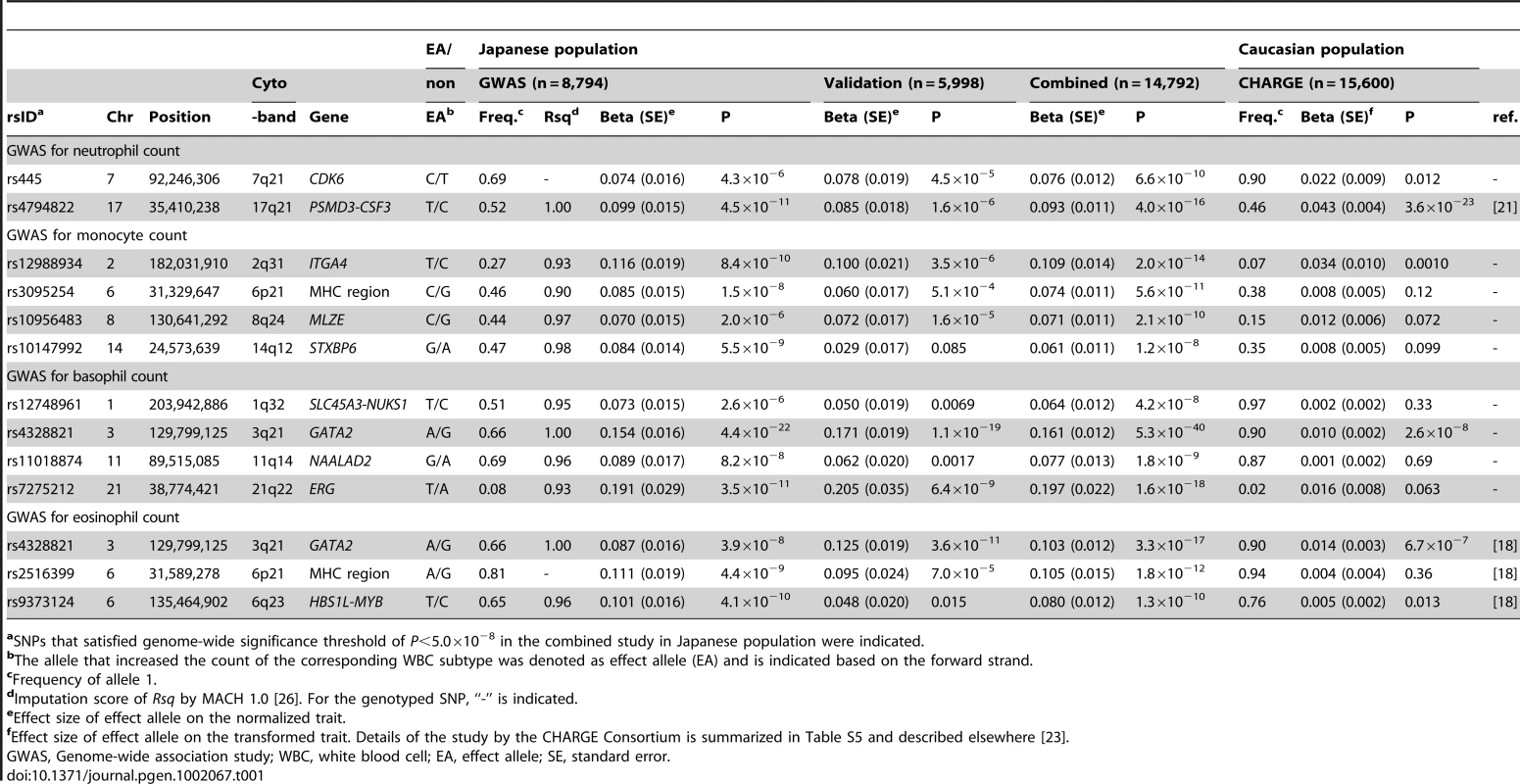
aSNPs that satisfied genome-wide significance threshold of P<5.0×10−8 in the combined study in Japanese population were indicated. Among the identified loci in the combined study, 4 loci were the replication for the previous studies: rs4794822 in the PSMD3-CSF3 locus for the neutrophil count [21], rs4328821 in the GATA2 locus, rs2516399 in the MHC region, and rs9373124 in the HBS1L-MYB locus for the eosinophil count [18]. Associations in the other 9 loci were novel findings to our knowledge (Figure 2). Specifically, we identified associations in one locus for the neutrophil count (rs445 in the CDK6 locus), 4 loci for the monocyte counts (rs12988934 in the ITGA4 locus, rs3095254 in the MHC region, rs10956483 in the MLZE locus, and rs10147992 in the STXBP6 loci), and 4 loci for the basophil count (rs12748961 in the SLC45A3-NUCKS1 locus, rs4328821 in the GATA2 locus, rs11018874 in the NAALAD2 locus, and rs7275212 in the ERG loci) (Figure 2). Of the associated SNPs located in the MHC region, rs3095254 was reported to be in linkage disequilibrium (LD) with HLA-Cw*0702 allele (D′ = 1 and r2 = 0.24), and rs2516399 was in LD with HLA-DRB1*0405 and HLA-DQB1*0401(D′>0.7 and r2>0.2) [29].
Fig. 2. Regional plots of the novel genetic loci associated with the WBC subtypes. 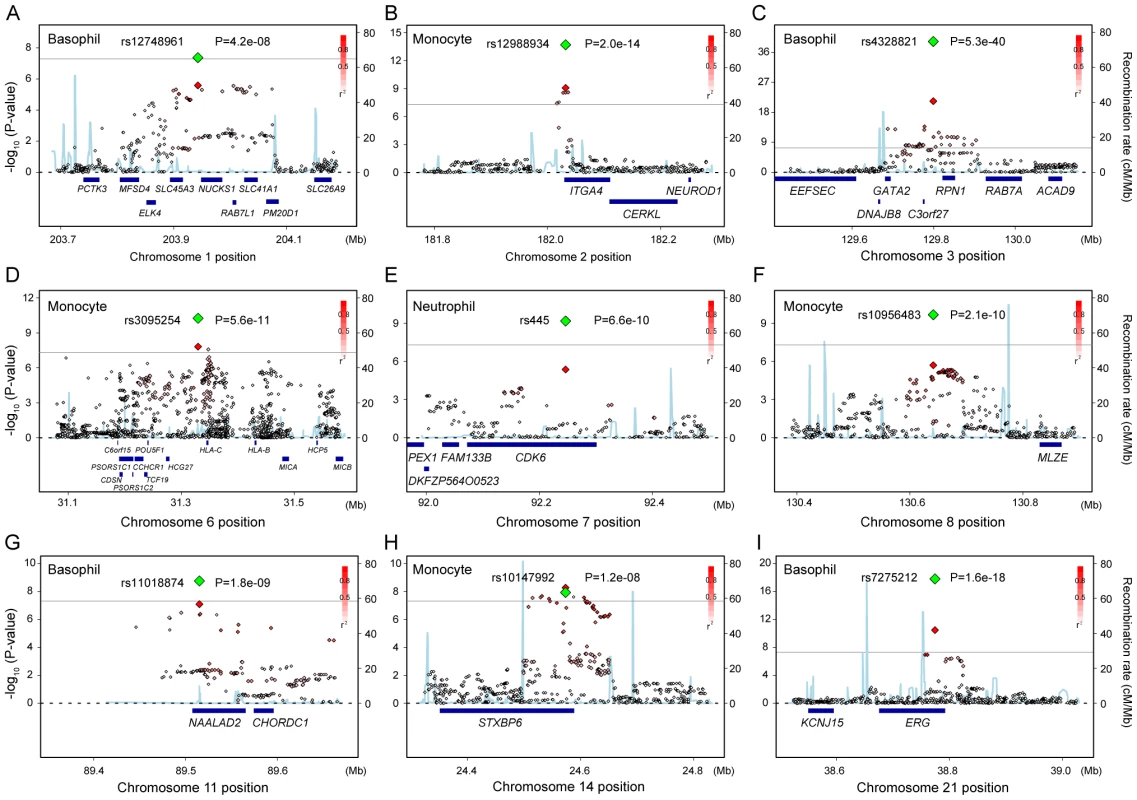
(A–I) Regional plots of the SNPs in the 9 novel loci identified in the GWAS for neutrophil, lymphocyte, monocyte, basophil, and eosinophil counts. Diamond-shaped dots represent -log10 (P-values) of the SNPs. The green dot indicates the P-value of the most significantly associated SNP in each of the loci in the combined study, and the red dot indicates its P-value in the GWAS. The density of the red color in the small-sized dots represents the r2 value with the most significantly associated SNP of the large-sized red dot. The blue line shows the recombination rates given by the HapMap database. The gray horizontal line represents the genome-wide significance threshold of P = 5.0×10−8. The lower part indicates the RefSeq genes in the locus. The plots were drawn using SNAP, version 2.1 [48]. Associations of the identified loci in Caucasian populations
To highlight the ethnically common and divergent genetic backgrounds of the WBC subtypes, the associations of the 12 identified loci were further evaluated in Caucasian populations by using 15,600 subjects in cohorts of the CHARGE Consortium (Table S5) [23]. The CHARGE Consortium consists of multiple community-based and prospectively designed cohorts from the United States and Europe [30] and has performed association studies for hematological traits, including the WBC subtypes [23]. We observed the same directional effects of the alleles in all 12 loci evaluated in the CHARGE Consortium. Furthermore, significant associations were observed in 4 loci (the PSMD3-CSF3 locus for the neutrophil count, the ITGA4 locus for the monocyte count, and the GATA2 locus for both the basophil and eosinophil counts; P<0.004). We also observed the suggestive associations in 2 loci (the CDK6 locus for the neutrophil count and the HBS1L-MYB locus for the eosinophil count; P<0.05).
Pleiotropic association study for WBC subtype-associated loci
We next evaluated the pleiotropic associations of the WBC subtype-associated loci. For the top-associated SNPs in each of the loci that indicated significant associations in our study (P<5.0×10−8), we evaluated the associations with the counts of the other WBC subtypes, total WBC count, RBC-related traits (RBC count, hemoglobin [Hb], hematocrit [Ht], mean corpuscular hemoglobin [MCH], mean corpuscular hemoglobin concentration [MCHC], mean corpuscular volume [MCV]), and PLT count using 30,454 Japanese subjects (Table S6).
We found various patterns of pleiotropic associations for the loci associated with the WBC subtypes (Figure 3A and Figure 4). Three loci demonstrated specific associations with the original WBC subtypes identified in the GWAS (rs12748961 in the SLC45A3-NUCKS1 locus, rs12988934 in the ITGA4 locus, rs11018874 in the NAALAD2 locus), although 9 other loci demonstrated pleiotropic associations with other traits. The most pleiotropic associations were observed in the HBS1L-MYB locus, which indicated significant associations with all of the evaluated hematological traits (P<0.005). The T allele of rs9373124 that increased the eosinophil count also increased the counts of the other WBC subtypes, total WBC count, RBC count, the Hb and Ht levels, and conversely decreased MCH, MCHC, MCV, and PLT count, validating its substantial role in hematopoiesis [15], [31].
Fig. 3. Pleiotropic associations of the genetic loci associated with the WBC subtypes. 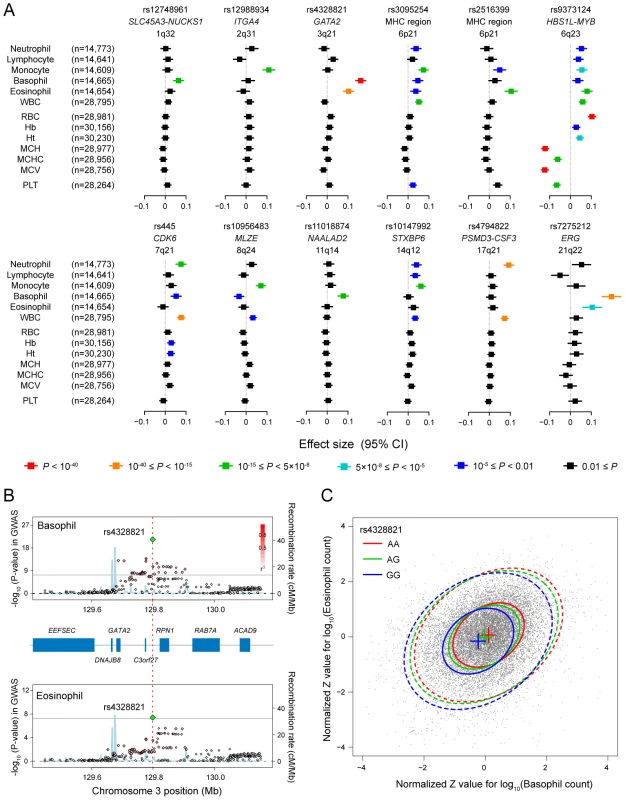
(A) Associations of the SNPs identified in the GWAS for the WBC subtypes with other WBC subtypes and hematological traits. Effect sizes of each SNP on the normalized traits are aligned vertically in each of the loci, and their color corresponds to the P-values of the associations according to the legend. WBC, total white blood cell count; RBC, red blood cell count; Hb, hemoglobin; Ht, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet count; CI confidence interval. (B) Regional plots of the SNPs in the GWAS for the basophil (upper) and eosinophil (lower) counts in the GATA2 locus. Diamond-shaped dots represent -log10 (P-values) of the SNPs in the GWAS; green indicates the most significantly associated SNP, and the density of the red color represents the r2 value with the most significantly associated SNP. The blue line shows recombination rates given by the HapMap database. The gray horizontal line represents the genome-wide significance threshold of P = 5.0×10−8. The middle part indicates the RefSeq genes in the locus. The red vertical dashed line represents the concordance of the peaks of the association at rs4328821 in the GWAS. (C) Scatter plot of the subjects enrolled in the GWAS and the replication study based on the normalized Z values for the basophil and eosinophil counts. The center, 50% probable ellipse, and 95% probable ellipse of the subjects with AA (red) / AG (green) / GG (blue) genotypes of rs4328821 are indicated as crosses, solid ellipses, and dashed ellipses, respectively. Fig. 4. Venn diagram of the pleiotropic associations among the WBC subtypes. 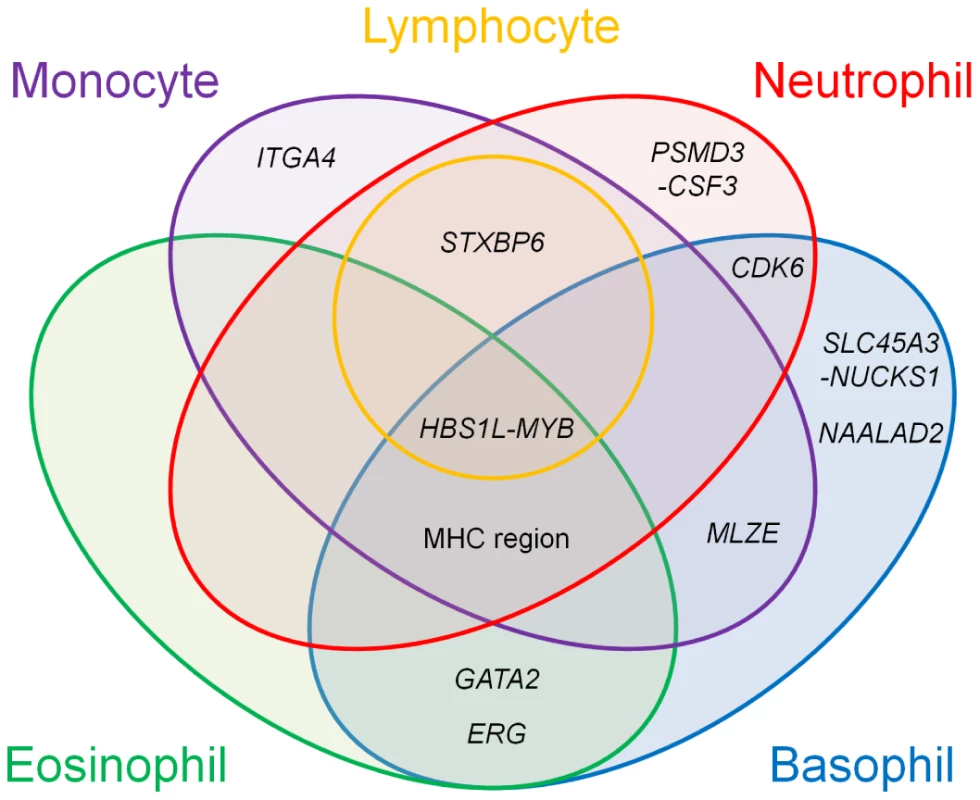
Genetic loci identified in the GWAS for the WBC subtypes are classified based on the results of the pleiotropic association study among the WBC subtypes. The colors in the Venn diagram (red, orange, purple, aqua, and green) correspond to each of the WBC subtypes (neutrophils, lymphocytes, monocytes, basophils, and eosinophils, respectively). Pleiotropic associations with P<0.01 are included. In the GATA2 locus, we observed significant associations with both the basophil and eosinophil counts (P<5.0×10−8; Figure 3B). This locus encompassed several genes, although GATA2 seemed to be most responsible for regulating eosinophils and basophils from a functional standpoint [3], [32]. Interestingly, the peaks of the associations of the SNPs in the basophil and eosinophil GWAS showed concordance at rs4328821. Possession of the A allele of rs4328821 increased both the basophil and eosinophil counts (Figure 3C). The subjects who were homozygous for the A allele had 1.28-fold and 1.19-fold higher basophil and eosinophil counts, respectively, compared with the corresponding levels of the subjects who were homozygous for the G allele. Moreover, rs4328821 significantly explained 2.7% of the correlation between the basophil and eosinophil counts (permutation P<1.0×10−9). Upon combining the effects of the SNPs in the identified loci, up to 2.1% of the variations of the counts of the WBC subtypes was explained, and up to 8.0% of the correlation between the WBC subtypes was explained (Table S2).
Discussion
Through a GWAS and a replication study consisting of 14,792 Japanese subjects, our study identified 12 loci that were significantly associated with the counts of WBC subtypes. Among the identified loci, 9 loci are reported for the first time in this study. The identified loci demonstrated a variety of patterns of pleiotropic associations within the WBC subtypes and/or with total WBC count, RBC-related traits, and PLT count, which suggest they have both unique and common roles in the processes of hematopoiesis. Comparison of the loci identified in the Japanese population with those in Caucasian populations demonstrated the ethnically common and divergent genetic backgrounds of the various WBC subtypes.
Two loci identified in the GWAS for the neutrophil count (the CDK6 and PSMD3-CSF3 loci) have previously been reported to be associated with the total WBC count [16], [17]. Since neutrophils are the most abundant subtype of WBCs and typically comprise 50–70% of the total WBC count, the associations between these loci with the total WBC count would have reflected the associations with the neutrophil count [21].
We identified 4 novel loci associated with monocyte count (the ITGA4, MLZE, and STXBP6 locus and the MHC region). The landmark SNP in the MHC region was located near the HLA-C gene and in moderate LD with the particular HLA-C allele, which belongs to MHC class I molecules. ITGA4 encodes the α4 chain of the integrins, which mediate migration of the WBCs [33]. Previous reports have demonstrated that STXBP6 (also known as amisyn) binds to the components of the SNARE complex, which mediates membrane fusions including phagocytosis [34], [35]. In response to inflammation, monocytes differentiate into macrophages and migrate into affected tissues of inflammation, and subsequently perform phagocytosis and antigen presentation using MHC molecules expressed on the cell surface [6]. Therefore, associations of the SNPs in these loci with the monocyte count would be plausible from a biological perspective. Recently, clinical benefits of inhibition of α4 integrin have been demonstrated in the treatment of autoimmune diseases [36]. Although further functional investigation is necessary, the SNP in the ITGA4 locus that was identified in our GWAS may be a promising target for pharmacogenomics of anti-α4 integrin therapy. MLZE (also known as GCDMC) belongs to the Gasdermin family of genes [37], and its role in the regulation of the monocyte count should be further explored.
In addition, we identified 4 novel loci associated with the basophil count (the SLC45A3-NUCKS1, GATA2, NAALAD2, and ERG loci) and replicated associations in 3 previously-reported loci associated with the eosinophil count (the GATA2 and HBS1L-MYB loci and the MHC region). Basophils and eosinophils coordinately mediate allergic inflammation [3]–[5], and the correlation of these counts [7] suggested the existence of genetic factors that are shared between them. Our pleiotropic study demonstrated overlap of the associated loci between the basophil and eosinophil counts, which was most highlighted in the GATA2 locus. GATA2 is a well-known zinc-finger transcription factor and plays an essential role in hematopoiesis, particularly in the regulation of basophils and eosinophils [3], [32]. The landmark SNP in the GATA2 locus was concordant in the GWAS for both the basophil and eosinophil counts, and significantly explained part of their correlation in the counts. Pleiotropic associations of the SNP with the basophil and eosinophil counts were further replicated in the Caucasian populations. These results suggested an ethnically-shared substantial functional role of the SNP in the etiology of GATA2.
ERG encodes a member of the Ets family of transcription factors, and is known to be included in the Down syndrome critical region on chromosome 21 [38]. Although its functional role(s) in the regulation of basophils has not been investigated to date, an essential role of ERG for definitive hematopoiesis has been demonstrated [38]. The SLC45A3-NUCKS1 locus in the present study encompassed several genes, and we submit that the functional origin of this locus should be further investigated. Interestingly, the fusion transcript of SLC45A3 and ERG is observed in prostate cancers, which have been characterized by the overexpression of ERG mRNA [39], [40], although we did not find any significant gene-gene interaction in the SNPs in these two loci for basophil count (data not shown). Finally, NAALAD2 is a member of the N-acetylated α-linked acidic dipeptidase gene family [41], and its role in the regulation of the basophil counts should be a topic to be further investigated in the future.
In contrast to the WBC subtypes mentioned above, no significant association was detected for the lymphocyte count. One probable explanation for this finding is that lymphocytes can be further divided into a variety of subsets, such as natural killer (NK) cells, T cells, and B cells, which were not examined specifically in this study. Therefore, future GWAS that focus on those specific subsets of lymphocytes [20] are necessary to efficiently investigate the genetic backgrounds of the lymphocytes.
Several points about this study bear discussion. First, since our study populations consisted of the disease patients, it would be useful to assess the possibility that the disease status might have confounded the results. When the respective disease groups were analyzed separately and evaluated through meta-analysis, all the identified loci satisfied the significant associations (P<5.0×10−8) without significant heterogeneities of the effects (α = 0.01). None of the identified loci has been reported to be associated with the risk of the diseases enrolled in the study population, except for the MHC region with Rheumatoid Arthritis (RA) [42]. Moreover, after the subjects affected with RA were excluded, the significant associations of the SNPs in the MHC region with the monocyte and eosinophil counts were observed (P = 1.7×10−10 for rs3095254 and P = 9.6×10−12 for rs2516399, respectively). It would be of note that we observed concordance of the associations in the identified loci with those by CHARGE consortium, which is consisted of multiple community-based and prospective cohorts incorporating normal populations. Although further validation study using non-affected subjects would be desirable, these observations suggested that the utilization of disease patients have not induced substantial bias in our study.
Second, the counts of the WBC subtypes were based on medical records. Although the data collection protocol is enormously standardized [22], there is a possibility that unstandardized discrepancy among the medical institutes might induce bias in the phenotype distributions and impair statistical power of the study.
Third, the explained proportion of the WBC subtypes by the identified loci would be estimated conservatively. Because of the stringent significance threshold adopted in the study, a number of associated loci with moderate effect sizes would be still unidentified. Further approaches, such as considering the entire SNPs simultaneously [43], are necessary for the accurate estimation of the explained variations. Candidate gene analysis based on the biological pathway of the WBC subtypes would also be a promising approach to uncover these unidentified loci [44].
Fourth, since the correlation among the traits could modulate the pattern of the pleiotropic associations, further distinction of actual pleiotropic associations from simple associations induced by the correlations would be a topic to be investigated.
In summary, our study identified 9 novel loci that are associated with the counts of the WBC subtypes. The pleiotropic association study of the identified loci demonstrated unique and common genetic backgrounds underlying the WBC subtypes. Our study should contribute to the general understanding of the etiology and regulation of the WBC subtypes.
Materials and Methods
Subjects
The subjects enrolled in the GWAS, and in the replication study for WBC subtypes (n = 14,792), and in the pleiotropic association study for hematological traits (n = 30,454) consisted of patients that were classified into 27 disease groups (Tables S1 and S6). The subjects in the pleiotropic association study included the subjects in the GWAS and the replication study. All subjects were collected under the support of the BioBank Japan Projects [22]. Subjects who were determined to be of non-Japanese origin by either self-report or by PCA in GWAS were excluded from analysis. Some of the subjects in this study have also been included in our previous studies [17], [21], [45], [46]. All participants provided written informed consent as approved by the ethical committees of the BioBank Japan Project [22] and the University of Tokyo. Clinical information of the subjects including age, gender, and smoking history were collected by self-report on the questionnaire. The laboratory data including the counts of the WBC subtypes and other hematological traits were collected from medical records by the professional medical coordinators according to the standardized protocol [22]. The details of the study enrolled by the CHARGE Consortium, including subject details and the study design, are described at length elsewhere [23], and are summarized in Table S5.
Genotyping and quality control
In the GWAS for the WBC subtypes, 592,232 SNPs were genotyped for 8,943 subjects using Illumina HumanHap610-Quad Genotyping BeadChip. We excluded 77 subjects with call rates <0.98 in the process of genotyping. After this initial exclusion, SNPs with call rates <0.99 or with ambiguous clustering of the intensity plots, or non-autosomal SNPs, were excluded. We excluded 67 closely related subjects based on the identity-by-descent (IBD), which was estimated using the “–genome” option implemented in PLINK version 1.06 [47]. For each pair with a 1st or 2nd degree of kinship, we excluded the one member of the pair with lower call rates than the other. We then excluded subjects whose ancestries were estimated to be distinct from East-Asian populations using PCA performed by EIGENSTRAT version 2.0 [24]. We performed PCA for the genotype data of our GWAS along with the genotype data of Phase II HapMap populations (unrelated European (CEU), African (YRU), and East-Asian (JPT + CHB) individuals) (release 24) [25]. Based on the PCA plot of the subjects, we visually identified and excluded 5 outliers in terms of ancestry from JPT + CHB clusters. Subsequently, the SNPs with MAF <0.01 or with an exact P-value of the Hardy-Weinberg equilibrium test <1.0×10−7 were excluded. Finally, we obtained 481,110 SNPs for 8,794 subjects.
After the quality control criteria mentioned above were applied, genotype imputation was performed using MACH 1.0 [26] in a two-step procedure [46]. The genotype data of Phase II HapMap JPT and CHB individuals (release 24) [25] were adopted as references. In the first step of the imputation, recombination and error rate maps were estimated using 500 randomly selected subjects from those enrolled in the GWAS. In the second step, genotype imputation of all subjects was performed using the estimated recombination and error rate maps. Quality control filters of MAF ≥0.01 and Rsq values ≥0.7 were applied for the imputed SNPs.
The genotype data of the SNPs enrolled in the replication or pleiotropic association study were obtained from the genome-wide screening data of the BioBank Japan Project [22]. Genotyping was performed using either Illumina HumanHap550v3 Genotyping BeadChip or Illumina HumanHap610-Quad Genotyping BeadChip, and the same quality control filters and imputation procedure were applied.
Statistical analysis
The common log-transformed values of the counts of each of the WBC subtypes were adjusted for gender, age, smoking history, and the affection statuses of the subjects with the disease groups (Table S1), using linear regression by R statistical software (version 2.11.0). Then the residuals were normalized as Z scores, and the subjects with Z score >4.0 or <−4.0 were excluded in each of the traits. Associations of the SNPs with the counts of the WBC subtypes were assessed by linear regression assuming the additive effects of the allele dosages on the Z scores, using mach2qtl software [26]. In the replication and pleiotropic association studies, the association of the SNPs with the normalized residuals were also evaluated by the linear regression as univariate analysis for each of the phenotypes. The transformation methods used for the hematological traits in the pleiotropic association study are summarized in Table S6. Combined study of the results of the GWAS and the replication study was performed using an inverse-variance method from the summary statistics of beta and standard error (SE). Through the combined study of the GWAS and the replication study, the locus which satisfied the genome-wide significance threshold of P<5.0×10−8 was considered to be significant. We did not account for multiple comparisons among the traits. These significantly associated loci were subsequently enrolled in the pleiotropic association study. For the selection of the loci that were evaluated in the replication study, we adopted less stringent threshold of P<5.0×10−6 to include potentially associated loci. For the evaluation of the identified loci using the Caucasian populations, Bonferroni correction based on the number of the evaluated loci were adopted (α = 0.05, n = 12, P<0.004). LD between the SNPs in the MHC region and HLA alleles were estimated using the genotype data of the SNPs and the high-resolution HLA alleles for Phase II HapMap JPT and CHB individuals [25], [29]. Explained proportions of the variations of the WBC subtypes by the combination of the associated SNPs were estimated based on the differences of the coefficient of determination, R2, in the multivariate linear regression model for common-log transformed counts of the respective WBC subtypes, including the associated SNPs as covariates, and the model additionally including age, gender, smoking history, and the affection statuses of the subjects as covariates. Explained proportions of the correlation between the two WBC subtypes by the associated SNPs were estimated based on the following statistics: (R2resi1 - R2resi2) / R2nomi, where R2nomi is the R2 between the log-transformed values of the counts of the WBC subtypes, R2resi1 is the R2 between the residuals of the values adjusted for gender, age, smoking history, and the affection statuses of the subjects, and R2resi2 is the R2 between the residuals of the values adjusted for gender, age, smoking history, the affection statuses of the subjects, and the SNPs. Significance of the statistics was evaluated using permutation procedure (× 109 iteration steps).
Web resources
The URLs for data presented herein are as follows.
BioBank Japan Project, http://biobankjp.org
MACH and mach2qtl software, http://www.sph.umich.edu/csg/abecasis/MACH/index.html
International HapMap Project, http://www.hapmap.org
PLINK software, http://pngu.mgh.harvard.edu/~purcell/plink/index.shtml
EIGENSTRAT software, http://genepath.med.harvard.edu/~reich/Software.htm
R statistical software, http://cran.r-project.org
SNAP, http://www.broadinstitute.org/mpg/snap/index.php
Supporting Information
Zdroje
1. RonaldHEdwardJBSanfordJSBruceFLeslieE 2009 Hematology: basic principles and practice (5th edition) Churchill: Livingstone/Elsevie.
2. FahyJV 2009 Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc 6 256 259
3. ArinobuYIwasakiHAkashiK 2009 Origin of basophils and mast cells. Allergol Int 58 21 28
4. SullivanBMLocksleyRM 2009 Basophils: a nonredundant contributor to host immunity. Immunity 30 12 20
5. GauvreauGMEllisAKDenburgJA 2009 Haemopoietic processes in allergic disease: eosinophil/basophil development. Clin Exp Allergy 39 1297 1306
6. Ziegler-HeitbrockLAncutaPCroweSDalodMGrauV 2010 Nomenclature of monocytes and dendritic cells in blood. Blood 116 e74 e80
7. ElvebackLGullyRJHalbergFHamerstonO 1956 Correlation of absolute basophil and eosinophil counts in blood from institutionalized human subjects. J Appl Physiol 9 205 207
8. EvansDMFrazerIHMartinNG 1999 Genetic and environmental causes of variation in basal levels of blood cells. Twin Res 2 250 257
9. HallMAAhmadiKRNormanPSniederHMacGregorAJ 2000 Genetic influence on peripheral blood T lymphocyte levels. Genes Immun 1 423 427
10. AndreoliCGreggEOPuntoniRGobbiVNunziataA 2010 Cross-sectional study of biomarkers of exposure and biological effect on monozygotic twins discordant for smoking. Clin Chem Lab Med 2010 Nov 18
11. MeisingerCProkischHGiegerCSoranzoNMehtaD 2009 A genome-wide association study identifies three loci associated with mean platelet volume. Am J Hum Genet 84 66 71
12. SoranzoNRendonAGiegerCJonesCIWatkinsNA 2009 A novel variant on chromosome 7q22.3 associated with mean platelet volume, counts, and function. Blood 113 3831 3837
13. BenyaminBFerreiraMAWillemsenGGordonSMiddelbergRP 2009 Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat Genet 41 1173 1175
14. ChambersJCZhangWLiYSehmiJWassMN 2009 Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat Genet 41 1170 1172
15. GaneshSKZakaiNAvan RooijFJSoranzoNSmithAV 2009 Multiple loci influence erythrocyte phenotypes in the CHARGE Consortium. Nat Genet 41 1191 1198
16. SoranzoNSpectorTDManginoMKuhnelBRendonA 2009 A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nat Genet 41 1182 1190
17. KamataniYMatsudaKOkadaYKuboMHosonoN 2010 Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat Genet 42 210 215
18. GudbjartssonDFBjornsdottirUSHalapiEHelgadottirASulemP 2009 Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet 41 342 347
19. FerreiraMAHottengaJJWarringtonNMMedlandSEWillemsenG 2009 Sequence variants in three loci influence monocyte counts and erythrocyte volume. Am J Hum Genet 85 745 749
20. FerreiraMAManginoMBrummeCJZhaoZZMedlandSE 2010 Quantitative trait loci for CD4:CD8 lymphocyte ratio are associated with risk of type 1 diabetes and HIV-1 immune control. Am J Hum Genet 86 88 92
21. OkadaYKamataniYTakahashiAMatsudaKHosonoN 2010 Common variations in PSMD3-CSF3 and PLCB4 are associated with neutrophil count. Hum Mol Genet 19 2079 2085
22. NakamuraY 2007 The BioBank Japan Project. Clin Adv Hematol Oncol 5 696 697
23. MichaelANDavidJCouperToshikoTanakaFrankJAvan RooijMing HueiChen 2113 Multiple loci are associated with white blood cell phenotypes. PLoS Genet 7 e1002113 doi:10.1371/journal.pgen.1002113
24. PriceALPattersonNJPlengeRMWeinblattMEShadickNA 2006 Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 904 909
25. The International HapMap Consortium 2003 The International HapMap Project. Nature 426 789 796
26. LiYWillerCSannaSAbecasisG 2009 Genotype imputation. Annu Rev Genomics Hum Genet 10 387 406
27. DevlinBRoederK 1999 Genomic control for association studies. Biometrics 55 997 1004
28. Yamaguchi-KabataYNakazonoKTakahashiASaitoSHosonoN 2008 Japanese population structure, based on SNP genotypes from 7003 individuals compared to other ethnic groups: effects on population-based association studies. Am J Hum Genet 83 445 456
29. de BakkerPIMcVeanGSabetiPCMirettiMMGreenT 2006 A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat Genet 38 1166 1172
30. PsatyBMO'DonnellCJGudnasonVLunettaKLFolsomAR 2009 Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet 2 73 80
31. WahlbergKJiangJRooksHJawaidKMatsudaF 2009 The HBS1L-MYB intergenic interval associated with elevated HbF levels shows characteristics of a distal regulatory region in erythroid cells. Blood 114 1254 1262
32. ZonLIYamaguchiYYeeKAlbeeEAKimuraA 1993 Expression of mRNA for the GATA-binding proteins in human eosinophils and basophils: potential role in gene transcription. Blood 81 3234 3241
33. RoseDMHanJGinsbergMH 2002 Alpha4 integrins and the immune response. Immunol Rev 186 118 124
34. ScalesSJHesserBAMasudaESSchellerRH 2002 Amisyn, a novel syntaxin-binding protein that may regulate SNARE complex assembly. J Biol Chem 277 28271 28279
35. WeedonMNLettreGFreathyRMLindgrenCMVoightBF 2007 A common variant of HMGA2 is associated with adult and childhood height in the general population. Nat Genet 39 1245 1250
36. von AndrianUHEngelhardtB 2003 Alpha4 integrins as therapeutic targets in autoimmune disease. N Engl J Med 348 68 72
37. TamuraMTanakaSFujiiTAokiAKomiyamaH 2007 Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics 89 618 629
38. LoughranSJKruseEAHackingDFde GraafCAHylandCD 2008 The transcription factor Erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cells. Nat Immunol 9 810 819
39. PfluegerDRickmanDSSbonerAPernerSLaFargueCJ 2009 N-myc downstream regulated gene 1 (NDRG1) is fused to ERG in prostate cancer. Neoplasia 11 804 811
40. EsguevaRPernerSJ LaFargueCSchebleVStephanC 2010 Prevalence of TMPRSS2-ERG and SLC45A3-ERG gene fusions in a large prostatectomy cohort. Mod Pathol 23 539 546
41. LambertLAMitchellSL 2007 Molecular evolution of the transferrin receptor/glutamate carboxypeptidase II family. J Mol Evol 64 113 128
42. StahlEARaychaudhuriSRemmersEFXieGEyreS 2010 Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet 42 508 514
43. YangJBenyaminBMcEvoyBPGordonSHendersAK 2010 Common SNPs explain a large proportion of the heritability for human height. Nat Genet 42 565 569
44. WangKLiMHakonarsonH 2010 Analysing biological pathways in genome-wide association studies. Nat Rev Genet 11 843 854
45. OkadaYKamataniYTakahashiAMatsudaKHosonoN 2010 A genome-wide association study in 19 633 Japanese subjects identified LHX3-QSOX2 and IGF1 as adult height loci. Hum Mol Genet 19 2303 2312
46. OkadaYTakahashiAOhmiyaHKumasakaNKamataniY 2011 Genome-wide association study for C-reactive protein levels identified pleiotropic associations in the IL6 locus. Hum Mol Genet 20 1224 1231
47. PurcellSNealeBTodd-BrownKThomasLFerreiraMA 2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
48. JohnsonADHandsakerREPulitSLNizzariMMO'DonnellCJ 2008 SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics 24 2938 2939
Štítky
Genetika Reprodukční medicína
Článek Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding SitesČlánek Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene SilencingČlánek Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse GutČlánek FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field SizeČlánek Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian SpermatogenesisČlánek Differential Effects of and Risk Variants on Association with Diabetic ESRD in African AmericansČlánek Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 6
-
Všechny články tohoto čísla
- Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding Sites
- Statistical Inference on the Mechanisms of Genome Evolution
- Revisiting Heterochromatin in Embryonic Stem Cells
- A Two-Stage Meta-Analysis Identifies Several New Loci for Parkinson's Disease
- Identification of a Sudden Cardiac Death Susceptibility Locus at 2q24.2 through Genome-Wide Association in European Ancestry Individuals
- Genomic Prevalence of Heterochromatic H3K9me2 and Transcription Do Not Discriminate Pluripotent from Terminally Differentiated Cells
- Epistasis between Beneficial Mutations and the Phenotype-to-Fitness Map for a ssDNA Virus
- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Telomere DNA Deficiency Is Associated with Development of Human Embryonic Aneuploidy
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Unexpected Role for DNA Polymerase I As a Source of Genetic Variability
- Transportin-SR Is Required for Proper Splicing of Genes and Plant Immunity
- How Chromatin Is Remodelled during DNA Repair of UV-Induced DNA Damage in
- Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene Silencing
- Two Evolutionary Histories in the Genome of Rice: the Roles of Domestication Genes
- Natural Allelic Variation Defines a Role for : Trichome Cell Fate Determination
- Multiple Common Susceptibility Variants near BMP Pathway Loci , , and Explain Part of the Missing Heritability of Colorectal Cancer
- Pathogenic Mechanism of the FIG4 Mutation Responsible for Charcot-Marie-Tooth Disease CMT4J
- A Functional Variant in Promoter Modulates Its Expression and Confers Disease Risk for Systemic Lupus Erythematosus
- Drift and Genome Complexity Revisited
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
- Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse Gut
- Pathways of Distinction Analysis: A New Technique for Multi–SNP Analysis of GWAS Data
- Web-Based Genome-Wide Association Study Identifies Two Novel Loci and a Substantial Genetic Component for Parkinson's Disease
- Chk2 and p53 Are Haploinsufficient with Dependent and Independent Functions to Eliminate Cells after Telomere Loss
- Exome Sequencing Identifies Mutations in High Myopia
- Distinct Functional Constraints Partition Sequence Conservation in a -Regulatory Element
- CorE from Is a Copper-Dependent RNA Polymerase Sigma Factor
- A Single Sex Pheromone Receptor Determines Chemical Response Specificity of Sexual Behavior in the Silkmoth
- FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field Size
- Maps of Open Chromatin Guide the Functional Follow-Up of Genome-Wide Association Signals: Application to Hematological Traits
- Increased Susceptibility to Cortical Spreading Depression in the Mouse Model of Familial Hemiplegic Migraine Type 2
- Differential Gene Expression and Epiregulation of Alpha Zein Gene Copies in Maize Haplotypes
- Parallel Adaptive Divergence among Geographically Diverse Human Populations
- Genetic Analysis of Genome-Scale Recombination Rate Evolution in House Mice
- Mechanisms for the Evolution of a Derived Function in the Ancestral Glucocorticoid Receptor
- Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian Spermatogenesis
- Interferon Regulatory Factor 8 Regulates Pathways for Antigen Presentation in Myeloid Cells and during Tuberculosis
- High-Resolution Analysis of Parent-of-Origin Allelic Expression in the Arabidopsis Endosperm
- Specific SKN-1/Nrf Stress Responses to Perturbations in Translation Elongation and Proteasome Activity
- Graded Nodal/Activin Signaling Titrates Conversion of Quantitative Phospho-Smad2 Levels into Qualitative Embryonic Stem Cell Fate Decisions
- Genome-Wide Analysis Reveals PADI4 Cooperates with Elk-1 to Activate Expression in Breast Cancer Cells
- Trait Variation in Yeast Is Defined by Population History
- Meiosis-Specific Loading of the Centromere-Specific Histone CENH3 in
- A Genome-Wide Survey of Imprinted Genes in Rice Seeds Reveals Imprinting Primarily Occurs in the Endosperm
- Multiple Regulatory Mechanisms to Inhibit Untimely Initiation of DNA Replication Are Important for Stable Genome Maintenance
- SIRT1 Promotes N-Myc Oncogenesis through a Positive Feedback Loop Involving the Effects of MKP3 and ERK on N-Myc Protein Stability
- Bacteriophage Crosstalk: Coordination of Prophage Induction by Trans-Acting Antirepressors
- Role of the Single-Stranded DNA–Binding Protein SsbB in Pneumococcal Transformation: Maintenance of a Reservoir for Genetic Plasticity
- Genomic Convergence among ERRα, PROX1, and BMAL1 in the Control of Metabolic Clock Outputs
- Genome-Wide Association of Bipolar Disorder Suggests an Enrichment of Replicable Associations in Regions near Genes
- Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese Population
- DNA Ligase III Promotes Alternative Nonhomologous End-Joining during Chromosomal Translocation Formation
- Differential Effects of and Risk Variants on Association with Diabetic ESRD in African Americans
- Finished Genome of the Fungal Wheat Pathogen Reveals Dispensome Structure, Chromosome Plasticity, and Stealth Pathogenesis
- Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
- Extracellular Matrix Dynamics in Hepatocarcinogenesis: a Comparative Proteomics Study of Transgenic and Null Mouse Models
- Integrating 5-Hydroxymethylcytosine into the Epigenomic Landscape of Human Embryonic Stem Cells
- Vive La Différence: An Interview with Catherine Dulac
- Multiple Loci Are Associated with White Blood Cell Phenotypes
- Nuclear Accumulation of Stress Response mRNAs Contributes to the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- A New Mutation Affecting FRQ-Less Rhythms in the Circadian System of
- Cryptic Transcription Mediates Repression of Subtelomeric Metal Homeostasis Genes
- A New Isoform of the Histone Demethylase JMJD2A/KDM4A Is Required for Skeletal Muscle Differentiation
- Genetic Determinants of Lipid Traits in Diverse Populations from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- A Genome-Wide RNAi Screen for Factors Involved in Neuronal Specification in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Statistical Inference on the Mechanisms of Genome Evolution
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



