-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHigh-Resolution Analysis of Parent-of-Origin Allelic Expression in the Arabidopsis Endosperm
Genomic imprinting is an epigenetic phenomenon leading to parent-of-origin specific differential expression of maternally and paternally inherited alleles. In plants, genomic imprinting has mainly been observed in the endosperm, an ephemeral triploid tissue derived after fertilization of the diploid central cell with a haploid sperm cell. In an effort to identify novel imprinted genes in Arabidopsis thaliana, we generated deep sequencing RNA profiles of F1 hybrid seeds derived after reciprocal crosses of Arabidopsis Col-0 and Bur-0 accessions. Using polymorphic sites to quantify allele-specific expression levels, we could identify more than 60 genes with potential parent-of-origin specific expression. By analyzing the distribution of DNA methylation and epigenetic marks established by Polycomb group (PcG) proteins using publicly available datasets, we suggest that for maternally expressed genes (MEGs) repression of the paternally inherited alleles largely depends on DNA methylation or PcG-mediated repression, whereas repression of the maternal alleles of paternally expressed genes (PEGs) predominantly depends on PcG proteins. While maternal alleles of MEGs are also targeted by PcG proteins, such targeting does not cause complete repression. Candidate MEGs and PEGs are enriched for cis-proximal transposons, suggesting that transposons might be a driving force for the evolution of imprinted genes in Arabidopsis. In addition, we find that MEGs and PEGs are significantly faster evolving when compared to other genes in the genome. In contrast to the predominant location of mammalian imprinted genes in clusters, cluster formation was only detected for few MEGs and PEGs, suggesting that clustering is not a major requirement for imprinted gene regulation in Arabidopsis.
Published in the journal: . PLoS Genet 7(6): e32767. doi:10.1371/journal.pgen.1002126
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002126Summary
Genomic imprinting is an epigenetic phenomenon leading to parent-of-origin specific differential expression of maternally and paternally inherited alleles. In plants, genomic imprinting has mainly been observed in the endosperm, an ephemeral triploid tissue derived after fertilization of the diploid central cell with a haploid sperm cell. In an effort to identify novel imprinted genes in Arabidopsis thaliana, we generated deep sequencing RNA profiles of F1 hybrid seeds derived after reciprocal crosses of Arabidopsis Col-0 and Bur-0 accessions. Using polymorphic sites to quantify allele-specific expression levels, we could identify more than 60 genes with potential parent-of-origin specific expression. By analyzing the distribution of DNA methylation and epigenetic marks established by Polycomb group (PcG) proteins using publicly available datasets, we suggest that for maternally expressed genes (MEGs) repression of the paternally inherited alleles largely depends on DNA methylation or PcG-mediated repression, whereas repression of the maternal alleles of paternally expressed genes (PEGs) predominantly depends on PcG proteins. While maternal alleles of MEGs are also targeted by PcG proteins, such targeting does not cause complete repression. Candidate MEGs and PEGs are enriched for cis-proximal transposons, suggesting that transposons might be a driving force for the evolution of imprinted genes in Arabidopsis. In addition, we find that MEGs and PEGs are significantly faster evolving when compared to other genes in the genome. In contrast to the predominant location of mammalian imprinted genes in clusters, cluster formation was only detected for few MEGs and PEGs, suggesting that clustering is not a major requirement for imprinted gene regulation in Arabidopsis.
Introduction
Genomic imprinting is an epigenetic phenomenon present in mammals and flowering plants that leads to differential expression of alleles of the same gene dependent on the parent-of-origin. Imprinted genes are differentially marked in the gametes, making maternal and paternal alleles functionally different [1]. Whereas in mammals imprinting occurs in the placenta as well as the embryo and tissues of the adult organism, most examples of imprinted genes in plants to date are confined to the endosperm [2]. Although examples of imprinted genes in the plant embryo exist [3], they seem to be rare. The endosperm is a functional analog of the mammalian placenta and serves to support embryo growth [4]. It is a triploid tissue that is derived after fertilization of the homodiploid central cell with a haploid sperm cell, whereas the second sperm cell will fertilize the haploid egg cell, leading to the formation of the diploid embryo [5].
Genome-wide studies of DNA methylation in embryo and endosperm have revealed transposon and repeat sequences to be largely hypomethylated in the endosperm compared to the embryo [6], [7], with virtually all CG sequences methylated in the embryo having reduced methylation levels in the endosperm [7]. Methylation levels at CG sites are partially restored in the endosperm of mutants where the DNA glycosylase DEMETER (DME) is disrupted [7], implicating DME to be largely responsible for genome-wide CG demethylation in the endosperm. Repression of the DNA methyltransferase MET1 in the central cell is also considered to contribute to the establishment of differential DNA methylation in the endosperm [8]. Transposon insertions or local sequence duplications are known to recruit DNA methylation and to initiate silencing of neighbouring genes in vegetative tissues [9]. This process is likely to render the targeted genes to be imprinted, as the maternal alleles can escape silencing by DME-mediated DNA demethylation in the endosperm. Based on the idea that DNA demethylation will activate genes in the endosperm that are silenced in vegetative tissues, Gehring and colleagues identified five novel imprinted genes and predicted around 50 imprinted genes in Arabidopsis, with many such genes encoding transcription factors and proteins with chromatin-related functions [6].
Although DNA methylation is widely recognized as a major mechanism for imprinted gene regulation, there are several examples suggesting that DNA methylation is not in all cases sufficient to establish imprinted gene expression. For instance, silencing of the maternal alleles of PHERES1 (PHE1) and the paternal alleles of MEDEA (MEA) and ARABIDOPSIS FORMIN HOMOLOGUE 5 (AtFH5) depend on repressive activity of the FERTILIZATION INDEPENDENT SEED (FIS) Polycomb group (PcG) complex [10]–[14]. The FIS PcG complex is a chromatin modifying complex that by trimethylating its target genes on histone H3 at lysine 27 (H3K27me3) causes gene repression [15]. MEA itself is a subunit of the FIS PcG complex and autoregulates its expression by repressing the paternal MEA allele [11]–[13], whereas activity of the maternal MEA allele requires DME-mediated DNA demethylation [16], [17]. Similarly, imprinted expression of PHE1 depends on both, the FIS PcG complex and DME-mediated DNA demethylation [6], [18], [19]. Demethylation of a helitron remnant located 2.5 kbps downstream of the PHE1 locus as well as binding of the FIS PcG complex to the PHE1 promoter region are required for silencing of the maternal PHE1 alleles, suggesting long-range interactions between the repeat region and PcG proteins [19].
As demethylation of repeat elements and transposons in the endosperm is a major mechanism giving rise to imprinted gene expression, it has been proposed that imprinting arose as a by-product of a silencing mechanism targeting invading foreign DNA [6], [7], [20]. Another view on the evolution of imprinted genes states that imprinting arose as a consequence of a conflict over the distribution of resources from the mother to the offspring [21], [22]. This theory predicts that there will be a selection for paternally active genes that can maximize the transfer of nutrients to the developing embryo, whereas the mother protects herself against the demands of the embryo by suppressing the growth induced by the paternally active genes. In line with this theory, imprinting occurs in placental mammals and flowering plants, both contributing maternal resources to the progeny. Furthermore, many imprinted genes in mammals affect both the demand and supply of nutrients across the placenta [23]. From the few known imprinted genes in plants, some do affect endosperm growth [24]–[26] and there is evidence that some imprinted genes may be fast evolving [27]–[29]. This suggests that imprinted gene expression, although being a likely by-product of a genome defence mechanism, may confer a selective advantage.
The discussion about the origin and evolution of imprinted gene expression in plants has been restricted by the sparse knowledge of imprinted loci. In this study we report on the identification of more than 60 genes in Arabidopsis with predicted parent-of-origin specific expression, greatly extending the number of potential imprinted loci in plants. Our study also revealed that specifically maternally and paternally expressed genes are regulated by different molecular mechanisms that rely on DNA methylation and FIS PcG function, respectively. Finally, we find that imprinted genes in plants are more rapidly evolving when compared to all other genes in the genome, and we propose that transposons may have been a driving force for the evolution of imprinted gene expression in Arabidopsis.
Results
Genome-Wide Identification of Genes with Parentally-Biased Expression
We performed reciprocal crosses of the two Arabidopsis thaliana accessions Col-0 and Bur-0 that offer a sufficiently high number of small nucleotide polymorphisms (SNPs) to define the parent-of-origin expression of the majority of genes [30]. Seeds containing globular stage embryos were harvested at 4 days after pollination (DAP). Microscopic analysis of seeds derived from four siliques developing after reciprocal crosses of Col-0 and Bur-0 accessions did not reveal obvious developmental differences (Figure 1A), suggesting that Col-0 and Bur-0 accessions have similar properties when used as maternal plant or pollen donor. We generated mRNA-sequencing libraries of seeds derived from Col-0 × Bur-0 and Bur-0×Col-0 crosses, which we sequenced to 80-fold and 67-fold transcriptome coverage, respectively (see Materials and Methods). We identified 12041 genes (q<0.05) with maternally biased expression (maternally expressed genes, MEGs; Table S1) and 119 genes (q<0.05) with paternally biased expression (paternally expressed genes, PEGs; Table S2; see Materials and Methods for details). Within this dataset we identified seven MEGs and six PEGs that were previously predicted to be regulated by genomic imprinting [6]. We also identified the known imprinted genes FWA [31], MYB3R2 [6], and At3g25260 [6] in our MEG dataset as well as PHE1 [10] and At5g62110 [6] in the PEG dataset. Several known imprinted genes were not identified either due to low numbers of sequence reads (MEA and FIS2), lack of SNPs between Col-0 and Bur-0 (MPC [26]), or lack of significant q values (ATFH5, HDG3, HDG8, and HDG9 [6]. Several of the previously identified imprinted genes (HDG3, HDG8, HDG9) had significantly deviating read numbers from a predicted 2m∶1p ratio only in one direction of the cross but not in the reciprocal cross and therefore failed to pass our significance threshold. This suggests that genomic imprinting can be accession-dependent and many genes may be imprinted in one accession but biallelically expressed in another accession, as was previously described for different alleles of the maize R and dzr1 loci [32]–[33]. Together, based on SNP distributions deviating from the expected 2m∶1p genome ratio we successfully identified six out of twelve previously identified imprinted genes, indicating that using this approach we can successfully identify novel imprinted genes on a genome-wide scale.
Fig. 1. Scheme of Experimental Procedures Leading to the Identification of MEGs and PEGs in Arabidopsis. 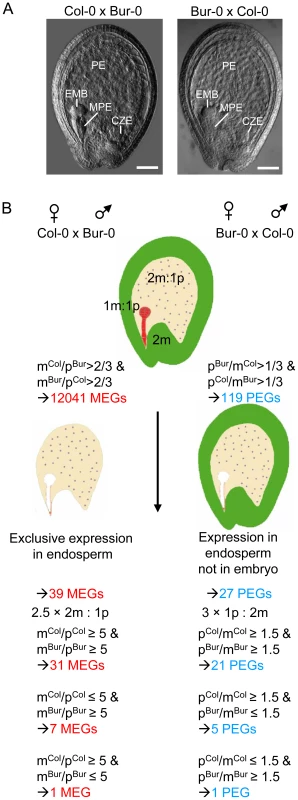
(A) Seeds derived after reciprocal crosses of Col-0 and Bur-0 accessions are phenotypically indistinguishable. Embyro (EMB), chalazal (CZE), micropylar (MPE) and peripheral (PE) endosperm are indicated. Scale bars, 50 µM. (B) Outline of filtering procedures leading to the identification of MEGs and PEGs. Identification of Imprinted Genes in the Endosperm
Genes that are regulated by genomic imprinting are expected to be expressed in zygotic tissues, excluding genes that contribute long lived RNAs from gametophytic tissues or that are expressed in the maternally-derived seed coat. As we used RNA isolated from seeds, many of the MEGs were likely to be seed coat-expressed genes and not necessarily imprinted genes. Therefore, we identified a stringent group of 400 genes that were preferentially expressed in the endosperm, selecting for genes with fivefold or greater signal log ratios (SLRs) in one of the endosperm domains compared with the seed coat and which were not significantly expressed in vegetative tissues (Table S3, Figure 1B and Materials and Methods). To avoid a bias towards strongly expressed genes, we included MEGs with low mRNA levels (read counts higher or equal to 10 and smaller or equal to 30) having only threefold or higher SLRs in one of the endosperm domains compared with the seed coat and which were not significantly expressed in vegetative tissues. After this selection we identified 39 candidate MEGs with maternally-biased expression (Table S4), among them FWA, MYB3R2 and three predicted imprinted genes (At2g19400, At3g23060, At4g00540 [6]). For simplicity, candidate MEGs and PEGs will be referred to as “MEGs” and “PEGs” throughout this manuscript. In contrast, MEGs and PEGs that passed additional PCR-based tests will be referred to as “confirmed MEGs and PEGs”. We considered the possibility that some MEGs are not transcribed in the endosperm but instead contribute long-lived maternally-inherited RNAs. This would predict that the mRNA levels of these genes should be reduced in seeds at 4 DAP compared to flowers containing developed female gametophytes. Out of 39 tested genes only three genes (At1g54280, At3g26590, At4g26140) had lower expression levels in 4 DAP seeds than in stage 12 flowers (containing eight-nucleate/seven-celled female gametophytes [34]), implicating that maternally stored mRNAs do not extensively bias the identification of imprinted genes at 4 DAP. Selection for parentally-biased expression was based on deviating sequence reads from the expected 2m∶1p ratio of maternal Col-0 (mCol) to paternal Bur-0 (pBur) as well as maternal Bur-0 (mBur) and paternal Col-0 (pCol) alleles. However, when analyzing the ratio of mCol to pCol as well as mBur to pCol alleles, we noted that seven of the identified MEGs were likely to be imprinted only in the Bur-0 accession, but not in the Col-0 accession and one of the identified MEGs was likely to be imprinted only in the Col-0 accession, but not in the Bur-0 accession (Table S4), considering a fivefold higher expression of both maternal alleles over the paternal allele as maternally-biased expression. This suggests that there is considerable accession-dependency underlying the regulation of imprinted genes in Arabidopsis.
Many PEGs were strongly expressed in pollen but only weakly expressed in the endosperm (Figure S1A, S1B), implicating that transcripts loaded after fertilization from the sperm cells into the seed remained detectable in seeds at 4 DAP, as suggested by previous findings [35]. Therefore, we selected for PEGs that were present in a group of 12190 genes that we identified as being significantly expressed in the endosperm (Table S5). After this selection we obtained 38 genes (Table S6). Genes with predominant expression in the embryo are expected to mimic genes with paternally-biased expression and were excluded from the PEG list, resulting in 27 PEGs (Table S7, Figure 1B), among them previously identified genes PHE1 [10] and At5g62110 [6] as well as five predicted imprinted genes (At4g11400, At1g48910, At5g50470, At3g19160, At1g23320 [6]). Considering a threefold higher expression of the paternal allele over maternal alleles as parentally-biased expression (Figure 1B), we identified five PEGs that were predominantly paternally expressed when inherited from the Col-0 parent, but biallelically expressed when inherited from Bur-0 and one PEG that was predominantly expressed when inherited from the Bur-0 parent but biallelically expressed when inherited from Col-0 (Table S7).
We considered the possibility that some of the MEGs and PEGs are regulated by parental-specific splicing. MEGs and PEGs had on average 6–8 SNPs per gene, making it rather unlikely that for genes with numbers of SNPs this large a single-exon splice variant could lead to the statistically very significant differences in overall read numbers. Nevertheless, we analyzed for every candidate gene its female and male read distributions over all SNPs of that gene over all SNPs of that gene with Pearsons's chi-square test. All MEGs and PEGs had p-values larger than 0.05, indicating that parental-specific splicing is not a major confounding factor in our analysis.
We tested allele-specific expression of selected MEGs and PEGs by restriction-based allele-specific PCR analysis as well as sequencing analysis and found eleven out of twelve MEGs tested to be predominantly expressed from the maternal alleles in reciprocal crosses (At1g52460, At3g23060, At5g03020, At1g60970, At2g19400, At4g29570, At5g46300, At3g10590, At3g21830, At1g51000, AGL36; Figure 2A). One MEG, At1g20730, was specifically maternally expressed in the Col-0×Bur-0 cross, whereas it was biallelically expressed in the Bur-0×Col-0 cross (Figure 2B). Two MEGs with predicted accession-specific imprinting (AGL28 and AGL96) were similarly regulated (Figure 2B), indicating that these three genes are exclusively maternally expressed in Bur-0, but biallelically expressed in Col-0. We also confirmed paternal-preferential expression for eight out of twelve tested PEGs (At4g31900, At1g49290, At5g54350, At3g50720, At1g11810, At5g50470, At3g62230, At3g49770; Figure 2C). Three of the twelve tested PEGs (AGL23, At1g66630, At1g11810) were preferentially paternally expressed in one direction of the cross but biallelically expressed in the reciprocal cross, indicating a significant accession-dependency in the regulation of PEGs (Figure 2D). Biallelic expression of AGL23 in Col-0 is consistent with the previously proposed role of AGL23 for female gametophyte development in the Col-0 accession [36]. However, our data suggest that the functional roles of AGL23 differ between different Arabidopsis accessions. We furthermore confirmed the predicted accession-specific expression of At4g11940, which was paternally expressed in the cross Bur-0×Col-0, but biallelically expressed in the reciprocal cross (Figure 2D). Together, with 19 out of 24 predicted reciprocally imprinted genes being experimentally confirmed and experimental confirmation of three out of three predicted accession-dependent imprinted genes, we conclude that the majority of the newly predicted MEGs and PEGs are indeed regulated by genomic imprinting.
Fig. 2. Allele-Specific Expression Analysis of MEGs and PEGs. 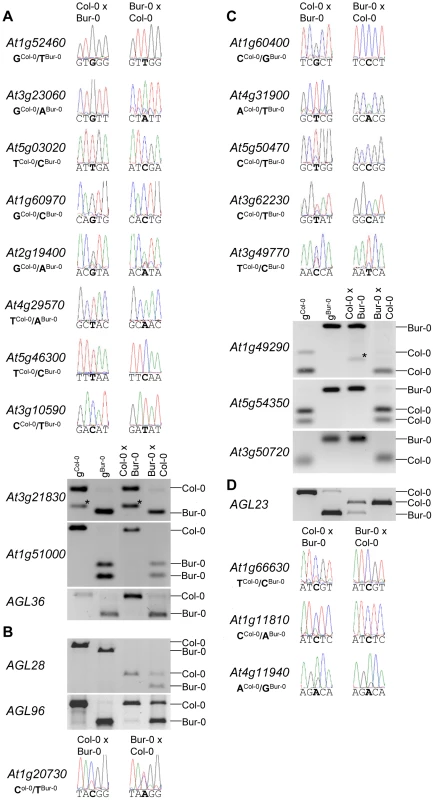
Seeds of reciprocal crosses of Col-0 and Bur-0 accessions were harvested at 4 DAP and allele-specific expression was tested by restriction-based allele-specific PCR analysis or sequencing. MEGs and PEGs that are imprinted in both directions of the cross are shown in (A) and (C), MEGs and PEGs that are accession-dependently imprinted are shown in (B) and (D). Asterisks indicate unspecific PCR bands. Size differences between controls and cDNA samples are caused by the presence of introns in amplified regions. Among reciprocally imprinted and accession-dependent imprinted MEGs and PEGs we detected a significant enrichment of nuclear localized proteins and transcription factors (Table S8), with many of them belonging to AGL MADS-box transcription factors (AGL36, AGL28, AGL96, PHE1, AGL23) that in yeast two-hybrid studies were shown to directly or indirectly interact with each other [37] as well as with AGL62 [38] (Figure S2). AGL62 has been proposed to be a major regulator of endosperm cellularization [38], suggesting a major regulatory role of imprinting genes in timing the onset of endosperm cellularization. Furthermore, MEGs were enriched for genes encoding cytidine deaminases (p = 3 E−4) that in zebrafish as well as in mammals have been proposed to be required for DNA demethylation [39], [40]. Whether cytidine deaminases play a similar role in the plant endosperm remains to be tested.
Dynamics of MEG and PEG Expression during Seed and Vegetative Development
Whereas MEGs were filtered based on the absence of expression in vegetative tissues, these filtering criteria were not applied for PEGs. However, expression patterns of MEGs and PEGs were very similar, both types of imprinted genes had a preference for being expressed in pollen, but were mainly excluded from vegetative tissues (Figure 3A). The expression profile of MEGs and PEGs in seeds was very comparable as well, both types of genes were not expressed in the seed coat, rarely expressed in the embryo, but most MEGs and PEGs were strongly expressed in the chalazal region of the endosperm (Figure 3B). Whereas expression of most MEGs was confined to the chalazal region of the endosperm, PEG expression was less restricted and extended to the peripheral and micropylar regions of the endosperm. MEG and PEG expression was clearly detectable in the endosperm of seeds containing preglobular stage embryos and expression in the chalazal endosperm remained detectable until seeds contained cotyledon stage embryos. Expression in the micropylar and peripheral region of the endosperm was only detectable until seeds contained heart stage embryos, after this stage expression remained confined to the chalazal endosperm. Average expression levels of MEGs and PEGs in the chalazal endosperm region were clearly above average expression levels of all genes, with expression being highest at the preglobular stage and declining towards the heart stage of seed development (Figure 3C).
Fig. 3. Expression Analysis of MEGs and PEGs in Vegetative and Seed Tissues. 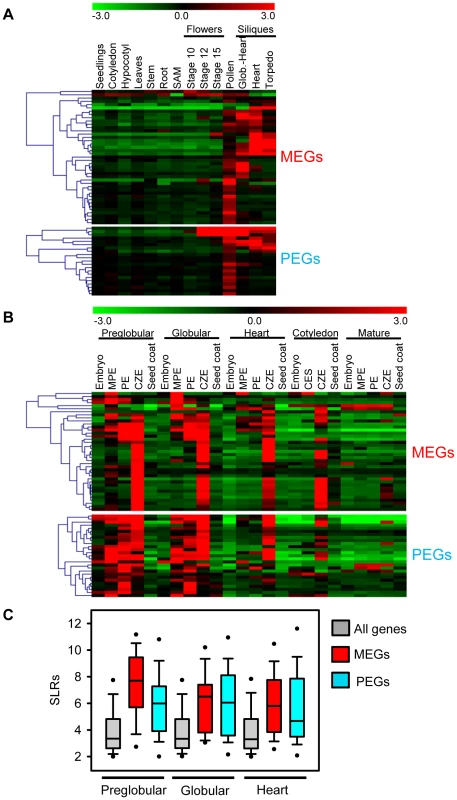
(A) Cluster analysis of MEGs and PEGs (including accession-dependent MEGs and PEGs) based on their expression in vegetative tissues and seeds. Each row represents a gene, and each column represents a tissue type. Tissue types are: seedlings, cotyledons, hypocotyl, leaves, stems, roots, shoot apical meristem (SAM), flowers at stages 10, 12, 15, siliques containing seeds with embryos in the globular to heart stage, heart stage and torpedo stage. Red or green indicate tissues in which a particular gene is highly expressed or repressed, respectively. (B) Cluster analysis of MEGs and PEGs (including accession-dependent MEGs and PEGs) based on their expression in embryo, endosperm and seed coat during different stages of seed development. Each row represents a gene, and each column represents a tissue type. Tissue types are: embryos from the preglobular stage to the mature stage, micropylar (MPE), peripheral (PE) and chalazal (CZE) endosperm derived from seeds containing embryos of the preglobular stage to the mature stage, and seed coat derived from seeds containing embryos of the preglobular stage to the mature stage. Red or green indicate tissues in which a particular gene is highly expressed or repressed, respectively. (C) Box plots of expression levels of MEGs (including accession-dependent MEGs; red) and PEGs (including accession-dependent PEGs; blue) compared to all genes (gray) in the chalazal endosperm region of seeds containing preglobular, globular and heart stage embryos. SLRs, Signal Log Ratios based on ATH1 microarray signals after RMA normalization. Different Localization and Regulatory Impact of DNA Methylation at MEG and PEG Loci
Previous studies predicted imprinted genes based on the assumption that DNA demethylation in the central cell causes activation of maternal alleles of genes, whereas paternal alleles remain methylated and silenced [6]. Whereas this assumption should predict MEGs, it is unlikely to successfully predict PEGs. However, in our PEG dataset we found five previously predicted imprinted genes [6], indicating that DNA methylation is important, but not the only regulator of imprinted gene expression. We analyzed the CG DNA methylation status of MEGs and PEGs in vegetative tissues and in the endosperm at 7–9 DAP using previously published data [7], [41]. Allele-specific DNA methylation patterns are established during gametogenesis and immediately after fertilization [42], [43], implicating that the endosperm DNA methylation profile at 7–9 DAP is similar to the profile at 4 DAP (time point of this study). There are two main classes of MEGs distinguishable based on the CG DNA methylation profile: MEGs without substantial CG DNA methylation in immediate vicinity to the genic regions (Figure 4 and Figure S3A–S3C) and MEGs with high levels of CG DNA methylation surrounding genic regions (Figure 5 and Figure S3D–S3F).
Fig. 4. Impact of DNA Methylation and FIS PcG Function on the Regulation of Confirmed MEGs without Prominent Genic CG DNA Methylation. 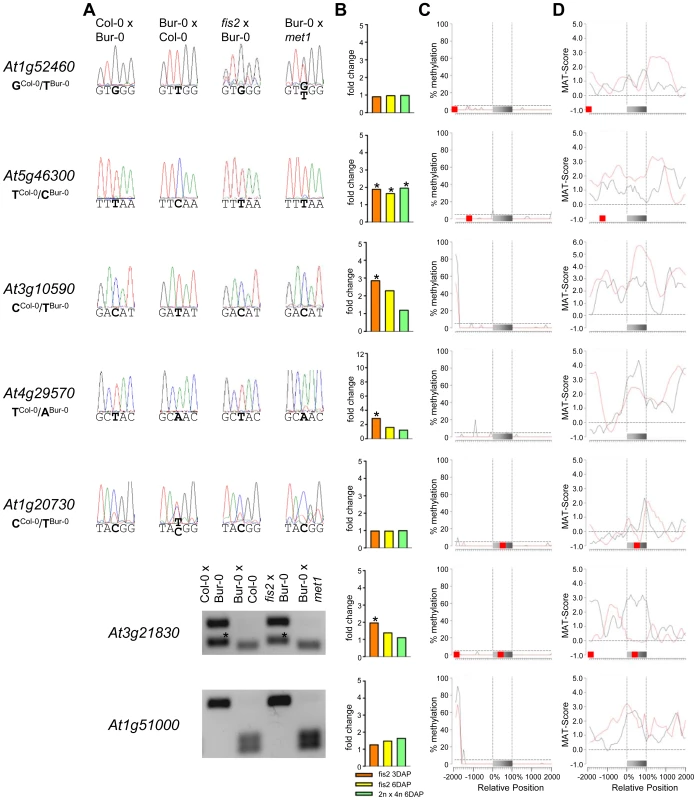
(A) Allele-specific expression analysis of indicated MEGs in seeds derived from crosses of Col-0×Bur-0, Bur-0×Col-0, fis2×Bur-0 and Col-0×met1. Seeds were harvested at 4 DAP and allele-specific expression was tested by restriction-based allele-specific PCR analysis or sequencing. Asterisks indicate unspecific PCR bands. (B) Fold-changes of MEG expression in fis2 mutant seeds at 3 and 6 days after pollination (DAP) and from seeds derived from pollination with tetraploid pollen donors at 6 DAP compared to wild-type seeds at the corresponding time points. Data are based on ATH1 microarray signals after RMA normalization. Significantly deregulated genes are marked by an asterisk. (C) CG DNA methylation profiles of indicated MEGs in vegetative tissues (black line) or endosperm (red line) based on data published by [7], [41]. The gray bar represents the annotated gene body from transcription start (left) to transcription end (right). Red boxes represent transposable elements. Profiles are shown for 5% length intervals along the gene body and for 100 bp sequence intervals for the 2-kb regions upstream and downstream of each gene. The vertical dotted lines mark the gene body. The horizontal dashed line marks the DNA methylation level in vegetative tissues of TAIR8-annotated genes at the transcriptional start site. (D) H3K27me3 profiles of indicated MEGs in vegetative tissues (black line) or endosperm (red line) based on data published by [52], [64]. The gray bar represents the annotated gene body from transcription start (left) to transcription end (right). Red boxes represent transposable elements. Profiles are shown for 5% length intervals along the gene body and for 100 bp sequence intervals for the 2-kb regions upstream and downstream of each gene. The vertical dotted lines mark the gene body. The horizontal dashed line marks the H3K27me3 level of TAIR8-annotated genes at the transcriptional start site. Fig. 5. Impact of DNA Methylation and FIS PcG Function on the Regulation of Confirmed MEGs with Prominent Genic CG DNA Methylation. 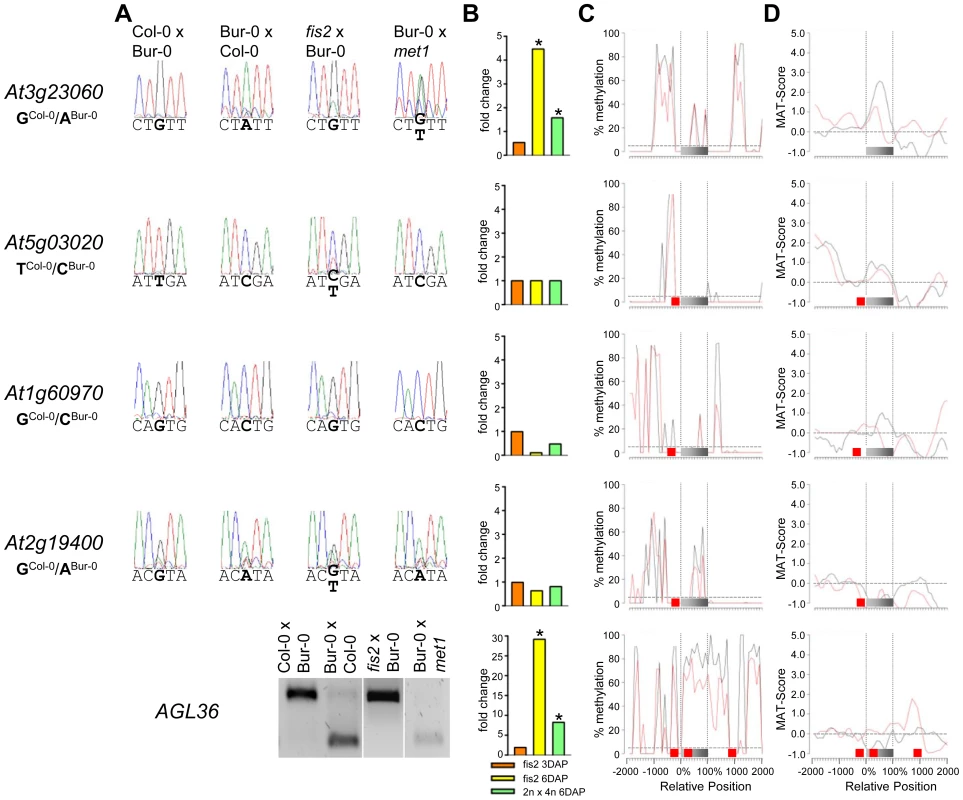
(A) Allele-specific expression analysis of indicated MEGs in seeds derived from crosses of Col-0×Bur-0, Bur-0×Col-0, fis2×Bur-0 and Col-0×met1. Seeds were harvested at 4 DAP and allele-specific expression was tested by restriction-based allele-specific PCR analysis or sequencing. (B) Fold-changes of MEG expression in fis2 mutant seeds at 3 and 6 days after pollination (DAP) and from seeds derived from pollination with tetraploid pollen donors at 6 DAP compared to wild-type seeds at the corresponding time points. Data are based on ATH1 microarray signals after RMA normalization. Significantly deregulated genes are marked by an asterisk. (C) CG DNA methylation profiles of indicated MEGs in vegetative tissues (black line) or endosperm (red line) based on data published by [7], [41]. The gray bar represents the annotated gene body from transcription start (left) to transcription end (right). Red boxes represent transposable elements. Profiles are shown for 5% length intervals along the gene body and for 100 bp sequence intervals for the 2-kb regions upstream and downstream of each gene. The vertical dotted lines mark the gene body. The horizontal dashed line marks the DNA methylation level in vegetative tissues of TAIR8-annotated genes at the transcriptional start site. (D) H3K27me3 profiles of indicated MEGs in vegetative tissues (black line) or endosperm (red line) based on data published by [52], [64]. The gray bar represents the annotated gene body from transcription start (left) to transcription end (right). Red boxes represent transposable elements. Profiles are shown for 5% length intervals along the gene body and for 100 bp sequence intervals for the 2-kb regions upstream and downstream of each gene. The vertical dotted lines mark the gene body. The horizontal dashed line marks the H3K27me3 level of TAIR8-annotated genes at the transcriptional start site. The CG DNA methylation profile of PEGs was clearly distinguishable from the MEG CG profiles; almost all PEGs had CG methylation peaks on average about 700 bps up - or downstream of the coding regions, whereas coding regions and the immediate up - and downstream regions were mostly devoid of CG DNA methylation (Figure 6 and Figure S4), suggesting that low levels of DNA methylation in this region are important for keeping PEGs transcriptionally active when paternally inherited and that DNA methylation at this region is unlikely to be responsible for keeping maternal alleles of PEGs silenced. The level of CG methylation in MEGs and PEGs was reduced in the endosperm providing an explanation why MEGs and PEGs were predicted in a previous study [6].
Fig. 6. Impact of FIS PcG Function on the Regulation of Confirmed PEGs. 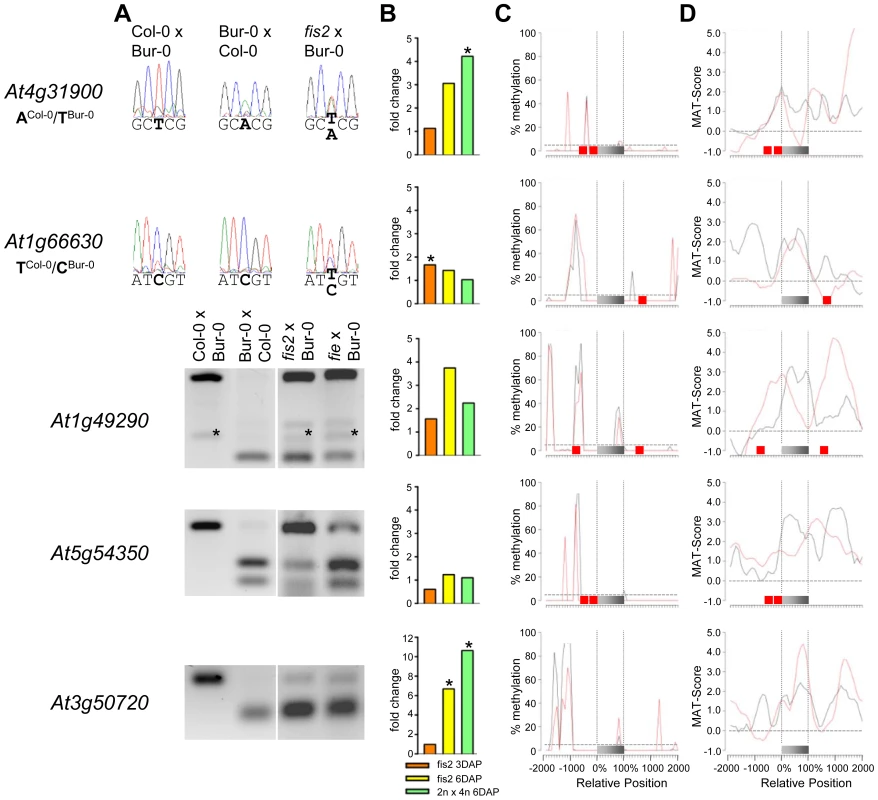
(A) Allele-specific expression analysis of indicated PEGs in seeds derived from crosses of Col-0×Bur-0, Bur-0×Col-0, fis2×Bur-0, and fie×Bur-0. Seeds were harvested at 4 DAP and allele-specific expression was tested by restriction-based allele-specific PCR analysis or sequencing. Asterisks indicate unspecific PCR bands. (B) Fold-changes of PEG expression in fis2 mutant seeds at 3 and 6 days after pollination (DAP) and from seeds derived from pollination with tetraploid pollen donors at 6 DAP compared to wild-type seeds at the corresponding time points. Data are based on ATH1 microarray signals after RMA normalization. Significantly deregulated genes are marked by an asterisk. (C) CG DNA methylation profiles of indicated PEGs in vegetative tissues (black line) or endosperm (red line) based on data published by [7], [41]. The gray bar represents the annotated gene body from transcription start (left) to transcription end (right). Red boxes represent transposable elements. Profiles are shown for 5% length intervals along the gene body and for 100 bp sequence intervals for the 2-kb regions upstream and downstream of each gene. The vertical dotted lines mark the gene body. The horizontal dashed line marks the DNA methylation level in vegetative tissues of TAIR8-annotated genes at the transcriptional start site. (D) H3K27me3 profiles of indicated PEGs in vegetative tissues (black line) or endosperm (red line) based on data published by [52], [64]. The gray bar represents the annotated gene body from transcription start (left) to transcription end (right). Red boxes represent transposable elements. Profiles are shown for 5% length intervals along the gene body and for 100 bp sequence intervals for the 2-kb regions upstream and downstream of each gene. The vertical dotted lines mark the gene body. The horizontal dashed line marks the H3K27me3 level of TAIR8-annotated genes at the transcriptional start site. Demethylation of the paternal genome by mutations in the DNA methyltransferase MET1 has been demonstrated to cause biallelic expression of several MEGs [26], [31], [44], [45]. Therefore, we tested allele-specific expression of confirmed MEGs in seeds derived after pollination with pollen of met1/MET1 plants. Out of six tested MEGs that were not substantially CG methylated in the genic region, there were three genes that became either biallelically expressed upon pollination with met1 pollen (At1g52460) or exclusively paternally expressed (At5g46300, At3g10590; Figure 4). The accession-dependent imprinted gene At1g20730 which was preferentially maternally expressed in the cross Col-0×Bur-0 but biallelically expressed in the reciprocal cross became as well paternally expressed upon met1 pollination (Figure 4). Analysis of non-CG DNA methylation revealed that within a distance of 2 kbps upstream of the transcriptional start site MEGs At5g46300, At3g10590 and At1g20730 were substantially marked by CHG or CHH methylation (Figure S5A), suggesting that non-CG DNA methylation might be involved in the repression of paternal MEG alleles. Non-CG DNA methylation levels were higher in the endosperm compared to vegetative tissues, in agreement with previous reports on frequent small interfering RNA–targeted hypermethylation of CHG and CHH target sites in the endosperm [7]. Out of five tested MEGs with substantial CG DNA methylation in the vicinity of genic regions only one MEG became reactivated upon pollination with met1 pollen (At3g23060; Figure 5), suggesting that prominent CG DNA methylation marks are not a decisive criterion for DNA methylation-dependent repression of the paternal MEG alleles. Conversely, the absence of prominent CG DNA methylation in vicinity of genic regions does not exclude a regulatory role of DNA methylation on the activity status of paternal MEG alleles.
MEGs and PEGs Are Regulated by the FIS PcG Complex
As DNA methylation seemed unlikely to be responsible for repression of the paternal alleles of many MEGs as well as the maternal alleles of PEGs, we further investigated by which alternative mechanism imprinting of MEGs and PEGs is regulated. Almost all PEGs (25 out of 27 PEGs and accession-dependent PEGs) and many MEGs (31 out of 39 MEGs and accession-dependent MEGs) were PcG target genes in vegetative tissues (Tables S4, S7). In the endosperm, the average H3K27me3 levels of MEGs and PEGs were clearly increased over the H3K27me3 levels of all genes, with the H3K27me3 levels of PEGs being twice as high as the H3K27me3 levels of MEGs (Figure S6), suggesting that silencing of the maternally inherited alleles of PEGs is mediated by the FIS PcG complex. This hypothesis would predict increased expression of PEGs upon loss of FIS function. We tested this hypothesis by analyzing expression levels of PEGs in fis2 mutants at 3 DAP and 6 DAP. Indeed, half of all PEGs and accession-dependent PEGs were significantly upregulated in the fis2 mutant (Figure 6 and Figure S4). Furthermore, we tested allele-specific expression of four confirmed PEGs (At4g31900, At1g49290, At5g54350, At3g50720) and one accession-dependent confirmed PEG (At1g66630) in fis2 and fie mutants lacking maternal FIS function. For all four PEGs as well as the accession-dependent PEG loss of FIS function caused activation of maternal PEG alleles, adding further support to our hypothesis (Figure 6).
Also 13 out of 39 MEGs and accession-dependent MEGs were significantly upregulated in the fis2 mutant (Figure 4, Figure 5 and Figure S3). However, allele-specific expression analysis revealed reactivation of the paternal MEG allele in only two out of eleven tested MEGs (At5g03020, At2g19400; Figure 4 and Figure 5), indicating that increased expression of MEGs in fis2 mutants is not necessarily a consequence of paternal MEG allele activation, but is likely caused by an increased expression of the maternal MEG alleles.
Previous studies revealed global deregulation of FIS PcG target genes in response to interploidy crosses (2n × 4n) [46], [47]. Global deregulation of imprinted genes was furthermore proposed to account for interploidy seed defects [48]. Therefore, if the FIS PcG complex plays a major role in the regulation of imprinted genes, imprinted genes should become largely deregulated in response to interploidy crosses. We tested this hypothesis by analyzing MEG and PEG expression in seeds derived after pollination of diploid plants with tetraploid pollen donors. Indeed, most MEGs and PEGs that were deregulated in fis2 were as well significantly deregulated in triploid seeds derived from interploidy crosses, adding support to this hypothesis (Figure 4, Figure 5, Figure 6, Figure S3 and Figure S4).
Together, our data reveal that maternal and paternal alleles of a subset of MEGs and maternal alleles of PEGs are regulated by the FIS PcG complex. The FIS PcG complex confers tight repression of the maternal PEG alleles and some paternal MEG alleles, whereas it mainly modulates expression of many maternal MEG alleles.
MEGs and PEGs Are Often Neighboured by Transposable Elements
Transposable elements have been implicated as the driving force for the evolution of imprinted gene expression [6], [7], [20]. Therefore, we addressed the question whether MEGs and PEGs have an increased likelihood to contain transposable elements in their vicinity compared to other detectable genes. Indeed, this test revealed a significantly increased likelihood for MEGs (p<0.009) as well as PEGs (p<1.3E-6). We also tested whether a particular subclass of transposable elements is enriched in the vicinity of MEGs and PEGs. Among the tested elements we noted a significant enrichment for RC/helitrons in PEGs (p<2.7 E-5). MEGs also had more RC/helitrons than expected by chance (8 versus 5), which was, however, not statistically significant (p<0.08). The presence of helitrons was previously reported within the 5′ region of imprinted genes MEA [49], FWA [31], HDG3 and HDG9 [6], implicating a functional association between the presence of helitrons and imprinted gene expression. Among MEGs we also noted a significant enrichment for MuDR (p = 0.01) and DNA transposable elements (p = 0.024), however, it is possible that (due to the relatively small sample size) enrichments for other elements were not detected. Correlating with a different CG DNA methylation pattern of MEGs and PEGs, the location of transposable elements in relation to the transcriptional start or stop differed between MEGs and PEGs (Figure 7B). PEGs had a much greater median distance of transposable elements in relation to the transcriptional start and stop compared to MEGs (Figure 7B), supporting previous observations of a distally located helitron remnant being required for imprinted expression of the PEG PHE1 [19].
Fig. 7. Types of Transposable Elements in the Vicinity of MEGs and PEGs. 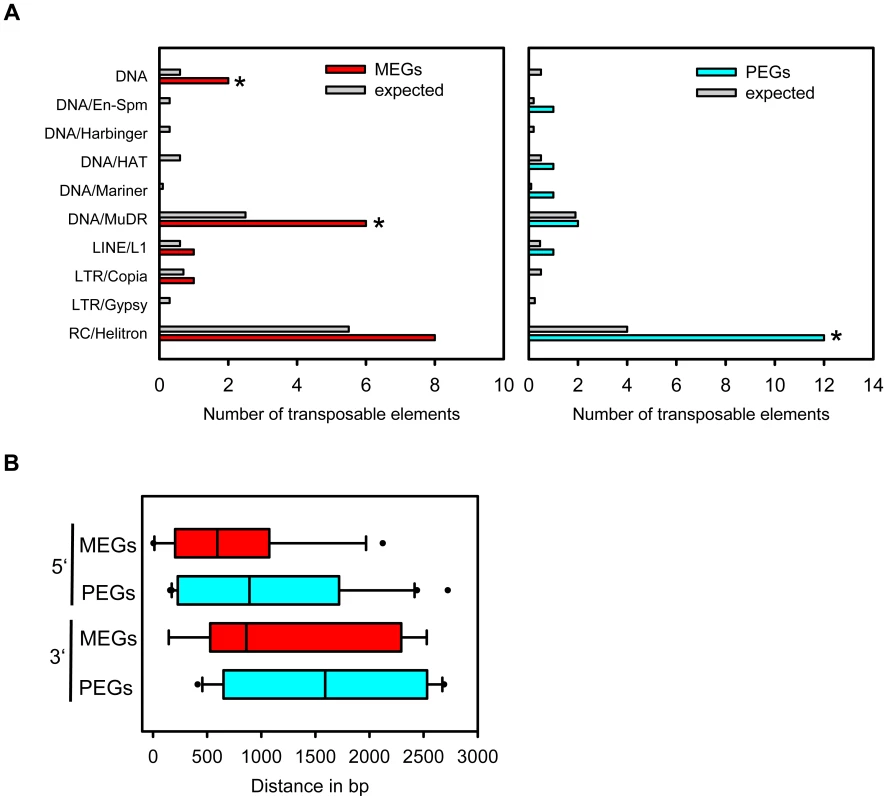
(A) Type of transposable elements present in MEGs (left panel) and PEGs (right panel) in comparison to their representation among detectable genes in our dataset (gray bars). TE superfamilies are as defined by TAIR (www.arabidopsis.org). (B) Distance of transposable elements in relation to the transcriptional start (5′ location) or stop (3′ location) of MEGs and PEGs. MEGs and PEGs Are Faster Evolving When Compared to the Rest of the Genes in the Arabidopsis thaliana Genome
A number of studies of imprinted genes in both mammals and plants have found evidence that imprinted genes are rapidly evolving under positive Darwinian selection [27]–[29]. To determine whether either MEGs or PEGs displayed any evidence of fast evolution, pairwise dN/dS calculations were performed for the entire genome of Arabidopsis thaliana. Using reciprocal BLASTP searches, 19,965 orthologous pairs of proteins (gene models) were identified between Arabidopsis thaliana and Arabidopsis lyrata from the 27,235 Arabidopsis thaliana gene models tested. These included 27 (69.23%) of the MEGs and accession-dependent MEGs and 19 (70.37%) of the PEGs and accession-dependent PEGs identified in this study. Gene models not returning reciprocal BLAST results were not considered further for dN/dS analysis. Those gene models tested were then split into MEGs, PEGs and a third group representing the remaining genes tested. All three classes were then compared to determine whether there was any difference in relation to dN/dS values which measure rate of evolution of a protein coding-locus (Table S9).
In all three classes most genes have a dN/dS of less than one suggesting some level of purifying selection. However, the dN/dS value (as calculated here) is an average across the whole CDS sequence and masks potential heterogeneity of selection pressures across the gene. Despite this caveat, the dN/dS differences between imprinted and biallelically expressed genes is quite striking. For both MEGs and PEGs the reported median dN/dS is significantly higher than that of the background dN/dS for the remainder of the genome (p = 1.184e-05 and p = 2.991e-08 respectively, Wilcoxon Rank Sum), indicating that uniparentally expressed imprinted genes are fast evolving when compared to biallelically expressed genes (as represented by the background dN/dS values). However, although the median dN/dS for the MEGs is observed to be a third higher than that of the PEGs this difference is not reported as significant (p = 0.1614, Wilcoxon Rank Sum).
Also notable within the MEGs is that eight (∼30%) of all the MEGs tested (i.e. At1g61090, At3g57250, At1g51000, At5g46300, At4g29570 At1g12180, At1g52460 and At1g07690) reported a dN/dS value greater than one providing particularly strong evidence of fast evolution for these genes.
Within the PEGs, only one of the genes (∼5%; At2g20160) displayed a dN/dS value greater than one. However, statistical testing did not reveal a significant difference between the number of fast evolving MEGs and PEGs (p = 0.0851, Fisher2019s exact test). Full details of the dN/dS analysis for both MEGs and PEGs is presented in Tables S10 and S11.
A Subset of MEGs and PEGs Are Located in Clusters
Imprinted loci in mammals are clustered over megabase regions in the genome and this clustering is essential to their imprinted regulation [50]; raising the question whether imprinted loci in Arabidopsis are located within clusters as well. We searched for clustered MEGs and PEGs (including accession-dependent MEGs and PEGs) by applying a sliding window analysis. Using window sizes of 50 genes, significantly higher numbers of MEGs and PEGs were found to occur in clusters than expected by chance (p<0.05; Figure S7). We identified five MEG clusters containing two to three genes as well as three PEG clusters containing two genes (Figure 8A, 8B). Interestingly, most clusters contained either homologous MEGs or PEGs, or non-imprinted homologs of MEGs and PEGs (Figure 8A, 8B), implicating local sequence duplications as a driving force for the formation of imprinted genes. If so, there should be a higher incidence of imprinted genes having close sequence homologs compared to the genome-wide frequency of homologous genes. We tested this hypothesis by analyzing the number of close homologs to MEGs and PEGs and found indeed that MEGs and PEGs have an increased frequency of close homologs in comparison to the genome-wide frequency (p<0.05, Table S12), suggesting that gene duplications are in most cases connected with the formation of imprinted genes in Arabidopsis. Therefore, it seems possible that cluster formation of MEGs and PEGs is a consequence of local gene duplication and not essential for imprinted gene regulation, in agreement with the finding that only a subset of imprinted genes is localized in clusters.
Fig. 8. Some MEGs and PEGs Are Located in Clusters. 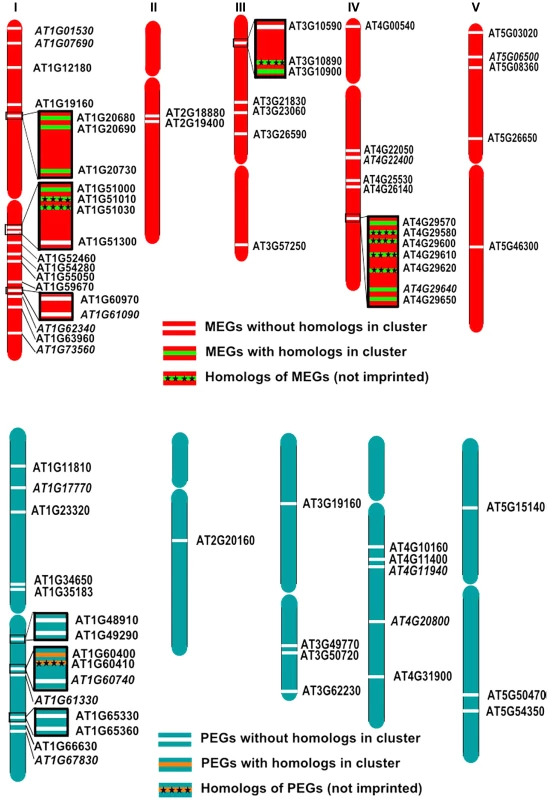
Chromosomal distribution of MEGs and accession-dependent MEGs (A) and PEGs and accession-dependent PEGs (B) along the chromosomes. Accession-dependent MEGs and PEGs are italicized. Genes located in clusters are boxed. Clustered MEGs and PEGs having homologs within the cluster are highlighted in green and orange, respectively. Non-imprinted homologs of clustered MEGs and PEGs are indicated by cross signs. Discussion
Unravelling the biological significance of genomic imprinting is crucially dependent on the identification of the majority of imprinted gene loci. In our study we succeeded in identifying more than 60 potentially imprinted loci, with a similar ratio of specifically maternally and paternally expressed imprinted genes. We successfully identified six out of twelve previously known imprinted genes, proving that our strategy can successfully identify novel imprinted genes. Six previously identified imprinted genes were not identified either because these genes are only weakly expressed at 4 DAP (MEA and FIS2) [46], they lack polymorphic sites between Col-0 and Bur-0 or because of accession-dependent imprinting. We only considered a gene to be imprinted if it had significantly deviating read numbers from the expected maternal to paternal ratio in both directions of the crosses. Those genes that passed this significance threshold were again tested for significant deviating read numbers when comparing the maternal and paternal Col-0 alleles versus the maternal and paternal Bur-0 alleles. Based on this comparison about 10% of the identified MEGs and 20% of the identified PEGs are likely to be imprinted only in one accession, however, many accession-dependent imprinted genes did not pass our first significance threshold (e.g. HDG3, HDG8, HDG9), suggesting that the number of accession-dependent imprinted genes is significantly higher. It has been noted that there is a difference between Arabidopsis accessions in their tolerance to interploidy crosses [51], whether accession-dependent imprinted genes are the underlying cause for this phenomenon is an attractive hypothesis.
Using publicly available microarray datasets we stringently filtered our MEG dataset for genes that are not expressed in vegetative tissues and the seed coat. This filtering allowed us to predict genes with allele-specific expression in the endosperm, but using this strategy we lost genes that are either not present on the ATH1 microarray (about 25%) or that are expressed in vegetative tissues but are still regulated by genomic imprinting. However, the vast majority of known imprinted genes are not significantly expressed during vegetative development, suggesting that we have identified a significant number of MEGs present in the genome. Although PEGs were not filtered against vegetative or seed coat expression, the majority of PEGs were similarly excluded from expression in vegetative tissues, indicating that imprinted genes in Arabidopsis have mainly endosperm-restricted functions.
Different Experimental Strategies Result in the Identification of Complementary Sets of Imprinted Genes
We compared the MEGs and PEGs identified in our study with MEGs and PEGs identified by a similar approach using the accession combinations Landsberg erecta (Ler) and Col [45]. Whereas the majority of unfiltered MEGs identified by Hsieh and colleagues were present in our unfiltered MEG dataset (84%; 549 genes; Tables S13 and S14), only six genes were commonly identified as MEGs after filtering (Table S17; Figure S8A). The majority of MEGs defined by Hsieh and colleagues as being expressed in the endosperm were also present in our unfiltered MEG dataset (78%, 89 genes, Figure S8A; Table S15). However, when analyzing the expression of these genes within different seed tissues we found the majority of them being strongly expressed in the seed coat (Figure S9) and did, therefore, not pass our stringent filtering criteria. Only eight out of 39 predicted MEGs identified in this study were as well present in the unfiltered dataset of Hsieh and colleagues (Figure S8A; Table S16) [45], indicating that differences in the filtering cannot sufficiently explain the differences between the identified MEG datasets. Similarly, only seven out of 119 predicted unfiltered PEGs overlapped with the unfiltered PEG dataset of Hsieh and colleagues (Table S18 and Table S19, Figure S8B), supporting the view that differences in filtering strategies do not fully account for the different datasets. It thus seems likely that the different experimental setup between this study and the study by Hsieh and colleagues, including different accession combinations and different developmental stages (4 DAP versus 7–9 DAP in [45]) resulted in the identification of complementary datasets.
Regulation of MEGs and PEGs by DNA Methylation and the FIS PcG Complex
Most PEGs were devoid of CG DNA methylation around the transcriptional start site, indicating that DNA methylation is not responsible for silencing of maternal PEG alleles. Instead, we provide evidence that silencing of at least some maternal PEG alleles is mediated by the FIS PcG complex. We found that PEG loci have high H3K27me3 levels in the endosperm, and importantly, many PEGs were activated upon loss of FIS function, which is likely contributed by reactivation of maternal PEG alleles. We also detected increased expression of MEGs upon loss of FIS function. However, this was not a necessary consequence of paternal MEG allele reactivation, but often caused by an activation of maternal MEG alleles, suggesting that endosperm hypomethylation makes maternal MEG alleles vulnerable to FIS silencing. This hypothesis is supported by previous findings from our group showing that genes and transposable elements are targeted by the FIS PcG complex in the endosperm, which are protected from PcG targeting by DNA methylation in vegetative tissues [52]. Which mechanism prevents the FIS complex from targeting paternal alleles of PEGs? We previously showed that DNA demethylation of a distally located region together with the promoter-localized FIS PcG complex are required for silencing of maternal PHE1 alleles, suggesting that long-range interactions of sequence elements are required for efficient silencing of maternal PHE1 alleles [19]. Here, we show that PEGs are flanked by regions of high CG DNA methylation levels, suggesting that upon DNA demethylation in the endosperm these regions are targeted by the FIS PcG complex, conferring tight repression of maternal PEG alleles. In support of this hypothesis, we found high H3K27me3 levels in PEG flanking regions, which were much higher than the H3K27me3 levels present in vegetative tissues (Figure 6, Figure S4 and Figure S6).
The impact of hypomethylation on the activity status of the paternal MEG alleles was contrasted by the lack of CG DNA methylation in the immediate vicinity of several MEGs. However, MEGs lacking CG methylation often contained substantial levels of non-CG DNA methylation (Figure S5), implicating that non-CG DNA methylation regulates paternal MEG alleles. Reactivation of these alleles upon loss of MET1 function might be a consequence of a proposed feedback between CG and non-CG DNA methylation [53], [54]. Therefore, it seems possible that the paternal alleles of some MEGs are silenced by non-CG methylation established specifically in the endosperm and reactivation of the paternal alleles requires loss of non-CG methylation.
In stark contrast to silencing of MEGs and PEGs during vegetative development, many MEGs and PEGs were expressed in pollen. The vegetative cell of pollen has low levels of DNA methylation [55], suggesting that reduced levels of DNA methylation in the vegetative cell cause activation of MEGs, similar to the activation of maternal MEG alleles in the endosperm. Whether activation of PEGs in pollen is caused by reduced PcG protein activity in the vegetative cell of pollen remains to be tested.
Evolution of Imprinted Genes
Our study also sheds light on the evolution of imprinted genes, as we found a significant enrichment of transposon insertions in vicinity to MEGs and PEGs. In particular helitrons were strongly enriched in the vicinity of PEGs and were also overrepresented (albeit not statistically significant) in the vicinity of MEGs. Helitrons are eukaryotic DNA transposons which constitute >2% of the Arabidopsis genome. A striking feature of helitrons is their capacity to capture and propagate host genes, making them powerful factors shaping the evolution of genomes [56]. Although the rate of gene capture in Arabidopsis is predicted be to low compared to the major occurrence of gene capture in maize [57], it is possible that these predictions are a drastic underestimation due to rapid purging of helitron elements in Arabidopsis [58]. Thus, it is possible that helitron-mediated gene duplications which generate increased gene dosage may set the ground for imprinted gene evolution. Interestingly, we found a higher incidence of MEGs and PEGs having close sequence homologs, however, whether these duplications are a consequence of helitron activity remains to be shown.
If parental conflicts involving imprinted genes are mediated by amino-acid changes in the gene products of imprinted loci under antagonistic co-evolution, such imprinted loci may be subject to rapid evolution, possibly under positive Darwinian selection [27]–[29]. The identification of 60 potentially imprinted genes in Arabidopsis provided the opportunity for an initial exploration of whether the MEGs and PEGs identified displayed any evidence of rapid evolution. When the dN/dS values of the imprinted genes (MEGs and PEGs and accession-dependent MEGs and PEGs) were compared with the average dN/dS values for all other genes in the genome it is clear that imprinted genes in Arabidopsis are more rapidly evolving. Furthermore, a significant proportion of the MEGs displayed dN/dS values greater than 1 which is indicative of fast evolving genes. Further sequencing of these imprinted genes in populations and outgroup species will determine whether these genes are undergoing positive Darwinian selection or are under relaxed constraints.
Imprinted genes are predominantly expressed in the endosperm, implicating a specific role of these genes during endosperm development. Alternatively, it is possible that imprinted genes are on the trajectory to become pseudogenes and therefore, are silenced during vegetative development. However, the fact that many imprinted genes have functional roles as transcription factors or have chromatin modifying activity supports a proposed functional role of imprinted genes for endosperm development. To identify these functions and to test whether MEGs and PEGs have indeed antagonistic roles in controlling endosperm growth as it has been proposed previously [21], [22], will remain the challenge of future investigations.
Materials and Methods
Plant Material and Growth Conditions
The Arabidopsis thaliana accessions used in this study were Col-0 and Bur-0. The fis2-5 and met1-3 mutants (both in Col accession) were described previously [52], . The newly identified fie-12 allele (GK_362D08; Col-0 accession) contains a T-DNA insertion within the third exon. The fie-12 seed abortion ratio and mutant seed phenotypes were analyzed and found to be similar to previously published fie alleles (data not shown). All mutants were heterozygous and the genotype confirmed by PCR analysis. Plants were grown in a growth cabinet under long day photoperiods (16 h light and 8 h dark) at 22°C. After 10 days, seedlings were transferred to soil and plants were grown in a growth chamber at 60% humidity and daily cycles of 16 h light at 22°C and 8 h darkness at 18°C. For reciprocal crosses, designated female partners were emasculated, and the pistils hand-pollinated two days after emasculation.
Phenotypic Analysis of Seed Development in Col-0 and Bur-0 Accessions
To control for variations in seed development between Col-0 and Bur-0 accessions, siliques were harvested 4 DAP and fixed overnight in 9 : 1 ethanol:acetic acid. Siliques were dissected to release seeds into clearing solution (67% chloralhydrate in 8% glycerol) for overnight incubation. Microscopy imaging was performed using a Leica DM 2500 microscope using DIC optics (Leica Microsystems, Wetzlar, Germany), images were captured using a Leica DFC300 FX digital camera (Leica) and exported using Leica Application Suite Version 2.4.0.R1 (Leica Microsystems) and processed using Photoshop 7.0 (Adobe).
RNA Extraction and cDNA Synthesis
Seeds of at least 40 siliques per sample were harvested into 50 µl RNAlater (Sigma, Buchs, Switzerland) at 4 DAP. Glass beads (1.25–1.55 mm) were added, and the samples were ground unfrozen in a Silamat S5 (Ivoclar Vivadent, Ellwangen, Germany). RNA was extracted using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. For cDNA synthesis residual DNA was removed using the Qiagen RNase-free DNase Set and cDNA was synthesized using the Fermentas First strand cDNA synthesis kit (Fermentas, Burlington, Canada) according to the manufactureŕs instruction.
Preparation of mRNA-Sequencing Libraries
Sequencing libraries were prepared with the Illumina mRNA-Seq Sample Prep Kit (Illumina, San Diego, USA) according to the manufacturer's instructions. After adapter ligation library fragments of ∼250 bp were isolated from an agarose gel. The DNA was PCR amplified with Illumina primers for 15 cycles, purified and loaded on an Illumina flow cell for cluster generation. Libraries were sequenced on the Illumina Genome Analyzer II following the manufacturer's protocols.
Bioinformatic Analysis
Identification of SNP-associated reads
TAIR8 chromosome sequences were downloaded from TAIR (ftp.arabidopsis.org/home/tair/Genes/TAIR8_genome_release/tair8.at.chromosomes.fas, chromosomes 1 to5), together with the TAIR8 genome annotation (ftp.arabidopsis.org/home/tair/Genes/TAIR8_genome_release/TAIR8_gff3/TAIR8_GFF3_genes.gff). Bur-0 SNPs [30], 569,859 SNPs in total for chromosomes 1 to 5, were downloaded from the 1001 genomes website (http://1001genomes.org/data/MPI/MPIOssowski2008/releases/2008_06_05/strains/Bur-0/Bur-0_homozygous_snp_080605.txt). For each of the 569,859 Col-0/Bur-0 SNPs, we extracted a 71nt genomic window around the SNP (SNP position plus/minus 35nt) from the Col-0 reference sequence and annotated it as a Col-0 window. The nucleotide in the SNP was then replaced by the Bur-0 variant and the resulting sequence annotated as a Bur-0 window.
A suffix array was constructed from the union of Col-0 and Bur-0 windows with the mkvtree program (http://www.vmatch.de/) [60]. RNAseq reads were mapped with the vmatch program (http://www.vmatch.de/) in both forward and reverse complementary orientation (options -d and -p), allowing up to two mismatches (option -h 2), requiring the whole read to map (option -l 36), and generating maximal substring matches that are unique in the Col-0/Bur-0 window dataset (option -mum cand). This procedure resulted in 8,576,779 matches for Bur-x-Col reads (out of 102,705,076 total reads) and 6,773,239 matches for Col-x-Bur reads (out of 122,367,092 total reads). SNP windows were associated with gene ids via the TAIR8 genome annotation by overlapping with or being included in a gene region (gene start to end, ignoring exon/intron structure). SNP windows that were associated with more than one gene were discarded. From the remaining SNP windows, the grand total associated with a gene was defined as that gene's expression level. 147,349 SNP windows were matched by at least one read in one of the two reciprocal experiments, accounting for 19,161 genes, out of 25,189 genes that had at least one SNP in at least one exon, out of 28,523 annotated genes in total. Sequencing raw data generated in this study are available at GEO, accession number GSE27292.
Testing for allele-specific expression
For each gene and cross (Col-0×Bur-0 and Bur-0×Col-0), we performed a binomial one-sided test against the null-hypothesis of 2m∶1p expression. The resulting p-values were the probabilities of deviation from the expected 2m∶1p ratio towards either larger maternal expression or larger paternal expression under the null-hypothesis of an unbiased 2m∶1p expression. The two p-values for maternal expression from the two reciprocal crosses (p1, p2) were summarized in a joint p-value based on the distribution of the second-order statistic by calculating p = max(p1,p2)∧2. Joint p-values for paternal expression were calculated analogously. Joint p-values, either describing reciprocal maternal expression or reciprocal paternal expression, were sorted in ascending order (from significant to insignificant), and for each joint p-value the false-discovery rate (FDR) [61] was calculated, as q = p*n/i, where n was the overall number of joint p-values and i was the rank of a given p-value. Genes with a q-value of 0.05 or less were selected as maternally or paternally expressed genes.
Parental-specific splicing was tested by analyzing for every candidate gene the numbers of reads across all SNPs of that gene, using Pearson's chi-square test (R function chisq.test with parameter simulate.p.value = T).
Filtering for endosperm-expressed genes and analysis of MEGs and PEGs
Filtering for endosperm-specific gene expression was performed by using data from endosperm transcript profiles generated in the laboratories of Bob Goldberg (UCLA), John Harada (UC Davis), Brandon Le (UCLA), Anhthu Bui (UCLA), and Julie Pelletier (UC Davis) that are available under http://estdb.biology.ucla.edu/genechip/project [62]. The same data were used to generate Figure 3. Reference transcript profiles from vegetative tissues (seedlings, cotyledons, hypocotyl, leaves, stems, roots, shoot apical meristem), flowers and siliques were published by [63]. Genes were considered as preferentially expressed in the endosperm if the SLRs in one of the endosperm domains were at least fivefold higher than the SLRs of the seed coat and SLRs were below five in vegetative tissues. MEGs with low mRNA levels (read counts higher or equal 10 and smaller or equal 30) were considered as being endosperm-preferred expressed if SLRs in one of the endosperm domains were at least threefold higher compared to the seed coat and SLRs were below five in vegetative tissues (Table S3). Genes were considered as being expressed in the endosperm with SLRs>4.5 in at least one of the endosperm domains (Table S5). The transcript profiles of wild-type and fis2 seeds at 3 and 6 DAP as well as seeds derived from interploidy crosses were published by [46], [52]. H3K27me3 profiling data from vegetative tissues and the endosperm were published by [52], [64]. Clustering analysis of expression profiles was performed using TM4 software [65]. DNA methylation profiles were taken from [7], [41] and were visualized using R software (http://www.r-project.org/). Enrichment of GO categories (obtained from TAIR) was tested based on the hypergeometric test and multiple-testing correction according to [61] with a critical p-value of 5.0E-03. Homologous genes were identified using the blastp program from blastall (http://www.ncbi.nlm.nih.gov/staff/tao/URLAPI/blastall/) by applying matrix BLOSUM62 and a critical e-value of 0.01. Identification of MEG and PEG clusters was performed by establishing the frequency of MEGs and PEGs present in windows of a defined size using a sliding window analysis. p values were calculated from a reference distribution that was based on an identical number of randomly sampled genes. Transposable elements were identified based on information in the TAIR database (ftp://ftp.arabidopsis.org/home/tair/Genes/TAIR8_genome_release/TAIR8_Transposable_Elements.txt). Statistical testing was performed using a hypergeometric test as well as a permutation test. Both tests gave the same result.
Testing for evidence of rapid evolution of MEGs and PEGs
Reciprocal BLASTP searches were performed between Arabidopsis thaliana versus Arabidopsis lyrata (i.e. Arabidopsis thaliana peptides versus Arabidopsis lyrata peptide database and Arabidopsis lyrata peptides versus Arabidopsis thaliana peptide database) of all MEGs and PEGs listed in Tables S4 and S7, respectively. The reciprocal top hit sequences were then aligned at the peptide level using MUSCLE [66]. Using the peptide alignment as a template the reciprocal top hit CDS sequences were then aligned using the tranalign [67]. Pairwise dN/dS analysis was then performed on the CDS alignments using both the CodeML program (using model 0 and runmode -2) and yn00 both from the PAML package [68]. The values from CodeML are reported in the main text, values for both CodeML and yn00 are found in Tables S9, S10 and S11.
Validation of RNA-Sequencing Results
Selected loci were validated using independently prepared RNAs from reciprocal crosses between Col-0 and Bur-0. Primers used for allele specific expression analysis of selected genes are specified in Table S20. The amplified products were either digested with indicated restriction enzymes (Table S20) and analyzed on agarose gels or by DNA sequencing.
Supporting Information
Zdroje
1. KöhlerCWeinhofer-MolischI 2010 Mechanisms and evolution of genomic imprinting in plants. Heredity 105 57 63
2. JullienPEBergerF 2009 Gamete-specific epigenetic mechanisms shape genomic imprinting. Curr Opin Plant Biol 12 637 642
3. JahnkeSScholtenS 2009 Epigenetic resetting of a gene imprinted in plant embryos. Curr Biol 19 1677 1681
4. BergerF 2003 Endosperm: the crossroad of seed development. Curr Opin Plant Biol 6 42 50
5. DrewsGNYadegariR 2002 Development and function of the angiosperm female gametophyte. Annu Rev Genet 36 99 124
6. GehringMBubbKLHenikoffS 2009 Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science 324 1447 1451
7. HsiehTFIbarraCASilvaPZemachAEshed-WilliamsL 2009 Genome-wide demethylation of Arabidopsis endosperm. Science 324 1451 1454
8. JullienPEMosqunaAIngouffMSakataTOhadN 2008 Retinoblastoma and its binding partner MSI1 control imprinting in Arabidopsis. PLoS Biol 6 e194 doi:10.1371/journal.pbio.0060194
9. TeixeiraFKColotV 2010 Repeat elements and the Arabidopsis DNA methylation landscape. Heredity 105 14 23
10. KöhlerCPageDRGagliardiniVGrossniklausU 2005 The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat Genet 37 28 30
11. BarouxCGagliardiniVPageDRGrossniklausU 2006 Dynamic regulatory interactions of Polycomb group genes: MEDEA autoregulation is required for imprinted gene expression in Arabidopsis. Genes Dev 20 1081 1086
12. GehringMHuhJHHsiehTFPentermanJChoiY 2006 DEMETER DNA glycosylase establishes MEDEA Polycomb gene self-imprinting by allele-specific demethylation. Cell 124 495 506
13. JullienPEKatzAOlivaMOhadNBergerF 2006 Polycomb group complexes self-regulate imprinting of the Polycomb group gene MEDEA in Arabidopsis. Curr Biol 16 486 492
14. Fitz GeraldJNHuiPSBergerF 2009 Polycomb group-dependent imprinting of the actin regulator AtFH5 regulates morphogenesis in Arabidopsis thaliana. Development 136 3399 3404
15. HennigLDerkachevaM 2009 Diversity of Polycomb group complexes in plants: same rules, different players? Trends Genet 25 414 423
16. ChoiYGehringMJohnsonLHannonMHaradaJJ 2002 DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in Arabidopsis. Cell 110 33 42
17. XiaoWGehringMChoiYMargossianLPuH 2003 Imprinting of the MEA Polycomb gene is controlled by antagonism between MET1 methyltransferase and DME glycosylase. Dev Cell 5 891 901
18. MakarevichGVillarCBErilovaAKöhlerC 2008 Mechanism of PHERES1 imprinting in Arabidopsis. J Cell Sci 121 906 912
19. VillarCBErilovaAMakarevichGTröschRKöhlerC 2009 Control of PHERES1 imprinting in Arabidopsis by direct tandem repeats. Mol Plant 2 654 660
20. BarlowDP 1993 Methylation and imprinting: from host defense to gene regulation? Science 260 309 310
21. HaigDWestobyM 1989 Parent specific gene expression and the triploid endosperm. Am Nature 134 147 155
22. TriversRBurtA 1999 Kinship and genomic imprinting. Results Probl Cell Differ 25 1 21
23. ReikWConstanciaMFowdenAAndersonNDeanW 2003 Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J Physiol 547 35 44
24. ChaudhuryAMMingLMillerCCraigSDennisES 1997 Fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci USA 94 4223 4228
25. KiyosueTOhadNYadegariRHannonMDinnenyJ 1999 Control of fertilization-independent endosperm development by the MEDEA Polycomb gene in Arabidopsis. Proc Natl Acad Sci U S A 96 4186 4191
26. TiwariSSchulzRIkedaYDythamLBravoJ 2008 MATERNALLY EXPRESSED PAB C-TERMINAL, a Novel Imprinted Gene in Arabidopsis, Encodes the Conserved C-Terminal Domain of Polyadenylate Binding Proteins. Plant Cell 20 2387 2398
27. SpillaneCSchmidKJLaoueille-DupratSPienSEscobar-RestrepoJM 2007 Positive darwinian selection at the imprinted MEDEA locus in plants. Nature 448 349 352
28. MiyakeTTakebayashiNWolfDE 2009 Possible diversifying selection in the imprinted gene, MEDEA, in Arabidopsis. Mol Biol Evol 26 843 857
29. O'ConnellMJLoughranNBWalshTADonoghueMTSchmidKJ 2010 A phylogenetic approach to test for evidence of parental conflict or gene duplications associated with protein-encoding imprinted orthologous genes in placental mammals. Mamm Genome 21 486 498
30. OssowskiSSchneebergerKClarkRMLanzCWarthmannN 2008 Sequencing of natural strains of Arabidopsis thaliana with short reads. Genome Res 18 2024 2033
31. KinoshitaTMiuraAChoiYKinoshitaYCaoX 2004 One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science 303 521 523
32. KermicleJ 1970 Dependence of the R-mottled aleurone phenotype in maize on the mode of sexual transmission. Genetics 66 69 85
33. ChaudhuriSMessingJ 1994 Allele-specific parental imprinting of dzr1, a posttranscriptional regulator of zein accumulation. Proc Natl Acad Sci U S A 91 4867 4871
34. ChristensenCKingEJordanJDrewsGN 1997 Megagametogenesis in Arabidopsis wild type and the Gf mutant. Sexual Plant Reproduction 10 49 64
35. BayerMNawyTGiglioneCGalliMMeinnelT 2009 Paternal control of embryonic patterning in Arabidopsis thaliana. Science 323 1485 1488
36. ColomboMMasieroSVanzulliSLardelliPKaterMM 2008 AGL23, a type I MADS-box gene that controls female gametophyte and embryo development in Arabidopsis. Plant J 54 1037 1048
37. de FolterSImminkRGKiefferMParenicovaLHenzSR 2005 Comprehensive interaction map of the Arabidopsis MADS Box transcription factors. Plant Cell 17 1424 1433
38. KangIHSteffenJGPortereikoMFLloydADrewsGN 2008 The AGL62 MADS domain protein regulates cellularization during endosperm development in Arabidopsis. Plant Cell 20 635 647
39. RaiKHugginsIJJamesSRKarpfARJonesDA 2008 DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell 135 1201 1212
40. PoppCDeanWFengSCokusSJAndrewsS 2010 Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature 463 1101 1105
41. ZilbermanDGehringMTranRKBallingerTHenikoffS 2007 Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat Genet 39 61 69
42. Bourc'hisDVoinnetO 2010 A small-RNA perspective on gametogenesis, fertilization, and early zygotic development. Science 330 617 622
43. FengSJacobsenSEReikW 2010 Epigenetic reprogramming in plant and animal development. Science 330 622 627
44. JullienPEKinoshitaTOhadNBergerF 2006 Maintenance of DNA methylation during the Arabidopsis life cycle is essential for parental imprinting. Plant Cell 18 1360 1372
45. HsiehTFShinJUzawaRSilvaPCohenS 2011 Inaugural Article: Regulation of imprinted gene expression in Arabidopsis endosperm. Proc Natl Acad Sci U S A
46. ErilovaABrownfieldLExnerVRosaMTwellD 2009 Imprinting of the Polycomb group gene MEDEA serves as a ploidy sensor in Arabidopsis. PLoS Genet 5 e1000663 doi:10.1371/journal.pgen.1000663
47. TiwariSSpielmanMSchulzROakeyRJKelseyG 2010 Transcriptional profiles underlying parent-of-origin effects in seeds of Arabidopsis thaliana. BMC Plant Biol 10 72
48. JullienPEBergerF 2010 Parental genome dosage imbalance deregulates imprinting in Arabidopsis. PLoS Genet 6 e1000885 doi:10.1371/journal.pgen.1000885
49. SpillaneCBarouxCEscobar-RestrepoJMPageDRLaoueilleS 2004 Transposons and tandem repeats are not involved in the control of genomic imprinting at the MEDEA locus in Arabidopsis. Cold Spring Harb Symp Quant Biol 69 465 475
50. WanLBBartolomeiMS 2008 Regulation of imprinting in clusters: noncoding RNAs versus insulators. Adv Genet 61 207 223
51. DilkesBPSpielmanMWeizbauerRWatsonBBurkart-WacoD 2008 The maternally expressed WRKY transcription factor TTG2 controls lethality in interploidy crosses of Arabidopsis. PLoS Biol 6 e308 doi:10.1371/journal.pbio.0060308
52. WeinhoferIHehenbergerERoszakPHennigLKöhlerC 2010 H3K27me3 profiling of the endosperm implies exclusion of polycomb group protein targeting by DNA methylation. PLoS Genet 6 e1001152 doi:10.1371/journal.pgen.1001152
53. ChanSWHendersonIRZhangXShahGChienJS 2006 RNAi, DRD1, and histone methylation actively target developmentally important non-CG DNA methylation in arabidopsis. PLoS Genet 2 e83 doi:10.1371/journal.pgen.0020083
54. ListerRO'MalleyRCTonti-FilippiniJGregoryBDBerryCC 2008 Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 133 523 536
55. SlotkinRKVaughnMBorgesFTanurdzicMBeckerJD 2009 Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136 461 472
56. KapitonovVVJurkaJ 2007 Helitrons on a roll: eukaryotic rolling-circle transposons. Trends Genet 23 521 529
57. HollisterJDGautBS 2007 Population and evolutionary dynamics of Helitron transposable elements in Arabidopsis thaliana. Mol Biol Evol 24 2515 2524
58. SweredoskiMDeRose-WilsonLGautBS 2008 A comparative computational analysis of nonautonomous helitron elements between maize and rice. BMC Genomics 9 467
59. SazeHScheidOMPaszkowskiJ 2003 Maintenance of CpG methylation is essential for epigenetic inheritance during plant gametogenesis. Nat Genet 34 65 69
60. AbouelhodaMKurtzSOhlebuschE 2004 Replacing suffix trees with enhanced suffix arrays. Journal of Discrete Algorithms 2 53 86
61. BenjaminiYHochbergY 1995 Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B 57 289 300
62. LeBHChengCBuiAQWagmaisterJAHenryKF 2010 Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc Natl Acad Sci U S A 107 8063 8070
63. SchmidMDavisonTSHenzSRPapeUJDemarM 2005 A gene expression map of Arabidopsis thaliana development. Nat Genet 37 501 506
64. ZhangXClarenzOCokusSBernatavichuteYVPellegriniM 2007 Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol 5 e129 doi:10.1371/journal.pbio.0050129
65. SaeedAISharovVWhiteJLiJLiangW 2003 TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34 374 378
66. EdgarRC 2004 MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32 1792 1797
67. RicePLongdenIBleasbyA 2000 EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16 276 277
68. YangZ 1997 PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci 13 555 556
Štítky
Genetika Reprodukční medicína
Článek Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding SitesČlánek Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene SilencingČlánek Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse GutČlánek FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field SizeČlánek Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian SpermatogenesisČlánek Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese PopulationČlánek Differential Effects of and Risk Variants on Association with Diabetic ESRD in African AmericansČlánek Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 6
-
Všechny články tohoto čísla
- Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding Sites
- Statistical Inference on the Mechanisms of Genome Evolution
- Revisiting Heterochromatin in Embryonic Stem Cells
- A Two-Stage Meta-Analysis Identifies Several New Loci for Parkinson's Disease
- Identification of a Sudden Cardiac Death Susceptibility Locus at 2q24.2 through Genome-Wide Association in European Ancestry Individuals
- Genomic Prevalence of Heterochromatic H3K9me2 and Transcription Do Not Discriminate Pluripotent from Terminally Differentiated Cells
- Epistasis between Beneficial Mutations and the Phenotype-to-Fitness Map for a ssDNA Virus
- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Telomere DNA Deficiency Is Associated with Development of Human Embryonic Aneuploidy
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Unexpected Role for DNA Polymerase I As a Source of Genetic Variability
- Transportin-SR Is Required for Proper Splicing of Genes and Plant Immunity
- How Chromatin Is Remodelled during DNA Repair of UV-Induced DNA Damage in
- Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene Silencing
- Two Evolutionary Histories in the Genome of Rice: the Roles of Domestication Genes
- Natural Allelic Variation Defines a Role for : Trichome Cell Fate Determination
- Multiple Common Susceptibility Variants near BMP Pathway Loci , , and Explain Part of the Missing Heritability of Colorectal Cancer
- Pathogenic Mechanism of the FIG4 Mutation Responsible for Charcot-Marie-Tooth Disease CMT4J
- A Functional Variant in Promoter Modulates Its Expression and Confers Disease Risk for Systemic Lupus Erythematosus
- Drift and Genome Complexity Revisited
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
- Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse Gut
- Pathways of Distinction Analysis: A New Technique for Multi–SNP Analysis of GWAS Data
- Web-Based Genome-Wide Association Study Identifies Two Novel Loci and a Substantial Genetic Component for Parkinson's Disease
- Chk2 and p53 Are Haploinsufficient with Dependent and Independent Functions to Eliminate Cells after Telomere Loss
- Exome Sequencing Identifies Mutations in High Myopia
- Distinct Functional Constraints Partition Sequence Conservation in a -Regulatory Element
- CorE from Is a Copper-Dependent RNA Polymerase Sigma Factor
- A Single Sex Pheromone Receptor Determines Chemical Response Specificity of Sexual Behavior in the Silkmoth
- FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field Size
- Maps of Open Chromatin Guide the Functional Follow-Up of Genome-Wide Association Signals: Application to Hematological Traits
- Increased Susceptibility to Cortical Spreading Depression in the Mouse Model of Familial Hemiplegic Migraine Type 2
- Differential Gene Expression and Epiregulation of Alpha Zein Gene Copies in Maize Haplotypes
- Parallel Adaptive Divergence among Geographically Diverse Human Populations
- Genetic Analysis of Genome-Scale Recombination Rate Evolution in House Mice
- Mechanisms for the Evolution of a Derived Function in the Ancestral Glucocorticoid Receptor
- Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian Spermatogenesis
- Interferon Regulatory Factor 8 Regulates Pathways for Antigen Presentation in Myeloid Cells and during Tuberculosis
- High-Resolution Analysis of Parent-of-Origin Allelic Expression in the Arabidopsis Endosperm
- Specific SKN-1/Nrf Stress Responses to Perturbations in Translation Elongation and Proteasome Activity
- Graded Nodal/Activin Signaling Titrates Conversion of Quantitative Phospho-Smad2 Levels into Qualitative Embryonic Stem Cell Fate Decisions
- Genome-Wide Analysis Reveals PADI4 Cooperates with Elk-1 to Activate Expression in Breast Cancer Cells
- Trait Variation in Yeast Is Defined by Population History
- Meiosis-Specific Loading of the Centromere-Specific Histone CENH3 in
- A Genome-Wide Survey of Imprinted Genes in Rice Seeds Reveals Imprinting Primarily Occurs in the Endosperm
- Multiple Regulatory Mechanisms to Inhibit Untimely Initiation of DNA Replication Are Important for Stable Genome Maintenance
- SIRT1 Promotes N-Myc Oncogenesis through a Positive Feedback Loop Involving the Effects of MKP3 and ERK on N-Myc Protein Stability
- Bacteriophage Crosstalk: Coordination of Prophage Induction by Trans-Acting Antirepressors
- Role of the Single-Stranded DNA–Binding Protein SsbB in Pneumococcal Transformation: Maintenance of a Reservoir for Genetic Plasticity
- Genomic Convergence among ERRα, PROX1, and BMAL1 in the Control of Metabolic Clock Outputs
- Genome-Wide Association of Bipolar Disorder Suggests an Enrichment of Replicable Associations in Regions near Genes
- Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese Population
- DNA Ligase III Promotes Alternative Nonhomologous End-Joining during Chromosomal Translocation Formation
- Differential Effects of and Risk Variants on Association with Diabetic ESRD in African Americans
- Finished Genome of the Fungal Wheat Pathogen Reveals Dispensome Structure, Chromosome Plasticity, and Stealth Pathogenesis
- Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
- Extracellular Matrix Dynamics in Hepatocarcinogenesis: a Comparative Proteomics Study of Transgenic and Null Mouse Models
- Integrating 5-Hydroxymethylcytosine into the Epigenomic Landscape of Human Embryonic Stem Cells
- Vive La Différence: An Interview with Catherine Dulac
- Multiple Loci Are Associated with White Blood Cell Phenotypes
- Nuclear Accumulation of Stress Response mRNAs Contributes to the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- A New Mutation Affecting FRQ-Less Rhythms in the Circadian System of
- Cryptic Transcription Mediates Repression of Subtelomeric Metal Homeostasis Genes
- A New Isoform of the Histone Demethylase JMJD2A/KDM4A Is Required for Skeletal Muscle Differentiation
- Genetic Determinants of Lipid Traits in Diverse Populations from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- A Genome-Wide RNAi Screen for Factors Involved in Neuronal Specification in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Statistical Inference on the Mechanisms of Genome Evolution
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



