-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
article has not abstract
Published in the journal: . PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004745
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004745Summary
article has not abstract
PrPSc, a misfolded, aggregation-prone isoform of the cellular prion protein (PrPC), is the infectious prion agent responsible for incurable brain diseases such as scrapie of sheep, bovine spongiform encephalopathy, and its human counterpart, variant Creutzfeldt-Jakob disease. In these disorders, collectively known as prion diseases, exogenous PrPSc propagates in the infected host by imprinting its aberrant conformation onto endogenous PrPC, eventually triggering a rapidly progressing neurodegenerative process that invariably leads to death. But what is the function of PrPC besides serving as a substrate for the generation of PrPSc? And how does PrPC misfolding cause neurological disease?
The Cellular Prion Protein
PrPC is a cell surface glycoprotein expressed in neurons and many other body cells. It is synthesized in the endoplasmic reticulum (ER), where it undergoes oxidative folding, N-linked glycosylation, and addition of a glycosyl-phosphatidyl-inositol (GPI) anchor that attaches the protein’s C terminus to the lipid bilayer. After transit in the Golgi, PrPC is delivered to the plasma membrane, where it resides in lipid rafts, which are membrane microdomains rich in cholesterol and sphingolipids. Some PrPC molecules are constitutively endocytosed and either recycled to the plasma membrane or delivered to lysosomes for degradation. PrPC has a flexible N terminus (residues 23–127, mouse PrP numbering) that can interact with copper and zinc ions, and a C-terminal globular domain (residues 128–231) comprising three α-helices and two short anti-parallel β-strands.
Inactivation of the PrPC gene in living organisms produced variable phenotypes. Knockdown of the PrPC-related genes PrP-1 and PrP-2 in the zebrafish Danio rerio caused, respectively, gastrulation arrest and malformed brains and eyes, indicative of essential roles in the fish’s development [1,2]. In contrast, PrPC knockout mice and cows had no major developmental or neuroanatomical defects (reviewed in [3]), indicating non-essential or redundant functions in higher vertebrates.
Based on the analysis of mild phenotypic traits that develop in PrPC knockout mice and on cell culture studies, mammalian PrPC has been assigned roles in many biological processes, including neurotransmission, olfaction, proliferation and differentiation of neural precursor cells, myelin maintenance, copper and zinc ion transport, and calcium homeostasis, as well as neuroprotective activities against several toxic insults, such as oxidative and excitotoxic damage [2–4]. How can PrPC serve so many different functions? Perhaps the answer lies in its ability to interact with a number of membrane proteins, potentially influencing their cellular localization and activity [4]. These include glutamate receptors of the N-methyl-D-aspartate (NMDA) subclass [5] and voltage-gated calcium channels (VGCCs) [6]. Interaction with these channels may account for some of the functional activities of PrPC, but may also activate toxic responses when PrPC misfolds (see below).
PrPC Mediates PrPSc Neurotoxicity
The fact that inactivation of the PrPC gene in mice or cows does not cause neurodegeneration indicates that prion pathogenesis is not due to loss of PrPC function, but to a gain of toxicity upon its conversion to PrPSc. Interestingly, extracellular PrPSc kills only neurons that express PrPC. This was first shown by a neurografting experiment in which neural tissue from PrPC-expressing mice was transplanted into the brains of PrP knockout mice, which do not replicate prions since they lack the PrPC substrate for PrPSc production [7]. After intracerebral prion infection, the transplanted mice developed neuropathology in the PrPSc-replicating graft but not in the surrounding PrP knockout tissue, even though this tissue accumulated substantial amounts of graft-derived PrPSc [8]. Consistently with this, switching off neuronal PrPC expression in mice with established prion infection rescued clinical disease and prevented neuronal loss, despite continuous production of PrPSc by surrounding astrocytes [9]. Moreover, prion-infected mice expressing a form of PrPC that lacks the GPI anchor and is secreted into the extracellular space did not develop the typical prion pathology despite large amounts of extracellular PrPSc [10,11]. Thus, PrPSc is not directly toxic to neurons; it is the endogenous PrPC conversion that causes neuronal dysfunction and death.
Conformational conversion of PrPC starts on the neuronal surface, where PrPC interacts with exogenous PrPSc, and proceeds within endocytic compartments. Thus, neurotoxicity may be triggered by PrPC misfolding at the cell surface or inside the cell.
Toxicity Induced by PrPC Misfolding at the Neuronal Surface
Two kinds of evidence suggest that alterations in the structure of cell surface PrPC can lead to neuronal death. PrPC molecules with certain internal deletions, including Δ94–134 and Δ105–125, induce dramatic neurodegeneration when expressed in transgenic mice [12,13]. These mutant molecules are efficiently trafficked to lipid raft regions of the plasma membrane, suggesting that their toxicity stems from abnormal activity at the neuronal surface rather than from mislocalization or intracellular retention.
PrPC attenuates the activity of NMDA receptors (NMDARs), protecting neurons from glutamate-induced excitotoxicity [5]. Supporting the idea that the internal deletions may corrupt this function, PrPΔ105–125 sensitized neurons to glutamate-induced, calcium-mediated cell death [14]. It was also found that PrPΔ105–125 induced non-selective ionic currents that depended on the integrity of the N-terminal 23–31 region [15]. A possible interpretation is that the toxic deletions promote a conformational change of the PrPC N terminus, altering its interaction with NMDARs and enabling the 23–31 segment to interact abnormally with the lipid bilayer, generating pores in the plasma membrane. Thus, a structural change in cell surface PrPC would simultaneously corrupt NMDAR function and plasma membrane permeability, leading to dysregulation of ion homeostasis and neuronal death.
Another set of experiments showed that monoclonal antibodies against specific epitopes in the C-terminal globular domain of PrPC induce rapid neurodegeneration when injected into the mouse brain or applied to cultured cerebellar slices [16]. Neurodegeneration was prevented by deleting the PrPC N terminus or by antibodies against this region. The latter also attenuated the toxicity of PrPΔ94–134 [16], suggesting that the globular domain antibodies and the internal deletions activate a similar pathogenic cascade, involving a structural rearrangement of the N terminus.
Supporting the idea that PrPSc docking onto cell surface PrPC may elicit a similar structural change and downstream toxic effects (Fig 1), it was found that when PrPSc was exogenously presented to cultured neurons, the resulting neurotoxicity was blocked by NMDAR antagonists or by deletion of the N-terminal domain of neuronal PrPC [17,18].
Fig. 1. Theoretical model for how cell surface PrPC misfolding could result in neurotoxicity. 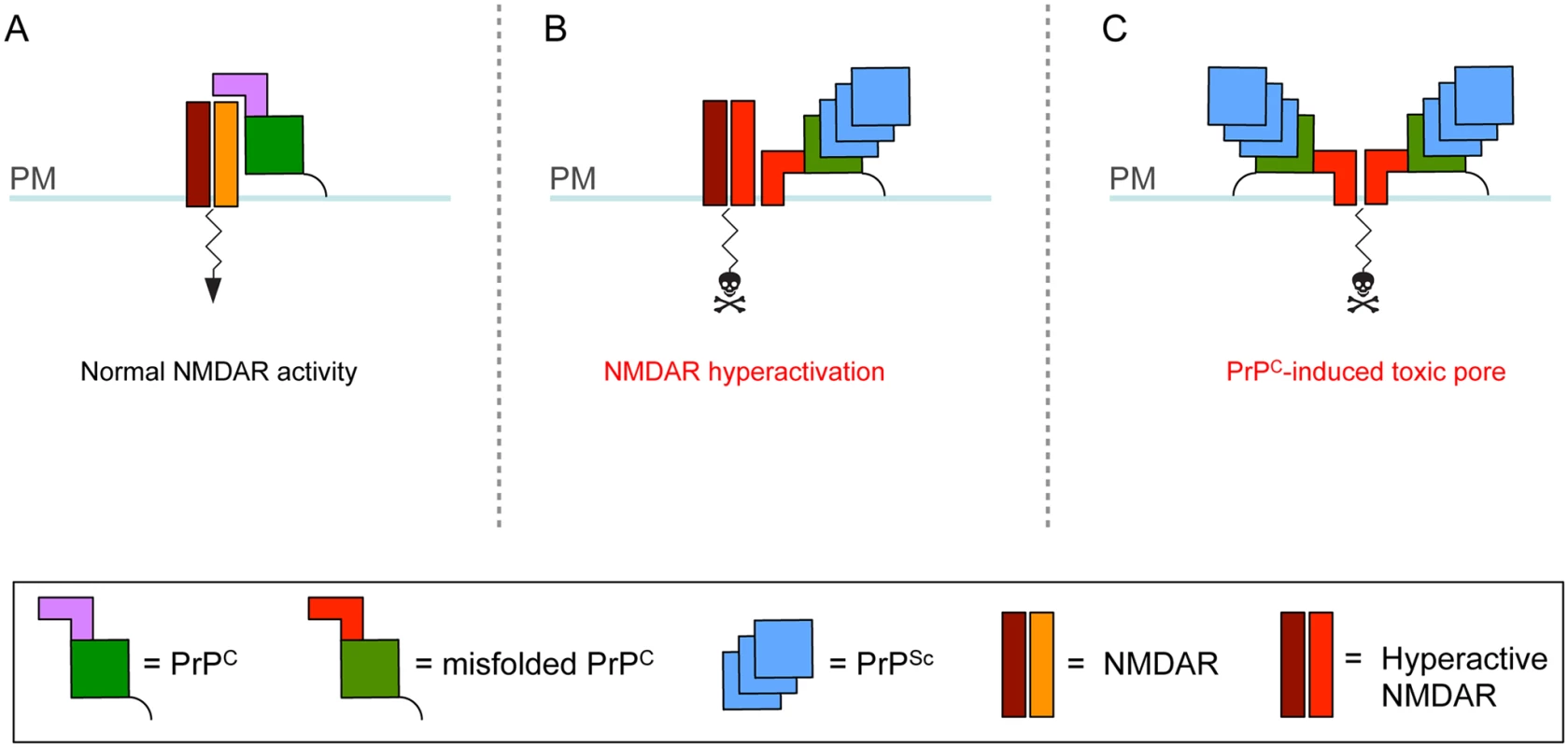
(A) PrPC consists of a flexible N terminus (mauve) and a globular C-terminal domain (green) attached to the plasma membrane (PM) by a GPI anchor (black line). PrPC associates with NMDARs, attenuating their activity [5]. (B–C) Interaction with extracellular PrPSc causes the N terminus of PrPC to undergo a structural rearrangement. This leads to aberrant interaction of PrPC with NMDARs and their hyperactivation (B) and/or abnormal insertion of the PrPC N terminus into the lipid bilayer with generation of a toxic pore (C). In addition to NMDARs, PrPC misfolding at the cell surface may corrupt the activity of other PrPC-interacting ion channels or signaling complexes. Neurotoxicity Induced by Intracellular PrPC Misfolding
A study in prion-infected mice gave information about a neurotoxic mechanism potentially triggered by intracellular accumulation of misfolded PrPC. Prions inoculated into the mouse hippocampus activates the translational repression pathway of the unfolded protein response (UPR). The UPR is a signal transduction cascade set in motion when misfolded proteins accumulate in the ER. A crucial step is auto-phosphorylation of the ER-associated kinase PERK, which phosphorylates the α subunit of the eukaryotic translation initiation factor 2 (eIF2α). This inhibits protein translation, reducing the overload of misfolded proteins. In the case of protracted UPR, however, sustained translational attenuation can have detrimental effects. In prion-infected mice, prolonged activation of the PERK/eIF2α pathway caused drops in the levels of pre - and post-synaptic proteins in the hippocampus, deficits in hippocampal synaptic transmission, and behavioral decline [19].
But what activates the UPR? PrPSc is unlikely to be the instigating factor, since it accumulates in the extracellular space or in endocytic compartments, rather than in the ER. The level of PrPC mRNA rises during prion infection, and the PrPC mRNA molecules escape eIF2α-P-induced translational inhibition [19]. Thus, ER overload with misfolded PrPC due to increased biosynthesis may be the actual cause of UPR activation. Alternatively, the UPR could be triggered by ER accumulation of CtmPrP, a transmembrane form of PrPC whose biogenesis at the ER membrane increases in prion-infected mice [20].
Prion infections are extremely rare in humans, in whom approximately 99% of all cases occur sporadically or are inherited because of mutations in the gene encoding PrPC. In these illnesses, PrPC misfolds spontaneously without the need for contact with exogenous PrPSc. When expressed in transgenic mice, PrPC molecules with certain genetic prion disease-associated mutations cause neurological syndromes that recapitulate key features of the corresponding human disorders [21–23]. These mutant PrPs misfold spontaneously in the ER lumen and are partly retained in the secretory pathway; surprisingly, however, they do not trigger the UPR [24,25]. How do they cause neurological disease? PrPC interacts physically with the α2δ-1 subunit of VGCCs [6]. This is a GPI-anchored protein which promotes the anterograde trafficking and correct synaptic localization and function of the channel complex. Owing to ER retention of mutant PrP, α2δ-1 accumulates intracellularly, impairing delivery of VGCCs to synapses. This leads to inefficient depolarization-induced calcium influx, abnormal cerebellar neurotransmission, and motor disease [6]. Since PrPC interacts with a number of other proteins that transit the secretory pathway, such as glutamate receptors and signaling complexes, its intracellular retention may have broader effects on neuronal function (Fig 2) [25].
Fig. 2. A role for intracellular PrPC retention in neuronal dysfunction. 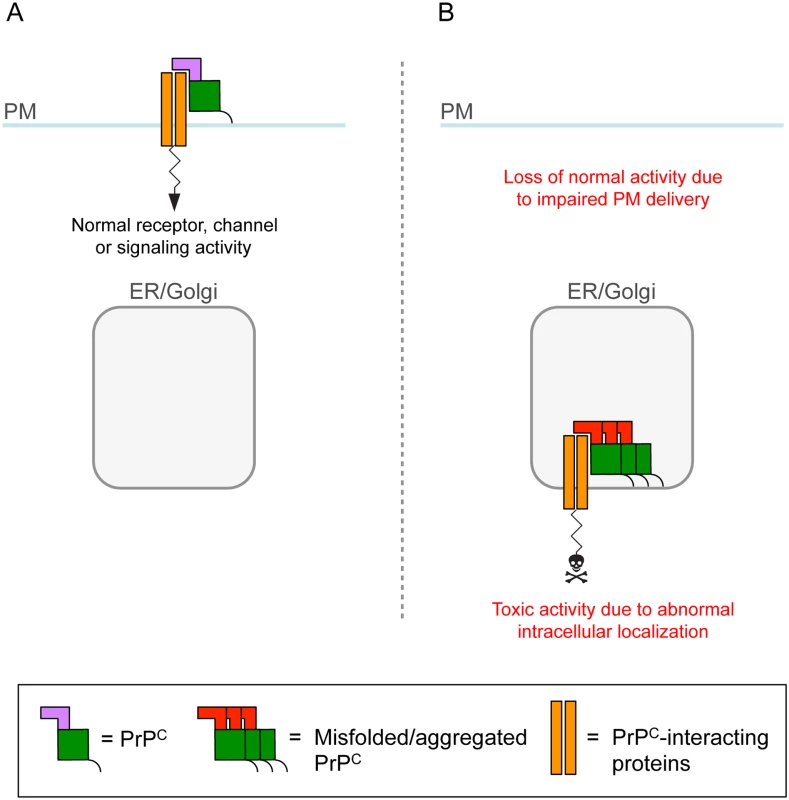
(A) PrPC on the plasma membrane (PM) influences the activity of neurotransmitter receptors, ion channels, and signaling complexes with which it interacts. (B) Owing to retention in transport organelles (ER/Golgi), misfolded/aggregated PrPC sequesters the interacting protein in intracellular compartments, leading to loss of normal function on the cell membrane [6]. Intracellular retention might also cause the complex to function abnormally and generate a toxic signal. Thus, in both acquired and genetic prion diseases, intracellular PrPC misfolding would ultimately alter synaptic proteostasis, either through an indirect, UPR-mediated mechanism, or by directly interfering with secretory trafficking of PrPC-interacting cargoes.
A Complex Interplay of Extracellular and Intracellular Toxicities
The experimental studies outlined above indicate different neurotoxic mechanisms that may be activated by misfolded PrPC in distinct cellular compartments, including corruption of PrPC interactions on the cell surface, disruption of plasma membrane permeability, impairment of secretory protein transport, and dysregulation of generic proteostatic pathways, such as the UPR.
These mechanisms are likely to co-exist, but may contribute differently to pathogenesis in different prion diseases. UPR-induced transcriptional attenuation may account for the synaptic dysfunction and degeneration that precedes neuronal death in the early stages of prion infection. As the disease progresses and PrPSc accumulates in the extracellular space, additional mechanisms may be engaged. PrPSc-induced misfolding of cell surface PrPC may be a key mediator of cell death [10] and cause rapid neuron demise by corrupting ion channel or signaling activities, and/or by generating toxic pores (Fig 1). In sporadic and genetic prion diseases, in which PrPSc formation is not obligatory for pathogenesis [26–28], spontaneous accumulation of misfolded PrPC molecules in transport organelles may be more important. Misfolded/aggregated PrPC may sequester ion channels or signaling complexes in intracellular compartments, leading to loss of their normal functions on the cell membrane and/or gain of toxic intracellular activities (Fig 2) [6].
This neurotoxic modality may contribute to the clinical variability of prion diseases. Different misfolded PrPC variants may be produced in different prion disorders, which may have different effects on neuronal function—hence, on the clinical presentation of disease—depending on their propensity to accumulate in intracellular organelles and interfere with the transport of the molecules with which they interact [25].
In view of their complex pathogenesis, what would be the best therapeutic option for prion diseases? Several compounds inhibit PrPSc propagation in cultured cells, but show little or no efficacy in vivo, and no therapeutically useful drug is currently available. The “mutability” of prions [29] means that molecules that target PrPSc can lead to the selection of drug-resistant variants that propagate more efficiently in the presence of the drug [30]. Given the emerging role of PrPC misfolding in neurotoxicity, drugs that stabilize its native conformation or down-regulate its expression may prove more effective, and applicable to the sporadic, genetic, and acquired forms.
Zdroje
1. Malaga-Trillo E, Solis GP, Schrock Y, Geiss C, Luncz L, et al. (2009) Regulation of embryonic cell adhesion by the prion protein. PLoS Biol 7: e55. doi: 10.1371/journal.pbio.1000055 19278297
2. Chiesa R, Harris DA (2009) Fishing for prion protein function. PLoS Biol 7: e75. doi: 10.1371/journal.pbio.1000075 19338390
3. Steele AD, Lindquist S, Aguzzi A (2007) The prion protein knockout mouse: a phenotype under challenge. Prion 1 : 83–93. 19164918
4. Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, et al. (2008) Physiology of the prion protein. Physiol Rev 88 : 673–728. doi: 10.1152/physrev.00007.2007 18391177
5. Khosravani H, Zhang Y, Tsutsui S, Hameed S, Altier C, et al. (2008) Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. J Cell Biol 181 : 551–565. doi: 10.1083/jcb.200711002 18443219
6. Senatore A, Colleoni S, Verderio C, Restelli E, Morini R, et al. (2012) Mutant PrP suppresses glutamatergic neurotransmission in cerebellar granule neurons by impairing membrane delivery of VGCC α2δ-1 subunit. Neuron 74 : 300–313. doi: 10.1016/j.neuron.2012.02.027 22542184
7. Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, et al. (1993) Mice devoid of PrP are resistant to scrapie. Cell 73 : 1339–1347. 8100741
8. Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, et al. (1996) Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature 379 : 339–343. 8552188
9. Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, et al. (2003) Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302 : 871–874. 14593181
10. Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, et al. (2005) Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 308 : 1435–1439. 15933194
11. Chesebro B, Race B, Meade-White K, Lacasse R, Race R, et al. (2010) Fatal transmissible amyloid encephalopathy: a new type of prion disease associated with lack of prion protein membrane anchoring. PLoS Pathog 6: e1000800. doi: 10.1371/journal.ppat.1000800 20221436
12. Baumann F, Tolnay M, Brabeck C, Pahnke J, Kloz U, et al. (2007) Lethal recessive myelin toxicity of prion protein lacking its central domain. Embo J 26 : 538–547. 17245436
13. Li A, Christensen HM, Stewart LR, Roth KA, Chiesa R, et al. (2007) Neonatal lethality in transgenic mice expressing prion protein with a deletion of residues 105–125. Embo J 26 : 548–558. 17245437
14. Biasini E, Unterberger U, Solomon IH, Massignan T, Senatore A, et al. (2013) A mutant prion protein sensitizes neurons to glutamate-induced excitotoxicity. J Neurosci 33 : 2408–2418. doi: 10.1523/JNEUROSCI.3406-12.2013 23392670
15. Solomon IH, Khatri N, Biasini E, Massignan T, Huettner JE, et al. (2011) An N-terminal polybasic domain and cell surface localization are required for mutant prion protein toxicity. J Biol Chem 286 : 14724–14736. doi: 10.1074/jbc.M110.214973 21385869
16. Sonati T, Reimann RR, Falsig J, Baral PK, O'Connor T, et al. (2013) The toxicity of antiprion antibodies is mediated by the flexible tail of the prion protein. Nature 501 : 102–106. doi: 10.1038/nature12402 23903654
17. Muller WE, Ushijima H, Schroder HC, Forrest JM, Schatton WF, et al. (1993) Cytoprotective effect of NMDA receptor antagonists on prion protein (PrionSc)-induced toxicity in rat cortical cell cultures. Eur J Pharmacol 246 : 261–267. 7901042
18. Resenberger UK, Harmeier A, Woerner AC, Goodman JL, Muller V, et al. (2011) The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication. EMBO J 30 : 2057–2070. doi: 10.1038/emboj.2011.86 21441896
19. Moreno JA, Radford H, Peretti D, Steinert JR, Verity N, et al. (2012) Sustained translational repression by eIF2αα-P mediates prion neurodegeneration. Nature 485 : 507–511. doi: 10.1038/nature11058 22622579
20. Hegde RS, Tremblay P, Groth D, DeArmond SJ, Prusiner SB, et al. (1999) Transmissible and genetic prion diseases share a common pathway of neurodegeneration. Nature 402 : 822–826. 10617204
21. Chiesa R, Piccardo P, Ghetti B, Harris DA (1998) Neurological illness in transgenic mice expressing a prion protein with an insertional mutation. Neuron 21 : 1339–1351. 9883727
22. Dossena S, Imeri L, Mangieri M, Garofoli A, Ferrari L, et al. (2008) Mutant prion protein expression causes motor and memory deficits and abnormal sleep patterns in a transgenic mouse model. Neuron 60 : 598–609. doi: 10.1016/j.neuron.2008.09.008 19038218
23. Bouybayoune I, Mantovani S, Del Gallo F, Bertani I, Restelli E, et al. (2015) Transgenic fatal familial insomnia mice indicate prion infectivity-independent mechanisms of pathogenesis and phenotypic expression of disease. PLoS Path 11: e1004796.
24. Quaglio E, Restelli E, Garofoli A, Dossena S, De Luigi A, et al. (2011) Expression of mutant or cytosolic PrP in transgenic mice and cells is not associated with endoplasmic reticulum stress or proteasome dysfunction. PLoS One 6: e19339. doi: 10.1371/journal.pone.0019339 21559407
25. Senatore A, Restelli E, Chiesa R (2013) Synaptic dysfunction in prion diseases: a trafficking problem? Int J Cell Biol 2013 : 543803. doi: 10.1155/2013/543803 24369467
26. Tateishi J, Kitamoto T (1995) Inherited prion diseases and transmission to rodents. Brain Pathol 5 : 53–59. 7767491
27. Mead S, Gandhi S, Beck J, Caine D, Gajulapalli D, et al. (2013) A novel prion disease associated with diarrhea and autonomic neuropathy. N Engl J Med 369 : 1904–1914. doi: 10.1056/NEJMoa1214747 24224623
28. Diack AB, Ritchie DL, Peden AH, Brown D, Boyle A, et al. (2014) Variably protease-sensitive prionopathy, a unique prion variant with inefficient transmission properties. Emerg Infect Dis 20 : 1969–1979. doi: 10.3201/eid2012.140214 25418327
29. Li J, Browning S, Mahal SP, Oelschlegel AM, Weissmann C (2010) Darwinian evolution of prions in cell culture. Science 327 : 869–872. doi: 10.1126/science.1183218 20044542
30. Berry DB, Lu D, Geva M, Watts JC, Bhardwaj S, et al. (2013) Drug resistance confounding prion therapeutics. Proc Natl Acad Sci U S A 110: E4160–4169. doi: 10.1073/pnas.1317164110 24128760
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR SelectionČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



