-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
Chagas disease, caused by Trypanosoma cruzi infection, represents the third greatest tropical disease burden in the world. No vaccine or suitable treatment is available for control of this infection. Based upon several studies we have conducted, we believe that TcG2 and TcG4 candidate antigens that are highly conserved in T. cruzi, expressed in clinically relevant forms of the parasite, and recognized by both B and T cell responses in multiple hosts, are an excellent choice for subunit vaccine development. In this study, we demonstrate that the delivery of TcG2 and TcG4 as a DNA-prime/protein-boost vaccine provided long-term protection from challenge infection, and this protection was associated with elicitation of long-lived CD8+ effector T cells. The longevity and efficacy of vaccine could be enhanced by booster immunization. We believe that this is the first report demonstrating a) a subunit vaccine can be useful in achieving long-term protection against T. cruzi infection and Chagas disease, and b) the effector T cells can be long-lived and play a role in vaccine elicited protection from parasitic infection.
Published in the journal: . PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004828
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004828Summary
Chagas disease, caused by Trypanosoma cruzi infection, represents the third greatest tropical disease burden in the world. No vaccine or suitable treatment is available for control of this infection. Based upon several studies we have conducted, we believe that TcG2 and TcG4 candidate antigens that are highly conserved in T. cruzi, expressed in clinically relevant forms of the parasite, and recognized by both B and T cell responses in multiple hosts, are an excellent choice for subunit vaccine development. In this study, we demonstrate that the delivery of TcG2 and TcG4 as a DNA-prime/protein-boost vaccine provided long-term protection from challenge infection, and this protection was associated with elicitation of long-lived CD8+ effector T cells. The longevity and efficacy of vaccine could be enhanced by booster immunization. We believe that this is the first report demonstrating a) a subunit vaccine can be useful in achieving long-term protection against T. cruzi infection and Chagas disease, and b) the effector T cells can be long-lived and play a role in vaccine elicited protection from parasitic infection.
Introduction
Chagas disease is prevalent in almost all Latin American countries, including Mexico and Central America [1]. Currently, ~11–18 million individuals are infected worldwide, and ~13,000 children and adults die annually because of the clinical complications of T. cruzi-induced heart disease and lack of effective treatments [2]. The vectorial, autochthonous, and congenital transmission of T. cruzi exists in the United States, where >300,000 infected individuals can potentially transfer infection through blood or organ donation [3–5]. When considered from a global perspective, Chagas disease represents the third greatest tropical disease burden after malaria and schistosomiasis [6].
Before setting the goal of vaccine development against any disease, an important question is whether vaccination is an economically viable approach with desirable health benefits. With regard to T. cruzi infection, the research community has pushed for a vaccine that can achieve complete parasite elimination from the host. However, several studies, including our published reports (reviewed in [7]), testing the efficacy of subunit vaccines have resulted in findings that vaccine-induced immunity can provide a reduction in tissue parasite burden associated with variable degrees of control of acute or chronic disease symptoms. The vaccine mediated control of infection and disease in experimental studies generally resembled that noted in 60–70% of the chagasic patients that remained seropositive and maintained residual parasites for their entire lives, but did not develop a clinically symptomatic form of the disease [2]. Further, recent computer simulation modeling of the impact of a prophylactic vaccine for Chagas disease showed that a vaccine would provide net cost savings (along with health benefits), even when the risk of infection is only 1%, vaccine efficacy is only 25%, and the cost of a vaccine is US$20 or lower [8]. Thus, it is ethically appropriate to consider a satisfactory vaccination goal to reduce the frequency and severity of clinical disease by decreasing the extent of persistent parasite burden; and accordingly, continuing efforts towards developing a vaccine against T. cruzi infection and Chagas disease are economically justifiable.
We have employed a computational/bioinformatics approach for unbiased screening of the T. cruzi genome database and identification of 11 potential candidates [9,10]. Through rigorous analysis over a period of several years, we determined that three candidates (TcG1, TcG2, TcG4) were maximally relevant for vaccine development [11]. These candidates were highly conserved in clinically relevant T. cruzi strains, expressed (mRNA/protein) in infective trypomastigote and intracellular amastigote stages of T. cruzi, and recognized by immunoglobulins and CD8+T cells in multiple T. cruzi-infected hosts [10,11].
We have examined the protective efficacy of TcG1, TcG2 and TcG4 (individually or in combination) in mice. Our data showed that co-delivery of the three antigens elicited additive immunity and protection from T. cruzi infection than was noted with individual candidate antigens [11]. Delivery of the 3-component vaccine by a DNA-prime/DNA-boost approach was less effective than the heterologous DNA-prime/protein-boost (D/P) approach in eliciting protective immunity [11–13]. Mice challenged with T. cruzi immediately after immunization with the 3-component D/P vaccine were capable of controlling 90–97% of the acute parasitemia and tissue parasite burden, and, subsequently, inflammatory infiltrate and tissue fibrosis were particularly absent in the heart and skeletal muscle of vaccinated mice [13].
In this study, we have sought to determine the long-term efficacy of the subunit vaccine against T. cruzi infection. We included TcG2 and TcG4 in the vaccine, as these antigens were most potent in eliciting parasite-specific antibody and CD8+T cell immunity [11–13]. Mice were immunized with TcG2/TcG4 vaccine delivered by the D/P approach, and we examined whether a) the 2-component D/P vaccine primed TH1 CD4+T cells and generated a stable pool of CD8+T memory cells, and b) the vaccine-primed T cells were capable of rapid expansion and intercepting the infecting T. cruzi. We also determined if c) the 2-component D/P vaccine primed immunity is enhanced by co-delivery of IL-12 and GM-CSF cytokine adjuvants, or d) a booster immunization (bi) several months after the second vaccine dose was effective in providing better protection from T. cruzi infection.
Results
Two-component DNA-prime/protein-boost (D/P) vaccine elicited long-lived anti-T. cruzi T cell immunity
D/P vaccination resulted in 60–70% expansion of splenic cell number, observed at day 14 and 120 post-vaccination (S1 Table). To examine the T cell profile primed by the TcG2 - and TcG4-encoding D/P vaccine, splenocytes from immunized mice were submitted to flow cytometry analysis before and after in vitro stimulation with recombinant antigens. Splenocytes were gated for CD4+ and CD8+ T cells and analyzed for surface markers of effector/effector memory (TEM, CD44+CD62L-) and central memory (TCM, CD44+CD62L+) phenotype. The immediate early T cell response to D/P vaccine, measured at 14 days post vaccination (dpv), was evidenced by a significant increase in ex vivo levels of CD4+ (36–39% of total, 3.8–4.7-fold) and CD8+ (34–43% of total, 2.5–3.3-fold) TEM and TCM cells, respectively, in D/P vaccinated mice when compared to that noted in non-vaccinated controls (Fig 1A and 1B, p<0.01). The vaccine-primed CD4+ and CD8+ T cells exhibited antigen-specific activation and proliferation (Ki67+), and were positive for IFNγ (10–12% and 18–20%, respectively) and TNFα (3.2–4.4% and 7.6–9.2%, respectively) cytokines (Fig 1C and 1D, p<0.05–0.01). Transport of CD107a/b integral membrane proteins to the plasma membrane of effector T cells is required for the cytolytic activity mediated by perforin and granzyme and the release of IFNγ which exerts pleiotropic effects to suppress intracellular pathogens. Flow cytometry studies showed a high frequency of the vaccine-primed CD8+T cells were CD107a+IFNγ+perforin+ (30–34%, Fig 1E, p<0.001).
Fig. 1. Two-component, DNA-prime/protein-boost (D/P) vaccine elicits poly-functional T cell response in mice. 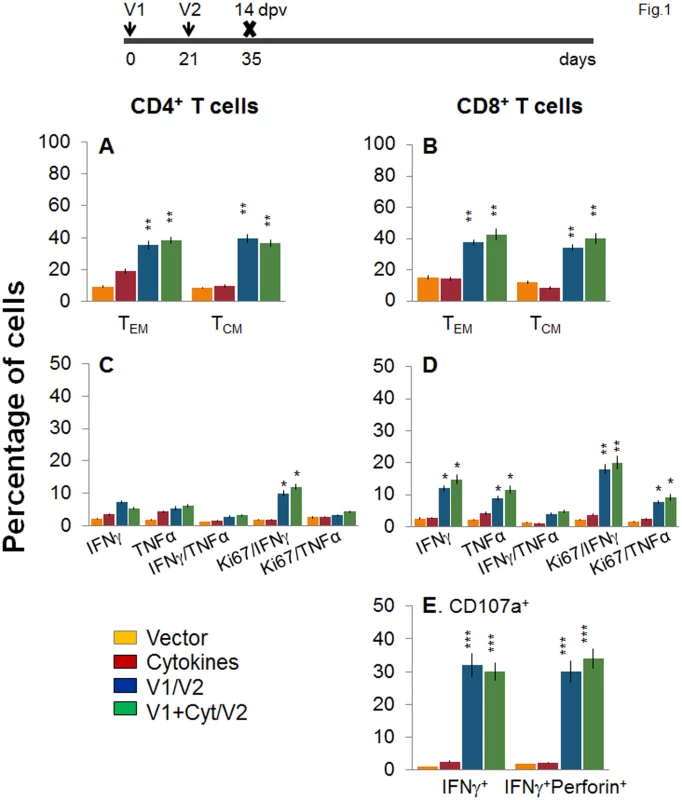
C57BL/6 mice were immunized with an empty vector, cytokines only, or D/P vaccine (V1 dose: TcG2- and TcG4-encoding plasmids ± IL-12- and GMCSF-expression plasmids; and V2 dose: recombinant TcG2 and TcG4 proteins), as detailed in Materials and Methods. (A&B) Splenocytes were obtained at 14 days post-vaccination (dpv) and ex vivo labeled with PE-conjugated anti-CD4, FITC-conjugated anti-CD8, PerCPCy5.5-conjugated anti-CD62L, and APC-conjugated anti-CD44 antibodies. Shown are the PE+CD4+ (A) and FITC+CD8+ (B) T cell subsets that were of effector/effector memory (TEM: CD44+CD62L-) and central memory (TCM, CD44+CD62L+) phenotype, determined by flow cytometry. (C-E) Splenocytes were in vitro stimulated for 48 h with TcG2 and TcG4 recombinant antigens and labeled with fluorescent-conjugated antibodies. The mean percentage of PE+CD4+ (C) and FITC+CD8+ (D) T cells that were IFNγ+ (e-Fluor) and/or TNFα+ (Cy5) with or without Ki67+ (PerCPCy 5.5) phenotype are shown. (E) The percentage of antigen-specific CD8+IFNγ+ T cells that were CD107a+ (Alexa-Fluor 488) and/or perforin+ (APC) was acquired by flow cytometry analysis. In all figures, data are presented as mean ± SD (n = 8/group, triplicate observations per experiment). Significance is presented as *p<0.05, **p<0.01, ***p<0.001 (vaccinated versus non-vaccinated or vaccinated/infected versus non-vaccinated/infected). Next, we examined the stability and effector phenotype of T cells in vaccinated mice at 120 dpv. The ex vivo frequency of CD4+ TEM and TCM (28–35%) was slightly decreased, while that of CD8+ TEM cells (48–52%) was increased, with a simultaneous decline in CD8+ TCM cells (22–26%) in vaccinated mice harvested at 120 dpv (compare Fig 2A and 2B with Fig 1A and 1B). The vaccine-elicited long-lived T cells proliferated upon antigenic stimulation (Ki67+, insets in Fig 2A and 2B), though the frequency of cytokine-producing CD4+T cells (Ki67+IFNγ+: 6–8% versus 10–12%; Ki67+TNFα+: 2.2–2.4% versus 3–4%) and CD8+T cells (Ki67+IFNγ+: 12–14% versus 18–20%; Ki67+TNFα+: 3.6–5.2% versus 8–9%) at 120 dpv, as compared to that noted at 14 dpv, was significantly decreased (compare Fig 2C and 2D with Fig 1C and 1D). Yet, 62–68% of the IFNγ producing CD4+ and CD8+ T cells were CD44+CD62L (TEM phenotype, Fig 2E and 2F, p<0.01–0.001), and 25% and 16–18% of the CD8+IFNγ+ T cells were CD107a+ and CD107a+perforin+, respectively, indicating their cytolytic phenotype (Fig 1G, p<0.05–0.01). Together, the data presented in Figs 1 and 2 suggested that the 2-component D/P vaccine primed CD4+ and CD8+ TEM/TCM cells were long-lived, and responded to antigenic stimulus with type 1 cytokine production and CTL activity. Co-delivery of cytokine adjuvants did not change the frequency or phenotype of the vaccine-primed T cells in immunized mice. No antigen-specific expansion of CD4+ and CD8+ T cells was observed in control mice given empty vector or cytokine adjuvants only.
Fig. 2. Stability and effector phenotype of D/P vaccine elicited T cells in mice. 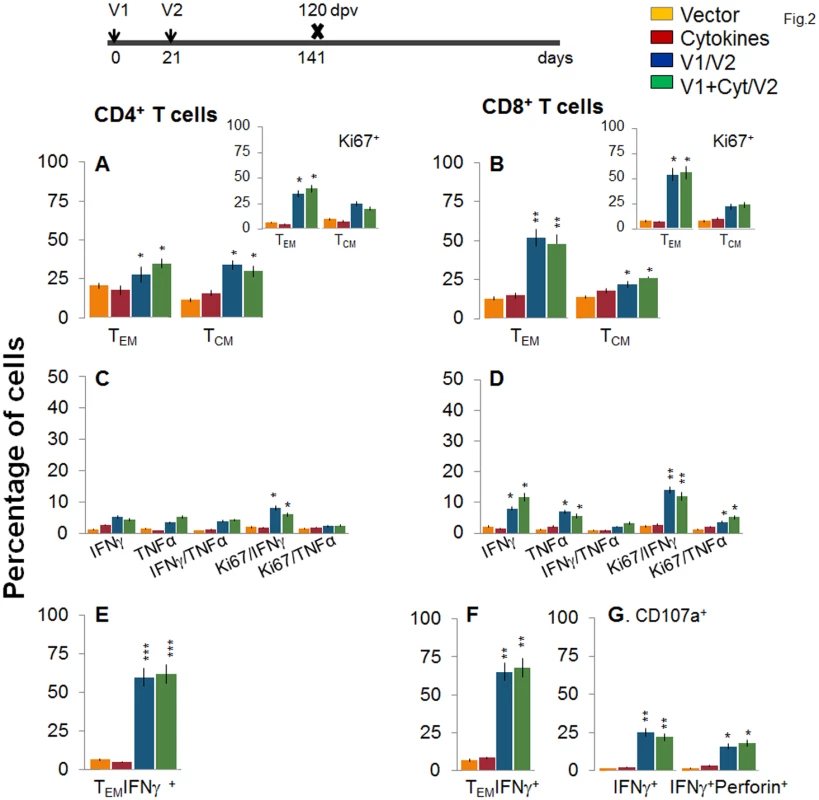
Mice were vaccinated as in Fig 1 and harvested at 120 days after the 2nd vaccine dose. (A&B) Splenocytes were ex-vivo labeled with fluorescence-conjugated antibodies and analyzed for CD4+ (A) and CD8+ (B) T cell subsets (TEM: CD44+CD62L-; TCM: CD44+CD62L+) by flow cytometry. Insets show D/P vaccine-primed T cell subsets that proliferated (Ki67+) in response to antigenic stimulus. (C-G) Splenocytes were in vitro stimulated for 48 h in the presence of TcG2 and TcG4 recombinant antigens, and incubated with fluorescent-conjugated antibodies as described in Fig 1. Shown are bar graphs of antigen-specific CD4+ (C&E) and CD8+ (D&F) T cell subsets that were proliferative (Ki67+, C&D) with T effector (E&F) phenotype and produced IFNγ and/or TNFα cytokines. The percentage is shown of antigen-specific CD8+CD107a+ T cells that were IFNγ+perforin- or IFNγ+perforin+ (G). To determine if the vaccine-elicited, long-lived T cells were responsive to T. cruzi, mice were challenged at 120 dpv and harvested at 10 days pi. Irrespective of vaccination status, all mice exhibited a significant (15-20-fold) expansion of splenic cell number, the maximum expansion being observed in vaccinated/infected mice (S1 Table). Ex vivo flow cytometry analysis of splenocytes showed the expansion of proliferating (Ki67+) CD4+ and CD8+ TEM cells by 1.3–1.6-fold in response to T. cruzi infection in vaccinated mice (Fig 3A and 3B and insets, compare with Fig 2A and 2B, p<0.05–0.01). Further, vaccinated mice exhibited up to 57%, 29% and 79% increase in antigen-specific IFNγ+, TNFα+, and IFNγ+TNFα+ CD4+T cells, respectively, following challenge infection (compare Fig 3C with 2C); and a majority (70–72%) of the IFNγ+CD4+T cells were proliferative (Ki67+) with effector (CD44+CD62L-) phenotype (Fig 3C and 3E, p<0.05–0.01). An expansion of vaccine-induced CD8+T cells in response to challenge infection was evidenced by a >2-fold increase in IFNγ+ and TNFα+ and a 4-fold increase in IFNγ+TNFα+ CD8+T cells (compare Fig 3D with Fig 2D, p<0.05–0.01); of which 80–84% exhibited proliferative (Ki67+) and effector (CD44+CD62L-) phenotype (Fig 3D and 3F, p<0.01). Likewise, vaccinated mice, in response to challenge infection, exhibited a 40–91% expansion of antigen-specific CD8+IFNγ+T cells that were also CD107a+ or CD107a+perforin+ (compare Fig 3G with Fig 2G, p<0.05). When compared to non-vaccinated/infected mice, vaccinated/infected mice exhibited an 8 - to 10-fold higher frequency of antigen-specific IFNγ+ effector (CD44+CD62L-) CD4+ and CD8+ T cells and CD8+ cytolytic T cells (Fig 3E–3G, p<0.001). Quantitative PCR showed, respectively, 2.2-fold and 2-3-fold decline in peripheral (S1A Fig) and tissue (spleen, heart, skeletal muscle; Fig 3H, p<0.05–0.01) levels of T. cruzi in vaccinated/infected mice when compared to that noted in non-vaccinated/infected controls. Together, these data suggested that the D/P vaccine-elicited, long-lived T cells rapidly expanded (CD8>CD4) with a type 1 effector phenotype in response to challenge infection, and were capable of controlling T. cruzi infection.
Fig. 3. Recall of the D/P vaccine elicited, long-lived T cells after challenge infection with T. cruzi. 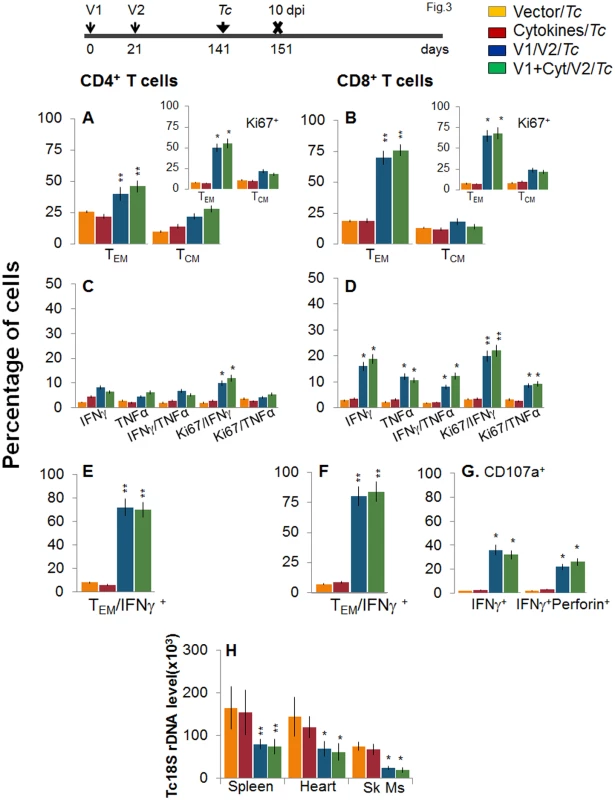
Mice were vaccinated as in Fig 2, and at 120 days post-vaccination, challenged with T. cruzi trypomastigotes (10,000 parasites/mouse), as described in Materials and Methods. Mice were harvested at 10 days post-infection (dpi). (A&B) Bar graphs show ex vivo percentage of PE+CD4+ (A) and FITC+CD8+ (B) splenic T cell subsets (TEM: CD44+CD62L-; TCM: CD44+CD62L+) in vaccinated/infected mice. Insets show D/P vaccine-primed T cell subsets that proliferated (Ki67+) in response to T. cruzi infection (controls: non-vaccinated/infected mice). (C-F) Splenic cells from vaccinated/infected and non-vaccinated/infected mice were in vitro stimulated for 48 h in the presence of TcG2 and TcG4 recombinant antigens, and then labeled with fluorescent-conjugated antibodies, as above. Shown are the mean percentage of CD4+ (C&E) and CD8+ (D&F) T cells that responded to T. cruzi infection with production of IFNγ and/or TNFα and were proliferative (Ki67+, C&D) with T effector (E&F) phenotype. (G) The percentage of T. cruzi-specific CD8+IFNγ+T cells that were CD107a+perforin- or CD107a+perforin+ are shown (± D/P vaccine). (H) Tissue parasite burden. Mice were challenged at 120 days post D/P vaccination and harvested at 10 dpi. Total DNA was isolated from spleen, heart and skeletal muscle (Sk Ms) tissue sections and submitted to real-time PCR amplification of Tc18SrDNA sequence (normalized to murine GAPDH). Booster immunization (bi) enhanced the recall T cell immunity against T. cruzi
Next, we determined if a bi would enhance the vaccine elicited T cell immunity and provide better control of T. cruzi infection. For this, mice were immunized with the 2-component D/P vaccine as above, and given a booster dose of TcG2/TcG4 recombinant proteins before challenge infection. The booster-immunized mice exhibited 2-3-fold higher number of splenic cells as compared to non-vaccinated mice (S1 Table). When analyzing splenic T cell frequency, we observed an overall decline in the splenic frequency of CD4+ (TEM: 21.3–22.2% versus 28–35%, TCM: 10.5–12.8% versus 30–34%) and CD8+ (TEM: 34.5–36.8% versus 48–52%, TCM: 14.4–16% versus 22–26%) T cells at 14 days post bi, when compared to that noted before bi (compare Fig 4A and 4B with Fig 2A and 2B). However, splenocytes from vaccinated/bi mice in vitro stimulated with recombinant TcG2/TcG4 proteins exhibited 1.6–3.4-fold and 1.4–3.2-fold higher frequencies of the cytokine (IFNγ and/or TNFα) producing CD4+ and CD8+ T cells, respectively, as compared to that noted in vaccinated (but not bi) mice (compare Fig 4C and 4D with Fig 2C and 2D, all p<0.05–0.01). Approximately, 60–83% of the antigen-specific IFNγ - or TNFα-producing CD4+T cells and 80–90% of the IFNγ-producing CD8+T cells exhibited a proliferative (Ki67+), effector (CD44+CD62L-) phenotype (Fig 4E and 4F, p<0.01). Importantly the frequency of CD8+CD107a+ and CD8+CD107a+perforin+ cells secreting IFNγ was increased by 37.5–45% post bi (compare Fig 4G with Fig 2G, p<0.05–0.01).
Fig. 4. D/P vaccine elicited, long-lived T cells exhibited enhanced antigen-specific, poly-functional phenotype after booster immunization (bi). 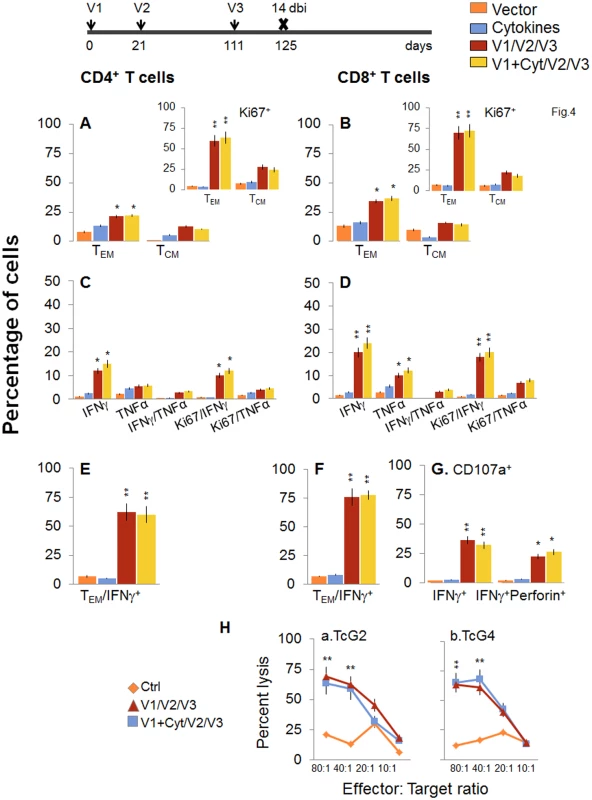
C57BL/6 mice were immunized at day 0 with V1 (TcG2- and TcG4-encoding plasmids ± IL-12- and GMCSF-expression plasmids), at day 21 with V2 (recombinant TcG2 and TcG4 proteins), and then at day 111 with V3 (booster dose, recombinant TcG2 and TcG4 proteins). Mice were harvested at 14 days after the booster immunization (dbi). Splenocytes were stimulated in the presence or absence of TcG2 and TcG4 recombinant antigens, labeled with fluorescent-conjugated antibodies, and analyzed by flow cytometry. (A&B) Ex vivo percentage of CD4+ (A) and CD8+ (B) splenic T cell subsets (TEM: CD44+CD62L-; TCM: CD44+CD62L+) in vaccinated/bi mice (Insets: antigen-specific Ki67+ T cell subsets in vaccinated/bi mice). (C-G) Bar graphs of antigen-specific CD4+ (C&E) and CD8+ (D&F) T cell subsets with their intracellular cytokine (IFNγ+, TNFα+) profile that were proliferative (Ki67+, C&D) with T effector (E&F) phenotype. The percentage of antigen-specific CD8+CD107a+ T cells that were IFNγ+perforin- and IFNγ+Perforin+ (G) in vaccinated/bi mice are shown. (H) Cytolytic activity. Splenocytes were in vitro stimulated with recombinant antigens and used as effectors for their ability to lyse EL4 target cells exposed to GFP+ recombinant MVA encoding TcG2 (H.a) or TcG4 (H.b) antigens (controls: target cells incubated with empty MVA). To validate the antigen-specific cytotoxic activity of the CD8+T lymphocytes in vaccinated/bi mice, spleen cells in vitro stimulated with recombinant antigens were used as effectors and tested for their ability to lyse EL4 cells exposed to GFP+ rMVA encoding TcG2 or TcG4 antigens or empty MVA. A significant level of antigen-specific cytolytic activity (range 56–69%, effector-to-target cell ratio, 80 : 1 or 40 : 1) was evident in vaccinated/bi mice (Fig 4H, p<0.01. Splenocytes from mice immunized with vector only or cytokines only did not show CTL activity against any of the antigen-sensitized target cells (Fig 4H).
When mice were challenged with T. cruzi immediately after bi, a potent expansion of parasite-specific T cells was evidenced by a 1.4-2-fold increase in ex vivo frequency of CD4+ and CD8+ TEM cell populations (compare Fig 5A and 5B with Fig 4A and 4B, p<0.05–0.01). Further, splenocytes of vaccinated/bi mice, in response to challenge infection, exhibited a strong increase in T. cruzi-specific IFNγ+ or TNFα+ (2.0–3.1-fold) and IFNγ+TNFα+ (4.3–7.8-fold) CD4+ and CD8+ T cells, that were also Ki67+ with effector (CD44+CD62L-) phenotype (compare Fig 5C–5F with Fig 4C–4F, p<0.05–0.01). The D/P-vaccinated/bi mice exhibited a larger expansion of total splenic cells (S1 Table) and T cells than was noted in the D/P-vaccinated mice in response to challenge infection, as was evidenced by a significantly higher (1.6–2.8-fold, p<0.05–0.01) frequency of proliferating and non-proliferating IFNγ+, TNFα+, and IFNγ+TNFα+ CD4+ and CD8+ T cells (compare Fig 5 with Fig 3). Subsequently, vaccinated/bi mice exhibited a 2.5-fold decline in peripheral (S1B Fig) and 3–3.8-fold decline in tissue (spleen, heart and skeletal muscle) levels of Tc18SrDNA in comparison to that noted in non-vaccinated/infected mice (Fig 5H, p<0.05–0.01). Together, the data presented in Figs 4 and 5, along with that presented in Figs 2 and 3, suggested that bi was effective in expanding the 2-component D/P vaccine-elicited type 1 cytokine producing T cells and CD8+ CTLs that provided better protection from challenge infection than was observed with 2-component D/P vaccine only.
Fig. 5. Booster immunization (bi) expanded the parasite-specific recall T cell response that provided superior protection from T. cruzi infection. 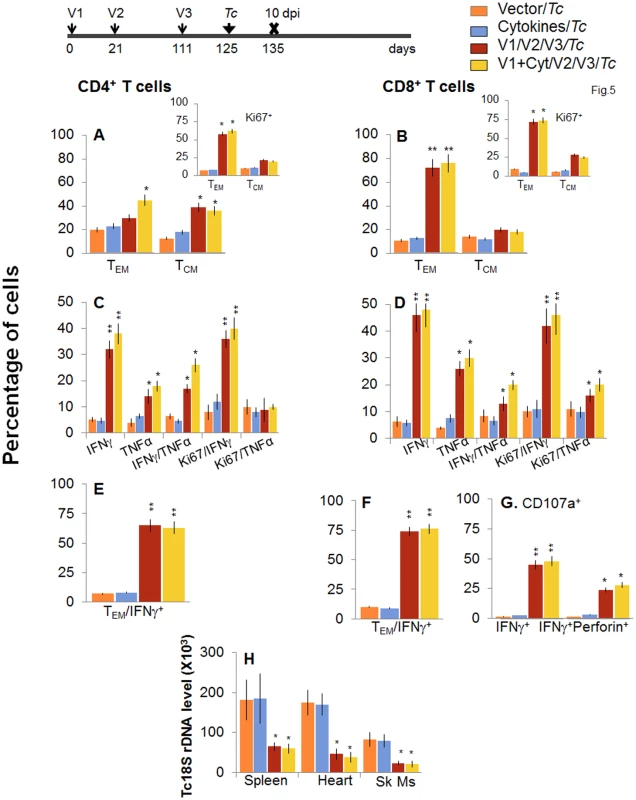
Mice were immunized with D/P vaccine followed by a booster dose as in Fig 4, and then, 14 days later, challenged with T. cruzi. Mice were harvested at 10 days pi. Splenocytes were in vitro stimulated in the presence or absence of TcG2 and TcG4 recombinant antigens, labeled with fluorescence-conjugated antibodies and analyzed by flow cytometry. (A&B) T. cruzi-specific CD4+ (A) and CD8+ (B) splenic T cell subsets in vaccinated/bi mice at 10 days pi. Insets show antigen-specific T cell subsets that proliferated (Ki67+) in response to T. cruzi infection in vaccinated/bi mice. (C-F) Shown are the mean percentage of CD4+ (C&E) and CD8+ (D&F) T cells that responded to T. cruzi infection with production of IFNγ and/or TNFα cytokines and were proliferative (Ki67+, C&D) with T effector (E&F) phenotype in vaccinated/bi mice. (G) Percentage of T. cruzi-specific CD8+CD107a+ that were IFNγ+perforin- or IFNγ+perforin+ in booster-immunized/infected mice. (H) D/P vaccine/bi was highly effective in controlling tissue parasite burden. Total DNA was isolated from spleen, heart and skeletal muscle (Sk Ms) tissue sections of vaccinated/bi/infected mice, and real time PCR amplification of Tc18SrDNA sequence was performed. Bar graphs show the Tc18SrDNA level normalized to murine GAPDH. Longevity and functional capability of vaccine/bi elicited T cells in providing protection from T. cruzi infection
To evaluate the stability and effector phenotype of the T cells elicited by the 2-component D/P vaccine and bi, mice were harvested at 120 or 180 days post bi. As above, the booster-immunized mice continued to maintain 2-3-fold higher number of splenic cells at 120 or 180 days post bi as compared to non-vaccinated mice (S1 Table). The ex vivo frequency of CD4+ TCM cells was increased by 3.1–3.4-fold and 2.1–3.2-fold at 120 and 180 days post-bi, respectively, while that of CD4+ TEM cells was increased modestly (1.4-2-fold) at 120 days post bi only, when compared to that noted in mice harvested at 14 days post bi (compare Figs 6A and 7A with Fig 4A). Ex vivo CD8+T cells of TEM phenotype at 120 days post bi (72–76%, Fig 6B) contracted by 2.4-fold at 180 days post bi (Fig 7B). In contrast, CD8+T cells of TCM phenotype were increased by 1.4-fold at 120–180 days post bi compared to that noted in mice harvested at 14 days post bi (compare Figs 6B and 7B with Fig 4B).
Fig. 6. Two-component D/P vaccine/bi elicited T cells retain the antigen-specific functional profile at 120 days post bi. 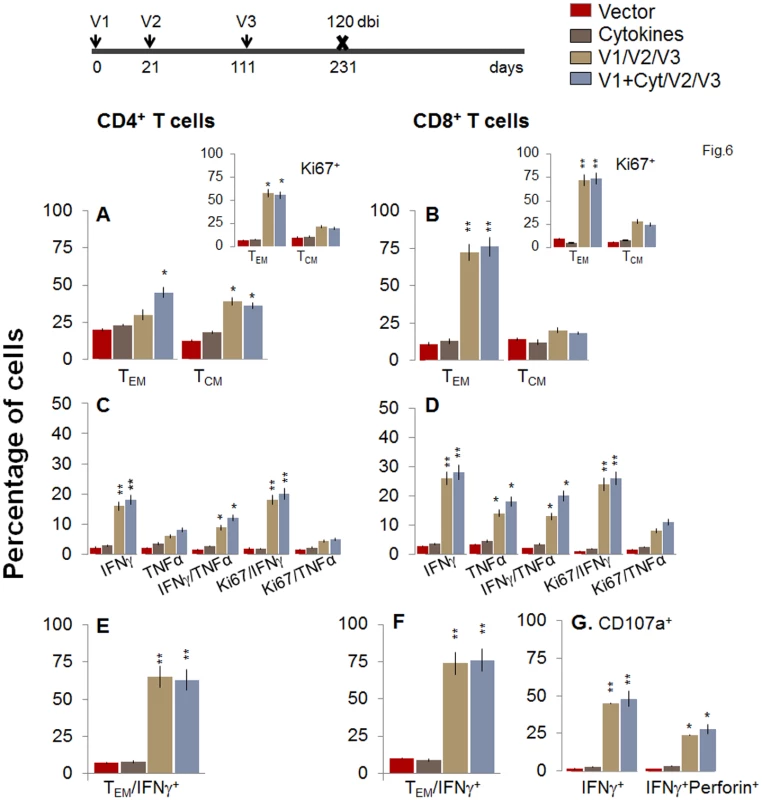
Mice were immunized with D/P vaccine followed by a booster dose as in Fig 4, and then harvested at 120 days after booster dose. Splenocytes were stimulated in the presence or absence of TcG2 and TcG4 recombinant antigens, labeled with fluorescent-conjugated antibodies, and analyzed by flow cytometry. (A&B) Ex vivo percentage of CD4+ (A) and CD8+ (B) splenic T cell subsets (TEM: CD44+CD62L-; TCM: CD44+CD62L+) at 120 days post bi (Insets: antigen-specific Ki67+ T cell subsets). (C-G) Bar graphs of CD4+ (C&E) and CD8+ (D&F) T cell subsets that proliferated (Ki67+, C&D) or exhibited TEM (E&F) phenotype with production of intracellular cytokines (IFNγ, TNFα) in vaccinated mice at 120 days post bi. Shown in (G) are the percentages of antigen-specific CD8+CD107a+T cells that were IFNγ+perforin- and IFNγ+perforin+ (G) in D/P-vaccinated mice at 120 days post bi. Fig. 7. A decline in the frequency of D/P vaccine/bi elicited, long-lived CD4+ and CD8+ T cells occurred by 180 days post bi. 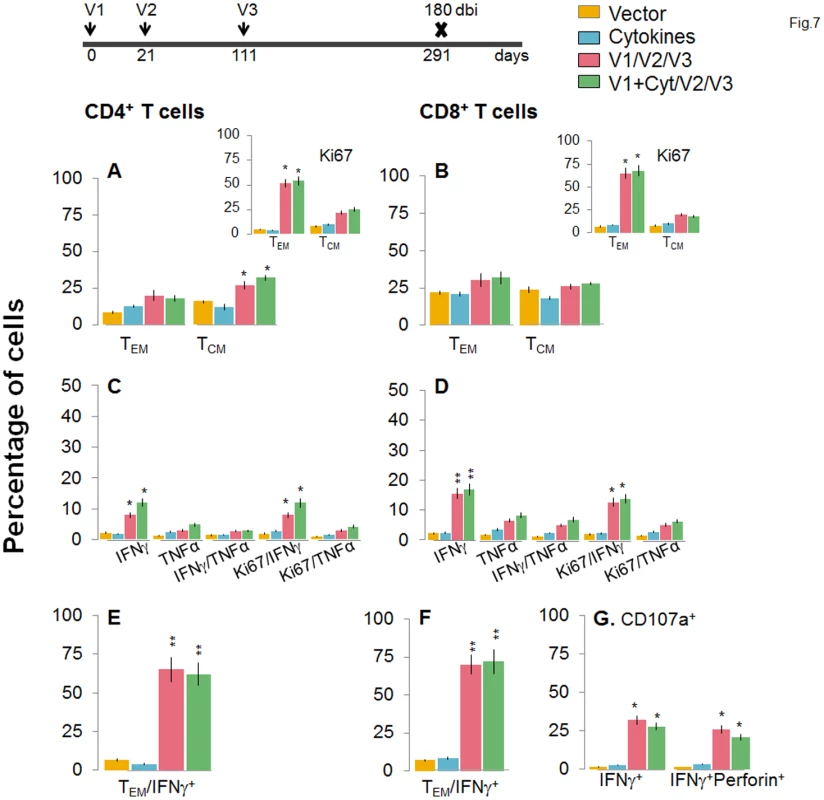
Mice were immunized with D/P vaccine followed by a booster dose as in Fig 4, and then harvested 180 days later. Splenocytes were stimulated in the presence or absence of TcG2 and TcG4 recombinant antigens, labeled with fluorescent-conjugated antibodies, and analyzed by flow cytometry. (A&B) Ex vivo percentage of CD4+ (A) and CD8+ (B) splenic T cell subsets (TEM: CD44+CD62L-; TCM: CD44+CD62L+) at 180 day post bi (Insets: antigenic-specific Ki67+ T cell subsets). (C-G) Bar graphs of CD4+ (C&E) and CD8+ (D&F) T cell subsets that proliferated (Ki67+, C&D) or exhibited TEM (E&F) phenotype with production of intracellular cytokines (IFNγ and/or TNFα) in vaccinated mice at 180 days post bi. Shown in (G) are the percentages of antigen-specific CD8+CD107a+ T cells that were IFNγ+perforin- and IFNα+perforin+ (G) in vaccinated mice at 180 days post bi. To determine if long-lived vaccine/bi elicited T cells are functional, splenocytes from vaccinated/bi mice were harvested at 120 or 180 days post bi, in vitro stimulated with recombinant antigens, and analyzed by flow cytometry. We noted a modest decline in the frequency of antigen-specific Ki67+CD4+T cells at 120 days (56–58%) and 180 days (52–54%) post bi when compared to that noted at 14 days (60–64%) post bi (compare Figs 6A and 7A with Fig 4A). The splenic cells from vaccinated mice harvested at 120 days post bi exhibited a ~2-4-fold larger expansion of proliferative (Ki67+) and non-proliferative (Ki67-) IFNγ+ and IFNγ+TNFα+ CD4+T cells in response to in vitro antigenic stimulus than was observed with splenic cells from mice harvested at 14 or 180 days post bi (compare Fig 6C with Figs 7C and 4C, p<0.05–0.01). The CD8+T cells, at 120 days post bi, exhibited a similar level of antigen-specific proliferative (Ki67+) capacity, but a significantly higher capacity for type 1 cytokine (IFNγ and/or TNFα) production and lytic activity (IFNγ+CD107a+perforin- or IFNγ+CD107a+perforin+) than was noted at 14 days post bi (compare Fig 6D, 6F, and 6G with Fig 4D, 4F, and 4G, p<0.05–0.01). At 180 days post bi, the percentage of CD8+T cells capable of proliferating and producing type 1 cytokines with lytic activity contracted by at least 30% in comparison to that noted at 120 days post bi (compare Fig 7D, 7F, and 7G with Fig 6D, 6F, and 6G). Together, the data presented in Figs 6 and 7 (along with Fig 4) suggested that the D/P vaccine/bi elicited CD4+ and CD8+ T cells were stable, the frequency of TCM cells was enhanced at 120–180 days post bi; and these CD4+ and CD8+ T cells were capable of rapidly responding to antigenic stimulus with proliferation and activation of type 1 cytokine production and cytolytic profile.
Finally, we determined whether long-lived T cells present in vaccinated/bi mice were capable of responding to T. cruzi infection. For this, at 120 or 180 days post bi, mice were challenged with T. cruzi and splenic cell characterization performed at day 10 pi. When challenged at 120 days post bi, mice exhibited a similar expansion of CD4+TEM cells capable of producing IFNγ and/or TNFα cytokines as was noted in mice challenged at 14 days post bi (compare Fig 8A, 8C, and 8E with Fig 5A, 5C and 5E, p<0.05–0.01). The CD8+T cells were maintained at a very high frequency (72–76%) in vaccinated mice at 120 days post bi (Fig 6B), and expanded only by 15–21% post challenge infection (Fig 8B). Importantly, in mice challenged at 120 days post bi, 36–38%, 24–28%, and 23–30% of the antigen-specific CD8+T cells were IFNγ+, TNFα+ or IFNγ+TNFα+, respectively, and 48–52% of the IFNγ+CD8+T cells were also CD107+perforin+ (Fig 8D, 8F, and 8G, p<0.05–0.01). These results suggested a significant expansion of cytokine producing (35–77%) CD8+T cells with a cytolytic phenotype (2-fold increase) in mice challenged at 120 days post bi in comparison to that noted in mice challenged at 14 days post bi (compare Fig 8D, 8F, and 8G with Fig 5D, 5F, and 5G, p<0.05–0.01). Subsequently, vaccinated/bi mice challenged 120 days post bi exhibited a significant control (1.5–2.3-fold) of peripheral (S1B Fig) and tissue (Fig 8H, p<0.05–0.01) parasites in comparison to the non-vaccinated/infected mice.
Fig. 8. Booster-immunized mice expand the recall T cell response capable of controlling T. cruzi infection at 120 days post bi. 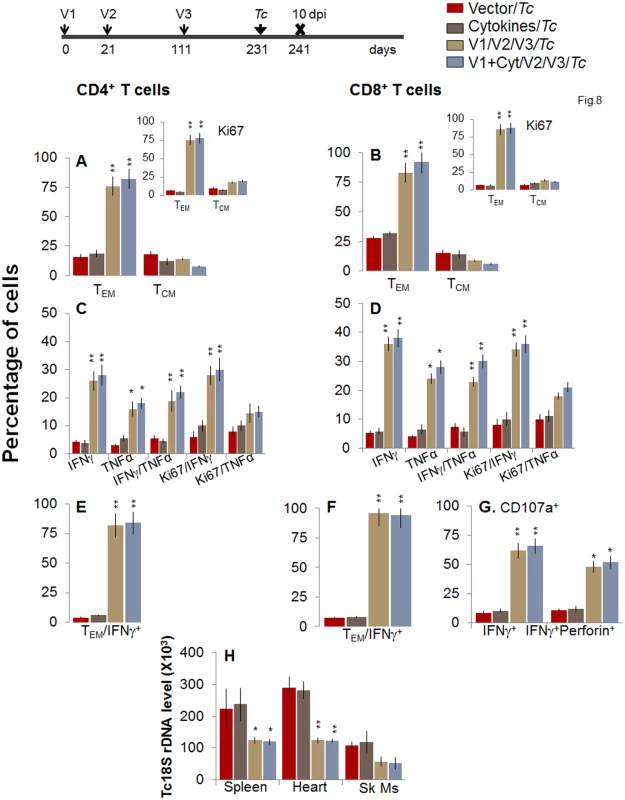
Mice were immunized with D/P vaccine followed by a booster dose as in Fig 4, and then, at 120 days post bi, infected with T. cruzi. Mice were harvested 10 days after the challenge infection, and splenocytes were stimulated in the presence or absence of TcG2 and TcG4 recombinant antigens, labeled with fluorescent-conjugated antibodies, and analyzed by flow cytometry. (A&B) T. cruzi-specific CD4+ (A) and CD8+ (B) splenic T cell subsets in vaccinated/bi mice infected at 120 days post bi. Insets show T. cruzi-specific, proliferating (Ki67+) T cell subsets in vaccinated mice challenged at 120 days post bi. (C-F) Shown are the mean percentage of CD4+ (C&E) and CD8+ (D&F) T cells that responded to T. cruzi infection by producing IFNγ and/or TNFα cytokines and were proliferative (Ki67+, C&D) with a T effector (E&F) phenotype in mice challenged at 120 days post bi. (G) The percentage of T. cruzi-specific CD8+CD107a+ that were IFNγ+perforin−- or IFNγ+perforin+ in mice that were infected at 120 days post bi. (H) Tissue parasite burden. Bar graphs of Tc18SrDNA levels, normalized to murine GAPDH, were developed by using a real time quantitative PCR approach. At 180 days post bi, splenic ex vivo CD4+ and CD8+ TEM cells increased by 20–44% only in response to challenge infection (with or without in vitro antigenic stimulation) with no change in the TCM population (Fig 9A and 9B). Yet, the effector CD4+ and CD8+ T cells generated post-challenge infection were poly-functional as was evidenced by 1.8-4-fold increase in IFNγ - and/or TNFα - producing CD4+T cells (compare Fig 9C with Fig 7C, p<0.01) and up to 2-fold increase in type 1 cytokine-producing CD8+T cells of cytolytic phenotype (compare Fig 9D, 9F, and 9G with Fig 7D, 7F, and 7G, p<0.01). The vaccinated mice, challenged at 180 days post bi, were able to achieve a 1.5–1.8-fold control of T. cruzi infection as compared to that noted in non-vaccinated/infected mice (Figs 9H and S1B, p<0.05). Together, the data presented in Figs 8 and 9 suggested that the D/P vaccine/bi induced T cell immunity was stable, and capable of rapidly expanding with a poly-functional phenotype in response to challenge infection at least until 180 days post bi, and provided significant control of parasite dissemination and replication.
Fig. 9. D/P vaccine/bi generated CD8+ TCM cells are capable of responding to challenge infection at 180 days post bi. 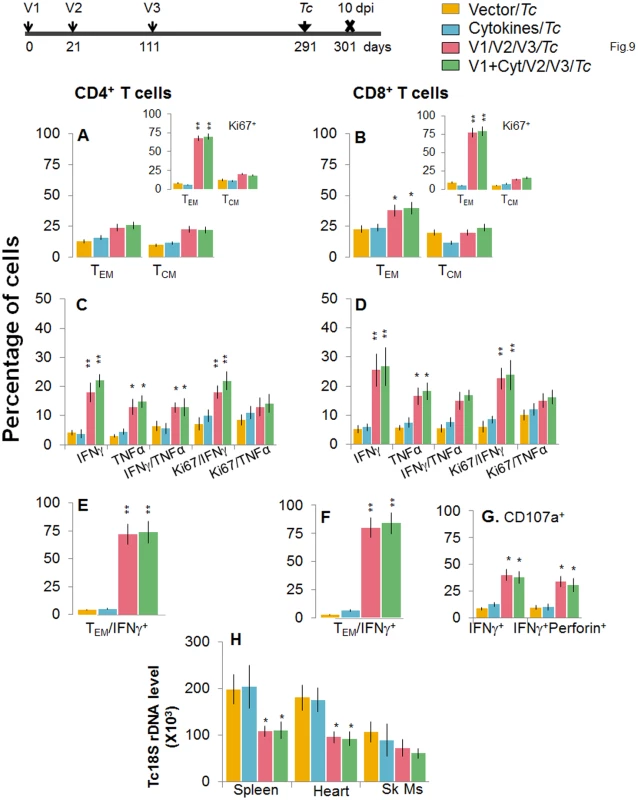
Mice were immunized with D/P vaccine followed by a booster dose as in Fig 4, and then 180 days post bi, infected with T. cruzi. Mice were harvested 10 days after the challenge infection, and splenocytes were stimulated in the presence or absence of TcG2 and TcG4 recombinant antigens, labeled with fluorescent-conjugated antibodies and analyzed by flow cytometry. (A&B) T. cruzi-specific CD4+ (A) and CD8+ (B) splenic T cell subsets in vaccinated mice infected at 180 days post bi. Insets show T. cruzi-specific, proliferating (Ki67+) T cell subsets in mice challenged at 180 days post bi. (C-F) Shown are mean percentage of CD4+ (C&E) and CD8+ (D&F) T cells that responded to T. cruzi infection by producing IFNγ and/or TNFα cytokines and were proliferative (Ki67+, C&D) with a T effector (E&F) phenotype in mice challenged at 180 days post bi. (G) The percentage of T. cruzi-specific CD8+CD107a+ that were IFNγ+perforin- or IFNγ+perforin+ in mice that were infected at 180 days post bi. (H) Tissue parasite burden. Real time PCR evaluation of Tc18SrDNA level, normalized to murine GAPDH, in spleen, heart and skeletal muscle tissue of vaccinated/bi mice that were infected at 180 days post bi. Discussion
The pathology of Chagas disease presents a complicated and diverse picture in humans [14]. The major complications and destructive evolutionary outcomes of chronic infection by T. cruzi in humans include ventricular fibrillation, thromboembolism, and congestive heart failure [15,16]. Studies in animal models and human patients have revealed the pathogenic mechanisms found during disease progression, and the features of protective immunity [17]. Parasite-specific CD4+T cells are suggested to assist in T. cruzi control through secretion of Th1 cytokines (e.g., IFNγ, IL2), amplification of the phagocytic activity of macrophages, stimulation of B cell proliferation, and antibody production and differentiation and activation of CD8+ T cells (reviewed in [17]). T. cruzi antigen-specific CD8+T cells contribute to parasite control, either by cytolysis of the infected cells or by secretion of Th1 cytokines (IFNγ) that induce trypanocidal activity (reviewed in [18,19]). Accordingly, several antigens (e.g. GP90, cruzipain, GP82, ASP2, TSA1, Tc24) have been tested to elicit protective immunity to T. cruzi in experimental animals (reviewed in [7,20,21]). A majority of the candidate antigens were selected based upon their potential to be recognized by antibodies in infected mice and have proved to be efficacious as vaccine in providing some degree of protection from T. cruzi infection. In parallel, efforts to enhance the protective efficacy of subunit vaccines against T. cruzi have included testing the use of adjuvants, e.g. saponin, CpGODN, IL-12 and GMCSF cytokines; [22] attenuated strain of Salmonella [23] or adenovirus [24] for antigen delivery, and heterologous prime-boost protocols [25].
Based upon several studies that we have conducted, we believe TcG2 and TcG4 candidate antigens are an excellent choice for subunit vaccine development, and a heterologous prime/boost approach for vaccine delivery is highly efficacious against T. cruzi infection. The selected candidates TcG2 and TcG4 tested in this study (and TcG1 tested in other studies) are highly conserved in clinically relevant T. cruzi strains, expressed (mRNA/protein) in infective trypomastigote and intracellular amastigote stages of T. cruzi, and released during parasite differentiation in host cell cytoplasm, a characteristic required for antigen presentation for T cell activation [10]. These antigens showed antigen-specific antibody (IgG1, IgG2a and IgG2b) and/or CD8+ T cell responses in T. cruzi-infected mice and dogs. Three candidate antigens (TcG1,TcG2 and TcG4) were also recognized by antibody response in chagasic patients from distinct study sites (Argentina-Bolivia and Mexico-Guatemala) and expressed in diverse strains of the circulating parasites [12,13,26]. Further, we noted that immunization with candidate antigens as a DNA vaccine provided T cell immunity (TcG2 = TcG4>TcG1) that was additive when the antigens were co-delivered and achieved a significant (but modest) control of challenge infection in mice and dogs [10–12]. The delivery of candidate antigens as heterologous prime/boost vaccine [13,27,28] provided protective immunity consisting of parasite - and antigen-specific lytic antibodies and type 1 CD8+ cytotoxic T lymphocytes against challenge infection and chronic disease that was significantly better than that observed with DNA-prime/DNA-boost vaccine [10,11]. The enhanced efficacy of a heterologous prime/boost approach for vaccine delivery could be because delivery of antigens as DNA vaccines elicits robust T-cell responses, which are critical for the development of T-cell-dependent antibody responses [29,30], and DNA immunization is also highly effective in priming antigen-specific memory B cells. Delivery of vaccine candidates as recombinant proteins is generally more effective at eliciting antibody responses and may directly stimulate antigen-specific memory B cells to differentiate into antibody-secreting cells, resulting in production of high-titer, antigen-specific antibodies [31,32]. Studies testing the protective efficacy of Tc24, TSA1 (individually or in combination, [33]) or other antigens (reviewed in [7,20,21]) have shown the protection associated with the induction of CD8+ T cell activity and IFN-γ production; however, in these studies, challenge infection was conducted immediately after vaccination. To the best of our knowledge, this is the first report documenting that a) a subunit vaccine can be useful in achieving long-term protection against T. cruzi infection and Chagas disease, and b) the effector T cells can be long-lived and play a role in vaccine elicited protection from parasitic infection.
Approximately, ~30–40% of the infected individuals develop symptomatic clinic disease presented as severe inflammatory myocarditis, and extensive destruction and fibrosis of the heart. T. cruzi antigen-specific T lymphocytes producing inflammatory cytokines (e.g. IFNγ, IL17) can be detected in most individuals during chronic infection [34]; however, their role in host resistance versus chronic pathology is debated [35–38]. In experimental studies using a variety of genetically modified mice, both innate and adaptive immune responses were found to play an important role in providing resistance to T. cruzi infection. With regard to innate immune responses, a compromised signaling through toll-like receptors (TLR-3, -7, -9) and their adaptor molecules MyD88 and TRIF, and inhibition of IL-1-activating inflammasomes resulted in increased susceptibility to T. cruzi infection [39–41]. Likewise, mice genetically deficient in CD4+ or CD8+ T cell function were extremely susceptible to infection [42], and it was shown that T lymphocytes act by producing type 1 cytokines, such as IFNγ, and CD8+ T cell cytotoxicity mediated by perforin is important for resistance against infection [9,25,43,44]. The question then arises as to how to determine that vaccination was effective since both vaccine and T. cruzi infection elicited potent T cell responses. With respect to this issue, our data in the present study provide clues to the mechanisms relevant to vaccine efficacy. One, immunization with the 2-component D/P vaccine resulted in the generation of antigen-specific, IFNγ-producing CD4+ and CD8+ T cells and CD8+ cytotoxic T lymphocytes that were long-lived and could be further enhanced by bi (Figs 2 and 4). Two, vaccinated mice exhibited an expansion of T cell responses within 7–10 days post-infection. Immune responses elicited by vaccination followed by infection were >3-orders of magnitude higher than that generated by infection only (at 10 dpi) and were mediated by multifunctional lymphocytes abundantly secreting pro-inflammatory cytokines, such as IFNγ and TNFα, capable of translocating the CD107a and perforin molecules to the cell surface, and producing cytotoxic activity against infected target cells. The T cell immunity elicited in D/P vaccinated (± booster-immunized) mice was similar to that noted against self-limiting infections. For example, influenza, LCMV, or Listeria have been reported to result in the highest T cell immunity, as measured by the frequency and function of specific CD8+ T lymphocytes, within 7–15 days pi [45]. In comparison, in experimental models of T. cruzi infection and human patients, parasite-specific T cell immunity is delayed (peaks at ≥30 day pi [44,46]); and when improved protective immunity was generated, it correlated with significantly higher numbers of antigen-specific IFN-γ producing total and CD8+ T cells that were better able to expand after in vitro re-stimulation [47]. Thus, we propose that vaccine-induced protection to T. cruzi infection and Chagas disease is associated with an increased frequency of highly competent CD8+ T lymphocytes at an early stage of parasite infection and replication. Further studies will be required to identify the underlying mechanisms for a delayed T cell response in natural T. cruzi infection, though a recent study suggests that accelerated apoptosis of CD8+T cells, resulting in inability to clear parasites, might be the key reason for chronic disease development [48].
Finally, we propose that our studies challenge the immunological paradigm of vaccine development. It is suggested that following pathogen control (or clearance), 90–95% of the T effector cells reportedly die (contraction phase), leaving behind a population of pathogen-specific CD8+ T central memory cells (TCM) that undergo antigen-independent/cytokine-dependent homeostatic proliferation which supports their long-term maintenance [45]. Moreover, these cells were found to efficiently increase cytokine and chemokine production, acquire cytotoxic ability and proliferate intensely during recall responses [49]. Thus, after an infectious challenge, heightened precursor frequency, expanded anatomical distribution, enhanced proliferative capacity, and rapid recall ability were reported as the hallmark attributes of protective CD8+ TCM cells [50,51]. During chronic Leishmania infection, TEF cells were maintained at high frequencies via reactivation of TCM and the TEF themselves and the short lived effector T cells were shown as the critical cells that mediate concomitant immunity [52]. In our studies, we have used a heterologous, prime-boost vaccination regimen consisting of priming with plasmid DNA, followed by a bi with either replication-defective MVA [27,28] or recombinant proteins (this study), in all instances using the TcG2 and TcG4 as vaccine candidates. In all these studies, we observed that our immunization protocol induced a stable pool of functional CD8+TEM cells (CD62L-CD44+), and these cells expanded in response to challenge infection at 14 days pi. In this study, a D/P vaccine (± bi) produced a stable pool of T effector memory (TEM) cells that exhibited rapid recall response to challenge infection with a potent increase in antigen-specific inflammatory cytokine production and CTL activity against specific target cells (Figs 3, 5, and 8). We did notice the generation of a strong pool of TCM cells in vaccinated mice after 120 or 180 days post bi, the time period equivalent to chronic infection; however, this pool of TCM was smaller than the TEM cells until 120 days post bi, and became predominant pool only after 180 days post bi (Figs 3, 5, 8, and 9). Others have shown that TEM cells, induced by ASP2 vaccine, were the main source of the anti-parasitic mediators IFNγ and TNFα, and these cells did not need to proliferate (rather increase the function) to provide protective immunity [53].
In patients with severe chagasic disease, a lower frequency of specific CD8+T cells with fewer early memory cells (CD45RA-, CD27+, and CD28+) and a higher number of late memory cells (CD45RA-, CD27-, and CD28-) that lack the capability to respond to parasite stimulus was noted [54]. Further, frequency of CD8+ TEM lymphocytes remains high for long periods of time after infection, likely due to parasite persistence. Indeed, treatment with an anti-parasite drug in the chronic disease phase led to the contraction of CD8+ T cells and a change in their phenotype to CD62Llow [55]. Further studies will be required to establish the implications of these observations on the fate of CD8+T cells in parasitic infections in the development of new vaccines [56].
In summary, we have demonstrated that our DNA-prime/protein-boost vaccine constituted of TcG2 and TcG4 candidate antigens provides long-lived, rapid recall immunity to T. cruzi infection. Mice immunized with a 2-component D/P vaccine elicited long-lived CD4+ and CD8+ effector memory T cells capable of producing type 1 cytokines, and cytotoxic T lymphocyte profile in an antigen-specific manner. The vaccinated mice responded to challenge infection with a rapid and potent expansion of type 1 cytokines producing CD4+ and CD8+ T cells and cytotoxic T lymphocyte activity against infected target cells, resulting in a >2-3-fold control of acute parasitemia and tissue parasite burden. Importantly, vaccine-induced immunity could be enhanced by bi that helped to maintain a high level of T cell population capable of efficiently expanding and providing an up to five-fold control of an invading pathogen. The vaccine-induced immunity waned slightly after 6 months post bi, but was still sufficient to provide 2-fold control of invading pathogens that, according to mathematical modeling, is sufficient to break the parasite transmission cycle [57] and prevent disease progression [8]. Many of the studies discussed highlight the importance of a preventive or therapeutic vaccine to control T. cruzi infection by at least decreasing parasite burden, cardiac tissue inflammation and damage or increasing survival if not providing sterile immunity.
Materials and Methods
Ethics statement
All animal experiments were conducted following NIH guidelines for housing and care of laboratory animals and in accordance with protocols approved by the Institutional Animal Care and Use Committee (protocol number 08-05-029) at The University of Texas Medical Branch at Galveston.
T. cruzi genes and generation of recombinant plasmids, proteins, and viruses
The cDNAs for TcG2 and TcG4 (SylvioX10 isolate, Genbank: AY727915 and AY727917, respectively; >99% homologous to CL Brenner (reference strain) sequences XM_806323 and XM_816508, respectively) [10] were cloned in eukaryotic expression plasmid pCDNA3.1 [10,11]. Plasmids encoding murine IL-12 (pcDNA3.msp35 and pcDNA3.msp40) and GM-CSF (pCMVI.GM-CSF) were previously described [11]. Recombinant plasmids were transformed into E. coli DH5-alpha-competent cells, grown in L-broth containing 100-μg/ml ampicillin, and purified by anion exchange chromatography by using the Qiagen maxi prep kit (Qiagen, Chatsworth, CA).
The cDNAs for TcG2 and TcG4 were cloned in-frame with a C-terminal His-tag into a pET-22b plasmid (Novagen, Gibbstown, NJ). Plasmids were transformed in BL21 (DE3) pLysS-competent cells, and recombinant proteins purified by using the poly-histidine fusion, peptide-metal chelation chromatography system [13].
The pLW44 vector consists of a green fluorescent protein (GFP) and multiple cloning site (MCS) cassette flanked by a pair of genomic sequences of the Modified Vaccinia Ankara (MVA) virus which allows homologous recombination and incorporation of both GFP and the gene of interest into the deletion III locus of the wild-type MVA genome [58,59]. The cDNAs for TcG2 and TcG4 were cloned at the Xma1/Sbf1 sites of pLW44, and recombinant plasmids were transformed and amplified in E. coli, and purified by using the Qiagen maxi prep kit. BHK-21 cells at 70% confluency (six-well plate) were infected with wild-type MVA (multiplicity of infection: 0.05) for one h, and then transfected with recombinant pLW44 plasmids encoding TcG2 or TcG4 (2 μg DNA) by using Lipofectamine 2000 reagent (Invitrogen, Grand Island, NY). After 48 h of incubation, cells were harvested, and cell lysates added at 10-fold dilutions to new BHK-21 cell monolayers that were then overlaid with 2% methylcellulose (Sigma, St. Louis, MO), and incubated for 48 h. At least three GFP+ fluorescent plaques were picked for each recombinant MVA (rMVA). The plaque purification procedure was repeated 4–6 times to ensure removal of wild-type MVA contamination. Purified plaques of rMVA.TcG2 and rMVA.TcG4 were then amplified in BHK-21 cell monolayers, and viral pellets were stored in 1 mM Tris-HCl (pH 9) at -80°C [27].
Immunization and challenge infection in mice
T. cruzi trypomastigotes (Sylvio X10/4 strain) were maintained and propagated by continuous in vitro passage in C2C12 cells. C57BL/6 female mice (6-week-old) were obtained from Harlan Labs (Indianapolis, IN). Mice (7-8-weeks-old) were immunized with the DNA prime dose consisting of the TcG2 - and TcG4-encoding plasmids with or without IL-12 - and GM-CSF-expression plasmids (25 μg each plasmid DNA/mouse, intramuscularly). Twenty-one days later, mice were given recombinant proteins (TcG2 and TcG4, 25 μg of each protein emulsified in 5 μg saponin/100 μl PBS/mouse, intra-dermally). Mice were harvested at 14 and 120 days after vaccination to determine early and long-term vaccine-induced T cell immunity, respectively. In some experiments, 90 days after the DNA-prime/protein-boost (D/P) vaccination, mice were given a booster immunization (bi) with recombinant proteins as above, and then sacrificed at 14, 120 and 180 days later to evaluate if bi provided stable, long-term anti-T. cruzi T cell immunity.
To evaluate the recall response to T. cruzi, mice in each group, i.e., 120 days after D/P vaccination or 14, 120, and 180 days after D/P/P vaccination, were challenged with T. cruzi (10,000 trypomastigotes/mouse, i.p.), and sacrificed 10 days post infection (dpi). Some mice were also sacrificed at >90 dpi to evaluate the vaccine efficacy in controlling chronic parasite burden.
In vitro stimulation of splenocytes, T cell characterization, and intracellular cytokines
Single-cell suspensions of spleen cells were prepared and cell number counted (S1 Table). Splenocytes (105 cells/100 μl RPMI) were distributed in 24-well plates, and incubated in the presence of Con A (5 μg/ml), recombinant proteins (10 μg/ml), or T. cruzi trypomastigotes lysate (TcTL, 25 μg protein/ml) at 37°C, 5% CO2 for 48 h. Un-stimulated or in vitro- stimulated splenocytes were washed in staining buffer (2% BSA/0.02% sodium azide in PBS) and incubated for 15 min in Fc Block (anti-CD16/CD32; BD Pharmingen). To evaluate the surface staining of effector and memory markers, we incubated the cells with the fluorescence - conjugated PE-αCD4, FITC-αCD8, PerCPCy5.5-αCD62L and APC-αCD44 antibodies (BD Pharmingen) for 30 min at 4°C in the dark. Then cells were washed twice in PBS and fixed in 2% paraformaldehyde. Fluorescent cells were visualized by using a FACSCalibur Cell Analyzer (BD Biosciences), acquiring >30,000 events in a live lymphocyte gate, and further analyzed by using FlowJo software (version 7.6.5, Tree-Star, San Carlo, CA) [27].
For the measurement of intracellular cytokines, splenocytes were stimulated as above except that brefeldin A (10-μg/ml, Sigma) or monensin (5 μg/ml) (BD Pharmingen) was added for the final 6 h of culture to block protein secretion. Cells were labeled with PE-αCD4 and FITC-αCD8 antibodies, fixed with 2% paraformaldehyde, re-suspended in 100 μl permeabilization buffer (0.1% saponin/1% FBS in PBS), and then utilized for intracellular staining with APC-αIL4, PerCPCy5.5-αIL10, e-Fluor-αIFNγ, Cy5-αTNFα and PerCP-Cy5.5-αKi67 antibodies (0.5-2-μg/100-μl, e-Biosciences). In some experiments, splenocytes were also incubated with APC-anti-perforin or Alexa-Fluor 488-anti-CD107 antibodies to determine the cytolytic activity of the activated/proliferating T cell subpopulations. Samples were analyzed by flow cytometry as above. In all experiments, cells stained with isotype-matched IgGs were used as controls [13,27].
Cytotoxic T lymphocyte (CTL) activity
Splenocytes (5×106 cells/2 ml/well in 24-well plates) were incubated with recombinant TcG2 or TcG4 proteins (10-μg/ml) at 37°C, 5% CO2 for 4–5 days to generate effector T cells. Semi-confluent EL-4 monolayers were exposed to rMVA encoding TcG2 or TcG4 (10 pfu/cell) for 1 h (controls: WT MVA), washed, and incubated in complete medium for 12 h at 37°C. Target EL-4 cells infected with GFP+ rMVA were co-cultured with effector cells (Effector: target cell ratio: 80 : 1–20 : 1) in 200 μl of RPMI medium at 37°C, 5% CO2 for 4 h. Cells were mixed with 2.5 mM EDTA to reduce the number of cell-cell conjugates, and 5 μl propidium iodide (PI) to discriminate viable and nonviable cells, and analyzed by flow cytometry for a fixed period of 60 sec/sample. The forward scatter (FSC) acquisition threshold was set to include nonviable events. The CTL activity was calculated using [GFP+ target cells after incubation with effector cells x 100 / Total GFP+ target cells].
Blood and tissue parasite burden
Blood DNA was isolated with a QiAamp Blood DNA mini kit (Qiagen, Chatsworth, CA). Skeletal muscle, spleen, and heart tissue (50 mg) were subjected to proteinase K lysis, and total DNA was purified by phenol/chloroform extraction and ethanol precipitation. Total DNA (50 ng) was used as a template, and real-time PCR performed on an iCycler thermal cycler with SYBR Green Supermix (Bio-Rad) and Tc18SrDNA-specific oligonucleotides. Data were normalized to murine GAPDH and fold change in parasite burden (i.e. Tc18SrDNA level) calculated as 2-ΔCt, where ΔCt represents the Ct (infected)—Ct (control) [60,61].
Statistical analysis
Data are expressed as mean ± SD (n = 8/group, triplicate observations per experiment). Data were analyzed by the Student’s t test (comparison of 2 groups) and 1-way analysis of variance (ANOVA) with Tukey’s post-hoc test (comparison of multiple groups) by using an SPSS (version 14.0, SPSS Inc, Chicago, Illinois) or Graph Pad InStat ver.3 software. Significance is presented as *p<0.05, **p<0.01, or ***p<0.001 (vaccinated versus non-vaccinated or vaccinated/infected versus non-vaccinated/infected).
Supporting Information
Zdroje
1. World Health Organization (2010) Chagas disease: control and elimination. UNDP/World Bank/WHO. http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_17-en.pdf, accessed on Mar 5, 2015.
2. Cunha-Neto E, Chevillard C (2014) Chagas disease cardiomyopathy: immunopathology and genetics. Mediators Inflamm 2014 : 683230. doi: 10.1155/2014/683230 25210230
3. Cantey PT, Stramer SL, Townsend RL, Kamel H, Ofafa K, et al. (2012) The United States Trypanosoma cruzi Infection Study: evidence for vector-borne transmission of the parasite that causes Chagas disease among United States blood donors. Transfusion.
4. Bern C, Kjos S, Yabsley MJ, Montgomery SP (2011) Trypanosoma cruzi and Chagas' Disease in the United States. Clin Microbiol Rev 24 : 655–681. doi: 10.1128/CMR.00005-11 21976603
5. Bern C, Montgomery SP (2009) An estimate of the burden of Chagas disease in the United States. Clin Infect Dis 49: e52–54. doi: 10.1086/605091 19640226
6. Tanowitz HB, Weiss LM, Montgomery SP (2011) Chagas disease has now gone global. PLoS Negl Trop Dis 5: e1136. doi: 10.1371/journal.pntd.0001136 21572510
7. Vázquez-Chagoyán JC, Gupta S, Garg NJ (2011) Vaccine development against Trypanosoma cruzi and Chagas disease. Advanced parasitol 75 : 121–146. doi: 10.1016/B978-0-12-385863-4.00006-X 21820554
8. Lee BY, Bacon KM, Connor DL, Willig AM, Bailey RR (2010) The potential economic value of a Trypanosoma cruzi (Chagas disease) vaccine in Latin America. PLoS Negl Trop Dis 4: e916. doi: 10.1371/journal.pntd.0000916 21179503
9. Garg NJ, Tarleton RL (2002) Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infection & Immunity 70 : 5547–5555.
10. Bhatia V, Sinha M, Luxon B, Garg NJ (2004) Utility of Trypanosoma cruzi sequence database for the identification of potential vaccine candidates: In silico and in vitro screening. Infect Immun 72 : 6245–6254. 15501750
11. Bhatia V, Garg NJ (2008) Previously unrecognized vaccine candidates control Trypanosoma cruzi infection and immunopathology in mice. Clin Vaccine Immunol 15 : 1158–1164. doi: 10.1128/CVI.00144-08 18550728
12. Aparicio-Burgos JE, Ochoa-Garcia L, Zepeda-Escobar JA, Gupta S, Dhiman M, et al. (2011) Testing the efficacy of a multi-component DNA-prime/DNA-boost vaccine against Trypanosoma cruzi infection in dogs. PLoS Negl Trop Dis 5: e1050. doi: 10.1371/journal.pntd.0001050 21625470
13. Gupta S, Garg NJ (2010) Prophylactic efficacy of TcVac2 against Trypanosoma cruzi in mice. PLoS Negl Trop Dis 4: e797. doi: 10.1371/journal.pntd.0000797 20706586
14. Tanowitz HB, Machado FS, Jelicks LA, Shirani J, de Carvalho AC, et al. (2009) Perspectives on Trypanosoma cruzi-induced heart disease (Chagas disease). Prog Cardiovasc Dis 51 : 524–539. doi: 10.1016/j.pcad.2009.02.001 19410685
15. Ribeiro AL, Nunes MP, Teixeira MM, Rocha MO (2012) Diagnosis and management of Chagas disease and cardiomyopathy. Nat Rev Cardiol 9 : 576–589. doi: 10.1038/nrcardio.2012.109 22847166
16. Machado FS, Tyler KM, Brant F, Esper L, Teixeira MM, et al. (2012) Pathogenesis of Chagas disease: time to move on. Front Biosci (Elite Ed) 4 : 1743–1758. 22201990
17. Machado FS, Dutra WO, Esper L, Gollob KJ, Teixeira MM, et al. (2012) Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Seminars in Immunopathology 34 : 753–770. doi: 10.1007/s00281-012-0351-7 23076807
18. Tarleton RL (2007) Immune system recognition of Trypanosoma cruzi. Curr Opin Immunol 19 : 430–434. 17651955
19. Padilla AM, Bustamante JM, Tarleton RL (2009) CD8+ T cells in Trypanosoma cruzi infection. Curr Opin Immunol 21 : 385–390. doi: 10.1016/j.coi.2009.07.006 19646853
20. Bhatia V, Garg NJ (2005) Current status and future prospects for a vaccine against American trypanosomiasis. Expert Rev Vaccines 4 : 867–880. 16372882
21. Bhatia V, Wen J-J, Zacks MA, Garg NJ (2009) American trypanosomiasis and perspectives on vaccine development. In: Stanberry LR, Barrett AD, editors. Vaccines for Biodefense and Emerging and Neglected Diseases. New York: Academic Press. pp. 1407–1434.
22. Vazquez-Chagoyan JC, Gupta S, Garg NJ (2011) Vaccine development against Trypanosoma cruzi and Chagas disease. Adv Parasitol 75 : 121–146. doi: 10.1016/B978-0-12-385863-4.00006-X 21820554
23. Cazorla SI, Becker PD, Frank FM, Ebensen T, Sartori MJ, et al. (2008) Oral vaccination with Salmonella enterica as a cruzipain-DNA delivery system confers protective immunity against Trypanosoma cruzi. Infect Immun 76 : 324–333. 17967857
24. Miyahira Y, Takashima Y, Kobayashi S, Matsumoto Y, Takeuchi T, et al. (2005) Immune responses against a single CD8+-T-cell epitope induced by virus vector vaccination can successfully control Trypanosoma cruzi infection. Infect Immun 73 : 7356–7365. 16239534
25. de Alencar BC, Persechini PM, Haolla FA, de Oliveira G, Silverio JC, et al. (2009) Perforin and gamma interferon expression are required for CD4+ and CD8+ T-cell-dependent protective immunity against a human parasite, Trypanosoma cruzi, elicited by heterologous plasmid DNA prime-recombinant adenovirus 5 boost vaccination. Infect Immun 77 : 4383–4395. doi: 10.1128/IAI.01459-08 19651871
26. Gupta S, Wan X-X, Zago MP, Martinez VCS, Silva TS, et al. (2013) Antigenicity and diagnostic potential of vaccine candidates in human Chagas disease,. Plos NTD 7: e2018. doi: 10.1371/journal.pntd.0002018 23350012
27. Gupta S, Garg NJ (2012) Delivery of antigenic candidates by a DNA/MVA heterologous approach elicits effector CD8+T cell mediated immunity against Trypanosoma cruzi. Vaccine 12 : 1464–1478
28. Gupta S, Garg NJ (2013) TcVac3 induced control of Trypanosoma cruzi infection and chronic myocarditis in mice. PLOS ONE 8: e59434. doi: 10.1371/journal.pone.0059434 23555672
29. Huygen K (2005) Plasmid DNA vaccination. Microbes Infect 7 : 932–938. 15878683
30. Donnelly JJ, Wahren B, Liu MA (2005) DNA vaccines: progress and challenges. J Immunol 175 : 633–639. 16002657
31. Scharpe J, Peetermans WE, Vanwalleghem J, Maes B, Bammens B, et al. (2009) Immunogenicity of a standard trivalent influenza vaccine in patients on long-term hemodialysis: an open-label trial. Am J Kidney Dis 54 : 77–85. doi: 10.1053/j.ajkd.2008.11.032 19339089
32. Zeng R, Zhang Z, Mei X, Gong W, Wei L (2008) Protective effect of a RSV subunit vaccine candidate G1F/M2 was enhanced by a HSP70-Like protein in mice. Biochem Biophys Res Commun 377 : 495–499. doi: 10.1016/j.bbrc.2008.10.002 18851947
33. Limon-Flores AY, Cervera-Cetina R, Tzec-Arjona JL, Ek-Macias L, Sanchez-Burgos G, et al. (2010) Effect of a combination DNA vaccine for the prevention and therapy of Trypanosoma cruzi infection in mice: role of CD4+ and CD8+ T cells. Vaccine 28 : 7414–7419. doi: 10.1016/j.vaccine.2010.08.104 20850536
34. Dutra WO, Gollob KJ (2008) Current concepts in immunoregulation and pathology of human Chagas disease. Curr Opin Infect Dis 21 : 287–292. doi: 10.1097/QCO.0b013e3282f88b80 18448974
35. Gomes JA, Bahia-Oliveira LM, Rocha MO, Martins-Filho OA, Gazzinelli G, et al. (2003) Evidence that development of severe cardiomyopathy in human Chagas' disease is due to a Th1-specific immune response. Infect Immun 71 : 1185–1193. 12595431
36. Laucella SA, Postan M, Martin D, Hubby Fralish B, Albareda MC, et al. (2004) Frequency of interferon - gamma-producing T cells specific for Trypanosoma cruzi inversely correlates with disease severity in chronic human Chagas disease. J Infect Dis 189 : 909–918. 14976609
37. Gomes JA, Bahia-Oliveira LM, Rocha MO, Busek SC, Teixeira MM, et al. (2005) Type 1 chemokine receptor expression in Chagas' disease correlates with morbidity in cardiac patients. Infect Immun 73 : 7960–7966. 16299288
38. Magalhaes LM, Villani FN, Nunes Mdo C, Gollob KJ, Rocha MO, et al. (2013) High interleukin 17 expression is correlated with better cardiac function in human Chagas disease. J Infect Dis 207 : 661–665. doi: 10.1093/infdis/jis724 23204182
39. Silva GK, Gutierrez FR, Guedes PM, Horta CV, Cunha LD, et al. (2010) Cutting edge: nucleotide-binding oligomerization domain 1-dependent responses account for murine resistance against Trypanosoma cruzi infection. J Immunol 184 : 1148–1152. doi: 10.4049/jimmunol.0902254 20042586
40. Rodrigues MM, Oliveira AC, Bellio M (2012) The Immune Response to Trypanosoma cruzi: Role of Toll-Like Receptors and Perspectives for Vaccine Development. J Parasitol Res 2012 : 507874. doi: 10.1155/2012/507874 22496959
41. Goncalves VM, Matteucci KC, Buzzo CL, Miollo BH, Ferrante D, et al. (2013) NLRP3 controls Trypanosoma cruzi infection through a caspase-1-dependent IL-1R-independent NO production. PLoS Negl Trop Dis 7: e2469. doi: 10.1371/journal.pntd.0002469 24098823
42. Tarleton RL, Grusby MJ, Postan M, Glimcher LH (1996) Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I - and class II-restricted T cells in immune resistance and disease. Int Immunol 8 : 13–22. 8671585
43. Nickell SP, Sharma D (2000) Trypanosoma cruzi: roles for perforin-dependent and perforin-independent immune mechanisms in acute resistance. Exp Parasitol 94 : 207–216. 10831388
44. Tzelepis F, de Alencar BC, Penido ML, Gazzinelli RT, Persechini PM, et al. (2006) Distinct kinetics of effector CD8+ cytotoxic T cells after infection with Trypanosoma cruzi in naive or vaccinated mice. Infect Immun 74 : 2477–2481. 16552083
45. Masopust D, Ahmed R (2004) Reflections on CD8 T-cell activation and memory. Immunol Res 29 : 151–160. 15181278
46. Tzelepis F, de Alencar BC, Penido ML, Claser C, Machado AV, et al. (2008) Infection with Trypanosoma cruzi restricts the repertoire of parasite-specific CD8+ T cells leading to immunodominance. J Immunol 180 : 1737–1748. 18209071
47. Eickhoff CS, Vasconcelos JR, Sullivan NL, Blazevic A, Bruna-Romero O, et al. (2011) Co-administration of a plasmid DNA encoding IL-15 improves long-term protection of a genetic vaccine against Trypanosoma cruzi. PLoS Negl Trop Dis 5: e983. doi: 10.1371/journal.pntd.0000983 21408124
48. Vasconcelos JR, Bruna-Romero O, Araujo AF, Dominguez MR, Ersching J, et al. (2012) Pathogen-induced proapoptotic phenotype and high CD95 (Fas) expression accompany a suboptimal CD8+ T-cell response: reversal by adenoviral vaccine. PLoS Pathog 8: e1002699. doi: 10.1371/journal.ppat.1002699 22615561
49. Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, et al. (2009) Memory CD8 T-cell compartment grows in size with immunological experience. Nature 457 : 196–199. doi: 10.1038/nature07486 19005468
50. DiSpirito JR, Shen H (2010) Quick to remember, slow to forget: rapid recall responses of memory CD8+ T cells. Cell Res 20 : 13–23. doi: 10.1038/cr.2009.140 20029390
51. Zanetti M, Castiglioni P, Ingulli E (2010) Principles of memory CD8 T-cells generation in relation to protective immunity. Adv Exp Med Biol 684 : 108–125. 20795544
52. Peters NC, Pagan AJ, Lawyer PG, Hand TW, Henrique Roma E, et al. (2014) Chronic parasitic infection maintains high frequencies of short-lived Ly6C+CD4+ effector T cells that are required for protection against re-infection. PLoS Pathog 10: e1004538. doi: 10.1371/journal.ppat.1004538 25473946
53. Rigato PO, de Alencar BC, de Vasconcelos JR, Dominguez MR, Araujo AF, et al. (2011) Heterologous plasmid DNA prime-recombinant human adenovirus 5 boost vaccination generates a stable pool of protective long-lived CD8(+) T effector memory cells specific for a human parasite, Trypanosoma cruzi. Infect Immun 79 : 2120–2130. doi: 10.1128/IAI.01190-10 21357719
54. Albareda MC, Laucella SA, Alvarez MG, Armenti AH, Bertochi G, et al. (2006) Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas' disease patients. Int Immunol 18 : 465–471. 16431876
55. Bustamante JM, Bixby LM, Tarleton RL (2008) Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med 14 : 542–550. doi: 10.1038/nm1744 18425131
56. Dos Santos Virgilio F, Pontes C, Dominguez MR, Ersching J, Rodrigues MM, et al. (2014) CD8(+) T cell-mediated immunity during Trypanosoma cruzi infection: a path for vaccine development? Mediators Inflamm 2014 : 243786. doi: 10.1155/2014/243786 25104879
57. Cohen JE, Gurtler RE (2001) Modeling household transmission of American trypanosomiasis. Science 293 : 694–698. 11474111
58. Earl PL MB, Wyatt LS, Carroll MW. (1998) Generation of recombinant vaccinia viruses. In: Current Protocols in Molecular Biology. Ausubel FM, Brent R, Kingston RE, Moore DD, Seidmen ZG, Smith ZA, Skuhl LX (eds). John Wiley and Sons: NewYork, pp 16.17.11–16.19.11.
59. Sandstrom E, Nilsson C, Hejdeman B, Brave A, Bratt G, et al. (2008) Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J Infect Dis 198 : 1482–1490. doi: 10.1086/592507 18808335
60. Garg NJ, Bhatia V, Gerstner A, deFord J, Papaconstantinou J (2004) Gene expression analysis in mitochondria from chagasic mice: Alterations in specific metabolic pathways. Biochemical J 381 : 743–752. 15101819
61. Wen J-J, Gupta S, Guan Z, Dhiman M, Condon D, et al. (2010) Phenyl-alpha-tert-butyl-nitrone and benzonidazole treatment controlled the mitochondrial oxidative stress and evolution of cardiomyopathy in chronic chagasic rats. J Am Coll Cardiol 55 : 2499–2508. doi: 10.1016/j.jacc.2010.02.030 20510218
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR SelectionČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



