-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEvidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
Error-prone polymerase function of RNA viruses can result in expression of defective RNAs harboring internal deletions of various sizes. Small subgenomic RNAs are strong inducers of the antiviral response by serving as pathogen-associated patterns that are predominantly detected by cellular sensors. Recently, it has been shown that influenza A virus defective RNAs are not only generated upon passages in cell culture, but also in infected humans, indicating that these subgenomic RNAs may also be relevant in infections in vivo. Here, we characterize a novel defective RNA derived from the PB2 segment of a highly pathogenic H5N1 influenza A virus. This RNA encodes a 10 kDa peptide (PB2Δ) which activates type I interferon (IFN) responses through direct interaction with the adapter protein MAVS, a key component of the RIG-I-dependent IFN induction. This is the first time that such a function was described for a defective RNA-encoded protein, a finding that has several important implications with regard to deciphering viral protein functions and options for immunostimulatory approaches. Furthermore, this is an example of how influenza viruses may acquire novel polypeptides with altered functions from its limited genome.
Published in the journal: . PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004924
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004924Summary
Error-prone polymerase function of RNA viruses can result in expression of defective RNAs harboring internal deletions of various sizes. Small subgenomic RNAs are strong inducers of the antiviral response by serving as pathogen-associated patterns that are predominantly detected by cellular sensors. Recently, it has been shown that influenza A virus defective RNAs are not only generated upon passages in cell culture, but also in infected humans, indicating that these subgenomic RNAs may also be relevant in infections in vivo. Here, we characterize a novel defective RNA derived from the PB2 segment of a highly pathogenic H5N1 influenza A virus. This RNA encodes a 10 kDa peptide (PB2Δ) which activates type I interferon (IFN) responses through direct interaction with the adapter protein MAVS, a key component of the RIG-I-dependent IFN induction. This is the first time that such a function was described for a defective RNA-encoded protein, a finding that has several important implications with regard to deciphering viral protein functions and options for immunostimulatory approaches. Furthermore, this is an example of how influenza viruses may acquire novel polypeptides with altered functions from its limited genome.
Introduction
Influenza A viruses (IAV) belong to the family of Orthomyxoviridae and are characterized by a segmented single-stranded RNA genome with negative orientation. These eight segments encode nine structural and up to seven nonstructural proteins [1–5]. For the production of infectious virus particles at least one copy of each of the segments needs to be packaged into the progeny particle during the virion assembly step [6].
Non-infectious particles exhibiting an incomplete viral genome e.g. due to a large internal deletion within at least one segment, are defined as defective interfering particles (DIPs) [7, 8]. These DIPs need the presence of a helper virus for replication of their defective RNA [9]. The generation of IAV DI RNAs has been linked to the ribonucleoprotein tertiary structure that leads to jumping of the viral polymerase making transitions between adjacent regions of the RNA template. Furthermore, there are indications that polymerase skipping is facilitated by short sequence repeats within viral segments [10]. It has been shown that the competition for viral RNA polymerases and preferential packaging of over-abundant DI RNA segments interferes with replication and packaging of full-length segments of replication competent helper virus [7, 8, 11–13].
IAV DI particles have been observed primarily after multiple passages of viruses in cell culture at high multiplicity of infection or in experimentally infected embryonated chicken eggs [10, 14, 15] and therefore were considered as experimental artifacts. However, subgenomic RNAs were isolated recently in vivo from naso-pharyngeal swabs of human patients [16]. The same study demonstrated that identical in vivo-derived defective RNAs were present in patients linked by direct contact which might suggest efficient co-transmission between them. It is thus reasonable to assume that defective RNAs are common byproducts also in natural infections albeit their function in the biology of influenza viruses and its interactions with the host remains elusive.
Defective RNAs have been shown to act as efficient pathogen-associated molecular patterns (PAMPs) recognized more potently by the sensor molecule retinoic acid-inducible gene-I (RIG-I) than viral genomic segments [17]. This activation of RIG-I leads to the induction of interferon beta (IFNβ) which is one of the key cytokines orchestrating a broadly reactive antiviral program upon infection [18]. Recently, Tapia and colleagues provided evidence that the appearance of defective RNAs coincides with the production of cytokines during IAV infection [19].
It has been shown previously that some subgenomic RNAs harbor open reading frames that might be expressed in the presence of a helper virus or could be in vitro translated into polypeptides [20, 21]. But, so far, a putative biological function of these proteins has never been analyzed, although they might share sequence similarities with their parental proteins and might therefore exhibit related or even different functions. In the past years, the discovery of novel IAV-encoded proteins led to new insights into viral pathogenicity [1–4, 22]. Surprisingly, all viral nonstructural proteins discovered since 2001 have been shown to be nonessential for viral replication [1–4] or even possess antiviral activities under certain conditions [23]. However, several analyses demonstrated that changes in expression levels of these proteins were linked to virulence in vivo [3, 23, 24]. Therefore, defective RNA-encoded proteins may also contribute to the course of infections in vivo and could exemplify a mechanism on how influenza viruses acquire novel polypeptides with altered functions from its limited genome.
The present study describes for the very first time a defective RNA-encoded functional polypeptide, named PB2∆. The subgenomic RNA was identified in the H5N1 strain A/Thailand/KAN-1/2004 (KAN-1). It belongs to the group of defective RNAs derived from the PB2 segment and potently restricts viral replication by a mechanism independent of DI RNA-mediated interference. The present study demonstrates that the defective RNA-encoded polypeptide PB2Δ directly interacts with the mitochondrial antiviral signaling protein (MAVS). In contrast to the effects of PB2-MAVS interaction, this leads to the induction of IFNβ expression, thereby diminishing viral replication. Furthermore, the presence of this particular defective RNA-encoded protein in KAN-1 infection leads to higher expression levels of antiviral acting genes also in vivo, resulting in a more severe disease phenotype.
Results
Characterization of PB2∆ defective interfering (DI)-like RNA
In recent years, new influenza encoded non-structural proteins were discovered which could be either linked to viral polymerase activity [25] or host cell response [3, 22, 26, 27] and are therefore determinants of viral virulence.
During analysis of a cDNA library generated from total RNAs of A549 cells infected with different IAV subtypes, cDNAs representing a 618 nt-long RNA were identified in H5N1 strain A/Thailand/KAN-1/2004-infected cultures. This RNA has sequence identity to the 5’ and 3’ regions of the viral PB2 gene but lacks 1715 nt from the PB2 internal region. Hence, the PB2Δ RNA retains viral promoter and packaging information required for efficient viral replication (Fig 1A) and thus possesses a structure that is typical for viral defective interfering RNAs [7]. The internal deletion junction site of the PB2∆ RNA occurs at nucleotide position 240 as observed previously for other defective RNAs with junction sites located around positions 200–300 in vitro and in vivo [10, 16]. Furthermore, this RNA harbors characteristic defective RNA sequence motifs as described by Jennings and colleagues (1983) such as the frequent occurrence of the sequences 5’..GAA..3’ and 5’..CAA..3’ near the junction site, the number of adenosine residues varying from 2 to 3. In addition, it has been described that identification of the precise junction is frequently not possible due to local sequence similarities [10]. Here, short repeat sequences at the junction site were also observed, although these nucleotides are not deleted but still present at both ends (5’..AGGAAT/AGGAAT..3’) (Fig 1A). Therefore, it can be concluded that the PB2∆ RNA structurally belongs to the group of viral defective interfering (DI)-like RNAs.
Fig. 1. Characterization of the H5N1 KAN-1-expressed PB2∆ DI-like RNA. 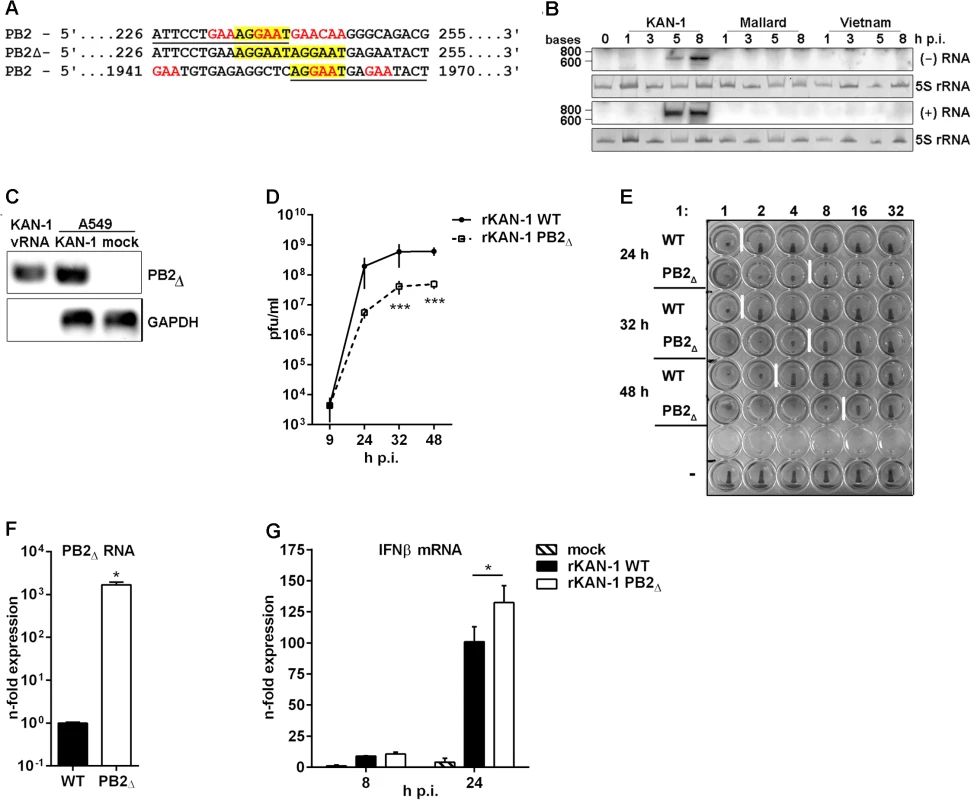
A) Sequence analysis of the PB2∆ DI-like RNA (middle lane) in comparison to the parental PB2 segment (upper and lower lane). Sequence identity is underlined in PB2 sequences and characteristic DI RNA sequence motives are displayed in color. B) Northern blot analysis of total RNA isolated from A549 infected with 5 MOI of different H5N1 isolates (KAN-1, Mallard and Vietnam) for the indicated time points. PB2∆ vRNA and c/mRNA were detected by hybridization probes covering the PB2∆ junction site. Visualization was achieved by autoradiography. As loading control serves 5S rRNA in ethidiumbromide stained PAA gels. C) A549 cells were infected with 5 MOI of KAN-1 for 8 h, subsequently viral particles were purified from cell culture supernatants and total RNA was isolated. Total RNA from uninfected or KAN-1-infected A549 served as controls. Detection of PB2∆ RNA and GAPDH mRNA was performed by qRT PCR and analyzed by agarose gel. B, C) Northern blot and agarose gel are representative of three independent experiments. D) A549 cells were infected with 0.01 MOI rKAN-1 WT or PB2∆ and virus-containing supernatants were harvested at the time points indicated. Infectivity titers were determined by a standard plaque assay and are depicted as mean (±SD) of one representative of three independent experiments. ***p≤0.001; two-way ANOVA, Sidak’s multiple comparisons test. E) Analysis of the relative amount of viral particles (infectious and noninfectious) in supernatants from (D) by hemagglutination assay. To exclude differences due to different replication abilities of the two viruses 1x106 infectious particles were used for the assay which is representative for three independent experiments. F) A549 cells were infected with 5 MOI of rKAN-1 WT or PB2∆ and 2 h p.i. total RNA was isolated. Expressional changes of PB2∆ RNA were detected by qRT-PCR and are depicted as mean n-fold (±SD) of one representative out of two independent experiments normalized to rKAN-1 WT. *p≤0.05; unpaired two-tailed Student’s t-test. G) A549 cells were infected with 1 MOI of rKAN-1 WT or PB2∆ and 8 or 24 h p.i. total RNA was isolated. Expressional changes of IFNβ mRNA were detected by qRT-PCR and are depicted as mean n-fold (±SD) of one representative out of two independent experiments normalized to control. *≤0.05; two-way ANOVA, Tukey’s multiple comparisons test. Significances between the different time points and to mock infection are not indicated. To test whether PB2∆ RNA was expressed also by closely related H5N1 viruses total RNA was isolated from A549 cells infected with A/Thailand/1(KAN-1)/2004, A/Vietnam/1203/2004 or A/Mallard/Bavaria/1/2006. A specific Northern blot probe complementary to the junction site was designed, thus preventing cross-hybridization with the PB2 genomic vRNA under the hybridization conditions applied. The PB2∆ RNA was detectable only in KAN-1-infected cells (Fig 1B), although especially the Vietnam strain shows high sequence similarity and sequence repetition at the junction site within the PB2 gene (S1 Table, NCBI’s Influenza Virus Resource [28] KAN-1: CY111595.1 and Vietnam: HM006756.1). Interestingly, sequence alignments showed that this repeat is present in 53% of all known human and 79% of all avian H5N1 strains compared (S1 and S2 Tables), while all other strains show in general only a single nucleotide substitution. To exclude that the generation of the PB2Δ RNA was a KAN-1 stock-specific event, the ability of de novo PB2Δ RNA synthesis was examined for the H5N1 strains KAN-1 and Vietnam. Undiluted passages of plaque-purified clones from PB2Δ-free virus preparations performed as described by Nayak and colleagues [29] uncovered PB2Δ RNA expression in different passages to various extents for both strains (e.g. KAN-1 clones 8 and 12 as well as Vietnam clones 3 and 7; S3 Table). Although PB2Δ RNA expression is a nonessential event and not specific for KAN-1, this strain seems to exhibit higher PB2Δ expression levels compared to Vietnam virus.
DI dependent interference is characterized by competition for viral polymerases during replication of vRNA into cRNA and preferential packaging of over-abundant DI segments [12, 13]. To test whether PB2∆ RNA can be replicated by the viral polymerase complex, Northern blot hybridization was performed with a specific probe complementary to PB2∆ (+)RNA. As expected, the presence of PB2∆ (+)RNA upon infection with the KAN-1 isolate was confirmed, suggesting that the PB2 DI-like RNA is a substrate for the viral polymerase and is amplified upon viral replication (Fig 1B). This was further verified by strand-specific qRT-PCR, uncovering the existence of all three viral RNA species (S1 Fig).
One further aspect of the interfering activity of defective viral RNAs is the preferential packaging of RNAs which exist at high copy numbers within the cell [12]. The retained 5’ and 3’ regions of the PB2 segment within the PB2∆ RNA suggest that packaging of this smaller RNA fragment into virions is possible. The presence of PB2∆ vRNA in KAN-1 viral particles was confirmed by qRT-PCR (Fig 1C). Total RNA from KAN-1 - and mock-infected A549 cells served as controls, showing higher amounts of PB2∆ RNA in infected cells compared to viral particles which might be due to the detection of PB2∆ RNA in negative and positive orientation. To exclude contamination of viral RNA with cellular RNA, the presence of GAPDH mRNA was analyzed, showing no PCR product from total RNA of viral particles (Fig 1C).
Hence, specific expression of PB2∆ DI-like RNA was confirmed and conversion into (+)RNA was shown, arguing that PB2∆ DI-like RNA serves as a substrate for the viral polymerase. Furthermore, it is packaged into viral particles and, thus, possesses all crucial features of defective RNAs owning interfering activity.
To answer the question as to whether the PB2∆ DI-like RNA exhibits interfering activity, a recombinant KAN-1 virus was produced by reverse genetics [30] harboring PB2∆ DI-like RNA as additional segment. This method has the advantage that the artificially introduced RNA predominantly persists over newly originated defective RNAs which are in an inferior position due to copy numbers [11, 31]. Furthermore, the generation of wild type virus allows the comparative analysis with a PB2∆ RNA-free virus.
To investigate whether the PB2∆ DI-like RNA exhibits interfering activity, replication of the recombinant PB2∆ RNA-expressing KAN-1 (rKAN-1 PB2∆) and of wild type virus (rKAN-1 WT) were compared (Fig 1D). Although 9 h p.i. there were no differences in viral replication, infectivity titers were significantly reduced by one log step at all further time points analyzed. Furthermore, rKAN-1 PB2∆ indeed induces the production of non-infectious particles as confirmed by hemagglutination assay (Fig 1E). This method allows the determination of the relative amount of particles in a virus suspension with a predefined number of infectious particles due to the same ability of infectious and non-infectious influenza particles to agglutinate avian erythrocytes. Here, an increased amount of virus particles was observed at all time points analyzed which was elevated by a factor of 4 for rKAN-1 PB2∆ infection compared to wild type virus (Fig 1E). To evaluate whether these differences are due to non-infectious particles induced by the PB2∆ DI-like RNA, whole genomes of both recombinant viruses were sequenced. This analysis revealed no differences in viral segment sequences neither among each other nor to the PB2Δ-expressing KAN-1 isolate, whereas a strong expression of PB2∆ m/cRNA for rKAN-1 PB2∆ was observed (Fig 1F), confirming that the presence of PB2Δ defective RNA indeed induces the production of non-infectious particles.
In recent years, increased attention has been paid to defective RNA-induced interferon expression. The first evidence of an influenza DI RNA inducing IFN expression came from DI 244 which protected wild type mice from lethal infections with heterologous paramyxo - and influenza B virus, but failed in protection of type I IFN receptor-deficient mice [32, 33]. The main PAMP that is sensed by different pattern-recognition receptors (PRRs) to induce the type I IFN response in viral infections is viral RNA. Particularly, detection of the 5’-triphosphate (5’-ppp) structure in viral RNAs by the cytoplasmic helicase RIG-I plays an important role in influenza A virus infection [34]. It has been shown that RIG-I preferentially binds 5’-ppp-containing small genomic segments and subgenomic RNAs [17]. Moreover, defective RNA generation coincides with the production of cytokines during IAV infection [19]. In addition, double-stranded RNAs of more than 30 base pairs in length, often found in snap - or copyback DI RNAs of Paramyxo- and Rhabdoviridae due to long double-stranded regions, also can trigger IFN responses [11]. To analyze whether PB2∆ DI-like RNA affects viral replication via enhancing virus-induced innate immune responses, the expression of IFNβ was determined by qRT-PCR. IFNβ is the most crucial mediator of the innate type I IFN response upon IAV infection [35]. No differences in IFNβ mRNA expression were detectable 8 h p.i. between the two recombinant viruses (Fig 1G), although PB2∆ RNA was expressed in high amounts as early as 2 h p.i. (Fig 1F). Interestingly, 24 h p.i. IFNβ mRNA levels were significantly increased upon infection with rKAN-1 PB2∆, suggesting that the reduced infectivity titers may be in part due to the induction of a strong host cell response.
PB2∆ protein impairs viral protein expression without affecting the viral polymerase complex
Internal deletion of nucleotides within influenza segments during the synthesis of defective RNAs in general results in truncated RNAs possessing all important sequences for transcription and translation. Therefore, the presence of an intact open reading frame (ORF) can result in the expression of polypeptides from subgenomic RNAs, as previously described [20, 21]. However, so far there are no studies delineating a potential function of these polypeptides in IAV infection. The PB2∆ RNA comprises the PB2 translational initiation codon followed by a frameshift downstream of the junction site, leading to early termination after 15 additional amino acid codons (Fig 2A). To test whether this potential ORF leads to the generation of a polypeptide from the PB2∆ mRNA, viral protein expression was analyzed. Antibodies directed against the PB2 N-terminus were used, a region that would be identical in both proteins. A distinct protein band around 10 kDa emerged 5 h p.i. that was not observed upon infection with other IAV isolates of various subtypes (Fig 2B). Furthermore, the existence of this protein in KAN-1-infected cells was confirmed by mass spectrometry (S2 Fig). To verify that it is encoded by PB2∆ mRNA, a specific siRNA was designed covering the conserved regions flanking the junction site (Fig 1A) thus limiting interference with viral PB2 RNA. Viral protein expression was analyzed in the presence or absence of PB2∆-specific siRNA (Fig 2C). As expected, the small PB2-like protein encoded by the PB2∆ RNA was down-regulated, whereas the expression of PB2 was not reduced. Interestingly, especially 5 h p.i. there was a slight increase in viral protein expression observable when PB2∆ was knocked down which might suggest a function of the small protein in viral protein expression. Another explanation would be a simultaneous knockdown of PB2∆ v/cRNA leading to a more efficient replication of KAN-1 due to the prevention of non-infectious particles. To analyze whether viral replication is affected by siRNA-mediated knockdown of PB2∆ RNA species, A549 cells were infected with KAN-1 for 8 h (Fig 2D, above). Although the KAN-1 isolate is well adapted to cell culture and replicates efficiently in A549 to high viral titers, there was a slight increase in viral replication observable when PB2∆ was knocked down (Fig 2D, below). Furthermore, expression of PB2Δ protein was compared in rKAN-1 WT and rKAN-1 PB2Δ infection. As expected, the PB2Δ protein was only detectable in rKAN-1 PB2Δ-infected cells concomitant with decreased expression levels of other viral proteins (S3 Fig).
Fig. 2. The PB2∆ mRNA encodes a 10 kDa protein that impairs viral gene expression. 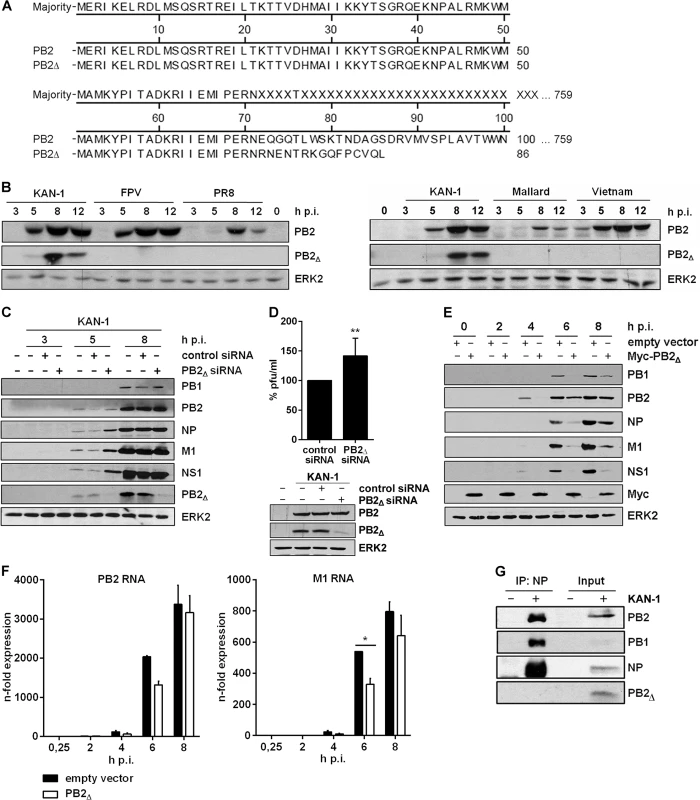
A) Sequence alignment of the PB2∆ protein in comparison to PB2. B) Western blot analysis of total lysates of A549 infected with 5 MOI of different influenza viruses of the subtypes H5N1 (KAN-1, Mallard, Vietnam; right), H7N7 (FPV) and H1N1 (PR8) (left). PB2∆ and PB2 were detected 3, 5, 8 and 12 h p.i.. Equal loading was verified by the detection of total ERK2. C, D) A549 cells were transfected with PB2∆-specific or scrambled siRNA and subsequently infected with KAN-1 (C: MOI 5, D: MOI 2) for the indicated time points. C) Expression of viral proteins PB1, PB2, NP, M1 and NS1 upon PB2∆ knockdown was analyzed by Western blot. Knockdown efficiency was verified by using PB2-specific antibodies. ERK2 expression served as loading control. D) Changes in infectivity titers upon PB2∆ knockdown were determined by standard plaque assay and are depicted as mean (±SD) of six independent experiments (above). **p≤0.01, unpaired two-tailed Student’s t-test. Efficient knockdown of PB2∆ was verified by Western blot analysis (below). Efficient infection was confirmed by immunodetection of viral PB2 and equal loading was verified by analysis of total ERK2 expression. E, F) A549 cells were transfected with PB2∆ or empty vector and subsequently infected with 5 MOI KAN-1 for the indicated time points. E) Expression of viral proteins PB1, PB2, NP, M1 and NS1 was analyzed by Western blot. Overexpression of PB2∆ was confirmed by using Myc-specific antibodies. Equal loading was verified by detection of total ERK2. F) Expression levels of viral m/cRNAs were detected by qRT-PCR and are depicted as mean n-fold (±SD) of one representative out of two independent experiments normalized to 15 min infection of empty vector-transfected cells. *p≤0.05, two-way ANOVA, Sidak’s multiple comparisons test. G) A549 cells were infected with 5 MOI KAN-1 for 8 h. Subsequently, co-immunoprecipitation of the viral polymerase complex was performed by using NP-specific antibodies. Efficient immunoprecipitation was confirmed by detection of viral proteins NP, PB1 and PB2. Presence of PB2∆ was analyzed by PB2-specific antibodies. B, C, E, G) Blots are representative of three independent experiments. Thus, the viral PB2∆ defective RNA is efficiently transcribed into mRNA that is translated to a truncated 10 kDa protein. The data further suggest that this polypeptide might fulfill an antiviral function in influenza virus replication.
In order to analyze a potential function of the PB2∆ protein, an expression vector containing the PB2∆ ORF was generated. Initially, viral protein expression was analyzed in the presence or absence of PB2∆ in A549 cells infected with KAN-1. Interestingly, overexpression of PB2∆ led to a significant reduction in viral protein expression (Fig 2E). This could be attributed at least in part to the fact that the expression of viral mRNAs such as PB2 and M1 was reduced in the presence of PB2∆ (Fig 2F), although not significant for PB2 and effects on protein levels were more pronounced (Fig 2E).
Due to its function as part of the viral polymerase complex, the IAV protein PB2 has been linked to viral pathogenicity and host adaptation [36, 37]. There are several mutations known that verifiably affect viral polymerase activity and thus have an impact on viral replication [38, 39]. Comparative analysis of the viral proteins PB2 and PB2∆ showed sequence identity within the N-termini (Fig 2A), comprising the binding sites for the viral PB1 protein. Nuclear interaction with PB1 is essential for the generation of an active viral polymerase complex. Therefore, the decreased expression of viral mRNAs might be due to a reduced viral polymerase activity caused by direct interaction of PB2∆ with the polymerase complex. To test this hypothesis, co-immunoprecipitation of the viral polymerase complex was performed by using antibodies specific for the viral nucleoprotein (NP). The polymerase subunits PB1 and PB2 were efficiently co-immunoprecipitated, whereas PB2∆ was only detectable in the input control (Fig 2G). Therefore, reduced levels of viral mRNA expression in the presence of PB2∆ are unlikely to be caused by a modulation of viral polymerase activity induced by direct interaction of PB2∆ with the polymerase complex.
PB2∆ interacts with the adapter protein MAVS
Another more indirect mode of interference with viral protein expression would be the enhancement of the IAV-induced antiviral host cell response. One of the most potent mediators of this response is IFNβ. Beside its role in viral replication, PB2 also participates in the evasion of the antiviral immune response mediated by direct interaction with the mitochondrial antiviral signaling protein (MAVS) [40]. The latter is of particular importance in the RIG-I-mediated signal transduction upon detection of viral RNA which results in the expression of type I IFNs [41–43]. It has been shown that the protein-protein interaction of MAVS and PB2 is mediated by N-terminal amino acids 1–37 of the viral PB2 protein [44] which are present in PB2∆ (Fig 2A). Therefore, an interaction with the cellular MAVS protein affecting IAV-induced IFNβ expression is likely to occur.
To test this hypothesis, HEK293 cells were transfected with plasmids expressing HA-tagged MAVS in combination with expression plasmids for Myc-tagged PB2∆ or Myc-tagged PB2, respectively. 24 h p.t. HA-tagged MAVS was precipitated with HA-specific antibodies and the presence of co-precipitating proteins was analyzed. As expected, co-precipitation with cellular MAVS was confirmed not only for viral PB2 but also for the PB2∆ protein (Fig 3A).
Fig. 3. PB2∆ interacts with MAVS at mitochondria thereby inducing IFNβ expression. 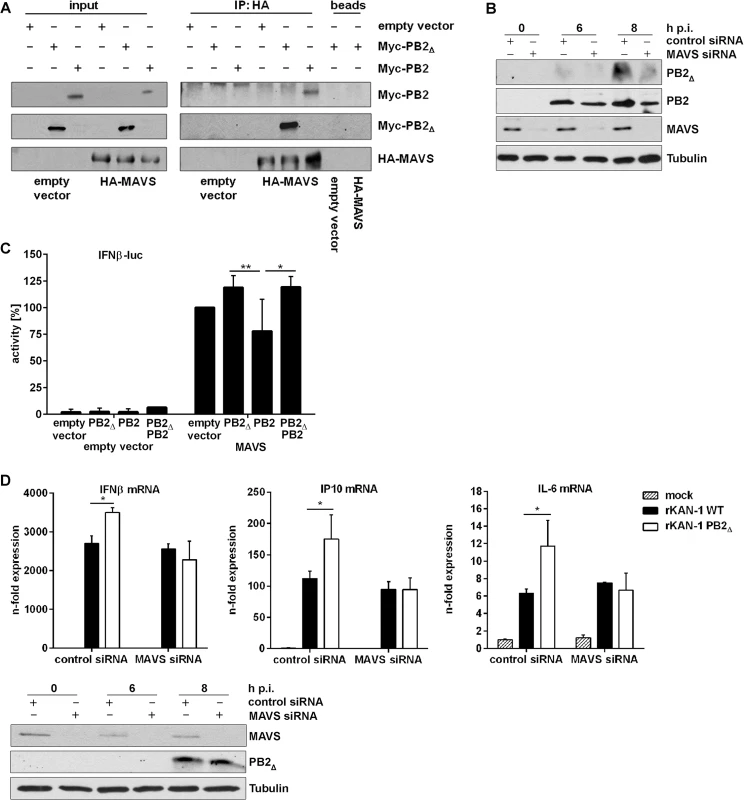
A) Hek293 cells were transfected with HA-MAVS or empty vector in combination with Myc-PB2∆, Myc-PB2 or empty vector. 24 h p.t. co-immunoprecipitation was performed by using HA-specific antibodies. Analysis of the co-immunoprecipitation of PB2 and PB2∆ was performed by detection of the Myc-tag. Blots are representative of three independent experiments. B) A549 cells were transfected with MAVS-specific or scrambled siRNA and subsequently infected with 5 MOI KAN-1. At the indicated time points, mitochondria were isolated and the presence of viral proteins PB2Δ and PB2 was analyzed by Western blot. Knockdown efficiency was verified by using MAVS-specific antibodies. Tubulin expression served as loading control. C) Hek293 cells were transfected with the IFNβ promoter in combination with HA-MAVS or empty vector as well as with PB2∆, PB2, PB2∆/PB2 or empty vector. 24 h p.t. promoter activity was measured by luciferase assay. Depicted are mean percentages (±SD) of four independent experiments. *p≤0.05, **p≤0.01; two-way ANOVA, Tukey’s multiple comparisons test. D) A549 cells were transfected with MAVS-specific or scrambled siRNA and subsequently infected with 0.5 MOI rKAN-1 WT or PB2Δ for 24 h. Expression levels of cytokines and ISGs were detected by qRT-PCR and are depicted as mean n-fold (±SD) of one representative out of three independent experiments normalized to non-infected control cells. *p≤0.05, two-way ANOVA, Sidak’s multiple comparisons test. Knockdown efficiency was verified by Western blot analysis using MAVS-specific antibodies. Presence of PB2∆ was analyzed by PB2-specific antibodies and Tubulin expression served as loading control. Blots are representative of two independent experiments. It has been shown that the IAV PB2 protein exhibits an N-terminal mitochondrial-targeting signal, leading to the association of PB2 with mitochondria where it can interact with MAVS which needs mitochondrial localization to fulfill its signaling functions [40, 42, 45]. To analyze whether PB2Δ is also associated with mitochondria and whether this localization is dependent on the presence of MAVS, mitochondria were isolated from MAVS siRNA-transfected A549 cells that were subsequently infected with KAN-1. As expected, knockdown of MAVS led to a reduced mitochondrial localization of PB2Δ that was even more pronounced compared to that of viral PB2 protein (Fig 3B).
To analyze whether this interaction might affect MAVS-induced IFNβ expression, reporter gene assays were performed. A construct containing the luciferase gene under control of the entire IFNβ promoter harboring all transcription factor binding sites responsible for the formation of the IFNβ enhanceosome was used. As expected, empty vector-transfected cells exhibited no luciferase activity when viral proteins were co-expressed (Fig 3C). The presence of the MAVS protein led to a strong induction of the IFNβ promoter activity that was even increased when PB2∆ was co-expressed. In clear contrast, the full-length PB2 protein resulted in a decreased promoter activity, confirming earlier findings [40]. Interestingly, the presence of PB2∆ in PB2/MAVS-expressing cells compensated for the PB2-mediated decrease in IFNβ promoter activity, resulting in the same level of luciferase expression as observed for MAVS/PB2∆-expressing cells. This MAVS-mediated mechanism of antiviral gene expression induced by PB2Δ was confirmed on endogenous levels by siRNA-mediated knockdown of MAVS. In this MAVS deficient situation, infection with a PB2Δ-positive virus led to the same levels of IFNβ and ISG mRNAs as observed in WT virus infection (Fig 3D). Since MAVS does not only participate in IFN induction but leads to the activation of different transcription factors inducing the expression of pro - and anti-inflammatory cytokines [41–43, 46], the expression of IL-6, a primarily NFκB-dependent cytokine, was analyzed in the presence of PB2Δ. As expected, IL-6 mRNA expression was also increased in presence of PB2Δ showing the same dependency on MAVS signaling as observed for IFN and ISGs, suggesting that PB2Δ leads to the activation of the full set of MAVS effector functions.
The results collectively demonstrate that PB2∆ can interact with MAVS, forming a functional complex that induces the expression of IFNβ, ISGs and other cytokines.
PB2∆ induces the expression of IFNβ, thereby restricting viral replication
It is well known that influenza virus proteins such as the nonstructural proteins NS1 and PB1-F2 or the components of the heterotrimeric polymerase complex modulate IFNβ induction [26, 27, 47, 48], although in an inhibitory manner.
To analyze whether overexpression of PB2∆ protein would interfere with IAV-mediated expression of IFNβ and alter the induction of interferon-stimulated genes (ISGs), KAN-1-induced mRNA levels of different antiviral acting genes were analyzed (Fig 4A). Indeed, overexpression of PB2∆ led to a strong interference with IFNβ mRNA induction that was detectable even in mock-infected cells, however, expression of the cytokine was strongly increased rather than inhibited. According to this, the expression of ISGs such as IP10 was also enhanced.
Fig. 4. PB2∆ increases virus-induced expression of IFNβ, thereby restricting viral replication of IFN-sensitive viruses. 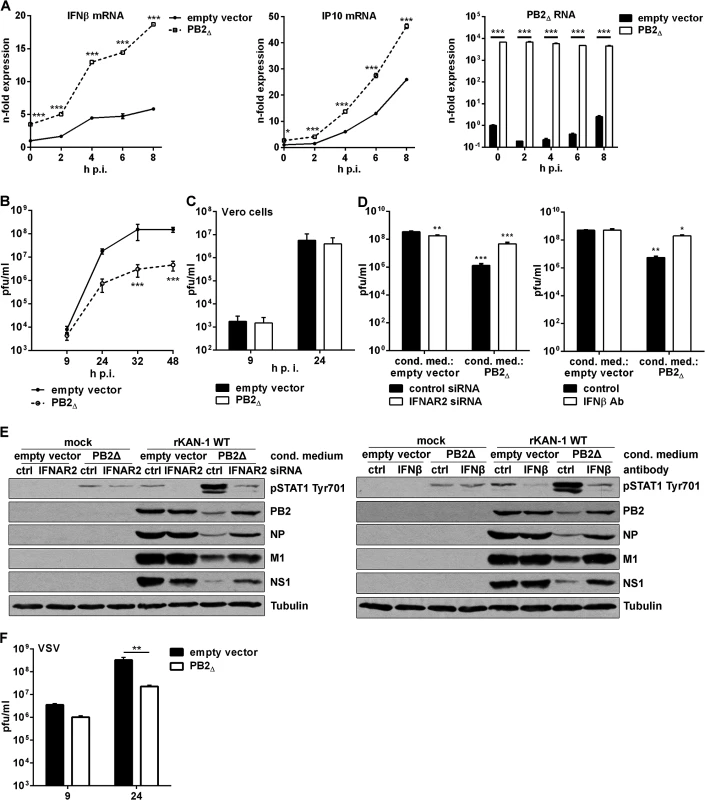
A) A549 cells were transfected with PB2∆ or empty vector and subsequently infected with 5 MOI KAN-1. At the time points indicated total RNA was isolated and expressional changes of IFNβ or IP10 mRNAs were analyzed by qRT-PCR and are depicted as mean n-fold (±SD) of one representative out of two independent experiments normalized to empty vector-transfected control. Efficient transfection was confirmed by detection of PB2∆ mRNA. *p≤0.05; ***p≤0.001; two-way ANOVA, Sidak’s multiple comparisons test. B, F) A549 or C) Vero cells were transfected with PB2∆ or empty vector and subsequently infected with B) 0.01 MOI KAN-1, C) 0.001 MOI KAN-1 or F) 0.01 MOI VSV. At the time points indicated virus-containing supernatants were harvested and infectivity titers were determined by a standard plaque assay and are depicted as mean (±SD) of three independent experiments. **p≤0.01, ***p≤0.001; two-way ANOVA, Sidak’s multiple comparisons test. D, E) A549 cells were transfected with PB2Δ or empty vector and 32 h p.t. conditioned media were transferred to IFNAR2 siRNA-transfected A549 cells or were enriched with neutralizing IFNβ antibody and transferred to untreated A549 cells. 16 h p.s. acceptor cells were infected with 0.1 MOI rKAN-1 WT for 24 h. D) Infectivity titers were determined by standard plaque assay and are depicted as mean (±SD) of one representative out of three independent experiments. *p≤0.05; **p≤0.01; ***p≤0.001; two-way ANOVA, Dunnett’s multiple comparisons test relating to empty vector-conditioned media-treated control cells, respectively. E) Expression of viral proteins PB2, NP, M1 and NS1 in conditioned medium-treated acceptor cells upon IFNAR2 knockdown or IFNβ neutralization was analyzed by Western blot. Knockdown and neutralization efficiencies were verified by using phosphor-STAT1 Tyr701 antibodies. Tubulin expression served as loading control. Blots are representative of three independent experiments. To gain insight into the role of PB2∆-induced stimulation of the innate immune response in viral replication, infectivity titers were determined in the presence or absence of PB2∆. KAN-1 replication was significantly reduced by more than 10-fold when PB2∆ was overexpressed (Fig 4B). Furthermore, this impaired viral propagation was most likely a consequence of the induction of type I IFN as demonstrated by infection of type I IFN-deficient Vero cells, where PB2∆ expression did not decrease infectivity titers (Fig 4C). This was confirmed by pretreatment of A549 cells with conditioned media of PB2Δ-transfected cells leading to a significant decrease in infectivity titers around 100-fold (Fig 4D). This suppression was recovered by specific knockdown of IFNα/β receptor chain 2 (IFNAR2) in the acceptor cells or enrichment of conditioned media with neutralizing IFNβ antibodies. Efficient interference with PB2Δ-induced type I IFN is demonstrated by reduced STAT1 Tyr701 phosphorylation which led to recovery of viral protein expression (Fig 4E). To analyze whether this PB2∆-dependent IFN induction is sufficient to inhibit replication of other IFN-sensitive viruses as well, A549 cells were infected with vesicular stomatitis virus (VSV), showing significantly reduced replication in the presence of PB2∆ (Fig 4F).
In summary, the defective RNA-encoded protein PB2∆ acts antiviral by the induction of type I IFN and ISGs, thereby inhibiting viral propagation of IFN-sensitive viruses.
Presence of PB2Δ leads to a more severe in vivo phenotype by enhancing early innate immune responses
The induced cytokine storm during severe influenza infections leads to major morbidity and mortality. A significant association between excessive early cytokine response, immune cell recruitment and poor outcome has been documented for highly pathogenic avian H5N1 virus infections [49]. To investigate the role of PB2Δ in the induction of the innate immune response in vivo, BALB/c mice were infected with 50 pfu rKAN-1 WT in comparison to a PB2Δ-positive virus. Two days post infection, mouse lungs were extracted and total RNA was isolated for qRT-PCR. Fig 5A shows lung ISG mRNA levels normalized to PBS-treated control mice. Although differences in IFNβ mRNA levels between PB2Δ-negative and—positive KAN-1 viruses were not significant at the time point analyzed (S4A Fig), different ISGs such as OAS-1 and IRF7 showed considerably increased expression levels in presence of PB2Δ, while virus replication was not significantly altered at that time (S4B Fig).
Fig. 5. Effects of PB2Δ on viral pathogenesis in vivo. 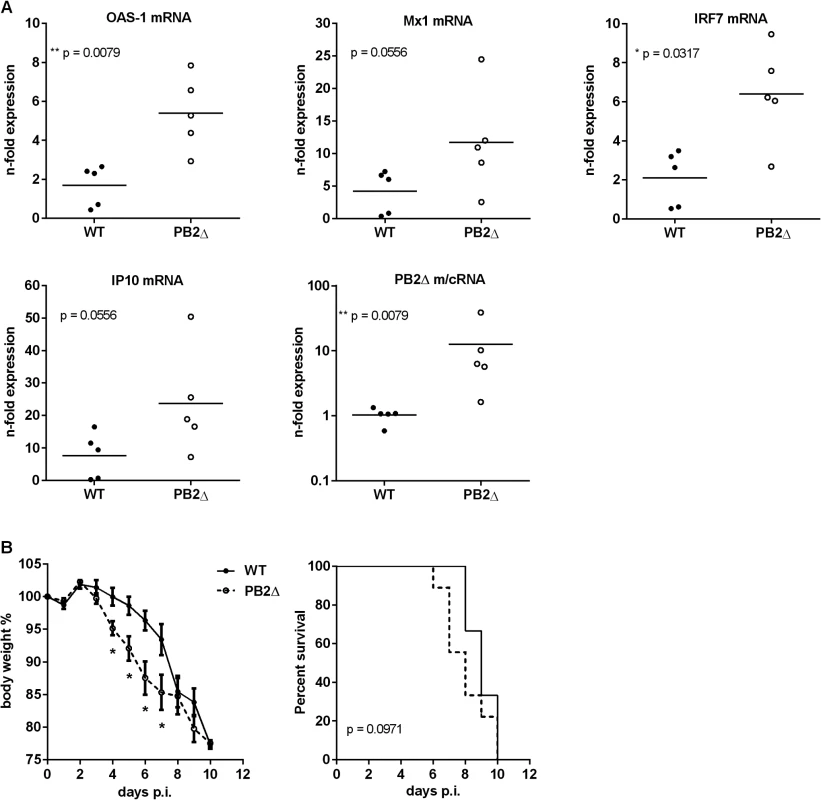
BALB/c mice were infected with 50 pfu rKAN-1 wild type or PB2Δ-expressing virus. A) Expression changes of ISG mRNAs in lungs were analyzed two days p.i. by qRT-PCR. N-fold expression in individual animals normalized to uninfected PBS control mice is depicted. *p≤0.05, **p≤0.01: Mann-Whitney test. B) Body weight curves; mean % body weight of 1–9 (initial group size) animals normalized to initial weight +/-SEM is depicted. *p-values range between 0.014 and 0.049, Mann-Whitney test. Animals were excluded from the analysis when reaching less than 80% of the initial body weight. Survival curves; %-survival of 1–9 (initial group size) animals is depicted. Statistical significance was analyzed by Gehan-Breslow-Wilcoxon test. Dysregulation of the innate cytokine response indicates disease severity and death during HPAIV infection [49, 50]. To analyze whether PB2Δ-induced ISG expression has an impact on disease severity of KAN-1 infection, BALB/c mice were infected with 50 pfu of wild type or PB2Δ-expressing virus. While in presence of PB2Δ mice lost body weight as early as 4 days post infection, weight loss in WT-infected mice was delayed and was not detectable until day 6 p.i. (Fig 5B, left). This is supported by the survival curves which show the tendency to earlier death of PB2Δ-infected mice, although this result is not significant.
These findings demonstrate that defective RNA-encoded PB2Δ protein-induced amplification of early innate immune responses leads to enhanced disease severity in KAN-1 infection and impressively emphasizes the role defective RNA-encoded proteins could play in viral pathogenicity of IAV.
Discussion
The generation of IAV defective interfering particles upon serial passages at high multiplicity of infection in vitro has been well described and in recent years there is increasing evidence that influenza defective RNAs are naturally occurring also in vivo [16]. It is still discussed why non-infectious particles are produced upon infection in many or possibly all animal virus systems and defective viruses have been ignored as possible important determinants in the outcome of natural virus infections for years [9]. Generation of non-infectious particles might be induced by the host, thereby restricting viral replication and boosting innate immune responses. One further possibility is that DI particles have evolved as an adaptive measure of the virus, thereby enabling persistent infections to allow further spread in a population, especially in the case of highly pathogenic viruses whose infections are associated with high mortality rates. Furthermore, the non-infectious particle population contains transcriptionally competent gene segments that can be complemented through coinfection [51]. Natural infection of an animal host involves interplay between physically and genetically heterogeneous virions and a mixed collection of wildly different cell types of varying susceptibility in combination with a dynamic and complex immune response. Therefore, genetic variability induced by error-prone polymerases is an evolutionary advantage that is strongly promoted by non-infectious particles [52]. Recently, Saira and colleagues showed that in vivo-derived DI-like RNAs were similar to those generated in vitro and that the presence of identical DI-like RNAs in patients linked by direct contact is compatible with transmission between them [16]. Therefore, a functional role of DI-like RNAs in natural infections seems not unlikely. Here, we investigated the molecular mechanisms by which defective particles, harboring a specific PB2-derived subgenomic RNA that encodes a 10kDa protein, inhibit H5N1 KAN-1 viral replication.
Beside DIP-induced interference mediated by competition for viral polymerases and preferential packaging of over-abundant DI segments [12, 13], recent reports show that subgenomic RNAs can act as potent immune stimulators [17, 19, 32, 33]. It has been observed that the cytoplasmic PRR RIG-I preferentially binds small genomic and subgenomic RNAs [17] and there is evidence that the appearance of defective RNAs coincides with the production of cytokines during IAV infection [19]. Here, overexpression of the defective RNA-encoded protein PB2∆ per se was sufficient to induce transcription of IFNβ mRNA, whereupon subsequent infection led to a further increase in IFNβ expression. In addition, KAN-1-induced expression of IFNβ and subsequently that of ISGs seems to be primarily induced by the PB2∆ protein and not by the detection of the defective RNA itself. This was shown by the recombinant PB2∆-expressing virus which induced increased IFNβ levels only late upon infection although considerable expression of PB2∆ RNA was detectable already 2 h p.i.. These data suggest that not only defective RNA but also some defective RNA-encoded proteins can act immunostimulatory. Furthermore, the PB2Δ protein-mediated IFN induction is completely independent of the detection of subgenomic RNA by the cellular sensor RIG-I but comprises complex formation with the adapter protein MAVS.
MAVS is of primary importance for RIG-I-mediated signal transduction upon sensing of viral PAMPs resulting in the expression of type I IFNs [41–43]. It has been shown that the PB2 protein interferes with IFNβ expression by direct interaction with MAVS via its binding site located within aa 1–37 [44]. It was hypothesized that PB2 binding leads to the inactivation of the MAVS complex by inhibition of intermolecular conformational changes needed for oligomerisation [44]. Here, PB2∆ binding of MAVS per se activates MAVS-mediated IFN induction. This suggests that not the binding of PB2 itself inhibits MAVS signaling but rather leads to a transition of the cellular protein into the active form, whereas the C terminal part of the viral protein interferes with induction of signal transduction. Thus, it is very likely that the C-terminus of PB2 inhibits MAVS oligomerisation or the interaction with downstream acting kinases such as TBK-1, TAK-1 or IKK or different transcription factors like IRF3 or IRF7 which are normally recruited by the adapter protein [41, 43, 46].
Interestingly, there was no interaction of PB2∆ with the viral polymerase complex observable although the binding site within PB2 mediating the interaction with MAVS is also involved in PB1 binding [44, 53]. This might be attributed to the tertiary structure of PB2∆. The binding site for MAVS and PB1 consists of 3 α-helices, of which the first establishes the interaction between PB1 and PB2 [53], whereas in the case of MAVS, helix 3 carries this function [44]. Therefore, specific folding of PB2∆ or steric inhibition by its C-terminus might lead to masking of helix 1, thereby inducing preferential binding of MAVS or even disabling PB1 binding. One further possibility would be the primary mediation of the PB1-PB2 interaction by an alternative PB1 binding site identified within the C-terminus of the PB2 protein [54]. Therefore, the present study reveals that functional analysis of defective RNA-encoded proteins can help to understand the biological characteristics of the parental viral protein and its interactions with viral and/or host factors.
The interaction of MAVS and PB2Δ at mitochondria increases IAV-induced type I IFN expression leading to reduced infectivity titers in the presence of PB2∆. Therefore, the present study links for the first time a biological function to an IAV defective RNA-encoded protein. In addition, PB2∆ is an antiviral acting protein whose action is not restricted to influenza A virus infection, but also limits the replication of other IFN-sensitive viruses as shown by VSV infection. Therefore, this peptide or a low-molecular peptidomimetic thereof might be useful as antiviral agent for IFN-sensitive viruses.
Interestingly, sequence alignments of human and avian H5N1 isolates showed that 53% or 79%, respectively, harbor the short sequence repeat at the junction site as observed for PB2∆ RNA which could be responsible for polymerase skipping, suggesting that these IAV isolates might also evolve a defective RNA similar to KAN-I PB2∆ upon passaging and/or transmission in vivo. This was supported by the finding that serial undiluted passaging of PB2Δ-free propagations of H5N1 strains KAN-1 and Vietnam led to the de novo generation of PB2Δ RNA to various extents. These data highlight different expression patterns of the same RNA within different preparations of the same virus strain and possibly explains different phenotypes of the same virus strain propagated in different laboratories.
Whether there are additional cis - or trans-mutations needed to facilitate generation of these defective RNAs has to be further studied. The apparent late expression of the PB2∆ protein compared to its RNA might suggest that there are other sequences or structures needed that support the translation of PB2∆.
In summary, PB2∆ is a virulence factor in H5N1 KAN-1 infections that restricts viral replication by modulation of the antiviral host gene response. Infections with highly pathogenic avian influenza viruses are characterized by a hyperactivation of the host immune response leading to an excessive expression of proinflammatory cytokines. This so called ‘cytokine burst’ has been discussed to be decisive for disease outcome [49, 50, 55]. Therefore, although PB2∆ is an antiviral acting protein in cell culture, its presence in KAN-1 infections in vivo enhances the induction of innate immune responses and thereby is involved in increased pathogenicity. Graef and colleagues hypothesized that the reduced PB2-induced suppression of MAVS-mediated signal transduction upon infection with avian influenza viruses in comparison to human subtypes might play a role in HPAIV-induced dysregulation of cytokine expression [40]. The adapter protein MAVS is not only essential for the induction of IFNβ but plays a role directly and indirectly in the induction of interferon-stimulated response element (ISRE)-regulated and proinflammatory genes by activation of transcription factors IRF3 (interferon regulatory factor 3) and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B-cells) [41–43, 46]. In this context, it has already been shown that the early suppression of cytokine amplification significantly leads to the protection of mice from lethal IAV infection [56, 57]. Therefore, in context of KAN-1 infection, targeting PB2∆ might be a promising approach to reduce disease manifestation by the suppression of immunopathology.
Overall, the present study provides evidence for the very first time that polypeptides encoded by influenza virus defective RNAs can fulfill biological functions that are associated but must not coincide with the activities of the parental full-length protein. Furthermore, subgenomic RNA-encoded polypeptides seem to play a prominent role in influenza virus pathogenicity, therefore the analysis of their functions leads to deeper understanding of the biology of influenza virus infections and its interactions with the host.
Materials and Methods
Ethics statement
All animal studies were performed in compliance with animal welfare regulations of the German Society for Laboratory Animal Science (GV-SOLAS) and the European Health Law of the Federation of Laboratory Animal Science Associations (FELASA). The protocol was approved by the relevant authorities in Hamburg (Behörde für Gesundheit und Verbraucherschutz) under the license number Az 42/13.
Viruses and cells
A/Thailand/KAN-1/2004 (H5N1; KAN-1) was used with kind permission from P. Puthavathana (Bangkok, Thailand). Recombinant A/Vietnam/1203/2004 (H5N1; Vietnam) and A/Puerto-Rico/8/34 (H1N1; PR8) were described before [58, 59]. A/FPV/Bratislava/79 (H7N7; fowl plaque virus, FPV) was a kind gift from S. Pleschka (Institute of Virology, Giessen, Germany) and A/Mallard/Bavaria/1/2006 (H5N1, Mallard) was obtained from O. Planz (Interfaculty Institute for Cell Biology, Tübingen, Germany). All experiments and handling of samples containing H5N1 or H7N7 infectious particles were performed in a biological safety level 3 containment. Influenza viruses as well as vesicular stomatitis virus strain Indiana (VSV) were propagated on Madin-Darby canine kidney (MDCKII) cells cultured in minimal essential medium (MEM, PAA Laboratories) containing 10% v/v FCS (Invitrogen) as described elsewhere [25]. Human alveolar epithelial cells (A549), green monkey epithelial cells (Vero) and human embryonic kidney 293 cells (HEK293) were cultured in Dulbecco’s modified eagle medium (DMEM, PAA Laboratories) containing 10% v/v FCS. All cell lines were originally purchased from ATCC and have been passaged in the laboratory. At regular intervals cells are checked for their identity by SNP-profiling (Multiplexion).
Plasmids
The luciferase reporter construct pTATA-IFNβ-luc containing the whole IFNβ enhanceosome upstream of a luciferase gene was a kind gift from J. Hiscott (Vaccine & Gene Therapy Institute of Florida, Port Saint Lucie, Florida, USA). pHW2000-PB2∆ was obtained by reverse transcription of the A/Thailand/KAN-1/2004 PB2∆ DI RNA by using influenza A segment one specific universal 12 primers [60] and subsequent PCR amplification. PB2∆ DI DNA was then cloned into the bidirectional pHW2000 plasmid [30] by using Eco31I restriction enzyme. pcDNA3-6xMyc-PB2∆ was obtained by PCR amplification of the open reading frame of PB2∆ that was subsequently cloned into pcDNA3-6xMyc by using EcoRV and XhoI restriction enzymes. Cloning of the PB2 gene into pcDNA3-6xMyc was performed the same way by Ludmilla Wixler (Institute of Molecular Virology, Muenster, Germany). Primer sequences are included in S4 Table. pCAGGS-HA-MAVS was a kind gift from B. Dauber (Robert Koch Institute, Berlin, Germany).
Generation of recombinant viruses
A set of eight plasmids based on the bidirectional pHW2000 plasmid allowing the rescue of the recombinant wild-type (WT) of influenza A/Thailand/KAN-1/2004 was used with kind permission from J. Stech (Friedrich-Loeffler-Institute, Riems, Germany). The generation of the recombinant viruses was carried out as described elsewhere [25, 30].
Plaque titration
Plaque forming units of a given virus suspension were determined by a standard plaque assay as described earlier [61].
Hemagglutination assay
Serial 2-fold dilutions of virus supernatants (1x106 infectious particles, according to 1 HA unit of rKAN-1 WT stock) were prepared in V-bottomed microtiter plates in a total volume of 50 μl PBS, the latter also serving as negative control. 50 μl of solution of 1% chicken erythrocytes (Rockland Immunochemicals, Inc) in PBS were added to the wells and microtiter plates were incubated for 30 min at 4°C. Hemagglutination was monitored by photography.
Transfection of siRNA and plasmid DNA
PB2∆ siRNA (5’-AGGAAUAGGAAUGAGAAUA-3’), MAVS siRNA (5’-GCUGAAGACAAGACCUAUA-3’), IFNAR2 siRNA (5’-GAAGCAUAAACCCGAAAUA-3’) and control siRNA (5’-UUCUCCGAACGUGUCACGU-3’) were synthesized by MWG-Biotech AG. Transfection with PB2Δ siRNA was performed with HiPerFect (Qiagen), MAVS and IFNAR2 siRNAs were transfected by the use of Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocols. Infections were carried out 16–48 h p.t. and conditioned medium experiments 36 h p.t.. Plasmid DNA was transfected using Lipofectamine 2000 (Invitrogen) consistent with manufacturer’s instructions and infections were carried out 24 h p.t..
Western blot analysis and antibodies
Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors [61]. Mitochondria were isolated by using the Mitochondria Fractionation Kit from BioVision according to the manufacturer’s protocol. RIPA protein lysates were cleared by centrifugation, lysates were separated by SDS-PAGE and blotted onto nitrocellulose membranes. Antisera directed against ERK2 (C-14) and influenza A PB1 (vK-20) were purchased from Santa Cruz Biotechnology and anti-α-Tubulin from Sigma-Aldrich. Antibody against influenza A PB2 protein was a kind gift of E. Fodor (Sir William Dunn School of Pathology, Oxford, UK [45]. Influenza A M1 and NP antibodies were obtained from AbD Serotec and mouse monoclonal antibody against influenza A NS1 (23–1) was developed at the Institute of Molecular Virology (Muenster, Germany) and can be purchased from Santa Cruz Biotechnology. MAVS (AT107) antibody was obtained from Alexis Biochemicals and anti-Myc (9E10) from ATCC. Antibody against phosphor-STAT1 Tyr701 (clone 14) was purchased from BD Transduction Laboratories. Neutralizing IFNβ antibody was obtained from Abcam and used in a concentration of 4 μg/ml conditioned medium.
Mass spectrometry
For proteome analyses 100 μg of protein mixtures from KAN-1-infected A549 cells were separated on a one-dimensional gel, the lanes were cut into twelve slices each and in-gel digested by trypsin as described previously [62]. Peptide fractions were collected and desalted separately using C18 StageTips [63]. LC-MS/MS analyses were performed on an EasyLC nano-HPLC (Proxeon Biosystems) coupled to an LTQ Orbitrap Elite mass spectrometer (Thermo Scientific) as described previously [64]. Briefly, the peptide mixtures were injected onto the column in HPLC solvent A (0.5% acetic acid) at a flow rate of 500 nl/min and subsequently eluted with an 87-min segmented gradient of 5–90% HPLC solvent B (80% ACN in 0.5% acetic acid). During peptide elution the flow rate was kept constant at 200 nl/min. For proteome analysis the 20 most intense precursor ions were sequentially fragmented by CID in each scan cycle. Sequenced precursor masses were excluded from further selection for 90 s. The target values for the LTQ were 5000 charges (MS/MS) and 106 charges (MS).
The MS data were processed using default parameters of the MaxQuant software (v1.2.2.9) [65]. Extracted peak lists were submitted to database search using the Andromeda search engine [66] to query a target-decoy databases [67] consisting of the uniprot human proteome database (88,692 protein entries, downloaded on the 25th of February 2014), of H5N1 virus database (13 entries, including the sequence of PB2Δ) and 248 commonly observed contaminants. In database search, full tryptic specificity was required and up to two missed cleavages were allowed. Carbamidomethylation of cysteine was set as fixed modification; protein N-terminal acetylation, and oxidation of methionine were set as variable modifications. Mass tolerances were set to 6 ppm at the precursor and 0.5 Da at the fragment ion level, respectively. False discovery rates were set to 5% at peptide, and protein group level and the minimum peptide length was set to five amino acids.
Co-immunoprecipitation
Cells were lysed with RIPA or Triton lysis (TLB) buffer [68] containing protease and phosphatase inhibitors. Lysates were cleared by centrifugation and supernatants were incubated o/n at 4°C with the antibodies indicated coupled to protein A/G-conjugated agarose (Roche). Complexes were washed three times with lysis buffer (5 min overhead shaking, 4°C) and resolved by SDS-PAGE with subsequent electrotransfer onto nitrocellulose membranes.
Luciferase reporter gene assays
Transfection of HEK293 with the IFNβ luciferase reporter plasmid (0.25 μg) in combination with the different expression plasmids (1 μg in total) was performed with polyethylenimine (PEI) as described elsewhere [69]. Luciferase assays were carried out 24 h p.t. as previously described [70]. Relative light units were normalized to protein concentrations determined with a standard Bradford assay.
RNA isolation, cDNA synthesis and qRT-PCR
Total RNA from cells was isolated using the RNeasy Kit (Qiagen) according to the manufacturer’s instructions. Lungs from mice were collected at the time points indicated and total RNA was isolated using TRIzol reagent (Invitrogen). TRIzol lysis was performed according to the manufacturer's protocol, introducing a secondary phase separation step as described previously [57].
Three micrograms of total RNA were reverse transcribed with RevertAid H Minus Reverse Transcriptase (MBI Fermentas) and oligo(dT) (MWG-Biotech AG) or random hexamer (Fermentas) primers according to the manufacturer’s protocol.
Strand-specific qRT PCR for distinguishing influenza v/c/mRNAs was performed as described previously [71]. Briefly, 200 ng of total RNA were reverse transcribed with RevertAid Premium Reverse Transcriptase (MBI Fermentas) and 10 pmol of each primer (specific for v/c/mRNAs and Oligo(dT)) according to the manufacturer’s instructions.
The cDNA was used for qRT-PCR, which was performed using a Roche LightCycler 480 and Brilliant SYBR Green III Mastermix (Agilent) according to the manufacturer’s instructions. Primer sequences are included in S4 Table. Relative changes in expression levels (n-fold) were calculated according to the 2-∆∆CT method [72].
Northern blot analysis
Total RNA (10 μg) was separated on 8% (w/v) polyacrylamide [29 : 1 acrylamide/bisacrylamide], 7 M urea gels and electro-transferred onto positively charged nylon membranes (Roche). Hybridization probes (50 pmol; PB2∆ vRNA: 5’-TGAAAGGAATAGGAATGAGAAT-3’; PB2∆ c/mRNA: 5’-ATTCTCATTCCTATTCCTTTCA-3’) were radioactively labeled using [γ-32P]-ATP (PerkinElmer) and T4 Polynucleotide Kinase (Fermentas) according to the manufacturer’s instructions. Northern blot analysis was performed as described previously [73].
Mouse experiments
All animal experiments were performed in the BSL-3 animal facility of the Heinrich-Pette-Institute, Leibniz Institute in Hamburg. BALB/c mice were obtained from the Harlan-Winkelmann animal breeding facilities. Eight-week-old mice were anaesthetized by intraperitoneal injection of 100 mg/kg Ketavet and 10 mg/kg Xylazin. Mice were infected by the intranasal route in a 50 μL volume as indicated. Health status of the animals was monitored daily according to the animal protocols approved by the Hamburg authorities.
Accession numbers
Supporting Information
Zdroje
1. Wise HM, Foeglein A, Sun J, Dalton RM, Patel S, Howard W, Anderson EC, Barclay WS, Digard P. A complicated message: Identification of a novel PB1-related protein translated from influenza A virus segment 2 mRNA. J Virol. 2009 Aug;83(16):8021–31. doi: 10.1128/JVI.00826-09 19494001
2. Wise HM, Hutchinson EC, Jagger BW, Stuart AD, Kang ZH, Robb N, Schwartzman LM, Kash JC, Fodor E, Firth AE, Gog JR, Taubenberger JK, Digard P. Identification of a novel splice variant form of the influenza a virus m2 ion channel with an antigenically distinct ectodomain. PLoS Pathog. 2012 Nov;8(11):e1002998. doi: 10.1371/journal.ppat.1002998 23133386
3. Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science. 2012 Jul 13;337(6091):199–204. doi: 10.1126/science.1222213 22745253
4. Muramoto Y, Noda T, Kawakami E, Akkina R, Kawaoka Y. Identification of novel influenza A virus proteins translated from PA mRNA. J Virol. 2013 Mar;87(5):2455–62. doi: 10.1128/JVI.02656-12 23236060
5. Bouvier NM, Palese P. The biology of influenza viruses. Vaccine. 2008 Sep 12;26 Suppl 4:D49–53. 19230160
6. Hutchinson EC, von Kirchbach JC, Gog JR, Digard P. Genome packaging in influenza A virus. J Gen Virol. 2010 Feb;91(Pt 2):313–28.
7. Davis AR, Hiti AL, Nayak DP. Influenza defective interfering viral RNA is formed by internal deletion of genomic RNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):215–9. 6928614
8. Nayak DP, Sivasubramanian N, Davis AR, Cortini R, Sung J. Complete sequence analyses show that two defective interfering influenza viral RNAs contain a single internal deletion of a polymerase gene. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2216–20. 6954536
9. Huang AS, Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–7. 5439728
10. Jennings PA, Finch JT, Winter G, Robertson JS. Does the higher order structure of the influenza virus ribonucleoprotein guide sequence rearrangements in influenza viral RNA? Cell. 1983 Sep;34(2):619–27. 6616623
11. Marriott AC, Dimmock NJ. Defective interfering viruses and their potential as antiviral agents. Rev Med Virol. 2010 Jan;20(1):51–62. doi: 10.1002/rmv.641 20041441
12. Odagiri T, Tashiro M. Segment-specific noncoding sequences of the influenza virus genome RNA are involved in the specific competition between defective interfering RNA and its progenitor RNA segment at the virion assembly step. J Virol. 1997 Mar;71(3):2138–45. 9032347
13. Odagiri T, Tominaga K, Tobita K, Ohta S. An amino acid change in the non-structural NS2 protein of an influenza A virus mutant is responsible for the generation of defective interfering (DI) particles by amplifying DI RNAs and suppressing complementary RNA synthesis. J Gen Virol. 1994 Jan;75 (Pt 1)(Pt 1):43–53.
14. Janda JM, Davis AR, Nayak DP, De BK. Diversity and generation of defective interfering influenza virus particles. Virology. 1979 May;95(1):48–58. 442544
15. Von Magnus P. Propagation of the PR8 strain of influenza A virus in chick embryos. III. properties of the incomplete virus produced in serial passages of undiluted virus. Acta Pathol Microbiol Scand. 1951;29(2):157–81. 14902470
16. Saira K, Lin X, DePasse JV, Halpin R, Twaddle A, Stockwell T, Angus B, Cozzi-Lepri A, Delfino M, Dugan V, Dwyer DE, Freiberg M, Horban A, Losso M, Lynfield R, Wentworth DN, Holmes EC, Davey R, Wentworth DE, Ghedin E, INSIGHT FLU002 Study Group, INSIGHT FLU003 Study Group. Sequence analysis of in vivo defective interfering-like RNA of influenza A H1N1 pandemic virus. J Virol. 2013 Jul;87(14):8064–74. doi: 10.1128/JVI.00240-13 23678180
17. Baum A, Sachidanandam R, Garcia-Sastre A. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc Natl Acad Sci U S A. 2010 Sep 14;107(37):16303–8. doi: 10.1073/pnas.1005077107 20805493
18. Randall RE, Goodbourn S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008 Jan;89(Pt 1):1–47. 18089727
19. Tapia K, Kim WK, Sun Y, Mercado-Lopez X, Dunay E, Wise M, Adu M, Lopez CB. Defective viral genomes arising in vivo provide critical danger signals for the triggering of lung antiviral immunity. PLoS Pathog. 2013 Oct;9(10):e1003703. doi: 10.1371/journal.ppat.1003703 24204261
20. Penn CR, Mahy BW. Novel polypeptides encoded by influenza virus subgenomic (DI type) virion RNAs. Virus Res. 1985 Nov;3(4):311–21. 4072398
21. Akkina RK, Chambers TM, Nayak DP. Expression of defective-interfering influenza virus-specific transcripts and polypeptides in infected cells. J Virol. 1984 Aug;51(2):395–403. 6205168
22. Chen W, Calvo PA, Malide D, Gibbs J, Schubert U, Bacik I, Basta S, O'Neill R, Schickli J, Palese P, Henklein P, Bennink JR, Yewdell JW. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med. 2001 Dec;7(12):1306–12. 11726970
23. Tauber S, Ligertwood Y, Quigg-Nicol M, Dutia BM, Elliott RM. Behaviour of influenza A viruses differentially expressing segment 2 gene products in vitro and in vivo. J Gen Virol. 2012 Apr;93(Pt 4):840–9. doi: 10.1099/vir.0.039966-0 22190016
24. Schmolke M, Manicassamy B, Pena L, Sutton T, Hai R, Varga ZT, Hale BG, Steel J, Perez DR, Garcia-Sastre A. Differential contribution of PB1-F2 to the virulence of highly pathogenic H5N1 influenza A virus in mammalian and avian species. PLoS Pathog. 2011 Aug;7(8):e1002186. doi: 10.1371/journal.ppat.1002186 21852950
25. Mazur I, Anhlan D, Mitzner D, Wixler L, Schubert U, Ludwig S. The proapoptotic influenza A virus protein PB1-F2 regulates viral polymerase activity by interaction with the PB1 protein. Cell Microbiol. 2008 May;10(5):1140–52. doi: 10.1111/j.1462-5822.2008.01116.x 18182088
26. Dudek SE, Wixler L, Nordhoff C, Nordmann A, Anhlan D, Wixler V, Ludwig S. The influenza virus PB1-F2 protein has interferon antagonistic activity. Biol Chem. 2011 Dec;392(12):1135–44. doi: 10.1515/BC.2011.174 22050228
27. Varga ZT, Ramos I, Hai R, Schmolke M, Garcia-Sastre A, Fernandez-Sesma A, Palese P. The influenza virus protein PB1-F2 inhibits the induction of type I interferon at the level of the MAVS adaptor protein. PLoS Pathog. 2011 Jun;7(6):e1002067. doi: 10.1371/journal.ppat.1002067 21695240
28. Bao Y, Bolotov P, Dernovoy D, Kiryutin B, Zaslavsky L, Tatusova T, Ostell J, Lipman D. The influenza virus resource at the national center for biotechnology information. J Virol. 2008 Jan;82(2):596–601. 17942553
29. Nayak DP, Tobita K, Janda JM, Davis AR, De BK. Homologous interference mediated by defective interfering influenza virus derived from a temperature-sensitive mutant of influenza virus. J Virol. 1978 Oct;28(1):375–86. 702654
30. Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A. 2000 May 23;97(11):6108–13. 10801978
31. Dimmock NJ, Rainsford EW, Scott PD, Marriott AC. Influenza virus protecting RNA: An effective prophylactic and therapeutic antiviral. J Virol. 2008 Sep;82(17):8570–8. doi: 10.1128/JVI.00743-08 18579602
32. Easton AJ, Scott PD, Edworthy NL, Meng B, Marriott AC, Dimmock NJ. A novel broad-spectrum treatment for respiratory virus infections: Influenza-based defective interfering virus provides protection against pneumovirus infection in vivo. Vaccine. 2011 Mar 24;29(15):2777–84. doi: 10.1016/j.vaccine.2011.01.102 21320545
33. Scott PD, Meng B, Marriott AC, Easton AJ, Dimmock NJ. Defective interfering influenza A virus protects in vivo against disease caused by a heterologous influenza B virus. J Gen Virol. 2011 Sep;92(Pt 9):2122–32. doi: 10.1099/vir.0.034132-0 21632569
34. Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5'-phosphates. Science. 2006 Nov 10;314(5801):997–1001. 17038589
35. Koerner I, Kochs G, Kalinke U, Weiss S, Staeheli P. Protective role of beta interferon in host defense against influenza A virus. J Virol. 2007 Feb;81(4):2025–30. 17151098
36. Brown EG, Liu H, Kit LC, Baird S, Nesrallah M. Pattern of mutation in the genome of influenza A virus on adaptation to increased virulence in the mouse lung: Identification of functional themes. Proc Natl Acad Sci U S A. 2001 Jun 5;98(12):6883–8. 11371620
37. Gabriel G, Dauber B, Wolff T, Planz O, Klenk HD, Stech J. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host. Proc Natl Acad Sci U S A. 2005 Dec 20;102(51):18590–5. 16339318
38. Hatta M, Gao P, Halfmann P, Kawaoka Y. Molecular basis for high virulence of hong kong H5N1 influenza A viruses. Science. 2001 Sep 7;293(5536):1840–2. 11546875
39. Mehle A, Doudna JA. An inhibitory activity in human cells restricts the function of an avian-like influenza virus polymerase. Cell Host Microbe. 2008 Aug 14;4(2):111–22. doi: 10.1016/j.chom.2008.06.007 18692771
40. Graef KM, Vreede FT, Lau YF, McCall AW, Carr SM, Subbarao K, Fodor E. The PB2 subunit of the influenza virus RNA polymerase affects virulence by interacting with the mitochondrial antiviral signaling protein and inhibiting expression of beta interferon. J Virol. 2010 Sep;84(17):8433–45. doi: 10.1128/JVI.00879-10 20538852
41. Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005 Sep 16;19(6):727–40. 16153868
42. Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005 Sep 9;122(5):669–82. 16125763
43. Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005 Oct 20;437(7062):1167–72. 16177806
44. Patel D, Schultz LW, Umland TC. Influenza A polymerase subunit PB2 possesses overlapping binding sites for polymerase subunit PB1 and human MAVS proteins. Virus Res. 2012 Dec 12
45. Carr SM, Carnero E, Garcia-Sastre A, Brownlee GG, Fodor E. Characterization of a mitochondrial-targeting signal in the PB2 protein of influenza viruses. Virology. 2006 Jan 20;344(2):492–508. 16242167
46. Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I - and Mda5-mediated type I interferon induction. Nat Immunol. 2005 Oct;6(10):981–8. 16127453
47. Kochs G, Garcia-Sastre A, Martinez-Sobrido L. Multiple anti-interferon actions of the influenza A virus NS1 protein. J Virol. 2007 Jul;81(13):7011–21. 17442719
48. Iwai A, Shiozaki T, Kawai T, Akira S, Kawaoka Y, Takada A, Kida H, Miyazaki T. Influenza A virus polymerase inhibits type I interferon induction by binding to interferon beta promoter stimulator 1. J Biol Chem. 2010 Oct 15;285(42):32064–74. doi: 10.1074/jbc.M110.112458 20699220
49. de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006 Oct;12(10):1203–7. 16964257
50. Cilloniz C, Shinya K, Peng X, Korth MJ, Proll SC, Aicher LD, Carter VS, Chang JH, Kobasa D, Feldmann F, Strong JE, Feldmann H, Kawaoka Y, Katze MG. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS Pathog. 2009 Oct;5(10):e1000604. doi: 10.1371/journal.ppat.1000604 19798428
51. Carter MJ, Mahy BW. Incomplete avian influenza virus contains a defective non-interfering component. Arch Virol. 1982;71(1):13–25. 7065901
52. Brooke CB. Biological activities of 'noninfectious' influenza A virus particles. Future Virol. 2014 Jan;9(1):41–51. 25067941
53. Sugiyama K, Obayashi E, Kawaguchi A, Suzuki Y, Tame JR, Nagata K, Park SY. Structural insight into the essential PB1-PB2 subunit contact of the influenza virus RNA polymerase. EMBO J. 2009 Jun 17;28(12):1803–11. doi: 10.1038/emboj.2009.138 19461581
54. Poole E, Elton D, Medcalf L, Digard P. Functional domains of the influenza A virus PB2 protein: Identification of NP - and PB1-binding sites. Virology. 2004 Mar 30;321(1):120–33. 15033571
55. Cheung CY, Poon LL, Lau AS, Luk W, Lau YL, Shortridge KF, Gordon S, Guan Y, Peiris JS. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: A mechanism for the unusual severity of human disease? Lancet. 2002 Dec 7;360(9348):1831–7. 12480361
56. Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MB, Rosen H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011 Sep 16;146(6):980–91. doi: 10.1016/j.cell.2011.08.015 21925319
57. Börgeling Y, Schmolke M, Viemann D, Nordhoff C, Roth J, Ludwig S. Inhibition of p38 mitogen-activated protein kinase impairs influenza virus-induced primary and secondary host gene responses and protects mice from lethal H5N1 infection. J Biol Chem. 2014 Jan 3;289(1):13–27. doi: 10.1074/jbc.M113.469239 24189062
58. Abt M, de Jonge J, Laue M, Wolff T. Improvement of H5N1 influenza vaccine viruses: Influence of internal gene segments of avian and human origin on production and hemagglutinin content. Vaccine. 2011 Jul 18;29(32):5153–62. doi: 10.1016/j.vaccine.2011.05.036 21624413
59. Zielecki F, Semmler I, Kalthoff D, Voss D, Mauel S, Gruber AD, Beer M, Wolff T. Virulence determinants of avian H5N1 influenza A virus in mammalian and avian hosts: Role of the C-terminal ESEV motif in the viral NS1 protein. J Virol. 2010 Oct;84(20):10708–18. doi: 10.1128/JVI.00610-10 20686040
60. Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol. 2001 Dec;146(12):2275–89. 11811679
61. Seyer R, Hrincius ER, Ritzel D, Abt M, Mellmann A, Marjuki H, Kuhn J, Wolff T, Ludwig S, Ehrhardt C. Synergistic adaptive mutations in the hemagglutinin and polymerase acidic protein lead to increased virulence of pandemic 2009 H1N1 influenza A virus in mice. J Infect Dis. 2012 Jan 15;205(2):262–71. doi: 10.1093/infdis/jir716 22102733
62. Borchert N, Dieterich C, Krug K, Schutz W, Jung S, Nordheim A, Sommer RJ, Macek B. Proteogenomics of pristionchus pacificus reveals distinct proteome structure of nematode models. Genome Res. 2010 Jun;20(6):837–46. doi: 10.1101/gr.103119.109 20237107
63. Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc. 2007;2(8):1896–906. 17703201
64. Carpy A, Krug K, Graf S, Koch A, Popic S, Hauf S, Macek B. Absolute proteome and phosphoproteome dynamics during the cell cycle of schizosaccharomyces pombe (fission yeast). Mol Cell Proteomics. 2014 Aug;13(8):1925–36. doi: 10.1074/mcp.M113.035824 24763107
65. Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008 Dec;26(12):1367–72. doi: 10.1038/nbt.1511 19029910
66. Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011 Apr 1;10(4):1794–805. doi: 10.1021/pr101065j 21254760
67. Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 2007 Mar;4(3):207–14. 17327847
68. Luig C, Kother K, Dudek SE, Gaestel M, Hiscott J, Wixler V, Ludwig S. MAP kinase-activated protein kinases 2 and 3 are required for influenza A virus propagation and act via inhibition of PKR. FASEB J. 2010 Oct;24(10):4068–77. doi: 10.1096/fj.10-158766 20484669
69. Ehrhardt C, Schmolke M, Matzke A, Knoblauch A, Will C, Wixler V, Ludwig S. Polyethylenimine, a cost-effective transfection reagent. Signal Transduction. 2006;6(3):179–84.
70. Ehrhardt C, Kardinal C, Wurzer WJ, Wolff T, von Eichel-Streiber C, Pleschka S, Planz O, Ludwig S. Rac1 and PAK1 are upstream of IKK-epsilon and TBK-1 in the viral activation of interferon regulatory factor-3. FEBS Lett. 2004 Jun 4;567(2–3):230–8. 15178349
71. Kawakami E, Watanabe T, Fujii K, Goto H, Watanabe S, Noda T, Kawaoka Y. Strand-specific real-time RT-PCR for distinguishing influenza vRNA, cRNA, and mRNA. J Virol Methods. 2011 Apr;173(1):1–6. doi: 10.1016/j.jviromet.2010.12.014 21185869
72. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001 Dec;25(4):402–8. 11846609
73. Mo D, Raabe CA, Reinhardt R, Brosius J, Rozhdestvensky TS. Alternative processing as evolutionary mechanism for the origin of novel nonprotein coding RNAs. Genome Biol Evol. 2013;5(11):2061–71. doi: 10.1093/gbe/evt155 24132753
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR SelectionČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání