-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHow Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
article has not abstract
Published in the journal: . PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004724
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1004724Summary
article has not abstract
Hematodinium Infections: A Problem for the Sustainability of Crustacean Fisheries and Aquaculture Worldwide
Dinoflagellates of the genus Hematodinium infect over 40 species of marine crustaceans. Since the initial description of this parasite in France in the 1930s [1], it appears to have spread globally, causing economic loss in some species, including Tanner crabs (Chionoecetes bairdi) in Alaska [2], blue crabs (Callinectes sapidus) in the United States [3], edible crabs (Cancer pagurus) and Norway lobsters (Nephrops norvegicus) in Europe [4–7], sand crabs (Portunus armatus) in Australia [8], and Chinese swimming crabs (Portunus trituberculatus) in northern China [9]. Because this disease is thought to be fatal, it may be of great significance to the sustainability of both captive shellfish fisheries and aquaculture [10,11]. For instance, the decline in blue crabs from the Atlantic to Gulf Coasts in the US appears to be linked to epizootic outbreaks of disease caused by Hematodinium [12]. Similarly, the reduction in velvet swimmer crab (Necora puber) numbers in Brittany in the 1980s has been attributed to the high prevalence of such infections [13]. Finally, the recent reports of Hematodinium infections in crabs and shrimp raised under aquaculture conditions in China [9,14,15] highlight the danger to additional, cultured crustacean populations.
The number and host range of species of Hematodinium is unclear. Chatton and Poisson [1] identified one species of this parasite that they termed H. perezi. Notably, this species of parasite was reported in two different species of crabs from several locations around the French coast. A second species, H. australis, was first described in sand crabs from Moreton Bay in Australia [8]. Recent studies making use of the variability of the ITS1 rDNA region of H. perezi suggest that there are three clades (genotypes), I–III, of this species (see [11] for further details). Pagenkopp Lohan et al. [16] concluded that blue crab populations collected from the Atlantic to the Gulf coasts of the US were all affected by one genotype, H. perezi (III), which implies a large geographic range. Importantly, other species of crabs collected from the same region were also infected by the same genotype of H. perezi [16]. In Europe, there may be two clades of Hematodinium infecting a range of crabs. H. perezi genotype I has been reported in Liocarcinus depurator [17] and Carcinus maenas. The second species of Hematodinium, currently unnamed (Hematodinium sp.) infects a wider range of crustaceans, including edible crabs (C. pagurus). Importantly, these observations imply that this parasite is a host generalist and may be able to “jump” from host species to species, thereby making it a significant threat to a wide variety of commercially important decapods.
What Is the Interaction between the Host and Hematodinium?
Reports on the pathology of Hematodinium spp. infections in a variety of crustacean hosts are all similar. While the mode of transmission of the parasite to its hosts is unclear, it is thought that susceptible animals may become infected after moulting [18,19], suggesting an integumentary route of invasion when the cuticle is soft and vulnerable. The parasites gain entry to the underlying tissues, including the main body space, termed the haemocoel, and multiply in tissues and organ systems including the heart, hepatopancreas, connective tissue, and the haemolymph (“blood”) [10]. The timescale of infection is protracted in some host species of crustacean, such as those living in colder waters, where the period from infection to death may be several months [2], whereas in others, it can be much more rapid [20]. Early phases of infection (see “Early Phase” in Fig 1) are characterised by limited changes in the number of circulating haemocytes and only modest elevation in parasite abundance in this tissue. One of the hallmarks of advanced Hematodinium infections (“Late Phase” in Fig 1) is the marked multiplication of the parasites in all tissues and a simultaneous reduction in the number of circulating haemocytes, leaving the affected animals unable to mount effective cellular defence and haemostatic responses [20]. Accompanying the increase in the number of parasites in advanced infections comes muscle necrosis [21] and “metabolic exhaustion” [22], characterised by significant reduction in plasma proteins, including haemocyanin that serves as both a respiratory pigment and as an immunologically active molecule [23]. Interestingly, these pathological changes may be reflected in alteration in the general behaviour of infected animals [24,25], leaving them more susceptible to predation and loss from the fishery.
Fig. 1. Interaction of Hematodinium with its crustacean host. 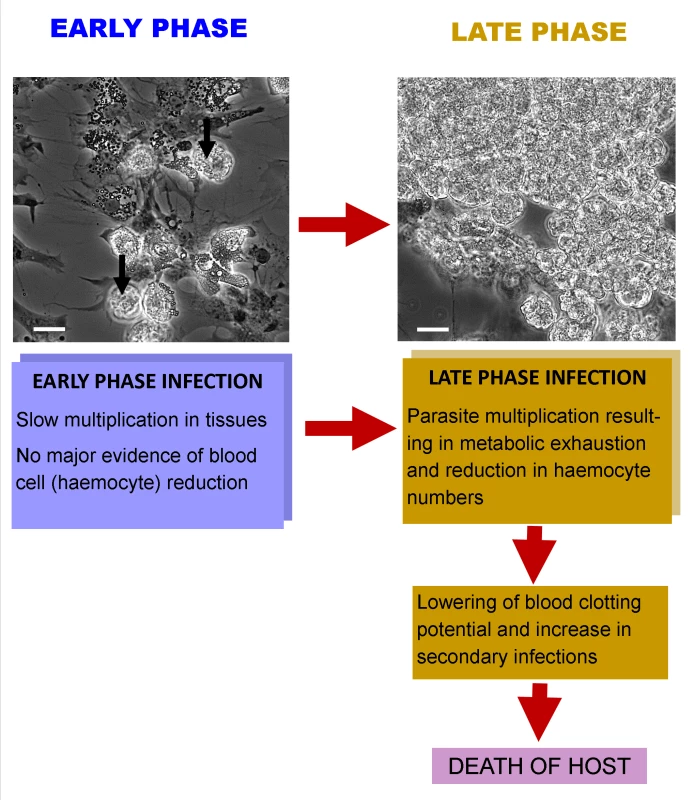
At an early phase of infection there is a small number of parasites in the haemolymph (unlabelled arrows) and a large number of haemocytes in circulation, as found in uninfected individuals (spread cells in micrograph). In late-phase infections, there are often large masses of Hematodinium in the haemolymph and very few circulating haemocytes. This reduction in haemocytes results in an increased likelihood of secondary infections and prolonged bleeding times following wounding. Death probably ensues as a result of parasite development and utilisation of host’s metabolic products, as well as a heightened chance of secondary infections. Is There Evidence of Immune Reactivity to the Presence of Hematodinium in Crustacean Hosts?
The immune system of invertebrates has been extensively studied, especially in insects and crustaceans. However, our understanding of the interaction between pathogens and parasites and their hosts is less well understood, with the notable exception of those agents that infect humans while in their invertebrate vectors. For a brief description of the main features of the crustacean immune system, the reader is directed to Box 1 in this article and recent reviews [26,27].
Box 1. The Invertebrate Immune System: A Brief Primer
The invertebrate immune system consists of cellular and humoral defences that are principally mediated by blood cells, which in arthropods are termed haemocytes. Non-self is recognised by a variety of immune factors, including lectins and products of the prophenoloxidase cascade [26,27]. The main cellular defence reactions are phagocytosis and a process in which pathogens and parasites are walled off, called nodule formation or encapsulation. The blood (haemolymph) contains a wide variety of antimicrobial factors including hydrolytic enzymes (e.g., lysozyme) and antimicrobial peptides (e.g., defensins). Although the defence reactions of invertebrates are considered to be non-specific, recent studies have questioned whether a unique form of immune memory is present at least in some invertebrates [27].
A significant number of studies have examined the pathology of Hematodinium infections in a wide range of crustacean hosts (see [10] for review). Although in some cases there has been evidence provided of a cellular host response to this parasite, such as the presence of haemocytic nodules [6], close examination reveals there is little or no evidence for the presence of the various stages of this parasite within the nodules, implying that their generation is not a direct response to the parasite. A potential explanation for the presence of nodules is as a result of other co-infections—which are common in some crustaceans [28–31]—or tissue damage caused by Hematodinium in late-phase infections (Fig 1) [31]. One report has produced convincing evidence for the uptake of Hematodinium by the fixed phagocytes of N. norvegicus [32], but to our knowledge no other studies have found such an association.
To further investigate this apparent lack of host response to the presence of Hematodinium, we injected juvenile edible crabs (C. pagurus) with Hematodinium and monitored changes in the haemogramme. These experiments found no evidence for reduction in the number of circulating haemocytes that might have resulted from localised nodule formation, and histological examination of various tissues, including the gills, failed to show the presence of nodules indicative of such a host response. Furthermore, seven days post-challenge, 100% of experimental animals had stages of the Hematodinium parasite free in the haemolymph (Fig 2A) implying that they had not been cleared from circulation by either fixed or free phagocytes. These studies support the concept of a failure of the immune defences to recognise and clear these parasites from the haemocoel. Hence, it can be concluded that Hematodinium spp., regardless of the host species studied, are not effectively recognised as foreign by the immune system.
Fig. 2. Experimental exploration of the interaction between Hematodinium and its host. 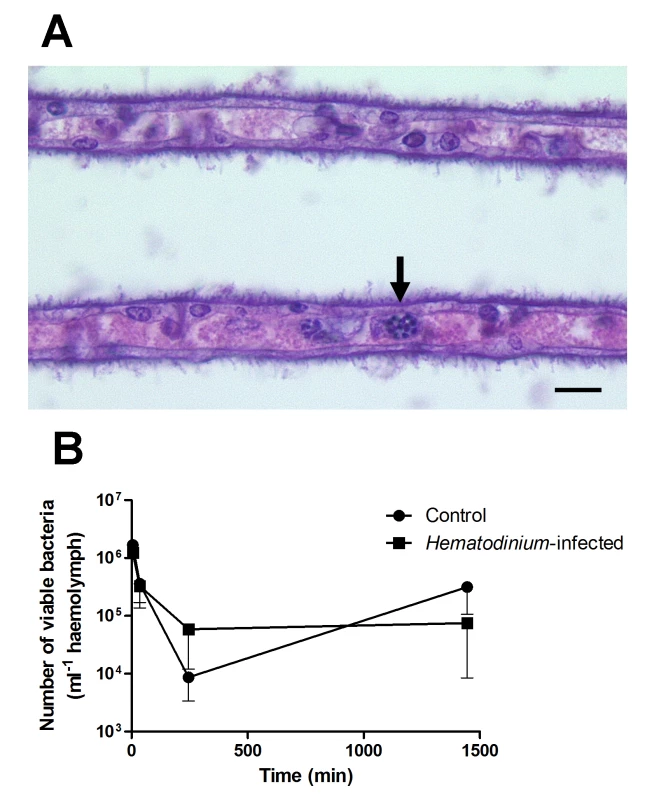
(A). Histological appearance of gill lamellae of a crab seven days post-injection of Hematodinium. Note these parasites in the gill space (unlabelled arrow). Scale bar = 10 μm. (B). Clearance of the gram-positive bacterium, Bacillus subtilis from Hematodinium-infected (early-stage infection) and uninfected juvenile edible crabs (C. pagurus). Crabs were injected intrahaemocoelically with 105 live bacteria and the numbers of remaining viable bacteria (cfu) in the circulation determined by standard plate counting on tryptone soya agar + 2% NaCl plates. Values shown are means of cfu with standard error of the mean (SEM), n = 5. The values are not statistically different between animals of differing disease status. What Are the Potential Mechanisms Employed by Hematodinium to Avoid Immune Recognition and Elimination?
There are several recognised strategies employed by parasites and pathogens to successfully colonise their hosts without their elimination by the cellular and humoral components of the immune system (see Box 1). Table 1 summarises and speculates on some of the more likely mechanisms that Hematodinium could use, and some of these are now explored.
Tab. 1. An exploration of potential mechanisms employed by <i>Hematodinium</i> to avoid, circumvent, or suppress the crustacean immune system. 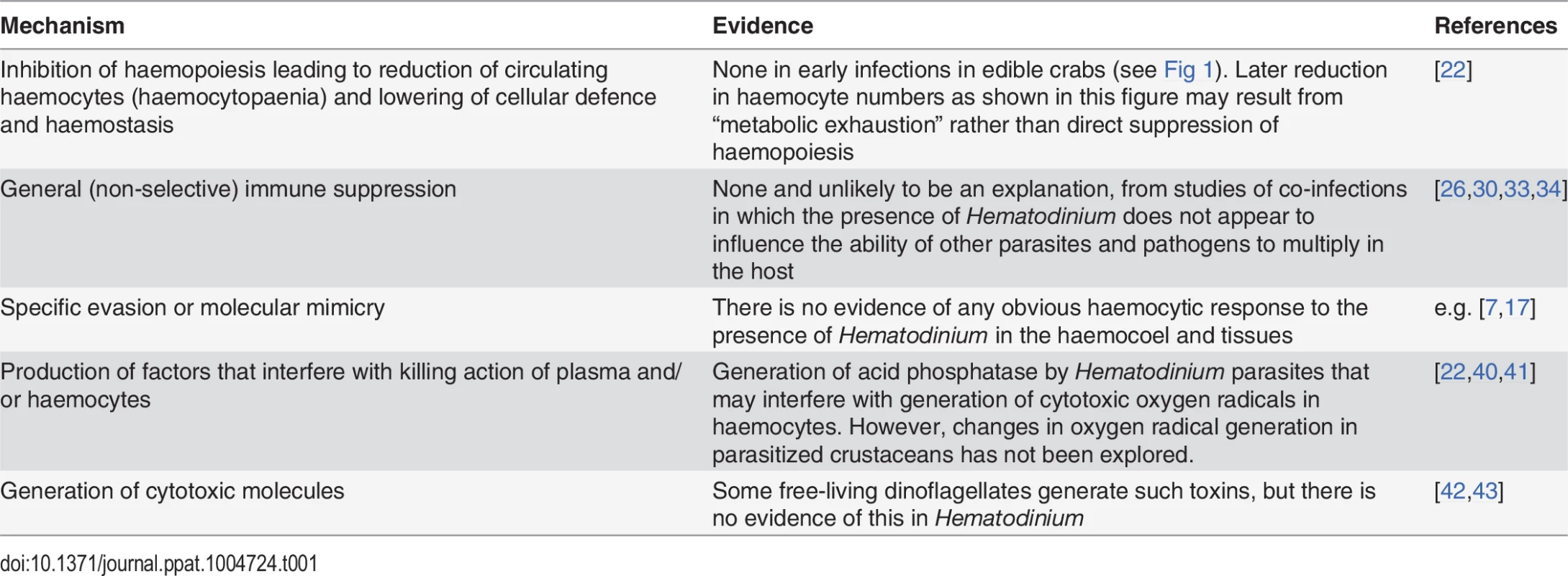
As haemocytes are the cells at the centre of the invertebrate immune system and are also responsible for blood coagulation, any reduction in their numbers has a profound effect on the survival of the infected host. One of the hallmarks of late-phase Hematodinium infections is the reduction in the number of circulating haemocytes that leaves the host unable to mount an effective cellular defence and haemostatic response [20]. The paucity of haemocytes in circulation could reflect their loss due to extensive infiltration of infected tissues but there is no evidence for this event from histological studies (e.g. [7]) and it is more likely that it results from suppression of haemopoietic activity. As we have shown that haemocytopaenia is a relatively late response to advanced infections, at least in the edible crab (Fig 1), this implies that the principal mechanism of pathogenicity does not involve suppression of haemopoiesis. Furthermore, we would argue that this loss of haemocytes is probably linked to the state of metabolic exhaustion [20,22] caused by the rapidly multiplying parasites “stealing” host resources, rather than any parasite-driven direct inhibition of haemopoiesis.
Some crustaceans with Hematodinium have been found to be susceptible to secondary infections caused by other parasites and pathogens [10,28,29]. One example of this interaction comes from studies with naturally Hematodinium-infected crabs that were found to have a secondary fungal infection [28]. These authors concluded that the presence of Hematodinium caused the host to be susceptible to these infections because they only found the fungus in Hematodinium-affected crabs. They also discussed the possibility that host immunosuppression caused by the presence of Hematodinium left these animals susceptible to this and other secondary infections. To investigate this putative interaction further, Smith et al. [33] challenged Hematodinium-infected and uninfected crabs with the fungus (now identified as a member of the Ophiocordyceps clade). Unexpectedly, the presence of Hematodinium apparently caused a reduction in the multiplication of the fungus in the haemocoel in comparison to Hematodinium-free crabs. However, all crabs, regardless of their infection status, died as a result of the fungal infection. Furthermore, the fungal infection was found in small numbers of edible crabs in the wild that were uninfected by Hematodinium, reflecting that the association between the two pathogens was not obligate [33]. These co-infections also reveal that crustaceans infected by Hematodinium are able to mount an efficient immune response to other pathogens that is not apparently different to that seen in uninfected hosts. Hence, these observations advocate that Hematodinium does not elicit general immune suppression. However, a recent study in which Chinese swimming crabs (Portunus trituberculatus) were challenged with Hematodinium, has provided evidence of an apparent immunosuppressive action by these parasites [34]. The study revealed that artificially infected crabs showed temporal changes in prophenoloxidase gene expression post-challenge. The prophenoloxidase activating system is a key component of the crustacean immune response (Box 1) [26], and therefore, changes in this are likely to adversely affect host survival. Although the authors concluded that Hematodinium showed the ability to cause potential inhibition of the prophenoloxidase activating system, levels of enzyme (i.e., phenoloxidase) activity varied post-challenge from no significant changes through to both elevation and suppression at the different time points studied.
To further investigate the possibility that Hematodinium causes generalised immunosuppression, we challenged juvenile edible crabs, with or without low-severity (i.e., early-phase) Hematodinium infections, with the non-pathogenic bacterium Bacillus subtilis, and its clearance from circulation was monitored by standard plate counts. These experiments demonstrated that crabs with low-severity Hematodinium infections showed no changes in their ability to clear the bacteria from circulation in comparison to uninfected crabs (Fig 2B). This implies that crabs with early-phase Hematodinium infections are not immunocompromised towards other potential bacterial pathogens. Overall, it is our opinion that generalised immune suppression is unlikely to be the main strategy utilised by Hematodinium to overwhelm its host.
The apparent lack of any cell–mediated response directed to the presence of large numbers of Hematodinium in the haemolymph points to the possibility that this parasite utilises molecular mimicry or evasion to “hide” from the host’s immune system. While there may be other strategies employed, this would seem the most likely explanation (Table 1). Several host–parasite relationships in which molecular mimicry or specific evasion operates have been investigated using invertebrate models and these provide us with mechanistic explanations that may shed light on the current crustacean–Hematodinium interaction. Two good examples of such models include the human malaria parasite, Plasmodium, in its invertebrate vectors, the anopheline mosquitoes [35,36], and Schistosoma mansoni in the freshwater snail, Biomphalaria glabrata [37,38].
Lectins are key immune recognition molecules in a number of invertebrates, which operate by binding unusual carbohydrate residues associated with pathogens and parasites [39]. Larval schistosomes may share glycans with the host haemocytes, leaving these cells unable to recognise the presence of such parasites via lectin-based recognition systems [37,38]. Probing for the presence of shared surface carbohydrates between parasites and haemocytes using biotinylated lectins has proven to be a useful and rapid initial approach to look for their role in molecular mimicry [39]. Such approaches may be a useful first point of investigation in the crustacean–Hematodinium model.
Conclusion
In this Opinion article, we have reviewed and discussed evidence that gives new insight into the host relationship of this key dinoflagellate parasite. This assemblage, belonging to the genus Hematodinium, has the capacity to successfully invade and multiply in the tissues of many different crustacean hosts without eliciting any obvious cellular immune response such as parasite encapsulation. There are very few parasites or pathogens that have the ability to circumvent the cellular defence reactions of several different hosts, making this of wide interest to immunologists. Whatever the mechanism(s) employed, these parasites have emerged as a significant threat to the future sustainability of shellfish fisheries and aquaculture worldwide, and hence, they warrant research focus.
Zdroje
1. Chatton É, Poisson R. Sur l’existence dans le sang des crabs, de péridiniens parasites: Hematodinium perezi n. g., n. sp. (Syndinidae). C R Séances Soc Biol. 1931;105 : 553–557. doi: 10.1039/c4sm01112k 25115726
2. Meyers TR, Koeneman TM, Botelho C, Short S. Bitter crab disease: a fatal dinoflagellate infection and marketing problem for Alaskan Tanner crabs Chionoecetes bairdi. Dis Aquat Org. 1987;3 : 195–216.
3. Messick GA. Hematodinium perezi infections in adult and juvenile blue crabs Callinectes sapidus from coastal bays of Maryland and Virginia, USA. Dis Aquat Org. 1994;19 : 77–82.
4. Latrouite D, Morizur T, Nöel P, Chagot D, Wilhelm G. Mortalite du tourteau Cancer pagurus provoquee par le dinoflagellate parasite: Hematodinium sp. Conseil Int. pour L’Exploration de la Mer. CM. 1988/K:32.
5. Field RH, Chapman CJ, Taylor AC, Neil DM, Vickerman K. Infection of the Norway lobster Nephrops norvegicus by a Hematodinium-like species of dinofiagellate on the west coast of Scotland. Dis Aquat Org. 1992;13 : 1–15.
6. Stentiford GD, Green M, Bateman K, Small HJ, Neil DM, Feist SW. Infection by a Hematodinium-like parasitic dinoflagellate causes Pink Crab Disease (PCD) in the edible crab Cancer pagurus. J Invertebr Pathol. 2002;79 : 179–191. 12133707
7. Smith AL, Hirschle L, Vogan C., Rowley AF. Parasitisation of juvenile edible crabs (Cancer pagurus) by the dinoflagellate, Hematodinium sp.: pathobiology, seasonality and its potential effects on commercial fisheries. Parasitology 2015; 142 : 428–438. doi: 10.1017/S0031182014001255 25118672
8. Hudson DA, Shields JD. Hematodinium australis n. sp., a parasitic dinoflagellate of the sand crab Portunus pelagicus from Moreton Bay, Australia. Dis Aquat Org. 1994;19 : 109–119.
9. Xu WJ, Shi H, Xu HX, Small HJ. Preliminary study on the Hematodinium infection in cultured Portunus triberculatus. Acta Hydrobiol Sin. 2007;31 : 640–647.
10. Stentiford GD, Shields JD. A review of the parasitic dinoflagellates Hematodinium species and Hematodinium-like infections in marine crustaceans. Dis Aquat Org. 2005;66 : 47–70. 16175968
11. Small HJ. Advances in our understanding of the global diversity and distribution of Hematodinium spp.—significant pathogens of commercially exploited crustaceans. J Invertebr Pathol. 2012;110 : 234–246. doi: 10.1016/j.jip.2012.03.012 22433998
12. Lee RF, Frischer ME. The decline of the blue crab. Amer Scientist 2004;92 : 548–553.
13. Wilhelm G, Mialhe E. Dinoflagellate infection associated with the decline of Necora puber crab populations in France. Dis Aquat Org. 1996;26 : 213–219.
14. Xu WJ, Xie J, Shi H, Li C. Hematodinium infections in cultured ridgetail white prawns, Exopalaemon carinicauda, in eastern China. Aquaculture 2010;300 : 25–31.
15. Li C, Song S, Liu Y, Chen T. Hematodinium infections in cultured Chinese swimming crab, Portunus trituberculatus in northern China. Aquaculture 2013;396–399 : 59–65.
16. Pagenkopp Lohan KM, Small HJ, Shields JD, Place AR, Reece K. Conservation of the first internal transcribed spacer (ITS1) region of Hematodinium perezi (genotype III) from Callinectes sapidus. Dis Aquat Org. 2013;103 : 65–75. doi: 10.3354/dao02559 23482386
17. Small HJ, Shields JD, Reece KS, Bateman K, Stentiford GD. Morphological and molecular characterization of Hematodinium perezi (Dinophyceae: Syndininiales), a dinoflagellete parasite of the harbour crab, Liocarcinus depurator. J Eukaryot Microbiol. 2012;59 : 54–66. doi: 10.1111/j.1550-7408.2011.00592.x 22092696
18. Shields JD, Taylor DM, Sutton SG, O’Keefe PG, Ings D, Pardy AL. Epidemiology of bitter crab disease (Hematodinium sp.) in snow crabs Chionoecetes opilio from Newfoundland, Canada. Dis Aquat Org. 2005;64 : 253–264. 15997824
19. Shields JD, Taylor DM, O’Keefe PG, Colbourne E, Hynick E. Epidemiological determinants in outbreaks of bitter crab disease (Hematodinium sp.) in snow crabs Chionoecetes opilio from Conception Bay, Newfoundland, Canada. Dis Aquat Org. 2007;77 : 61–72. 17933398
20. Shields JD, Squyars CM. Mortality and hematology of blue crabs, Callinectes sapidus, experimentally infected with the parasitic dinoflagellate Hematodinium perezi. Fish Bull. 2000;98 : 139–152.
21. Stentiford GD, Neil DM. A rapid onset, post-capture muscle necrosis in Norway lobster, Nephrops norvegicus (L.) from the west coast of Scotland, United Kingdom. J Fish Dis. 2000;23 : 251–264.
22. Shields JD, Scanlon C, Volety A. Aspects of the pathophysiology of blue crabs, Callinectes sapidus, infected with the parasitic dinoflagellate Hematodinium perezi. Bull Mar Sci. 2003;72 : 519–535.
23. Coates C, Nairn J. Diverse immune functions of hemocyanins. Dev Comp Immunol, 2014;45 : 43–55. doi: 10.1016/j.dci.2014.01.021 24486681
24. Butler MJ IV, Tiggelaar JM II, Shields JD, Butler MJ V. Effects of the parasitic dinoflagellate Hematodinium perezi on blue crab (Callinectes sapidus) behavior and predation. J Exp Mar Biol Ecol. 2014;461 : 381–388.
25. Stentiford GD, Neil DM, Albalat A, Milligan RJ, Bailey N. The effect of parasitic infection by Hematodinium sp. on escape swimming and subsequent recovery in the Norway lobster, Nephrops norvegicus (L.). J Crust Biol. 2015 (in press)
26. Cerenius L, Jiravanichpaisal P, Liu H-p, Söderhäll I. Crustacean Immunity In: Söderhäll K, editor. Invertebrate Immunity, Landes Bioscience; 2010. pp. 239–259.
27. Rowley AF, Powell A. Invertebrate immune systems: specific, quasi-specific or nonspecific? J Immunol. 2007;179 : 7209–7214. 18025161
28. Stentiford GD, Evans M, Bateman K, Feist SW. Co-infection by a yeast-like organism in Hematodinium-infected European edible crabs Cancer pagurus and velvet swimming crabs Necora puber from the English Channel. Dis Aquat Org. 2003;54 : 195–202. 12803383
29. Stentiford GD. Diseases of the European edible crab (Cancer pagurus): a review. ICES J Mar Sci. 2008;65 : 1578–1592.
30. Smith AL, Whitten MMA, Hirschle L, Pope EC, Wootton EC, Vogan CL, et al. Bacterial septicaemia in prerecruit edible crabs, Cancer pagurus L. J Fish Dis. 2014;37 : 729–737. doi: 10.1111/jfd.12163 23962351
31. Wheeler K, Shields JD, Taylor DM. Pathology of Hematodinium infections in snow crabs (Chionoecetes opilio) from Newfoundland, Canada. J Invertebr Pathol. 2007;95 : 93–100. 17336326
32. Field RH, Appleton PL. A Hematodinium-like dinoflagellate infection of the Norway lobster Nephrops norvegicus: observations on pathology and progression of infection. Dis Aquat Org. 1995;22 : 115–128.
33. Smith AL, Hamilton KM, Hirschle L, Wootton EC, Vogan CL, Pope EC, et al. Characterization and molecular epidemiology of a fungal infection of edible crabs (Cancer pagurus) and interaction of the fungus with the dinoflagellate parasite Hematodinium. Appl Env Microbiol. 2013;79 : 783–793.
34. Li M, Li C, Wang J, Song S. Immune response and gene expression in hemocytes of Portunus trituberculatus inoculated with the parasitic dinoflagellate Hematodinium. Mol Immunol. 2015;65 : 113–122. doi: 10.1016/j.molimm.2015.01.002 25659082
35. Marois E. The multifaceted mosquito anti-Plasmodium response. Curr Opin Microbiol. 2011;14 : 429–435. doi: 10.1016/j.mib.2011.07.016 21802348
36. Molina-Cruz A, Garver LS, Alabaster A, Bangiolo L. Haile A, Winikor J, et al. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science 2013;340 : 984–987. doi: 10.1126/science.1235264 23661646
37. Yoshino TP, Wu X-J, Gonzale L.A, Hooke CH. Circulating Biomphalaria glabrata hemocyte subpopulations possess shared schistosome glycans and receptors capable of binding larval glycoconjugates. Exp Parasitol. 2013;133 : 28–36. doi: 10.1016/j.exppara.2012.10.002 23085445
38. Yoshino TP, Wu X-J, Liu H, Gonzalez LA, Deelder AM, Hokke CH. Glycotope sharing between snail hemolymph and larval schistosomes: larval transformation products alter shared glycan patterns of plasma proteins. PLoS Negl Trop Dis. 2012;6: e1569. doi: 10.1371/journal.pntd.0001569 22448293
39. Kawasaki M, Delamare-Deboutteville J, Dang C, Barnes AC. Hemiuroid trematode sporocysts are undetected by hemocytes of the intermediate host, the ark cockle Anadara trapezia: potential role of surface carbohydrates in successful parasitism. Fish Shellfish Immunol. 2013;35 : 1937–1947. doi: 10.1016/j.fsi.2013.09.040 24161777
40. Hoppes JH. Effects of the dinoflagellate parasite Hematodinium sp. on the immune response of its crustacean hosts. PhD thesis, University of Southampton, U.K., 2011.
41. Small HJ, Shields JD, Neil DM, Taylor AC, Coombs GH. Differences in enzyme activities between two species of Hematodinium, parasitic dinoflagellates of crustaceans. J Invertebr Pathol. 2007;94 : 175–183. 17156792
42. Shumway SE. A review of the effects of algal blooms on shellfish and aquaculture. J World Aquac Soc. 1990;21 : 65–104.
43. Mello DF, de Oliveira ES, Vieira RC, Simoes E, Trevisan R, Liuz Dafre A, et al. Cellular and transcriptional responses of Crassostrea gigas hemocytes exposed in vitro to bevetoxin (PbTx-2). Mar Drugs 2012;10 : 583–597. doi: 10.3390/md10030583 22611355
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR SelectionČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



