-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaActivation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
In this manuscript, we describe, for the first time, a potential role for regulatory T cells (Treg) as an important factor in determining disease outcome in humans following exposure to wild-type S. Typhi. We studied in considerable depth the modulation of Treg activation characteristics and their homing potential in the development of typhoid disease following a wild-type S. Typhi challenge in a unique human infection model. We show that S. Typhi-specific up-regulation of the gut homing molecule integrin α4β7 pre-challenge is associated with subsequent development of typhoid disease. We further demonstrate that increased S. Typhi-specific expression of molecules associated with Treg activation as well as distinct kinetics of the expression of key activation molecules involved in Treg function are present in volunteers diagnosed with typhoid disease. We also provide the first evidence that Treg can functionally suppress S. Typhi-specific CD8+ T cells in vitro. These intriguing results suggest that Treg are likely to play a role in the development of typhoid fever and potentially other enteric infections.
Published in the journal: . PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004914
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004914Summary
In this manuscript, we describe, for the first time, a potential role for regulatory T cells (Treg) as an important factor in determining disease outcome in humans following exposure to wild-type S. Typhi. We studied in considerable depth the modulation of Treg activation characteristics and their homing potential in the development of typhoid disease following a wild-type S. Typhi challenge in a unique human infection model. We show that S. Typhi-specific up-regulation of the gut homing molecule integrin α4β7 pre-challenge is associated with subsequent development of typhoid disease. We further demonstrate that increased S. Typhi-specific expression of molecules associated with Treg activation as well as distinct kinetics of the expression of key activation molecules involved in Treg function are present in volunteers diagnosed with typhoid disease. We also provide the first evidence that Treg can functionally suppress S. Typhi-specific CD8+ T cells in vitro. These intriguing results suggest that Treg are likely to play a role in the development of typhoid fever and potentially other enteric infections.
Introduction
Salmonella enterica serovar Typhi (S. Typhi), the causative agent of typhoid fever, is a major public health threat throughout the developing world. An estimated 26.9 million cases resulting in approximately 217,000 deaths occur annually [1,2]. Furthermore, antibiotic resistance increasingly limits treatment options in many areas [3,4]. Current typhoid vaccines licensed in the US provide modest protection and are only moderately immunogenic [5,6]. In order to effectively develop new vaccine candidates that will provide robust, long-lasting protection, an improved understanding of the immune correlates of protection is desirable. The recent re-establishment of the human challenge model with wild-type S. Typhi provides a unique opportunity to investigate in detail the immune responses following exposure to this pathogen [7].
While multiple studies have investigated cell-mediated immune (CMI) responses against S. Typhi immunization and infection [8,9,10,11,12,13,14,15], to date there are no published studies of the potential role of regulatory T cell (Treg) responses against this organism. Treg are a specialized subset of CD4+ T cells that are responsible for regulating other immune cells [16,17,18]. They are characterized by expression of interleukin (IL)-2 receptor α (CD25) and the transcription factor Forkhead box protein (Fox)P3 [18]. Treg may be derived in vivo in the thymus (tTreg) or the periphery (pTreg) as well as following in vitro activation (iTreg) [19]. At present, there is considerable controversy regarding the expression of specific molecules (e.g., Helios for tTreg) that enable the distinction among these subsets [19]. For simplicity, since we did not measure expression of the considerable number of molecules required to potentially differentiate among these subsets, in the current studies we refer to circulating Treg as those which were obtained ex vivo from the peripheral blood and are likely to represent a combination of tTreg and pTreg. Activated Treg may traffic to the sites of specific immune responses and exert their regulatory functions via cytotoxic T-lymphocyte-associated protein 4 (CTLA-4; CD152) competition for co-stimulatory molecules (CD80 and CD86) on antigen presenting cells, consumption of IL-2, and production of suppressive cytokines [17]. Alterations in homing molecules/chemokine receptors expressed by Treg affect their ability to traffic to the site of specific immune responses [16,20,21,22,23]. In addition to their roles in autoimmunity and cancer biology, Treg have been shown to play a role in suppression of immune responses against multiple pathogens, potentially contributing to disease [24,25].
In the present studies we have evaluated the characteristics and kinetics of Treg homing potential and activation, as well as the functional capacity of Treg to suppress S. Typhi-specific T cell responses following wild-type challenge of healthy adult volunteers. Of importance, we identified distinct homing potential and activation patterns associated with typhoid diagnosis indicating that Treg may play an important role in the development of typhoid fever. In fact, it is likely that immune homeostasis between suppressive and inflammatory responses is critical to the prevention of disease. These studies describe for the first time, the role of S. Typhi-specific modulation of Treg homing potential and activation characteristics in typhoid disease, a role which may be broadly applicable to other enteric infections.
Results
Similar levels of circulating Treg and ex vivo Treg proliferation were observed in volunteers diagnosed with typhoid (TD) and those who were not (No TD)
Peripheral blood mononuclear cells (PBMC) from healthy adult volunteers were obtained prior to and at multiple time-points following challenge with ~2 x 104 colony forming units (cfu) of wild-type S. Typhi (S1 Fig) [7]. Volunteers who developed a fever ≥38°C sustained for ≥12 hours and/or blood culture-confirmed S. Typhi bacteremia were diagnosed with typhoid as described [7]. In the present study, randomly selected volunteers meeting criteria for typhoid diagnosis (TD, n = 6) and volunteers who did not meet criteria (No TD, n = 6) were assessed for Treg phenotype, activation status, and homing potential. In the randomly selected TD volunteers, the time of typhoid diagnosis ranged from 6–9 days post-challenge. Flow cytometry was used to detect the percentages of circulating CD4+ FoxP3+ Treg as well as the more stringently defined CD4+ FoxP3+ CTLA-4+ CD25+ Treg subset in unstimulated PBMC. The gating strategy is shown in S2 Fig. Percentages of FoxP3+ cells ranged from <1% to 3.5% of total CD4+ T cells, a finding consistent with previous reports [26,27]. CTLA-4+ CD25+ Treg ranged from 23–88% of CD4+ FoxP3+ Treg. There was considerable variation among volunteers in both groups and we found no significant difference between TD and No TD volunteers in the pre - or post-challenge percentages of circulating Treg as defined by either strategy (Fig 1A, 1B, 1D and 1E). Furthermore, no statistically significant differences in the percentage of circulating Treg over time were noted in either group (Fig 1A, 1B, 1D and 1E). Furthermore, in a subset of volunteers, we measured Ki67 expression ex vivo as a surrogate of proliferation. While circulating Treg expressed Ki67 indicating that a small proportion of them were proliferating in vivo, there was no difference in the magnitude or kinetics of Ki67 expression between TD and No TD volunteers (Fig 1C and 1F).
Fig. 1. Percentages of circulating and proliferating Treg in TD and No TD volunteers. 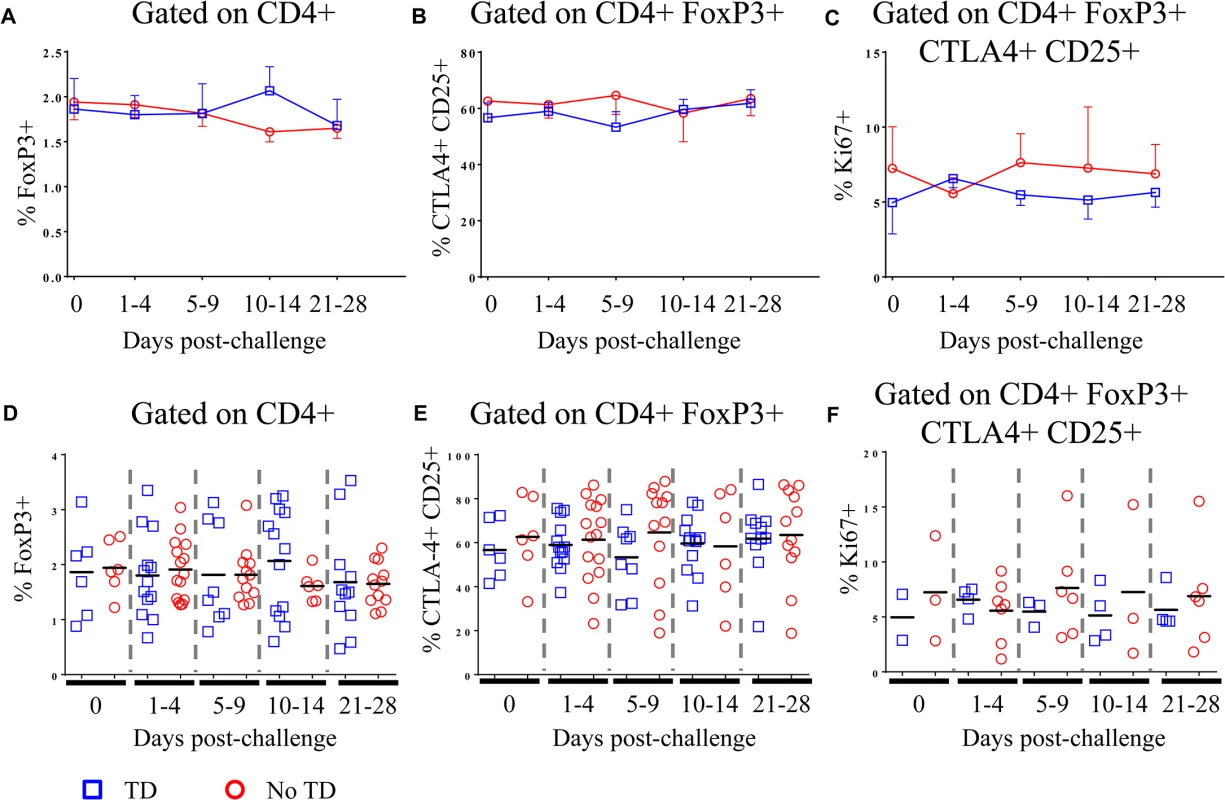
A) Percentage of CD4+ T cells positive for FoxP3 expression and B) percentage of CD4+ FoxP3+ T cells positive for CTLA-4 and CD25 in PBMC obtained pre-challenge and at multiple time-points after challenge (TD n = 6; No TD n = 6). C) Percentage of CD4+ FoxP3+ CTLA-4+ CD25+ T cells expressing Ki67 (a marker of proliferation) ex vivo (TD n = 2, No TD n = 3). Values are shown as the mean +/- SEM. Scatter plots showing D) the percentage of CD4+ T cells positive for FoxP3 expression and E) the percentage of CD4+ FoxP3+ T cells positive for CTLA-4 and CD25 in PBMC obtained pre-challenge and at multiple time-points after challenge (TD n = 6; No TD n = 6). F) the percentage of CD4+ FoxP3+ CTLA-4+ CD25+ T cells expressing Ki67 (a marker of proliferation) ex vivo (TD n = 2, No TD n = 3). Means are indicated with a black horizontal line. P-values were determined using a mixed effects regression model. TD (blue squares); No TD (red circles). Values from multiple time-points were grouped together in time segments (1–4, 5–9, 10–14, and 21–28 days post-challenge) to account for variability in the samples available from each volunteer. Some volunteers had samples from multiple time-points in a time-segment resulting in more data points than the corresponding number of volunteers. Differential expression of homing molecules in circulating Treg from TD and No TD volunteers
Although no differences were identified between TD and No TD volunteers in the total percentage of circulating Treg, we hypothesized that differences in the S. Typhi-specific modulation of the homing potential of Treg might be associated with typhoid diagnosis. To evaluate this possibility, PBMC from volunteers challenged with wild-type S. Typhi (as described above) were stimulated with S. Typhi-infected autologous Epstein Barr Virus (EBV)-transformed B lymphoblastoid cell lines (B-LCL) or non-infected B-LCL (negative control). Flow cytometry was utilized to detect expression of the gut homing molecule integrin α4β7, as well as CXCR3 (homing to sites of inflammation) and CCR6 (homing to sites of TH17 inflammation). Relative (net) S. Typhi-specific modulation of the expression of homing molecules was determined by subtracting the values obtained following stimulation with non-infected B-LCL from stimulation with S. Typhi-infected B-LCL.
As an enteric pathogen, the gut is the first site of immune encounter with S. Typhi, and therefore, we conjectured that the ability of S. Typhi to up-regulate the expression of integrin α4β7 (a gut homing molecule) on circulating Treg might be associated with suppression of protective host responses contributing to disease. We observed that pre-challenge levels of S. Typhi-specific modulation of integrin α4β7 expression were indeed higher on circulating Treg isolated from volunteers who were subsequently diagnosed with typhoid (TD; p = 0.054—mixed effects regression model (Fig 2A and 2D)). Of note, S. Typhi-specific expression of integrin α4β7 was significantly down-regulated in TD volunteers in the 1–4 day time-frame after challenge (p = 0.047—mixed effects regression model), remaining at relatively stable levels thereafter (Fig 2A and 2D). Interestingly, there was an opposite trend in No TD volunteers with up-regulation of S. Typhi-specific integrin α4β7 expression in the 10–14 and 21–28 days post-challenge time frames; however, these changes did not reach statistical significance.
Fig. 2. S. Typhi-specific homing potential of circulating Treg. 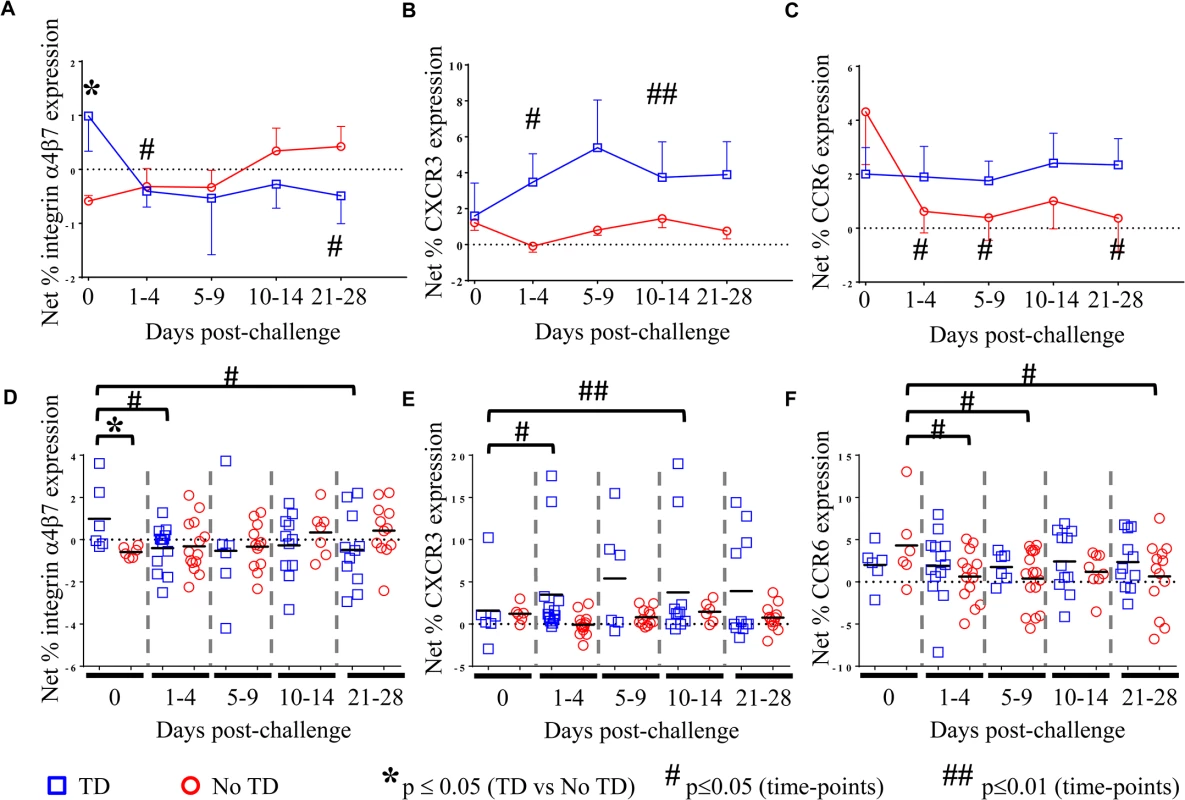
Net S. Typhi-specific expression of A) integrin 47, (TD n = 6, No TD n = 6) B) CXCR3, (TD n = 6, No TD n = 6) C) CCR6 (TD n = 6, No TD n = 6) Values are shown as the mean +/- SEM. Scatter plots showing the net expression of D) integrin α4β7, E) CXCR3, and F) CCR6 on S. Typhi-specific Treg. Means are indicated with a black horizontal line. Time points with statistically significant differences between TD and No TD volunteers (*) or among time-points within each group (#) are identified. P-values were determined using a mixed effects regression model. TD (blue squares); No TD (red circles). Values from multiple time-points were grouped together in time segments (1–4, 5–9, 10–14, and 21–28 days post-challenge) to account for variability in the numbers of samples available from each volunteer. Some volunteers had samples from multiple time-points in a time-segment resulting in more data points than the corresponding number of volunteers. We further investigated whether differences in CXCR3 expression are able to distinguish TD and No TD volunteers as S. Typhi-specific Treg homing to sites of active inflammation could also suppress a protective immune response. Both TD and No TD volunteers exhibited S. Typhi-specific modulation of CXCR3 expression prior to challenge; however, there was no difference observed between the groups (Fig 2B and 2E). Following challenge, CXCR3 expression was significantly up-regulated on S. Typhi-specific Treg in TD volunteers (p = 0.04—mixed effects regression model) with 2 volunteers showing particularly high levels of S. Typhi-specific up-regulation of CXCR3 expression (Fig 2E). Expression of CXCR3 upon exposure to S. Typhi-infected targets remained relatively constant over time in No TD volunteers.
Additionally, because TC17 responses have been identified following Ty21a immunization [8], we hypothesized that expression of CCR6 may also lead to homing of Treg to sites of S. Typhi-induced inflammation. While S. Typhi-specific modulation of the expression of CCR6 by circulating Treg was detected, we did not find significant differences between TD and No TD volunteers prior to challenge (Fig 2C and 2F). S. Typhi-specific up-regulation of CCR6 expression by circulating Treg remained relatively constant in TD volunteers with no significant differences in the mean pre-challenge expression compared to later time-points. In contrast, a significant decrease in S. Typhi-specific expression of CCR6 by circulating Treg was evidenced after challenge in No TD volunteers (p = 0.02–0.03—mixed effects regression model) (Fig 2C and 2F). Of interest, no differences were detected in S. Typhi-specific modulation of CCR6 expression between TD and No TD volunteers following challenge.
Activation of peripheral S. Typhi-specific Treg after challenge is associated with typhoid diagnosis
We hypothesized that increases in S. Typhi-specific expression of additional activation molecules on circulating Treg might be associated with suppression of protective responses resulting in typhoid diagnosis. To test this hypothesis, modulation of S. Typhi-specific expression levels of activation molecules, including HLA-DR, CD11a (lymphocyte function-associated antigen-1; LFA-1), T cell immunoglobulin mucin (Tim)-3, CD304 (neuropilin-1; NRP-1), CD279 (programmed cell death-1; PD-1), CD27, and CD39 (ectonucleoside triphosphate diphosphohydrolase 1; ENTPD1) on Treg were measured using flow cytometry. As for the measurement of homing potential, PBMC from volunteers challenged with wild-type S. Typhi were stimulated with S. Typhi-infected autologous B-LCL or non-infected B-LCL. Relative (net) S. Typhi-specific modulation of the expression of the activation molecules listed above was determined by subtracting the values obtained following stimulation with non-infected B-LCL from stimulation with S. Typhi-infected B-LCL.
There was considerable variability among volunteers in the S. Typhi-specific modulation of the expression of all activation molecules. We observed that S. Typhi-specific up-regulation of PD-1, CD27, LFA-1, NRP-1, and Tim-3 was present before challenge in many volunteers (Fig 3B, 3C, 3E and 3F, Fig 4A and 4B and S3B Fig). However, we identified no significant differences in the S. Typhi-specific expression of activation molecules prior to challenge in TD volunteers compared with No TD volunteers (Fig 3A and 3F, and S3 Fig). At early time-points (days 1–4) following challenge, however, we observed a notable increase in S. Typhi-specific expression of HLA-DR resulting in significantly higher expression in TD than in No TD volunteers (p = 0.015—mixed effects regression model) (Fig 3A and 3D). In contrast, S. Typhi-specific HLA-DR expression on circulating Treg in No TD volunteers decreased slightly after challenge (days 1–4), returning to baseline levels by 21–28 days post-challenge (Fig 3A and 3D).
Fig. 3. S. Typhi-specific activation of circulating Treg. 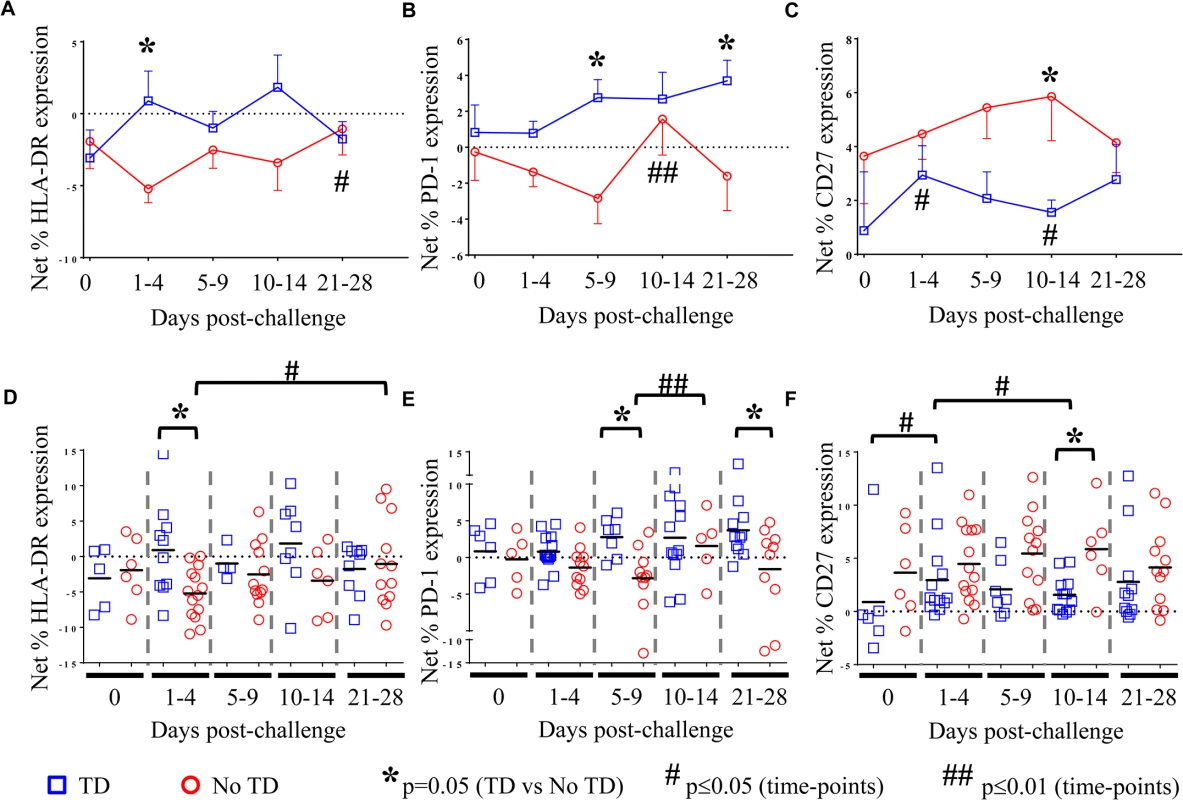
Net S. Typhi-specific expression of A) HLA-DR, (TD n = 5, No TD n = 4) B) PD-1, (TD n = 6, No TD n = 6) and C) CD27, (TD n = 6, No TD n = 6) on Treg. Values are shown as the mean +/- SEM. Scatter plots showing the net expression of D) HLA-DR, (TD n = 5, No TD n = 4) E) PD-1, (TD n = 6, No TD n = 6) and F) CD27, (TD n = 6, No TD n = 6) on S. Typhi-specific Treg. Means are indicated with a black horizontal line. Time points with statistically significant differences between TD and No TD volunteers (*) or among time-points within each group (#) are identified. P-values were determined using a mixed effects regression model. TD (blue squares); No TD (red circles). Values from multiple time-points were grouped together in time segments (1–4, 5–9, 10–14, and 21–28 days post-challenge) to account for variability in the numbers of samples available from each volunteer. Some volunteers had samples from multiple time-points in a time-segment resulting in more data points than the corresponding number of volunteers. Fig. 4. Kinetics of S. Typhi-specific modulation of LFA-1 and NRP-1 expression on circulating Treg following challenge. 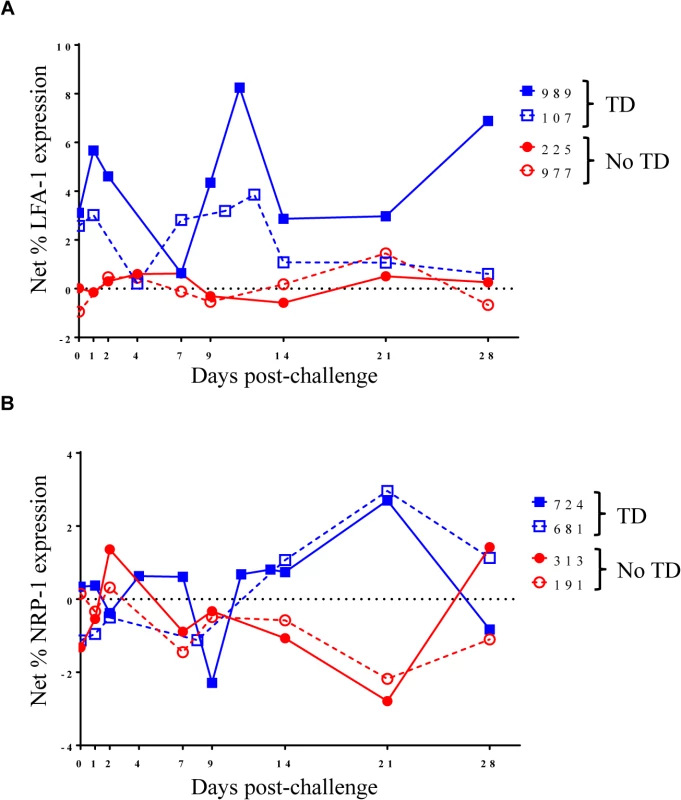
Kinetic curves from representative volunteers showing net S. Typhi-specific modulation of the expression of A) LFA-1 and B) NRP-1 from day 0 (pre-challenge) until day 28 post-challenge. We also identified marked up-regulation of the expression of PD-1 by S. Typhi-infected targets in circulating Treg isolated from TD compared to No TD volunteers post-challenge (Fig 3B and 3E). S. Typhi-specific up-regulation of PD-1 expression increased gradually in TD volunteers following challenge with the highest levels identified on days 21–28 post-challenge (Fig 3B and 3E). Distinctly, however, among those volunteers who did not develop disease we noted a general down-regulation of S. Typhi-specific PD-1 expression in circulating Treg 1–9 days post-challenge with a significant increase between the days 5–9 and days 10–14 post-challenge time groups (Fig 3B and 3E). S. Typhi-specific PD-1 expression returned to baseline levels by days 21–28 post-challenge (Fig 3B and 3E). These opposite trends resulted in significantly higher up-regulation of S. Typhi-specific expression of PD-1 in circulating Treg in TD compared to No TD volunteers at days 5–9 (p = 0.0097—mixed effects regression model) and 21–28 post-challenge (p = 0.0092—mixed effects regression model) (Fig 3B and 3E).
Interestingly, we observed increased up-regulation of the expression of CD27 on S. Typhi-specific Treg in No TD compared to TD volunteers (Fig 3C and 3F). While the trend was present at most time-points, this difference was statistically significant only in the day 10–14 time frame (after typhoid diagnosis and initiation of antibiotics), (p = 0.031—mixed effects regression model). In a subset of volunteers, S. Typhi-specific CD39 expression was also measured. Although there were only a small number of samples tested, we identified up-regulation of S. Typhi-specific CD39 expression on circulating Treg following challenge in TD volunteers. This increase peaked at days 10–14 post-challenge and was significantly higher than pre-challenge (p = 0.02—mixed effects regression model) (S3A Fig). While S. Typhi-specific Tim-3 expression was present on circulating Treg there was no difference noted between TD and No TD volunteers or in either group over time (S3B Fig).
Differential kinetics of activation of circulating S. Typhi-specific Treg between TD and No TD volunteers
To further explore changes in S. Typhi-specific modulation of the expression of activation molecules over time, we examined kinetic curves of individual volunteers. While significant differences were not detected in the mean expression of LFA-1 between TD and No TD volunteers, the kinetic patterns were remarkably different in volunteers diagnosed, or not, with typhoid following challenge. Despite considerable variation among volunteers, a pattern of increased expression of LFA-1 around the time of disease was identified in a majority of TD volunteers (4/5) while expression remained relatively constant for most No TD volunteers (Fig 4A). We also identified differences in the kinetic patterns of S. Typhi-specific NRP-1 expression. Unlike LFA-1, we observed S. Typhi-specific up-regulation of NRP-1 expression in TD volunteers after diagnosis and initiation of antibiotics (days 14–21 post-challenge) (Fig 4B).
Circulating Treg suppress S. Typhi-specific Teff responses
To further assess the functionality of Treg in the setting of typhoid disease, we performed CD25 depletion assays. PBMC from 4 TD volunteers were either mock depleted (pan anti-mouse IgG) or CD25 depleted (anti-human CD25) using magnetic bead separation. Time-points were selected based on known S. Typhi-specific cytokine responses. A total of 7 independent volunteer-time points were used for depletion studies. Depletion resulted in a 55–74% reduction in FoxP3+ CD4+ T cells (Fig 5A and 5B). Following stimulation with S. Typhi-infected B-LCL, CD8+ T effector memory (TEM) were evaluated for S. Typhi-specific cytokine production in the presence (mock-depleted) or absence (CD25-depleted) of Treg using mass cytometry. We found higher percentages of IFN-γ and TNF-α single cytokine producing and multi-functional (IFN-γ+ TNF-α+) S. Typhi-specific CD8+ TEM when Treg were depleted (Fig 5C and 5E). Interestingly, increases in cytokine production were observed in S. Typhi-specific CD8+ TEM with or without gut homing potential (integrin α4β7+ and integrin α4β7-, respectively) (Fig 5E).
Fig. 5. S. Typhi-specific cytokine production following Treg depletion. 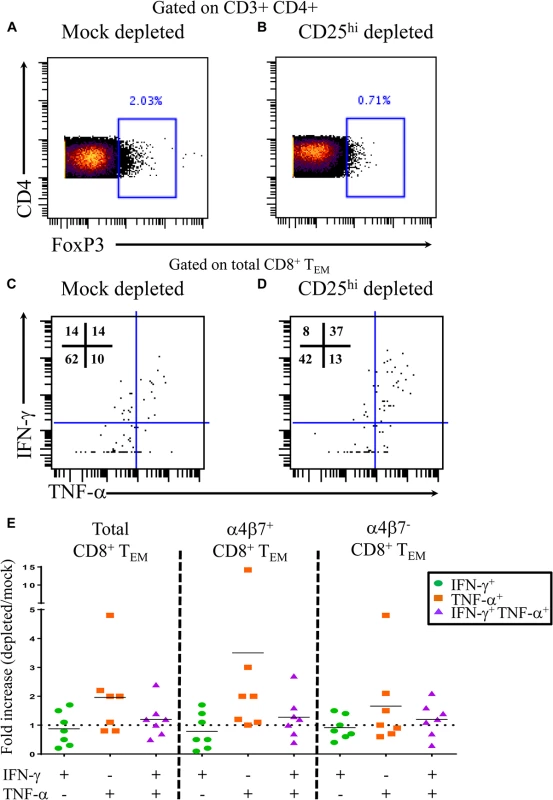
Percentage of CD4+ FoxP3+ T cells following A) mock depletion or B) CD25 depletion in a representative volunteer. Production of IFN-γ and/or TNF-α by S. Typhi-specific CD8+ TEM following C) mock or D) CD25 depletion. E) Data are presented as fold increases in IFN-γ and/or TNF-α production by S. Typhi-specific total CD8+ TEM in depleted vs mock-depleted cultures, as well as by CD8+ TEM expressing, or not, integrin α4β7. Discussion
Effective immune responses must balance the need for pathogen-specific inflammatory responses to fight infection with the need to protect the host from the consequences of excessive inflammation. Homeostasis between regulatory and effector T cells is a major component of this balance. Treg suppress Teff by multiple mechanisms including contact dependent mechanisms, such as CTLA-4, as well as contact-independent mechanisms such as IL-10 production. We aimed to investigate the characteristics, kinetics, and functionality of Treg responses in an S. Typhi human controlled infection model. Homing of Treg to sites of specific inflammation has been previously shown [16,20,21,22,23]. Integrin α4β7 is an important molecule associated with homing of lymphocytes to the gut, the site of initial encounter with S. Typhi [28]. Here we identified, for the first time, significantly higher pre-challenge gut homing potential of circulating Treg (up-regulation of S. Typhi-specific integrin α4β7 expression) in volunteers who were subsequently diagnosed with typhoid disease compared to those who were not. Following challenge, however, there was a significant decrease in S. Typhi-specific integrin α4β7 expression on circulating Treg, suggesting that these Treg left the peripheral blood, presumably as a result of homing to the gut microenvironment. It is currently unclear why S. Typhi-specific Treg expressing differential levels of integrin α4β7 were observed among volunteers before challenge. Participants were recruited in a non-endemic area and are, therefore, unlikely to have previously encountered S. Typhi. However, the S. Typhi genome has a high degree of homology with other Enterobacteriaceae. Thus, differences in baseline Treg responses could be the result of previous encounters with other enteric Gram negative bacilli, including those present in the normal gut microbiota. We have previously reported that oral immunization of volunteers with attenuated oral S. Typhi vaccines elicits S. Typhi-specific TEM which expressed, or not, the gut homing molecule integrin α4β7 [11,29]. It has been shown that T cells activated in the gut preferentially express high levels of integrin α4β7 compared to T cells primed in peripheral lymph nodes [30]. Therefore, it is possible that Treg initially primed in the gut would express higher levels of integrin α4β7 upon re-stimulation resulting in recirculation to the site of initial antigen encounter. It is thus reasonable to speculate that higher levels of Treg homing to the gut may suppress local Teff responses resulting in ineffectual control of the infection ultimately leading to typhoid diagnosis. This hypothesis is further supported by our findings showing the capacity of Treg to suppress S. Typhi–specific responses by integrin α4β7+ TEM elicited in volunteers following exposure to wild-type S. Typhi. Of note, we have also observed in these volunteers, that Treg suppress S. Typhi–specific responses by integrin α4β7 - TEM, suggesting that specific Treg might also exert their regulatory activity at systemic sites.
In addition to early homing to the gut, we identified S. Typhi-specific up-regulation of the expression of both CCR6 and CXCR3. CXCR3 expression on Treg is associated with homing to sites of TH1/TC1 inflammation [21]. It is known that immunization with S. Typhi vaccines, as well as natural infection with S. Typhi, induce predominantly TH1/TC1 type responses [8,9,10,11,12,13,14,15,29]. While not significant, there was a trend toward higher levels of S. Typhi-specific up-regulation of CXCR3 expression on circulating Treg in TD volunteers compared to No TD volunteers. However, this was primarily driven by two volunteers. Future studies with additional volunteers will help to establish the validity of these findings. CCR6 is responsible for homing of lymphocytes, including Treg, to sites of TH17/Tc17 inflammation [20,22]. We have previously identified S. Typhi-specific production of IL-17A by CD8+ TEM following Ty21a immunization [8]. While no significant differences were noted in S. Typhi-specific up-regulation of CCR6 expression on circulating Treg in TD versus No TD volunteers prior to or following challenge, No TD volunteers exhibited a significant S. Typhi-specific decrease in the levels of expression of CCR6 on Treg following challenge, then remained at relatively constant levels through day 28 post challenge. These results suggest that suppression of S. Typhi-specific TH17/Tc17 responses plays a role in protection from typhoid disease. TH17/Tc17 responses are known to produce inflammation including recruitment of neutrophils [31] and increased TH17 infiltration has been identified in gut inflammatory conditions, such as in Crohn’s disease [32]. It is possible that excessive inflammation could result in increased gut permeability and subsequent dissemination of S. Typhi. It is important to note however, that TH17 cells also play an important role in gut mucosal integrity [33]. Therefore, it is likely that the balance of Treg and TH17/Tc17 effector responses may be critical in determining disease outcome.
Taken together, these results highlight the likely importance of Treg localization in the development of typhoid fever. Interestingly, integrin α4β7 is the only molecule measured which showed significant pre-challenge differences in S. Typhi-specific expression on circulating Treg between TD and No TD volunteers, highlighting the potential importance of the local responses early in infection.
In addition to homing to appropriate sites, the activation status of Treg is likely to affect their potential to suppress S. Typhi-specific inflammatory responses. Expression of the activation molecule HLA-DR has been associated with increased contact-dependent activity of human Treg [34]. Furthermore, it has been shown that HLA-DR+ Treg, while more active, are also more susceptible to apoptosis [35]. We identified significant increases in HLA-DR expression on circulating S. Typhi-specific Treg in TD compared to No TD volunteers in the early time-points (day 1–4) post-challenge suggesting that increased S. Typhi-specific activation of circulating Treg may play a role in the development of typhoid fever. Furthermore, we also identified significant differences in the intracellular PD-1 content in S. Typhi-specific circulating Treg between TD and No TD volunteers. While only a small percentage of natural Treg express PD-1 on the surface, higher levels of PD-1 transcript have been associated with suppressive function, suggesting that PD-1 expression is also involved in the development of typhoid fever [36]. Similarly, we observed up-regulation of S. Typhi–specific surface expression of CD39 in TD volunteers, albeit at later time points. Treg expression of CD39 has been associated with suppression of TH17/TC17 responses [37] which, as previously mentioned, have been identified following S. Typhi immunization [8]. It is therefore possible that CD39 expressing Treg in TD volunteers exert their activity, at least in part, by modulating TH17/TC17 responses. In contrast, we did not identify differences in S. Typhi-specific Tim-3 expression between TD and No TD volunteers. The fact that Tim-3 expression on Treg has been associated with increased suppressive Treg activity [38], but no differences were observed between TD and No TD, suggests that the mechanism(s) of Treg activation may vary depending on the model studied. The observations that increased levels of Treg activation appear to play a role in the development of typhoid fever are supported by the results of depletion studies that show increased S. Typhi-specific cytokine production by TEM following depletion of Treg.
CD27 has been proposed as a marker for Treg suppressive activity as well as a marker for CD4+ T memory phenotype [39,40]. We identified higher levels of S. Typhi-specific CD27 expression in No TD volunteers, particularly at days 10–14 post-challenge. This is in striking contrast to other markers of activation which were all increased in TD volunteers. Both CD27+ and CD27 - populations displaying suppressive characteristics have been identified in expanded human Treg [41]. Interestingly, CD27+ Treg were predominantly CD62L+ compared to CD27 - Treg suggesting that CD27+ Treg may be localizing to peripheral lymph nodes [41]. Therefore, the tissue distribution of activated Treg, their characteristics and levels of activation may constitute important determining factors in protection from typhoid fever by contributing to an appropriate balance between suppressive and inflammatory responses.
In addition to S. Typhi-specific increase in HLA-DR expression and up-regulation of PD-1 and CD39 in Treg, we also identified differences in the kinetic patterns of other molecules associated with Treg activation including LFA-1 and NRP-1. LFA-1 plays an important role in the formation of Treg aggregates that block access of responder T cells to dendritic cells [18]. In contrast, the precise function of NRP-1 up-regulation on human Treg remains to be elucidated and in some studies NRP-1 expression was not identified on human Treg [42]. However, other studies have shown NRP-1 up-regulation to be associated with Treg activation in humans [43]. Furthermore, in mice, NRP-1 has been suggested as a marker for tTreg; however, this has not been definitively shown in humans [19,42]. Here we identified S. Typhi-specific up-regulation of NRP-1 in TD volunteers and report differences in the observed kinetics of S. Typhi-specific NRP-1 expression between TD and No TD volunteers. The difference in kinetics of S. Typhi-specific expression of both LFA-1 and NRP-1 molecules in TD compared to No TD volunteers may indicate that multiple mechanisms of increased Treg activation play a role in Treg responses following S. Typhi challenge. Furthermore, these findings suggest that not only the precise balance, but also the timing of Treg responses with inflammatory responses might ultimately determine disease outcome.
While there are no animal models for typhoid fever that fully recapitulate human disease, there have been studies in mice using infection with S. Typhimurium which reveal a potential role for Treg in suppressing specific Teff responses [44]. In this mouse model, increased Treg suppressive capacity, including upregulated CTLA-4 (CD152) expression, is associated with higher S. Typhimurium bacterial burden. Furthermore, Treg ablation results in enhanced Teff activation leading to reduced pathogen burden. These results support our findings that increased Treg activation is associated with typhoid disease and that Treg are capable of suppressing S. Typhi-specific Teff in humans.
In summary, we have shown that S. Typhi-specific up-regulation of the gut homing molecule integrin α4β7 prior to challenge is associated with typhoid diagnosis. Moreover, despite differences in the kinetics of the responses among various Treg activation molecules, with the notable exception of CD27, there was a clear trend for circulating Treg from TD volunteers to display increased levels of S. Typhi-specific activation. Of great importance, we have also demonstrated that Treg are functionally capable of suppressing S. Typhi-specific CD8+ TEM cytokine responses. While the small sample size is a limitation, these studies provide an important first description of Treg responses following S. Typhi exposure in humans. Further investigation into how these responses may relate to protection following immunization with attenuated strains of S. Typhi will provide much needed information to inform and accelerate the development of novel vaccines for typhoid and other enteric fevers, as well as other enteric infections. For example, strategies to identify vaccines that activate Teff without the concomitant activation of suppressive Treg responses, or that elicit an optimal balance between Teff and Treg responses may result in improved protective efficacy.
Materials and Methods
Volunteers and isolation of peripheral blood mononuclear cells (PBMC)
Healthy adult volunteers aged 18–60 were recruited by the Centre for Clinical Vaccinology and Tropical Medicine, Oxford, UK, to participate in this study. Volunteers with history of typhoid fever or immunization against typhoid fever were excluded [7]. Volunteers were orally challenged with 1–5x104 CFU of wt-S. Typhi (Quailes strain) suspended in sodium bicarbonate at Oxford University in compliance with the National Research Ethic Service (NRES), Oxford Research Ethics Committee A [7]. Close monitoring was performed throughout the study, and at the time of typhoid fever diagnosis (TD, as determined by blood culture-confirmed S. Typhi bacteremia or development of a fever ≥38°C for ≥12 hours), volunteers were treated with a 2-week course of antibiotics (Ciprofloxacin, 500mg twice daily). Those volunteers who did not developed typhoid fever (No TD) received a 2-week course of antibiotics at day 14 post-challenge. PBMC collected from 12 randomly selected volunteers (TD n = 6, No TD n = 6) participating in the challenge trial were used in this study. Selection was made based on the number of available PBMC with those volunteers having more PBMC utilized for the studies. PBMC were isolated prior to challenge and at 9–11 time-points following challenge (S1 Fig). Isolation was performed by Lymphoprep gradient centrifugation (Axis-Shield, Oslo, Norway) and PBMC were cryopreserved in liquid nitrogen following standard techniques within four hours of initial blood draw. Viability of cryopreserved PBMC was assessed after thawing of cells and an overnight rest at 37°C with 5% CO2 (as described in ex vivo stimulation).
Target/stimulator cells
B-LCL were generated from autologous PBMC for each volunteer as previously described [45]. Briefly, B-LCL were established using supernatant from the B95.8 cell line (ATCC CRL1612; American Type Culture Collection) as the source of EBV. PBMC from each volunteer were incubated with EBV containing supernatant and cyclosporine (0.5 μg/mL; Sigma, St. Louis, MO) at 37°C with 5% CO2 for 2–3 weeks. B-LCL were maintained in culture or cryopreserved until use.
Infection of target/stimulator cells
Target cells were infected by incubation with wild-type S. Typhi strain ISP1820 in RPMI 1640 media (Gibco, Carlsbad, CA) without antibiotics for 3 hours at 37°C with 5% CO2 as previously described [45]. On the day following infection the cells were gamma irradiated (6000 rad). To confirm that targets were infected with S. Typhi, cells were stained with anti-Salmonella common structural Ag (CSA-1)-FITC (Kierkegaard & Perry, Gaithersburg, MD) and analyzed by flow cytometry on an LSRII flow cytometer (BD Biosciences, San Jose, CA) [8]. The percentage of cells infected with S. Typhi was recorded for each experiment and the infected targets were only used if infection rates were >30% of viable cells.
Ex vivo stimulation
PBMC were thawed and rested overnight at 37°C. Cells were then resuspended in RPMI 1640 media (Gibco) supplemented with 100 U/mL penicillin (Sigma), 100 μg/mL streptomycin (Sigma), 50 μg/mL gentamicin (Gibco), 2 mM L-glutamine (Gibco), 2.5 mM sodium pyruvate (Gibco), 10 mM HEPES buffer (Gibco), and 10% fetal bovine serum (Gemini Bioproducts, West Sacramento, CA) at a concentration of 1x106 cells/mL in sterile 5 mL round bottom tubes (BD Falcon, Franklin Lakes, NJ). PBMC were stimulated with S. Typhi-infected B-LCL or B-LCL alone (negative control). After 2 hours, Golgi Stop (containing monensin) and Golgi Plug (containing brefeldin A) from BD were added at concentrations of 0.5 μl/mL and cultures continued overnight at 37°C in 5% CO2. Media alone was used as an additional negative control.
Conventional flow cytometric analyses
Following stimulation as described above, cells were plated in 96-well V-bottom plates for staining. Cells were washed once with staining buffer (phosphate buffered saline with 0.5% BSA and 0.1% sodium azide) and stained for live/dead discrimination using Invitrogen LIVE/DEAD fixable yellow dead cell stain kit (Invitrogen, Carlsbad, CA). Fc receptor blocking was performed with human immunoglobulin (Sigma; 3 μg/mL) followed by surface staining, performed as previously described.[8] Briefly, cells were surface stained with panels that included the following fluorochrome-conjugated monoclonal antibodies against: CD14-BV570 (M5E2, Biolegend, San Diego, CA), CD19-BV570 (HIB19, Biolegend), CD3-BV650 (OKT3, Biolegend), CD4-APC-H7 (RPA-T4, BD), CD25-PECy7 (M-A251, BD), CCR6/CD196-PE (11A9, BD), HLA-DR-Qdot 800 (Life technologies, Grand Island, NY), integrin α4β7-Alexa 647 (clone ACT-1, conjugated in-house), CXCR3/CD183-Alexa 700 (1C6/CXCR3, BD), LFA-1/CD11a-Alexa 488 (HI111, Biolegend), NRP-1/CD304-APC (12C2, Biolegned), CD27-BV605 (4S.B3, Biolegend), CD39-BV421 (A1, Biolegend), and Tim-3-Alexa 700 (344823, R&D, Minneapolis, MN) at 4°C for 30 minutes. The cells were then fixed and permeabilized using FoxP3 IC fixation and permeabilization buffers from eBiosciences according to manufacturer’s recommendations. Intracellular staining with FoxP-PerCP-Cy5.5 (236A/E7, BD), CTLA-4/CD152-PECy5 (BNI3, BD), PD-1/CD279-BV421 (EH12.1, BD) and Ki67-BV605 (Ki67, Biolegend) was performed for 20 minutes at room temperature. After staining, cells were fixed in 1% paraformaldehyde and stored at 4°C until analyzed. Flow cytometry was performed using a customized LSRII flow cytometer (BD). Flow cytometry data were analyzed using WinList version 7 (Verity Software House, Topsham, ME) software package. Graphs were generated using GraphPad Prism version 6 (Graphpad Software, San Diego, CA).
CD25-depletion studies
In a subset of volunteers, CD25 cells were depleted - or mock-depleted using anti-CD25 or pan anti-mouse IgG Dynabeads, respectively (Invitrogen) as previously described [25]. Briefly, thawed PBMC were rested overnight as described above. Following the overnight rest, PBMC were divided into two aliquots consisting of 2.1–3 x 106 cells and either mock-depleted or depleted of CD25 cells using magnetic bead separation. Depleted (mock and CD25) PBMC were stimulated with S. Typhi-infected B-LCL or non-infected B-LCL (negative control) as described above.
Mass cytometry
Following CD25 - or mock-depletion and stimulation with S. Typhi-infected B-LCL, cells were stained for mass cytometry with a panel of 22 metal-conjugated mAb to detect both Treg and responder T cells. A table of the mAb used is shown in supplementary materials (S1 Table). Viability staining was performed with cisplatinum (Sigma; 25 μM) for 60 seconds. Following cisplatinum, samples were Fc-blocked with human immunoglobulin (Sigma; 3 μg/mL) followed by surface staining, performed as previously described. Fixation and permeabilization were performed with FoxP3 IC fixation and permeabilization buffers (eBiosciences) followed by intracellular staining. Samples were stained with an Ir191/193 DNA intercalator for cell detection by mass cytometry within 48 hours of sample acquisition and re-suspended in EQ4 normalization beads (Fluidigm, Sunnyvale, CA). Acquisition was performed using a CyTOF mass cytometer (Fluidigm, formerly DVS Sciences). Data were analyzed with Fluidigm Cytobank.
Statistical analyses
Observations were grouped by day following challenge in the following periods: pre-challenge, days 1 to 4, days 5 to 9, days 10 to 14, and days 21 to 28 (there were no observations between days 14 and 21). Volunteers often contributed more than one observation to these time periods. To compare mean values by time period and group, while accounting for the lack of independence between multiple measures from the same volunteer at the same time period and across time periods, we used mixed effects models, including a random effect for subject, fit by restricted maximum likelihood. Through simulation experiments we confirmed that this approach provided valid statistical inference for data sets of this size.
Ethics statement
The human challenge study was performed in compliance with the National Research Ethic Service (NRES), and approved by the Oxford Research Ethics Committee A. All volunteers provided written informed consent.
Supporting Information
Zdroje
1. Crump JA, Luby SP, Mintz ED (2004) The global burden of typhoid fever. Bull World Health Organ 82 : 346–353. 15298225
2. Buckle GC, Walker CL, Black RE (2012) Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J Glob Health 2 : 010401. doi: 10.7189/jogh.02.010401 23198130
3. Bhutta ZA (1996) Impact of age and drug resistance on mortality in typhoid fever. Arch Dis Child 75 : 214–217. 8976660
4. Rowe B, Ward LR, Threlfall EJ (1997) Multidrug-resistant Salmonella typhi: a worldwide epidemic. Clin Infect Dis 24 Suppl 1: S106–109. 8994789
5. Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, et al. (1999) Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine 17 Suppl 2: S22–27. 10506405
6. Sur D, Ochiai RL, Bhattacharya SK, Ganguly NK, Ali M, et al. (2009) A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med 361 : 335–344. doi: 10.1056/NEJMoa0807521 19625715
7. Waddington CS, Darton TC, Jones C, Haworth K, Peters A, et al. (2014) An outpatient, ambulant-design, controlled human infection model using escalating doses of salmonella typhi challenge delivered in sodium bicarbonate solution. Clin Infect Dis 58 : 1230–1240. doi: 10.1093/cid/ciu078 24519873
8. McArthur MA, Sztein MB (2012) Heterogeneity of multifunctional IL-17A producing S. Typhi-specific CD8+ T cells in volunteers following Ty21a typhoid immunization. PLoS One 7: e38408. doi: 10.1371/journal.pone.0038408 22679502
9. Salerno-Goncalves R, Fernandez-Vina M, Lewinsohn DM, Sztein MB (2004) Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol 173 : 5852–5862. 15494539
10. Salerno-Goncalves R, Pasetti MF, Sztein MB (2002) Characterization of CD8(+) effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol 169 : 2196–2203. 12165550
11. Salerno-Goncalves R, Wahid R, Sztein MB (2005) Immunization of volunteers with Salmonella enterica serovar Typhi strain Ty21a elicits the oligoclonal expansion of CD8+ T cells with predominant Vbeta repertoires. Infect Immun 73 : 3521–3530. 15908381
12. Salerno-Goncalves R, Wahid R, Sztein MB (2010) Ex Vivo kinetics of early and long-term multifunctional human leukocyte antigen E-specific CD8+ cells in volunteers immunized with the Ty21a typhoid vaccine. Clin Vaccine Immunol 17 : 1305–1314. doi: 10.1128/CVI.00234-10 20660136
13. Salerno-Goncalves R, Wyant TL, Pasetti MF, Fernandez-Vina M, Tacket CO, et al. (2003) Concomitant induction of CD4+ and CD8+ T cell responses in volunteers immunized with Salmonella enterica serovar typhi strain CVD 908-htrA. J Immunol 170 : 2734–2741. 12594304
14. Sztein MB (2007) Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica Serovar Typhi strains used as live oral vaccines in humans. Clin Infect Dis 45 Suppl 1: S15–19. 17582562
15. Sztein MB, Salerno-Goncalves R, McArthur MA (2014) Complex adaptive immunity to enteric fevers in humans: lessons learned and the path forward. Front Immunol 5 : 516. doi: 10.3389/fimmu.2014.00516 25386175
16. Wing K, Sakaguchi S (2010) Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol 11 : 7–13. doi: 10.1038/ni.1818 20016504
17. Sakaguchi S, Wing K, Miyara M (2007) Regulatory T cells—a brief history and perspective. Eur J Immunol 37 Suppl 1: S116–123. 17972355
18. Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T (2009) Regulatory T cells: how do they suppress immune responses? Int Immunol 21 : 1105–1111. doi: 10.1093/intimm/dxp095 19737784
19. Dhamne C, Chung Y, Alousi AM, Cooper LJ, Tran DQ (2013) Peripheral and thymic foxp3(+) regulatory T cells in search of origin, distinction, and function. Front Immunol 4 : 253. doi: 10.3389/fimmu.2013.00253 23986762
20. Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, et al. (2007) Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med 204 : 2803–2812. 18025126
21. Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, et al. (2009) The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 10 : 595–602. doi: 10.1038/ni.1731 19412181
22. Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, et al. (2008) CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol 181 : 8391–8401. 19050256
23. Engelhardt BG, Sengsayadeth SM, Jagasia M, Savani BN, Kassim AA, et al. (2012) Tissue-specific regulatory T cells: biomarker for acute graft-vs-host disease and survival. Exp Hematol 40 : 974–982 e971. doi: 10.1016/j.exphem.2012.08.002 22885125
24. Li L, Lao SH, Wu CY (2007) Increased frequency of CD4(+)CD25(high) Treg cells inhibit BCG-specific induction of IFN-gamma by CD4(+) T cells from TB patients. Tuberculosis (Edinb) 87 : 526–534. 17851131
25. Lyke KE, Dabo A, Arama C, Daou M, Diarra I, et al. (2012) Reduced T regulatory cell response during acute Plasmodium falciparum infection in Malian children co-infected with Schistosoma haematobium. PLoS One 7: e31647. doi: 10.1371/journal.pone.0031647 22348117
26. Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA (2001) CD4+CD25high regulatory cells in human peripheral blood. J Immunol 167 : 1245–1253. 11466340
27. Presicce P, Moreno-Fernandez ME, Lages CS, Orsborn KI, Chougnet CA (2010) Association of two clones allows for optimal detection of human FOXP3. Cytometry A 77 : 571–579. doi: 10.1002/cyto.a.20875 20162533
28. Mora JR, Iwata M, Eksteen B, Song SY, Junt T, et al. (2006) Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314 : 1157–1160. 17110582
29. Wahid R, Salerno-Goncalves R, Tacket CO, Levine MM, Sztein MB (2008) Generation of specific effector and memory T cells with gut - and secondary lymphoid tissue - homing potential by oral attenuated CVD 909 typhoid vaccine in humans. Mucosal Immunol 1 : 389–398. doi: 10.1038/mi.2008.30 19079203
30. Stagg AJ, Kamm MA, Knight SC (2002) Intestinal dendritic cells increase T cell expression of alpha4beta7 integrin. Eur J Immunol 32 : 1445–1454. 11981833
31. Witowski J, Ksiazek K, Jorres A (2004) Interleukin-17: a mediator of inflammatory responses. Cell Mol Life Sci 61 : 567–579. 15004696
32. Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, et al. (2009) Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med 206 : 525–534. doi: 10.1084/jem.20081712 19273624
33. Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, et al. (2008) Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 14 : 421–428. doi: 10.1038/nm1743 18376406
34. Baecher-Allan C, Wolf E, Hafler DA (2006) MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol 176 : 4622–4631. 16585553
35. Ashley CW, Baecher-Allan C (2009) Cutting Edge: Responder T cells regulate human DR+ effector regulatory T cell activity via granzyme B. J Immunol 183 : 4843–4847. doi: 10.4049/jimmunol.0900845 19801510
36. Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, et al. (2004) In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med 199 : 1455–1465. 15184499
37. Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, et al. (2009) CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol 183 : 7602–7610. doi: 10.4049/jimmunol.0901881 19917691
38. Gupta S, Thornley TB, Gao W, Larocca R, Turka LA, et al. (2012) Allograft rejection is restrained by short-lived TIM-3+PD-1+Foxp3+ Tregs. J Clin Invest 122 : 2395–2404. doi: 10.1172/JCI45138 22684103
39. Duggleby RC, Shaw TN, Jarvis LB, Kaur G, Gaston JS (2007) CD27 expression discriminates between regulatory and non-regulatory cells after expansion of human peripheral blood CD4+ CD25+ cells. Immunology 121 : 129–139. 17425604
40. Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, et al. (2005) Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med 201 : 1793–1803. 15939793
41. Koenen HJ, Fasse E, Joosten I (2005) CD27/CFSE-based ex vivo selection of highly suppressive alloantigen-specific human regulatory T cells. J Immunol 174 : 7573–7583. 15944257
42. Yadav M, Stephan S, Bluestone JA (2013) Peripherally induced tregs—role in immune homeostasis and autoimmunity. Front Immunol 4 : 232. doi: 10.3389/fimmu.2013.00232 23966994
43. Chaudhary B, Khaled YS, Ammori BJ, Elkord E (2014) Neuropilin 1: function and therapeutic potential in cancer. Cancer Immunol Immunother 63 : 81–99. doi: 10.1007/s00262-013-1500-0 24263240
44. Johanns TM, Ertelt JM, Rowe JH, Way SS (2010) Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog 6: e1001043. doi: 10.1371/journal.ppat.1001043 20714351
45. Sztein MB, Tanner MK, Polotsky Y, Orenstein JM, Levine MM (1995) Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J Immunol 155 : 3987–3993. 7561107
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR SelectionČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



