-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNatural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
Type I interferons (IFN-I) are innate cytokines crucial for vertebrate antiviral defenses. IFN-I exert antiviral effector functions and orchestrate antiviral immunity. IFN-I are induced early after infection, upon sensing of viral particles or infected cells by immune receptors. Intracellular Toll-like receptors (TLR) are selectively expressed in specialized immune cell types such as plasmacytoid dendritic cells (pDC), enabling them to copiously produce IFN-I upon detection of engulfed viral nucleic acids. pDC or intracellular TLR have been reported to be crucial for resistance to experimental infections with many viruses in mice but dispensable for resistance to natural infections in humans. Our aim was to investigate this puzzling difference. Mice deficient for TLR activity mounted strong IFN-I responses despite producing very low IFN-I levels and controlled the infection by a moderate dose of murine cytomegalovirus much better than mice deficient for IFN-I responses. Deficient TLR responses could be compensated by direct recognition of infected cells by natural killer cells. Hence, we identified experimental conditions in mice mimicking the lack of requirement of TLR functions for antiviral defense observed in humans. We used these experimental models to advance our basic understanding of antiviral immunity in a way that might help improve treatments for patients.
Published in the journal: . PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004897
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004897Summary
Type I interferons (IFN-I) are innate cytokines crucial for vertebrate antiviral defenses. IFN-I exert antiviral effector functions and orchestrate antiviral immunity. IFN-I are induced early after infection, upon sensing of viral particles or infected cells by immune receptors. Intracellular Toll-like receptors (TLR) are selectively expressed in specialized immune cell types such as plasmacytoid dendritic cells (pDC), enabling them to copiously produce IFN-I upon detection of engulfed viral nucleic acids. pDC or intracellular TLR have been reported to be crucial for resistance to experimental infections with many viruses in mice but dispensable for resistance to natural infections in humans. Our aim was to investigate this puzzling difference. Mice deficient for TLR activity mounted strong IFN-I responses despite producing very low IFN-I levels and controlled the infection by a moderate dose of murine cytomegalovirus much better than mice deficient for IFN-I responses. Deficient TLR responses could be compensated by direct recognition of infected cells by natural killer cells. Hence, we identified experimental conditions in mice mimicking the lack of requirement of TLR functions for antiviral defense observed in humans. We used these experimental models to advance our basic understanding of antiviral immunity in a way that might help improve treatments for patients.
Introduction
Type I interferons (IFN-I) orchestrate vertebrate antiviral defenses through two complementary mechanisms [1]. These cytokines induce multiple Interferon Stimulated Genes (ISG) coding for effector molecules of cell-intrinsic antiviral immunity. IFN-I instruct antiviral innate and adaptive immunity, in part by promoting the maturation of dendritic cells (DC) for potent activation of natural killer (NK) cells and CD8 T lymphocytes. Genetic deficiencies compromising IFN-I responses dramatically increase susceptibility to viral infections in mice and men [2]. In addition to IFN-I, type III interferons (IFN-III) also appear critical for antiviral defense, based on the analysis of mutant mice, and on the strong association between resistance to viral infections and polymorphisms affecting these genes in humans [1, 3, 4]. IFN-I and IFN-III share the same signaling pathways and downstream target genes. However, while the IFN-I receptor (IFNAR) is ubiquitously expressed, the receptor for IFN-III is selectively expressed in epithelial cells [1].
The contribution of different cell types and molecular sensors to IFN-I induction during viral infections is the subject of debate. IFN-I can be induced by two major mechanisms in infected hosts [1]. Potentially all host cell types are equipped with innate immune sensors of endogenous viral replication that can trigger IFN-I production. Certain immune cell types are also able to sense viral infection in their surroundings and consequently produce high levels of IFN-I without being infected. This ability is especially strong in the plasmacytoid subset of DC (pDC). pDC recognize and engulf viral particles or material derived from infected cells. Subsequent detection of nucleic acids by toll-like receptors (TLR) 7 and 9 in specialized endosomes leads to MyD88 - and IRAK4-dependent IFN-I induction [5]. On the other hand, while patients genetically deficient for MYD88 or IRAK4 show enhanced susceptibility to mycobacteria, they are resistant to most viral infections [6–8]. MyD88 is critical for signaling not only by all TLRs except TLR3 but also by the receptors for all members of the interleukin-1 (IL-1) cytokine family [9]. Hence, in humans, pDC, TLR7/8/9 and all the IL-1 cytokine family are largely redundant for antiviral defense, in particular with regards to induction of protective IFN-I responses [8]. In contrast, mice genetically deficient for Tlr7, Tlr9 or Myd88 show enhanced susceptibility to a broad range of pathogens including many viruses [8]. pDC production of IFN-I has been proposed to be essential in mice for the control of acute systemic viral infections in particular with herpes viruses [10–12] or coronaviruses [13]. pDC may also contribute to prevent the establishment of chronic infections with certain viruses [14–16]. Here, we designed experimental studies to investigate what could explain this reported discrepancy between humans and mice for the importance of MyD88 responses in antiviral defense. During viral infections in mice, the impact of MyD88 inactivation or pDC depletion had only been assessed on the basis of IFN-I production. Here, we also examined how it affected the induction of protective IFN-I responses. We also investigated whether NK cell responses could compensate for MyD88 deficiency for host resistance.
We used experimental infection by murine cytomegalovirus (MCMV), a natural pathogen of mice [17], for which IFN-I responses are critical for protection both in vitro in macrophages [18–21] and in vivo [22, 23]. pDC sense MCMV infection through TLR7/9 [24–27] and constitute the major source of IFN-I in vivo [10, 24, 28, 29]. However, infected stromal cells have also been described as potent IFN-I producers in the spleen, 8 hrs after infection [30]. NK cells sense MCMV infection through their activating receptor Ly49H, allowing them to specifically recognize and kill infected cells through binding to m157, a viral protein expressed at their surface. pDC depletion prior to infection leads to a dramatic decrease of serum IFN-I levels but only to a modest and transient increase in viral loads in most organs [5, 10, 24, 29]. In contrast, either NK cell depletion, or deficiency in endosomal TLR activity, decreases the ability of C57BL/6 mice to control viral replication and increases morbidity and mortality [24–27, 31]. It is assumed that the enhanced susceptibility to certain systemic viral infections of mice deficient for endosomal TLR activity is largely due to their decreased IFN-I production [8, 10–13, 24, 27]. However, whether tissue responses to IFN-I are reduced in these animals has not been examined. Moreover, other immune functions are dampened or lost in these animals, including IL-12 production [5] and, in MyD88-deficient mice, responses to all member of the IL-1 cytokine family [9]. Moreover, the respective contributions of IFN-I-, MyD88 - and Ly49H-dependent responses to overall resistance to MCMV infection has not been rigorously investigated in parallel in mice of the same genetic background, deficient for one or more of these responses. We thus designed a study to rigorously explore how MyD88 genetic deficiency in mice affects their responses to MCMV infection, in particular their ability to mount strong type I IFN responses and to resist disease development, for comparison with the analysis previously published in patients genetically deficient for MYD88 [7]. In both species, MyD88 deficiency does not only affect direct viral sensing by TLRs but also signaling by all IL-1 family cytokines including IL-18. We also wanted to explore whether the consequences of MyD88 deficiency could be modulated by mutations or polymorphisms in other immune genes, for example those encoding NK cell activating receptors. In steady state conditions, in mice, IL-18 has been reported to be crucial for NK cell functional maturation, enabling them to respond to activation with synthetic stimuli [32]. Hence, using MyD88-deficient animals to examine the interaction between DC and NK cell direct sensing of infected cells might not seem optimal since MyD88-deficiency could affect NK cell responses in a cell-intrinsic manner. During MCMV infection in vivo, contradictory results have been reported regarding the role of IL-18 in the promotion of the proliferation of Ly49H+ NK cells, with no role observed in one study [33], an absolute requirement reported in a another study [34], and an important contribution but not an absolute requirement in a third study [35]. In any case, IL-18 has been described as a cytokine crucial for IFN-γ production by NK cells in the spleen but not in the liver and appears to be dispensable for resistance to MCMV infection under conditions where IL-12 and NK cells are critical [36]. In other words, IL-18 does not seem to be required for NK cell-dependent protection against primary MCMV infection. CD8 T cell responses are also important for immune defense against CMV infection in human and mice [5]. TLR or IL-1 stimulation of DC are considered to be critical for the induction of protective antiviral cellular adaptive immunity [37, 38]. CD8 T cell responses against MCMV are altered in mice affected in their MyD88 or NK cell activities [24, 39–42]. However, how NK and MyD88 responses are integrated in the shaping of antiviral CD8 T cells responses has not been examined.
We used a series of mutant BALB/c mice expressing or not Ly49H, and deficient or not for MyD88 or for the receptor for IFNAR. We characterized their immune response and overall resistance to MCMV infection. We used BALB/c congenic animals, C.B6-Klra8Cmv1-r/UwaJ mice referred to as BALB/c-Ly49H+ mice in the manuscript, which carry most of the C57BL/6 NK gene complex, not only limited to Ly49h [43]. Contrary to the situation observed in BALB/c mice, in BALB/c-Ly49H+ mice NK cells play a major role in the control of an in vitro grown WT MCMV Smith virus. This protective NK cell activity is almost completely abrogated when using an in vitro grown MCMV Smith virus strain lacking the m157 gene [44]. Here, we compared viral control and mortality between BALB/c and BALB/c-Ly49H+ mice upon infection with a K181 virus strain lacking the m157 gene, Δm157 MCMV [45]. Upon infection with a moderate dose of salivary gland-extracted Δm157 MCMV, BALB/c-Ly49H+ mice did not control viral replication more efficiently or survive better than BALB/c animals (S1A–S1C Fig). Altogether, these observations strongly suggest that most of the differences observed in the increased resistance to MCMV infection of BALB/c-Ly49H+ mice as compared to BALB/c animal likely result from the much more efficient recognition of infected cells by their NK cells, due to the interaction of the Ly49H NK cell activation receptor with the viral m157 protein expressed at the surface of infected cells. Nevertheless, some contributions for other genes encoded in the NK gene complex cannot be totally excluded. We chose to inject MCMV intraperitoneally because it is the most frequent route of inoculation used with MCMV and because it rapidly causes a systemic infection, which resolution is thought to more stringently depend of systemic production of IFN-I by pDC than for local infections. Indeed, with several other viral infections, including infections with the herpesviruses HSV-1 and HSV-2, pDC were shown to contribute to IFN-I production only under condition of systemic viral spread and not under conditions where the infections is performed and controlled in epithelia [12, 46]. Moreover, in mice, pDC depletion compromises the control of HSV-1 or HSV-2 only for systemic but not local infections [12]. Our results demonstrate that, in our experimental settings, MyD88 but not IFN-I responses are largely redundant for control of a systemic herpes virus infection in mice. This contradicts current thinking but reconciles the requisite of mice and men for innate antiviral defense. We identify direct recognition of infected cells by NK cells as one innate immune sensing mechanism able to compensate in part for the loss of MyD88 activity. Our results also highlight an unexpected ability of low levels of IFN-I to induce strong cell-intrinsic antiviral immunity in vivo, emphasizing the importance of measuring responses to, rather than production of, cytokines to assess their physiological role.
Results
pDC or MyD88 deficiency dramatically decreases IFN-I production but still allows a strong ISG induction during MCMV infection
To assess the contribution of pDC activation and endosomal TLR7/9 triggering to IFN-I responses during MCMV infection, we analyzed the induction of IFN-I and ISG in the spleen or blood of BALB/c mice knocked-out for MyD88 or TLR9, or depleted of pDC through administration of the 120G8 mAb directed against Bst2. 120G8 mAb injection dramatically reduced circulating IFN-I titers and splenic Ifnb1 expression at d1.5 after infection (Fig 1A and 1B). However, unexpectedly, only a slight impairment was observed in splenic induction of 3 canonical ISG at d1.5 and 3 after infection (Fig 1B). Consistently, 120G8 mAb injection in BALB/c mice did not compromise splenic viral control at d6 (S2A and S2B Fig). Because the administration of the 120G8 mAb resulted in an efficient and selective, even though not entirely specific, depletion of pDC during MCMV infection (see materials and methods), we can rigorously conclude that pDC and their high systemic production of type I IFN are not required for induction of strong type I IFN responses and relatively efficient control of MCMV infection in our experimental settings. Similar results were obtained for splenic induction of Ifnb1 and ISG in BALB/c MyD88-/- and BALB/c TLR9-/- mice (Fig 1B and S2C Fig). In contrast, ISG induction was completely abrogated in IFNAR-/- animals (Fig 1B). Thus induction of strong IFN-I responses in the spleen of MCMV infected mice seems not to require pDC, MyD88 or TLR9 functions and can be induced by very low levels of IFN-I.
Fig. 1. pDC or MyD88 deficiency impairs IFN-I production but not splenic IFN-I responses and virus control. 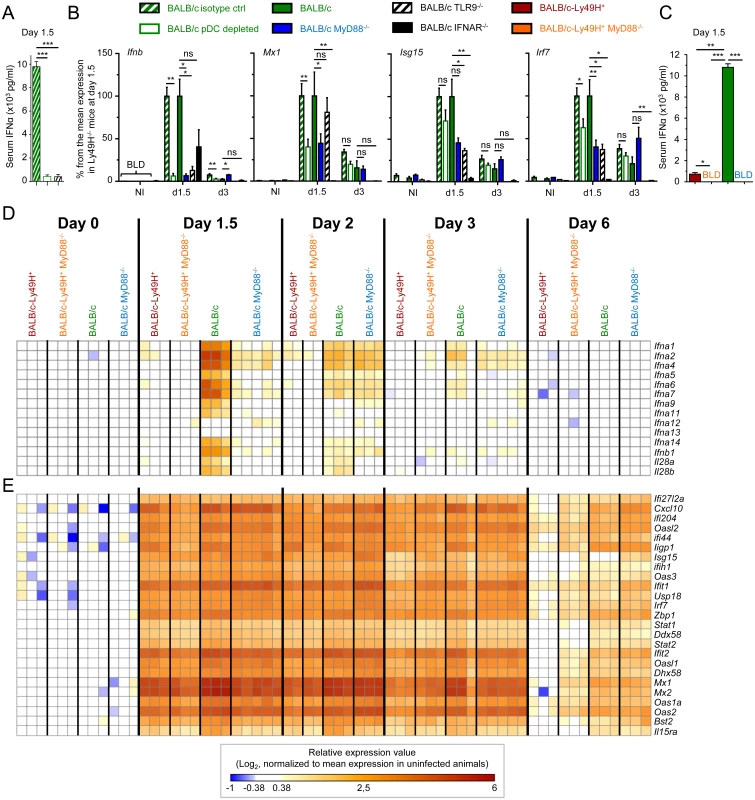
Control (Rat IgG), pDC-depleted (αBST2) or untreated BALB/c mice and untreated BALB/c, BALB/c MyD88-/-, BALB/c IFNAR-/- and BALB/c TLR9-/- mice were infected with 2.5 x 103 pfu MCMV or left uninfected (NI). (A) IFN-α serum titers were measured at d1.5 post-infection by ELISA. Results (mean±SEM) are shown from one experiment representative of 3 independent ones. (B) RT-PCR analysis of the expression of selected genes in the spleen at d1.5 or 3 post-infection. Data are normalized to mean expression of d1.5 BALB/c mice (100% reference level). Results (mean±SEM) are shown from 2 pooled independent experiments, each with 2 to 3 mice per group. (C) Serum IFN-α levels were measured at d1.5 post-infection by ELISA in BALB/c-Ly49H+, BALB/c-Ly49H+ MyD88-/-, BALB/c and BALB/c MyD88-/- mice. Results (mean±SEM) are shown from one experiment representative of 3 independent ones, each with 3 mice per group. BLD: below limit of detection. (D-E) Microarray analyses were performed on total mRNA extracted from spleen at d0, 1.5, 2, 3 and 6 post-infection. Heatmaps show the relative expression value for IFN-I/III genes (D) and for 25 ISG (E). The expression pattern of ISG is examined globally in S2E–S2G Fig. Results shown are from 2 pooled independent experiments, each with 1 to 3 mice per group. High IFN-I responses are observed in MCMV-infected mice, irrespective of pDC activation and IFN-I serum titers
Systemic levels of IFN-I during MCMV infection are controlled by a balance between pDC and NK cell activation [5]. High systemic IFN-I production not only requires the ability of pDC to sense viral nucleic acids through functional endosomal TLR, but is also promoted by viral replication, which is normally limited by NK cell activity. Hence, to generalize our observation that very low levels of IFN-I production are sufficient to induce strong IFN-I responses, we next measured pangenomic ISG induction in mice with low serum IFN-I titers (Fig 1C) and splenic pDC IFN-I expression (S2A and S2D Fig) resulting either from a primary immune deficiency (MyD88-/- animals) or on the contrary from early control of the virus by NK cells (Ly49H+ animals). A strong induction of most IFN-I/III genes was observed in BALB/c mice at d1.5 after MCMV infection. This progressively decreased over time to become undetectable by d6 (Fig 1D). In contrast, hardly any induction of IFN-I/III genes was seen in BALB/c-Ly49H+ and BALB/c-Ly49H+ MyD88-/- animals, and only a weaker and delayed induction was observed in BALB/c MyD88-/- mice (Fig 1D). However, strikingly, a very strong ISG induction was observed in all mouse strains, already at d1.5 after MCMV infection. This was maintained until d3 in all mouse strains and was still strong at d6 in BALB/c and BALB/c MyD88-/- mice (Fig 1E and S2E–S2G Fig). Thus, induction of strong IFN-I responses in the spleen of MCMV infected mice does not require pDC, MyD88 and TLR9 function and can be induced optimally under conditions where IFN-I/III are undetectable not only in the circulation but also in the spleen both at protein and mRNA levels. Hence, contrary to the commonly accepted dogma, pDC, MyD88 and TLR9 are dispensable in mice for induction of strong IFN-I responses against systemic infection with a herpes virus, in our experimental settings.
IFN-I responses are essential to promote resistance to MCMV infection irrespective of Ly49H expression and confer a significant level of protection even in MyD88-/- deficient animals
We next examined to what extent IFN-I responses are required for defense against MCMV under our experimental conditions. We tested susceptibility to MCMV infection of 6 different mouse strains, with deficiencies in either IFNAR or MyD88, and expressing or not Ly49H (Fig 2), or selectively depleted for pDC (S3 Fig). We infected these animals with serial doses of MCMV, to define the LD50 inoculum at which 50% of the animals were killed (Fig 2A). As expected, BALB/c-Ly49H+ mice were the most resistant to infection. BALB/c IFNAR-/- and BALB/c-Ly49H+ IFNAR-/- mice were the most susceptible, with an LD50 more than 50 times lower than that of BALB/c-Ly49H+ animals. Thus, IFN-I responses are critical for defense against MCMV infection even in mice bearing the Ly49H activating receptor allowing direct sensing of infected cells by NK cells. Strikingly, BALB/c-Ly49H+ MyD88-/- and BALB/c MyD88-/- mice harbored clearly higher LD50 than IFNAR-/- animals. Hence, MyD88-/- mice were significantly more resistant than IFNAR-/- animals, despite their low to undetectable production of IFN-I. In addition, pDC depletion did not increase the susceptibility of BALB/c mice after infection with the LD50 (S3 Fig). Thus, the induction of IFN-I responses to levels allowing a significant control of MCMV infection does not require MyD88 and pDC functions.
Fig. 2. IFN-I responses are essential but MyD88 and Ly49H partly redundant to protect against MCMV infection. 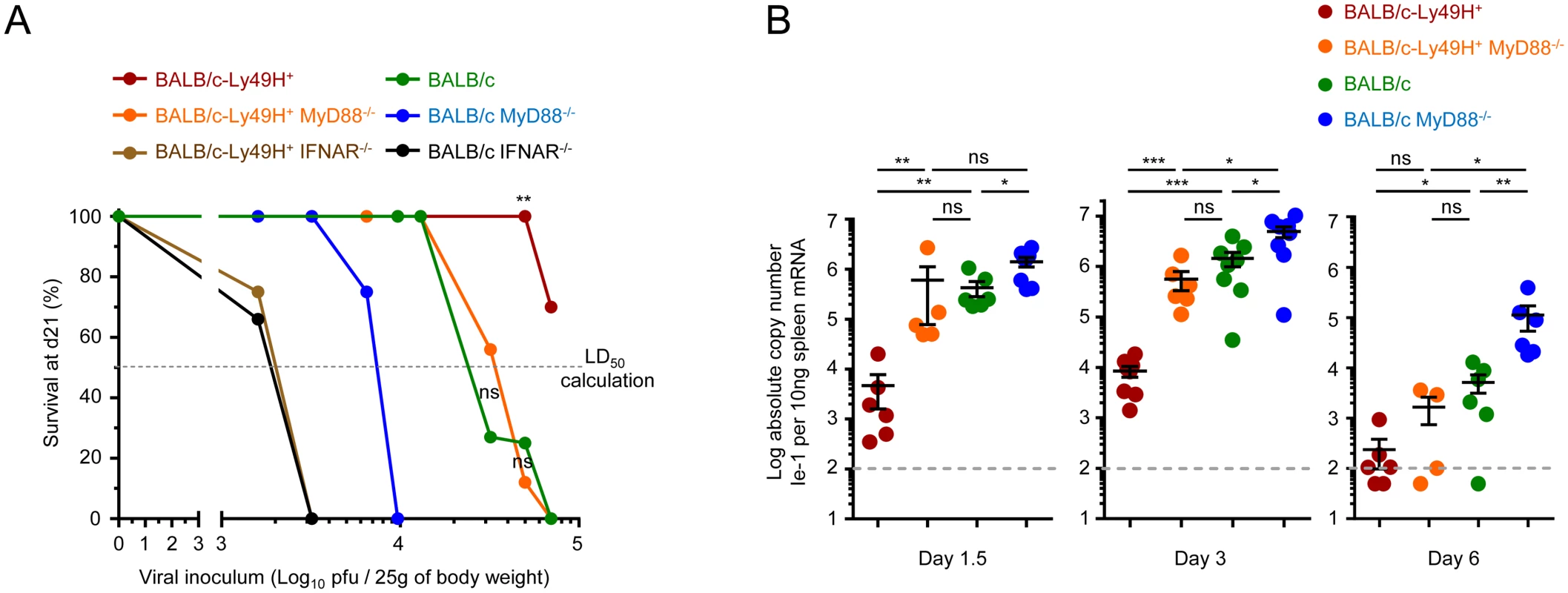
(A) Survival of mice at d21 post-infection (Y-axis) as a function of the doses of MCMV inoculum (X-axis). Indicated mice were infected with between 1.6x103 and 7x104 PFU of MCMV, with overlaps for several doses between strains of mice, and their survival was followed for 21d. Data is shown as percentage of survival. In total, 8 different doses were tested in 9 different experiments in order to determine the susceptibility of each mouse strain, with 2 to 4 mouse strains simultaneously studied in each experiment. Data was derived from the following numbers of mice and tested doses of MCMV inoculum: BALB/c-Ly49H+: 24 mice for 4 doses; BALB/c-Ly49H+ MyD88-/-: 37 mice for 6 doses; BALB/c-Ly49H+ IFNAR-/-: 8 mice for 2 doses; BALB/c: 43 mice for 5 doses; BALB/c MyD88-/-: 15 mice for 4 doses; BALB/c IFNAR-/-: 6 mice for 2 doses. The median lethal dose that causes 50% of lethality in each mouse strain (LD50) was calculated from the graph as the dose on the X-axis that corresponds to 50% mortality on the Y-axis as represented by the horizontal dotted line labeled “LD50 calculation”. LD50 BALB/c-Ly49H+: ≥105 pfu/mouse; LD50 BALB/c-Ly49H+ MyD88-/-: 3.3x104 pfu; LD50 BALB/c-Ly49H+ IFNAR-/-: 2x103 pfu; LD50 BALB/c: 2.2x104 pfu; LD50 BALB/c MyD88-/-: 8x103 pfu; LD50 BALB/c IFNAR-/-: 2x103 pfu. (B) Splenic viral titers were measured at d1.5, 3 and 6 post-infection. Data (mean±SEM) are shown from 2 to 4 pooled independent experiments, each with 2 to 3 mice per group. Contrary to IFNAR deficiency, MyD88 deficiency can be compensated at least in part by direct sensing of infected cells by NK cells
The sensitivity to MCMV infection conferred by IFNAR deficiency was independent of the expression of Ly49H. This was not the case for MyD88 deficiency. BALB/c-Ly49H+ MyD88-/- mice were clearly more resistant than BALB/c MyD88-/- mice (Fig 2A). Conversely, the sensitivity to MCMV infection associated with Ly49H deficiency was aggravated by MyD88 deficiency, since BALB/c mice were more resistant than BALB/c MyD88-/- mice (Fig 2A). BALB/c and BALB/c-Ly49H+ MyD88-/- mice exhibited similar LD50. Hence, the deficiency in TLR-mediated MCMV sensing by DC and in responses to IL-1 cytokine family can be compensated at least in part by direct sensing of infected cells by NK cells and vice versa. Indeed, under conditions of infection with a moderate MCMV dose, below the LD50 of BALB/c MyD88-/- mice, control of viral replication was significantly less efficient in these animals than in the three other mouse strains examined (Fig 2B). NK cell activation during MCMV infection depends on IL-12, IL-18 and IL-15 [5, 35]. The induction and/or activity of IL-12 and IL-18 are both strongly decreased in MyD88-/- C57BL/6 mice. This correlates with a significant impairment of NK cell responses under conditions of high viral inoculum [5]. In contrast, here, the survival and viral titers observed suggested that functional antiviral NK cell responses were induced in BALB/c-Ly49H+ MyD88-/- mice. Thus, we examined NK cell functions in BALB/c mice deficient or competent for MyD88, and expressing or not Ly49H (Fig 3). At d3 after MCMV infection, the Ly49H+ NK cells from BALB/c-Ly49H+ MyD88-/- mice were clearly activated although to a significantly lesser extent than in BALB/c-Ly49H+ animals (Fig 3A–3D and S4A and S4C Fig). Diminished IL-12 and type I IFN production by DC as well as cell-intrinsic loss of IL-18 signaling could all have contributed to the decrease in NK cell proliferation, production of IFN-gamma and expression of Granzyme B observed in MyD88-/- mice, according to previous reports which analyzed the contribution of these different responses during MCMV infection [23–26, 35, 36, 47, 48]. In any case, at d6 after MCMV infection, the activation of Ly49H+ NK cells was higher in BALB/c-Ly49H+ MyD88-/- mice as compared to BALB/c-Ly49H+ animals (Fig 3A–3D). Moreover, NK cell depletion led to a significant increase in viral replication not only in BALB/c-Ly49H+ mice but also in BALB/c-Ly49H+ MyD88-/- animals (Fig 3E and S4D Fig). Thus, NK cells can be sufficiently activated so as to contribute significantly to the control of MCMV infection despite a primary immune deficiency that abrogates viral sensing by DC through TLR7/9, as well as the responses to IL-18 and all other IL-1 family cytokines, provided that the NK cells can directly recognize virally infected cells through triggering of a dedicated NK cell activating receptor. Ly49H+ NK cells from BALB/c-Ly49H+ IFNAR-/- mice were significantly activated at d3 and d6 post infection (Fig 3A–3D), to levels similar to those observed in BALB/c-Ly49H+ MyD88-/- mice. However, in contrast to the situation in the latter animals, in BALB/c-Ly49H+ IFNAR-/- mice, viral replication did not appear to be curtailed by NK cell activity, since it was already very high in untreated mice and was not increased further upon NK cell depletion (Fig 3E). These results suggest that Ly49H+ NK cells are similarly activated in BALB/c-Ly49H+ IFNAR-/- and BALB/c-Ly49H+ MyD88-/- mice but fail to control viral replication in the former because the absence of IFN-I response allows fast and widespread virus replication overwhelming the antiviral functions of Ly49H+ NK cell. Thus, MyD88 but not IFNAR deficiency can be compensated at least in part by direct sensing of infected cells by NK cells in our experimental model of systemic MCMV infection.
Fig. 3. MyD88 deficiency does not completely abrogate NK cell-mediated protection. 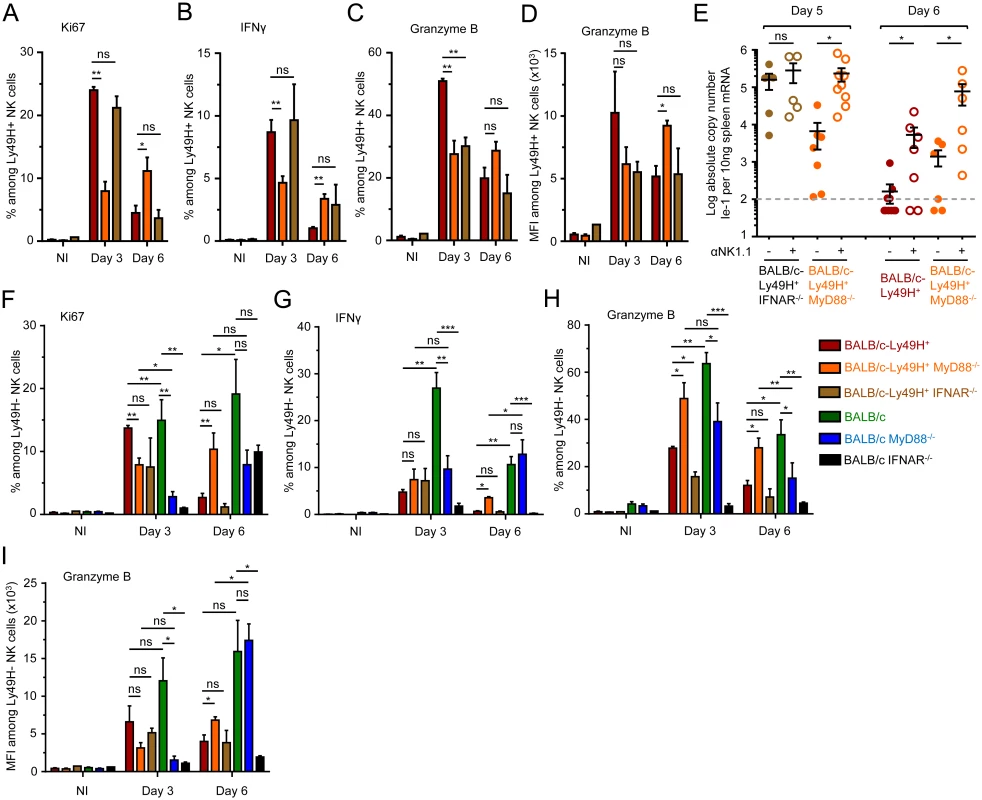
(A-D) Ly49H+ NK cell activation in BALB/c-Ly49H+, BALB/c-Ly49H+ MyD88-/- and BALB/c-Ly49H+ IFNAR-/- mice at d0, 3 and 6 post infection. Proliferation was assessed by Ki67 expression (A). Antiviral effector functions were assessed by intracellular staining for IFN-γ (B) and Granzyme B (C-D). (E) Impact of NK cell depletion on viral replication in the spleen of BALB/c-Ly49H+, BALB/c-Ly49H+ MyD88-/- and BALB/c-Ly49H+ IFNAR-/- mice. Mice were depleted of NK cells and splenic viral titers were measured 5 or 6d post infection. (F-I) Ly49H- NK cell activation in BALB/c-Ly49H+, BALB/c-Ly49H+ MyD88, BALB/c-Ly49H+ IFNAR-/-, BALB/c, BALB/c MyD88-/- and BALB/c IFNAR-/- mice. Data (mean±SEM) are shown from 3 pooled independent experiments each with 3 mice per group. NK cell activation in MyD88-/- mice does not require cell-intrinsic Ly49H signals but likely relies on residual IL-12 responses
A strong activation of Ly49H- NK cells was observed in all infected mice (Fig 3F–3I and S4A and S4B Fig). Hence, NK cell activation in MyD88-/- mice did not require cell-intrinsic Ly49H signals. IL-12 and IL-18 production were decreased in MCMV-infected BALB/c MyD88-/- and BALB/c-Ly49H+ MyD88-/- mice (S4E Fig). However, similarly to what happened with the IFN-I response, IL-12/18 stimulated genes were strongly induced in infected MyD88-/- mice (S4F and S4G Fig). Hence, persistence of NK cell activation in MyD88-/- mice likely resulted from residual IL-12 production and subsequent IL-12-dependent responses.
The combined deficiency for Ly49H and MyD88 prevents the expansion of antiviral CD8 T cells
We next investigated the functions and numbers of antiviral CD8 T cells in the different mouse strains under study. Contrary to our expectations, the in vivo cytotoxic activity of anti-MCMV CD8 T cells was similar in the four mouse strains examined at d6 after infection (Fig 4A and S5A Fig). The CD8 T cells from Ly49H-/-MyD88-/- mice even exhibited a significantly stronger expression of IFN-γ and Granzyme B directly ex vivo without any restimulation (Fig 4A and S5B Fig). Moreover, depletion of CD8 T cells led to a significant increase of viral replication both in BALB/c and BALB/c MyD88-/- animals (Fig 4B and S5B Fig). Thus, during MCMV infection, CD8 T cells are hyperactivated in BALB/c MyD88-/- animals and contribute to the control of viral replication. Hyperactivated CD8 T cell responses can contribute to liver immunopathology and death during MCMV infection [49]. However, CD8 T cell depletion led to a higher mortality of BALB/c MyD88-/- mice (Fig 4C), ruling out a deleterious role of CD8 T cells in our experimental settings. Upon infection, there was a strong and significant increase in the numbers of total and anti-MCMV CD8 T cells in all of the three mouse strains expressing MyD88 and/or Ly49H, but not in BALB/c MyD88-/-animals (Fig 4D). Hence, since the efficiency of viral control in vivo by CD8 T cells depends on the local effector-to-target ratio in infected tissues [50], the enhanced susceptibility of BALB/c MyD88-/- mice to MCMV infection likely resulted in part from their failure to expand their antiviral CD8 T cells. As compared to the other mouse strains, the BALB/c MyD88-/- mice exhibited a significant increase in their splenic expression of genes associated with CD8 T cell exhaustion or with fibrosis and a decrease in the expression of the gene signature of red pulp macrophages (S5C and S5D Fig). This is consistent with the early and strong depletion of those cells previously reported in mice lacking efficient antiviral NK cell responses and infected with a high dose of MCMV [51]. At d4 post infection, BALB/c MyD88-/- and BALB/c IFNAR-/- mice also harbored a marked disruption of their spleen architecture (Fig 4E). Altogether, these observations suggest that early, strong and irreversible damage is caused to secondary lymphoid organs upon infection in Ly49H-/-MyD88-/-animals. This possibly is a consequence of persistently high levels of viral replication that cause a loss of the support function of these organs for all T cells and favor exhaustion of antiviral CD8 T cells. The early and profound loss of splenic red pulp macrophages and stromal cells occurring in BALB/c MyD88-/-animals, as witnessed by microarray analysis, likely contribute significantly to the loss of the specific micro-anatomical niches required for sustaining high numbers of antiviral CD8 T cells. Thus, either efficient antiviral NK cell activity or MyD88 functions are necessary to promote antiviral CD8 T cell responses while simultaneous failure of both responses strongly compromises antiviral adaptive immunity.
Fig. 4. Impact of MyD88 or Ly49H deficiencies on antiviral CD8 T cell responses. 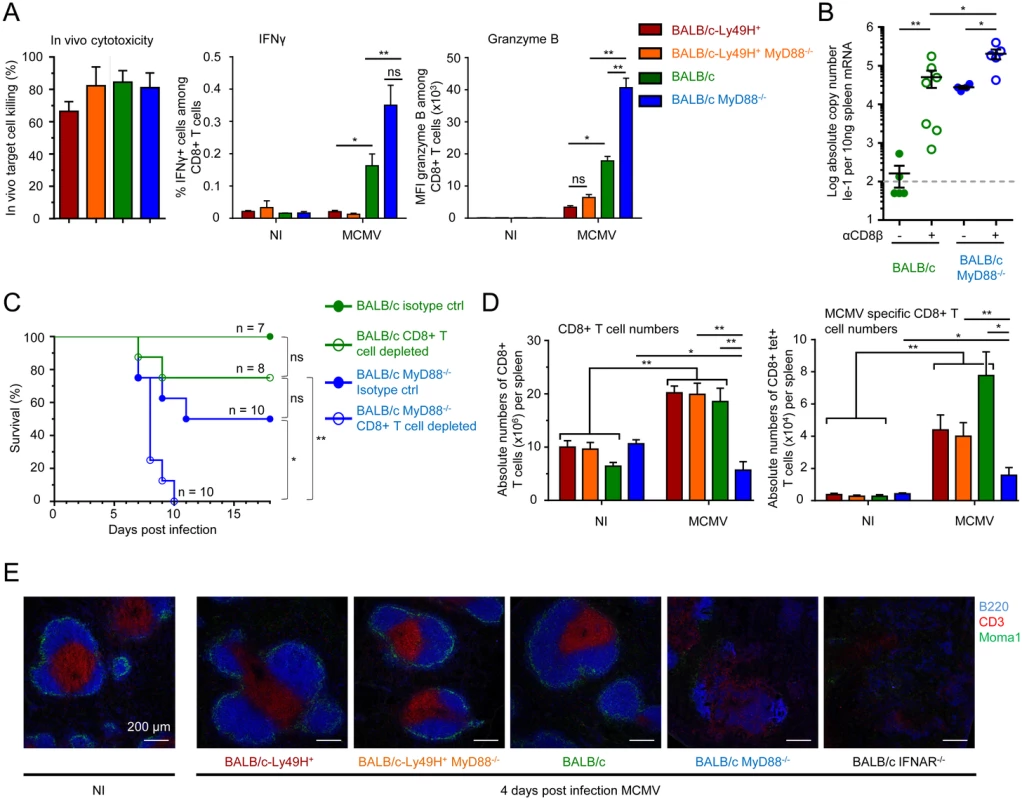
(A-C) Analysis of antiviral CD8 T cell effector functions. (A) In vivo cytotoxicity, ex vivo IFN-γ production and Granzyme B expression by splenic CD8 T cells from BALB/c-Ly49H+, BALB/c-Ly49H+ MyD88-/-, BALB/c and BALB/c MyD88-/- mice at d0 and 6 post infection. Data (mean ± SEM) are shown from 3 pooled independent experiments each with 2 or 3 mice per group. (B-C) Impact of CD8 T cell depletion on disease in BALB/c and BALB/c MyD88-/- mice. Mice were depleted of CD8 T cells and infected with 2.5x103 pfu MCMV (B) or with 8x103 pfu MCMV (C). Splenic viral titers were measured at d6 post infection (B) or mortality was monitored daily (C). For B, data (mean±SEM) are represented from 2 pooled independent experiments each with 2 to 4 mice per group. For C, data show the percent survival from 2 pooled independent experiments each with 3 to 5 mice per group, n represents the total number of mice per group. (D) Enumeration of total (left panel) and anti-IE-1 (right panel) CD8 T cells in the spleens of d5 MCMV-infected mice. Results (mean±SEM) are shown from 4 pooled independent experiments each with 3 mice per group. (E) Immunohistological analysis of tissue damage to the spleen in d4 MCMV infected mice. Mice were infected with 104 pfu MCMV. Spleen were harvested at d4 and stained to evaluate the integrity of the T cell zone as assessed by examining its marginal zone boundary (CD169) and its T cell (CD3) and B cell (B220) zones. Results are shown for one representative mouse per experimental group from 3 independent experiments each with 3 mice per group. Discussion
IFN-I responses are essential for defense against most viral infections in mice and men [2, 52–54]. However, it has been claimed that the requirements for pDC and endosomal TLR in IFN-I production differ fundamentally during natural infections of men and experimental challenges of laboratory mice [8], since IRAK4 or MyD88 genetic deficiencies only increase susceptibility to viruses in mice. However, whether IFN-I responses are impaired in infected MyD88-/- mice and whether this contributes to their enhanced susceptibility to viral infections had not previously been rigorously examined. Moreover, it is unknown whether other immune responses could compensate for MyD88 deficiency during resistance to viral infections in mice as must occur in humans.
Our results show that IFN-I responses are critical for defense against MCMV infection in mice. However, in our experimental settings, pDC and MyD88 are not required for the induction of protective IFN-I responses while they are crucial for high systemic production of these cytokines. Contrary to expectations [8, 11, 12], in mice, high systemic production of IFN-I resulting from endosomal sensing of viruses by pDC is dispensable for relatively efficient intrinsic, innate and adaptive immune responses to our model of systemic infection by a herpes virus. This observation is consistent with the redundancy of pDC and TLR7/9 functions but not IFN-I responses for antiviral defense in humans [2, 6–8, 53, 54]. Moreover, even if mice deficient for MyD88 are more susceptible than wild-type animals to MCMV infection, this can be compensated by other modalities of innate sensing of the infection, namely in our experimental set-up by direct NK cell recognition of infected cells. Hence, while IFN-I, IFN-γ and cytotoxic responses are all necessary for immune defenses against MCMV infection and most other experimental viral infections in mice, access to these functions can be promoted by a number of complementary and/or partly redundant pathways. These include the triggering of different innate immune recognition receptors and the mobilization of distinct cell types with overlapping antiviral activities (Fig 5). Indeed, Ly49H+ NK cells can promote early control of MCMV replication in the absence of CD8 T cells [55, 56] and vice versa [57, 58], likely because they exert partly overlapping functions including IFN-γ production and cytotoxicity [59–62] (Fig 5). However, in RAG-deficient C57BL/6 mice lacking B and T cell responses, immune escape variants mutated for m157 are selected over time and ultimately cause the death of the mice after several weeks [55, 56]. Hence, context-dependent redundancies in innate and adaptive antiviral immune responses likely contribute to the robustness of host defenses under physiological conditions despite the immune evasion strategies developed by many viruses, as illustrated also by the demonstration of redundancies and complementarities between NK cells, CD8 T cells and CD4 T cells in the prevention of MCMV reactivation in latently infected mice lacking humoral immunity [63]. It is likely that other innate immune effector cells contribute to context-dependent redundancies in antiviral defense mechanisms in mice, including NK T cells [64–68] or γδ T cells [69, 70]. Similarly, the absence of overtly increased susceptibility to viruses in patients genetically deficient for endosomal TLR activity due to loss-of-function mutations in MYD88 or IRKA4 likely results from their preserved ability to mount protective intrinsic and innate immune responses that efficiently control primary infections. These involve not only IFN-I responses but also innate cytotoxic responses by NK, NK T or γδ T cells [71–73], before adaptive immunity induction. However, it is possible that pDC and MyD88 are necessary for efficient host defense under specific conditions of viral infections, including during herpesvirus infections consecutive to bone marrow transplantation where adaptive immunity and some innate immune responses are suppressed and the conditions of viral replication may be analogous to a high dose challenge.
Fig. 5. Model of redundancies and complementarities between molecular sensors and cell types for mounting the IFN-I, IFN-γ and cytotoxic cellular immune responses which are necessary for control of MCMV infection. 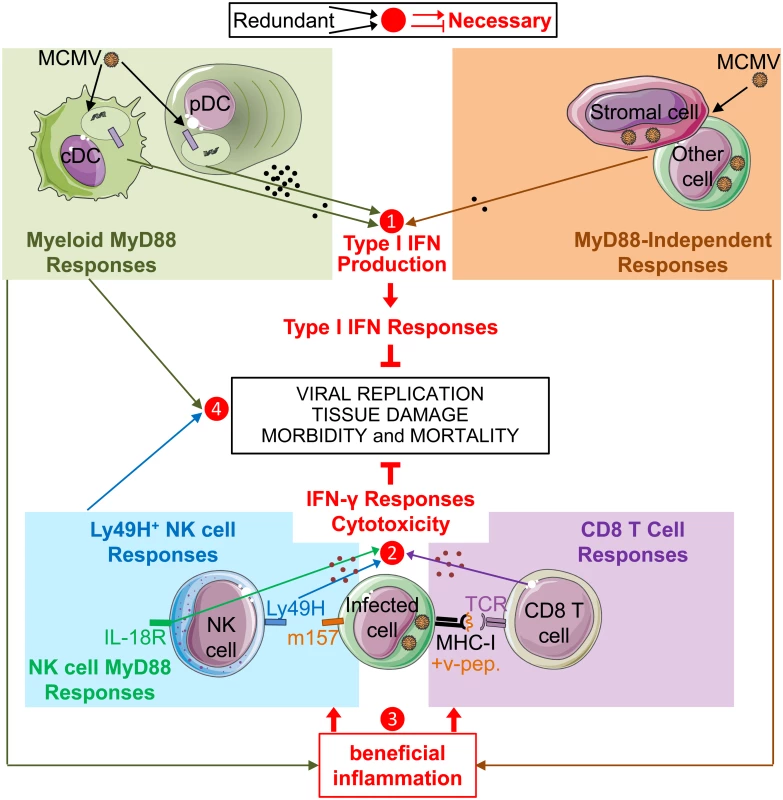
Based on our own data as well as on many other studies published previously, we propose a model whereby IFN-I, IFN-γ and cytotoxic responses are all necessary for immune defenses against MCMV infection (red lines and text), but access to these functions can be promoted by a number of partly redundant and/or complementary pathways (arrows converging towards a number). IFN-I, IFN-γ and cytotoxic cellular immune responses are critical for control of viral replication, prevention of excessive tissue damage and overall resistance of the host in terms of morbidity and mortality. Several cell types and pathways can lead to IFN-I production. Early after intra-peritoneal injection, MCMV infects stromal cells and other cell types, inducing an IFN-I production by those cells independently of MyD88 and TLRs. In addition, viral particles or material derived from infected cells can be engulfed by cDC and pDC to promote their production of high levels of IFN-I or other cytokines upon triggering of the TLR7/9-to-MyD88 signaling cascade. In our experimental settings, MyD88/TLR9 responses of DC and MyD88-independent responses of infected cells are redundant for the induction of strong IFN-I responses, even though only very low to undetectable levels of IFN-I are produced in the absence of MyD88 responses (❶). In parallel, NK cells and CD8 T cells are able to produce IFN-γ and to specifically recognize and kill MCMV infected cells, via the activation receptor Ly49H or via their TCR, respectively. Cell-mediated immune control of viral replication can thus be performed by both NK and CD8 T cells which can largely compensate one another for this function (❷). However, the antiviral functions of NK and CD8 T cells are not strictly redundant but partly complementary, since, for example, the absence of CD8 T cell responses might increase the risk of selection of viral mutants able to escape NK cell control. MyD88 responses can promote the activation of NK and CD8 T cells via TLR-dependent activation of myeloid cells and/or through cell-intrinsic effects. However, MyD88 responses are not necessary for this function in mice expressing NK cell activation receptors able to directly sense MCMV-infected cells. In these mice, in addition to IFN-I production, MyD88-independent responses from infected cells might provide the other beneficial inflammatory signals necessary for this function (❸). Functional redundancies might also exist between MyD88 responses and NK cell activity for prevention of excessive damage to lymphoid tissues (❹), in order to preserve their micro-anatomical niches supporting the proliferation and survival of CD8 T cells. Our study demonstrates that even undetectably low levels of IFN-I/III are sufficient to induce a strong and widespread expression of genes involved in cell-intrinsic antiviral immunity during a systemic viral infection, including in MyD88-/- mice. This observation is consistent with a recent report showing that strongly increasing IFN-I production by pDC during MCMV infection, while preserving other antiviral immune responses, does not improve control of viral replication [74]. On the contrary, high systemic IFN-I production by pDC might even delay the induction of antiviral adaptive immunity due to negative effects on the DC and CD8 T cell compartments [39, 75]. Hence, delivery of small amounts of IFN-I in a tightly controlled spatio-temporal manner could be a better way to treat certain viral infections or cancers than administration of high systemic doses of cytokines. The latter treatment may not induce better cell-intrinsic immunity but rather induce systemic responses associated with severe side effects [1]. In addition, given current technological limits to cytokine detection, our results suggest that to investigate whether a cytokine plays a physiological role in a disease, one should quantitate the effect of abrogating cytokine-mediated signaling on disease evolution, and functionally assess how perturbing cytokine activity affects disease outcome, rather than only titrating cytokine concentration.
One may wonder why infected hosts produce high systemic levels of IFN-I if very low to undetectable levels of these cytokines are sufficient to induce a strong expression of ISG and their downstream protective antiviral functions. One possibility is that high systemic production of IFN-I by spleen pDC in the absence of protective NK cell responses is necessary to promote defense or homeostasis of distant organs. It was recently published that elevated levels of IFN-I has systemic effects which promote epithelial turnover and wound repair [76]. However, since MyD88-deficient mice harbor only a mild increase in susceptibility to MCMV infection as compared to IFNAR-deficient mice, these types of systemic IFN-I responses might be rather required to prevent concomitant heterologous infections or for defense against viruses able to cause chronic infections, by allowing a systemic state of pathogen alert with induction of protective responses in all tissues. Indeed, a similar role was recently reported for IFN-γ production by tissue-resident memory CD8 T cells, whereby it induced tissue-wide responses able to protect the skin against viral variants able to escape adaptive immunity or against heterologous infections [77, 78].
In d6 infected BALB/c-Ly49H+ MyD88-/- mice, not only Ly49H+ but also Ly49H- NK cells are significantly more activated than in BALB/c-Ly49H+ mice. NK cells are also significantly activated in infected animals deficient in Ly49H and MyD88 signaling. Hence, NK cells do not require direct sensing of infected cells for their activation, even at late time points after infection. Rather Ly49H protects mice against MCMV by allowing cytokine-activated NK cells to specifically recognize and kill infected cells. The mechanisms promoting NK cell activation in MyD88-/- mice merit further study. IL-12 and IL-18 play important roles in this process in wild type animals [35, 36, 47]. The analysis of the expression pattern of target genes of these cytokines in the spleen of infected mice showed that a significant and sustained induction of cytokine responses are still observed in MyD88-/- animals despite a dramatic decrease in the expression of the cytokines themselves. Hence, similarly to IFN-I, IL-12 requires MyD88 for high level production but not for functional induction of NK cell activation.
Our result showed that NK cell sensing and killing of infected cells can compensate MyD88 but not IFNAR deficiency for the control of systemic MCMV infection in BALB/c mice. In contrast, others have reported that IFN-I responses are not required for NK cell activation and antiviral activity in C57BL/6 mice [42, 79]. This might be due in part to differences between C57BL/6 and BALB/c mice. Alternatively, this might be due to differences between virus strains. In the other studies, relatively low inoculum doses of in vitro produced viruses were used. Viruses deriving from the pSM3fr parental strain are highly attenuated in vivo due to a mutation in the viral MCK-2 chemokine [80]. Under these experimental settings, Ly49H+ NK cell responses were shown to completely or moderately compensate deficient IFN-I responses to promote control of viral replication. Further, NK cell activity was reported to be dispensable for late (d7) control of viral replication in C57BL/6 mice competent for IFN-I responses in contrast to our observations using BALB/c-Ly49H+ mice infected with moderate doses of salivary gland virus. The morbidity and mortality associated with the infections was not documented [42, 79]. Hence, it is possible that the previously reported compensation between IFNAR deficiency and NK cell activity resulted in part from the use of highly attenuated conditions of infection allowing clearance of MCMV and survival even in mice deficient for both IFNAR and Ly49H.
In our study, the higher susceptibility to MCMV of BALB/c MyD88-/- mice as compared to BALB/c-Ly49H+ MyD88-/- or BALB/c mice was associated with a dramatic decrease in the number of antiviral and total CD8 T cells in the spleen. BALB/c MyD88-/- mice harbor a striking disruption of their spleen architecture as early as d4 after infection. Microarray analyses showed a strong induction of genes associated with fibrosis and a downregulation of the red pulp macrophage transcriptomic signature at d6 post-infection in the spleen of BALB/c MyD88-/- animals. Hence, in the absence of efficient antiviral NK cell responses, MyD88 responses are required to protect the secondary lymphoid organs from extensive tissue damage. This includes preventing the loss of cell types and micro-anatomical structures necessary to support protective antiviral immune adaptive responses.
In summary, our study shows that MyD88 responses are dispensable for the induction of protective IFN-I responses against a systemic herpesvirus infection in mice. We show that the enhanced susceptibility of MyD88-deficient mice to MCMV infection is not attributable to loss of IFN-I responses, to decreased NK cell activation or to compromised CD8 T cell priming. Rather, it results from severe tissue damage to the spleen leading to a failure to sufficiently amplify the compartment of effector antiviral CD8 T cells. Moreover, we show that direct recognition and sensing of virus-infected cells by NK cells can compensate for MyD88 - but not IFNAR-deficiency and promote resistance to a systemic MCMV infection in our experimental settings. These results challenge our current understanding of the requirements for the induction of protective responses to systemic viral infections in mice, in particular the role of pDC and endosomal TLR7/9 [8, 10–12, 24–27]. These results are consistent with the conclusions drawn from the studies of human patients suffering from primary immunodeficiencies [2, 6–8, 53, 54]. Hence, immune responses might not differ between mice and men as much as is sometimes suggested. Rather, it is important to define experimental settings in mice that best reflect clinical observations in humans, in order to take advantage of this versatile animal model to advance basic immunological knowledge in a way that might benefit human health [81]. Early disruption of lymphoid organ architecture during human immunodeficiency virus infection has been proposed as an important cause for the failure of the host to control viral replication and for disease development [82]. Understanding the mechanisms leading to the disruption of the architecture of lymphoid organs early during viral infections in mice and how MyD88 or NK cell responses can prevent this process could open novel avenues to treat viral infections in humans.
Materials and Methods
Ethics statement
The animal care and use protocols (ID no. 11-09/09/2011) were designed in accordance with national and international laws for laboratory animal welfare and experimentation (EEC Council Directive 2010/63/EU, September 2010), and approved by the Marseille Ethical Committee for Animal Experimentation (registered by the Comité National de Réflexion Ethique sur l’Expérimentation Animale under no. 14).
Mice and in vivo treatments
8 to 12 week old mice of different strains were used for experiments (see supplemental materials and methods in S1 File). Animals were bred under pathogen-free conditions at CIML. Infections were initiated at d0 by i.p. injection of 2.5 x 103 pfu salivary gland-extracted MCMV K181 v70 [23, 49] or Δm157 [45] strains (third and second in vivo passages, respectively), unless specified otherwise. pDC, NK cells or CD8 T cells were depleted in vivo by intraperitoneal delivery of 500μg anti-Bst2 (120G8) mAb, 100μg anti-NK1.1 mAb or 150μg anti-CD8β mAb. Antibodies were injected on d-1 before MCMV infection, followed by injections on d1, 3 and 5 for NK cell and pDC depletion, and on d2 and 7 for CD8 T cell depletion. Control mice were treated with Rat IgG. Cell depletion efficiency was assessed by flow cytometry as detailed in S1 File. Bst2 expression was increased on B cells, NK cells and cDC in infected mice. However, even under these conditions, the levels of Bst2 expressed by these cells were much lower than that observed on pDC. No depletion of B cells, T cells and NK cells was observed in our experimental conditions, although we cannot completely exclude that a very small fraction of some of these cell types was affected due to a higher expression of Bst2, for example plasmocytes. However, the numbers of cDC in infected mice treated with anti-Bst2 antibody was half that of infected animals treated with isotype control (S6 Fig).
Spleen preparation and flow cytometry analysis
Spleen leukocyte suspensions were prepared using DNAse I and collagenase D [23], stained as detailed in S1 File, and acquired on a FACSCanto II Flow Cytometer (BD Bioscience).
mRNA preparation, real-time PCR experiments, quantification of viral titers and microarray experiments
Pieces of spleen were harvested and stabilized overnight in RNAlater solution (Qiagen). High-quality total RNA was prepared and used for qRT-PCR or microarrays as previously described [23]. Relative gene expression was calculated with the ΔΔCt method using Hprt as housekeeping gene for normalization. Viral titers were measured as absolute levels of expression of the Ie1 gene [23]. Microarray analyses were performed as previously described [23]. Gene set compositions are given in S1 Table. Data have been deposited in the GEO database under reference GSE62729.
Quantification of serum IFN-α levels
Serum IFN-α2/α4 levels were determined by ELISA (eBioscience) according to the manufacturer’s instructions.
In vivo cytotoxicity assay
Antigen-specific CD8 T cell–mediated in vivo cytotoxicity was assayed as described [83].
Enumeration of, and effector molecule expression by, antiviral CD8 T cells
Splenic lymphocytes from mice infected with MCMV were isolated at d6 post infection and CD8 T cells were analyzed for H-2L(d)/IE-1(168YPHFMPTNL176) binding and intracellular IFN-γ or Granzyme B expression.
Morbidity and mortality
Infected mice were monitored daily for signs of morbidity (weight loss, piloerection, hunched posture and lethargy). Imminent death was defined as loss of 20% initial body weight or development of severe lethargy (unresponsiveness to touch) established in a preliminary experiment using death as the endpoint.
Histology
Spleen section were prepared, stained and imaged as previously described [28] and detailed in S1 File.
Statistical analyses
Statistical analyses were performed using a nonparametric Mann-Whitney test performed with Prism 6 (GraphPad Software) for all experiments except survival analyses where a Mantel-Cox test was used. ns, non significant (p > 0.05); *p < 0.05; **p < 0.01; ***p < 10–3.
Supporting Information
Zdroje
1. Tomasello E, Pollet E, Vu Manh T-P, Uzé G, Dalod M. Harnessing mechanistic knowledge on beneficial versus deleterious IFN-I effects to design innovative immunotherapies targeting cytokine activity to specific cell types. Frontiers in Immunology. 2014;5.
2. Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, et al. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev. 2008;226 : 29–40. doi: 10.1111/j.1600-065X.2008.00698.x 19161414
3. Mordstein M, Kochs G, Dumoutier L, Renauld JC, Paludan SR, Klucher K, et al. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS pathogens. 2008;4(9):e1000151. doi: 10.1371/journal.ppat.1000151 18787692
4. Pott J, Mahlakoiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, et al. IFN-lambda determines the intestinal epithelial antiviral host defense. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(19):7944–9. doi: 10.1073/pnas.1100552108 21518880
5. Alexandre YO, Cocita CD, Ghilas S, Dalod M. Deciphering the role of DC subsets in MCMV infection to better understand immune protection against viral infections. Frontiers in microbiology. 2014;5 : 378. doi: 10.3389/fmicb.2014.00378 25120535
6. Ku CL, von Bernuth H, Picard C, Zhang SY, Chang HH, Yang K, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. The Journal of experimental medicine. 2007;204(10):2407–22. 17893200
7. von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321(5889):691–6. doi: 10.1126/science.1158298 18669862
8. von Bernuth H, Picard C, Puel A, Casanova JL. Experimental and natural infections in MyD88 - and IRAK-4-deficient mice and humans. European journal of immunology. 2012;42(12):3126–35. doi: 10.1002/eji.201242683 23255009
9. Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–18. doi: 10.1016/j.immuni.2013.11.010 24332029
10. Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, et al. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. The Journal of experimental medicine. 2002;195(4):517–28. 11854364
11. Baranek T, Zucchini N, Dalod M. Plasmacytoid dendritic cells and the control of herpesvirus infections. Viruses. 2009;1(3):383–419. doi: 10.3390/v1030383 21994554
12. Swiecki M, Wang Y, Gilfillan S, Colonna M. Plasmacytoid dendritic cells contribute to systemic but not local antiviral responses to HSV infections. PLoS pathogens. 2013;9(10):e1003728. doi: 10.1371/journal.ppat.1003728 24204273
13. Cervantes-Barragan L, Zust R, Weber F, Spiegel M, Lang KS, Akira S, et al. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109(3):1131–7. 16985170
14. Cervantes-Barragan L, Lewis KL, Firner S, Thiel V, Hugues S, Reith W, et al. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(8):3012–7. doi: 10.1073/pnas.1117359109 22315415
15. Blasius AL, Krebs P, Sullivan BM, Oldstone MB, Popkin DL. Slc15a4, a gene required for pDC sensing of TLR ligands, is required to control persistent viral infection. PLoS pathogens. 2012;8(9):e1002915. doi: 10.1371/journal.ppat.1002915 23028315
16. Macal M, Lewis GM, Kunz S, Flavell R, Harker JA, Zuniga EI. Plasmacytoid dendritic cells are productively infected and activated through TLR-7 early after arenavirus infection. Cell host & microbe. 2012;11(6):617–30.
17. Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 2003;5(13):1263–77. 14623023
18. Strobl B, Bubic I, Bruns U, Steinborn R, Lajko R, Kolbe T, et al. Novel functions of tyrosine kinase 2 in the antiviral defense against murine cytomegalovirus. Journal of immunology. 2005;175(6):4000–8. 16148148
19. Kropp KA, Robertson KA, Sing G, Rodriguez-Martin S, Blanc M, Lacaze P, et al. Reversible inhibition of murine cytomegalovirus replication by gamma interferon (IFN-gamma) in primary macrophages involves a primed type I IFN-signaling subnetwork for full establishment of an immediate-early antiviral state. Journal of virology. 2011;85(19):10286–99. doi: 10.1128/JVI.00373-11 21775459
20. Blanc M, Hsieh WY, Robertson KA, Watterson S, Shui G, Lacaze P, et al. Host defense against viral infection involves interferon mediated down-regulation of sterol biosynthesis. PLoS Biol. 2011;9(3):e1000598. doi: 10.1371/journal.pbio.1000598 21408089
21. Blanc M, Hsieh WY, Robertson KA, Kropp KA, Forster T, Shui G, et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity. 2013;38(1):106–18. doi: 10.1016/j.immuni.2012.11.004 23273843
22. Presti RM, Pollock JL, Dal Canto AJ, O'Guin AK, Virgin HWt. Interferon gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. The Journal of experimental medicine. 1998;188(3):577–88. 9687534
23. Baranek T, Manh TP, Alexandre Y, Maqbool MA, Cabeza JZ, Tomasello E, et al. Differential responses of immune cells to type I interferon contribute to host resistance to viral infection. Cell host & microbe. 2012;12(4):571–84.
24. Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21(1):107–19. 15345224
25. Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(10):3516–21. 14993594
26. Delale T, Paquin A, Asselin-Paturel C, Dalod M, Brizard G, Bates EE, et al. MyD88-dependent and-independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. Journal of immunology. 2005;175(10):6723–32. 16272328
27. Zucchini N, Bessou G, Traub S, Robbins SH, Uematsu S, Akira S, et al. Cutting edge: Overlapping functions of TLR7 and TLR9 for innate defense against a herpesvirus infection. Journal of immunology. 2008;180(9):5799–803. 18424698
28. Zucchini N, Bessou G, Robbins SH, Chasson L, Raper A, Crocker PR, et al. Individual plasmacytoid dendritic cells are major contributors to the production of multiple innate cytokines in an organ-specific manner during viral infection. International immunology. 2008;20(1):45–56. 18000008
29. Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33(6):955–66. doi: 10.1016/j.immuni.2010.11.020 21130004
30. Schneider K, Loewendorf A, De Trez C, Fulton J, Rhode A, Shumway H, et al. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell host & microbe. 2008;3(2):67–76.
31. Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, et al. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292(5518):934–7. 11340207
32. Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, et al. Cutting edge: Priming of NK cells by IL-18. Journal of immunology. 2008;181(3):1627–31. 18641298
33. French AR, Sjolin H, Kim S, Koka R, Yang L, Young DA, et al. DAP12 signaling directly augments proproliferative cytokine stimulation of NK cells during viral infections. Journal of immunology. 2006;177(8):4981–90. 17015680
34. Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4(2):175–81. 12496964
35. Madera S, Sun JC. Cutting edge: stage-specific requirement of IL-18 for antiviral NK cell expansion. Journal of immunology. 2015;194(4):1408–12. doi: 10.4049/jimmunol.1402001 25589075
36. Pien GC, Satoskar AR, Takeda K, Akira S, Biron CA. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-gamma responses during viral infection. Journal of immunology. 2000;165(9):4787–91.
37. Pang IK, Ichinohe T, Iwasaki A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8(+) T cell responses to influenza A virus. Nat Immunol. 2013;14(3):246–53. doi: 10.1038/ni.2514 23314004
38. Beutler B, Eidenschenk C, Crozat K, Imler JL, Takeuchi O, Hoffmann JA, et al. Genetic analysis of resistance to viral infection. Nat Rev Immunol. 2007;7(10):753–66. 17893693
39. Robbins SH, Bessou G, Cornillon A, Zucchini N, Rupp B, Ruzsics Z, et al. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS pathogens. 2007;3(8):e123. 17722980
40. Andrews DM, Estcourt MJ, Andoniou CE, Wikstrom ME, Khong A, Voigt V, et al. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. The Journal of experimental medicine. 2010;207(6):1333–43. doi: 10.1084/jem.20091193 20513749
41. Stadnisky MD, Xie X, Coats ER, Bullock TN, Brown MG. Self MHC class I-licensed NK cells enhance adaptive CD8 T-cell viral immunity. Blood. 2011;117(19):5133–41. doi: 10.1182/blood-2010-12-324632 21436069
42. Mitrovic M, Arapovic J, Traven L, Krmpotic A, Jonjic S. Innate immunity regulates adaptive immune response: lessons learned from studying the interplay between NK and CD8+ T cells during MCMV infection. Medical microbiology and immunology. 2012;201(4):487–95. doi: 10.1007/s00430-012-0263-0 22965169
43. Scalzo AA, Brown MG, Chu DT, Heusel JW, Yokoyama WM, Forbes CA. Development of intra-natural killer complex (NKC) recombinant and congenic mouse strains for mapping and functional analysis of NK cell regulatory loci. Immunogenetics. 1999;49(3):238–41. 9914339
44. Bubic I, Wagner M, Krmpotic A, Saulig T, Kim S, Yokoyama WM, et al. Gain of virulence caused by loss of a gene in murine cytomegalovirus. Journal of virology. 2004;78(14):7536–44. 15220428
45. Handke W, Luig C, Popovic B, Krmpotic A, Jonjic S, Brune W. Viral inhibition of BAK promotes murine cytomegalovirus dissemination to salivary glands. Journal of virology. 2013;87(6):3592–6. doi: 10.1128/JVI.02657-12 23302869
46. Kumagai Y, Takeuchi O, Kato H, Kumar H, Matsui K, Morii E, et al. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007;27(2):240–52. 17723216
47. Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, et al. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. Journal of immunology. 2002;169(8):4279–87. 12370359
48. Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. The Journal of experimental medicine. 2012;209(5):947–54. doi: 10.1084/jem.20111760 22493516
49. Livingston-Rosanoff D, Daley-Bauer LP, Garcia A, McCormick AL, Huang J, Mocarski ES. Antiviral T cell response triggers cytomegalovirus hepatitis in mice. Journal of virology. 2012;86(23):12879–90. doi: 10.1128/JVI.01752-12 22993151
50. Li Q, Skinner PJ, Ha SJ, Duan L, Mattila TL, Hage A, et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009;323(5922):1726–9. doi: 10.1126/science.1168676 19325114
51. Jordan S, Ruzsics Z, Mitrovic M, Baranek T, Arapovic J, Krmpotic A, et al. Natural killer cells are required for extramedullary hematopoiesis following murine cytomegalovirus infection. Cell host & microbe. 2013;13(5):535–45.
52. Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264(5167):1918–21. 8009221
53. Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nature genetics. 2003;33(3):388–91. 12590259
54. Hambleton S, Goodbourn S, Young DF, Dickinson P, Mohamad SM, Valappil M, et al. STAT2 deficiency and susceptibility to viral illness in humans. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(8):3053–8. doi: 10.1073/pnas.1220098110 23391734
55. French AR, Pingel JT, Wagner M, Bubic I, Yang L, Kim S, et al. Escape of mutant double-stranded DNA virus from innate immune control. Immunity. 2004;20(6):747–56. 15189739
56. French AR, Pingel JT, Kim S, Yang L, Yokoyama WM. Rapid emergence of escape mutants following infection with murine cytomegalovirus in immunodeficient mice. Clin Immunol. 2005;115(1):61–9. 15870022
57. Reddehase MJ, Mutter W, Munch K, Buhring HJ, Koszinowski UH. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. Journal of virology. 1987;61(10):3102–8. 3041033
58. Pahl-Seibert MF, Juelch M, Podlech J, Thomas D, Deegen P, Reddehase MJ, et al. Highly protective in vivo function of cytomegalovirus IE1 epitope-specific memory CD8 T cells purified by T-cell receptor-based cell sorting. Journal of virology. 2005;79(9):5400–13. 15827154
59. Tay CH, Welsh RM. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. Journal of virology. 1997;71(1):267–75. 8985346
60. Loh J, Chu DT, O'Guin AK, Yokoyama WM, Virgin HWt. Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. Journal of virology. 2005;79(1):661–7. 15596864
61. van Dommelen SL, Sumaria N, Schreiber RD, Scalzo AA, Smyth MJ, Degli-Esposti MA. Perforin and granzymes have distinct roles in defensive immunity and immunopathology. Immunity. 2006;25(5):835–48. 17088087
62. Sumaria N, van Dommelen SL, Andoniou CE, Smyth MJ, Scalzo AA, Degli-Esposti MA. The roles of interferon-gamma and perforin in antiviral immunity in mice that differ in genetically determined NK-cell-mediated antiviral activity. Immunol Cell Biol. 2009;87(7):559–66. doi: 10.1038/icb.2009.41 19564888
63. Polic B, Hengel H, Krmpotic A, Trgovcich J, Pavic I, Luccaronin P, et al. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. The Journal of experimental medicine. 1998;188(6):1047–54. 9743523
64. van Dommelen SL, Tabarias HA, Smyth MJ, Degli-Esposti MA. Activation of natural killer (NK) T cells during murine cytomegalovirus infection enhances the antiviral response mediated by NK cells. Journal of virology. 2003;77(3):1877–84. 12525622
65. Wesley JD, Tessmer MS, Chaukos D, Brossay L. NK cell-like behavior of Valpha14i NK T cells during MCMV infection. PLoS pathogens. 2008;4(7):e1000106. doi: 10.1371/journal.ppat.1000106 18636102
66. Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. Journal of immunology. 2008;181(7):4452–6. 18802047
67. Reilly EC, Thompson EA, Aspeslagh S, Wands JR, Elewaut D, Brossay L. Activated iNKT cells promote memory CD8+ T cell differentiation during viral infection. PloS one. 2012;7(5):e37991. doi: 10.1371/journal.pone.0037991 22649570
68. Tyznik AJ, Verma S, Wang Q, Kronenberg M, Benedict CA. Distinct requirements for activation of NKT and NK cells during viral infection. Journal of immunology. 2014;192(8):3676–85. doi: 10.4049/jimmunol.1300837 24634489
69. Khairallah C, Netzer S, Villacreces A, Juzan M, Rousseau B, Dulanto S, et al. gammadelta T Cells Confer Protection against Murine Cytomegalovirus (MCMV). PLoS pathogens. 2015;11(3):e1004702. doi: 10.1371/journal.ppat.1004702 25747674
70. Sell S, Dietz M, Schneider A, Holtappels R, Mach M, Winkler TH. Control of murine cytomegalovirus infection by gammadelta T cells. PLoS pathogens. 2015;11(2):e1004481. doi: 10.1371/journal.ppat.1004481 25658831
71. Lafarge X, Merville P, Cazin MC, Berge F, Potaux L, Moreau JF, et al. Cytomegalovirus infection in transplant recipients resolves when circulating gammadelta T lymphocytes expand, suggesting a protective antiviral role. The Journal of infectious diseases. 2001;184(5):533–41. 11494158
72. Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon ME, et al. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood. 2008;112(4):1317–24. doi: 10.1182/blood-2008-01-136713 18539896
73. Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annual review of immunology. 2013;31 : 163–94. doi: 10.1146/annurev-immunol-032712-100001 23298212
74. Puttur F, Arnold-Schrauf C, Lahl K, Solmaz G, Lindenberg M, Mayer CT, et al. Absence of Siglec-H in MCMV infection elevates interferon alpha production but does not enhance viral clearance. PLoS pathogens. 2013;9(9):e1003648. doi: 10.1371/journal.ppat.1003648 24086137
75. Wikstrom ME, Fleming P, Comerford I, McColl SR, Andoniou CE, Degli-Esposti MA. A chemokine-like viral protein enhances alpha interferon production by plasmacytoid dendritic cells but delays CD8+ T cell activation and impairs viral clearance. Journal of virology. 2013;87(14):7911–20. doi: 10.1128/JVI.00187-13 23658453
76. Sun L, Miyoshi H, Origanti S, Nice TJ, Barger AC, Manieri NA, et al. Type I interferons link viral infection to enhanced epithelial turnover and repair. Cell host & microbe. 2015;17(1):85–97.
77. Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi: 10.1126/science.1254536 25170049
78. Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, et al. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346(6205):101–5. doi: 10.1126/science.1254803 25278612
79. Geurs TL, Zhao YM, Hill EB, French AR. Ly49H engagement compensates for the absence of type I interferon signaling in stimulating NK cell proliferation during murine cytomegalovirus infection. Journal of immunology. 2009;183(9):5830–6. doi: 10.4049/jimmunol.0901520 19828630
80. Jordan S, Krause J, Prager A, Mitrovic M, Jonjic S, Koszinowski UH, et al. Virus progeny of murine cytomegalovirus bacterial artificial chromosome pSM3fr show reduced growth in salivary Glands due to a fixed mutation of MCK-2. Journal of virology. 2011;85(19):10346–53. doi: 10.1128/JVI.00545-11 21813614
81. O'Garra A. Systems Approach to Understand the Immune Response in Tuberculosis: An Iterative Process between Mouse Models and Human Disease. Cold Spring Harb Symp Quant Biol. 2013;78 : 173–7. doi: 10.1101/sqb.2013.78.020172 24100583
82. Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33(6):306–14. doi: 10.1016/j.it.2012.04.002 22613276
83. Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. Journal of immunology. 2003;171(1):27–31.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR SelectionČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



