-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaExpression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
The events leading to a successful encapsulation of parasitoid wasp eggs in the larvae of the fruit fly Drosophila melanogaster are insufficiently understood. The formation of a capsule seals off the wasp egg, and this process is often functionally compared to the formation of granulomas in vertebrates. Like granuloma formation in humans, the encapsulation process in fruit flies requires the activation, mobilization, proliferation and differentiation of different blood cell types. Here, we have studied the role of Edin (elevated during infection) in the immune defense against the parasitoid wasp Leptopilina boulardi in Drosophila larvae. We demonstrate that edin expression in the fat body (an immune-responsive organ in Drosophila functionally resembling the mammalian liver) is required for a normal defense against wasp eggs. Edin is required for the release of blood cells from larval tissues and for the subsequent increase in circulating blood cell numbers. Our results provide new knowledge of how the encapsulation process is regulated in Drosophila, and how blood cells are activated upon wasp parasitism. Understanding of the encapsulation process in invertebrates may eventually lead to a better knowledge of the pathophysiology of granuloma formation in human diseases, such as tuberculosis.
Published in the journal: . PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004895
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004895Summary
The events leading to a successful encapsulation of parasitoid wasp eggs in the larvae of the fruit fly Drosophila melanogaster are insufficiently understood. The formation of a capsule seals off the wasp egg, and this process is often functionally compared to the formation of granulomas in vertebrates. Like granuloma formation in humans, the encapsulation process in fruit flies requires the activation, mobilization, proliferation and differentiation of different blood cell types. Here, we have studied the role of Edin (elevated during infection) in the immune defense against the parasitoid wasp Leptopilina boulardi in Drosophila larvae. We demonstrate that edin expression in the fat body (an immune-responsive organ in Drosophila functionally resembling the mammalian liver) is required for a normal defense against wasp eggs. Edin is required for the release of blood cells from larval tissues and for the subsequent increase in circulating blood cell numbers. Our results provide new knowledge of how the encapsulation process is regulated in Drosophila, and how blood cells are activated upon wasp parasitism. Understanding of the encapsulation process in invertebrates may eventually lead to a better knowledge of the pathophysiology of granuloma formation in human diseases, such as tuberculosis.
Introduction
Parasitoid wasps are natural enemies of insects such as the fruit fly Drosophila melanogaster. In the course of a successful wasp infection, a female wasp lays an egg in a fruit fly larva and the wasp larva hatches. Thereafter, the wasp larva develops inside the Drosophila larva using the host tissue as a source of nutrition to ultimately emerge as an adult wasp, unless the wasp larva is eliminated by the host’s immune response [1].
The initial oviposition of a wasp egg triggers changes in gene expression in the fruit fly and activates both humoral and cellular defense mechanisms [2–4]. The role of the humoral defense, i.e. the production of antimicrobial peptides by the fat body, via the Imd and Toll pathways in response to a microbial challenge, is well characterized in response to microbial challenge (reviewed in [5, 6]). However, in the context of wasp parasitism, cellular immunity is more striking than the humoral response. The cellular immune responses are mediated by three types of blood cells, or hemocytes: plasmatocytes, lamellocytes and crystal cells (reviewed for example in [7, 8]). The round and small plasmatocytes are the most abundant type tallying up to 95% of all of the larval hemocytes. Plasmatocytes are responsible for phagocytosing invading microorganisms and apoptotic particles and are also required for a normal resistance against bacteria [9–12]. Crystal cells comprise around 5% of all hemocytes and they contain phenoloxidase-containing crystals that are released in the melanization response [13]. Lamellocytes, on the other hand, are solely found in larvae and are rarely present in individuals that are not immune-challenged. The main task of lamellocytes is to participate in encapsulating objects that are too large to be phagocytosed, such as the eggs of parasitoids wasps. However, the encapsulation of wasp eggs requires the concerted action of all three types of hemocytes [7].
Upon a wasp infection, the presence of a wasp egg is first recognized. Plasmatocytes are the first cells that adhere to the wasp egg and they spread around the surface of the egg forming the first layer of the capsule [14]. A wasp infection also leads to the differentiation of a large number of lamellocytes [15–17], which migrate towards the wasp egg and attach onto the plasmatocyte-covered egg. During a successful immune response lamellocytes, together with plasmatocytes, form a multilayered capsule that surrounds the wasp egg. The capsule is melanized, phenol oxidases and reactive oxygen species are released within the capsule [18], and the wasp is ultimately killed.
Although many pathways, such as the Toll and JAK/STAT pathway, have been shown to have a role in the encapsulation response [3], the phenomenon is still insufficiently understood. In this current study, we investigate the role of Edin (elevated during infection) in a wasp infection. Edin is a small peptide that is secreted into the hemolymph upon infection [19, 20], and it is required for the immune response against Listeria monocytogenes [21]. Earlier, we have shown that the expression of edin is induced after a bacterial infection, and it has a minor role in the resistance against Enterococcus faecalis [20]. In this study, we investigated whether edin expression is induced by a wasp infection using the Leptopilina boulardi strain G486. We also examined the role of Edin in the encapsulation response and in the activation and formation of hemocytes upon a wasp infection. We report that edin expression is required in the fat body upon a wasp infection in order to mount an effective encapsulation response, and that knocking down edin in the fat body causes defects in hemocyte mobilization in Drosophila larvae.
Results
Edin is induced upon a wasp infection
We have previously shown that edin is induced both in vitro and in vivo upon a microbial infection, but were unable to find any essential role for Edin in this context [20]. To test whether a wasp infection induces the expression of edin, we infected Canton S larvae with the parasitoid wasp Leptopilina boulardi strain G486, and determined the expression levels of edin in whole larvae three hours after infection using qRT-PCR. As is seen in Fig 1A, the wasp infection led to a 7-fold induction in the expression levels of edin compared to uninfected larvae. Because the fat body is the main immune-responsive organ in the fruit fly, we next looked at edin mRNA levels in the fat bodies of wasp-infected larvae 24 hours post-infection. As is shown in Fig 1B, the expression of edin was more highly induced in the fat bodies of the wasp-infected larvae than in whole larvae (80-fold induction). Our results indicate that edin is upregulated after a wasp infection in larvae and that the fat body is a main source for its expression.
Fig. 1. Edin expression is induced upon a wasp infection. 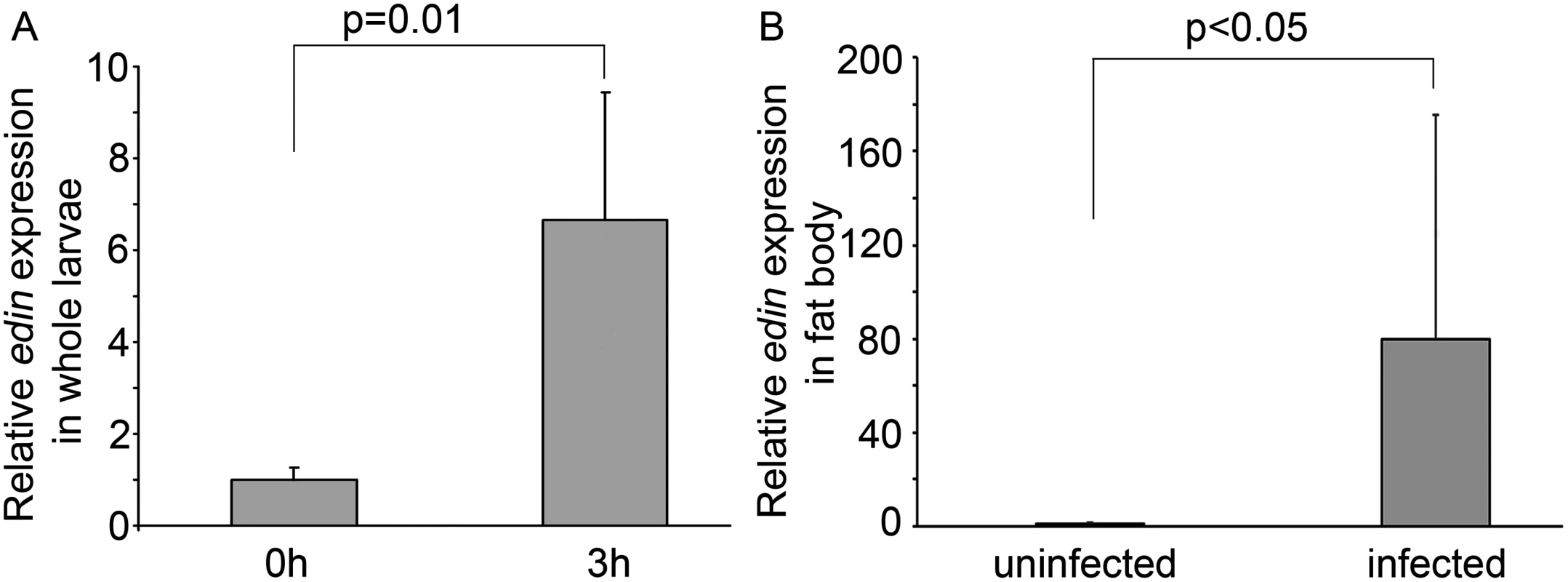
(A) Wasp infection causes a 6.7-fold increase in edin expression in 2nd instar Canton S larvae. Data are pooled from two independent experiments, n = 2 for each experiment, where one sample represents 10 larvae. (B) Edin expression is induced in the fat bodies of Canton S larvae 24 hours post infection. The data are pooled from four independent experiments, and each experiment consisted of two samples, where one sample represents 8–10 larval fat bodies. Edin expression in the fat body is required for the normal encapsulation of wasp eggs
Fruit fly larvae can mount an effective immune response against invading parasitoids by encapsulating the wasp egg. To address the functional significance of edin expression for the encapsulation process upon an L. boulardi infection, we used the UAS-GAL4 system to knock down edin expression. The normal response against the wasp egg is the formation of a visible melanized capsule around the parasitoid egg, and in our hands, 45–66% of control larvae had a melanized capsule. First, we crossed edin14289 RNAi flies (#14289, hereafter referred to as edin14289) with flies carrying the C564-GAL4 driver, which is expressed in many organs, including the fat body, salivary glands and lymph glands [22], and looked for the presence of melanized capsules 27–29 hours after the wasp parasitization (Fig 2A). Parasitized w1118 controls showed an encapsulation rate of 47%. Similarly, w1118 crossed with C564-GAL4 or edin14289 showed encapsulation rates of 52% and 53%, respectively, while only 15% of edin14289 crossed with C564-GAL4 showed melanized capsules. To ensure that the observed phenotype was caused by reduced edin expression, we analyzed the encapsulation response of another edin RNAi line (#109528, hereafter referred to as edin109528). Similarly to the edin14289 line, edin109528 crossed with the driver line showed a clearly decreased encapsulation efficiency of 7% (Fig 2A), when compared to edin109528 crossed with w1118.
Fig. 2. Knock down of edin in the fat body decreases the encapsulation and killing ability of Drosophila larvae. 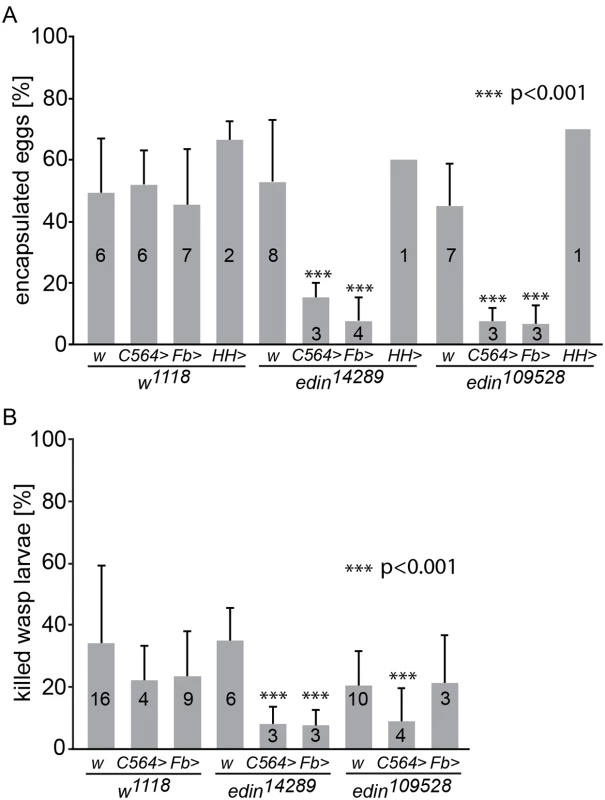
(A) The encapsulation response of two different edin RNAi lines (edin14289 and edin109528) was analyzed 27-29h after a wasp infection. The C564-GAL4 (C564>), Fb-GAL4 (Fb>) and HmlΔ;He-GAL4 (HH>) drivers were used to drive the expression of the RNAi constructs. w1118 (w) was used as control. Data were pooled from one to eight individual experiments, as depicted on each column, each experiment with at least 50 analyzed individual infected larvae. (B) The ability of Drosophila larvae to kill wasp eggs was assessed with two different edin RNAi lines (edin14289 and edin109528) 48-50h after infection. The C564-GAL4 (C564>) and Fb-GAL4 (Fb>) drivers were used to drive the expression of the RNAi constructs. w1118 (w) was used as control. Data are pooled from three to sixteen independent experiments, as indicated on each column, and at least 50 infected larvae were scored per experiment. Error bars in A and B show standard deviations. Knocking down the expression of edin in several tissues including the fat body or in the fat body alone caused a significant decrease in the encapsulation activity and killing response of Drosophila larvae compared to controls, whereas knocking down edin in hemocytes had no effect. We next used a fat body-specific driver to examine specifically whether the lowered encapsulation response was due to the role of edin in the fat body. We crossed both the edin14289 and edin109528 RNAi lines with the Fb-GAL4 driver line and examined the encapsulation response of the offspring. Fb-GAL4 crossed with w1118 showed encapsulation levels of 45% (Fig 2A), whereas edin RNAi flies crossed with Fb-GAL4 showed an encapsulation activity of only 8% (edin14289) and 7% (edin109528). In addition, similar results were also obtained with another fat body-specific driver, Lsp2-GAL4 (edin109528, S1 Fig).
We also analyzed the encapsulation activity of edin RNAi larvae crossed with the pan-hemocyte driver HmlΔ;He-GAL4 and were not able to see any effect with either of the RNAi lines (60% and 70% encapsulation, Fig 2A). Together, these data suggest that Edin is required for a normal encapsulation response after parasitization, and that its expression is required in the larval fat body but not in the hemocytes.
Edin expression is required for the resistance against wasp parasitism in Drosophila larvae
Scoring for the ability of the fly larva to melanize the wasp egg does not indicate whether the fruit fly larva is actually able to overcome the parasitization. Therefore, we replicated the experimental setting in Fig 2A, but scored for the presence of living or dead wasp larvae 48–50 hours post infection. The parasite was scored as killed by the fruit fly larva if a melanized wasp egg was found in the hemocoel in the absence of a living wasp larva. As is seen in Fig 2B, the percentage of dead wasps in control larvae varied between 20–34%. When edin14289 RNAi was induced with either the C564-GAL4 or Fb-Gal4 driver, the percentage of dead wasps was significantly reduced (8% in both cases). A significant decrease was also observed with the combination of the edin109528 RNAi line and the C564-GAL4 driver (9% killing rate). These results, together with the encapsulation phenotype, indicate that edin is required for the resistance against wasp parasitism in Drosophila larvae.
Edin expression is not required for lamellocyte differentiation in Drosophila larvae upon L. boulardi parasitism
Lamellocytes have a central role in the resistance against L. boulardi parasitism. They are not found in the hemocoel of healthy, unchallenged Drosophila larvae, but they are formed in response to a wasp infection [15–17]. To investigate whether the expression of edin in the fat body is required for lamellocyte formation, we bled hemocytes of wasp-challenged larvae 48–50 hours after infection. Plasmatocytes and lamellocytes were visualized using the eaterGFP (green) and msnCherry (red) reporters, respectively. As is shown in Fig 3A and 3B, all of the hemocytes in the unchallenged larvae express the eaterGFP reporter and are msnCherry-negative, indicating that only plasmatocytes are present. Lamellocytes are msnCherry-positive, large, and flat cells. They are present only in the infected larvae (Fig 3A’ and 3B’) and are found both in RNAi treated and control larvae, indicating that edin expression in the fat body is not required for lamellocyte formation upon a wasp infection (Fig 3B’). It is noteworthy that the infected larvae contain cells that express both eaterGFP and msnCherry reporters, showing that some of the cells are undergoing plasmatocyte to lamellocyte transition and are not yet fully differentiated lamellocytes (Fig 3A’ and S2 Fig).
Fig. 3. Quantification of hemocytes in edin RNAi larvae after a wasp infection. 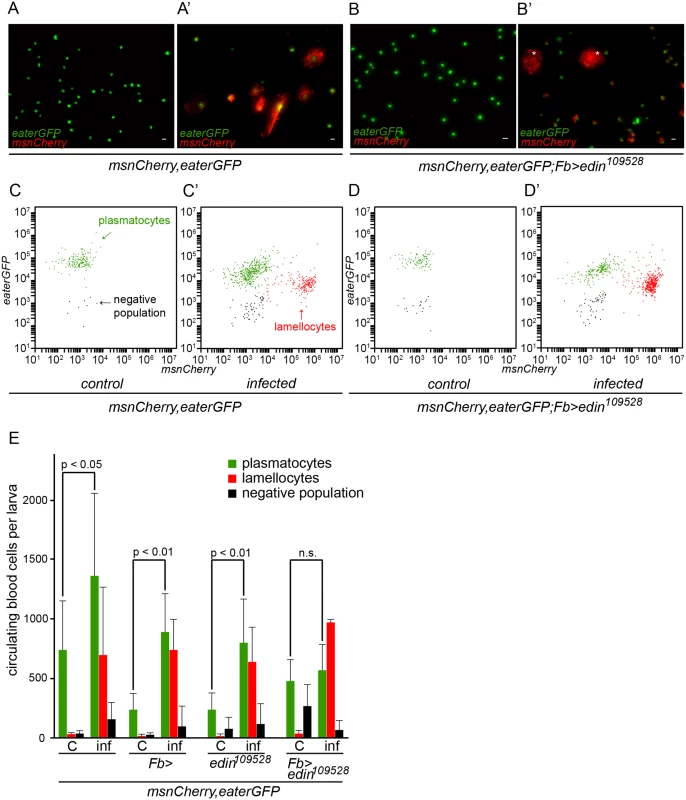
(A-B) Hemocytes of infected larvae were bled 48–50 hours post-infection and visualized with the eaterGFP (green) and msnCherry (red) reporters. Uninfected controls contained only GFP-positive cells that corresponded to plasmatocytes (green). (A’ and B’) msnCherry expression was detected in the infected samples and this included lamellocytes (asterisks) and cells that express both eaterGFP and msnCherry indicating that they were undergoing lamellocyte transition. Lamellocytes were present also in the infected edin RNAi larvae suggesting that edin expression is not necessary for lamellocyte differentiation. Scale bars are 10 μm (C-E) Flow cytometry was carried out to quantify the amount of hemocytes in the unchallenged and the wasp infected edin RNAi larvae. (C = control, inf = infected) In order to obtain additional information about the role of Edin after wasp infection, we used flow cytometry and the msnCherry,eaterGFP reporter to analyze hemocytes of larvae, where edin was knocked down in the fat body. Fig 3C–3D’ show representative scatter plots of hemocytes of uninfected and infected larvae with edin RNAi in the fat body as well as age-matched uninfected and infected control larvae at the 27–29 hour time point. Lamellocytes were induced in spite of edin depletion in the fat body. When comparing hemocyte numbers of uninfected and infected control larvae and edin RNAi larvae, we found that although lamellocyte numbers of infected animals did not differ (p = 0.061, Fig 3E), the plasmatocyte numbers generally increased approximately two to three fold after infection in controls but remained constant in edin knock-down larvae (Fig 3E). Taken together, Edin was dispensable for lamellocyte formation but seemed to be necessary to increase plasmatocyte numbers after a wasp infection.
Edin expression in fat body is not necessary for plasmatocyte spreading and adhesion
In order to properly encapsulate wasp eggs, blood cells must adhere and spread on the egg surface until the egg is finally encapsulated. The Rac GTPase Rac2 regulates the actin cytoskeleton that mediates the spreading of plasmatocytes on the wasp egg [23]. To ensure that the defect in encapsulation is not caused by a defective plasmatocyte function, we tested whether plasmatocytes adhere and spread normally on glass slides and on wasp eggs. In our experimental setting, lamellocytes appear 20 hours after parasitization. To get only plasmatocytes, we bled larvae 14 hours after wasp infection and stained the microtubules and the actin cytoskeleton (Fig 4A and 4B”). We measured the tubulin to actin ratio from approximately 120 hemocytes of larvae with edin RNAi in fat body and control larvae, and found no significant difference in the spreading behavior (control: tubulin/actin = 0.46, standard deviation = 0.18; edin RNAi: tubulin/actin = 0.42, standard deviation = 0.21; p = n.s., S1 Table). Another way of looking at spreading behavior is assaying the distribution of the NimC1 protein that is specific for plasmatocytes. The NimC1 protein forms a cytoplasmic ring in control cells, whereas it accumulates in the center of the cell in Rac2 mutants [23]. NimC1 antibody staining of plasmatocytes on the wasp egg 14 hours after parasitization of edin RNAi larvae was indistinguishable from controls (Fig 4C and 4D) indicating normal adhesion and spreading of plasmatocytes in vivo.
Fig. 4. Edin expression in fat body is dispensable for normal hemocyte attachment to and spreading on glass and wasp eggs, but is necessary to increase blood cell numbers in circulation early after wasp infection. 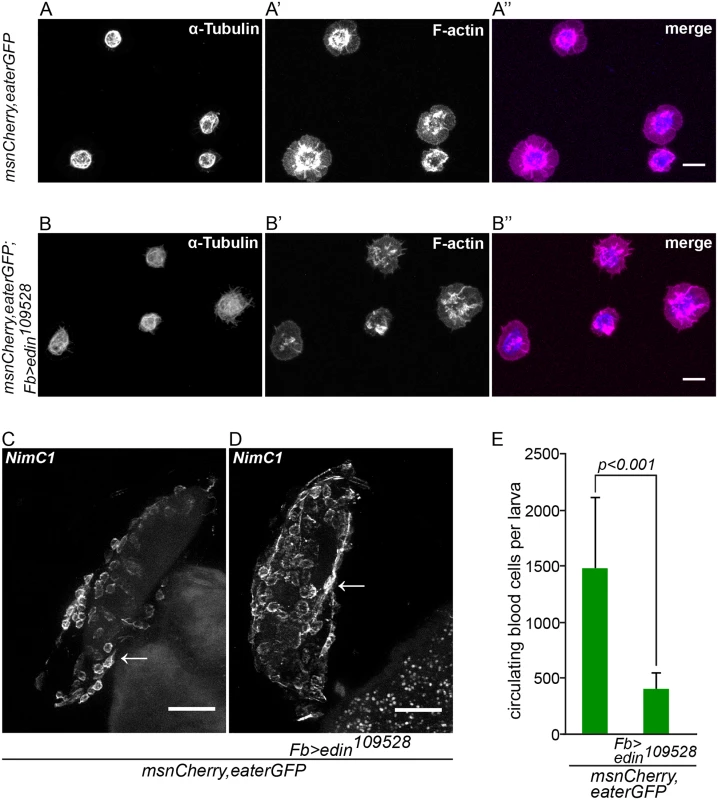
(A-B”) Hemocytes from infected control larvae (msnCherry,eaterGFP, A-A”) and from infected larvae in which edin was knocked down in the fat body (msnCherry,eaterGFP;Fb>edin109528, B-B”) spread normally on glass 14 hours after wasp infection despite knock down of edin in fat body. The spreading ability of hemocytes was assayed by staining α-Tubulin (blue) and F-actin (magenta). The size bar denotes 10 μm. (C and D). Wasp eggs from infected control larvae (msnCherry,eaterGFP, C) and from infected larvae in which edin was knocked down in the fat body (msnCherry,eaterGFP;Fb>edin109528, D) were stained with the anti-plasmatocyte antibody NimC1. The wasp eggs were dissected 14 hours after parasitization and are still attached to the gut. Plasmatocytes spread normally on the eggs irrespective of edin RNAi in the fat body. Arrows denote examples of plasmatocytes spreading and adhering normally on the surface of the wasp egg. The scale bar depicts 50 μm. (E) Edin RNAi in the fat body (msnCherry,eaterGFP;Fb>edin109528) reduced the number of circulating cells after wasp infection in comparison to control larvae (msnCherry,eaterGFP) 14 hours after infection. Circulating blood cell numbers were obtained with flow cytometry. Edin expression in the fat body is required for the increase of plasmatocyte numbers in circulation after a wasp infection
The defining early events of capsule formation are the recognition of the wasp egg by plasmatocytes [14] and a significant increase of hemocytes in circulation. [24]. To study whether edin expression is required to increase plasmatocyte numbers in the early stages of an infection, we counted plasmatocytes 14 hours after wasp infection using flow cytometry. As is shown in Fig 4E, edin RNAi in the fat body resulted in more than three times fewer cells compared to controls (p<0.001). Taken together, Edin is dispensable for lamellocyte formation but it is necessary to increase plasmatocyte numbers in circulation in the early stages of a wasp infection.
Knocking down edin in the fat body causes an altered hemocyte phenotype in wasp-infected larvae
Sessile plasmatocytes reside attached to the skin of Drosophila larvae and form a hematopoietic compartment that releases blood cells in response to a wasp infection [25, 26]. In order to see if the decreased numbers of plasmatocytes were due to a defect in releasing the sessile plasmatocytes into circulation, we imaged the Fb-GAL4-driven edin RNAi larvae and the respective control crosses 27–29 hours after the wasp parasitization, and again used the msnCherry,eaterGFP reporter line to allow the visualization of plasmatocytes (green) and lamellocytes (red). In the uninfected controls (Fig 5A–5D, top row), the banded pattern of plasmatocytes and the lymph gland could been seen. The bands represented plasmatocytes that resided in the sessile compartment in the absence of an immune stimulus. When the larvae were infected by wasps, the green banded pattern disappeared (Fig 5E–5G) and lamellocytes appeared in the hemolymph (Fig 5E’–5G’). This was due to the activation of the hemocytes in the sessile compartment in response to the wasp infection, which causes the cells to leave the compartment and enter the circulation, where many differentiate into lamellocytes [25, 26]. Consistent with our flow cytometry data (Fig 3), when edin was knocked down in the fat body, lamellocytes still appeared in the circulation showing that Edin did not affect the formation of lamellocytes (Fig 5H’). However, in the edin knockdown larvae the banded pattern of plasmatocytes was not disrupted as in the controls (Fig 5H and 5H’). Of note, overexpression of edin in the fat body did not disrupt the banded pattern indicating that the overexpression of edin alone was not sufficient for releasing the sessile hemocytes into the circulation (S3 Fig). In conclusion, our data suggest that edin expression in the fat body affects plasmatocyte activation and release from the sessile compartment. This suggests that the silencing of edin results in a compromised response to L. boulardi parasitism in the early stages of the infection, and that the altered resistance is due to insufficient plasmatocyte numbers in circulation.
Fig. 5. Edin expression in the fat body is required for the activation of plasmatocytes upon a wasp attack 27–29 hours after infection. 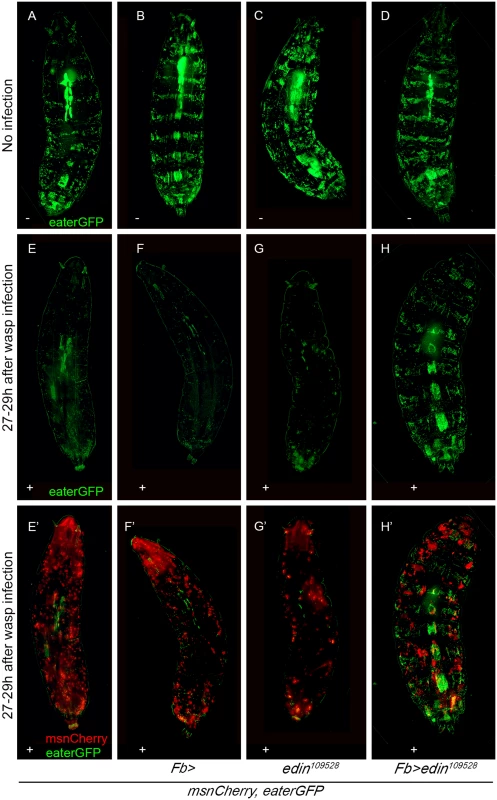
The in vivo phenotype of wasp infected edin RNAi larvae was studied using the eaterGFP (green = plasmatocytes) and mCherry (red = lamellocytes) reporters. Imaging was performed 27–29 hours post infection with living Drosophila larvae. (A-D) Uninfected larvae show an uninterrupted banding pattern formed by sessile plasmatocytes (green). (E-H) Shows only the green channel (eaterGFP) of infected larvae and (E’-H’) both the green and the red (msnCherry) channel. Infected larvae have lost the banding pattern and lamellocytes have appeared, but infected msnCherry,eaterGFP;Fb>edin109528 larvae still show a visible banding pattern formed by the sessile cells.– = uninfected larvae, + = wasp infected larvae. Fig 5 shows representative images of at least 10 larvae per condition and per genotype. Discussion
Encapsulation is a complex response against a wasp attack in fruit fly larvae and it requires the concerted action of activated hemocytes. In the course of the encapsulation response, plasmatocytes and the encapsulation-specific lamellocytes form a multilayered capsule around the wasp egg and sequester the invading parasite from the hemocoel of the larva. In addition to inducing the encapsulation response, a wasp infection causes changes in the expression profile of the fruit fly genes [3, 4]. Our results show that edin was rapidly induced in response to an infection by the endoparasitoid wasp Leptopilina boulardi and that edin expression in the fat body, but not in hemocytes, was required to mount a normal encapsulation response against the wasp. Encapsulation was not blocked entirely, however, as approximately 10% of the larvae encapsulated the wasp egg, when edin was knocked down in the fat body. Nevertheless, lamellocyte numbers were unaffected and plasmatocyte spreading behavior was normal. Instead, in larvae where edin was knocked down in the fat body, fewer plasmatocytes were present in circulation, while more hemocytes were retained within the sessile compartment. These data indicate that the presence of lamellocytes alone is not enough for the fruit fly larva to kill the wasp egg. Sufficient numbers of plasmatocytes are also needed.
We discovered that knocking down edin in the fat body did not affect lamellocyte differentiation but compromised the increase of plasmatocyte numbers after a wasp infection. The impaired encapsulation response observed in our study could be therefore due to the misregulation of hemocyte proliferation and/or activation. Because plasmatocyte function was not impaired, as the cells were able to attach and spread normally onto glass slides and wasp eggs, the lowered plasmatocyte number could be the cause of the defects observed in the encapsulation response. Other studies have shown that high hemocyte numbers are associated with an increased resistance against parasitoid wasps in D. melanogaster as well as in other Drosophila species [27–30], although the molecular mechanisms behind this phenomenon are not understood. In our study, the lowered numbers of plasmatocytes are observed already early on during the wasp infection (14 h post infection), suggesting that the function of Edin is critical at the onset of an immune response. This might be the case also in the context of an antimicrobial response, where edin knock down seems to have a modest effect on the levels of some antimicrobial peptides during the early phases of a bacterial infection [20].
Studies have shown that, when hemocytes are activated after an immune stimulus, the banded pattern formed by plasmatocytes is disrupted and the cells are released into the circulation [25, 26, 31], where they can differentiate into lamellocytes [16, 17, 26]. The mobilization of sessile cells occurs prior to the release of hemocytes from the lymph gland [17, 26], and this disruption of the banded pattern is caused by changes in the adhesive properties of the cells. Several genes have been reported to be involved in the attachment of the sessile hemocytes to the sessile compartment [25, 32]. For example, the conserved Rho family of GTPases, namely Rac1 and Rho, regulate the release of sessile cells through the regulation of the adhesive properties of the cells [33, 34]. It has also been suggested that sessile hemocytes adhere to laminin under the larval integument in a syndecan-dependent manner [35]. Additionally, the EGF-repeat containing receptor Eater, which was originally identified for its role in the phagocytosis of bacteria [36], was recently reported to be required in plasmatocytes for the adhesion of hemocytes to the sessile compartment [37]. In our current study, we show that sessile plasmatocytes of edin RNAi larvae did not leave the sessile bands, and the numbers of circulating plasmatocytes did not change after a wasp infection, yet normal amounts of lamellocytes were formed. Despite comparatively normal amounts of lamellocytes, the encapsulation response was impaired when the sessile plasmatocytes could not be mobilized. Hence, besides forming the first layer of the capsule and giving rise to lamellocytes, plasmatocytes have other functions in the encapsulation response that are dependent on edin expression in the fat body.
Our results imply that the effect of Edin is non-cell autonomous and that it seems to act as a molecule that signals from fat body to hemocytes either directly or indirectly. Although the humoral and cellular aspects of Drosophila immunity are often depicted as separate, several studies have provided evidence of the interaction between hemocytes and the fat body. For example, the antimicrobial peptide response to an E. coli infection in domino mutants which lack hemocytes, is normal, but these mutants fail to induce Diptericin during a gut infection by Erwinia carotovora suggesting that hemocytes mediate a signal from the gut to the fat body [38, 39]. In line with these data, Brennan et al. have shown that Psidin acts in the hemocytes to activate the production of Defensin in the fat body [40]. Another example of crosstalk between hemocytes and the fat body is the requirement of Upd3 expression in hemocytes to activate the JAK-STAT pathway in the fat body of adult flies [41]. Furthermore, in larvae, the production of the cytokine Spätzle by hemocytes is needed for the activation of Toll-mediated AMP production in the fat body [42]. Hemocytes are also mediators of the transport of the nitric oxide from its site of production in the gut epithelia to the fat body, where AMP production via the Imd pathway is activated [43, 44]. However, contradicting data also exist for adult flies showing that the ablation of hemocytes by apoptosis does not affect AMP induction in the fat body [45, 46]. A more recent study has shown that the interaction between the fat body and hemocytes is crucial in controlling tumor cell death [47]. Recently, we also showed that Toll signaling in the fat body controlled hemocyte differentiation and activation, but that it did not play a major role in the immune response against L. boulardi as the wasps were able to suppress Toll signaling in the fat body [48]. These examples point to the existence of active tissue-to-tissue signaling that orchestrates appropriate immune responses against different immune challenges. According to our results, Edin functions as a cytokine-like molecule, but the receptor for Edin and its localization remain to be studied. Edin might signal directly from the fat body to the hemocytes, but it may also signal to other tissues or cells that then affect the function of the hemocytes in the sessile compartment (Fig 6). Although Edin is not structurally conserved outside brachyrecan flies [20], its cytokine-like function might be conserved, as in the case of the Spätzle-like function of the vertebrate nerve growth factor β [49], for example.
Fig. 6. A schematic presentation of the function of Edin. 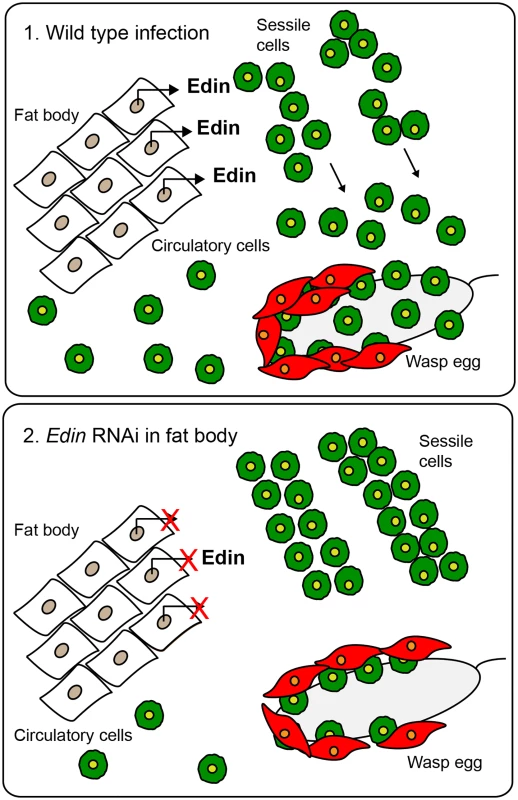
(1.) Edin is induced in the fat body shortly after wasp infection and secreted into the hemolymph. There, Edin directly or indirectly induces the release of plasmatocytes from the sessile hemocyte compartment. These cells go into circulation, find the wasp egg and participate in forming the capsule around the parasitoid egg. (2.) If the expression of edin is knocked down in the fat body in the context of a wasp infection, plasmatocytes are retained in the sessile compartment instead of being released into circulation, causing a defect in the encapsulation of the wasp egg. Based on our results Edin appears to be a key regulator in the cross-talk between fat body and hemocytes in the context of a wasp infection. As in the encapsulation response, the granuloma formation in vertebrates also requires the recruitment of many different cell types. For example, the adult zebrafish responds to a Mycobacterium marinum infection by enclosing the infectious foci in granulomas [50, 51], but also the intracellular bacterium Listeria monocytogenes is sequestered inside granulomas to constrain the infection [52]. Whether information obtained from genetically tractable model organisms such as Drosophila melanogaster, will lead to a better understanding of the pathophysiology of granuloma formation remains to be studied.
Materials and Methods
Drosophila stocks
UAS-edin RNAi (CG32185) flies #109528 and #14289 (hereafter called edin109528 and edin14289) were obtained from the Vienna Drosophila Resource Center. The driver lines used in this study were the fat body-specific driver Fb-GAL4, the hemocyte-specific driver HmlΔ;He-GAL4 [48] and C564-GAL4, which was obtained from Prof. Bruno Lemaitre (Global Health Institute, EPFL, Switzerland). The C564-GAL4 driver is expressed in many tissues such as the fat body, lymph gland, salivary glands, gut and brain but not in hemocytes [22].
The hemocyte reporter lines eaterGFP (for plasmatocytes) [53] and MSNF9mo-mCherry (for lamellocytes, hereafter called msnCherry) [54] were obtained from Robert Schulz’s laboratory. The lines were crossed to create the msnCherry,eaterGFP reporter line. The mCherry,eaterGFP reporter was further crossed with Fb-GAL4 and edin RNAi109528 to obtain the mCherry,eaterGFP;Fb-GAL4 and mCherry,eaterGFP;edin109528 lines. Canton S flies were used for RNA extractions.
Wasp infection
Ten GAL4-driver virgin females were crossed with five RNAi male flies and allowed to lay eggs at +25°C. w1118 flies and GAL4-driver virgin females crossed with w1118 males and w1118 virgin females crossed with RNAi males were used as controls. The flies were transferred daily into fresh vials and the vials containing eggs were transferred to +29°C. On the third day after egg-laying, the larvae were infected with 20 female and 10 male wasps of the Leptopilina boulardi strain G486. The larvae were infected for 2 hours at room temperature after which the wasps were removed and the larvae were transferred back to +29°C.
The encapsulation properties were assayed 27–29 hours after the infection and the killing ability of the larval immune system 48–50 hours after the wasp infection. The egg was scored as encapsulated when traces of melanin were found on it. To analyze the killing ability of the Drosophila larva, three types of phenotypes were scored. The wasp was scored as killed if a melanized wasp egg or melanized wasp larva without other living wasp larvae was found in the hemolymph, whereas the wasp was scored as living when a living wasp that had escaped a melanized capsule was present or when a living wasp larva without any melanized particles was found in the hemocoel.
RNAi extraction from larvae and fat bodies
Eight to ten Canton S larvae per sample were snap frozen on dry ice at 0 hours or 3 hours after the wasp infection. The fat bodies were dissected in 1x PBS 24 hours after the wasp infection and kept on ice. Both larvae and fat bodies were homogenized in TRIsure reagent (Bioline, London, UK) and total RNAs were extracted according to the manufacturer’s instructions.
Quantitative real-time PCR
Quantitative RT-PCR was carried out using the iScript One-Step RT-PCR kit with SYBR Green (Bio-Rad, Hercules, CA, USA) and the Bio-Rad CFX96 (Bio-Rad) instrument according to the manufacturer’s instructions. Results were analyzed with the Bio-Rad CFX Manager software version 1.6. Actin5C was used as a housekeeping gene. The following primers were used: Forward 5’-CTCGTGTCCTGCTGTCTG-3’ and reverse 5’-GCCTTCGTAGTTGTTCCG-3' for edin and forward 5’-CGAAGAAGTTGCTGCTCTGG-3’ and reverse 5’-AGAACGATACCGGTGGTACG-3’ for Actin5C.
Microscopy
Drosophila larvae were imaged using 3rd instar larvae 27–29 hours after the wasp infection. The larvae were washed three times in H2O and embedded on microscope slides in a drop of ice-cold glycerol. The larvae were immobilized at -20°C before imaging. The Zeiss ApoTome.2 was used for live imaging of larvae. For hemocyte imaging, the larvae were washed three times in H2O, and the hemocytes were bled into 1 x PBS 48–50 hours after the wasp infection. Uninfected controls of the same age were also used. The hemocytes were let to adhere to the glass surface of a microscope slide for 30 minutes, after which they were fixed with 3.7% paraformaldehyde for 5 minutes. The samples were washed with PBS and mounted with the Prolong Gold Anti-Fade reagent with DAPI (Molecular Probes). Hemocyte imaging was carried out with the Zeiss AxioImager.M2 microscope with Zeiss AxioCam and the Zen Blue 2011 software and with the Zeiss LSM780 in the case of the antibody-stained hemocytes. The hemocyte images were processed with ImageJ 1.49p (Rasband WS, ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, imagej.nih.gov/ij, 1997–2012).
Quantification of larval hemocytes with flow cytometry
Hemocytes from infected and control larvae were bled into 1 x PBS with 8% BSA to obtain the hemocytes. Flow cytometry was used to detect eaterGFP-positive and msnCherry-positive cells in these samples. The Accuri C6 flow cytometer (BD, Franklin Lakes, NJ, USA) was used to run the samples, and the data was analyzed using the BD Accuri C6 software. The gating strategy is explained in S2 Fig.
Immunofluorescence
For F-actin and α-tubulin stainings, hemocytes were bled from 15 larvae per cross into 20 μl of 1 x PBS with 8% BSA in pools of three larvae per well and allowed to spread on a glass slide for 45 minutes. Cells were fixed with 3.7% paraformaldehyde/PBS solution for 10 minutes, washed three times with PBS and permeabilized for 5 minutes with 0.1% Triton X-100 before antibody staining. Cells were incubated for 2 hours with an unconjugated mouse α-tubulin monoclonal antibody (Life Technologies, 1μg/ml concentration) followed by one hour incubation with the Alexa Fluor 405 goat anti-mouse secondary antibody (Life Technologies, a 1 : 500 dilution in 1% BSA in PBS). F-actin was visualized by incubating the cells for 30 minutes with the Alexa Fluor 680 nm Phalloidin stain (Invitrogen) diluted to 1 : 50 in 1x PBS with 1% BSA. After this, the cells were washed 3 times with PBS and mounted using the ProLong Gold antifade mountant (Life Technologies). We measured the area of Phalloidin and α-tubulin staining with ImageJ 1.49p and calculated the ratio of α-tubulin to Phalloidin areas.
Wasp eggs with hemocytes attached onto them were collected from fly larvae 12–14 hours after infection in a drop of 8% BSA in 1 x PBS, fixed with 3.7% paraformaldehyde/PBS solution for 10 minutes, washed three times with PBS, and stained for 4 hours with an undiluted mixture of monoclonal P1a and P1b (NimC1) plasmatocyte-specific antibodies [55]. Thereafter, the samples were washed 3 times with PBS and incubated with the Alexa Fluor 405 goat anti-mouse secondary antibody (Life Technologies, 1 : 500 dilution). The eggs were mounted with 50% glycerol prior to imaging. Three eggs per cross were imaged.
Statistical analyses
Edin expression data was analyzed using an independent samples two-tailed T-test, with unequal variances assumed. The analysis was carried out using Microsoft Office Professional Plus Excel 2013. The threshold for statistical significance was established as p<0.05.
We applied a Generalized Linear Model (glm) in R 3.1.2 (2014-10-31) — “Pumpkin Helmet” (R Development Core, 2003) to analyze the encapsulation and parasite killing data (R Core Team 2014, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/). The categorical explanatory variable was “Cross” and the binary response variable was numbers of “successful encapsulation” or “killed parasites” and numbers of “failed encapsulation” or “failed parasite killing”. Differences between specific crosses were analyzed by Chi-square tests.
We analyzed the cell spreading data and cell numbers 14–16 hours post infection with Welch’s T-test implemented in R 3.1.2 (2014-10-31) (R Development Core, 2003). The data were log-transformed prior to the analyses to obtain normal distribution.
Full factorial analysis of variance (ANOVA) was applied to data on plasmatocyte and lamellocyte numbers 27–29 hours after infection with cross and infection status (infected or not infected) as explanatory variables. The data did not meet the requirement for normal distribution and was log transformed prior to the analyses. In the analysis of plasmatocyte numbers, a significant interaction term was found between cross and infection status and therefore plasmatocyte numbers were further analyzed conducting ANOVAs separately for each cross with infection status as explanatory variable. This data was analyzed using IBM SPSS Statistics version 22.
Supporting Information
Zdroje
1. Keebaugh ES, Schlenke TA. Insights from natural host-parasite interactions: The drosophila model. Dev Comp Immunol 2014; 42(1): 111–123. doi: 10.1016/j.dci.2013.06.001 23764256
2. Benassi V, Coustau C, Carton Y. Insect immunity: A genetic factor (hrtp) is essential for antibacterial peptide expression in drosophila after infection by parasitoid wasps. Arch Insect Biochem Physiol 2000; 43(2): 64–71. 10644970
3. Schlenke TA, Morales J, Govind S, Clark AG. Contrasting infection strategies in generalist and specialist wasp parasitoids of drosophila melanogaster. PLoS Pathog 2007; 3(10): 1486–1501. 17967061
4. Wertheim B, Kraaijeveld AR, Schuster E, Blanc E, Hopkins M, et al. Genome-wide gene expression in response to parasitoid attack in drosophila. Genome Biol 2005; 6(11): R94. 16277749
5. Valanne S, Wang JH, Rämet M. The drosophila toll signaling pathway. J Immunol 2011; 186(2): 649–656. doi: 10.4049/jimmunol.1002302 21209287
6. Myllymäki H, Valanne S, Rämet M. The drosophila imd signaling pathway. J Immunol 2014; 192(8): 3455–3462. doi: 10.4049/jimmunol.1303309 24706930
7. Honti V, Csordas G, Kurucz E, Márkus R, Andó I. The cell-mediated immunity of drosophila melanogaster: Hemocyte lineages, immune compartments, microanatomy and regulation. Dev Comp Immunol 2014; 42(1): 47–56. doi: 10.1016/j.dci.2013.06.005 23800719
8. Meister M, Lagueux M. Drosophila blood cells. Cell Microbiol 2003; 5(9): 573–580. 12925127
9. Elrod-Erickson M, Mishra S, Schneider D. Interactions between the cellular and humoral immune responses in drosophila. Curr Biol 2000; 10(13): 781–784. 10898983
10. Franc NC, Heitzler P, Ezekowitz RA, White K. Requirement for croquemort in phagocytosis of apoptotic cells in drosophila. Science 1999; 284(5422): 1991–1994. 10373118
11. Manaka J, Kuraishi T, Shiratsuchi A, Nakai Y, Higashida H, et al. Draper-mediated and phosphatidylserine-independent phagocytosis of apoptotic cells by drosophila hemocytes/macrophages. J Biol Chem 2004; 279(46): 48466–48476. 15342648
12. Ulvila J, Vanha-aho LM, Kleino A, Vähä-Mäkilä M, Vuoksio M, et al. Cofilin regulator 14-3-3zeta is an evolutionarily conserved protein required for phagocytosis and microbial resistance. J Leukoc Biol 2011; 89(5): 649–659. doi: 10.1189/jlb.0410195 21208897
13. Meister M. Blood cells of drosophila: Cell lineages and role in host defence. Curr Opin Immunol 2004; 16(1): 10–15. 14734104
14. Russo J, Dupas S, Frey F, Carton Y, Brehelin M. Insect immunity: Early events in the encapsulation process of parasitoid (leptopilina boulardi) eggs in resistant and susceptible strains of drosophila. Parasitology 1996; 112 (Pt 1)(Pt 1): 135–142. 8587797
15. Rizki TM, Rizki RM. Lamellocyte differentiation in drosophila larvae parasitized by leptopilina. Dev Comp Immunol 1992; 16(2–3): 103–110. 1473596
16. Stofanko M, Kwon SY, Badenhorst P. Lineage tracing of lamellocytes demonstrates drosophila macrophage plasticity. PLoS One 2010; 5(11): e14051. doi: 10.1371/journal.pone.0014051 21124962
17. Honti V, Csordas G, Márkus R, Kurucz E, Jankovics F, et al. Cell lineage tracing reveals the plasticity of the hemocyte lineages and of the hematopoietic compartments in drosophila melanogaster. Mol Immunol 2010; 47(11–12): 1997–2004. doi: 10.1016/j.molimm.2010.05.291 20691478
18. Nappi AJ, Vass E. Hydrogen peroxide production in immune-reactive drosophila melanogaster. J Parasitol 1998; 84(6): 1150–1157. 9920305
19. Verleyen P, Baggerman G, D'Hertog W, Vierstraete E, Husson SJ, et al. Identification of new immune induced molecules in the haemolymph of drosophila melanogaster by 2D-nanoLC MS/MS. J Insect Physiol 2006; 52(4): 379–388. 16510152
20. Vanha-aho LM, Kleino A, Kaustio M, Ulvila J, Wilke B, et al. Functional characterization of the infection-inducible peptide edin in drosophila melanogaster. PLoS One 2012; 7(5): e37153. doi: 10.1371/journal.pone.0037153 22606343
21. Gordon MD, Ayres JS, Schneider DS, Nusse R. Pathogenesis of listeria-infected drosophila wntD mutants is associated with elevated levels of the novel immunity gene edin. PLoS Pathog 2008; 4(7): e1000111. doi: 10.1371/journal.ppat.1000111 18654628
22. Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N. Activation of a drosophila janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J 1995; 14(12): 2857–2865. 7796812
23. Williams MJ, Andó I, Hultmark D. Drosophila melanogaster Rac2 is necessary for a proper cellular immune response. Genes Cells 2005; 10(8): 813–823. 16098145
24. Russo J, Brehelin M, Carton Y. Haemocyte changes in resistant and susceptible strains of D. melanogaster caused by virulent and avirulent strains of the parasitic wasp leptopilina boulardi. J Insect Physiol 2001; 47(2): 167–172. 11064023
25. Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, et al. A directed screen for genes involved in drosophila blood cell activation. Proc Natl Acad Sci U S A 2004; 101(39): 14192–14197. 15381778
26. Márkus R, Laurinyecz B, Kurucz E, Honti V, Bajusz I, et al. Sessile hemocytes as a hematopoietic compartment in drosophila melanogaster. Proc Natl Acad Sci U S A 2009; 106(12): 4805–4809. doi: 10.1073/pnas.0801766106 19261847
27. Kacsoh BZ, Schlenke TA. High hemocyte load is associated with increased resistance against parasitoids in drosophila suzukii, a relative of D. melanogaster. PLoS One 2012; 7(4): e34721. doi: 10.1371/journal.pone.0034721 22529929
28. Moreau SJ, Guillot S, Populaire C, Doury G, Prevost G, et al. Conversely to its sibling drosophila melanogaster, D. simulans overcomes the immunosuppressive effects of the parasitoid asobara citri. Dev Comp Immunol 2005; 29(3): 205–209. 15572069
29. Prevost G, Eslin P. Hemocyte load and immune resistance to asobara tabida are correlated in species of the drosophila melanogaster subgroup. J Insect Physiol 1998; 44(9): 807–816. 12769876
30. Kraaijeveld AR, Hutcheson KA, Limentani EC, Godfray HC. Costs of counterdefenses to host resistance in a parasitoid of drosophila. Evolution 2001; 55(9): 1815–1821. 11681736
31. Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in drosophila. Dev Biol 2001; 230(2): 243–257. 11161576
32. Stofanko M, Kwon SY, Badenhorst P. A misexpression screen to identify regulators of drosophila larval hemocyte development. Genetics 2008; 180(1): 253–267. doi: 10.1534/genetics.108.089094 18757933
33. Williams MJ, Wiklund ML, Wikman S, Hultmark D. Rac1 signalling in the drosophila larval cellular immune response. J Cell Sci 2006; 119(Pt 10): 2015–2024. 16621891
34. Williams MJ, Habayeb MS, Hultmark D. Reciprocal regulation of Rac1 and Rho1 in drosophila circulating immune surveillance cells. J Cell Sci 2007; 120(Pt 3): 502–511. 17227793
35. Narita R, Yamashita H, Goto A, Imai H, Ichihara S, et al. Syndecan-dependent binding of drosophila hemocytes to laminin alpha3/5 chain LG4-5 modules: Potential role in sessile hemocyte islets formation. FEBS Lett 2004; 576(1–2): 127–132.
36. Kocks C, Cho JH, Nehme N, Ulvila J, Pearson AM, et al. Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in drosophila. Cell 2005; 123(2): 335–346. 16239149
37. Bretscher AJ, Honti V, Binggeli O, Burri O, Poidevin M, et al. The nimrod transmembrane receptor eater is required for hemocyte attachment to the sessile compartment in drosophila melanogaster. Biol Open 2015.
38. Braun A, Hoffmann JA, Meister M. Analysis of the drosophila host defense in domino mutant larvae, which are devoid of hemocytes. Proc Natl Acad Sci U S A 1998; 95(24): 14337–14342. 9826701
39. Basset A, Khush RS, Braun A, Gardan L, Boccard F, et al. The phytopathogenic bacteria erwinia carotovora infects drosophila and activates an immune response. Proc Natl Acad Sci U S A 2000; 97(7): 3376–3381. 10725405
40. Brennan CA, Delaney JR, Schneider DS, Anderson KV. Psidin is required in drosophila blood cells for both phagocytic degradation and immune activation of the fat body. Curr Biol 2007; 17(1): 67–72. 17208189
41. Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in drosophila JAK/STAT-dependent response to septic injury. Dev Cell 2003; 5(3): 441–450. 12967563
42. Shia AK, Glittenberg M, Thompson G, Weber AN, Reichhart JM, et al. Toll-dependent antimicrobial responses in drosophila larval fat body require spatzle secreted by haemocytes. J Cell Sci 2009; 122(Pt 24): 4505–4515. doi: 10.1242/jcs.049155 19934223
43. Foley E, O'Farrell PH. Nitric oxide contributes to induction of innate immune responses to gram-negative bacteria in drosophila. Genes Dev 2003; 17(1): 115–125. 12514104
44. Dijkers PF, O'Farrell PH. Drosophila calcineurin promotes induction of innate immune responses. Curr Biol 2007; 17(23): 2087–2093. 18060786
45. Charroux B, Royet J. Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the drosophila immune response. Proc Natl Acad Sci U S A 2009; 106(24): 9797–9802. doi: 10.1073/pnas.0903971106 19482944
46. Defaye A, Evans I, Crozatier M, Wood W, Lemaitre B, et al. Genetic ablation of drosophila phagocytes reveals their contribution to both development and resistance to bacterial infection. J Innate Immun 2009; 1(4): 322–334. doi: 10.1159/000210264 20375589
47. Parisi F, Stefanatos RK, Strathdee K, Yu Y, Vidal M. Transformed epithelia trigger non-tissue-autonomous tumor suppressor response by adipocytes via activation of toll and eiger/TNF signaling. Cell Rep 2014; 6(5): 855–867. doi: 10.1016/j.celrep.2014.01.039 24582964
48. Schmid MR, Anderl I, Vesala L, Vanha-aho LM, Deng XJ, et al. Control of drosophila blood cell activation via toll signaling in the fat body. PLoS One 2014; 9(8): e102568. doi: 10.1371/journal.pone.0102568 25102059
49. Hepburn L, Prajsnar TK, Klapholz C, Moreno P, Loynes CA, et al. Innate immunity. A spaetzle-like role for nerve growth factor beta in vertebrate immunity to staphylococcus aureus. Science 2014; 346(6209): 641–646. doi: 10.1126/science.1258705 25359976
50. Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, et al. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity 2002; 17(6): 693–702. 12479816
51. Parikka M, Hammaren MM, Harjula SK, Halfpenny NJ, Oksanen KE, et al. Mycobacterium marinum causes a latent infection that can be reactivated by gamma irradiation in adult zebrafish. PLoS Pathog 2012; 8(9): e1002944. doi: 10.1371/journal.ppat.1002944 23028333
52. Mielke ME, Peters C, Hahn H. Cytokines in the induction and expression of T-cell-mediated granuloma formation and protection in the murine model of listeriosis. Immunol Rev 1997; 158 : 79–93. 9314076
53. Sorrentino RP, Tokusumi T, Schulz RA. The friend of GATA protein U-shaped functions as a hematopoietic tumor suppressor in drosophila. Dev Biol 2007; 311(2): 311–323. 17936744
54. Tokusumi T, Sorrentino RP, Russell M, Ferrarese R, Govind S, et al. Characterization of a lamellocyte transcriptional enhancer located within the misshapen gene of drosophila melanogaster. PLoS One 2009; 4(7): e6429. doi: 10.1371/journal.pone.0006429 19641625
55. Kurucz E, Márkus R, Zsamboki J, Folkl-Medzihradszky K, Darula Z, et al. Nimrod, a putative phagocytosis receptor with EGF repeats in drosophila plasmatocytes. Curr Biol 2007; 17(7): 649–654. 17363253
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR SelectionČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



