-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaWhy HIV Virions Have Low Numbers of Envelope Spikes: Implications for Vaccine Development
article has not abstract
Published in the journal: . PLoS Pathog 10(8): e32767. doi:10.1371/journal.ppat.1004254
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004254Summary
article has not abstract
Introduction
The major structural proteins of most viruses, including both naked icosahedral and enveloped types, are present in a dense array on the virion surface. This pattern has likely evolved to promote structural integrity, maximize cell binding and entry, and minimize genome size. HIV and related simian lentiviruses are unusual in having a low density of envelope protein spikes on their surfaces (Figure 1) [1]. Why has HIV evolved this exceptional virion structure? We believe that studies of human papillomavirus (HPV) virus-like particles (VLPs), which are the basis of the highly successful HPV vaccine, may provide the conceptual underpinning for answering this question and key insights for developing effective HIV prophylactic vaccines.
Fig. 1. What's different about HIV virions? 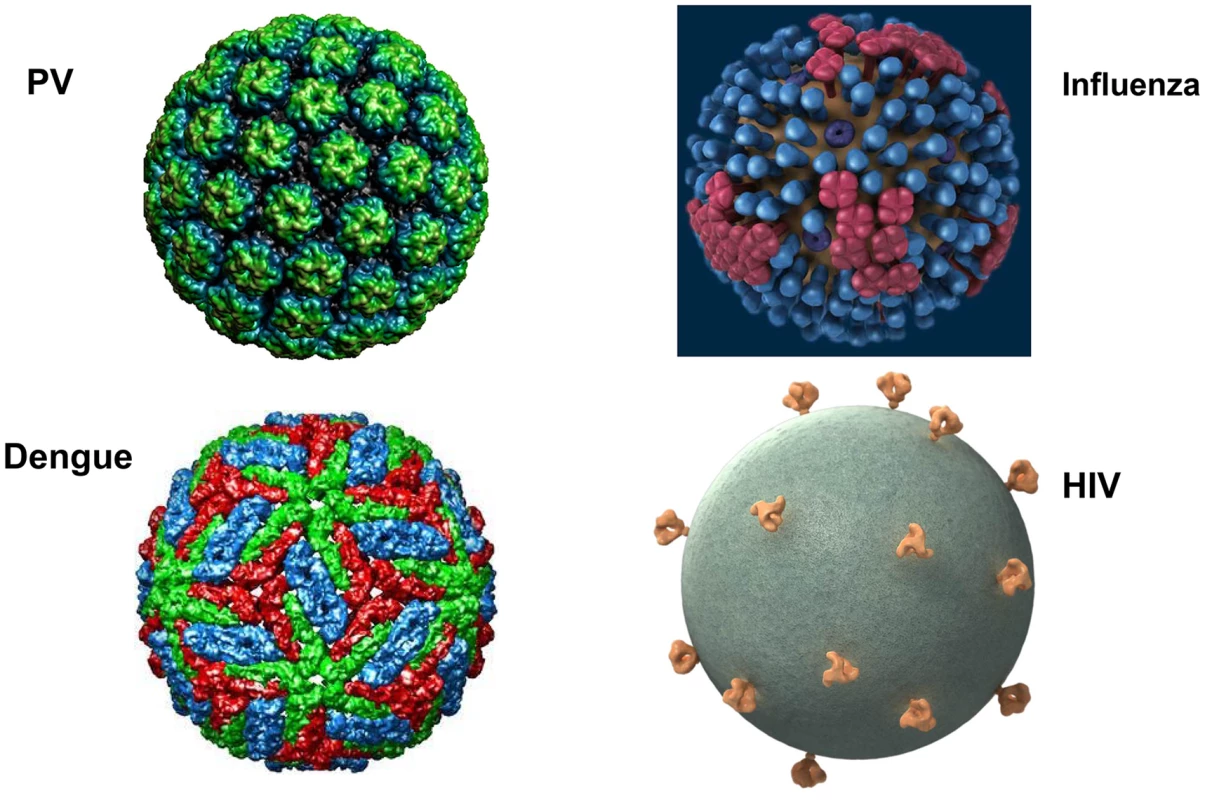
Surface projections of bovine papillomavirus (BPV), HIV, dengue virus, and influenza virus are shown (not to scale). The images of papillomavirus (PV) and dengue virus were obtained from the Viper database (PMID: 18981051). The image of influenza virus is courtesy of cdc.gov. The HIV image was generously provided by Sriram Subramaniam, National Cancer Institute. Why Does HIV Have So Few Virion Spikes?
HIV is notably inefficient at transmission—it has been estimated that 200–2,000 encounters are required per heterosexual transmission event [2]. In large part this may be due to the low number (estimated to be 14, on average) of envelope spikes per virion, especially since increasing the number of envelope spikes per virion increases infectivity, at least in an in vitro simian immunodeficiency virus (SIV) model [1], [3]. It seems unlikely that HIV evolved a low number of envelope spikes to limit its transmission efficiency. Low density of env spikes may have evolved, in part, because it prevents bivalent binding of immunoglobulin G (IgG) antibodies to the virion, thus reducing antibody avidity and impeding neutralization [4]. However, we think that an alternative explanation for the selection for low numbers of envelope spikes is that this feature retards the induction of a broad spectrum antibody response.
Why Is It Important to Understand How a Virus Counteracts the Host's Antibody Response?
To be successful, mammalian viruses must overcome the host's humoral immune response. One strategy is to essentially outrun immunity by rapidly replicating to produce sufficient virions for transmission before neutralizing antibody can be generated. Alternatively, viruses that establish persistent infections must evade or delay induction of virus neutralizing antibodies so that infectious virions can be shed for extended periods [5]. The evasion mechanisms used by persisting viruses to circumvent humoral immunity vary depending upon the specifics of the viral life cycle. Understanding the major mechanisms of humoral immune evasion for a virus can provide key insights into development of effective antibody-mediated prophylactic vaccines against that virus, as recently demonstrated for HPVs. HPVs only produce virions in terminally differentiated layers of a stratified epithelium and release their virions to the environment during sloughing of the surface cells. As a consequence, antibody responses to natural HPV infection are weak [6]. However, by evolving an escape mechanism that relies on ignorance of the virions by the humoral immune system, HPVs have not evolved strategies to evade induction of potent neutralizing antibody responses if the virions are exposed to the systemic immune system, as is readily accomplished by standard parenteral injection. Thus, it is not surprising, at least in retrospect, that intramuscular injection of HPV L1 VLPs (which mimic the outer surface of the authentic virion) induces a virion antibody response that is highly protective against HPV infection and associated neoplasia [7].
Why Do HPV Vaccines Induce Such Potent Neutralizing Antibody Responses?
Vaccination with HPV VLPs induces exceptionally strong antibody responses in humans. Even a single dose consistently induces neutralizing antibody titers that plateau well above the levels induced by natural infection, and they remain stable for years [8]. This type of humoral response, which is not seen with simple subunit vaccines, is thought to depend on the high-density repetitive display of the neutralizing epitopes on the VLP surface. Multivalent and dense (50–100 Å) spacing of surface determinants is a common feature of many microbial surfaces, including virions (Figure 1) and bacterial pili, and this pattern is a key determinant for recognition of an antigen as foreign by the humoral immune system [9]. This spacing of epitopes is rarely found on mammalian body surfaces routinely exposed to the systemic immune system. Clustering of B cell receptors by antigens with this spacing presumably sends exceptionally strong downstream activation and survival signals to the B cells, promoting high-titer and durable antibody responses. Remarkably, recognition of this epitope pattern is dominant over the mechanisms that normally promote B cell tolerance to self. For example, we found that display of tumor necrosis factor alpha (TNFα), a normally tolerogenic self-antigen, in a high-density array on an HPV VLP leads to long-lasting IgG responses against TNFα that are essentially equivalent to those against the foreign epitopes on the VLP [10]. The key determinant for breaking tolerance is the density of the epitope spacing [11]. Neither strong foreign T helper epitopes, high doses, nor potent adjuvants can substitute for high-density display for breaking self-tolerance. Vaccination with self-antigen displayed on VLPs, but not the same antigen in a low-valency form, can even reactivate self-specific anergic B cells [12]. The concept of virus-like display for breaking B cell tolerance to self-antigens has been well validated in clinical trials targeting several chronic disease targets [13].
Why Has the Mammalian Immune System Evolved to Tolerate the Potential for Autoantibody Induction by Virus-Like Display?
One could imagine induction of autoantibodies occurring, for instance, if virions become coated by their cell surface receptor during lytic infections. It is possible that virus-like display of self-antigens occurs so rarely that there has been no evolutionary pressure against it. However, we postulate that there has been positive selection for this potentially detrimental outcome because of the overwhelming importance of inducing a rapid neutralizing antibody response to lytic viruses. A majority of newly produced naive B cells are autoreactive [14] and functionally anergic [15], and many more B cells are likely to go through autoreactive intermediates during the process of somatic hypermutation that leads to affinity maturation. It follows that generating high-affinity neutralizing antibodies against high-density determinants on virion surfaces should be able to proceed along many immunoglobulin gene lineages because they could involve self-reactive progenitors and intermediates, thus ensuring their rapid and consistent appearance in a genetically diverse population (Figure 2). This conjecture is consistent with the observation that HPV VLPs induce essentially 100% seroconversion rates in humans, even after a single dose [8].
Fig. 2. Model for the induction of neutralizing antibodies by high-density versus low-density epitope display. 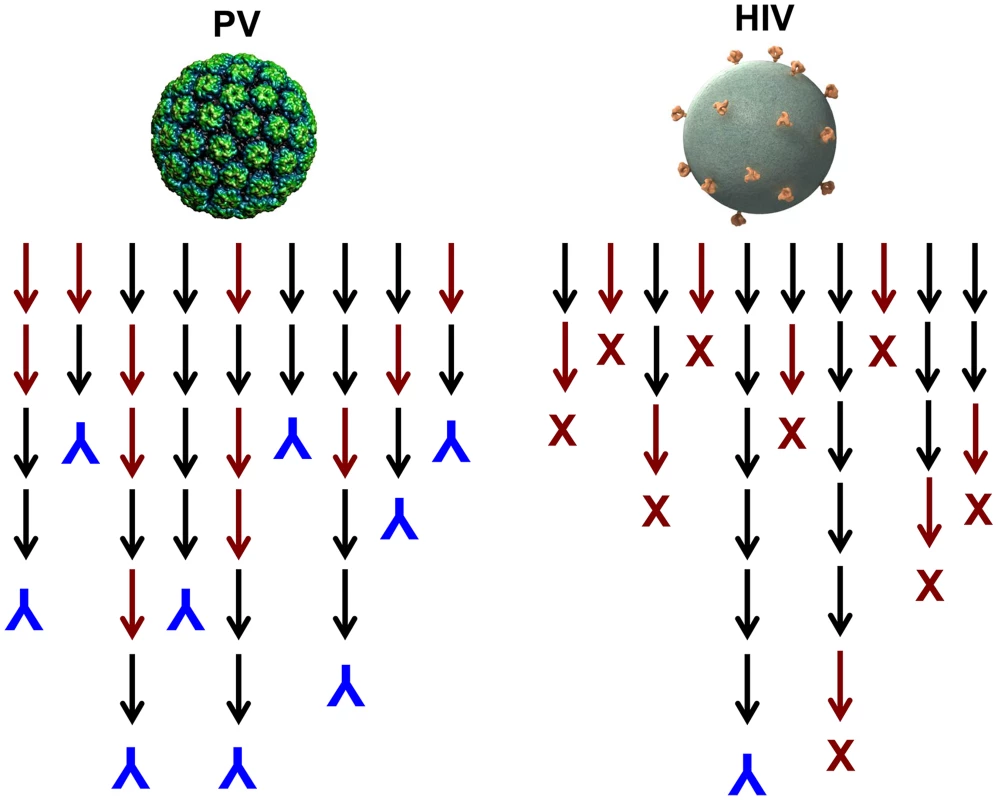
Black arrows represent antibody species during lineage evolution that are not reactive with self; red arrows represent species that are reactive with self. Inverted blue Ys denote B cell lineages that result in production of a virion binding antibody; red Xs denote deletion or tolerization of a self-reactive B cell lineage. Why Are Neutralizing Antibody Responses to HIV Infection Delayed?
In contrast to HPV VLP vaccination, it is well documented that neutralizing antibodies are exceptionally slow to develop during HIV infection, and nearly all broadly neutralizing antibodies invariably have undergone a large number of hypersomatic mutations [16]. Why is this the case for HIV and presumably not for most other viruses? Although the well-documented mechanisms involving glycan shields and conformational masking of key conserved functional sites likely contribute [17], we believe that the answer may primarily lie in the exceptionally low number of envelope spikes on the virion surface. In experimental systems, it has been shown that virus-like display of self-antigens can only overcome B cell tolerance when the mean epitope spacing is less than 100–150 Å [11]. The average spacing of HIV spikes has been estimated to be approximately 230 Å [1]. Therefore, in contrast to most other viruses, HIV neutralizing antibodies will likely have a much more constrained number of immunoglobulin gene developmental pathways because they cannot readily involve self-reactive intermediates (Figure 2). The low density of virion epitopes also raises the potential for HIV to evolve critical envelope determinates that are partially cross-reactive with self, so that mechanisms of self-tolerance can hinder induction of antibodies to them. Supporting this hypothesis is a recent study identifying a self-antigen that displays the epitope recognized by 2F5, a broadly neutralizing gp41 monoclonal antibody. Polypeptides bearing the epitope recognized by 2F5 are poorly immunogenic across species in which this epitope is conserved, but high-titer antibodies are readily induced in opossums, which naturally carry a variant peptide in the self-antigen that is not recognized by the antibody [18]. This immune evasion mechanism would not be available to viruses with high-density display of their critical virion determinants since the cross-reactive B cells would not be tolerized. This conjecture provides an explanation for the observation that most of the rare broadly neutralizing HIV antibodies isolated to date have an exceptionally high degree of self cross-reactivity and polyreactivity [19]. Given the need to develop through intermediates that are not strongly cross-reactive and an evolutionary drive to mimic self, it should not be surprising that broadly neutralizing HIV antibodies have complex lineages involving a high number of somatic mutations and develop late, if at all, in HIV-infected individuals.
How Can Insights into HIV's Mechanism for Retarding Induction of Neutralizing Antibodies Be Translated into an Effective HIV Prophylactic Vaccine?
Recently, there have been suggestions for developing HIV prophylactic vaccines based on an in-depth understanding of the specific set of complex intermediates involved in the generation of broadly neutralizing antibodies during natural HIV infection [16], [19]. The observations outlined above suggest an alternative approach, bypassing the need to go through these specific intermediates by generating a virus-like display vaccine that could potentially generate broadly neutralizing antibodies through a larger set of lineages involving self-reactive intermediates. The key would be to generate high-density virus-like display of the HIV antigen. We favor display of conformationally correct forms of trimeric gp160, gp120, or gp41 over short peptides or mimotopes because it would be more difficult for the virus to escape a neutralizing antibody response encompassing multiple epitopes. Display of these large, complex antigens at sufficient density to break B cell self-tolerance may not be easy to achieve. In our opinion, this goal should be a major focus of HIV prophylactic vaccine development. Since HIV env epitopes may be more self cross-reactive than the capsid proteins of most viruses, it will be critical to carefully evaluate the possibility that pathogenic autoantibodies are generated by the vaccines.
It is important to note that not all “virus-like” particles are equally immunogenic in humans. For example, in contrast to HPV VLPs, the hepatitis B surface antigen particles that are the basis of all current hepatitis B virus (HBV) vaccines generate detectable antibodies in only a minority of subjects after one dose and titers do not routinely stabilize, even after three doses [20]. To inform design of prophylactic vaccines for HIV, it seems worthwhile to determine the key differences that account for the variability in the intrinsic immunogenicity of various VLP platforms and to evaluate their ability to break B cell tolerance to displayed self-antigens.
In summary, we postulate that HIV has evolved an exceptionally low number of envelope spikes so that effective neutralizing antibodies can't be generated through self-reactive intermediates and therefore may not have evolved effective defenses against vaccines that can. In trading low efficiency of transmission for delayed induction of an effective neutralizing antibody response, the virus may have created an Achilles heel that might be exploited by a vaccine that relies on high-density virus-like display.
Zdroje
1. ZhuP, LiuJ, BessJJr, ChertovaE, LifsonJD, et al. (2006) Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441 : 847–852.
2. PowersKA, PooleC, PettiforAE, CohenMS (2008) Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis 8 : 553–563.
3. YusteE, ReevesJD, DomsRW, DesrosiersRC (2004) Modulation of Env content in virions of simian immunodeficiency virus: correlation with cell surface expression and virion infectivity. J Virol 78 : 6775–6785.
4. KleinJS, BjorkmanPJ (2010) Few and far between: how HIV may be evading antibody avidity. PLoS Pathog 6: e1000908.
5. HangartnerL, ZinkernagelRM, HengartnerH (2006) Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol 6 : 231–243.
6. StanleyM (2010) HPV - immune response to infection and vaccination. Infect Agent Cancer 5 : 19.
7. SchillerJT, LowyDR (2012) Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol 10 : 681–692.
8. SafaeianM, PorrasC, PanY, KreimerA, SchillerJT, et al. (2013) Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 6 : 1242–1250.
9. BachmannMF, ZinkernagelRM (1996) The influence of virus structure on antibody responses and virus serotype formation. Immun Today 17 : 553–558.
10. ChackerianB, LowyDR, SchillerJT (2001) Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J Clin Invest 108 : 415–423.
11. ChackerianB, LenzP, LowyDR, SchillerJT (2002) Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J Immunol 169 : 6120–6126.
12. ChackerianB, DurfeeMR, SchillerJT (2008) Virus-like display of a neo-self antigen reverses B cell anergy in a B cell receptor transgenic mouse model. J Immunol 180 : 5816–5825.
13. BachmannMF, WhiteheadP (2013) Active immunotherapy for chronic diseases. Vaccine 31 : 1777–1784.
14. WardemannH, YurasovS, SchaeferA, YoungJW, MeffreE, et al. (2003) Predominant autoantibody production by early human B cell precursors. Science 301 : 1374–1377.
15. YarkoniY, GetahunA, CambierJC (2010) Molecular underpinning of B-cell anergy. Immunol Rev 237 : 249–263.
16. MascolaJR, HaynesBF (2013) HIV-1 neutralizing antibodies: understanding nature's pathways. Immunol Rev 254 : 225–244.
17. WestAPJr, ScharfL, ScheidJF, KleinF, BjorkmanPJ, et al. (2014) Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell 156 : 633–648.
18. YangG, HollTM, LiuY, LiY, LuX, et al. (2013) Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J Exp Med 210 : 241–256.
19. VerkoczyL, DiazM (2014) Autoreactivity in HIV-1 broadly neutralizing antibodies: implications for their function and induction by vaccination. Curr Opin HIV AIDS 9 : 224–234.
20. WistromJ, AhlmC, LundbergS, SettergrenB, TarnvikA (1999) Booster vaccination with recombinant hepatitis B vaccine four years after priming with one single dose. Vaccine 17 : 2162–2165.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial PathogensČlánek A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during TransmissionČlánek The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil ChemotaxisČlánek Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Regulatory RNAs Involved in Bacterial Antibiotic Resistance
- From Dandruff to Deep-Sea Vents: -like Fungi Are Ecologically Hyper-diverse
- Pathogenicity and Epithelial Immunity
- Mother–Infant HIV Transmission: Do Maternal HIV-Specific Antibodies Protect the Infant?
- Hell's BELs: acterial 3 igases That Exploit the Eukaryotic Ubiquitin Machinery
- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Primary Seronegative but Molecularly Evident Hepadnaviral Infection Engages Liver and Induces Hepatocarcinoma in the Woodchuck Model of Hepatitis B
- TLR2 Signaling Decreases Transmission of by Limiting Bacterial Shedding in an Infant Mouse Influenza A Co-infection Model
- Production of an Attenuated Phenol-Soluble Modulin Variant Unique to the MRSA Clonal Complex 30 Increases Severity of Bloodstream Infection
- Inhibition of the TRAIL Death Receptor by CMV Reveals Its Importance in NK Cell-Mediated Antiviral Defense
- Early Mucosal Sensing of SIV Infection by Paneth Cells Induces IL-1β Production and Initiates Gut Epithelial Disruption
- Limited HIV Infection of Central Memory and Stem Cell Memory CD4+ T Cells Is Associated with Lack of Progression in Viremic Individuals
- Virus-Specific Regulatory T Cells Ameliorate Encephalitis by Repressing Effector T Cell Functions from Priming to Effector Stages
- A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during Transmission
- The HIV-1 Envelope Transmembrane Domain Binds TLR2 through a Distinct Dimerization Motif and Inhibits TLR2-Mediated Responses
- Infection with MERS-CoV Causes Lethal Pneumonia in the Common Marmoset
- VGIII Isolates Causing Infections in HIV/AIDS Patients in Southern California: Identification of the Local Environmental Source as Arboreal
- Diverse Host-Seeking Behaviors of Skin-Penetrating Nematodes
- Capsid Protein VP4 of Human Rhinovirus Induces Membrane Permeability by the Formation of a Size-Selective Multimeric Pore
- The Murine Gammaherpesvirus Immediate-Early Rta Synergizes with IRF4, Targeting Expression of the Viral M1 Superantigen to Plasma Cells
- Characterization of an Insecticidal Toxin and Pathogenicity of against Insects
- The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil Chemotaxis
- Histone Deacetylase Inhibitors Impair the Elimination of HIV-Infected Cells by Cytotoxic T-Lymphocytes
- A Locus Encompassing the Epstein-Barr Virus Kinase Regulates Expression of Genes Encoding Viral Structural Proteins
- Distinct APC Subtypes Drive Spatially Segregated CD4 and CD8 T-Cell Effector Activity during Skin Infection with HSV-1
- Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
- Adoptive Transfer of EBV Specific CD8 T Cell Clones Can Transiently Control EBV Infection in Humanized Mice
- Schistosome Feeding and Regurgitation
- EVM005: An Ectromelia-Encoded Protein with Dual Roles in NF-κB Inhibition and Virulence
- Rabies Virus Hijacks and Accelerates the p75NTR Retrograde Axonal Transport Machinery
- Why HIV Virions Have Low Numbers of Envelope Spikes: Implications for Vaccine Development
- Identification of Anti-virulence Compounds That Disrupt Quorum-Sensing Regulated Acute and Persistent Pathogenicity
- HIV-1 Receptor Binding Site-Directed Antibodies Using a VH1-2 Gene Segment Orthologue Are Activated by Env Trimer Immunization
- Cooperation between Epstein-Barr Virus Immune Evasion Proteins Spreads Protection from CD8 T Cell Recognition across All Three Phases of the Lytic Cycle
- Parasite Extracellular Vesicles: Mediators of Intercellular Communication
- RC1339/APRc from Is a Novel Aspartic Protease with Properties of Retropepsin-Like Enzymes
- Cyclic di-GMP-dependent Signaling Pathways in the Pathogenic Firmicute
- Non-random Escape Pathways from a Broadly Neutralizing Human Monoclonal Antibody Map to a Highly Conserved Region on the Hepatitis C Virus E2 Glycoprotein Encompassing Amino Acids 412–423
- Neutrophil Elastase Causes Tissue Damage That Decreases Host Tolerance to Lung Infection with Species
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- SGNH Hydrolase-Like Proteins AlgJ and AlgX Have Similar Topology but Separate and Distinct Roles in Alginate Acetylation
- Why Sexually Transmitted Infections Tend to Cause Infertility: An Evolutionary Hypothesis
- Late Engagement of CD86 after Influenza Virus Clearance Promotes Recovery in a FoxP3 Regulatory T Cell Dependent Manner
- Determinants of Influenza Transmission in South East Asia: Insights from a Household Cohort Study in Vietnam
- A Novel Signal Transduction Pathway that Modulates Quorum Sensing and Bacterial Virulence in
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- A Cysteine Protease Inhibitor of Is Essential for Exo-erythrocytic Development
- EBNA3C Augments Pim-1 Mediated Phosphorylation and Degradation of p21 to Promote B-Cell Proliferation
- On the Front Line: Quantitative Virus Dynamics in Honeybee ( L.) Colonies along a New Expansion Front of the Parasite
- Assembly and Architecture of the EBV B Cell Entry Triggering Complex
- NLR-Associating Transcription Factor bHLH84 and Its Paralogs Function Redundantly in Plant Immunity
- The PDZ-Binding Motif of Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Is a Determinant of Viral Pathogenesis
- Strain-Specific Properties and T Cells Regulate the Susceptibility to Papilloma Induction by Papillomavirus 1
- Human Cytomegalovirus pUL79 Is an Elongation Factor of RNA Polymerase II for Viral Gene Transcription
- The GAP Activity of Type III Effector YopE Triggers Killing of in Macrophages
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- Pathogenicity and Epithelial Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



