-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFrom Dandruff to Deep-Sea Vents: -like Fungi Are Ecologically Hyper-diverse
article has not abstract
Published in the journal: . PLoS Pathog 10(8): e32767. doi:10.1371/journal.ppat.1004277
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004277Summary
article has not abstract
Introduction
As the dominant component of the mycobiota on human skin [1] —both healthy and diseased [2] —the genus Malassezia has received a fair amount of attention. Since the middle of the 19th century, researchers have linked these fungi with skin maladies such as dandruff and eczema [3], but their difficulty to culture axenically long hampered studies of their systematics and diversity [4]. Malassezia is the sole genus within the fungal order Malasseziales, contained within the proposed subphylum Malasseziomycetes (anonymous reviewer; personal communication). Although Malassezia is sister to the so-called “smut” plant pathogens, they are markedly divergent in ecological terms. A hallmark of Malassezia species is their incomplete fatty acids synthesis metabolic pathway, and reliance, instead, on a suite of extracellular lipases, phospholipases, and acid sphingomyelinases [5]. In fact, only a single species, M. pachydermatis, is able to survive in axenic culture lacking lipid amendment [6].
Until recently, it was assumed that Malassezia evolved into a specialized and narrow niche associated with the skin of mammalian hosts. However, culture-independent studies of fungi from environmental samples show that Malassezia are exceedingly widespread and ecologically diverse [3]. Recent studies in little-characterized marine environments point to extensive diversification of Malassezia-like organisms, providing exciting opportunities to explore the ecology, evolution and diversity of this enigmatic group.
What Do We Know about the Diversity and Distribution of Putative Malassezia spp. from Environmental Sequences?
Despite being difficult to cultivate, putative Malassezia are readily detected in environmental DNA samples using standard fungal “barcoding” approaches. Scanning GenBank and the scientific literature, therefore, is useful for approximating occurrence patterns. DNA sequences identical to M. globosa and M. restricta, which are both well characterized as human skin associates, appear to be cosmopolitan. M. restricta may be particularly widespread, and DNA sequences similar to these species have been detected in habitats as diverse as deep-sea sediments [7], hydrothermal vents [8], stony corals [9], lobster larval guts [10], Japanese Eel (Anguilla japonica) gut and muscle tissue [11], Antarctic soils [12], [13], on the exoskeleton of soil nematodes [14], and various plant roots including mycoheterotrophic species such as orchids (e.g., [15]). Remarkably, the ribosomal DNA sequences of Malassezia in these studies are nearly identical to those of human associates, suggesting either a very recent divergence in habitat or else that these organisms are highly tolerant to some of the planet's most extreme environments. Unsurprisingly, Malassezia sequences are not uncommon in studies of human dwellings [16], where human skin contributes substantially to house dust.
Both putatively familiar and novel Malassezia-like organisms are abundant on living marine hosts. Pollock and colleagues [17] report Malassezia dermatitis in captive pinnipeds. Two recent studies of marine biotrophic fungi show that Malassezia-like organisms can numerically dominate fungal communities on invertebrates. A cultivation-independent study of marine sponges from Hawaii [18] revealed a high diversity of Malassezia-like sequences, and indicated that a subset of these differed from the adjacent water column. The authors' analysis further suggested that some of these putative Malassezia taxa are host specific at the species level. In a study of the scleractinian coral Acropora hyacinthus, Amend and colleagues [9] found that a phylogenetically diverse suite of Malassezia-like DNA sequences comprised the majority of fungi on apparently healthy colonies. A single taxon, most closely resembling Malassezia globosa, was significantly more abundant amongst corals located in warmer water.
Are Marine Malassezia Related to Terrestrial Species?
The evolutionary origins of marine Malassezia and their relatedness to better - characterized terrestrial species is a matter of speculation. A phylogeny compiled from environmental samples and sequenced isolates (Figure 1) demonstrates a tremendous amount of phylogenetic novelty contained within and adjacent to the Malassezia lineage. Evidence from both large and small subunit loci of the ribosomal cistron demonstrate well-supported clades from various environments, including a large monophyletic group of marine water column mycoplankton, sequences from separate studies of marine anoxic environments, and combinations of host-associated (coral and coralline algae) Malassezia that group with presumably free-living taxa in various marine and terrestrial habitats. The relatively long branch lengths separating some of these isolates from their sister taxa suggest either a particularly rapid diversification, or, alternatively, that intermediate taxa remain to be sampled and sequenced.
Fig. 1. Phylogenetic Tree of Malassezia-like sequences derived from environmental DNA sequences and isolates. 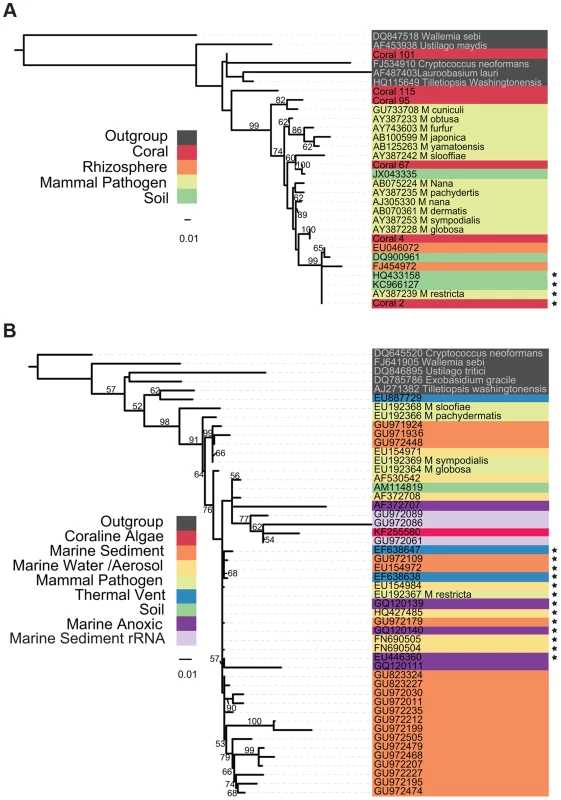
Tree topology was inferred using the best of 1,000 bootstrap replicates of a Maximum Likelihood tree building algorithm (RAxML). Bootstrap support values >50% are indicated. Organism origin is indicated by highlight coloring. Sequences with 99% or greater identity to M. restricta are starred. (A) Tree containing sequences from the D1 and D2 variable domains of the large ribosomal subunit. Coral sequences are deposited in the NCBI SRA, accession #SRP005168. (B) Tree containing sequences spanning the V4 hypervariable region of the ribosomal small subunit; most sequences were compiled in [19]. A phylogeny of environmental sequences from putative Malassezia shows that marine taxa are interdigitated amongst those from human hosts and other terrestrial substrates and do not form a single monophyletic clade. This topology suggests repeated transitions between marine and terrestrial habitats, a pattern not atypical of marine fungi within other Dikarya lineages, including yeasts [19]. The relative ease with which fungi transition between such different environments may owe to the strength of chitinous cell walls, which withstands increased water and osmotic pressures in deep saline habitats [19].
What Can We Infer about the Ecology and Trophic Status of Marine Malassezia?
The tremendous diversity of habitats in which Malassezia-like organisms are found suggests that marine species of this group may incorporate a spectrum of trophic strategies ranging from saprotrophy to biotrophy. Resident Malassezia-like organisms on seemingly healthy coral and sponge hosts may be commensals, latent pathogens awaiting host immunosuppression, or both, depending on host and environmental context. A study of crustose coralline algae around Palmyra Atoll found that a Malassezia phylotype was abundant in banding disease lesions [20]. Incidence of the disease increased by an order of magnitude following an el Niño event. A laboratory manipulation study showed that disease virulence correlated with an interaction between increases in CO2 and temperature. Despite efforts, the authors were unable to cultivate the fungus, and it remains to be tested if Malassezia is the cause or merely a symptom of the banding disease. Nevertheless, the study presents the possibility that a putative Malassezia may act as a pathogen in nonmammal hosts under certain environmental contexts. The high incidence and virulence of the disease raises the possibility that when combined with environmental perturbations, marine Malassezia may even exert bottom-up control on reef community structure.
How Do We Know that Malassezia Detected in Marine Environmental DNA Aren't Contaminants?
Given the high incidence of Malassezia species on human skin [1], [2], a healthy skepticism is warranted since mammalian skin cells from terrestrial sources could potentially accumulate in marine samples, or contamination by lab personnel could result in false positives. Potential for contamination is particularly high when environmental DNA sequences are generated using sensitive, high-throughput methods. Nevertheless, multiple lines of evidence support the position of Malassezia-like organisms as true marine residents. Edgcomb and colleagues [21] reported a high proportion of Malassezia-like sequences in deep-sea sediments detected by sequencing environmental RNA. Because single stranded RNA degrades quickly in situ, its presence supports the notion of active growth as opposed to DNA “contamination” in this habitat. Furthermore, the RNA sequences were distinct from those of any organism known to associate with mammalian hosts, excluding the possibility of lab contamination. A follow-up study using even more stringent protocols and negative controls to exclude exogenous nucleic acids detected Malassezia-like sequences in samples located at depths of 1.6 and 45.1 meters below the sea floor [22]. Fungal community composition overall was highly correlated with site geochemistry, suggesting the environmental selection of a metabolically active assemblage. Similarly, an analysis of actively transcribed genes (mRNA) from a coral habitat identified components of multiple metabolic pathways allied with sequenced Malassezia genomes [9] —further evidence that these fungi are alive and metabolically active underwater. The fact that Malassezia-like organisms are frequently found in remote marine locations far from humans (e.g., [7], [8], [21], [23]–[27], and many others) also renders the terrestrial input hypothesis less likely.
What Are Future Directions for Research into Marine Malassezia?
The remarkable environmental plasticity of M. restricta lends itself to population-level studies of adaptation and acclimatization among the Earth's most extreme environments. How do differences in gene content and transcription correlate with residence in arctic soils versus deep-sea vents? What traits mark the transition from saprobic to pathogenic lifestyles? How many times has a marine (or terrestrial) lifestyle evolved independently?
As a model system, the genus Malassezia has much to offer: three sequenced genomes, M. globosa, M. restricta, and M. sympodialis, contain fewer than 9 Mb and 5,000 genes [28], [29], placing them amongst the smallest free-living genomes in the kingdom Fungi. Furthermore, although the sexual cycle has not been observed in this group, a genomic signature of bipolar mating exists [3], [5], [29], [30]. The genus Malassezia contains a rich and potentially novel suite of enzymes and metabolites [2] from a variety of inhospitable and relatively unexplored habitats.
Arguably, the greatest challenge to studying marine Malassezia is in obtaining axenic cultures, and no marine isolates, to my knowledge, have been recovered to date. Even among species associated with the better-characterized terrestrial mammalian hosts, lab cultivation can be a hit-or-miss affair, involving media that requires a variety of specialized and exotic fatty acids [2], [3], [6]. Studying marine environments introduces additional complexities, such as requiring specialized pressure and salinity growth conditions,that can further complicate cultivation efforts.
Nevertheless, there is much to learn about the basic biology and physiology of host-associated marine Malassezia-like organisms independent of culturing. Some of these questions can be addressed using microscopy in conjunction with labeling techniques such as fluorescence in situ hybridization (FISH). Even basic questions about where Malassezia-like organisms reside on corals remains to be answered. Are Malassezia-like organisms associated with coral mucus, for example, or are they more closely associated with the dinoflagellate symbionts? Does their exclusion affect host fitness? Is there evidence of host specificity or co-evolution?
Conclusions
Analysis of environmental sequences demonstrates that putative members of the Malassezia lineage likely rank among the most widespread fungi on the planet. They are found in a startling diversity of habitats and locations, from polar regions to deep-sea vents. Malassezia-like species appear to dominate certain marine habitats, which should most certainly be the focus of future research into the diversity and distribution of this enigmatic group. Clearly, considering Malassezia a mere epidermis-commensal is a definition that is only skin deep.
Zdroje
1. FindleyK, OhJ, YangJ, ConlanS, DemingC, et al. (2013) Topographic diversity of fungal and bacterial communities in human skin. Nature 498 : 367–370 doi:10.1038/nature12171
2. AshbeeHR (2007) Update on the genus Malassezia. Med Mycol 45 : 287–303 doi:10.1080/13693780701191373
3. Boekhout T, Guého E, Mayser P, Velegraki A, editors (2010) Malassezia and the Skin: Science and Clinical Practive. Berlin: Springer. 1 pp. doi:10.1007/978-3-642-03616-3
4. GuéhoE, MidgleyG, GuillotJ (1996) The genus Malassezia with description of four new species. Antonie Van Leeuwenhoek 69 : 337–355 doi:10.1007/BF00399623
5. XuJ, SaundersCW, HuP, GrantRA, BoekhoutT, et al. (2007) Dandruff-associated Malassezia genomes reveal convergent and divergent virulence traits shared with plant and human fungal pathogens. Proc Natl Acad Sci U S A 104 : 18730–18735 doi:10.1073/pnas.0706756104
6. CabañesFJ (2014) Malassezia Yeasts: How many species infect humans and animals? PLoS Pathog 10: e1003892 doi:10.1371/journal.ppat.1003892.t001
7. LaiX, CaoL, TanH, FangS, HuangY, et al. (2007) Fungal communities from methane hydrate-bearing deep-sea marine sediments in South China Sea. ISME J 1 : 756–762 doi:10.1038/ismej.2007.51
8. Le CalvezT, BurgaudG, MaheS, BarbierG, VandenkoornhuyseP (2009) Fungal diversity in deep-sea hydrothermal ecosystems. Appl Environ Microbiol 75 : 6415–6421 doi:10.1128/AEM.00653-09
9. AmendAS, BarshisDJ, OliverTA (2012) Coral-associated marine fungi form novel lineages and heterogeneous assemblages. ISME J 6 : 1291–1301 doi:10.1038/ismej.2011.193
10. O'RorkeR, LaverySD, WangM, NodderSD, JeffsAG (2013) Determining the diet of larvae of the red rock lobster (Jasus edwardsii) using high-throughput DNA sequencing techniques. Mar Biol 161 : 551–563 doi:10.1007/s00227-013-2357-7
11. TeraharaT, ChowS, KurogiH, LeeS-H, TsukamotoK, et al. (2011) Efficiency of peptide nucleic acid-directed PCR clamping and Its application in the investigation of natural diets of thejapanese eel Leptocephali. PLoS ONE 6: e25715 doi:10.1371/journal.pone.0025715
12. ArenzBE, HeldBW, JurgensJA, FarrellRL, BlanchetteRA (2006) Fungal diversity in soils and historic wood from the Ross Sea Region of Antarctica. Soil Biol Biochem 38 : 3057–3064 doi:10.1016/j.soilbio.2006.01.016
13. FellJW, ScorzettiG, ConnellL, CraigS (2006) Biodiversity of micro-eukaryotes in Antarctic Dry Valley soils with. Soil Biol Biochem 38 : 3107–3119 doi:10.1016/j.soilbio.2006.01.014
14. RenkerC, AlpheiJ, BuscotF (2003) Soil nematodes associated with the mammal pathogenic fungal genus Malassezia (Basidiomycota: Ustilaginomycetes) in Central European forests. Biol Fertil Soils 37 : 70–72 doi:10.1007/s00374-002-0556-3
15. RoyM, WatthanaS, StierA, RichardF, VessabutrS, et al. (2009) Two mycoheterotrophic orchids from Thailand tropical dipterocarpacean forests associate with a broad diversity of ectomycorrhizal fungi. BMC Biol 7 : 51 doi:10.1186/1741-7007-7-51
16. PitkarantaM, MeklinT, HyvarinenA, PaulinL, AuvinenP, et al. (2007) Analysis of fungal flora in indoor dust by ribosomal DNA sequence analysis, quantitative PCR, and culture. Appl Environ Microbiol 74 : 233–244 doi:10.1128/AEM.00692-07
17. PollockCG, RohrbachB, RamsayEC (2000) Fungal dermatitis in captive pinnipeds. J Zoo Wildl Med 31 : 374–378 doi:%%;10.1638/1042-7260(2000)031%5B0374:FDICP%5D2.0.CO;2
18. GaoZ, LiB, ZhengC, WangG (2008) Molecular Detection of Fungal Communities in the Hawaiian Marine Sponges Suberites zeteki and Mycale armata. Appl Environ Microbiol 74 : 6091–6101 doi:10.1128/AEM.01315-08
19. RichardsTA, JonesMDM, LeonardG, BassD (2012) Marine Fungi: Their Ecology and Molecular Diversity. Annu Rev Marine Sci 4 : 495–522 doi:10.1146/annurev-marine-120710-100802
20. WilliamsGJ, PriceNN, UshijimaB, AebyGS, CallahanS, et al. (2014) Ocean warming and acidification have complex interactive effects on the dynamics of a marine fungal disease. Proc R Soc Lond B Biol Sci 281 : 20133069–20133069 doi:10.1098/rspb.2013.3069
21. EdgcombVP, BeaudoinD, GastR, BiddleJF, TeskeA (2011) Marine subsurface eukaryotes: the fungal majority. Environ Microbiol 13 : 172–183 doi:10.1111/j.1462-2920.2010.02318.x
22. OrsiW, OrsiW, BiddleJF, BiddleJF, EdgcombV, et al. (2013) Deep Sequencing of Subseafloor Eukaryotic rRNA Reveals Active Fungi across Marine Subsurface Provinces. PLoS ONE 9: e56335.
23. WilliamsNM, CroneEE, RoulstonTH, MinckleyRL, PackerL, et al. (2010) Biological Conservation. Biol Conserv 143 : 2280–2291 doi:10.1016/j.biocon.2010.03.024
24. SinghP, RaghukumarC, VermaP, ShoucheY (2010) Fungal Community Analysis in the Deep-Sea Sediments of the Central Indian Basin by Culture-Independent Approach. Microb Ecol 61 : 507–517 doi:10.1007/s00248-010-9765-8
25. JebarajCS, RaghukumarC, BehnkeA, StoeckT (2010) Fungal diversity in oxygen-depleted regions of the Arabian Sea revealed by targeted environmental sequencing combined with cultivation. FEMS Microbiol Ecol 71 : 399–412 doi:10.1111/j.1574-6941.2009.00804.x
26. BassD, HoweA, BrownN, BartonH, DemidovaM, et al. (2007) Yeast forms dominate fungal diversity in the deep oceans. Proc R Soc Lond B Biol Sci 274 : 3069–3077 doi:10.1093/nar/28.23.4698
27. GaoZ, JohnsonZI, WangG (2009) Molecular characterization of the spatial diversity and novel lineages of mycoplankton in Hawaiian coastal waters. ISME J 4 : 111–120 doi:10.1038/ismej.2009.87
28. SaundersCW, ScheyniusA, HeitmanJ (2012) Malassezia Fungi Are Specialized to Live on Skin and Associated with Dandruff, Eczema, and Other Skin Diseases. PLoS Pathog 8: e1002701 doi:10.1371/journal.ppat.1002701.g001
29. GiotiA, NystedtB, LiW, XuJ, AnderssonA, et al. (2013) Genomic insights into the atopic eczema-associated skin commensal yeast Malassezia sympodialis. MBio 4: e00572–12 doi:10.1128/mBio.00572-12
30. CoelhoMA, SampaioJP, GoncalvesP (2013) Living and Thriving on the Skin: Malassezia Genomes Tell the Story. MBio 4: e00117–13–e00117–13 doi:10.1128/mBio.00117-13
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial PathogensČlánek A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during TransmissionČlánek The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil ChemotaxisČlánek Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Regulatory RNAs Involved in Bacterial Antibiotic Resistance
- From Dandruff to Deep-Sea Vents: -like Fungi Are Ecologically Hyper-diverse
- Pathogenicity and Epithelial Immunity
- Mother–Infant HIV Transmission: Do Maternal HIV-Specific Antibodies Protect the Infant?
- Hell's BELs: acterial 3 igases That Exploit the Eukaryotic Ubiquitin Machinery
- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Primary Seronegative but Molecularly Evident Hepadnaviral Infection Engages Liver and Induces Hepatocarcinoma in the Woodchuck Model of Hepatitis B
- TLR2 Signaling Decreases Transmission of by Limiting Bacterial Shedding in an Infant Mouse Influenza A Co-infection Model
- Production of an Attenuated Phenol-Soluble Modulin Variant Unique to the MRSA Clonal Complex 30 Increases Severity of Bloodstream Infection
- Inhibition of the TRAIL Death Receptor by CMV Reveals Its Importance in NK Cell-Mediated Antiviral Defense
- Early Mucosal Sensing of SIV Infection by Paneth Cells Induces IL-1β Production and Initiates Gut Epithelial Disruption
- Limited HIV Infection of Central Memory and Stem Cell Memory CD4+ T Cells Is Associated with Lack of Progression in Viremic Individuals
- Virus-Specific Regulatory T Cells Ameliorate Encephalitis by Repressing Effector T Cell Functions from Priming to Effector Stages
- A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during Transmission
- The HIV-1 Envelope Transmembrane Domain Binds TLR2 through a Distinct Dimerization Motif and Inhibits TLR2-Mediated Responses
- Infection with MERS-CoV Causes Lethal Pneumonia in the Common Marmoset
- VGIII Isolates Causing Infections in HIV/AIDS Patients in Southern California: Identification of the Local Environmental Source as Arboreal
- Diverse Host-Seeking Behaviors of Skin-Penetrating Nematodes
- Capsid Protein VP4 of Human Rhinovirus Induces Membrane Permeability by the Formation of a Size-Selective Multimeric Pore
- The Murine Gammaherpesvirus Immediate-Early Rta Synergizes with IRF4, Targeting Expression of the Viral M1 Superantigen to Plasma Cells
- Characterization of an Insecticidal Toxin and Pathogenicity of against Insects
- The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil Chemotaxis
- Histone Deacetylase Inhibitors Impair the Elimination of HIV-Infected Cells by Cytotoxic T-Lymphocytes
- A Locus Encompassing the Epstein-Barr Virus Kinase Regulates Expression of Genes Encoding Viral Structural Proteins
- Distinct APC Subtypes Drive Spatially Segregated CD4 and CD8 T-Cell Effector Activity during Skin Infection with HSV-1
- Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
- Adoptive Transfer of EBV Specific CD8 T Cell Clones Can Transiently Control EBV Infection in Humanized Mice
- Schistosome Feeding and Regurgitation
- EVM005: An Ectromelia-Encoded Protein with Dual Roles in NF-κB Inhibition and Virulence
- Rabies Virus Hijacks and Accelerates the p75NTR Retrograde Axonal Transport Machinery
- Why HIV Virions Have Low Numbers of Envelope Spikes: Implications for Vaccine Development
- Identification of Anti-virulence Compounds That Disrupt Quorum-Sensing Regulated Acute and Persistent Pathogenicity
- HIV-1 Receptor Binding Site-Directed Antibodies Using a VH1-2 Gene Segment Orthologue Are Activated by Env Trimer Immunization
- Cooperation between Epstein-Barr Virus Immune Evasion Proteins Spreads Protection from CD8 T Cell Recognition across All Three Phases of the Lytic Cycle
- Parasite Extracellular Vesicles: Mediators of Intercellular Communication
- RC1339/APRc from Is a Novel Aspartic Protease with Properties of Retropepsin-Like Enzymes
- Cyclic di-GMP-dependent Signaling Pathways in the Pathogenic Firmicute
- Non-random Escape Pathways from a Broadly Neutralizing Human Monoclonal Antibody Map to a Highly Conserved Region on the Hepatitis C Virus E2 Glycoprotein Encompassing Amino Acids 412–423
- Neutrophil Elastase Causes Tissue Damage That Decreases Host Tolerance to Lung Infection with Species
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- SGNH Hydrolase-Like Proteins AlgJ and AlgX Have Similar Topology but Separate and Distinct Roles in Alginate Acetylation
- Why Sexually Transmitted Infections Tend to Cause Infertility: An Evolutionary Hypothesis
- Late Engagement of CD86 after Influenza Virus Clearance Promotes Recovery in a FoxP3 Regulatory T Cell Dependent Manner
- Determinants of Influenza Transmission in South East Asia: Insights from a Household Cohort Study in Vietnam
- A Novel Signal Transduction Pathway that Modulates Quorum Sensing and Bacterial Virulence in
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- A Cysteine Protease Inhibitor of Is Essential for Exo-erythrocytic Development
- EBNA3C Augments Pim-1 Mediated Phosphorylation and Degradation of p21 to Promote B-Cell Proliferation
- On the Front Line: Quantitative Virus Dynamics in Honeybee ( L.) Colonies along a New Expansion Front of the Parasite
- Assembly and Architecture of the EBV B Cell Entry Triggering Complex
- NLR-Associating Transcription Factor bHLH84 and Its Paralogs Function Redundantly in Plant Immunity
- The PDZ-Binding Motif of Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Is a Determinant of Viral Pathogenesis
- Strain-Specific Properties and T Cells Regulate the Susceptibility to Papilloma Induction by Papillomavirus 1
- Human Cytomegalovirus pUL79 Is an Elongation Factor of RNA Polymerase II for Viral Gene Transcription
- The GAP Activity of Type III Effector YopE Triggers Killing of in Macrophages
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- Pathogenicity and Epithelial Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



