-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPathogenicity and Epithelial Immunity
article has not abstract
Published in the journal: . PLoS Pathog 10(8): e32767. doi:10.1371/journal.ppat.1004257
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004257Summary
article has not abstract
Candida species are one of the most common fungal pathogens of humans and the causative agents of superficial and invasive candidiasis. The vast majority of Candida infections are mucosal, manifesting as vaginal or oral candidiasis, which together account for an estimated 40 million infections per year. High-level Candida colonisation is also associated with several gut diseases, including Crohn's disease and ulcerative colitis, and reducing fungal burdens reduces disease severity [1]. Additionally, Candida species are an ever-increasing problem in immunocompromised patients. Furthermore, in common with the vast majority of life-threatening systemic infections, systemic Candida infections are usually acquired through mucosal surfaces. Therefore, it is of paramount importance to understand how epithelial tissues detect and restrict these pathogens to mucosal surfaces.
Which Candida albicans Factors Are Required for Mucosal Infections?
The most common Candida species that causes human mucosal infections is Candida albicans, an endogenous commensal in approximately 50% of individuals. C. albicans is able to undergo morphological switching between a yeast and hyphal form, and the ability to switch to the hyphal form is a critical feature of pathogenicity at mucosal surfaces. Several unique hyphal proteins such as hyphal wall protein 1 (Hwp1p) and agglutinin-like sequence 3 (Als3p) have been identified as virulence attributes by promoting epithelial attachment and invasion [2]. In addition, other virulence factors that promote C. albicans pathogenicity (e.g., biofilm formation, hydrolytic enzyme production) are also linked to hypha formation. Furthermore, C. albicans strains unable to produce hyphae or maintain hypha formation are non-invasive and avirulent in vitro and in murine models of mucosal infection [3], [4], indicating a key role for hypha formation in C. albicans pathogenicity.
How Is C. albicans Recognised by Epithelial Cells?
Mucosal surfaces comprise epithelial cells, which are the first line of defence against C. albicans. However, the epithelial receptors that trigger immune responses in response to this fungus are largely unknown. In oral epithelial cells, recognition of yeast and hyphal cells can occur via conventional fungal pathogen-associated molecular patterns (PAMPs) (e.g., mannans, β-glucans) and pattern recognition receptors (PRRs) (e.g., toll-like receptors, C-type lectin receptors), but activation of an immune response appears to be independent of these PAMPs and PRRs [5]. Rather, C. albicans appears to interact with epithelial-associated proteins such as E-cadherin and human epidermal growth factor receptor 2 (Her2) [6]. This recognition event triggers the induced endocytosis of C. albicans, providing a mechanism of epithelial cell entry and promoting pathogenicity. Although endocytosis is required for invasion, there is only circumstantial evidence to suggest that endocytosis directly contributes to immune activation [7]. Accordingly, the epithelial PRRs or receptors involved in the induction of pro-inflammatory responses by C. albicans remain to be elucidated.
How Is “Pathogenic” C. albicans Identified?
Given that mucosal surfaces are in constant contact with the microbiome, an important function of epithelial cells is to respond to opportunistic microbes when they become pathogenic in order to raise an appropriate host response. This is achieved predominantly through the activation of cellular signalling mechanisms, including mitogen-activated protein kinase (MAPK), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and phosphatidylinositide 3-kinase (PI3K) pathways. With regard to C. albicans, oral and vaginal epithelial cells detect both the yeast and hyphal form of this fungus (Figure 1) [5], [8], [9]. Despite not inducing damage, C. albicans yeast cells weakly activate all three MAPK pathways (p38, c-Jun N-terminal kinases [JNK], extracellular signal-regulated protein kinases 1 and 2 [ERK1/2]), together with NF-κB and PI3K signalling. This drives the activation of the transcription factors NF-κB and c-Jun (via ERK1/2 and JNK) but is insufficient to induce immune activation (Figure 1). In contrast to yeast cells, C. albicans hyphae also activate MAPK signalling but specifically induce the transcription factor c-Fos (via p38). Furthermore, C. albicans hyphae activate MAPK phosphatase 1 (MKP1) via the ERK1/2 pathway, which is known to regulate MAPK-mediated immune responses. This combination of c-Fos and MKP1 activation is specifically associated with hypha formation and correlates with immune activation. In contrast, activation of the PI3K pathway is involved in protection against hypha-induced damage. While hypha-induced damage triggers host immune defences, the mechanisms that induce these protective PI3K signals are currently unknown.
Fig. 1. C. albicans-induced epithelial cell signalling. 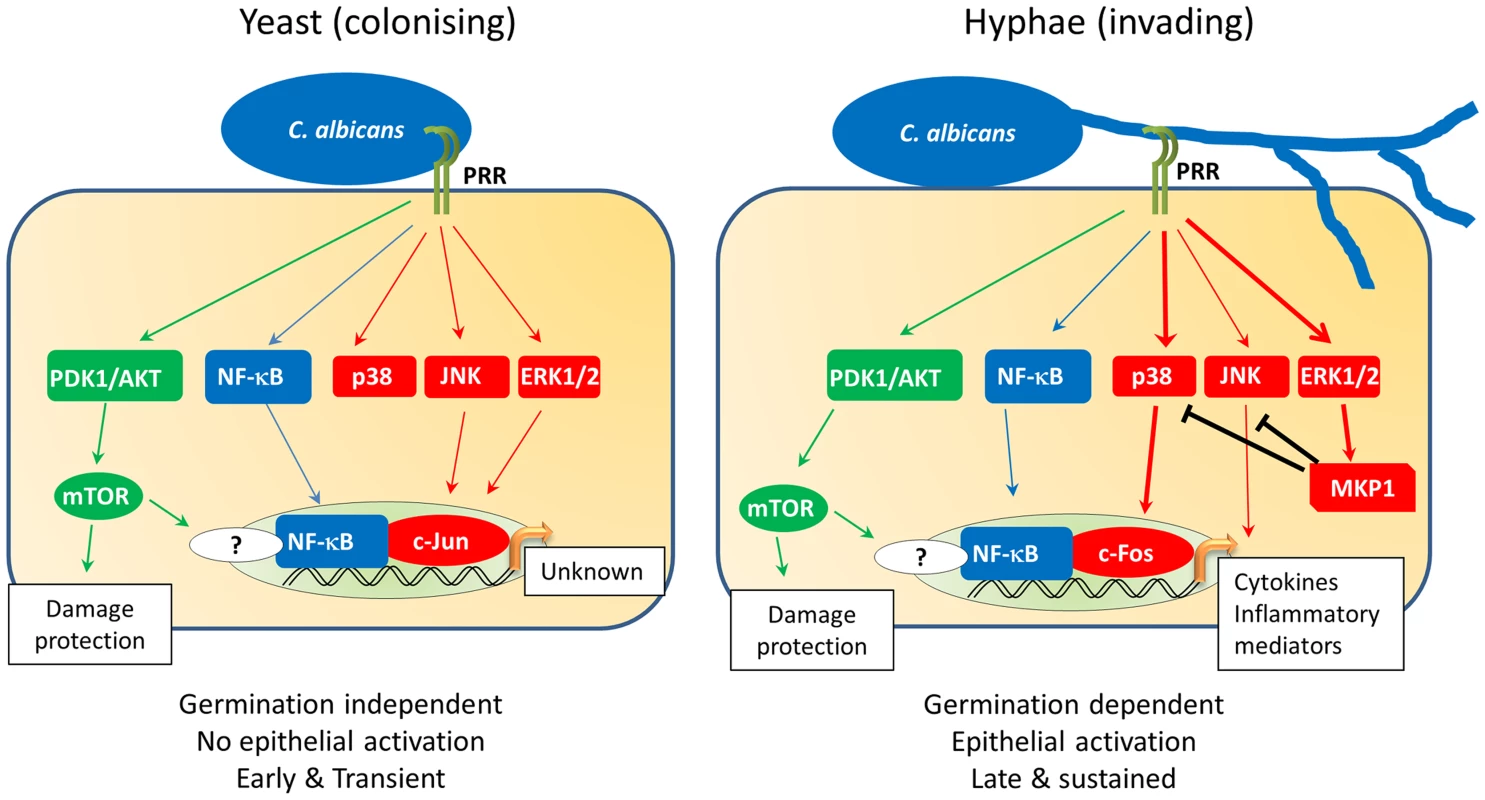
Oral epithelial cell discrimination of C. albicans yeast from hyphae is enabled via differential MAPK signalling. Recognition of yeast triggers activation of PI3K (green) and NF-κB (blue) as well as weak, transient activation of all three MAPK pathways (red). This MAPK activation leads to a transient activation of the c-Jun transcription factor via JNK/ERK1/2 signalling, with as-yet-unknown transcriptional effects. Activation of the PI3K pathway leads to activation of the epithelial damage protection and/or prevention response. Exposure of epithelial cells to C. albicans hyphae leads to the strong activation of MAPK signalling, resulting in the activation of the c-Fos transcription factor via the p38 pathway. At this point, regulation of MAPK signalling is initiated by the induction and stabilisation of the MAPK phosphatase, MKP1 (via the ERK1/2 pathway), which acts to regulate p38 and JNK signalling. Activation of c-Fos in the presence of NF-κB and PI3K signalling leads to the production of cytokines and inflammatory mediators, thereby activating immune responses to C. albicans. How Does Epithelial Activation Induce Innate Immunity?
Epithelial activation by C. albicans hyphae induces pro-inflammatory immune responses, which results in the recruitment of innate immune cells, particularly neutrophils (Figure 2). Neutrophils protect against C. albicans infection directly through phagocytosis and neutrophil extracellular trap (NET) formation [10] and indirectly via immunological cross-talk with the epithelium [11]. However, neutrophils do not play an obvious protective role during vaginal infection and might even exacerbate disease in humans [12]. Other key epithelial responses include the production of antimicrobial peptides (e.g., β-defensins, cathelicidin), alarmins (e.g., S100A8/9), and matrix metalloproteases; together these help combat fungal infections, initiate other immune responses, and promote epithelial remodelling and barrier repair [13]. In addition, oral and vaginal epithelial cells possess direct antifungal activity [14], suggesting that the uppermost epithelial layers are able to naturally prevent C. albicans hypha formation and growth at mucosal surfaces, thereby helping to maintain C. albicans in the commensal state.
Fig. 2. C. albicans recognition and protection at mucosal surfaces. 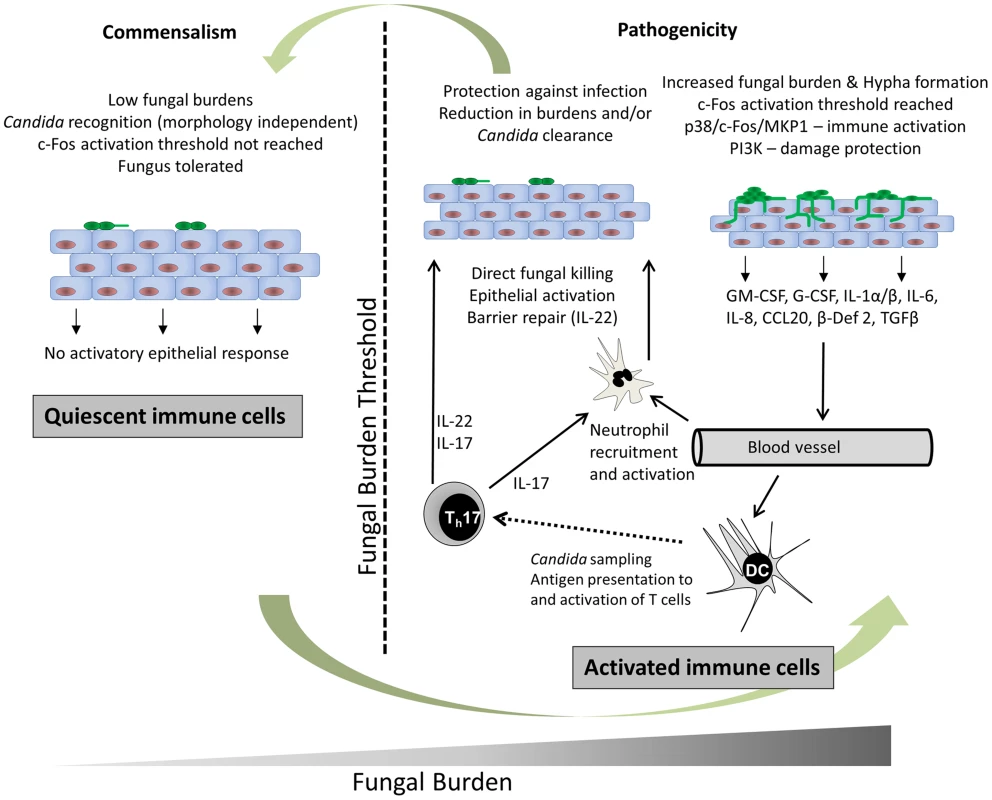
In health (left panel) C. albicans resides in the commensal state, which is characterised by low fungal burdens. C. albicans is recognised but an activation threshold is not reached; thus, the fungus is tolerated without activating epithelial immune responses. During infection (right panel), C. albicans burdens increase and an activation threshold is reached when a sufficient hyphal biomass is present. Immune recognition of C. albicans hyphae occurs via unknown PRR mechanisms but results in the activation of NF-κB, MAPK, and PI3K signalling pathways. Signalling via p38/c-Fos enables discrimination between yeast and hyphae whilst all three pathways (NF-κB, MAPK, and PI3K) promote immune activation, particularly via p38/c-Fos. Finally, PI3K signalling activates epithelial damage protection/prevention mechanisms. Cytokines and chemokines secreted by epithelial cells in response to C. albicans hypha invasion and damage recruit and activate immune cells. IL-8 recruits neutrophils that are in turn activated by GM-CSF, G-CSF, and IL-1 family members. Neutrophils protect directly through phagocytosis and NET (neutrophil extracellular trap) formation and indirectly via immunological cross-talk with epithelial TLR4. CCL20 and β-defensin 2 secretion recruits mucosal-homing CCR6-expressing dendritic cells, which will process fungal antigens and activate Th immunity, including Th17 cells. TGFβ may also act with IL-1α and IL-6 to induce Th17 differentiation. IL-17 production by Th17 cells increases neutrophil activity and IL-22 production promotes epithelial barrier function. Together, these innate and adaptive immune response mechanisms ultimately clear the fungus or reduce fungal burdens below the activation threshold, thereby re-establishing the commensal phenotype. How Does Epithelial Activation Induce Adaptive Immunity?
Epithelial cells initiate adaptive immunity via the production of pro-inflammatory molecules, which act as chemoattractants to recruit mucosal-homing dendritic cells. Dendritic cells recognise C. albicans through PAMPs such as mannans and β-glucan using conventional PRRs, which ultimately results in the activation of different T helper cells (e.g., Th1, Th2, Th17, Tregulatory) in the local draining lymph nodes (Figure 2). The most recent advances in adaptive fungal immunity relate to the recently identified Th17 cells and many of the functions previously ascribed to Th1 cells are now regarded as being functions of this new T cell phenotype. Th17 cells secrete interleukins IL-17A and IL-17F, which stimulate a variety of cells (e.g., epithelial cells and fibroblasts) to produce antimicrobial peptides, metalloproteases, and chemokines, which promotes neutrophil recruitment and activation [15], ultimately resulting in fungal clearance. Th17 cells also secrete IL-22, which limits fungal growth and maintains epithelial barrier function (Figure 2) [16]. Notably, patients with impaired IL-17 production or hyper-IgE syndrome (HIES) are unable to clear mucosal C. albicans infections and develop chronic mucocutaneous candidiasis [17]. Indeed, many studies investigating patients with autoimmune conditions have highlighted the importance of Th17 responses in protection against mucosal candidiasis.
Summary
Mucosal immune responses to C. albicans are highly diverse because of the variety of fungal PAMPs and antigens recognised by different host cells at multiple infection sites. The key function of epithelial cells appears to be discrimination between the morphological status and between the potentially commensal and pathogenic states of C. albicans. Epithelial activation initiates a complex network of immune interactions between host and fungus, which determines the downstream innate and adaptive response that ultimately resolves the infection. Whilst much progress has been made in deciphering the key proteins, cells, and mechanisms contributing to host immunity against Candida, the next few years should provide a leap forward in clinical and translational applications with regard to how Candida infections can be managed and controlled at mucosal surfaces without recourse to antimycotic agents.
Zdroje
1. KumamotoCA (2011) Inflammation and gastrointestinal Candida colonization. Curr Opin Microbiol 14 : 386–391.
2. ZhuW, FillerSG (2010) Interactions of Candida albicans with epithelial cells. Cell Microbiol 12 : 273–282.
3. KamaiY, KubotaM, HosokawaT, FukuokaT, FillerSG (2001) New model of oropharyngeal candidiasis in mice. Antimicrob Agents Chemother 45 : 3195–3197.
4. RahmanD, MistryM, ThavarajS, ChallacombeSJ, NaglikJR (2007) Murine model of concurrent oral and vaginal Candida albicans colonization to study epithelial host-pathogen interactions. Microbes Infect 9 : 615–622.
5. MoyesDL, RunglallM, MurcianoC, ShenC, NayarD, et al. (2010) A Biphasic Innate Immune MAPK Response Discriminates between the Yeast and Hyphal Forms of Candida albicans in Epithelial Cells. Cell Host Microbe 8 : 225–235.
6. ZhuW, PhanQT, BoontheungP, SolisNV, LooJA, et al. (2012) EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc Natl Acad Sci U S A 109 : 14194–14199.
7. VillarCC, KashlevaH, MitchellAP, Dongari-BagtzoglouA (2005) Invasive phenotype of Candida albicans affects the host proinflammatory response to infection. Infect Immun 73 : 4588–4595.
8. MoyesDL, ShenC, MurcianoC, RunglallM, RichardsonJP, et al. (2014) Protection Against Epithelial Damage During Candida albicans Infection Is Mediated by PI3K/Akt and Mammalian Target of Rapamycin Signaling. J Infect Dis 209 : 1816–1826.
9. MoyesDL, MurcianoC, RunglallM, IslamA, ThavarajS, et al. (2011) Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS ONE 6: e26580.
10. UrbanCF, ErmertD, SchmidM, bu-AbedU, GoosmannC, et al. (2009) Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida albicans. PLoS Pathog 5: e1000639.
11. WeindlG, NaglikJR, KaeslerS, BiedermannT, HubeB, et al. (2007) Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling. J Clin Invest 117 : 3664–3672.
12. FidelPLJr, BarousseM, EspinosaT, FicarraM, SturtevantJ, et al. (2004) An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun 72 : 2939–2946.
13. YanoJ, NoverrMC, FidelPLJr (2012) Cytokines in the host response to Candida vaginitis: Identifying a role for non-classical immune mediators, S100 alarmins. Cytokine 58 : 118–128.
14. LillyEA, YanoJ, FidelPLJr (2010) Annexin-A1 identified as the oral epithelial cell anti-Candida effector moiety. Mol Oral Microbiol 25 : 293–304.
15. KornT, BettelliE, OukkaM, KuchrooVK (2009) IL-17 and Th17 Cells. Annu Rev Immunol 27 : 485–517.
16. De LucaA, ZelanteT, D'AngeloC, ZagarellaS, FallarinoF, et al. (2010) IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol 3 : 361–373.
17. LilicD (2012) Unravelling fungal immunity through primary immune deficiencies. Curr Opin Microbiol 15 : 420–426.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial PathogensČlánek A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during TransmissionČlánek The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil ChemotaxisČlánek Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Regulatory RNAs Involved in Bacterial Antibiotic Resistance
- From Dandruff to Deep-Sea Vents: -like Fungi Are Ecologically Hyper-diverse
- Pathogenicity and Epithelial Immunity
- Mother–Infant HIV Transmission: Do Maternal HIV-Specific Antibodies Protect the Infant?
- Hell's BELs: acterial 3 igases That Exploit the Eukaryotic Ubiquitin Machinery
- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Primary Seronegative but Molecularly Evident Hepadnaviral Infection Engages Liver and Induces Hepatocarcinoma in the Woodchuck Model of Hepatitis B
- TLR2 Signaling Decreases Transmission of by Limiting Bacterial Shedding in an Infant Mouse Influenza A Co-infection Model
- Production of an Attenuated Phenol-Soluble Modulin Variant Unique to the MRSA Clonal Complex 30 Increases Severity of Bloodstream Infection
- Inhibition of the TRAIL Death Receptor by CMV Reveals Its Importance in NK Cell-Mediated Antiviral Defense
- Early Mucosal Sensing of SIV Infection by Paneth Cells Induces IL-1β Production and Initiates Gut Epithelial Disruption
- Limited HIV Infection of Central Memory and Stem Cell Memory CD4+ T Cells Is Associated with Lack of Progression in Viremic Individuals
- Virus-Specific Regulatory T Cells Ameliorate Encephalitis by Repressing Effector T Cell Functions from Priming to Effector Stages
- A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during Transmission
- The HIV-1 Envelope Transmembrane Domain Binds TLR2 through a Distinct Dimerization Motif and Inhibits TLR2-Mediated Responses
- Infection with MERS-CoV Causes Lethal Pneumonia in the Common Marmoset
- VGIII Isolates Causing Infections in HIV/AIDS Patients in Southern California: Identification of the Local Environmental Source as Arboreal
- Diverse Host-Seeking Behaviors of Skin-Penetrating Nematodes
- Capsid Protein VP4 of Human Rhinovirus Induces Membrane Permeability by the Formation of a Size-Selective Multimeric Pore
- The Murine Gammaherpesvirus Immediate-Early Rta Synergizes with IRF4, Targeting Expression of the Viral M1 Superantigen to Plasma Cells
- Characterization of an Insecticidal Toxin and Pathogenicity of against Insects
- The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil Chemotaxis
- Histone Deacetylase Inhibitors Impair the Elimination of HIV-Infected Cells by Cytotoxic T-Lymphocytes
- A Locus Encompassing the Epstein-Barr Virus Kinase Regulates Expression of Genes Encoding Viral Structural Proteins
- Distinct APC Subtypes Drive Spatially Segregated CD4 and CD8 T-Cell Effector Activity during Skin Infection with HSV-1
- Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
- Adoptive Transfer of EBV Specific CD8 T Cell Clones Can Transiently Control EBV Infection in Humanized Mice
- Schistosome Feeding and Regurgitation
- EVM005: An Ectromelia-Encoded Protein with Dual Roles in NF-κB Inhibition and Virulence
- Rabies Virus Hijacks and Accelerates the p75NTR Retrograde Axonal Transport Machinery
- Why HIV Virions Have Low Numbers of Envelope Spikes: Implications for Vaccine Development
- Identification of Anti-virulence Compounds That Disrupt Quorum-Sensing Regulated Acute and Persistent Pathogenicity
- HIV-1 Receptor Binding Site-Directed Antibodies Using a VH1-2 Gene Segment Orthologue Are Activated by Env Trimer Immunization
- Cooperation between Epstein-Barr Virus Immune Evasion Proteins Spreads Protection from CD8 T Cell Recognition across All Three Phases of the Lytic Cycle
- Parasite Extracellular Vesicles: Mediators of Intercellular Communication
- RC1339/APRc from Is a Novel Aspartic Protease with Properties of Retropepsin-Like Enzymes
- Cyclic di-GMP-dependent Signaling Pathways in the Pathogenic Firmicute
- Non-random Escape Pathways from a Broadly Neutralizing Human Monoclonal Antibody Map to a Highly Conserved Region on the Hepatitis C Virus E2 Glycoprotein Encompassing Amino Acids 412–423
- Neutrophil Elastase Causes Tissue Damage That Decreases Host Tolerance to Lung Infection with Species
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- SGNH Hydrolase-Like Proteins AlgJ and AlgX Have Similar Topology but Separate and Distinct Roles in Alginate Acetylation
- Why Sexually Transmitted Infections Tend to Cause Infertility: An Evolutionary Hypothesis
- Late Engagement of CD86 after Influenza Virus Clearance Promotes Recovery in a FoxP3 Regulatory T Cell Dependent Manner
- Determinants of Influenza Transmission in South East Asia: Insights from a Household Cohort Study in Vietnam
- A Novel Signal Transduction Pathway that Modulates Quorum Sensing and Bacterial Virulence in
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- A Cysteine Protease Inhibitor of Is Essential for Exo-erythrocytic Development
- EBNA3C Augments Pim-1 Mediated Phosphorylation and Degradation of p21 to Promote B-Cell Proliferation
- On the Front Line: Quantitative Virus Dynamics in Honeybee ( L.) Colonies along a New Expansion Front of the Parasite
- Assembly and Architecture of the EBV B Cell Entry Triggering Complex
- NLR-Associating Transcription Factor bHLH84 and Its Paralogs Function Redundantly in Plant Immunity
- The PDZ-Binding Motif of Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Is a Determinant of Viral Pathogenesis
- Strain-Specific Properties and T Cells Regulate the Susceptibility to Papilloma Induction by Papillomavirus 1
- Human Cytomegalovirus pUL79 Is an Elongation Factor of RNA Polymerase II for Viral Gene Transcription
- The GAP Activity of Type III Effector YopE Triggers Killing of in Macrophages
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- Pathogenicity and Epithelial Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



