-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDiverse Host-Seeking Behaviors of Skin-Penetrating Nematodes
Parasitic worms are a significant public health problem. Skin-penetrating worms such as hookworms and the human threadworm Strongyloides stercoralis dwell in the soil before infecting their host. However, how they locate and identify appropriate hosts is not understood. Here we investigated the host-seeking behavior of Str. stercoralis. We found that Str. stercoralis moves quickly and actively searches for hosts to infect. We also found that Str. stercoralis is attracted to human skin and sweat odorants, including many that also attract mosquitoes. We then compared olfactory behavior across parasitic worm species and found that parasites with similar hosts respond similarly to odorants even when they are not closely related, suggesting parasitic worms use olfactory cues to select hosts. A better understanding of host seeking in skin-penetrating worms may lead to novel control strategies.
Published in the journal: . PLoS Pathog 10(8): e32767. doi:10.1371/journal.ppat.1004305
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004305Summary
Parasitic worms are a significant public health problem. Skin-penetrating worms such as hookworms and the human threadworm Strongyloides stercoralis dwell in the soil before infecting their host. However, how they locate and identify appropriate hosts is not understood. Here we investigated the host-seeking behavior of Str. stercoralis. We found that Str. stercoralis moves quickly and actively searches for hosts to infect. We also found that Str. stercoralis is attracted to human skin and sweat odorants, including many that also attract mosquitoes. We then compared olfactory behavior across parasitic worm species and found that parasites with similar hosts respond similarly to odorants even when they are not closely related, suggesting parasitic worms use olfactory cues to select hosts. A better understanding of host seeking in skin-penetrating worms may lead to novel control strategies.
Introduction
Skin-penetrating nematodes such as the threadworm Str. stercoralis and the hookworms Ancylostoma duodenale and Necator americanus (Figure 1A) are intestinal parasites that infect approximately 1 billion people worldwide. Infection with skin-penetrating worms can cause chronic gastrointestinal distress as well as stunted growth and long-term cognitive impairment in children. Moreover, Str. stercoralis infection can be fatal for immunocompromised individuals and infants [1]. Str. stercoralis is endemic in tropical and sub-tropical regions throughout the world, including the United States, and is estimated to infect 30–100 million people worldwide [2]. Infection rates in rural and semi-rural areas are often high, particularly among children. For example, a recent study found that 25% of school children in semi-rural Cambodia were infected with Str. stercoralis [3]. A better understanding of how skin-penetrating worms target human hosts could lead to new strategies for preventing infections.
Fig. 1. Phylogenetic relationships and life cycles of parasitic nematodes. 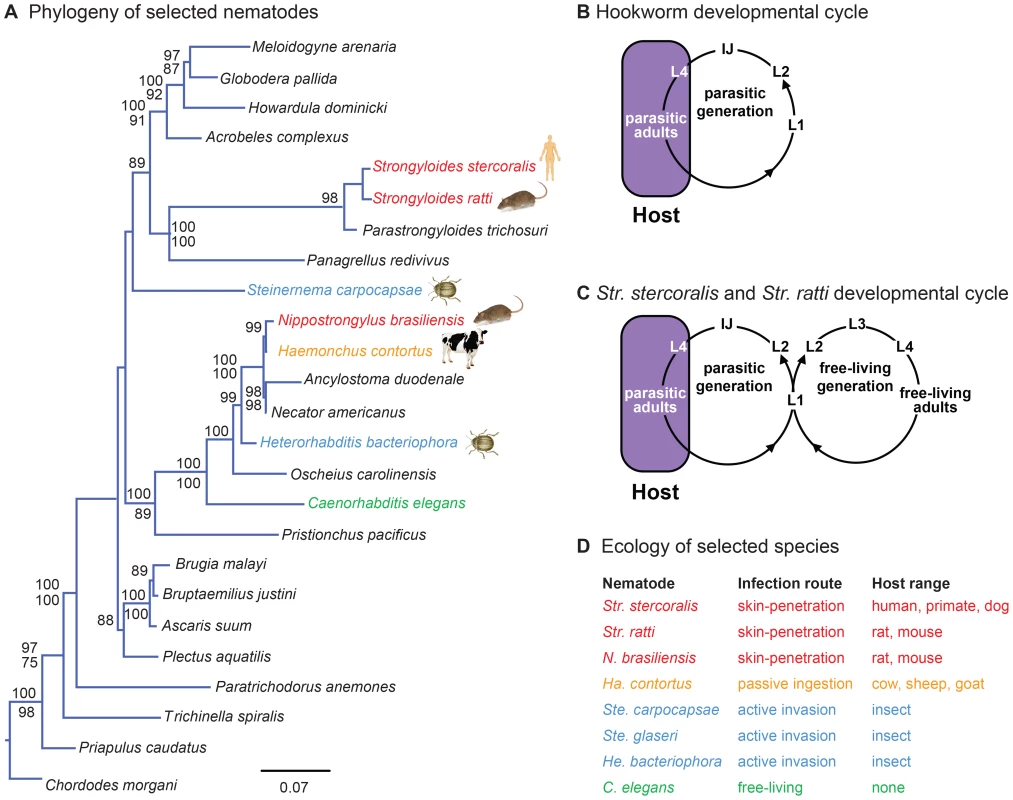
A. Phylogeny of selected nematode species. Phylogenetic analysis is from Dillman et al., 2012 [22]. Species used in the present study are highlighted. Red = skin-penetrating mammalian-parasitic nematode; gold = passively ingested mammalian-parasitic nematode; blue = entomopathogenic nematode; green = free-living nematode. For each of the selected species, icons depict one of their common hosts (human, rat, beetle, or cow). Phylogenetic relationships are based on ML and Bayesian analyses of nearly complete SSU sequences. Values above each branch represent Bayesian posterior probabilities; ML bootstrap indices appear below each branch. Values lower than 75 are not reported. Priapulus caudatus and Chordodes morgani were defined as outgroups. Detailed methods for phylogenetic tree construction are provided in Dillman et al., 2012 [22]. B–C. Life cycles of skin-penetrating nematodes. B. Hookworms must infect a new host every generation. IJs infect hosts by skin-penetration. Nematodes develop to adulthood, reproduce, and lay eggs inside the host. Eggs are excreted in host feces and develop into IJs, which find and infect new hosts. C. Str. stercoralis and Str. ratti can develop through a single generation outside the host. Some larvae excreted in host feces develop into IJs; others develop into free-living adults that mate and reproduce outside the host. All progeny of free-living adults develop into IJs, which find and infect new hosts. L1–L4 are larval stages; IJ = infective juvenile. D. Ecology of selected nematode species. Skin-penetrating nematodes are infective only during a particular stage of their life cycle called the infective juvenile (IJ), a developmentally arrested third larval stage analogous to the C. elegans dauer [4]. IJs inhabit the soil and infect by skin penetration, often through the skin between the toes. Inside the host, IJs migrate through the circulatory system to the lungs, are coughed up and swallowed, and develop to adulthood in the intestine [1]. IJs may also reach the intestine using other migratory routes [5]. Adult nematodes reproduce in the intestine, and eggs or young larvae are excreted in feces. In the case of hookworms, young larvae develop into IJs, which find and infect new hosts (Figure 1B). In the case of Strongyloides species, some larvae develop into IJs and others develop into free-living adults. In the human parasite Str. stercoralis and the rat parasite Str. ratti, which are subjects of this study, all progeny of free-living adults develop into IJs (Figure 1C). Some species of Strongyloides, such as the dog and cat parasite Str. planiceps, can undergo a limited number of sequential free-living generations [6]. Thus, Strongyloides can develop through at least one free-living generation outside the host. Str. stercoralis can also cycle through multiple parasitic generations in the same host, resulting in a potentially fatal disseminated infection [1].
Little is known about the process by which skin-penetrating nematodes find hosts [7]. IJs of some skin-penetrating species respond to heat and sodium chloride [8]–[12], suggesting a role for thermosensation and gustation in host seeking. In addition, Str. stercoralis is attracted to human blood serum and sweat [10], [12], while Str. ratti is attracted to mammalian blood serum [13]. It has long been speculated that olfaction may be important for host seeking, since animals emit unique odor blends that could confer species-specificity [7]. However, the only specific odorant that has so far been found to elicit a response from a skin-penetrating nematode is urocanic acid, a component of mammalian skin that attracts Str. stercoralis [14]. Thus, the extent to which skin-penetrating nematodes use olfactory cues to locate hosts is unclear.
Here we examined the host-seeking strategies and sensory behaviors of the human parasite Str. stercoralis as well as two other species of skin-penetrating nematodes, the rat parasites Str. ratti and Nippostrongylus brasiliensis (Figure 1A, D). We compared their behaviors to those of five other nematode species with diverse lifestyles and ecological niches: the passively ingested ruminant-parasitic nematode Haemonchus contortus; the entomopathogenic nematodes (EPNs) Heterorhabditis bacteriophora, Steinernema glaseri, and Steinernema carpocapsae; and the free-living nematode Caenorhabditis elegans (Figures 1A, D). This across-species analysis was used to fit the behaviors of skin-penetrating nematodes into an ecological framework, and to identify species-specific behavioral differences that reflect differences in phylogeny, host range, or infection route. We found that different species of mammalian-parasitic nematodes employ diverse host-seeking strategies, with the human parasite Str. stercoralis being a cruiser that actively seeks out hosts. We found that Str. stercoralis and the other skin-penetrating nematodes are attracted to skin and sweat odorants, while the passively ingested ruminant parasite Ha. contortus is attracted to the smell of grass. By comparing odor response profiles across species, we found that olfactory preferences reflect host specificity rather than phylogeny, suggesting a critical role for olfaction in the process of host finding and appropriate host selection. Our results provide insight into how skin-penetrating nematodes locate hosts to infect.
Results/Discussion
Mammalian-parasitic nematodes vary in their movement patterns
To gain insight into the host-seeking strategies used by mammalian-parasitic nematodes, we first examined their movement patterns in the absence of chemosensory stimuli. We compared their movement patterns to those of EPNs, which use well-characterized host-seeking strategies: some are “cruisers” that actively search for hosts, some are “ambushers” that wait for passing hosts, and some use an intermediate strategy [9], [15]. We first examined motility using an assay in which IJs were allowed to distribute on an agar plate in the absence of chemosensory stimuli for one hour and the location of IJs on the plate was recorded. We found that the motility of skin-penetrating IJs resembled that of EPN cruisers, with the human parasite Str. stercoralis being the most active (Figure 2A). By contrast, the motility of Ha. contortus resembled that of the ambushing EPN Ste. carpocapsae (Figure 2A). Thus, skin-penetrating IJs appear to be more active than passively ingested IJs.
Fig. 2. Foraging behaviors of skin-penetrating nematodes. 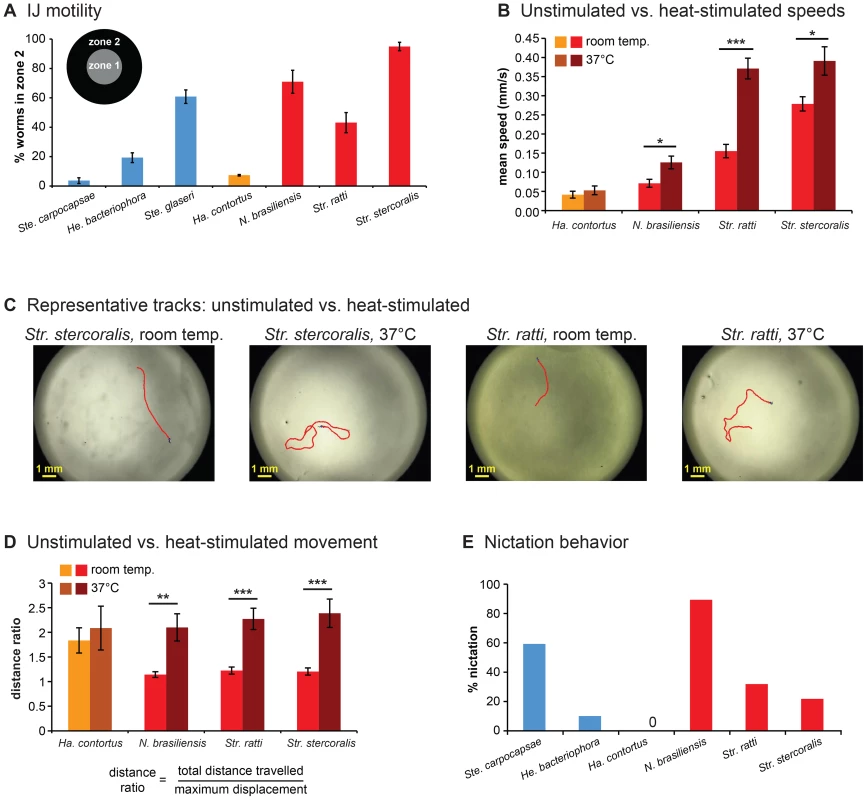
A. IJ motility in the absence of chemosensory stimulation. Motility varies across species (P<0.0001, one-way ANOVA), with Str. stercoralis being the most active (P<0.01, one-way ANOVA with Tukey-Kramer post-test). n = 6–9 trials for each species. For this graph and subsequent graphs with multiple species, red = skin-penetrating; gold = passively ingested; blue = entomopathogenic. Of the three entomopathogenic species, Ste. carpocapsae is considered an ambusher, Ste. glaseri is considered an active cruiser, and He. bacteriophora is considered a less active cruiser [15]. Statistical analysis is shown in Table S1. B. Unstimulated vs. heat-stimulated mean speeds of mammalian-parasitic IJs. Heat-stimulated IJs were exposed to an acute 37°C stimulus and tracked at 37°C. ***, P<0.001; *, P<0.01, unpaired t test or Mann-Whitney test. n = 5–10 trials for each species. C–D. Heat stimulates local search behavior. C. Representative tracks for Str. stercoralis and Str. ratti from 20 s recordings at room temperature versus 37 s recordings at room temperature versus 37°C. D. Movement patterns at room temperature versus 37°C. Distance ratios were calculated as the total track length divided by the maximum displacement attained during the 20 s recording period. A distance ratio of 1 indicates travel in a straight line s recording period. A distance ratio of 1 indicates travel in a straight line; a distance ratio of >1 indicates a curved trajectory. ***, P<0.001; **, P<0.01, Mann-Whitney test. n = 5–10 trials. E. Nictation frequencies of IJs. Nictation was defined as standing or waving behavior of at least 5 s in duration over the course of a 2 min period. Nictation frequencies varied among species (P<0.0001, chi-square test). N. brasiliensis showed a nictation frequency comparable to Ste. carpocapsae (P>0.05, chi-square test with Bonferroni correction) and greater than Str. stercoralis or Str. ratti (P<0.01, chi-square test with Bonferroni correction). Statistical analysis is shown in Table S4. n = 20–28 IJs for each species. For all graphs, error bars indicate SEM. To investigate the host-seeking strategies of skin-penetrating nematodes in more detail, we examined unstimulated movement of IJs using automated worm tracking [16]. We found that parasitic IJs vary dramatically in their crawling speeds, with the human parasite Str. stercoralis moving more rapidly than the other species tested (Figure S1A). The mean speeds of the skin-penetrating rat parasites were comparable to that of the most active EPN, Ste. glaseri, while the mean speed of Ha. contortus resembled that of the less active EPNs (Figure S1A). Turn probability also varied among species but did not correlate with speed (Figure S1B). Some but not all species crawled significantly faster following mechanical stimulation, and in fact the maximum speeds attained by Str. stercoralis, Str. ratti, and Ste. glaseri following mechanical stimulation were similar (Figure S1C–D, Movies S1 and S2). Thus, at least some of the differences in basal crawling speeds among species reflect differences in movement strategy rather than differences in the inherent speeds at which the IJs are capable of crawling.
The fact that Str. stercoralis has a higher basal speed than Str. ratti and N. brasiliensis is consistent with the possibility that host-seeking strategy evolved independently in these species to accommodate host behavior and ecology. Str. ratti and N. brasiliensis are parasites of nesting rodents, which are highly focal with circumscribed resting places. Since parasite transmission likely occurs within the confines of the nest, rapid mobility may not provide an adaptive advantage for these parasites. By contrast, Str. stercoralis is a parasite of humans, primates, and dogs, all of which are highly mobile. Rapid mobility may be necessary for Str. stercoralis to accommodate the mobility of its hosts.
Heat increases crawling speed and stimulates local searching in skin-penetrating nematodes
Heat is emitted by all mammals and is a known sensory cue for some mammalian-parasitic nematodes, including Str. stercoralis [11]. We therefore examined the responses of the mammalian-parasitic IJs to a 37°C heat stimulus. We found that the skin-penetrating nematodes increased their crawling speed in response to thermal stimulation, while the passively ingested nematode Ha. contortus did not (Figure 2B). Skin-penetrating nematodes may increase their speed in response to heat to maximize the likelihood of encountering host skin.
A comparison of IJ movement patterns at room temperature versus 37°C revealed that skin-penetrating IJs show dramatically different movement patterns at the different temperatures. The trajectories of individual IJs were relatively straight at room temperature but highly curved at 37°C (Figure 2C). To quantify these differences, we calculated a distance ratio consisting of the total distance travelled divided by the maximum displacement achieved. We found that all three species of skin-penetrating nematodes showed greater distance ratios at 37°C compared to room temperature (Figure 2D). These results suggest that heat may act as a cue that signifies host proximity and stimulates local searching. However, we note that the temperature at the surface of human skin is 32–35°C [17], and IJ movement within this temperature range remains to be examined.
Nictation behavior varies among mammalian-parasitic nematodes
An important component of host-seeking strategy for many parasitic nematodes is nictation, a behavior in which the worm stands on its tail and waves its head to facilitate attachment to passing hosts [9]. We examined the nictation behavior of mammalian-parasitic nematodes by performing nictation assays on an “artificial dirt” substrate consisting of dense agar with near-microscopic pillars [18], since IJs are not capable of standing on standard agar plates due to the high surface tension on the plates [18]. We found that nictation frequencies varied among species. N. brasiliensis showed a high nictation frequency comparable to that of the ambushing EPN Ste. carpocapsae (Figure 2E and Movie S3), suggesting that it spends most of its foraging time nictating. By contrast, the Strongyloides species showed much lower rates of nictation (Figure 2E and Movie S4), suggesting they spend most of their foraging time crawling. Ha. contortus did not nictate on the artificial dirt substrate or any other substrate tested (see Materials and Methods), suggesting it may not be capable of nictating.
Mammalian-parasitic nematodes utilize diverse host-seeking strategies
Taken together, our results suggest that mammalian-parasitic nematodes employ diverse host-seeking strategies. The skin-penetrating Strongyloides species appear to be cruisers that are highly mobile and tend to crawl rather than nictate. By contrast, the passively ingested nematode Ha. contortus appears to be an ambusher that displays little unstimulated movement. N. brasiliensis can exhibit rapid, prolonged movement comparable to that of the cruisers but tends to nictate rather than crawl, suggesting it is also an ambusher. However, we note that foraging strategy is in some cases substrate-dependent, and different strains of a species can exhibit different host-seeking behaviors [19], [20]. Thus, we cannot exclude the possibility that the host-seeking strategies of these species may vary under conditions not tested here.
Str. stercoralis is attracted to human-emitted odorants
EPNs have been shown to use a diverse array of insect volatiles and herbivore-induced plant volatiles for host finding [21]–[30]. By contrast, only one odorant has so far been identified as an attractant for Str. stercoralis [14]. We therefore tested the extent to which Str. stercoralis displays directed movement in response to human-emitted volatiles. We examined the responses of Str. stercoralis IJs to a large panel of odorants, most of which are known to be emitted by human skin, sweat, and skin microbiota (Table S5). Responses were examined using a chemotaxis assay (Figures S2 and S3) [21], [22]. We found that Str. stercoralis was strongly attracted to a number of these odorants (Figure 3A). Nearly all of the attractants we identified for Str. stercoralis also attract anthropophilic mosquitoes (Figure 3A), suggesting that nematodes and mosquitoes target humans using many of the same olfactory cues. While many of the human-emitted odorants that attracted Str. stercoralis are also emitted by other mammals, 7-octenoic acid is thought to be human-specific [31] and may be used by Str. stercoralis to target humans. Str. stercoralis and disease-causing mosquitoes are co-endemic throughout the world [2], and our results raise the possibility of designing traps that are effective against both parasites.
Fig. 3. Olfactory responses of mammalian-parasitic nematodes. 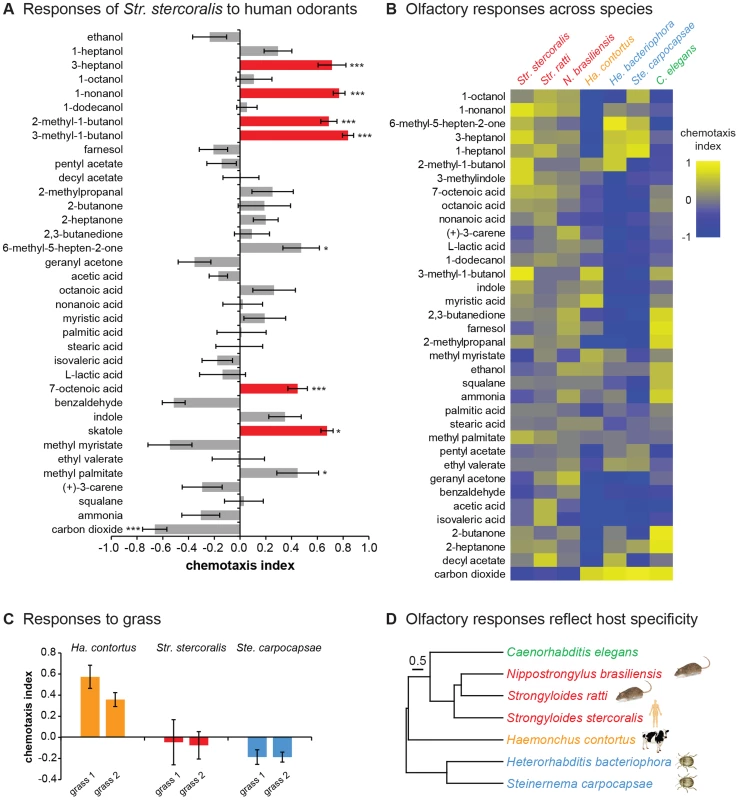
A. Str. stercoralis is attracted to a number of human-emitted odorants. Red = attractants for Str. stercoralis that also attract anthropophilic mosquitoes [31], [52]–[58]. n = 6–23 trials per odorant. Str. stercoralis did not respond to the chemotaxis controls (Figure S3). *, P<0.05; ***, P<0.001 relative to control, t-test (CO2 vs. air and L-lactic acid vs. H2O) or one-way ANOVA with Bonferroni post-test (all other odorants vs. paraffin oil). B. Olfactory responses across species. Response magnitudes are color-coded according to the scale shown to the right of the heat map, and odorants are ordered based on hierarchical cluster analysis. n = 6–14 trials for each odorant-species combination. Each species exhibited a unique odor response profile (P<0.0001, two-way ANOVA with Tukey's post-test). Data for responses of EPNs and C. elegans to 10% CO2 are from Dillman et al., 2012 [22]. Red = skin-penetrating; gold = passively ingested; blue = insect-parasitic; green = free-living. C. Responses of Ha. contortus to grass odor. Responses to the odors of two different grass samples were examined. n = 8–17 trials for each sample. D. Olfactory preferences reflect host specificity rather than phylogeny. The behavioral dendrogram was constructed based on the odor response profiles of each species. Hierarchical cluster analysis was performed using UPGMA (Unweighted Pair Group Method with Arithmetic Mean). Euclidean distance was used as a similarity measure. Hosts (humans, ruminants, rodents, or insects) for each species are indicated. Coph. Corr. = 0.96. For all graphs, error bars indicate SEM. We also examined responses to carbon dioxide (CO2), which is emitted by aerobic organisms in exhaled breath and is an attractant for many parasites, including EPNs [9], [21], [22]. We found that Str. stercoralis was repelled by CO2 at high concentrations and neutral to CO2 at low concentrations, suggesting that CO2 is not a host attractant (Figure 3A and Figure S4A). These results are consistent with the fact that Str. stercoralis infects by skin penetration, and only low levels of CO2 are emitted from skin [32]. However, some EPNs respond synergistically to mixtures of CO2 and other odorants [33], and we cannot exclude the possibility that Str. stercoralis is attracted to CO2 in mixtures or under conditions not tested here.
Olfactory preferences of parasitic nematodes reflect host specificity
The fact that Str. stercoralis responds to human-emitted odorants suggests that olfaction plays an important role in host finding. However, the extent to which Str. stercoralis or any other mammalian-parasitic nematode uses olfactory cues for host selection is not known. To gain insight into whether olfaction contributes to host choice, we compared the olfactory responses of Str. stercoralis to those of six other species: Str. ratti, N. brasiliensis, Ha. contortus, He. bacteriophora, Ste. carpocapsae, and C. elegans. We found that all species responded to a wide array of odorants, indicating that as is the case for EPNs [21], [22], even ambushers are capable of robust chemotaxis (Figure 3B and Figure S4). Moreover, each species exhibited a unique odor response profile, indicating that olfactory responses are species-specific even among closely related species such as Str. stercoralis and Str. ratti (Figure 3B). CO2 response varied greatly among species. Like Str. stercoralis, Str. ratti and N. brasiliensis were repelled by CO2 at high concentrations and neutral to CO2 at low concentrations (Figure 3B and Figure S4B–C). By contrast, Ha. contortus IJs, like EPN IJs and C. elegans dauers [21], [22], were attracted to CO2 (Figure 3B and Figure S4D). To confirm that the observed responses to odorants were olfactory rather than gustatory, we examined the responses of Str. stercoralis and Str. ratti to a subset of odorants in a modified chemotaxis assay in which odorants were placed on the plate lid rather than the plate surface. We found that attractive responses were still observed when the odorants were placed on the plate lid, although the response of Str. stercoralis to one odorant was slightly reduced (Figure S5). Thus, the observed behavioral responses are primarily olfactory, but in some cases may include a gustatory component.
The olfactory preferences of the passively ingested mammalian parasite, Ha. contortus, are consistent with its known ecology. Ha. contortus IJs migrate from the feces of their ruminant hosts to grass blades, where they are ingested by grazing ruminants [34]. The fact that 5% CO2, which approximates the concentration found in exhaled breath [35], was strongly attractive to Ha. contortus (Figure S4D) suggests that Ha. contortus may use exhaled CO2 to migrate toward the mouths of potential hosts. By contrast, Ha. contortus was repelled by many of the skin and sweat odorants tested (Figure 3B), consistent with a lack of attraction to mammalian skin. Of the few attractive odorants we identified for Ha. contortus, two – methyl myristate and myristic acid – are known constituents of cow and goat milk [36]–[38] and may be used by Ha. contortus to migrate toward cows and goats. To test whether Ha. contortus also responds to plant-emitted odorants, we examined responses to freshly cut grass. We found that Ha. contortus is attracted to the smell of grass, while Str. stercoralis and Ste. carpocapsae are not (Figure 3C). These results suggest that Ha. contortus uses CO2 in combination with other ruminant-emitted odorants and grass odorants to position itself for passive ingestion.
We then quantitatively compared odor response profiles across species, and found that species with similar hosts responded more similarly to odorants despite their phylogenetic distance (Figure 3D). For example, the distantly related rat parasites Str. ratti and N. brasiliensis responded similarly to odorants, as did the distantly related insect parasites He. bacteriophora and Ste. carpocapsae. The three skin-penetrating species responded more similarly to each other than to the other species tested, while the passively ingested mammalian parasite Ha. contortus responded very differently from all of the other species tested (Figure 3D). These results indicate that olfactory preferences reflect host specificity and infection mode rather than phylogeny, consistent with a key role for olfaction in host selection.
Olfactory preferences of Strongyloides species are life stage-specific
Skin-penetrating nematodes exit from hosts in feces as eggs or young larvae and subsequently develop into infective larvae outside the host. Thus, both infective and non-infective life stages are present in the environment (Figure 1B–C). This raises the question of whether host attraction is specific to the infective stage. We compared olfactory responses of free-living larvae, free-living adults, and IJs for both Str. stercoralis and Str. ratti in response to a subset of host odorants. We found that all three life stages were robustly attracted to host odorants, suggesting that host attraction is not downregulated in non-infective life stages (Figure 4). The free-living life stages of skin-penetrating worms are thought to reside primarily on host fecal matter, where they feed on bacteria present in the feces [39]. We therefore compared the responses of free-living larvae, free-living adults, and IJs to host feces. We found that responses differed dramatically across life stages: free-living larvae and adults were strongly attracted to feces, while IJs were neutral to host feces (Figure 4). Moreover, while Str. ratti IJs were neutral to both host and non-host feces, Str. stercoralis IJs were neutral to host feces but repelled by non-host feces (Figure 4).
Fig. 4. Olfactory responses of Strongyloides species vary across life stages. 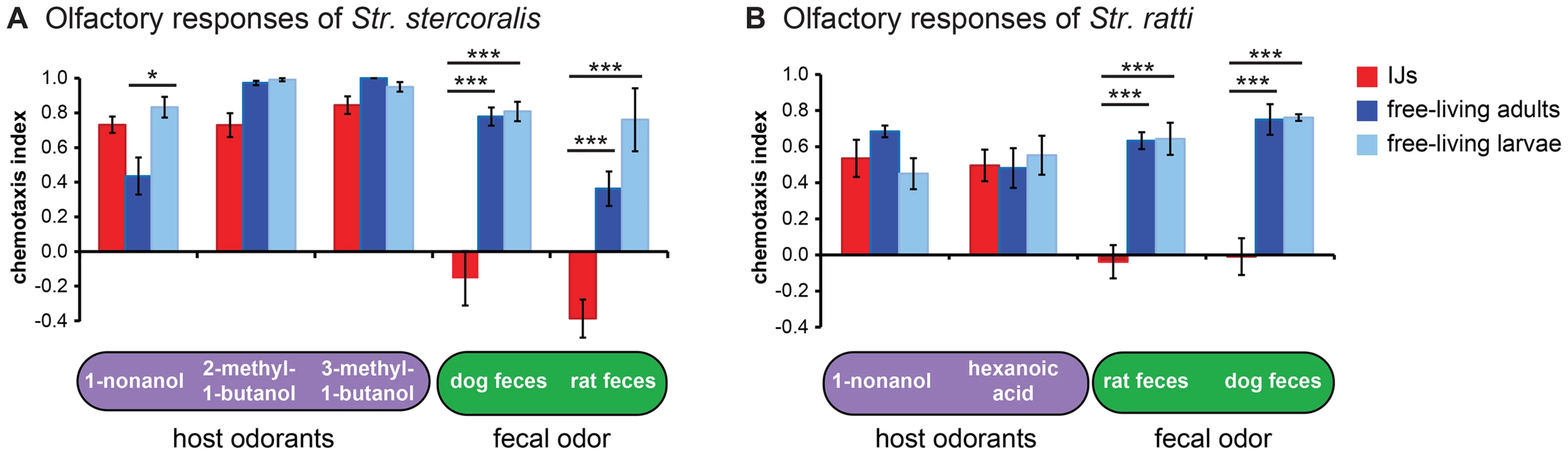
A–B. Responses of either Str. stercoralis (A) or Str. ratti (B) IJs, free-living adults, and free-living larvae to host odorants and fecal odor. *, P<0.05; ***, P<0.001, two-way ANOVA with Tukey's post-test. n = 4–12 trials for Str. stercoralis and n = 6–26 trials for Str. ratti for each condition. Error bars indicate SEM. Our results suggest a model in which all life stages are attracted to host skin odor, but strong attraction to host fecal odor by the free-living life stages causes them to remain on feces. Attraction to fecal odor is downregulated at the infective stage, enabling the IJs to migrate away from the feces in search of hosts. Repulsion of Str. stercoralis IJs from non-host feces may serve as an additional mechanism to prevent foraging in close proximity to non-hosts. To gain insight into the individual odorants that confer changes in sensitivity to feces, we examined responses to two components of fecal odor, skatole and indole [40]. We found that the free-living stages of Str. ratti were highly attracted to both skatole and indole, while the IJs were neutral to both odorants (Figure S6A). Thus, altered sensitivity to these odorants may contribute to the developmental change in the response to fecal odor. By contrast, Str. stercoralis IJs were more attracted to skatole than the free-living life stages and all three life stages were relatively unresponsive to indole (Figure S6B), suggesting that other as yet unidentified odorants mediate the sensitivity of Str. stercoralis to fecal odor.
Implications for nematode control
Str. stercoralis infection is a worldwide cause of chronic morbidity and mortality. Current drugs used to treat nematode infections are inadequate for nematode control: some are toxic, drug resistance is a growing concern, and reinfection rates are high [41]. Our data suggest that Str. stercoralis IJs are fast-moving cruisers that actively search for hosts using a chemically diverse array of human-emitted odorants. The identification of odorants that attract or repel Str. stercoralis and other parasitic nematodes lays a foundation for the design of targeted traps or repellents, which could have broad implications for nematode control.
Materials and Methods
Ethics statement
Gerbils were used for host passage of Str. stercoralis, and rats were used for host passage of Str. ratti and N. brasiliensis. All protocols and procedures were approved by the UCLA Office of Animal Research Oversight (Protocol No. 2011-060-03B), which adheres to the AAALAC standards for laboratory animal use, and were in strict accordance with the Guide for the Care and Use of Laboratory Animals.
Nematodes, vertebrate animals, and insects
Strongyloides stercoralis UPD strain and Strongyloides ratti ED321 strain were provided by Dr. James Lok (University of Pennsylvania). Nippostrongylus brasiliensis was provided by Dr. Edward Platzer (University of California, Riverside). Haemonchus contortus was provided by Dr. Adrian Wolstenholme and Mr. Bob Storey (University of Georgia). Heterorhabditis bacteriophora Oswego strain and Steinernema glaseri VS strain were provided by David Shapiro-Ilan (USDA). Steinernema carpocapsae were from the ALL strain [21], [22], [42]. C. elegans dauers were from the wild isolate CB4856 (“Hawaii”). Male Mongolian gerbils for culturing Str. stercoralis were obtained from Charles River Laboratories. Male or female Long-Evans or Sprague Dawley rats for culturing Str. ratti and N. brasiliensis were obtained either from Harlan Laboratories or second-hand from other investigators at UCLA through the UCLA Internal Animal Transfer supply system for surplus animals. Galleria mellonella larvae for culturing EPNs were obtained from American Cricket Ranch (Lakeside, CA).
Maintenance of Str. stercoralis
Str. stercoralis was serially passaged in gerbils and maintained on fecal-charcoal plates. Inoculation of gerbils with Str. stercoralis was performed essentially as previously described [43]. Briefly, Str. stercoralis IJs were isolated from fecal-charcoal plates using a Baermann apparatus [43]. Each gerbil was subcutaneously injected with 2000 IJs in 200 µl sterile PBS. Gerbils became patent (as defined by the presence of nematodes in gerbil feces) on day 12 post-inoculation and remained patent for approximately 70 days. At 28 and 35 days post-inoculation, each gerbil received 2 mg methylprednisolone (Depo-Medrol, Pfizer) subcutaneously to induce an auto-infective cycle. To harvest infested feces, gerbils were housed overnight in cages containing a wire rack on the bottom of the cage. Fecal pellets fell below the rack onto damp cardboard and were collected the following morning. Feces were mixed with dH2O and autoclaved charcoal (bone char from Ebonex Corp., Cat # EBO.58BC.04) in an approximately 1∶1 ratio of charcoal to feces. The fecal-charcoal mixtures were poured into Petri dishes (10 cm diameter, 20 mm height) lined with wet filter paper, and were stored at 23°C until use. Nematodes used for behavioral analysis were isolated from fecal-charcoal plates using a Baermann apparatus [43] or from plate lids. To obtain free-living larvae (primarily post-parasitic L2s) for chemotaxis assays, nematodes were collected from fecal-charcoal plates after approximately 18 hrs. To obtain free-living adults for chemotaxis assays, nematodes were collected from fecal-charcoal plates after 48 hrs. To obtain IJs, nematodes were collected from fecal-charcoal plates starting at day 5 post-collection. IJs were used for behavioral assays within 2 weeks of fecal collection.
Maintenance of Str. ratti
Str. ratti was serially passaged in rats and maintained on fecal-charcoal plates. Inoculation of rats with Str. ratti was performed essentially as previously described [44]. Briefly, Str. ratti IJs were isolated from fecal-charcoal plates using a Baermann apparatus. Each rat was subcutaneously injected with 700 IJs in 300 µl sterile PBS. Rats became patent on day 6 post-inoculation and remained patent for up to 28 days post-inoculation. To harvest infested feces, rats were housed overnight in cages containing a wire rack on the bottom of the cage. Fecal pellets fell below the rack onto damp cardboard and were collected the following morning. Fecal-charcoal plates were prepared as described above for Str. stercoralis and stored at 23°C until use. Nematodes used for behavioral analysis were isolated from fecal-charcoal plates using a Baermann apparatus [43] or from plate lids. Free-living larvae, adults, and IJs were obtained from fecal-charcoal plates as described above for Str. stercoralis.
Maintenance of N. brasiliensis
N. brasiliensis was serially passaged in rats and maintained on fecal-charcoal plates. To inoculate rats, N. brasiliensis IJs were isolated from fecal-charcoal plates using a Baermann apparatus. Each rat was subcutaneously injected with 4000 IJs in 300 µl sterile PBS. Rats became patent on day 6 post-inoculation and remained patent for up to 14 days. Infested feces were collected as described above for Str. ratti. Fecal-charcoal plates were prepared as described above for Str. stercoralis, except that vermiculite (Fisher catalog # S17729) was added to the feces and charcoal in an approximately 1∶1∶1 ratio of vermiculite to charcoal to feces. Plates were stored at 23°C until use. In some cases, either Nystatin (Sigma catalog # N6261) at a concentration of 200 U/ml or Fungizone (Gibco catalog #15290-018) at a concentration of 1 µg/ml was added to the filter paper on the bottom of the plate to inhibit mold growth. Nematodes used for behavioral analysis were isolated from fecal-charcoal plates using a Baermann apparatus [43] or from plate lids. To obtain IJs, nematodes were collected from fecal-charcoal plates starting at day 7 post-collection. IJs were used for behavioral assays within 2 weeks of fecal collection.
Maintenance of Ha. contortus
Ha. contortus was stored in dH2O at 8°C prior to use. IJs were tested within 6 months of collection. No differences in IJ movement or behavior were observed in freshly collected versus 6 month old IJs. IJ behavior declined after 6 months, so IJs older than 6 months were not tested.
Maintenance of entomopathogenic nematodes (EPNs)
EPNs were cultured as previously described [21]. Briefly, 5 last instar Galleria mellonella larvae were placed in a 5 cm Petri dish with a 55 mm Whatman 1 filter paper acting as a pseudo-soil substrate in the bottom of the dish. Approximately 250 µl containing 500–1000 IJs suspended in water was evenly distributed on the filter paper. After 7–10 days the insect cadavers were placed on White traps [45]. Emerging IJs were collected from the White trap, rinsed 3 times with dH2O, and stored in dH2O until use. Ste. carpocapsae and He. bacteriophora were maintained at 25°C, while Ste. glaseri was maintained at room temperature. IJs were used for behavioral assays within 7 days of collection from the White trap.
Maintenance of C. elegans
C. elegans was cultured on NGM plates seeded with E. coli OP50 according to standard methods [46]. Dauer larvae were collected from the lids of plates from which the nematodes had consumed all of the OP50 and stored in dH2O at room temperature prior to use. Dauer larvae were used for behavioral assays within 2 weeks of collection from plate lids.
Motility assays
30–100 IJs were placed in the center of a chemotaxis plate [47]. IJs were allowed to distribute over the agar surface for 1 hr, after which the percentage of IJs in the outer zone (Zone 2) was determined. Zone 1 was a 4 cm diameter circle centered in the middle of the plate. Zone 2 consisted of the rest of the plate and included the edges of the plate, which acted as a trap since IJs that crawled onto the plate edge desiccated and could not return to the agar surface.
Recording worm movement for automated tracking
Recordings of worm movement were obtained with an Olympus E-PM1 digital camera attached to a Leica S6 D microscope. To quantify unstimulated movement, 4–5 IJs were placed in the center of a chemotaxis plate [47] and allowed to acclimate for 10 min. 20 s recordings were then obtained. Worms that either did not move, that stopped moving during the recording, or that crawled off the assay plate during the recording were excluded from the analysis. To quantify movement before and after mechanical stimulation, IJs were placed on chemotaxis plates and allowed to acclimate for 10 min. prior to tracking. Baseline movement was recorded for approximately 15 s. The plate lid was then removed, the IJ was gently agitated using a worm pick, and post-agitation movement was recorded for approximately 30 s. 5 s recording clips directly following agitation were used to calculate the maximum speeds shown in Figure S1D, and 5 s recording clips directly preceding and following agitation were used to generate the sample tracks shown in Figure S1C. Maximum speeds were calculated in WormAnalyzer (see below) based on changes in worm position over a seven frame (or 0.23 second) window. To quantify movement following thermal stimulation, assays were performed in a 37°C warm room. Chemotaxis assay plates were kept in the warm room prior to use. Individual IJs were transported into the warm room, transferred to assay plates, and immediately recorded for 20 s. For the room temperature control, IJs were similarly transferred to assay plates and immediately recorded for 20 s. Locomotion was quantified using WormTracker and WormAnalyzer multi-worm tracker software (Miriam Goodman lab, Stanford University) [16]. The following WormTracker settings were adjusted from the default settings (designed for C. elegans adults) for analysis of IJ movement: min. single worm area = 20 pixels; max. size change by worm between successive frames = 250 pixels; shortest valid track = 30 frames; auto-thresholding correction factor = 0.001. To calculate turn frequencies, the following WormAnalyzer settings were adjusted from the default settings for analysis of IJ speed: sliding window for smoothing track data = 30 frames; minimum run duration for pirouette identification = 2.9 s for Str. stercoralis, 5.3 s for Ste. glaseri, and 6 s for all other species (to compensate for differences in speed among species). All turns were confirmed by visual observation of worm tracks; turns not confirmed by visual observation were not counted. For calculations of maximum displacement in Figure 2D, the distance between the worm's start point and the farthest point the worm reached during the 20 s recording was calculated in ImageJ.
Nictation assays
Nictation was quantified on “micro-dirt” agar chips cast from polydimethylsiloxane (PDMS) molds as previously described [18], except that chips were made from 5% agar dissolved in dH2O and were incubated at 37°C for 2 hr and then room temperature for 1 hr before use. The micro-dirt chip consisted of agar with near-microscopic pillars covering its surface (pillar height of 25 µm with a radius of 25 µm and an interval between pillars of 25 µm), which allowed IJs to nictate on top of the pillars. For each assay, 3–10 IJs were transferred to the micro-dirt chip and allowed to acclimate on the chip for 10 min. Each IJ was then monitored for 2 min. An IJ was scored as “nictating” if it raised its head off the surface of the chip for a period of at least 5 s during the 2 min assay period. Nictation behavior was also tested on sand. Sand nictation assays were performed essentially as previously described [21], [48]. Sand (silicon dioxide, >230 mesh, CAS 60676-86-0) was distributed onto the surface of a chemotaxis plate using a sieve. IJs were transferred to the plate surface and allowed to acclimate for 10 min. Nictation behavior was then observed for two minutes. In all cases, nictation behavior on sand was consistent with nictation behavior on micro-dirt chips. In the case of Ha. contortus, we also tested for nictation on grass and vermiculite; no nictation was observed on any substrate tested. To test for nictation on grass, grass samples were collected from a lawn seeded with UC Verde Buffalo grass and perennial rye grass (the same lawn as for sample 1 below). The grass was cut into small chunks (∼2.5 mm×2.5 mm) and distributed onto the surface of a chemotaxis plate. IJs were transferred onto the plate surface or directly onto blades of grass, and nictation was scored after a 10 min. acclimation period. Nictation was also scored after 20, 30, or 60 min., or the next day. No nictation was observed with Ha. contortus at any time point.
Odor chemotaxis assays
Odor chemotaxis assays were performed essentially as described [21], [22] (Figure S2). Assays were performed on chemotaxis assay plates [47]. Scoring regions consisted of 2 cm diameter circles on each side of the plate along the diameter with the center of the circle 1 cm from the edge of the plate, as well as the rectangular region extending from the edges of the circle to the edge of the plate. Either 2 µl (for mammalian-parasitic IJs) or 1 µl (for insect-parasitic IJs and C. elegans dauers) of 5% sodium azide was placed in the scoring region as anesthetic. 5 µl of odorant was then placed on the surface of the assay plate in the center of one scoring region, and 5 µl of control (paraffin oil, dH2O, or ethanol) was placed on the surface of the assay plate in the center of the other scoring region. Approximately 200 worms were placed in the center of the assay plate and left undisturbed on a vibration-reducing platform for 3 hours at room temperature. A chemotaxis index (CI) was then calculated as: CI = (# worms at odorant−# worms at control)/(# worms at odorant+control) (Figure S2). A positive CI indicates attraction; a negative CI indicates repulsion. A 3 hour assay duration was used because 3 hour assays were found to be most effective for EPNs [21], [49]. However, 1 hour assays were also performed with Str. ratti, and no significant differences were observed in 1 hour vs. 3 hour assays (Table S6). Two identical assays were always performed simultaneously with the odor gradient in opposite directions on the two plates to control for directional bias due to room vibration; assays were discarded if the difference in the CIs for the two plates was ≥0.9 or if fewer than 7 worms moved into the scoring regions on one or both of the plates. Liquid odorants were tested undiluted unless otherwise indicated. Solid odorants were prepared as follows: 1-dodecanol, methyl palmitate, and methyl myristate were diluted 0.05 g in 2.5 ml paraffin oil; palmitic acid was diluted 10 g in 200 ml ethanol; myristic acid, skatole, and indole were diluted 0.05 g in 2.5 ml ethanol; and L-lactic acid was diluted 0.05 g in 2.5 ml dH2O. Ammonia was purchased as a 2 M solution in ethanol. Solid odorants were tested at these concentrations unless otherwise indicated. For assays in which odorants were placed on the plate lid rather than the plate surface (Figure S5), filter paper squares of approximately 0.5 cm in width were attached to the plate lid using double-stick tape. Odorant or control was then pipetted onto the filter paper, and chemotaxis was examined as described above.
CO2 chemotaxis assays
CO2 chemotaxis assays were performed essentially as described [21], [22]. Assays were performed on chemotaxis assay plates [47], and scoring regions were as described above for odor chemotaxis assays (Figure S2). Gases were delivered at a rate of 0.5 ml/min through holes in the plate lids from gastight syringes filled with either a CO2 mixture containing the test concentration of CO2, 10% O2, and the balance N2, or a control air mixture containing 10% O2 and 90% N2. Certified gas mixtures were obtained from Air Liquide or Airgas. Assays were performed and scored as described above for odor chemotaxis assays, except that the assay duration was 1 hour.
Grass chemotaxis assays
Fresh grass samples were collected from the campus of the University of California, Los Angeles. Sample 1 was collected from a lawn seeded with UC Verde Buffalo grass and perennial rye grass, and sample 2 was collected from a lawn seeded with a custom blend of annual ryegrass, Festuca, Bonsai dwarf fescue, Bermuda grass, and bluegrass. 200 µl of dH2O was added to 0.1 g grass. Grass was then ground in a small weigh boat, and 5 µl of the grass suspension was used in a chemotaxis assay with 5 µl dH2O as a control. Grass was either used immediately for chemotaxis assays or stored at 4°C for no more than 3 days.
Fecal chemotaxis assays
Uninfected rat or dog feces was collected from animals in the UCLA vivarium. Responses to feces were tested using a modified chemotaxis assay in which feces was placed on the plate lid rather than the plate surface. Filter paper squares of approximately 0.5 cm in width were attached to the plate lid using double-stick tape. Fecal matter was moistened with dH2O, smeared onto filter paper, and tested in a chemotaxis assay as described above for odor chemotaxis assays. We note that similar attraction to feces was observed when filter paper with feces was tested against filter paper with dH2O, and no attraction was observed to wet filter paper when wet filter paper was tested against dry filter paper (data not shown).
Data analysis
Statistical analysis was performed using either GraphPad Instat, GraphPad Prism, or PAST [50]. The heatmap was generated using Heatmap Builder [51].
Supporting Information
Zdroje
1. SchaferTW, SkopicA (2006) Parasites of the small intestine. Curr Gastroenterol Rep 8 : 312–320.
2. ScharF, TrostdorfU, GiardinaF, KhieuV, MuthS, et al. (2013) Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis 7: e2288.
3. KhieuV, ScharF, MartiH, SayasoneS, DuongS, et al. (2013) Diagnosis, treatment and risk factors of Strongyloides stercoralis in schoolchildren in Cambodia. PLoS Negl Trop Dis 7: e2035.
4. HotezP, HawdonJ, SchadGA (1993) Hookworm larval infectivity, arrest and amphiparatenesis: the Caenorhabditis elegans Daf-c paradigm. Parasitol Today 9 : 23–26.
5. SchadGA, AikensLM, SmithG (1989) Strongyloides stercoralis: is there a canonical migratory route through the host? J Parasitol 75 : 740–749.
6. YamadaM, MatsudaS, NakazawaM, ArizonoN (1991) Species-specific differences in heterogenic development of serially transferred free-living generations of Strongyloides planiceps and Strongyloides stercoralis. J Parasitol 77 : 592–594.
7. AshtonFT, LiJ, SchadGA (1999) Chemo - and thermosensory neurons: structure and function in animal parasitic nematodes. Vet Parasitol 84 : 297–316.
8. BhopaleVM, KupprionEK, AshtonFT, BostonR, SchadGA (2001) Ancylostoma caninum: the finger cell neurons mediate thermotactic behavior by infective larvae of the dog hookworm. Exp Parasitol 97 : 70–76.
9. ChaissonKE, HallemEA (2012) Chemosensory behaviors of parasites. Trends Parasitol 28 : 427–436.
10. ForbesWM, AshtonFT, BostonR, ZhuX, SchadGA (2004) Chemoattraction and chemorepulsion of Strongyloides stercoralis infective larvae on a sodium chloride gradient is mediated by amphidial neuron pairs ASE and ASH, respectively. Vet Parasitol 120 : 189–198.
11. LopezPM, BostonR, AshtonFT, SchadGA (2000) The neurons of class ALD mediate thermotaxis in the parasitic nematode, Strongyloides stercoralis. Int J Parasitol 30 : 1115–1121.
12. KogaM, NuamtanongS, DekumyoyP, YoonuanT, MaipanichW, et al. (2005) Host-finding behavior of Strongyloides stercoralis infective larvae to sodium cation, human serum, and sweat. Southeast Asian J Trop Med Public Health 36 : 93–98.
13. KogaM, TadaI (2000) Strongyloides ratti: chemotactic responses of third-stage larvae to selected serum proteins and albumins. J Helminthol 74 : 247–252.
14. SaferD, BrenesM, DunipaceS, SchadG (2007) Urocanic acid is a major chemoattractant for the skin-penetrating parasitic nematode Strongyloides stercoralis. Proc Natl Acad Sci USA 104 : 1627–1630.
15. DownesMJ, GriffinCT (1996) Dispersal behavior and transmission strategies of the entomopathogenic nematodes Heterorhabditis and Steinernema. Biocontrol Sci Techn 6 : 347–356.
16. RamotD, JohnsonBE, BerryTL, CarnellL, GoodmanMB (2008) The parallel worm tracker: a platform for measuring average speed and drug-induced paralysis in nematodes. PLoS ONE 3: e2208.
17. LiuY, WangL, LiuJ, DiY (2013) A study of human skin and surface temperatures in stable and unstable thermal environments. J Therm Biol 38 : 440–448.
18. LeeH, ChoiMK, LeeD, KimHS, HwangH, et al. (2012) Nictation, a dispersal behavior of the nematode Caenorhabditis elegans, is regulated by IL2 neurons. Nat Neurosci 15 : 107–112.
19. GriffinCT (2012) Perspectives on the behavior of entomopathogenic nematodes from dispersal to reproduction: traits contributing to nematode fitness and biocontrol efficacy. J Nematol 44 : 177–184.
20. WilsonMJ, EhlersR-U, GlazerI (2012) Entomopathogenic nematode foraging strategies – is Steinernema carpocapsae really an ambush forager? Nematol 14 : 389–394.
21. HallemEA, DillmanAR, HongAV, ZhangY, YanoJM, et al. (2011) A sensory code for host seeking in parasitic nematodes. Curr Biol 21 : 377–383.
22. DillmanAR, GuillerminML, LeeJH, KimB, SternbergPW, et al. (2012) Olfaction shapes host-parasite interactions in parasitic nematodes. Proc Natl Acad Sci USA 109: E2324–2333.
23. LaznikZ, TrdanS (2013) An investigation on the chemotactic responses of different entomopathogenic nematode strains to mechanically damaged maize root volatile compounds. Exp Parasitol 134 : 349–355.
24. AliJG, AlbornHT, StelinskiLL (2010) Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. J Chem Ecol 36 : 361–368.
25. AliJG, AlbornHT, Campos-HerreraR, KaplanF, DuncanLW, et al. (2012) Subterranean, herbivore-induced plant volatile increases biological control activity of multiple beneficial nematode species in distinct habitats. PLoS ONE 7: e38146.
26. AliJG, AlbornHT, StelinskiLL (2011) Constitutive and induced subterranean plant volatiles attract both entomopathogenic and plant-parasitic nematodes. J Ecol 99 : 26–35.
27. HiltpoldI, BaroniM, ToepferS, KuhlmannU, TurlingsTC (2010) Selection of entomopathogenic nematodes for enhanced responsiveness to a volatile root signal helps to control a major root pest. J Exp Biol 213 : 2417–2423.
28. KollnerTG, HeldM, LenkC, HiltpoldI, TurlingsTC, et al. (2008) A maize (E)-β-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell 20 : 482–494.
29. RasmannS, KollnerTG, DegenhardtJ, HiltpoldI, ToepferS, et al. (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434 : 732–737.
30. RasmannS, AliJG, HelderJ, van der PuttenWH (2012) Ecology and evolution of soil nematode chemotaxis. J Chem Ecol 38 : 615–628.
31. QiuYT, SmallegangeRC, van LoonJJ, TakkenW (2011) Behavioural responses of Anopheles gambiae sensu stricto to components of human breath, sweat and urine depend on mixture composition and concentration. Med Vet Entomol 25 : 247–255.
32. AlkalayI, SuetsuguS, ConstantineH, SteinM (1971) Carbon dioxide elimination across human skin. Am J Physiol 220 : 1434–1436.
33. TurlingsTC, HiltpoldI, RasmannS (2012) The importance of root-produced volatiles as foraging cues for entomopathogenic nematodes. Plant Soil 358 : 51–60.
34. ZajacAM (2006) Gastrointestinal nematodes of small ruminants: life cycle, anthelmintics, and diagnosis. Vet Clin North Am Food Anim Pract 22 : 529–541.
35. PleilJD, LindstromAB (1995) Measurement of volatile organic compounds in exhaled breath as collected in evacuated electropolished canisters. J Chromatogr B Biomed Appl 665 : 271–279.
36. ManssonHL (2008) Fatty acids in bovine milk fat. Food Nutr Res 52 : 10.
37. PovoloM, PelizzolaV, RaveraD, ContariniG (2009) Significance of the nonvolatile minor compounds of the neutral lipid fraction as markers of the origin of dairy products. J Agric Food Chem 57 : 7387–7394.
38. HaenleinGFW (2004) Goat milk in human nutrition. Small Ruminant Res 51 : 155–163.
39. Viney ME, Lok JB (2007) Strongyloides spp. In WormBook, www.WormBook.org.
40. MooreJG, JessopLD, OsborneDN (1987) Gas-chromatographic and mass-spectrometric analysis of the odor of human feces. Gastroenterology 93 : 1321–1329.
41. DiawaraA, SchwenkenbecherJM, KaplanRM, PrichardRK (2013) Molecular and biological diagnostic tests for monitoring benzimidazole resistance in human soil-transmitted helminths. Am J Trop Med Hyg 88 : 1052–1061.
42. BilgramiAL, GaugerR, Shapiro-IlanDI, AdamsBJ (2006) Source of trait deterioration in entomopathogenic nematodes Heterorhabditis bacteriophora and Steinernema carpocapsae during in vivo culture. Nematology 8 : 397–409.
43. Lok JB (2007) Strongyloides stercoralis: a model for translational research on parasitic nematode biology. In WormBook, www.WormBook.org.
44. ShaoH, LiX, NolanTJ, MasseyHCJr, PearceEJ, et al. (2012) Transposon-mediated chromosomal integration of transgenes in the parasitic nematode Strongyloides ratti and establishment of stable transgenic lines. PLoS Pathog 8: e1002871.
45. WhiteGF (1927) A method for obtaining infective nematode larvae from cultures. Science 66 : 302–303.
46. BrennerS (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94.
47. BargmannCI, HartwiegE, HorvitzHR (1993) Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74 : 515–527.
48. CampbellJF, KayaHK (2000) Influence of insect-associated cues on the jumping behavior of entomopathogenic nematodes (Steinernema spp.). Behavior 137 : 591–609.
49. O'HalloranDM, BurnellAM (2003) An investigation of chemotaxis in the insect parasitic nematode Heterorhabditis bacteriophora. Parasitol 127 : 375–385.
50. HammerØ, HarperDAT, RyanPD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electronica 4 : 9pp.
51. KingJY, FerraraR, TabibiazarR, SpinJM, ChenMM, et al. (2005) Pathway analysis of coronary atherosclerosis. Physiol Genomics 23 : 103–118.
52. MukabanaWR, MweresaCK, OtienoB, OmusulaP, SmallegangeRC, et al. (2012) A novel synthetic odorant blend for trapping of malaria and other African mosquito species. J Chem Ecol 38 : 235–244.
53. VerhulstNO, MbadiPA, KissGB, MukabanaWR, van LoonJJ, et al. (2011) Improvement of a synthetic lure for Anopheles gambiae using compounds produced by human skin microbiota. Malar J 10 : 28.
54. SmallegangeRC, Bukovinszkine-KissG, OtienoB, MbadiPA, TakkenW, et al. (2012) Identification of candidate volatiles that affect the behavioural response of the malaria mosquito Anopheles gambiae sensu stricto to an active kairomone blend: laboratory and semi-field assays. Physiol Entomol 37 : 60–71.
55. MathewN, AyyanarE, ShanmugaveluS, MuthuswamyK (2013) Mosquito attractant blends to trap host seeking Aedes aegypti. Parasitol Res 112 : 1305–1312.
56. MboeraLE, TakkenW, MdiraKY, PickettJA (2000) Sampling gravid Culex quinquefasciatus (Diptera: Culicidae) in Tanzania with traps baited with synthetic oviposition pheromone and grass infusions. J Med Entomol 37 : 172–176.
57. LealWS, BarbosaRM, XuW, IshidaY, SyedZ, et al. (2008) Reverse and conventional chemical ecology approaches for the development of oviposition attractants for Culex mosquitoes. PLoS ONE 3: e3045.
58. MillarJG, ChaneyJD, MullaMS (1992) Identification of oviposition attractants for Culex quiquefasciatus from fermented Bermuda grass infusions. J Am Mosq Control Assoc 8 : 11–17.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial PathogensČlánek A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during TransmissionČlánek The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil ChemotaxisČlánek Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Regulatory RNAs Involved in Bacterial Antibiotic Resistance
- From Dandruff to Deep-Sea Vents: -like Fungi Are Ecologically Hyper-diverse
- Pathogenicity and Epithelial Immunity
- Mother–Infant HIV Transmission: Do Maternal HIV-Specific Antibodies Protect the Infant?
- Hell's BELs: acterial 3 igases That Exploit the Eukaryotic Ubiquitin Machinery
- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Primary Seronegative but Molecularly Evident Hepadnaviral Infection Engages Liver and Induces Hepatocarcinoma in the Woodchuck Model of Hepatitis B
- TLR2 Signaling Decreases Transmission of by Limiting Bacterial Shedding in an Infant Mouse Influenza A Co-infection Model
- Production of an Attenuated Phenol-Soluble Modulin Variant Unique to the MRSA Clonal Complex 30 Increases Severity of Bloodstream Infection
- Inhibition of the TRAIL Death Receptor by CMV Reveals Its Importance in NK Cell-Mediated Antiviral Defense
- Early Mucosal Sensing of SIV Infection by Paneth Cells Induces IL-1β Production and Initiates Gut Epithelial Disruption
- Limited HIV Infection of Central Memory and Stem Cell Memory CD4+ T Cells Is Associated with Lack of Progression in Viremic Individuals
- Virus-Specific Regulatory T Cells Ameliorate Encephalitis by Repressing Effector T Cell Functions from Priming to Effector Stages
- A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during Transmission
- The HIV-1 Envelope Transmembrane Domain Binds TLR2 through a Distinct Dimerization Motif and Inhibits TLR2-Mediated Responses
- Infection with MERS-CoV Causes Lethal Pneumonia in the Common Marmoset
- VGIII Isolates Causing Infections in HIV/AIDS Patients in Southern California: Identification of the Local Environmental Source as Arboreal
- Diverse Host-Seeking Behaviors of Skin-Penetrating Nematodes
- Capsid Protein VP4 of Human Rhinovirus Induces Membrane Permeability by the Formation of a Size-Selective Multimeric Pore
- The Murine Gammaherpesvirus Immediate-Early Rta Synergizes with IRF4, Targeting Expression of the Viral M1 Superantigen to Plasma Cells
- Characterization of an Insecticidal Toxin and Pathogenicity of against Insects
- The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil Chemotaxis
- Histone Deacetylase Inhibitors Impair the Elimination of HIV-Infected Cells by Cytotoxic T-Lymphocytes
- A Locus Encompassing the Epstein-Barr Virus Kinase Regulates Expression of Genes Encoding Viral Structural Proteins
- Distinct APC Subtypes Drive Spatially Segregated CD4 and CD8 T-Cell Effector Activity during Skin Infection with HSV-1
- Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
- Adoptive Transfer of EBV Specific CD8 T Cell Clones Can Transiently Control EBV Infection in Humanized Mice
- Schistosome Feeding and Regurgitation
- EVM005: An Ectromelia-Encoded Protein with Dual Roles in NF-κB Inhibition and Virulence
- Rabies Virus Hijacks and Accelerates the p75NTR Retrograde Axonal Transport Machinery
- Why HIV Virions Have Low Numbers of Envelope Spikes: Implications for Vaccine Development
- Identification of Anti-virulence Compounds That Disrupt Quorum-Sensing Regulated Acute and Persistent Pathogenicity
- HIV-1 Receptor Binding Site-Directed Antibodies Using a VH1-2 Gene Segment Orthologue Are Activated by Env Trimer Immunization
- Cooperation between Epstein-Barr Virus Immune Evasion Proteins Spreads Protection from CD8 T Cell Recognition across All Three Phases of the Lytic Cycle
- Parasite Extracellular Vesicles: Mediators of Intercellular Communication
- RC1339/APRc from Is a Novel Aspartic Protease with Properties of Retropepsin-Like Enzymes
- Cyclic di-GMP-dependent Signaling Pathways in the Pathogenic Firmicute
- Non-random Escape Pathways from a Broadly Neutralizing Human Monoclonal Antibody Map to a Highly Conserved Region on the Hepatitis C Virus E2 Glycoprotein Encompassing Amino Acids 412–423
- Neutrophil Elastase Causes Tissue Damage That Decreases Host Tolerance to Lung Infection with Species
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- SGNH Hydrolase-Like Proteins AlgJ and AlgX Have Similar Topology but Separate and Distinct Roles in Alginate Acetylation
- Why Sexually Transmitted Infections Tend to Cause Infertility: An Evolutionary Hypothesis
- Late Engagement of CD86 after Influenza Virus Clearance Promotes Recovery in a FoxP3 Regulatory T Cell Dependent Manner
- Determinants of Influenza Transmission in South East Asia: Insights from a Household Cohort Study in Vietnam
- A Novel Signal Transduction Pathway that Modulates Quorum Sensing and Bacterial Virulence in
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- A Cysteine Protease Inhibitor of Is Essential for Exo-erythrocytic Development
- EBNA3C Augments Pim-1 Mediated Phosphorylation and Degradation of p21 to Promote B-Cell Proliferation
- On the Front Line: Quantitative Virus Dynamics in Honeybee ( L.) Colonies along a New Expansion Front of the Parasite
- Assembly and Architecture of the EBV B Cell Entry Triggering Complex
- NLR-Associating Transcription Factor bHLH84 and Its Paralogs Function Redundantly in Plant Immunity
- The PDZ-Binding Motif of Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Is a Determinant of Viral Pathogenesis
- Strain-Specific Properties and T Cells Regulate the Susceptibility to Papilloma Induction by Papillomavirus 1
- Human Cytomegalovirus pUL79 Is an Elongation Factor of RNA Polymerase II for Viral Gene Transcription
- The GAP Activity of Type III Effector YopE Triggers Killing of in Macrophages
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- Pathogenicity and Epithelial Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



