-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaVGIII Isolates Causing Infections in HIV/AIDS Patients in Southern California: Identification of the Local Environmental Source as Arboreal
The environmentally-acquired human pathogen C. gattii is responsible for ongoing and expanding outbreaks in the Western United States and Canada. C. gattii comprises four distinct molecular types: VGI, VGII, VGIII, and VGIV. Molecular types VGI, VGII, and VGIII have been isolated from patients and animals throughout the Western US. The Pacific Northwest and Canadian outbreak is primarily caused by C. gattii VGII. VGIII is responsible for ongoing infections in HIV/AIDS patients in Southern California. However, only two environmental C. gattii isolates have ever been identified from the Californian environment: CBS7750 (VGII) and WM161 (VGIII). We sought to collect environmental samples from areas that had confirmed reports of clinical or veterinary infections. Here we report the isolation of C. gattii VGI and VGIII from environmental soil and tree samples. C. gattii isolates were obtained from three novel tree species: Canary Island pine, American sweetgum, and a Pohutukawa tree. Genetic analysis provides robust evidence that these environmental isolates are the source of human infections.
Published in the journal: . PLoS Pathog 10(8): e32767. doi:10.1371/journal.ppat.1004285
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004285Summary
The environmentally-acquired human pathogen C. gattii is responsible for ongoing and expanding outbreaks in the Western United States and Canada. C. gattii comprises four distinct molecular types: VGI, VGII, VGIII, and VGIV. Molecular types VGI, VGII, and VGIII have been isolated from patients and animals throughout the Western US. The Pacific Northwest and Canadian outbreak is primarily caused by C. gattii VGII. VGIII is responsible for ongoing infections in HIV/AIDS patients in Southern California. However, only two environmental C. gattii isolates have ever been identified from the Californian environment: CBS7750 (VGII) and WM161 (VGIII). We sought to collect environmental samples from areas that had confirmed reports of clinical or veterinary infections. Here we report the isolation of C. gattii VGI and VGIII from environmental soil and tree samples. C. gattii isolates were obtained from three novel tree species: Canary Island pine, American sweetgum, and a Pohutukawa tree. Genetic analysis provides robust evidence that these environmental isolates are the source of human infections.
Introduction
Outbreaks of infectious diseases caused by all major classes of microbial pathogens occur globally, annually, and these outbreaks pose public health challenges [1]–[4]. Advancing understanding of forces driving outbreaks to enhance our ability to predict, contain, and blunt their impact include: 1) identification of environmental sources and vectors, and 2) defining genetic mechanisms that give rise to infectious microbes with altered virulence or transmissibility.
Over 200 species of fungi are recognized as human/animal pathogens [5]. Fungal pathogens also cause outbreaks of disease. This includes clusters of infections caused by Coccidioides immitis/posadasii, Histoplasma capsulatum, or Apophysomyces trapeziformis following soil perturbations (earthquakes, tornadoes, dust storms, construction, landscaping) [6]–[13]. The Cryptococcus pathogenic species complex includes the globally distributed human pathogens C. neoformans and C. gattii, which cause significant fungal disease burden and increasing public health costs in immunocompromised and immunocompetent individuals worldwide [14]–[19]. Cryptococcus is annually responsible for >1,000,000 infections, >620,000 deaths, and one-third of all AIDS related deaths [17]. Cryptococcus neoformans and C. gattii have been isolated from various environmental sources (soil, trees, bird guano) but reports genetically linking specific environmental reservoirs to individual cases are limited because: 1) infections often take considerable time to diagnose, 2) are not reportable diseases in the USA and abroad, 3) C. neoformans and C. gattii are not often distinguished by species or by molecular type, and 4) retrospective environmental surveys are rare and scattered, may lack molecular analysis and corresponding clinical isolates, and/or follow many years after reported clinical infections [13], [20].
Cryptococcus gattii comprises four distinct molecular types: VGI, VGII, VGIII, and VGIV, without evidence of genetic exchange of nuclear genomes, providing evidence the four molecular types represent distinct, related species [16], [21]–[25]. C. gattii VGII and VGIII are associated with two distinct expanding outbreaks in the Western US [22], [26]. Phylogenetic analysis suggests that C. gattii and C. neoformans diverged ∼40 million years ago and VGII is the ancestral molecular type to the C. gattii clade [23], [24], [27]–[30]. Genetic rearrangement between the VG types could actively suppress recombination, acting as reproductive barriers, and limiting productive recombination. However, analysis of the mitochondrial genomes of C. gattii VGI and VGII lineages indicates a highly clonal mitochondrial genome within each lineage consistent with uniparental mitochondrial inheritance but different genealogies support the hypothesis that examples of mitochondrial genome transmission from VGII into VGI isolates have occurred [31], [32]. Furthermore, transmission of hypervirulence traits within and between different molecular types was recently demonstrated in laboratory crosses [33]. Thus, given evidence linking mitochondrial function to enhanced intracellular proliferation of VGII outbreak isolates in macrophages, and previous studies linking mitochondria to virulence of plant fungal pathogens, these findings illustrate how genetic exchange can impact virulence of pathogenic fungi [33]–[35].
C. gattii molecular type VGII, which is highly virulent and has a predilection for infecting apparently healthy hosts, is causing an outbreak on Vancouver Island that has expanded to the Canadian mainland and also Washington, Oregon, and possibly California [21], [22], [36], [37]. As a result of increased sampling and molecular analysis, three sub-molecular types are now recognized: VGIIa, VGIIb, and VGIIc. The Vancouver, BC and Pacific Northwest (PNW) outbreak is characterized by infections caused predominantly by VGIIa, the major genotype, with a lower frequency of incidence of the VGIIb minor genotype. A novel, distinct, highly virulent molecular type designated as VGIIc is presently restricted to Oregon [26], [38]. Prior to the now recognized outbreak in the Pacific Northwest, C. gattii VGIIa was isolated in 1970 from a human sputum sample (Seattle, Washington, NIH444) and in 1992 a closely related VGII isolate was obtained from an environmental sample from San Francisco, California (CBS7750) [21], [39], [40]. Sporadic isolates possibly related to the PNW VGIIa major genotype at a limited number of MLST loci have been reported outside the USA [25], [41], [42]. Due to the scarcity of these VGIIa related genotypes outside the USA, the environmental reservoir and source has not been established. On the other hand, while isolates related to the VGIIb minor genotype at a limited number of MLST loci have been reported from several geographical regions, VGIIb isolates that are indistinguishable across 30 MLST loci have only been reported from Australia, thus providing robust evidence that this is the likely geographic source of the VGIIb outbreak isolates [21], [25], [27], [42], [43].
In contrast, C. gattii molecular type VGIII is responsible for ongoing infections in immunocompromised HIV/AIDS patients in Southern California and the Southwestern US [18], [22], [37], [44], [45]. Outside the US VGIII has been associated with sporadic infections in Brazil, Colombia, Mexico, India, Germany, and Korea [14], [23], [37], [46]–[51]. Despite the high preponderance of infections caused by C. gattii VGIII in the HIV/AIDS population of California and the Southwestern USA, only one environmental VGIII isolate (WM161) has ever been recovered from California [23], [37], [44], [52]. On the other hand, in Colombia and Argentina VGIII isolates have been isolated from the environment, yet clinical prevalence appears low in those localities [47], [53]–[55].
C. gattii VGIII has been further classified into two groups, VGIIIa and VGIIIb, based on MLST analysis [22]. Analysis of the Californian VGIII clinical population indicated that VGIIIa is more clonal with only one identified MATa isolate in comparison to VGIIIb, in which both MATα and MATa isolates were frequently identified [22], [45]. Unlike other VG molecular types or C. neoformans, MATa isolates are frequently isolated from both clinical and environmental populations of VGIIIb, suggesting that the VGIIIb population may be fertile and actively undergoing a-α sexual recombination in nature [56]–[58]. VGIII environmental isolates have been reported from Tipuana tipu trees in Argentina, Terminalia catappa, Corymbia ficifolia, Eucalyptus sp, and Ficus sp. in Colombia, and Manilkara hexandra in India [47], [49], [53]–[55]. Analysis of 60 VGIII isolates from California resulted in the identification of only four alleles shared between VGIIIa and VGIIIb in four independent isolates, CAP10 and TEF1 appear to be ancestral, while the shared PLB1 allele appears to have been introgressed between VGIIIa and VGIIIb [22]. The MLST analysis, haplotype analysis, paired allele graphs, percentages of compatible loci, and indices of association all support the divergence of the VGIIIa and VGIIIb subtypes, limited recombination within VGIIIa, and more frequent recombination in VGIIIb [22].
Prior to the Pacific Northwest outbreak caused by C. gattii VGII, California was historically recognized as the oldest known region associated with the occurrence of cryptococcosis within the USA. Early studies denoted California as the most prominent site of serotype C infections, later identified as C. gattii VGIII. Byrnes et al. 2011 was the first to establish the prevalence of C. gattii VGIII within the HIV/AIDS clinical population of California but did not obtain nor identify any VGIII isolates directly from the environment [22]. Of note, the MLST profile of the historically identified environmental isolate WM161 did not match the MLST profiles of any of the reported clinical isolates obtained from California; therefore, the infectious environmental reservoir of C. gattii VGIII remained unknown. Previous environmental surveys within the Pacific Northwest, USA have identified the environmental niche of C. gattii VGII [59], [60]. The lack of genetic exchange between the molecular types, genomic rearrangements confined within molecular types, and differences in host predilection strongly suggest that the molecular types recognized within C. gattii comprise cryptic species. We therefore sought to identify the environmental reservoir of C. gattii VGIII in California by sampling plants and soil in areas near residents with confirmed C. gattii infections.
As a result of this study we have identified the local environmental source of C. gattii VGI and VGIII associated with trees and soil debris in the greater Los Angeles area of California. Molecular typing and whole genome analysis of the Californian C. gattii VGIII environmental isolates indicates a high amount of molecular diversity exists in this region, VGIIIa and VGIIIb can colonize similar and overlapping niches, and some environmental isolates are closely related to clinical isolates and the likely source of previously reported human infections, and a potential reservoir for inciting new infections. Furthermore, our results suggest that many of these isolates are sexually competent and capable of intra - and inter-molecular mating which might facilitate dispersal and propagate altered infectious characteristics such as virulence or transmissibility. Further work is needed to fully elucidate the links between Cryptococcus molecular types, environmental distributions, and the propensity to initiate disease in exposed hosts.
Results
Identification of the environmental source of C. gattii VGIII in Southern California
The long term and extensive clinical prevalence of C. gattii in California has implicated a local endogenous reservoir but even with increased environmental sampling the environmental source of infections has remained undefined. We therefore sought to collect environmental samples from areas that had confirmed reports of clinical and/or veterinary infections. From soil and swab samples collected at 24 locations throughout the greater Los Angeles area over a two year period (Figure S1), we screened 146 environmental (n = 107) and clinical (n = 39) isolates utilizing canavanine glycine bromothymol blue (CGB) and niger seed (NGS) indicator media. We identified 30 (20.6%) potential C. gattii isolates, 11 clinical and 19 environmental isolates obtained from 4 out of 24 sites (16.7%) that were melanin positive on NGS and produced a blue color reaction on CGB agar (Figure 1 and Table S4). Eleven of 39 clinical (28.2%) and 19 of 107 (17.8%) environmental isolates were also confirmed as C. gattii via differences in ATP6 PCR product length and MLST analysis (Figure 1, Tables S4 and 5) [61]. C. gattii was isolated in association with three novel host tree species: Pinus canariensis (Canary Island pine, isolates LMES-3A, MCP-1A, MCPR1-X, USC-X, USC2-SIC), Liquidamar styraciflua (American sweetgum, isolates 78-1-S3A, BHPP3-X), and Metrosideros excels (Pohutukawa tree, isolate BHPP1-X). All C. gattii isolates were haploid by FACS analysis (Table S4). An additional 100 isolates were further identified as C. neoformans based on positive pigmentation on NGS agar, negative blue color formation/no growth on CGB agar, and IGS1 sequence (data not shown).
Fig. 1. Global molecular analysis illustrates a diverse population of C. gattii VGIII isolates are endemic to Southern California. 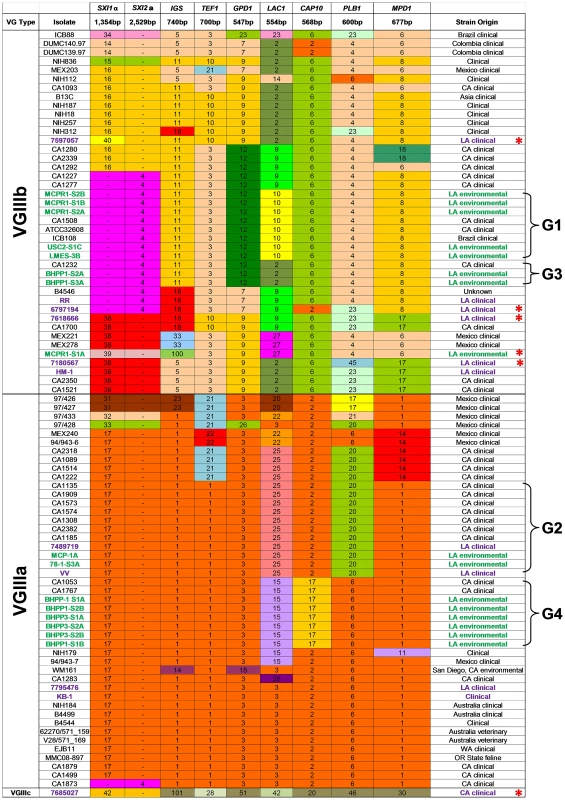
Multilocus sequence typing was performed on 8 loci. Unique alleles were assigned a distinct color for each marker. New environmental (green, n = 13) and clinical (Purple, n = 5) VGIII isolates from California are compared to previously analyzed VGIII isolates (Table adapted from [22]). Seven VGIIIa and five VGIIIb environmental isolates representing four MLST groups shared indistinguishable MLST profiles with previously reported clinical isolates as indicated (brackets, denoted as Groups 1–4 (G1, G2, G3, and G4). New MLST sequence types were observed for both VGIIIa (n = 3) and VGIIIb (n = 3) and are denoted with red asterisks. MLST analysis indicates a diverse population of C. gattii VGIII are endemic to Southern California
To characterize the molecular types prevalent in the Californian isolates, MLST analysis was performed on 11 unlinked loci for the 11 clinical and 19 environmental C. gattii strains obtained from Los Angeles, California USA. The data were compared to previously reported VGIII isolates [22] to determine the presence of novel or shared MLST profiles between environmental and clinical isolates (Figure 1 and Table S4). Isolates primarily clustered into three previously recognized molecular types: VGI, VGIIIa, and VGIIIb. The VGI molecular type found in California shares the most common MLST type observed worldwide (Table S5). Both VGIIIb MATα (5/14, 36%) and MATa (9/14, 64%) mating types were obtained from clinical and environmental isolates, suggesting the potential for an actively sexually recombining population in Southern California within this molecular type subgroup. In contrast, all of the VGIIIa isolates characterized were found to be MATα, suggesting that opposite sex mating may be rare among isolates of this subtype. Overall, nine isolates typed as VGIIIb MATa (2 clinical and 7 environmental), five as VGIIIb MATα (4 clinical and 1 environmental), 12 as VGIIIa MATα (4 clinical and 8 environmental), and three isolates typed as VGI MATα (Tables S4 and S5). These results reveal a, widespread reservoir of C. gattii in the greater Los Angeles area as the likely source of frequent cryptococcal infections in HIV/AIDS patients in Southern California.
Sixteen isolates, 14 environmental (7 VGIIIa and 7 VGIIIb) and 2 clinical (1 VGIIIa and 1 VGIIIb), shared four different MLST profiles (designated groups G1 to G4, represented with brackets) with previously reported clinical isolates (Figure 1), indicating shared descent between recently identified environmental isolates and previously reported clinical isolates spanning an isolation period of up to 12 years. Six new MLST sequence types were identified: five VGIIIb (4 clinical and 1 environmental) and one uncategorized VGIII type (7685027) (Figure 1). Additional analysis of the CAP59, TOR1, SOD1, and URA5 loci did not improve resolution of isolates that shared an indistinguishable 8-locus MLST profile. Thus, there was a high level of diversity in these environmental isolates reflected by the recovery of a multitude of distinct MLST profiles (Figure 1 and Table S4). Furthermore, we obtained both VGIIIa and VGIIIb molecular sub-types from the same sample (BHPP1 designation) or environmental location (MCP/MCPR or BHPP1/BHPP3 designations) suggesting that both molecular types can colonize the same ecological niche in California fostering the potential for hybridization. Thus, their lack of frequent genetic exchange suggests that the two molecular types could represent distinct species in which mating is limited by genetic and/or temporal barriers limiting productive introgressions between the molecular types.
One clinical isolate (7685027) did not align with either of the two previously recognized VGIIIa or VGIIIb molecular types and either represents a novel VGIII subgroup that has been undersampled or a novel hypermutator isolate [62]. This isolate contains many completely novel MLST alleles (URA5, TOR1, PLB1, MPD1, LAC1, CAP10, GPD1, and IGS1) not observed previously (Figures 1 and 2), consistent with a possible origin via action of a hypermutator phenotype strain resulting in the rapid emergence of novel MLST alleles [62]. On the other hand, other MLST alleles (SOD1, CAP59, and TEF1) are shared with two additional isolates (IHEM14941S and IHEM14941W) obtained from a Mexican immigrant diagnosed in Spain and thus they may represent a novel VGIIIc sub-molecular type.
Fig. 2. Phylogenetic analysis of newly identified C. gattii isolates from California. 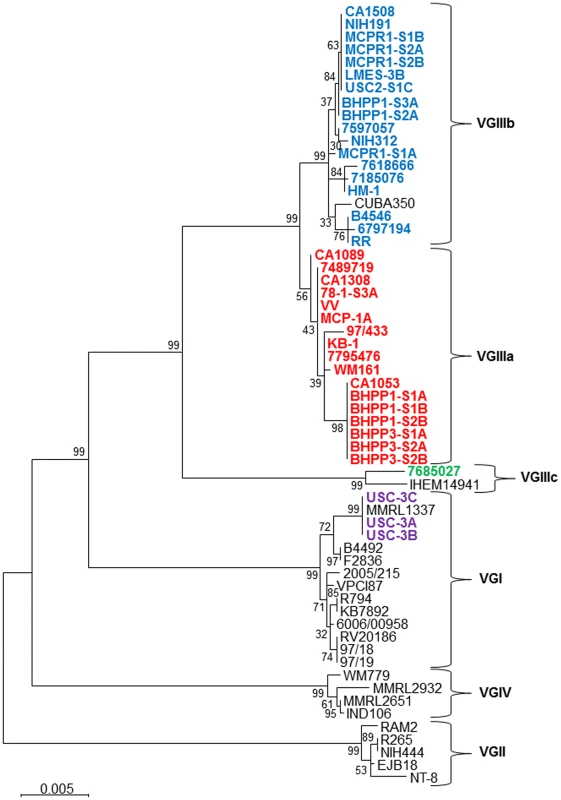
Phylogenetic tree obtained by analysis of 7 concatenated MLST loci (IGS1, TEF1, GPD1, LAC1, CAP10, PLB1, and MPD1) using 500 bootstrap replicates and the Maximum Likelihood (MLC) method based on Tamura-Nei model applying Neighbor-Join and BioNL algorithms. The tree with the highest log likelihood is shown. The analysis involved 61 sequences and a total of 4252 positions. Scale bar indicated 0.005 substitutions per nucleotide. In addition to MLST analysis of nuclear loci, we expanded our analysis to explore the mitochondrial genome because the mitochondria are primarily uniparentally inherited from the MATa parent, uniparental/biparental mitochondrial inheritance rates may differ between molecular types, and their genome evolves independently from the nuclear genome [32], [33], [63]–[66].
Phylogenetic analysis indicated that the MtLrRNA locus was indistinguishable between VGIIIa and VGIIIb isolates, suggesting closely related shared ancestry (data not shown). Utilizing ATP6-Byrnes primers, we observed distinct PCR size polymorphisms between VGI (300 bp), VGII (200 bp), and VGIII (≥600 bp) [61]. Additional PCR and MLST analysis of all VGIII isolates indicated a distinct size polymorphism between VGIIIa (600 bp) and VGIIIb (700 bp) except for a small subset of C. gattii VGIIIb isolates that exhibited a PCR product equivalent to the VGIIIa (600 bp) isolates. Large differences between the ATP6-Byrnes [33], [61] PCR product size limited informative phylogenetic sequence analysis between C. gattii molecular types so we utilized additional ATP6-Bovers primers previously used for mitochondrial analysis in Cryptococcus species [32]. Phylogenetic analysis of ATP6-Bovers primer sequences indicated two clades mostly segregating VGIIIa from VGIIIb, and MATa from MATα isolates as expected with the exception of a small group of clinical VGIIIb isolates (71805076, DUMC140.97, and HM-1) that share mitochondrial sequences with VGIIIa (Figure S2a). DUMC140.97 also shares nuclear sequence of CAP10 (allele 2) with VGIIIb but no evidence of shared nuclear sequence is evident in the MLST analysis of HM-1 and 7180567. Haplotype network analysis indicates the MATa VGIIIb allele as ancestral and the shared MATα allele as derived suggesting further evidence of a rare introgression event with mitochondrial recombination occurring between VGIIIa and VGIIIb isolates (Figure S2b).
Haplotype network analysis indicates rare introgression events between VGIIIa and VGIIIb molecular groups
We further examined the presence and distribution of shared MLST alleles between VGIIIa and VGIIIb by paired allele and haplotype network analysis, determining the evolutionary history of each allele and testing for evidence of recombination within or between molecular types in California. Two clinical VGIIIb isolates (6797194 and 7618666) share CAP10 allele 2 with known VGIIIa isolates. Paired allele diagrams between the MAT locus and LAC1, or LAC1 and TEF1, illustrate recombination occurring in the Californian VGIII population (Figure 3). Evidence of recombination is present within VGIIIb and the more clonal VGIIIa population (Figure S3). Especially notable is the evidence for recombination between the MAT locus and LAC1 in VGIIIb within the Californian isolates, suggesting that a-α mating is ongoing between isolates harboring SXI2a allele 4 and SXI1α allele 48.
Fig. 3. Evidence for recombination in Californian VGIII isolates. 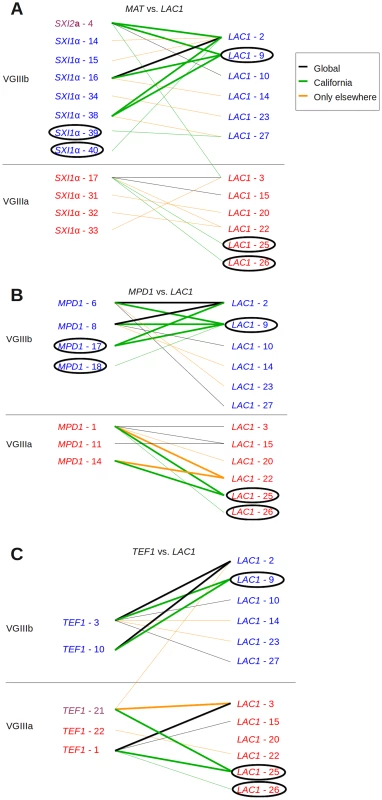
Informative paired allele diagrams depicting MLST data are presented. An hourglass shape indicates the presence of all four allele combinations, providing evidence for recombination. Alleles unique to California are circled, while black lines indicate combinations found both in Californian isolates and those from elsewhere. Green lines indicate combinations found only in California, and orange lines indicate combinations only found outside of California. (A) MAT versus LAC1 paired allele analysis provides an example of recombination within the VGIIIb group in California, as well as one including one allele combination found additionally in Brazil and four in California. (B) MPD1 versus LAC1 and (C) TEF1 versus LAC1 provide examples of recombination within the VGIIIb group (top) and within VGIIIa (bottom). Additional informative comparisons are presented in Figure S3. Haplotype network analysis indicates that for two loci, PLB1 and CAP10, the alleles shared between VGIIIa and VGIIIb isolates represent recent introgression events because the shared allele occupies a position other than the ancestral haplotype network allele (represented within the rectangle, Figure 4). In contrast haplotype network analysis of the TEF1 share allele 21 represents the origin of the allele as ancestral. Additional haplotype network analysis of the evolutionary history of 9 additional MLST loci not shared between VGIIIa and VGIIIb robustly supports genetic isolation and provides evidence for cryptic speciation although evidence of additional shared alleles may emerge in the VGIII population with increased sampling and analysis of a broader set of nuclear loci (Figure S4).
Fig. 4. Haplotype network analysis suggests recent introgression between VGIIIa and VGIIIb. 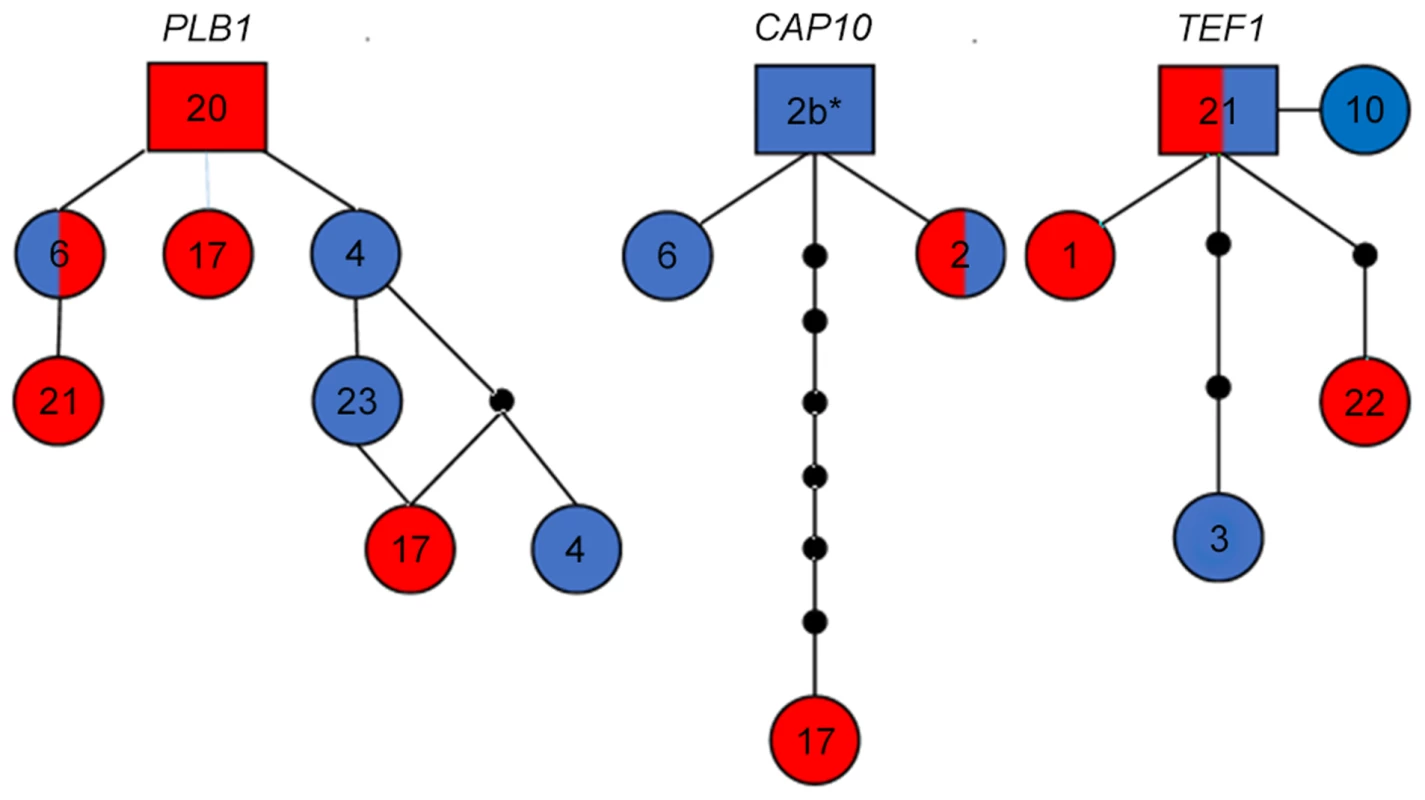
Alleles for each respective locus are indicated numerically. Alleles are colored red representing VGIIIa, blue representing VGIIIb, and dual-coloration representing alleles shared between the VGIIIa and VGIIIb molecular types. Alleles in squares represent the proposed ancestral allele, circles represent alleles present in the population, lines between alleles represent one predicted evolutionary event, and smaller black circles represent alleles that have not been recovered, or which may no longer be represented in the population. Haplotype networks for PLB1 and CAP10 exemplify a derived non-ancestral origin of alleles shared between the VGIIIa and VGIIIb populations. CAP10 Allele 2b* (6797194) is represented as allele 2 in Figure 2 2 because over the reported and defined 611 bp the sequence identically matches. But from additional extended sequence reads of approximately 50 bp we observe a 1 bp substitution (T→C) at 23 bp downstream of the current reported CAP10 allele sequence that distinguishes this allele from the standard allele 2. The TEF1 haplotype network demonstrates ancestral origins of the shared allele 21. Whole genome sequences of MLST matched isolates indicates that environmental C. gattii isolates were the likely reservoirs associated with historical clinical infections
We next determined that the newly isolated environmental strains are extremely closely related to the previously reported clinical strains based on whole genome sequencing. We sequenced matched clinical and environmental isolates representing 3 of 4 matched MLST groups, VGIIIa (group 1, CA1308, MCP-1A, 78-1-S3A and group 2, CA1053 and BHPP3-S1A) and VGIIIb (group 3, CA1508 and MCPR1-S1B) to determine whether environmental isolates could represent the source population for previously reported clinical infections. Whole genome sequence analysis of MLST matched clinical and environmental strains indicates that the genomes of grouped isolates are remarkably similar and very closely related (Figure 5 and Table S6, S7, and S8). The genomic sequences for these strains were aligned to the closest reference genome available, the VGII outbreak strain R265. SNP calling indicated a very high level of diversity in the total set of sequenced strains with a total of 773,900 polymorphic sites relative to the VGII isolate R265 [67]. This corresponds to approximately 4.4% of the genome. Within the VGIII sequenced set, there was ∼10-fold less diversity with 87,219 polymorphic sites corresponding to 0.49% of the ∼20 MB genome, which is still substantial. However, in spite of this diversity, the MLST matched isolates were remarkably similar to each other, ranging from a minimum of 46 differentiating sites (0.00026%) between VGIIIa isolates CA1053 (clinical) and BHPP3-S1A (environmental) to a maximum of 183 (0.0010%) between VGIIIb isolates CA1508 (clinical) and MCPR1-S1B (environmental, Figure 5). The SNPs identified within each matched set were characterized based on potential impact and the findings are summarized in Tables S6, S7, and S8. Variants altered three splice sites and introduced a moderate number of nonsynonymous SNPs. These nonsynonymous sites were also characterized based on predicted function, and a number of candidate pathogenesis factors were identified, including some with putative roles in heat tolerance, oxidative stress resistance, and stress response. Whole genome sequencing analysis indicates that the three identified environmental C. gattii populations are the likely environmental reservoirs for recent clinical infections and could serve as the source of additional infections.
Fig. 5. MLST matched clinical and environmental isolates are highly related. 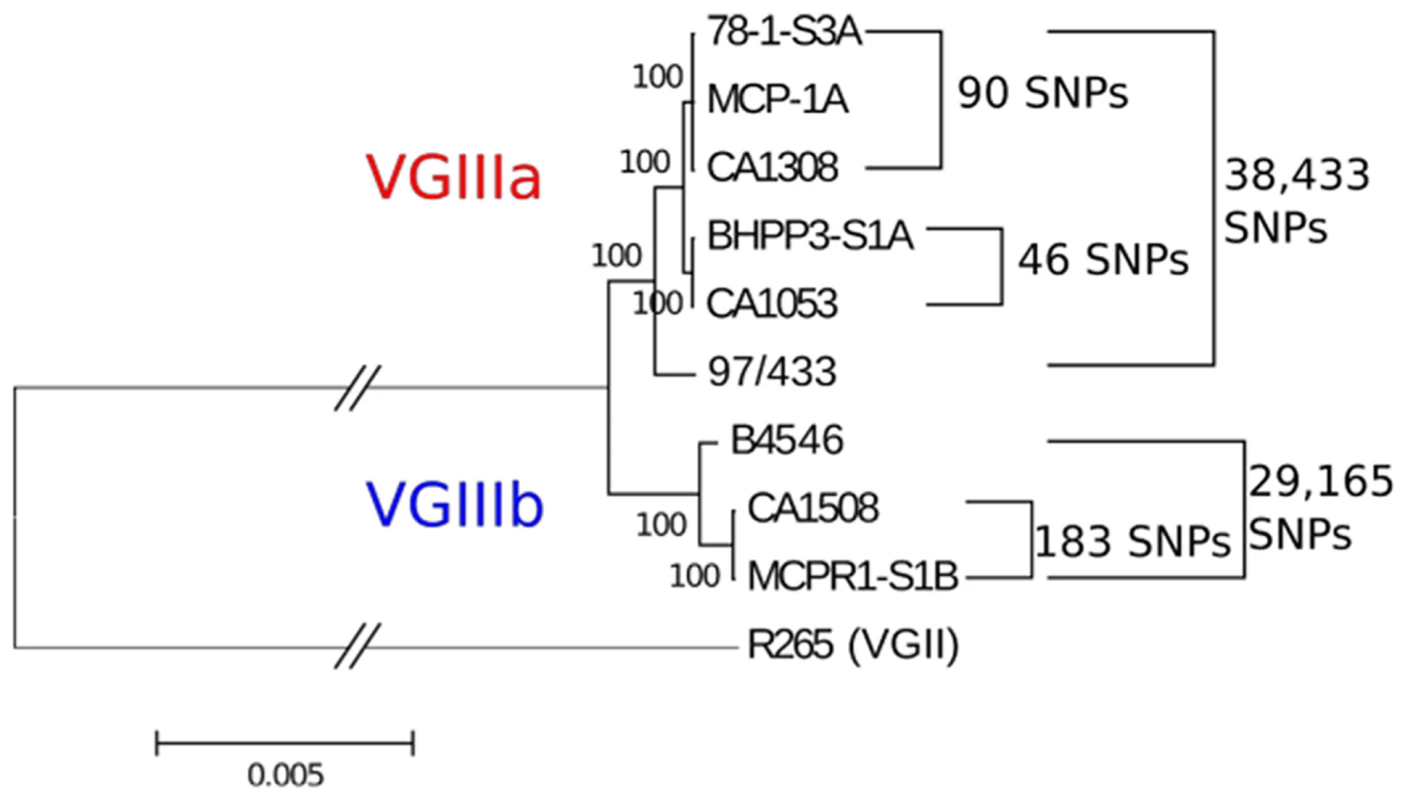
Maximum likelihood tree generated from a reference-based alignment to bases 310,000–410,000 from Supercontig 5 of VGII isolate R265 using 500 bootstrap replicates. Branch length has been truncated, and this is marked with hashes between the outgroup and cluster of VGIII isolates to improve readability. Scale bar indicates 0.005 substitutions per nucleotide position over this window of Supercontig 5. Total numbers of polymorphic sites differentiating each sub-clade of VGIII across the entire genome are indicated to the right of the tree. Environmental C. gattii VGIII isolates are fertile
In addition to MLST analysis and population analysis, mating assays were conducted that established these environmental and clinical VGIII isolates are fertile with C. neoformans (VNI, VNII) and C. gattii (VGII, VGIII) isolates (Table S9a and S9b). Both MATa and MATα isolates produced positive mating reactions as assessed by light and electron microscopy with lateral hypha emanating from the colony (Figure 6), fused clamp connections (Figure S5a and S5b), basidia, and basidiospores (Figure 6 and Figure S5). All strains tested with the exception of two clinical strains (7618666 and 7795476) were fertile with at least one tester strain, although this did not always include mating with a VGIII strain (Table S7b). Both VGIIIb MATα and MATa isolates were fertile, and all VGIIIa MATα environmental isolates were fertile with VNI, VNII, or VGIII tester strains (Table S9b). Environmental isolates were generally more fertile than clinical isolates; however, spore viability was not assessed to determine if these matings produced viable progeny. Clinical isolates are in general less fertile and may have undergone genetic or epigenetic modifications in the host that reduce their fertility. These results suggest that 1) VGIIIb isolates may undergo a-α opposite-sex mating in the environment, 2) many VGIIIa isolates are fertile and could participate in opposite-sex mating when a fertile MATa isolate is present, and 3) interactions between molecular types could play a role in mating in the environment.
Fig. 6. Environmental C. gattii isolates are fertile. 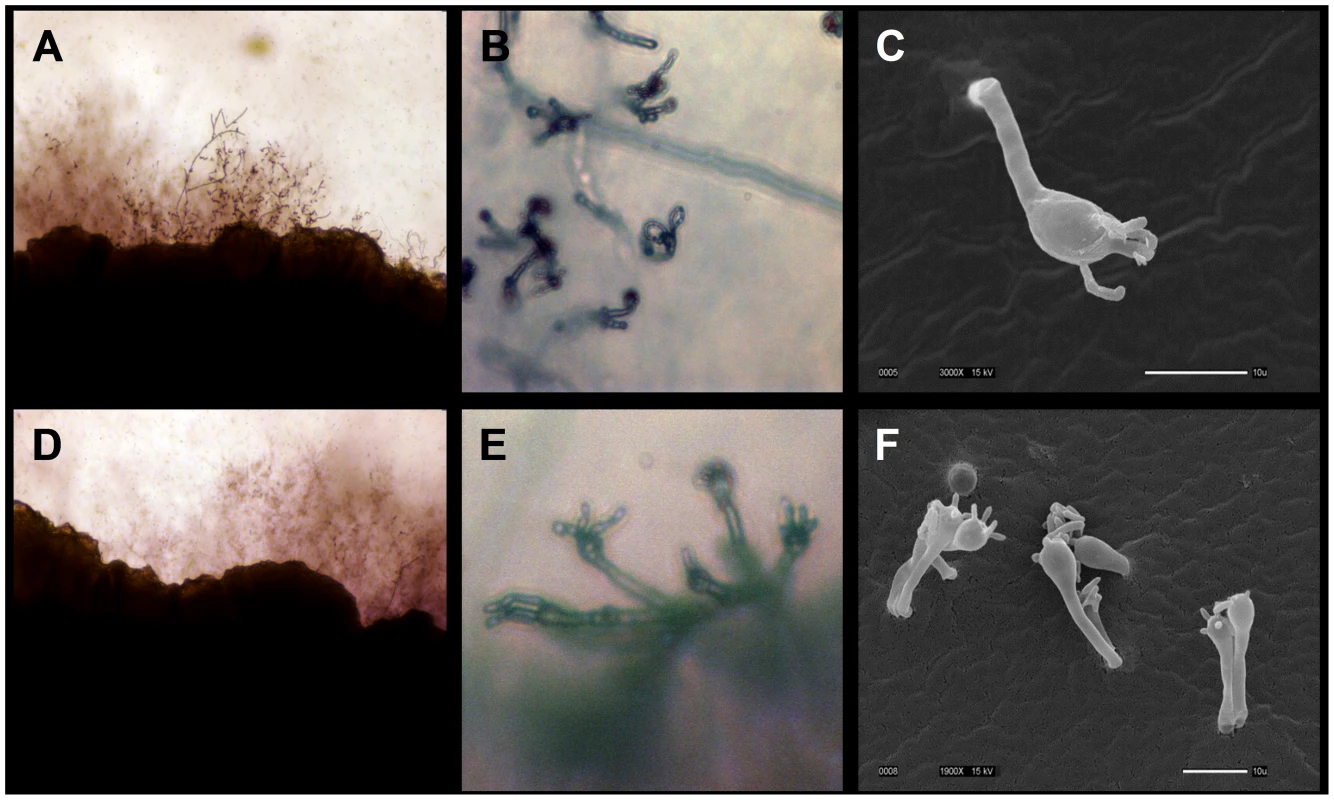
(A, B, C) Isolate MCP-1A or (D, E, F) 78-1 S3A was mated with VGIII B4565a tester strain on V8 agar pH = 5 for 14 days at room temperature in the dark. (A, D) Hyphal growth along the mating patch edges is illustrated (100×). (B, E) Prolific basidia with chains of basidiospores are illustrated (400×). (C, F) Scanning electron micrographs show basidia and spores formed during sexual reproduction (Scale bar = 10 µm). Intracellular proliferation and mitochondrial tubularization appear decoupled in VGIII isolates
Virulence attributes of clinical and environmental C. gattii isolates were ascertained based on intracellular proliferation rates (IPR) and percent mitochondrial tubularization within J774 BALB/c macrophage cell lines. An IPR≥2 indicates a high replication rate within macrophages and is frequently correlated with higher virulence. An IPR score <1 indicates low proliferation levels were observed in macrophages, which have been correlated with a lower potential for virulence in whole animal models in previous studies. An IPR between 1 and 2 indicates moderate proliferation in macrophages and moderate virulence attributes. We observed moderate to low IPR for selected VGIIIb and VGIIIa isolates in direct comparison to the highly virulent VGIIa C. gattii major Pacific Northwest outbreak isolate R265 (Figure 7a and 7b). Percent yeast cells observed with tubularized mitochondria ranged from 9 to 37%, which suggests mitochondrial tubularization is decoupled from IPR in macrophages for C. gattii VGIII isolates compared to the VGIIa isolate R265 [21], [34] (Figure 7b). The coefficient of determination (R2 = 0.2593 is <1, p = 0.0155) indicates a weak positive linear correlation exists between IPR and the presence of tubular mitochondria in all VGIII isolates. A positive but weakly linear correlation is observed for VGIIIa (R2 = 0.3692 is <1, p = 0.0624) but not for VGIIIb (R2 = 0.001 is <1, p = 0.9226) suggesting differences in IPR and mitochondrial regulation may be associated with different molecular sub-types. Our results demonstrate that survival and replication in macrophages (IPR) is similar between environmental and clinical VGIIIa and VGIIIb isolates of both mating types. Notably some VGIII strains have high rates of tubularization prior to a known encounter with the macrophage niche (environmental isolates) while others do not display increased tubularization after encountering the macrophage niche (clinical isolates). The presence of tubular mitochondria did not appear to be strongly correlated with IPR in VGIII isolates, in contrast with previous studies on VGII Pacific northwest outbreak isolates [21], [34]. These data may reflect that VGIII isolates commonly infect HIV/AIDS patients versus VGII isolates that rarely occur in HIV/AIDS patients, and hence VGIII isolates may be in general less virulent than VGII isolates.
Fig. 7. Intracellular proliferation rates (IPR) illustrate moderate proliferation levels and suggest mitochondrial tubularization is decoupled from IPR in macrophages for C. gattii VGIII isolates. 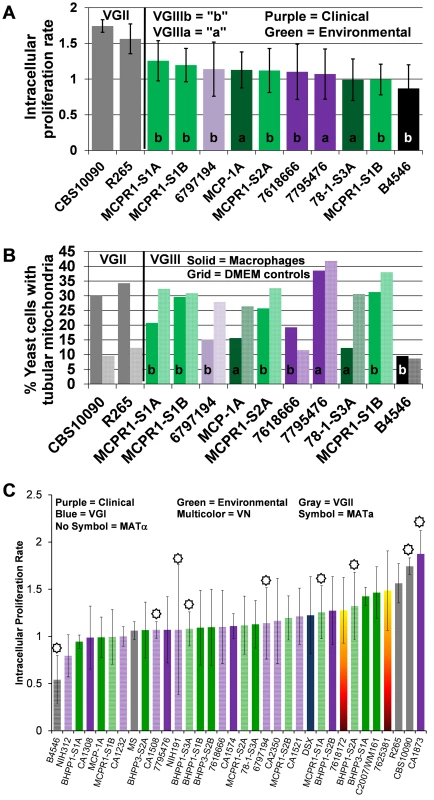
(A) Intracellular C. gattii proliferation rates in macrophages were similar between environmental and clinical isolates. (B) Percent tubular mitochondria as a result of co-incubation with macrophages (solid bars) in comparison to DMEM controls (hatched bars). Percent tubular mitochondria does not correlate with IPR or virulence. Clinical isolates (VGIIIa dark-purple; VGIIIb light-purple), environmental isolates (VGIIIa dark-green; VGIIIb light-green), and previously reported isolates VGIIIa reference strain B4546 (Black) and VGII (Grey). (C) Ranked IPR of tested C. gattii isolates. C. gattii VGIII isolates are virulent in the murine model
The virulence attributes of VGIII C. gattii isolates were further addressed in vivo utilizing the murine intranasal model. The first two environmental isolates, MCP-1A and 78-1-S3C (both identified in late 2011), were assessed for virulence in the BALB/c mouse model and the average length of survival ranged from 56 to more than 250 days post infection (Figure S6a and S6b). Mean survival ranked from shortest to longest is as follows: CA1232 (84 days), CA1053 (90.5 days), CA1308 (96 days), CA1508 (136 days), BHPP1-S2B (149 days), NIH191 (173.5 days), BHPP3-S1A (197 days) and all others were undetermined because some mice were still surviving at the termination of the experiment. MCP-1A and 78-1-S3A were less virulent than the VGIII clinical isolates RR and VV (p<0.002, Figure S6b). Environmental isolate 78-1-S3A was similar in virulence to clinical isolate CA1089 (p = 0.6409) and somewhat more virulent than the historical NIH312 isolate (p = 0.034) (Figure S6b). Our results indicate that environmental isolate MCP-1A is less virulent than environmental isolate 78-1-S3A (p = 0.0083) with which it shares an indistinguishable MLST profile, IPR, and differs by less than 90 SNPs at the whole genome level (Figure 5, Table S8), suggesting that relatively few genetic differences could be responsible for differences in virulence between two very closely related isolates. At a minimum, no more than 46 SNPs seemed to be necessary to confer a phenotypic difference (Figure 5, Table S7). Additionally, differences in epigenetic or transcriptional modifications could contribute to phenotypic differences observed between matched MLST isolates. C. gattii VGIII environmental isolates exhibited attenuated virulence in comparison to VGIII clinical isolates in the intranasal murine mouse model. Virulence observed in the mouse model correlated with previously determined moderate IPR and was decoupled from mitochondrial phenotype suggesting roles of intracellular proliferation and mitochondrial program in the divergence in virulence of C. gattii molecular types.
Virulence attributes of closely related environmental versus clinical genotypes were further examined in A/JCr mice, which are typically more permissive to cryptococcal infection than BALB/c mice. Four groups of MLST matched strains representing VGIIIa (group 2, CA1308, CA1574, and MCP-1A; group 3, CA1053 and BHPP3-S1A) and VGIIIb (group 1, CA1232 and BHPP1-S3A; group 4, CA1508, MCPR1-S1B, NIH191/ATCC32608) were compared in the A/JCr murine model (Figure 8a). Clinical isolates exhibited reduced survival time and higher virulence in comparison to MLST matched environmental isolates. More virulent clinical isolates were associated with more virulent environmental isolates (Figure 8a). MCP-1A and BHPP1-S3A were severely attenuated for virulence in comparison to other virulent strains with 9 of 10 infected animals surviving to the conclusion of the study. Two isolates, one environmental (MCPR1-S1B) and one clinical (CA1574), were avirulent for the duration of study (250+ days post infection) in A/JCr mice (all 10 of 10 animals infected surviving). Tissue burdens ranged from 107 to 108 cells/gram tissue in the lungs, 102 to 106 cells/gram tissue in the spleen, and 102 to 106 cells/gram tissue in the brain and varied greatly between isolates. Consistently, and in agreement with previously published studies, higher organ loads were observed in the lung tissue in comparison to the brain tissues and may suggest that pulmonary cryptococcosis could contribute to the observed mortality [22], [68]. Granulomas were observed macroscopically in the lungs post necropsy and microscopically in histopathological sections for both VGIIIa and VGIIIb isolates (data not shown). Although we observed differences in mean survival time between VGIII strains, high tissue burdens were observed for all strains (of both high and low virulence) post mortem (Figure 8b). This suggests that determination of virulence is not just a static predetermined trait but also dependent on duration of exposure and host status, which could play a more prominent role in disease development in longer-lived human and animal populations.
Fig. 8. Environmental isolates that share an indistinguishable MLST profile with clinical isolates are less virulent in mice. 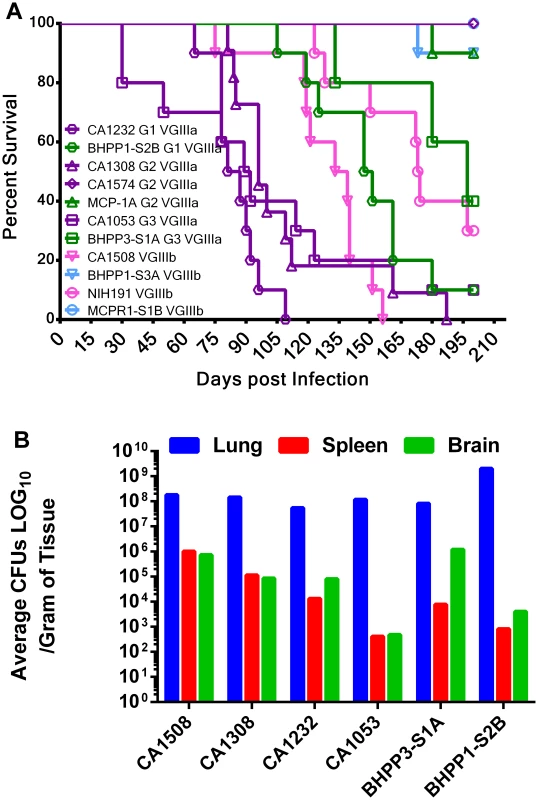
(A) 11 matched isolates representing four distinct MLST groups, three VGIIIa (G1, G2, G3, two strains each) and one VGIIIb (4 strains, 2 clinical, and 2 environmental), were tested for virulence in the murine animal model. Ten male A/JCr mice per strain were intranasally infected with 106 cells and survival was recorded for 200 days. MLST groups are represented by shared symbol and group number. Clinical isolates (VGIIIa dark-purple; VGIIIb light purple), environmental isolates (VGIIIa dark-green; VGIIIb light green). Median survival CA1232 = 90 days, CA1308 = 100 days, CA1053 = 103 days, CA1508 = 127 days, and BHPP1-S2B = 138 days. (B) Mice were assessed for tissue burden postmortem. Response of C. gattii VGIIIa and VGIIIb molecular types to antifungal drugs
Cryptococcus is an environmentally acquired fungal pathogen of humans and infections caused by differing molecular types may result in differences in clinical presentations, treatment, and therapeutic outcomes. In this study, the antifungal susceptibilities to amphotericin B, fluconazole, flucytosine, and ketoconazole were assessed for C. gattii VGIII isolates to examine if differences in antifungal susceptibility might be associated with molecular type and/or origin of the isolates (environmental or clinical). Our results provide evidence that the MIC of VGIIIa isolates can be higher than VGIIIb for recommended drug therapies, amphotericin B (p = 0.0178, Figure 9a) and flucytosine (p<0.0001, Figure 9b), but not for the maintenance antifungal drug fluconazole (p = 0.1059, Figure 9c), or the second line drug ketoconazole (p = 0.0685, Figure 9d). No significant differences were observed between clinical and environmental isolates within VGIIIa or VGIIIb isolates suggesting the genetic propensity for antifungal resistance may exist in environmental populations (Figure 9e–h, p>0.05). However, resistance was observed in response to exposure to fluconazole and/or flucytosine in VGIIIb clinical (HM-1, 7180567, 7618666, CA1508) and environmental isolates (MCPR1-S1B, MCPR1-S2B, MCPR1-S2A, BHPP1-S2A, BHPP1-S3A) as well as VGIIIa clinical (KB-1, CA1308) and environmental (MCP-1A, BHPP1-S2B, BHPP3-S2A) isolates suggesting some isolates may have a greater propensity for the microevolution of drug resistance within the host, possibly influencing long term treatment outcomes. In summary, we observed the emergence of resistance (fluconazole and flucytosine) and higher MICs (amphotericin B and flucytosine) among different subsets of the C. gattii VGIII population for the recommended first line treatment drugs, amphotericin B and flucytosine, suggesting molecular type and the genetic propensity for resistance could possibly impact treatment and long-term outcomes.
Fig. 9. C. gattii VGIIIb isolates display higher antifungal susceptibility values to Amphotericin B and flucytosine in contrast to VGIIIa isolates. 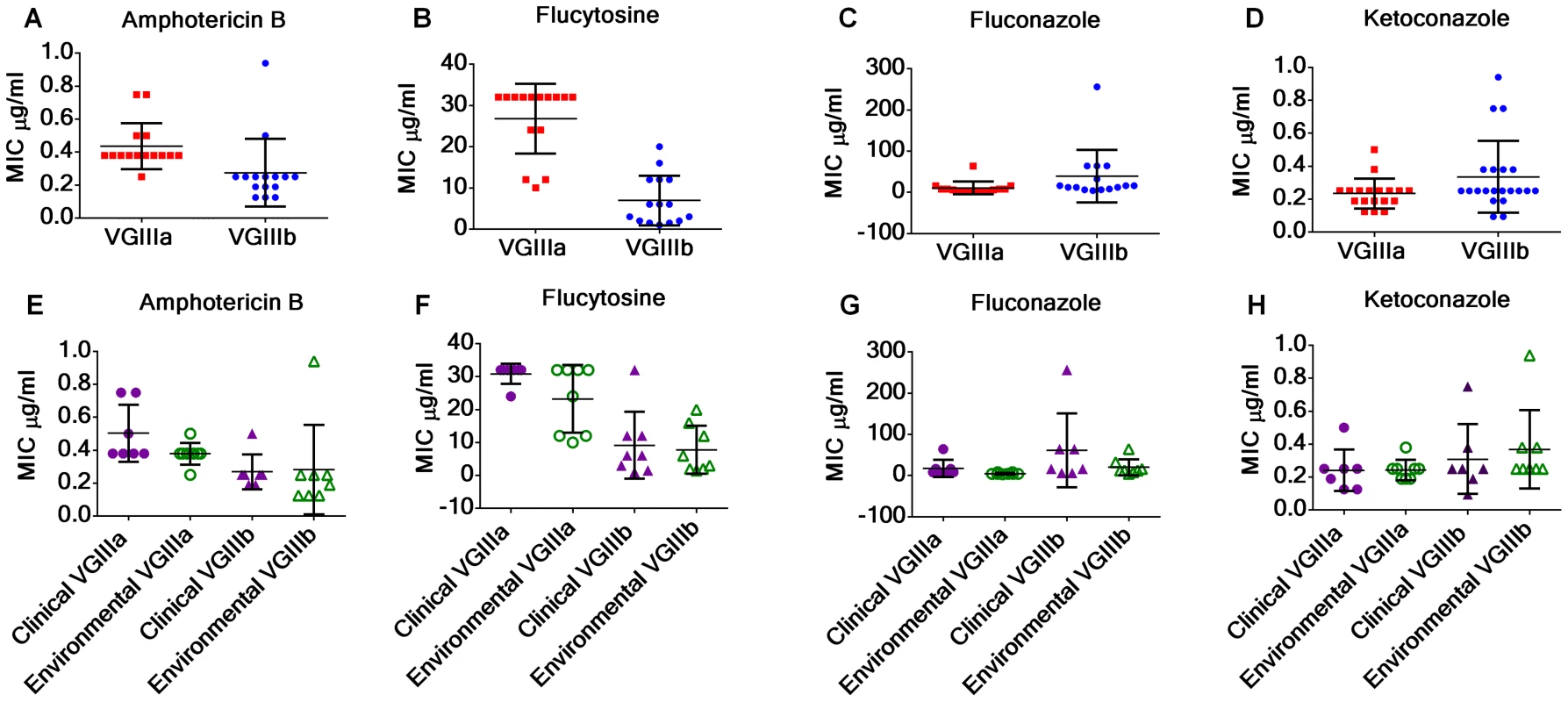
(A, E) MIC of VGIIIa (red) versus VGIIIb (blue) for amphotericin B (A), flucytosine (B), fluconazole (C), and ketoconazole (D). MIC of clinical (purple) and environmental (green) isolates of VGIIIa (triangles) and VGIIIb (circles). The MIC of VGIIIa isolates was significantly higher than VGIIIb isolates for amphotericin B (p = 0.0178) and flucytosine (p<0.0001) but not for fluconazole (p = 0.1059) or ketoconazole (p = 0.0685). No significant differences were observed between environmental and clinical isolates of VGIIIa or of VGIIIb in response to amphotericin B (E), flucytosine (F), fluconazole (G), or ketoconazole (H) (Table S10a, S10b and S10c). MIC values, which exceeded the maximum concentration for each Etest, were assigned as the maximum concentration tested for averaging and graphing (Table S1). Maximum concentrations of Etest amphotericin B (32 µg/ml), fluconazole (256 µg/ml), flucytosine (32 µg/ml), and ketoconazole (32 µg/ml). Discussion
The clinical and veterinary prevalence of cryptococcosis and the identification of C. gattii as the causative agent in the PNW outbreak stimulated increased environmental sampling that resulted in the identification of the ecological niche of C. gattii in the environment in association with native trees on Vancouver Island and in the Pacific Northwest [59]. Increased molecular typing has resulted in the identification of both C. gattii VGIIa and VGIIb molecular types with shared MLST profiles between clinical and environmental isolates, indicating the endogenous environmental reservoir for the ongoing C. gattii PNW outbreak [22], [26], [60]. The outbreak is caused by three largely clonal lineages: VGIIa/major, VGIIb/minor, and VGIIc/novel which are members of a global, fertile, recombining population that gave rise to the hypervirulent outbreak strains [21].
Previous studies have demonstrated the presence and molecular diversity of C. gattii in the clinical population of Southern California but only one historical environmental VGIII isolate (WM161) had been reported from this region, even with increased sampling [22], [45], [69]–[71]. The historical VGIII environmental isolate (WM161) differed in MLST profile (due to unique IGS and GPD1 alleles) compared to all known VGIII clinical isolates from California. Ongoing epidemic incidences of C. gattii that have emerged in the Western USA are cause for concern due to clinical infections in both immunocompetent individuals and populations with immunocompromised status, including HIV/AIDS. C. gattii is an environmentally acquired pathogen and thus the paucity of environmental isolates is incongruent with its known presence in the clinical population in California. The identification and environmental isolation reported herein of C. gattii VGI and VGIII from four independent sampling sites in the greater Los Angeles area mimics the patchy and often sporadic isolation reported for other environmentally acquired fungal pathogens (Blastomyces, Coccidioides) associated with periodic outbreaks in the USA [6], [72]–[74].
In this study we report the environmental isolation of 19 C. gattii VGIIIa, VGIIIb, and VGI isolates from Los Angeles, California from samples obtained from three novel tree species Pinus canariensis (Canary Island pine), Liquidamar styraciflua (American sweetgum), and Metrosideros excels (Pohutukawa tree). These environmental isolates were obtained from areas in close proximity to the residences of patients. We have identified an environmental source of the most common C. gattii VGI molecular type, which constitutes a smaller proportion of C. gattii infections in California and elsewhere in the US [22], [45], [75], [76]. Of the 19 environmental isolates, 16 share indistinguishable MLST profiles with previously reported clinical isolates from the Southern California region identifying endogenous environmental reservoirs for both VGIIIa and VGIIIb infections in the Los Angeles, metropolitan area, USA [22]. Therefore, we have the rare opportunity to identify and genetically link known environmental reservoirs to individual cases in Southern California.
In this study we report the presence of fertile MATa and MATα environmental isolates and demonstrate evidence of recombination within the VGIIIb population in the Southern California region. This is notable, at least in part, because a-α mating is relatively rare in the Cryptococcus species complex, with a few notable exceptions, because of the paucity of MATa isolates [56], [58], [77], [78]. In the case of C. neoformans var. grubii, the majority of MATa isolates are part of a specific population restricted to Botswana that is also hypothesized to be the origin of the species [79]. The population of VGIIIb MATa isolates could similarly represent a locally restricted founder population in California. It is also possible that the paucity of MATa isolates within the VGIIIa population, may be indicative of a high frequency of α-α mating in the population. As in VGIIIb, there is evidence for recombination within the VGIIIa population, but in this case may be occurring without the involvement of MATa isolates. While we cannot rule out the existence of MATa isolates that have remained unsampled, our current evidence suggests this population may reassort without bisexual mating. Further sampling will increase the power of this analysis Sex within C. gattii populations is important for two reasons. First, the sexual cycle produces spores that can be aerosolized as potential infectious propagules [80]. Second, sex can aid in the evolution of virulence. Same-sex mating has been proposed as a factor in the development of the VGIIa major outbreak strain in the Pacific Northwest, while a-α sexual reproduction aids in the transmission of hypervirulence [25], [33]. Through both routes, sex can contribute to the development and persistence of an outbreak. In addition, sexual reproduction led to the emergence of the modern pathogenic lineage for the common human parasite Toxoplasma gondii and selfing has recently been shown to drive outbreaks [81], [82].
C. gattii VGIII clinical and environmental isolates share similar in vitro IPR and mitochondrial phenotypes. Moderate ranges of IPR appear unrelated to the extent of mitochondrial tubularization in contrast to C. gattii VGII isolates, suggesting these VGIII isolates have reduced virulence in comparison to the VGII outbreak strains [21], [33]. We observed reduced virulence of VGIII in comparison to VGII in the murine model, consistent with the observed predilection of VGIII to infect immunocompromised individuals with HIV/AIDS compared to VGII that commonly infects otherwise healthy patients. An alternative explanation is that C. gattii VGIII causes dormant infections that can be reactivated at the time of immunosuppression whereas VGII causes primary infections. However, this alternative explanation seems less likely because VGII has also been associated with the potential to cause dormant infections [14]. We tested paired clinical and environmental isolates that shared indistinguishable MLST profiles, and although WGS indicated relatively few genetic differences, all clinical isolates were more virulent in comparison to the environmentally isolated paired MLST strains except for VGIIIa CA1574. Furthermore, in the paired MLST matched isolates that were analyzed in murine virulence assays we found that all VGIIIa strains were more virulent than VGIIIb strains, in agreement with previous findings [22]. Tissue burden and histopathological analyses indicated significantly higher fungal burdens in the lungs in comparison to the spleen or brain, consistent with previous reports of a predilection of VGIII to initiate prolonged pulmonary infections [22], [32]. Thus, although clinical and environmental isolates shared indistinguishable MLST profiles, IPR, and >99.9% sequence identity, their virulence potential differed in the murine model.
Whole genome sequencing of three different MLST matched groups representing both VGIIIa and VGIIIb populations indicated that previously reported clinical isolates (circa 2000–2005) and newly identified environmental isolates (2011–2012) are extremely similar, differing at only 46 SNPs between VGIIIa clinical isolate CA1053 and environmental isolate BHPP3-S1A, and 183 SNPs between the VGIIIb clinical isolate CA1508 and the environmental isolate MCPR1-S1B. This is all the more poignant given that the clinical and environmental isolates were ascertained over an isolation period spanning 5 to 12 years, further forging a link between the environmental isolates and clinical infections in Southern California. This can be compared to H99 lab passaged isolates obtained over a ten year period, which are distinguished by 11 SNPs and 11 indels [83]. Furthermore, a recent report on Staphylococcus aureus (genome size 2.4 Mb) used a cutoff of 40 single nucleotide variants (SNVs) to decide whether two strains were indistinguishable or different [84]. We aligned our sequences to R265 with a total genome size of 17.5 Mb, which would allow for 6.2-fold more variants and up to 249 SNVs. Thus, by even this strict comparison the VGIIIa/b clinical and environmental isolates are closely related than bacterial isolates concluded to be causally related in chains of transmission.
Whole genome sequencing allows new approaches to pathogenesis. However, comparative genomic approaches have limitations. When comparing highly pathogenic and less pathogenic isolates, finding a genetic change important for pathogenesis is challenging because of unrelated incidental genetic diversity. One method to avoid this is use strains that have a common, recent origin. This reduces genomic differences that could obscure causative genotypic changes and increases the probability of linking genotype to phenotype. In this study, environmental isolates that we propose directly gave rise to the infectious isolate were chosen via MLST matching; however, similar approaches could be used for longitudinal studies of individuals with recurrent fungal infections or for other environmental infections where a source population is readily available. While beyond the scope of this study, resource populations established through MLST matching of isolates with disparate phenotypes will provide an avenue for linkage between genotype and phenotype. Substantial differences in virulence were observed in our studies between two very similar isolates. At a minimum, no more than 46 SNPs seems necessary to confer a phenotypic difference between the clinical isolate CA1053 and the environmental isolate BHPP3-S1A; only 15 of these SNPs result in nonsynonymous changes. We sequenced three closely related VGIIIa isolates from group 2, representing two independent environmental isolates obtained from different locations and a previously identified clinical isolate. The three isolates differ by only 90 SNPs but exhibit differences in pathogenicity and antifungal sensitivity. Furthermore this genotype is noteworthy because it is represented in the current clinical population by isolate 7489719. This suggests that adaptation from an environmental lifestyle to a pathogenic lifestyle may be a very rapid event, involving epigenetic modification, changes in transcriptional regulation, or requiring few genetic changes. Passage of Cryptococcus through amoeba, nematodes, slime molds, plants, and animals yields passaged strains with enhanced virulence [68], [85]–[89]. Prolonged lab culture without host exposure can attenuate virulence [90]. Passage of strain H99 resulted in a lineage with enhanced or attenuated virulence with only 4 associated SNPs, two of which could contribute to attenuated virulence [83]. Also notable is that none of the SNPs from our three matched sets was shared between sets or in common genes. This suggests that either there are multiple independent routes of adaptation to produce a more virulent organism, or that transcriptional and epigenetic changes may also contribute.
C. gattii is an environmentally acquired opportunistic fungal pathogen that can cause acute or latent infections in both immunocompromised and immunocompetent hosts and increased recognition of ongoing outbreaks in humans, pets, and wildlife is emerging as a serious public health concern and financial burden. We report the isolation of fertile C. gattii VGIIIa, and VGIIIb isolates from environmental soil and swab samples from non-Eucalyptus host trees in the greater Los Angeles area of Southern California. MLST and WGS analysis coupled with mating and virulence studies demonstrate that environmental C. gattii VGIII isolates are actively recombining and the likely source of human acquired infections in this region and can further contribute to ongoing infections in Southern California. There is now a substantial body of accumulated data that suggest that C. gattii is associated in the environment with many tree species (native and non-native) worldwide and these hosts function as environmental reservoirs for disease outbreaks. Future environmental and epidemiological studies can further incorporate molecular and whole genome analysis to connect known clinical/veterinary incidences of cryptococcosis with newly identified ecological niches to further address the link between genotype, risk of exposure, and propensity for initiating disease.
Materials and Methods
Environmental sampling and isolates utilized in this study
Swabs of individual tree trunks and soil samples from around the base of trees were collected during the summers of 2011 and 2012 utilizing BD BBL Single application CultureSwabs with Liquid Amies (VWR#90001-036). In summary, over two years 24 sites were sampled to obtain 109 tree swabs of over 30 tree species and 58 soil samples from collected from the greater Los Angeles area. In 2011 samples were obtained from 9 locations, 64 trees (30 different species, including 10 Eucalyptus trees from four independent sites.), and 25 soil samples in the greater Los Angeles area. In 2012 the two sites that had previously yielded C. gattii were resampled and 15 additional sites were sampled focusing on Pinus canariensis and Liquidambar styraciflua. From these trees 45 trees were swabbed and 33 soil samples were collected. The swabs were streaked onto Niger seed (NGS) agar containing chloramphenicol (0.5 g/L, Sigma C0378-100G) Two grams of soil were suspended in 10 mL of sterile ddH20 and allowed to settle. Three 100 µl aliquots were plated on NGS agar. Plates were incubated at 30°C for 1 to 3 days. Yeast colonies producing brown pigmentation were selected and colony purified. All clinical and environmental isolates were streaked onto NGS and canavanine-glycine bromothymol blue (CGB) agar and incubated for 1 to 3 days to identify C. gattii isolates. Clinical isolates were obtained from 48 patients who were treated at the University of Southern California, Harbor-UCLA Medical Center, or Kaiser Permanente Downey Hospital between February 2008 and January 2013. Of these patients, 32 were considered to be immunocompromised due to HIV/AIDS or other causes. The clinical isolates were de-identified and linked to major cross streets near the patients' homes. Stocks were maintained at −80°C in 25% glycerol. All strains utilized in this study are listed in Table S1. Genomic DNA was prepared by the CTAB method for all isolates. Potential C. gattii isolates were screened for molecular type by size differences of the ATP6 PCR product [33], [61].
Multilocus sequence typing analysis
Multilocus sequence typing was performed on 12 loci (SXI1α or SXI2a, IGS, TEF1, GPD1, LAC1, CAP10, PLB1, MPD1, CAP59, TOR1, SOD1, and URA5) [15], [21], [25]. For each isolate, genomic regions were PCR amplified, purified (ExoSAP-IT, Qiagen), sequenced, and both forward and reverse strands were assembled with complete double-strand coverage. All primers utilized in this study are listed in Table S2. MLST sequences were viewed and edited by Sequencher; alignment and phylogenetic analyses were conducted using MEGA version 5 [91]. Each allele was assigned a corresponding number, or given a new number if the sequence was not already assigned an allele number in the GenBank database (NCBI), International Society for Human and Animal Mycoses MLST Database (ISHAM), or previously published reports [15], [22], [27], [45]. GenBank accession numbers with corresponding allele numbers are listed in Table S3. Haplotype network analysis was performed using TCS software (version 1.21) [92].
Ploidy analysis
Ploidy was determined by fluorescence activated cell sorting (FACS) as previously described [93]. Briefly, cells were grown, passaged twice in 25 ml YPD broth at 25°C, collected by centrifugation, and washed and resuspended in 5 mL of 1x PBS. 1 mL of cells was collected by centrifugation and fixed in 70% ETOH overnight with gentle shaking at 4°C. Cells were collected by centrifugation, washed, and resuspended in 1 mL of 1x NS buffer. Cells were centrifuged and resuspended 200 µl (180 µl NS buffer, 14 µl RNase A, 6 µl propidium iodide and incubated overnight at room temperature. 50 µl of each strain (10,000 cells) was mixed with 500 µL of Tris-PI mix (482 µl 1M Tris pH 7.5+18 µl propidium iodide) and analyzed using the FL1 channel (slow laser scan) on a Becton-Dickinson FACScan. C. gattii NIH444 and C. neoformans XL143 were utilized as haploid and diploid reference controls.
Sequencing and assembly of VGIII genomes
Whole Genome Sequencing (WGS) was done by Illumina paired end reads. Genomes of strains CA1053, CA1308, CA1508, BHPP3-S1A, MCP-1A, 78-1-S3A, MCPR1-S1B, and 97/433 were sequenced using HiSeq2500 using paired end reads with an insert size of approximately 300 bp and a read length of 100 bp. Initial processing was performed using the Illumina Pipeline (v.1.8.2) [94]. The genome of B4546 was previously sequenced [33]. Reads were aligned to the published VGII reference genome supercontigs using BWA-sampe [95] and SNPs were called using the Genome Analysis Toolkit (GATK V2.4-9) Unified Genotyper with the haploid ploidy setting. The resulting calls were then filtered to remove calls with a quality score below 30, and individual depths below 5 reads. SNPs common to each pair were removed and the remaining SNPs differentiating the subset were manually examined using the BAM files to remove erroneous calls based on poor mapping or repetitive sequences. The resulting variants were analyzed using SnpEff to determine impact of SNPs [96]. Assignment of gene function was carried out using a combination of BLAST to the S. cerevisiae genome and CD search through NCBI.
Whole genome trees were generated from a 100 kb region aligning to supercontig 5 of the VGII reference genome. SNPs were manually filtered to eliminate erroneous calls. The resulting SNPs were used to generate alternate reference sequences using GATK's FastaAlternateReferenceMaker that were then aligned using Kalign [97]. The resulting alignment was used to produce a maximum likelihood tree using MEGA5 with 500 bootstraps [91].
Allele compatibility tests
Allele information derived from MLST analysis was used to construct paired allele networks, where alleles present in combination were connected with a solid line. The presence of all four combinations of two alleles was represented as an hourglass shape, with the lines darkened to indicate evidence for recombination [22]. This analysis relies on the assumption that homoplasy is a rare event.
Mating assays
MATa and MATα isolates were grown on YPD agar at room temperature for two days. Cells were scraped off YPD agar, washed, and suspended in autoclaved ddH2O. Cells were spotted alone or in combination (MATα and MATa) on V8 pH = 5 and V8 pH = 7 mating agar and incubated in the dark at room temperature. Plates were checked once a week for 24 weeks for hyphae and/or the formation of basidia and basidiospores. To examine mating reactions for morphological features associated with mating, scanning electron microscopy studies were conducted. One centimeter square blocks were excised from the agar plates and fixed in 2% glutaraldehyde (Electron Microscopy Sciences, EMS, Hatfield, PA, USA) with 0.05% malachite green oxalate (EMS) in 0.1 M sodium cacodylate buffer and incubated at 4°C until further processing. The fixation buffer was removed, blocks were dehydrated by ethanol series, critical point dried (Pelco CPD2, Ted Pella, Inc., Redding, California, USA), sputter coated, and imaged with the FEI XL30 SEM-FEG (FEI Company, Hillsboro, Oregon, USA) at the electron microscopy facility at North Carolina State University.
In vitro macrophage intracellular proliferation rate and formation of tubular mitochondria
Intracellular proliferation rate (IPR) was determined as previously described utilizing J774 macrophages [21], [33], [34]. Macrophages were co-incubated for 2 hours with opsonized cryptococcal cells (18B7 antibody) as described previously [21], [34]. Wells were washed with phosphate-buffered saline (PBS) four times to remove excess extracellular yeast cells and 1 ml of fresh serum-free DMEM was then added. For the control time point T = 0, the DMEM was discarded and 200 µl of sterile ddH2O was added to lyse the macrophages. After 30 minutes, lysed macrophages released intracellular yeast cells, which were then collected in an additional 200 µl ddH2O. For the additional time points T = 18 hrs and 24 hrs, intracellular cryptococcal cells were collected and independently counted with a hemocytometer.
The IPR assay was replicated at least three times for each strain tested using independently propagated batches of macrophages. The IPR value was calculated by dividing the maximum intracellular yeast number by the initial intracellular yeast number at T = 0. We confirmed that Trypan Blue stains 100% of the cryptococcal cells in a heat-killed culture, but only approximately 5% of cells from a standard overnight culture. Compared to a conventional colony counting method, this method was shown to be more sensitive in detecting the clustered yeast population or yeast cells undergoing budding.
Tubularized mitochondria were determined as previously described [33], [34]. C. gattii cells were grown overnight at 37°C in DMEM, harvested, washed twice in PBS, and re-suspended in PBS plus Mito-Tracker Red CMXRos (Invitrogen) at a final concentration of 20 nM for 15 min at 37°C. Cells were washed three times and re-suspended in PBS. 100 yeast cells in three replicates per strain were randomly chosen, imaged using a Zeiss Axiovert 135 TV microscope with a 100X oil immersion Plan-Neofluor objective or a Nikon Eclipse Ti Plan Apo VC 60X oil immersion objective, images were collected, and percent tubularized mitochondria were calculated. All images were processed identically in ImageJ and mitochondrial morphologies were analyzed and counted blindly. IPR and tubularization data were analyzed for statistically significant differences using one-way ANOVA analysis with multiple comparisons by Tukey's Honestly Significant Difference (HSD) posthoc test. A p-value of <0.05 after controlling for multiplicity was considered to be statistically significant. IPR and percent yeast cells with tubular mitochondria were plotted and linear regression, residual plot, and R2 analysis was completed with GraphPad Prism version 6.03 (Windows, GraphPad Software, La Jolla California USA, www.graphpad.com.).
In vivo murine model
Six-week-old female A/JCr mice (Cat. No. 01A24, NCI-Frederick) or male BALB/c mice (Cat. No. 01B05, NCI-Frederick) were used. Mice were acclimatized in the facility for one week prior to infection by intranasal instillation and were housed in cages at 21°C and 50% humidity with a 12 hr light/12 hr dark cycle. Cells were grown in YPD broth with two successive passages and collected by centrifugation, washed with autoclaved ddH2O, and suspended in autoclaved ddH2O. Mice were inoculated intranasally with 106 cells in 40 µl. At the first signs of poor health or discomfort, mice were euthanized with CO2. Kaplan-Meier survival curves were constructed by GraphPad Prism version 6.03 (Windows, GraphPad Software, La Jolla California USA, www.graphpad.com.). Additional data on colonization was obtained from lung, brain, and spleen tissues postmortem. Tissues were aseptically harvested postmortem. Tissue homogenates were serially diluted and plated on YPD agar, incubated at 30°C for 2 to 3 days, and colony forming units (CFUs) were determined. Two mice were chosen at random from each treatment group, and lung, spleen, or brain tissues were fixed in 10% buffered formalin (lung and spleen tissues) or Bouin's fixative (brain tissues), processed into paraffin blocks, sectioned, and stained with hematoxylin and eosin (H & E) and Mayer's mucicarmine for histopathological examination.
Ethics statement
All animal studies were conducted in the Division of Laboratory Animal Resources (DLAR) facilities at Duke University Medical Center (DUMC) and animals were handled according to the guidelines defined by the United States Animal Welfare Act and in full compliance with the DUMC Institutional Animal Care Use Committee (IACUC). Animal models were reviewed and approved by DUMC IACUC under IACUC protocol # A217-11-08.
Antifungal drug sensitivity
C. gattii isolates were grown and passaged two times in YPD broth at 30°C. Cells were collected by centrifugation and washed and suspended in 0.85% NaCl to an OD600 = 2 (equivalent to 1 McFarland turbidity) following BioMerieux recommended protocol (http://www.biomerieux-diagnostics.com). Two RPMI agar plates (RPMI 1640+MOPS+2% Glucose+1.5% agar) per strain were swabbed in three contrasting directions and allowed to dry at room temperature. Amphotericin B, fluconazole, flucytosine, and ketoconazole BioMerieux Etest strips were place on plates in pairs, and incubated 48–72 hours at 35°C with 5% CO2 and MIC values were reported as indicated in the Etest instructions.
Supporting Information
Zdroje
1. ReedC, BiggerstaffM, FinelliL, KooninLM, BeauvaisD, et al. (2013) Novel framework for assessing epidemiologic effects of influenza epidemics and pandemics. Emerg Infect Dis 19 : 85–91.
2. DawoodFS, IulianoAD, ReedC, MeltzerMI, ShayDK, et al. (2012) Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 12 : 687–695.
3. JutlaA, WhitcombeE, HasanN, HaleyB, AkandaA, et al. (2013) Environmental factors influencing epidemic cholera. Am J Trop Med Hyg 89 : 597–607.
4. ChristianKA, IjazK, DowellSF, ChowCC, ChitaleRA, et al. (2013) What we are watching–five top global infectious disease threats, 2012: a perspective from CDC's Global Disease Detection Operations Center. Emerg Health Threats J 6 : 20632.
5. de Hoog GS, Guarro J, Gene J, Figueras MJ (2000) Atlas of Clinical Fungi. Utrecht: Centraalbureau voor Schimmelcultures/Universitat Rovira i Virgili.
6. FisherFS, BultmanMW, JohnsonSM, PappagianisD, ZaborskyE (2007) Coccidioides niches and habitat parameters in the southwestern United States: a matter of scale. Ann N Y Acad Sci 1111 : 47–72.
7. PappagianisD (1988) Epidemiology of coccidioidomycosis. Curr Top Med Mycol 2 : 199–238.
8. HowardDH (1984) The epidemiology and ecology of blastomycosis, coccidioidomycosis and histoplasmosis. Zentralbl Bakteriol Mikrobiol Hyg A 257 : 219–227.
9. BarkerBM, JewellKA, KrokenS, OrbachMJ (2007) The population biology of coccidioides: epidemiologic implications for disease outbreaks. Ann N Y Acad Sci 1111 : 147–163.
10. Neblett FanfairR, BenedictK, BosJ, BennettSD, LoYC, et al. (2012) Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med 367 : 2214–2225.
11. ChamanyS, MirzaSA, FlemingJW, HowellJF, LenhartSW, et al. (2004) A large histoplasmosis outbreak among high school students in Indiana, 2001. Pediatr Infect Dis J 23 : 909–914.
12. CairnsL, BlytheD, KaoA, PappagianisD, KaufmanL, et al. (2000) Outbreak of coccidioidomycosis in Washington state residents returning from Mexico. Clin Infect Dis 30 : 61–64.
13. BrownGD, DenningDW, GowNA, LevitzSM, NeteaMG, et al. (2012) Hidden killers: human fungal infections. Sci Transl Med 4 : 165rv113.
14. HagenF, ColomMF, SwinneD, TintelnotK, IattaR, et al. (2012) Autochthonous and dormant Cryptococcus gattii infections in Europe. Emerging Infectious Diseases 18 : 1618–1624.
15. MeyerW, AanensenDM, BoekhoutT, CogliatiM, DiazMR, et al. (2009) Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med Mycol 47 : 1–14.
16. CogliatiM (2013) Global molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii: An atlas of the molecular types. Scientifica 2013 : 23.
17. ParkBJ, WannemuehlerKA, MarstonBJ, GovenderN, PappasPG, et al. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23 : 525–530.
18. PyrgosV, SeitzAE, SteinerCA, PrevotsDR, WilliamsonPR (2013) Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLOS ONE 8: e56269.
19. DixonDM, McNeilMM, CohenML, GellinBG, La MontagneJR (1996) Fungal infections: a growing threat. Public Health Rep 111 : 226–235.
20. SpringerDJ, ChaturvediV (2010) Projecting global occurrence of Cryptococcus gattii. Emerg Infect Dis 16 : 14–20.
21. ByrnesEJ3rd, LiW, LewitY, MaH, VoelzK, et al. (2010) Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the Northwest United States. PLOS Pathogens 6: e1000850.
22. ByrnesEJ3rd, LiW, RenP, LewitY, VoelzK, et al. (2011) A diverse population of Cryptococcus gattii molecular type VGIII in Southern Californian HIV/AIDS patients. PLOS Pathogens 7: e1002205.
23. MeyerW, CastanedaA, JacksonS, HuynhM, CastanedaE (2003) Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerging Infectious Diseases 9 : 189–195.
24. NgamskulrungrojP, GilgadoF, FaganelloJ, LitvintsevaAP, LealAL, et al. (2009) Genetic diversity of the Cryptococcus species complex suggests that Cryptococcus gattii deserves to have varieties. PLOS ONE 4: e5862.
25. FraserJA, GilesSS, WeninkEC, Geunes-BoyerSG, WrightJR, et al. (2005) Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437 : 1360–1364.
26. ByrnesEJ3rd, BildfellRJ, FrankSA, MitchellTG, MarrKA, et al. (2009) Molecular evidence that the range of the Vancouver Island outbreak of Cryptococcus gattii infection has expanded into the Pacific Northwest in the United States. J Infect Dis 199 : 1081–1086.
27. HagenF, CeresiniPC, PolacheckI, MaH, van NieuwerburghF, et al. (2013) Ancient dispersal of the human fungal pathogen Cryptococcus gattii from the Amazon rainforest. PLOS ONE 8: e71148.
28. BoversM, HagenF, KuramaeEE, BoekhoutT (2008) Six monophyletic lineages identified within Cryptococcus neoformans and Cryptococcus gattii by multi-locus sequence typing. Fungal Genet Biol 45 : 400–421.
29. SharptonTJ, NeafseyDE, GalaganJE, TaylorJW (2008) Mechanisms of intron gain and loss in Cryptococcus. Genome Biol 9: R24.
30. XuJ, VilgalysR, MitchellTG (2000) Multiple gene genealogies reveal recent dispersion and hybridization in the human pathogenic fungus Cryptococcus neoformans. Mol Ecol 9 : 1471–1481.
31. XuJ, YanZ, GuoH (2009) Divergence, hybridization, and recombination in the mitochondrial genome of the human pathogenic yeast Cryptococcus gattii. Mol Ecol 18 : 2628–2642.
32. BoversM, HagenF, KuramaeEE, BoekhoutT (2009) Promiscuous mitochondria in Cryptococcus gattii. FEMS Yeast Res 9 : 489–503.
33. VoelzK, MaH, PhadkeS, ByrnesEJ, ZhuP, et al. (2013) Transmission of hypervirulence traits via sexual reproduction within and between lineages of the human fungal pathogen Cryptococcus gattii. PLOS Genetics 9: e1003771.
34. MaH, HagenF, StekelDJ, JohnstonSA, SionovE, et al. (2009) The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc Natl Acad Sci U S A 106 : 12980–12985.
35. OlsonA, StenlidJ (2001) Plant pathogens. Mitochondrial control of fungal hybrid virulence. Nature 411 : 438.
36. ByrnesEJ3rd, BildfellRJ, DearingPL, ValentineBA, HeitmanJ (2009) Cryptococcus gattii with bimorphic colony types in a dog in western Oregon: additional evidence for expansion of the Vancouver Island outbreak. J Vet Diagn Invest 21 : 133–136.
37. WalravenCJ, GersteinW, HardisonSE, WormleyF, LockhartSR, et al. (2011) Fatal disseminated Cryptococcus gattii infection in New Mexico. PLOS ONE 6: e28625.
38. MacDougallL, KiddSE, GalanisE, MakS, LeslieMJ, et al. (2007) Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerg Infect Dis 13 : 42–50.
39. UptonA, FraserJA, KiddSE, BretzC, BartlettKH, et al. (2007) First contemporary case of human infection with Cryptococcus gattii in Puget Sound: Evidence for spread of the Vancouver Island outbreak. J Clin Microbiol 45 : 3086–3088.
40. ChaturvediS, RenP, NarasipuraSD, ChaturvediV (2005) Selection of optimal host strain for molecular pathogenesis studies on Cryptococcus gattii. Mycopathologia 160 : 207–215.
41. OkamotoK, HatakeyamaS, ItoyamaS, NukuiY, YoshinoY, et al. (2010) Cryptococcus gattii genotype VGIIa infection in man, Japan, 2007. Emerging Infectious Diseases 16 : 1155–1157.
42. NgamskulrungrojP, SerenaC, GilgadoF, MalikR, MeyerW (2011) Global VGIIa isolates are of comparable virulence to the major fatal Cryptococcus gattii Vancouver Island outbreak genotype. Clinical Microbiology and Infection 17 : 251–258.
43. KaocharoenS, NgamskulrungrojP, FiracativeC, TrillesL, PiyabongkarnD, et al. (2013) Molecular epidemiology reveals genetic diversity amongst isolates of the Cryptococcus neoformans/C. gattii species complex in Thailand. PLoS Negl Trop Dis 7: e2297.
44. ChaturvediS, DyavaiahM, LarsenRA, ChaturvediV (2005) Cryptococcus gattii in AIDS patients, southern California. Emerging Infectious Diseases 11 : 1686–1692.
45. LockhartSR, IqbalN, HarrisJR, GrossmanNT, DebessE, et al. (2013) Cryptococcus gattii in the United States: genotypic diversity of human and veterinary isolates. PLOS ONE 8: e74737.
46. TrillesL, Lazera MdosS, WankeB, OliveiraRV, BarbosaGG, et al. (2008) Regional pattern of the molecular types of Cryptococcus neoformans and Cryptococcus gattii in Brazil. Mem Inst Oswaldo Cruz 103 : 455–462.
47. FiracativeC, TorresG, RodriguezMC, EscandonP (2011) First environmental isolation of Cryptococcus gattii serotype B, from Cucuta, Colombia. Biomedica 31 : 118–123.
48. ChoiYH, NgamskulrungrojP, VarmaA, SionovE, HwangSM, et al. (2010) Prevalence of the VNIc genotype of Cryptococcus neoformans in non-HIV-associated cryptococcosis in the Republic of Korea. FEMS Yeast Res 10 : 769–778.
49. ChowdharyA, PrakashA, RandhawaHS, KathuriaS, HagenF, et al. (2013) First environmental isolation of Cryptococcus gattii, genotype AFLP5, from India and a global review. Mycoses 56 : 222–228.
50. OlivaresLR, MartinezKM, CruzRM, RiveraMA, MeyerW, et al. (2009) Genotyping of Mexican Cryptococcus neoformans and C. gattii isolates by PCR-fingerprinting. Med Mycol 47 : 713–721.
51. GrosseP, TintelnotK, SollnerO, SchmitzB (2001) Encephalomyelitis due to Cryptococcus neoformans var gattii presenting as spinal tumour: case report and review of the literature. J Neurol Neurosurg Psychiatry 70 : 113–116.
52. ByrnesEJ3rd, MarrKA (2011) The outbreak of Cryptococcus gattii in western North America: epidemiology and clinical issues. Current Infectious Disease Reports 13 : 256–261.
53. EscandonP, SanchezA, FiracativeC, CastanedaE (2010) Isolation of Cryptococcus gattii molecular type VGIII, from Corymbia ficifolia detritus in Colombia. Med Mycol 48 : 675–678.
54. MazzaM, RefojoN, Bosco-BorgeatME, TavernaCG, TroveroAC, et al. (2013) Cryptococcus gattii in urban trees from cities in North-eastern Argentina. Mycoses
55. CallejasA, OrdonezN, RodriguezMC, CastanedaE (1998) First isolation of Cryptococcus neoformans var. gattii, serotype C, from the environment in Colombia. Med Mycol 36 : 341–344.
56. HallidayCL, BuiT, KrockenbergerM, MalikR, EllisDH, et al. (1999) Presence of alpha and a mating types in environmental and clinical collections of Cryptococcus neoformans var. gattii strains from Australia. J Clin Microbiol 37 : 2920–2926.
57. SaracliMA, YildiranST, SenerK, GonlumA, DoganciL, et al. (2006) Genotyping of Turkish environmental Cryptococcus neoformans var. neoformans isolates by pulsed field gel electrophoresis and mating type. Mycoses 49 : 124–129.
58. MontagnaMT (2002) A note on the isolation of Cryptococcus neoformans serotype A MATa strain from the Italian environment. Med Mycol 40 : 593–595.
59. KiddSE, HagenF, TscharkeRL, HuynhM, BartlettKH, et al. (2004) A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci USA 101 : 17258–17263.
60. KiddSE, ChowY, MakS, BachPJ, ChenH, et al. (2007) Characterization of environmental sources of the human and animal pathogen Cryptococcus gattii in British Columbia, Canada, and the Pacific Northwest of the United States. Applied Environmental Microbiology 73 : 1433–1443.
61. Byrnes EJ, III (2010) Examination of the molecular epidemiology, expansion, population structure, and virulence of the emerging fungal pathogen Cryptococcus gattii in the United States [Ph.D.]. Ann Arbor: Duke University. 311 p.
62. MagditchDA, LiuTB, XueC, IdnurmA (2012) DNA mutations mediate microevolution between host-adapted forms of the pathogenic fungus Cryptococcus neoformans. PLoS Pathog 8: e1002936.
63. ToffalettiDL, NielsenK, DietrichF, HeitmanJ, PerfectJR (2004) Cryptococcus neoformans mitochondrial genomes from serotype A and D strains do not influence virulence. Curr Genet 46 : 193–204.
64. ZhuP, ZhaiB, LinX, IdnurmA (2013) Congenic strains for genetic analysis of virulence traits in Cryptococcus gattii. Infect Immun 81 : 2616–2625.
65. YanZ, XuJ (2003) Mitochondria are inherited from the MATa parent in crosses of the basidiomycete fungus Cryptococcus neoformans. Genetics 163 : 1315–1325.
66. YanZ, SunS, ShahidM, XuJ (2007) Environment factors can influence mitochondrial inheritance in the fungus Cryptococcus neoformans. Fungal Genet Biol 44 : 315–322.
67. D'SouzaCA, KronstadJW, TaylorG, WarrenR, YuenM, et al. (2011) Genome variation in Cryptococcus gattii, an emerging pathogen of immunocompetent hosts. mBio 2: e00342–00310.
68. SpringerDJ, RenP, RainaR, DongY, BehrMJ, et al. (2010) Extracellular fibrils of pathogenic yeast Cryptococcus gattii are important for ecological niche, murine virulence and human neutrophil interactions. PLOS ONE 5: e10978.
69. Kwon-ChungKJ, BennettJE (1984) Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol 120 : 123–130.
70. Kwon-ChungKJ, BennettJE (1984) High prevalence of Cryptococcus neoformans var. gattii in tropical and subtropical regions. Zentralbl Bakteriol Mikrobiol Hyg [A] 257 : 213–218.
71. PfeifferT, EllisD (1991) Environmental isolation of Cryptococcus neoformans var. gattii from California. J Infect Dis 163 : 929–930.
72. ReedKD, MeeceJK, ArcherJR, PetersonAT (2008) Ecologic niche modeling of Blastomyces dermatitidis in Wisconsin. PLoS ONE 3: e2034.
73. McCulloughMJ, DiSalvoAF, ClemonsKV, ParkP, StevensDA (2000) Molecular epidemiology of Blastomyces dermatitidis. Clin Infect Dis 30 : 328–335.
74. MandelMA, BarkerBM, KrokenS, RounsleySD, OrbachMJ (2007) Genomic and population analyses of the mating type loci in Coccidioides species reveal evidence for sexual reproduction and gene acquisition. Eukaryot Cell 6 : 1189–1199.
75. ByrnesEJ3rd, LiW, LewitY, PerfectJR, CarterDA, et al. (2009) First reported case of Cryptococcus gattii in the Southeastern USA: implications for travel-associated acquisition of an emerging pathogen. PLOS ONE 4: e5851.
76. SellersB, HallP, Cine-GowdieS, HaysAL, PatelK, et al. (2012) Cryptococcus gattii: an emerging fungal pathogen in the Southeastern United States. Am J Med Sci 343 : 510–511.
77. LitvintsevaAP, KestenbaumL, VilgalysR, MitchellTG (2005) Comparative analysis of environmental and clinical populations of Cryptococcus neoformans. J Clin Microbiol 43 : 556–564.
78. Loperena-AlvarezY, RenP, LiX, Schoonmaker-BoppDJ, RuizA, et al. (2010) Genotypic characterization of environmental isolates of Cryptococcus gattii from Puerto Rico. Mycopathologia 170 : 279–285.
79. LitvintsevaAP, ThakurR, VilgalysR, MitchellTG (2006) Multilocus sequence typing reveals three genetic subpopulations of Cryptococcus neoformans var. grubii (serotype A), including a unique population in Botswana. Genetics 172 : 2223–2238.
80. VelagapudiR, HsuehYP, Geunes-BoyerS, WrightJR, HeitmanJ (2009) Spores as infectious propagules of Cryptococcus neoformans. Infection and Immunity 77 : 4345–4355.
81. SuC, EvansD, ColeRH, KissingerJC, AjiokaJW, et al. (2003) Recent expansion of Toxoplasma through enhanced oral transmission. Science 299 : 414–416.
82. WendteJM, MillerMA, LambournDM, MagargalSL, JessupDA, et al. (2010) Self-mating in the definitive host potentiates clonal outbreaks of the apicomplexan parasites Sarcocystis neurona and Toxoplasma gondii. PLoS Genet 6: e1001261.
83. JanbonG, OrmerodKL, PauletD, ByrnesEJ3rd, YadavV, et al. (2014) Analysis of the genome and transcriptome of Cryptococcus neoformans var. grubii reveals complex RNA expression and microevolution leading to virulence attenuation. PLoS Genet 10: e1004261.
84. PriceJR, GolubchikT, ColeK, WilsonDJ, CrookDW, et al. (2014) Whole-genome sequencing shows that patient-to-patient transmission rarely accounts for acquisition of Staphylococcus aureus in an intensive care unit. Clin Infect Dis 58 : 609–618.
85. SteenbergenJN, ShumanHA, CasadevallA (2001) Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci U S A 98 : 15245–15250.
86. SteenbergenJN, NosanchukJD, MalliarisSD, CasadevallA (2003) Cryptococcus neoformans virulence is enhanced after growth in the genetically malleable host Dictyostelium discoideum. Infect Immun 71 : 4862–4872.
87. MylonakisE, AusubelFM, PerfectJR, HeitmanJ, CalderwoodSB (2002) Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc Natl Acad Sci U S A 99 : 15675–15680.
88. McClellandEE, PerrineWT, PottsWK, CasadevallA (2005) Relationship of virulence factor expression to evolved virulence in mouse-passaged Cryptococcus neoformans lines. Infect Immun 73 : 7047–7050.
89. CurrieB, SanatiH, IbrahimAS, EdwardsJEJr, CasadevallA, et al. (1995) Sterol compositions and susceptibilities to amphotericin B of environmental Cryptococcus neoformans isolates are changed by murine passage. Antimicrob Agents Chemother 39 : 1934–1937.
90. FranzotSP, MukherjeeJ, CherniakR, ChenL, HamdanJS, et al. (1998) Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect Immun 66 : 89–97.
91. TamuraK, PetersonD, PetersonN, StecherG, NeiM, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28 : 2731–2739.
92. ClementM, PosadaD, CrandallKA (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9 : 1657–1659.
93. TanakaR, TaguchiH, TakeoK, MiyajiM, NishimuraK (1996) Determination of ploidy in Cryptococcus neoformans by flow cytometry. J Med Vet Mycol 34 : 299–301.
94. DePristoMA, BanksE, PoplinR, GarimellaKV, MaguireJR, et al. (2011) A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43 : 491–498.
95. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
96. CingolaniP, PlattsA, Wang leL, CoonM, NguyenT, et al. (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6 : 80–92.
97. LassmannT, SonnhammerEL (2005) Kalign–an accurate and fast multiple sequence alignment algorithm. BMC Bioinformatics 6 : 298.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial PathogensČlánek A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during TransmissionČlánek The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil ChemotaxisČlánek Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Regulatory RNAs Involved in Bacterial Antibiotic Resistance
- From Dandruff to Deep-Sea Vents: -like Fungi Are Ecologically Hyper-diverse
- Pathogenicity and Epithelial Immunity
- Mother–Infant HIV Transmission: Do Maternal HIV-Specific Antibodies Protect the Infant?
- Hell's BELs: acterial 3 igases That Exploit the Eukaryotic Ubiquitin Machinery
- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Primary Seronegative but Molecularly Evident Hepadnaviral Infection Engages Liver and Induces Hepatocarcinoma in the Woodchuck Model of Hepatitis B
- TLR2 Signaling Decreases Transmission of by Limiting Bacterial Shedding in an Infant Mouse Influenza A Co-infection Model
- Production of an Attenuated Phenol-Soluble Modulin Variant Unique to the MRSA Clonal Complex 30 Increases Severity of Bloodstream Infection
- Inhibition of the TRAIL Death Receptor by CMV Reveals Its Importance in NK Cell-Mediated Antiviral Defense
- Early Mucosal Sensing of SIV Infection by Paneth Cells Induces IL-1β Production and Initiates Gut Epithelial Disruption
- Limited HIV Infection of Central Memory and Stem Cell Memory CD4+ T Cells Is Associated with Lack of Progression in Viremic Individuals
- Virus-Specific Regulatory T Cells Ameliorate Encephalitis by Repressing Effector T Cell Functions from Priming to Effector Stages
- A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during Transmission
- The HIV-1 Envelope Transmembrane Domain Binds TLR2 through a Distinct Dimerization Motif and Inhibits TLR2-Mediated Responses
- Infection with MERS-CoV Causes Lethal Pneumonia in the Common Marmoset
- VGIII Isolates Causing Infections in HIV/AIDS Patients in Southern California: Identification of the Local Environmental Source as Arboreal
- Diverse Host-Seeking Behaviors of Skin-Penetrating Nematodes
- Capsid Protein VP4 of Human Rhinovirus Induces Membrane Permeability by the Formation of a Size-Selective Multimeric Pore
- The Murine Gammaherpesvirus Immediate-Early Rta Synergizes with IRF4, Targeting Expression of the Viral M1 Superantigen to Plasma Cells
- Characterization of an Insecticidal Toxin and Pathogenicity of against Insects
- The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil Chemotaxis
- Histone Deacetylase Inhibitors Impair the Elimination of HIV-Infected Cells by Cytotoxic T-Lymphocytes
- A Locus Encompassing the Epstein-Barr Virus Kinase Regulates Expression of Genes Encoding Viral Structural Proteins
- Distinct APC Subtypes Drive Spatially Segregated CD4 and CD8 T-Cell Effector Activity during Skin Infection with HSV-1
- Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
- Adoptive Transfer of EBV Specific CD8 T Cell Clones Can Transiently Control EBV Infection in Humanized Mice
- Schistosome Feeding and Regurgitation
- EVM005: An Ectromelia-Encoded Protein with Dual Roles in NF-κB Inhibition and Virulence
- Rabies Virus Hijacks and Accelerates the p75NTR Retrograde Axonal Transport Machinery
- Why HIV Virions Have Low Numbers of Envelope Spikes: Implications for Vaccine Development
- Identification of Anti-virulence Compounds That Disrupt Quorum-Sensing Regulated Acute and Persistent Pathogenicity
- HIV-1 Receptor Binding Site-Directed Antibodies Using a VH1-2 Gene Segment Orthologue Are Activated by Env Trimer Immunization
- Cooperation between Epstein-Barr Virus Immune Evasion Proteins Spreads Protection from CD8 T Cell Recognition across All Three Phases of the Lytic Cycle
- Parasite Extracellular Vesicles: Mediators of Intercellular Communication
- RC1339/APRc from Is a Novel Aspartic Protease with Properties of Retropepsin-Like Enzymes
- Cyclic di-GMP-dependent Signaling Pathways in the Pathogenic Firmicute
- Non-random Escape Pathways from a Broadly Neutralizing Human Monoclonal Antibody Map to a Highly Conserved Region on the Hepatitis C Virus E2 Glycoprotein Encompassing Amino Acids 412–423
- Neutrophil Elastase Causes Tissue Damage That Decreases Host Tolerance to Lung Infection with Species
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- SGNH Hydrolase-Like Proteins AlgJ and AlgX Have Similar Topology but Separate and Distinct Roles in Alginate Acetylation
- Why Sexually Transmitted Infections Tend to Cause Infertility: An Evolutionary Hypothesis
- Late Engagement of CD86 after Influenza Virus Clearance Promotes Recovery in a FoxP3 Regulatory T Cell Dependent Manner
- Determinants of Influenza Transmission in South East Asia: Insights from a Household Cohort Study in Vietnam
- A Novel Signal Transduction Pathway that Modulates Quorum Sensing and Bacterial Virulence in
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- A Cysteine Protease Inhibitor of Is Essential for Exo-erythrocytic Development
- EBNA3C Augments Pim-1 Mediated Phosphorylation and Degradation of p21 to Promote B-Cell Proliferation
- On the Front Line: Quantitative Virus Dynamics in Honeybee ( L.) Colonies along a New Expansion Front of the Parasite
- Assembly and Architecture of the EBV B Cell Entry Triggering Complex
- NLR-Associating Transcription Factor bHLH84 and Its Paralogs Function Redundantly in Plant Immunity
- The PDZ-Binding Motif of Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Is a Determinant of Viral Pathogenesis
- Strain-Specific Properties and T Cells Regulate the Susceptibility to Papilloma Induction by Papillomavirus 1
- Human Cytomegalovirus pUL79 Is an Elongation Factor of RNA Polymerase II for Viral Gene Transcription
- The GAP Activity of Type III Effector YopE Triggers Killing of in Macrophages
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- Pathogenicity and Epithelial Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



