-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCharacterization of an Insecticidal Toxin and Pathogenicity of against Insects
Most entomopathogenic bacteria can produce toxin proteins and proliferate in the intestines of insects after natural oral ingestion. These bacteria can evade the systemic and local immune responses of insects. Here, we used insect larva of the diamond back moth (Plutella xylostella), a cruciferous crop pest to study the pathogenic mechanism of infection by novel bacterium Psudomonas taiwanensis. We examined how P. taiwanensis colonizes and escapes the immune response in the gut of P. xylostella and the mechanism of pathogenesis after oral ingestion of P. taiwanensis. Oral ingestion of P. taiwanensis induced severe damage in intestinal cells of P. xylostella and disrupted intestinal epithelial integrity. A toxin protein, the C component of insecticidal toxin protein complex (TccC) contributed to the pathogenicity of P. taiwanensis by thwarting oxidative stress and phagocytosis and inducing apoptosis in the gut cells of the host. Taken together our results shed light on the bacterial pathogenic processes in insect hosts, particularly the mechanism of pathogenesis of P. taiwanensis.
Published in the journal: . PLoS Pathog 10(8): e32767. doi:10.1371/journal.ppat.1004288
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004288Summary
Most entomopathogenic bacteria can produce toxin proteins and proliferate in the intestines of insects after natural oral ingestion. These bacteria can evade the systemic and local immune responses of insects. Here, we used insect larva of the diamond back moth (Plutella xylostella), a cruciferous crop pest to study the pathogenic mechanism of infection by novel bacterium Psudomonas taiwanensis. We examined how P. taiwanensis colonizes and escapes the immune response in the gut of P. xylostella and the mechanism of pathogenesis after oral ingestion of P. taiwanensis. Oral ingestion of P. taiwanensis induced severe damage in intestinal cells of P. xylostella and disrupted intestinal epithelial integrity. A toxin protein, the C component of insecticidal toxin protein complex (TccC) contributed to the pathogenicity of P. taiwanensis by thwarting oxidative stress and phagocytosis and inducing apoptosis in the gut cells of the host. Taken together our results shed light on the bacterial pathogenic processes in insect hosts, particularly the mechanism of pathogenesis of P. taiwanensis.
Introduction
The pathogenic ability of many insecticidal bacteria depends on toxin complexes that consist of proteins encoded by gene clusters scattered around their genomes [1], [2]. Toxin complexes (Tcs) comprise three different classes of functional components, TcA, TcB and TcC, which display different insecticidal functions in several entomopathogenic bacteria [2]. However, the mechanism by which Tcs cause insect lethality remains largely unknown. Most studies have reported that full insecticidal activities require intact toxin complexes, which are formed by cross-linking of the A, B and C components [3]–[5]. In the bacterium Photorhabdus luminescens, toxicity and insecticidal activity of the A component (TcdA) was enhanced by co-expression of the B (TcdB) and C components (TccC) [4], [5]. When TcdA1 or TcdB2/TccC3 or TcdB2/TccC5 fusion proteins were used alone for infection of Galleria mellonella hemocytes, phagocytosis was ineffective. However, a combination of TcdA1 and TcdB2/TccC3 or TcdB2/TccC5 produced toxicity [5].
Nevertheless, the A component (TcdA) of P. luminescences W14 expressed at a high level in transgenic Arabidopsis is sufficient to cause high toxicity to Manduca sexta [6]. Lee et al. analyzed the 3-D structure of the A component TcA-like protein (XptA1) of Xenorhabdus nematophila PMFI296 and found that this protein formed a bottle-shaped tetrameric complex and bound to target cell membranes, and might be responsible for delivering the toxin complex [7]. Fragment analysis of TcdA showed that its N-terminus causes rearrangement of actin cytoskeleton and its C-terminus of coiled-coil domain promotes protein-protein interactions in mammalian tissue culture cells [4]. In the B component, the N-terminus of TcdB1 contains SpvB-like domain and C-terminus contains RCC1-like domains, which also causes actin contraction [4]. The SpvB domain encodes a mono-ADP-ribosyltransferase [8] and the RCC1-like domain mediates chromatin condensation [9]. In Tcs, the B component may interact with the A and C components and modify the C component [4]. Although TccC from Xenorhabdus alone has a high toxicity using a microsyringe injecting method against the wax moth Galleria [10], TccC recombinant protein isolated from E.coli expressing Photorhabdus tccC gene displays little oral activity alone [3], [4]. TcdA1/TcdB2/TccC3 fusion protein modifies threonine-148 of actin by ADP-ribosylation and induces actin clustering in G. mellonella hemocytes and HeLa cells [5]. However, TcdA1/TcdB2/TccC5 fusion protein modifies glutamine-61 and glutamine-63 of RhoA by ADP-ribosylation [5]. This suggests that different amino acid regions in TccC3 and TccC5 are responsible for their biological activities and diverse toxin components might derive different functional activities from variable compositions of domains and motifs in different entomopathoenic bacteria. Also, the possibility that a single component of the Tcs has oral toxicity against insets cannot be excluded.
Despite extensive studies on functions of the Tc components, little is known about the relationship between pathogenicity caused by Tc components and insect immune responses. Reactive oxygen species (ROS), anti-microbial peptides (AMPs), lysozymes, pattern recognition proteins, circulating recognition molecules and phagocytes are involved in the defense mechanisms of the insect immune system [11]. After oral ingestion, the local immune system is triggered in the intestinal tract cells of Drosophila to produce AMPs and ROS, important and complementary contributors to defense against ingested microbes [12], [13]. Pathogens have developed strategies to counteract host immune responses, evading the local immune system to promote pathogenicity [14]. Pseudomonas entomophila, which lacks a type III secretion system, develops multiple virulence factors to promote its pathogenicity [15]. Among these virulence factors, AprA metalloprotease plays an important role in fighting against the AMPs of Drosophila [14]. In addition, P. entomophila causes changes in expression levels in the genes that modulate the cytoskeleton components of the gut epithelium of the host through the JNK pathway [16]. Oxidative burst increases epithelium renewal and boosts the gut homeostasis system, which induces stem cell proliferation. In the Drosophila midgut, epithelium renewal is essential to defend against bacterial oral infection and is controlled by the immune response. The JNK (c-JNK NH2 terminal kinase) pathway is required for intestinal stem cells to maintain and proliferate after human pathogen Pseudomonas aeruginosa [17], [18] and insect pathogen Pseudomonas entomophila infection [19].
Pseudomonas taiwanensis is a novel Gram-negative bacterium isolated from soils that can grow on medium with shrimp shell powder as the sole carbon and nitrogen source [20]. Interestingly, P. taiwanensis displays high levels of extracellular chitinasae, chitosanase, and nattokinase activities under shrimp shell medium [21], [22]. P. taiwanensis also has a broad-host range of insecticidal activity against a Dipteran species (Drosophila melanogaster) and a number of Lepidopteran species (Plutella xylostella, Spodoptera exigua, and Trichoplusia ni). Recombinant TccC from P. taiwanensis alone caused mortality of Drosophila larvae, indicating that the TccC of P. taiwanensis has toxic properties independent of other components [23]. Comparison of amino acid sequences showed that the N-terminal fragments of different TccC components are highly conserved, while the C-terminal regions of TccC components are hypervariable [5], [24]. In this study, we investigated the mechanisms underlying the colonization of P. taiwanensis in the guts of insect larvae, its evasion of host immune response and its disruption of the intestinal barrier after oral ingestion. We analyzed the damage caused by P. taiwanensis and examined the signaling pathways triggered by P. taiwanensis in larval guts after oral infection. In addition, we characterized the function of the virulence factor TccC of P. taiwanensis and showed that the C terminus of TccC from P.taiwanensis might play an important role in its pathogenicity.
Results
Insecticidal activity of TccC of P. taiwanensis toward P. xylostella
In a previous study, the tccC gene from P. taiwanensis was overexpressed in E. coli and the recombinant TccC was able to increase the mortality in Drosophila larvae [23]. In addition to Drosophila melanogaster, we found that P. taiwanensis has insecticidal activity against a number of Lepidopteran species, including several vegetable pests Plutella xylostella, Spodoptera exigua and Trichoplusia ni (Table S1). Here, we investigated the in vivo insecticidal activities of the P. taiwanensis TccC against the Lepidopteran species P. xylostella. The expression level of TccC in P. taiwanensis was highest when bacterial cells reached the stationary phase (24 h) (Figure 1A). Therefore, we collected P. taiwanensis cells at this stage and determined their toxicity. The P. taiwanensis cells were orally administered to the P. xylostella larvae. The larvae in the treatment group exhibited slower growth and were melanized, dehydrated, and rigid in comparison with those in the control group (Figure 1).
Fig. 1. Relative expression levels of tccC and toxicity of P. taiwanensis to P. xylostella larvae. 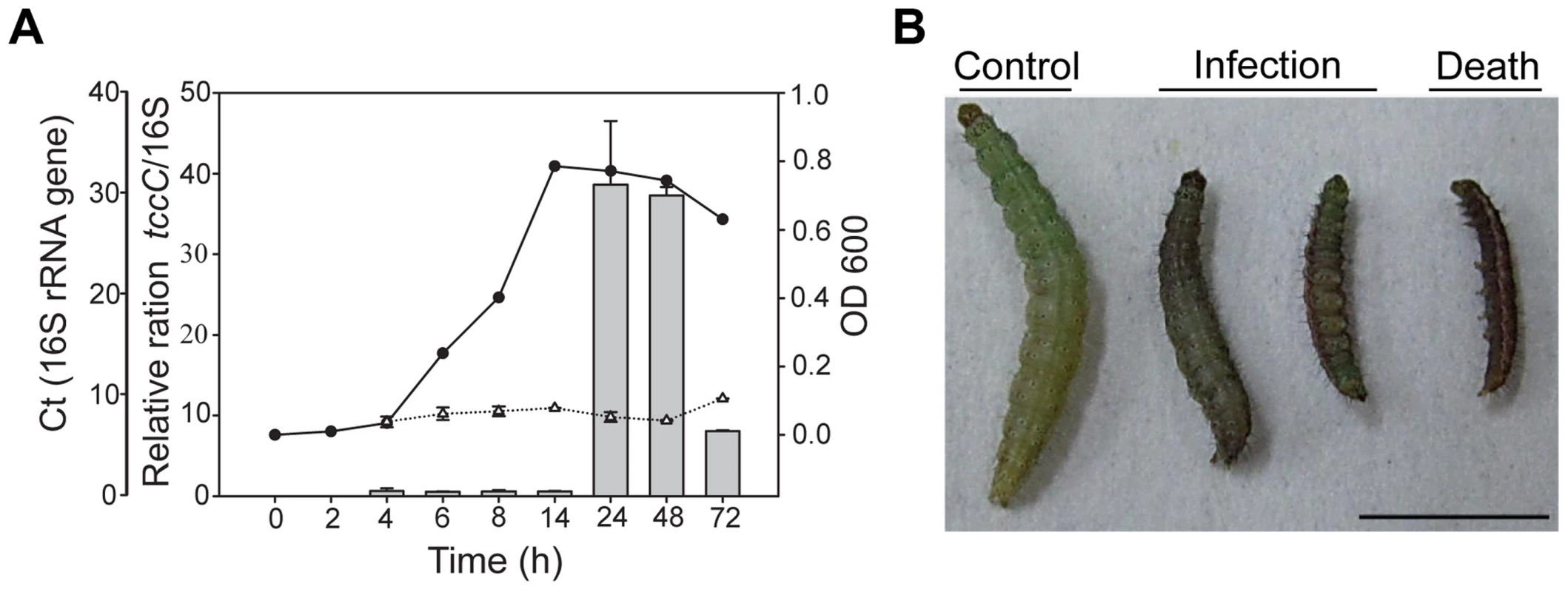
(A) The tccC expression levels in different growth phases (grey bar) of P. taiwanensis were analyzed by real-time PCR and compared with internal control 16S rRNA gene (white triangle). Growth curves of P. taiwanensis were measured by OD600 (black circle). (B) P. xylostella larvae were infected after 3 days of oral ingestion by P. taiwanensis. Left, control (non-infection). Center, infection. Right, death. Larvae, three instar. Ingestion does: 50 µl OD = 1 cells/0.5×1 cm2 vegetable block. Scale bar = 0.5 cm. We compared the amino acid sequences of several TccC-like proteins from different pathogens, and found that all of them had an N-terminal conserved RhsA-like domain and a C-terminal hypervariable fragment (Figure S1). Interestingly, the TccC of P. taiwanensis has a unique sodium/glutamate symporter-like domain and a TraT-like domain in the C-terminal region (Figure S1). In order to evaluate the function of the TccC protein, we generated an isogenic tccC gene knockout mutant, designated ΔtccC, of P. taiwanensis (Figure S2). Table 1 shows the mortality rates of P. xylostella larvae orally administered with whole cells or different cell fractions of wild-type or ΔtccC P. taiwanensis. The mortality of P. xylostella larvae infected with P. taiwanensis ΔtccC strain (OD = 2.0) was only 42.4% while those infected with wild-type P. taiwanensis was 94.5% (Table 1). We further prepared different cellular fractions of P. taiwanensis (Figure S3) and tested their effects on P. xylostella larvae. More than 50% of P. xylostella larvae infected with cell lysates, insoluble lysates (cell membranes and cell wall pellets) and extracellular supernatants of wild-type P. taiwanensis died at the end of the 5-day feeding period (Table 1). Moreover, the mortalities of P. xylostella larvae infected with cell lysates and insoluble pellets of P. taiwanensis ΔtccC were lower than those infected with wild-type lysates (Table 1). These results indicate that the insecticidal activity of P. taiwanenesis might be attributable, at least in part, to the TccC.
Tab. 1. P. xylostella mortality after oral infection with P. taiwanensis wild-type and ΔtccC strains or their cell lysate, sonicated pellet, sonicated supernatant, or culture supernatant on day 5. 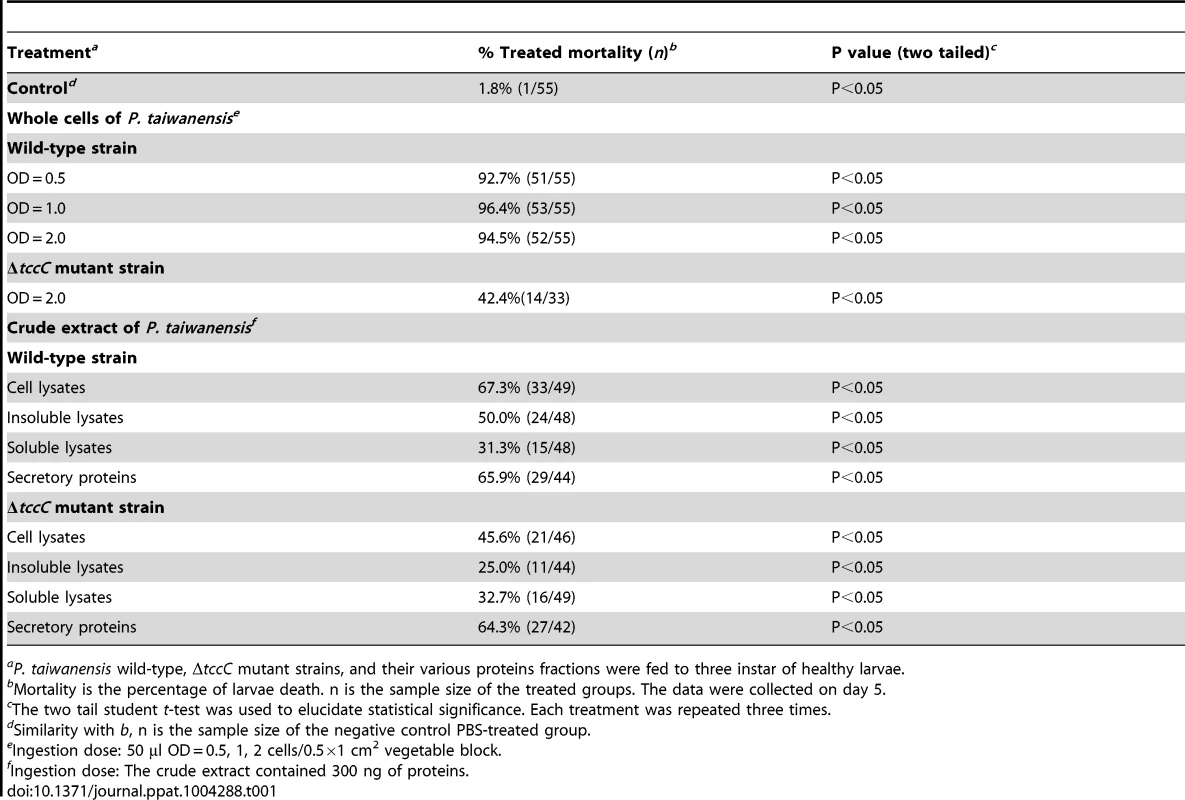
aP. taiwanensis wild-type, ΔtccC mutant strains, and their various proteins fractions were fed to three instar of healthy larvae. Infection of Lepidopteran larvae with toxins, bacteria or viruses caused the appearance of apical protrusion and protrusion ruptures in the damaged enterocytes [25]. Therefore, we performed histological analyses to assess the effect of P. taiwanensis infection on the intestinal tracts of P. xylostella. The ultrastructure of the midgut of P. xylostella larva showed that oral infection with P. taiwanensis had a strong impact on gut cells (Figure 2). After infection with P. taiwanensis for 48 h, apical protrusion of enterocytes, abnormal microvilli and cell lysis were induced in the guts in P. xylostella (Figure 2B compared with 2A) indicating that P. taiwanensis infection caused serious injury to the midgut epithelial cells, which could not be repaired in the homeostatic process and finally caused the death of the host. Similarly, ultrastructure sections of P. xylostella larvae that ingested 100 ng toxin complex (Tc)/cm2 food, showed columnar cells in the guts containing many vesicle-like structures [26]. In contrast, ingestion of the ΔtccC mutant only resulted in abnormal microvilli in P. xylostella intestinal tracts, without any apical protrusions or cell lysis (Figure 2C).
Fig. 2. TccC of P. taiwanensis contributes to damage to the midgut of P. xylostella larvae. 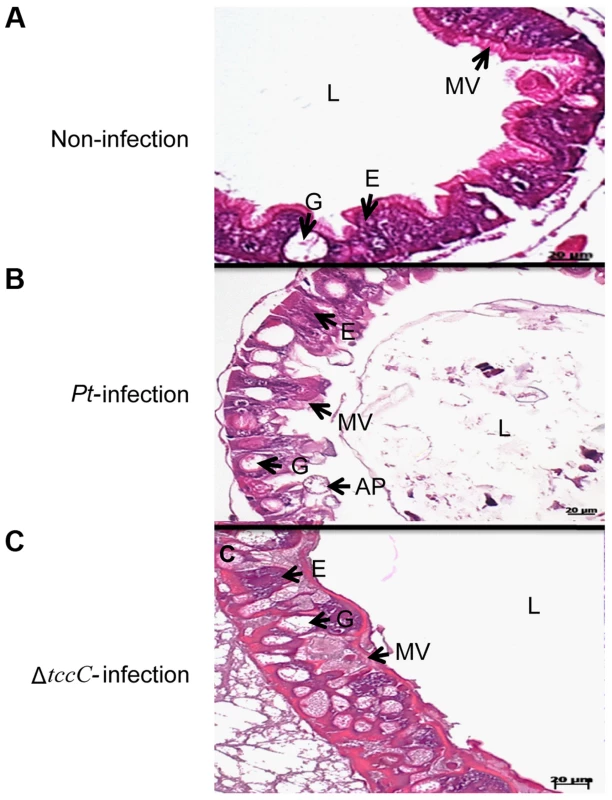
Histological tissue of midgut sections were collected with (A) non-infection or (B) 48 h after oral infection by wild-type or (C) tccC mutant P. taiwanensis. In non-infection larva, the larval stage epithelium presents enterocytes (E, commonly called columnar cells), goblets cells (G), microvilli (MV). After 48-h oral infection with P. taiwanensis considerable damage to gut cells such as apical protrusion (AP), cell lysis, and abnormal microvilli can be seen. Although tccC mutant also causes abnormal microvilli, infection with tccC mutant did not result in any apical protrusion or cell lysis. In surviving larva, the damaged gut induced stem cell proliferation and differentiation and promoted gut cell renewal, resulting in tccC mutant-induced morphological alterations in goblet cells that are not seen in non-infected or wild-type P. taiwanensis infection insects. Scale bar = 20 µm. Damage to the gut can induce stem cells to proliferate and differentiate to replace the damaged cells, producing a higher number of goblet cells with a larger size than the control group [25], [27], [28]. Homeostasis of gut cells, through which damaged cells are replaced with newly differentiated enterocytes and goblet cells, is very important during infection [18], [25]. Lethal pathogens are capable of overcoming host immune defenses, and eventually blocking gut homeostasis [17]. Of particular interest, in this study, we observed that oral infection of P. xylostella with P. taiwanensis ΔtccC resulted in a greater number of goblet cells in the midgut system (Figure 2C) as compared with the non-infected or wild-type P. taiwanensis-infected P. xylostella (Figure 2A and 2B) indicating that only infection with ΔtccC, but not the wild-type, could induce the differentiation of damaged cells and the formation of many goblets in the midgut system. On the other hand, we also found that enlargement of the goblet cavity did not occur in ΔtccC infection (Figure 2C). It is likely that over-proliferation of differentiated goblet cells replaced damaged gut cells when P. xylostella was infected by the ΔtccC mutant strain. Wild-type P. taiwanensis, however, displayed severe damage, inducing collapse of the epithelial layer, enlargement of goblet cells, and rupture of apical protrusion (Figure 2B). This suggests that the toxicity of P. taiwanensis ΔtccC was lower than that of the wild-type strain, and the midgut epithelial cells could be repaired (Figure 2C).
The colonization and invasion of midgut epithelial cells of P. xylostella by P. taiwanensis were further confirmed by bacterial quantification and histological examination. After oral infection for 48 h, the bacterial counts of P. taiwanensis ΔtccC were lower than those of wild-type strain in the midgut of P. xylostella (Figure 3A). In addition, the midgut epithelial cells were seriously disrupted by wild-type P. taiwanensis after oral infection for 48 h (Figure 3B).
Fig. 3. TccC is instrumental in P. taiwanensis persistence in the gut of P. xylostella and in invasion of the epithelium cells. 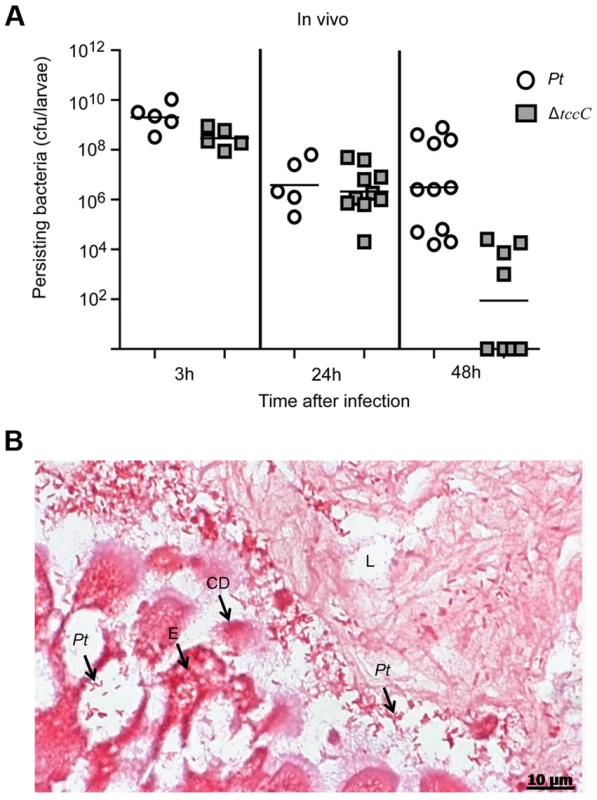
(A) Comparison of colonization through bacteria persistence in P. xylostella larvae. After oral infection by P. taiwanensis wild-type strain and tccC mutant strain, the numbers of colony-forming-units (cfu) were calculated at different time points. (B) Gram stain of P. xylostella midgut tissue section was observed after oral infection by P. taiwanensis for 48 h. The epithelial cells were invaded and lysed by P. taiwanensis. At 48 h, P. taiwanensis crossed the epithelium barrier of midgut into the cells. Pt, P. taiwanensis; CD, cell debris; E, enterocytes; L, lumen. Scale Bar = 10 µm. The insecticidal activity of the TccC was further confirmed by treatment of Sf9 insect cells with different P. taiwanensis cell fractions (Figure 4). The survival rates of Sf9 insect cells exposed to the intact cells (P. taiwanensis alive), cell lysate (total proteins), soluble lysate (cytosolic proteins) and insoluble lysate (cell wall and cell membrane) of wild-type P. taiwanensis were significantly lower than those exposed to PBS buffer (Figure 4A–D). On the other hand, the survival rates of Sf9 insect cells exposed to the intact cells or cell wall pellets of P. taiwanensis ΔtccC were not significantly different from those exposed to PBS buffer, only those exposed to the cell lysates or soluble lysate of P. taiwanensis ΔtccC were significantly decreased. Since P. taiwanensis ΔtccC did not express TccC, it was likely that some other virulence factors were present in the cell lysates of P. taiwanensis ΔtccC. Furthermore, active phagocytosis was found in Sf9 viable cells, a characteristic phenomenon during in vivo apoptosis but uncommon for in vitro cultures. Sf9 cells are phagocytic and contain unusually high numbers of phagosomes, particularly after glucose depletion [29]. In the early infection stage (after incubation for 1 h), RFP-labeled P. taiwanensis was phagocytosed by Sf9 cells (Figure S4A). After incubation for 3 h, lysis of Sf9 cells infected with P. taiwanensis was observed, as compared with no lysis in non-infected cells (Figure S4B and S4C).
Fig. 4. Toxicity of P. taiwanensis and various cell fractions on Spodoptera frugiperda Sf9 insect cells. 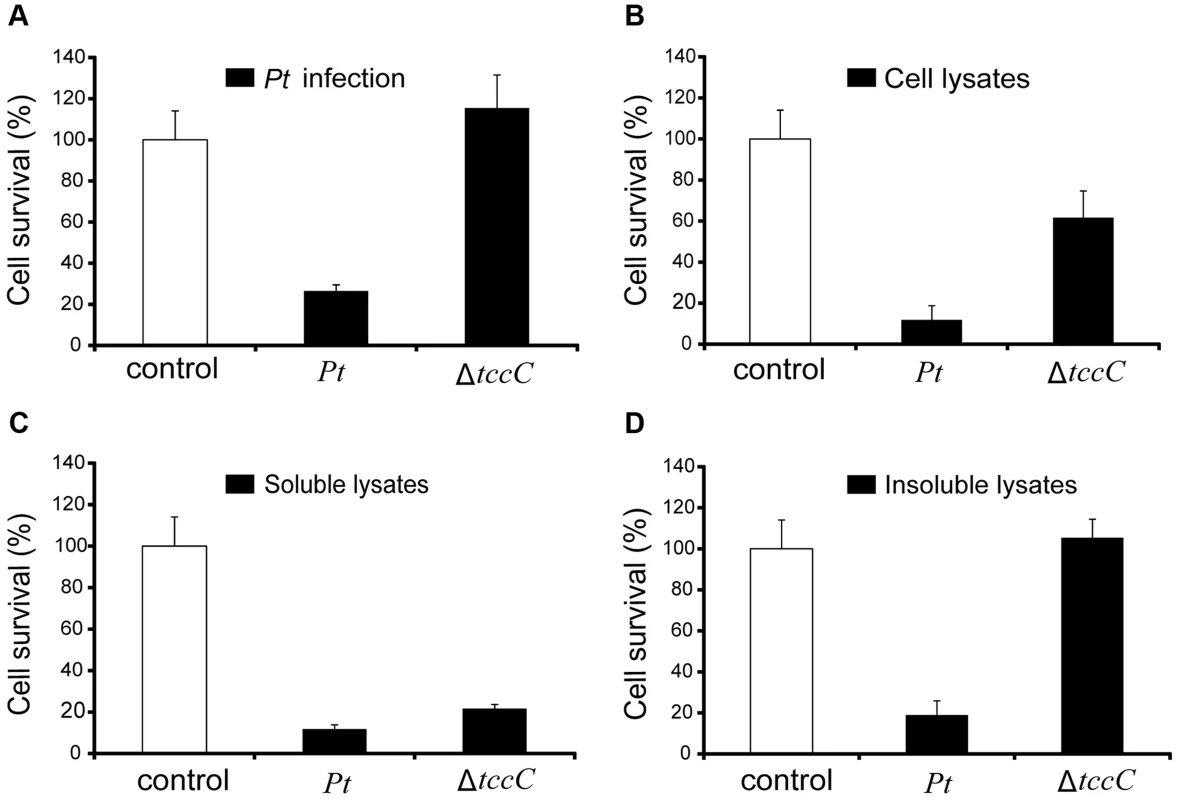
(A) Survival rates of Sf9 cells after infection of P. taiwanensis wild-type and ΔtccC (MOI = 1000) and protein fractions (10 µg/ml) derived from (B) cell lysates, (C) soluble lysates and (D) insoluble lysates of P. taiwanensis. Every well in a 96-well plate contained 5000 Sf9 cells. The results are obtained by XTT proliferation assay P. taiwanensis infection or protein treatment for 72 h. Induction of apoptotic cell death by TccC of P. taiwanensis
In a previous study, both the JNK and JAK-STAT pathways and several cell proliferation genes of adult Drosophila were significantly induced after oral infection with P. entomophila [16]. The JNK pathway was activated in apoptotic or proliferative cells [30]. To determine whether P. taiwanensis infection induces apoptosis in Lepidopteran Sf-9 and LD-5d cells, we used Annexin V-FITC to stain for apoptotic cells and DAPI staining to determine total cell numbers. Apoptosis was detected in Lepidopteran Sf-9 and LD-5d cells after 10 h of infection with P. taiwanensis (Figure 5A and 5B) and significantly higher mortality rates were observed than in the non-infection control (Figure 5C). Furthermore, the JNK pathway of the gut epithelial cells of P. xylostella larvae was triggered by P. taiwanensis infection (Figure 6). In addition to the JNK pathway, we also examined the expression of the caspase genes, which can also induce apoptotic cell death [31]. After 48-h oral infection with P. taiwanensis, the expression level of cleaved-caspase-3 was increased in the midgut cells (Figure 6). The expression levels of JNK-2 and cleaved-caspase-3 in P. xylostella larva infected with P. taiwanensis ΔtccC were lower (Figure 5) than in the wild-type strain of P. taiwanensis, indicating that TccC might induce apoptosis and play an important role in cell death of the gut epithelial cells of P. xylostella larvae.
Fig. 5. Apoptosis-induced by P. taiwanensis infection in Sf9 and LD-5d cell lines. 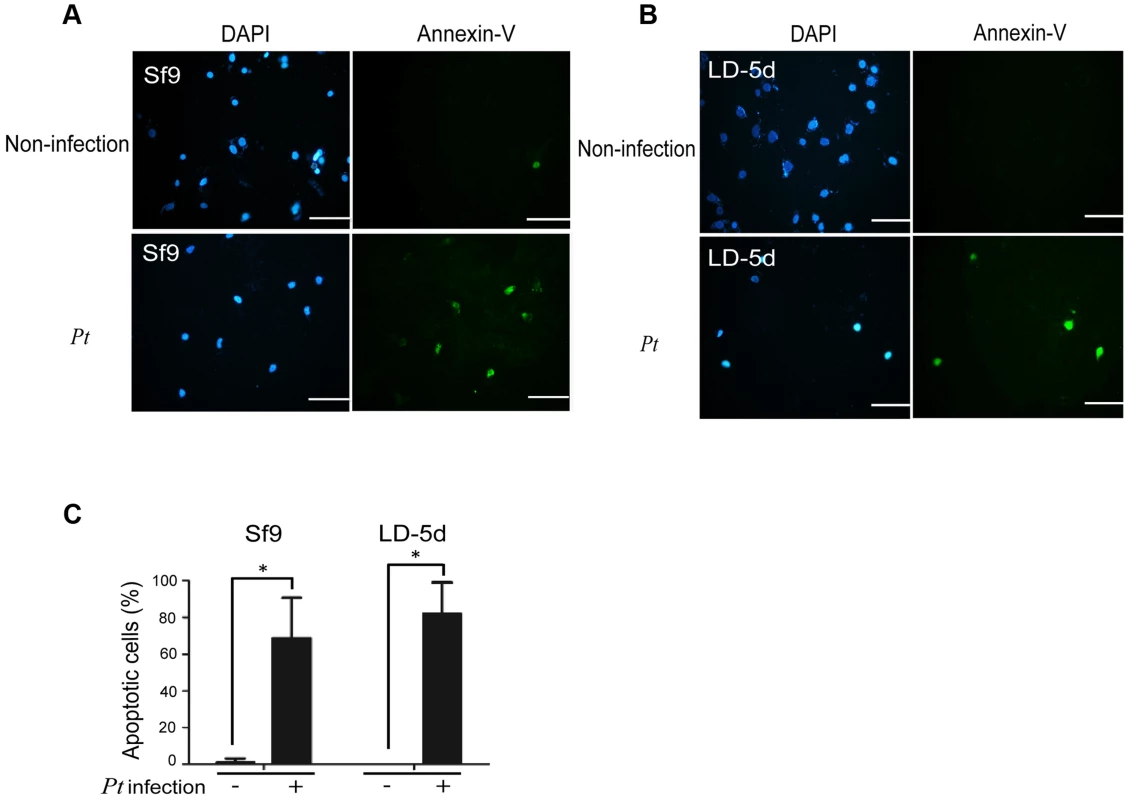
After P. taiwanensis (MOI = 1000) infection for 10 h, the insect cell lines Sf9 (A) and LD-5d (B) were stained with Annexin V-FITC and DAPI staining. P. taiwanensis can induce apoptosis in Sf9 and LD-5d cells after infection for 10 h. (C) The frequencies of apoptopic cells in (A) and (B) were quantified by calculating the ratio of Sf9 and LD-5d to DAPI positive numbers. Scale bar = 20 µm. Fig. 6. Immunohistochemical analysis of cleaved-caspase-3 and JNK-2 protein expression level in the gut tissue of P. xylostella larva. 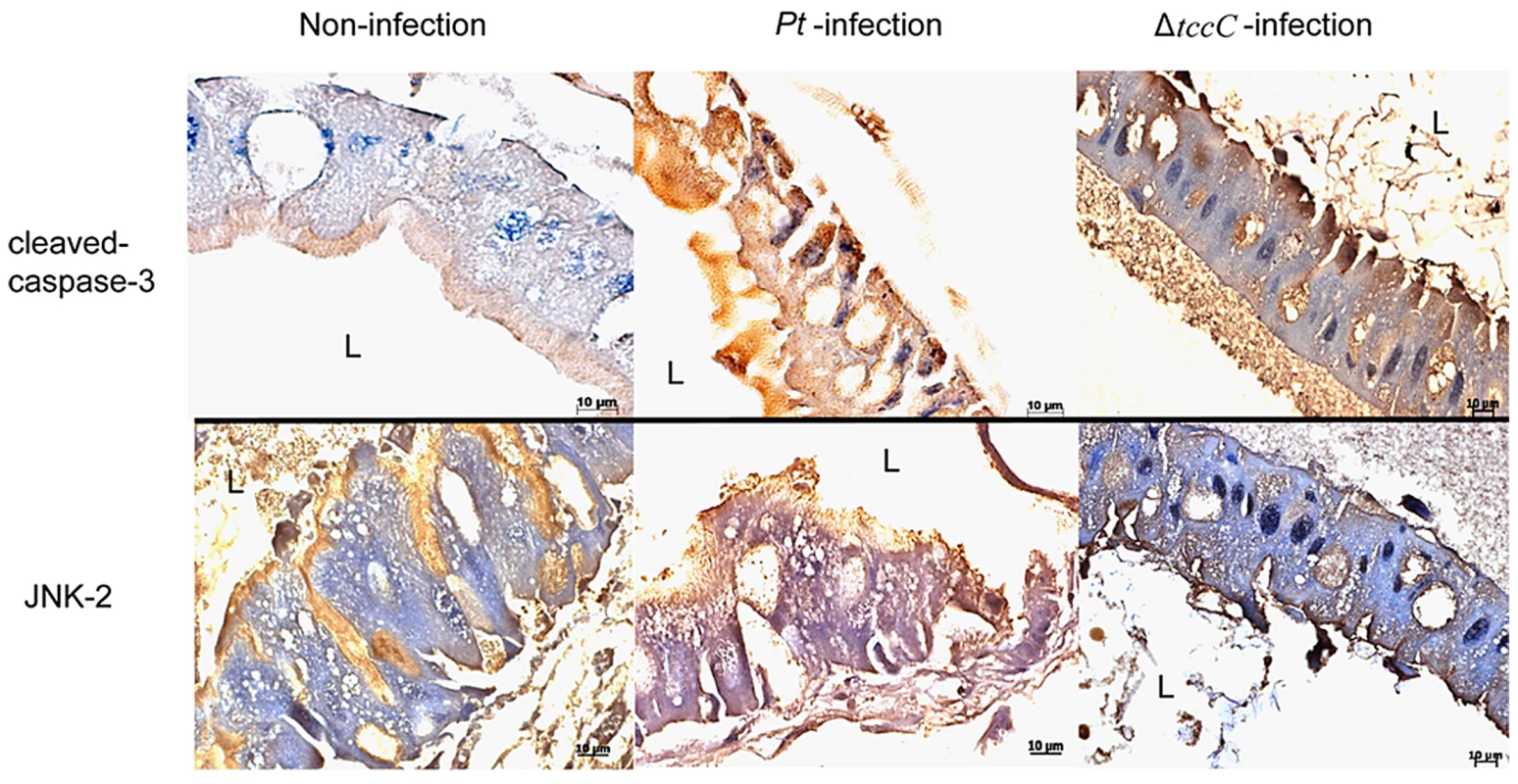
Expression level of caspase-3 was detected by immunohistochemical analysis using cleaved-caspase-3 or JNK-2 antibodies. Samples of larvae were collected after oral infection for 48 h by P. taiwanensis wild-type or ΔtccC strain. Effect of TccC on the antioxidant response of P. taiwanensis
The digestive tracts of healthy insects are protected against bacterial disruption by an intact gut epithelial barrier and the host immune defense system. In the insect gut system, antimicrobial peptides (AMPs) and reactive oxygen species (ROS) are important elements of the defense system against invading pathogens [11]. In order to overcome the attack of AMPs, P. entomophilas secretes an abundant protease (AprA) that degrades AMPs [14]. We analyzed the protease and antioxidant responses of P. taiwanensis strains to evaluate their resistance against the insect gut immune system. At the stationary phase of bacterial growth, P. taiwanensis secreted large amounts of proteases (Figure S5) and showed high antioxidant response (Figure S6). Interestingly, the antioxidant response of P. taiwanensis ΔtccC was significantly lower than that of wild-type P. taiwanensis (Figure S6), indicating that the antioxidant response of P. taiwanenesis might be directly or indirectly regulated by the TccC.
In order to confirm the involvement of the TccC in antioxidant response, wild-type and ΔtccC P. taiwenansis were exposed to different concentrations of hydrogen peroxide and the bacterial counts were determined. The results showed that wild-type P. taiwenansis had a higher survival rate than ΔtccC (Figure 7A), demonstrating that TccC also played a role in the protection of bacterial cells against ROS. ROS induced greater damage in the tccC mutant at high concentrations of H2O2 treatment (Figure 7A). The P. taiwanensis TccC protein contains a sodium/glutamate symporter Glts–like domain in its C-terminal, which might function in glutamate transport. To compare the glutamate uptake activity of wild-type and ΔtccC mutant, P. taiwanensis cells were cultured in medium containing 250 µM of 15N-L-glutamate for 4 hours. Uptake activity of 15N-L-glutamate was defined as enrichment of 15N content in P. taiwanensis cells. The results showed that glutamate uptake activity was much lower in ΔtccC than in the wild-type (Figure S7), which is consistent with the hypothesis that TccC has glutamate import activity. Since L-glutamate can be converted to glutathione, TccC might play a role in defense against ROS attack and maintain the intracellular redox potential in P. taiwanensis. We, therefore, next determined whether P. taiwanensis possesses the ability to degrade hydrogen peroxide (H2O2). We found that 1 mM H2O2 was quickly degraded after incubation with wild-type P. taiwanensis for 2 min. In contrast, it took 15 min to completely decompose when incubated with tccC mutant (Figure S6). Together, our results suggest that wild-type P. taiwanensis has higher H2O2 detoxification activity, and is, therefore, more resistant to ROS attack generated by the host immune response than the tccC mutant.
Fig. 7. TccC contributes to increases in oxidant stress defense and inhibits phagocytosis. 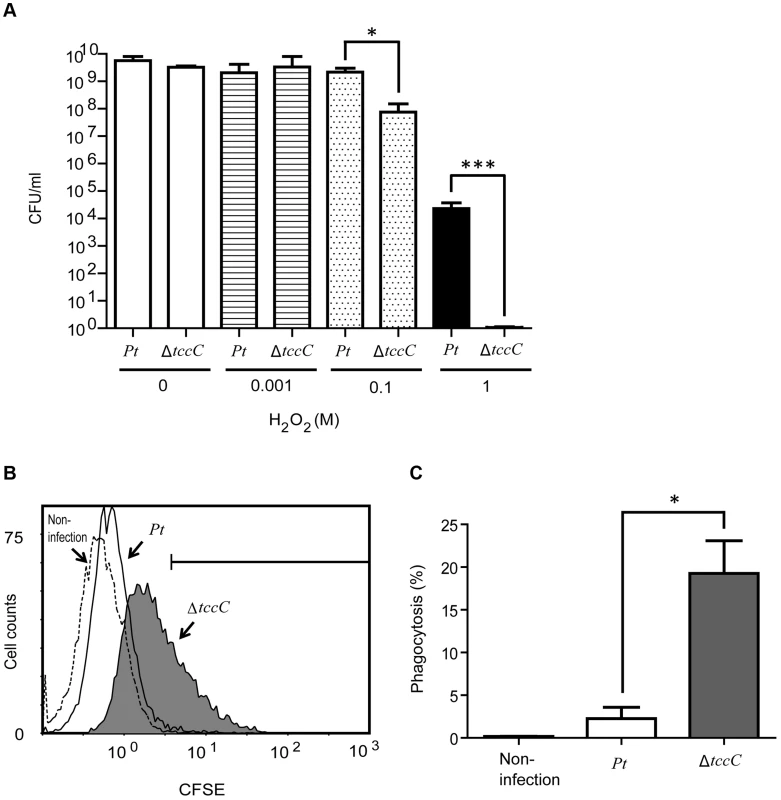
(A) Antioxidant response was measured by different H2O2 concentration treatments. Wild-type and tccC mutant of P. taiwanensis were exposed to different concentrations of H2O2 for 3 h and surviving cells were detected by CFU. (B–C) Phagocytosis of CFSE-stained wild-type or tccC mutant of P. taiwanensis by macrophages was detected. CFSE-stained wild-type or tccC mutant of P. taiwanensis incubated with macrophage (MOI = 1000) for 30 min and (B) following analysis of phagocytosis and (C) quantification by flow cytometry. Untreated macrophage cells were used as a negative control. Antiphagocytic activity of TccC
To evaluate the antiphagocytic activity of TccC, we performed a phagocytosis assay in which wild-type and ΔtccC P. taiwanensis cells were fluorescent-labeled with CFSE and then incubated with mouse macrophage cells. As seen in the scatter plot in Figure 7B, macrophage cells incubated with fluorescent-labeled P. taiwanensis ΔtccC for 30 min showed a shift in the peak position toward higher fluorescence intensity, indicating that the amount of phagocytized ΔtccC was larger than that of phagocytized wild-type P. taiwanensis. To substantiate the findings of the scatter plot analysis, the percentage of phagocytized P. taiwanensis was calculated (Figure 7C). The mouse macrophages engulfed fewer wild-type cells than the ΔtccC cells, suggesting that wild-type P. taiwanensis possessed antiphagocytic activity that might be partly attributable to TccC. We also analyzed the cytotoxicity of P. taiwanensis wild-type and ΔtccC toward mouse macrophages and found that the survival rate of mouse marcophages in the presence of the wild-type was not different from that in the presence of ΔtccC, suggesting that P. taiwanensis does not have a cytotoxic effect on mouse macrophages (Figure S8).
Processing and location of TccC in vivo
Based on Pfam domain prediction, TccC is predicted to possess an RhsA domain (11-673), an Rhs repeat-associated core (600-680), and sodium/glutamate symporter-like (726-825) and TraT complement resistance-like domains (736-781). In addition, three transmembrane regions (718-742, 744-758, 760-778) were predicted at the C-terminal region (Figure 8A, vertical red bars). Western blot analyses were performed to determine the subcellular localization of the TccC protein in P. taiwanensis (Figure 8B). Three cellular fractions were prepared according to the method outlined in Figure S3. Surprisingly, two protein bands were detected in the total cellular protein fraction, a ∼70 kD and a ∼40 kD bands, representing a processed form of TccC protein (Figure 8B, lane CL). In the soluble protein fraction, only the ∼70 kD band was detected (lane SL), whereas in the insoluble pellet fraction that contained cell wall and membrane proteins only, the processed ∼40 kD band was detected (lane IL). This suggests that TccC protein was processed when it was inserted into the membrane of P. taiwanensis cells. Because the C-terminal of the TccC contains three transmembrane regions, we suspect that the C-terminal domain of TcCC was integrated into the membrane.
Fig. 8. TccC processing and locations of fragments in P. taiwanensis. 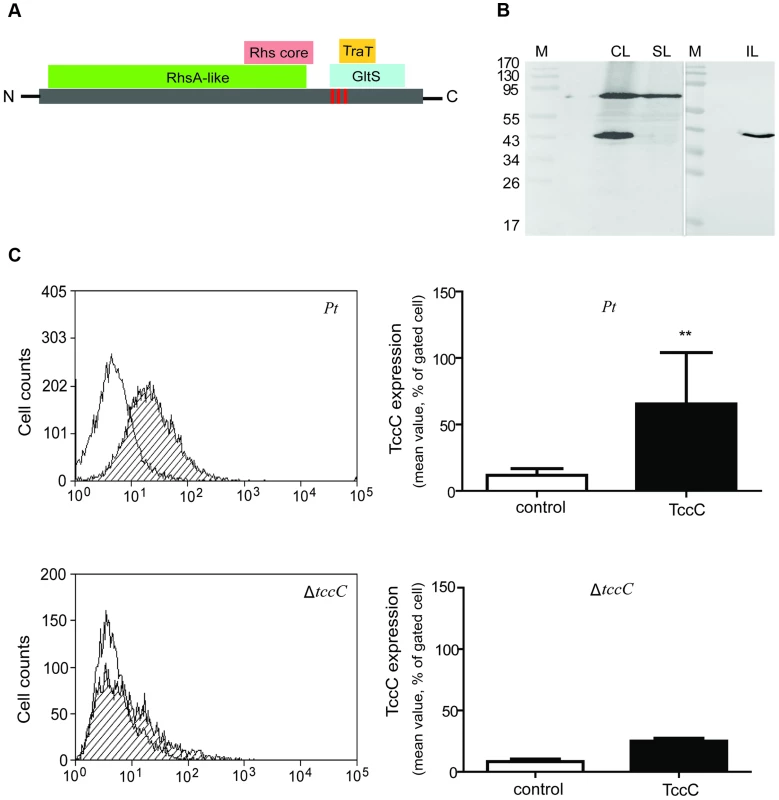
(A) According to the predicted protein domains, TccC possesses a highly conserve RhsA protein (11-673) in the N-terminus and Rhs repeat-associated core domain (600-680), sodium/glutamate symporter-like (726-825), and TraT complement resistance-like proteins (736-781) in the C-terminus. The vertical line indicates three transmembrane fragments at the C-terminal hypervariable region (IIYVLMSVVLEALATTIGMAGGLLGGAAGGAIGGAVGGVIANVPGAAVGATWGASVGGLVG). The amino acid sequence of the transmembrane helix was predicted by Philius transmembrane prediction server. (B) TccC fragments were detected in different locations in P. taiwanensis by western blotting using TccC antibody. Cell lysate (CL), soluble lysate (SL), and insoluble lysate (IL) to confirm TccC location was obtained according to the experimental steps outlined in Figure S3. (C) TccC was measured by flow cytometry after staining with TccC-FITC antibody. Wild-type and ΔtccC were compared with the non-stained control of P. taiwanensis. We have observed that the recombinant TccC protein also was similarly processed in E. coil expression system. To further characterize the cleavage process, TccC with 6xHis-tag was cloned into a broad host range vector pCPP30, and overexpressed in P. taiwanensis and E.coli (BL21) (Figure S9). The His-tagged TccC proteins were purified using a nickel ion column. Western blot analysis showed that processed forms of TccC proteins with similar molecular weight were purified from both E. coli and P. taiwanensis (Figure S9). This result suggests that the TccC has a similar cleavage site in E.coli and P. taiwanensis.
To test whether the TccC was indeed integrated into cell membrane, the TccC was labeled with FITC to trace the outer membrane fraction by staining with TccC-FITC antibody. Flow cytometry analysis showed that the fluorescence signal of TccC on the cell surface of P. taiwanensis had significantly higher density than the non-stained control (Figure 8C). In contrast, no significant fluorescence density was detected in the tccC mutant.
Discussion
Although toxin complex (Tc) genes that encode insect toxins are found in many entomopathogenic bacteria, the mechanisms of action of these toxin complexes are not well understood. In our previous study, we found that oral ingestion of the purified recombinant C component of the toxin complex TccC-like protein from P. taiwanensis caused high mortality in Drosophila [23]. In this study, we demonstrate that tccC mutant has lower toxicity toward larvae of the agricultural pest P. xylostella than the wild-type (Table 1). However, tccC mutant still induces a 42.4% mortality rate. Therefore, in addition to TccC as an insecticidal factor, P. taiwanensis also triggers others virulence factors or cofactors to produce the toxic effect. From our unpublished whole genome sequencing data, we found many virulence-related factors, such as other components of insecticidal toxin complexes (tccA, tccB, tcdB), lipases, proteases, chitinase, nonribosomal peptides and repeat-in-toxin (RTX), which might have toxic effects on insects.
In the genome of P. taiwanensis there are two homologus tccC genes located in different gene clusters. TccC (846 aa) and TccC2 (896 aa) have 57% amino acid sequence identity in the RhsA domain (Figure S1), but have little similarity in other regions. TccC has predicted Glt and TraT domains in the C-terminus, while TccC2 has no predicted domains in the same region. We, therefore, propose that TccC and TccC2 have different functions in P. taiwanensis. TccC is induced in the stationary phase in P. taiwanensis (Figure 1A), and like the toxin complex genes of Clostridium botulinum is upregulated in the early stationary phase [15], [32], [33]. In P. entomophila, which also infects Drosophila [15], the GacS/GacA system regulates secondary metabolite formation, and influences sigma factor σs accumulation to respond to stress of Pseudomonas spp. in the stationary phase [34]. Virulence and pathogenicity are also regulated by the GacS/GacA two-component system [35]. Based on the similar expression patterns of these toxin genes, we propose that TccC is also regulated by GacS/GacA in P. taiwanensis.
Histological tissues of midgut sections of P. xylostella displayed severe damage caused by the ingestion of wild-type P. taiwanensis, but ingestion of tccC mutant did not cause dramatic damage (Figure 2). The tccC mutant induced P. xylostella larvae gut stem cell proliferation and cell renewal (Figure 2C compared to 2A). Conversely, wild-type P. taiwanensis disrupted gut cell renewal (Figure 2B compared to 2A) and entered into gut cells (Figure 3B). These results indicate that P. taiwanensis can evade insect immune responses and colonize in the gut (Figure 3). Drosophila melanogaster produces antimicrobial peptides (AMPs), reactive oxygen species (ROS) and hemocytes in local and systemic responses to defend against pathogens [11], [14], [36]. Pathogens can degrade AMPs by zinc metalloprotease (AprA), protect cells from ROS-induced damage by antioxidant enzymes, and inhibit phagocytosis by hemocytes [11], [14], [36]. Because Drosophila immune responses are shared with other higher organisms [36]–[38], we used the information gained from the Drosophila studies to investigate the interaction between agricultural pest P. xylostella and P. taiwanensis. Our results showed that P. taiwanensis is involved in resistance to a burst of high H2O2 in the environment (Figure 7A) and is capable of degrading H2O2 (Figure S6). P. taiwanensis also produces proteinase activity in the stationary phase (Figure S5). In phagocytosis, P. taiwanensis-RFP labeled cells were engulfed by Sf9 cells to form phagosomes, resulting in the lysis of Sf9 cells (Figure S4). These mechanisms together enable P. taiwanensis to effectively colonize in the gut of P. xylostella larvae. In an interaction between P. taiwanensis and mouse macrophages, P. taiwanensis displayed antiphagocytosis activity (Figure 7B and 7C). However, P. taiwanensis did not cause mouse macrophage death (Figure S8). Similar to entomopathogenic P. entomophila [15], P. taiwanensis did not infect macrophages because it lacks a type III secretion system (unpublished whole genome database).
The Tc has been well characterized from the insect pathogen P. luminescens, and the TcC component that has biological activity in the C-terminal hypervariable region have been identified as ADP-ribosyltransfeases [39]. A previous study showed that TcC-like (TccC) protein may be cleaved and coexpressed with TcB (TcdB) by an unclear mechanisms [4]. Recently, Aktories and colleagues showed that TcC was autoproteolytically cleaved and secreted the C-terminal region into host cells by the TcA channel of the tripartite Tc toxin complex [39]. However, in the P. taiwanensis system, we found that TccC was autoproteolytically cleaved and the cleaved-C-terminus was integrated into the cell membrane (Figure 8B and S9). This result is consistent with the observation that the C-terminus of TccC consists of a transmembrane GltS-like domain and a TraT-like domain (Figure 8A). The Tc mechanism seems to be very different in P. taiwanensis and P. luminescens. The biological activity of TccC depends on C-terminal functional regions. Therefore, there may be functional differences in Tcs across pathogens that correlate with the differences in the amino acid sequences in the hypervariable regions of TccCs (Figure S1).
Analysis of the tccC mutant indicated that TccC, which is associated with defense against local immune response, significantly contributes to the colonization of P. taiwanensis in the gut (Figure 3A). Considerably less damage was seen in the intestinal tract after ingestion of the tccC mutant. The gltS gene encodes the sodium-dependent glutamate symporter which transporters L-glutamate into bacterial cells [40], [41]. Nitrogen metabolism of L-gultamate plays an important role in the colonization and pathogenicity of some bacteria such as Streptococcus pneumonia, Helicobacter pylori and Neisseria meningitidis [42]–[44]. In the L-glutamate uptake system of N. meningitidis, L-glutamate is converted into glutathione, which can detoxify ROS produced by polymorphonuclear neutrophil leucocytes [44]. In the insect gut system, ROS is an important element in the defense against invading pathogens [11]. In an in vitro analysis, we showed that the tccC mutant had less H2O2 detoxification activity, which might be attributed to the function of the GltS-like domain (Figure 7A). Conversely, we propose that the TraT-like domain in the C-terminus of TccC might have other functions. TraT is an outer membrane protein that mediates the barrier function, which excludes toxic compounds such as bile salts, lysophosphatides, lysozymes, phosphlipases and proteases in the environments from entering cells [45], [46]. TraT can also inhibit phagocytosis by macrophages [47]. The tccC mutant of P. taiwanensis increased phagocytosis by mouse macrophage cells (Figure 7). However, we cannot exclude the possibility that reduced toxicity of the tccC mutant per se resulted in enhanced phagocytosis. Previous studies showed that P. luminescens inhibited phagocytosis by utilizing the toxin complex rather than a single component [5], [48]. Therefore, it is likely that the Tc complex is not formed in the tccC mutant, affecting the antiphagocytosis activity of the mutant. Outermembrane proteins and lipoproteins are known to be involved in host-pathogen interactions, such as adhesion and permeability barrier, maintaining intracellular physiological functions, and promoting host immune responses and barrier disruption by pathogens [49], [50]. Among the Tcc genes with known sequences, only the C - terminal of TccC from P. taiwanensis has Glts and TraTdomians with potential permease functions. These permease activities might contribute to the insecticidal activity after oral infection by P. taiwanensis.
A number of studies have demonstrated that Tc toxin complexes are involved in pathogenicity in insects; however, none has shown whether signal transduction pathways are triggered by Tc toxin complexes or by a single component. Our results showed that P. taiwanensis triggers the JNK and caspase pathways in the gut system, which mediate inflammation and apoptosis of damaged cells, respectively (Figure 6). The tccC mutant did not induce the JNK and caspase pathways in gut cells, suggesting that TccC plays a role in triggering the cell homeostasis and apoptosis signaling pathways. Consistently, infection of P. taiwanensis induced apoptosis in insect cell lines (Figure 5). Histological analysis of the midgut showed that the epithelium cells sustained less damage and displayed cell renewal after infection by the tccC mutant (Figure 2C). These data demonstrate that TccC has a significant effect on the P. taiwanensis and P. xylostella interaction and contributes to cell death mediated by JNK - and caspase-depend pathways. Cleaved-caspase-3 is the active form of caspase-3 in damaged cells and is regarded as an apoptotic initiator in mammalian cells. Insects like Drosophila and Choristoneura fumiferana are also known to have a similar mechanism [31], [51]. In this study, our IHC analysis proved that cleaved-caspase-3 accumulates in the damaged gut cells of P. xylostella after infection by P. taiwanensis and thus induces apoptosis. Previously, many studies have focused on using recombinant proteins to examine the toxicity of Tcs, but the mechanism by which Tc complexes work and the host cells respond are still poorly understood. The results presented here demonstrate that the TccC in P. taiwanensis can induce host damage by triggering signal transduction of the apoptosis pathway after oral infection.
Materials and Methods
Bacterial strains, culture condition, and antibiotics
P. taiwanensis BCRC 17751 [23] was used as the entomopathogenic species. Escherichia coli DH5α was used in all construction experiments. E. coli S17-1 [52] was used for biparental mating with P. taiwanensis, and E.coli BL21 was used to express recombinant protein. P. taiwanensis and E. coli were grown in Luria-Bertani (LB) broth or on an agar plate. P. taiwanensis cultures were grown at 30°C and E. coli cultures were grown at 37°C. Antibiotics were applied at the following concentrations: rifampicin (34 µg/ml), ampicillin (100 µg/ml), and spectinomycin (100 µg/ml) for P. taiwanensis wild-type cultured media; and kanamycin (30 µg/ml), tetracycline (20 µg/ml) for P. taiwanensis mutant strain and overexpression strain, respectively; kanamycin (50 µg/ml), ampicillin (100 µg/ml), and tetracycline (20 µg/ml) for E.coli strain.
Cell culture
Both the Lepidoptera insect Spodoptera frugiperda Sf9 cell line and Lymantria dispar IPLB LD-652Y-5d cell line were provided by Dr. C.H. Wang (Department of Entomology, National Taiwan University). The gypsy moth (Lymantria dispar) cell line, IPLB LD-652Y-5d was subcloned from IPLB LD-652Y [53]. They were grown in Sf-900 II SFM (Gibco) medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin/glutamine (PSG) (Invitrogen) at 27°C.
Construction of the P. taiwanensis ΔtccC knockout mutant
An tccC (GenBank database accession number, HQ260745) knockout mutant of P. taiwanensis, designated ΔtccC was constructed by double recombination of the suicide vector pEX100T [54] containing the tccC fragment with a kanamycin resistance cassette inserted [55]. A tccC-kan-tccC fragment was generated by inserting a 1345-bp kanamycin resistance cassette into an 852-bp fragment that contains the coding sequence of tccC. The tccC-kan-tccC fragment was cloned into pEX100T suicide vector, and then transformed into E.coli S17-1 for conjugation with wild-type P. taiwanensis. The double recombination tccC mutant strain was selected on LB plates containing 5% sucrose, 30 µg/ml kanamycin, 34 µg/ml rifampicin, and 100 µg/ml spectinomycin. The resulting ΔtccC mutant was confirmed by PCR and sequencing.
Bioassay of infection experiments and effective protein fractions
Bioassays of bacteria infection of larvae were performed by natural oral infection. P. taiwanensis was grown for 24 hours to the stationary phase and collected. Subsequently, the cell pellet was washed three times in 5 ml PBS (pH 7.4) and resuspended in PBS, adjusted to different concentrations (OD). Different concentrations of bacteria (50 µl) were applied to surface of 0.5×1 cm2 vegetable pieces, which were used for feeding larvae of vegetable moth Plutella xylostella and incubated at 25°C. Each infected larva was observed at day 5 after oral infection and the mortality rate was calculated. Healthy third-instar P. xylostella larvae were provided by the Taiwan Agricultural Chemicals and Toxic Substances Research Institute.
To determine the protein fractions that cause mortality against P. xylostella, P. taiwanensis was cultured for 24 hours. The cell culture was harvested by centrifugation (15 min at 4,600g, 4°C), and supernatants and cell pellets were collected separately. For culture supernatants, the secreted proteins were filtered through a 0.22 µm PVDF filter (Millipore) and concentrated using a Vivaspin 20 concentrator (10 kDa MWCO, GE Healthcare). The harvested cell pellets were washed with PBS two times and resuspended in PBS with protease inhibitor and lysed with sonication (cell lysates). The cell lystaes were separated into insoluble lysates and soluble lysates by centrifugation (30 min at 26,000g, 4°C), and the soluble lysates were filtered by a 0.22 µm PVDF filter. The insoluble lysates were washed with PBS two times and resuspended in PBS. A schematic of the experimental procedure for extraction of the various protein fractions of P. taiwanensis is shown in Figure S3. For toxicity analysis of protein fractions from P. taiwanensis, 300 ng of proteins dissolved in 10 µl PBS were used for insect larvae treatment. Protein extracts were quantified by Pierce 660 nm protein assay method (Pierce).
Cell survival assay
To investigate the effect of P. taiwanensis on insect cells, proliferation of Spodoptera frugiperda Sf9 cells was determined by a colorimetric XTT assay [56]. For cytotoxicity assay, Sf9 cells were seeded at 5,000 per well in 96-well culture plates supplemented with 10 µg/ml of the various fraction proteins of P. taiwanensis (extraction procedure shown in Figure S3), or a multiplicity of infection (MOI) of 1000 Pt/cell was added in antibiotic-free medium. After 72-h treatment, cell proliferation was quantified by Cell Proliferation Assay Kit (XTT) (Biological Industries).
Apoptotic assay
Cell early stage apoptosis was detected by Annexin V-FITC assay [57]. The percentages of apoptosis of human or insect cells were determined by counting visible annexin V-positive cells under the fluorescence microscope. Cells (5,000 cells well−1) were incubated with protein fractions of P. taiwanensis at 10 µg/ml or with P. taiwanensis (MOI = 1000) for 72 h on the well in 24-well plates. After treatment for 72 h, the cells were washed twice in PBS and detected using the ApoAlert Annexin V-FITC Kit (BD) according to the manufacturer's instructions. The DNA in the nuclei was stained with 4′,6-diamidino-2-phenylindole dilactate (DAPI) for 5 min. Finally, the stained cells were washed twice in PBS, fixed with 4% paraformaldehyde for 10 minutes, and then observed under a fluorescence microscope (Zeiss Axiovert 100M, Carl Zeiss, Germany). Annexin V positive cells were counted and identified as P. taiwanensis-induced early stage apoptotic cells.
Sectioning and HE, gram, immunohistochemistry staining
After bacteria oral infection for 48 h, third instar larvae were fixed in 10% buffered formalin (pH 7.0) for at least 48 h. After fixation, larvae were sent to the Laboratory of Pathological Section of National Taiwan University for sectioning. The tissue sections were analyzed by hematoxylin–eosin, Gram's, or immunohistochemistry staining. Immunohistochemical (IHC) staining was performed using anti-JNK-2 [N1C3] (GTX105523, Genetex; 80% [276/398] sequence identity to c-Jun NH2-terminal kinase of Bombyx mori, NP_001103396) and anti caspase-3 p17 (GTX123678, Genetex; 36% [46/129] sequence identity to caspase 3 of Bombyx mori, AAW79564 [58]) antibodies, followed by diaminobenzidine (DAB) for color development and counterstained with hematoxylin from the Laboratory Animal Center of National Taiwan University Hospital.
Purification of TccC
Full-length TccC-His6 fusion fragment was cloned into the broad host range Pcpp30 vector and transformed into E. coli (BL21) and P. taiwanensis. Overexpressed TccC-His6 fusion protein was purified by His SpinTrap columns (GE Healthcare) after P. taiwanensis and E. coli growth into stationary phase (24 h), and the results were displayed by western blotting using the anti-TccC antibody.
Analysis of TccC location
Western immunoblotting
For SDS PAGE, 20 µg proteins of different cellular fractions from P. taiwanensis were dissolved in loading buffer with SDS and then applied to gel electrophoresis. After electrophoresis, the proteins were transferred to nitrocellulose membranes under 40 mA for 12 h. TccC was detected with specific anti-TccC antibody, using rabbit polyclonal antibodies raised against P. taiwanensis TccC full-length recombinant protein purified from E.coli BL21 expression. After first antibody binding, the color was developed with horseradish peroxidase-coupled anti-rabbit secondary antibody binding and chemiluminescent detection reagent (Pierce).
Flow cytometry
Flow cytometry was used to determine membrane localization of TccC. Wild-type and ΔTccC mutant strains of P. taiwanensis were grown overnight and collected at stationary phase (24 h). The cultures were adjusted 109 CFU/ml, and then 100 µl adjusted-bacteria was centrifuged to collect pellets. The bacteria pellets were washed three times with PBS at 4°C and resuspended in 200 µl PBS with 1% BSA. The polyclonal anti-TccC antibody (1/100 dilution) was added to the bacteria suspension on ice for 1 h. The bacteria was washed three times with PBS again and stained with goat FITC-conjugated anti-rabbit IgG secondary antibody (1/100 dilution) (Jackson Immunoresearch) on ice for 1 h. After staining, the bacteria were washed three times and resuspended in 1 ml PBS and analyzed by flow cytometry. Flow cytometry was performed by MoFlo XDP Cell Sorter (Beckman Coulter) using Summit 5.2 software (Beckman Coulter).
Phagocytosis assay
P. taiwanensis cells were collected in the early stationary phase and washed twice with PBS, and resuspended in PBS to OD = 1 (4×109 cells). One milliliter of resuspended cells was added to CFSE (final concentration of 5 µM) and incubated at 30°C in the dark for 30 min. The cells were washed three times with PBS and observed under fluorescent microscope. For phagocytosis assays, CSFE labeled P. taiwanensis cells were added to macrophage cells (MOI = 1000) for 30 min at 37°C in the dark, and then washed three times with PBS. Quantification and observation of phagocytosis was measured by flow cytometry and fluorescent microscope respectively. Flow cytometry was performed by Cytomics FC500 (Beckman Coulter) using CXP software (Beckman Coulter). Ten thousand cells were collected for analyses. Non-infected macrophage cells were used as a negative control.
Quantitative H2O2 assay and proliferation assay of P. taiwanensis
P. taiwanensis cells grown to stationary phase (24 h) were collected, washed three times in PBS, and resuspended in PBS to 109 cells per ml and subsequently incubated with 1M H2O2. The concentration of H2O2 remaining was detected at different time points after treatments using a PeroX-Oquant Quantitative Peroxide Assay Kits (Pierce).
Visualization of the proliferation effect of hydroxyl radicals in P. taiwanensis was performed as described in Huang and Chiou (2011) [59]. P. taiwanensis was grown in LB broth for 24 h and then incubated with different concentrations of H2O2 for 3 h. Proliferation was determined by counting the colony-forming units.
Quantitative 15N-L-glutamate uptake assay in P. taiwanensis
P. taiwanensis cells grown to the stationary phase (24 h) were collected, and cultured with LB broth containing 250 µM of 15N-L-glutamate for additional 4 hours. The cells were washed three times with PBS and dried at 80°C for 2 days. The dried cells were weighed and the15N content was analyzed using a continuous-flow isotope ratio mass spectrometer coupled with a carbon nitrogen elemental analyzer (ANCA-GSL 20/20; PDZ Europa).
Supporting Information
Zdroje
1. WaterfieldNR, BowenDJ, FetherstonJD, PerryRD, ffrench-ConstantRH (2001) The tc genes of Photorhabdus: a growing family. Trends Microbiol 9 : 185–191.
2. Ffrench-ConstantR, WaterfieldN (2006) An ABC guide to the bacterial toxin complexes. Adv Appl Microbiol 58 : 169–183.
3. WaterfieldN, DowlingA, SharmaS, DabornPJ, PotterU, et al. (2001) Oral toxicity of Photorhabdus luminescens W14 toxin complexes in Escherichia coli. Appl Environ Microbiol 67 : 5017–5024.
4. WaterfieldN, HaresM, YangG, DowlingA, ffrench-ConstantR (2005) Potentiation and cellular phenotypes of the insecticidal Toxin complexes of Photorhabdus bacteria. Cellular Microbiology 7 : 373–382.
5. LangAE, SchmidtG, SchlosserA, HeyTD, LarrinuaIM, et al. (2010) Photorhabdus luminescens Toxins ADP-Ribosylate Actin and RhoA to Force Actin Clustering. Science 327 : 1139–1142.
6. LiuD, BurtonS, GlancyT, LiZ-S, HamptonR, et al. (2003) Insect resistance conferred by 283-kDa Photorhabdus luminescens protein TcdA in Arabidopsis thaliana. Nature Biotechnology 21 : 1222–1228.
7. LeeSC, Stoilova-McphieS, BaxterL, FülöpV, HendersonJ, et al. (2007) Structural Characterisation of the Insecticidal Toxin XptA1, Reveals a 1.15 MDa Tetramer with a Cage-like Structure. Journal of Molecular Biology 366 : 1558–1568.
8. OttoH, Tezcan-MerdolD, GirischR, HaagF, RhenM, et al. (2000) The spvB gene-product of the Salmonella enterica virulence plasmid is a mono(ADP-ribosyl)transferase. Mol Microbiol 37 : 1106–1115.
9. MooreJD (2001) The Ran-GTPase and cell-cycle control. Bioessays 23 : 77–85.
10. Joo LeeP, AhnJ-Y, KimY-H, Wook KimS, KimJ-Y, et al. (2004) Cloning and heterologous expression of a novel insecticidal gene (tccC1) from Xenorhabdus nematophilus strain. Biochemical and Biophysical Research Communications 319 : 1110–1116.
11. LemaitreB, HoffmannJ (2007) The Host Defense of Drosophila melanogaster. Annual Review of Immunology 25 : 697–743.
12. HaEM, OhCT, BaeYS, LeeWJ (2005) A direct role for dual oxidase in Drosophila gut immunity. Science 310 : 847–850.
13. RyuJH, HaEM, OhCT, SeolJH, BreyPT, et al. (2006) An essential complementary role of NF-kappaB pathway to microbicidal oxidants in Drosophila gut immunity. EMBO J 25 : 3693–3701.
14. LiehlP, BlightM, VodovarN, BoccardF, LemaitreB (2006) Prevalence of Local Immune Response against Oral Infection in a Drosophila/Pseudomonas Infection Model. PLoS Pathogens 2: e56.
15. VodovarN, VallenetD, CruveillerS, RouyZ, BarbeV, et al. (2006) Complete genome sequence of the entomopathogenic and metabolically versatile soil bacterium Pseudomonas entomophila. Nature Biotechnology 24 : 673–679.
16. VodovarN (2005) Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proceedings of the National Academy of Sciences 102 : 11414–11419.
17. BuchonN, BroderickNA, ChakrabartiS, LemaitreB (2009) Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev 23 : 2333–2344.
18. ApidianakisY, PitsouliC, PerrimonN, RahmeL (2009) Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc Natl Acad Sci U S A 106 : 20883–20888.
19. JiangH, PatelPH, KohlmaierA, GrenleyMO, McEwenDG, et al. (2009) Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137 : 1343–1355.
20. WangLT, TaiCJ, WuYC, ChenYB, LeeFL, et al. (2009) Pseudomonas taiwanensis sp. nov., isolated from soil. International Journal of Systematic and Evolutionary Microbiology 60 : 2094–2098.
21. WangS-L, ChenS-J, WangC-L (2008) Purification and characterization of chitinases and chitosanases from a new species strain Pseudomonas sp. TKU015 using shrimp shells as a substrate. Carbohydrate Research 343 : 1171–1179.
22. WangS-L, ChenH-J, LiangT-W, LinY-D (2009) A novel nattokinase produced by Pseudomonas sp. TKU015 using shrimp shells as substrate. Process Biochemistry 44 : 70–76.
23. LiuJ-R, LinY-D, ChangS-T, ZengY-F, WangS-L (2010) Molecular Cloning and Characterization of an Insecticidal Toxin from Pseudomonas taiwanensis. Journal of Agricultural and Food Chemistry 58 : 12343–12349.
24. LangAE, SchmidtG, SheetsJJ, AktoriesK (2011) Targeting of the actin cytoskeleton by insecticidal toxins from Photorhabdus luminescens. Naunyn Schmiedebergs Arch Pharmacol 383 : 227–235.
25. HakimRS, BaldwinK, SmaggheG (2010) Regulation of Midgut Growth, Development, and Metamorphosis. Annual Review of Entomology 55 : 593–608.
26. HurstMRH, JonesSA, BinglinT, HarperLA, JacksonTA, et al. (2011) The Main Virulence Determinant of Yersinia entomophaga MH96 Is a Broad-Host-Range Toxin Complex Active against Insects. Journal of Bacteriology 193 : 1966–1980.
27. LoebMJ, MartinPA, HakimRS, GotoS, TakedaM (2001) Regeneration of cultured midgut cells after exposure to sublethal doses of toxin from two strains of Bacillus thuringiensis. J Insect Physiol 47 : 599–606.
28. TanakaS, YoshizawaY, SatoR (2012) Response of midgut epithelial cells to Cry1Aa is toxin-dependent and depends on the interplay between toxic action and the host apoptotic response. FEBS Journal 279 : 1071–1079.
29. Meneses-AcostaA, MendoncaR, MerchantH, CovarrubiasL, RamirezO (2001) Comparative characterization of cell death between Sf9 insect cells and hybridoma cultures. Biotechnol Bioeng 72 : 441–457.
30. RyooHD, GorencT, StellerH (2004) Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell 7 : 491–501.
31. Shahidi-NoghabiS, DammeEJMV, IgaM, SmaggheG (2010) Exposure of insect midgut cells to Sambucus nigra L. agglutinins I and II causes cell death via caspase-dependent apoptosis. Journal of Insect Physiology 56 : 1101–1107.
32. BradshawM, DineenSS, MaksND, JohnsonEA (2004) Regulation of neurotoxin complex expression in Clostridium botulinum strains 62A, Hall A-hyper, and NCTC 2916. Anaerobe 10 : 321–333.
33. SaujetL, MonotM, DupuyB, SoutourinaO, Martin-VerstraeteI (2011) The key sigma factor of transition phase, SigH, controls sporulation, metabolism, and virulence factor expression in Clostridium difficile. Journal of bacteriology 193 : 3186–3196.
34. WhistlerCA, CorbellNA, SarniguetA, ReamW, LoperJE (1998) The two-component regulators GacS and GacA influence accumulation of the stationary-phase sigma factor sigmaS and the stress response in Pseudomonas fluorescens Pf-5. J Bacteriol 180 : 6635–6641.
35. De la Torre-ZavalaS, AguileraS, Ibarra-LacletteE, Hernandez-FloresJL, Hernandez-MoralesA, et al. (2011) Gene expression of Pht cluster genes and a putative non-ribosomal peptide synthetase required for phaseolotoxin production is regulated by GacS/GacA in Pseudomonas syringae pv. phaseolicola. Res Microbiol 162 : 488–498.
36. HaEM, OhCT, RyuJH, BaeYS, KangSW, et al. (2005) An antioxidant system required for host protection against gut infection in Drosophila. Developmental cell 8 : 125–132.
37. TzouP, De GregorioE, LemaitreB (2002) How Drosophila combats microbial infection: a model to study innate immunity and host-pathogen interactions. Current opinion in microbiology 5 : 102–110.
38. HultmarkD (2003) Drosophila immunity: paths and patterns. Current opinion in immunology 15 : 12–19.
39. MeuschD, GatsogiannisC, EfremovRG, LangAE, HofnagelO, et al. (2014) Mechanism of Tc toxin action revealed in molecular detail. Nature 508 : 61–65.
40. KalmanM, GentryDR, CashelM (1991) Characterization of the Escherichia coli K12 gltS glutamate permease gene. Mol Gen Genet 225 : 379–386.
41. TolnerB, PoolmanB, KoningsWN (1992) Characterization and functional expression in Escherichia coli of the sodium/proton/glutamate symport proteins of Bacillus stearothermophilus and Bacillus caldotenax. Mol Microbiol 6 : 2845–2856.
42. ShibayamaK, WachinoJ, ArakawaY, SaidijamM, RutherfordNG, et al. (2007) Metabolism of glutamine and glutathione via gamma-glutamyltranspeptidase and glutamate transport in Helicobacter pylori: possible significance in the pathophysiology of the organism. Mol Microbiol 64 : 396–406.
43. HendriksenWT, KloostermanTG, BootsmaHJ, EstevaoS, de GrootR, et al. (2008) Site-specific contributions of glutamine-dependent regulator GlnR and GlnR-regulated genes to virulence of Streptococcus pneumoniae. Infect Immun 76 : 1230–1238.
44. TalaA, MonacoC, NagorskaK, ExleyRM, CorbettA, et al. (2011) Glutamate utilization promotes meningococcal survival in vivo through avoidance of the neutrophil oxidative burst. Molecular microbiology 81 : 1330–1342.
45. DonaldsonDM, RobertsRR, LarsenHS, TewJG (1974) Interrelationship between serum beta-lysin, lysozyme, and the antibody-complement system in killing Escherichia coli. Infect Immun 10 : 657–666.
46. Patterson-DelafieldJ, MartinezRJ, LehrerRI (1980) Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect Immun 30 : 180–192.
47. AgueroME, AronL, DeLucaAG, TimmisKN, CabelloFC (1984) A plasmid-encoded outer membrane protein, TraT, enhances resistance of Escherichia coli to phagocytosis. Infect Immun 46 : 740–746.
48. AuC, DeanP, ReynoldsSE, ffrench-ConstantRH (2004) Effect of the insect pathogenic bacterium Photorhabdus on insect phagocytes. Cell Microbiol 6 : 89–95.
49. SukupolviS, O'ConnorCD (1990) TraT lipoprotein, a plasmid-specified mediator of interactions between gram-negative bacteria and their environment. Microbiol Rev 54 : 331–341.
50. TombJF, WhiteO, KerlavageAR, ClaytonRA, SuttonGG, et al. (1997) The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388 : 539–547.
51. FanY, BergmannA (2010) The cleaved-Caspase-3 antibody is a marker of Caspase-9-like DRONC activity in Drosophila. Cell Death Differ 17 : 534–539.
52. SimonR, PrieferU, PuhlerA (1983) A Broad Host Range Mobilization System for In Vivo Genetic Engineering: Transposon Mutagenesis in Gram Negative Bacteria. Nat Biotech 1 : 784–791.
53. McClintockJT, DoughertyEM, WeinerRM (1986) Semipermissive Replication of a Nuclear Polyhedrosis Virus of Autographa californica in a Gypsy Moth Cell Line. J Virol 57 : 197–204.
54. SchweizerHP, HoangTT (1995) An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158 : 15–22.
55. DatsenkoKA (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proceedings of the National Academy of Sciences 97 : 6640–6645.
56. RoehmNW, RodgersGH, HatfieldSM, GlasebrookAL (1991) An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods 142 : 257–265.
57. van EngelandM, NielandLJ, RamaekersFC, SchutteB, ReutelingspergerCP (1998) Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31 : 1–9.
58. PortaH, Muñoz-MinuttiC, SoberónM, BravoA (2011) Induction of Manduca sexta Larvae Caspases Expression in Midgut Cells by Bacillus thuringiensis Cry1Ab Toxin. Psyche: A Journal of Entomology 2011 : 1–7.
59. HuangC-H, ChiouS-H (2011) Proteomic analysis of upregulated proteins in Helicobacter pylori under oxidative stress induced by hydrogen peroxide. The Kaohsiung Journal of Medical Sciences 27 : 544–53.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial PathogensČlánek A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during TransmissionČlánek The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil ChemotaxisČlánek Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Regulatory RNAs Involved in Bacterial Antibiotic Resistance
- From Dandruff to Deep-Sea Vents: -like Fungi Are Ecologically Hyper-diverse
- Pathogenicity and Epithelial Immunity
- Mother–Infant HIV Transmission: Do Maternal HIV-Specific Antibodies Protect the Infant?
- Hell's BELs: acterial 3 igases That Exploit the Eukaryotic Ubiquitin Machinery
- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Primary Seronegative but Molecularly Evident Hepadnaviral Infection Engages Liver and Induces Hepatocarcinoma in the Woodchuck Model of Hepatitis B
- TLR2 Signaling Decreases Transmission of by Limiting Bacterial Shedding in an Infant Mouse Influenza A Co-infection Model
- Production of an Attenuated Phenol-Soluble Modulin Variant Unique to the MRSA Clonal Complex 30 Increases Severity of Bloodstream Infection
- Inhibition of the TRAIL Death Receptor by CMV Reveals Its Importance in NK Cell-Mediated Antiviral Defense
- Early Mucosal Sensing of SIV Infection by Paneth Cells Induces IL-1β Production and Initiates Gut Epithelial Disruption
- Limited HIV Infection of Central Memory and Stem Cell Memory CD4+ T Cells Is Associated with Lack of Progression in Viremic Individuals
- Virus-Specific Regulatory T Cells Ameliorate Encephalitis by Repressing Effector T Cell Functions from Priming to Effector Stages
- A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during Transmission
- The HIV-1 Envelope Transmembrane Domain Binds TLR2 through a Distinct Dimerization Motif and Inhibits TLR2-Mediated Responses
- Infection with MERS-CoV Causes Lethal Pneumonia in the Common Marmoset
- VGIII Isolates Causing Infections in HIV/AIDS Patients in Southern California: Identification of the Local Environmental Source as Arboreal
- Diverse Host-Seeking Behaviors of Skin-Penetrating Nematodes
- Capsid Protein VP4 of Human Rhinovirus Induces Membrane Permeability by the Formation of a Size-Selective Multimeric Pore
- The Murine Gammaherpesvirus Immediate-Early Rta Synergizes with IRF4, Targeting Expression of the Viral M1 Superantigen to Plasma Cells
- Characterization of an Insecticidal Toxin and Pathogenicity of against Insects
- The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil Chemotaxis
- Histone Deacetylase Inhibitors Impair the Elimination of HIV-Infected Cells by Cytotoxic T-Lymphocytes
- A Locus Encompassing the Epstein-Barr Virus Kinase Regulates Expression of Genes Encoding Viral Structural Proteins
- Distinct APC Subtypes Drive Spatially Segregated CD4 and CD8 T-Cell Effector Activity during Skin Infection with HSV-1
- Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
- Adoptive Transfer of EBV Specific CD8 T Cell Clones Can Transiently Control EBV Infection in Humanized Mice
- Schistosome Feeding and Regurgitation
- EVM005: An Ectromelia-Encoded Protein with Dual Roles in NF-κB Inhibition and Virulence
- Rabies Virus Hijacks and Accelerates the p75NTR Retrograde Axonal Transport Machinery
- Why HIV Virions Have Low Numbers of Envelope Spikes: Implications for Vaccine Development
- Identification of Anti-virulence Compounds That Disrupt Quorum-Sensing Regulated Acute and Persistent Pathogenicity
- HIV-1 Receptor Binding Site-Directed Antibodies Using a VH1-2 Gene Segment Orthologue Are Activated by Env Trimer Immunization
- Cooperation between Epstein-Barr Virus Immune Evasion Proteins Spreads Protection from CD8 T Cell Recognition across All Three Phases of the Lytic Cycle
- Parasite Extracellular Vesicles: Mediators of Intercellular Communication
- RC1339/APRc from Is a Novel Aspartic Protease with Properties of Retropepsin-Like Enzymes
- Cyclic di-GMP-dependent Signaling Pathways in the Pathogenic Firmicute
- Non-random Escape Pathways from a Broadly Neutralizing Human Monoclonal Antibody Map to a Highly Conserved Region on the Hepatitis C Virus E2 Glycoprotein Encompassing Amino Acids 412–423
- Neutrophil Elastase Causes Tissue Damage That Decreases Host Tolerance to Lung Infection with Species
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- SGNH Hydrolase-Like Proteins AlgJ and AlgX Have Similar Topology but Separate and Distinct Roles in Alginate Acetylation
- Why Sexually Transmitted Infections Tend to Cause Infertility: An Evolutionary Hypothesis
- Late Engagement of CD86 after Influenza Virus Clearance Promotes Recovery in a FoxP3 Regulatory T Cell Dependent Manner
- Determinants of Influenza Transmission in South East Asia: Insights from a Household Cohort Study in Vietnam
- A Novel Signal Transduction Pathway that Modulates Quorum Sensing and Bacterial Virulence in
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- A Cysteine Protease Inhibitor of Is Essential for Exo-erythrocytic Development
- EBNA3C Augments Pim-1 Mediated Phosphorylation and Degradation of p21 to Promote B-Cell Proliferation
- On the Front Line: Quantitative Virus Dynamics in Honeybee ( L.) Colonies along a New Expansion Front of the Parasite
- Assembly and Architecture of the EBV B Cell Entry Triggering Complex
- NLR-Associating Transcription Factor bHLH84 and Its Paralogs Function Redundantly in Plant Immunity
- The PDZ-Binding Motif of Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Is a Determinant of Viral Pathogenesis
- Strain-Specific Properties and T Cells Regulate the Susceptibility to Papilloma Induction by Papillomavirus 1
- Human Cytomegalovirus pUL79 Is an Elongation Factor of RNA Polymerase II for Viral Gene Transcription
- The GAP Activity of Type III Effector YopE Triggers Killing of in Macrophages
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- Pathogenicity and Epithelial Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



