-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDeterminants of Influenza Transmission in South East Asia: Insights from a Household Cohort Study in Vietnam
Influenza causes an estimated three to five million severe illnesses worldwide each year. In order to guide control policies it is important to determine the key risk factors for transmission. This is often done by studying transmission in households but in the past, analysis of such data has suffered from important simplifying assumptions (for example not being able to account for the effect of biological markers of protection like pre-season antibody titers). We have developed new statistical methods that address these limitations and applied them to a large household cohort study conducted in 2007–2010 in Vietnam. By comparing a large range of model variants, we have obtained unique insights into the patterns and determinants of transmission of seasonal (H1N1, H3N2, B) and pandemic H1N1pdm09 influenza in South East Asia. This includes estimating the proportion of cases attributed to household transmission, the average household transmission probability, the protection afforded by pre-season HI titers, and the effect of age on infection risk after correcting for pre-season HI titers.
Published in the journal: . PLoS Pathog 10(8): e32767. doi:10.1371/journal.ppat.1004310
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004310Summary
Influenza causes an estimated three to five million severe illnesses worldwide each year. In order to guide control policies it is important to determine the key risk factors for transmission. This is often done by studying transmission in households but in the past, analysis of such data has suffered from important simplifying assumptions (for example not being able to account for the effect of biological markers of protection like pre-season antibody titers). We have developed new statistical methods that address these limitations and applied them to a large household cohort study conducted in 2007–2010 in Vietnam. By comparing a large range of model variants, we have obtained unique insights into the patterns and determinants of transmission of seasonal (H1N1, H3N2, B) and pandemic H1N1pdm09 influenza in South East Asia. This includes estimating the proportion of cases attributed to household transmission, the average household transmission probability, the protection afforded by pre-season HI titers, and the effect of age on infection risk after correcting for pre-season HI titers.
Introduction
Three to five millions severe illnesses and 250,000 to 500,000 deaths worldwide are due to the influenza virus each year [1]. To guide control policies, it is important that the determinants of influenza transmission are fully characterized. Such assessment is complex because the risk of influenza infection is multifaceted. For each individual, it depends on immunity that was acquired naturally or via vaccination; but also on the level of exposure to influenza the individual has in the community or in the household, which may vary by season, household and individual. Here, from the analysis of original data and relying on new and innovative statistical methods, we ascertain in a unifying and integrative framework the relative contribution of variables that influence these different mechanisms.
This task is challenging because both protection and exposure are imperfectly characterized; and uncertainties about one may affect estimates for the other. For example, for haemagglutination-inhibition (HI) assays which are extensively used in the approval process for influenza vaccines [2], [3], it is generally accepted that a HI titer of 1∶40 is associated with a 50% reduction in the risk of infection [4], [5]. However, it has long been acknowledged that HI titers are only an imperfect correlate of protection. For example, in 2009, the proportion of elderly people estimated to be protected against H1N1pdm09 influenza was much higher than had been suggested by pre-pandemic HI titers [6]. In the first study that characterized the protective effect of HI titers, Hobson et al [4] used a challenge design to ensure all subjects in the study had the same level of exposure to influenza; but such approach is expensive and can only be applied to healthy adults. In non-experimental settings, however, it is harder to control for heterogeneity in individual exposures to influenza due to the difficulty to track down all potential sources of infection.
Case-ascertained household transmission studies have been extensively used to quantify exposure in the household setting [7]–[13]. In this design, community-based influenza cases, that are labelled as index cases, are recruited via primary care practices or outpatient clinics. Symptoms of the index case and their household members are then monitored for one to two weeks following symptoms onset in the index case; virological samples may also be collected. However, since the follow-up of each household starts with an influenza case, this approach cannot be used to reliably quantify exposure from the community or to estimate the relative contributions of households and the community in the general epidemic. Furthermore, as index cases must have sufficiently severe symptoms to make contact with a healthcare provider and then have sufficiently high viral loads to be detected by laboratory tests for influenza, there may be a selection bias towards more infectious cases, which may lead the probability of transmission in the household to be overestimated.
An alternative, less common design offers a more representative view of the role of households in influenza transmission. It is based on a cohort of households that are recruited prior to an epidemic and followed up during the epidemic [14], [15]. Although the timing and source of infection is typically unobserved, collection of serum samples at baseline and after the epidemic makes it possible to determine serologically which subjects were infected. Statistical methods exist to estimate from such data the probability of transmission from other household members and from the community [16]–[18]. However, they become cumbersome and numerically intractable as the number of categories of individuals (e.g. child/adult or low/intermediate/high HI titers) or the size of the social unit of interest (e.g. here households) increase [17], [19]. As a consequence, to our knowledge, it has never been possible to evaluate the protective effect associated with HI titers in such a framework, preventing a more integrated analysis of the determinants of influenza transmission.
Here, from the analysis of the large Ha Nam household cohort study [20] conducted from 2007 to 2010 in Vietnam and relying on new and innovative statistical methods [19], we ascertain in a unifying and integrative framework the protective effects associated with HI titers and age, along with the relative contributions of households and the community in influenza transmission. Differences by subtype are also investigated. The analysis makes it possible to ascertain potential biases in case-ascertained household transmission studies which are extensively used for early assessment at the start of influenza pandemics [8], [10]–[12]. The analysis also documents influenza household transmission in South East Asia, which has received somewhat less attention than in Western countries [9], [15], [21]–[23].
Materials and Methods
Data
Samples were collected from a household-based cohort of 940 participants in 270 households in a single community in semi-rural northern Vietnam as previously described [20]. None of the participants had ever received influenza immunisation. Participants aged 5 years or older were asked to provide serial blood samples at times when national influenza surveillance data indicated that influenza circulation was minimal. The samples described here were collected over a period of three consecutive influenza seasons, from December 2007 through April 2010. Serological samples were collected between 1st–7th December 2007 (bleed 1), 9th–15th December 2008 (bleed 2), 2nd–4th June 2009 (bleed 3), and on the 3rd April 2010 (bleed 4). This provided three sets of paired samples either side of an influenza transmission season: 548 paired samples for season 1 (2008), 501 paired samples for season 2 (Spring 2009), and 540 paired samples for season 3 (Autumn 2009). In season 1, the influenza A virus strains detected in the cohort through ILI surveillance were A/H1N1/Brisbane/59/2007-like and A/H3N2/Brisbane/10/2007-like; in season 2, they were A/H1N1/Brisbane/59/2007-like and A/H3N2/Perth/16/2009-like; and in season 3, it was A/H1N1/California/7/2009-like. There was co-circulation of influenza B Yamagata lineage and Victoria lineage in both season 1 and season 2, with a predominance of Yamagata lineage in season 1 and Victoria lineage in season 2.
For each season and subtype, analysis was restricted to households with at most 1 individual for whom paired serum samples were missing.
Laboratory methods
Influenza hemagglutination inhibition (HI) assays were performed according to standard protocols [WHO 2011 manual]. The seasonal influenza A viruses used were isolated from participants' swabs or from swabs taken from patients presenting in Ha Noi in the same season and propagated in embryonated hen's eggs or in MDCK cells (ATCC). A reference antigen supplied by WHO (A/H1N1/California/7/2009-like) was used to assess season 3/pandemic sera. A single influenza B virus isolated from a participant during 2008 was used to assess serum for both the first and second seasons. The virus had a titer of 320 with B/Wisconsin/1/2010 (Yamagata) reference antisera and of <10 with B/Brisbane/60/2008 (Victoria) antisera. Each virus was first assessed for haemagglutination of erythrocytes from chickens, guinea pigs and turkeys then titrated with optimal erythrocytes. Serum was treated with receptor destroying enzyme (Denka Seiken, Japan) then heat inactivated and adsorbed against packed erythrocytes. Eight 2-fold dilutions of serum were made starting from 1∶10 and incubated with 4 HA units/25 µl of virus. Appropriate erythrocytes were added and plates read when control cells had settled. Virus, serum and positive controls were included in each assay. Pre - and post-season sera were tested in pairs. Each serum was tested in a single dilution series. The HI titer was read as the reciprocal of the highest serum dilution causing complete inhibition of RBC agglutination, partial agglutination was not scored as inhibition of agglutination. If there was no inhibition of HI at the highest serum concentration (1∶10 dilution) the titer was designated as 5.
Case definition
Influenza virus infection was defined as a ≥4-fold increase in antibody titer from pre-season to post-season titers, with post season titers ≥40. For the purposes of analysis low, intermediate, and high pre-season HI titers were defined as ≤1∶10, 1∶20–1∶40, and ≥1∶80 respectively.
Notations
Data were collected for 3 different seasons s = 1…3 : 2008 (s = 1), Spring 2009 (s = 2) and Autumn 2009 (s = 3). We classify the influenza virus into 4 different categories v = 1…4: seasonal A(H1N1) (v = 1); seasonal A(H1N1) (v = 2); seasonal B (v = 3); pandemic A(H1N1) (v = 4). A set of k = 1…K households are under study. Household k ( = 1…K) is of size nk. Individuals are categorized in two types: children i.e. aged ≥5 to ≤15 y.o. and adults.
Transmission model
A subject may be infected by influenza subtype v in the community (i.e. outside the household) or by another household member. Here, we define a generic model for the occurrence of these events.
During season s, the probability that subject i from household k has contacts in the community that would lead to infection by influenza subtype v is defined as . The force of infection from the community is modelled as:
where measures the force of infection for subtype v during season s, captures the susceptibility of adults relative to children (i.e children are the reference group) and captures the effect of pre-season titers, with 3 categories low (≤10), intermediate (20–40) and high (≥80) (reference category: ≤10).The probability that subject i gets infected if household member j is infected is defined as with
where measures the transmission rate as a function of household size nk (the rate can be inversely proportional to nk [7] or independent of nk, depending on model variant), captures the infectivity of adults relative to children (i.e. children are the reference group).Inference
It is challenging to estimate parameters of the transmission model from final size data because the chains of transmission are not observed. Here, we consider a simplified version of the approach developed by Demiris and O'Neill [19] to tackle the problem. A household of size n is represented by a random directed graph with n vertices (Figure 1). Each vertex represents a household member. Edges are added to represent the unobserved chain of transmission. Two types of edges are possible. If there is an edge between subject j and subject i, it means that subject i is infected if subject j gets infected. If there is an edge between the community and subject i, it means that subject i gets infected.
Fig. 1. Final size data and methods to estimate transmission parameters. 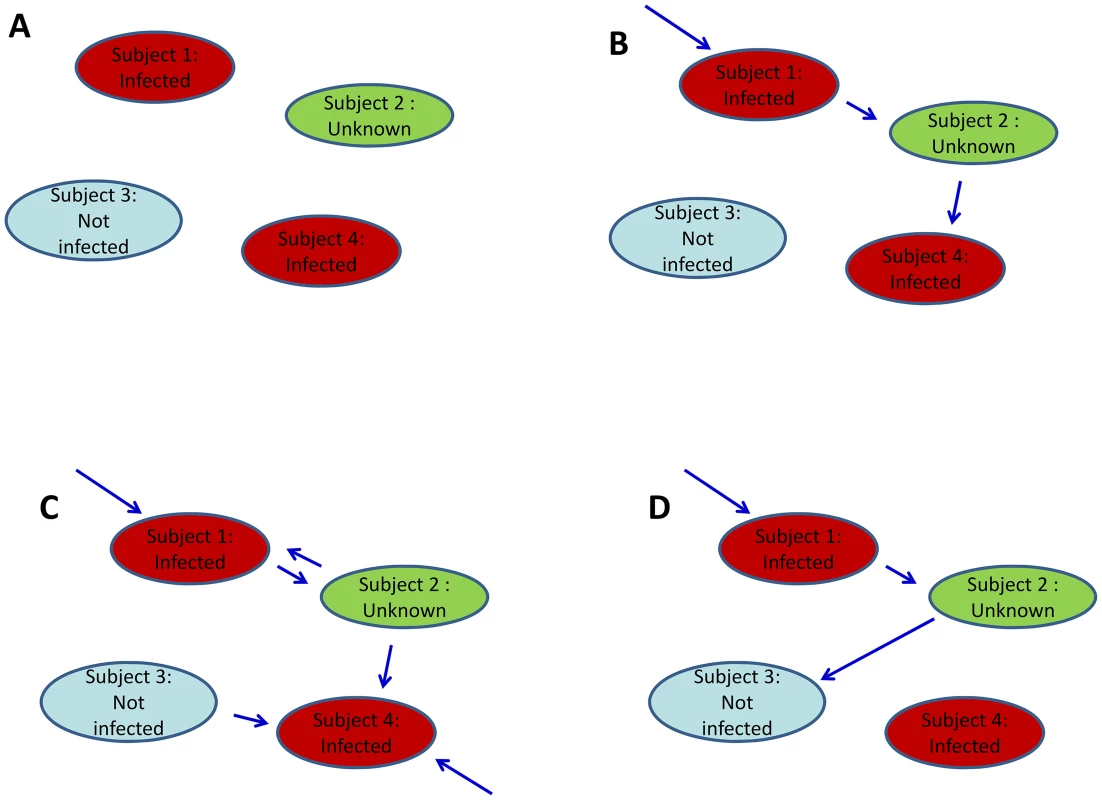
A. Example of final size data for a household of size 4. Subjects 1 and 4 were infected; subject 3 was not; diagnostic for subject 2 was missing. B. Example of digraph consistent with the final size data. For inference, data are augmented with a digraph (blue arrows) that informs on the transmission process. If there is an edge from the community to subject i, subject i was infected (this is the case for subject 1). If there is an edge from subject j to subject i, it means that if subject j was infected then subject i was infected too. C. Another example of digraph consistent with the data. We note that certain digraphs may allow more than 1 possible route of transmission. For example, subject 4 could have been infected in the community or by subject 2. D. Example of digraph that is not consistent with the data. This is because this digraph would imply that subject 3 was infected but subject 4 was not. For a given digraph, it is possible to derive the likelihood function [19]. However, since the chains of transmission are unobserved, different configurations for the edges of the digraph may be consistent with the final size data (Figure 1). The digraph is therefore considered as ‘augmented data’ [24]. The joint posterior distribution of parameters and augmented data is explored by Markov chain Monte Carlo sampling. The algorithm explores the set of digraphs consistent with the data and estimates therefore correctly capture uncertainty about the digraph (see Text S1 for technical details).
We use a Uniform prior U([0; 10,000]) for all parameters except those characterising relative infectivity or relative susceptibility (i.e. to a reference group). For this latter class of parameters, following [8], we choose a log-Normal prior LN(0,1). This prior satisfies the invariance condition that for example the ratio (adult susceptibility/child susceptibility) has the same prior as the ratio (child susceptibility/adult susceptibility). In particular, it gives equal probabilities to the relative susceptibility of children versus adults being larger or smaller than 1.
Since the households under study represent only a fraction of households in the study area [20], we assume here that households are independent of each other. The assumption of independence, which is standard in this type of analysis [8], [14], [16], [25], [26], substantially reduces the computational burden compared with that of the more general model of Demeris et al [19].
A simulation study was carried out to investigate the performances of the statistical approach.
Model comparison
Once the model structure has been defined and methods to estimate the parameters of the model from that data are available, different model variants may be considered. For example, the effect of pre-season HI titers may be the same for all subtypes, may vary by subtype, by age group etc… Here we consider a large number of possible model variants. Each of them is fitted to the data and we determine the model variant that provides the best fit to the data. This model comparison exercise is essential to better characterize key dependencies in household transmission. We use the Deviance Information Criterion (DIC) for model comparison [27]. The smaller the DIC, the better the model. A DIC difference of 5 is considered to be a substantial improvement.
For each variable of interest, we explore the following variants:
-
Effect of pre-season HI titers on susceptibility: i) no effect; ii) one threshold value (i.e. intermediate and high HI titers have a different effect than low titers); iii) two threshold values (i.e. low, intermediate and high HI titers each lead to a different protective effect). We also consider model variants in which the effect of pre-season HI titers varies with age.
-
Effect of age on susceptibility: i) no effect; ii) age effect similar for all subtypes; iii) age effect different for seasonal and pandemic influenza; iv) age effect different for each subtype.
-
Effect of age on infectivity: i) no effect; ii) age effect similar for all subtypes.
-
Effect of household size on the household transmission rate β: i) β is independent of the household size; ii) β is inversely proportional to the household size. We also consider models in which β varies with the subtype.
-
Risk of community infection of children with low pre-season HI titers: i) constant; ii) varies by season; iii) varies by subtype; iv) varies by season and subtype.
In general, no satisfying version of the criterion exists for data augmentation frameworks such as the one used here [28]. This is because the likelihood of the observed data is not available. To solve this problem, we use importance sampling [29] to estimate the likelihood of the observed data and be able to derive the DIC. The likelihood is derived as follows. For each household, we simulate N = 2,000 epidemics in the household. The contribution of a household to the likelihood is then equal to the proportion of simulations where simulated infection statuses in the household perfectly match the observed ones (to avoid computational issues of likelihoods equal to zero, we assume that the sensitivity Se and specificity Sp of the diagnostic is not perfect, i.e. Se = 0.999 and Sp = 0.999).
Estimating the proportion of cases attributed to household transmission and the average household transmission probability
In order to estimate the proportion of influenza cases that may be attributed to household transmission, we simulate epidemics in households where i) all parameters are drawn from the posterior distribution and ii) all parameters are drawn from the posterior distribution except the within household transmission rate which is assumed to be null. The case counts difference between i) and ii) gives the proportion of cases that may be attributed to household transmission.
For each pair of case-household contact in the dataset, we calculate the associated probability of transmission under the assumption that the case was the first or the only infected in the pair and derive the average household transmission probability across all pairs.
Model adequacy
We compare the observed final size distribution with the one simulated with parameters drawn from the posterior distribution.
Ethics statement
The research was approved by the institutional review board of the National Institute of Hygiene and Epidemiology, Vietnam; the Oxford Tropical Research Ethics Committee, University of Oxford, UK; and the Ethics Committee of the London School of Hygiene and Tropical Medicine, UK. All participants provided written informed consent.
Results
Between 140 and 155 households (439–502 subjects including 95–121 children and 344–393 adults) were eligible for analysis, depending on the season and subtype. The average household size was 2.9.
Of all the model variants explored in our extensive model comparison exercise, Figure 2 summaries the characteristics of the model that had the best fit based on the DIC. The best fitting model had the following properties. The community risk of infection of children with low pre-season titers varied both with the subtype and the season (Figure 2A). It was minimum for H3N2 in 2008 and maximum for A(H1N1)pdm09 in Autumn 2009. The DIC substantially worsened if the community risk of infection of children varied with the subtype but was assumed to constant from one season to the next (ΔDIC = 24.7).
Fig. 2. Determinants of influenza transmission in and out of the household. 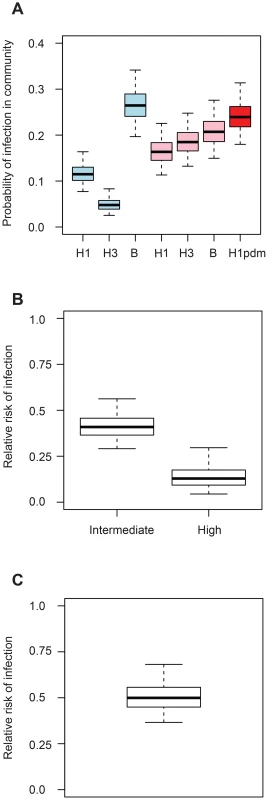
A. Probability of influenza infection from the community for children with low pre-season titres. The season is indicated by the color (blue: 2008; pink: Spring 2009; red: Autumn 2009). B. Relative risk of infection for intermediate (1∶20–1∶40) and high (≥1∶80) pre-season HI titres relative to low (≤1∶10) pre-season HI titres (in the household and in the community). C. Relative risk of infection of adults relative to children after correcting for pre-season HI titres (in the household and in the community). We found that high pre-season titers were protective against infection, with a reduction in the hazard of infection of 59% (95% CI, 44%–71%) for intermediate titers (20–40) and 87% (95% CI, 70%–96%) for high titers (≥80) (Figure 2B). DIC substantially worsened if the number of titer categories was reduced to 2 (ΔDIC = 20.8) or if pre-season titers were not accounted for (ΔDIC = 44.0).
Even after correcting for pre-season titers, we found that adults had half the risk of acquiring infection in the household compared to children (reduction in the hazard of infection of adults relative to children: 50%; 95% CI 32%–63%) (Figure 2C). Adding an age effect for each subtype did not improve the fit (ΔDIC = 0.2). Distinguishing pandemic versus seasonal influenza only provided a marginal improvement to the DIC (ΔDIC = −4.0) (reduction in the hazard of infection of adults relative to children for seasonal influenza: 41%, 95% CI 15%–58%; reduction in the hazard of infection of adults relative to children for pandemic influenza: 68%, 95% CI 42%–82%). Assuming the effect of age varied by subtype did not improve the fit (Figure S1; ΔDIC = 0.2). Ignoring the effect of the age of the subject on the risk of infection substantially worsened the fit (ΔDIC = 37.7). Assuming that infectivity changed with the age of the case did not improve the fit (ΔDIC = 13.2). Assuming the effect of pre-season HI titers could change with age, we found that a rise in HI titers had a slightly more pronounced effect on children than on adults (Figure S2). However, the fit of this model was not as good as that of our best fitting model (ΔDIC = 6.9).
The probability of transmission from an infected individual to another household member was 8% (95% CI, 6%, 10%) on average, and varied with pre-season titer, age and household size. In a households of size 3, the probability of transmission from an infected individual to a child with low, intermediate and high pre-season titers was estimated to be 27% (95% CI 21%–35%), 12% (95% CI, 8%, 17%) and 4% (95% CI, 1%, 9%), respectively. These probabilities dropped to 15% (95% CI 9%–23%), 6% (95% CI 4%–11%) and 2% (95% CI 0–5%), respectively, if the recipient was an adult. As has been found in studies of households in Western developed countries [7], [8], the best fitting model assumed that household transmission hazard decreased with increasing household size. Ignoring this dependency worsened the fit substantially (ΔDIC = 40.7). After correcting for these variables, estimating an effect of subtype on the probability of transmission in the household did not improve the fit (ΔDIC = 13.1). We estimated that 26% (95% CI: 21%, 30%) of cases may be attributed to household transmission. Figure S3 shows the prevalence of infection along with the estimated contribution of household transmission by season and subtype (NB: Figure 2A captures only partially variations in the prevalence of infection as the distribution of pre-season HI titers vary for each season and subtype and by age group).
The fit of the model to the data was adequate (Table 1).
Tab. 1. Observed and expected final size distribution in households with completed diagnostics. 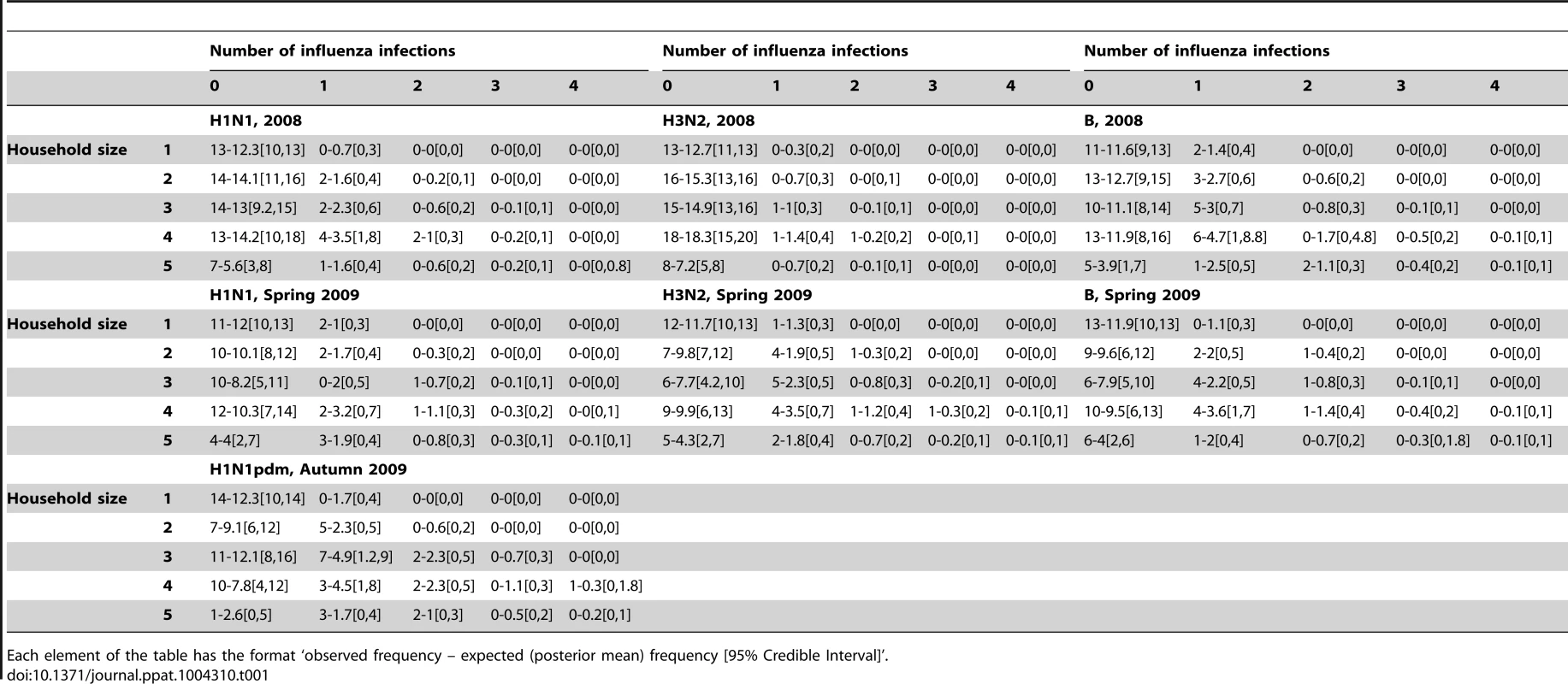
Each element of the table has the format ‘observed frequency – expected (posterior mean) frequency [95% Credible Interval]’. In a simulation study we found all parameters could be estimated from the data and no important systematic bias was detected (Table S1). Out of 10 simulated datasets and 11 parameters, there was 94% probability that the simulation value was in the 95% CI.
Discussion
We have characterised the determinants of transmission of seasonal (H1N1, H3N2, B) and pandemic H1N1pdm09 influenza from a household cohort study conducted in 2007–2010 in Vietnam.
We estimated that the household Secondary Infection Risk (proportion of household contacts infected by an index case, SIR) was approximately 8% on average. This is broadly consistent with estimates of SAR derived from case-ascertained studies, when diagnosis of contact cases is based on RT-PCR laboratory confirmation (median SIRPCR: 8%; range: 3%, 38%; n = 12) or on a clinical case definition of Febrile Acute Respiratory Illness (median SARFARI: 11%; range: 3%, 37%; n = 18) [12]. Lau et al [12] also reported two estimates of the proportion of household contacts who seroconverted of 20% [30] and 27% [31]. As expected, these proportions are larger than 8% since they capture transmission from the index case but also from the community for the whole duration of the epidemic. The similarity between our estimates and those derived from case-ascertained studies validates the use of case-ascertained studies as a way to obtain representative estimates of influenza household transmission. Overall, we estimated that 26% (95% CI: 21%, 30%) of influenza infections may be attributed to household transmission. This is consistent with other estimates in the literature [32].
We also estimated the risk factors for household transmission and the risk of infection. Pre-season titer and age had a strong impact on the risk of infection. An HI titer of 40 is generally accepted to give a 50% reduction in the risk of infection [5]. Here we found a slightly more subtle effect of pre-season titer, with the risk of infection decreasing incrementally with HI titer and the reduction being as high as 90% for HI titer ≥80. Even after correcting for pre-season titers, we found that adults had half the risk of acquiring infection compared to children. This supports the idea that HI titer is an imperfect correlate of protection. There is growing evidence that antibodies directed at the stalk domain of the HA protein may be important mediators of protection that accumulates with repeated exposure to influenza viruses but which is not detectable by the HI assay [33]. Consistent with other studies [7], [8], [34], we found that the household person-to-person transmission probability decreased with increasing household size.
Ours is the only contemporary study to prospectively assess the transmission of influenza in a random selection of all households (including those without children) in an unimmunised community over multiple seasons. The use of a final-size model based on serology minimizes the under-ascertainment inherent in studies that detect only symptomatic cases. As such we believe these results are the best available assessment of the risk of acquisition of influenza in the household and the community.
The earlier analysis of this dataset [20] simply reported empirical infection rates by age based on a four-fold or greater increase in HI titers between paired sera, and did not estimate any other transmission parameters nor influences on the probability of transmission. The analysis presented in this manuscript therefore adds substantial new insights including estimates of the probability of transmission from an infected individual to another household member, the proportion of infections acquired in the household and the community, and how the probability of infection is affected by pre-season HI titers, age and household size
This study has some limitations. First, the HI assay has imperfect sensitivity and specificity [35], [36]. As a consequence, the infection status of some individuals may be incorrectly classified. The use of microneutralization assay to detect pH1N1 seroconversions would have increased the sensitivity. The average number of households per season was relatively small (about 150). However, the study was run over 3 seasons and looked at multiple different subtypes (H1N1, H3N2, B, H1N1pdm09), for a total of 6 pairs season/subtype. This means that the amount of information contained in these data is roughly that of a study of 6×150 = 900 households run over 1 season and for 1 subtype. This explains why the credible intervals for most parameters are relatively narrow. We were unable to assess transmission risks in children aged less than 5 years, since serum samples were not obtained from these subjects.
Here, we disentangled the relative contributions of households and the community in the risk of influenza infection. This was made under the assumption that households were independent of each other and that all individuals of an age group were exposed to the same risk of infection in the community. Although standard in such analyses [8], [14], [16], [25], [26], in practice, the risk of infection in the community may have a spatial component, potentially leading to higher transmission rates between households that are close to each other. However, we were unable to test this assumption here since our dataset was not spatially structured. Estimating the effect of space on influenza transmission will be an important step forward. This can for example be done from the analysis of household serological cohort studies in which the spatial location of each household is be documented [37]. Ideally, one would like to integrate such analysis in the framework of Demiris and O'Neill [19], so that the contributions of households and space can be characterized in a single and coherent framework. This is an important subject for future research.
This study considerably extends previously limited evidence on influenza transmission in non-Western countries. It also validates the use of case-ascertained studies as a way to obtain representative estimates of influenza household transmission. This has important implications for early assessment of household transmission in future pandemics, as case-ascertained studies are the only household design that can be used close to real-time.
Supporting Information
Zdroje
1. WHO Influenza (Seasonal). Available at www.who.int/mediacentre/factsheets/fs211/en/index.html. Accessed on 30 May 2012.
2. Committee for Proprietary Medicinal Products (1997) Note for guidance on harmonisation of requirements for influenza vaccines. EMEA
3. WoodJM, LevandowskiRA (2003) The influenza vaccine licensing process. Vaccine 21 : 1786–1788.
4. HobsonD, CurryRL, BeareAS, Ward-GardnerA (1972) The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 70 : 767–777.
5. CoudevilleL, BailleuxF, RicheB, MegasF, AndreP, et al. (2010) Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol 10 : 18.
6. DorigattiI, CauchemezS, FergusonNM (2013) Increased transmissibility explains the third wave of infection by the 2009 H1N1 pandemic virus in England. Proceedings of the National Academy of Sciences of the United States of America 110 : 13422–13427.
7. CauchemezS, CarratF, ViboudC, ValleronAJ, BoellePY (2004) A Bayesian MCMC approach to study transmission of influenza: application to household longitudinal data. Statistics in Medicine 23 : 3469–3487.
8. CauchemezS, DonnellyCA, ReedC, GhaniAC, FraserC, et al. (2009) Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. New England Journal of Medicine 361 : 2619–2627.
9. CowlingBJ, ChanKH, FangVJ, LauLL, SoHC, et al. (2010) Comparative epidemiology of pandemic and seasonal influenza A in households. New England Journal of Medicine 362 : 2175–2184.
10. GhaniAC, BaguelinM, GriffinJ, FlascheS, PebodyR, et al. (2009) The Early Transmission Dynamics of H1N1pdm Influenza in the United Kingdom. PLoS Curr Influenza 1: RRN1130.
11. HPA (2009) The First Few Hundred (FF100) project: Epidemiological protocols for comprehensive assessment of early swine influenza cases in the United Kingdom. Available at http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1257260453727. Last accessed on 14 Dec 2012.
12. LauLLH, NishiuraH, KellyH, IpDKM, LeungGM, et al. (2012) Household Transmission of 2009 Pandemic Influenza A (H1N1) A Systematic Review and Meta-analysis. Epidemiology 23 : 531–542.
13. TsangTK, CauchemezS, PereraRA, FreemanG, FangVJ, et al. (2014) Association between antibody titers and protection against influenza virus infection within households. J Infect Dis doi:10.1093/infdis/jiu186
14. LonginiIMJr, KoopmanJS, MontoAS, FoxJP (1982) Estimating household and community transmission parameters for influenza. Am J Epidemiol 115 : 736–751.
15. KlickB, NishiuraH, NgS, FangVJ, LeungGM, et al. (2011) Transmissibility of Seasonal and Pandemic Influenza in a Cohort of Households in Hong Kong in 2009. Epidemiology 22 : 793–796.
16. AddyCL, LonginiIMJr, HaberM (1991) A generalized stochastic model for the analysis of infectious disease final size data. Biometrics 47 : 961–974.
17. Andersson H, Britton T (2000) Stochastic epidemic models and their statistical analysis. New-York: Springer-Verlag.
18. FraserC, CummingsDAT, KlinkenbergD, BurkeDS, FergusonNM (2011) Influenza Transmission in Households During the 1918 Pandemic. American Journal of Epidemiology 174 : 505–514.
19. DemirisN, O'NeillPD (2005) Bayesian inference for stochastic multitype epidemics in structured populations via random graphs. Journal of the royal Statistical Society Series B 67 : 731–745.
20. HorbyP, Mai leQ, FoxA, ThaiPQ, Thi Thu YenN, et al. (2012) The epidemiology of interpandemic and pandemic influenza in Vietnam, 2007–2010: the ha nam household cohort study I. American Journal of Epidemiology 175 : 1062–1074.
21. CowlingBJ, IpDK, FangVJ, SuntarattiwongP, OlsenSJ, et al. (2013) Aerosol transmission is an important mode of influenza A virus spread. Nat Commun 4 : 1935.
22. KiefferA, PaboribouneP, CrepeyP, FlaissierB, SouvongV, et al. (2013) 2009 A(H1N1) seroconversion rates and risk factors among the general population in Vientiane Capital, Laos. PLoS One 8: e61909.
23. LapidusN, de LamballerieX, SalezN, SetbonM, FerrariP, et al. (2012) Integrative study of pandemic A/H1N1 influenza infections: design and methods of the CoPanFlu-France cohort. BMC Public Health 12 : 417.
24. O'NeillPD, BaldingDJ, BeckerNG, EerolaM, MollisonD (2000) Analyses of infectious disease data from household outbreaks by Markov chain Monte Carlo methods. Journal of the Royal Statistical Society Series C 49 : 517–542.
25. LonginiIMJr, KoopmanJS, HaberM, CotsonisGA (1988) Statistical inference for infectious diseases. Risk-specific household and community transmission parameters. Am J Epidemiol 128 : 845–859.
26. LonginiIMJr, MontoAS, KoopmanJS (1984) Statistical procedures for estimating the community probability of illness in family studies: rhinovirus and influenza. Int J Epidemiol 13 : 99–106.
27. SpiegelhalterDJ, BestNG, CarlinBP, van der LindeA (2002) Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society Series B 64 : 583–639.
28. CeleuxG, ForbesF, RobertCP, TitteringtonDM (2006) Deviance Information Criteria for Missing Data Models. Bayesian Analysis 1 : 651–673.
29. Liu JS (2001) Monte Carlo strategies in scientific computing. New York: Springer-Verlag.
30. ChangLY, ChenWH, LuCY, ShaoPL, FanTY, et al. (2011) Household transmission of Pandemic (H1N1) 2009 Virus, Taiwan. Emerg Infect Dis 17 : 1928–1931.
31. JacksonML, FranceAM, HancockK, LuX, VeguillaV, et al. (2011) Serologically confirmed household transmission of 2009 pandemic influenza A (H1N1) virus during the first pandemic wave–New York City, April–May 2009. Clinical Infectious Diseases 53 : 455–462.
32. FergusonNM, CummingsDA, CauchemezS, FraserC, RileyS, et al. (2005) Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature 437 : 209–214.
33. KrammerF, PaleseP (2013) Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol 3 : 521–530.
34. CauchemezS, BhattaraiA, MarchbanksTL, FaganRP, OstroffS, et al. (2011) Role of social networks in shaping disease transmission during a community outbreak of 2009 H1N1 pandemic influenza. Proceedings of the National Academy of Sciences of the United States of America 108 : 2825–2830.
35. RothbarthPH, GroenJ, BohnenAM, de GrootR, OsterhausADME (1999) Influenza virus serology - a comparative study. Journal of Virological Methods 78 : 163–169.
36. CauchemezS, HorbyP, FoxA, MaiLQ, ThanhLT, et al. (2012) Influenza Infection Rates, Measurement Errors and the Interpretation of Paired Serology. Plos Pathogens 8: e1003061.
37. LesslerJ, CummingsDA, ReadJM, WangS, ZhuH, et al. (2011) Location-specific patterns of exposure to recent pre-pandemic strains of influenza A in southern China. Nat Commun 2 : 423.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial PathogensČlánek A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during TransmissionČlánek The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil ChemotaxisČlánek Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Regulatory RNAs Involved in Bacterial Antibiotic Resistance
- From Dandruff to Deep-Sea Vents: -like Fungi Are Ecologically Hyper-diverse
- Pathogenicity and Epithelial Immunity
- Mother–Infant HIV Transmission: Do Maternal HIV-Specific Antibodies Protect the Infant?
- Hell's BELs: acterial 3 igases That Exploit the Eukaryotic Ubiquitin Machinery
- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Primary Seronegative but Molecularly Evident Hepadnaviral Infection Engages Liver and Induces Hepatocarcinoma in the Woodchuck Model of Hepatitis B
- TLR2 Signaling Decreases Transmission of by Limiting Bacterial Shedding in an Infant Mouse Influenza A Co-infection Model
- Production of an Attenuated Phenol-Soluble Modulin Variant Unique to the MRSA Clonal Complex 30 Increases Severity of Bloodstream Infection
- Inhibition of the TRAIL Death Receptor by CMV Reveals Its Importance in NK Cell-Mediated Antiviral Defense
- Early Mucosal Sensing of SIV Infection by Paneth Cells Induces IL-1β Production and Initiates Gut Epithelial Disruption
- Limited HIV Infection of Central Memory and Stem Cell Memory CD4+ T Cells Is Associated with Lack of Progression in Viremic Individuals
- Virus-Specific Regulatory T Cells Ameliorate Encephalitis by Repressing Effector T Cell Functions from Priming to Effector Stages
- A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during Transmission
- The HIV-1 Envelope Transmembrane Domain Binds TLR2 through a Distinct Dimerization Motif and Inhibits TLR2-Mediated Responses
- Infection with MERS-CoV Causes Lethal Pneumonia in the Common Marmoset
- VGIII Isolates Causing Infections in HIV/AIDS Patients in Southern California: Identification of the Local Environmental Source as Arboreal
- Diverse Host-Seeking Behaviors of Skin-Penetrating Nematodes
- Capsid Protein VP4 of Human Rhinovirus Induces Membrane Permeability by the Formation of a Size-Selective Multimeric Pore
- The Murine Gammaherpesvirus Immediate-Early Rta Synergizes with IRF4, Targeting Expression of the Viral M1 Superantigen to Plasma Cells
- Characterization of an Insecticidal Toxin and Pathogenicity of against Insects
- The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil Chemotaxis
- Histone Deacetylase Inhibitors Impair the Elimination of HIV-Infected Cells by Cytotoxic T-Lymphocytes
- A Locus Encompassing the Epstein-Barr Virus Kinase Regulates Expression of Genes Encoding Viral Structural Proteins
- Distinct APC Subtypes Drive Spatially Segregated CD4 and CD8 T-Cell Effector Activity during Skin Infection with HSV-1
- Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
- Adoptive Transfer of EBV Specific CD8 T Cell Clones Can Transiently Control EBV Infection in Humanized Mice
- Schistosome Feeding and Regurgitation
- EVM005: An Ectromelia-Encoded Protein with Dual Roles in NF-κB Inhibition and Virulence
- Rabies Virus Hijacks and Accelerates the p75NTR Retrograde Axonal Transport Machinery
- Why HIV Virions Have Low Numbers of Envelope Spikes: Implications for Vaccine Development
- Identification of Anti-virulence Compounds That Disrupt Quorum-Sensing Regulated Acute and Persistent Pathogenicity
- HIV-1 Receptor Binding Site-Directed Antibodies Using a VH1-2 Gene Segment Orthologue Are Activated by Env Trimer Immunization
- Cooperation between Epstein-Barr Virus Immune Evasion Proteins Spreads Protection from CD8 T Cell Recognition across All Three Phases of the Lytic Cycle
- Parasite Extracellular Vesicles: Mediators of Intercellular Communication
- RC1339/APRc from Is a Novel Aspartic Protease with Properties of Retropepsin-Like Enzymes
- Cyclic di-GMP-dependent Signaling Pathways in the Pathogenic Firmicute
- Non-random Escape Pathways from a Broadly Neutralizing Human Monoclonal Antibody Map to a Highly Conserved Region on the Hepatitis C Virus E2 Glycoprotein Encompassing Amino Acids 412–423
- Neutrophil Elastase Causes Tissue Damage That Decreases Host Tolerance to Lung Infection with Species
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- SGNH Hydrolase-Like Proteins AlgJ and AlgX Have Similar Topology but Separate and Distinct Roles in Alginate Acetylation
- Why Sexually Transmitted Infections Tend to Cause Infertility: An Evolutionary Hypothesis
- Late Engagement of CD86 after Influenza Virus Clearance Promotes Recovery in a FoxP3 Regulatory T Cell Dependent Manner
- Determinants of Influenza Transmission in South East Asia: Insights from a Household Cohort Study in Vietnam
- A Novel Signal Transduction Pathway that Modulates Quorum Sensing and Bacterial Virulence in
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- A Cysteine Protease Inhibitor of Is Essential for Exo-erythrocytic Development
- EBNA3C Augments Pim-1 Mediated Phosphorylation and Degradation of p21 to Promote B-Cell Proliferation
- On the Front Line: Quantitative Virus Dynamics in Honeybee ( L.) Colonies along a New Expansion Front of the Parasite
- Assembly and Architecture of the EBV B Cell Entry Triggering Complex
- NLR-Associating Transcription Factor bHLH84 and Its Paralogs Function Redundantly in Plant Immunity
- The PDZ-Binding Motif of Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Is a Determinant of Viral Pathogenesis
- Strain-Specific Properties and T Cells Regulate the Susceptibility to Papilloma Induction by Papillomavirus 1
- Human Cytomegalovirus pUL79 Is an Elongation Factor of RNA Polymerase II for Viral Gene Transcription
- The GAP Activity of Type III Effector YopE Triggers Killing of in Macrophages
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- Pathogenicity and Epithelial Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





