-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaStrain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
Transmissible spongiform encephalopathies (TSE) or prion diseases are neurodegenerative disorders associated with conversion of normal host prion protein (PrP) to a misfolded, protease-resistant form (PrPres). Genetic variations of prion protein in humans and animals can alter susceptibility to both familial and infectious prion diseases. The N171S PrP polymorphism is found mainly in humans of African descent, but its low incidence has precluded study of its possible influence on prion disease. Similar to previous experiments of others, for laboratory studies we created a transgenic model expressing the mouse PrP homolog, PrP-170S, of human PrP-171S. Since PrP polymorphisms can vary in their effects on different TSE diseases, we tested these mice with four different strains of mouse-adapted scrapie. Whereas 22L and ME7 scrapie strains induced typical clinical disease, neuropathology and accumulation of PrPres in all transgenic mice at 99-128 average days post-inoculation, strains RML and 79A produced clinical disease and PrPres formation in only a small subset of mice at very late times. When mice expressing both PrP-170S and PrP-170N were inoculated with RML scrapie, dominant-negative inhibition of disease did not occur, possibly because interaction of strain RML with PrP-170S was minimal. Surprisingly, in vitro PrP conversion using protein misfolding cyclic amplification (PMCA), did not reproduce the in vivo findings, suggesting that the resistance noted in live mice might be due to factors or conditions not present in vitro. These findings suggest that in vivo conversion of PrP-170S by RML and 79A scrapie strains was slow and inefficient. PrP-170S mice may be an example of the conformational selection model where the structure of some prion strains does not favor interactions with PrP molecules expressing certain polymorphisms.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002275
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002275Summary
Transmissible spongiform encephalopathies (TSE) or prion diseases are neurodegenerative disorders associated with conversion of normal host prion protein (PrP) to a misfolded, protease-resistant form (PrPres). Genetic variations of prion protein in humans and animals can alter susceptibility to both familial and infectious prion diseases. The N171S PrP polymorphism is found mainly in humans of African descent, but its low incidence has precluded study of its possible influence on prion disease. Similar to previous experiments of others, for laboratory studies we created a transgenic model expressing the mouse PrP homolog, PrP-170S, of human PrP-171S. Since PrP polymorphisms can vary in their effects on different TSE diseases, we tested these mice with four different strains of mouse-adapted scrapie. Whereas 22L and ME7 scrapie strains induced typical clinical disease, neuropathology and accumulation of PrPres in all transgenic mice at 99-128 average days post-inoculation, strains RML and 79A produced clinical disease and PrPres formation in only a small subset of mice at very late times. When mice expressing both PrP-170S and PrP-170N were inoculated with RML scrapie, dominant-negative inhibition of disease did not occur, possibly because interaction of strain RML with PrP-170S was minimal. Surprisingly, in vitro PrP conversion using protein misfolding cyclic amplification (PMCA), did not reproduce the in vivo findings, suggesting that the resistance noted in live mice might be due to factors or conditions not present in vitro. These findings suggest that in vivo conversion of PrP-170S by RML and 79A scrapie strains was slow and inefficient. PrP-170S mice may be an example of the conformational selection model where the structure of some prion strains does not favor interactions with PrP molecules expressing certain polymorphisms.
Introduction
TSE or prion diseases are transmissible neurodegenerative diseases occurring in a variety of mammalian species including domestic and wild animals as well as humans [1]. Examples include scrapie in sheep, chronic wasting disease (CWD) in cervids and bovine spongiform encephalopathy (BSE) in cattle. Human diseases include sporadic and variant Creutzfeldt-Jakob disease (sCJD and vCJD) and familial diseases such as Gerstmann–Sträussler–Scheinker syndrome (GSS), Fatal Familial Insomnia (FFI) and familial CJD. A hallmark of prion diseases is the conversion of the normal protease-sensitive host prion protein (PrPsen) into a misfolded partially protease-resistant form (PrPres) which may be in part responsible for the generation of the disease [2]. PrP expression is required for prion disease, and PrP knockout mice are resistant to both infection and disease [3].
PrP gene polymorphisms occur naturally in many species. In both sheep and in mice such polymorphisms have been found to influence susceptibility to scrapie infection and disease. For example, sheep expressing Alanine, Arginine, Arginine at positions 136, 154 and 171 (A136R154R171) respectively, are highly resistant to most strains of sheep scrapie [4], [5], [6]. However, recent observations indicate that sheep with the A136R154R171 genotype can be susceptible to atypical scrapie (Nor98) suggesting that resistance might be strain specific [7], [8]. Similarly in inbred mice there are two known PrP alleles (Prnpa and Prnpb) which are characterized by amino acid differences at PrP residues 108 and 189 [9], [10], [11]. Mice with Prnpa have short incubation times with one set of scrapie strains and prolonged incubation times with another set, while mice with Prnpb show an opposite pattern of incubation times with these same scrapie strains [12], [13]. Thus there is a different strain-specific pattern of susceptibility associated with each of these Prnp genotypes.
In humans, the methionine vs. valine polymorphism at PrP residue 129 appears to influence the clinical phenotype of familial prion disease associated with the D178N PrP mutation [14], [15], as well as susceptibility to sCJD, vCJD and kuru [16], [17], [18], [19], [20]. In addition, the human polymorphism E219K may be associated with resistance to sCJD [21], but does not appear to correlate with resistance to vCJD in humans or mouse models [22], [23]. The human PrP polymorphism, G127V, was recently shown to be associated with resistance to kuru [24]. Other naturally occurring human polymorphisms, such as G142S and N171S, have not been tested for prion disease susceptibility [25].
N171S is a PrP polymorphism found in Sub-Saharan Africans, Jamaicans and Sardinians [26]. The rarity of this polymorphism and the fact that it is present mainly in geographical regions with limited CJD surveillance make it difficult to detect possible associations with prion disease. Therefore, in order to initiate laboratory studies of the possible effects of this polymorphism on TSE diseases and possibly also other CNS disorders, we generated transgenic mice expressing mouse PrP-170S, the mouse homolog of human PrP-171S (Table 1).
Tab. 1. Comparison of PrP residues in humans, mice and transgenic mice generated in the present study. 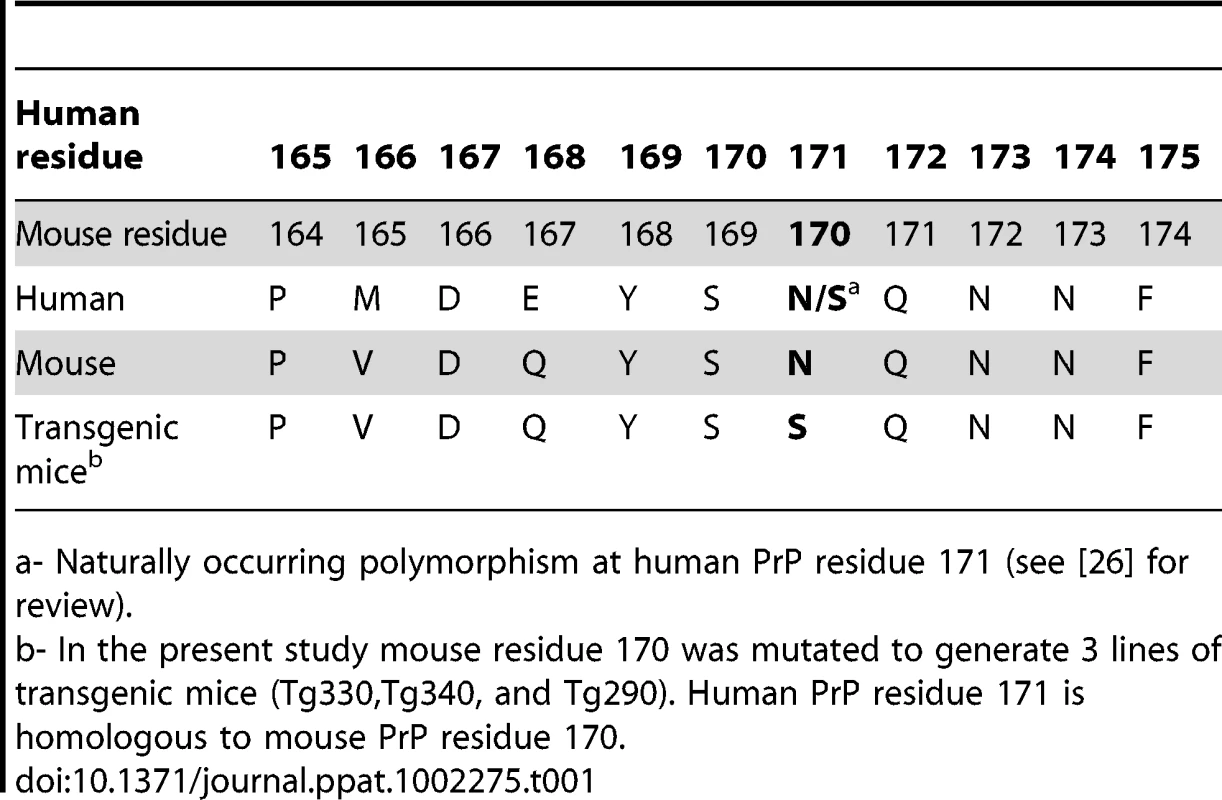
a- Naturally occurring polymorphism at human PrP residue 171 (see [26] for review). This approach of using transgenic mice expressing mouse or hamster PrP with human PrP mutations and/or polymorphic residues at homologous sites has been taken in numerous previous studies of prion diseases. These include important studies of models of Gerstmann-Sträussler-Scheinker syndrome (GSS) [27], [28], [29], [30], [31], [32], FFI [33] and familial CJD [34], [35], as well as a non-infectious neurodegenerative disease associated with expression of prion protein with a nine octapeptide insertion [36]. In addition, transgenic mice expressing mouse PrP with cervid PrP residues associated with a “rigid loop” in PrP have also revealed interesting in vivo pathogenic effects [37], [38].
Because PrP variations in animals and humans can have different effects on different TSE strains, we tested our mice with four different scrapie strains which have been maintained in continuous mouse passage for many years. In these studies PrP-170S expressing mice were highly susceptible to scrapie strains 22L and ME7, but were markedly resistant to scrapie strains RML and 79A. In contrast, control mice were susceptible to all four strains. This in vivo strain-specific influence on scrapie susceptibility was not reproduced in cell-free in vitro PrP conversion studies, suggesting that in vivo conditions not replicated in our in vitro system were required. PrP-170S transgenic mice appear to be an interesting new model to study the interactions between TSE agent strains and PrP.
Materials and Methods
Ethics statement
All mice were housed at the Rocky Mountain Laboratories (RML) in an AAALAC-accredited facility and experimentation followed NIH RML Animal Care and Use Committee approved protocols (NIH/RML Protocol #2007-31). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Generation of transgenic mice
To study the effect of the human N171S PrP polymorphism (Ref SNP#rs16990018) we constructed transgenic mice expressing serine instead of asparagine at mouse PrP residue 170 (homologous to human PrP residue 171) (Table 1). The mutation was made at residue 170 in mouse PrP using a cDNA clone of mouse PrP, p1-5 (E48-16) [39] using oligonucleotides 2077U (5′ - CCA GTG GAT CAG TAC AGC AGC CAG AAC AAC TTC GTG C -3′) and 2078L (5′ - GCA CGA AGT TGT TCT GGC TGC TGT ACT GAT CCA CTG G -3′) with a site directed mutagenesis kit (Stratagene/Agilent, Santa Clara, CA). The plasmid with the mutation was recloned, and the mutation was confirmed by sequencing. A DNA fragment from PshAI to SfoI with PrP sequences containing the mutation was excised, purified and religated into a subclone, p44-3 (E58-6) [39], [40], derived from the pHGPrP half-genomic clone [41] by digesting with AgeI and SfoI to remove a portion of the PrP ORF and replacing this segment with an oligonucleotide polylinker containing these and other sites. Upstream sequences previously excised between two BamHI sites to remove an unwanted SfoI site were replaced by digestion with adjacent sites NotI and BspEI and the original 6.2kb fragment from PHGPrP was reinserted. The resulting plasmid, p188-6, was digested with NotI and SbfI to separate mouse sequences from bacterial plasmid sequences, and the fragment containing the mouse sequences was used to inoculate C57BL/6 mouse eggs to generate transgenic mice expressing PrP-170S [39]. Of the five transgenic founding lines produced, three lines (Tg330, Tg340 and Tg290) were selected for experimentation. These transgenic lines were hemizygous for the transgene and homozygous for Prnp, the gene encoding normal mouse PrP (MoPrP). The Prnp gene encoding PrP-170N in C57BL/6 mice was removed by serial backcrossing to C57BL/10Sn mice with a knocked-out Prnp gene (B10 PrP-/-) derived from the original Edinburgh Prnp knockout mouse as previously described [39], [42]. Transgenic lines were maintained as transgene heterozygotes by crossing to C57BL/10 PrP-/ - mice and selection of transgene positive mice by PCR analysis of tail DNA.
Genotyping was conducted using standard PCR reactions as previously described [39]. Briefly, detection of the modified knock-out version of Prnp and the neo cassette in the PrP-/ - mice was accomplished using primers RK1 and Mut217 as previously described [43]. These primers yielded a C57BL/10 MoPrP product of approximately 700 bp and a PrP-/ - neomycin cassette gene product of approximately 1700bp. Detection of the half-genomic PrP transgene, expressing the N170S PrP construct, was accomplished using primers pE2+ and Mut217 as described previously [43]. PCR products were visualized via electrophoresis in a 2% agarose gel.
Laboratory animals
All mice were housed at the Rocky Mountain Laboratories (RML) in an AAALAC-accredited facility and experimentation followed NIH RML Animal Care and Use Committee approved protocols (NIH/RML Protocol #2007-31). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Mice were bred and genotyped at RML. For use as controls, weanling C57BL/10Hsd mice were obtained from Harlan Sprague Dawley, Madison, WI. Transgenic tga20 mice were obtained from EMMA (Munich, Germany) [41].
Scrapie inoculations and animal observations
Mice were injected intracerebrally (i.c.) with 50μl of a 1% (wt/vol) dilution of brain homogenate pools from C57BL mice terminally ill from 22L, RML, 79A or ME7 scrapie. Two stocks of RML scrapie (RML-06, RML-81) were used. Titers of scrapie stocks were determined in previous i.c. endpoint titration experiments and were as follows; 22L = 2.5×109, ME7 = 4.0×108, RML-06 = 3.2×108, RML-81 = 4.8×108, 79A = 1.6×108 (units = 50% infectious dose (ID50)/gm of brain). Brain homogenates were diluted for inoculation in phosphate buffered balanced saline (PBBS) pH 7.2, supplemented with 2% fetal bovine serum (Hyclone, Logan, UT).
Observations were made daily to assess clinical signs of scrapie disease, which included ataxia, altered gait, wasting, kyphosis, hind limb weakness, aimless wandering, somnolence, immobility and leg clasping reflex. Mice with clinical signs were euthanized and brain tissue was analyzed for PrPres by immunoblotting. Mice with both clinical signs and PrPres by immunoblot were defined as diseased, and the day of euthanasia was recorded as the incubation period in the data presented. Uninoculated control transgenic mice were followed up to 700 days of age and showed no clinical signs of scrapie differing from normal signs of senescence. Several uninoculated mice were analyzed by histopathology after euthanasia at 465 days of age, and brain tissue had no detectable grey matter vacuolation or abnormal PrPres deposition.
Statistical analysis of data in the coexpression experiment was done by a one way ANOVA with Dunnett's multiple comparison test using GraphPad Prism software.
Immunoblot analysis
Tissue samples from mice were analyzed for PrPres and PrPsen by immunoblot. Brain homogenates (20%w/v) were prepared in 10 mM Tris-HCl [pH 7.4] using a mini-beadbeater (Biospec products, Bartlesville, OK). All homogenates were sonicated for 1 min using a Vibracell cup-horn sonicator (Sonics, Newtown, NJ) as previously described [40]. To test for PrPres, samples were proteinase K treated as follows; 20 µl of a 20% (w/v) tissue homogenate was adjusted to 100 mM Tris HCl (pH 8.3), 1% Triton X-100,1% sodium deoxycholate and 50 µg/ml proteinase K (PK), in a total volume of 31 µl. Tubes were mixed and incubated for 45 minutes at 37°C. The reaction was stopped by adding 2 µl of 100 mM Pefabloc (Roche Diagnostics, Indianapolis, Indiana) and placed on ice for 5 min. Samples tested for PrPsen were treated with proteinase inhibitors: 10 µM leupeptin, 1 µM pepstatin A, and 7 µg/ml aprotinin. PrPsen samples were not treated with PK. An equal volume of 2X Laemmli sample buffer (Biorad, Hercules, CA) was added to both PrPsen and PrPres samples, and then tubes were boiled 5 minutes. Samples were frozen at −20°C until the day of analysis, when samples were thawed, reboiled for 5 minutes and then electrophoresed on a 16% Tris-Glycine SDS-PAGE gel (Invitrogen, Carlsbad,CA) and blotted to PVDF membranes (Biorad) using a 7 minute transfer, program 3 (P3) on an iBlot (Invitrogen) device. Immunoblots were blocked in a solution of 2.5% milk (Biorad) in 0.1 M Tris, 1.5M NaCl and 0.05% Tween20, for 1 hour. Then blots were probed with D13 anti-PrP monoclonal antibody [44], (InPro, San Francisco, CA) at a final concentration of 0.2 µg/ml, followed by a peroxidase-conjugated anti-human IgG secondary antibody (Sigma, St. Louis, MO) at a final dilution of 1∶5000 in the blocking buffer described above. Bands were detected using enhanced chemiluminescence substrate (ECL) as directed by manufacturer (GE Healthcare, Pittsburgh, PA).
Histology and immunohistochemistry
For histopathological analysis mice were deeply anesthetized and euthanized by cervical dislocation. Tissues were removed and placed in 3.7% phosphate-buffered formalin for 3 to 5 days before dehydration and embedding in paraffin. Serial 5 µm sections were cut using a standard Leica microtome, placed on positively charged glass slides and dried overnight at 43°C. Slides were then deparaffinized using standard procedures. Slides were stained with hematoxylin and eosin and analyzed for pathological changes. Immunohistochemical detection of PrPres using DAB chromogen (DAB Map kit; Ventana Medical Systems, Tucson, AZ.) was done as follows: antigen retrieval and staining were performed using the Ventana automated Discovery XT stainer. PrP antigens were exposed by incubation in CC1 buffer (Ventana) containing Tris-Borate-EDTA, pH 8.0 for 20 minutes at 95°C. Staining for PrP was done using human anti-mouse PrP monoclonal antibody D13 at a dilution of 1∶500 at 37°C for 2 hours, followed by a biotinylated anti-human IgG at 1∶500 (Jackson ImmunoResearch, West Grove, PA.), and avidin-horseradish peroxidase with DAB as chromogen. Slides were examined and photomicrographs were taken observed using an Olympus BX51 microscope and Microsuite FIVE software.
Protein misfolding cyclic amplification (PMCA) assay
For preparation of normal brain homogenates containing PrPsen to be used as the substrate for PMCA reactions healthy transgenic mice expressing either PrP-170S (Tg330) or PrP-170N (Tga20) [41] were deeply anesthetized and then perfused with PBS containing 5 mM EDTA. Brains were removed and homogenized in a beadbeater at a concentration of 20% (w/v) in PMCA conversion buffer (PBS containing 4 mM EDTA, 1% Triton X-100 and complete mini-protease inhibitor cocktail (Roche), sterilized by filtration with a 0.2 µm filter) then cooled on ice for 10–15 min. Homogenates were diluted with PMCA conversion buffer to 10% (w/v) then clarified by a brief 1500 X g spin [45]. Supernatants were stored at −80° C in 1 ml aliquots until used in PMCA reactions.
Brain homogenates used as seeds for the PMCA reactions came from clinically sick 22L or RML-infected C57BL/6 mice. Brains were homogenized at a 20% (w/v) concentration using 0.1 M Tris pH 7.4, then diluted in the same buffer to a final concentration of 10% (w/v) prior to storage at −80°C. Scrapie-positive brain homogenates were used to “seed” normal brain homogenate in the following manner. A 10% scrapie-positive brain homogenate (seed) was added to normal brain homogenate (substrate) at desired dilutions and then these master mixes were aliquoted into multiple 0.2ml reaction tubes (GeneMate, ISCBioexpress, Kaysville, Utah). One of these tubes was frozen as an unsonicated control and the remaining tubes were repeatedly sonicated and incubated as described below. Tubes were positioned in a plastic tube rack PMCA adapter (Misonix, Farmingdale, NY) and placed on the rim of a microplate horn of a Misonix Model 3000 microsonicator so that the 50 µl samples were immersed in the sonicator bath. The microplate horn was covered with a plastic lid to minimize evaporation from the water bath. The sonicator was located inside an incubator set to 37°C and was programmed to perform cycles, each consisting of a 40 second pulse of sonication set at 60% maximum followed by a 30 min incubation. Forty-eight cycles (i.e. 24 h) constituted one round of PMCA.
PMCA reaction tubes were removed from the sonicator and vortexed and 10 µl of the 50 µl volume was sampled and mixed with 10 µl PK at 100 µg/ml for a final PK concentration of 50 µg/ml. Samples were maintained at 37°C in a water bath for one hour. The PK digestions were halted with 2 µl of 100 mM Pefabloc (Roche Diagnostics) and placed on ice for 5 min. An equal volume of 2X Laemmli sample buffer (Biorad, Hercules, CA) was added to the PMCA samples, and then tubes were boiled 5 minutes. Samples were frozen at −20°C until the day of analysis, when samples were thawed, and reboiled for 5 minutes. PMCA products were visualized by SDS page gel electrophoresis and immunoblotting as described above for brain tissues, with the following exceptions. Gels were blotted to HyBond ECL nitrocellulose membranes (GE Healthcare Life Sciences) instead of PVDF membrane. Immunoblots were blocked in Near-Infrared Fluorescence Western Blotting Blocking Buffer (Rockland Immunochemicals Inc., Gilbertsville, PA) and PBS mixed in equal parts. Primary antibody was D13 1∶100 diluted supernatant derived from CHO cells expressing the D13 antibody construct [44]. These cells were obtained from R. Anthony Williamson, The Scripps Research Institute, La Jolla, CA. The secondary antibody was IRDye800CW-conjugated goat-anti-mouse IgG (LiCor, Lincoln, NE) diluted at 1∶10,000. Both antibodies were diluted in the blocking buffer described above with the addition of 0.2% Tween 20. Finally, bands were detected using an Odyssey near-infrared fluorescence scanner (LiCor). Groups were compared using a Mann-Whitney test with GraphPad Prism software.
Results
Generation, breeding and PrP expression of transgenic mice expressing mouse PrP-170S
To study the effect of the human PrP polymorphism, N171S, on susceptibility to prion disease we generated transgenic mice expressing mouse PrP-170S, the mouse homolog of the human PrP-171S (Table 1). Five founder lines were produced on the C57BL/6 background, and these were crossed serially to C57BL/10 mice homozygous for the Edinburgh version of the PrP null gene (Prnp-/-) [42]. Three lines of the transgenic mice (Tg290, Tg330, and Tg340) with the Prnp-/ - gene, and hemizygous for the PrP-170S transgene, were obtained and used for further study.
Because PrPsen expression is known to influence scrapie incubation period [41], [46], we determined PrPsen levels in brain by immunoblot. Both Tg330 and Tg340 mice expressed PrPsen at levels 2 to 3-fold higher than were seen in non-transgenic control C57BL/10 mice (Prnp+/+) which express PrP-170N (Figure 1). Tg290 expressed approximately 10-fold lower PrP levels than did control mice (data not shown). All three strains of PrP-170S mice were next tested for susceptibility to scrapie infection.
Fig. 1. Immunoblot detection of PrPsen from C57BL/10 and transgenic mice. 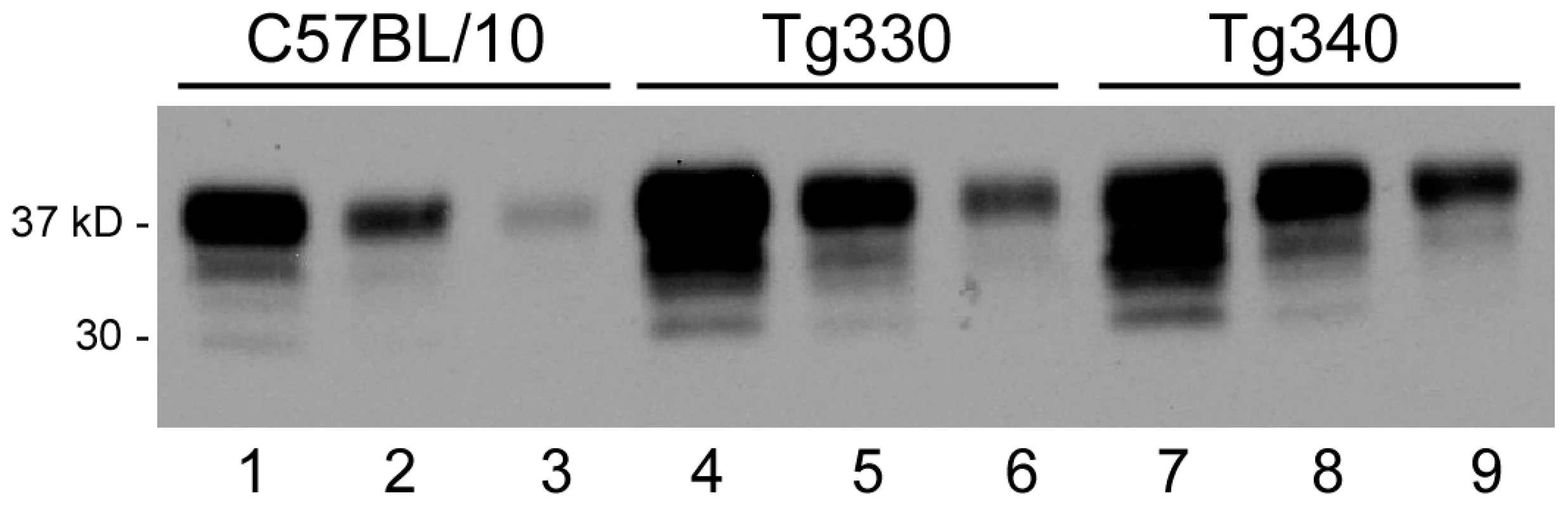
Blot was developed using anti-PrP monoclonal antibody D13 and enhanced chemiluminescence exposure for 1 minute. Lanes 1-3 show brain PrP-170N expression level in a C57BL/10 mouse, lanes 4-6 and lanes 7-9 show brain PrP-170S expression in Tg330 and Tg340 mice. For each mouse, three lanes beginning on the left were loaded with 0.5, 0.25 and 0.125 mg tissue equivalents, respectively. Comparison of these groups of samples suggested 2-3-fold greater PrP expression in the transgenic mice versus C57BL/10 mice. Several different individual transgenic mouse brains were also studied, and results confirmed the data presented here. Tg330 and Tg340 also expressed detectable levels of PrPsen in kidney, cecum, adrenal gland, heart, spleen and skeletal muscle (immunoblots not shown). Resistance to scrapie strains RML and 79A in PrP-170S transgenic mice
Four strains of scrapie were used to test the influence of the N170S polymorphism on susceptibility to TSE disease. In Tg330 and Tg340 mice, the 22L and ME7 scrapie strains produced 100% incidence of typical fatal prion disease with clinical signs similar to control C57BL/10 mice (Table 2). Shorter incubation periods were observed in the Tg330 and Tg340 mice than in C57BL/10 mice, probably due to higher PrPsen expression levels in the transgenic mice. The diagnosis of scrapie was confirmed by the detection of PrPres in brain by immunoblotting. Brain PrPres levels in transgenic mice were slightly less than those in control mice, possibly due to shorter incubation periods (Figure 2A). In Tg290 mice inoculation with scrapie strain 22L produced disease in 6 of 8 mice at an average incubation period of 615 dpi (data not shown). This lower incidence of disease and slower tempo appeared to be related to the low PrPsen expression in Tg290 mice, and this line was not studied further.
Fig. 2. Immunoblot analysis of PrPres in scrapie-infected mice. 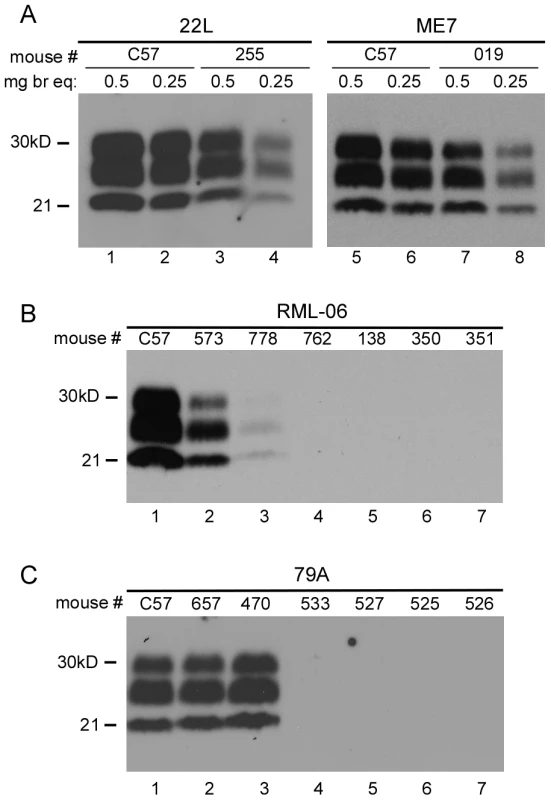
(A) Panel A shows immunoblots of brain homogenates tested using both 0.5mg and 0.25mg brain equivalents per lane. PrPres levels in clinical Tg330 mice (#255 and #019) were slightly lower than levels seen in clinical C57BL/10 mice. All C57BL/10 and Tg330 mice inoculated with 22L and ME7 scrapie were positive for PrPres by western blot at the time of clinical disease. In panels B and C, lanes 1 and 2 had 0.5 mg brain equivalents and other lanes had 1.0mg. (B) RML (stock 06) inoculations. Of fifteen transgenic mice tested, two (#573 and 778) were positive for PrPres at 451 and 518 days respectively. Four of the 13 PrPres-negative mice are also shown. RML (stock 81) inoculation of 14 mice resulted in none positive for PrPres by immunoblot (not shown). (C) 79A inoculations. Of fourteen transgenic mice tested, two mice (#657 and 470) were positive for PrPres at 439 and 545 dpi respectively. Four of the 12 PrPres-negative mice are also shown. Tab. 2. Susceptibility of transgenic and C57BL/10 mice to four strains of scrapie. 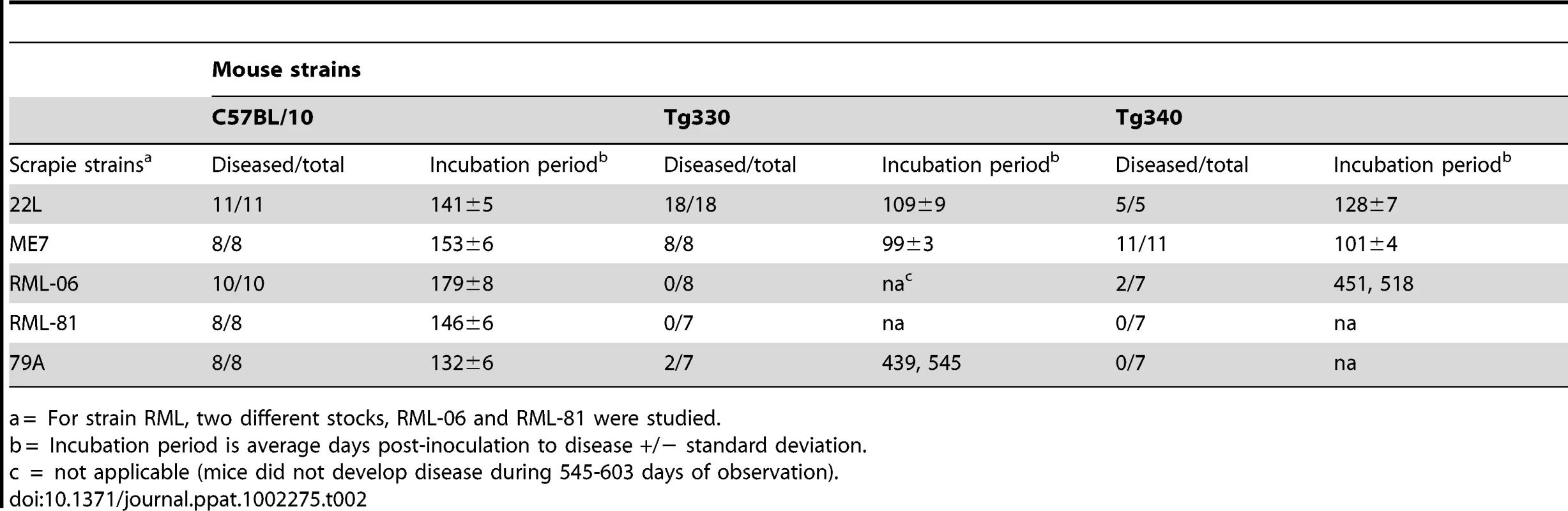
a = For strain RML, two different stocks, RML-06 and RML-81 were studied. Unexpected results were observed upon inoculation of RML and 79A scrapie strains. Whereas control C57BL/10 mice were uniformly susceptible to these two strains, transgenic Tg 330 and Tg340 mice were quite resistant. Only two mice inoculated with RML and two mice inoculated with 79A had clinical disease and PrPres detectable by immunoblot (Figure 2), and all four occurred at late times ranging from 439-545dpi (Table 2).
Brains were also examined microscopically for pathology and presence of PrPres. In Tg mice infected with strains ME7 or 22L, at the time of clinical disease vacuolation and PrPres deposition were widespread in many brain regions (Figure 3A,B,C) similar to non-transgenic control mice (not shown). In contrast, after infection with strains 79A or RML, Tg mice with clinical signs or PrPres detectable by immunoblot showed localized vacuolation and PrPres deposition (Figures 3E and 3H) mostly limited to the thalamus, hippocampus and pons. In addition, ten RML or 79A-infected Tg mice, who were negative for PrPres by immunoblot, had subclinical infection as shown by brain PrPres deposits mainly localized to the vestibular nuclei in the pons or to the anterodorsal region of the thalamus detected at 545-603dpi (Figure 3I,J,K,L). In these same areas gray matter vacuolation was either absent or minimal (Figure 3F). Detection of these subclinical mice provided evidence of a higher incidence of infection than was shown by standard clinical observation and analysis of brain PrPres by immunoblotting. Perhaps these mice should be considered preclinical as they might have developed clinical disease if allowed to survive for a longer time. These results suggested that infection of Tg PrP-170S mice by RML or 79A scrapie occurred at slow and reduced levels, and this appeared to account for the rare presence of clinical signs in this experiment.
Fig. 3. Histopathological analysis of brain tissue from transgenic mice inoculated with various scrapie strains. 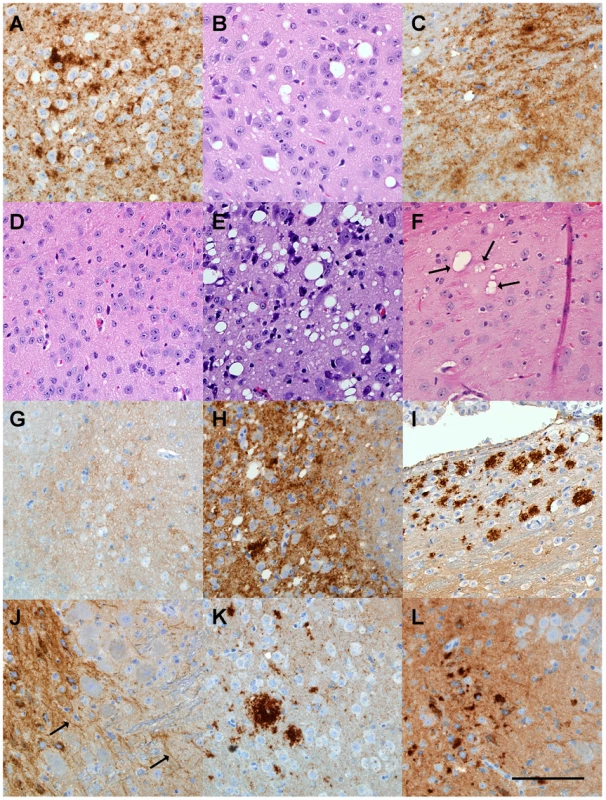
(A) (B) ME7-inoculated transgenic mouse with clinical signs at 98 dpi showing cerebral cortex with extensive PrPres deposition by D13 staining (A) and vacuolation by H&E staining (B). (C) 22L-inoculated transgenic mouse with clinical signs at 123dpi showing PrPres deposition in hypothalamus. (D) H&E stained area of pons from control uninoculated 465 day old mouse. (E) Vacuolation of pons in 79A-inoculated transgenic mouse with clinical disease at 545dpi. (F) Several pleomorphic and multiloculated vacuoles (arrows) seen by H&E staining in anterodorsal area of thalamus of RML-inoculated mouse at 603dpi. Vacuoles were not seen in other brain regions of this mouse. Mouse was negative for clinical signs and for PrPres by immunoblot. (G) D13 anti-PrP staining of pons from a 465 day old uninoculated control mouse. Note light brown PrPsen staining in background. (H) D13-stained pons from a 79A-inoculated mouse positive for clinical signs and PrPres by immunoblot at 545 dpi. Localized areas with similar stained aggregates were also seen in the thalamus, hypothalamus and hippocampus of this mouse. (I) D13-positive PrPres in anterodorsal area of thalamus of an RML-inoculated mouse negative for both PrPres by immunoblot and clinical signs at 603 dpi. Deposits were not detected in other brain regions. Light staining in lower part of panel is PrPsen which was also seen in uninoculated mice. (J) Positive D13 staining around cells and along linear structure of extracellular space or possibly axons (arrows) in pons from an RML-inoculated mouse negative for both PrPres by immunoblot and clinical signs at 545dpi. No other brain regions had detectable staining. (K) Stained PrPres aggregates in anterodorsal thalamus from 79A-inoculated mouse negative for clinical signs and for PrPres by immunoblot at 545dpi. Similar aggregates were also seen in the pons. (L) Stained PrPres aggregates in pons from a 79A-inoculated mouse negative for clinical signs and for PrPres by immunoblot at 545 dpi. PrPres was detected only in a small area of the pons. Diffuse staining in background is PrPsen. Scale bar is 100 µm and applies to all panels. In vitro generation of RML and 22L PrPres using protein misfolding cyclic amplification (PMCA)
Our in vivo experiments showed a dramatic difference in the tempo and levels of PrPres generation in brains of Tg330 and Tg340 mice after infection with scrapie strains RML and 79A compared to strains 22L and ME7. Possibly a delay in PrP conversion and subsequent accumulation of PrPres might account for our results. Therefore to test PrP conversion under in vitro conditions we used the PMCA cell-free system [47]. As a source of PrPsen, brain homogenates derived from either Tga20 mice expressing PrP-170N or Tg330 mice expressing PrP-170S were used, and these reactions were initiated by seeding with PrPres from PrP-170N mice infected with scrapie strains 22L or RML.
Surprisingly, RML PrPres seed generated PrPres conversion with both PrP-170N and PrP-170S brain homogenates. Quantification of PrPres produced in the PMCA revealed slightly greater amounts of product in the PrP-170N reactions, but these differences were not statistically significant (Figure 4A, 4B and 4C). However, the positive conversion of PrP-170S by RML PrPres indicated that there was not a basic inability of RML scrapie PrPres to convert PrP-170S. This was in contrast to the slow conversion in vivo requiring over 400 days when Tg330 and Tg340 mice were inoculated with RML scrapie (Figures 2 and 3).
Fig. 4. PMCA reactions comparing PrP-170N and PrP-170S as PrPsen substrates. 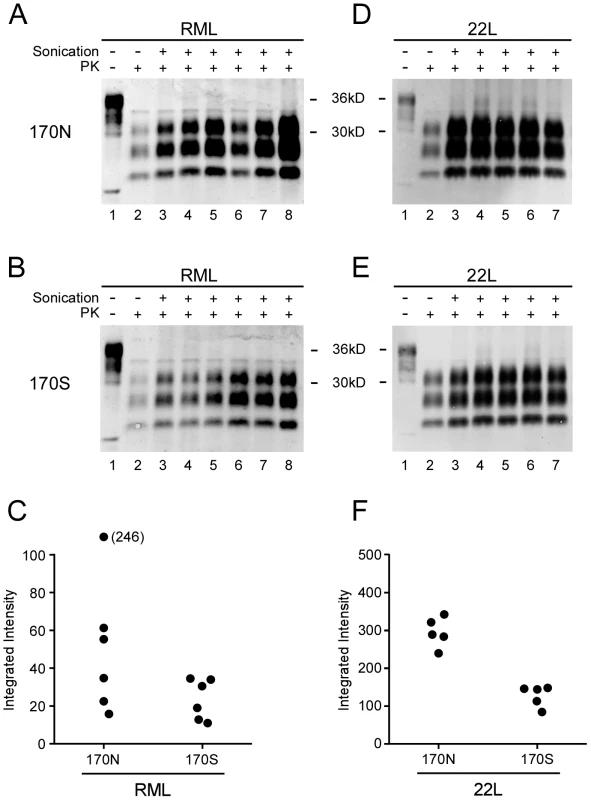
Panels A, B, D, and E show immunoblots of PMCA products visualized on the LiCOR Odyssey Scanner. RML (A and B) or 22L (D and E) scrapie-infected brain homogenates diluted 1∶400 were used to seed two different brain homogenate substrates: 170N from Tga20 mice (A and D) and 170S from Tg330 mice (B and E). For all gels, lane 1 contains unseeded Tga20 brain homogenate (5 µg brain equivalent of Tga20 brain homogenate), which was used as a control to show differences in detection sensitivity (gain setting) on the Odyssey scanner. For example, gain was increased for panels A and B compared to D and E because of the lower signal obtained with the RML seed in both 170N and 170S substrates. Other lanes contain 3.5 µl of PK-digested sonicated reaction product (lanes 3–8) or unsonicated freeze–control (lane 2). Amplification is evident by comparison of the unsonicated lanes with corresponding sonicated lanes. Results shown are representative of several independent experiments. Panels C and F show quantitation of PrPres as integrated intensity, i.e. the quantitative measure of protein detected by the scanner, from each lane of experiments shown in A, B, D, and E (using LyCor Odyssey scanner and software). Closed circles represent values for sonicated samples minus values for unsonicated freeze-control samples. Groups were compared by a 2-tailed nonparametric Mann Whitney test. For the 22L seed, the 170N and 170S substrates were significantly different (P<0.01), and for the RML seed, the 170N and 170S were not significantly different (P = 0.093). In similar PMCA reactions 22L PrPres seed also gave higher PrPres generation using PrP-170N compared to PrP-170S substrate. These differences were statistically different (Figure 4D, 4E, 4F), and might in part be due to the higher PrP expression in substrates from tga20 versus Tg330 mice. In summary, comparison of PrP-170N and PrP-170S mice by seeding with PrPres from RML or 22L gave no evidence that in vitro conversion of PrP-170S by RML PrPres was abnormal as detected by PMCA.
Infectivity studies in mice expressing both PrP-170S and PrP-170N
In numerous previous studies expression of two different PrP alleles from the same or different species has been shown to reduce the level of prion infection as well as the incidence and tempo of disease [22], [48], [49]. The resistance of the Tg330 and Tg340 mice to RML and 79A scrapie prompted us to test whether the presence of both PrP-170S and PrP-170N in the same mouse would result in lower disease incidence or increased incubation times.
To investigate this question, 22L or RML scrapie was inoculated into mice expressing two PrP alleles, one allele of the PrP-170S transgene and one allele of the mouse Prnp gene expressing PrP-170N. Mice expressing only one allele of PrP-170N (Prnp+/−) were inoculated as controls. With the 22L strain, mice expressing both 170S and 170N PrP had significantly faster incubation times (106–115dpi) than mice expressing only 170N (259 dpi) (Table 3). Therefore interference between these PrP variants did not seem to occur. The decrease in incubation period seen when both PrP alleles were expressed could be explained if the PrP-170S variant could contribute to a more rapid incubation time, as would be predicted by the susceptibility of the original Tg330 and Tg340 mice to 22L infection.
Tab. 3. Effect of coexpression of 170N and 170S PrPsen on prion disease after inoculation with scrapie strains 22L or RML. 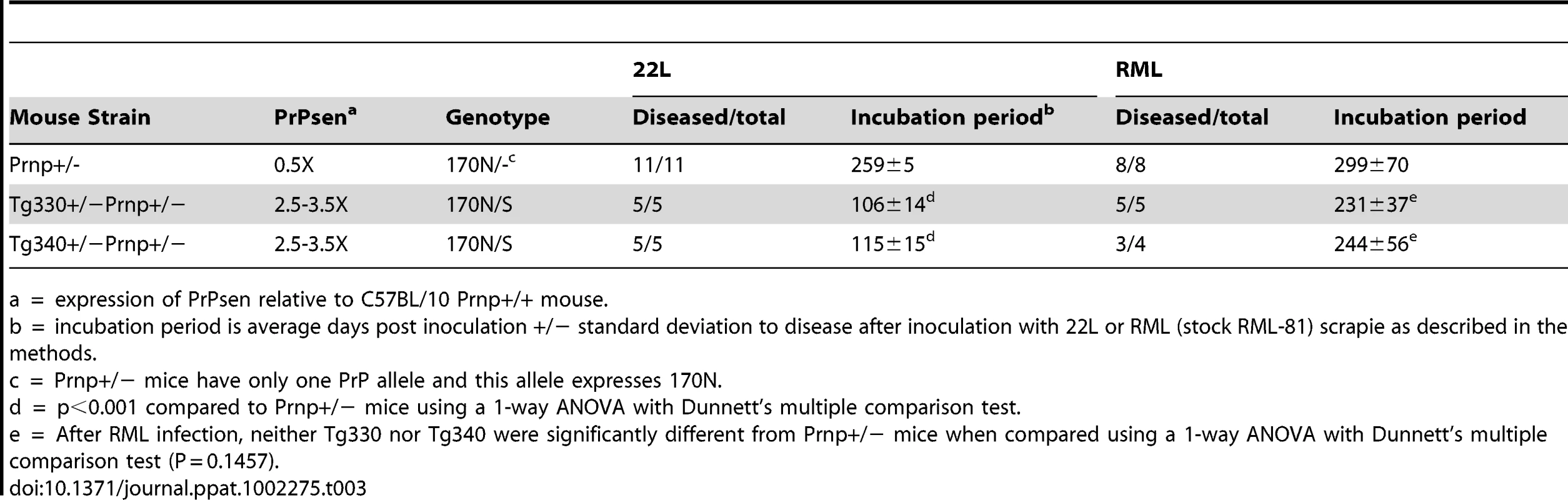
a = expression of PrPsen relative to C57BL/10 Prnp+/+ mouse. In the case of strain RML, the expression of both alleles was associated with a decrease in incubation time compared to expression of PrP-170N alone, but these differences were not statistically significant (Table 3). Therefore, the more highly expressed PrP-170S protein appeared to contribute very little towards accelerating the disease tempo in these mice, and also there was no evidence for interference. This outcome might be predicted from the very low susceptibility of the PrP-170S transgenic mice to RML scrapie infection (Table 2). In summary, the PrP-170S variant appeared to be “neutral” during the infection by RML scrapie, showing no interference with the PrP-170N in the co-expression experiment.
Discussion
In the present experiments the influence of a normal human prion protein gene allelic variation, N171S, on prion disease susceptibility was studied in a mouse system using transgenic mice expressing the mouse homolog, N170S. Susceptibility to prion disease induced by mouse scrapie strains, 22L and ME7, was identical in control mice expressing PrP-170N versus transgenic mice expressing PrP-170S. In contrast, after inoculation with scrapie strains, RML or 79A, mice expressing PrP-170S were markedly resistant compared to control mice expressing PrP-170N. Of the 43 transgenic mice inoculated with these two scrapie strains, disease occurred only after 439 days and this was seen in only 4 mice. An additional 10 mice had subclinical infection after 545–603 days, as shown by detection of PrPres by IHC, however the remaining 29 mice had no evidence of clinical signs or PrPres accumulation in brain after observation up to 603 days. These results demonstrated that mice expressing PrP-170S were highly resistant to infection by strains RML and 79A, but this resistance was strain-specific since there was no resistance to two other strains (ME7 and 22L).
One explanation for the scrapie strain-specific differences seen in our experiments may lie in the origins of the scrapie strains used (Figure 5). Strain ME7 was the result of a passage of natural scrapie in Suffolk sheep directly to mice and therefore was not related to other scrapie strains[50]. On the other hand, 22L, RML and 79A share a common origin in that they were all derived from the Moredun Institute's sheep scrapie brain pool 1(SSBP/1)[51]. However, there were major differences in the passage history of each of these 3 strains, and strains 79A and RML were more closely related to each other than to strain 22L [51], [52] (Figure 5). This may explain the similar resistance pattern of strains 79A and RML in PrP-170S mice.
Fig. 5. History of scrapie strains used. 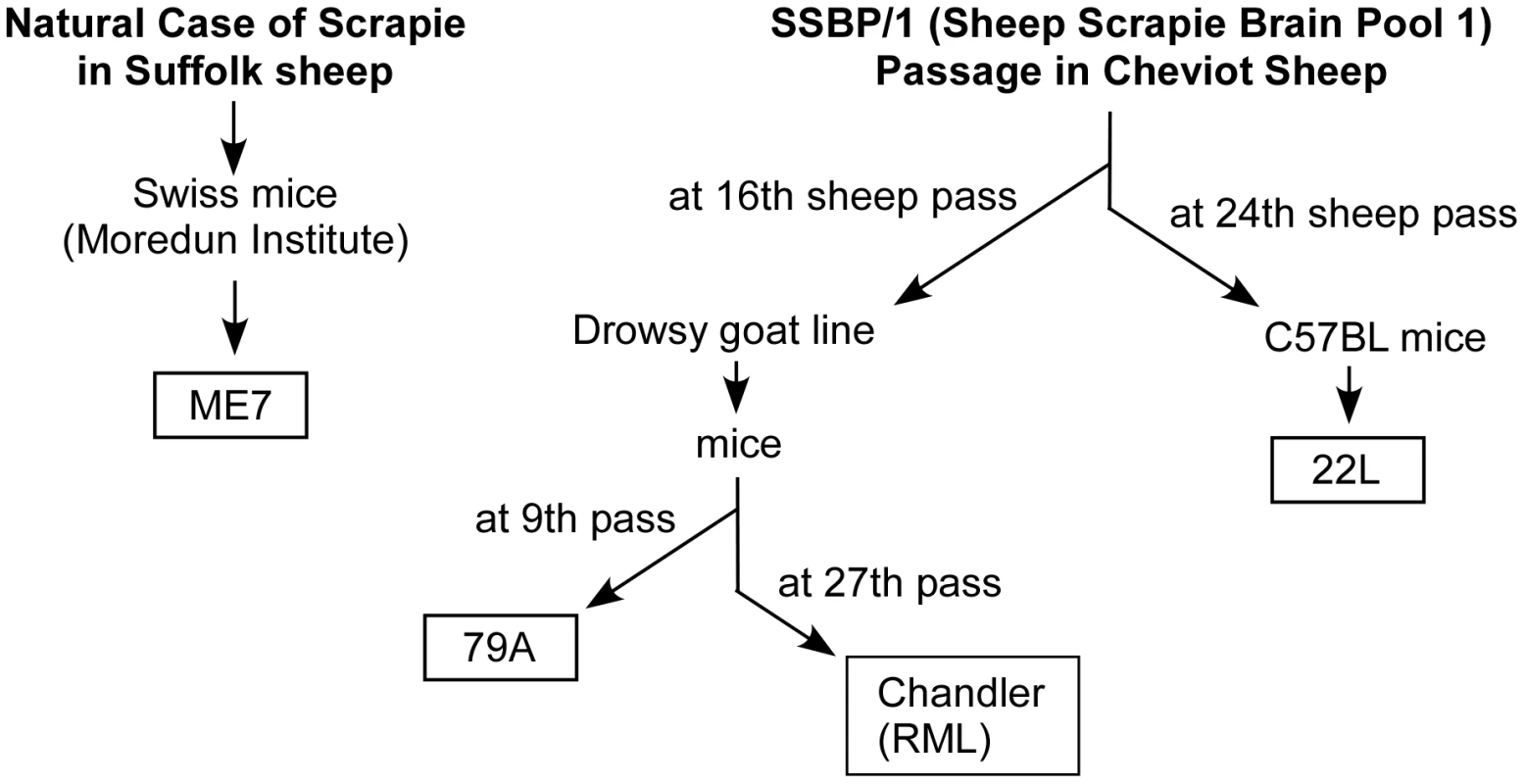
The derivations and passage history of strains ME7 [50], 79A [51], Chandler (RML) [52] and 22L [51] are shown. Chandler (RML) and 79A are both derived from the “drowsy goat” line [82], and therefore are likely to be more closely related to each other than to strains ME7 or 22L. Although the biochemical explanation for scrapie strains in general remains a mystery at this time, it has been hypothesized that PrPres from each strain adopts a slightly different conformation which is conferred on successive PrPsen molecules as they are converted to PrPres [53]. Accordingly, our current data might be explained by the conformational selection model of prion strains and species barriers [54], [55] which was first developed to explain prion strain differences involving the Sup35 protein in yeast [56], [57], [58]. This model suggests that only a subset of all possible PrPres conformations is compatible with any individual PrP primary structure. Thus incompatibility between the infecting prion strain and the host PrP would result in a transmission barrier [54]. For example, mouse PrP-170S might easily assume the conformations required by strains ME7 or 22L, but might be less adept at assuming the conformations associated with strains RML or 79A. In practice most mutations studied lead to altered incubation periods [30], [31], but when strong species barriers exist, either no transmission or low level subclinical cross-species transmission has been observed [59], [60], [61].
Another possible explanation for our results is that in vivo conversion of PrP-170S by RML or 79A strains might generate PrPres with a lower than usual stability. This might be due to either high dissociation of PrPres into smaller oligomers or increased susceptibility of PrPres to catabolic degradation. However, we were not able to detect any evidence for the presence of PrPres with increased susceptibility to proteinase K in PrP-170S transgenic mice infected by either RML or 79A scrapie strains (data not shown).
Effects of PrP amino acid variations on prion disease species and strain transmission barriers in some cases appear to correlate with differences in PrP conversion. For example, sheep homozygous for PrP with V136R154Q 171 or A136R154Q 171 show opposite patterns of susceptibility to scrapie strains SSBP1 and CH1641 [62], [63], and in vitro generation of PrPres in PMCA reactions agreed with the in vivo resistance observed [64]. However, when we tested PrP conversion in vitro using PMCA, both 22L and RML PrPres were able to seed the generation of PrPres derived from PrP-170S (Figure 4). This conversion of PrP-170S by RML scrapie was in contrast to the ineffective production of disease and slow and low PrPres generation in PrP-170S mice infected with RML scrapie. Biological and biochemical differences between conditions in brain tissue of live mice and PMCA test tube reactions might account for the discrepancies between our in vivo and in vitro results. Similar discrepancies between PrPres generation by PMCA in vitro and clinical susceptibility have been noted previously, and in some cases strong in vivo transmission barriers between species have been easily overcome by using PMCA with minor alterations in conditions [65]. Thus PrP conversion by PMCA would appear to be less selective than in vivo infection by TSE agents.
Interestingly, the N171S polymorphism in humans (homologous to the N170S change in our Tg330 and Tg340 mice) occurs near other PrP residues implicated in influencing PrP structure and folding as well as susceptibility to prion diseases in animals. For example, alterations in PrP folding in vitro have been noted after mutations homologous to human residues 168 [66] and 170 [67]. At the structural level, PrP mutation at human residue 170 (S170N) appears to create a stabilized loop structure located near residues 165-175 [68], and this change may influence susceptibility to CWD and other prion agents [37], [38]. However, others have reported no effect of S170N on species-specific PrP conversion in vitro in a mouse-hamster system [69], [70], [71]. In contrast, resistance of rabbits to prion disease appears to be associated with a serine at human PrP residue 174 [72]. Similarly, the sheep polymorphism at residue 171 (human residue 168) is important in the resistance of sheep to classical scrapie strains [6], [73], [74]. Possibly the N171S polymorphism examined in the present study might be able to modulate prion disease because of its location near the PrP loop structure and other nearby influential residues.
In humans, familial prion diseases have been associated with PrP mutations in the near vicinity of the PrP loop structure, i.e. Q160X [75], Y163X [76], D178N [77] and V180I [78]. The N171S polymorphism is not by itself associated with familial prion disease [26]. However, a previous study identified a family with an unusual psychiatric disorder associated with PrP N171S [79]. More recently, in an African-American family with unusual psychiatric signs and sleep abnormalities preceding onset of familial CJD, disease was linked to expression of a PrP molecule containing both PrP N171S and D178N mutations [80]. Interestingly this is the first family with African ancestry where the D178N mutation has been detected, as the 12 previously reported families were of European or Japanese descent [78], [80]. This family might be an example where the N171S polymorphism altered the clinical disease signs when expressed in combination with a known pathogenic PrP mutation. It remains unclear whether this effect is mediated by a direct influence of these mutations on PrP misfolding or whether indirect effects involving other non-PrP molecules might also play a role. Nevertheless, the fact that the N171S polymorphism is present in healthy populations of humans [81], suggests that N171S is likely non-pathogenic by itself and that there may even be a selective advantage for maintaining its presence in human genomes.
Zdroje
1. SejvarJJSchonbergerLBBelayED 2008 Transmissible spongiform encephalopathies. J Am Vet Med Assoc 233 1705 1712
2. MooreRATaubnerLMPriolaSA 2009 Prion protein misfolding and disease. Curr Opin Struct Biol 19 14 22
3. BuelerHAguzziASailerAGreinerRAAutenriedP 1993 Mice devoid of PrP are resistant to scrapie. Cell 73 1339 1347
4. GoldmannWHunterNSmithGFosterJHopeJ 1994 PrP genotype and agent effects in scrapie: change in allelic interaction with different isolates of agent in sheep, a natural host of scrapie. J Gen Virol 75 (Pt 5) 989 995
5. HunterN 1997 PrP genetics in sheep and the applications for scrapie and BSE. Trends Microbiol 5 331 334
6. LaplancheJLChatelainJWestawayDThomasSDussaucyM 1993 PrP polymorphisms associated with natural scrapie discovered by denaturing gradient gel electrophoresis. Genomics 15 30 37
7. BenestadSLArsacJNGoldmannWNoremarkM 2008 Atypical/Nor98 scrapie: properties of the agent, genetics, and epidemiology. Vet Res 39 19
8. BenestadSLSarradinPThuBSchonheitJTranulisMA 2003 Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet Rec 153 202 208
9. CarlsonGAKingsburyDTGoodmanPAColemanSMarshallST 1986 Linkage of prion protein and scrapie incubation time genes. Cell 46 503 511
10. WestawayDGoodmanPAMirendaCAMcKinleyMPCarlsonGA 1987 Distinct prion proteins in short and long scrapie incubation period mice. Cell 51 651 662
11. MooreRCHopeJMcBridePAMcConnellISelfridgeJ 1998 Mice with gene targetted prion protein alterations show that Prnp, Sinc and Prni are congruent. Nat Genet 18 118 125
12. BruceMEMcConnellIFraserHDickinsonAG 1991 The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: implications for the nature of the agent and host control of pathogenesis. J Gen Virol 72 Pt 3 595 603
13. DickinsonAGMeikleVM 1969 A comparison of some biological characteristics of the mouse-passaged scrapie agents, 22A and ME7. Genet Res 13 213 225
14. GoldfarbLGPetersenRBTabatonMBrownPLeBlancAC 1992 Fatal familial insomnia and familial Creutzfeldt-Jakob disease: disease phenotype determined by a DNA polymorphism. Science 258 806 808
15. MeadS 2006 Prion disease genetics. Eur J Hum Genet 14 273 281
16. BishopMTWillRGMansonJC 2010 Defining sporadic Creutzfeldt-Jakob disease strains and their transmission properties. Proc Natl Acad Sci U S A 107 12005 12010
17. HeadMWBunnTJBishopMTMcLoughlinVLowrieS 2004 Prion protein heterogeneity in sporadic but not variant Creutzfeldt-Jakob disease: UK cases 1991-2002. Ann Neurol 55 851 859
18. LeeHSBrownPCervenakovaLGarrutoRMAlpersMP 2001 Increased susceptibility to Kuru of carriers of the PRNP 129 methionine/methionine genotype. J Infect Dis 183 192 196
19. WadsworthJDAsanteEADesbruslaisMLinehanJMJoinerS 2004 Human prion protein with valine 129 prevents expression of variant CJD phenotype. Science 306 1793 1796
20. PalmerMSDrydenAJHughesJTCollingeJ 1991 Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature 352 340 342
21. ShibuyaSHiguchiJShinRWTateishiJKitamotoT 1998 Codon 219 Lys allele of PRNP is not found in sporadic Creutzfeldt-Jakob disease. Ann Neurol 43 826 828
22. HizumeMKobayashiATeruyaKOhashiHIronsideJW 2009 Human prion protein (PrP) 219K is converted to PrPSc but shows heterozygous inhibition in variant Creutzfeldt-Jakob disease infection. J Biol Chem 284 3603 3609
23. LukicABeckJJoinerSFearnleyJSturmanS 2010 Heterozygosity at polymorphic codon 219 in variant creutzfeldt-jakob disease. Arch Neurol 67 1021 1023
24. MeadSWhitfieldJPoulterMShahPUphillJ 2009 A novel protective prion protein variant that colocalizes with kuru exposure. N Engl J Med 361 2056 2065
25. MeadSStumpfMPWhitfieldJBeckJAPoulterM 2003 Balancing selection at the prion protein gene consistent with prehistoric kurulike epidemics. Science 300 640 643
26. BeckJAPoulterMCampbellTAAdamsonGUphillJB 2010 PRNP allelic series from 19 years of prion protein gene sequencing at the MRC Prion Unit. Hum Mutat 31 E1551 1563
27. YangWCookJRassbachBLemusADeArmondSJ 2009 A New Transgenic Mouse Model of Gerstmann-Straussler-Scheinker Syndrome Caused by the A117V Mutation of PRNP. J Neurosci 29 10072 10080
28. HsiaoKKScottMFosterDGrothDFDeArmondSJ 1990 Spontaneous neurodegeneration in transgenic mice with mutant prion protein. Science 250 1587 1590
29. NazorKEKuhnFSewardTGreenMZwaldD 2005 Immunodetection of disease-associated mutant PrP, which accelerates disease in GSS transgenic mice. Embo J 24 2472 2480
30. BarronRMThomsonVKingDShawJMeltonDW 2003 Transmission of murine scrapie to P101L transgenic mice. J Gen Virol 84 3165 3172
31. BarronRMThomsonVJamiesonEMeltonDWIronsideJ 2001 Changing a single amino acid in the N-terminus of murine PrP alters TSE incubation time across three species barriers. Embo J 20 5070 5078
32. MansonJCJamiesonEBaybuttHTuziNLBarronR 1999 A single amino acid alteration (101L) introduced into murine PrP dramatically alters incubation time of transmissible spongiform encephalopathy. Embo J 18 6855 6864
33. JacksonWSBorkowskiAWFaasHSteeleADKingOD 2009 Spontaneous generation of prion infectivity in fatal familial insomnia knockin mice. Neuron 63 438 450
34. MastrianniJACapellariSTellingGCHanDBosqueP 2001 Inherited prion disease caused by the V210I mutation: transmission to transgenic mice. Neurology 57 2198 2205
35. DossenaSImeriLMangieriMGarofoliAFerrariL 2008 Mutant prion protein expression causes motor and memory deficits and abnormal sleep patterns in a transgenic mouse model. Neuron 60 598 609
36. ChiesaRPiccardoPGhettiBHarrisDA 1998 Neurological illness in transgenic mice expressing a prion protein with an insertional mutation. Neuron 21 1339 1351
37. SigurdsonCJNilssonKPHornemannSHeikenwalderMMancoG 2009 De novo generation of a transmissible spongiform encephalopathy by mouse transgenesis. Proc Natl Acad Sci U S A 106 304 309
38. SigurdsonCJNilssonKPHornemannSMancoGFernandez-BorgesN 2010 A molecular switch controls interspecies prion disease transmission in mice. J Clin Invest 120 2590 2599
39. ChesebroBTrifiloMRaceRMeade-WhiteKTengC 2005 Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 308 1435 1439
40. Meade-WhiteKRaceBTrifiloMBossersAFavaraC 2007 Resistance to chronic wasting disease in transgenic mice expressing a naturally occurring allelic variant of deer prion protein. J Virol 81 4533 4539
41. FischerMRulickeTRaeberASailerAMoserM 1996 Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. Embo J 15 1255 1264
42. MansonJCClarkeARHooperMLAitchisonLMcConnellI 1994 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol Neurobiol 8 121 127
43. ShmerlingDHegyiIFischerMBlattlerTBrandnerS 1998 Expression of amino-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell 93 203 214
44. MatsunagaYPeretzDWilliamsonABurtonDMehlhornI 2001 Cryptic epitopes in N-terminally truncated prion protein are exposed in the full-length molecule: dependence of conformation on pH. Proteins 44 110 118
45. MeyerettCMichelBPulfordBSprakerTRNicholsTA 2008 In vitro strain adaptation of CWD prions by serial protein misfolding cyclic amplification. Virology 382 267 276
46. WestawayDDeArmondSJCayetano-CanlasJGrothDFosterD 1994 Degeneration of skeletal muscle, peripheral nerves, and the central nervous system in transgenic mice overexpressing wild-type prion proteins. Cell 76 117 129
47. SaaPCastillaJSotoC 2005 Cyclic amplification of protein misfolding and aggregation. Methods Mol Biol 299 53 65
48. PerrierVKanekoKSafarJVergaraJTremblayP 2002 Dominant-negative inhibition of prion replication in transgenic mice. Proc Natl Acad Sci U S A 99 13079 13084
49. PriolaSACaugheyBRaceREChesebroB 1994 Heterologous PrP molecules interfere with accumulation of protease-resistant PrP in scrapie-infected murine neuroblastoma cells. J Virol 68 4873 4878
50. ZlotnikIRennieJC 1963 Further observations on the experimental transmission of scrapie from sheep and goats to laboratory mice. J Comp Pathol 73 150 162
51. KimberlinRHWalkerCAFraserH 1989 The genomic identity of different strains of mouse scrapie is expressed in hamsters and preserved on reisolation in mice. J Gen Virol 70 Pt 8 2017 2025
52. KimberlinRHWalkerCA 1978 Evidence that the transmission of one source of scrapie agent to hamsters involves separation of agent strains from a mixture. J Gen Virol 39 487 496
53. CaugheyBBaronGSChesebroBJeffreyM 2009 Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions. Annu Rev Biochem 78 177 204
54. CollingeJClarkeAR 2007 A general model of prion strains and their pathogenicity. Science 318 930 936
55. WadsworthJDAsanteEACollingeJ 2010 Review: contribution of transgenic models to understanding human prion disease. Neuropathol Appl Neurobiol 36 576 597
56. TanakaMChienPNaberNCookeRWeissmanJS 2004 Conformational variations in an infectious protein determine prion strain differences. Nature 428 323 328
57. TanakaMChienPYonekuraKWeissmanJS 2005 Mechanism of cross-species prion transmission: an infectious conformation compatible with two highly divergent yeast prion proteins. Cell 121 49 62
58. TanakaMCollinsSRToyamaBHWeissmanJS 2006 The physical basis of how prion conformations determine strain phenotypes. Nature 442 585 589
59. RaceRChesebroB 1998 Scrapie infectivity found in resistant species. Nature 392 770
60. RaceRMeade-WhiteKRainesARaymondGJCaugheyB 2002 Subclinical scrapie infection in a resistant species: persistence, replication, and adaptation of infectivity during four passages. J Infect Dis 186 Suppl 2 S166 170
61. RaceRRainesARaymondGJCaugheyBChesebroB 2001 Long-term subclinical carrier state precedes scrapie replication and adaptation in a resistant species: analogies to bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease in humans. J Virol 75 10106 10112
62. HoustonEFHallidaySIJeffreyMGoldmannWHunterN 2002 New Zealand sheep with scrapie-susceptible PrP genotypes succumb to experimental challenge with a sheep-passaged scrapie isolate (SSBP/1). J Gen Virol 83 1247 1250
63. JeffreyMGonzalezLChongAFosterJGoldmannW 2006 Ovine infection with the agents of scrapie (CH1641 isolate) and bovine spongiform encephalopathy: immunochemical similarities can be resolved by immunohistochemistry. J Comp Pathol 134 17 29
64. ThorneLTerryLA 2008 In vitro amplification of PrPSc derived from the brain and blood of sheep infected with scrapie. J Gen Virol 89 3177 3184
65. CastillaJGonzalez-RomeroDSaaPMoralesRDe CastroJ 2008 Crossing the species barrier by PrP(Sc) replication in vitro generates unique infectious prions. Cell 134 757 768
66. RobinsonPJPinheiroTJ 2009 The unfolding of the prion protein sheds light on the mechanisms of prion susceptibility and species barrier. Biochemistry 48 8551 8558
67. GorfeAACaflischA 2007 Ser170 controls the conformational multiplicity of the loop 166-175 in prion proteins: implication for conversion and species barrier. Faseb J 21 3279 3287
68. ChristenBPerezDRHornemannSWuthrichK 2008 NMR structure of the bank vole prion protein at 20 degrees C contains a structured loop of residues 165-171. J Mol Biol 383 306 312
69. PriolaSAChesebroB 1995 A single hamster PrP amino acid blocks conversion to protease-resistant PrP in scrapie-infected mouse neuroblastoma cells. J Virol 69 7754 7758
70. KanekoKZulianelloLScottMCooperCMWallaceAC 1997 Evidence for protein X binding to a discontinuous epitope on the cellular prion protein during scrapie prion propagation. Proc Natl Acad Sci U S A 94 10069 10074
71. PriolaSAChabryJChanK 2001 Efficient conversion of normal prion protein (PrP) by abnormal hamster PrP is determined by homology at amino acid residue 155. J Virol 75 4673 4680
72. VorbergIGroschupMHPfaffEPriolaSA 2003 Multiple amino acid residues within the rabbit prion protein inhibit formation of its abnormal isoform. J Virol 77 2003 2009
73. GoldmannWHunterNFosterJDSalbaumJMBeyreutherK 1990 Two alleles of a neural protein gene linked to scrapie in sheep. Proc Natl Acad Sci U S A 87 2476 2480
74. O'RourkeKIHolyoakGRClarkWWMickelsonJRWangS 1997 PrP genotypes and experimental scrapie in orally inoculated Suffolk sheep in the United States. J Gen Virol 78 ) Pt 4 975 978
75. FinckhUMuller-ThomsenTMannUEggersCMarksteinerJ 2000 High frequency of mutations in four different disease genes in early-onset dementia. Ann N Y Acad Sci 920 100 106
76. ReveszTHoltonJLLashleyTPlantGFrangioneB 2009 Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol 118 115 130
77. GoldfarbLGBrownPHaltiaMCathalaFMcCombieWR 1992 Creutzfeldt-Jakob disease cosegregates with the codon 178Asn PRNP mutation in families of European origin. Ann Neurol 31 274 281
78. NozakiIHamaguchiTSanjoNNoguchi-ShinoharaMSakaiK 2010 Prospective 10-year surveillance of human prion diseases in Japan. Brain 133 3043 3057
79. SamaiaHBMariJJValladaHPMouraRPSimpsonAJ 1997 A prion-linked psychiatric disorder. Nature 390 241
80. ApplebyBSApplebyKKHallRCWallinMT 2010 D178N, 129Val and N171S, 129Val genotype in a family with Creutzfeldt-Jakob disease. Dement Geriatr Cogn Disord 30 424 431
81. SchatzlHMDa CostaMTaylorLCohenFEPrusinerSB 1995 Prion protein gene variation among primates. J Mol Biol 245 362 374
82. ChandlerRL 1961 Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet 1 1378 1379
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



