-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
CD36 is the major receptor mediating nonopsonic phagocytosis of Plasmodium falciparum-parasitized erythrocytes by macrophages. Its expression on macrophages is mainly controlled by the nuclear receptor PPARγ. Here, we demonstrate that inflammatory processes negatively regulate CD36 expression on human and murine macrophages, and hence decrease Plasmodium clearance directly favoring the worsening of malaria infection. This CD36 downregulation in inflammatory conditions is associated with a failure in the expression and activation of PPARγ. Interestingly, using siRNA mediating knock down of Nrf2 in macrophages or Nrf2 - and PPARγ-deficient macrophages, we establish that in inflammatory conditions, the Nrf2 transcription factor controls CD36 expression independently of PPARγ. In these conditions, Nrf2 activators, but not PPARγ ligands, enhance CD36 expression and CD36-mediated Plasmodium phagocytosis. These results were confirmed in human macrophages and in vivo where only Nrf2 activators improve the outcome of severe malaria. Collectively, this report highlights that the Nrf2 transcription factor could be an alternative target to PPARγ in the control of severe malaria through parasite clearance.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002254
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002254Summary
CD36 is the major receptor mediating nonopsonic phagocytosis of Plasmodium falciparum-parasitized erythrocytes by macrophages. Its expression on macrophages is mainly controlled by the nuclear receptor PPARγ. Here, we demonstrate that inflammatory processes negatively regulate CD36 expression on human and murine macrophages, and hence decrease Plasmodium clearance directly favoring the worsening of malaria infection. This CD36 downregulation in inflammatory conditions is associated with a failure in the expression and activation of PPARγ. Interestingly, using siRNA mediating knock down of Nrf2 in macrophages or Nrf2 - and PPARγ-deficient macrophages, we establish that in inflammatory conditions, the Nrf2 transcription factor controls CD36 expression independently of PPARγ. In these conditions, Nrf2 activators, but not PPARγ ligands, enhance CD36 expression and CD36-mediated Plasmodium phagocytosis. These results were confirmed in human macrophages and in vivo where only Nrf2 activators improve the outcome of severe malaria. Collectively, this report highlights that the Nrf2 transcription factor could be an alternative target to PPARγ in the control of severe malaria through parasite clearance.
Introduction
Mononuclear phagocytes represent the first line of innate immune defense against pathogens through mechanisms involving recognition by pattern-recognition receptors (PRRs) of highly structurally conserved microbial structures, known as pathogen-associated molecular patterns [1]. Among the PRRs family, the class B scavenger receptor CD36, initially known as a receptor for the uptake of oxidatively low density lipoprotein, also mediates the recognition and the elimination of apoptotic cells and bacteria [2], [3]. Additionally, the CD36 receptor specifically recognizes Plasmodium falciparum parasitized-erythrocytes (PfPEs), resulting in a CD36-dependent nonopsonic phagocytosis of PfPEs and a decrease in parasite-induced TNF-α secretion [4]. Consistently, CD36-deficient macrophages displayed a marked phagocytic defect for parasitized erythrocytes compared with wild-type macrophages [5]. Furthermore, a recent study demonstrates in vivo the importance of CD36 receptor expression on macrophages during malaria infection. Indeed, CD36−/− mice present a defect in parasite clearance [5].
CD36 expression is under the transcriptional control of a PPARγ nuclear receptor. As a consequence, PPARγ ligands, such as thiazolidinediones, or IL4 and IL13 Th2 cytokines, promote CD36 expression on macrophages [6]–[8]. Moreover, rosiglitazone and IL13 have been shown to promote in vitro an increase in CD36-mediated phagocytosis and a decrease in malaria parasite-induced TNF-α release both on murine macrophage and human monocytes [4], [8], [9]. More recently, rosiglitazone treatment has been shown in vivo to reduce parasitemia level in the Plasmodium chabaudi chabaudi AS murine experimental model through the CD36 pathway [9]. Pharmacological modulation of CD36 expression on macrophages might therefore contribute to enhance parasite elimination and limit host inflammatory deleterious response to malaria infection.
Nevertheless, much of the pathology associated with malaria infection is a result of excessive and uncontrolled production of proinflammatory markers and cytokines [10], [11]. In this acute malaria inflammatory context, we previously demonstrated that CD36 receptor expression was reduced on the surface of circulating monocytes from P. falciparum infected patients [12]. In line with this, Th1 cytokines, such as TNF-α and IFNγ decrease CD36 expression both on monocytes and macrophages [13], [14]. Interestingly, this CD36 downregulation was correlated with a marked reduction in PPARγ activation upon TNF-α stimulation [13]. Collectively, all these data suggest that inflammatory processes might negatively regulate PPARγ expression and activation in macrophages.
Surprisingly, PPARγ−/− macrophages did not present a totally abolished CD36 phenotype [8], [15]. This data suggests the existence of alternative pathways controlling CD36 expression on macrophages. In this study, we focused on NF-E2 related factor 2 (Nrf2), a transcription factor involved in the prevention of severe inflammatory diseases [16], that is activated in response to oxidative stress and electrophiles agents, such as sulforaphane or diethylmaleate. We previously demonstrated that the anti-TNFα antibody treatment increased CD36 expression on human monocytes through the enhancement of reactive oxygen species production independently of PPARγ [13]. Nrf2 was also shown to play an important role in the regulation of CD36 expression [17]–[19]. We therefore postulated that Nrf2 transcription factor might substitute PPARγ to promote CD36 expression and hence CD36-mediated phagocytosis of PfPEs during acute inflammatory processes.
In this study, we show in vitro on murine and human monocytes-derived macrophages (hMDMs) and in vivo in murine inflammatory-induced severe malaria model, that inflammatory processes downregulate CD36 expression and CD36-mediated Plasmodium clearance, exacerbating the development of severe malaria infection. In acute inflammatory conditions, PPARγ ligands were unable to promote CD36 expression and subsequently to restore the loss of CD36-mediated Plasmodium clearance. Interestingly, we demonstrate the existence of an alternative pathway controlling CD36 expression in inflammatory conditions independently of PPARγ both on murine and human inflammatory macrophages. We established in vitro and in vivo that the Nrf2 transcription factor is essential to promote CD36 expression and Plasmodium clearance and therefore control malaria infection. This report highlights that Nrf2 transcription factor could be a therapeutic target in the control of severe malaria infection.
Results
Inflammatory conditions decrease CD36 expression and CD36 mediated-Plasmodium PEs phagocytosis through the downregulation of PPARγ
To determine the effect of inflammation on the modulation of CD36 expression, murine peritoneal macrophages were treated during 24 h for the quantification of CD36 protein level and during 5 h for mRNA level with TNF-α, peptidoglycan (PGN), or were incubated in presence of P. falciparum culture supernatant (P.f. cs) to mimic a more physiological malaria inflammatory context. The analysis of CD36 protein level on cells was evaluated on a selected R1 region, in which 92,2% of the cells were F4/80 and CD36 double-positive (Fig. S1A). Fig. 1A shows that TNF-α, PGN and P.f. cs treatments significantly decreased CD36 protein level. Consistent with this data, CD36 mRNA level was also downregulated following TNF-α, PGN and P.f. cs treatments (Fig. 1B). To assess whether TLR signaling directly leads to reduced CD36 expression or if the effects mediated by PGN or P.f. cs are dependent on TNF-α production, we evaluated CD36 protein level in presence of an anti-TLR2 antibody or etanercept, a potent TNF-α inhibitor. We demonstrated that PGN and P.f. cs treatments downregulate CD36 expression through TLR2 (Fig. 1C). The use of etanercept, unequivocally prove that the effects mediated by TLR2 activators on macrophage CD36 expression are independent of TNF-α production (Fig. 1C).
Fig. 1. Inflammatory conditions decrease CD36 expression and CD36-mediated PfPEs phagocytosis through downregulation of PPARγ. 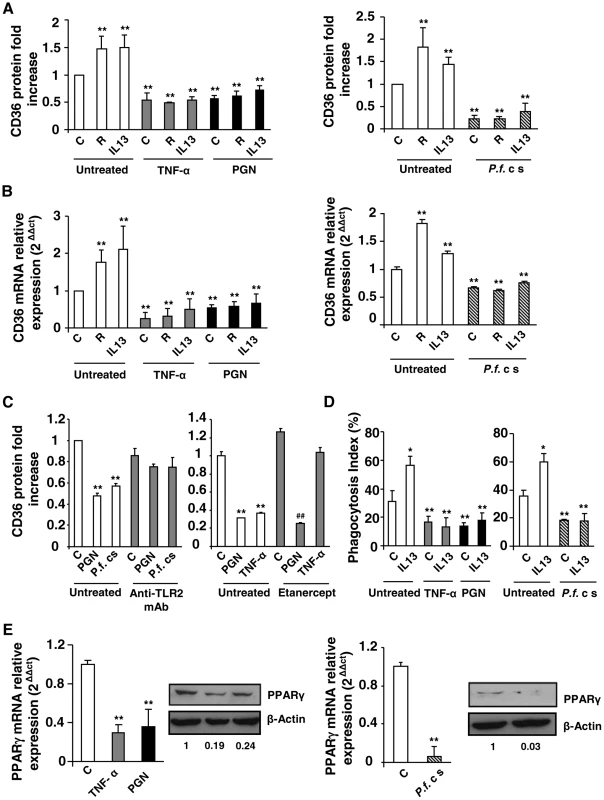
(A–B) CD36 protein and mRNA levels detected by flow cytometry or qRT-PCR on Swiss murine peritoneal macrophages firstly treated during 24 h with TNF-α (10 ng/mL), peptidoglycan (PGN) (1 µg/mL) or Plasmodium falciparum culture supernatant (P.f. c s) and incubated during 20 supplementary hours with rosiglitazone (R) (5 µM) or IL13 (50 ng/mL) for protein quantification or 5 supplementary hours for mRNA detection. Data are represented as a mean ± SD of three independent experiments. **p<0.01 compared with control cells (untreated). (C) CD36 protein level detected by flow cytometry on macrophages pre-incubated with a TL2 blocking monoclonal antibody (Anti-TLR2 mAb) (10 µg/mL) or Etanercept, a TNF-α inhibitor (10 µg/mL), and stimulated with TNF-α (10 ng/mL), PGN (1 µg/mL) or P.f. cs. Data are from a representative experiment performed in triplicate ± SD. **p<0.01 compared with control cells (untreated). ##p<0.01 compared with the respective control (Etanercept treated cells). (D) Phagocytosis index of P. falciparum unopsonized erythrocytes by Swiss murine macrophages stimulated as described in (A). Data are from a representative experiment performed in triplicate ± SD. The experiment was repeated three times. *p<0.05 and **p<0.01 compared with control cells (untreated). (E) PPARγ protein and mRNA levels in Swiss macrophages were determined by qRT-PCR after treatment of cells with TNF-α, PGN or P.f. cs. Data are represented as a mean ± SD of three independent experiments for mRNA quantification. **p<0.01 compared with control cells (C). We then evaluated whether rosiglitazone and IL13, known to promote CD36 expression via an activation of the nuclear receptor PPARγ, could reverse the downregulation of CD36 receptor induced by inflammatory conditions. Murine peritoneal macrophages were firstly treated for 24 h with TNF-α, peptidoglycan (PGN) or P.f. cs and were then incubated for an additional time with rosiglitazone or IL13. Fig. 1A and FACS profiles (Fig. S1B, S1C) showed that rosiglitazone and IL13 increased CD36 protein level in noninflammatory conditions (control). However, in an inflammatory context both rosiglitazone and IL13 treatments failed to increase the CD36 protein and mRNA levels (Fig. 1A, 1B, S1B, S1C). These data demonstrate that inflammation negatively regulates CD36 expression and reveals the failure of PPARγ ligands to promote CD36 expression on macrophages in these conditions.
To determine whether these inflammatory conditions were also associated with an impaired P. falciparum clearance, the ability of macrophages to phagocytose PfPEs was assessed. Fig. 1D showed a significantly reduced ability of macrophages to eliminate PfPEs in inflammatory conditions (TNF-α, PGN, P.f. cs). In addition, IL13 was not able to restore the decrease of CD36-mediated phagocytosis of PfPEs observed in inflammatory conditions (Fig. 1D). Therefore, the failure of PPARγ activators to promote CD36 expression and its PfPEs phagocytosis-associated function in inflammatory conditions strongly suggests that PPARγ is no longer able to exert its transcriptional activity on the CD36 promoter.
To investigate whether the failure in CD36-dependent PPARγ transcriptional activity was correlated with a lower level of PPARγ expression, we evaluated PPARγ protein and mRNA levels in an inflammatory context. Interestingly, TNF-α, PGN and P.f. cs significantly decreased PPARγ protein and mRNA expressions (Fig. 1E). All these observations indicate that the inability of PPARγ ligands to enhance CD36 expression and its antimalarial associated functions in inflammatory conditions was associated with a marked decrease of PPARγ expression.
Nrf2 activators promote CD36 expression and enhance CD36-mediated Plasmodium falciparum-PEs phagocytosis in inflammatory conditions
We hypothesized that the Nrf2 transcription factor, recently known to control the CD36 expression, could substitute the deficiency of PPARγ in acute inflammatory conditions to enhance the expression of the CD36 receptor. Murine macrophages were firstly treated over 24 h with TNF-α, PGN or P.f. cs and then incubated for an additional time with Nrf2 activators sulforaphane (SFN) or diethylmaleate (DEM). Fig. 2A and FACS profiles (Fig. S2A, S2B) demonstrate that sulforaphane (SFN) and diethylmaleate (DEM) increase the CD36 protein level both in noninflammatory (control) and in an inflammatory context (TNF-α, PGN or Pfcs). Consistent with this data, the same profiles of CD36 mRNA were obtained (Fig. 2B). Then, the study of CD36 protein level after the administration of Nrf2 or PPARγ activators before the onset of inflammation demonstrate that both Nrf2 and PPARγ ligands prevent the downregulation of CD36 expression. (Fig. S2C).
Fig. 2. Nrf2 activators promote CD36 expression and enhance CD36-mediated PfPEs phagocytosis in inflammatory conditions. 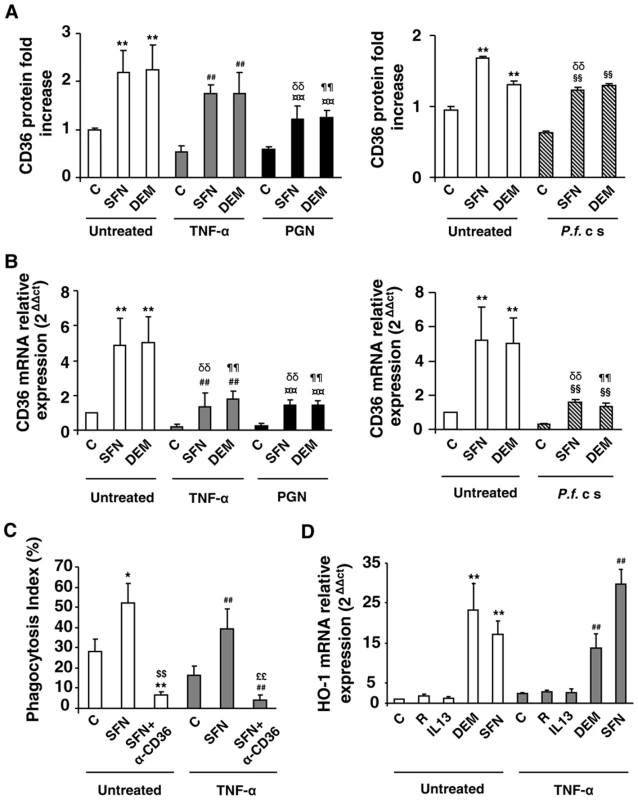
(A–B) CD36 protein and mRNA levels detected by flow cytometry or qRT-PCR on Swiss murine peritoneal macrophages firstly treated during 24 h with TNF-α (10 ng/mL), PGN (1 µg/mL) or P. falciparum culture (P.f. c s) and then incubated during 20 h with sulforaphane (SFN) (10 µM) or diethylmaleate (DEM) (100 µM) for protein quantification or 5 supplementary hours for mRNA detection. Data are represented as a mean ± SD of three independent experiments. **p<0.01 compared with the respective control (untreated); ##p<0.01 compared with the respective control (TNF-α treated cells); ¤¤p<0.01 compared with the respective control (PGN treated cells); §§p<0.01 compared with the respective control (P.f. cs treated cells); δδ p<0.01compared with control SFN-treated cells; ¶¶ p<0.01compared to control DEM-treated cells. (C) Phagocytosis index of Pf PEs by Swiss murine macrophages stimulated as described in (A). Data are represented as a mean ± SD of three independent experiments. *p<0.05 and **p<0.01 compared with the respective control (untreated); ##p<0.01 compared with the respective control (TNF-α treated cells); $$p<0.01 compared with the respective control (SFN treated cells); ££p<0.01 compared with the respective control (TNF+SFN treated cells) (D) HO-1 mRNA level in Swiss peritoneal macrophages determined by qRT-PCR after treatment of cells during 24 h with TNF-α (10 ng/mL) and then during 5 h with rosiglitazone (R) (5 µM), IL13 (50 ng/mL) or SFN (10 µM). Data are represented as a mean ± SD of three independent experiments. **p<0.01 compared with the respective control (untreated); ##p<0.01 compared with the respective control (TNF-α treated cells). In parallel, we showed that Nrf2 activators also up-regulate PPARγ mRNA levels in an Nrf2-dependent manner (Fig. S2E, S2F). However, no synergistic effect between PPARγ and Nrf2 activators has been observed in vitro on macrophage CD36 expression in inflammatory conditions (Fig. S2D).
We then explored whether this CD36 up-regulation by Nrf2 activators during inflammatory conditions could restore the decrease of CD36-mediated phagocytosis of PfPEs observed during inflammation. Fig. 2C reveals that an SFN treatment both in noninflammatory (control) and inflammatory conditions (TNF-α) enhanced the phagocytosis of PfPEs. These inductions were abolished by the use of a CD36 specific antibody (α-CD36), demonstrating that these phagocytic processes were dependent of the CD36 receptor.
To validate that the transcriptional activity of Nrf2 was still effective under inflammatory conditions, we next studied the modulation of HO-1 gene expression, a specific Nrf2 target gene [18]. Both in noninflammatory (control) and inflammatory (TNF-α) conditions, SFN and DEM significantly increased HO-1 mRNA expression, while PPARγ activators (IL13 or rosiglitazone) did not change HO-1 mRNA level (Fig. 2D), demonstrating that Nrf2 transcriptional activity was still efficient in inflammatory conditions. Altogether, these results suggest that the Nrf2 transcriptional factor could be involved in the regulation of CD36 expression and hence in CD36-mediated PfPEs phagocytosis in inflammatory conditions.
Nrf2 activators promote CD36 expression independently of PPARγ
To establish that the Nrf2 transcription factor may promote CD36 expression in absence of the PPARγ nuclear receptor, we performed experiments on the RAW 264.7 macrophage murine cell line which expresses a very low level of PPARγ, as demonstrated in Fig. 3A. Fig. 3B shows that PPARγ specific activators, IL13 and rosiglitazone, did not change the CD36 protein level, whereas the Nrf2 activators (SFN or DEM) strongly enhanced CD36 protein expression (Fig. 3B). These results show that in absence of PPARγ, Nrf2 activators are able to promote CD36 expression.
Fig. 3. Nrf2 activators promote CD36 expression independently of PPARγ. 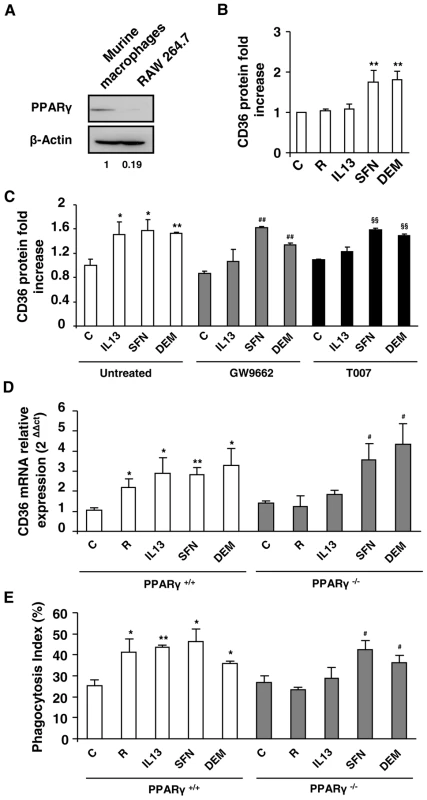
(A) PPARγ protein level on Swiss murine peritoneal macrophages and on RAW264.7 cells. The experiments were repeated three times. (B) CD36 protein level on murine RAW264.7 cells after treatment of cells with IL13 (50 ng/mL), rosiglitazone (R) (5 µM), sulforaphane (SFN) (10 µM) or diethylmaleate (DEM) (100 µM). Data are represented as a mean ± SD of three independent experiments. **p<0.01 compared with control cells. (C) CD36 protein level detected by flow cytometry on Swiss murine peritoneal macrophages firstly treated during 1 h with the PPARγ antagonists GW9662 (5 µM) and T007 (2 µM) and then incubated during 20 h with IL13, SFN or DEM. Data are represented as a mean ± SD of three independent experiments. **p<0.01 compared with the respective control (untreated); ##p<0.01 compared with the respective control (GW9662 treated cells); §§p<0.01 compared with the respective control (T007 treated cells). (D) CD36 mRNA level on PPARγ+/+ and PPARγ−/− C57BL/6 murine peritoneal macrophages after treatment of cells with rosiglitazone, IL13, SFN or DEM. Data are from a representative experiment performed in triplicate ± SD. The experiment was repeated three times. *p<0.05 and **p<0.01 compared with the respective control (PPARγ+/+); ##p<0.01 compared with the respective control (PPARγ−/−). (E) Phagocytosis index of P. falciparum unopsonized erythrocytes by murine PPARγ+/+ and PPARγ−/− C57BL/6 macrophages stimulated as described in (E). Data are from a representative experiment performed in triplicate ± SD. The experiment was repeated three times. *p<0.05 and **p<0.01 compared with the respective control (PPARγ+/+); #p<0.05 compared with the respective control (PPARγ−/−). To further confirm that PPARγ was not involved in the regulation of CD36 by Nrf2 activators, murine macrophages were incubated in the presence of GW9662 or T007, two specific irreversible antagonists of PPARγ. Fig. 3C shows that the macrophages treated by GW9662 or T007 failed to up-regulate CD36 expression after exposure to IL13. In contrast, GW9662 or T007 treatments did not affect CD36 over-expression observed with SFN or DEM treatments. Altogether these data prove that the induction of CD36 by Nrf2 activators is independent of PPARγ.
Finally, to unequivocally prove that Nrf2 activators could promote CD36 expression in absence of PPARγ, we studied CD36 mRNA expression in macrophages in which PPARγ had been selectively disrupted. As expected, the expression of CD36 was promoted by rosiglitazone, IL13, SFN or DEM treatments in macrophages from PPARγ+/+ mice. Interestingly, the CD36 over-expression following rosiglitazone or IL13 treatments failed in PPARγ deficient macrophages (PPARγ−/−), while SFN or DEM treatments enhanced CD36 expression in PPARγ−/− cells (Fig. 3D). In line, the PfPEs phagocytosis level by PPARγ−/− macrophages was only enhanced following SFN or DEM treatments and not by rosiglitazone or IL13 (Fig. 3E). Altogether these data establish that only Nrf2 activators contribute to the enhancement of CD36 expression and CD36 mediated-P. falciparum phagocytosis in absence of PPARγ.
Nrf2 activation is involved in CD36 induction during inflammatory conditions
To confirm the specific involvement of Nrf2 transcription factor in the regulation of CD36 expression under inflammatory conditions, TNF-α activated macrophages were transiently transfected with siRNA specifically targeting Nrf2. As predicted, the siRNA-mediated knock down of Nrf2 decreased HO-1 expression in TNF-α treated macrophages following SFN or DEM treatments (Fig. S3A, S3B). Interestingly, the increase of CD36 mRNA level both in TNF-α treated macrophages (Fig. 4A) and RAW cells (Fig. 4B) following SFN or DEM treatments was abolished after transfection with siRNA targeting Nrf2. These data strongly suggest that the up-regulation of CD36 in inflammatory context is mediated by Nrf2 transcription factor.
Fig. 4. Nrf2 transcription factor is involved in CD36 overexpression during inflammatory processes. 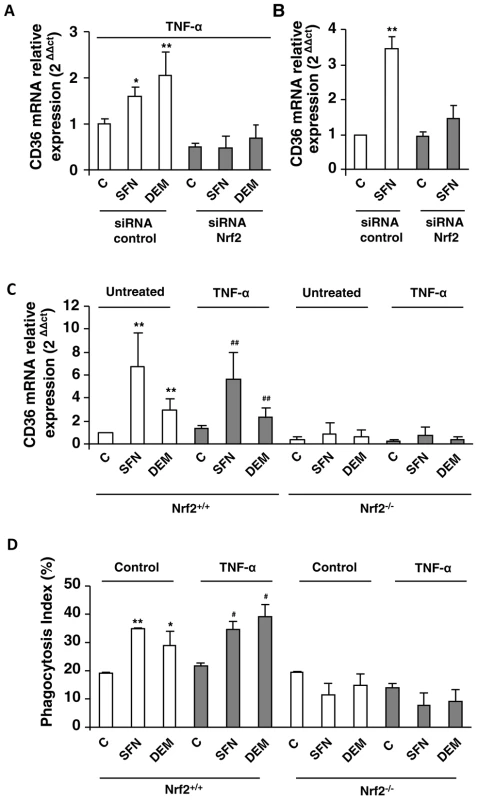
CD36 mRNA level on Swiss peritoneal macrophages treated during 24 h with TNF-α (10 ng/mL) (A) and on RAW264.7 cells (B) and transfected with siRNA targeting Nrf2 (siRNA Nrf2) or control siRNA (siRNA control) and stimulated with sulforaphane SFN (10 µM) or diethylmaleate (DEM) (100 µM). Data are represented as a mean ± SD of three independent experiments. *p<0.05 and **p<0.01 compared with the respective control (cells transfected with siRNA control), (C) CD36 mRNA level on Nrf2+/+ and Nrf2−/− C57BL/6 murine peritoneal macrophages after treatment during 24 h with TNF-α and then incubated during 5 h with SFN or DEM. Data are from a representative experiment performed in triplicate ± SD. The experiment was repeated three times **p<0.01 compared with the respective control (Nrf2+/+), ##p<0.01 compared with the respective control (Nrf2+/+ cells treated with TNF-α). (D) Phagocytosis index of PfPEs by murine Nrf2+/+ and Nrf2−/− C57BL/6 macrophages stimulated as described in (C). Data are from a representative experiment performed in triplicate ± SD. The experiment was repeated three times. *p<0.05 and **p<0.01 compared with the respective control (Nrf2+/+ control cells); #p<0.05 compared with the respective control (Nrf2+/+ cells treated with TNF-α). To consolidate our hypothesis, we studied CD36 and HO-1 mRNA levels in macrophages from Nrf2−/− mice. The induction of CD36 (Fig. 4C) and HO-1 (Fig. S3C) mRNA levels by SFN or DEM treatments in macrophages from Nrf2+/+ mice both in normal (control) and in inflammatory conditions (TNF-α) was not observed in macrophages from Nrf2−/− mice. Consistently, the levels of P. falciparum phagocytosis were not enhanced by SFN or DEM treatments in Nrf2−/− macrophages (Fig 4D). Altogether, these data clearly revealed that Nrf2 plays a crucial role in the activation of CD36 macrophage gene expression and in its phagocytosis-associated functions during acute inflammatory processes.
To evaluate the DNA-binding activity of Nrf2 transcription factor in inflammatory conditions, a DNA-binding ELISA-based assay using a specific Nrf2 antibody was performed. Fig. 5A demonstrates that Nrf2 was specifically activated by SFN treatment and bound to its ARE-binding sequence both in noninflammatory and in inflammatory (TNF-α) conditions. Fig. 5B reveals that the Nrf2 protein level was increased in the nucleus of SFN-treated cells both in noninflammatory and in inflammatory (TNF-α) conditions. Then, to evaluate this nuclear localization of Nrf2 following SFN treatment, confocal laser scanning microscopy analysis was performed. Nrf2 transcription factor was localized both in the nucleus and in the cytoplasm of control and TNF-α treated cells (Fig. 5C). Interestingly, Nrf2 was exclusively located in the nucleus following SFN treatment both in noninflammatory and in inflammatory (TNF-α) conditions. Altogether these results confirm that after SFN treatment Nrf2 translocates to the nucleus and exerts its transcriptional activity during inflammatory conditions.
Fig. 5. Nrf2 activation by sulforaphane occurs even under inflammatory conditions. 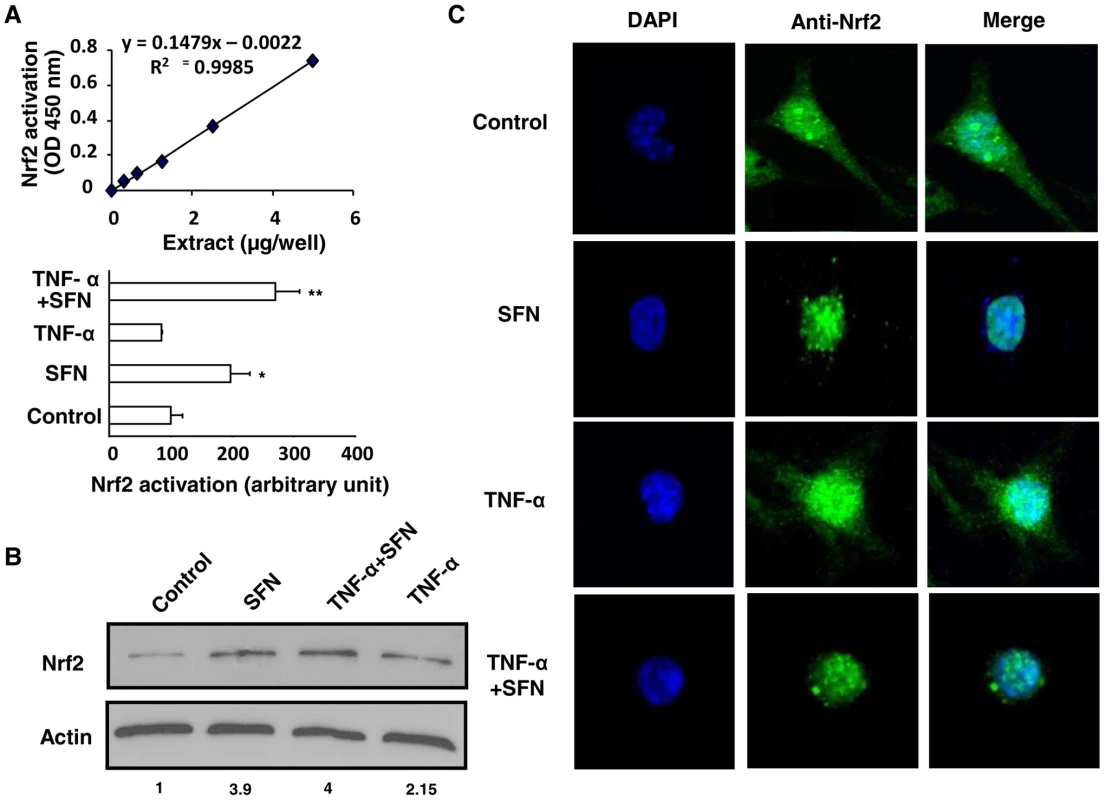
(A) Quantification of the ARE-nuclear binding of Nrf2 by DNA-binding ELISA TransAM kit after treatment of Swiss peritoneal macrophages with TNF-α during 24 h and then during 1 h with SFN. The data are expressed as relative arbitrary units. Data are from a representative experiment performed in triplicate ± SD. The experiment was repeated three times. *p<0.05 and **p<0.01 compared with control cells. (B) Nrf2 protein level in nuclei extracts of cells described in (A) (C) Confocal laser microscopy on Swiss macrophages described in (A). The blue color represents the nucleus; the green color represents Nrf2. Merged images of the blue and green colors are shown in the right-hand panels. The data are representative of three independent experiments. Nrf2 activators up-regulate CD36 and increase phagocytosis of P. falciparum-PEs on human monocytes-derived macrophages in inflammatory conditions
To extend our results to human monocytes-derived macrophages (hMDMs), we evaluated the CD36 protein level on inflammatory hMDMs following rosiglitazone, IL13, SFN or DEM treatments. Cells were gated on the R1 region, corresponding to the hMDMs population highly expressing CD36 (Fig. S4C). TNF-α, PGN and P.f. cs downregulated CD36 protein and mRNA levels on hMDMs (Fig. 6A, S4A, S4B). The increase of CD36 protein level in control hMDMs following treatments by rosiglitazone, IL13, SFN and DEM was only observed in TNF-α and PGN treated hMDMs after SFN or DEM treatments. Indeed, rosiglitazone and IL13 did not promote CD36 protein level in these hMDMs (Fig. 6A, S4D). These data confirm in humans that during inflammatory processes only Nrf2 activators were able to promote CD36 expression. The failure to promote CD36 expression on hMDMS via the PPARγ signaling pathway was associated with a marked reduction of PPARγ mRNA and protein levels (Fig. 6B) during acute inflammatory processes.
Fig. 6. Nrf2 activators promote CD36 and increase P.falciparum clearance by human MDMs in inflammatory conditions. 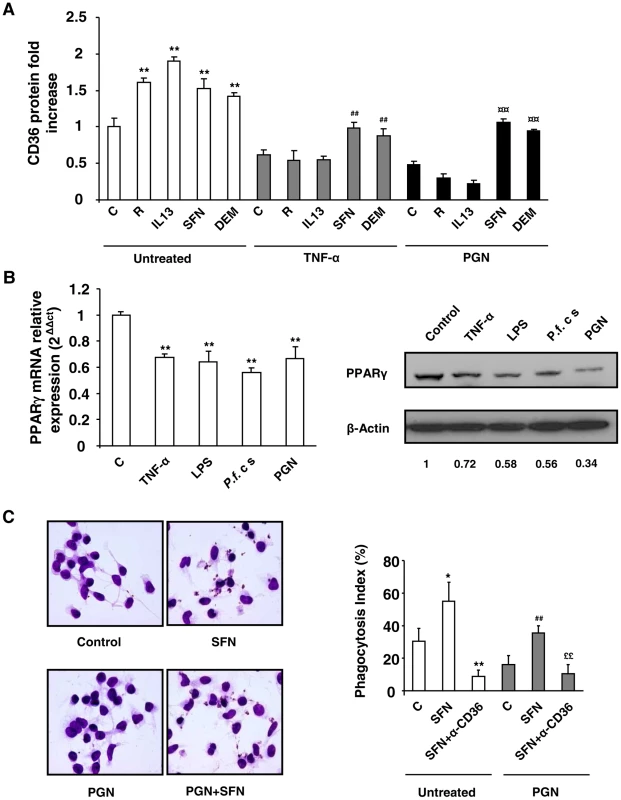
(A) CD36 protein level detected by flow cytometry on human-monocytes derived macrophages (hMDMs) firstly treated during 24 h with TNF-α (10 ng/mL) or PGN (1 µg/mL) and then incubated during 20 h with rosiglitazone (R) (5 µM), IL13 (50 ng/mL), SFN (10 µM) or DEM (100 µM). Data are represented as a mean ± SD of three independent experiments. **p<0.01 compared with the respective control (untreated); #p<0.05 compared with the respective control (TNF-α treated cells); ¤¤p<0.01 compared with the respective control (PGN treated cells). (B) PPARγ protein and mRNA levels on hMDMs treated with TNF-α, LPS, P.f. cs and PGN. PPARγ western blot is representative of three independent experiments and qPCR data are represented as a mean ± SD of three independent experiments. **p<0.01 compared with the respective control (untreated). (C) Phagocytosis index of P. falciparum unopsonized erythrocytes by hMDMs stimulated as described in (A). Data are represented as a mean ± SD of three independent experiments. **p<0.01 compared with the respective control (untreated); ##p<0.01 compared with the respective control (PGN treated cells); $$p<0.01 compared with the respective control (SFN treated cells); ££p<0.01 compared with the respective control (TNF+SFN treated cells). Finally, the ability of hMDMs to mediate the phagocytosis of PfPEs under acute inflammatory processes was determined. Fig. 6C reveals that SFN treatment promoted the phagocytosis of P. falciparum both in noninflammatory (control) and inflammatory (PGN) hMDMs. Phagocytosis both in normal and inflammatory contexts was significantly inhibited by a CD36 specific antibody, demonstrating that the induction of P. falciparum phagocytosis by SFN treated hMDMs was dependent of CD36 receptor (Fig. 6C). These data indicate that in humans during inflammatory processes, only Nrf2 activators up-regulate CD36 expression on hMDMs and P. falciparum phagocytosis-associated function.
Nrf2 but not PPARγ activators improve in vivo severe malaria outcome
Since we have demonstrated that Nrf2 regulates similarly CD36 receptor expression in inflammatory conditions both in human monocytes-derived macrophages and in Swiss murine peritoneal macrophages, we developed an induced-inflammatory severe malaria model in Swiss genetic background to validate that Nrf2 could be a therapeutic target in the prevention of severe malaria.
To establish this malaria model, Swiss mice were pre-treated with peptidoglycan (PGN), a TLR2 activator, and then infected with P. berghei. As expected, PGN-treated mice had a significantly lower survival rate compared with control mice (p = 0,043) which did not succumb in the early phase of infection, demonstrating that PGN-induced inflammatory processes strongly worsen the outcome of infection in the Swiss mice model (Fig. 7A). In addition, PGN treated mice presented significant higher parasitemia levels than control animals (Fig. 7B), but did not succumb from anemia (data not shown). Altogether, these results indicate that installed acute inflammatory processes before infection by P. berghei in mice clearly worsen the severity of the infection.
Fig. 7. Nrf2 activator sulforaphane improves the outcome of induced-severe malaria trough a reduction in parasite burden. 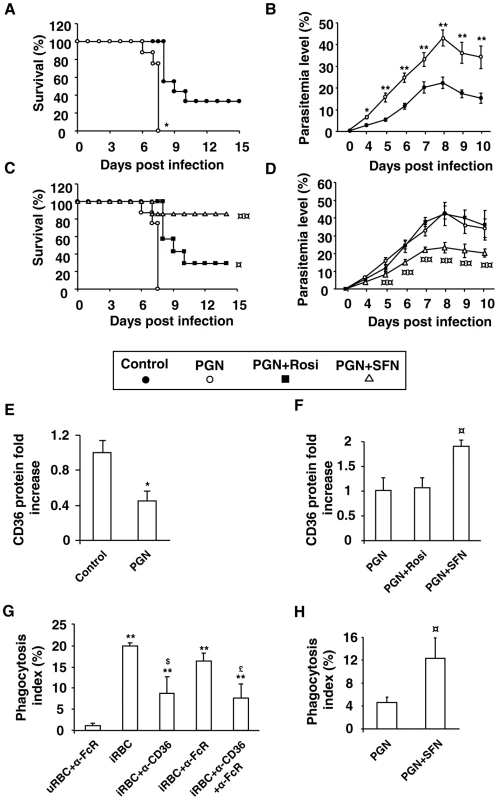
Swiss mice receiving PGN (200 µg/mouse) (subcutaneous route) two days before infection and d,L-sulforaphane (75 mg/kg) or rosiglitazone (3 mg/kg) (oral route) during five days postinfection were infected with 1×106 Plasmodium berghei parasites via intra-peritoneal injection. (A) and (C) Survival was assessed twice daily. **p<0.01 compared with untreated mice (control), ¤¤p<0.01 compared with PGN-treated mice. (B) and (D) Parasitemia levels were assessed daily. **p<0.01 compared with untreated macrophages (control); ¤p<0.05 and ¤¤p<0.01 compared with PGN-treated cells. (E) and (F) CD36 expression on macrophages was measured the day of infection by flow cytometry on three independent mice. *p<0.05 compared with untreated macrophages (control); ¤p<0.05 compared with PGN-treated cells. (G) Phagocytosis index of P. berghei unopsonized erythrocytes by Swiss murine macrophages incubated with FcR blocking antibodies (20 µg/mL) and CD36 blocking antibodies (10 µg/mL). Data are presented as mean ± SD of one experiment performed in triplicate. **p<0.01 compared to uRBC+α−FcR, $p<0.05 compared with iRBC+α−CD36, £p<0.05 compared with iRBC+α−FcR. (H) Phagocytosis index of P. berghei unopsonized erythrocytes by 3 days-infected macrophages from Swiss mice treated with PGN and SFN as described above. ¤p<0.05 compared with PGN-treated macrophages. To determine whether the PGN-induced increase in parasite burden observed in our severe malaria model was associated with macrophage CD36 downregulation, we evaluated the CD36 protein level on peritoneal macrophages. PGN treatment significantly decreased CD36 protein level (Fig. 7E). Altogether these data demonstrate that inflammatory processes induced by PGN treatment in infected mice downregulate the macrophage CD36 expression and contribute to worsen the severity of malaria infection in mice.
To assess whether Nrf2 activators or PPARγ ligands were able to improve the outcome of severe malaria, PGN-induced severe malaria mice were treated with SFN or rosiglitazone. The in vivo oral treatment of mice with SFN initiated the day of infection and followed 5 days post infection greatly increases the survival rates of mice (p = 0,0001) (Fig. 7C) and contributes to limit the parasite burden in the first days of infection (Fig. 7D). The rosiglitazone treatment increases the survival rates of PGN treated mice (p = 0,032) (Fig. 7C) and does not affect their blood parasitemia level (Fig. 7D). Interestingly, the CD36 protein level on macrophages only increased after in vivo SFN treatment (Fig. 7F), demonstrating that in an in vivo acute inflammatory context only Nrf2 activators and not PPARγ ligands are able to up-regulate CD36 expression. Finally, we demonstrate that macrophages from infected SFN-treated mice enhance P. berghei clearance (Fig. 7H). The use of anti-CD36 and anti-FcR antibodies demonstrates that P. berghei-PEs were internalized in a CD36 dependent manner (Fig. 7G). Altogether, these results strongly suggest that targeting Nrf2 in vivo contributes to improve the outcome against inflammatory-induced severe malaria in mice.
Discussion
Mononuclear phagocytes play an important role in the clearance of blood-stage malaria parasites resulting in an early control of parasite proliferation during acute infection [5], [20]. The importance of CD36 receptor expression on macrophages in malaria parasite clearance and in the regulation of parasite-induced inflammatory processes has been demonstrated both in vitro and in vivo on CD36−/− mice [5]. Recent data provide evidence that rosiglitazone PPARγ ligand can in vitro and in vivo improves the outcome of experimental malaria in mice, enhancing CD36-mediated PEs phagocytic processes and limiting parasite-induced inflammatory processes [9]. Nevertheless, much of the pathology associated with malaria infections is a result of excessive and uncontrolled production of proinflammatory markers [11], [21]. In this study, we demonstrated that inflammatory processes induced by TNF-α or TLR2 ligands downregulate CD36 expression on Swiss murine and human macrophages. These data are consistent with other studies, highlighting the deleterious effect of LPS, IFNγ or TNF-α treatment on CD36 expression [13], [14], [22], [23]. We also show that a similar CD36 downregulation was observed on murine and human macrophages following Pfcs treatment, which contains soluble factors released from infected erythrocytes rupture such as PfGPI anchors (glycophosphatidylinositol), described as TLR2 ligands [24]. In addition, we have previously demonstrated that circulating human monocytes presented a loss of CD36 expression during the acute phase of plasmodial infection [12]. In parallel, we demonstrated that PGN and P.f. cs treatments downregulate CD36 expression specifically through TLR2. Surprisingly, the TLR2 activators-mediated CD36 dowregulation is independent of TNF-α release. In addition, we demonstrate in this study that this decrease in CD36 expression on macrophages impairs CD36-mediated PfPEs phagocytosis. Consistent with these data, prostaglandin E2, a pro-inflammatory eicosanoid, was shown to downregulate CD36 expression on macrophage directly resulting in a reduced CD36-phagocytic ability leading to the development of endometriosis [25].
PPARγ ligands thiazolidinediones and IL4 or IL13, two Th2 cytokines known to activate PPARγ, were previously shown in vitro to promote CD36 expression and enhance CD36-mediated PEs phagocytosis [4], [6], [8], [9], [26]. Surprisingly, our current results revealed that PPARγ ligands and IL13 have no effect on the modulation of CD36 expression in inflammatory macrophages. Consistent with this, TNF-α was previously shown to downregulate CD36 expression on human monocytes involving a direct reduction in PPARγ activation [13]. In line with these results, it has been reported that inflammatory processes reduce PPARγ expression and activation, highlighting the deleterious effect of inflammatory processes on PPARγ [27]–[29]. Collectively, our data provides compelling evidence that inflammatory processes negatively regulate the expression of PPARγ in swiss murine macrophages and human monocyte-derived macrophages, resulting in a failure to trigger this pathway to promote CD36 expression and Plasmodium clearance. Interestingly, inflammatory stimuli did not negatively regulate the expression of PPARγ and hence CD36 expression in C57BL/6 murine macrophages, suggesting the importance of genetic background in their regulation. Therefore, it seems that the Swiss mice model better mimics the human model. Indeed, inflammatory processes inhibit PPARγ and CD36 in hMDMS, and monocytes from Plasmodium-infected patients exhibit a CD36 dowregulation [12].
In our study, we demonstrated that PPARγ−/− macrophages did not present a totally abolished CD36 phenotype, suggesting the existence of alternative pathways controlling CD36 expression on macrophages. We also previously demonstrated that the increase of the CD36 receptor following an anti-TNF-α antibody treatment occurred independently of PPARγ and involved radical oxygen species production (ROS) via NADPH oxidase activation [13]. The ROS are potent inducers of Nrf2 activation, a transcription factor involved in the prevention of severe inflammatory diseases [16]. Interestingly, this transcription factor was shown to play an important role in the regulation of CD36 expression on murine macrophages [18]. Thus, we postulated that Nrf2 transcription factor might substitute PPARγ to promote CD36 expression during acute inflammatory processes. Consistent with this hypothesis, we demonstrated that SFN or DEM, Nrf2 activators, upregulate CD36 expression both on murine and hMDMs during inflammatory processes. This CD36 overexpression leads to a higher elimination rate of PfPEs by macrophages. CD36 induction following SFN or DEM treatments was shown to be PPARγ independent and Nrf2 dependent. Altogether, these data unequivocally demonstrate that the Nrf2 transcription factor regulate CD36 expression in absence or in the presence of PPARγ nuclear receptor.
Serghides et al. have recently focused on the effects of PPARγ ligand treatment in an inflammatory cerebral malaria murine model. The rosiglitazone treatment improves the survival rate of mice with cerebral malaria. Nevertheless, time course administration of rosiglitazone revealed that the rosiglitazone treatment was a lot more efficient when administered one week before infection than one day after the onset of the disease [9]. In our induced-inflammatory severe malaria model, we demonstrated that rosiglitazone administered after the onset of infection, when the inflammatory processes are already triggered, has a very slight effect compared with SFN effect on mice survival rate and no effect on the modulation of parasitemia level. The positive in vivo effect of rosiglitazone on Swiss mice survival may be associated to a refractory population of macrophages to the inflammatory mediated CD36 downregulation. The differences observed between our study and the data published by the Kain's group are certainly related to the genetic background of the murine models used. Indeed, we did not in fact observe in vitro the downregulations of PPARγ and CD36 on macrophages from C57BL/6 mice in inflammatory conditions while PPARγ and CD36 were greatly impaired in swiss macrophages, as in hMDMs. These data suggest that the benefit of using rosiglitazone depends on the modulation of PPARγ expression which seems to be dependent on individual genetic background. The variability of rosiglitazone effectiveness in inflammatory processes has already been observed in other studies. Preventive administration of thiazolidinediones did in fact provide beneficial effects in murine models of ulcerative colitis but was less efficient when administered after the onset of the disease because PPARγ was shown to be downregulated by colitis-induced inflammatory processes [30]. Similar results were observed in human patients with colitis, which present a modest improvement in the outcome of the disease after rosiglitazone treatment [31]. This rosiglitazone inefficiency could be directly related to an impairment of PPARγ expression observed in patients with inflammatory colitis [32].
Interestingly, as opposed to the rosiglitazone treatment which slightly decreases severe malaria infection, we demonstrated here for the first time that the SFN, an Nrf2 activator, strongly contributes to control the elevation of parasite burden and hence consistently improves the outcome of severe inflammatory induced-malaria. This was correlated to an induction of CD36 expression on macrophage following SFN treatment and with a higher phagocytic capacity for PEs. In addition, SFN treatment did not alter major pro - and anti-inflammatory cytokines involved in malaria physiopathology (Fig. S5A, S5B), suggesting that SFN-induced protection is essentially due to the uptake of parasites through CD36 receptor. In addition, we showed that SFN treatment also reduces parasitemia in Swiss infected mice without prior PGN treatment (data not shown), indicating that the Nrf2 pathway is also important under more natural infection conditions.
Recently, a clinical study in humans described the effectiveness of rosiglitazone as an adjunct treatment to standard therapy for non severe malaria [33]. Nevertheless, although the effects of rosiglitazone in combination with conventional antimalarial treatments were effective in a non severe P. falciparum infection associated with moderated and controlled inflammatory processes, one question may subsist on the effectiveness of rosiglitazone when administered during severe malaria when acute inflammatory processes are engaged. We showed on human inflammatory MDMs that the rosiglitazone treatment was ineffective on CD36-mediated parasite clearance due to a downregulation of PPARγ. Interestingly, the Nrf2 activators treatments were able to promote CD36 expression on human MDMs and hence participate actively to the clearance of Plasmodium.
In conclusion, the present results provide direct evidence that inflammatory processes and particularly malaria parasite-induced inflammatory processes impair CD36 expression on Swiss murine as on human MDMs and CD36-mediated phagocytosis, favoring the worsening of malaria infection. This observation is correlated to a failure in PPARγ expression and activation. However, in inflammatory conditions, we demonstrated that Nrf2 pathway controls CD36 expression and improves the outcome of severe malaria independently of PPARγ. Thus, the results suggest the possibility that Nrf2 may be a therapeutic target for the control of severe malaria.
Materials and Methods
Ethics statement
This study was carried out in accordance with Approval No B3155503 and all animal experiments followed the guiding principles of animal care and use defined by the Conseil Scientifique du Centre de Formation et de Recherche Experimental Médico Chirurgical (CFREMC) with the rules of Decree 87–848 dated 10/19/1987 (modified by Decree 2001-464 and Decree 2001-131 relative to European Convention, EEC Directive 86/609 dated 24/11/1986). The experiments were approved by the ethics board of the Midi-Pyrénées ethic committee for animal experimentation (Experimentation permit number 31-067).
Monocytes were obtained from healthy blood donors (Etablissement Français du Sang, Toulouse). Written informed consents were obtained from the donors under EFS contract n°21/PVNT/TOU/UPS04/2010–0025. Following articles L1243-4 and R1243-61 of the French Public Health Code, the contract was approved by the French Ministry of Science and Technology (agreement n°AC 2009-921).
Animals
Female Swiss and C57BL/6 transgenic 6–12-week-old mice were used both for in vitro and in vivo experiments. The generation of the PPARγ−/− [8] and Nrf2−/− [34] mouse lines were previously described.
Mouse peritoneal macrophages isolation
Resident peritoneal cells were harvested by washing the peritoneal cavity with sterile NaCl 0,9%. Collected cells were centrifuged and the cell pellet was suspended in Macrophage-Serum Free Medium (M-SFM) (Gibco Invitrogen). Cells were allowed to adhere for 2 h at 37°C, 5% CO2. Non-adherent cells were then removed by washing with PBS.
Human monocytes isolation and differentiation into macrophages
Human peripheral blood mononuclear cells were isolated from the blood of healthy volunteers by a density gradient centrifugation method on Lymphoprep (Abcys). Monocytes were isolated by adherence to plastic for 2 h in M-SFM at 37°C, 5% CO2. Monocytes were cultured for 5–7 supplementary days in M-SFM containing 50 ng/mL M-CSF (eBiosciences) to allow for differentiation into human monocyte-derived macrophages.
P. falciparum culture and supernatant collection
The laboratory P. falciparum strain FcB1-Columbia presenting the phenotype Knobs+ at the erythrocyte surface was continuously cultured according to Trager and Jensen [35] with modifications [36]. To obtain P. falciparum culture supernatant (P.f. cs) for in vitro stimulations, parasites were highly synchronized by 5% D-Sorbitol treatment (Sigma). After schizont stage-infected erythrocyte rupture occurred, the culture medium was collected and centrifuged before being used for in vitro experiments. For phagocytosis assays, trophozoite-stage infected erythrocytes were washed and used at a PEs macrophage ratio of 20∶1. For P.berghei phagocytosis experiments, we used a PEs macrophage ratio of 10∶1.
Reagents
Murine peritoneal macrophages and hMDMs were stimulated by TNF-α (10 ng/mL) (eBiosciences), LPS (100 ng/mL) (Sigma Aldrich), Peptidoglycan (PGN) (1 µg/mL) (Sigma Aldrich), rosifoglitazone (5 µM) (Cayman Chemical), IL-13 (50 ng/mL) (eBiosciences), sulforaphane (SFN) (10 µM) (Sigma Aldrich), diethylmaleate (DEM) (100 µM) (Sigma Aldrich), and by 500 µL of P.f. cs. Macrophages were incubated with the specific inhibitors of PPARγ, GW9662 (5 µM) and T0070907 (2 µM) (Cayman Chemical), 30 min before the addition of PPARγ or Nrf2 activators.
Flow cytometry assay
Murine macrophage surface expression of CD36 was detected as previously described [37]. Briefly, murine macrophage CD36 expression was detected using a PE-monoclonal CD36 antibody (Santacruz, sc-13572) and compared with an irrelevant appropriate isotype control (Santa Cruz, sc-3600). hMDMs macrophages were stained with mouse IgM,κ CD36-APC antibody (BD Pharmingen). A mouse IgM,κ isotype-matched antibody conjugated to APC (BD Pharmingen) was used as a control. A minimum population of 3000 cells was analyzed for each data point. All analyses were performed on a FACScan using CellQuestPro software (Becton Dickinson).
Quantitative real-time PCR experiments
RNA and cDNA preparation were as described [38]. Quantitative RT-PCR was performed on a LightCycler 480 system using LightCycler 480 SYBR GREEN I MASTER (Roche Diagnostics). β-actin was used as the invariant control. The sequences of primers were listed in supplementary Table S1. The N-fold differential expression of mRNA gene samples was expressed as 2ΔΔCt.
Fluorescence imaging confocal microscopy
Cells were fixed with PBS containing 4% paraformaldehyde and were then incubated in a glycine solution (100 mM). After permeabilization and blocking, cells were then incubated overnight at 4°C with anti-Nrf2 antibody (Santacruz, sc-13032). Cells were then incubated with Alexa 488-conjugated anti rabbit antibody (Invitrogen) for 1 h at room temperature. Nuclei were stained with DAPI. Treated cells were covered with glass slips using Perma Fluor (Thermo Scientific). All microscopy imagery was performed with a LEICA SP2 laser scanning confocal microscope. For each condition, 40–50 cells were analyzed. The staining is representative of three independent experiments.
Phagocytosis assays
Phagocytosis assays were performed as already described [8]. The phagocytic index was calculated as the percentage of macrophages with PEs phagocytosed.
Transfection assays
Nrf2 siRNA (Santacruz sc-37049) and control siRNA (Santacruz sc-37007) were transfected into RAW cells or Swiss murine peritoneal macrophages using Lipofectamine 2000 reagent (Invitrogen) as described in the manufacturer's protocol.
Nuclear protein extraction and DNA-binding activity
Nuclear proteins were isolated with NE-PER kit (Thermo Scientific). Nrf2 TransAM ELISA-kit (Active Motif) was used to evaluate Nrf2 DNA-binding activity. The final A450 was read on a microplate reader (Wallac 1420 Victor2).
Western blot and antibodies
Westen Blot experiments were performed as described [38]. Rabbit polyclonal IgG anti-PPARγ (Santa Cruz Biotechnology, sc-7196), rabbit polyclonal IgG anti-Nrf2 (Santacruz Biotechnology, sc-13032) and goat polyclonal IgG anti-actin (Santacruz biotechnology, sc-1615) were used. Secondary polyclonal anti-goat or -rabbit IgG HRP coupled Abs were used (Cell Signaling).
Murine malaria models
In vivo assays were performed with Plasmodium berghei. Parasites were administered intraperitoneally (1.106 parasites/mouse) in Swiss female 12 week-old mice. Studies were performed on separate groups of 7 mice each infected and were repeated twice. Two days before infection, mice were treated subcutaneously with PGN (200 µg/mouse). Mice were then treated by oral route with d,L-sulforaphane (Santacruz Bio) (75 mg/kg), rosiglitazone (3 mg/kg) or with vehicule 5 days following the infection. The mice were then monitored daily for parasitemia levels with thin blood smears and survival was assessed twice daily.
Statistical analysis
For each in vitro experiments, the data were subjected to one-way analysis of variance followed by the means multiple comparison method of Bonferroni-Dunnet. p<0.05 was considered as the level of statistical significance. Survival studies were done using 7 mice per group and were repeated twice. Statistical significance was determined by a log-rank test.
Supporting Information
Zdroje
1. TrinchieriGSherA 2007 Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol 7 179 190
2. GreenbergMESunMZhangRFebbraioMSilversteinR 2006 Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med 203 2613 2625
3. BaranovaINKurlanderRBocharovAVVishnyakovaTGChenZ 2008 Role of human CD36 in bacterial recognition, phagocytosis, and pathogen-induced JNK-mediated signaling. J Immunol 181 7147 7156
4. SerghidesLKainKC 2001 Peroxisome proliferator-activated receptor gamma-retinoid X receptor agonists increase CD36-dependent phagocytosis of Plasmodium falciparum-parasitized erythrocytes and decrease malaria-induced TNF-alpha secretion by monocytes/macrophages. J Immunol 166 6742 6748
5. PatelSNLuZAyiKSerghidesLGowdaDC 2007 Disruption of CD36 impairs cytokine response to Plasmodium falciparum glycosylphosphatidylinositol and confers susceptibility to severe and fatal malaria in vivo. J Immunol 178 3954 3961
6. NagyLTontonozPAlvarezJGChenHEvansRM 1998 Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 93 229 240
7. HuangJTWelchJSRicoteMBinderCJWillsonTM 1999 Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature 400 378 382
8. BerryABalardPCosteAOlagnierDLaganeC 2007 IL-13 induces expression of CD36 in human monocytes through PPARgamma activation. Eur J Immunol 37 1642 1652
9. SerghidesLPatelSNAyiKLuZGowdaDC 2009 Rosiglitazone modulates the innate immune response to Plasmodium falciparum infection and improves outcome in experimental cerebral malaria. J Infect Dis 199 1536 1545
10. StevensonMMRileyEM 2004 Innate immunity to malaria. Nat Rev Immunol 4 169 180
11. LykeKEBurgesRCissokoYSangareLDaoM 2004 Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun 72 5630 5637
12. BerryACheneGBenoit-VicalFLepertJCBernadJ 2005 Ex vivo and in vitro impairment of CD36 expression and tumor necrosis factor-alpha production in human monocytes in response to Plasmodium falciparum-parasitized erythrocytes. J Parasitol 91 316 322
13. BoyerJFBalardPAuthierHFauconBBernadJ 2007 Tumor necrosis factor alpha and adalimumab differentially regulate CD36 expression in human monocytes. Arthritis Res Ther 9 R22
14. NakagawaTNozakiSNishidaMYakubJMTomiyamaY 1998 Oxidized LDL increases and interferon-gamma decreases expression of CD36 in human monocyte-derived macrophages. Arterioscler Thromb Vasc Biol 18 1350 1357
15. NecelaBMSuWThompsonEA 2008 Toll-like receptor 4 mediates cross-talk between peroxisome proliferator-activated receptor gamma and nuclear factor-kappaB in macrophages. Immunology 125 344 358
16. ThimmulappaRKLeeHRangasamyTReddySPYamamotoM 2006 Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest 116 984 995
17. D'ArchivioMScazzocchioBFilesiCVariRMaggiorellaMT 2008 Oxidised LDL up-regulate CD36 expression by the Nrf2 pathway in 3T3-L1 preadipocytes. FEBS Lett 582 2291 2298
18. IshiiTItohKRuizELeakeDSUnokiH 2004 Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res 94 609 616
19. SussanTEJunJThimmulappaRBedjaDAnteroM 2008 Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS One 3 e3791
20. SuZFortinAGrosPStevensonMM 2002 Opsonin-independent phagocytosis: an effector mechanism against acute blood-stage Plasmodium chabaudi AS infection. J Infect Dis 186 1321 1329
21. Artavanis-TsakonasKTongrenJERileyEM 2003 The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clin Exp Immunol 133 145 152
22. MemonRAFeingoldKRMoserAHFullerJGrunfeldC 1998 Regulation of fatty acid transport protein and fatty acid translocase mRNA levels by endotoxin and cytokines. Am J Physiol 274 E210 217
23. YesnerLMHuhHYPearceSFSilversteinRL 1996 Regulation of monocyte CD36 and thrombospondin-1 expression by soluble mediators. Arterioscler Thromb Vasc Biol 16 1019 1025
24. KrishnegowdaGHajjarAMZhuJDouglassEJUematsuS 2005 Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem 280 8606 8616
25. ChuangPCWuMHShojiYTsaiSJ 2009 Downregulation of CD36 results in reduced phagocytic ability of peritoneal macrophages of women with endometriosis. J Pathol 219 232 241
26. CosteADubourdeauMLinasMDCassaingSLepertJC 2003 PPARgamma promotes mannose receptor gene expression in murine macrophages and contributes to the induction of this receptor by IL-13. Immunity 19 329 339
27. AfifHBenderdourMMfuna-EndamLMartel-PelletierJPelletierJP 2007 Peroxisome proliferator-activated receptor gamma1 expression is diminished in human osteoarthritic cartilage and is downregulated by interleukin-1beta in articular chondrocytes. Arthritis Res Ther 9 R31
28. MracekTCannonBHoustekJ 2004 IL-1 and LPS but not IL-6 inhibit differentiation and downregulate PPAR gamma in brown adipocytes. Cytokine 26 9 15
29. ZhouMWuRDongWJacobAWangP 2008 Endotoxin downregulates peroxisome proliferator-activated receptor-gamma via the increase in TNF-alpha release. Am J Physiol Regul Integr Comp Physiol 294 R84 92
30. KatayamaKWadaKNakajimaAMizuguchiHHayakawaT 2003 A novel PPAR gamma gene therapy to control inflammation associated with inflammatory bowel disease in a murine model. Gastroenterology 124 1315 1324
31. LewisJDLichtensteinGRSteinRBDerenJJJudgeTA 2001 An open-label trial of the PPAR-gamma ligand rosiglitazone for active ulcerative colitis. Am J Gastroenterol 96 3323 3328
32. DubuquoyLJanssonEADeebSRakotobeSKarouiM 2003 Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology 124 1265 1276
33. BoggildAKKrudsoodSPatelSNSerghidesLTangpukdeeN 2009 Use of peroxisome proliferator-activated receptor gamma agonists as adjunctive treatment for Plasmodium falciparum malaria: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 49 841 849
34. ItohKChibaTTakahashiSIshiiTIgarashiK 1997 An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236 313 322
35. TragerWJensenJB 1976 Human malaria parasites in continuous culture. Science 193 673 675
36. Benoit-VicalFLelievreJBerryADeymierCDechy-CabaretO 2007 Trioxaquines are new antimalarial agents active on all erythrocytic forms, including gametocytes. Antimicrob Agents Chemother 51 1463 1472
37. GalèsAConducheABernadJLefevreLOlagnierD 2010 PPARgamma controls dectin-1 expression required for host antifungal defense against Candida albicans. PLoS Pathog 6 e1000714
38. LefèvreLGalèsAOlagnierDBernadJPerezL 2010 PPARgamma ligands switched high fat diet-induced macrophage M2b polarization toward M2a thereby improving intestinal Candida elimination. PLoS One 5 e12828
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



