-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
article has not abstract
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002208
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1002208Summary
article has not abstract
Fifteen years ago, in a series of elegant studies, Hackstadt and colleagues showed that the obligate intracellular bacteria Chlamydia trachomatis save on their lipid needs by incorporating sphingomyelins (SMs) made by their host [1]–[3]. Shortly after, Hatch and McClarty's teams reported that several eukaryotic glycerophospholipids are also trafficked from the host to the bacteria, which replace host-synthesized straight-chain fatty acids by their own branched-chain fatty acids [4]. Even cholesterol, a lipid rarely found in bacteria, was shown to accumulate in Chlamydia [5]. As a result of this intense exploitation of host lipids, the composition of the bacterial membrane is closer to that of a eukaryotic cell than to that of a prokaryote.
Throughout their developmental cycle, chlamydiae reside within a membrane-bounded compartment, the inclusion. How they acquire host lipids remains an open question. Possible mechanisms studied so far involve vesicular trafficking from host compartments, including vesicular traffic out of the Golgi apparatus, fusion with multivesicular body–derived vesicles, and engulfment of lipid droplets [6]. Two papers recently published in PLoS Pathogens show that non-vesicular traffic is also involved [7], [8].
SMs are synthesized by the transfer of phosphorylcholine to a ceramide in a reaction catalyzed by SM synthases. When added to infected cells, the fluorescent probe C6-NBD-ceramide traffics through the Golgi apparatus and rapidly accumulates in the bacteria, in the form of SM and not ceramide [1], indicating that the probe is converted to SM by host SM synthases before transport to the bacteria. However, understanding SM acquisition by the bacteria requires going one step back, into ceramide transport. Both studies show that CERT, a lipid transfer protein involved in non-vesicular endoplasmic reticulum (ER) to Golgi transport of ceramide [9], and VAPA and VAPB, its ER-resident partners, are enriched around the inclusion membrane [7], [8]. At the ultrastructural level, Derré and colleagues observed CERT on the inclusion membrane and VAPB on ER tubules in close proximity to the bacteria-filled compartment. By analogy with the ER-Golgi membrane contact sites described for non-vesicular transport of ceramide by CERT (Figure 1), Derré proposes that ER-inclusion membrane contact sites allow for direct transfer of ceramide to the inclusion. The group identified the inclusion protein of bacterial origin IncD as a specific binding partner for CERT [7].
Fig. 1. Direct transport of ceramide from the ER to C. trachomatis inclusion. 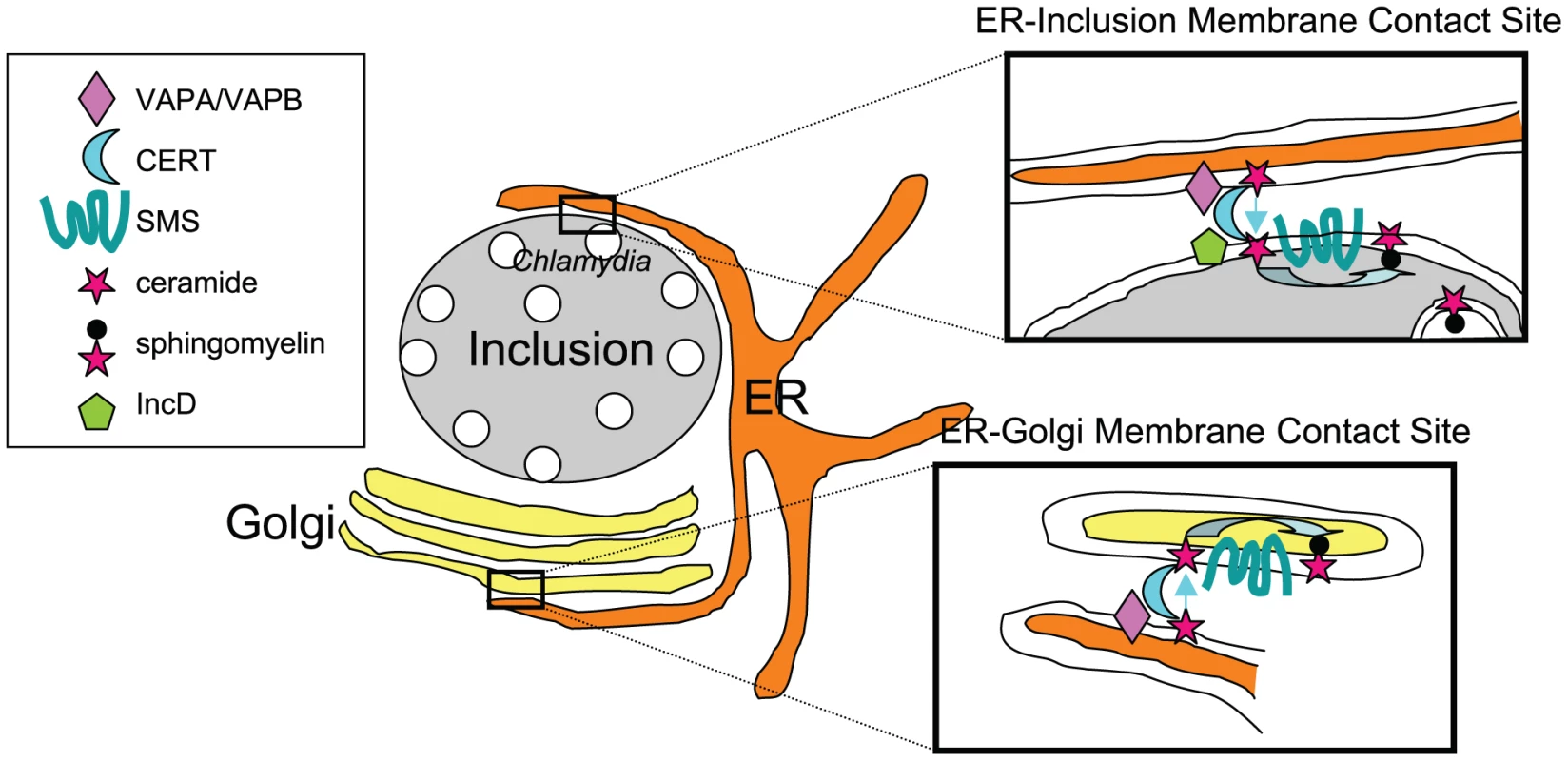
At ER-Golgi membrane contact sites the ceramide transfer protein CERT associates to the ER-resident proteins VAPA/VAPB and, via its PH domain, to PI4P at the trans-Golgi. Upon transfer by CERT, ceramide is converted to SM by a SM synthase (SMS). In Chlamydia-infected cells, ER-inclusion membrane contact sites involving VAPA/VAPB and CERT are observed. CERT interacts with the inclusion-anchored bacterial protein IncD through its PH domain but independently of PI4P. Upon transfer to the inclusion membrane, ceramide might be converted to SM by host SMS, which is enriched around the inclusion, and incorporated by the bacteria. Because the catalytic site of SMS is in the lumenal site of the Golgi apparatus, it would imply that the enzyme traffics to the inclusion membrane to convert ceramide, by a mechanism that remains to be determined. Other possibilities for SM acquisition by the bacteria are discussed [8]. Alternative routes for the transfer of SM and other lipids to the Chlamydia are discussed in an excellent recent review [6]. For what purpose does ceramide traffic to the inclusion? Bacteria accumulate an estimated 50% of SM synthesized from exogenously added ceramide [1]. Therefore, while a role for ceramide per se on the inclusion is not excluded, it is expected that its conversion to SM should strongly benefit the bacteria. There are SM synthase genes in humans identified as SMS1 and SMS2. SMS1 is found in the trans-Golgi apparatus while SMS2 is predominantly associated with the plasma membrane. Elwell and colleagues show that both enzymes are in close proximity to the inclusion membrane, and propose that the recruitment of CERT, its ER binding partner VAPA, and SM synthases establish an “on-site SM factory” [8].
Like CERT, other lipid transfer/binding proteins have been described as functional components of ER-Golgi membrane contact sites. Future studies need to address whether these non-vesicular lipid transfer systems are involved in the acquisition of phospholipids and sterols by the inclusion. Such a direct transfer could explain why transfer of host phospholipids to the bacteria was unaffected by brefeldin A, which inhibits Arf1-dependent vesicular transit through the Golgi apparatus. It is also consistent with the observation that traffic of glycoproteins out of the Golgi is not disrupted by infection [3].
In the presence of brefeldin A, SM acquisition by the bacteria is reduced and inclusions are smaller [1]. This observation and others argue for the existence of a vesicular-mediated access of SM to the inclusion [2]. The new data presented in PLoS Pathogens do not speak against this possibility, which can operate alongside non-vesicular traffic. In fact, Elwell et al. also provide data showing that depletion of the brefeldin A target GBF1 reproduces the effect of the drug on Chlamydia infection, implicating GBF1 in the vesicular route for SM acquisition [8].
Interestingly, while brefeldin A (or GBF1 depletion) only affect inclusion size, and not bacterial proliferation, CERT (or VAP) depletion have an impact on both [7], [8]. Does this mean that the non-vesicular process makes a greater contribution to total SM acquisition? This will be difficult to assess with the methods used currently. Due to rapid photobleaching, quantification of the accumulation of fluorescent probes by imaging is technically challenging. Incidentally, the two studies report divergent results on the effect of CERT depletion on SM accumulation in the inclusion assessed by this technique. In addition to not being quantitative with the probes currently available, imaging does not give information on the possible modifications of the fluorescent-tagged lipid in the host or in the bacteria [4]. But more than quantity, the site of SM acquisition at the inclusion might determine its fate. Elwell's data suggest that the two pathways contribute to different aspects of the developmental cycle of Chlamydia, CERT being important for bacterial replication and the vesicular pathway being essential for inclusion growth and stability [8]. This would imply that the SMs of different origin constitute two distinct pools, either because they consist of different molecules and/or because they do not diffuse freely on the inclusion and cannot be equally taken up by the bacteria.
Both studies were conducted on the human pathogen Chlamydia trachomatis. Surprisingly, Derré and colleagues report that the guinea pig strain Chlamydia caviae does not recruit CERT to its inclusion, consistent with the absence of IncD in this strain [7]. Is that so unexpected? We already know that these obligate intracellular bacteria have adopted multiple redundant mechanisms to enter cells and to intercept host intracellular traffic, to give only two well-studied examples [6]. It is hard to imagine that chlamydiae have not put the same energy into exploiting all possible steps of lipid transport in eukaryotic cells.
Zdroje
1. HackstadtTScidmoreMARockeyDD 1995 Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc Natl Acad Sci U S A 92 4877 4881
2. HackstadtTRockeyDDHeinzenRAScidmoreMA 1996 Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J 15 964 977
3. ScidmoreMAFischerERHackstadtT 1996 Sphingolipids and glycoproteins are differentially trafficked to the Chlamydia trachomatis inclusion. J Cell Biol 134 363 374
4. WylieJLHatchGMMcClartyG 1997 Host cell phospholipids are trafficked to and then modified by Chlamydia trachomatis. J Bacteriol 179 7233 7242
5. CarabeoRAMeadDJHackstadtT 2003 Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc Natl Acad Sci U S A 100 6771 6776
6. ScidmoreMA 2011 Recent advances in Chlamydia subversion of host cytoskeletal and membrane trafficking pathways. Microbes Infect 13 527 535
7. DerréISwissRAgaisseH 2011 The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites. PLoS Pathog 7 6 e1002092 doi:10.1371/journal.ppat.1002092
8. ElwellCAJiangSKimJHLeeAWittmannT 2011 Chlamydia trachomatis co-opts GBF1 and CERT to acquire host sphingomyelin for distinct roles during intracellular development. PLoS Pathog 7 7 e1002198 doi:10.1371/journal.ppat.1002198
9. HanadaKKumagaiKTomishigeNYamajiT 2009 CERT-mediated trafficking of ceramide. Biochim Biophys Acta 1791 684 691
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



