-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPhosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
Tuberculosis (TB) treatment is hampered by the long duration of antibiotic therapy required to achieve cure. This indolent response has been partly attributed to the ability of subpopulations of less metabolically active Mycobacterium tuberculosis (Mtb) to withstand killing by current anti-TB drugs. We have used immune modulation with a phosphodiesterase-4 (PDE4) inhibitor, CC-3052, that reduces tumor necrosis factor alpha (TNF-α) production by increasing intracellular cAMP in macrophages, to examine the crosstalk between host and pathogen in rabbits with pulmonary TB during treatment with isoniazid (INH). Based on DNA microarray, changes in host gene expression during CC-3052 treatment of Mtb infected rabbits support a link between PDE4 inhibition and specific down-regulation of the innate immune response. The overall pattern of host gene expression in the lungs of infected rabbits treated with CC-3052, compared to untreated rabbits, was similar to that described in vitro in resting Mtb infected macrophages, suggesting suboptimal macrophage activation. These alterations in host immunity were associated with corresponding down-regulation of a number of Mtb genes that have been associated with a metabolic shift towards dormancy. Moreover, treatment with CC-3052 and INH resulted in reduced expression of those genes associated with the bacterial response to INH. Importantly, CC-3052 treatment of infected rabbits was associated with reduced ability of Mtb to withstand INH killing, shown by improved bacillary clearance, from the lungs of co-treated animals compared to rabbits treated with INH alone. The results of our study suggest that changes in Mtb gene expression, in response to changes in the host immune response, can alter the responsiveness of the bacteria to antimicrobial agents. These findings provide a basis for exploring the potential use of adjunctive immune modulation with PDE4 inhibitors to enhance the efficacy of existing anti-TB treatment.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002262
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002262Summary
Tuberculosis (TB) treatment is hampered by the long duration of antibiotic therapy required to achieve cure. This indolent response has been partly attributed to the ability of subpopulations of less metabolically active Mycobacterium tuberculosis (Mtb) to withstand killing by current anti-TB drugs. We have used immune modulation with a phosphodiesterase-4 (PDE4) inhibitor, CC-3052, that reduces tumor necrosis factor alpha (TNF-α) production by increasing intracellular cAMP in macrophages, to examine the crosstalk between host and pathogen in rabbits with pulmonary TB during treatment with isoniazid (INH). Based on DNA microarray, changes in host gene expression during CC-3052 treatment of Mtb infected rabbits support a link between PDE4 inhibition and specific down-regulation of the innate immune response. The overall pattern of host gene expression in the lungs of infected rabbits treated with CC-3052, compared to untreated rabbits, was similar to that described in vitro in resting Mtb infected macrophages, suggesting suboptimal macrophage activation. These alterations in host immunity were associated with corresponding down-regulation of a number of Mtb genes that have been associated with a metabolic shift towards dormancy. Moreover, treatment with CC-3052 and INH resulted in reduced expression of those genes associated with the bacterial response to INH. Importantly, CC-3052 treatment of infected rabbits was associated with reduced ability of Mtb to withstand INH killing, shown by improved bacillary clearance, from the lungs of co-treated animals compared to rabbits treated with INH alone. The results of our study suggest that changes in Mtb gene expression, in response to changes in the host immune response, can alter the responsiveness of the bacteria to antimicrobial agents. These findings provide a basis for exploring the potential use of adjunctive immune modulation with PDE4 inhibitors to enhance the efficacy of existing anti-TB treatment.
Introduction
Despite effective chemotherapy available for over 50 years, and development of a control strategy of directly observed therapy short-course (DOTS), tuberculosis (TB) remains the leading cause of adult mortality attributable to a single infectious disease [1]. M. tuberculosis (Mtb), the causative agent of TB, is an intracellular pathogen that is well adapted to survive in host phagocytes within lung granulomas in humans and experimentally infected animals [2]–[4]. The outcome of Mtb infection is largely determined by a delicate balance between the host immune response and bacterial evasion and/or subversion of this response, resulting in successful control of the infection or manifestations of active disease of different severity [5], [6]. In the presence of an optimal host immune response, growth of the infecting Mtb is controlled efficiently and the bacilli often cannot be detected in infected tissues by the conventional colony-forming unit (CFU) assay. However, during growth arrest, not all the infecting bacilli are necessarily killed. Rather, they can adapt to survive in a viable latent state, serving as a reservoir for potential reactivation TB in the host, when immunity weakens [7]–[10]. Following Mtb infection of a host with a suboptimal immune response, the bacilli, internalized into the phagosome of macrophages and dendritic cells, replicate and grow [11], [12]. Within the phagosome, Mtb must resist the bactericidal molecules of the host cell, including abundant reactive oxygen (ROS) and nitrogen species (RNS), hydrolytic enzymes and an acidic pH [13]–[15]. The invading bacteria counteract this hostile host environment by dampening the process of phagosome maturation, inhibiting lysosome-phagosome fusion and limiting acidification, and shifting from a reliance on high oxygen and a predominantly carbohydrate carbon source for growth. Several mycobacterial genes involved in the bacterial response to phagosomal stresses, such as hypoxia, starvation, iron depletion, acid shock and alternate carbon metabolism, have been reported to contribute to the metabolic adaptation of the bacteria to intracellular survival and growth [16].
Activation of the immune response following Mtb infection affects the expression of many host genes that are involved in the production of cytokines, chemokines, surface receptors, and molecules associated with intracellular signaling [6], [17]. These early changes affect subsequent cellular events, including extravasation of leukocytes from the circulation, migration of immune cells to affected tissues and lymphoid organs, and proliferation of effector cells of the innate and adaptive immune response, in an orchestrated response to fight the infection [18]–[20]. An association between the level of macrophage activation and Mtb intracellular survival has been reported previously [12], [21]. In particular, RNS produced by activated macrophages inhibit the growth of Mtb and have been implicated as an environmental cue directing the physiologic shift of the bacilli towards a state of dormancy [10], [22], [23]. Thus, both the host cells and the bacilli alter their gene expression during Mtb infection. However, the specific nature and the interdependence of these events, and the links between the host-pathogen crosstalk and the outcome of Mtb infection, are not fully understood.
Tumor necrosis factor-alpha (TNF-α), produced by activated macrophages and other cells of the immune system, is required for the protective host response against Mtb infection. This inflammatory cytokine renders the macrophages more capable of controlling the growth of or killing intracellular Mtb [24]–[26]. In addition, TNF-α plays a vital role in coordinating and driving the host inflammatory response [27]. While complete inhibition of TNF-α production results in exacerbation or reactivation of TB in animals and humans, excessive TNF-α levels can lead to severe inflammation and damage to host cells and tissues [28], [29]. Since intracellular cyclic AMP (cAMP) regulates TNF-α production, the total cellular TNF-α level and the resulting tissue inflammation are determined in part by the level of this molecule [30]. Consequently, agents that increase cellular cAMP levels have anti-inflammatory properties [31]–[33]. Consistent with these findings, inhibitors of PDE4 (PDE4i) that increase cellular cAMP levels have been shown to reduce TNF-α production and dampen inflammation [34]–[36].
Pharmacologic inhibition of TNF-α production has recently been considered as a therapeutic modality in inflammatory diseases [37]–[39]. Inhibition of TNF-α production by thalidomide treatment in vivo and in vitro has been reported earlier [40], [41]. Additional studies have shown that thalidomide treatment, in combination with antibiotics, reduces TNF-α production in patients with pulmonary TB and can improve treatment outcome [42]. To avoid the side effects of thalidomide, analogs of the drug have been synthesized and screened for their safety and TNF-α inhibitory capacity [43], [44]. One class of synthetic compounds acts as effective PDE4i, which reduce TNF-α production by increasing intracellular cAMP levels. These PDE4i are non-emetic, non-teratogenic, and several of them have been found to be well tolerated by humans in Phase I and II clinical studies [45]. One of these PDE4i, used in the present study, CC-3052, is water soluble, more stable in human plasma and ∼200-fold more potent in reducing TNF-α production, compared to the parent drug [46]. In peripheral blood mononuclear cells (PBMC), the effect of CC-3052 on TNF-α inhibition is dose-dependent [46]. Importantly, at pharmacologically active doses, CC-3052 affects mainly monocyte and macrophage TNF-α production, i.e. the innate immune response, and does not have a significant effect on T cell activation, suggesting a highly cell-specific mechanism of action [47]–[49].
Isoniazid (INH) is one of the first-line antibiotics used in TB treatment. Though INH efficiently kills actively growing Mtb, dormant and non-replicating bacilli are killed only poorly [50], [51]. This observation has been reported in humans, mouse and guinea pig models of experimental pulmonary TB, as well as in vitro, using broth culture and Mtb infected macrophages [52], [53]. Exposure to INH results in the expression of a number of Mtb genes involved in multiple stress and/or toxic responses [54]–[56]. In addition, a specific group of INH-responsive Mtb genes, involved in fatty acid metabolism and cell wall biosynthesis, have been reported to be differentially expressed between actively growing versus dormant bacilli, during acute and chronic mouse infection, respectively [57]. These findings highlight the close links between the regulation of Mtb gene expression, bacterial metabolism and the response of the bacilli to INH treatment.
As mentioned above, activation of macrophages by Mtb infection creates a more hostile intracellular environment for the bacilli to survive and grow [18]–[20]. It has been postulated that robust macrophage activation drives a sub-population of infecting organisms into a metabolic state that is recalcitrant to antibiotic killing [10], [22], [23]. Consequently, we hypothesize that dampening macrophage activation will alleviate the stress on the intracellular bacilli, thereby creating a more permissive environment for bacterial metabolism, resulting in improved efficacy of antibiotic killing. To test this hypothesis, using a rabbit model of pulmonary Mtb infection, we examined the impact of CC-3052 treatment on the interactions between the host and pathogen, as revealed by the changes in the expression of host and pathogen genes. We used the rabbit model because, upon Mtb infection, rabbits develop progressive pulmonary cavitary TB that, in contrast to the mouse, is similar to the pathologic process seen in humans. We determined which immune response genes in the infected rabbit were specifically affected by treatment with CC-3052. In addition, we evaluated corresponding changes in the expression profile of bacterial genes, with particular emphasis on the stress and INH response genes. Finally, we examined the impact of CC-3052 treatment on bacillary load in the lungs of Mtb infected rabbits with and without INH co-treatment. The results of our study provide data to support the idea that combining anti-TB drugs with an adjunctive immune modulator may enhance the efficacy of current TB therapy regimens and shorten the duration of treatment if applied appropriately to humans.
Results
Effect of CC-3052 treatment on differential host gene expression in Mtb infected rabbit lungs
Owing to the current paucity in immunologic reagents to analyze the cellular function and soluble mediators of immunity in rabbits, we used microarray technology to investigate the rabbit immune response to pulmonary Mtb infection. Ingenuity Pathway Analysis (IPA) was used for functional classification and pathway construction of differentially expressed rabbit genes during Mtb infection and CC-3052 treatment. Since an annotated rabbit genome database for functional analysis is currently unavailable, we utilized the homologues of rabbit genes from the annotated human, mouse and rat genomes available in the IPA knowledge base for our analysis. Rabbits were infected with Mtb by the respiratory route, and the development of the host immune response in the lungs was evaluated at 4, 8 and 12 weeks post-infection using microarrays (Table 1). Of the 43,603 oligonucleotide probes represented on the rabbit array and analyzed by global gene expression patterns, 5,332 were significantly differentially expressed (3,075 up; 2,257 down) in response to 4 weeks of Mtb infection of the lungs, compared to uninfected, naïve animals (significant change defined as ±2 fold with p≤0.05). Progressive up-regulation of host genes was noted as the infection progressed for 8 weeks and 12 weeks, with 8,597 (5,344 up; 3,253 down) and 13,783 (4,260 up; 9,523 down) genes differentially regulated, respectively. It is important to note that with time, as the granulomas mature and differentiate following Mtb infection in rabbit lungs, the profile of gene expression also changes. In addition, of the total number of differentially regulated genes only a subset overlap (1,269, 5,212 and 1,584 genes respectively for 4 vs. 8, 8 vs. 12 and 4 vs.12 weeks, data not shown), consistent with the histologic observation of heterogeneity of the differentiating granulomas [58].
Tab. 1. Number of differentially expressed host genes in Mtb infected rabbit lungs with or without CC-3052 treatment. 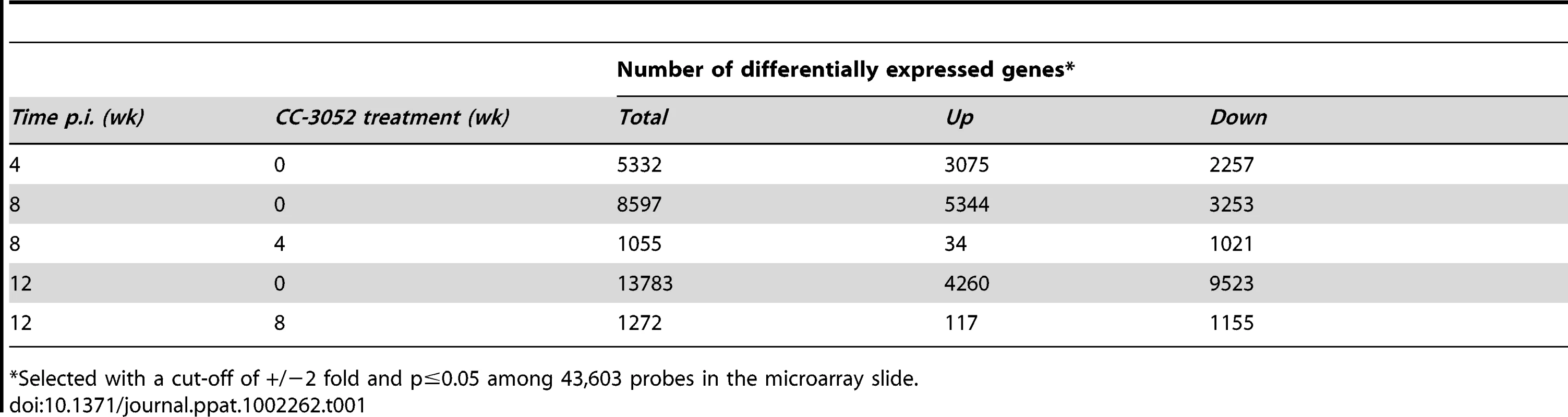
*Selected with a cut-off of +/−2 fold and p≤0.05 among 43,603 probes in the microarray slide. CC-3052 treatment was started at 4 weeks post-infection, and lung tissue was collected after 4 and 8 weeks of treatment (i.e. at 8 and 12 weeks post-infection). To study the changes in host gene expression induced by CC-3052 treatment, we compared the gene expression profiles in the Mtb infected lungs of CC-3052 treated rabbits to untreated infected control animals at the same time points. The complete list of rabbit genes differentially expressed by CC-3052 treatment at the time points tested has been submitted to the Gene Expression Omnibus (GEO) database (GSE27992). Among the rabbit genes differentially expressed by Mtb infection, statistically significant changes in the expression of 1,055 (34 genes up; 1,021 down) and 1,272 (117 genes up; 1,155 genes down) genes were observed after 4 and 8 weeks of CC-3052 treatment, respectively (Table 1). Expression of 20 overlapping genes was affected at both 4 (1 up; 19 down) and 8 weeks (9 up; 11 down) of CC-3052 treatment. The distribution, among various cellular immune response pathways, of the genes differentially expressed in response to 4 and 8 weeks of CC-3052 treatment, is shown in Table 2. About 30% of the genes differentially expressed following 4 weeks of CC-3052 treatment, compared to no treatment, were involved in host immune response-related pathways. These included immune cell growth and proliferation, cell morphology and movement, cell death/apoptosis, immune cell trafficking and hematologic development and function (Table 2). At 8 weeks of CC-3052 treatment, somewhat higher numbers of genes, involved in immune cell growth/proliferation, cell death, molecular transport, hematologic development/function, cell trafficking, were modulated compared to 4 weeks of treatment. In contrast, the number of rabbit genes involved in tissue morphology/development and immune cell signaling/interaction were reduced at 8 weeks compared to 4 weeks of CC-3052 treatment. In summary, the microarray analysis reveals differential expression of many host genes during Mtb infection in the rabbit lung as well as the modulation of a subset of those genes, involved in key host immune response pathways, following CC-3052 treatment.
Tab. 2. Classification of differentially expressed host genes in <i>Mtb</i> infected rabbit lungs treated with CC-3052. 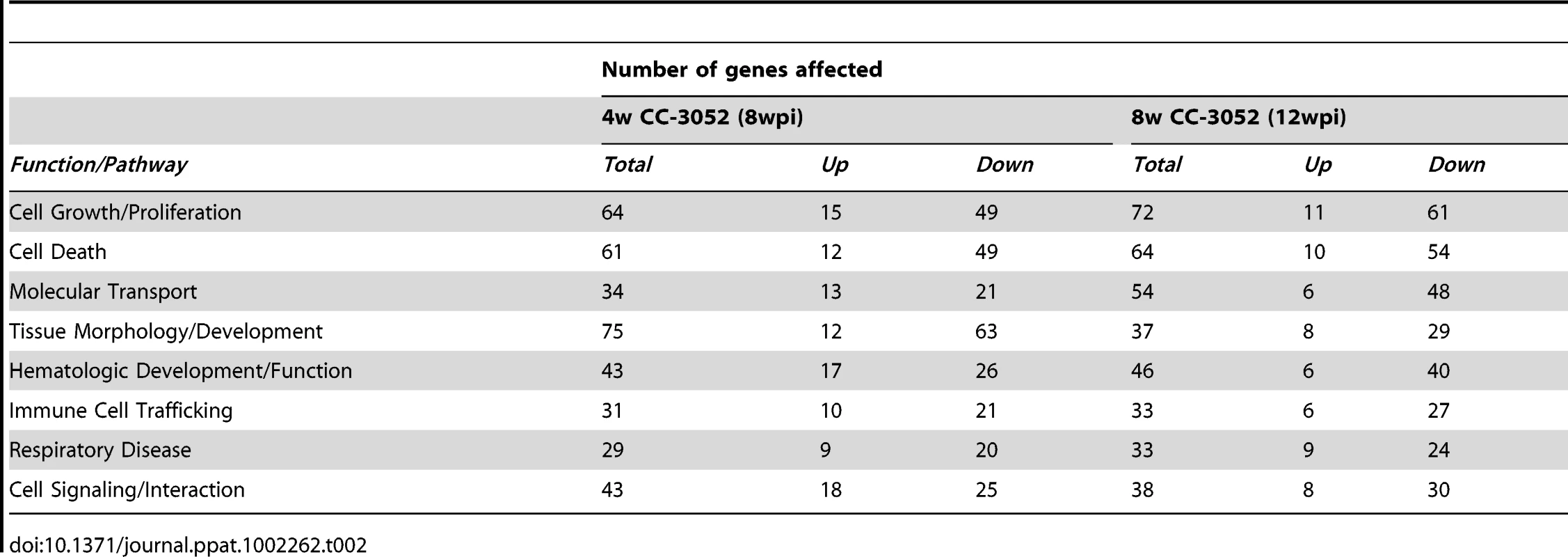
Modulation of the TNF-α network genes by CC-3052 treatment in Mtb infected rabbit lungs
Since inhibition of PDE4 reduces TNF-α expression, a key regulator of innate and acquired immunity, we studied the effect of CC-3052 treatment on the genes of the TNF-α network. The expression patterns of a subset of genes that directly regulate or are regulated by TNF-α, in Mtb infected rabbit lungs, at 0, 4 and 8 weeks of CC-3052 treatment were analyzed by microarray and compared to levels of expression in lungs of infected untreated animals (Figure 1). The fold changes in gene expression between CC-3052 treated and untreated animals were used for the pathway construction using IPA. In order to determine absolute expression levels, we set no cut-off values for the expression levels of individual genes in the network.
Fig. 1. Differential expression of genes involved in the TNF-α network between untreated and CC-3052 treated, Mtb infected rabbits. 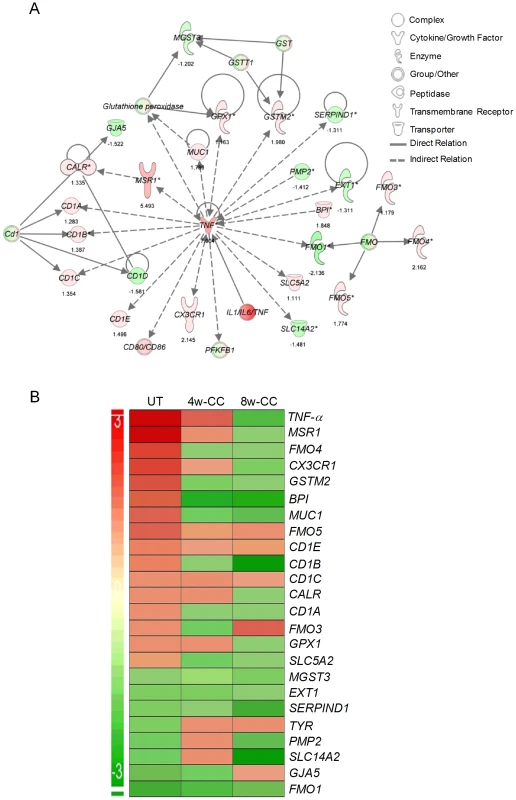
The absolute expression level of rabbit genes, from the microarray experiments on the lungs of Mtb infected and CC-3052 treated or untreated rabbits, was used in Ingenuity Pathway Analysis (IPA) software program to construct pathway maps. (A) Pathway map of rabbit genes regulating- or regulated by- TNF-α at 4 weeks (No CC-3052 treatment) The numbers at the bottom of each gene represent the absolute expression levels. (B) Intensity plot of TNF-a network genes before (UT) or after 4weeks (4w-CC) or 8 weeks (8w-CC) of CC-3052 treatment. For both A and B, green color represents down-regulation and red color represents up-regulation. The level of up- or down-regulation is related to the intensity of respective colors. In the untreated rabbits, Mtb infection up-regulated more than half of the genes (18 out of 32) in the TNF-α network, including TNF-α itself (Figure 1A). Only 14 out of 32 genes were down-regulated at 4 weeks post-infection compared to uninfected, control rabbits. After 4 and 8 weeks of CC-3052 treatment, the level of expression of the majority of these genes, specifically those involved in tissue inflammation and the innate immune response, including TNF-α, were progressively reduced (about 54% of genes at 4 weeks and 75% at 8 weeks of CC-3052 treatment) compared to untreated animals (Figure 1B). Not all of the genes in the TNF-α network were affected by CC-3052 treatment. Eight genes that are indirectly related to the TNF-α network (CD1c, CD1e, FMO-1, EXT-1, MGST-3, and SERPIN-D1) were expressed at comparable levels in untreated and CC-3052 treated, infected rabbits at 4 and 8 weeks post-treatment. Overall, the expression pattern of key genes of the TNF-α network impacted by CC-3052 treatment suggests a gradual reduction in the activation of the TNF-α network in the lungs of Mtb infected rabbits.
Effect of PDE4 blockade on expression of representative host genes in Mtb infected rabbit lungs
To evaluate the impact of CC-3052 treatment on the expression of host genes associated with PDE4, the target of CC-3052, we analyzed mRNA levels from the lungs of Mtb infected rabbits by quantitative real-time PCR (qRT-PCR) (Figure 2). At 4 weeks post-infection, low basal levels of expression of TNF-α and PDE4A were noted in comparison with uninfected animals. TNF-α mRNA levels increased to about 30 - and 45-fold, respectively, in response to 8 and 12 weeks of infection, but were not significantly different between these two time points. The expression of PDE4A was similarly elevated at 8 weeks and was somewhat lower at 12 weeks post-infection. Treatment of infected rabbits with CC-3052 (for 4 or 8 weeks) significantly reduced the expression levels of both TNF-α and PDE4A (Figure 2). On the other hand, while the lungs of Mtb infected animals showed lower PKA expression at all time points in comparison with uninfected controls, CC-3052 treatment reversed this effect significantly, leading to about 3-fold higher levels of PKA mRNA at 4 and 8 weeks of treatment (i.e. 8 and 12 weeks post-infection) (Figure 2).
Fig. 2. qRT-PCR of rabbit genes that are directly related to the mode of action of CC-3052. 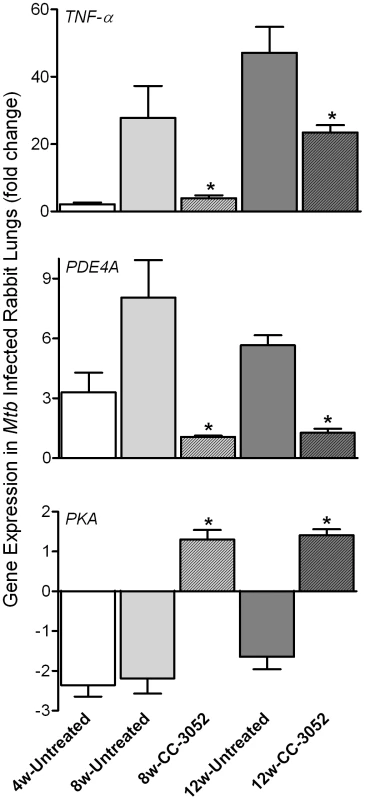
Total host and bacterial RNA was isolated from lungs of the rabbits from various treatment groups (mentioned in the Materials and Methods section) of Mtb infected and uninfected rabbits at 4, 8 and 12 weeks post-infection and used to enumerate the transcript levels in qRT-PCR experiments with gene specific primers. The difference in specific gene expression was calculated as fold change in expression between CC-3052 treated vs. untreated or Mtb infected vs. uninfected rabbits at each time point. The X-axis legend is common for all four figures. Changes in the rabbit gene expression by Mtb infection are represented as relative fold change, compared to the levels in uninfected rabbit lungs, and normalized to housekeeping GAPDH levels. Results shown are mean ± standard deviation from at least 2 experiments done in duplicate (total of at least 4 data points) with samples from two to four animals per group per time point. *Statistically significant difference between CC-3052 treated and untreated animals at the same time point (p≤0.05). We then evaluated by qRT-PCR the mRNA levels of a subset of genes, including cytokines (IFN-γ, IL10, IL13, IL6 and TNF-β), growth factors, chemokines and their receptors (CXCR3, GMCSF, MIF and CCL4), signaling molecules and receptors (NFκB, APRIL1, PI3K3 and TLR2) and intracellular trafficking and apoptosis-related genes (RAB7, LAMP2, BCL-X, FAS and RAB11) (Figure 3A and B). Many of these gene products are known markers of macrophage activation and maturation, endosomal trafficking and/or Th1/Th2 immunity [59]–[60]. In untreated rabbits, most of these genes were progressively induced by Mtb infection from 4 to 8 weeks, and many had reached a plateau (IFN-γ, IL13, IL6, MIF, CCL4, NFκB, APRIL, TLR2, RAB7 and LAMP2) or were even reduced (IL10, CXCR3, GMCSF and PI3K3) by 12 weeks post-infection. In contrast, expression of the anti-apoptotic gene BCL-X was progressively down-regulated by infection, and there were no significant changes in the expression of other selected genes, including TNF-β and RAB11 (Figure 3A and B). Following 4 or 8 weeks of CC-3052 treatment, the expression of many of these same genes were significantly reduced in comparison to Mtb infected untreated animals. The expression of genes that were not regulated by Mtb infection, for example RAB11 and TNF-β, were also unaffected by CC-3052 treatment (Figure 3). In summary, the expression profile of the host immune response genes in the lungs of Mtb infected rabbits suggests that CC-3052 treatment of rabbits with pulmonary TB resulted in a dampening of macrophage activation in the lungs.
Fig. 3. Effect of CC-3052 treatment on rabbit gene expression during Mtb infection. 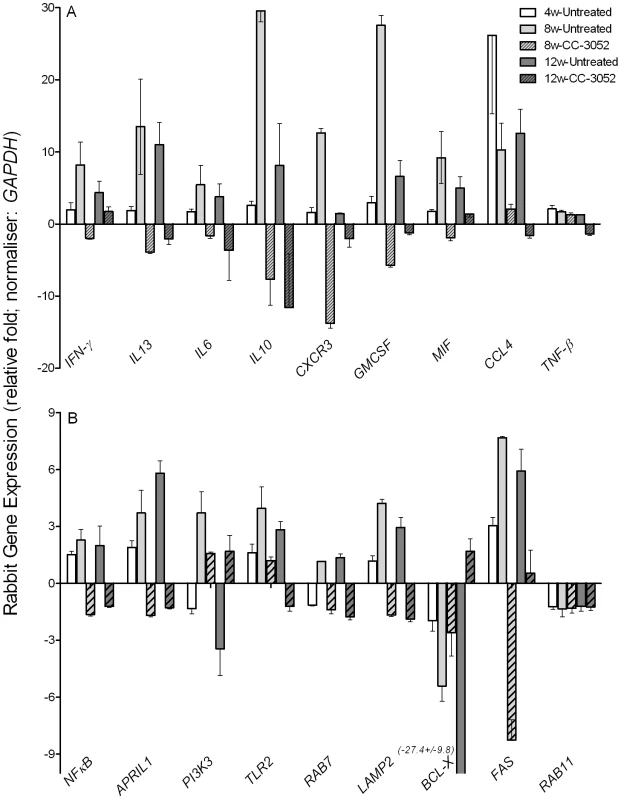
Total RNA isolated from the lungs of Mtb infected and uninfected rabbits that were either treated with CC-3052 or left untreated were used to determine the abundance of transcripts using qRT-PCR with gene specific primers. (A) Level of expression of rabbit genes encoding for cytokines (IFN-γ, IL13, IL6, IL10 and TNF-β) and chemokines (CXCR3, GMCSF, MIF and CCL4). (B) Expression level of rabbit genes encoding for intracellular signaling molecules (NFκB, APRIL1, PI3K3 and TLR2), endosomal trafficking markers (RAB7, LAMP2 and RAB11) or apoptosis pathways (BCL-X and FAS) in the lungs of Mtb infected rabbits. Change in gene expression levels in the CC-3052 treated rabbits were calculated as relative fold difference after normalization to the level of expression of the housekeeping gene, GAPDH, and calibrated against the levels in uninfected or untreated animals. The difference in the level of expression of all tested genes between CC-3052 treated (CC) and untreated (UT) animals were statistically significant (p≤0.05) at 8 and 12 weeks post-infection except for the following: TNF-βat 8 weeks and IFN-γ at 12 weeks. Expression of RAB11 was not statistically different at both 8 and 12 weeks post-infection. The results shown are mean ± standard deviation from at least 2 experiments done in duplicate (total of at least 4 data points) with samples from two to four animals per group per time point. Effect of CC-3052 treatment on Mtb gene expression in infected rabbit lungs
We analyzed the impact of CC-3052 treatment on gene expression of the infecting bacilli. Total RNA from Mtb isolated from infected rabbit lungs was prepared, and the expression levels of selected hypoxic/oxidative stress response genes (narX, narK2, devR, sodA and sodC) were evaluated by qRT-PCR. Expression of these genes was induced progressively in the rabbit lungs from 4 to 12 weeks post-infection, with the exception of narK2 which reached a plateau after 8 weeks (Figure 4), consistent with our observation of progressive activation of macrophages in the granulomas. This notion is also supported by previous demonstrations that rabbit granulomas are hypoxic [61] and exposure of Mtb to hypoxia differentially regulates several genes of the dosR regulon [62], [63]. Treatment of Mtb infected rabbits with CC-3052 significantly reduced the expression of narX, narK2, devR, sodA and sodC from 5 - to 150 - folds (p<0.05), compared to infected but untreated animals. These results are consistent with our hypothesis that CC-3052-mediated reduction in macrophage activation would be accompanied by changes in the extent of the Mtb stress response during intracellular survival (Figure 4). However, treatment of log-phase Mtb culture with equimolar amount (compared to in vivo) of CC-3052 had no impact on the mRNA levels of these and other dormancy/stress response-related Mtb genes, compared to untreated controls (Supporting Information Figure S1), indicating that the effect of CC-3052 on Mtb gene expression was specific to the in vivo conditions.
Fig. 4. Effect of CC-3052 treatment on the expression of Mtb stress response genes in rabbit lungs. 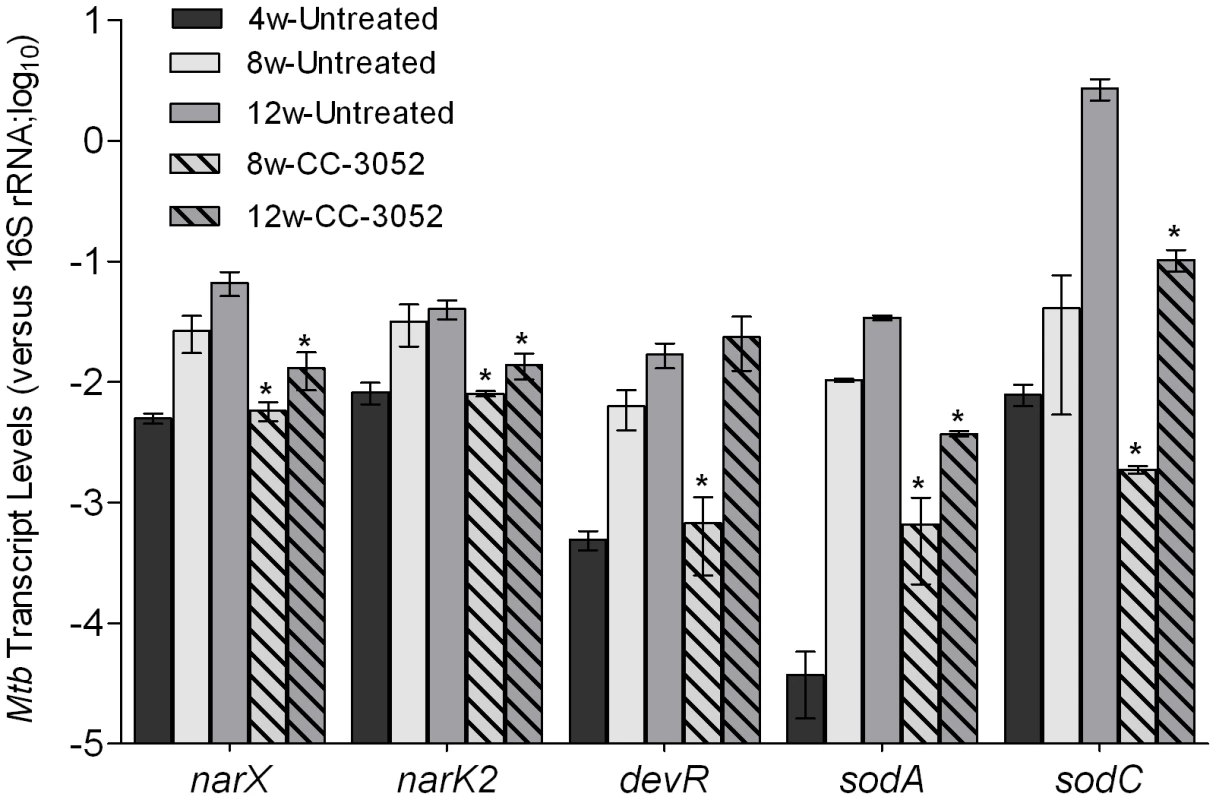
Total Mtb RNA was isolated from the lungs of rabbits from various treatment groups (mentioned in the Materials and Methods section) of Mtb infected rabbits at 4, 8 and 12 weeks post-infection and used to quantify the amount of transcripts in qRT-PCR experiments with primers specific for those Mtb genes that are involved in the stress response. The values plotted in the graph are absolute transcript levels of each Mtb gene after normalization to 16S rRNA levels. The mycobacterial genes represented in the graph are: narX- probable nitrate reductase; narK2- possible nitrate/nitrite transporter; devR- two component transcriptional regulatory protein; sodA- superoxide dismutase A; sodC-superoxide dismutase C. The results are shown as mean ± standard deviation from at least 2 experiments done in duplicate (total of at least 4 data points) with samples from two to four animals per group per time point. *Statistically significant difference between CC-3052 treated and untreated animals (p≤0.05). Recently, a comprehensive analysis of the gene expression profiles of Mtb isolates, representing diverse lineages, grown in vitro within activated and resting murine macrophages was reported [64]. We selected for study a subset of genes from the Homolka et.al. report, including genes previously reported as differentially expressed by Mtb in response to environmental stresses, such as metabolic adaptation to dormancy [65], [66]. The genes were functionally grouped as follows: a) protein synthesis (rpsT, rpsR, rpsP, rpsL, rpsG, rplL and rplU); b) iron metabolism (mbtI, mbtF, mbtH, mbtE, mbtD, mbtC, mbtG, mbtA, mbtB and bfrA); c) cell wall/lipid metabolism (ppsD, drrA, papA5, dfrA, fbpB, pckA, lipF, mmpL8, pcaA, tgs1, icl and drrC); d) general stress response (hspX, sigF, sigH, dnaE2, relA, mprA, groEL1, groEL2, groES and dnaJ); e) ESX-3/secretion system (Rv0284-Rv0286, Rv0289, Rv0290 and Rv0292); and f) histone-like proteins (hns and lsr2) (Figure 5). Comparative gene expression profiling revealed that many of the Mtb genes involved in protein synthesis, ESX-3/secretion, iron metabolism and histone-like proteins were up-regulated by 4 weeks of CC-3052 treatment in rabbit lungs (Figure 5). In contrast, many of the general stress response and cell wall/lipid metabolism associated genes were down-regulated by CC-3052 treatment. When the Mtb gene expression pattern from the lungs of CC-3052 treated rabbits was compared to the functional classifications of Homolka et.al., the bacteria from the rabbits showed a gene expression profile closer to that seen in resting, rather than activated, macrophages (Figure 5) [13], [64], [67]. Thus, our functional analysis of the Mtb gene expression profile in the lungs of infected rabbits during CC-3052 treatment suggested that the bacilli were in a different metabolic state than in the lungs of untreated, control rabbits, and that this metabolic state corresponded with that observed in suboptimally activated macrophages.
Fig. 5. Effect of CC-3052 treatment on the expression of Mtb metabolic genes in rabbit lungs. 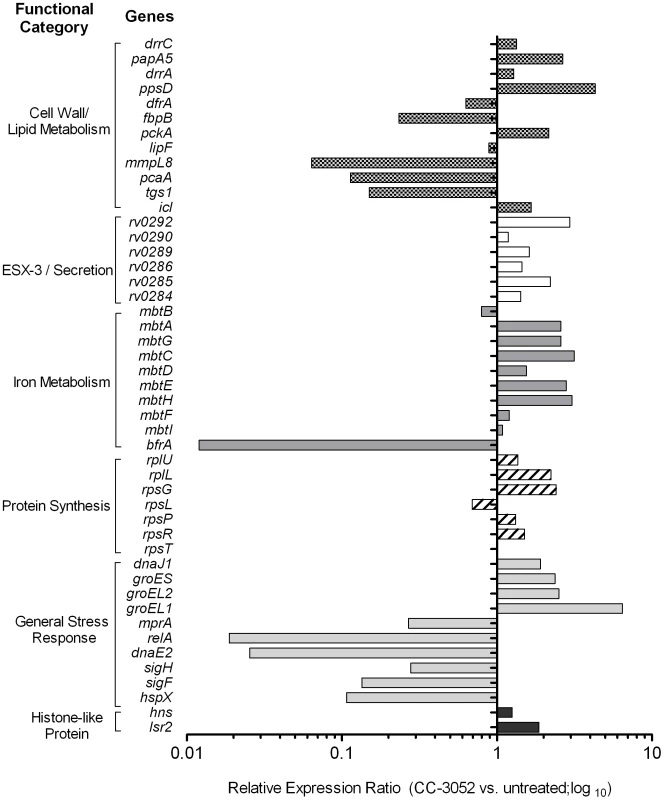
Total Mtb RNA was isolated from the lungs of rabbits from various treatment groups (mentioned in the Materials and Methods section) of Mtb infected rabbits at 8 weeks post-infection and used to measure the transcript levels using qRT-PCR with primers specific for Mtb genes involved in various metabolic processes. The change in Mtb gene expression is represented as relative ratio between untreated and CC-3052 treated samples, after the levels of expression of each gene was normalized to 16S rRNA levels. Description of individual Mtb genes is included in Table S1. Results shown are average values from at least 2 experiments done in duplicate (total of at least 4 data points) with samples from two to four animals per group per time point. Effect of CC-3052 treatment on INH-mediated Mtb killing in infected rabbit lungs
To study the effect of CC-3052 treatment on the ability of INH to kill Mtb in the lungs of infected rabbits, animals were treated from 4 weeks post-infection with high dose INH (50 mg/kg body weight/day) or CC-3052 (25 mg/kg body weight/day), neither or both for 4 or 8 weeks (i.e., 8 or 12 weeks post-infection). In the untreated animals, implantation of about 3.2 log10 bacilli (on day 0) into the lungs resulted in progressive, active disease with exponential bacterial growth up to 4 weeks of infection, after which the bacterial counts stabilized and then declined slightly. The control of bacillary load in the lungs of rabbits was not significantly affected by CC-3052 treatment, as indicated by the similar numbers of CFU in untreated and CC-3052 treated animals at all time points tested (Figure 6). There were also no significant differences in numbers of CFU in the liver and spleen between these two groups (data not shown). INH treatment of infected rabbits did not significantly reduce the CFU numbers in the lungs of treated rabbits for the first 4 weeks. However, 8 weeks of INH treatment (i.e. 12 weeks post-infection), the bacillary load in the lungs was reduced by about 1 log10 (Figure 6).
Fig. 6. Effect of CC-3052 and INH treatment on the growth of Mtb in rabbit lungs. 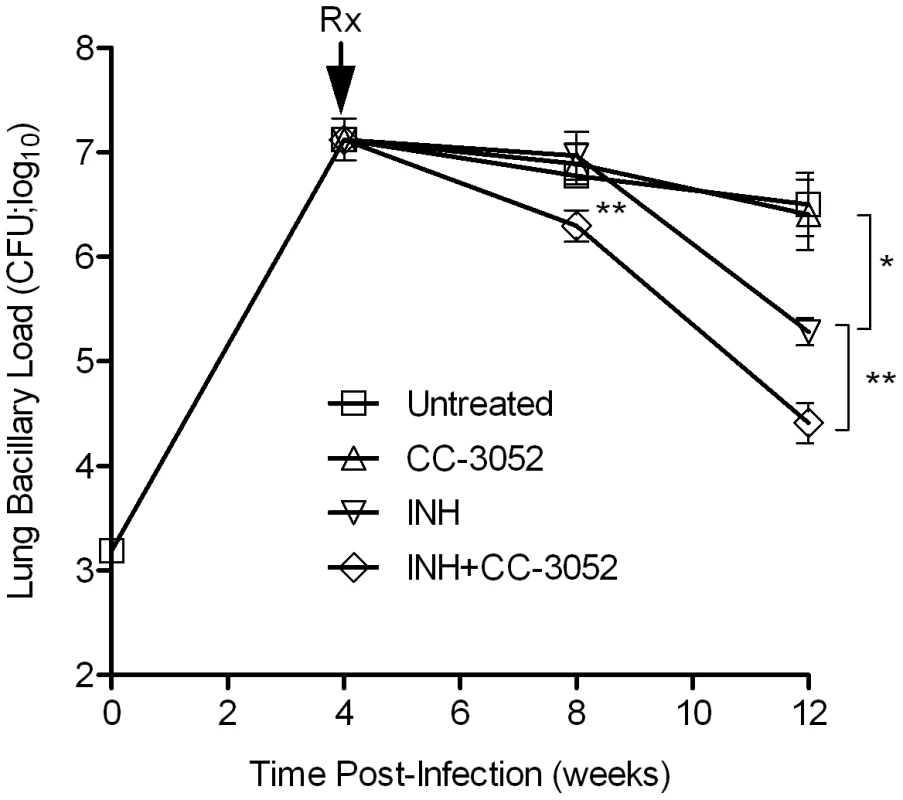
Rabbits were infected with Mtb HN878 by aerosol challenge to implant 3.2 log10 bacilli on day 0. Starting at 4 weeks post-infection, groups of rabbits were treated with CC-3052, INH, both or left untreated, and treatment continued until 12 weeks post-infection. At 0, 4, 8 and 12 weeks post-infection, groups of 2–4 rabbits per treatment condition, were necropsied and segments of lung tissue were homogenized and plated on 7H9 agar plates to enumerate the numbers of CFU (detailed in the Materials and Methods section). The total numbers of CFU (in log10 scale) was calculated for the entire rabbit lungs. Rx represents the starting time point (4 weeks) for CC-3052 and/or INH treatment. The experiment was repeated at least twice with 2–4 animals per time point per treatment group. Results shown are means ± standard deviation from 6–11 animals. *Statistically significant difference between untreated and INH treated animals at 12 weeks post-infection (p<0.05). **Statistically significant difference between INH plusCC-3052- and INH alone- treated animals at 8 and 12 weeks post-infection (p<0.05). The limited efficacy of INH monotherapy in Mtb infected rabbits during the first 4 weeks prompted us to evaluate the bioavailability of the drug. To assess the extent to which INH was absorbed into the circulation, we measured the plasma INH concentration in animals that received either 25 mg/kg or 50 mg/kg of INH per day by gavage administration. Both doses of the drug resulted in similar plasma levels up to 8 hours post-administration (Figure 7A). However, only the 50 mg/kg dose of INH resulted in detectable INH levels in the plasma up to 24 hours. To compare INH availability when administered in drinking water to that of the gavage route of delivery, rabbits were treated with 50 mg/kg of INH by either route, and the pharmacokinetics of INH in the plasma were compared (Figure 7B). The parameters for INH in drinking water were calculated, assuming a steady state dosing during a 24-hour interval. Compared to INH in drinking water, a single dose of 50 mg/kg of INH administered by gavage showed about 100-fold increase in plasma drug levels within 2 hours of administration. However, both routes of administration showed similar INH levels in the plasma at 24 hours post-administration and similar bioavailability, as measured by the area under the curve (Figure 7B). This observation supported the use of gavage administration for appropriate dosing of INH, thereby avoiding the uncertainty of drug dosing through drinking water which arises from the fact that rabbits do not consume reproducible volumes of water daily.
Fig. 7. Pharmacokinetics and dynamics of INH uptake into the serum of treated rabbits. 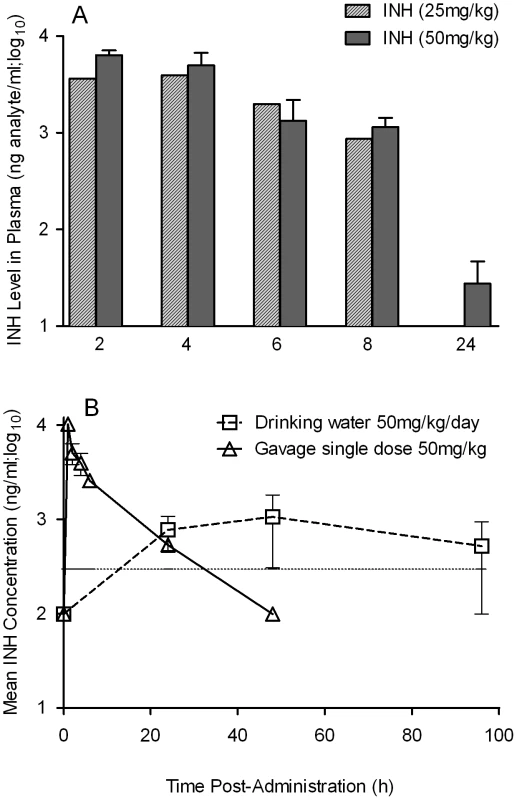
(A) Level of INH detected in the plasma of rabbits treated with a bolus dose of either 25 or 50 mg/kg of INH by gavage. INH levels were estimated by HPLC-mass spectrometry as explained in the Materials and Methods section (B) Effect of different modes of administration of INH on rabbit plasma INH levels. Rabbits were treated with INH either by gavage using a rubber feeding tube, or in drinking water. Plasma INH from treated rabbits was separated from the blood collected at indicated time points, post-administration, to measure the INH concentration by HPLC-mass spectrometry as explained in the Methods section. The horizontal dotted line in (B) represents the optimal level of quantification of INH. When rabbits were treated with CC-3052 in combination with INH for 4 weeks, a statistically significant reduction in the numbers of CFU was noted, compared with INH alone (p = 0.027, Figure 6). An additional 4 weeks of co-treatment resulted in a 10-fold reduction in bacillary load compared to INH alone (p = 0.003, Figure 6). Thus, while the rate of Mtb killing was similar during the second month of treatment, the absolute numbers of CFU in CC-3052 co-treated animals were significantly lower than in the INH alone group by the end of experiment. It is important to note that treatment of infected rabbits with INH alone or in combination with CC-3052 for up to 2 months did not produce INH resistant bacilli, as determined from the CFU assay by plating the lung homogenates in the presence and absence of INH. The effect of CC-3052, either alone or in combination with INH, on Mtb was specific to the in vivo conditions present in the lungs of infected rabbits, since the addition of up to 50X molar excess of CC-3052 and 0.2 µg/ml of INH (the minimal inhibitory concentration) to a growing Mtb culture in vitro did not affect the growth or INH killing significantly (Figure S2). Future experiments, including prolonging the duration of treatment of infected rabbits (more than 8 weeks) and varying the starting time of treatment, will determine if combined administration of INH and CC-3052 can fully eliminate the bacteria from rabbit lungs.
Effect of CC-3052 treatment on INH-mediated Mtb gene expression in infected rabbit lungs
We next analyzed the impact of INH treatment on the expression of INH-associated Mtb genes in the presence and absence of CC-3052 in rabbit lungs (Figure 8). Total Mtb RNA was isolated from the lungs of infected rabbits and analyzed by qRT-PCR. Similar to results reported in several studies, after 4 weeks of INH treatment, we observed significant increases in the mRNA levels of the Mtb genes, katG, ahpC, inhA, kasA, iniB and efpA (Figure 8). [55], [57], [68]. There was no statistically significant differences in the level of expression of fadD26 between Mtb RNA pools from INH treated and untreated animals. The gene induction profile was specific for INH treatment, since these genes were not affected by treatment of similarly infected rabbits with rifampicin (RIF), another important antibiotic for TB treatment (Figure 8). The fold change in expression for these genes, after normalization to the 16S rRNA, ranged from 2 - (for inhA) to about 20-fold (for katG and ahpC) (p≤0.05) in comparison to levels observed in untreated animals. The levels of expression of these genes in the lungs of untreated and CC-3052 alone-treated animals were comparable and significantly less than what was observed in the INH only treated animals. Thus, although 4 weeks of INH treatment of infected rabbits did not reduce the bacillary load significantly, exposure to the antibiotic clearly affected the physiology of the infecting bacteria, as manifested by differential gene expression, consistent with previous reports [7], [8], [55], [57], [68]. Interestingly, co-treatment with INH plus CC-3052 reduced the expression of INH–induced genes in Mtb to levels similar to those observed in untreated rabbit lungs.
Fig. 8. Effect of CC-3052 treatment on the expression of Mtb INH responsive genes in rabbit lungs. 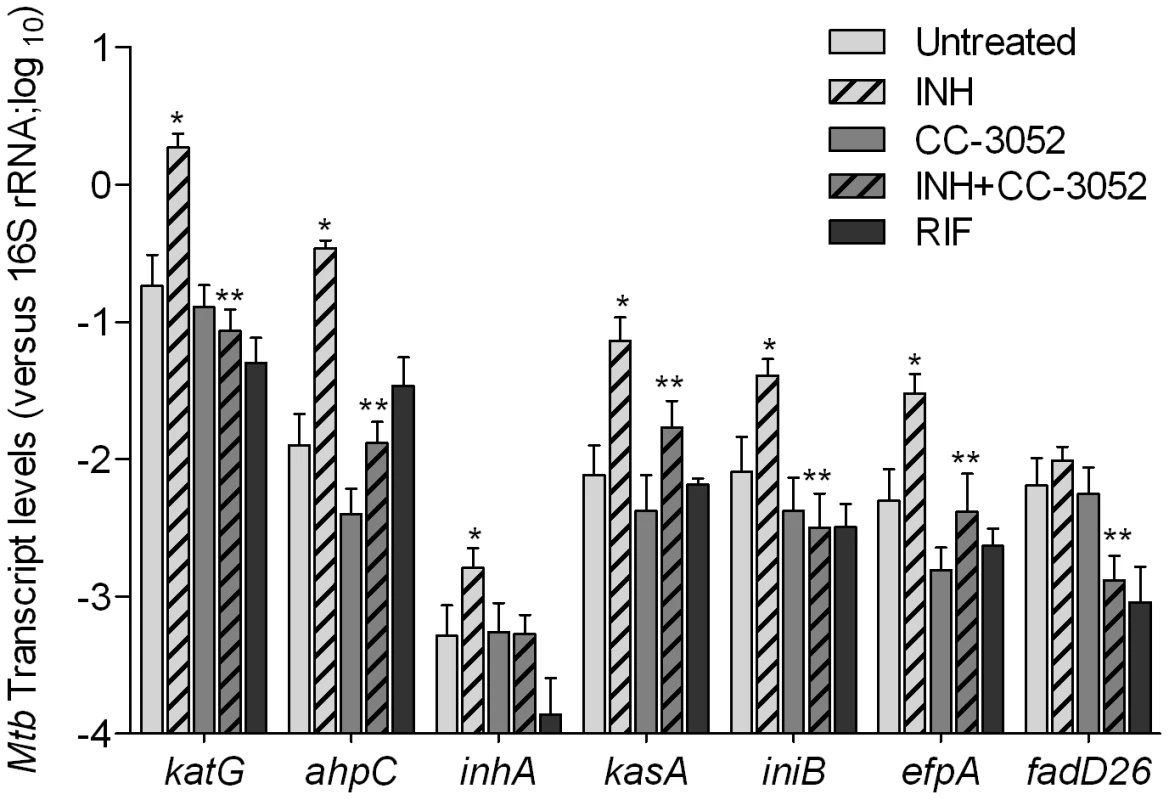
Total Mtb RNA was isolated from the lungs of rabbits from various treatment groups (mentioned in the Materials and Methods section) of Mtb infected rabbits at 8 weeks post-infection and used to quantify the amount of transcripts by qRT-PCR with primers specific for those Mtb genes that respond to exposure to INH. The difference in Mtb gene expression was calculated as relative ratio (in log10 scale) between untreated and CC-3052 treated rabbits, after the level of expression of each gene was normalized to 16S rRNA levels. RIF represents samples from rabbits treated with rifampicin. The mycobacterial genes represented in the graph are: katG- catalase-peroxidase-peroxynitritase; ahpC- alkyl hydroperoxidase C; inhA- NADH-dependant enoyl-ACP reductase; kasA- beta-ketoacyl-ACP-synthase; iniB- isoniazid inducible gene; efpA- possible integral membrane efflux protein; fadD26- fatty acid CoA synthase. Results shown are mean ± standard deviation from at least 2 experiments done in duplicate (total of at least 4 data points) with samples from two to four animals per group per time point. * Statistically significant difference between untreated and INH treated animals (p<0.05). ** Statistically significant difference between INH plusCC-3052- and INH alone- treated animals (p<0.05). Discussion
In the present study, we describe the effect of PDE4 inhibition on the immune response during Mtb infection in a rabbit model of pulmonary TB and show how changes in the expression of immune response genes affect the crosstalk between the host and pathogen (Figure 9). During the course of Mtb infection in the lungs of untreated rabbits, expression levels of many host genes involved in the innate immune response were up - regulated. Corresponding changes were observed in the expression of many Mtb genes known to be responsible for successful intracellular survival of the bacilli, including genes involved in protecting the bacteria against ROS - and RNS-induced damage and bacillary response to stress induced by nutritional deprivation and acid shock. These genes were up-regulated gradually with disease progression in the lungs of untreated, Mtb infected rabbits. Treatment of the Mtb infected rabbits with CC-3052 led to significantly reduced expression of many of the host innate immune response genes, including TNF-α and IL-6. Concomitant with these PDE4-induced changes in host gene expression, the levels of expression of many bacterial stress response genes were reduced.
Fig. 9. Schematic representation of the mechanism by which CC-3052 treatment affects macrophage activation and improve INH-mediated <i>Mtb</i> killing in the lungs of infected rabbits. 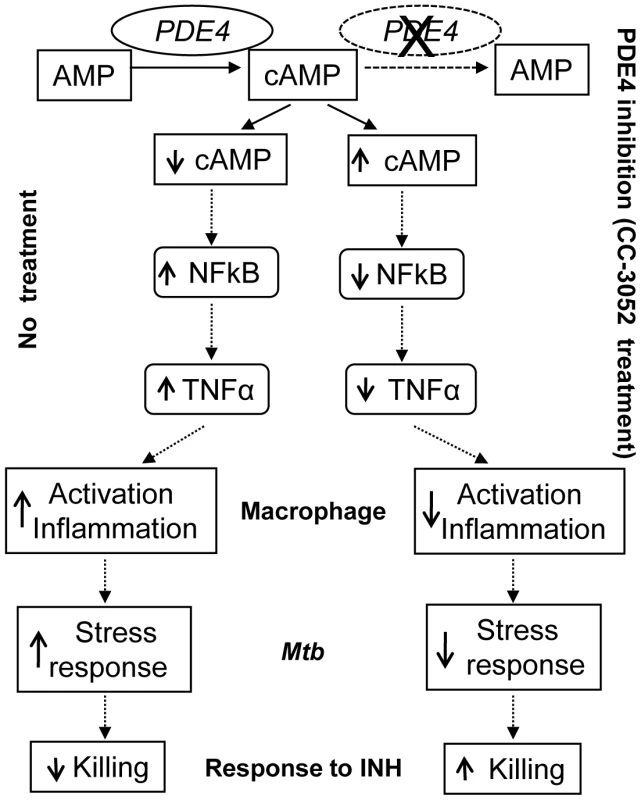
Importantly, CC-3052 treatment of infected rabbits was associated with a loss in the ability of the bacilli to withstand INH killing, and bacillary clearance was improved starting from the first month of treatment compared to INH alone. These results contrast with our previously reported study in the murine TB infection model [49], in which we showed that co-treatment with CC-3052 extended the duration of INH-mediated bacillary clearance from the lungs at later time points, when INH effectiveness began to fail, but did not significantly affect the CFU numbers during early treatment [49]. The differences in the kinetics of improved INH-mediated Mtb clearance from the lungs in the presence of immune modulation likely reflect differences in the pathogenesis of Mtb infection in these two animal models. While rabbit lungs develop fully matured granulomas by 4 weeks post-infection that progress to necrotizing granulomas and cavities, similar to humans, the mouse immune response to Mtb infection involves the accumulation of mononuclear leukocytes that do not differentiate into structured granulomas [69]. Our observations suggest that, in the rabbit, INH fails to kill the bacilli efficiently in the early well-differentiated granulomas of the lungs (4 to 8 weeks post-infection). Moreover, CC-3052 co-treatment improves INH-mediated bacillary clearance at these early time points. Thus, in the present study, we have demonstrated the effect of PDE4 inhibition on Mtb killing within a mature granuloma, in contrast to our findings in the mouse, where the impact of immune modulation was observed on residual persisting bacilli towards the end of treatment. It is possible that, in the rabbit model, CC-3052 treatment would further improve Mtb killing at later time points (after 12 weeks) when INH alone fails to kill persisters. Indeed, it has been reported that the proportion of physiologically quiescent bacilli and their vulnerability to INH killing determines the overall response to INH treatment [70], [71]. Hence, the differential outcome of INH treatment with and without CC-3052 could be attributed to changes in the metabolism of a sub-population of bacilli [56], [72]. This suggests the presence of a higher percent of bacilli that are dormant and/or non-responsive to INH at 4 weeks post-infection of the rabbit lung, compared to the mouse lung. Since the nature and extent of the host immune response to Mtb infection significantly affect the physiology of the infecting bacilli [73], and rabbit granulomas are hypoxic and more mature compared to lesions in the mouse lung, it is expected that the number of bacilli that are non-responsive to INH treatment in the 4-week rabbit granulomas may be relatively high. Taken together, studies in the rabbit provided a useful model that is more similar to human disease than the infection seen in the mouse lung.
The changes observed in host gene expression during CC-3052 treatment of Mtb infected rabbits support a link between PDE4 inhibition and specific modulation of the innate immune response. So far, at least 11 families of class 1 PDEs (PDE1-11) have been identified in humans [74]. However, only 3 of the 11 (PDEs 4, 7 and 8) are involved in hydrolyzing cAMP [75]. Of these, PDE4 is the predominant isoform of phosphodiesterase found in monocyte/macrophages [76]. Thus, inhibition of PDE4 would be expected to target monocytes and macrophages, but not T cells and other cells of the immune response. The ability of the rabbits to control bacillary growth in the lungs in the presence of CC-3052 supports the conclusion that this drug does not suppress the acquired immune response. Moreover, in our previous study, the mouse TB model enabled us to directly demonstrate that CC-3052 does not inhibit T cell activation in Mtb infected animals [49]. PDE4 hydrolyses cAMP in the phagocyte, decreasing the levels of this important second messenger, which regulates essential cell signaling events that determine many cell functions [77], [78]. Previous studies have shown that increased levels of cAMP modulate immune cell functions, including the respiratory burst, chemotaxis, phagocytosis and phagosome maturation [79], [80]. Consistent with these findings, treatment of Mtb infected rabbits with CC-3052 significantly reduced the steady state mRNA levels of TNF-α and other pro-inflammatory, Th1 and Th2 cytokines, chemokines and their receptors, apoptosis-associated genes, surface receptors, and signaling molecules in macrophages. TNF-α plays a central role in the host innate immune response and specifically in the activation of macrophages [81]. This cytokine is primarily regulated via TLR signaling through activation of the NFκB pathway [82], [83]. Thus, the reduced level of expression of both the TNF-α and NFκB genes in the lungs of Mtb infected rabbits treated with CC-3052 suggests a suboptimal activation of macrophages in the lesions of these animals [84].
Suboptimal activation of macrophages has been shown to affect the cellular organization in the lung granulomas of Mtb infected animals [49]. Briefly, in Mtb infected animals, there were no significant differences either in the total number of subpleural lung granulomas or in the area of lung involved, between the untreated and CC-3052 treated rabbits up to 12 weeks post-infection. However, the granulomas in the lungs of CC-3052 treated rabbits appeared less necrotic, less fibrotic and more cellular compared to the untreated rabbits, most strikingly at 12 weeks post-infection (8 weeks of CC-3052 treatment). In mice, CC-3052 treatment affected the distribution of T cells in the lung lesions without affecting their total numbers. When Mtb infected animals (both mice and rabbits) were treated with INH plus CC-3052, a significant reduction in the size and number of lung granulomas was noted, compared to INH treatment alone. Whether these morphologic changes affected drug penetration and/or exposure of the bacilli to INH in the granulomas is as yet unknown and will be investigated in the future.
Macrophage activation during infection is associated with phagosome maturation, as indicated by the acquisition of RAB7 and LAMP2 on the organelle surface [59], [85]. The reduced expression of RAB7 and LAMP2 in response to CC-3052 treatment suggests that there may indeed have been impaired or suboptimal macrophage phagosome maturation in the immune modulated rabbits. In addition, macrophage activation during Mtb infection is characterized by the production of a number of pro-inflammatory cytokines, chemokines, growth factors and their receptors. Increased TNF-α production by activated macrophages leads to increased expression of apoptotic genes, such as FAS, and reduced expression of anti-apoptotic gene expression, such as BCL-X [60]. The reduced expression of FAS and increased expression of BCL-X observed in rabbits treated with CC-3052 suggests reduced cell apoptosis during treatment with the PDE4i. Overall, the pattern of change in host gene expression in the lungs of Mtb infected rabbits treated with CC-3052, compared to untreated rabbits, was similar to that seen in resting macrophages cultured in vitro, where expression of the genes encoding for proinflammatory cytokines, chemokines, and their receptors was relatively low [86]–[88].
The modulation of host innate immunity by CC-3052 treatment appeared to be sensed by the infecting intracellular Mtb, resulting in corresponding changes in the expression of many bacterial genes. How would changes in macrophage activation be expected to affect the physiology of the infecting bacilli? First, the observation that expression of many of the Mtb stress response genes induced during infection was significantly reduced by CC-3052 treatment supports our hypothesis that Mtb adapted to perturbations in the host environment [14], [23], [73], [89]–[92]. The metabolic shift-down of selected Mtb genes during non-replicating persistence is mediated by the activation of devR, a global transcriptional regulator, that up-regulates at least 53 Mtb genes involved in the bacterial adaptation to dormancy [93], [62]. Among these are the Mtb stress response genes, narX and narK2, which are induced when the infecting bacilli enter into dormancy [65], [94], [95]. In the present study, CC-3052 treatment reduced the expression of devR, narX, and narK2 in the infected rabbit lungs, suggesting that the bacilli sensed the reduction in environmental pressure within suboptimally activated macrophages. Treatment with the PDE4i also lead to reduced expression of the transcriptional regulator relA, which is essential for Mtb survival under stress conditions, as well as for infection, persistence and dissemination in mice and guinea pigs [96], [97]. Consistent with our findings, loss of relA expression in Mtb has been shown to be associated with reduced lung inflammation and pathology in infected guinea pigs [97]. Similarly, mycobacterial genes encoding superoxide dismutases (sodA and sodC), heat shock protein (hspX) and stress response sigma factors (sigF and sigH) have been shown to be associated with adaptation of the bacilli to a stressful environment, such as hypoxia, within activated macrophages [98]–[100]. The mRNA levels of these mycobacterial genes (sodA, sodC, sigF and sigH) were down-regulated during CC-3052 treatment, consistent with adaptation of the bacilli to a less stressful environment. Differential regulation of the sigma factors ensures the regulation of a distinct set of Mtb genes essential for survival under various environmental conditions such as availability of nutrients, oxygen status, and the presence of antibacterial molecules. [101]. Moreover, the up-regulation of a set of Mtb genes involved in protein synthesis, cell wall/lipid metabolism, iron metabolism and ESX-3/secretion pathways in response to CC-3052 treatment was similar to the pattern observed for intracellular Mtb inside resting macrophages [64]. Interestingly, cycloproponation of Mtb cell wall mycolates by pcaA has been shown to be essential for the immune recognition of Mtb through trehalose dimycolate (TDM), an inflammatory glycolipid unique for pathogenic Mtb [102], [103]. Furthermore, lack of mycobacterial pcaA gene expression was shown to reduce the level of TNF-α and other proinflammatory cytokines in Mtb infected murine bone marrow macrophages [102]. Taken together, the results of our study support the existence of mutually regulated host-pathogen interactions during Mtb infection. Both the infected host cells and the infecting bacilli appear to fine-tune their gene expression during adaptation to a state of intracellular infection. When the natural infection-induced macrophage activation was dampened in CC-3052 treated animals, as evidenced by the reduced expression of innate immune response genes, the bacilli sensed the change and reset their metabolism by altering their own gene expression.
Remarkably, changes in the gene expression profile of the bacilli, albeit limited in magnitude, appeared to affect the ability of INH to kill the bacteria. It is conceivable that, when the transcriptional regulation of Mtb genes, such as those governed by devR, relA and alternate sigma factors, associated with the dormant/non-replicative state is altered, the bacilli are forced out of the non-replicating, persistent drug tolerant state [104], [105]. Thus, a change in the microenvironment wherein the infecting Mtb reside would significantly influence the bacterial vulnerability to antibiotic killing. Consistent with this hypothesis, we observed improved bacterial killing in the lungs of Mtb infected rabbits treated with INH plus CC-3052 compared to INH alone. Increased expression of the INH-induced Mtb genes following 4 weeks of INH treatment was an indication of bacterial exposure to INH, despite the minimal bactericidal activity observed at this time point. In contrast, 4 weeks of INH treatment in the presence of CC-3052 led to reduced levels of expression of these genes. Though the exact mechanism of down-regulation of INH-responsive Mtb gene expression during CC-3052 co-treatment is currently unknown, this observation may be linked to the improved killing of Mtb by INH plus CC-3052. The results of a number of previously published studies may offer alternative non-exclusive explanations for our findings. INH is a pro-drug that is activated by the bacterial catalase-peroxidase (KatG) to form functional adducts with NAD, which inhibit InhA, an important enzyme involved in the cell wall synthesis of Mtb [106]–[107]. In addition to INH activation, KatG also possesses peroxynitritase and NADH oxidase activities that are vital for bacterial defense against oxidative stress [108]–[109]. Thus, as we have observed in the CC-3052 treated rabbits, under reduced oxidative stress conditions, katG expression would be expected not to be up-regulated. Furthermore, induction of mycobacterial efflux pumps and their regulators, in response to intracellular stress conditions during macrophage infection of Mtb, has been reported [110]–[112]. Recently, increased expression of an efflux pump was reported to be associated with tolerance of M. marinum to INH in a zebrafish model of mycobacterial infection [113]. Moreover, up-regulation of efpA, encoding for an efflux pump in Mtb, has been reported in MDR-TB [114]. In the present study, the reduced expression of efpA in CC-3052 treated rabbits may contribute to the improved INH-mediated killing observed. Recently, by screening a chemical library of 300 compounds for their inhibitory activity against enoyl reductase (InhA), Vilcheze et.al identified two potential targets, CD39 and CD117, that exhibited significantly increased Mtb killing when combined with INH, compared to treatment by either of the compounds separately or INH alone [115]. This finding suggests that chemicals that reduce/inhibit InhA could facilitate improved Mtb killing when co-administered with INH. A similar mechanism may be operative in our studies where inhibition of expression of inhA by CC-3052 treatment of the rabbits was associated with an increase in INH-killing of Mtb.
Studies from our group and others have shown that generalized immune suppression by anti-TNF-α antibody treatment in the mouse model of pulmonary TB compromised the ability of the animals to control bacillary growth, and exacerbated the pathology in the lungs [84], [116], [117]. Unlike anti-TNF-α antibody treatment and similar to our data in the mouse, CC-3052-mediated PDE4 inhibition had no effect on bacillary growth in the lungs of infected rabbits [49]. The use of PDE4 inhibition as a means to reduce, but not abolish, TNF-α levels has the selective advantage of targeting the level of production of the cytokine in monocytes and macrophages. Thus, T cells and other cells of the immune response would retain their ability to produce TNF-α, facilitating control of Mtb infection in the lungs. In contrast, complete neutralization of TNF-α production by treatment with an antibody against the cytokine would be expected to render the animals immune suppressed, thereby leading to uncontrolled bacillary growth and death of the animals [118]–[121], [94]. In addition, in vitro culture of Mtb in the presence of CC-3052 had no effect on the growth of the bacilli, confirming that the PDE4i had no direct effect on bacillary growth or killing, but rather affected the bacilli in vivo via modifying the host cell (Figure S2). Thus, dampening innate immunity without interfering with the development of the acquired immune response was associated with changes in the metabolic activity of Mtb but not with loss of control of bacillary growth. Previous reports have demonstrated the usefulness of TNF-α inhibition by CC-3052 treatment, both in acute and chronic HIV-1 infection in vitro [122], [123]. In addition, the safe administration of several PDE4i has been demonstrated in clinical trials for the treatment of other inflammatory diseases, including asthma and chronic obstructive pulmonary disease [124]–[126]. An adjunctive therapeutic approach, such as the one described in this study, could be used to treat humans with TB, providing a means for shortening treatment and improving clinical outcome in patients with active disease. The absence of significant immune suppression or other toxicities supports the idea that such an approach may safely contribute to improved treatment of TB.
Taken together, our observations support the hypothesis that changes in the physiology of the bacteria, in response to changes in the host immune response can alter the susceptibility of the bacteria to antimicrobial agents. This is a novel strategy to combat Mtb infection, facilitating the use of the existing drugs more efficiently. As different anti-TB drugs target both overlapping and diverse bacterial functions, it is important to evaluate immune modulation during other single as well as multidrug treatments. Currently we are testing this approach by combining CC-3052 with other anti-TB drugs in our rabbit model of pulmonary TB.
Materials and Methods
Ethics statement
All rabbit experiments were performed according to the procedures and policies of the Animal Welfare Act guidelines for housing and care of laboratory animals and conducted in accordance with Public Health Service Policy Institutional regulations. Animal ethics approval for rabbit aerosol infection with Mtb, treatment, post-treatment care, euthanasia and necropsy was obtained from the Institutional Animal Care and Use Committee (IACUC) and Institutional Biosafety Committee (IBC) of the Public Health Research Institute (PHRI) at University of Medicine and Dentistry of New Jersey (UMDNJ) (IACUC approval numbers 070,124 and 125). All procedures with the infected animals/tissues were performed in a Biosafety level three (BSL-3) containment facilities according to the approved protocols.
Mycobacterial culture conditions and chemicals
Mycobacterium tuberculosis HN878 (Mtb) (a gift from Dr. Musser, TX, USA), a member of Beijing strains, was grown to mid-log phase (OD600 = 0.6–0.7) in Middlebrook 7H9 liquid media (Difco, MI, USA) supplemented with 0.5% glycerol, 10% OADC enrichment (oleic acid, albumin, dextrose and catalase; BD Biosciences, MD, USA) and 0.25% Tween 80 at 37°C and 5% CO2 as static culture. The culture was gently mixed once a day for proper aeration. Serial dilutions of the culture were enumerated for the number of colony forming units (CFU) by plating on 7H10 agar plates (Difco, MI, USA) followed by incubation of the plates at 37°C and 5% CO2 for 4–5 weeks. All the stock cultures were stored as aliquots at −80°C until use. A vial of frozen stock culture was thawed at 37°C, sonicated three times in 5-seconds pulses on ice to disrupt bacterial clumps, diluted to 5×106 CFU/ml in sterile saline with 0.05% Tween 80 and used for rabbit aerosol infection [127]. The PDE4 inhibitor used in this study, CC-3052, was a kind gift from Celgene Corporation (Celgene Corporation, NJ, USA). All other chemicals were purchased from Sigma (St. Louis, MI, USA) unless otherwise mentioned.
To study the effect of CC-3052 and/or INH on Mtb growth in vitro, bacterial pellets from mid-log culture were washed with sterile PBS and resuspended in complete 7H9 media. About 1×106 CFU/ml of the bacteria in complete 7H9 media was seeded in to 24 well plates and various concentrations of INH (0.0125 µg or 0.2 µg per ml), CC-3052 (4 or 40 or 200 µM) or a combination of both (CC-3052+INH) were added every day up to 4 days. The number of bacterial CFU was enumerated for the initial inoculum and after every 24 hours of culture by plating serially diluted bacterial suspensions (treated and untreated) on 7H10 agar plates followed by incubation of the plates at 37°C and 5% CO2 for 4–5 weeks.
Rabbit aerosol infections
A total of 84 male and female, specific pathogen-free, New Zealand White rabbits of approximately 2.5 kg in weight, purchased from Millbrook Farms (Millbrook Farms, MA,USA) were used for all the experiments reported in this study. The animals were acclimatized for a week after arrival at the PHRI Research Animal Facility before exposure to aerosol challenge. Groups of 6 rabbits (per round of infection) were infected with Mtb, using a nose-only aerosol exposure system (CH Technologies, Inc., NJ, USA) as described earlier [127]. Twelve to twenty-four rabbits were infected for each experiment. After 3 hours post-exposure, one group of infected animals were sedated with a combination of Ketamine and Xylazine and euthanized by Euthasol and necropsy performed to determine the bacillary load (T = 0) implanted in the lungs as described earlier [128]. For all the experiments, the bacterial inoculum, exposure time and conditions were standardized so as to implant approximately 3.2 log10 CFU in the lungs at T = 0. Infected rabbits were housed individually in a BSL-3 facility, with an unrestricted food and water supply. At defined time points (T = 4, 8 and 12 weeks post-infection), groups of 2–4 rabbits, per treatment and time point, were euthanized and the lung, liver and spleen aseptically removed and portions of each organs used for CFU enumeration and isolation of host and bacterial RNA. The tissue segments for RNA isolation were frozen immediately in liquid nitrogen and stored at −80°C.
Treatment of Mtb infected rabbits
The Mtb infected rabbits were classified into the following treatment groups: 1. CC-3052 Treatment. The immune modulatory drug used in this study, CC-3052, is an analog of thalidomide. Solutions of CC-3052 were prepared, freshly every day, in sterile water and administered through gavage at a dose of 25 mg/kg body weight using a flexible rubber feeding tube 5 days per week. Treatment with CC-3052 started at 4 weeks post-infection and continued until 12 weeks after infection (8 weeks of treatment). 2. Isoniazid (INH) Chemotherapy. Freshly prepared INH, at 25 or 50 mg/kg body weight was administered to rabbits through oral administration using a flexible rubber feeding tube 5 days a week. The INH treatment was started at 4 weeks post-infection and continued until 12 weeks after Mtb infection (8 weeks of treatment). 3. INH plus CC-3052 Combination Therapy. Rabbits were treated with a combination of INH (50 mg/kg body weight) plus CC-3052 (25 mg/kg body weight) using a flexible rubber feeding tube 5 days per week. Treatment was initiated concurrently at 4 weeks post-infection and continued until 12 weeks after Mtb infection (8 weeks of treatment).
All three treatments were carried out in parallel. One group each of uninfected and infected but untreated rabbits served as controls for each time point of the experiment.
Bacillary load estimation
Bacterial loads in the lungs, liver and spleens of the Mtb infected rabbits were enumerated by CFU assay in respective organs at each time point of the experiment. Briefly, random portions of lungs (about 1/3 of the entire lung), liver (about 1/10) and spleen (about half) tissue homogenates in sterile saline were serially diluted and plated onto Middlebrook 7H11 agar plates (Difco, MI, USA) with or without INH supplement (0.2 µg/ml). The plates were incubated at 37°C for 4 to 5 weeks. Colonies were counted, and results were calculated for total numbers of CFU in the whole organ.
Host and mycobacterial total RNA isolation
Total RNA of the host and Mtb were isolated from the lung tissues of all four groups of rabbit (infected, infected and CC-3052-, INH - or INH+CC-3052 - treated and uninfected) at each time point of necropsy (T = 0, 4, 8 and 12 weeks post-infection). Tissue sections from each rabbit were processed separately for host and bacterial RNA extraction. To prepare host RNA, lung tissue slices were homogenized in 10 volumes (wt/vol) of TRIzol (Invitrogen, CA, USA) using a PolyTron homogenizer (Kinematica, Lucerne, Switzerland), and extracted with 0.3 vol (vol/vol) of chloroform. The mixture was centrifuged at 13,000 rpm for 20 minutes at 4°C and the cleared supernatant was added to an equal volume of precipitation solution (Macherey-Nagel, GmbH) and eluted through the NucleoSpin kit as per the manufacturer's protocol (Macherey-Nagel, GmbH). For bacterial total RNA isolation from infected rabbit lungs, a modified differential lysis method was used [129]. Briefly, the tissue sections were homogenized in 10 volumes of sterile 0.01% SDS solution, followed by centrifugation at 13,000 rpm for 20 minutes at 15°C to pellet the bacteria. The bacterial pellet was resuspended in 10 volume of TRIzol (wt/vol) and the mixture was subjected to bead beating with Ribo-lyser (MP Biosciences, OK, USA) for 2 minutes in 30-seconds pulses with 1-minute ice-incubation in between the pulses. The lysate was extracted with equal volume of chloroform (vol/vol) followed by centrifugation and the cleared supernatant was eluted using NucleoSpin kit as per the manufacturer's protocol (Macherey-Nagel, GmbH). Both host and bacterial RNA were subjected to DNase I digestion before final purification through RNeasy mini kit (Qiagen, MD, USA). The quantity and quality of the total RNA was estimated by NanoDrop (NanoDrop Products, DE, USA) and agarose gel electrophoresis as reported elsewhere [130].
Rabbit microarray analysis
The rabbit microarray slides and the recommended reagents were purchased from Agilent Technologies (Agilent Technologies, CA, USA). According to the manufacturer, each rabbit microarray slide contains quadruplicates of 43,604 probes (4 arrays per slide) of rabbit genome, derived from public domain databases, corresponding to 43,604 open reading frames (ORFs). The total lung RNA from individual rabbits (2–4 animals per time point per group) was processed separately for each microarray experiment. Dye-swap was done for every group, to avoid dye bias during cDNA labeling, hybridization and post-hybridization procedures. The conditions for cDNA synthesis, labeling and hybridization were according to the standard operating protocol of the Center for Advanced Genomics of PHRI (refer <http://www.cag.icph.org/downloads_page.htm>). For the 4 weeks time point, the gene expression values of the Mtb infected rabbits were calculated relative to uninfected rabbits (Infected vs. Uninfected) and for all other time points, the gene expression values of CC-3052 treated rabbits were calibrated against untreated (but Mtb infected) rabbits at the same time point (CC-3052 treated vs. untreated). The slides were scanned and analyzed by Agilent Scanner and Feature Extraction Software (Agilent Technologies, CA, USA) and the acquired data was loaded into Partek Genomics Suite (PARTEK, MO, USA) for further analysis after appropriate statistical analysis to adjust for the errors. A 2.0 fold difference in expression value and a p value of less than 0.05 was set as cut-off to select differentially expressed genes between various comparison groups. The selected list of differentially expressed rabbit genes from PARTEK Genomics Suite was further analyzed for functional classification including derivation of networks and pathways using Ingenuity Pathway Analysis (IPA) software (Ingenuity Pathway Analysis, CA, USA). Since the information on the functional characterization of rabbit genes is currently unavailable with any of the commercial software including IPA, we used the gene information from human, mouse and rat database, from various microarray platforms, available in IPA. We searched the IPA knowledge base for conserved homologous of rabbit genes that perform similar functions and mostly share common pathways, across the 3 different species (mouse, rat and human). The filtered list of rabbit genes, containing “pathway eligible genes” was classified according to their functions in specific pathways and used to construct corresponding networks in IPA. The complete list of rabbit genes differentially expressed by CC-3052 treatment has been submitted to the GEO database (GSE27992).
Quantitative Real Time PCR Analysis (qRT-PCR)
Total RNA of the host and Mtb, isolated from the uninfected/infected rabbit lungs were subjected to cDNA synthesis using Sprint RT Complete kit as described by the manufacturer (Clontech, CA, USA). The cDNA was amplified with gene specific primers and SYBR green mix as per the manufacturer's instructions (Clontech, CA, USA) in a MxPro4000 real time PCR machine (Stratagene, CA, USA). The SYBR green qRT-PCR mix also contains ROX as an internal reference dye. The primers for specific rabbit and Mtb genes were designed using Clone Manager Suite (Sci-Ed software, NC, USA). The DNA (for Mtb) and mRNA (for rabbit) sequences of specific Mtb and rabbit genes were obtained from Tuberculist (for Mtb genes) or GenBank (for rabbit genes) data base. The DNA sequences of the primers used for qRT-PCR can be found in Table S1 and S2. The threshold cycle (Ct) for each amplified target gene was calculated using MxPro4000 software (Stratagene, CA, USA). Uniform baseline fluorescence was set for all the genes in each experiment and across different experiments. The Mtb gene for 16S rRNA and the transcripts for rabbit GAPDH gene were used to normalize the Ct values of the target genes. Fold change was calculated using the formula 2−ΔΔCt and represented either as absolute transcript levels or as relative expression after normalization to uninfected or untreated groups. The experiments were repeated at least 3 times with RNA samples from two to four animals per group per time point per treatment group.
Isoniazid measurement in rabbit plasma
The concentration of INH in the plasma of Mtb infected and treated rabbits were measured using Sciex API4000 (Applied Biosystems, CA, USA) mass spectrometer coupled to Symbiosis Pharma HPLC system (Spark Holland B.V, The Netherlands). Briefly, one part of the plasma or homogenized lung tissue was extracted with nine parts (vol/vol) of acetonitrile containing 0.2% acetic acid. Ten microliter of the supernatant was injected into a Phenomenex Gemini C6-Phenyl column (4.6×150 mm) (Phenomenex, CA, USA) and eluted with a gradient of mobile phase A (0.2% acetic acid in deionized water) and B (0.2% acetic acid in methanol) at a flow rate of 1 ml/min. Presence of the INH analytes and quantification of drug levels were performed by monitoring multiple reactions of parent/daughter transitions in electro-spray positive ionization mode. The test samples, standards and quality control samples were spiked with Warfarin, which was used as an internal standard. The calibration curve was designed to cover the expected concentration range (10.2 ng/ml to 10.4 µg/ml) of samples delivered and was derived from standard solutions of INH. The data obtained was processed using Analyst software v 1.4.2 (Applied Biosystems, CA, USA) and regression for pharmacokinetic parameters was performed as non-compartmental analysis using WinNonLin 5.0 (Pharsight, CA, USA).
Statistical analysis
The independent Student t-test or the Mann-Whitney test for nonparametric independent data was used for analysis (SPSS software). p≤0.05 was considered significant for all the experiments.
Supporting Information
Zdroje
1. DyeCWilliamsBG 2010 The population dynamics and control of tuberculosis. Science 328 856 861
2. de ChastellierC 2009 The many niches and strategies used by pathogenic mycobacteria for survival within host macrophages. Immunobiology 214 526 542
3. SaundersBMBrittonWJ 2007 Life and death in the granuloma: immunopathology of tuberculosis. Immunol Cell Biol 85 103 111
4. CosmaCLShermanDRRamakrishnanL 2003 The secret lives of the pathogenic mycobacteria. Annu Rev Microbiol 57 641 676
5. PietersJ 2008 Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe 3 399 407
6. JordaoLBleckCKMayorgaLGriffithsGAnesE 2008 On the killing of mycobacteria by macrophages. Cell Microbiol 10 529 548
7. PaigeCBishaiWR 2009 Penitentiary or penthouse condo: the tuberculous granuloma from the microbe's point of view. Cell Microbiol 12 301 309
8. StokesRWWaddellSJ 2009 Adjusting to a new home: Mycobacterium tuberculosis gene expression in response to an intracellular lifestyle. Future Microbiol 4 1317 1335
9. SteinbergBEGrinsteinS 2008 Pathogen destruction versus intracellular survival: the role of lipids as phagosomal fate determinants. J Clin Invest 118 2002 2011
10. ChanJFlynnJ 2004 The immunological aspects of latency in tuberculosis. Clin Immunol 110 2 12
11. FlannaganRSCosioGGrinsteinS 2009 Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol 7 355 366
12. RohdeKHAbramovitchRBRussellDG 2007 Mycobacterium tuberculosis invasion of macrophages: linking bacterial gene expression to environmental cues. Cell Host Microbe 2 352 364
13. SchnappingerDEhrtSVoskuilMILiuYManganJA 2003 Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J Exp Med 198 693 704
14. DuttaNKMehraSDidierPJRoyCJDoyleLA 2010 Genetic requirements for the survival of tubercle bacilli in primates. J Infect Dis 201 1743 1752
15. TailleuxLWaddellSJPelizzolaMMortellaroAWithersM 2008 Probing host pathogen cross-talk by transcriptional profiling of both Mycobacterium tuberculosis and infected human dendritic cells and macrophages. PLoS One 3 e1403
16. HampshireTSonejiSBaconJJamesBWHindsJ 2004 Stationary phase gene expression of Mycobacterium tuberculosis following a progressive nutrient depletion: a model for persistent organisms? Tuberculosis (Edinb) 84 228 238
17. FlynnJLChanJ 2001 Immunology of tuberculosis. Annu Rev Immunol 19 93 129
18. KoulAHergetTKleblBUllrichA 2004 Interplay between mycobacteria and host signalling pathways. Nat Rev Microbiol 2 189 202
19. SahiratmadjaEAlisjahbanaBde BoerTAdnanIMayaA 2007 Dynamic changes in pro - and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease activity and response to curative treatment. Infect Immun 75 820 829
20. SalgameP 2005 Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr Opin Immunol 17 374 380
21. MacMickingJD 2009 Recognizing macrophage activation and host defense. Cell Host Microbe 5 405 407
22. LiuPTModlinRL 2008 Human macrophage host defense against Mycobacterium tuberculosis. Curr Opin Immunol 20 371 376
23. EhrtSSchnappingerD 2009 Mycobacterial survival strategies in the phagosome: defence against host stresses. Cell Microbiol 11 1170 1178
24. QuesniauxVFJacobsMAllieNGrivennikovSNedospasovSA 2010 TNF in host resistance to tuberculosis infection. Curr Dir Autoimmun 11 157 179
25. LyLHMcMurrayDN 2009 The Yin-Yang of TNFalpha in the guinea pig model of tuberculosis. Indian J Exp Biol 47 432 439
26. BekkerLGFreemanSMurrayPJRyffelBKaplanG 2001 TNF-alpha controls intracellular mycobacterial growth by both inducible nitric oxide synthase-dependent and inducible nitric oxide synthase-independent pathways. J Immunol 166 6728 6734
27. HaoSBaltimoreD 2009 The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat Immunol 10 281 288
28. BothaTRyffelB 2003 Reactivation of latent tuberculosis infection in TNF-deficient mice. J Immunol 171 3110 3118
29. BekkerLGHaslettPMaartensGSteynLKaplanG 2000 Thalidomide-induced antigen-specific immune stimulation in patients with human immunodeficiency virus type 1 and tuberculosis. J Infect Dis 181 954 965
30. KastRE 2000 Tumor necrosis factor has positive and negative self regulatory feed back cycles centered around cAMP. Int J Immunopharmacol 22 1001 1006
31. ZidekZ 1999 Adenosine - cyclic AMP pathways and cytokine expression. Eur Cytokine Netw 10 319 328
32. EiglerASiegmundBEmmerichUBaumannKHHartmannG 1998 Anti-inflammatory activities of cAMP-elevating agents: enhancement of IL-10 synthesis and concurrent suppression of TNF production. J Leukoc Biol 63 101 107
33. SerezaniCHBallingerMNAronoffDMPeters-GoldenM 2008 Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol 39 127 132
34. SounessJEGriffinMMaslenCEbsworthKScottLC 1996 Evidence that cyclic AMP phosphodiesterase inhibitors suppress TNF alpha generation from human monocytes by interacting with a ‘low-affinity’ phosphodiesterase 4 conformer. Br J Pharmacol 118 649 658
35. SounessJEAldousDSargentC 2000 Immunosuppressive and anti-inflammatory effects of cyclic AMP phosphodiesterase (PDE) type 4 inhibitors. Immunopharmacology 47 127 162
36. DereeJMartinsJOMelbostadHLoomisWHCoimbraR 2008 Insights into the regulation of TNF-alpha production in human mononuclear cells: the effects of non-specific phosphodiesterase inhibition. Clinics (Sao Paulo) 63 321 328
37. LeeTWFedorakRN 2010 Tumor necrosis factor-alpha monoclonal antibodies in the treatment of inflammatory bowel disease: clinical practice pharmacology. Gastroenterol Clin North Am 39 543 557
38. TaylorPC 2010 Pharmacology of TNF blockade in rheumatoid arthritis and other chronic inflammatory diseases. Curr Opin Pharmacol 10 308 315
39. MurdacaGColomboBMPuppoF 2009 Anti-TNF-alpha inhibitors: a new therapeutic approach for inflammatory immune-mediated diseases: an update upon efficacy and adverse events. Int J Immunopathol Pharmacol 22 557 565
40. SampaioEPSarnoENGalillyRCohnZAKaplanG 1991 Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med 173 699 703
41. MoreiraALSampaioEPZmuidzinasAFrindtPSmithKA 1993 Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med 177 1675 1680
42. TramontanaJMUtaipatUMolloyAAkarasewiPBurroughsM 1995 Thalidomide treatment reduces tumor necrosis factor alpha production and enhances weight gain in patients with pulmonary tuberculosis. Mol Med 1 384 397
43. CorralLGKaplanG 1999 Immunomodulation by thalidomide and thalidomide analogues. Ann Rheum Dis 58 Suppl 1 I107 113
44. MarriottJBMullerGStirlingDDalgleishAG 2001 Immunotherapeutic and antitumour potential of thalidomide analogues. Expert Opin Biol Ther 1 675 682
45. BartlettJBMichaelAClarkeIADredgeKNicholsonS 2004 Phase I study to determine the safety, tolerability and immunostimulatory activity of thalidomide analogue CC-5013 in patients with metastatic malignant melanoma and other advanced cancers. Br J Cancer 90 955 961
46. MarriottJBWestbyMCooksonSGuckianMGoodbournS 1998 CC-3052: a water-soluble analog of thalidomide and potent inhibitor of activation-induced TNF-alpha production. J Immunol 161 4236 4243
47. CorralLGHaslettPAMullerGWChenRWongLM 1999 Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol 163 380 386
48. MarriottJBClarkeIADredgeKMullerGStirlingD 2002 Thalidomide and its analogues have distinct and opposing effects on TNF-alpha and TNFR2 during co-stimulation of both CD4(+) and CD8(+) T cells. Clin Exp Immunol 130 75 84
49. KooMSMancaCYangGO'BrienPSungN 2011 Phosphodiesterase 4 inhibition reduces innate immunity and improves isoniazid clearance of Mycobacterium tuberculosis in the lungs of infected mice. PLoS One 6 e17091
50. SirgelFADonaldPROdhiamboJGithuiWUmapathyKC 2000 A multicentre study of the early bactericidal activity of anti-tuberculosis drugs. J Antimicrob Chemother 45 859 870
51. FoxWEllardGAMitchisonDA 1999 Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946-1986, with relevant subsequent publications. Int J Tuberc Lung Dis 3 S231 279
52. MitchisonDA 2003 Role of isoniazid in once-weekly rifapentine treatment of pulmonary tuberculosis. Am J Respir Crit Care Med 167 1298 1299
53. WeinerMBurmanWVernonABenatorDPeloquinCA 2003 Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am J Respir Crit Care Med 167 1341 1347
54. BettsJCMcLarenALennonMGKellyFMLukeyPT 2003 Signature gene expression profiles discriminate between isoniazid-, thiolactomycin-, and triclosan-treated Mycobacterium tuberculosis. Antimicrob Agents Chemother 47 2903 2913
55. BoshoffHIMyersTGCoppBRMcNeilMRWilsonMA 2004 The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J Biol Chem 279 40174 40184
56. WilsonMDeRisiJKristensenHHImbodenPRaneS 1999 Exploring drug-induced alterations in gene expression in Mycobacterium tuberculosis by microarray hybridization. Proc Natl Acad Sci U S A 96 12833 12838
57. KarakousisPCWilliamsEPBishaiWR 2008 Altered expression of isoniazid-regulated genes in drug-treated dormant Mycobacterium tuberculosis. J Antimicrob Chemother 61 323 331
58. JoAnneLFlynnLTIzzoAngeloKaplanGilla 2008 Experimental Animal Models of Tuberculosis. KGaA, Weinheim Wiley-VCH Verlag GmBH&Co 389 426
59. LeeBYJethwaneyDSchillingBClemensDLGibsonBW The Mycobacterium bovis bacille Calmette-Guerin phagosome proteome. Mol Cell Proteomics 9 32 53
60. MaYLiuHTu-RappHThiesenHJIbrahimSM 2004 Fas ligation on macrophages enhances IL-1R1-Toll-like receptor 4 signaling and promotes chronic inflammation. Nat Immunol 5 380 387
61. ViaLELinPLRaySMCarrilloJAllenSS 2008 Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun 76 2333 2340
62. ParkHDGuinnKMHarrellMILiaoRVoskuilMI 2003 Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol 48 833 843
63. BartekILRutherfordRGruppoVMortonRAMorrisRP 2009 The DosR regulon of M. tuberculosis and antibacterial tolerance. Tuberculosis (Edinb) 89 310 316
64. HomolkaSNiemannSRussellDGRohdeKH 2010 Functional genetic diversity among Mycobacterium tuberculosis complex clinical isolates: delineation of conserved core and lineage-specific transcriptomes during intracellular survival. PLoS Pathog 6 e1000988
65. SohaskeyCDWayneLG 2003 Role of narK2X and narGHJI in hypoxic upregulation of nitrate reduction by Mycobacterium tuberculosis. J Bacteriol 185 7247 7256
66. HumpelAGebhardSCookGMBerneyM 2010 The SigF regulon in Mycobacterium smegmatis reveals roles in adaptation to stationary phase, heat, and oxidative stress. J Bacteriol 192 2491 2502
67. SchnappingerD Schoolnik GK, Ehrt S 2006 Expression profiling of host pathogen interactions: how Mycobacterium tuberculosis and the macrophage adapt to one another. Microbes Infect 8 1132 1140
68. WaddellSJStablerRALaingKKremerLReynoldsRC 2004 The use of microarray analysis to determine the gene expression profiles of Mycobacterium tuberculosis in response to anti-bacterial compounds. Tuberculosis (Edinb) 84 263 274
69. KaplanGTsenovaL 2010 Pulmonary Tuberculosis in the Rabbit. Leong F.JDVDickT A color atlas of comparative pathology of pulmonary tuberculosis Singapore CRC Press 107 130
70. KarakousisPCYoshimatsuTLamichhaneGWoolwineSCNuermbergerEL 2004 Dormancy phenotype displayed by extracellular Mycobacterium tuberculosis within artificial granulomas in mice. J Exp Med 200 647 657
71. JindaniAAberVREdwardsEAMitchisonDA 1980 The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis 121 939 949
72. VilchezeCJacobsWRJr 2007 The mechanism of isoniazid killing: clarity through the scope of genetics. Annu Rev Microbiol 61 35 50
73. RussellDG 2001 Mycobacterium tuberculosis: here today, and here tomorrow. Nat Rev Mol Cell Biol 2 569 577
74. BeavoJAContiMHeaslipRJ 1994 Multiple cyclic nucleotide phosphodiesterases. Mol Pharmacol 46 399 405
75. ContiMBeavoJ 2007 Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76 481 511
76. ShepherdMCBaillieGSStirlingDIHouslayMD 2004 Remodelling of the PDE4 cAMP phosphodiesterase isoform profile upon monocyte-macrophage differentiation of human U937 cells. Br J Pharmacol 142 339 351
77. AgarwalNLamichhaneGGuptaRNolanSBishaiWR 2009 Cyclic AMP intoxication of macrophages by a Mycobacterium tuberculosis adenylate cyclase. Nature 460 98 102
78. TaskenKAandahlEM 2004 Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev 84 137 167
79. Bengis-GarberCGruenerN 1996 Protein kinase A downregulates the phosphorylation of p47 phox in human neutrophils: a possible pathway for inhibition of the respiratory burst. Cell Signal 8 291 296
80. KalamidasSAKuehnelMPPeyronPRybinVRauchS 2006 cAMP synthesis and degradation by phagosomes regulate actin assembly and fusion events: consequences for mycobacteria. J Cell Sci 119 3686 3694
81. LinPLPlessnerHLVoitenokNNFlynnJL 2007 Tumor necrosis factor and tuberculosis. J Investig Dermatol Symp Proc 12 22 25
82. AkiraSTakedaK 2004 Toll-like receptor signalling. Nat Rev Immunol 4 499 511
83. BonizziGKarinM 2004 The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25 280 288
84. LyLHJeevanAMcMurrayDN 2009 Neutralization of TNFalpha alters inflammation in guinea pig tuberculous pleuritis. Microbes Infect 11 680 688
85. RobertsEAChuaJKyeiGBDereticV 2006 Higher order Rab programming in phagolysosome biogenesis. J Cell Biol 174 923 929
86. ThuongNTDunstanSJChauTTThorssonVSimmonsCP 2008 Identification of tuberculosis susceptibility genes with human macrophage gene expression profiles. PLoS Pathog 4 e1000229
87. VolpeECappelliGGrassiMMartinoASerafinoA 2006 Gene expression profiling of human macrophages at late time of infection with Mycobacterium tuberculosis. Immunology 118 449 460
88. ShuiWGilmoreSASheuLLiuJKeaslingJD 2009 Quantitative proteomic profiling of host-pathogen interactions: the macrophage response to Mycobacterium tuberculosis lipids. J Proteome Res 8 282 289
89. RohdeKYatesRMPurdyGERussellDG 2007 Mycobacterium tuberculosis and the environment within the phagosome. Immunol Rev 219 37 54
90. CappelliGVolpeEGrassiMLiseoBColizziV 2006 Profiling of Mycobacterium tuberculosis gene expression during human macrophage infection: upregulation of the alternative sigma factor G, a group of transcriptional regulators, and proteins with unknown function. Res Microbiol 157 445 455
91. DebCLeeCMDubeyVSDanielJAbomoelakB 2009 A novel in vitro multiple-stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen. PLoS One 4 e6077
92. KaplanGPostFAMoreiraALWainwrightHKreiswirthBN 2003 Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun 71 7099 7108
93. VoskuilMISchnappingerDViscontiKCHarrellMIDolganovGM 2003 Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med 198 705 713
94. SirakovaTDDubeyVSDebCDanielJKorotkovaTA 2006 Identification of a diacylglycerol acyltransferase gene involved in accumulation of triacylglycerol in Mycobacterium tuberculosis under stress. Microbiology 152 2717 2725
95. ShilohMUManzanilloPCoxJS 2008 Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe 3 323 330
96. PrimmTPAndersenSJMizrahiVAvarbockDRubinH 2000 The stringent response of Mycobacterium tuberculosis is required for long-term survival. J Bacteriol 182 4889 4898
97. KlinkenbergLGLeeJHBishaiWRKarakousisPC 2011 The stringent response is required for full virulence of Mycobacterium tuberculosis in guinea pigs. J Infect Dis 202 1397 1404
98. RosenkrandsISlaydenRACrawfordJAagaardCBarryCE3rd 2002 Hypoxic response of Mycobacterium tuberculosis studied by metabolic labeling and proteome analysis of cellular and extracellular proteins. J Bacteriol 184 3485 3491
99. ConversePJKarakousisPCKlinkenbergLGKesavanAKLyLH 2009 Role of the dosR-dosS two-component regulatory system in Mycobacterium tuberculosis virulence in three animal models. Infect Immun 77 1230 1237
100. KesavanAKBrooksMTufarielloJChanJManabeYC 2009 Tuberculosis genes expressed during persistence and reactivation in the resistant rabbit model. Tuberculosis (Edinb) 89 17 21
101. SachdevaPMisraRTyagiAKSinghY 2009 The sigma factors of Mycobacterium tuberculosis: regulation of the regulators. FEBS J 277 605 626
102. RaoVFujiwaraNPorcelliSAGlickmanMS 2005 Mycobacterium tuberculosis controls host innate immune activation through cyclopropane modification of a glycolipid effector molecule. J Exp Med 201 535 543
103. RaoVGaoFChenBJacobsWRJrGlickmanMS 2006 Trans-cyclopropanation of mycolic acids on trehalose dimycolate suppresses Mycobacterium tuberculosis -induced inflammation and virulence. J Clin Invest 116 1660 1667
104. LeistikowRLMortonRABartekILFrimpongIWagnerK 2009 The Mycobacterium tuberculosis DosR regulon assists in metabolic homeostasis and enables rapid recovery from nonrespiring dormancy. J Bacteriol 192 1662 1670
105. ChauhanSSharmaDSinghASuroliaATyagiJS 2011 Comprehensive insights into Mycobacterium tuberculosis DevR (DosR) regulon activation switch. Nucleic Acids Res. E-pub ahead of print doi:10.1093/nar/gkr37
106. Saint-JoanisBSouchonHWilmingMJohnssonKAlzariPM 1999 Use of site-directed mutagenesis to probe the structure, function and isoniazid activation of the catalase/peroxidase, KatG, from Mycobacterium tuberculosis. Biochem J 338 Pt 3 753 760
107. ParikhSLXiaoGTongePJ 2000 Inhibition of InhA, the enoyl reductase from Mycobacterium tuberculosis, by triclosan and isoniazid. Biochemistry 39 7645 7650
108. WengenackNLJensenMPRusnakFSternMK 1999 Mycobacterium tuberculosis KatG is a peroxynitritase. Biochem Biophys Res Commun 256 485 487
109. TimminsGSDereticV 2006 Mechanisms of action of isoniazid. Mol Microbiol 62 1220 1227
110. FontanPAArisVAlvarezMEGhannySChengJ 2008 Mycobacterium tuberculosis sigma factor E regulon modulates the host inflammatory response. J Infect Dis 198 877 885
111. MorrisRPNguyenLGatfieldJViscontiKNguyenK 2005 Ancestral antibiotic resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 102 12200 12205
112. NguyenLThompsonCJ 2006 Foundations of antibiotic resistance in bacterial physiology: the mycobacterial paradigm. Trends Microbiol 14 304 312
113. AdamsKNTakakiKConnollyLEWiedenhoftHWingleeK 2011 Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell 145 39 53
114. GuptaAKKatochVMChauhanDSSharmaRSinghM 2011 Microarray analysis of efflux pump genes in multidrug-resistant Mycobacterium tuberculosis during stress induced by common anti-tuberculous drugs. Microb Drug Resist 16 21 28
115. VilchezeCBaughnADTufarielloJLeungLKuoM 2011 Novel inhibitors of InhA efficiently kill Mycobacterium tuberculosis under aerobic and anaerobic conditions. Antimicrob Agents Chemother 55 3889 98
116. FlynnJLGoldsteinMMChanJTrieboldKJPfefferK 1995 Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2 561 572
117. ChakravartySDZhuGTsaiMCMohanVPMarinoS 2008 Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs. Infect Immun 76 916 926
118. BernsteinDMLaneyCMorrisEKLoftusEF 2005 False beliefs about fattening foods can have healthy consequences. Proc Natl Acad Sci U S A 102 13724 13731
119. LynchMJHillEVHouslayMD 2006 Intracellular targeting of phosphodiesterase-4 underpins compartmentalized cAMP signaling. Curr Top Dev Biol 75 225 259
120. PasipanodyaJGMillerTLVecinoMMunguiaGGarmonR 2007 Pulmonary impairment after tuberculosis. Chest 131 1817 1824
121. SaliuOYSoferCSteinDSSchwanderSKWallisRS 2006 Tumor-necrosis-factor blockers: differential effects on mycobacterial immunity. J Infect Dis 194 486 492
122. La MaestraLZaninoniAMarriottJBLazzarinADalgleishAG 2000 The thalidomide analogue CC-3052 inhibits HIV-1 and tumour necrosis factor-alpha (TNF-alpha) expression in acutely and chronically infected cells in vitro. Clin Exp Immunol 119 123 129
123. GuckianMDransfieldIHayPDalgleishAG 2000 Thalidomide analogue CC-3052 reduces HIV+ neutrophil apoptosis in vitro. Clin Exp Immunol 121 472 479
124. SpinaD 2008 PDE4 inhibitors: current status. Br J Pharmacol 155 308 315
125. BartlettJBDredgeKDalgleishAG 2004 The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer 4 314 322
126. SchaferPHPartonAGandhiAKCaponeLAdamsM 2010 Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol 159 842 855
127. TsenovaLBergtoldAFreedmanVHYoungRAKaplanG 1999 Tumor necrosis factor alpha is a determinant of pathogenesis and disease progression in mycobacterial infection in the central nervous system. Proc Natl Acad Sci U S A 96 5657 5662
128. TsenovaLSokolKFreedmanVHKaplanG 1998 A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. J Infect Dis 177 1563 1572
129. TalaatAMLyonsRHowardSTJohnstonSA 2004 The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proc Natl Acad Sci U S A 101 4602 4607
130. SubbianSMehtaPKCirilloSLCirilloJD 2007 The Mycobacterium marinum mel2 locus displays similarity to bacterial bioluminescence systems and plays a role in defense against reactive oxygen and nitrogen species. BMC Microbiol 7 4
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



