-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaStructure of the Vesicular Stomatitis Virus N-P Complex
Replication of non-segmented negative-strand RNA viruses requires the continuous supply of the nucleoprotein (N) in the form of a complex with the phosphoprotein (P). Here, we present the structural characterization of a soluble, heterodimeric complex between a variant of vesicular stomatitis virus N lacking its 21 N-terminal residues (NΔ21) and a peptide of 60 amino acids (P60) encompassing the molecular recognition element (MoRE) of P that binds RNA-free N (N0). The complex crystallized in a decameric circular form, which was solved at 3.0 Å resolution, reveals how the MoRE folds upon binding to N and competes with RNA binding and N polymerization. Small-angle X-ray scattering experiment and NMR spectroscopy on the soluble complex confirms the binding of the MoRE and indicates that its flanking regions remain flexible in the complex. The structure of this complex also suggests a mechanism for the initiation of viral RNA synthesis.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002248
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002248Summary
Replication of non-segmented negative-strand RNA viruses requires the continuous supply of the nucleoprotein (N) in the form of a complex with the phosphoprotein (P). Here, we present the structural characterization of a soluble, heterodimeric complex between a variant of vesicular stomatitis virus N lacking its 21 N-terminal residues (NΔ21) and a peptide of 60 amino acids (P60) encompassing the molecular recognition element (MoRE) of P that binds RNA-free N (N0). The complex crystallized in a decameric circular form, which was solved at 3.0 Å resolution, reveals how the MoRE folds upon binding to N and competes with RNA binding and N polymerization. Small-angle X-ray scattering experiment and NMR spectroscopy on the soluble complex confirms the binding of the MoRE and indicates that its flanking regions remain flexible in the complex. The structure of this complex also suggests a mechanism for the initiation of viral RNA synthesis.
Introduction
Negative-sense RNA viruses include numerous major human pathogens such as influenza virus, rabies virus, measles virus and respiratory syncytial virus. The (−)RNA genome of these viruses is condensed by a viral nucleoprotein (N) into a helical nucleocapsid [1], that associates with the polymerase complex and serves as the template for RNA replication and transcription [2]. Replication of the genome thus requires a continuous supply of N molecules to encapsidate both the (+)RNA intermediate copies and the newly synthesized (−)RNA genomes in single-stranded forms [3]. For non-segmented (−)RNA viruses of the Rhabdoviridae and Paramyxoviridae, N is assisted by the viral phosphoprotein (P). P binds to nascent RNA-free N, forming a N0-P complex (the superscript 0 denotes the absence of RNA) that prevents the polymerization of N and the non-specific encapsidation of host cell RNAs [4], [5], [6], [7], [8], [9]. These processes are independent of each other [10] and, therefore, P has to fulfill two chaperone activities; blocking both RNA binding and self-assembly of N. P is a modular protein comprising a long N-terminal disordered region and two folded domains, a central oligomerization domain and a C-terminal nucleocapsid-binding domain, separated by a flexible linker [11], [12]. The N0-binding region is localized in the N-terminal disordered region [7], [8], [9], and it has been demonstrated that in vesicular stomatitis virus (VSV), a prototypical rhabdovirus, this region of P contains transient helical elements and may thus constitute a short molecular recognition element (MoRE) that folds upon binding to its partner [13].
In rhabdovirus nucleocapsids, every N molecule binds nine nucleotides in a positively charged cavity at the interface between its N - (NNTD) and C-terminal (NCTD) domains [14], [15]. The N-RNA complex is stabilized by multiple salt bridges between the sugar-phosphate backbone of the RNA and basic residues of N, by contacts between neighboring N molecules involving hydrophobic side-to-side interactions, mainly between adjacent NCTD, and by the exchange of N - and C-terminal sub-domains between adjacent N protomers (NNT-arm, aa 1–21 and NCT-loop, aa 340–375, respectively) [14], [15]. Once formed, the N-RNA complex is stable and cannot be disassembled by full-length P [16]. However, on the basis of the N-RNA structure, we hypothesized that deletion of the NNT-arm may sufficiently destabilize the N-RNA complex so that P or a peptide fragment of P containing the MoRE that binds N0, could displace the RNA molecule.
In this study, we report the reconstitution of complexes between a recombinant N of VSV lacking the 21 N-terminal residues, NΔ21, and either full-length P dimer [11] or a peptide encompassing the N0-binding MoRE of P [13], named here P60, that comprises the first 60 amino acids of P, a two-amino acid linker and a C-terminal His6-tag. The characterization by absorbance spectroscopy and size-exclusion chromatography (SEC) combined with static light scattering (MALLS) demonstrates that both NΔ210-P60 and NΔ210-P dimer complexes are free of RNA in solution, forming soluble heterodimers or heterotrimers, respectively. Therefore, P60 fulfills both chaperone activities of full-length P. The heterodimer NΔ210-P60 crystallized, but under the crystallization conditions, it assembled into a circular decamer of heterodimers very similar to the previously crystallized decameric N-RNA ring. The crystal structure of the decameric form of the NΔ210-P60 complex reveals the molecular mechanisms by which the N0-binding MoRE of P attaches to N. NMR spectroscopy confirms that the MoRE of P binds to N in the NΔ210-P60 complex in solution as in the crystal structure and shows that the regions of P flanking this MoRE remain flexible in the complex. Finally, these results suggest mechanisms for the encapsidation of newly synthesized RNA and for the initiation of RNA synthesis by the viral polymerase.
Results
Strategy for reconstituting the NΔ210-P and NΔ210-P60 complexes
Production of a mutant of N deleted of its 21 first N-terminal residues (NΔ21) in Escherichia coli led to the formation of inclusion bodies and of poorly soluble complexes, which could not be purified. In order to improve the solubility of the NΔ21 mutant, it was produced in E. coli in fusion with an N-terminal maltose binding protein (MBP) tag. The purified MBP-NΔ21 formed soluble, oligomeric N-RNA complexes, which eluted next to the exclusion volume of a Superdex S200 column (Figures S1A and S1B in Text S1). The presence of RNA was demonstrated by the absorbance ratio at 280 nm and 260 nm of 1.05 (A280 nm/A260 nm) (Figure S1C in Text S1). The MBP-NΔ21 monomer migrated as a single protein of about 100 kDa on a denaturing 4–20% gradient PAGE (Figure S1B in Text S1). Incubation of MBP-NΔ21 at 20°C overnight in the presence of P60 resulted in the displacement of the bacterial RNA from N, the dissociation of the oligomeric N-RNA complexes and the formation of an new species that eluted at 14.1 mL (Figure S2A in Text S1). The analysis by SEC-MALLS indicated a weight-averaged molecular mass of 92±2 kDa in agreement with the calculated molecular mass of the MBP-NΔ210-P60 complex (calculated mass: 88,326 Da (MBP-NΔ21)+8,053 Da (P60) = 96,379 Da). The co-elution of MBP-NΔ21 and P60 was confirmed by denaturing 4–20% gradient PAGE (Figure S2B in Text S1). The complex contained much less RNA as shown by the absorbance spectrum (A280 nm/A260 nm = 1.60) (Figure S2C in Text S1). After cleavage of the MBP tag with the TEV protease, the resulting NΔ210-P60 complex was purified by Ni2+ chelate affinity chromatography followed by SEC. The complex of NΔ21 with full-length P dimer [11] was then prepared by incubating the purified NΔ210-P60 complex overnight with the intact P dimer.
Solution properties of the NΔ210-P and NΔ210-P60 complexes
The molecular mass of the NΔ210-P dimer complex determined by SEC-MALLS was constant throughout the chromatographic peak indicating that the complex was monodisperse (Mw/Mn = 1.00±0.01), and the molecular mass of 104±4 kDa was consistent with that of a heterotrimer composed of one NΔ21 and an intact P dimer (calculated mass: 45,377 Da (NΔ21)+2×30,976 Da = 107,329 Da) (Figure 1) in accordance with the dimeric state of P in solution [11] and with a previous determination for the rabies virus N0-P complex by native mass spectrometry [17]. The hydrodynamic radius (RS) of 5.8±0.1 nm is about 1.5 fold larger than that for a globular particle of the same molecular mass (calculated RS = 4.0 nm) reflecting the elongated shape of the complex and the existence of a long N-terminal disordered region (aa 1–106) [11], [12].
Fig. 1. Heterotrimeric VSV NΔ210-P dimer complex in solution. 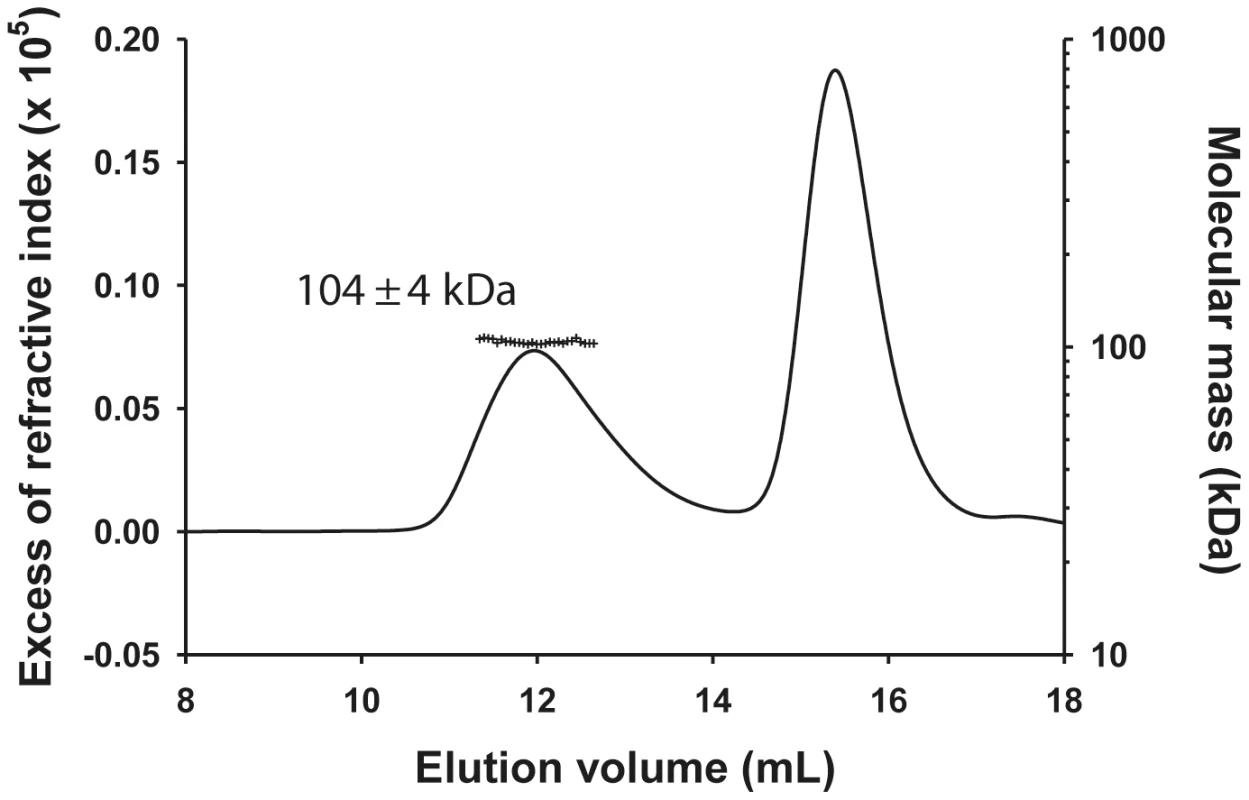
The complex formed between NΔ21 and full-length P dimer in solution was analyzed by SEC-MALLS. The NΔ210-P complex elutes at 12.0 mL and the remaining NΔ210-P60 complex elutes at 15.6 mL (line). The molecular mass of 104±4 kDa (crosses) indicates a 1∶2 complex between one RNA-free NΔ21 and two P molecules in accordance with the previous observation that P forms exclusively dimers in solution and with the N0-P2 complex determined for rabies virus. The NΔ210-P60 complex contained no RNA (Figure 2A), and its RS of 3.2±0.1 nm and molecular mass of 53±3 kDa indicated a globular 1∶1 complex (Figure 2B), which agrees with the fact that P60 does not contain the dimerization domain of P [12], [18]. The radius of gyration (Rg) of the NΔ210-P60 complex determined from SAXS data (2.7±0.1 nm) (Figure S3 and Table S1 in Text S1) was similar to that of a single N protomer extracted from the N-RNA complex (2.8 nm) [15], but the calculated curve of the extracted protein poorly fitted the experimental curve of NΔ210-P60, probably because of the presence of P60 (Figures S4A and S4B in Text S1). Ab initio bead models reconstructed from SAXS data [19] (Figure 2C) could easily accommodate the structure of an isolated N protomer deleted of its NNT-arm, except for the NCT-loop, which is likely to adopt a different conformation in solution (Figure 2D). Although the low resolution of the model precluded the precise localization of P60, the absence of an empty groove at the interface between NNTD and NCTD suggests that P60 could bind in this region. These results clearly show that P60 fulfils both chaperone functions of P in maintaining N0 soluble and free of RNA, and because the size and flexibility of full-length P render the NΔ210-P complex unsuitable for X-ray crystallography and NMR studies, the NΔ210-P60 complex was used for further structural characterization.
Fig. 2. Heterodimeric VSV NΔ210-P60 complex in solution. 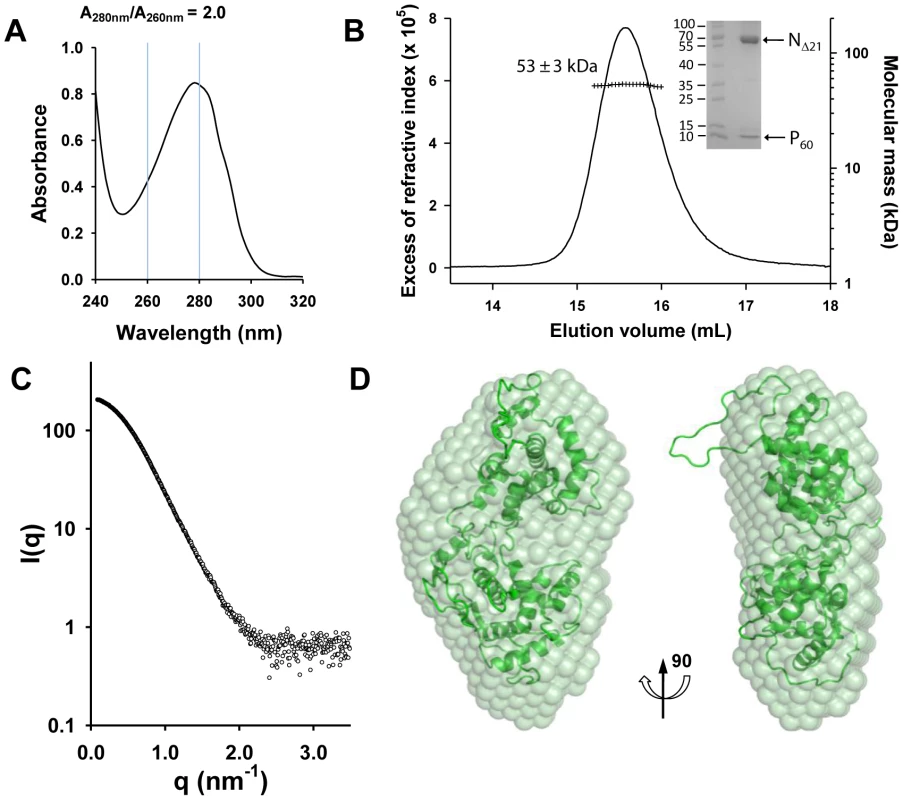
(A) The absence of RNA in the complex is revealed by the UV absorbance spectrum, which exhibits an A280 nm/A260 nm ratio of 2.0. (B) Analysis of the NΔ210-P60 complex by SEC-MALLS. The complex elutes as a single peak at 15.6 mL (line), and the presence of the two proteins in the complex is demonstrated by SDS-PAGE analysis of the peak fraction using Coomassie blue staining (inset). The molecular mass of 53±2 kDa (crosses) indicates a 1∶1 complex between NΔ21 and P60 (calculated molecular mass = 45,377 Da (NΔ21)+8,053 Da (P60) = 53,430 Da). (C) Experimental SAXS data (open circles) up to 3.5 nm−1. The SAXS curve recorded at ESRF beamline ID 14-3 shows the scattering intensity I(q) as a function of the scattering vector, . (D) Average ab initio bead model of the NΔ210-P60 complex. The N protomer extracted from the circular N-RNA complex (2GIC chain E) fits to the SAXS-derived model, except for the NCT-loop. Crystal structure of a decameric NΔ210-P60 complex
The NΔ210-P60 complex crystallized at low pH as a decameric circular complex. The structure was solved at a resolution of 3.0 Å by molecular replacement using the structure of an N protomer derived from the N-RNA complex [15] (Table 1). The structure of N in the NΔ210-P60 complex was almost identical to that of N in the N-RNA complex (rmsd = 0.96 Å) (Figure 3A and Figure S5 in Text S1) [15]. The complex contained no RNA, but instead, in each protomer, residues 6 to 35 of P60 were visible in a groove formed by residues of the hinge region of N (aa 200–300) at the junction between NNTD and NCTD (Figure 3B). Previous observations showed that the isolated N0-binding region of P transiently populates α-helical conformers in the region 2–12 and 25–31 [13], and that residues 11 to 30 of P are essential for forming the N0-P complex [7]. Upon binding to NΔ21, the second fluctuating helix is stabilized and extends from amino acids 17 to 31 (Figure 3B). The theoretical SAXS curve calculated from the crystal structure of one protomer of the NΔ210-P60 complex fits adequately the experimental curve of the soluble complex (Figure S4A and S4C in Text S1), and the structure is perfectly accommodated within the ab initio bead model (Figure S4D in Text S1) showing that the structure of the NΔ210-P60 complex in solution is the same as that in the crystal.
Fig. 3. Crystal structure of a decameric form of the NΔ210-P60 complex. 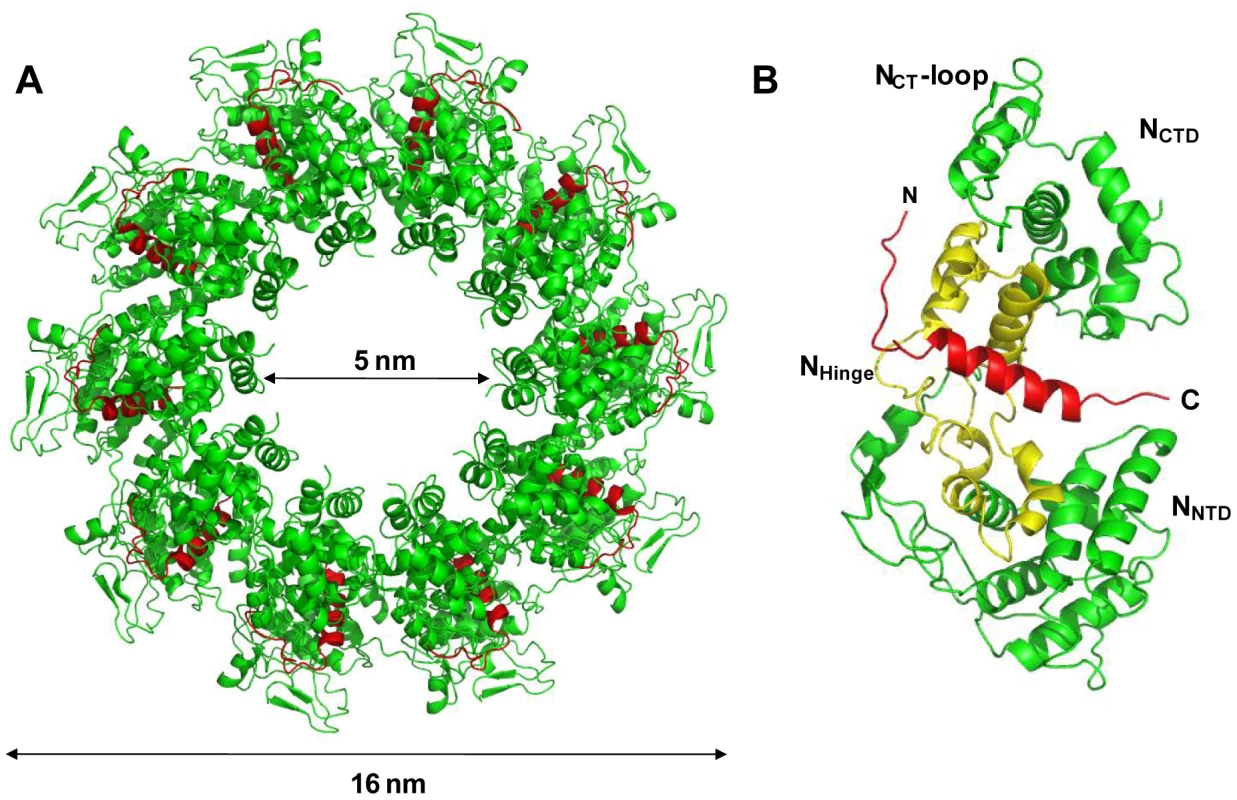
(A) Overall structure of the decamer of NΔ210-P60 complex. NΔ21 is shown in green and P60 in red. (B) Ribbon representation of one protomer of NΔ210-P60. The central hinge region of N (aa 200–300) is shown in yellow and P60 (aa 6–35) is shown in red. Tab. 1. Data collection and refinement statistics (molecular replacement). 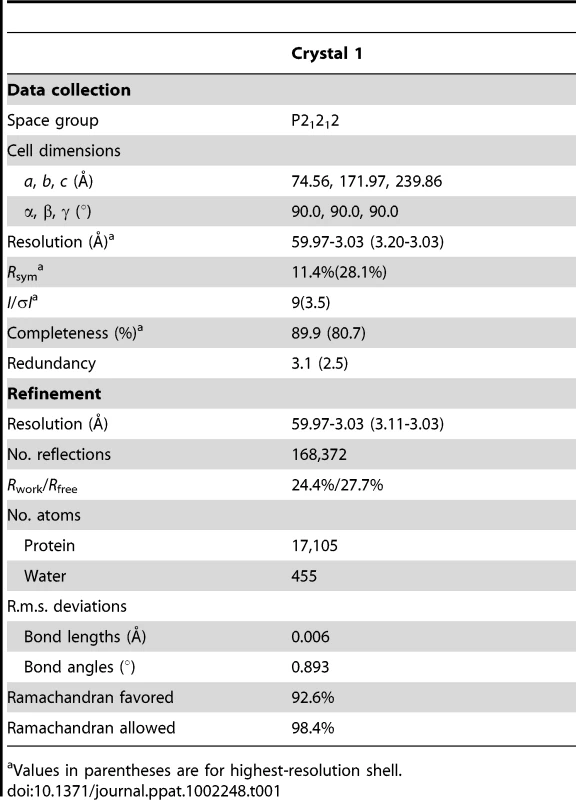
Values in parentheses are for highest-resolution shell. The structure of the NΔ210-P60 complex shows how the N0-binding MoRE of P prevents both the interaction with RNA and the self-assembly of soluble RNA-free N. The binding site of P is different from that of RNA but both sites do overlap (Figures 4A, 4B and Figure S5 in Text S1), and the C-terminal turn of the α-helix of P60 (aa 27–31) together with the following residues (aa 32–35) block the RNA binding cavity (Figure 4A) and inhibit RNA binding. Concomitantly, the other extremity of the MoRE (aa 6–13) docks in a shallow groove on the backside of the N protomer which, in the multimeric N-RNA complex, is occupied by the NNT-arm of the adjacent Ni−1 protomer and whose bottom is made up of the NCT-loop of the Ni+1 protomer (Figure 4C and 4D). With w.t. N, the N-terminal part of the MoRE of P will compete with the NNT-arm of a neighboring N molecule and therefore interferes with the polymerization of N in the absence of RNA.
Fig. 4. P binding hinders RNA binding and self-assembly of soluble N. 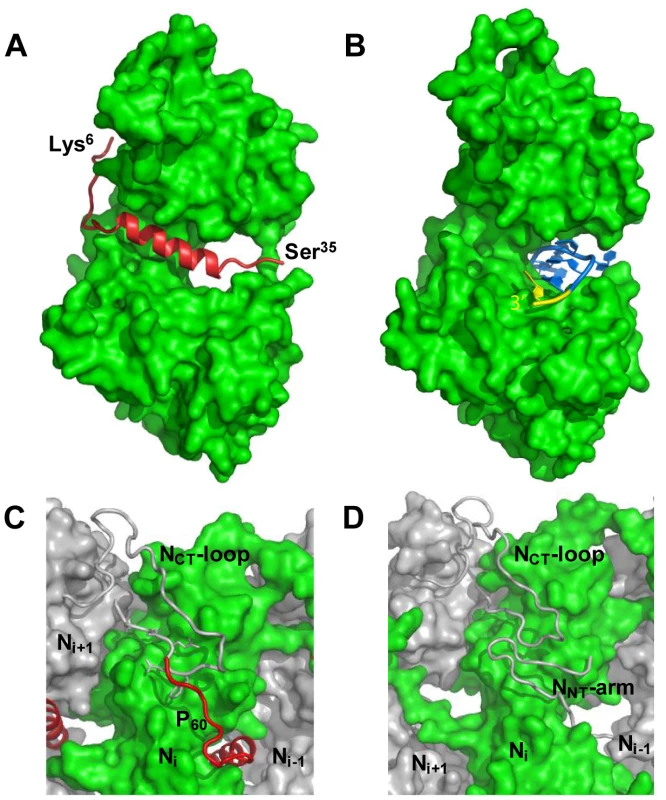
(A) Representations of one protomer from the NΔ210-P60 complex. The N protomer is shown as space filling model in green and P60 is shown as a cartoon representation in red. The visible N- and C-terminal residues of P60 are labeled. (B) Representations of one protomer from the w.t. N-RNA complex. The 3′ terminal nucleotide of the RNA molecule is shown in yellow. These representations show that P60 fills the RNA-binding cavity on the side of N that accommodates the 3′-end of the RNA molecule. (C, D) Close-up of the interactions between exchangeable sub-domains in the circular NΔ210-P60 and N-RNA complexes. In the w.t. N-RNA complex (D), the NNT-arm of protomer Ni−1 contacts the NCT-loop of protomer Ni+1 while both sub-domains are docked on the back-side of protomer Ni (in green). In the NΔ210-P60 complex (C), the N-terminal extremity of P60 docks on protomer Ni (in green) at the position of the NNT-arm of protomer Ni−1 and contacts the NCT-loop of protomer Ni+1. These representations suggest that P60 interferes with the assembly of N in the absence of RNA. The MoRE of VSV P (aa 6–35) binds to the central hinge region of N mainly through hydrophobic interactions. The amphipathic α-helix of P, together with residues 14 to 16, inserts into a hydrophobic groove of N. Tyr14 perfectly fits into a small hydrophobic pocket (Figure 5A). In addition, the complex is stabilized by three intermolecular salt bridges (Figure 5A). This region of N (aa 200–300), which also plays a central role in binding RNA is highly conserved among VSV serotypes as well as in rabies virus (RAV) [20], [21] (Figure S6 in Text S1). The hinge region of VSV N exhibits 30% identity in amino acid sequence with that of RAV N, as compared with 21% and 13% for the N - and C-terminal lobes, respectively. Figure 5B shows that several hydrophobic residues lining the binding groove of P are conserved between VSV and RAV N, as well as Arg312, suggesting that a similar complex forms in RAV (Figure S6 in Text S1). In the N-RNA complex, the RNA molecule interacts with N through electrostatic interactions between phosphate groups of the RNA backbone and basic residues of the protein, while the bases of three nucleotides (nt. 5, 7 and 8) are docked onto an hydrophobic surface of the RNA binding groove [15], which is also part of the MoRE binding site (Figure 5B). However, none of the basic residues of VSV N directly contacting the RNA backbone in the N-RNA complex is involved in the interaction with the MoRE of P. A similar mode of RNA binding was observed in the RAV N-RNA complex [14], but only two arginines out of the six residues involved in direct interactions with phosphate groups in the VSV complex are conserved in the RAV complex [20].
Fig. 5. Surface properties and amino acid conservation in the P binding site. 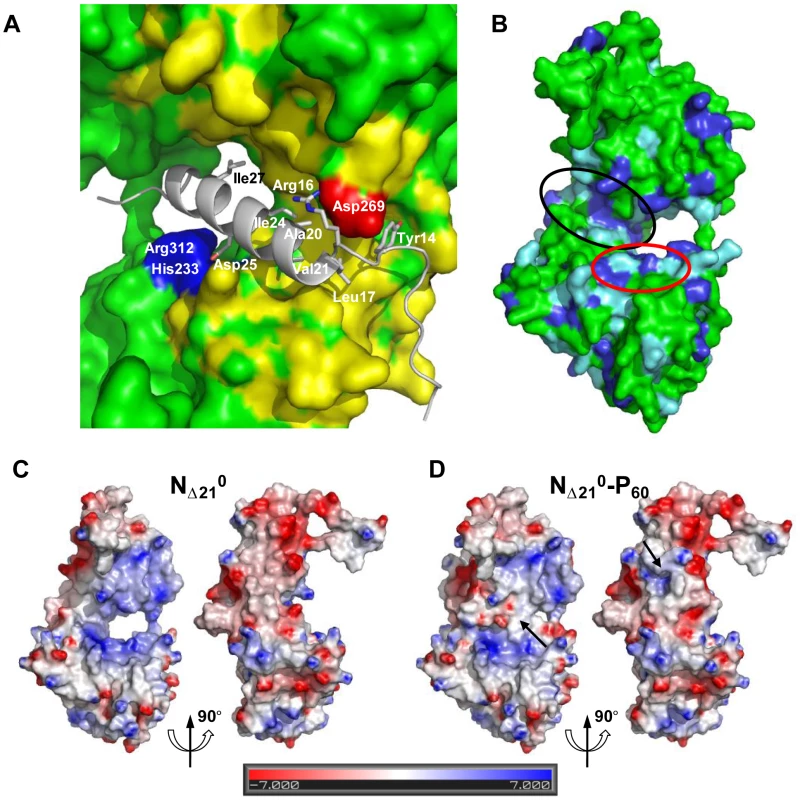
(A) Close up of the interface between RNA-free NΔ21 and P60 showing the hydrophobic contacts and salt bridges. Residues 17 to 31 of P60 fold into an amphipathic α-helix that lies in a hydrophobic cavity formed by residues of the hinge region of N and is stabilized by hydrophobic contacts involving residues of P60 spaced i+3 or i+4 (Leu17, Val21, Ile24 and Ile27). Tyr14 docks into a small cavity lined with hydrophobic residues. Hydrophobic side chains in N are colored in yellow and hydrophobic side chains of P60 are labeled. The complex is also stabilized by salt bridges between Asp25 of P60 and Arg312 and His233 of N (in blue) and between Arg16 of P60 and Asp269 of N (in red). (B) Amino acid sequence conservation between VSV and RAV N. Identical residues are shown in dark blue and similar residues are shown in light blue. The surface area circled in black shows the binding groove of P and the surface area circle in red shows the hydrophobic site common to both P and the bases at the 3′ end of the RNA. (C, D) Electrostatic surface potential of the NΔ210 protein (C) compared with that of the NΔ210-P60 complex (D). Both panels show in the same orientations the two sides of the NΔ210 protein involved in binding the MoRE of P. The arrows indicate regions in which the electrostatic surface potential of N is modified by the presence of the peptide. The surface potentials were calculated with the Delphi program and are color-coded on the surface from red (negatively charged residues, −7 kcal/mol) to blue (positively charged residues, +7 kcal/mol). The RNA binding groove of N is rich in basic residues forming a highly positive surface area (Figure 5C), while the backside of NCTD harbors a negative surface potential (Figure 5C). The MoRE of P (aa 6–35) has a bipolar distribution of charges with a positive pole at its N-terminus and a negative pole its C-terminus. In the NΔ210-P60 complex, the negative pole of P localizes in the RNA binding groove, while the positive pole docks on the backside of NCTD, modifying the distribution of electrostatic potentials on these surfaces of N and suggesting that electrostatics could play a role in orientating P before binding (Figures 5C and 5D). The crystallization is at pH 4.6 and it is likely that protonation of acidic groups reduces repulsion forces that keep the NΔ210-P60 complex in its isolated form at pH 7.0.
NMR spectroscopy
In the crystal structure of the NΔ210-P60 complex residues 6 to 13 and 32 to 35 of P60 exhibit conformational heterogeneity (Figure S7 in Text S1), while residues 1 to 5 and 36 to 68 are not visible. To further characterize the conformational dynamics of these parts of P60 in the soluble NΔ210-P60 complex, we used nuclear magnetic resonance (NMR) spectroscopy. Initially, spectra of 15N, 13C, 2H-labeled P60 in complex with unlabeled NΔ21 were recorded. In a complex of this size (53 kDa) NMR signals are significantly broadened, precluding their detection, but in the HSQC spectrum of the NΔ210-P60 complex, resonances corresponding to the last 28 amino acids of P60 (aa 41–60+linker+His6 tag) are clearly visible (Figure 6A), suggesting that this tail remains free and flexible in the complex. Comparison with the free peptide showed that most resonances superimpose. Small chemical shift differences were observed for residues Q41 to G44, probably due to the proximity of the bulk complex, and for two aromatic residues (Y53, F54) suggesting weak interactions of these residues with N. The amide backbone 15N transverse relaxation rate constant (R2) is sensitive to rapid fluctuations at the pico - to nanosecond time scale, as well as to chemical shift exchange on the micro - to millisecond time scale. Transverse relaxation of the visible resonances increased from the C-terminus to the region containing the bound helix, indicating a corresponding increase in rigidity of the backbone (Figure 6B). Very weak additional peaks up-field shifted in the amide proton dimension were detected in a 15N-1H TROSY spectrum further supporting folding of the helical element upon binding (data not shown).
Fig. 6. The N-terminal and C-terminal region of P60 flanking the MoRE exhibits conformational flexibility in the soluble complex. 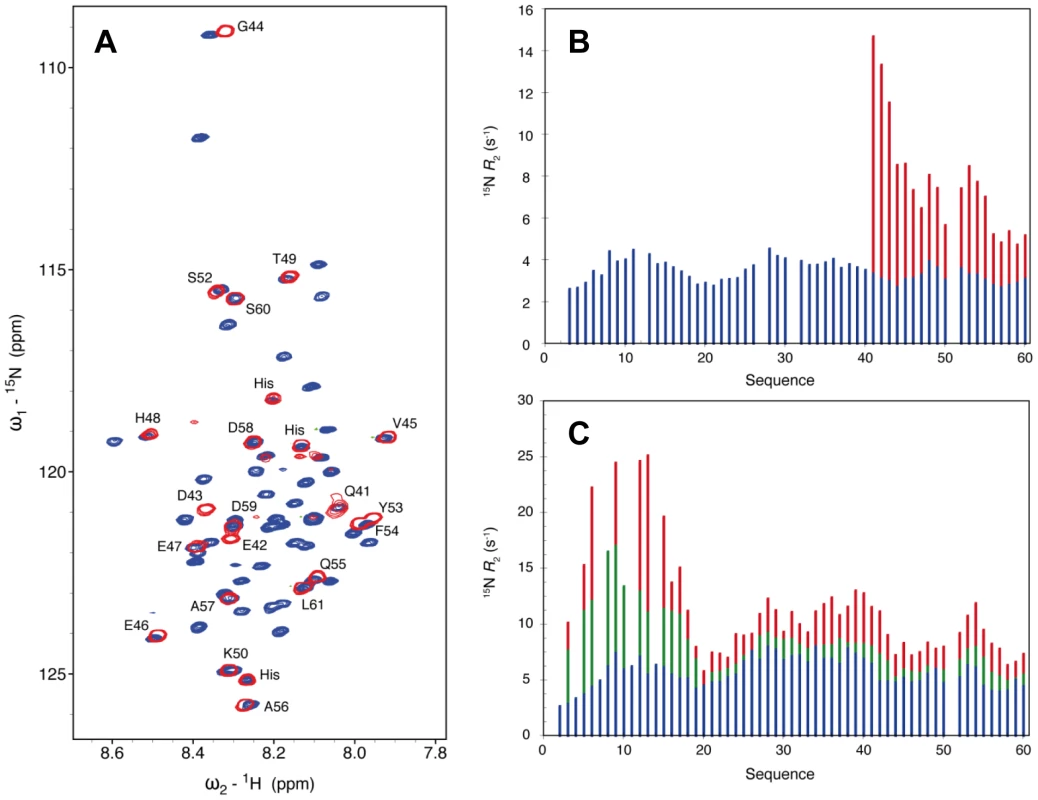
(A) Comparison of the 2D 1H-15N HSQC NMR spectra of free 15N, 13C, 2H-labelled P60 (blue) and in complex with NΔ21 (red). Both spectra were recorded at 14.1 T and 25°C in 20 mM Tris-HCl, 150 mM NaCl, 50 mM Glu, 50 mM Arg with 10% D2O adjusted to pH 6.0. The labels indicate the assignment of the resonances of the complex. (B) 15N R2 spin relaxation rates measured under the same conditions as (A) for the free 15N-labeled P60 (blue) and the NΔ210-P60 complex (red). (C) 15N R2 spin relaxation rates measured at 14.1 T and 10°C of the free 15N-labeled P60 (blue), a mixture of 0.27 mM 15N-labeled P60 and 0.09 mM unlabeled MBP-NΔ21 (green) and a mixture of 0.24 mM 15N-labeled P60 and 0.17 mM unlabeled MBP-NΔ21 (red). In a second experiment, addition of sub-stoichiometric amounts of unlabeled NΔ21 attached to MBP (to maintain solubility) to 15N-labeled P60 resulted in an overall reduction in the intensity of the peaks in the HSQC spectrum, in proportion of the amount of added MBP-NΔ21 indicating that a fraction of P60 formed a complex with MBP-NΔ21. Spin relaxation measurements of equilibrium mixtures of free and bound peptide refine our understanding of the dynamics of the system. Systematically larger R2 values observed for residues 1 to 17 reveal additional contributions from chemical shift exchange (Rex) that are not present in the free form of the peptide and that increase significantly upon increasing the molar ratio of MBP-NΔ21 to P60 (Figure 5C). This indicates that the N-terminal part of P60 experiences conformational exchange when in complex with NΔ21 with an interconversion rate on the micro to millisecond timescale. In the crystal structure, residues 6 to 13 bind in place of the NNT-arm of the protomer Ni−1 and pack onto the NCT-loop of protomer Ni+1 (Figures 4C and 4D), but in the isolated form of the complex, the NCT-loop binding surface for these residues is missing. These results confirmed that in the solution, like in the crystal, residues 17 to 35 of P form a stable complex with N, while the flanking N - and C-terminal regions remain dynamic. The flanking C-terminal part (aa 40–60) behaves as a flexible tail and shows little evidence of interaction with N. The flanking N-terminal region (aa 1–16) interacts with N but undergoes conformational exchange. These flexible regions seem dispensable for the chaperone activities of P since a shorter peptide encompassing residues 7 to 40 of P is also capable of displacing bacterial RNA from NΔ21 and of forming a soluble heterodimeric 1∶1 complex with this protein (data not shown).
Discussion
In the current study, we have determined the structure of the N0-binding MoRE of P bound to N and demonstrated that the regions of P flanking the MoRE conserve some flexibility in the N0-P complex. Because the N0-P complex is required for the replication of the virus [5], [22], inhibition of its formation might represent an interesting target for blocking viral replication and could explain the recent observations that a homologous peptide (P60) from rabies virus P inhibits viral replication [23].
The NΔ210-P60 complex as a model of the N0-P complex
Our results demonstrate that the reconstituted NΔ210-P60 complex is a suitable model for the viral N0-P complex in agreement with previous studies. Both the crystal structure and the NMR spectroscopy experiments show that the MoRE of P, which adopts a stable conformation upon binding to N, includes residues 6 to 35 and corresponds closely to the fragment that was previously identified as essential and sufficient for maintaining N in a soluble form (aa 11–30) [7].
Previous studies revealed that VSV P is a dimeric and modular protein in which the N-terminal part (aa 1–106) is globally disordered [11], [12], [13], [18], [24], [25]. The dimerization domain of P is localized in the central region of the protein (aa 107–177) [18] and is therefore not present in P60, which is monomeric [13]. The stoichiometry of the NΔ210-P60 complex (1∶1) shows that one MoRE of P is capable of binding one N molecule. The stoichiometry of the complex formed between NΔ21 and full-length P (1∶2) in the concentration range used here suggests that a single NΔ21 is bound to P dimer. The remaining part of the P dimer is tethered to NΔ21 through a flexible linker, in agreement with the large hydrodynamic radius measured here for the NΔ210-P dimer complex. In isolation, the N-terminal region of P contains two transient α helices (aa 2–12 and 25–38) [13]. In the crystal structure, the second helix is stabilized and extends from residue 17 to residue 35, whereas the first helix is not present. Residues 1 to 5 are not visible and residues 6 to 13 adopt different conformations in the different protomers of the circular complex. Because NMR spectroscopy reveals that this N-terminal region of P (aa 1–17) is in chemical shift exchange, it is possible that in solution it adopts different conformations bound in different orientations on the surface of N, but that only those docked into the backside groove of N allow the packing of the NΔ210-P60 complex into crystals and were thus selected during the crystallization process.
The truncated form of N (NΔ21) conserves the ability of self-association in the presence of RNA and, like w.t. N, forms oligomeric N-RNA complexes when expressed in bacteria. As assumed from the structure of the oligomeric N-RNA complex [15], the NNT-arm stabilizes the multimeric N-RNA complexes by linking together adjacent N protomers. The N-RNA complex formed with w.t. N could not be dissociated by the addition of full-length P or of a fragment of P encompassing the N0-binding region. However, the deletion of the N-terminal sub-domain destabilized the complex and allowed P60 to displace the RNA molecule and disassemble the multimeric N-RNA complex. In a previous study, the co-expression of P with a similar variant of VSV N lacking the first 22 amino acids (NΔ22) led to the production of complexes of different sizes containing NΔ22 and P but not of N-RNA complexes, suggesting a role for the N-terminal region of N in the encapsidation of the RNA [10]. Assuming an equilibrium between the N0-P complex and the multimeric N-RNA complex, with w.t. N, the stabilization brought by the NNT-arm to the multimeric assembly would displace the equilibrium towards the formation of the N-RNA complex. In the absence of the NNT-arm, the truncated N molecules assemble onto cellular RNAs as seen in our expression system, but in the presence of co-expressed P, like upon addition of P60 to our purified MBP-NΔ21-RNA complexes, the equilibrium is displaced towards the formation of the N0-P complexes. The absence of N-RNA complex in cells co-expressing NΔ22 and P may not result from a default of encapsidation but rather from the displacement of the equilibrium towards N0-P.
Unexpectedly, the NΔ210-P60 complex failed to crystallize as a heterodimer but crystallized into circular decamers of heterodimers. With the exception of the missing NNT-arm, the structure of NΔ21 in the NΔ210-P60 complex is very similar to that of N in the decameric N-RNA complex, with less than 1 Å r.m.s.d. between the two structures. Different explanations why multimerization occurs under crystallization conditions can be proposed. Firstly, the NΔ210-P60 complex crystallized at pH 4.6 like VSV circular N-RNA complexes [15], while solution SEC-MALLS and SAXS experiments were performed at pH 7.5 and NMR experiments at pH 6.0. A modification of the electrostatic surface potential (Figures 5C and 5D) could affect the equilibrium between heterodimeric and multimeric NΔ210-P60 complex. No evidence of multimerization was, however, found in solution at pH 4.6 in the concentration range used for SEC-MALLS and SAXS experiments. Secondly, the ring-like structure of ten protomers appears as a favored organization of VSV N, likely reflecting on some geometrical and/or surface properties of the protein. VSV N forms ring-like structures mostly containing ten N subunits in the presence of non-specific RNA when expressed in a recombinant system [15], [26]. The RNA can be removed from these ring-like structures without disrupting the multimeric assembly [27]. A single amino acid variant of VSV N that is no longer capable of binding RNA also crystallized into a decameric assembly of empty N molecules [10]. These circular N-RNA complexes are artifacts of the crystallization process because the actual nucleocapsid is very long and cannot form rings. However, in the virion, the nucleocapsid adopts a bullet-shaped structure composed of a trunk in which the nucleocapsid regularly spirals into superposed turns of 37.5 subunits of N and of a tip which is formed of seven turns containing varying numbers of subunits [1]. The upper turn of the bullet tip, which may represent the nucleation centre from which the particle assembles, resembles a decameric ring, suggesting that the assembly in ten members ring or spiral corresponds to an optimal side-by-side orientation between adjacent N subunits. The RNA-free NΔ210-P60 complex is capable of assembling into circular multimers, and it seems likely that an increase of the concentration of the NΔ210-P60 complex under the crystallization conditions together with a change in pH shift the equilibrium towards the multimers.
This raises the question of the effect of crystallization on the structure of the NΔ210-P60 complex. The SAXS curve calculated for NΔ210-P60 protomer extracted from the crystal structure perfectly reproduced the experimental curve of the soluble complex, while NMR spectroscopy clearly shows that the same segment of P (aa 17–35) is involved in a stable complex with N in solution and in the crystal, arguing that crystallization has no major effect on the structure of the more rigid part of the complex. In solution, the N-terminal part of the MoRE of P (aa 1–16) appears to be in conformational exchange and could thus exist in different conformers including those observed in the crystal in which residues 6 to 16 are docked onto the backside groove of N. Crystallization of the NΔ210-P60 complex may thus select the more compact conformers and therefore not reproduce the conformational diversity of this region that is found in solution. In addition, the high conservation rate of residues of N forming the binding surface for the MoRE of P, both within VSV serotypes and between the evolutionarily more distant VSV and RAV, supports the localization of the interface between the two proteins and hints at the formation of a similar complex in RAV.
Mechanisms of chaperone activities
The characterizations in solution indicate that the NΔ210-P60 complex is RNA-free heterodimer and that, therefore, P60 or a shorter fragment of P (aa 7–40) fulfill both chaperone activities of P. The crystal structure of the NΔ210-P60 complex clearly shows how the N0-binding region of P inhibits RNA binding by filling the RNA-binding groove of N. In solution, this part of P also forms a stable complex with N as seen by NMR spectroscopy. The structure also suggests how P prevents the self-assembly of N in the absence of RNA. In the bound form observed in the crystal structure, the N-terminal extremity of the MoRE (aa 6–16) directly competes with the NNT arm of a neighboring N molecule. Assuming this region of P fluctuates between bound and free forms in the soluble N0-P complex, the free form may act as an entropic bristle, thereby also preventing the oligomerization of N. With full-length P dimer, the flexibility and the bulkiness of the remainder of the protein may also contribute to this effect by masking the binding interfaces for RNAs or other N molecules. In addition, the MoRE of P exhibits a bipolar distribution of charges, with a positive pole at its N-terminal extremity and a negative pole at its C-terminal extremity. Binding of the MoRE of P modifies the electrostatic surface potential of N, notably reducing the positive surface potential on one side of the molecule, and may thereby affect the side-by-side interaction with another N molecule.
From the results presented here, we propose a hypothesis for the encapsidation of a newly synthesized RNA molecule during viral genome replication. By forming a complex with P, a nascent N molecule is prevented from binding to host-cell RNA and is preserved in a soluble form. During RNA replication, N is transferred to a growing RNA molecule and P is released. Little is known about the mechanism of this reaction or about the role played by the polymerase complex in this process. Our results show that the NNT-arm stabilizes the multimeric N-RNA complex and therefore suggest that the multimeric N-RNA complex is more stable than the N0-P complex. The transfer of N from the N0-P complex to the growing N-RNA complex could simply be driven by a higher stability of the N-RNA complex. Upon transfer of N onto the RNA and release of P, the backside groove of N of the last added N molecule is liberated and becomes available for accepting the NNT-arm of the next incoming N molecule (Figure 7A). By blocking the backside groove of N, the N-terminal part of P ensures that N molecules do not assemble into empty N polymers but assemble only onto an RNA molecule. It is also noteworthy that, in VSV, a high affinity binding site for the L protein was localized in the second half of the N-terminal disordered region of P [7], [28]. The dynamic nature of the N-terminal region of P and the proximity of the two binding sites may have significance for the mechanism of action of the transcription/replication machinery. The binding of N0 to P may prevent the simultaneous binding of L, or conversely, the simultaneous binding of N0 and L may modify the activity of the polymerase.
Fig. 7. Schematic representations of the mechanism of RNA replication of VSV. 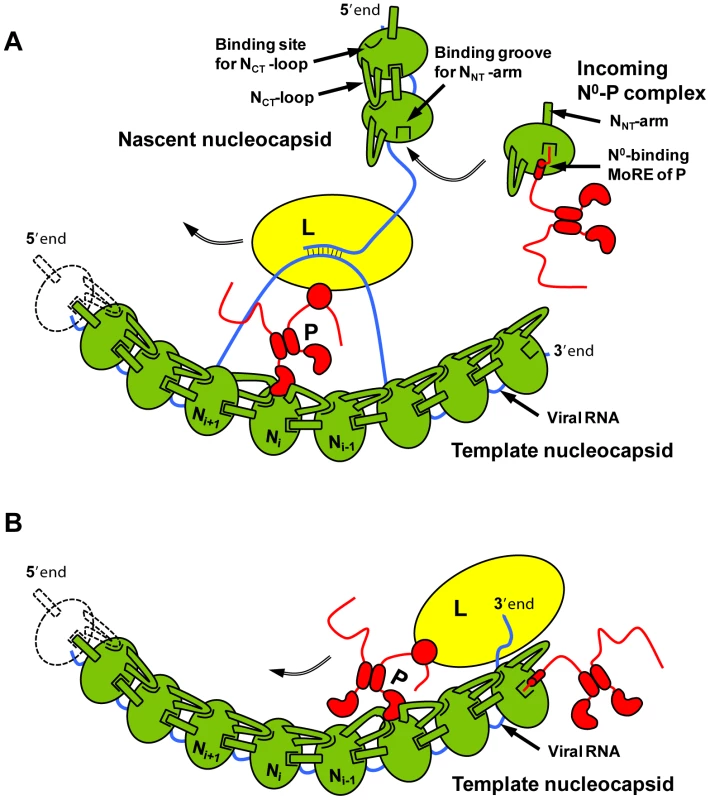
The nucleoprotein (in green) forms with the RNA genome (blue line) the active template for the polymerase complex comprising the L (in yellow) and P (in red) proteins. (A) Encapsidation during RNA replication. During replication, the newly synthesized antigenomic or genomic RNA is encapsidated by nascent N molecules that are transferred from the soluble N0-P complex. In the N0-P complex, the N-terminal MoRE of P prevents host-cell RNA binding by obstructing the RNA binding groove and the self-assembly of N by interfering with the docking of the NNT-arm of another N. Upon the transfer of N to the growing viral RNA P is released, the binding groove for the NNT-arm is freed in the RNA-bound form and can accept the next incoming N molecule. (B) Initiation of RNA synthesis. By binding at the 3′ extremity of the nucleocapsid, the N-terminal MoRE of P might displace nucleotides from the N molecule and allow the polymerase to initiate RNA synthesis. In addition to its role in RNA encapsidation, the binding of the N-terminal region of P to N may also provide a mechanism for the initiation of (+)RNA synthesis at the genome 3′ end and of (−)RNA synthesis at the antigenome 3′ end [29], [30]. Encapsidated RNA genome and antigenome are completely covered with the nucleoprotein and are not accessible to the RNA polymerase. However, the first N molecule at the 3′ extremity of nucleocapsids exposes its binding site for the N-terminal MoRE of P (Figure 7B). By binding to this surface, P may destabilize the N-RNA complex sufficiently to displace several nucleotides from the first N protomer and allow the polymerase access to the RNA.
Materials and Methods
Reconstitution of the NΔ210-P and NΔ210-P60 complexes
The cDNAs encoding vesicular stomatitis virus nucleoprotein or a fragment of this protein deleted of the 21 N-terminal residues were amplified by PCR and introduced into the pET-M40 plasmid (EMBL) using NcoI and XhoI restriction sites. The resulting constructs code for chimeric proteins that comprise an N-terminal maltose binding protein tag (MBP) and a tobacco etch virus (TEV) cleavage site. The cDNA encoding the 60 first N-terminal amino acids of VSV P (P60) was amplified by PCR and cloned into the pET28a plasmid containing a C-terminal His6-tag and a linker of two amino-acids (EL) using NcoI and XhoI restriction sites. All constructions were checked by DNA sequencing.
The plasmids were transformed into Escherichia coli Rosetta (DE3) cells and the expression of the recombinant proteins was induced with 1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) for 18 h at 16°C. Cells were harvested by centrifugation, suspended in buffer A (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 mM DTT) containing protease inhibitors (Complete EDTA-Free, Roche Diagnostics) and disrupted by sonication. The extract was centrifuged at 20,000 g during 30 min at 4°C and the supernatant was filtered (0.45 µm). The MBP-fusion proteins were purified by affinity chromatography on amylose resin (New England Biolabs) followed by size exclusion chromatography (SEC) on a Superdex S200 column (GE Healthcare) equilibrated in buffer A. P60 was purified by affinity chromatography on a Ni2+ resin column (Quiagen) followed by SEC on a Superdex S75 column (GE Healthcare) equilibrated in buffer A supplemented with 50 mM Glu and 50 mM Arg. Samples for NMR spectroscopy were produced in M9 minimal medium containing MEM vitamins (Gibco). For producing 15N-labeled P60, the medium was supplemented with 1.0 g.L−1 of 15NH4Cl and 2.0 g.L−1 of unlabeled glucose, while for producing 15N, 13C, 2H-labeled P60 the minimal medium was prepared in D2O and supplemented with 1.0 g.L−1 of 15NH4Cl and 2.0 g.L−1 of 13C glucose.
The NΔ210-P60 complex was prepared by incubating overnight at 4°C an excess of P60 with the MBP-NΔ21-RNA complexes. The MBP-NΔ210-P60 complex was purified by Ni2+ chelate affinity chromatography by using the His-tag present on P60 to remove the excess of free MBP-NΔ21-RNA complex, followed by SEC on a Superdex S200 column equilibrated in buffer A and amylose affinity chromatography to eliminate unbound P60.
The MBP tag was removed by incubating the protein with the TEV protease overnight at 4°C. The N protein contains the additional N-terminal tripeptide GAM. The NΔ210-P60 complex was then purified using a Ni2+ chelate affinity chromatography followed by SEC on a Superdex S200 column equilibrated in buffer A. This procedure yielded pure NΔ210-P60 complex. The samples were checked by SDS-PAGE using denaturing 4–20% gradient PAGE (Biorad).
Size exclusion chromatography (SEC) combined with detection by multi-angle laser light scattering (MALLS) and refractometry: SEC-MALLS
SEC was performed with a Superdex S200 column (GE Healthcare) equilibrated in 20 mM Tris–HCl, pH 7.5, 150 mM NaCl. Separations were performed at 20°C with a flow rate of 0.5 ml.min−1. 50 µL of a protein solution at a concentration ranging from 2.7 to 8.0 mg.mL−1 were injected. On-line multi-angle laser light scattering (MALLS) detection was performed with a DAWN-EOS detector (Wyatt Technology Corp., Santa Barbara, CA) using a laser emitting at 690 nm. Protein concentration was measured on-line by refractive index measurements using a RI2000 detector (Schambeck SFD) and a refractive index increment dn/dc = 0.185 mL.g−1. Data were analyzed and weight-averaged molecular masses (Mw) were calculated using the software ASTRA V (Wyatt Technology Corp., Santa Barbara, CA) as described previously [11]. For size determination, the column was calibrated with proteins of known Stokes' radius (RS) [31].
Small angle X-ray scattering (SAXS) and ab initio modeling
SAXS data were collected at the European Synchrotron Radiation Facility (E.S.R.F., Grenoble, France) on beamline ID14-3. The sample-to-detector distance was 1 m and the wavelength of the X-rays was 0.931 Å. Samples were contained in a 1.9 mm wide quartz capillary. The exposition time was optimized for reducing radiation damage. Data acquisition was performed at 20°C. Data reduction was performed using the established procedure available at ID14-3 and buffer background runs were subtracted from sample runs.
The SAXS profile of the NΔ210-P60 complex was recorded for scattering vectors, , in the range 0.05 nm−1<q<3.5 nm−1. The profiles obtained at three different protein concentrations 2.7–8.0 mg.mL−1 had the same shape and were flat at low q values indicating the absence of significant aggregation (Figure S4A). The radius of gyration and forward intensity at zero angle (I(0)) were determined with the programs PRIMUS [32] by using the Guinier approximation at low q values (Figure S4B), in a range up to 1.3:(1)The forward scattering intensity was calibrated using bovine serum albumin and lysozyme as references. The radius of gyration, Rg, and the pair distance distribution function, P(r), were calculated with the program GNOM [33] (Figure S4C). The maximum dimension (Dmax) value was adjusted so that the Rg value obtained from GNOM agreed with that obtained from the Guinier analysis. The Rg values showed no significant dependence on protein concentration, confirming the absence of aggregation or intermolecular interactions in the concentration range used in this study. The measured Rg value of 2.7±0.1 nm (Table S1 in Text S1), and the molecular mass of 65±15 kDa derived from the scattering intensity at zero angle, I0, were in agreement with SEC-MALLS results. Additionally, the distance distribution function (Figure S4C) and the Kratky plot (Figure S4D) were typical of globular, folded proteins.
Ab initio low-resolution bead model reconstructions of the NΔ210-P60 complex were performed from the scattering curves using the program DAMMIF [19]. This program restores a low-resolution shape of the protein as a volume filled with densely packed spheres (dummy atoms) that reproduces the experimental scattering curve by a simulated annealing minimization procedure. DAMMIF minimizes the interfacial area between the molecule and the solvent by imposing compactness and connectivity constraints. 20 independent models were generated with DAMMIF with no symmetry restriction and were combined with the program DAMAVER [34] yielding an average model that exhibited the common structural features of all reconstructions. The models were aligned pairwise by minimizing the normalized spatial discrepancy (NSD) score with the program SUPCOMB [35]. The mean NSD score of 0.75 (values ranging from 0.74 to 0.77) indicated an adequate convergence of the models [34]. All figures were generated with PyMOL (http://www.pymol.org).
Crystallization, data collection and structure determination and refinement
Crystallization conditions were screened by the hanging drop vapor diffusion method using a PixSys4200 Cartesian robot (high-throughput crystallization laboratory at EMBL Grenoble, France). The screen was performed by combining 0.1 µl of protein solution at 8 mg.mL−1 in buffer A with 0.1 µl of Hampton Crystal Screen solutions. Hits were reproduced manually. The NΔ210-P60 complex crystallized at 20°C in 0.1 M sodium acetate buffer, pH 4.6, containing 4% (w/v) of PEG4000. Single crystals were harvested from the drop, briefly soaked in the reservoir solution supplemented with 25% glycerol and flash frozen in liquid nitrogen at 100 K before data collection. X-ray diffraction data were collected at a wavelength of 0.933 Å on the ID14-2 beamline at the ESRF (Grenoble, France).
The data were processed using the program iMosflm [36] and scaled with the program Scala from the ccp4 suite [37]. The structure was solved by molecular replacement with the program Phaser [38] using residues 22 to 422 of a protomer of N (2GIC, chain E) extracted from the N-RNA crystal structure [15] as a search model. The visible part of P60 was assigned and constructed with the program Buccaneer [39], and the overall structure was refined to 3.0 Å resolution using Coot [40] and Refmac5 [41]. The quality of the model was checked with PROCHECK [42]. Data collection and refinement statistics are summarized in Table 1.
The NΔ210-P60 complex was crystallized in space group P21212. The crystallographic asymmetric unit contained five protomers of NΔ210-P60 complex. Each protomer includes residues 22 to 422 of N and residues 6 to 33 of P60. In some protomers, residues 34 and 35 could also be constructed.
NMR spectroscopy
NMR experiments were performed on a Varian spectrometer operating at a 1H frequency of 600 MHz. All samples contained 20 mM Tris-HCl, 150 mM NaCl, 50 mM Glu, 50 mM Arg with 10% D2O adjusted to pH 6.0. The concentration of free 15N, 13C, 2H-labeled P60 was 0.9 mM and the concentration of the NΔ210-P60 complex (15N, 13C, 2H-labeled P1–60 in complex with unlabeled N) was 0.28 mM. In the titration experiment, 15N-labeled P60 was initially at 0.28 mM. MBP - NΔ21 was added at final concentrations of 0.09 mM or 0.17 mM. 15N R2 (CPMG) relaxation experiments were acquired using standard pulse sequences [43]. The spectra were acquired with a sweep width of 8.0 kHz and 512 complex points in the 1H dimension, and a sweep width of 1.2 kHz and 200 complex points in the 15N dimension. The magnetization decay was sampled at 10, 30, 50, 70, 90, 130, 170, 210 and 250 ms and the peak heights were used to extract the relaxation rates. To obtain estimates of the errors on the relaxation rates, a repeat measurement of one of the relaxation delays (70 ms) was carried out.
Supporting Information
Zdroje
1. GePTsaoJScheinSGreenTJLuoM 2010 Cryo-EM model of the bullet-shaped vesicular stomatitis virus. Science 327 689 693
2. ArnheiterHDavisNLWertzGSchubertMLazzariniRA 1985 Role of the nucleocapsid protein in regulating vesicular stomatitis virus RNA synthesis. Cell 41 259 267
3. PattonJTDavisNLWertzGW 1984 N protein alone satisfies the requirement for protein synthesis during RNA replication of vesicular stomatitis virus. J Virol 49 303 309
4. PelusoRWMoyerSA 1988 Viral proteins required for the in vitro replication of vesicular stomatitis virus defective interfering particle genome RNA. Virology 162 369 376
5. HowardMWertzG 1989 Vesicular stomatitis virus RNA replication: a role for the NS protein. J Gen Virol 70 Pt 10 2683 2694
6. MastersPSBanerjeeAK 1988 Complex formation with vesicular stomatitis virus phosphoprotein NS prevents binding of nucleocapsid protein N to nonspecific RNA. J Virol 62 2658 2664
7. ChenMOginoTBanerjeeAK 2007 Interaction of vesicular stomatitis virus P and N proteins: Identification of two overlapping domains at the N-terminus of P that are involved in N0-P complex formation and encapsidation of viral genome RNA. J Virol 81 13478 13485
8. MavrakisMMehouasSRealEIseniFBlondelD 2006 Rabies virus chaperone: identification of the phosphoprotein peptide that keeps nucleoprotein soluble and free from non-specific RNA. Virology 349 422 429
9. CurranJMarqJBKolakofskyD 1995 An N-terminal domain of the Sendai paramyxovirus P protein acts as a chaperone for the NP protein during the nascent chain assembly step of genome replication. J Virol 69 849 855
10. ZhangXGreenTJTsaoJQiuSLuoM 2008 Role of intermolecular interactions of vesicular stomatitis virus nucleoprotein in RNA encapsidation. J Virol 82 674 682
11. GérardFCARibeiroEAlbertiniAZaccaiGEbelC 2007 Unphosphorylated Rhabdoviridae phosphoproteins form elongated dimers in solution. Biochemistry 46 10328 10338
12. GérardFCARibeiroEALeyratCIvanovIBlondelD 2009 Modular organization of rabies virus phosphoprotein. J Mol Biol 388 978 996
13. LeyratCJensenMRRibeiroEAGérardFRuigrokR 2011 The N0-binding region of the vesicular stomatitis virus phosphoprotein is globally disordered but contains transient α-helices. Prot Sci 20 542 556
14. AlbertiniAAWernimontAKMuziolTRavelliRBClapierCR 2006 Crystal structure of the rabies virus nucleoprotein-RNA complex. Science 313 360 363
15. GreenTJZhangXWertzGWLuoM 2006 Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. Science 313 357 360
16. RibeiroEALeyratCGérardFCAlbertiniAAFalkC 2009 Binding of rabies virus polymerase cofactor to recombinant circular nucleoprotein-RNA complexes. J Mol Biol 394 558 575
17. MavrakisMIseniFMazzaCSchoehnGEbelC 2003 Isolation and characterisation of the rabies virus N degrees-P complex produced in insect cells. Virology 305 406 414
18. DingHGreenTJLuSLuoM 2006 Crystal structure of the oligomerization domain of the phosphoprotein of vesicular stomatitis virus. J Virol 80 2808 2814
19. FrankeDSvergunDI 2009 DAMMIF, a program for rapid ab-initio shape determination in small-angle scattering. J Appl Cryst 42 342 346
20. LuoMGreenTJZhangXTsaoJQiuS 2007 Conserved characteristics of the rhabdovirus nucleoprotein. Virus Res 129 246 251
21. NayakDPandaDDasSCLuoMPattnaikAK 2009 Single-amino-acid alterations in a highly conserved central region of vesicular stomatitis virus N protein differentially affect the viral nucleocapsid template functions. J Virol 83 5525 5534
22. GuptaAKBanerjeeAK 1997 Expression and purification of vesicular stomatitis virus N-P complex from Escherichia coli: role in genome RNA transcription and replication in vitro. J Virol 71 4264 4271
23. CastelGChteouiMCaignardGPrehaudCMehouasS 2009 Peptides that mimic the amino-terminal end of the rabies virus phosphoprotein have antiviral activity. J Virol 83 10808 10820
24. DingHGreenTJLuoM 2004 Crystallization and preliminary X-ray analysis of a proteinase-K-resistant domain within the phosphoprotein of vesicular stomatitis virus (Indiana). Acta Crystallogr D Biol Crystallogr 60 2087 2090
25. RibeiroEAJrFavierAGerardFCLeyratCBrutscherB 2008 Solution structure of the C-terminal nucleoprotein-RNA binding domain of the vesicular stomatitis virus phosphoprotein. J Mol Biol 382 525 538
26. GreenTJMacphersonSQiuSLebowitzJWertzGW 2000 Study of the assembly of vesicular stomatitis virus N protein: role of the P protein. J Virol 74 9515 9524
27. GreenTJRowseMTsaoJKangJGeP 2010 Access of RNA encapsidated in the nucleocapsid of vesicular stomatitis virus. J Virol 85 2714 2722
28. EmersonSUSchubertM 1987 Location of the binding domains for the RNA polymerase L and the ribonucleocapsid template within different halves of the NS phosphoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A 84 5655 5659
29. AbrahamGBanerjeeAK 1976 Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A 73 1504 1508
30. EmersonSU 1982 Reconstitution studies detect a single polymerase entry site on the vesicular stomatitis virus genome. Cell 31 635 642
31. UverskyVN 1993 Use of fast protein size-exclusion liquid chromatography to study the unfolding of proteins which denature through the molten globule. Biochemistry 32 13288 13298
32. KonarevPVVolkovVVSokolovaAM.H.J.KSvergunDI 2003 PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J Appl Cryst 36 1277 1282
33. SemenyukAVSvergunD 1991 GNOM - a program package for small-angle scattering data processing. J Appl Crystallog 24 537 540
34. VolkovVVSvergunDI 2003 Uniqueness of ab initio shape determination in small-angle scattering. J Appl Cryst 36 860 864
35. KozinMBSvergunDI 2001 Automated matching of high - and low-resolution structural models. J Appl Cryst 34 33 41
36. PowellHR 1999 The Rossmann Fourier autoindexing algorithm in MOSFLM. Acta Crystallogr D Biol Crystallogr 55 1690 1695
37. PottertonEBriggsPTurkenburgMDodsonE 2003 A graphical user interface to the CCP4 program suite. Acta Crystallogr D Biol Crystallogr 59 1131 1137
38. McCoyAJGrosse-KunstleveRWAdamsPDWinnMDStoroniLC 2007 Phaser crystallographic software. J Appl Crystallogr 40 658 674
39. CowtanKD 2006 The Buccaneer software for automated model building. Acta Crystallogr D Biol Crystallogr 62 1002 1011
40. EmsleyPLohkampBScottWGCowtanK 2010 Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66 486 501
41. MurshudovGNVaginAADodsonEJ 1997 Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53 240 255
42. LaskowskiRAMacarthurMWMossDSThorntonJM 1993 PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst 26 283 291
43. FarrowNAMuhandiramRSingerAUPascalSMKayCM 1994 Backbone dynamics of a free and phosphopeptide-complexed Src homology 2 domain studied by 15N NMR relaxation. Biochemistry 33 5984 6003
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



