-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPlague and Climate: Scales Matter
Plague is enzootic in wildlife populations of small mammals in central and eastern Asia, Africa, South and North America, and has been recognized recently as a reemerging threat to humans. Its causative agent Yersinia pestis relies on wild rodent hosts and flea vectors for its maintenance in nature. Climate influences all three components (i.e., bacteria, vectors, and hosts) of the plague system and is a likely factor to explain some of plague's variability from small and regional to large scales. Here, we review effects of climate variables on plague hosts and vectors from individual or population scales to studies on the whole plague system at a large scale. Upscaled versions of small-scale processes are often invoked to explain plague variability in time and space at larger scales, presumably because similar scale-independent mechanisms underlie these relationships. This linearity assumption is discussed in the light of recent research that suggests some of its limitations.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002160
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1002160Summary
Plague is enzootic in wildlife populations of small mammals in central and eastern Asia, Africa, South and North America, and has been recognized recently as a reemerging threat to humans. Its causative agent Yersinia pestis relies on wild rodent hosts and flea vectors for its maintenance in nature. Climate influences all three components (i.e., bacteria, vectors, and hosts) of the plague system and is a likely factor to explain some of plague's variability from small and regional to large scales. Here, we review effects of climate variables on plague hosts and vectors from individual or population scales to studies on the whole plague system at a large scale. Upscaled versions of small-scale processes are often invoked to explain plague variability in time and space at larger scales, presumably because similar scale-independent mechanisms underlie these relationships. This linearity assumption is discussed in the light of recent research that suggests some of its limitations.
Introduction
Plague is a rapidly progressing infectious disease that is infamous for having caused the death of millions of people in large historic pandemics [1] as well as numerous other deadly but localized outbreaks [2]. Plague, caused by the pathogenic bacterium Y. pestis is transmitted from host to host by fleas via blood feeding, through consumption or handling of infectious host tissues, or through inhalation of infectious materials. Plague is thought to persist for long periods of time at low to very low levels of prevalence in so-called enzootic cycles that cause little host mortality and involve partially resistant rodents (often called enzootic or maintenance hosts). These long periods are punctuated by occasional outbursts or epizootics (i.e., spreading die-offs) among these hosts or epidemics, when the incidence among humans increases. Figure 1 illustrates these intertwined cycles.
Fig. 1. Schematic of the plague cycle with small mammals as hosts and fleas as vectors. 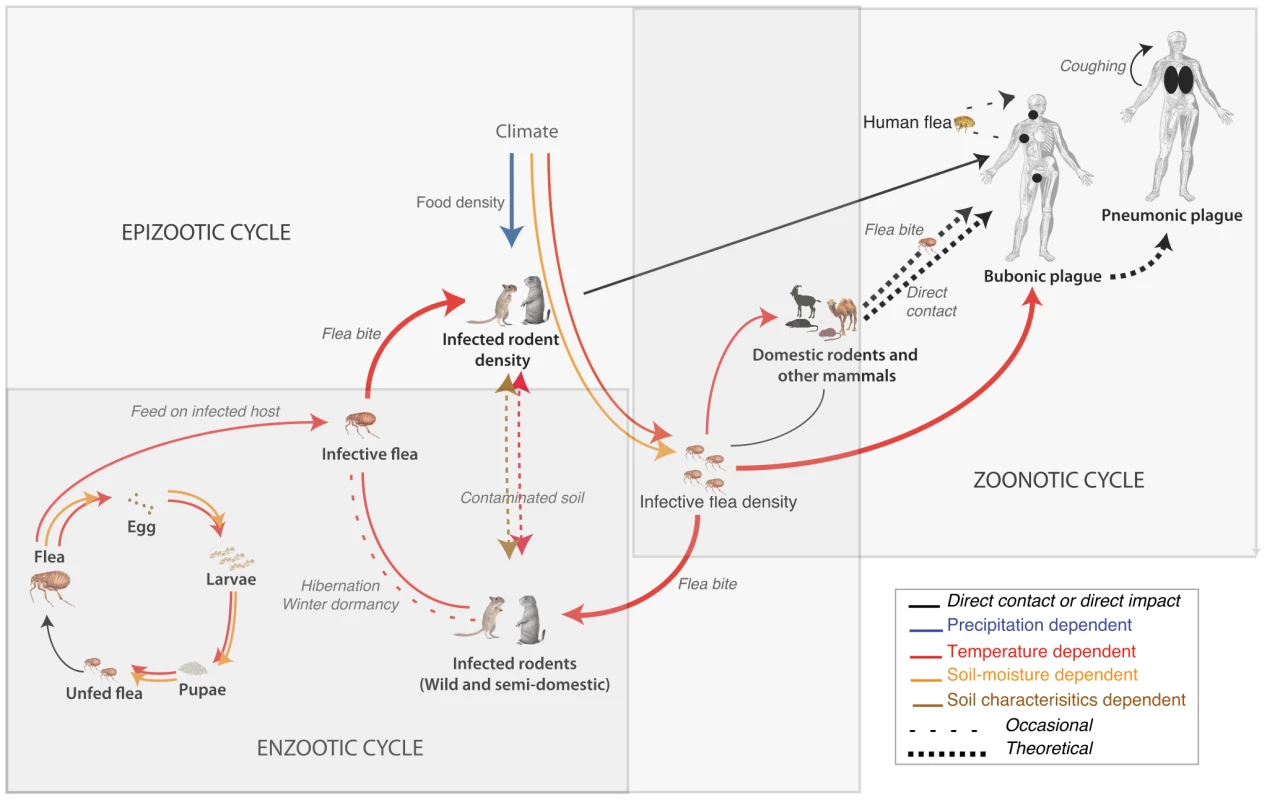
Arrows represent connections affected by climate with a color-coding depending on the most influential climate variable on this link (i.e., precipitation, temperatures, and other variables indirectly depending on them such as soil characteristics and soil moisture). Grey rectangles somewhat arbitrarily delimit epizootic, enzootic, and zoonotic cycles. Note that despite their location at the far end of the cycle, humans often provide the only available information on plague dynamics. Climate has long been suspected to be a key factor in the alternation between quiescent and active periods of plague. Rogers (1928) suggested seasonal variations in temperature and humidity to be responsible for the seasonal patterns of human plague incidence in India [3]. Decades later, Davis showed that human plague outbreaks in several African countries were less frequent when the weather was too hot (>27°C) or cold (<15°C) [4]. Subsequent studies showed an increased plague incidence in Vietnam during the hot, dry season, when following a period of high seasonal rainfall [5], [6]. Nowadays, several studies, as we report here, demonstrate climate's impacts on plague incidence spatial and temporal variability.
In the context of public health and wildlife conservation, we need an improved understanding of the mechanisms underlying the association between plague outbreaks and climate. As we will show in this review, this understanding is only partially available at present. There are several reasons for this. First and perhaps most importantly, the plague cycle is complex. It is composed of three components that interact with each other and all are influenced by climate variables with a broad range of times lags. Also, climate variability manifests itself at numerous temporal and spatial scales that condition the form of the response in plague dynamics. To cope with this series of difficulties, we break down the problem as follows: in section 1 we review individual effects of climate variables on each of the plague components. Our knowledge of these effects is primarily based on small-scale studies that are useful because they provide conceptual models for how larger scale climate variability may force the plague system. The way these conceptual models are most often used raises the issue of upscaling conclusions by inference from the results of small scale studies, a subject on which we focus on in section 2. Also, in the latter we review likely impacts of climate change on plague incidence.
Climate Dependence of the Flea Vectors and Rodent Hosts
The plague system is the result of complex interactions between its components, the densities, life cycle, dynamics and geographical distributions all of which are individually influenced by climate variables. Climate variables influence the dynamics of flea vectors and rodent hosts with responses varying considerably among species [7], [8]. Figure 1 illustrates the plague cycle in relation to those climate variables known to be important (namely temperature, humidity and precipitation) to the main plague hosts and vectors.
It is accepted that abundance of rodent fleas is affected by ambient temperature, rainfall, and relative humidity, with warm-moist weather providing a likely explanation for higher flea indices [4], [5]. Indeed, temperature, rainfall, and relative humidity have direct effects on development and survival, as well as the behavior and reproduction of fleas and their populations [9]–[12]. For example, the rate of metamorphosis of Xenopsylla cheopis (as a primary flea of the black rat Rattus rattus, X. cheopis is likely the main vector of plague in foci affecting humans), from egg to adult is regulated by temperature. Fleas are ecto-thermic and hence sensitive to temperature fluctuations; this is enhanced by the fact that all of the immature flea stages occur off host. Flea development rates increase with temperature until they reach a critical value; then the survival of immature stages decreases if high temperatures are combined with low humidity [13]. Temperature and relative humidity impact flea survival [5]: survival is in fact inversely proportional to air saturation deficit at a constant temperature [14]. Flea larvae are susceptible to desiccation [15] and typically acquire water from adult excreta. Survival of immature stages of fleas in rodent burrows is also affected by soil moisture that is partly controlled by outside precipitation [16] even though detrimental moisture losses and temperature swings are reduced by living underground [9]. Conversely, when coupled with a high organic load, excessively wet conditions in rodent burrows (e.g., relative humidity >95%) can promote the growth of destructive fungi that diminish larval and egg survival [5], [17].
Rodent survival and population dynamics are also affected by climate. A direct effect occurs when high intensity rainfall causes flooding of rodent burrows [5] but the effects of precipitation on rodent densities are mostly bottom-up [18]. Indeed, rainfall controls primary production which limits rodent abundances [19]. Reproduction and recruitment periods often follow wet seasons when increases of primary production can be used to build up juvenile populations [20]. Accordingly, rodent population densities show clear association with annual rainfall and its seasonal distribution e.g., in Chile [21]–[23], Tanzania [24], and Australia [25], [26]. But the relation between precipitation patterns and rodent densities can be complex, localized, and dependent on the timing and the intensity of precipitation events (see also below) [8], [27]. Temperature effects on rodent populations are less clear in part because rodents are homeothermic and hence do not respond immediately to changes in ambient temperatures. In temperate areas, low temperatures in winter can nonetheless negatively affect rodent populations either directly or through low food availability [28]. Nevertheless, under some circumstances, conditions detrimental to hosts or vectors can favor the maintenance of plague. Evidence of hibernation as a factor of prolongation or modification of Y. pestis infection in rodents would need further elucidation. The flea Citellophilus tesquorum altaicus for instance, is supposedly able to maintain a plague infection over the winter by feeding on hibernating long-tailed Siberian souslik (Citellus undulatus) [29]. Also, populations of Tatera indica aestivating during adverse conditions in India presumably continue to act as hosts for infected fleas, thereby promoting the persistence of plague infection within the area [30]. Hence, the survival of Y. pestis in relatively plague-resistant burrowing rodents that interrupt their activities to hibernate through winter or aestivate in summer could influence or prevent the transient temporal and spatial extinction of plague occurrence.
Human Plague Incidence Is Not Unrelated to Human Factors
Human activities and behavior in plague-infected areas are also to be considered as important determinants of plague transmission to humans [8]. When occurrences of plague are due to human intrusions in natural plague areas, it is thus important to consider climate as a second order variable that influences disease incidence through human behavior (drought, famine, war, or other events). In Argentina, plague transmission reportedly occurred during the harvesting season [31]. In Lushoto, a plague endemic region in Tanzania, daily and gender activities seem to impact plague levels [32], although plague tends to peak during the season with the least agricultural activity, which is a time when people usually gather in houses.
What Plague Niches Reveal about Plague's Environmental Preferences
Plague foci are present over an expanded geographical range that includes the Western US, portions of South America, East and South Africa, and Southeast Asia [33]. Long-term maintenance of plague in defined ecological niches may inform us about the environmental conditions that are required for plague to establish in permanent foci. Unfortunately, enzootic plague is poorly described; in many foci, local reservoirs have yet to be identified. The geographical limits of plague territories are hence rarely defined or only by occurrence in domestic rodents and humans [34]. It is reasonable to assert that plague manifests itself under various ecological conditions [35], [36]. It is noteworthy though, that modern plague foci in North and East Africa, Western North America, parts of South America, and many scattered regions in Asia (notably China and Kazakhstan) occur primarily in either semi-arid to arid areas or low humidity forest types of habitat, and the disease apparently fails to persist for long periods in humid tropical lowland areas (except from occasional invasion through movement of infected humans or transportation of infected rodents or fleas) [37]–[39]. Also, plague is almost invariably absent from the hottest and driest desert regions like the Sahara or Sonoran deserts [37], [40].
The Complexity Introduced by Interactions among Scales and Other Nonlinearity
The previous section reviewed reported effects of climate variables on the components of the plague cycle, indicating that climate affects rodents and fleas individually and their population dynamics and structures. Consequently, climate impacts the natural cycle of plague as a whole and in ways that are not likely to reflect simply the sum of these individual effects. In this section, we review studies on the effects of climate (including the environment) on plague at different spatial levels and occurrences ranging from rodent burrows to areas that form a coherent and apparently self-sustaining system (a single niche or focus), or even to larger regions that could comprise many such systems (several foci). We emphasize the fact that (i) scales relevant to plague ecology are nested within each other, as shown in Figure 2, so that climate effects at a given scale may not be simply extrapolated from those observed at a smaller scale, and (ii) processes exist that prevent the plague system from responding to climate fluctuations in a way that simply reflects the sum of the responses of its individual component.
Fig. 2. Illustration of the abiotic environment impact on the plague cycle as a function of spatial scale. 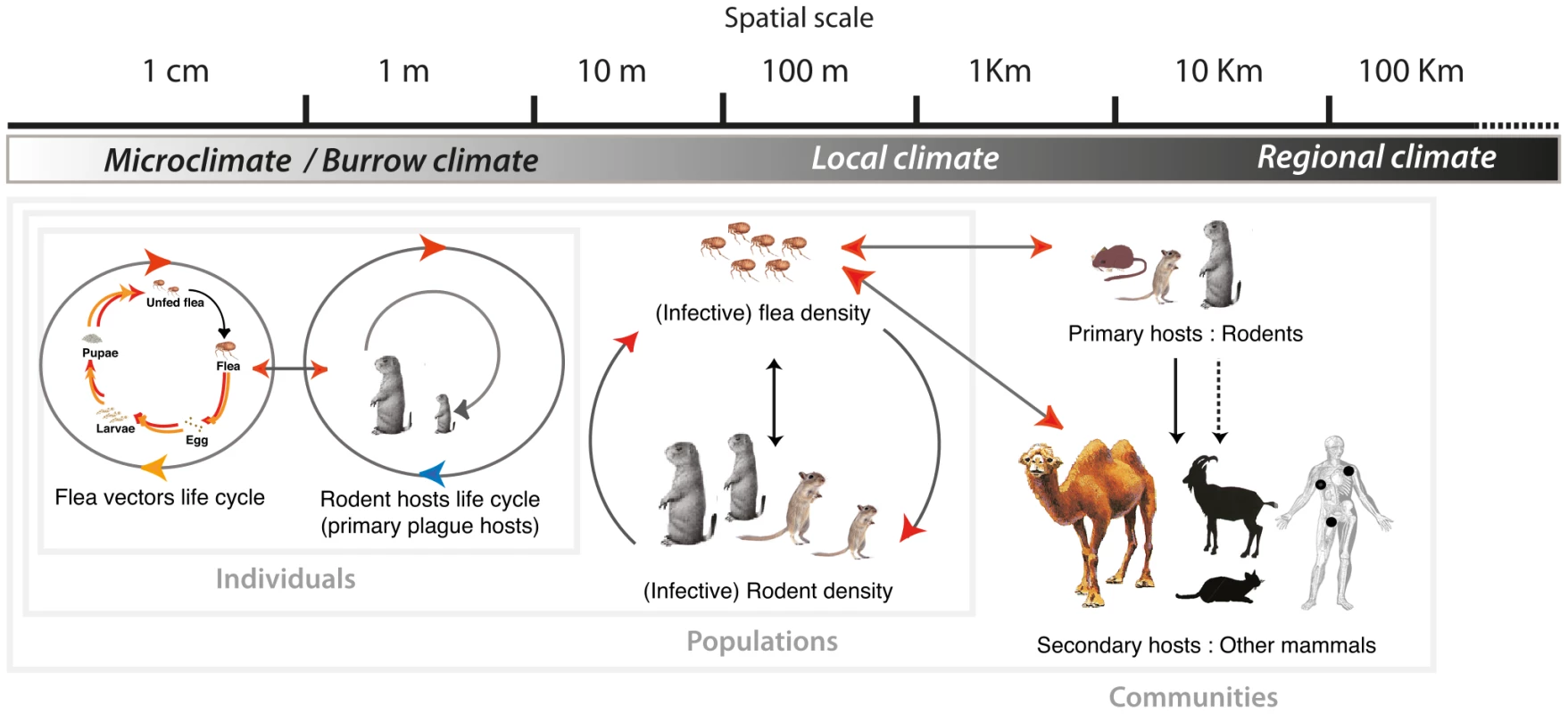
Arrows represent connections affected by climate (see Figure 1 for the meaning of color coding). Most climate variables act over a wide range of scales and only the effects we deemed most important are represented. At the level of individuals, populations and communities hosts and vectors are influenced by climate variability at the relevant scale (local or regional). At the smaller scale, the burrow acts as a filter on climate variables. Note that secondary hosts are placed at the kilometer and larger scale on the basis of the type of information generally available regarding their infection. It is readily apparent that associations exist between large-scale climate such as climate indices and plague/plague hosts dynamics; associations that can be consistently explained by processes detailed in the first section. Effects of precipitation patterns on plague incidence are for instance assumed to be the results of climate's bottom-up effects on plague hosts. In Peru, climate fluctuations associated with El Niño were related to a bubonic plague outbreak in 1999 [41]. In northern Colorado, prairie dog colony extinctions attributed to plague were weakly associated with El Niño southern oscillation [42]. In the Western US, spatial synchrony of periods with low and high number of human plague occurrences throughout the west revealed large scale synchrony [43]. In most foci of the southwestern part of this region, above normal precipitation in winter and spring was used to explain increases in human plague cases [44], and high summer temperatures, decreases of its incidence in the same area [45]. The El Niño/Southern Oscillation (ENSO) and the Pacific Decadal Oscillation were similarly related to precipitation, temperatures, food availability, and the number of plague cases themselves [46]. Finally, the increase rate of human plague in China is associated at the province level with the Southern Oscillation Index and Sea Surface Temperature of the tropic Pacific east equator [47]. An important assumption to the above-cited studies is that large-scale climate variability produces coherent and synchronous effects on rodent hosts populations. Few studies actually investigate the reality of the invoked synchronous trophic forcing on plague hosts' population dynamics so as to demonstrate such a climate induced bottom-up control. At this point, it is worth mentioning a counterexample provided by Kausrud et al. [48], who investigated the population dynamics of great gerbils Rhombomys opimus—the main plague host in Kazakhstan—and demonstrated spatial synchrony of their populations over areas larger than the ones expected by migration processes. Their results indicate that the observed synchrony of great gerbil's densities was most probably due to the effects of climate and that similar bottom-up processes could also synchronize plague activity in this focus.
In any case, there are caveats to inferring processes occurring at small scale as a means to explain the ones observed at larger scales. First, causal relations can typically only be suggested (e.g., by a chain of supposedly causal processes from climate indices to relevant climate variables and from these to plague incidence) but not demonstrated. More problematically, a variety of processes can be expected to interfere with each other in the transition from small to large scale. Tentatively, we propose below a classification of these processes into two categories. The first ones pertain to the complexity of the environment itself and the second ones to population interactions.
An example in the first category can be provided by studies showing that rodent burrows could be considered a “climate filter”. Conditions in rodent burrows are subject to environmental factors from nearby surroundings such as temperature, humidity, vegetation, various soil properties (e.g., soil texture and structure, soil organic carbon content, etc.), and other landscape factors (e.g., slope, orientation with respect to dominant winds, sun exposure, etc.). But, the underground location of most burrows implies that conditions inside these structures fluctuate only moderately [49], [50]. In fact, temperatures inside and outside burrow systems are highly correlated, but inside humidity is a complex function of past rainfall and soil characteristics [51] rather than of present ambient humidity [52]. Note that investigations on burrow micro-climate have been conducted in different parts of the world [51], [53], [54], including in plague-endemic regions, but these measurements were generally obtained from only a small number of natural or, in some instances, artificial burrows, allowing investigators to draw only tentative conclusions from the results of these studies [49], [55]. Perhaps more importantly for our present purposes, climate conditions inside the burrows could specifically influence the distribution of plague vectors so that hosts' distribution becomes insufficient to explain vectors distribution, a situation that has been observed in plague areas in Madagascar [56]–[58] or plague-free areas in Israel [59], [60]. These examples emphasize the difficulty of upscaling processes by simple extrapolation. Among the numerous other examples of environment-related complications are the landscape heterogeneities within plague areas. In particular, strong orographic forcings frequent in plague areas locally affect large-scale climate variability [61]. In fact, accurate plague prediction models seem to require high resolution environmental and climate representation (e.g., 250-m resolution images accurately predict plague distribution when 10-km resolution images fails in sub-Saharan Africa). According to the authors, plague focality can not be explained by fragmented environmental conditions at this coarse scale [35], [62].
In the second category are the complications introduced by interactions within and between plague host and plague vector populations in their response to climate variability. It is commonly expected that fluctuations in abundance of rodent hosts, e.g. induced by climate variability, will translate into plague prevalence fluctuations. However, the relationship between host abundance and host prevalence is complex and scale dependent. Field studies of rodent reservoirs show a negative correlation between host abundance and host prevalence at seasonal time scale. The finding has been explained by the juvenile dilution effect, that is, the arrival of numerous healthy juveniles in a population [63]; an effect likely to be relevant for diseases with no vertical transmission such as plague (or Hantavirus [64]). In contrast, a few longer field studies (>5 years) show a positive, although delayed relationship between reservoir abundance and prevalence [63]. These antagonist responses to variability at different time scales are typical of systems in which nonlinear interactions play an essential role. The different responses of rodent and flea populations to climate (fast for fleas while rodents tend to integrate environmental conditions over some years) provide another reason for questioning the existence of a straightforward link between abundance and prevalence. A more complex model was proposed for the Western US, in which human plague incidence depends both on time-lagged precipitation events, which presumably increase rodent numbers and favor plague epizootic, and on relatively cool summer temperatures during the plague transmission season, which are favorable to infectious fleas [45]. This model has been coined “trophic cascade hypothesis,” although it has yet to be tested rigorously for plague under field conditions [65] (see also [66] for a discussion on the accuracy of the use of the term “trophic” for the cascade hypothesis). In particular, the scale at which the trophic cascade model is valid needs to be addressed. Parmenter [44], who first introduced this model, shows its relevance at a local scale (i.e., by demonstrating significant associations between plague and nearby precipitation), but could not extend this result to a state-wide level. Interestingly, a cascade relationship was recovered at an even larger scale by Ben-Ari [46], possibly because the integration of delayed density-dependence effects of large-scale climate on rodents were taken into account at decadal time scales. The study by Stenseth et al. [67] illustrates the challenge that needs to be confronted when addressing cascading effects of climate on plague prevalence in nature. There are numerous relationships between climate elements (temperature and precipitation with or without lags) and various ways these elements can impact the plague components (in this particular case rodent density, prevalence, and flea burden). The type of dataset that would let us isolate/untangle the mechanisms and the spatio-temporal scales at which processes operate may not be available.
These examples do not necessarily invalidate studies that scale up small-scale results (individual measurements, lab experiments, local correlations, etc.) in the simplest way, but we believe they illustrate the need for more investigation on the impact of complex interactions and environment heterogeneities at intermediate scales.
Implications for Climate Change
The need to understand disease responses in relation to climate variability is made particularly acute by ongoing global climate change. Effects of temperature rise on vector-borne diseases and notably the ones involving free-living stages of terrestrial animals are expected [68]. Beyond that, a lot of uncertainty remains on whether or how climate change might affect pathogens and disease transmission, i.e., changes in population size (for vectors or hosts), overall prevalence, timing and seasonality, or shifts in geographical distribution. There are various choices to be made when addressing climate change impact on a disease like plague, particularly with respect to the degree of complexity that should be chosen for climate models and any associated biological models and the relation between them.
Stenseth et al. [67] performed a sensitivity study to a one-degree increase in temperature into a statistical host vector plague model developed specifically for Kazakhstan. They show that this (simple) climate change scenario would lead to a 50% increase in plague prevalence among great gerbils. Nakazawa et al. [69] used two Global Circulation Models (GCMs) to infer mean temperature and precipitation changes between the present and a 30-year period centered around 2055. These changes were then applied to a higher resolution present state GCM that provided a climate changed forcing state, which was fed into an Ecological Niche Model (ENM) predicting plague occurrences in the US from a set of environmental variables. They show subtle shifts of plague habitats (generally northward). Snall et al. [70] used an elaborate procedure to downscale climate scenarios from several GCMs before using these data to force an explicit model for the joint host-parasite dynamics of black-tailed prairie dogs and plague in the Western US. A related decrease in the number of infected prairie dog colonies (leading to an increase in prairie dog colonies) is predicted, presumably, as a consequence of the negative impact of increased frequency of abnormally hot days on plague transmission.
These studies are difficult to compare with each other because of the specificities of their methodologies even when they lead to contrasting results over the same region [70]. Admittedly, more investigations are required. Among the numerous possible approaches, the safest arguably would rely on using climate-forcing sensitivities of intermediate complexity where biological models are forced by existing modes of climate variability. This makes sense not only because climate change will in large part occur through a modification of these modes (which can be extracted from Intergovernmental Panel on Climate Change [IPCC] GCMs [61]), but also because biological data are then available for evaluation.
Conclusion
Climate impacts all components of the plague cycle (host, vector, and pathogen) in various ways and over a wide range of scales (from micro—individual flea life cycle—to macro—a plague area composed of several disjoint foci). Several studies have established links between plague occurrence and climate factors that can a posteriori be justified by assuming the validity at a large scale of relationships that have only been observed at a small scale (assumption of linearity). We have reviewed both the successes and the limitations of this assumption. To go beyond simple inferences on how climate fluctuations (including long-term climate change) affect plague, it might be necessary to select a (or a few) preferential scale, on the basis of the fact that they would be the most informative and/or relevant for public health policies. In this regard, intrinsic dynamics of plague hosts and vectors should be kept in mind as it alone greatly contributes to the entanglement of scales that drive the overall dynamics of plague prevalence [71]. Further, assessing plague risks for humans at such scales may in fact require investigating plague dynamics on a much wider range of scales and presumably include a fuller description of the plague system in its environment, as we have tried to outline in this review.
Zdroje
1. StensethNCAtshabarBBBegonMBlemainSRBertheratE 2008 Plague: past, present and future. PloS Med 5 e3 doi:10.1371/journal.pmed.0050003
2. CatanachIJ 2001 The “globalization” of disease? India and the plague. Journal of World History 12 131 153
3. RogersL 1928 The yearly variations in plague in India in relation to climate: forecasting epidemics. Proc Roy Soc Ser B 103 42 72
4. DavisDH 1953 Plague in Africa from 1935 to 1949; a survey of wild rodents in African territories. Bull World Health Organ 5 665 700
5. CavanaughDCMarshallJD 1972 The influence of climate on the seasonal prevalence of plague in the Republic of Vietnam. J Wildl Dis 8 85 94
6. CavanaughDDangerfieldHHunterDJoyRMarshallJDJ 1968 Some observations on the current plague outbreak in the Republic of Vietnam. Am J Public Health Nations 58 742 752
7. MeservePLYungerJAGutiérrezJRContrerasLCMilsteadWB 1995 Heterogeneous responses of small mammals to an El Niño Southern Oscillation event in north central semiarid Chile and the importance of ecological scale. J Mammal 76 580 595
8. GublerDJReiterPEbiKLYapWNasciR 2001 Climate variability and change in the United States: potential impacts on vector - and rodent-borne diseases. Environ Health Persp 109 223 33
9. KrasnovBRKhokhlovaISFieldenLJBurdelovaNV 2001 Effect of air temperature and humidity on the survival of pre-imaginal stages of two flea species (Siphonaptera: Pulicidae). J Med Entomol 38 629 37
10. KrasnovBRKhokhlovaISFieldenLJBurdelovaNV 2001 Development rates of two Xenopsylla flea species in relation to air temperature and humidity. Med Vet Entomol 15 249 258
11. KrasnovBRBurdelovaNVShenbrotGIKhokhlovaIS 2002 Annual cycles of four flea species in the central Negev desert. Med Vet Entomol 16 266 276
12. GageKLBurkotTREisenRJHayesEB 2008 Climate and vector-borne diseases. Am J Prev Med 35 436 50
13. GageKLBurkotTREisenRJHayesEB 2008 Climate and vector-borne diseases. Am J Prev Med 35 436 50
14. BacotAWMartinCJ 1924 The respective influence of temperature and moisture upon the survival of the rat flea. J Hyg 23 98 105
15. CavanaughDC 1971 Specific effect of temperature upon transmission of the plague bacillus by the oriental rat flea, Xenopsylla Cheopis. J Trop Med Hyg 20 264 273
16. EisenRJGageKL 2009 Adaptive strategies of Yersinia pestis to persist during inter-epizootic and epizootic periods. Vet Res 40 01
17. ParmenterRRYadavEPParmenterCAEttestadPGageKL 1999 Incidence of plague associated with increased winter-spring precipitation in New Mexico. Am J Trop Med Hyg 61 814 821
18. MeservePLMilsteadWBGutierrezJR 2001 Results of a food addition experiment in a north-central Chile small mammal assemblage: evidence for the role of “bottom-up” factors. Oïkos 94 548 556
19. LetnicMTamayoBDickmanCR 2005 The responses of mammals to La Nina (El Nino Southern Oscillation) associated rainfall, predation, and wildfire in central Australia 86 689 703
20. JaksicFMLimaM 2003 Myths and facts on ratadas: bamboo blooms, rainfall peaks and rodent outbreaks in South America. Aust J Ecol 28 237 251
21. LimaMJaksicFM 1999 Population rate of change in the leaf-eared mouse: the role of density-dependence, seasonality and rainfall. Aust J Ecol 24 110 116
22. LimaMKeymerJEJaksicFM 1999 El Nino-southern oscillation-driven rainfall variability and delayed density dependence cause rodent outbreaks in western South America: linking demography and population dynamics. Am Nat 153 476 491
23. LimaMMarquetPAJaksicFM 1999 El Nino events, precipitation patterns, and rodent outbreaks are statistically associated in semiarid Chile. Ecography 22 213 218
24. LeirsHVerhagenRVerheyenWMwanjabePMbiseT 1996 Forecasting rodent outbreaks in Africa: an ecological basis for Mastomys control in Tanzania. J Appl Ecol 33 937 943
25. DickmanCRHaythornthwaiteASMcNaughtGHMahonPSTamayoB 2001 Population dynamics of three species of dasyurid marsupials in arid central Australia: a 10-year study. Wildl Res 28 493 506
26. MillsJN 2005 Regulation of rodent-borne viruses in the natural host: implications for human disease. Arch Virol 19 45 57
27. BrownJHMorgan ErnestSK 2002 Rain and rodents: complex dynamics of desert consumers. Bioscience 52 979 987
28. KorslundLSteenH 2006 Small rodent winter survival: snow conditions limit access to food resources. J Anim Ecol 75 156 166
29. BazanovaLPNikitinAYPopkovAFMaevskiiMP 2007 Seasonal peculiarities of plague agent (Yersinia pestis) transmission to the long-tailed suslik by fleas (Citellophilus tesquorum) in Tuva [in Russian]. Zoologichesky Zhurnal 86 846 852
30. BaltazardMBahmanyarM 1960 Research on plague in India. Bull World Health Organ 23 169 215
31. MacchiavelloA 1946 A focus of sylvatic plague on the Peruvian-Ecuadorian frontier. Science 104 522
32. KilonzoBSMvenaZSMachanguRSMbiseTJ 1997 Preliminary observations on factors responsible for long persistence and continued outbreaks of plague in Lushoto district, Tanzania. Acta tropica 68 215 27
33. WHO (World Health Organization) 2008 Interregional meeting on prevention and control of plague. Antananarivo, Madagascar 7–11 April 2006. 1–65. Available: http://www.who.int/csr/resources/publications/WHO_HSE_EPR_2008_3w.pdf. Accessed 16 August 2011
34. NeerinckxSBPetersonATGulinckHDeckersJLeirsH 2008 Geographic distribution and ecological niche of plague in sub-Saharan Africa. Int J Health Geogr 7 54
35. PrenticeMBRahalisonL 2007 Plague. Lancet 369 1196 1207
36. DennisDTGageKLGratzNGPolandJDTikhomirovE 1999 Plague manual: epidemiology, distribution, surveillance and control. Bull World Health Organ 1 171
37. PerryRDFetherstonJD 1997 Yersinia pestis - etiologic agent of plague. Clin Microbiol Rev 10 35 66
38. GageKLKosoyMY 2005 Natural history of plague: perspectives from more than a century of research. Ann Rev Entomol 50 505 28
39. BarnesA 1982 Surveillance and control of bubonic plague in the United States. Symp Zool Soc Lond 50 237 270
40. DavalosVATorresMAMauricciCOLaguna-TorresVAChinarroMP 2001 Outbreak of bubonic plague in Jacocha, Huancabamba, Peru. Rev Soc Bras Med Trop 34 87 90
41. StappPAntolinMFBallM 2004 Patterns of extinction in prairie dog metapopulations: plague outbreaks follow El Ninño events. Front Ecol Environ 2 235 240
42. Ben AriTGershunovAGageKLSnällTEttestadP 2008 Human plague in the USA: the importance of regional and local climate. Biol lett 4 737 740
43. PollitzerR 1954 Plague. World Health Organization monograph series number 22. Geneva World Health Organization 698
44. ParmenterRRYadavEPParmenterCEttestadPGageKL 1999 Incidence of plague associated with increased winter-spring precipitation in New Mexico. Am J Trop Med Hyg 61 814 821
45. EnscoreREBiggerstaffBJBrownTLFulghamREReynoldsPJ 2002 Modeling relationships between climate and the frequency of human plague cases in the southwestern United States, 1960-1997. Am J Trop Med Hyg 66 186 196
46. Ben AriTGershunovATristanRCazellesBGageK 2010 Interannual variability of human plague occurrence in the Western United States explained by tropical and North Pacific Ocean climate variability. Am J Trop Med Hyg 83 624 632
47. ZhangZLiZTaoYChenMWenX 2007 Relationship between increase rate of human plague in China and global climate index as revealed by cross-spectral and cross-wavelet analyses. Int Zool 2 144 153
48. KausrudKLViljugreinHFrigessiABegonMDavisS 2007 Climatically driven synchrony of gerbil populations allows large-scale plague outbreaks. P Roy Soc B-Biol Sci 274 1963 1969
49. LonganeckerDSBurroughsAL 1952 Studies of the microclimate of the California ground squirrel burrow and its relation to seasonal changes in the flea population. 33 488 499
50. SumberaRChitaukaliWNElichovaMKubovaJBurdaH 2004 Microclimatic stability in burrows of an Afrotropical solitary bathyergid rodent, the silvery mole-rat (Heliophobius argenteocinereus). J Zool 263 409 416
51. HallLOMyersK 1978 Variations in the microclimate in rabbit warrens in semi-arid New South Wales. Aust J Ecol 3 187 194
52. Osacar-JimenezJJLucientes-CurdiJCalvete-MargolleC 2001 Abiotic factors influencing the ecology of wild rabbit fleas in north-eastern Spain. Med Vet Entomol 15 157 66
53. HaasGE 1965 Comparative suitability of the four murine rodents of Hawaii as hosts for Xenopsylla vexabilis and X. cheopis (Siphonaptera). J Med Entomol 275-83
54. ShenbrotGKrasnovBKhokhlovaIDemidovaTFieldenL 2002 Habitat-dependent differences in architecture and microclimate of the burrows of Sundevall's jird (Meriones crassus) (Rodentia: Gerbillinae) in the Negev Desert, Israel. J Arid Environ 51 265 279
55. RyckmanRE 1971 Plague vector studies. II. The role of climatic factors in determining seasonal fluctuations of flea species associated with the California ground squirrel. J Med Entomol 8 541 549
56. KleinJM 1966 Donnees ecologiques et biologiques sur Synopsyllus fonquerniei, puce de rat peridomestique dans la region de Tanarive [In French]. Cahiers Orstom Entomologie medicale 4 3 29
57. KleinJMUilenbergG 1966 Donnees faunistiques et ecologiques sur les puces de Madagascar (Siphonaptera) [In French]. Cahiers Orstom Entomologie 16 31 60
58. ChanteauS 2006 Atlas de la peste a Madagascar. Paris IRD Editions
59. KrasnovBKhokhlovaIFieldenLBurdelovaNV 2002 The effect of substrate on survival and development of two species of desert fleas (Siphonaptera: Pulicidae). Parasite 9 135 142
60. AdjemianJCZGirvetzEHBeckettLFoleyJE 2006 Analysis of Genetic Algorithm for Rule-Set Production (GARP) modeling approach for predicting distributions of fleas implicated as vectors of plague, Yersinia pestis, in California. J Med Entomol 43 93 103
61. IPPC IP onCC 2007 Climate Change 2007: the physical science basis - summary for policymakers. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva IPPC
62. NeerinckxSPetersonATGulinckHDeckersSKimaroD 2010 Predicting potential risk areas of human plague for the Western Usambara mountains, Lushoto district, Tanzania. Am J Trop Med Hyg 82 492 500
63. DavisSCalvetELeirsH 2005 Fluctuating rodent populations and risk to humans from rodent-borne zoonoses. Vect Born Zoo Dis 5 305 314
64. MillsJNKsiazekTGPetersCJChildsJE 1999 Long-term studies of hantavirus reservoir populations in the southwestern United States: a synthesis. Emerg Infect Dis 5 135 42
65. CollingeSKJohnsonWCRayCMatchettRGrenstenJ 2005 Testing the generality of a trophic-cascade model for plague. EcoHealth 2 102 112
66. StappP 2007 Trophic cascades and disease ecology. EcoHealth 4 121 124
67. StensethNCSamiaNIViljugreinHKausrudKLBegonM 2006 Plague dynamics are driven by climate variation. Proc Natl Acad Sci U S A 103 13110 13115
68. HarvellCDMitchellCEWardJRAltizerSDobsonAP 2002 Climate warming and disease risks for terrestrial and marine biota. Science 296 2158 2162
69. NakazawaYWilliamsRPetersonATMeadPStaplesE 2007 Climate change effects on plague and tularemia in the United States. Vect Born Zoo Dis 7 529 540
70. SnallTBenestadREStensethNC 2009 Expected future plague levels in a wildlife host under different scenarios of climate change. Glob Change Biol 15 500 507
71. DavisSTrapmanPLeirsHBegonMHeesterbeekJAP 2008 The abundance threshold for plague as a critical percolation phenomenon. Nature 454 635 637
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



