-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSignificant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
Antiretroviral therapy (ART) has reduced morbidity and mortality in HIV-1 infection; however HIV-1-associated neurocognitive disorders (HAND) persist despite treatment. The reasons for the limited efficacy of ART in the brain are unknown. Here we used functional genomics to determine ART effectiveness in the brain and to identify molecular signatures of HAND under ART. We performed genome-wide microarray analysis using Affymetrix U133 Plus 2.0 Arrays, real-time PCR, and immunohistochemistry in brain tissues from seven treated and eight untreated HAND patients and six uninfected controls. We also determined brain virus burdens by real-time PCR. Treated and untreated HAND brains had distinct gene expression profiles with ART transcriptomes clustering with HIV-1-negative controls. The molecular disease profile of untreated HAND showed dysregulated expression of 1470 genes at p<0.05, with activation of antiviral and immune responses and suppression of synaptic transmission and neurogenesis. The overall brain transcriptome changes in these patients were independent of histological manifestation of HIV-1 encephalitis and brain virus burdens. Depending on treatment compliance, brain transcriptomes from patients on ART had 83% to 93% fewer dysregulated genes and significantly lower dysregulation of biological pathways compared to untreated patients, with particular improvement indicated for nervous system functions. However a core of about 100 genes remained similarly dysregulated in both treated and untreated patient brain tissues. These genes participate in adaptive immune responses, and in interferon, cell cycle, and myelin pathways. Fluctuations of cellular gene expression in the brain correlated in Pearson's formula analysis with plasma but not brain virus burden. Our results define for the first time an aberrant genome-wide brain transcriptome of untreated HAND and they suggest that antiretroviral treatment can be broadly effective in reducing pathophysiological changes in the brain associated with HAND. Aberrantly expressed transcripts common to untreated and treated HAND may contribute to neurocognitive changes defying ART.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002213
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002213Summary
Antiretroviral therapy (ART) has reduced morbidity and mortality in HIV-1 infection; however HIV-1-associated neurocognitive disorders (HAND) persist despite treatment. The reasons for the limited efficacy of ART in the brain are unknown. Here we used functional genomics to determine ART effectiveness in the brain and to identify molecular signatures of HAND under ART. We performed genome-wide microarray analysis using Affymetrix U133 Plus 2.0 Arrays, real-time PCR, and immunohistochemistry in brain tissues from seven treated and eight untreated HAND patients and six uninfected controls. We also determined brain virus burdens by real-time PCR. Treated and untreated HAND brains had distinct gene expression profiles with ART transcriptomes clustering with HIV-1-negative controls. The molecular disease profile of untreated HAND showed dysregulated expression of 1470 genes at p<0.05, with activation of antiviral and immune responses and suppression of synaptic transmission and neurogenesis. The overall brain transcriptome changes in these patients were independent of histological manifestation of HIV-1 encephalitis and brain virus burdens. Depending on treatment compliance, brain transcriptomes from patients on ART had 83% to 93% fewer dysregulated genes and significantly lower dysregulation of biological pathways compared to untreated patients, with particular improvement indicated for nervous system functions. However a core of about 100 genes remained similarly dysregulated in both treated and untreated patient brain tissues. These genes participate in adaptive immune responses, and in interferon, cell cycle, and myelin pathways. Fluctuations of cellular gene expression in the brain correlated in Pearson's formula analysis with plasma but not brain virus burden. Our results define for the first time an aberrant genome-wide brain transcriptome of untreated HAND and they suggest that antiretroviral treatment can be broadly effective in reducing pathophysiological changes in the brain associated with HAND. Aberrantly expressed transcripts common to untreated and treated HAND may contribute to neurocognitive changes defying ART.
Introduction
HAND is a common complication of HIV-1 infection in the nervous system presenting a varied spectrum of clinical manifestations with cognitive, motor and behavioral symptoms. Currently three conditions of increasing severity are recognized as components of HAND: HIV-1-associated asymptomatic neurocognitive impairment, HIV-1-associated mild neurocognitive disorders (MND), and HIV-1-associated dementia (HIV-D or HAD) [1]. HAND remains prevalent in populations with access to highly active ART, despite the efficacy of these therapies in controlling viral load and ameliorating viral load-associated clinical and neuroradiologic abnormalities [2]–[6]. By some accounts, up to 50% of HIV-1-infected individuals will develop some form of HAND regardless of access to currently available ART [7]–[9]. Under ART, HAND has became milder, its course more protracted and variable in symptoms, and it now overlaps with aging processes and potentially with other neurodegenerative diseases in AIDS patients [7], [10], [11]. The etiology of persistent cognitive deficits in patients on ART remains unclear. Studies of cerebrospinal fluid from individuals with HAND have sometimes supported contradictory conclusions; in the absence of viral replication, some authors observe associations among dementia, abnormal neurometabolites, and inflammatory phenotypes; others, with non-inflammatory states or markers of neurodegeneration [12]–[16]. There have been limited studies of brain tissues from treated patients with HAND to evaluate what biologic pathways remain abnormally regulated under ART, with studies largely focused on single cell types or molecules [17]. Comprehensive analysis of the spectrum of molecular abnormalities in the brain that may underlie HAND in the presence of ART has not yet been undertaken.
Here we used functional genomics to conduct comparative analysis of genome-wide gene expression profiles in brain tissues from treated and untreated patients who died with HAND. Functional genomics has been applied with success to identification of complex molecular pathways in carcinogenesis and to better detection, classification, and prognosis of some cancers [18]–[23]. This approach has also been used extensively to investigate the transcriptome correlates of HIV-1 infection in peripheral tissues from HIV-1-infected patients including lymph nodes [24]–[26], CD4+ T cells [27], [28], monocytes [29], B cells [30], and gastrointestinal mucosa [31]. Some of these studies determined the effects of ART on HIV-1 transcriptomes in patients, revealing categories of treatment-responsive genes as well as aberrantly expressed transcripts that may serve as targets for future therapies [24], [27]–[31]. In a related approach, a recent study evaluated gene expression profiles of blood monocytes as a function of ART and neuropsychological impairment of HIV-1-infected patients [32]. Interestingly in this case, there was no correlation between changes in blood monocyte transcriptomes under treatment and clinical HAND [32]. Generally, this research benefited from the ability to serially sample peripheral tissues and individual cell types in living individuals, allowing ongoing evaluation of treatment.
In contrast, analyses of human brain tissues by functional genomics can only be conducted retrospectively in autopsy tissues and are complicated by the multicellular interactions underlying central nervous system diseases [33]. Nonetheless, large-scale, cross-sectional gene expression profiling of brain tissues has revealed potential pathogenic pathways in Alzheimer's disease (AD) [34], [35], Parkinson's disease [36], [37], chronic schizophrenia [38], multiple sclerosis [39], and viral encephalitis [40]. With respect to HIV-1 infection in the brain, investigators reported transcriptional changes in selected gene categories such as anion channels in the frontal cortex of patients who died with HAND [41], [42]. Another group reported aberrant expression of genes specific to HIV-1 encephalitis (HIVE) [43], established a correlation between use of methamphetamine and up-regulation of interferon genes in these patients [44], and investigated the role of microRNA in gene regulation in HIV-1-infected brain [45]. To our knowledge, these studies did not consider the effects of ART on brain gene dysregulation in HAND.
The Manhattan HIV Brain Bank (MHBB; member of the National NeuroAIDS Tissue Consortium) follows a cohort of advanced-stage, HIV-1-infected individuals with a high prevalence of well-characterized cognitive dysfunction; with entry to the study, participants agree to be organ donors upon death. The antiviral treatment status of study participants is monitored while in the program. Thus, brain tissues obtained by this program provide an opportunity to examine the potentially diverse processes underlying HAND in the ART era. We report herein the gene expression profiles of individuals with HAND focusing on the impact of ART on these profiles.
Results
Effect of therapy on HIV-1 brain burdens
We assayed virus burden and cellular gene expression on archived brain tissues from 15 HIV-1-infected patients with HAND and six HIV-1-negative subjects with no neurological or neuropathological abnormalities. Information for this study group is summarized in Table 1 and Methods. All assays were performed on parallel samples from deep white matter within the anterior frontal lobe, an area implicated in HAND and HIV-1-associated neuropathologies [46], [47]. Custom consensus primers based on sequences of HIV-1 amplified from the brain were used for reliable measurement of HIV-1 in the brain by quantitative real-time PCR (QPCR) (see Methods); the results were confirmed by standard PCR and hybridization with a specific probe (Table 1 and Supplementary Figure S1). Pre-mortem plasma HIV-1 burdens are plotted for comparison (Supplementary Figure S1). With the exception of patient 30015, the HIV-1 brain burdens in untreated patients correlated with presentation of HIVE; on average, these patients had about 180-fold more viral RNA per µg total RNA than patients without HIVE. Patient 30015 had limited HIVE pathology and no detectable virus in the brain (Table 1). Patients on ART, both with and without HIVE, had lower virus burdens in the brain than untreated patients, results consistent with a previous study in a different cohort [48]. In patients with HIVE the reduction was 50% and 95% at the DNA and RNA levels respectively, while virus was undetectable in treated patients without HIVE. Notably, there was no correlation between brain and plasma viral loads and brain virus burdens were independent of patient's age, gender, ethnic background, or postmortem interval.
Tab. 1. Study subject information and HIV burdens in different compartments. 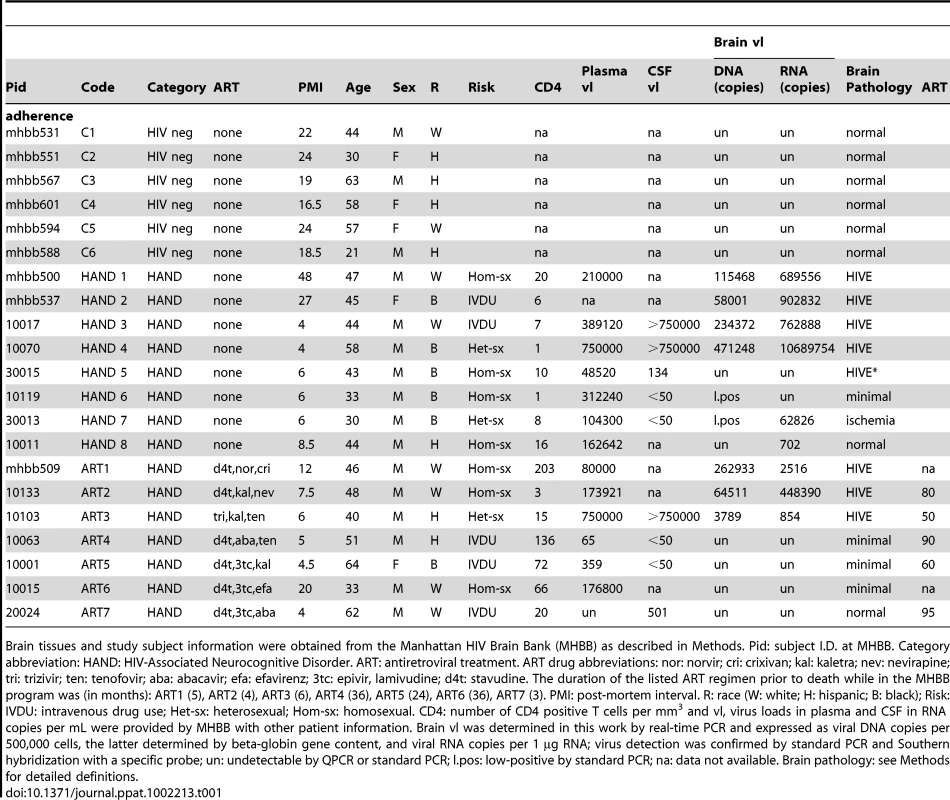
Brain tissues and study subject information were obtained from the Manhattan HIV Brain Bank (MHBB) as described in Methods. Pid: subject I.D. at MHBB. Category abbreviation: HAND: HIV-Associated Neurocognitive Disorder. ART: antiretroviral treatment. ART drug abbreviations: nor: norvir; cri: crixivan; kal: kaletra; nev: nevirapine; tri: trizivir; ten: tenofovir; aba: abacavir; efa: efavirenz; 3tc: epivir, lamivudine; d4t: stavudine. The duration of the listed ART regimen prior to death while in the MHBB program was (in months): ART1 (5), ART2 (4), ART3 (6), ART4 (36), ART5 (24), ART6 (36), ART7 (3). PMI: post-mortem interval. R: race (W: white; H: hispanic; B: black); Risk: IVDU: intravenous drug use; Het-sx: heterosexual; Hom-sx: homosexual. CD4: number of CD4 positive T cells per mm3 and vl, virus loads in plasma and CSF in RNA copies per mL were provided by MHBB with other patient information. Brain vl was determined in this work by real-time PCR and expressed as viral DNA copies per 500,000 cells, the latter determined by beta-globin gene content, and viral RNA copies per 1 µg RNA; virus detection was confirmed by standard PCR and Southern hybridization with a specific probe; un: undetectable by QPCR or standard PCR; l.pos: low-positive by standard PCR; na: data not available. Brain pathology: see Methods for detailed definitions. Untreated HAND patients with dementia have similar brain tissue transcriptomes irrespective of their HIVE histopathology and virus burdens
Global gene expression profiles of patient brain tissues were determined on Affymetrix GeneChip Array Human Genome U133 Plus 2.0 Arrays. Three independent array experiments were performed, each comprising a subset of HIV-1-positive samples and controls, with most samples tested either in duplicates or repeated in independent runs and some samples tested three times. Replicate gene sets of the same samples were averaged, yielding 21 final brain tissue datasets for 21 subjects. Preliminary hierarchical cluster analysis of complete datasets from HAND patients indicated that the primary biological variable in clustering of these datasets was whether or not patients were on ART at the time of death (not shown). Interestingly, this analysis also indicated that brain transcriptomes from untreated patients with HIVE did not cluster independently of non-HIVE transcriptomes (Figure 1A), suggesting that they are statistically similar across their entire datasets. This result was surprising because untreated patients with HIVE had on average higher brain virus burdens than patients without HIVE (Table 1 and Supplementary Figure S1) and HIV-1 infection is known to induce cellular gene expression [49]. To confirm findings of cluster analysis we performed global gene set analysis using GAzer software [50] to identify biological pathways that were most significantly altered in HIVE positive and negative groups compared to uninfected controls (Figure 1B). GAzer employs parametric analysis of sets of co-regulated genes across complete microarray datasets, independent of arbitrary fold change (FC) value limits, thus increasing statistical power of detection of biological differences between datasets [50], [51]. Figure 1B depicts the eight most altered pathways in HAND and HAND/HIVE datasets versus controls and Supplementary Table S1 lists all aberrant pathways and detailed statistics of GAzer analysis including Z-score, p-and q-values, and Bonferroni correction value. Consistent with previous array studies in HIVE patients [43], [44], datasets of patients with HIVE showed greater dysregulation of immune responses and endogenous antigen presentation pathways than those without HIVE (Figure 1B), possibly reflecting effects of high virus burdens in the brain in untreated HIVE (Table 1). However, the two HAND patient groups were generally similar with respect to the ranking and extent of change (indicated by all four statistical measures) of the majority of altered gene sets (Figure 1B and Supplementary Table S1). Because of their overall similarities in both cluster and gene set analyses, we chose to pool microarray datasets from HAND patients with and without encephalitis for analysis of antiviral drug effects.
Fig. 1. Similarity of brain transcriptomes of untreated HAND patients with and without encephalitis. 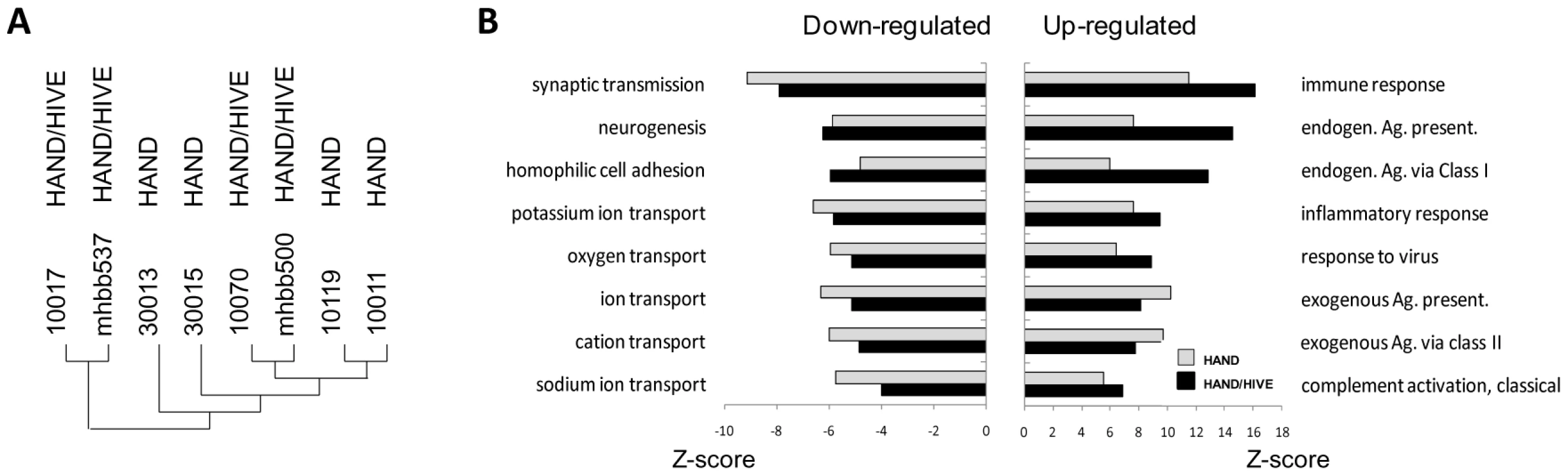
A. Using Affymetrix microarrays we evaluated gene expression in brain tissue. Normalized data from HAND patients with or without encephalitis were analyzed by Hierarchical Clustering using Genesis software. B. Gene set analysis was conducted by GAzer software to identify biological pathways most significantly up-regulated (right panel) or down-regulated (left panel) in untreated HAND without or with encephalitis (HAND/HIVE) vs. uninfected controls. Patients with HAND on antiretroviral therapy have many fewer and milder gene expression changes in the brain than untreated patients
Seven of the 15 patients in our cohort were treated using different antiretroviral drug combinations (Table 1) enabling investigation of the extent of HIV-1-induced changes in the brain and potential differences between treated and untreated patients at the level of brain transcriptomes. Patient microarray datasets were pooled separately into untreated (HAND, n = 8) and treated (ART, n = 7) groups and each group was compared to the uninfected control pool C (n = 6). We used a cut-off of 1.5 FC and p-value<0.05 to identify significantly dysregulated genes. For some analyses, we also established a subgroup ARTa excluding low adherence subjects ART3 and ART5. The complete lists of FC values and accompanying statistics for HAND, ART, and ARTa are provided in Supplementary Tables S2 and S3. Overall, we identified 2073 dysregulated transcripts in the untreated HAND group and 333 and 145 transcripts in the ART and ARTa groups, respectively (Table S2). The Venn diagram in Figure 2A depicts the number of genes dysregulated in brain tissues of untreated and treated patients with HAND and the overlap in the dysregulated genes among the three groups tested. Excluding multiple probes for the same gene and transcripts of undefined function at the time of this writing, the HAND group had 1470 genes with significantly altered expression compared to 260 in ART and 107 in ARTa (Figure 2A, Supplementary Tables S2 and S3). About two-thirds of dysregulated genes (947) in untreated patients were up-regulated and 95 of these had FC values of ≥3.0 and p = 10−2–10−7, among down-regulated genes, 58 had FC of ≤−3.0 and p of 10−2–10−4, suggesting that HIV-1 infection profoundly alters the brain transcriptome in untreated patients with HAND and indicating significant conformity of molecular profiles of disease in this cohort. In treated patients, down-modulated genes predominated, the FC values ranged from 3.95 to 1.5 for up-regulated genes and from −1.5 to −2.87 for down-regulated genes, and p-values were 5×10−2–7.3×10−5 (Supplementary Tables S2 and S3), indicating less uniformity of brain gene expression in this group. These results indicate marked differences between aberrant brain transcriptome profiles of untreated and treated patients, the latter showing 6–14-fold (depending on treatment compliance) fewer dysregulated genes with generally lesser dysregulation of expression than in untreated patients.
Fig. 2. The pattern of changes in brain cell gene expression relative to uninfected subjects differs significantly between untreated HAND patients and HAND patients treated with ART. 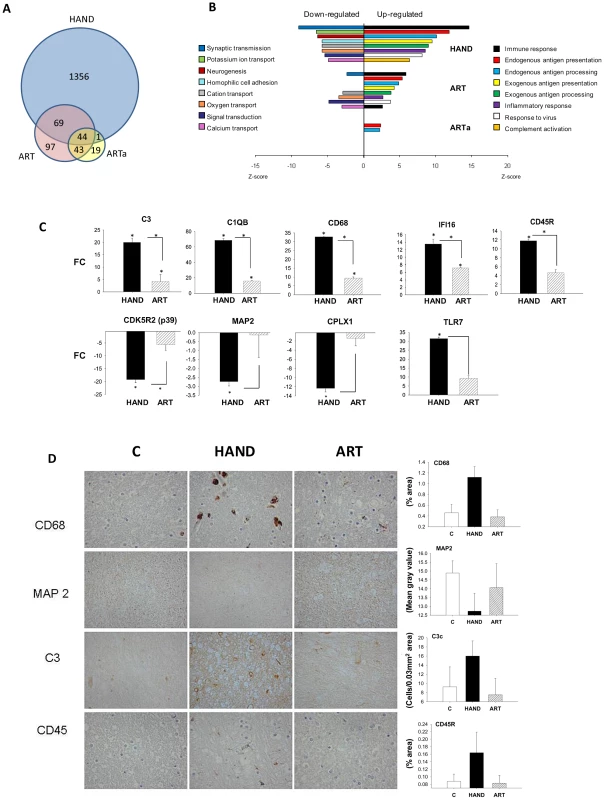
A. The Venn diagram displays the number and overlap of transcripts differentially expressed relative to uninfected subjects from HAND, ART, and HAND patients excluding low adherence patients ART3 and ART5 (ARTa). B. GAzer was employed to identify the eight most dysregulated biological pathways relative to uninfected subjects in the HAND dataset and the extent of change in the same pathways in ART and ARTa datasets are shown for comparison with Z-scores plotted for the significantly up-regulated (right panel) and down-regulated (left panel) pathways. C. QPCR analysis of gene expression in HAND and ARTa patients plotting fold change of expression uninfected subjects. Asterisks represent p-value<0.05 in t-test analysis, asterisks over column indicate comparison to values from uninfected subjects, asterisks between columns represent their comparison. D. Immunostaining and quantitation of selected proteins in brain tissue of uninfected subjects (C), HAND and ART patients. To confirm this observation we conducted gene ontology analysis using GAzer to examine cellular processes affected by HIV-1 infection in the brain in our patient groups. Altered gene sets (biological pathways) were identified by comparing each patient group to HIV-1-negative controls; Supplementary Table S4 lists these pathways for HAND, ART, and ARTa in the order of their significance as determined by the Z-score, p and q values, and Bonferroni statistics [50], [51]. Figure 2B shows the eight most dysregulated biological pathways in the HAND datasets, displaying the extent of dysregulation in the same pathways in ART and ARTa datasets. Up-regulated pathways in untreated HAND patients included immune responses, inflammation, response to virus, and complement activation while synaptic transmission, neurogenesis, ion transport, cell adhesion, and signal transduction were down-regulated. The statistical significance for the eight major pathway changes reached Z-scores of 6–14 and p, q and Bonferroni values of ≥10−8 (Figure 2B and Supplementary Table S4). In contrast, samples from treated patients showed either fewer significantly altered pathways (ART and ARTa panels in Figure 2B) or lesser extent of dysregulation of the remaining pathways displayed (e.g., the ART panel in Figure 2B). The extent and kind of changes in gene ontology processes in the treatment compliant ARTa group were limited compared to changes seen in untreated patients: of the 8 most up-regulated HAND pathways only endogenous antigen presentation and processing were also up-regulated in ARTa with Z-scores less than 2.5, low significance compared to other pathways (p = 0.018 and 0.026, respectively), and no down-regulated gene sets in these categories were identified (Figure 2B and Supplementary Table S4). Notably, some pathways that were significantly down-regulated in HAND were significantly up-regulated in ARTa including neurotransmitter secretion (Z = 4.26; p = 2×10−5) and synaptic transmission (Z = 2.9; p = 0.0037) (Supplementary Table S4).
Dysregulation of selected genes detected by microarrays was confirmed in adjacent tissues by QPCR. Genes were chosen by previous demonstration of their link to HAND [52]–[56]. Representative gene expression values are shown in Figure 2C and the complete list is provided in Supplementary Table S5. Consistent with microarray data, brain tissues from untreated patients showed significant up-regulation of complement component 3 (C3), macrophage antigen CD68 (CD68), and protein-tyrosine phosphatase receptor-type C PTPRC (also known as CD45R); and down-regulation of neuronal cyclin-dependent kinase 5, regulatory subunit 2 (CDK5R2) (only significant for p39), neuronal marker microtubule-associate protein 2 (MAP2) and the SNARE protein complexin 1 (CPLX1), compared to brains of patients on ART. As also noted in other studies [57], confirmatory QPCR analyses generally yielded higher FC results than parallel microarray analyses (Supplementary Table S5). Four of the changes in gene expression in the brain were also tested at the protein level by immunohistochemistry (Figure 2D). We observed increased expression of CD68, C3c and CD45R proteins in untreated HAND compared to uninfected control tissue, and an amelioration of the protein dysregulation in patients with HAND on ART. MAP2 protein was down-regulated in HAND brains compared with brains from uninfected subjects, and it was partially restored in patients under treatment for HIV-1 infection. Taken together, our findings parallel recent reports of the effects of ART on gene expression in peripheral tissues [24], [27], [29] in that treatment of HIV-1 infection by ART is also accompanied by marked reduction of the virally-induced dysregulation of gene expression in brain tissues. Treatment adherence further reduces the number of genes and biological pathways affected.
Gene expression profiles from treated HAND patients cluster with profiles from HIV-1-negative controls
In the next level of analysis, we conducted unsupervised hierarchical clustering for 2073 dysregulated transcripts implicated in HAND (Figure 2A and Supplementary Table S2) to visualize and group individual gene expression profiles from all 21 subjects in this study (Figure 3A). We used normalized Robust Microarray Average (RMA) values from duplicate samples for each subject for greater statistical power (Methods). Three main clusters of subjects were identified. With the exception of HAND5, untreated HAND patients clustered as one group (cluster 2 in Figure 3A) distinct from the other two clusters. ART-treated HAND patients clustered with uninfected controls in two co-mingled clusters, cluster 1 containing subjects C1, C2, C3 and ART1 and cluster 3 including subjects C4, C5, C6, ART2, ART3, ART4, ART5, ART6, and the remaining untreated HAND5. Compared to cluster 3, cluster 1 was more phylogenetically distant from untreated HAND, but overall clusters 1 and 3, separately or combined, were significantly different from cluster 2. The t-test values computed using RMA values for all transcripts in cluster 1 vs. cluster 2, cluster 3 vs. cluster 2, and cluster 1+cluster 3 vs. cluster 2 were 1×10−221, 7.8×10−25, and 1.96×10−24, respectively. These results demonstrate that for the 2073 transcripts tested, gene expression in brain tissues of patients on ART tends to resemble that of uninfected subjects, indicating that ART is associated with a profound reduction in the extent of dysregulation of HAND-related genes in the brain. These results also suggest that the differences related to HIV-1 disease (and treatment) override potential differences resulting from genetic heterogeneity of individual donors.
Fig. 3. Extent and kind of differences in brain cell gene expression in untreated HAND patients and HAND patients receiving ART. 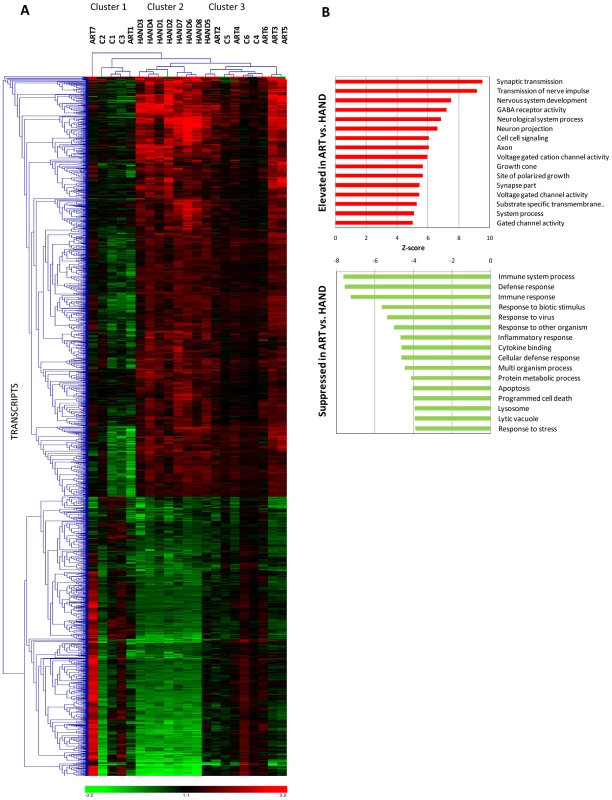
A. Selecting the transcripts differentially expressed in brain tissue from HAND patients relative to uninfected subjects, hierarchical cluster analysis was performed on extent of expression from all subjects using Genesis software. Red represents up-regulation, green represents down-regulation. B. Gene ontology analysis of ART dataset vs. HAND dataset was performed using GAzer software. The upper panel shows pathways elevated in ART and the lower panel shows pathways suppressed in ART relative to HAND brain samples. The statistical similarity of ART transcriptomes with HIV-1-negative controls with respect to presumptive HIV-1-impacted genes (Figure 3A) prompted us to directly compare gene expression changes of ART brain tissue to expression in HAND brain tissue, without filtering microarray results through HIV-1-negative controls. This analysis inquires whether control of HIV-1 replication by ART removed viral perturbations to cellular gene expression in the brain, analogous to longitudinal studies of peripheral tissues from HIV-1-infected subjects pre and post-ART [24] which are not feasible for the brain. Using normalized RMA datasets for over 54,000 transcripts detected by the U133 chipset, we compared 7 treated patients to 8 untreated patients delimiting the results by FC of 1.5 and t-test value of 0.05; the complete list these differentially expressed transcripts is shown in Supplementary Table S6. In calculating the ratio of gene expression in ART to HAND, positive FC values in ART indicate an association of increased gene expression with treatment and negative FC indicate reduced cellular gene expression associated with treatment. Overall, we identified 640 significantly up-regulated and 276 down-regulated genes in this analysis (Supplementary Table S6). Samples from patients with treated HAND show increased expression (relative to untreated HAND) of many genes involved in neuronal functions including synaptoporin (SYNPR, FC 6.55 and p = 6.5×10−4), neurofilament, light polypeptide (NFEL, FC 6.15, p = 4.6×10−4), and synaptotagmin IV (SYT4, FC 4.16, p = 5.8×10−4) compared to samples from untreated patients. Conversely, genes involved in immune activation including CD74 antigen (CD74, FC −2.49, p-0.013), complement component 1, q subcomponent, C chain (C1QC, FC −2.47, p = 0.022), and interferon-induced protein with tetratricopeptide repeats 2 (IFIT2, FC −2.35, p = 0.007) were reduced in expression in treated HAND patient samples relative to samples from untreated patients. For reference, Supplementary Table S6 also lists respective ART microarray data normalized to HIV-1-negative controls. Notably, all but 7 of the genes in treated patients that increased in expression relative to untreated HAND were unchanged in the ART/HIV-negative control comparison (Supplementary Table S6), suggesting that treated patients express these genes at normal (control) levels. Similar normalization of expression (272 out of 276) was found for genes that were down-modulated in ART versus HAND.
To put these findings in the context of the biological pathways potentially affected by ART, we employed GAzer to compare the complete ART microarray datasets to those from HAND. Figure 3B depicts 16 biological processes that were most significantly changed in ART relative to HAND and Supplementary Table S7 provides complete statistics for this analysis. Twelve up-regulated processes in treated versus untreated HAND, with Z-scores ranging from 9.54 to 5.02, were related to neuronal function and repair. The down-modulated pathways included, in decreasing order of significance, immune responses, inflammatory responses, apoptosis, and responses to stress. These results suggest that ART reverses many dysfunctional processes of untreated HAND represented in gene ontology analysis. To provide an alternative view of the extensive up-regulation of cellular processes in ART relative to HAND, up-regulated genes with p-values of ≤0.01 included in Supplementary Table S6 were analyzed by STRING to identify predicted gene interaction networks (Figure 4). In this analysis ART was associated with improved synaptic transmission including synaptic vesicle system, nervous system development including cytoskeleton associated proteins, and GABA neurotransmission networks. These findings strongly suggest that by reducing HIV-1 replication, ART also reduces triggers to aberrant gene expression in the brain.
Fig. 4. Predicted interaction networks of brain cell genes significantly downmodulated in untreated HAND and expressed at close to control levels in HAND patients under ART. 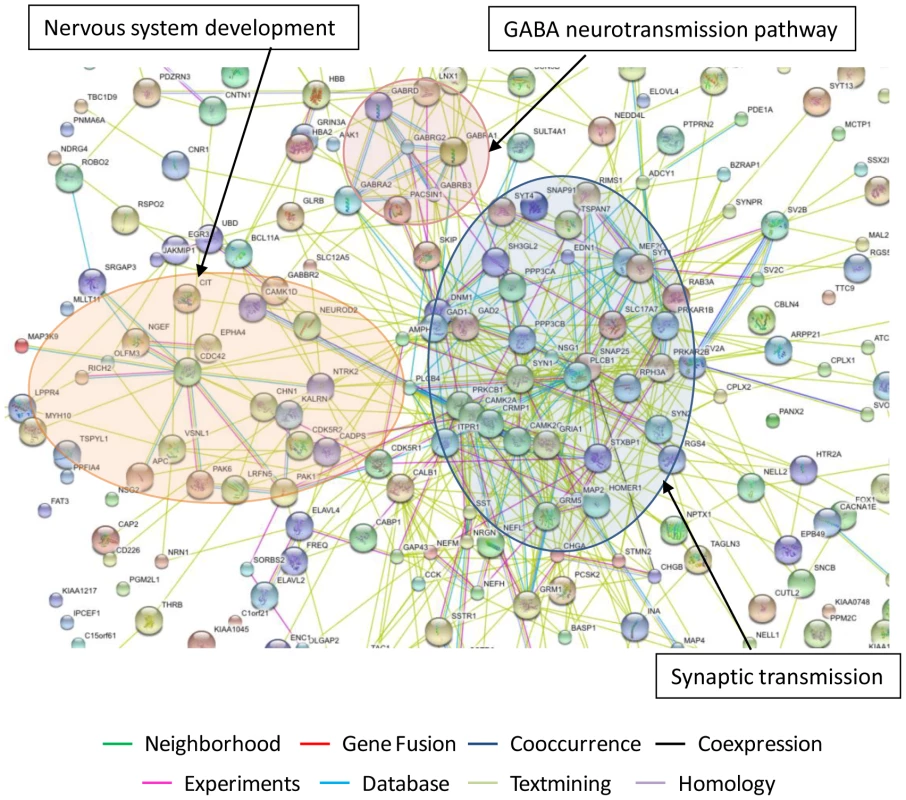
The interactions between genes were identified using STRING software with each type of interaction distinguished by color. The most significantly regulated pathways identified in this analysis are synaptic transmission, nervous system development and GABA neurotransmission pathway. Identification of genes that remain significantly dysregulated in the brain despite treatment
The HAND patients in this study share cognitive dysfunction whether they were untreated or treated with ART (Table 1). To begin to identify transcripts that may contribute to HAND development or persistence despite ART, we used t-test to compare significantly changed genes in untreated (Supplementary Table S2) and treated (Supplementary Table S3) patients with HAND; a gene whose expression was not significantly different in the HAND versus ART comparison (p>0.05) was considered similarly dysregulated in both groups of patients relative to uninfected subjects. We have identified 43 such up-regulated and 42 down-regulated genes; they are listed grouped into biological categories in Supplemental Table S8, selected genes with their expression statistics are listed in Figure 5A, and their heatmap expression profiles in all subjects in this study are shown in Figure 5B. Considering functional characterization, genes related to immune responses were up-regulated in both HAND and ART samples including complement receptor 1 (CR1), chemokine, CXC motif, ligand 2 (CXCL2), major histocompatibility complex, class II HLA-DQB1 and interferon-mediated antiviral responses including interferon-induced protein with tetratricopeptide repeats 1 (IFIT1); interferon-induced protein 44 (IFI44); myxovirus resistance 1 (MX1); 2′,5′-oligoadenylate synthetase 1 (OAS1), and signal transducer and activator of transcription 1 (STAT1). Cell cycle pathway was dysregulated in both treated and untreated HAND patients, with some sets of genes up-regulated and others down-regulated (Figure 5A and 5B). Over-expression of selected transcripts in interferon or chemokine pathways in both HAND and ART brain samples was confirmed by real-time PCR (Figure 5C), over-expression of HLA Class II alleles and proliferating cell nuclear antigen (PCNA) was also demonstrated by immunohistochemistry (Figure 5D). Of particular interest, common down-regulated genes in treated and untreated patients with HAND included myelin-related genes myelin-associated oligodendrocyte basic protein (MOBP), myelin transcription factor 1 (MYT1) and myelin basic protein (MBP). Down-regulation of MOBP and MYT1 was confirmed by real-time PCR, with the MYT1 gene being particularly suppressed in the ART group (FC = −38.38, p = 1×10−5) (Supplementary Table S5). This result is consistent with histopathological detection of myelin pallor in autopsy brain tissues from some patients with HAND [58], [59], although other explanations also exist [60].
Fig. 5. Common dysregulated genes in brain tissues of treated and untreated HAD patients. 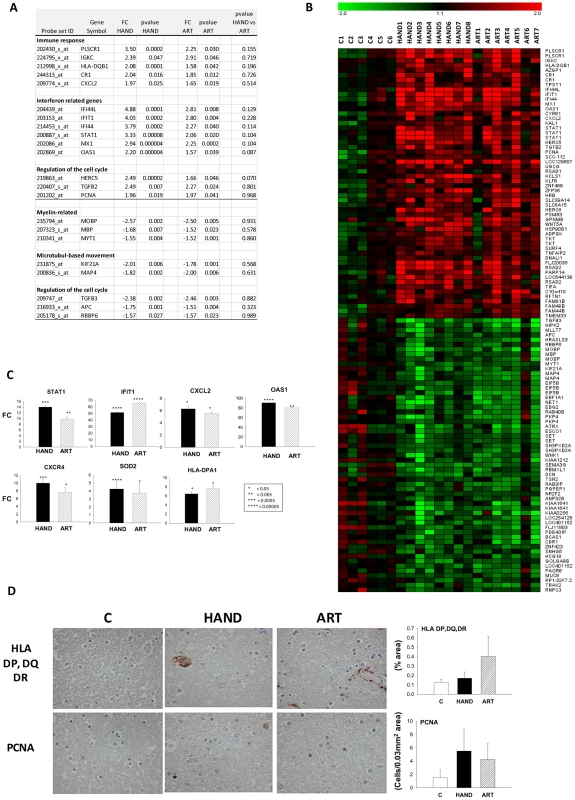
A. Selected genes significantly up or down-regulated in HAND or ART patients compared to uninfected subjects are tabulated (FC: Fold-change, pv: t-test p-value). B. Heatmap representation of significantly dysregulated genes in both HAND and ART datasets relative to uninfected subjects. C. QPCR analysis of expression of selected genes in HAND and ART patients relative to uninfected subjects. D. Immunostaining and quantitation of selected proteins in brain tissue. Correlation of gene expression changes in the brain and HIV-1 load in different compartments
Pearson's formula was applied to determine the correlation of the level of expression of each transcript with HIV-1 load in plasma, cerebrospinal fluid (CSF) and brain. Examples of genes correlating positively with plasma viral load are shown in Figure 6A, including histocompatibility loci HLA-B-G and F, interferon-gamma-inducible protein 30 (IFI30), OAS1, and Cathepsin S (CTSS). Transcripts with a positive (>0.5) or negative (<−0.5) correlation with viral load were analyzed using the gene ontology software Expression Analysis Significance Explorer (EASE) to identify the pathways that correlated most with viral load (Figure 6B). Pathways positively correlated with viral load were similar in the three compartments (brain, CSF, plasma), including several immune activation responses. The main difference among the three compartments was the level of significance of the changes, as represented by the EASE score. All the pathways implicated in immune response correlated better with plasma viral load than with brain viral load. Even though CSF data were available for only 9 of the 15 infected patients, the positive correlations for CSF were higher than those for brain, although less strong than those observed for plasma. The categories of antigen presentation and processing were correlated only with viral load in plasma. Pathways down-regulated in correlation with viral load differed depending on the compartment tested. No biological pathway correlated negatively with brain viral load. Pathways negatively correlated with plasma viral load included synaptic transmission, cell communication, transmission of nerve impulse, organogenesis and neurogenesis. A wide variety of metabolic pathways negatively correlated with CSF viral load. Overall, grouping genes engaged in similar biological functions indicates that gene groups induced in the brain correlated best with virus burden in the periphery and that virus burden in the brain, unlike viral load in plasma or CSF, was uncorrelated to suppression of expression of any gene group.
Fig. 6. Global gene expression changes in HAND brain correlated the most with plasma and less with CSF and brain virus load. 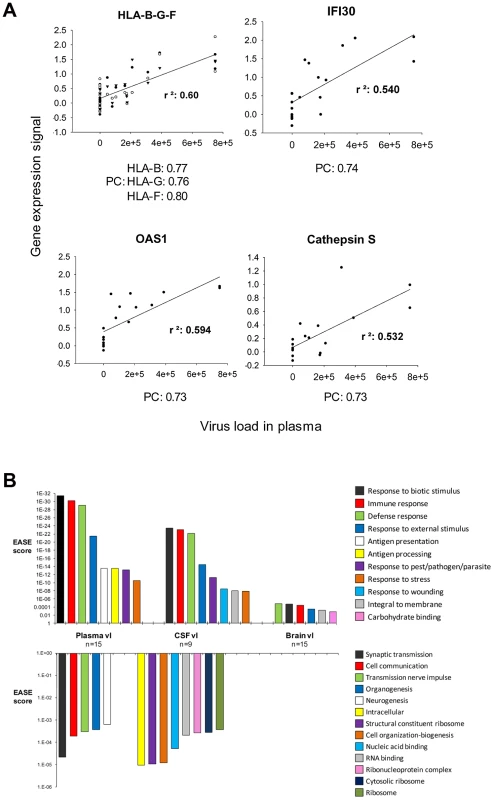
A. Examples of individual genes whose expression positively correlates with plasma virus load (r2: Coefficient of determination, PC: Pearson correlation). B. The upper panel shows positive correlations and the lower panel shows negative correlations with virus load (vl) in plasma, CSF, or brain respectively and gene expression in the brain. Genes that correlated positively and highly with virus load were analyzed using EASE software also negative correlations. The EASE score represent the significance of the regulated pathways. Discussion
We employed functional genomics to investigate the potential effects of antiretroviral treatment on brain pathophysiology in a cohort of patients who died with HAND. Our results suggest that ART profoundly, but not completely, alleviates aberrant gene expression in brain tissues of these patients. These findings may shed light on the molecular basis of HAND persistence despite treatment. Several points should be made about this work.
Microarray profiles of HAND
The foundation of this work is a new comprehensive database of global gene expression profiles in brain tissues of patients with HAND. The profiles described here complement published datasets from previous array studies in HAND [41]–[44] with important differences. For the first time in this disease, we analyzed brain transcriptomes on the basis of the antiretroviral treatment of patients, revealing two distinct, largely non-overlapping groups of aggregate gene expression profiles termed ART and untreated HAND. The ART and untreated HAND profiles also formed separate clusters in unsupervised hierarchical cluster analysis [61] of individual patient datasets, confirming that they are phylogenetically distant from each other based on a large sets of aberrantly expressed genes used in this analysis (Figure 3A). Importantly, the hierarchical clustering distinction between treated and untreated patients was statistically more prominent than potential distinctions in gene expression related to other patient characteristics in our cohort including HIVE and intravenous drug use (Figure 3A and Supplementary Figure S2). Therefore, in most analyses in this work we considered datasets from patients with and without HIVE as one disease category, stratified only on the basis of ART. These results suggest that ART, through effects on HIV-1-associated changes in cellular gene expression [27], [62], [63], is one of the key biological variables governing the extent and pattern of gene dysregulation in molecular profiles of HIV-1 brain disease.
Another important difference with previous HAND array studies concerns the histological regions of the brain tested. We evaluated frontal deep white matter, whereas most of the previous studies focused on the neighboring cortical gray matter [41]–[44] or small gene sets in both brain regions [41]. White matter is the primary site of HIV-1 infection and HIV-1-associated neuropathologies [46], [47] and frontal cortex is one of the sites of synaptic and dendritic damage consequent to this infection [64], [65]. Transcriptomes from these two areas reflect different regional physiologies and therefore different aspects of HIV-1 neuropathogenesis. With these caveats, a meta-analysis summarized in Supplementary Table S9 identified a small number of genes involved in interferon-related responses (IFIT1, IFITM1, IFI44, MX1), synaptic functions (SYN1, SYN2, GABRG2, MAP2), and cell cycle (CDC42, CDK5R1, R2) that were dysregulated in common in the present and previously published HAND datasets. These genes and biological pathways may represent features of HIV-1-associated neuropathogenesis common to white and gray matter.
Brain tissues of untreated patients with HIV-1 dementia show extensive dysregulation of gene expression independent of presence of HIVE
During the last twenty years, individual inflammatory and neurodegenerative mediators in HAND brains were demonstrated by immunocytochemistry, in situ hybridization, and other methods (reviewed in [66]–[69]). The bulk of this work was conducted with brain tissues from untreated patients, as brain autopsies after introduction of ART have become less frequent [70]. Consistent with these observations, the untreated HAND profile defined here reveals a broad and extensive dysregulation of cellular gene expression in brain tissues, with 1470 HAND-associated aberrantly expressed genes, up-regulation of immune activation, antiviral responses, and inflammation, and down-modulation of neuronal functions, neuronal repair, and cell cycle. It should be noted that our untreated HAND profile included transcriptomes from patients with and without HIVE (Figure 2 and 3). HIVE is a characteristic histopathology associated with high HIV-1 burdens in the brain [71], [72], and previous array studies documented differentially expressed transcripts and biological pathways potentially attributable to the extensive infection in the brain [43]–[45], [73]. We confirmed some of these differences in our untreated group with and without HIVE in gene ontology analysis but it is noteworthy that they differed mainly in the degree of dysregulation and not the biological pathways affected (Figure 1). Thus, at the level of a transcriptome analysis, the altered expression of some transcripts attributed to high HIV-1 burdens in HIVE [43], [44] contributes to but does not change the overall statistical characteristics of the molecular phenotype of HAND. Our results are consistent with reports of limited correlation between clinical manifestations of HAND and HIVE histopathology [58], [74], [75]. Rather, untreated HAND appears to correlate better with presence of inflammatory mediators and diffusely activated macrophages and microglial cells in the brain than with virus burdens in the tissue per se [17], [58], [75]–[78].
Independent microarray studies in other patient populations are needed to determine whether these gene expression profiles are fully representative of untreated HAND. In general, transcriptome profiles of disease can serve as a platform for verification of results obtained in studies of individual physiological processes [33], [79], [80] and as a tool for discovery of new ones. In this context, a number of “novel” (i.e., relatively new to the HAND literature) dysregulated genes in our untreated HAND database may shed light on the process of HAND pathogenesis and merit further investigation. For example, transcripts encoding apolipoprotein C-I and C-II (APOC-I and APOC-II) were among the most up-regulated in the untreated HAND dataset, with FC of 5.71 (p = 9.7×10−5) and 3.35 (p = 0.009), respectively (Supplementary Table S2). Dysregulation of lipid metabolism linked to APOE polymorphism is a marker of AD [81] and was indicated in HIV-1 dementia [82], [83]. While we could not find reports on APOC-I in HAND, this lipoprotein was found in association with beta-amyloid plaques in AD brains and expression of human APOC-I allele in native APOC-I null mice was shown to impair learning and memory [84]. Conversely, hemoglobin α-2 and hemoglobin β (HBA-2 and HBB) were among the most down-regulated transcripts in our untreated HAND dataset (FC of −5.31 and −4.22; Supplementary Tables S2 and S5). Neuronal hemoglobins are members of the globin superfamily which are predominantly expressed in neurons and may play an important role in neuroprotection [85]. Consistent with our findings, expression of neuronal hemoglobin is reduced or absent in disease affected brain regions in patients with several neurodegenerative conditions including AD and Parkinson's disease [86].
ART efficiently mitigates aberrant gene expression in brain tissues of patients with HAND
The major finding of this work is the profound difference between the extensive global gene dysregulation observed in brain tissues of untreated patients with HAND and muted gene changes in their treated counterparts. The magnitude of ART effects in the brain suggested by our results was surprising given the limited clinical outcomes of ART on HAND [7], [87], including in the cohort evaluated here (Table 1), and variable findings in the CSF of treated patients [12]–[16]. The ART effects on the brain were inferred because we could not test brain RNA in the same individuals before and after initiation of therapy, as is possible in microarray studies with peripheral tissues [24], [27], [29], [31]. However, the overall effects of ART on altered gene expression were remarkably similar in the periphery and brain. This was evident in markedly fewer dysregulated genes in treated compared to untreated patients, in our case 253 versus 1470; and in a global shift in gene expression patterns from aberrant in the absence of treatment to muted dysregulation under treatment, for example in peripheral CD4+ T cells [27], [29], lymphoid tissue [26], and brain here (Figure 3A). Importantly, both in the CD4+ T lymphocyte study [27] and in the present work, gene expression profiles of treated patients were statistically similar to those of HIV-1-negative controls, suggesting a trend toward normalization of gene expression under ART. We confirmed this trend for selected gene products in the present work by real-time PCR and immunocytochemistry (Figure 2). These results suggest that ART regimens, which generally include at least one brain penetrant antiviral compound (Table 1), are similarly effective in mitigating global molecular changes in the brain and in peripheral tissues.
We noted two reciprocal effects of ART on gene dysregulation in brain tissues illuminating the systemic and brain-specific aspects of HAND pathogenesis. One is a significant and broad moderation of up-regulated genes linked to HIV-1 induced antiviral and inflammatory responses thought to drive HAND pathogenesis, including interferon-related ISG15 and IFIT3, macrophage markers CD68, CD163, and CD14, and chemokines and chemokine receptors CCL8, CCR1, and CXCR4 [67]–[69] (Figure 2 and 3). Interestingly, this effect of ART was common to diverse tissues examined by microarrays including brain (this study) and CD4+ T cells, macrophages, lymph nodes, and intestinal mucosa tested by others [24], [27], [29], [31], and thus it likely represents a system-wide response to suppression of HIV-1 replication. Although gene ontology pathways containing these genes in patients under ART were still up-regulated compared to controls (Figure 2B and Figure 5), our results suggest that ART can alleviate a surprisingly large number of deleterious responses in the brain that have been linked previously to HIV-1 infection in model systems [88]. This causal link is further strengthened by an apparent correlation in this work between treatment compliance and extent of gene dysregulation in the brain (Figure 2).
The other effect of ART in the brain we observed was specific to the nervous system and it involved normalization of a large number of down-regulated genes and biological pathways linked to nervous system functions and by extension to neurocognitive disease. For example, the bioinformatics tool STRING [89] identified nervous system development, synaptic transmission, and GABA-neurotransmission pathway as the three major predicted interaction networks of genes that approached normal expression in treated patients (Figure 4). On a smaller scale, our confirmatory tests showed that products such as MAP2 and complexin-1 (CPLX1) were significantly down-regulated in untreated patients at RNA and protein levels and they were expressed at control-like levels in tissues from treated patients (Figure 2). It is conceivable that restoration of normal expression of at least some of these genes under ART would restore some aspects of normal brain physiology [7]. Although treated patients in our cohort still manifested HAND prior to death, our results suggest that they may have already shifted to a milder molecular profile in the brain that had more in common with HIV-1-negative controls than untreated patients.
Of interest, the global changes in brain cell gene expression seen by microarrays correlated positively with plasma but not brain virus burdens (Figure 6). This association may be analogous to the clustering of brain transcriptomes independently of encephalitis and brain HIV-1 burden by commonly dysregulated biological pathways. In any case, the single measurement of brain virus burden at autopsy may not capture the chronic insult to brain function suffered by patients living years with HIV-1 infection, albeit with some control exerted by ART.
Genes dysregulated in common under ART and in untreated HAND
Perhaps the most intriguing contribution of the present array analysis lies in the ability to discern patterns of abnormality that persist in cognitively-impaired patients who are on central nervous system penetrant ART, and to distinguish these patterns from HAND in the untreated state. In the bioinformatics sense, we used ART as a biological filter to reduce the overall gene expression disturbance in brain transcriptomes of patients with HAND and through that determine whether continuing dysregulation of gene expression could play a role in continuing brain disease. The results indicate that continuing up-regulation of innate and adaptive immune responses are an important part of brain abnormalities in ART-treated dementia. Over-expression of Class II MHC in the brain persists despite therapy in our study; it has been associated with many neurodegenerative diseases [90]. Defects in myelin metabolism, shown for HAND at the gene expression level here and indicated previously by neuropathological observation of myelin pallor in brains of patients with HAD [58], are also common to many other neurodegenerative diseases [38], [91]. Activation of interferon-related genes often found in symptomatic HIV-1 infection may underlie abnormalities in cell function [28]. Such changes, coupled with cell cycle perturbations, may support emerging magnetic resonance spectroscopy studies that have demonstrated persistent white matter inflammation in patients with HIV-1-related cognitive impairment [92]. However, it is unclear what particular aspects of immune activation or response are relevant to nervous system dysfunction, and how to distinguish deleterious gene products from those that may function in a neuroprotective manner. In simian immunodeficiency virus infection, innate immunity, IL-6, and interferon responses are important elements in brain viral control [56], [93], but in a recent study expression of interferon-α in the brain was conclusively linked to neuronal dysfunction in a mouse model of HIVE [94]. Careful analysis of individual genes identified here may begin to clarify the mechanism of HAND persistence under treatment.
Materials and Methods
Ethics statement
Human brain samples and clinical data were obtained from the Manhattan HIV Brain Bank (MHBB), a member of the National NeuroAIDS Tissue Consortium, under an Institutional Review Board-approved protocol at the Mount Sinai School of Medicine. Written informed consent was obtained from all subjects in this study or their primary next-of-kin. HIV-1 and gene expression analyses were conducted on de-identified brain samples under an “exempt” status approved by an Institutional Review Board of St. Luke's-Roosevelt Hospital Center.
Study subjects and brain regions used for analysis
Study subject information is listed in Table 1. Fifteen HIV-1-positive subjects used in this study were chosen on the basis of having HAND and the presence or absence of HIVE as determined by neuromedical or neuropsychological evaluation and postmortem neuropathology [95], and then were further categorized as either dying on or off ART. Fourteen of HIV-1-positive patients in this study died with HAD; one patient designated ART2 in Table 1 was classified as MND based on his lack of emotive/behavioral criteria [96]. Patients who displayed HAND without HIVE histopathology at autopsy met American Academy of Neurology criteria for HAND regardless of ART status. This definition requires demonstration of cognitive and functional impairments, and the presence of emotive or motoric phenomena [96]. Except for patient ART2, patients with HIVE were similarly impaired, regardless of ART status, or had histories of HAND on medical record review. Patients felt to have cognitive impairments) due to non-HIV-1 causes (neuropsychological impairment – other, as described in [95] were excluded. All patients dying on ART had substantive treatment histories, ranging 1 to 7 years prior to demise. The ART regimens at death had a mean duration of 16 months (range, 3 months to 3 years). For five of seven patients on ART, treatment compliance estimates were made by self-reported 4 day recall [97]. HIV-1-negative subjects were chosen on the basis of normal neurological function (as determined by chart review) and normal neurohistology. All neurohistologic diagnoses were rendered by a board-certified neuropathologist (SM) and a minimum of 50 sections were examined for each brain. The following brain pathology definitions were used in the present work (Table 1): Normal: no brain pathology; HIVE: HIV encephalitis; HIVE*: HIVE was limited to the basal ganglia (pid 30015); Minimal: minimal histopathological changes including trivial microscopic abnormalities such as an isolated vermal scar (pid 10119), a venous ectasia (pid 10063), atherosclerosis and minimal perivascular inflammation sub-threshold for diagnosis (pid 10001), and minimal perivascular inflammation sub-threshold for diagnosis (pid 10015). At the time of autopsy, coronal sections of brain were snap-frozen and maintained in −85°C until sub-dissection. Effort was made to keep the post mortem interval (PMI) to a minimum; the PMI for subjects in this study are listed in Table 1. Brain samples for this analysis were obtained from the centrum semiovale (deep white matter) at the coronal level of the genu of the corpus callosum. Multiple samples from the same region were dissected for gene expression profiling, real-time PCR (QPCR), and protein assays. Equivalent regions from the contralateral hemisphere were formalin fixed and utilized for immunohistochemistry.
DNA and RNA isolation from human brain and reverse transcription
Total DNA and RNA were isolated from human brain tissue by, respectively, DNeasy Blood and Tissue Kit and RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. RNA was quantified by spectrophotometry and RNA quality was verified by spectrophotometry and agarose gel electrophoresis. RNA was then treated with DNAse I (Fisher Healthcare, Houston, TX). cDNA was synthesized using the Superscript First-Strand Kit (Invitrogen, Carlsbad, CA) for quantitative analysis and the WT-Ovation™ RNA Amplification System (NuGEN Technologies, Inc., San Carlos, CA) for relative analysis according to the manufacturer's protocol.
Virus load in the brain
Viral RNA and DNA burdens in human brain were determined by quantitative real-time PCR using primers designed based on HIV-1 consensus sequences for the group of patients in this study. We first screened patient brain samples for the presence of HIV-1 content by a standard nested PCR for viral DNA or RNA (cDNA) as previously described [98]. To achieve broad detection of diverse Clade B HIV-1 species, first-round PCR was performed using custom designed primers for a conserved Clade B gag consensus sequences: SQ5 (+) 5′-CAA ATG GTA CAT CAG GCC ATA TCA CC-3′ and SQ3′ (−) 5′-CCC TGA CAT GCT GTC ATC ATT TCT TC-3′. For nested PCR step we used primers SQ5′ (above) and SK39 [99]; the PCR products were resolved on an agarose gel and detected by Southern Blot hybridization with (32P)-labeled probe SK19 [99] (Supplementary Figure S1). The HIV-1 gag nested PCR amplicons from individual HIV-1 DNA positive patients were sequenced and used to design patient consensus primers (5′ (+) QSQ5 5′-ACC CAT GTT T(T/A)C AGC ATT ATC AGA-3′ and 3′ (−) QSQ3 5′-GAT GTC CCC CCA CTG TGT TT-3′) and Taqman probe (HSQP 6FAM-AGC CAC CCC ACA AGA-MGBNFQ). For real-time PCR amplification, 2 µl of brain tissue DNA or 5 µl of cDNA were combined with 2× Universal Master Mix (Applied Biosystems, Carlsbad, CA), 900 nM consensus primer (custom synthesized by Invitrogen) and 200 nM probe (synthesized by Applied Biosystems; QPCR conditions were essentially as described [100]. Standard curve for viral DNA and cDNA quantification was constructed from graded amounts of HIV-1 NL4-3 plasmid DNA. Viral DNA burdens were normalized to total cellular DNA content by β-globin amplification and expressed as HIV-1 DNA copies/number of cells calculated from a β-globin DNA standard curve, with 2 copies of β-globin gene equaling one cell. Viral RNA burdens were normalized by tissue glyceraldehydes-3-phosphate dehydrogenase (GAPDH) content and expressed as number of viral copies in 1 µg tissue RNA [100].
Microarray hybridization
Microarray experiments were conducted at the Bionomics Research and Technology Center in EOSHI University of Medicine and Dentistry of New Jersey. Total RNA were extracted from tissue samples using the RNeasy Mini Kit (Qiagen) followed by DNase I treatment. RNA qualities were assessed by electrophoresis using the Agilent Bioanalyzer 2100 and spectrophotometric analysis prior to cDNA synthesis. Fifty nanograms of total RNA from each sample were used to generate a high fidelity cDNA for array hybridization using NuGen WT-Ovation Pico RNA Amplification. Detailed protocols for sample preparation can be found at http://www.nugeninc.com. After fragmentation and biotin labeling using NuGen Encore Biotin Module, the samples were hybridized to Affymetrix Human Genome 133 plus 2.0 arrays. Washing and staining of all arrays were carried out in the Affymetrix fluidics module as per the manufacturer's protocol. The detection and quantitation of target hybridization was performed with an Affymetrix GeneChip Scanner. Data were assessed for array performance prior to analysis. The majority of patient samples were analyzed in duplicates starting from the cDNA synthesis step; in some cases second analysis was on an adjoining brain sample.
Microarray data analysis
The .cel data files generated by the Affymetrix microarray hybridization platform were analyzed by the ArrayAssist software (Stratagene, Santa Clara, CA). Probe level analysis was performed using the RMA algorithm. After verification of data quality by Affymetrix internal controls and signal distribution analysis as described in Stratagene ArrayAssist Protocol, data was transformed using variance stabilization and logarithm transformation with a base of 2. Fluorescence values were normalized by mean intensities of all chip samples. Means of normalized expression values were calculated for duplicate samples from each individual, and these values were either used directly in some analytical programs (see below) or employed to calculate fold change (FC) in the transcript compared to HIV-1-negative controls. Genes showing FC values above 1.5 or below −1.5 and unpaired t-test p-values of <0.05 were defined as significantly changed. In some analyses we applied a t-test cutoff of p<0.01. To test for effect of antiretroviral treatment, we calculated the FC for significantly modulated transcripts separately from untreated and treated patients, the latter subdivided into all-treated and a subset without two known low-compliant patients, versus uninfected controls. We also compared directly normalized expression values of selected transcripts from treated and untreated patients to generate the treated versus untreated FC values that were not filtered through HIV-1-negative controls.
To compare brain samples of all the individuals included in the study we clustered the expression profiles using unsupervised Hierarchical Clustering (Average Linkage Clustering) and heatmap visualization software from Genesis [101] available at http://genome.tugraz.at/. This type of analysis allows us to identify the similarities and differences in the expression patterns of groups of patients and/or transcripts. For broad characterization of gene expression changes in untreated and treated patients we conducted gene set and gene ontology analysis using GAzer [50] (http://expressome.kobic.re.kr/GAzer/index.faces). GAzer is a web-based tool that identifies, by a parametric statistical analysis of complete primary normalized microarray data, over-represented sets of genes (functionally related genes) rather than individual genes. This type of analysis compensates for the fact that small changes not seen at the gene level are often detected when the gene set as a whole is examined [50], [51]. When analysis was limited to smaller sets of genes defined as differentially expressed, we performed functional categorization of gene families by EASE [102], available at the NIH web site (http://david.abcc.ncifcrf.gov/). The predicted biological pathway and network relationships among differentially expressed genes in our array datasets were identified using the Search Tool for the Retrieval if Interacting Genes/Proteins (STRING) (http://string-db.org/) [89].
Gene expression validation by QPCR
Changes in expression of selected genes identified by microarrays analysis were validated in the same or adjoining brain samples by QPCR using Taqman chemistry and probes from the Universal Probe Library (Roche, Indianapolis, IN). Primers were designed using the online ProbeFinder software available at the Roche Universal Probe Library Assay Design Center (http://www.roche-applied-science.com). The QPCR reactions contained 2 µl of cDNA generated from tissue RNA obtained as described above, 10 µl of 2× Universal Master Mix (Applied Biosystems-ABI), 0.2 µl of each forward and reverse primers at 200 nM, 0.2 µl of probe at 100 nM, and RNAse/DNAase-free water. All reactions were performed in duplicate and were run in a 7500 real-time PCR system (ABI). Raw data was analyzed using the 7500 System SDS Software (ABI). Data was normalized using 2 housekeeping genes, GAPDH and ribosomal protein S18 (RPS18) to assure reproducibility. Relative quantification employed the comparative threshold cycle method (Applied Biosystems Technical Bulletin n°2).
Immunohistochemistry
For immunohistochemical analysis, formalin fixed blocks were taken from the frontal white matter of the autopsy brains of 4 normal, 6 HIV-1-non-treated, and 4 HIV-1-ART-treated individuals. A microarray block consisting of 3 tissue punches diameter of 1 mm) from each block was constructed. 5 µM serial sections were cut and immunohistochemistry performed with an array of antibodies listed in the Table 2.
Tab. 2. Antibodies used for immunohistochemistry. 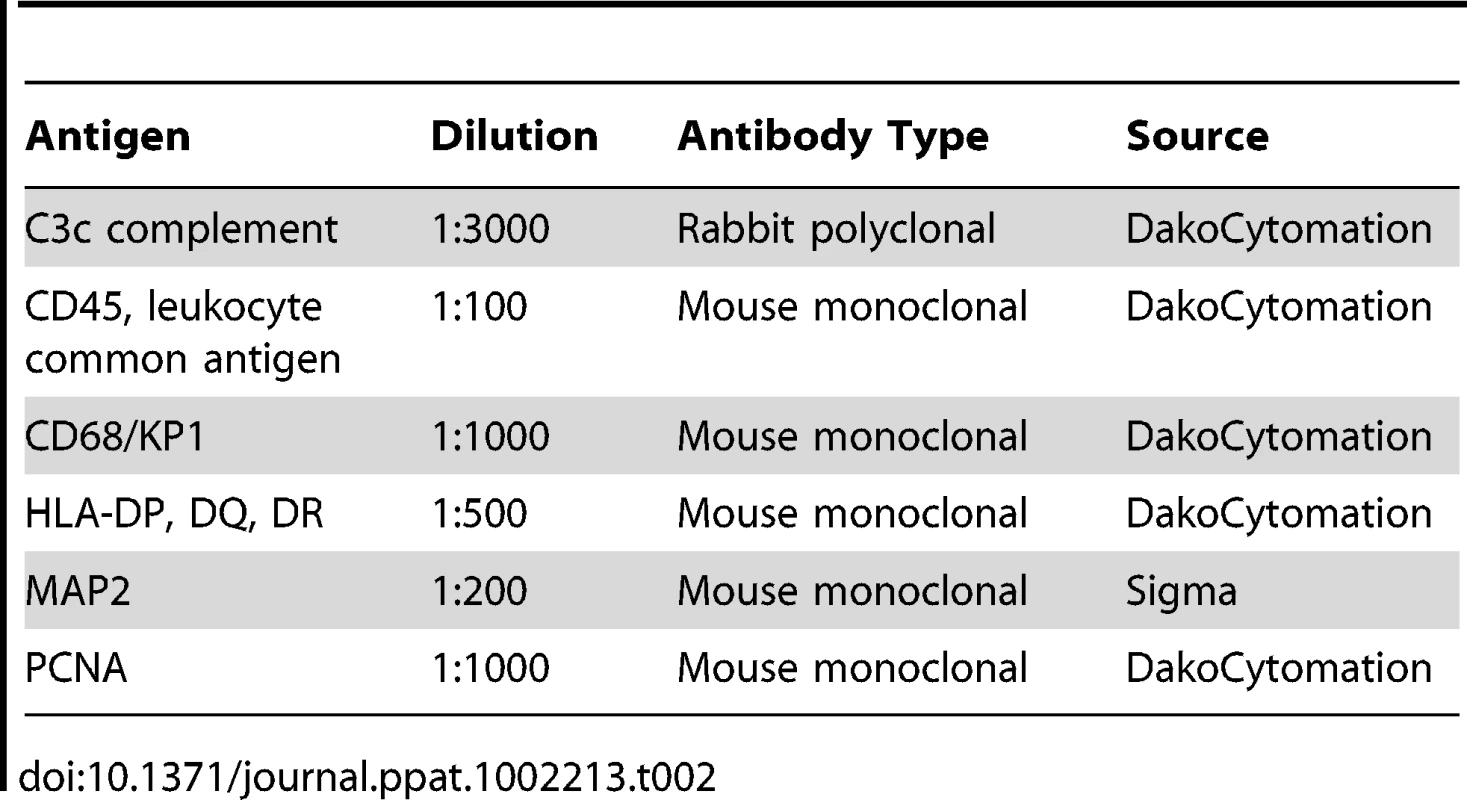
Formalin-fixed, paraffin-embedded sections were deparaffinized with xylenes, hydrated in graded alcohols, and incubated with 3% H2O2 in methanol. Following washing, sections were boiled in Target retrieval solution (DAKO Corp., Carpinteria, CA), subsequently incubated in a serum free protein blocking solution and then incubated with the primary antibodies indicated in Table 2. CD45 (PTPRC) was incubated overnight at 4°C and the all the remaining antibodies for 1 h at room temperature. Primary antibodies were detected with peroxidase anti rabbit or mouse IgG ImmPRESS (DAKO Corp.) reagent and counterstained with hematoxylin. Slides were visualized in a light microscope and either one, three or six 0.03 mm2 areas of white matter staining from each case were photographed using a 40× objective and a Nikon Coolpix II digital camera attached to the microscope by a Coolpix MDC lens. The intensity of illumination and position of sub-stage condenser on the microscope were constant for all images. The number of areas taken depended on the type of quantitative analysis that followed. The percentage area occupied by cells immunoreactive for CD68, CD45 and HLA DP, DQ, DR was quantitated by analysis of six 0.03 mm2 images using a proprietary automated morphometric analysis software [103]. The mean intensity of MAP2 and STAT1 staining was analyzed on one 0.03 mm2 image, utilizing Image J software (http://rsbweb.nih.gov/ij/). Each image was converted to 8-bit grayscale and a mean gray value of the pixels in the full photographic image was measured after a background subtraction and inversion were performed. For C3c and PCNA, cell counts were done by eye using either three 0.03 mm2 (C3c) or the full punch (PCNA). Statistical analysis was performed using StatView (V.5.0.1) (Adept Scientific, Bethesda, MD). The principal statistical test used was the Analysis of Variance for single comparisons (ANOVA). Follow-up post-hoc tests were conducted when required. Significance values were set at 0.05 and below.
Correlation analysis
Correlation analysis between virus loads in plasma, CSF, or brain and gene expression in the brain was performed using the Pearson's correlation formula in Microsoft Excel software (Microsoft Corporation, Redmond, WA). Transcripts showing positive (>0.5) or negative correlation (<−0.5) were categorized into biological functional pathways using EASE software.
Microarray data repository
The microarray results presented here are available in the Gene Expression Omnibus database (www.ncbi.nlm.nih.gov/geo) in a MIAME compliant format under accession number GSE28160.
Gene accession numbers
The Gene ID numbers for the genes mentioned in the text are listed below. The ID number corresponds to the ‘National Center for Biotechnology Information database’ (http://www.ncbi.nlm.nih.gov/gene): CD4 (920), C3 (718), CD68 (968), PTPRC (5788), CDK5R2 (8941), MAP2 (4133), CPLX1 (10815), SYNPR (132204), NFEL (4747), SYT4 (6860), CD74 (972), C1QC (714), IFIT2 (3433), CR1 (1378), CXCL2 (2920), HLA-DQB1 (3119), IFIT1 (3434), IFI44 (10561), MX1 (4599), OAS1 (4938), STAT1 (6772), PCNA (5111), MOBP (4336), MYT1 (4661), MBD (4155), IFI30 (10437), CTSS (1520), IFITM1 (8519), B2M (567), CD14 (929), APOC-I (341), APOC-II (344), HBA-2 (3040), HBB (3043), IFI16 (3428), TLR7 (51284), SYN1 (6853), SYN2 (6854), GABRG2 (2566), CDC42 (998), CDK5R1 (8851), GAPDH (2597), RPS18 (6222).
Supporting Information
Zdroje
1. AntinoriAArendtGBeckerJTBrewBJByrdDA 2007 Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69 1789 1799
2. ChangLErnstTLeonido-YeeMWittMSpeckO 1999 Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology 53 782 789
3. RobertsonKRRobertsonWTFordSWatsonDFiscusS 2004 Highly active antiretroviral therapy improves neurocognitive functioning. J Acquir Immune Defic Syndr 36 562 566
4. RobertsonKRSmurzynskiMParsonsTDWuKBoschRJ 2007 The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 21 1915 1921
5. SacktorNMcDermottMPMarderKSchifittoGSelnesOA 2002 HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol 8 136 142
6. TozziVBalestraPBellagambaRCorpolongoASalvatoriMF 2007 Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 45 174 182
7. EllisRLangfordDMasliahE 2007 HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 8 33 44
8. HarezlakJBuchthalSTaylorMSchifittoGZhongJ 2011 Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 25 625 633
9. SimioniSCavassiniMAnnoniJMRimbault AbrahamABourquinI 2010 Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 24 1243 1250
10. HeatonRKCliffordDBFranklinDRJrWoodsSPAkeC 2010 HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75 2087 2096
11. McArthurJC 2004 HIV dementia: an evolving disease. J Neuroimmunol 157 3 10
12. ChangLErnstTSt HillaireCConantK 2004 Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther 9 431 440
13. CysiqueLBrewBJHalmanMCatalanJSacktorN 2005 Undetectable cerebrospinal fluid HIV RNA and beta-2 microglobulin do not indicate inactive AIDS dementia complex in highly active antiretroviral therapy-treated patients. J Acquir Immune Defic Syndr 39 426 429
14. EdénAPriceRWSpudichSFuchsDHagbergL 2007 Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis 196 1779 1783
15. McArthurJCMcDermottMPMcClernonDSt HillaireCConantK 2004 Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch Neurol 61 1687 1696
16. NeuenburgJKFurlanSBacchettiPPriceRWGrantRM 2005 Enrichment of activated monocytes in cerebrospinal fluid during antiretroviral therapy. AIDS 19 1351 1359
17. AnthonyICRamageSNCarnieFWSimmondsPBellJE 2005 Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol 64 529 536
18. BildAHYaoGChangJTWangQPottiA 2006 Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 439 353 357
19. GolubTRSlonimDKTamayoPHuardCGaasenbeekM 1999 Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 286 531 537
20. KöbelMKallogerSEBoydNMcKinneySMehlE 2008 Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med 5 e232
21. ParmigianiGGarrett-MayerESAnbazhaganRGabrielsonE 2004 A cross-study comparison of gene expression studies for the molecular classification of lung cancer. Clin Cancer Res 10 2922 2927
22. RhodesDRYuJShankerKDeshpandeNVaramballyR 2004 Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci USA 101 9309 9314
23. StratfordJKBentremDJAndersonJMFanCVolmarKA 2010 A six-gene signature predicts survival of patients with localized pancreatic ductal adenocarcinoma. PLoS Med 7 e1000307
24. LiQSchackerTCarlisJBeilmanGNguyenP 2004 Functional genomic analysis of the response of HIV-1-infected lymphatic tissue to antiretroviral therapy. J Infect Dis 189 572 582
25. LiQSmithAJSchackerTWCarlisJVDuanL 2009 Microarray analysis of lymphatic tissue reveals stage-specific, gene expression signatures in HIV-1 infection. J Immunol 183 1975 1982
26. SmithAJLiQWietgrefeSWSchackerTWReillyCS 2010 Host genes associated with HIV-1 replication in lymphatic tissue. J Immunol 185 5417 5424
27. RotgerMDangKKFellayJHeinzenELFengS 2010 Genome-wide mRNA expression correlates of viral control in CD4+ T-cells from HIV-1-infected individuals. PLoS Pathog 6 e1000781
28. WuJQDwyerDEDyerWBYangYHWangB 2008 Transcriptional profiles in CD8+ T cells from HIV+ progressors on HAART are characterized by coordinated up-regulation of oxidative phosphorylation enzymes and interferon responses. Virology 380 124 135
29. Van den BerghRFlorenceEVliegheEBoonefaesTGrootenJ 2010 Transcriptome analysis of monocyte-HIV interactions. Retrovirology 7 53
30. RichardYAmielCJeantilsVMestivierDPortierA 2010 Changes in blood B cell phenotypes and Epstein-Barr virus load in chronically human immunodeficiency virus-infected patients before and after antiretroviral therapy. J Infect Dis 202 1424 1434
31. GuadalupeMSankaranSGeorgeMDReayEVerhoevenD 2006 Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol 80 8236 8247
32. SunBAbadjianLRempelHCalosingCRothlindJ 2010 Peripheral biomarkers do not correlate with cognitive impairment in highly active antiretroviral therapy-treated subjects with human immunodeficiency virus type 1 infection. J Neurovirol 16 115 124
33. GlanzerJGHaydonPGEberwineJH 2004 Expression profile analysis of neurodegenerative disease: advances in specificity and resolution. Neurochem Res 29 1161 1168
34. BlalockEMGeddesJWChenKCPorterNMMarkesberyWR 2004 Incipient Alzheimer's disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci USA 101 2173 2178
35. LukiwWJ 2004 Gene expression profiling in fetal, aged, and Alzheimer hippocampus: a continuum of stress-related signaling. Neurochem Res 29 1287 1297
36. MillerRMFederoffHJ 2006 Microarrays in Parkinson's disease: a systematic approach. NeuroRx 3 319 326
37. SutherlandGTMatigianNAChalkAMAndersonMJSilburnPA 2009 A cross-study transcriptional analysis of Parkinson's disease. PLoS One 4 e4955
38. HakakYWalkerJRLiCWongWHDavisKL 2001 Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA 98 4746 4751
39. LockCHermansGPedottiRBrendolanASchadtE 2002 Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 8 500 508
40. Gebicke-HaerterPJ 2005 Microarrays and expression profiling in microglia research and in inflammatory brain disorders. J Neurosci Res 81 327 341
41. GelmanBBSoukupVMSchuenkeKWKeherlyMJHolzerC3rd 2004 Acquired neuronal channelopathies in HIV-associated dementia. J Neuroimmunol 157 111 119
42. ShapshakPDuncanRTorres-MuñozJEDuranEMMinagarA 2004 Analytic approaches to differential gene expression in AIDS versus control brains. Front Biosci 9 2935 2946
43. MasliahERobertsESLangfordDEverallICrewsL 2004 Patterns of gene dysregulation in the frontal cortex of patients with HIV encephalitis. J Neuroimmunol 157 163 175
44. EverallISalariaSRobertsECorbeilJSasikR 2005 Methamphetamine stimulates interferon inducible genes in HIV infected brain. J Neuroimmunol 170 158 171
45. TatroETScottERNguyenTBSalariaSBanerjeeS 2010 Evidence for Alteration of Gene Regulatory Networks through MicroRNAs of the HIV-infected brain: novel analysis of retrospective cases. PLoS One 5 e10337
46. Lopez-VillegasDLenkinskiREFrankI 1997 Biochemical changes in the frontal lobe of HIV-infected individuals detected by magnetic resonance spectroscopy. Proc Natl Acad Sci USA 94 9854 9859
47. SailasutaNShrinerKRossB 2009 Evidence of reduced glutamate in the frontal lobe of HIV-seropositive patients. NMR Biomed 22 326 331
48. LangfordDMarquie-BeckJde AlmeidaSLazzarettoDLetendreS 2006 Relationship of antiretroviral treatment to postmortem brain tissue viral load in human immunodeficiency virus-infected patients. J Neurovirol 12 100 107
49. WangZTrillo-PazosGKimSYCankiMMorgelloS 2004 Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J Neurovirol 10 Suppl 1 25 32
50. KimS-BYangSKimS-KKimSCWooHG 2007 GAzer: gene set analyzer. Bioinformatics 23 1697 1699
51. KimSYVolskyDJ 2005 PAGE: parametric analysis of gene set enrichment. BMC Bioinformatics 6 144
52. Fischer-SmithTCroulSSverstiukAECapiniCL'HeureuxD 2001 CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 7 528 541
53. McManusCMWeidenheimKWoodmanSENunezJHesselgesserJ 2000 Chemokine and chemokine-receptor expression in human glial elements: induction by the HIV protein, Tat, and chemokine autoregulation. Am J Pathol 156 1441 1453
54. MooreDJMasliahERippethJDGonzalezRCareyCL 2006 Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 20 879 887
55. YangBSinghSBressaniRKanmogneGD 2010 Cross-talk between STAT1 and PI3K/AKT signaling in HIV-1-induced blood-brain barrier dysfunction: Role of CCR5 and implications for viral neuropathogenesis. J Neurosci Res 88 3090 3101
56. RobertsESBurudiEMEFlynnCMaddenLJRoinickKL 2004 Acute SIV infection of the brain leads to upregulation of IL6 and interferon-regulated genes: expression patterns throughout disease progression and impact on neuroAIDS. J Neuroimmunol 157 81 92
57. LoganJMJEdwardsKJ 2009 An overview of PCR platforms. LoganJEdwardsKSaundersN Real-Time PCR: Current Technology and Applications Portland, OR Caister Academic Press
58. GlassJDWesselinghSLSelnesOAMcArthurJC 1993 Clinical-neuropathologic correlation in HIV-associated dementia. Neurology 43 2230 2237
59. WohlschlaegerJWengerEMehraeinPWeisS 2009 White matter changes in HIV-1 infected brains: a combined gross anatomical and ultrastructural morphometric investigation of the corpus callosum. Clin Neurol Neurosurg 111 422 429
60. PowerCKongPACrawfordTOWesselinghSGlassJD 1993 Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier. Ann Neurol 34 339 350
61. KapetanovicIMRosenfeldSIzmirlianG 2004 Overview of commonly used bioinformatics methods and their applications. Ann NY Acad Sci 1020 10 21
62. ChunTWJustementJSLempickiRAYangJDennisGJr 2003 Gene expression and viral prodution in latently infected, resting CD4+ T cells in viremic versus aviremic HIV-infected individuals. Proc Natl Acad Sci USA 100 1908 1913
63. van't WoutABLehrmanGKMikheevaSAO'KeeffeGCKatzeMG 2003 Cellular gene expression upon human immunodeficiency virus type 1 infection of CD4+-T-cell lines. J Virol 77 1392 1402
64. BudkaH 1991 Neuropathology of human immunodeficiency virus infection. Brain Pathol 1 163 175
65. EverallILuthertPLantosP 1993 A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J Neuropathol Exp Neurol 52 561 566
66. KolsonDLPomerantzRJ 1996 AIDS dementia and HIV-1-induced neurotoxicity: possible pathogenic associations and mechanisms. J Biomed Sci 3 389 414
67. KaulMGardenGALiptonSA 2001 Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410 988 994
68. NathA 2002 Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis 186 Suppl 2 S193 198
69. Kraft-TerrySDBuchSJFoxHSGendelmanHE 2009 A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron 64 133 145
70. ScaravilliFBazilleCGrayF 2007 Neuropathologic contributions to understanding AIDS and the central nervous system. Brain Pathol 17 197 208
71. SharerLRSaitoYEpsteinLGBlumbergBM 1994 Detection of HIV-1 DNA in pediatric AIDS brain tissue by two-step ISPCR. Adv Neuroimmunol 4 283 285
72. NaviaBAChoESPetitoCKPriceRW 1986 The AIDS dementia complex: II. Neuropathology. Ann Neurol 19 525 535
73. RobertsESZandonattiMAWatryDDMaddenLJHenriksenSJ 2003 Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol 162 2041 2057
74. BellJE 1998 The neuropathology of adult HIV infection. Rev Neurol (Paris) 154 816 829
75. GrayFAdle-BiassetteHChretienFLorin de la GrandmaisonGForceG 2001 Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol 20 146 155
76. Kraft-TerrySDStothertARBuchSGendelmanHE 2010 HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol Dis 37 542 548
77. XingHQHayakawaHGelpiEKubotaRBudkaH 2009 Reduced expression of excitatory amino acid transporter 2 and diffuse microglial activation in the cerebral cortex in AIDS cases with or without HIV encephalitis. J Neuropathol Exp Neurol 68 199 209
78. YadavACollmanRG 2009 CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmune Pharmacol 4 430 447
79. D'AgataVCavallaroS 2004 Genomic portraits of the nervous system in health and disease. Neurochem Res 29 1201 1212
80. HoheiselJD 2006 Microarray technology: beyond transcript profiling and genotype analysis. Nat Rev Genet 7 200 210
81. StefaniMLiguriG 2009 Cholesterol in Alzheimer's disease: unresolved questions. Curr Alzheimer Res 6 15 29
82. CutlerRGHaugheyNJTammaraAMcArthurJCNathA 2004 Dysregulation of sphingolipid and sterol metabolism by ApoE4 in HIV dementia. Neurology 63 626 630
83. HaugheyNJBandaruVVBaeMMattsonMP 2010 Roles for dysfunctional sphingolipid metabolism in Alzheimer's disease neuropathogenesis. Biochim Biophys Acta 1801 878 886
84. AbildayevaKBerbeeJFBloklandAJansenPJHoekFJ 2008 Human apolipoprotein C-I expression in mice impairs learning and memory functions. J Lipid Res 49 856 869
85. HuaSAntaoSTCorbettAWittingPK 2010 The significance of neuroglobin in the brain. Curr Med Chem 17 160 172
86. FerrerIGomezACarmonaMHuesaGPortaS 2011 Neuronal hemoglobin is reduced in Alzheimer's disease, argyrophilic grain disease, Parkinson's disease, and dementia with Lewy bodies. J Alzheimers Dis 23 537 550
87. ValcourVSithinamsuwanPLetendreSAncesB 2011 Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep 8 54 61
88. LiptonSGendelmanHE 1995 Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med 233 934 940
89. JensenLJKuhnMStarkMChaffronSCreeveyC 2009 STRING 8–a global view on proteins and their functional interactions in 630 organisms. Nucl Acids Res 37 D412 D416
90. PiehlFOlssonT 2009 Inflammation and susceptibility to neurodegeneration: the use of unbiased genetics to decipher critical regulatory pathways. Neuroscience 158 1143 1150
91. StadelmannCBruckW 2008 Interplay between mechanisms of damage and repair in multiple sclerosis,. J, Neurol 255 Suppl 1 12 18
92. GongvatanaASchweinsburgBCTaylorMJTheilmannRJLetendreSL 2009 White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol 15 187 195
93. BarberSAHerbstDSBullockBTGamaLClementsJE 2004 Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system. J Neurovirol 10 Suppl. 1 15 20
94. SasARBimonte-NelsonHSmothersCTWoodwardJTyorWR 2009 Interferon-α causes neuronal dysfunction in encephalitis. J Neurosci 29 3948 3955
95. WoodsSPRippethJDFrolABLevyJKRyanE 2004 Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV-1. J Clin Exp Neuropsych 26 759 778
96. Report of a Working Group of the American Academy of Neurology AIDS Task Force 1991 Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Neurology 41 778 785
97. ReynoldsNRSunJNagarajaHNGiffordALWuAW 2007 Optimizing measurement of self-reported adherence with the ACTG Adherence Questionnaire: a cross-protocol analysis. J Acquir Immune Defic Syndr 46 402 409
98. ChowdhuryIHChaoWPotashMJSovaPGendelmanHE 1996 vif-negative human immunodeficiency virus type 1 persistently replicates in primary macrophages, producing attenuated progeny virus. J Virol 70 5336 5345
99. OuC-YKwokSMitchellSMackDSninskyJ 1988 DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science 239 295 297
100. HadasEBorjabadAChaoWSainiMIchiyamaK 2007 Testing antiretroviral drug efficacy in conventional mice infected with chimeric HIV-1. AIDS 21 905 909
101. SturnAQuackenbushJTrajanoskiZ 2002 Genesis: cluster analysis of microarray data. Bioinformatics 18 207 208
102. HosackDADennisGJrShermanBTLaneHCLempickiRA 2003 Identifying biological themes within lists of genes with EASE. Genome Biol 4 R70
103. WuHSMurrayJMorgelloS 2008 Segmentation of Brain Immunohistochemistry Images Using Clustering of Linear Centroids and Regional Shapes. J Imaging Sci Technol 52 405021 4050211
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



