-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaWhole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
Although autism has a clear genetic component, the high genetic heterogeneity of the disorder has been a challenge for the identification of causative genes. We used homozygosity analysis to identify probands from nonconsanguineous families that showed evidence of distant shared ancestry, suggesting potentially recessive mutations. Whole-exome sequencing of 16 probands revealed validated homozygous, potentially pathogenic recessive mutations that segregated perfectly with disease in 4/16 families. The candidate genes (UBE3B, CLTCL1, NCKAP5L, ZNF18) encode proteins involved in proteolysis, GTPase-mediated signaling, cytoskeletal organization, and other pathways. Furthermore, neuronal depolarization regulated the transcription of these genes, suggesting potential activity-dependent roles in neurons. We present a multidimensional strategy for filtering whole-exome sequence data to find candidate recessive mutations in autism, which may have broader applicability to other complex, heterogeneous disorders.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002635
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002635Summary
Although autism has a clear genetic component, the high genetic heterogeneity of the disorder has been a challenge for the identification of causative genes. We used homozygosity analysis to identify probands from nonconsanguineous families that showed evidence of distant shared ancestry, suggesting potentially recessive mutations. Whole-exome sequencing of 16 probands revealed validated homozygous, potentially pathogenic recessive mutations that segregated perfectly with disease in 4/16 families. The candidate genes (UBE3B, CLTCL1, NCKAP5L, ZNF18) encode proteins involved in proteolysis, GTPase-mediated signaling, cytoskeletal organization, and other pathways. Furthermore, neuronal depolarization regulated the transcription of these genes, suggesting potential activity-dependent roles in neurons. We present a multidimensional strategy for filtering whole-exome sequence data to find candidate recessive mutations in autism, which may have broader applicability to other complex, heterogeneous disorders.
Introduction
Autism is a neurodevelopmental disorder characterized by impaired communication skills, social behavior abnormalities, and stereotypies, with a prevalence of ∼1/150 children [1]. It is considered to be one of the most highly genetic neuropsychiatric disorders with a heritability of 40–80% [2], [3]. Family studies show that siblings of autistic children are at a ∼25-fold higher risk to develop autism than the general population [4], and twin studies show concordance of the autism phenotype in 20–30% of dizygotic twins and ∼60% of monozygotic twins [3], [4]. Genome-wide linkage and association studies, and candidate gene approaches have identified several susceptibility loci and implicated potential autism genes [5]–[7]. The fact that no single genetic aberration accounts for more than 1% of cases suggests extreme genetic heterogeneity [8], [9], posing a major challenge to identifying causative genes. To date genes have been identified on the basis of overlap with other syndromic neurodevelopmental disorders (e.g. Fragile X syndrome, Angelman syndrome, Rett syndrome), chromosomal abnormalities and copy number variation, and as causes for nonsyndromic autism (e.g. NRXN1, NLGN3/4X, SHANK3) [4], [10]. In a few cases, autism has been shown to be caused by homozygous recessive mutations due to recent shared ancestry [11], although the contribution of recessive mutations in outbred populations remains unexplored.
Recessive mutations in autism may behave like other rare recessive traits, thus allowing gene mapping using homozygosity analysis. Homozygosity mapping is frequently employed to isolate disease genes in families where the parents are known to be definably related, typically as cousins, which increases the risk for recessive disease [12]–[14]. However homozygous recessive “founder” mutations are also common in patients whose parents share only distant ancestry, common ethnicity, or in some cases no apparent ancestry at all [15], and population analysis of runs of homozygosity has been used to define genomic loci that may harbor such mutations in diseases characterized by genetic heterogeneity [16]–[18]. Here we surveyed the mutational spectrum in individuals with autism from nonconsanguineous populations who were selected for the high degree of homozygosity in the genome, since high levels of homozygosity suggest distant or cryptic shared ancestry of the parents. We identified several patients with potentially new autism mutations, and found that a surprising number of these mutations occurred in genes that are regulated by neuronal depolarization.
Results/Discussion
To sort the genetic heterogeneity of autism, we used homozygosity analysis [19] to identify a subset of patients likely to be enriched for recessive mutations. We performed a homozygosity-based analysis of 1000 families (5,431 individuals) in the Autism Genetic Research Exchange (AGRE) [20] cohort. Though most American families in this cohort are of mixed European ancestry and share no acknowledged near ancestors, we hypothesized that a small proportion of European-American parents share a traceable common ancestor, or may share common ethnic ancestry through both parental lines, which in either case may result in homozygosity for rare recessive founder mutations, as has been demonstrated for a host of known Mendelian recessive diseases [21]. We identified a small subset of “outlier” AGRE families (<2% of the total) in which the affected children show runs of homozygosity totaling up to ∼9% of their genome. This low proportion of families with elevated homozygosity is consistent with low reported rates of consanguinity in the AGRE collection. Nonetheless, in the few outlier families, rates of homozygosity are far higher than generally observed in individuals whose parents have no common ancestry (≤1.6%), and overlap or exceed in some cases the predicted range of homozygosity expected in offspring of first cousin parents (6.25%) [22] (Figure 1A). The sizes of homozygous blocks in probands from these outlier families ranged from ∼5–19 cM on average (Figure 1B), suggesting ancient shared ancestry in these families compared to larger blocks of homozygosity seen in consanguineous families (≥20 cM) [22]. Since the AGRE dataset provides no specific information about shared ancestry or consanguinity between parents, we explored the level of shared ancestry between parents, by performing tests to estimate relatedness between individuals based on identical-by-state (IBS) and identical-by-descent (IBD) genotype information [23], [24]. We find that for 16 families where probands had the largest amount of homozygosity in their genomes, some of the parental pairs were more closely related than average (Figure S1), but that parental relatedness by itself, as analyzed by these methods, did not always predict the degree of homozygosity in the offspring.
Fig. 1. Homozygosity analysis in the AGRE collection. 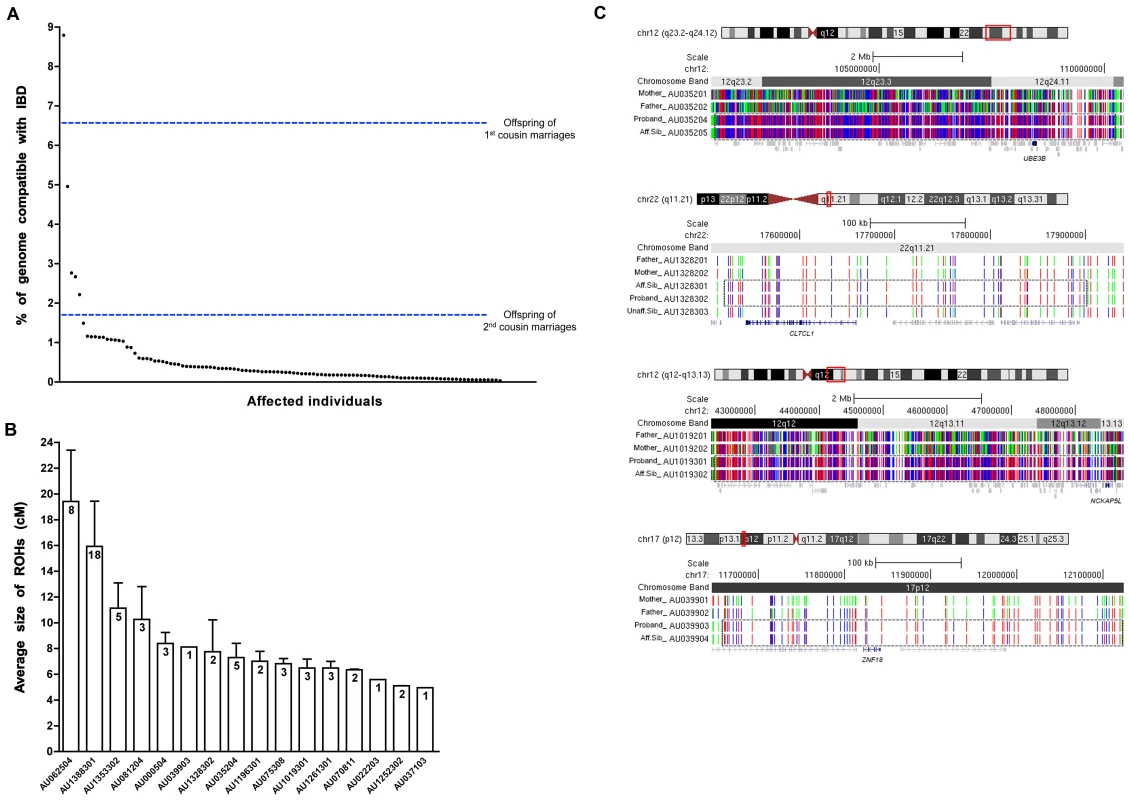
(A) A plot of the percent homozygosity in the genome of probands from the entire AGRE collection. All affected individuals with runs of homozygosity (ROHs) >5 cM are plotted. Offspring of first cousin marriages are expected to have 6.25% homozygosity in their genomes, while those of second cousin marriages are expected to have 1.6%. IBD: identity by descent. (B) The average sizes of the ROHs in cM are plotted for each of the 16 AGRE samples that were sequenced. The number of the ROHs is shown in each bar. Values are mean ± SEM. (C) ROHs containing candidate disease variants are shared by affected individuals and absent from unaffected individuals. Sample names are indicated on the left (Aff.Sib: affected sibling, Unaff.Sib: unaffected sibling). Homozygous SNPs are shown in red or blue and heterozygous SNPs are shown in green. ROHs are enclosed in the dotted box. The candidate autism gene in each family is shown in navy below the ROHs. All other genes in grey did not contain rare, potentially pathogenic variants. No whole genome SNP data is available for individual AU035203, but we genotyped the sample for all homozygous variants identified by the whole exome sequencing of AU035204. We performed whole exome sequencing in 16 AGRE patients, selected because they showed the largest total proportion of their genome homozygous (∼1%–9%) of all patients in the collection. We reasoned that some of the runs of homozygosity would contain homozygous causative mutations. Whole exome sequencing allows for the high-throughput, unbiased survey of all exonic variation in a patient, including any known mutations. Sequencing was performed using the Illumina Genome Analyzer II platform following enrichment of exonic sequences using Agilent's SureSelect Human Exome Kit. We obtained an average coverage of 92% at 20X (Table S1), and identified an average of 34,615 total variants per exome (Table S2), subsequently filtering them to identify rare, likely deleterious changes. Since we wanted to identify rare private mutations, common variants identified by the 1000 Genomes project and dbSNP130 were filtered out, and remaining variants were subject to an in-house bioinformatics pipeline to annotate variants that may disrupt gene function (by altering the coding sequence, the splice sites, or truncating the protein). On average, 735 variants per exome were potentially pathogenic, and out of these, 39 per genome (on average) were homozygous (Table S2). The availability of whole exome sequence allowed us to test each patient systematically for mutations in known autism genes on the autosomes as well as the X chromosome, and no inherited mutations that were predicted to be damaging in well-documented autism genes were found in the 16 patients.
To rule out variants that arose from spontaneous cell line artifacts, somatic mosaic mutations, or sequencing errors, we validated all homozygous variants in all family members using Sequenom technology. Genotyping candidate variants in the 16 probands allowed us to examine inheritance of variants as well as segregation with disease, since many families had multiple affected individuals as well as unaffected siblings (Figure S2). Variants that did not validate with Sequenom genotyping despite high sequencing depth (≥100) generally occurred in regions of the genome that were not uniquely mappable. For uniquely mapped variants, the rate of validation correlated well with sequencing depth (Pearson's correlation = 0.532, P = 0.001×10−30, t-test) (Figure S3). Analysis of segregation further permitted us to focus on bona fide inherited mutations as we only considered those variants that were homozygous in the proband (by whole exome sequencing and Sequenom confirmation), heterozygous or absent in unaffected siblings, and transmitted from heterozygous parents. This validation step thus eliminates any possible sequencing errors or somatic mutations that complicate many high-throughput sequencing studies. We overlaid the validated variants with the result of our homozygosity analysis and further focused our attention on that subset of variants that fell within runs of homozygosity shared by affected siblings and absent from unaffected siblings. This allowed us to narrow down the number of candidate variants per exome, and for four families only 1 variant segregated with the disease (Table 1, Figure 1C). For some families our approach did not yield any candidate recessive variants as expected, since homozygous variants will not necessarily be causative even in some families selected based upon homozygosity. We then further examined the prevalence of candidate homozygous mutations in a control population of ∼700 normal individuals. We were able to exclude homozygous variants based on several criteria including: prevalence in controls, the genes not being expressed in brain, or the genes being mutated in other disorders (Table S3). Under this variant prioritization model (Figure 2), candidate autism mutations were identified in four of the 16 probands (Table 2, Figure 1C), with these candidate disease variants falling within runs of homozygosity shared by affected siblings and absent from unaffected siblings.
Fig. 2. A four-dimensional approach to identifying autism candidate genes. 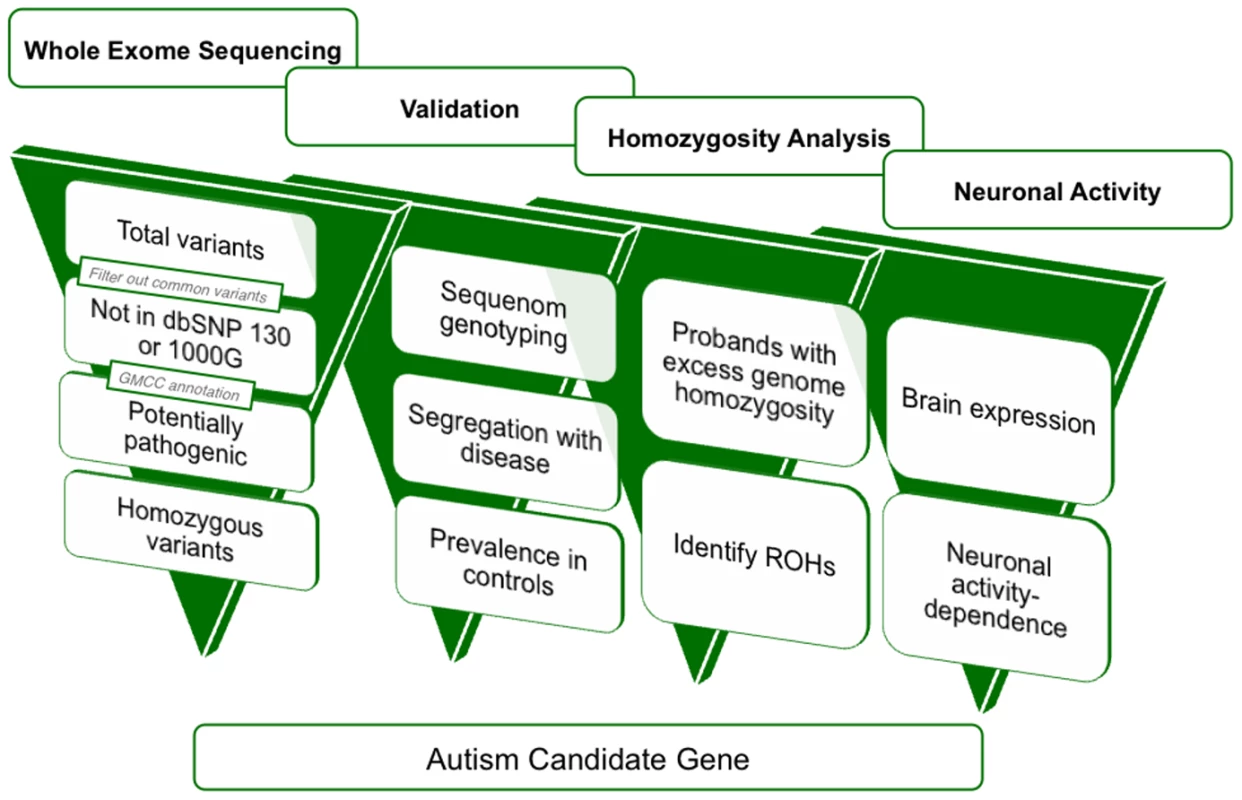
Overview of variant filtration and prioritization of whole exome sequencing data. Results from variant validation and homozygosity analysis were combined with neuronal activity data to identify candidate autism genes from whole exome sequence. 1000G: 1000 Genomes Project, GMCC: genomic mutation consequence calculator, ROHs: runs of homozygosity. Tab. 1. Whole-exome sequencing identifies rare, previously unreported homozygous variants in 16 AGRE autism patients. 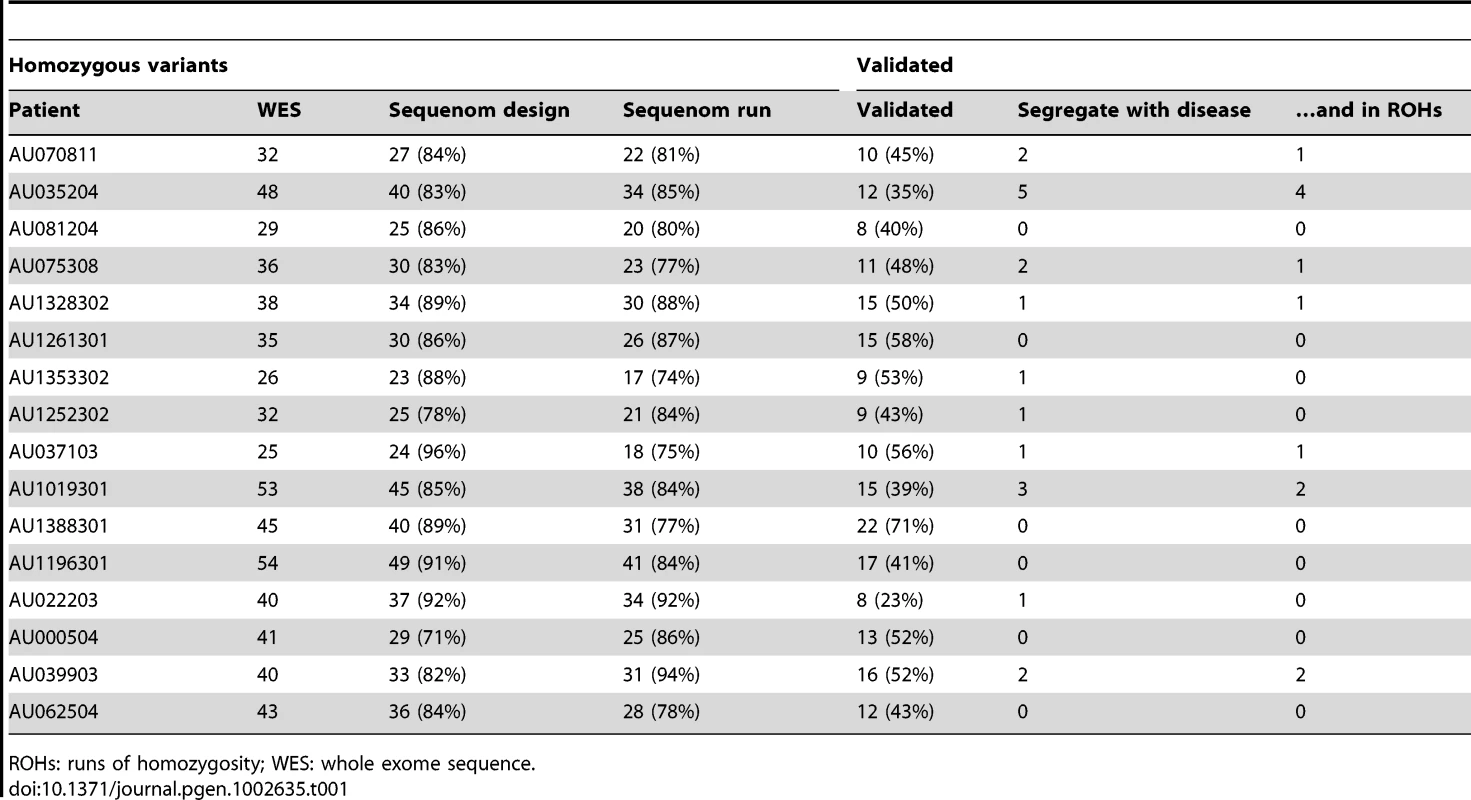
ROHs: runs of homozygosity; WES: whole exome sequence. Tab. 2. Candidate autism genes identified in 4 AGRE patients. 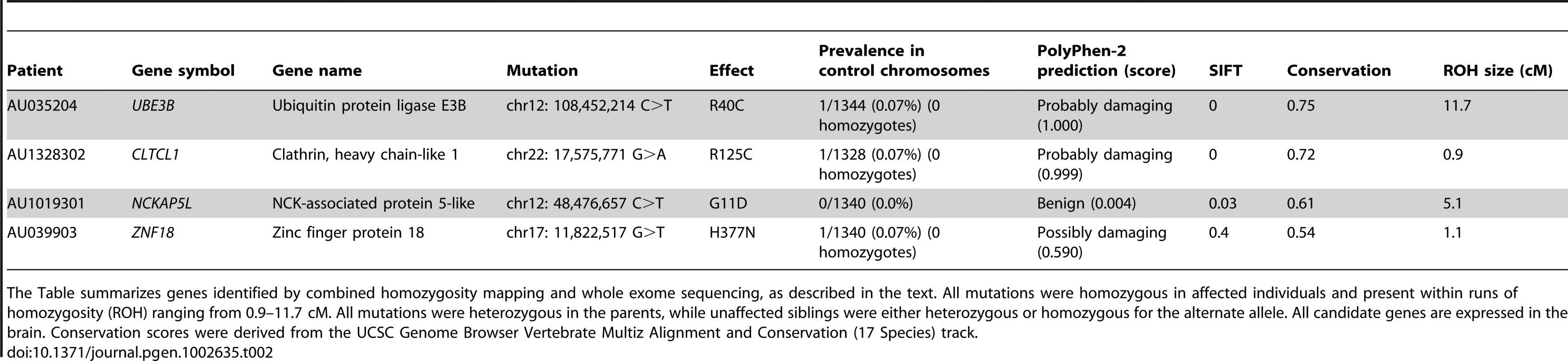
The Table summarizes genes identified by combined homozygosity mapping and whole exome sequencing, as described in the text. All mutations were homozygous in affected individuals and present within runs of homozygosity (ROH) ranging from 0.9–11.7 cM. All mutations were heterozygous in the parents, while unaffected siblings were either heterozygous or homozygous for the alternate allele. All candidate genes are expressed in the brain. Conservation scores were derived from the UCSC Genome Browser Vertebrate Multiz Alignment and Conservation (17 Species) track. The candidate mutations identified in this study implicate several candidate genes in autism that encode proteins involved in small GTPase mediated signal transduction, transcriptional regulation, and protein modification processes (Table 2). Among the mutations we identified is a homozygous c.144 C>T change that creates an R40C mutation in ubiquitin protein ligase E3B (UBE3B), a member of the E3 ubiquitin-conjugating enzyme family. UBE3B is highly expressed in the brain and may play a role in stress response [25]. The UBE3B R40C mutation identified in AU035204 is predicted to be damaging, was homozygous in both affected children (monozygotic twins), heterozygous in the parents and unaffected sibling (Figure S2), and was absent in the homozygous state in 1344 control chromosomes. UBE3B is highly conserved across species and belongs to the same family as UBE3A, the gene disrupted in Angelman syndrome, a neurodevelopmental disorder characterized by intellectual disability, movement or balance problems, abnormal behaviors, and speech and language impairment. Recent work has shown that experience-driven neuronal activity induces Ube3a transcription and that it regulates excitatory synapse development and function through targeting the key synaptic molecules Arc and Ephexin5 [26], [27].
We also narrowed down the candidate genes to only one in AU1328302. An R125C mutation in CLTCL1, encoding clathrin heavy chain-like 1, was homozygous in both affected children, heterozygous in the parents and unaffected sibling, and predicted to be damaging (Table 2 and Figure S2). CLTCL1 is disrupted in a patient with features of DiGeorge syndrome, including intellectual disability, facial dysmorphia, long slender digits, and genital anomalies [28]. It encodes a member of the clathrin heavy chain family, representing a major structural component of coated pits and vesicles involved in intracellular trafficking, which are important to glutamate receptor turnover.
Since resequencing of candidate genes in a larger cohort is an important validation step in evaluation of any candidate gene, we screened a larger independent cohort of whole exome data from 418 autism cases and 371 controls, sequenced as part of the ARRA Autism Sequencing Consortium. DNA from these cases and controls underwent whole exome capture, cloning and sequencing in the same fashion that our 16 cases did at the Broad Institute. For all four genes, we compared the rate of mutations under a recessive model, looking for either homozygous or compound heterozygous mutations in cases versus controls. As a group, the 4 genes showed a higher number of recessive mutations (homozygous or compound heterozygous) in cases (24/418, 5.7%) compared to controls (11/371, 3.0%) (P = 0.042, Fisher's exact test, one-tailed). These mutations were all missense changes and were relatively rare, all with allele frequencies of ≤5% (Table 3). One gene, CLTCL1, especially stood out compared to the other four genes, having 17 mutations in cases versus 6 mutations in controls (Table 3).
Tab. 3. Whole-exome screen identifies additional potential recessive mutations in the four candidate autism genes. 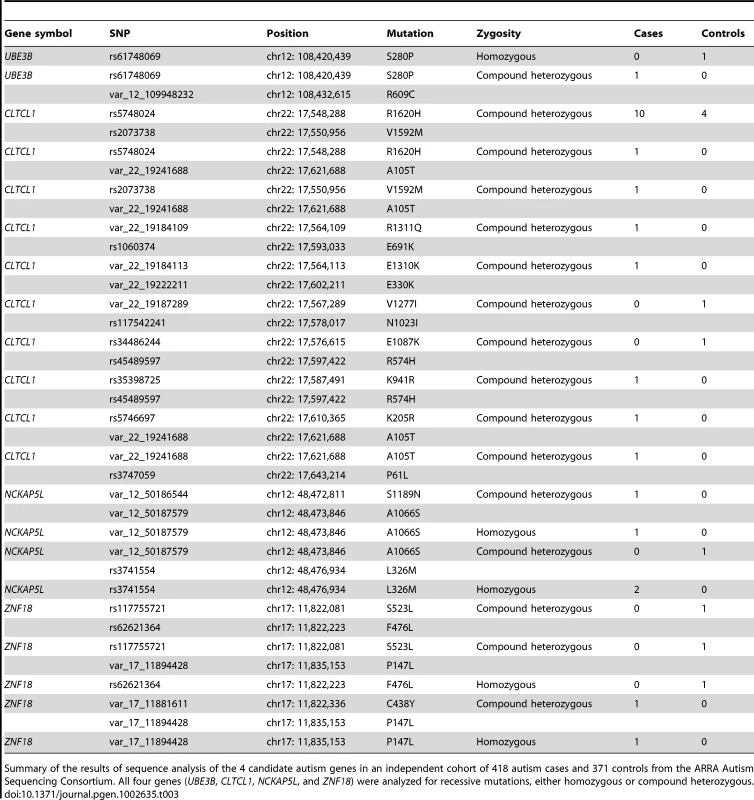
Summary of the results of sequence analysis of the 4 candidate autism genes in an independent cohort of 418 autism cases and 371 controls from the ARRA Autism Sequencing Consortium. All four genes (UBE3B, CLTCL1, NCKAP5L, and ZNF18) were analyzed for recessive mutations, either homozygous or compound heterozygous. Genes with essential roles in synaptic plasticity have been implicated as an important cause of autism (e.g. NRXN1, NLGN3/4X, SHANK2/3) [29], [30], and since many synaptic plasticity genes are regulated by neuronal depolarization [11], [31], we tested the degree to which our autism candidate genes showed expression that could be modulated by neuronal activity. We depolarized mouse cortical neuron cultures and assayed changes in gene expression levels. We found that four out of four of the mouse homologs of our candidate genes are upregulated in response to neuronal activity (UBE3B, CLTCL1/Cltc, NCKAP5L, and ZNF18/Zkscan6) (Figure 3). This is particularly interesting because in general only about 1000 transcripts, or about 3% of the transcriptome, manifest such depolarization-regulated gene transcription [32]. The upregulation of Ube3b in response to depolarization resembles the activity-dependent transcription of its close paralog Ube3a, which has well-documented roles in synaptic plasticity [26], [27]. The regulation of expression of several potential recessive autism genes by neuronal depolarization suggests that the candidate genes are likely to be involved in neuronal function and/or development, and mutations in these genes might lead to nervous system dysfunction in the context of autism spectrum disorders (ASDs).
Fig. 3. Regulation of four candidate autism genes by neuronal activity. 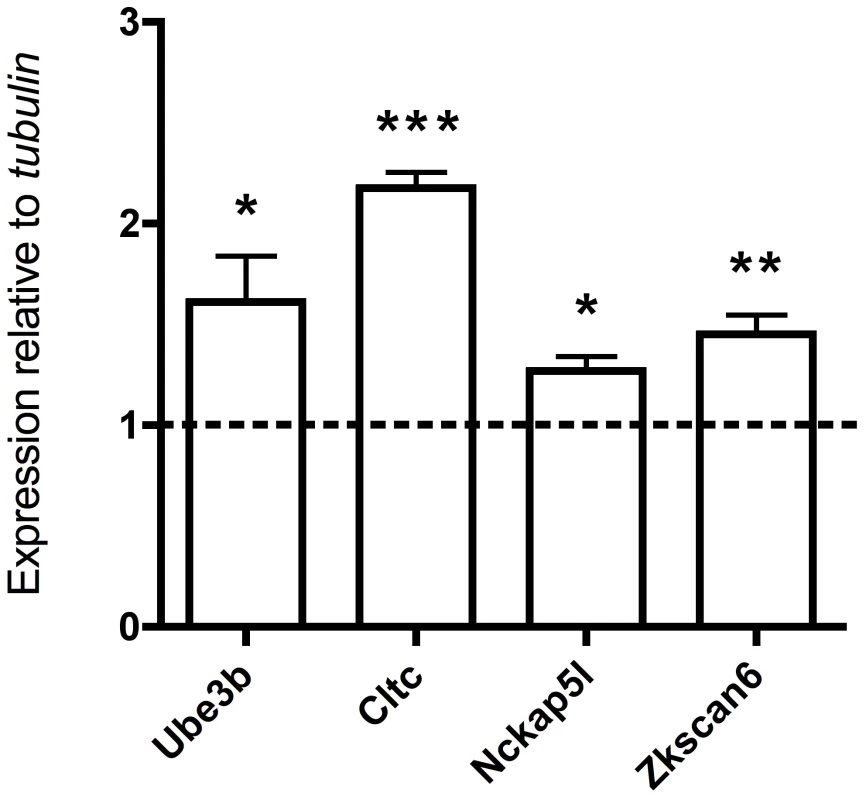
qRT-PCR analysis of total RNA from depolarized mouse cortical neurons stimulated with KCl for 6 hours (the dashed line represents no KCl treatment, values are mean ± SEM from three independent experiments, each experiment was performed in triplicate, ***P<0.0001, **P<0.004, *P<0.04, t-test). In the 12/16 patients for whom we did not identify homozygous candidate mutations, we examined the mutational spectrum under different models of inheritance. Out of an average of 696 rare, heterozygous, and potentially deleterious variants per exome, we identified 67 candidate compound heterozygous changes (at least two deleterious variants in the same gene). Sequenom genotyping validated an average of 27 of these variants, and phasing of the resulting set in trios revealed ∼4 true compound heterozygotes with one allele inherited from each parent. Genotyping of unaffected siblings when available reduced this number to ∼2 variants per individual consistent with fully penetrant, recessive disease (Table S4). For three patients, we narrowed down the candidates to 1 gene and for 8 patients there were no candidate genes with compound heterozygous variants (Table S5). Analysis of X-linked mutations did not identify mutations in well-validated X-linked autism genes, though 11/14 male patients carried rare hemizygous X-linked variants, three of which occurred in genes associated with intellectual disability (ARHGEF6, AFF2, and OCRL). The first variant in ARHGEF6, which encodes Rac/Cdc42 guanine nucleotide exchange factor 6, results in an I444N mutation. The second variant in AFF2, encoding Fragile X mental retardation 2, causes a P847A mutation that is predicted to be benign by PolyPhen-2. The third variant disrupts a splice donor site in OCRL (oculocerebrorenal syndrome of Lowe gene) (Table S6). Splicing mutations in OCRL have been identified in patients with Lowe oculocerebrorenal syndrome [33]–[36], characterized by hydrophthalmia, cataract, intellectual disability, vitamin D-resistant rickets, amino aciduria, and reduced ammonia production by the kidney. Since patient AU1019301 is not known to exhibit a renal phenotype or any other Lowe syndrome phenotypes, it is unlikely that this mutation is causative of the neurological condition of the patient. Segregation analysis showed that these three X-linked mutations were inherited from heterozygous mothers, confirming that they are not cell line artifacts. Since our study design enriched for families with potential shared inheritance, it does not permit confident determination of the causative nature of these potential compound heterozygous or X-linked mutations, which could only be tested by analysis of additional cases.
Our results illustrate both the challenges and the potential of whole exome sequencing in an extremely genetically heterogeneous condition such as autism. Each exome contains large numbers of variants that initially challenge analysis. We present a systematic method to approach whole exome data, by filtering for variants compatible with identity by descent, surveying prevalence in controls, segregation analysis, and incorporating functional information (Figure 2). Almost all instances in which new genetic syndromes have been identified using whole exome or whole genome sequencing have involved families with recessive disorders generally (Miller syndrome) [37], [38] and/or shared parental ancestry specifically (WDR62-associated cortical malformations) [39], because the analysis of homozygous mutations provides tremendous power to improve “signal to noise” caused by sequencing errors, spontaneous cell line mutations, somatic mutations, etc. Hence, tracing ancestry may be an important tool to define genetic causes in a subset of autism patients. Our study further emphasizes the power of whole exome and whole genome approaches in allowing a complete survey of all potential mutations in the patient genome, and the systematic screening of all major modes of inheritance. Recent studies have confirmed the contribution of de novo point mutations (5–20% of cases) [40] and de novo copy number variants (5–10% of cases) [41] to autism.
Our data suggest a potentially important role for recessive mutations in autism. Though our pre-selection of 16 patients for whole exome sequencing, and our limited analysis of whole exome data from >400 cases in the ARRA Autism Sequencing Consortium, does not allow us to calculate the proportion of cases likely attributable to recessive as opposed to other causes (e.g. de novo, X-linked), our data do suggest that a systematic analysis of recessive causes of autism would be worthwhile. Homozygous null mutations appear to be exceedingly rare in autism, while homozygous missense changes were found in several candidate genes (Table 2), consistent with the possibility that some cases of ASD may reflect hypomorphic mutations in genes that have more severe phenotypes when completely disabled [11]. On the other hand, compound heterozygous recessive mutations could be more common in the outbred families represented by the AGRE.
Furthermore, we find that different patients showed candidate mutations in different ASD candidate genes, confirming that recessive autism genes are likely to be highly heterogeneous. On the other hand, several of the genes we identified represent new neuronal depolarization-dependent genes, further supporting a role of defective synaptic transmission and neuronal plasticity in the pathogenesis of ASD.
Finally, the approach employed here might be of value to the dissection of other complex traits where extreme genetic heterogeneity is suspected or confirmed. Since many neuropsychiatric conditions - including schizophrenia, intellectual disability, and epilepsy - often (albeit not exclusively) arise from loss of gene function, it is reasonable to suppose that recessive loss of gene function may play detectable roles in other conditions. Despite the rich variation in the human exome, our study design and approach to variant prioritization allowed identification of candidate autism genes from a relatively small sample.
Materials and Methods
Subjects
Whole exome sequencing was performed on DNA samples from the AGRE collection available at the Broad Institute. All human studies were reviewed and approved by the institutional review board of the Children's Hospital Boston, the Broad Institute, Cambridge, and the local institutions.
Homozygosity analysis
The analysis was performed using the Illumina 550 SNP genotype data for 1000 families from the AGRE collection. The data was obtained with permission from the AGRE [42]. Runs of homozygosity were calculated using custom scripts, allowing for no more than 2 consecutive heterozygous SNPs in a run and 3 heterozygous calls in every 10 consecutive SNPs. Intervals homozygous for the same haplotype and shared by all affected individuals were used to narrow the locus in each family.
Estimating relatedness
We used PLINK [23] to calculate the probability that one allele is shared IBD (Z1), and we calculated IBS2*_ratio and the percent of informative SNPs as described by Stevens et al. [24]. Briefly, IBS2*_ratio is equal to (IBS2*)/(IBS2*+IBS0), and the percent of informative SNPs is equal to (IBS0+IBS2*)/(IBS0+IBS1+IBS2), where IBS0 is the total number of observations in which two discordant homozygotes are present, and IBS2* results when two concordant heterozygotes are compared between any pair of individuals.
Whole-exome sequencing and data analysis
Exome enrichment was performed on 3 µg of genomic DNA, using the SureSelect Human Exome Kit (Agilent Technologies, Inc., Santa Clara, CA), according to the manufacturer's protocol. The kit covers exonic sequences of ∼18,500 genes and a total of ∼33 Mb of target territory. The captured, purified and amplified library targeting the exome from each patient was sequenced on the Illumina GA II. Paired-end sequences were obtained at a read length of 72 bp.
High-throughput sequence analysis was performed according to a customized bioinformatic pipeline for tracking sequence data, aligning reads, calculating coverage, calling variants, annotating variants with respect to functional effect, filtering out benign variation and flagging candidate rare, pathogenic mutations. Briefly, BWA version 0.5.7 (ref. 3) was employed to align reads to the human genome (reference build hg18). Consensus and variant base calls were made with SAMtools version 0.1.7 (pileup), filtered for quality (mapping quality >10 for insertions and deletions, and >25 for SNPs), and loaded into a MySQL database for storage and further processing, including annotation of the predicted consequences (noncoding, coding synonymous, coding nonsynonymous or frameshift, splice site) of each variant using GMCC [43] (Genomic mutation consequence calculator). Candidate mutations were identified by starting with a list of all variants, removing those present either in dbSNP130 or the 1000 Genomes Project database, and selecting for coding nonsynonymous, frameshift or splice site changes. Sequence data were visualized using either the UCSC Genome Browser or the Broad Institute Integrated Genome Viewer. All genomic base positions are presented in reference to the human genome NCBI build 36 (hg18). The functional effect of the mutation on the protein was assessed using PolyPhen-2 [44].
Sequenom genotyping
Sequenom genotyping of variants in the probands and their family members was performed on the iPLEX Gold platform at the Broad Institute. Variants were genotyped in control individuals also using the Sequenom iPLEX Gold assay at the Molecular Genetics Core Facility at Children's Hospital Boston. The controls collection consisted of 704 neurologically normal samples obtained from the Coriell Cell Repositories (Camden, NJ; 584 Caucasian samples), or available in our lab (80 Saudi and 40 Bedouin samples).
Resequencing analysis of candidate genes in the ARRA Autism Sequencing Consortium
We screened whole exome sequencing data from a total of 789 exomes (418 autism cases and 371 controls) that were sequenced at the Broad Institute (as described above) as part of a case-control study by the ARRA Autism Sequencing Consortium. Recessive mutations (homozygous and compound heterozygous) were counted in cases and in controls and a Fisher's exact test was used to determine whether the number of mutations in cases was significantly different than the number in controls. Samples in this study are of European ancestry from the AGRE collection, the Autism Sequencing Consortium (ASC), and the National Institute of Mental Health (NIMH).
Mouse cortical cultures
E16.5 C57B6 mouse embryo cortices were dissected and then dissociated in 1× Hank's Balanced Salt Solution (HBSS), 20 mg/ml trypsin (Worthington Biochemicals, Lakewood, NJ), and 0.32 mg/ml L-cysteine (Sigma, St. Louis, MO) for 10 minutes. Trypsin treatment was terminated with three two-minute washes in 1× HBSS with 10 mg/ml trypsin inhibitor (Sigma, St. Louis, MO). Trituration of cells was performed with a flame-narrowed Pasteur pipette to fully dissociate cells. Neurons were seeded at an approximate density of 1×106/well on 6-well culture plates. The dishes were pre-coated overnight with poly-ornithine (30 µg/mL, Sigma) in water, washed three times with water, and washed once with Neurobasal Medium (Life Technologies, Carlsbad, CA) before use. Neurons were maintained in 2 ml/well Neurobasal Medium containing B27 Supplement (2%; Invitrogen, Carlsbad, CA), penicillin-streptomycin (50 µg/ml penicillin, 50 U/ml streptomycin, Sigma) and glutamine (1 mM, Sigma, St. Louis, MO). Neurons were grown in vitro for 7 days. 8 ml of the medium was replaced with 10 ml fresh warm medium on the 4th and 6th days in vitro (DIV).
Membrane depolarization and quantitative RT–PCR detection of activity induction
For KCl depolarization of neurons, DIV 6 neurons were quieted overnight in 1 µM TTX and 100 µM APV, and they were incubated for 0 or 6 hours in 55 mM KCl. Total RNA was isolated from cultures using 1 ml Trizol/well according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Isolated RNA was treated with DNAseI Amplification Grade (Invitrogen, Carlsbad, CA) and cDNA library was synthesized by cDNA High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA). The cDNA was the source of input for quantitative PCR, using a Step One Plus Real-Time PCR Instrument and SYBR Green reagents (Applied Biosystems, Carlsbad, CA). The relative expression plot was constructed using concentration values that were normalized to corresponding tubulin concentrations.
Accession numbers
The whole exome sequence data is available online (The National Database for Autism Research (NDAR) Collection ID: NDARCOL0001918).
Supporting Information
Zdroje
1. Autism and Developmental Disabilities Monitoring Network Surveillance Year 2006 Principal Investigators, Centers for Disease Control and Prevention (CDC) 2009 Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ 58 1 20
2. GeschwindDH 2011 Genetics of autism spectrum disorders. Trends Cogn Sci 15 409 416
3. HallmayerJClevelandSTorresAPhillipsJCohenB 2011 Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch Gen Psychiatry 68 1095 1102
4. GeschwindDH 2009 Advances in autism. Annu Rev Med 60 367 380
5. O'RoakBJStateMW 2008 Autism genetics: strategies, challenges, and opportunities. Autism Res 1 4 17
6. SzatmariPPatersonADZwaigenbaumLRobertsWBrianJ 2007 Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet 39 319 328
7. WeissLAArkingDEDalyMJChakravartiA 2009 A genome-wide linkage and association scan reveals novel loci for autism. Nature 461 802 808
8. MitchellKJ 2011 The genetics of neurodevelopmental disease. Curr Opin Neurobiol 21 197 203
9. BetancurC 2011 Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res 1380 42 77
10. MilesJH 2011 Autism spectrum disorders–a genetics review. Genet Med 13 278 294
11. MorrowEMYooSYFlavellSWKimTKLinY 2008 Identifying autism loci and genes by tracing recent shared ancestry. Science 321 218 223
12. HamamyHAMasriATAl-HadidyAMAjlouniKM 2007 Consanguinity and genetic disorders. Profile from Jordan. Saudi Med J 28 1015 1017
13. HoodfarETeebiAS 1996 Genetic referrals of Middle Eastern origin in a western city: inbreeding and disease profile. J Med Genet 33 212 215
14. StollCAlembikYDottBFeingoldJ 1994 Parental consanguinity as a cause of increased incidence of birth defects in a study of 131,760 consecutive births. Am J Med Genet 49 114 117
15. NallsMAGuerreiroRJSimon-SanchezJBrasJTTraynorBJ 2009 Extended tracts of homozygosity identify novel candidate genes associated with late-onset Alzheimer's disease. Neurogenetics 10 183 190
16. Schuurs-HoeijmakersJHHehir-KwaJYPfundtRvan BonBWde LeeuwN 2011 Homozygosity mapping in outbred families with mental retardation. Eur J Hum Genet 19 597 601
17. CollinRWvan den BornLIKleveringBJde Castro-MiroMLittinkKW 2011 High-resolution homozygosity mapping is a powerful tool to detect novel mutations causative for autosomal recessive RP in the Dutch population. Invest Ophthalmol Vis Sci 52 2227 2239
18. LenczTLambertCDeRossePBurdickKEMorganTV 2007 Runs of homozygosity reveal highly penetrant recessive loci in schizophrenia. Proc Natl Acad Sci U S A 104 19942 19947
19. LanderESBotsteinD 1987 Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science 236 1567 1570
20. GeschwindDHSowinskiJLordCIversenPShestackJ 2001 The autism genetic resource exchange: a resource for the study of autism and related neuropsychiatric conditions. Am J Hum Genet 69 463 466
21. KrawitzPMSchweigerMRRodelspergerCMarcelisCKolschU 2010 Identity-by-descent filtering of exome sequence data identifies PIGV mutations in hyperphosphatasia mental retardation syndrome. Nat Genet 42 827 829
22. WoodsCGCoxJSpringellKHampshireDJMohamedMD 2006 Quantification of homozygosity in consanguineous individuals with autosomal recessive disease. Am J Hum Genet 78 889 896
23. PurcellSNealeBTodd-BrownKThomasLFerreiraMA 2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
24. StevensELHeckenbergGRobersonEDBaugherJDDowneyTJ 2011 Inference of relationships in population data using identity-by-descent and identity-by-state. PLoS Genet 7 e1002287 doi:10.1371/journal.pgen.1002287
25. GongTWHuangLWarnerSJLomaxMI 2003 Characterization of the human UBE3B gene: structure, expression, evolution, and alternative splicing. Genomics 82 143 152
26. GreerPLHanayamaRBloodgoodBLMardinlyARLiptonDM 2010 The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 140 704 716
27. MargolisSSSalogiannisJLiptonDMMandel-BrehmCWillsZP 2010 EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell 143 442 455
28. HolmesSERiaziMAGongWMcDermidHESellingerBT 1997 Disruption of the clathrin heavy chain-like gene (CLTCL) associated with features of DGS/VCFS: a balanced (21;22)(p12;q11) translocation. Hum Mol Genet 6 357 367
29. WalshCAMorrowEMRubensteinJL 2008 Autism and brain development. Cell 135 396 400
30. RamockiMBZoghbiHY 2008 Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature 455 912 918
31. FlavellSWGreenbergME 2008 Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci 31 563 590
32. FlavellSWKimTKGrayJMHarminDAHembergM 2008 Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron 60 1022 1038
33. MonnierNSatreVLerougeEBerthoinFLunardiJ 2000 OCRL1 mutation analysis in French Lowe syndrome patients: implications for molecular diagnosis strategy and genetic counseling. Hum Mutat 16 157 165
34. ChouYYChaoSCChiouYYLinSJ 2005 Identification of OCRL1 mutations in two Taiwanese Lowe syndrome patients. Acta Paediatr Taiwan 46 226 229
35. SchrammLGalAZimmermannJNetzerKOHeidbrederE 2004 Advanced renal insufficiency in a 34-year-old man with Lowe syndrome. Am J Kidney Dis 43 538 543
36. CauMAddisMCongiuRMeloniCCaoA 2006 A locus for familial skewed X chromosome inactivation maps to chromosome Xq25 in a family with a female manifesting Lowe syndrome. J Hum Genet 51 1030 1036
37. NgSBBuckinghamKJLeeCBighamAWTaborHK 2010 Exome sequencing identifies the cause of a mendelian disorder. Nat Genet 42 30 35
38. RoachJCGlusmanGSmitAFHuffCDHubleyR 2010 Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science 328 636 639
39. BilguvarKOzturkAKLouviAKwanKYChoiM 2010 Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature 467 207 210
40. O'RoakBJDeriziotisPLeeCVivesLSchwartzJJ 2011 Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet 43 585 589
41. SandersSJErcan-SencicekAGHusVLuoRMurthaMT 2011 Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70 863 885
42. GlessnerJTWangKCaiGKorvatskaOKimCE 2009 Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 459 569 573
43. MajorJE 2007 Genomic mutation consequence calculator. Bioinformatics 23 3091 3092
44. AdzhubeiIASchmidtSPeshkinLRamenskyVEGerasimovaA 2010 A method and server for predicting damaging missense mutations. Nat Methods 7 248 249
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



