-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
Escherichia coli translation initiation factor 2 (IF2) performs the unexpected function of promoting transition from recombination to replication during bacteriophage Mu transposition in vitro, leading to initiation by replication restart proteins. This function has suggested a role of IF2 in engaging cellular restart mechanisms and regulating the maintenance of genome integrity. To examine the potential effect of IF2 on restart mechanisms, we characterized its influence on cellular recovery following DNA damage by methyl methanesulfonate (MMS) and UV damage. Mutations that prevent expression of full-length IF2-1 or truncated IF2-2 and IF2-3 isoforms affected cellular growth or recovery following DNA damage differently, influencing different restart mechanisms. A deletion mutant (del1) expressing only IF2-2/3 was severely sensitive to growth in the presence of DNA-damaging agent MMS. Proficient as wild type in repairing DNA lesions and promoting replication restart upon removal of MMS, this mutant was nevertheless unable to sustain cell growth in the presence of MMS; however, growth in MMS could be partly restored by disruption of sulA, which encodes a cell division inhibitor induced during replication fork arrest. Moreover, such characteristics of del1 MMS sensitivity were shared by restart mutant priA300, which encodes a helicase-deficient restart protein. Epistasis analysis indicated that del1 in combination with priA300 had no further effects on cellular recovery from MMS and UV treatment; however, the del2/3 mutation, which allows expression of only IF2-1, synergistically increased UV sensitivity in combination with priA300. The results indicate that full-length IF2, in a function distinct from truncated forms, influences the engagement or activity of restart functions dependent on PriA helicase, allowing cellular growth when a DNA–damaging agent is present.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002648
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002648Summary
Escherichia coli translation initiation factor 2 (IF2) performs the unexpected function of promoting transition from recombination to replication during bacteriophage Mu transposition in vitro, leading to initiation by replication restart proteins. This function has suggested a role of IF2 in engaging cellular restart mechanisms and regulating the maintenance of genome integrity. To examine the potential effect of IF2 on restart mechanisms, we characterized its influence on cellular recovery following DNA damage by methyl methanesulfonate (MMS) and UV damage. Mutations that prevent expression of full-length IF2-1 or truncated IF2-2 and IF2-3 isoforms affected cellular growth or recovery following DNA damage differently, influencing different restart mechanisms. A deletion mutant (del1) expressing only IF2-2/3 was severely sensitive to growth in the presence of DNA-damaging agent MMS. Proficient as wild type in repairing DNA lesions and promoting replication restart upon removal of MMS, this mutant was nevertheless unable to sustain cell growth in the presence of MMS; however, growth in MMS could be partly restored by disruption of sulA, which encodes a cell division inhibitor induced during replication fork arrest. Moreover, such characteristics of del1 MMS sensitivity were shared by restart mutant priA300, which encodes a helicase-deficient restart protein. Epistasis analysis indicated that del1 in combination with priA300 had no further effects on cellular recovery from MMS and UV treatment; however, the del2/3 mutation, which allows expression of only IF2-1, synergistically increased UV sensitivity in combination with priA300. The results indicate that full-length IF2, in a function distinct from truncated forms, influences the engagement or activity of restart functions dependent on PriA helicase, allowing cellular growth when a DNA–damaging agent is present.
Introduction
Translation Initiation Factor 2 (IF2; for a review, see [1]) is an essential cellular protein that brings mRNA, the 30S ribosome, and the initiator fMet-tRNA together into the 30S initiation complex and then promotes association with the 50S ribosomal unit to form the 70S initiation complex [2]–[4]. We have previously identified it as an essential component for reconstituting bacteriophage Mu replication by transposition in vitro, a process in which IF2 makes way for initiation of DNA synthesis by the cellular restart proteins [5]. This finding raises the question whether IF2 could play an important function in the maintenance of genome integrity by regulating the engagement or activity of restart proteins.
For bacteriophage Mu transposition in vitro [6], IF2 plays a critical part [5] during the transition from strand exchange catalyzed by MuA transposase [7], [8] to the assembly of the replisome by the host replication restart proteins [9] (Figure 1; for a review, see [10]). IF2 binds to Mu DNA only upon disassembly of the oligomeric MuA transpososome that remains tightly bound to Mu ends after strand exchange [11]–13. This process begins as ClpX weakens the transpososome assembly [14]–[16] and is completed by host factors which promote transition to replisome assembly [5], [9], [15], [17]. Strand exchange creates a fork at each Mu end, creating a potential site for initiating Mu DNA replication. However, the Mu forks retain a block to initiation of DNA replication even after transpososome disassembly, and IF2 appears to play a key role in unlocking this complex [5]. Restart proteins are subsequently assembled, beginning with the displacement of the IF2 by PriA helicase. The reaction in vitro specifically requires the E. coli replication restart proteins PriA, PriC, and DnaT but not PriB, indicating that the mode of Mu replication reconstituted in this system is through the PriA-PriC restart system [18], [19]. (The PriA-PriC pathway is one of the two major cellular restart pathways, the other being the PriA-PriB pathway, which requires PriA, PriB, and DnaT [18].) Additionally, only truncated forms of IF2 (IF2-2 and IF2-3; Mr of 79.7 and 78.8 k compared to 97.3 k for full-length IF2-1), synthesized from two internal, in-frame start codons within the infB gene, have been found to be active in this in vitro system.
Fig. 1. Transition from transpososome to replisome during bacteriophage Mu transposition. 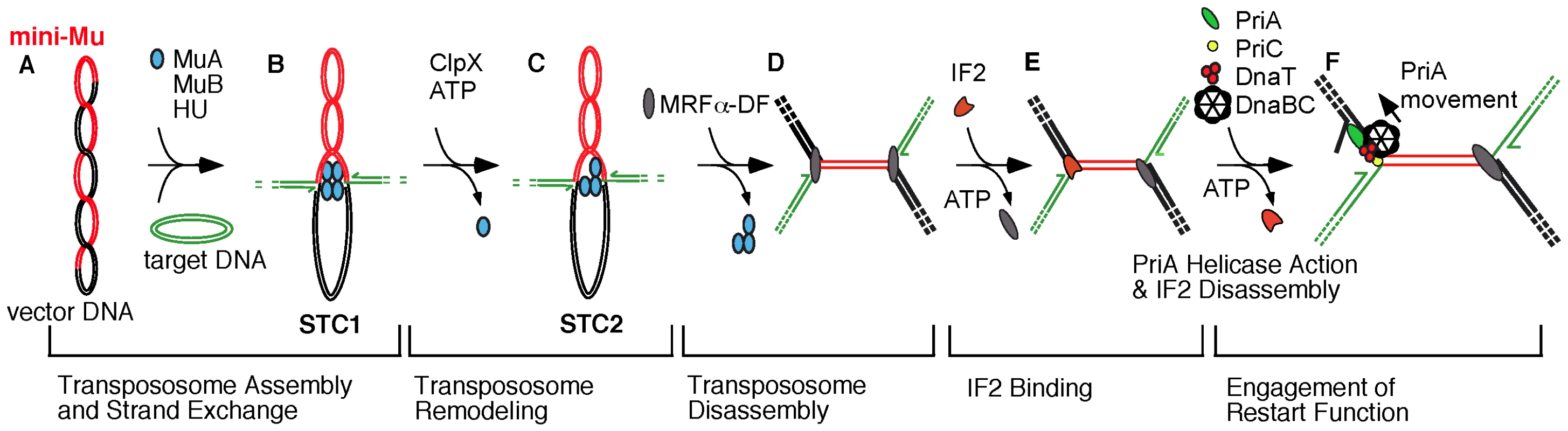
The model reflects changes in nucleoprotein complexes at the Mu ends as the transpososome, assembled from MuA protomers, is sequentially remodeled to a replisome [5], [9], [15]. A) A supercoiled plasmid bearing a miniature version of the Mu genome serves as the donor for transposition in vitro; a second plasmid is used as the target for transposition. B) The phage-encoded MuA is assembled into an oligomeric transpososome, tightly bound to the Mu ends, and this transpososome-DNA complex is preserved as it catalyzes the transfer of Mu ends to target DNA, forming a DNA fork at each Mu end (Strand Transfer Complex 1 or STC1). The half arrows depict the 3′-OH ends of DNA at each fork, which is a potential site for initiation of Mu DNA replication. C) ClpX remodels the transpososome (STC2), weakening its interaction with DNA [14], [15] and preparing the complex for disassembly. D) An unknown host factor (MRFα-DF) completes transpososome disassembly, forming a new nucleoprotein assembly that still does not permit access of the Mu fork to replication and restart proteins. E) IF2 binds to Mu DNA and unlocks the replication block at one or both forks. F) PriA binds to the Mu fork, and its helicase action promotes the disassembly of IF2, leading to the loading of the major replicative helicase DnaB from the DnaB-DnaC complex for replisome assembly. The indicated movement of PriA on the lagging strand template may serve the dual function of promoting IF2 disassembly and unwinding the DNA helix for DnaB loading [5], [29]. Indeed, the role of the various IF2 forms in translation remains unclear. Full-length (IF2-1) and truncated (IF2-2/3) forms are present in nearly equimolar amounts under normal growth conditions [20], [21], and IF2-2/3 levels increase with respect to IF2-1 during cold shock [22]. Mutations that prevent expression of IF2-1 or IF2-2/3 elicit cold sensitivity [21]. However, even IF2 with one-third of its residues deleted from the N-terminal end has intact activities in vitro as translation factor and supports cell viability when present in excess [23], [24].
IF2's role in Mu DNA replication by transposition in vitro raises the question whether it can influence or regulate the engagement of cellular restart mechanisms. The apparent function implied by the Mu replication system is that by binding to forked DNA templates, it may promote or regulate the action of restart proteins. IF2's molecular chaperone activity [25] potentially plays a function similar to ClpX, promoting remodeling of the nucleoprotein assembly at the Mu ends for the transition to a new complex [5] or plays a key part in the activation of enzymatic functions necessary for replication restart. Moreover, IF2's major function as translation factor as well as its possible function as a transcriptional activator [26], [27] also indicate its potential to influence restart mechanisms by promoting expression of proteins needed for this process. Indeed, the role of IF2 in Mu replication may be an idiosyncrasy of Mu as a parasite exploiting host proteins to promote its own propagation; alternatively, it may reflect IF2's cellular role in regulating engagement of restart functions, a function that Mu exploits as a parasite.
In this work, we examined whether IF2 function can affect specific pathways for replication restart by perturbing its function with mutations that prevent expression of IF2-1 or IF2-2/3. Only truncated forms of IF2 have been found to be active in the reconstituted Mu replication system by the PriA-PriC pathway [5]. While this result does not necessarily indicate that only the truncated forms of IF2 may be involved in restart mechanisms (the in vitro system may have lacked factors needed to engage IF2-1), it nevertheless suggests functional differences between isoforms that may be examined in vivo.
Here, we demonstrate that the loss of IF2-1 or IF2-2/3 results in different defects in restart mechanisms that cope with DNA damage during cell growth. In particular, the loss of IF2-1 elicits a phenotype that is analogous to a certain restart mutant. No matter the mechanism by which IF2 influences restart mechanisms, the results indicate a new function of IF2 in influencing the engagement of restart mechanisms, the relative levels of IF2 isoforms having the potential to affect the choice or course of the restart mechanism. We discuss the potential for IF2 to regulate maintenance of genome integrity with respect to cell physiology, suggesting a means for coordinating replication, recombination, and repair with translation status.
Results
IF2 binds to Mu ends in vivo upon induction of Mu development
In the in vitro Mu replication system, binding of IF2-2 can be detected after strand exchange just prior to the binding of the restart protein PriA [5]. Since this is the major basis for suspecting that IF2 may serve a function that affects activity of restart functions, we wished to confirm that IF2 indeed binds at or near Mu ends in vivo when Mu development is induced. Chromatin immunoprecipitation (ChIP) analysis was conducted with extracts of induced lysogens expressing IF2 with an N-terminal S tag (S-IF2) after extensive RNase treatment.
Mu DNA was co-precipitated with S-IF2-1 and S-IF2-2 in induced GTN373 (a thermoinducible Mu lysogen) at 35 min postinduction (Figure 2A, S-IF2-1 and S-IF2-2), using antibody against the S tag. In contrast, relatively little Mu DNA was precipitated with S-IF2-2 upon inducing the isogenic lysogen that has a clpX knockout mutation (Figure 2A, S-IF2-2 ClpX−) and thus cannot support Mu replication [28]. This result parallels findings in vitro that the omission of molecular chaperone ClpX from the reaction system does not permit binding of IF2-2 to Mu DNA and the initiation of Mu replication [5], [15]. As it appeared that Mu ends were being enriched in immunoprecipitations when cells were undergoing Mu replication, we repeated the ChIP with 5-fold less antibody to ascertain whether bound S-IF2-2 in induced GTN373 is concentrated around Mu ends. In the immunoprecipitated samples, the Mu ends sequences were enriched over the center sequences (18 kb from either end) as well as host DNA (Figure 2C). In the control PCR amplification of total DNA, the Mu end and center sequences were amplified to the same extent. Mu PCR products were produced at higher levels than the host thrA PCR product at 35 min postinduction, reflecting the replication of Mu during lytic development. IF2 does have some nonspecific DNA binding activity [27]. Thus, the enrichment of Mu end sequences with respect to Mu center sequences by immunoprecipitation is the best indicator of preferred IF2 binding at or near Mu ends although the enrichment of Mu end sequences with respect to host DNA is also clear in this analysis.
Fig. 2. Binding of S-IF2 to Mu ends upon induction of phage replication by transposition. 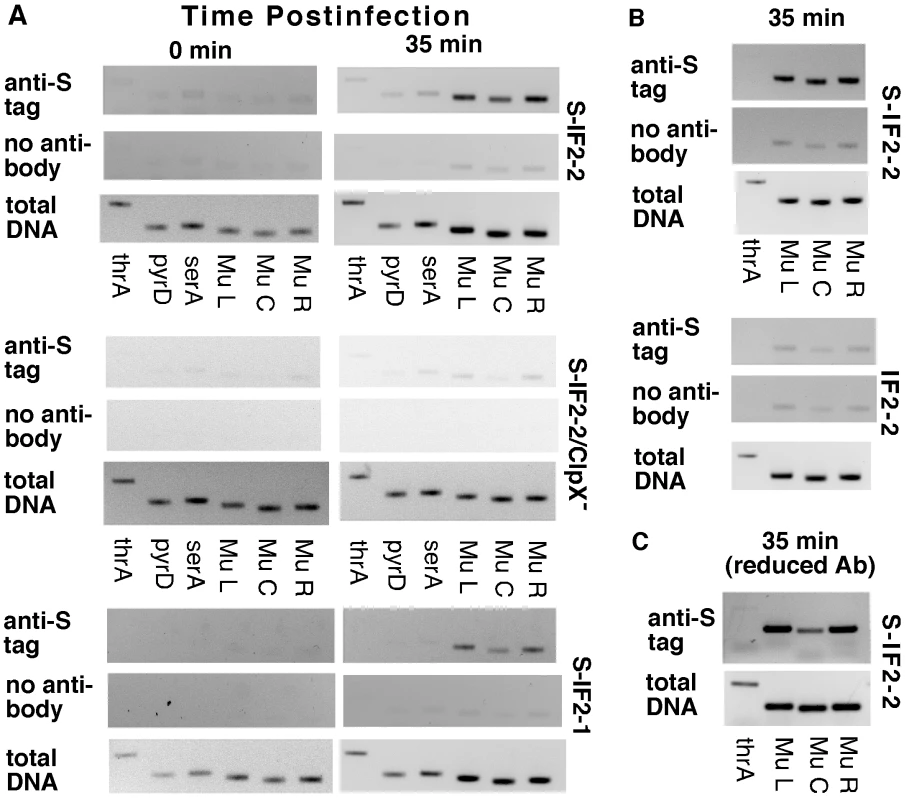
Mu development in GTN373 (his::Mucts62 priA300 del(gpt-lac)5) and GTN622 (his::Mucts62 priA300 clpX::kan del(gpt-lac)5) transformed with pBAD24-S-IF2-1, pBAD24-S-IF2-2, or pBAD24-IF2-2 were induced by incubation at 42°C, and immunoprecipitation with anti-S-tag monoclonal antibody was conducted with samples from 0 and 35 min postinduction. The presence of host and Mu DNA sequences in immunocomplexes were detected by amplifying 200–400 bp segments of template DNA (2 µl of indicated DNA dilutions in 10-µl reactions) in a 26-cycle PCR, using primers for amplifying thrA, pyrD, serA, Mu left end (L), Mu center (C), and Mu right end (R). A) Pull-down of Mu sequences by anti-S-tag antibody during Mu development. 1:20 dilutions of the immunoprecipitation (IP) and the no antibody control and 1∶2500 dilutions of total DNA were used. B) Comparison of IP with S-tagged and untagged IF2-2. GTN373 transformed with the indicated plasmids were used. C) Binding of S-IF2-2 concentrated at or near Mu ends. Analysis was conducted with induced pBAD24-S-IF2-2/GTN373, using one-fifth the standard amount of antibody. To ensure that the anti-S tag antibody was specifically precipitating Mu DNA bound to S-IF2-2, we compared the co-precipitation of Mu DNA (35 min postinduction) in induced lysogens expressing S-IF2-2 and untagged IF2-2 (Figure 2B). When the IF2-2 had no S tag, no more Mu DNA was captured in the immunoprecipitation than in the no-antibody control.
The results indicate that not only truncated IF2-2/3 but also full-length IF2-1 bind at or near Mu ends upon induction of Mu development, corroborating the role IF2 plays in vitro in promoting initiation of Mu DNA replication by restart proteins. In vitro, IF2 makes way for the binding of PriA [5], which binds to forked DNA structures [29], [30] such as the Mu fork, and PriA subsequently displaces IF2 from Mu DNA. The ChIP analysis by itself can only indicate a preponderance of IF2 binding around the Mu ends and does not rule out the possibility that IF2 binds at nearby sites. Nevertheless, these results together with the role IF2 plays in vitro strongly suggest that there are IF2 molecules bound at the Mu fork during lytic development. The role played by IF2 in Mu replication raises the question whether IF2 function can regulate the engagement or activity of restart functions.
A deletion mutant that cannot express the full-length IF2 is unable to grow in the presence of MMS
We constructed a series of strains with infB alleles that only allow expression of full-length IF2-1 or the truncated forms IF2-2/3 to examine their effect on restart functions. The infB alleles were introduced into the chromosome where a transposon vector was inserted, and then the natural infB allele was knocked out by introduction of the del(infB)1::tet allele, which precisely deletes the natural cistron for IF2 (Figure 3A–3B). To prevent the expression of IF2-1, we deleted sequences around the translation initiation start site for IF2-1. Sequences from 14 nucleotides upstream of the IF2-1 start codon to 32 nucleotides upstream of the IF2-2 start codon were deleted (Figure 3B); this is known to permit expression of the truncated IF2 forms while eliminating IF2-1 expression [21]. The resulting allele, denoted as infB(del1) to indicate that the deletion prevents expression of IF2-1, supports the synthesis of only IF2-2 and IF2-3. Expression of the truncated IF2 forms were prevented by changing the start codons of IF2-2 and IF2-3, gug to guc (g474c) and aug to acg (t494c); these mutations have previously been shown to eliminate expression of the truncated forms while leaving a functional IF2-1 [21]. We shall refer to this allele as infB(del2/3) to indicate that the mutations prevent expression of IF2-2 and IF2-3 even though del2/3 is not a deletion mutation. The resulting infB del1, del2/3, and wild-type (wt) alleles were introduced into the transposon site as part of the nusA infB operon (<nusAinfB> to signify that this is encoded within the transposon).
Fig. 3. Introduction of a second single-copy infB allele and knockout of the natural allele. 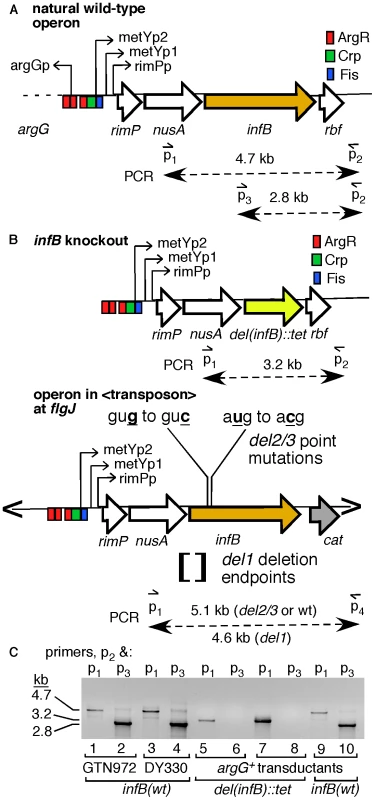
A) The natural nusAinfB operon. The operon is driven by the three indicated promoters, and the multiple cistrons are, starting from promoter proximal genes, metY, rimP, nusA, infB, rbfA, truB, rpsO, and pnp (not all shown). PCR primers used to specifically amplify the natural infB and not other copies of infB introduced in the cell are shown. B) The del(infB)1::tet allele and nusA infB allele harbored on an EZ-Tn5 transposon. The knockout allele precisely excises the infB cistron and replaces it with the tet cistron. The essential infB function is provided in single-copy form in a transposon (“<” and “>” represents transposon ends) integrated in flgJ. The transposon encodes chloramphenicol resistance (cat), allowing easy transfer to other cells, and the nusAinfB operon that includes the three ArgR binding sites up to the stop codon for infB. C) PCR analysis to detect knockout of the natural infB allele. DY330 is the strain used to engineer the del(infB)1::tet allele; GTN972 (GTN932 del(argG)781::kan) is the recipient strain for P1vir transduction from DY330del(infB)1::tet<infB>. Three typical GTN932 ArgG+ transductants are shown, two that coinherited del(infB)1::tet and one that did not (infB(wt)). The infB alleles of all mutants were amplified using the locus-specific primers and sequenced for verification. The natural infB allele could be readily knocked out by introducing the del(infB)1::tet allele when the operon in the transposon had infB(wt), infB(del1), and infB(del2/3) alleles. (The procedures for verifying deletion of the natural infB allele as illustrated in Figure 3C and for verifying infB alleles by PCR and sequencing will be described under Materials and Methods.) The <infB(del2/3)> and especially the <infB(del1)> strains display some measure of cold sensitivity, growing very slowly at 25°C and below, consistent with previous reports about strains with analogous alleles [21]. We determined that the strain with the single copy <infB(del1)> as sole allele was highly sensitive to MMS whereas the strains with <infB(wt)> and <infB(del2/3)> as sole alleles were not (Figure 4A and Figure S1A).
Fig. 4. MMS sensitivity of the <infB(del1)> mutant and complementation by other infB alleles. 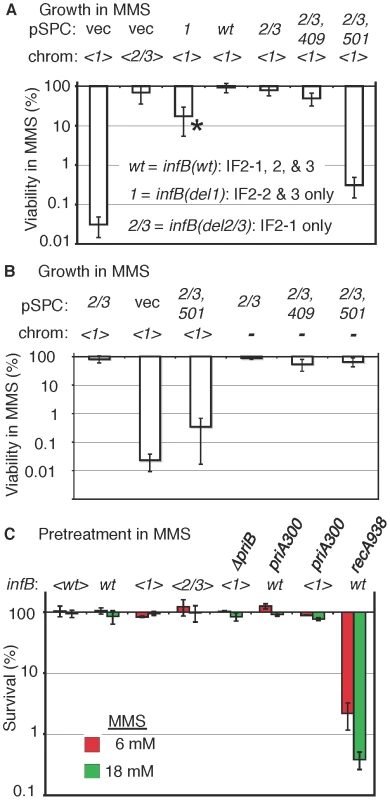
A) GTN1114 del(argA)743::kan (GTN1156) and GTN1115 del(argA)743::kan (GTN1157) bearing pSPCnusAinfB plasmids that contain infB(wt), infB(del1) (1), infB(del1) (2/3), or infB(del2/3,D409E) (2/3,409), or no infB (vec) allele. The asterisk (*) indicates that the strain required 40–42 h incubation (37°C) on MMS plates to yield sufficiently large colonies that could be counted, indicating especially slow growth on these plates (all other strains required 16–20 h incubation). The results are the average of 6–7 independent experiments. B) Multicopy infB(del2/3,D501N) allele can support growth in MMS provided that the chromosomal <infB(del1)> allele is not present. Viability of GTN932 infB(del1) del(infB)1::tet (GTN1114) and GTN932 del(infB)1::tet bearing the indicated pSPCnusAinfB plasmids (see the legend to panel A for the key) with and without MMS was determined (5–6 independent experiments). C) Sensitivity measured by 15-min exposure of cells at 6 mM and 18 mM MMS and plating in the absence of MMS (3–4 independent experiments). Strains with the indicated infB allele in the chromosome or transposon (<>) were used: GTN1050 (<wt>), GTN932 (wt), GTN1114 (<1>), GTN1115 (<2/3>), GTN1117 (<1> and del(priB)302), GTN381 (wt and priA300), GTN1323 (<1> and priA300), and GTN1376 (wt and recA938). The results indicate that the del1 mutation causes the inability to grow in the presence of MMS. The question is whether this is due to a general deficiency in repair, recombination, and restart functions, resulting from a generally deficient translation initiation function, or whether there is any specificity of the defect. We should note that the <infB(del1)> strain (ArgA−) was at least moderately proficient in homologous recombination measured by P1 transduction, although the frequency of Arg+ transductants was reduced approximately 5 fold compared to <infB(wt)> and <infB(del2/3)> strains (Figure S1B). While some reduction in homologous recombination frequency may be part of the phenotype of this strain, the reduction seen here is modest compared to the 20–50 fold reduction in P1 transduction demonstrated for the priA knockout strain [31].
The full-length IF2-1 is necessary for growth in the presence of MMS
To determine whether it is indeed IF2-1 that is needed to maintain MMS-resistance, we complemented the <infB(del1)> allele of strain GTN1156 with the infB(del2/3) allele, harbored as part of a nusA infB operon on a plasmid with a pSC101 replicon, pSPCnusAinfB(del2/3). While the empty plasmid vector could not confer MMS-resistance and homologous recombination proficiency, IF2-1 expressed from pSPCnusAinfB(del2/3) did restore high viability on MMS plates (Figure 4A). In contrast, IF2-2/3 expressed from the plasmid-borne infB(del1) allele only partially restored viability on MMS plates (Figure 4A). While the multicopy infB(del1) allele did increase dramatically the viable count on MMS plates, the colonies grew up very slowly, and the viable count on these plates was still 5–10 fold lower than that of the strain with the multicopy infB(del2/3) allele (Figure 4A). These results illustrate functional differences between IF2-1 and IF2-2/3 in promoting recovery after MMS treatment. They also indicate that IF2-2/3 when expressed from a multicopy vector may compensate for the lack of IF2-1, albeit inefficiently.
To confirm that it was not just the DNA segment deleted in the infB(del1) allele but the full-length IF2-1 protein that was needed for complementation, we introduced IF2 G domain mutations, infB(c1227a) or (c1501a), which result in the IF2 D409E and D501N alterations, respectively, into pSPCnusAinfB(del2/3). The infB(D409E) allele is an example of a viable G mutant that is functional at 37°C [32] whereas infB(D501N) is a recessive allele that is lethal as a single-copy gene [33]. Introduction of pSPCnusAinfB(del2/3,D409E) into GTN1156, but not pSPCnusAinfB(del2/3,D501N), restored MMS-resistance (Figure 4A). IF2-1 must therefore be providing the function needed for viability in MMS. The level of homologous recombination in the <infB(del1)> mutant, examined by P1 transduction, could also be increased by supplying the various infB alleles on the plasmid vector (Figure S1C). Due to the relatively modest effect on homologous recombination, this aspect of the infB(del1) mutant was not further examined.
Dominant negative effect of the <infB(del1)> allele over multicopy infB(del2/3,D501N)
The <infB(del1)> strain, which produces only IF2-2/3 and has extremely low viability in the presence of 6 mM MMS, attains high viability when complemented with the plasmid-borne infB(del2/3) allele, which restores IF2-1 production (Figure 4A and 4B). This indicates that the multicopy infB(del2/3) allele is dominant over the <infB(del1)> allele. The inability of pSPCnusAinfB(del2/3,D501N) to restore efficient growth of the <infB(del1)> strain in MMS could indicate the inactivation of a necessary function of IF2-1 by the D501N mutation. Alternatively, the <infB(del1)> allele may be dominant negative over the multicopy infB(del2/3,D501N) allele in terms of supporting growth in MMS.
Although the D501N mutation is lethal when present as a single-copy infB allele, this mutation is recessive to the wild-type allele [33]. We therefore tested whether the multicopy infB(del2/3,D501N) allele on the plasmid could support viability by itself. The natural infB allele in strain GTN932 that bears plasmids pSPCnusAinfB(del2/3), pSPCnusAinfB(del2/3,D409E), or pSPCnusAinfB(del2/3, D501N) could readily be knocked out, leaving the infB on the plasmid as the sole allele in the cell. This allowed us to test whether or not IF2-1(D501N), expressed from multicopy <infB(del2/3, D501N)>, is defective in a function that IF2-1 provides but IF2-2/3 fails to perform. Although the strain with the multicopy infB(del2/3,D501N) as the sole allele grew relatively slowly, requiring at least twice the incubation time as the other two strains for growth, it was clearly viable and also retained significant viability on MMS plates, comparable to viability of analogous strains with infB(del2/3) and infB(del2/3,D409E) as sole alleles (Figure 4B). That is, the multicopy infB(del2/3,D501N) is able to support high viability in MMS so long as the <infB(del1)> allele is absent. Introduction of the D501N mutation to the multicopy infB(del2/3) allele thus results in loss of dominance over <infB(del1)>, not in the loss of a function needed to maintain viability in MMS. These results suggest that IF2-2/3, at levels produced from the single-copy <infB(del1)> allele, is performing a function in a way that aggravates problems which the cells encounter during growth in MMS, outcompeting IF2-1(D501N) that is able to carry out the function appropriately to maintain viability. In other words, IF2-2/3 does not necessarily lack the capacity to perform the IF2-1 function. Rather, it appears to carry it out in a way that dramatically reduces viability. That is, the recessive properties of infB(D501N) with respect to the infB(del1) allele, including its ability to support resistance to MMS as the sole multicopy allele, suggest that MMS sensitivity of the <infB(del1)> strain is not simply due to a general deficiency in translation initiation function when only IF2-2/3 is present.
Characteristics of MMS sensitivity of the <infB(del1)> mutant, an attribute shared by the priA300 mutant
We next determined whether the MMS sensitivity of the <infB(del1)> mutant reflected deficiency in the levels of repair or restart proteins in these mutants. In the analysis described above, MMS resistance was measured by growth of cells on plates containing MMS. By this analysis, cells must not only survive initial exposure to the DNA-damaging agent but also grow into colonies in its presence. We also measured the ability of strains exposed to MMS to recover and grow in the absence of MMS in order to assess their capacity to repair DNA lesions and restart DNA replication. Strains that are defective in genes such as priA, recA, and polA that participate in DNA repair or replication restart are known to be quite sensitive as measured by initial exposure for 15 min in MMS and plating without MMS to determine the number of survivors; the alkA tag mutant, which is defective in a major mechanism for repairing alkylated bases (base excision repair), is also sensitive to MMS by this criteria [34]. The <infB(del1)> mutant was as resistant to MMS as infB(wt) strains by this criteria (Figure 4C), with MMS resistance comparable to strains with natural infB, <infB(wt)>, and <infB(del2/3)> alleles; in contrast the recA938 mutant was highly sensitive by this criteria (Figure 4C). It should be noted that when cells were deficient in both the PriA-PriB and PriA-PriC pathway (deficient in both PriB and PriC), they had very low viability even without MMS treatment (Figure S2A). As restart mutants tend to have very low viability even without MMS, we measured MMS sensitivity of a del(dnaT)759::kan mutant with a dnaC(a491t) suppressor mutation, which greatly increases cell viability. Even with the suppressor mutation, the dnaT knockout strain was significantly more sensitive to the 15-min MMS treatment (Figure S2B) than the <infB(del1)> mutant. These results indicate that levels of repair and restart factors in the <infB(del1)> strain are sufficient for the recovery of DNA replication and cell growth after DNA damage by MMS. However, there was a 1000-fold reduction in viability of the <infB(del1)> mutant on 6 mM MMS plates (Figure 5A). That is, the <infB(del1)> strain is proficient in repairing DNA damage and resuming DNA replication after the 15-min exposure in MMS, but it is severely defective in its ability to sustain growth in MMS. Thus, the <infB(del1)> mutant is not able to cope with the sustained damage to DNA during cell growth. This could indicate that a repair or restart factor, although not deficient, is sufficiently low such that it cannot keep up with constant DNA damage inflicted on MMS plates; alternatively, it is possible that the regulation of repair and restart processes are not appropriate for efficiently supporting DNA replication under these conditions.
Fig. 5. Characteristics of MMS sensitivity exhibited by <infB(del1)> mutants and similarity to priA300. 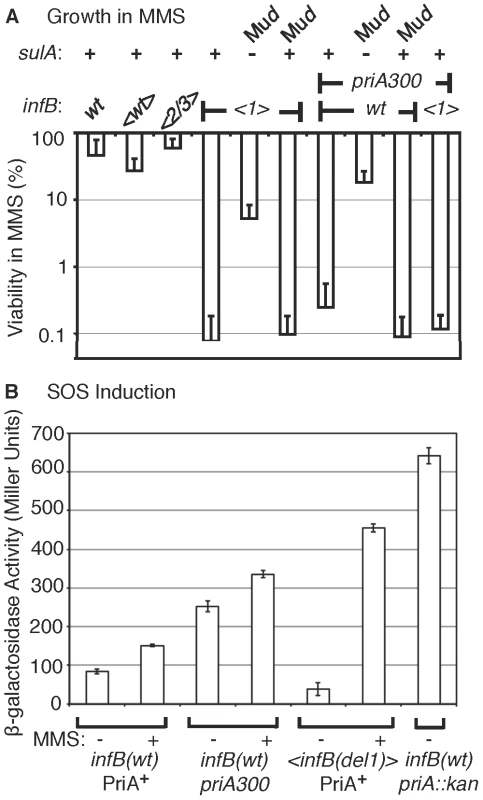
A) Sensitivity of <infB(del1)> and priA300 mutants for growth in MMS and suppression by a sulA mutation. The indicated strains are the same as those listed in the legend to Figure 4C. In addition, the sulA::Mud(lac,Ap,B::Tn9) allele is present in GTN1387 (<infB(del1)> SulA−) and GTN1384 (priA300 SulA−), and the Mud(lac,Ap,B::Tn9) is integrated in a site other than sulA in GTN1399 (infB(del1) SulA+) and GTN1396 (priA300 SulA+). Viability of GTN1376 (recA938) on MMS plates was less than 10−5%. Results are the average of at least 3 independent determinations. B) SOS induction monitored using the sulA::lacZ reporter. GTN1385 (infB(wt) PriA+), GTN1384 (priA300), and GTN1387 (<infB(del1) PriA+) were grown in LB to OD600 of 0.3. To 2-ml portions of each culture, MMS was added to 18 mM final concentration. β-galactosidase activity in MMS-treated (+) and untreated (−) cultures was measured. β-galactosidase activity of untreated GTN1639 (GTN1385 priA2::kan) is shown for comparison. Introduction of the sulA::Mud(lac, Ap, B::Tn9) allele greatly restored viability of the <infB(del1)> mutant in MMS (Figure 5A). The sulA gene, which is a component of the SOS system induced by DNA damage, is a cell division inhibitor [35]. In mutants such as the priA null strain, which has a constitutively induced SOS system, the high expression of sulA results in loss of viability, which can be largely restored by sulA mutations [36]. It is important to note that the sulA::Mud(lac, Ap, B::Tn9) allele did not fully restore viability to the <infB(del1)> mutant. Moreover, scorable colonies on MMS plates required incubation for over 36 hours at 37°C whereas infB(wt) colonies readily arose in 16 hours. That is, the sulA mutation did not fully revert <infB(del1)> to the wild-type phenotype.
Interestingly, the priA300 mutant had a phenotype much like the <infB(del1)> mutant, resistant to MMS when exposed to MMS and plated in its absence but highly sensitive when plated on 6 mM MMS plates (cf. Figure 4C with Figure 5A). The priA300 allele encodes for a helicase-deficient PriA that is fully proficient in primosome and replisome assembly by the PriA-PriB pathway [19], [37]. The priA300 mutant has previously been shown to have essentially a wild-type phenotype unless that mutation is combined with mutations affecting other restart functions such as priB; wild-type properties of the priA300 mutant include homologous recombination proficiency and relatively high UV resistance [19], [38]. As with the <infB(de11)> mutant, the sulA::Mud(lac,Ap,B::Tn9) allele could restore viability of the priA300 mutant in MMS (Figure 5A). In addition, priA300 was epistatic with the infB(del1) allele, causing no significant increase in MMS sensitivity (Figure 4C and Figure 5A). In contrast, the <infB(del1)> del(priB)302 combination (GTN1117) was synergistic, reducing viability to 0.010±0.002% on the MMS plates. The priB knockout alone did not have such a severe effect; the <infB(wt)> del(priB)302 strain (GTN1133) had a viability of 43±8% on MMS plates. In addition, knockout of priC did not increase UV sensitivity; the <infB(wt)> del(priC)752::kan strain (GTN1059) had a viability of 84±7% on MMS plates. These results indicate that the PriA-PriC pathway, which requires PriA helicase, is not solely responsible for allowing cell growth in the presence of MMS and that the PriA-PriB pathway most likely makes a significant contribution to mechanisms dependent on PriA helicase as well. We shall further examine the interactions of priA300 and del(priB)302 with the <infB(de11)> and <infB(de12/3)> alleles by UV sensitivity. The epistatic relationship between the infB(del1) and priA300 alleles suggests that the loss of IF2-1 specifically affects the activity or engagement of factors in restart pathways that require PriA helicase.
Despite the high MMS sensitivity of the <infB(del1)> strain, it did not resemble the priA knockout mutant in terms of having constitutively high levels of SOS induction (Figure 5B). Expression from the sulA::lacZ SOS reporter was significantly lower than the strain with wild-type priA and infB and the priA300 strain. The latter strain had moderate basal levels of SOS induction, which was significantly less than that of the priA knockout. Treatment of the wild-type and priA300 strains with 18 mM MMS elicited moderate increases in SOS expression; in contrast, treatment of the <infB(del1)> strain elicited over a 10-fold increase in SOS expression, consistent with the role of SOS induction reducing the strain's viability upon MMS treatment,
UV sensitivity of <infB(wt)>, <infB(del1)>, and <infB(del2/3)> strains and epistasis analysis with restart functions
Although the <infB(del1)> strain was sensitive to growth in MMS, it was slightly more resistant to UV light than the <infB(wt)> strain (Figure 6A). In fact, the <infB(del2/3)> mutant, which was found to be the most MMS-resistant, was slightly more UV sensitive than the <infB(wt)> strain (Figure 6A). These results do not rule out the possibility that the del1 and del2/3 mutations impair or knock out restart mechanisms engaged after UV irradiation. As there are multiple restart pathways in the cell, the PriA-PriB and PriA-PriC pathways being the two major ones [18], the del1 or del2/3 mutation may predominantly affect only one pathway but not the other. To test this possibility, we examined the effect of the infB alleles in combination with priB or priC knockout alleles.
Fig. 6. UV sensitivity of <infB(del1)> and <infB(del2/3)> and epistasis analysis with priB and priC. 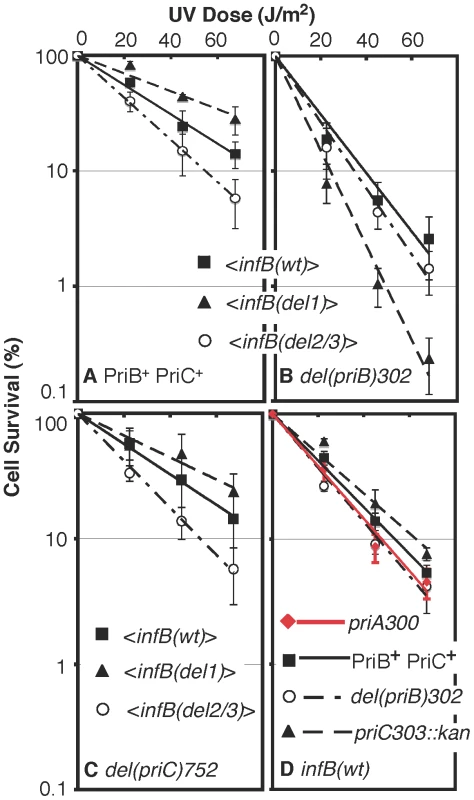
A) UV sensitivity of <infB(wt)> (GTN1050), <infB(del1)> (GTN1114), and <infB(del2/3)> (GTN1115) mutants. B) UV sensitivity of mutants with del(priB)302 and the <infB(wt)> (GTN1133), <infB(del1)> (GTN1117), or <infB(del2/3)> (GTN1119) alleles. C) UV sensitivity of mutants with del(priC)752 and the <infB(wt)> (GTN1059), <infB(del1)> (GTN1135), or <infB(del2/3)> (GTN1137) alleles. D) As reference, the effect of priA300 (GTN381), del(priB)302 (GTN394) and priC303::kan (GTN387) alleles in the GTN932 genetic background was examined. The priB and priC knockout alleles were the first knockout mutations of these genes to be characterized [39]. All results are the average of at least 3 independent determinations. It is well established that the knockout of priB or priC has little to no effect by itself [39] in contrast to the priA or dnaT knockouts, which affects both major restart pathways and elicits high sensitivity to DNA-damaging agents and low viability [18], [36], [40]. As expected, neither the priB nor priC knockout had any effect on UV sensitivity when introduced into the parent strain (GTN932) used to construct the various <infB> mutants (Figure 6D). While the del(priC)752 allele had absolutely no effect on single-copy <infB(wt)>, <infB(del1)>, and <infB(del2/3)> strains (Figure 6C; cf. with Figure 6A), the del(priB)302 clearly had a synergistic effect with the infB(del1) mutation to elicit relatively high UV sensitivity (Figure 6B). This finding that the priB knockout, but not the priC knockout, is synergistic with the <infB(del1)> allele to increase UV sensitivity indicates that the loss of full-length IF2-1 diminishes the PriA-PriC pathway for recovery after UV irradiation. Introduction of a pBAD24-priB plasmid into the del(priB)302 <infB(del1)> strain (GTN1117), allowing the expression of PriB driven by the PBAD promoter with arabinose as inducer, increased its UV resistance to levels comparable to the del(priB)302 <infB(wt)> strain (GTN1133; Figure 7A), confirming that the deficiency of GTN1117 can be reversed by expressing PriB. This indicates that the activity of repair and restart proteins needed for recovery after UV irradiation in GTN1117, which has the <infB(del1)> allele, is comparable to that in GTN1133, which has the <infB(wt)> allele. Therefore, the increased UV sensitivity of GTN1117 with respect to GTN1133 is most likely due to some type of deficiency in the PriC-dependent pathway. We were unable to measurably increase UV resistance by expressing PriC from pBAD24-priC (Figure 7A). Indeed, PriC in its active form must be present in GTN1117. When the chromosomal priC was knocked out in pBAD24-priC/GTN1117 (GTN1566), expression of PriC from the plasmid vector became essential for viability with or without pre-treatment with MMS (Figure S2A), viability being less than 0.1% in the presence of glucose. In the presence of arabinose, viability of GTN1566 with or without MMS treatment was comparable to the strain with an intact chromosomal priC. That is, active PriC can be expressed from pBAD24-priC or the chromosomal priC gene in the <infB(del1)> genetic background, and supplementation of PriC expression in GTN1117 from the plasmid cannot restore any measure of UV resistance. These results suggest that its relatively high UV sensitivity is not caused by a deficiency in PriC, PriA, and DnaT.
Fig. 7. Epistasis analysis of infB alleles with del(priB)302 and priA300. 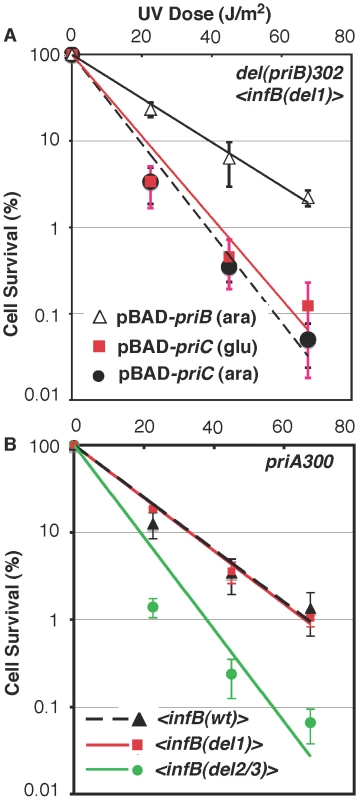
A) UV sensitivity of GTN1117 expressing priB or priC from pBAD24. GTN1117, which is del(priB)302 <infB(del1)>, bearing plasmid pBAD24-priB or pBAD24-priC were grown up in LB medium containing 100 µg/ml ampicillin and 0.02% L-arabinose or 0.2% D-glucose as indicated. After UV irradiation, cells were plated on 0.02% arabinose/LB and plain LB plates for viability, both of which produced identical results. B) Interaction of infB alleles with priA300. Survival of priA300 strains with the <infB(wt)> (GTN1298), <infB(del1)> (GTN1323), and <infB(del2/3)> (GTN1297) after UV irradiation was measured. Although the del(priB)302 <infB(del1)> strain (GTN1117) has high UV sensitivity, its ability to recover after a 15-min exposure to MMS was comparable to the wild-type control (Figure 4C). Moreover, Mu plating efficiency on this strain is not dramatically reduced, indicating that the PriA-PriC pathway can promote Mu replication in the absence of IF2-1 (Figure S3), a result consistent with properties of Mu replication in vitro [5]. In general, the Mu plating efficiencies on the various <infB(wt, del1, or del2/3)> strains, whether in the PriB+PriC+, del(priB)302, or del(priC)752::kan genetic backgrounds, were nearly the same. These results indicate that restart proteins needed to promote Mu replication by the PriA-PriC pathway are present at sufficient levels to support lytic development. They also suggest that the defect of the infB(del1) allele is not a deficiency in restart activity needed for recovery but rather in the regulation of restart activity needed to maintain replication in the presence of the DNA-damaging agent.
Although the effect is not as much as in the <infB(del1)> background, the del(priB)302 allele also did significantly increase UV sensitivity when introduced into the <infB(wt)> background (cf. the solid square data points in Figure 6A and 6B) whereas it had essentially no effect in the natural infB(wt) background (Figure 6D). This may reflect a small change in relative or absolute levels of full-length and truncated IF2 when the infB allele is expressed from the transposon site, a change that has no discernible effect unless specific restart mechanisms are inactivated as with the del(priB)302 mutation. Interestingly, in the <infB(wt)> background both the priA300 (Figure 7B) and the del(priB)302 (Figure 6B) allele increased UV sensitivity to the same level. Like the del(priB)302, the priA300 allele is known to have little effect on UV sensitivity [19], and indeed we found essentially no effect of the priA300 allele in the GTN932 background (Figure 6D), which has the natural infB(wt) allele. As we described above, the priA300 and <infB(del1)> alleles both independently elicit sensitivity to growth in MMS, and the two mutations are epistatic for this trait, consistent with a model in which PriA helicase and IF2-1 function in the same pathway to maintain efficient growth in MMS. In the UV sensitivity analysis, the infB(del1) allele was also found to be epistatic with priA300, not being able to elicit further UV sensitivity in the priA300 background (Figure 7B). That is, loss of IF2-1 attenuates pathways dependent on PriA helicase such as the PriA-PriC pathway. In contrast, the infB(del2/3) allele was synergistic with priA300 to increase UV sensitivity (Figure 7B). The results indicate that loss of IF2-2/3 from the infB(del2/3) allele results in deficiency of a restart pathway that is distinct from the IF2-1/PriA helicase-dependent pathway. Mu plating efficiency on the three priA300 strains with the <infB(wt)>, <infB(del1)>, and <infB(del2/3)> were essentially the same, the titer obtained on the latter two strains being greater than 90% of the titer on the priA300 <infB(wt)> strain. Thus, as with the del(priB)302 <infB(del1)> combination, which also synergistically contributes to high UV sensitivity, the priA300 <infB(del2/3)> combination does not lead to an inability to initiate Mu replication by the available host restart machinery.
Sporadic SOS induction of the del(priB)302 <infB(del1)> mutant
What is notable about the UV sensitivity analysis is that the combination of priA300 <infB(del2/3)> or del(priB)302 <infB(del1)> mutations does not produce the extremely severe phenotype of the priA300 del(priB)302 combination, which elicits a phenotype analogous to the priA knockout [19]. That is, loss of IF2-1 or IF2-2/3 does not result in the inability to promote replication restart by the respective pathways they influence, but rather the loss of each IF2 isoform affects some mechanism needed to maintain high viability when the restart mechanism is engaged after DNA damage. However, under normal growth conditions or if cells are allowed to recover after MMS treatment or UV irradiation without the presence of DNA damaging agents, there is little effect of knocking out IF2 isoforms, and a mild effect is seen when these mutations are combined with the restart mutation del(priB)302 or priA300, which by itself has little effect under normal growth conditions. We examined the cell morphology of the various infB mutants to examine whether there is an increased incidence of sporadic SOS induction, leading to filamentation [41] of a small fraction of the cells in the population.
The strains with the single del(priB)302 or <infB(del2/3)> mutant had essentially wild-type morphology (Figure S4A), 100% of cells being 0–8 µm in length when at least 40,000 cells were analyzed. Cells with the single <infB(del1)> or priA300 mutation (GTN1114 and GTN1298, respectively) tended to be longer in size, with a higher incidence of moderate sized filaments (examples of moderate filaments are indicated by white arrows). In a sample of 1000 cells, 1% of the cells were in the 8–30 µm range for GTN1114 and GTN1298. Consistent with the relatively low basal levels of SOS expression measured for the <infB(del1)> mutant at the macroscopic level (Figure 5B), the level of its filamentation was quite low compared with that of the priA knockout mutant (Figure S4B), but the moderate filamentation suggests an increased incidence of sporadic SOS induction.
What was notable for the synergistic <infB(del1)> del(priB)302 combination (GTN1117) was that it gave rise to a low but significant frequency of very large filaments greater than 30 µm (Figure S4A). The incidence of filaments over 30 µm in size was found to be 0.13% in a screening of 33,000 total cells, most of these large filaments (0.10% of total cells) being over 50 µm in length. Only one other combination of an infB allele with the priA300, del(priB)302, or wild-type restart functions (Figure S4A) yielded any filaments over 50 µm in 100,000 cells screened. The mutant with the synergistic <infB(del2/3)> priA300 combination (GTN1297) produced filaments greater than 30 µm at a significantly lower frequency of 0.02% in a screening of 100,000 cells, of which only 3 were greater than 50 µm. Filaments in the 30–50 µm range also arose with the single <infB(del1)> or priA300 mutants (GTN1114 and GTN1298, respectively) but with a frequency of no more than one in 40,000 cells. No filaments of greater than 30 µm were detected with the <infB(wt)> (GTN1050), <infB(del2/3)> (GTN1115), del(priB)302 (GTN1133), and the <infB(del1)> priA300 (GTN1323) strains when at least 100,000 cells were examined. The results indicate that the <infB(del1)> del(priB)302 mutant (and, to a lesser extent, the <infB(del2/3)> priA300 mutant) has an increased incidence of very high SOS induction (leading to the formation of giant filaments) in a small fraction of the cell population growing in LB, suggesting a reduced capacity to cope with accidents that might occur during DNA replication for normal cell growth. However, these mutants clearly do not have the characteristics of extensive SOS induction as with a priA knockout strain such as GTN430 (Figure S4B; 3% of cells producing filaments greater than 30 µm in a sample of 4000 cells, 2% greater than 50 µm).
Characteristics of a restart mutant with a suppressor mutation in dnaC
The characteristics of strains such as the <infB(del1)> or priA300 mutant are more akin to a priA knockout strain that has acquired a suppressor mutation in dnaC (Table 1). GTN412, which is a Mucts62 lysogen, can support Mu replication upon thermoinduction to yield a high level of infective centers, has a high level of viability on MMS plates, and has a relatively low level of expression from its SOS reporter gene (dinD::lacZ). Introduction of the priA knockout decreased viability on MMS and formation of Mu infective centers by several orders of magnitude. The presence of a suppressor mutation in dnaC (GTN522) did diminish cell filamentation (Figure S4B; the number of filaments >30 µm are reduced to 0.05% from 3%, measured in a sample of 15,000 cells), reduce the level of SOS induction as indicated by the dinD::lacZ reporter, and restore the ability to form Mu infective centers, but this strain retained the severe sensitivity to growth in the presence of MMS, a central feature of the both the <infB(del1)> and priA300 mutants. This is consistent with the ability of the dnaC suppressor mutation to bypass the requirement for PriA to initiate DNA synthesis at forked DNA structures [38], [42]; however, without PriA the mechanism for promoting replication restart and promoting high viability in the presence of MMS (the IF2-1/PriA helicase-dependent pathway) appears to be compromised. In the same way, a priA300 or <infB(del1)> mutant may be able to promote replication restart by a less preferred pathway, which may permit replication restart to proceed but does not do so in a way that supports high viability during growth in the presence of MMS.
Tab. 1. Characteristics of a priA knockout mutant with a dnaC suppressor. 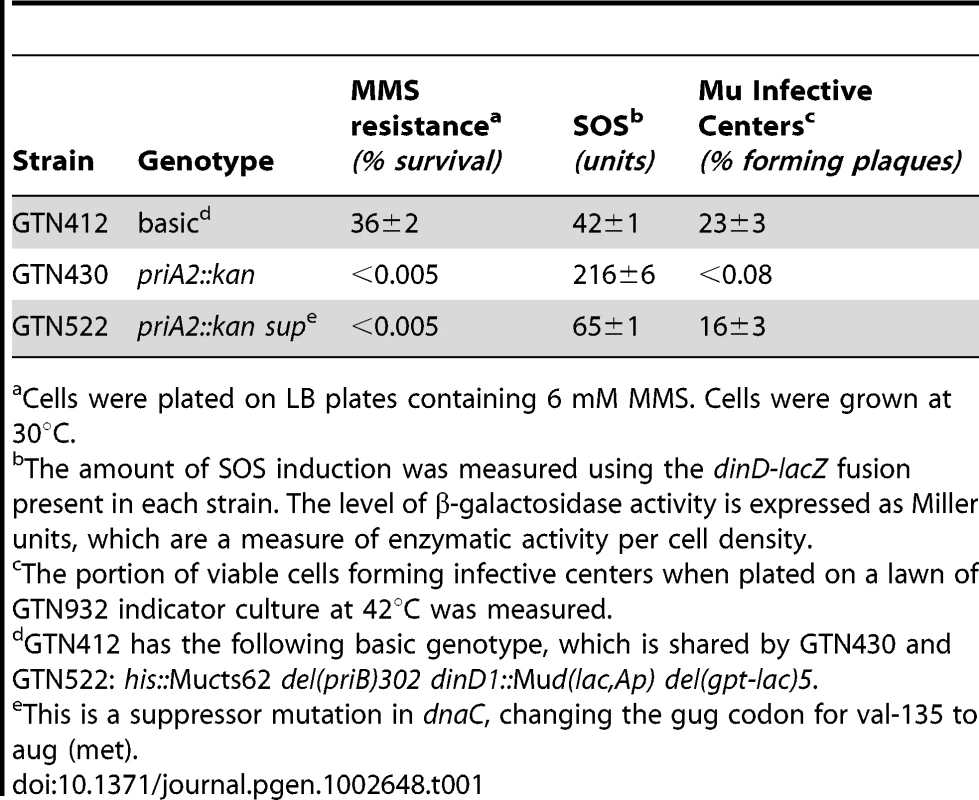
Cells were plated on LB plates containing 6 mM MMS. Cells were grown at 30°C. Discussion
Conclusions
The present work indicates a special relationship between the PriA helicase function and IF2-1 (see Table 2). Both the PriA helicase function and IF2-1 are required to allow cells to grow with maximal viability in the presence of MMS. Nevertheless, neither of these mutants display the severe characteristics of the priA knockout, having UV resistance that is comparable to wild type and being able to recover from MMS treatment with very high viability provided that it can do so in the absence of MMS. The defect of the priA300 mutant, previously shown to have nearly a wild-type phenotype [19], is a surprising new phenotype, being defective in the ability to grow in the presence of MMS but not in its ability to recover from MMS treatment. Even more surprising is the finding that the loss of the IF2-1 function elicits the same phenotype. Another characteristic which indicates that the infB(del1) allele affects some aspect of replication restart is the suppressing effect of knocking out sulA, a mutation that greatly increases viability of both the infB(del1) and priA300 mutant on MMS plates. Moreover, MMS treatment of inf(del1) mutant promotes an especially high level of SOS induction compared to the level promoted in wild type.
Tab. 2. Comparison of Attributes of <i>priA300</i> and <i>infB(del1)</i> mutant. 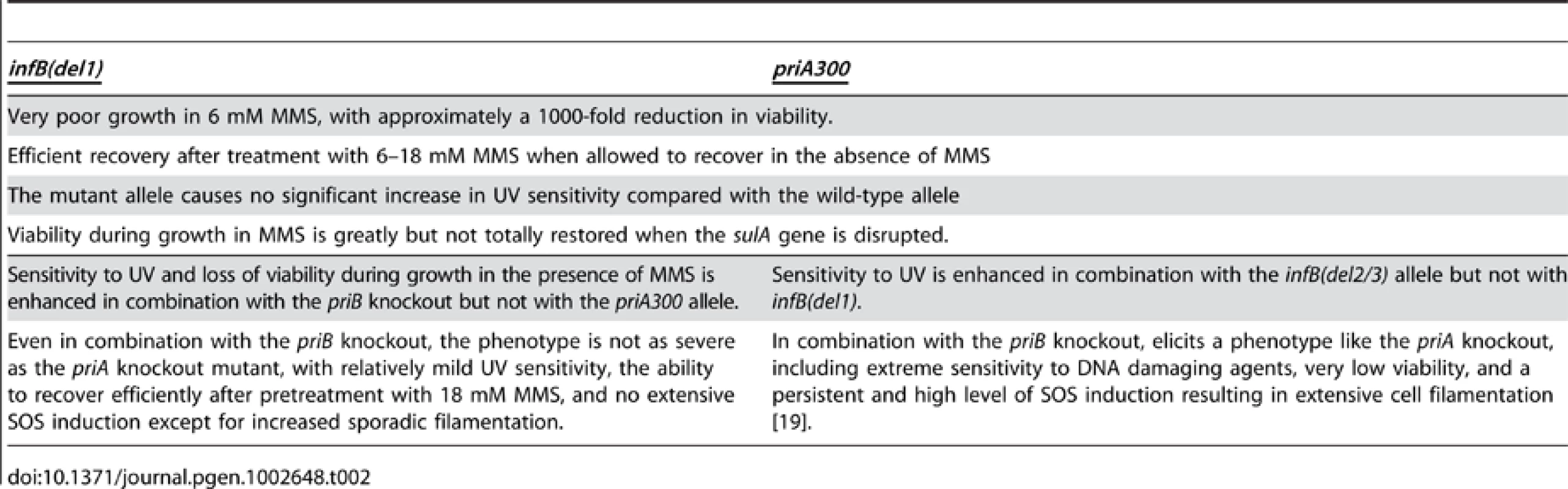
A relationship between full-length and truncated IF2 isoforms and replication restart functions is further indicated by UV sensitivity analysis. Both the <infB(del1)> and <infB(del2/3)> exhibit UV resistance comparable to wild type, but the combinations of <infB(del1)> del(priB)302 and <infB(del2/3)> priA300 significantly enhance UV sensitivity. Moreover, the <infB(del1)> del(priB)302 mutant (and, to a lesser extent, the <infB(del2/3)> priA300 mutant) display an increased frequency of sporadic SOS induction, indicated by the increased frequency of very long filaments over 30 µm. Clearly, the general population of these cells do not display the same high level of SOS induction of the priA knockout cells at the macroscopic level. The sporadic nature of filamentation is consistent with the thinking that these cells are mostly proficient in coping with accidents of DNA replication which may arise during normal growth conditions, unlike the priA knockout that copes with such accidents poorly. One would expect that only a small minority of cells would need to cope with a large number of DNA lesions during growth in LB unless a DNA-damaging agent such as MMS is present. The combination of <infB(del1)> del(priB)302 and <infB(del2/3)> priA300 alleles may sufficiently attenuate the major pathways that lead from DNA damage to replication restart, thus manifesting a modest but significant increase in sensitivity to UV irradiation.
The epistatic relationship between the priA300 and infB(del1) alleles revealed by both UV sensitivity and viability on MMS plates indicates that IF2-1 and PriA helicase function in common pathways as proposed in Figure 8A. This includes mechanisms in both the PriA-PriB and PriA-PriC pathway, for neither the priB or priC knockout has the severe effect of priA300 for growth on MMS plates. What remains of the major restart pathways when PriA helicase is inactive are mechanisms in the PriA-PriB pathway that can operate in the priA300 background [19]. Thus, the effect of the infB(del2/3) allele in this genetic background (increased UV sensitivity and increased incidence of sporadic cell filamentation) suggests that IF2-2/3 plays a role in this pathway. However, we have yet to find a phenotype for the infB(del2/3) allele alone, comparable to MMS sensitivity of the infB(del1) mutant, and whether IF2-2/3 is a key participant in PriA helicase-independent restart mechanisms (Figure 8A) remains be determined.
Fig. 8. Role of IF2 isoforms in the major replication restart pathways. 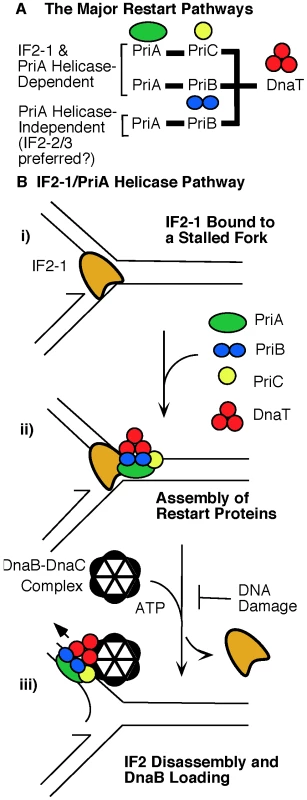
A) The major restart pathways and the influence of IF2 isoforms. Genetic analysis indicates that IF2-1 influences the restart pathways dependent on PriA helicase, including not only PriA-PriC pathway but also part of the PriA-PriB pathway. IF2-2/3 may play a prominent role in the pathways that do not require PriA helicase. The diagram should not be interpreted to indicate that IF2-2/3 cannot participate in reactions involving PriA helicase or that IF2-1 cannot participate in reactions where PriA's helicase is inactive. However, under these circumstances restart pathways do not function optimally to maintain maximal cell viability. The minor, less robust pathways such as the Rep-PriC pathway are not shown here. These PriA-independent pathways require suppressor mutations in dnaC to support a significant level of cell viability [18], [38], [39]. B) Model for the IF2-2/PriA helicase pathway. i) The starting point is a stalled replication fork with bound IF2-1. ii) Binding of restart proteins. The key protein to bind at this stage is PriA, which is poised to displace IF2-1 upon activation of its helicase activity. iii) Replisome assembly. PriA helicase action disassembles IF2-1 from the template, making way for initiation of DNA replication. It is hypothesized that removal of IF2-1 by PriA helicase can be regulated, extensive damage to the DNA template being able to inhibit this process and prevent replication restart. Removal of IF2-2/3 by PriA helicase bypasses this regulation, and IF2-2/3 may also be removed from the DNA by other mechanisms. Finally, the characteristics of the <infB(del1)> and priA300 mutants and especially the infB(del1) del(priB)302 double mutant are more like the priA knockout with a suppressor mutation in dnaC rather than the priA knockout with no suppressor. The <infB(del1)> and priA300 mutants, like the priA knockout with suppressor, do not exhibit the extreme sensitivity to UV irradiation, the massive cell filamentation, and the inability to support Mu replication that is characteristic of the priA knockout. Nevertheless, all of these mutants are not able to grow efficiently on media containing 6 mM MMS, their viability on MMS plates being approximately 0.1% or less. For the priA knockout, the dnaC suppressor allows replication restart to proceed, but the bypass of the restart proteins compromises maintenance of high cell viability when DNA replication proceeds during relatively high rates of DNA damage. Similarly, replication restart mechanisms can still operate in the <infB(del1)> mutant, and the lack of IF2-1 may bypass the preferred pathway that maintains high cell viability during growth in the presence of MMS. As IF2-1 and IF2-2/3 share 726 common residues, IF2-1 having 157–164 extra residues at the N-terminal end, it is quite conceivable that IF2-2/3 can replace IF2-1 in the IF2-1/PriA helicase-dependent pathway, allowing replication restart to proceed but lacking the function need to maintain high cell viability. The ability to grow under conditions that damage DNA at elevated levels could provide cells with the selective advantage that conserves the function of restart proteins despite the fact that suppressor mutations can bypass the need for these proteins. For example, the fact that the helicase motif of PriA is highly conserved among diverse bacteria [43] has been puzzling in light of the fact that its inactivation by the priA300 mutation seemed to have little effect on the cell phenotype, but the ability of cells with active PriA helicase to grow under conditions that damage DNA at a relatively high rate would indeed be a selective advantage that would conserve this motif.
The potential defect in restart function promoted by loss of IF2-1 and IF2-2/3
The phenotype of the <infB(del1)> mutant raises the question of what IF2-1 could be doing to influence cellular recovery after DNA damage by a PriA helicase-dependent pathway. First, IF2-1 and IF2-2/3 could have different preferences for mRNAs such that IF2-1 specifically promotes the translation of factors needed to support this pathway. Such a mechanism would be novel as such a role of the various IF2 isoforms in promoting differential gene expression has yet to be described. Second, IF2 may act as a transcription factor and the various IF2 isoforms may have different activity in this regard such that IF2-1 is specifically needed to regulate expression of genes needed for PriA helicase-dependent pathways. The finding that IF2 can selectively promote transcription of rRNA by RNA polymerase in vitro [26] and the identification of a region in the carboxy terminal region of IF2 with nonspecific DNA binding activity [27] have prompted the proposal that IF2 has activity influencing transcription. Third, IF2 has been shown to have molecular chaperone activity [25]. The IF2 isoforms may ensure that specific factors in their respective pathways are active when required. We have previously proposed a role of IF2 as a chaperone performing a function much like ClpX (Figure 1B–1C) where IF2 binds to a Mu end and prepares the DNA template for assembly of restart proteins, a process beginning with displacement of IF2 from DNA by PriA helicase. The analysis of this present work cannot definitively establish that any one of these possibilities is the basis for IF2's influence on cellular restart mechanisms; however, we favor the third mechanism in which IF2 acts as molecular chaperone, based on the role of IF2 in bacteriophage Mu replication in vitro [5], [17], the phenotype of the infB(del1) mutant, and the relationship of this allele with priA300.
A key question regarding the function of IF2-1 is, why does its loss lead to a severe decrease in viability during growth on MMS despite the fact that the cell remains proficient for supporting replication restart? We suspect that the loss of the preferred IF2 isoform for a restart mechanism, loss of PriA helicase activity, or the complete loss of PriA in the presence of a dnaC suppressor results in the inability to fine-tune the progression of restart pathways, a level of regulation that becomes essential when cells must grow under conditions that damage DNA at a high rate. If we speculate that the role of IF2 in Mu replication in vitro is applicable for cellular restart mechanisms, we can illustrate the type of regulation that IF2 might exert (Figure 8B).
An important difference between IF2-1 and the truncated forms IF2-2/3 for the assembly of restart proteins at stalled forks may be the mechanism by which they respond to a hypothetical go-ahead signal for restarting DNA replication. When DNA damage is accumulating at a relatively high rate, a mechanism that regulates restart by preventing re-establishment of the replication fork until the template is relatively free of DNA damage may ensure efficient DNA replication in the presence of a DNA-damaging agent. For example, restarting DNA replication before the DNA is relatively free of lesions will only result in the stalling of the fork again, causing delay in establishing a productive replication fork and thus inducing a high level of SOS response that may become toxic.
These considerations are reminiscent of the findings of Flores et al. [44], who determined that priA300 greatly diminishes viability of the holDG10 mutant. The holD gene encodes the Psi unit of DNA polymerase III holoenzyme, and the mutant Psi causes frequent replication fork stalling. That is, the effect of priA300 becomes discernible only when the rate of replication fork stalling becomes high. As noted by Flores et al. [44], the deficiency in PriA helicase caused by the priA300 mutation may lead to the inability to promote duplex opening on the DNA substrate for DnaB helicase loading and replisome assembly [29]; alternatively, another function of PriA besides the helicase could be inactivated by the priA300 mutation, leading to the inability to cope with frequent fork arrest in the holDG10 mutant. The PriA function needed to sustain high viability of the holDG10 mutant may be related to the pathway in which both IF2-1 and PriA helicase play a role. When cells must grow in the presence of MMS, the action of PriA helicase to displace IF2-1 may play a critical function to ensure maximal cell viability, or conceivably, the inactivation or attenuation of another function by priA300 may prevent what we call the IF2-1/PriA helicase pathway from operating optimally. This example underscores the possibility that PriA helicase as well as IF2-1 play multiple roles for replication restart, some of which may be part of their mutual participation in the IF2-1/PriA helicase pathway and some of which may not. PriA helicase may play important roles in duplex opening for DnaB loading as well as displacement of IF2 to initiate replication restart, but only the latter may be essential for the IF2-1/PriA helicase pathway.
The role of IF2 isoforms in influencing replication restart mechanisms has important implications for how replication restart and the maintenance of genome stability may be regulated with respect to cell physiology. As a translation factor, IF2 has a strong influence on cell growth and progression through the cell cycle while responding to cellular signals such as the alarmone (p)ppGpp [45], which is an indicator of nutritional deprivation. Depending upon the physiological status, how replication restart is carried out can be critical in determining cell viability, and IF2 may respond to cellular signals to determine the conditions for restart. The IF2 function in translation is a highly conserved one found in all living cells [46], [47]. Its role in influencing pathways for maintaining genome integrity prompts the question whether this general function has been conserved in other organisms to play some function in coordinating replication, recombination, and repair functions with respect to growth conditions.
Materials and Methods
Bacterial strains
All experimental analysis was conducted with derivatives of GTN932 (Hfr del(gpt-lac)5; see Table S1), an E. coli K-12 strain that is a derivative of PK191 [48]. We have conducted PCR and sequencing analysis to verify that this line of strains have wild-type relA, not the relA1 allele [49] as sometimes reported for PK191 strains. The del(priB)302 and priC303::kan alleles from JC19272 [39], priA2::kan from PN104 [36], del(priC)752::kan from JW0456-1, del(dnaT)759::kan dnaC(a491t) from JW4336-2, and del(argA)743::kan from JW2786-1 [50] were introduced into bacterial strains by P1vir transduction as previously described [39]. Inheritance of del(priB)752::kan was screened by PCR analysis with primers PriBupper and PriBlower (Table S2). The priA300 was introduced by P1 transduction, first transferring the metB1 allele by selecting for the closely linked btuB3191::Tn10 from CAG5052; the priA300 was then transferred from SS97 by selecting for Met+ transductants (tetracycline-sensitive transductants were chosen) [19], which were screened by PCR amplification with primers PriA-Nseq and PriA-Cseq and sequenced with revPriA820 primer. The sulA::Mud(lac,Ap,B::Tn9) from SS97 [18] or dinD1::Mud1(lac,Ap) from PN104 [36] was introduced into strains by P1 transduction and selection on ampicillin plates; transductants were screened for disruption of the sulA or dinD genes with primers sulAupper and sulAlower or dinDupper and dinDlower, respectively. The clpX::kan strain was constructed as previously described [15].
The del(infB)1::tet allele was constructed by first integrating a single copy nusAinfB operon into a random site of the host chromosome as part of the EZ-Tn5 transposon. The natural infB cistron was precisely excised and replaced with a tetR cistron from pACYC184 [51], using recombineering methods [52] to generate the del(infB)::tet allele. As recombination events at the natural infB site were very difficult to isolate, we created a PCR template to generate the del(infB)::tet allele, with approximately 1-kb of DNA from upstream and downstream of infB to flank the tet cistron. This template on the pGEM-Teasy vector (Promega) was amplified using PfuUltra High Fidelity DNA polymerase (Stratagene) using the primers nusLower and rbfUp2, and the PCR product was used to transform heat-induced DY330 flgJ::<nusAinfB-kan>.
The various flgJ::<nusAinfB-cat> alleles were constructed by introducing infB mutations into the nusAinfB operon harbored on the EZ-Tn5 transposon. The transposon was from the pMOD-6<KAN-2/MCS> purchased from Epicentre, and it was introduced into DY330 as a transpososome according to the instructions of the manufacturer. The transposon was determined to be integrated in the flgJ gene by a single primer PCR and sequencing method [53]. For introduction of various infB alleles at the transposon site, the transposon was modified by recombineering [52]. Heat-induced DY330<KAN-2/MCS> was transformed with a PCR product made by amplifying the cat gene of pACYC184 with primers DelMOD6Cat and lowerKanCat (see Table S2). The resulting strain DY330<del(kan)::cat)>, which is chloramphenicol-resistant and kanamycin sensitive, serves as the strain for introducing various alleles at this site.
PCR products for introducing the nusA infB operon at the transposon were made using pMOD-6<KAN-2/MCS> constructs as template. The nusA infB operon, amplified from the E. coli chromosome using PfuUltra High Fidelity with primers argRmetYp2 and IF2BamHI, was cloned between the SphI and XbaI site of pMOD-6<KAN-2/MCS> (promoter side of the operon is proximal to the SphI site). Various infB mutations were introduced into the resulting plasmid. The operon was then amplified using primers lowerMod6Tn and antiSqRP, and the PCR product was used to transform heat-induced DY330<del(kan)::cat)>, selecting transformed cells on LB plates containing 25 µg/ml kanamycin and screening for chloramphenicol sensitivity. To construct versions of these flgJ::<nusAinfB> alleles that encode chloramphenicol rather than kanamycin resistance, heat-induced DY330 flgJ::<nusAinfBkan> strains were transformed with PCR products made by amplifying the cat gene of pACYC184 with primers upperKanCat and lowerKanCat. This inactivates the kan gene while leaving intact the nusAinfB contained within transposons. The resulting constructs were always verified by sequencing as described below.
We could readily knock out the natural infB allele of a strain with the <nusAinfB(wt, del1 or del2/3)> cassette by introducing the del(infB)::TetR allele. As the expression of tetracycline resistance was relatively feeble from this allele, introduction of the knockout was most conveniently done by co-transduction with the closely linked argG; Arg+ transductants of a del(argG)781::kan recipient strain co-inherited the del(infB)::TetR allele at a frequency greater than 80%, provided that an infB allele which supports cell viability was provided from another site. Even when the second infB function was supplied by pSPCnusAinfB(del2/3,D501N), greater than 80% of the Arg+ transductants coinherited del(infB)1::tet allele, indicating that the multicopy infB(del2/3,D501N) can maintain cell viability (the presence of the D501N mutation in the sole infB allele was verified by sequencing). When the second nusAinfB operon was present on the chromosome, it was introduced into the transposon inserted in flgJ. The various flgJ::<nusAinfB-cat> alleles were constructed by recombineering methods in DY330 as described above and transferred to other strains by P1vir transduction. The nusAinfB operon contained within the transposon includes all three ArgR binding sites (see Figure 3A) and extends to the stop codon for infB.
As the nusAinfB operon in the transposon lacks downstream genes such as rbf in the natural operon, the infB alleles at the natural site and the transposon in flgJ can be separately amplified for DNA sequencing (Figure 3A–3C; primers p1 and p2 for the natural site and p1 and p4 for the transposon site). Thus, the presence of infB at the natural site could readily be detected by primers (p1 and p2) annealing to sites flanking infB to yield a 4.7-kb band (Figure 3C, lanes 1, 3, and 9), confirmed by 2.8-kb band yielded by one primer (p3) annealing within infB and one (p2) downstream of the gene (lanes 2, 4, and 9). (See the list of primers in Table S2.) Knockout of the natural infB, in contrast, could be detected with the formation of a 3.2-kb band with primers p1 and p2 (lanes 5 and 7) and no bands (lanes 6 and 8; cf. with lanes 2, 4, and 10) with p3 and p2.
We found this to be the best method for constructing strains with various single-copy infB alleles, for the replacement of the wild-type infB allele at the natural site proved to be very difficult. As constructed strains were suspected to be potential restart mutants, their dnaC allele was sequenced to determine whether any suppressor mutations have accumulated there [39]. None of the mutants we isolated had as severe a phenotype as the priA null mutant, and no suppressor mutations in dnaC were detected.
Plasmids
All pSPCnusAinfB plasmids with various infB alleles were constructed using the pBAD43 plasmid vector (a gift from Dr. Jonathan Beckwith, Harvard Medical School) [54]. This plasmid is a relatively low copy plasmid, having a pSC101 plasmid origin and conferring spectinomycin resistance. The nusAinfB operon, amplified by PCR using primers p1nusAinfB and IF2BamHI (see Table S2) and PfuUltra High Fidelity DNA polymerase, was inserted into the NsiI-BamHI site of the pBAD43 vector. The ara and PBAD sequences required for arabinose-based gene expression by this plasmid were deleted by digestion with NsiI-BamHI and replaced with the nusAinfB operon, which begins downstream of the metYp2 promoter, including the last 5 nucleotides of the Fis binding site and ending with the stop codon for infB. As a vector control for the pSPCnusAinfB plasmids, pBAD43 was used.
Construction of pBAD24 plasmids [55] that express IF2-1, IF2-2, and S-tagged IF2-2 (S-IF2-2) has been described previously [5]. The plasmid for expressing S-IF2-1 was similarly constructed by amplifying the infB gene using primers Stag-IF2-1 and IF2BamHI, which introduce the S-tag coding sequence. The coding sequence was ligated into the NdeI-BamHI site of a pBAD24 vector whose NcoI site has been modified to an NdeI site. The priB and priC genes were cloned into pBAD24, amplifying these genes using the NdeI-priB/PstI-priB and NdeI-priC/PstI-priC oligonucleotides and ligating into the NdeI/PstI site of the pBAD24 vector.
Site-specific mutagenesis was carried out using the QuikChange Lightning Multi-Site-Directed Mutagenesis Kit purchased from Stratagene, using primers listed for this purpose in Table S2. The infB(del1) deletion was generated by amplifying the nusAinfB operon harbored on a plasmid vector with 5′-phosphorylated primers delIF2-1UP and delIF2-LOW (see Table S2), with PfuUltra High Fidelity and circularizing the linear PCR product with T4 DNA ligase. All mutations were verified by sequencing.
Immunoprecipitation of IF2-DNA complexes
ChIP analysis was conducted by modification of previously published procedures [56], [57]. The major change was the incubation of cell lysate with 50 µg/ml RNase A at 37°C for 30 min just before the immunoprecipitation step. Additional details are described in Protocol S1.
Other methods
Sensitivity of strains to MMS was measured both by direct plating on LB plates containing 6 mM MMS and by 15 min exposure to 0–18 mM MMS, the latter based on the procedure by Nowosielska et al. [34]. β-galactosidase activity was measured according to the procedure of Miller [58]. Mu was plated on LB plates at 37°C with 10 mM magnesium sulfate on a background of indicator cultures. Mu infective centers from thermoinducible lysogens were plated on a background GTN932 indicator at 42°C. Mucts62 lysogens were grown at 30°C. Cultures of priA2::kan strains were maintained in Davis minimal medium (Difco) containing glucose, thiamine, proline, and histidine, and the viable count was determined on plates containing the same media.
All results from measuring MMS and UV sensitivity, homologous recombination proficiency, enzyme assays, and Mu plating efficiency are indicated with error expressed as the standard deviation from the mean (at least three independent experiments; the number of independent experiments is indicated). See Protocol S1 for additional details.
Supporting Information
Zdroje
1. LaursenBSSorensenHPMortensenKKSperling-PetersenHU 2005 Initiation of protein synthesis in bacteria. Microbiol Mol Biol Rev 69 101 123
2. CanonacoMACalogeroRAGualerziCO 1986 Mechanism of translational initiation in prokaryotes. Evidence for a direct effect of IF2 on the activity of the 30 S ribosomal subunit. FEBS letters 207 198 204
3. Godefroy-ColburnTWolfeADDondonJGrunberg-ManagoMDessenP 1975 Light-scattering studies showing the effect of initiation factors on the reversible dissociation of Escherichia coli ribosomes. J Mol Biol 94 461 478
4. WintermeyerWGualerziC 1983 Effect of Escherichia coli initiation factors on the kinetics of N-Acphe-tRNAPhe binding to 30S ribosomal subunits. A fluorescence stopped-flow study. Biochemistry 22 690 694
5. NorthSHKirtlandSENakaiH 2007 Translation factor IF2 at the interface of transposition and replication by the PriA-PriC pathway. Mol Microbiol 66 1566 1578
6. MizuuchiK 1983 In vitro transposition of bacteriophage Mu: A biochemical approach to a novel replication reaction. Cell 35 785 794
7. CraigieRArndt-JovinDJMizuuchiK 1985 A defined system for the DNA strand-transfer reaction at the initiation of bacteriophage Mu transposition: Protein and DNA substrate requirements. Proc Natl Acad Sci USA 82 7570 7574
8. CraigieRMizuuchiK 1985 Mechanism of transposition of bacteriophage Mu: Structure of a transposition intermediate. Cell 41 867 876
9. JonesJMNakaiH 1997 The φX174-type primosome promotes replisome assembly at the site of recombination in bacteriophage Mu transposition. EMBO J 16 6886 6895
10. ChaconasGHarsheyRM 2002 Transposition of phage Mu DNA. CraigNLCraigieRGellertMLambowitzAM Mobile DNA II Washington, D.C. ASM Press 384 402
11. LavoieBDChanBSAllisonRGChaconasG 1991 Structural aspects of a higher order nucleoprotein complex: Induction of an altered DNA structure at the Mu-host junction of the Mu Type 1 transpososome. EMBO J 10 3051 3059
12. MizuuchiMBakerTAMizuuchiK 1992 Assembly of the active form of the transposase-Mu DNA complex: A critical control point in Mu transposition. Cell 70 303 311
13. SuretteMGBuchSJChaconasG 1987 Transpososomes: Stable protein-DNA complexes involved in the in vitro transposition of bacteriophage Mu DNA. Cell 49 253 262
14. BurtonBMWilliamsTLBakerTA 2001 ClpX-mediated remodeling of Mu transpososomes: selective unfolding of subunits destabilizes the entire complex. Mol Cell 8 449 454
15. KruklitisRWeltyDJNakaiH 1996 ClpX protein of Escherichia coli activates bacteriophage Mu transposase in the strand transfer complex for initiation of Mu DNA synthesis. EMBO J 15 935 944
16. LevchenkoILuoLBakerTA 1995 Disassembly of the Mu transposase tetramer by the ClpX chaperone. Genes Dev 9 2399 2408
17. NorthSHNakaiH 2005 Host factors that promote transpososome disassembly and the PriA-PriC pathway for restart primosome assembly. Mol Microbiol 56 1601 1616
18. SandlerSJ 2000 Multiple genetic pathways for restarting DNA replication forks in Escherichia coli K-12. Genetics 155 487 497
19. SandlerSJMcCoolJDDoTTJohansenRU 2001 PriA mutations that affect PriA-PriC function during replication restart. Mol Microbiol 41 697 704
20. HoweJGHersheyJW 1983 Initiation factor and ribosome levels are coordinately controlled in Escherichia coli growing at different rates. J Biol Chem 258 1954 1959
21. SacerdotCVachonGLaalamiSMorel-DevilleFCenatiempoY 1992 Both forms of translational initiation factor IF2 (alpha and beta) are required for maximal growth of Escherichia coli. Evidence for two translational initiation codons for IF2 beta. J Mol Biol 225 67 80
22. GiuliodoriAMBrandiAGualerziCOPonCL 2004 Preferential translation of cold-shock mRNAs during cold adaptation. RNA 10 265 276
23. CenatiempoYDevilleFDondonJGrunberg-ManagoMSacerdotC 1987 The protein synthesis initiation factor 2 G-domain. Study of a functionally active C-terminal 65-kilodalton fragment of IF2 from Escherichia coli. Biochemistry 26 5070 5076
24. LaalamiSPutzerHPlumbridgeJAGrunberg-ManagoM 1991 A severely truncated form of translational initiation factor 2 supports growth of Escherichia coli. J Mol Biol 220 335 349
25. CaldasTLaalamiSRicharmeG 2000 Chaperone properties of bacterial elongation factor EF-G and initiation factor IF2. J Biol Chem 275 855 860
26. TraversAADebenhamPGPongsO 1980 Translation initiation factor 2 alters transcriptional selectivity of Escherichia coli ribonucleic acid polymerase. Biochemistry 19 1651 1656
27. VachonGRaingeaudJDerijardBJulienRCenatiempoY 1993 Domain of E. coli translational initiation factor IF2 homologous to lambda cI repressor and displaying DNA binding activity. FEBS Lett 321 241 246
28. Mhammedi-AlaouiAPatoMGamaM-JToussaintA 1994 A new component of bacteriophage Mu replicative transposition machinery: The Escherichia coli ClpX protein. Mol Microbiol 11 1109 1116
29. JonesJMNakaiH 1999 Duplex opening by primosome protein PriA for replisome assembly on a recombination intermediate. J Mol Biol 289 503 515
30. McGlynnPAl-DeibAALiuJMariansKJLloydRG 1997 The DNA replication protein PriA and the recombination protein RecG bind D-loops. J Mol Biol 270 212 221
31. KogomaTCadwellGWBarnardKGAsaiT 1996 The DNA replication priming protein, PriA, is required for homologous recombination and double-strand break repair. J Bacteriol 178 1258 1264
32. LaursenBSSiwanowiczILarigauderieGHedegaardJItoK 2003 Characterization of mutations in the GTP-binding domain of IF2 resulting in cold-sensitive growth of Escherichia coli. J Mol Biol 326 543 551
33. LaalamiSTimofeevAVPutzerHLeauteyJGrunberg-ManagoM 1994 In vivo study of engineered G-domain mutants of Escherichia coli translation initiation factor IF2. Mol Microbiol 11 293 302
34. NowosielskaASmithSAEngelwardBPMarinusMG 2006 Homologous recombination prevents methylation-induced toxicity in Escherichia coli. Nucleic Acids Res 34 2258 2268
35. GottesmanSHalpernETrislerP 1981 Role of sulA and sulB in filamentation by lon mutants of Escherichia coli K-12. J Bacteriol 148 265 273
36. NursePZavitzKHMariansKJ 1991 Inactivation of the Escherichia coli PriA DNA replication protein induces the SOS response. J Bacteriol 173 6686 6693
37. ZavitzKHMariansKJ 1992 ATPase-deficient mutants of the Escherichia coli DNA replication protein PriA are capable of catalyzing the assembly of active primosomes. J Biol Chem 267 6933 6940
38. SandlerSJSamraHSClarkAJ 1996 Differential suppression of priA2::kan phenotypes in Escherichia coli K-12 by mutations in priA, lexA, and dnaC. Genetics 143 5 13
39. SandlerSJMariansKJZavitzKHCoutuJParentMA 1999 DnaC mutations suppress defects in DNA replication and recombination functions in priB and priC double mutants in E. coli K-12. Mol Microbiol 34 91 101
40. McCoolJDFordCCSandlerSJ 2004 A dnaT mutant with phenotypes similar to those of a priA2::kan mutant in Escherichia coli K-12. Genetics 167 569 578
41. HuismanOD'AriRGottesmanS 1984 Cell-division control in Escherichia coli: specific induction of the SOS function SfiA protein is sufficient to block septation. Proceedings of the National Academy of Sciences of the United States of America 81 4490 4494
42. LiuJXuLSandlerSMariansKJ 1999 Replication fork assembly at recombination intermediates is required for bacterial growth. Proc Natl Acad Sci USA 96 3552 3555
43. JonesJMNakaiH 2000 PriA and T4 gp59: factors that promote DNA replication on forked DNA substrates. Mol Microbiol 36 519 527
44. FloresMJEhrlichSDMichelB 2002 Primosome assembly requirement for replication restart in the Escherichia coli holDG10 replication mutant. Mol Microbiol 44 783 792
45. MilonPTischenkoETomsicJCasertaEFolkersG 2006 The nucleotide-binding site of bacterial translation initiation factor 2 (IF2) as a metabolic sensor. Proc Natl Acad Sci U S A 103 13962 13967
46. KyrpidesNCWoeseCR 1998 Universally conserved translation initiation factors. Proc Natl Acad Sci U S A 95 224 228
47. KyrpidesNCWoeseCR 1998 Archaeal translation initiation revisited: the initiation factor 2 and eukaryotic initiation factor 2B alpha-beta-delta subunit families. Proc Natl Acad Sci USA 95 3726 3730
48. LowB 1973 Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol 113 798 812
49. MetzgerSSchreiberGAizenmanECashelMGlaserG 1989 Characterization of the relA1 mutation and a comparison of relA1 with new relA null alleles in Escherichia coli. J Biol Chem 264 21146 21152
50. BabaTAraTHasegawaMTakaiYOkumuraY 2006 Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2 1 11
51. ChangACYCohenSN 1978 Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 134 1141 1156
52. YuDEllisHMLeeECJenkinsNACopelandNG 2000 An efficient recombination system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci U S A 97 5978 5983
53. HermannSRMillerJAO'NeillSTsaoTTHardingRM 2000 Single-primer amplification of flanking sequences. Biotechniques 29 1176 1180
54. StewartEJKatzenFBeckwithJ 1999 Six conserved cysteines of the membrane protein DsbD are required for the transfer of electrons from the cytoplasm to the periplasm of Escherichia coli. EMBO J 18 5963 5971
55. GuzmanLMBelinDCarsonMJBeckwithJ 1995 Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177 4121 4130
56. LinDCGrossmanAD 1998 Identification and characterization of a bacterial chromosome partitioning site. Cell 92 675 685
57. SokolskyTDBakerTA 2003 DNA gyrase requirements distinguish the alternate pathways of Mu transposition. Mol Microbiol 47 397 409
58. MillerJH 1972 Experiments in molecular genetics Cold Spring Harbor, NY Cold Spring Harbor Laboratory 352 355
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



