-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCommon Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
Optic nerve degeneration caused by glaucoma is a leading cause of blindness worldwide. Patients affected by the normal-pressure form of glaucoma are more likely to harbor risk alleles for glaucoma-related optic nerve disease. We have performed a meta-analysis of two independent genome-wide association studies for primary open angle glaucoma (POAG) followed by a normal-pressure glaucoma (NPG, defined by intraocular pressure (IOP) less than 22 mmHg) subgroup analysis. The single-nucleotide polymorphisms that showed the most significant associations were tested for association with a second form of glaucoma, exfoliation-syndrome glaucoma. The overall meta-analysis of the GLAUGEN and NEIGHBOR dataset results (3,146 cases and 3,487 controls) identified significant associations between two loci and POAG: the CDKN2BAS region on 9p21 (rs2157719 [G], OR = 0.69 [95%CI 0.63–0.75], p = 1.86×10−18), and the SIX1/SIX6 region on chromosome 14q23 (rs10483727 [A], OR = 1.32 [95%CI 1.21–1.43], p = 3.87×10−11). In sub-group analysis two loci were significantly associated with NPG: 9p21 containing the CDKN2BAS gene (rs2157719 [G], OR = 0.58 [95% CI 0.50–0.67], p = 1.17×10−12) and a probable regulatory region on 8q22 (rs284489 [G], OR = 0.62 [95% CI 0.53–0.72], p = 8.88×10−10). Both NPG loci were also nominally associated with a second type of glaucoma, exfoliation syndrome glaucoma (rs2157719 [G], OR = 0.59 [95% CI 0.41–0.87], p = 0.004 and rs284489 [G], OR = 0.76 [95% CI 0.54–1.06], p = 0.021), suggesting that these loci might contribute more generally to optic nerve degeneration in glaucoma. Because both loci influence transforming growth factor beta (TGF-beta) signaling, we performed a genomic pathway analysis that showed an association between the TGF-beta pathway and NPG (permuted p = 0.009). These results suggest that neuro-protective therapies targeting TGF-beta signaling could be effective for multiple forms of glaucoma.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002654
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002654Summary
Optic nerve degeneration caused by glaucoma is a leading cause of blindness worldwide. Patients affected by the normal-pressure form of glaucoma are more likely to harbor risk alleles for glaucoma-related optic nerve disease. We have performed a meta-analysis of two independent genome-wide association studies for primary open angle glaucoma (POAG) followed by a normal-pressure glaucoma (NPG, defined by intraocular pressure (IOP) less than 22 mmHg) subgroup analysis. The single-nucleotide polymorphisms that showed the most significant associations were tested for association with a second form of glaucoma, exfoliation-syndrome glaucoma. The overall meta-analysis of the GLAUGEN and NEIGHBOR dataset results (3,146 cases and 3,487 controls) identified significant associations between two loci and POAG: the CDKN2BAS region on 9p21 (rs2157719 [G], OR = 0.69 [95%CI 0.63–0.75], p = 1.86×10−18), and the SIX1/SIX6 region on chromosome 14q23 (rs10483727 [A], OR = 1.32 [95%CI 1.21–1.43], p = 3.87×10−11). In sub-group analysis two loci were significantly associated with NPG: 9p21 containing the CDKN2BAS gene (rs2157719 [G], OR = 0.58 [95% CI 0.50–0.67], p = 1.17×10−12) and a probable regulatory region on 8q22 (rs284489 [G], OR = 0.62 [95% CI 0.53–0.72], p = 8.88×10−10). Both NPG loci were also nominally associated with a second type of glaucoma, exfoliation syndrome glaucoma (rs2157719 [G], OR = 0.59 [95% CI 0.41–0.87], p = 0.004 and rs284489 [G], OR = 0.76 [95% CI 0.54–1.06], p = 0.021), suggesting that these loci might contribute more generally to optic nerve degeneration in glaucoma. Because both loci influence transforming growth factor beta (TGF-beta) signaling, we performed a genomic pathway analysis that showed an association between the TGF-beta pathway and NPG (permuted p = 0.009). These results suggest that neuro-protective therapies targeting TGF-beta signaling could be effective for multiple forms of glaucoma.
Introduction
Glaucoma is a leading cause of blindness worldwide [1]. Primary open angle glaucoma (POAG), the most common form of glaucoma in the Western world, is an age-related, complex disease characterized by progressive irreversible degeneration of the optic nerve due to apoptotic retinal ganglion cell death [2]. In addition to age, epidemiologic studies have revealed multiple risk factors for the condition including elevated intraocular pressure (IOP), African-American race, family history and low ocular perfusion pressure [3]–[5]. Of these, elevated intraocular pressure (IOP) is the only treatable risk factor; however, many individuals have IOP elevation without optic nerve disease [6], and at least 33% of affected individuals have progressive retinal ganglion cell loss despite IOP measurements in the normal range (less than 22 mmHg), a condition defined as normal-pressure glaucoma (NPG) [7]. Preventative or neuro-protective therapies for glaucoma are not yet available and little is known about the molecular events that influence susceptibility to glaucomatous optic nerve degeneration.
POAG is genetically complex [8]. Linkage studies have identified over 20 genomic regions likely to contain POAG-related genes [9]. Importantly, genes that influence POAG risk overall may specifically contribute to separate independent biological processes affecting the disease outcome including regulation of IOP and retinal ganglion cell physiology. Individuals with elevated IOP without optic nerve disease may only carry genetic variants that influence IOP regulation, while individuals with NPG may primarily carry genetic variants that predispose to retinal ganglion cell death as the nerve degenerates in these patients without the added stress of elevated IOP.
Recent genome-wide association studies (GWAS) have identified several genetic risk factors for POAG overall, including single-nucleotide polymorphisms (SNPs) located in the CAV1/CAV2 intergenic region [10]–[11], and in the genomic regions containing the TMCO1 and CDKN2BAS genes [12] in a study of glaucoma patients with advanced optic nerve disease. Linkage studies have identified two genes that contribute to rare familial forms of NPG, OPTN (optineurin) [13] and TBK1 (TANK-binding kinase) [14], and an intronic SNP in SRBD1 (S1 RNA binding domain) has been associated with NPG in a GWAS of 355 Japanese cases [15]. Genome-wide association studies have not yet identified genes commonly associated with normal-pressure glaucoma or with optic nerve disease in glaucoma. The identification of genes that influence glaucoma-related optic nerve degeneration is an important step toward the development of neuro-protective therapies that could substantially reduce the morbidity caused by this common disease, and such therapies could be relevant to optic nerve degeneration occurring in many chronic forms of glaucoma.
To identify genes that predispose to glaucomatous optic nerve disease, we completed two GWAS for POAG: the GLAUGEN (Glaucoma Genes and Environment) GWAS that is part of the GENEVA (GENEVA Genes Environment Association) studies [16] and the NEIGHBOR (NEI Glaucoma Human genetics collaBORation) GWAS [17]. We then performed a meta-analysis as well as normal-pressure and high-pressure subgroup analyses of the combined dataset. To determine if the observed associations were specific to POAG or could generalize more broadly to optic nerve disease in other forms of glaucoma we selected the lead SNPs showing significant association in the normal-pressure glaucoma subgroup analysis, and tested them for association with glaucoma in a population of unrelated individuals with exfoliation syndrome glaucoma.
Results
Genome-wide association studies and meta-analyses
After data cleaning, 976 cases and 1,140 controls collected from three study sites [Nurses' Health Study, Health Professionals Follow-up Study and the Genetic Etiologies of Primary-Open angle Glaucoma Study (GEP)] were analyzed for the GLAUGEN study, and 2,170 cases and 2,347 controls collected from 12 sites (Table S1) were analyzed for the NEIGHBOR study. All cases and controls for both studies were residents of the continental United States and were of mainly European ancestry, which was confirmed by principal component analysis. The general characteristics for the cases and controls are shown in Table 1. For POAG overall in the GLAUGEN GWAS, there were no SNPs with p-values that reached the genome-wide significance level of p = 5×10−8 (Figure S1 and Figure S2).
Tab. 1. Features of cases and controls. 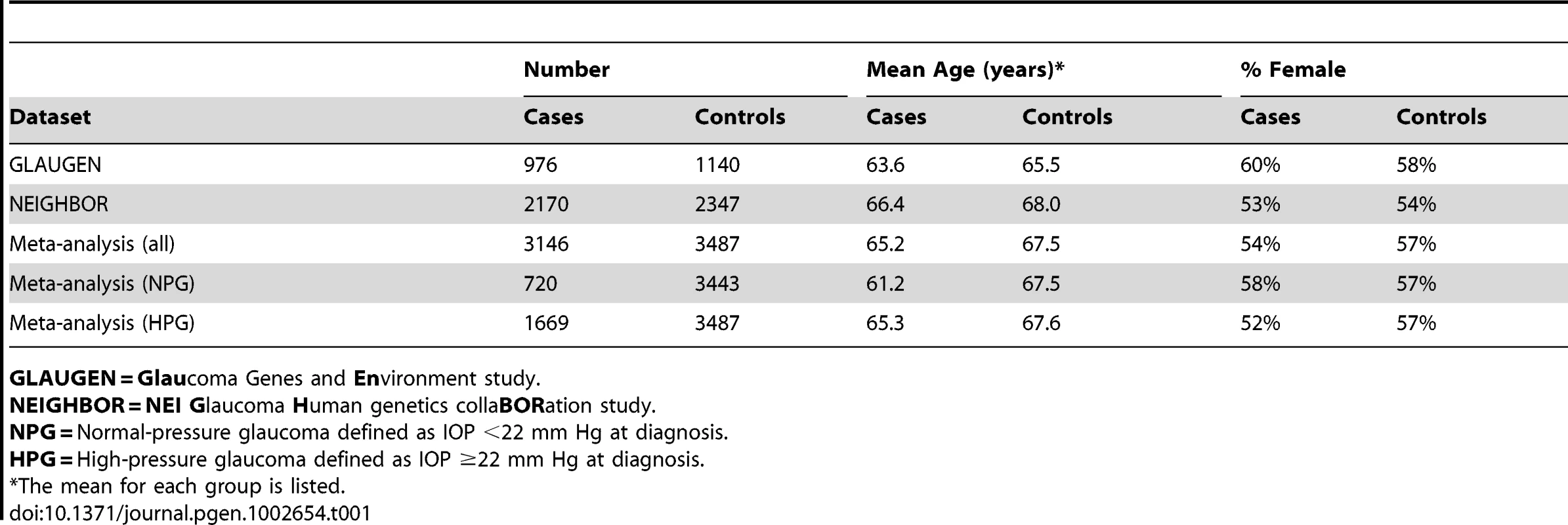
GLAUGEN = Glaucoma Genes and Environment study. In the NEIGHBOR overall POAG GWAS, there were seventeen SNPs that reached the genome-wide significance level of p = 5×10−8. Sixteen of the significant SNPs were found in the CDKN2BAS gene region on chromosome 9p21.3 (top SNP rs4977756 (OR = 0.66 [95% CI 0.59–0.73], p value = 7.4×10−16). The remaining significant SNP rs10483727 was located in the SIX1/SIX6 region on chromosome 14 (OR = 1.32 [95% CI 1.20–1.46], p value = 3.1×10−8). (Figure S3 and Figure S4; Table S2).
Using METAL [18], we conducted a meta-analysis of the GLAUGEN and NEIGHBOR datasets that included a total of 3,146 cases and 3,487 controls and found nineteen SNPs that achieved genome-wide significance for POAG overall (Figure S5): seventeen of them in the CDKN2BAS region, with the most significant SNP being rs2157719 (OR = 0.69 [95%CI 0.63–0.75], p = 1.86×10−18, and two SNPs in the SIX1/SIX6 region on chromosome 14q23 (most significant SNP: rs10483727, OR = 1.32 [95%CI 1.21–1.43], p = 3.87×10−11) (Table S3). A number of genomic regions, including the CDKN2BAS and SIX1/SIX6 regions have been previously associated with optic nerve quantitative parameters (cup-to-disc ratio (CDR) and optic nerve area) [19]–[24]. Of these, only the CDKN2BAS and SIX1/SIX6 regions were significantly associated with glaucoma in the meta-analysis, although several other previously identified gene regions demonstrated suggestive associations including SALL1, LRP1B and SIRPA (Table 2, Table S4).
Tab. 2. Glaucoma association results for SNPs in gene regions associated with quantitative optic nerve parameters optic nerve area and cup-to-disc ratio (CDR). 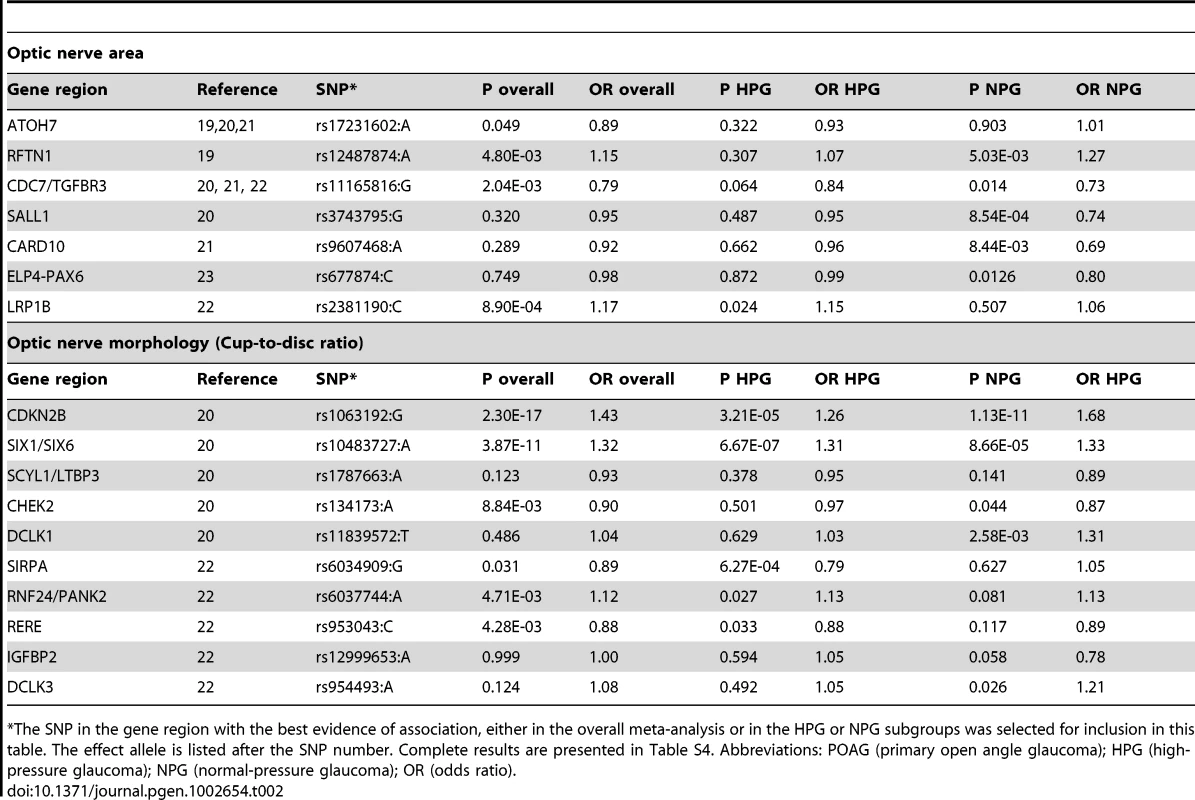
The SNP in the gene region with the best evidence of association, either in the overall meta-analysis or in the HPG or NPG subgroups was selected for inclusion in this table. The effect allele is listed after the SNP number. Complete results are presented in Table S4. Abbreviations: POAG (primary open angle glaucoma); HPG (high-pressure glaucoma); NPG (normal-pressure glaucoma); OR (odds ratio). Normal-pressure and high-pressure subgroup analyses
To identify genetic risk factors primarily associated with optic nerve disease in glaucoma, we performed separate meta-analyses for NPG (720 cases) and high-pressure glaucoma (HPG, 1669 cases with IOP ≥22 mm Hg), (757 cases did not have untreated IOP data available). In the HPG meta-analysis, no SNPs, even those in the CDKN2BAS and SIX1/SIX6 regions, reached genome-wide significance (Figure S6). In the NPG meta-analysis, SNPs in two regions reached genome-wide significance, (Figure 1, Table 3): thirteen SNPs in the CDKN2BAS region on 9p21 (most significant SNP was rs2157719, (OR = 0.58 [95% CI 0.50–0.67], p = 1.17×10−12) (Figure 2, Figure S7) and three SNPs in an evolutionarily conserved region on chromosome 8q22 (most significant SNP was rs284489, OR = 0.62 [95% CI 0.53–0.72], p = 8.88×10−10) (Figure 3, Figure S8).
Fig. 1. Genome-wide association results with normal pressure glaucoma (NPG) (IOP <22 mm Hg) in the GLAUGEN-NEIGHBOR meta-analysis (720 cases and 3487 controls). 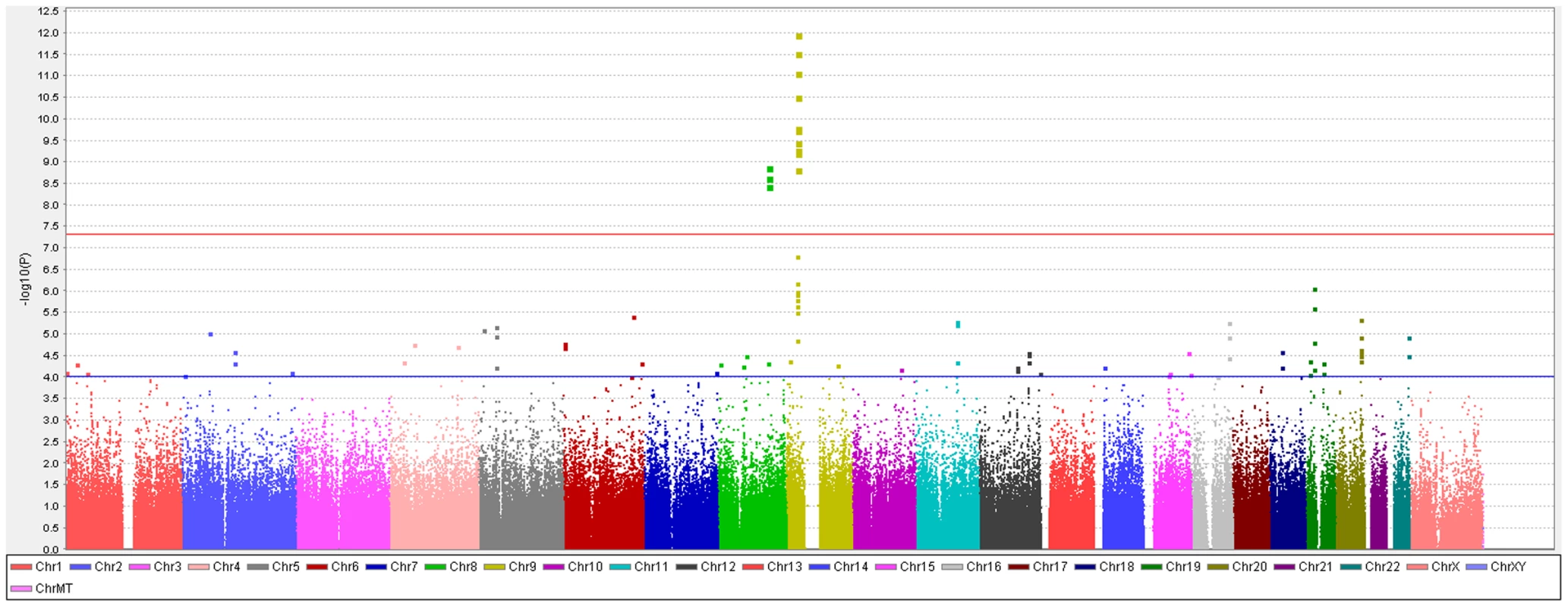
The red line identifies a p-value of 5×10−8. Covariates include: (NEIGHBOR) age, gender, study site and eigenvectors 1 and 2; (GLAUGEN) age, gender, study site, DNA extraction method, DNA specimen type and eigenvectors 1, 2 and 6. Fig. 2. Meta-analysis results for SNPs associated with normal pressure glaucoma (NPG) (IOP <22 mmHg) located in the CDKN2BAS region on chromosome 9p21. 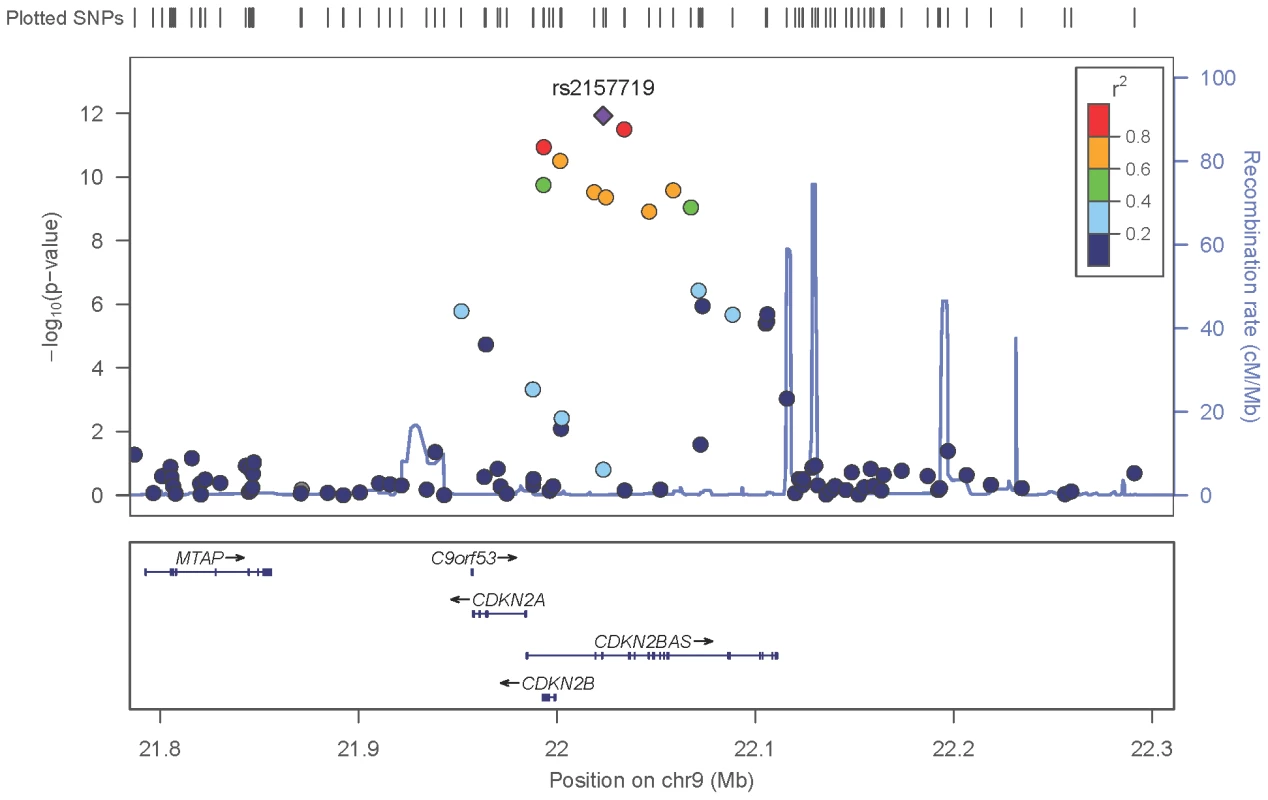
The most significant SNP (rs2157719, p = 1.17×10−12) is indicated with a solid diamond. Fig. 3. Meta-analysis results for SNPs associated with normal pressure glaucoma (NPG) (IOP <22 mm Hg) located in the 8q22 region. 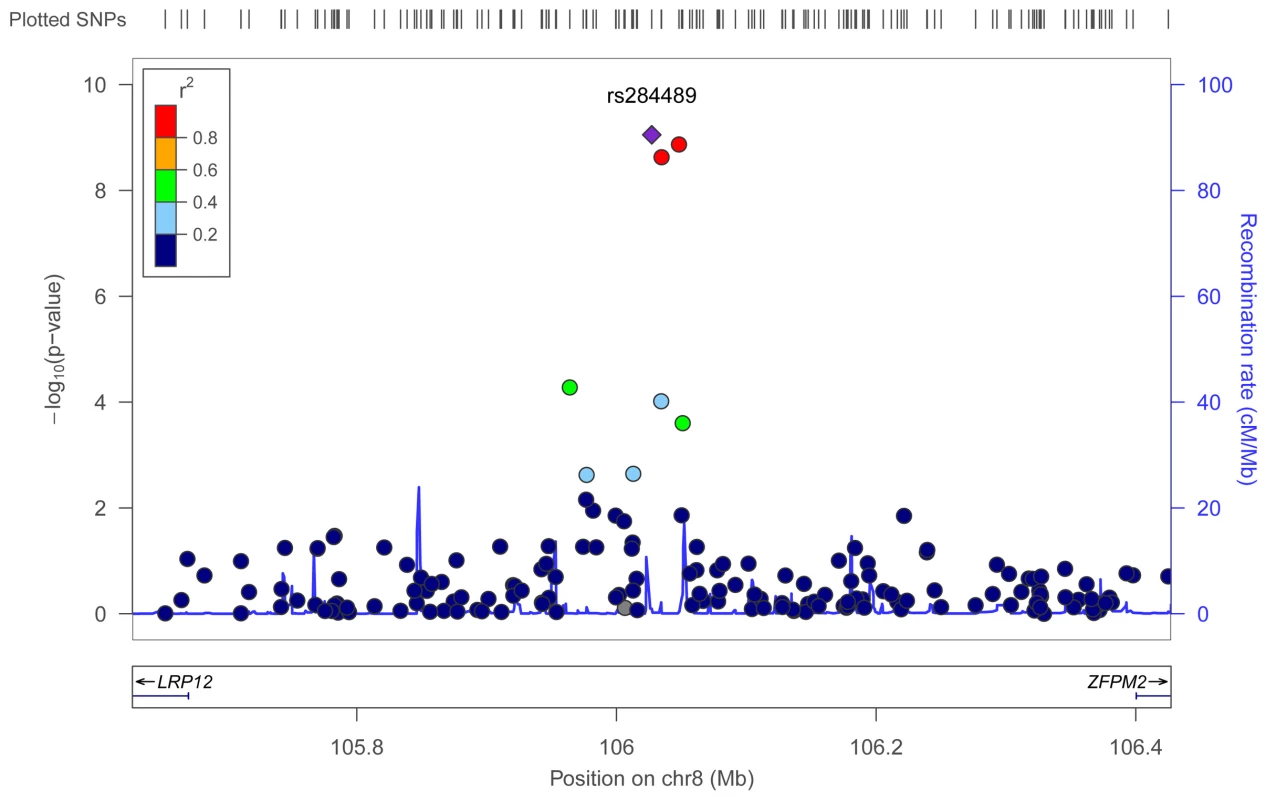
The most significant SNP (rs284489, p = 8.8×10−10) is indicated with a solid diamond. Tab. 3. Association results for genome-wide significant SNPs for the Normal Pressure Glaucoma (NPG) analyses. 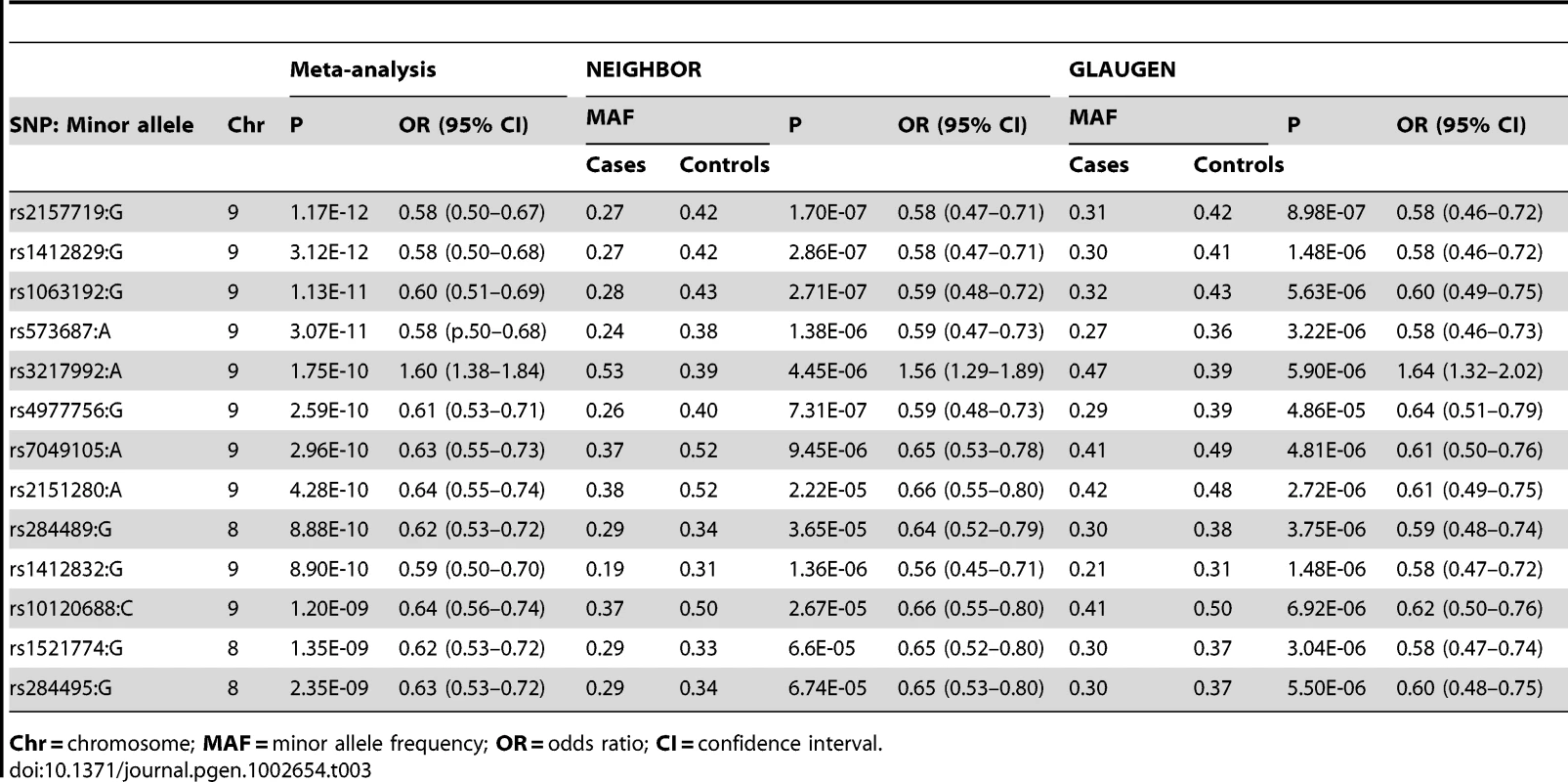
Chr = chromosome; MAF = minor allele frequency; OR = odds ratio; CI = confidence interval. Single–SNP associations
Chromosome 9p21 region
After imputation 90 SNPs in the 9p21 region were significantly associated with NPG (Table S5), although conditional analysis showed that none of these were independent of rs2157719, the most significantly associated SNP in the meta-analysis. The minor allele for rs2157719 showed a protective effect, and is located within the genomic region coding for the noncoding RNA, CDKN2BAS, also known as ANRIL, which regulates expression of CDKN2B as well as other genes [25]. rs2157719 falls within a DNase I hypersensitivity site [26] that has a significant signal in a variety of cell types [27] (Figure S9). Targeted re-sequencing of the CDKN2BAS gene in a subset of 11 cases carrying a protective haplotype that includes rs2157719 (Table S6) identified two rare variants (rs7341786 [A]; rs7341791 [A]) in 8 of the 11 individuals. After imputation we found that these variants are significantly associated with glaucoma (p = 2.73×10−9, OR = 0.75; p = 3.09×10−9, OR = 0.75 respectively). These SNPs have been previously shown to affect RNA splicing [28], suggesting that altering CDKN2BAS expression may contribute to glaucoma.
Chromosome 8q22 region
The three SNPs located in the 8q22 region that were significantly associated with NPG in the subgroup analysis are in strong linkage disequilibrium (r2>0.8) (Figure S8), and imputation followed by conditional analysis did not reveal additional independent associations. The genomic region containing the associated SNPs includes highly conserved DNA sequences and several DNaseI hypersensitivity sites [26], [27] (Figure S10). An imputed SNP (rs284492) in strong LD with the lead SNP (rs284489) falls within or proximal to DNaseI hypersensitivity sites with significant activity in choroid plexus epithelial cells, as well as two ocular tissues (nonpigmented ciliary epithelial cells, and iris pigment epithelial cells) (Figure S10, Table S5). Haplotypes significantly associated with NPG (Table S7) include these DNaseI hypersensitivity sites. This putative regulatory region is located within a 630 Kb gene desert flanked by LRP12 (Low-density lipoprotein receptor related protein 12) and ZFPM2 (Zinc finger protein multitype 2) and could influence expression of either of these genes.
Ocular expression of chromosome 8 genes LRP12 and ZFPM2
Using RT-PCR, we found that both LRP12 and ZFPM2 are expressed in the human optic nerve, as well as in other ocular tissues relevant to glaucoma (Figure S11). In mouse experiments using laser microdissection to selectively study specific layers of the retina, both LRP12 and ZFPM2 were expressed in the retinal ganglion cell and inner nuclear layers (Figure S11), making them good candidates for optic nerve susceptibility genes.
DNA sequencing of LRP12 and ZFPM2
We performed targeted resequencing of the LRP12 and ZFPM2 genes in 16 NPG patients and also analyzed the exomes of 50 POAG cases and 18 controls for DNA sequence variants in both genes (Table S8). None of the variants identified are expected to significantly affect gene expression or protein function.
Association of 9p21 and 8q22 SNPs with exfoliation glaucoma
To determine if SNPs associated with NPG were associated with optic nerve degeneration in other types of glaucoma, we examined the association of the top SNPs in both the 9p21 and 8q22 genomic regions with optic nerve degeneration in exfoliation glaucoma, another form of open-angle glaucoma. Exfoliation syndrome, a condition predisposing to elevated intraocular pressure, is associated with genetic variants in LOXL1 [29], however, these variants appear to contribute to the development of the exfoliation syndrome and not to optic nerve disease in POAG [30]. We found that rs2157719 in 9p21 was significantly associated with exfoliation glaucoma (Table 4), and rs284489 in 8q22 was also nominally associated with the same directionality of the association observed for NPG.
Tab. 4. Association results for exfoliation glaucoma and 9p21 and 8q22 SNPs. 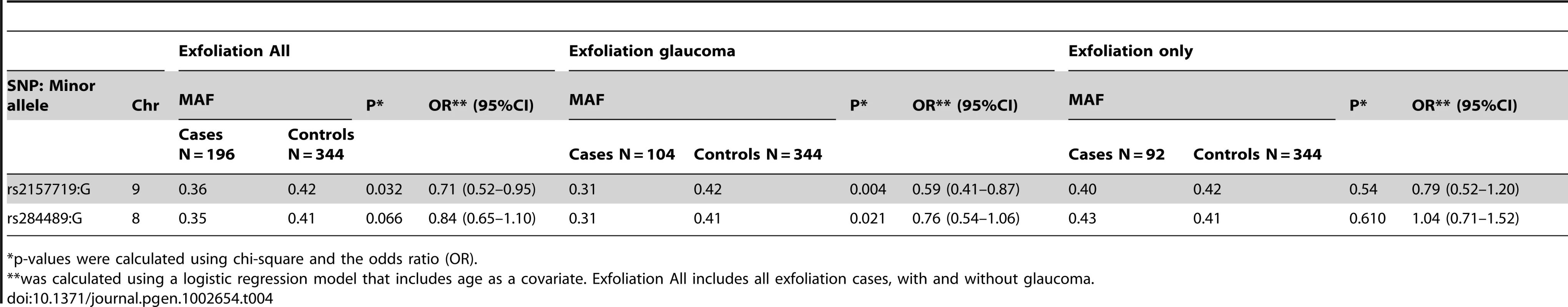
p-values were calculated using chi-square and the odds ratio (OR). TGF–beta pathway analysis
Both the 9p21 and 8q22 regions could contribute to TGF-beta signaling. The 9p21 SNPs could effect expression of CDKN2BAS, a noncoding RNA that influences expression of CDKN2B, a member of the TGF-beta signaling pathway [31]. Using the SCAN database [32], we found that the three 8q22 SNPs associated with NPG (rs284489, rs284495 and rs1521774) influence the expression of TSC22 (TGF-beta stimulated clone 22), which also modulates signaling by TGF-beta [33]. Using the PARIS (Pathway Analysis by Randomization Incorporating Structure) algorithm [34], we found that the TGF-beta pathway (KEGG, hsa04350) Kyoto Encyclopedia of Genes and Genomes) overall was associated with NPG in our combined dataset (permuted p = 0.009).
Discussion
Using two large POAG case-control datasets, we have completed a meta-analysis for POAG followed by subgroup analyses for NPG and HPG. We have identified two genomic regions that are associated with NPG and showed that the lead SNP in each region is also associated with optic nerve disease in a second type of open-angle glaucoma (exfoliation syndrome related glaucoma).
The first region includes the CDKN2BAS gene on chromosome 9p21, previously associated with cup-to-disc ratio (CDR) an optic nerve quantitative parameter, as well as POAG in candidate gene studies [20], [35], [36] and more recently in a GWAS using a sample of severely affected POAG patients [12]. In this study, we show that this gene region is associated with NPG suggesting that CDKN2BAS contributes to optic nerve degeneration in glaucoma. CDKN2BAS codes for an antisense RNA that regulates the expression of CDKN2B, which is an inhibitor of cyclin-dependent kinase 4 (CDK4), a protein kinase that has a pivotal role in cell cycle progression [37]. The minor alleles of the NPG associated SNPs are protective, suggesting that these SNPs, or other variants in linkage disequilibrium with these SNPs, could influence CDKN2BAS and CDKN2B expression with corresponding changes in cyclin-dependent kinase activity that could result in retinal ganglion cell apoptosis. CDKN2BAS expression can also be influenced by interferon alpha [38]. As inflammation and autoimmunity may be factors contributing to glaucoma pathogenesis [39], the regulation of CDKN2BAS expression by interferon could suggest a direct role for CDKN2BAS in glaucoma pathogenesis. CDKN2BAS also regulates the expression of CDKN2A, a gene previously shown to be down-regulated in other neurodegenerative disorders, including Alzheimer's disease, suggesting that regulation of CDKN2A expression by CDKN2BAS could also contribute to degeneration of the optic nerve in glaucoma [40].
The second region associated with NPG in this study is an evolutionarily conserved DNA segment on chromosome 8q22 with predicted regulatory function. This region partially overlaps with a POAG locus defined by a linkage study of a single large family with low intraocular pressure (GLC1D) [41], and a second linkage study using multiple affected families stratified by age of disease onset [42]. The conserved region falls within a 630 Kb gene desert flanked by the LRP12 and ZFPM2 genes. ZFPM2, also known as FOG2, codes for a zinc finger protein that appears to contribute to cardiovascular development, and may have a role in ocular development [43]. ZFPM2 is expressed in the eye, and expression was observed to increase after injury to the optic nerve [44]. The LRP12 gene is a member of the LRP (low-lipoprotein receptor) gene family, with several members previously implicated in glaucoma. LRP1 is decreased in the optic neuropathy associated with Alzheimer's disease [45], LRP1B is associated with optic nerve area [22], a knock-out of LRP2 causes a glaucoma-like phenotype in zebrafish [46], LRP4 expression is increased in response to retinal ischemia [47], and expression of LRP10 and LRP11 is up-regulated in a rat model of glaucoma [48], [49]. Importantly, LRP12, previously known as ST7, is a receptor for SMAD4, a major regulator of the TGF-beta signaling pathway [50]. Interestingly, SMAD4 influences activity of TSC22 [51].
The 8q22 genomic region associated with NPG contains putative regulatory sites that appear to be active in two cell types that could contribute to glaucoma-related optic nerve disease, choroid plexus epithelial cells and non-pigmented ciliary body epithelial cells. The choroid plexus is responsible for formation of cerebral spinal fluid, and recent studies have suggested that low cerebral spinal fluid pressure, which could be caused by decreased formation of cerebral spinal fluid, may create a deleterious gradient across the lamina cribrosa in NPG mimicking a similar gradient induced by higher IOP in HPG [52], [53]. The 8q22 regulatory region is also active in non-pigmented ciliary body epithelial cells, which affect ocular intraocular pressure through the production of aqueous humor (a fluid that is similar in composition to cerebral spinal fluid).
Both genomic regions identified in this study could contribute to regulation of TGF-beta signaling and our pathway analysis provides additional support for a role for TGF-beta signaling in glaucomatous optic nerve disease and retinal ganglion cell death. TGF-beta and other members of the TGF-beta signaling pathway have been previously implicated in glaucoma [54], [55], and genes participating in TGF-beta signaling are expressed in ocular structures that are involved in glaucoma, including the anatomic structures regulating IOP and the optic nerve [56]–[58]. TGF-beta1 is up-regulated in optic nerve tissue in an animal model of glaucoma [59], and mutations in LTBP2 (latent transforming growth factor beta binding protein 2) cause a rare autosomal recessive form of congenital glaucoma [60]. Collectively, these results suggest that therapies directed toward the regulation of TGF-beta signaling could protect the optic nerve from degeneration in glaucoma. The discovery of POAG genetic risk factors, especially the factors that predispose to glaucoma-related optic neuropathy, is a critical first step toward understanding the pathophysiology of POAG and the development of gene-based screening tests and neuro-protective therapies for this common blinding disease.
Methods
Subjects
The institutional review boards of the Massachusetts Eye and Ear Infirmary, Harvard School of Public Health, the Brigham and Women's Hospital, University of Pittsburgh, Johns Hopkins University, Duke University, University of West Virginia, University of Miami, University of Michigan, Stanford University, Marshfield Clinic, and the University of California, San Diego. approved this study.
Clinical definitions
POAG cases were defined as individuals for whom reliable visual field (VF) tests show characteristic VF defects consistent with glaucomatous optic neuropathy. Individuals were classified as affected if the VF defects were reproduced on a subsequent test or if a single qualifying VF was accompanied by a cup-disc ratio (CDR) of 0.7 or more in at least one eye. The examination of the ocular anterior segment did not show signs of secondary causes for elevated IOP such as exfoliation syndrome or pigment dispersion syndrome and the filtration structures were deemed to be open based on clinical measures. Elevation of IOP was not a criterion for inclusion; however, 67% of cases did have a history of elevated IOP (≥22 mm Hg) measured in a clinical setting (typically between the hours of 8AM and 5PM) and were classified as high-pressure glaucoma (HPG). Cases with IOP <22 mm Hg measured in the clinic at the time of study enrollment (without treatment) were classified as normal-pressure glaucoma (NPG). Cases undergoing IOP-lowering therapy at the time of enrollment were included in the HPG group if they had a documented history of IOP >22 prior to treatment and cases undergoing IOP-lowering therapy at the time of enrollment were included in the NPG if they did not have recorded pressures >22 mmHg before treatment. As glaucoma patients are long-term patients with several clinic visits each year, for most of the glaucoma cases in this study the IOP measurements were made at least twice on multiple occasions. Exfoliation glaucoma cases had evidence of characteristic fibrillar deposits as previously described [61] in addition to glaucoma as defined above. Controls had normal optic nerves (cup-disc ratios ≤0.6) and normal intraocular pressure (≤21 mm Hg).
Dataset descriptions
The GLAUGEN dataset included 976 cases and 1,140 controls drawn from three different studies: the Genetic Etiologies of Primary-Open angle Glaucoma (GEP), the Nurses' Health Study (NHS) and the Health Professionals Follow-up study (HPFS). The GEP is a clinic-based case-control set, and the NHS and HPFS are case-control sets nested within population-based studies. Additional information about the case identification and control selection process in GLAUGEN can be found at The Primary Open-Angle Glaucoma Genes and Environment (GLAUGEN) Study. Study Accession: phs000308.v1.p1. www.ncbi.nlm.nih.gov/projects/gap. December 21, 2010. The majority of DNA samples were prepared using Qiagen extraction kits (Invitrogen). More than half of the samples were derived from buccal cells, and we previously demonstrated the feasibility of genotyping buccal cell DNA on the Illumina 660W Quad platform [62].
Cases and controls for the NEIGHBOR study were collected from 12 sites (Table S1). Additional information about the NEIGHBOR consortium can be found in reference 17.
Genome-wide association studies
Genotyping and quality control
Genotyping for the GLAUGEN and NEIGHBOR samples was completed using the Illumina Human660W_Quad_v1 array. (Illumina, San Diego, CA) at the Broad Institute (GLAUGEN) and the Center for Inherited Diseases Research (CIDR) (NEIGHBOR). At both of the genotyping centers, samples were plated to allow equal representation of cases and controls per plate from each study site in order to minimize batch effects.
For the GLAUGEN samples genotyping calls were generated using Illumina's BeadStudio, GenomeStudio and Autocall software along with genotype cluster definitions based on study samples. SNPs with a GenTrain score <0.636, cluster separation score <0.4 and call rate <97% were considered technical failures at the genotyping center and were automatically deleted before release for further quality control. Data was released for 2,241 study samples (95% of attempted samples). Subsequent data quality control measures consisted of identifying and removing samples with gender misidentification, unexpected duplicates and unexpected relatedness. Analysis of connectivity removed samples that appeared to be related to other samples and/or suggestive of contamination. Any SNP with missing call rate >2% or with Hardy Weinberg p-value<10−4 in the control population was excluded. Logistic regression analysis indicated that study site (GEP, NHS or HPFS), DNA source (blood or cheek cell), and DNA extraction method (DNAzol, Qiagen or GENTRA) were independent predictors of genotyping call rate for the GLAUGEN samples. Hence, these variables along with age and gender were used in the logistic regression models.
For the NEIGHBOR samples data from CIDR was released for 5,155 study samples (97% of attempted samples). Study samples, including 117 study duplicates, were plated and genotyped together with 227 HapMap controls (208 CEU; 11 YRI, 4 JPT, 4 CHB). Genotyping was performed using Illumina Human660W-Quadv1_C BeadChips (Illumina, San Diego, CA, USA) and the Illumina Infinium II assay protocol [63]. Allele cluster definitions for each SNP were determined using Illumina GenomeStudio Genotyping Module version 1.7.4, GenTrain version 1.0 and the combined intensity data from 99.9% of the samples. The resulting cluster definitions were used on all samples. Genotypes were not called if the quality threshold (Gencall score) was below 0.15. Genotypes were released by CIDR for 557,029 SNPs (99.58% of attempted). Genotypes were not released for SNPs that had call rates less than 85%, more than 1 HapMap replicate error, cluster separation less than 0.2, more than a 3% (autosomal) or 2.2% (X chromosome) difference in call rate between genders, more than 0.4% (X chromosome) male heterozygosity, or more than a 8% (autosomal) difference in AB frequency. XY, Y and mitochondrial SNPs were manually reviewed and clusters adjusted or genotypes dropped as appropriate. Intensity data was released for all attempted SNPs. The mean non-Y SNP call rate and mean sample call rate was 99.9% for the released CIDR dataset. Study duplicate reproducibility was 99.99%. After applying quality control filters, 495,132 SNPs were analyzed in 976 cases and 1140 controls for GLAUGEN and 523,528 SNPs were analyzed in 2170 cases and 2347 controls for NEIGHBOR.
Association analysis
Logistic regression to assess the association between individual SNPs and POAG was done using PLINK v1.07 [64]. For GLAUGEN, the logistic regression model included age, gender, study site, DNA source, DNA extraction method and 3 eigenvectors (EV 1, 2 and 6). For NEIGHBOR, the logistic regression model included age, gender, study site and 2 eigenvectors (EV1 and 2). Quantile-quantile plots (Figures S1 and S3) were used to estimate genomic inflation factors which were 1.009 for GLAUGEN and 1.034 for NEIGHBOR.
Meta-analysis
Combined meta-analysis of the GLAUGEN and NEIGHBOR datasets was done using the METAL [18] software package. We analyzed each study using logistic regression as described previously. Then, we combined the results using the inverse weighted variance method based on the regression coefficients and standard errors estimated from each study as implemented in the program METAL [18]. The GENOMICCONTROL option was set to ON to adjust for genomic inflation differences between the studies.
Haplotype analysis
Haplotype analysis (logistic regression) was performed with PLINK 1.07 [64] using sliding windows of 2–6 SNPs across the associated regions. Covariates in the model were the same as for the single-allele analyses.
Pathway analysis
Pathway analysis was done using the Pathway Analysis by Randomization Incorporating Structure (PARIS) pathway analysis software package [34]. Genes comprising the transforming growth factor beta (TGF-beta) pathway were identified using the KEGG database (hsa:04350). SNPs were considered to reside in a pathway gene if the SNP fell within the ENSEMBLE genomic interval+/−50 kb to either side of the gene. If the overlap included another gene, the overlapping SNP(s) were counted once. A single-allele p-value of <0.05 was considered to be nominally significant and included in the PARIS analysis.
Imputation
Focused regional imputation was used to infer genotypes for SNPs not directly genotyped using MACH 1.0 [65], [66] to impute to the 1000 genomes data, release 2010–06. SNPs with a quality score (Rsq) of <0.5 were discarded before analysis. The resulting genotypes were analyzed using PLINK with the same covariates for each dataset as the non-imputed analyses. The meta-analysis procedure was also identical to that described above.
Ocular expression study of LRP12 and ZFPM2 in an animal model
Mouse laser capture microdissection
Retinas from adult mice were isolated, processed, and subjected to laser capture microdissection. cDNA purified from the respective cell layers was synthesized and analyzed by quantitative PCR as previously described [67].
Human ocular expression
Total RNA was extracted from dissected tissues (cornea, trabecular meshwork, retina, optic nerve) from normal human donor eyes as previously described [68]. Primer sequences were designed to specifically amplify the LRP12 and ZFPM2 genes. Amplification products were visualized by gel electrophoresis.
DNA sequencing
Targeted resequencing using Sanger methods as described [69] was carried out on genes from the associated loci on chromosome 8 and chromosome 9. All exons of LRP12 and ZFPM2 were sequenced in 16 NPG patients, and all exons of CDKN2BAS were sequenced in 11 cases carrying a protective haplotype including rs2157719 (Table S6). Genomic DNA was sequenced using primers designed to amplify the coding exons as well as the adjacent splice sites for both genes. PCR products were directly sequenced on the ABI PRISM 3100 Genetic Analyzer (Applied Biosystems) with BigDye Terminators (Applied Biosystems) according to standard protocols. Methods and analysis for exome sequencing are described in Text S1.
Association with exfoliation glaucoma
Exfoliation cases were collected from the Massachusetts Eye and Ear infirmary glaucoma clinic (cases) and the comprehensive eye service (controls in GLAUGEN or NEIGHBOR). All cases and controls were examined by a board certified ophthalmologist prior to study enrollment. The lead SNPs for each region associated with NPG were analyzed in 196 Caucasian patients with exfoliation syndrome, 104 of who also had glaucoma as defined as above. Single-SNP associations were analyzed using chi-square and logistic regression models adjusting for age.
Web resources
PLINK (http://pngu.mgh.harvard.edu/~purcell/plink/); EIGENSOFT (http://genepath.med.harvard.edu/~reich/Software.htm); ENCODE (http://genome.ucsc.edu/ENCODE/); SCAN (http://www.scandb.org/newinterface/about.html); KEGG (http://www.genome.jp/kegg/); UCSC genome (http://genome.ucsc.edu/); ENSEMBL (http://useast.ensembl.org/index.html).
Supporting Information
Zdroje
1. QuiqleyHABromanAT 2006 The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90 262 267
2. CaprioliJVarmaR 2011 Intraocular pressure: modulation as treatment for glaucoma. Am J Ophthalmol 152 340 344
3. GirkinCASamplePALiebmannJMJainSBowdC 2010 African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol 128 541 550
4. GroverDSBudenzDL 2011 Ocular perfusion pressure and glaucoma. Int Ophthalmol Clin 5 19 25
5. WangXHarmonJZabrieskieNChenYGrobSWilliamsB 2010 Using the Utah Population Database to assess familial risk of primary open angle glaucoma. Vision Res 50 2391 2395
6. MedeirosFAWeinrebRNSamplePAGomiCFBowdC 2005 Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol 123 1351 1360
7. AndersonDRDranceSMSchulzerM Collaborative Normal-Tension Glaucoma Study Group 2001 Natural history of normal-tension glaucoma. Ophthalmology 108 247 253
8. FanBJWiggsJL 2010 Glaucoma: genes, phenotypes, and new directions for therapy. J Clin Invest 120 3064 3072
9. FingertJH 2011 Primary open-angle glaucoma genes. Eye (Lond) 25 587 595
10. ThorleifssonGWaltersGBHewittAWMassonGHelgasonA 2010 Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet 42 906 909
11. WiggsJLKangJHYaspanBLMirelDBLaurieC 2011 Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum Mol Genet 20 4707 4713
12. BurdonKPMacgregorSHewittAWSharmaSChidlowG 2011 Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet 43 574 578
13. AungTRezaieTOkadaKViswanathanACChildAH 2005 Clinical features and course of patients with glaucoma with the E50K mutation in the optineurin gene. Invest Ophthalmol Vis Sci 46 2816 2822
14. FingertJHRobinALStoneJLRoosBRDavisLK 2011 Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Hum Mol Genet 20 2482 2494
15. MeguroAInokoHOtaMMizukiNBahramS Writing Committee for the Normal Tension Glaucoma Genetic Study Group of Japan Glaucoma Society 2010 Genome-wide association study of normal tension glaucoma: common variants in SRBD1 and ELOVL5 contribute to disease susceptibility. Ophthalmology 117 1331 1338
16. CornelisMCAgrawalAColeJWHanselNNBarnesKC 2010 The Gene, Environment Association Studies consortium (GENEVA): maximizing the knowledge obtained from GWAS by collaboration across studies of multiple conditions. Genet Epidemiol 34 364 372
17. WiggsJLHauserMAAbdrabouWAllinghamRRBudenzDL 2011 The NEIGHBOR Consortium Primary Open Angle Glaucoma Genome-wide Association Study: Rationale, Study design and Clinical variables. In press Journal of Glaucoma
18. WillerCJLiYAbecasisGR 2010 METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26 2190 2191
19. MacgregorSHewittAWHysiPGRuddleJBMedlandSE 2010 Genome-wide association identifies ATOH7 as a major gene determining human optic disc size. Hum Mol Genet 19 2716 2724
20. RamdasWDvan KoolwijkLMIkramMKJansoniusNMde JongPT 2010 A genome-wide association study of optic disc parameters. PLoS Genet 6 e1000978 doi:10.1371/journal.pgen.1000978
21. KhorCCRamdasWDVithanaENCornesBKSimX 2011 Genome-wide association studies in Asians confirm the involvement of ATOH7 and TGFBR3, and further identify CARD10 as a novel locus influencing optic disc area. Hum Mol Genet 20 1864 1872
22. AxenovichTZorkoltsevaIBelonogovaNvan KoolwijkLBorodinP 2011 Linkage and association analyses of glaucoma related traits in a large pedigree from a Dutch genetically isolated population. J Med Genet 48 802 809
23. GastenARamdasWBroerLvan KoolwijkLIkramM 2012 A genetic epidemiologic study of candidate genes involved in the optic nerve head morphology. Invest Ophthalmol Vis Sci 2012 Jan 20. [Epub ahead of print]
24. RamdasWDvan KoolwijkLMLemijHGPasuttoFCreeAJ 2011 Common genetic variants associated with open-angle glaucoma. Hum Mol Genet 20 2464 2471
25. CunningtonMSSantibanez KorefMMayosiBMBurnJKeavneyB 2010 Chromosome 9p21 SNPs Associated with Multiple Disease Phenotypes Correlate with ANRIL Expression. PLoS Genet 6 e1000899 doi:10.1371/journal.pgen.1000899
26. HesselberthJRChenXZhangZSaboPJSandstromRReynoldsAPThurmanRENephSKuehnMSNobleWSFieldsSStamatoyannopoulosJA 2009 Global mapping of protein-DNA interactions in vivo by digital genomic footprinting. Nat Methods 6 283 289
27. BirneyEStamatoyannopoulosJADuttaAGuigóR ENCODE Project Consortium 2007 Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447 799 816
28. BurdCEJeckWRLiuYSanoffHKWangZSharplessNE 2010 Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet 6 e1001233 doi:10.1371/journal.pgen.1001233
29. ThorleifssonGMagnussonKPSulemPWaltersGBGudbjartssonDF 2007 Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science 317 1397 1400
30. LiuYSchmidtSQinXGibsonJHutchinsK 2008 Lack of association between LOXL1 variants and primary open-angle glaucoma in three different populations. Invest Ophthalmol Vis Sci 49 3465 3468
31. HannonGJBeachD 1994 p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 371 257 261
32. GamazonERZhangWKonkashbaevADuanSKistnerEO 2010 SCAN: SNP and copy number annotation. Bioinformatics 26 259 262
33. UchidaDOmoteharaFNakashiroKTateishiYHinoS 2003 Posttranscriptional regulation of TSC-22 (TGF-beta-stimulated clone-22) gene by TGF-beta 1. Biochem Biophys Res Commun 305 846 854
34. YaspanBLBushWSTorstensonESMaDPericak-VanceMA 2011 Genetic analysis of biological pathway data through genomic randomization. Hum Genet 129 563 571
35. FanBJWangDYPasqualeLRHainesJLWiggsJL 2011 Genetic variants associated with optic nerve vertical cup-to-disc ratio are risk factors for primary open angle glaucoma in a US Caucasian population. Invest Ophthalmol Vis Sci 52 1788 1792
36. RamdasWDvan KoolwijkLMLemijHGPasuttoFCreeAJ 2011 Common genetic variants associated with open-angle glaucoma. Hum Mol Genet 20 2464 2471
37. GreeneLALiuDXTroyCMBiswasSC 2007 Cell cycle molecules define a pathway required for neuron death in development and disease. Biochim Biophys Acta 1772 392 401
38. HarismendyONotaniDSongXRahimNGTanasaB 2011 9p21 DNA variants associated with coronary artery disease impair interferon-γ signaling response. Nature 470 264 268
39. HowellGRMacalinaoDGSousaGLWaldenMSotoI 2011 Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest 21 1429 1444
40. PogueAICuiJGLiYYZhaoYCulicchiaFLukiwWJ 2010 Micro RNA-125b (miRNA-125b) function in astrogliosis and glial cell proliferation. Neuroscience Letters 476 18 22
41. TrifanOCTraboulsiEIStoilovaDAlozieINguyenR 1998 A third locus (GLC1D) for adult-onset primary open-angle glaucoma maps to the 8q23 region. Am J Ophthalmol 126 17 28
42. CrooksKRAllinghamRRQinXLiuYGibsonJR 2011 Genome-wide Linkage Scan for Primary Open Angle Glaucoma: Influences of Ancestry and Age at Diagnosis. PLoS ONE 6 e21967 doi:10.1371/journal.pone.0021967
43. FossettNTevosianSGGajewskiKZhangQOrkinSHSchulzRA 2001 The Friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc Natl Acad Sci U S A 98 7342 7347
44. PanagisLZhaoXGeYRenLMittagTWDaniasJ 2011 Retinal gene expression changes related to IOP exposure and axonal loss in DBA/2J mice. Invest Ophthalmol Vis Sci 52 7807 7816
45. CuzzoLMRoss-CisnerosFNYeeKMWangMYSadunAA 2011 Low-density lipoprotein receptor-related protein is decreased in optic neuropathy of Alzheimer disease. J Neuroophthalmol 31 139 146
46. VethKNWillerJRColleryRFGrayMPWillerGB 2011 Mutations in zebrafish lrp2 result in adult-onset ocular pathogenesis that models myopia and other risk factors for glaucoma. PLoS Genet 7 e1001310 doi:10.1371/journal.pgen.1001310
47. PrasadSSKojicLWenYHChenZXiongW 2010 Retinal gene expression after central retinal artery ligation: effects of ischemia and reperfusion. Invest Ophthalmol Vis Sci 51 6207 6319
48. WangDYRayARodgersKErgorulCHymanBT 2010 Global gene expression changes in rat retinal ganglion cells in experimental glaucoma. Invest Ophthalmol Vis Sci 51 4084 4095
49. JohnsonECJiaLCepurnaWODoserTAMorrisonJC 2007 Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci 48 3161 3177
50. BattleMAMaherVMMcCormickJJ 2003 ST7 is a novel low-density lipoprotein receptor-related protein (LRP) with a cytoplasmic tail that interacts with proteins related to signal transduction pathways. Biochemistry 42 7270 7282
51. ChoiSJMoonJHAhnYWAhnJHKimDUHanTH 2005 Tsc-22 enhances TGF-beta signaling by associating with Smad4 and induces erythroid cell differentiation. Mol Cell Biochem 1 23 28
52. KillerHEMillerNRFlammerJMeyerPWeinrebRN 2011 Cerebrospinal fluid exchange in the optic nerve in normal-tension glaucoma. Br J Ophthalmol 2011 Nov 24. [Epub ahead of print]
53. BerdahlJPAllinghamRRJohnsonDH 2008 Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology 115 763 768
54. HanHWeckerTGrehnFSchlunckG 2011 Elasticity-Dependent Modulation of TGF-{beta} Responses in Human Trabecular Meshwork Cells. Invest Ophthalmol Vis Sci 52 2889 2896
55. PasqualeLRDorman-PeaseMELuttyGAQuigleyHAJampelHD 1993 Immunolocalization of TGF-beta 1, TGF-beta 2, and TGF-beta 3 in the anterior segment of the human eye. Invest Ophthalmol Vis Sci 34 23 30
56. MiaoHCrabbAWHernandezMRLukasTJ 2010 Modulation of factors affecting optic nerve head astrocyte migration. Invest Ophthalmol Vis Sci 51 4096 4103
57. KirwanRPLeonardMOMurphyMClarkAFO'BrienCJ 2005 Transforming growth factor-beta-regulated gene transcription and protein expression in human GFAP-negative lamina cribrosa cells. Glia 52 309 324
58. SethiAMaoWWordingerRJClarkAF 2011 Transforming Growth Factor Beta Induces Extracellular Matrix Protein Crosslinking Lysyl Oxidase (LOX) Genes in Human Trabecular Meshwork Cells. Invest Ophthalmol Vis Sci May 5 [Epub ahead of print] (2011)
59. ZodeGSClarkAFWordingerRJ 2009 Bone morphogenetic protein 4 inhibits TGF-beta2 stimulation of extracellular matrix proteins in optic nerve head cells: role of gremlin in ECM modulation. Glia 57 755 766
60. AliMMcKibbinMBoothAParryDAJainP 2009 Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet 84 664 671
61. FanBJPasqualeLRRheeDLiTHainesJLWiggsJL 2011 LOXL1 promoter haplotypes are associated with exfoliation syndrome in a U.S. Caucasian population. Invest Ophthalmol Vis Sci 52 2372 2378
62. LoomisSJOlsonLMPasqualeLRWiggsJMirelD 2010 Feasibility of High-Throughput Genome-Wide Genotyping using DNA from Stored Buccal Cell Samples. Biomark Insights 5 49 55
63. GundersonKLSteemersFJRenHNgPZhouL 2006 Whole-genome genotyping. Methods Enzymol 410 359 376
64. PurcellSNealeBTodd-BrownKThomasLFerreiraMA 2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
65. LiYWillerCJSannaSAbecasisGR 2009 Genotype Imputation. Annu Rev Genomics Hum Genet 10 387 406
66. LiYWillerCJDingJScheetPAbecasisGR 2010 MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol 34 816 834
67. WahlinKJHacklerLJrAdlerRZackDJ 2010 Alternative splicing of neuroligin and its protein distribution in the outer plexiform layer of the chicken retina. J Comp Neurol 518 4938 4962
68. LiuWLiuYQinXJSchmidtSHauserMAAllinghamRR 2010 AQP1 and SLC4A10 as candidate genes for primary open-angle glaucoma. Mol Vis 16 93 97
69. DesronvilTLogan-WyattDAbdrabouWTrianaMJonesR 2010 Distribution of COL8A2 and COL8A1 gene variants in Caucasian primary open angle glaucoma patients with thin central corneal thickness. Mol Vis 16 2185 2191
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



