-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
Mu is both a transposable element and a temperate bacteriophage. During lytic growth, it amplifies its genome by replicative transposition. During infection, it integrates into the Escherichia coli chromosome through a mechanism not requiring extensive DNA replication. In the latter pathway, the transposition intermediate is repaired by transposase-mediated resecting of the 5′ flaps attached to the ends of the incoming Mu genome, followed by filling the remaining 5 bp gaps at each end of the Mu insertion. It is widely assumed that the gaps are repaired by a gap-filling host polymerase. Using the E. coli Keio Collection to screen for mutants defective in recovery of stable Mu insertions, we show in this study that the gaps are repaired by the machinery responsible for the repair of double-strand breaks in E. coli—the replication restart proteins PriA-DnaT and homologous recombination proteins RecABC. We discuss alternate models for recombinational repair of the Mu gaps.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002642
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002642Summary
Mu is both a transposable element and a temperate bacteriophage. During lytic growth, it amplifies its genome by replicative transposition. During infection, it integrates into the Escherichia coli chromosome through a mechanism not requiring extensive DNA replication. In the latter pathway, the transposition intermediate is repaired by transposase-mediated resecting of the 5′ flaps attached to the ends of the incoming Mu genome, followed by filling the remaining 5 bp gaps at each end of the Mu insertion. It is widely assumed that the gaps are repaired by a gap-filling host polymerase. Using the E. coli Keio Collection to screen for mutants defective in recovery of stable Mu insertions, we show in this study that the gaps are repaired by the machinery responsible for the repair of double-strand breaks in E. coli—the replication restart proteins PriA-DnaT and homologous recombination proteins RecABC. We discuss alternate models for recombinational repair of the Mu gaps.
Introduction
Transposable elements drive genome evolution in many ways – increasing DNA content, rearranging and mutating genes, as well as altering gene regulation [1]. Temperate phage Mu has played a pivotal role in our current understanding of how movable elements move [2]. A unique aspect of Mu is that, depending on the phase of its life cycle, it moves using either replicative or non-replicative modes of DNA transposition [3]. Most of our knowledge of Mu transposition is derived for the replicative pathway, where during lytic growth, Mu amplifies its genome by repeated transposition-replication events which exploit the host replication apparatus [4], [5]. In vitro experiments have established that in this pathway, the Mu transposase (MuA protein) mediates single-strand cleavages at Mu ends followed by strand transfer of the cleaved ends into target DNA; the latter reaction is greatly assisted by MuB protein (Figure 1). The resulting branched strand transfer joint is resolved by target-primed replication, which is initiated by the PriA primosome and completed by the Pol III holoenzyme, and results in duplication of the Mu genome after every round of integration. At the end of the lytic cycle, Mu genomes are packaged into phage heads such that they include host sequences (flaps) from both sides of a Mu insertion.
Fig. 1. Known steps in replicative and non-replicative (repair) pathways of Mu transposition. 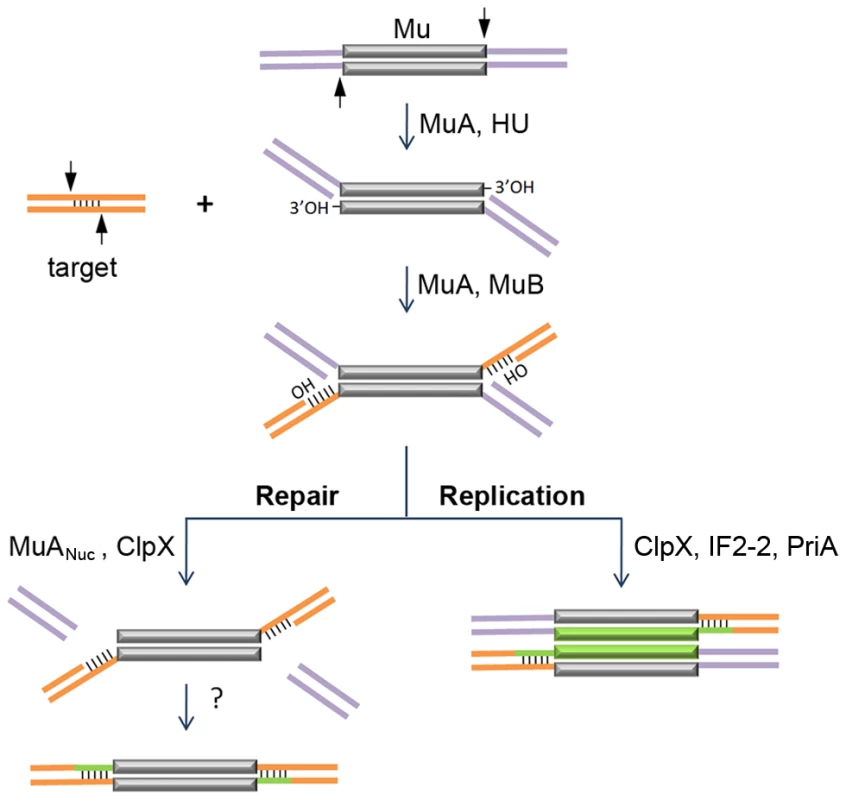
The transposase MuA, in the presence of E. coli protein HU, first introduces single-stranded cleavages at the 3′ ends. With assistance from MuB, the 3′OHs at the cleaved ends are transferred by MuA to phosphodiester bonds spaced 5 bp apart in the target [3], [4]. The resultant branched strand transfer intermediate is processed alternately. During the lytic cycle, Mu is inserted in the chromosome, the target is also in the chromosome, so the purple flanking DNA is continuous with the orange target; transposition is intramolecular. The target OHs found in the strand transfer intermediate are used as primers to replicate Mu (green lines). ClpX, IF2-2 and other uncharacterized factors are required for disassembly of the transpososome followed by assembly of the PriA restart primosome on the Mu ends [5]. During integration of infecting Mu, the purple flanking DNA on the incoming Mu genome is non-covalently joined to itself via phage N protein; transposition into the chromosome target is intermolecular [9], [10], [11]. The branched strand transfer intermediate is resolved/repaired by MuANuc-mediated resection of the flap DNA [13], [14]. ClpX is required for this reaction. The remaining gaps are thought to be filled by host enzymes. The non-replicative pathway of Mu transposition is only used when progeny phage infect new hosts [6], [7], [8]. Along with Mu DNA, the phage also inject into the host the phage N protein, which binds at the termini and converts the linear Mu genome into a non-covalently closed supercoiled circle [9], [10], [11]. Integration of the infecting Mu into the host genome follows the same initial nick-join steps of transposition established for the replicative mechanism in vitro; however, instead of target-primed Mu replication, the host flaps are resected and the gaps are repaired by unknown mechanisms [12] (Figure 1). Flap resection has not yet been demonstrated in vitro. This reaction is dependent in vivo on the cryptic endonuclease activity harbored within the C-terminal domain of the transposase MuA (designated MuANuc in this study), as well as on the chaperone protein ClpX [13], [14]. ClpX is known to play an essential role during Mu replication, remodeling the Mu transpososome and enabling its transition to a replisome [5], [15] (Figure 1). The alternative choices for resolving the transposition intermediate, i.e. repair versus replication, must involve additional phage and host factors whose identity is not yet established.
The current study was undertaken to identify host factors involved in the repair of Mu insertions during the non-replicative infection pathway. To do so we used the Keio Collection, which is a set of 3,985 precisely defined, single-gene deletions of all nonessential genes in Escherichia coli K-12 [16], and screened for mutants defective in recovery of Mu::Cm insertions. Among the several mutants that gave a poor yield of CmR integrants, a majority of those that allowed Mu entry showed normal integration and replication of wild type Mu. By using two additional phage variants to re-screen/re-test in order to eliminate those defective in maintenance of a stable prophage state, we narrowed the search to a small subset of the mutants. Included among these were mutants in the homologous recombination pathway - recA, recB, recC. Two mutants - priA and dnaT – were defective in Mu replication as expected, but were unexpectedly defective in the recovery of insertions despite being proficient in Mu integration. The data show that Mu insertions are repaired by the replication restart machinery and homologous recombination proteins.
Results
E. coli Keio mutant screen with wild-type Mu
A functional map of the Mu genome is shown in Figure 2A. A ∼1 kb cat cassette encoding chloramphenicol (Cm) resistance was inserted into a non-essential region of the prophage genome (see Materials and Methods). Phage derived from this strain were used to infect the Keio mutant collection (see Table 1 for strain information), which occupies forty-eight 96-well plates, and spotted on agar slabs containing chloramphenicol to select for Mu lysogens as described in Methods. The control panel in Figure 2B shows results expected for known hosts where Mu integrates, but either does or does not replicate. In our standard wild type host BU1384 where Mu replicates, ∼90% of the infected cells undergo lytic growth and lysis, and ∼10% of the survivors (i.e. ∼1% of input cells) are lysogens. Mu fails to replicate in isogenic strains carrying either a himA or a clpX null mutant allele. himA(ihfA) codes for one of the two subunits of the regulatory protein IHF, which is required for early Mu gene transcription [2], [17], and ClpX is essential for Mu replication [5], [18]. Both of these mutant strains support Mu integration [12], [13], [19]. A larger number of CmR colonies are recovered in these strains compared to wild type because Mu does not undergo lytic development. Similar differences in the recovery of CmR colonies were seen in the wild type Keio strain BW25113 and its isogenic himA and clpX derivatives. In our screen for repair-defective mutants, we expected to identify mutant spots with either no CmR colonies or with fewer colonies than wild-type.
Fig. 2. Identification of E. coli mutants in the Keio library defective in recovery of Mu::Cm insertions. 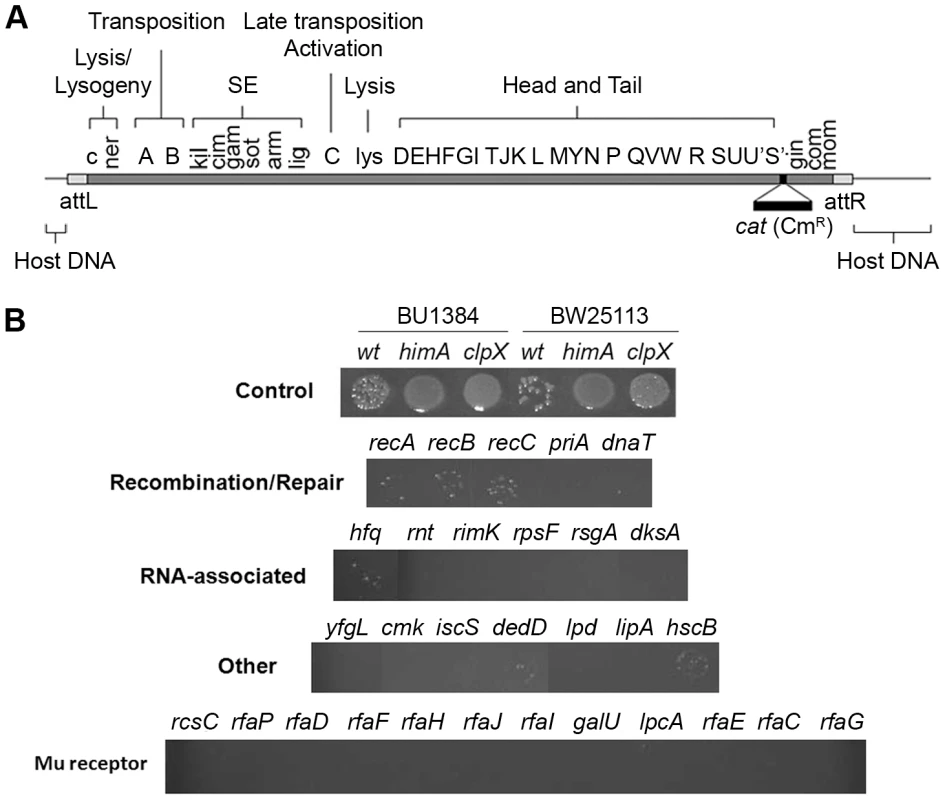
(A) Functional map of the Mu genome packaged within a phage particle, showing position of inserted CmR cassette, and host or flap DNA attached to both ends. The SE (semi-essential) region contains 14 orfs [27]; only those assigned a phenotype/function are indicated [2]. (B) Cultures infected with Mu::Cm were spotted on Cm plates as described in Methods. Control panel: Expected results from infection of two different wild type and their derivative mutant strains - clpX and himA - that do not support Mu replication. Bottom four panels: Final set of mutants from the Keio screen showing lower Mu::Cm lysogen recovery compared to the wild type strain, grouped into indicated categories. Tab. 1. Strains used in this study. 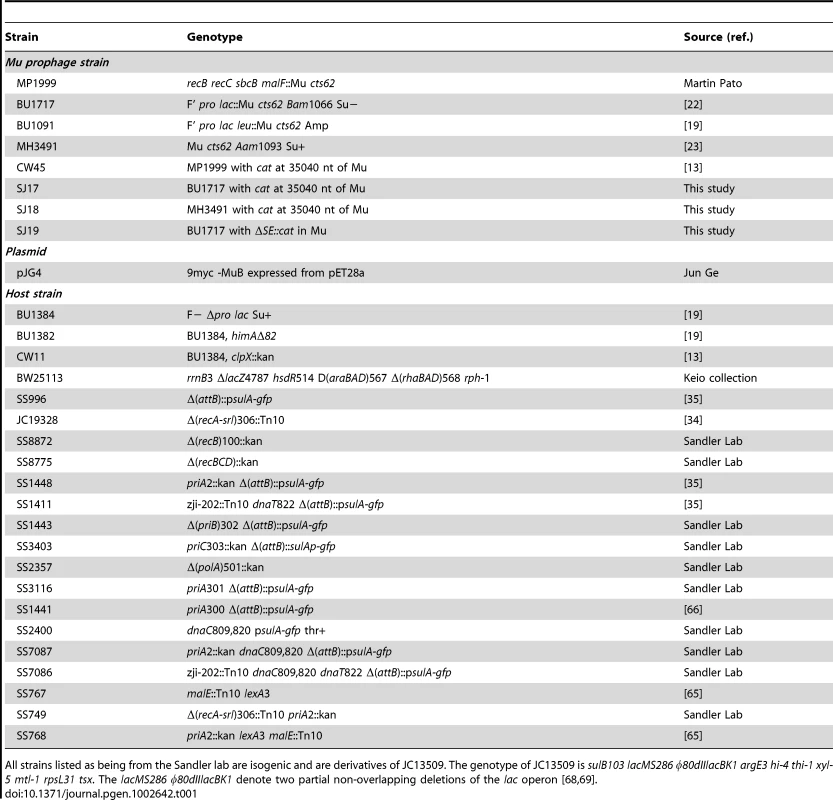
All strains listed as being from the Sandler lab are isogenic and are derivatives of JC13509. The genotype of JC13509 is sulB103 lacMS286 φ80dIIlacBK1 argE3 hi-4 thi-1 xyl-5 mtl-1 rpsL31 tsx. The lacMS286 φ80dIIlacBK1 denote two partial non-overlapping deletions of the lac operon [68], [69]. The majority of mutant strains behaved like wild type in this screen. Known host mutants that do not support replication were easily identified (Figure S1, see plate #1), but no new candidates with this phenotype were observed. Several mutants displayed the phenotype of interest i.e. showed fewer or no colonies in the spots compared to wild type (Figure S1, see plate #1 and #9). The phenotype of these latter mutants was re-confirmed by infecting with Mu phage carrying a different antibiotic resistance marker (Mu::Amp) to ensure that the phenotype was independent of the antibiotic used for selection. The final set of 30 mutants displaying this phenotype is arranged in four panels below the control panel in Figure 2B. The mutants are classified broadly into genes known to affect DNA recombination/Repair, RNA-associated functions, ‘Other’ functions, and Mu receptor function. A more detailed description of gene function is listed in Table S1.
Mu integration and replication in E. coli mutants defective in lysogen recovery
The poor yield of CmR colonies in the mutants shown in Figure 2B could be due to defects in Mu entry, integration, stable maintenance of lysogeny, or repair. To distinguish between some of these possibilities a PCR assay was first employed to test for Mu integration (Figure 3A). Two primers were chosen to amplify covalent junctions between the left end of Mu DNA and an arbitrarily chosen target gene purH. A PCR product is expected once the 3′ ends of Mu are joined to the target regardless of the fate of 5′ ends (see Figure 1). PCR products of different lengths are expected since Mu integration is essentially random [20], [21]. Using this method, a control experiment first followed the time course of wild type as well as mutant Bam and Aam Mu phage infections in the wild type strain. The particular Bam mutation used here (Bam1066) is reported to be fairly proficient in integration but defective in replicative transposition of Mu [22]. The Aam mutant (Aam1093) is defective in integration [23]. The integration patterns obtained during these infection experiments were consistent with the known transposition properties of these phages (Figure 3A).
Fig. 3. PCR assay for Mu integration in mutants defective in lysogen recovery. 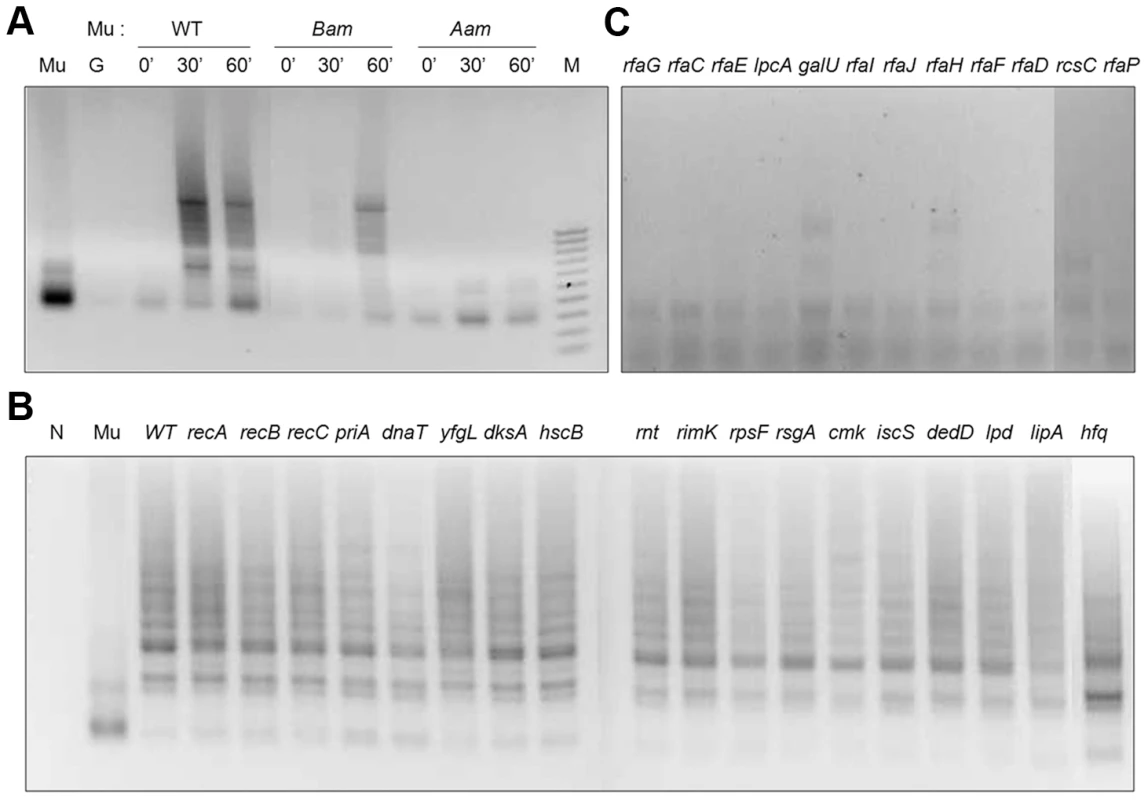
(A) Control PCR reactions monitoring integration at different time points after infection of wild type BW25113 with Mu::Cm, Mu::Cm(Bam1066) and Mu::Cm(Aam1093) phage. These phages can integrate-replicate, integrate but not replicate, or not integrate, respectively. (B) PCR results for wild type Mu::Cm integration 30 min after infection of mutants in the first three categories shown in Figure 2B. (C) As in (B) but with mutants in the Mu Receptor category. Control reactions with either no template (N), Mu, or genomic DNA templates from uninfected BW25113 host (G) are indicated, along with size markers (M). Reaction products were run on agarose gels and stained with ethidium bromide as described under Methods. Wild type Mu was used to infect the 30 mutants obtained in the initial screen for repair-defective mutants (Figure 2B). Mutants grouped under Recombination-Repair, RNA and Other categories all showed similar levels as well as patterns of integration compared to the wild type strain (Figure 3B). Quantitative PCR with a subset of these mutants (priA, recA) validated the results with normal PCR (Figure S2; we note that Southern blots used in earlier studies also showed similar levels of Mu integration in wild type and priA mutants [24]). Thus, these mutants were not defective in either Mu entry or integration. A majority of the mutants with defects in the LPS biosynthesis pathway, however, showed little or no integration (Figure 3C). This is likely due to a block in Mu entry, since the receptor for Mu is located within the LPS [25], [26].
To test if mutants that supported integration also supported Mu replication, cell lysis and phage production were monitored. Growth of the strains with and without Mu infection is shown in Figure S3. The LPS mutants in Figure 2B all grew as well as wild type; only a representative mutant rfaF is shown in Figure S3A. Neither this mutant, nor others in this category were susceptible to lysis by Mu infection (Figure S3B), supporting the conclusion that this group of mutants is defective in Mu entry. They were therefore not studied further. The remaining mutants showed varying degrees of growth impairment compared to wild type (Figure S3A). With the exception of priA and dnaT, which are essential for Mu replication [5], cell lysis and phage production were observed in all of the infected strains (Figure S3B). Thus, the majority of these mutants supported both Mu integration and replication. Their defect in yielding stable lysogens could therefore be due to an inability to maintain lysogeny or defects in repair of the insertions.
Defects in maintenance of the prophage state or lysogeny might be discerned by examining Mu plaque morphologies on these mutants. These would be expected to have a ‘clear’ rather than the ‘turbid’ phenotype observed for wild type Mu, which can be maintained in a lysogenic state. dksA, hfq, rnt and rpsF gave turbid plaque morphologies somewhat similar to the wild type strain, dedD was apparently clear, while the remaining mutants had clear centers and clear edges with turbid rings in-between (Figure S4). In the latter set of mutants with the mixed clear-turbid phenotype, it was difficult to ascertain whether the lysogeny-maintenance function might be affected.
Keio mutant screen with replication-defective Mu
To eliminate scoring mutants as repair-defective because they were unable to maintain the lysogenic state and were therefore going lytic, we re-screened the Keio library with a Mu::Cm variant defective in replication. This phage carries the Bam1066 mutation, which allows integration but does not support replicative transposition (see Figure 3A; [22]). The same set of mutants was isolated in this screen as well. In the spot test results shown in Figure S5, it appears that some of the mutants have more CmR colonies than obtained with wild type phage (see Figure 2B). This is because a higher proportion of cells survive during infection with this phage due to absence of lytic growth. Lysogen recovery was therefore quantified as described under Methods (Figure 4A). Among mutants in the Recombination-Repair category, priA and dnaT mutants were the most severely affected in lysogen recovery (0.04%), followed by recA (0.2%), recB (0.7%) and recC (0.9%). Among mutants in the RNA and Other category, with the exception of yfgL, dksA, hfq, rimK and lpd, the remainder had lysogen frequencies similar to or even better than wild type.
Fig. 4. Mutant screen using replication-defective Mu. 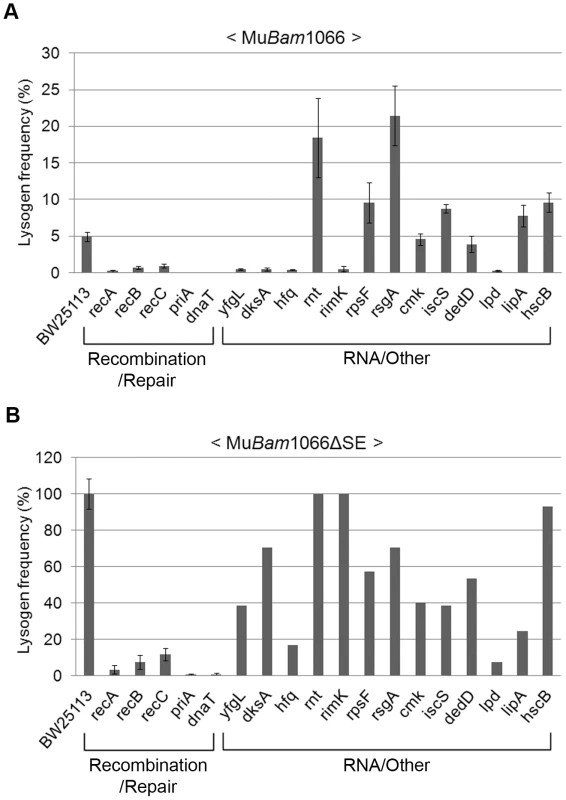
Lysogenization efficiencies (calculated as CmR cells/infected cells×100) of the mutant strains infected with either (A) Mu::Cm(Bam1066) or (B) MuBam1066ΔSE::Cm. Mutant categories as in Figure 2B. Error bars indicate standard deviation from the mean of triplicate data sets obtained from three independent colonies of the same strain. In (B), data for RNA/Other mutants are from a single colony/experiment. See Methods for details. A surprising aspect of the data shown in Figure 4A is that lysogen recovery in the wild type was only ∼5% with MuBam phage, and that cell viability after infection was only ∼20% (Figure S6A). Similar low cell viability was observed even after infection with integration-defective MuAam phage (Figure 3A and Figure S6A), which gave no CmR colonies. To test if this was due to expression of the cell killing function kil or to other function(s) specified by the unknown orfs in the SE (semi-essential) region [27], which is transcribed as part of a long early transcript that includes the A and B genes [2] (see Figure 2A), we deleted the SE region in the MuBam phage (see Methods). Indeed, infection with MuBam1066ΔSE::Cm phage improved both lysogen recovery and cell viability in the wild type to 100% (Figure 4B and Figure S6B, respectively). Under these conditions, all the mutants in the Recombination-Repair category still remained impaired (<15% of wild type) for lysogen recovery. In the RNA/Other category, hfq, lpd and lipA were also still substantially impaired (18–25% of wild type). Since hfq shows wild type plaque morphology (Figure S4) and since there is no obvious relationship of the known functions of these three genes to DNA repair, we will not consider them further here.
We conclude that a majority of the E. coli genes required for recovery of stable Mu insertions provide functions that apparently allow host survival in the presence of lethal phage functions specified by the SE region of Mu. The group of five genes that remain defective - priA, dnaT, recA, recB and recC – is significant in that this group is known to participate in recombinational repair. The isolation of this group of genes must be related to the repair of Mu insertions and not to repair of random double strand breaks generated upon Mu infection, because (1) they are dependent on Mu integration (i.e. infection with MuAam1093 phage does not significantly affect the viability of the priA and recA hosts as compared to wild type; Figure S6A), and (2) Mu-induced mutations are known to be tightly linked to Mu i.e. they are not random [28].
Role of replication restart in the non-replicative pathway of Mu transposition
PriA and DnaT play a central role in the repair of nicks and gaps created by DNA damaging agents in E. coli by promoting replication restart after fork collapse, either with or without the involvement of recombination [29]. There are multiple pathways for replication restart that require PriA, PriB, PriC, DnaT and Rep [29]. These proteins identify the correct substrate, process it if necessary, and then aid DnaC in loading the replicative helicase DnaB during pre-primosome formation. PriA and DnaT are required for the two main pathways of ‘Restart’ where PriB and PriC have redundant roles. Thus priA and dnaT null mutants have extreme phenotypes whereas priB and priC null mutants have none. dnaC809,820 is a priC/rep-independent suppressor that restores all known phenotypes of priA and dnaT null mutants [30]. During the lytic cycle of Mu growth, PriA restarts Mu replication without the involvement of homologous recombination ([24], [31] and Figure S3B). The data reported in Figure 2, Figure 3, and Figure 4 in this study show that PriA and DnaT are also required during the non-replicative event, along with a requirement for homologous recombination proteins.
To confirm the phenotype of priA, dnaT, and the rec genes and to dissect the role of PriA further, we tested these and several different mutant alleles of these genes in a different strain background. The priA, dnaT, recA, recB (and recBCD) mutants all showed defects in Mu lysogen recovery in this strain background as well (Figure 5A). priA and dnaT mutants show poor growth (Figure S3A and [32], [33], [34]) and many cells in the population have high levels of SOS expression [35]. SOS genes are normally kept silent by the repressor LexA, and activated only when LexA is cleaved by RecA in response to DNA damage [36]. SOS induction can be prevented by removing recA or by introducing a non-cleavable lexA3 allele [37]. To test if SOS expression is responsible for the low recovery of Mu lysogens, we tested priA lexA3 and priA recA double mutants; both mutants remained defective (Figure 5B). A lexA3 mutant alone supported efficient recovery of Mu insertions, showing additionally that the SOS response is not required, but that the recombination function of RecA is needed. We note that recA1, a recombination-defective missense allele of recA, was not seen to affect recovery of Mu insertions in Salmonella [38], [39]. This allele can bind ssDNA in vitro [40], and perhaps has residual activity in vivo that allows it to function in Mu repair. We also note that several genes in the Keio collection were recently reported to be partially duplicated [41]. Of these, priB and polA are of interest to this study. These gene deletions as well as priC were therefore re-tested in the same strain background as the priA alleles. They were found to not affect Mu recovery (Figure 5B).
Fig. 5. Behavior of various priA, dnaT, and rec alleles in a different strain background. 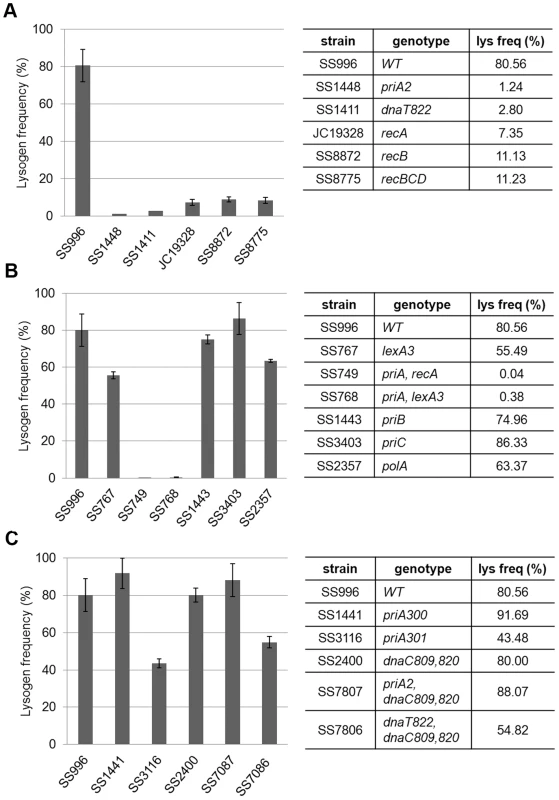
MuBam1066ΔSE::Cm was used for infection of indicated strains to assess their role in recovery of Mu insertions. Other descriptions as in Figure 4. PriA has at least four types of activities: ATPase, helicase, the ability to load the replisome, and the ability to interact with other proteins. PriA300 (K230R) inactivates the ATPase and helicase activities, yet primosome assembly can occur both in vivo and in vitro [42], [43]. PriA301 (C479Y) mutates a residue in the cysteine-rich region of PriA thought to be important for protein-protein interactions and helicase activity [44]. Like priA300, priA301 maintains wild-type growth and recombination proficiency [45], [46]. Lack of the helicase activity of PriA has been reported to impair Mu replication both in vivo and in vitro [31]. Using the helicase-defective strains priA300 and priA301, we observed that the helicase and protein-protein interaction activities of PriA are largely dispensable (Figure 5C), indicating that it is the primosome activity of PriA that is essential for recovery of Mu insertions. This is further supported by the observation that combining priA and dnaT null mutations with dnaC809,820 restores the ability of strains to recover lysogens (Figure 5C). Both in vivo and in vitro experiments have suggested that mutant DnaC proteins suppress the absence of PriA/DnaT complex by bypassing its role in helping DnaC to load DnaB/PolIII directly onto a recombinational intermediate [30], [47].
To confirm that all of these data point to a critical role for replication restart in repair of Mu insertions, we sequenced fifteen independent insertions which were recovered at a low frequency in the priA mutant (see Materials and Methods) (Figure 6). Of these, five insertions had rearranged the Mu-host junctions in various ways, and their precise location could not be determined. Two insertions had symmetrical additions (at both ends) of a nucleotide not found in the wild type host, likely due to repair by an error-prone polymerase, and one of these strains had two copies of Mu. Eight insertions had normal Mu-host junctions. We note that the sequencing strategy included cloning of CmR Mu DNA fragments, favoring recovery of R end fragments that had not been deleted or rearranged, and therefore underestimating the fraction of incorrectly repaired insertions. Overall, these results show that in the absence of PriA, Mu insertions are repaired inefficiently and often incorrectly by alternate pathways. Thus, PriA is indeed required for normal repair of Mu insertions.
Fig. 6. Sequence of Mu-host junctions at 15 insertions recovered in a priA mutant infected with Mu::Cm(Bam1066). 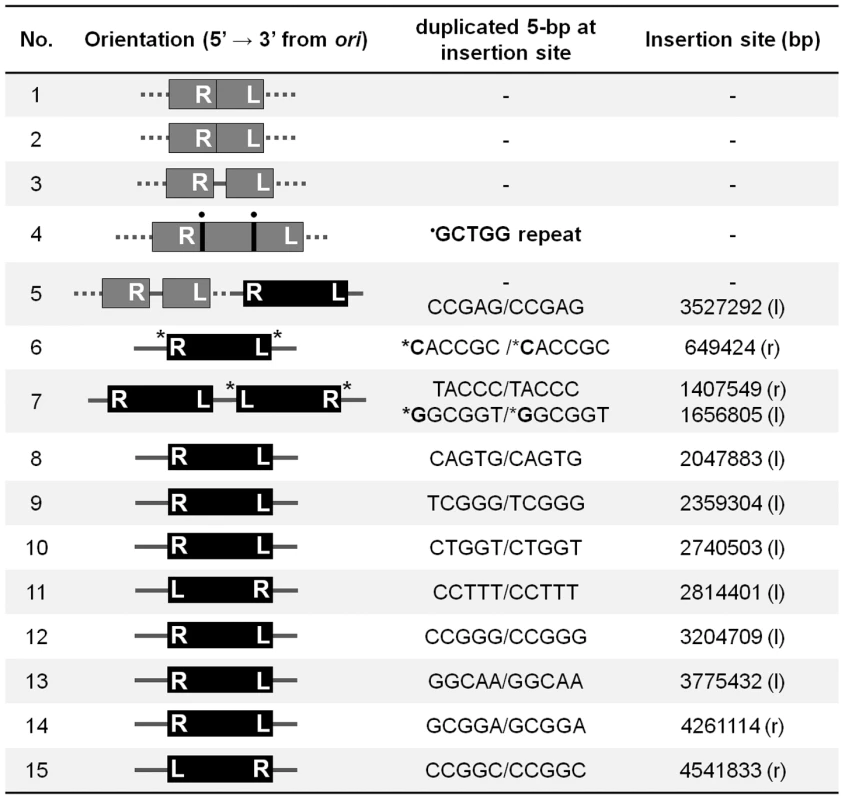
See Methods for sequencing details. Orientation refers to clockwise positions of Mu from oriC, which is at ∼3.92 Mb; ter is at ∼1.59 Mb. The numbers in the Insertion site column refer to nucleotides on the E. coli genome. Black bars, intact Mu with L an R ends indicated; Gray bars, truncated/duplicated Mu with only one end identified; Dotted lines, undetermined host DNA sequence; • a repeated sequence; * insertion of nucleotides not found in the host DNA; l, r, position of insertions in the left and right replicores, respectively. Discussion
Most transposable elements generate characteristic target site duplications flanking their insertion sites as a result of staggered cuts in the target initially made by the transposase [1]. For the large majority of known transposable elements whose transposition is not coupled to replication, it is not known how the single-stranded gaps left in the target after strand transfer are filled. For retroviruses and Line retroelements, double-strand break repair pathways (NHEJ, ATM, ATR) have been implicated [48], [49], [50], [51]. The present study finds that for Mu, in the non-replicative pathway, the gaps are repaired by the primary machinery for double-strand break repair in E. coli – the PriA primosome and homologous recombination proteins. This finding represents a radical change in thinking regarding Mu transposition in particular, and the transposition field in general. In the case of Mu, this is because one did not expect replicative functions to be involved in a transposition event that had been labeled ‘non-replicative’ in early studies. The original label was somewhat of a misnomer in that it described the replication status of Mu prior to integration [6], [7], [8]. However, discovery of flap DNA removal upon Mu integration [12], [13] meant that a second round of transposition could not occur until the gapped strand transfer intermediate was repaired. This event is therefore clearly different from the target-primed replication that immediately follows strand transfer during the replicative pathway. Early experiments that established the non-replicative transposition pathway found limited replication near the ends shortly after integration of infecting Mu, consistent with the idea of gap-filling repair [7]. We note that simple inserts generated using crude extracts and mini-Mu plasmids in vitro were also seen to have some replication associated with ends of Mu DNA, although it is not clear whether these simple insertion events are representative of the first integration event after Mu infection [52]. The identification of replication restart proteins in the present study suggests a new pathway for gap repair. These findings should spur a re-examination of similar assumptions made for other transposons that transpose by non-replicative mechanisms.
Requirement for PriA in both replicative and non-replicative Mu transposition
There are three pathways for replication restart in E. coli: PriA–PriB, PriA–PriC, and PriC–Rep, which differ in their recognition of stalled forked structures [29]. PriA plays an essential role in initiation of replication on the forked DNA intermediates generated during the lytic phase of Mu growth, using either the PriA–PriB or PriA–PriC pathway, in addition to the proteins that are required for E. coli chromosomal replication [24], [31], [53], [54]. During Mu transposition, the transition from strand transfer to DNA replication can be divided into a number of discrete steps [3], [5]. MuA initially remains tightly bound to the Mu fork as a multi-subunit complex called transpososome. In a highly choreographed series of steps, host proteins dislodge this transpososome and assemble a replisome. In the first step of this transition, ClpX alters MuA subunit interactions to weaken interaction of the transpososome with DNA [55], [56], [57]. Next, as yet unidentified cellular factors called Mu Replication Factor α2 (MRF α2) displace the transpososome and exchange it with the translation initiation factor IF2-2 to produce a pre-replisome [58]. Finally, the helicase activity of PriA is required to displace IF2-2, remodeling the template to permit replisome assembly, which includes DnaT, DnaB, DnaC and the DNA polymerase III holoenzyme [5]. PriA has distinct replisome assembly and 3′ to 5′ helicase activities [29]. Helicase-defective PriA supports little or no Mu replication in vitro, and shows a partial defect in Mu replication in vivo [31]. These data indicate that PriA's replisome assembly activity is essential for initiation of Mu DNA replication and that the helicase activity also promotes this process. PriA is thought to bind to the lagging strand template at the fork and unwind it in a 3′ to 5′ direction, promoting loading of DnaB, thus coupling its replisome assembly and helicase activities.
The surprising requirement of PriA and DnaT in the non-replicative pathway of Mu transposition as reported in this study, suggests strongly that the 5 bp gaps generated upon Mu insertion are repaired by the replication restart machinery. This shared requirement for the PriA primosome in both pathways might imply that the PriA loading steps after strand transfer are similar in both. What apparently distinguishes the two pathways is non-requirement of the helicase activity of PriA, and requirement for homologous recombination proteins. We discuss two alternate models for recombinational gap repair below.
Models for recombinational repair
Nicks and gaps in DNA are normally repaired when their encounter with a traveling replication fork converts them into a double strand break, collapsing the fork [36]. The broken end serves as an entry point for RecBCD, generating single strands for RecA binding, followed by invasion of the intact sister chromosome, thus reconstituting a forked structure for restarting replication via the PriA primosome [59], [60]. In such a scenario for Mu repair, an oriC-initated fork will cause a double strand break when, arriving at the site of a Mu insertion, it encounters the flanking gap (Figure 7A). The double-strand break will be on the chromosomal DNA flanking the Mu insertion, which is expected to be processed by RecBCD, followed by restoration of the fork by recombination, and restart of replication by the primosome. Two considerations make this scenario unappealing. First, Mu does not insert near replication forks [61], so the unrepaired intermediate would be potentially vulnerable to degradation while it waits for the oriC-initiated fork to arrive. Second, the passing fork would encounter only one of the two gaps at each Mu end that need repair, so the entire Mu would have to be replicated, generating a second double strand break at the distal Mu end, reiterating RecA-mediated invasion and primosome assembly before repair of the second gap can be completed. A parsimonious alternative model takes advantage of the PriA replisome already present at the forked strand transfer joints at both Mu ends, recruited there in the normal course of transpososome disassembly (see Figure 1). In this model, the initial steps of PriA recruitment and replication are common to both the repair and replication pathways (Figure 7B). The pathways differ in the flap cleavage step, which ensues concomitant with replication restart, leaving double-strand breaks on the Mu lagging strand. These breaks allow RecBCD entry, creating single-stranded 5′ Mu ends on which RecA polymerizes [62]. Although 3′ end strand invasion is generally preferred with purified RecA, 5′ ends can be used for strand exchange in vitro [63], and in vivo recombination data also fit models that invoke 5′ strand invasion [64]. The Holliday junction so created can then be resolved by Ruv proteins or endonucleases. This model reverses the steps normally associated with recombinational repair, with replication preceding recombination. According to this model, there will be limited replication near the two Mu ends in this largely non-replicative event.
Fig. 7. Models for recombinational repair of Mu insertions in the non-replicative pathway. 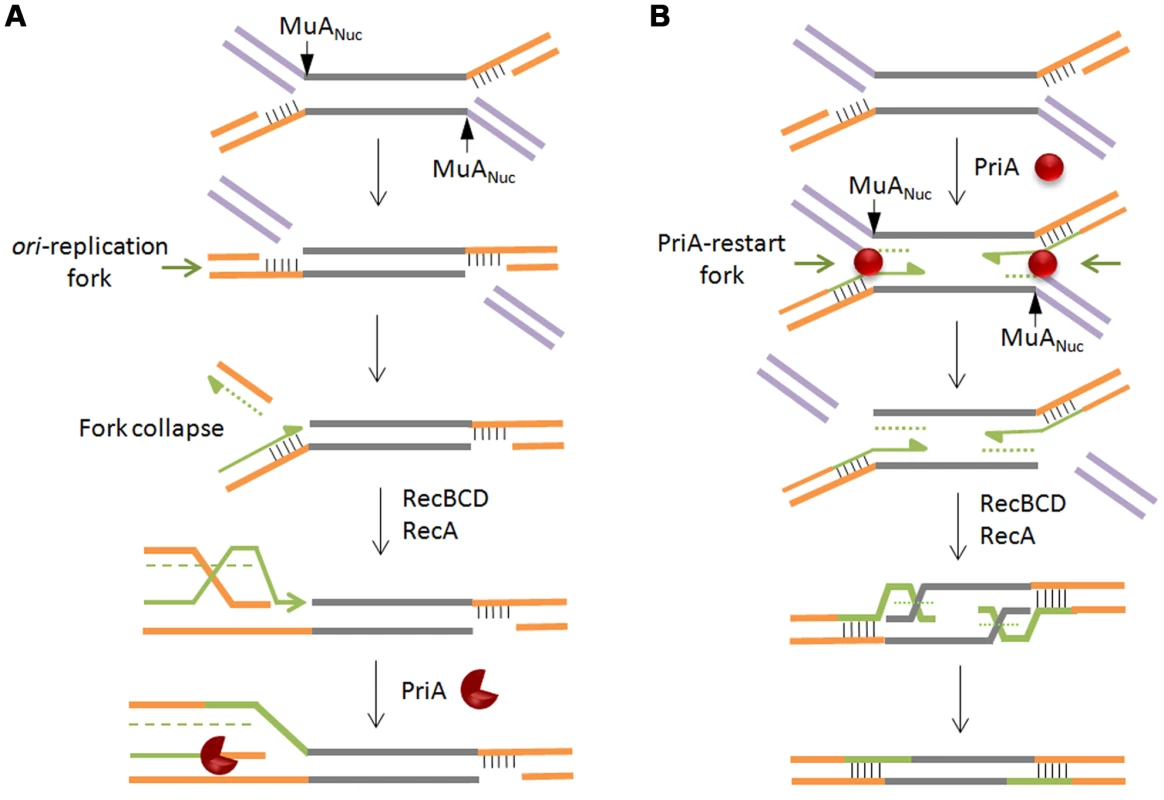
Both models presented rely on repair of double strand breaks by homologous recombination and replication restart proteins, but differ in the location of the break and the order of the recombination/restart-replication events that follow. In (A), the break is on the chromosomal DNA flanking the Mu insertion. Here, homologous recombination is followed by restart replication. In (B), the break is on the Mu lagging strand. Here, restart replication precedes homologous recombination. Alternate shapes for PriA denote uni- or bi-directional replication. See text for details. What signals flap cleavage in one pathway and not in the other? We speculate that the MuN protein, which normally protects the ends of infecting Mu DNA from degradation, dissociates from the ends, perhaps upon interaction with the transpososome assembled on the strand transfer complex. This allows RecBC to enter and peel away the 3′ strand of the flap, engaging and activating MuANuc on the 5′ strand.
Summary
This is the first report of specific host processes involved in repair of transposon insertions in bacteria. We find that the PriA primosome and homologous recombination proteins, which are essential for repair of double-strand breaks in E. coli, play a critical role in the repair of Mu insertions. We favor a model for recombinational repair in which PriA restart of Mu replication is followed by RecA-mediated resolution of double-strand breaks on the Mu lagging strands created by the flap endonuclease activity of the transposase. Given that the predominant route taken by Mu upon infection is to enter lytic growth, it is plausible that Mu first co-opted the PriA system for replication, and later used it for repair. It will be interesting to see whether other transposons use these same processes for repair of their insertions.
Materials and Methods
Strain construction
All strains used in this work are derivatives of E. coli K-12 and are listed in Table 1 [13], [19], [22], [23], [34], [35], [65], [66]. The Keio Collection (single-gene knockout library of 3,985 nonessential genes in E. coli) was obtained from the National BioResource Project, Japan. The wild type strain in this collection is BW25113. E. coli Mu lysogen strains BU1717 or MH3491 were used to construct strains SJ17 – SJ19 (Table 1), where a ∼1 kb cat cassette was inserted downstream of the invertible G-segment on the Mu genome at nt 35,040, before gin, by the method of Datsenko and Wanner [67]. The SE deletion was similarly constructed; it removes nt 4,319–7,954 from the Mu genome, substituting the cat cassette in its place. All Mu phages used in this study carry the temperature-sensitive ts62 allele of the lysogenic repressor gene c. Primers used in this study are listed in Table S2.
High-throughput screening of the Keio library
Cultures from the Keio collection stocked in 96-well plates were inoculated into new sterilized 96-well plates with 0.2 ml of Luria broth (LB) by using the 12-multichannel pipette (Biohit). They were incubated at 37°C overnight without shaking. 4 µl of saturated overnight cultures were transferred to 0.2 ml of fresh LB media supplemented with 2.5 mM CaCl2 and 5 mM MgSO4 in 96-well plates and incubated at 37°C until OD600 reached around 0.5, measured directly in the plates by DTX880 microplate reader (Beckman). Mu phage was added to the cultures at a multiplicity of infection (moi) of 5, mixed briefly, and incubated at 30°C for 1 hr. 4 µl of infected cultures were spotted on slab agar plates having dimensions similar to the 96-well plates and containing 25 µg/ml chloramphenicol; plates were incubated overnight at 30°C.
PCR–based assay for Mu DNA integration
50 µl overnight cultures were transferred to 5 ml of fresh LB media supplemented with 2.5 mM CaCl2 and 5 mM MgSO4 and grown to 0.5 at an OD600. Phage were added to the cultures at 5 moi and incubated at 30°C for 30 min. Infected cells were harvested and the total DNA were isolated by Wizard Genomic DNA purification kit (Promega). PCR was conducted with 50 ng DNA as a template, 10 pmol primers, 1× Go Taq master mix (Promega), and distilled water up to 50 µl. Primers were designed to anneal to the left end of Mu DNA and the purH gene of E. coli. PCR conditions were: 94°C for 2 min, 30 cycles of - 94°C for 30 sec, 50°C for 30 sec, 72°C for 2 min 30 sec - and a final extension at 72°C for 2 min. PCR amplification primers used in this study are listed in Table S2. The reaction products were electrophoresed on 1% agarose gels and visualized by staining with ethidium bromide.
Quantitative real-time PCR analysis
This method measures DNA amounts based on the fluorescence signal from SYBR-bound DNA. PCR reactions were conducted with the same templates and primers as used for normal PCR, with the additional inclusion of 1× Power SYBR Green PCR Master Mix (Applied Biosystems), and distilled water up to 25 µl. The PCR program in the 7900HT sequence detector (Applied Biosystems) was as follows: 95°C for 10 min, followed by several cycles of - 95°C for 30 sec, 50°C for 30 sec, 72°C for 2 min 30 sec. Cumulative fluorescence was measured at the beginning of the exponential phase of the PCR reaction to determine the fractional cycle number (CT). The level of integrated Mu DNA was normalized to a chromosomal locus dnaC, amplified with appropriate primers listed in Table S2.
Growth curves
100 µl of saturated overnight cultures were transferred to 10 ml of fresh LB media and incubated at 37°C until OD600 reached around 0.5 for all cultures. From then on, growth was monitored by measuring OD600 at various times for 2 hr. A similar procedure was followed for obtaining lytic growth curves, except that the LB media was supplemented with 2.5 mM CaCl2 and 5 mM MgSO4. At OD600 of around 0.5, Mu phage was added at 5 moi, mixed briefly, and incubated at 37°C for 3 hr until most cultures were completely lysed. In all cases where priA2::kan, dnaT822 (without dnaC mutations) or polA::kan strains were used, these were grown overnight in minimal media, followed by dilution into fresh LB media, and then allowed to grow into log phase before infection with the different Mu phages.
Phage
These were prepared by induction of the prophage strains by thermal inactivation of the temperature-sensitive (ts) phage repressor c, and concentrated by CsCl gradient centrifugation as described [12]. For strains BU1717 (MuBam1066), SJ17 (Mu::Cm(Bam1066)) and SJ18 (MuBam1066ΔSE::Cm), the prophages were induced in the presence of pJG4 (c-myc MuB expressed from pET28(a) without IPTG induction) to supplement MuB protein. Typical phage titers after concentration were ∼1011 pfu (plaque forming units) for wild type Mu, and ∼1010 pfu for the Bam or BamΔSE phage. Phage titers for wild type Mu with and without the cat insertion were similar, showing that the insertion did not affect phage yields.
Lysogenization/survival frequency
Cultures were infected with Mu::Cm(Bam1066), MuBam1066ΔSE::Cm or Mu::Cm(Aam1093) phage as described under ‘PCR-based assay for Mu integration’. Before and after infection, appropriate dilutions of cells in LB media were spread onto agar plates with or without 25 µg/ml chloramphenicol to obtain cell counts for input cells, survivors after infection, and lysogens. Plates were incubated at 30°C overnight, and colonies were counted the next day. Lysogenization efficiency was calculated as CmR cells/input cells×100, and survival efficiency was calculated as survivors (on non-antibiotic plate)/input cells×100.
Sequencing Mu insertion sites in the priA mutant
priA lysogens were selected as CmR colonies after infection with Mu::Cm(Bam1066) phage. After overnight culture into LB media, chromosomal DNA was isolated by Wizard Genomic DNA purification kit and digested by restriction enzyme BamHI and PstI. Digested DNA fragments were purified and ligated with similarly digested pUC19 plasmid. CmR transformants were isolated and digested by BamHI and PstI to ascertain that the insert size was larger than 4 kb, so that it included DNA flanking the insertion. R1 primer (Table S2) was annealed to Mu DNA right end to obtain sequence of the flanking DNA. Based on this sequence, appropriate primers were used to PCR-amplify DNA flanking the left end of the insertion using the L1 primer. DNA sequencing was performed at our core sequencing facility.
Plaque morphology
10 µl of an appropriate dilution of phage suspension were mixed with 100 µl of host cells grown to 0.5–0.6 at OD600 in LB including 2.5 mM CaCl2 and 5 mM MgSO4. The mixture was added to 3 ml of 0.3% molten soft agar at 42°C, and poured on top of an LB agar plate containing 2.5 mM CaCl2 and 5 mM MgSO4. Plates were incubated overnight at 37°C.
Supporting Information
Zdroje
1. CraigNLCraigieRGellertMLambowitzAM 2002 Mobile DNA II ASM Press Washington, D.C.
2. SymondsNToussaintAVan de PuttePHoweMM 1987 Phage Mu Cold Spring Harbor, New York Cold Spring Harbor Laboratory
3. ChaconasGHarsheyRM 2002 Transposition of phage Mu DNA. MobileDNAIICraigNLCraigieRGellertMLambowitzAM Washington DC ASM Press 384 402
4. MizuuchiK 1992 Transpositional recombination: mechanistic insights from studies of Mu and other elements. Annu Rev Biochem 61 1011 1051
5. NakaiHDoseevaVJonesJM 2001 Handoff from recombinase to replisome: insights from transposition. Proc Natl Acad Sci U S A 98 8247 8254
6. AkroydJESymondsN 1983 Evidence for a conservative pathway of transposition of bacteriophage Mu. Nature 303 84 86
7. HarsheyRM 1984 Transposition without duplication of infecting bacteriophage Mu DNA. Nature 311 580 581
8. LiebartJCGhelardiniPPaolozziL 1982 Conservative integration of bacteriophage Mu DNA into pBR322 plasmid. Proc Natl Acad Sci U S A 79 4362 4366
9. HarsheyRMBukhariAI 1983 Infecting bacteriophage Mu DNA forms a circular DNA-protein complex. J Mol Biol 167 427 441
10. PuspursAHTrunNJReeveJN 1983 Bacteriophage Mu DNA circularizes following infection of Escherichia coli. EMBO J 2 345 352
11. GloorGChaconasG 1986 The bacteriophage Mu N gene encodes the 64-kDa virion protein which is injected with, and circularizes, infecting Mu DNA. J Biol Chem 261 16682 16688
12. AuTKAgrawalPHarsheyRM 2006 Chromosomal integration mechanism of infecting Mu virion DNA. J Bacteriol 188 1829 1834
13. ChoiWHarsheyRM 2010 DNA repair by the cryptic endonuclease activity of Mu transposase. Proc Natl Acad Sci U S A 107 10014 10019
14. WuZChaconasG 1995 A novel DNA binding and nuclease activity in domain III of Mu transposase: evidence for a catalytic region involved in donor cleavage. EMBO J 14 3835 3843
15. AbdelhakimAHOakesECSauerRTBakerTA 2008 Unique contacts direct high-priority recognition of the tetrameric Mu transposase-DNA complex by the AAA+ unfoldase ClpX. Mol Cell 30 39 50
16. BabaTAraTHasegawaMTakaiYOkumuraY 2006 Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2 2006.0008
17. HigginsNPCollierDAKilpatrickMWKrauseHM 1989 Supercoiling and integration host factor change the DNA conformation and alter the flow of convergent transcription in phage Mu. J Biol Chem 264 3035 3042
18. Mhammedi-AlaouiAPatoMGamaMJToussaintA 1994 A new component of bacteriophage Mu replicative transposition machinery: the Escherichia coli ClpX protein. Mol Microbiol 11 1109 1116
19. ChaconasGGloorGMillerJLKennedyDLGiddensEB 1984 Transposition of bacteriophage Mu DNA: expression of the A and B proteins from lambda pL and analysis of infecting Mu DNA. Cold Spring Harb Symp Quant Biol 49 279 284
20. MannaDBreierAMHigginsNP 2004 Microarray analysis of transposition targets in Escherichia coli: the impact of transcription. Proc Natl Acad Sci U S A 101 9780 9785
21. GeJLouZCuiHShangLHarsheyRM 2011 Analysis of phage Mu DNA transposition by whole-genome Escherichia coli tiling arrays reveals a complex relationship to distribution of target selection protein B, transcription and chromosome architectural elements. J Biosci 36 587 601
22. ChaconasGGiddensEBMillerJLGloorG 1985 A truncated form of the bacteriophage Mu B protein promotes conservative integration, but not replicative transposition, of Mu DNA. Cell 41 857 865
23. O'DayKJSchultzDWEricsenWRawlukkLHoweMM 1978 A search for integration deficient mutants of bacteriophage Mu-1. Microbiology. SchlessingerD Washington, D.C. ASM Publications 48 51
24. JonesJMNakaiH 1997 The φX174-type primosome promotes replisome assembly at the site of recombination in bacteriophage Mu transposition. EMBO J 16 6886 6895
25. MullerKHTrustTJKayWW 1988 Unmasking of bacteriophage Mu lipopolysaccharide receptors in Salmonella enteritidis confers sensitivity to Mu and permits Mu mutagenesis. J Bacteriol 170 1076 1081
26. SandulacheRPrehmPKampD 1984 Cell wall receptor for bacteriophage Mu G(+). J Bacteriol 160 299 303
27. MorganGJHatfullGFCasjensSHendrixRW 2002 Bacteriophage Mu genome sequence: analysis and comparison with Mu-like prophages in Haemophilus, Neisseria and Deinococcus. J Mol Biol 317 337 359
28. TaylorAL 1963 Bacteriophage-induced mutations in E. coli. Proc Natl Acad Sci 50 1043 1051
29. GabbaiCBMariansKJ 2010 Recruitment to stalled replication forks of the PriA DNA helicase and replisome-loading activities is essential for survival. DNA Repair (Amst) 9 202 209
30. SandlerSJMariansKJZavitzKHCoutuJParentMA 1999 dnaC mutations suppress defects in DNA replication - and recombination-associated functions in priB and priC double mutants in Escherichia coli K-12. Mol Microbiol 34 91 101
31. JonesJMNakaiH 1999 Duplex opening by primosome protein PriA for replisome assembly on a recombination intermediate. J Mol Biol 289 503 516
32. LeeEHKornbergA 1991 Replication deficiencies in priA mutants of Escherichia coli lacking the primosomal replication n' protein. Proc Natl Acad Sci U S A 88 3029 3032
33. NursePZavitzKHMariansKJ 1991 Inactivation of the Escherichia coli priA DNA replication protein induces the SOS response. J Bacteriol 173 6686 6693
34. McCoolJDFordCCSandlerSJ 2004 A dnaT mutant with phenotypes similar to those of a priA2::kan mutant in Escherichia coli K-12. Genetics 167 569 578
35. McCoolJDLongEPetrosinoJFSandlerHARosenbergSM 2004 Measurement of SOS expression in individual Escherichia coli K-12 cells using fluorescence microscopy. Mol Microbiol 53 1343 1357
36. KuzminovA 1999 Recombinational repair of DNA damage in Escherichia coli and bacteriophage lambda. Microbiol Mol Biol Rev 63 751 813
37. LittleJWEdmistonSHPacelliLZMountDW 1980 Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A 77 3225 3229
38. HughesKTOliveraBMRothJR 1987 Rec dependence of Mu transposition from P22-transduced fragments. J Bacteriol 169 403 409
39. SontiRVKeatingDHRothJR 1993 Lethal transposition of Mud phages in Rec - strains of Salmonella typhimurium. Genetics 133 17 28
40. LauderSDKowalczykowskiSC 1993 Negative co-dominant inhibition of recA protein function. Biochemical properties of the recA1, recA13 and recA56 proteins and the effect of recA56 protein on the activities of the wild-type recA protein function in vitro. J Mol Biol 234 72 86
41. YamamotoNNakahigashiKNakamichiTYoshinoMTakaiY 2009 Update on the Keio collection of Escherichia coli single-gene deletion mutants. Mol Syst Biol 5 335
42. SandlerSJ 2005 Requirements for replication restart proteins during constitutive stable DNA replication in Escherichia coli K-12. Genetics 169 1799 1806
43. ZavitzKHMariansKJ 1992 ATPase-deficient mutants of the Escherichia coli DNA replication protein PriA are capable of catalyzing the assembly of active primosomes. J Biol Chem 267 6933 6940
44. ZavitzKHMariansKJ 1993 Helicase-deficient cysteine to glycine substitution mutants of Escherichia coli replication protein PriA retain single-stranded DNA-dependent ATPase activity. Zn2+ stimulation of mutant PriA helicase and primosome assembly activities. J Biol Chem 268 4337 4346
45. SandlerSJMcCoolJDDoTTJohansenRU 2001 PriA mutations that affect PriA-PriC function during replication restart. Mol Microbiol 41 697 704
46. SandlerSJSamraHSClarkAJ 1996 Differential suppression of priA2::kan phenotypes in Escherichia coli K-12 by mutations in priA, lexA, and dnaC. Genetics 143 5 13
47. LiuJXuLSandlerSJMariansKJ 1999 Replication fork assembly at recombination intermediates is required for bacterial growth. Proc Natl Acad Sci U S A 96 3552 3555
48. SmithJADanielR 2006 Following the path of the virus: the exploitation of host DNA repair mechanisms by retroviruses. ACS Chem Biol 1 217 226
49. SuzukiJYamaguchiKKajikawaMIchiyanagiKAdachiN 2009 Genetic evidence that the non-homologous end-joining repair pathway is involved in LINE retrotransposition. PLoS Genet 5 e1000461 doi:10.1371/journal.pgen.1000461
50. GasiorSLWakemanTPXuBDeiningerPL 2006 The human LINE-1 retrotransposon creates DNA double-strand breaks. J Mol Biol 357 1383 1393
51. YangYXGuenVRichardJCohenEABerthouxL 2010 Cell context-dependent involvement of ATR in early stages of retroviral replication. Virology 396 272 279
52. MizuuchiK 1984 Mechanism of transposition of bacteriophage Mu: polarity of the strand transfer reaction at the initiation of transposition. Cell 39 395 404
53. NorthSHNakaiH 2005 Host factors that promote transpososome disassembly and the PriA-PriC pathway for restart primosome assembly. Mol Microbiol 56 1601 1616
54. JonesJMNakaiH 2000 PriA and phage T4 gp59: factors that promote DNA replication on forked DNA substrates Mol Microbiol 36 519 527
55. LevchenkoILuoLBakerTA 1995 Disassembly of the Mu transposase tetramer by the Clpx chaperone. Genes Dev 9 2399 2408
56. KruklitisRWeltyDJNakaiH 1996 Clpx protein of Escherichia coli activates bacteriophage Mu transposase in the strand transfer complex for initiation of Mu DNA synthesis. EMBO J 15 935 944
57. AbdelhakimAHSauerRTBakerTA 2010 The AAA+ ClpX machine unfolds a keystone subunit to remodel the Mu transpososome. Proc Natl Acad Sci U S A 107 2437 2442
58. NorthSHKirtlandSENakaiH 2007 Translation factor IF2 at the interface of transposition and replication by the PriA-PriC pathway. Mol Microbiol 66 1566 1578
59. McGlynnPLloydRG 2002 Recombinational repair and restart of damaged replication forks. Nat Rev Mol Cell Biol 3 859 870
60. MichelBGromponeGFloresMJBidnenkoV 2004 Multiple pathways process stalled replication forks. Proc Natl Acad Sci U S A 101 12783 12788
61. NakaiHTaylorAL 1985 Host DNA replication forks are not preferred targets for bacteriophage Mu transposition. J Bacteriol 163 282 290
62. RegisterJC3rdGriffithJ 1985 The direction of RecA protein assembly onto single strand DNA is the same as the direction of strand assimilation during strand exchange. J Biol Chem 260 12308 12312
63. BorkJMCoxMMInmanRB 2001 The RecOR proteins modulate RecA protein function at 5′ ends of single-stranded DNA. EMBO J 20 7313 7322
64. Gumbiner-RussoLMRosenbergSM 2007 Physical analyses of E. coli heteroduplex recombination products in vivo: on the prevalence of 5′ and 3′ patches. PLoS ONE 2 e1242 doi:10.1371/journal.pone.0001242
65. McCoolJDSandlerSJ 2001 Effects of mutations involving cell division, recombination, and chromosome dimer resolution on a priA2::kan mutant. Proc Natl Acad Sci U S A 98 8203 8210
66. BoonsombatRYehSPMilneASandlerSJ 2006 A novel dnaC mutation that suppresses priB rep mutant phenotypes in Escherichia coli K-12. Mol Microbiol 60 973 983
67. DatsenkoKAWannerBL 2000 One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97 6640 6645
68. KonradEB 1977 Method for the isolation of Escherichia coli mutants with enhanced recombination between chromosomal duplications. J Bacteriol 130 167 172
69. ZiegJKushnerSR 1977 Analysis of genetic recombination between two partially deleted lactose operons of Escherichia coli K-12. J Bacteriol 131 123 132
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



