-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaImproved Statistics for Genome-Wide Interaction Analysis
Recently, Wu and colleagues [1] proposed two novel statistics for genome-wide interaction analysis using case/control or case-only data. In computer simulations, their proposed case/control statistic outperformed competing approaches, including the fast-epistasis option in PLINK and logistic regression analysis under the correct model; however, reasons for its superior performance were not fully explored. Here we investigate the theoretical properties and performance of Wu et al.'s proposed statistics and explain why, in some circumstances, they outperform competing approaches. Unfortunately, we find minor errors in the formulae for their statistics, resulting in tests that have higher than nominal type 1 error. We also find minor errors in PLINK's fast-epistasis and case-only statistics, although theory and simulations suggest that these errors have only negligible effect on type 1 error. We propose adjusted versions of all four statistics that, both theoretically and in computer simulations, maintain correct type 1 error rates under the null hypothesis. We also investigate statistics based on correlation coefficients that maintain similar control of type 1 error. Although designed to test specifically for interaction, we show that some of these previously-proposed statistics can, in fact, be sensitive to main effects at one or both loci, particularly in the presence of linkage disequilibrium. We propose two new “joint effects” statistics that, provided the disease is rare, are sensitive only to genuine interaction effects. In computer simulations we find, in most situations considered, that highest power is achieved by analysis under the correct genetic model. Such an analysis is unachievable in practice, as we do not know this model. However, generally high power over a wide range of scenarios is exhibited by our joint effects and adjusted Wu statistics. We recommend use of these alternative or adjusted statistics and urge caution when using Wu et al.'s originally-proposed statistics, on account of the inflated error rate that can result.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002625
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002625Summary
Recently, Wu and colleagues [1] proposed two novel statistics for genome-wide interaction analysis using case/control or case-only data. In computer simulations, their proposed case/control statistic outperformed competing approaches, including the fast-epistasis option in PLINK and logistic regression analysis under the correct model; however, reasons for its superior performance were not fully explored. Here we investigate the theoretical properties and performance of Wu et al.'s proposed statistics and explain why, in some circumstances, they outperform competing approaches. Unfortunately, we find minor errors in the formulae for their statistics, resulting in tests that have higher than nominal type 1 error. We also find minor errors in PLINK's fast-epistasis and case-only statistics, although theory and simulations suggest that these errors have only negligible effect on type 1 error. We propose adjusted versions of all four statistics that, both theoretically and in computer simulations, maintain correct type 1 error rates under the null hypothesis. We also investigate statistics based on correlation coefficients that maintain similar control of type 1 error. Although designed to test specifically for interaction, we show that some of these previously-proposed statistics can, in fact, be sensitive to main effects at one or both loci, particularly in the presence of linkage disequilibrium. We propose two new “joint effects” statistics that, provided the disease is rare, are sensitive only to genuine interaction effects. In computer simulations we find, in most situations considered, that highest power is achieved by analysis under the correct genetic model. Such an analysis is unachievable in practice, as we do not know this model. However, generally high power over a wide range of scenarios is exhibited by our joint effects and adjusted Wu statistics. We recommend use of these alternative or adjusted statistics and urge caution when using Wu et al.'s originally-proposed statistics, on account of the inflated error rate that can result.
Introduction
Genome-wide association studies (GWAS) have been remarkably successful at identifying the genomic locations of variants involved in a variety of complex diseases [2]–[7]. In spite of this success, some researchers have expressed disquiet at the issue of the ‘missing heritability’ [8], namely the fact that the disease-associated single nucleotide polymorphisms (SNPs) identified through GWAS often account for only a small proportion of the the observed correlations in phenotype between relatives. This suggests that additional genetic factors remain to be found. Several explanations for this phenomenon have been suggested. Firstly, the SNPs identified through GWAS are likely to be surrogates in (imperfect) linkage disequilibrium (LD) with the true causal variants, and thus cannot be expected to fully account for their effects, particularly if the true causal variants are rare. Secondly, the low power of GWAS to detect loci of small effect means that many specific true loci remain undiscovered, even though the fact of their (combined) existence may be detectable from the observed genetic data [9], [10]. Finally (and the main focus of this communication) is the fact that the single-locus (SNP by SNP) testing strategy generally undertaken as the primary analysis tool in a GWAS may be underpowered to detect loci that interact with other genetic or enviromental factors, since effects at such loci may not be visible unless the contributing interacting factors are also taken into account.
The relationship between biological and statistical interaction has been hotly debated over many years [11]–[19]. It is now generally accepted that the lack of direct correspondence between statistical and biologial interaction makes it difficult to make strong inferences concerning biological mechanism from the existence of interaction terms in a statistical model. Nevertheless, the existence of such terms does imply that the interacting factors should at least both be ‘involved’ in disease in some way. Detection of statistical interaction thus provides a good starting point for a more focussed investigation of the joint involvement of the relevant factors, which can perhaps be better addressed through other types of experimental data. In addition, the increased detection power provided by statistical models that include interaction terms, when such terms do in fact operate [20], motivates the development of improved methods for detecting and modelling statistical interaction, particularly in the context of GWAS. The hope is that such methods will be useful for detecting effects that may be missed in standard single-locus analysis, thus providing a complementary strategy to standard GWAS analysis approaches for detecting loci involved in disease.
In case/control studies, statistical interaction is generally modelled as departure from a simple linear model describing the individual (main) effects of predictor variables on the predicted log odds of disease [17]. Consider two binary variables, and , whose presence/absence (coded 0/1) is believed be associated with a disease outcome. Logistic regression models the main effects ( and ) and interaction term () between the variables via the linear model(1)where represents the probability that an individual in the study is a case rather than a control. Applying this idea to genetic predictor variables (such as SNP genotypes) is complicated by the fact that genetic predictors are not binary, but rather take 3 levels according to the number of copies (0,1,2) of the susceptibility allele possessed. However, we can easily convert to a binary coding by assuming a recessive or dominant model for each of the factors considered (thus collapsing two genotype categories to one at each locus). Alternatively, we can fit the above regression model using predictor variables coded (0,1,2), according to the number of susceptibility allele possessed, thus imposing an additive model (on the log odds scale) within each locus for the effect of each susceptibility allele. Yet another approach would be to fit a more general nine parameter (saturated) genotype model [17], that includes effects due to one or two copies of the susceptibility allele at locus 1 (, ), at locus 2 (, ), and four interaction parameters (, , , ) representing the additional contribution to risk from combinations of these effects, resulting in the following model:(2)(where here represents an indicator variable for the occurence of event ).
Given the simpler logistic regression model (1), a variety of tests can be performed to assess the effects of the two contributing factors. (Similar tests can be derived for logistic regression model (2)). A 3df test of tests for association at both loci, allowing for their possible interaction. A 2df test of tests for association at locus 2, allowing for possible interaction with locus 1. Such a test has been shown to be a powerful approach when interactions exist, while losing very little power when no interactions exist [20]. In the current communication, we will focus on the 1df test of i.e. a test of the interaction term alone. This test has the disadvantage of being generally underpowered compared to tests of main effects [21]. However, we might hope that loci with reasonably large main effects will be potentially detectable via a single-locus scan. We are interested in detecting loci that will be missed via single-locus analysis, i.e. those for which the interaction term is likely to be particularly important. Moreover, assuming we can construct a good test of , this test can potentially be combined with tests of the main effects [22], allowing the construction of joint tests of association while allowing for interaction, if desired.
Methods
Wu et al. (2010) statistic
Recently, Wu and colleagues [1] proposed two novel statistics for genome-wide (pairwise) interaction analysis using case/control or case-only data. The statistics proposed by [1] were based on considering ‘haplotypes’ at two diallelic loci, and , with locus having alleles and and locus having alleles and . For linked loci, the concept of ‘haplotype’ corresponded to its usual interpretation in terms of the physical coupling of alleles on the DNA strand inherited from a single parent. For unlinked loci, the concept of ‘haplotype’ referred simply to the fact the alleles involved were inherited from the same parent (a concept sometimes referred to as gametic phase disequilibrium), without necessarily implying any physical coupling of the alleles.
Wu et al. [1] propose to detect interaction via consideration of the log odds ratio(3)where is the haplotype frequency of haplotype − (i.e. the probability of this haplotype) in some sample under consideration. We define a parameter vector , chosen to reparameterise the 4 haplotype frequencies in terms of the allele frequencies, , , , and , and a ‘linkage disequilibrium’ (LD) (or more generally, for unlinked loci, allelic association) parameter, , such thatNote that the odds ratio in (3) relates to the odds of a allele appearing on a ‘haplotype’ in coupling with a allele (and a allele with ) i.e. it acts a measure of correlation between alleles at the two loci, rather than relating to the odds of disease. No correlation () corresponds to the situation where the allelic association parameter . Wu et al. (2010) propose that, under the null hypothesis of no interaction, , where and refer to calculating within the sample of cases and controls respectively. If, in addition, there is no population-level allelic association between alleles at and , then .
Wu et al. [1] give a complicated description motivating their use of , however this quantity can perhaps more easily be motivated by analogy with classical ‘case-only’ analysis [23], [24], [25]. Case-only analysis stems from the observation that, for binary predictor variables, a test of the interaction term in the logistic regresssion model (1) can be obtained by noticing that it equals the ‘ratio of odds ratios’:where and are the joint probabilities that binary variables and take values and , i.e. , calculated within the sample of cases and controls, respectively. If variables and are uncorrelated in the controls (or, equivalently, in the general population under a rare disease assumption) then the denominatorand a test of interaction can be constructed by testing whetherThis test has the advantage [23], [24], [25] of being substantially more powerful than the usual logistic regression test of . If we are not willing to assume that variables and are uncorrelated in the controls, then a natural test of interaction can instead be constructed by testing whetheror, equivalently, whetherConsidered in this light, the log odds ratio considered by Wu et al. [1] can be seen as analagous to the quantity used in case-only analysis, if the unit of analysis is defined to be a ‘haplotype’ (rather than an individual) and if binary variables and are defined as indicator variables for the two possible alleles at each locus on the haplotype.
To test for interaction, Wu et al. [1] propose two test statistics, one for case-only and one for case/control analysis, which we denote as and (4)(5)Here is the log OR (for − and − alleles being in coupling, as opposed to − and −), is its estimated variance (calculated using the delta method), and and refer to quantities calculated within the sample of cases and controls respectively. The case-only test should be suitable provided there is no correlation (e.g. due to LD) between alleles at the two loci. The case/control test is more suitable if we expect correlation between alleles at the two loci due to the fact they are linked, or induced by other influences such population stratification [26]).
In order to actually calculate and , we need to know (or estimate) the ‘haplotype’ frequencies in cases and controls. Even for linked loci, haplotypes are not generally observed, but luckily many programs exist to estimate haplotype frequencies (often via an EM algorithm) given unphased genotype data. Most if not all such programs assume Hardy-Weinberg equilibrium (HWE) in order to perform the calculation. We expect HWE to hold in the general population (and thus in controls, under a rare disease assumption). Under the global null hyothesis of no association between disease status and the loci in question (via either main effects or interactions), haplotype frequencies in cases should be identical to those in controls, and HWE should also hold in the cases. However, under the alternative hypothesis of association and/or interaction, HWE will not necessarily hold in the cases [27] (unless the disease model is assumed to result from multiplicative haplotype effects [28]), meaning that haplotypes in cases cannot be considered to come together independently. We return to this point later.
Wu et al. [1] provide the following formulae for their proposed statistics:(6)(7)where and are the number of sampled case and control individuals, and and are estimators of the haplotype frequencies in cases and controls, respectively. However, the denominators in these formulae (based on calculating the asymptotic variances of and ) are only correct if haplotypes are actually observed i.e. there is no phase uncertainty. Consequently, we expect these variance estimates to be too small if haplotype frequencies are estimated from unphased genotype data, resulting in test statistics that are too large. In Text S1 we use results from Brown [29] and application of the delta method to calculate the correct asymptotic variances of and . We refer to our corresponding resulting test statistics as ‘adjusted’ Wu statistics:(8)(9)(where now relates to the correct asymptotic variance of as given in Text S1). Interestingly, if one calculates this variance under the null hypothesis that (as might be reasonable when performing case-only analysis, where this assumption is in any case required), it turns out that the resulting variance is exactly double that derived by Wu et al. [1]. In these circumstances, our case-only statistic would be exactly half of the original Wu case-only statistic. This suggests that another way to construct a valid version of Wu's case-only statistic would be to simply divide the original statistic by two. In computer simulations (data not shown), we found negligible differences between between our ‘adjusted’ statistic and , and thus, in our Results section, we only report results for .
PLINK's fast-epistasis statistics
Two fast approaches for testing interaction (in addition to a slower logistic regression based approach) are implemented in the computer program PLINK [30]. For a set of individuals (either cases or controls), PLINK takes unphased genotype data as shown in Table 1 and expands it out to the 22 allelic table shown in Table 2. The log odds ratio in this table can be calculated as with estimated variance . PLINK's fast-epistasis tests test whether correlation between alleles at the two loci exists (case-only test) or is different between cases and controls (case/control test) via the following test statistics:(10)(11)Here and again refer to quantities calculated within the sample of cases and controls respectively. These statistics are seen to have exactly the same form as the Wu and adjusted Wu statistics, but with the log odds ratio and its estimated variance relating to slightly different quantities, namely those quantities shown in Table 2.
Tab. 1. Multilocus genotype counts at two SNPs in a set of genotyped individuals. 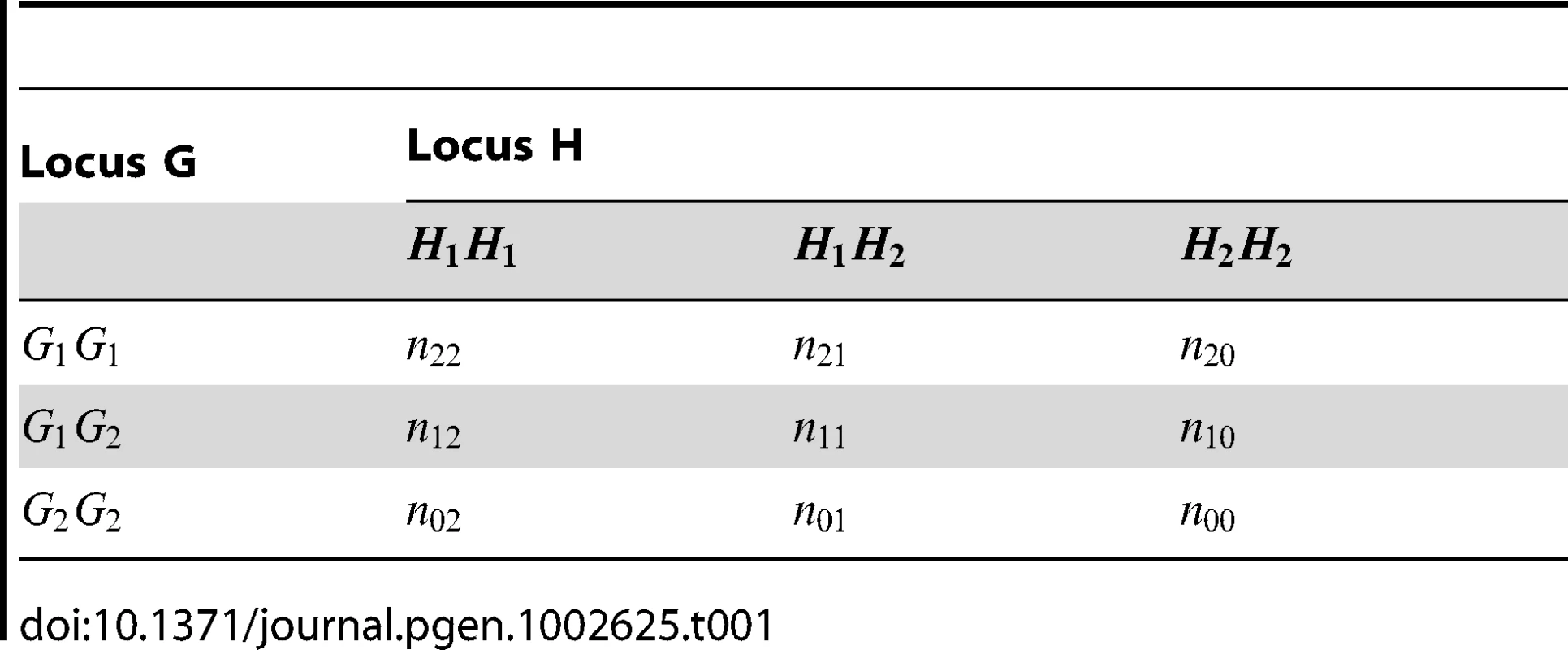
Tab. 2. Allele counts derived from <em class="ref">Table 1</em>, as calculated by PLINK. 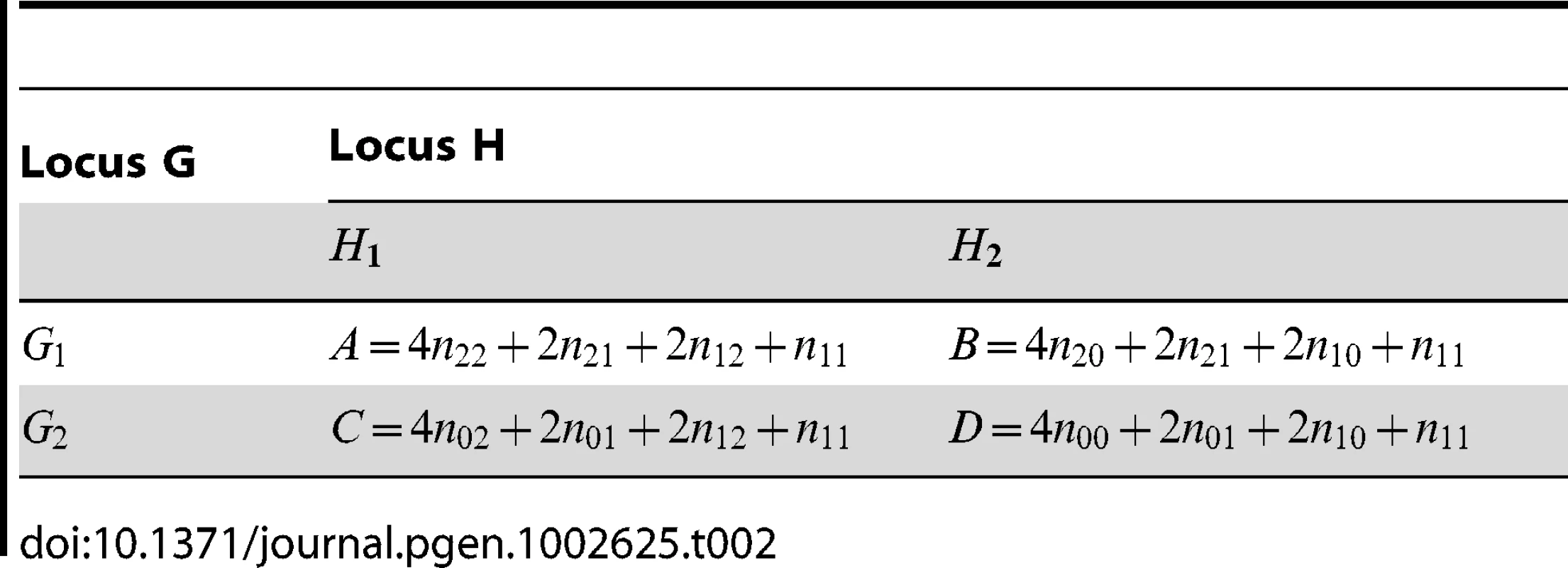
Apart from the difference in , the main difference between PLINK's statistics and those proposed by Wu et al. is that fact that, in PLINK, no estimation of phased haplotype frequencies is performed. Nevertheless, the log odds ratio can be shown to be exactly that which would be obtained if one did estimate haplotype counts, assuming that the middle cell () in Table 1 resolves into phased genotypes −/ − or −/ − with equal frequencies. The haplotype counts implicitly utilized by PLINK are therefore similar to what would be obtained from an EM algorithm, except that in PLINK the middle cell is resolved assuming no correlation between alleles at the two loci, resulting (presumably) in a set of estimated haplotype frequencies that will be biased towards showing lower levels of allelic association. We hypothesise that this bias towards lower levels of allelic association might partly account for the inferior performance of PLINK observed by Wu et al. [1].
Although the log odds ratio in PLINK corresponds to what would be obtained from attempting to resolve phase while assuming no correlation between alleles at the two loci, the variance estimate is based on counting independent alleles rather than haplotypes (where is the total number of individuals in Table 1). The formula for the variance estimate assumes that there are 3 independent cell probabilities in Table 2. However, since the data in Table 2 was originally derived from Table 1, considering these data as realisations from a multinomial distribution, we can see that in fact there should be 8 parameters corresponding to 8 independent cell probabilities. In Text S1, we use the delta method to calculate the correct asymptotic variances of and , based on the multinomial data in Table 1. We refer to the corresponding resulting test statistics as ‘adjusted’ fast-epistasis statistics:(12)(13)where now relates to the correct asymptotic variance of as given in Text S1, and and again refer to quantities calculated within the sample of cases and controls respectively.
Wellek and Ziegler (2009) statistics
Both the methods of Wu et al. [1] and the fast-epistasis tests implemented in PLINK operate by turning a question about statistical interaction into a question about allelic association (or correlation), namely, whether association between alleles two loci exists (case-only test) or is different between cases and controls (case/control test)). However, many different measures of allelic association (usually calculated for linked loci, and thus assumed to reflect LD) have been proposed. Arguably the most popular are Lewontin's [31] and Pearson's product-moment correlation coefficient (or the square of it, ) [32]. In most current genetic applications, these measures are calculated based on known or estimated haplotype frequencies. Wellek and Ziegler [33] pointed out that one advantage of is that it may be calculated without estimating phase, simply by applying it to two variables, and , coded (0,1,2) according to the number of susceptibility alleles possessed at each locus. Wellek and Ziegler [33] examined the performance of as a measure of LD using either estimated (phased) haplotype frequencies or using unresolved genotype data and showed that, if HWE holds, the loss of precision for estimating was negligible when using unphased genotypes rather than (phased) haplotypes.
If HWE does not hold, Wellek and Ziegler found the genotype-based estimator of to be unbiased but the haplotype-based estimator to be strongly biased, i.e. to not reflect the ‘true’ value of based on the true haplotype frequencies. This would seem an unappealing property of the haplotype-based estimator, if the goal is to accurately estimate the true level of allelic association (or LD) between two loci. However, if the purpose is rather to test for interaction (via testing whether correlation between alleles two loci exists (case-only test) or is different between cases and controls (case/control test)), it is possible that such a bias could be advantageous in terms of improving power. Since the method of Wu et al. [1] relies on estimating ‘haplotypes’ within the sample of cases (under a potentially incorrect HWE assumption), we hypothesise that the bias pointed out by [33] might also contribute to the superior performance observed by Wu et al. [1] for their approach compared to PLINK.
Given a genotype-based estimator of , Wellek and Ziegler [33] propose using Fisher's transformation to calculate a quantityand its estimated variance. A natural pair of statistics for testing interaction based on might therefore be:In computer simulations (data not shown), we found the performance of these statistics to be virtually identical to statistics based on the correlation coefficient itself. We therefore instead define our Wellek and Ziegler inspired statistics based on the correlation coefficient as:(14)(15)where again and refer to quantities calculated within the sample of cases and controls respectively. Formulae for the correlation coefficient and its estimated variance are given by Wellek and Ziegler [33]. Note that the test based on the difference in the correlation coefficients between cases and controls, , was also proposed by Kam-Thong et al. [34] and implemented in a program called EPIBLASTER. In EPIBLASTER, is used as a screening step, prior to performing a full logistic regression analysis on the subset of pairs of loci showing some loose level of significance with .
New “joint effects” tests
Although designed to test specifically for (statistical) interaction, several of the test statistics proposed above can be shown to be sensitive to the situation where there is, in fact, no interaction, but one or both of the loci display main effects (see details in Text S2). This is rather unsatisfactory as, even if one of the loci does have a genuine main effect, this phenomenon could lead to potentially increased false positive rates with respect to detection of the other locus (through its apparent – but false – interaction with the locus that has genuine main effects). Ideally, one would hope that detection of a significant interaction effect would indicate genuine interaction, but, even if this is not the case, one would at least hope that both loci identified have some involvement in disease (with their precise joint effects - interactive or otherwise - being determinable through further, more focussed, statistical or biological investigation).
In order to address this issue, we propose two new ‘joint effects’ tests that are sensitive only to either a) a genuine interaction effect or b) (if the disease is not sufficiently rare), main effects present at both loci. Our tests are motivated by a desire to test the same interaction parameter as tested by Wu et al. [1]. However, unlike some previously-proposed tests, our new tests can be shown to have the advantage of not being sensitive to main effects at a single locus. Moreover, under a rare disease assumption, our new tests can also be shown to be insensitive to main effects at both loci, thus reflecting genuine interaction. Thus, application of our joint effects tests will not result in an inflated type 1 error rate with respect to the detection of loci that are not involved in the disease (even though, for a common disease, our tests could potentially result in an inflated type 1 error with respect to whether the pair of loci actually interact, in the usual statistical sense).
Our new tests are based on the counts in Table 1, calculated separately within the sample of cases and controls. Consider using each of the four top left cells in Table 1 in turn, to estimate four odds ratios relative to the baseline (bottom right) cell:In Text S3 we show that, under a rare disease assumption, these estimated odds ratios can be considered as estimates of the following functions of , where refers to to the log odds ratio estimated in the method of Wu et al. [1]:(16)
To construct our proposed tests, we therefore propose to use the four relationships in (16) as four estimating equations for , and test the hypothesis that (case/only test) or that is equal for cases and controls (case/control test). Further motivation for our tests is provided in Text S3. Note that corresponds to the situation where all four of the ‘interaction’ odds ratios (, , , ) equal 1.
We construct two separate estimates of , using the data in Table 1 as tabulated for either cases or controls. Equation (16) implies that we can estimate via a weighted average:where relates to the estimate of obtained from Table 1, and the weights are chosen to sum to 1 and make the variance of minimum (see Text S1 for details). Having estimated and its variance (see Text S1) separately using data from either cases or controls, we can then construct ‘joint effects’ tests:(17)(18)where again and refer to the quantities calculated within cases and controls respectively.
A difficulty with estimation arises when . If this occurs, we replace the objective quantity bywhich reduces to zero if (i.e. possesses the same desirable property under the null hypothesis). Writing in terms of , we obtain four estimating equations for instead of , and we estimate as:with optimal weights chosen to make the variance minimum as before. Estimating the variance of as , this results in alternate versions of our joint effects tests:(19)(20)where again and refer to quantities calculated within cases and controls respectively.
Relationship to standard regression approaches
Text S3 motivates our ‘joint effects’ tests through consideration of the relationship between the original Wu et al. [1] method and standard logistic regression. A natural question of interest is the relationship between the other two methods described here (FE, WZ) and standard regression approaches – and, in particular, to what extent the different odds ratios () estimated by these methods correspond to the usual interaction parameters ( and , , , ) in Equations (1) and (2). In Text S4 we show, for each of these methods, the relationship between the parameters estimated in that method and those estimated in standard logistic or linear regression. In addition, in Text S5, we show that the WZ case-only statistic can be viewed equivalently as a score test with respect to the interaction parameter . It would be of interest to determine whether a similar relationship holds for the other statistics considered here. However, providing this derivation for the remaining statistics is beyond the scope of the current manuscript, and we defer it to future work.
Simulation study
We performed computer simulations to evaluate the performance (type 1 error and power) of the various test statistics described above. For the Wu and adjusted Wu methods, haplotype frequencies in cases and controls were calculated from unphased genotype data using an EM algorithm as implemented in either PLINK or the R library ‘Genetics’. The general structure of the disease models we considered is shown in Table 3, assuming two loci G and H, each having two alleles and . We simulated 1000 cases and 1000 controls from a general population assumed to be in HWE. Writing the haplotype frequencies in the general population as (−) for , we considered the same two sets of haplotype frequencies considered by [1]:
-
Loci not in LD: , , and .
-
Loci in LD: , , and .
Tab. 3. Structure of log odds in disease models used for simulation study. 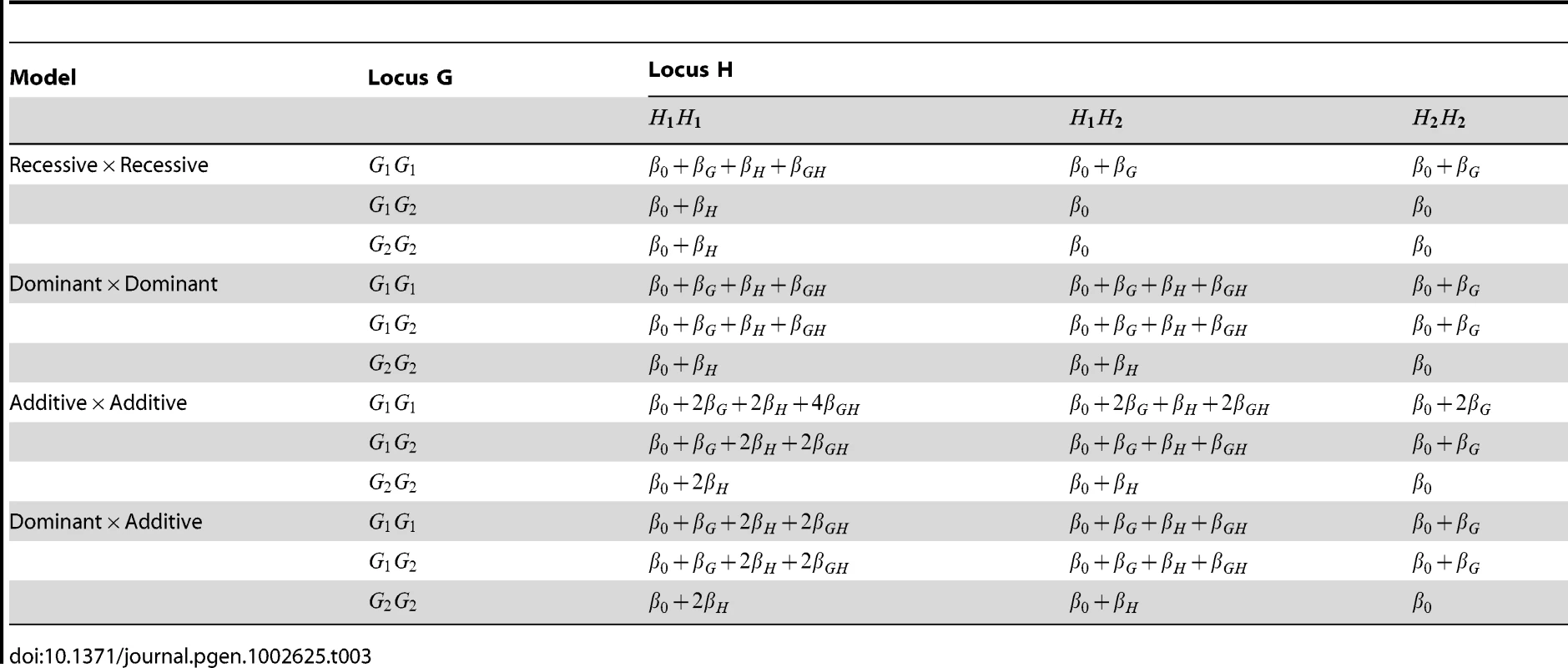
When the two SNPs were not in LD, we examined the performance of both case/control and case-only statistics. When the SNPs were simulated to be in LD, we examined only the performance of case/control statistics (since we know that case-only statistics will show inflated type 1 error in this situation). To investigate type 1 error we considered 8 scenarios, each using 10,000 data replications. To investigate power we considered a further 4 scenarios, each using 1,000 data replications. The structure of the simulated models and the parameter values assumed are given in Tables 3 and 4. Note that in Tables 3 and 4 we denote the baseline, main effect and interaction parameter values (, , , in Equation (1)) as (, , , ) respectively. In each scenario apart from 5c and 5d, the baseline regression coefficient was chosen to equal , corresponding to a baseline penetrance of 2%. For Scenarios 5c and 5d we assumed a rarer disease, with baseline penetrance 0.0001. For each power scenario, we increased from 0 (no interaction) to a value at which the power to detect an effect (at significance level 0.01) was close to 100% for the best-performing statistics.
Tab. 4. Description of simulation scenarios. 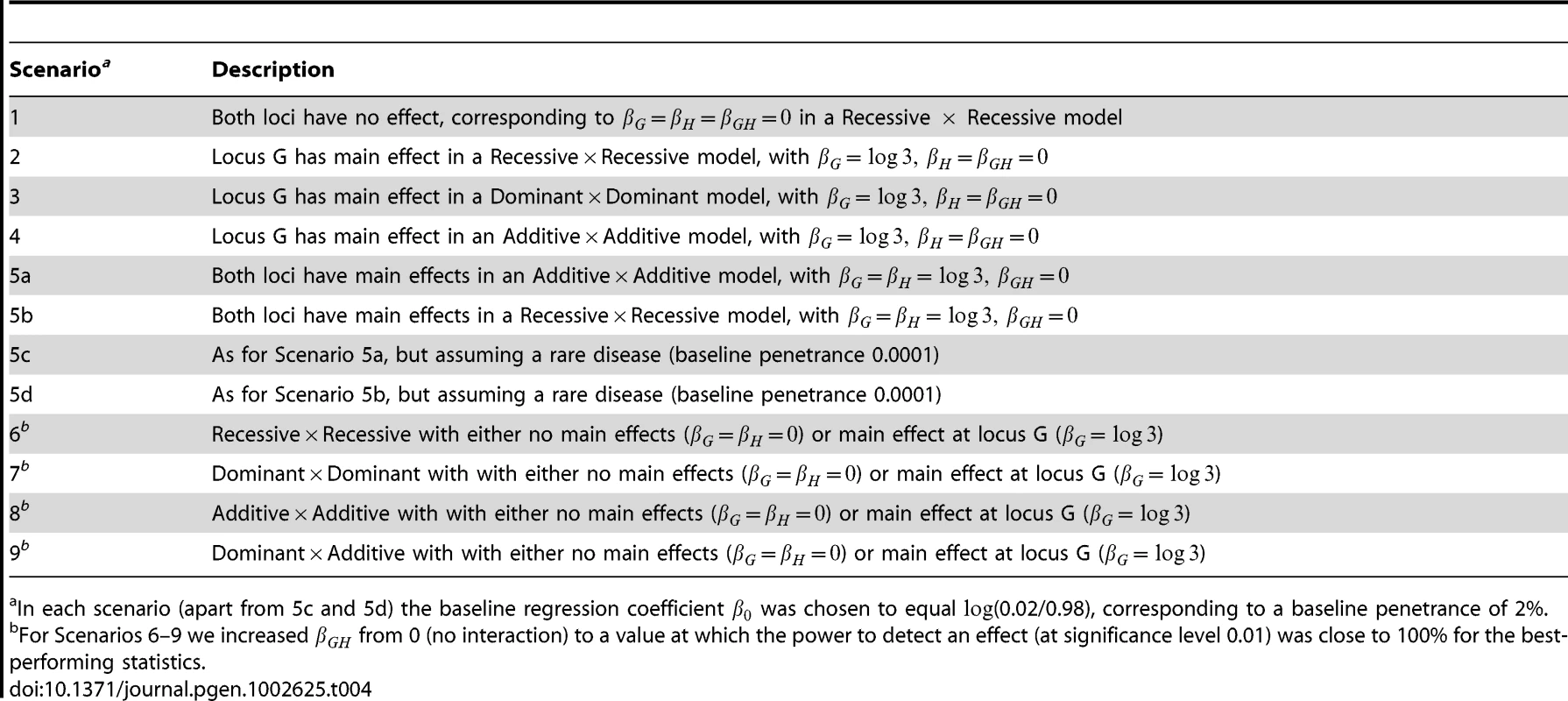
In each scenario (apart from 5c and 5d) the baseline regression coefficient was chosen to equal (0.02/0.98), corresponding to a baseline penetrance of 2%. In addition to the test statistics described above, when comparing power (Scenarios 6–9) we also calculated several additional statistics. Firstly, as an ‘optimal’ test we considered analysing the data assuming the ‘correct’ model (i.e. imposing the correct structure in terms of whether a model was assumed to be additive, dominant or recessive at each locus, see Table 4). For case/control data this was achieved by using logistic regression with the correct coding of predictor variables at each locus, and then comparing models in which an interaction term was or was not included via a likelihood ratio test. For case-only data, the ‘optimal’ analysis was implemented by using the Wellek and Ziegler statistic (14) with the correct coding of predictor variables (corresponding to an additive, dominant or recessive model) at each locus. For comparison, we also considered ‘sub-optimal’ tests where an incorrect coding for the simulation model was used. Secondly, we considered an ‘ideal’ version of the Wu et al. statistics (Equations (6) and (7)), in which we assumed haplotypes could be inferred without error. In this case, the formulae proposed by Wu et al. [1] should be correct, as there is no increase in the asymptotic variances used in the denominator due to phase uncertainty. Although not achievable in practice, for theoretical interest we investigated the performance of the Wu et al. statistics (with respect to both type 1 error and power) in this ‘ideal’ situation.
To gain additional insight into the properties of the methods considered, for Scenario 7 we noted the ‘haplotype’ frequencies and resulting LD measures , and obtained from the EM algorithm applied (separately) to cases and controls (as used in the Wu and adjusted Wu approaches). These were compared to the true haplotype frequencies and correlation measures (as implied by the generating model), the genotype-based correlation coefficient (as used in the Wellek and Ziegler inspired approaches), and the haplotype frequencies and correlation measures calculated from Table 2 (which are, effectively, those used by PLINK).
Data application
As an illustration of the methods described, we also applied them to real data from a publicly available genome-wide data set consisting of 1748 cases of Crohn's disease and 2938 population-based controls obtained from the Wellcome Trust Case Control Consortium (WTCCC) [2]. Since this exercise was purely for illustrative purposes, in the interests of time we limited our analysis to that of a single chromosome, chromosome 22. We used the same quality control procedures as the WTCCC [2] to remove poor-quality SNPs and samples prior to analysis. This generated 5750 SNPs across chromosome 22, resulting in 16,528,375 pairwise combinations to be tested for interaction.
Results
Evaluation of type 1 error
Figure 1 shows quantile-quantile (QQ) plots of the distribution of the different test statististics calculated in Scenario 1 (so under the global null of no effects at either locus). For a test that is performing correctly (i.e. with well-calibrated type 1 error), we would expect to see all points lying on the line with slope equal to 1. We find this to be true for all methods except the original Wu et al. [1] statistics (Equations (6) and (7)), which show strong departure from the line, indicating a severe inflation in type 1 error.
Fig. 1. Chi-squared (1 df) Q-Q plot for Scenario 1 (Global Null). 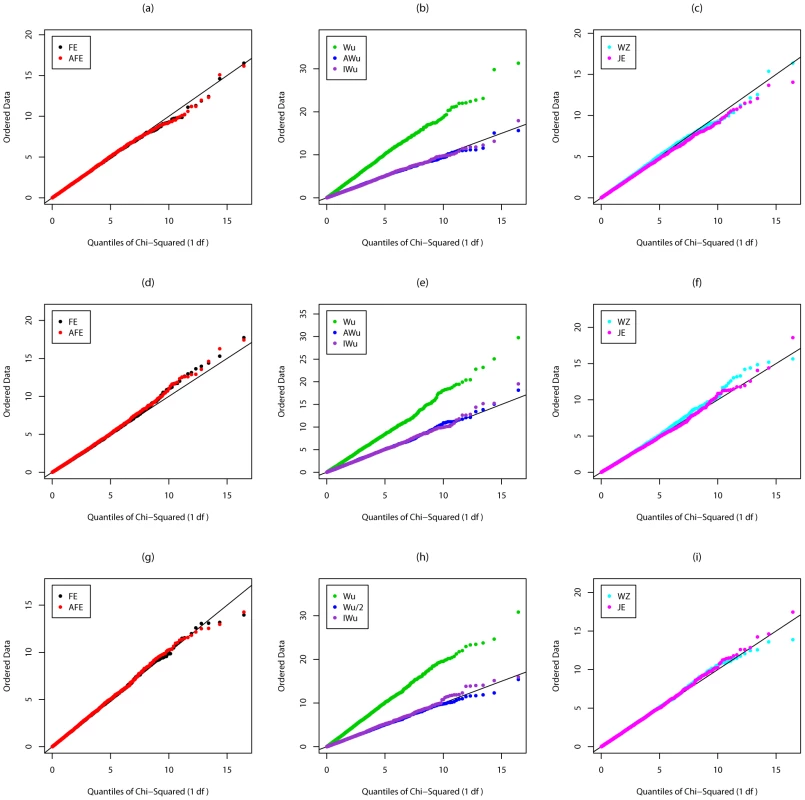
Top panels ((a), (b) and (c)): Case/Control not in LD; Middle panels ((d), (e) and (f)): Case/Control in LD; Bottom panels ((g), (h) and (i)): Case-Only not in LD; FE: Fast-Epistasis; AFE: Adjusted FE; Wu: Wu et al. statistic; AWu: Adjusted Wu statistic; IWu: Ideal Wu statistic; WZ: Wellek and Ziegler statistic; JE: Joint Effects statistic. Figure 2 shows QQ plots for Scenario 2 in which locus G has a recessive main effect. Again the original Wu et al. [1] statistics show a severe inflation in type 1 error. A severe inflation is also seen for the Wellek and Ziegler inspired statistics and PLINK's fast-epistasis tests (both the original and our adjusted version) in case/control analysis, when the two SNPs considered are in LD in the general population. (Some theoretical explanation for these results can be found in Text S2). This inflation in the presence of LD is not seen for the ideal Wu statistics or for our new joint effects statistics. For case-only analysis, we see a small inflation in type 1 error for PLINK's fast-epistasis test, which is corrected through use of our adjusted version of this test. We also see a slight deflation in type 1 error (indicating the method is conservative) for our adjusted Wu statistic.
Fig. 2. Chi-squared (1 df) Q-Q plot for Scenario 2 (Recessive effect at locus G). 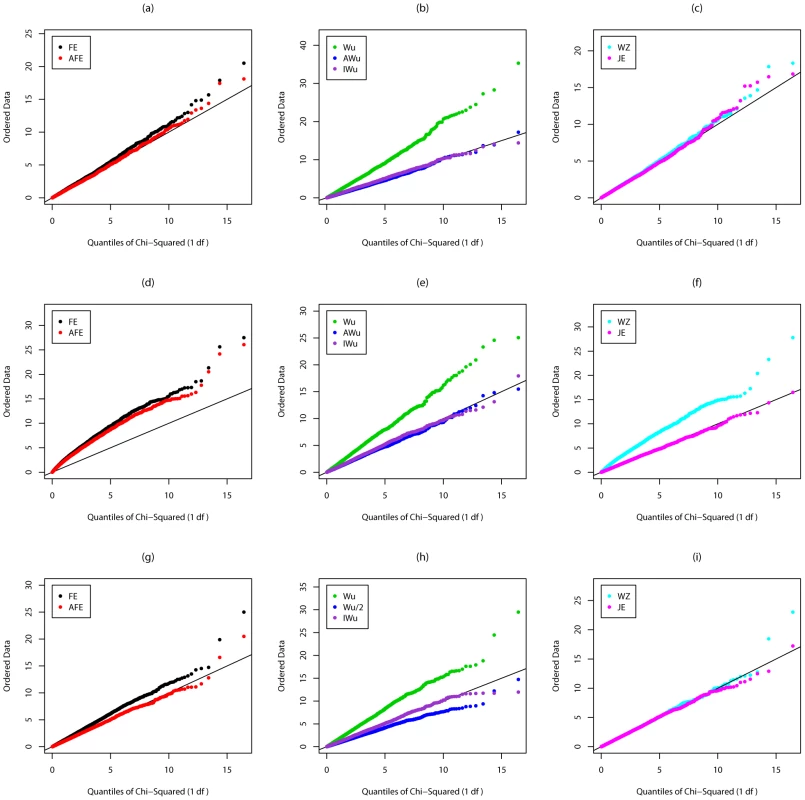
Top panels ((a), (b) and (c)): Case/Control not in LD; Middle panels ((d), (e) and (f)): Case/Control in LD; Bottom panels ((g), (h) and (i)): Case-Only not in LD; FE: Fast-Epistasis; AFE: Adjusted FE; Wu: Wu et al. statistic; AWu: Adjusted Wu statistic; IWu: Ideal Wu statistic; WZ: Wellek and Ziegler statistic; JE: Joint Effects statistic. A similar pattern is seen for Scenario 3 (in which locus G has a dominant main effect, see Figure S1) except that, in this case, the Wellek and Ziegler inspired case/control statistic does not appear to show inflated type 1 error in the presence of LD, and, for case-only analysis, PLINK's fast-epistasis test shows a slight deflation (rather than inflation) in type 1 error, while our adjusted Wu statistic shows a slight inflation. Correct type 1 errors are achieved by the ideal Wu statistics and by our new joint effects statistics. Results from Scenario 4 (in which locus G has an additive main effect) are shown in Figure S2. In this case, all methods appear to have correct type 1 error except the original Wu et al. [1] statistics and the Wellek and Ziegler inspired case/control statistic in the presence of LD.
Figures S3, Figure S4, Figure 3, and Figure 4 show the results from Scenarios 5a, 5b, 5c, 5d, in which both loci have main effects. Provided the disease is rare (Figure 3 and Figure 4), our joint effects statistics show correct type 1 error, while the adjusted fast-epistasis and Wellek and Ziegler methods can show inflated type 1 errors, particularly in the presence of LD. (Some theoretical explanation for these results can be found in Text S2). The Adjusted Wu method has generally correct type 1 error although it appears to be slightly conservative for case/only analysis in Figure 4. When the disease is more common (Figures S3 and S4), the presence of main effects appears to have an impact on the type 1 error of virtually all methods, indicating that none are completely immune from detecting pairs of loci that are both involved in disease, but which do not, in fact, require any statistical interaction term to describe their action. The only method that appears immune to this problem is the ideal Wu statistic applied to case/control (but not to case-only) data.
Fig. 3. Chi-squared (1 df) Q-Q plot for Scenario 5c (Rare disease, Additive effects at both loci). 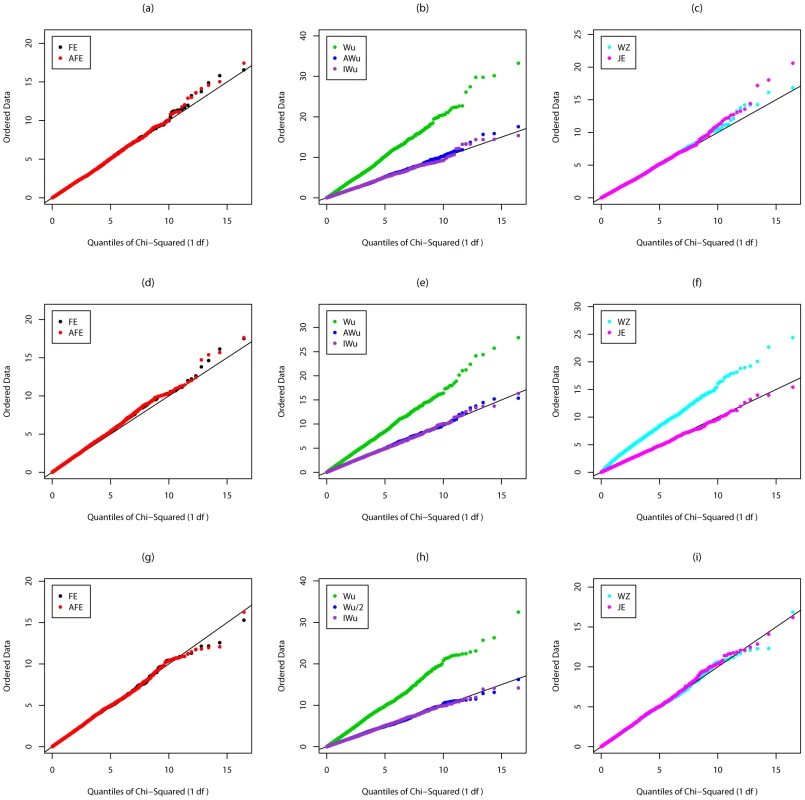
Top panels ((a), (b) and (c)): Case/Control not in LD; Middle panels ((d), (e) and (f)): Case/Control in LD; Bottom panels ((g), (h) and (i)): Case-Only not in LD; FE: Fast-Epistasis; AFE: Adjusted FE; Wu: Wu et al. statistic; AWu: Adjusted Wu statistic; IWu: Ideal Wu statistic; WZ: Wellek and Ziegler statistic; JE: Joint Effects statistic. Fig. 4. Chi-squared (1 df) Q-Q plot for Scenario 5d (Rare disease, Recessive effects at both loci). 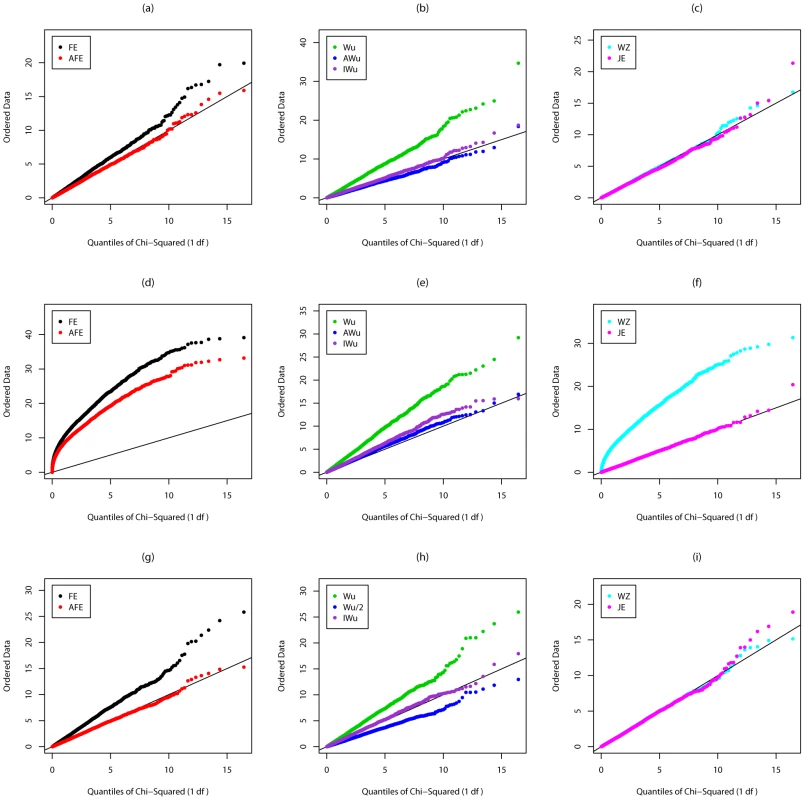
Top panels ((a), (b) and (c)): Case/Control not in LD; Middle panels ((d), (e) and (f)): Case/Control in LD; Bottom panels ((g), (h) and (i)): Case-Only not in LD; FE: Fast-Epistasis; AFE: Adjusted FE; Wu: Wu et al. statistic; AWu: Adjusted Wu statistic; IWu: Ideal Wu statistic; WZ: Wellek and Ziegler statistic; JE: Joint Effects statistic. Evaluation of power
Figure 5 shows power curves for Scenario 6 (RecessiveRecesssive model) for all methods considered, including methods that assume ‘correct’ or ‘incorrect’ knowledge of the true structure of the underlying generating model. The left hand panels show results when there are no main effects, while the right hand panels show results in the presence of a main effect at locus G. We use solid lines to represent methods that have been shown (Figure 1, Figure 2, Figure 3, Figure 4; Figures S1, S2, S3, S4) or would be expected on theoretical grounds to have correct type 1 error. We use dashed lines to represent methods that have been shown (Figure 1, Figure 2, Figure 3, Figure 4; Figures S1, S2, S3, S4) to have incorrect type 1 error under the relevant generating model (and whose ‘power’ should therefore be interpreted cautiously in the light of that fact). In all cases, we find that the highest power among methods that correctly control the type 1 error is seen for ‘optimal’ tests that impose the correct structure, while the lowest power is seen for ‘sub-optimal’ tests that impose the incorrect structure, as might be expected from standard statistical theory. Amongst the other tests, no method consistently outperforms the others; in some cases our joint effects test has highest power, in other cases the adjusted Wu or adjusted or original fast-epistasis tests perform best. The ideal Wu test (in which we assume haplotypes can be estimated without uncertainty) shows generally lower power than the other tests considered, in this scenario.
Fig. 5. Power curves for Scenario 6 (Recessive Recessive). 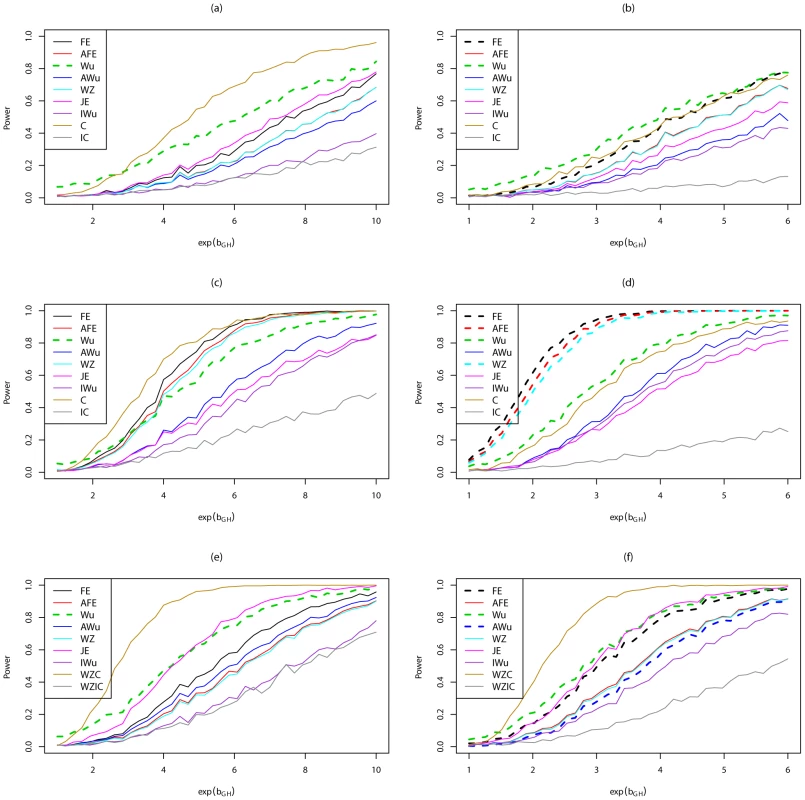
Power to achieve significance level . Top panels ((a) and (b)): Case/Control not in LD; Middle panels ((c) and (d)): Case/Control in LD; Bottom panels ((e) and (f)): Case-Only not in LD; Left hand panels ((a), (c) and (e)): No main effect; Right hand panels ((b), (d) and (f)): Locus G has main effect; FE: Fast-Epistasis; AFE: Adjusted FE; Wu: Wu et al. statistic; AWu: Adjusted Wu statistic; WZ: Wellek and Ziegler statistic; JE: Joint Effects statistic; IWu: Ideal Wu statistic; C: Logistic regression using correct coding; IC: Logistic regression using incorrect ( = RecessiveDominant) coding; WZC: Wellek and Ziegler case-only statistic using correct coding; WZIC: Wellek and Ziegler case-only statistic using incorrect ( = RecessiveDominant) coding. Figure 6 shows power curves for Scenario 7 (DominantDominant model). The original Wu statistic shows apparent highest power, but this observation is tempered by the fact that we know it has inflated type 1 error. Again, highest power among methods that correctly control the type 1 error is generally obtained for ‘optimal’ tests that impose the correct structure, although in some cases this power is closely matched by the adjusted Wu or joint effects tests. The original and adjusted fast-epistasis tests show low power when applied to case/control data. The ideal Wu test also shows generally low power when applied to either case/control or case-only data.
Fig. 6. Power curves for Scenario 7 (Dominant Dominant). 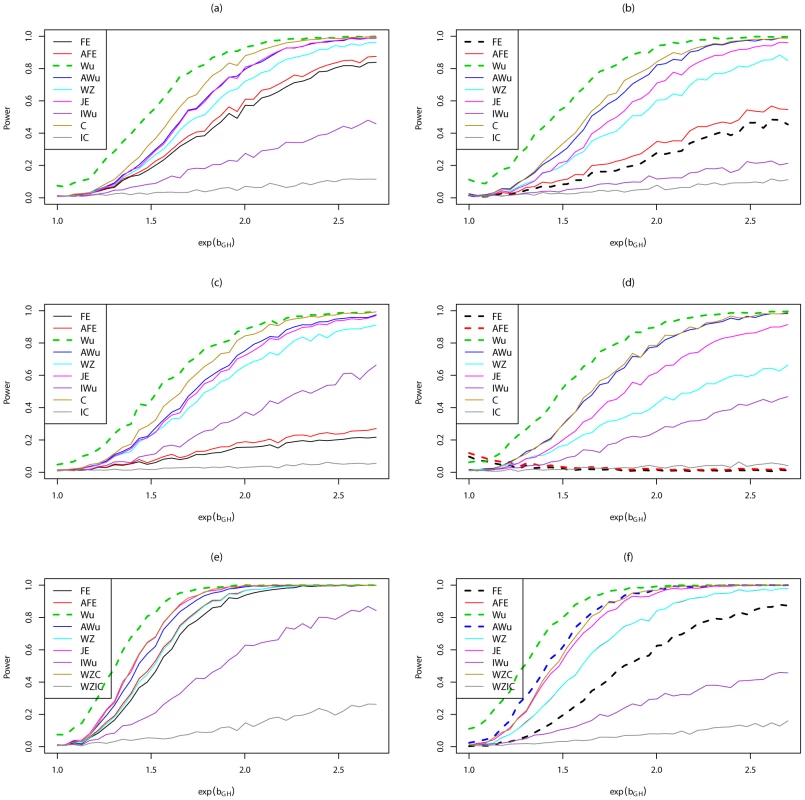
Power to achieve significance level . Top panels ((a) and (b)): Case/Control not in LD; Middle panels ((c) and (d)): Case/Control in LD; Bottom panels ((e) and (f)): Case-Only not in LD; Left hand panels ((a), (c) and (e)): No main effect; Right hand panels ((b), (d) and (f)): Locus G has main effect; FE: Fast-Epistasis; AFE: Adjusted FE; Wu: Wu et al. statistic; AWu: Adjusted Wu statistic; WZ: Wellek and Ziegler statistic; JE: Joint Effects statistic; IWu: Ideal Wu statistic; C: Logistic regression using correct coding; IC: Logistic regression using incorrect ( = DominantRecessive) coding; WZC: Wellek and Ziegler case-only statistic using correct coding; WZIC: Wellek and Ziegler case-only statistic using incorrect ( = DominantRecessive) coding. Figure S5 shows power curves for Scenario 8 (AdditiveAdditive model). Most methods perform fairly similarly, except for analysis under an incorrect model and the ideal Wu test, which both show lower power. For case/control data, in this scenario, the Wellek and Ziegler test slightly outperforms most other tests.
Figure S6 shows power curves for Scenario 9 (DominantAdditive model). Again we find that the highest power among methods that correctly control the type 1 error is seen for ‘optimal’ tests that impose the correct structure, while the lowest power is seen for either for the ideal Wu statistic, or for ‘sub-optimal’ tests that impose the incorrect structure. Amongst the other tests, no method consistently outperforms the others; in some cases the Wellek and Ziegler test shows highest power, whereas in other cases the joint effects or adjusted Wu statistics show highest power.
Table 5 shows the true and estimated haplotype frequencies and correlation measures, as used by several different methods, under one particular setting for simulation Scenario 7. When data is simulated without LD between the loci, we see that, in controls, both the EM algorithm (as used in the Wu et al. and adjusted Wu methods) and the allele counting algorithm (used in PLINK's fast-epistasis method) give very similar results with respect to estimated haplotype frequencies and resulting correlation measures. The correlation measures (along with the Wellek and Ziegler genotype-based correlation coefficient) are correctly estimated as being close to 0. The slight departure from 0 results from the fact that the disease is not particularly rare, and so the presence of an interaction effect will cause unaffected controls, as well as cases, to show some slight correlation between alleles at the two loci.
Tab. 5. True and estimated haplotype frequencies and correlation measures used by different methods. 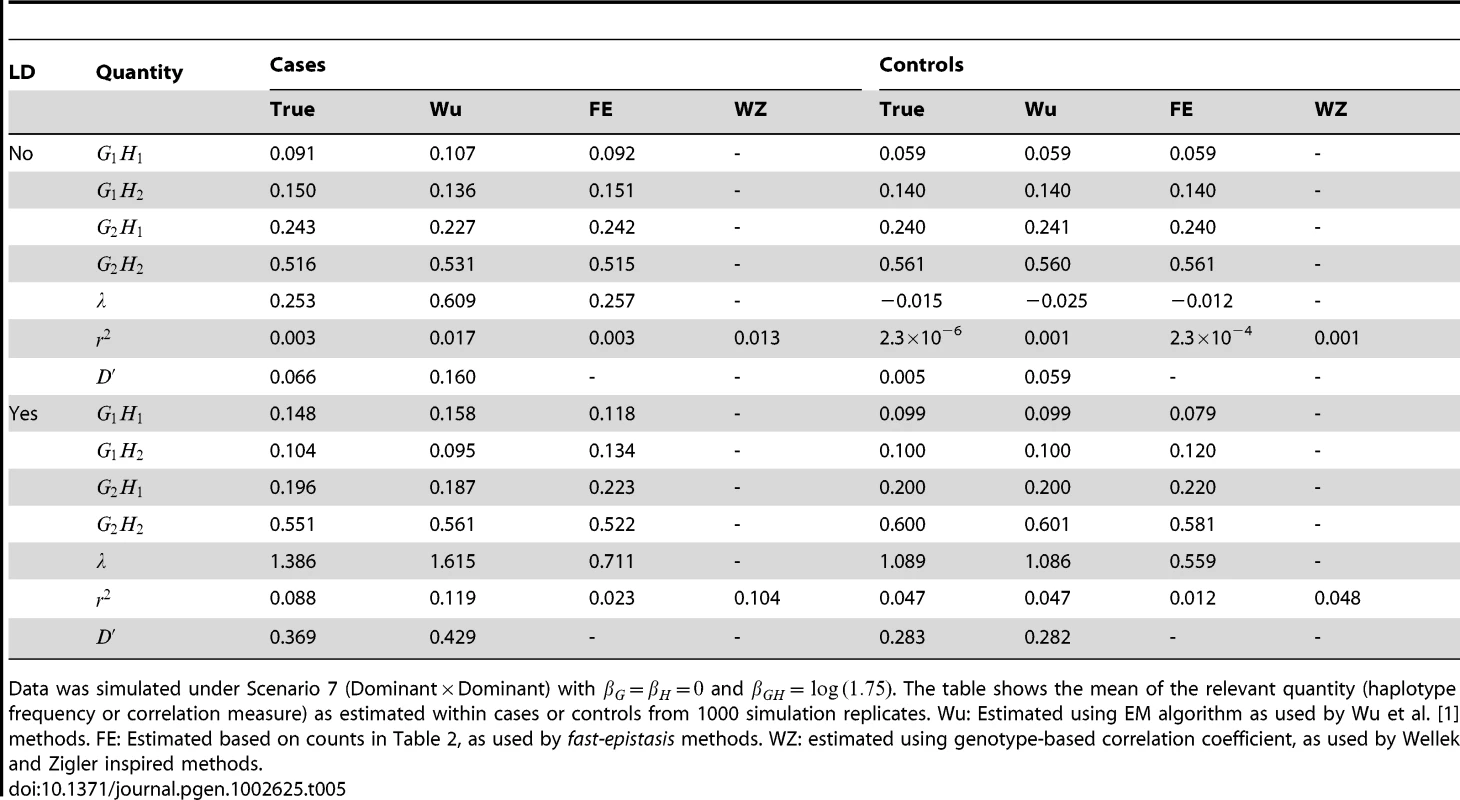
Data was simulated under Scenario 7 (DominantDominant) with and . The table shows the mean of the relevant quantity (haplotype frequency or correlation measure) as estimated within cases or controls from 1000 simulation replicates. Wu: Estimated using EM algorithm as used by Wu et al. [1] methods. FE: Estimated based on counts in Table 2, as used by fast-epistasis methods. WZ: estimated using genotype-based correlation coefficient, as used by Wellek and Zigler inspired methods. In cases (with no LD) however, the story is very different. All methods show correlation between alleles at the two loci, however the haplotype frequencies and resulting correlation measures estimated using PLINK's allele counting algorithm seem to be much closer to the true generating values. The EM algorithm (as used in the Wu et al. and adjusted Wu methods) produces upwardly biased estimates, presumably because of the incorrect (within cases) HWE assumption made. This results in much higher apparent correlation, which could plausibly increase power when testing whether correlation between alleles two loci exists (case-only test) or is different between cases and controls (case/control test)). However, the power of any given test will depend not just on the level of apparent correlation, but also on the estimated variance of the correlation measure used, and our results overall suggest that the bias induced by the incorrect HWE asssumption does not necessarily always translate to a substantially improved power.
In the presence of LD, for controls the EM algorithm (as used in the Wu et al. and adjusted Wu methods) appears to better capture the true haplotype frequencies and resulting correlation measures, while the PLINK's allele counting algorithm produces results that are biased downwards (i.e. towards showing lower levels of correlation). For cases, PLINK's allele counting algorithm produces correlation measures that are biased downwards from the true values, while the EM algorithm produces correlation measures that biased upwards. Given that any analysis in the presence of LD needs to be based on the difference in correlations between cases and controls, it is unclear to what extent these biases will operate to improve power for one method over another, although the results shown in Figure 6 suggest that these bias may partly account for the high power of the adjusted Wu methods in that scenario.
Data application
Figure S7 shows the results from applying the different methods to 5750 SNPs across chromosome 22 genotyped in the WTCCC Crohn's disease dataset. Since SNPs on the same chromosome are likely to be in LD, we limited our analysis to the case/control version of all statistics considered. Given the large number of potential tests performed (16,528,375 pairwise combinations), for the joint effects, fast-epistasis and Wellek and Ziegler inspired methods, we only output results passing a value threshold of 0.001 (although note that, for the fast-epistasis statistic, PLINK in fact only performed a total of 13,818,410 tests that passed its validity criteria).
The QQ plots (Figure S7) show that the joint effects, fast-epistasis and Wellek and Ziegler inspired statistics all follow the expected distribution under the null hypothesis, even in this tail () of the distribution. We also noted that, for these three methods, the proportion of tests falling into this tail was , as expected (data not shown). The most computationally efficient implementation was PLINK, which took approximately 20 minutes to perform 13,818,410 tests. The Wellek and Ziegler and joint effects methods were considerably slower, each taking 20 hours (on the same computer system) to perform 16,528,375 tests. We implemented the Wellek and Ziegler and joint effects statistics through code written by ourselves in R, and so these times could be considerably reduced by re-writing the code (e.g. in C++) and making use of mechanisms for efficient binary data storage.
The original and adjusted Wu methods were prohibitively slow to calculate for all 16,528,375 pairwise combinations, most likely because of the requirement of these methods to estimate haplotype frequencies from unphased genotype data (e.g. via an EM algorithm). (We implemented these methods through code written by ourselves in R; calculation might be achievable in reasonable time through use of more efficient programming in C++, binary data storage and parallel execution on a computer cluster). Figure S7 therefore shows the results for the original and adjusted Wu methods for a subset of 10813 SNP pairs consisting of the first and the thousandth SNP, each paired with all others. Even in this reduced data set, we can see that the adjusted Wu statistic follows the expected distribution under the null hypothesis while the original Wu statistic shows an inflated distribution, in line with the results we found in our computer simulations.
The results in Figure S7 do not provide any strong evidence for the existence of interactions between SNPs on chromosome 22 in the WTCCC Crohn's data. However, it is of interest to see to what extent the different methods implicate the same ‘top SNP pairs’. Figure S8 plots the observed test statistics for the joint effects, fast-epistasis and Wellek and Ziegler inspired statistics against one another. The results from these three methods are seen to be broadly correlated, with the same SNP pairs tending to fall at the extreme of the distribution, regardless of which method is used.
Since we were unable to calculate the Wu and adjusted Wu statistics for all pairs of SNPs, at the suggestion of a reviewer, we used another approach for calculating these statistics, which we hoped would be computationally quicker. We used a phasing algorithm to infer haplotypes across chromosome 22, for each individual. We carried out this step using the program SHAPEIT [35], which has the advantage of outputting for each individual not just a single “most likely” haplotype configuration, but additionally allows one to store the uncertainty and sample a set of haplotype configurations. We sampled 100 replicate haplotype configurations for each individual. Since the idea of the Wu method is to compare ‘apparent LD’ within cases to that within controls, we initially carried out the phasing in case and control groups separately, although we later compared our results to those obtained when phasing the cases and controls together.
Having generated 100 replicates of phased haplotypes, we then calculated, for each pair of SNPs, the mean (over the 100 replicates) haplotype frequencies in cases and controls. (The haplotype frequencies within each replicate were calculated simply by counting resolved case and control haplotypes). We used these mean haplotype frequencies in the formulae for the Wu and adjusted Wu statistics (Equations 7 and 9 respectively). Note that these formulae were derived on the basis of sampling theory under the assumption of a certain number of observed haplotypes, and it is unclear whether the same theoretical arguments should apply to haplotype frequencies that have been estimated in a different way. In particular, SHAPEIT uses a hidden Markov model that is motivated by population genetics principles, resulting in a greater borrowing of information across SNPs and individuals than is used in the other approaches. This fact, together with the fact we averaged (over 100 replicates), suggests that the haplotype frequencies (and thus and ) estimated from SHAPEIT may be more accurate and less variable than those estimated in the other approaches, thus requiring a smaller variance in the denominator of the test statistic. To address this issue, we used an additional strategy of calculating the variance directly from the 100 replicates. Within each replicate, we calculated the haplotype frequencies and log odds ratios and . We then calculated the sample mean and variance of and over the 100 replicates and constructed a ‘SHAPEIT variance-based Wu (SVBW) test statistic’:
Figure S9 shows QQ plots for the Wu and adjusted Wu test statistics (Equations 7 and 9) applied to the mean estimated haplotype frequencies from SHAPEIT, for the subset of 10813 SNP pairs consisting of the first and the thousandth SNP, each paired with all others. The test statistics (shown in red and black) are seen to be considerably deflated in comparison to the expected distribution, suggesting that the variance of the SHAPEIT-derived haplotype frequencies is indeed considerably lower than that implied by Equations 7 and 9. We noticed, however, that the test statistics appeared to be approximately half their expected value. We therefore constructed an alternative ‘SHAPEIT mean-based Wu (SMBW) test statistic’:(21)which can be seen to be equivalent to the original Wu case/control statistic, but under the assumption of double the number of haplotypes. The SMBW test statistics (shown in green) are seen to closely follow the expected distribution, suggesting that variance of the SHAPEIT-derived haplotype frequencies is indeed equivalent to what would be obtained from observing twice the number of haplotypes. We consulted the description of the algorithm used by SHAPEIT [35] and noticed that it involves an iterative procedure of updating an individual's current haplotype configuration by sampling haplotypes from a set of currently resolved haplotypes (for the other individuals in the data set), in such a way that recombination and mutation events are allowed for. This means that, for SNPs close together, the sampling procedure would effectively be sampling alleles from currently-resolved haplotypes (where is the number of individuals in the data set) while for SNPs that are far apart, a recombination event is virtually guarranteed and so the sampling procedure is effectively sampling from haplotypes constructed by sampling the alleles at each SNP independently. Since the majority of our pairwise tests involve SNPs that are far apart, the majority of the tests will indeed closely correspond to effectively observing haplotypes. Note that this argument is quite similar to the argument that could be used to justify the construction of PLINK's fast-epistasis statistic on the basis of alleles.
Logically, one would expect that the variance could be estimated even better by allowing for the actual recombination distance between each pair of SNPs, so that SNPs that are closer together are considered to have a probability of undergoing a recombination and thus being sampled from haplotypes, and probability of not undergoing a recombination and thus being sampled from haplotypes. (For definition of , see [35]). However, we found implementation of this approach resulted in test statistics that did not follow the expected (on 1 df) distribution quite as well as simply assuming or haplotypes (data not shown). One possible explanation is that the iterative nature of the SHAPEIT algorithm means that even SNPs that lie close together are likely to be subject to a recombination event at some point during the procedure, generating closer to effective haplotypes. Further work, beyond the scope of this paper, would be required to follow up the explanation for these observations in more detail.
Figure S10 (Panel (a)) shows the QQ plot for the SHAPEIT variance-based Wu (SVBW) test statistic, for the subset of 10813 SNP pairs consisting of the first and the thousandth SNP, each paired with all others. Although the majority of the points do lie on the expected line, there are a number of outliers. We noticed that the most severe outliers corresponded to pairs of SNPs that lie within 1 cM of one another (shown in red), suggesting that the variance of the haplotype frequencies within short regions may perhaps be under-estimated by the SHAPEIT algorithm. (Another explanation is that these are true interactions and/or haplotype effects, however this seems a little unlikely given that they are not identified by any other method). We removed all pairs of SNPs that lie within 1 cM of one another from both the SVBW and SMBW results, which resulted in test statistics that followed the expected distribution more closely (Panels (b) and (c)). Panel (d) shows a comparison between the resulting SMBW and SVBW test statistics, showing how extremely similar they are. Figure S11 Panels (a) and (b) show a comparison between the SMBW and SVBW statistics and the AWu statistic, while Panels (c) and (d) show a comparison between the SMBW and SVBW statistics and the JE statistic. Although these different test statistics are by no means identical, they are seen to be broadly correlated, as expected.
Figure S12 shows a comparison of the SMBW (left hand panels) and SVBW (right hand panels) results from haplotypes estimated by applying SHAPEIT to cases and controls separately (y axes) or together (x axes). Points marked in red on the top panels correspond to SNP pairs where the SNPs are less than 1 cM apart; these pairs are seen to generate outliers for the SVBW test regardless of whether SHAPEIT is applied to cases and controls separately or together. These outliers do not occur with the SMBW test when SHAPEIT is applied to cases and controls together. The bottom panels repeat these plots, but with SNP pairs where the SNPs are less than 1 cM apart removed. Overall the results from applying SHAPEIT to cases and controls separately (y axes) or together (x axes) are seen to be highly correlated, particularly for the SMBW test. We investigated the outliers (where the results were very different according to whether cases and controls were phased separately or together) and noticed that the vast majority of these corresponded to SNPs whose minor allele frequency is close to 0.5, and for which there had been a swap with respect to which allele was designated as the minor allele between the case and control groups, when phased separately. This resulted in an incorrect matching of haplotypes between case and control groups, resulting in an incorrect test statistic. (Interestingly, the 7 outliers for which the test statistic is close to 0 when the cases and controls were phased separately are also seen as outliers when compared to the AWu and JE tests (Figure S11), indicating that the results from SHAPEIT applied to cases and controls together are concordant with the AWu and JE results). We found that the allele swap problem had occurred in 46 out of the 5750 SNPs considered i.e. just under 1% of the results presented from applying SHAPEIT to cases and controls separately were incorrect. This might suggest that the strategy of phasing cases and controls together is more reliable, although in practice one could avoid this problem when phasing cases and controls separately by performing a more careful check at the analysis stage. Intuitively, one might expect that the strategy of phasing cases and controls separately might be more powerful when constructing tests that are based on haplotype differences between cases and controls, but a detailed comparison of the relative power of these two approaches would required further investigation.
Although the SHAPEIT approaches appear to result in more accurate haplotype estimation than the EM algorithm-based Wu and AWu approaches, generating haplotype frequency estimates that can (with care) be translated into Wu-like interaction tests, in our hands, implementation of these approaches was not computationally faster than the original Wu and AWu methods. Although generation of 100 replicates of phased chromosome 22 haplotypes in SHAPEIT was relatively fast (taking around 28 hours on our system), our program for generating the resulting SMBW and SVBW test statistics ended up taking about 3 seconds per SNP pair. (For each SNP pair we needed to read in – or store in memory – 100 replicates of phased haplotypes for each individual, in order to pick out the required alleles at the two SNPs, and then calculate haplotype frequencies, and , within each replicate, followed by the mean and variance of these quantities across replicates). No doubt more efficient programming, binary data storage and implementation on a computer cluster could considerably speed up this procedure. Given the close correspondence between the SMBW and SVBW tests, together with the better performance of SMBW for SNPs that lie close together, a natural first step might be to initially focus on SMBW alone, for which and within each replicate, and all variances across replicates, would not need to be calculated.
Discussion
Here we have investigated, through theoretical derivation, computer simulations and a real data example, the properties of several previously-proposed statistics for performing genome-wide interaction analysis using case/control or case-only data [1], [30], together with a number of alternative statistics proposed by ourselves and others [33], [34]. Our main finding is that the statistics proposed by Wu and colleagues [1] show substantially increased type 1 error due to the incorrect variance estimates used (Equations (6) and (7)) which do not account for the uncertainty induced when estimating phased haplotype frequencies from unphased genotype data. This inflation in type 1 error can be corrected by using a variance estimate that accounts for this uncertainty, as in our adjusted Wu statistics. All other methods investigated appear to show adequate control of type 1 error under the null hypothesis of no genetic effects (main effects or interactions), although several methods (including the fast-epistasis method implemented in PLINK [30] and the Wellek and Ziegler method [33], [34]) can show increased type 1 error when there is a main effect at one or both loci, particularly if there is also LD. Only the ideal Wu method and our new joint effects statistics achieve consistent control of type 1 error in the presence of a main effect at just one of the loci.
In terms of power, comparison of the different methods is somewhat complicated by the fact that several of the methods show increased type 1 error in different circumstances. However, even when comparing methods that control the type 1 error rate in a given situation, no method consistently outperforms all others. Generally high power over a range of scenarios is exhibited by the Wellek and Ziegler statistics [33], [34] and by our new joint effects statistics and adjusted Wu statistics. Given that, out of these options, only the joint effects statistics achieve adequate control of type 1 error in the presence of a single main effect, this might suggest that the joint effects tests would be the overall preferred option. Although the ideal Wu method also shows adequate control of type 1 error in the presence of a single main effect, observation of known haplotypes, as required by this method, is unachievable in practice. Even if it were achievable, e.g. through experimental assays that allow determination of haplotypes, or through the use of larger numbers of markers to help infer phase between the two SNPs in question, Figure 5 and Figure 6 and Figures S5 and S6 show that the power achieved by the ideal Wu approach is generally lower than for other approaches. This slightly counter-intuitive result might be due to the fact that the ideal Wu method is not affected by the bias that results from incorrectly assuming HWE when estimating haplotype frequencies in cases, a bias that can potentially increase power.
Somewhat surprisingly, many of our results appear to contradict results presented by Wu and colleagues [1] who found in simulations (using similar generating models to those considered here) and application to real data that their method gave adequate control of type 1 error and higher power than competing methods (including logistic regression analysis under the correct model). We have been unable to fully determine the reason for these discrepencies, even after discussion with the authors of [1], although our discussions have highlighted some possible explanations. With respect to the simulation results, our current understanding is that the simulations performed by Wu et al. did not, in fact, include any consideration of haplotype uncertainty (their simulations simply assumed haplotypes could be observed without error – as, in a simulated data set, they can). This explains the apparently correct type 1 error observed by Wu et al. but it means that all their simulations (of both type 1 error and power) are highly misleading with respect to illustrating how their method might perform in practice (where haplotype uncertainty will invariably exist, particularly at loci that are not in strong LD). It also does not explain the difference in power we see compared to Wu et al. when we also assume haplotypes can be observed without error (our ‘ideal’ Wu statistics). We speculate that one possible explanation for this difference might be that Wu et al. assumed in their simulations that haplotypes come together independently in cases (which is true under a multiplicative haplotype model [28], but not under recessive or dominant models). It is unclear what effect such an erroneous assumption would have on the power of the different methods, but it might possbly explain why Wu et al. found their method to give consistently higher power than logistic regression analysis under a correct model, whereas we find (as might be expected from statistical theory) that logistic regression analysis under a correct model gives generally higher power than the adjusted or ideal Wu statistics.
The explanation of these simulation discrepencies also does not explain why Wu et al. found correct (or possibly slightly deflated) type 1 error in analysis of real data (see QQ plot shown in Figure 1 of Wu et al. (2010) [1]), whereas in our own application of the original Wu et al. (2010) method to real data (Figure S7), we found the same general inflation of test statistics as we observe in computer simulations. One possibility is that Wu et al. inadvertedly divided by a factor of two when using their formulae (our Equations (6) and (7)) to calculate the desired test statistics. This would result in a test that would approximately correspond to our adjusted Wu statistic. In any case, unless or until these issues can be resolved, we recommend use of our new joint effects or adjusted statistics, and urge caution when using Wu et al.'s [1] originally-proposed statistics, on account of the inflated error rate that can result.
We have focussed in this communication on methods that test for interaction per se i.e. that test (or attempt to test) the interaction term in a linear model (such as Equation 1). As mentioned previously, if one prefers to test combinations of terms (e.g. in order to implement tests of association allowing for interaction [20], [17]) one may do so by combining a test of the interaction term with some test of the other terms [22]. It is well-known (and indeed can be seen from Figure 5 and Figure 6 and Figures S5 and S6) that case-only tests are more powerful than case/control tests for testing interaction, provided there is no population-level correlation between the two variables being tested. Although such an assumption should in principal be reasonable when testing genetic variants that are located sufficiently far apart as to be expected not to show LD, in practice GWAS data often does display long-range allelic association [17], possibly due to population structure [26] or other confounding influences. This suggests that, in application to GWAS data, the case/control versions of the statistics described here might be preferred over the case-only versions, in spite of their lower power. Alternatively, construction of weighted combinations of the case-only and case/control statistics [36]) might prove a more powerful approach. Several authors have recently proposed the use of retrospective likelihoods [37], [26] that can increase power by exploiting an assumption of gene-gene independence in the underlying population (or in controls, if the disease is rare or controls unselected). These methods have been used, for example, in a conditional search exercise exploiting known loci for prostate cancer in a multi-stage GWAS [38]. The advantage of these frameworks is that they allow the incorporation of covariates (such as principal components scores) to account for population stratification, as well as allowing a wider class of tests. Since the methods described here can all be formulated in terms of (prospective) linear or logistic regression models (see Text S3 and S4), in theory such approaches could be applied to the tests described here. However, an advantage of the current formulations is that closed-form expressions for the tests are available, which makes them attractive when carrying out all pairwise interaction scans in GWAS, on account of the fact that the tests are rapidly computed.
R code for implementing the joint effects, Wellek and Ziegler and adjusted Wu statistics described in this manuscript is available on request from the authors.
Supporting Information
Zdroje
1. WuXDongHLuoLZhuYPengG 2010 A novel statistic for genome-wide interaction analysis. PLoS Genet 6 e1001131 doi:10.1371/journal.pgen.1001131
2. WTCCC 2007 Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447 661 678
3. FraylingTMTimpsonNJWeedonMNZegginiEFreathyRM 2007 A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316 889 894
4. ToddJWalkerNCooperJSmythDDownesK 2007 Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 39 857 864
5. ZegginiEScottLJSaxenaRVoightBFMarchiniJL 2008 Meta-analysis of genomewide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 40 638 645
6. BarrettJCHansoulSNicolaeDLChoJHDuerrRH 2008 Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet 40 955 962
7. MellsGFFloydJAMorleyKICordellHJFranklinCS 2011 Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet 43 329 332
8. ManolioTACollinsFSCoxNJGoldsteinDBHindorffLA 2009 Finding the missing heritability of complex diseases. Nature 461 747 753
9. YangJBenyaminBMcEvoyBPGordonSHendersAK 2010 Common SNPs explain a large proportion of the heritability for human height. Nat Genet 42 565 569
10. YangJManolioTAPasqualeLRBoerwinkleECaporasoN 2011 Genome partitioning of genetic variation for complex traits using common SNPs. Nat Genet 43 519 525
11. SiemiatyckiJThomasDC 1981 Biological models and statistical interactions: an example from multistage carcinogenesis. International Journal of Epidemiology 10 383 387
12. ThompsonWD 1991 Effect modification and the limits of biological inference from epidemiologic data. Journal of Clinical Epidemiology 44 221 232
13. PhillipsPC 1998 The language of gene interaction. Genetics 149 1167 1171
14. CordellHJ 2002 Epistasis: what it means, what it doesn't mean, and statistical methods to detect it in humans. Hum Molec Genet 11 2463 2468
15. McClayJLvan den OordEJ 2006 Variance component analysis of polymorphic metabolic systems. J Theor Biol 240 149 159
16. PhillipsPC 2008 Epistasis–the essential role of gene interactions in the structure and evolution of genetic systems. Nat Rev Genet 9 855 867
17. CordellHJ 2009 Detecting gene-gene interactions that underlie human diseases. Nat Rev Genet 10 392 404
18. ClaytonDG 2009 Prediction and interaction in complex disease genetics: experience in type 1 diabetes. PLoS Genet 5 e1000540 doi:10.1371/journal.pgen.1000540
19. WangXElstonRZhuX 2010 The meaning of interaction. Hum Hered 70 269 277
20. KraftPYenYCStramDOMorrisonJGaudermanWJ 2007 Exploiting gene-environment interaction to detect genetic associations. Hum Hered 63 111 119
21. GaudermanWJ 2002 Sample size requirements for association studies of gene-gene interaction. Am J Epidemiol 155 478 484
22. ChapmanJClaytonD 2007 Detecting association using epistatic information. Genet Epidemiol 31 894 909
23. PiegorschWWWeinbergCRTaylorJA 1994 Non-hierarchical logistic models and case-only designs for assessing susceptibility in population-based case-control studies. Statistics in Medicine 13 153 162
24. YangQKhouryMJSunFFlandersWD 1999 Case-only design to measure gene-gene interaction. Epidemiology 10 167 170
25. WeinbergCRUmbachDM 2000 Choosing a retrospective design to assess joint genetic and environmental contributions to risk. Am J Epidemiol 152 197 203
26. BhattacharjeeSWangZCiampaJKraftPChanockS 2010 Using principal components of genetic variation for robust and powerful detection of gene-gene interactions in case-control and case-only studies. Am J Hum Genet 86 331 342
27. ZaykinDVMengZEhmMG 2006 Contrasting linkage-disequilibrium patterns between cases and controls as a novel association-mapping method. Am J Hum Genet 78 737 746
28. SasieniP 1997 From genotypes to genes: doubling the sample size. Biometrics 53 1253 1261
29. BrownA 1975 Sample sizes required to detect linkage disequilibrium between two or three loci. Theoretical Population Biology 8 184 201
30. PurcellSNealeBTodd-BrownKThomasLFerreiraMA 2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
31. LewontinR 1998 On measures of gametic disequilibrium. Genetics 120 849 852
32. BaldingDJ 2006 A tutorial on statistical methods for population association studies. Nat Rev Genet 7 781 791
33. WellekSZieglerA 2009 A genotype based approach to assessing the association between single nucleotide polymorphisms. Hum Hered 67 128 139
34. Kam-ThongTCzamaraDTsudaKBorgwardtKLewisCM 2011 EPIBLASTER-fast exhaustive two-locus epistasis detection strategy using graphical processing units. Eur J Hum Genet 19 465 471
35. DelaneauOMarchiniJZaguryJF 2011 A linear complexity phasing method for thousands of genomes. Nature Methods doi:10.1038/nmeth.1785
36. MukherjeeBChatterjeeN 2008 Exploiting gene-environment independence for analysis of casecontrol studies: an empirical Bayes-type shrinkage estimator to trade-off between bias and efficiency. Biometrics 64 685 94
37. ChatterjeeNCarrollRJ 2005 Semiparametric maximum likelihood estimation exploiting geneenvironment independence in case-control studies. Biometrika 92 399 418
38. CiampaJYeagerMAmundadottirLJacobsKKraftP 2011 Large-scale exploration of gene-gene interactions in prostate cancer using a multistage genome-wide association study. Cancer Res 71 3287 3295
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



